Abstract
Aim:
Our study aimed to evaluate epicardial adipose tissue (EAT) thickness in old adults, identify potential factors influencing it, and explore the association between EAT and left ventricular hypertrophy (LVH) in the older adult population.
Methods:
We analyzed cross-sectional data from the population-based AugUR study, including subjects aged ≥70 years. Cardiac geometry and EAT thickness were measured by echocardiography.
Results:
Among 988 participants aged 70–95 years (55.8% men), LVH was present in 368 individuals (37.2%). EAT thickness was similar between women and men (mean ± standard deviation: 4.1 ± 2.0 mm vs. 4.2 ± 1.9 mm, p = 0.29) but increased with age group (70–79 years: 4.0 ± 1.8 mm, >79 years 4.5 ± 2.0 mm, p < 0.001). Linear regression models of potential risk factors influencing EAT thickness showed associations with age [β = 0.038 mm per year; 95% confidence interval (CI) = 0.014–0.063, p < 0.05], body mass index (BMI) (β = 0.097 mm per kg/m2, 95% CI = 0.070–0.124, p < 0.05), and low-density lipoprotein-cholesterol (LDL, β = 0.008 mm per mg/dL, 95% CI = 0.004–0.012, p < 0.05). Given the reported causal relationship between EAT and LVH in experimental studies, multivariable regression models were performed to evaluate the association between EAT thickness and left ventricular mass index (LVMi). EAT thickness was associated with LVMi independent of sex, age, BMI, LDL, smoking, hypertension, diabetes, and chronic kidney disease (LVMi ß = 2.43 g/m2/mm, 95% CI 1.26–3.59, p = <0.001; LVH OR = 1.36/mm, 95% CI 1.01–1.84, p < 0.05). This association was strong in men but not in women.
Conclusion:
EAT thickness continues to increase with age beyond 69 years. Age, BMI, and LDL-cholesterol are associated with increasing EAT thickness. Our study strengthens the experimental hypothesis of a link between EAT and LVH by translational, population-based data. However, this association is more pronounced in men than in women.
Introduction
Cardiac metabolism plays a crucial role in maintaining continuous function of the heart. Alterations in cardiac metabolism have been implicated in the pathogenesis of different cardiomyopathies, highlighting the central role of energy production and utilization (1–3).
In recent years, epicardial adipose tissue (EAT) has received increasing attention due to its potential influence on cardiac metabolic function (4, 5). EAT is located between the myocardium and the visceral layer of the pericardium, with no membrane separating it from the myocardium. This unique anatomical position allows EAT to exert direct paracrine and vasocrine effects on cardiomyocytes by releasing inflammatory cytokines, adipokines, and free fatty acids (4). These signaling molecules can affect myocardial metabolism, inflammation, and remodeling, suggesting a potential mechanistic link between EAT and structural changes in the heart (4–8).
Experimental studies in guinea pigs have provided further evidence supporting this link (9). Specifically, it has been shown that EAT can drive left ventricular hypertrophy (LVH) through the release of cytokines (9). These findings suggest that EAT not only reflects underlying metabolic and inflammatory changes but may also actively contribute to myocardial remodeling and hypertrophy. Epidemiological studies in younger populations have confirmed an association between increased EAT thickness and LVH, further supporting the hypothesis that EAT plays a role in cardiac remodeling (10–18).
However, it remains unclear whether EAT thickness continues to increase with age in older and very old individuals—the age group with the highest prevalence of LVH. Given the rising proportion of older individuals in the population and the increasing burden of cardiovascular disease in this group, understanding the relationship between EAT and LVH in older adults is of particular clinical interest.
We aimed to establish reference values for EAT thickness in older and very old individuals, identify potential risk factors influencing EAT thickness, and assess the association between EAT and LVH in this high-risk population. To address these scientific needs, we analyzed cross-sectional population-based data from the AugUR study (Age-related diseases: understanding genetic and non-genetic influences—a study at the University of Regensburg), including participants aged >69 years, living in/around the city of Regensburg, Germany (19, 20). By translating experimental findings into an epidemiological context, this study aims to improve understanding of the role of EAT in age-related cardiac remodeling.
Methods
Study sample
The study protocol of the AugUR study has previously been described (19, 21, 22). In brief, the AugUR study is a prospective, population-based cohort study that recruited mobile people aged 69 years or older in the city of Regensburg, Germany, and nearby counties using a random sample provided by the local registries of residence. For the first recruitment wave between 2013 and 2015 (AugUR1), 1,133 of 5,644 contacted persons provided their consent to participate (overall response rate 20.1%). All participants acompleted a 2-h study program that included a physical examination and standardized personal interviews. For participants who received echocardiography not at recruitment but during a follow-up visit, data collected during the follow-up visit were considered baseline for this analysis.
Ethics statement
The study protocol, study procedures, and data protection strategy were approved by the Ethics Committee of the University of Regensburg, Germany (vote 12-101-0258). Written informed consent was obtained from each included individual after being informed about the study but before study inclusion. The study was conducted in accordance with the Declaration of Helsinki.
Assessment of medical conditions, medication intake, and blood biomarkers
Trained staff collected information on medical history, cardiovascular diseases—including presence, time of onset, and interventions—current medical regimen, smoking status, and sociodemographic factors using a standardized interview. Participants were requested to take their medication packages/blisters and medication lists to the study center. Trained staff recorded all medications currently taken by each participant (21, 23).
Heart rate and blood pressure were measured three times (using an automatic device: Omron M10-IT; Omron Healthcare, Kyoto, Japan), and the average of the second and third measurements was used in these analyses.
Biomarker analyses have been previously described (23). In brief, non-fasting blood samples were drawn in a sitting position after at least 5 min of resting. Low-density lipoprotein-cholesterol (LDL) was determined on a Beckman AU 5,400 analyzer with enzymatic tests (OSR6183, OSR6187, and OSR6116; Beckman Coulter, Krefeld, Germany). Serum creatinine was determined enzymatically using a Siemens Dimension Vista 1500 (assay ECREA, Siemens Healthcare, Erlangen, Germany).
Diabetes mellitus was defined by the prescription of oral antihyperglycemic agents or insulin, in accordance with previous studies (24, 25). Heart failure was determined by self-report. Coronary artery disease (CAD) was defined as a self-reported history of myocardial infarction, coronary bypass surgery, or percutaneous coronary intervention. Body mass index (BMI) was calculated using measured height and weight with participants wearing light clothing. LDL-cholesterol was measured from fresh blood samples, and estimated glomerular filtration rate (eGFR) was calculated using serum creatinine using the CKD-EPI 2009 formula (23).
Evaluation of cardiac geometry and epicardial adipose tissue by echocardiography
Echocardiographic assessments in the AugUR study followed two protocols. The standard program, conducted by a trained study nurse, assessed left ventricular function and morphology of all four chambers. In 1,018 participants, transthoracic echocardiography was performed by trained staff according to a standardized operating procedure using a commercially available ultrasound unit (HP Sonos 5500 with a 2–4 MHz probe; Philips, Eindhoven, The Netherlands) (21). Recorded images were post-hoc analyzed with Xcelera (version R3.2LI V.3.2.1.520-2011, Philips Medical Systems, Amsterdam, The Netherlands) following a standardized protocol. Left ventricular mass was calculated using M-mode in parasternal long-axis view and the Devereux formula (26). Left ventricular end-diastolic diameter, left ventricular end-systolic diameter, interventricular septum thickness, and posterior wall thickness were measured using 2D-guided M-Mode. Epicardial adipose tissue was measured in the parasternal long axis along the free RV wall at the level of the aortic valve during end systole (Supplementary Figure S1). All EAT measurements were performed post-hoc by a single investigator to minimize interoperator variability. For each participant, measurements were averaged over three cardiac cycles in normal heart rhythm and over 10 cardiac cycles in arrhythmia to enhance measurement reliability. Left ventricular mass was related to body surface area according to the DuBois formula (27).
Left ventricular hypertrophy was defined as left ventricular mass index >115 mg/m2 in men and >95 g/m2 in women according to the American College of Cardiology Cardiovascular Imaging Committee and European Association of Echocardiography consensus paper (28).
Left ventricular ejection fraction (LVEF) was measured using the monoplane method of discs (modified Simpson's rule) in the apical four-chamber view (28).
Cardiac cycles were defined as the interval between the onsets of transmitral inflows, measured by pulsed-wave Doppler. Heart rate was calculated as follows:Random errors were minimized by repeating all measurements three times in regular heart rhythm and 10 times in arrhythmia.
An extended echocardiographic protocol was performed in a subgroup by a cardiology-trained physician, including additional assessments of right ventricular function and heart valves. Therefore, valvular disease data are available for only 327 participants (19). High-grade valvular disease was defined as the presence of either severe aortic valve stenosis or severe mitral valve insufficiency. Valvular diseases were assessed and graded at study inclusion according to current guidelines (29, 30).
Statistics
Continuous variables are presented as median with 25th and 75th percentiles or as mean and standard deviation. Non-continuous variables are presented as absolute numbers and percentages of non-missing values. To evaluate factors influencing EAT, univariable linear regression analysis was performed. Subsequently, multivariable linear regression analyses were performed including variables significantly associated with EAT, with additional adjustment for age and sex.
Multivariable linear regression analysis was performed to evaluate EAT as a risk factor for increased LVMi. Therefore, known risk factors for LVH were chosen as covariates (BMI, LDL-cholesterol, smoking, hypertension, diabetes, and eGFR <60 mL/min/1.72 m2), alongside adjustment for age and sex (31). This model was also used for both sexes separately, thereby removing sex as a covariate. The same model was used in multivariable binary regression analyses to evaluate the association between EAT and LVH, followed by separate analyses for both sexes. Regression results are presented as ß estimators, with corresponding 95% confidence intervals (CIs) and p-values. Statistical significance was defined by a two-sided p-value of 0.05 or less. IBM SPSS statistics version 29 was used for statistical analyses.
Results
Baseline characteristics of the study sample
In 1,018 participants, transthoracic echocardiographic recordings were analyzed. In 30 cases, EAT could not be measured due to insufficient image quality (feasibility 97.1%). As a consequence, the study sample for all analyses comprised 988 participants. The median age of the participants was 77 years (P25 74 years; P75 81 years; minimum 70 years, maximum 95 years), and 44.2% were women. Diabetes was the most frequent comorbidity in both men and women (Table 1). Left ventricular hypertrophy was highly prevalent, affecting 368 participants (51.2%). Severely reduced LVEF (below 30%) was observed in only four participants. Overall, the prevalence of left ventricular hypertrophy and diastolic dysfunction was quite high in this older study population, whereas systolic dysfunction was rare.
Table 1
| Parameter | Women | Men | ||
|---|---|---|---|---|
| mean ± SD | n | mean ± SD | n | |
| Sociodemographic | ||||
| Age (years) | 77.6 ± 4.9 | 437 | 78.3 ± 5.1 | 551 |
| Comorbidities | ||||
| Uncontrolled hypertension | 29 (6.7%) | 436 | 50 (9.1%) | 551 |
| Diabetes | 87 (19.9%) | 437 | 132 (24.0%) | 551 |
| Coronary artery disease | 38 (8.7%) | 437 | 130 (23.6%) | 550 |
| Heart failure | 68 (15.6%) | 435 | 67 (12.2%) | 548 |
| Stroke | 25 (5.7%) | 437 | 65 (11.9%) | 551 |
| Smoking | 114 (26.1%) | 437 | 327 (59.3%) | 551 |
| BMI (kg/m2) | 27.8 ± 5.0 | 435 | 28.2 ± 3.9 | 545 |
| Examination results | ||||
| Heartrate (bpm) | 64.5 ± 10.5 | 407 | 62.0 ± 11.1 | 523 |
| Systolic blood pressure (mmHg) | 130.6 ± 18.2 | 436 | 133.1 ± 18.6 | 551 |
| Diastolic blood pressure (mmHg) | 77.9 ± 11.1 | 436 | 76.7 ± 10.9 | 551 |
| LDL-cholesterol (mg/dL) | 151.9 ± 34.7 | 436 | 139.5 ± 33.6 | 548 |
| eGFRCrea (mL/min/1.73 m2) | 68.6 ± 16.6 | 429 | 66.0 ± 17.2 | 541 |
| Echocardiography | ||||
| EAT (mm) | 4.1 ± 2.0 | 437 | 4.2 ± 1.9 | 551 |
| LVMi (g/m2) | 94.3 ± 25.2 | 358 | 117.9 ± 32.6 | 418 |
| LVEF (%) | 61.9 ± 6.9 | 424 | 59.3 ± 8.0 | 528 |
| LVH | 194 (54.2%) | 358 | 204 (48.8%) | 418 |
| Medication | ||||
| Antiplatelet agents | 141 (32.3%) | 429 | 231 (42.1%) | 524 |
| Vitamin K antagonists | 28 (6.4%) | 429 | 51 (9.3%) | 524 |
| DOAC | 19 (4.4%) | 429 | 37 (6.7%) | 524 |
| ACE inhibitors | 120 (27.5%) | 429 | 181 (33.0%) | 524 |
| Angiotensin-II receptor blockers | 112 (25.7%) | 429 | 123 (22.4%) | 524 |
| ß-blockers | 182 (41.7%) | 429 | 223 (40.6%) | 524 |
| Mineralocorticoid receptor antagonist | 16 (3.7%) | 429 | 18 (3.3%) | 524 |
| Digitalis glycosides | 10 (2.3%) | 429 | 11 (2.0%) | 524 |
| Metformin | 51 (11.7%) | 429 | 67 (12.2%) | 524 |
| SGLT2 inhibitor | 2 (0.5%) | 429 | 1 (0.2%) | 524 |
| Statins | 126 (28.9%) | 429 | 209 (38.1%) | 524 |
Baseline characteristics of 988 participants of the AugUR study.
Continuous parameters are presented as mean ± SD, and non-continuous parameters are presented as absolute numbers (% non-missing); the number of paticipiants with known parameter (n). SD, standard deviation; BMI, body mass index; LDL, low-density lipoprotein; eGFR, estimated glomerular filtration rate based on creatinine clearance; EAT, epicardial adipose tissue; LMVi, left ventricular mass index; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; DOACs, direct oral anticoagulants; ACE, angiotensin converting enzyme.
EAT thickness was 4.1 ± 1.9 mm in the entire study sample, showing a mostly even distribution (Figure 1). EAT was similar in both women (4.1 ± 2.0 mm) and men (4.2 ± 1.9 mm, p for difference 0.29) but differed between age groups (4.0 ± 1.8 mm 70–79years, 4.5 ± 2.0 mm >80 years, p < 0.001).
Figure 1
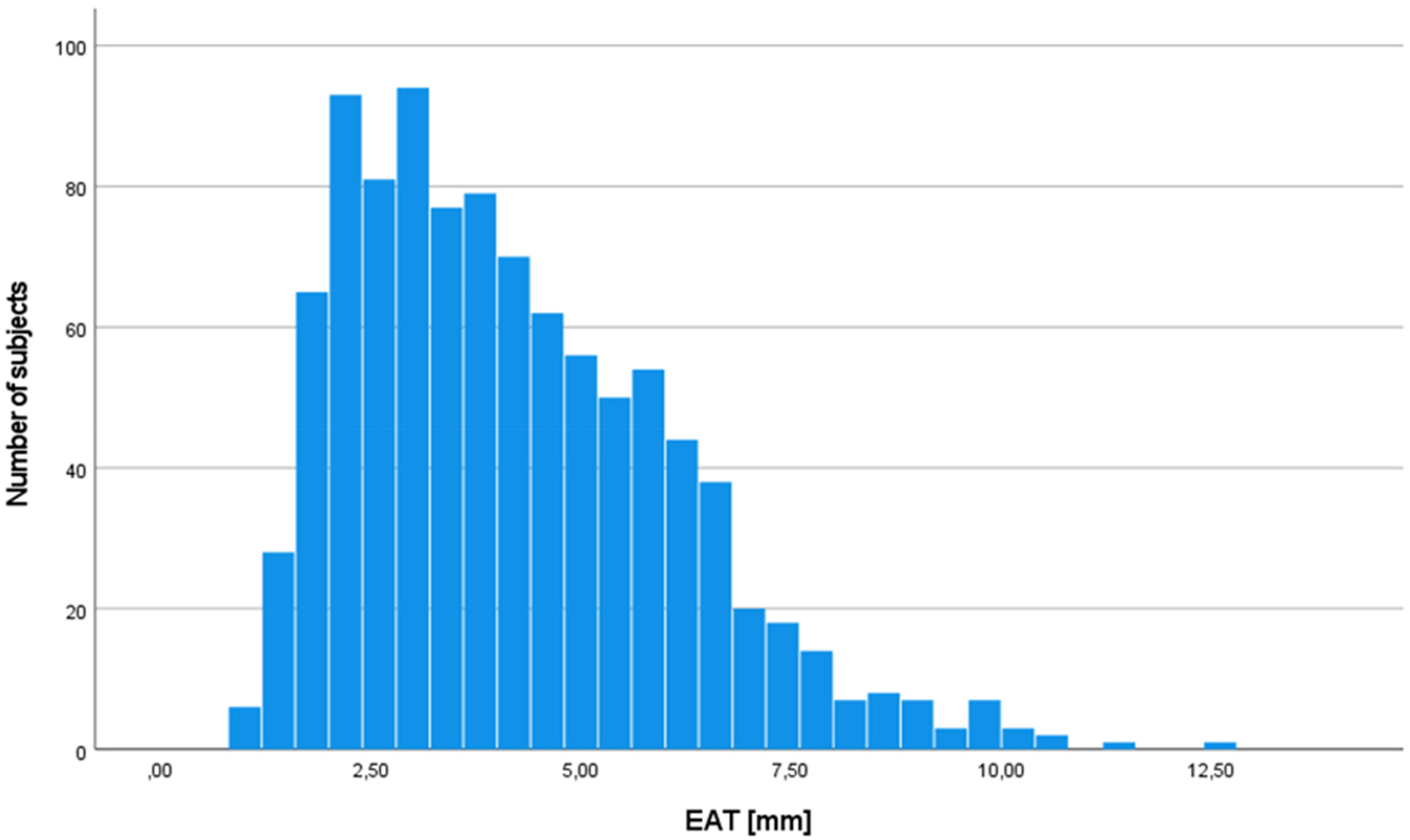
Distribution of epicardial adipose tissue in the study population (n = 988). EAT, epicardial adipose tissue.
Factors influencing epicardial adipose tissue
To assess factors influencing EAT thickness , we evaluated established cardiovascular risk factors, which have been associated with EAT in previous trials including younger participants (hypertension, BMI, LDL-cholesterol, C-reactive protein (CRP)). In addition, the intake of metformin or statins was included, as these agents have been reported to affect EAT thickness (32–35). SGLT-2 inhibitors were not considered because only three participants were using these agents at the time of recruitment. LDL-cholesterol was included as the primary lipid parameter owing on its well-established role in cardiovascular risk and its relevance to EAT (36–38). In univariable linear regression analyses, BMI (ß = 0.102 mm/kg/m2, 95% CI 0.076–0.128, p < 0.001), LDL-cholesterol (ß = 0.004 mm/mg/dL, 95% CI 0.000–0.007, p = 0.045), and eGFR <60 mL/min/1.73 m2 (ß = 0.388 mm, 95% CI 0.131–0.644, p = 0.003) were associated with higher EAT thickness, in contrast to hypertension (ß = 0.126 mm/mmHg, 95% CI −0.143–0.395, p = 0.357), history of coronary artery disease (ß = 0.148 mm, 95% CI −0.168–0.465, p = 0.358), hs-CRP (ß = −0.097 mm/mg/dL, 95% CI −0.173–0.157, p = 0.923), or the presence of severe aortic valve stenosis (ß = 0.188 mm, 95% CI −2.185–2.561, p = 0.876) (Supplementary Table S1). Intake of metformin or statins was also associated with increased EAT thickness (metformin ß = 0.476 mm, 95% CI 0.11–0.842, p = 0.01; statin ß = 0.279, 95% CI 0.028–0.53, p = 0.03). These factors were included in a multivariable regression analysis (Table 2). BMI and LDL level remained associated with EAT thickness, independent of each other and of age and statin intake. Together, BMI and LDL level, but not hypertension, sex, ejection fraction (EF), or chronic kidney disease, are the main determinants of EAT thickness in participants aged 70 years and older, which may point to EAT thickness as a metabolic marker.
Table 2
| Parameter | ß | 95% CI | p-Value |
|---|---|---|---|
| Total study population (n = 969) | |||
| Age (years) | 0.038 mm/year | 0.014–0.063 | 0.002 |
| Sex (O/1 f/m) | 0.086 mm | −0.151–0.323 | 0.477 |
| BMI (kg/m2) | 0.097 mm/kg/m2 | 0.070–0.124 | <0.001 |
| LDL (mg/dL) | 0.008 mm/mg/dl | 0.004–0.012 | <0.001 |
| eGFR <60 (O/1 n/y) | 0.168 mm | −0.097–0.432 | 0.213 |
| Diabetes (O/1 n/y) | 0.149 mm | −0.234–0.531 | 0.446 |
| Metformin (O/1 n/y) | 0.090 mm | −0.385–0.566 | 0.710 |
| Statin (O/1 n/y) | 0.353 mm | 0.080–0.626 | 0.011 |
| Women (n = 429) | |||
| Age (ears) | 0.035 mm/year | −0.003–0.073 | 0.072 |
| BMI (kg/m2) | 0.082 mm/kg/m2 | 0.045–0.119 | <0.001 |
| LDL (mg/dL) | 0.010 mm/mg/dl | 0.005–0.016 | <0.001 |
| eGFR <60 (O/1 n/y) | 0.234 mm | −0.177–0.644 | 0.265 |
| Diabetes (O/1 n/y) | 0.497 mm | −0.120–1.114 | 0.114 |
| Metformin (O/1 n/y) | −0.018 mm | −0.772–0.736 | 0.962 |
| Statin (O/1 n/y) | 0.462 mm | 0.025–0.900 | 0.038 |
| Men (n = 540) | |||
| Age (Years) | 0.042 | 0.010–0.074 | 0.011 |
| BMI (kg/m2) | 0.113 | 0.073–0.154 | <0.001 |
| LDL (mg/dL) | 0.006 | 0.001–0.011 | 0.023 |
| eGFR <60 (O/1 n/y) | 0.139 | −0.209–0.487 | 0.432 |
| Diabetes (O/1 n/y) | −0.102 | −0.595–0.392 | 0.685 |
| Metformin (O/1 n/y) | 0.189 | −0.430–0.808 | 0.548 |
| Statin (O/1 n/y) | 0.251 | −0.101–0.602 | 0.161 |
Factors influencing epicardial adipose tissue.
BMI and LDL-cholesterol are independently associated with EAT thickness in multivariable linear regression analyses. EAT, epicardial adipose tissue; BMI, body mass index; LDL, low-density lipoprotein; eGFR, estimated glomerular filtration rate; 95% CI, 95% confidence interval of ß.
Association between epicardial adipose tissue and left ventricular mass and hypertrophy
As the paracrine activity of EAT has been shown to promote increases in LVMi and therefore LVH in animal models (9) and EAT has also been associated with left ventricular hypertrophy in younger, healthy populations (13, 15), we sought to determine whether EAT remains associated with LVMi and LVH in an older age group in whom LVH and resulting diastolic dysfunction represent common challenges in daily clinical practice. Consequently, we performed multivariable linear regression analyses to evaluate the association between EAT and LVMi. We selected established risk factors for left ventricular hypertrophy—and therefore increased LVMi—together with age and sex as covariates. In detail, the resulting multivariable regression model included EAT, sex, and age; LDL-level and BMI, both of which were associated with EAT thickness in our previous model; and coronary artery disease, hypertension, smoking, diabetes, and chronic kidney disease (eGFR <60 mL/min/1.73 m2), all of which are known risk factors for LVH (31, 39). EAT thickness was independently associated with left ventricular mass (ß = 2.43 g/m2/mm, 95% CI 1.26–3.59, p < 0.001, Table 3). Since sex was strongly associated with LVMi, we also performed regression analyses for men and women separately. Of note, EAT was independently associated with left ventricular mass in 409 men with a reasonable effect size (ß = 3.95 g/m2/mm, 95% CI 2.17–5.73, p < 0.001), but not at all in 350 women (ß = 0.79 g/m2/mm, 95% CI −0.67–2.25, p = 0.29). Overall, EAT thickness is strongly associated with LVMi in older participants, among whom left ventricular hypertrophy (51.2%) is considerably prevalent. However, the association is strong among men but not among women. These effects persisted after adjusting for median systolic and diastolic blood pressure instead of hypertension, respectively.
Table 3
| Parameter | ß | 95% CI | p-Value |
|---|---|---|---|
| Total study population (n = 759) | |||
| EAT | 2.356 | 1.200–3.511 | <0.001 |
| Sex | 21.496 | 17.108–25.883 | <0.001 |
| Age | 0.601 | 0.167–1.035 | <0.05 |
| CAD | 11.073 | 5.214–16.932 | <0.001 |
| BMI | 1.185 | 0.685–1.684 | <0.001 |
| LDL | −0.007 | −0.071–0.058 | 0.833 |
| Smoking | −0.576 | −4.905–3.754 | 0.794 |
| Hypertension | 9.459 | 2.346–16.573 | <0.05 |
| Diabetes | 0.191 | −5.065–5.446 | 0.943 |
| eGFR <60 | −3.231 | −7.887–1.425 | 0.173 |
| Women (n = 350) | |||
| EAT | 0.813 | −0.647–2.274 | 0.274 |
| Age | 0.823 | 0.253–1.393 | <0.05 |
| CAD | 9.206 | −0.552–18.964 | 0.064 |
| BMI | 0.711 | 0.130–1.292 | <0.05 |
| LDL | −0.064 | −0.144–0.016 | 0.115 |
| Smoking | −0.216 | −6.238–5.806 | 0.944 |
| Hypertension | 4.066 | −6.087–14.219 | 0.431 |
| Diabetes | 0.809 | −6.238–7.856 | 0.821 |
| eGFR <60 | −2.771 | −8.861–3.318 | 0.371 |
| Men (n = 409) | |||
| EAT | 3.706 | 1.953–5.459 | <0.001 |
| Age | 0.501 | −0.138–1.141 | 0.124 |
| CAD | 12.476 | 4.839–20.113 | <0.05 |
| BMI | 1.786 | 0.947–2.624 | <0.001 |
| LDL | 0.054 | −0.046–0.154 | 0.287 |
| Smoking | −1.249 | −7.404–4.907 | 0.690 |
| Hypertension | 11.781 | 1.804–21.758 | <0.05 |
| Diabetes | −0.454 | −8.126–7.217 | 0.907 |
| eGFR <60 | −3.404 | −10.246–3.438 | 0.329 |
Multivariable linear regression showing association between EAT and left ventricular mass index.
In linear regression, EAT is independently associated with left ventricular mass. This effect is more pronounced in male than in female participants. CI, confidence interval of ß; EAT, epicardial adipose tissue; BMI, body mass index; LDL, low-density lipoprotein; CAD, coronary artery disease; eGFR, estimated glomerular filtration rate.
Next, we examined whether EAT was not only associated with LVMi but also with pathologic LVH. We performed multivariable binary logistic regression analyses using the same set of variables. Consistent with our findings for LVMi, epicardial adipose tissue was associated with LVH (Table 4). The association between EAT per mm and LVH was more pronounced than that observed between diabetes or chronic kidney disease and LVH. Thus, EAT emerged a stronger risk marker for LVH than some usually suspected comorbidities contributing to LVH. Only hypertension was more strongly associated with LVH than EAT (Figure 2). This effect was well evident in men but not in women.
Table 4
| Parameter | OR | 95% CI | p-Value |
|---|---|---|---|
| Total study population (n = 759) | |||
| EAT > median | 1.452 | 1.076–1.959 | <0.05 |
| Sex | 1.058 | 0.779–1.438 | 0.717 |
| Age > median | 1.102 | 0.801–1.515 | 0.551 |
| CAD | 1.579 | 1.125–2.217 | <0.05 |
| Obesity | 1.040 | 0.606–1.786 | 0.886 |
| Hypercholesterinemia | 0.783 | 0.570–1.075 | 0.131 |
| Smoking | 2.780 | 1.616–4.779 | <0.001 |
| Hypertension | 1.274 | 0.872–1.862 | 0.210 |
| Diabetes | 1.042 | 0.745–1.457 | 0.811 |
| eGFR <60 | 2.100 | 1.363–3.234 | <0.001 |
| Women (n = 350) | |||
| EAT > median | 0.993 | 0.638–1.547 | 0.977 |
| Age > median | 1.077 | 0.685–1.693 | 0.748 |
| CAD | 1.806 | 0.778–4.191 | 0.169 |
| Obesity | 1.839 | 1.116–3.031 | <0.05 |
| Hypercholesterinemia | 1.014 | 0.368–2.793 | 0.978 |
| Smoking | 0.862 | 0.523–1.422 | 0.562 |
| Hypertension | 2.378 | 0.997–5.670 | 0.051 |
| Diabetes | 1.704 | 0.950–3.057 | 0.074 |
| eGFR <60 | 1.113 | 0.672–1.842 | 0.678 |
| Men (n = 409) | |||
| EAT > median | 2.023 | 1.338–3.059 | <0.001 |
| Age > median | 1.063 | 0.696–1.624 | 0.776 |
| CAD | 2.332 | 1.394–3.903 | <0.05 |
| Obesity | 1.397 | 0.871–2.243 | 0.166 |
| Hypercholesterinemia | 1.100 | 0.572–2.115 | 0.775 |
| Smoking | 0.744 | 0.488–1.132 | 0.167 |
| Hypertension | 3.297 | 1.621–6.705 | <0.001 |
| Diabetes | 1.020 | 0.610–1.706 | 0.940 |
| eGFR <60 | 1.009 | 0.639–1.595 | 0.968 |
Multivariable binary logistic regression showing association between EAT and left ventricular hypertrophy.
In binary logistic regression, EAT is independently associated with left ventricular hypertrophy. This effect is pronounced in men but absent in women. Median EAT thickness was 4.1 mm (4.1 mm in women, 4.2 mm in men), and median age was 78 years (77.6 years in women, 78.3 years in men). CI, confidence interval of OR; EAT, epicardial adipose tissue; CAD, coronary artery disease; eGFR, estimated glomerular filtration rate.
Figure 2
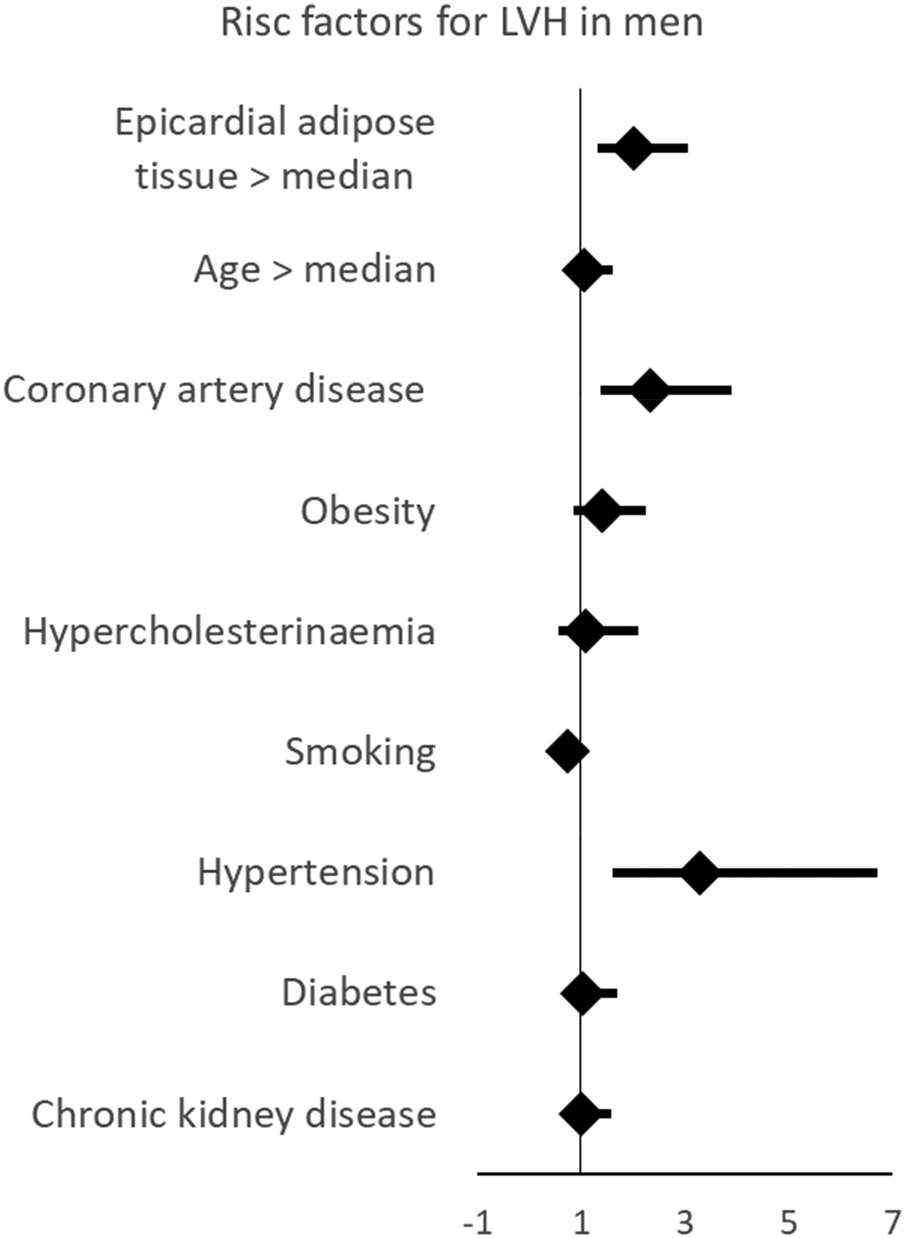
Multivariable linear regression showing that EAT is associated with left ventricular hypertrophy, and linear regression showing that EAT is independently associated with left hypertrophy. This effect is more pronounced in male than in female participants. LVH, left ventricular hypertrophy.
Aortic valve stenosis and mitral valve insufficiency are established risk factors for LVH (40, 41). Echocardiography was performed using two protocols: a standard program for all participants and an extended program with detailed valve assessment in a subset of 327 participants (19). To assess potential confounding, high-grade valvular disease was included in multivariable linear and logistic regression models as a sensitivity analysis (Supplementary Tables S3, S4). The association between EAT and LVMi remained significant, whereas the association with LVH was significant only in men, likely reflecting the reduced sample size.
Discussion
In 988 participants aged 70 years and older, increased EAT thickness is associated with advancing age. EAT thickness was dependent on age and metabolic markers, such as BMI and LDL-cholesterol, in older and very old individuals. Neither hypertension nor chronic kidney disease was associated with EAT thickness. As the paracrine activity of EAT has been shown to promote LVH in animal models (9) and EAT has been associated with LVH in younger, healthy populations (13, 15), we investigated whether this association persists in an older population in whom LVH and the resulting diastolic dysfunction represent common clinical challenges. Although EAT thickness was similar between women and men, an association between EAT and both LVMi and LVH was observed only in men.
EAT thickness in young people
Although methodologies for quantifying EAT thickness differ—most often relying on CT imaging in smaller cohorts—the reported median values span a considerable range. In populations under 74 years of age, thickness measurements have been as low as 0.9 mm (42). In contrast, larger-scale investigations have documented median EAT depths of 4.0 mm and even 5.4 mm, respectively (43, 44). Cohorts with particularly high prevalence of metabolic syndrome (up to 50%) consistently exhibit correspondingly greater EAT accumulation (43). In our study cohort, EAT thickness was independent of sex but increased with advancing age, yielding median values slightly higher than those reported in earlier studies involving younger participants.
EAT as a component of metabolic disease
EAT is metabolically active and capable of releasing proinflammatory cytokines, adipokines, and other bioactive molecules, which can influence both local myocardial tissue and systemic metabolic processes (5). The observed association between EAT and BMI and LDL-cholesterol in our study aligns with prior research in younger population-based samples, showing an association between EAT and high levels of LDL-cholesterol and high BMI (33, 44–48). The Jackson Heart Study demonstrated a strong association between EAT and metabolic syndrome (n = 1,414, OR = 1.48), and Zimmermann et al. showed an association between increased EAT and BMI as well as increased EAT and hyperlipidemia [n = 1,217, OR (BMI) = 1.19, OR (hyperlipidemia) = 2.84] (49, 50). Overall, previous and our studies suggest that EAT accumulation reflects broader patterns of metabolic dysfunction.
The association between statin use and EAT thickness observed in this study contrasts with findings from previous reports (35). This effect was predominantly observed in women and may be influenced by the strong association between LDL-cholesterol and EAT in our cohort, as well as the relatively low prevalence of statin use among women (28.9%) despite a median LDL-cholesterol of 151.9 mg/dl.
Sex differences in the association between EAT and LVH
Given its close anatomical proximity to the myocardium, EAT is positioned to affect cardiac structure and function through paracrine signaling (6, 8). Previous experimental studies have shown that EAT promotes LVH by releasing specific signaling molecules, such as inflammatory cytokines and free fatty acids (4, 5, 9). Epidemiological studies have likewise reported associations between EAT and LVMi, reinforcing the hypothesis that EAT contributes to cardiac remodeling through metabolic and inflammatory pathways (10–18). Our findings extend these experimental and epidemiologic observations by demonstrating a significant association between EAT thickness and LVMi in a population-based sample of older and very old individuals. This suggests that EAT activity may remain relevant in advanced age and continue to contribute to cardiac hypertrophy even in later life.
Interestingly, the association between EAT and LVMi, as well as LVH, was observed only in men and not in women. Other sex-specific differences in EAT have already been shown, such as a stronger association with body fat in women than in men; however, to our knowledge, a difference between men and women regarding the association between EAT and LVH has not been reported yet (44). A possible explanation might be the higher levels of testosterone in men, which are associated with an increased risk of developing left ventricular hypertrophy (51). Molecular evidence also supports sex-dependent differences in myocardial response to pressure overload. In human hearts subjected to chronic pressure overload, transcriptomic profiling and confirmatory analyses have demonstrated that maladaptive left ventricular remodeling is more common in men. In this setting, profibrotic and proinflammatory pathways are upregulated in male myocardium, whereas genes related to extracellular matrix and inflammatory are relatively suppressed in female myocardium. These differences are accompanied by greater histological fibrosis in men than in women, indicating distinct sex-specific regulation of fibrosis and inflammation in left ventricular remodeling (52). This mechanism may also underlie a differential effect of EAT on left ventricular hypertrophy in men; however, direct data supporting this theory are currently lacking, and further research is required.
This sex-specific pattern raises important questions about the underlying mechanisms driving these differences. It is tempting to speculate that the renin–angiotensin system (RAS) may play a role in this discrepancy. In the population-based CARTaGENE study of 19.996 Canadian residents (Québec), 1,284 underwent cardiac MRI as part of the CAHHM study (mean age 55 years). The analyses revealed that renin (but not aldosterone) was associated with cardiac adiposity in women, whereas aldosterone (but not renin) was associated with cardiac adiposity in men (53). The underlying mechanisms are barely understood.
Further research is needed to clarify the contribution of the RAS or other sex-specific factors to the relationship between EAT and LVH.
Translational implications
Our findings suggest a potential role for EAT as a modifiable target in the prevention and treatment of LVH, particularly in men. Recent cardiometabolic medications, such as glucagon-like peptide 1 receptor agonists and sodium–glucose cotransporter 2 (SGLT2) inhibitors, have been shown to modulate adipose tissue metabolism and improve cardiovascular outcomes.
Given the established links between EAT, metabolic dysfunction, and cardiac remodeling, interventions aimed at reducing EAT thickness or modifying its metabolic activity may offer therapeutic benefits in preventing or reversing LVH. The observed sex-specific differences further suggest that targeted therapies may need to account for these biological differences to optimize outcomes. This consideration is particularly important in the analysis of preclinical animal studies, which often rely exclusively on male or female animals.
Limitations
Our study has several limitations. Due to the recruitment strategy of our study, there is a bias toward healthier individuals, as participants needed to visit our outpatient clinic. This led to a disproportionately healthy study population, both physically and mentally. Notably, individuals with severely reduced EF (≤30%) were underrepresented in the study population (n = 4). Therefore, our findings may not be generalizable to patients with severely impaired systolic function. Severe valve diseases were also underrepresented (n = 9/327).
Furthermore, the diagnosis of diabetes in this study was based on medication intake. In case of providers ordering metformin for patients with prediabetes, this study might overestimate the proportion of patients with diabetes in the elderly population.
EAT thickness was measured using echocardiography. This evaluation is time and cost-effective and is not associated with relevant risks but is more dependent on the experience of examiners than MRI or CT scan. All echocardiographic examinations were performed by one of two experienced echocardiographers to minimize interobserver variability, and each measurement was repeated three times in normal heart rhythm and 10 times in arrhythmia to reduce random errors.
Another limitation is the absence of endocrine measurements, which could have provided insights into the hormonal and metabolic pathways underlying the sex-specific differences in the association between EAT and LVH. Future studies should aim to explore the contributions of endocrine factors, including the RAS and sex hormones, to EAT-mediated cardiac remodeling.
Conclusion
In summary, our study confirms and extends previous findings by demonstrating that EAT thickness increases with age and is influenced by metabolic factors such as BMI and LDL cholesterol. The observed association between EAT and LVH in older adults supports the hypothesis that EAT is involved in cardiac remodeling. The finding that this association is restricted to men suggests the potential presence of sex-specific mechanisms, which warrants further investigation.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethikommission Universität Regensburg. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AS: Formal analysis, Writing – original draft, Writing – review & editing. KS: Data curation, Investigation, Methodology, Project administration, Supervision, Writing – review & editing. MZ: Data curation, Formal analysis, Methodology, Project administration, Validation, Writing – review & editing. MH: Data curation, Formal analysis, Validation, Writing – review & editing. LM: Investigation, Project administration, Resources, Validation, Writing – review & editing. AL: Validation, Writing – review & editing. IH: Formal analysis, Methodology, Project administration, Resources, Validation, Writing – review & editing. AD: Methodology, Project administration, Validation, Writing – review & editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. The AugUR study was supported by grants from the German Federal Ministry of Education and Research (BMBF 01ER1206 and BMBF 01ER1507) to IH, by the Deutsche Forschungsgemeinschaft (DFG HE 3690/7-1) to IH, and by institutional budget (University of Regensburg). Part of this work was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation—DI 2876/2-1; 519332007 to AD).
Acknowledgments
The authors greatly appreciate the outstanding study assistance of Ms. Lydia Mayerhofer, Ms. Magdalena Scharl, and Ms. Sabine Schelter. In addition, the authors would like to thank Mr. Josef Simon for his excellent technical help. We thank all study participants for contributing to the AugUR study.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence, and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2026.1705319/full#supplementary-material
References
1.
Paulus MG Renner K Nickel AG Brochhausen C Limm K Zügner E et al Tachycardiomyopathy entails a dysfunctional pattern of interrelated mitochondrial functions. Basic Res Cardiol. (2022) 117(1):45. 10.1007/s00395-022-00949-0
2.
Dietl A Winkel I Deutzmann R Schröder J Hupf J Riegger G et al Interatrial differences of basal molecular set-up and changes in tachycardia-induced heart failure—a proteomic profiling study. Eur J Heart Fail. (2014) 16(8):835–45. 10.1002/ejhf.122
3.
Dietl A Maack C . Targeting mitochondrial calcium handling and reactive oxygen species in heart failure. Curr Heart Fail Rep. (2017) 14(4):338–49. 10.1007/s11897-017-0347-7
4.
Conte M Petraglia L Poggio P Valerio V Cabaro S Campana P et al Inflammation and cardiovascular diseases in the elderly: the role of epicardial adipose tissue. Front Med (Lausanne). (2022) 9:844266. 10.3389/fmed.2022.844266
5.
Iacobellis G . Epicardial adipose tissue in contemporary cardiology. Nat Rev Cardiol. (2022) 19(9):593–606. 10.1038/s41569-022-00679-9
6.
Perez-Miguelsanz J Jiménez-Ortega V Cano-Barquilla P Garaulet M Esquifino AI Varela-Moreiras G et al Early appearance of epicardial adipose tissue through human development. Nutrients. (2021) 13(9):2906. 10.3390/nu13092906
7.
Iacobellis G . Epicardial Adipose Tissue: From Cell to Clinic. Cham: Humana imprint (2020). Available online at:https://link.springer.com/content/pdf/10.1007/978-3-030-40570-0.pdf(Accessed July 27, 2025).
8.
Anan M Uchihashi K Aoki S Matsunobu A Ootani A Node K et al A promising culture model for analyzing the interaction between adipose tissue and cardiomyocytes. Endocrinology. (2011) 152(4):1599–605. 10.1210/en.2010-1106
9.
Swifka J Weiss J Addicks K Eckel J Rösen P . Epicardial fat from Guinea pig: a model to study the paracrine network of interactions between epicardial fat and myocardium?Cardiovasc Drugs Ther. (2008) 22(2):107–14. 10.1007/s10557-008-6085-z
10.
Konishi M Sugiyama S Sugamura K Nozaki T Matsubara J Akiyama E et al Accumulation of pericardial fat correlates with left ventricular diastolic dysfunction in patients with normal ejection fraction. J Cardiol. (2012) 59(3):344–51. 10.1016/j.jjcc.2012.01.006
11.
Shah RV Anderson A Ding J Budoff M Rider O Petersen SE et al Pericardial, but not hepatic, fat by CT is associated with CV outcomes and structure: the multi-ethnic study of atherosclerosis. JACC Cardiovasc Imaging. (2017) 10(9):1016–27. 10.1016/j.jcmg.2016.10.024
12.
Liu J Fox CS Hickson D Sarpong D Ekunwe L May WD et al Pericardial adipose tissue, atherosclerosis, and cardiovascular disease risk factors: the Jackson heart study. Diabetes Care. (2010) 33(7):1635–9. 10.2337/dc10-0245
13.
Fox CS Gona P Hoffmann U Porter SA Salton CJ Massaro JM et al Pericardial fat, intrathoracic fat, and measures of left ventricular structure and function: the Framingham heart study. Circulation. (2009) 119(12):1586–91. 10.1161/CIRCULATIONAHA.108.828970
14.
Silaghi A Piercecchi-Marti M-D Grino M Leonetti G Alessi MC Clement K et al Epicardial adipose tissue extent: relationship with age, body fat distribution, and coronaropathy. Obesity (Silver Spring). (2008) 16(11):2424–30. 10.1038/oby.2008.379
15.
Pucci G Battista F de Vuono S Boni M Scavizzi M Ricci MA et al Pericardial fat, insulin resistance, and left ventricular structure and function in morbid obesity. Nutr Metab Cardiovasc Dis. (2014) 24(4):440–6. 10.1016/j.numecd.2013.09.016
16.
Al-Talabany S Mordi I Graeme Houston J Colhoun HM Weir-McCall JR Matthew SZ et al Epicardial adipose tissue is related to arterial stiffness and inflammation in patients with cardiovascular disease and type 2 diabetes. BMC Cardiovasc Disord. (2018) 18(1):31. 10.1186/s12872-018-0770-z
17.
Granér M Seppälä-Lindroos A Rissanen A Hakkarainen A Lundbom N Kaprio J et al Epicardial fat, cardiac dimensions, and low-grade inflammation in young adult monozygotic twins discordant for obesity. Am J Cardiol. (2012) 109(9):1295–302. 10.1016/j.amjcard.2011.12.023
18.
Yilmaz S Demirtas INCİ S Coşansu K Gündüz H Kiliç H . Association between epicardial adipose tissue thickness and left ventricular diastolic functions. Sakarya Tıp Dergisi. (2020) 10(3):390–6. 10.31832/smj.732042
19.
Stark K Olden M Brandl C Dietl A Zimmermann ME Schelter SC et al The German AugUR study: study protocol of a prospective study to investigate chronic diseases in the elderly. BMC Geriatr. (2015) 15(1):130. 10.1186/s12877-015-0122-0
20.
Brandl C Zimmermann ME Günther F Dietl A Küchenhoff H Loss J et al Changes in healthcare seeking and lifestyle in old aged individuals during COVID-19 lockdown in Germany: the population-based AugUR study. BMC Geriatr. (2022) 22(1):34. 10.1186/s12877-021-02677-x
21.
Dietl A Zimmermann ME Brandl C Wallner S Burkhardt R Maier LS et al Distribution and specificity of high-sensitivity cardiac troponin T in older adults without acute cardiac conditions: cross-sectional results from the population-based AugUR study. BMJ Open. (2021) 11(11):e052004. 10.1136/bmjopen-2021-052004
22.
Heinrich MA Stark KJ Zimmermann ME Herold JM Brandl C Schober AD et al NT-proBNP in the very old population: reference values and heart failure specificity from the AugUR-study. Clin Res Cardiol. (2025):1–12. 10.1007/s00392-025-02785-3
23.
Donhauser FJ Zimmermann ME Steinkirchner AB Wiegrebe S Dietl A Brandl C et al Cardiovascular risk factor control in 70- to 95-year-old individuals: cross-sectional results from the population-based AugUR study. J Clin Med. (2023) 12(6):2102. 10.3390/jcm12062102
24.
Dietl A Stark K Zimmermann ME Meisinger C Schunkert H Birner C et al NT-proBNP Predicts cardiovascular death in the general population independent of left ventricular mass and function: insights from a large population-based study with long-term follow-up. PLoS One. (2016) 11(10):e0164060. 10.1371/journal.pone.0164060
25.
Steinkirchner AB Zimmermann ME Donhauser FJ Dietl A Brandl C Koller M et al Self-report of chronic diseases in old-aged individuals: extent of agreement with general practitioner medical records in the German AugUR study. J Epidemiol Community Health. (2022) 76(11):931–8. 10.1136/jech-2022-219096
26.
Devereux RB Reichek N . Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. (1977) 55(4):613–8. 10.1161/01.CIR.55.4.613
27.
Dubois D . A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. (1916) 17:863–71. 10.1001/archinte.1916.00080130010002
28.
Lang RM Bierig M Devereux RB Flachskampf FA Foster E Pellikka PA et al Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. (2005) 18(12):1440–63. 10.1016/j.echo.2005.10.005
29.
Lancellotti P Tribouilloy C Hagendorff A Popescu BA Edvardsen T Pierard LA et al Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. (2013) 14(7):611–44. 10.1093/ehjci/jet105
30.
Baumgartner H Hung J Bermejo J Chambers JB Evangelista A Griffin BP et al Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. (2009) 22(1):1–23; quiz 101–2. 10.1016/j.echo.2008.11.029
31.
Frohlich ED . Potential mechanisms explaining the risk of left ventricular hypertrophy. Am J Cardiol. (1987) 59(2):A91–7. 10.1016/0002-9149(87)90183-4
32.
Tok D Kadife I Turak O Ozcan F Başar N Cağlı K et al Increased epicardial fat thickness is associated with low grade systemic inflammation in metabolic syndrome. Turk Kardiyol Dern Ars. (2012) 40(8):690–5. 10.5543/tkda.2012.60207
33.
Rosito GA Massaro JM Hoffmann U Ruberg FL Mahabadi AA Vasan RS et al Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. (2008) 117(5):605–13. 10.1161/CIRCULATIONAHA.107.743062
34.
Ziyrek M Kahraman S Ozdemir E Dogan A . Metformin monotherapy significantly decreases epicardial adipose tissue thickness in newly diagnosed type 2 diabetes patients. Rev Port Cardiol. (2019) 38(6):419–23. 10.1016/j.repc.2018.08.010
35.
Jamialahmadi T Simental-Mendia LE Eid AH Almahmeed W Salehabadi S Al-Rasadi K et al Efficacy of statin therapy in reducing epicardial adipose tissue: a systematic review and meta-analysis. Arch Med Sci. (2024) 20(3):997–1001. 10.5114/aoms/189575
36.
Gao B Li C Liao Q Pan T Ren C Cao Q . Epicardial fat volume evaluated with multidetector computed tomography and other risk factors for prevalence of three-vessel coronary lesions. Eur J Med Res. (2022) 27(1):308. 10.1186/s40001-022-00956-w
37.
Colom C Viladés D Pérez-Cuellar M Leta R Rivas-Urbina A Carreras G et al Associations between epicardial adipose tissue, subclinical atherosclerosis and high-density lipoprotein composition in type 1 diabetes. Cardiovasc Diabetol. (2018) 17(1):156. 10.1186/s12933-018-0794-9
38.
Yılmaz M Mirzaoğlu Ç . Monocyte to HDL cholesterol ratio and smoking are predictors of epicardial fat tissue thickness. World J Biol Pharm Health Sci. (2024) 18(1):12–20. 10.30574/wjbphs.2024.18.1.0160
39.
Park SK Ryoo J-H Kang JG Jung JY . Smoking status, intensity of smoking, and their relation to left ventricular hypertrophy in working aged Korean men. Nicotine Tob Res. (2021) 23(7):1176–82. 10.1093/ntr/ntab020
40.
Dweck MR Joshi S Murigu T Gulati A Alpendurada F Jabbour A et al Left ventricular remodeling and hypertrophy in patients with aortic stenosis: insights from cardiovascular magnetic resonance. J Cardiovasc Magn Reson. (2012) 14(1):50. 10.1186/1532-429X-14-50
41.
Gaasch WH Meyer TE . Left ventricular response to mitral regurgitation: implications for management. Circulation. (2008) 118(22):2298–303. 10.1161/CIRCULATIONAHA.107.755942
42.
Albuquerque FN Somers VK Blume G Miranda W Korenfeld Y Calvin AD et al Usefulness of epicardial adipose tissue as predictor of cardiovascular events in patients with coronary artery disease. Am J Cardiol. (2012) 110(8):1100–5. 10.1016/j.amjcard.2012.06.003
43.
Calabuig Á Barba J Guembe MJ Díez J Berjón J Martínez-Vila E et al Epicardial adipose tissue in the general middle-aged population and its association with metabolic syndrome. Rev Esp Cardiol (Engl Ed). (2017) 70(4):254–60. 10.1016/j.recesp.2016.07.025
44.
Seo K-W Lim H-S Shin J-H Park J-S . Gender difference in the relationship between epicardial adipose tissue and central obesity. Medicine (Baltimore). (2024) 103(48):e40756. 10.1097/MD.0000000000040756
45.
Ansaldo AM Montecucco F Sahebkar A Dallegri F Carbone F . Epicardial adipose tissue and cardiovascular diseases. Int J Cardiol. (2019) 278:254–60. 10.1016/j.ijcard.2018.09.089
46.
Si Y Cui Z Liu J Ding Z Han C Wang R et al Pericardial adipose tissue is an independent risk factor of coronary artery disease and is associated with risk factors of coronary artery disease. J Int Med Res. (2020) 48(6):300060520926737. 10.1177/0300060520926737
47.
Pierdomenico SD Pierdomenico AM Cuccurullo F Iacobellis G . Meta-analysis of the relation of echocardiographic epicardial adipose tissue thickness and the metabolic syndrome. Am J Cardiol. (2013) 111(1):73–8. 10.1016/j.amjcard.2012.08.044
48.
Braescu L Sturza A Aburel OM Sosdean R Muntean D Luca CT et al Assessing the relationship between indexed epicardial adipose tissue thickness, oxidative stress in adipocytes, and coronary artery disease complexity in open-heart surgery patients. Medicina (Kaunas). (2024) 60(1):177. 10.3390/medicina60010177
49.
Liu J Fox CS Hickson DA May WL Ding J Carr JJ et al Pericardial fat and echocardiographic measures of cardiac abnormalities: the Jackson Heart Study. Diabetes Care. (2011) 34(2):341–6. 10.2337/dc10-1312
50.
Zimmermann GS Ruether T von Ziegler F Greif M Tittus J Schenzle J et al Increased pericardial adipose tissue in smokers. J Clin Med. (2021) 10(15):3382. 10.3390/jcm10153382
51.
Ikeda Y Aihara K Yoshida S Akaike M Matsumoto T . Effects of androgens on cardiovascular remodeling. J Endocrinol. (2012) 214(1):1–10. 10.1530/JOE-12-0126
52.
Kararigas G Dworatzek E Petrov G Summer H Schulze TM Baczko I et al Sex-dependent regulation of fibrosis and inflammation in human left ventricular remodelling under pressure overload. Eur J Heart Fail. (2014) 16(11):1160–7. 10.1002/ejhf.171
53.
Hundemer GL Agharazii M Madore F Piché M-E Gagnon C Bussières A et al Sex-specific associations of aldosterone and renin with body composition: a population-based cohort study. J Clin Endocrinol Metab. (2025) 110(3):801–10. 10.1210/clinem/dgae566
Summary
Keywords
epicardial adipose tissue, hypertrophy, left ventricular mass, older adults, population-based
Citation
Schober AD, Stark KJ, Zimmermann ME, Heinrich MA, Maier LS, Luchner A, Heid IM and Dietl A (2026) Sex-specific association of epicardial adipose tissue thickness and left ventricular hypertrophy in the older adults—cross-sectional results from the population-based AugUR study. Front. Cardiovasc. Med. 13:1705319. doi: 10.3389/fcvm.2026.1705319
Received
30 September 2025
Revised
05 January 2026
Accepted
21 January 2026
Published
18 February 2026
Volume
13 - 2026
Edited by
Daniela Trabattoni, Monzino Cardiology Center (IRCCS), Italy
Reviewed by
Francisco Rodrigues, Polytechnic Institute of Castelo Branco, Portugal
Stefano Quarta, National Research Council (CNR), Italy
Updates
Copyright
© 2026 Schober, Stark, Zimmermann, Heinrich, Maier, Luchner, Heid and Dietl.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Alexander Dietl alexander.dietl@ukr.de Alexander D. Schober alexander.schober@ukr.de
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.