Abstract
Background:
Patients with rheumatoid arthritis (RA) face a substantially increased risk of cardiovascular disease (CVD), yet existing risk prediction models often perform poorly in this population. QRISK3 and RA-adapted SCORE2 incorporate RA in their frameworks, but their validity in Asian cohorts remains uncertain.
Methods:
We conducted a retrospective observational study using electronic hospital records from The First People's Hospital of Zhangjiagang City in China (2020–2025). Adults with confirmed RA who subsequently experienced a first major CVD event (coronary heart disease, ischemic stroke, or transient ischemic attack) were included. QRISK3 and RA-adapted SCORE2 were applied to the conventional thresholds of ≥10% and ≥5% respectively, to define high risk. Agreement between tools was assessed with Cohen's kappa and McNemar's test. Adjusted logistic regression examined demographic, RA-related, and traditional risk factors associated with risk underestimation.
Results:
A total of 249 patients with RA and CVD were included. Both tools substantially underestimated risk, with underestimation more frequent for QRISK3 than RA-adapted SCORE2. Agreement between the two was moderate (κ = 0.44), with discordance most marked across age, glucocorticoid exposure, and disease activity subgroups. Patients with high baseline DAS28 scores were particularly likely to be misclassified as low risk. In adjusted models, diabetes, chronic kidney disease, and systemic steroid use were associated with greater underestimation.
Conclusions:
QRISK3 and RA-adapted SCORE2substantially underestimated cardiovascular risk in Chinese patients with RA, especially those with active disease. European-derived tools may not be reliable in this setting, underscoring the need for recalibrated or RA-specific models.
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterised by chronic inflammation and accelerated atherosclerosis, resulting in an increased burden of cardiovascular disease (CVD) (1). Past evidence has shown that patients with RA experience approximately 1.5 to 2 times higher rates of atherosclerotic CVD compared with the general population (2), and about half of deaths are related to CVD causes (3). This elevated burden arises from a combination of conventional risk factors, systemic inflammation, and genetic susceptibility.
Before most CVD prediction tools were developed and they incorporated traditional risk factors in the general population. Previous studies indicated that these tools systematically underestimate risk in patients with RA, reflecting the failure to account for disease-specific factors such as chronic inflammation and RA-related comorbidities (4). In response, some RA-specific CVD risk models have been proposed. For example, the modified SCORE algorithm, recommended by the European League Against Rheumatism (EULAR), applies a 1.5 multiplication factor to SCORE estimates in patients with defined RA characteristics (5). Similarly, QRISK3, derived from a large UK primary care database, incorporates RA and systemic lupus erythematosus as independent predictors, alongside conventional risk factors (6).
However, the performance of RA-adapted cardiovascular risk algorithms remains incompletely characterised, especially outside their original derivation settings. Asian populations, including China, typically have lower baseline CVD incidence and distinct risk factor distributions compared with Western cohorts (7). The Framingham equations and the ACC/AHA Pooled Cohort Equations (PCEs) have been evaluated in Asian populations, with notable underestimation in females (8–10). But the performance of RA-specific algorithms in these populations is limited. QRISK3 and RA-adapted SCORE2, developed and validated in European or UK cohorts, have not been systematically examined in Chinese patients with RA (11).
To address these gaps, we conducted a retrospective cohort study in China to evaluate the performance of QRISK3 and RA-adapted SCORE2 against observed CVD outcomes in patients with established RA. We examined how QRISK3 and RA-adapted SCORE2 classify cardiovascular risk in this population, quantified the proportion of patients classified above or below commonly used clinical thresholds, and explored patient characteristics associated with potential misclassification.
Method
Study design and setting
We carried out a retrospective observational study at Zhangjiagang First People's Hospital in China. Data were routinely collected from electronic hospital records, which included details from outpatient rheumatology visits, inpatient admissions, diagnostic reports, and laboratory results. The observation window was from 1 May 2020 to 31 May 2025. Ethical approval was obtained from the institutional review board, and individual patient consent was waived because all data were anonymised and analysed retrospectively.
Study population
We retrospectively identified adult patients (≥18 years) with rheumatoid arthritis (RA) from electronic hospital records between 1 May 2020 and 31 May 2025. Within this RA cohort, we then ascertained patients who had a documented cardiovascular disease (CVD) diagnosis in the same records. Both RA and CVD diagnoses were confirmed by review of clinical documentation, serological findings, and specialist reports. Patients with a prior history of CVD or baseline statin use were excluded. From within this RA population, we included patients who had a clinician-confirmed diagnosis of CVD during the study period.
Cardiovascular outcomes
In this study, cardiovascular outcomes were defined in line with the QRISK3 development studies. The composite endpoint comprised the first recorded occurrence of fatal or non-fatal coronary heart disease, ischemic stroke, or transient ischemic attack. Events were initially identified from hospital diagnosis records and then confirmed by detailed clinical review, which incorporated clinical summaries, relevant imaging, and cardiology reports.
Data collection
Demographic, clinical, medication and laboratory information were extracted from hospital records. Demographic data included age, sex, and smoking status. Clinical data include the comorbidities history (diabetes, chronic kidney disease, atrial fibrillation, migraine, systemic lupus erythematosus, and severe mental illness) and RA-related data [baseline disease activity score (DAS28: Remission-low/moderate/high), rheumatoid factor and anti-cyclic citrullinated peptide antibody status]. Treatment information included the use of disease-modifying antirheumatic drugs, antihypertensive therapy, atypical antipsychotics, and treatment for erectile dysfunction. Physiological and laboratory measures comprised systolic blood pressure, within-person variation in systolic blood pressure (calculated as the standard deviation of the two most recent readings), total cholesterol, HDL cholesterol, cholesterol/HDL ratio, height, weight, and body mass index. Where baseline medical history or lifestyle information was incomplete, these data were updated or confirmed during the clinical assessment at the time of the cardiovascular diagnosis.
Statistical analysis
Continuous variables were presented as means with standard deviations or medians with interquartile ranges, depending on distribution. Categorical variables were reported as counts and percentages.
Predicted risks were calculated for each patient using QRISK3 and RA-adapted SCORE2. QRISK3 estimates the 10-year risk of a first major cardiovascular event. Following the UK practice guideline, patients were classified as being at high risk if their predicted risk was 10% or greater. The RA-adapted SCORE2 estimate was calculated as the 10-year risk of cardiovascular death, with SCORE2 values multiplied by 1.5 to account for rheumatoid arthritis following the EULAR suggestion. And a threshold of 5% was used to define high risk. Concordance between QRISK3 and RA-adapted SCORE2 classifications was assessed using Cohen's kappa (12), and McNemar's test (13) for paired proportions.
This was a case-only study restricted to patients with rheumatoid arthritis who experienced a first cardiovascular event. Consequently, traditional model performance metrics such as discrimination (C-statistics) and calibration could not be assessed. Analyses therefore focused on threshold-based risk classification, examining whether patients with confirmed events would have been classified above or below commonly used clinical risk thresholds. Underestimation was defined in our cohort as cases in which patients with confirmed cardiovascular outcomes had predicted low risks. The primary analysis quantified the risk distribution and the proportion of underestimation by each tool. Subgroup analyses repeated this calculation within sex, age group (<50, ≥50 years), glucocorticoid exposure, baseline DAS28 score, type of cardiovascular outcome (coronary heart disease, ischemic stroke, transient ischemic attack), and time from RA diagnosis to cardiovascular event (≤10 years vs. >10 years. Crude and age- and sex-adjusted logistic regression models were used to examine patient characteristics, a range of demographic, RA-related, and traditional cardiovascular risk factors associated with underestimation. Results were presented as odds ratios (OR) with 95% confidence intervals (CI).
All analyses were conducted using R software (version 4.4.1, R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was defined as a two-sided p-value of <0.05.
Result
A total of 249 patients with RA and subsequent CVD were included, of whom 131 (52.6%) were female. The median age at RA diagnosis was 55 years (IQR, 51–60), with a median age at first CVD event of 64 years (IQR, 60–68). Current smoking was more common among men (36%) than women (8%). And 64% of patients had moderate baseline disease activity (DAS28). Coronary heart disease was the most frequent event, followed by ischemic stroke and transient ischemic attack. More information is reported in Table 1.
Table 1
| Characteristic | Overall (N = 249) | Female (n = 131) | Male (n = 118) |
|---|---|---|---|
| RA diagnosis age | 55 (51–60) | 57 (54–63) | 53 (48–57) |
| CVD age, | 64 (60–68) | 67 (63–71) | 62 (58–65) |
| Smoking status, n (%) | |||
| Never | 138 (55) | 91 (69) | 47 (40) |
| Former | 58 (23) | 29 (22) | 29 (25) |
| Current | 53 (21) | 11 (8.4) | 42 (36) |
| Regular steroid use, n (%) | 128 (51) | 73 (56) | 55 (47) |
| Blood pressure treatment, n (%) | 76 (31) | 40 (31) | 36 (31) |
| Antihypertensive treatment, n (%) | 65 (26) | 27 (21) | 38 (32) |
| Extra-articular disease, n (%) | 44 (18) | 21 (16) | 23 (19) |
| Erosive disease, n (%) | 57 (23) | 29 (22) | 28 (24) |
| DAS28 category, n (%) | |||
| Remission/Low | 49 (20) | 23 (18) | 26 (22) |
| Moderate | 159 (64) | 86 (66) | 73 (62) |
| High | 41 (16) | 22 (17) | 19 (16) |
| Hypertension, n (%) | 70 (28) | 36 (27) | 34 (29) |
| Diabetes, n (%) | 78 (31) | 46 (35) | 32 (27) |
| Obesity, n (%) | 19 (7.6) | 9 (6.9) | 10 (8.5) |
| Hyperlipidaemia, n (%) | 40 (16) | 17 (13) | 23 (19) |
| Atrial fibrillation, n (%) | 11 (4.4) | 6 (4.6) | 5 (4.2) |
| Chronic kidney disease, n (%) | 30 (12) | 19 (15) | 11 (9.3) |
| Chronic lung disease, n (%) | 45 (18) | 24 (18) | 21 (18) |
| Chronic liver disease, n (%) | 12 (4.8) | 7 (5.3) | 5 (4.2) |
Baseline characteristics of the study cohort.
QRISK3 classified 126 patients (50.6%) as high risk (≥10%) and 123 (49.4%) as low risk (<10%). In contrast, RA-adapted SCORE2 identified 183 patients (73.5%) as high risk (≥5%) and 66 (26.5%) as low risk (<5%). Overall, QRISK3 underestimated cardiovascular risk in 123 patients (49.4%), while RA-adapted SCORE2 underestimated risk in 66 patients (26.5%). Cross-classification demonstrated substantial discordance between the tools in Figure 1.
Figure 1
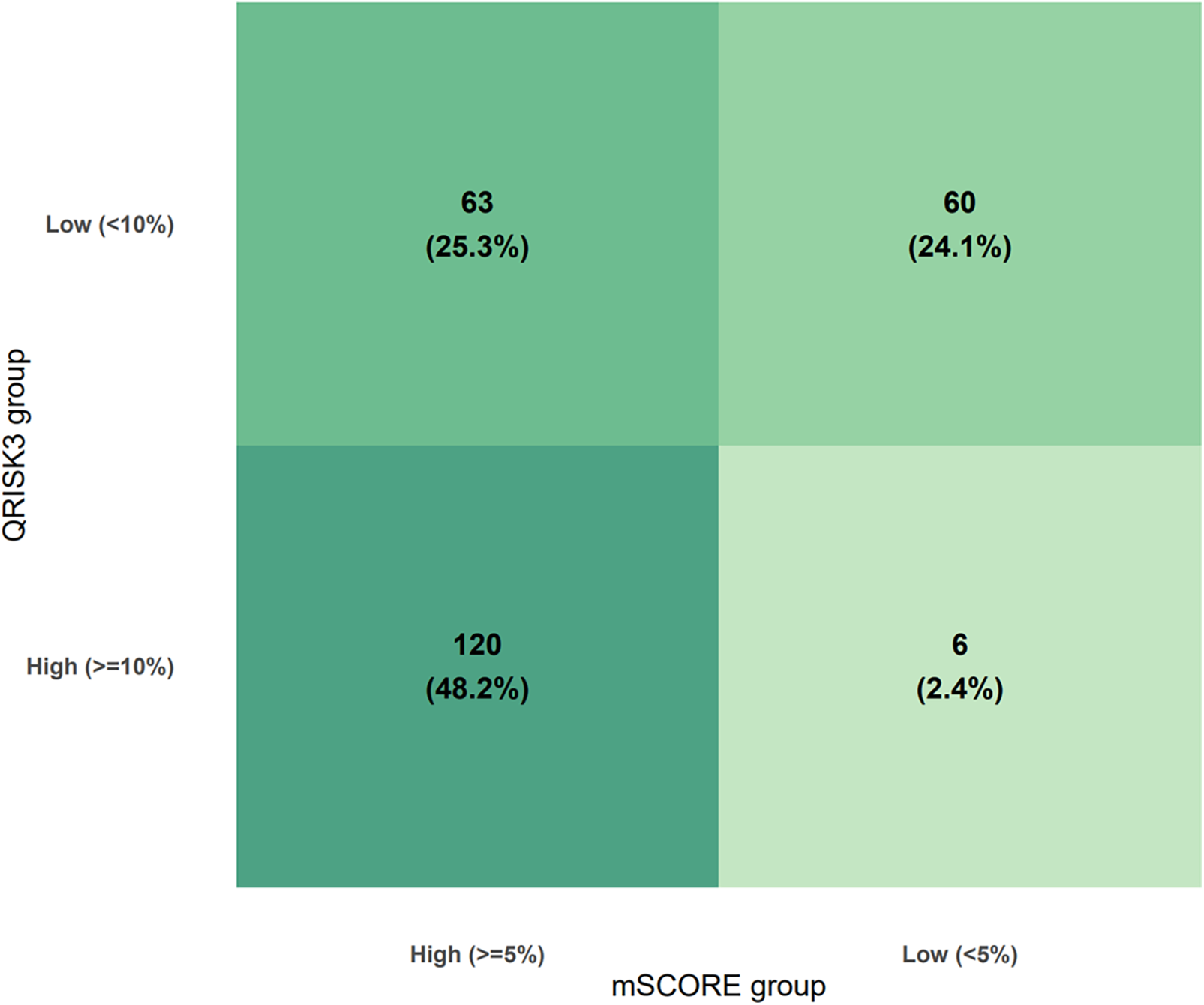
Cross-classification of RA patients by QRISK3 and RA-adapted SCORE2.
Agreement between QRISK3 and RA-adapted SCORE2 classifications was moderate. Overall, the two tools assigned patients to the same risk category in 72.3% of cases. Cohen's κ was 0.44, indicating moderate agreement beyond chance (p < 0.001). McNemar's test showed significant asymmetry in discordant classifications (χ2 = 45.4, p < 0.001), with RA-adapted SCORE2 more frequently classifying patients as high risk compared with QRISK3.
Among patients diagnosed with RA before the age of 50 years, QRISK3 classified only 11 (23.9%) as high risk, with 22 (47.8%) misclassified as low risk by both tools. In contrast, patients diagnosed at age ≥50 were more frequently identified as high risk by both algorithms. Steroid use also influenced classification: 61% of glucocorticoid users were identified as high risk by both tools, compared with only 35% among non-users. By disease activity, patients with high DAS28 scores were most frequently classified as high risk (63.4% by both tools), while discordance was greatest in the moderate disease group. Differences were also observed by CVD subtype, with RA-adapted SCORE2 more frequently identifying patients with ischemic stroke as high risk compared with QRISK3. When stratified by time to cardiovascular event, QRISK3 underestimated risk in 43.6% (95% CI: 36.2–51.2) of patients who developed CVD within 10 years of RA diagnosis, increasing to 64.3% (95% CI: 51.9–75.4) among those with events occurring after 10 years; corresponding estimates for RA-adapted SCORE2 were 20.7% (95% CI: 15.0–27.3) and 41.4% (95% CI: 29.8–53.8), respectively. All subgroup results are reported in Figure 2 and Table 2.
Figure 2
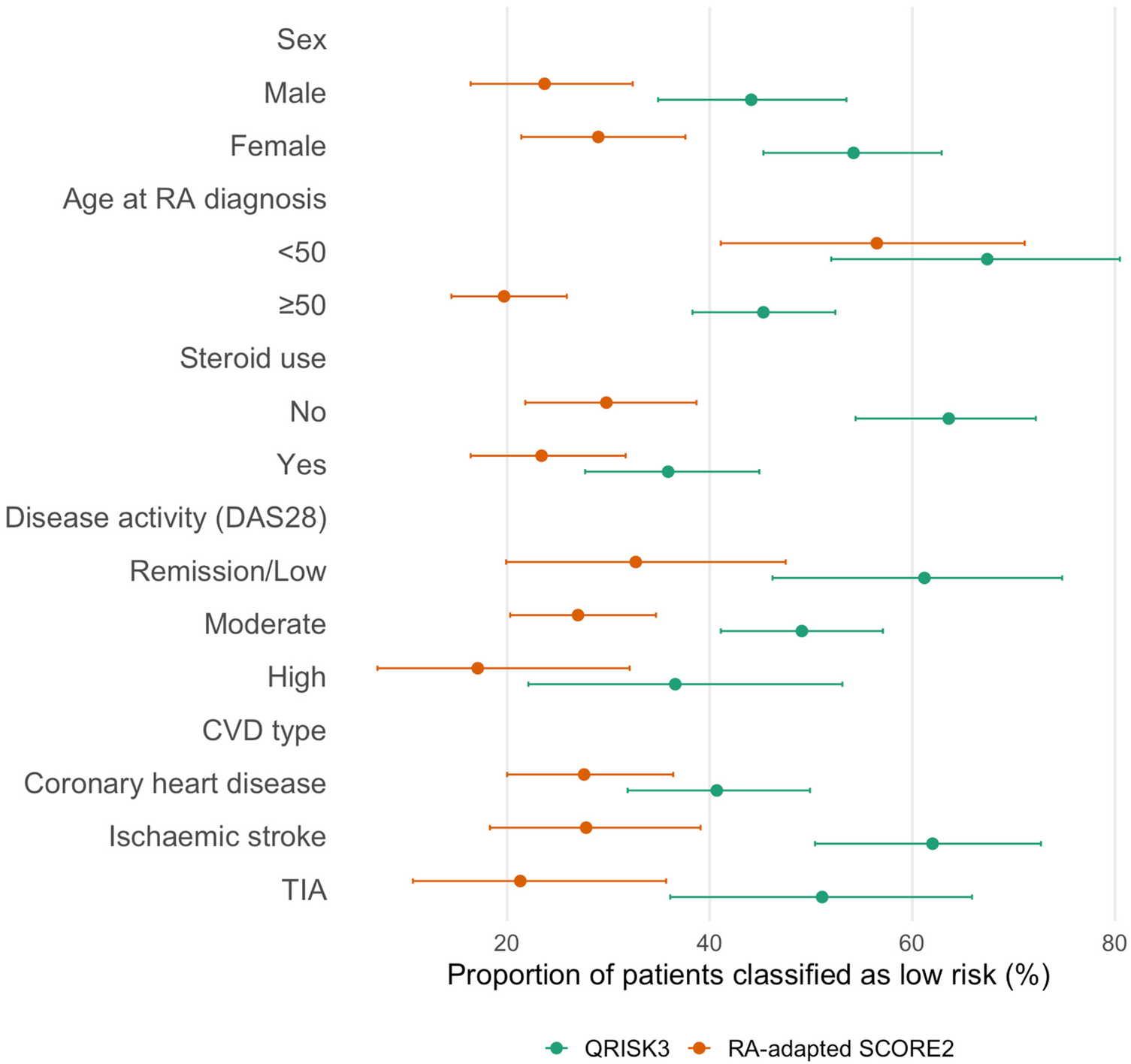
Risk underestimation by QRISK3 and RA-adapted SCORE2 across subgroups. Points represent proportions and horizontal bars indicate 95% confidence intervals. Subgroup sample sizes were: sex (female n = 131, male n = 118); age at RA diagnosis (<50 years n = 46, ≥50 years n = 203); steroid use (no n = 121, yes n = 128); disease activity by DAS28 (remission/low n = 49, moderate n = 159, high n = 41); and cardiovascular event type (coronary heart disease n = 123, ischaemic stroke n = 79, transient ischaemic attack n = 47).
Table 2
| Subgroup | Category | Both high n (%) | QRISK3 high only n (%) | RA-adapted SCORE2 high only n (%) | Both low n (%) |
|---|---|---|---|---|---|
| Sex | Female | 58 (44.3) | 2 (1.5) | 35 (26.7) | 36 (27.5) |
| Male | 62 (52.5) | 4 (3.4) | 28 (23.7) | 24 (20.3) | |
| Age at RA diagnosis | <50 years | 11 (23.9) | 4 (8.7) | 9 (19.6) | 22 (47.8) |
| ≥50 years | 109 (53.7) | 2 (1.0) | 54 (26.6) | 38 (18.7) | |
| Steroid use | No | 42 (34.7) | 2 (1.7) | 43 (35.5) | 34 (28.1) |
| Yes | 78 (60.9) | 4 (3.1) | 20 (15.6) | ||
| DAS28 category | Remission/Low | 19 (38.8) | 0 | 14 (28.6) | 16 (32.7) |
| Moderate | 75 (47.2) | 6 (3.8) | 41 (25.8) | 37 (23.3) | |
| High | 26 (63.4) | 0 | 8 (19.5) | 7 (17.1) | |
| CVD type | Coronary heart disease | 69 (56.1) | 4 (3.3) | 20 (16.3) | 30 (24.4) |
| Ischemic stroke | 28 (35.4) | 2 (2.5) | 29 (36.7) | 20 (25.3) | |
| TIA | 23 (48.9) | 0 | 14 (29.8) | 10 (21.3) |
QRISK3 and RA-adapted SCORE2 classification within subgroups.
In multivariable analyses adjusted for age and sex (Table 3), several predictors of risk underestimation were identified. For QRISK3, patients with traditional CVD risk factors, like diabetes (OR, 0.04; 95% CI, 0.02–0.10), hyperlipidaemia (OR, 0.33; 95% CI, 0.14–0.74), and chronic kidney disease (OR, 0.07; 95% CI, 0.02–0.22), were associated with a lower likelihood of underestimation. Similar patterns were observed for RA-adapted SCORE2, with high disease activity (OR, 0.27; 95% CI, 0.07–0.96) linked to underestimation, whereas diabetes (OR, 0.01; 95% CI, 0.00–0.04) was strongly protective. The crude regression results are reported in Supplementary Table S1.
Table 3
| Factor | Underestimation in QRISK3 | Underestimation in RA-adapted SCORE2 | Underestimation in both tools |
|---|---|---|---|
| DAS28 (Ref: Remission/Low) | |||
| Moderate | 0.65 (0.31–1.38), p = 0.268 | 1.02 (0.43–2.51), p = 0.969 | 0.74 (0.31–1.78), p = 0.490 |
| High | 0.26 (0.09–0.69), p = 0.008 | 0.27 (0.07–0.96), p = 0.053 | 0.28 (0.07–0.96), p = 0.051 |
| Extra-articular disease | 1.10 (0.52–2.32), p = 0.808 | 0.71 (0.23–1.97), p = 0.532 | 0.85 (0.28–2.34), p = 0.769 |
| Erosive disease | 0.79 (0.39–1.59), p = 0.515 | 1.08 (0.46–2.46), p = 0.850 | 1.04 (0.44–2.35), p = 0.926 |
| RA duration (per year) | 1.02 (0.94–1.11), p = 0.674 | 0.99 (0.89–1.10), p = 0.869 | 0.99 (0.89–1.09), p = 0.817 |
| Hypertension | 1.11 (0.60–2.09), p = 0.736 | 0.49 (0.20–1.10), p = 0.094 | 0.63 (0.27–1.40), p = 0.269 |
| Diabetes | 0.04 (0.02–0.10), p < 0.001 | 0.01 (0.00–0.04), p < 0.001 | 0.01 (0.00–0.07), p < 0.001 |
| Obesity | 2.14 (0.70–7.35), p = 0.199 | 0.46 (0.12–1.63), p = 0.250 | 0.64 (0.16–2.18), p = 0.491 |
| Hyperlipidaemia | 0.33 (0.14–0.74), p = 0.009 | 0.44 (0.15–1.19), p = 0.121 | 0.45 (0.14–1.22), p = 0.134 |
| Family history of CHD | 0.59 (0.29–1.18), p = 0.139 | 0.45 (0.17–1.09), p = 0.089 | 0.59 (0.23–1.42), p = 0.259 |
| Chronic kidney disease | 0.07 (0.02–0.22), p < 0.001 | 0.34 (0.08–1.14), p = 0.101 | 0.02 (0.00–0.18), p = 0.005 |
| Chronic lung disease | 0.68 (0.31–1.44), p = 0.315 | 0.53 (0.18–1.39), p = 0.211 | 0.42 (0.14–1.14), p = 0.104 |
Adjusted regression of factors associated with risk underestimation.
Discussion
This study provides one of the evaluations of QRISK3 and RA-adapted SCORE2 in a Chinese cohort of patients with rheumatoid arthritis and established cardiovascular disease. This threshold-based approach reflects real-world use of cardiovascular risk calculators, which guide preventive treatment decisions based on predefined cut-offs rather than continuous risk estimates. We found that both tools substantially underestimated cardiovascular risk, with underestimation more pronounced for QRISK3 than RA-adapted SCORE2. Agreement between the two algorithms was only moderate, and discordance was particularly evident across age groups, steroid use, and disease activity categories. These findings suggest that existing RA-adapted cardiovascular risk models, which were developed and validated in European primary care populations, may not adequately capture the risk profile of Asian patients with RA. By directly comparing two widely used algorithms against observed cardiovascular outcomes, our study highlights the need for better-calibrated risk prediction tools tailored to Asian populations.
Our findings are in keeping with earlier work showing that conventional risk factors do not fully explain the excess cardiovascular burden in rheumatoid arthritis. Even when smoking, hypertension, and diabetes are accounted for, patients with RA continue to experience excess events, pointing to the role of systemic inflammation, cumulative glucocorticoid exposure, and disease activity as additional drivers (14). Existing tools attempt to address this in different ways: QRISK3 incorporates RA as a binary variable, while RA-adapted SCORE2 applies a simple multiplier. Neither approach, however, reflects the heterogeneity of RA severity or treatment histories, which likely contributes to the underestimation we observed. Prior studies that attempted to integrate inflammatory markers such as CRP into general population algorithms reported little improvement in discrimination, underscoring the difficulty of operationalising RA-specific risk in prediction models (15, 16). Furthermore, active systemic inflammation accelerates atherosclerosis and contributes to excess cardiovascular morbidity in RA (17). Our analysis found that patients with high DAS28 scores were more likely to underestimate their cardiovascular risk. Although our analyses relied on baseline disease activity at the time of diagnosis, remission is the primary target of modern RA management, and many patients subsequently achieve lower disease activity with treatment. Nonetheless, our results highlight that disease activity at presentation remains an important signal of cardiovascular risk, and that patients with uncontrolled disease may require closer monitoring beyond what current calculators provide. Nevertheless, because disease activity was assessed using a single baseline DAS28 measurement, our analysis does not capture cumulative or time-varying inflammatory burden. As sustained inflammation over time is a key determinant of cardiovascular risk in rheumatoid arthritis, reliance on baseline DAS28 may underestimate the contribution of inflammation to cardiovascular risk misclassification.
Notably, several established cardiovascular risk factors, including diabetes, chronic kidney disease, and hyperlipidaemia, were associated with a lower likelihood of risk underestimation. This pattern reflects how these variables are handled within the QRISK3 and RA-adapted SCORE2 algorithms rather than any protective biological effect. Because these conditions are explicitly incorporated and heavily weighted, their presence increases the probability that patients exceed predefined risk thresholds. Consequently, individuals with prominent traditional risk factors are less likely to be classified as low risk, even within a cohort restricted to patients who subsequently developed cardiovascular disease. In contrast, RA-related contributors such as disease activity and inflammatory burden are incompletely represented, leaving patients without major conventional risk factors particularly susceptible to risk underestimation.
Several mechanisms may further explain the systematic underestimation observed in our study. First, both QRISK3 and RA-adapted SCORE2 were developed and validated using composite outcomes that included fatal cardiovascular events. In contrast, our study was restricted to non-fatal endpoints. This definitional mismatch is important. Fatal and non-fatal events differ in both temporal occurrence and risk factor associations, and exclusion of mortality may partly explain the gap between predicted and observed risks. We did not capture cardiovascular death events in our study. Second, neither model perfectly captures key RA-specific contributors to cardiovascular risk. Chronic systemic inflammation, cumulative glucocorticoid exposure, and longer disease duration have consistently been linked with excess CVD risk in RA (18, 19). However, these features are either absent from RA-adapted SCORE2 or reduced to a single binary term in QRISK3. Third, differences in background population risk profiles are relevant. For example, smoking prevalence is substantially higher among Chinese men than in European populations (20). But lipid levels tend to be lower in Chinese patients, with both LDL-C and triglycerides typically below those observed in the Western population (21–23). Because both QRISK3 and RA-adapted SCORE2 place considerable weight on cholesterol measures, lower lipid concentrations may deflate predicted risk estimates despite high cardiovascular event rates. This discrepancy highlights how applying coefficients derived from Western cohorts may introduce systematic bias when used in Asian populations.
Future work should focus on recalibration of existing calculators using contemporary RA cohorts, ideally incorporating biomarkers of inflammation and longitudinal treatment data. Given population differences in risk factor profiles and background event rates, external validation across diverse healthcare settings, including Asian populations, is essential before these tools can be confidently applied in routine care. Ultimately, the value of any prediction tool will depend not only on its statistical performance but also on its ability to guide timely and effective prevention in high-risk patients.
This study was conducted in a hospital-based rheumatology service, which inevitably shapes the case mix of patients observed. In routine clinical practice, patients followed in secondary or tertiary care tend to have more active disease, greater treatment complexity, and a higher burden of comorbidity than those whose RA is well controlled in the community. Many are referred because of persistent symptoms, treatment escalation, or complications, rather than stable disease alone. It is therefore likely that our cohort over-represents patients with more severe or difficult-to-control RA, in whom cardiovascular risk is already elevated. As a consequence, the degree of risk underestimation observed in this study may not directly reflect that seen in community-managed RA populations with milder disease. At the same time, this setting mirrors where cardiovascular risk assessment and preventive decisions are most often revisited in practice, suggesting that underestimation may be most clinically relevant in hospital-managed patients rather than in lower-risk community cohorts.
This study has several limitations. First, the relatively small sample size substantially limits statistical power, particularly for subgroup analyses. Second, the single-centre, hospital-based design restricts generalisability, as patients there often represent more severe or complex cases than those managed in community settings. Third, as all participants had experienced a cardiovascular event, we were unable to perform standard calibration analyses or evaluate discrimination in the general RA population; instead, we were limited to assessing relative underestimation and agreement between tools. Although this case-only design does not permit assessment of discrimination or calibration, it allows evaluation of potential underestimation at clinically relevant decision thresholds. Conceptually, if a substantial proportion of patients who subsequently experienced a cardiovascular event are classified below recommended high-risk thresholds, this suggests that the risk tool may underestimate risk in this population when applied in routine clinical practice. Fourth, we relied on cross-sectional baseline data without repeated measures of disease activity, inflammatory markers, or treatment exposure, which may underestimate the contribution of dynamic RA-related factors. Fifth, important unmeasured confounders, such as diet, socioeconomic status, physical activity, and cumulative glucocorticoid exposure, were not available and may have influenced risk classification. Another limitation is the lack of detailed longitudinal data on the duration, severity, and cumulative burden of both rheumatoid arthritis and major cardiovascular risk factors, including diabetes, hypertension, dyslipidaemia, chronic kidney disease, and smoking. Future studies with prospective follow-up and time-updated exposures are needed to better capture cumulative risk.
Conclusion
In summary, we found that QRISK3 and RA-adapted SCORE2 systematically underestimated cardiovascular risk in Chinese patients with RA and established CVD, reflecting both limitations in model design and differences in population risk profiles. These findings caution against uncritical application of Western-developed calculators in Asian settings and emphasise the importance of integrating RA-specific disease features into future prediction models. For clinicians, risk calculators should be viewed as supportive tools rather than definitive guides, with preventive strategies informed by broader clinical judgment that considers inflammation, treatment exposure, and comorbidities. For researchers, the priority is to recalibrate or redesign models using contemporary RA cohorts that reflect local practice and patient characteristics. Only through this iterative process can prediction tools move beyond statistical metrics to meaningfully guide prevention in high-risk RA populations.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Zhangjiagang First People's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because in this study in accordance with the national legislation and the institutional requirements.
Author contributions
XL: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. XN: Formal analysis, Resources, Validation, Writing – original draft, Writing – review & editing. WQ: Investigation, Software, Validation, Writing – original draft, Writing – review & editing. CS: Formal analysis, Resources, Visualization, Writing – original draft, Writing – review & editing. XY: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. YanZ: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. YabZ: Conceptualization, Formal analysis, Validation, Writing – original draft, Writing – review & editing. ZY: Investigation, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declared that financial support was not received for this work and/or its publication.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2026.1712088/full#supplementary-material
References
1.
Hansildaar R Vedder D Baniaamam M Tausche A-K Gerritsen M Nurmohamed MT . Cardiovascular risk in inflammatory arthritis: rheumatoid arthritis and gout. Lancet Rheumatol. (2021) 3(1):e58–70. 10.1016/S2665-9913(20)30221-6
2.
van Halm VP Peters MJ Voskuyl AE Boers M Lems WF Visser M et al Rheumatoid arthritis versus diabetes as a risk factor for cardiovascular disease: a cross-sectional study, the CARRE investigation. Ann Rheum Dis. (2009) 68(9):1395–400. 10.1136/ard.2008.094151
3.
Semb AG Ikdahl E Wibetoe G Crowson C Rollefstad S . Atherosclerotic cardiovascular disease prevention in rheumatoid arthritis. Nat Rev Rheumatol. (2020) 16(7):361–79. 10.1038/s41584-020-0428-y
4.
Crowson CS Rollefstad S Ikdahl E Kitas GD van Riel PLCM Gabriel SE et al Impact of risk factors associated with cardiovascular outcomes in patients with rheumatoid arthritis. Ann Rheum Dis. (2018) 77(1):48–54. 10.1136/annrheumdis-2017-211735
5.
Agca R Heslinga SC Rollefstad S Heslinga M McInnes IB Peters MJL et al EULAR Recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. (2017) 76(1):17–28. 10.1136/annrheumdis-2016-209775
6.
Hippisley-Cox J Coupland C Brindle P . Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. Br Med J. (2017) 357:j2099. 10.1136/bmj.j2099
7.
Yiu K-H Tse H-F Mok M-Y Lau C-S . Ethnic differences in cardiovascular risk in rheumatic disease: focus on Asians. Nat Rev Rheumatol. (2011) 7(10):609–18. 10.1038/nrrheum.2011.126
8.
Zhiting G Jiaying T Haiying H Yuping Z Qunfei Y Jingfen J . Cardiovascular disease risk prediction models in the Chinese population- a systematic review and meta-analysis. BMC Public Health. (2022) 22(1):1608. 10.1186/s12889-022-13995-z
9.
Yang X Li J Hu D Chen J Li Y Huang J et al Predicting the 10-year risks of atherosclerotic cardiovascular disease in Chinese population: the China-PAR project (prediction for ASCVD risk in China). Circulation. (2016) 134(19):1430–40. 10.1161/CIRCULATIONAHA.116.022367
10.
Kim JH Lee G Hwang J Kim JW Kwon JW Song YK . Performance of cardiovascular risk prediction models in Korean patients with new-onset rheumatoid arthritis: national cohort study. J Am Heart Assoc. (2023) 12(22):e030604. 10.1161/JAHA.123.030604
11.
Corrales A Vegas-Revenga N Atienza-Mateo B Corrales-Selaya C Prieto-Peña D Rueda-Gotor J et al Combined use of QRISK3 and SCORE as predictors of carotid plaques in patients with rheumatoid arthritis. Rheumatology. (2020) 60(6):2801–7. 10.1093/rheumatology/keaa718
12.
Cohen J . A coefficient of agreement for nominal scales. Educ Psychol Meas. (1960) 20(1):37–46. 10.1177/001316446002000104
13.
Fagerland MW Lydersen S Laake P . The McNemar test for binary matched-pairs data: mid-p and asymptotic are better than exact conditional. BMC Med Res Methodol. (2013) 13(1):91. 10.1186/1471-2288-13-91
14.
Kalogeropoulos A Georgiopoulou V Psaty BM Rodondi N Smith AL Harrison DG et al Inflammatory markers and incident heart failure risk in older adults: the health ABC (health, aging, and body composition) study. J Am Coll Cardiol. (2010) 55(19):2129–37. 10.1016/j.jacc.2009.12.045
15.
Rajagopalan V Alemao E Kawabata H Solomon D . SAT0069 Performance of the framingham cardiovascular risk prediction model with and without C-reactive protein or erythrocyte sedimentation rate in RA: analysis of US electronic medical records database. Ann Rheum Dis. (2014) 73:615. 10.1136/annrheumdis-2014-eular.1833
16.
Alemao E Cawston H Bourhis F Al M Rutten-van Molken M Liao KP et al Comparison of cardiovascular risk algorithms in patients with vs without rheumatoid arthritis and the role of C-reactive protein in predicting cardiovascular outcomes in rheumatoid arthritis. Rheumatology. (2017) 56(5):777–86. 10.1093/rheumatology/kew440
17.
Meng H Cheng IT Yan BPY Lee AP So H Tam L-S . Moderate and high disease activity levels increase the risk of subclinical atherosclerosis progression in early rheumatoid arthritis: a 5-year prospective study. RMD Open. (2024) 10(1):e003488. 10.1136/rmdopen-2023-003488
18.
So H Lam TO Meng H Lam SHM Tam L-S . Time and dose-dependent effect of systemic glucocorticoids on major adverse cardiovascular event in patients with rheumatoid arthritis: a population-based study. Ann Rheum Dis. (2023) 82(11):1387–93. 10.1136/ard-2023-224185
19.
van der Valk ES Mohseni M Iyer AM van den Hurk MJB Lengton R Kuckuck S et al Long-term glucocorticoid exposure and incident cardiovascular diseases—the lifelines cohort. J Clin Endocrinol Metab. (2024) 109(10):2520–9. 10.1210/clinem/dgae081
20.
Liu Z Li Y-h Cui Z-y Li L Nie X-q Yu C-d et al Prevalence of tobacco dependence and associated factors in China: findings from nationwide China health literacy survey during 2018–19. Lancet Reg Health West Pac. (2022) 24:100464. 10.1016/j.lanwpc.2022.100464
21.
Karthikeyan G Teo KK Islam S McQueen MJ Pais P Wang X et al Lipid profile, plasma apolipoproteins, and risk of a first myocardial infarction among Asians: an analysis from the INTERHEART study. J Am Coll Cardiol. (2009) 53(3):244–53. 10.1016/j.jacc.2008.09.041
22.
Truesdale KP Stevens J Cai J . Impact of body mass index levels on lipid abnormalities in Chinese Asians, American blacks and American whites: the People’s Republic of China (PRC) and atherosclerosis risk in communities (ARIC) studies. Atherosclerosis. (2011) 218(2):517–23. 10.1016/j.atherosclerosis.2011.06.052
23.
Goff DC Jr. Bertoni AG Kramer H Bonds D Blumenthal RS Tsai MY et al Dyslipidemia prevalence, treatment, and control in the multi-ethnic study of atherosclerosis (MESA): gender, ethnicity, and coronary artery calcium. Circulation. (2006) 113(5):647–56. 10.1161/CIRCULATIONAHA.105.552737
Summary
Keywords
cardiovascular disease, disease activity(DAS28), QRISK3 algorithm, RA-adapted SCORE2 algorithm, rheumatoid arthritis
Citation
Li X, Ni X, Qian W, Sun C, Yuan X, Zhang Y, Zhang Y and Yuan Z (2026) Underestimation of cardiovascular risk by QRISK3 and RA-adapted SCORE2 in a Chinese rheumatoid arthritis population. Front. Cardiovasc. Med. 13:1712088. doi: 10.3389/fcvm.2026.1712088
Received
24 September 2025
Revised
22 January 2026
Accepted
26 January 2026
Published
12 February 2026
Volume
13 - 2026
Edited by
Alberto Lo Gullo, Garibaldi Hospital, Italy
Reviewed by
Arianna Toscano, University Hospital of Policlinico G. Martino, Italy
Borui Li, Peking University, China
Updates
Copyright
© 2026 Li, Ni, Qian, Sun, Yuan, Zhang, Zhang and Yuan.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Zening Yuan 17625125608@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.