Abstract
Hematopoiesis is a finely orchestrated process, whereby hematopoietic stem cells give rise to all mature blood cells. Crucially, they maintain the ability to self-renew and/or differentiate to replenish downstream progeny. This process starts at an embryonic stage and continues throughout the human lifespan. Blood cancers such as leukemia occur when normal hematopoiesis is disrupted, leading to uncontrolled proliferation and a block in differentiation of progenitors of a particular lineage (myeloid or lymphoid). Although normal stem cell programs are crucial for tissue homeostasis, these can be co-opted in many cancers, including leukemia. Myeloid or lymphoid leukemias often display stem cell-like properties that not only allow proliferation and survival of leukemic blasts but also enable them to escape treatments currently employed to treat patients. In addition, some leukemias, especially in children, have a fetal stem cell profile, which may reflect the developmental origins of the disease. Aberrant fetal stem cell programs necessary for leukemia maintenance are particularly attractive therapeutic targets. Understanding how hijacked stem cell programs lead to aberrant gene expression in place and time, and drive the biology of leukemia, will help us develop the best treatment strategies for patients.
Introduction
Stem cells perform a complex balancing act between self-renewal and differentiation throughout ontogeny. To perform these functions, stem cells proliferate rapidly and repair DNA damage. However, these “stemness” properties present a vulnerability as, if hijacked, they provide cancer cells with the pathways required for growth and survival (Taipale and Beachy, 2001; Hanahan and Weinberg, 2011).
Hematopoietic stem cells
Human hematopoiesis is a dynamic process beginning at day 18 in the yolk sac (Palis and Yoder, 2001), with definitive hematopoietic stem cells (HSCs) originating in the aorta–gonad–mesonephros from 4 post-conception weeks (pcw) (Tavian and Peault, 2005; Ivanovs et al., 2011). HSCs subsequently migrate to fetal liver (FL), the main site of hematopoiesis until birth (Ivanovs et al., 2017), with contribution from fetal bone marrow (FBM) 10–12 pcw (Charbord et al., 1996; O’Byrne et al., 2019). After birth, BM becomes the sole site of hematopoiesis.
Fetal HSCs are molecularly and functionally distinct from postnatal HSCs
Fetal HSCs are more proliferative than their postnatal counterparts (Lansdorp et al., 1993; Muench et al., 1994; Bowie et al., 2006; Popescu et al., 2019; Roy et al., 2021) with higher self-renewal capacity (Copley and Eaves, 2013; Copley et al., 2013), better in vivo engraftment (Harrison et al., 1997; Holyoake et al., 1999), and a distinct metabolic profile (Manesia et al., 2015). Adult HSCs show a myeloid lineage bias (Benz et al., 2012); additionally, innate lymphoid cells such as B1a B-cells are derived exclusively from embryonic/fetal HSCs (Zhou et al., 2015; Montecino-Rodriguez et al., 2016). These functional differences may be a consequence of distinct developmental gene expression programs.
Stem cell programs in leukemia
In leukemia, stem cell programs may be inappropriately reactivated or retained and/or co-opted from fetal development. This may be a consequence of some leukemias originating in utero, especially in children (Greaves, 2005).
In this review, we discuss stem cell programs that are aberrantly active in the wrong cellular context (“place”) or stage of ontogeny (“time”) in pediatric leukemia and their potential applications in developing targeted therapies (Figure 1).
FIGURE 1
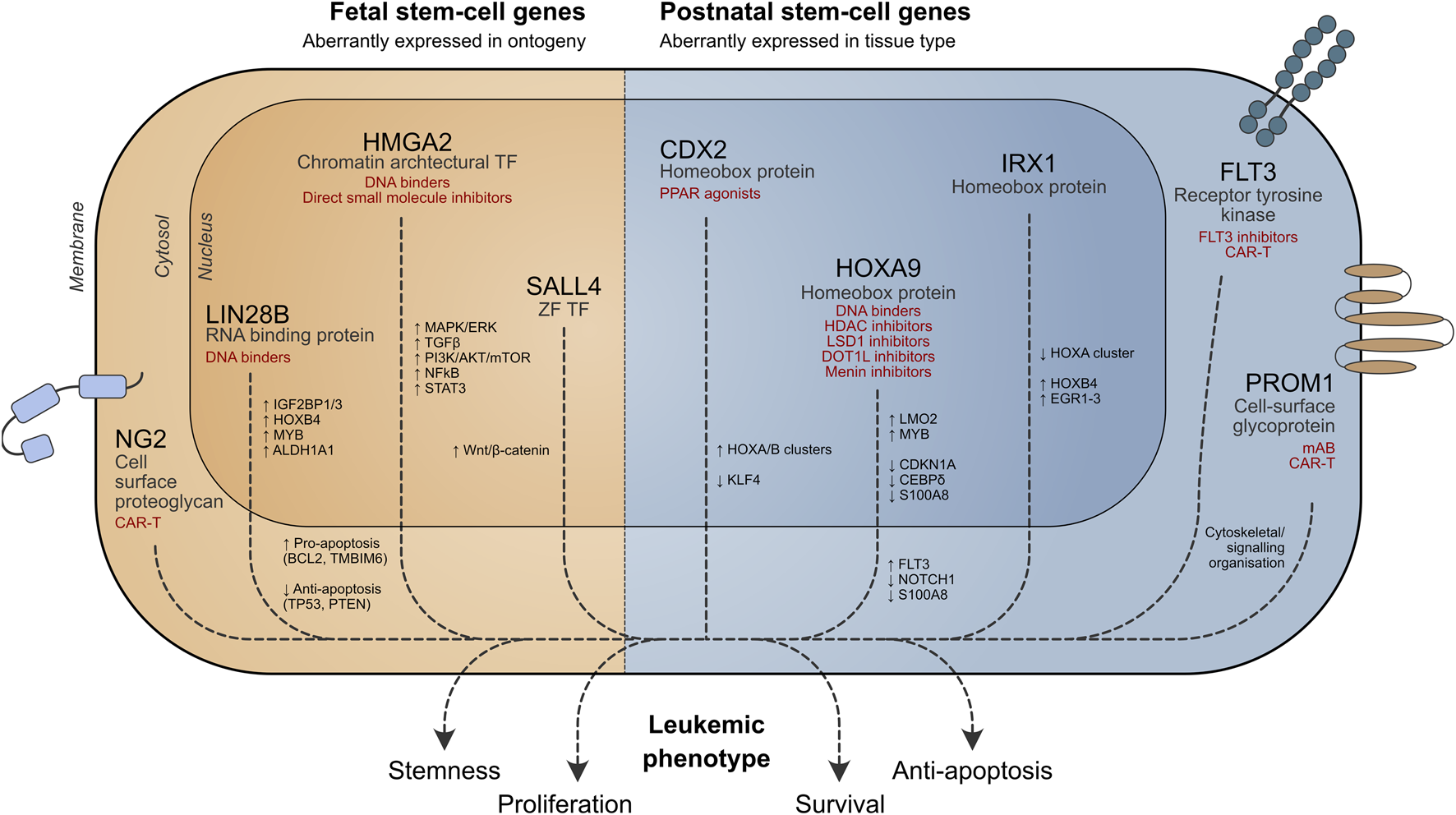
Role of stem cell genes in leukemia cell survival. Potential therapeutic strategies targeting aberrant stem cell-associated pathways are shown in red. TF = transcription factor, ZF = zinc finger.
Stem cell programs in the wrong place
Aberrant stem cell genes in leukemia
Leukemic blasts may exhibit stem cell properties, conferring a more aggressive phenotype. Several stem cell genes are important for leukemia biology (Table 1), and some key examples are discussed below.
TABLE 1
| Stem cell gene | Normal place of and time of expression | Normal function | Aberrant expression and function | Therapeutic potential | Key reference |
|---|---|---|---|---|---|
| Transcription factors | |||||
| HOXA9 | Thoraco-caudal neural tube and somites; limb buds | Expression of stemness genes, including FLT3, MYB, CDK6, and RUNX1 | KMT2A rearranged ALL and AML (70% of cases) NPM1c-mutated AML | Pre-clinical: co-factor (MEIS1) inhibitors and DNA binders | Vey et al. (2017), Stein et al. (2018), Sonoda et al. (2021), Abedin et al. (2022), Issa et al. (2023), Perner et al. (2023) |
| Fetal and adult HSCs | Drives self-renewal and differentiation block | Clinical: HDAC inhibitors (abexinostat and pracinostat); LSD1 inhibitors (bomedemstat); and DOT1L inhibitors (pinometostat); menin inhibitors (revumenib) | |||
| CDX2 | Embryonic trophectoderm development and gut patterning adult intestinal stem cells | Activation of trophectoderm and intestinal development programs | AML and ALL | Pre-clinical: PPARγ agonists to derepress KLF4 | Esmaeili et al. (2021) |
| Activates HOX genes and represses KLF4 expression | |||||
| LMO2 | Early yolk sac and fetal definitive hematopoiesis HSCs and angiogenesis | Transcription factor complex with TAL1, GATA1, 2, and 3 | Blocks differentiation in T-ALL | Preclinical: macromolecule inhibition of LMO2 is not efficacious as monotherapy | Yamada et al. (1998), Ferrando et al. (2002), Malumbres et al. (2011), Riddell et al. (2014) |
| Can induce pluripotency in fibroblasts | |||||
| SALL4 | ESCs, postnatal HSCs | Transcription factor and epigenetic regulator | Pediatric AML | Preclinical | Zhang et al. (2006), Ballerini et al. (2008), Yang et al. (2008), Aguila et al. (2011), Jeong et al. (2011), Ueno et al. (2014), Hodeib et al. (2023) |
| Pediatric B-ALL | |||||
| Can induce pluripotency in fibroblasts | |||||
| SOX17 | Fetal HSCs | Transcription factor | Limited evidence in pediatric AML. | Early-phase clinical trials of tazemetostat | Kim et al. (2007), Kormish et al. (2010), Tang et al. (2014) |
| Regulates endoderm and hemopoietic differentiation and inhibits Wnt signaling | |||||
| RUNX1 (Hsa21) | Embryonic/fetal HSCs | Essential for establishment of definitive hematopoiesis | RUNX1A isoform important in ML-DS cooperates with GATA1s and miR-125b to upregulate MYC | Preclinical MYC inhibitor: MYCi361 | North et al. (2002), Gialesaki et al. (2023) |
| Epigenetic modifiers | |||||
| HMGA 1 and 2 | ESC | Binding to the minor groove of AT-rich DNA sequences alters the chromatin structure to regulate transcription | KMT2A rearranged ALL | Preclinical data: competitive DNA minor groove binders | Roy et al. (2013), Wu et al. (2015), Cinkornpumin et al. (2017), Kumar et al. (2019), Roy et al. (2021) |
| Fetal HSC and MPP. | Relapsed B-ALL | Downstream pathway modulation | |||
| Direct small-molecule inhibition | |||||
| HMGN1 (Hsa21) | Fetal and adult stem and progenitor cells | De-compacts chromatin and acts in opposition to histone H1 | DS-ALL: overexpression promotes PreB-cell expansion and upregulates CRLF2 | Pre-clinical: GSK-J4, which targets HMGN1 via inhibition of histone demethylases | Cabal-Hierro et al. (2020), Page et al. (2022) |
| AML | |||||
| DNMT3A and B | ESC adult HSCs | DNA methyltransferase to silence HSC regulatory genes | Pediatric AML | Phase 1 trial DNMT inhibitor azacytidine in pediatric r/r AML. | Challen et al. (2011), Liang et al. (2013), Ueno et al. (2014), Liao et al. (2015), Sun et al. (2018) |
| DMNT3B included in pLSC6 | Ongoing trial in treatment-naive pediatric AML (NCT03164057) | ||||
| EZH2 | ESCs | Component of the PRC2 complex to initiate gene repression | High-risk pediatric AML | EZH2 inhibitor tazemetostat | Ezhkova et al. (2009), Mochizuki-Kashio et al. (2011), D'Angelo et al. (2015), Schäfer et al. (2016), Bond et al. (2018), Italiano et al. (2018) |
| Fetal and adult HSCs | B-ALL | Early-phase clinical trial for non-Hodgkin lymphoma and solid malignancies | |||
| T-ALL | |||||
| LSC gene sets | |||||
| LSC17 | Stem cell gene set | DNMT3B, GPR56, CD34, SOCS2, FAM30A, ZBTB46, NYNRIN, ARHGAP22, LAPTM4B, MMRN1, DPYSL3, CDK6, CPXM1, SMIM24, EMP1, NGFRAP1, and AKR1C3 | AML | Prognostic in adult and pediatric AML cohorts | Ng et al. (2016b) |
| pLSC6 | Stem cell gene set | DNMT3B, GPR56, CD34, SOCS2, SPINK2, and FAM30A | AML | Prognostic in pediatric AML cohorts | Elsayed et al. (2020) |
| RNA-binding proteins (RBPs) | |||||
| LIN28B | ESCs, fetal HSCs, MPP, and LMPP. | Maintains stem cell pluripotency | Pediatric AML | Preclinical data for small-molecule inhibitors including 1,632 | Yuan et al. (2012), Copley et al. (2013), Helsmoortel et al. (2016a), Helsmoortel et al. (2016b), Zhang et al. (2016), Wang et al. (2019) |
| JMML | |||||
| Acts as negative regulator of Let-7 micro RNAs (which suppress many oncogenes) | B-ALL | ||||
| Can induce pluripotency | |||||
| IGF2BP1 and IGF2BP3 | Fetal HSCs, MPP, and LMPP | Posttranscriptional regulation of genes in fetal life | Pediatric AML | Potential for induction of let-7 miRNA to reduce IGF2BP1/3 levels | Alajez et al. (2012), Elagib et al. (2017), Elcheva et al. (2020), Lin et al. (2023), Sharma et al. (2023) |
| Pediatric ALL | |||||
| miRNA | |||||
| miR-99a/99b | HSCs | Self-renewal | Pediatric AML and ALL | Moqadam et al. (2013), Zhang et al. (2013), Khalaj et al. (2017) | |
| miR-125b (Hsa21) | HSCs | Anti-apoptotic. Confers lymphoid bias to HSCs | Pediatric ALL and Ph + ALL | Prognostic marker in ALL and APML | Klusmann et al. (2010), Ooi et al. (2010), Zhang et al. (2011), Piatopoulou et al. (2017), Alejo-Valle et al. (2022) |
| AMKL and APML | Suppresses ARID3A and cooperates with GATA1s in ML-DS models | ||||
| ML-DS | |||||
| miR-128a | HSCs | Maintains stem cell pluripotency | Pediatric AML, ALL, and KMTA2Ar ALL | Georgantas et al. (2007), Mi et al. (2007), De Luca et al. (2017), Malouf et al. (2021) | |
| miR-155 | HSCs | Bias toward B lymphoid commitment | Pediatric ALL and AML | Synthetic miR-155 phase I T cell lymphoma and ATLL trial (NTC02580552) | Georgantas et al. (2007), Yan et al. (2015), Bayraktar and Van Roosbroeck (2018), Liang et al. (2021) |
| miR-181 | HSCs | Regulates HSC differentiation | Pediatric ALL and AML | Zhu et al. (2017), Egyed et al. (2020) | |
| miR-196b | HSCs | Increased cell survival and proliferation | Pediatric AML, KMT2Ar ALL, and T-ALL | Popovic et al. (2009), Schotte et al. (2009), Yan et al. (2015) | |
| 24-miRNA | miRNA signature | mir-20b, mir-223, miR-193, miR-24, miR-128, miR-17, miR-199b, miR-181c, miR-181b, miR-181a, miR-21, miR-222, miR-331, miR-373, miR-708, miR-34b, miR-195, miR-151a, miR-30b, miR-22, let7g, let7i, miR-1290, and miR-9 | Pediatric AML | Risk stratification | Esperanza-Cebollada et al. (2023) |
| Adhesion proteins | |||||
| CD44 | Widespread, including HSCs | Osteopontin/fibronectin/hyaluronan receptor. Adhesion and migration | AML LSC homing to BM | Pre-clinical: CD44 targeting mAb | Jin et al. (2006), Amanzadeh et al. (2018) |
| Apoptotic resistance | |||||
| Integrins | Widespread, including HSCs | BM microenvironment adhesion and signaling | AML LSC homing to BM | Preclinical | Jäger et al. (2007), Hsieh et al. (2013), Maeda et al. (2015), Darwish et al. (2021), Sudha et al. (2021) |
| ITGAM (CD11b) | Anti-apoptotic signaling | α4 targeting mAb | |||
| ITGAV (CD51) | Chemoresistance | β2 targeting CAR-T | |||
| ITGA2/4/6 | Thyrointegrin (αvβ3) targeting drugs | ||||
| ITGB1/2/3 | VLA-4 (α4β1) targeting peptide | ||||
| Selectins | Endothelial cells, leukocytes, and platelets | Permit hematopoietic cell adhesion and rolling | Supports AML LSC survival via PI3K/Akt signaling | Clinical: E-selectin antagonist (uproleselan) | DeAngelo et al. (2022) |
| SELE (CD62E) | |||||
| SELL (CD62L) | Supports HSC proliferation in the BM niche | ||||
| SELP (CD62P) | |||||
| Other/surface proteins | |||||
| PROM1 | Fetal and adult stem cell populations, including HSCs | Unclear; roles in Wnt signaling, PI3K signaling, and regulating membrane topology | KMT2A-r ALL | Pre-clinical: monoclonal antibody, bispecific CD19/CD133 CAR-T | Wang et al. (2018b), Li et al. (2018), Dai et al. (2020), Vora et al. (2020) |
| Cancer stem cells | Clinical: monospecific CD133 CAR-T | ||||
| CRLF2 (Hsa21) | Hematopoiesis | Dimerizes with IL7RA to form the receptor for thymic stromal lymphopoietin | Overexpressed and rearranged in | Tyrosine kinase inhibitors | Bagashev et al. (2022), Tian et al. (2023) |
| Fetal lymphopoiesis | DS-ALL BCR-ABL like ALL | Preclinical: TSLPR CAR-T; IB7/CD3 bispecific antibody | |||
| DYRK1A (Hsa21) | Neural tissues | Serine/threonine kinase. Required for normal lymphoid maturation | ML-DS | Preclinical: small-molecule DYRK1A inhibitor, EHT1610 | Malinge et al. (2012), Thompson et al. (2015), Bhansali et al. (2021), Carey-Smith et al. (2024) |
Summary of developmental/stem cell genes involved in the pathophysiology of pediatric leukemia.
Italicsed words are gene names.
Homeobox genes
Homeobox genes form a group of transcription factors (TFs) with 235 functional genes in humans (Holland et al., 2007).
HOXA9
The largest class of homeobox genes, the HOX genes (Holland, 2013), play important roles in hematopoiesis, such as development and maintenance of HSCs (Magnusson et al., 2007; Ramos-Mejía et al., 2014). HOXA9 can reprogram tissues to a hematopoietic fate (Ng E. S. et al., 2016; Sugimura et al., 2017).
Over 50% of AML cases overexpress HOXA9, which correlates with poor survival (Drabkin et al., 2002; Andreeff et al., 2008; Tholouli et al., 2012). HOXA9 directly binds target genes along with PBX3/MEIS1 (Wong et al., 2007; Li et al., 2016), upregulating oncogenes such as FLT3, LMO2, and MYB(Huang et al., 2012; Collins et al., 2014). HOXA9 also suppresses apoptotic/differentiation factors, promoting leukemia cell survival and maintaining a more stem-like state (Agrawal-Singh et al., 2023).
HOXA9 plays a key role in KMT2A-rearranged (KMT2A-r) leukemia (Faber et al., 2009; Orlovsky et al., 2011), mediated by binding of the KMT2A fusion protein to HOX gene promoters (Milne et al., 2002; Milne et al., 2005) and recruitment of DOT1L H3K79 methyltransferases (Bernt et al., 2011; Kerry et al., 2017). Dysregulated HOXA9 expression is also seen in NPM1c-mutated AML (Brunetti et al., 2018; Uckelmann et al., 2023).
CDX2
CDX2 of the ParaHox family of homeobox genes (Brooke et al., 1998) is not normally expressed in hematopoietic cells and inhibits the hematopoietic potential of murine embryonic stem cells (ESCs) (McKinney-Freeman et al., 2008).
Over 90% of AML cases overexpress CDX2 (Chase et al., 1999; Scholl et al., 2007; Rawat et al., 2008), and ectopic expression of Cdx2 confers oncogenic properties to murine HSCs (Rawat et al., 2004; Scholl et al., 2007). CDX2 overexpression studies suggest upregulation of HOX genes as the oncogenic mechanism (Rawat et al., 2008), although at least one alternative pathway is through direct suppression of KLF4 (Faber et al., 2013).
Aberrant CDX2 expression is also frequently seen in ALL (Riedt et al., 2009; Thoene et al., 2009) and confers a poor prognosis (Yasuda et al., 2022).
IRX genes
The Iroquois genes (IRX1-6) belong to the TALE group of homeobox genes. IRX1 is required for normal development of kidney and neural tissues (Alarcón et al., 2008; Freese et al., 2014) but not expressed in most hematopoietic precursors (Nagel et al., 2022). IRX3 and IRX5 are not expressed in hematopoiesis.
In KMT2A-r infant ALL, IRX1 or HOXA9 expression defines two distinct subgroups (Stam et al., 2010; Symeonidou and Ottersbach, 2021). IRX1 prevents KMT2A::AFF1 from activating HOXA9 expression; instead, expression of HOXB4 causes persistence of HSC factors (Kuhn et al., 2016). IRX1+(HOXA-) KMT2A-r ALL has a poorer prognosis (Agraz-Doblas et al., 2019; Isobe et al., 2022).
In AML, IRX1/3/5 is aberrantly expressed (Nagel and Meyer, 2022). In AML cell lines, expressions of IRX1 and IRX3/5 are mutually exclusive with opposing effects on GATA1/2 activity (Somerville et al., 2018).
PROM1
Prominin-1 (PROM1/CD133) is a membrane pentaspan glycoprotein, identified in the mouse neuroepithelium (Weigmann et al., 1997) and human HSCs (Miraglia et al., 1997; Yin et al., 1997). Its function remains enigmatic; and its role in cytoskeletal organization is postulated. In solid organ malignancies, PROM1 regulates extracellular vesicle formation and release (Rappa et al., 2013; Kang et al., 2019; Zhao et al., 2020).
PROM1 is expressed in many cancers (Reya et al., 2001; Singh et al., 2004; Bao et al., 2006; Ferrandina et al., 2009), including leukemia, where the expression is associated with poor prognosis (Tolba et al., 2013). It is a common feature of KMT2A-r leukemias and essential for survival of KMT2A::AFF1 cell lines (Godfrey et al., 2021; Wang L.-l. et al., 2022).
Leukemic stem cells
In addition to inappropriate expression of stem cell genes, there may be very rare leukemic stem cells (LSCs) within a leukemia that can propagate disease in serial transplantation (Lapidot et al., 1994; Hope et al., 2004; Hong et al., 2008). This concept is well-established in AML (Dick, 2008), but less so in ALL (Cox et al., 2009). AML LSCs share the key feature of self-renewal with normal HSCs alongside similarities in gene expression (Sachs et al., 2020), immunophenotype (e.g., CD133+(Tolba et al., 2013), CD123+(Testa et al., 2002)), and metabolism (Manesia et al., 2015).
A stem cell signature (LSC17) has been used to risk stratify adult (Ng S. W. K. et al., 2016) and some pediatric AMLs (Duployez et al., 2019), where it confers a worse prognosis. A six-gene pediatric AML signature (pLSC6) (Elsayed et al., 2020) was derived from a cohort of 163 pediatric AMLs (Table 1). The adult and pediatric signatures share five genes (DNMT3B, GPR56, CD34, FAM30A/KIAA012, and SOCS2), although with different gene weightings, and pLSC6 includes a further unique gene, SPINK2.
Single-cell RNA-seq identified LSC clusters in pediatric AML; these LSC markers require functional validation (Zhang Y. et al., 2023). Fetal-specific genes (HMGA2) have been identified in a rare HSC-like fraction of KMT2A-r infant leukemia (Chen et al., 2022), and IGF2BP1 maintains LSC by regulating HOXB4, MYB, and ALDH1A1 in pediatric leukemia cell lines (Elcheva et al., 2020).
Aberrant stem cell gene expression in ontogeny
Fetal stem cell programs in pediatric leukemia
Pediatric leukemias [and solid malignancies such as neuroblastoma (Molenaar et al., 2012)] may exhibit properties of fetal HSCs. This may represent a fetal cell of origin or indicate reactivation of fetal programs (Monk and Holding, 2001; Sharma et al., 2022; Solé et al., 2022). Either way, the inappropriate expression of fetal genes is important for cancer biology. Leukemic blasts, especially LSCs (Somervaille et al., 2009), show fetal-specific gene expression profiles (Wu et al., 2015; Helsmoortel et al., 2016b; Elcheva et al., 2020; Bai et al., 2021; Tran et al., 2021). Down syndrome (DS)-associated leukemias and juvenile myelomonocytic leukemia (JMML) are two examples resulting from perturbation of fetal HSPCs.
Trisomy 21 causes global perturbation of fetal hematopoiesis, with increased phenotypic HSCs and megakaryocyte–erythroid progenitors (MEPs), as well as a marked skew to erythropoiesis with a concomitant decrease in B-lymphopoiesis (Chou et al., 2008; Roy et al., 2012; Jardine et al., 2021). DS fetal HSPCs and stromal cells also display increased inflammatory signatures (Jardine et al., 2021). Chromosome 21 (Hsa21) stem cell genes dysregulated/overexpressed in DS include transcription factor GABPA, which affects HSC maintenance/differentiation (Yu et al., 2011), and chromatin modifier HMGN1.
Children with DS have an increased risk of AML and ALL (Hasle et al., 2000). Mutations in the megakaryocyte–erythroid transcription factor GATA1 in fetal life lead to transient abnormal myelopoiesis (TAM) in the fetal/neonatal period (Roberts et al., 2013; Wagenblast et al., 2021). Additional mutations, most commonly in the cohesin complex genes (Labuhn et al., 2019), are required for myeloid leukemia of DS (Roberts and Izraeli, 2014). DS-ALL also likely stems from perturbed lymphopoiesis, which begins in utero, and is characterized by CRLF2/TSLPR overexpression in 50% and JAK2 mutations in 20% (Li et al., 2023). Key Hsa21 genes important for leukemogenesis are listed in Table 1.
JMML, an HSC-derived leukemia (Cooper et al., 2000; Louka et al., 2021), has a fetal molecular profile (Roy et al., 2021; Hartmann et al., 2023). As the oncogenic hit probably occurs in fetal HSCs, developmental stem cell programs are hijacked for leukemogenesis.
Fetal oncogenes relevant to stem cell activity and implicated in pediatric leukemia act via a diverse range of mechanisms and are discussed below (Figure 1).
Fetal genes with transcription factor activity implicated in pediatric leukemia
SCL/TAL1 was originally identified as overexpressed in T-ALL (Ferrando et al., 2002). Ablation of the gene causes embryonic death (Porcher et al., 2017), but it is dispensable for adult HSCs (Mikkola et al., 2003).
LMO2 is essential for fetal hematopoiesis (Yamada et al., 1998). LMO2 expression is seen in a subset of pediatric T-ALL (Ferrando et al., 2002) and B-ALL (Malumbres et al., 2011). In gene therapy trials for SCID, two patients developed T-ALL through off-target activation of LMO2 (Hacein-Bey-Abina et al., 2003).
SALL4 is expressed in ESCs (Zhang et al., 2006), but downregulated postnatally with only low-level expression in HSCs (Gao et al., 2013). It is aberrantly expressed in pediatric AML (Ballerini et al., 2008) and ALL (Den Boer et al., 2009; Ueno et al., 2014), with overexpression conferring poor prognosis (Harvey et al., 2010; Jeong et al., 2011).
Fetal genes important for post-transcriptional regulation
The bulk of post-transcriptional control is exerted by RNA-binding proteins (RBPs). Some RBPs such as LIN28B and insulin-like growth factor 2 mRNA-binding proteins (IGF2BP1/IGF2BP3) have a fetal expression pattern, a role in stem cell biology (Copley and Eaves, 2013; Degrauwe et al., 2016; Zhang et al., 2016) and pediatric leukemia.
LIN28B
LIN28B has wide-ranging physiological roles in fetal tissues; however, the expression after birth is limited to the placenta and testis (Uhlén et al., 2015). In hematopoiesis, FL HSCs have the highest expression of LIN28B (Roy et al., 2021), and expression in postnatal cells can reactivate fetal-like erythropoiesis (Lee et al., 2013; Basak et al., 2020) and B-lymphopoiesis (Yuan et al., 2012). LIN28B can reprogram somatic cells to induce pluripotency (Zhang et al., 2016); however, this can be oncogenic in other contexts (Wuputra et al., 2020).
The main action of LIN28B is to prevent maturation of let-7 miRNAs (Rybak et al., 2008; Viswanathan and Daley, 2010). LIN28B also directly stabilizes many mRNAs in conjunction with IGF2BP1/IGF2BP3. In murine B progenitors, this includes Pax5 and Arid3a, thereby driving fetal B-lymphopoiesis (Wang et al., 2019).
LIN28B is frequently aberrantly expressed in cancers, including leukemia (Viswanathan et al., 2009; Balzeau et al., 2017). Suppression of let-7 miRNAs by LIN28B leads to de-repression of oncogenes (MYC, RAS, MYB, and ARID3A) and epigenetic regulators, HMGA2 and CBX2 (Wang D. et al., 2022).
A meta-analysis showed that 7.5% of pediatric leukemias express LIN28B (Helsmoortel et al., 2016b). Aberrant LIN28B expression defines a poor prognosis subgroup in JMML (Helsmoortel et al., 2016a), where H19, a fetal oncogene (Matouk et al., 2014), is stabilized in the presence of LIN28B (Helsmoortel et al., 2016b). AML in children <3 years has higher levels of LIN28B (and IGF2BP1/3) expression than in children >3 years (Bolouri et al., 2021). Although LIN28B has predominantly been reported to have a pro-leukemic role in AML (Zhou et al., 2017), one study on a murine KMT2A::MLLT3 AML model suggests that LIN28B abrogates perinatal leukemia development (Eldeeb et al., 2023). Given >50% of human neonatal leukemias are of myeloid lineage, these findings seem counterintuitive, although it is possible that neonatal AML arises from LIN28B negative progenitors. Given the role of LIN28B in fetal B-lymphopoiesis, it may also be important for ALL initiation or maintenance.
IGF2BP1 and IGF2BP3
IGF2BP1 and IGF2BP3 are important for fetal organogenesis and are expressed in FL HSCs, but not in adult HSCs (Wang D. et al., 2022). Induction of IGF2BP3 in adult HSCs induces a fetal-type output (Palanichamy et al., 2016; Wang et al., 2019).
IGF2BP1 and IGF2BP3 have been linked to leukemia, as well as solid malignancies, and are often co-expressed with LIN28B (Elcheva et al., 2020; Tran et al., 2021). The mechanism of action for IGF2BP3 in oncogenesis is segregation of mRNA transcripts from the cytoplasmic RNA-induced silencing complex, including the let-7 miRNA family.
IGF2BP1 is linked to pediatric AML and ETV6/RUNX1 B-ALL, while IGF2BP3 is linked to AML, KMT2A::AFF1 ALL, and BCR::ABL1 ALL (Stoskus et al., 2011; Palanichamy et al., 2016; Elcheva et al., 2020; Zhang et al., 2022). IGF2BP1 supports an LSC phenotype in AML (Elcheva et al., 2020). In AML cell lines, knockdown of IGF2BP3 leads to reduced cell proliferation in an N6-methyladenosine (m6A)-dependent fashion (Zhang et al., 2022). Depletion of the murine paralog IGF2BP3 increases the latency of leukemia in murine models of KMT2A::AFF1 AML (Tran et al., 2021).
Fetal genes important for epigenetic regulation
HMGA1 and 2
HMGA1 and HMGA2 are fetal oncogenes affecting epigenetic regulation. The HMGA family encodes proteins with AT hooks which interact with DNA to alter the chromatin architecture. These genes have much lower expression in adult tissues than in the fetal counterparts (Kumar et al., 2019; Roy et al., 2021), and HMGA1 can promote a pluripotent state (Shah et al., 2012). HMGA2 is expressed mainly in fetal HSC/MPP and influences both differentiation and proliferation of stem cells (Battista et al., 2003; Li et al., 2007; Copley et al., 2013), as well as promoting long-term in vivo reconstitution by cord blood CD34+ cells (Kumar et al., 2019).
Reactivation of HMGA1 and HMGA2 has been demonstrated in a wide range of malignancies (Huso and Resar, 2014; Mansoori et al., 2021) including leukemia (Efanov et al., 2014). HMGA1 expression has been linked to risk of relapse in pediatric B-ALL (Roy et al., 2013). In pediatric and adult AML, high expression of HMGA2 is linked to poor prognosis, and knockdown of the gene has induced differentiation in primary blasts (Marquis et al., 2018; Tan et al., 2018). HMGA2 induces T-ALL in a Eμ-HMGA2 transgenic mouse (Efanov et al., 2014).
microRNAs in leukemia
Aberrant expression of microRNAs (miRNAs) specific to fetal life and stem cell compartment (O'Connell et al., 2010) is implicated in pediatric leukemia (Grobbelaar and Ford, 2019; Gaur et al., 2020) (Table1). Pediatric AML can be risk-stratified by a 24-miRNA signature (Esperanza-Cebollada et al., 2023). Eight of these have target genes within the pLSC6 signature and includes let-7 miRNAs (known repressors of oncogenes), with lower let-7g/let-7i expression in high-risk AML. One of the pLSC6 genes (FAM30A) is an lncRNA. Signatures based on lncRNA differentiate pediatric leukemia subtypes, but do not inform prognosis (Buono et al., 2022).
Targeting aberrant stem cell programs in leukemia for therapy
The inappropriate expression of stem cell genes, while conferring survival advantage to leukemic cells, can also render them dependent on specific proteins or pathways, and thus vulnerable to targeted disruption. Fetal stem cell genes are the most attractive targets as they are not expressed in healthy postnatal tissues, ameliorating concerns about off-target effects. Genes expressed in leukemic and healthy postnatal stem cells present more of a challenge. However, excessive leukemic reliance on the aberrant pathway, the so-called “oncogenic addiction,” can generate a therapeutic window, whereby leukemic cells can be killed while sparing normal stem cells. Potential targeting strategies are summarized in Table 1. Specific approaches relating to stem cell genes discussed in this review are explored below.
Small-molecule inhibitors
Many stem cell genes code for TFs or other DNA-binding proteins, considered “undruggable,” owing to their intrinsically disordered nature. Recent improvements in screening methods have identified HMGA2-binding compounds, including the antimicrobials sumarin and ciclopirox (Huang et al., 2019; Su et al., 2020) and MEIS1/2 inhibitors (Turan et al., 2020).
An alternative approach employs small molecules that bind the minor groove of the TF cognate sequence. DNA binders of this type can inhibit HOXA9 (Depauw et al., 2019), with in vitro activity against HOXA9-dependent cells lines (Sonoda et al., 2021). Similarly, netropsin and trabectedin demonstrate antitumor activity in HMGA2+ neoplasia. Treatment with both drugs shows a synergistic anti-proliferative effect in infant ALL cell lines (Wu et al., 2015). Other approaches to target HMGA2 include targeting downstream pathways such as G2M transition (Moison et al., 2022) and PI3K/Akt/mTOR (Tan et al., 2016).
TF function can also be impaired by preventing their expression. HOXA9 transcription is dependent on DOT1L-mediated H3K79 methylation in KMT2A-r leukemia. DOT1L has been successfully targeted with small molecules, pinometostat/EPZ5676 (Basavapathruni et al., 2014; Waters et al., 2016). Early-phase clinical trials suggest modest activity against KMT2A-r leukemia (Stein et al., 2018). Newer DOT1L inhibitors with oral availability and improved pharmacokinetics have been developed (Stauffer et al., 2019; Perner et al., 2020).
HOXA9 is also dependent on the scaffold protein menin for expression in KMT2A-r leukemias (Yokoyama et al., 2005) and NPM1c-mutated AML (Kühn et al., 2016). The small-molecule revumenib prevents the interaction of menin with its target proteins. Revumenib induces remission in 30% of relapsed/refractory leukemia patients (Issa et al., 2023), although mutations in MEN1 can lead to drug resistance (Perner et al., 2023).
Direct inhibition of CDX2 has not yet been possible; however, the observation that PPARγ signaling restores KLF4 expression offers a potential therapeutic route to partially opposing CDX2 activity. PPARγ agonists are toxic to CDX2 overexpressing leukemia cell lines in vitro (Faber et al., 2013; Esmaeili et al., 2021).
Targeting RNA-binding proteins
Like DNA-binding proteins, small-molecule inhibition of RBPs is difficult, although recent high-throughput approaches have generated candidates (Wu, 2020). The most promising LIN28(A/B) inhibitor is C1632, which targets LIN28B + cell lines both by disruption of LIN28B–let-7 interaction (Franses et al., 2020; Zhang Q. et al., 2023; Shahab et al., 2023) and in Ewing sarcoma by disruption of the interaction between EWS-FLI1 mRNA and LIN28B (Keskin et al., 2020). Other molecules such as LI71 bind the cold shock domain and have efficacy against LIN28B + cancer cell lines (Wang L. et al., 2018).
Small-molecule inhibitors of IGF2BP1 and IGF2BP3 are at the preclinical stage. BTYNB destabilizes oncogenic transcripts by disrupting the IGF2BP1–mRNA association (Müller et al., 2020; Jamal et al., 2023; Sharma et al., 2023). Combining menin inhibitors with depletion of IGF2BP3 impairs cell growth and increases differentiation of KMT2A::AFF1 leukemia (Lin et al., 2023).
Another potential strategy to boost let-7 miRNA expression, thus inhibiting several oncogenes (Cinkornpumin et al., 2017), has yet to be applied in leukemia.
Immune effector cell therapy
Stem cell markers as targets for immunotherapy: The cell surface marker PROM1 is a highly attractive target for immunotherapy. A CD19/CD133 tandem CAR-T (Li et al., 2018) and CD19/133 bispecific CAR-iNKT (Ren et al., 2023) show efficacy in vivo against KMT2A-r cell lines. However, valid concerns about stem cell toxicity when targeting CD133 in patients have been raised (Bueno et al., 2019). Preclinical testing and early-phase trials using CD133-CAR-T in solid malignancies (Wang et al., 2018b; Dai et al., 2020; Vora et al., 2020) revealed no BM aplasia and only transient, reversible hematological toxicities. Longer-term follow-up and assessment will be required to confirm the safety of CD133 targeting. CAR-T directed against NG2 has shown promise in mobilizing leukemic blasts and rendering them more sensitive to chemotherapy in mouse models (Lopez-Millan et al., 2019). Anti-CD117 CAR-T therapy shows preclinical efficacy (Myburgh et al., 2020) but also eliminates healthy HSCs, necessitating novel approaches such as terminating “safety switches” (Magnani et al., 2023). Both CAR-T (Wang et al., 2018c) and CAR-NK (Mansour et al., 2023) cells have been used to target FLT3. Anti-CD123 CAR-T therapy has been used in early-stage clinical trials, appearing safe and potentially effective (Yao et al., 2019; Wermke et al., 2021).
Immunotherapy is particularly attractive in DS-associated leukemias where conventional treatments cause significant toxicities. In DS-ALL patients, CD19-, CD22-, and TSLPR-directed immunotherapies could yield promising results (Bagashev et al., 2022; Laetsch et al., 2023).
Discussion
Stem cells possess unique properties allowing the expansion, self-renewal, and differentiation required for tissue homeostasis. These programs are frequently co-opted by leukemias, where they provide growth and survival advantages. Although there is renewed interest in reprogramming adult HSCs to become more “fetal-like”, the potential of fetal stem cell genes to also promote oncogenesis must be considered.
Understanding stem cell programs in leukemia, including oncofetal genes, is vital to disentangling the biology of leukemias, including treatment resistance/relapse, and identifying mechanisms vulnerable to novel targeted therapies.
Statements
Author contributions
RL: conceptualization, writing–original draft, and writing–review and editing. JC: conceptualization, writing–original draft, and writing–review and editing. AR: conceptualization, supervision, and writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. REL was supported by a CRUK Oxford DPhil in Cancer Sciences studentship (DCS-CRUK-CRTF20-RL). JWC was supported by a CCLG LPT Research Grant (CCLGA/2020/23/AR). AR was supported by the Wellcome Trust Clinical Research Career Development Fellowship (216632/Z/19/Z) and Medical Research Council Funding (MC_UU_00029/7).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abedin S. M. Badar T. Gauger K. Michaelis L. C. Runaas L. Carlson K.-S. et al (2022). Safety and efficacy of pracinostat in combination with gemtuzumab ozogamicin (PraGO) in patients with relapsed/refractory acute myeloid leukemia. Leukemia Res.123, 106984. 10.1016/j.leukres.2022.106984
2
Agrawal-Singh S. Bagri J. Giotopoulos G. Azazi D. M. A. Horton S. J. Lopez C. K. et al (2023). HOXA9 forms a repressive complex with nuclear matrix-associated protein SAFB to maintain acute myeloid leukemia. Blood141 (14), 1737–1754. 10.1182/blood.2022016528
3
Agraz-Doblas A. Bueno C. Bashford-Rogers R. Roy A. Schneider P. Bardini M. et al (2019). Unraveling the cellular origin and clinical prognostic markers of infant B-cell acute lymphoblastic leukemia using genome-wide analysis. Haematologica104 (6), 1176–1188. 10.3324/haematol.2018.206375
4
Aguila J. R. Liao W. Yang J. Avila C. Hagag N. Senzel L. et al (2011). SALL4 is a robust stimulator for the expansion of hematopoietic stem cells. Blood118 (3), 576–585. 10.1182/blood-2011-01-333641
5
Alajez N. M. Shi W. Wong D. Lenarduzzi M. Waldron J. Weinreb I. et al (2012). Lin28b promotes head and neck cancer progression via modulation of the insulin-like growth factor survival pathway. Oncotarget3 (12), 1641–1652. 10.18632/oncotarget.785
6
Alarcón P. Rodríguez-Seguel E. Fernández-González A. Rubio R. Gómez-Skarmeta J. L. (2008). A dual requirement for Iroquois genes during Xenopus kidney development. Dev. Camb. Engl.135 (19), 3197–3207. 10.1242/dev.023697
7
Alejo-Valle O. Weigert K. Bhayadia R. Ng M. Issa H. Beyer C. et al (2022). The megakaryocytic transcription factor ARID3A suppresses leukemia pathogenesis. Blood139 (5), 651–665. 10.1182/blood.2021012231
8
Amanzadeh A. Heidarnejad F. Abdollahpour-Alitappeh M. Molla-Kazemiha V. Yari S. Hadizadeh-Tasbiti A. et al (2018). Development of high-affinity monoclonal antibody using CD44 overexpressed cells as a candidate for targeted immunotherapy and diagnosis of acute myeloid leukemia. Hum. Antibodies26 (1), 7–15. 10.3233/HAB-170315
9
Andreeff M. Ruvolo V. Gadgil S. Zeng C. Coombes K. Chen W. et al (2008). HOX expression patterns identify a common signature for favorable AML. Leukemia22 (11), 2041–2047. 10.1038/leu.2008.198
10
Bagashev A. Loftus J. Niswander L. Ross S. Falkenstein C. D. Junco J. et al (2022). P1421: bimodal targeting of cytokine receptor-like factor 2 (crlf2) with jak inhibition and chimeric antigen receptor t cell immunotherapy in down syndrome acute lymphoblastic leukemia. HemaSphere6, 1305–1306. 10.1097/01.Hs9.0000848544.25019.34
11
Bai J. Yokomizo-Nakano T. Kubota S. Sun Y. Kanai A. Iimori M. et al (2021). Overexpression of Hmga2 activates Igf2bp2 and remodels transcriptional program of Tet2-deficient stem cells in myeloid transformation. Oncogene40 (8), 1531–1541. 10.1038/s41388-020-01629-w
12
Ballerini P. Boelle P.-Y. Deswarte C. Auvrignon A. Couchy G. Parker J. L. et al (2008). Prognostic significance of SALL4 expression levels in paediatric acute myeloid leukaemia (AML). Blood112 (11), 2243. 10.1182/blood.V112.11.2243.2243
13
Balzeau J. Menezes M. R. Cao S. Hagan J. P. (2017). The LIN28/let-7 pathway in cancer. Front. Genet.8, 31. 10.3389/fgene.2017.00031
14
Bao S. Wu Q. McLendon R. E. Hao Y. Shi Q. Hjelmeland A. B. et al (2006). Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature444 (7120), 756–760. 10.1038/nature05236
15
Basak A. Munschauer M. Lareau C. A. Montbleau K. E. Ulirsch J. C. Hartigan C. R. et al (2020). Control of human hemoglobin switching by LIN28B-mediated regulation of BCL11A translation. Nat. Genet.52 (2), 138–145. 10.1038/s41588-019-0568-7
16
Basavapathruni A. Olhava E. J. Daigle S. R. Therkelsen C. A. Jin L. Boriack-Sjodin P. A. et al (2014). Nonclinical pharmacokinetics and metabolism of EPZ-5676, a novel DOT1L histone methyltransferase inhibitor. Biopharm. Drug Dispos.35 (4), 237–252. 10.1002/bdd.1889
17
Battista S. Pentimalli F. Baldassarre G. Fedele M. Fidanza V. Croce C. M. et al (2003). Loss of Hmga1 gene function affects embryonic stem cell lympho-hematopoietic differentiation. FASEB J.17 (11), 1496–1498. 10.1096/fj.02-0977fje
18
Bayraktar R. Van Roosbroeck K. (2018). miR-155 in cancer drug resistance and as target for miRNA-based therapeutics. Cancer Metastasis Rev.37, 33–44. 10.1007/s10555-017-9724-7
19
Benz C. Copley M. R. Kent D. G. Wohrer S. Cortes A. Aghaeepour N. et al (2012). Hematopoietic stem cell subtypes expand differentially during development and display distinct lymphopoietic programs. Cell Stem Cell10 (3), 273–283. 10.1016/j.stem.2012.02.007
20
Bernt K. M. Zhu N. Sinha A. U. Vempati S. Faber J. Krivtsov A. V. et al (2011). MLL-rearranged leukemia is dependent on aberrant H3K79 methylation by DOT1L. Cancer Cell20 (1), 66–78. 10.1016/j.ccr.2011.06.010
21
Bhansali R. S. Rammohan M. Lee P. Laurent A. P. Wen Q. Suraneni P. et al (2021). DYRK1A regulates B cell acute lymphoblastic leukemia through phosphorylation of FOXO1 and STAT3. J. Clin. Invest.131 (1), e135937. 10.1172/JCI135937
22
Bolouri H. Ries R. Pardo L. Hylkema T. Zhou W. Smith J. L. et al (2021). A B-cell developmental gene regulatory network is activated in infant AML. PloS One16 (11), e0259197. 10.1371/journal.pone.0259197
23
Bond J. Labis E. Marceau-Renaut A. Duployez N. Labopin M. Hypolite G. et al (2018). Polycomb repressive complex 2 haploinsufficiency identifies a high-risk subgroup of pediatric acute myeloid leukemia. Leukemia32 (8), 1878–1882. 10.1038/s41375-018-0187-9
24
Bowie M. B. McKnight K. D. Kent D. G. McCaffrey L. Hoodless P. A. Eaves C. J. (2006). Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. J. Clin. Invest.116 (10), 2808–2816. 10.1172/jci28310
25
Brooke N. M. Garcia-Fernàndez J. Holland P. W. (1998). The ParaHox gene cluster is an evolutionary sister of the Hox gene cluster. Nature392 (6679), 920–922. 10.1038/31933
26
Brunetti L. Gundry M. C. Sorcini D. Guzman A. G. Huang Y.-H. Ramabadran R. et al (2018). Mutant NPM1 maintains the leukemic state through HOX expression. Cancer Cell34 (3), 499–512. 10.1016/j.ccell.2018.08.005
27
Bueno C. Velasco-Hernandez T. Gutiérrez-Agüera F. Zanetti S. R. Baroni M. L. Sánchez-Martínez D. et al (2019). CD133-directed CAR T-cells for MLL leukemia: on-target, off-tumor myeloablative toxicity. Leukemia33 (8), 2090–2125. 10.1038/s41375-019-0418-8
28
Buono L. Iside C. De Matteo A. Stellato P. Beneduce G. de Vera d’Aragona R. P. et al (2022). Specific lncRNA signatures discriminate childhood acute leukaemias: a pilot study. Cancer Cell Int.22 (1), 373. 10.1186/s12935-022-02789-3
29
Cabal-Hierro L. van Galen P. Prado M. A. Higby K. J. Togami K. Mowery C. T. et al (2020). Chromatin accessibility promotes hematopoietic and leukemia stem cell activity. Nat. Commun.11 (1), 1406. 10.1038/s41467-020-15221-z
30
Carey-Smith S. L. Simad M. H. Panchal K. Aya-Bonilla C. Smolders H. Lin S. et al (2024). Efficacy of DYRK1A inhibitors in novel models of Down syndrome acute lymphoblastic leukemia. Haematologica. 10.3324/haematol.2023.284271
31
Challen G. A. Sun D. Jeong M. Luo M. Jelinek J. Berg J. S. et al (2011). Dnmt3a is essential for hematopoietic stem cell differentiation. Nat. Genet.44 (1), 23–31. 10.1038/ng.1009
32
Charbord P. Tavian M. Humeau L. Péault B. (1996). Early ontogeny of the human marrow from long bones: an immunohistochemical study of hematopoiesis and its microenvironment [see comments]. Blood87 (10), 4109–4119. 10.1182/blood.v87.10.4109.bloodjournal87104109
33
Chase A. Reiter A. Burci L. Cazzaniga G. Biondi A. Pickard J. et al (1999). Fusion of ETV6 to the caudal-related homeobox gene CDX2 in acute myeloid leukemia with the t(12;13)(p13;q12). Blood93 (3), 1025–1031. 10.1182/blood.V93.3.1025
34
Chen C. Yu W. Alikarami F. Qiu Q. Chen C.-h. Flournoy J. et al (2022). Single-cell multiomics reveals increased plasticity, resistant populations, and stem-cell–like blasts in KMT2A-rearranged leukemia. Blood, J. Am. Soc. Hematol.139 (14), 2198–2211. 10.1182/blood.2021013442
35
Chou S. T. Opalinska J. B. Yao Y. Fernandes M. A. Kalota A. Brooks J. S. et al (2008). Trisomy 21 enhances human fetal erythro-megakaryocytic development. Blood112 (12), 4503–4506. 10.1182/blood-2008-05-157859
36
Cinkornpumin J. Roos M. Nguyen L. Liu X. Gaeta X. Lin S. et al (2017). A small molecule screen to identify regulators of let-7 targets. Sci. Rep.7 (1), 15973. 10.1038/s41598-017-16258-9
37
Collins C. Wang J. Miao H. Bronstein J. Nawer H. Xu T. et al (2014). C/EBPα is an essential collaborator in Hoxa9/Meis1-mediated leukemogenesis. Proc. Natl. Acad. Sci. U. S. A.111 (27), 9899–9904. 10.1073/pnas.1402238111
38
Cooper L. J. Shannon K. M. Loken M. R. Weaver M. Stephens K. Sievers E. L. (2000). Evidence that juvenile myelomonocytic leukemia can arise from a pluripotential stem cell. Blood, J. Am. Soc. Hematol.96 (6), 2310–2313. 10.1182/blood.v96.6.2310
39
Copley M. R. Babovic S. Benz C. Knapp D. J. Beer P. A. Kent D. G. et al (2013). The Lin28b-let-7-Hmga2 axis determines the higher self-renewal potential of fetal haematopoietic stem cells. Nat. Cell Biol.15 (8), 916–925. 10.1038/ncb2783
40
Copley M. R. Eaves C. J. (2013). Developmental changes in hematopoietic stem cell properties. Exp. Mol. Med.45 (11), e55. 10.1038/emm.2013.98
41
Cox C. V. Diamanti P. Evely R. S. Kearns P. R. Blair A. (2009). Expression of CD133 on leukemia-initiating cells in childhood ALL. Blood113 (14), 3287–3296. 10.1182/blood-2008-04-154187
42
Dai H. Tong C. Shi D. Chen M. Guo Y. Chen D. et al (2020). Efficacy and biomarker analysis of CD133-directed CAR T cells in advanced hepatocellular carcinoma: a single-arm, open-label, phase II trial. Oncoimmunology9 (1), 1846926. 10.1080/2162402X.2020.1846926
43
D'Angelo V. Iannotta A. Ramaglia M. Lombardi A. Zarone M. R. Desiderio V. et al (2015). EZH2 is increased in paediatric T-cell acute lymphoblastic leukemia and is a suitable molecular target in combination treatment approaches. J. Exp. Clin. cancer Res. CR34 (1), 83. 10.1186/s13046-015-0191-0
44
Darwish N. H. E. Glinsky G. V. Sudha T. Mousa S. A. (2021). Targeting thyrointegrin αvβ3 using fluorobenzyl polyethylene glycol conjugated tetraiodothyroacetic acid (NP751) in acute myeloid leukemia. Front. Oncol.11, 793810. 10.3389/fonc.2021.793810
45
DeAngelo D. J. Jonas B. A. Liesveld J. L. Bixby D. L. Advani A. S. Marlton P. et al (2022). Phase 1/2 study of uproleselan added to chemotherapy in patients with relapsed or refractory acute myeloid leukemia. Blood139 (8), 1135–1146. 10.1182/blood.2021010721
46
Degrauwe N. Suvà M. L. Janiszewska M. Riggi N. Stamenkovic I. (2016). IMPs: an RNA-binding protein family that provides a link between stem cell maintenance in normal development and cancer. Genes Dev.30 (22), 2459–2474. 10.1101/gad.287540.116
47
De Luca L. Trino S. Laurenzana I. Tagliaferri D. Falco G. Grieco V. et al (2017). Knockdown of miR-128a induces Lin28a expression and reverts myeloid differentiation blockage in acute myeloid leukemia. Cell death Dis.8 (6), e2849. 10.1038/cddis.2017.253
48
Den Boer M. L. van Slegtenhorst M. De Menezes R. X. Cheok M. H. Buijs-Gladdines J. G. C. A. M. Peters S. T. C. J. M. et al (2009). A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study. Lancet. Oncol.10 (2), 125–134. 10.1016/S1470-2045(08)70339-5
49
Depauw S. Lambert M. Jambon S. Paul A. Peixoto P. Nhili R. et al (2019). Heterocyclic diamidine DNA ligands as HOXA9 transcription factor inhibitors: design, molecular evaluation, and cellular consequences in a HOXA9-dependant leukemia cell model. J. Med. Chem.62 (3), 1306–1329. 10.1021/acs.jmedchem.8b01448
50
Dick J. E. (2008). Stem cell concepts renew cancer research. Blood112 (13), 4793–4807. 10.1182/blood-2008-08-077941
51
Drabkin H. A. Parsy C. Ferguson K. Guilhot F. Lacotte L. Roy L. et al (2002). Quantitative HOX expression in chromosomally defined subsets of acute myelogenous leukemia. Leukemia16 (2), 186–195. 10.1038/sj.leu.2402354
52
Duployez N. Marceau-Renaut A. Villenet C. Petit A. Rousseau A. Ng S. W. K. et al (2019). The stem cell-associated gene expression signature allows risk stratification in pediatric acute myeloid leukemia. Leukemia33 (2), 348–357. 10.1038/s41375-018-0227-5
53
Efanov A. Zanesi N. Coppola V. Nuovo G. Bolon B. Wernicle-Jameson D. et al (2014). Human HMGA2 protein overexpressed in mice induces precursor T-cell lymphoblastic leukemia. Blood Cancer J.4 (7), e227. 10.1038/bcj.2014.46
54
Egyed B. Kutszegi N. Sági J. C. Gézsi A. Rzepiel A. Visnovitz T. et al (2020). MicroRNA-181a as novel liquid biopsy marker of central nervous system involvement in pediatric acute lymphoblastic leukemia. J. Transl. Med.18 (1), 250–312. 10.1186/s12967-020-02415-8
55
Elagib K. E. Lu C.-H. Mosoyan G. Khalil S. Zasadzińska E. Foltz D. R. et al (2017). Neonatal expression of RNA-binding protein IGF2BP3 regulates the human fetal-adult megakaryocyte transition. J. Clin. investigation127 (6), 2365–2377. 10.1172/JCI88936
56
Elcheva I. A. Wood T. Chiarolanzio K. Chim B. Wong M. Singh V. et al (2020). RNA-binding protein IGF2BP1 maintains leukemia stem cell properties by regulating HOXB4, MYB, and ALDH1A1. Leukemia34 (5), 1354–1363. 10.1038/s41375-019-0656-9
57
Eldeeb M. Yuan O. Guzzi N. Thi Ngoc P. C. Konturek-Ciesla A. Kristiansen T. A. et al (2023). A fetal tumor suppressor axis abrogates MLL-fusion-driven acute myeloid leukemia. Cell Rep.42 (2), 112099. 10.1016/j.celrep.2023.112099
58
Elsayed A. H. Rafiee R. Cao X. Raimondi S. Downing J. R. Ribeiro R. et al (2020). A six-gene leukemic stem cell score identifies high risk pediatric acute myeloid leukemia. Leukemia34 (3), 735–745. 10.1038/s41375-019-0604-8
59
Esmaeili S. Salari S. Kaveh V. Ghaffari S. H. Bashash D. (2021). Alteration of PPAR-GAMMA (PPARG; PPARγ) and PTEN gene expression in acute myeloid leukemia patients and the promising anticancer effects of PPARγ stimulation using pioglitazone on AML cells. Mol. Genet. Genomic Med.9 (11), e1818. 10.1002/mgg3.1818
60
Esperanza-Cebollada E. Gómez-González S. Perez-Jaume S. Vega-García N. Vicente-Garcés C. Richarte-Franqués M. et al (2023). A miRNA signature related to stemness identifies high-risk patients in paediatric acute myeloid leukaemia. Br. J. Haematol.202 (1), 96–110. 10.1111/bjh.18746
61
Ezhkova E. Pasolli H. A. Parker J. S. Stokes N. Su I. h. Hannon G. et al (2009). Ezh2 orchestrates gene expression for the stepwise differentiation of tissue-specific stem cells. Cell136 (6), 1122–1135. 10.1016/j.cell.2008.12.043
62
Faber J. Krivtsov A. V. Stubbs M. C. Wright R. Davis T. N. van den Heuvel-Eibrink M. et al (2009). HOXA9 is required for survival in human MLL-rearranged acute leukemias. Blood113 (11), 2375–2385. 10.1182/blood-2007-09-113597
63
Faber K. Bullinger L. Ragu C. Garding A. Mertens D. Miller C. et al (2013). CDX2-driven leukemogenesis involves KLF4 repression and deregulated PPARγ signaling. J. Clin. Investigation123 (1), 299–314. 10.1172/JCI64745
64
Ferrandina G. Petrillo M. Bonanno G. Scambia G. (2009). Targeting CD133 antigen in cancer. Expert Opin. Ther. Targets13 (7), 823–837. 10.1517/14728220903005616
65
Ferrando A. A. Neuberg D. S. Staunton J. Loh M. L. Huard C. Raimondi S. C. et al (2002). Gene expression signatures define novel oncogenic pathways in T cell acute lymphoblastic leukemia. Cancer Cell1 (1), 75–87. 10.1016/s1535-6108(02)00018-1
66
Franses J. W. Philipp J. Missios P. Bhan I. Liu A. Yashaswini C. et al (2020). Pancreatic circulating tumor cell profiling identifies LIN28B as a metastasis driver and drug target. Nat. Commun.11 (1), 3303. 10.1038/s41467-020-17150-3
67
Freese N. H. Lam B. A. Staton M. Scott A. Chapman S. C. (2014). A novel gain-of-function mutation of the proneural IRX1 and IRX2 genes disrupts Axis elongation in the araucana rumpless chicken. PLOS ONE9 (11), e112364. 10.1371/journal.pone.0112364
68
Gao C. Kong N. R. Li A. Tatetu H. Ueno S. Yang Y. et al (2013). SALL4 is a key transcription regulator in normal human hematopoiesis. Transfusion53 (5), 1037–1049. 10.1111/j.1537-2995.2012.03888.x
69
Gaur V. Chaudhary S. Tyagi A. Agarwal S. Sharawat S. K. Sarkar S. et al (2020). Dysregulation of miRNA expression and their prognostic significance in paediatric cytogenetically normal acute myeloid leukaemia. Br. J. Haematol.188 (6), e90–e94. 10.1111/bjh.16375
70
Georgantas R. W. III Hildreth R. Morisot S. Alder J. Liu C.-g. Heimfeld S. et al (2007). CD34+ hematopoietic stem-progenitor cell microRNA expression and function: a circuit diagram of differentiation control. Proc. Natl. Acad. Sci.104 (8), 2750–2755. 10.1073/pnas.0610983104
71
Gialesaki S. Brauer-Hartmann D. Issa H. Bhayadia R. Alejo-Valle O. Verboon L. et al (2023). RUNX1 isoform disequilibrium promotes the development of trisomy 21-associated myeloid leukemia. Blood141 (10), 1105–1118. 10.1182/blood.2022017619
72
Godfrey L. Crump N. T. O’Byrne S. Lau I. J. Rice S. Harman J. R. et al (2021). H3K79me2/3 controls enhancer–promoter interactions and activation of the pan-cancer stem cell marker PROM1/CD133 in MLL-AF4 leukemia cells. Leukemia35 (1), 90–106. 10.1038/s41375-020-0808-y
73
Greaves M. (2005). In utero origins of childhood leukaemia. Early Hum. Dev.81 (1), 123–129. 10.1016/j.earlhumdev.2004.10.004
74
Grobbelaar C. Ford A. M. (2019). The role of MicroRNA in paediatric acute lymphoblastic leukaemia: challenges for diagnosis and therapy. J. Oncol.2019, 8941471. 10.1155/2019/8941471
75
Hacein-Bey-Abina S. Von Kalle C. Schmidt M. McCormack M. P. Wulffraat N. Leboulch P. et al (2003). LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Sci. (New York, N.Y.)302 (5644), 415–419. 10.1126/science.1088547
76
Hanahan D. Weinberg R. A. (2011). Hallmarks of cancer: the next generation. Cell144 (5), 646–674.
77
Harrison D. E. Zhong R. K. Jordan C. T. Lemischka I. R. Astle C. M. (1997). Relative to adult marrow, fetal liver repopulates nearly five times more effectively long-term than short-term. Exp. Hematol.25 (4), 293–297.
78
Hartmann M. Schoenung M. Rajak J. Maurer V. Hai L. Bauer K. et al (2023). Oncogenic RAS-pathway activation drives oncofetal reprogramming and creates therapeutic vulnerabilities in juvenile myelomonocytic leukemia. bioRxiv. Avail;able at:10.1101/2023.10.27.563754
79
Harvey R. C. Mullighan C. G. Wang X. Dobbin K. K. Davidson G. S. Bedrick E. J. et al (2010). Identification of novel cluster groups in pediatric high-risk B-precursor acute lymphoblastic leukemia with gene expression profiling: correlation with genome-wide DNA copy number alterations, clinical characteristics, and outcome. Blood116 (23), 4874–4884. 10.1182/blood-2009-08-239681
80
Hasle H. Clemmensen I. H. Mikkelsen M. (2000). Risks of leukaemia and solid tumours in individuals with Down's syndrome. Lancet355 (9199), 165–169. 10.1016/S0140-6736(99)05264-2
81
Helsmoortel H. H. Bresolin S. Lammens T. Cavé H. Noellke P. Caye A. et al (2016a). LIN28B overexpression defines a novel fetal-like subgroup of juvenile myelomonocytic leukemia. Blood127 (9), 1163–1172. 10.1182/blood-2015-09-667808
82
Helsmoortel H. H. De Moerloose B. Pieters T. Ghazavi F. Bresolin S. Cavé H. et al (2016b). LIN28B is over-expressed in specific subtypes of pediatric leukemia and regulates lncRNA H19. Haematologica101 (6), e240–e244. 10.3324/haematol.2016.143818
83
Hodeib H. El Amrousy D. Youssef A. Khedr R. Al-Asy H. Shabana A. et al (2023). Acute lymphoblastic leukemia in children and SALL4 and BMI-1 gene expression. Pediatr. Res.94 (4), 1510–1515. 10.1038/s41390-021-01854-3
84
Holland P. W. H. (2013). Evolution of homeobox genes. Wiley Interdiscip. Rev. Dev. Biol.2 (1), 31–45. 10.1002/wdev.78
85
Holland P. W. H. Booth H. A. F. Bruford E. A. (2007). Classification and nomenclature of all human homeobox genes. BMC Biol.5 (1), 47. 10.1186/1741-7007-5-47
86
Holyoake T. L. Nicolini F. E. Eaves C. J. (1999). Functional differences between transplantable human hematopoietic stem cells from fetal liver, cord blood, and adult marrow. Exp. Hematol.27 (9), 1418–1427. 10.1016/s0301-472x(99)00078-8
87
Hong D. Gupta R. Ancliff P. Atzberger A. Brown J. Soneji S. et al (2008). Initiating and cancer-propagating cells in TEL-AML1-associated childhood leukemia. Science319 (5861), 336–339. 10.1126/science.1150648
88
Hope K. J. Jin L. Dick J. E. (2004). Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat. Immunol.5 (7), 738–743. 10.1038/ni1080
89
Hsieh Y.-T. Gang E. J. Geng H. Park E. Huantes S. Chudziak D. et al (2013). Integrin alpha4 blockade sensitizes drug resistant pre-B acute lymphoblastic leukemia to chemotherapy. Blood121 (10), 1814–1818. 10.1182/blood-2012-01-406272
90
Huang Y. Sitwala K. Bronstein J. Sanders D. Dandekar M. Collins C. et al (2012). Identification and characterization of Hoxa9 binding sites in hematopoietic cells. Blood119 (2), 388–398. 10.1182/blood-2011-03-341081
91
Huang Y.-M. Cheng C.-H. Pan S.-L. Yang P.-M. Lin D.-Y. Lee K.-H. (2019). Gene expression signature-based approach identifies antifungal drug ciclopirox as a novel inhibitor of HMGA2 in colorectal cancer. Biomolecules9 (11), 688. 10.3390/biom9110688
92
Huso T. H. Resar L. M. S. (2014). The high mobility group A1 molecular switch: turning on cancer - can we turn it off?Expert Opin. Ther. Targets18 (5), 541–553. 10.1517/14728222.2014.900045
93
Isobe T. Takagi M. Sato-Otsubo A. Nishimura A. Nagae G. Yamagishi C. et al (2022). Multi-omics analysis defines highly refractory RAS burdened immature subgroup of infant acute lymphoblastic leukemia. Nat. Commun.13 (1), 4501. 10.1038/s41467-022-32266-4
94
Issa G. C. Aldoss I. DiPersio J. Cuglievan B. Stone R. Arellano M. et al (2023). The menin inhibitor revumenib in KMT2A-rearranged or NPM1-mutant leukaemia. Nature615 (7954), 920–924. 10.1038/s41586-023-05812-3
95
Italiano A. Soria J.-C. Toulmonde M. Michot J.-M. Lucchesi C. Varga A. et al (2018). Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study. Lancet. Oncol.19 (5), 649–659. 10.1016/S1470-2045(18)30145-1
96
Ivanovs A. Rybtsov S. Ng E. S. Stanley E. G. Elefanty A. G. Medvinsky A. (2017). Human haematopoietic stem cell development: from the embryo to the dish. Development144 (13), 2323–2337. 10.1242/dev.134866
97
Ivanovs A. Rybtsov S. Welch L. Anderson R. A. Turner M. L. Medvinsky A. (2011). Highly potent human hematopoietic stem cells first emerge in the intraembryonic aorta-gonad-mesonephros region. J. Exp. Med.208 (12), 2417–2427. 10.1084/jem.20111688
98
Jäger S. Jahnke A. Wilmes T. Adebahr S. Vögtle F. N. deLima-Hahn E. et al (2007). Leukemia targeting ligands isolated from phage display peptide libraries. Leukemia21 (3), 411–420. 10.1038/sj.leu.2404548
99
Jamal A. Hassan Dalhat M. Jahan S. Choudhry H. Imran Khan M. (2023). BTYNB, an inhibitor of RNA binding protein IGF2BP1 reduces proliferation and induces differentiation of leukemic cancer cells. Saudi J. Biol. Sci.30 (3), 103569. 10.1016/j.sjbs.2023.103569
100
Jardine L. Webb S. Goh I. Quiroga Londono M. Reynolds G. Mather M. et al (2021). Blood and immune development in human fetal bone marrow and Down syndrome. Nature598 (7880), 327–331. 10.1038/s41586-021-03929-x
101
Jeong H.-W. Cui W. Yang Y. Lu J. He J. Li A. et al (2011). SALL4, a stem cell factor, affects the side population by regulation of the ATP-binding cassette drug transport genes. PloS One6 (4), e18372. 10.1371/journal.pone.0018372
102
Jin L. Hope K. J. Zhai Q. Smadja-Joffe F. Dick J. E. (2006). Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat. Med.12 (10), 1167–1174. 10.1038/nm1483
103
Kang M. Kim S. Ko J. (2019). Roles of CD133 in microvesicle formation and oncoprotein trafficking in colon cancer. FASEB J.33 (3), 4248–4260. 10.1096/fj.201802018R
104
Kerry J. Godfrey L. Repapi E. Tapia M. Blackledge N. P. Ma H. et al (2017). MLL-AF4 spreading identifies binding sites that are distinct from super-enhancers and that govern sensitivity to DOT1L inhibition in leukemia. Cell Rep.18 (2), 482–495. 10.1016/j.celrep.2016.12.054
105
Keskin T. Bakaric A. Waszyk P. Boulay G. Torsello M. Cornaz-Buros S. et al (2020). LIN28B underlies the pathogenesis of a subclass of ewing sarcoma LIN28B control of EWS-FLI1 stability. Cell Rep.30 (13), 4567–4583. 10.1016/j.celrep.2019.12.053
106
Khalaj M. Woolthuis C. M. Hu W. Durham B. H. Chu S. H. Qamar S. et al (2017). miR-99 regulates normal and malignant hematopoietic stem cell self-renewal. J. Exp. Med.214 (8), 2453–2470. 10.1084/jem.20161595
107
Kim I. Saunders T. L. Morrison S. J. (2007). Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell130 (3), 470–483. 10.1016/j.cell.2007.06.011
108
Klusmann J. H. Li Z. Bohmer K. Maroz A. Koch M. L. Emmrich S. et al (2010). miR-125b-2 is a potential oncomiR on human chromosome 21 in megakaryoblastic leukemia. Genes Dev.24 (5), 478–490. 10.1101/gad.1856210
109
Kormish J. D. Sinner D. Zorn A. M. (2010). Interactions between SOX factors and Wnt/beta-catenin signaling in development and disease. Dev. Dyn. Official Publ. Am. Assoc. Anatomists239 (1), 56–68. 10.1002/dvdy.22046
110
Kuhn A. Loscher D. Marschalek R. (2016). The IRX1/HOXA connection: insights into a novel t(4;11)- specific cancer mechanism. Oncotarget7 (23), 35341–35352. 10.18632/oncotarget.9241
111
Kühn M. W. M. Song E. Feng Z. Sinha A. Chen C.-W. Deshpande A. J. et al (2016). Targeting chromatin regulators inhibits leukemogenic gene expression in NPM1 mutant leukemia. Cancer Discov.6 (10), 1166–1181. 10.1158/2159-8290.CD-16-0237
112
Kumar P. Beck D. Galeev R. Thoms J. A. I. Talkhoncheh M. S. de Jong I. et al (2019). HMGA2 promotes long-term engraftment and myeloerythroid differentiation of human hematopoietic stem and progenitor cells. Blood Adv.3 (4), 681–691. 10.1182/bloodadvances.2018023986
113
Labuhn M. Perkins K. Matzk S. Varghese L. Garnett C. Papaemmanuil E. et al (2019). Mechanisms of progression of myeloid preleukemia to transformed myeloid leukemia in children with down syndrome. Cancer Cell36 (3), 340. 10.1016/j.ccell.2019.08.014
114
Laetsch T. W. Maude S. L. Rives S. Hiramatsu H. Bittencourt H. Bader P. et al (2023). Three-year update of tisagenlecleucel in pediatric and young adult patients with relapsed/refractory acute lymphoblastic leukemia in the ELIANA trial. J. Clin. Oncol.41 (9), 1664–1669. 10.1200/JCO.22.00642
115
Lansdorp P. M. Dragowska W. Mayani H. (1993). Ontogeny-related changes in proliferative potential of human hematopoietic cells. J. Exp. Med.178 (3), 787–791. 10.1084/jem.178.3.787
116
Lapidot T. Sirard C. Vormoor J. Murdoch B. Hoang T. Caceres-Cortes J. et al (1994). A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature367 (6464), 645–648. 10.1038/367645a0
117
Lee Y. T. de Vasconcellos J. F. Yuan J. Byrnes C. Noh S. J. Meier E. R. et al (2013). LIN28B-mediated expression of fetal hemoglobin and production of fetal-like erythrocytes from adult human erythroblasts ex vivo. Blood122 (6), 1034–1041. 10.1182/blood-2012-12-472308
118
Li D. Hu Y. Jin Z. Zhai Y. Tan Y. Sun Y. et al (2018). TanCAR T cells targeting CD19 and CD133 efficiently eliminate MLL leukemic cells. Leukemia32 (9), 2012–2016. 10.1038/s41375-018-0212-z
119
Li O. Li J. Dröge P. (2007). DNA architectural factor and proto-oncogene HMGA2 regulates key developmental genes in pluripotent human embryonic stem cells. FEBS Lett.581 (18), 3533–3537. 10.1016/j.febslet.2007.06.072
120
Li Z. Chang T. C. Junco J. J. Devidas M. Li Y. Yang W. et al (2023). Genomic landscape of Down syndrome-associated acute lymphoblastic leukemia. Blood142 (2), 172–184. 10.1182/blood.2023019765
121
Li Z. Chen P. Su R. Hu C. Li Y. Elkahloun A. G. et al (2016). PBX3 and MEIS1 cooperate in hematopoietic cells to drive acute myeloid leukemias characterized by a core transcriptome of the MLL-rearranged disease. Cancer Res.76 (3), 619–629. 10.1158/0008-5472.CAN-15-1566
122
Liang C. Li Y. Wang L.-N. Zhang X.-L. Luo J.-S. Peng C.-J. et al (2021). Up-regulated miR-155 is associated with poor prognosis in childhood acute lymphoblastic leukemia and promotes cell proliferation targeting ZNF238. Hematology26 (1), 16–25. 10.1080/16078454.2020.1860187
123
Liang D.-C. Liu H.-C. Yang C.-P. Jaing T.-H. Hung I.-J. Yeh T.-C. et al (2013). Cooperating gene mutations in childhood acute myeloid leukemia with special reference on mutations of ASXL1, TET2, IDH1, IDH2, and DNMT3A. Blood121 (15), 2988–2995. 10.1182/blood-2012-06-436782
124
Liao J. Karnik R. Gu H. Ziller M. J. Clement K. Tsankov A. M. et al (2015). Targeted disruption of DNMT1, DNMT3A and DNMT3B in human embryonic stem cells. Nat. Genet.47 (5), 469–478. 10.1038/ng.3258
125
Lin T. L. Jaiswal A. K. Ritter A. J. Reppas J. Tran T. M. Neeb Z. T. et al (2023). Targeting IGF2BP3 enhances antileukemic effects of menin-MLL inhibition in MLL-AF4 leukemia. Blood Adv.8, 261–275. 10.1182/bloodadvances.2023011132
126
Lopez-Millan B. Sanchéz-Martínez D. Roca-Ho H. Gutiérrez-Agüera F. Molina O. Diaz de la Guardia R. et al (2019). NG2 antigen is a therapeutic target for MLL-rearranged B-cell acute lymphoblastic leukemia. Leukemia33 (7), 1557–1569. 10.1038/s41375-018-0353-0
127
Louka E. Povinelli B. Rodriguez-Meira A. Buck G. Wen W. X. Wang G. et al (2021). Heterogeneous disease-propagating stem cells in juvenile myelomonocytic leukemia. J. Exp. Med.218 (2), e20180853. 10.1084/jem.20180853
128
Maeda N. Ohashi T. Chagan-Yasutan H. Hattori T. Takahashi Y. Harigae H. et al (2015). Osteopontin-integrin interaction as a novel molecular target for antibody-mediated immunotherapy in adult T-cell leukemia. Retrovirology12 (1), 99. 10.1186/s12977-015-0225-x
129
Magnani C. F. Myburgh R. Brunn S. Chambovey M. Ponzo M. Volta L. et al (2023). Anti-CD117 CAR T cells incorporating a safety switch eradicate human acute myeloid leukemia and hematopoietic stem cells. Mol. Ther. Oncolytics30, 56–71. 10.1016/j.omto.2023.07.003
130
Magnusson M. Brun A. C. M. Lawrence H. J. Karlsson S. (2007). Hoxa9/hoxb3/hoxb4 compound null mice display severe hematopoietic defects. Exp. Hematol.35 (9), 1421–1428. 10.1016/j.exphem.2007.05.011
131
Malinge S. Bliss-Moreau M. Kirsammer G. Diebold L. Chlon T. Gurbuxani S. et al (2012). Increased dosage of the chromosome 21 ortholog Dyrk1a promotes megakaryoblastic leukemia in a murine model of Down syndrome. J. Clin. Invest.122 (3), 948–962. 10.1172/JCI60455
132
Malouf C. Antunes E. T. B. O’Dwyer M. Jakobczyk H. Sahm F. Landua S.-L. et al (2021). miR-130b and miR-128a are essential lineage-specific codrivers of t(4;11) MLL-AF4 acute leukemia. Blood138 (21), 2066–2092. 10.1182/blood.2020006610
133
Malumbres R. Fresquet V. Roman-Gomez J. Bobadilla M. Robles E. F. Altobelli G. G. et al (2011). LMO2 expression reflects the different stages of blast maturation and genetic features in B-cell acute lymphoblastic leukemia and predicts clinical outcome. Haematologica96 (7), 980–986. 10.3324/haematol.2011.040568
134
Manesia J. K. Xu Z. Broekaert D. Boon R. van Vliet A. Eelen G. et al (2015). Highly proliferative primitive fetal liver hematopoietic stem cells are fueled by oxidative metabolic pathways. Stem Cell Res.15 (3), 715–721. 10.1016/j.scr.2015.11.001
135
Mansoori B. Mohammadi A. Ditzel H. J. Duijf P. H. G. Khaze V. Gjerstorff M. F. et al (2021). HMGA2 as a critical regulator in cancer development. Genes (Basel)12 (2), 269. 10.3390/genes12020269
136
Mansour A. G. Teng K.-Y. Li Z. Zhu Z. Chen H. Tian L. et al (2023). Off-the-shelf CAR–engineered natural killer cells targeting FLT3 enhance killing of acute myeloid leukemia. Blood Adv.7 (20), 6225–6239. 10.1182/bloodadvances.2022007405
137
Marquis M. Beaubois C. Lavallée V. P. Abrahamowicz M. Danieli C. Lemieux S. et al (2018). High expression of HMGA2 independently predicts poor clinical outcomes in acute myeloid leukemia. Blood Cancer J.8 (8), 68. 10.1038/s41408-018-0103-6
138
Matouk I. J. Raveh E. Abu-lail R. Mezan S. Gilon M. Gershtain E. et al (2014). Oncofetal H19 RNA promotes tumor metastasis. Biochim. Biophys. Acta1843 (7), 1414–1426. 10.1016/j.bbamcr.2014.03.023
139
McKinney-Freeman S. L. Lengerke C. Jang I.-H. Schmitt S. Wang Y. Philitas M. et al (2008). Modulation of murine embryonic stem cell-derived CD41+c-kit+ hematopoietic progenitors by ectopic expression of Cdx genes. Blood111 (10), 4944–4953. 10.1182/blood-2007-11-124644
140
Mi S. Lu J. Sun M. Li Z. Zhang H. Neilly M. B. et al (2007). MicroRNA expression signatures accurately discriminate acute lymphoblastic leukemia from acute myeloid leukemia. Proc. Natl. Acad. Sci.104 (50), 19971–19976. 10.1073/pnas.0709313104
141
Mikkola H. K. Klintman J. Yang H. Hock H. Schlaeger T. M. Fujiwara Y. et al (2003). Haematopoietic stem cells retain long-term repopulating activity and multipotency in the absence of stem-cell leukaemia SCL/tal-1 gene. Nature421 (6922), 547–551. 10.1038/nature01345
142
Milne T. A. Briggs S. D. Brock H. W. Martin M. E. Gibbs D. Allis C. D. et al (2002). MLL targets SET domain methyltransferase activity to Hox gene promoters. Mol. Cell10 (5), 1107–1117. 10.1016/s1097-2765(02)00741-4
143
Milne T. A. Martin M. E. Brock H. W. Slany R. K. Hess J. L. (2005). Leukemogenic MLL fusion proteins bind across a broad region of the Hox a9 locus, promoting transcription and multiple histone modifications. Cancer Res.65 (24), 11367–11374. 10.1158/0008-5472.CAN-05-1041
144
Miraglia S. Godfrey W. Yin A. H. Atkins K. Warnke R. Holden J. T. et al (1997). A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood90 (12), 5013–5021. 10.1182/blood.v90.12.5013
145
Mochizuki-Kashio M. Mishima Y. Miyagi S. Negishi M. Saraya A. Konuma T. et al (2011). Dependency on the polycomb gene Ezh2 distinguishes fetal from adult hematopoietic stem cells. Blood118 (25), 6553–6561. 10.1182/blood-2011-03-340554
146
Moison C. Spinella J.-F. Chagraoui J. Lavallée V.-P. Lehnertz B. Thiollier C. et al (2022). HMGA2 expression defines a subset of human AML with immature transcriptional signature and vulnerability to G2/M inhibition. Blood Adv.6 (16), 4793–4806. 10.1182/bloodadvances.2021005828
147
Molenaar J. J. Domingo-Fernández R. Ebus M. E. Lindner S. Koster J. Drabek K. et al (2012). LIN28B induces neuroblastoma and enhances MYCN levels via let-7 suppression. Nat. Genet.44 (11), 1199–1206. 10.1038/ng.2436
148
Monk M. Holding C. (2001). Human embryonic genes re-expressed in cancer cells. Oncogene20 (56), 8085–8091. 10.1038/sj.onc.1205088
149
Montecino-Rodriguez E. Fice M. Casero D. Berent-Maoz B. Barber C. L. Dorshkind K. (2016). Distinct genetic networks orchestrate the emergence of specific waves of fetal and adult B-1 and B-2 development. Immunity45 (3), 527–539. 10.1016/j.immuni.2016.07.012
150
Moqadam F. A. Lange-Turenhout E. Aries I. Pieters R. Den Boer M. (2013). MiR-125b, miR-100 and miR-99a co-regulate vincristine resistance in childhood acute lymphoblastic leukemia. Leukemia Res.37 (10), 1315–1321. 10.1016/j.leukres.2013.06.027
151
Muench M. O. Cupp J. Polakoff J. Roncarolo M. G. (1994). Expression of CD33, CD38, and HLA-DR on CD34+ human fetal liver progenitors with a high proliferative potential. Blood83 (11), 3170–3181. 10.1182/blood.v83.11.3170.3170
152
Müller S. Bley N. Busch B. Glaß M. Lederer M. Misiak C. et al (2020). The oncofetal RNA-binding protein IGF2BP1 is a druggable, post-transcriptional super-enhancer of E2F-driven gene expression in cancer. Nucleic Acids Res.48 (15), 8576–8590. 10.1093/nar/gkaa653
153
Myburgh R. Kiefer J. D. Russkamp N. F. Magnani C. F. Nuñez N. Simonis A. et al (2020). Anti-human CD117 CAR T-cells efficiently eliminate healthy and malignant CD117-expressing hematopoietic cells. Leukemia34 (10), 2688–2703. 10.1038/s41375-020-0818-9
154
Nagel S. Meyer C. (2022). Normal and aberrant TALE-class homeobox gene activities in pro-B-cells and B-cell precursor acute lymphoblastic leukemia. Int. J. Mol. Sci.23 (19), 11874. 10.3390/ijms231911874
155
Nagel S. Pommerenke C. Meyer C. MacLeod R. A. F. (2022). The hematopoietic TALE-code shows normal activity of IRX1 in myeloid progenitors and reveals ectopic expression of IRX3 and IRX5 in acute myeloid leukemia. Int. J. Mol. Sci.23 (6), 3192. 10.3390/ijms23063192
156
Ng E. S. Azzola L. Bruveris F. F. Calvanese V. Phipson B. Vlahos K. et al (2016a). Differentiation of human embryonic stem cells to HOXA+ hemogenic vasculature that resembles the aorta-gonad-mesonephros. Nat. Biotechnol.34 (11), 1168–1179. 10.1038/nbt.3702
157
Ng S. W. K. Mitchell A. Kennedy J. A. Chen W. C. McLeod J. Ibrahimova N. et al (2016b). A 17-gene stemness score for rapid determination of risk in acute leukaemia. Nature540 (7633), 433–437. 10.1038/nature20598
158
North T. E. de Bruijn M. F. Stacy T. Talebian L. Lind E. Robin C. et al (2002). Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity16 (5), 661–672. 10.1016/s1074-7613(02)00296-0
159
O’Byrne S. Elliott N. Rice S. Buck G. Fordham N. Garnett C. et al (2019). Discovery of a CD10-negative B-progenitor in human fetal life identifies unique ontogeny-related developmental programs. Blood, J. Am. Soc. Hematol.134 (13), 1059–1071. 10.1182/blood.2019001289
160
O'Connell R. M. Chaudhuri A. A. Rao D. S. Gibson W. S. Balazs A. B. Baltimore D. (2010). MicroRNAs enriched in hematopoietic stem cells differentially regulate long-term hematopoietic output. Proc. Natl. Acad. Sci.107 (32), 14235–14240. 10.1073/pnas.1009798107
161
Ooi A. L. Sahoo D. Adorno M. Wang Y. Weissman I. L. Park C. Y. (2010). MicroRNA-125b expands hematopoietic stem cells and enriches for the lymphoid-balanced and lymphoid-biased subsets. Proc. Natl. Acad. Sci.107 (50), 21505–21510. 10.1073/pnas.1016218107
162
Orlovsky K. Kalinkovich A. Rozovskaia T. Shezen E. Itkin T. Alder H. et al (2011). Down-regulation of homeobox genes MEIS1 and HOXA in MLL-rearranged acute leukemia impairs engraftment and reduces proliferation. Proc. Natl. Acad. Sci. U. S. A.108 (19), 7956–7961. 10.1073/pnas.1103154108
163
Page E. C. Heatley S. L. Eadie L. N. McClure B. J. de Bock C. E. Omari S. et al (2022). HMGN1 plays a significant role in CRLF2 driven Down Syndrome leukemia and provides a potential therapeutic target in this high-risk cohort. Oncogene41 (6), 797–808. 10.1038/s41388-021-02126-4
164
Palanichamy J. K. Tran T. M. Howard J. M. Contreras J. R. Fernando T. R. Sterne-Weiler T. et al (2016). RNA-binding protein IGF2BP3 targeting of oncogenic transcripts promotes hematopoietic progenitor proliferation. J. Clin. Invest.126 (4), 1495–1511. 10.1172/jci80046
165
Palis J. Yoder M. C. (2001). Yolk-sac hematopoiesis: the first blood cells of mouse and man. Exp. Hematol.29 (8), 927–936. 10.1016/s0301-472x(01)00669-5
166
Perner F. Gadrey J. Y. Xiong Y. Hatton C. Eschle B. K. Weiss A. et al (2020). Novel inhibitors of the histone methyltransferase DOT1L show potent antileukemic activity in patient-derived xenografts. Blood136 (17), 1983–1988. 10.1182/blood.2020006113
167
Perner F. Stein E. M. Wenge D. V. Singh S. Kim J. Apazidis A. et al (2023). MEN1 mutations mediate clinical resistance to menin inhibition. Nature615 (7954), 913–919. 10.1038/s41586-023-05755-9
168
Piatopoulou D. Avgeris M. Marmarinos A. Xagorari M. Baka M. Doganis D. et al (2017). miR-125b predicts childhood acute lymphoblastic leukaemia poor response to BFM chemotherapy treatment. Br. J. cancer117 (6), 801–812. 10.1038/bjc.2017.256
169
Popescu D.-M. Botting R. A. Stephenson E. Green K. Webb S. Jardine L. et al (2019). Decoding human fetal liver haematopoiesis. Nature574 (7778), 365–371. 10.1038/s41586-019-1652-y
170
Popovic R. Riesbeck L. E. Velu C. S. Chaubey A. Zhang J. Achille N. J. et al (2009). Regulation of mir-196b by MLL and its overexpression by MLL fusions contributes to immortalization. Blood, J. Am. Soc. Hematol.113 (14), 3314–3322. 10.1182/blood-2008-04-154310
171
Porcher C. Chagraoui H. Kristiansen M. S. (2017). SCL/TAL1: a multifaceted regulator from blood development to disease. Blood129 (15), 2051–2060. 10.1182/blood-2016-12-754051
172
Ramos-Mejía V. Navarro-Montero O. Ayllón V. Bueno C. Romero T. Real P. J. et al (2014). HOXA9 promotes hematopoietic commitment of human embryonic stem cells. Blood124 (20), 3065–3075. 10.1182/blood-2014-03-558825
173
Rappa G. Mercapide J. Anzanello F. Pope R. M. Lorico A. (2013). Biochemical and biological characterization of exosomes containing prominin-1/CD133. Mol. Cancer12, 62. 10.1186/1476-4598-12-62
174
Rawat V. P. S. Cusan M. Deshpande A. Hiddemann W. Quintanilla-Martinez L. Humphries R. K. et al (2004). Ectopic expression of the homeobox gene Cdx2 is the transforming event in a mouse model of t(12;13)(p13;q12) acute myeloid leukemia. Proc. Natl. Acad. Sci. U. S. A.101 (3), 817–822. 10.1073/pnas.0305555101
175
Rawat V. P. S. Thoene S. Naidu V. M. Arseni N. Heilmeier B. Metzeler K. et al (2008). Overexpression of CDX2 perturbs HOX gene expression in murine progenitors depending on its N-terminal domain and is closely correlated with deregulated HOX gene expression in human acute myeloid leukemia. Blood111 (1), 309–319. 10.1182/blood-2007-04-085407
176
Ren H. Elliott N. Lye B. Cross J. Field L. Ponnusamy K. et al (2023). Bispecific CAR-iNKT immunotherapy for high risk MLL-rearranged acute lymphoblastic leukemia. Blood142 (1), 766. 10.1182/blood-2023-186442
177
Reya T. Morrison S. J. Clarke M. F. Weissman I. L. (2001). Stem cells, cancer, and cancer stem cells. Nature414 (6859), 105–111. 10.1038/35102167
178
Riddell J. Gazit R. Garrison B. S. Guo G. Saadatpour A. Mandal P. K. et al (2014). Reprogramming committed murine blood cells to induced hematopoietic stem cells with defined factors. Cell157 (3), 549–564. 10.1016/j.cell.2014.04.006
179
Riedt T. Ebinger M. Salih H. R. Tomiuk J. Handgretinger R. Kanz L. et al (2009). Aberrant expression of the homeobox gene CDX2 in pediatric acute lymphoblastic leukemia. Blood113 (17), 4049–4051. 10.1182/blood-2008-12-196634
180
Roberts I. Alford K. Hall G. Juban G. Richmond H. Norton A. et al (2013). GATA1-mutant clones are frequent and often unsuspected in babies with Down syndrome: identification of a population at risk of leukemia. Blood122 (24), 3908–3917. 10.1182/blood-2013-07-515148
181
Roberts I. Izraeli S. (2014). Haematopoietic development and leukaemia in Down syndrome. Br. J. Haematol.167 (5), 587–599. 10.1111/bjh.13096
182
Roy A. Cowan G. Mead A. J. Filippi S. Bohn G. Chaidos A. et al (2012). Perturbation of fetal liver hematopoietic stem and progenitor cell development by trisomy 21. Proc. Natl. Acad. Sci.109 (43), 17579–17584. 10.1073/pnas.1211405109
183
Roy A. Wang G. Iskander D. O’Byrne S. Elliott N. O’Sullivan J. et al (2021). Transitions in lineage specification and gene regulatory networks in hematopoietic stem/progenitor cells over human development. Cell Rep.36 (11), 109698. 10.1016/j.celrep.2021.109698
184
Roy S. Di Cello F. Kowalski J. Hristov A. C. Tsai H.-L. Bhojwani D. et al (2013). HMGA1 overexpression correlates with relapse in childhood B-lineage acute lymphoblastic leukemia. Leukemia Lymphoma54 (11), 2565–2567. 10.3109/10428194.2013.782610
185
Rybak A. Fuchs H. Smirnova L. Brandt C. Pohl E. E. Nitsch R. et al (2008). A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat. Cell Biol.10 (8), 987–993. 10.1038/ncb1759
186
Sachs K. Sarver A. L. Noble-Orcutt K. E. LaRue R. S. Antony M. L. Chang D. et al (2020). Single-cell gene expression analyses reveal distinct self-renewing and proliferating subsets in the leukemia stem cell compartment in acute myeloid leukemia. Cancer Res.80 (3), 458–470. 10.1158/0008-5472.Can-18-2932
187
Schäfer V. Ernst J. Rinke J. Winkelmann N. Beck J. F. Hochhaus A. et al (2016). EZH2 mutations and promoter hypermethylation in childhood acute lymphoblastic leukemia. J. Cancer Res. Clin. Oncol.142 (7), 1641–1650. 10.1007/s00432-016-2174-8
188
Scholl C. Bansal D. Döhner K. Eiwen K. Huntly B. J. P. Lee B. H. et al (2007). The homeobox gene CDX2 is aberrantly expressed in most cases of acute myeloid leukemia and promotes leukemogenesis. J. Clin. Investigation117 (4), 1037–1048. 10.1172/JCI30182
189
Schotte D. Chau J. C. K. Sylvester G. Liu G. Chen C. van der Velden V. H. J. et al (2009). Identification of new microRNA genes and aberrant microRNA profiles in childhood acute lymphoblastic leukemia. Leukemia23 (2), 313–322. 10.1038/leu.2008.286
190
Shah S. N. Kerr C. Cope L. Zambidis E. Liu C. Hillion J. et al (2012). HMGA1 reprograms somatic cells into pluripotent stem cells by inducing stem cell transcriptional networks. PLOS ONE7 (11), e48533. 10.1371/journal.pone.0048533
191
Shahab S. W. Roggeveen C. M. Sun J. Kunhiraman H. McSwain L. F. Juraschka K. et al (2023). The LIN28B-let-7-PBK pathway is essential for group 3 medulloblastoma tumor growth and survival. Mol. Oncol.17 (9), 1784–1802. 10.1002/1878-0261.13477
192
Sharma A. Blériot C. Currenti J. Ginhoux F. (2022). Oncofetal reprogramming in tumour development and progression. Nat. Rev. Cancer22 (10), 593–602. 10.1038/s41568-022-00497-8
193
Sharma G. Tran T. M. Bansal I. Beg M. S. Bhardwaj R. Bassi J. et al (2023). RNA binding protein IGF2BP1 synergizes with ETV6-RUNX1 to drive oncogenic signaling in B-cell Acute Lymphoblastic Leukemia. J. Exp. Clin. cancer Res. CR42 (1), 231. 10.1186/s13046-023-02810-1
194
Singh S. K. Hawkins C. Clarke I. D. Squire J. A. Bayani J. Hide T. et al (2004). Identification of human brain tumour initiating cells. Nature432 (7015), 396–401. 10.1038/nature03128
195
Solé L. Lobo-Jarne T. Álvarez-Villanueva D. Alonso-Marañón J. Guillén Y. Guix M. et al (2022). p53 wild-type colorectal cancer cells that express a fetal gene signature are associated with metastasis and poor prognosis. Nat. Commun.13 (1), 2866. 10.1038/s41467-022-30382-9
196
Somervaille T. C. Matheny C. J. Spencer G. J. Iwasaki M. Rinn J. L. Witten D. M. et al (2009). Hierarchical maintenance of MLL myeloid leukemia stem cells employs a transcriptional program shared with embryonic rather than adult stem cells. Cell stem Cell4 (2), 129–140. 10.1016/j.stem.2008.11.015
197
Somerville T. D. D. Simeoni F. Chadwick J. A. Williams E. L. Spencer G. J. Boros K. et al (2018). Derepression of the Iroquois homeodomain transcription factor gene IRX3 confers differentiation block in acute leukemia. Cell Rep.22 (3), 638–652. 10.1016/j.celrep.2017.12.063
198
Sonoda Y. Itoh M. Tohda S. (2021). Effects of HOXA9 inhibitor DB818 on the growth of acute myeloid leukaemia cells. Anticancer Res.41 (4), 1841–1847. 10.21873/anticanres.14950
199
Stam R. W. Schneider P. Hagelstein J. A. P. van der Linden M. H. Stumpel D. J. P. M. de Menezes R. X. et al (2010). Gene expression profiling-based dissection of MLL translocated and MLL germline acute lymphoblastic leukemia in infants. Blood115 (14), 2835–2844. 10.1182/blood-2009-07-233049
200
Stauffer F. Weiss A. Scheufler C. Möbitz H. Ragot C. Beyer K. S. et al (2019). New potent DOT1L inhibitors for in vivo evaluation in mouse. ACS Med. Chem. Lett.10 (12), 1655–1660. 10.1021/acsmedchemlett.9b00452
201
Stein E. M. Garcia-Manero G. Rizzieri D. A. Tibes R. Berdeja J. G. Savona M. R. et al (2018). The DOT1L inhibitor pinometostat reduces H3K79 methylation and has modest clinical activity in adult acute leukemia. Blood131 (24), 2661–2669. 10.1182/blood-2017-12-818948
202
Stoskus M. Gineikiene E. Valceckiene V. Valatkaite B. Pileckyte R. Griskevicius L. (2011). Identification of characteristic IGF2BP expression patterns in distinct B-ALL entities. Blood Cells, Mol. Dis.46 (4), 321–326. 10.1016/j.bcmd.2011.02.005
203
Su L. Bryan N. Battista S. Freitas J. Garabedian A. D’Alessio F. et al (2020). Identification of HMGA2 inhibitors by AlphaScreen-based ultra-high-throughput screening assays. Sci. Rep.10 (1), 18850. 10.1038/s41598-020-75890-0
204
Sudha T. Godugu K. Darwish N. H. E. Nazeer T. Mousa S. A. (2021). Novel polyethylene glycol-conjugated triazole derivative with high thyrointegrin αvβ3 affinity in acute myeloid leukemia management. Cancers13 (16), 4070. 10.3390/cancers13164070
205
Sugimura R. Jha D. K. Han A. Soria-Valles C. da Rocha E. L. Lu Y.-F. et al (2017). Haematopoietic stem and progenitor cells from human pluripotent stem cells. Nature545 (7655), 432–438. 10.1038/nature22370
206
Sun W. Triche T. Malvar J. Gaynon P. Sposto R. Yang X. et al (2018). A phase 1 study of azacitidine combined with chemotherapy in childhood leukemia: a report from the TACL consortium. Blood131 (10), 1145–1148. 10.1182/blood-2017-09-803809
207
Symeonidou V. Ottersbach K. (2021). HOXA9/IRX1 expression pattern defines two subgroups of infant MLL-AF4-driven acute lymphoblastic leukemia. Exp. Hematol.93, 38–43.e5. 10.1016/j.exphem.2020.10.002
208
Taipale J. Beachy P. A. (2001). The Hedgehog and Wnt signalling pathways in cancer. Nature411 (6835), 349–354.
209
Tan L. Wei X. Zheng L. Zeng J. Liu H. Yang S. et al (2016). Amplified HMGA2 promotes cell growth by regulating Akt pathway in AML. J. Cancer Res. Clin. Oncol.142 (2), 389–399. 10.1007/s00432-015-2036-9
210
Tan L. Xu H. Chen G. Wei X. Yu B. Ye J. et al (2018). Silencing of HMGA2 reverses retardance of cell differentiation in human myeloid leukaemia. Br. J. Cancer118 (3), 405–415. 10.1038/bjc.2017.403
211
Tang C.-Y. Lin J. Qian W. Yang J. Ma J.-C. Deng Z.-Q. et al (2014). Low SOX17 expression: prognostic significance in de novo acute myeloid leukemia with normal cytogenetics. Clin. Chem. Laboratory Med.52 (12), 1843–1850. 10.1515/cclm-2014-0487
212
Tavian M. Peault B. (2005). Embryonic development of the human hematopoietic system. Int. J. Dev. Biol.49 (2-3), 243–250. 10.1387/ijdb.041957mt
213
Testa U. Riccioni R. Militi S. Coccia E. Stellacci E. Samoggia P. et al (2002). Elevated expression of IL-3Ralpha in acute myelogenous leukemia is associated with enhanced blast proliferation, increased cellularity, and poor prognosis. Blood, J. Am. Soc. Hematol.100 (8), 2980–2988. 10.1182/blood-2002-03-0852
214
Thoene S. Rawat V. P. S. Heilmeier B. Hoster E. Metzeler K. H. Herold T. et al (2009). The homeobox gene CDX2 is aberrantly expressed and associated with an inferior prognosis in patients with acute lymphoblastic leukemia. Leukemia23 (4), 649–655. 10.1038/leu.2008.355
215
Tholouli E. MacDermott S. Hoyland J. Yin J. L. Byers R. (2012). Quantitative multiplex quantum dot in-situ hybridisation based gene expression profiling in tissue microarrays identifies prognostic genes in acute myeloid leukaemia. Biochem. Biophysical Res. Commun.425 (2), 333–339. 10.1016/j.bbrc.2012.07.092
216
Thompson B. J. Bhansali R. Diebold L. Cook D. E. Stolzenburg L. Casagrande A. S. et al (2015). DYRK1A controls the transition from proliferation to quiescence during lymphoid development by destabilizing Cyclin D3. J. Exp. Med.212 (6), 953–970. 10.1084/jem.20150002
217
Tian Z. Shi C. Yang G. Allen J. K. Shi Q. Al-Shami A. et al (2023). Preclinical development of 1B7/CD3, a novel anti-TSLPR bispecific antibody that targets CRLF2-rearranged Ph-like B-ALL. Leukemia37 (10), 2006–2016. 10.1038/s41375-023-02010-y
218
Tolba F. M. Foda M. E. kamal H. M. Elshabrawy D. A. (2013). Expression of CD133 in acute leukemia. Med. Oncol.30 (2), 527. 10.1007/s12032-013-0527-6
219
Tran T. M. Philipp J. Bassi J. S. Nibber N. Draper J. M. Lin T. L. et al (2021). The RNA-binding protein IGF2BP3 is critical for MLL-AF4-mediated leukemogenesis. Leukemia36, 68–79. 10.1038/s41375-021-01346-7
220
Turan R. D. Albayrak E. Uslu M. Siyah P. Alyazici L. Y. Kalkan B. M. et al (2020). Development of small molecule MEIS inhibitors that modulate HSC activity. Sci. Rep.10 (1), 7994. 10.1038/s41598-020-64888-3
221
Uckelmann H. J. Haarer E. L. Takeda R. Wong E. M. Hatton C. Marinaccio C. et al (2023). Mutant NPM1 directly regulates oncogenic transcription in acute myeloid leukemia. Cancer Discov.13 (3), 746–765. 10.1158/2159-8290.CD-22-0366
222
Ueno S. Lu J. He J. Li A. Zhang X. Ritz J. et al (2014). Aberrant expression of SALL4 in acute B cell lymphoblastic leukemia: mechanism, function, and implication for a potential novel therapeutic target. Exp. Hematol.42 (4), 307–316. 10.1016/j.exphem.2014.01.005
223
Uhlén M. Fagerberg L. Hallström B. M. Lindskog C. Oksvold P. Mardinoglu A. et al (2015). Proteomics. Tissue-based map of the human proteome. Sci. (New York, N.Y.)347 (6220), 1260419. 10.1126/science.1260419
224
Vey N. Prebet T. Thalamas C. Charbonnier A. Rey J. Kloos I. et al (2017). Phase 1 dose-escalation study of oral abexinostat for the treatment of patients with relapsed/refractory higher-risk myelodysplastic syndromes, acute myeloid leukemia, or acute lymphoblastic leukemia. Leukemia Lymphoma58 (8), 1880–1886. 10.1080/10428194.2016.1263843
225
Viswanathan S. R. Daley G. Q. (2010). Lin28: a MicroRNA regulator with a macro role. Cell140 (4), 445–449. 10.1016/j.cell.2010.02.007
226
Viswanathan S. R. Powers J. T. Einhorn W. Hoshida Y. Ng T. L. Toffanin S. et al (2009). Lin28 promotes transformation and is associated with advanced human malignancies. Nat. Genet.41 (7), 843–848. 10.1038/ng.392
227
Vora P. Venugopal C. Salim S. K. Tatari N. Bakhshinyan D. Singh M. et al (2020). The rational development of cd133-targeting immunotherapies for glioblastoma. Cell Stem Cell26 (6), 832–844. 10.1016/j.stem.2020.04.008
228
Wagenblast E. Araujo J. Gan O. I. Cutting S. K. Murison A. Krivdova G. et al (2021). Mapping the cellular origin and early evolution of leukemia in Down syndrome. Science373 (6551), eabf6202. 10.1126/science.abf6202
229
Wang D. Tanaka-Yano M. Meader E. Kinney M. A. Morris V. Lummertz da Rocha E. et al (2022a). Developmental maturation of the hematopoietic system controlled by a Lin28b-let-7-Cbx2 axis. Cell Rep.39 (1), 110587. 10.1016/j.celrep.2022.110587
230
Wang L. Rowe R. G. Jaimes A. Yu C. Nam Y. Pearson D. S. et al (2018a). Small-molecule inhibitors disrupt let-7 oligouridylation and release the selective blockade of let-7 processing by LIN28. Cell Rep.23 (10), 3091–3101. 10.1016/j.celrep.2018.04.116
231
Wang L.-l. Tang X. Zhou G. Liu S. Wang Y. Chen F. et al (2022b). PROM1 and CTGF expression in childhood MLL-rearrangement acute lymphoblastic leukemia. J. Oncol.2022, 5896022. 10.1155/2022/5896022
232
Wang S. Chim B. Su Y. Khil P. Wong M. Wang X. et al (2019). Enhancement of LIN28B-induced hematopoietic reprogramming by IGF2BP3. Genes and Dev.33 (15-16), 1048–1068. 10.1101/gad.325100.119
233
Wang Y. Chen M. Wu Z. Tong C. Dai H. Guo Y. et al (2018b). CD133-directed CAR T cells for advanced metastasis malignancies: A phase I trial. Oncoimmunology7 (7), e1440169. 10.1080/2162402X.2018.1440169
234
Wang Y. Xu Y. Li S. Liu J. Xing Y. Xing H. et al (2018c). Targeting FLT3 in acute myeloid leukemia using ligand-based chimeric antigen receptor-engineered T cells. J. Hematol. Oncol.11 (1), 60. 10.1186/s13045-018-0603-7
235
Waters N. J. Smith S. A. Olhava E. J. Duncan K. W. Burton R. D. O'Neill J. et al (2016). Metabolism and disposition of the DOT1L inhibitor, pinometostat (EPZ-5676), in rat, dog and human. Cancer Chemother. Pharmacol.77 (1), 43–62. 10.1007/s00280-015-2929-y
236
Weigmann A. Corbeil D. Hellwig A. Huttner W. B. (1997). Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc. Natl. Acad. Sci. U. S. A.94 (23), 12425–12430. 10.1073/pnas.94.23.12425
237
Wermke M. Kraus S. Ehninger A. Bargou R. C. Goebeler M.-E. Middeke J. M. et al (2021). Proof of concept for a rapidly switchable universal CAR-T platform with UniCAR-T-CD123 in relapsed/refractory AML. Blood137 (22), 3145–3148. 10.1182/blood.2020009759
238
Wong P. Iwasaki M. Somervaille T. C. P. So C. W. E. Cleary M. L. (2007). Meis1 is an essential and rate-limiting regulator of MLL leukemia stem cell potential. Genes and Dev.21 (21), 2762–2774. 10.1101/gad.1602107
239
Wu P. (2020). Inhibition of RNA-binding proteins with small molecules. Nat. Rev. Chem.4 (9), 441–458. 10.1038/s41570-020-0201-4
240
Wu Z. Eguchi-Ishimae M. Yagi C. Iwabuki H. Gao W. Tauchi H. et al (2015). HMGA2 as a potential molecular target in KMT2A-AFF1-positive infant acute lymphoblastic leukaemia. Br. J. Haematol.171 (5), 818–829. 10.1111/bjh.13763
241
Wuputra K. Ku C.-C. Wu D.-C. Lin Y.-C. Saito S. Yokoyama K. K. (2020). Prevention of tumor risk associated with the reprogramming of human pluripotent stem cells. J. Exp. Clin. Cancer Res.39 (1), 100–124. 10.1186/s13046-020-01584-0
242
Yamada Y. Warren A. J. Dobson C. Forster A. Pannell R. Rabbitts T. H. (1998). The T cell leukemia LIM protein Lmo2 is necessary for adult mouse hematopoiesis. Proc. Natl. Acad. Sci. U. S. A.95 (7), 3890–3895. 10.1073/pnas.95.7.3890
243
Yan W. Xu L. Sun Z. Lin Y. Zhang W. Chen J. et al (2015). MicroRNA biomarker identification for pediatric acute myeloid leukemia based on a novel bioinformatics model. Oncotarget6 (28), 26424–26436. 10.18632/oncotarget.4459
244
Yang J. Chai L. Gao C. Fowles T. C. Alipio Z. Dang H. et al (2008). SALL4 is a key regulator of survival and apoptosis in human leukemic cells. Blood112 (3), 805–813. 10.1182/blood-2007-11-126326
245
Yao S. Jianlin C. Yarong L. Botao L. Qinghan W. Hongliang F. et al (2019). Donor-derived cd123-targeted CAR T cell serves as a RIC regimen for haploidentical transplantation in a patient with FUS-ERG+ AML. Front. Oncol.9, 1358. 10.3389/fonc.2019.01358
246
Yasuda T. Sanada M. Kawazu M. Kojima S. Tsuzuki S. Ueno H. et al (2022). Two novel high-risk adult B-cell acute lymphoblastic leukemia subtypes with high expression of CDX2 and IDH1/2 mutations. Blood139 (12), 1850–1862. 10.1182/blood.2021011921
247
Yin A. H. Miraglia S. Zanjani E. D. Almeida-Porada G. Ogawa M. Leary A. G. et al (1997). AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood90 (12), 5002–5012. 10.1182/blood.v90.12.5002.5002_5002_5012
248
Yokoyama A. Somervaille T. C. P. Smith K. S. Rozenblatt-Rosen O. Meyerson M. Cleary M. L. (2005). The menin tumor suppressor protein is an essential oncogenic cofactor for MLL-associated leukemogenesis. Cell123 (2), 207–218. 10.1016/j.cell.2005.09.025
249
Yu S. Cui K. Jothi R. Zhao D. M. Jing X. Zhao K. et al (2011). GABP controls a critical transcription regulatory module that is essential for maintenance and differentiation of hematopoietic stem/progenitor cells. Blood117 (7), 2166–2178. 10.1182/blood-2010-09-306563
250
Yuan J. Nguyen C. K. Liu X. Kanellopoulou C. Muljo S. A. (2012). Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science335 (6073), 1195–1200. 10.1126/science.1216557
251
Zhang H. Luo X.-Q. Feng D.-D. Zhang X.-J. Wu J. Zheng Y.-S. et al (2011). Upregulation of microRNA-125b contributes to leukemogenesis and increases drug resistance in pediatric acute promyelocytic leukemia. Mol. cancer10, 108–113. 10.1186/1476-4598-10-108
252
Zhang J. Ratanasirintrawoot S. Chandrasekaran S. Wu Z. Ficarro S. B. Yu C. et al (2016). LIN28 regulates stem cell metabolism and conversion to primed pluripotency. Cell Stem Cell19 (1), 66–80. 10.1016/j.stem.2016.05.009
253
Zhang J. Tam W.-L. Tong G. Q. Wu Q. Chan H.-Y. Soh B.-S. et al (2006). Sall4 modulates embryonic stem cell pluripotency and early embryonic development by the transcriptional regulation of Pou5f1. Nat. Cell Biol.8 (10), 1114–1123. 10.1038/ncb1481
254
Zhang L. Li X. Ke Z. Huang L. Liang Y. Wu J. et al (2013). MiR-99a may serve as a potential oncogene in pediatric myeloid leukemia. Cancer Cell Int.13 (1), 110–111. 10.1186/1475-2867-13-110
255
Zhang N. Shen Y. Li H. Chen Y. Zhang P. Lou S. et al (2022). The m6A reader IGF2BP3 promotes acute myeloid leukemia progression by enhancing RCC2 stability. Exp. Mol. Med.54 (2), 194–205. 10.1038/s12276-022-00735-x
256
Zhang Q. Shi M. Zheng R. Han H. Zhang X. Lin F. (2023a). C1632 inhibits ovarian cancer cell growth and migration by inhibiting LIN28 B/let-7/FAK signaling pathway and FAK phosphorylation. Eur. J. Pharmacol.956, 175935. 10.1016/j.ejphar.2023.175935
257
Zhang Y. Jiang S. He F. Tian Y. Hu H. Gao L. et al (2023b). Single-cell transcriptomics reveals multiple chemoresistant properties in leukemic stem and progenitor cells in pediatric AML. Genome Biol.24 (1), 199. 10.1186/s13059-023-03031-7
258
Zhao H. Chen S. Fu Q. (2020). Exosomes from CD133+ cells carrying circ-ABCC1 mediate cell stemness and metastasis in colorectal cancer. J. Cell. Biochem.121 (5-6), 3286–3297. 10.1002/jcb.29600
259
Zhou J. Bi C. Ching Y. Q. Chooi J.-Y. Lu X. Quah J. Y. et al (2017). Inhibition of LIN28B impairs leukemia cell growth and metabolism in acute myeloid leukemia. J. Hematol. Oncol.10 (1), 138. 10.1186/s13045-017-0507-y
260
Zhou Y. Li Y. S. Bandi S. R. Tang L. Shinton S. A. Hayakawa K. et al (2015). Lin28b promotes fetal B lymphopoiesis through the transcription factor Arid3a. J. Exp. Med.212 (4), 569–580. 10.1084/jem.20141510
261
Zhu R. Zhao W. Fan F. Tang L. Liu J. Luo T. et al (2017). A 3-miRNA signature predicts prognosis of pediatric and adolescent cytogenetically normal acute myeloid leukemia. Oncotarget8 (24), 38902–38913. 10.18632/oncotarget.17151
Summary
Keywords
leukemia, stem cells, development genes, gene regulation, fetal oncogenes
Citation
Ling RE, Cross JW and Roy A (2024) Aberrant stem cell and developmental programs in pediatric leukemia. Front. Cell Dev. Biol. 12:1372899. doi: 10.3389/fcell.2024.1372899
Received
18 January 2024
Accepted
11 March 2024
Published
27 March 2024
Volume
12 - 2024
Edited by
Antonella Lettieri, University of Milan, Italy
Reviewed by
Anilkumar Gopalakrishnapillai, Alfred I. duPont Hospital for Children, United States
Updates
Copyright
© 2024 Ling, Cross and Roy.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anindita Roy, anindita.roy@paediatrics.ox.ac.uk
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.