Abstract
Polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, and skin changes (POEMS) syndrome is frequently mistaken for chronic inflammatory demyelinating polyneuropathy, as its clinical profile is highly variable and neurological symptoms often dominate the initial course. In the present case, the disorder initially presented as cardiovascular dysfunction, which delayed decision-making related to the original diagnosis. As the condition advanced, the patient developed progressive limb weakness, splenomegaly, and abnormalities in lipid metabolism. Whole-body bone scintigraphy revealed osteolytic lesions in association with a plasmacytoma, whereas the serum vascular endothelial growth factor levels were markedly elevated. Based on the cumulative findings, POEMS syndrome was diagnosed. Treatment with interventional chemotherapy combined with adjunctive symptomatic care resulted in a marked reduction in symptoms and promoted functional recovery. This case provides detailed clinical evidence highlighting the critical importance of early recognition and accurate diagnostic evaluation in POEMS syndrome. By aligning our observations with the existing literature, we emphasize strategies that promote timely diagnosis, minimize diagnostic error, and improve therapeutic outcomes.
Introduction
POEMS syndrome is an uncommon plasma cell disorder, and its name is derived from the acronym representing its characteristic features: Polyneuropathy (P), Organomegaly (O), Endocrinopathy (E), Monoclonal protein (M), and Skin changes (S) (1). Beyond these classic features, the clinical picture may be broadened to include sclerotic bone lesions, Castleman disease, papilledema, peripheral or generalized edema, ascites, elevated platelet counts, polycythemia, thrombotic complications, and multiorgan involvement affecting the kidneys, lungs, and heart (2). From a pathophysiological perspective, two diagnostic hallmarks are particularly notable: markedly elevated vascular endothelial growth factor (VEGF) levels, reflecting a cytokine-mediated inflammatory cascade, and the presence of a monoclonal lambda (λ) immunoglobulin (3, 4). When compared with chronic inflammatory demyelinating polyneuropathy (CIDP), POEMS syndrome demonstrates more pronounced axonal injury in the peripheral nerves, with the lower limbs more severely affected than the upper limbs (5). Neuropathy typically begins with sensory disturbances such as paresthesia or hyperesthesia and gradually progresses to profound motor deficits, which include distal muscle weakness (e.g., foot drop), muscle atrophy, areflexia, and gait abnormalities (6, 7). This relentless neurological decline is a key determinant of both morbidity and mortality in the affected individuals (8). Because this disorder is rare, with a prevalence estimated at only 0.3 per 100,000 individuals (9) and presents with heterogeneous features, misdiagnosis is common. It is frequently mistaken for CIDP, which often results in diagnostic delays and missed opportunities for timely intervention (10). To minimize these risks, accurate recognition requires a meticulous diagnostic work-up that integrates detailed medical history, comprehensive clinical examination, and a collaborative, multidisciplinary assessment (11). In this report, we have described a case of POEMS syndrome that first manifested with cardiovascular abnormalities to provide a focused clinical analysis.
Case presentation
A 34-year-old man presented to our hospital with a 2-month history of persistent lower back pain, progressively accompanied by numbness and weakness in both lower limbs; 10 days prior, he had been evaluated at a local hospital, where he was diagnosed with “lumbar disc herniation,” for which he received inpatient care. Owing to inadequate symptom relief following the standard therapy, he was referred to our institution for further assessment and management.
The patient's medical history was notable for a prior admission to the Cardiology Department of our hospital from January 29 to February 2, 2024, prompted by chest tightness lasting for >10 days. At that time point, the working diagnoses included “suspected coronary artery disease under observation” and hyperlipidemia. Physical examination revealed no hepatosplenomegaly, absence of lower limb edema, and intact neurological function. He was discharged with a prescription for aspirin enteric-coated tablets and atorvastatin calcium tablets.
Upon current evaluation, physical examination revealed multiple enlarged, firm lymph nodes palpable in the bilateral submandibular and inguinal regions. The lumbar spine maintained normal physiological alignment; however, sensory testing demonstrated a diminished perception of pain and light touch in both lower limbs, accompanied by mild tenderness over the L5–S1 paravertebral area. Neurological assessment revealed the complete absence of deep tendon reflexes in the brachioradialis, biceps, triceps, patellar, and Achilles tendons bilaterally. Manual muscle testing revealed a strength of 3/5 in the tibialis anterior, extensor hallucis longus, and gastrocnemius muscles bilaterally, and 4/5 in the triceps brachii. No pathological reflexes were elicited. Electromyography indicated impaired conduction across the peripheral nerves in the upper and lower limbs, which is consistent with moderate generalized peripheral neuropathy.
Auxiliary examinations
Laboratory examinations
At the time of readmission, following an earlier misdiagnosis of “lumbar disc herniation” for bilateral lower limb numbness, the patient underwent repeat evaluation of cardiovascular parameters on November 11, 2024. The findings were as follows: lipid profile: total cholesterol = 2.62 mmol/L, high-density lipoprotein cholesterol = 0.61 mmol/L, and low-density lipoprotein cholesterol = 1.38 mmol/L; inflammatory markers: C-reactive protein = 9.8 mg/L and interleukin (IL)-6 = 9.50 pg/mL; coagulation and hematology: fibrinogen = 4.41 g/L and erythrocyte sedimentation rate = 21 mm/h; complete blood count: platelets = 354 × 109/L, mean corpuscular hemoglobin = 26.6 pg, monocytes count = 0.67 × 109/L, and red blood cell count = 5.91 × 1012/L; and VEGF = 1,262.34 pg/mL. With lumbar disc herniation effectively excluded, POEMS syndrome became the primary diagnostic consideration. Additional laboratory tests performed at this stage, including hematologic indices, coagulation profile, myocardial enzyme assays, and serum electrolytes, are presented in Table 1.
Table 1
| Test category | Parameter | Result | Test category | Parameter | Result |
|---|---|---|---|---|---|
| Coagulation panel | Plasma prothrombin time | 10.2 S | Electrolyte panel | Potassium level | 4.6 mol/L |
| Activated partial thromboplastin time | 25.0 S | Sodium level | 139.3 mol/L | ||
| Thrombin time | 18.5 S | Chloride level | 101.6 mol/L | ||
| Plasma fibrinogen | 2.27 g/L | Calcium level | 2.29 mol/L | ||
| Complete blood count (differential) | White blood cell count | 10.92 × 109/L | Lymphocyte count | 5.13 × 109/L | |
| Neutrophil percentage | 42% | Monocyte count | 0.85 × 109/L | ||
| Lymphocyte percentage | 47% | Eosinophil count | 0.18 × 109/L | ||
| Monocyte percentage | 7.8% | Basophil count | 0.17 × 109/L | ||
| Eosinophil percentage | 1.60% | Red blood cell count | 5.65 × 1012/L | ||
| Basophil percentage | 1.60% | Hemoglobin level | 153.00 g/L | ||
| Neutrophil count | 4.59 × 109/L | ||||
| Cardiac enzyme panel | Serum creatine kinase level | 71 U/L | Renal function panel | Urea level | 5.99 uoml/L |
| Serum creatine kinase-MB Isoenzyme activity | 19 U/L | Creatinine level | 73 uoml/L | ||
| α-Hydroxybutyrate Dehydrogenase level | 102 U/L | Urea-to-creatinine ratio | 0.08 | ||
| Lactate dehydrogenase level | 163 U/L | Serum bicarbonate (HCO₃⁻) level | 29.1 uoml/L | ||
| Serum lactate dehydrogenase isoenzyme level | 31 U/L | Uric acid level | 544 uoml/L | ||
| Serum β2-microglobulin level | 2.3 mg/L |
Laboratory test outcomes during November 10–20, 2024.
Boldface type indicates values out of range.
Imaging examinations
Radiographic Imaging: Plain x-ray demonstrated diffusely distributed, small nodular areas of slightly increased density involving the vertebral bodies, their appendages, pelvic bones, and ribs, with indistinct margins and no associated surrounding soft tissue masses. Marginal osteophytes were observed along the cervical vertebrae, and multiple nodules of varying sizes with low signal intensity were detected across all sequences in the vertebrae and their appendages, exhibiting relatively well-defined boundaries (Figure 1A).
Figure 1
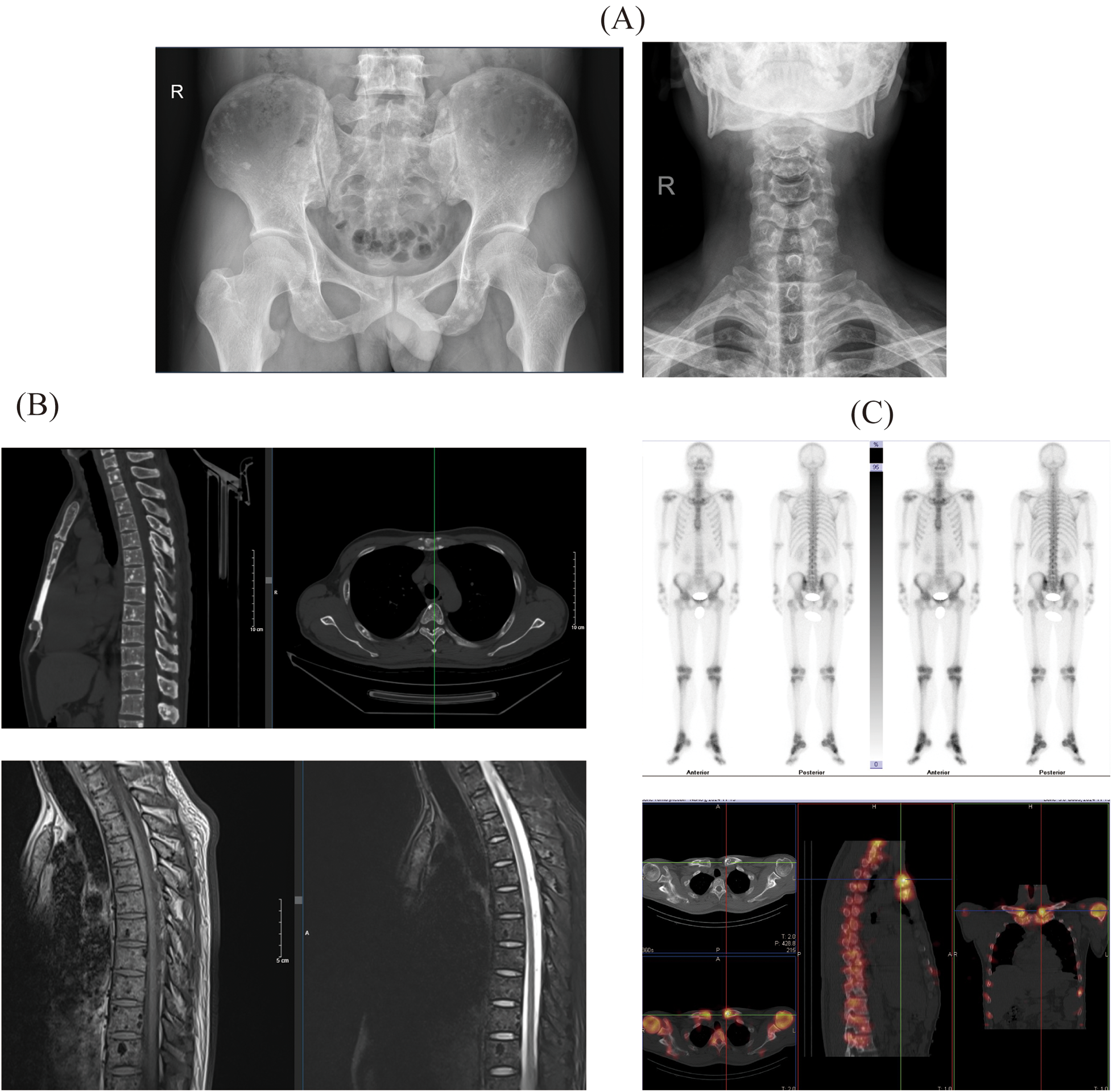
The results of the patient's whole-body imaging examination include: (A) x-ray images of the pelvis and cervical spine; (B) computed tomography (CT) and magnetic resonance imaging (MRI) results of the spine; (C) single-photon emission computed tomography (SPECT) results of the patient.
Computed Tomography (CT): CT imaging identified diffusely scattered punctate and nodular hyperdense lesions with clear margins affecting the spine, its appendages, and the pelvic bones. Magnetic Resonance Imaging (MRI): MRI revealed multiple nodules of varying size, exhibiting low signal intensity across all sequences, which were noted within the vertebral bodies and appendages, with no enhancement following contrast administration (Figure 1B).
Single-Photon Emission Computed Tomography (SPECT): SPECT imaging demonstrated diffusely distributed punctate, nodular, and larger patchy hyperdense areas within the spine and its appendages, sternum, both shoulder joints, and bilateral ribs, clavicles, and humeri (Figure 1C).
Pathology and Immunohistochemistry: Histopathological analysis of the left inguinal lymph node, performed on November 14, 2024, revealed chronic inflammation with reactive hyperplasia (Figure 2B). The examination of the left inguinal skin demonstrated chronic inflammatory changes, thinning of the squamous epithelium, and basal cell layer pigmentation (Figure 2C).
Figure 2
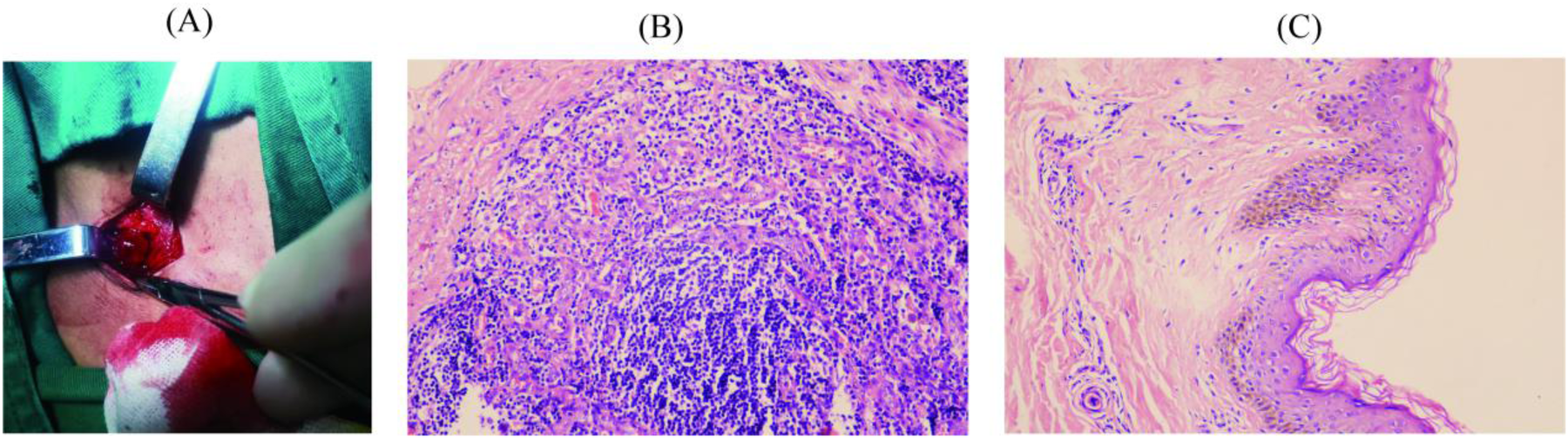
Clinical samples and hematoxylin-eosin staining results of the patient. (A) Inguinal lymph node sample; (B) Pathological findings of the left inguinal lymph node; (C) Pathological findings of the left inguinal skin.
Laboratory and Diagnostic Investigations: Serum immunofixation electrophoresis identified a monoclonal immunoglobulin of the IgA-λ subtype (Figure 3A). Urinary Bence–Jones protein analysis revealed precipitation bands corresponding to the λ-light chain and free λ-light-chain regions, confirming the presence of Bence–Jones protein of the λ-free light-chain type (Figure 3B). Immunohistochemical assessment revealed a mild increase in the plasma cells (approximately 2%), which raised suspicion for plasmacytoma (Figure 3C).
Figure 3
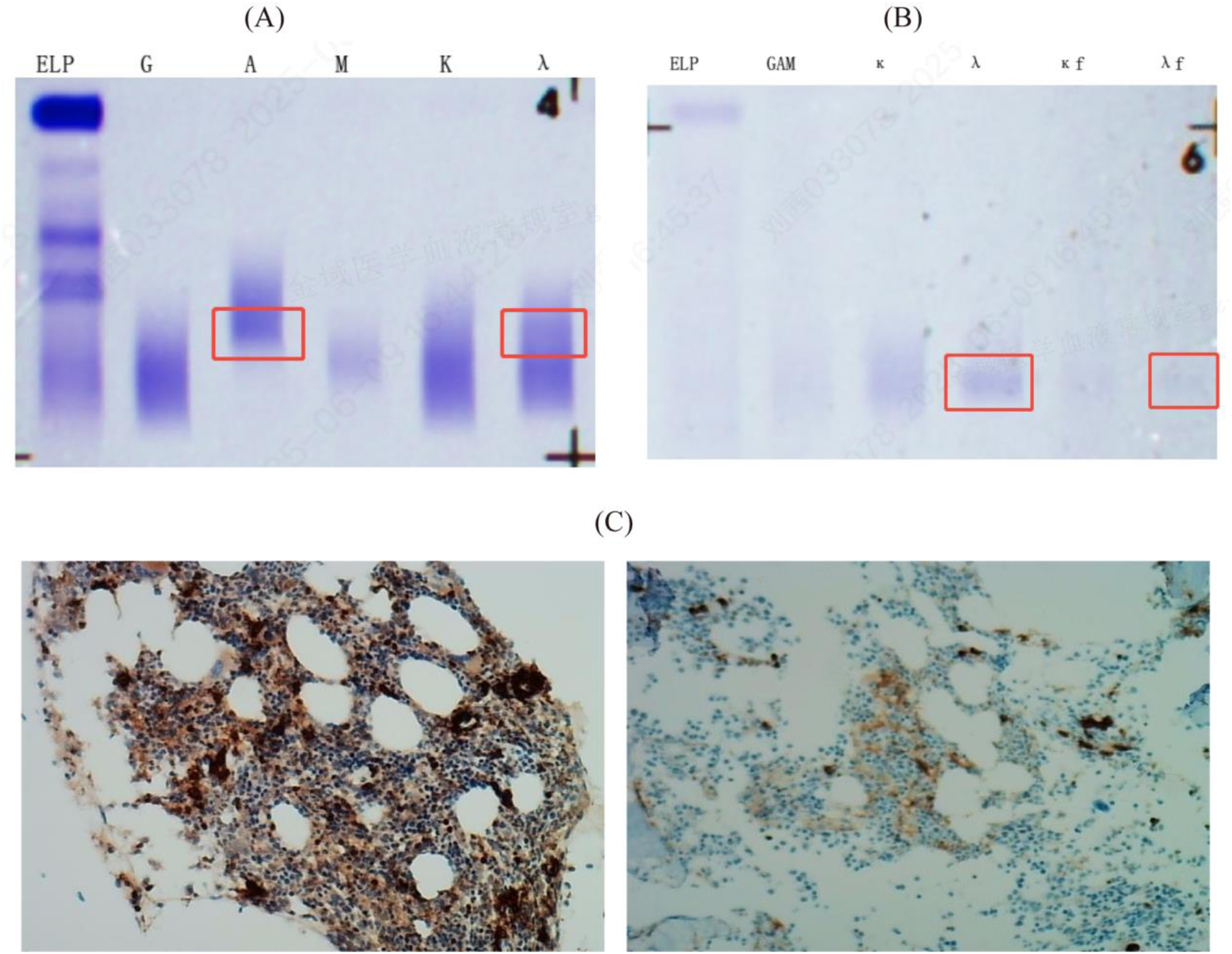
Results of serum immunofixation electrophoresis (IFE), urine bence-jones protein electrophoresis (BJP) and immunohistochemistry in the patient. (A) Serum IFE showed a monoclonal immunoglobulin type of IgA-λ in the IgA, IgG, IgM, κ, and λ lanes; (B) Urine BJP was positive, with the type being λ free light chain; BJP: Bence-Jones protein; ELP: Immunofixation electrophoresis; G: IgG; A: IgA; M: IgM; D: IgD; E: IgE; κ: κ chain; λ: λ chain; κf: Free κ chain; λf: Free λ chain. (C) CD38 scattered and small clusters (2%, strong +), Kappa scattered few (+), Lambda scattered and small clusters (+), CD19 scattered and 2 small nodules (+), Cyclin-D1 (−), MUM1 scattered and small clusters (+), BCMA (2%, moderate +), GPRC5D (1%, moderate +), P53 (5% +), BCL-2 scattered and 2 small nodules (+), Ki-67 (40% +).
Endocrine evaluation showed a serum prolactin level of 16.36 ng/mL and a testosterone level of 97.06 ng/dL. Electromyographic studies demonstrated abnormal nerve conduction in the upper and lower limbs, with neurogenic changes indicative of multiple peripheral nerve injuries. These findings indicated a combination of demyelination and axonal degeneration of moderate severity, consistent with the 2023 mandatory major diagnostic criteria for POEMS syndrome (12).
The imaging results corroborated these findings, confirming osteosclerotic lesions and markedly elevated serum VEGF levels. Fundoscopic examination revealed intact macular regions bilaterally, accompanied by optic disc edema (Figure 4A). CT of the abdomen revealed splenomegaly, with a maximal longitudinal dimension of 127.65 mm and a thickness of approximately 5.0 cm at the splenic hilum (Figure 4B).
Figure 4
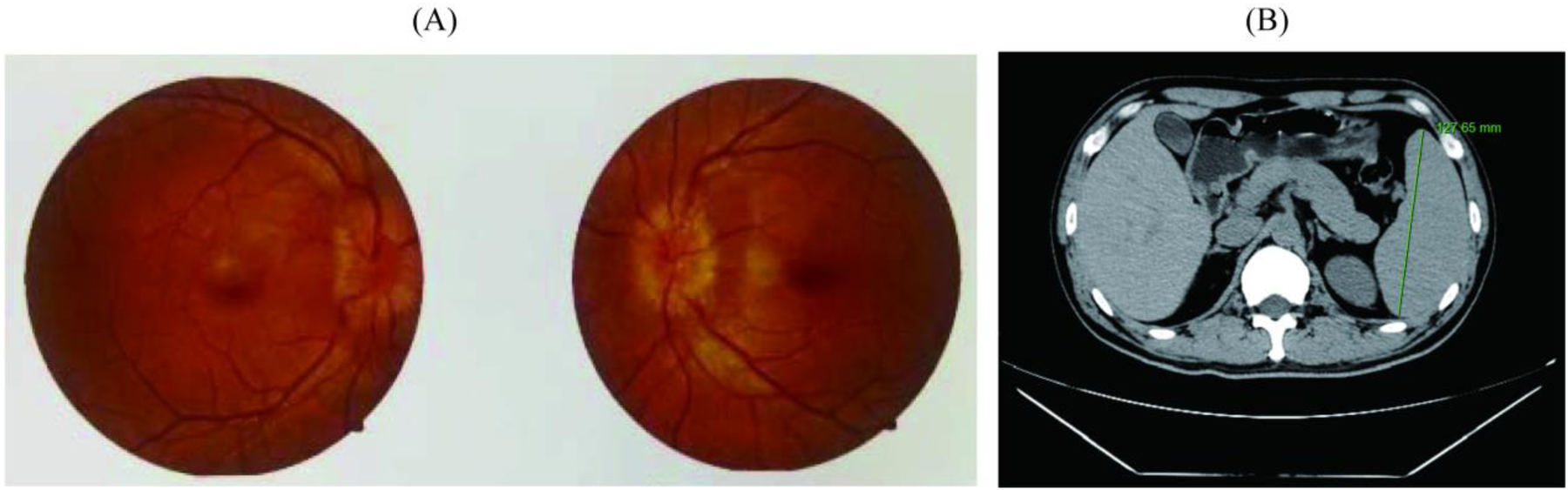
Examination results of patient-related complications. (A) Results of fundus optic nerve examination; (B) Results of spleen computed tomography scan.
Following a multidisciplinary consultation and comprehensive integration of clinical, laboratory, and imaging findings while systematically excluding alternative diagnoses, a definitive diagnosis of POEMS syndrome was established. Renal function parameters pre- and post-treatment are summarized in Table 2.
Table 2
| Time of assessment | Uric acid level | β2-microglobulin level |
|---|---|---|
| After Treatment | 243 uoml/L | 4.8 mg/L |
| Before Treatment | 496 uoml/L | 2.3 mg/L |
Renal function parameters of the study patients before and after treatment.
Boldface type indicates values out of range.
Treatment and outcome
Following confirmation of the POEMS syndrome, the patient was initiated on a therapeutic regimen on November 20, 2024, comprising lenalidomide (25 mg orally on days 1–21) and dexamethasone (20 mg intravenously on days 1, 8, 15, and 22 of each 28-day cycle). Adjunctive supportive measures included aspirin enteric-coated tablets to inhibit platelet aggregation, atorvastatin calcium tablets for plaque stabilization, mecobalamin to support peripheral nerve repair, rabeprazole enteric-coated tablets for acid suppression and gastric mucosal protection, and sucralfate suspension gel to reinforce gastric mucosal protection. Follow-up neurological assessments conducted in March and June 2025 revealed substantial clinical improvement, as evidenced by increased muscle strength in both lower limbs, enhanced gait stability, and a notable reduction in fall frequency.
Discussion
POEMS syndrome is a rare and complex plasma cell disorder that is frequently misdiagnosed in medically underserved regions owing to its broad spectrum of clinical manifestations, often leading to delayed treatment and progressive morbidity (13). Management primarily focuses on two objectives: eradication of the underlying clonal plasma cell population and provision of supportive care to address the syndrome's diverse systemic effects (14).
In our patient, the initial presentation consisted of chest tightness accompanied by a paroxysmal “obstructive” sensation beneath the lower sternum. Coronary artery disease was initially suspected, and symptomatic treatment with Compound Danshen tablets, fluvastatin, and atorvastatin calcium tablets provided only transient relief, with symptoms recurring intermittently. Notably, POEMS syndrome cases that initially present with cardiac involvement tend to follow a more aggressive clinical course (15, 16), potentially progressing to cardiomyopathy, pericarditis, pericardial effusion, or heart failure. Among these complications, heart failure accounts for up to one-third of disease-related mortality (17, 18). Vascular endothelial growth factor (VEGF), a pivotal cytokine in angiogenesis, increases vascular permeability, promotes neovascularization, and contributes to osteoblast activation (19). Elevated VEGF levels play a central role in the development of the characteristic clinical manifestations of POEMS syndrome (2). Therefore, in Latin America, measurement of VEGF is a key component of the diagnostic criteria for POEMS syndrome (20). The majority of patients with POEMS syndrome exhibit clonal proliferation of λ-type plasma cells, often with restricted VJ region usage in the bone marrow, reflecting the monoclonal origin of the disease (21). Certain λ light chains may engage in molecular mimicry with receptors involved in VEGF secretion, thereby directly promoting VEGF oversecretion (22). Therefore, the combination of unexplained cardiac abnormalities and elevated serum VEGF levels should prompt heightened clinical suspicion for POEMS syndrome.
Skeletal lesions in POEMS syndrome typically progress slowly through sclerotic or reparative processes (23). Therapeutic interventions directed at the underlying plasma cell clone can promote re-ossification of osteolytic lesions and stabilization of sclerotic bone changes, potentially reducing compressive effects on adjacent neural structures (24). The neurological manifestations in this case were prototypical and provided critical diagnostic clues. The patient presented with bilateral lower limb numbness and weakness, and electromyography demonstrated a mixed pattern of moderate demyelination and axonal injury, consistent with the characteristic peripheral neuropathy of POEMS syndrome. It has been established that dysregulated VEGF levels are closely associated with systemic skeletal abnormalities and vascular pathology, including coronary atherosclerosis. Effective reduction of VEGF can simultaneously ameliorate multiple clinical features, such as papilledema and cardiac dysfunction, while normalizing bone metabolism (25, 26). Conversely, VEGF also serves as a sensitive biomarker of the therapeutic response. In clinical studies of POEMS syndrome, extravascular volume overload has been observed in at least one-third of patients, most commonly manifesting as peripheral edema or serous cavity effusions, including ascites or pleural effusion (27). However, their secondary impact on peripheral nerves, whether through mechanical compression or VEGF-induced endoneurial edema, may be reversible with appropriate therapy. Furthermore, involvement of the motor central nervous system may be more common than previously recognized (28). In studies by Maroun et al., systemic therapy not only achieved hematologic remission but also produced a rapid decline in serum VEGF levels (29, 30). This biochemical improvement was subsequently associated with measurable recovery of neurological function, often accompanied by stabilization or partial restoration of neurophysiological parameters (31). The underlying mechanism likely involves a reduction in VEGF-mediated vascular permeability, resulting in decreased endoneurial edema and the restoration of a microenvironment conducive to nerve repair (32). In cases where clinical evaluation reveals suggestive features, such as cutaneous changes or pedal edema, assessment of VEGF levels should be strongly prioritized (33). Concurrently, vigilant monitoring of organ involvement is essential, as pathological changes may arise in the spleen, liver, or lymph nodes, which are components of the ancillary diagnostic criteria, as the disease progresses (34).
Given the broad and variable presentation of POEMS syndrome, patients who initially present with lower limb weakness are at high risk of being misdiagnosed with other neurological disorders, particularly chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) (35). Therefore, key strategies to minimize early stage misdiagnosis include maintaining a high index of suspicion for POEMS in patients with non-specific polyneuropathy syndromes; incorporating serum VEGF measurement and M-protein screening into the routine workup for neuropathy workup; and recognizing POEMS as a systemic disorder that necessitates multidisciplinary collaboration spanning neurology, hematology, endocrinology, and radiology, for diagnosis and effective management.
Early involvement of a multidisciplinary team, together with improved clinician expertise in distinguishing POEMS syndrome from other disorders and a deeper understanding of its pathophysiology, can substantially reduce the risk of delayed or missed diagnosis. Additionally, enhancing patient education and increasing disease awareness can facilitate earlier recognition and timely intervention. Collectively, these strategies may transform the management of POEMS syndrome from a historically challenging condition into one that is increasingly identifiable, controllable, and responsive to precision therapies.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Hechi Hospital, The First Affiliated Hospital of Guangxi Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a subset of the patients' case data was extracted for research purposes. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XT: Funding acquisition, Writing – original draft. GF: Writing – original draft. JY: Investigation, Writing – original draft. CL: Supervision, Writing – review & editing. ZQ: Funding acquisition, Supervision, Writing – original draft.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This research was funded by Hechi Science and Technology Plan Project Task Document (grant number AB240803); Guangxi Medical and Health Self-funded Research Project (grant number Z-M20221848), and Institutional Research Project at Youjiang Medical University for Nationalities (grant number yy2024ky035).
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2026.1716667/full#supplementary-material
References
1.
Jurczyszyn A Olszewska-Szopa M Vesole D . POEMS syndrome-clinical picture and management. Current knowledge. Clin Lymphoma Myeloma Leuk. (2023) 23(8):575–82. 10.1016/j.clml.2023.04.008
2.
Suichi T Misawa S . POEMS syndrome. Brain Nerve. (2024) 76(5):547–54. 10.11477/mf.1416202642
3.
Watanabe O Arimura K Kitajima I Osame M Maruyama I . Greatly raised vascular endothelial growth factor (VEGF) in POEMS syndrome. Lancet (London, England). (1996) 347(9002):702. 10.1016/S0140-6736(96)91261-1
4.
Soubrier M Dubost J Serre AF Ristori J Sauvezie B Cathebras P et al Growth factors in POEMS syndrome: evidence for a marked increase in circulating vascular endothelial growth factor. Arthritis Rheum. (1997) 40(4):786–7. 10.1002/art.1780400430
5.
Nasu S Misawa S Sekiguchi Y Shibuya K Kanai K Fujimaki Y et al Different neurological and physiological profiles in POEMS syndrome and chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatr. (2012) 83(5):476–9. 10.1136/jnnp-2011-301706
6.
Koike H Iijima M Mori K Yamamoto M Hattori N Watanabe H et al Neuropathic pain correlates with myelinated fibre loss and cytokine profile in POEMS syndrome. J Neurol Neurosurg Psychiatr. (2008) 79(10):1171–9. 10.1136/jnnp.2007.135681
7.
Keddie S Lunn MP . POEMS syndrome. Curr Opin Neurol. (2018) 31(5):551–8. 10.1097/WCO.0000000000000610
8.
Koike H Iijima M Mori K Yamamoto M Hattori N Watanabe H et al A case report of POEMS syndrome with lower limb numbness and weakness. Asian J Surg. (2023) 46(11):4905–6. 10.1016/j.asjsur.2023.05.161
9.
Twigg J Methley A Lavin T Dickinson G Teager A . Living with polyneuropathy organomegaly endocrinopathy monoclonal gammopathy skin changes (POEMS) syndrome: a case study of healthcare experiences and quality of life. Disabil Rehabil. (2021) 43(17):2502–10. 10.1080/09638288.2019.1700563
10.
Kuwabara S Suichi T Misawa S . ‘Early VEGF testing in inflammatory neuropathy avoids POEMS syndrome misdiagnosis and associated costs’ by Marsh et al. J Neurol Neurosurg Psychiatr. (2021) 92(2):118–9. 10.1136/jnnp-2020-323528
11.
Ozden Y Gursoy S . From hepatomegaly to POEMS syndrome: a case report. Hepatology Forum. (2024) 5(1):44–6. 10.14744/hf.2022.2022.0035
12.
Dispenzieri A . POEMS Syndrome: update on diagnosis, risk-stratification, and management. Am J Hematol. (2023) 98(12):1934–50. 10.1002/ajh.27081
13.
D’Sa S Khwaja J Keddie S Keh RY Smyth D Ronneberger R et al Comprehensive diagnosis and management of POEMS syndrome. HemaSphere. (2022) 6(11):e796. 10.1097/HS9.0000000000000796
14.
Khouri J Nakashima M Wong S . Update on the diagnosis and treatment of POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes) syndrome: a review. JAMA Oncol. (2021) 7(9):1383–91. 10.1001/jamaoncol.2021.0586
15.
Takahashi Y Iwano H Nakano I Fukushima A Naya M Shimizu A et al POEMS Syndrome showing left ventricular dysfunction and extracellular edema assessed by cardiac magnetic resonance imaging. Intern Med (Tokyo, Japan). (2019) 58(17):2539–43. 10.2169/internalmedicine.2842-19
16.
Levene J Murray N Desai S Simpson TF Karam C Silbermann R et al Pericardial tamponade and other cardiac complications in POEMS syndrome. JACC Case Rep. (2021) 3(2):286–90. 10.1016/j.jaccas.2020.12.027
17.
Dispenzieri A Kyle RA Lacy MQ Rajkumar SV Therneau TM Larson DR et al POEMS Syndrome: definitions and long-term outcome. Blood. (2003) 101(7):2496–506. 10.1182/blood-2002-07-2299
18.
Nakanishi T Sobue I Toyokura Y Nishitani H Kuroiwa Y Satoyoshi E et al The crow-fukase syndrome: a study of 102 cases in Japan. Neurology. (1984) 34(6):712–20. 10.1212/WNL.34.6.712
19.
Wang C Huang X-F Cai Q-Q Cao X-X Cai H Zhou D et al Remarkable expression of vascular endothelial growth factor in bone marrow plasma cells of patients with POEMS syndrome. Leuk Res. (2016) 50:78–84. 10.1016/j.leukres.2016.09.017
20.
Gallardo-Pérez MM Negrete-Rodríguez P Gertz MA Peña C Riva E Gilli V et al The Latin-American experience in POEMS syndrome: a study of the GELAMM (Grupo de Estudio Latinoamericano de Mieloma Múltiple). Acta Haematol. (2025) 148:249–57. 10.1159/000540890
21.
Merlini G Stone MJ . Dangerous small B-cell clones. Blood. (2006) 108(8):2520–30. 10.1182/blood-2006-03-001164
22.
Jaccard A Pascal V Magy L Roussel M . POEMS Syndrome. Presse Med (Paris, France: 1983). (2025) 54(1):104270. 10.1016/j.lpm.2025.104270
23.
Adam Z Řehák Z Keřkovský M Povýšil C Ezer E Buliková A et al Monoclonal gammopathy of clinical signifi cance with osteosclerotic lesions—a case report and a literature review. Klin Onkol. (2024) 37(3):209–19. 10.48095/ccko2024209
24.
Gao X-M Li A-A Zhao H Shen K-N Li J . Long-term outcomes of newly diagnosed POEMS syndrome patients who received first-line lenalidomide-based therapy. Haematologica. (2024) 109(11):3776–80. 10.3324/haematol.2024.285282
25.
Kastritis E Terpos E Anagnostopoulos A Xilouri I Dimopoulos MA . Angiogenetic factors and biochemical markers of bone metabolism in POEMS syndrome treated with high-dose therapy and autologous stem cell support. Clin Lymphoma Myeloma. (2006) 7(1):73–6. 10.3816/CLM.2006.n.043
26.
Li J Zhou DB . New advances in the diagnosis and treatment of POEMS syndrome. Br J Haematol. (2013) 161(3):303–15. 10.1111/bjh.12236
27.
Dispenzieri A . POEMS Syndrome: 2019 update on diagnosis, risk-stratification, and management. Am J Hematol. (2019) 94(7):812–27. 10.1002/ajh.25495
28.
Uyanık HU Yıldız FG Gülmez B Tan E Temuçin ÇM . Polyneuropathy with motor conduction block in POEMS. Muscle Nerve. (2025) 71(2):159–65. 10.1002/mus.28302
29.
Bou Zerdan M George TI Bunting ST Chaulagain CP . Recent advances in the treatment and supportive care of POEMS syndrome. J Clin Med. (2022) 11(23):7011. 10.3390/jcm11237011
30.
Ocampo-Navia MI Noreña MAN Santos LRC Afanador JRM Farías RAQ Sánchez JLB . POEMS Syndrome diagnosis in a patient with mixed polyneuropathy: case report. Prague Med Rep. (2022) 123(1):27–34. 10.14712/23362936.2022.3
31.
Nobile-Orazio E Terenghi F Giannotta C Gallia F Nozza A . Serum VEGF levels in POEMS syndrome and in immune-mediated neuropathies. Neurology. (2009) 72(11):1024–6. 10.1212/01.wnl.0000344569.13496.ff
32.
Misawa S . Electrophysiologic aspects of crow-fukase (POEMS) syndrome–significance in early diagnosis and insights into the pathophysiology. Brain Nerve/Shinkei Kenkyu Shinpo. (2008) 60(6):595–601.
33.
Marsh ES Keddie S Terris-Prestholt F D’Sa S Lunn MP . Early VEGF testing in inflammatory neuropathy avoids POEMS syndrome misdiagnosis and associated costs. J Neurol Neurosurg Psychiatr. (2021) 92(2):172–6. 10.1136/jnnp-2020-324012
34.
León AA Urteaga MG Correa KM Garcia WJ Crovetto MG Correa KM et al A case report of polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes (POEMS) syndrome: a diagnostic iceberg. Cureus. (2024) 16(3):e56229. 10.7759/cureus.56229
35.
Arkhipov IE Vergunova IY Malkova NA Korobko DS . POEMS-syndrome. Zh Nevrol Psikhiatr Im S.S. Korsakova. (2023) 123:15–21. 10.17116/jnevro202312307215
Summary
Keywords
cardiovascular lesions, peripheral neuropathy, POEMS syndrome, polycythemia, sclerotic bone lesion
Citation
Tan X, Fan G, Yao J, Lu C and Qin Z (2026) POEMS syndrome with cardiovascular lesions as the initial manifestation: a case report and literature review. Front. Cardiovasc. Med. 13:1716667. doi: 10.3389/fcvm.2026.1716667
Received
30 September 2025
Revised
03 January 2026
Accepted
06 January 2026
Published
30 January 2026
Volume
13 - 2026
Edited by
Ye Zhang, Fourth Military Medical University, China
Reviewed by
Shuai Tan, Capital Medical University, China
Moisés M. Gallardo-Pérez, Universidad Popular Autónoma del Estado de Puebla, Mexico
Updates
Copyright
© 2026 Tan, Fan, Yao, Lu and Qin.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Zhaojie Qin qinzhaojie163@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.