- 1Department of Otorhinolaryngology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Otolaryngology Head and Neck, Nanjing Tongren Hospital, School of Medicine, Southeast University, Nanjing, China
- 3State Key Laboratory of Bioelectronics, School of Life Sciences and Technology, Jiangsu Province High-Tech Key Laboratory for Bio-Medical Research, Southeast University, Nanjing, China
- 4Co-Innovation Center of Neuroregeneration, Nantong University, Nantong, China
- 5Institute of Stem Cell and Regeneration, Chinese Academy of Sciences, Beijing, China
- 6Beijing Key Laboratory of Neural Regeneration and Repair, Capital Medical University, Beijing, China
Sensorineural deafness is mainly caused by damage to the tissues of the inner ear, and hearing impairment has become an increasingly serious global health problem. When the inner ear is abnormally developed or is damaged by inflammation, ototoxic drugs, or blood supply disorders, auditory signal transmission is inhibited resulting in hearing loss. Forkhead box G1 (FoxG1) is an important nuclear transcriptional regulator, which is related to the differentiation, proliferation, development, and survival of cells in the brain, telencephalon, inner ear, and other tissues. Previous studies have shown that when FoxG1 is abnormally expressed, the development and function of inner ear hair cells is impaired. This review discusses the role and regulatory mechanism of FoxG1 in inner ear tissue from various aspects – such as the effect on inner ear development, the maintenance of inner ear structure and function, and its role in the inner ear when subjected to various stimulations or injuries – in order to explain the potential significance of FoxG1 as a new target for the treatment of hearing loss.
Introduction
The number of people suffering from hearing impairment in the world was approximately 500 million in 2015, ranking fourth among all disability factors and ahead of diabetes and dementia (GBD, 2016). Factors that cause hearing disability include congenital, infectious, noise exposure, drugs/medications, age-related, traumatic, and immune-mediated causes. Most of these factors induce damage to the inner ear tissue and eventually cause sensorineural deafness (Brown et al., 2018). The nuclear transcription factor FoxG1 has been shown to affect the process of cell proliferation and differentiation (Ariani et al., 2008; Florian et al., 2012), and in the study of Rett syndrome it was found that FoxG1 might indirectly affect oxidative damage to erythrocytes (Ciccoli et al., 2015; Valacchi et al., 2017). In addition, researchers have found that FoxG1 has an important regulatory effect on mitochondrial energy metabolism and biosynthesis in neuroepithelial cells (Pancrazi et al., 2015). In the inner ear, FoxG1 is mainly involved in regulating the formation and differentiation of hair cells (HCs), supporting cells, and cochlear spiral neurons, thereby maintaining cochlear function and morphology (Jahan et al., 2018; He et al., 2019; Zhang et al., 2020). In addition, hearing loss caused by aging, noise exposure, and ototoxic drugs is mainly due to damage to inner ear cells caused by oxidative stress (Wang et al., 2002; Kujawa and Liberman, 2009; Liberman et al., 2015; Francis and Cunningham, 2017; Jang et al., 2018), and FoxG1 can affect the reactive oxygen species (ROS) level in cells by maintaining mitochondrial function. Therefore, exploration of the role of FoxG1 in the inner ear will increase the use of FoxG1 as a target in the treatment of hearing loss.
The Role of FoxG1 in the Neural Stem Cells
Neural stem cells (NSCs) are self-renewable multipotent cells that can differentiate into different types of nerve cells (Chandwani et al., 2019). Because neural progenitor cells (NPCs) have limited life span and poor self-renewal ability, NSCs regulate the balance of pro-death and pro-survival signals to ensure the number of progenitor cell pools during development (Yadirgi et al., 2011; Sierra et al., 2015). As one of the markers of NPCs in the brain, FoxG1 was used to detect the differentiation level of pluripotent stem cells and embryonic stem cells (Yahata et al., 2011; Yamamizu et al., 2013). During embryonic development, FoxG1 is mainly expressed in the progenitor cells of the cerebral cortex, basal ganglia, and olfactory bulb (Dou et al., 1999). With the extension of development time, the expression area of FoxG1 changes. At mouse E12.5, FoxG1 is still expressed in the NPCs of the telencephalon, but no longer expressed in other neural tubes (Hettige and Ernst, 2019). In the mature mouse brain, FoxG1 is only expressed in neuroepithelial cells such as the cerebral cortex and hippocampus (Hettige and Ernst, 2019). These indicate that FoxG1 can regulate the telencephalic development through spatio-temporal patterning and interaction with different signaling. In the telencephalon of Foxg1 null mice, the dorsal neuroepithelial cells proliferation reduced and differentiate prematurely, and lead to depletion of the NPC pool (Xuan et al., 1995; Hanashima et al., 2002; Martynoga et al., 2005). This indicate that FoxG1 is involved in regulating the neuroepithelial cell proliferation and differentiation time during the morphogenesis and development of the telencephalon. Researchers used reprogramming approach to transduces FoxG1 and other transcription factors into mouse fibroblasts and astrocytes and they successfully converted somatic cells into proliferative NPCs (Ma et al., 2019). Brancaccio et al. (2010) found that overexpression of Foxg1 gene can maintain the ability of cells self-renewal and promote the increase in the number of NSCs. The self-renewal activity of NSCs decreases with age. Nakatani et al. (2019) found that the expression of Ecrg4 gene was significantly increased in aging NSC. When the Ecrg4 was overexpressed in NSC, the proliferation ability of NSC was significantly reduced, and when the expression of Ecrg4 was deleted, the decline in the proliferation ability of NSC caused by age was recovered. The NSC proliferation caused by the deletion of Ecrg4 expression was achieved by activating the expression of Foxg1 (Nakatani et al., 2019).
In the inner ear, after conditional knockout Foxg1 in the HCs, we found that the number of HCs in the apex turn of the cochlea of newborn mice increased significantly, indicating that the deletion of Foxg1 expression caused the disorder of HC proliferation and differentiation (He et al., 2019). By transcriptome sequencing analysis of HCs, we found that the knockout of Foxg1 caused abnormal expression of multiple signaling pathways and related genes. The knockout of Foxg1 caused the inhibition of the Notch signaling pathway in HCs, which probably led to the premature differentiation of NPCs, and ultimately resulted in the increase of HCs in the apex turn of the cochlea of newborn mice (He et al., 2019). After conditional knockout Foxg1 in supporting cells and inner ear stem cells using Sox2-CreER mice and Lgr5-EGFP-CreERT2 mice, we also found that the number of HCs increased significantly (Zhang et al., 2020). Through EDU assay and in vitro sphere-forming assay, we found that this phenotype was mainly due to the knockout of Foxg1 promoting the trans-differentiation of supporting cells to HCs, and the expression of genes related to the cell cycle and Notch signaling pathway was also affected (Zhang et al., 2020). The above findings indicated that FoxG1 can affect the differentiation and proliferation of inner ear NPCs through the regulation of multiple signal pathways and related factors expression.
The Role of FoxG1 in Other Tissues
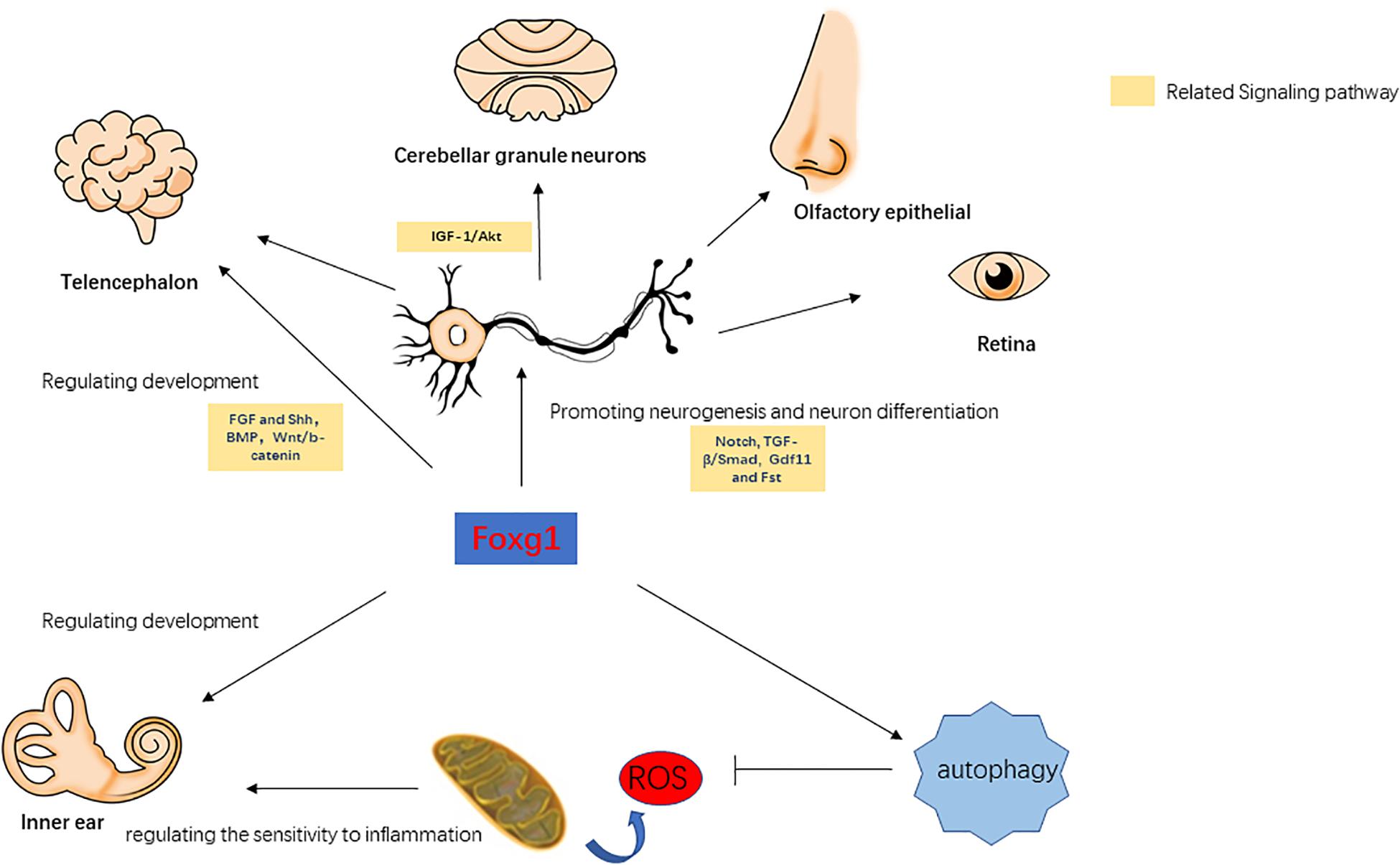
Forkhead box G1 belongs to the Fox transcription factor gene family, and is involved in the regulation of telencephalon development, cortical neuron differentiation, neurogenesis, and axonal exogenous growth (Brancaccio et al., 2010; Manuel et al., 2011; Figure 1). In Foxg1 knockout mice, it was found that when the expression of FoxG1 in the embryonic stage was suppressed the volume of the cerebral hemisphere was severely reduced and the mice died soon after birth, and large numbers of precursor cells differentiated into Cajal-Retzius cells and the differentiation into cortical neurons was inhibited, resulting in thinning of the cortex and abnormal neuronal stratification (Hanashima et al., 2004, 2007; Cargnin et al., 2018; Testa et al., 2019). In the development of the ventral telencephalon, the absence of FoxG1 expression causes abnormal expression of FGF and Shh signaling-related pathways, which affect the development of the ventral telencephalon (Manuel et al., 2010). This indicates that FoxG1 may affect the development of the telencephalon by regulating other signal pathways. In the telencephalon, dentate gyrus, and cerebral cortex, FoxG1 has been found to play an important role in regulating the proliferation and differentiation of neural progenitor cells. When FoxG1 expression is absent, this leads to cell cycle disruption in the progenitor cells of the telencephalon (Manuel et al., 2011), a decrease in the population of cortical intermediate progenitor cells (Siegenthaler et al., 2008), and a decrease in the number of stem cells due to the loss of the self-renewal capacity of neural stem cells (Brancaccio et al., 2010). In addition, FoxG1 is also involved in regulating the integration of multipolar pyramidal neuronal precursors into cortical plates (Miyoshi and Fishell, 2012). In the development of the olfactory epithelium, FoxG1 can affect the proliferation and differentiation of olfactory epithelial cells in cooperation with the Gdf11 (Growth differentiation factor 11) and Fst (Follistatin) proteins (Kawauchi et al., 2009). In the development of the chicken brain, the overexpression of FoxG1 can lead to the massive outgrowths of telencephalon and mesencephalon. This phenotype was not due to the activation of cell proliferation after the increase in FoxG1 expression, but due to the inhibition of apoptosis (Ahlgren et al., 2003). When FoxG1 expression is abnormal, nerve development in tissues such as the cerebral cortex, telencephalon, ear, retina, and olfactory epithelium is inhibited (Pauley et al., 2006). Since the inner ear and the above tissues have the same neurodevelopmental process, and FoxG1 has an important regulatory role in other tissues, we believe that it may also have a similar important regulatory role in the inner ear.
FoxG1-Related Signaling Pathways
Forkhead box G1 plays a cooperative regulatory role with the IGF-1/Akt, TGF-β/Smad, BMP, Wnt/β-catenin, Notch, and other signaling pathways. Dastidar et al. (2012) found that normal expression levels of FoxG1 can inhibit apoptosis in mouse cerebellar granule neurons, and when FoxG1 is overexpressed it has an antagonistic effect on the pro-survival factor IGF-1. TGF-β can inhibit the proliferation of a variety of embryonic epithelial cells, and FoxG1 was found to have an antagonistic effect on TFG-β (Pelton et al., 1991; Feijen et al., 1994; Furuta et al., 1997). When FoxG1 expression is inhibited, TGF-β or other growth inhibitory factors enhance the inhibitory effect on brain precursor cells (Pelton et al., 1991). In the TGF-β/Smad pathway, the binding of Smad to its ligands is competitively inhibited by FoxG1, thereby inhibiting the function of Smad. When FoxG1 competitively binds with Smad, this also suppresses the expression of TGF-β target genes, thereby blocking its downstream pathway (Dou et al., 2000). BMP can enhance the differentiation efficiency of precursors in the telencephalon, while FoxG1 can affect cellular differentiation by inhibiting the expression of BMP (Liu et al., 2018). Wnt/β-catenin and Shh can regulate the differentiation of the ventral and dorsal telencephalon, and Danesin et al. (2009) found that FoxG1 can directly or indirectly inhibit the expression of the Wnt/β-catenin pathway, thereby affecting cellular differentiation in the telencephalon. In addition, FoxG1 also has a regulatory relationship with Hes, Groucho/TLE, and other Notch signaling pathways, and FoxG1 can prevent the premature differentiation of precursor cells by inhibiting the expression of genes related to neurogenesis targeted by the Notch signaling pathway (Dali et al., 2018; Chiola et al., 2019; Richard and Jia-Hao, 2020; Zhang et al., 2020). In summary, FoxG1 affects multiple physiological processes and is associated with multiple signaling pathways. FoxG1 maintains the normal growth and development of various tissues by regulating cell proliferation and apoptosis (Figure 1). In the survival and development of inner ear HCs, the above-mentioned signaling pathways also play an important regulatory role. For example, the Wnt and Notch signaling pathway are related to the development of the inner ear, the generation and differentiation of supporting cells, HCs and neurons (Jayasena et al., 2008; Jacques et al., 2012; Brown et al., 2020). BMP signaling pathway affects inner ear morphogenesis, nerve fiber formation, and HC development (Yang et al., 1999; Blauwkamp et al., 2007). Therefore, FoxG1 is likely to affect the development of the inner ear and the survival of HCs through interaction with these pathways.
The Role of FoxG1 in the Inner Ear
The Role of FoxG1 in Inner Ear Development
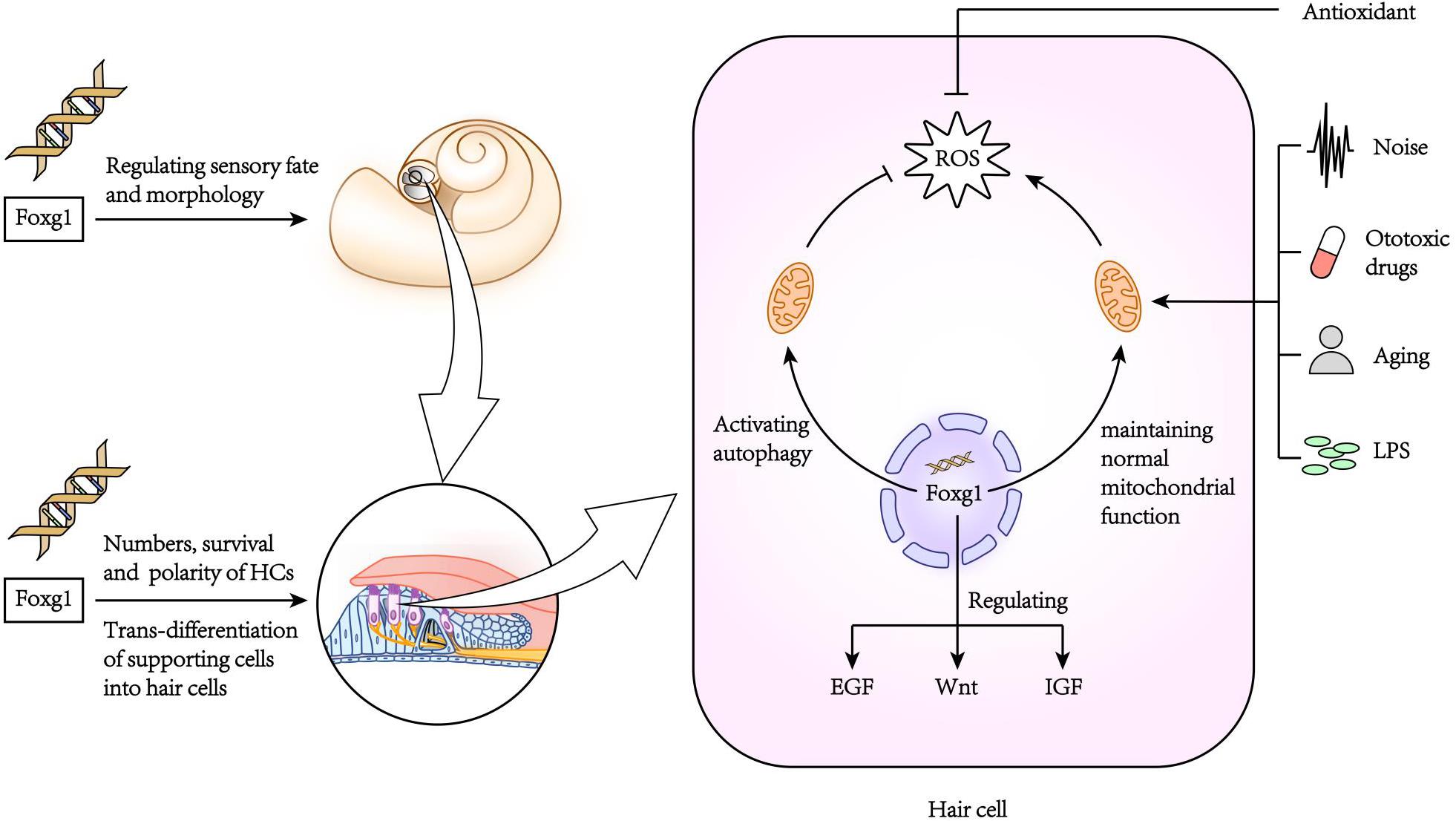
Pauley et al. (2006) found that FoxG1 was expressed in most types of cells in the crista, endolymphatic vessels and organ of Corti of the inner ear, including HCs, supporting cells, border cells, and Hensen cells, etc. FoxG1-deletion mice had significantly shorter cochlear ducts than normally developing mice and lacked the formation of the horizontal crista and ampulla (Pauley et al., 2006). In addition, the distribution of inner ear nerve fibers in FoxG1-deletion mice also showed abnormalities (Harasztosi et al., 2019). Hwang et al. found that FoxG1 is essential for the formation and separation of the sensory cristae, indicating that FoxG1 has a function in regulating sensory fate in the inner ear (Hwang et al., 2009). Deletion of FoxG1 can also cause the polarity of HCs to change (Pauley et al., 2006). Our previous research found that after specifically knocking out FoxG1 in HCs, the cochlear ducts length did not change after birth, but there was an increase in the number of HCs (He et al., 2019). In addition, we also found that the Wnt, IGF, and EGF signaling pathways were inhibited in HCs absent FoxG1 expression, and the survival time of adult mouse HCs was shortened (Figure 2; He et al., 2019).
The Role of FoxG1 in Cell Survival in the Inner Ear
Due to the non-renewability of mammalian cochlear HCs, their damage is the main cause of hearing loss. When FoxG1 expression is inhibited, it will not only cause morphogenesis and functional defects of the auditory system, but also affect the survival of HCs and ultimately lead to hearing loss. In newborn mouse cochleae with conditional knockout Foxg1 in HCs, we found that the HCs in the apex turn of the mouse cochlea increased significantly at P1–P7, and then gradually returned to normal levels with the increase of time, and there was no significant change in the hearing of the mice before P21 (He et al., 2019). But after P21, the HCs in the basal turn appeared to be lost, and as the mouse ages, the number of HCs lost gradually increases and develops toward the apex turn. In addition, the results of RNA sequencing analysis showed that the deletion of Foxg1 expression cause the inhibition of IGF signaling pathway in HCs (Figure 2). Previous studies have found that FoxG1 and IGF1 have a synergistic regulatory effect. IGF1 plays an important role in the protection of nerve cell damage, that is, the inner ear cells of IGF1 knockout mice will appear apoptosis and hearing loss (Camarero et al., 2002; Murillo-Cuesta et al., 2011). Therefore, FoxG1 may affect cell proliferation and apoptosis sensitivity by regulating multiple signal pathways such as IGF and Notch. de Iriarte Rodríguez et al. (2015) found that abnormal expression of C-Raf caused hearing loss and increased sensitivity to noise in mice. In the embryonic cochlea of C-Raf null mice, the expression of Foxg1 increased. It is speculated that this phenomenon is due to the body promotes the survival of auditory neurons in the inner ear through activating the expression of Foxg1, thereby reducing the cochlea development abnormal caused by the deletion of C-Raf expression.
The Role of FoxG1 in Inflammation in the Inner Ear
The inner ear is often stimulated by various factors leading to an inflammatory reaction. A mild inflammatory reaction can remove toxins and pathogenic microorganisms, and thus has a protective effect on tissues and cells, but excessive inflammatory reactions can cause serious damage to the inner ear (Kalinec et al., 2017). In the model of inflammation of the inner ear caused by LPS (lipopolysaccharide), inflammatory cells accumulate in the inner ear causing the stria vascularis to swell, which in turn damages the auditory HCs (Hirose et al., 2014; Hirose and Li, 2019). In our previous research, we found that FoxG1 has an important regulatory role in inhibiting the sensitivity of aging HCs to inflammation (He et al., 2020b). When the HCs were treated with low concentration of LPS, the expression level of FoxG1 and autophagy increased, on the contrary, when treated with high concentration of LPS, the levels of both decreased significantly, which indicated that FoxG1 may play its role in promoting survival through the regulation of autophagy. When the expression of FoxG1 was inhibited, the level of autophagy in HCs was also inhibited, and the level of apoptosis was significantly increased. We also found that in D-galactose-induced aging HCs, FoxG1 inhibits the increase in ROS in cells induced by LPS by activating autophagy, thereby regulating the sensitivity of aging HCs to inflammation and maintaining the function and survival of HCs (He et al., 2020a; Figure 2).
Discussion
Our research on FoxG1 in the inner ear suggests that FoxG1 may be involved in protecting the inner ear from damage (He et al., 2020a). Ototoxic drugs and aging are the two main causes of inner ear damage and mainly include aminoglycoside antibiotics (such as neomycin and gentamicin) and anti-tumor platinum-based drugs (such as cisplatin) (Ryals et al., 2018). For mechanism of ototoxic drugs inducing deafness, Schacht, 1999 found that ototoxic drugs mainly damage the inner ear cells by generating excessive oxygen free radicals, which would injury hearing function finally, and the use of antioxidants can reduce such damage. In addition, related studies on noise-induced hearing loss have found that oxidative stress caused by noise is also an important cause of cellular damage in the inner ear (Ohlemiller, 2008), and Shuhei and Xiangxin found that IGF-1 can effectively inhibit neomycin-induced damage to HCs (Yoshida et al., 2015; Xiangxin Lou et al., 2015 Jan). It is known that FoxG1 not only inhibits the increase in ROS level by activating autophagy, but also has a close regulatory relationship with IGF-1. Therefore, FoxG1 might be involved in regulating the processes through which ototoxic drugs and noise exposure damage the inner ear.
It is now generally accepted that age-related oxidative stress is one of the factors leading to hearing loss (da Costa et al., 2016). When mitochondrial function is abnormal, the excessive ROS in the cell will disrupt gene expression, protein renewal, and other biological functions, which will lead to disrupted biosynthesis and energy metabolism and eventually lead to cell death. It is known that FoxG1 affects mitochondrial membrane potential and mitochondrial division and fusion (Pancrazi et al., 2015), and when FoxG1 expression is abnormal mitochondrial energy metabolism, biosynthesis, and membrane potential are disturbed, which in turn affects cell proliferation and differentiation. Therefore, normal expression of FoxG1 is a key factor in maintaining normal mitochondrial function and ROS levels. Rodriguez-de la Rosa et al. found that IGF-1 not only has anti-apoptotic effects, but also can activate cell renewal (Rodriguez-de la Rosa et al., 2017). Mariño et al. found that IGF-1 can extend the lifespan of premature aging mice (Zmpste24-deficient mice) by restoring somatotroph axis function (Mariño et al., 2010). Therefore, FoxG1 might affect the occurrence and development of age-related hearing loss by regulating multiple pathways. Foxg1 as one of the marker genes of inner ear progenitor cells and plays an important role in the process of inducing pluripotent stem cells to differentiate into inner ear cells (Boddy et al., 2020). Therefore, the Foxg1-related reprogramming technology has great application value to regenerate cells in the inner ear that are affected by pathology or damage. C-MYC is a regulatory factor that plays an important role in cell proliferation, growth and apoptosis (Han et al., 2009). In the study of wound repair, Zhan et al. (2018) found that nitric oxide can induce the transcription of c-myc to promote the proliferation of epidermal stem cells, and the c-myc promoter activity is regulated by FoxG1 during this process. In the related research of the inner ear, it was found that c-myc can not only protect the inner ear from noise damage but also promote the self-renewal of otic progenitor cells (Han et al., 2009; Kwan et al., 2015). The loss of sensory HCs and neurons in the inner ear is the main cause of sensorineural hearing loss, and this loss is irreversible. The promoters of c-myc and Sox2 are highly similar, and the target genes include kinases that regulate the cell cycle (Kwan et al., 2015). Therefore, FoxG1 may play an important role in the regeneration of HCs by regulating the c-myc signaling pathway. However, there are relatively few studies on FoxG1 in the inner ear. Moreover, FoxG1 may have different regulatory mechanisms in different organs and tissues, as well as in the growth and development of different types of cells. Therefore, the regulation mechanism of FoxG1 in the inner ear remains to be studied in the future.
Conclusion
Forkhead box G1 not only plays a key role in the development of the cerebral cortex and neurons, but also has a close regulatory relationship with the development of the inner ear, the survival of HCs, and the protection of HCs against injury. FoxG1 is essential for the maintenance of NSCs and NPCs, and directly regulates the differentiation process of cells. It has been reported that FoxG1 can promote the survival of inner ear HCs by regulating autophagy, mitochondrial function, and related signaling pathways. Thus the in-depth exploration of the role of FoxG1 in the inner ear will improve its use as a target for the regeneration of HCs and the treatment of sensorineural hearing loss.
Author Contributions
YD, WM, and ZH conceived and wrote the manuscript. WK, ZH, and RC modified the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA16010303), National Natural Science Foundation of China (Nos. 81873700, 81800915, 82030029, and 81970882), National Key R&D Program of China (Nos. 2017YFA0103903 and 2019YFA0111400), Natural Science Foundation of Jiangsu Province (BE2019711), and Shenzhen Fundamental Research Program (JCYJ2019081 4093401920).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
FoxG1, Forkhead box G1; ROS, reactive oxygen species; IGF-1, insulin like growth factor-1; TGF-β, transforming growth factor beta; BMP, bone morphogenetic protein; HCs, hair cells; LPS, lipopolysaccharide; NPCs, neural progenitor cells; NSCs, neural stem cells.
References
Ahlgren, S., Vogt, P., and Bronner-Fraser, M. (2003). Excess FoxG1 causes overgrowth of the neural tube. J. Neurobiol. 57, 337–349. doi: 10.1002/neu.10287
Ariani, F., Hayek, G., Rondinella, D., Artuso, R., Mencarelli, M. A., Spanhol-Rosseto, A., et al. (2008). FOXG1 is responsible for the congenital variant of Rett syndrome. Am. J. Hum. Genet 83, 89–93. doi: 10.1016/j.ajhg.2008.05.015
Blauwkamp, M. N., Beyer, L. A., Kabara, L., Takemura, K., Buck, T., King, W. M., et al. (2007). The role of bone morphogenetic protein 4 in inner ear development and function. Hear Res. 225, 71–79. doi: 10.1016/j.heares.2006.12.010
Boddy, S. L., Romero-Guevara, R., Ji, A. R., Unger, C., Corns, L., Marcotti, W., et al. (2020). Generation of Otic Lineages from Integration-Free Human-Induced Pluripotent Stem Cells Reprogrammed by mRNAs. Stem Cells Int. 2020:3692937. doi: 10.1155/2020/3692937
Brancaccio, M., Pivetta, C., Granzotto, M., Filippis, C., and Mallamaci, A. (2010). Emx2 and Foxg1 inhibit gliogenesis and promote neuronogenesis. Stem Cells 28, 1206–1218. doi: 10.1002/stem.443
Brown, C. S., Emmett, S. D., Robler, S. K., and Tucci, D. L. (2018). Global Hearing Loss Prevention. Otolaryngol. Clin. North Am. 51, 575–592. doi: 10.1016/j.otc.2018.01.006
Brown, R., Groves, A. K., and Hear (2020). Hear, Hear for Notch: Control of Cell Fates in the Inner Ear by Notch Signaling. Biomolecules 10:370. doi: 10.3390/biom10030370
Camarero, G., Villar, M. A., Contreras, J., Fernández-Moreno, C., Pichel, J. G., Avendaño, C., et al. (2002). Cochlear abnormalities in insulin-like growth factor-1 mouse mutants. Hear Res. 170, 2–11. doi: 10.1016/s0378-5955(02)00447-1
Cargnin, F., Kwon, J. S., Katzman, S., Chen, B., Lee, J. W., and Lee, S. K. (2018). FOXG1 Orchestrates Neocortical Organization and Cortico-Cortical Connections. Neuron 100, 1083–1096e5. doi: 10.1016/j.neuron.2018.10.016
Chandwani, M. N., Creisher, P. S., and O’Donnell, L. A. (2019). Understanding the Role of Antiviral Cytokines and Chemokines on Neural Stem/Progenitor Cell Activity and Survival. Viral. Immunol. 32, 15–24. doi: 10.1089/vim.2018.0091
Chiola, S., Do, M. D., Centrone, L., and Mallamaci, A. (2019). Foxg1 Overexpression in Neocortical Pyramids Stimulates Dendrite Elongation Via Hes1 and pCreb1 Upregulation. Cereb. Cortex 29, 1006–1019. doi: 10.1093/cercor/bhy007
Ciccoli, L., De Felice, C., Leoncini, S., Signorini, C., Cortelazzo, A., Zollo, G., et al. (2015). Red blood cells in Rett syndrome: oxidative stress, morphological changes and altered membrane organization. Biol. Chem. 396, 1233–1240. doi: 10.1515/hsz-2015-0117
da Costa, J. P., Vitorino, R., Silva, G. M., Vogel, C., Duarte, A. C., and Rocha-Santos, T. (2016). A synopsis on aging-Theories, mechanisms and future prospects. Ageing Res. Rev. 29, 90–112. doi: 10.1016/j.arr.2016.06.005
Dali, R., Verginelli, F., Pramatarova, A., Sladek, R., and Stifani, S. (2018). Characterization of a FOXG1:TLE1 transcriptional network in glioblastoma-initiating cells. Mol. Oncol. 12, 775–787. doi: 10.1002/1878-0261.12168
Danesin, C., Peres, J. N., Johansson, M., Snowden, V., Cording, A., Papalopulu, N., et al. (2009). Integration of telencephalic Wnt and hedgehog signaling center activities by Foxg1. Dev. Cell 16, 576–587. doi: 10.1016/j.devcel.2009.03.007
Dastidar, S. G., Bardai, F. H., Ma, C., Price, V., Rawat, V., Verma, P., et al. (2012). Isoform-specific toxicity of Mecp2 in postmitotic neurons: suppression of neurotoxicity by FoxG1. J. Neurosci. 32, 2846–2855. doi: 10.1523/JNEUROSCI.5841-11.2012
de Iriarte Rodríguez, R., Magariños, M., Pfeiffer, V., Rapp, U. R., and Varela-Nieto, I. (2015). C-Raf deficiency leads to hearing loss and increased noise susceptibility. Cell Mol. Life Sci. 72, 3983–3998. doi: 10.1007/s00018-015-1919-x
Dou, C. L., Li, S., and Lai, E. (1999). Dual role of brain factor-1 in regulating growth and patterning of the cerebral hemispheres. Cereb. Cortex 9, 543–550. doi: 10.1093/cercor/9.6.543
Dou, C., Lee, J., Liu, B., Liu, F., Massague, J., Xuan, S., et al. (2000). BF-1 interferes with transforming growth factor beta signaling by associating with Smad partners. Mol. Cell Biol. 20, 6201–6211. doi: 10.1128/mcb.20.17.6201-6211.2000
Feijen, A., Goumans, M. J., and van den Eijnden-van Raaij, A. J. (1994). Expression of activin subunits, activin receptors and follistatin in postimplantation mouse embryos suggests specific developmental functions for different activins. Development 120, 3621–3637.
Florian, C., Bahi-Buisson, N., and Bienvenu, T. (2012). FOXG1-Related Disorders: From Clinical Description to Molecular Genetics. Mol. Syndromol. 2, 153–163. doi: 10.1159/000327329
Francis, S. P., and Cunningham, L. L. (2017). Non-autonomous Cellular Responses to Ototoxic Drug-Induced Stress and Death. Front. Cell Neurosci. 11:252. doi: 10.3389/fncel.2017.00252
Furuta, Y., Piston, D. W., and Hogan, B. L. (1997). Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development 124, 2203–2212.
GBD (2016). Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1545–1602. doi: 10.1016/s0140-6736(16)31678-6
Han, Y., Zhong, C., Hong, L., Wang, Y., Qiao, L., and Qiu, J. (2009). Effect of c-myc on the ultrastructural structure of cochleae in guinea pigs with noise induced hearing loss. Biochem. Biophys. Res. Commun. 390, 458–462. doi: 10.1016/j.bbrc.2009.09.091
Hanashima, C., Fernandes, M., Hebert, J. M., and Fishell, G. (2007). The role of Foxg1 and dorsal midline signaling in the generation of Cajal-Retzius subtypes. J. Neurosci. 27, 11103–11111. doi: 10.1523/jneurosci.1066-07.2007
Hanashima, C., Li, S. C., Shen, L., Lai, E., and Fishell, G. (2004). Foxg1 suppresses early cortical cell fate. Science 303, 56–59. doi: 10.1126/science.1090674
Hanashima, C., Shen, L., Li, S. C., and Lai, E. (2002). Brain factor-1 controls the proliferation and differentiation of neocortical progenitor cells through independent mechanisms. J. Neurosci. 22, 6526–6536. doi: 10.1523/jneurosci.22-15-06526.2002
Harasztosi, C., Wolter, S., Gutsche, K., Duran-Alonso, M. B., Lopez-Hernandez, I., Pascual, A., et al. (2019). Differential deletion of GDNF in the auditory system leads to altered sound responsiveness. J. Neurosci. Res. 98, 1764–1779. doi: 10.1002/jnr.24544
He, Z. H., Zou, S. Y., Li, M., Liao, F. L., Wu, X., Sun, H. Y., et al. (2020a). The nuclear transcription factor FoxG1 affects the sensitivity of mimetic aging hair cells to inflammation by regulating autophagy pathways. Redox Biol. 28:101364.
He, Z. H., Zou, S. Y., Li, M., Liao, F. L., Wu, X., Sun, H. Y., et al. (2020b). The nuclear transcription factor FoxG1 affects the sensitivity of mimetic aging hair cells to inflammation by regulating autophagy pathways. Redox Biol. 28, 101364–101364. doi: 10.1016/j.redox.2019.101364
He, Z., Fang, Q., Li, H., Shao, B., Zhang, Y., Zhang, Y., et al. (2019). The role of FOXG1 in the postnatal development and survival of mouse cochlear hair cells. Neuropharmacology 144, 43–57. doi: 10.1016/j.neuropharm.2018.10.021
Hettige, N. C., and Ernst, C. (2019). FOXG1 Dose in Brain Development. Front. Pediatr. 7:482. doi: 10.3389/fped.2019.00482
Hirose, K., and Li, S. Z. (2019). The role of monocytes and macrophages in the dynamic permeability of the blood-perilymph barrier. Hear Res. 374, 49–57. doi: 10.1016/j.heares.2019.01.006
Hirose, K., Li, S. Z., Ohlemiller, K. K., and Ransohoff, R. M. (2014). Systemic lipopolysaccharide induces cochlear inflammation and exacerbates the synergistic ototoxicity of kanamycin and furosemide. J. Assoc. Res. Otolaryngol. 15, 555–570. doi: 10.1007/s10162-014-0458-8
Hwang, C. H., Simeone, A., Lai, E., and Wu, D. K. (2009). Foxg1 is required for proper separation and formation of sensory cristae during inner ear development. Dev. Dyn. 238, 2725–2734. doi: 10.1002/dvdy.22111
Jacques, B. E., Puligilla, C., Weichert, R. M., Ferrer-Vaquer, A., Hadjantonakis, A. K., Kelley, M. W., et al. (2012). A dual function for canonical Wnt/β-catenin signaling in the developing mammalian cochlea. Development 1, 4395–4404. doi: 10.1242/dev.080358
Jahan, I., Elliott, K. L., and Fritzsch, B. (2018). Understanding Molecular Evolution and Development of the Organ of Corti Can Provide Clues for Hearing Restoration. Integr. Comp. Biol. 58, 351–365. doi: 10.1093/icb/icy019
Jang, J. Y., Blum, A., Liu, J., and Finkel, T. (2018). The role of mitochondria in aging. J. Clin. Invest. 128, 3662–3670. doi: 10.1172/jci120842
Jayasena, C. S., Ohyama, T., Segil, N., and Groves, A. K. (2008). Notch signaling augments the canonical Wnt pathway to specify the size of the otic placode. Development 135, 2251–2261. doi: 10.1242/dev.017905
Kalinec, G. M., Lomberk, G., Urrutia, R. A., and Kalinec, F. (2017). Resolution of Cochlear Inflammation: Novel Target for Preventing or Ameliorating Drug-, Noise- and Age-related Hearing Loss. Front. Cell Neurosci. 11:192. doi: 10.3389/fncel.2017.00192
Kawauchi, S., Kim, J., Santos, R., Wu, H. H., Lander, A. D., and Calof, A. L. (2009). Foxg1 promotes olfactory neurogenesis by antagonizing Gdf11. Development 136, 1453–1464. doi: 10.1242/dev.034967
Kujawa, S. G., and Liberman, M. C. (2009). Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J. Neurosci. 29, 14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009
Kwan, K. Y., Shen, J., and Corey, D. P. (2015). C-MYC transcriptionally amplifies SOX2 target genes to regulate self-renewal in multipotent otic progenitor cells. Stem cell Rep. 4, 47–60. doi: 10.1016/j.stemcr.2014.11.001
Liberman, L. D., Suzuki, J., and Liberman, M. C. (2015). Dynamics of cochlear synaptopathy after acoustic overexposure. J. Assoc. Res. Otolaryngol. 16, 205–219. doi: 10.1007/s10162-015-0510-3
Liu, B., Xiao, H., and Zhao, C. (2018). Forced Expression of Foxg1 in the Cortical Hem Leads to the Transformation of Cajal-Retzius Cells into Dentate Granule Neurons. J. Dev. Biol. 6:16. doi: 10.3390/jdb6030016
Lou, X., Yuan, H., Xie, J., Wang, X., Yang, L., and Zhang, Y. (2015). Jan. “Growth Factors Have a Protective Effect on Neomycin-Induced Hair Cell Loss.”. Cell Biol. Int. 39, 65–73. doi: 10.1002/cbin.10347
Ma, Y., Wang, K., Pan, J., Fan, Z., Tian, C., Deng, X., et al. (2019). Induced neural progenitor cells abundantly secrete extracellular vesicles and promote the proliferation of neural progenitors via extracellular signal-regulated kinase pathways. Neurobiol. Dis. 124, 322–334. doi: 10.1016/j.nbd.2018.12.003
Manuel, M. N., Martynoga, B., Molinek, M. D., Quinn, J. C., Kroemmer, C., Mason, J. O., et al. (2011). The transcription factor Foxg1 regulates telencephalic progenitor proliferation cell autonomously, in part by controlling Pax6 expression levels. Neural Dev. 6:9. doi: 10.1186/1749-8104-6-9
Manuel, M., Martynoga, B., Yu, T., West, J. D., Mason, J. O., and Price, D. J. (2010). The transcription factor Foxg1 regulates the competence of telencephalic cells to adopt subpallial fates in mice. Development 137, 487–497. doi: 10.1242/dev.039800
Mariño, G., Ugalde, A. P., Fernández, A. F., Osorio, F. G., Fueyo, A., Freije, J. M., et al. (2010). Insulin-like growth factor 1 treatment extends longevity in a mouse model of human premature aging by restoring somatotroph axis function. Proc. Natl. Acad. Sci. U S A. 107, 16268–16273. doi: 10.1073/pnas.1002696107
Martynoga, B., Morrison, H., Price, D. J., and Mason, J. O. (2005). Foxg1 is required for specification of ventral telencephalon and region-specific regulation of dorsal telencephalic precursor proliferation and apoptosis. Dev. Biol. 283, 113–127. doi: 10.1016/j.ydbio.2005.04.005
Miyoshi, G., and Fishell, G. (2012). Dynamic FoxG1 expression coordinates the integration of multipolar pyramidal neuron precursors into the cortical plate. Neuron 74, 1045–1058. doi: 10.1016/j.neuron.2012.04.025
Murillo-Cuesta, S., Rodríguez-de la Rosa, L., Cediel, R., Lassaletta, L., and Varela-Nieto, I. (2011). The role of insulin-like growth factor-I in the physiopathology of hearing. Front. Mol. Neurosci. 4:11. doi: 10.3389/fnmol.2011.00011
Nakatani, Y., Kiyonari, H., and Kondo, T. (2019). Ecrg4 deficiency extends the replicative capacity of neural stem cells in a Foxg1-dependent manner. Development 146:168120. doi: 10.1242/dev.168120
Ohlemiller, K. K. (2008). Recent findings and emerging questions in cochlear noise injury. Hear Res. 245, 5–17. doi: 10.1016/j.heares.2008.08.007
Pancrazi, L., Di Benedetto, G., Colombaioni, L., Della Sala, G., Testa, G., Olimpico, F., et al. (2015). Foxg1 localizes to mitochondria and coordinates cell differentiation and bioenergetics. Proc. Natl. Acad. Sci. U S A. 112, 13910–13915. doi: 10.1073/pnas.1515190112
Pauley, S., Lai, E., and Fritzsch, B. (2006). Foxg1 is required for morphogenesis and histogenesis of the mammalian inner ear. Dev. Dyn. 235, 2470–2482. doi: 10.1002/dvdy.20839
Pelton, R. W., Saxena, B., Jones, M., Moses, H. L., and Gold, L. I. (1991). Immunohistochemical localization of TGF beta 1, TGF beta 2, and TGF beta 3 in the mouse embryo: expression patterns suggest multiple roles during embryonic development. J. Cell Biol. 115, 1091–1105. doi: 10.1083/jcb.115.4.1091
Richard, S. A., and Jia-Hao, Z. (2020). Elucidating the pathogenic and biomarker potentials of FOXG1 in glioblastoma. Oncol. Rev. 14:444. doi: 10.4081/oncol.2020.444
Rodriguez-de la Rosa, L., Lassaletta, L., Calvino, M., Murillo-Cuesta, S., and Varela-Nieto, I. (2017). The Role of Insulin-Like Growth Factor 1 in the Progression of Age-Related Hearing Loss. Front. Aging Neurosci. 9:411. doi: 10.3389/fnagi.2017.00411
Ryals, M., Morell, R. J., Martin, D., Boger, E. T., Wu, P., Raible, D. W., et al. (2018). The Inner Ear Heat Shock Transcriptional Signature Identifies Compounds That Protect Against Aminoglycoside Ototoxicity. Front. Cell Neurosci. 12:445. doi: 10.3389/fncel.2018.00445
Schacht, J. (1999). Biochemistry and pharmacology of aminoglycoside-induced hearing loss. Acta Physiol. Pharmacol. Ther. Latinoam 49, 251–256.
Siegenthaler, J. A., Tremper-Wells, B. A., and Miller, M. W. (2008). Foxg1 haploinsufficiency reduces the population of cortical intermediate progenitor cells: effect of increased p21 expression. Cereb. Cortex 18, 1865–1875. doi: 10.1093/cercor/bhm209
Sierra, A., Martín-Suárez, S., Valcárcel-Martín, R., Pascual-Brazo, J., Aelvoet, S. A., Abiega, O., et al. (2015). Neuronal hyperactivity accelerates depletion of neural stem cells and impairs hippocampal neurogenesis. Cell Stem Cell 16, 488–503. doi: 10.1016/j.stem.2015.04.003
Testa, G., Olimpico, F., Pancrazi, L., Borello, U., Cattaneo, A., Caleo, M., et al. (2019). Cortical Seizures in FoxG1(+/-) Mice are Accompanied by Akt/S6 Overactivation, Excitation/Inhibition Imbalance and Impaired Synaptic Transmission. Int. J. Mol. Sci. 20:4127. doi: 10.3390/ijms20174127
Valacchi, G., Pecorelli, A., Cervellati, C., and Hayek, J. (2017). 4-hydroxynonenal protein adducts: Key mediator in Rett syndrome oxinflammation. Free Radic. Biol. Med. 111, 270–280. doi: 10.1016/j.freeradbiomed.2016.12.045
Wang, Y., Hirose, K., and Liberman, M. C. (2002). Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J. Assoc. Res. Otolaryngol. 3, 248–268. doi: 10.1007/s101620020028
Xuan, S., Baptista, C. A., Balas, G., Tao, W., Soares, V. C., and Lai, E. (1995). Winged helix transcription factor BF-1 is essential for the development of the cerebral hemispheres. Neuron 14, 1141–1152. doi: 10.1016/0896-6273(95)90262-7
Yadirgi, G., Leinster, V., Acquati, S., Bhagat, H., Shakhova, O., and Marino, S. (2011). Conditional activation of Bmi1 expression regulates self-renewal, apoptosis, and differentiation of neural stem/progenitor cells in vitro and in vivo. Stem Cells 29, 700–712. doi: 10.1002/stem.614
Yahata, N., Asai, M., Kitaoka, S., Takahashi, K., Asaka, I., Hioki, H., et al. (2011). Anti-Aβ drug screening platform using human iPS cell-derived neurons for the treatment of Alzheimer’s disease. PloS one 6:e25788. doi: 10.1371/journal.pone.0025788
Yamamizu, K., Piao, Y., Sharov, A. A., Zsiros, V., Yu, H., Nakazawa, K., et al. (2013). Identification of transcription factors for lineage-specific ESC differentiation. Stem cell Rep. 1, 545–559. doi: 10.1016/j.stemcr.2013.10.006
Yang, X., Castilla, L. H., Xu, X., Li, C., Gotay, J., Weinstein, M., et al. (1999). Angiogenesis defects and mesenchymal apoptosis in mice lacking SMAD5. Development 126, 1571–1580.
Yoshida, S., Sugahara, K., Hashimoto, M., Hirose, Y., Shimogori, H., and Yamashita, H. (2015). The minimum peptides of IGF-1 and substance P protect vestibular hair cells against neomycin ototoxicity. Acta Otolaryngol. 135, 411–415. doi: 10.3109/00016489.2014.979438
Zhan, R., Wang, F., Wu, Y., Wang, Y., Qian, W., Liu, M., et al. (2018). Nitric oxide promotes epidermal stem cell proliferation via FOXG1-c-Myc signalling. Nitric Oxide 73, 1–8. doi: 10.1016/j.niox.2017.12.002
Keywords: FoxG1, hearing loss, inner ear hair cells, autophagy, development
Citation: Ding Y, Meng W, Kong W, He Z and Chai R (2020) The Role of FoxG1 in the Inner Ear. Front. Cell Dev. Biol. 8:614954. doi: 10.3389/fcell.2020.614954
Received: 07 October 2020; Accepted: 18 November 2020;
Published: 03 December 2020.
Edited by:
Chen Zhang, Capital Medical University, ChinaCopyright © 2020 Ding, Meng, Kong, He and Chai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weijia Kong, ZW50d2prb25nQGh1c3QuZWR1LmNu; Zuhong He, aGV6dWhvbmdAMTYzLmNvbQ==; Renjie Chai, cmVuamllY0BzZXUuZWR1LmNu
 Yanyan Ding
Yanyan Ding Wei Meng
Wei Meng Weijia Kong
Weijia Kong Zuhong He
Zuhong He Renjie Chai
Renjie Chai