Abstract
O-GlcNAcylation is an essential post-translational modification that adds O-linked β-N-acetylglucosamine (O-GlcNAc) to numerous proteins’ serine or threonine residues. Several studies have indicated O-GlcNAcylation regulates various processes related to cancer, including signal transduction, transcription, cell division, metabolism, and cytoskeletal regulation. Programmed cell death (PCD) is a regulated and organized form of cell death controlled by genes, including apoptosis, autophagy, pyroptosis, necroptosis, and ferroptosis. As research on PCD has become increasingly in-depth, a potential link between O-GlcNAcylation and PCD has emerged. This review will focus on the complex relationships between O-GlcNAcylation and different PCD pathways, which are closely tied to the onset, progression, and resistance of cancer. By clarifying the relationship between O-GlcNAcylation and PCD, we aim to create a theoretical basis for improving anti-cancer treatments, with promising potential for clinical application.
Introduction
Programmed cell death (PCD) is a crucial terminal pathway for cells in multicellular organisms. It plays a significant role in various biological events, including morphogenesis, tissue homeostasis, and eliminating harmful cells (Sun and Peng, 2009). PCD dysregulation contributes to the pathogenesis of many diseases including cancer (Pilátová et al., 2023). Certain forms of PCD, such as apoptosis, autophagy-dependent cell death, pyroptosis, ferroptosis, and necroptosis, are closely linked to cancer and various other diseases (Wang et al., 2023). Therefore, understanding the mechanisms that regulate PCD is crucial for developing strategies to manipulate it in the management of cancer.
Protein post-translational modifications (PTMs) refer to the covalent alterations made to proteins through the addition of small functional groups or complex biomolecules to specific amino acid residues (Wa et al., 2005). In recent decades, there has been a growing recognition of the critical roles that PTMs, such as phosphorylation, glycosylation, acetylation, and ubiquitylation, play in regulating various cellular processes. As a result, these modifications have attracted considerable interest in the field of molecular biology (Yang and Qian, 2017). O-GlcNAc protein modification, also known as O-GlcNAcylation, is commonly found in the cytoplasm, nucleus and mitochondria. It was first identified on the surface of mouse lymphocytes by Carmen-Rosa Torres and Gerald Hart in 1983 (Torres and Hart, 1984; Hanover et al., 2010). This modification is a type of post-translational modification that involves glycosylation, where a single GlcNAc molecule is attached to the serine or threonine residues on proteins via an O-linked β-glycosidic bond (Holt et al., 1987). Unlike conventional protein glycosylation, O-GlcNAcylation is a dynamic and reversible process. O-GlcNAcylation is highly responsive to a wide range of extrinsic stimuli, including osmotic, oxidative, hyperthermic, and genotoxic stresses (Yang and Qian, 2017; Chatham et al., 2021). These mechanisms for sensing cellular stress are closely related to PCD (Zuppini et al., 2007; Li et al., 2024). However, the role of O-GlcNAcylation in PCD remains underexplored. This review aims to systematically organize O-GlcNAcylation-regulated PCD mechanism and to propose novel strategies for tumor therapy.
An overview of O-GlcNAcylation
Glycosylation is one of the most common and variable forms of PTMs (Xu et al., 2024). The two primary types of glycosylation are N-glycosylation and O-glycosylation. In N-glycosylation, the core of N-linked glycans is always attached to asparagine residues in the protein backbone. These glycans typically have a common pentasaccharide core consisting of two N-acetylglucosamine (GlcNAc) residues and three mannose residues (Higel et al., 2016). In contrast, O-glycosylation begins with the addition of a single sugar molecule to serine or threonine residues (Calvete et al., 2008). There are several types of protein O-glycosylation. Mucin-type O-glycosylation starts with N-acetylgalactosamine (GalNAc) and forms a diverse glycan chain, which is typically found in mucins and other secreted proteins. O-GlcNAcylation occurs in the cytoplasm and nucleus and plays a role in regulating transcription, metabolism, and the stress response. O-linked fucose (O-fucose) and O-linked glucose (O-Glc) function as parts of the Notch receptor and are involved in regulating the Notch signaling pathway. O-Xylosylation is catalyzed by xylosyltransferase, leading to the formation of a glycosaminoglycan (GAG) chain (Li et al., 2023; Zhang and Ten Hagen, 2019).
O-GlcNAcylation acts as a nutrient sensor through the hexosamine biosynthetic pathway (HBP). This pathway is crucial for sensing metabolic status and regulates the production of uridine diphosphate N-acetylglucosamine (UDP-GlcNAc) (Lam et al., 2021). The HBP utilizes metabolites from several key metabolic pathways, including glucose (derived from carbohydrate metabolism), glutamine (from protein and amino acid metabolism), acetyl-CoA (from lipid and fatty acid metabolism), and uridine triphosphate (UTP) (from nucleic acid and nucleotide metabolism) to produce the uridine diphosphate N-acetylglucosamine (UDP-GlcNAc) (Paneque et al., 2023). This nucleotide sugar is the substrate of protein O-GlcNAcylation (Figure 1) (Tran and Wang, 2019).
FIGURE 1
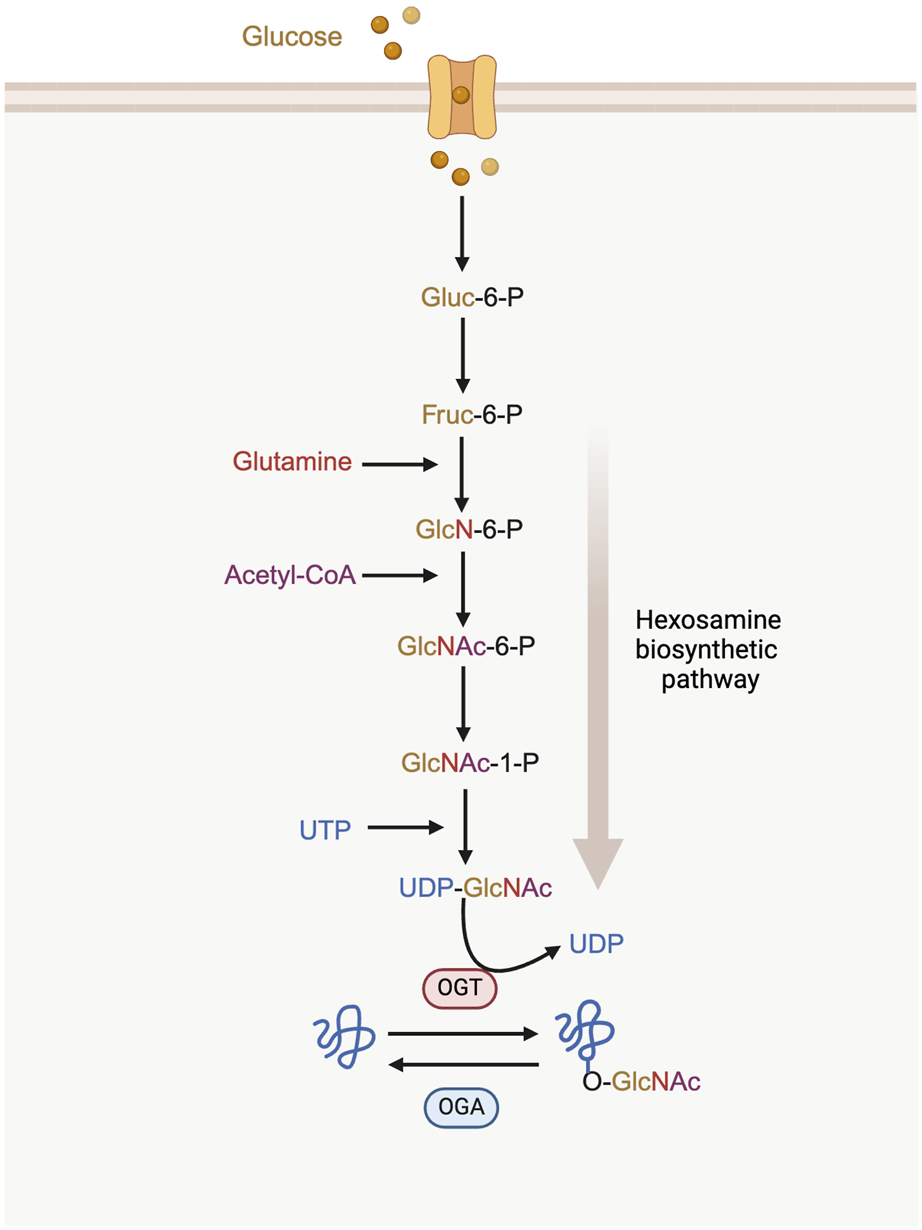
The process of hexosamine biosynthetic pathway (HBP) and protein O-GlcNAcylation. UDP-GlcNAc synthesis requires the incorporation of glucose, glutamine, acetyl-CoA and UTP. O-GlcNAc on proteins is cycled between the addition of OGT and the removal of OGA. Gluc-6-P: glucose-6-phosphatase; Fruc-6-P: fructose-6-phosphate; GlcN-6-P: glucosamine-6-phosphate; GlcNAc-6-P: N-acetylglucosamine-6-phosphate; GlcNAc-1-P: N-acetylglucosamine-1-phosphate.
A single N-acetylglucosamine (GlcNAc) moiety is attached to the hydroxyl oxygen atom of serine or threonine residues on proteins. This modification is catalyzed by O-GlcNAc transferase (OGT), while its removal is carried out by O-GlcNAcase (OGA) (Torres and Hart, 1984).
Synergistic interactions between OGT and OGA are critical for maintaining O-GlcNAcylation levels within an optimum range (Ciraku et al., 2022). Additionally, fluctuations in the availability of UDP-GlcNAc also affect O-GlcNAc levels. When the levels of glucose, glucosamine or free fatty acids increase, or when the key enzymes of the HBP are overexpressed, UDP-GlcNAc levels rise correspondingly. This increase ultimately leads to a higher overall level of O-GlcNAcylation in intracellular proteins (Lazarus et al., 2011; Ong et al., 2018).
Altered O-GlcNAcylation has been observed in cell lines of various cancers (Slawson and Hart, 2011; de Queiroz et al., 2014; Trinca and Hagan, 2018). One potential explanation for this phenomenon is a change in the metabolic state of the cells, shifting from oxidative phosphorylation to aerobic glycolysis, a process known as the Warburg effect (Pavlova and Thompson, 2016). In cancer cells, excess glucose primarily enters glycolysis, which increases the flow toward alternative glucose pathways, such as HBP (Ouyang et al., 2022). In addition, imbalanced enzymatic activity of OGT and OGA due to somatic mutations or altered protein stability (Tang et al., 2023; Peng et al., 2021) is also a contributing factor.
Imbalanced O-GlcNAcylation can crosstalk with other PTMs to promote malignant tumor progression. O-GlcNAcylation interacts extensively with phosphorylation by regulating the phosphorylation of adjacent residues or competing for the same serine or threonine residues (Hart et al., 2011). This interaction has been shown to regulate the activation of AMPK and alter the substrate selectivity of OGT in several cell lines (Bullen et al., 2014), potentially affecting cellular gene expression, cell growth, and apoptotic cell death (Hart et al., 2011). For instance, the O-GlcNAcylation of AMPK reduces levels of phospho-AMPK and its activation, which may subsequently decrease the levels of p21 and p27, both of which are cell cycle inhibitors dependent on AMPK, as well as apoptosis in cervical cancer cells (Kim et al., 2019). In addition to phosphorylation, researchers are exploring the complex interactions between O-GlcNAcylation and other PTMs. OGT-mediated O-GlcNAcylation of YTHDF2 on Ser263 enhances its protein stability and oncogenic activity by preventing its ubiquitination (Yang et al., 2023). O-GlcNAcylation of SIRT1 at the Ser549 site directly enhances the deacetylase activity of SIRT1, protecting cells from stress-induced apoptosis (Han et al., 2017).
OGT and OGA
OGT is encoded by a single gene in Xq13 of the human genome. This genome is spliced and translated into three distinct isoforms: nucleocytoplasmic OGT (ncOGT), mitochondrial OGT (mOGT), and short OGT (sOGT) (Nolte and Müller, 2002). The N-terminus of OGT contains a tetratricopeptide repeat (TPR) domain, which is essential for recognizing and binding to protein substrates (Love et al., 2003). In contrast, the C-terminal domain is responsible for glycosyltransferase activity (Zhang et al., 2022). Opposing OGT is OGA, an enzyme that is predominantly localized in the cytosol, with some presence in the nucleus (Wells et al., 2002). OGA is categorized as a member of CAZY glycoside hydrolase (GH) family 84 (GH84), which includes two major splice isoforms known as long (lOGA) and short (sOGA) (Alteen et al., 2021). The catalytic activity of OGA primarily relies on its N-terminal structural domain (Stephen et al., 2021).
Both OGT and OGA are evolutionarily conserved (Love et al., 2010) and expressed throughout mammalian cells. Their structures have been resolved (Roth et al., 2017; Meek et al., 2021). Early research in mammals has shown that the genetic knockout of OGT leads to embryonic lethality, while the knockout of OGA results in perinatal lethality. This suggests that O-GlcNAcylation is crucial for the development of organisms (Shafi et al., 2000; Yang et al., 2012). Furthermore, dysregulation of OGT and OGA is associated with various pathological conditions (Chatham et al., 2021). Aberrant expression of OGT and OGA are often found in many tumors, suggesting a role in tumor promotion (Liu et al., 2024).
In recent years, the O-GlcNAc modification has emerged as a key regulator of various cellular processes (Martinez et al., 2017; Bacigalupa et al., 2018). However, the potential significance of protein O-GlcNAcylation in mediating both pathological and physiological processes in numerous human diseases—such as cancer, diabetes, neurodegenerative disorders, and cardiovascular diseases—has only recently been reported and remains largely unexplored (Nie and Yi, 2019).
O-GlcNAcylation and programmed cell death
PTMs significantly affect almost all cellular biological processes. The diversity and crosstalk have been linked to PCD in cancer such as apoptosis, autophagy and ferroptosis (Di et al., 2024; Ai et al., 2023; Liu et al., 2022). Research on PTMs has become a vital focus in cancer studies, aiming to enhance our understanding of cancer biology and to identify new biomarkers and therapeutic targets (Pan and Chen, 2022).
Glycosylation is a key mode of PTMs in living organisms, playing a crucial role in regulating PCD by influencing protein folding, transport, and localization. For instance, N-glycosylation in the α-I domain of integrin plays a pivotal role in collagen and laminin binding. Abolished N-glycosylation results in downregulation of focal adhesion signaling and increased cellular apoptosis (Huang et al., 2021). Additionally, inhibiting N-glycosylation of mTRAIL-R leads to increased formation of the death-inducing signaling complex (DISC) and subsequent activation of caspase-8. Blocking the N-glycosylation of 4F2hc could reduce 4F2hc protein stability and sensitize PDAC cells to ferroptosis (Estornes et al., 2018; Ma et al., 2023).
As a dual sensor for nutrient availability and cellular stress, O-GlcNAcylation is highly dynamic (Wells et al., 2002; Vosseller et al., 2001), suggesting that O-GlcNAcylation is closely related to PCD. In this discussion, we will focus on the relationship between five common types of PCD: apoptosis, autophagy, necroptosis, ferroptosis and pyroptosis, and their connection to O-GlcNAcylation (Figure 2). Investigating O-GlcNAcylation may provide new avenues for treating related diseases.
FIGURE 2
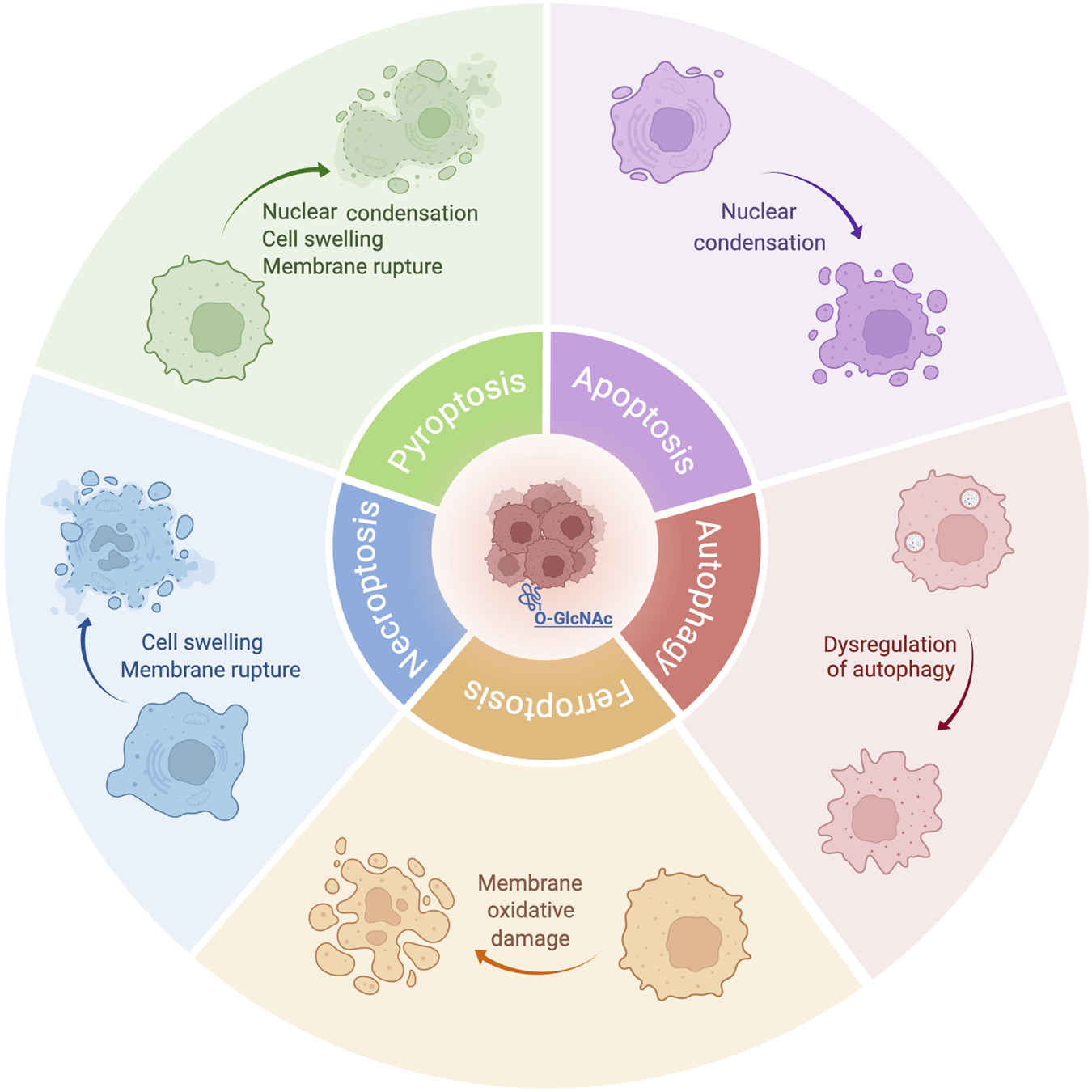
O-GlcNAcylation-mediated five PCDs play a role in cancer. O-GlcNAcylation is an essential post-translational modification that plays a role in cancer by adding O-GlcNAc to numerous proteins’ serine or threonine residues affecting PCD in cancer cells including apoptosis, autophagy, ferroptosis, necroptosis and pyroptosis.
O-GlcNAcylation and apoptosis
Apoptosis is an ordered and orchestrated cellular process that takes place under both normal physiological and pathological conditions (Wong, 2011). This process is genetically controlled and is primarily categorized into three pathways: endogenous pathways (the mitochondrial pathway), exogenous pathways (the death receptor pathway), and pathways induced by endoplasmic reticulum (ER) stress (Hu et al., 2018). Since increased levels of protein O-GlcNAc were reported to reduce cardiomyocyte apoptosis in 2007 (Champattanachai et al., 2007),a growing number of studies have shown that apoptosis is regulated by O-GlcNAcylation. This modification plays a crucial role in the onset and progression of cancer by either promoting or suppressing apoptosis. In neuroblastoma N2a cells, O-GlcNAcylation inhibits oxidative stress-induced apoptosis by modulating the expression and activity of signal transducer and activator of transcription 3 (STAT3) and forkhead box protein O 1 (FOXO1) (Zhang C. C. et al., 2024). In human lung carcinoma, O-GlcNAcylation of p53/c-Myc regulates cisplatin (CDDP)-induced apoptosis in lung cancer cells. Under conditions of high p53 activation, O-GlcNAcylation of p53 promotes its ubiquitin-mediated proteasomal degradation, leading to an increase in oncogenic and anti-apoptotic functions (Luanpitpong et al., 2017). By contrast, O-GlcNAcylation of c-Myc exerts the opposite effect. Additionally, during liver cancer progression, O-GlcNAcylation of β-catenin increases its expression, stability, and nuclear accumulation, promoting liver cancer cell proliferation while inhibiting apoptosis (Table 1) (Gao et al., 2019).
TABLE 1
| Target gene | Type of PCD | Disease models | Biofunction | References |
|---|---|---|---|---|
| STAT3/FOXO1 | apoptosis | Neuroblastoma | O-GlcNAcylation inhibits oxidative stress-induced apoptosis by modulating the expression and activity of STAT3 and FOXO1 | Zhang et al. (2024a) |
| p53/c-Myc | apoptosis | Lung carcinoma | O-GlcNAcylation of p53 leads to an increase in oncogenic and anti-apoptotic functions. O-GlcNAcylation of c-Myc exerts the opposite effect | Luanpitpong et al. (2017) |
| β-catenin | apoptosis | Liver cancer | O-GlcNAcylation of β-catenin increases its expression, stability, and nuclear accumulation, inhibiting liver cancer cell apoptosis | Gao et al. (2019) |
| DR4 | apoptosis | Gastric cancer | O-GlcNAcylation of DR4 enables both apoptotic and necroptotic tumor cell death | Lee et al. (2019) |
| SNAP29 | autophagy | Glioblastoma, pancreatic ductal adenocarcinomas, etc. | Enhanced the O-GlcNAcylation of SNAP29 leads to the blockage of autophagic flux and cell death | Pellegrini et al. (2023) |
| ULK1 | autophagy | Head and neck squamous cell carcinomas | ULK1 O-GlcNAcylation promotes ULK1 stability and autophagosome-lysosome fusion, which could promote HNSCC survival | Shi et al. (2022) |
| AMPK | autophagy | Bladder cancer | O-GlcNAcylation of AMPK suppresses the activity of this regulator, thereby inhibiting autophagic flux | Jin et al. (2020) |
| TFRC | ferroptosis | Hepatocellular carcinoma | O-GlcNAcylation of TFRC at Ser687 reduces the TFRC protein level and decreases the resistance of HCC cells to ferroptosis | Zhou et al. (2024b) |
| TFRC | ferroptosis | Hepatocellular carcinoma | O-GlcNAcylation increases the sensitivity of HCC cells to ferroptosis by enhancing the t the expression of TFRC | Zhu et al. (2021) |
| ZEB1 | ferroptosis | Pancreatic cancers | O-GlcNAc modification of ZEB1 enhances its stabilization and nuclear translocation, thus decreasing ferroptosis | Wang et al. (2022) |
| p53 | pyroptosis | Lung carcinomatous, euroblastoma | De-O-GlcNAcylation of p53 enhances its stability and promotes pyroptosis | Wang et al. (2024) |
O-GlcNAcylation-PCD axis in diverse cancers.
Interestingly, O-GlcNAcylation is regarded as both an apoptotic inhibitor and an activator, highlighting its contradictory role in regulation. For example, tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is acknowledged for its ability to trigger selective apoptosis in tumor cells (Micheau, 2018). The O-GlcNAcylation of human TRAIL receptor with a death domain, TRAIL-R1 (DR4), enables both apoptotic and necroptotic tumor cell death (Lee et al., 2019). Furthermore, AKT is a well-known key regulator of cell death and survival, and O-GlcNAcylation negatively regulates the activation of AKT signaling, ultimately triggering apoptosis (Table 2) (Shi et al., 2015).
TABLE 2
| Related gene | Type of PCD | Disease models | Biofunction | References |
|---|---|---|---|---|
| AKT | apoptosis | Cerebral ischaemia-related diseases | O-GlcNAcylation negatively regulates the activation of AKT signaling, triggering apoptosis | Shi et al. (2015) |
| ULK1 | autophagy | Autophagy-related diseases | ULK1 O-GlcNAcylation leads to the production of PI(3)P, which is necessary for the initiation of autophagy | Pyo et al. (2018) |
| FTH | ferroptosis | Diseases related to iron overload | De-O-GlcNAcylation of FTH promotes its interaction with NCOA4 and activates ferroptosis | Yu et al. (2022) |
| RIPK3 | necroptosis | Septic inflammation | O-GlcNAcylation of the RIPK3 prevents RIPK3-RIPK3 homo-interaction and inhibited necroptosis signaling | Li et al. (2019) |
| RIPK3 | necroptosis | Alzheimer’s disease | By modifying RIPK3, O-GlcNAcylation suppresses the phosphorylation of RIPK3 and the interaction between RIPK1 and RIPK3 | Park et al. (2021) |
| RIPK1 | necroptosis | Erythrocyte necroptosis-related diseases | O-GlcNAcylation of RIPK1 inhibits its phosphorylation at Ser166 and prevents the formation of the RIPK1-RIPK3 complex | Seo et al. (2023) |
| NEK7 | pyroptosis | Osteoarthritis | O-GlcNAcylation of NEK7 induced by OGT enhances chondrocyte pyroptosis through the suppressive interaction between NEK7 and NLRP3 | He et al. (2024) |
| NLRP3 | pyroptosis | Periodontitis | LPS induces pyroptosis in HGFs by increasing OGT expression and promoting the O-GlcNAcylation of NLRP3 | Zhou et al. (2024b), Yang et al. (2024) |
| NLRP3 | pyroptosis | Non-alcoholic fatty liver disease | BPA enhances OGT-mediated O-GlcNAcylation of NLRP3, leads to abnormal lipid accumulation, and induces pyroptosis in HepG2 cells | Zhang et al. (2024b) |
O-GlcNAcylation-PCD axis in no-cancer diseases.
O-GlcNAcylation and autophagy
Autophagy is the process by which cells degrade and recycle proteins and organelles to maintain intracellular homeostasis (Liu et al., 2023). While autophagy is crucial for cellular quality control and survival, dysregulation of autophagy, including a lack of coordination between autophagosome formation and lysosomal degradation, can lead to autophagy-dependent cell death (Nah et al., 2022). Previous studies have highlighted the significance of O-GlcNAcylation in cancer initiation and progression through its regulation of autophagy.
Cytotoxic small molecule (SM15) is a small molecule that acts as a potent autophagy inhibitor. SM15 is demonstrated to enhance the O-GlcNAcylation of SNAP29 and inhibits the formation of the SNARE fusion complex, which leads to the blockage of autophagic flux and ultimately results in cell death (Pellegrini et al., 2023). The unc-51-like-kinase 1 (ULK1) complex is an essential regulator of mammalian autophagy, and its function is largely conserved across all eukaryotes, highlighting its significance (Zachari and Ganley, 2017). The O-GlcNAcylation of ULK1 is essential for its binding to and phosphorylation of ATG14L. This process activates the lipid kinase VPS34, which subsequently leads to the production of phosphatidylinositol-(3)-phosphate (PI(3)P). PI(3)P is necessary for phagophore formation and the initiation of autophagy (Pyo et al., 2018). A similar result indicated that ULK1 O-GlcNAcylation at Ser409 and Ser410 promotes ULK1 stability and autophagosome-lysosome fusion, which could promote HNSCC survival by enhancing autophagy (Shi et al., 2022). In contrast to the findings of the two studies mentioned above, the O-GlcNAcylation of AMP activated protein kinase (AMPK) suppresses autophagic flux by targeting the AMPK-ULK1 pathway in bladder cancer cell lines. This suppression, in turn, promotes the development and progression of bladder cancer (Table 1) (Jin et al., 2020).
Given that Autophagy and O-GlcNAcylation play critical roles in tumors, understanding the specific mechanisms of O-GlcNAcylation in regulating autophagy could provide valuable insights for potential cancer therapies.
O-GlcNAcylation and ferroptosis
Ferroptosis is a non-apoptotic form of regulated cell death characterized by the lethal accumulation of iron-dependent membrane-localized lipid peroxides (Zhou Q. et al., 2024). Since its discovery in 2012, ferroptosis has been viewed as a potential strategy for cancer treatment (Dixon et al., 2012).
Recent studies have revealed a connection between O-GlcNAcylation and ferroptotic cell death. Yu’s group discovered that inhibiting O-GlcNAcylation enhances both ferritinophagy and mitophagy, leading to increased sensitivity to ferroptosis. Specifically, reduced O-GlcNAcylation promotes the interaction between ferritin heavy chain (FTH) and NCOA4, the receptor for ferritinophagy. This interaction facilitates ferritin degradation, leading to the release of labile iron. The released iron accumulates in the mitochondria, which amplifies lipid peroxidation and promotes ferroptosis (Yu et al., 2022). Transferrin receptor (TFRC) is a key protein that facilitates iron import, which promotes ferroptosis by increasing cellular iron uptake (Hong et al., 2021; Tang et al., 2021). The de-O-GlcNAcylation of TFRC at Ser687 has been confirmed to reduce polyubiquitination on Lys665, thus enhancing the TFRC protein stability and increasing the ferroptosis sensitivity of HCC cells (Zhou X. et al., 2024). However, Zhu et al. found that O-GlcNAcylation increased the sensitivity of HCC cells to ferroptosis by significantly enhancing the transcriptional activity of YAP and the expression of TFRC (Zhu et al., 2021). These findings illustrate the different roles of O-GlcNAcylation in regulating iron metabolism mutations in HCC. TGF-ZEB1 pathway has been reported to exhibit increased cancer cells susceptibility to ferroptosis (Viswanathan et al., 2017). In pancreatic cancers, glucose-activated O-GlcNAc modification of ZEB1 at Ser555 enhances its stabilization and nuclear translocation, thus decreasing lipid peroxidation and ferroptosis in mesenchymal pancreatic cancer cells (Table 1) (Wang et al., 2022). Therefore, targeting O-GlcNAcylation to induce ferroptosis could be a potential therapeutic strategy for ferroptosis-based therapy.
O-GlcNAcylation and necroptosis
Necroptosis is the result of mitochondrial changes and plasma membrane permeabilization, leading to the release of cytoplasmic contents into the extracellular space and triggering an inflammatory response (Beretta and Zaffaroni, 2022).
During necroptotic cell death, the formation of receptor-interacting protein kinases 1/3 (RIPK1/3) induces the phosphorylation of pseudo kinase mixed lineage kinase domain-like protein (MLKL), leading to cell membrane destruction (Cho et al., 2009; He et al., 2009; Grootjans et al., 2017). Recently, it was reported that OGT-mediated O-GlcNAcylation to be involved in the necroptosis of inflammatory diseases. O-GlcNAcylation of the RIPK3 at Thr 467 prevented RIPK3-RIPK1 hetero-and RIPK3-RIPK3 homo-interaction and inhibited innate immunity and necroptosis signaling (Li et al., 2019). Wu-Mei-Wan (WMW) is a classic traditional Chinese herbal medicine that has been one of the key formulations for treating digestive diseases from ancient times to the present (Wu et al., 2020). Wu et al. identified 11 manufacturer compounds in WMW using high-performance liquid chromatography (HPLC). They found that hesperidin, coptisine and ginsenoside Rb1 promoted RIPK3 O-GlcNAcylation by increasing OGT levels and decreasing OGA activity. This process inhibits necroptosis and ultimately helps alleviate TNBS-induced colitis (Wu F. et al., 2021). Similar results observed by Park et al. indicated the protective role of O-GlcNAcylation in Alzheimer’s disease (AD). O-GlcNAcylation can inhibit necroptosis by modifying RIPK3, which alleviates AD pathology, including Aβ accumulation, neuronal loss, neuroinflammation, and microglial dysfunction (Park et al., 2021). In addition, O-GlcNAcylation of RIPK1 inhibits its phosphorylation at Ser166 and prevents the formation of the RIPK1-RIPK3 complex, thereby protecting red blood cells (RBCs) from necroptotic cell death (Table 2) (Seo et al., 2023).
While there is no direct evidence that O-GlcNAcylation regulates cancer cells through the mechanisms described above, these studies suggest new directions for targeted cancer therapy.
O-GlcNAcylation and pyroptosis
Pyroptosis is a type of cell death that is dependent on caspases. It involves the formation of pores in the cell membrane, leading to cell swelling, rupture of the plasma membrane, and the release of all intracellular contents (Huang et al., 2022). p53 is a crucial tumor suppressor, and the loss of p53 function often precedes cancer development (Zhang et al., 2020). Wang et al. show that the de-O-GlcNAcylation of p53 enhances its stability. This increased stability leads to the transcriptional upregulation of genes related to the Bcl-2 family and death receptors, promoting pyroptosis in tumor cells (Table 1) (Wang et al., 2024).
While numerous studies have shown the crucial role of pyroptosis and O-GlcNAcylation in various cancers, research on the interplay between the O-GlcNAcylation and pyroptosis axis in cancer remains limited, as most studies concentrate on certain chronic diseases (Table 2). He et al. recently revealed that O-GlcNAcylation of NEK7 induced by OGT promotes the progression of osteoarthritis (OA) by enhancing chondrocyte pyroptosis through the suppressive interaction between NEK7 and NLRP3 (He et al., 2024). Additionally, lipopolysaccharide (LPS) induces pyroptosis in human gingival fibroblasts (HGFs) by increasing OGT expression, which promotes the O-GlcNAcylation of NLRP3. This indicates that O-GlcNAcylation of NLRP3 was a driving factor for periodontitis (Yang et al., 2024). Another study showed that bisphenol A (BPA) enhances OGT-mediated O-GlcNAcylation of NLRP3, leads to abnormal lipid accumulation, and induces pyroptosis in HepG2 cells, thus accelerating the progression of non-alcoholic fatty liver disease (NAFLD) in vitro (Zhang Y. et al., 2024). Thus, studying pyroptosis in other diseases enhances our understanding of the O-GlcNAcylation-pyroptosis axis in cancer.
Potential therapeutic applications of O-GlcNAcylation-modified programmed cell death
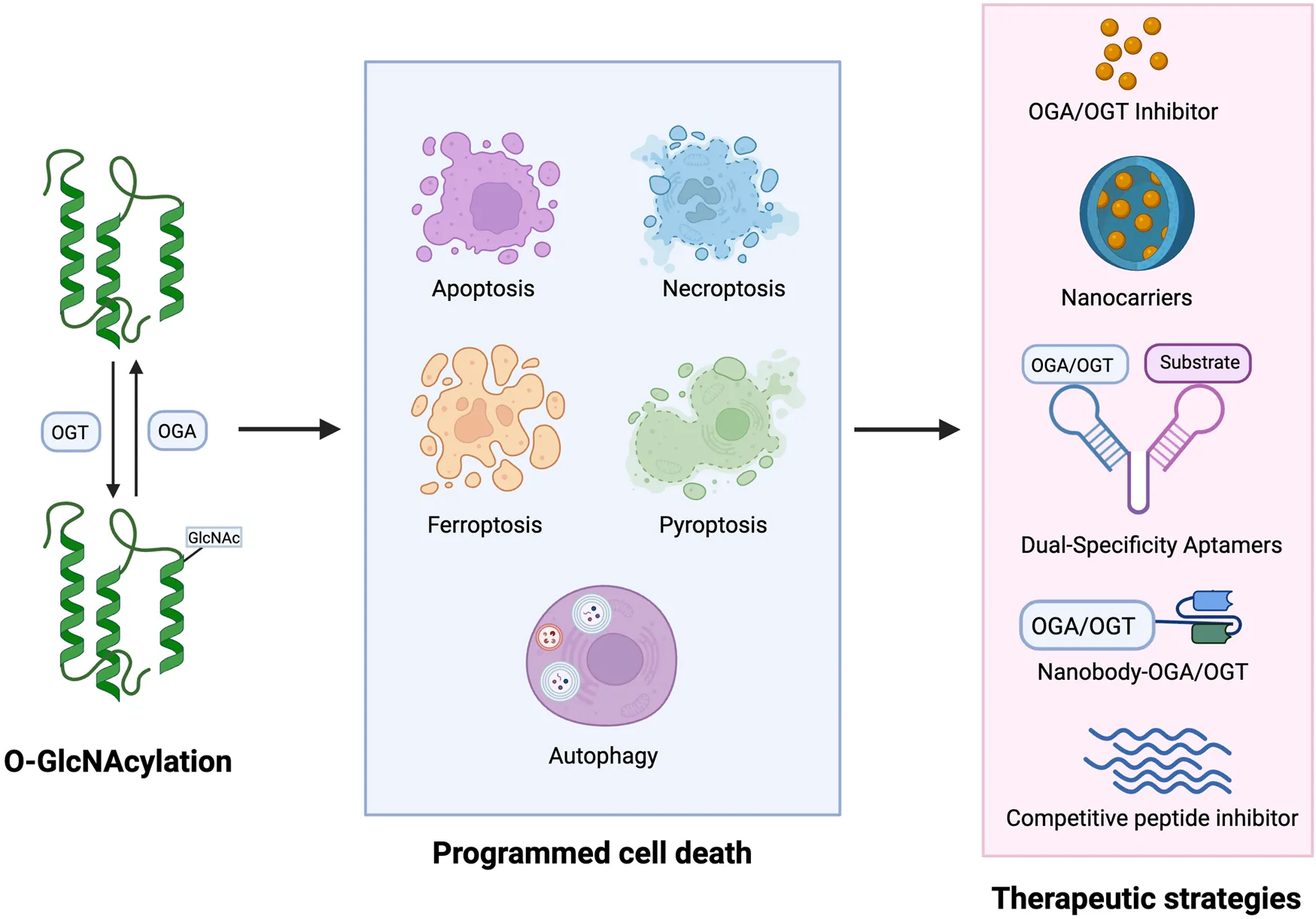
Given the adverse roles of dysregulated O-GlcNAcylation, particularly hyper-O-GlcNAc in cancers (Lu et al., 2022), targeting O-GlcNAcylation to modulate key proteins involved in PCD presents a promising strategy for clinical anti-cancer therapies.
Several small-molecule inhibitors have been developed to either directly inhibit the activity of OGT or OGA, thereby manipulating the O-GlcNAcylation of target proteins (Zhang D. et al., 2024). OSMI-1 is a small molecule that acts as a highly specific inhibitor of OGT from the quinolinone-6-sulfonamide (Q6S) class (Very and El Yazidi-Belkoura, 2022). This compound shows potential for therapy, particularly as it can enhance the effectiveness of certain cancer treatments. Inositol-requiring enzyme 1 α (IRE1α), a sensors for ER stress, plays a role in apoptosis through the IRE1α/JNK pathway. Recent studies have shown that combinatorial treatment of colon cancer cell with metformin and OSMI-1 leads to a more pronounced induction of apoptosis. This enhancement occurs through the activation of the IRE1α/JNK pathway, which is facilitated by a reduction in O-GlcNAcylation (Le et al., 2023). In addition, the combination of TRAIL and OSMI-1 shows promise as a therapeutic strategy for overcoming TRAIL resistance in the treatment of colon cancer (Lee S. J. et al., 2021). TRAIL activates NF-κB signaling for survival and growth, which causes resistance to apoptosis. However, when OSMI-1 is introduced, it decreases the O-GlcNAcylation of IkappaB kinase (IKK), inhibiting IKK activity and the downstream signaling pathway. This results in reduced phosphorylation and nuclear translocation of the NF-κB p65 subunit and enhanced apoptosis in cancer cells (Figure 3). Additionally, the cleavage of Gasdermin-D (GSDMD) is crucial for initiating pyroptosis. Treatment with the OGA inhibitor Thiamet-G, which raises global O-GlcNAc levels, alleviates LPS-induced endothelial injury by inhibiting GSDMD cleavage and decreasing markers of pyroptosis, ultimately improving outcomes in sepsis (Yu et al., 2024).
FIGURE 3
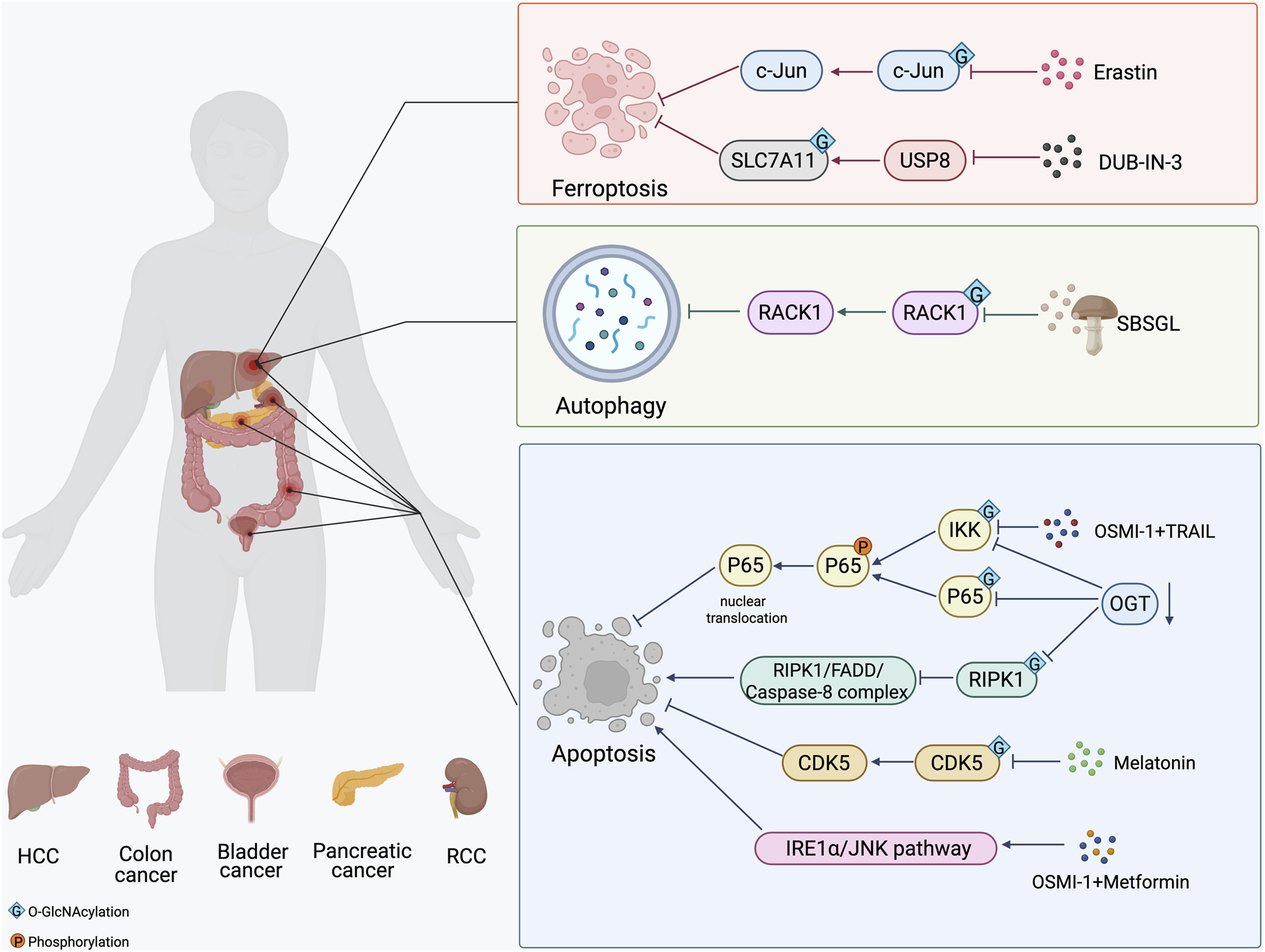
The strategy of O-GlcNAcylation in the treatment of in different cancers. The O-GlcNAcylation of proteins plays a crucial role in cancer development by affecting apoptosis, autophagy and ferroptosis. Small molecule inhibitors of OGT and OGA, several natural and synthetic compounds, or targeting OGT may provide a novel approach to cancer treatment.
In addition to small molecule inhibitors of OGT and OGA, several natural and synthetic compounds could reduce the proliferation of cancer cells by regulating O-GlcNAcylation modification. Melatonin’s biological functions extend beyond the regulation of the circadian rhythm (Moloudizargari et al., 2021). It is crucial for maintaining glucose homeostasis and energy metabolism. Research has shown that melatonin can inhibit the proliferation of bladder cancer cells and promote their apoptosis by suppressing the O-GlcNAcylation of cyclin-dependent-like kinase 5 (CDK5) and reducing the expression level of CDK5 (Wu J. et al., 2021). The mechanism by which melatonin reduced O-GlcNAc levels of CDK5 involved a decrease in the expression of GFAT, the rate-limiting enzyme of the HBP, leading to a significant decrease in UDP-GlcNAc levels following melatonin treatment. In liver cancer, Ferroptosis inducer erastin inhibits O-GlcNAcylation of c-Jun, decreases its protein expression, transcriptional activity, and nuclear accumulation. c-Jun activity reduction promoted ferroptosis and reduced the malignancy of liver cancer cells (Chen et al., 2019). Ganoderma lucidum, a therapeutic fungus, is a significant target for cancer treatments involving abnormal levels of O-GlcNAcylation. Sporoderm-broken spores of G. lucidum (SBSGL), which contain primarily triterpenoids and polysaccharides, have been shown to effectively inhibit hepatoblastoma malignancy and modulate autophagic flux by decreasing O-GlcNAc modifications in the Receptor for activated C kinase 1 (RACK1) protein and its protein levels. This finding suggests that SBSGL could be a promising complement to conventional therapies (Shen et al., 2024). Additionally, DUB-IN-3, the inhibitor of ubiquitin specific peptidase 8 (USP8), shows effective anti-cancer responses. Mechanistic studies reveal that USP8 stabilizes OGT via inhibiting poly-ubiquitination process on OGT protein, thus increasing the Ser26 O-GlcNAcylation of solute carrier family 7, member 11 (SLC7A11) (Figure 3). Thus, pharmacological inhibition of USP8 induces ferroptosis by reducing the stability of OGT and ultimately inhibits the progression of HCC (Tang et al., 2023).
Targeting OGT, OGA, and HBP pathways to modulate PCD may provide a novel approach to cancer treatment. Knocking down OGT has been shown to increase sensitivity to sunitinib in renal cell carcinoma (RCC). Specifically, reduced OGT expression inhibits RIPK1 O-GlcNAcylation and promotes the formation of RIPK1/FADD/Caspase-8 complex, thereby enhancing RIPK1-dependent apoptosis induced by sunitinib (Zeng et al., 2024). In pancreatic cancer, OGT knockdown in PDAC cells leads to a decrease in the O-GlcNAcylation of both IKKα and p65. This reduction is accompanied by lower levels of phosphorylated IKK and p65, decreased nuclear localization of p65, and diminished activation of NF-κB signaling (Ma et al., 2013). Inhibition of NF-κB signaling has been shown to result in PDAC cell apoptosis (Liptay et al., 2003). In addition, the inhibition of OGT, in combination with low-dose chemotherapy, can cause p53-proficient colon cancer cells to switch from senescence to apoptosis (Figure 3). This shift has the potential to enhance the efficacy of chemotherapy for colon cancer while reducing side effects (Loison et al., 2024). Although we have explored some of these important mechanistic details, there is limited research on treating cancer by modulating O-GlcNAcylation. Therefore, integrative mechanisms of O-GlcNAcylation need more studies to identify more targets beneficial to drug research and development.
Conclusion and perspectives
Since the initial discovery of O-GlcNAcylation in 1984 (Torres and Hart, 1984), significant efforts have been made to uncover the functions and roles of this PTM. Recent evidence highlights the essential role of O-GlcNAcylation in the development of various cancers. However, the impact of O-GlcNAcylation on PCD in cancer remains largely unclear.
Developing tools and approaches to deepen our understanding of O-GlcNAcylation as an epigenetic mark is a major challenge in the field (Dupas et al., 2023). For instance, the development of liquid chromatography-mass spectrometry (LC-MS) has enabled accurate and large-scale prediction of O-GlcNAcylation sites in specific proteins (Xu et al., 2021). The advancement of single-cell isolation and analysis can provide more detailed profiling of individual cell-specific responses, ranging from gene expression to proteomics (Lee B. E. et al., 2021). As a result, the field is poised for rapid discoveries that will further elucidate the mechanisms of O-GlcNAcylation in PCD.
There are still many barriers to the clinical use of OGT or OGA inhibitors. Altering global O-GlcNAcylation levels in cells can impact the O-GlcNAcylation of numerous proteins unrelated to the disease, potentially leading to severe side effects or the development of new conditions (Lu et al., 2022). Moreover, these inhibitors often exhibit high toxicity, low efficacy, poor water solubility, and limited cell permeability, making in vivo studies challenging (Yang et al., 2022). Therefore, there is an urgent need for methods that specifically target the modulation of O-GlcNAcylation on PCD proteins for cancer therapy (Zhang D. et al., 2024).
A well-designed nanocarrier can enhance in vivo studies in animal models by improving the solubility and cell permeability of certain compounds (Yang et al., 2022). Ge et al. demonstrated the nanobody-fused split OGA, designed to serve as an O-GlcNAc eraser, successfully deglycosylated a broad range of target proteins. It has high selectivity and little effect on overall O-GlcNAc levels (Chen et al., 2024). Dual-specificity (DS) aptamers are modular RNA molecules designed to connect two aptamer motifs through a linker domain. In cells, they induce proximity between OGT and a specific protein, resulting in increased O-GlcNAcylation of the substrate (Zhu and Hart, 2023). Additionally, a noteworthy strategy involves using short peptides that contain glycosylation sites to competitively inhibit glycosylation in specific proteins (Zhu et al., 2023). This approach opens up future opportunities for the development of targeted drug therapies. While these technologies are relatively new and the pathway to put them into clinical practice is long, O-GlcNAcylation-regulated PCD will provide novel targets for cancer treatment.
Statements
Author contributions
XY: Conceptualization, Writing – original draft. WR: Writing – review and editing, Funding acquisition. ZZ: Conceptualization, Validation, Writing – original draft. SL: Data curation, Validation, Funding acquisition, Writing – review and editing. RS: Validation, Visualization, Writing – review and editing. KS: Software, Supervision, Writing – review and editing. KZ: Conceptualization, Supervision, Funding acquisition, Writing – review and editing. LG: Funding acquisition, Supervision, Writing – review and editing. JZ: Data curation, Supervision, Funding acquisition, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the National Natural Science Foundation of China (No.42176096 [L.G.], 42176097 [K.Q.Z.]), the Natural Science Foundation of Shandong Province (No. ZR2021MD065 [K.Q.Z.], ZR2021MH305 [J.J.Z.], ZR2022MH223 [W.H.R]), the TaiShan Scholars Foundation of Shandong Province (No. tsqn202306397 [L.G.]), and the Shandong Province medical health science and technology development plan project (202208020829 [S.M.L]), Science and Technology Project of Qingdao West Coast New Area (No. 2022-47 [L.G.], 2022-46 [J.J.Z.]).
Acknowledgments
We thank BioRender (https://app.biorender.com/) for creating figures.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fcell.2025.1712372.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
- O-GlcNAc
O-linked β-N-acetylglucosamine
- PCD
programmed cell death
- PTMs
protein post-translational modifications
- HBP
hexosamine biosynthetic pathway
- UDP-GlcNAc
uridine diphosphate N-acetylglucosamine
- UTP
uridine triphosphate
- UDP-GlcNAc
UDP-N-acetylglucosamine
- GlcNAc
N-acetylglucosamine
- OGT
O-GlcNAc transferase
- OGA
O-GlcNAcase
- ncOGT
nucleocytoplasmic OGT
- mOGT
mitochondrial OGT
- sOGT
short OGT
- TPR
tetratricopeptide repeat
- GH84
glycoside hydrolase (GH) family 84
- ER
endoplasmic reticulum
- STAT3
signal transducer and activator of transcription 3
- FOXO1
forkhead box protein O 1
- TRAIL
tumor necrosis factor (TNF)-related apoptosis-inducing ligand
- ULK1
unc-51-like-kinase 1
- PI(3)P
production of phosphatidylinositol-(3)-phosphate
- AMPK
AMP activated protein kinase
- TFRC
transferrin receptor
- HCC
hepatocellular carcinoma
- RIPK1/3
receptor-interacting protein kinases 1/3; mixed lineage kinase domain-like protein
- WMW
Wu-Mei-Wan
- AD
Alzheimer’s disease
- RBCs
red blood cells
- LPS
lipopolysaccharide
- HGFs
human gingival fibroblasts
- BPA
bisphenol A
- NAFLD
non-alcoholic fatty liver disease
- Q6S
quinolinone-6-sulfonamide
- GSDMD
Gasdermin-D
- CDK5
cyclin-dependent-like kinase 5
- SBSGL
sporoderm-broken spores of G. lucidum
- RACK1
receptor for activated C kinase 1
- USP8
ubiquitin specific peptidase 8;
- SLC7A11
solute carrier family 7, member 11
- RCC
renal cell carcinoma
- LC-MS
liquid chromatography-mass spectrometry
- POI
protein of interest
References
1
Ai Y. L. Wang W. J. Liu F. J. Fang W. Chen H. Z. Wu L. Z. et al (2023). Mannose antagonizes GSDME-Mediated pyroptosis through AMPK activated by metabolite GlcNAc-6P. Cell Res.33, 904–922. 10.1038/s41422-023-00848-6
2
Alteen M. G. Tan H. Y. Vocadlo D. J. (2021). Monitoring and modulating O-GlcNAcylation: assays and inhibitors of O-GlcNAc processing enzymes. Curr. Opin. Struct. Biol.68, 157–165. 10.1016/j.sbi.2020.12.008
3
Bacigalupa Z. A. Bhadiadra C. H. Reginato M. J. (2018). O-GlcNAcylation: key regulator of glycolytic pathways. J. Bioenerg. Biomembr.50, 189–198. 10.1007/s10863-018-9742-3
4
Beretta G. L. Zaffaroni N. (2022). Necroptosis and prostate cancer: molecular mechanisms and therapeutic potential. Cells, 11. 10.3390/cells11071221
5
Bullen J. W. Balsbaugh J. L. Chanda D. Shabanowitz J. Hunt D. F. Neumann D. et al (2014). Cross-talk between two essential nutrient-sensitive enzymes: O-GlcNAc transferase (OGT) and AMP-activated protein kinase (AMPK). J. Biol. Chem.289, 10592–10606. 10.1074/jbc.M113.523068
6
Calvete J. J. Sanz L. (2008). Analysis of O-glycosylation. Methods Mol. Biol.446, 281–292. 10.1007/978-1-60327-084-7_20
7
Champattanachai V. Marchase R. B. Chatham J. C. (2007). Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein-associated O-GlcNAc. Am. J. Physiol. Cell Physiol.292, C178–C187. 10.1152/ajpcell.00162.2006
8
Chatham J. C. Zhang J. Wende A. R. (2021). Role of O-Linked N-Acetylglucosamine protein modification in cellular (patho)Physiology. Physiol. Rev.101, 427–493. 10.1152/physrev.00043.2019
9
Chen Y. Zhu G. Liu Y. Wu Q. Zhang X. Bian Z. et al (2019). O-GlcNAcylated c-Jun antagonizes ferroptosis via inhibiting GSH synthesis in liver cancer. Cell Signal63, 109384. 10.1016/j.cellsig.2019.109384
10
Chen L. Hu M. Chen L. Peng Y. Zhang C. Wang X. et al (2024). Targeting O-GlcNAcylation in cancer therapeutic resistance: the sugar Saga continues. Cancer Lett.588, 216742. 10.1016/j.canlet.2024.216742
11
Cho Y. S. Challa S. Moquin D. Genga R. Ray T. D. Guildford M. et al (2009). Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell137, 1112–1123. 10.1016/j.cell.2009.05.037
12
Ciraku L. Esquea E. M. Reginato M. J. (2022). O-GlcNAcylation regulation of cellular signaling in cancer. Cell Signal90, 110201. 10.1016/j.cellsig.2021.110201
13
de Queiroz R. M. Carvalho E. Dias W. B. (2014). O-GlcNAcylation: the sweet side of the cancer. Front. Oncol.4, 132. 10.3389/fonc.2014.00132
14
Di Y. Zhang X. Wen X. Qin J. Ye L. Wang Y. et al (2024). MAPK signaling-mediated RFNG phosphorylation and nuclear translocation restrain oxaliplatin-induced apoptosis and ferroptosis. Adv. Sci. (Weinh)11, e2402795. 10.1002/advs.202402795
15
Dixon S. J. Lemberg K. M. Lamprecht M. R. Skouta R. Zaitsev E. M. Gleason C. E. et al (2012). Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell149, 1060–1072. 10.1016/j.cell.2012.03.042
16
Dupas T. Lauzier B. McGraw S. (2023). O-GlcNAcylation: the sweet side of epigenetics. Epigenetics Chromatin16, 49. 10.1186/s13072-023-00523-5
17
Estornes Y. Dondelinger Y. Weber K. Bruggeman I. Peall A. MacFarlane M. et al (2018). N-glycosylation of mouse TRAIL-R restrains TRAIL-Induced apoptosis. Cell Death Dis.9, 494. 10.1038/s41419-018-0544-7
18
Gao S. Miao Y. Liu Y. Liu X. Fan X. Lin Y. et al (2019). Reciprocal regulation between O-GlcNAcylation and β-Catenin facilitates cell viability and inhibits apoptosis in liver cancer. DNA Cell Biol.38, 286–296. 10.1089/dna.2018.4447
19
Grootjans S. Vanden Berghe T. Vandenabeele P. (2017). Initiation and execution mechanisms of necroptosis: an overview. Cell Death Differ.24, 1184–1195. 10.1038/cdd.2017.65
20
Han C. Gu Y. Shan H. Mi W. Sun J. Shi M. et al (2017). O-GlcNAcylation of SIRT1 enhances its deacetylase activity and promotes cytoprotection under stress. Nat. Commun.8, 1491. 10.1038/s41467-017-01654-6
21
Hanover J. A. Krause M. W. Love D. C. (2010). The hexosamine signaling pathway: O-GlcNAc cycling in feast or famine. Biochim. Biophys. Acta1800, 80–95. 10.1016/j.bbagen.2009.07.017
22
Hart G. W. Slawson C. Ramirez-Correa G. Lagerlof O. (2011). Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu. Rev. Biochem.80, 825–858. 10.1146/annurev-biochem-060608-102511
23
He S. Wang L. Miao L. Wang T. Du F. Zhao L. et al (2009). Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-Alpha. Cell137, 1100–1111. 10.1016/j.cell.2009.05.021
24
He C. Wu Q. Zeng Z. Yang Y. He H. Hu M. et al (2024). OGT-Induced O-GlcNAcylation of NEK7 protein aggravates osteoarthritis progression by enhancing NEK7/NLRP3 axis. Autoimmunity57, 2319202. 10.1080/08916934.2024.2319202
25
Higel F. Seidl A. Sörgel F. Friess W. (2016). N-glycosylation heterogeneity and the influence on structure, function and pharmacokinetics of monoclonal antibodies and Fc fusion proteins. Eur. J. Pharm. Biopharm.100, 94–100. 10.1016/j.ejpb.2016.01.005
26
Holt G. D. Snow C. M. Senior A. Haltiwanger R. S. Gerace L. Hart G. W. (1987). Nuclear pore complex glycoproteins contain cytoplasmically disposed O-linked N-acetylglucosamine. J. Cell Biol.104, 1157–1164. 10.1083/jcb.104.5.1157
27
Hong X. Roh W. Sullivan R. J. Wong K. H. K. Wittner B. S. Guo H. et al (2021). The lipogenic regulator SREBP2 induces transferrin in circulating melanoma cells and suppresses ferroptosis. Cancer Discov.11, 678–695. 10.1158/2159-8290.CD-19-1500
28
Hu H. Tian M. Ding C. Yu S. (2018). The C/EBP homologous protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection. Front. Immunol.9, 3083. 10.3389/fimmu.2018.03083
29
Huang Y. L. Liang C. Y. Labitzky V. Ritz D. Oliveira T. Cumin C. et al (2021). Site-specific N-glycosylation of integrin α2 mediates collagen-dependent cell survival. iScience24, 103168. 10.1016/j.isci.2021.103168
30
Huang C. Li J. Zhang C. (2022). What role does pyroptosis play in cancer?Mol. Metab.65, 101587. 10.1016/j.molmet.2022.101587
31
Jin L. Yuan F. Dai G. Yao Q. Xiang H. Wang L. et al (2020). Blockage of O-linked GlcNAcylation induces AMPK-Dependent autophagy in bladder cancer cells. Cell Mol. Biol. Lett.25, 17. 10.1186/s11658-020-00208-x
32
Kim M. Y. Kim Y. S. Kim M. Choi M. Y. Roh G. S. Lee D. H. et al (2019). Metformin inhibits cervical cancer cell proliferation via decreased AMPK O-GlcNAcylation. Anim. Cells Syst. Seoul.23, 302–309. 10.1080/19768354.2019.1614092
33
Lam C. Low J. Y. Tran P. T. Wang H. (2021). The hexosamine biosynthetic pathway and cancer: current knowledge and future therapeutic strategies. Cancer Lett.503, 11–18. 10.1016/j.canlet.2021.01.010
34
Lazarus M. B. Nam Y. Jiang J. Sliz P. Walker S. (2011). Structure of human O-GlcNAc transferase and its complex with a peptide substrate. Nature469, 564–567. 10.1038/nature09638
35
Lee D. E. Lee G. Y. Lee H. M. Choi S. Y. Lee S. J. Kwon O. S. (2023). Synergistic apoptosis by combination of metformin and an O-GlcNAcylation inhibitor in Colon cancer cells. Cancer Cell Int.23, 108. 10.1186/s12935-023-02954-2
36
Lee H. Oh Y. Jeon Y. J. Lee S. Y. Kim H. Lee H. J. et al (2019). DR4-Ser424 O-GlcNAcylation promotes sensitization of TRAIL-tolerant persisters and TRAIL-resistant cancer cells to death. Cancer Res.79, 2839–2852. 10.1158/0008-5472.CAN-18-1991
37
Lee S. J. Lee D. E. Choi S. Y. Kwon O. S. (2021a). OSMI-1 enhances TRAIL-induced apoptosis through ER stress and NF-κB signaling in Colon cancer cells. Int. J. Mol. Sci.22, 11073. 10.3390/ijms222011073
38
Lee B. E. Suh P. G. Kim J. I. (2021b). O-GlcNAcylation in health and neurodegenerative diseases. Exp. Mol. Med.53, 1674–1682. 10.1038/s12276-021-00709-5
39
Li X. Gong W. Wang H. Li T. Attri K. S. Lewis R. E. et al (2019). O-GlcNAc transferase suppresses inflammation and necroptosis by targeting receptor-interacting Serine/Threonine-Protein kinase 3. Immunity50, 1115–590.e6. 10.1016/j.immuni.2019.03.008
40
Li X. Pinou L. Du Y. Chen X. Liu C. (2023). Emerging roles of O-glycosylation in regulating protein aggregation, phase separation, and functions. Curr. Opin. Chem. Biol.75, 102314. 10.1016/j.cbpa.2023.102314
41
Li Q. Yang L. Zhang C. Yuan J. Zhang J. Tao W. et al (2024). METTL16 deficiency attenuates apoptosis through translational control of extrinsic death receptor during nutrient deprivation. Biochem. Biophys. Res. Commun.708, 149802. 10.1016/j.bbrc.2024.149802
42
Liptay S. Weber C. K. Ludwig L. Wagner M. Adler G. Schmid R. M. (2003). Mitogenic and antiapoptotic role of constitutive NF-kappaB/Rel activity in pancreatic cancer. Int. J. Cancer105, 735–746. 10.1002/ijc.11081
43
Liu L. Li H. Hu D. Wang Y. Shao W. Zhong J. et al (2022). Insights into N6-methyladenosine and programmed cell death in cancer. Mol. Cancer21, 32. 10.1186/s12943-022-01508-w
44
Liu S. Yao S. Yang H. Liu S. Wang Y. (2023). Autophagy: regulator of cell death. Cell Death Dis.14, 648. 10.1038/s41419-023-06154-8
45
Liu X. Wang J. Xiang Y. Wang K. Yan D. Tong Y. (2024). The roles of OGT and its mechanisms in cancer. Cell Biosci.14, 121. 10.1186/s13578-024-01301-w
46
Loison I. Pioger A. Paget S. Metatla I. Vincent A. Abbadie C. et al (2024). O-GlcNAcylation inhibition redirects the response of Colon cancer cells to chemotherapy from senescence to apoptosis. Cell Death Dis.15, 762. 10.1038/s41419-024-07131-5
47
Love D. C. Kochan J. Cathey R. L. Shin S. H. Hanover J. A. Kochran J. (2003). Mitochondrial and nucleocytoplasmic targeting of O-linked GlcNAc transferase. J. Cell Sci.116, 647–654. 10.1242/jcs.00246
48
Love D. C. Krause M. W. Hanover J. A. (2010). O-GlcNAc cycling: emerging roles in development and epigenetics. Semin. Cell Dev. Biol.21, 646–654. 10.1016/j.semcdb.2010.05.001
49
Lu Q. Zhang X. Liang T. Bai X. (2022). O-GlcNAcylation: an important post-translational modification and a potential therapeutic target for cancer therapy. Mol. Med.28, 115. 10.1186/s10020-022-00544-y
50
Luanpitpong S. Angsutararux P. Samart P. Chanthra N. Chanvorachote P. Issaragrisil S. (2017). Hyper-O-GlcNAcylation induces cisplatin resistance via regulation of p53 and c-Myc in human lung carcinoma. Sci. Rep.7, 10607. 10.1038/s41598-017-10886-x
51
Ma Z. Vocadlo D. J. Vosseller K. (2013). Hyper-O-GlcNAcylation is anti-apoptotic and maintains constitutive NF-κB activity in pancreatic cancer cells. J. Biol. Chem.288, 15121–15130. 10.1074/jbc.M113.470047
52
Ma H. Chen X. Mo S. Zhang Y. Mao X. Chen J. et al (2023). Targeting N-glycosylation of 4F2hc mediated by glycosyltransferase B3GNT3 sensitizes ferroptosis of pancreatic ductal adenocarcinoma. Cell Death Differ.30, 1988–2004. 10.1038/s41418-023-01188-z
53
Martinez M. R. Dias T. B. Natov P. S. Zachara N. E. (2017). Stress-induced O-GlcNAcylation: an adaptive process of injured cells. Biochem. Soc. Trans.45, 237–249. 10.1042/BST20160153
54
Meek R. W. Blaza J. N. Busmann J. A. Alteen M. G. Vocadlo D. J. Davies G. J. (2021). Cryo-EM structure provides insights into the dimer arrangement of the O-linked β-N-acetylglucosamine transferase OGT. Nat. Commun.12, 6508. 10.1038/s41467-021-26796-6
55
Micheau O. (2018). Regulation of TNF-related apoptosis-inducing ligand signaling by glycosylation. Int. J. Mol. Sci.19, 715. 10.3390/ijms19030715
56
Moloudizargari M. Moradkhani F. Hekmatirad S. Fallah M. Asghari M. H. Reiter R. J. (2021). Therapeutic targets of cancer drugs: modulation by melatonin. Life Sci.267, 118934. 10.1016/j.lfs.2020.118934
57
Nah J. Zablocki D. Sadoshima J. (2022). The role of autophagic cell death in cardiac disease. J. Mol. Cell Cardiol.173, 16–24. 10.1016/j.yjmcc.2022.08.362
58
Nie H. Yi W. (2019). O-GlcNAcylation, a sweet link to the pathology of diseases. J. Zhejiang Univ. Sci. B20, 437–448. 10.1631/jzus.B1900150
59
Nolte D. Müller U. (2002). Human O-GlcNAc transferase (OGT): genomic structure, analysis of splice variants, fine mapping in Xq13.1. Mamm. Genome13, 62–64. 10.1007/s00335-001-2108-9
60
Ong Q. Han W. Yang X. (2018). O-GlcNAc as an integrator of signaling pathways. Front. Endocrinol. (Lausanne)9, 599. 10.3389/fendo.2018.00599
61
Ouyang M. Yu C. Deng X. Zhang Y. Zhang X. Duan F. (2022). O-GlcNAcylation and its role in cancer-associated inflammation. Front. Immunol.13, 861559. 10.3389/fimmu.2022.861559
62
Pan S. Chen R. (2022). Pathological implication of protein post-translational modifications in cancer. Mol. Asp. Med.86, 101097. 10.1016/j.mam.2022.101097
63
Paneque A. Fortus H. Zheng J. Werlen G. Jacinto E. (2023). The hexosamine biosynthesis pathway: regulation and function. Genes (Basel)14, 933. 10.3390/genes14040933
64
Park J. Ha H. J. Chung E. S. Baek S. H. Cho Y. Kim H. K. et al (2021). O-GlcNAcylation ameliorates the pathological manifestations of Alzheimer's disease by inhibiting necroptosis. Sci. Adv.7, eabd3207. 10.1126/sciadv.abd3207
65
Pavlova N. N. Thompson C. B. (2016). The emerging hallmarks of cancer metabolism. Cell Metab.23, 27–47. 10.1016/j.cmet.2015.12.006
66
Pellegrini F. R. De Martino S. Fianco G. Ventura I. Valente D. Fiore M. et al (2023). Blockage of autophagosome-lysosome fusion through SNAP29 O-GlcNAcylation promotes apoptosis via ROS production. Autophagy19, 2078–2093. 10.1080/15548627.2023.2170962
67
Peng K. Liu R. Jia C. Wang Y. Jeong G. H. Zhou L. et al (2021). Regulation of O-Linked N-Acetyl glucosamine transferase (OGT) through E6 stimulation of the ubiquitin ligase activity of E6AP. Int. J. Mol. Sci.22, 10286. 10.3390/ijms221910286
68
Pilátová M. B. Solárová Z. Mezencev R. Solár P. (2023). Ceramides and their roles in programmed cell death. Adv. Med. Sci.68, 417–425. 10.1016/j.advms.2023.10.004
69
Pyo K. E. Kim C. R. Lee M. Kim J. S. Kim K. I. Baek S. H. (2018). ULK1 O-GlcNAcylation is crucial for activating VPS34 via ATG14L during autophagy initiation. Cell Rep.25, 2878–2890. 10.1016/j.celrep.2018.11.042
70
Roth C. Chan S. Offen W. A. Hemsworth G. R. Willems L. I. King D. T. et al (2017). Structural and functional insight into human O-GlcNAcase. Nat. Chem. Biol.13, 610–612. 10.1038/nchembio.2358
71
Seo J. Kim Y. Ji S. Kim H. B. Jung H. Yi E. C. et al (2023). O-GlcNAcylation of RIPK1 rescues red blood cells from necroptosis. Front. Immunol.14, 1160490. 10.3389/fimmu.2023.1160490
72
Shafi R. Iyer S. P. Ellies L. G. O'Donnell N. Marek K. W. Chui D. et al (2000). The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc. Natl. Acad. Sci. U. S. A.97, 5735–5739. 10.1073/pnas.100471497
73
Shen R. Ge Y. Qin Y. Gao H. Yu H. Wu H. et al (2024). Sporoderm-broken spores of Ganoderma lucidum modulate hepatoblastoma malignancy by regulating RACK1-mediated autophagy and tumour immunity. J. Cell Mol. Med.28, e18223. 10.1111/jcmm.18223
74
Shi J. Gu J. H. Dai C. L. Gu J. Jin X. Sun J. et al (2015). O-GlcNAcylation regulates ischemia-induced neuronal apoptosis through AKT signaling. Sci. Rep.5, 14500. 10.1038/srep14500
75
Shi Y. Yan S. Shao G. C. Wang J. Jian Y. P. Liu B. et al (2022). O-GlcNAcylation stabilizes the autophagy-initiating kinase ULK1 by inhibiting chaperone-mediated autophagy upon HPV infection. J. Biol. Chem.298, 102341. 10.1016/j.jbc.2022.102341
76
Slawson C. Hart G. W. (2011). O-GlcNAc signalling: implications for cancer cell biology. Nat. Rev. Cancer11, 678–684. 10.1038/nrc3114
77
Stephen H. M. Adams T. M. Wells L. (2021). Regulating the regulators: mechanisms of substrate selection of the O-GlcNAc cycling enzymes OGT and OGA. Glycobiology31, 724–733. 10.1093/glycob/cwab005
78
Sun Y. Peng Z. L. (2009). Programmed cell death and cancer. Postgrad. Med. J.85, 134–140. 10.1136/pgmj.2008.072629
79
Tang L. J. Zhou Y. J. Xiong X. M. Li N. S. Zhang J. J. Luo X. J. et al (2021). Ubiquitin-specific protease 7 promotes ferroptosis via activation of the p53/TfR1 pathway in the rat hearts after ischemia/reperfusion. Free Radic. Biol. Med.162, 339–352. 10.1016/j.freeradbiomed.2020.10.307
80
Tang J. Long G. Hu K. Xiao D. Liu S. Xiao L. et al (2023). Targeting USP8 inhibits O-GlcNAcylation of SLC7A11 to promote ferroptosis of hepatocellular carcinoma via stabilization of OGT. Adv. Sci. (Weinh)10, e2302953. 10.1002/advs.202302953
81
Torres C. R. Hart G. W. (1984). Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J. Biol. Chem.259, 3308–3317. 10.1016/s0021-9258(17)43295-9
82
Tran D. H. Wang Z. V. (2019). Glucose metabolism in cardiac hypertrophy and heart failure. J. Am. Heart Assoc.8, e012673. 10.1161/JAHA.119.012673
83
Trinca G. M. Hagan C. R. (2018). O-GlcNAcylation in women's cancers: breast, endometrial and ovarian. J. Bioenerg. Biomembr.50, 199–204. 10.1007/s10863-017-9730-z
84
Very N. El Yazidi-Belkoura I. (2022). Targeting O-GlcNAcylation to overcome resistance to anti-cancer therapies. Front. Oncol.12, 960312. 10.3389/fonc.2022.960312
85
Viswanathan V. S. Ryan M. J. Dhruv H. D. Gill S. Eichhoff O. M. Seashore-Ludlow B. et al (2017). Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature547, 453–457. 10.1038/nature23007
86
Vosseller K. Wells L. Hart G. W. (2001). Nucleocytoplasmic O-glycosylation: O-GlcNAc and functional proteomics. Biochimie83, 575–581. 10.1016/s0300-9084(01)01295-0
87
Walsh C. T. Garneau-Tsodikova S. Gatto G. J. Jr. (2005). Protein posttranslational modifications: the chemistry of proteome diversifications. Angew. Chem. Int. Ed. Engl.44, 7342–7372. 10.1002/anie.200501023
88
Wang X. Liu M. Chu Y. Liu Y. Cao X. Zhang H. et al (2022). O-GlcNAcylation of ZEB1 facilitated mesenchymal pancreatic cancer cell ferroptosis. Int. J. Biol. Sci.18, 4135–4150. 10.7150/ijbs.71520
89
Wang M. Yu F. Zhang Y. Li P. (2023). Programmed cell death in tumor immunity: mechanistic insights and clinical implications. Front. Immunol.14, 1309635. 10.3389/fimmu.2023.1309635
90
Wang J. Wang Y. Xiao H. Yang W. Zuo W. You Z. et al (2024). Dynamic O-GlcNAcylation coordinates etoposide-triggered tumor cell pyroptosis by regulating p53 stability. J. Biol. Chem.301, 108050. 10.1016/j.jbc.2024.108050
91
Wells L. Gao Y. Mahoney J. A. Vosseller K. Chen C. Rosen A. et al (2002). Dynamic O-glycosylation of nuclear and cytosolic proteins: further characterization of the nucleocytoplasmic beta-N-acetylglucosaminidase, O-GlcNAcase. J. Biol. Chem.277, 1755–1761. 10.1074/jbc.m109656200
92
Wong R. S. (2011). Apoptosis in cancer: from pathogenesis to treatment. J. Exp. Clin. Cancer Res.30, 87. 10.1186/1756-9966-30-87
93
Wu F. Shao Q. Hu M. Zhao Y. Dong R. Fang K. et al (2020). Wu-mei-wan ameliorates chronic colitis-associated intestinal fibrosis through inhibiting fibroblast activation. J. Ethnopharmacol.252, 112580. 10.1016/j.jep.2020.112580
94
Wu F. Shao Q. Cheng Z. Xiong X. Fang K. Zhao Y. et al (2021a). Traditional herbal formula Wu-Mei-Wan alleviates TNBS-Induced colitis in mice by inhibiting necroptosis through increasing RIPK3 O-GlcNAcylation. Chin. Med.16, 78. 10.1186/s13020-021-00493-4
95
Wu J. Tan Z. Li H. Lin M. Jiang Y. Liang L. et al (2021b). Melatonin reduces proliferation and promotes apoptosis of bladder cancer cells by suppressing O-GlcNAcylation of cyclin-dependent-like kinase 5. J. Pineal Res.71, e12765. 10.1111/jpi.12765
96
Xu S. Sun F. Tong M. Wu R. (2021). MS-based proteomics for comprehensive investigation of protein O-GlcNAcylation. Mol. Omics17, 186–196. 10.1039/d1mo00025j
97
Xu X. Peng Q. Jiang X. Tan S. Yang W. Han Y. et al (2024). Altered glycosylation in cancer: molecular functions and therapeutic potential. Cancer Commun. (Lond)44, 1316–1336. 10.1002/cac2.12610
98
Yang X. Qian K. (2017). Protein O-GlcNAcylation: emerging mechanisms and functions. Nat. Rev. Mol. Cell Biol.18, 452–465. 10.1038/nrm.2017.22
99
Yang Y. R. Song M. Lee H. Jeon Y. Choi E. J. Jang H. J. et al (2012). O-GlcNAcase is essential for embryonic development and maintenance of genomic stability. Aging Cell11, 439–448. 10.1111/j.1474-9726.2012.00801.x
100
Yang R. Wang L. Wu Z. Yin Y. Jiang S. W. (2022). How nanotechniques Could vitalize the O-GlcNAcylation-Targeting approach for cancer therapy. Int. J. Nanomedicine17, 1829–1841. 10.2147/ijn.s360488
101
Yang Y. Yan Y. Yin J. Tang N. Wang K. Huang L. et al (2023). O-GlcNAcylation of YTHDF2 promotes HBV-related hepatocellular carcinoma progression in an N(6)-methyladenosine-dependent manner. Signal Transduct. Target Ther.8, 63. 10.1038/s41392-023-01316-8
102
Yang H. Xiao L. Wu D. Zhang T. Ge P. (2024). O-GlcNAcylation of NLRP3 contributes to lipopolysaccharide-induced pyroptosis of human gingival fibroblasts. Mol. Biotechnol.66, 2023–2031. 10.1007/s12033-023-00846-4
103
Yu F. Zhang Q. Liu H. Liu J. Yang S. Luo X. et al (2022). Dynamic O-GlcNAcylation coordinates ferritinophagy and mitophagy to activate ferroptosis. Cell Discov.8, 40. 10.1038/s41421-022-00390-6
104
Yu F. Zhang Z. Leng Y. Chen A. F. (2024). O-GlcNAc modification of GSDMD attenuates LPS-induced endothelial cells pyroptosis. Inflamm. Res.73, 5–17. 10.1007/s00011-023-01812-1
105
Zachari M. Ganley I. G. (2017). The mammalian ULK1 complex and autophagy initiation. Essays Biochem.61, 585–596. 10.1042/EBC20170021
106
Zeng X. Chen Z. Zhu Y. Liu L. Zhang Z. Xiao Y. et al (2024). O-GlcNAcylation regulation of RIPK1-dependent apoptosis dictates sensitivity to sunitinib in renal cell carcinoma. Drug Resist Updat77, 101150. 10.1016/j.drup.2024.101150
107
Zhang L. Ten Hagen K. G. (2019). O-Linked glycosylation in Drosophila melanogaster. Curr. Opin. Struct. Biol.56, 139–145. 10.1016/j.sbi.2019.01.014
108
Zhang C. Liu J. Xu D. Zhang T. Hu W. Feng Z. (2020). Gain-of-function mutant p53 in cancer progression and therapy. J. Mol. Cell Biol.12, 674–687. 10.1093/jmcb/mjaa040
109
Zhang N. Jiang H. Zhang K. Zhu J. Wang Z. Long Y. et al (2022). OGT as potential novel target: structure, function and inhibitors. Chem. Biol. Interact.357, 109886. 10.1016/j.cbi.2022.109886
110
Zhang C. C. Li Y. Jiang C. Y. Le Q. M. Liu X. Ma L. et al (2024a). O-GlcNAcylation mediates H(2)O(2)-induced apoptosis through regulation of STAT3 and FOXO1. Acta Pharmacol. Sin.45, 714–727. 10.1038/s41401-023-01218-z
111
Zhang Y. Han S. Li T. Zhu L. Wei F. (2024b). Bisphenol A induces non-alcoholic fatty liver disease by promoting the O-GlcNAcylation of NLRP3. Arch. Physiol. Biochem.130, 814–822. 10.1080/13813455.2023.2288533
112
Zhang D. Qi Y. Inuzuka H. Liu J. Wei W. (2024c). O-GlcNAcylation in tumorigenesis and its implications for cancer therapy. J. Biol. Chem.300, 107709. 10.1016/j.jbc.2024.107709
113
Zhou Q. Meng Y. Li D. Yao L. Le J. Liu Y. et al (2024a). Ferroptosis in cancer: from molecular mechanisms to therapeutic strategies. Signal Transduct. Target Ther.9, 55. 10.1038/s41392-024-01769-5
114
Zhou X. Wang Y. Li X. Zhou J. Yang W. Wang X. et al (2024b). O-GlcNAcylation regulates the stability of transferrin receptor (TFRC) to control the ferroptosis in hepatocellular carcinoma cells. Redox Biol.73, 103182. 10.1016/j.redox.2024.103182
115
Zhu Y. Hart G. W. (2023). Dual-specificity RNA aptamers enable manipulation of target-specific O-GlcNAcylation and unveil functions of O-GlcNAc on β-catenin. Cell186, 428–445.e27. 10.1016/j.cell.2022.12.016
116
Zhu G. Murshed A. Li H. Ma J. Zhen N. Ding M. et al (2021). O-GlcNAcylation enhances sensitivity to RSL3-induced ferroptosis via the YAP/TFRC pathway in liver cancer. Cell Death Discov.7, 83. 10.1038/s41420-021-00468-2
117
Zhu Q. Wang H. Chai S. Xu L. Lin B. Yi W. et al (2023). O-GlcNAcylation promotes tumor immune evasion by inhibiting PD-L1 lysosomal degradation. Proc. Natl. Acad. Sci. U. S. A.120, e2216796120. 10.1073/pnas.2216796120
118
Zuppini A. Andreoli C. Baldan B. (2007). Heat stress: an inducer of programmed cell death in Chlorella saccharophila. Plant Cell Physiol.48, 1000–1009. 10.1093/pcp/pcm070
Summary
Keywords
O-GlcNAcylation, cancer, programmed cell death, apoptosis, autophagy, pyroptosis, ferroptosis, necroptosis
Citation
Yan X, Ren W, Zhu Z, Li S, Shi R, Sun K, Zhi K, Gao L and Zheng J (2025) Insights into O-GlcNAcylation and programmed cell death in cancer. Front. Cell Dev. Biol. 13:1560491. doi: 10.3389/fcell.2025.1560491
Received
14 January 2025
Accepted
05 September 2025
Published
22 September 2025
Corrected
23 October 2025
Volume
13 - 2025
Edited by
Shyamala Maheswaran, Massachusetts General Hospital, United States
Reviewed by
Futoshi Suizu, Kagawa Prefectural University of Health Sciences, Japan
Mansoor-Ali Vaali-Mohammed, King Saud University, Saudi Arabia
Updates
Copyright
© 2025 Yan, Ren, Zhu, Li, Shi, Sun, Zhi, Gao and Zheng.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Gao, dentist_gl@qdu.edu.cn; Keqian Zhi, zhikeqianqd@qdu.edu.cn; Jingjing Zheng, zhengjingjing.1984@163.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.