Abstract
Introduction:
Despite advances in stent design, pharmacotherapy, and procedural techniques that have improved percutaneous coronary intervention (PCI) outcomes and reduced PCI-related complications, these events still occur and are associated with adverse outcomes. Moreover, complex PCI procedures, which predispose to increased risk of complications, are increasingly performed. Understanding risk factors, underlying mechanisms, evidence-based management, and preventive strategies are essential to optimize procedural outcomes. This review aims to summarize current evidence and highlight gaps in knowledge related to PCI-associated complications.
Methods:
This narrative review used a focused PubMed search through October 2025, prioritizing randomized trials, large observational studies, guidelines, and consensus statements.
Results:
Acute vessel closure, which most commonly results from dissection or thrombosis and perforation are frequently associated with hemodynamic compromise and increased procedural mortality. Device-related complications such as entrapment and fracture, although rare, can potentially lead to significant morbidity and mortality. Preventive strategies emphasize appropriate lesion preparation, proper device selection and sizing, gentle manipulation, and the use of adjunctive imaging modalities such as intravascular ultrasound and optical coherence tomography to minimize risk. Early recognition and prompt management of these complications are essential to decreased adverse outcomes of PCI in both short and long term. However, due to the low incidence of these events, current management strategies are largely based on case reports, observational studies, and expert consensus.
Conclusions:
Future large-scale studies and registry data, along with artificial intelligence-guided risk modeling are warranted to facilitate individualized prediction, enhance procedural safety, and advance precision management across preprocedural, intraprocedural and postprocedural phases.
Graphical Abstract
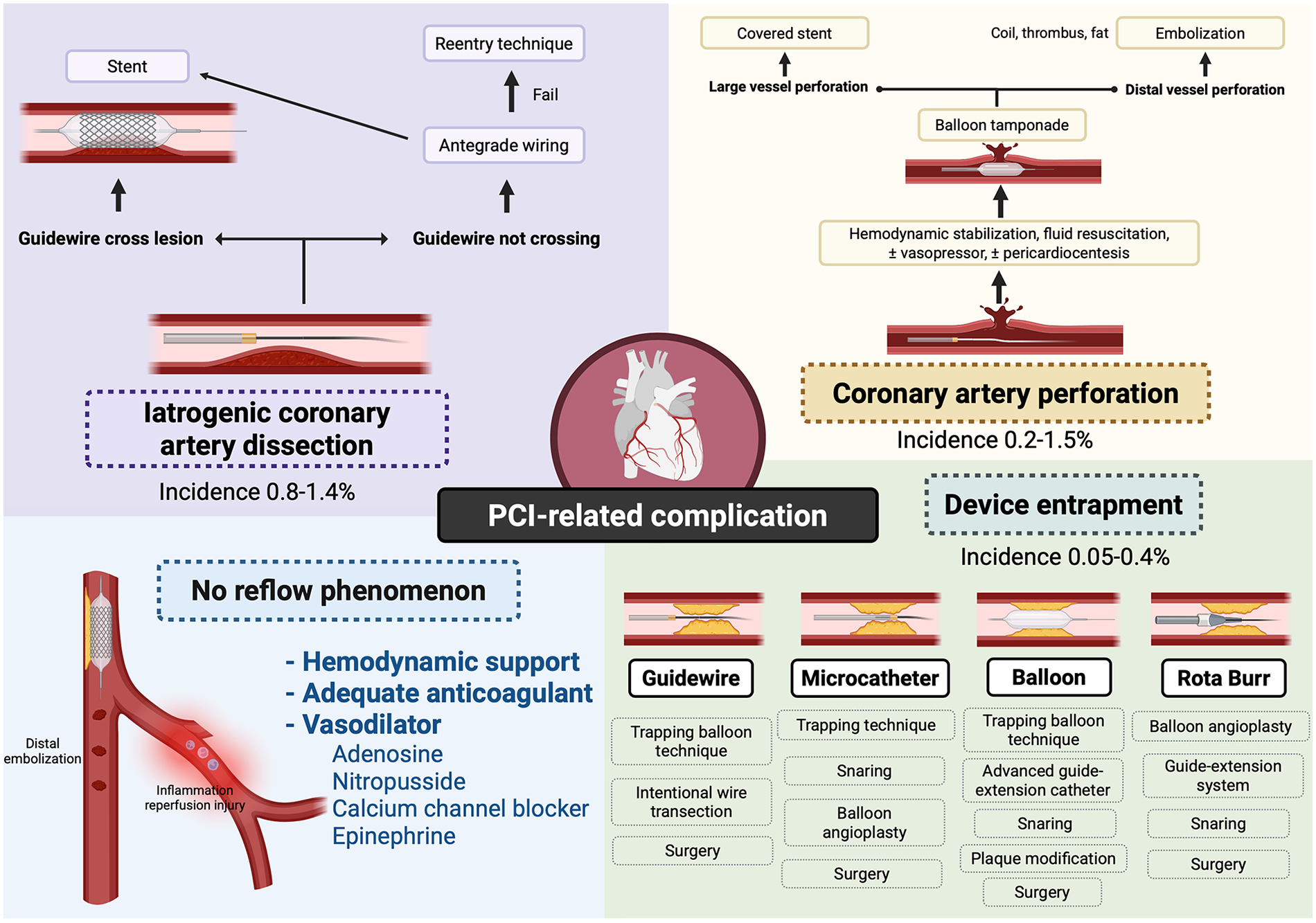
PCI-Related Complications and Management Strategies. This schematic summarizes major complications associated with percutaneous coronary intervention (PCI), including iatrogenic coronary artery dissection, coronary perforation, no-reflow phenomenon, and device entrapment. Created in BioRender. Attachaipanich T. (2026) https://BioRender.com/mpuf12a, licensed under Academic License.
1 Introduction
Percutaneous coronary intervention (PCI) has become one of the most frequently performed cardiovascular procedures, with over 600,000 PCI cases performed annually at more than 1,600 centers across the United States (1). Advances in stent design, procedural techniques, and the use of potent antithrombotic therapies have markedly improved clinical outcomes and reduced procedural complications (2). Despite these improvements, PCI-related complications still occur and remain clinically significant, contributing to increased morbidity and mortality (3). Furthermore, the rising prevalence of complex PCI procedures has been associated with a higher risk of adverse events (4). A comprehensive understanding of the underlying mechanisms, risk factors, management approaches, and preventive strategies is essential to minimize these complications and optimize patient outcomes. The aim of this review is to summarize current evidence on PCI-related complications, focusing on intraprocedural coronary-related complications, including acute vessel closure, coronary dissection, coronary perforation, device entrapment or fracture, stent loss, and air embolism. These include the underlying pathophysiologic mechanisms to evidence-based management and prevention strategies, with the goal of guiding clinical decision-making and improving PCI outcomes in contemporary practice. This narrative review was based on a focused literature search of PubMed from inception through October 2025 using terms related to PCI-related complications. Priority was given to randomized clinical trials, large observational studies, registry data, consensus statements, and contemporary guideline documents, with inclusion of relevant case series and expert opinion when higher-level evidence was limited.
2 Acute vessel closure
2.1 Description
Acute vessel closure refers to the abrupt cessation of anterograde coronary perfusion, leading to myocardial ischemia. In the NHLBI Dynamic Registry, an observational registry study, the incidence of acute vessel closure declined from approximately 3% during the balloon angioplasty era to 0.3% in the contemporary PCI era, largely due to advances in stent technology and potent antithrombotic therapy (5). Acute vessel closure may result from coronary dissection, thrombosis, distal embolization, side branch occlusion after stenting, vasospasm, pseudolesion formation, equipment entrapment, intramural hematoma, and no-reflow, with dissection and intracoronary thrombus being the predominant causes (6).
2.2 Risk factors
Risk factors for acute vessel closure include patient-related factors such as female sex, prior myocardial infarction (MI), presentation with acute MI, and high surgical risk (6). Angiographic and procedural factors include multivessel disease, eccentric or complex lesions, multiple lesions within the same vessel, long lesion length, and bifurcation or branch-point lesions (6).
2.3 Management strategies
Management of acute vessel closure involves maintaining wire access, promptly identifying the underlying mechanism, and performing targeted intervention based on identified mechanism, while maintaining hemodynamic stability (6). Understanding these mechanisms, particularly dissection and thrombosis, is essential for prevention, early recognition, and timely management.
3 Coronary artery dissection
3.1 Iatrogenic coronary artery dissection
3.1.1 Description
Iatrogenic coronary artery dissection is a rare but serious complication of PCI and represents one of the major mechanisms of acute vessel closure. The reported incidence of iatrogenic coronary dissection during PCI ranges from 0.8% to 1.4% (7–9). Iatrogenic coronary artery dissection most commonly results from noncoaxial catheter or guide engagement, forceful contrast injection against a dampened pressure waveform, inadvertent deep seating of the guide catheter, or excessive manipulation of stiff guiding catheters (7–9). Aggressive advancement or withdrawal of interventional devices and the use of polymer-jacketed guidewires may further propagate a dissection flap. In addition, balloon rupture, particularly in the presence of a calcified lesion, can result in an aortocoronary dissection (7–9). Importantly, although mechanical injury typically initiates the dissection, subsequent contrast injections may propagate a hydraulic dissection, further extending the intramural hematoma and potentially leading to vessel occlusion (10).
3.1.2 Risk factors
Patient-related factors include advanced age, female sex, and presentation with ACS (11). Angiographic risk factors include calcified, eccentric, long, or complex lesions (ACC/AHA type B or C), as well as vessel tortuosity (11). Procedural risk factors include a large balloon-to-artery ratio (>1.2), noncoaxial guide alignment, and deep seating of the catheter (11). Dissection is typically visualized on coronary angiography. Once a dissection is identified, further antegrade contrast injection should be avoided (10). Intravascular imaging, particularly IVUS, can facilitate accurate diagnosis and delineation of the dissection's extent (10).
In addition, underlying connective tissue disorders and pathogenic collagen or extracellular matrix gene variants have been associated with an increased risk of spontaneous coronary artery dissection, and syndromic conditions such as Loeys-Dietz syndrome have been reported as rare causes of SCAD (12, 13). However, the extent to which these genetic conditions independently increase the risk of iatrogenic coronary dissection during PCI remains unclear.
3.1.3 Classification
Iatrogenic coronary dissection is classified by the National Heart, Lung, and Blood Institute (NHLBI) into six types (A-F) as shown in Figure 1 (14). Type A represents a minor radiolucent line within the vessel lumen without persistent contrast staining after dye clearance. Type B is characterized by parallel tracts or a double-lumen appearance that also disappear following dye clearance, similar to Type A. Type C involves extraluminal contrast staining that persists after dye clearance, indicating deeper vessel wall involvement. Type D is identified by a spiral luminal filling defect that compromises flow within the true lumen, while Type E presents as a persistent luminal filling defect within the coronary artery lumen. Type F represents the most severe form, defined by total vessel occlusion with complete loss of antegrade flow (14). The NHLBI classification correlates with both clinical outcomes and management strategies. Types C through F are typically clinically significant and often require intervention, whereas Types A and B are usually benign and may be managed conservatively (14). The risk of acute vessel closure increases with the severity of dissection, progressing from Type A to Type F (14). Types B and C are associated with relatively low incidences of acute vessel closure (3% and 10%, respectively), while Types D, E, and F carry substantially higher risks, with incidences of approximately 30%, 37%, and 69%, respectively (14). The specific management strategies for coronary dissection are discussed in the dedicated dissection section below.
Figure 1
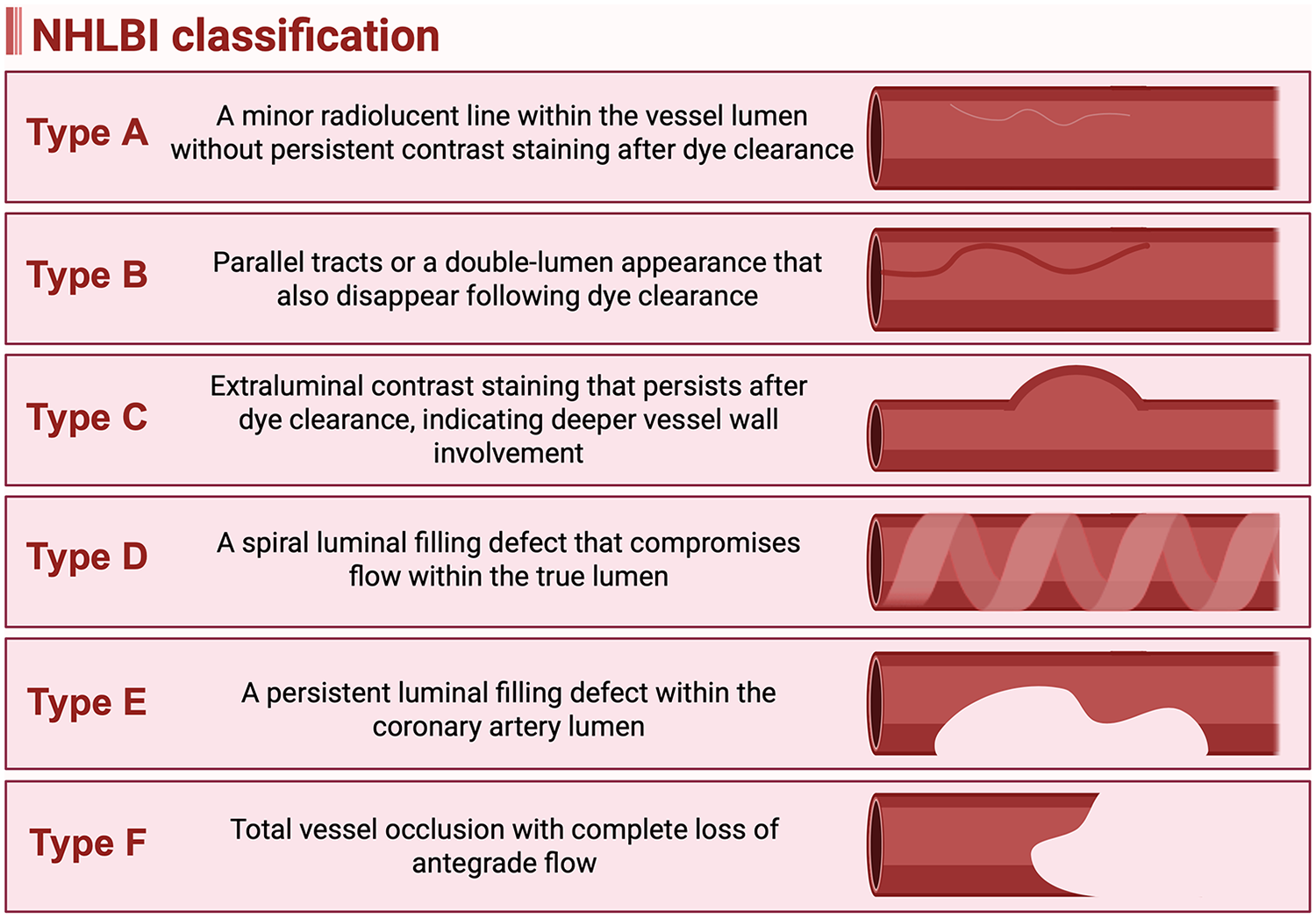
National heart, lung, and blood institute (NHLBI) classification of iatrogenic coronary artery dissection. (Created in BioRender. Attachaipanich, T. (2026) https://BioRender.com/mtbbyj5, licensed under Academic License).
3.1.4 Management strategies
Management of guide catheter-induced dissection generally requires stent implantation, and the procedural approach depends on whether the guidewire has already crossed the lesion (10, 15). IVUS can help confirm whether the wire is positioned in the true or false lumen (10). If the wire is already across the lesion and located in the true lumen, a stent should be placed proximally to seal the entry point of the dissection and compress the hematoma (10). When the distal extent of the dissection is visualized, consider stenting the distal edge first to prevent further propagation (10, 15). If TIMI flow remains <3 after proximal stenting, this suggests persistent distal hematoma or occlusion; in such cases, balloon dilation or use of a cutting balloon may be considered to decompress the hematoma and restore flow (10, 15). If rewiring is necessary, IVUS should be used to ensure entry into the true lumen. IVUS is also useful for determining the length of the dissection, appropriate stent sizing, and optimization of stent deployment (10). Although optical coherence tomography (OCT) has been successfully used in some cases of catheter-induced dissection, its use should be approached with caution, as forceful contrast injection required for blood clearance can exacerbate the dissection (16).
If the wire has not crossed the lesion, an antegrade wiring attempt should be performed, followed by stenting once true lumen access is accessed (10, 15). When the initial wire lies within the false lumen, IVUS may be left in place alongside the first wire to provide real-time imaging guidance during the parallel wire technique. This approach allows the subintimal wire to tamponade the false lumen while a second wire is advanced into the true lumen (10). The subintimal transcatheter withdrawal (STRAW) technique may also be used to aspirate and decompress a subintimal hematoma that prevents distal reentry (17).
If antegrade wiring fails, alternative reentry techniques can be performed, including wire-based dissection reentry, reentry using a Stingray reentry system, or a retrograde approach (10, 15). The subintimal tracking and reentry (STAR) technique involves advancing a knuckled polymer-jacketed wire through the subintimal space until it spontaneously reenters the distal true lumen (18). Modified antegrade reentry methods, such as mini-STAR and limited antegrade subintimal tracking (LAST), have been developed to achieve earlier and more controlled reentry, minimizing the dissection plane and vessel trauma (18). The Stingray balloon system facilitates controlled subintimal navigation and targeted reentry into the true lumen using a flat, dual-lumen balloon and a specialized reentry wire (18). In selected cases with suitable collateral vessels, a retrograde approach may be performed.
3.1.5 Preventive strategies
Prevention of iatrogenic coronary dissection includes avoiding contrast injection when a dampened pressure waveform is observed, preventing deep catheter engagement, maintaining coaxial guide alignment, minimizing aggressive wire manipulation, and exercising caution with polymer-jacketed guidewires (19). Preprocedural IVUS imaging can assist in lesion assessment and guide appropriate lesion preparation to minimize the risk of dissection (10).
3.2 Iatrogenic aortocoronary dissection
3.2.1 Description
Aortocoronary dissection may extend into the aortic cusp or ascending aorta. The reported incidence of iatrogenic aortocoronary dissection ranges from 0.02% to 0.2%, with most cases originating from the right coronary cusp (7, 20). The majority of these dissections can be managed with ostial stenting, which seals the entry tear (20).
3.2.2 Classification and management strategies
The Dunning classification provides an angiographic framework for characterizing aortocoronary dissections based on the extent of aortic involvement: Class I dissections are confined to the aortic cusp, Class II extend less than 4 cm into the ascending aorta, and Class III extend more than 4 cm into the ascending aorta (21). This classification correlates with both prognosis and management strategy. Higher Dunning classes are associated with an increased likelihood of surgical intervention and higher mortality, reported as 0% for Class I, 4.3% for Class II, and 6.5% for Class III (8). Most Class I cases can be successfully treated conservatively or with ostial stenting, whereas 17.4% of Class II and 40.3% of Class III cases require surgical repair (8). IVUS should be used to guide stent placement to ensure complete ostial coverage and seal the dissection entry point (22).
4 Intracoronary thrombus
4.1 Description and risk factors
Intracoronary thrombus is another common cause of acute vessel closure (6). It can result from inadequate antithrombotic therapy, a hypercoagulable state, or stent underexpansion (23). Several risk factors have been identified. Patient-related factors include male sex, smoking, hypercoagulable states, hyperglycemia, hypercholesterolemia, vasculitis, delayed presentation, and cardiogenic shock (23). Procedural- and lesion-related factors include acute plaque rupture, complex or long lesions, slow coronary flow, large vessel diameter (>4 mm), right coronary artery (RCA) involvement, old saphenous vein graft (SVG) lesions, inadequate anticoagulation, suboptimal dual antiplatelet therapy (DAPT), and heparin-induced thrombocytopenia (HIT) (23). Importantly, failure to confirm a therapeutic ACT before wiring the vessel is a preventable cause of intracoronary thrombus formation.
4.2 Management strategies
Optimal pharmacologic therapy plays a key role in reducing thrombus burden and improving procedural outcomes. DAPT, including aspirin and a potent P2Y12 inhibitor, along with intraprocedural anticoagulation, can reduce thrombus burden during PCI (24). In large randomized controlled trials, including ISAR-REACT 2 and EARLY ACS, routine pretreatment or intraprocedural administration of glycoprotein IIb/IIIa inhibitors (GPI) does not reduce ischemic events but is associated with an increased risk of bleeding complications (25–27). Consequently, current guidelines recommend against the routine upstream use of GPI; however, adjunctive intravenous or intracoronary GPI may be considered in the setting of a large thrombus burden or no-reflow to reduce infarct size and improve procedural success (24). In the INFUSE-AMI trial, a randomized controlled study of patients with large anterior STEMI, intracoronary abciximab was associated with a lower thrombus burden and smaller infarct size (28). Moreover, previous studies have shown that GPI administration is associated with a reduced thrombus burden in both ST-elevation MI (STEMI) and non-ST-elevation acute coronary syndrome (NSTE-ACS) (29, 30). Regarding the route of administration, randomized studies have demonstrated no significant difference between intravenous and intracoronary GPI in reducing thrombus burden (31, 32).
In addition to pharmacologic strategies, several mechanical techniques have been investigated for managing large thrombus burdens. Thrombus aspiration aims to remove intracoronary thrombus in this setting. In early randomized trials such as TAPAS study, thrombus aspiration was associated with improved TIMI flow and better clinical outcomes (33, 34). However, subsequent large randomized controlled trials, including TASTE and TOTAL trials, found that routine thrombus aspiration did not improve clinical outcomes and was associated with an increased risk of stroke (35, 36). A pooled analysis of three large randomized studies similarly showed no clinical benefit with routine thrombus aspiration (37). In contrast, subgroup analyses suggested that in patients with a high thrombus burden, thrombus aspiration was associated with a significantly lower risk of cardiovascular death, though it was offset by an increased risk of stroke (37). Overall, current guidelines recommend against the routine use of thrombus aspiration in STEMI; however, it may be considered in cases of a large thrombus burden, risk of no-reflow, or as a bailout strategy following failed balloon angioplasty or stent deployment (24). A recent prospective study reported that mechanical thrombus aspiration in acute coronary syndrome (ACS) with a high thrombus burden achieved a high rate of thrombus removal and improved myocardial perfusion (38). Nonetheless, randomized data supporting mechanical thrombus aspiration remains limited.
Intracoronary fibrinolytic therapy is another potential treatment option for large thrombus burden. Several small randomized trials have demonstrated the potential efficacy and safety of intracoronary fibrinolytic agents in STEMI populations (39–41). In the DISSOLUTION trial, a small randomized study of STEMI patients with a high thrombus burden showed that intracoronary urokinase improved myocardial blood flow without increasing bleeding risk (42). However, current evidence remains limited to small randomized studies, and further large-scale trials are needed.
4.3 Preventive strategies
Because procedural thrombus formation arises from mechanical disruption, inadequate anticoagulation, or suboptimal stent deployment, the primary focus in PCI-related thrombosis should be on optimized intraprocedural anticoagulation, careful lesion preparation, and proper stent expansion, with selective use of adjunctive therapies in cases where thrombus develops despite these preventive measures. Overall, evidence from STEMI studies may guide the treatment of iatrogenic intracoronary thrombus, but its occurrence during PCI should be recognized as a distinct procedural complication requiring prompt identification and management.
5 No-reflow phenomenon
5.1 Description and risk factors
The no-reflow phenomenon refers to impaired myocardial reperfusion despite successful epicardial coronary revascularization. Although advances in intervention techniques have reduced its incidence, no-reflow remains associated with adverse outcomes, including heart failure, arrhythmia, cardiogenic shock, and mortality (43). The no-reflow phenomenon is more likely to occur in the setting of STEMI, degenerated SVG, and during rotational atherectomy (44, 45). Predictors included patient-related factors such as advanced age, male sex, smoking, dyslipidemia, chronic kidney disease (CKD), diabetes mellitus, hypertension, CHA2DS2-VASc ≥ 5, elevated inflammatory and cardiac biomarkers, and higher Killip class (44, 46–48). Procedural- and lesion-related factors include lesion length >15 mm, prolonged reperfusion time, SVG PCI, low pre-procedural TIMI flow grade, high thrombus burden, and intra-aortic balloon pump (IABP) use, (49–52). Among patients with STEMI, the presence of atrial fibrillation, late presentation, and prolonged door-to-balloon time also increase the risk of no-reflow (45, 53).
The mechanisms underlying the no-reflow phenomenon include distal embolization, ischemic injury, and reperfusion injury (44). Distal embolization is the most common cause, resulting from platelet activation, thrombus fragmentation, and the release of plaque debris during catheter manipulation (44). Ischemic injury leads to cellular swelling, loss of vascular tone, and interstitial edema, which together cause microvascular obstruction and impaired perfusion (44). Reperfusion injury involves inflammatory cell infiltration, cytokine release, and oxidative stress, resulting in endothelial dysfunction and tissue injury (44).
The diagnosis of no-reflow is made angiographically, defined as a TIMI flow grade <3 and a myocardial blush grade (MBG) < 3 in the absence of other causes. These findings indicate abnormal myocardial perfusion despite epicardial coronary artery revascularization (44, 45). Other potential causes of impaired flow, such as macrovascular dissection, vasospasm, or thrombus, should be excluded using intravascular imaging modalities such as intravascular ultrasound (IVUS) (54).
5.2 Management strategies
The management of coronary no-reflow includes the administration of vasodilators, assessment of anticoagulation adequacy through activated clotting time (ACT) monitoring, and hemodynamic stabilization with circulatory support as needed (55). Intracoronary administration of vasodilators, delivered via a microcatheter, dual lumen catheter, or an over-the-wire balloon, is preferred to minimize systemic side effects and ensure targeted delivery to the microcirculation. Commonly used vasodilators include adenosine, sodium nitroprusside, and calcium channel blockers (CCBs) such as verapamil, diltiazem, and nicardipine, as well as intracoronary epinephrine as shown in Table 1 (56).
Table 1
| Vasodilator | Route | Dose |
|---|---|---|
| Adenosine | Intracoronary | 50–200 μg |
| Intravenous | 70 μg/kg/min | |
| Diltiazem | Intracoronary | 400 μg |
| Epinephrine | Intracoronary | 50–200 μg |
| Nicardipine | Intracoronary | 50–200 μg |
| Nitroprusside | Intracoronary | 50–200 μg |
| Verapamil | Intracoronary | 100–250 μg |
Pharmacotherapy vasodilators in the management of coronary no-reflow.
While adenosine and nitroprusside have been shown to improve angiographic TIMI flow, data demonstrating a corresponding reduction in major adverse cardiac events (MACE) are limited. In the AMISTAD and AMISTAD-II randomized controlled trials, adenosine administration was associated with a significant reduction in infarct size; however, no improvement in clinical outcomes was observed (57, 58). A randomized study in STEMI patients following thrombus aspiration compared adenosine, nitroprusside, and saline, and demonstrated that only adenosine was associated with greater ST-segment resolution (59). Angiographic microvascular obstruction and 30-day MACE were numerically lower with adenosine but did not reach statistical significance, while no difference was observed in the nitroprusside group (59). A subsequent randomized study using higher doses of adenosine reported no significant improvement in infarct size or microvascular obstruction, but did show an increased incidence of adverse events, including heart failure. Notably, the adenosine dose used in this trial (1–2 mg) was substantially higher than the recommended intracoronary dose of 50–200 μg (60, 61). A meta-analysis of nitroprusside in STEMI patients demonstrated a significant reduction in the incidence of no-reflow with nitroprusside therapy (62).
Current evidence for CCBs in the management of no-reflow remains limited. Only small randomized studies have evaluated the efficacy of CCBs in this setting (63–65). Meta-analyses demonstrated that intracoronary verapamil and diltiazem were associated with a reduced incidence of no-reflow and lower rates of MACE at 6 months, although without improvement in left ventricular ejection fraction (66, 67). A network meta-analysis comparing various intracoronary agents in STEMI showed that verapamil, nitroprusside, and adenosine were associated with lower rates of no-reflow compared with control (68). Further large-scale randomized trials are needed to clarify the benefits of CCBs in this context.
Intracoronary epinephrine has also been investigated as a treatment option for no-reflow that results in coronary microvascular vasodilation through β2-adrenergic receptor activation, which promotes smooth-muscle relaxation and reduces intracellular calcium overload. A small retrospective study demonstrated that intracoronary epinephrine improved coronary blood flow in STEMI patients with no-reflow refractory to conventional therapy (69). Similarly, a small randomized trial comparing intracoronary epinephrine with adenosine found that epinephrine achieved a significantly higher frequency of TIMI grade 3 flow and a lower corrected TIMI frame count compared with adenosine (70). An important advantage of epinephrine over traditional coronary vasodilators is its ability to augment systemic blood pressure, which is particularly beneficial in no-reflow accompanied by hypotension or cardiogenic shock, where other vasodilators may worsen hemodynamics.
5.3 Preventive strategies
Preventive strategies for no-reflow should address both precatheter (71) ization and intraprocedural factors based on patient risk profiles (56). Precatheterization measures include optimization of comorbidities and strict glycemic control, while minimizing door-to-balloon time remains essential. Intraprocedural prevention includes the prophylactic use of intracoronary vasodilators and selective thrombus aspiration in cases of high thrombus burden (56). Use of embolic protection devices may also be considered in setting of SVG PCI, since SVG PCI are associated with an increased risk of no-reflow compared with native vessel PCI (71). Early randomized studies in SVG PCI demonstrated that distal embolic protection was associated with a significantly lower incidence of no-reflow (72). Similarly, a randomized study in ACS patients with attenuated plaque ≥5 mm in length identified by IVUS found that distal embolic protection was associated with a lower risk of no-reflow compared with control (73). Therefore, current guidelines recommend considering embolic protection devices for SVG PCI as a Class IIa indication (71, 74).
6 Coronary artery perforation
6.1 Description
Coronary artery perforation is an uncommon but potentially life-threatening complication of PCI, with a reported incidence ranging from 0.2% to 1.5% of all PCI procedures (75–77). In chronic total occlusion (CTO) interventions, the incidence is substantially higher, reaching up to 4.8% (78). Coronary perforation is associated with an increased risk of MACE and in-hospital mortality of up to 17.8% in some studies, as well as with increased long-term mortality (75–77).
6.2 Risk factors
Predictors include patient-related factors such as advanced age, female sex, CKD, hypertension, current smoking, prior MI, previous coronary artery bypass grafting (CABG), lower body mass index, presentation with cardiogenic shock, and prior PCI (75–77, 79). Anatomical factors include CTO lesions, high-risk type C morphology, bifurcation lesions, heavy calcification, long lesion length, small vessel diameter, and severe vessel tortuosity (75–77, 79–81). Procedural factors include the use of hydrophilic-coated, polymer-jacketed or stiff-tip guidewires, oversized balloons and stents, left main or left anterior descending (LAD) artery intervention, use of laser or rotational atherectomy, preprocedural TIMI flow <2, and preexisting coronary dissection (75–77, 79–81). In setting of CTO interventions, predictors of coronary perforation include advanced age, use of anterograde dissection and re-entry or retrograde approaches, presence of blunt or no stump morphology, and moderate-to-severe calcification (82). Importantly, the use of intracoronary imaging-guided PCI has been shown to significantly reduce the risk of coronary perforation, particularly in the context of CTO or heavy calcification (83).
Coronary artery perforation can be classified based on the severity of the lesion and the size of the affected vessel. Angiographic classification systems describe the severity according to the extent of extravasation, which correlates with the underlying etiology, clinical impact, and prognosis. Classification based on the vessel size also guides management strategies.
6.3 Classification
The Ellis classification was proposed to categorize coronary artery perforation based on the angiographic severity of contrast extravasation and remains the most widely used grading system as shown in Figure 2 and Table 2 (84). Type I refers to a concealed perforation, characterized by an extraluminal crater without any evidence of contrast extravasation. Type II denotes a limited perforation, in which localized or perivascular contrast staining is present but without a visible jet of contrast extravasation. Type III represents a severe perforation, defined by a free-flowing stream of contrast extravasation through an exit hole ≥1 mm in diameter, with contrast filling and clearing outside the vessel lumen (84). In Type III perforations, contrast may extravasate into the pericardial space, potentially leading to cardiac tamponade, or into a cardiac chamber, which generally results in better hemodynamic tolerance. When extravasation extends into an anatomical cardiac chamber, the perforation is subclassified as Type III cavity spilling.
Figure 2
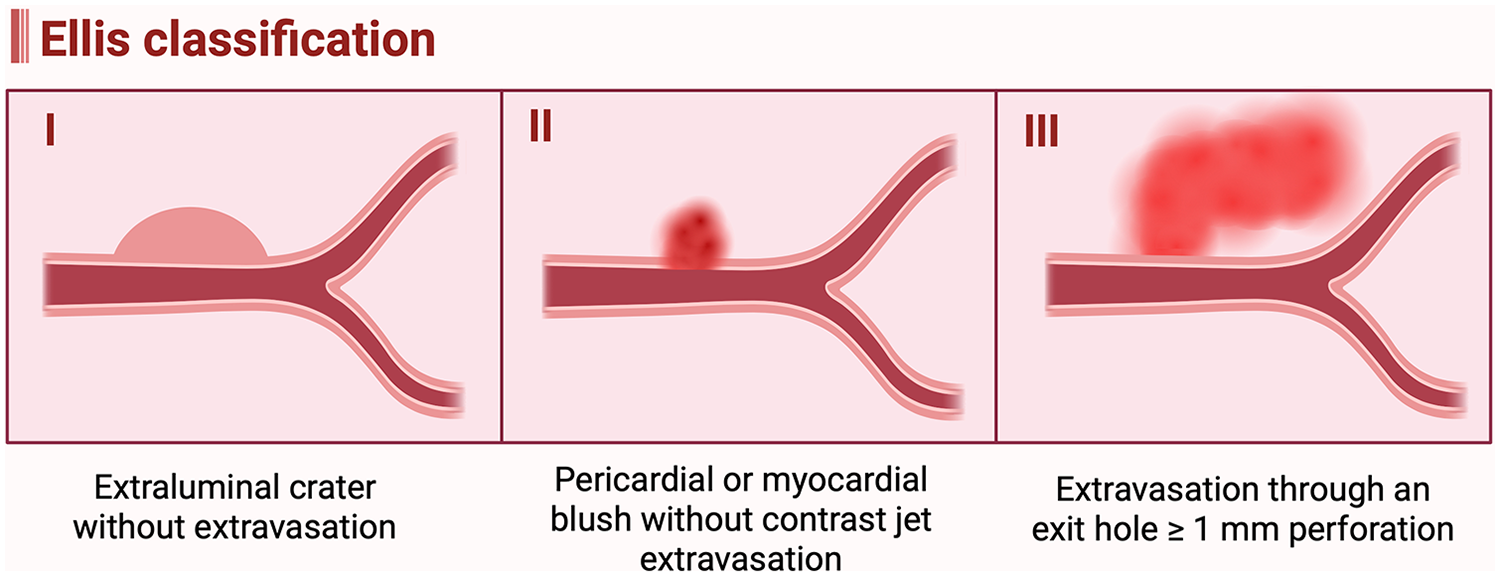
Ellis classification of coronary artery perforation. (Created in BioRender. Attachaipanich, T. (2026) https://BioRender.com/rbq4kvi, licensed under Academic License).
Table 2
| Ellis type | I | II | III | III CS |
|---|---|---|---|---|
| Definition | Extraluminal crater without extravasation | Pericardial or myocardial blush without contrast jet extravasation | Extravasation through an exit hole ≥1 mm perforation | Perforation into an anatomical cavity chamber |
| Clinical outcome | Usually benign, hemodynamic stable | Usually benign, hemodynamic stable, risk of delay tamponade | Hemodynamic compromise, high risk of tamponade and mortality | Can be benign, better hemodynamic tolerance |
| Management |
|
|
|
|
Ellis classification of coronary artery perforation: definitions, clinical outcomes, and recommended management strategies.
The Ellis classification correlates with both the mechanism of perforation and clinical outcomes. Types I and II typically result from small-vessel or distal guidewire perforations and are usually hemodynamically stable. In contrast, Type III perforations are often associated with large-vessel injuries, hemodynamic compromise, and a significantly higher risk of major complications and mortality (85). A retrospective study reported in-hospital mortality rates of 0%, 1.7%, and 21% for Ellis Types I, II, and III, respectively. During long-term follow-up, Ellis Type III perforations were associated with a threefold increase in all-cause mortality compared with Types I and II (85). The incidence of acute cardiac tamponade was also significantly higher in Type III perforations (40.4%) compared with Types I and II (3.8%), whereas the rate of delayed tamponade was higher in Types I and II (7.7%) than in Type III (3.5%) (85).
6.4 Large-vessel perforation
6.4.1 Description
Large-vessel coronary perforation most commonly occurs due to the use of oversized or overinflated balloons and stents, or as a complication of atherectomy devices such as rotational atherectomy (86). These perforations are typically classified as Ellis Type III, representing the most severe form of coronary perforation (85, 86). Clinically, large-vessel perforation can lead to rapid hemodynamic deterioration due to cardiac tamponade, often requiring emergent pericardiocentesis and hemodynamic support (86). The sudden onset of acute chest pain or hemodynamic instability during balloon inflation or stent deployment should raise suspicion for coronary artery perforation, which can be promptly confirmed by angiographic visualization of contrast extravasation.
6.4.2 Management strategies
Management of coronary artery perforation involves both general supportive measures applicable to all cases and specific strategies based on the vessel size and perforation severity. General management includes hemodynamic stabilization with fluid resuscitation, vasopressor support if needed, and pericardiocentesis in cases of cardiac tamponade (81). Importantly, in cases of ongoing extravasation or persistent localized tamponade, particularly in patients with prior CABG, where the pericardial space may not be amenable to complete percutaneous drainage, emergent surgical intervention, including creation of a pericardial window or operative repair, may be required to restore and maintain hemodynamic stability (87).
Balloon tamponade remains the first-line procedural intervention and should be performed by inflating a balloon at or just proximal to the perforation site for 5–10 min using a balloon-to-artery ratio of 1:1 at low inflation pressure (81). Because prolonged balloon inflation can compromise distal myocardial perfusion and lead to ischemia or infarction, a specialized device known as the Ringer perfusion balloon has been developed. This helical-shaped inflatable balloon opposes the vessel wall while maintaining antegrade coronary perfusion through a hollow central lumen (88). In a recent study, the Ringer perfusion balloon demonstrated a high procedural success rate, achieving cessation of contrast extravasation in 84.6% of cases and maintenance of TIMI grade 2–3 flow in 100% of patients (88). Intravenous anticoagulant and antiplatelet therapies should be withheld immediately following the recognition of a coronary artery perforation. Reversal of anticoagulation may be considered once all interventional equipment has been withdrawn from the coronary artery. Recent evidence further supports the safety of this approach (89). In a single-center retrospective study 160 patients with coronary perforations during CTO PCI, protamine administration after complete device removal was associated with significantly lower rates of in-hospital mortality and cardiac tamponade, with no observed cases of acute stent thrombosis (89). These findings reinforce that appropriately timed heparin reversal can be safely utilized in select cases of coronary artery perforation.
If balloon tamponade fails to control the bleeding, covered stent implantation should be performed (15). The goal of a covered stent is to seal the perforation using an impermeable barrier layer, thereby restoring vessel integrity and preventing further extravasation (90, 91). Two main types of covered stents are currently available. The Graftmaster (Abbott Vascular) has a traditional “sandwich” design, consisting of a bilayer metallic framework with a thin, biocompatible polytetrafluoroethylene (PTFE) membrane between the layers. It typically has a larger diameter and requires a larger guide catheter for delivery (91). The PK Papyrus (Biotronik) has a single-layer cobalt-chromium platform covered with a highly elastic polyurethane membrane, offering a smaller profile, improved flexibility, and compatibility with smaller guide catheters and standard 0.014-inch guidewires (90, 91).
The deployment technique depends on the guide catheter size and stent type. If the guide catheter is large enough, the “block-and-deliver” technique can be used with a single guide catheter (92). In this method, a second guidewire is advanced while the balloon remains inflated at the perforation site. The balloon is briefly deflated to allow guidewire passage, then reinflated to maintain hemostasis while the covered stent is advanced proximally. Once positioned, the balloon is deflated and withdrawn, allowing deployment of the covered stent at the perforation site (92).
When the initial guide is too small, the “ping-pong” (dual-guide) technique can be performed (93). In this approach, a second guide catheter is introduced while maintaining balloon inflation. The balloon is briefly deflated to allow passage of the second guidewire and covered stent, to the perforation site. After the stent is positioned, the first guidewire and balloon are withdrawn, and the covered stent is deployed to seal the perforation (93).
Despite their efficacy, covered stents cause side-branch occlusion, which may result in myocardial ischemia. Evidence comparing different covered stent types remains limited. Observational study in patients with large-vessel perforations successfully sealed with a covered stent reported that the PK Papyrus was associated with a significantly lower rate of target lesion revascularization at 30 days compared with the Graftmaster, although no differences were observed in 30-day or 1-year MACE (94).
Although covered stents provide rapid and effective sealing of large-vessel perforations, several concerns remain regarding their long-term performance, including higher rates of restenosis and stent thrombosis (91). While these devices were conceptually designed to reduce in-stent restenosis by creating a mechanical barrier that limits neointimal proliferation through the stent struts, randomized data in SVG-PCI have not demonstrated a reduction in restenosis compared with bare-metal stents (95, 96). A retrospective study reported a definite stent thrombosis rate of 8.6% among patients with Ellis Grade III perforation treated with a covered stent, and registry data similarly show a higher incidence of stent thrombosis within one year in covered stents compared with other stent platforms (96, 97). These findings underscore the need for optimal deployment technique and high-pressure post-dilation.
Perforations occurring in or near coronary bifurcations represent a particularly challenging scenario, as management must balance rapid hemostasis with preservation of side-branch flow. Initial treatment follows standard principles, including immediate balloon tamponade and hemodynamic stabilization, with precise localization of the perforation relative to the bifurcation determined using angiography and intravascular imaging. When bleeding persists, covered stent implantation is often required; however, bifurcation perforations may necessitate intentional side-branch jailing to achieve complete sealing (98). A systematic review of Ellis grade III perforations involving left main distal bifurcation lesions demonstrated that crossover covered stenting is the most commonly employed strategy, while alternative approaches, including covered stent implantation with the jailed balloon technique or simultaneous kissing covered stents, may improve side-branch preservation in selected cases (98). Side-branch sacrifice is sometimes unavoidable to achieve definitive hemostasis, with subsequent reaccess using high tip-load guidewires and dual-lumen catheters when clinically indicated. These principles are illustrated by a recent case report describing a complex bifurcation core perforation managed with a dual-covered stent strategy, in which intentional side-branch jailing was required for sealing, followed by advanced rewiring techniques to restore coronary blood flow (99).
6.5 Distal-vessel perforation
6.5.1 Description
Distal-vessel perforation typically occurs in distal coronary segments, side branches or collaterals in patients undergoing CTO PCI, most commonly resulting from guidewire-related injury. Previous studies have shown that the majority of guidewire-associated perforations are caused by hydrophilic guidewires (100). Compared with large-vessel perforation, small-vessel perforation carries a lower risk of immediate hemodynamic compromise, but it may lead to delayed cardiac tamponade, which can develop hours to days after PCI (101). Diagnosis is usually established by coronary angiography, which should include prolonged image acquisition with panning of the distal vessel to visualize subtle or delayed contrast extravasation. Adjunctive imaging with transthoracic echocardiography, including contrast-enhanced echocardiography, can determine the presence and location of ongoing extravasation, differentiate pericardial from intracardiac leakage, and guide the need for additional interventions such as coil embolization or covered stent placement.
6.5.2 Management strategies
Management principles are similar to those used in large-vessel perforation and begin with prolonged balloon inflation (15). If balloon tamponade fails to achieve hemostasis, a microcatheter should be advanced as close as possible to the perforation site for embolization therapy (15). Coil embolization is the preferred therapeutic option for sealing distal or small-vessel perforations vessel (81, 102, 103). Based on their delivery mechanism, coils are categorized into two main types. Pushable coils are non-retrievable once deployed and have a less predictable final position, whereas detachable coils permit controlled, reversible delivery, allowing for more precise placement at the target site (81, 103). During coil delivery, a second guidewire and balloon can be inflated proximally to the perforated branch to control bleeding, using the block-and-deliver technique (92).
In case where coils are unavailable, several alternative embolic agents may be used to achieve hemostasis. These include autologous fat, typically harvested from the abdomen or groin and delivered through a microcatheter; autologous blood clot, which serves as a natural occlusive material; and thrombin, which promotes localized thrombosis at the site of perforation to effectively seal the vessel (81). A negative suction technique using a microcatheter connected to a syringe has also been reported to achieve successful hemostasis by collapsing the perforated distal vessel (104). More recently, a novel technique involving the delivery of silk or absorbable suture fragments via microcatheter has been reported, effectively occluding distal perforations and achieving durable hemostasis (105). If embolization fails to seal the perforated branch, a covered stent can be deployed in the main vessel to seal the ostium of the perforated side branch, thereby preventing further extravasation (15).
6.6 Reversal of anticoagulation
Reversal of anticoagulation can facilitate hemostasis in cases of coronary artery perforation, but it must be balanced against the increased risk of thrombotic complications, particularly when interventional devices remain within the coronary artery. Reversal is generally recommended only after all equipment has been removed from the coronary system (15). Several studies have reported that a notable proportion of patients undergo anticoagulation reversal while devices are still positioned within the coronary artery (75, 106). However, evidence regarding the safety of this practice remains limited. A recent retrospective study demonstrated that although heparin reversal during active instrumentation was associated with successful hemostasis, it also resulted in coronary thrombosis in 7.6% of cases (107). Furthermore, a minimum ACT <150 s was strongly correlated with the occurrence of coronary thrombosis (107). These findings highlight the importance of careful timing of anticoagulant reversal.
More recently, additional evidence has demonstrated that protamine administration can be safely performed once all intracoronary equipment has been removed. In a single-center retrospective study of 160 patients who developed coronary perforation during CTO PCI, protamine administered after complete device withdrawal was associated with substantially lower rates of in-hospital mortality and cardiac tamponade, with no cases of acute stent thrombosis observed (89). These data support the safety of anticoagulation reversal when undertaken at the appropriate procedural stage.
For reversal, unfractionated heparin can be partially or fully neutralized with protamine sulfate, administered at a dose of 1 mg intravenously for every 100 units of heparin given, with a typical total dose of 25–50 mg (15, 108). In cases where bivalirudin is used, although it has a short plasma half-life, fresh frozen plasma may be considered to partially reverse its effect and promote more rapid hemostasis (108).
7 Entrapped equipment
Entrapped equipment is rare and can result in abrupt vessel closure, MI, coronary perforation, thrombosis, or arrhythmia (109). In severe cases, device entrapment may necessitate emergency surgical retrieval (109). The reported incidence of device entrapment ranged between 0.05% and 0.4% (109). Risk factors for device entrapment include severe vessel angulation, heavy calcification, and marked vessel tortuosity (109). The use of intravascular imaging (IVUS or OCT) to assess lesion characteristics before intervention can help guide appropriate lesion preparation and potentially reduce the risk of device entrapment. However, these imaging catheters themselves can become entrapped in tight or heavily calcified lesions, and therefore, forceful advancement should be avoided (110).
7.1 Coronary guidewires entrapment
7.1.1 Description
The incidence of guidewire entrapment or fracture ranges from 0.1%–0.2%, increasing to approximately 0.5% in CTO interventions (109, 111, 112). Risk factors for guidewire entrapment and fracture include guidewire jailing, excessive rotation (>180°), aggressive wire manipulation, use of multiple guidewires, and advancing an atherectomy burr over a kinked wire (113, 114). Anatomical features include marked vessel angulation, heavy calcification, bifurcation lesions, and CTO morphology further increase the risk of entrapment (113, 114). Entrapment may lead to wire fracture and retained fragments, particularly when excessive traction is applied. Advancing a rotational atherectomy burr against kink or immobile wire can also cause transection (113, 114).
Diagnosis of guidewire fracture is typically made by identifying loss of wire continuity on fluoroscopy or confirmed using IVUS (115). Retained guidewire fragments can result in serious complications, including thrombosis, coronary dissection, perforation, arrhythmia, or systemic embolization (116). Structurally, guidewires consist of inner and outer components; unraveling of the outer coil increases thrombogenicity and may cause luminal obstruction or distal embolism (109).
7.1.2 Management strategies
Management of an entrapped guidewire typically begins with percutaneous retrieval techniques. A microcatheter can be advanced as distally as possible over the trapped wire, followed by gradual withdrawal to facilitate release (15, 117). Alternatively, a small-profile balloon may be advanced, inflated at the site of entrapment, and gently pulled back to dislodge the trapped segment (117). Excessive traction should be avoided, as the guidewire core may unravel proximally and extend into the aorta. This unravelling can be difficult to visualize angiographically and is highly thrombogenic, emphasizing the importance of controlled retrieval rather than forceful pulling. If percutaneous retrieval fails, intentional wire transection can be performed. This technique has been described using rotational atherectomy to cut the entrapped guidewire, retrieve the proximal broken end, and leave the remaining distal fragment securely jailed beneath a stent, thereby reducing the risk of embolization (118). Surgical removal considered if the fragment poses thrombotic or embolic risk (117).
In setting of retained guidewire fragments, management should be individualized based on the location and extent of the retained wire, given its thrombogenic potential and the limited evidence regarding long-term outcomes. Conservative management may be appropriate in selected cases, particularly when the fragment is confined to a small, distal, or chronically occluded vessel, or when the risks of percutaneous or surgical retrieval outweigh potential benefits (111). Several studies have reported a benign clinical course in patients with retained fragments confined to chronically occluded or distal vessels, or in cases where retrieval attempts were unsuccessful (111, 119).
However, if the wire fragment extends into the aorta, removal is recommended due to the risk of thrombus formation and systemic embolization (111). Percutaneous retrieval techniques include the snare technique and the double- or triple-wire twisting method, in which additional guidewires are advanced alongside the fractured segment and rotated using a torquer to entangle and retrieve the wire (111, 120, 121). The knuckle-twister technique, a modification using a polymer-jacketed tapered wire formed into a knuckle, can be advanced distal to the fracture, then rotated and withdrawn to capture and extract the fragment, with high reported success and reproducibility (122). If percutaneous retrieval fails, surgical removal should be considered (111, 120).
For fragments confined within the coronary artery, several percutaneous strategies are available, including the snare technique, wire-twisting technique, small-balloon inflation and withdrawal, or stent deployment to seal and exclude the fractured wire from the lumen (111, 117, 120). When these percutaneous approaches are unsuccessful or carry excessive procedural risk, surgical removal should be pursued, whereas conservative management may be reserved for high-risk or clinically stable cases (111, 117).
7.1.3 Preventive strategies
Coronary guidewire entrapment can be reduced by careful lesion assessment, appropriate wire selection, and avoidance of excessive wire rotation (>180°), particularly with hydrophilic or polymer-jacketed guidewires. Resistance during manipulation should prompt reassessment rather than forceful advancement, guidewire jailing should be minimized, and rotational atherectomy should not be performed over kinked or immobile guidewires, with frequent fluoroscopic confirmation of wire position in complex anatomy.
7.2 Rota burr entrapment
7.2.1 Description
Previous studies have reported an incidence of approximately 0.4%–1% of procedures (123). The rotational burr, coated with diamond particles, ablates plaque in a unidirectional manner. When burring is halted within a tight or calcified segment, particularly distal to the lesion, the burr may become irretrievably trapped (124). One common mechanism is the “Kokesi phenomenon”, named after the Japanese doll with a bulb-shaped head and hollow body (124). This occurs when a small burr is advanced at high rotational speed through a narrow, rigidly calcified lesion, mimicking the insertion of the doll's neck into its body, allowing rotation but preventing withdrawal (124). Conversely, using an oversized burr in a severely calcified vessel or maintaining a high burr-to-artery ratio can also increase the risk of entrapment (125). Additionally, performing rotational atherectomy on a recently implanted, underexpanded stent may predispose to burr entrapment, as the stent struts impede burr movement (125).
7.2.2 Management strategies
When burr entrapment occurs, percutaneous retrieval should be attempted prior to emergency surgery. The initial management involves balloon dilation of the lesion to enlarge the lumen and release the burr. This can be achieved by introducing a second guidewire, either via a second guiding catheter or by removing the rotablation advancer sheath to allow wire passage (125, 126). After successful wiring, balloon angioplasty at or proximal to the burr may facilitate its retrieval (125). Additional techniques include pulling the RotaWire, burr, and guiding catheter as a single unit, using the distal wire bulb to transmit traction and dislodge the burr, as well as disconnecting the burr from the housing and rotating it in reverse while maintaining gentle traction to free it from the lesion. If unsuccessful, deep guide catheter intubation or the use of a guide-extension (“mother-and-child”) system, advancing a guide-extension or smaller guide catheter toward the burr, may assist in extraction (125, 127, 128). The snare technique has also been reported as an effective percutaneous retrieval method (129). If all percutaneous attempts fail, emergent surgical removal should be performed (125, 130).
7.2.3 Preventive strategies
Preventive strategies include avoiding excessive forward force, refraining from stopping burr rotation within or distal to the lesion, and preventing abrupt deceleration of rotational speed (125). Appropriate burr sizing and gradual advancement using a pecking motion remain essential procedural principles to minimize the risk of entrapment (125).
7.3 Balloon rupture and entrapment
7.3.1 Description
Balloon entrapment is an uncommon but can lead to abrupt vessel closure and myocardial ischemia. It may occur secondary to balloon shaft fracture, balloon rupture, failure to deflate, or interaction with a previously implanted stent struts (131). The mechanisms underlying failure to deflate include elastic recoil of a severely calcified lesion compressing the balloon, premature withdrawal into the guide catheter before complete deflation, or kinking or damage to the hypotube (132).
7.3.2 Management strategies
Management of failure to deflate includes using an indeflator filled with normal saline rather than contrast, attempting gentle balloon withdrawal without full deflation, or puncturing the balloon with another guidewire (133). Increasing the proportion of saline in the contrast mixture reduces viscosity and shortens balloon deflation time (132). If these steps are unsuccessful, high-pressure inflation to intentionally rupture the balloon may be used; however, this approach should be reserved for large-caliber vessels due to the high risk of coronary dissection or perforation (132).
When balloon entrapment occurs, and the cause is failure to deflate, the above steps should be followed. Otherwise, strategies include repeated gentle inflation and deflation, or gently forward-backward manipulation to release the trapped balloon (15, 131). Several techniques can be performed for retrieval of an entrapped balloon. The balloon-trapping technique involves advancing a second balloon into the guide catheter and inflating it to secure the entrapped balloon shaft, allowing the entire system to be withdrawn as a single unit. Alternatively, advancement of a guide-extension catheter over the trapped balloon shaft can facilitate its release by providing additional support and coaxial alignment. The snare technique may also be used when the balloon is accessible (15, 131). In cases where entrapment is caused by a heavily calcified lesion, plaque modification using a second guidewire and balloon to dilate the vessel distal to the trapped segment can modify the and release the entrapped balloon (134). The mini-STAR technique has also been described as an effective bailout option (135).
Balloon shaft fracture may occur as a result of kinking or excessive manipulation. Management depends on the location of the retained fragment (131). If a portion of the fragment remains within the guide catheter, a balloon-trapping maneuver or snare device may be used for retrieval (131). Surgical removal should be considered if percutaneous efforts are unsuccessful. When the fragment extends beyond the guide catheter, retrieval can be attempted by inflating another balloon alongside the retained segment to facilitate extraction or by using a snare system (131). Rotational atherectomy has also been successfully used to modify calcified plaque surrounding a retained balloon fragment, followed by stent implantation to secure the material and prevent distal embolization (136).
7.3.3 Preventive strategies
Preventive strategies include adequate lesion preparation, particularly with atherectomy for severely calcified lesions, avoiding very high-pressure balloon inflation, and avoiding forceful advancement of a balloon through resistant or underprepared segments.
7.4 Microcatheters entrapment
7.4.1 Description
The mechanisms include over-torquing, forceful advancement through heavily calcified or tortuous vessels, and use of the microcatheter without an internal guidewire for support (131). These factors increase friction and mechanical stress along the shaft, predisposing to device entrapment or structural failure.
7.4.2 Management strategies
The management approach is generally similar to that used for other types of device entrapment (15, 131). Management options include percutaneous retrieval, surgical removal, or conservative management, depending on the fragment's location and clinical impact. Among percutaneous strategies, the trapping technique is often effective (15, 131, 137). Additional options include the snare technique or plaque-modification maneuvers, such as advancing a second guidewire with balloon angioplasty to relieve mechanical impingement and facilitate removal (15, 131). If percutaneous retrieval fails, surgical extraction may be required, though conservative management can be reasonable when a small fragment remains confined to a distal branch and the procedural risk outweighs the benefit (15, 131).
7.4.3 Preventive strategies
Preventive strategies focus on minimizing mechanical stress during catheter manipulation. Operators should avoid excessive rotation and refrain from aggressive advancement through resistant or heavily calcified segments. Selecting microcatheters with reinforced or stiffer distal tips may further reduce the risk of tip fracture or deformation (138).
8 Stent loss
Stent loss is a rare complication of PCI that can lead to systemic embolization, emergency CABG, or even death (139). The incidence of stent loss has declined with the introduction of premounted stents and improvements in device design, but it remains clinically relevant, with reported rates ranging from 0.3% to 1.3% (139, 140). Lesion-related risk factors include severe calcification and pronounced proximal angulation (139). Procedural contributors include inadequate vessel preparation or direct stenting without predilation, forceful advancement or withdrawal, use of guide-extension catheters, and stent delivery through a previously implanted stent (139, 140). Based on these risk factors, stent loss can be minimized by ensuring thorough lesion preparation, avoiding forceful advancement when resistance is encountered, using guide-extension catheters to facilitate controlled stent delivery across calcified or tortuous segments, and delaying negative balloon preparation until the stent has reached the intended deployment site.
Stent types used in contemporary PCI include bare-metal stents (BMS), drug-eluting stents (DES), and bioresorbable scaffolds (BRS). BRS are designed to provide temporary mechanical support followed by complete resorption, with the theoretical advantage of avoiding long-term complications associated with permanent metallic stents, such as in-stent restenosis and late stent thrombosis (141). Compared with metallic stents, BRS have thicker struts and reduced radial strength, which may increase susceptibility to deformation during delivery and predispose to complications such as scaffold unloading or detachment. Case reports have described successful management of BRS unloading with immediate in-situ balloon expansion and post-dilation, although the relative risk of stent loss across different stent platforms remains unclear (142, 143).
8.1 Partial stent loss
Partial stent loss occurs when the stent becomes partially dislodged from its balloon delivery system while crossing a resistant or tight lesion, or when the stent deforms and disengages from the balloon during withdrawal.
Management options include retrieval, deployment, or crushing of the lost stent (139, 144). Initial management generally begins with percutaneous retrieval. One common approach involves inflating the partially engaged balloon within the lost stent and carefully withdrawing the system into the guide catheter. Another option is the small-balloon technique, where a guidewire is advanced distal to the unexpanded stent, and a small balloon is positioned, inflated at low pressure, and gently pulled back to retrieve the stent into the guide catheter (145). If balloon inflation causes stent deformation that prevents guide catheter entry, the balloon, stent, and guide catheter may be removed together as a single unit (145).
If this method fails, assessment of stent-vessel size compatibility is necessary. When the stent size is appropriate for the vessel, deployment in situ can be considered (139, 144). In cases of size mismatch or poor positioning, other options include retrieval techniques similar to those used for complete stent loss, crushing with another stent, or advancing and redeploying the stent to a more suitable location (139, 144). The stent-crush technique involves deploying a second stent at the same site to compress the displaced stent. However, this should be avoided in critical segments, particularly the left main coronary artery, due to the risk of acute thrombosis or in-stent restenosis (139, 144). Adequate stent apposition is crucial to prevent thrombosis; therefore, the use of intravascular imaging such as OCT or IVUS is recommended to assess expansion and apposition (146). Stent advancement and deployment can be performed by slightly inflating the balloon to stabilize the stent, carefully advancing it to the target site, and then deploying it (145, 147).
8.2 Total stent loss
Management of total stent loss depends on whether the guidewire remains in situ. When the guidewire is still positioned across the lesion, the procedure begins with the small-balloon technique, similar to the approach used for partial stent loss. If this fails, stent-vessel sizing should be assessed. When the stent size is appropriate for the vessel, deployment at the current location can be considered (139, 144). If there is a size mismatch, stent advancement and redeployment using a balloon to reposition the dislodged stent to a more suitable site may be attempted (139, 144).
If the stent cannot be successfully deployed, snaring, guidewire twisting, or stent-crush techniques can be considered as alternative bailout strategies (139). The snare technique involves engaging the proximal end of the dislodged stent with a snare loop and withdrawing the captured stent into the guide sheath (139). In the twisting-guidewire technique, one or more additional guidewires are advanced through the stent struts and rotated together using a torquing device to entangle and retrieve the stent (121). If retrieval attempts are unsuccessful, the crush technique may be performed by deploying another stent at the same site to compress the dislodged stent against the vessel wall. In cases of total stent loss with guidewire loss, a new guidewire should first be advanced distally to secure vessel access. Subsequently, the snare technique should be attempted; if retrieval fails, the crush technique remains a reasonable alternative.
Extra-coronary stent dislodgement can also occur, with management determined by the location of the lost stent. When the stent migrates into large peripheral arteries, such as the iliac or common femoral artery, snaring or crushing with a peripheral stent may be appropriate (139, 148). In contrast, stent dislodgement into smaller branches below the common femoral artery is typically benign, and conservative management has been reported successful in such cases (139, 145, 149).
9 Air embolism
9.1 Description
Air embolism is an uncommon but serious complication of PCI that can result in abrupt vessel closure. The reported incidence ranges from 0.1% to 0.3% (150). Clinical manifestations vary from asymptomatic or transient ischemic symptoms to severe presentations, such as cardiogenic shock, malignant arrhythmias, or sudden death, particularly in cases of massive air embolism (150). Electrocardiographic findings in intracoronary air embolism include ST-segment elevation, nonspecific ST-T wave changes, T-wave inversion, atrioventricular block, and ventricular tachyarrhythmias (150). Evidence from in vivo studies have demonstrated that the volume of air introduced into the coronary circulation directly correlates with the degree of myocardial dysfunction and mortality in animal models (151). The most common cause of air embolism is inadequate catheter preparation, particularly incomplete aspiration of the guide or delivery system before device insertion. Other contributing factors include balloon rupture or leakage, prolonged negative suction, device exchanges through the guide catheter, deep intubation or rapid withdrawal of the guiding catheter, and leaky manifold or connector systems (150, 152). Diagnosis is typically established by direct angiographic visualization of round, mobile radiolucent bubbles within the coronary lumen. In more complex or static lesions where bubbles may be immobile or subtle, OCT can be used for confirmation (153).
9.2 Management strategies
Management focuses on prompt hemodynamic stabilization and oxygen therapy. Administration of 100% oxygen accelerates the reabsorption of intravascular air (150). In hemodynamically unstable patients, inotropic support or mechanical circulatory assistance should be initiated as needed. Treatment options include forceful injection of saline or blood, or using a guidewire to disrupt the air embolus, promoting dispersion and distal blood flow (150). Balloon aspiration may also be performed (150, 154). In cases where vasospasm is present or when air bubbles obstruct distal vessels or the microcirculation, leading to slow flow and myocardial blush, intracoronary adenosine can be administered to facilitate coronary blood flow (154).
10 Unintended stent deformation
10.1 Description
Unintended stent deformation (USD) refers to unplanned mechanical distortion of an implanted coronary stent, leading to deformation, fracture, or mechanical obstruction. If unrecognized or untreated, USD may result in stent thrombosis, loss of coronary access, and an increased risk of MACE (155, 156).
USD is frequently underrecognized because it may be angiographically occult. Intracoronary imaging is essential in aiding diagnosis. OCT, due to its high spatial resolution, is effective for detecting subtle deformation patterns, overlapping or diverging struts, abluminal wire position, and longitudinal stent deformation (155–158).
Mechanistically, USD most commonly results from accidental abluminal rewiring and guide catheter or guide-extension catheter collision with the stent edge, particularly during repeated rewiring and device manipulation in bifurcation PCI (155). In the OCTOBER trial substudy, USD was identified by OCT in 9.3% of bifurcation PCI cases and in 18.5% of left main bifurcation interventions; abluminal rewiring and guide catheter collision accounted for approximately 44% and 40% of cases, respectively (155). Stent design features, including reduced longitudinal strength in thin-strut platforms, especially in the setting of complex lesions including calcification, tortuosity, long and ostial disease, may further predispose to deformation (159). Importantly, untreated USD was associated with more than a twofold increase in the risk of MACE at 2 year, whereas treated USD was not associated with an increased risk of MACE (155).
10.2 Management strategies
Management of USD is guided by intracoronary imaging and includes high-pressure balloon post-dilation, focal optimization of the deformed segment, correction of abluminal rewiring, and additional stent implantation (155).
10.3 Preventive strategies
Preventive strategies emphasize proper bifurcation technique, routine verification of wire position, avoidance of deep guide catheter intubation, cautious use of guide-extension devices, and use of intracoronary imaging, particularly in complex PCI.
11 Coronary anatomical variations and PCI risk
Coronary anatomical variations contribute to procedural complexity and the risk of PCI-related complications. Variations in vessel size, tortuosity, angulation, calcification, and bifurcation geometry can significantly influence guide catheter engagement, guidewire manipulation, device deliverability, lesion preparation, and stent deployment (160). Complex anatomical features such as severe calcification, long or eccentric lesions, bifurcations, vessel tortuosity, and CTO increase procedural difficulty and predispose to acute vessel closure, coronary dissection, perforation, device entrapment, and stent loss (6, 11, 75–77, 79–81, 109). Careful pre-procedural anatomical assessment, appropriate lesion preparation, and intracoronary imaging-guided PCI are important for mitigating anatomy-predisposed complications and improving PCI outcomes.
12 Role of intracoronary imaging in PCI-related complications
Intracoronary imaging with IVUS and OCT plays an important role in guiding PCI in contemporary practice, from pre-intervention assessment and intraprocedural guidance to post-intervention evaluation. In the setting of PCI-related complications, these modalities aid in diagnosis, guide management, and potentially prevent adverse events by providing detailed assessment of vessel size, plaque morphology, and stent-vessel interaction beyond angiography alone (158).
IVUS, with deeper tissue penetration, is particularly useful for evaluating complex coronary anatomy and heavily calcified lesions, guiding lesion preparation, vessel sizing, and stent expansion. IVUS guidance has been associated with lower rates of major complications, including coronary perforation, especially in complex PCI and CTO interventions (83). IVUS is also effective for identifying PCI-related complications such as coronary dissection and intramural hematoma, intracoronary thrombus, coronary perforation, and retained or fractured intracoronary equipment (10, 115).
OCT provides higher spatial resolution using near-infrared light, enabling detailed visualization of stent malapposition, underexpansion, deformation or collapse, and edge dissection. OCT also assists in diagnosing PCI-related complications, including intracoronary thrombus and subtle vessel injury or air embolism (146, 153). However, due to the need for contrast injection and limited penetration depth, OCT should be used cautiously in the setting of active dissection, perforation, or advanced chronic kidney disease (16).
13 Failure to rescue after PCI-related complications
Failure to rescue (FTR), defined as death following a procedural complication, has emerged as an important quality metric in procedural outcomes (161). Data from the National Cardiovascular Data Registry, a large U.S. observational registry, demonstrated that although the overall incidence of PCI complications is low, in-hospital mortality approaches 20% among patients who experience a complication, compared with 1.3% in those without complications (3). Coronary artery perforation and coronary dissection were associated with high FTR rates, approximately 12.3% and 7%, respectively. These findings underscore the importance of preventive strategies, as well as timely recognition and management. The study also demonstrated a significant hospital-level variation in FTR, highlighting the influence of institutional factors such as catheterization laboratory protocols, complication detection pathways, availability of rescue tools, and team-based response systems (3). Understanding patient- and procedure-related risk factors, the pathophysiology of PCI complications, and evidence-based management strategies can support the development of standardized rescue protocols and postprocedural monitoring practices, ultimately improving procedural outcomes.
14 Conclusions
PCI has been increasingly performed with a lower incidence of complications. However, PCI-related complications still occur and can lead to adverse clinical outcomes. These include vessel closure, perforation, dissection, entrapment, stent loss, and embolization. Preprocedural and intraprocedural risk stratification, an understanding of the underlying mechanisms, and evidence-based management, along with the use of adjunctive tools such as intracoronary imaging, can mitigate the incidence and improve outcomes of PCI-related complications. An evidence-based approach is essential to achieve optimal short- and long-term results; however, due to the low incidence of many of these complications, current management strategies are often derived from case reports, observational studies, and expert opinion. Further large-scale studies utilizing clinical databases and artificial intelligence-guided risk stratification are warranted to enable individualized prediction, enhance procedural safety, and advance precision management aimed at reducing complications and improving patient outcomes.
Statements
Author contributions
TA: Writing – original draft, Writing – review & editing. HV: Supervision, Writing – review & editing. MK: Supervision, Writing – review & editing. MA: Supervision, Writing – review & editing. RH: Supervision, Writing – review & editing. JD: Supervision, Writing – review & editing. CK: Investigation, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declared that financial support was not received for this work and/or its publication.
Conflict of interest
CK is the founder of HumanX, a company that focuses on artificial intelligence in precision medicine and innovation in healthcare; he declares that his affiliation with HumanX is not a competing interest.
The remaining author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Masoudi FA Ponirakis A de Lemos JA Jollis JG Kremers M Messenger JC et al Executive summary: trends in U.S. Cardiovascular care: 2016 report from 4 ACC national cardiovascular data registries. J Am Coll Cardiol. (2017) 69(11):1424–6. 10.1016/j.jacc.2016.12.004
2.
Asano T Ono M Dai Z Saito A Kanie T Takaoka Y et al Temporal trends in clinical outcomes after percutaneous coronary intervention: a systematic review of 66,327 patients from 25 all-comers trials. EuroIntervention. (2022) 17(16):1318–29. 10.4244/EIJ-D-21-00192
3.
Doll JA Kataruka A Manandhar P Wojdyla DM Yeh RW Wang TY et al Failure to rescue after percutaneous coronary intervention: insights from the national cardiovascular data registry. Circ Cardiovasc Interv. (2024) 17(8):e013670. 10.1161/CIRCINTERVENTIONS.123.013670
4.
Kheifets M Vons SA Bental T Vaknin-Assa H Greenberg G Samara A et al Temporal trends in complex percutaneous coronary interventions. Front Cardiovasc Med. (2022) 9:913588. 10.3389/fcvm.2022.913588
5.
Venkitachalam L Kip KE Selzer F Wilensky RL Slater J Mulukutla SR et al Twenty-year evolution of percutaneous coronary intervention and its impact on clinical outcomes: a report from the national heart, lung, and blood institute-sponsored, multicenter 1985–1986 PTCA and 1997–2006 dynamic registries. Circ Cardiovasc Interv. (2009) 2(1):6–13. 10.1161/CIRCINTERVENTIONS.108.825323
6.
Bergelson BA Fishman RF Tommaso CL . Abrupt vessel closure: changing importance, management, and consequences. Am Heart J. (1997) 134(3):362–81. 10.1016/S0002-8703(97)70069-3
7.
Ramasamy A Bajaj R Jones DA Amersey R Mathur A Baumbach A et al Iatrogenic catheter-induced ostial coronary artery dissections: prevalence, management, and mortality from a cohort of 55,968 patients over 10 years. Catheter Cardiovasc Interv. (2021) 98(4):649–55. 10.1002/ccd.29382
8.
Sanchez-Jimenez E Levi Y Roguin A . Iatrogenic aortocoronary dissection during right coronary artery procedures: a systematic review of the published literature. J Soc Cardiovasc Angiogr Interv. (2022) 1(6):100443. 10.1016/j.jscai.2022.100443
9.
Eshtehardi P Adorjan P Togni M Tevaearai H Vogel R Seiler C et al Iatrogenic left main coronary artery dissection: incidence, classification, management, and long-term follow-up. Am Heart J. (2010) 159(6):1147–53. 10.1016/j.ahj.2010.03.012
10.
Harding SA Fairley SL . Catheter-induced coronary dissection: keep calm and don't inject. JACC Case Rep. (2019) 1(2):113–5. 10.1016/j.jaccas.2019.07.002
11.
Rogers JH Lasala JM . Coronary artery dissection and perforation complicating percutaneous coronary intervention. J Invasive Cardiol. (2004) 16(9):493–9.
12.
MacCarrick G Black JH 3rd Bowdin S El-Hamamsy I Frischmeyer-Guerrerio PA Guerrerio AL et al Loeys-Dietz syndrome: a primer for diagnosis and management. Genet Med. (2014) 16(8):576–87. 10.1038/gim.2014.11
13.
Zekavat SM Chou EL Zekavat M Pampana A Paruchuri K Cardenas L et al Fibrillar collagen variants in spontaneous coronary artery dissection. JAMA Cardiol. (2022) 7(4):396–406. 10.1001/jamacardio.2022.0001
14.
Huber MS Mooney JF Madison J Mooney MR . Use of a morphologic classification to predict clinical outcome after dissection from coronary angioplasty. Am J Cardiol. (1991) 68(5):467–71. 10.1016/0002-9149(91)90780-O
15.
Doll JA Hira RS Kearney KE Kandzari DE Riley RF Marso SP et al Management of percutaneous coronary intervention complications: algorithms from the 2018 and 2019 seattle percutaneous coronary intervention complications conference. Circ Cardiovasc Interv. (2020) 13(6):e008962. 10.1161/CIRCINTERVENTIONS.120.008962
16.
Hayman S Lavi S . Healing of iatrogenic coronary dissection and intramural hematoma: insights from OCT. J Invasive Cardiol. (2018) 30(1):E12–e3.
17.
Smith EJ Di Mario C Spratt JC Hanratty CG de Silva R Lindsay AC et al Subintimal TRAnscatheter withdrawal (STRAW) of hematomas compressing the distal true lumen: a novel technique to facilitate distal reentry during recanalization of chronic total occlusion (CTO). J Invasive Cardiol. (2015) 27(1):E1–4.
18.
Michael TT Papayannis AC Banerjee S Brilakis ES . Subintimal dissection/reentry strategies in coronary chronic total occlusion interventions. Circ Cardiovasc Interv. (2012) 5(5):729–38. 10.1161/CIRCINTERVENTIONS.112.969808
19.
Boyle AJ Chan M Dib J Resar J . Catheter-induced coronary artery dissection: risk factors, prevention and management. J Invasive Cardiol. (2006) 18(10):500–3.
20.
Kostantinis S Rempakos A Simsek B Karacsonyi J Allana SS Alaswad K et al Aortocoronary dissection during percutaneous coronary interventions for chronic total occlusion: insights from the PROGRESS-CTO registry. Catheter Cardiovasc Interv. (2023) 102(1):56–63. 10.1002/ccd.30680
21.
Dunning DW Kahn JK Hawkins ET O'Neill WW . Iatrogenic coronary artery dissections extending into and involving the aortic root. Catheter Cardiovasc Interv. (2000) 51(4):387–93. 10.1002/1522-726x(200012)51:4%3C387::aid-ccd3%3E3.0.co;2-b
22.
Abdou SM Wu CJ . Treatment of aortocoronary dissection complicating anomalous origin right coronary artery and chronic total intervention with intravascular ultrasound guided stenting. Catheter Cardiovasc Interv. (2011) 78(6):914–9. 10.1002/ccd.23021
23.
Topaz O Topaz A Owen K . Thrombus grading for coronary interventions: the role of contemporary classifications. Interv Cardiol. (2011) 3:705–12. 10.2217/ica.11.76
24.
Rao SV O'Donoghue ML Ruel M Rab T Tamis-Holland JE Alexander JH et al Correction to: 2025 ACC/AHA/ACEP/NAEMSP/SCAI guideline for the management of patients with acute coronary syndromes: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2025) 151(13):e771–862. 10.1161/CIR.0000000000001328
25.
Giugliano RP White JA Bode C Armstrong PW Montalescot G Lewis BS et al Early versus delayed, provisional eptifibatide in acute coronary syndromes. N Engl J Med. (2009) 360(21):2176–90. 10.1056/NEJMoa0901316
26.
Kastrati A Neumann FJ Schulz S Massberg S Byrne RA Ferenc M et al Abciximab and heparin versus bivalirudin for non-ST-elevation myocardial infarction. N Engl J Med. (2011) 365(21):1980–9. 10.1056/NEJMoa1109596
27.
Kastrati A Mehilli J Neumann FJ Dotzer F ten Berg J Bollwein H et al Abciximab In patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment: the ISAR-REACT 2 randomized trial. JAMA. (2006) 295(13):1531–8. 10.1001/jama.295.13.joc60034
28.
Stone GW Maehara A Witzenbichler B Godlewski J Parise H Dambrink JH et al Intracoronary Abciximab and aspiration thrombectomy in patients with large anterior myocardial infarction: the INFUSE-AMI randomized trial. JAMA. (2012) 307(17):1817–26. 10.1001/jama.2012.421
29.
Zhao XQ Théroux P Snapinn SM Sax FL . Intracoronary thrombus and platelet glycoprotein IIb/IIIa receptor blockade with tirofiban in unstable angina or non-Q-wave myocardial infarction. Angiographic results from the PRISM-PLUS trial (platelet receptor inhibition for ischemic syndrome management in patients limited by unstable signs and symptoms). PRISM-PLUS investigators. Circulation. (1999) 100(15):1609–15. 10.1161/01.cir.100.15.1609
30.
Ohlmann P Reydel P Jacquemin L Adnet F Wolf O Bartier JC et al Prehospital abciximab in ST-segment elevation myocardial infarction: results of the randomized, double-blind MISTRAL study. Circ Cardiovasc Interv. (2012) 5(1):69–76. 10.1161/CIRCINTERVENTIONS.111.961425
31.
Sanati HR Zahedmehr A Firouzi A Farrashi M Amin K Peighambari MM et al Intracoronary versus intravenous eptifibatide during percutaneous coronary intervention for acute ST-segment elevation myocardial infarction; a randomized controlled trial. Cardiovasc Interv Ther. (2017) 32(4):351–7. 10.1007/s12928-016-0418-9
32.
Thiele H Wöhrle J Hambrecht R Rittger H Birkemeyer R Lauer B et al Intracoronary versus intravenous bolus abciximab during primary percutaneous coronary intervention in patients with acute ST-elevation myocardial infarction: a randomised trial. Lancet. (2012) 379(9819):923–31. 10.1016/S0140-6736(11)61872-2
33.
Svilaas T Vlaar PJ van der Horst IC Diercks GF de Smet BJGL van den Heuvel AF et al Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med. (2008) 358(6):557–67. 10.1056/NEJMoa0706416
34.
Vlaar PJ Svilaas T van der Horst IC Diercks GFH Fokkema ML de Smet BJGL et al Cardiac death and reinfarction after 1 year in the thrombus aspiration during percutaneous coronary intervention in acute myocardial infarction study (TAPAS): a 1-year follow-up study. Lancet. (2008) 371(9628):1915–20. 10.1016/S0140-6736(08)60833-8
35.
Fröbert O Lagerqvist B Olivecrona GK Omerovic E Gudnason T Maeng M et al Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med. (2013) 369(17):1587–97. 10.1056/NEJMoa1308789
36.
Jolly SS Cairns JA Yusuf S Meeks B Pogue J Rokoss MJ et al Randomized trial of primary PCI with or without routine manual thrombectomy. N Engl J Med. (2015) 372(15):1389–98. 10.1056/NEJMoa1415098
37.
Jolly SS James S Džavík V Cairns JA Mahmoud KD Zijlstra F et al Thrombus aspiration in ST-segment-elevation myocardial infarction: an individual patient meta-analysis: thrombectomy trialists collaboration. Circulation. (2017) 135(2):143–52. 10.1161/CIRCULATIONAHA.116.025371
38.
Mathews SJ Parikh SA Wu W Metzger DC Chambers JW Ghali MGH et al Sustained mechanical aspiration thrombectomy for high thrombus burden coronary vessel occlusion: the multicenter CHEETAH study. Circ Cardiovasc Interv. (2023) 16(2):e012433. 10.1161/CIRCINTERVENTIONS.122.012433
39.
Sezer M Oflaz H Gören T Okçular I Umman B Nişanci Y et al Intracoronary streptokinase after primary percutaneous coronary intervention. N Engl J Med. (2007) 356(18):1823–34. 10.1056/NEJMoa054374
40.
Sezer M Çimen A Aslanger E Elitok A Umman B Buğra Z et al Effect of intracoronary streptokinase administered immediately after primary percutaneous coronary intervention on long-term left ventricular infarct size, volumes, and function. J Am Coll Cardiol. (2009) 54(12):1065–71. 10.1016/j.jacc.2009.04.083
41.
Geng W Zhang Q Liu J Tian X Zhen L Song D et al A randomized study of prourokinase during primary percutaneous coronary intervention in acute ST-segment elevation myocardial infarction. J Interv Cardiol. (2018) 31(2):136–43. 10.1111/joic.12461
42.
Greco C Pelliccia F Tanzilli G Tinti MD Salenzi P Cicerchia C et al Usefulness of local delivery of thrombolytics before thrombectomy in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention [the delivery of thrombolytics before thrombectomy in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention (DISSOLUTION) randomized trial]. Am J Cardiol. (2013) 112(5):630–5. 10.1016/j.amjcard.2013.04.036
43.
Morishima I Sone T Okumura K Tsuboi H Kondo J Mukawa H et al Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction. J Am Coll Cardiol. (2000) 36(4):1202–9. 10.1016/S0735-1097(00)00865-2
44.
Rezkalla SH Kloner RA . No-reflow phenomenon. Circulation. (2002) 105(5):656–62. 10.1161/hc0502.102867
45.
Rezkalla SH Dharmashankar KC Abdalrahman IB Kloner RA . No-reflow phenomenon following percutaneous coronary intervention for acute myocardial infarction: incidence, outcome, and effect of pharmacologic therapy. J Interv Cardiol. (2010) 23(5):429–36. 10.1111/j.1540-8183.2010.00561.x
46.
Jaffe R Charron T Puley G Dick A Strauss BH . Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation. (2008) 117(24):3152–6. 10.1161/CIRCULATIONAHA.107.742312
47.
Huang X Zheng W Zhao XD Nie SP . CHA2DS2-VASc Score predicts the slow flow/no-reflow phenomenon in ST-segment elevation myocardial infarction patients with multivessel disease undergoing primary percutaneous coronary intervention. Medicine. (2021) 100(21):e26162. 10.1097/MD.0000000000026162
48.
Fajar JK Heriansyah T Rohman MS . The predictors of no reflow phenomenon after percutaneous coronary intervention in patients with ST elevation myocardial infarction: a meta-analysis. Indian Heart J. (2018) 70(3):S406–18. 10.1016/j.ihj.2018.01.032
49.
Wang CH Chen YD Yang XC Wang LF Wang HS Sun ZJ et al A no-reflow prediction model in patients with ST-elevation acute myocardial infarction and primary drug-eluting stenting. Scand Cardiovasc J. (2011) 45(2):98–104. 10.3109/14017431.2011.558209
50.
Iwakura K Ito H Ikushima M Kawano S Okamura A Asano K et al Association between hyperglycemia and the no-reflow phenomenon in patients with acute myocardial infarction. J Am Coll Cardiol. (2003) 41(1):1–7. 10.1016/S0735-1097(02)02626-8
51.
Barman HA Kahyaoglu S Durmaz E Atici A Gulsen K Tugrul S et al The CHADS-VASc score is a predictor of no-reflow in patients with non-ST-segment elevation myocardial infarction. Coron Artery Dis. (2020) 31(1):7–12. 10.1097/MCA.0000000000000781
52.
Rezkalla SH Stankowski RV Hanna J Kloner RA . Management of no-reflow phenomenon in the catheterization laboratory. JACC Cardiovasc Interv. (2017) 10(3):215–23. 10.1016/j.jcin.2016.11.059
53.
Magro M Springeling T van Geuns RJ Zijlstra F . Myocardial ‘no-reflow’ prevention. Curr Vasc Pharmacol. (2013) 11(2):263–77.
54.
Klein LW Kern MJ Berger P Sanborn T Block P Babb J et al Society of cardiac angiography and interventions: suggested management of the no-reflow phenomenon in the cardiac catheterization laboratory. Catheter Cardiovasc Interv. (2003) 60(2):194–201. 10.1002/ccd.10620
55.
Tamis-Holland JE Abbott JD Al-Azizi K Barman N Bortnick AE Cohen MG et al SCAI expert consensus statement on the management of patients with STEMI referred for primary PCI. J Soc Cardiovasc Angiogr Interv. (2024) 3(11):102294. 10.1016/j.jscai.2024.102294
56.
Caiazzo G Musci RL Frediani L Umińska J Wanha W Filipiak KJ et al State of the art: no-reflow phenomenon. Cardiol Clin. (2020) 38(4):563–73. 10.1016/j.ccl.2020.07.001
57.
Mahaffey KW Puma JA Barbagelata NA DiCarli MF Leesar MA Browne KF et al Adenosine as an adjunct to thrombolytic therapy for acute myocardial infarction: results of a multicenter, randomized, placebo-controlled trial: the acute myocardial infarction STudy of ADenosine (AMISTAD) trial. J Am Coll Cardiol. (1999) 34(6):1711–20. 10.1016/s0735-1097(99)00418-0
58.
Ross AM Gibbons RJ Stone GW Kloner RA Alexander RW . A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II). J Am Coll Cardiol. (2005) 45(11):1775–80. 10.1016/j.jacc.2005.02.061
59.
Niccoli G Rigattieri S De Vita MR Valgimigli M Corvo P Fabbiocchi F et al Open-label, randomized, placebo-controlled evaluation of intracoronary adenosine or nitroprusside after thrombus aspiration during primary percutaneous coronary intervention for the prevention of microvascular obstruction in acute myocardial infarction: the REOPEN-AMI study (intracoronary nitroprusside versus adenosine in acute myocardial infarction). JACC Cardiovasc Interv. (2013) 6(6):580–9. 10.1016/j.jcin.2013.02.009
60.
Nazir S Khan J Mahmoud I Greenwood J Blackman D Kunadian V et al The REFLO-STEMI (REperfusion facilitated by LOcal adjunctive therapy in ST-elevation myocardial infarction) trial: a randomised controlled trial comparing intracoronary administration of adenosine or sodium nitroprusside with control for attenuation of microvascular obstruction during primary percutaneous coronary intervention. Efficacy and Mechanism Evaluation. (2016) 3:1–48. 10.3310/eme03090
61.
Nazir SA Khan JN Mahmoud IZ Greenwood JP Blackman DJ Kunadian V et al Efficacy and Mechanism Evaluation. the REFLO-STEMI (REperfusion Facilitated by LOcal Adjunctive Therapy in ST-Elevation Myocardial Infarction) Trial: A Randomised Controlled Trial Comparing Intracoronary Administration of Adenosine or Sodium Nitroprusside with Control for Attenuation of Microvascular Obstruction During Primary Percutaneous Coronary Intervention. Southampton (UK): NIHR Journals Library (2016). Copyright © Queen’s Printer and Controller of HMSO 2016. This work was produced by Nazir et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre. Southampton SO16 7NS, UK: Alpha House, University of Southampton Science Park.
62.
Zhao S Qi G Tian W Chen L Sun Y . Effect of intracoronary nitroprusside in preventing no reflow phenomenon during primary percutaneous coronary intervention: a meta-analysis. J Interv Cardiol. (2014) 27(4):356–64. 10.1111/joic.12133
63.
Michaels AD Appleby M Otten MH Dauterman K Ports TA Chou TM et al Pretreatment with intragraft verapamil prior to percutaneous coronary intervention of saphenous vein graft lesions: results of the randomized, controlled vasodilator prevention on no-reflow (VAPOR) trial. J Invasive Cardiol. (2002) 14(6):299–302.
64.
Huang RI Patel P Walinsky P Fischman DL Ogilby JD Awar M et al Efficacy of intracoronary nicardipine in the treatment of no-reflow during percutaneous coronary intervention. Catheter Cardiovasc Interv. (2006) 68(5):671–6. 10.1002/ccd.20885
65.
Huang D Qian J Ge L Jin X Jin H Ma J et al REstoration of COronary flow in patients with no-reflow after primary coronary interVEntion of acute myocaRdial infarction (RECOVER). Am Heart J. (2012) 164(3):394–401. 10.1016/j.ahj.2012.06.015
66.
Su Q Nyi TS Li L . Adenosine and verapamil for no-reflow during primary percutaneous coronary intervention in people with acute myocardial infarction. Cochrane Database Syst Rev. (2015) 2015(5):Cd009503. 10.1002/14651858.CD009503.pub3
67.
Wang L Cheng Z Gu Y Peng D . Short-term effects of verapamil and diltiazem in the treatment of No reflow phenomenon: a meta-analysis of randomized controlled trials. Biomed Res Int. (2015) 2015:382086. 10.1155/2015/382086
68.
Niu X Zhang J Bai M Peng Y Sun S Zhang Z . Effect of intracoronary agents on the no-reflow phenomenon during primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction: a network meta-analysis. BMC Cardiovasc Disord. (2018) 18(1):3. 10.1186/s12872-017-0722-z
69.
Navarese EP Frediani L Kandzari DE Caiazzo G Cenname AM Cortese B et al Efficacy and safety of intracoronary epinephrine versus conventional treatments alone in STEMI patients with refractory coronary no-reflow during primary PCI: the RESTORE observational study. Catheter Cardiovasc Interv. (2021) 97(4):602–11. 10.1002/ccd.29113
70.
Khan KA Qamar N Saghir T Sial JA Kumar D Kumar R et al Comparison of intracoronary epinephrine and adenosine for No-reflow in normotensive patients with acute coronary syndrome (COAR trial). Circ Cardiovasc Interv. (2022) 15(2):e011408. 10.1161/CIRCINTERVENTIONS.121.011408
71.
Lawton JS Tamis-Holland JE Bangalore S Bates ER Beckie TM Bischoff JM et al 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: executive summary: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2022) 145(3):e4–e17. 10.1161/CIR.0000000000001039
72.
Baim DS Wahr D George B Leon MB Greenberg J Cutlip DE et al Randomized trial of a distal embolic protection device during percutaneous intervention of saphenous vein aorto-coronary bypass grafts. Circulation. (2002) 105(11):1285–90. 10.1161/01.CIR.0000012783.63093.0C
73.
Hibi K Kozuma K Sonoda S Endo T Tanaka H Kyono H et al A randomized study of distal filter protection versus conventional treatment during percutaneous coronary intervention in patients with attenuated plaque identified by intravascular ultrasound. JACC Cardiovasc Interv. (2018) 11(16):1545–55. 10.1016/j.jcin.2018.03.021
74.
Neumann FJ Sousa-Uva M Ahlsson A Alfonso F Banning AP Benedetto U et al 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. (2019) 40(2):87–165. 10.1093/eurheartj/ehy394
75.
Shaukat A Tajti P Sandoval Y Stanberry L Garberich R Nicholas Burke M et al Incidence, predictors, management and outcomes of coronary perforations. Catheter Cardiovasc Interv. (2019) 93(1):48–56. 10.1002/ccd.27706
76.
Nairooz R Parzynski CS Curtis JP Mohsen A McNulty E Uretsky BF et al Contemporary trends, predictors and outcomes of perforation during percutaneous coronary intervention (from the NCDR cath PCI registry). Am J Cardiol. (2020) 130:37–45. 10.1016/j.amjcard.2020.06.014
77.
Kinnaird T Kwok CS Kontopantelis E Ossei-Gerning N Ludman P deBelder M et al Incidence, determinants, and outcomes of coronary perforation during percutaneous coronary intervention in the United Kingdom between 2006 and 2013: an analysis of 527 121 cases from the British cardiovascular intervention society database. Circ Cardiovasc Interv. (2016) 9(8):1–8. 10.1161/CIRCINTERVENTIONS.115.003449
78.
Sapontis J Salisbury AC Yeh RW Cohen DJ Hirai T Lombardi W et al Early procedural and health Status outcomes after chronic total occlusion angioplasty: a report from the OPEN-CTO registry (outcomes, patient health Status, and efficiency in chronic total occlusion hybrid procedures). JACC Cardiovasc Interv. (2017) 10(15):1523–34. 10.1016/j.jcin.2017.05.065
79.
Mikhail P Howden N Monjur M Jeyaprakash P Said C Bland A et al Coronary perforation incidence, outcomes and temporal trends (COPIT): a systematic review and meta-analysis. Open Heart. (2022) 9(2):e002076. 10.1136/openhrt-2022-002076
80.
Shimony A Joseph L Mottillo S Eisenberg MJ . Coronary artery perforation during percutaneous coronary intervention: a systematic review and meta-analysis. Can J Cardiol. (2011) 27(6):843–50. 10.1016/j.cjca.2011.04.014
81.
Lemmert ME van Bommel RJ Diletti R Wilschut JM de Jaegere PP Zijlstra F et al Clinical characteristics and management of coronary artery perforations: a single-center 11-year experience and practical overview. J Am Heart Assoc. (2017) 6(9):1–8. 10.1161/JAHA.117.007049
82.
Kostantinis S Simsek B Karacsonyi J Alaswad K Jaffer FA Khatri JJ et al Development and validation of a scoring system for predicting clinical coronary artery perforation during percutaneous coronary intervention of chronic total occlusions: the PROGRESS-CTO perforation score. EuroIntervention. (2023) 18(12):1022–30. 10.4244/EIJ-D-22-00593
83.
Sawayama Y Sasaki K Taninobu N Ikuta A Osakada K Kubo S et al The effect of intravascular imaging-guided percutaneous coronary intervention on coronary artery perforation. JACC Asia. (2025) 5(1):46–55. 10.1016/j.jacasi.2024.09.004
84.
Ellis SG Ajluni S Arnold AZ Popma JJ Bittl JA Eigler NL et al Increased coronary perforation in the new device era. Incidence, classification, management, and outcome. Circulation. (1994) 90(6):2725–30. 10.1161/01.CIR.90.6.2725
85.
Ford TJ Adamson C Morrow AJ Rocchiccioli P Collison D McCartney PJ et al Coronary artery perforations: glasgow natural history study of covered stent coronary interventions (GNOCCI) study. J Am Heart Assoc. (2022) 11(19):e024492. 10.1161/JAHA.121.024492
86.
Hendry C Fraser D Eichhofer J Mamas MA Fath-Ordoubadi F El-Omar M et al Coronary perforation in the drug-eluting stent era: incidence, risk factors, management and outcome: the UK experience. EuroIntervention. (2012) 8(1):79–86. 10.4244/EIJV8I1A13
87.
Karatasakis A Akhtar YN Brilakis ES . Distal coronary perforation in patients with prior coronary artery bypass graft surgery: the importance of early treatment. Cardiovasc Revasc Med. (2016) 17(6):412–7. 10.1016/j.carrev.2016.05.014
88.
Kandzari DE Alqarqaz M Nicholson WJ Kearney KE Buller CE Cristea E et al Clinical experience with a novel perfusion balloon catheter in patients with coronary artery perforation: primary results from the ringer clinical study. J Soc Cardiovasc Angiogr Interv. (2025) 4(7):103575. 10.1016/j.jscai.2025.103575
89.
Kataruka A Parhar K Daniels D Maynard C Kearney KE Mahmoud A et al Protamine utilization and clinical outcomes for coronary artery perforation in chronic total occlusion percutaneous coronary intervention procedures. J Soc Cardiovasc Angiography Interv. (2025) 2025:104000. 10.1016/j.jscai.2025.104000
90.
Kandzari DE Birkemeyer R . PK Papyrus covered stent: device description and early experience for the treatment of coronary artery perforations. Catheter Cardiovasc Interv. (2019) 94(4):564–8. 10.1002/ccd.28306
91.
Kilic ID Fabris E Serdoz R Caiazzo G Foin N Abou-Sherif S et al Coronary covered stents. EuroIntervention. (2016) 12(10):1288–95. 10.4244/EIJV12I10A210
92.
Tarar MN Christakopoulos GE Brilakis ES . Successful management of a distal vessel perforation through a single 8-French guide catheter: combining balloon inflation for bleeding control with coil embolization. Catheter Cardiovasc Interv. (2015) 86(3):412–6. 10.1002/ccd.25939
93.
Ben-Gal Y Weisz G Collins MB Genereux P Dangas GD Teirstein PS et al Dual catheter technique for the treatment of severe coronary artery perforations. Catheter Cardiovasc Interv. (2010) 75(5):708–12. 10.1002/ccd.22331
94.
Bartuś J Januszek R Hudziak D Kołodziejczak M Kuźma Ł Tajstra M et al Clinical outcomes following large vessel coronary artery perforation treated with covered stent implantation: comparison between polytetrafluoroethylene- and polyurethane-covered stents (CRACK-II registry). J Clin Med. (2021) 10(22):5441. 10.3390/jcm10225441
95.
Stankovic G Colombo A Presbitero P van den Branden F Inglese L Cernigliaro C et al Randomized evaluation of polytetrafluoroethylene-covered stent in saphenous vein grafts: the randomized evaluation of polytetrafluoroethylene COVERed stent in saphenous vein grafts (RECOVERS) trial. Circulation. (2003) 108(1):37–42. 10.1161/01.CIR.0000079106.71097.1C
96.
Al-Lamee R Ielasi A Latib A Godino C Ferraro M Mussardo M et al Incidence, predictors, management, immediate and long-term outcomes following grade III coronary perforation. JACC Cardiovasc Interv. (2011) 4(1):87–95. 10.1016/j.jcin.2010.08.026
97.
Harnek J James SK Lagerqvist B . Very long-term outcome of coronary covered stents: a report from the SCAAR registry. EuroIntervention. (2019) 14(16):1660–7. 10.4244/EIJ-D-18-00855
98.
Takagi K Yoshida R Fujita T Noguchi T . Bail-out techniques in percutaneous intervention for ellis grade III coronary perforation in left main distal bifurcation lesions. J Soc Cardiovasc Angiogr Interv. (2023) 2(3):100609. 10.1016/j.jscai.2023.100609
99.
Holck EN Andreasen LN Hansen EK Thomsen JS Brüel A Johansen P et al Complex treatment of a coronary bifurcation perforation with a dual-covered stent strategy. JACC Case Rep. (2025) 30(7):103382. 10.1016/j.jaccas.2025.103382
100.
Kiernan TJ Yan BP Ruggiero N Eisenberg JD Bernal J Cubeddu RJ et al Coronary artery perforations in the contemporary interventional era. J Interv Cardiol. (2009) 22(4):350–3. 10.1111/j.1540-8183.2009.00469.x
101.
Al Mawed M Vlachojannis M Pula A Gielen S . Delayed coronary perforation four days after percutaneous coronary intervention with subsequent cardiac tamponade: a case report. Catheter Cardiovasc Interv. (2023) 102(6):1061–5. 10.1002/ccd.30861
102.
Senguttuvan NB Ramakrishnan S Gulati GS Seth S Bhargava B . How should I treat guidewire-induced distal coronary perforation?EuroIntervention. (2012) 8(1):155–63. 10.4244/EIJV8I1A23
103.
Shemisa K Karatasakis A Brilakis ES . Management of guidewire-induced distal coronary perforation using autologous fat particles versus coil embolization. Catheter Cardiovasc Interv. (2017) 89(2):253–8. 10.1002/ccd.26542
104.
Yasuoka Y Sasaki T . Successful collapse vessel treatment with a syringe for thrombus-aspiration after the guidewire-induced coronary artery perforation. Cardiovasc Revasc Med. (2010) 11(4):263.e1–3. 10.1016/j.carrev.2009.04.003
105.
Li Y Wang G Sheng L Xue J Sun D Gong Y . Silk suture embolization for sealing distal coronary artery perforation: report of two cases. Rev Cardiovasc Med. (2015) 16(2):165–9. 10.3909/ricm0768
106.
Shirakabe A Takano H Nakamura S Kikuchi A Sasaki A Yamamoto E et al Coronary perforation during percutaneous coronary intervention lessons from our experiences. Int Heart J. (2007) 48(1):1–9. 10.1536/ihj.48.1
107.
Sawayama Y Sasaki K Taninobu N Ikuta A Osakada K Kubo S et al Heparin reversal for coronary artery perforation. Catheter Cardiovasc Interv. (2025) 105(2):464–74. 10.1002/ccd.31339
108.
Giannini F Candilio L Mitomo S Ruparelia N Chieffo A Baldetti L et al A practical approach to the management of complications during percutaneous coronary intervention. JACC Cardiovasc Interv. (2018) 11(18):1797–810. 10.1016/j.jcin.2018.05.052
109.
Iturbe JM Abdel-Karim AR Papayannis A Mahmood A Rangan BV Banerjee S et al Frequency, treatment, and consequences of device loss and entrapment in contemporary percutaneous coronary interventions. J Invasive Cardiol. (2012) 24(5):215–21.
110.
Sasseen BM Burke JA Shah R Costa MA Zenni M Gilmore P et al Intravascular ultrasound catheter entrapment after coronary artery stenting. Catheter Cardiovasc Interv. (2002) 57(2):229–33. 10.1002/ccd.10244
111.
Hartzler GO Rutherford BD McConahay DR . Retained percutaneous transluminal coronary angioplasty equipment components and their management. Am J Cardiol. (1987) 60(16):1260–4. 10.1016/0002-9149(87)90604-7
112.
Gasparini GL Sanz-Sanchez J Regazzoli D Boccuzzi G Oreglia JA Gagnor A et al Device entrapment during percutaneous coronary intervention of chronic total occlusions: incidence and management strategies. EuroIntervention. (2021) 17(3):212–9. 10.4244/EIJ-D-20-00781
113.
Ghosh PK Alber G Schistek R Unger F . Rupture of guide wire during percutaneous transluminal coronary angioplasty. Mechanics and management. J Thorac Cardiovasc Surg. (1989) 97(3):467–9. 10.1016/S0022-5223(19)34588-X
114.
Danek BA Karatasakis A Brilakis ES . Consequences and treatment of guidewire entrapment and fracture during percutaneous coronary intervention. Cardiovasc Revasc Med. (2016) 17(2):129–33. 10.1016/j.carrev.2015.12.005
115.
Zhang ZW Pan XJ Zou JH Qi F . Attempted retrieval of guidewire fragment using the twisting wire technique causes coronary perfusion: case report. Medicine. (2024) 103(16):e37842. 10.1097/MD.0000000000037842
116.
Keltai M Bartek I Biró V . Guidewire snap causing left main coronary occlusion during coronary angioplasty. Cathet Cardiovasc Diagn. (1986) 12(5):324–6. 10.1002/ccd.1810120509
117.
Al-Moghairi AM Al-Amri H . Management of retained intervention guide-wire: a literature review. Curr Cardiol Rev. (2013) 9(3):260–6. 10.2174/1573403X11309030010
118.
Li C Chen Z Wang M . Retrieving entrapped guidewire using rotablation technique: case series and literature review. Eur Heart J Case Rep. (2022) 6(7):ytac261. 10.1093/ehjcr/ytac261
119.
Kaplan S Kaplan ST Kutlu M . An unusual case of guide wire fractured during primary percutaneous coronary intervention, and two year follow-up. Kardiol Pol. (2010) 68(11):1291–3.
120.
Owens CG Spence MS . How should I treat a patient to remove a fractured jailed side branch wire?. EuroIntervention. (2011) 7(4):520–7. 10.4244/EIJV7I4A83
121.
Devidutta S Lim ST . Twisting wire technique: an effective method to retrieve fractured guide wire fragments from coronary arteries. Cardiovasc Revasc Med. (2016) 17(4):282–6. 10.1016/j.carrev.2016.01.013
122.
Leibundgut G Achim A Krivoshei L . Safe and predictable transcatheter removal of broken coronary guidewires: the ‘knuckle-twister’ technique: a case series report. Eur Heart J Case Rep. (2023) 7(8):ytad311. 10.1093/ehjcr/ytad311
123.
Sharma SK Tomey MI Teirstein PS Kini AS Reitman AB Lee AC et al North American expert review of rotational atherectomy. Circ Cardiovasc Interv. (2019) 12(5):e007448. 10.1161/CIRCINTERVENTIONS.118.007448
124.
Kaneda H Saito S Hosokawa G Tanaka S Hiroe Y . Trapped rotablator: kokesi phenomenon. Catheter Cardiovasc Interv. (2000) 49(1):82–4. discussion 5. 10.1002/(sici)1522-726x(200001)49:1%3C82::aid-ccd18%3E3.0.co;2-x
125.
Sulimov DS Abdel-Wahab M Toelg R Kassner G Geist V Richardt G . Stuck rotablator: the nightmare of rotational atherectomy. EuroIntervention. (2013) 9(2):251–8. 10.4244/EIJV9I2A41
126.
Grise MA Yeager MJ Teirstein PS . A case of an entrapped rotational atherectomy burr. Catheter Cardiovasc Interv. (2002) 57(1):31–3. 10.1002/ccd.10263
127.
Kimura M Shiraishi J Kohno Y . Successful retrieval of an entrapped rotablator burr using 5 Fr guiding catheter. Catheter Cardiovasc Interv. (2011) 78(4):558–64. 10.1002/ccd.22995
128.
Cunnington M Egred M . Guideliner, a child-in-a-mother catheter for successful retrieval of an entrapped rotablator burr. Catheter Cardiovasc Interv. (2012) 79(2):271–3. 10.1002/ccd.23032
129.
Prasan AM Patel M Pitney MR Jepson NS . Disassembly of a rotablator: getting out of a trap. Catheter Cardiovasc Interv. (2003) 59(4):463–5. 10.1002/ccd.10611
130.
Alexiou K Kappert U Knaut M Matschke K Tugtekin SM . Entrapped coronary catheter remnants and stents: must they be surgically removed?. Tex Heart Inst J. (2006) 33(2):139–42.
131.
Sanz-Sánchez J Mashayekhi K Agostoni P Egred M Avran A Kalyanasundaram A et al Device entrapment during percutaneous coronary intervention. Catheter Cardiovasc Interv. (2022) 99(6):1766–77. 10.1002/ccd.30160
132.
Leibundgut G Degen C Riede F . Transcutaneous puncture of an undeflatable coronary angioplasty balloon catheter. Case Rep Cardiol. (2018) 2018:6252809. 10.1155/2018/6252809
133.
Girish MP Gupta MD Mittal A . Percutaneous retrieval of entrapped partially inflated broken coronary angioplasty balloon by modified fogarty technique. J Invasive Cardiol. (2011) 23(7):E173–6.
134.
Nosair M Hayek A Avram R . Get out of jail: managing an entrapped balloon using subintimal plaque modification. JACC Case Rep. (2022) 4(19):1252–5. 10.1016/j.jaccas.2022.07.004
135.
Morales-Victorino N Rojas-Chavez M Osnaya-Martinez JC Alvarado-Montes de Oca M Garcia-Garcia F Alcantara-Melendez MA . STEMI under-expanded stent, stuck rotablator burr and successful retrieval by using a mini STAR technique. Cardiovasc Revasc Med. (2018) 19(8s):16–9. 10.1016/j.carrev.2018.04.023
136.
Balasubramaniam K Elbarouni B Kass M Minhas K Ravandi A . Rotational atherectomy in the management of ruptured and entrapped coronary angioplasty balloon. Cardiovasc Revasc Med. (2021) 28:140–3. 10.1016/j.carrev.2020.09.039
137.
Tsujimura T Ishihara T Iida O Asai M Masuda M Okamoto S et al Successful percutaneous retrieval of a detached microcatheter tip using the guide-extension catheter trapping technique: a case report. J Cardiol Cases. (2019) 20(5):168–71. 10.1016/j.jccase.2019.07.007
138.
Vemmou E Nikolakopoulos I Xenogiannis I Megaly M Hall A Wang Y et al Recent advances in microcatheter technology for the treatment of chronic total occlusions. Expert Rev Med Devices. (2019) 16(4):267–73. 10.1080/17434440.2019.1602039
139.
Brilakis ES Best PJ Elesber AA Barsness GW Lennon RJ Holmes DR Jr et al Incidence, retrieval methods, and outcomes of stent loss during percutaneous coronary intervention: a large single-center experience. Catheter Cardiovasc Interv. (2005) 66(3):333–40. 10.1002/ccd.20449
140.
Alomar ME Michael TT Patel VG Altomare CG Rangan BV Cipher D et al Stent loss and retrieval during percutaneous coronary interventions: a systematic review and meta-analysis. J Invasive Cardiol. (2013) 25(12):637–41.
141.
Katagiri Y Stone GW Onuma Y Serruys PW . State of the art: the inception, advent and future of fully bioresorbable scaffolds. EuroIntervention. (2017) 13(6):734–50. 10.4244/EIJ-D-17-00499
142.
Eid N Abdel Wahab M Thanu AS . Bioresorbable stent unloading during percutaneous coronary intervention: early detection and management. World J Cardiol. (2024) 16(10):616–8. 10.4330/wjc.v16.i10.616
143.
Sun T Zhang MX Zeng Y Ruan LH Zhang Y Yang CL et al Unloading and successful treatment with bioresorbable stents during percutaneous coronary intervention: a case report. World J Cardiol. (2024) 16(8):484–90. 10.4330/wjc.v16.i8.484
144.
Malik SA Brilakis ES Pompili V Chatzizisis YS . Lost and found: coronary stent retrieval and review of literature. Catheter Cardiovasc Interv. (2018) 92(1):50–3. 10.1002/ccd.27464
145.
Eggebrecht H Haude M von Birgelen C Oldenburg O Baumgart D Herrmann J et al Nonsurgical retrieval of embolized coronary stents. Catheter Cardiovasc Interv. (2000) 51(4):432–40. 10.1002/1522-726x(200012)51:4%3C432::aid-ccd12%3E3.0.co;2-1
146.
Candilio L Mitomo S Carlino M Colombo A Azzalini L . Stent loss during chronic total occlusion percutaneous coronary intervention: optical coherence tomography-guided stent ‘crushing and trapping’. Cardiovasc Revasc Med. (2017) 18(7):531–4. 10.1016/j.carrev.2017.03.017
147.
Wongpraparut N Yalamachili V Leesar MA . Novel implication of combined stent crushing and intravascular ultrasound for dislodged stents. J Invasive Cardiol. (2004) 16(8):445–6.
148.
Meisel SR DiLeo J Rajakaruna M Pace B Frankel R Shani J . A technique to retrieve stents dislodged in the coronary artery followed by fixation in the iliac artery by means of balloon angioplasty and peripheral stent deployment. Catheter Cardiovasc Interv. (2000) 49(1):77–81. 10.1002/(sici)1522-726x(200001)49:1%3C77::aid-ccd17%3E3.0.co;2-y
149.
Alfonso F Martinez D Hernández R Goicolea J Segovia J Fernández-Ortíz A et al Stent embolization during intracoronary stenting. Am J Cardiol. (1996) 78(7):833–5. 10.1016/S0002-9149(96)00433-X
150.
Khan M Schmidt DH Bajwa T Shalev Y . Coronary air embolism: incidence, severity, and suggested approaches to treatment. Cathet Cardiovasc Diagn. (1995) 36(4):313–8. 10.1002/ccd.1810360406
151.
Van Blankenstein JH Slager CJ Schuurbiers JC Strikwerda S Verdouw PD . Heart function after injection of small air bubbles in coronary artery of pigs. J Appl Physiol. (1993) 75(3):1201–7. 10.1152/jappl.1993.75.3.1201
152.
Kahn JK Hartzler GO . The spectrum of symptomatic coronary air embolism during balloon angioplasty: causes, consequences, and management. Am Heart J. (1990) 119(6):1374–7. 10.1016/S0002-8703(05)80188-7
153.
Maruri-Sánchez R Rivero F Bastante T Cuesta J García-Guimaraes M Alfonso F . Intracoronary bubbles: iatrogenic air embolism assessed with optical coherence tomography. JACC Cardiovasc Interv. (2017) 10(16):e153–4. 10.1016/j.jcin.2017.06.031
154.
Yew KL Razali F . Massive coronary air embolism successfully treated with intracoronary catheter aspiration and intracoronary adenosine. Int J Cardiol. (2015) 188:56–7. 10.1016/j.ijcard.2015.04.040
155.
Andreasen LN Neghabat O Laanmets P Kumsars I Bennett J Olsen NT et al Unintended deformation of stents during bifurcation PCI: an OCTOBER trial substudy. JACC Cardiovasc Interv. (2024) 17(9):1106–15. 10.1016/j.jcin.2024.03.013
156.
Würtz M Christiansen EH Kristensen SD Holm NR . Accidentally crushed stent during complex bifurcation treatment. A potential cause of very late stent thrombosis. Int J Cardiol. (2015) 197:113–5. 10.1016/j.ijcard.2015.06.045
157.
Leibundgut G Löffelhardt N Toma A Neumann FJ Gick M . Optical coherence tomography of longitudinal stent compression. EuroIntervention. (2012) 8(8):989. 10.4244/EIJV8I8A149
158.
Truesdell AG Alasnag MA Kaul P Rab ST Riley RF Young MN et al Intravascular imaging during percutaneous coronary intervention. JACC state-of-the-art review. J Am Coll Cardiol. (2023) 81(6):590–605. 10.1016/j.jacc.2022.11.045
159.
Mamas MA Williams PD . Longitudinal stent deformation: insights on mechanisms, treatments and outcomes from the food and drug administration manufacturer and user facility device experience database. EuroIntervention. (2012) 8(2):196–204. 10.4244/EIJV8I2A33
160.
Chawla R Ahamad W Sharma V . Techniques to overcome difficulty in device deliverability to lesion in Complex PCI. Curr Cardiol Rev. (2020) 16(2):117–24. 10.2174/1573403X15666191018105627
161.
Silber JH Williams SV Krakauer H Schwartz JS . Hospital and patient characteristics associated with death after surgery. A study of adverse occurrence and failure to rescue. Med Care. (1992) 30(7):615–29. 10.1097/00005650-199207000-00004
Summary
Keywords
ACS—ACS/NSTEMI, PCI, percutaneous coronary intervention (PCI), complex PCI, PCI complications
Citation
Attachaipanich T, Virk HUH, Khawaja M, Alam M, Hira RS, Doll JA and Krittanawong C (2026) A practical approach to the management of percutaneous coronary intervention complications. Front. Cardiovasc. Med. 13:1760313. doi: 10.3389/fcvm.2026.1760313
Received
04 December 2025
Revised
22 December 2025
Accepted
16 January 2026
Published
05 February 2026
Volume
13 - 2026
Edited by
Tommaso Gori, Johannes Gutenberg University Mainz, Germany
Reviewed by
Nabil Eid, IMU University, Malaysia
Lene Dohn Andreasen, Aarhus University Hospital, Denmark
Updates
Copyright
© 2026 Attachaipanich, Virk, Khawaja, Alam, Hira, Doll and Krittanawong.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Chayakrit Krittanawong Chayakrit.krittanawong@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.