Abstract
Background and aims:
Hypertrophic cardiomyopathy (HCM) is characterized by left ventricular hypertrophy and diastolic dysfunction. While sodium-glucose cotransporter 2 inhibitors (SGLT-2i) have demonstrated efficacy in heart failure (HF), their role in HCM remains underexplored. This real-world study aimed to evaluate the clinical efficacy of SGLT-2i in HCM patients.
Methods and results:
A retrospective analysis was conducted on HCM patients admitted between January 2021 and December 2024. After PSM, 94 patients initiating SGLT-2i were compared with 94 controls. Primary endpoints included changes (Δ) in echocardiographic parameters and NYHA class at 6-month follow-up. Secondary endpoint was readmission for HF by June 2025. At 6-month follow-up, patients treated with SGLT-2i showed significantly greater improvements in key parameters compared to controls: septal e′ (Δ 0.7 ± 1.3 vs. Δ 0.04 ± 1.6 cm/s, p = 0.002), E/e′ (Δ −5.1 ± 8.7 vs. Δ 0.4 ± 6.4, p < 0.001), and IVST (Δ −1.3 vs. Δ −0.2 mm, p = 0.005), alongside a greater reduction in NYHA class [−1 (−1 to −0.25) vs. −1 (−1 to 0), p = 0.031]. Multivariate analysis confirmed sustained differences in improvements of septal e′ (t = 2.26, p = 0.025), E/e′ (t = −3.75, p < 0.001) and NYHA class (p = 0.038). No significant difference was found in HF readmission (20 events in SGLT-2i group vs. 17 in control group; log-rank p = 0.73) after 16.3-month median follow-up. No hypoglycemic events occurred and there was no significant deterioration in renal function.
Conclusion:
SGLT-2i administration was associated with improved left ventricular diastolic function and NYHA class in HCM patients without increasing risks of renal dysfunction or hypoglycemia, supporting its potential therapeutic value in this population.
Introduction
Hypertrophic cardiomyopathy (HCM) is a hereditary cardiomyopathy characterized by left ventricular (LV) hypertrophy, often accompanied by diastolic dysfunction, myocardial fibrosis and microvascular dysfunction (1–4). Its prevalence is estimated at 1:500 in the general population, and may reach 1:200 when undiagnosed or asymptomatic cases are considered, highlighting its substantial clinical and public health impact (1).
Conventional treatment strategies for HCM have focused on symptom control and risk reduction through β-blockers, calcium channel blockers, and invasive procedures. Septal reduction therapy (SRT) is reserved for patients with significant left ventricular outflow tract obstruction (LVOTO), whereas implantable cardioverter-defibrillators (ICDs) are employed for preventing sudden cardiac death both primarily and secondarily (2–4). Although advances in understanding have reduced annual mortality from 6% to approximately 0.5%—now comparable to the general population (1)—many patients continue to experience impaired quality of life due to dynamic LVOTO, diastolic impairment, arrhythmias, and progression to HF (1), emphasizing the demand for targeted therapies.
Mavacamten, a novel cardiac myosin inhibitor, represents a therapeutic breakthrough by specifically attenuating LVOTO and postponing SRT in suitable candidates (5, 6). Despite its clinical benefits, widespread use is limited by high costs and restricted availability.
Sodium-glucose cotransporter 2 inhibitor (SGLT-2i) is originally used to treat diabetes mellitus (DM) through inhibiting the reabsorption of glucose by the epithelial cells of the renal proximal tubule, increasing the excretion of glucose in the urine, and thus lowering blood sugar (7). Apart from glycemic benefits, SGLT-2i have exhibited considerable cardioprotective properties in heart failure patients—including those with reduced or preserved ejection fraction (HFrEF and HFpEF)— regardless of DM (8, 9). Mechanistic studies suggest these drugs improve myocardial energetics, reduce oxidative stress and inflammatory responses, suppress fibrotic processes, promote reverse remodeling, and lessen calcium accumulation (10). Given that these actions correspond to central pathological features of HCM, SGLT-2i may offer potential in enhancing diastolic performance and alleviating hypertrophy and fibrosis (3).
Notably, pivotal randomized controlled trials (RCTs) establishing the cardiovascular benefits of SGLT-2i systematically excluded patients with HCM (11, 12). Consequently, the current evidence guiding their use in HCM is extrapolated from studies in HFpEF, a population with distinct pathophysiology. This has created a critical evidence gap regarding the efficacy of SGLT-2i on HCM-specific pathological features, such as left ventricular hypertrophy and diastolic dysfunction. To address this gap, we conducted this real-world, propensity score-matched (PSM) study with two primary objectives: first, to evaluate the effects of SGLT-2i on echocardiographic markers of cardiac structure and diastolic function specific to HCM; and second, to assess their safety and preliminary impact on functional status in this understudied population.
Methods
Study population
This was a single-center retrospective cohort study. We searched inpatients from January 2021 to December 2024 from electronic medical records in our department. Patients were included meeting the following criteria: ① diagnosed with HCM (3), which was defined echocardiographically as a maximal left ventricular wall thickness ≥15 mm in the absence of other cardiac or systemic diseases that could account for the hypertrophy; ② ≥18 years old; ③ patients with both obstructive (left ventricular outflow tract pressure gradient ≥30 mmHg) and non-obstructive forms of HCM were eligible for inclusion.
Exclusion criteria were as follows: ① previously used SGLT-2i; ② combined with severe renal insufficiency (eGFR <30 mL/min/1.73 m2); ③ echocardiographic data were not available at 6 (±1) months after discharge; ④ STR was performed during the follow-up period.
Given that this was a retrospective observational study and no patient intervention was required, informed consent from subjects could be waived, which was approved by the ethics committee. In conducting the study, we maintained a strong focus on the protection of patients’ personal interests, rights, privacy, and image rights.
Group assignment
Based on SGLT-2i exposure, patients selected were classified to two groups: the SGLT-2i group (newly initiated during the admission) and the control group (no SGLT-2i use). Medication history was obtained from electronic prescription records.
Outcomes
Our primary endpoints were the echocardiographic parameters and NYHA class, the former including mitral annular tissue velocity (e′), early diastolic mitral inflow velocity (E)/e′, and interventricular septal thickness (IVST) at 6-month (±1 month) follow-up.
Secondary endpoint was unplanned HF readmission (based on ICD-10 codes) by June 2025. Hospitalizations where HF was not the primary driver (e.g., for arrhythmia without acute HF, pneumonia, or elective surgery) were excluded. This endpoint was adjudicated by two independent cardiologists (F.L and L.W).
Safety endpoints included adverse reactions such as hypoglycemia and urinary tract infection.
Data collection
Baseline data from HCM patients were collected, including medical history, concomitant medications, laboratory parameters—such as serum creatinine, HbA1c, fasting plasma glucose (FPG), B-type natriuretic peptide (BNP)—as well as echocardiographic measures at baseline and 6 months (±1 month) after discharge. We also recorded endpoint events including HF readmission, hypoglycemia and urinary tract infection.
For patients not followed up at our institution, telephone follow-up was made to obtain follow-up information from other healthcare facilities and to ascertain whether endpoint events had occurred. Those without any post-discharge follow-up data were excluded from the study.
Statistical analysis
We employed 1:1 PSM to balance baseline characteristics between the groups. The propensity score model incorporated clinically relevant confounding factors, including sex, age, body mass index (BMI), history of hypertension, and DM. Matching was performed using a 1:1 nearest-neighbor algorithm with a caliper width set to 0.2 standard deviations of the logit propensity score. Covariate balance was assessed by calculating absolute standardized mean differences (SMD) for all baseline variables.
Numbers (frequencies) were used to describe categorical variables, mean ± standard deviation to describe normal distribution continuous variables while median (interquartile range, IQR) to describe non-normal distribution. χ2 test was used for comparison between categorical variables, while T-test was used for normally distributed continuous variables and Mann–Whitney U test for non-normal continuous variables. Multivariable analysis was performed using linear regression or a bootstrap multiple linear regression model (1,000 repetitions), depending on whether the continuous variables were normally distributed or not. All fitted models were subjected to diagnostic checks, which confirmed the adherence to key statistical assumptions: residual independence (Durbin-Watson statistic), homoscedasticity, and approximate normality of residuals. Furthermore, the absence of significant multicollinearity was verified (all variance inflation factors <5). Model fit was reported using the adjusted R2.
Kaplan–Meier curve was used to evaluate differences in HF readmission events. We also performed subgroup analysis of obstructive hypertrophic cardiomyopathy (oHCM) and non-obstructive hypertrophic cardiomyopathy (noHCM) at low-risk.
We used IBM SPSS version 26.0 (SPSS Inc., Chicago, IL, USA) and R version 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria) to perform the statistical analyses described above.
Results
Baseline characteristics
Full cohort
A total of 395 patients with HCM were enrolled from January 2021 to December 2024. After excluding 142 individuals based on the criteria outlined in Figure 1, 253 eligible patients were included in the analysis. Among them, 163 (64.4%) had hypertension, 96 (37.9%) had DM, and 148 (58.4%) were diagnosed with HF. The mean age was 65.0 ± 14.7 years, and 67.2% (170/253) were male. Baseline characteristics are presented in Table 1. Within the full cohort, 94 patients (37.2%) received SGLT-2i. Compared to non-users, those treated with SGLT-2i were generally older and showed a higher prevalence of hypertension, DM, HF, and AF. They also had elevated baseline values of BNP, HbA1c, FPG, septal e′, and E/e′.
Figure 1
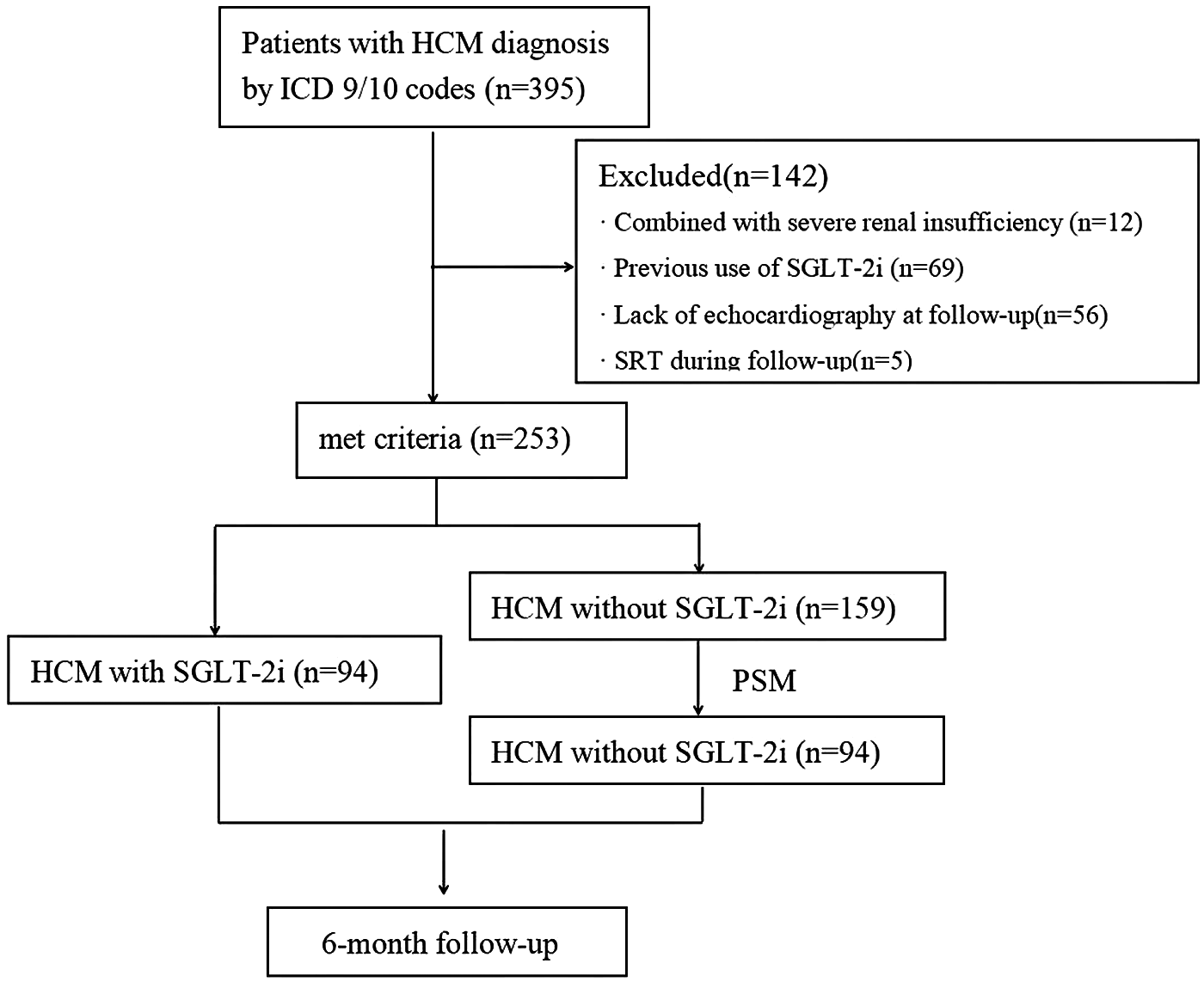
Flowchart depicting the participant selection process for the study. HCM, hypertrophic cardiomyopathy; ICD, international Classification of diseases; SGLT-2i, sodium-glucose cotransporter-2 inhibitors; SRT, Septal reduction therapy; PSM, propensity score matching.
Table 1
| Parameter | Full cohort | PSM cohort | |||||
|---|---|---|---|---|---|---|---|
| SGLT-2i (+) n = 94 | SGLT-2i (−) N = 159 | p value | SGLT-2i (+) n = 94 | SGLT-2i (−) n = 94 | p value | SMD | |
| Age, years | 69.0 ± 14.0 | 64.0 ± 14.7 | 0.007 | 69.0 ± 14.0 | 65.4 ± 14.8 | 0.092 | 0.24 |
| Male sex, n (%) | 62 (66.0%) | 108 (67.9%) | 0.75 | 62 (66.0%) | 65 (69.1%) | 0.64 | 0.074 |
| Smoking, n (%) | 49 (52.1%) | 75 (47.2%) | 0.45 | 49 (52.1%) | 43 (45.7%) | 0.38 | 0.13 |
| BMI, kg/m2a | 23.2 (21.9 to 26.1) | 24.1 (20.5 to 26.4) | 0.28 | 23.2 (21.9 to 26.1) | 23.6 (21.8 to 25.8) | 0.77 | 0.13 |
| Blood pressure, mmHg | |||||||
| Systolic | 131.8 ± 21.7 | 139.0 ± 25.2 | 0.048 | 131.8 ± 21.7 | 137.5 ± 24.9 | 0.096 | 0.24 |
| Diastolic | 74.9 ± 15.8 | 78.5 ± 17.4 | 0.13 | 74.9 ± 15.8 | 78.0 ± 16.7 | 0.19 | 0.19 |
| HR, bpm | 74.9 ± 15.3 | 75.7 ± 16.3 | 0.76 | 74.9 ± 15.3 | 76.6 ± 16.4 | 0.46 | 0.11 |
| NYHA class | 0.001 | 0.16 | 0.28 | ||||
| NYHA II | 35 (37.2%) | 95 (59.7%) | – | 35 (37.2%) | 48 (51.1%) | – | – |
| NYHA III | 48 (51.1%) | 46 (28.9%) | – | 48 (51.1%) | 38 (40.4%) | – | – |
| NYHA IV | 11 (11.7%) | 18 (11.3%) | – | 11 (11.7%) | 8 (8.5%) | – | – |
| Comorbidities | |||||||
| Hypertension, n (%) | 68 (72.3%) | 95 (59.7%) | 0.043 | 68 (72.3%) | 71 (75.5%) | 0.64 | 0.078 |
| CAD, n (%) | 39 (41.5%) | 60 (37.7%) | 0.75 | 39 (41.5%) | 37 (39.4%) | 0.77 | 0.064 |
| DM, n (%) | 46 (48.9%) | 50 (31.4%) | 0.006 | 46 (48.9%) | 40 (41.7%) | 0.42 | 0.13 |
| HF, n (%) | 75 (79.8%) | 73 (45.9%) | <0.001 | 75 (79.8%) | 59 (62.8%) | 0.01 | 0.37 |
| Af, n (%) | 45 (53.2%) | 49 (30.9%) | 0.007 | 45 (53.2%) | 36 (38.3%) | 0.19 | 0.20 |
| Stroke, n (%) | 20 (21.3%) | 21 (13.2%) | 0.12 | 20 (21.3%) | 16 (17.0%) | 0.46 | 0.11 |
| CKD, n (%) | 30 (31.9%) | 38 (23.9%) | 0.18 | 30 (31.9%) | 26 (27.7%) | 0.53 | 0.09 |
| COPD, n (%) | 6 (6.4%) | 6 (3.8%) | 0.38 | 6 (6.4%) | 3 (3.2%) | 0.31 | 0.15 |
| Laboratory data | |||||||
| Hemoglobin, mg/dL | 136.3 ± 19.2 | 137.6 ± 19.6 | 0.63 | 136.3 ± 19.2 | 134.4 ± 18.4 | 0.48 | 0.10 |
| Creatinine, mg/dL | 95.1 ± 23.4 | 86.9 ± 36.9 | 0.018 | 95.1 ± 23.4 | 91.7 ± 35.1 | 0.44 | 0.11 |
| BNP, pg/mLa | 446.0 (289.0 to 1,007.1) | 110.0 (10.0 t o870.3) | <0.001 | 446.0 (289.0 to 1,007.1) | 209.3 (55.5 to 565.3) | 0.005 | 0.51 |
| FPG, mmol/L | 6.7 ± 2.6 | 5.9 ± 2.0 | 0.006 | 6.7 ± 2.6 | 6.2 ± 2.1 | 0.18 | 0.21 |
| HbA1c, % | 6.7 ± 1.6 | 6.1 ± 1.1 | <0.001 | 6.7 ± 1.6 | 6.6 ± 1.3 | 0.71 | 0.069 |
| SUA, mg/dL | 413.2 ± 104.8 | 401.8 + 105.6 | 0.41 | 413.2 ± 104.8 | 408.6 ± 98.1 | 0.76 | 0.14 |
| LDL, mmol/L | 2.4 ± 0.9 | 2.7 ± 1.0 | 0.022 | 2.4 ± 0.9 | 2.5 ± 1.0 | 0.66 | 0.11 |
| Echocardiographic parameters | |||||||
| IVST, mma | 18.2 (15.0 to 21.7) | 16.4 (13.0 to 20.6) | 0.20 | 18.2 (15.0 to 21.7) | 16.0 (14.0 to 19.6) | 0.18 | 0.48 |
| LAD, mm | 48.3 ± 7.9 | 40.7 ± 10.1 | <0.001 | 48.3 ± 7.9 | 42.2 ± 7.3 | 0.013 | 0.80 |
| LVEDD, mm | 47.2 ± 7.5 | 47.1 ± 6.6 | 0.90 | 47.2 ± 7.5 | 46.2 ± 7.3 | 0.37 | 0.14 |
| LVEF, % | 57.8 ± 10.3 | 61.0 ± 10.7 | 0.016 | 57.8 ± 10.3 | 60.2 ± 6.4 | 0.007 | 0.28 |
| Septal e′ | 4.2 ± 1.4 | 5.0 ± 1.6 | <0.001 | 4.2 ± 1.4 | 4.8 ± 1.3 | 0.004 | 0.44 |
| E/e′ | 19.2 ± 9.5 | 14.9 ± 7.6 | <0.001 | 19.2 ± 9.5 | 14.4 ± 5.7 | <0.001 | 0.61 |
| LVOTPG at resta | 6.0 (3.0 to 12.0) | 7.0 (3.0 to 13.0) | 0.38 | 6.0 (3.0 to 12.0) | 6.0 (4.0 to 11.5) | 0.43 | 1.15 |
| LVOT obstruction, n (%) | 11 (11.7%) | 18 (11.3%) | 0.91 | 11 (11.7%) | 13 (13.8%) | 0.66 | 0.062 |
| Therapy | |||||||
| Beta-Blocker, n (%) | 69 (73.4%) | 122 (76.7%) | 0.59 | 69 (73.4%) | 76 (80.9%) | 0.23 | 0.18 |
| ACEI/ARB/ARNI, n (%) | 71 (75.5%) | 98 (61.6%) | 0.035 | 71 (75.5%) | 69 (73.4%) | 0.74 | 0.05 |
| ARNI, n (%) | 63 (67.0%) | 78 (49.1%) | 0.003 | 63 (67.0%) | 59 (62.8%) | 0.54 | 0.09 |
| SGLT2-i, n (%) | 94 (100%) | – | NA | 94 (100%) | – | NA | NA |
| Dapagliflozin | 69 (73.4%) | – | NA | 69 (73.4%) | – | NA | NA |
| Empagliflozin | 19 (20.2%) | – | NA | 19 (20.2%) | – | NA | NA |
| Others | 6 (6.4%) | – | NA | 6 (6.4%) | – | NA | NA |
| MRA, n (%) | 59 (62.8%) | 58 (36.5%) | <0.001 | 59 (62.8%) | 39 (41.4%) | 0.003 | 0.44 |
| Loop Diuretic, n (%) | 65 (69.1%) | 78 (49.1%) | <0.001 | 65 (69.1%) | 48 (51.1%) | 0.01 | 0.37 |
| CCB, n (%) | 70 (74.5%) | 116 (73.0%) | 0.79 | 70 (74.5%) | 68 (72.3%) | 0.74 | 0.05 |
| Antiplatelet drug, n (%) | 40 (42.6%) | 61 (38.4%) | 0.51 | 40 (42.6%) | 50 (53.2%) | 0.15 | 0.21 |
| OACs, n (%) | 48 (57.4%) | 57 (35.8%) | 0.018 | 48 (51.1%) | 36 (38.3%) | 0.080 | 0.26 |
| Statin, n (%) | 69 (73.4%) | 110 (69%) | 0.48 | 69 (73.4%) | 67 (71.3%) | 0.75 | 0.05 |
| Metformin, n (%) | 18 (19.1%) | 45(28.3%) | 0.11 | 18 (19.1%) | 24 (25.5%) | 0.29 | 0.15 |
| DPP4i, n (%) | 8 (8.5%) | 26 (16.4%) | 0.083 | 8 (8.5%) | 16 (17.0%) | 0.080 | 0.26 |
Baseline characteristics for matched study population.
SGLT-2i, sodium-glucose cotransporter-2 inhibitors; BMI, body mass index; HR, heart rate; CAD, coronary artery disease; MI, myocardial infarction; DM, diabetes mellitus; HF, heart failure; Af, atrial fibrillation; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; BNP, B-type natriuretic peptide; FPG, fasting plasma glucose; SUA, serum uric acid; LDL, low density lipoprotein cholesterol; IVST, Interventricular septal thickness; LAD, left atrial diameter; LVEDD, left ventricular end diastolic diameter; LVEF, left ventricular ejection fraction; E, early diastolic mitral inflow velocity; e′, mitral annular tissue velocity; LVOTPG, left ventricular outflow tract pressure gradient; LVOT, left ventricular outflow tract; ACEI/ARB/ARNI, angiotensin converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor neprilysin inhibitor; MRA, mineralcorticoid recept antagonist; CCB, calcium channel blocker; OACs, oral anticoagulants; DPP4i: Dipeptidyl peptidase 4 inhibitors.
Median (IQR).
PSM cohort
To reduce potential confounding bias from baseline characteristics, we conducted 1:1 PSM based on sex, age, BMI, hypertension, and DM, as shown in Figure 2. A total of 94 HCM patients not using SGLT-2i were selected as controls and matched with 94 patients initiating SGLT-2i therapy. The baseline characteristics of the PSM cohort are summarized in Table 1. The cohort had a mean age of 67.2 ± 14.4 years, median BMI of 23.4 (21.3–26.2), and 67.6% were male. Comorbidities were common, including hypertension (73.9%), HF (71.3%), DM (41.5%), atrial fibrillation (AF) (45.8%), coronary artery disease (CAD) (40.4%), and chronic kidney disease (CKD) (29.8%). Median IVST was 16.2 (15.0–20.5) mm, mean LVEF was 59.0 ± 8.4%, and 12.8% of patients exhibited left ventricular outflow tract obstruction (LVOTO) at rest.
Figure 2
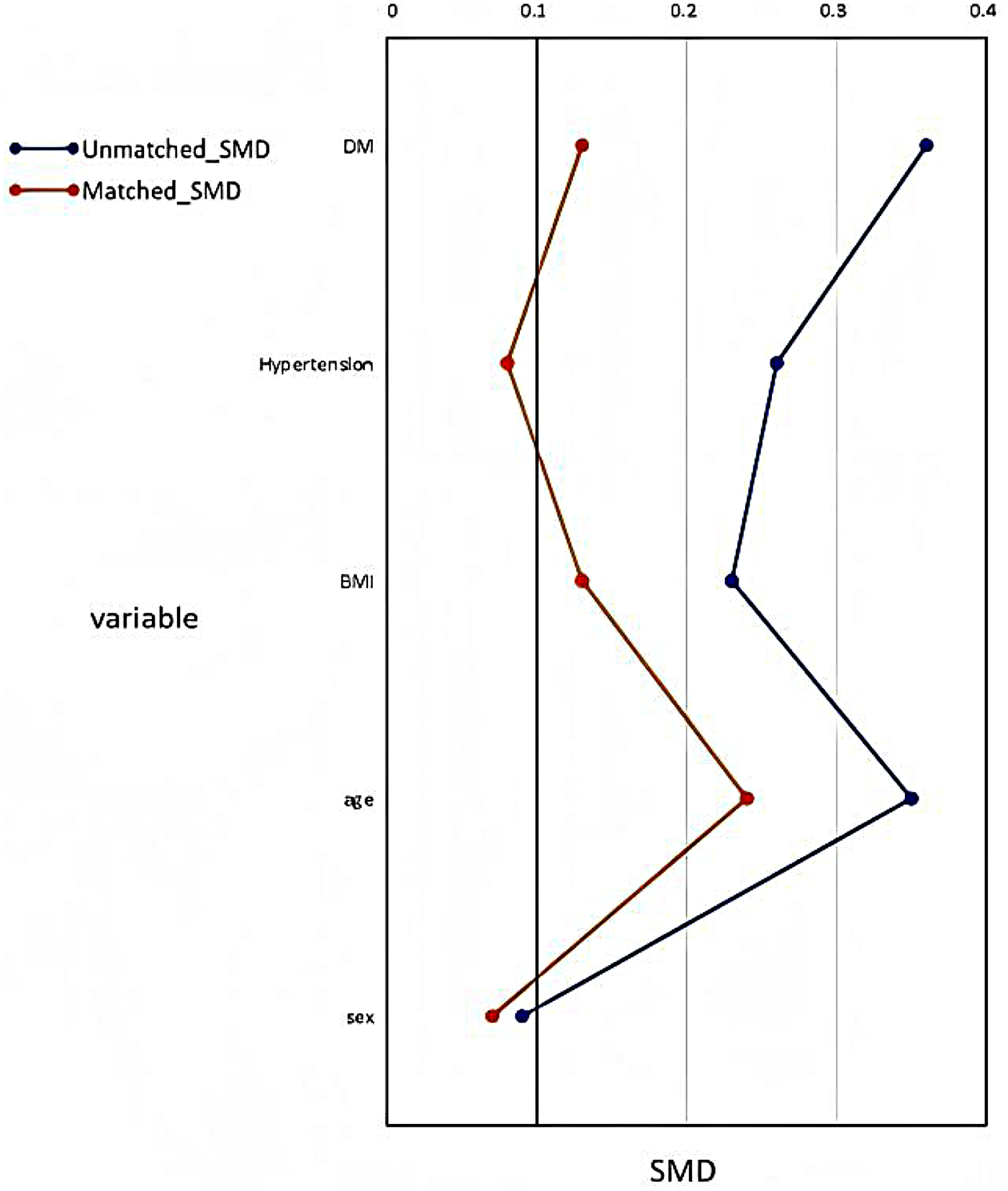
Propensity Score Matching. BMI, body mass index; SMD, Standardized mean difference.
After PSM, aside from a higher prevalence of HF in the SGLT-2i group (79.8% vs. 62.8%, p = 0.01), no other comorbidities differed significantly between the two groups. However, baseline BNP levels, echocardiographic parameters (including LAD, LVEF, septal e′, and E/e′), as well as the use of mineralocorticoid receptor antagonists (MRA) and loop diuretics, still showed significant differences between groups.
Outcomes
Primary endpoints
Table 2 summarizes the changes in echocardiographic parameters, laboratory indicators and NYHA class for both groups. At 6-month follow-up, in the SGLT-2i group, significant improvements from baseline were observed in septal e′ (4.9 ± 1.4 vs. 4.2 ± 1.4, p < 0.001), and E/e′ (13.9 ± 5.8 vs. 19.1 ± 9.5, p < 0.001). IVST, LVOTPG at rest, HR, BNP and NYHA class were significantly reduced in both groups compared to values recorded during hospitalization.
Table 2
| Parameter | SGLT-2i (+) (n = 94) | SGLT-2i (−) (n = 94) | Inter-group p value | Cohen’s d/δ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Change | p value | Baseline | Follow-up | Change | p value | |||
| Septal e′ | 4.2 ± 1.4 | 4.9 ± 1.4 | 0.7 ± 1.3 | <0.001 | 4.8 ± 1.3 | 4.8 ± 1.4 | 0.04 ± 1.6 | 0.82 | 0.002 | 0.45 |
| E/e′ | 19.2 ± 9.5 | 13.9 ± 5.8 | −5.1 ± 8.7 | <0.001 | 14.4 ± 5.7 | 14.8 ± 6.0 | 0.4 ± 6.4 | 0.58 | <0.001 | 0.72 |
| IVST, mm | 18.2 (15.0 to 21.7) | 16.8 (14.2 to 19.0) | −1.3 (−3.1 to 0) | <0.001* | 16.0 (14.0 to 19.6) | 15.8 (13.5 to 19.0) | −0.2 (−2.0 to 0.9) | 0.01* | 0.005* | 0.35** |
| LAD, mm | 48.3 ± 7.9 | 47.2 ± 7.4 | −1.1 ± 4.5 | 0.016 | 42.2 ± 7.3 | 42.3 ± 7.0 | 0.08 ± 5.5 | 0.89 | 0.11 | 0.24 |
| LVEDD, mm | 47.2 ± 7.5 | 46.9 ± 6.8 | −0.3 ± 5.5 | 0.63 | 46.2 ± 7.3 | 46.6 ± 6.7 | 0.4 ± 6.5 | 0.56 | 0.45 | 0.12 |
| LVEF, % | 57.8 ± 10.3 | 59.1 ± 8.5 | 1.3 ± 8.8 | 0.14 | 61.2 ± 6.4 | 64.1 ± 7.2 | 2.9 ± 6.7 | <0.001 | 0.18 | 0.21 |
| LVOTPG at rest | 6.0 (3.0 to 12.3) | 6.0 (3.0 to 10.25) | 0 (−3.0 to 1.3) | 0.023* | 6.0 (4.0 to 10.0) | 6.0 (4.0 to 11.0) | 0.5 (−3.0 to 2.0) | 0.025* | 0.40* | 0.20** |
| HR, bpm | 74.9 ± 15.3 | 65.8 ± 4.3 | −9.1 ± 14.2 | <0.001 | 76.6 ± 16.4 | 66.6 ± 6.3 | −10.0 ± 14.7 | <0.001 | 0.68 | 0.062 |
| FPG, mmol/L | 6.7 ± 2.6 | 6.1 ± 1.6 | −0.58 ± 2.1 | 0.008 | 6.2 ± 2.1 | 6.1 ± 1.4 | 0.15 ± 1.5 | 0.33 | 0.007 | 0.40 |
| Creatinine, mg/dL | 95.1 ± 23.4 | 97.4 ± 24.3 | 2.3 ± 15.7 | 0.16 | 91.7 ± 35.1 | 92.1 ± 41.3 | 0.3 ± 15.4 | 0.84 | 0.38 | 0.13 |
| BNP, pg/mL | 424.7 (205.0 to 919.7) | 270.5 (125.8 to 419.4) | 0 (−607.5 to 0) | <0.001* | 191.2 (46.1 to 512.0) | 126.0 (35.6 to 334.2) | 0 (−105.7 to 0) | <0.001* | 0.023* | 0.25** |
| NYHA class | 3 (2 to 3) | 2 (2 to 2) | −1 (−1 to −0.25) | <0.001* | 2 (2 to 3) | 2 (1 to 2) | −1 (−1 to 0) | <0.001* | 0.031* | 0.22** |
| HF readmission | 6 (6.4%) | 4 (4.3%) | 0.71 | 0.096 | ||||||
Comparison of laboratory data changes at baseline and 6-month follow-up.
GLT-2i, sodium glucose cotransporter-2 inhibition; HR, heart rate; FPG, fasting plasma glucose; BNP, B-type natriuretic peptide; IVST, Interventricular septal thickness; LAD, left atrial diameter; LVEDD, left ventricular end diastolic diameter; LVEF, left ventricular ejection fraction; E, early diastolic mitral inflow velocity; e′, mitral annular tissue velocity; LVOTPG, left ventricular outflow tract pressure gradient; HF, heart failure.
Z value by Mann–Whitney U test.
δ value by Cliff's Delta.
When compared to the control group, the SGLT-2i group exhibited more pronounced improvements in septal e′ (Δ0.7 ± 1.3 vs. Δ0.04 ± 1.6, p = 0.002) and E/e′ (Δ–5.1 ± 8.7 vs. Δ 0.4 ± 6.4, p < 0.001), as illustrated in Figures 3a,b. Similarly, greater reductions were noted in IVST [Δ–1.3 (–3.1 to 0) mm vs. Δ −0.2 (–2.0 to 0.9) mm, p = 0.005; Figure 3c], NYHA class [Δ −1(−1 to −0.25) vs. Δ −1 (−1 to 0), p = 0.031] and BNP levels [Δ0 (–607.5 to 0) pg/mL vs. Δ 0 (–105.7 to 0) pg/mL, p = 0.023].
Figure 3
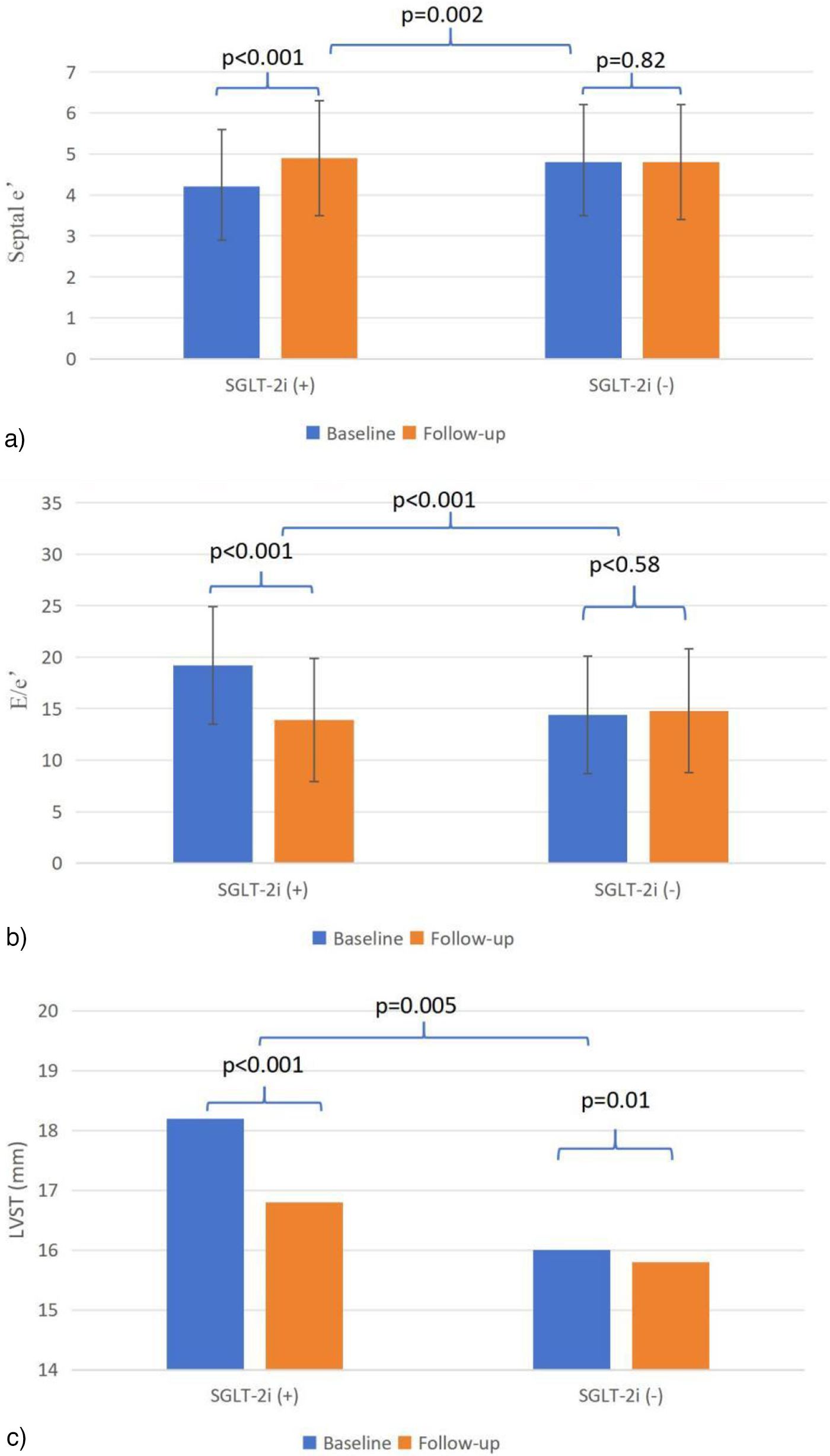
(a–c) Echocardiographic parameters from baseline to follow-up. e′, mitral annular tissue velocity; E, early diastolic mitral inflow velocity; IVST, Interventricular septal thickness.
Multivariate analysis, after adjustment for comorbidity of HF, baseline BNP, LAD, LVEF, septal e′, E/e′, use of MRA and loop diuretic (Table 3), confirmed that the improvements in septal e′ (t = 2.26, p = 0.025), E/e′ (t = −3.75, p < 0.001), and NYHA class (p = 0.038) remained significantly greater in the SGLT-2i group compared to the control group.
Table 3
| Parameter | SGLT-2i(+) (n = 94) | SGLT-2i(−) (n = 94) | t | Adjusted difference between groups (95% CI) | P value | R 2 values | DW |
|---|---|---|---|---|---|---|---|
| Δseptal e′ | 0.7 ± 1.3 | 0.04 ± 1.6 | 2.26 | 0.059 to 0.86 | 0.025 | 0.31 | 1.96 |
| ΔE/e′ | −5.1 ± 8.7 | 0.4 ± 6.4 | −3.75 | −4.96 to −1.54 | <0.001 | 0.57 | 2.27 |
| ΔIVSTa | −1.3 (−3.1 to 0) | −0.2 (−2.0 to 0.9) | – | −1.66 to 0.57 | 0.34 | 0.007 | 2.17 |
| ΔLAD | −1.1 ± 4.5 | 0.08 ± 5.5 | 0.18 | −1.37 to 1.63 | 0.86 | 0.16 | 1.92 |
| ΔLVEF | 1.3 ± 8.8 | 2.9 ± 6.7 | −1.96 | −4.14 to 0.019 | 0.052 | 0.33 | 2.19 |
| ΔLVEDD | −0.3 ± 5.5 | 0.4 ± 6.5 | 0.036 | −1.88 to 1.95 | 0.97 | 0.042 | 2.20 |
| ΔLVOTPG at resta | 0 (−3.0 to 1.3) | 0.5 (−3.0 to 2.0) | – | −2.13 to 10.67 | 0.28 | 0.026 | 2.09 |
| ΔBNPa | 0 (−607.5 to 0) | 0 (−105.7 to 0) | – | −167.34 to 16.48 | 0.13 | 0.75 | 2.03 |
| ΔFPG | −0.58 ± 2.1 | 0.15 ± 1.5 | −1.63 | −1.08 to 0.10 | 0.11 | −0.009 | 2.00 |
| ΔNYHA classa | −1 (−1 to −0.25) | −1 (−1 to 0) | – | −0.50 to −0.018 | 0.038 | 0.008 | 2.11 |
Multivariable analysis.
HF, heart failure; BNP, B-type natriuretic peptide; MRA, mineralcorticoid recept antagonist; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; E, early diastolic mitral inflow velocity; e′, mitral annular tissue velocity; SGLT-2i, Sodium glucose cotransporter-2 inhibition; IVST, Interventricular septal thickness; LVEDD, left ventricular end diastolic diameter; LVOTPG, left ventricular outflow tract pressure gradient; FPG, fasting plasma glucose; DW: Durbin-Watson statistic. #No significant multicollinearity was detected, as all variance inflation factors were <5 (HF comorbidity: 1.69, baseline BNP: 1.55, LAD: 1.46, LVEF: 1.19, septal e′: 1.47, E/e′: 1.56, MRA use: 2.45, loop diuretic use: 2.40). (Adjusted for comorbidity of HF, baseline BNP, LAD, LVEF, septal e′, E/e′, use of MRA and loop diuretic#).
Bootstrap multiple linear regression model.
Secondary endpoint
At the 6-month follow-up, HF readmission occurred in 6 patients in the SGLT-2i group and 4 in the control group (p = 0.71) (Table 2). Over a median follow-up of 16.3 months, the Kaplan–Meier analysis demonstrated no significant difference in HF readmission between the two groups (20 events in the SGLT-2i group vs. 17 in the control group; log-rank p = 0.73), as illustrated in Figure 4.
Figure 4
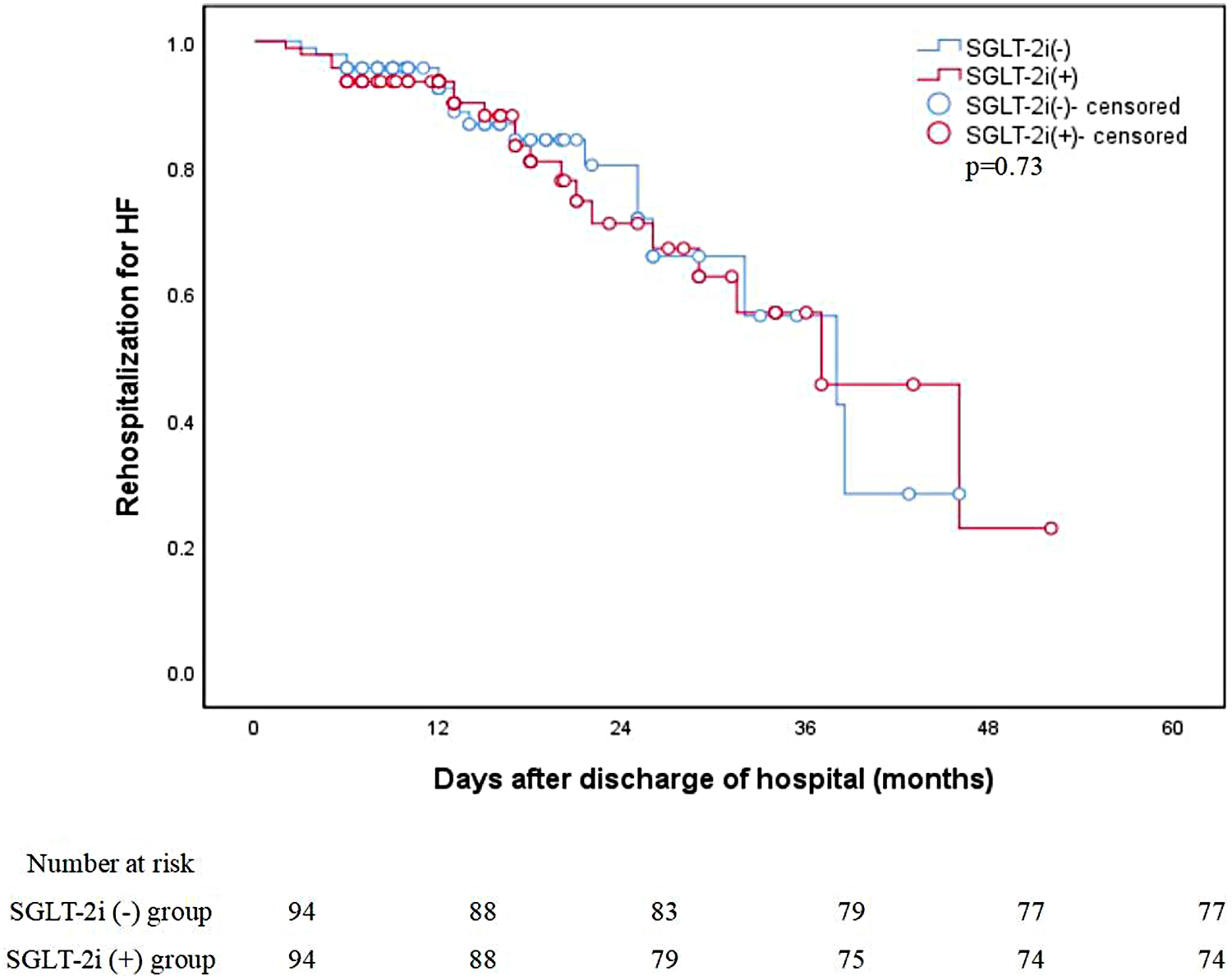
Unadjusted Kaplan–Meier curves: Rehospitalization for HF. HF, heart failure; SGLT-2i, sodium-glucose cotransporter-2 inhibitors.
Safety endpoints
No hypoglycemia and urinary tract infection events were recorded in the patients' outpatient visits and telephone follow-up. And no significant difference in the changes of creatinine (2.3 ± 15.7 vs. 0.3 ± 15.4 mg/dL, inter-group p = 0.38) was observed during follow-up between groups (Table 2).
Subgroup analysis
A subgroup of 24 oHCM patients with resting LVOTO (13 receiving SGLT-2i and 11 controls) was analyzed. As shown in Table 4, SGLT-2i treatment was associated with a significant improvement in septal e′ compared with the control group [1.2 (–0.2 to 1.6) vs. −0.2 (–1.0 to 0.7), p = 0.018]. However, no significant between-group difference was observed in the change of resting LVOTPG [–21.0 (–35.5 to −8.0) vs. −28.0 (–61.0 to −13.0), p = 0.19].
Table 4
| Parameter | oHCM subgroup | noHCM subgroup without DM or HF | ||||||
|---|---|---|---|---|---|---|---|---|
| SGLT-2i (+) (n = 13) | SGLT-2i (−) (n = 11) | Z value | p | SGLT-2i (+) (n = 4) | SGLT-2i (−) (n = 25) | Z value | p | |
| Δseptal e′ | 1.2 (−0.2 to 1.6) | −0.2 (−1.0 to 0.7) | 2.35* | 0.02 | 1.9 (1.7 to 3.0) | 0.3 (−0.8 to 0.9) | 2.66* | 0.005 |
| ΔE/e′ | −3.4 (−22.0 to 0.02)) | −0.5 (−5.8 to 2.7) | −1.59* | 0.12 | −7.3 (−13.5 to −0.5) | 1.7 (−0.2 to 3.8) | −2.77* | 0.003 |
| ΔIVST | −2.2 (−4.0 to −0.4) | −2.0 (−3.5 to 1.3) | −0.75* | 0.46 | −1.7 (−4.4 to 2.0) | 0 (−1.8 to 1.1) | −0.64* | 0.56 |
| ΔLAD | −1.2 (−2.6 to 2.1) | −0.5 (−4.2 to 3.0) | −0.12* | 0.91 | −2.4 (−4.6 to −1.4) | 0.9 (−2.6 to 3.0) | −1.65* | 0.10 |
| ΔLVOTPG at rest | −21.0 (−35.5 to −8.0) | −28.0 (−61.0 to −13.0) | 1.36* | 0.19 | 1.0 (−1.0 to 6.0) | 1.0 (−2.0 to 2.0) | 0.99* | 0.35 |
Subgroup analysis of oHCM and noHCM at Low-risk.
oHCM, obstructive hypertrophic cardiomyopathy; noHCM, non-obstructive hypertrophic cardiomyopathy; DM, diabetes mellitus; HF, heart failure; E, early diastolic mitral inflow velocity; e′, mitral annular tissue velocity; IVST, interventricular septal thickness; LAD, left atrial diameter; LVOTPG, left ventricular outflow tract pressure gradient.
Z value by Mann–Whitney U test.
Among the 188 included HCM patients, only 29 did not have comorbid DM or HF, and among these, only 4 were treated with SGLT-2i. In this small subgroup, patients receiving SGLT-2i showed significantly greater improvement in septal e′ [1.9 (1.7 to 3.0) vs. 0.3 (–0.8 to 0.9), p = 0.005] and E/e′ [–7.3 (–13.5 to −0.5) vs. 1.7 (–0.2 to 3.8), p = 0.003] compared with controls (Table 4).
Discussion
To our knowledge, this represents one of the first PSM studies to systematically evaluate SGLT-2i specifically in a dedicated cohort of patients with HCM, with particular emphasis on clinically relevant subgroups, including those with LVOTO. Our findings extend the potential therapeutic utility of this drug class from the well-established HF populations to the distinct context of HCM. Notably, we demonstrate that SGLT-2i treatment is associated with a concordant improvement across a triad of disease-specific parameters: a trend towards reduction in IVST, enhancement of early diastolic relaxation (septal e′), and a significant decrease in left ventricular filling pressure (E/e′). This pattern of “structure-function-hemodynamics” benefit was observed in the overall population as well as in key subgroups, without compromising renal function or increasing hypoglycemic risk, and suggests a targeted impact on the core pathophysiology of HCM.
Given that current standard therapies for HCM often leave many patients with persistent symptoms (2), SGLT-2i present a novel therapeutic opportunity. Their pleiotropic effects—including benefits on myocardial energetics, calcium handling, and reverse remodeling (10, 13, 14)—directly target the pathological hallmarks of HCM (1, 2), such as myocardial fibrosis (15) and diastolic dysfunction (16), providing a mechanistic rationale for the improvements observed in our study.
LV hypertrophy and diastolic function
HCM symptoms primarily stem from LV diastolic dysfunction due to hypertrophy and fibrosis, a pathology shared with HFpEF (3). Initial clinical evidence from Subramanian et al. (17) in non-obstructive HCM patients and supporting preclinical data (18) indicate that SGLT-2i can improve diastolic function, aligning with our findings. Furthermore, SGLT-2i may mitigate key pathological processes like myocardial fibrosis and promote reverse remodeling (10, 13–16). The DAPA-LVH trial (15) demonstrated that dapagliflozin significantly reduced left ventricular mass in patients with diabetes and LV hypertrophy, confirming its capacity to reverse this key structural abnormality.
This study provides evidence of a multi-dimensional and physiologically coherent benefit associated with SGLT-2i therapy in HCM (17). Although the absolute changes in some echocardiographic parameters were modest, their clinical relevance must be interpreted within the context of a chronic, slowly progressive disease where reversal of cardiac abnormalities is inherently limited (1, 3). The minimal change observed in the control group aligns with this expected natural history (1). In contrast, the SGLT-2i group demonstrated a concordant pattern of improvement: a trend toward structural change with reduced IVST (ΔIVST: −1.3 vs. −0.2 mm), a significant enhancement in early diastolic relaxation velocity (Δ septal e′: 0.7 vs. 0.04 cm/s), and a marked reduction in left ventricular filling pressure (ΔE/e′: −5.1 vs. 0.4). These parameters are physiologically interlinked, suggesting that attenuation of hypertrophy (15) may facilitate improved relaxation, which in turn lowers filling pressures (16, 17). Notably, the improvement in E/e′ was substantial (Cohen's d = 0.72), a parameter with direct clinical relevance to symptoms and prognosis in HCM (3). The coherent “structure-function-hemodynamics” improvement observed in this study suggests that SGLT-2i may modulate the pathological milieu in HCM through pathways such as improved myocardial energetics and attenuated fibrosis. This pharmacological strategy intriguingly resonates with fundamental discoveries in cardiovascular remodeling—for instance, recent work highlighting the pivotal role of signaling axes like MrgD/PIM1 in regulating pathological hypertrophy and fibrosis (19). While the specific molecular targets of SGLT-2i differ, they share the core biological principle of modulating the adverse cellular and extracellular matrix environment to facilitate functional recovery (19, 20).
A nuanced interpretation of the structural data remains warranted, however. Specifically, the between-group difference in IVST reduction did not reach statistical significance after multivariable adjustment. This likely reflects the biological reality that structural reversal lags behind functional improvement (16), especially in HCM where hypertrophy is a fundamental, genetically driven pathology and a particularly challenging therapeutic target (2, 3). Therefore, the consistent trend observed here should be regarded as an important preliminary signal, providing a rationale for investigating whether longer-term SGLT-2i treatment can translate these early functional benefits into clinically meaningful structural remodeling, thereby potentially interrupting the cascade from hypertrophy to overt HF.
Thus, our findings not only support the potential disease-modifying value of SGLT-2i in HCM but also situate it within the broader therapeutic endeavor to reverse maladaptive cardiac remodeling. This holistic evidence base strengthens the therapeutic rationale for SGLT-2i in HCM beyond isolated parameter changes.
Cardiovascular events
Large real-world studies support SGLT-2i's benefits in HCM. A TriNetX analysis (21) of 872 matched patients showed a 76% lower all-cause mortality and 37% fewer cardiovascular events with SGLT-2i after two years. Similarly, a Korean cohort study (22) of 4,126 HCM patients with diabetes associated SGLT-2i use with significantly reduced risks of heart failure hospitalization (18%), all-cause mortality (45%), and sudden cardiac death (50%). These cohorts had high comorbidity rates, aligning with established high-risk profiles in HCM (23).
In our study, most patients initiated SGLT-2i due to comorbid DM or HF, which align with its approved clinical indications (8). It is noteworthy that the observed significant improvements in LV diastolic function and filling pressure did not translate into a significant reduction in HF hospitalization over a median follow-up of approximately 16.3 months in this study. This finding warrants careful interpretation within the context of the study design and disease pathophysiology. First, the follow-up duration may have been insufficient. In large randomized trials (11, 24) of SGLT-2i in HFpEF (a phenotype akin to HCM-related HF), the benefit on HF hospitalization typically requires more than 2 years to fully emerge. The early functional and structural improvements captured in our study may thus represent a potential harbinger of longer-term clinical benefit. Second, HF hospitalization is a multifactorial composite endpoint, particularly influenced in HCM by complex mechanisms such as dynamic outflow tract obstruction and arrhythmias (1, 2). The statistical power of our study, given its sample size and follow-up period, was limited for detecting potentially modest differences in this endpoint. Finally, this aligns with the recognized mode of action of SGLT-2i—the biological benefits mediated through improved myocardial energetics, reduced fibrosis, etc., are progressive and cumulative (10, 13, 14). Consequently, a temporal dissociation between short-term functional improvement (a “disease-modifying” signal) and a future reduction in hard endpoints is plausible. Future studies with longer follow-up, larger sample sizes, and possibly incorporating intermediate clinical endpoints such as exercise capacity, are needed to fully elucidate the long-term clinical value of SGLT-2i in HCM.
LVOTO and therapeutic implications
LVOTO is a key determinant of symptoms in oHCM (25). While first-line therapies like beta-blockers or myosin inhibitors (2, 5, 6, 26) primarily aim to reduce the gradient, our study found that SGLT-2i did not confer additional reduction in LVOT gradient compared to controls. This important finding helps define the therapeutic niche of SGLT-2i: its benefit in HCM appears largely independent of mechanical relief of obstruction.
Consequently, in oHCM, SGLT-2i should be viewed not as a treatment for obstruction, but as a potential adjunctive therapy targeting the underlying myocardial pathophysiology (e.g., diastolic dysfunction, fibrosis) (10, 13, 14), particularly in patients with concomitant HF or DM. For the substantial population with noHCM, where specific disease-modifying drugs are scarce, the improvements in diastolic function and structure observed in our study suggest SGLT-2i may hold particular promise, warranting dedicated investigation.
Arrhythmia
Arrhythmias, particularly AF, are major complications in HCM, driving HF and stroke risk (1, 2). The SHaRe registry (N = 4,591) reported high incidence rates of AF (20%) and ventricular arrhythmias (6%) in HCM cohort (27, 28). While guidelines recommend anticoagulation for all HCM patients with AF, SGLT-2i has been associated with a 26% reduction in ischemic stroke (26), though a large meta-analysis found it did not significantly lower AF incidence (29).
Ventricular arrhythmias, a leading cause of sudden cardiac death (SCD), stem from structural and electrical remodeling in HCM (1). SGLT-2i may mitigate this risk by attenuating fibrosis, reversing adverse remodeling, and stabilizing electrical activity through mechanisms such as improved myocardial energetics and calcium handling (10, 13–15, 30). Clinical trials in HF populations have demonstrated that SGLT-2i significantly reduces ventricular arrhythmias (31, 32), including sustained ventricular tachycardia/ventricular fibrillation recorded in patients with ICDs (32).
Critically, a recent meta-analysis (33), extended this benefit to SCD prevention. This meta-analysis by Matteucci et al., encompassing 58,569 patients across 8 RCTs, demonstrated that SGLT-2i significantly reduced the risk of SCD (OR: 0.82; 95% CI: 0.72–0.94; P = 0.0104) in broad populations with DM, HF, or CKD, thereby expanding the spectrum of their cardiovascular benefits. The prevention of SCD remains a major unmet clinical challenge, especially in individuals without high-risk features amenable to device therapy, as pharmacological strategies specifically approved for SCD primary prevention are currently lacking. In this context, the emerging evidence for SGLT-2i (33), alongside their proposed mechanisms of attenuating fibrosis, reversing adverse remodeling, and potentially stabilizing cardiomyocyte electrophysiology (10, 14), represents a paradigm-shifting advance in arrhythmic risk management. Although not yet studied specifically in HCM, SGLT-2i represents a particularly promising therapeutic option to address the residual risk of SCD in this population.
Adverse events
SGLT-2i demonstrate a favorable safety profile in heart failure and diabetes populations. While known class effects include urinary tract infections and diabetic ketoacidosis, a large meta-analysis (34) confirmed no significant increase in hypoglycemia or fracture risk. Real-world data from the TriNetX study (19) and our findings support its overall safety and good tolerability in HCM patients with clinical indications. Expert consensus (35) emphasizes patient evaluation and education prior to initiation.
Future perspectives
This study provides real-world evidence for the benefit of SGLT-2i in HCM using conventional clinical imaging and functional biomarkers. Looking forward, advancing this field will require a deeper mechanistic understanding and dynamic monitoring of the myocardial microenvironment. Emerging biosensing technologies—such as electrochemical methods for the real-time detection of mitochondrial oxidative stress markers (36)—exemplify the next generation of tools for precise risk stratification and therapy monitoring in cardiomyopathy. Integrating such precision biomarkers with traditional endpoints in future research is a crucial step toward personalized and dynamic management of HCM.
Limitations
Despite the findings presented, this study has several limitations that should be acknowledged: (1) Selection bias: As a single-center retrospective analysis, the study included a high proportion of patients with multiple comorbidities, many of whom were already on guideline-directed therapies for HF or diabetes. This may limit the generalizability of the results, particularly to low-risk HCM populations, which were underrepresented in our cohort. (2) Short follow-up duration: The relatively short follow-up period precludes definitive conclusions regarding the long-term efficacy and safety of SGLT-2i in HCM. (3) Unmeasured medication adherence: As a retrospective study, direct monitoring of adherence (e.g., via pill count) was not feasible. Adherence was assessed indirectly using available clinical data, including the frequency of prescription refills in outpatient records and physician documentation of patient-reported medication intake during follow-up visits. (4) Residual confounding: Despite statistical adjustments, unmeasured or unknown confounders inherent to observational designs may affect the results. (5) Data completeness: Retrospective data collection is susceptible to missing or inconsistently recorded information, potentially introducing bias. (6) Limited power for subgroup analyses: The small sample size in certain subgroups (e.g., low-risk patients) reduces the reliability of subgroup-specific conclusions.
Conclusion
In this real-world, PSM study, initiation of SGLT-2i therapy was associated with significant improvements in LV diastolic function and NYHA functional class in patients with HCM, without increasing renal or hypoglycemic risk. These findings support the potential therapeutic value of SGLT-2i, particularly in HCM patients with comorbidities like HF or diabetes where these agents are already indicated. However, given the observational design and single-center nature of our study, large-scale, prospective randomized trials are warranted to confirm efficacy, identify optimal patient subgroups, and elucidate underlying mechanisms before broader clinical recommendations can be made.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Committee of Clinical Investigation of Zhejiang Hospital (2024-130K) and conducted with the guidelines of the Declaration of Helsinki. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because this was a retrospective observational study and no patient intervention was required, informed consent from subjects could be waived, which was approved by the ethics committee. In conducting the study, we maintained a strong focus on the protection of patients' personal interests, rights, privacy, and image rights.
Author contributions
CD: Writing – review & editing, Data curation, Software. FL: Supervision, Writing – review & editing. LW: Data curation, Conceptualization, Writing – review & editing. XX: Writing – original draft, Writing – review & editing, Project administration.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This study was supported by the Traditional Chinese Scientific and Technological Projects for Medicine and Health of Zhejiang Province (Grant No. 2025ZL004); Zhejiang Medical Association Special Fund Program for Clinical Medicine (Grant No. 2024ZYC-A259).
Acknowledgments
We thank all the staff and all the reviewers who participated in the review during the preparation of this manuscript.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Barry J Desai MY Nishimura RA Spirito P Rakowski H Towbin JA et al Management of hypertrophic cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. (2022) 79(4):390–414. 10.1016/j.jacc.2021.11.021
2.
Ammirati E Contri R Coppini R Cecchi F Frigerio M Olivotto I . Pharmacological treatment of hypertrophic cardiomyopathy: current practice and novel perspectives. Eur J Heart Fail. (2016) 18(9):1106–18. 10.1002/ejhf.541
3.
Ommen SR Ho CY Asif IM Balaji S Burke MA Day SM et al 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR guideline for the management of hypertrophic cardiomyopathy: a report of the American Heart Association/American College of Cardiology joint committee on clinical practice guidelines. Circulation. (2024) 149:e1239–311. 10.1161/CIR.0000000000001250
4.
Arbelo E Protonotarios A Gimeno JR Arbustini E Barriales-Villa R Basso C et al 2023 ESC guidelines for the management of cardiomyopathies. Eur Heart J. (2023) 44(37):3503–626. 10.1093/eurheartj/ehad194
5.
Olivotto I Oreziak A Barriales-Villa R Abraham TP Masri A Garcia-Pavia P et al Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2020) 396(10253):759–69. 10.1016/S0140-6736(20)31792-X
6.
Desai MY Owens A Wolski K Geske JB Saberi S Wang A et al Mavacamten in patients with hypertrophic cardiomyopathy referred for septal reduction: week 56 results from the VALOR-HCM randomized clinical trial. JAMA Cardiol. (2023) 8(10):968–77. 10.1001/jamacardio.2023.3342
7.
Davies MJ D’Alessio DA Fradkin J Kernan WN Mathieu C Mingrone G et al Management of hyperglycemia in type 2 diabetes, 2018. a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. (2018) 41(12):2669–701. 10.2337/dci18-0033
8.
Heidenreich PA Bozkurt B Aguilar D Allen LA Byun JJ Colvin MM et al 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2022) 79(17):e263–421. 10.1016/j.jacc.2021.12.012
9.
Khunti K . SGLT2 inhibitors in people with and without T2DM. Nat Rev Endocrinol. (2021) 17(2):75–6. 10.1038/s41574-020-00453-2
10.
Panico C Bonora B Camera A Chilelli NC Prato GD Favacchio G et al Pathophysiological basis of the cardiological benefits of SGLT-2 inhibitors: a narrative review. Cardiovasc Diabetol. (2023) 22(1):164. 10.1186/s12933-023-01855-y
11.
Anker SD Butler J Filippatos G Shahzeb Khan M Ferreira JP Bocchi E et al Baseline characteristics of patients with heart failure with preserved ejection fraction in the EMPEROR-preserved trial. Eur J Heart Fail. (2020) 22(12):2383–92. 10.1002/ejhf.2064
12.
Scheffer M Driessen-Waaijer A Hamdani N Landzaat JWD Jonkman NH Paulus WJ et al Stratified treatment of heart failure with preserved ejection fraction: rationale and design of the STADIA-HFpEF trial. ESC Heart Fail. (2020) 7(6):4478–87. 10.1002/ehf2.13055
13.
Kubota Y Shimizu W . Clinical benefits of sodium-glucose cotransporter 2 inhibitors and the mechanisms underlying their cardiovascular effects. JACC Asia. (2022) 2(3):287–93. 10.1016/j.jacasi.2022.03.009
14.
Wu YJ Wang SB Wang LS . SGLT2 inhibitors: new hope for the treatment of acute myocardial infarction?Am J Cardiovasc Drugs. (2022 Nov) 22(6):601–13. 10.1007/s40256-022-00545-6
15.
Brown AJM Gandy S McCrimmon R Houston JG Struthers AD Lang CC . A randomized controlled trial of dapagliflozin on left ventricular hypertrophy in people with type two diabetes: the DAPALVH trial. Eur Heart J. (2020) 41(36):3421–32. 10.1093/eurheartj/ehaa419
16.
Rau M Thiele K Hartmann N-UK Schuh A Altiok E Möllmann J et al Empagliflozin does not change cardiac index nor systemic vascular resistance but rapidly improves left ventricular filling pressure in patients with type 2 diabetes: a randomized controlled study. Cardiovasc Diabetol. (2021) 20(1):6. 10.1186/s12933-020-01175-5
17.
Subramanian M Sravani V Krishna SP Bijjam S Sunehra C Yalagudri S et al Efficacy of SGLT2 inhibitors in patients with diabetes and nonobstructive hypertrophic cardiomyopathy. Am J Cardiol. (2023) 188:80–6. 10.1016/j.amjcard.2022.10.054
18.
Wijnker PJM Dinani R van der Laan NC Algül S Knollmann BC Verkerk AO et al Hypertrophic cardiomyopathy dysfunction mimicked in human engineered heart tissue and improved by sodium-glucose cotransporter 2 inhibitors. Cardiovasc Res. (2024) 120(3):301–17. 10.1093/cvr/cvae004
19.
Zhong H Yao L An H Fang L Liu X Wang Q et al Mrgd as a novel modeling and treatment target for pulmonary hypertension. Arterioscler Thromb Vasc Biol. (2025) 45(5):e164–83. 10.1161/ATVBAHA.124.322337
20.
Yao L An H Fan C Lan Q Zhong H Zhang Y et al Injectable BMSC-based extracellular matrix-mimicking microtissue for myocardial infarction repair. Adv Sci (Weinh). (2026) 13(3):e00299. 10.1002/advs.202500299
21.
Aglan A Fath AR Eldaly AS Anderson AS Phillips JS Maron BJ et al Impact of sodium glucose cotransporter 2 inhibitors on mortality in hypertrophic cardiomyopathy. JACC Adv. (2024) 3(3):100843. 10.1016/j.jacadv.2024.100843
22.
Jung MH Cho JS Lee SY Youn JC Choi Y Chung WB et al Sodium–glucose cotransporter-2 inhibitors and clinical outcomes in patients with hypertrophic cardiomyopathy and diabetes: a population-based cohort study. Eur J Prev Cardiol. (2025) 32:1103–11. 10.1093/eurjpc/zwae345
23.
Wasserstrum Y Barriales-Villa R Fernández-Fernández X Adler Y Lotan D Peled Y et al The impact of diabetes mellitus on the clinical phenotype of hypertrophic cardiomyopathy. Eur Heart J. (2019) 40(21):1671–7. 10.1093/eurheartj/ehy625
24.
Solomon SD McMurray JJV Claggett B de Boer RA DeMets D Hernandez AF et al Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. (2022) 387(12):1089–98. 10.1056/NEJMoa2206286
25.
Moon I Lee S-Y Kim H-K Han K-D Kwak S Kim M et al Trends of the prevalence and incidence of hypertrophic cardiomyopathy in Korea: a nationwide population-based cohort study. PLoS One. (2020) 15(1):e0227012. 10.1371/journal.pone.0227012
26.
Braunwald E Saberi S Abraham TP Elliott PM Olivotto I . Mavacamten: a first-in-class myosin inhibitor for obstructive hypertrophic cardiomyopathy. Eur Heart J. (2023) 44(44):4622–33. 10.1093/eurheartj/ehad637
27.
Ho CY Day SM Ashley EA Michels M Pereira AC Jacoby D et al Genotype and lifetime burden of disease in hypertrophic cardiomyopathy: insights from the sarcomeric human cardiomyopathy registry (SHaRe). Circulation. (2018) 138(14):1387–98. 10.1161/CIRCULATIONAHA.117.033200
28.
Zelniker TA Bonaca MP Furtado RHM Mosenzon O Kuder JF Murphy SA et al Effect of dapagliflozin on atrial fibrillation in patients with type 2 diabetes mellitus: insights from the DECLARE-TIMI 58 trial. Circulation. (2020) 141:1227–34. 10.1161/CIRCULATIONAHA.119.044183
29.
Zhang H-D Ding L Mi L-J Zhang A-K Zhang K Jiang Z-H et al Sodium-glucose co-transporter-2 inhibitors for the prevention of atrial fibrillation: a systemic review and meta-analysis. Eur J Prev Cardiol. (2024) 31(7):770–9. 10.1093/eurjpc/zwad356
30.
Lim VG He H Lachlan T Ng GA Kyrou I Randeva HS et al Impact of sodium-glucose co-transporter inhibitors on cardiac autonomic function and mortality: no time to die. Europace. (2022) 24:1052–7. 10.1093/europace/euab321
31.
Curtain JP Docherty KF Jhund PS Petrie MC Inzucchi SE Køber L et al Effect of dapagliflozin on ventricular arrhythmias, resuscitated cardiac arrest, or sudden death in DAPA-HF. Eur Heart J. (2021) 42(36):3727–38. 10.1093/eurheartj/ehab560
32.
von Lewinski D Tripolt NJ Sourij H Pferschy PN Oulhaj A Alber H et al Ertugliflozin to reduce arrhythmic burden in ICD/CRT patients (ERASe-trial)—a phase III study. Am Heart J. (2022) 246:152–60. 10.1016/j.ahj.2022.01.008
33.
Matteucci A Pandozi C Bonanni M Mariani MV Sgarra L Nesti L et al Impact of empagliflozin and dapagliflozin on sudden cardiac death: a systematic review and meta-analysis of adjudicated randomized evidence. Heart Rhythm. (2025) S1547-5271(25)02890-5. 10.1016/j.hrthm.2025.09.023
34.
Zheng C Lin M Chen Y Xu H Yan L Dai H . Effects of sodium-glucose cotransporter type 2 inhibitors on cardiovascular, renal, and safety outcomes in patients with cardiovascular disease: a meta-analysis of randomized controlled trials. Cardiovasc Diabetol. (2021) 20(1):83. 10.1186/s12933-021-01272-z
35.
Wang S Fang L Xie S Wei L Liu C . Interpretation and discussion of the 2023 expert consensus statement on practical considerations for the use of SGLT-2 inhibitors in the Asia-Pacific countries. Int J Endocrinol Metab. (2024) 44(4):279–83. 10.3760/cma.j.cn121383-20230730-07045
36.
Hao P Liu Y Wang P Li M Li X . Electrochemical regulation of mitochondrial respiratory chain protein redox states using transmembrane copper peptides for myocardial infarction risk assessment. Bioelectrochemistry. (2026) 167:109071. 10.1016/j.bioelechem.2025.109071
Summary
Keywords
heart failure, hypertrophic cardiomyopathy, left ventricular diastolic function, real-world study, sodium-glucose cotransporter 2 inhibitor
Citation
Ding C, Lv F, Wang L and Xu X (2026) SGLT-2 inhibitors improve cardiac function in hypertrophic cardiomyopathy: a real-world propensity score-matched study. Front. Cardiovasc. Med. 13:1742682. doi: 10.3389/fcvm.2026.1742682
Received
09 November 2025
Revised
11 January 2026
Accepted
19 January 2026
Published
12 February 2026
Volume
13 - 2026
Edited by
Ting Yuan, Goethe-Universität Frankfurt, Germany
Reviewed by
Alexandre A. da Silva, University of Mississippi Medical Center, United States
Panpan Hao, Shandong University, China
Nicola Pierucci, Sapienza University of Rome, Italy
Updates
Copyright
© 2026 Ding, Lv, Wang and Xu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Xiaohong Xu syxuxiaohong@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.