- 1Department of Critical Care Medicine, People's Hospital of Ningxia Hui Autonomous Region, Ningxia Medical University, Yinchuan, China
- 2Department of Cardiovascular Medicine, Cardiovascular and Cerebrovascular Disease Hospital of General Hospital of Ningxia Medical University, Yinchuan, China
- 3Department of Cardiovascular Medicine, The Fifth People's Hospital of Ningxia, Shizuishan, China
Purpose: To develop a risk prediction model for left ventricular thrombus (LVT) formation in patients with acute ST-segment elevation myocardial infarction (STEMI).
Patients and methods: We performed a retrospective analysis of patients with STEMI in our hospital between October 2017 to October 2020. According to transthoracic echocardiography, these patients were included in the LVT group (n = 50) or no-LVT group (n = 130). Clinical data were collected from both groups. The comparison between groups, single-factor logistic regression analysis, Lasso regression analysis and multi-factor logistic regression analysis were performed successively to screen the risk factors and to establish risk prediction models. After evaluation and internal verification, we obtained an optimal risk prediction model. A nomogram was constructed to visualize the optimal model.
Results: The risk prediction model contained seven variables including MCV (OR = 1.251, 95% CI = 1.021–1.531, P = 0.030), D-Dimer (OR = 9.798, 95% CI = 2.630–36.503, P = 0.001), CRP (OR = 1.033, 95% CI = 1.011–1.055, P = 0.003), LVEF (OR = 0.903, 95% CI = 0.819–0.995, P = 0.040), A wave velocity (OR = 0.044, 95% CI = 0.002–0.906, P = 0.043), pericardial effusion (OR = 16.926, 95% CI = 2.767–103.522, P = 0.002) and anterior wall infarction (OR = 12.275, 95% CI = 2.136–70.548, P = 0.005). The C-index value was 0.966 and the area under the ROC curve was 96.6% (95% CI = 0.9399–0.9914). Simple cross-validation, k-fold, leave-one-out, and bootstrap analyses were used to internally verify the model. The accuracy of the model were 0.926, 0.9, 0.9, and 0.899, and Kappa values were 0.807, 0.744, 0.744, and 0.739. The area under the ROC curve was 94.7%, as verified by bootstrapping.
Conclusion: MCV, D-dimer level, CRP level, LVEF, A-wave velocity, pericardial effusion, and anterior wall infarction were independently related to the occurrence of LVT in STEMI at the acute stage. The multivariate logistic regression risk prediction model developed in this study demonstrates good discrimination and calibration, enabling preliminary risk stratification for LVT in patients with acute STEMI.
1 Introduction
ST-segment elevation myocardial infarction (STEMI) is a clinical syndrome characterized by myocardial ischemia symptoms, persistent ST segment elevation and elevated myocardial injury markers (1). With the early revascularization such as thrombolysis and percutaneous transluminal coronary intervention (PCI), the mortality rate of patients decreases, but its complications, such as papillary muscle rupture, heart rupture and ventricular aneurysm, still affect the long-term prognosis of patients (2). LVT is one of the complications of STEMI (3). The formation of LVT is related to the classic Virchow's triad, that is, vascular endothelial injury, hypercoagulable state of blood and abnormal hemodynamics. These three preconditions of thrombosis are reflected to different degrees in patients with STEMI (4). It has been reported that, before thrombolytic therapy, the incidence of LVT was 54% (5), and the incidence of LVT decreased to 28% after thrombolysis (6). In recent years, with the improvement in early vascular recanalization technology and patient management, the incidence of LVT has further decreased, ranging from 0.7% to 8.4% (7, 8).
LVT can fall off the wall of the ventricle and reach various tissues and organs with blood flow, which obviously increases the risk of systemic embolism, ischemic stroke, and cardiovascular adverse events, and has a profound impact on the survival and prognosis of patients. It is reported that LVT is an independent predictor of major cardiovascular adverse events in one year, and patients with LVT have a higher incidence of major cardiovascular adverse events than those without LVT (21.7% vs. 10.3%) (9). Patients with LVT often have no characteristic clinical manifestations, which are mostly found in clinical routine echocardiography. They can also be found when infarctions in other organs occur, such as stroke, which may have caused serious adverse clinical outcomes. Therefore, it is extremely important to identify LVT-high-risk patients early and actively intervene clinically.
There have been many studies on the risk factors of LVT formation, and the reported risk factors include extensive anterior wall infarction, ventricular aneurysm, decreased left ventricular ejection fraction, and severe abnormal wall motion (7, 8, 10). However, at present, there is no recognized index with satisfactory prediction ability or a related risk prediction model. The risk prediction model based on logistic regression or COX regression provides a certain value for early identification, intervention, and prognosis evaluation of patients at high risk of some diseases in the clinic and is widely used in related research in medical and biological fields. In this study, based on LASSO regression and logistic regression, a risk prediction model of LVT in acute STEMI patients was established, and a nomogram was drawn to visualize the model for clinical use, which provided a reference for the clinical prediction of LVT.
2 Materials and methods
2.1 Patients and design
We retrospectively analyzed patients with STEMI at the Ningxia Medical University General Hospital and the Department of Cardiology Heart-Brain-Vascular Hospital between October 2017 and October 2020. The clinical data of the two groups were collected from the electronic medical record system. The diagnostic criteria for STEMI are at least two contiguous leads with ST-segment elevation ≥ 2.5 mm in men, ≥1.5 mm in women in leads V2–V3 and/or ≥1 mm in the other leads, and symptoms of myocardial ischemia or an increase and/or decrease in cTn values with at least one value above the 99th percentile URL. According to in-hospital transthoracic echocardiography, the patients were diagnosed with LVT. (Echocardiography showed an echo-dense mass in the left ventricle, which had a clear edge and was adjacent to the non-energetic myocardium. It could be clearly identified in the whole cardiac cycle, and could be distinguished from the muscle trabecula) (11). Patients with myocardial infarction, arterial or venous thrombosis, thrombotic hematological diseases, dilated cardiomyopathy and severe valve diseases in the past were excluded.
2.2 Variable extraction
The clinical data of the two groups were collected from the electronic medical record system. Information on the following indices was extracted: baseline data (including age, sex, body mass index, blood pressure at admission, and basic diseases such as hypertension, diabetes, abnormal lipid metabolism, smoking history, and drinking history), laboratory data (blood routine, biochemical indexes, coagulation indices, thyroid function, CRP, glycosylated hemoglobin, NT-Pro BNP), echocardiographic data (LVEDD, LVEDS, LVEF, LVFS, abnormal regional wall motion, pericardial effusion, left ventricular aneurysm, mitral regurgitation, and echocardiographic diagnosis of LVT from the onset time of chest pain), PCI, and other information (location of criminal blood vessels, number of criminal blood vessels, infarct location, and Killip grade at admission).
2.3 Statistical analysis
The collected data were sorted and statistically analyzed using SPSS 25.0, and R version 4.0.4. Continuous variables are expressed as mean ± standard deviation (x bar ± SD), and normality tests were performed. The t-test was used for distributed continuous variables, and the nonparametric rank-sum test was used for non-normally distributed continuous variables to compare the differences between groups. Categorical data are expressed as frequencies (percentages), and the chi-square test was used to compare differences between groups. P < 0.05 was considered statistically significant. The univariate logistic regression, Lasso logistic regression and multivariate logistic regression were successively performed to preliminarily establish two logistic regression models. Internal evaluation and verification were performed using the two established risk prediction models to obtain the optimal risk prediction model. Based on the optimal model, a nomogram was drawn to visualize the optimal model.
3 Results
There were about 3,096 cases with STEMI and 50 were diagnosed with LVT. The 50 patients diagnosed with LVT were included in the case group. And we Use the SORTBY and RANDARRAY functions in Excel to randomly select 130 STEMI patients without LVT as the control group.
3.1 Comparison of clinical date between groups
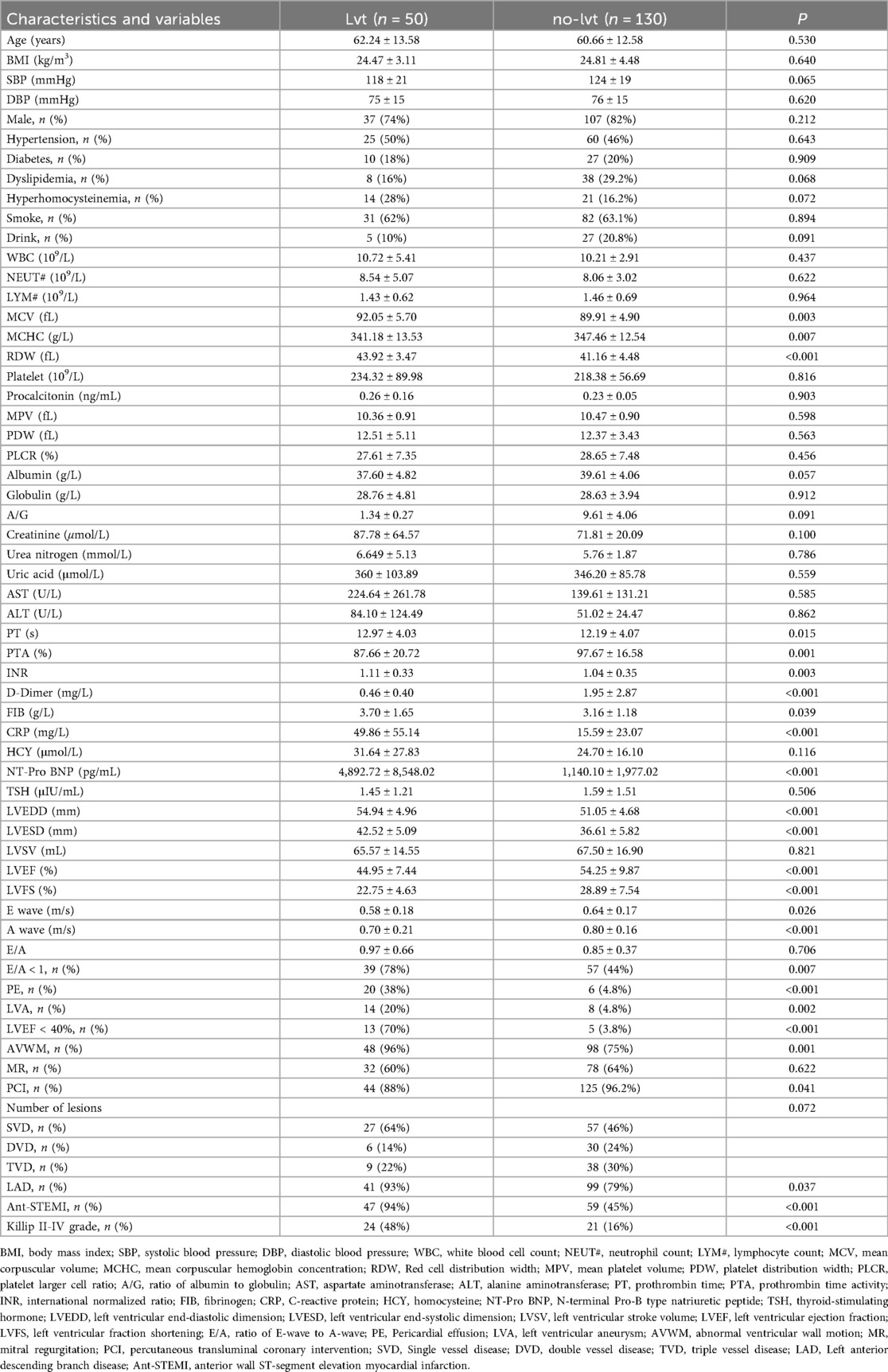
The case group included 50 patients and the control group included 130 patients. There were no significant differences in age, sex, body mass index, blood pressure at admission, hypertension, diabetes, dyslipidemia, hyperhomocysteinemia, smoking, or drinking history between the two groups. Mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), red cell distribution width (RDW), prothrombin time (PT), prothrombin time activity (PTA), D-Dimer, international normalized ratio (INR), fibrinogen (FIB), C-reactive protein (CRP), N-terminal Pro-B type natriuretic peptide (NT-Pro BNP), left ventricular end-diastolic dimension (LVEDD), left ventricular end-systolic dimension (LVESD), left ventricular ejection fraction (LVEF), LVEF <40%, left ventricular fraction shortening (LVFS), A-wave velocity, E-wave velocity, E/A <1, abnormal wall motion, pericardial effusion, left ventricular aneurysm, percutaneous transluminal coronary intervention (PCI), left anterior descending branch lesion, anterior wall infarction and Killip grade II-IV at admission showed significant differences between the two groups (P < 0.05) (Table 1).
3.2 Univariate logistic regression
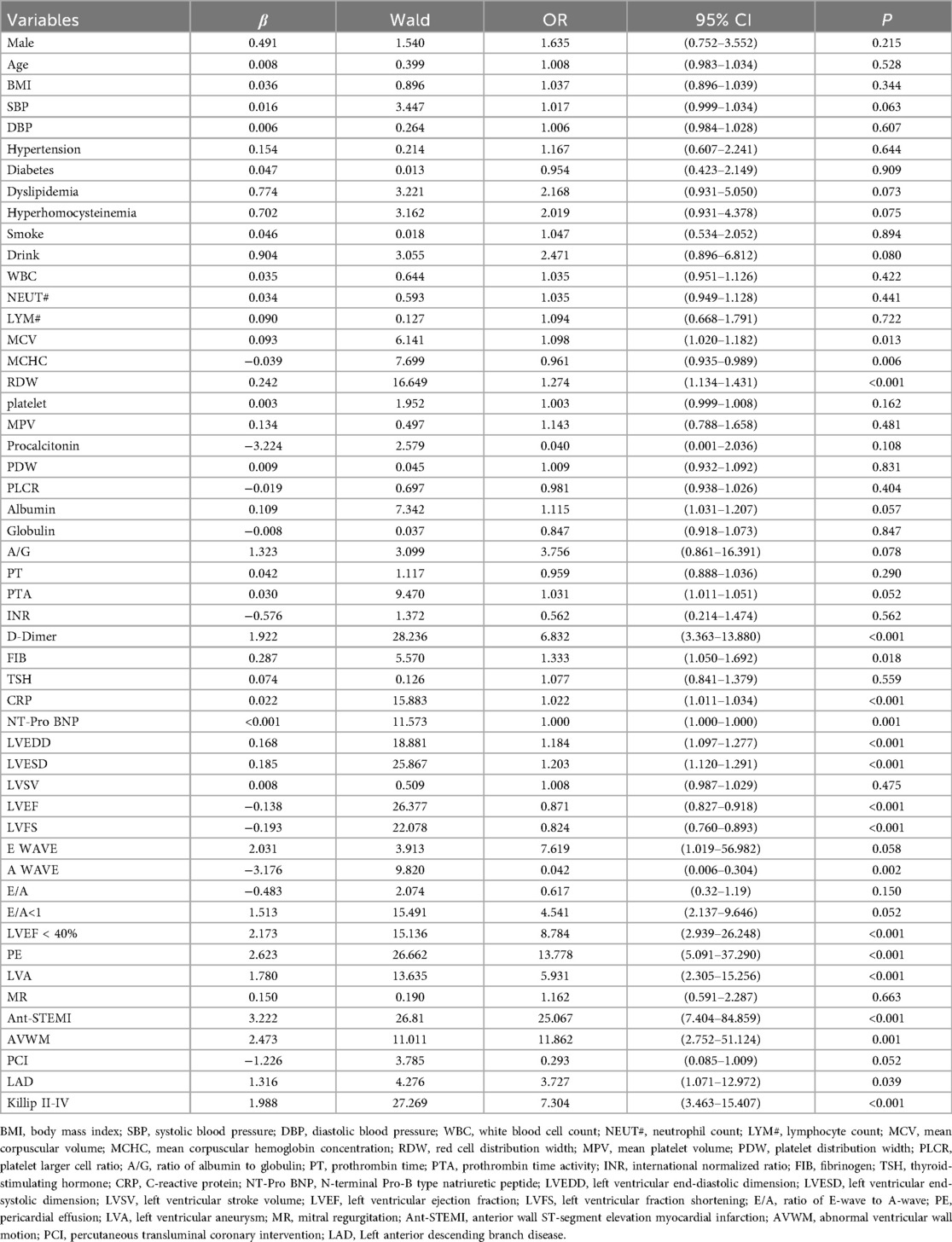
The results of the univariate logistic regression analysis showed that 19 variables, including MCV, MCHC, RDW, D-dimer, FIB, CRP, NT-pro BNP, LVEDD, LVESD, LVEF, LVEF < 40%, LVFS, A wave velocity, pericardial effusion, left ventricular aneurysm, abnormal wall motion, anterior wall infarction, left anterior descending artery disease, and Killip classification II–IV on admission, were associated with LVT formation in STEMI patients at the acute stage (Table 2).
3.3 Construction of the risk prediction model
3.3.1 Lasso logistic regression
The LASSO regression for the 19 variables and the cross-validation of the LASSO regression results for all lambda values was shown in Figure 1. The left dashed line is the λ value with the smallest MSE, and the right dashed line is the λ value corresponding to the combination of variables with the least MSE in the variance range. According to the two λ values, the regression coefficient of each variable after regularization can be obtained, in which some variable coefficients are compressed to zero, and the variables with different coefficients corresponding to the two λ values are retained. Two variable combinations were formed, which are represented by combinations 1 and 2.
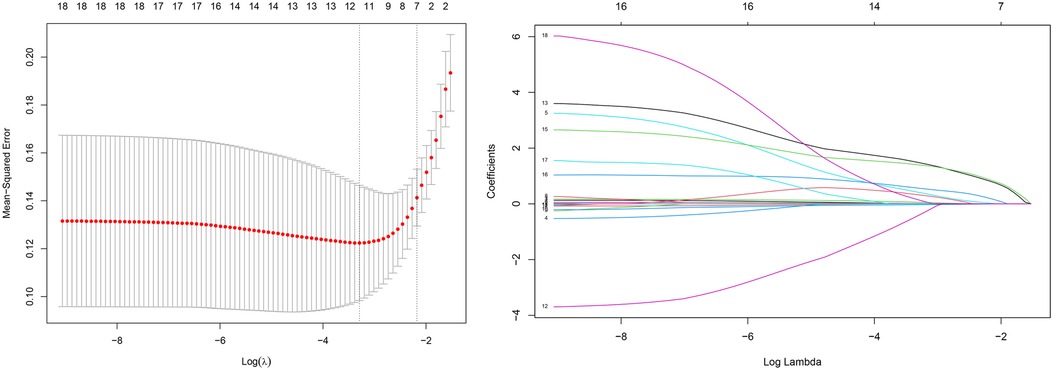
Figure 1. Variable screening based on lasso regression. (left) Characterization of the variation of variable coefficients; (right) The process of selecting the optimal value of the parameter λ in the Lasso regression model by the cross-validation method.
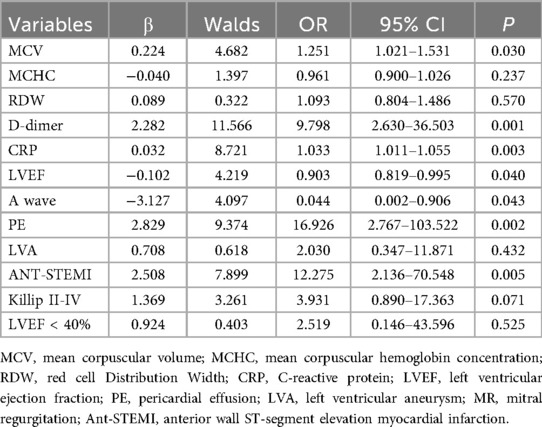
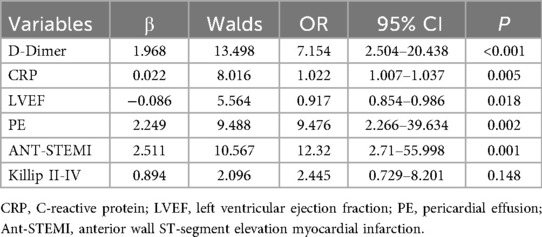
Combination 1 contained 12 variables (MCV, MCHC, RDW, D-dimer, CRP, LVEF, A wave, PE, LVA, ANT-STEMI, Killip II-IV, and LVEF < 40%), and combination 2 contained six variables (D-dimer, CRP, LVEF, PE, ANT-STEMI, and Killip II–IV).
3.3.2 Multivariate logistic regression model construction
Multivariate logistic regression analysis was performed on two variable combinations. The results of multivariate logistic regression analysis in combination 1 showed that MCV (OR = 1.251, 95% CI = 1.021–1.531, P = 0.030), D-Dimer (OR = 9.798, 95% CI = 2.630–36.503, P = 0.001), CRP (OR = 1.033, 95% CI = 1.011–1.055, P = 0.003), LVEF (OR = 0.903, 95% CI = 0.819–0.995, P = 0.040), A wave velocity (OR = 0.044, 95% CI = 0.002–0.906, P = 0.043), pericardial effusion (OR = 16.926, 95% CI = 2.767–103.522, P = 0.002), and anterior wall infarction (OR = 12.275, 95% CI = 2.136–70.548, P = 0.005) were independently related to the occurrence of LVT in STEMI patients (Table 3).
The results of multivariate logistic regression analysis in combination 2 showed that D-Dimer (OR = 7.154, 95% CI = 2.504–20.438, P < 0.001), CRP (OR = 1.022, 95% CI = 1.007–1.037, P = 0.005), LVEF (OR = 0.917, 95% CI = 0.854–0.986, P = 0.018), pericardial effusion (OR = 9.476, 95% CI = 2.266–39.634, P = 0.002) and anterior wall infarction (OR = 12.32, 95% CI = 2.71–55.998, P = 0.001) were independently related to the occurrence of LVT in STEMI patients (Table 4).
According to the logistic regression analysis of the combination of the two variables, two logistic regression risk prediction models were established: Models 1 and 2. Model 1 contained seven variables: MCV, D-dimer, CRP, EF, A-wave velocity, pericardial effusion, and anterior wall infarction, while model 2 contained five variables: D-dimer, CRP, LVEF, pericardial effusion, and anterior wall infarction.
3.4 Optimal model selection
3.4.1 Internal evaluation
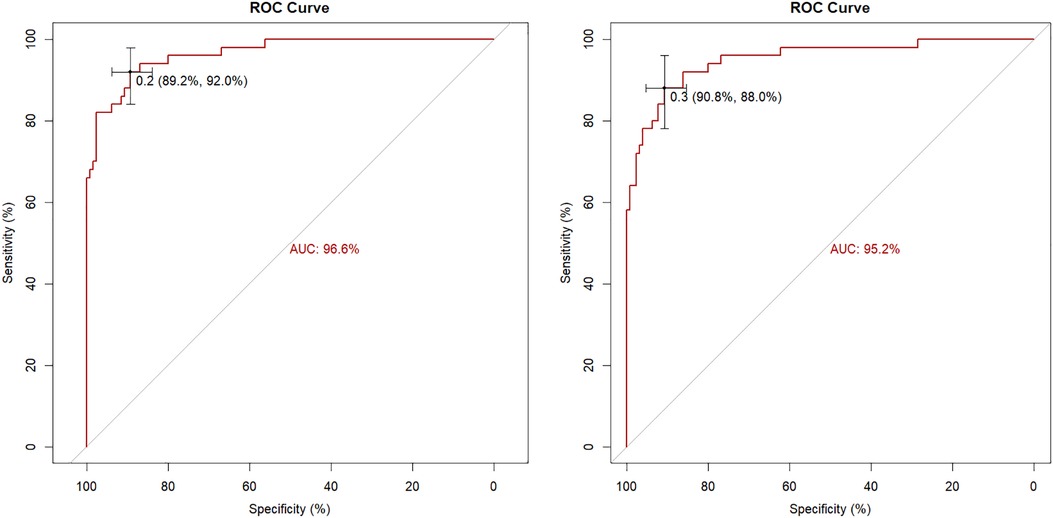
Internal evaluation and verification of the two risk prediction models were performed. The internal evaluations included discrimination and calibration. The C-index value of Model 1 was 0.966, and the area under the ROC curve was 96.6% (95% CI = 0.9399–0.9914. The C-index value of Model 2 was 0.952, and the area under the ROC curve was 95.2% (95% CI = 0.9158–0.9873 (Figure 2).
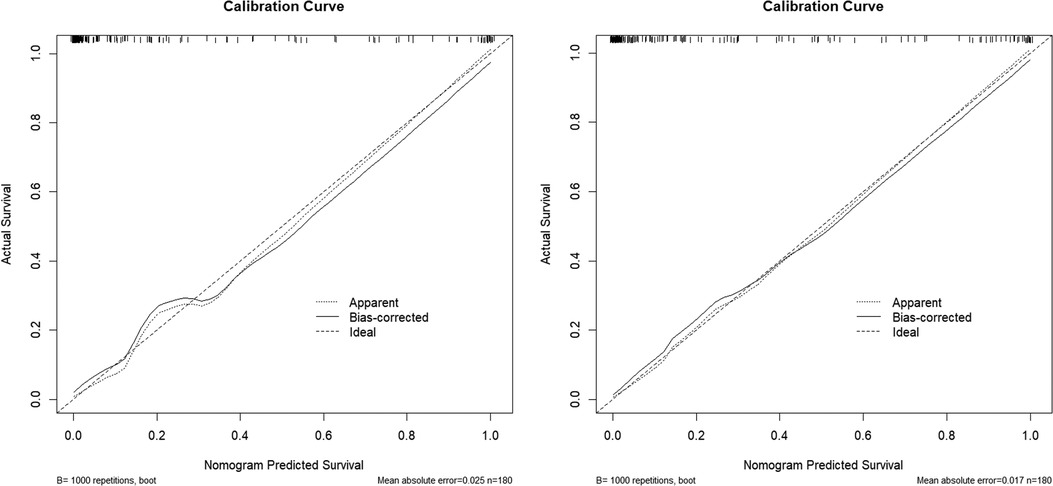
The calibration curves of the two models were drawn using the R software. The predicted results of the two models are in good agreement with those of the ideal model (Figure 3).
3.4.2 Internal verification
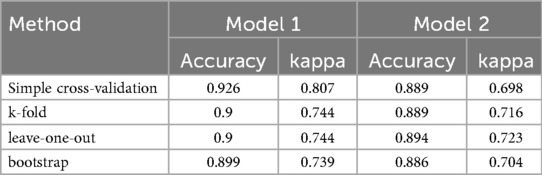
Simple cross-validation, k-fold, leave-one-out, and bootstrap analyses were used to internally verify the model. The accuracy of two models were 0.926, 0.9, 0.9, 0.899 and 0.889, 0.889, 0.894, 0.886, and Kappa values were 0.807, 0.744, 0.744, 0.739 and 0.698, 0.716, 0.723, 0.704, respectively. The areas under the ROC curves of the two models were 94.7% and 92.8%, respectively, as verified by the bootstrap analysis (Table 5).
Through internal evaluation and verification of the two models, we believe that the prediction ability of Model 1 is better than that of Model 2, and Model 1 is taken as the optimal risk prediction model. The variables included in the optimal model were MCV, D-dimer, CRP, LVEF, A-wave velocity, anterior wall infarction, and pericardial effusion.
3.5 Nomogram for optimal model
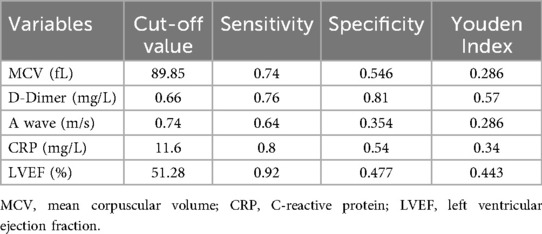
Before drawing the nomogram, we calculated the optimal cut-off value for continuous variables. ROC curves of five continuous variables in model 1, such as red blood cell distribution width, D-dimer, CRP, LVEF, and A-wave velocity, were drawn using SPSS. The Jordan index of the best cut-off value of each variable was calculated, and the best cut-off value and the corresponding specificity and sensitivity of the five continuous variables were obtained (Table 6).
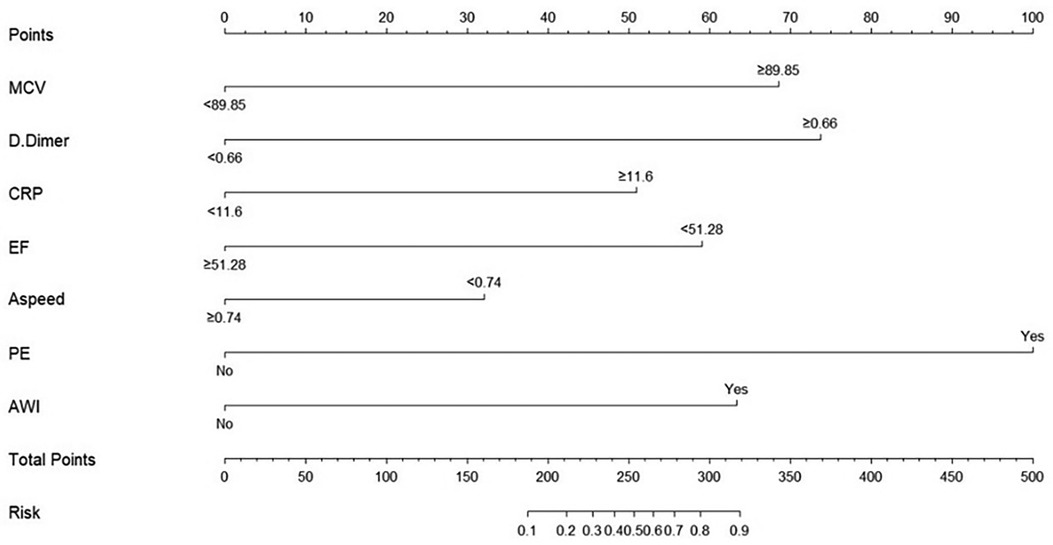
According to the best cutoff value, the continuous variables were converted into binary variables, and the nomogram of the best risk prediction model was drawn using the R software, which visualizes the risk prediction model and is convenient for clinical application (Figure 4).
4 Discussion
Prior to the widespread adoption of percutaneous coronary intervention (PCI), the incidence of left ventricular thrombus (LVT) in patients with ST-segment elevation myocardial infarction (STEMI) was considerably high. For instance, an Italian study published in 1986 reported an LVT incidence of 54% (5)—diagnosed by two-dimensional echocardiography—in STEMI patients who received neither anticoagulant nor antiplatelet therapy. Similarly, a 1989 Italian study found that among patients with anterior STEMI treated with thrombolysis, the LVT incidence was 28% (6). With the advent of early reperfusion strategies—particularly primary PCI—and the routine use of potent antithrombotic and anticoagulant therapies, the incidence of LVT has declined substantially. In a recent multicenter study using cardiac magnetic resonance (CMR) imaging, LVT was detected in 26 of 738 STEMI patients, yielding an overall incidence of 3.5%, with a higher rate of 7.1% observed specifically in those with anterior STEMI (12). Notably, CMR is currently regarded as the gold standard for LVT diagnosis due to its superior sensitivity and specificity. In contrast, transthoracic echocardiography (TTE)—the modality used in our study—may underestimate the true prevalence of LVT, potentially introducing bias in the sensitivity of prediction models based on TTE findings. Nevertheless, TTE remains the most widely used clinical tool for LVT screening owing to its accessibility, low cost, and feasibility at the bedside—advantages that are especially valuable in resource-limited settings. Our institution is located in an economically underdeveloped region where CMR is rarely utilized in routine practice due to its high cost, prolonged acquisition time, and the logistical challenges of transporting critically ill patients. Consequently, we elected to use TTE as the diagnostic criterion for LVT in this study to enhance the real-world relevance and preliminary translational potential of our findings. Among the 3,096 eligible STEMI patients included in our analysis, TTE identified LVT in 50 cases, corresponding to an incidence of 1.61%. This figure falls within the range of 0.7% (7) to 8.4% (8) reported in studies from the past decade that also relied on TTE for LVT diagnosis, supporting the consistency of our results with contemporary literature. In summary, advances in reperfusion therapy and antithrombotic management have markedly reduced the incidence of LVT in STEMI patients.
Despite the declining incidence, left ventricular thrombus (LVT) persists as a clinically significant complication of ST-elevation myocardial infarction (STEMI), adversely affecting long-term outcomes. However, there is a lack of effective predictive indicators to help clinicians identify high-risk patients early. In this study, we found that MCV, D-dimer level, CRP level, LVEF, A-wave velocity, pericardial effusion, and anterior wall infarction were independent risk factors for LVT.
The MCV is an index used to measure the volume of red blood cells and is mainly used in the differential diagnosis of anemia. In recent years, some studies have suggested that MCV is related to the mortality of patients with end-stage renal disease (13), all-cause mortality of acute heart failure (14), severity of coronary artery disease (15), adverse outcomes after PCI (16), and endothelial cell function (17), but the specific mechanism remains unclear. The research results of Yalcin Solak et al. show that endothelial function is negatively linearly correlated with MCV, revealing that endothelial dysfunction may be the internal mechanism between MCV and the occurrence of complex cardiovascular events in patients with end-stage renal disease (17). The research results of Wang Huaiyu showed that MCV is positively correlated with Gensini score, suggesting that MCV is significantly correlated with the severity of coronary artery disease (15); Masahiro Myojo et al. thought that risk stratification based on MCV may be valuable for evaluating the prognosis of patients after PCI (16); Tomaueda et al. think that MCV > 100 fl is an independent predictor of all-cause cardiovascular death in ADHF patients (18). The possible mechanisms of MCV and the above cardiovascular events include inflammatory reaction, malnutrition, abnormal bone marrow function, endothelial dysfunction, oxidative stress (13, 17), but further exploration is needed. However, there have been no reports of MCV related to thrombosis. The results of the comparison between the groups in this study suggested that the MCV in the case group was significantly higher than that in the control group. Multivariate regression analysis suggested that there is an independent correlation between them, which may suggest that patients with LVT have more severe vascular disease, more serious inflammatory and oxidative stress reactions, and more significant endothelial dysfunction. However, this hypothesis requires further investigation.
D-dimer is a soluble degradation product of fibrin, which is a product of cross-linked fibrin degraded by fibrinolytic enzyme (19). It is often used as a marker of activation of coagulation and fibrinolysis systems in clinics and is widely used in the diagnosis of venous thromboembolism (VTE). In recent years, it has been found that the D-dimer level in patients with acute coronary syndrome is closely related to the cardiac function and prognosis of patients after PCI (20). In a previous study, it was found that, in patients with left ventricular dysfunction after PCI, the elevation of D-dimer level was an independent predictor of left ventricular wall thrombus (21). Similarly, our study found that D-dimer was associated with LVT formation in STEMI patients, regardless of whether patients had cardiac dysfunction or whether they underwent PCI.
CRP is a sensitive non-specific inflammatory response marker in acute inflammation, which is mainly synthesized in the liver (22). Its transcription level is regulated by the cytokine interleukin-6, and its level can be increased 1,000 times when acute inflammation occurs (22). CRP is also considered an independent biomarker of cardiovascular risk (23). A large number of studies have shown that plasma CRP is not only involved in anti-inflammatory and pro-inflammatory processes, but is also related to endothelial dysfunction, leukocyte activation, angiogenesis, and other processes, which promote the pathogenesis of atherosclerosis, and can also induce platelet aggregation and activation and participate in thrombosis (24). It has been found that the change rate of CRP 24 h after admission is independently related to microvascular embolism and early left ventricular dysfunction after STEMI (25). The results of this study show that CRP level is independently related to the formation of LVT in STEMI patients, which is consistent with previously reported results (25).
Many studies have reported a correlation between LVEF and LVT in STEMI patients, and the LVEF decrease was independently related to LVT occurrence, which has been confirmed in many studies (7, 8, 10, 26). Our study results were consistent with those of previous studies, but the best cutoff value of LVEF for predicting LVT occurrence was different. Some studies suggest that LVEF <40% is an independent risk factor for LVT occurrence in acute extensive anterior wall or extensive anterior wall STEMI patients (26). Niazi et al. think that LVT is more common in patients with acute myocardial infarction whose ejection fraction is less than 30% (8). The different LVEF cutoff values may be related to sample selection, because different infarct sites have different effects on patients' cardiac function. Determining the best cutoff value of LVEF for predicting LVT formation requires a large-sample, multicenter clinical study.
E-wave and A-wave represent the mitral valve blood flow velocity in early and late diastole, respectively, and are often used to evaluate left ventricular diastolic function (27). In this study, the comparison between groups showed that the A-wave velocity and E-wave velocity in the case group were significantly lower than those in the control group (0.70, 0.21 vs. 0.80, 0.16, P < 0.001, 0.58, 0.18 vs. 0.64, 0.17, P = 0.026), and there was no statistical difference between the E/A groups. Multiple logistic regression analysis showed that the A-wave velocity was independently related to LVT formation in STEMI patients and that the diastolic function of patients with left ventricular thrombosis decreased more obviously than that of the control group. This result is also supported by previous studies. Pankaj Garg et al. conducted a prospective study by using four-dimensional blood flow cardiovascular magnetic resonance imaging and studied the related factors of LVT formation in STEMI patients by calculating the magnitude of the decrease of ventricular kinetic energy (KE) in different periods. The results showed that the decrease in A-wave KE from the middle of the ventricle to the apex of the heart in patients with LVT was significantly higher than that in patients without LVT (87% vs. 78%, P < 0.01), which suggests that late diastolic blood flow filling velocity plays an important role in left ventricular apical scour (28).
In previous studies, LVT was found to be more likely to occur in patients with anterior wall infarction and extensive anterior wall infarction. Therefore, in our study, the infarct site was divided into the anterior and non-anterior walls for comparison between the groups, and the difference was statistically significant. Logistic regression analysis showed that anterior wall infarction was independently related to LVT in patients with STEMI, consistent with previous research results (7, 8, 29).
This study found that there were significantly more patients with pericardial effusion in the case group than in the control group, and this difference was statistically significant. The number of patients with pericardial effusion ranged from low to moderate. No large pericardial effusions were observed. Pericardial effusion is a common complication in STEMI patients. A moderate–large amount of pericardial effusion often indicates more serious myocardial infarction, and the incidence of MACE increases significantly within one year after STEMI, which is a sign of poor prognosis (30). Jiang Yongxin et al. found that pericardial effusion was related to LVT after AMI by univariate analysis, but further multivariate analysis failed to prove that pericardial effusion was independently related to LVT formation (7). However, our study suggests that pericardial effusion is independently related to the occurrence of LVT, and eventually becomes an important index in the risk prediction model.
Based on the results of multivariate regression analysis, we established a multivariate regression model to predict the occurrence of left ventricular mural thrombus. The model contained these seven indicators, which were independently related to the occurrence of LVT. These seven indicators are easy to obtain in clinical practice with strong repeatability and low acquisition costs. For convenience of clinical use, we drew the nomogram of model 1 in R software, which can simply and quickly calculate the total score of each patient and evaluate the risk of LVT formation. Through internal evaluation and internal verification, the prediction efficiency of the model was found to be satisfactory.
5 Conclusion
MCV, D-dimer level, CRP level, LVEF, A-wave velocity, pericardial effusion, and anterior wall infarction were independently related to the occurrence of LVT in STEMI at the acute stage. The multivariate logistic regression risk prediction model developed in this study demonstrates good discrimination and calibration, enabling preliminary risk stratification for LVT in patients with acute STEMI; however, further multicenter prospective studies are warranted to validate and refine this model based on the current findings.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the ethical review board at the General Hospital of Ningxia Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
JW: Data curation, Visualization, Writing – original draft. PM: Conceptualization, Methodology, Writing – original draft. QX: Methodology, Writing – original draft, Writing – review & editing. LH: Writing – original draft. SZ: Writing – original draft.
Funding
The author(s) declared that financial support was not received for this work and/or its publication.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, De Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology foundation/American Heart Association task force on practice guidelines. Circulation. (2013) 127(4):e362–425. doi: 10.1161/CIR.0b013e3182742cf6
2. McManus DD, Gore J, Yarzebski J, Spencer F, Lessard D, Goldberg RJ. Recent trends in the incidence, treatment, and outcomes fried of patients with STEMI and NSTEMI. Am J Med. (2011) 124(1):40–7. doi: 10.1016/j.amjmed.2010.07.023
3. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2018) 39(2):119–77. doi: 10.1093/eurheartj/ehx393
4. Delewi R, Zijlstra F, Piek JJ. Left ventricular thrombus formation after acute myocardial infarction. Heart. (2012) 98(23):1743–9. doi: 10.1136/heartjnl-2012-301962
5. Vecchio C, Chiarella F, Lupi G, Bellotti P, Domenicucci S. Left ventricular thrombus in anterior acute myocardial infarction after thrombolysis. A GISSI-2 connected study. Circulation. (1991) 84(2):512–9. doi: 10.1161/01.CIR.84.2.512
6. Domenicucci S, Bellotti P, Chiarella F, Lupi G, Vecchio C. Spontaneous morphologic changes in left ventricular thrombi: a prospective two-dimensional echocardiographic study. Circulation. (1987) 75(4):737–43. doi: 10.1161/01.CIR.75.4.737
7. Jiang YX, Jing LD, Jia YH. Clinical characteristics and risk factors of left ventricular thrombus after acute myocardial infarction: a matched case-control study. Chin Med J (Engl). (2015) 128(18):2415–9. doi: 10.4103/0366-6999.164869
8. Niazi AK, Kassem H, Shalaby G, Khaled S, Alzahrani MS, Ali HM, et al. Incidence and predictors of left ventricular (LV) thrombus after ST-elevation myocardial infarction (STEMI) in the holy capital of Saudi Arabia. J Saudi Heart Assoc. (2021) 33(2):101–8. doi: 10.37616/2212-5043.1243
9. Zhang Q, Wang CM, Shi ST, Chen H, Zhou Y. Relationship of left ventricular thrombus formation and adverse outcomes in acute anterior myocardial infarction in patients treated with primary percutaneous coronary intervention. Clin Cardiol. (2019) 42(1):69–75. doi: 10.1002/clc.23106
10. Shacham Y, Leshem-Rubinow E, Ben AE, Ben Assa E, Rogowski O, Topilsky Y, et al. Frequency and correlates of early left ventricular thrombus formation following anterior wall acute myocardial infarction treated with primary percutaneous coronary intervention. Am J Cardiol. (2013) 111(5):667–70. doi: 10.1016/j.amjcard.2012.11.016
11. Weinsaft JW, Kim HW, Crowley AL, Klem I, Shenoy C, Van Assche L, et al. LV Thrombus detection by routine echocardiography: insights into performance characteristics using delayed enhancement CMR. JACC Cardiovasc Imaging. (2011) 4(7):702–12. doi: 10.1016/j.jcmg.2011.03.017
12. Poss J, Pöss J, Desch S, Eitel C, de Waha S, Thiele H, et al. Left ventricular thrombus formation after ST-segment-elevation myocardial infarction: insights from a cardiac magnetic resonance multicenter study. Circ Cardiovasc Imaging. (2015) 8(10):e003417. doi: 10.1161/CIRCIMAGING.115.003417
13. Hsieh YP, Chang CC, Kor CT, Yang Y, Wen Y-K, Chiu P-F. Mean corpuscular volume and mortality in patients with CKD. Clin J Am Soc Nephrol. (2017) 12(2):237–44. doi: 10.2215/CJN.00970116
14. Ueda T, Kawakami R, Horii M, Sugawara Y, Matsumoto T, Okada S, et al. High mean corpuscular volume is a new indicator of prognosis in acute decompensated heart failure. Circ J. (2013) 77(11):2766–71. doi: 10.1253/circj.CJ-13-0718
15. Wang H, Yang G, Zhao J, Wang M. Association between mean corpuscular volume and severity of coronary artery disease in the northern Chinese population: a cross-sectional study. J Int Med Res. (2020) 48(3):300060519896713. doi: 10.1177/0300060519896713
16. Myojo M, Iwata H, Kohro T, Sato H, Kiyosue A, Ando J, et al. Prognostic implication of macrocytosis on adverse outcomes after coronary intervention. Atherosclerosis. (2012) 221(1):148–53. doi: 10.1016/j.atherosclerosis.2011.11.044
17. Solak Y, Yilmaz MI, Saglam M, Demirbas S, Verim S, Unal HU, et al. Mean corpuscular volume is associated with endothelial dysfunction and predicts composite cardiovascular events in patients with chronic kidney disease. Nephrology (Carlton). (2013) 18(11):728–35. doi: 10.1111/nep.12130
18. Hatamian H, Saberi A, Pourghasem M. The relationship between stroke mortality and red blood cell parameters. Iran J Neurol. (2014) 13(4):237–40.25632337
19. Johnson ED, Schell JC, Rodgers GM. The D-dimer assay. Am J Hematol. (2019) 94(7):833–9. doi: 10.1002/ajh.25482
20. Chen R, Liu C, Zhou P, Sheng Z, Song L, Zhao H, et al. Prognostic value of D-dimer in patients with acute coronary syndrome treated by percutaneous coronary intervention: a retrospective cohort study. Thromb J. (2021) 19(1):30. doi: 10.1186/s12959-021-00281-y
21. You J, Wang X, Wu J, Gao L, Du P, Liu H, et al. Predictors and prognosis of left ventricular thrombus in post-myocardial infarction patients with left ventricular dysfunction after percutaneous coronary intervention. J Thorac Dis. (2018) 10(8):4912–22. doi: 10.21037/jtd.2018.07.69
22. Black S, Kushner I, Samols D. C-reactive protein. J Biol Chem. (2004) 279(47):48487–90. doi: 10.1074/jbc.R400025200
23. Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the centers for disease control and prevention and the American Heart Association. Circulation. (2003) 107(3):499–511. doi: 10.1161/01.CIR.0000052939.59093.45
24. Melnikov IS, Kozlov SG, Saburova OS, Avtaeva YN, Prokofieva LV, Gabbasov ZA. Current position on the role of monomeric C-reactive protein in vascular pathology and atherothrombosis. Curr Pharm Des. (2020) 26(1):37–43. doi: 10.2174/1381612825666191216144055
25. Holzknecht M, Tiller C, Reindl M, Lechner I, Fink P, Lunger P, et al. Association of C-reactive protein velocity with early left ventricular dysfunction in patients with first ST-elevation myocardial infarction. J Clin Med. (2021) 10(23):5494. doi: 10.3390/jcm10235494
26. Zhang GB. Clinical features and treatment of left ventricular thrombus after acute myocardial infarction). Peking Union Medical College (2019).
27. Pai RG, Stoletniy LN. An integrated measure of left ventricular diastolic function based on relative rates of mitral E and A wave propagation. J Am Soc Echocardiogr. (1999) 12(10):811–6. doi: 10.1016/S0894-7317(99)70185-9
28. Garg P, van der Geest RJ, Swoboda PP, Crandon S, Fent GJ, Foley JRJ, et al. Left ventricular thrombus formation in myocardial infarction is associated with altered left ventricular blood flow energetics. Eur Heart J Cardiovasc Imaging. (2019) 20(1):108–17. doi: 10.1093/ehjci/jey121
29. Tan S, Thakur U, Chow KY, Lee S, Ngoi A, Nerlekar N, et al. Predictive utility of left heart catheterization indices for left ventricular thrombus formation after anterior ST-elevation myocardial infarction. Cardiovasc Revasc Med. (2022) 34:106–11. doi: 10.1016/j.carrev.2021.01.013
Keywords: acute ST-segment elevation myocardial infarction, left ventricular thrombus, nomogram, risk factors, risk prediction model
Citation: Wang J, Ma P, Xu Q, Hai L and Zhang S (2026) Development of a risk prediction model for left ventricular thrombosis in STEMI patients. Front. Cardiovasc. Med. 13:1704792. doi: 10.3389/fcvm.2026.1704792
Received: 13 September 2025; Revised: 11 January 2026;
Accepted: 16 January 2026;
Published: 4 February 2026.
Edited by:
Carlos Jerjes-Sanchez, Escuela de Medicina y Ciencias de la Salud Tec Salud, Tecnológico de Monterrey, MexicoReviewed by:
Luis Mariano De La Torre Fonseca, Hospital Docente Comandante Manuel Fajardo, CubaWenjie Chen, Capital Medical University, China
Copyright: © 2026 Wang, Ma, Xu, Hai and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: QingBin Xu, RHIud2FuZzIwODlAZ21haWwuY29t
†These authors share first authorship
 Jingjing Wang
Jingjing Wang Ping Ma2,†
Ping Ma2,†