Abstract
Ubiquitination is a critical type of protein post-translational modification playing an essential role in many cellular processes. To date, more than eight types of ubiquitination exist, all of which are involved in distinct cellular processes based on their structural differences. Studies have indicated that activation of the ubiquitination pathway is tightly connected with inflammation-related diseases as well as cancer, especially in the non-proteolytic canonical pathway, highlighting the vital roles of ubiquitination in metabolic programming. Studies relating degradable ubiquitination through lys48 or lys11-linked pathways to cellular signaling have been well-characterized. However, emerging evidence shows that non-degradable ubiquitination (linked to lys6, lys27, lys29, lys33, lys63, and Met1) remains to be defined. In this review, we summarize the non-proteolytic ubiquitination involved in tumorigenesis and related signaling pathways, with the aim of providing a reference for future exploration of ubiquitination and the potential targets for cancer therapies.
1 Introduction
1.1 The Ubiquitin-Proteasome System
Ubiquitination, also known as ubiquitylation, refers to the process by which ubiquitin (Ub, a small and highly conserved protein), with the help of a series of special enzymes, classifies proteins in cells, selects target proteins, and modifies those proteins (McDowell and Philpott, 2016; Seeler and Dejean, 2017; Rape, 2018). Ubiquitination plays fundamental roles in many cellular events such as cell proliferation (Kwon and Ciechanover, 2017; Werner et al., 2017; Senft et al., 2018; Song and Luo, 2019), cell cycle (Teixeira and Reed, 2013; Darling et al., 2017; Gilberto and Peter, 2017), DNA repair (Alpi and Patel, 2009; Abbas and Dutta, 2011), immune response (Heaton et al., 2016; Rudnicka and Yamauchi, 2016; Manthiram et al., 2017), transcription (Zhou et al., 2018; Imam et al., 2019; Sun et al., 2019), angiogenesis (Zhang et al., 2022), metastasis (Rossi and Rossi, 2022), and apoptosis (Xu et al., 2017; Zhou et al., 2017).
Protein ubiquitination requires three different enzymes: E1 ubiquitin-activating enzymes, E2 ubiquitin-conjugating enzymes, and E3 ubiquitin ligases (Heap et al., 2017; O'Connor and Huibregtse, 2017). E1 enzymes activate the ubiquitin polypeptide in an ATP-dependent manner, and the activated forms are then conjugated to E2 enzymes through the formation of thioester bonds (Wang et al., 2018). Finally, E3 ubiquitin ligases recognize both E2 enzymes and specific target substrates that confer specificity to the system, and then the Ub can be transferred from E2 enzymes to the target substrates to complete the ubiquitination process (Rittinger and Ikeda, 2017; Yau et al., 2017). The coordination of E1, E2, and E3 enzymes earmarks target proteins with a wide variety of ubiquitin modifications such that distinct ubiquitin modifications transmit different cellular signals (Khaminets et al., 2016; Venuto and Merla, 2019). Specific ubiquitin-binding domains (UBDs) can identify ubiquitylated substrate proteins (Kristariyanto et al., 2015; Kung et al., 2019; Polykratis et al., 2019; Spiliotopoulos et al., 2019). UBDs utilize diverse mechanisms to interact with various surface patches on ubiquitin molecules or different ubiquitin linkages (Hicke et al., 2005; Dikic et al., 2009; Komander and Rape, 2012). The hydrophobic surfaces of ubiquitin molecules, such as isoleucine 36 (Ile36) and isoleucine 44 (Ile44), are the structural basis for the recognition of ubiquitination signals. Different ubiquitin chains have various spatial structures and thus expose different hydrophobic surfaces, which can be recognized by specific UBDs. Proteins containing UBDs recognize and transmit the functional signals represented by ubiquitin chains (Buetow and Huang, 2016). Similar to many other protein post-translational modifications (PTMs), ubiquitination can be preserved through cleavage by deubiquitylating enzymes (DUBs) (Herhaus and Sapkota, 2014; Nishi et al., 2014; Clague et al., 2019) (Figure 1).
FIGURE 1
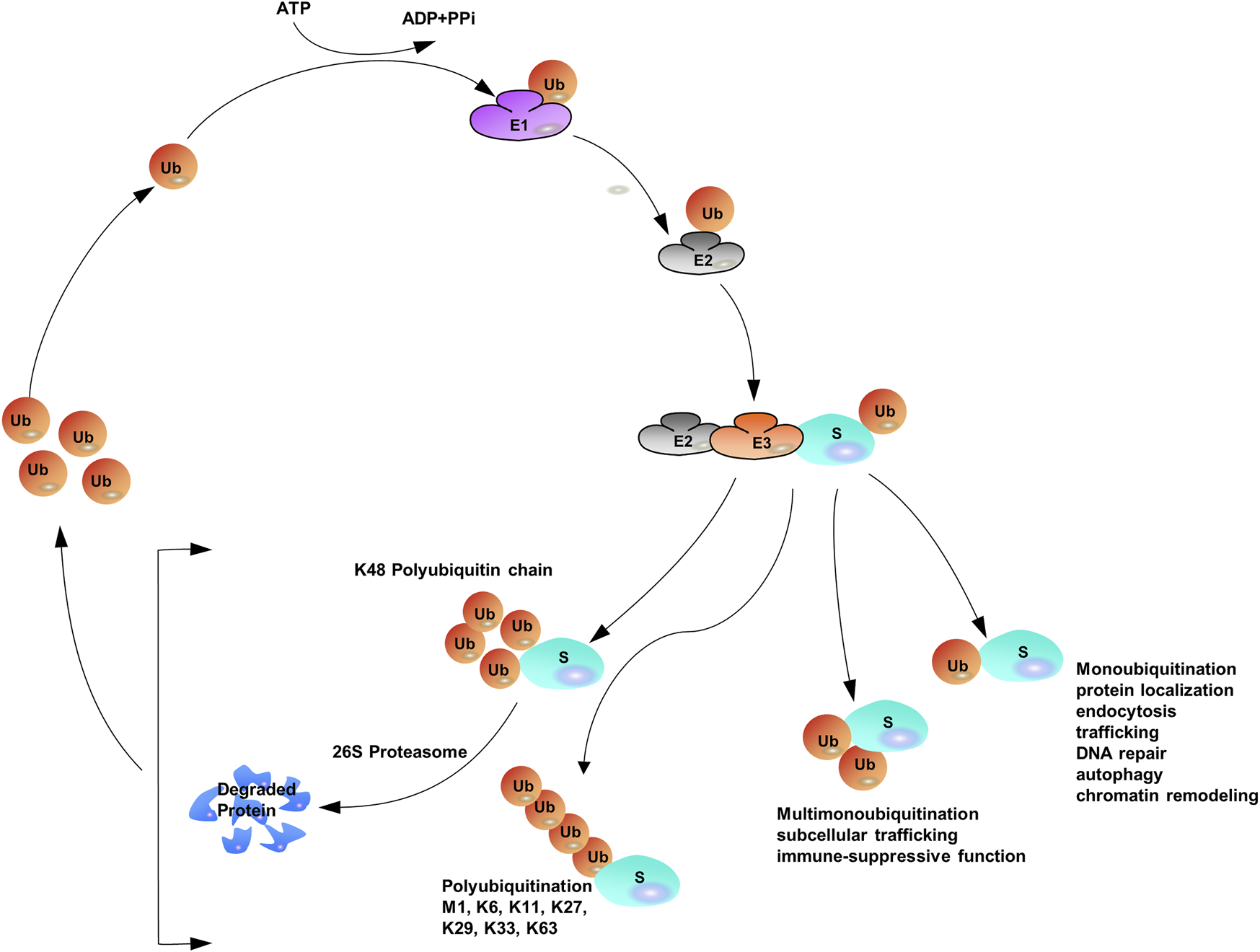
The Ubiquitination Machinery: ubiquitin conjugates can differ in structure and function.
The versatility of ubiquitination is determined by the complex assembly pattern of ubiquitin molecules on the target protein. Ubiquitins can be attached to one or multiple lysine residues with either a single ubiquitin molecule (mono- and multi-mono-ubiquitination, respectively) or ubiquitin polymers (poly-ubiquitination) (Eger et al., 2010; Akutsu et al., 2016; Chitale and Richly, 2017; van der Heden van Noort et al., 2017). Poly-ubiquitin chains comprising only one single linkage are often assumed to be homotypic (Jeusset and McManus, 2017; Leestemaker and Ovaa, 2017), whereas heterotypic chains adopt multiple linkages within the same polymer (branched or non-branched) (Akturk et al., 2018). In a poly-ubiquitin chain, ubiquitin moieties can be linked through any of the seven lysine residues (K6, K11, K27, K29, K33, K48, and K63) or N-terminal methionine (Met1) (Dittmar and Winklhofer, 2019; Spit et al., 2019; Liao et al., 2022), resulting in an almost unlimited number of poly-ubiquitin chain topologies (Varadan et al., 2004; Sadowski and Sarcevic, 2010; Dondelinger et al., 2016; O'Connor and Huibregtse, 2017; Padala et al., 2017). Further complexity is added to ubiquitination when the ubiquitin polypeptide is modified by phosphorylation (Dong et al., 2017) or acetylation (Choudhary et al., 2009; Ohtake et al., 2015). Given the sophisticated assembly of protein ubiquitination, it has been often referred to as the “ubiquitin code” (Komander and Rape, 2012). Proteomics studies have shown that Lys48-linked chains are predominant in cells (>50% of all linkages), and Lys63-linked chains rank the second abundant chain form, however, researchers have begun to characterize the remain chain types, which were considered to be “atypical” ubiquitin modifications (linked through Lys6, Lys11, Lys27, Lys29, Lys33 and Met1) (Xu et al., 2009; Dammer et al., 2011; Kim et al., 2011; Wagner et al., 2011; Ziv et al., 2011).
1.2 Structural Features of Poly-Ubiquitin Chains
Poly-ubiquitin chains occur when a single ubiquitin molecule is repeatedly connected in series with another ubiquitin lysine residue. Substrate proteins can be distinguished by poly-ubiquitin chains by attaching between different types of deubiquition formations, single mono-ubiquitination events, multiple mono-ubiquitinations events, homotypic ubiquitination events and heterotypic ubiquitination events (branched and non-branched ubiquitination) (Sokratous et al., 2014; Yau and Rape, 2016; Zhao et al., 2017). This also led to the formation of eight different homotypic chains. The key distinguishing feature of how this can be achieved is the specific combination of E2/E3 enzymes, thereby triggering distinct cellular fates of substrate proteins. However, some of the reported E2/E3 enzyme combinations were not 100% specific for targeted linkage. It has been reported that two E1 enzymes were selected for ubiquitin in humans: UBA1 and UBA6 (Barghout and Schimmer, 2021). Humans also encoded 40 E2 conjugation enzymes cooperate with approximately 600 E3 ligase enzymes (Hodson et al., 2014). The E3s were categorized into three groups: RING/U-box, HECT, and RING between RING (RBR) (Wang et al., 2020).
With the assistance of E1, E2, and E3, mono-ubiquitination occurs when a single ubiquitin is attached to its target proteins, then Ub molecules are added to the model in linear ways one by one (Torres et al., 2009; Tang et al., 2011). The sequential addition model of Ub on the substrate contributes to the elongation of the Ub lines. When secondary Ub molecules are connected to specific lysine residues, they are called homotypic chains. If any of the attached adjacent Ub molecules are linked to each other by different lysine residues (mixed or branched model), a heteropic structure is formed. For homotypic chains, reports have found that different chain types are closely related to the confirmation of the structure, either “compact” or “open”. Generally, non-proteolytic ubiquitination Lys63 linkages and Met1 linkages, adopt “open” ones. Contrast to the aforementioned linkages, internal structural molecules interact with each other among degradable linkages, like Lys6, Lys11 and Lys48 linkages, and those linkages display “open” conformation. Furthermore, all ubiquitin moieties can be modified by acetylation or phosphorylation to add additional layers of complexity (Kane et al., 2014; Kazlauskaite et al., 2014; Koyano et al., 2014; Ordureau et al., 2014; Ohtake et al., 2015; Swaney et al., 2015) (Figure 1).
Numerous studies have found that ubiquitin acetylation inhibits poly-ubiquitination elongation, and phosphoubiquitin leads to mitophagy. Any PTM chain can be changed to ubiquitin chains, which may prevent or facilitate ubiquitin interactions. Protein phosphorylation is linked to ubiquitination for proteasomal degradation. Reports have shown that the phosphorylation of ULK1 by MAPK1/3 kinase interacts with BTRC, which leads to subsequent proteasomal degradation and attenuates breast cancer bone metastasis (Deng et al., 2020). However, the stability of some proteins is also regulated by phosphorylation. Reports have also shown that Aurora B-mediated phosphorylation of ubiquitin specific protease 13 (USP13) at Serine 114 promoted the stability of Aurora B.
1.3 Encoding and Decoding the Ubiquitin Code
Encoding and decoding the ubiquitin code is performed by factors that recognize Ub chains and connect the substrate proteins to the downstream response (Ji and Kwon, 2017; Kwon and Ciechanover, 2017). Recognition of chains occurs through discrete domains and affinity binders with specificity for a particular Ub substrate and chain type (Fu et al., 2012; Suryadinata et al., 2014; Kniss et al., 2018; Michel et al., 2018). This complex system consists of the conjugation of diverse mono, multi-mono and polymeric chains (Rösner et al., 2015; Ji and Kwon, 2017). The interpretation of how, when, and why the ubiquitin codes are written, read, and erased emerged to be characterized. Ubiquitination is a powerful decoration process of proteins and is typically actualized by “ubiquitinase” (Zientara-Rytter and Subramani, 2019). Ubiquitin can be successfully linked to one of the seven lysine residues, all of which can be characterized as poly-ubiquitin chains (Laplantine et al., 2009; Regev et al., 2015; Bax et al., 2019). Poly-ubiquitin chains with different topologies depended upon the lysine residues (which were chosen to be attached) and the substantial chain length, determine the lucky chance of the target proteins and regulate diverse cellular processes, known as “ubiquitin code” (Dittmar and Selbach, 2017; Chatr-Aryamontri et al., 2018; Fottner et al., 2019; O'Donnell, 2019; Song and Luo, 2019). Recent discoveries have deepened our understanding of the whole picture of the ubiquitin code, the interplay between “writers” (E1/E2/E3s), “erasers” (DUBs) and “readers” (ubiquitin binding domain containing proteins) (Tanno and Komada, 2013; Heride et al., 2014; Rogerson et al., 2015; Di Lello and Hymowitz, 2016; Smeenk and Mailand, 2016). Studies have highlighted that mono-ubiquitination can be catalyzed by different E2 and E3 enzymes, acting either individually or together to determine specific substrates. Notably, the linkage specificity of E3s containing RING or U-box domains is likely dictated by E2. As for the HECT E3s class of enzymes, HECT domain swaps can activate the acceptor lysine and are sufficient to determine the linkage specificity. RBR E3s, however, are somewhat complex. RBR E3s display linkage specificity in multiple chains, Met1-, Lys63-, Lys48-, and Lys27-linked chains, as well as mono-ubiquitination, while cooperating with E2 to synthesize Lys-linked or Met1-linked chains. The Ub tag attached to a certain substrate, which is preferably achieved through the cooperation of specific E2/E3 enzyme pairs, represents a complex yet specific message encoded by the cell (Kim and Huibregtse, 2009; David et al., 2011; Turek et al., 2018). Interestingly, this is achieved by Ub receptors which are equipped with one or more UBDs (Mattern et al., 2019). Ubiquitin recognition achieved by UBDs can translate written code into specific outcomes. There are more than 20 families of UBDs that bind to different patches on Ub surrounding hydrophobic patches, either lle44 or lle36 (Dikic et al., 2009; Hendriks et al., 2018). Moreover, UBDs are able to sense unique 3D conformations of distinct chain types, which are associated with diverse biological activities, meaning that this small motif-containing protein can help recognize versatile signals and affect the desired effect (Lee et al., 2006; Penengo et al., 2006; Reyes-Turcu et al., 2006; Bomar et al., 2010). In addition, ubiquitin-interacting proteins which served as “decoders” of the ubiquitin message, participated in the downstream regulation of the ubiquitinated substrate (Heideker and Wertz, 2015; Leznicki and Kulathu, 2017; Mevissen and Komander, 2017). These “decoders” may specifically reverse the ubiquitination process or function as receptors for the transfer of the targeted substrate toward downstream signaling components and/or subcellular compartments (Hu et al., 2002; Lin et al., 2008; Komander et al., 2009). To date, 55 ubiquitin specific proteases (USPs), 14 ovarian tumor DUBs (OTUs), 10 JAMM family DUBs, 4 ubiquitin C-terminal hydrolases (UCHs) and 4 Josephin domain DUBs have been identified. Encoding and decoding ubiquitin codes are responsible for all levels of epigenetic changes, and by changing substrate protein activities, they can also activate and repress effects on gene transcription depending on their target proteins and the ubiquitin chain types, all of which are connectively related to the process of tumor proliferation (Kim and Baek, 2006; Zheng et al., 2008; Fradet-Turcotte et al., 2013; Yeh et al., 2018). Ubiquitin code signaling is frequently dysregulated in numerous cancer types and can function as a tumor suppressor or tumor promoter, suggesting a potential target for cancer therapy (Loch and Strickler, 2012; Choudhry et al., 2018; Emanuelli et al., 2019).
1.4 Physiological Functions of Non-proteolytic Poly-Ubiquitin Chains
The simplest version of ubiquitination or mono-ubiquitination confers non-degradative activities including protein localization (Yang et al., 2017), endocytosis (Shih et al., 2002; Windheim et al., 2008), trafficking, DNA repair (Xie et al., 2014; Whiteaker et al., 2018), autophagy (Chen S. et al., 2017; Zheng et al., 2020; Leng et al., 2021) and chromatin remodeling (Cole et al., 2021). When mono-ubiquitination is further modified, multiple lysine residues of the substrate are yielded to induce multi-mono-ubiquitination. Emerging investigations have found that this process connects ubiquitin with subcellular trafficking (Yin et al., 2010; Cooray et al., 2011) and immune-suppressive functions (Zhu et al., 2018). As for poly-ubiquitination, Lys48-linked poly-ubiquitination leads to the degradation of substrates (Komander and Rape, 2012). In contrast, Lys63-linked poly-ubiquitination exerts critical signaling functions in regulating protein stability, including nuclear factor κB (NF-κB) signaling (Syed et al., 2006; Wu Z. et al., 2014; Gallo et al., 2014), endocytosis (Galan and Haguenauer-Tsapis, 1997), DNA damage responses, and immune responses (Wu and Karin, 2015; Hrdinka et al., 2016; Liu et al., 2017; Paul and Wang, 2017). Emerging experiments have shown that incorrect regulation of cellular processes (either tumour inhibitors or promoters) contributes to cancer pathogenesis and progression (Shirane et al., 1999). Additionally, the cellular functions of atypical ubiquitin linkages (except Lys11-linked ubiquitin) are supposed to be non-degradable (Kulathu and Komander, 2012; Iwai, 2014, 2015; Meza Gutierrez et al., 2018) and in most cases, activities involved in Lys63-linked poly-ubiquitin chains are also considered to be non-degradable (Liu et al., 2015).
Of note, other chains also regulate specific physiological functions: Lys6-linked poly-ubiquitin was supposed to be indirectly linked to DNA damage response with the help of heterodimeric ubiquitin E3 ligase BRCA1–BARD1 (Wu-Baer et al., 2003; Wu-Baer et al., 2010). Linear (M1) chains can regulate NF-κB activation (Behrends and Harper, 2011; Chen et al., 2015; Borghi et al., 2018), whereas Lys11 linkage acts as a powerful degradation signal in heterotypic ubiquitin conjugates (Locke et al., 2014; Mevissen et al., 2016) and lys27 linkage can prompt mitochondrial depolarization and mediate translocation of the E3 ligase Parkin, which accumulates Lys27-linked linkages on mitochondrial protein voltage-dependent anion-selective channel protein1 (VDAC1). This exact Lys27-linked translocation leads to Parkinson’s disease in the presence of Parkin (Geisler et al., 2010; Glauser et al., 2011). Lys27 linkage has also been demonstrated to be related with the DNA damage response and innate immune response (Xue et al., 2018). The propagation of Wnt/β-catenin signaling through Lys29-or Lys11-linked ubiquitin chains is closely associated with cancer pathogenesis and is involved in protein ubiquitination at multiple levels (Hay-Koren et al., 2011). Several studies have reported the functions of both Lys29-and Lys33-linked chains in the regulation of AMPK-related protein kinases (Al-Hakim et al., 2008), and the Lys33 linkage is negatively regulated by T-cell antigen receptors (TCRs), which indirectly affect cellular activities in tumors (Huang et al., 2010). In this review, we will focus on recent progresses in non-degradable ubiquitin chains in tumorigenesis and the essential proteins involved in non-proteolytic ubiquitination.
2 The Roles of Non-Proteolytic Ubiquitination in Tumorigenesis
Non-proteolytic ubiquitination, including both mono-ubiquitination and poly-ubiquitination (mainly K6-, K27-, K29-, K33-, K63-, and M1-linked poly-ubiquitination), has become key regulators in a variety of cancers. How they function as signaling entities in the pathogenesis and progression of cancer will be beneficial to gain an in-depth understanding of ubiquitination (Figure 2).
FIGURE 2
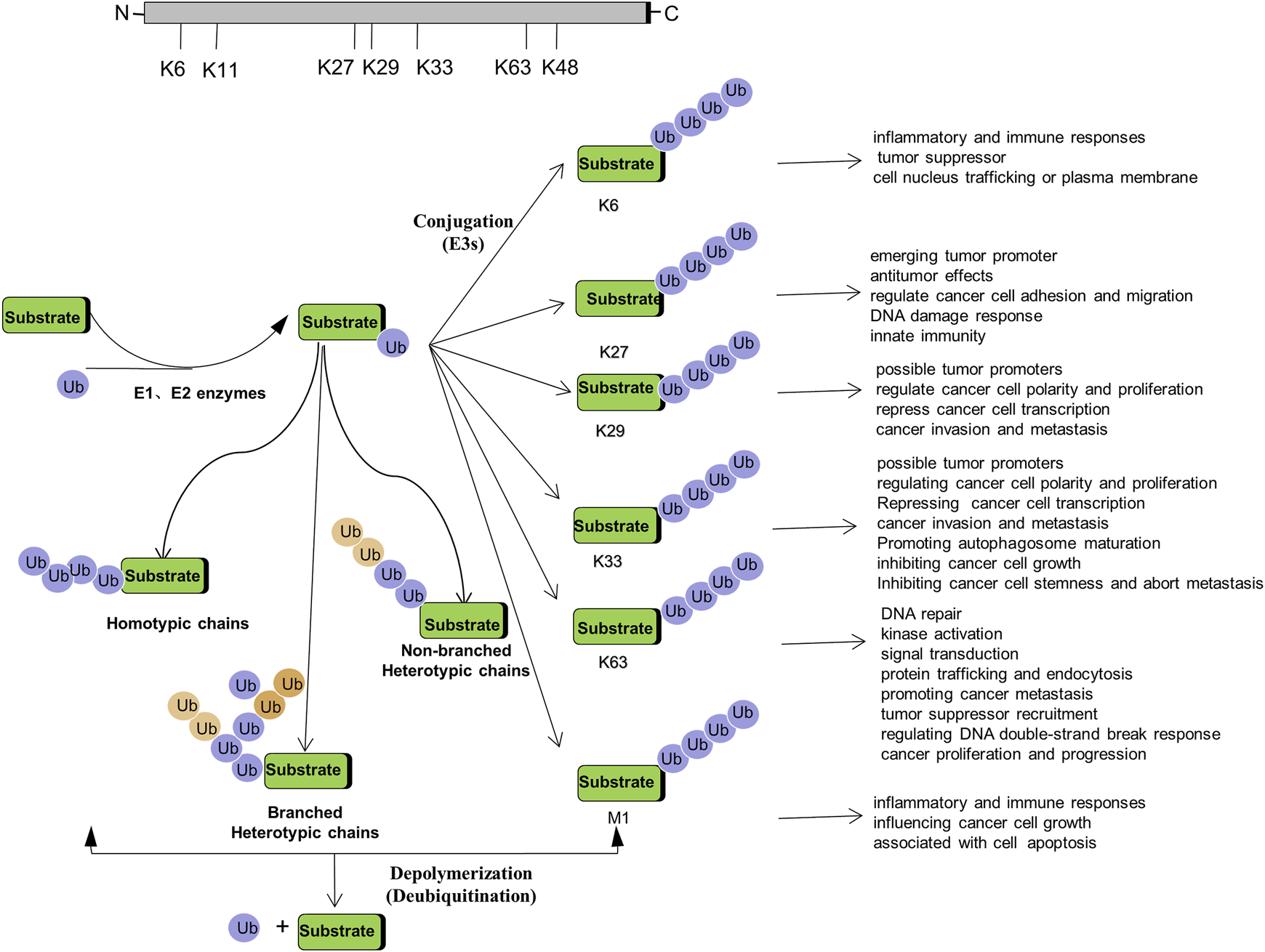
Regulation of Tumorigenesis by Non-proteolytic Ubiquitination: Linked through Lys6, Lys27, Lys 29, Lys 33, Lys 63, M1 poly-ubiquitination.
2.1 Mono-Ubiquitination
Mono-ubiquitination has been suggested to be even more dynamic than previously thought, and its functions have been deciphered by various ubiquitin-binding proteins. It has been demonstrated that the cellular functionality of ubiquitin is mediated by mono-ubiquitin and/or poly-ubiquitin. In fact, mono-ubiquitination is associated with tumorigenesis, not limited to membrane transportation, endocytosis, receptor internalization, degradation in lysosomes, and protein reprocessing (Haglund et al., 2003). BMI1 interacts with histone H2A through mono-ubiquitination, repressing multiple genes, such as INK4A/ARF, which function in the pRb and p53 pathways, thereby facilitating cancer progression (Lin et al., 2015). Interestingly, in the presence of UbE2E1, PRC1 catalyzes the mono-ubiquitination of H2A, contributing to cancer cell proliferation (Wheaton et al., 2017). Mono-ubiquitination is also involved in the process of USP22 regulating histone H2B and exhibits both oncogenic and tumor-suppresser roles in cancer development (Jeusset and McManus, 2017). In addition, reversible mono-ubiquitination activity plays an essential role in balancing TGF-β/SMAD signaling, which is involved in cancer initiation and progression (Xie et al., 2014). Mono-ubiquitination also regulates forkhead box O (FOXO) proteins, which control specific gene expression programs that are vital for slowing the onset of cancer in aging individuals (Greer and Brunet, 2008). Strikingly, FANCL cooperates with, UBE2T and catalyzes mono-ubiquitination, which participated in the regulation of Fanconi Anemia pathway, leading to chromosome instability and promoting tumorigenesis (Machida et al., 2006; Hodson et al., 2014; Miles et al., 2015; Sun et al., 2020; Wang S. et al., 2021) (Table 1, 2).
TABLE 1
| E2 | Alias | Accompanied E3 | Linkage | Phenotypic Characteristics | Neoplastic Implications | Substrate | Mechanism Summary |
|---|---|---|---|---|---|---|---|
| UBE2N | UBC13 | TRAF2/TRAF6? | K63 | preventing tumor formation and metastasis | modulating breast cancer metastasis | NEMO? | UEV1A, together with Ubc13, promote breast cancer metastasis through Lys63-linked polyubiquitination of target proteins and NF-кB-mediated MMP1 expression (Wu et al., 2014b) |
| UBE2N | UBC13 | TRAF6 | K63 | DNA damage repair and protein kinase activation | metastatic spread and lung colonization by breast cancer cells | p38 | Ubc13 catalyzes K63-linked proteins, accompnied by TAK1-p38 activation, whose activity is essential for breast cancer metastasis (Wu et al., 2014a) |
| Ubc13:Uev1A/Uev2 | UBE2N:UBE2V1/Uev2 | RNF8 | K63 | DNA damage repair and cytokinesis | genomic instability in adult T-cell leukemia (ATL) | TAK1?IKK? | Ubc13/Uev1A and RNF8 interact with each other and generate K63-pUb, which is recognized by Tax, stimulating TAK1 and IKK activation (Ho et al., 2015) |
| Ubc13 | UBE2N | TRAF6 | K63 | activating NF-κB signaling | elicit anti-tumour responses | RANK? | STAT3 negatively regulates Ubc1 involving K63-linked ubiquitination, and suppress pro-inflammatory cytokines by modulating NF-κB signaling (Zhang et al., 2014) |
| Ubc13 | UBE2N | RNF8 | K63 | DNA double-strand break (DSB) responses | BRCA1 Tumor Suppressor Recruitment | histone | RNF8 stimulates Ubc13-dependent Lys-63 ubiquitination, which is pivotal for DNA damage response and recruitment of BRCA1 (Hodge et al., 2016) |
| Ubc13 | UBE2N | TRAF6 | K63 | innate and adaptive immunity | osteoclast differentiation | TRAF6 (autoubiquitination) | Ubc13/Uev1A interacts and binds to the active RING domain of TRAF6, which is essential for the formation of Lys63-linked pUb, thus triggering NF-κB activation and osteoclast differentiation (Lamothe et al., 2008) |
| Ubc13/Uev1A | UBE2N/UBE2V1 | TRAF6 | K63 | activating NF-κB signaling | osteoclast differentiation | TRAF6 (autoubiquitination) | TRAF6 in combination with Ubc/Uev1A catalyzes TRAF6 auto-ubiquitination through Lys63-linked poly-Ub chains, which controls NF-κB signaling and osteoclast differentiation (Lamothe et al., 2007b) |
| Ubc13/Uev1A | UBE2N/UBE2V1 | TRAF6 | K63 | spontaneous osteoclast differentiation | TRAF6 (auto-ubiquitination) | TRAF6 interacts with Ubc13/Uev1A, facilating Lys-linked quto-ubiquitination of TRAF in a RING domain-dependent fashion, and modulating downstream NF-κB signaling (Lamothe et al., 2007a) | |
| UBE2O | UBE2O | TRAF6 | K63 | activating NF-κB signaling | modulating NF-κB signaling associated cancers | TRAF6 (auto-polyubiquitination) | UBE2O negatively regulates the recruitment of TRAF6, inducing TRAF6 auto-ubiquitination through binding to K63 residue, and subsequently prevents NF-κB activation by the IL-1R/TLR complex (Zhang et al., 2013b) |
| UBE2T | UBE2T | FANCL | monoubiquitination | maintenance of chromosome stability | disrupting DNA repair pathways | FANCD2 | In the presence of FANCL, UBE2T stimulates monoubiquitination of FANCD2, which is vital for disrupting abnormal chromosomes and efficient DNA damage repair (Machida et al., 2006) |
| UBE2T | UBE2T | FANCL | automonoubiquitination | maintenance of chromosome stability | disrupting DNA repair pathways | UBE2T | Automonoubiquitination of UBE2T inhibits own conjugation activity (Machida et al., 2006) |
| UBE2T | UBE2T | FANCL | monoubiquitination | DNA repair | leading to leukemia and bone marrow failure | FANCD2 | FANCL interacts with UBE2T in an ELF-domain-dependent fashion, which regulates DNA damage-induced FANCD2 monoubiquitination (Miles et al., 2015) |
| UBE2T | UBE2T | FANCL | monoubiquitination | DNA interstrand crosslink repair | genomic instabilities | FANCD2 | FANCL specifically interacts with UBE2T, leading to FANCD2 ubiquitination, which is involved in Fanconi Anemia pathway (Hodson et al., 2014) |
| RAD6 | UBE2B | in absence of E3 | K63? | promoting DNA repair | promoting recurrence and metastasis in ovarian cancer | β-catenin | Rad6 facilitates DNA repair and stem cell gene transcription, through mediating H2B ubiquitination (Somasagara et al., 2017) |
| RAD6B | UBE2B | in absence of E3 | K63 | DNA repair and mutagenesis | h-catenin modification in breast cancer | β-catenin | Rad6B ubiquitinates b-catenin through K63-linked ubiquitination, which regulates transcriptional activity in breast cancer (Shekhar et al., 2008) |
| UBE2B | UBE2B | BRE1 | monoubiquitination | promoting the G1-S transition and cell proliferation | promoting G1-S transition and cell proliferation | H2B | UBE2B modulates CCND1 transcription level by regulating the levels of H2B monoubiquitination, promoting cell cycle progression and proliferation (Cai et al., 2014) |
| Ube2w | UBE2W | RNF4 | monoubiquitination | DNA damage repair | potential prostate, breast and lung cancer target | SUMO | Ube2w associated with RNF4, mediating mono-ubiquitination of SUMO chains. Those chains can be further ubiquitinated through K63 chains in response to DNA damager (Maure et al., 2016) |
| Ubc13 | UBE2N | TRAF6? | K63 | Autoimmunity and aberrant T cell activation | modulating NF-κB associated cancer | IKK | Ubc13 conjugates K63-linked ubiquitin chains involving Ubc13-IKK signaling axis, which have a robust evidence in regulating T cell function (Chang et al., 2012) |
| UBC13 | UBE2N | Bcl10 | K63 | activating NF-kappaB pathway | modulating NF-κB associated cancer | NEMO | UBC13 is dependent in Bcl10 modulating NEMO lysine-63-linked ubiquitination, and subsequent NF-kappaB activation (Zhou et al., 2004) |
| UBE2N | UBE2N | MEKK1 | K63 | embryonic survival | promoting ES-cell differentiation and tumour formation | TAB1 | Together with UBE2N, MEKK1 could tag TAB1 with Lys63-linked poly-Ub, promoting ES-cell differentiation and tumourigenesis (Charlaftis et al., 2014) |
| UbcH6 | UBE2E1 | TRAF4 | K63 | DNA damage | overcome chemoresistance in colorectal cancer | CHK1 | UbcH6 combined with TRAF4, which is critical for CHK1 K63-linked ubiquitination and essential for cell proliferation, colony formation (Yu et al., 2020b) |
| UBE2T | UBE2T | RNF8 | monoubiquitination | facilitating cell cycle arrest activation | conferring HCC radioresistance | H2AX | UBE2T/RNF8 complex, monoubiquitinated H2AX/γH2AX, facilitating cell cycle arrest activation, thus inducing HCC radioresistance (Sun et al., 2020) |
| Ubc13:Uev1A/Uev2 | UBE2N:UBE2V1/UEV2 | RNF8 | K63 | DNA damage repair and cytokinesis | genomic instability of ATL cells | NEMO and TAB2/3 | RNF8and Ubc13:Uev1A/Uev2assemble K63-pUb chains on NEMO and TAB2/3 respectively, allowing TAK1 and IKK activation (Ho et al., 2015) |
| UbE2E1 | PRC1 | monoubiquitination | maintenance of stem cell proliferation | promoting cancer cell proliferation | H2A | UbE2E1 interacts with PRC1 complex, catalyzing monoubiquitination of H2A (Wheaton et al., 2017) | |
| Ubc13 | UBE2N | TRAF6 | K63 | regulating immune signaling | NF-κB signaling related cancer | Ubc13, together with TRAF6, mediates K63-linked polyubiquitin signaling pathway, including NF-κB signaling (Lenoir et al., 2021) | |
| Uev1A/Ubc13 | UBE2N/UBE2V1 | TRAF6 | K63 | regulating AKT signaling pathway | promoting breast cancer cell migration and EMT signaling | AKT | Uev1A/Ubc13 interact with TRAF6, ubiquitinates AKT with K63-linked ubiquitination, which is required for AKT activation, promoting cell migration and EMT in breast cancer (Niu et al., 2021) |
| UBE2T | FANCL | monoubiquitination | involving in FA pathway-induced chromosome instability | functions in cancer predisposition | ID | UBE2T/FANCI-FANCD2 complex remodeling the ID-DNA complex, preventing clamp opening after monoubiquitination (Wang et al., 2021a) | |
| UbcH6 | NEDD4 | K63 | regulating cell-cell adhesion, mechanosensing and autophagy | involving in angiogenesis and tumor growth | IGPR-1 | NEDD4 and UbcH6 are involved in the K63-linked ubiquitination of IGPR-1, regulating different cellular activities, such as cell adhesion, autophagy, mechanosensing, angiogenesis and tumor growth (Sun et al., 2021) | |
| Ubc13 | UBE2N | RNF213 | K63 | angiogenic activity | regulating cell mobility and invasion | RNF213 interacts with Ubc13 and promotes its own autoubiquitination, controling inflammatory responses and angiogenic activities (Habu and Harada, 2021) |
Summary of the combined E2/E3 enzymes in Tumorigenesis.
TABLE 2
| Type | DUBs | Linkage | Phenotypic Characteristics | Neoplastic Implications | Substrate | Mechanism Summary |
|---|---|---|---|---|---|---|
| OTU | TRABID (ZRANB1) | K63 | stem cell self-renewal or differentiation | Wnt-induced transcription in colorectal cancer cell | APC | Trabid preferentially binding to K63-linked ubiquitination chains, which is required for Wnt-induced transcription (Tran et al., 2008) |
| OTU | TRABID (ZRANB1) | K29, K33 | inhibiting autophagy flux | Promoting autophagosome maturation and inhibiting hepatocellular carcinoma growth | UVRAG | ZRANB1 precisely cleaves K29 and K33-linked polyubiquitin chains from UVRAG, regulating autophagy system (Feng et al., 2019) |
| OTU | OTUD1 | K33 | restricting the TGF-β signaling | inhibiting breast cancer stemness and metastasis | SMAD7 | OTUD1 directly deubiquitinates the SMAD7, shuts off TGF-β signals, thereby suppressing metastasis in breast cancer (Zhang et al., 2017) |
| OTU | OTUD1 | K63 | regulating organ growth, tissue regeneration | Regulating tumorigenesis, cancer cell survival and chemoresistance | YAP | OTUD1 cleaves K63-linked poly Ub from YAP, which is assembled by SKP2 E3 ligase, regulating tumorigenesis (Yao et al., 2018) |
| OTU | OTUD1 | K63? | suppressing colony formation and increasing apoptosis | arresting cell growth and inducing apoptosis | p53 | OTUD1 interacts with and stabilizes p53. Its overexpression significantly suppress colony formation, and increases apoptosis (Piao et al., 2017) |
| OTU | OTULIN | M1 | activating NF-κB and promoting pro-inflammatory cytokines and restricting bacterial proliferation | NF-κB signaling associated cancers | cytosolic salmonella | OTULIN dissolutes linear Ub chains on cytosolic S, resulting in ultimately NF-κB activation (van Wijk et al., 2017) |
| OTU | OTULIN | M1 | regulating NF-κB signaling and sensitizing cell death | NF-κB signaling associated cancers | RIPK1 | OTULIN interacts with LUBAC, balancing Met1-polyUb chains, thereby regulating NF-κB signaling (Keusekotten et al., 2013) |
| OTU | OTUD1 | K63? | decreases cell proliferation and increases cell apoptosis | regulating tumor-suppressor p53 | p53 | OTUD1 interacts with and deubiquitinates p53, regulates p53 stability and activity (Piao et al., 2017) |
| OTU | A20 | K63 | NF-κB transcriptional activity-mediated cell death and chronic inflammation | NF-kB signaling-related cancers | RIP | A20 erases K63-linked ubiquitin chains from RIP, and it also polyubiquitnate RIP with K48-linked ubiquitin chains in a carboxy-terminal-domain-dependent manner, which downregulating NF-kB signalling, (Wertz et al., 2004) |
| OTU | A20 | K63 | downregulating NF-kB pathway | NF-kB signaling-related cancers | TRAF6/RIP | A20 display dual ubiquitin-editing functions, mediating both non-proteolytic Lys63-linked ubiquitin chains and degradative Lys48-linked ubiquitin chains, thus regulating NF-κB activities (Lin et al., 2008) |
| OTU | OTUD7B | K63 | regulating mTORC2 signalling, thus relating to cell growth and metabolic disorders | activates Akt signaling and Kras-driven lung tumorigenesis in vivo | GβL | OTUD7B and TRAF2 regulate stability of GβL, which plays critical roles in mTORC2 signaling (Wang et al., 2017a) |
| UCH | BAP1 | monoubiquitination | inhibiting cell apoptosis | mediating tumor suppression in both mice and humans | H2A | Inactivation of BAP1 causes apoptosis through regulating H2A monoubiquitination, regulating tumor suppression (He et al., 2019) |
| USP | USP4 | K63 | activating inflammation and immune response | inhibiting TNFα-induced cancer cell migration | TRAF2/TRAF6 | USP4 negatively regulates the TRAF2- and TRAF6-stimulated NF-κB activation, and inhibits cancer cell migration (Xiao et al., 2012) |
| USP | CYLD | K63 | regulating NF-κB-mediated inflammation | associating the development of head and neck squamous cell carcinomas | NEMO | TRAF3/CYLD complex regulate NF-κB transcriptional level, which is associated with head and neck squamous cell carcinomas with HPV infection (Chen et al., 2017b) |
| USP | CYLD | K63/M1 | regulating Innate Immune Signaling | tumor suppressor | RIPK2 | CYLD counteracts Met1-Ub and Lys63-Ub conjugated to Ripk2, and this deubiquitinase activity plays an important role in innate immune regulation (Hrdinka et al., 2016) |
| USP | USP8 | K63 | DNA damage response | genomic instability in cancer | BRIT1 | USP8 rescues BRIT1 from K63 ubiquitin and regulates its recruitment to DNA double-strand break sites (Ge et al., 2015) |
| USP | USP10 | K63 | controlling cell cycle | promoting proliferation of t chronic myeloid leukemia cells | Bcr-Abl | SKP2 acts as co-regulator of K63-linked ubiquitination of Bcr-Abl for its activation. While USP10 deubiquitinates and stabilizes SKP protein levels and amplifies Bcr-Abl activation in chronic myeloid leukemia cells (Liao et al., 2019) |
| USP | USP20 | K63 | negatively regulating inflammation, cell proliferation, and apoptosis | promoting adult T cell leukemia (ATL) development | Tax | USP20 targets and deubiquitinates TRAF6 and TAX, negatively regulating NF-κB signaling (Yasunaga et al., 2011) |
| USP | USP17 | K63 | enhancing inflammation and promoting macrophage recruitment | promoting lung cancer growth | cIAP1/2 | USP17 interacted with and disrupted the TRAF2/TRAF3 complex through reducing K63-linked ubiquitination of TRAF2 and TRAF6. This activity positively drives stemness and inflammation in lung cancer (Lu et al., 2018) |
| USP | CYLD | K63 | regulating inflammation | promoting tumor growth | TAK1 | Itch-Cyld complex sequentially cleaving K63-linked ubiquitin chain on Tak1 thus terminating the inflammatory response (Ahmed et al., 2011) |
| USP | CYLD | K63 | negative regulate the NF-κB pathway | tumor suppressor | E6 | HPV E6 suppresses the CYLD under hypoxic conditions, promoting unrestricted NF-κB activation and allowing for malignant progression of tumors (An et al., 2008) |
| USP | CYLD | K63 | controling inflammation | inhibiting tumor formation | Bcl-3 | Cyld erases K63-linked polyubiquitin chains from Bcl-3, inactivating NF-κB signaling (Massoumi et al., 2006) |
| USP | CYLD | K63 | controls survival and inflammation | inhibiting tumor cell Proliferation | TRAF2 | Cyld regulates inflammation through deubiquitinating TRAF2 and blocking NF-κB pathway (Massoumi et al., 2006) |
| USP | USP14 | K63 | inflammation | acute colitis and colitis-associated colon cancer development | p100/p52 | TRAM14 recruits USP14 to cleave K63-linked ubiquitin chains of p100/p52, regulating NF-κB-mediated autophagy and innate immunity. (Chen et al., 2020) |
| USP | USP1 | K63 | regulating macroautophagy/autophagy | affecting breast cancer cell growth | ULK1 | USP1 modulates ULK1 K63-linked deubiquitination, and regulates autophagy, also affects breast cancer cell growth relying on autophagy (Raimondi et al., 2019) |
| USP | USP1 | K63 | Double-strand breaks (DSBs) | potential tumour suppressor | histones | USP1 actively destorys K63-linked poly-ubiquitin chains on histones. And its recruitment to damage sites has a close link with genome stability and double-strand breaks (Ha et al., 2017) |
| USP | USP34 | K63 | genome stability maintenance | promoting ES-cell differentiation and tumour formation | H2A | USP34 stabilizes RNF168, recruiting repair proteins at DSBs, which, is critical for genome stability (Sy et al., 2013) |
| USP | CYLD | K63/M1 | DNA damage-induced apoptosis | enhancing sensitivity to chemodrug in cancer cells | NEMO | CYLD downregulates K63-linked and linear ubiquitination of NEMO, promoting apoptosis (Niu et al., 2011) |
| USP | CYLD | K63 | activating the cell death pathway | regulating ATLL cell death | RIPK1 | CYLD erases k63-linked ubiquitin chains from RIPK1, which activates the cell death pathway and activates CYLD and RIPK1-dependent tumor cell death in ATLL (Xu et al., 2020) |
| USP | USP38 | K63 | restoring genome stability | regulating cancer cell response to genotoxic insults | HDAC1 | USP38 preferentially removed the K63-linked ubiquitin chains from HDAC1, regulating genomic stability (Yang et al., 2020) |
| UBQLN4 | K63/K48 | homologous recombination repair | predictor of poor survival in various cancer entities | MRE11 | Overexpression of UBQLN4 represses homologous recombination activity through inhibiting MRE11 ubiquitination, thus presenting close relationship with survival rates in various cancer (Jachimowicz et al., 2019) | |
| OTU | ZRANB1 | K29/K33 | promoting autophagosome maturation | inhibiting cell growth in hepatocellular carcinoma | UVRAG | ZRANB1 specifically cleaves SMURF1-induced K29 and K33-linked polyubiquitin chains from UVRAG, regulating autophagosome maturation and HCC growth (Feng et al., 2019) |
| JAMM | POH1 | K63 | double-strand DNA break responses, embryonic stem cell differentiation | promoting tumour formation in human hepatocellular carcinomas (HCCs) | E2F1 | POH1 binds to and stabilizes the E2F1, upregulating Survivin and FOXM1 protein levels, accompanied by accelerating tumor growth (Wang et al., 2015) |
| USP | USP30 | K6 | regulating mitophagy and neurodegenerative disease | functions in hepatocellular carcinoma | USP30 specifically cleaves the Lys6 linked ubiquitin chains, regulating mitophagy, apoptosis and tumorigenesis | |
| OTUD1 | OTUD1 | K63 | suppressing intestinal inflammation | NF-kB signaling-related cancers | RIPK1 | OTUD1 prefercially cleaves K63-linked polyubiquitin chains from RIPK1, inhibiting colonic inflammation and NF-κB signaling |
| USP | CYLD | K63 | regulating ERK activation | regulating cancer cell growth, proliferation, migration | ERK1/2 | CYLD cleaves K63-linked ubiquitination mediated by TRIM1, regulating ERK signaling and the assocaited cancer development |
| USP | USP10 | K63 | inhibiting NSCLC cell proliferation and migration | PTEN | USP10 suppress NSCLC cell proliferation and migration through abolishing PTEN from K63-linked polyubiquitination mediated by TRIM25 | |
| OTU | A20 | K63 | anti-inflammatory effects | tumor suppressor | TBK1 | A20 inhibits TBK1 activation through reducing K63-linked ubiquitination of Nrdp1, regulating inflammation |
Summary of the Identified DUBs Involving in Tumorigenesis.
2.2 Linear (M1) Linkage
2.2.1 The LUBAC Complex Encodes the M1 Linkage
Emerging evidences connect Met1-linked ubiquitin chains to NF-κB signaling, which enables physiological regulation of inflammation and immune responses (Emmerich et al., 2011; Borghi et al., 2018). Indeed, mutations and deficiencies involved in the formation and dissolution of Met1-linked poly-ubiquitin chains have been extensively illustrated in immune-related disorders (Tokunaga and Iwai, 2012; Fiil et al., 2013; Fiil and Gyrd-Hansen, 2014). Until now, linear ubiquitin chain assembly complex (LUBAC) is the only known E3 ubiquitin ligase assembling this type of chain (Kirisako et al., 2006; Walczak et al., 2012). The multi-subunit E3 ligase comprises catalytically active hybrid organic-inorganic perovskite (HOIP, also known as RNF31) and two adaptor proteins, HOIP1L (also known as RBCK1) and SHANK-associated RH domain-interacting protein (SHARPIN) (Tokunaga et al., 2011). Both HoIL-1 and SHARPIN can co-operate with HOIP, which might be a central part of the LUBAC complex (Ikeda et al., 2011; Elton et al., 2015). The HOIP orthologue, linear ubiquitin E3 ligase (LUBEL), modifies Kenny with M1-linked linear ubiquitin chains in Drosophila and is indispensable for inflammatory responses by activating Imd pathway (Aalto et al., 2019). Additionally, HOIP, with the help of cIAP1, is recruited to the linear ubiquitination of the TNFR2 signaling complex and activates canonical NF-κB, thereby facilitating cancer progression (Borghi et al., 2018). Multiple investigations found that HOIP, presumably through M1-linked ubiquitination, was proved to be connected with sorts of malignancies, including breast and prostate cancer (Guo et al., 2015; Zhu et al., 2016). Furthermore, previous experiments highlight that HOIL-1 interacts with HOIP, which adds a Mi-linked poly-ubiquitin chain to specific NF-κB signaling proteins, suggesting its link to a diversity of immune disorders, antiviral signaling (Elton et al., 2015), iron and xenobiotic metabolism (Elton et al., 2015), apoptosis (Emmerich et al., 2011; Lewis et al., 2015; Sasaki and Iwai, 2015), and cancer (Queisser et al., 2014; Taminiau et al., 2016). Another member of the LUBAC family, SHARPIN, is a novel component of the LUBAC complex. Spontaneous mutation of this tiny gene led to dysregulation of the NF-κB signaling pathway through linear ubiquitinate of NEMO (the key modulator of NF-κB). This systematic linear ubiquitination is obvious and can induce immune system disorders in SHARPIN-deficient mice (Tokunaga et al., 2011). Interestingly, SHARPIN-containing complexes can also interact with NEMO to activate NF-κB pathway (Ikeda et al., 2011). Moreover, NEMO was identified to be modified by LUBAC, generating M1-linked chains that were recognized by the UBAN domain of NEMO, causing conformational changes in the intertwined helices of NEMO dimers (Haas et al., 2009; Belgnaoui et al., 2012; Tokunaga and Iwai, 2012; Noad et al., 2017). NF-κB signaling regulates human cellular activities in different ways and is balanced by ubiquitination and deubiquitination (Adhikari et al., 2007; Lork et al., 2017) (Table 2).
2.2.2 OTULIN Dissembles M1-Linked Poly-Ubiquitin Chains
OTULIN, a methionine 1 (M1)-specific deubiquitinase (DUB), is a rare member of the OTU family of DUBs. Its proximal ubiquitin moiety cannot break down isopeptide linkages of ubiquitin chains, but can efficiently cleave peptide bonds present in the linear chains (Keusekotten et al., 2013; Rivkin et al., 2013). OTULIN presents negative regulation in the cellular process of immune homeostasis and inflammation (Damgaard et al., 2019). Depletion of OTULIN resulted in an increase in the formation of linear Ub chains and demonstrated proteasome dysregulation as the cause of NF-κB positive activation, which in turn restricts bacterial proliferation (Takiuchi et al., 2014; van Wijk et al., 2017). Moreover, the deficiency of OTULIN led to the inability to remove M1-linked poly-ubiquitin signals, which are typically conjugated by the LUBAC, resulting in LUBAC degradation and dysregulation of TNF signaling and cell death (Tokunaga, 2013; Damgaard et al., 2016). Notably, the function of LUBAC is controlled by cylindromatosis (CYLD), which interacts with the LUBAC core subunit HOIP to generate Met1-linked ubiquitin. However, this interaction can be weakened by the Met1-Ub-specific deubiquitinase OTULIN. In addition, through the deubiquitinase function of OTULIN, LUBAC can regulate Met1-Ub to ensure an advisable response to innate immune activity (Elliott et al., 2016; Hrdinka et al., 2016). The CYLD/TRAF3 complex has also been reported to regulate NF-κB-mediated inflammation and interferon signaling, which defines a subset of head and neck cancers that harbor human papillomavirus (Chen T. et al., 2017). Notably, accumulating evidence has manifested that linear ubiquitin chains play essential roles in ensuring appropriate activity of inflammatory responses and innate immune signaling (Tokunaga and Iwai, 2012; Tokunaga, 2013; Jing et al., 2017). The linear ubiquitination of cFLIP is directly induced by RNF31, a catalytic subunit of LUBAC, at Lys-351 and Lys-353, contributing to TNFα-induced apoptosis, thereby protecting cells from apoptosis (Tang et al., 2018) (Table 3).
TABLE 3
| E3 | Linkage | Phenotypic Characteristics | Neoplastic Implications | Substrate | Mechanism Summary |
|---|---|---|---|---|---|
| RNF8 | K63 | regulating DNA double-strand break responses | BRCA1 Tumor Suppressor Recruitment | histone | RNF8 interact with Ubc13, generating K63-linked ubiquitin chains on histone, which positively regulate DNA double-strand break and BRCA1 recruitment (Hodge et al., 2016) |
| RNF8 | K63 | DNA damage repair and cytokinesis | genomic instability of ATL cells | Tax | Stimulated by Tax, RNF8 and Ubc13:Uev1A function together, generating K63-pUb chains, whichactivated TAK and NF-κB signaling (Zhi et al., 2020) |
| HOIL-1 | M1 | immune regulation | NF-kB activation in cancers | NEMO | HOIL-1 modifies NF-κB core proteins with linear ubiquitin chains, regulating NF-κB pathway signaling (Elton et al., 2015) |
| CHIP | K6 | suppressing of cell death | FADD | CHIP triggers K6-linked polyubiquitylation of FADD, leading to the suppression of cell death (Seo et al., 2018) | |
| BRCA1 | K6 | DNA damage response | tumor suppressor | BRCA1 autoubiquitination | UBXN1binds to BRCA1 active site and decorate it with K6-linked polyubiquitin chains in a UBA-domain-dependent manner and BRCA1/BARD1 complex is regulated by the ubiquitinate status of BRCA1 (Wu-Baer et al., 2010) |
| BRCA1 | K6 | DNA repair, transcrip- tional regulation, and cell cycle checkpoint control | tumor suppressor | BRCA1 autoubiquitination | BRCA1 mediates autoubiquitination by conjugating to K6-linked polymers, which impart cellular properties (Wu-Baer et al., 2003) |
| BRCA1 | K6 | DNA double-stranded breaks repair | tumor suppressor | might be BRCA1 autoubiquitination? | BRCA1 recruits its autoubiquitination at DNA damage sites, which is dependented on K6-linked linkage. BRCA1:BARD1 enzyme activity is regulated by BRCA1 ubiquitin status. (Morris and Solomon, 2004) |
| BRCA1 | K6 | regulating DNA repair, transcriptional leves, cell cycle and cell apoptosis | tumor suppressor | BRCA1 autoubiquitination | BRCA1-BARD1 regulate BRCA1 autoubiquitination by preferentially mediating K6-linked polyubiquitin chains (Nishikawa et al., 2004) |
| Hectd3 | K27, K29 | leading to NF-kB activation | NF-κB associated cancer | Malt1 | Hectd3 promotes K27 and K29 polyubiquitination on Malt1, regulating autoimmunity and other Th17-related diseases (Cho et al., 2019) |
| WWP1 | K27 | suppressing the dimerization, membrane recruitment | restoring tumor-suppressive activity | PTEN | WWP1 triggers K27-linked polyubiquitination of PTEN to regulate subcellular localization cancer susceptibility syndromes (Lee et al., 2019c) |
| TRAF4 | K27, K29 | facilitating immune cell migration | promoting cancer cell invasion | TrkA | TRAF4 promotes K27 and K29-linked ubiquitin linkages on TrkA, facilitating prostate cell invasion (Singh et al., 2018) |
| RNF4 | K63 | DNA damage repair | potential prostate, breast and lung cancer target | Trim5α | Ube2w interacts with RNF4, promoting monoubiquitination of SUMO chains, which are further modified to form K63-linked ubiquitin chains (Maure et al., 2016) |
| RNF8 | K63 | DNA damage response | breast cancer predisposition | H2A/H2AX | RNF8 activated with Ubc13, promoting K63-linked polyubiquitin conjugation to histones H2A/H2AX, then contributing to breast cancer predisposition (Vuorela et al., 2011) |
| Skp2 | K63 | promoting survival and Akt-mediated glycolysis | restricting cancer cell progression | Akt | Skp2/SCF complex catalyzes K63-linked ubiquitination chains on Akt, which is required for glycolysis and cancer development (Chan et al., 2013) |
| Skp2 | K63 | controling cell cycle | promoting proliferation of chronic myeloid leukemia cells | Bcr-Abl | SKP2 triggers K63-linked ubiquitination of Bcr-Abl, regulating downstream signaling, and is vital for chronic myeloid leukemia development and progression (Liao et al., 2019) |
| RNF113A | K63 | DNA repair | potentially associating with tumor progression | BRR2 | RNF113A interacts with BRR2 through K63-linked polyubiquitin, mediating repairment of DNA alkylation damage (Brickner et al., 2017) |
| TRAF2 | K63 | mediating several cell growth and metabolic pathways | facilitating tumorigenesis | GβL | TRAF2 promotes K63-linked polyubiquitination of GβL, and regulats mTORC2 signalling, thus mediating several cell growth and metabolic pathways (Wang et al., 2017a) |
| TRIM37 | mono-ubiquitination | regulating transcriptional repression | promoting transformation in breast cancer | H2A | TRIM37 mono-ubiquitinates histone H2A, thus associating with transcriptional repression (Bhatnagar et al., 2014) |
| RNF8/RNF168 | K63 | DNA double-strand breaks (DSBs) | mediating ATM-dependent carcinogenesis | H2A/H2AX | RNF8 and RNF168 combined together to catalyze K63-linked poly-Ub chains on H2A/H2AX, which is important for transcription and DNA double-strand breaks (Shanbhag et al., 2010) |
| HectH9 | K63 | regulating transcriptional activation and repression | tumor cell Proliferation | Myc | HectH9 recruits 63-linked polyubiquitin chains to Myc, modulating cell proliferation in various tumor cells (Adhikary et al., 2005) |
| Bcl10 | K63 | activating the NF-κB pathway | NF-κB associated cancer | NEMO | UBC13 and Bcl10function together inducing NEMO ubiquitination through lysine-63-linked ubiquitination, and subsequent NF-κB activation (Zhou et al., 2004) |
| RNF8 | K63 | DNA repair | tumour-promoting | probably hisotne H1 | In p97–ATX3 activated conditions, RNF8 mediates K63-Ub at sites of DNA lesions, regulating genome instability, cell invasion and metastasis (Singh et al., 2019) |
| PARK2/Parkin | K33 | fine-tune necroptosis and inflammation | tumor suppressor/inflammation-associated tumorigenesis | RIPK3 | AMPK activated Parkin/RIPK3 complex through K33-linked polyubiquitination, which negatively regulates necroptosis and inflammation-associated tumorigenesis (Lee et al., 2019b) |
| TRAF6 | K63/K27 | maintaining nuclear genome integrity | promoting cancer progression | hDNA2 | hTRAF6 catalyzes the K27- and K63-linked polyubiquitination of hDNA2, maintaining nuclear genome integrity and the associated cancer biology (Meng et al., 2019) |
| HectH9 | K63 | integrating glycolysis activation and apoptosis resilience | regulating tumor metabolism and cancer stem cell expansion | HK2 | HectH9 catalyzes HK2’s K63-linked ubiquitination, regulating stem cell expansion and CSC-induced chemoresistance in prostate cancer (Lee et al., 2019a) |
| TRAF6 | K63 | regulating inflammation and immunity | promoting liver tumorigenesis and correlates with poor prognosis | HDAC3 | TRAF6 ubiquitinates HDAC3 with K63-linked ubiquitin chains, regulating inflammation and malignant transformation and progression in HCC(Wu et al., 2020) |
| ITCH | K27 | immune response | promoting proliferation and invasion of melanoma cells | BRAF | Activated ITCH maintains BRAF activity and subsequent MEK/ERK signaling through Lysine 27-linked ubiquitination, enhancing proliferation and invasion of melanoma cells (Yin et al., 2019) |
| TRAF6 | K63 | immunity | anti-tumor immunity in the cancer setting | FOXP3 | TRAF6 bind to and facilitates Regulatory T cells (Tregs) activties through K63-linked ubiquitination at lysine 262, acting as aTreg-stabilizing regulator and playing crucial roles in immune control and anti-tumor immunity (Ni et al., 2019) |
| SMURF1 | K29/K33 | promoting autophagosome maturation | inhibiting cell growth in hepatocellular carcinoma | UVRAG | SMURF1 mediates K29 and K33-linked polyubiquitin chains on UVRAG, promoting autophagosome maturation and inhibiting hepatocellular carcinoma growth (Feng et al., 2019) |
| TRAF6 | K63 | NF-κB activation and autophagy activation | cancer cell migration, cell invasion | BECN1/TRAF6 | PRDX1 negatively regulates TRAF6 ubiquitin-ligase activity, leading to NF-κB inactivation and autophagy activation (Min et al., 2018) |
| RNF8 | K63 | DNA double-strand break repair | regulating L3MBTL2 mutation in leukemia | L3MBTL2 | MDC1 recruites L3MBTL2 to sites of DNA lesion and is then ubiquitylated by RNF8, promoting DNA DSB repair and regulating L3MBTL2-induced cancers (Nowsheen et al., 2018) |
| Itch | K63 | regulating tissue patterning, stem cell maintenance | modulating medulloblastoma tumorigenesis | SuFu | Itch/β-arrestin2 complex mediates Lys63-linked polyubiquitylation on SuFu, thus controling Hedgehog signalling and medulloblastoma tumorigenesis (Infante et al., 2018) |
| FBXO32 | K63 | brain development | promoting tumorigenicity and metastasis in humans | CtBP1 | FBXO32 directly ubiquitinates CtBP1 with K63-linked ubiquitin chains, and this interaction activity regulates downstream EMT signaling and is essential for tumor metastasis and brain development (Sahu et al., 2017) |
| Cullins | K29 | promoting cell motility | modulating cell migration | hnRNP A1 | SPSB1 catalyzes K29-linked polyUb chains on hnRNP A1, modulating cell migration and cell motility in EGF signaling (Wang et al., 2017b) |
| HUWE1 | K63 | preventing DNA damage accumulation | colonic tumour suppressor | Myc | Huwe1 mediates MYC transactivation activity via K63-linked ubiquitination, inhibiting accumulation of DNA damage and preventing tumour initiation especially in colonic cancers (Myant et al., 2017) |
| HectH9 | K63 | relating to embryonic lethal | promoting hypoxia-induced tumour progression | HAUSP(USP7) | HectH9 mediates K63-polyubiquitin chains conjugated to HAUSP. HAUSP then deubiquitinates HIF-1α, promoting hypoxia-induced tumour progression (Wu et al., 2016) |
| RNF8 | K63 | conferring chemoresistance | tumor-promoting function | Twist | RNF8 activates and ubiquitinate Twist, leading to subsequent EMT and CSC functions, thus exerting tumor-promoting functions such as cell migration and invasion (Lee et al., 2016) |
| FBXW7 | K63 | genome integrity | tumor suppressor | XRCC4 | FBXW7 firstly phosphorylated by ATM and then it ubiquitylates XRCC4 via K63-linkage, promoting NHEJ repair, which is closely related to DSB and genomic stability (Zhang et al., 2016) |
| Trim7 | K63 | regulating proliferation and apoptosis | promoting Ras-mediated lung adenocarcinoma | RACO-1 | Trim7 catalyzes Lys63-linked ubiquitination of RACO-1 in response to RAS signaling, and Trim7 overexpression increases lung tumour burden while knockdown of Trim7 reduces tumour growth in xenografts models (Chakraborty et al., 2015) |
| Skp2 | K63 | regulating energy metabolism, proliferation, apoptosis, and cell polarity | tumor growth in HCC | LKB1 | Skp2-dependent activation of LKB1 through K63-linked Ubiquitination is essential for HCC tumor growth and related to poor survival outcomes (Lee et al., 2015) |
| TRAF6 | K63 | enhancing chemotherapeutic efficacy | promoting T-ALL progression | MCL1 | IRAK1/4 signaling activated TRAF6, mediating K63-linked ubiquitination of MCL1, promoting T-ALL progression (Li et al., 2015) |
| PELI1 | K63 | maintenance of autoimmunity | promoting lymphomagenesis | BCL6 | PELI1 specifically binds to BCL6 and induces lysine 63-linked ubiquitin chains on BCL6, promoting lymphomagenesis, modulating the maintenance of autoimmunity through TLR and TCR signaling (Park et al., 2014) |
| MEKK1 | K63 | embryonic survival | promoting ES-cell differentiation and tumour formation | TAB1 | MEKK1 ubiquitylates TAB1 with Lys63-linked polyubiquitin in a PHD motif-dependent manner, inducing TAK1 and MAPK activation, which are crucial for ES-cell differentiation and tumourigenesis (Charlaftis et al., 2014) |
| TRAF4 | K63 | regulating immunity | driving Breast Cancer Metastasis | TβRI | TβRI-receptor TRAF4 interacts with each other, triggering Lys 63-linked TRAF4 polyubiquitylation and TAK1 activation, promoting cell migration and metastasis in breast cancer (Zhang et al., 2013a) |
| RNF8/RNF168 | K63 | maintaining genome stability | suppressing tumourigenesis | BLM | RNF8/RNF168, triggers BLM activation, leading to BLM recruiting to the ubiquitin-interacting motifs of RAP80, which is vital to maintain genome stability and suppressing tumourigenesis (Tikoo et al., 2013) |
| RFP | K27 | inhibiting apoptosis and promoting cell survival and proliferation |
tumor suppression | PTEN | E3 ubiquitin ligase RFP interacts with PTEN through K27-linked ubiquitination diminishing the effect of AKT signaling, involving in tumor suppression regulation (Lee et al., 2013) |
| FAAP20 | K63 | DNA damage repair and genome maintenance | leading to hematologic defects and cancer in patients | FANCA | FAAP20 binds K-63–linked ubiquitin chains in a UBZ domain-dependent manner, modulating DNA damage repair and genome maintenance (Ali et al., 2012) |
| LUBAC | M1 | DNA damage-induced apoptosis | enhancing sensitivity to chemodrug in cancer cells | NEMO | LUBAC-mediated NEMO linear ubiquitination promotes TAK1 and IKK activation, protecting cells from DNA damage-induced apoptosis (Niu et al., 2011) |
| TRAF4 (RNF83) | K63 | DNA damage | overcome chemoresistance in colorectal cancer | CHK1 | CHK1 K63-linked ubiquitination is mediated by TRAF4, which is essential for CHK1phosphorylation and activation during DNA damage response, and is close to cell proliferation, colony formation in colorectal cancer (Yu et al., 2020b) |
| LUBAC | M1 | regulating cell activation and death | promoting breast cancer | NEMO | Epsins 1 and 2 interact with LUBAC, promoting NEMO linear ubiquitination and resulting in breast cancer development (Song et al., 2021) |
| DZIP3 | K63 | regulating cell cycle | promoting cancer cell growth, migration, and invasion | Cyclin D1 | DZIP3/hRUL138 stabilizes and ubiquitinated Cyclin D1 protein through K63-linked ubiquitination, and closely related with cell cycle progression, cancer cell growth, invasion, migration (Kolapalli et al., 2021) |
| RNF138 | K63 | driving NF-kB activation and innate immunity | promoting NF-kB activation in lymphomas | MYD88L265P | RNF138 triggers K63-linked polyubiquitination of MYD88L265P, resulting in constitutive activation of NF-κB and the associated lymphomagenesis (Yu et al., 2020a) |
| RNF181 | K63 | endocrine resistance | facilitating breast cancer progression | ERα protein | RNF181 functions as E3 enzymes through K63-linked ubiquitination, which facilitates breast cancer progression (Zhu et al., 2020) |
| TRIM11 | mono-ubiquitination | regulating estrogen-dependent gene expression | promoting cell growth and migration | Erα | TRIM11 interacts with the N terminal of ERα and maintains ERα stability through mono-ubiquitination, thus promoting cell growth and proliferation in breast cancer (Tang et al., 2020) |
| HUWE1 | K27-, K29- | regulating DNA damage response | promoting radio-resistance of prostate cancer cells | JMJD1A | HUWE1 mediates the K27-/K29-linked ubiquitination of JMJD1A, enhancing c-Myc activity, promoting DSB repair and sensitizing the response of prostate cancer (Fan et al., 2020) |
| RNF6 | K63 | maintaining nuclear receptors | promoting cell proliferation | glucocorticoid receptor (GR) | RNF6 stabilize GR genes and enhances its transcriptional activity by catalyzing its K63-linked polyubiquitination, promoting MM cell proliferation and survival (Ren et al., 2020) |
| TRIM27 | suppressing cell senescence | cell cycle dysregulation, tumor cell proliferation and migration | p21 | TRIM27 regulates cell apoptosis, cell senescence through mediating the ubiquitination of p21 in breast cancer (Xing et al., 2020) | |
| SPOP | K27 | increasing DNA replication stress | sensitizing cancer cells to ATR inhibition | Geminin | SPOP binding Geminin catalyzes K27-linked poly-ubiquitination of Geminin, preventing DNA replication over-firing and sensitizing cancer cells to ATR inhibition (Ma et al., 2021) |
| NF-X1 | K33 | regulating glycine metabolism | preventing glioma tumor growth | GLDC | Acetylation of GLDC inhibits its enzymes activity, and facilitates K33-linked ubiquitination by NF-X1, regulating glycine metabolism and tumorgenesis (Liu et al., 2021) |
| HUWE1 | K63 | promoting c-Myc activity | promoting retinoblastoma cell proliferation | c-Myc | HELZ2 triggers K63-linked ubiquitination activity of c-Myc by HUWE1 to mediate retinoblastoma tumorgenesis (Dai et al., 2021) |
| RNF8 | K63 | activating AKT pathway | promoting lung cancer cell proliferation and resistance to chemotherapy | Akt | RNF8 mediates K63-linked ubiquitination of Akt, promoting lung cancer cell proliferation and resistance to DNA damage (Xu et al., 2021) |
| TRIM31 | K63 | stabilizing and activating p53 | inhibiting breast cancer progression | p53 | TRIM31 directly ubiquitinates p53 with K63-linked ubiquitination through its RING domain, activating p53 pathway, suppressing breast cancer progression (Guo et al., 2021) |
| TRIM15 | K63 | activating NF-κB and Akt signaling pathway | regulating cancer cell growth, proliferation, migration | ERK1/2 | TRIM15 mediates K63-linked polyubiquitination of ERK1/2, then activating ERK signaling, leading to cell proliferation, migration and differentiation (Zhu et al., 2021) |
| NEDD4 | K63 | regulating cell-cell adhesion, mechanosensing and autophagy | involving in angiogenesis and tumor growth | IGPR-1 | NEDD4 and UbcH6 are involved in the K63-linked ubiquitination of IGPR-1, regulating different cellular activities (Sun et al., 2021) |
| TRIM25 | K63 | activating AKT/mTOR signaling | promoting NSCLC cell survival and tumor growth | PTEN | TRIM25 directly interacts with PTEN and catalyzes its K63-linked ubiquitination, modulating PTEN signaling and involving in cell survival and tumor growth in NSCLC (He et al., 2022) |
| TRIM41 | K63 | innate antiviral response | NF-κB associated cancer | BCL10 | TRIM41 modifies K63-linked polyubiquitination of BCL10, activating NF-κB and TBK1 signaling pathway (Yu et al., 2021) |
| HECTD3 | K63 | regulating inflammation | NF-κB associated cancer | TRAF3 | HECTD3 interacts with TRAF3 via K63-linked polyubiquitination, reducing inflammation and faciliating NF-κB inflammation pathway (Zhou et al., 2021) |
| DZIP3 | K63 | driving cell cycle | promoting cancer progression | Cyclin D1 | DZIP3 stabilizes Cyclin D1 by promoting K63-linked ubiquitination of Cyclin D1, driving cell cycle and cancer progression (Kolapalli et al., 2021) |
| TRIM22 | K63 | activating NF-κB signaling | promoting glioblastoma tumor growth | IKKγ | TRIM22 promotes K63-linked ubiquitination of IKKγ, leading to degradation of IκBα and NF-κB activation (Ji et al., 2021) |
Summary of the E3 enzymes in Tumoregenesis.
2.2.3 Linear Ubiquitination in Tumorigenesis
M1-linked ubiquitination, specifically N-terminal Met1-linked ubiquitination, is able to form eight different inter-ubiquitin linkages via its N-terminal methionine (M1). It can be specifically catalyzed by LUBAC. For instance, EGFR recruits PKP2 to the plasma membrane and cooperates with LUBAC (HOIP), activating linear ubiquitination of NEMO, which is critical for tumor cell proliferation (Hua et al., 2021). Also, Epsins 1/2 promotes NEMO linear ubiquitination via LUBAC, driving breast cancer development (Song et al., 2021). Moreover, LUBAC (SHARPIN) regulated β-catenin activity through linear ubiquitination, promoting gastric tumorigenesis (Zhang et al., 2021). Interestingly, OTULIN exclusively cleaves M1-linked ubiquitination and exhibits a high affinity for linear ubiquitination. LUBAC and linear ubiquitination have been found to be relevant to TNF signaling. Interestingly, OTULIN was shown to remove linear ubiquitination from LUBAC-modified proteins, which is critical for various cellular activities (Draber et al., 2015). Moreover, the deubiquitinating enzyme, CYLD, was also identified for disassembly Met1-linked-Ub (mostly the immune system). A previous report demonstrated that modification of proliferating cell nuclear antigen (PCNA) induces apoptosis and inhibits tumor growth through the linear ubiquitin chain (Qin et al., 2018).
2.3 Lys63 Linkage
2.3.1 The Writer Enzymes for the Lys63 Linkage
It has been well established that Lys63 chains (ubiquitin chains topology lysine 63 poly-ubiquitin linkages) regulate and trigger distinct cellular signaling, including kinase activation, signal transduction, protein trafficking, endocytosis and DNA repair (Spence et al., 1995; Hofmann and Pickart, 1999; Komander and Rape, 2012; Wu and Karin, 2015; Hrdinka et al., 2016). The E2 conjugating enzyme complex Ubc13/Uev1A preferentially assembles the K63-pUb chain (Smith et al., 2013; Zhang et al., 2018). Furthermore, Tax can be recruited to K63-pUb by E3 ligase RNF8 and Ubc13/Uev1A, which allows the activation of TGFβ-activating kinase 1 (TAK1), followed by multiple downstream signaling pathways such as the IKK and JNK pathways. These ultimately lead to DNA damage repair, cytokinesis, and the genomic instability in ATL cells (Ho et al., 2015; Lee et al., 2017). Similarly, tumor necrosis factor receptor associated factor 6 (TRAF6) can interact with the E2 conjunction enzyme Ubc13/Uev1A in a RING-dependent manner, catalyzing Lys63-linked TRAF6 auto-ubiquitination. This activates IKK and NF-κB, thereby promoting TAK1 and IKK to trigger spontaneous osteoclast differentiation (Lamothe et al., 2007a; Lenoir et al., 2021). Another report also showed that TRAF6, in a RING-dependent fashion, catalyzed auto-ubiquitination by conjugating with ubc13/Uev1A, activating the AKT pathway, and promoting cell migration in breast cancer (Lamothe et al., 2007b; Niu et al., 2021).
The Ubc13/Uev1A complex has been shown to conjugate Lys63-linked poly-ubiquitination of substrate proteins, which contribute to breast cancer metastasis via NF-кB signaling regulation (Wu Z. et al., 2014; 2017). It is also reported that Ubc13 can catalyze K63-linked protein poly-ubiquitination, which is indispensable for the activation of non-SMAD signaling by TAK1 and p38, whose activity controls breast cancer metastatic spread and lung colonization (Wu X. et al., 2014). Interacting with Ubc13, RNF213 mediates autoubiquitination and controls inflammatory responses and angiogenic activities (Habu and Harada, 2021). RNF8 was demonstrated to activate Ubc13 and recruit K63-linked poly-ubiquitin conjugation to histones H2A/H2AX, thus contributing to breast cancer predisposition (Vuorela et al., 2011). Moreover, RNF8 utilizes the RING domain, mediating Lys63-linked Ub chains, which is required for DNA double-strand break (DSB) signaling and the downstream BRCA1 tumor suppressor recruitment or lung cancer cell proliferation (Hodge et al., 2016; Xu et al., 2021). Inhibiting the Ubc13/Uev1A complex specifically, which is critical for Lys63-linked ubiquitination, promotes ubiquitin conjugation at the Lys147 site, thereby upregulating NF-кB signaling in multiple myeloma and other cancers (Gallo et al., 2014). UbcH6 and NEDD4 regulate angiogenesis and tumor growth (Sun et al., 2021). Ube2w accompanies the E3 ligase RNF4 function in distinct DNA repair pathways through Lys63-linked chains and BRIC6 (also named BRUCE) acting on K63-linked ubiquitinylate in unstimulated cells, which regulates the DNA double-strand break response through bridging USP8 and BRCT-repeat inhibitor of hTERT expression (BRIT1) in a deubiquitination manner (Ge et al., 2015; Maure et al., 2016). Poly-ubiquitination of histone H1 depends on Ubc13 and RNF8, which prolong pre-existing ubiquitin modifications to K63-linked chains, thereby stimulating RNF8-Ubc13 mediated DNA damage response (Mandemaker et al., 2017). SKP2 triggers non-proteolytic K63-linked ubiquitination, which is crucial for cancer initiation and progression by positively regulating cancer cell survival and glycolysis. The depletion of SKP2 restricts cancer stem cell proliferation and survival (Chan et al., 2013). However, the non-proteolytic K63-linked ubiquitination triggered by SKP2 can be reversed and modulated by debiquitinase ovarian tumor domain-containing protein 1 (OTUD1) (Yao et al., 2018) and USP10 (Liao et al., 2019). USP10 has been identified as a novel deubiquitinase of SKP2 that modulates and stabilizes SKP2. Indeed, USP10 can recognize and remove Lys63-linked ubiquitin chains from Bcr-Abl, leading to positive activities in chronic myeloid leukemia cells. OTUD1 interacts with p53 and is essential for constant stabilization of p53. Its overexpression dramatically induces the cell cycle and apoptosis (Mevissen et al., 2013; Piao et al., 2017). In addition, several more DUBs have been defined as having linkage specificity for Lys63-linked ubiquitination. CYLD, a tumor suppressor, inhibits NF-κB signaling by cleaving K63-linked ubiquitination of NEMO/IKKγ, thus reducing its stability and averting the IKK complex from phosphorylation of IκB (Chen T. et al., 2017). Furthermore, CYLD was associated with the catalytic LUBAC subunit HOIP to counteract Lys63-Ub and Met1-Ub conjugation to receptor-interacting protein kinase 2 (RIPK2), leading to restriction of innate immune signaling and cytokine production (Hrdinka et al., 2016). TRAF-binding protein domain (TRABID) was demonstrated to bind and cleave Lys63-linked ubiquitin moieties on APC tumor suppressor substrates, which led to the disruption of APC and activation of Wnt signaling in colorectal cancer cell lines (Tran et al., 2008). The USP17/TRAF2/TRAF3 complex acts to stabilize its client proteins, enhancing inflammatory responses and stemness in lung cancer cells (Lu et al., 2018). USP20 deubiquitinates TRAF6 and Tax, and may function as a key regulator of adult T cell leukemia (ATL) leukemogenesis through suppressing NF-κB activation (Yasunaga et al., 2011). In terms of actively removing Lys63-linked poly-ubiquitin chains on GβL, OTUD7B appears to be the primary regulator in governing mTOR2 integrity, which is essential for NF-κB activation in cancer biology (Wang B. et al., 2017). Further detailed analysis of Lys63-linked ubiquitination is required to better understand how ubiquitin chains function in numerous cellular events. However, identification of this linkage will provide an ideal point to understand the potential mechanism in the cellular regulation of the incoming ubiquitin research (Table 1, 2).
2.3.2 Disassembly of Lys63 Poly-Ubiquitin Chains by DUBs
Although a number of DUBs have been identified, the disassembly ability of the substrate and its physiological role, especially for Lys63 poly-ubiquitin chains, is poorly defined. Previous studies have shown that K63-specific DUBs, A20, and CYLD present the anti-tumor effects by regulating NF-κB signaling (Heyninck and Beyaert, 2005; Sun, 2010; Meng et al., 2021; Zhu et al., 2021). USP10 reduces K63-linked ubiquitination chains and functions as a tumor suppressor (He et al., 2021). Stimulated by the pro-inflammatory cytokine TNF, A20 exerts two major functions: sequential de-ubiquitination and catalyzing receptor-interacting protein (RIP), resulting in downregulation of NF-κB signaling. Absence of A20 led to hyper-accumulation of Lys63 poly-ubiquitin in NF-κB signaling. Like A20, CYLD disassembles the Lys63 poly-ubiquitin recruiting signal and degrades IKKκ. In this way, USP4 and OTUD1 cleaves K63-linked ubiquitination chains, and regulates downstream NF-κB signaling (Fan et al., 2011; Liang et al., 2013; Wu et al., 2022) (Table 3).
2.3.3 The Multifaceted Functions of Lys63 Poly-Ubiquitin Chains in Cancer
Lys63-linked chains have been described as non-degradative signals and are the core of inflammatory system and the NF-κB pathway, which is involved in either mono-ubiquitination or poly-ubiquitination. TGFβ type I receptor (TBRI) was shown to interact with TRAF6 through Lys63-dependent poly-ubiquitination in order to promote tumor invasion (Mu et al., 2011). It has also been reported that polo-like kinase 1 (PLK1) phosphorylates kruppel-like factor 4 (KLF4), leading to the recruitment of TRAF6, resulting in Lys63-linked ubiquitination and promoting nasopharyngeal tumorigenesis (Mai et al., 2019). Moreover, TRAF6 functions in hepatocarcinogenesis by regulating TRAF6/HDAC3/c-Myc signal axis. Mechanistically, TRAF6 interacts with histone deacetylase 3 (HDAC3) via Lys63-linked ubiquitin chains, which enhances c-Myc stability, and its overexpression has been proposed to facilitate HCC progression (Wu et al., 2020). Similarly, HUWE1 promotes K63-linked ubiquitinatination of c-Myc, thereby promoting retinoblastoma cell proliferation (Dai et al., 2021). It was also demonstrated that ASK1-dependent phosphorylation and lys63-linked poly-ubiquitination of LKB1 is critical for its activation. Tankyrase and RNF146 act as upstream regulators of the LKB1/AMPK pathway to promote Lys63-linked ubiquitination resulting in AMPK activation and tumor suppression (Li et al., 2019). Lys63-linked non-proteolytic ubiquitination of Hexokinase 2 by HectH9 effectively disrupts glycolysis activation and facilitates apoptosis in prostate cancer cells (Lee H. J. et al., 2019). SET domain bifurcated histone lysine methyltransferase 1 (SETDB1) methylates Akt and functions as a scaffold to recruit JMJD2A, which then binds TRAF6 and Skp2-SCF to the Akt complex, thereby promoting tumor development in lung cancer (Wang et al., 2019). Notably, numbers of E3 ligase have been discovered to catalyze K63-linked ubiquitination chains, which are involved in activating NF-κB or Akt signaling pathway, acting as tumor promoters and facilitating tumor progression (Ji et al., 2021; Xu et al., 2021; Yu et al., 2021; Zhou et al., 2021; Zhu et al., 2021; He et al., 2022). Lys63 poly-ubiquitin chains were also found to be involved in driving the cell cycle and promoting cancer progression (Kolapalli et al., 2021). In contrast, tripartite motif-containing 31 (TRIM31) directly ubiquitinates p53 via K63-linked ubiquitination, resulting in tumor-suppressing effects (Guo et al., 2021) (Table 2).
2.4 Lys6 Linkages–Tumor Suppressors
Lys6-linked ubiquitin chains are less abundant in resting cells and their functional implications are unclear (Durcan et al., 2014; Michel et al., 2017). It has been reported that BRCA1 can be autoubiquitinated and then bound by UBX domain containing protein 1 (UBXN1) through K6-linked poly-ubiquitin chains. Interestingly, UBXN1 regulates BRCA1 expression upon ubiquitination. Autoubiquitinated forms of BRCA1 act as tumor suppressors and inhibit their enzymatic function (Wu-Baer et al., 2010). Moreover, BRCA1 auto-ubiquitination occurs in a way that the BRCA1/BARD1 complex conducts polymerization by conjugation with K6-linked polymers, which imparts cellular properties to its natural enzymatic substrates (Wu-Baer et al., 2003), further linking BRCA1 auto-ubiquitination to the tumor suppressor. C terminus HSC70-interacting protein (CHIP) was reported to bind to fas-associated protein with death domain (FADD) to induce K6-linked poly-ubiquitination of FADD, which was demonstrated to be essential for the prevention of cell death (Seo et al., 2018).
It has also been revealed that community-based learning collaborative (CBLC) assembles K6- and K11-linked poly-ubiquitin on EGFR and positively regulates its stability. The sustained activation of EGFR is largely dependent on CBLC-mediated ubiquitination, and its dysregulation is preferentially destined for membrane recycling, which plays an important role in non-small-cell lung carcinoma (NSCLC) progression (Hong et al., 2018).
Structural findings indicated that USP30 efficiently cleaves Lys6-linked Ub chains, which abrogates parkin-mediated Ub-chain formation in mitochondria. Dysfunction of this novel distal Ub-recognition mechanism is associated with physiological disorders such as hepatocellular carcinoma (Sato et al., 2017; Wang et al., 2022). Although an extraordinary progress has been made over the last 2 decades, the detailed functional consequences of Lys6 modifications require further investigation (Tables 2, 3).
2.5 Lys27 linkages—emerging Tumor Promoter
Emerging investigations have demonstrated that K27-linked poly-ubiquitination is crucial for promoting cancer development, such as facilitating cell proliferation, invasion, and metastasis (Peng et al., 2011; Yin et al., 2019). ITCH, an E3 ubiquitin ligase, was shown to generate Lys27-linked poly-ubiquitination of the transcription factor TIEG1, which inhibits TIEG1 nuclear translocation and subsequent Treg development (Peng et al., 2011). Additionally, TGF-β, in the presence of cytokine IL-6, can efficiently promote mono- and poly-ubiquitination of TIEG1 and modulate Treg/Th17 differentiation (Peng et al., 2011). Yet, it seems that tumor immunity in TIEG1−/− mice were apparently enhanced by hampering Treg development and increasing Th17 response, suggesting its pro-tumor effects (Peng et al., 2011). In the presence of proinflammatory cytokines, ITCH can catalyze BRAF to disrupt 14–3–3-mediated inhibition of BRAF kinase activity, resulting in ΜEK/ERK signaling activation. This ubiquitin function plays an essential role in supporting the proliferation and invasion abilities of melanoma cells (Yin et al., 2019). Another report connected Lys27-linked ubiquitination to melanoma cell invasive properties, in which HECT domain and ankyrin repeat containing E3 ubiquitin protein ligase 1 (HACE1) decorated fibronection with Lys27 Ub moieties (El-Hachem et al., 2018). As mentioned in this study, upregulation of fibronectin in turn modulates the transcription levels of integrin subunit alpha V (ITGAV) and integrin β1 (ITGB1), leading to an increased invasive power of melanoma cells (El-Hachem et al., 2018). TRAF4 was found to increase TrkA kinase activity through K27- and K29-linked ubiquitination upon nerve growth factor (NGF) stimulation, followed by the recruitment of downstream adaptor proteins and increased metastasis in prostate cancer (Singh et al., 2018).
Strikingly, reports have also revealed that Lys27-linked ubiquitination is implicated in the DNA damage response (DDR) and innate immunity. A previous study demonstrated that RNF168 can target the N-terminal tail of histones H2A/H2A.X, generating Lys27-linked ubiquitin chains. In response to DNA damage, K27 ubiquitination is the major source of PTMs that mark chromatin. Meanwhile, the activition of DDR can be inhibited by mutation of K27, which hinders the localization of 53BP1 and BRCA1 to DDR foci (Gatti et al., 2015). DDR is responsible for the recognition, signal transduction and repair of DNA damage. The inactivation of DDR will result in the accumulation of cell mutations and the increase of genomic instability, which play an essential role in cancer initiation (Klinakis et al., 2020). It is also indicated that K27 and the related DDR mediators may be the potential targets for the development of anti-tumor drugs. cGAS is subjected to K27 poly-ubiquitination by RNF185, facilitating cGAS-mediated innate immune response. TRIM-mediated ubiquitination by binding to residue K27 activates TBK1 recruitment to mitochondrial antiviral signaling (MAVS) and promotes innate immunity (Wang Q. et al., 2017). TRIM31 and TRIM40 can also mediate K27-linked ubiquitination, thereby regulating innate and adaptive immunity (Wang X. et al., 2021; Shen et al., 2021). Specifically, TRIM40 interacts with Riok3, resulting in RIG-I and MDA5 degradation through K27-linked ubiquitination, and negatively regulates innate immunity (Shen et al., 2021). Nevertheless, TRIM31 catalyzes K27-linked ubiquitination of SYK, facilitating antifungal immunity (Wang X. et al., 2021). Additionally, auto-ubiquitination of TRIM23 through K27-linked ubiquitination was found to mediate autophagy via activation of TBK1 (Sparrer et al., 2017). Moreover, E3 ubiquitin ligase Hectd3 decorates Stata3 with non-degradative K27-linked poly-ubiquitin chains and Malt1 with K27/K29-linked poly-ubiquitin chains, leading to signaling-related ubiquitination in neuroinflammation (Cho et al., 2019) (Table 3). In fact, the innate immune system plays a key role in the formation of tumor. The response of it is generally affected by a variety of immune cells and cytokines in tumor microenvironment (Wenbo and Wang, 2017). In this way, it is beneficial to clarify the regulatory effects of K27 on tumor innate immunity for understanding the mechanisms of cancer.
2.6 Lys29 and Lys33 linkage—possible Tumor Promoters
Recent proteomic data have identified the role of Lys29-and Lys33-linked ubiquitin chains in various biological processes, including the control of AMPK-mediated mitochondrial function and Wnt-induced transcription signaling (Jiang et al., 2015; Nusse and Clevers, 2017). AMPK-related kinases, AMPK-related kinase 5 (ARK5, also known as NUAK1) and microtubule-affinity-regulating kinase 4 (MARK4), which are mediated by unconventional Lys29/Lys33 linkage, are involved in cell polarity and proliferation (Al-Hakim et al., 2008). One of the underlying mechanisms is that USP9X specifically identifies Lys29/Lys33-conjugated ubiquitin chains on NUAK1 and MARK4 (Al-Hakim et al., 2008). However, it should be noted that the ubiquitination of NUAK1 and MARK4 suppresses their phosphorylations rather than restores their stability and facilitates LKB1 activation. Interestingly, the TRABID core domain N-terminal Npl4-like zinc finger (NAZF1) preferentially hydrolyzes K29/K33-linked diUb, and this novel AnkUBD displays TRABID linkage specificity (Licchesi et al., 2011). Additionally, TRABID interacts with APC tumor suppressor protein, recruits TCF target genes, and activates their transcription in colorectal cancer cells (Tran et al., 2008). The OUT family DUB TRABID preferentially abolishes Smad ubiquitination regulatory factor 1-induced K29/K33-linked poly-ubiquitin chains from UV radiation resistance associated gene (UVRAG), thereby promoting autophagosome maturation and inhibiting cell proliferation in hepatocellular cancer (Feng et al., 2019). Considering its linkage specificity, it would be insightful to explore the potential positive regulation of TRABID in cancer tumorigenesis via these two pathways.
K29-linked ubiquitin chains play important roles in driving cancer invasion and metastasis, as well as in the positive regulation of immunity (Singh et al., 2018; Cho et al., 2019; Gao et al., 2021). Several studies have also manifested that, with the assistance of Cbl-b and ITCH, T cell receptor-zeta (TCR-zeta) was decorated with a K33 linage, accompanied by positively activated T cells and immune responses (Huang et al., 2010). In addition, OTUD1 directly deubiquitinates the inhibitor SMAD7 of TGF-β pathway and aborates Lys33-linked poly-ubiquitin chains, which inhibits cell stemness and suppresses metastasis (Zhang et al., 2017). Acetylation of GLDC inhibits its enzymatic activity and facilitates K33-linked ubiquitination by NF-X1, thereby suppressing glioma tumor growth (Liu et al., 2021). Ultimately, Lys29-and Lys33-linked ubiquitin chains appear to have more complicated cellular functions that remain to be characterized (Table 2, 3).
2.7 Mixed Linkage and Branched Poly-Ubiquitination
Mixed linkage chains send mixed signaling messages that can be identified by different linkage-specific receptors. Our understanding of the mixed linkages and branched poly-ubiquitin is limited. Previous reports have elucidated that Tax in combination with UbcH2, Uhc5c, or UbcH7, can catalyze the construction of free mixed-linkage poly-ubiquitin chains, which are responsible for IKK-NF-κB activation and induction of T cell transformation (Wang et al., 2016). Furthermore, Brcc 36 isopeptidase complex (BRISC), the JAMM/MPN + family of DUBs, preferentially cleaves K63 linkages within mixed-linkage chains. In addition, RING1B was found to generate atypical mixed poly-ubiquitin chains and mediate mono-ubiquitination of H2A (Ben-Saadon et al., 2006). A recent study showed that SPOP triggered mixed-linkage ubiquitination of MyD88 in human lymphoma cells and mouse HSCs, suggesting that the SPOP-MyD88 pathway plays a critical role in hematopoietic neoplasms (Jin et al., 2020).
3 Conclusion and Furture Perspectives
Non-proteolytic ubiquitination, the molecular switch in cell fate regulation, plays a crucial role in post-translational protein modifications. Diverse ubiquitination enzymes are essential for ubiquitination linkages that are necessary for normal metabolism and physiological functions. Meanwhile, it is also the root cause of physiological disorders, such as cancer. The aberrant regulation of the UPS is typically achieved by ubiquitination enzymes, DUBs, 20S proteasome catalytic core particles and 19S proteasome regulatory particles (Rape, 2018). In general, specific ubiquitination enzymes determine ubiquitin linking with one of the seven lysine residues to form distinctive styles of poly-ubiquitin chains, deciding the fate of substrate proteins. It is noteworthy that the ubiquitin proteasome system functions as a theoretical target for drug screening, and the study of ubiquitination will provide more insights into the development of anti-tumor drugs. Recently, the quantities of E3s inhibitors have already been processed in preclinical models of cancer immunotherapy. It has been shown that E3 enzyme Smac mimetics (SMs) were the promising immune modulators for cancer therapy as the antagonists targeting E3 ligases IAPs (Cossu et al., 2019). The small molecule inhibitor AMG-232, targeting another E3-ubiquitin ligase oncogenic mouse double minute 2 homolog (MDM2), was shown to strengthen T cell killing of cancer cells, especially when combined with an anti-PD-1 monoclonal antibody (Sahin et al., 2020). Several other proteasome inhibitors have been confirmed to be clinically effective against malignancies as well. The neddylation (NAE) inhibitor pevonedistat has already been tested in multiple clinical trials and has shown positive effects in patients with AML or advanced solid tumors (Barghout and Schimmer, 2021). Small molecule inhibitors based on deubiquitinase have been widely used in experimental anti-tumor therapy, most of which are still in the preclinical research stage. Due to dose-limiting toxicity, the first deubiquitinase inhibitor VLX1570 was terminated in the clinical trial phase. No other deubiquitinase inhibitors have been approved for clinical studies since then. Also, the discovered deubiquitinase inhibitors related to tumor treatment are mainly concentrated in the USP family, and the relationship between the inhibitors of non-USP family members and the treatment of malignant tumors needs to be further studied (Zhang et al., 2022). Interestingly, the innovative approaches of proteolysis targeting chimeras (PROTACs) and molecular glues might facilitate clinical cancer therapy (Cruz Walma et al., 2022; Kung and Weber, 2022; Zhou and Xu, 2022). Consequently, it is essential further to investigate the role of ubiquitination enzymes in tumorigenesis. Meanwhile, targeting different ubiquitination, including K6-, K27-, K29-, K33-, K63-, and M1-linked poly-ubiquitination, may also be one of the directions for cancer drug discovery. Although great progresses have been achieved with the development of anti-cancer drugs aimed at the UPS, numerous challenges still stand in the way. Some candidate inhibitors have emerged as drug resistant or have limited efficacy in patients. Despite these questions, the discovery of new drugs targeting single or multiple segments of the UPS is still worthy of future research.
Statements
Author contributions
Conceptualization, SJ and JH; writing original draft preparation, XY; writing review and editing, QL, FL, SJ and JH; visualization: XT and TY; supervision: SJ and JH; funding acquisition: SJ and XY. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by the National Natural Science Foundation of China (grant nos. 81873249 and 82074360) and the Young Taishan Scholars Program of Shandong Province (grant no. tsqn201909200) and Shandong Province Medicine and Health Project (grant no2019WS091).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Aalto A. L. Mohan A. K. Schwintzer L. Kupka S. Kietz C. Walczak H. (2019). M1-linked Ubiquitination by LUBEL Is Required for Inflammatory Responses to Oral Infection in Drosophila. Cell Death Differ.26 (5), 860–876. 10.1038/s41418-018-0164-x
2
Abbas T. Dutta A. (2011). CRL4Cdt2: Master Coordinator of Cell Cycle Progression and Genome Stability. Cell Cycle10 (2), 241–249. 10.4161/cc.10.2.14530
3
Adhikari A. Xu M. Chen Z. J. (2007). Ubiquitin-mediated Activation of TAK1 and IKK. Oncogene26 (22), 3214–3226. 10.1038/sj.onc.1210413
4
Adhikary S. Marinoni F. Hock A. Hulleman E. Popov N. Beier R. et al (2005). The Ubiquitin Ligase HectH9 Regulates Transcriptional Activation by Myc and Is Essential for Tumor Cell Proliferation. Cell123 (3), 409–421. 10.1016/j.cell.2005.08.016
5
Ahmed N. Zeng M. Sinha I. Polin L. Wei W. Z. Rathinam C. et al (2011). The E3 Ligase Itch and Deubiquitinase Cyld Act Together to Regulate Tak1 and Inflammation. Nat. Immunol.12 (12), 1176–1183. 10.1038/ni.2157
6
Akturk A. Wasilko D. J. Wu X. Liu Y. Zhang Y. Qiu J. et al (2018). Mechanism of Phosphoribosyl-Ubiquitination Mediated by a Single Legionella Effector. Nature557 (7707), 729–733. 10.1038/s41586-018-0147-6
7
Akutsu M. Dikic I. Bremm A. (2016). Ubiquitin Chain Diversity at a Glance. J. Cell Sci.129 (5), 875–880. 10.1242/jcs.183954
8
Al-Hakim A. K. Zagorska A. Chapman L. Deak M. Peggie M. Alessi D. R. (2008). Control of AMPK-Related Kinases by USP9X and Atypical Lys(29)/Lys(33)-Linked Polyubiquitin Chains. Biochem. J.411 (2), 249–260. 10.1042/bj20080067
9
Ali A. M. Pradhan A. Singh T. R. Du C. Li J. Wahengbam K. et al (2012). FAAP20: a Novel Ubiquitin-Binding FA Nuclear Core-Complex Protein Required for Functional Integrity of the FA-BRCA DNA Repair Pathway. Blood119 (14), 3285–3294. 10.1182/blood-2011-10-385963
10
Alpi A. F. Patel K. J. (2009). Monoubiquitylation in the Fanconi Anemia DNA Damage Response Pathway. DNA Repair (Amst)8 (4), 430–435. 10.1016/j.dnarep.2009.01.019
11
An J. Mo D. Liu H. Veena M. S. Srivatsan E. S. Massoumi R. et al (2008). Inactivation of the CYLD Deubiquitinase by HPV E6 Mediates Hypoxia-Induced NF-kappaB Activation. Cancer Cell14 (5), 394–407. 10.1016/j.ccr.2008.10.007
12
Barghout S. H. Schimmer A. D. (2021). E1 Enzymes as Therapeutic Targets in Cancer. Pharmacol. Rev.73 (1), 1–58. 10.1124/pharmrev.120.000053
13
Bax M. McKenna J. Do-Ha D. Stevens C. H. Higginbottom S. Balez R. et al (2019). The Ubiquitin Proteasome System Is a Key Regulator of Pluripotent Stem Cell Survival and Motor Neuron Differentiation. Cells8 (6). 10.3390/cells8060581
14
Behrends C. Harper J. W. (2011). Constructing and Decoding Unconventional Ubiquitin Chains. Nat. Struct. Mol. Biol.18 (5), 520–528. 10.1038/nsmb.2066
15
Belgnaoui S. M. Paz S. Samuel S. Goulet M. L. Sun Q. Kikkert M. et al (2012). Linear Ubiquitination of NEMO Negatively Regulates the Interferon Antiviral Response through Disruption of the MAVS-TRAF3 Complex. Cell Host Microbe12 (2), 211–222. 10.1016/j.chom.2012.06.009
16
Ben-Saadon R. Zaaroor D. Ziv T. Ciechanover A. (2006). The Polycomb Protein Ring1B Generates Self Atypical Mixed Ubiquitin Chains Required for its In Vitro Histone H2A Ligase Activity. Mol. Cell24 (5), 701–711. 10.1016/j.molcel.2006.10.022
17
Bhatnagar S. Gazin C. Chamberlain L. Ou J. Zhu X. Tushir J. S. et al (2014). TRIM37 Is a New Histone H2A Ubiquitin Ligase and Breast Cancer Oncoprotein. Nature516 (7529), 116–120. 10.1038/nature13955
18
Bomar M. G. D'Souza S. Bienko M. Dikic I. Walker G. C. Zhou P. (2010). Unconventional Ubiquitin Recognition by the Ubiquitin-Binding Motif within the Y Family DNA Polymerases Iota and Rev1. Mol. Cell37 (3), 408–417. 10.1016/j.molcel.2009.12.038
19
Borghi A. Haegman M. Fischer R. Carpentier I. Bertrand M. J. M. Libert C. et al (2018). The E3 Ubiquitin Ligases HOIP and cIAP1 Are Recruited to the TNFR2 Signaling Complex and Mediate TNFR2-Induced Canonical NF-Κb Signaling. Biochem. Pharmacol.153, 292–298. 10.1016/j.bcp.2018.01.039
20
Brickner J. R. Soll J. M. Lombardi P. M. Vagbo C. B. Mudge M. C. Oyeniran C. et al (2017). A Ubiquitin-dependent Signalling axis Specific for ALKBH-Mediated DNA Dealkylation Repair. Nature551 (7680), 389–393. 10.1038/nature24484
21
Buetow L. Huang D. T. (2016). Structural Insights into the Catalysis and Regulation of E3 Ubiquitin Ligases. Nat. Rev. Mol. Cell Biol.17 (10), 626–642. 10.1038/nrm.2016.91
22
Cai F. Chen P. Chen L. Biskup E. Liu Y. Chen P. C. et al (2014). Human RAD6 Promotes G1-S Transition and Cell Proliferation through Upregulation of Cyclin D1 Expression. PLoS One9 (11), e113727. 10.1371/journal.pone.0113727
23
Chakraborty A. Diefenbacher M. E. Mylona A. Kassel O. Behrens A. (2015). The E3 Ubiquitin Ligase Trim7 Mediates C-Jun/AP-1 Activation by Ras Signalling. Nat. Commun.6, 6782. 10.1038/ncomms7782
24
Chan C. H. Morrow J. K. Li C. F. Gao Y. Jin G. Moten A. et al (2013). Pharmacological Inactivation of Skp2 SCF Ubiquitin Ligase Restricts Cancer Stem Cell Traits and Cancer Progression. Cell154 (3), 556–568. 10.1016/j.cell.2013.06.048
25
Chang J. H. Xiao Y. Hu H. Jin J. Yu J. Zhou X. et al (2012). Ubc13 Maintains the Suppressive Function of Regulatory T Cells and Prevents Their Conversion into Effector-like T Cells. Nat. Immunol.13 (5), 481–490. 10.1038/ni.2267
26
Charlaftis N. Suddason T. Wu X. Anwar S. Karin M. Gallagher E. (2014). The MEKK1 PHD Ubiquitinates TAB1 to Activate MAPKs in Response to Cytokines. EMBO J.33 (21), 2581–2596. 10.15252/embj.201488351
27
Chatr-Aryamontri A. van der Sloot A. Tyers M. (2018). At Long Last, a C-Terminal Bookend for the Ubiquitin Code. Mol. Cell70 (4), 568–571. 10.1016/j.molcel.2018.05.006
28
Chen M. Zhao Z. Meng Q. Liang P. Su Z. Wu Y. et al (2020). TRIM14 Promotes Noncanonical NF-kappaB Activation by Modulating P100/p52 Stability via Selective Autophagy. Adv. Sci. (Weinh)7 (1), 1901261. 10.1002/advs.201901261
29
Chen S. Jing Y. Kang X. Yang L. Wang D. L. Zhang W. et al (2017a). Histone H2B Monoubiquitination Is a Critical Epigenetic Switch for the Regulation of Autophagy. Nucleic Acids Res.45 (3), 1144–1158. 10.1093/nar/gkw1025
30
Chen T. Zhang J. Chen Z. Van Waes C. (2017b). Genetic Alterations in TRAF3 and CYLD that Regulate Nuclear Factor kappaB and Interferon Signaling Define Head and Neck Cancer Subsets Harboring Human Papillomavirus. Cancer123 (10), 1695–1698. 10.1002/cncr.30659
31
Chen Y. He L. Peng Y. Shi X. Chen J. Zhong J. et al (2015). The Hepatitis C Virus Protein NS3 Suppresses TNF-α-Stimulated Activation of NF-Κb by Targeting LUBAC. Sci. Signal8 (403), ra118. 10.1126/scisignal.aab2159
32
Chitale S. Richly H. (2017). Timing of DNA Lesion Recognition: Ubiquitin Signaling in the NER Pathway. Cell Cycle16 (2), 163–171. 10.1080/15384101.2016.1261227
33
Cho J. J. Xu Z. Parthasarathy U. Drashansky T. T. Helm E. Y. Zuniga A. N. et al (2019). Hectd3 Promotes Pathogenic Th17 Lineage through Stat3 Activation and Malt1 Signaling in Neuroinflammation. Nat. Commun.10 (1), 701. 10.1038/s41467-019-08605-3
34
Choudhary C. Kumar C. Gnad F. Nielsen M. L. Rehman M. Walther T. C. et al (2009). Lysine Acetylation Targets Protein Complexes and Co-regulates Major Cellular Functions. Science325 (5942), 834–840. 10.1126/science.1175371
35
Choudhry H. Zamzami M. A. Omran Z. Wu W. Mousli M. Bronner C. et al (2018). Targeting microRNA/UHRF1 Pathways as a Novel Strategy for Cancer Therapy. Oncol. Lett.15 (1), 3–10. 10.3892/ol.2017.7290
36
Clague M. J. Urbé S. Komander D. (2019). Breaking the Chains: Deubiquitylating Enzyme Specificity Begets Function. Nat. Rev. Mol. Cell Biol.20 (6), 338–352. 10.1038/s41580-019-0099-1
37
Cole A. J. Dickson K. A. Liddle C. Stirzaker C. Shah J. S. Clifton-Bligh R. et al (2021). Ubiquitin Chromatin Remodelling after DNA Damage Is Associated with the Expression of Key Cancer Genes and Pathways. Cell Mol. Life Sci.78 (3), 1011–1027. 10.1007/s00018-020-03552-5
38
Cooray S. N. Guasti L. Clark A. J. (2011). The E3 Ubiquitin Ligase Mahogunin Ubiquitinates the Melanocortin 2 Receptor. Endocrinology152 (11), 4224–4231. 10.1210/en.2011-0147
39
Cossu F. Milani M. Mastrangelo E. Lecis D. (2019). Targeting the BIR Domains of Inhibitor of Apoptosis (IAP) Proteins in Cancer Treatment. Comput. Struct. Biotechnol. J.17, 142–150. 10.1016/j.csbj.2019.01.009
40
Cruz Walma D. A. Chen Z. Bullock A. N. Yamada K. M. (2022). Ubiquitin Ligases: Guardians of Mammalian Development. Nat. Rev. Mol. Cell Biol.23 (5), 350–367. 10.1038/s41580-021-00448-5
41
Dai H. Zeng W. Zeng W. Yan M. Jiang P. Li Y. et al (2021). HELZ2 Promotes K63-Linked Polyubiquitination of C-Myc to Induce Retinoblastoma Tumorigenesis. Med. Oncol.39 (1), 11. 10.1007/s12032-021-01603-w
42
Damgaard R. B. Elliott P. R. Swatek K. N. Maher E. R. Stepensky P. Elpeleg O. et al (2019). OTULIN Deficiency in ORAS Causes Cell Type-specific LUBAC Degradation, Dysregulated TNF Signalling and Cell Death. EMBO Mol. Med.11 (3). 10.15252/emmm.201809324
43
Damgaard R. B. Walker J. A. Marco-Casanova P. Morgan N. V. Titheradge H. L. Elliott P. R. et al (2016). The Deubiquitinase OTULIN Is an Essential Negative Regulator of Inflammation and Autoimmunity. Cell166 (5), 1215–1230. 10.1016/j.cell.2016.07.019
44
Dammer E. B. Na C. H. Xu P. Seyfried N. T. Duong D. M. Cheng D. et al (2011). Polyubiquitin Linkage Profiles in Three Models of Proteolytic Stress Suggest the Etiology of Alzheimer Disease. J. Biol. Chem.286 (12), 10457–10465. 10.1074/jbc.M110.149633
45
Darling S. Fielding A. B. Sabat-Pośpiech D. Prior I. A. Coulson J. M. (2017). Regulation of the Cell Cycle and Centrosome Biology by Deubiquitylases. Biochem. Soc. Trans.45 (5), 1125–1136. 10.1042/bst20170087
46
David Y. Ternette N. Edelmann M. J. Ziv T. Gayer B. Sertchook R. et al (2011). E3 Ligases Determine Ubiquitination Site and Conjugate Type by Enforcing Specificity on E2 Enzymes. J. Biol. Chem.286 (51), 44104–44115. 10.1074/jbc.M111.234559
47
Deng R. Zhang H. L. Huang J. H. Cai R. Z. Wang Y. Chen Y. H. et al (2020). MAPK1/3 Kinase-dependent ULK1 Degradation Attenuates Mitophagy and Promotes Breast Cancer Bone Metastasis. Autophagy, 1–19. 10.1080/15548627.2020.1850609
48
Di Lello P. Hymowitz S. G. (2016). Unveiling the Structural and Dynamic Nature of the Ubiquitin Code. Structure24 (4), 498–499. 10.1016/j.str.2016.03.013
49
Dikic I. Wakatsuki S. Walters K. J. (2009). Ubiquitin-binding Domains - from Structures to Functions. Nat. Rev. Mol. Cell Biol.10 (10), 659–671. 10.1038/nrm2767
50
Dittmar G. Selbach M. (2017). Deciphering the Ubiquitin Code. Mol. Cell65 (5), 779–780. 10.1016/j.molcel.2017.02.011
51
Dittmar G. Winklhofer K. F. (2019). Linear Ubiquitin Chains: Cellular Functions and Strategies for Detection and Quantification. Front. Chem.7, 915. 10.3389/fchem.2019.00915
52
Dondelinger Y. Darding M. Bertrand M. J. Walczak H. (2016). Poly-ubiquitination in TNFR1-Mediated Necroptosis. Cell Mol. Life Sci.73 (11-12), 2165–2176. 10.1007/s00018-016-2191-4
53
Dong X. Gong Z. Lu Y. B. Liu K. Qin L. Y. Ran M. L. et al (2017). Ubiquitin S65 Phosphorylation Engenders a pH-Sensitive Conformational Switch. Proc. Natl. Acad. Sci. U. S. A.114 (26), 6770–6775. 10.1073/pnas.1705718114
54
Draber P. Kupka S. Reichert M. Draberova H. Lafont E. de Miguel D. et al (2015). LUBAC-recruited CYLD and A20 Regulate Gene Activation and Cell Death by Exerting Opposing Effects on Linear Ubiquitin in Signaling Complexes. Cell Rep.13 (10), 2258–2272. 10.1016/j.celrep.2015.11.009
55
Durcan T. M. Tang M. Y. Pérusse J. R. Dashti E. A. Aguileta M. A. McLelland G. L. et al (2014). USP8 Regulates Mitophagy by Removing K6-Linked Ubiquitin Conjugates from Parkin. Embo J.33 (21), 2473–2491. 10.15252/embj.201489729
56
Eger S. Scheffner M. Marx A. Rubini M. (2010). Synthesis of Defined Ubiquitin Dimers. J. Am. Chem. Soc.132 (46), 16337–16339. 10.1021/ja1072838
57
El-Hachem N. Habel N. Naiken T. Bzioueche H. Cheli Y. Beranger G. E. et al (2018). Uncovering and Deciphering the Pro-invasive Role of HACE1 in Melanoma Cells. Cell Death Differ.25 (11), 2010–2022. 10.1038/s41418-018-0090-y
58
Elliott P. R. Leske D. Hrdinka M. Bagola K. Fiil B. K. McLaughlin S. H. et al (2016). SPATA2 Links CYLD to LUBAC, Activates CYLD, and Controls LUBAC Signaling. Mol. Cell63 (6), 990–1005. 10.1016/j.molcel.2016.08.001
59
Elton L. Carpentier I. Verhelst K. Staal J. Beyaert R. (2015). The Multifaceted Role of the E3 Ubiquitin Ligase HOIL-1: beyond Linear Ubiquitination. Immunol. Rev.266 (1), 208–221. 10.1111/imr.12307
60
Emanuelli A. Manikoth Ayyathan D. Koganti P. Shah P. A. Apel-Sarid L. Paolini B. et al (2019). Altered Expression and Localization of Tumor Suppressive E3 Ubiquitin Ligase SMURF2 in Human Prostate and Breast Cancer. Cancers (Basel)11 (4). 10.3390/cancers11040556
61
Emmerich C. H. Schmukle A. C. Walczak H. (2011). The Emerging Role of Linear Ubiquitination in Cell Signaling. Sci. Signal4 (204), re5. 10.1126/scisignal.2002187
62
Fan L. Xu S. Zhang F. Cui X. Fazli L. Gleave M. et al (2020). Histone Demethylase JMJD1A Promotes Expression of DNA Repair Factors and Radio-Resistance of Prostate Cancer Cells. Cell Death Dis.11 (4), 214. 10.1038/s41419-020-2405-4
63
Fan Y. H. Yu Y. Mao R. F. Tan X. J. Xu G. F. Zhang H. et al (2011). USP4 Targets TAK1 to Downregulate TNFalpha-Induced NF-kappaB Activation. Cell Death Differ.18 (10), 1547–1560. 10.1038/cdd.2011.11
64
Feng X. Jia Y. Zhang Y. Ma F. Zhu Y. Hong X. et al (2019). Ubiquitination of UVRAG by SMURF1 Promotes Autophagosome Maturation and Inhibits Hepatocellular Carcinoma Growth. Autophagy15 (7), 1130–1149. 10.1080/15548627.2019.1570063
65
Fiil B. K. Damgaard R. B. Wagner S. A. Keusekotten K. Fritsch M. Bekker-Jensen S. et al (2013). OTULIN Restricts Met1-Linked Ubiquitination to Control Innate Immune Signaling. Mol. Cell50 (6), 818–830. 10.1016/j.molcel.2013.06.004
66
Fiil B. K. Gyrd-Hansen M. (2014). Met1-linked Ubiquitination in Immune Signalling. Febs J.281 (19), 4337–4350. 10.1111/febs.12944
67
Fottner M. Brunner A. D. Bittl V. Horn-Ghetko D. Jussupow A. Kaila V. R. I. et al (2019). Site-specific Ubiquitylation and SUMOylation Using Genetic-Code Expansion and Sortase. Nat. Chem. Biol.15 (3), 276–284. 10.1038/s41589-019-0227-4
68
Fradet-Turcotte A. Canny M. D. Escribano-Díaz C. Orthwein A. Leung C. C. Huang H. et al (2013). 53BP1 Is a Reader of the DNA-Damage-Induced H2A Lys 15 Ubiquitin Mark. Nature499 (7456), 50–54. 10.1038/nature12318
69
Fu Q. S. Song A. X. Hu H. Y. (2012). Structural Aspects of Ubiquitin Binding Specificities. Curr. Protein Pept. Sci.13 (5), 482–489. 10.2174/138920312802430581
70
Galan J. M. Haguenauer-Tsapis R. (1997). Ubiquitin Lys63 Is Involved in Ubiquitination of a Yeast Plasma Membrane Protein. Embo J.16 (19), 5847–5854. 10.1093/emboj/16.19.5847
71
Gallo L. H. Meyer A. N. Motamedchaboki K. Nelson K. N. Haas M. Donoghue D. J. (2014). Novel Lys63-Linked Ubiquitination of IKKβ Induces STAT3 Signaling. Cell Cycle13 (24), 3964–3976. 10.4161/15384101.2014.988026
72
Gao P. Ma X. Yuan M. Yi Y. Liu G. Wen M. et al (2021). E3 Ligase Nedd4l Promotes Antiviral Innate Immunity by Catalyzing K29-Linked Cysteine Ubiquitination of TRAF3. Nat. Commun.12 (1), 1194. 10.1038/s41467-021-21456-1
73
Gatti M. Pinato S. Maiolica A. Rocchio F. Prato M. G. Aebersold R. et al (2015). RNF168 Promotes Noncanonical K27 Ubiquitination to Signal DNA Damage. Cell Rep.10 (2), 226–238. 10.1016/j.celrep.2014.12.021
74
Ge C. Che L. Ren J. Pandita R. K. Lu J. Li K. et al (2015). BRUCE Regulates DNA Double-Strand Break Response by Promoting USP8 Deubiquitination of BRIT1. Proc. Natl. Acad. Sci. U. S. A.112 (11), E1210–E1219. 10.1073/pnas.1418335112
75
Geisler S. Holmström K. M. Skujat D. Fiesel F. C. Rothfuss O. C. Kahle P. J. et al (2010). PINK1/Parkin-mediated Mitophagy Is Dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol.12 (2), 119–131. 10.1038/ncb2012
76
Gilberto S. Peter M. (2017). Dynamic Ubiquitin Signaling in Cell Cycle Regulation. J. Cell Biol.216 (8), 2259–2271. 10.1083/jcb.201703170
77
Glauser L. Sonnay S. Stafa K. Moore D. J. (2011). Parkin Promotes the Ubiquitination and Degradation of the Mitochondrial Fusion Factor Mitofusin 1. J. Neurochem.118 (4), 636–645. 10.1111/j.1471-4159.2011.07318.x
78
Greer E. L. Brunet A. (2008). FOXO Transcription Factors in Ageing and Cancer. Acta Physiol. (Oxf)192 (1), 19–28. 10.1111/j.1748-1716.2007.01780.x
79
Guo J. Liu X. Wang M. (2015). miR-503 Suppresses Tumor Cell Proliferation and Metastasis by Directly Targeting RNF31 in Prostate Cancer. Biochem. Biophys. Res. Commun.464 (4), 1302–1308. 10.1016/j.bbrc.2015.07.127
80
Guo Y. Li Q. Zhao G. Zhang J. Yuan H. Feng T. et al (2021). Loss of TRIM31 Promotes Breast Cancer Progression through Regulating K48- and K63-Linked Ubiquitination of P53. Cell Death Dis.12 (10), 945. 10.1038/s41419-021-04208-3
81
Ha K. Ma C. Lin H. Tang L. Lian Z. Zhao F. et al (2017). The Anaphase Promoting Complex Impacts Repair Choice by Protecting Ubiquitin Signalling at DNA Damage Sites. Nat. Commun.8, 15751. 10.1038/ncomms15751
82
Haas T. L. Emmerich C. H. Gerlach B. Schmukle A. C. Cordier S. M. Rieser E. et al (2009). Recruitment of the Linear Ubiquitin Chain Assembly Complex Stabilizes the TNF-R1 Signaling Complex and Is Required for TNF-Mediated Gene Induction. Mol. Cell36 (5), 831–844. 10.1016/j.molcel.2009.10.013
83
Habu T. Harada K. H. (2021). UBC13 Is an RNF213-Associated E2 Ubiquitin-Conjugating Enzyme, and Lysine 63-linked Ubiquitination by the RNF213-UBC13 axis Is Responsible for Angiogenic Activity. FASEB Bioadv3 (4), 243–258. 10.1096/fba.2019-00092
84
Haglund K. Sigismund S. Polo S. Szymkiewicz I. Di Fiore P. P. Dikic I. (2003). Multiple Monoubiquitination of RTKs Is Sufficient for Their Endocytosis and Degradation. Nat. Cell Biol.5 (5), 461–466. 10.1038/ncb983
85
Hay-Koren A. Caspi M. Zilberberg A. Rosin-Arbesfeld R. (2011). The EDD E3 Ubiquitin Ligase Ubiquitinates and Up-Regulates Beta-Catenin. Mol. Biol. Cell22 (3), 399–411. 10.1091/mbc.E10-05-0440
86
He M. Chaurushiya M. S. Webster J. D. Kummerfeld S. Reja R. Chaudhuri S. et al (2019). Intrinsic Apoptosis Shapes the Tumor Spectrum Linked to Inactivation of the Deubiquitinase BAP1. Science364 (6437), 283–285. 10.1126/science.aav4902
87
He Y. Jiang S. Mao C. Zheng H. Cao B. Zhang Z. et al (2021). The Deubiquitinase USP10 Restores PTEN Activity and Inhibits Non-small Cell Lung Cancer Cell Proliferation. J. Biol. Chem.297 (3), 101088. 10.1016/j.jbc.2021.101088
88
He Y. M. Zhou X. M. Jiang S. Y. Zhang Z. B. Cao B. Y. Liu J. B. et al (2022). TRIM25 Activates AKT/mTOR by Inhibiting PTEN via K63-Linked Polyubiquitination in Non-small Cell Lung Cancer. Acta Pharmacol. Sin.43 (3), 681–691. 10.1038/s41401-021-00662-z
89
Heap R. E. Gant M. S. Lamoliatte F. Peltier J. Trost M. (2017). Mass Spectrometry Techniques for Studying the Ubiquitin System. Biochem. Soc. Trans.45 (5), 1137–1148. 10.1042/bst20170091
90
Heaton S. M. Borg N. A. Dixit V. M. (2016). Ubiquitin in the Activation and Attenuation of Innate Antiviral Immunity. J. Exp. Med.213 (1), 1–13. 10.1084/jem.20151531
91
Heideker J. Wertz I. E. (2015). DUBs, the Regulation of Cell Identity and Disease. Biochem. J.465 (1), 1–26. 10.1042/bj20140496
92
Hendriks I. A. Lyon D. Su D. Skotte N. H. Daniel J. A. Jensen L. J. et al (2018). Site-specific Characterization of Endogenous SUMOylation across Species and Organs. Nat. Commun.9 (1), 2456. 10.1038/s41467-018-04957-4
93
Herhaus L. Sapkota G. P. (2014). The Emerging Roles of Deubiquitylating Enzymes (DUBs) in the TGFβ and BMP Pathways. Cell Signal26 (10), 2186–2192. 10.1016/j.cellsig.2014.06.012
94
Heride C. Urbé S. Clague M. J. (2014). Ubiquitin Code Assembly and Disassembly. Curr. Biol.24 (6), R215–R220. 10.1016/j.cub.2014.02.002
95
Heyninck K. Beyaert R. (2005). A20 Inhibits NF-kappaB Activation by Dual Ubiquitin-Editing Functions. Trends Biochem. Sci.30 (1), 1–4. 10.1016/j.tibs.2004.11.001
96
Hicke L. Schubert H. L. Hill C. P. (2005). Ubiquitin-binding Domains. Nat. Rev. Mol. Cell Biol.6 (8), 610–621. 10.1038/nrm1701
97
Ho Y. K. Zhi H. Bowlin T. Dorjbal B. Philip S. Zahoor M. A. et al (2015). HTLV-1 Tax Stimulates Ubiquitin E3 Ligase, Ring Finger Protein 8, to Assemble Lysine 63-Linked Polyubiquitin Chains for TAK1 and IKK Activation. PLoS Pathog.11 (8), e1005102. 10.1371/journal.ppat.1005102
98
Hodge C. D. Ismail I. H. Edwards R. A. Hura G. L. Xiao A. T. Tainer J. A. et al (2016). RNF8 E3 Ubiquitin Ligase Stimulates Ubc13 E2 Conjugating Activity that Is Essential for DNA Double Strand Break Signaling and BRCA1 Tumor Suppressor Recruitment. J. Biol. Chem.291 (18), 9396–9410. 10.1074/jbc.M116.715698
99
Hodson C. Purkiss A. Miles J. A. Walden H. (2014). Structure of the Human FANCL RING-Ube2T Complex Reveals Determinants of Cognate E3-E2 Selection. Structure22 (2), 337–344. 10.1016/j.str.2013.12.004
100
Hofmann R. M. Pickart C. M. (1999). Noncanonical MMS2-Encoded Ubiquitin-Conjugating Enzyme Functions in Assembly of Novel Polyubiquitin Chains for DNA Repair. Cell96 (5), 645–653. 10.1016/s0092-8674(00)80575-9
101
Hong S. Y. Kao Y. R. Lee T. C. Wu C. W. (2018). Upregulation of E3 Ubiquitin Ligase CBLC Enhances EGFR Dysregulation and Signaling in Lung Adenocarcinoma. Cancer Res.78 (17), 4984–4996. 10.1158/0008-5472.Can-17-3858
102
Hrdinka M. Fiil B. K. Zucca M. Leske D. Bagola K. Yabal M. et al (2016). CYLD Limits Lys63- and Met1-Linked Ubiquitin at Receptor Complexes to Regulate Innate Immune Signaling. Cell Rep.14 (12), 2846–2858. 10.1016/j.celrep.2016.02.062
103
Hu M. Li P. Li M. Li W. Yao T. Wu J. W. et al (2002). Crystal Structure of a UBP-Family Deubiquitinating Enzyme in Isolation and in Complex with Ubiquitin Aldehyde. Cell111 (7), 1041–1054. 10.1016/s0092-8674(02)01199-6
104
Hua F. Hao W. Wang L. Li S. (2021). Linear Ubiquitination Mediates EGFR-Induced NF-kappaB Pathway and Tumor Development. Int. J. Mol. Sci.22 (21). 10.3390/ijms222111875
105
Huang H. Jeon M. S. Liao L. Yang C. Elly C. Yates J. R. 3rd et al (2010). K33-linked Polyubiquitination of T Cell Receptor-Zeta Regulates Proteolysis-independent T Cell Signaling. Immunity33 (1), 60–70. 10.1016/j.immuni.2010.07.002
106
Ikeda F. Deribe Y. L. Skånland S. S. Stieglitz B. Grabbe C. Franz-Wachtel M. et al (2011). SHARPIN Forms a Linear Ubiquitin Ligase Complex Regulating NF-Κb Activity and Apoptosis. Nature471 (7340), 637–641. 10.1038/nature09814
107
Imam S. Kömürlü S. Mattick J. Selyutina A. Talley S. Eddins A. et al (2019). K63-Linked Ubiquitin Is Required for Restriction of HIV-1 Reverse Transcription and Capsid Destabilization by Rhesus TRIM5α. J. Virol.93 (14). 10.1128/jvi.00558-19
108
Infante P. Faedda R. Bernardi F. Bufalieri F. Lospinoso Severini L. Alfonsi R. et al (2018). Itch/beta-arrestin2-dependent Non-proteolytic Ubiquitylation of SuFu Controls Hedgehog Signalling and Medulloblastoma Tumorigenesis. Nat. Commun.9 (1), 976. 10.1038/s41467-018-03339-0
109
Iwai K. (2014). Diverse Roles of the Ubiquitin System in NF-Κb Activation. Biochim. Biophys. Acta1843 (1), 129–136. 10.1016/j.bbamcr.2013.03.011
110
Iwai K. (2015). “Linear Polyubiquitination: A Crucial Regulator of NF-Κb Activation,” in Innovative Medicine: Basic Research and Development. Editors NakaoK.MinatoN.UemotoS. (Tokyo: Springer Copyright), 51–59. 10.1007/978-4-431-55651-0_4
111
Jachimowicz R. D. Beleggia F. Isensee J. Velpula B. B. Goergens J. Bustos M. A. et al (2019). UBQLN4 Represses Homologous Recombination and Is Overexpressed in Aggressive Tumors. Cell176 (3), 505–519. e522. 10.1016/j.cell.2018.11.024
112
Jeusset L. M. McManus K. J. (2017). Ubiquitin Specific Peptidase 22 Regulates Histone H2B Mono-Ubiquitination and Exhibits Both Oncogenic and Tumor Suppressor Roles in Cancer. Cancers (Basel)9 (12). 10.3390/cancers9120167
113
Ji C. H. Kwon Y. T. (2017). Crosstalk and Interplay between the Ubiquitin-Proteasome System and Autophagy. Mol. Cells40 (7), 441–449. 10.14348/molcells.2017.0115
114
Ji J. Ding K. Luo T. Zhang X. Chen A. Zhang D. et al (2021). TRIM22 Activates NF-kappaB Signaling in Glioblastoma by Accelerating the Degradation of IkappaBalpha. Cell Death Differ.28 (1), 367–381. 10.1038/s41418-020-00606-w
115
Jiang S. Park D. W. Gao Y. Ravi S. Darley-Usmar V. Abraham E. et al (2015). Participation of Proteasome-Ubiquitin Protein Degradation in Autophagy and the Activation of AMP-Activated Protein Kinase. Cell Signal27 (6), 1186–1197. 10.1016/j.cellsig.2015.02.024
116
Jin X. Shi Q. Li Q. Zhou L. Wang J. Jiang L. et al (2020). CRL3-SPOP Ubiquitin Ligase Complex Suppresses the Growth of Diffuse Large B-Cell Lymphoma by Negatively Regulating the MyD88/NF-kappaB Signaling. Leukemia34 (5), 1305–1314. 10.1038/s41375-019-0661-z
117
Jing H. Fang L. Ding Z. Wang D. Hao W. Gao L. et al (2017). Porcine Reproductive and Respiratory Syndrome Virus Nsp1α Inhibits NF-Κb Activation by Targeting the Linear Ubiquitin Chain Assembly Complex. J. Virol.91 (3). 10.1128/jvi.01911-16
118
Kane L. A. Lazarou M. Fogel A. I. Li Y. Yamano K. Sarraf S. A. et al (2014). PINK1 Phosphorylates Ubiquitin to Activate Parkin E3 Ubiquitin Ligase Activity. J. Cell Biol.205 (2), 143–153. 10.1083/jcb.201402104
119
Kazlauskaite A. Kondapalli C. Gourlay R. Campbell D. G. Ritorto M. S. Hofmann K. et al (2014). Parkin Is Activated by PINK1-dependent Phosphorylation of Ubiquitin at Ser65. Biochem. J.460 (1), 127–139. 10.1042/bj20140334
120
Keusekotten K. Elliott P. R. Glockner L. Fiil B. K. Damgaard R. B. Kulathu Y. et al (2013). OTULIN Antagonizes LUBAC Signaling by Specifically Hydrolyzing Met1-Linked Polyubiquitin. Cell153 (6), 1312–1326. 10.1016/j.cell.2013.05.014
121
Khaminets A. Behl C. Dikic I. (2016). Ubiquitin-Dependent and Independent Signals in Selective Autophagy. Trends Cell Biol.26 (1), 6–16. 10.1016/j.tcb.2015.08.010
122
Kim H. C. Huibregtse J. M. (2009). Polyubiquitination by HECT E3s and the Determinants of Chain Type Specificity. Mol. Cell Biol.29 (12), 3307–3318. 10.1128/mcb.00240-09
123
Kim K. I. Baek S. H. (2006). SUMOylation Code in Cancer Development and Metastasis. Mol. Cells22 (3), 247–253.
124
Kim W. Bennett E. J. Huttlin E. L. Guo A. Li J. Possemato A. et al (2011). Systematic and Quantitative Assessment of the Ubiquitin-Modified Proteome. Mol. Cell44 (2), 325–340. 10.1016/j.molcel.2011.08.025
125
Kirisako T. Kamei K. Murata S. Kato M. Fukumoto H. Kanie M. et al (2006). A Ubiquitin Ligase Complex Assembles Linear Polyubiquitin Chains. Embo J.25 (20), 4877–4887. 10.1038/sj.emboj.7601360
126
Klinakis A. Karagiannis D. Rampias T. (2020). Targeting DNA Repair in Cancer: Current State and Novel Approaches. Cell Mol. Life Sci.77 (4), 677–703. 10.1007/s00018-019-03299-8
127
Kniss A. Schuetz D. Kazemi S. Pluska L. Spindler P. E. Rogov V. V. et al (2018). Chain Assembly and Disassembly Processes Differently Affect the Conformational Space of Ubiquitin Chains. Structure26 (2), 249–258. e244. 10.1016/j.str.2017.12.011
128
Kolapalli S. P. Sahu R. Chauhan N. R. Jena K. K. Mehto S. Das S. K. et al (2021). RNA-binding RING E3-Ligase DZIP3/hRUL138 Stabilizes Cyclin D1 to Drive Cell-Cycle and Cancer Progression. Cancer Res.81 (2), 315–331. 10.1158/0008-5472.CAN-20-1871
129
Komander D. Clague M. J. Urbé S. (2009). Breaking the Chains: Structure and Function of the Deubiquitinases. Nat. Rev. Mol. Cell Biol.10 (8), 550–563. 10.1038/nrm2731
130
Komander D. Rape M. (2012). The Ubiquitin Code. Annu. Rev. Biochem.81, 203–229. 10.1146/annurev-biochem-060310-170328
131
Koyano F. Okatsu K. Kosako H. Tamura Y. Go E. Kimura M. et al (2014). Ubiquitin Is Phosphorylated by PINK1 to Activate Parkin. Nature510 (7503), 162–166. 10.1038/nature13392
132
Kristariyanto Y. A. Abdul Rehman S. A. Campbell D. G. Morrice N. A. Johnson C. Toth R. et al (2015). K29-selective Ubiquitin Binding Domain Reveals Structural Basis of Specificity and Heterotypic Nature of K29 Polyubiquitin. Mol. Cell58 (1), 83–94. 10.1016/j.molcel.2015.01.041
133
Kulathu Y. Komander D. (2012). Atypical Ubiquitylation - the Unexplored World of Polyubiquitin beyond Lys48 and Lys63 Linkages. Nat. Rev. Mol. Cell Biol.13 (8), 508–523. 10.1038/nrm3394
134
Kung C. P. Weber J. D. (2022). It's Getting Complicated-A Fresh Look at P53-MDM2-ARF Triangle in Tumorigenesis and Cancer Therapy. Front. Cell Dev. Biol.10, 818744. 10.3389/fcell.2022.818744
135
Kung W. W. Ramachandran S. Makukhin N. Bruno E. Ciulli A. (2019). Structural Insights into Substrate Recognition by the SOCS2 E3 Ubiquitin Ligase. Nat. Commun.10 (1), 2534. 10.1038/s41467-019-10190-4
136
Kwon Y. T. Ciechanover A. (2017). The Ubiquitin Code in the Ubiquitin-Proteasome System and Autophagy. Trends Biochem. Sci.42 (11), 873–886. 10.1016/j.tibs.2017.09.002
137
Lamothe B. Besse A. Campos A. D. Webster W. K. Wu H. Darnay B. G. (2007a). Site-specific Lys-63-Linked Tumor Necrosis Factor Receptor-Associated Factor 6 Auto-Ubiquitination Is a Critical Determinant of I Kappa B Kinase Activation. J. Biol. Chem.282 (6), 4102–4112. 10.1074/jbc.M609503200
138
Lamothe B. Campos A. D. Webster W. K. Gopinathan A. Hur L. Darnay B. G. (2008). The RING Domain and First Zinc Finger of TRAF6 Coordinate Signaling by Interleukin-1, Lipopolysaccharide, and RANKL. J. Biol. Chem.283 (36), 24871–24880. 10.1074/jbc.M802749200
139
Lamothe B. Webster W. K. Gopinathan A. Besse A. Campos A. D. Darnay B. G. (2007b). TRAF6 Ubiquitin Ligase Is Essential for RANKL Signaling and Osteoclast Differentiation. Biochem. Biophys. Res. Commun.359 (4), 1044–1049. 10.1016/j.bbrc.2007.06.017
140
Laplantine E. Fontan E. Chiaravalli J. Lopez T. Lakisic G. Véron M. et al (2009). NEMO Specifically Recognizes K63-Linked Poly-Ubiquitin Chains through a New Bipartite Ubiquitin-Binding Domain. Embo J.28 (19), 2885–2895. 10.1038/emboj.2009.241
141
Lee B. L. Singh A. Mark Glover J. N. Hendzel M. J. Spyracopoulos L. (2017). Molecular Basis for K63-Linked Ubiquitination Processes in Double-Strand DNA Break Repair: A Focus on Kinetics and Dynamics. J. Mol. Biol.429 (22), 3409–3429. 10.1016/j.jmb.2017.05.029
142
Lee H. J. Li C. F. Ruan D. He J. Montal E. D. Lorenz S. et al (2019a). Non-proteolytic Ubiquitination of Hexokinase 2 by HectH9 Controls Tumor Metabolism and Cancer Stem Cell Expansion. Nat. Commun.10 (1), 2625. 10.1038/s41467-019-10374-y
143
Lee H. J. Li C. F. Ruan D. Powers S. Thompson P. A. Frohman M. A. et al (2016). The DNA Damage Transducer RNF8 Facilitates Cancer Chemoresistance and Progression through Twist Activation. Mol. Cell63 (6), 1021–1033. 10.1016/j.molcel.2016.08.009
144
Lee J. T. Shan J. Zhong J. Li M. Zhou B. Zhou A. et al (2013). RFP-Mediated Ubiquitination of PTEN Modulates its Effect on AKT Activation. Cell Res.23 (4), 552–564. 10.1038/cr.2013.27
145
Lee S. B. Kim J. J. Han S. A. Fan Y. Guo L. S. Aziz K. et al (2019b). The AMPK-Parkin axis Negatively Regulates Necroptosis and Tumorigenesis by Inhibiting the Necrosome. Nat. Cell Biol.21 (8), 940–951. 10.1038/s41556-019-0356-8
146
Lee S. Tsai Y. C. Mattera R. Smith W. J. Kostelansky M. S. Weissman A. M. et al (2006). Structural Basis for Ubiquitin Recognition and Autoubiquitination by Rabex-5. Nat. Struct. Mol. Biol.13 (3), 264–271. 10.1038/nsmb1064
147
Lee S. W. Li C. F. Jin G. Cai Z. Han F. Chan C. H. et al (2015). Skp2-dependent Ubiquitination and Activation of LKB1 Is Essential for Cancer Cell Survival under Energy Stress. Mol. Cell57 (6), 1022–1033. 10.1016/j.molcel.2015.01.015
148
Lee Y. R. Chen M. Lee J. D. Zhang J. Lin S. Y. Fu T. M. et al (2019c). Reactivation of PTEN Tumor Suppressor for Cancer Treatment through Inhibition of a MYC-WWP1 Inhibitory Pathway. Science364 (6441). 10.1126/science.aau0159
149
Leestemaker Y. Ovaa H. (2017). Tools to Investigate the Ubiquitin Proteasome System. Drug Discov. Today Technol.26, 25–31. 10.1016/j.ddtec.2017.11.006
150
Leng S. Huang W. Chen Y. Yang Y. Feng D. Liu W. et al (2021). SIRT1 Coordinates with the CRL4B Complex to Regulate Pancreatic Cancer Stem Cells to Promote Tumorigenesis. Cell Death Differ.28 (12), 3329–3343. 10.1038/s41418-021-00821-z
151
Lenoir J. J. Parisien J. P. Horvath C. M. (2021). Immune Regulator LGP2 Targets Ubc13/UBE2N to Mediate Widespread Interference with K63 Polyubiquitination and NF-kappaB Activation. Cell Rep.37 (13), 110175. 10.1016/j.celrep.2021.110175
152
Lewis M. J. Vyse S. Shields A. M. Boeltz S. Gordon P. A. Spector T. D. et al (2015). UBE2L3 Polymorphism Amplifies NF-Κb Activation and Promotes Plasma Cell Development, Linking Linear Ubiquitination to Multiple Autoimmune Diseases. Am. J. Hum. Genet.96 (2), 221–234. 10.1016/j.ajhg.2014.12.024
153
Leznicki P. Kulathu Y. (2017). Mechanisms of Regulation and Diversification of Deubiquitylating Enzyme Function. J. Cell Sci.130 (12), 1997–2006. 10.1242/jcs.201855
154
Li N. Wang Y. Neri S. Zhen Y. Fong L. W. R. Qiao Y. et al (2019). Tankyrase Disrupts Metabolic Homeostasis and Promotes Tumorigenesis by Inhibiting LKB1-AMPK Signalling. Nat. Commun.10 (1), 4363. 10.1038/s41467-019-12377-1
155
Li Z. Younger K. Gartenhaus R. Joseph A. M. Hu F. Baer M. R. et al (2015). Inhibition of IRAK1/4 Sensitizes T Cell Acute Lymphoblastic Leukemia to Chemotherapies. J. Clin. Invest125 (3), 1081–1097. 10.1172/JCI75821
156
Liang L. Fan Y. Cheng J. Cheng D. Zhao Y. Cao B. et al (2013). TAK1 Ubiquitination Regulates Doxorubicin-Induced NF-kappaB Activation. Cell Signal25 (1), 247–254. 10.1016/j.cellsig.2012.09.003
157
Liao Y. Liu N. Xia X. Guo Z. Li Y. Jiang L. et al (2019). USP10 Modulates the SKP2/Bcr-Abl axis via Stabilizing SKP2 in Chronic Myeloid Leukemia. Cell Discov.5, 24. 10.1038/s41421-019-0092-z
158
Liao Y. Sumara I. Pangou E. (2022). Non-proteolytic Ubiquitylation in Cellular Signaling and Human Disease. Commun. Biol.5 (1), 114. 10.1038/s42003-022-03060-1
159
Licchesi J. D. Mieszczanek J. Mevissen T. E. Rutherford T. J. Akutsu M. Virdee S. et al (2011). An Ankyrin-Repeat Ubiquitin-Binding Domain Determines TRABID's Specificity for Atypical Ubiquitin Chains. Nat. Struct. Mol. Biol.19 (1), 62–71. 10.1038/nsmb.2169
160
Lin S. C. Chung J. Y. Lamothe B. Rajashankar K. Lu M. Lo Y. C. et al (2008). Molecular Basis for the Unique Deubiquitinating Activity of the NF-kappaB Inhibitor A20. J. Mol. Biol.376 (2), 526–540. 10.1016/j.jmb.2007.11.092
161
Lin X. Ojo D. Wei F. Wong N. Gu Y. Tang D. (2015). A Novel Aspect of Tumorigenesis-BMI1 Functions in Regulating DNA Damage Response. Biomolecules5 (4), 3396–3415. 10.3390/biom5043396
162
Liu B. Zhang M. Chu H. Zhang H. Wu H. Song G. et al (2017). The Ubiquitin E3 Ligase TRIM31 Promotes Aggregation and Activation of the Signaling Adaptor MAVS through Lys63-Linked Polyubiquitination. Nat. Immunol.18 (2), 214–224. 10.1038/ni.3641
163
Liu R. Zeng L. W. Gong R. Yuan F. Shu H. B. Li S. (2021). mTORC1 Activity Regulates Post-translational Modifications of glycine Decarboxylase to Modulate glycine Metabolism and Tumorigenesis. Nat. Commun.12 (1), 4227. 10.1038/s41467-021-24321-3
164
Liu Z. Gong Z. Jiang W. X. Yang J. Zhu W. K. Guo D. C. et al (2015). Lys63-linked Ubiquitin Chain Adopts Multiple Conformational States for Specific Target Recognition. Elife4. 10.7554/eLife.05767
165
Loch C. M. Strickler J. E. (2012). A Microarray of Ubiquitylated Proteins for Profiling Deubiquitylase Activity Reveals the Critical Roles of Both Chain and Substrate. Biochim. Biophys. Acta1823 (11), 2069–2078. 10.1016/j.bbamcr.2012.05.006
166
Locke M. Toth J. I. Petroski M. D. (2014). Lys11- and Lys48-Linked Ubiquitin Chains Interact with P97 during Endoplasmic-Reticulum-Associated Degradation. Biochem. J.459 (1), 205–216. 10.1042/bj20120662
167
Lork M. Verhelst K. Beyaert R. (2017). CYLD, A20 and OTULIN Deubiquitinases in NF-Κb Signaling and Cell Death: So Similar, yet So Different. Cell Death Differ.24 (7), 1172–1183. 10.1038/cdd.2017.46
168
Lu C. H. Yeh D. W. Lai C. Y. Liu Y. L. Huang L. R. Lee A. Y. et al (2018). USP17 Mediates Macrophage-Promoted Inflammation and Stemness in Lung Cancer Cells by Regulating TRAF2/TRAF3 Complex Formation. Oncogene37 (49), 6327–6340. 10.1038/s41388-018-0411-0
169
Ma J. Shi Q. Cui G. Sheng H. Botuyan M. V. Zhou Y. et al (2021). SPOP Mutation Induces Replication Over-firing by Impairing Geminin Ubiquitination and Triggers Replication Catastrophe upon ATR Inhibition. Nat. Commun.12 (1), 5779. 10.1038/s41467-021-26049-6
170
Machida Y. J. Machida Y. Chen Y. Gurtan A. M. Kupfer G. M. D'Andrea A. D. et al (2006). UBE2T Is the E2 in the Fanconi Anemia Pathway and Undergoes Negative Autoregulation. Mol. Cell23 (4), 589–596. 10.1016/j.molcel.2006.06.024
171
Mai J. Zhong Z. Y. Guo G. F. Chen X. X. Xiang Y. Q. Li X. et al (2019). Polo-like Kinase 1 Phosphorylates and Stabilizes KLF4 to Promote Tumorigenesis in Nasopharyngeal Carcinoma. Theranostics9 (12), 3541–3554. 10.7150/thno.32908
172
Mandemaker I. K. van Cuijk L. Janssens R. C. Lans H. Bezstarosti K. Hoeijmakers J. H. et al (2017). DNA Damage-Induced Histone H1 Ubiquitylation Is Mediated by HUWE1 and Stimulates the RNF8-Rnf168 Pathway. Sci. Rep.7 (1), 15353. 10.1038/s41598-017-15194-y
173
Manthiram K. Zhou Q. Aksentijevich I. Kastner D. L. (2017). The Monogenic Autoinflammatory Diseases Define New Pathways in Human Innate Immunity and Inflammation. Nat. Immunol.18 (8), 832–842. 10.1038/ni.3777
174
Massoumi R. Chmielarska K. Hennecke K. Pfeifer A. Fassler R. (2006). Cyld Inhibits Tumor Cell Proliferation by Blocking Bcl-3-dependent NF-kappaB Signaling. Cell125 (4), 665–677. 10.1016/j.cell.2006.03.041
175
Mattern M. Sutherland J. Kadimisetty K. Barrio R. Rodriguez M. S. (2019). Using Ubiquitin Binders to Decipher the Ubiquitin Code. Trends Biochem. Sci.44 (7), 599–615. 10.1016/j.tibs.2019.01.011
176
Maure J. F. Moser S. C. Jaffray E. G. F Alpi A. Hay R. T. (2016). Loss of Ubiquitin E2 Ube2w Rescues Hypersensitivity of Rnf4 Mutant Cells to DNA Damage. Sci. Rep.6, 26178. 10.1038/srep26178
177
McDowell G. S. Philpott A. (2016). New Insights into the Role of Ubiquitylation of Proteins. Int. Rev. Cell Mol. Biol.325, 35–88. 10.1016/bs.ircmb.2016.02.002
178
Meng Y. Liu C. Shen L. Zhou M. Liu W. Kowolik C. et al (2019). TRAF6 Mediates Human DNA2 Polyubiquitination and Nuclear Localization to Maintain Nuclear Genome Integrity. Nucleic Acids Res.47 (14), 7564–7579. 10.1093/nar/gkz537
179
Meng Z. Xu R. Xie L. Wu Y. He Q. Gao P. et al (2021). A20/Nrdp1 Interaction Alters the Inflammatory Signaling Profile by Mediating K48- and K63-Linked Polyubiquitination of Effectors MyD88 and TBK1. J. Biol. Chem.297 (1), 100811. 10.1016/j.jbc.2021.100811
180
Mevissen T. E. Hospenthal M. K. Geurink P. P. Elliott P. R. Akutsu M. Arnaudo N. et al (2013). OTU Deubiquitinases Reveal Mechanisms of Linkage Specificity and Enable Ubiquitin Chain Restriction Analysis. Cell154 (1), 169–184. 10.1016/j.cell.2013.05.046
181
Mevissen T. E. T. Komander D. (2017). Mechanisms of Deubiquitinase Specificity and Regulation. Annu. Rev. Biochem.86, 159–192. 10.1146/annurev-biochem-061516-044916
182
Mevissen T. E. T. Kulathu Y. Mulder M. P. C. Geurink P. P. Maslen S. L. Gersch M. et al (2016). Molecular Basis of Lys11-Polyubiquitin Specificity in the Deubiquitinase Cezanne. Nature538 (7625), 402–405. 10.1038/nature19836
183
Meza Gutierrez F. Simsek D. Mizrak A. Deutschbauer A. Braberg H. Johnson J. et al (2018). Genetic Analysis Reveals Functions of Atypical Polyubiquitin Chains. Elife7. 10.7554/eLife.42955
184
Michel M. A. Komander D. Elliott P. R. (2018). Enzymatic Assembly of Ubiquitin Chains. Methods Mol. Biol.1844, 73–84. 10.1007/978-1-4939-8706-1_6
185
Michel M. A. Swatek K. N. Hospenthal M. K. Komander D. (2017). Ubiquitin Linkage-specific Affimers Reveal Insights into K6-Linked Ubiquitin Signaling. Mol. Cell68 (1), 233–246. e235. 10.1016/j.molcel.2017.08.020
186
Miles J. A. Frost M. G. Carroll E. Rowe M. L. Howard M. J. Sidhu A. et al (2015). The Fanconi Anemia DNA Repair Pathway Is Regulated by an Interaction between Ubiquitin and the E2-like Fold Domain of FANCL. J. Biol. Chem.290 (34), 20995–21006. 10.1074/jbc.M115.675835
187
Min Y. Kim M. J. Lee S. Chun E. Lee K. Y. (2018). Inhibition of TRAF6 Ubiquitin-Ligase Activity by PRDX1 Leads to Inhibition of NFKB Activation and Autophagy Activation. Autophagy14 (8), 1347–1358. 10.1080/15548627.2018.1474995
188
Morris J. R. Solomon E. (2004). BRCA1 : BARD1 Induces the Formation of Conjugated Ubiquitin Structures, Dependent on K6 of Ubiquitin, in Cells during DNA Replication and Repair. Hum. Mol. Genet.13 (8), 807–817. 10.1093/hmg/ddh095
189
Mu Y. Sundar R. Thakur N. Ekman M. Gudey S. K. Yakymovych M. et al (2011). TRAF6 Ubiquitinates TGFbeta Type I Receptor to Promote its Cleavage and Nuclear Translocation in Cancer. Nat. Commun.2, 330. 10.1038/ncomms1332
190
Myant K. B. Cammareri P. Hodder M. C. Wills J. Von Kriegsheim A. Gyorffy B. et al (2017). HUWE1 Is a Critical Colonic Tumour Suppressor Gene that Prevents MYC Signalling, DNA Damage Accumulation and Tumour Initiation. EMBO Mol. Med.9 (2), 181–197. 10.15252/emmm.201606684
191
Ni X. Kou W. Gu J. Wei P. Wu X. Peng H. et al (2019). TRAF6 Directs FOXP3 Localization and Facilitates Regulatory T-Cell Function through K63-Linked Ubiquitination. EMBO J.38 (9). 10.15252/embj.201899766
192
Nishi R. Wijnhoven P. le Sage C. Tjeertes J. Galanty Y. Forment J. V. et al (2014). Systematic Characterization of Deubiquitylating Enzymes for Roles in Maintaining Genome Integrity. Nat. Cell Biol.16 (10), 10161011–10261018. 10.1038/ncb3028
193
Nishikawa H. Ooka S. Sato K. Arima K. Okamoto J. Klevit R. E. et al (2004). Mass Spectrometric and Mutational Analyses Reveal Lys-6-Linked Polyubiquitin Chains Catalyzed by BRCA1-BARD1 Ubiquitin Ligase. J. Biol. Chem.279 (6), 3916–3924. 10.1074/jbc.M308540200
194
Niu J. Shi Y. Iwai K. Wu Z. H. (2011). LUBAC Regulates NF-kappaB Activation upon Genotoxic Stress by Promoting Linear Ubiquitination of NEMO. EMBO J.30 (18), 3741–3753. 10.1038/emboj.2011.264
195
Niu T. Wu Z. Xiao W. (2021). Uev1A Promotes Breast Cancer Cell Migration by Up-Regulating CT45A Expression via the AKT Pathway. BMC Cancer21 (1), 1012. 10.1186/s12885-021-08750-3
196
Noad J. von der Malsburg A. Pathe C. Michel M. A. Komander D. Randow F. (2017). LUBAC-Synthesized Linear Ubiquitin Chains Restrict Cytosol-Invading Bacteria by Activating Autophagy and NF-Κb. Nat. Microbiol.2, 17063. 10.1038/nmicrobiol.2017.63
197
Nowsheen S. Aziz K. Aziz A. Deng M. Qin B. Luo K. et al (2018). L3MBTL2 Orchestrates Ubiquitin Signalling by Dictating the Sequential Recruitment of RNF8 and RNF168 after DNA Damage. Nat. Cell Biol.20 (4), 455–464. 10.1038/s41556-018-0071-x
198
Nusse R. Clevers H. (2017). Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell169 (6), 985–999. 10.1016/j.cell.2017.05.016
199
O'Connor H. F. Huibregtse J. M. (2017). Enzyme-substrate Relationships in the Ubiquitin System: Approaches for Identifying Substrates of Ubiquitin Ligases. Cell Mol. Life Sci.74 (18), 3363–3375. 10.1007/s00018-017-2529-6
200
O'Donnell M. A. (2019). Gustavo Silva: Translating the Ubiquitin Code. J. Cell Biol.218 (1), 3–4. 10.1083/jcb.201812083
201
Ohtake F. Saeki Y. Sakamoto K. Ohtake K. Nishikawa H. Tsuchiya H. et al (2015). Ubiquitin Acetylation Inhibits Polyubiquitin Chain Elongation. EMBO Rep.16 (2), 192–201. 10.15252/embr.201439152
202
Ordureau A. Sarraf S. A. Duda D. M. Heo J. M. Jedrychowski M. P. Sviderskiy V. O. et al (2014). Quantitative Proteomics Reveal a Feedforward Mechanism for Mitochondrial PARKIN Translocation and Ubiquitin Chain Synthesis. Mol. Cell56 (3), 360–375. 10.1016/j.molcel.2014.09.007
203
Padala P. Soudah N. Giladi M. Haitin Y. Isupov M. N. Wiener R. (2017). The Crystal Structure and Conformations of an Unbranched Mixed Tri-ubiquitin Chain Containing K48 and K63 Linkages. J. Mol. Biol.429 (24), 3801–3813. 10.1016/j.jmb.2017.10.027
204
Park H. Y. Go H. Song H. R. Kim S. Ha G. H. Jeon Y. K. et al (2014). Pellino 1 Promotes Lymphomagenesis by Deregulating BCL6 Polyubiquitination. J. Clin. Invest124 (11), 4976–4988. 10.1172/JCI75667
205
Paul A. Wang B. (2017). RNF8- and Ube2S-dependent Ubiquitin Lysine 11-Linkage Modification in Response to DNA Damage. Mol. Cell66 (4), 458–472. e455. 10.1016/j.molcel.2017.04.013
206
Penengo L. Mapelli M. Murachelli A. G. Confalonieri S. Magri L. Musacchio A. et al (2006). Crystal Structure of the Ubiquitin Binding Domains of Rabex-5 Reveals Two Modes of Interaction with Ubiquitin. Cell124 (6), 1183–1195. 10.1016/j.cell.2006.02.020
207
Peng D. J. Zeng M. Muromoto R. Matsuda T. Shimoda K. Subramaniam M. et al (2011). Noncanonical K27-Linked Polyubiquitination of TIEG1 Regulates Foxp3 Expression and Tumor Growth. J. Immunol.186 (10), 5638–5647. 10.4049/jimmunol.1003801
208
Piao S. Pei H. Z. Huang B. Baek S. H. (2017). Ovarian Tumor Domain-Containing Protein 1 Deubiquitinates and Stabilizes P53. Cell Signal33, 22–29. 10.1016/j.cellsig.2017.02.011
209
Polykratis A. Martens A. Eren R. O. Shirasaki Y. Yamagishi M. Yamaguchi Y. et al (2019). A20 Prevents Inflammasome-dependent Arthritis by Inhibiting Macrophage Necroptosis through its ZnF7 Ubiquitin-Binding Domain. Nat. Cell Biol.21 (6), 731–742. 10.1038/s41556-019-0324-3
210
Qin Z. Jiang W. Wang G. Sun Y. Xiao W. (2018). Linear Ubiquitin Chain Induces Apoptosis and Inhibits Tumor Growth. Apoptosis23 (1), 16–26. 10.1007/s10495-017-1433-8
211
Queisser M. A. Dada L. A. Deiss-Yehiely N. Angulo M. Zhou G. Kouri F. M. et al (2014). HOIL-1L Functions as the PKCζ Ubiquitin Ligase to Promote Lung Tumor Growth. Am. J. Respir. Crit. Care Med.190 (6), 688–698. 10.1164/rccm.201403-0463OC
212
Raimondi M. Cesselli D. Di Loreto C. La Marra F. Schneider C. Demarchi F. (2019). USP1 (Ubiquitin Specific Peptidase 1) Targets ULK1 and Regulates its Cellular Compartmentalization and Autophagy. Autophagy15 (4), 613–630. 10.1080/15548627.2018.1535291
213
Rape M. (2018). Ubiquitylation at the Crossroads of Development and Disease. Nat. Rev. Mol. Cell Biol.19 (1), 59–70. 10.1038/nrm.2017.83
214
Regev O. Roth Z. Korman M. Khalaila I. Gur E. (2015). A Kinetic Model for the Prevalence of Mono- over Poly-Pupylation. Febs J.282 (21), 4176–4186. 10.1111/febs.13413
215
Ren Y. Xu X. Mao C. Y. Han K. K. Xu Y. J. Cao B. Y. et al (2020). RNF6 Promotes Myeloma Cell Proliferation and Survival by Inducing Glucocorticoid Receptor Polyubiquitination. Acta Pharmacol. Sin.41 (3), 394–403. 10.1038/s41401-019-0309-6
216
Reyes-Turcu F. E. Horton J. R. Mullally J. E. Heroux A. Cheng X. Wilkinson K. D. (2006). The Ubiquitin Binding Domain ZnF UBP Recognizes the C-Terminal Diglycine Motif of Unanchored Ubiquitin. Cell124 (6), 1197–1208. 10.1016/j.cell.2006.02.038
217
Rittinger K. Ikeda F. (2017). Linear Ubiquitin Chains: Enzymes, Mechanisms and Biology. Open Biol.7 (4). 10.1098/rsob.170026
218
Rivkin E. Almeida S. M. Ceccarelli D. F. Juang Y. C. MacLean T. A. Srikumar T. et al (2013). The Linear Ubiquitin-specific Deubiquitinase Gumby Regulates Angiogenesis. Nature498 (7454), 318–324. 10.1038/nature12296
219
Rogerson D. T. Sachdeva A. Wang K. Haq T. Kazlauskaite A. Hancock S. M. et al (2015). Efficient Genetic Encoding of Phosphoserine and its Nonhydrolyzable Analog. Nat. Chem. Biol.11 (7), 496–503. 10.1038/nchembio.1823
220
Rösner D. Schneider T. Schneider D. Scheffner M. Marx A. (2015). Click Chemistry for Targeted Protein Ubiquitylation and Ubiquitin Chain Formation. Nat. Protoc.10 (10), 1594–1611. 10.1038/nprot.2015.106
221
Rossi F. A. Rossi M. (2022). Emerging Role of Ubiquitin-specific Protease 19 in Oncogenesis and Cancer Development. Front. Cell Dev. Biol.10, 889166. 10.3389/fcell.2022.889166
222
Rudnicka A. Yamauchi Y. (2016). Ubiquitin in Influenza Virus Entry and Innate Immunity. Viruses8 (10). 10.3390/v8100293
223
Sadowski M. Sarcevic B. (2010). Mechanisms of Mono- and Poly-Ubiquitination: Ubiquitination Specificity Depends on Compatibility between the E2 Catalytic Core and Amino Acid Residues Proximal to the Lysine. Cell Div.5, 19. 10.1186/1747-1028-5-19
224
Sahin I. Zhang S. Navaraj A. Zhou L. Dizon D. Safran H. et al (2020). AMG-232 Sensitizes High MDM2-Expressing Tumor Cells to T-Cell-Mediated Killing. Cell Death Discov.6, 57. 10.1038/s41420-020-0292-1
225
Sahu S. K. Tiwari N. Pataskar A. Zhuang Y. Borisova M. Diken M. et al (2017). FBXO32 Promotes Microenvironment Underlying Epithelial-Mesenchymal Transition via CtBP1 during Tumour Metastasis and Brain Development. Nat. Commun.8 (1), 1523. 10.1038/s41467-017-01366-x
226
Sasaki K. Iwai K. (2015). Roles of Linear Ubiquitinylation, a Crucial Regulator of NF-Κb and Cell Death, in the Immune System. Immunol. Rev.266 (1), 175–189. 10.1111/imr.12308
227
Sato Y. Okatsu K. Saeki Y. Yamano K. Matsuda N. Kaiho A. et al (2017). Structural Basis for Specific Cleavage of Lys6-Linked Polyubiquitin Chains by USP30. Nat. Struct. Mol. Biol.24 (11), 911–919. 10.1038/nsmb.3469
228
Seeler J. S. Dejean A. (2017). SUMO and the Robustness of Cancer. Nat. Rev. Cancer17 (3), 184–197. 10.1038/nrc.2016.143
229
Senft D. Qi J. Ronai Z. A. (2018). Ubiquitin Ligases in Oncogenic Transformation and Cancer Therapy. Nat. Rev. Cancer18 (2), 69–88. 10.1038/nrc.2017.105
230
Seo J. Lee E. W. Shin J. Seong D. Nam Y. W. Jeong M. et al (2018). K6 Linked Polyubiquitylation of FADD by CHIP Prevents Death Inducing Signaling Complex Formation Suppressing Cell Death. Oncogene37 (36), 4994–5006. 10.1038/s41388-018-0323-z
231
Shanbhag N. M. Rafalska-Metcalf I. U. Balane-Bolivar C. Janicki S. M. Greenberg R. A. (2010). ATM-Dependent Chromatin Changes Silence Transcription in Cis to DNA Double-Strand Breaks. Cell141 (6), 970–981. 10.1016/j.cell.2010.04.038
232
Shekhar M. P. Gerard B. Pauley R. J. Williams B. O. Tait L. (2008). Rad6B Is a Positive Regulator of Beta-Catenin Stabilization. Cancer Res.68 (6), 1741–1750. 10.1158/0008-5472.CAN-07-2111
233
Shen Y. Tang K. Chen D. Hong M. Sun F. Wang S. et al (2021). Riok3 Inhibits the Antiviral Immune Response by Facilitating TRIM40-Mediated RIG-I and MDA5 Degradation. Cell Rep.35 (12), 109272. 10.1016/j.celrep.2021.109272
234
Shih S. C. Katzmann D. J. Schnell J. D. Sutanto M. Emr S. D. Hicke L. (2002). Epsins and Vps27p/Hrs Contain Ubiquitin-Binding Domains that Function in Receptor Endocytosis. Nat. Cell Biol.4 (5), 389–393. 10.1038/ncb790
235
Shirane M. Hatakeyama S. Hattori K. Nakayama K. Nakayama K. (1999). Common Pathway for the Ubiquitination of IkappaBalpha, IkappaBbeta, and IkappaBepsilon Mediated by the F-Box Protein FWD1. J. Biol. Chem.274 (40), 28169–28174. 10.1074/jbc.274.40.28169
236
Singh A. N. Oehler J. Torrecilla I. Kilgas S. Li S. Vaz B. et al (2019). The P97-Ataxin 3 Complex Regulates Homeostasis of the DNA Damage Response E3 Ubiquitin Ligase RNF8. EMBO J.38 (21), e102361. 10.15252/embj.2019102361
237
Singh R. Karri D. Shen H. Shao J. Dasgupta S. Huang S. et al (2018). TRAF4-mediated Ubiquitination of NGF Receptor TrkA Regulates Prostate Cancer Metastasis. J. Clin. Invest128 (7), 3129–3143. 10.1172/JCI96060
238
Smeenk G. Mailand N. (2016). Writers, Readers, and Erasers of Histone Ubiquitylation in DNA Double-Strand Break Repair. Front. Genet.7, 122. 10.3389/fgene.2016.00122
239
Smith C. J. Berry D. M. McGlade C. J. (2013). The E3 Ubiquitin Ligases RNF126 and Rabring7 Regulate Endosomal Sorting of the Epidermal Growth Factor Receptor. J. Cell Sci.126 (Pt 6), 1366–1380. 10.1242/jcs.116129
240
Sokratous K. Hadjisavvas A. Diamandis E. P. Kyriacou K. (2014). The Role of Ubiquitin-Binding Domains in Human Pathophysiology. Crit. Rev. Clin. Lab. Sci.51 (5), 280–290. 10.3109/10408363.2014.915287
241
Somasagara R. R. Spencer S. M. Tripathi K. Clark D. W. Mani C. Madeira da Silva L. et al (2017). RAD6 Promotes DNA Repair and Stem Cell Signaling in Ovarian Cancer and Is a Promising Therapeutic Target to Prevent and Treat Acquired Chemoresistance. Oncogene36 (48), 6680–6690. 10.1038/onc.2017.279
242
Song K. Cai X. Dong Y. Wu H. Wei Y. Shankavaram U. T. et al (2021). Epsins 1 and 2 Promote NEMO Linear Ubiquitination via LUBAC to Drive Breast Cancer Development. J. Clin. Invest131 (1). 10.1172/JCI129374
243
Song L. Luo Z. Q. (2019). Post-Translational Regulation of Ubiquitin Signaling. J. Cell Biol.218 (6), 1776–1786. 10.1083/jcb.201902074
244
Sparrer K. M. J. Gableske S. Zurenski M. A. Parker Z. M. Full F. Baumgart G. J. et al (2017). TRIM23 Mediates Virus-Induced Autophagy via Activation of TBK1. Nat. Microbiol.2 (11), 1543–1557. 10.1038/s41564-017-0017-2
245
Spence J. Sadis S. Haas A. L. Finley D. (1995). A Ubiquitin Mutant with Specific Defects in DNA Repair and Multiubiquitination. Mol. Cell Biol.15 (3), 1265–1273. 10.1128/mcb.15.3.1265
246
Spiliotopoulos A. Blokpoel Ferreras L. Densham R. M. Caulton S. G. Maddison B. C. Morris J. R. et al (2019). Discovery of Peptide Ligands Targeting a Specific Ubiquitin-like Domain-Binding Site in the Deubiquitinase USP11. J. Biol. Chem.294 (2), 424–436. 10.1074/jbc.RA118.004469
247
Spit M. Rieser E. Walczak H. (2019). Linear Ubiquitination at a Glance. J. Cell Sci.132 (2). 10.1242/jcs.208512
248
Sun J. Zhu Z. Li W. Shen M. Cao C. Sun Q. et al (2020). UBE2T-regulated H2AX Monoubiquitination Induces Hepatocellular Carcinoma Radioresistance by Facilitating CHK1 Activation. J. Exp. Clin. Cancer Res.39 (1), 222. 10.1186/s13046-020-01734-4
249
Sun L. Amraei R. Rahimi N. (2021). NEDD4 Regulates Ubiquitination and Stability of the Cell Adhesion Molecule IGPR-1 via Lysosomal Pathway. J. Biomed. Sci.28 (1), 35. 10.1186/s12929-021-00731-9
250
Sun S. C. (2010). CYLD: a Tumor Suppressor Deubiquitinase Regulating NF-kappaB Activation and Diverse Biological Processes. Cell Death Differ.17 (1), 25–34. 10.1038/cdd.2009.43
251
Sun X. Ding Y. Zhan M. Li Y. Gao D. Wang G. et al (2019). Usp7 Regulates Hippo Pathway through Deubiquitinating the Transcriptional Coactivator Yorkie. Nat. Commun.10 (1), 411. 10.1038/s41467-019-08334-7
252
Suryadinata R. Roesley S. N. Yang G. Sarčević B. (2014). Mechanisms of Generating Polyubiquitin Chains of Different Topology. Cells3 (3), 674–689. 10.3390/cells3030674
253
Swaney D. L. Rodríguez-Mias R. A. Villén J. (2015). Phosphorylation of Ubiquitin at Ser65 Affects its Polymerization, Targets, and Proteome-wide Turnover. EMBO Rep.16 (9), 1131–1144. 10.15252/embr.201540298
254
Sy S. M. Jiang J. O W. S. Deng Y. Huen M. S. (2013). The Ubiquitin Specific Protease USP34 Promotes Ubiquitin Signaling at DNA Double-Strand Breaks. Nucleic Acids Res.41 (18), 8572–8580. 10.1093/nar/gkt622
255
Syed N. A. Andersen P. L. Warrington R. C. Xiao W. (2006). Uev1A, a Ubiquitin Conjugating Enzyme Variant, Inhibits Stress-Induced Apoptosis through NF-kappaB Activation. Apoptosis11 (12), 2147–2157. 10.1007/s10495-006-0197-3
256
Takiuchi T. Nakagawa T. Tamiya H. Fujita H. Sasaki Y. Saeki Y. et al (2014). Suppression of LUBAC-Mediated Linear Ubiquitination by a Specific Interaction between LUBAC and the Deubiquitinases CYLD and OTULIN. Genes cells..19 (3), 254–272. 10.1111/gtc.12128
257
Taminiau A. Draime A. Tys J. Lambert B. Vandeputte J. Nguyen N. et al (2016). HOXA1 Binds RBCK1/HOIL-1 and TRAF2 and Modulates the TNF/NF-κB Pathway in a Transcription-independent Manner. Nucleic Acids Res.44 (15), 7331–7349. 10.1093/nar/gkw606
258
Tang J. Luo Y. Tian Z. Liao X. Cui Q. Yang Q. et al (2020). TRIM11 Promotes Breast Cancer Cell Proliferation by Stabilizing Estrogen Receptor Alpha. Neoplasia22 (9), 343–351. 10.1016/j.neo.2020.06.003
259
Tang L. Y. Yamashita M. Coussens N. P. Tang Y. Wang X. Li C. et al (2011). Ablation of Smurf2 Reveals an Inhibition in TGF-β Signalling through Multiple Mono-Ubiquitination of Smad3. Embo J.30 (23), 4777–4789. 10.1038/emboj.2011.393
260
Tang Y. Joo D. Liu G. Tu H. You J. Jin J. et al (2018). Linear Ubiquitination of cFLIP Induced by LUBAC Contributes to TNFα-Induced Apoptosis. J. Biol. Chem.293 (52), 20062–20072. 10.1074/jbc.RA118.005449
261
Tanno H. Komada M. (2013). The Ubiquitin Code and its Decoding Machinery in the Endocytic Pathway. J. Biochem.153 (6), 497–504. 10.1093/jb/mvt028
262
Teixeira L. K. Reed S. I. (2013). Ubiquitin Ligases and Cell Cycle Control. Annu. Rev. Biochem.82, 387–414. 10.1146/annurev-biochem-060410-105307
263
Tikoo S. Madhavan V. Hussain M. Miller E. S. Arora P. Zlatanou A. et al (2013). Ubiquitin-dependent Recruitment of the Bloom Syndrome Helicase upon Replication Stress Is Required to Suppress Homologous Recombination. EMBO J.32 (12), 1778–1792. 10.1038/emboj.2013.117
264
Tokunaga F. Iwai K. (2012). Linear Ubiquitination: a Novel NF-Κb Regulatory Mechanism for Inflammatory and Immune Responses by the LUBAC Ubiquitin Ligase Complex. Endocr. J.59 (8), 641–652. 10.1507/endocrj.ej12-0148
265
Tokunaga F. (2013). Linear Ubiquitination-Mediated NF-Κb Regulation and its Related Disorders. J. Biochem.154 (4), 313–323. 10.1093/jb/mvt079
266
Tokunaga F. Nakagawa T. Nakahara M. Saeki Y. Taniguchi M. Sakata S. et al (2011). SHARPIN Is a Component of the NF-kappaB-Activating Linear Ubiquitin Chain Assembly Complex. Nature471 (7340), 633–636. 10.1038/nature09815
267
Torres M. P. Lee M. J. Ding F. Purbeck C. Kuhlman B. Dokholyan N. V. et al (2009). G Protein Mono-Ubiquitination by the Rsp5 Ubiquitin Ligase. J. Biol. Chem.284 (13), 8940–8950. 10.1074/jbc.M809058200
268
Tran H. Hamada F. Schwarz-Romond T. Bienz M. (2008). Trabid, a New Positive Regulator of Wnt-Induced Transcription with Preference for Binding and Cleaving K63-Linked Ubiquitin Chains. Genes Dev.22 (4), 528–542. 10.1101/gad.463208
269
Turek I. Tischer N. Lassig R. Trujillo M. (2018). Multi-tiered Pairing Selectivity between E2 Ubiquitin-Conjugating Enzymes and E3 Ligases. J. Biol. Chem.293 (42), 16324–16336. 10.1074/jbc.RA118.004226
270
van der Heden van Noort G. J. Kooij R. Elliott P. R. Komander D. Ovaa H. (2017). Synthesis of Poly-Ubiquitin Chains Using a Bifunctional Ubiquitin Monomer. Org. Lett.19 (24), 6490–6493. 10.1021/acs.orglett.7b03085
271
van Wijk S. J. L. Fricke F. Herhaus L. Gupta J. Hotte K. Pampaloni F. et al (2017). Linear Ubiquitination of Cytosolic Salmonella Typhimurium Activates NF-kappaB and Restricts Bacterial Proliferation. Nat. Microbiol.2, 17066. 10.1038/nmicrobiol.2017.66
272
Varadan R. Assfalg M. Haririnia A. Raasi S. Pickart C. Fushman D. (2004). Solution Conformation of Lys63-Linked Di-ubiquitin Chain Provides Clues to Functional Diversity of Polyubiquitin Signaling. J. Biol. Chem.279 (8), 7055–7063. 10.1074/jbc.M309184200
273
Venuto S. Merla G. (2019). E3 Ubiquitin Ligase TRIM Proteins, Cell Cycle and Mitosis. Cells8 (5). 10.3390/cells8050510
274
Vuorela M. Pylkas K. Winqvist R. (2011). Mutation Screening of the RNF8, UBC13 and MMS2 Genes in Northern Finnish Breast Cancer Families. BMC Med. Genet.12, 98. 10.1186/1471-2350-12-98
275
Wagner S. A. Beli P. Weinert B. T. Nielsen M. L. Cox J. Mann M. et al (2011). A Proteome-wide, Quantitative Survey of In Vivo Ubiquitylation Sites Reveals Widespread Regulatory Roles. Mol. Cell Proteomics10 (10), M111–M013284. 10.1074/mcp.M111.013284
276
Walczak H. Iwai K. Dikic I. (2012). Generation and Physiological Roles of Linear Ubiquitin Chains. BMC Biol.10, 23. 10.1186/1741-7007-10-23
277
Wang B. Jie Z. Joo D. Ordureau A. Liu P. Gan W. et al (2017a). TRAF2 and OTUD7B Govern a Ubiquitin-dependent Switch that Regulates mTORC2 Signalling. Nature545 (7654), 365–369. 10.1038/nature22344
278
Wang B. Ma A. Zhang L. Jin W. L. Qian Y. Xu G. et al (2015). POH1 Deubiquitylates and Stabilizes E2F1 to Promote Tumour Formation. Nat. Commun.6, 8704. 10.1038/ncomms9704
279
Wang C. Long W. Peng C. Hu L. Zhang Q. Wu A. et al (2016). HTLV-1 Tax Functions as a Ubiquitin E3 Ligase for Direct IKK Activation via Synthesis of Mixed-Linkage Polyubiquitin Chains. PLoS Pathog.12 (4), e1005584. 10.1371/journal.ppat.1005584
280
Wang F. Fu X. Chen P. Wu P. Fan X. Li N. et al (2017b). SPSB1-mediated HnRNP A1 Ubiquitylation Regulates Alternative Splicing and Cell Migration in EGF Signaling. Cell Res.27 (4), 540–558. 10.1038/cr.2017.7
281
Wang F. Gao Y. Zhou L. Chen J. Xie Z. Ye Z. et al (2022). USP30: Structure, Emerging Physiological Role, and Target Inhibition. Front. Pharmacol.13, 851654. 10.3389/fphar.2022.851654
282
Wang G. Long J. Gao Y. Zhang W. Han F. Xu C. et al (2019). SETDB1-mediated Methylation of Akt Promotes its K63-Linked Ubiquitination and Activation Leading to Tumorigenesis. Nat. Cell Biol.21 (2), 214–225. 10.1038/s41556-018-0266-1
283
Wang P. Dai X. Jiang W. Li Y. Wei W. (2020). RBR E3 Ubiquitin Ligases in Tumorigenesis. Semin. Cancer Biol.67 (Pt 2), 131–144. 10.1016/j.semcancer.2020.05.002
284
Wang Q. Huang L. Hong Z. Lv Z. Mao Z. Tang Y. et al (2017c). The E3 Ubiquitin Ligase RNF185 Facilitates the cGAS-Mediated Innate Immune Response. PLoS Pathog.13 (3), e1006264. 10.1371/journal.ppat.1006264
285
Wang S. Wang R. Peralta C. Yaseen A. Pavletich N. P. (2021a). Structure of the FA Core Ubiquitin Ligase Closing the ID Clamp on DNA. Nat. Struct. Mol. Biol.28 (3), 300–309. 10.1038/s41594-021-00568-8
286
Wang X. Zhang H. Shao Z. Zhuang W. Sui C. Liu F. et al (2021b). TRIM31 Facilitates K27-Linked Polyubiquitination of SYK to Regulate Antifungal Immunity. Signal Transduct. Target Ther.6 (1), 298. 10.1038/s41392-021-00711-3
287
Wang Y. Shi M. Feng H. Zhu Y. Liu S. Gao A. et al (2018). Structural Insights into Non-canonical Ubiquitination Catalyzed by SidE. Cell173 (5), 1231–1243. e1216. 10.1016/j.cell.2018.04.023
288
Wenbo L. Wang J. (2017). Uncovering the Underlying Mechanism of Cancer Tumorigenesis and Development under an Immune Microenvironment from Global Quantification of the Landscape. J. R. Soc. Interface14 (131). 10.1098/rsif.2017.0105
289
Werner A. Manford A. G. Rape M. (2017). Ubiquitin-Dependent Regulation of Stem Cell Biology. Trends Cell Biol.27 (8), 568–579. 10.1016/j.tcb.2017.04.002
290
Wertz I. E. O'Rourke K. M. Zhou H. Eby M. Aravind L. Seshagiri S. et al (2004). De-ubiquitination and Ubiquitin Ligase Domains of A20 Downregulate NF-kappaB Signalling. Nature430 (7000), 694–699. 10.1038/nature02794
291
Wheaton K. Sarkari F. Stanly Johns B. Davarinejad H. Egorova O. Kaustov L. et al (2017). UbE2E1/UBCH6 Is a Critical In Vivo E2 for the PRC1-Catalyzed Ubiquitination of H2A at Lys-119. J. Biol. Chem.292 (7), 2893–2902. 10.1074/jbc.M116.749564
292
Whiteaker J. R. Zhao L. Ivey R. G. Sanchez-Bonilla M. Moore H. D. Schoenherr R. M. et al (2018). Targeted Mass Spectrometry Enables Robust Quantification of FANCD2 Mono-Ubiquitination in Response to DNA Damage. DNA Repair (Amst)65, 47–53. 10.1016/j.dnarep.2018.03.003
293
Windheim M. Peggie M. Cohen P. (2008). Two Different Classes of E2 Ubiquitin-Conjugating Enzymes Are Required for the Mono-Ubiquitination of Proteins and Elongation by Polyubiquitin Chains with a Specific Topology. Biochem. J.409 (3), 723–729. 10.1042/bj20071338
294
Wu B. Qiang L. Zhang Y. Fu Y. Zhao M. Lei Z. et al (2022). The Deubiquitinase OTUD1 Inhibits Colonic Inflammation by Suppressing RIPK1-Mediated NF-kappaB Signaling. Cell Mol. Immunol.19 (2), 276–289. 10.1038/s41423-021-00810-9
295
Wu H. T. Kuo Y. C. Hung J. J. Huang C. H. Chen W. Y. Chou T. Y. et al (2016). K63-polyubiquitinated HAUSP Deubiquitinates HIF-1alpha and Dictates H3K56 Acetylation Promoting Hypoxia-Induced Tumour Progression. Nat. Commun.7, 13644. 10.1038/ncomms13644
296
Wu H. Yang T. Y. Li Y. Ye W. L. Liu F. He X. S. et al (2020). Tumor Necrosis Factor Receptor-Associated Factor 6 Promotes Hepatocarcinogenesis by Interacting with Histone Deacetylase 3 to Enhance C-Myc Gene Expression and Protein Stability. Hepatology71 (1), 148–163. 10.1002/hep.30801
297
Wu X. Karin M. (2015). Emerging Roles of Lys63-Linked Polyubiquitylation in Immune Responses. Immunol. Rev.266 (1), 161–174. 10.1111/imr.12310
298
Wu X. Zhang W. Font-Burgada J. Palmer T. Hamil A. S. Biswas S. K. et al (2014a). Ubiquitin-conjugating Enzyme Ubc13 Controls Breast Cancer Metastasis through a TAK1-P38 MAP Kinase Cascade. Proc. Natl. Acad. Sci. U. S. A.111 (38), 13870–13875. 10.1073/pnas.1414358111
299
Wu Z. Shen S. Zhang Z. Zhang W. Xiao W. (2017). Erratum to: Ubiquitin-Conjugating Enzyme Complex Uev1A-Ubc13 Promotes Breast Cancer Metastasis through Nuclear Factor-Кb Mediated Matrix Metalloproteinase-1 Gene Regulation. Breast Cancer Res.19 (1), 41. 10.1186/s13058-017-0833-6
300
Wu Z. Shen S. Zhang Z. Zhang W. Xiao W. (2014b). Ubiquitin-conjugating Enzyme Complex Uev1A-Ubc13 Promotes Breast Cancer Metastasis through Nuclear Factor-Small Ka, CyrillicB Mediated Matrix Metalloproteinase-1 Gene Regulation. Breast Cancer Res.16 (4), R75. 10.1186/bcr3692
301
Wu-Baer F. Lagrazon K. Yuan W. Baer R. (2003). The BRCA1/BARD1 Heterodimer Assembles Polyubiquitin Chains through an Unconventional Linkage Involving Lysine Residue K6 of Ubiquitin. J. Biol. Chem.278 (37), 34743–34746. 10.1074/jbc.C300249200
302
Wu-Baer F. Ludwig T. Baer R. (2010). The UBXN1 Protein Associates with Autoubiquitinated Forms of the BRCA1 Tumor Suppressor and Inhibits its Enzymatic Function. Mol. Cell Biol.30 (11), 2787–2798. 10.1128/MCB.01056-09
303
Xiao N. Li H. Luo J. Wang R. Chen H. Chen J. et al (2012). Ubiquitin-specific Protease 4 (USP4) Targets TRAF2 and TRAF6 for Deubiquitination and Inhibits TNFalpha-Induced Cancer Cell Migration. Biochem. J.441 (3), 979–986. 10.1042/BJ20111358
304
Xie F. Zhang Z. van Dam H. Zhang L. Zhou F. (2014). Regulation of TGF-Beta Superfamily Signaling by SMAD Mono-Ubiquitination. Cells3 (4), 981–993. 10.3390/cells3040981
305
Xing L. Tang X. Wu K. Huang X. Yi Y. Huan J. (2020). TRIM27 Functions as a Novel Oncogene in Non-triple-negative Breast Cancer by Blocking Cellular Senescence through P21 Ubiquitination. Mol. Ther. Nucleic Acids22, 910–923. 10.1016/j.omtn.2020.10.012
306
Xu L. Jia Y. Yang X. H. Han F. Zheng Y. Ni Y. et al (2017). MicroRNA-130b Transcriptionally Regulated by Histone H3 Deacetylation Renders Akt Ubiquitination and Apoptosis Resistance to 6-OHDA. Biochim. Biophys. Acta Mol. Basis Dis.1863 (6), 1678–1689. 10.1016/j.bbadis.2017.04.012
307
Xu P. Duong D. M. Seyfried N. T. Cheng D. Xie Y. Robert J. et al (2009). Quantitative Proteomics Reveals the Function of Unconventional Ubiquitin Chains in Proteasomal Degradation. Cell137 (1), 133–145. 10.1016/j.cell.2009.01.041
308
Xu X. Kalac M. Markson M. Chan M. Brody J. D. Bhagat G. et al (2020). Reversal of CYLD Phosphorylation as a Novel Therapeutic Approach for Adult T-Cell Leukemia/lymphoma (ATLL). Cell Death Dis.11 (2), 94. 10.1038/s41419-020-2294-6
309
Xu Y. Hu Y. Xu T. Yan K. Zhang T. Li Q. et al (2021). RNF8-mediated Regulation of Akt Promotes Lung Cancer Cell Survival and Resistance to DNA Damage. Cell Rep.37 (3), 109854. 10.1016/j.celrep.2021.109854
310
Xue B. Li H. Guo M. Wang J. Xu Y. Zou X. et al (2018). TRIM21 Promotes Innate Immune Response to RNA Viral Infection through Lys27-Linked Polyubiquitination of MAVS. J. Virol.92 (14). 10.1128/jvi.00321-18
311
Yang J. M. Schiapparelli P. Nguyen H. N. Igarashi A. Zhang Q. Abbadi S. et al (2017). Characterization of PTEN Mutations in Brain Cancer Reveals that Pten Mono-Ubiquitination Promotes Protein Stability and Nuclear Localization. Oncogene36 (26), 3673–3685. 10.1038/onc.2016.493
312
Yang Y. Yang C. Li T. Yu S. Gan T. Hu J. et al (2020). The Deubiquitinase USP38 Promotes NHEJ Repair through Regulation of HDAC1 Activity and Regulates Cancer Cell Response to Genotoxic Insults. Cancer Res.80 (4), 719–731. 10.1158/0008-5472.CAN-19-2149
313
Yao F. Zhou Z. Kim J. Hang Q. Xiao Z. Ton B. N. et al (2018). SKP2- and OTUD1-Regulated Non-proteolytic Ubiquitination of YAP Promotes YAP Nuclear Localization and Activity. Nat. Commun.9 (1), 2269. 10.1038/s41467-018-04620-y
314
Yasunaga J. Lin F. C. Lu X. Jeang K. T. (2011). Ubiquitin-specific Peptidase 20 Targets TRAF6 and Human T Cell Leukemia Virus Type 1 Tax to Negatively Regulate NF-kappaB Signaling. J. Virol.85 (13), 6212–6219. 10.1128/JVI.00079-11
315
Yau R. G. Doerner K. Castellanos E. R. Haakonsen D. L. Werner A. Wang N. et al (2017). Assembly and Function of Heterotypic Ubiquitin Chains in Cell-Cycle and Protein Quality Control. Cell171 (4), 918–933. 10.1016/j.cell.2017.09.040
316
Yau R. Rape M. (2016). The Increasing Complexity of the Ubiquitin Code. Nat. Cell Biol.18 (6), 579–586. 10.1038/ncb3358
317
Yeh C. H. Bellon M. Nicot C. (2018). FBXW7: a Critical Tumor Suppressor of Human Cancers. Mol. Cancer17 (1), 115. 10.1186/s12943-018-0857-2
318
Yin H. Gui Y. Du G. Frohman M. A. Zheng X. L. (2010). Dependence of Phospholipase D1 Multi-Monoubiquitination on its Enzymatic Activity and Palmitoylation. J. Biol. Chem.285 (18), 13580–13588. 10.1074/jbc.M109.046359
319
Yin Q. Han T. Fang B. Zhang G. Zhang C. Roberts E. R. et al (2019). K27-linked Ubiquitination of BRAF by ITCH Engages Cytokine Response to Maintain MEK-ERK Signaling. Nat. Commun.10 (1), 1870. 10.1038/s41467-019-09844-0
320
Yu X. Li W. Deng Q. Liu H. Wang X. Hu H. et al (2020a). MYD88 L265P Elicits Mutation-specific Ubiquitination to Drive NF-kappaB Activation and Lymphomagenesis. Blood. 10.1182/blood.2020004918
321
Yu X. Li W. Liu H. Deng Q. Wang X. Hu H. et al (2020b). Ubiquitination of the DNA-Damage Checkpoint Kinase CHK1 by TRAF4 Is Required for CHK1 Activation. J. Hematol. Oncol.13 (1), 40. 10.1186/s13045-020-00869-3
322
Yu Z. Li X. Yang M. Huang J. Fang Q. Jia J. et al (2021). TRIM41 Is Required to Innate Antiviral Response by Polyubiquitinating BCL10 and Recruiting NEMO. Signal Transduct. Target Ther.6 (1), 90. 10.1038/s41392-021-00477-8
323
Zhang H. Hu H. Greeley N. Jin J. Matthews A. J. Ohashi E. et al (2014). STAT3 Restrains RANK- and TLR4-Mediated Signalling by Suppressing Expression of the E2 Ubiquitin-Conjugating Enzyme Ubc13. Nat. Commun.5, 5798. 10.1038/ncomms6798
324
Zhang L. Liu Q. Liu K. W. Qin Z. Y. Zhu G. X. Shen L. T. et al (2021). SHARPIN Stabilizes Beta-Catenin through a Linear Ubiquitination-independent Manner to Support Gastric Tumorigenesis. Gastric Cancer24 (2), 402–416. 10.1007/s10120-020-01138-5
325
Zhang L. Zhou F. Garcia de Vinuesa A. de Kruijf E. M. Mesker W. E. Hui L. et al (2013a). TRAF4 Promotes TGF-Beta Receptor Signaling and Drives Breast Cancer Metastasis. Mol. Cell51 (5), 559–572. 10.1016/j.molcel.2013.07.014
326
Zhang Q. Karnak D. Tan M. Lawrence T. S. Morgan M. A. Sun Y. (2016). FBXW7 Facilitates Nonhomologous End-Joining via K63-Linked Polyubiquitylation of XRCC4. Mol. Cell61 (3), 419–433. 10.1016/j.molcel.2015.12.010
327
Zhang X. Meng T. Cui S. Liu D. Pang Q. Wang P. (2022). Roles of Ubiquitination in the Crosstalk between Tumors and the Tumor Microenvironment (Review). Int. J. Oncol.61 (1). 10.3892/ijo.2022.5374
328
Zhang X. Zhang J. Zhang L. van Dam H. ten Dijke P. (2013b). UBE2O Negatively Regulates TRAF6-Mediated NF-kappaB Activation by Inhibiting TRAF6 Polyubiquitination. Cell Res.23 (3), 366–377. 10.1038/cr.2013.21
329
Zhang Y. Li Y. Yang X. Wang J. Wang R. Qian X. et al (2018). Uev1A-Ubc13 Catalyzes K63-Linked Ubiquitination of RHBDF2 to Promote TACE Maturation. Cell Signal42, 155–164. 10.1016/j.cellsig.2017.10.013
330
Zhang Z. Fan Y. Xie F. Zhou H. Jin K. Shao L. et al (2017). Breast Cancer Metastasis Suppressor OTUD1 Deubiquitinates SMAD7. Nat. Commun.8 (1), 2116. 10.1038/s41467-017-02029-7
331
Zhao X. Lutz J. Höllmüller E. Scheffner M. Marx A. Stengel F. (2017). Identification of Proteins Interacting with Ubiquitin Chains. Angew. Chem. Int. Ed. Engl.56 (49), 15764–15768. 10.1002/anie.201705898
332
Zheng J. Wang B. Zheng R. Zhang J. Huang C. Zheng R. et al (2020). Linc-RA1 Inhibits Autophagy and Promotes Radioresistance by Preventing H2Bub1/USP44 Combination in Glioma Cells. Cell Death Dis.11 (9), 758. 10.1038/s41419-020-02977-x
333
Zheng Y. G. Wu J. Chen Z. Goodman M. (2008). Chemical Regulation of Epigenetic Modifications: Opportunities for New Cancer Therapy. Med. Res. Rev.28 (5), 645–687. 10.1002/med.20120
334
Zhi H. Guo X. Ho Y. K. Pasupala N. Engstrom H. A. A. Semmes O. J. et al (2020). RNF8 Dysregulation and Down-Regulation during HTLV-1 Infection Promote Genomic Instability in Adult T-Cell Leukemia. PLoS Pathog.16 (5), e1008618. 10.1371/journal.ppat.1008618
335
Zhou H. Liu Y. Zhu R. Ding F. Wan Y. Li Y. et al (2017). FBXO32 Suppresses Breast Cancer Tumorigenesis through Targeting KLF4 to Proteasomal Degradation. Oncogene36 (23), 3312–3321. 10.1038/onc.2016.479
336
Zhou H. Wertz I. O'Rourke K. Ultsch M. Seshagiri S. Eby M. et al (2004). Bcl10 Activates the NF-kappaB Pathway through Ubiquitination of NEMO. Nature427 (6970), 167–171. 10.1038/nature02273
337
Zhou L. Jiang H. Du J. Li L. Li R. Lu J. et al (2018). USP15 Inhibits Multiple Myeloma Cell Apoptosis through Activating a Feedback Loop with the Transcription Factor NF-κBp65. Exp. Mol. Med.50 (11), 1–12. 10.1038/s12276-018-0180-4
338
Zhou L. Xu G. (2022). The Ubiquitination-dependent and -Independent Functions of Cereblon in Cancer and Neurological Diseases. J. Mol. Biol.434 (5), 167457. 10.1016/j.jmb.2022.167457
339
Zhou W. Zhong Z. Lin D. Liu Z. Zhang Q. Xia H. et al (2021). Hypothermic Oxygenated Perfusion Inhibits HECTD3-Mediated TRAF3 Polyubiquitination to Alleviate DCD Liver Ischemia-Reperfusion Injury. Cell Death Dis.12 (2), 211. 10.1038/s41419-021-03493-2
340
Zhu F. Yi G. Liu X. Zhu F. Zhao A. Wang A. et al (2018). Ring Finger Protein 31-mediated Atypical Ubiquitination Stabilizes Forkhead Box P3 and Thereby Stimulates Regulatory T-Cell Function. J. Biol. Chem.293 (52), 20099–20111. 10.1074/jbc.RA118.005802
341
Zhu G. Herlyn M. Yang X. (2021). TRIM15 and CYLD Regulate ERK Activation via Lysine-63-Linked Polyubiquitination. Nat. Cell Biol.23 (9), 978–991. 10.1038/s41556-021-00732-8
342
Zhu J. Li X. Su P. Xue M. Zang Y. Ding Y. (2020). The Ubiquitin Ligase RNF181 Stabilizes ERalpha and Modulates Breast Cancer Progression. Oncogene39 (44), 6776–6788. 10.1038/s41388-020-01464-z
343
Zhu J. Zhuang T. Yang H. Li X. Liu H. Wang H. (2016). Atypical Ubiquitin Ligase RNF31: the Nuclear Factor Modulator in Breast Cancer Progression. BMC Cancer16, 538. 10.1186/s12885-016-2575-8
344
Zientara-Rytter K. Subramani S. (2019). The Roles of Ubiquitin-Binding Protein Shuttles in the Degradative Fate of Ubiquitinated Proteins in the Ubiquitin-Proteasome System and Autophagy. Cells8 (1). 10.3390/cells8010040
345
Ziv I. Matiuhin Y. Kirkpatrick D. S. Erpapazoglou Z. Leon S. Pantazopoulou M. et al (2011). A Perturbed Ubiquitin Landscape Distinguishes between Ubiquitin in Trafficking and in Proteolysis. Mol. Cell Proteomics10 (5), M111–M009753. 10.1074/mcp.M111.009753
Summary
Keywords
ubiquitin, atypical ubiquitination, ubiquitin E2 conjugating enzyme, ubiquitin E3 ligase, tumorigenesis, ubiquitin-proteasome system
Citation
Yin X, Liu Q, Liu F, Tian X, Yan T, Han J and Jiang S (2022) Emerging Roles of Non-proteolytic Ubiquitination in Tumorigenesis. Front. Cell Dev. Biol. 10:944460. doi: 10.3389/fcell.2022.944460
Received
15 May 2022
Accepted
15 June 2022
Published
06 July 2022
Volume
10 - 2022
Edited by
Haitao Wang, Center for Cancer Research (NIH), United States
Reviewed by
Jianping Guo, Sun Yat-sen University, China
Petro Starokadomskyy, University of Texas Southwestern Medical Center, United States
Jianlin Liu, Dana–Farber Cancer Institute, United States
Shivani Dixit, National Cancer Institute (NIH), United States
Updates
Copyright
© 2022 Yin, Liu, Liu, Tian, Yan, Han and Jiang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Han, hanj3669@163.com; Shulong Jiang, jnsljiang@163.com
This article was submitted to Epigenomics and Epigenetics, a section of the journal Frontiers in Cell and Developmental Biology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.