Abstract
Periprosthetic osteolysis (PPO) induced by wear particles is an important cause of aseptic loosening after artificial joint replacement, among which the imbalance of osteogenesis and osteoclastic processes occupies a central position. The cells involved in PPO mainly include osteoclasts (macrophages), osteoblasts, osteocytes, and fibroblasts. RANKL/RANK/OGP axis is a typical way for osteolysis. Autophagy, a mode of regulatory cell death and maintenance of cellular homeostasis, has a dual role in PPO. Although autophagy is activated in various periprosthetic cells and regulates the release of inflammatory cytokines, osteoclast activation, and osteoblast differentiation, its beneficial or detrimental role remains controversy. In particular, differences in the temporal control and intensity of autophagy may have different effects. This article focuses on the role of autophagy in PPO, and expects the regulation of autophagy to become a powerful target for clinical treatment of PPO.
1 Introduction
Clinically, joint replacement is an effective procedure for the treatment of joint trauma and end-stage joint diseases, such as osteoarthritis and rheumatoid arthritis (Price et al., 2018). It is estimated that by 2030, more than 4 million joint replacement surgeries will be performed annually in the United States (Charnley, 1971; Kurtz et al., 2007). With the continuous improvement of surgical methods and the further advancement of prosthetic materials, the quality of life of patients has been greatly improved (Shan et al., 2014). Nevertheless, there are still a certain number of patients who require more difficult revision surgery, the main causes of which are periprosthetic osteolysis (PPO) and aseptic loosening (AL) (Malchau et al., 1993; Learmonth et al., 2007). Artificial joint revision surgery is also called re-joint replacement. Generally, when the joint prosthesis becomes loose or damaged, or pain occurs and affects normal life, the orthopedic doctor will open the prosthesis of the original joint and then take it out, and then treat the soft tissue and bone appropriately according to the specific situation. Finally, a suitable new joint prosthesis is placed in the joint again. In the United States, revision surgery after joint replacement surgery due to PPO accounts for 20.3% and is one of the most common reasons (Desai and Bancroft, 2008; Yin et al., 2022). In a 10-year follow-up study, osteolysis occurred in 24% (32 of 133 hips) of patients with primary total hip replacement using standard polyethylene (Lachiewicz and Soileau, 2016). In a serial follow-up of 250 single-center ankle arthroplasties, the prevalence of osteolysis was quite high at 31.6% (79 of 250 ankles) and occurred within 3 years of surgery (Lee and Lee, 2022). These results collectively indicate that PPO after joint replacement surgery is a challenging problem. PPO arises from a chronic inflammatory response triggered by cellular phagocytosis of implant-derived particulate debris. Multiple cytokines lead to osteoclast recruitment and activation, bone resorption, and ultimately AL (Looney et al., 2006; Noordin and Masri, 2012). The pathophysiology of AL has not been fully elucidated. The etiology may be related to the material and design of the prosthesis, the procedure, and the way the prosthesis is used. At present, the common prosthesis materials include metal, polyethylene, polymethyl methacrylate and ceramics (Bozic et al., 2009; Bouhidel et al., 2015). Currently, titanium (Ti)-based composites are the most ideal prosthetic implants due to their excellent mechanical strength and good biocompatibility. However, Ti implants have some inherent drawbacks and are prone to the formation of wear debris due to their high coefficient of friction (Kang et al., 2017; Bressan et al., 2019). Titanium wear particles may cause damage to surrounding cells and exacerbate the spread of inflammation. How to alleviate the death and inflammatory response of periprosthetic cells is a key way to prevent PPO.
Periprosthetic membrane (PM) refers to the synovial-like interface membrane between the prosthesis and the surrounding bone tissue, which contains a variety of cell types, such as fibroblasts, macrophages, osteoclasts, osteoblasts, osteocytes and a small number of lymphocytes (Tuan et al., 2008; Camuzard et al., 2019). Wear particles from prosthetic materials are phagocytosed by cells within the PM and induce an inflammatory response, leading to an imbalance of osteoblasts and osteoclasts, which subsequently leads to osteolysis and AL (Catelas et al., 1999; Nich et al., 2013). The cell types and molecular biological mechanisms involved are very complex, including cytokines such as interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α), interferon (IFN)-β, interleukin 1β (IL-1β), prostaglandin E2 and macrophage-colony stimulating factor (M-CSF) production, cell-to-cell interactions and ultimately the recruitment and activation of osteoclasts (Okafor et al., 2006; Wang et al., 2017; Yang et al., 2016; Deng et al., 2017a; O'Neill et al., 2013; Purdue et al., 2007; Lochner et al., 2011; Ping et al., 2017). In this series of biological activities, the release of receptor activator of nuclear kappa-B ligand (RANKL) and its binding to receptor RANK are central events, which directly promote osteoclastogenesis and subsequent osteolysis (Zhai et al., 2014; Zhao et al., 2016; Ping et al., 2017). After being stimulated by wear particles, cells in PM can undergo multiple modes of regulated cell death (RCD), including autophagy (Chen et al., 2021), apoptosis (Deng et al., 2017b), pyroptosis (Zhang et al., 2022) and even ferroptosis (Xu et al., 2021). Among them, autophagy is a well-studied RCD mode, which plays a pivotal role in PPO.
Autophagy is a ubiquitous and highly conserved process of self-catabolism and energy dynamic cycle unique to eukaryotic cells. During the process of cell proliferation, differentiation and maturation. It maintains intracellular homeostasis and provides material as well as energy by degrading misfolded proteins or damaged organelles (Glick et al., 2010; Mizushima and Komatsu, 2011). Three types of autophagy have been described in mammals, namely,: macroautophagy, chaperone-mediated autophagy and microautophagy, depending on the mechanism by which cargo is delivered to the lysosome (Scrivo et al., 2018). The current research focuses on macroautophagy, and unless otherwise specified, autophagy refers to macroautophagy. Autophagy begins with a phagosome formed by a double-layered membrane, wrapping some substances to be degraded to form an autophagosome, which fuses with a lysosome to form an autophagolysosome, which is eventually degraded (Mizushima and Komatsu, 2011). Mitophagy is a special type of selective autophagy that degrades damaged or aged mitochondria through receptor-mediated mechanisms to maintain mitochondrial homeostasis in cells (Boya et al., 2013; Ploumi et al., 2017). Although necessary for the survival of eukaryotic cells, autophagy is a double-edged sword that can also cause cell damage or even death (Chen et al., 2012). A growing body of evidence has confirmed that autophagy plays a pivotal role in bone metabolism, and abnormal autophagy can disrupt the balance of bone metabolism. Wear particles accelerate the PPO process by affecting autophagy (Ha et al., 2014; Camuzard et al., 2019).
In this regard, a large number of studies have focused on RCD in PPO. Zhang R et al. (Zhang et al., 2021) conducted a bibliometric study on the current PPO research based on VOSviewer, and found that “autophagy”, “bone-resorbing cells” as well as “proinflammatory cytokines” are research hotspots in this field. Due to the duality of autophagy and the complexity of its regulatory mechanism, there is no exact consensus on its mechanism of action on PPO. Inappropriate levels of autophagy may lead to negative effects. Collectively, this review article focuses on the role of autophagy in the development and progression of PPO, so as to provide novel ideas for reducing the incidence of PPO and AL.
2 Cell types involved in PPO
2.1 Osteocytes
Osteocytes are embedded in the bone matrix and account for more than 95% of all osteocytes in adult bones. They are terminally differentiated cells of the osteoblast lineage and are involved in bone growth, bone modeling, and bone remodeling (Bonewald, 2007; O'Brien et al., 2013). Sclerostin (encoded by SOST), a potent Wnt/β-catenin inhibitor, is a soluble glycoprotein secreted only by osteocytes that controls bone formation and absorption by binding to the co-receptor low-density lipoprotein receptor-related protein (LRP5/6). When osteocytes are mechanically stimulated, sclerostin production is blocked to activate osteoblasts to promote new bone formation (Lu et al., 2018). In addition, Osteoprotegerin (OPG) and receptor activator of nuclear factor kappa B ligand (RANKL) expressed by osteocytes are also directly involved in the regulation of bone resorption (Nakashima et al., 2011).
2.2 Osteoblasts and osteoclasts
As important cellular components of bone, osteoblasts and osteoclasts work together to maintain bone metabolic homeostasis. Osteoblasts are differentiated from bone marrow mesenchymal stem cells (BMSCs), which is the important source of osteocytes. Osteoclasts, the only cells responsible for bone resorption, are multinucleated giant cells derived from monocytes or macrophages (Madel et al., 2019). Therefore, the generation and activity enhancement of osteoclasts play a key role in the pathogenesis of PPO (Jacome-Galarza et al., 2019). Osteoblasts and chondrocytes originated from BMSCs are responsible for the construction of bone. On the contrary, their “enemies” are osteoclasts derived from hematopoietic stem cells, responsible for bone resorption and destruction (osteolysis). They jointly maintain the homeostasis of the bone matrix (Zaidi, 2007). Due to the close expression profile of osteoclasts, the RAW 264.7 cell line is often used by scholars in experiments to demonstrate the characteristics of osteoclasts (Todde et al., 2009).
RANKL and M-CSF are essential cytokines for the activation and maturation of osteoclast precursors (Zauli et al., 2004; Chakraborty et al., 2017). M-CSF upregulates the expression of RANK, the cognate receptor for RANKL (Arai et al., 1999; Novack and Mbalaviele, 2016). RANKL is a member of the TNF family. After binding to the specific receptor RANK, it activates the downstream nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) signal transduction pathways through recruiting the intracellular adaptor protein TNF receptor-associated factor (TRAF) (Arai et al., 1999; Feng, 2005). The mechanisms of the interaction between osteoblasts and osteoclasts are complex. OPG, also known as osteoclastogenesis inhibitory factor, secreted by osteoblasts or BMSCs, is a secreted glycoprotein that also belongs to the TNF receptor family. OPG is a decoy receptor for RANKL and inhibits osteoclast differentiation and osteolysis by blocking the interaction of RANKL with RANK by competing with RANK for RANKL binding (Kobayashi et al., 2009).
2.3 Fibroblasts
In PM, the highest proportion is fibroblasts, reaching more than 70%, however, much less attention has been paid to its role in PPO (Wei et al., 2005). When stimulated by wear particles, fibroblasts or fibroblast-like synoviocytes (FLSs) can not only secrete inflammatory factors, including RANKL, IL-6, IL-8, IL-1β and TNF-α, etc., to promote the maturation of osteoclasts (Wei et al., 2005), but also can produce sclerostin (SOST), an antagonist of Wnt and BMP signaling, to inhibit the osteogenic capacity of osteoprogenitor cells, thereby exacerbating bone loss (Jagga et al., 2021). In addition, it was found that nano-Al2O3 particles reduced the expression of RANKL by inducing autophagy in fibroblasts, thereby alleviating PPO (Li et al., 2018). In-depth study of fibroblasts contributes to further understanding of the pathogenesis of PPO.
3 Autophagy
Autophagy has been extensively studied in osteolysis and PPO. Although most scholars believe that wear particles can induce an increase in the level of autophagy and accelerate osteolysis, some scholars hold the opposite opinion, arguing that autophagy has a protective effect, and enhancing autophagy can prevent PPO (Wang et al., 2017; Li et al., 2018). Autophagy is induced in a RANKL-dependent manner during osteoclastogenesis and also strongly influences the process of osteoclastic bone resorption (Zhang et al., 2019).
The term “autophagy” is originated from the Greek meaning for “eating of self”. In the 1950s, Belgian scientist Christian de Duve used electron microscopy to observe the structure of autophagosomes, and in 1962, Ashford et al. observed autophagy in mouse liver cells after insulin treatment using transmission electron microscopy (ASHFORD and PORTER, 1962; Deter and De Duve, 1967). Autophagy is a highly conserved autocatabolic cellular process unique to eukaryotic cells. It degrades cytoplasmic misfolded or aggregated proteins, damaged organelles, long-lived proteins or exogenous substances through the lysosomal system to achieve the metabolic needs of the cells themselves, which is important to maintain the homeostasis of cells (Pohl and Dikic, 2019). The process of autophagy occurrence can be divided into four stages: Ⅰ. formation of septum membrane; Ⅱ. formation of autophagosome; Ⅲ. transportation and fusion of autophagosome; Ⅳ. lysis of autophagosome (Figure 1). Under normal physiological conditions, the body keeps a low level of autophagy to maintain cell metabolism and organelle renewal. When stimulated by the external environment (such as lack of oxygen, sugar, amino acid, and energy, etc.), it will activate intracellular autophagy, and then encapsulate the degraded material with double membranes to form autophagosomes, which are transported to lysosomes. Subsequently they combine to form autophagolysosomes, which are digested and degraded by a variety of enzymes and at the same time provide raw materials for the synthesis of new organelles to maintain cell survival (Rosenfeldt and Ryan, 2009; Rubinsztein et al., 2012). However, when autophagy is overactivated, resulting in mitochondrial dissolution and denaturation, chromatin fragmentation, and nuclear disintegration, it will cause cell death, which is called autophagy-dependent cell death (Rabinovich-Nikitin and Kirshenbaum, 2021).
FIGURE 1

The process of autophagy.
ATG proteins transcribed and translated by autophagy related genes (Atg) are involved in the initiation, formation and eventual degradation of autophagosomes. Atg3, Atg5, Atg7, Atg10, Atg12 and LC3 (microtubule-associated protein 1 light chain 3, MAP1-LC3) are involved in two ubiquitin-like protein processing and modification processes (Alonso et al., 2007). Atg12 is localized on the bilayer isolation membrane of preautophagosomes and is associated with the formation of preautophagosomes. LC3, homologous to yeast Atg7/8, is localized to the autophagosome separation membrane (not bound to phosphatidylethanolamine [PE]), inner and outer membranes, and autophagolysosomal membranes (bound to PE) (Wu et al., 2006). After the LC3 precursor is formed, it is processed by the protease ATG4 into cytoplasmic soluble LC3-I (18 kDa), which can combine with PE on the surface of the autophagic vesicle to form the membrane-bound form LC3-II (16 kDa). LC3-II, a marker molecule of autophagosomes, is located in pre-autophagosomes and autophagosomes, and its content is proportional to the number of autophagosomes (Liu et al., 2017).
In mammals, autophagy is mainly divided into microautophagy, macroautophagy and chaperone-mediated autophagy (Parzych and Klionsky, 2014). The aforementioned autophagy refers to macroautophagy. Microautophagy and endosomal microautophagy refers to the process in which the cargoes are internalized directly by invaginations of lysosome and endosomal membranes (Mijaljica et al., 2011; Tekirdag and Cuervo, 2018). Molecular chaperone-mediated autophagy means that proteins are recognized by cytoplasmic partners one by one and bring them to the lysosomal surface for transmembrane translocation (Dice, 2007; Kaushik and Cuervo, 2018). Unless otherwise specified, the autophagy referred to in this article is macroautophagy.
Accumulating evidence suggests that autophagy is an important etiology of osteolysis. Autophagy can be elicited by wear particles in the many cell types involved in osteolysis, including osteoclasts, osteoblasts, osteocyte, fibroblasts and macrophages (Wang et al., 2020). Studies found that the level of autophagy increased during osteoblast differentiation. deletion of either Atg5 or Atg7 resulted in reduced osteoblast numbers in mice (Vrahnas et al., 2019). Interestingly, in an osteolysis model, Wang Z and his researchers demonstrated that apoptosis induced by CoCrMo metal particles exacerbated osteolysis by stimulating osteoblast autophagy (Wang et al., 2015a). The same research team also confirmed that TiAl6V4 particles induced autophagy in osteocytes mediated the downregulation of IFN-β, which in turn activated osteoclasts (Wang et al., 2017). In arthritis models, inhibition of autophagy limited macrophage differentiation to osteoclasts, thereby attenuating joint destruction (Shi et al., 2017). Nevertheless, nano-Al2O3-induced autophagy of fibroblasts limited osteoclast activation by reducing RANKL expression (Li et al., 2018). Therefore, modulation of autophagy on different types of cells in PM plays different roles in the treatment of PPO.
During the process of osteoblast differentiation, mitochondrial function and ATP content were significantly enhanced (Gao et al., 2018). Mitophagy was first proposed by Lemasters in 2005 (Lemasters, 2005). Autophagy is divided into selective autophagy and non-selective autophagy. Mitophagy belongs to the latter. PTEN-induced putative kinase 1 (PINK1), a serine/threonine (Ser/Thr) kinase, is required in the Parkin mediated mitophagy (Kawajiri et al., 2010; Narendra et al., 2010). Parkin is an E3-ubiquitin ligase selectively recruited to dysfunctional mitochondria by PINK1 via phosphorylation of Ser 65 in the UBL domain of Parkin (Narendra et al., 2008; Kondapalli et al., 2012). Similarly, mitophagy contributes to tissue homeostasis by reducing reactive oxygen species (ROS) and pro-inflammatory factors produced by damaged mitochondria. In vitro, 17β-estradiol enhances mitophagy in the mouse osteoblast cell line MC3T3-E1 to promote cell proliferation (Sun et al., 2018). However, mitophagy disturbances may also impair cellular energy metabolism and physiological functions (Goldman et al., 2010). One study unmasked that inhibition of autophagy is accompanied by endoplasmic reticulum and mitochondrial accumulation in Atg7-deleted osteoblasts, resulting in decreased bone mass and increased fractures in mice (Piemontese et al., 2016). Mitochondrial fusion proteins MFN1 (mitofusin1) and MFN2 are GTPases located on the outer mitochondrial membrane and play a key role in mitochondrial fusion. MFN2 can also promote mitophagy (Chen et al., 2003; Zhao et al., 2011). Ballard A et al. (Ballard et al., 2020) discovered that in osteoclast precursors with a double conditional knockout of MFN1 and MFN2, osteoclasts maturation was suppressed and bone mass was increased in female mice. In addition, MFN2-deficient female mice were resistant to Rankl-induced osteolysis and mitophagy was essential for osteoclast differentiation. The duality of autophagy remains controversy in the pathogenesis of osteolysis, but it is undeniable that the regulation of autophagy is a powerful weapon in the prevention and treatment of osteolysis.
4 Autophagy in osteolysis
4.1 Autophagy promotes osteolysis (negative effect)
Netrin-1 is an axon guidance protein involved in osteoclast activation and osteolysis (Purdue et al., 2007). L. Wang et al. (Wang et al., 2021) found that Ti particles activated autophagy in RAW 264.7 cells by increasing the expression of Netrin-1 and its receptor Unc5b, thereby promoting the expression of Atg5, Atg7, Atg12, Beclin-1 and LC3-II. Although the autophagy inhibitor 3-methyladenine (3-MA) reversed Ti particle-induced TRAP, RANKL and OPG expression and osteoclastogenesis, it did not affect Netrin-1. Netrin-1 promotes autophagy in RAW 264.7 via ERK phosphorylation, which subsequently induces osteoclastogenesis and increases in inflammatory factors such as IL-1β, IL-6, and TNF-α.
A certain level of transcription factor (NF)-κB is necessary to normal bone metabolism (Abu-Amer, 2013), however, under inflammatory conditions, high level of NF-κB causes osteoclast activation and bone destruction (Xing et al., 2005; Pasparakis, 2008). NF-κB is the principal mediator of RANK signaling, and its signaling pathway members include NF-κB1 (p50), NF-κB2 (P52), RelA (p65), IκB, IKKα, IKKβ, and NEMO (Franzoso et al., 1997; Boyce et al., 1999; Boyce et al., 2010). Normally, NF-κB exists as a dimer. When unstimulated, the nuclear localization sequence of NF-κB binds to inhibitory IκB proteins. Stimulatory signals, such as RANKL, cause IKKγ/NEMO and IKKβ, as well as other adaptor proteins, to assemble into IκB kinase (IKK), which phosphorylates the downstream substrate IκB (Franzoso et al., 1997; Boyce et al., 2005; Boyce et al., 2010), and lead to the release of the p65/p50 NF-κB subunits into the nucleus to exert their functions (Gilmore, 2006). Phosphorylated IκB are degraded through the proteasomal pathway. The ubiquitin-proteasome system plays a crucial role in regulating NF-κB activity (Skaug et al., 2009).
Stimulation of bone marrow derived macrophages (BMDMs) by RANKL is multifaceted. Apart from NF-κB, RANKL stimulation identically activates the MAPK signalling pathway including ERK, JNK and p38. The activation of the above two signaling pathways requires TRAF2, TRAF5 and TRAF6, especially TRAF6 (Feng, 2005). TRAF6 deficiency facilitates osteosclerosis in mice (Lomaga et al., 1999). RANKL activates RANK in a trimeric symmetric complex with TRAF6, a RING-domain E3 ubiquitin ligase (Yu et al., 2018), together with NFATc1, are essential for osteoclast maturation and differentiation (Kim et al., 2009). In addition, TRAF6 recruitment after RANKL stimulation resulted in further activation of NF-κB, MAPK (JNK, ERK and p38) and Akt (Kim et al., 2013). In a recent study, it was found that in the RANKL-RANK signaling pathway, TRAF6 binds and induces the ubiquitination of Beclin- 1, which promotes autophagy and induces osteoclast differentiation (Chu et al., 2020). One research conducted by B. Chu et al. (Chu et al., 2020) indicated that application of Nepetin, A flavonoid with anti-inflammatory activity, not only inhibited RANKL-induced activation of NF-κB and MAPK, but also impeded TEAF6-dependent Beclin-1 ubiquitination and autophagy induction, which are necessary for osteoclast differentiation. Nepetin prevented IκBα degradation as well as p65 nuclear localization by inhibiting IKK activation, thereby reducing the transcriptional activity of NF-κB and the induction of c-Fos and NFATc1. Intriguingly, the inducer of autophagy, rapamycin, counteracted the inhibitory effect of Nepetin on autophagy and accelerated osteoclast activation and bone resorption. This study further elucidated the potential therapeutic application of autophagy inhibition against osteoclast-dependent osteolysis.
Bortezomib (BTZ), a reversible proteasome inhibitor, is recognized to hinder nuclear transport of NF-κB by impeding the chymotryptic activity of polyubiquitinated protein degradation in 26S proteasome, thereby inhibiting NF-κB (Deng et al., 2017a; Grosset et al., 2019). Zhang Z and his research team (Zhang et al., 2020a) found that Ti particles caused MG-63 cells, a type of osteosarcoma-derived cells that resemble poorly differentiated osteoblasts, to activate the NF-κB signaling pathway and increase inflammatory mediators, such as IL-1β, IL-6, TNF-α, release along with activation of the autophagy marker LC3. The use of nano-aluminum particles and BTZ, a proteasome inhibitor, co-cultured with MG-63 blocked the degradation of IκBα induced by Ti particles and inhibited the activation of NF-κB. Intriguingly, Nano-Alumina (Al) inhibited autophagy and prevented apoptosis and osteolysis. Using nano-Al to improve the pro-inflammatory properties of Ti particles, combined with inflammatory factor inhibitors to regulate autophagy may be an effective way to alleviate osteolysis.
Although osteocytes are the most numerous cells in the bone, the relationship between osteocytes and osteoclasts in periprosthetic osteolysis studies has not been well studied. Previous studies have demonstrated that ultra-high molecular weight polyethylene (UHMWPE) particles stimulate osteocytes to secrete prostaglandin E2 to activate osteoclasts (Lohmann et al., 2002). IFN-β, a pleiotropic cytokine in cellular immunity, is also responsible for osteoclast differentiation and bone resorption (Abraham et al., 2009; Xiong et al., 2016). IFN-β can hinder RANKL-induced osteoclast activation through multiple signaling pathways, such as RANKL-c-fos-NFATc1 (Zhao et al., 2014). Wang Z et al. (Wang et al., 2017) reported that TiAl6V4 alloy particles enhanced the autophagy level of osteocytes and decreased the expression of IFN-β, which inhibited the differentiation of BMDMs into osteoclasts. Negatively modulation of autophagy with Atg5 siRNA blocked these effects. Another study by the same researching group unmasked that TiAl6V4 induced autophagy in macrophages and phosphorylates p38, thereby upregulating the expression of TNF-a and accelerating osteolysis. The autophagy inhibitor 3-MA or p38 inhibitor SB202190 hindered the above process (Liu et al., 2016). Intriguingly, another research by the same study group confirmed that CoCrMo particles promoted osteoblast apoptosis in osteoblastic cell line MC3T3-E1 by facilitating autophagy through ERN1-MAPK8 pathway. Similarly, Atg5 siRNA and 3-MA attenuated CoCrMo particles-induced osteoblast apoptosis and osteolysis by the impeding of the autophagy (Wang et al., 2015a). Another experiment using the MC3T3-E1 cell line implied that hyperoside pretreatment protects against Ti particle-induced damage by reducing osteoblast autophagy and apoptosis through the tumor necrosis factor ligand superfamily member 12 (TWEAK)-p38 pathway (Zhang and Zhang, 2019).
Earlier studies indicated that titanium particles induced increased expression of CD147, a transmembrane glycoprotein belonging to the immunoglobulin superfamily, in KG-1a cells, which activated autophagy, thereby increasing soluble RANKL levels and promoting osteoclast occurrence. These effects were reversed when siRNA-CD147 transfection or autophagy inhibitor chloroquine were performed (Su et al., 2018). Consequently, blocking autophagy may represent a potential therapeutic approach for treating particle-induced PPO.
4.2 Autophagy inhibits osteolysis (positive effect)
As important players in the inflammatory response, fibroblasts play an important role in chemokine synthesis and inflammation regulation (Smith et al., 1997; Flavell et al., 2008). Studies have confirmed that wear particles induce an increase in the RANKL/OPG ratio in fibroblasts (Wang et al., 2015b). When activated, cytokines secreted by fibroblasts induce the recruitment and differentiation of monocytes, a process involved in wear particle-mediated osteolysis (Goodman and Ma, 2010; Filer, 2013). Fibroblasts occupy a large amount in the pseudo-synovia around the joint after joint replacement, and their secretion of RANKL promotes the activation of osteoclasts under the stimulation of titanium particles (Wei et al., 2005). As touched upon earlier, Al reduced the inflammatory response of cells induced by Ti particles (Zhang et al., 2020a). Strikingly, unlike aluminum that inhibited autophagy, some scholars proposed that aluminum promoted autophagy, although the former was detected in osteoblast-like MG-63 cells (Zhang et al., 2020a) and the latter was in FLSs (Li et al., 2018). D Li and others (Li et al., 2018) isolated FLSs from the synovium of clinical revision arthroplasty patients and stimulated them with Al2O3, CoCr, and UHMWPE particles, respectively. showed lower levels of RANKL, autophagy, and inflammation. The results demonstrated that the increased autophagy intensity of FLSs was accompanied by lower RANKL expression after Al2O3 stimulation. Lentiviral interference or enhancement of Beclin-1 further confirmed that autophagy levels were inversely proportional to RANKL expression and the number of osteoclasts. Afterwards, in a rat model of femoral head replacement, Al2O3 reduced FLSs secretion of RANKL and osteoclast activation by enhancing autophagy. Although the regulation of autophagy by aluminum is controversial, these conclusions provide further consideration for the selection of joint prosthesis materials.
Wear particle-mediated monocyte recruitment and inflammatory responses are important pathogenesis of osteolysis. In an earlier study in an interface membrane specimen from clinical joint prosthesis revision, titanium particle stimulation inhibited autophagy in fibroblasts and resulted in ADAM metallopeptidase domain 10 (ADAM10), including the precursor form p-ADAM10 and its mature form m-ADAM10 increase. Subsequent secretion of C-X3-C motif chemokine ligand 1 (CX3CL1), a unique chemokine, increased and promoted the migration of the human myeloid leukemia mononuclear (THP-1) cells. The autophagy activator rapamycin reversed the effects of titanium particles (Wu et al., 2020). Accordingly, blocking monocyte recruitment and the inflammatory response it induces is also an important strategy to prevent osteolysis.
As touched upon above, NEMO, a scaffold protein lacking enzymatic activity, is a key molecule in the NF-κB signaling pathway. Adapala N et al. (Adapala et al., 2020) identified lysine K)270 as a target regulating RANKL signaling, and NEMOK270A mutation hinders autophagy in BMDMs and exacerbates rankl-induced osteoclastogenesis. Additionally, the molecular mechanisms and the treatment strategies mentioned above to regulate autophagy for the treatment of PPO and AL were summarized in Figure 2 and Table 1.
FIGURE 2
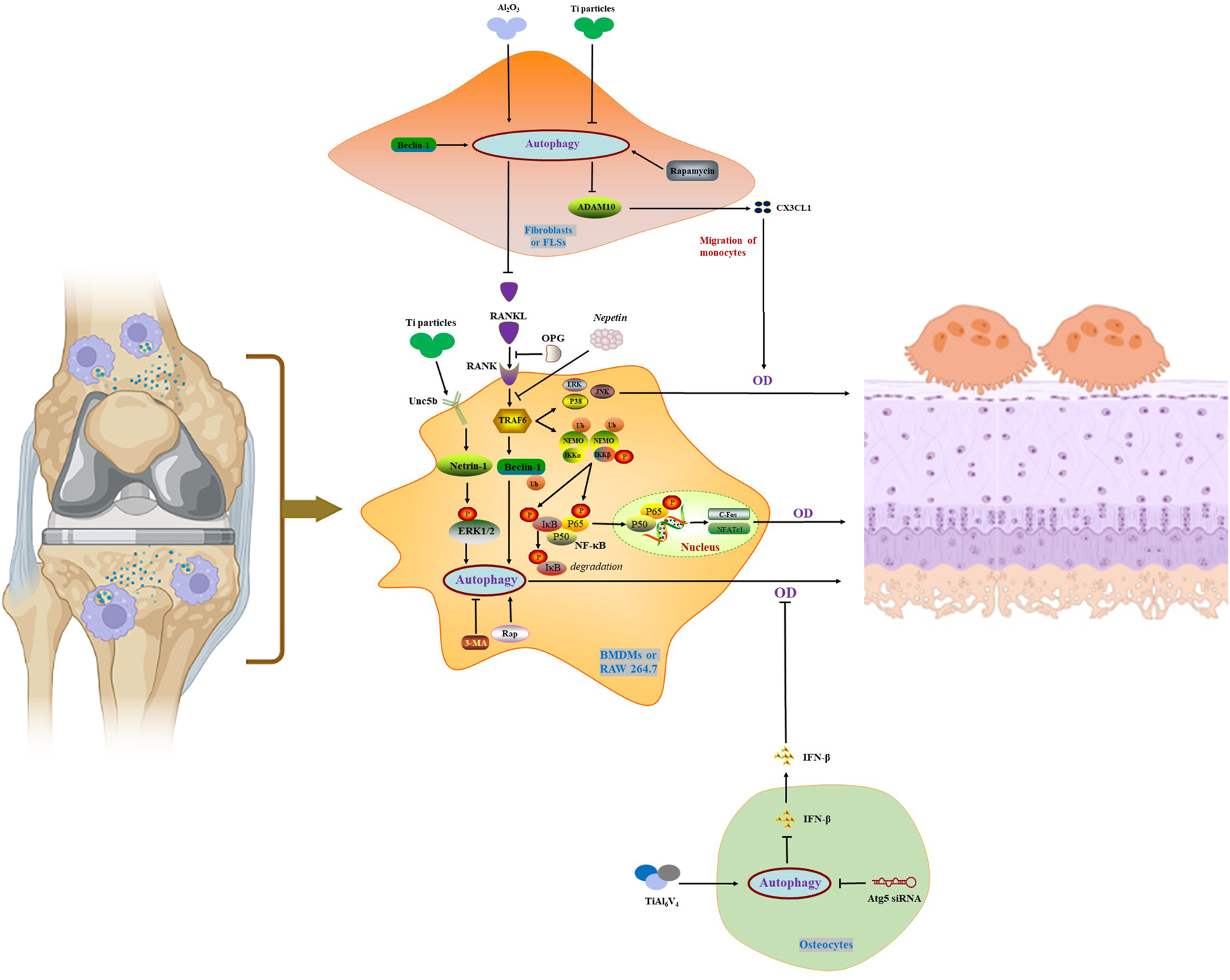
Autophagy in the activation and maturation of osteoclasts.
TABLE 1
| Intervention target/agents | Animal | Cell type | Animal model | In vitro cell model | Hallmark of autophagy | Molecular mechanism | Ref |
|---|---|---|---|---|---|---|---|
| Kawajiri et al., (2010) | |||||||
| Boyce et al., (1999) | |||||||
| Boyce et al., (2005) | |||||||
| Kang et al., (2017) | |||||||
| Kim et al., (2009) | |||||||
| Wu et al., (2006) | |||||||
| Chu et al., (2020) | |||||||
| Zauli et al., (2004) | |||||||
| Zhao et al., (2014) |
Experimental study of autophagy in PPO.
Precision therapy, such as targeted drug delivery, is a hot spot in modern medical research (Li et al., 2021). A recent experiment released reactive oxygen species (ROS) scavenger and autophagy activator rapamycin through a drug-loaded system on a nanoplatform to rescue chondrocyte degeneration after IL-1β stimulation for the treatment of osteoarthritis (Xue et al., 2021). Thus, At the same time of primary joint replacement, local implantation of drug release system to alleviate the inflammatory response caused by later prosthesis wear is still a therapeutic strategy worthy of further study.
5 Disscussion
Although joint replacement has greatly improved the quality of life of patients with severe end-stage joint disease, aseptic osteolysis and loosening of the prosthesis caused by wear particles of the prosthesis limit the service life of the artificial joint (Varnum, 2017). Various cell types, including osteoblasts, osteoclasts, macrophages, fibroblasts, and lymphocytes, are involved in the pathological process of PPO, and the direct factor is the imbalance between osteoblasts and osteoclasts. RANKL/RANK/OGP axis is a typical way for RANKL to participate in bone remodeling. The role of autophagy has aways been controversial, and there is no clear conclusion so far. It is undeniable that wear particles can induce the occurrence of autophagy in PM. Autophagy induces macrophages and osteoblasts to secrete proinflammatory cytokines, such as TNF-α, IL-6 and IL-8 or high mobility group box 1, which exacerbate the progression of PPO (Camuzard et al., 2019). However, autophagy can also inhibit the release of RANKL from fibroblasts or reduce the expression of IFN-β by osteoblasts, thereby hindering the activation of osteoclasts (Li et al., 2018; Wu et al., 2020). In addition, 17β-estradiol alleviates osteoporosis by enhancing osteoblast autophagy through G protein-coupled receptor 30-extracellular regulated protein kinase 1/2 (ERK1/2) signaling pathway, which also exhibits positive effect of autophagy (Sun et al., 2018). During the progression of PPO, complex regulatory mechanisms may exist between cells in the PM, including crosstalk in autophagy regulation. It is worth noting that different autophagy intensities and durations may have opposite effects. The research on autophagy in PPO mainly focuses on macroautophagy, chaperone-mediated autophagy and microautophagy have not yet been studied, and mitophagy is also a research gap, which warrants deeper examination. NF-κB activation is critical for the recruitment and maturation of macrophages, as well as the production of pro-inflammatory cytokines and chemokines (Lin et al., 2014). The regulation of autophagy on the NF-κB pathway in PPO deserves further study. Autophagy has a dual role in PPO, how to rationally regulate autophagy to prevent PPO remains to be fully elucidated.
In contrast, the pathogenesis of selective autophagy in PPO has been less studied. As the site of cellular energy conversion, the integrity and quantity of mitochondria play an active role in the physiological functions of cells. When mitochondrial dysfunction occurs, it is manifested by increased release of ROS and inflammatory factors, mitochondrial depolarization and enhanced mitophagy (Guo et al., 2011; Hui et al., 2016; Collins et al., 2018). Regulation of mitochondrial number and function through mitophagy is important for maintaining the biological activity of osteoblasts and osteoclasts. Another form of selective autophagy, ferroautophagy, is mediated by nuclear receptor coactivator 4 (NCOA4), enabling ferritin, mainly ferritin heavy chain 1, to degraded by autophagosomes. Eventually ferritin-bound iron is released as free iron. Excessive activation of ferroautophagy may induce intracellular iron overload and lead to cellular damage (Bauckman and Mysorekar, 2016; Nai et al., 2021). X Qin et al. revealed that ferroautophagy was necessary for zinc oxide nanoparticles-induced ferroptosis in vascular endothelial cells (Qin et al., 2021). Ferroautophagy is involved in processes including tumor development and erythropoiesis (Zhang et al., 2020b; Nai et al., 2021), however, its effect on PPO deserves further study.
Strikingly, most studies hinder the progress of PPO by enhancing or inhibiting autophagy, which further verifies the duality of autophagy. Nevertheless, it is difficult to achieve therapeutic effects by simply regulating autophagy because of complex cell differences as well as differences in time and space. Similarly, autophagy also has both a “dark” and a “bright” side in tumor therapy. Autophagy and mitophagy are thought to contribute to drug resistance and survival of cancer cells. Mitophagy reduces ROS formation by degrading damaged mitochondria, and anticancer drugs combined with autophagy inhibition have been validated in clinical trials of various human cancers. However, mitophagy inhibition also promotes cancer cell metastasis (Erkan et al., 2005; Ishaq et al., 2020). As a result, simply enhancing or inhibiting autophagy may not achieve satisfactory therapeutic effects. Interfering with autophagy at different times during the PPO process may have opposite effects. Even if autophagy is intervened at the same time, autophagy in different cells may play different roles in PM. The precision of time and space, the appropriate strength of autophagy, and the precise targeting of cellularity will be an important aspect of future research, especially the exact intervention of autophagy in a specific cell.
There is an urgent need to find safe and effective therapeutic drugs to relieve PPO in clinical practice. Anti-rankl antibodies (Denosumab), parathyroid hormones (Teriparatide and Abaloparatide), bisphosphonates (Fosamax) and selective estrogen receptor modulators (Raloxifene) have shown promising anti-osteolytic effects by inhibiting osteoclast activity. Sclerostin modulators are also actively entering clinical applications. SOST modulators (Romosozumab and Blosozumab) are also actively entering clinical applications. Nevertheless, long-term use of these drugs may bring serious adverse reactions, such as cardiovascular accidents, liver and kidney toxicity, bone necrosis, and malignant tumors, which limit their clinical use (Herrington et al., 2000; Berruti et al., 2006; Cao et al., 2014; Hayden et al., 2014). In terms of autophagy regulation, reducing RANKL production by modulating autophagy in fibroblasts is also a potential therapeutic strategy. By developing new drugs targeting autophagy of different cells in PM, as well as changing the time and dose of administration, in order to more accurately control the process of PPO development. Improvements in prosthetic design and surgical modalities are additional potential approaches. Interestingly, compared with titanium particles, ceramic particles had less effect on the release of inflammatory factors and stimulation of macrophages (Zhang et al., 2011; Ding et al., 2012; Jeffers and Walter, 2012). Al2O3 can also inhibit osteoclast maturation by enhancing autophagy (Li et al., 2018). Therefore, optimizing the prosthetic material is also crucial to reduce the incidence of PPO.
Collectively, we review the critical roles of autophagy, mitophagy and ferroautophagy in the pathophysiology of PPO. The occurrence of PPO is the result of various intercellular interactions. Autophagy has two sides, and how to adjust the time and intensity of autophagy to reduce the occurrence of osteolysis remains challenging. Although several studies support the important role of autophagy in PPO, there are still many unanswered questions about the regulation of autophagy in timing, intensity and target cells. Understanding the molecular mechanism of autophagy in PPO will provides insight for AL therapeutics.
Statements
Author contributions
ZY and GG wrote the draft paper. JY and BW planned the study and modified the article. XW and WL drew pictures.
Funding
We acknowledge financial support from the National Natural Science Foundation of China (81871795), Jiangsu Provincial Health Commission Project (LGY2020052, Z2022086), Nanjing Health Science and Technology Development Special Fund Project (YKK22222), the Key Projects of Youth Innovation and Scientific Research Fund of the Affiliated Jiangning Hospital with Nanjing Medical University (JNYYZXKY202201 and JNYYZXKY202115) and Lianyungang Science and Technology Bureau Project (SF2118).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2023.1123753/full#supplementary-material
References
1
Abraham A. Ramanathan M. Weinstock-Guttman B. Mager D. (2009). Mechanisms of interferon-beta effects on bone homeostasis. Biochem. Pharmacol.77 (12), 1757–1762. 10.1016/j.bcp.2009.01.007
2
Abu-Amer Y. (2013). NF-κB signaling and bone resorption. Osteoporos. Int. J. established as result Coop. between Eur. Found. Osteoporos. Natl. Osteoporos. Found. U. S. A.24 (9), 2377–2386. 10.1007/s00198-013-2313-x
3
Adapala N. Swarnkar G. Arra M. Shen J. Mbalaviele G. Ke K. et al (2020). Inflammatory osteolysis is regulated by site-specific ISGylation of the scaffold protein NEMO. eLife9, e56095. 10.7554/eLife.56095
4
Alonso S. Pethe K. Russell D. Purdy G. (2007). Lysosomal killing of Mycobacterium mediated by ubiquitin-derived peptides is enhanced by autophagy. Proc. Natl. Acad. Sci. U. S. A.104 (14), 6031–6036. 10.1073/pnas.0700036104
5
Arai F. Miyamoto T. Ohneda O. Inada T. Sudo T. Brasel K. et al (1999). Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J. Exp. Med.190 (12), 1741–1754. 10.1084/jem.190.12.1741
6
Ashford T. Porter K. (1962). Cytoplasmic components in hepatic cell lysosomes. J. Cell. Biol.12, 198–202. 10.1083/jcb.12.1.198
7
Ballard A. Zeng R. Zarei A. Shao C. Cox L. Yan H. et al (2020). The tethering function of mitofusin2 controls osteoclast differentiation by modulating the Ca2+-NFATc1 axis. J. Biol. Chem.295 (19), 6629–6640. 10.1074/jbc.RA119.012023
8
Bauckman K. Mysorekar I. (2016). Ferritinophagy drives uropathogenic Escherichia coli persistence in bladder epithelial cells. Autophagy12 (5), 850–863. 10.1080/15548627.2016.1160176
9
Berruti A. Tucci M. Generali D. Mosca A. Ardine M. Vana F. et al (2006). Management of the side-effects of intravenous bisphosphonates: Targeting the serum parathyroid hormone elevation. Ann. Oncol. official J. Eur. Soc. Med. Oncol.17 (12), 1854–1855. 10.1093/annonc/mdl181
10
Bonewald L. (2007). Osteocytes as dynamic multifunctional cells. Ann. N. Y. Acad. Sci.1116, 281–290. 10.1196/annals.1402.018
11
Bouhidel J. Wang P. Siu K. Li H. Youn J. Cai H. (2015). Netrin-1 improves post-injury cardiac function in vivo via DCC/NO-dependent preservation of mitochondrial integrity, while attenuating autophagy. Biochimica biophysica acta1852 (2), 277–289. 10.1016/j.bbadis.2014.06.005
12
Boya P. Reggiori F. Codogno P. (2013). Emerging regulation and functions of autophagy. Nat. Cell. Biol.15 (7), 713–720. 10.1038/ncb2788
13
Boyce B. Xing L. Franzoso G. Siebenlist U. (1999). Required and nonessential functions of nuclear factor-kappa B in bone cells. Bone25 (1), 137–139. 10.1016/s8756-3282(99)00105-2
14
Boyce B. Yamashita T. Yao Z. Zhang Q. Li F. Xing L. (2005). Roles for NF-kappaB and c-Fos in osteoclasts. J. bone mineral metabolism23, 11–15. 10.1007/bf03026317
15
Boyce B. Yao Z. Xing L. (2010). Functions of nuclear factor kappaB in bone. Ann. N. Y. Acad. Sci.1192, 367–375. 10.1111/j.1749-6632.2009.05315.x
16
Bozic K. Kurtz S. Lau E. Ong K. Vail T. Berry D. (2009). The epidemiology of revision total hip arthroplasty in the United States. J. bone Jt. Surg. Am. volume91 (1), 128–133. 10.2106/jbjs.H.00155
17
Bressan E. Ferroni L. Gardin C. Bellin G. Sbricoli L. Sivolella S. et al (2019). Metal nanoparticles released from dental implant surfaces: Potential contribution to chronic inflammation and peri-implant bone loss. Mater. (Basel, Switz.12 (12), 2036. 10.3390/ma12122036
18
Camuzard O. Breuil V. Carle G. Pierrefite-Carle V. (2019). Autophagy involvement in aseptic loosening of arthroplasty components. J. bone Jt. Surg. Am. volume101 (5), 466–472. 10.2106/jbjs.18.00479
19
Cao X. Xu P. Oyola M. Xia Y. Yan X. Saito K. et al (2014). Estrogens stimulate serotonin neurons to inhibit binge-like eating in mice. J. Clin. investigation124 (10), 4351–4362. 10.1172/jci74726
20
Catelas I. Petit A. Zukor D. Marchand R. Yahia L. Huk O. (1999). Induction of macrophage apoptosis by ceramic and polyethylene particles in vitro. Biomaterials20 (7), 625–630. 10.1016/s0142-9612(98)00214-2
21
Chakraborty S. Kloos B. Harre U. Schett G. Kubatzky K. (2017). Pasteurella multocida toxin triggers RANKL-independent osteoclastogenesis. Front. Immunol.8, 185. 10.3389/fimmu.2017.00185
22
Charnley J. (1971). Present status of total hip replacement. Ann. rheumatic Dis.30 (6), 560–564. 10.1136/ard.30.6.560
23
Chen G. Ke Z. Xu M. Liao M. Wang X. Qi Y. et al (2012). Autophagy is a protective response to ethanol neurotoxicity. Autophagy8 (11), 1577–1589. 10.4161/auto.21376
24
Chen H. Detmer S. Ewald A. Griffin E. Fraser S. Chan D. (2003). Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell. Biol.160 (2), 189–200. 10.1083/jcb.200211046
25
Chen W. Xian G. Gu M. Pan B. Wu X. Ye Y. et al (2021). Autophagy inhibitors 3-MA and LY294002 repress osteoclastogenesis and titanium particle-stimulated osteolysis. Biomaterials Sci.9 (14), 4922–4935. 10.1039/d1bm00691f
26
Chu B. Chen S. Zheng X. Ye J. Cheng X. Zhang L. et al (2020). Nepetin inhibits osteoclastogenesis by inhibiting RANKL-induced activation of NF-κB and MAPK signalling pathway, and autophagy. J. Cell. Mol. Med.24 (24), 14366–14380. 10.1111/jcmm.16055
27
Collins J. Diekman B. Loeser R. (2018). Targeting aging for disease modification in osteoarthritis. Curr. Opin. rheumatology30 (1), 101–107. 10.1097/bor.0000000000000456
28
Deng Z. Jin J. Wang Z. Wang Y. Gao Q. Zhao J. (2017). The metal nanoparticle-induced inflammatory response is regulated by SIRT1 through NF-κB deacetylation in aseptic loosening. Int. J. nanomedicine12, 3617–3636. 10.2147/ijn.S124661
29
Deng Z. Wang Z. Jin J. Wang Y. Bao N. Gao Q. et al (2017). SIRT1 protects osteoblasts against particle-induced inflammatory responses and apoptosis in aseptic prosthesis loosening. Acta biomater.49, 541–554. 10.1016/j.actbio.2016.11.051
30
Desai M. Bancroft L. (2008). The case. Diagnosis: Periprosthetic osteolysis. Orthopedics31 (6), 518–615. 10.3928/01477447-20080601-07
31
Deter R. De Duve C. (1967). Influence of glucagon, an inducer of cellular autophagy, on some physical properties of rat liver lysosomes. J. Cell. Biol.33 (2), 437–449. 10.1083/jcb.33.2.437
32
Dice J. (2007). Chaperone-mediated autophagy. Autophagy3 (4), 295–299. 10.4161/auto.4144
33
Ding Y. Qin C. Fu Y. Xu J. Huang D. (2012). In vitro comparison of the biological activity of alumina ceramic and titanium particles associated with aseptic loosening. Biomed. Mater. (Bristol, Engl.7 (4), 045019. 10.1088/1748-6041/7/4/045019
34
Erkan M. Kleeff J. Esposito I. Giese T. Ketterer K. Büchler M. et al (2005). Loss of BNIP3 expression is a late event in pancreatic cancer contributing to chemoresistance and worsened prognosis. Oncogene24 (27), 4421–4432. 10.1038/sj.onc.1208642
35
Feng X. (2005). RANKing intracellular signaling in osteoclasts. IUBMB life57 (6), 389–395. 10.1080/15216540500137669
36
Filer A. (2013). The fibroblast as a therapeutic target in rheumatoid arthritis. Curr. Opin. Pharmacol.13 (3), 413–419. 10.1016/j.coph.2013.02.006
37
Flavell S. Hou T. Lax S. Filer A. Salmon M. Buckley C. (2008). Fibroblasts as novel therapeutic targets in chronic inflammation. Br. J. Pharmacol.153, S241–S246. 10.1038/sj.bjp.0707487
38
Franzoso G. Carlson L. Xing L. Poljak L. Shores E. Brown K. et al (1997). Requirement for NF-kappaB in osteoclast and B-cell development. Genes. and Dev.11 (24), 3482–3496. 10.1101/gad.11.24.3482
39
Gao J. Feng Z. Wang X. Zeng M. Liu J. Han S. et al (2018). SIRT3/SOD2 maintains osteoblast differentiation and bone formation by regulating mitochondrial stress. Cell. death Differ.25 (2), 229–240. 10.1038/cdd.2017.144
40
Gilmore T. (2006). Introduction to NF-kappaB: Players, pathways, perspectives. Oncogene25 (51), 6680–6684. 10.1038/sj.onc.1209954
41
Glick D. Barth S. Macleod K. (2010). Autophagy: Cellular and molecular mechanisms. J. pathology221 (1), 3–12. 10.1002/path.2697
42
Goldman S. Taylor R. Zhang Y. Jin S. (2010). Autophagy and the degradation of mitochondria. Mitochondrion10 (4), 309–315. 10.1016/j.mito.2010.01.005
43
Goodman S. Ma T. (2010). Cellular chemotaxis induced by wear particles from joint replacements. Biomaterials31 (19), 5045–5050. 10.1016/j.biomaterials.2010.03.046
44
Grosset A. Ouellet V. Caron C. Fragoso G. Barrès V. Delvoye N. et al (2019). Validation of the prognostic value of NF-κB p65 in prostate cancer: A retrospective study using a large multi-institutional cohort of the Canadian prostate cancer biomarker network. PLoS Med.16 (7), e1002847. 10.1371/journal.pmed.1002847
45
Guo Y. Yang T. Liu Y. Shen H. Lei S. Yu N. et al (2011). Mitochondria-wide association study of common variants in osteoporosis. Ann. Hum. Genet.75 (5), 569–574. 10.1111/j.1469-1809.2011.00663.x
46
Ha S. Weitzmann M. Beck G. (2014). Bioactive silica nanoparticles promote osteoblast differentiation through stimulation of autophagy and direct association with LC3 and p62. ACS Nano8 (6), 5898–5910. 10.1021/nn5009879
47
Hayden R. Fortin J. Harwood B. Subramanian B. Quinn K. Georgakoudi I. et al (2014). Cell-tethered ligands modulate bone remodeling by osteoblasts and osteoclasts. Adv. Funct. Mater.24 (4), 472–479. 10.1002/adfm.201302210
48
Herrington D. Reboussin D. Brosnihan K. Sharp P. Shumaker S. Snyder T. et al (2000). Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N. Engl. J. Med.343 (8), 522–529. 10.1056/nejm200008243430801
49
Hui W. Young D. Rowan A. Xu X. Cawston T. Proctor C. (2016). Oxidative changes and signalling pathways are pivotal in initiating age-related changes in articular cartilage. Ann. rheumatic Dis.75 (2), 449–458. 10.1136/annrheumdis-2014-206295
50
Ishaq M. Ojha R. Sharma A. Singh S. (2020). Autophagy in cancer: Recent advances and future directions. Seminars cancer Biol.66, 171–181. 10.1016/j.semcancer.2020.03.010
51
Jacome-Galarza C. Percin G. Muller J. Mass E. Lazarov T. Eitler J. et al (2019). Developmental origin, functional maintenance and genetic rescue of osteoclasts. Nature568 (7753), 541–545. 10.1038/s41586-019-1105-7
52
Jagga S. Sharma A. Lee Y. Nam J. Lee S. (2021). Sclerostin-mediated impaired osteogenesis by fibroblast-like synoviocytes in the particle-induced osteolysis model. Front. Mol. Biosci.8, 666295. 10.3389/fmolb.2021.666295
53
Jeffers J. Walter W. (2012). Ceramic-on-ceramic bearings in hip arthroplasty: State of the art and the future. J. bone Jt. Surg. Br.94 (6), 735–745. 10.1302/0301-620x.94b6.28801
54
Kang C. Wei L. Song B. Chen L. Liu J. Deng B. et al (2017). Involvement of autophagy in tantalum nanoparticle-induced osteoblast proliferation. Int. J. nanomedicine12, 4323–4333. 10.2147/ijn.S136281
55
Kaushik S. Cuervo A. (2018). The coming of age of chaperone-mediated autophagy. Nat. Rev. Mol. Cell. Biol.19 (6), 365–381. 10.1038/s41580-018-0001-6
56
Kawajiri S. Saiki S. Sato S. Sato F. Hatano T. Eguchi H. et al (2010). PINK1 is recruited to mitochondria with parkin and associates with LC3 in mitophagy. FEBS Lett.584 (6), 1073–1079. 10.1016/j.febslet.2010.02.016
57
Kim H. Choi H. Shin J. Kim K. Huh J. Lee S. et al (2009). Selective inhibition of RANK blocks osteoclast maturation and function and prevents bone loss in mice. J. Clin. investigation119 (4), 813–825. 10.1172/jci36809
58
Kim H. Kim T. Jeong B. Cho I. Han D. Takegahara N. et al (2013). Tmem64 modulates calcium signaling during RANKL-mediated osteoclast differentiation. Cell. metab.17 (2), 249–260. 10.1016/j.cmet.2013.01.002
59
Kobayashi Y. Udagawa N. Takahashi N. (2009). Action of RANKL and OPG for osteoclastogenesis. Crit. Rev. Eukaryot. gene Expr.19 (1), 61–72. 10.1615/critreveukargeneexpr.v19.i1.30
60
Kondapalli C. Kazlauskaite A. Zhang N. Woodroof H. Campbell D. Gourlay R. et al (2012). PINK1 is activated by mitochondrial membrane potential depolarization and stimulates Parkin E3 ligase activity by phosphorylating Serine 65. Open Biol.2 (5), 120080. 10.1098/rsob.120080
61
Kurtz S. Ong K. Lau E. Mowat F. Halpern M. (2007). Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. bone Jt. Surg. Am. volume89 (4), 780–785. 10.2106/jbjs.F.00222
62
Lachiewicz P. Soileau E. (2016). Highly cross-linked polyethylene provides decreased osteolysis and reoperation at minimum 10-year follow-up. J. arthroplasty31 (9), 1959–1962. 10.1016/j.arth.2016.02.038
63
Learmonth I. Young C. Rorabeck C. (2007). The operation of the century: Total hip replacement. Lancet (London, Engl.370 (9597), 1508–1519. 10.1016/s0140-6736(07)60457-7
64
Lee G. Lee K. (2022). Periprosthetic osteolysis as a risk factor for revision after total ankle arthroplasty: A single-center experience of 250 consecutive cases. J. bone Jt. Surg. Am. volume104 (15), 1334–1340. 10.2106/jbjs.21.01093
65
Lemasters J. (2005). Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res.8 (1), 3–5. 10.1089/rej.2005.8.3
66
Li S. Li M. Huo S. Wang Q. Chen J. Ding S. et al (2021). Voluntary-opsonization-enabled precision nanomedicines for inflammation treatment. Adv. Mater. Deerf. Beach, Fla)33 (3), e2006160. 10.1002/adma.202006160
67
Li W. C. Li Z. Wang H. He J. Zhu J. (2018). Nano-sized Al2O3 particle-induced autophagy reduces osteolysis in aseptic loosening of total hip arthroplasty by negative feedback regulation of RANKL expression in fibroblasts. Cell. death Dis.9 (8), 840. 10.1038/s41419-018-0862-9
68
Lin T. Tamaki Y. Pajarinen J. Waters H. Woo D. Yao Z. et al (2014). Chronic inflammation in biomaterial-induced periprosthetic osteolysis: NF-κB as a therapeutic target. Acta biomater.10 (1), 1–10. 10.1016/j.actbio.2013.09.034
69
Liu K. Qiu W. Naveen Raj E. Liu H. Huang H. Lin Y. et al (2017). Ubiquitin-coated nanodiamonds bind to autophagy receptors for entry into the selective autophagy pathway. Autophagy13 (1), 187–200. 10.1080/15548627.2016.1254864
70
Liu N. Meng J. Wang Z. Zhou G. Shi T. Zhao J. (2016). Autophagy mediated TiAl₆V₄ particle-induced peri-implant osteolysis by promoting expression of TNF-α. Biochem. biophysical Res. Commun.473 (1), 133–139. 10.1016/j.bbrc.2016.03.065
71
Lochner K. Fritsche A. Jonitz A. Hansmann D. Mueller P. Mueller-Hilke B. et al (2011). The potential role of human osteoblasts for periprosthetic osteolysis following exposure to wear particles. Int. J. Mol. Med.28 (6), 1055–1063. 10.3892/ijmm.2011.778
72
Lohmann C. Dean D. Bonewald L. Schwartz Z. Boyan B. (2002). Nitric oxide and prostaglandin E2 production in response to ultra-high molecular weight polyethylene particles depends on osteoblast maturation state. J. bone Jt. Surg. Am. volume84 (3), 411–419. 10.2106/00004623-200203000-00012
73
Lomaga M. Yeh W. Sarosi I. Duncan G. Furlonger C. Ho A. et al (1999). TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes. and Dev.13 (8), 1015–1024. 10.1101/gad.13.8.1015
74
Looney R. Schwarz E. Boyd A. O'Keefe R. (2006). Periprosthetic osteolysis: An immunologist's update. Curr. Opin. rheumatology18 (1), 80–87. 10.1097/01.bor.0000198004.88568.96
75
Lu S. McGough M. Shiels S. Zienkiewicz K. Merkel A. Vanderburgh J. et al (2018). Settable polymer/ceramic composite bone grafts stabilize weight-bearing tibial plateau slot defects and integrate with host bone in an ovine model. Biomaterials179, 29–45. 10.1016/j.biomaterials.2018.06.032
76
Madel M. Ibáñez L. Wakkach A. de Vries T. Teti A. Apparailly F. et al (2019). Immune function and diversity of osteoclasts in normal and pathological conditions. Front. Immunol.10, 1408. 10.3389/fimmu.2019.01408
77
Malchau H. Herberts P. Ahnfelt L. (1993). Prognosis of total hip replacement in Sweden. Follow-up of 92,675 operations performed 1978-1990. Acta Orthop. Scand.64 (5), 497–506. 10.3109/17453679308993679
78
Mijaljica D. Prescott M. Devenish R. (2011). Microautophagy in mammalian cells: Revisiting a 40-year-old conundrum. Autophagy7 (7), 673–682. 10.4161/auto.7.7.14733
79
Mizushima N. Komatsu M. (2011). Autophagy: Renovation of cells and tissues. Cell.147 (4), 728–741. 10.1016/j.cell.2011.10.026
80
Nai A. Lidonnici M. Federico G. Pettinato M. Olivari V. Carrillo F. et al (2021). NCOA4-mediated ferritinophagy in macrophages is crucial to sustain erythropoiesis in mice. Haematologica106 (3), 795–805. 10.3324/haematol.2019.241232
81
Nakashima T. Hayashi M. Fukunaga T. Kurata K. Oh-Hora M. Feng J. et al (2011). Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med.17 (10), 1231–1234. 10.1038/nm.2452
82
Narendra D. Jin S. Tanaka A. Suen D. Gautier C. Shen J. et al (2010). PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol.8 (1), e1000298. 10.1371/journal.pbio.1000298
83
Narendra D. Tanaka A. Suen D. Youle R. (2008). Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell. Biol.183 (5), 795–803. 10.1083/jcb.200809125
84
Nich C. Takakubo Y. Pajarinen J. Ainola M. Salem A. Sillat T. et al (2013). Macrophages-Key cells in the response to wear debris from joint replacements. J. Biomed. Mater. Res. Part A101 (10), 3033–3045. 10.1002/jbm.a.34599
85
Noordin S. Masri B. (2012). Periprosthetic osteolysis: Genetics, mechanisms and potential therapeutic interventions. Can. J. Surg. J. Can. de Chir.55 (6), 408–417. 10.1503/cjs.003711
86
Novack D. Mbalaviele G. (2016). Osteoclasts-key players in skeletal health and disease. Microbiol. Spectr.4 (3), 15. 10.1128/microbiolspec.MCHD-0011-2015
87
O'Brien C. Nakashima T. Takayanagi H. (2013). Osteocyte control of osteoclastogenesis. Bone54 (2), 258–263. 10.1016/j.bone.2012.08.121
88
O'Neill S. Queally J. Devitt B. Doran P. O'Byrne J. (2013). The role of osteoblasts in peri-prosthetic osteolysis. bone and Jt. J.95-B (8), 1022–1026. 10.1302/0301-620x.95b8.31229
89
Okafor C. Haleem-Smith H. Laqueriere P. Manner P. Tuan R. (2006). Particulate endocytosis mediates biological responses of human mesenchymal stem cells to titanium wear debris. J. Orthop. Res. official Publ. Orthop. Res. Soc.24 (3), 461–473. 10.1002/jor.20075
90
Parzych K. Klionsky D. (2014). An overview of autophagy: Morphology, mechanism, and regulation. Antioxidants redox Signal.20 (3), 460–473. 10.1089/ars.2013.5371
91
Pasparakis M. (2008). IKK/NF-kappaB signaling in intestinal epithelial cells controls immune homeostasis in the gut. Mucosal Immunol.1, S54–S57. 10.1038/mi.2008.53
92
Piemontese M. Onal M. Xiong J. Han L. Thostenson J. Almeida M. et al (2016). Low bone mass and changes in the osteocyte network in mice lacking autophagy in the osteoblast lineage. Sci. Rep.6, 24262. 10.1038/srep24262
93
Ping Z. Wang Z. Shi J. Wang L. Guo X. Zhou W. et al (2017). Inhibitory effects of melatonin on titanium particle-induced inflammatory bone resorption and osteoclastogenesis via suppression of NF-κB signaling. Acta biomater.62, 362–371. 10.1016/j.actbio.2017.08.046
94
Ploumi C. Daskalaki I. Tavernarakis N. (2017). Mitochondrial biogenesis and clearance: A balancing act. FEBS J.284 (2), 183–195. 10.1111/febs.13820
95
Pohl C. Dikic I. (2019). Cellular quality control by the ubiquitin-proteasome system and autophagy. Sci. (New York, NY)366 (6467), 818–822. 10.1126/science.aax3769
96
Price A. Alvand A. Troelsen A. Katz J. Hooper G. Gray A. et al (2018). Knee replacement. Lancet (London, Engl.392 (10158), 1672–1682. 10.1016/s0140-6736(18)32344-4
97
Purdue P. Koulouvaris P. Potter H. Nestor B. Sculco T. (2007). The cellular and molecular biology of periprosthetic osteolysis. Clin. Orthop. Relat. Res.454, 251–261. 10.1097/01.blo.0000238813.95035.1b
98
Qin X. Zhang J. Wang B. Xu G. Yang X. Zou Z. et al (2021). Ferritinophagy is involved in the zinc oxide nanoparticles-induced ferroptosis of vascular endothelial cells. Autophagy17 (12), 4266–4285. 10.1080/15548627.2021.1911016
99
Rabinovich-Nikitin I. Kirshenbaum L. (2021). YAP/TFEB pathway promotes autophagic cell death and hypertrophic cardiomyopathy in lysosomal storage diseases. J. Clin. investigation131 (5), e146821. 10.1172/jci146821
100
Rosenfeldt M. Ryan K. (2009). The role of autophagy in tumour development and cancer therapy. Expert Rev. Mol. Med.11, e36. 10.1017/s1462399409001306
101
Rubinsztein D. Codogno P. Levine B. (2012). Autophagy modulation as a potential therapeutic target for diverse diseases. Nat. Rev. Drug Discov.11 (9), 709–730. 10.1038/nrd3802
102
Scrivo A. Bourdenx M. Pampliega O. Cuervo A. (2018). Selective autophagy as a potential therapeutic target for neurodegenerative disorders. Lancet Neurology17 (9), 802–815. 10.1016/s1474-4422(18)30238-2
103
Shan L. Shan B. Graham D. Saxena A. (2014). Total hip replacement: A systematic review and meta-analysis on mid-term quality of life. Osteoarthr. Cartil.22 (3), 389–406. 10.1016/j.joca.2013.12.006
104
Shi B. Huang Q. Birkett R. Doyle R. Dorfleutner A. Stehlik C. et al (2017). SNAPIN is critical for lysosomal acidification and autophagosome maturation in macrophages. Autophagy13 (2), 285–301. 10.1080/15548627.2016.1261238
105
Skaug B. Jiang X. Chen Z. (2009). The role of ubiquitin in NF-kappaB regulatory pathways. Annu. Rev. Biochem.78, 769–796. 10.1146/annurev.biochem.78.070907.102750
106
Smith R. Smith T. Blieden T. Phipps R. (1997). Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am. J. pathology151 (2), 317–322.
107
Su B. Li D. Xu J. Zhang Y. Cai Z. Kauther M. et al (2018). Wear particles enhance autophagy through up-regulation of CD147 to promote osteoclastogenesis. Iran. J. basic Med. Sci.21 (8), 806–812. 10.22038/ijbms.2018.29347.7093
108
Sun X. Yang X. Zhao Y. Li Y. Guo L. (2018). Effects of 17β-estradiol on mitophagy in the murine mc3t3-E1 osteoblast cell line is mediated via G protein-coupled estrogen receptor and the ERK1/2 signaling pathway. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res.24, 903–911. 10.12659/msm.908705
109
Tekirdag K. Cuervo A. (2018). Chaperone-mediated autophagy and endosomal microautophagy: Joint by a chaperone. J. Biol. Chem.293 (15), 5414–5424. 10.1074/jbc.R117.818237
110
Todde V. Veenhuis M. van der Klei I. (2009). Autophagy: Principles and significance in health and disease. Biochimica biophysica acta1792 (1), 3–13. 10.1016/j.bbadis.2008.10.016
111
Tuan R. Lee F. T Konttinen Y. Wilkinson J. Smith R. Implant Wear Symposium 2007 Biologic Work Group (2008). What are the local and systemic biologic reactions and mediators to wear debris, and what host factors determine or modulate the biologic response to wear particles?J. Am. Acad. Orthop. Surg.16, S42–S48. 10.5435/00124635-200800001-00010
112
Varnum C. (2017). Outcomes of different bearings in total hip arthroplasty - implant survival, revision causes, and patient-reported outcome. Dan. Med. J.64 (3), B5350.
113
Vrahnas C. Blank M. Dite T. Tatarczuch L. Ansari N. Crimeen-Irwin B. et al (2019). Increased autophagy in EphrinB2-deficient osteocytes is associated with elevated secondary mineralization and brittle bone. Nat. Commun.10 (1), 3436. 10.1038/s41467-019-11373-9
114
Wang L. Gao Z. Zhang J. Huo Y. Xu Q. Qiu Y. (2021). Netrin-1 regulates ERK1/2 signaling pathway and autophagy activation in wear particle-induced osteoclastogenesis. Cell. Biol. Int.45 (3), 612–622. 10.1002/cbin.11544
115
Wang S. Deng Z. Ma Y. Jin J. Qi F. Li S. et al (2020). The role of autophagy and mitophagy in bone metabolic disorders. Int. J. Biol. Sci.16 (14), 2675–2691. 10.7150/ijbs.46627
116
Wang Z. Deng Z. Gan J. Zhou G. Shi T. Wang Z. et al (2017). TiAl6V4 particles promote osteoclast formation via autophagy-mediated downregulation of interferon-beta in osteocytes. Acta biomater.48, 489–498. 10.1016/j.actbio.2016.11.020
117
Wang Z. Huang Z. Gan J. Liu N. Zhou G. Shi T. et al (2015). The fibroblast expression of RANKL in CoCrMo-particle-induced osteolysis is mediated by ER stress and XBP1s. Acta biomater.24, 352–360. 10.1016/j.actbio.2015.06.024
118
Wang Z. Liu N. Liu K. Zhou G. Gan J. Wang Z. et al (2015). Autophagy mediated CoCrMo particle-induced peri-implant osteolysis by promoting osteoblast apoptosis. Autophagy11 (12), 2358–2369. 10.1080/15548627.2015.1106779
119
Wei X. Zhang X. Zuscik M. Drissi M. Schwarz E. O'Keefe R. (2005). Fibroblasts express RANKL and support osteoclastogenesis in a COX-2-dependent manner after stimulation with titanium particles. J. bone mineral Res. official J. Am. Soc. Bone Mineral Res.20 (7), 1136–1148. 10.1359/jbmr.050206
120
Wu J. Dang Y. Su W. Liu C. Ma H. Shan Y. et al (2006). Molecular cloning and characterization of rat LC3A and LC3B-wo novel markers of autophagosome. Biochem. biophysical Res. Commun.339 (1), 437–442. 10.1016/j.bbrc.2005.10.211
121
Wu W. Wang L. Mao Y. Dai K. Hao Y. (2020). Impaired autophagy in the fibroblasts by titanium particles increased the release of CX3CL1 and promoted the chemotactic migration of monocytes. Inflammation43 (2), 673–685. 10.1007/s10753-019-01149-0
122
Xing L. Schwarz E. Boyce B. (2005). Osteoclast precursors, RANKL/RANK, and immunology. Immunol. Rev.208, 19–29. 10.1111/j.0105-2896.2005.00336.x
123
Xiong Q. Zhang L. Ge W. Tang P. (2016). The roles of interferons in osteoclasts and osteoclastogenesis. Jt. bone spine83 (3), 276–281. 10.1016/j.jbspin.2015.07.010
124
Xu Y. Sang W. Zhong Y. Xue S. Yang M. Wang C. et al (2021). CoCrMo-Nanoparticles induced peri-implant osteolysis by promoting osteoblast ferroptosis via regulating Nrf2-ARE signalling pathway. Cell. Prolif.54 (12), e13142. 10.1111/cpr.13142
125
Xue S. Zhou X. Sang W. Wang C. Lu H. Xu Y. et al (2021). Cartilage-targeting peptide-modified dual-drug delivery nanoplatform with NIR laser response for osteoarthritis therapy. Bioact. Mater.6 (8), 2372–2389. 10.1016/j.bioactmat.2021.01.017
126
Yang H. Xu Y. Zhu M. Gu Y. Zhang W. Shao H. et al (2016). Inhibition of titanium-particle-induced inflammatory osteolysis after local administration of dopamine and suppression of osteoclastogenesis via D2-like receptor signaling pathway. Biomaterials80, 1–10. 10.1016/j.biomaterials.2015.11.046
127
Yin J. Yin Z. Lai P. Liu X. Ma J. (2022). Pyroptosis in periprosthetic osteolysis. Biomolecules12 (12), 1733. 10.3390/biom12121733
128
Yu J. Qin B. Moyer A. Nowsheen S. Liu T. Qin S. et al (2018). DNA methyltransferase expression in triple-negative breast cancer predicts sensitivity to decitabine. J. Clin. investigation128 (6), 2376–2388. 10.1172/jci97924
129
Zaidi M. (2007). Skeletal remodeling in health and disease. Nat. Med.13 (7), 791–801. 10.1038/nm1593
130
Zauli G. Rimondi E. Nicolin V. Melloni E. Celeghini C. Secchiero P. (2004). TNF-related apoptosis-inducing ligand (TRAIL) blocks osteoclastic differentiation induced by RANKL plus M-CSF. Blood104 (7), 2044–2050. 10.1182/blood-2004-03-1196
131
Zhai Z. Qu X. Li H. Yang K. Wan P. Tan L. et al (2014). The effect of metallic magnesium degradation products on osteoclast-induced osteolysis and attenuation of NF-κB and NFATc1 signaling. Biomaterials35 (24), 6299–6310. 10.1016/j.biomaterials.2014.04.044
132
Zhang G. Wang Y. Tang G. Ma Y. (2019). Puerarin inhibits the osteoclastogenesis by inhibiting RANKL-dependent and -independent autophagic responses. BMC complementary Altern. Med.19 (1), 269. 10.1186/s12906-019-2691-5
133
Zhang Q. Zhang X. (2019). Hyperoside decreases the apoptosis and autophagy rates of osteoblast MC3T3-E1 cells by regulating TNF-like weak inducer of apoptosis and the p38mitogen activated protein kinase pathway. Mol. Med. Rep.19 (1), 41–50. 10.3892/mmr.2018.9622
134
Zhang R. Lin J. Chen F. Chen M. (2021). Worldwide trends of research on periprosthetic osteolysis: A bibliometric study based on VOSviewer. Indian J. Orthop.55 (5), 1326–1334. 10.1007/s43465-021-00462-x
135
Zhang Y. Yan M. Niu W. Mao H. Yang P. Xu B. et al (2022). Tricalcium phosphate particles promote pyroptotic death of calvaria osteocytes through the ROS/NLRP3/Caspase-1 signaling axis in amouse osteolysis model. Int. Immunopharmacol.107, 108699. 10.1016/j.intimp.2022.108699
136
Zhang Y. Zheng Y. Qin L. (2011). A comprehensive biological evaluation of ceramic nanoparticles as wear debris. Nanomedicine Nanotechnol. Biol. Med.7 (6), 975–982. 10.1016/j.nano.2011.04.005
137
Zhang Z. Fu X. Xu L. Hu X. Deng F. Yang Z. et al (2020). Nanosized alumina particle and proteasome inhibitor bortezomib prevented inflammation and osteolysis induced by titanium particle via autophagy and NF-κB signaling. Sci. Rep.10 (1), 5562. 10.1038/s41598-020-62254-x
138
Zhang Z. Guo M. Li Y. Shen M. Kong D. Shao J. et al (2020). RNA-binding protein ZFP36/TTP protects against ferroptosis by regulating autophagy signaling pathway in hepatic stellate cells. Autophagy16 (8), 1482–1505. 10.1080/15548627.2019.1687985
139
Zhao J. Liu T. Jin S. Wang X. Qu M. Uhlén P. et al (2011). Human MIEF1 recruits Drp1 to mitochondrial outer membranes and promotes mitochondrial fusion rather than fission. EMBO J.30 (14), 2762–2778. 10.1038/emboj.2011.198
140
Zhao R. Chen N. Zhou X. Miao P. Hu C. Qian L. et al (2014). Exogenous IFN-beta regulates the RANKL-c-Fos-IFN-beta signaling pathway in the collagen antibody-induced arthritis model. J. Transl. Med.12, 330. 10.1186/s12967-014-0330-y
141
Zhao Y. Wei J. Tian Q. Liu A. Yi Y. Einhorn T. et al (2016). Progranulin suppresses titanium particle induced inflammatory osteolysis by targeting TNFα signaling. Sci. Rep.6, 20909. 10.1038/srep20909
Summary
Keywords
periprosthetic osteolysis, autophagy, mitophagy, aseptic loosening, RANKL
Citation
Yin Z, Gong G, Wang X, Liu W, Wang B and Yin J (2023) The dual role of autophagy in periprosthetic osteolysis. Front. Cell Dev. Biol. 11:1123753. doi: 10.3389/fcell.2023.1123753
Received
14 December 2022
Accepted
16 March 2023
Published
24 March 2023
Volume
11 - 2023
Edited by
Ozgur Kutuk, Başkent University, Türkiye
Reviewed by
Qiangrong Liang, New York Institute of Technology, United States
Alexandrina Ferreira Mendes, University of Coimbra, Portugal
Updates
Copyright
© 2023 Yin, Gong, Wang, Liu, Wang and Yin.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Yin, yinjiandoc@163.com; Bin Wang, wangbin2495@163.com
†These authors have contributed equally to this work
This article was submitted to Cell Death and Survival, a section of the journal Frontiers in Cell and Developmental Biology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.