- 1Department for Women’s Health, University Hospital Tübingen, Tübingen, Germany
- 2Department of Physiology, University of Tübingen, Tübingen, Germany
- 3Department of Physiology, Jining Medical University, Jining, China
Introduction: A transient window of uterine receptivity ensures that embryos implant in an optimal endometrial environment. Failure to establish or premature closure of the implantation window is thought to be a major cause of infertility, which affects many couples globally. Embryos release trypsin, which designates its developmental potential and plays a crucial role in implantation. Calcium (Ca2+) signalling participates in receptivity and is thus a prerequisite for embryo implantation. Left-right determination factor 2 (LEFTY2) is a negative regulator of endometrial receptivity and is associated with unexplained infertility. We hypothesize that LEFTY2 impedes Ca2+ entry induced by trypsin in endometrial cells.
Methods: In silico analysis was performed to investigate classical trypsin pathway genes in human embryos. Trypsin levels from single human embryo conditioned medium were subject to ELISA. To determine if trypsin signals can modulate calcium entry, intracellular calcium [Ca2+]i was determined utilizing Fura-2 fluorescence in human endometrial epithelial cells (Ishikawa cells). Bioinformatic analysis on publicly available single cell sequencing data was used to investigate the expression of L-type calcium channel (CACNA1C) in endometrium. qRT-PCR and immunofluorescence were used to quantify L-type calcium channel abundance.
Results: We report that the trypsin machinery is established at the blastocyst stage and that high levels of trypsin are associated with a successful pregnancy. Treatment with LEFTY2 or combined treatment with LEFTY2 and trypsin blocked the increase of L-type Ca2+ channel levels and activity. Treatment of endometrial cells with trypsin was followed by an increase of [Ca2+]i, an effect that was significantly blunted by amiloride and LEFTY2. Further, the trypsin induced increase of [Ca2+]i was significantly blunted by L-type calcium channel inhibitor nifedipine. In the presence of nifedipine, LEFTY2 did not further modify trypsin induced increase of [Ca2+]i. LEFTY2 significantly decreased levels of L-type Ca2+ channel.
Discussion: Taken together, we demonstrate that high trypsin levels are associated with a positive pregnancy outcome and that infertility factor LEFTY2 downregulates trypsin induced Ca2+ increase due in part by interference with nifedipine sensitive Ca2+ entry. These findings contribute further to our knowledge of unexplained infertility and failed assisted reproductive technologies.
1 Introduction
Human reproduction is often considered surprisingly inefficient, echoed by the evidence that only 40%–60% of conceptions in young, healthy women result in a successful birth (Muter et al., 2023). The causes of female infertility are vast ranging from endometriosis, ovulation and fallopian tube disorders (Ehsani et al., 2019). Furthermore, in many industrialised countries, factors such as obesity, metabolic syndrome, vaping/smoking and advanced maternal age (AMA) also contribute to infertility (Emokpae and Brown, 2021). Despite these identifiable factors, approximately 30% of infertility cases remain unexplained (Dougherty et al., 2023). Failure of the endometrium to achieve a receptive state is thought to be a major cause of infertility and the rate-limiting step in assisted reproductive technology (Gellersen and Brosens, 2014). Furthermore, large clinical studies revealed that the implantation rate drops significantly in women with reduced fertility, even when using donor oocytes from young women pointing to a critical role of the endometrium or aberrant endometrial factor(s) as the key culprit (Toner et al., 2002; Soares et al., 2005; Loid et al., 2024). However, the mechanisms underlying these pregnancy failures are poorly understood.
Decidualization is the transformation of endometrial stromal fibroblasts into specialized secretory decidual cells and is critical for establishing a supportive environment essential for embryo implantation and subsequent placental development (Gellersen and Brosens, 2014). In humans, decidualization occurs in the mid-luteal phase of the menstrual cycle and occurs independently of pregnancy (Okada et al., 2018). In each menstrual cycle, the endometrium undergoes estrogen-driven proliferation followed by progesterone-induced differentiation, resulting in a brief window during which embryo implantation can occur. In humans, this receptive window opens approximately 6 days after the pre-ovulatory LH surge and lasts for about 4 days, typically aligning with days 19–22 of a typical 28-day menstrual cycle (Muter et al., 2023). The decidual micro-environment is dynamic and capable of responding and adapting to embryo-cross talk, and it is therefore proposed that the endometrium can act as a ‘bio-sensor’ of embryo quality. Interference by extrinsic factors or ‘malfunctioning’ of the maternal endometrial biosensor may lead to an inhibition of implantation or out-of-phase implantation of non-viable embryos known as the ‘selection hypothesis’ (Weimar et al., 2012; Teklenburg et al., 2010a). This notion has been further corroborated by the observation that endometrium from women suffering with unexplained infertility or recurrent implantation failure do not respond to intrinsic and embryonic cues (Simon and Laufer, 2012).
For the endometrium to function as a biosensor, human embryos must produce mechanical or chemical signals that convey their developmental potential to the maternal cells. Human blastocysts release various signals such as interleukins, microRNAs, mucins, growth factors, hormones, and trypsin-like proteases, which designate their developmental potential and play a role in the implantation process (Wang and Dey, 2006; Ruan et al., 2012; Brosens et al., 2014). Trypsin-like proteases are known to play a role in early embryo development in invertebrates and vertebrates, including mouse and rhesus monkey (Mishra and Seshagiri, 2000; Perona and Wassarman, 1986; Lin et al., 2006; Jiang et al., 2011). Trypsin, a serine protease secreted by the murine blastocyst, has been implicated in cross-talk with the uterine epithelium (Shmygol and Brosens, 2021). According to studies of Ruan et al. in a murine model, trypsin (released by embryos) cleaved the α-subunit epithelial sodium channel (ENaC) present on epithelial cells, leading to cell membrane depolarization. This depolarization activated the L-type voltage gated Ca2+ channel, resulting in a sustained cytosolic Ca2+ activity ([Ca2+]i) rise. The increase in [Ca2+]i resulted in a cyclooxygenase 2 (COX2) dependent rise in prostaglandin E2 (PGE2) release, thereby augmenting the process of implantation and decidualization (Ruan et al., 2012). Furthermore, in mice, using either a serine protease inhibitor or amiloride (ENaC inhibitor) was found to reduce the number of implantation sites (Sun et al., 2007).
Left-right determination factor 2 (LEFTY2) or endometrial bleeding associated factor (EBAF) is a member of the transforming growth factor (TGF)-β superfamily (Cornet et al., 2002; Ulloa and Tabibzadeh, 2001). LEFTY2 is initially produced as precursor, which is then cleaved to release the C-terminal monomeric active protein (Tabibzadeh and Hemmati-Brivanlou, 2006). LEFTY2 is highly expressed in decidualizing human endometrial stromal cells (HESCs) during the late luteal phase of the menstrual cycle, coinciding with the closure of the window of implantation (Gellersen and Brosens, 2014; Tabibzadeh et al., 1998). Enhanced LEFTY2 expression was associated with unexplained infertility (Salker et al., 2011), abnormal uterine bleeding (Tabibzadeh and Kothapalli, 1996; Kothapalli et al., 1997) and implantation failure (Tabibzadeh et al., 2000; Tang et al., 2005). Further, in vivo gene transfer of LEFTY2 in the mouse uterus led to implantation failure, though the mechanism remains to be defined. The present study determined trypsin activity from single human embryo conditioned media and tested whether infertility factor LEFTY2 can modify trypsin-induced Ca2+ entry in a model of human endometrial epithelial cells.
2 Materials and methods
2.1 Cell culture
Human endometrial epithelial cells (Ishikawa cells; ECACC-99040201; a widely used model for implantation) (Brosens et al., 2014; Jiang et al., 2017; Tang et al., 2018; Kumar et al., 2017; Salker et al., 2016a) were maintained in Dulbecco’s modified Eagle’s medium/F12 without phenol red (Invitrogen, Germany) supplemented with fetal bovine serum (FBS, Gibco, Germany), 1% (v/v) antibiotic-antimycotic solution (Gibco, United States), and 0.25% (v/v) L-glutamine (Gibco, United Kingdom). Cells were incubated at 37°C in a humid atmosphere maintained at 5% (v/v) CO2. Cells were tested for mycoplasma infection at regular intervals. Cells were normally seeded at 2 × 105 and allowed to recover for 24 h. Where indicated, the cells were treated with LEFTY2 for 6 h (25 ng mL−1; R&D Systems, Germany) as previously described (Salker et al., 2016a; Salker et al., 2015; Salker et al., 2016b) and/or trypsin for 24 h (Ruan et al., 2012) (20 μg mL−1; Invitrogen, Germany) in the absence and presence of the ENaC inhibitor amiloride (1 μM; Sigma, Germany), or L-type Ca2+ channel inhibitor nifedipine (10 μM; Sigma, Germany) for the indicated periods and with the indicated concentrations. To mimic the in vivo decidualization environment, the cells were treated with 0.5 μM 8-Bromo-cAMP (cAMP, Tocris, United Kingdom) and 1 μM Medroxyprogesterone 17-acetate (MPA, Sigma, Germany) for 6 days as described in the previous study (Salker et al., 2018), followed by treatment with LEFTY2 and trypsin as described above.
2.2 Ca2+ measurements
Fura-2 fluorescence was used to determine intracellular Ca2+ activity (Bhavsar et al., 2013). Cells were incubated with Fura-2/AM (2 μM, Invitrogen, Germany) for 20 min at 37°C. SOCE was determined by extracellular Ca2+ removal in the presence of sarco/endoplasmic Ca2+ ATPase inhibitor thapsigargin (1 μM, Invitrogen, Germany) and subsequent Ca2+ re-addition. For sodium-calcium exchanger (NCX)-induced calcium entry, extracellular Na+ was removed by replacing it with Li+ or Choline, promoting calcium influx through NCX. For potassium-dependent sodium-calcium exchanger (NCKX)-induced calcium entry, both extracellular Na+ was removed and extracellular K+ was added to enhance NCKX activity. Cells were excited alternatively at 340 nm and 380 nm through an objective (Fluor 40×/1.30 oil) built on an inverted phase-contrast microscope (Axiovert 100, Carl Zeiss, Germany). Emitted fluorescence intensity was recorded at 505 nm. Data were acquired using specialized computer software (Metafluor, Universal Imaging, United States). Cytosolic Ca2+ activity was calculated from the 340 nm/380 nm ratio (Bhavsar et al., 2013; Yang et al., 2014).
2.3 Flow cytometry
Ishikawa cells were treated as described in above. The trypsin-induced calcium entry was estimated by flow cytometry using Fluo-4 staining (F14201, Invitrogen, Germany) in accordance with the manufacturer’s instructions. Briefly, cells were collected by trypsin and washed with PBS, then suspended in calcium- containing PBS (1 mM CaCl2 and 0.49 mM MgCl2) with 5 µM Fluo-4. After that, cells were then incubated at room temperature for 40 min, protected from light, and washed again with calcium-containing PBS, and analysed by flow cytometry. Data were analysed using the Flowjo software (Flowjo LLC, Oregon, United States).
2.4 Quantitative real time-PCR (qRT-PCR)
Total mRNA was extracted from whole cell cultures using Trizol (Invitrogen, Germany) followed by the phenol-chloroform protocol. 2 μg of mRNA was reverse transcribed using the Maxima™ H Minus cDNA Synthesis Master Mix with dsDNase (M1681, ThermoFisher Scientific, Germany), following the manufacturer’s protocol. The resulting first-strand cDNA was diluted and stored at −20°C. Primers were designed using the NCBI, PrimerBlast software. Human ribosomal protein L19 (L19; RPL19) was used as the endogenous housekeeping gene, to normalize for variances in input cDNA. Primer sequences will be provided on request. Detection of gene expression was performed with PowerUp SYBR Green Master Mix (A25742, Thermofisher Scientific, Germany) and quantitative RT-PCR was performed on a QuantStudio 3 Real-Time PCR system (A28567, Thermofisher Scientific, Germany) using universal cycling conditions. Transcript levels were determined using the ΔΔCt method and expressed as arbitrary units (a.u). Non-template control (NTC) reactions (cDNA was substituted with DEPC water) and reverse transcriptase (RT) controls were also included. In NTC or RT control reactions PCR products were not detected (data not shown). Melting curve analysis and agarose gel electrophoresis confirmed amplification specificity.
2.5 Protein extraction and western blotting
Total protein samples were prepared by lysing the adherently cultured Ishikawa cells in Laemmli buffer containing 0.5 M Tris hydrochloride (Roth, Germany) pH 6.8, 20% Sodium dodecyl sulfate (SDS, Sigma, Germany), 0.1% Bromophenol blue (Serva, Germany),1% beta mercaptoethanol (Sigma, Germany), and 20% glycerol (Roth, Germany). Whole cell protein lysates were heated at 95°C for 5 min.
Extracts were loaded on to a 12% sodium dodecyl sulfate poly-acrylamide gel (SDS-PAGE) using the XCell SureLock® Mini-Cell apparatus (Invitrogen, Germany) followed by electrophoresis. The protein from the gel was transferred onto a poly-vinylidenefluoride membrane (Amersham Biosciences, Germany). After air drying, the membranes were activated in 100% methanol and subsequently blocked using 5% non-fat milk or bovine serum albumin (BSA) for 1–2 h at RT. Membranes were probed overnight at 4°C with antibodies: LEFTY2 (1:500, sc-365845, Santa Cruz, Germany), CACNA1C (1:200, ACC-013, Alomone Labs, Israel), COX2 (1:200, MA5-14568, ThermoFisher, Germany), GAPDH (1:1,000, #2118L, Cell Signaling, Germany) was used for loading control. After 3 washes with TBS-T, each for 10 min, the membranes were incubated with HRP-conjugated anti-rabbit secondary antibody (1:2000, #7074s, Cell Signaling, Germany) or HRP-conjugated anti-mouse secondary antibody (1:2000, 7076S, Cell Signaling, Germany) at RT for 1 h, followed by 3 washes with TBS-T. Protein bands were detected using a chemiluminescent detection kit (WesternBright™ ECL, ThermoFisher, Germany) and visualized by using iBrightTM Imaging System (Invitrogen, Germany). Bands were quantified with ImageJ Software (Schindelin et al., 2012). Full uncropped Western blotting images are provided in the Supplementary Figures 1, 2.
2.6 Immunofluorescence
Ishikawa cells were plated on 12 mm round coverslips at a density of 5,000 cells per coverslip. Treatment was performed as described above. Post treatment the cells were fixed for 15 min with 4% paraformaldehyde (PFA, Sigma, Germany), washed 3 times with PBS, and permeabilized for 10 min in 0.1% Triton X-100 (Sigma, Germany)/PBS. The coverslips were blocked with 5% BSA (Sigma, Germany) in 0.1% TritonX-100/PBS for 1 h at room temperature and were probed overnight at 4°C with primary antibody: anti-CACNA1C antibody (1:100, ab84814, Abcam, United Kingdom). After 3 washes, coverslips were probed overnight at 4°C with secondary antibody: Alexa Fluor 568 (2 µg/mL, #A-11004, Invitrogen, Germany). The coverslips were mounted with ProLong Gold antifade reagent with DAPI (#P36931, Invitrogen, Germany) on slides. Microscopy was performed with EVOS M7000 cell imaging system (ThermoFisher, Germany) with × 20 objective. Scale bar was 25 μm.
2.7 Enzyme-linked immunosorbent assay (ELISA)
Ishikawa cells were treated as stated above. The culture medium was harvested and stored at −80°C. The collected cultured medium was processed for ELISA by using human prostaglandin E2 (PGE2) ELISA Kit (#KHL1701, Invitrogen, Germany) following the manufacturer’s instructions. The absorbance was measured with Varioskan LUX spectrophotometer (ThermoFisher Scientific, Germany).
2.8 Ethical approval
This study (218/2023BO2) was approved by the Ethics Commission at the Medical Faculty of Eberhard-Karls University of Tübingen. Written informed consent was obtained from all participating patients/parents who were attending the in vitro fertilization (IVF) clinic, Universitätsklinikum Tübingen. The patient sample of 24 was determined using a power calculation (G*’Power; 80% power and alpha-type 1 error of 5%) based on a previous publication (Kang, 2021).
2.9 Embryo conditioned media collection and trypsin activity using ELISA based methods
Individual embryos were cultured in 25 µL droplets in Sage 1-step medium (Origio, CooperSurgical, Germany) media using the EmbryoScope® (Vitrolife GmbH, Germany). Embryos graded as ‘A’ were transferred back to the recipient and retrospectively determined for a positive pregnancy up to 12 weeks after. The embryo slide was collected and the embryo media was collected immediately and frozen at −80°C until further use. Wells which contained media but with no embryo contact was used as an internal control. Trypsin activity ELISA kit (AB102531, Abcam, United Kingdom) was used and results calculated precisely according to the manufacturer’s instructions. Samples were then correlated to a positive pregnancy test.
2.10 Data mining
In silico analysis was performed on the following publicly available datasets from the Gene Expression Omnibus (GEO): Pre-implantation embryonic development (Homo sapiens; ID:GDS3959) (Xie et al., 2010). Bioinformatic analysis was performed on publicly available single cell sequencing data from the Single Cell Expression Atlas (Vento-Tormo et al., 2018).
2.11 Statistical analysis
Data were analysed with the statistical package Graphpad Prism (Graphpad software Inc). Unpaired Student’s t-test and one-way ANOVA were used where appropriate. Statistical significance was assumed when P < 0.05. Data were exported to Microsoft Excel for analysis and graphs were generated and analysed using GraphPad Prism® Software.
3 Results
3.1 Expression patterns and impact of trypsin pathway genes in human preimplantation embryos
For the endometrium to act as a biosensor, the human blastocyst must signal its potential to the maternal cells. Trypsin-like proteases are known to play a role in early embryo development. Trypsin-like proteases are involved in early and pre-implantation embryo development in invertebrates such as drosophila, salmon, marine crab, Xenopus (Skern-Mauritzen et al., 2009; Misra et al., 1998; Xu et al., 2013; Yamada et al., 2000) and in vertebrates such as mouse, rat, rabbit, sheep, hamster and rhesus monkeys (Mishra and Seshagiri, 2000; Perona and Wassarman, 1986; Lin et al., 2006). Whether this plays a role in human embryos remains untested. To explore the expression patterns of genes implicated in the trypsin pathway, we mined publicly available transcriptomic data (GEO accession number: GDS3959) of human embryos at differing preimplantation developmental stages (1C; 1-cell embryo, 2C; 2-cell embryo, 4C; 4-cell embryo, 8C; 8-cell embryo, M; morula and B; blastocyst) (Xie et al., 2010). As shown in Figure 1, transcriptomic analyses of key classical trypsin pathway gene transcripts were present in human pre-implantation embryos, highlighting a mechanism of trypsin activity at the blastocyst stage. Trypsin is produced through the enzymatic breakdown of trypsinogen, and its activity can be regulated by various trypsin and protease inhibitors. Enteropeptidase, encoded by transmembrane serine protease 15 (TMPRSS15), is considered the ‘master regulator’ of trypsin activity due to its role in activating trypsinogen by cleavage (Smith and Johnson, 2013). In Figure 1a, we showed that TMPRSS15 expression significantly increases at the 8-cell stage in human embryos (P = 0.0043), whilst the protease serine 1 (PRSS1) gene (Figure 1b), which encodes trypsinogen, is not regulated during preimplantation development. Additionally, the highly regulated serine protease genes transmembrane protease serine subtype 2 (TMPRSS2) (P = 0.0004) and prostasin (PRSS8, P < 0.0001) are also notably upregulated at the blastocyst stage in human embryos (Figures 1c, d).
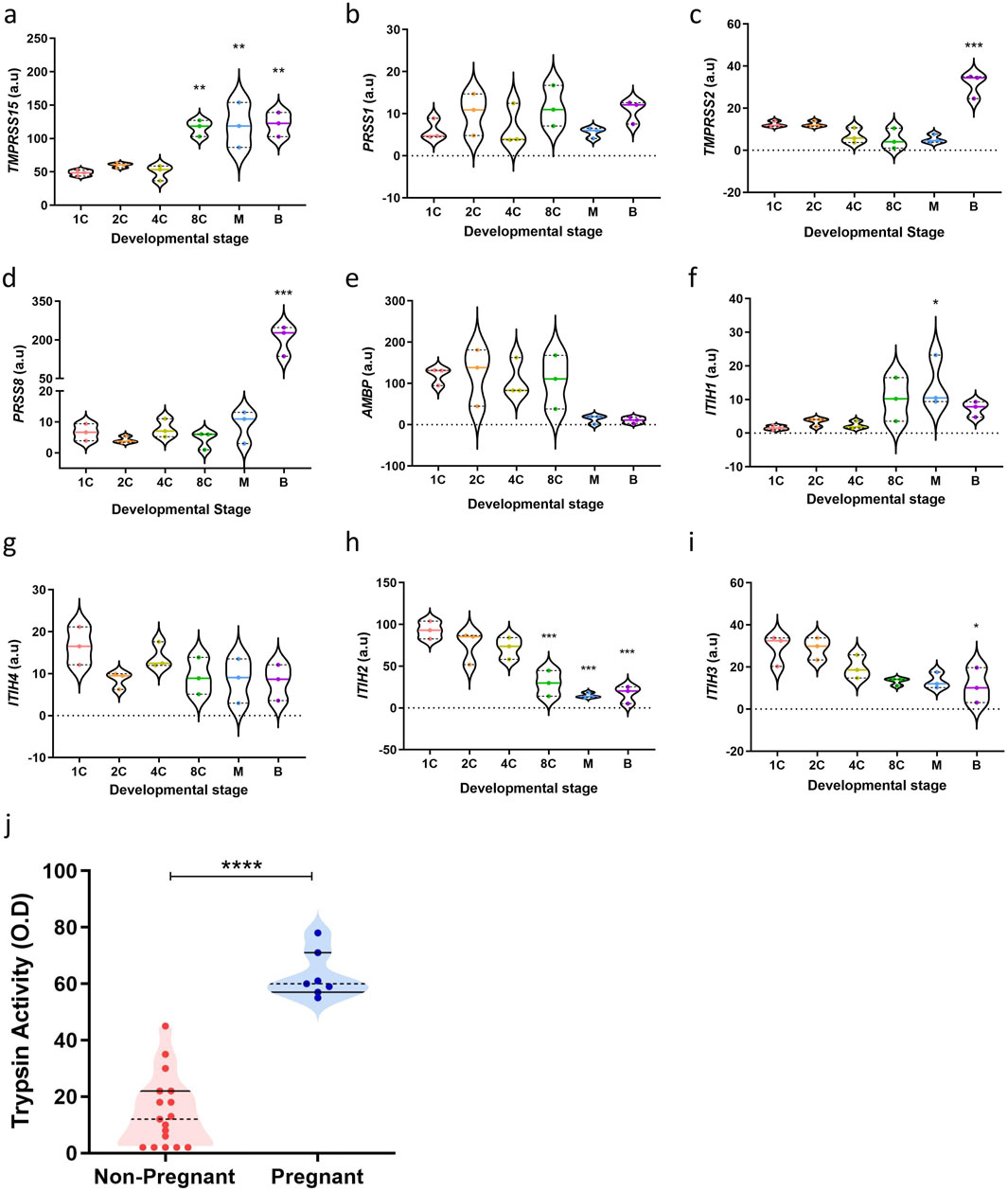
Figure 1. Trypsin activity increases with developmental maturation. (a–i) Key classical trypsin pathway genes expression in human pre-implantation embryos (n = 3) (GEO accession number: GDS3959) (1C: 1-cell embryo, 2C: 2-cell embryo, 4C: 4-cell embryo, 8C: 8-cell embryo, M: morula, (b) blastocyst). The data are presented as mean ± SEM. One-way ANOVA were used to calculate statistical significance. Asterisks (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001) indicate a significant difference compared to the 1-cell embryo stage. (j) Trypsin activity in individual droplets collected (n = 24). All measurements were performed in duplicate, normalised to unconditioned medium and are displayed as individual values. The data are presented as mean ± SEM. Unpaired Student’s t-test used to calculate statistical significance. ****P < 0.0001.
Critically, several trypsin inhibitors also play a role in regulating trypsin activity during embryo development. Alpha-1-microglobulin/bikunin precursor (AMBP) is a plasma protein that includes bikunin, which acts as a potent trypsin inhibitor via its Kunitz-type protease inhibitor domain (Sanchez et al., 2002). As Figure 1e showed, expression of AMBP decreases at the morula stage in human embryos (P = 0.1032). Inter-α-trypsin inhibitors (IαI), composed of heavy chains (ITIH1-4) and the light chain bikunin, also contribute to trypsin inhibition. The heavy chains stabilize the inhibitory function of bikunin by forming complexes with it (Lord et al., 2020; Zhuo and Kimata, 2008). The transcriptional regulation of ITIH genes during pre-implantation development may therefore influence embryo-derived trypsin activity. ITIH1 expression increases beyond the 4-cell stage (P = 0.0301), while ITIH4 expression is lowest at the 2-cell stage (P = 0.2276) (Figures 1f, g). Additionally, ITIH2 (P = 0.0007) and ITIH3 (P = 0.0247) expression progressively decreases beyond the 2-cell stage in human pre-implantation embryos (Figures 1h, i). These findings indicate decreasing trypsin inhibition at later stages of human pre-implantation embryo development, strengthening a role for trypsin as a vital embryonic signal at implantation.
To assess whether trypsin activity levels can serve as indicators of embryo implantation potential, trypsin activity was measured in embryo culture media (ECM) from individual day 5 single embryo transfer (SET) embryos. The criteria for SET in our unit include maternal age under 37 years, one high-quality blastocyst (the embryo having an “A” grade for both the inner cell mass and the trophectoderm) and no prior failed IVF cycles. By selecting ECM from SETs, we controlled for factors such as patient age, embryo quality, developmental stage, and prognosis, ensuring that the trypsin levels detected in the ECM were directly related to pregnancy outcomes.
ECM samples were subsequently collected and analysed for trypsin activity using an ELISA-based method. Notably, trypsin activity was found to be significantly higher in ECM from embryos that implanted following SET, compared with those that failed to implant (Figure 1j, P < 0.0001). The median absorbance was 14.65 ± 12.87 O.D. for the non-pregnant group and 63 ± 8.35 O.D. for the pregnant group, with ranges of values from 2 to 45 O.D. for the non-pregnant group and 55 to 78 O.D. for the pregnant group. Taken together, these findings confirm a role for trypsin as a potential embryonic signal and information on embryo competency.
3.2 LEFTY2 modulates trypsin-induced calcium influx in endometrial epithelial cells
Failure to establish the implantation window is thought to be a major cause of infertility. A putative candidate is LEFTY2, which we have shown to be involved in unexplained infertility (Salker et al., 2018). In the next series of experiments, we investigated the effect of trypsin on intracellular calcium ([Ca2+]i) levels and explored whether endometrial infertility factor, LEFTY2 could interfere with this process. As illustrated in Figures 2a–c, the addition of trypsin (closed circles, ●) led to a rapid increase in [Ca2+]i, characterized by a pronounced rise in both slope and peak. This trypsin-induced calcium influx was significantly attenuated by the ENaC blocker amiloride (10 µM), as shown in Figures 2a, b, d. Further analysis involved pre-treating endometrial epithelial cells with LEFTY2 (25 ng/mL, open circles ○) for 6 h, as previously described (Salker et al., 2016a). This pre-treatment resulted in a notable reduction in trypsin-induced Ca2+ entry, as depicted in Figures 2a, b, e. Interestingly, the presence of amiloride in combination with LEFTY2 pre-treatment showed a trend towards an even greater reduction in [Ca2+]i, though this combined effect did not achieve statistical significance, as indicated in Figures 2a, b, f.
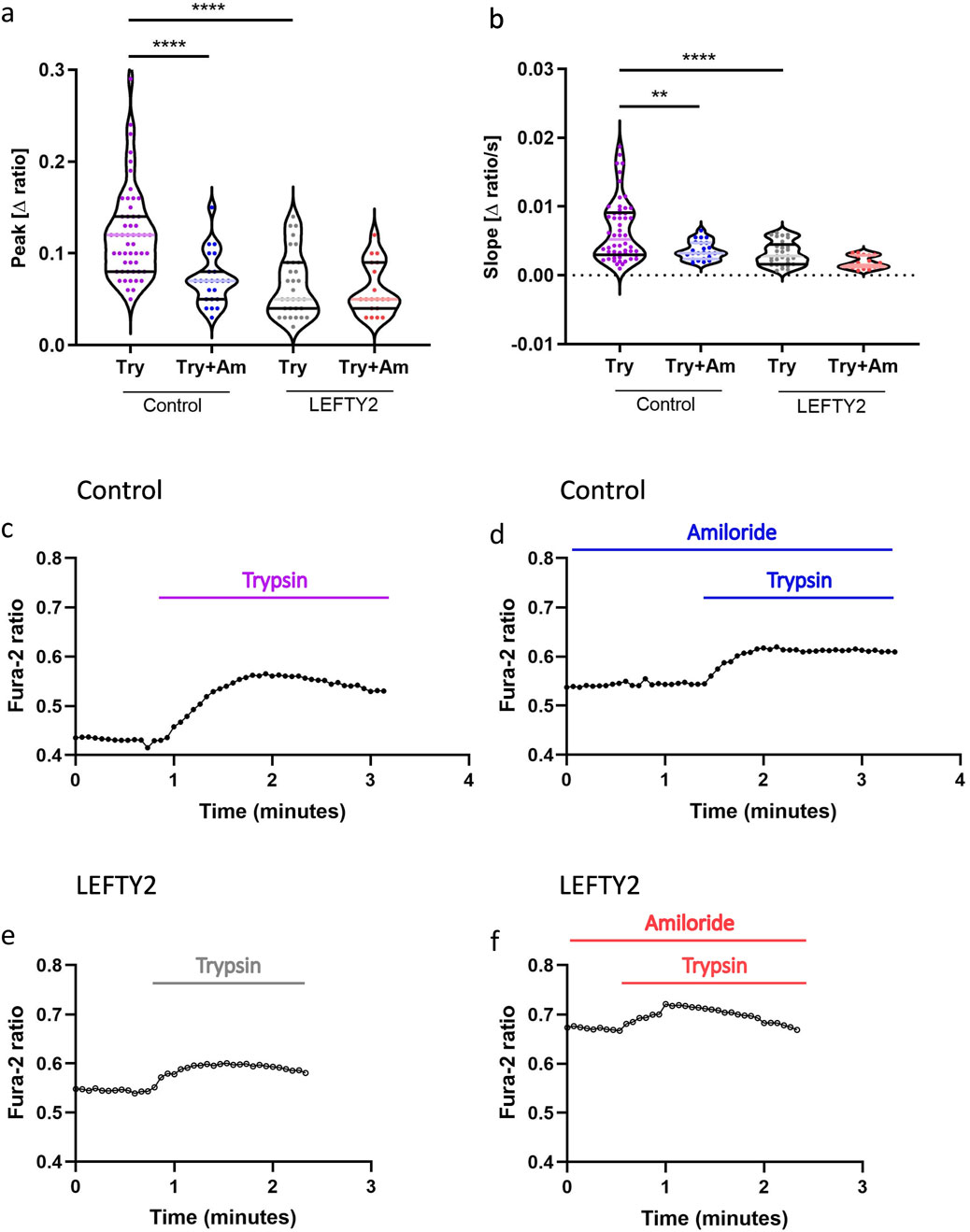
Figure 2. Trypsin induced Ca2+ entry in human endometrial epithelial cells is blocked by the presence of LEFTY2. (a, b) Arithmetic means (± SEM, n = 19–54 cells) of the peak value (a) and slope (b) of the change in intracellular Ca2+ concentrations following trypsin treatment (purple) without and with the presence of amiloride, without (left bars, Control) and with (right bars, LEFTY2) pretreatment with LEFTY2 (25 ng/mL, 6 h). (c–f) Representative original tracings showing intracellular Ca2+ concentrations in Fura-2/AM loaded human endometrial epithelial cells without (black circles) and with (open circles) pretreatment with LEFTY2 (25 ng/mL, 6 h) human endometrial epithelial cells prior to and following addition of trypsin (20 µg/mL) without (c, e) and with (d, f) the presence of amiloride (10 µM). The amplitude (peak) and the velocity (slope, calculated from the linear fit) of the Ca2+ entry was analysed. The data are presented as mean ± SEM. One-way ANOVA was used to calculate statistical significance. **P < 0.01, ****P < 0.0001.
3.3 LEFTY2 attenuates trypsin-induced upregulation of L-type calcium channels
The previous results showed that decrease of calcium does not occur by blocking ENaC. We next tested whether the inhibitory effect of LEFTY2 on Ca2+ entry was paralleled by altered L-type calcium channel levels. To determine whether L-type voltage gated Ca2+ channel (CACNA1C) is expressed in normal human endometrial tissue, we first performed bioinformatic analysis on publicly available single cell sequencing data (Vento-Tormo et al., 2018). We observed an expression of CACNA1C in human decidua, particularly in endometrial decidual cells (Figures 3a, b). As shown in Figure 3c, treatment of trypsin alone increased CACNA1C transcript levels, in keeping with previous findings (Ruan et al., 2012). Transcript levels were reduced in the presence of LEFTY2 and following co-treatment with LEFTY2 and trypsin. The induction of LEFTY2 was confirmed by western blotting (Figure 3d). Subsequently, immunofluorescence analysis demonstrated that the intensity of cytosolic CACNA1C increased upon trypsin treatment, while it decreased following treatment with LEFTY2 and co-treatment with LEFTY2 and trypsin (Figure 3e). The original images are provided in Supplementary Figure 3. Additionally, we also showed the same trend by western blot upon the co-treatment of LEFTY2 and trypsin (Supplementary Figure 4). Furthermore, to investigate the downstream factors regulated by trypsin-induced Ca2+ entry, we measured COX2 levels using Western blotting. As shown in Figure 3f, COX2 protein expression increased following trypsin treatment and decreased after treatment with LEFTY2. Since COX2 is a key enzyme in PGE2 synthesis, we further measured PGE2 levels using ELISA. As shown in Figure 3g, PGE2 levels followed the same pattern as COX2 under the same treatment conditions.
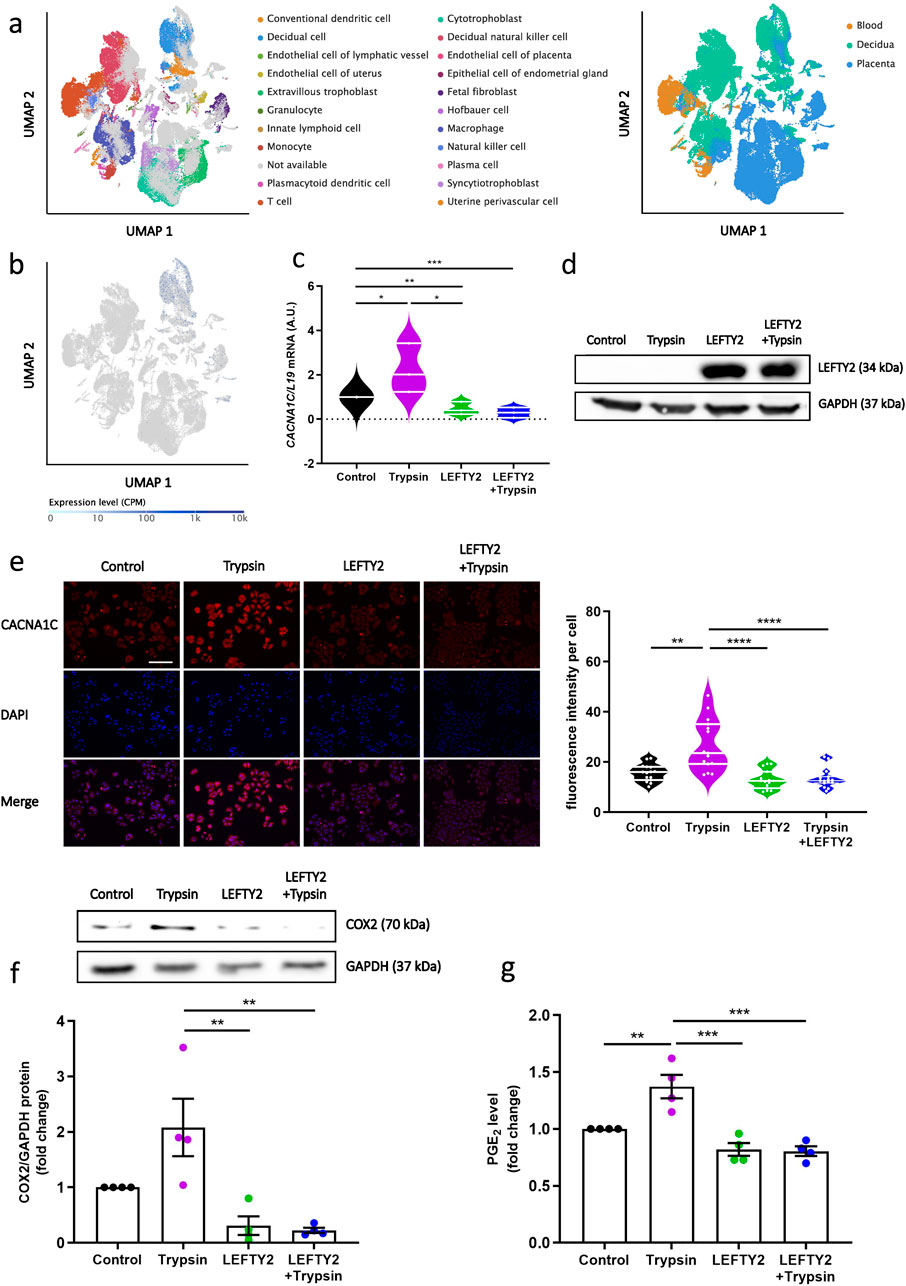
Figure 3. LEFTY2 decreases L-type ca2+ Channel abundance. Cell cultures were treated with or without LEFTY2 (25 ng/mL) for 6 h either in the presence or absence of Trypsin (20 µg/mL, 24 h). (a) Uniform manifold approximation and projection (UMAP) clustering of tissues and cell types; Tissue compartments and cell types were annotated in the Single Cell Expression Atlas. (b) Expression of CACNA1C in single cells, presented as counts per million (CPM), overlaid on the UMAP map. (c) Arithmetic means ± SEM (n = 6) of transcript levels encoding the human CACNA1C transcript levels were determined by qRT-PCR, normalized to the levels of L19 mRNA and expressed in arbitrary units (a.u.). (d) Western blot analysis of LEFTY2 expression in Ishikawa cells (n = 4). GAPDH was used as a loading control. (e) IF microscopy of Ishikawa cells treated with or without LEFTY2 (25 ng/mL) for 6 h either in the presence or absence of Trypsin (20 µg/mL, 24 h) showing CACNA1C subcellular localization. CACNA1C fluorescence intensity quantification results were shown (right). CACNA1C: Alexa Fluor 568 (red); nucleus: DAPI (blue). Quantification performed from 3 experiments with >15 cells quantified for each condition. Scale bar = 25 µm. (f) Western blot analysis of COX2 expression in Ishikawa cells (n = 4). GAPDH was used as a loading control. (g) PGE2 level determined by ELISA. The data are presented as mean ± SEM. One-way ANOVA was used to calculate statistical significance. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
3.4 LEFTY2 and nifedipine interaction in modulating trypsin-induced calcium entry
To evaluate whether the inhibitory effect of LEFTY2 on trypsin-induced Ca2+ entry is sensitive to nifedipine, a known L-type calcium channel blocker, we conducted experiments with trypsin in the absence and presence of nifedipine (10 µM) (Manohar et al., 2021). As shown in Figures 4a–d, both slope and peak of the [Ca2+]i increase induced by trypsin (closed circles, ●) were significantly attenuated when nifedipine was present. This indicates that nifedipine effectively blunts the trypsin-induced upregulation of [Ca2+]i. Additionally, the pre-treatment with LEFTY2 (25 ng/mL, open circles ○) resulted in a notable reduction in trypsin-induced Ca2+ entry, as depicted in Figures 4a, b, e. However, when LEFTY2 was applied in the presence of nifedipine, it did not significantly alter the trypsin-induced Ca2+ increase, as depicted in Figures 4a, b, f. These results suggest that the inhibitory action of LEFTY2 on Ca2+ entry is at least partially mediated through pathways sensitive to nifedipine.
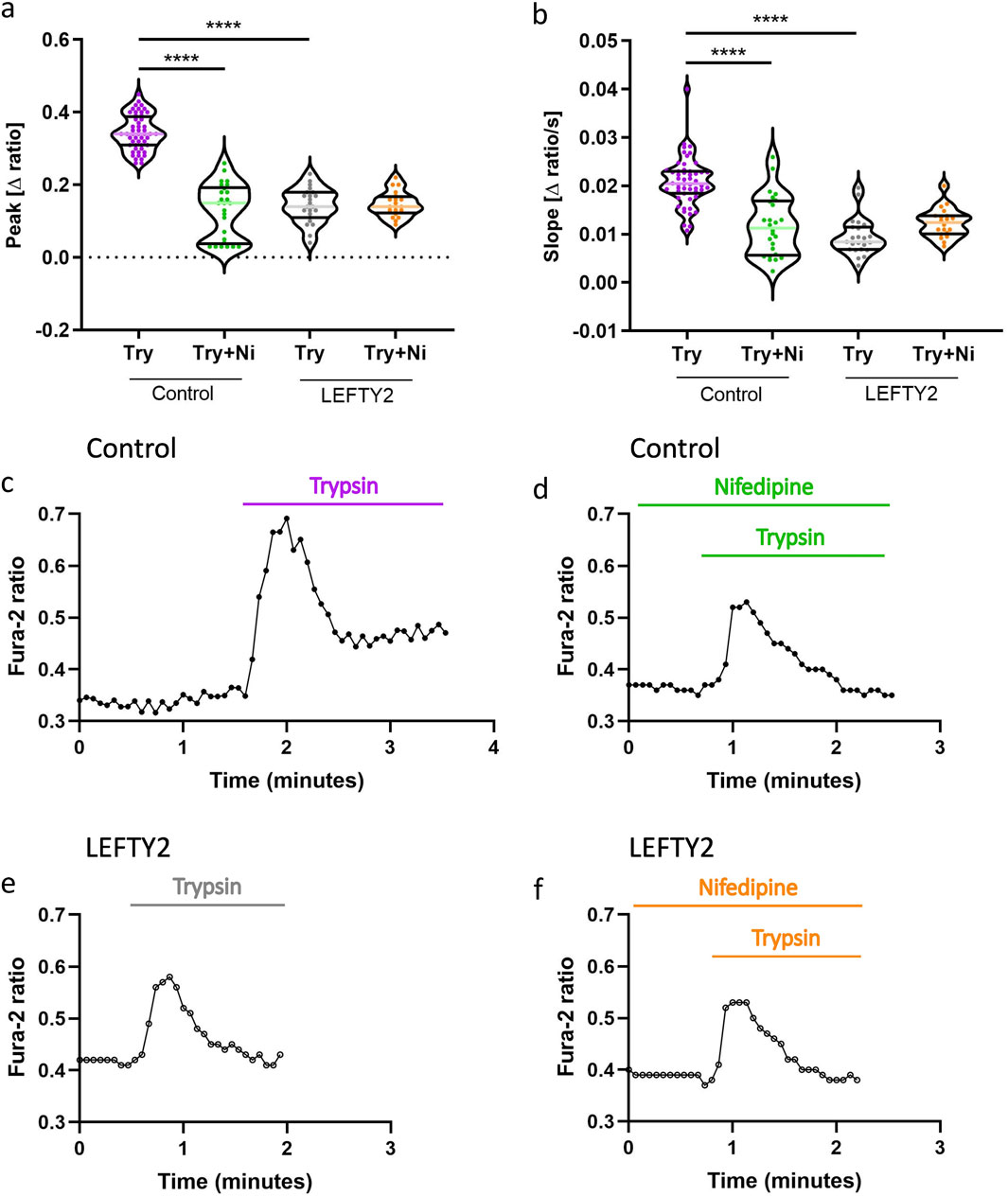
Figure 4. LEFTY2 decreases the trypsin induced Ca2+ entry by impeding the nifedipine sensitive L-type calcium channel. (a, b) Arithmetic means (± SEM, n = 19–54 cells) of the peak value (a) and slope (b) of the change in intracellular Ca2+ concentrations following trypsin treatment without and with the presence of nifedipine, without (left bars, Control) and with (right bars, LEFTY2) pretreatment with LEFTY2 (25 ng/mL, 6 h). (c-f) Representative original tracings showing intracellular Ca2+ concentrations in Fura-2/AM loaded human endometrial epithelial cells without (black circles) and with (open circles) pretreatment with LEFTY2 (25 ng/mL, 6 h) human endometrial epithelial cells prior to and following addition of trypsin (20 µg/mL) without (c, e) and with (d, f) the presence of nifedipine (10 µM). The nifedipine (peak) and the velocity (slope, calculated from the linear fit) of the Ca2+ entry was analysed. The data are presented as mean ± SEM. One-way ANOVA was used to calculate statistical significance. ****P < 0.0001.
4 Discussion
Unexplained infertility presents a major challenge for reproductive medicine professionals. Reduced endometrial receptivity during the implantation window for embryos may be a key factor contributing to unexplained infertility and failed IVF cycles (Stevens et al., 2022). It has been shown that the endometrium can act as a ‘bio-sensor’ of embryo quality, in order to limit maternal investment of non-viable embryos (Teklenburg et al., 2010b; Macklon and Brosens, 2014). The ‘selection hypothesis’ suggests that an excessive or pronounced decidual response can shorten the window of receptivity and enhance the elimination of embryos, thereby lowering the risk of miscarriage but potentially preventing conception (Brosens et al., 2014). Therefore, the human uterus has an intrinsic ability to adapt and can adjust its receptivity and selectivity traits (Gellersen and Brosens, 2014).
Embryos communicate via serine proteases including trypsin, as demonstrated in mammals where blastocysts secrete proteases to induce calcium signaling in endometrial cells during implantation (Hennes et al., 2023) and in Drosophila, where serine proteases like Easter are involved in patterning by activating a proteolytic cascade (Misra et al., 1998). Trypsin is formed through the enzymatic cleavage of trypsinogen and is primarily regulated by enteropeptidase, which serves as regulator by activating trypsinogen through cleavage (Zheng et al., 2009; Whitcomb et al., 1996; Szabo et al., 2003; Hegyi and Sahin-Toth, 2017). This initiation causes trypsin release, which is able to auto-activate trypsinogen thereby increasing trypsin production (Szabo et al., 2003). The gene encoding trypsinogen itself, PRSS1, is not regulated during pre-implantation development and the gene encoding PRSS8, located on chromosome 16 is a serine protease expressed highly by healthy embryos. Interestingly, chromosomes 16 and 22 are the most common chromosomes affected by trisomy (Munne et al., 2004). The augmented gene dosage in trisomic embryos could result in an over-dosage of embryonic trypsin thereby encouraging invasion of the chromosomally ‘abnormal’ embryo (Quenby et al., 2002; Ma et al., 2009). In this study, we show that trypsin activity was measurable in ECM from individually cultured human embryos and was found to be related to successful pregnancies. A limitation of our study was that the participating patients were selected based on their infertility. As a result, the generalizability of the findings to a broader population, including those without known fertility issues should be assessed. Further research involving a more diverse patient cohort is necessary to validate the observed association between trypsin activity from ECM and successful pregnancies to rule out the effects of genetics and ancestry. Further refinement of this analysis could provide a novel complementary approach to embryo grading and selection in IVF treatment by incorporating embryo-derived trypsin as a potential marker of implantation, alongside traditional morphology-based assessments.
ENaC, located on the epithelium, has been shown to be upregulated during the peri-implantation period in mice (Liu et al., 2014) where it controls water and electrolyte resorption and is known to contribute to uterine closure in mice (Li et al., 2023). ENaC is activated by a variety of mechanical stimuli, such as changes in shear stress or stretching of the epithelial tissue (Kleyman et al., 2018) and by serine proteases, which cleave specific segments of the channel to facilitate its activation (Shi et al., 2013). Serine proteases, including trypsin, are present at the embryo-endometrial interface, where they are released by the embryo and are essential for successful implantation (Brosens et al., 2014; Salamonsen and Nie, 2002). As previously proposed, trypsin likely facilitates its effects through the activation of ENaC, resulting in Na+ influx and subsequent membrane depolarization (Ruan et al., 2012).
Further, our present observations reveal that endometrial infertility factor LEFTY2 downregulates trypsin-induced Ca2+ entry. Treatment of endometrial epithelial cells with trypsin was followed by a rapid increase of [Ca2+]i, an effect that was significantly blunted by ENaC inhibitor amiloride. We acknowledge the limitations of using an in vitro model that uses a human carcinoma-derived endometrial epithelial cells as the responses, and underlying molecular mechanisms, may not faithfully recapitulate the in vivo situation. However, this cell line has been used in numerous studies to investigate receptivity and implantation (Wang et al., 2024; Ruane et al., 2024). Notwithstanding, our data reveals that the inhibitory effect of LEFTY2 on trypsin induced Ca2+ entry could not have been due to inhibition of ENaC, which is actually upregulated by LEFTY2 (Salker et al., 2016a). The trypsin induced [Ca2+]i increase was strongly and significantly blunted by nifedipine. In the presence of nifedipine, LEFTY2 did not further modify trypsin induced increase of [Ca2+]i. Thus, LEFTY2 downregulated trypsin induced [Ca2+]i increase is largely due to interference with nifedipine sensitive Ca2+ entry. Interestingly, there have been two clinical trials with the use of nifedipine prior to embryo transfers with the conjecture that a relaxed myometrium and vasodilation may serve to improve implantation rates. However, both studies showed a decrease in pregnancy rates compared with the placebo group. This observed negative effect may be due to blocking of the required rise in calcium necessary for implantation (Nataj Majd et al., 2022; Ng et al., 2019). We cannot rule the contribution of other calcium channels in the process of implantation and infertility. A study by Bahar et al. showed that there was a change in the methylation status and transcriptomic levels of several T-type calcium channels and was correlated with recurrent implantation failure (Davoodi et al., 2024). Additionally, progesterone is known to modulate calcium channels. According to our data (Supplementary Figure 5a) the addition of cAMP and MPA with and without LEFTY2 did not change (total) calcium entry in endometrial epithelial cells. Additionally, we also provide evidence that the store operated calcium entry (SOCE), NCX and NCKX are not involved in the effects of trypsin activity, pointing to a conserved role of L-type calcium channel function (Supplementary Figures 5b–d). Further work is required to validate whether LEFTY2 can alter additional transporters and ion channels in the endometrium.
Lifestyle factors contribute to obesity and metabolic syndrome which are known factors impairing endometrial function and decreasing fertility rates (Dag and Dilbaz, 2015; Vrhovac et al., 2021). Endometrial cells cannot synthesise glucose de novo and must take up glucose via transporters (Vrhovac et al., 2021). In the first stage of pregnancy, the endometrium provides nutritional support for the embryo, in a process known as histotrophic nutrition (Burton et al., 2002). Anaerobic glycolysis depends on the availability of glucose and valuable source of glucose is glycogen deposits within endometrial cells. During early development the embryo is dependent on anaerobic glycolysis for its energy supply, thereby a compromise in adequate storage may also result in poor pregnancy outcomes (Chen and Dean, 2023). The sodium-glucose transporter 1 (SGLT1 or SLC5A1) is a Na+-coupled glucose transporter responsible for taking up glucose against the electrochemical gradient and, thus glycogen storage in cells (Gyimesi et al., 2020). LEFTY2 can increase transcript levels and protein abundance of SGLT1 and glycogen abundance and may interfere with implantation and early pregnancy events (Zeng et al., 2020). Glucose uptake in endometrial cells is not solely dependent on SGLT1, but may also involve glucose transporters from the glucose transporter molecules (GLUT) family (Vrhovac et al., 2021). Studies have shown that GLUT1 protein levels are significantly reduced in endometrial biopsies from women with infertility (von Wolff et al., 2003). Obesity is associated with increased glycogen storage and also reduced fertility (Dag and Dilbaz, 2015). In keeping with this animal studies also suggest that high glucose levels are detrimental to endometrial function and lower fertility rates (Salker et al., 2017; Zhang et al., 2020). How altered LEFTY2 influences uterine glycogen metabolism and implantation is almost entirely unexplored and future studies are warranted.
Endometrial LEFTY2 appears to play a dual role in the regulation of Ca2+ entry. Firstly, the downregulation of Orai1 prevents the conversion of the endometrium into a receptive phenotype by attenuating the expression of Ca2+ sensitive receptivity genes (Salker et al., 2018). Secondly, LEFTY2 downregulates the effect of trypsin induced Ca2+ entry and could prevent embryo-induced Ca2+ entry. It is tempting to speculate that both Orai1 and SOCE are required for fine-tuning endometrial receptivity prior to embryo implantation and that trypsin-induced Ca2+ entry takes a leading role during embryo implantation (Ruan et al., 2012) suggesting that LEFTY2 is a potent inhibitor of both, SOCE and trypsin-induced Ca2+ entry.
Taken together, our data demonstrates that embryo-derived trypsin is produced by human embryos and that increasing amounts are associated with a successful pregnancy. Our study further uncovers a negative influence of LEFTY2 on trypsin-induced nifedipine sensitive Ca2+ entry, an effect contributing to the adverse impact of LEFTY2 on embryo implantation (Figure 5).
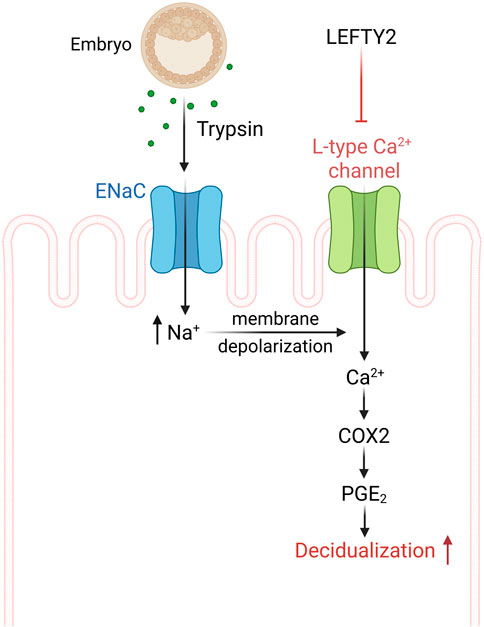
Figure 5. Schematic drawing of L-type calcium channel involvement in the process of implantation. ENaC activation by trypsin from the embryo causes epithelial cell membrane depolarization that activates L-type Ca2+ channel and Ca2+ influx. The endometrial epithelial Ca2+ influx can further activate cAMP-related pathways in stromal cells, leading to the process of implantation (Salker, 2025).
Data availability statement
The raw data supporting the conclusions of this article will be made available upon request to MSS.
Ethics statement
The study was approved by the Ethics Commission at the Medical Faculty of Eberhard-Karls University of Tübingen (218/2023BO2). The study was conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ZY: Data curation, Formal Analysis, Writing – original draft, Writing – review and editing, Investigation, Methodology, Visualization. JY: Data curation, Formal Analysis, Writing – original draft, Writing – review and editing, Investigation, Methodology, Visualization. SK: Writing – review and editing, Project administration, Resources. MA: Data curation, Formal Analysis, Writing – review and editing, Investigation, Methodology, Visualization. SYB: Funding acquisition, Project administration, Writing – review and editing, Resources. MH: Project administration, Writing – review and editing, Resources. FL: Project administration, Supervision, Writing – review and editing, Conceptualization, Resources. MSS: Conceptualization, Funding acquisition, Investigation, Project administration, Supervision, Writing – original draft, Writing – review and editing, Formal Analysis, Resources, Visualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by funding to M.S.S intramural funds of Tübingen University (IZKF), the Athene award by the Federal Ministry of Education and Research (BMBF) and the Baden-Württemberg Ministry of Science as part of the Excellence Strategy of the German Federal and State Governments and by the Margarete von Wrangell (MvW 31-7635.41/118/3) habilitation scholarship co-funded by the Ministry of Science, Research and the arts (MWK) of the state of Baden-Württemberg and by the European Social Funds. Z.Y is supported by the CSC scholarship fund from PR China.
Acknowledgments
We acknowledge the support from the Open Access Publishing Fund of Tübingen University. This work has been used as part of the MD thesis of Ms Zhiqi Yang.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2025.1499339/full#supplementary-material
References
Bhavsar, S. K., Schmidt, S., Bobbala, D., Nurbaeva, M. K., Hosseinzadeh, Z., Merches, K., et al. (2013). AMPKα1-sensitivity of Orai1 and Ca(2+) entry in T - lymphocytes. Cell Physiol. Biochem. 32 (3), 687–698. doi:10.1159/000354472
Brosens, J. J., Salker, M. S., Teklenburg, G., Nautiyal, J., Salter, S., Lucas, E. S., et al. (2014). Uterine selection of human embryos at implantation. Sci. Rep. 4, 3894. doi:10.1038/srep03894
Burton, G. J., Watson, A. L., Hempstock, J., Skepper, J. N., and Jauniaux, E. (2002). Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J. Clin. Endocrinol. Metab. 87 (6), 2954–2959. doi:10.1210/jcem.87.6.8563
Chen, Z., and Dean, M. (2023). Endometrial glucose metabolism during early pregnancy. Reprod. Fertil. 4 (4), e230016. doi:10.1530/RAF-23-0016
Cornet, P. B., Picquet, C., Lemoine, P., Osteen, K. G., Bruner-Tran, K. L., Tabibzadeh, S., et al. (2002). Regulation and function of LEFTY-A/EBAF in the human endometrium. mRNA expression during the menstrual cycle, control by progesterone, and effect on matrix metalloprotineases. J. Biol. Chem. 277 (45), 42496–42504. doi:10.1074/jbc.M201793200
Dag, Z. O., and Dilbaz, B. (2015). Impact of obesity on infertility in women. J. Turk Ger. Gynecol. Assoc. 16 (2), 111–117. doi:10.5152/jtgga.2015.15232
Davoodi, N. B., Hashemi Karoii, D., Favaedi, R., Ramazanali, F., Jahangiri, M., Movaghar, B., et al. (2024). Differential expression of ion channel coding genes in the endometrium of women experiencing recurrent implantation failures. Sci. Rep. 14 (1), 19822. doi:10.1038/s41598-024-70778-9
Dougherty, M. P., Poch, A. M., Chorich, L. P., Hawkins, Z. A., Xu, H., Roman, R. A., et al. (2023). Unexplained female infertility associated with genetic disease variants. N. Engl. J. Med. 388 (11), 1055–1056. doi:10.1056/NEJMc2211539
Ehsani, M., Mohammadnia-Afrouzi, M., Mirzakhani, M., Esmaeilzadeh, S., and Shahbazi, M. (2019). Female unexplained infertility: a disease with imbalanced adaptive immunity. J. Hum. Reprod. Sci. 12 (4), 274–282. doi:10.4103/jhrs.JHRS_30_19
Emokpae, M. A., and Brown, S. I. (2021). Effects of lifestyle factors on fertility: practical recommendations for modification. Reprod. Fertil. 2 (1), R13–R26. doi:10.1530/RAF-20-0046
Gellersen, B., and Brosens, J. J. (2014). Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr. Rev. 35 (6), 851–905. doi:10.1210/er.2014-1045
Gyimesi, G., Pujol-Gimenez, J., Kanai, Y., and Hediger, M. A. (2020). Sodium-coupled glucose transport, the SLC5 family, and therapeutically relevant inhibitors: from molecular discovery to clinical application. Pflugers Arch. 472 (9), 1177–1206. doi:10.1007/s00424-020-02433-x
Hegyi, E., and Sahin-Toth, M. (2017). Genetic risk in chronic pancreatitis: the trypsin-dependent pathway. Dig. Dis. Sci. 62 (7), 1692–1701. doi:10.1007/s10620-017-4601-3
Hennes, A., Devroe, J., De Clercq, K., Ciprietti, M., Held, K., Luyten, K., et al. (2023). Protease secretions by the invading blastocyst induce calcium oscillations in endometrial epithelial cells via the protease-activated receptor 2. Reprod. Biol. Endocrinol. 21 (1), 37. doi:10.1186/s12958-023-01085-7
Jiang, R., Ding, L., Zhou, J., Huang, C., Zhang, Q., Jiang, Y., et al. (2017). Enhanced HOXA10 sumoylation inhibits embryo implantation in women with recurrent implantation failure. Cell Death Discov. 3, 17057. doi:10.1038/cddiscovery.2017.57
Jiang, Y. H., Shi, Y., He, Y. P., Du, J., Li, R. S., Shi, H. J., et al. (2011). Serine protease inhibitor 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (AEBSF) inhibits the rat embryo implantation in vivo and interferes with cell adhesion in vitro. Contraception 84 (6), 642–648. doi:10.1016/j.contraception.2011.03.017
Kang, H. (2021). Sample size determination and power analysis using the G*Power software. J. Educ. Eval. Health Prof. 18, 17. doi:10.3352/jeehp.2021.18.17
Kleyman, T. R., Kashlan, O. B., and Hughey, R. P. (2018). Epithelial Na(+) channel regulation by extracellular and intracellular factors. Annu. Rev. Physiol. 80, 263–281. doi:10.1146/annurev-physiol-021317-121143
Kothapalli, R., Buyuksal, I., Wu, S. Q., Chegini, N., and Tabibzadeh, S. (1997). Detection of ebaf, a novel human gene of the transforming growth factor beta superfamily association of gene expression with endometrial bleeding. J. Clin. Invest 99 (10), 2342–2350. doi:10.1172/JCI119415
Kumar, V., Soni, U. K., Maurya, V. K., Singh, K., and Jha, R. K. (2017). Integrin beta8 (ITGB8) activates VAV-RAC1 signaling via FAK in the acquisition of endometrial epithelial cell receptivity for blastocyst implantation. Sci. Rep. 7 (1), 1885. doi:10.1038/s41598-017-01764-7
Li, Y., Martin, T. E., Hancock, J. M., Li, R., Viswanathan, S., Lydon, J. P., et al. (2023). Visualization of preimplantation uterine fluid absorption in mice using Alexa Fluor™ 488 Hydrazide. Biol. Reprod. 108 (2), 204–217. doi:10.1093/biolre/ioac198
Lin, H. Y., Zhang, H., Yang, Q., Wang, H. X., Wang, H. M., Chai, K. X., et al. (2006). Expression of prostasin and protease nexin-1 in rhesus monkey (Macaca mulatta) endometrium and placenta during early pregnancy. J. Histochem Cytochem 54 (10), 1139–1147. doi:10.1369/jhc.6A7005.2006
Liu, X. M., Zhang, D., Wang, T. T., Sheng, J. Z., and Huang, H. F. (2014). Ion/water channels for embryo implantation barrier. Physiol. (Bethesda) 29 (3), 186–195. doi:10.1152/physiol.00039.2013
Loid, M., Obukhova, D., Kask, K., Apostolov, A., Meltsov, A., Tserpelis, D., et al. (2024). Aging promotes accumulation of senescent and multiciliated cells in human endometrial epithelium. Hum. Reprod. Open 2024 (3), hoae048. doi:10.1093/hropen/hoae048
Lord, M. S., Melrose, J., Day, A. J., and Whitelock, J. M. (2020). The inter-alpha-trypsin inhibitor family: versatile molecules in biology and pathology. J. Histochem Cytochem 68 (12), 907–927. doi:10.1369/0022155420940067
Ma, X. J., Fu, Y. Y., Li, Y. X., Chen, L. M., Chai, K., and Wang, Y. L. (2009). Prostasin inhibits cell invasion in human choriocarcinomal JEG-3 cells. Histochem Cell Biol. 132 (6), 639–646. doi:10.1007/s00418-009-0652-7
Macklon, N. S., and Brosens, J. J. (2014). The human endometrium as a sensor of embryo quality. Biol. Reprod. 91 (4), 98. doi:10.1095/biolreprod.114.122846
Manohar, K., Gupta, R. K., Gupta, P., Saha, D., Gare, S., Sarkar, R., et al. (2021). FDA approved L-type channel blocker Nifedipine reduces cell death in hypoxic A549 cells through modulation of mitochondrial calcium and superoxide generation. Free Radic. Biol. Med. 177, 189–200. doi:10.1016/j.freeradbiomed.2021.08.245
Mishra, A., and Seshagiri, P. B. (2000). Evidence for the involvement of a species-specific embryonic protease in zona escape of hamster blastocysts. Mol. Hum. Reprod. 6 (11), 1005–1012. doi:10.1093/molehr/6.11.1005
Misra, S., Hecht, P., Maeda, R., and Anderson, K. V. (1998). Positive and negative regulation of Easter, a member of the serine protease family that controls dorsal-ventral patterning in the Drosophila embryo. Development 125 (7), 1261–1267. doi:10.1242/dev.125.7.1261
Munne, S., Bahce, M., Sandalinas, M., Escudero, T., Marquez, C., Velilla, E., et al. (2004). Differences in chromosome susceptibility to aneuploidy and survival to first trimester. Reprod. Biomed. Online 8 (1), 81–90. doi:10.1016/s1472-6483(10)60501-9
Muter, J., Lynch, V. J., McCoy, R. C., and Brosens, J. J. (2023). Human embryo implantation. Development 150 (10), dev201507. doi:10.1242/dev.201507
Nataj Majd, M., Moini, A., Samimi Sadeh, S., and Bastanhagh, E. (2022). The effect of Nifedipine on embryo transfer outcomes: a randomized clinical trial. Int. J. Reprod. Biomed. 20 (12), 1013–1018. doi:10.18502/ijrm.v20i12.12562
Ng, K. K. L., Rozen, G., Stewart, T., Agresta, F., and Polyakov, A. (2019). Does nifedipine improve outcomes of embryo transfer? interim analysis of a randomized, double blinded, placebo-controlled trial. Med. Baltim. 98 (4), e14251. doi:10.1097/MD.0000000000014251
Okada, H., Tsuzuki, T., and Murata, H. (2018). Decidualization of the human endometrium. Reprod. Med. Biol. 17 (3), 220–227. doi:10.1002/rmb2.12088
Perona, R. M., and Wassarman, P. M. (1986). Mouse blastocysts hatch in vitro by using a trypsin-like proteinase associated with cells of mural trophectoderm. Dev. Biol. 114 (1), 42–52. doi:10.1016/0012-1606(86)90382-9
Quenby, S., Vince, G., Farquharson, R., and Aplin, J. (2002). Recurrent miscarriage: a defect in nature's quality control? Hum. Reprod. 17 (8), 1959–1963. doi:10.1093/humrep/17.8.1959
Ruan, Y. C., Guo, J. H., Liu, X., Zhang, R., Tsang, L. L., Dong, J. D., et al. (2012). Activation of the epithelial Na+ channel triggers prostaglandin E₂ release and production required for embryo implantation. Nat. Med. 18 (7), 1112–1117. doi:10.1038/nm.2771
Ruane, P. T., Paterson, I., Reeves, B., Adlam, D., Berneau, S. C., Renshall, L., et al. (2024). Glucose influences endometrial receptivity to embryo implantation through O-GlcNAcylation-mediated regulation of the cytoskeleton. Am. J. Physiol. Cell Physiol. 327 (3), C634–C645. doi:10.1152/ajpcell.00559.2023
Salamonsen, L. A., and Nie, G. (2002). Proteases at the endometrial-trophoblast interface: their role in implantation. Rev. Endocr. Metab. Disord. 3 (2), 133–143. doi:10.1023/a:1015407012559
Salker, M. S. (2025). Created in BioRender. Available online at: https://BioRender.com/z6xq99a.
Salker, M. S., Christian, M., Steel, J. H., Nautiyal, J., Lavery, S., Trew, G., et al. (2011). Deregulation of the serum- and glucocorticoid-inducible kinase SGK1 in the endometrium causes reproductive failure. Nat. Med. 17 (11), 1509–1513. doi:10.1038/nm.2498
Salker, M. S., Hosseinzadeh, Z., Alowayed, N., Zeng, N., Umbach, A. T., Webster, Z., et al. (2016a). LEFTYA activates the epithelial Na+ channel (ENaC) in endometrial cells via serum and glucocorticoid inducible kinase SGK1. Cell Physiol. Biochem. 39 (4), 1295–1306. doi:10.1159/000447834
Salker, M. S., Schierbaum, N., Alowayed, N., Singh, Y., Mack, A. F., Stournaras, C., et al. (2016b). LeftyA decreases actin polymerization and stiffness in human endometrial cancer cells. Sci. Rep. 6, 29370. doi:10.1038/srep29370
Salker, M. S., Singh, Y., Durairaj, R. R. P., Yan, J., Alauddin, M., Zeng, N., et al. (2018). LEFTY2 inhibits endometrial receptivity by downregulating Orai1 expression and store-operated Ca(2+) entry. J. Mol. Med. Berl. 96 (2), 173–182. doi:10.1007/s00109-017-1610-9
Salker, M. S., Singh, Y., Zeng, N., Chen, H., Zhang, S., Umbach, A. T., et al. (2017). Loss of endometrial sodium glucose cotransporter SGLT1 is detrimental to embryo survival and fetal growth in pregnancy. Sci. Rep. 7 (1), 12612. doi:10.1038/s41598-017-11674-3
Salker, M. S., Zhou, Y., Singh, Y., Brosens, J., and Lang, F. (2015). LeftyA sensitive cytosolic pH regulation and glycolytic flux in Ishikawa human endometrial cancer cells. Biochem. Biophys. Res. Commun. 460 (3), 845–849. doi:10.1016/j.bbrc.2015.03.120
Sanchez, D., Martinez, S., Lindqvist, A., Akerstrom, B., and Falkenberg, C. (2002). Expression of the AMBP gene transcript and its two protein products, alpha(1)-microglobulin and bikunin, in mouse embryogenesis. Mech. Dev. 117 (1-2), 293–298. doi:10.1016/s0925-4773(02)00202-2
Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9 (7), 676–682. doi:10.1038/nmeth.2019
Shi, S., Carattino, M. D., Hughey, R. P., and Kleyman, T. R. (2013). ENaC regulation by proteases and shear stress. Curr. Mol. Pharmacol. 6 (1), 28–34. doi:10.2174/18744672112059990027
Shmygol, A., and Brosens, J. J. (2021). Proteinase activated receptors mediate the trypsin-induced Ca(2 +) signaling in human uterine epithelial cells. Front. Cell Dev. Biol. 9, 709902. doi:10.3389/fcell.2021.709902
Simon, A., and Laufer, N. (2012). Assessment and treatment of repeated implantation failure (RIF). J. Assist. Reprod. Genet. 29 (11), 1227–1239. doi:10.1007/s10815-012-9861-4
Skern-Mauritzen, R., Frost, P., Dalvin, S., Kvamme, B. O., Sommerset, I., and Nilsen, F. (2009). A trypsin-like protease with apparent dual function in early Lepeophtheirus salmonis (Kroyer) development. BMC Mol. Biol. 10, 44. doi:10.1186/1471-2199-10-44
Smith, E. T., and Johnson, D. A. (2013). Human enteropeptidase light chain: bioengineering of recombinants and kinetic investigations of structure and function. Protein Sci. 22 (5), 577–585. doi:10.1002/pro.2239
Soares, S. R., Troncoso, C., Bosch, E., Serra, V., Simon, C., Remohi, J., et al. (2005). Age and uterine receptiveness: predicting the outcome of oocyte donation cycles. J. Clin. Endocrinol. Metab. 90 (7), 4399–4404. doi:10.1210/jc.2004-2252
Stevens, B. L., Habets, D., Den Hartog, J., Al-Nasiry, S., Wieten, L., Morre, S., et al. (2022). Endometrial factors in the implantation failure spectrum: protocol of a MUltidisciplinary observational cohort study in women with Repeated Implantation failure and recurrent Miscarriage (MURIM Study). BMJ Open 12 (6), e056714. doi:10.1136/bmjopen-2021-056714
Sun, Z. G., Shi, H. J., Gu, Z., Wang, J., and Shen, Q. X. (2007). A single intrauterine injection of the serine protease inhibitor 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride reversibly inhibits embryo implantation in mice. Contraception 76 (3), 250–255. doi:10.1016/j.contraception.2007.05.084
Szabo, R., Wu, Q., Dickson, R. B., Netzel-Arnett, S., Antalis, T. M., and Bugge, T. H. (2003). Type II transmembrane serine proteases. Thromb. Haemost. 90 (2), 185–193. doi:10.1160/TH03-02-0071
Tabibzadeh, S., and Hemmati-Brivanlou, A. (2006). Lefty at the crossroads of “stemness” and differentiative events. Stem Cells 24 (9), 1998–2006. doi:10.1634/stemcells.2006-0075
Tabibzadeh, S., and Kothapalli, R. (1996). From steroid signals to local regulatory factors involved in endometrial bleeding. Eur. J. Obstet. Gynecol. Reprod. Biol. 70 (1), 25–27. doi:10.1016/s0301-2115(96)02572-9
Tabibzadeh, S., Lessey, B., and Satyaswaroop, P. G. (1998). Temporal and site-specific expression of transforming growth factor-beta4 in human endometrium. Mol. Hum. Reprod. 4 (6), 595–602. doi:10.1093/molehr/4.6.595
Tabibzadeh, S., Mason, J. M., Shea, W., Cai, Y., Murray, M. J., and Lessey, B. (2000). Dysregulated expression of ebaf, a novel molecular defect in the endometria of patients with infertility. J. Clin. Endocrinol. Metab. 85 (7), 2526–2536. doi:10.1210/jcem.85.7.6674
Tang, M., Mikhailik, A., Pauli, I., Giudice, L. C., Fazelabas, A. T., Tulac, S., et al. (2005). Decidual differentiation of stromal cells promotes Proprotein Convertase 5/6 expression and lefty processing. Endocrinology 146 (12), 5313–5320. doi:10.1210/en.2005-0684
Tang, M., You, J., Wang, W., Lu, Y., Hu, X., Wang, C., et al. (2018). Impact of galectin-1 on trophoblast stem cell differentiation and invasion in in vitro implantation model. Reprod. Sci. 25 (5), 700–711. doi:10.1177/1933719117725816
Teklenburg, G., Salker, M., Heijnen, C., Macklon, N. S., and Brosens, J. J. (2010b). The molecular basis of recurrent pregnancy loss: impaired natural embryo selection. Mol. Hum. Reprod. 16 (12), 886–895. doi:10.1093/molehr/gaq079
Teklenburg, G., Salker, M., Molokhia, M., Lavery, S., Trew, G., Aojanepong, T., et al. (2010a). Natural selection of human embryos: decidualizing endometrial stromal cells serve as sensors of embryo quality upon implantation. PLoS One 5 (4), e10258. doi:10.1371/journal.pone.0010258
Toner, J. P., Grainger, D. A., and Frazier, L. M. (2002). Clinical outcomes among recipients of donated eggs: an analysis of the U.S. national experience, 1996-1998. Fertil. Steril. 78 (5), 1038–1045. doi:10.1016/s0015-0282(02)03371-x
Ulloa, L., and Tabibzadeh, S. (2001). Lefty inhibits receptor-regulated Smad phosphorylation induced by the activated transforming growth factor-beta receptor. J. Biol. Chem. 276 (24), 21397–21404. doi:10.1074/jbc.M010783200
Vento-Tormo, R., Efremova, M., Botting, R. A., Turco, M. Y., Vento-Tormo, M., Meyer, K. B., et al. (2018). Single-cell reconstruction of the early maternal-fetal interface in humans. Nature 563 (7731), 347–353. doi:10.1038/s41586-018-0698-6
von Wolff, M., Ursel, S., Hahn, U., Steldinger, R., and Strowitzki, T. (2003). Glucose transporter proteins (GLUT) in human endometrium: expression, regulation, and function throughout the menstrual cycle and in early pregnancy. J. Clin. Endocrinol. Metab. 88 (8), 3885–3892. doi:10.1210/jc.2002-021890
Vrhovac, M. I., Karin-Kujundzic, V., Madunic, J., Sola, I. M., and Serman, L. (2021). Endometrial glucose transporters in health and disease. Front. Cell Dev. Biol. 9, 703671. doi:10.3389/fcell.2021.703671
Wang, H., and Dey, S. K. (2006). Roadmap to embryo implantation: clues from mouse models. Nat. Rev. Genet. 7 (3), 185–199. doi:10.1038/nrg1808
Wang, X., Sun, Y., Shi, H., and Xin, A. (2024). Establishment of an embryo implantation model in vitro. J. Vis. Exp. (208). doi:10.3791/66873
Weimar, C. H., Kavelaars, A., Brosens, J. J., Gellersen, B., de Vreeden-Elbertse, J. M., Heijnen, C. J., et al. (2012). Endometrial stromal cells of women with recurrent miscarriage fail to discriminate between high- and low-quality human embryos. PLoS One 7 (7), e41424. doi:10.1371/journal.pone.0041424
Whitcomb, D. C., Gorry, M. C., Preston, R. A., Furey, W., Sossenheimer, M. J., Ulrich, C. D., et al. (1996). Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat. Genet. 14 (2), 141–145. doi:10.1038/ng1096-141
Xie, D., Chen, C. C., Ptaszek, L. M., Xiao, S., Cao, X., Fang, F., et al. (2010). Rewirable gene regulatory networks in the preimplantation embryonic development of three mammalian species. Genome Res. 20 (6), 804–815. doi:10.1101/gr.100594.109
Xu, X., Liu, X., and Tao, J. (2013). Changes in biochemical composition and digestive enzyme activity during the embryonic development of the marine crab, Charybdis japonica (Crustacea: Decapoda). Zool. Sci. 30 (3), 160–166. doi:10.2108/zsj.30.160
Yamada, K., Takabatake, T., and Takeshima, K. (2000). Isolation and characterization of three novel serine protease genes from Xenopus laevis. Gene 252 (1-2), 209–216. doi:10.1016/s0378-1119(00)00225-0
Yang, W., Nurbaeva, M. K., Schmid, E., Russo, A., Almilaji, A., Szteyn, K., et al. (2014). Akt2-and ETS1-dependent IP3 receptor 2 expression in dendritic cell migration. Cell Physiol. Biochem. 33 (1), 222–236. doi:10.1159/000356664
Zeng, N., Okumura, T., Alauddin, M., Khozooei, S., Rajaxavier, J., Zhang, S., et al. (2020). LEFTY2/endometrial bleeding-associated factor up-regulates Na+ coupled glucose transporter SGLT1 expression and glycogen accumulation in endometrial cancer cells. PLoS One 15 (4), e0230044. doi:10.1371/journal.pone.0230044
Zhang, H., Qi, J., Wang, Y., Sun, J., Li, Z., Sui, L., et al. (2020). Progesterone regulates glucose metabolism through glucose transporter 1 to promote endometrial receptivity. Front. Physiol. 11, 543148. doi:10.3389/fphys.2020.543148
Zheng, X. L., Kitamoto, Y., and Sadler, J. E. (2009). Enteropeptidase, a type II transmembrane serine protease. Front. Biosci. Elite Ed. 1 (1), 242–249. doi:10.2741/E23
Keywords: LEFTY2, Ca2+ channels, endometrium, receptivity, unexplained reproductive failure
Citation: Yang Z, Yan J, Kull S, Alauddin M, Brucker SY, Henes M, Lang F and Salker MS (2025) Embryo-derived trypsin-induced calcium entry is inhibited by endometrial infertility factor, LEFTY2. Front. Cell Dev. Biol. 13:1499339. doi: 10.3389/fcell.2025.1499339
Received: 20 September 2024; Accepted: 04 April 2025;
Published: 29 May 2025.
Edited by:
Ivan Varga, Comenius University, SlovakiaReviewed by:
Suranga P. Kodithuwakku, University of Peradeniya, Sri LankaIgnacio Santiago Alvarez Miguel, University of Extremadura, Spain
Huanhuan Jiang, Anhui Medical University, China
Copyright © 2025 Yang, Yan, Kull, Alauddin, Brucker, Henes, Lang and Salker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Madhuri S. Salker, bWFkaHVyaS5zYWxrZXJAbWVkLnVuaS10dWViaW5nZW4uZGU=
 Zhiqi Yang
Zhiqi Yang Jing Yan2,3
Jing Yan2,3 Steffen Kull
Steffen Kull Md. Alauddin
Md. Alauddin Sara Y. Brucker
Sara Y. Brucker Melanie Henes
Melanie Henes Florian Lang
Florian Lang Madhuri S. Salker
Madhuri S. Salker