Abstract
Objective:
Concurrent pulmonary diseases are common in patients with gastroesophageal reflux disease (GERD). However, whether GERD increase the incidence of pulmonary diseases is uncertain because of a lack of quantitative evidence. We conducted a meta-analysis to determine whether GERD was associated with the increased incidence of subsequent of pulmonary diseases.
Methods:
The PubMed, Embase, Web of Science and Cochrane Library databases were searched through 12 July 2024. The primary outcomes were asthma and pneumonia, and the secondary outcomes were pulmonary fibrosis (PF), chronic obstructive pulmonary disease (COPD), lung cancer, interstitial lung disease (ILD), bronchiectasis, bronchitis, acute lung injury (ALI), pulmonary embolism, pulmonary tuberculosis (PTB) and nontuberculous mycobacterial pulmonary disease (NTMPD). Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to investigate the associations of prior GERD with the incidence of pulmonary diseases, and subgroup analyses based on the treatment of GERD, age and source of OR were performed.
Results:
A total of 45 cohort studies were included. The pooled results indicated that GERD was significantly linked to an increased incidence of asthma (OR = 1.50, P < 0.001) and pneumonia (OR = 1.53, P < 0.001), as did PF (OR = 1.43, P = 0.001), COPD (OR = 1.41, P = 0.004), lung cancer (OR = 1.51, P < 0.001), ILD (OR = 1.28, P = 0.015), bronchiectasis (OR = 1.63, P = 0.039), bronchitis (OR = 1.24, P < 0.001), ALI (OR = 2.07, P < 0.001), pulmonary embolism (OR = 1.33, P = 0.013), PTB (OR = 1.63, P = 0.015) and NTMPD (OR = 3.36, P < 0.001). Subgroup analyses stratified by age and source of OR yielded similar results. However, no significant associations between treated GERD and the incidence of asthma (OR = 1.27, P = 0.081) or lung cancer (OR = 1.01, P = 0.97) were observed.
Conclusion:
The presence of GERD is associated with an increased incidence of subsequent various pulmonary diseases, but regular treatment may eliminate this effect. These findings highlight the importance of screening and management for pulmonary diseases and of standardized therapy in patients with GERD.
Clinical trial registration no:
INPLASY202490013
Introduction
Gastroesophageal reflux disease (GERD) is a common digestive disorder characterized by the reflux of stomach contents into the esophagus, leading to a variety of symptoms and complications (Azer et al., 2024). Globally, the prevalence of GERD varies by region and diagnostic criteria, but GERD is generally estimated to affect approximately 10%–20% of adults (Richter and Rubenstein, 2018; Rubenstein and Chen, 2014). The prevalence of GERD is relatively high in Western countries but relatively low in other regions, such as Asia and Africa (Rubenstein and Chen, 2014; Jung, 2011). However, in recent years, the prevalence of GERD in China has been increasing due to changes in lifestyle and dietary habits (Liu et al., 2023; Li et al., 2023a; Zhang et al., 2022). The risk factors for GERD include advanced age, obesity, smoking, alcohol consumption, and a high-fat diet (Richter and Rubenstein, 2018). GERD can significantly impact quality of life and increase the risk of complications such as esophagitis, Barrett’s esophagus, and esophageal adenocarcinoma (Azer et al., 2024).
GERD is often overlooked and misdiagnosed in clinical practice, primarily because of atypical symptoms and a lack of awareness about the need for individuals with these symptoms to seek medical consultation and treatment. The standardized treatment of GERD requires long-term medication and improvements in lifestyles. However, a significant number of patients are unable to comply, leading to the development of long-term complications (Katzka, 2014). The impact of GERD on the risk of developing the abovementioned esophageal disorders has been well documented (Azer et al., 2024; Abdallah et al., 2019). In recent years, accumulating evidence has suggested that GERD may also have a significant effect on the development of lung diseases such as the asthma and pulmonary fibrosis which severely affect the quality of life and increase the mortality among GERD patients (Azer et al., 2024). However, this issue has not yet been thoroughly explored or systematically addressed. Clarifying the impact of GERD on pulmonary diseases can further reveal the underlying mechanisms of these diseases, while also highlighting the role of treating GERD in the prevention and management of respiratory diseases.
Therefore, this study aimed to clarify the impact of GERD on the incidence of pulmonary diseases on the basis of available evidence. The findings will contribute to the clinical management of patients with GERD, as well as to the prevention and early screening of pulmonary diseases.
Materials and methods
This meta-analysis was registered with the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY202490013, DOI: 10.37766/inplasy2024.9.0013).
Literature search
PubMed, Embase, Web of Science and Cochrane Library databases were searched from database inception through 12 July 2024 for the following terms: gastroesophageal reflux, gastro-oesophageal reflux, chronic obstructive pulmonary disease, COPD, asthma, pulmonary tuberculosis, pneumonia, pulmonary fibrosis, pulmonary embolism, lung cancer, bronchitis, bronchiectasis, pulmonary disease, respiratory disease, risk, incidence and morbidity. The search strategy is presented in Supplementary file 1. During the search, MeSH terms and free texts were applied, and references in the included studies were also reviewed.
Inclusion criteria
Studies that met the following criteria were included: 1) GERD was diagnosed on the basis of symptoms such as acid reflux and heartburn, as well as through analysis by gastroscopy, PPI test or 24-h esophageal pH monitoring both among the adults and children; 2) GERD was diagnosed before the occurrence of pulmonary diseases, but in cases where the original articles did not explicitly report whether GERD preceded pulmonary disease, we inferred the sequence based on the study’s hypothesis or statements such as “GERD increases the risk of” or “GERD is a risk factor for pulmonary disease”; 3) pulmonary diseases were diagnosed on the basis of symptoms, blood tests, etiology, imaging, and pathological examinations and/or bronchoscopy; 4) the incidence rates of pulmonary diseases were compared between the GERD and non-GERD groups, and represented as odds ratios (ORs) with 95% confidence intervals (CIs) or enough data were provided to calculate them; 5) full text was available; and 6) if the data severely overlapped or were duplicated, only the latest or most comprehensive studies were included.
Exclusion criteria
Studies that met the following criteria were excluded: 1) the presence of other confounding factors, such as gastrointestinal disorders; 2) ORs with 95% CIs were not available even after the authors were contacted; 3) the endpoints were not of interest, such as exacerbations of pulmonary diseases; and 4) letter, meeting abstract, editorial, animal trial, review or case report articles.
Literature selection and data collection
First, duplicated publications were removed automatically or manually by EndNote (version 21.3, Clarivate Analytics, London, England, United Kingdom) software. The titles and abstracts were subsequently screened for relevance, and the full texts of potentially relevant publications were further reviewed for suitability.
The following data were extracted from each included study: name of first author, publication year, data source, age, sample size, number of GERD cases, treatment history of GERD (treated vs untreated), source of OR (multivariate vs univariate), endpoint, and OR and 95% CI for the corresponding endpoint.
In this meta-analysis, based on the strength of the association between GERD and various pulmonary diseases, as well as the extent of their reporting in previous studies, the primary outcomes were defined as asthma and pneumonia, and the secondary outcomes were pulmonary fibrosis (PF), chronic obstructive pulmonary disease (COPD), lung cancer, interstitial lung disease (ILD), bronchiectasis, bronchitis, acute lung injury (ALI), pulmonary embolism, pulmonary tuberculosis (PTB) and nontuberculous mycobacterial pulmonary disease (NTMPD).
Methodological quality assessment
All the studies included in our meta-analysis were cohort studies. Therefore, the Newcastle–Ottawa scale (NOS) was used for quality evaluation, including cohort selection, comparability and outcome measurement. Studies with an NOS score >5 were regarded as high-quality studies (Stang, 2010).
The literature search, selection, data extraction, and quality evaluation were independently performed by two authors (Yao Wang and Yijie Bu), and all disagreements and discrepancies were resolved through team discussion.
Statistical analysis
All statistical analyses were conducted by STATA (version 15.0, StataCorp LLC, College Station, Texas, United States) software. The heterogeneity among included studies was assessed by the I2 statistic and Q test. When significant heterogeneity was observed, presented as I2>50% and/or P < 0.1, the random-effects model was used; or the fixed-effects model was applied (Barili et al., 2018). The ORs with 95% CIs were combined to evaluate the association between presence of GERD and incidence of pulmonary diseases. If ORs and 95% CIs were both reported in the multivariate and univariate analyses, data from multivariate analysis were extracted and applied preferentially. The sensitivity analysis was conducted to clarify the source of heterogeneity and evaluate the stability of the pooled results. Besides, Begg’s funnel plot and Egger’s test were performed to identify publication bias for the asthma and pneumonia (Begg and Mazumdar, 1994; Egger et al., 1997). Significant publication bias was defined as the noticeably asymmetric Begg’s funnel plot and P value < 0.05 of Egger’s test, and then the trim-and-fill method was applied to evaluate the impact of potentially unpublished studies on the stability of overall pooled results, with an inspection level of α = 0.05 (Wang et al., 2021).
Furthermore, subgroup analyses stratified by the treatment of GERD (treated vs. untreated), age (adult vs. child) and source of OR (multivariate vs. univariate) were also conducted to identify the impact of these factors on the association of GERD with incidence of pulmonary diseases.
Results
Literature search and selection
The literature search and selection process were presented in Figure 1. Initially, 10,996 records were identified from the databases, and 1,356 duplicated records were removed. A total of 9,139 and 398 publications were excluded after reviewing the titles and abstracts, respectively. After the full texts were carefully reviewed, 57 publications were excluded, thirty of which were due to GERD not being critically diagnosed before the occurrence of pulmonary disease. Eventually, 45 studies were included in the meta-analysis (el-Serag and Sonnenberg, 1997; El-Serag et al., 2001; Gislason et al., 2002; Jaspersen et al., 2003; Gunnbjömsdóttir et al., 2004; Ruigómez et al., 2005; Garcia Rodriguez et al., 2008; Gribbin et al., 2009; Uddenfeldt et al., 2010; García-Sancho et al., 2011; Emilsson et al., 2013; Patria et al., 2013; Martinez et al., 2014; Wang et al., 2014; Zhong et al., 2015; Fan et al., 2016; Hsu et al., 2016; Yang et al., 2016; Hsu et al., 2017; Zhang et al., 2017; Choi et al., 2019; Kim et al., 2020a; Kim et al., 2020b; Kuo et al., 2020; Wang et al., 2020; Abdel Baseer and Sakhr, 2021; Baqir et al., 2021; Cantarutti et al., 2021; Amarnath et al., 2022; Ahn et al., 2023; Cotton et al., 2023; Duncan et al., 2023; Jareebi et al., 2023; Kim et al., 2023; Li et al., 2023b; Reynolds et al., 2023; Sarwar Zubairi et al., 2023; Shen et al., 2023; Cleven et al., 2024; Dong et al., 2024; Fakhraei et al., 2024; Liu et al., 2024; Shan and Ge, 2024.
FIGURE 1
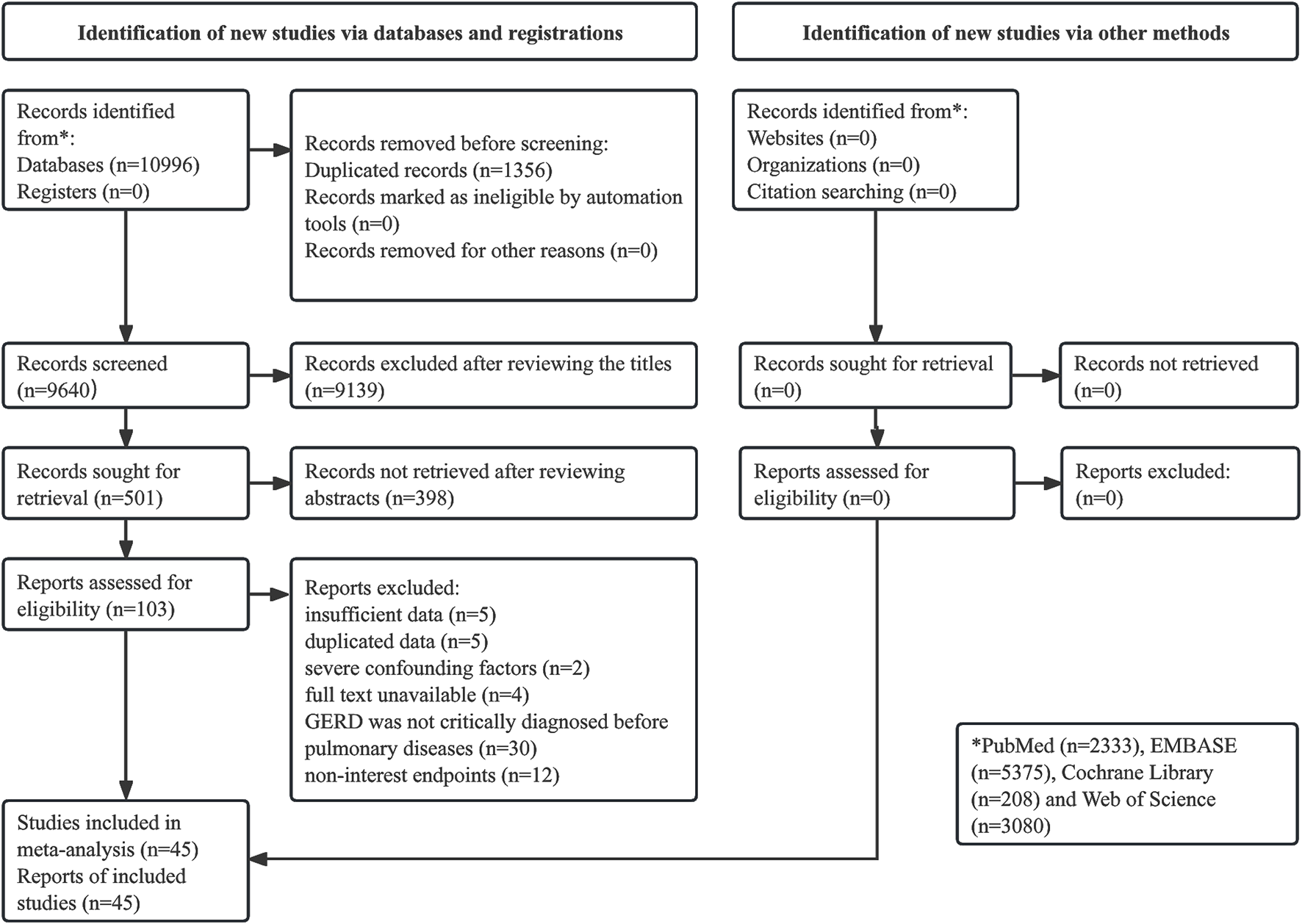
Prisma flow diagram of this meta-analysis.
Basic characteristics of the included studies
These 45 cohort studies were published from 1997-2024, with sample sizes ranging from 43-602,604. Most of the participants were from public databases and were adult patients. The associations of GERD with the incidence of asthma (el-Serag and Sonnenberg, 1997; El-Serag et al., 2001; Gislason et al., 2002; Jaspersen et al., 2003; Gunnbjömsdóttir et al., 2004; Ruigómez et al., 2005; Uddenfeldt et al., 2010; Emilsson et al., 2013; Martinez et al., 2014; Kim et al., 2020a; Kim et al., 2020b; Wang et al., 2020; Cantarutti et al., 2021; Ahn et al., 2023; Fakhraei et al., 2024; Yao et al., 2024), pneumonia (el-Serag and Sonnenberg, 1997; El-Serag et al., 2001; Patria et al., 2013; Martinez et al., 2014; Wang et al., 2014; Yang et al., 2016; Hsu et al., 2017; Zhang et al., 2017; Kuo et al., 2020; Abdel Baseer and Sakhr, 2021; Dong et al., 2024), PF (el-Serag and Sonnenberg, 1997; Gribbin et al., 2009; García-Sancho et al., 2011; Baqir et al., 2021; Cotton et al., 2023; Reynolds et al., 2023; Sarwar Zubairi et al., 2023; Wu et al., 2024), COPD (el-Serag and Sonnenberg, 1997; Garcia Rodriguez et al., 2008; Shen et al., 2023; Liu et al., 2024), lung cancer (Hsu et al., 2016; Choi et al., 2019; Amarnath et al., 2022; Li et al., 2023b), ILD (Jareebi et al., 2023; Cleven et al., 2024; Shan and Ge, 2024), bronchiectasis (el-Serag and Sonnenberg, 1997; El-Serag et al., 2001; Duncan et al., 2023) and bronchitis (el-Serag and Sonnenberg, 1997; Dong et al., 2024) were explored in 16, 11, 8, 4, 4, 3, 3 and 2 of the included studies, and 1 study explored the relationships between GERD and the incidence of ALI (Zhong et al., 2015), pulmonary embolism (Dong et al., 2024), PTB (Fan et al., 2016) and NTMPD (Kim et al., 2023). The data are shown in Table 1.
TABLE 1
| Author | Year | Country | Data source | Age (year-old) | Sample size | Number of GERD cases | Treatment of GERD | Source of OR | Endpoints |
|---|---|---|---|---|---|---|---|---|---|
| el-Serag and Sonnenberg (1997) | 1997 | United States | Department of Veterans Affairs | 60 ± 13/56 ± 15 | 202,732 | 101,366 | NR | M | Asthma, bronchiectasis, bronchitis, COPD, PF, pneumonia |
| El-Serag et al. (2001) | 2001 | United States | Texas Children’s Hospital | 9.16 ± 4.61/8.64 ± 4.92 | 9,900 | 1980 | NR | M | Asthma, bronchiectasis, pneumonia |
| Gislason et al. (2002) | 2002 | Iceland, Belgium, and Sweden | Iceland, Belgium, and Sweden | 33 ± 7/34 ± 7 | 2,197 | 101 | NR | M | Asthma |
| Jaspersen et al. (2003) | 2003 | Germany, Austria and Switzerland | Germany, Austria and Switzerland | 53.8 ± 14.0 | 4,179 | 2,114 | NR | M | Asthma |
| Gunnbjömsdóttir et al. (2004) | 2004 | Iceland, Norway, Denmark, Sweden and Estonia | Iceland, Norway, Denmark, Sweden and Estonia | 39.6 ± 7.1 | 14,552 | 1,422 | NR | M | Asthma |
| Ruigómez et al. (2005) | 2005 | United Kingdom | United Kingdom General Practice Research Database | 2–79 | 13,758 | 5,653 | NR | M | Asthma |
| Garcia Rodriguez et al. (2008) | 2008 | United Kingdom | United Kingdom General Practice Research Database | 40–79 | 9,509 | 4,391 | NR | M | COPD |
| Gribbin et al. (2009) | 2009 | United Kingdom | The Health Improvement Network primary care database | >40 | 4,513 | 387 | NR | U | Idiopathic PF |
| Uddenfeldt et al. (2010) | 2010 | Sweden | Gastrikland and Jamtland in the central part of Sweden | 16–69 | 8,150 | NR | NR | M | Asthma |
| García-Sancho et al. (2011) | 2011 | United States | National Institute of Respiratory Diseases | 67.8 ± 9.5 | 363 | 46 | NR | U | Idiopathic PF |
| Emilsson et al. (2013) | 2013 | Iceland, Sweden and Belgium | Iceland, Sweden and Belgium | 33.5 ± 7.2/34.0 ± 6.8/35.0 ± 7.3 | 1,560 | 262 | NR | U | Asthma |
| Patria et al. (2013) | 2013 | Italy | Department of Pathophysiology and Transplantation, University of Milan | 7.9 ± 4.5/8.1 ± 4.5 | 291 | 51 | NR | M | Pneumonia |
| Martinez et al. (2014) | 2014 | United States | COPDGene Study | 63.1 ± 8.6 | 4,483 | 1,307 | NR | U | Asthma, pneumonia |
| Wang et al. (2014) | 2014 | China | Zhejiang Tongde Hospital | 72.3 ± 5.6 | 876 | 314 | NR | M | Pneumonia |
| Zhong et al. (2015) | 2015 | China | People’s Hospital of Ganzhou | NR | 1,309 | 349 | NR | M | ALI |
| Fan et al. (2016) | 2016 | China | Taiwan’s National Health Insurance Research Database | 51 (39–64) | 63,930 | 31,965 | NR | M | PTB |
| Hsu et al. (2016) | 2016 | China | Taiwan’s National Health Insurance Research Database | 18–100 | 76,369 | 15,412 | NR | M | Lung cancer |
| Yang et al. (2016) | 2016 | China | Second Hospital of Tianjin Medical University | 76.1 ± 5.8 | 986 | 350 | NR | U | Pneumonia |
| Hsu et al. (2017) | 2017 | China | Taiwan’s National Health Insurance Research Database | 48.3 ± 16.0 | 31,430 | 15,715 | NR | M | Pneumonia |
| Zhang et al. (2017) | 2017 | China | Xianyang Central Hospital | 71.2 ± 5.3 | 120 | 38 | NR | M | Pneumonia |
| Choi et al. (2019) | 2019 | Republic of Korea | Korean National Health Insurance Database | ≥20 | 1,070 | 427 | NR | M | Lung cancer |
| Kim et al. (2020a) | 2020 | Korea | Korean Health Insurance Review and Assessment Service-National Sample Cohort |
≤14 | 1,596 | 532 | NR | M | Asthma |
| Kim et al. (2020b) | 2020 | Korea | Korean Health Insurance Review and Assessment Service-National Sample Cohort |
≥20 | 349,506 | 116,502 | NR | M | Asthma |
| Kuo et al. (2020) | 2020 | China | Taiwan’s National Health Insurance Research Database | 5.8 ± 4.6/3.9 ± 3.6/3.6 ± 3.9 | 6,356 | 220 | NR | M | Pneumonia |
| Wang et al. (2020) | 2020 | China | Taiwan’s National Health Insurance Research Database | 55.8 ± 15.5/56.7 ± 15.1 | 48,154 | 685 | NR | U | Asthma |
| Abdel Baseer and Sakhr (2021) | 2021 | Egypt | South Valley University hospital | 7.17 ± 1.72/6.59 ± 1.80 | 174 | 20 | NR | M | Pneumonia |
| Baqir et al. (2021) | 2021 | Minnesota | Rochester Epidemiology Project | 74 (67–80) | 339 | 113 | NR | M | PF |
| Cantarutti et al. (2021) | 2021 | Italy | Italian primary care database | Children | 454,113 | 9,020 | Treated/ untreated |
M | Asthma |
| Amarnath et al. (2022) | 2022 | United States | 17 Northwell healthcare facilities | 72.9 ± 13.1/71.7 ± 10.7 | 1,083 | 174 | Mixed/treated | M | Lung cancer |
| Ahn et al. (2023) | 2023 | United States | United Kingdom Biobank | NR | 332,601 | 71,522 | NR | U | Asthma |
| Cotton et al. (2023) | 2023 | United Kingdom | NR | NR | 11,259 | 2,668 | NR | M | PF |
| Duncan et al. (2023) | 2023 | United States | Aerodigestive Center at Boston Children’s Hospital | 7.9 ± 0.5 | 43 | 20 | NR | U | Bronchiectasis |
| Jareebi et al. (2023) | 2023 | SAU | Integrative Epidemiology Unit, FinnGen | NR | 602,604 | NR | NR | U | ILD |
| Kim et al. (2023) | 2023 | Republic of Korea | Korean National Health Insurance Service National Sample Cohort | ≥20 | 87,120 | 17,424 | NR | U | NTMPD |
| Li et al. (2023b) | 2023 | China | Integrative Epidemiology Unit, FinnGen | NR | 602,604 | 129,080 | NR | U | Lung cancer |
| Reynolds et al. (2023) | 2023 | United Kingdom | FinnGen, International Lung Cancer Consortium | NR | 367,441 | 78,707 | NR | U | Idiopathic PF |
| Sarwar Zubairi et al. (2023) | 2023 | Pakistan | Aga Khan University Hospital (AKUH) and Jinnah Postgraduate Medical Centre | 66.1 ± 10.9/64.6 ± 11.1 | 454 | 121 | NR | U | Idiopathic PF |
| Shen et al. (2023) | 2023 | China | Integrative Epidemiology Unit, FinnGen | NR | 602,604 | 129,080 | NR | U | COPD |
| Cleven et al. (2024) | 2024 | United States | Population of firefighters and EMS providers at the WTC-disaster site | 40 ± 9.5/53 ± 8.2 | 14,525 | 6,873 | NR | M | ILD |
| Dong et al. (2024) | 2024 | China | Integrative Epidemiology Unit, FinnGen | NR | 602,604 | 129,080 | NR | U | Bronchitis, pneumonia, pulmonary embolism |
| Fakhraei et al. (2024) | 2024 | Iceland | Respiratory Health in Northern Europe | 25–54 | 11,024 | 1,323 | NR | M | Asthma |
| Liu et al. (2024) | 2024 | China | United Kingdom Biobank | NR | 332,601 | 71,522 | NR | U | COPD |
| Shan and Ge (2024) | 2024 | China | China-Japan Friendship Hospital | 46 ± 14.5 | 59 | 33 | NR | M | ILD |
| Wu et al. (2024) | 2024 | China | Integrative Epidemiology Unit, FinnGen | NR | 602,604 | 129,080 | NR | U | Idiopathic PF |
| Yao et al. (2024) | 2024 | China | Integrative Epidemiology Unit, FinnGen | NR | 602,604 | 129,080 | NR | U | Asthma |
Basic characteristics of included studies.
NR, not reported; U, univariate analysis; M, multivariate analysis; GERD, gastroesophageal reflux disease; OR, odds ratio; COPD, chronic obstructive pulmonary disease; PF, pulmonary fibrosis; ALI, acute lung injury; PTB, pulmonary tuberculosis; ILD, interstitial lung disease; NTMPD, non-tuberculous mycobacteria pulmonary disease.
Detailed information on the methodological quality assessment is presented in Supplementary Table 1. All the included studies were of high quality with NOS scores ≥6.
Meta-analysis results for primary outcomes
The pooled results demonstrated that the presence of GERD was significantly associated with an increased incidence of asthma (OR = 1.50, 95% CI: 1.41–1.60, P < 0.001; I2 = 69.1%, P < 0.001) (Figure 2). In addition, subgroup analyses stratified by age (adult: OR = 1.48, 95% CI: 1.41–1.55, P < 0.001; child: OR = 1.65, 95% CI: 1.33–2.04, P < 0.001) (Supplementary Table S1A) and source of OR (multivariate: OR = 1.56, 95% CI: 1.44–1.69, P < 0.001; univariate: OR = 1.39, 95% CI: 1.23–1.58, P < 0.001) (Supplementary Table S1B) revealed similar findings. However, a subgroup analysis on the basis of treatment history revealed a significant association between GERD and the incidence of asthma only among untreated patients (OR = 1.57, 95% CI: 1.19–2.08; P = 0.002) (Table 2).
FIGURE 2
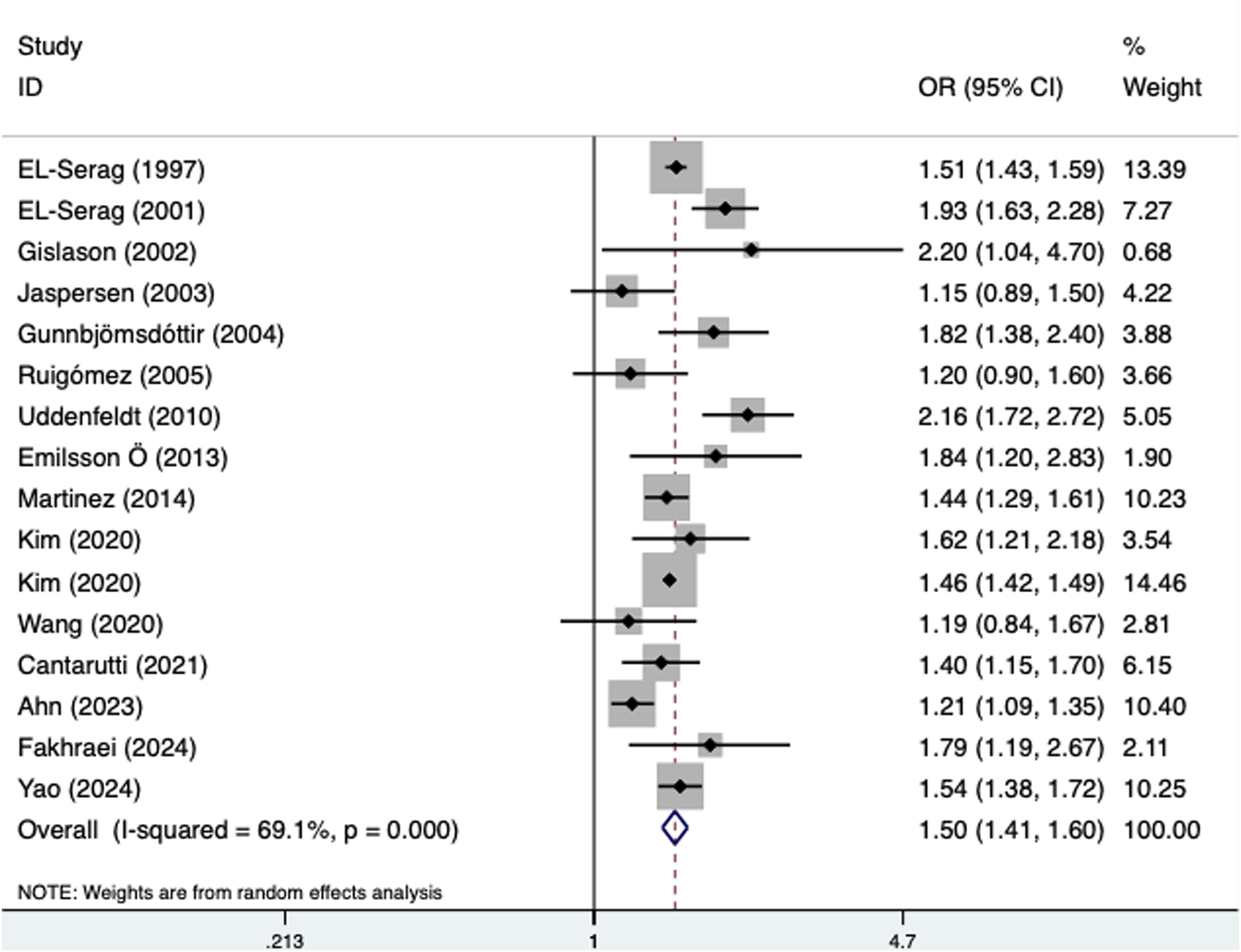
Association of the presence of gastroesophageal reflux disease with the incidence of asthma.
TABLE 2
Results of meta-analysis for primary outcomes.
OR, odds ratio; CI, confidence interval; GERD, gastroesophageal reflux disease.
Furthermore, the presence of GERD was also related to increased incidence of pneumonia (OR = 1.53, 95% CI: 1.35–1.74, P < 0.001; I2 = 89.3%, P < 0.001) (Figure 3), which was confirmed by subgroup analyses according to age (adult: OR = 1.45, 95% CI: 1.25–1.68, P < 0.001; child: OR = 2.04, 95% CI: 1.45–2.88, P < 0.001) (Supplementary Table S1C) and the source of OR (multivariate: OR = 1.84, 95% CI: 1.42–2.39, P < 0.001; univariate: OR = 1.31, 95% CI: 1.13–1.51, P < 0.001) (Supplementary Table S1D).
FIGURE 3
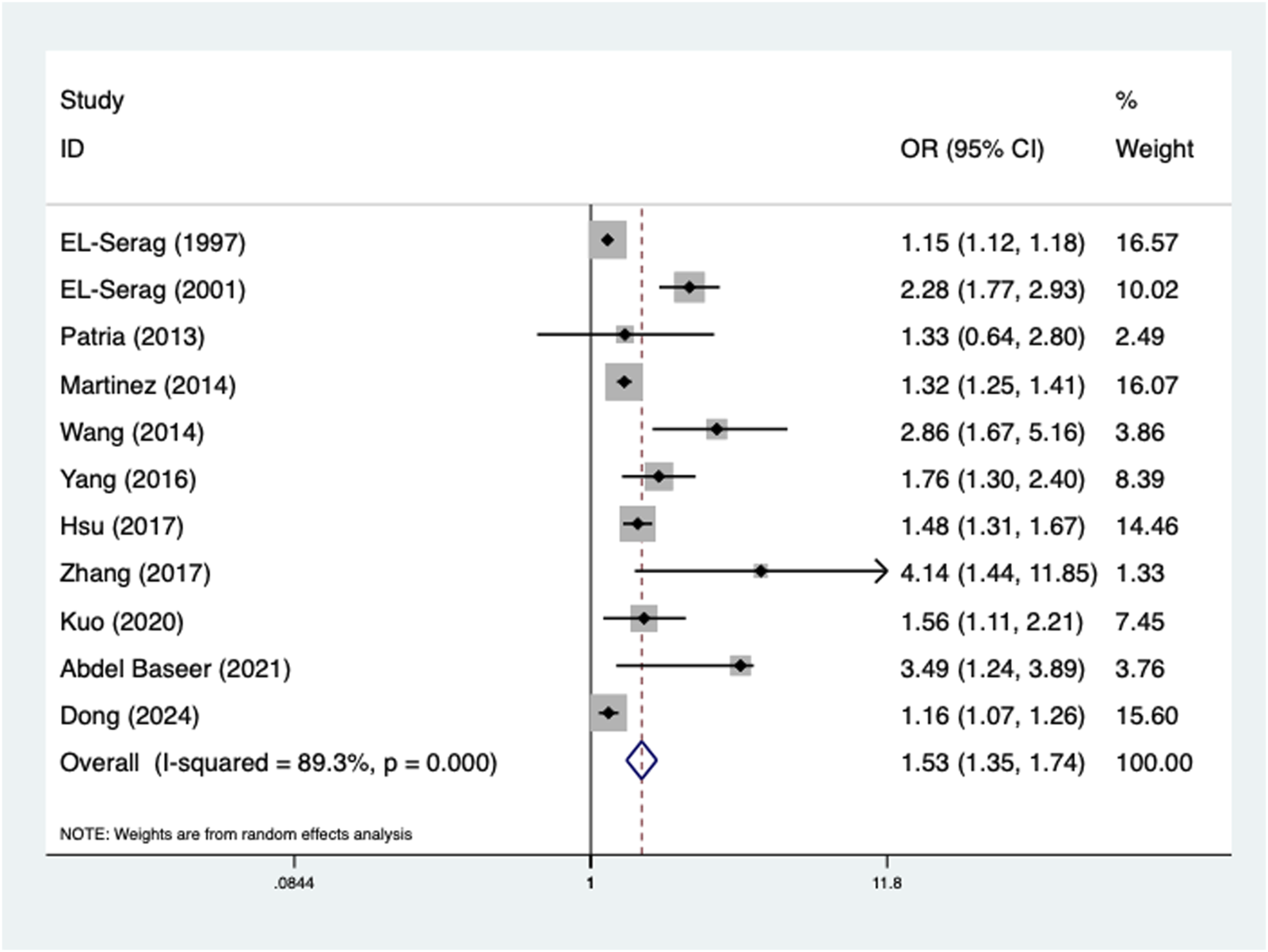
Association of the presence of gastroesophageal reflux disease with the incidence of pneumonia.
Meta-analysis results for secondary outcomes
According to the currently available data, GERD significantly increased the incidence of PF (OR = 1.43, 95% CI: 1.17–1.76, P = 0.001; I2 = 92.6%, P < 0.001), COPD (OR = 1.41, 95% CI: 1.12–1.78, P = 0.004; I2 = 90.6%, P < 0.001), lung cancer (OR = 1.51, 95% CI: 1.33–1.71, P < 0.001; I2 = 28.7%, P = 0.240), ILD (OR = 1.28, 95% CI: 1.05–1.57, P = 0.015; I2 = 94.4%, P < 0.001), bronchiectasis (OR = 1.63, 95% CI: 1.02–2.60, P = 0.039; I2 = 51.5%, P = 0.127), bronchitis (OR = 1.24, 95% CI: 1.16–1.33, P < 0.001; I2 = 64.4%, P = 0.094), ALI (OR = 2.07, 95% CI: 1.37–2.89, P < 0.001), pulmonary embolism (OR = 1.33, 95% CI: 1.12–1.58, P = 0.013), PTB (OR = 1.63, 95% CI: 1.10–2.40, P = 0.015) and NTMPD (OR = 3.36, 95% CI: 2.10–5.37, P < 0.001) (Table 3).
TABLE 3
Results of meta-analysis for secondary outcomes.
OR, odds ratio; CI, confidence interval; GERD, gastroesophageal reflux disease; COPD, chronic obstructive pulmonary disease; PF, pulmonary fibrosis; ALI, acute lung injury; PTB, pulmonary tuberculosis; ILD, interstitial lung disease; NTMPD, non-tuberculous mycobacteria pulmonary disease.
Subgroup analyses stratified by the source of OR for PF (multivariate: OR = 1.38, 95% CI: 1.27–1.50, P < 0.001; univariate: OR = 1.42, 95% CI: 1.03–1.96, P = 0.031) (Supplementary Figure S2A), COPD (multivariate: OR = 1.22, 95% CI: 1.17–1.27, P < 0.001; univariate: OR = 1.66, 95% CI: 1.15–2.40, P = 0.007) (Supplementary Figure S2B), lung cancer (multivariate: OR = 1.70, 95% CI: 1.40–2.05, P < 0.001; univariate: OR = 1.37, 95% CI: 1.16–1.62, P < 0.001) (Supplementary Figure S2C) and ILD (multivariate: OR = 4.96, 95% CI: 2.83–8.69, P < 0.001) (Supplementary Figure S2D) and stratified by age for bronchiectasis (adult: OR = 1.26, 95% CI: 1.08–1.46, P = 0.002; child: OR = 2.30, 95% CI: 1.31–4.05, P = 0.004) (Supplementary Figure S2E) further clarified the associations between the occurrence of GERD and increased incidence of the above pulmonary diseases.
Sensitivity analysis and publication bias
Sensitivity analyses for asthma (Figure 4A) and pneumonia (Figure 4B) indicated that the results were stable and reliable and that none of the included studies significantly affected the pooled results.
FIGURE 4
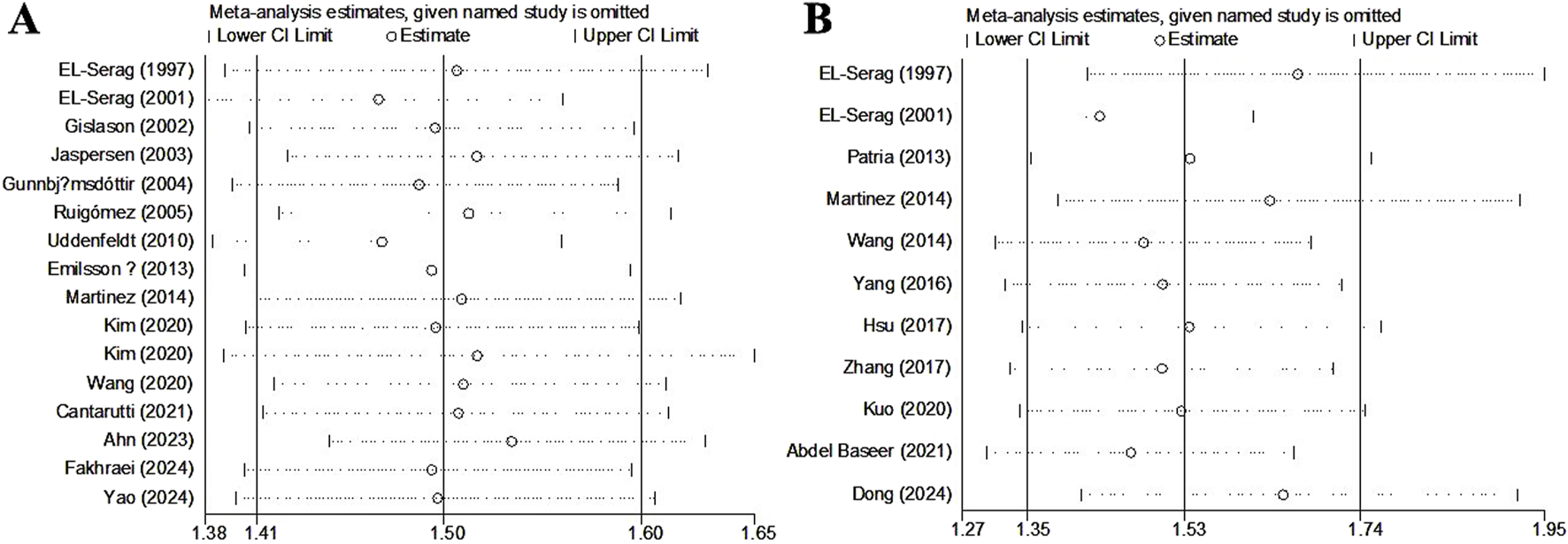
Sensitivity analysis about the association of gastroesophageal reflux disease with the incidence of asthma (A) and pneumonia (B).
According to Begg’s funnel plots and Egger’s test for asthma (Figure 5A, P = 0.462) and pneumonia (Figure 5B, P = 0.002), there was significant publication bias for the association between GERD and the incidence of pneumonia. Therefore, the trim-and-fill method was applied, and two potentially unpublished studies were identified (Supplementary Figure S3); however, these two studies did not affect the overall conclusion (fixed OR = 1.20, 95% CI: 1.17–1.22, P < 0.001; random OR = 1.46, 95% CI: 1.29–1.66, P < 0.001).
FIGURE 5
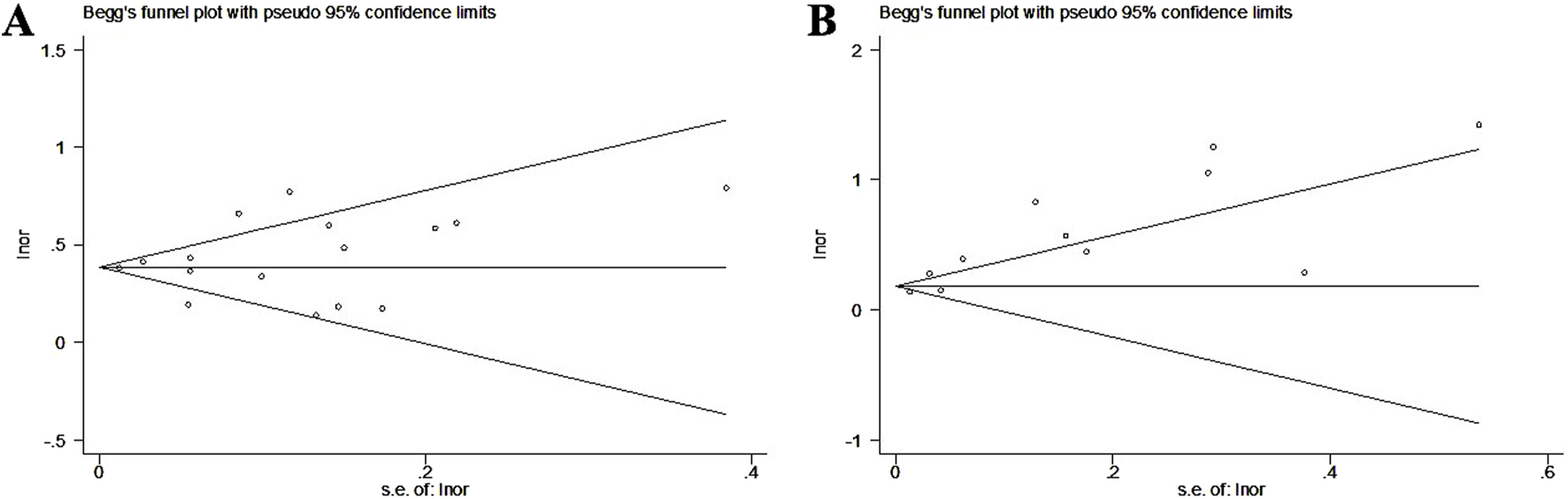
Begg’s funnel plots about the association of gastroesophageal reflux disease with the incidence of asthma (A) and pneumonia (B).
Discussion
Our meta-analysis is the first to comprehensively and systematically explore the impact of GERD on the incidence of pulmonary diseases. On the basis of our pooled results, it is believed that GERD are significantly associated with increased incidence of pulmonary diseases such as asthma, pneumonia, PF and lung cancer. However, standardized treatment might reduce this incidence of pulmonary diseases. The above findings highlight the importance of screening and management for pulmonary diseases and of regular therapy in patients with GERD.
The development of pulmonary diseases related to GERD is associated with various mechanisms. During GERD, stomach acid and gastric contents can reflux into the esophagus and eventually be aspirated into the airways. These small amounts of stomach acid and other gastric contents can irritate the airway mucosa, triggering an inflammatory response that leads to airway hyperreactivity and bronchoconstriction, which induces asthma and COPD (Nemzek and Kim, 2009; Houghton et al., 2016). Chronic microaspiration may also increase the risk of infections (Thomson et al., 2007). Gastric acid reflux stimulates nerve endings in the esophagus and upper airways, activating vagal nerve reflexes that cause airway constriction and inflammation. This reflexive response can lead to asthma symptoms and increase the risk of acute COPD exacerbation (Harding, 2001; Patterson and Harding, 1999). Additionally, the local inflammatory response triggered by stomach acid releases inflammatory mediators such as cytokines and histamine, which can circulate through the bloodstream and affect the lungs, exacerbating airway inflammation, worsening asthma and COPD, and increasing the risk of pulmonary infections (Azer et al., 2024). Long-term acid reflux can cause damage to the mucosa of both the upper and lower airways, weakening local defense mechanisms and increasing the susceptibility of the airways to pathogen infections. This mucosal damage can trigger or worsen pneumonia and may promote the development of pulmonary fibrosis (Houghton et al., 2016). The inflammation caused by GERD may extend downward into the airways, leading to chronic inflammation. Persistent chronic inflammation can not only cause pneumonia but also contribute to the development of lung cancer (Demb et al., 2019).
As mentioned above, the microaspiration of stomach acid and gastric contents into the airways is the core cause of the subsequent development of pulmonary diseases in patients with GERD. Therefore, standardized acid suppression therapy is crucial for reducing the risk of pulmonary diseases in GERD patients. This is consistent with our findings, as patients who complied with regular treatment were not at increased incidence of pulmonary diseases. Furthermore, we believe that improving lifestyle habits, such as dietary adjustments, maintaining proper postmeal positioning, weight management, and avoiding smoking and excessive alcohol consumption, could also help to control the symptoms of GERD, which would contribute to reducing the incidence of subsequent pulmonary diseases (Katz et al., 2022; Katzka and Kahrilas, 2020).
In our meta-analysis, the inclusion criteria were strictly defined and required that GERD be diagnosed prior to pulmonary diseases or similar descriptions in the articles were required to better evaluate the impact of the presence of GERD on the incidence of pulmonary diseases. Some pulmonary diseases can also affect the incidence and exacerbation of GERD. Patients with asthma, COPD and PF are at increased incidence of GERD (Ruigómez et al., 2005; Liang and Feng, 2012; Dziekiewicz et al., 2015; Ruaro et al., 2022). The key causes include the following. Asthma and COPD can affect patients’ breathing, especially during an asthma attack or an acute exacerbation of COPD. Forceful breathing and frequent coughing increase the pressure in the chest and abdomen, which pushes stomach contents back into the esophagus, triggering GERD (Ayazi et al., 2011; Solidoro et al., 2017). Asthmatic patients and COPD patients often experience frequent coughing and an increase in the volume of respiratory secretions. Repeated coughing exerts pressure on the lower esophageal sphincter (LES), which can cause it to relax (Azer et al., 2024). When the LES relaxes, its ability to prevent stomach acid from refluxing weakens, leading to an increased incidence of acid reflux (Azer et al., 2024). Patients with severe pulmonary diseases may have reduced physical activity and are more frequently in a reclining or semireclining position, which increases the risk of acid reflux, especially after eating or during sleep. Some studies suggest that lung diseases can trigger esophageal motility disorders through vagal nerve reflexes. This reflex response can lead to decreased LES function, thereby increasing the risk of acid reflux (Zhang et al., 2021). Therefore, studies in which pulmonary diseases were diagnosed prior to GERD and cross-sectional studies were excluded from our meta-analysis.
Our results suggested that standardized treatment could reduce the impact of GERD on the incidence of pulmonary diseases. However, we believe this pattern may be more applicable to GERD patients who have been certainly diagnosed through gastroscopy, PPI test, or 24-h esophageal pH monitoring rather than the symptoms, as well as patients with risk factors such as the elderly age and obesity for pulmonary diseases. Besides, several studies investigating the associations of the presence of GERD with exacerbdations of asthma, pneumonia and COPD among GERD patients have been conducted, and GERD has been reported to be a risk factor for poor disease control (Ferrera et al., 2024; Gaillet et al., 2015; Hozawa et al., 2022; Baumeler et al., 2016; Bigatao et al., 2018). However, few studies have explored the relationship between the treatment of GERD and pulmonary disease control. There are many GERD patients with pulmonary diseases in the clinic. Therefore, further clarification of the impact of standardized treatment for GERD on the exacerbation of lung diseases is crucial, as this information will help improve the clinical management of these patients.
In several studies, specific populations were involved, such as firefighters and EMS providers at the WTC disaster site (Cleven et al., 2024) and children and young people with cerebral palsy (Kuo et al., 2020), which may affect the generalizability of our conclusions. However, the majority of enrolled patients were from the general population and the inclusion of these studies was intended to capture a broad spectrum of GERD-related impacts across different contexts, thereby enriching our understanding of the association between GERD and development of pulmonary diseases in diverse populations. Substantial heterogeneity was observed in several analyses, which may be related to various factors such as diagnostic criteria, study populations, or designs. However, due to the lack of original data, this could not be confirmed. Therefore, more detailed analyses are needed in future related studies to further determine our findings. Furthermore, significant publication bias about the association between GERD and incidence of pneumonia was detected, which may be related to a bias toward positive results, sample selection bias (i.e., some studies focusing on populations more prone to pneumonia), and the impact of small-sample studies. However, the analysis of trim-and-fill method indicated that the potentially unpublished studies did not affect the results and our conclusion was stable and reliable.
There are several limitations in our meta-analysis. First, most of the included studies were retrospective, and the sample sizes of ten studies were fewer than one thousand, which might cause some bias. Second, in the eighteen included studies, only univariate analyses were performed, and we were unable to conduct subgroup analyses on the basis of other important parameters, such as gastrointestinal comorbidities, the degree of GERD and lifestyle habits, including smoking and alcohol consumption, due to the lack of original data. Therefore, the impacts of these confounding factors on the association of GERD with the incidence of pulmonary diseases need to be investigated. Third, few studies have explored the relationships between the presence of GERD and the incidences of COPD (n = 4), lung cancer (n = 4), ILD (n = 3), bronchiectasis (n = 3), ALI (n = 1), pulmonary embolism (n = 1), PTB (n = 1) and NTMPD (n = 1) and only three studies reported on GERD treatment, which should be evaluated by future studies. Fourth, our results indicate that standardized treatment may eliminate the impact of GERD on the incidence of pulmonary diseases. However, the drugs and treatment periods used were not further reviewed or analyzed. Fifth, not all patients were diagnosed by gastroscopy, PPI test or 24-h esophageal pH monitoring, rather, diagnoses were made based on symptoms, which may lead to a certain degree of bias. Sixth, in a few studies, specific populations were included, such as firefighters and EMS providers at the WTC disaster site (Cleven et al., 2024) and children and young people with cerebral palsy (Kuo et al., 2020), which may affect the generalizability of our conclusions. However, the majority of enrolled patients were from the general population. Seventh, some included studies lacked explicit information on the temporal sequence of GERD diagnosis and pulmonary disease onset. Although we inferred directionality from study design and language, the absence of concrete timing data may introduce uncertainty in causal interpretation. Eighth, furthermore, although many included studies reported GERD diagnosis prior to the diagnosis of pulmonary diseases, this does not guarantee that GERD preceded the actual onset of pulmonary pathology, given the possibility of diagnostic delays, especially in chronic or asymptomatic lung conditions.
Conclusion
Overall, GERD is associated with increased incidence of various pulmonary diseases, but regular treatment may eliminate this effect. These findings indicate the importance of screening and management for pulmonary diseases and standardized therapy in patients with GERD. However, owing to the limitations of our meta-analysis, further prospective high-quality studies are needed to verify the findings, and more detailed investigations should be performed.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
XW: Conceptualization, Formal Analysis, Supervision, Writing – original draft. YW: Conceptualization, Data curation, Software, Visualization, Writing – review and editing. YB: Investigation, Methodology, Software, Validation, Writing – review and editing. YL: Data curation, Formal Analysis, Methodology, Writing – review and editing. SG: Conceptualization, Supervision, Validation, Visualization, Writing – original draft. GC: Data curation, Investigation, Project administration, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Chengdu High-level Key Clinical Specialty Construction Project. This study was sponsored by “Qimingxing” Research Fund for Young Talents of West China Hospital, Sichuan University (ID: HXQMX0071), 1-3-5 Project of Center for High Altitude Medicine, West China Hospital, Sichuan University (ID: GYYX24008), Beijing CSCO Clinical Oncology Research Foundation (ID: Y-2022METAZQN-0116) and Noncommunicable Chronic Diseases—National Science and Technology Major Project (ID: 2023ZD0501706), 1-3-5 Project for Disciplines of Excellence, West China Hospital, Sichuan University, (ID: ZYAI24001 to GC).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fcell.2025.1719856.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abdallah J. George N. Yamasaki T. Ganocy S. Fass R. (2019). Most patients with gastroesophageal reflux disease who failed proton pump inhibitor therapy also have functional esophageal disorders. Clin. Gastroenterol. Hepatol.17 (6), 1073–1080. 10.1016/j.cgh.2018.06.018
2
Abdel Baseer K. A. Sakhr H. (2021). Clinical profile and risk factors of recurrent pneumonia in children at Qena governorate, Egypt. Int. J. Clin. Pract.75 (4), e13695. 10.1111/ijcp.13695
3
Ahn K. Penn R. B. Rattan S. Panettieri R. A. Jr. Voight B. F. (2023). An SS: Menmelian randomization analysis reveals a complex genetic interplay among atopic dermatitis, asthma, and gastroesophageal reflux Disease. Am. J. Respir. Crit. Care Med.207 (2), 130–137. 10.1164/rccm.202205-0951OC
4
Amarnath S. Starr A. Chukkalore D. Elfiky A. Abureesh M. Aqsa A. et al (2022). The association between gastroesophageal reflux disease and non-small cell lung cancer: a retrospective case-control study. Gastroenterol. Res.15 (4), 173–179. 10.14740/gr1537
5
Ayazi S. DeMeester S. R. Hsieh C. C. Zehetner J. Sharma G. Grant K. S. et al (2011). Thoraco-abdominal pressure gradients during the phases of respiration contribute to gastroesophageal reflux disease. Dig. Dis. Sci.56 (6), 1718–1722. 10.1007/s10620-011-1694-y
6
Azer S. A. Hashmi M. F. Reddivari A. K. R. (2024). Gastroesophageal reflux disease (GERD). In: StatPearls. edn. Treasure Island (FL): StatPearls Publishing LLC.
7
Baqir M. Vasirreddy A. Vu A. N. Moua T. Chamberlain A. M. Frank R. D. et al (2021). Idiopathic pulmonary fibrosis and gastroesophageal reflux disease: a population-based, case-control study. Respir. Med.178, 106309. 10.1016/j.rmed.2021.106309
8
Barili F. Parolari A. Kappetein P. A. Freemantle N. (2018). Statistical primer: heterogeneity, random- or fixed-effects model analyses?Interact. Cardiovasc Thorac. Surg.27 (3), 317–321. 10.1093/icvts/ivy163
9
Baumeler L. Papakonstantinou E. Milenkovic B. Lacoma A. Louis R. Aerts J. G. et al (2016). Therapy with proton-pump inhibitors for gastroesophageal reflux disease does not reduce the risk for severe exacerbations in COPD. Respirology21 (5), 883–890. 10.1111/resp.12758
10
Begg C. B. Mazumdar M. (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics50 (4), 1088–1101. 10.2307/2533446
11
Bigatao A. M. Herbella F. A. M. Del Grande L. M. Nascimento O. A. Jardim J. R. Patti M. G. (2018). Chronic obstructive pulmonary disease exacerbations are influenced by gastroesophageal reflux disease. Am. Surg.84 (1), 51–55. 10.1177/000313481808400122
12
Cantarutti A. Amidei C. B. Valsecchi C. Scamarcia A. Corrao G. Gregori D. et al (2021). Association of treated and untreated gastroesophageal reflux disease in the first year of life with the subsequent development of asthma. Int. J. Environ. Res. Public Health18 (18), 9633. 10.3390/ijerph18189633
13
Choi W.-I. Jeong J. Lee C. W. (2019). Association between EGFR mutation and ageing, history of pneumonia and gastroesophageal reflux disease among patients with advanced lung cancer. Eur. J. Cancer122, 101–108. 10.1016/j.ejca.2019.09.010
14
Cleven K. L. Zeig-Owens R. Mueller A. K. Vaeth B. Hall C. B. Choi J. et al (2024). Interstitial lung disease and progressive pulmonary fibrosis: a world trade center cohort 20-Year longitudinal study. Lung202 (3), 257–267. 10.1007/s00408-024-00697-z
15
Cotton C. Alton P. Hughes D. M. Zhao S. S. (2023). Genetic liability to gastro-esophageal reflux disease, obesity, and risk of idiopathic pulmonary fibrosis. Respir. Investig.61 (3), 335–338. 10.1016/j.resinv.2023.02.005
16
Demb J. Wei E. K. Izano M. Kritchevsky S. Swede H. Newman A. B. et al (2019). Chronic inflammation and risk of lung cancer in older adults in the health, aging and body composition cohort study. J. Geriatr. Oncol.10 (2), 265–271. 10.1016/j.jgo.2018.07.008
17
Dong R. Zhang Q. Peng H. (2024). Gastroesophageal reflux disease and the risk of respiratory diseases: a Mendelian randomization study. J. Transl. Med.22 (1), 60. 10.1186/s12967-023-04786-0
18
Duncan D. R. Cohen A. Golden C. Lurie M. Mitchell P. D. Liu E. et al (2023). Gastrointestinal factors associated with risk of bronchiectasis in children. Pediatr. Pulmonol.58 (3), 899–907. 10.1002/ppul.26276
19
Dziekiewicz M. A. Banaszkiewicz A. Urzykowska A. Lisowska A. Rachel M. Sands D. et al (2015). Gastroesophageal reflux disease in children with cystic fibrosis. Adv. Exp. Med. Biol.873, 1–7. 10.1007/5584_2015_154
20
Egger M. Davey Smith G. Schneider M. Minder C. (1997). Bias in meta-analysis detected by a simple, graphical test. Bmj315 (7109), 629–634. 10.1136/bmj.315.7109.629
21
El-Serag H. B. Gilger M. Kuebeler M. Rabeneck L. (2001). Extraesophageal associations of gastroesophageal reflux disease in children without neurologic defects. Gastroenterology121 (6), 1294–1299. 10.1053/gast.2001.29545
22
el-Serag H. B. Sonnenberg A. (1997). Comorbid occurrence of laryngeal or pulmonary disease with esophagitis in United States military veterans. Gastroenterology113 (3), 755–760. 10.1016/s0016-5085(97)70168-9
23
Emilsson Ö. I. Bengtsson A. Franklin K. A. Torén K. Benediktsdóttir B. Farkhooy A. et al (2013). Nocturnal gastro-oesophageal reflux, asthma and symptoms of OSA: a longitudinal, general population study. Eur. Respir. J.41 (6), 1347–1354. 10.1183/09031936.00052512
24
Fakhraei R. M. Lindberg E. Benediktsdottir B. Svanes C. Johannessen A. Holm M. et al (2024). Gastroesophageal reflux and snoring are related to asthma and respiratory symptoms: results from a nordic longitudinal population survey. Respir. Med.221, 107495. 10.1016/j.rmed.2023.107495
25
Fan W. C. Ou S. M. Feng J. Y. Hu Y. W. Yeh C. M. Su V. Y. et al (2016). Increased risk of pulmonary tuberculosis in patients with gastroesophageal reflux disease. Int. J. Tuberc. Lung Dis.20 (2), 265–270. 10.5588/ijtld.15.0251
26
Ferrera M. C. Lopez C. L. Murray S. Jain R. G. Labaki W. W. Make B. J. et al (2024). Risk factors for chronic obstructive pulmonary disease exacerbations among individuals without a history of recent exacerbations: a COPDGene analysis. Ann. Am. Thorac. Soc.21 (3), 421–427. 10.1513/AnnalsATS.202209-751OC
27
Gaillet G. Favelle O. Guilleminault L. de Muret A. Lemarie E. Lecomte T. et al (2015). Gastroesophageal reflux disease is a risk factor for severity of organizing pneumonia. Respiration89 (2), 119–126. 10.1159/000369470
28
Garcia Rodriguez L. A. Ruigomez A. Martin-Merino E. Johansson S. Wallander M.-A. (2008). Relationship between gastroesophageal reflux disease and COPD in UK primary care. Chest134 (6), 1223–1230. 10.1378/chest.08-0902
29
García-Sancho C. Buendía-Roldán I. Fernández-Plata M. R. Navarro C. Pérez-Padilla R. Vargas M. H. et al (2011). Familial pulmonary fibrosis is the strongest risk factor for idiopathic pulmonary fibrosis. Respir. Med.105 (12), 1902–1907. 10.1016/j.rmed.2011.08.022
30
Gislason T. Janson C. Vermeire P. Plaschke P. Björnsson E. Gislason D. et al (2002). Respiratory symptoms and nocturnal gastroesophageal reflux: a population-based study of young adults in three European countries. Chest121 (1), 158–163. 10.1378/chest.121.1.158
31
Gribbin J. Hubbard R. Smith C. (2009). Role of diabetes mellitus and gastro-oesophageal reflux in the aetiology of idiopathic pulmonary fibrosis. Respir. Med.103 (6), 927–931. 10.1016/j.rmed.2008.11.001
32
Gunnbjömsdóttir M. I. Omenaas E. Gíslason T. Norrman E. Olin A. C. Jogi R. et al (2004). Obesity and nocturnal gastro-oesophageal reflux are related to onset of asthma and respiratory symptoms. Eur. Respir. J.24 (1), 116–121. 10.1183/09031936.04.00042603
33
Harding S. M. (2001). Gastroesophageal reflux, asthma, and mechanisms of interaction. Am. J. Med.111 (Suppl. 8A), 8S-12S–12s. 10.1016/s0002-9343(01)00817-8
34
Houghton L. A. Lee A. S. Badri H. DeVault K. R. Smith J. A. (2016). Respiratory disease and the oesophagus: reflux, reflexes and microaspiration. Nat. Rev. Gastroenterol. Hepatol.13 (8), 445–460. 10.1038/nrgastro.2016.91
35
Hozawa S. Maeda S. Kikuchi A. Koinuma M. (2022). Exploratory research on asthma exacerbation risk factors using the Japanese claims database and machine learning: a retrospective cohort study. J. Asthma59 (7), 1328–1337. 10.1080/02770903.2021.1923740
36
Hsu C. K. Lai C. C. Wang K. Chen L. (2016). Risk of lung cancer in patients with gastro-esophageal reflux disease: a population-based cohort study. PeerJ4, e2753. 10.7717/peerj.2753
37
Hsu W. T. Lai C. C. Wang Y. H. Tseng P. H. Wang K. Wang C. Y. et al (2017). Risk of pneumonia in patients with gastroesophageal reflux disease: a population-based cohort study. PLoS One12 (8), e0183808. 10.1371/journal.pone.0183808
38
Jareebi M. A. Gharawi N. F. Shami M. O. Kariri A. M. Hakami T. F. Alamer N. M. et al (2023). Unraveling the complex relationship between gastroesophageal reflux disease, lifestyle factors, and interstitial lung disease: insights from two-sample mendelian randomization analyses. Cureus15 (12), e51220. 10.7759/cureus.51220
39
Jaspersen D. Kulig M. Labenz J. Leodolter A. Lind T. Meyer-Sabellek W. et al (2003). Prevalence of extra-oesophageal manifestations in gastro-oesophageal reflux disease: an analysis based on the ProGERD study. Aliment. Pharmacol. Ther.17 (12), 1515–1520. 10.1046/j.1365-2036.2003.01606.x
40
Jung H. K. (2011). Epidemiology of gastroesophageal reflux disease in Asia: a systematic review. J. Neurogastroenterol. Motil.17 (1), 14–27. 10.5056/jnm.2011.17.1.14
41
Katz P. O. Dunbar K. B. Schnoll-Sussman F. H. Greer K. B. Yadlapati R. Spechler S. J. (2022). ACG clinical guideline for the diagnosis and management of gastroesophageal reflux disease. Am. J. Gastroenterol.117 (1), 27–56. 10.14309/ajg.0000000000001538
42
Katzka D. A. (2014). The complex relationship between eosinophilic esophagitis and gastroesophageal reflux disease. Dig. Dis.32 (1-2), 93–97. 10.1159/000357080
43
Katzka D. A. Kahrilas P. J. (2020). Advances in the diagnosis and management of gastroesophageal reflux disease. Bmj371, m3786. 10.1136/bmj.m3786
44
Kim S. Y. Kim H.-R. Min C. Oh D. J. Park B. Choi H. G. (2020a). Bidirectional association between GERD and asthma in children: two longitudinal follow-up studies using a national sample cohort. Pediatr. Res.88 (2), 320–324. 10.1038/s41390-020-0749-1
45
Kim S. Y. Min C. Oh D. J. Choi H. G. (2020b). Bidirectional association between GERD and asthma: two longitudinal Follow-Up studies using a national sample cohort. J. Allergy Clin. Immunol. Pract.8 (3), 1005–1013. 10.1016/j.jaip.2019.10.043
46
Kim Y. Yoon J. H. Ryu J. Yang B. Chung S. J. Kang H. K. et al (2023). Gastroesophageal reflux disease increases susceptibility to nontuberculous mycobacterial pulmonary disease. Chest163 (2), 270–280. 10.1016/j.chest.2022.08.2228
47
Kuo T. J. Hsu C.-L. Liao P.-H. Huang S.-J. Hung Y.-M. Yin C.-H. (2020). Nomogram for pneumonia prediction among children and young people with cerebral palsy: a population-based cohort study. Plos One15 (7), e0235069. 10.1371/journal.pone.0235069
48
Li L. Ren Q. Zheng Q. Bai Y. He S. Zhang Y. et al (2023b). Causal associations between gastroesophageal reflux disease and lung cancer risk: a Mendelian randomization study. Cancer Med.12 (6), 7552–7559. 10.1002/cam4.5498
49
Li N. Yang W. L. Cai M. H. Chen X. Zhao R. Li M. T. et al (2023a). Burden of gastroesophageal reflux disease in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. BMC Public Health23 (1), 582. 10.1186/s12889-023-15272-z
50
Liang B. M. Feng Y. L. (2012). Association of gastroesophageal reflux disease symptoms with stable chronic obstructive pulmonary disease. Lung190 (3), 277–282. 10.1007/s00408-011-9365-5
51
Liu B. Chen M. You J. Zheng S. Huang M. (2024). The causal relationship between gastroesophageal reflux disease and chronic obstructive pulmonary disease: a bidirectional two-sample Mendelian randomization study. Int. J. Chron. Obstruct Pulmon Dis.19, 87–95. 10.2147/COPD.S437257
52
Liu Z. Gao X. Liang L. Zhou X. Han X. Yang T. et al (2023). Prevalence, general and periodontal risk factors of gastroesophageal reflux disease in China. J. Inflamm. Res.16, 235–244. 10.2147/JIR.S395777
53
Martinez C. H. Okajima Y. Murray S. Washko G. R. Martinez F. J. Silverman E. K. et al (2014). Impact of self-reported gastroesophageal reflux disease in subjects from COPDGene cohort. Respir. Res.15 (1), 62. 10.1186/1465-9921-15-62
54
Nemzek J. A. Kim J. (2009). Pulmonary inflammation and airway hyperresponsiveness in a mouse model of asthma complicated by acid aspiration. Comp. Med.59 (4), 321–330.
55
Patria F. Longhi B. Tagliabue C. Tenconi R. Ballista P. Ricciardi G. et al (2013). Clinical profile of recurrent community-acquired pneumonia in children. BMC Pulm. Med.13, 60. 10.1186/1471-2466-13-60
56
Patterson P. E. Harding S. M. (1999). Gastroesophageal reflux disorders and asthma. Curr. Opin. Pulm. Med.5 (1), 63–67. 10.1097/00063198-199901000-00011
57
Reynolds C. J. Del Greco F. Allen R. J. Flores C. Jenkins R. G. Maher T. M. et al (2023). The causal relationship between gastro-oesophageal reflux disease and idiopathic pulmonary fibrosis: a bidirectional two-sample Mendelian randomisation study. Eur. Respir. J.61 (5), 2201585. 10.1183/13993003.01585-2022
58
Richter J. E. Rubenstein J. H. (2018). Presentation and epidemiology of gastroesophageal reflux disease. Gastroenterology154 (2), 267–276. 10.1053/j.gastro.2017.07.045
59
Ruaro B. Pozzan R. Confalonieri P. Tavano S. Hughes M. Matucci Cerinic M. et al (2022). Gastroesophageal reflux disease in idiopathic pulmonary fibrosis: viewer or actor? To treat or not to treat?Pharm. (Basel)15 (8), 1033. 10.3390/ph15081033
60
Rubenstein J. H. Chen J. W. (2014). Epidemiology of gastroesophageal reflux disease. Gastroenterol. Clin. North Am.43 (1), 1–14. 10.1016/j.gtc.2013.11.006
61
Ruigómez A. García Rodríguez L. A. Wallander M. A. Johansson S. Thomas M. Price D. (2005). Gastroesophageal reflux disease and asthma: a longitudinal study in UK general practice. Chest128 (1), 85–93. 10.1378/chest.128.1.85
62
Sarwar Zubairi A. B. Rabbani U. Hassan M. Fatmi Z. Ahmed N. Ali A. S. et al (2023). Risk factors of idiopathic pulmonary fibrosis in Pakistani population: a matched case-control studyy. J. Pak. Med. Assoc.73 (9), 1782–1787. 10.47391/JPMA.6099
63
Shan X. Ge Y. (2024). Interstitial lung disease in patients with mixed connective tissue disease: a retrospective study. Int. J. General Med.17, 2091–2099. 10.2147/IJGM.S464704
64
Shen Z. Qiu B. Chen L. Zhang Y. (2023). Common gastrointestinal diseases and chronic obstructive pulmonary disease risk: a bidirectional Mendelian randomization analysis. Front. Genet.14, 1256833. 10.3389/fgene.2023.1256833
65
Solidoro P. Patrucco F. Fagoonee S. Pellicano R. (2017). Asthma and gastroesophageal reflux disease: a multidisciplinary point of view. Minerva Med.108 (4), 350–356. 10.23736/S0026-4806.17.05181-3
66
Stang A. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol.25 (9), 603–605. 10.1007/s10654-010-9491-z
67
Thomson R. M. Armstrong J. G. Looke D. F. (2007). Gastroesophageal reflux disease, acid suppression, and Mycobacterium avium complex pulmonary disease. Chest131 (4), 1166–1172. 10.1378/chest.06-1906
68
Uddenfeldt M. Janson C. Lampa E. Leander M. Norback D. Larsson L. et al (2010). High BMI is related to higher incidence of asthma, while a fish and fruit diet is related to a lower-results from a long-term follow-up study of three age groups in Sweden. Respir. Med.104 (7), 972–980. 10.1016/j.rmed.2009.12.013
69
Wang X. Gao Y. Wang Q. Zhang M. Chen J. Zhong L. (2014). Risk factors for aspiration pneumonia in elderly patients and countermeasures. Chin. J. Nosocomiology24 (5), 1161–1162. 10.11816/cn.ni.2014-131697
70
Wang Y. Li J. Chang S. Dong Y. Che G. (2021). Risk and influencing factors for subsequent primary lung cancer after treatment of breast cancer: a systematic review and two meta-analyses based on four million cases. J. Thorac. Oncol.16 (11), 1893–1908. 10.1016/j.jtho.2021.07.001
71
Wang Y. T. Tsai M. C. Wang Y. H. Wei J. C. (2020). Association between proton pump inhibitors and asthma: a population-based cohort study. Front. Pharmacol.11, 607. 10.3389/fphar.2020.00607
72
Wu X. Xiao X. Fang H. He C. Wang H. Wang M. et al (2024). Elucidating shared biomarkers in gastroesophageal reflux disease and idiopathic pulmonary fibrosis: insights into novel therapeutic targets and the role of angelicae sinensis radix. Front. Pharmacol.15, 1348708. 10.3389/fphar.2024.1348708
73
Yang L. Jiang Y. Zhang X. Li Q. Lin L. (2016). Factors analysis and prevention of aspiration pneumonia in elderly patients. Chin. J. Nosocomiology26 (13), 2948–2950. 10.11816/cn.ni.2016-153877
74
Yao P. Liao X. Huang J. Dang Y. Jiang H. (2024). Identifying causal relationships between gastroesophageal reflux and extraesophageal diseases: a Mendelian randomization study. Med. Baltim.103 (7), e37054. 10.1097/MD.0000000000037054
75
Zhang B. Hu Y. Shi X. Li W. Zeng X. Liu F. et al (2021). Integrative effects and vagal mechanisms of transcutaneous electrical acustimulation on gastroesophageal motility in patients with gastroesophageal reflux disease. Am. J. Gastroenterol.116 (7), 1495–1505. 10.14309/ajg.0000000000001203
76
Zhang D. Liu S. Li Z. Wang R. (2022). Global, regional and national burden of gastroesophageal reflux disease, 1990-2019: update from the GBD 2019 study. Ann. Med.54 (1), 1372–1384. 10.1080/07853890.2022.2074535
77
Zhang Y. Zhu P. Wang Y. (2017). Characteristics of pathogenic bacteria infection and related risk factors in elderly patients with aspiration pneumonia. Chin. J. Microecology29 (9), 1069–1072. 10.13381/j.cnki.cjm.201709019
78
Zhong B. Huang G. Li Y. Chen Y. Li Y. (2015). Risk factors of postoperative acute lung injury. J. Clin. Anesth.31 (9), 888–890.
Summary
Keywords
gastroesophageal reflux disease, pulmonary disease, meta-analysis, asthma, pneumonia
Citation
Wang X, Wang Y, Bu Y, Liu Y, Gong S and Che G (2025) Association of gastroesophageal reflux disease with the incidence of pulmonary disease. Front. Cell Dev. Biol. 13:1552126. doi: 10.3389/fcell.2025.1552126
Received
27 December 2024
Accepted
10 July 2025
Published
23 July 2025
Corrected
22 October 2025
Volume
13 - 2025
Edited by
Liang Zhao, Dalian University of Technology, China
Reviewed by
Edward Chan, Rocky Mountain Regional VA Medical Center, United States
Chenhui Yao, The First Affiliated Hospital of Dalian Medical University, China
Updates
Copyright
© 2025 Wang, Wang, Bu, Liu, Gong and Che.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sheng Gong, gongshengxw@163.com; Guowei Che, cheguoweixw@126.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.