Abstract
Transcorneal electrical stimulation (TES), a noninvasive therapeutic technique, has gained attention for its potential to treat retinal and optic nerve diseases. TES involves applying weak electrical currents via electrodes on the cornea to stimulate retinal ganglion cells (RGCs) without causing activation of photoreceptors, inducing phosphenes, and enabling the evaluation of inner retinal function. This is valuable for assessing residual retinal activity in patients with photoreceptor or RGC degeneration. Furthermore, TES has shown significant neuroprotective effects on RGCs and photoreceptors through mechanisms involving the upregulation of neurotrophic factors (e.g., insulin-like growth factor 1, brain-derived neurotrophic factor, and ciliary neurotrophic factor), reduction of inflammatory responses, and enhanced ocular blood flow. These findings are supported by extensive animal studies, showing its efficacy in mitigating retinal degeneration and optic nerve damage while promoting axonal regeneration. Clinically, TES has shown potential in improving visual function in diseases such as RP, optic neuropathies, and ischemic retinal conditions; however long-term benefits remain a challenge. Randomized controlled trials have indicated the safety and modest therapeutic effects of TES, suggesting its potential as an adjunct treatment for visual impairments. Moreover, TES may extend beyond ophthalmology into neurology. Because the retina is anatomically connected to the brain, TES can influence brain regions such as the visual cortex and hippocampus. Preliminary research proposes its potential for modulating brain, such as those with retinitis pigmentosa (RP). TES has demonstrated significant neuroprotective effects in networks, cognition, and emotional pathways, offering hope for treating neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease. In summary, TES represents a versatile and promising therapy for retinal and neurological disorders, and ongoing advancements will likely expand its applications in clinical practice. Further studies are warranted to optimize its parameters, enhance its efficacy, and explore its full therapeutic potential.
1 Introduction
Electrical stimulation (ES) is a promising therapeutic tool for treating various neurological disorders. Multiple studies have demonstrated significant beneficial effects of ES with optimal safety and feasibility.
Vagus nerve stimulation (VNS) is clinically applied for the treatment of epilepsy, depression, cluster headache, and migraine (Cheng et al., 2022; Austelle et al., 2024). Deep brain stimulation has been applied in clinical practice for over 25 years and is well-established as an effective treatment for Parkinson’s disease (PD), dystonia, and Tourette syndrome (Ranjan et al., 2024).
Transcranial electrical stimulation (tES) has also been extensively investigated to alter brain function noninvasively by applying current to electrodes on the scalp. tES can induce changes in synaptic excitability and is promising for enhancing recovery in patients with stroke (Motolese et al., 2022). In addition, tES is clinically applied for the treatment of Alzheimer’s disease (AD) (Pilloni et al., 2022), Cerebral vasospasm and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage are the leading causes of morbidity and mortality after aneurysmal subarachnoid hemorrhage (Budohoski et al., 2014). Several types of ES have been tested for the treatment of cerebral vasospasms and delayed cerebral ischemia, including trigeminal/vagus/facial nerve stimulation, sphenopalatine ganglion and spinal cord stimulation, tES, transcutaneous electrical neurostimulation, and electroacupuncture (Powell et al., 2022).
For the retina and optic nerve (ON), transcorneal ES (TES) modulates retinal neurons to evoke light sensations, commonly referred to as “phosphene.” This phenomenon has been utilized to evaluate the residual retinal function in individuals with visual impairments. Furthermore, owing to the neuroprotective effects of TES on injured retinal ganglion cells (RGCs) in vivo (Morimoto et al., 2005), basic and clinical research on TES and related ES methods have significantly progressed over the past two decades. These advancements have established ES as a promising treatment approach for ON and retinal diseases (Morimoto, 2012; Pardue et al., 2014; Tao et al., 2016; Liu et al., 2021; Li et al., 2024).
This review explores the fundamental and clinical studies conducted on TES to date and its potential future applications.
2 History of transcorneal electrical stimulation
ES of the eye can induce a light sensation known as “phosphine,” a phenomenon that later led to the development of retinal prostheses aimed at restoring vision in blind patients with advanced retinitis pigmentosa (RP).
TES is used to stimulate the retina and evoke phosphenes. The procedure involves placing a bipolar contact lens electrode with an inner and outer ring in the form of a contact lens, such as an electroretinogram (ERG) electrode, or DTL-electrode on the patient’s cornea (Figure 1). To stimulate the retina, a weak electric current is then applied through the electrodes. Numerous studies have investigated ES-induced phosphenes. Early research on electrically induced phosphenes primarily focused on psychophysical studies (Motokawa et al., 1951; Brindley, 1960).
FIGURE 1
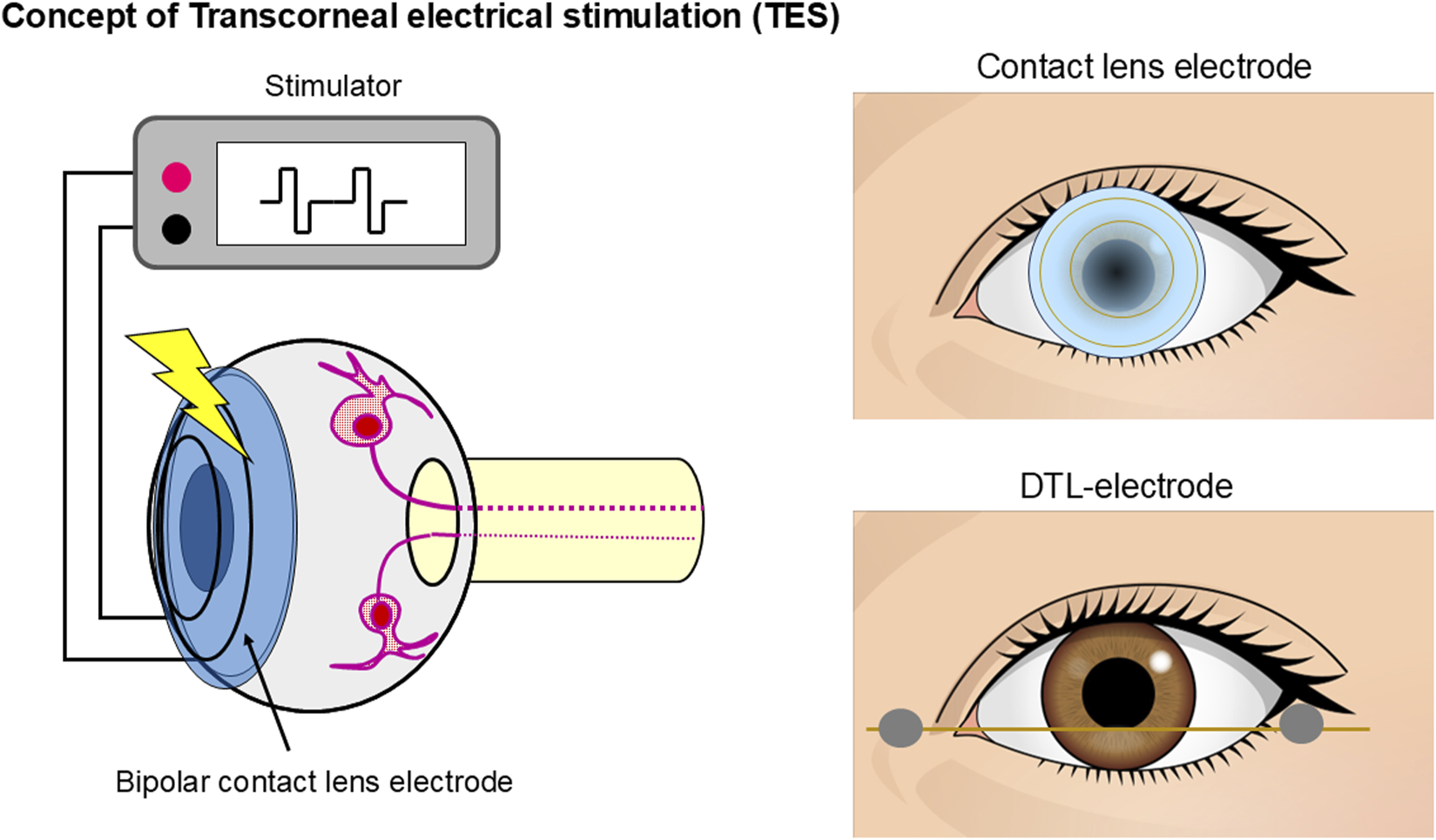
Schematic representation of TES. A bipolar contact lens electrode with an inner and outer ring in the form of a contact lens or DTL-electrode was placed on the participant’s cornea. Electric current pulses activate the retinal neurons.
Subsequently, Jarvik and Kopp (1967) developed a more convenient and less invasive method known as TES. TES was then employed in electrophysiological studies to investigate the relationship between ES and phosphene perception. Potts et al. (1968) were the first to report an “electrically evoked potentials (EER)” induced by TES. They found that the latency of EER was shorter than that of the visually evoked potentials, indicating that TES can evoke visual perception even without photoreceptor involvement (Potts et al., 1968; Potts and Inoue, 1969; 1970). Shimazu et al. (1996) and Shimazu et al. (1999) also reported similar findings using experiments with cats.
Subsequently, several human experiments have evaluated the characteristics of EERs in healthy participants (Miyake et al., 1980a; Dorfman et al., 1987; Takei et al., 1993) and patients with various retinal and ON diseases (Miyake et al., 1980b; Miyake et al., 1980c; Dorfman et al., 1987). Studies have reported that the EERs remained nearly normal in patients with functional disorders of the rod or cone visual pathways (Miyake et al., 1980b), whereas they were reduced in patients with central retinal artery occlusion or ON diseases (Miyake et al., 1980c). Despite these findings, basic research on TES made no significant progress, nor did it lead to clinical applications, and studies on TES came to a standstill for some time.
3 TES to assess inner retinal function
The advancement in retinal prostheses and regenerative medicine renders it possible to restore the vision of patients with blindness and retinal degenerative diseases such as RP. For the successful restoration of vision through such treatment, the function of the inner retinal neurons must remain intact.
As the prospect of clinical applications transitions into reality, robust and sophisticated methodologies are increasingly needed to assess residual inner retinal function in patients with blindness (Table 1), particularly those being considered candidates for these transformative treatments.
TABLE 1
| Study | Subject | Electrode | Parameters | Evaluation method |
|---|---|---|---|---|
| Potts et al. (1968) | Healthy (n = 8) | Contact lens electrode | Monophasic 0.3–2.3 mA, 5.0–50 ms duration | EER |
| Potts and Inoue. (1969) | RP (n = 4) | Contact lens electrode | Monophasic 2.0 mA, 5.0 ms duration | EER |
| Miyake et al. (1980a) | Healthy (n = 50) | Contact lens electrode | Monophasic, 0.1–2.0 mA, 5.0 ms duration, 1.98 Hz | EER |
| Miyake et al. (1980b) | IRD (n = 5) | Contact lens electrode | Monophasic, 0.1–2.0 mA, 5.0 ms duration, 1.98 Hz | EER |
| Miyake et al. (1980c) | CRAO (n = 8) | Contact lens electrode | Monophasic, 0.1–2.0 mA, 5.0 ms duration, 1.98 Hz | EER |
| Dorfman et al. (1987) | Ocular trauma (n = 17), Healthy (n = 4) | Contact lens electrode | Monophasic, 2.0–20 mA, 1.0 ms duration, 1.0 Hz | EER |
| Takei et al. (1993) | Healthy (n = 23), MH (n = 6), RAO (n = 3) | Contact lens electrode | Monophasic 0.3–2.0 mA 5 ms duration, 1.85 Hz | EER |
| Morimoto et al. (2006) | IRD (n = 20), Healty (n = 8) | Contact lens electrode | Biphasic, 0.05–2.0 mA, 10 ms/phase, 20 Hz 1.0 s | Subjective phosphene and pupillary reflex |
| Gekeler et al. (2006) | Healthy (n = 17), POAG (n = 9), RP (n = 14), Amblyopia (n = 3), Homonymous visual field loss (n = 4). | DTL electrode | Monophasic, 0–4.0 mA, 0.05–50.0 ms duration, 0.67 Hz, | Subjective phosphene |
| Fujikado et al. (2007) | Healty (n = 6), RP (n = 2) | Monopolar scleral electrode | Biphasic, 1.0–1.5 mA, 1.0 ms/phase+interpulse 1.0 ms , 20 Hz, 20 pulses or 0.5–4.0 ms/phase+interpulse 1.0 ms, 50 Hz, 20 pulses | Subjective phosphene and pupillary reflex |
| Huang et al. (2009) | RP (n = 17), Healthy (n = 15) | Contact lens electrode | Biphasic, 0.025–1.0 mA, 5, 7.5, 10 ms /phase, 20 Hz, 2 s | Subjective phosphene, OCT |
| Naycheva et al. (2012) | Healthy (n = 20), RP (n = 30) ,STG(n = 14), RAO (n = 20), NAION (n = 16), POAG (n = 17) | DTL electrode | Biphasic, 0–10 mA, 1–100 Hz, 5 ms/phase | Subjective phosphene |
| Kelbsch et al. (2017) | RP (n = 40), Healthy(n = 40) | DTL electrode | Biphasic, 0–1.2 mA, 10 ms/phase, 20 Hz | Subjective phosphene and pupillary reflex |
| Kelbsch et al. (2018) | Healthy (n = 14) | DTL electrode | Sinusoidal, 0.01, 0.02,0.05 mA, 10 or 20 Hz, envelope frequency 1.2 Hz | Subjective phosphene and pupillary reflex |
Summary of clinical studies on the evaluation of inner retinal function by TES and similar ES.
CRAO, central retinal arterial occlusion; DTL, Dawson–Trick–Litzkow; EER, electrically evoked response; ES, electrical stimulation; IRD, Inherited retinal degeneration; MH, macular hole; NAION, nonarteritic anterior ischemic optic neuropathy;OCT, optical coherence tomography; ON, optic neuropathy; POAG, primary open-angle glaucoma; RAO, retinal arterial occlusion; RP, retinitis pigmentosa; STG, Stargardt disease; TES, transcorneal electrical stimulation.
In particular, patients eligible for treatment often suffer from degeneration-induced photoreceptor loss, which makes it impossible to evaluate inner retinal function using conventional ophthalmic tests such as visual acuity (VA) tests, visual field (VF) tests, ERG, or VEP. The structural evaluation of the inner retinal layers using optical coherence tomography (OCT) is currently the sole method for assessing the inner retinal layers (Chader et al., 2009).
TES is considered an effective method for evaluating inner retinal functions because it can stimulate RGCs without activating photoreceptors (Potts and Inoue, 1970; Miyake et al., 1980b; Shimazu et al., 1999). This has brought TES back into the spotlight, leading to a resurgence of its research starting in the 2000s. A method combining TES-induced phosphenes and pupil responses to evaluate inner retinal function subjectively and objectively in healthy individuals and patients with inherited retinal degeneration has been reported (Morimoto et al., 2006; Kelbsch et al., 2017; Kelbsch et al., 2018).
Methods have been developed to evaluate the function of the inner retinal layers by analyzing the characteristics of ES-induced phosphenes, such as their position, size, shape, brightness, and color (Gekeler et al., 2006; Fujikado et al., 2007; Naycheva et al., 2012). Furthermore, a combined approach integrating OCT for retinal structural assessment with the evaluation of phosphenes was also proposed (Huang et al., 2009).
Given the rapidity, safety, and reliability of phosphene-based evaluation of inner retinal function using TES, this method shows great potential for assessing inner retinal layer function in patients with blindness and inherited retinal degeneration and could become a standard diagnostic test.
In the future, with the wide adoption of regenerative medicine, retinal prostheses, and optogenetic therapies, TES is expected to play an increasingly crucial role in assessing the functionality of the inner retinal layers in patients who have lost photoreceptors.
4 Neuroprotective effects of TES on the retinal neurons
ES can dose-dependently modulate the survival rates of isolated central nervous system (CNS) neurons in vitro (Kaplan et al., 1988). Many studies have investigated the neuroprotective effects of ES on injured neurons in vivo. Within the auditory system, the survival of spiral ganglion cells (SGCs) is a key factor that influences the performance of cochlear implants. Enhancing SGC survival is anticipated to improve sensitivity and enhance auditory discrimination. Chronic ES supported SGC survival that would otherwise degenerate following exposure to ototoxic drugs in vivo (Lousteau, 1987; Hartshorn et al., 1991).
Similarly, in the visual system, brief ES using monophasic pulses on the transected ON increased RGC survival in rats, demonstrating the neuroprotective effect of ES on the ON (Morimoto et al., 2002). Furthermore, the extent of this survival-promoting effect was dependent on the ES parameters (Okazaki et al., 2008).
Direct ES of the ON has demonstrated a neuroprotective effect; however, its highly invasive nature makes its clinical application challenging. Therefore, this study focused on TES, a less invasive and safer stimulation method than ON stimulation, which is also used to evaluate the function of inner retinal layers. This study revealed that TES exerts a neuroprotective effect on RGCs, similar to that of direct ON stimulation. TES increased RGC survival after ON transection in rats by upregulating endogenous IGF-1 (Morimoto et al., 2005). The survival-promoting effect of TES was dependent on ES parameters (Morimoto et al., 2010). TES was also neuroprotective for axons in crushed ONs (Miyake et al., 2007) and enhanced the axonal regeneration of RGCs through the activation of the IGF-1 pathway in the rat ON crush model (Tagami et al., 2009).
Moreover, TES exerts neuroprotective effects on photoreceptors. In animals with inherited photoreceptor degeneration, TES enhanced photoreceptor survival in Royal College of Surgeons rats (Morimoto et al., 2007; Gonzalez Calle et al., 2023), P347L transgenic rabbits (Morimoto et al., 2012), P23H rats (Rahmani et al., 2013), N-methyl-N-nitrosourea-administered mice (Tao et al., 2016), rd 10 mice (Liu et al., 2022), and phototoxic rats (Ni et al., 2009), rhodopsin knockout mice (Enayati et al., 2024). TES also exerted neuroprotective effects on ischemic damaged retinas in vivo (Wang et al., 2011). TES provided RGC axon protection and led to a reduction in inflammatory cells in a mouse glaucoma model (Jassim et al., 2021).
The results of numerous animal experiments have demonstrated the neuroprotective effects of TES in the eyes of patients with retinal degenerative diseases and ON disorders (Table 2).
TABLE 2
| Study | Animal | Model | Electrode | Parameters | Effect | |
|---|---|---|---|---|---|---|
| Morimoto et al. (2002) | Wistar rats (ON transection) | TON | Optic nerve monopolar electrodes | Monophasic 0.02–0.07 mA, 0.05 ms duration, 20 Hz 2 h, once | RGC survival | |
| Morimoto et al. (2005) | Wistar rats (ON transection) | TON | Contact lens electrode | Biphasic 0.1 mA, 0.5–3.0 ms/phase, 20 Hz, 1.0 h, once | RGC survival | |
| Morimoto et al. (2007) | RCS rats | RP | Contact lens electrode | Biphasic 0.05–0.1 mA, 1.0 ms/phase, 20 Hz, 1 h, once a week for 2–6 wk | PR survival | |
| Miyake et al. (2007) | Wistar rats (ON crush) | TON | Contact lens electrode | Biphasic 0.5 mA, 0.05 ms/phase, 20 Hz, 6 h, once | RGC survival | |
| Okazaki et al. (2008) | Wistar rats (ON transection) | TON | Optic nerve monopolar electrodes | Monophasic 0.05 mA, 0.05 ms duration, 10–50 Hz 10–120 min, once | RGC survival | |
| Tagami et al. (2009) | Wistar rats (ON crush) | TON | Contact lens electrode | Biphasic 0.1 mA, 1 ms/phase, 20 Hz, 1 h, 1,2,4,12 times for 12 d | Axonal regeneration of RGCs | |
| Ni et al. (2009) | SD rats (light-induced) | RP | Contact lens electrode | Pre: biphasic 0.1–0.5 mA, 3 ms/phase, 20–100 Hz, 1 hr, once Post: biphasic 0.2-0.3 mA, 3 ms/phase, 20 Hz, 1 hr, every 3 d for 1-2 wk |
PR survival | |
| Morimoto et al. (2010) | Wistar rats (ON transection) | TON | Contact lens electrode | Biphasic 0.1 mA, 1 ms/phase, 20 Hz, 1 h, once | RGC survival | |
| Wang et al. (2011) | SD rats(high IOP) | ION | Contact lens electrode | Biphasic 0.3 mA, 3 ms/phase, 20 Hz, 1 h, every 2 d for 2 wk | RGC survival | |
| Morimoto et al. (2012) | P347L transgenic rabbits | RP | Contact lens electrode | Biphasic 0.7 mA, 10 ms/phase, 20 Hz, 1 h, once a week for 6 wk | PR survival | |
| Rahmani et al. (2013) | P23H rats | RP | Sintered pellet electrodes, cornea & mouth | Sinusoidal, 4.7 mA, 5 Hz, 30 min, twice a week for 12 wks | PR function (ERG) | |
| Tao et al. (2016) | C57/BL mice(MNU treated) | RP | Contact lens electrode | Biphasic 0.1–0.2 mA, 20 Hz, 1 h, three times for a week | PR survival | |
| Jassim et al. (2021) | DBA/2J (D2) mice | Glaucoma | Contact lens electrode | Biphasic 0.1 mA, 1 ms/phase, 20 Hz, 10 min, every 3 d for 8 wk | RGC survival | |
| Liu et al. (2022) | rd10 mice | RP | Sclera electrode | Biphasic 0.05–0.1 mA, 2.5 ms/phase +interpulse 1 ms, 20 Hz, 1 hr, 3 or 5 times for 5 d | PR survival | |
| Gonzalez Calle et al. (2023) | RCS rats | RP | Cornea ring electrode | Biphasic 0.2–0.1 mA, 10 ms/phase, 6 Hz, 2 hr, once a week, 6 times | PR survival | |
| Enayati et al. (2024) | rhodopsin knockout mice | RP | Skin electrodes (upper and lower eye lids) | Monophasic, rectangular (0.1 mA, 2–200 Hz, 40 s/cycle, 160 s) + ramp waveform (0.1 mA, 20 Hz, 160 s), 5 d x 2 times | Improvement in retinal function and visual behavior | |
Summary of preclinical studies of TES and similar ES.
ERG, electroretinogram; ION, ischemic optic neuropathy; IOP, intraocular pressure; MNU, N-methyl-N-nitrosourea; ON, optic nerve; PR, photoreceptor; RCS, Royal College of Surgeon; RGC, retinal ganglion cell; RP, retinitis pigmentosa; TON, traumatic optic neuropathy.
5 Mechanism of the neuroprotective effects of TES on the retina and ON
The mechanism underlying the neuroprotective effects of ES has been extensively studied over time. As regards the neuroprotective and axonal outgrowth-promoting effects of ES, ES-induced depolarization via the activation of voltage-dependent Ca2+ channels is crucial.
Brief periods of ES applied to cultured Xenopus spinal neurons significantly increased intracellular Ca2+ and cAMP levels, which, in turn, play a crucial role in promoting the extension of growth cones (Ming et al., 2001). Various neurotrophic factors are reportedly induced by ES applied to RGCs and/or Müller cells, exerting neuroprotective effects in vitro.
RGC stimulation by ES from a silicon chip enhanced their survival and axonal growth in response to brain-derived neurotrophic factor (BDNF) in vitro (Goldberg et al., 2002).
Brief ES of cultured Müller cells increased the gene expression of IGF-1, BDNF, basic fibroblast growth factor (bFGF) (Sato et al., 2008a; Sato et al., 2008b; Sato et al., 2008c), and ciliary neurotrophic factor (CNTF) (Enyati et al., 2020) by activating L-type voltage-dependent Ca2+channels.
The neuroprotective effects of TES are considered to involve various neurotrophic and neuroprotective factors that are expressed within the retina in response to TES in vivo. TES enhanced retinal neuron survival by increasing endogenous neurotrophic factors, namely, IGF-1 (Morimoto et al., 2005), BDNF and CNTF (Ni et al., 2009; Tao et al., 2016), and bFGF (Yu et al., 2020). These neurotrophic factors increased significantly in Müller cells, which play a significant role in TES-induced neuroprotection.
Other neuroprotective factors were reported to be related to the neuroprotective effects of TES. Bcl-2 was upregulated, whereas Bax was downregulated (Ni et al., 2009; Tao et al., 2016), and the tumor necrosis factor superfamily was upregulated in the retina after TES (Willmann et al., 2011). DNA methylation changes with therapeutic effects were also induced by TES (Tew et al., 2024).
TES also affects the immune system. TES decreased the number of Iba-1-positive microglial cells, reduced interleukin-6 (IL-6) and COX-2 expression and NF-κB phosphorylation, and increased IL-10 levels (Fu et al., 2018). Microglial inhibition by TES was observed in genetic secondary glaucoma mouse model (Jassim et al., 2021).
Other effects of TES are thought to be associated with increased ocular blood flow. Numerous studies have investigated the potential relationship between TES and retinal blood flow, and growing evidence indicates a significant link. For instance, measurements of TES-induced retinal intrinsic reflective changes in cat eyes have revealed vascular changes caused by the activation of retinal neurons (Mihashi et al., 2011; Morimoto et al., 2014). Similarly, studies assessing blood flow in human eyes have demonstrated that TES increases chorioretinal blood flow in both healthy individuals (Kurimoto et al., 2010) and patients with RP (Bittner et al., 2018a). Furthermore, the TES-induced increase in retinal blood flow involves neurovascular coupling (NVC) (Su et al., 2020).
NVC is a phenomenon in which neurons, glial cells, and blood vessels in the CNS work together. When neurons become active, the blood flow in the corresponding region increases to meet the energy demands of the active neurons by delivering oxygen and glucose. NVC is essential for supporting RGC metabolism and survival (Haider et al., 2022). Many patients with glaucoma suffer from vascular deficits, including reduced blood flow, impaired autoregulation, NVC dysfunction, and breakdown of the blood–retina and blood–brain barriers (Alarcon-Martinez et al., 2023).
Based on the above findings, increased blood flow is inferred to exert a neuroprotective effect on the retina. The TES-induced increase in ocular and retinal blood flow may contribute to this neuroprotective effect.
TES also affects neuronal activity in the visual pathway and ameliorates retinal-genicular- cortical function in diseases involving the visual system (Castoldi et al., 2025).
In summary, TES is thought to exert neuroprotective effects on RGCs and photoreceptors through various mechanisms. These include the production of neurotrophic factors via Müller cells, DNA methylation, modulation of the immune system (e.g., suppression of macrophage activity), and an increase in ocular and retinal blood flow, and amelioration of retinal-genicular- cortical function in ocular diseases involving the visual system (Figure 2).
FIGURE 2
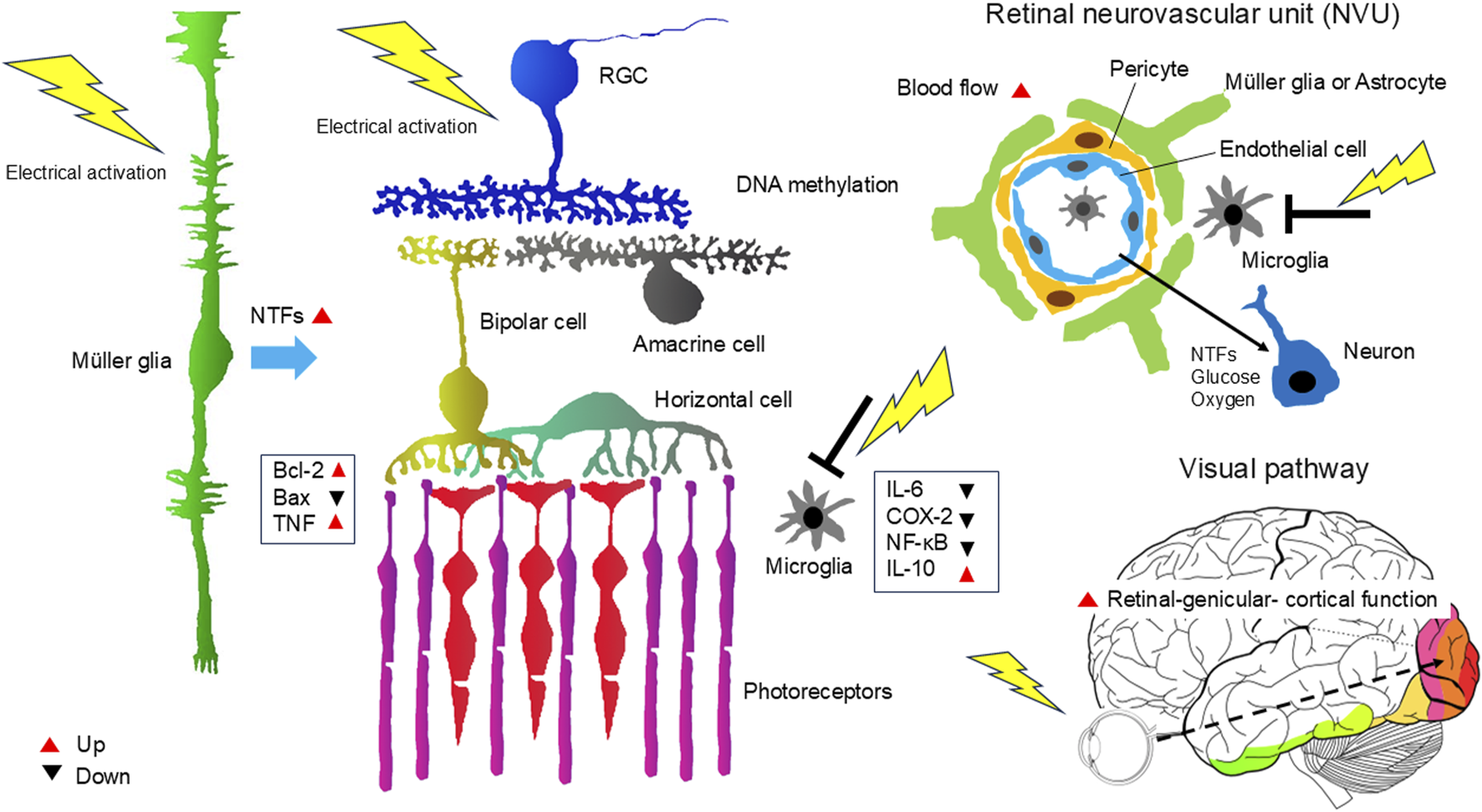
Mechanism of the neuroprotective effects of TES on the retina and ON. TES triggers various responses that act together to promote neuronal survival and improve neuronal function. These include the production of neurotrophic factors via Müller cells, DNA methylation, modulation of the immune system (e.g., suppression of macrophage activity), and an increase in ocular and retinal blood flow, and amelioration of retinal-genicular- cortical function in ocular diseases involving the visual system. NTFs, neurotrophic factors.
6 Clinical application of TES for various retinal and ON diseases
Numerous clinical studies have examined TES and similar ES therapies for various retinal and ON diseases (Table 3). Initial TES was performed for nonarteritic anterior ischemic optic neuropathy and traumatic optic neuropathy, and some patients reported improvements in VA and VFs (Fujikado et al., 2006). Since then, TES has been applied to diseases, including retinal artery occlusion (Inomata et al., 2007; Oono et al., 2011; Naycheva et al., 2013), Best vitelliform macular dystrophy (Ozeki et al., 2013), ON atrophy (Gall et al., 2010), and glaucoma (Ota et al., 2018). Despite the modest therapeutic effects, positive outcomes were observed, leading to the application of this treatment approach to various ocular diseases.
TABLE 3
| Study | Subject | Design | Type | Electrode | Parameters | Outcome |
|---|---|---|---|---|---|---|
| Fujikado et al. (2006) | ION (n = 3), TON (n = 5) | Case series | TES | Contact lens electrode | Biphasic 0.3-2 mA, 10 ms/phase, 20 Hz, 30 min, once | Improvement in VA and VF |
| Inomata et al. (2007) | CRAO (n = 2), BRAO (n = 1) | Case series | TES | Contact lens electrode | Biphasic 1.1 mA, 10 ms/phase, 20 Hz, 30 min, once a month for 3 mo | Improvement in VA and VF |
| Gall et al. (2010) | TON (n = 1) | Case report | rtACS | Skin electrode (upper eye lid) | Biphasic, current bursts, <0.6 mA, 10–30 Hz, 30–40-min for 10 d | Increase of detection ability and mean perimetric threshold |
| Schatz et al. (2011) | RP (n = 24) | Prospective, randomized, partially blinded study | TES | DTL electrode | Biphasic intensity 66% or 150% of EPT, 5 ms/phase, 20 Hz, 30 min, once a week for 6 wk | Improvement in VF at 150% of EPT |
| Oono et al. (2011) | BRAO (n = 5) | Case series | TES | Contact lens electrode | Biphasic 0.5–0.9 mA, 10 ms/phase, 20 Hz, 0.5 hour, once | Improvement in visual function (mERG, HFA) |
| Gall et al. (2011) | OND (n = 24), OND (placebo, n = 18) | Prospective, randomized, sham controlled study | rtACS | Skin electrode (upper eye lid) | Biphasic, current bursts, <1.0 mA, 5-20 Hz, 20-40 min daily for 10 d | Increase of detection ability |
| Sabel et al. (2011) | OND (n = 12), OND (placebo, n = 10) | Prospective, randomized, double-blind, placebo controlled study | rtACS | Skin electrode (upper eye lid) | Biphasic, current bursts, <1.0 mA, 5–20 Hz, 15 min daily for 10 d | Improvement of central visual field |
| Fedorov et al. (2011) | OND (n = 446) | Open-label, clinical observational study | rtACS | Skin electrode (upper eye lid) | Biphasic, current bursts, <1.0 mA, 5–20 Hz, 25–40 min daily for 10 d | Improvement in VF and VA |
| Naycheva et al. (2013) | CRAO (n = 10, sham n = 2), BRAO (sham n = 1) | Prospective, randomized, sham-controlled study | TES | DTL electrode | Biphasic intensity 66% or 150% of EPT, 5.0 ms/phase, 20 Hz, 30 min, once a week for 6 wk | Improvement in ERG response (a-wave) at 150% of EPT |
| Ozeki et al. (2013) | BVMD (n = 1) | Case report | TES | Contact lens electrode | Biphasic 0.17-0.25 mA, 10 ms/phase, 20 Hz, 30 min, 4 times | Improvement in VA |
| Gall et al. (2016) | OND (n = 45), OND (sham, n = 37) | Multicenter, prospective, randomized, double-blind, sham-controlled study | rtACS | Skin electrode (upper eye lid) | Biphasic, current bursts, 125% of EPT, 8–25 Hz, 50 min 10 d within 2 weeks | Improvement in VF |
| Schatz et al. (2017) | RP (n = 32), RP (sham, n = 20) | Prospective, randomized, partially masked study | TES | DTL electrode | Biphasic intensity 150% or 200% of EPT, 5 ms/phase, 20 Hz, 30 min per week for 52 consecutive wk | Improvement of retinal function (scotopic b-wave) at 200% of EPT |
| Wagner et al. (2017) | RP (n = 7) | Prospective open-label observational study | TES | DTL electrode | Biphasic intensity 150% of EPT or 1.0 mA, 5 ms/phase, 20 Hz, 30 min, once a week for 6 mo, 24 sessions | No improvement in visual function compared to the control eyes |
| Ota et al. (2018) | POAG (n = 3), NTG (n = 2) | Case series | TES | DTL electrode | Biphasic 0.3-0.5 mA, 10 ms/phase, 20 Hz, 30 min, every 3 mo for 11–68 mo | Improvement in VF (POAG) |
| Bittner and Seger (2018b) | RP (n = 7) | Prospective, randomized, controlled study | TES | DTL electrode | Biphasic 0.75 mA, 5 ms/phase, 20 Hz, 30 min, once a week for 6 wk | Prevention of slowly diminishing vision (ETDRS VA, GVF, qCSF) |
| Miura et al. (2019) | RP (n = 10) | Prospective, non-randomized, open-label, uncontrolled study | TdES | Skin electrode (lower eye lid) | Biphasic 1.0 mA, 10 ms/phase, 20 Hz, 30 min, every 2 weeks for 6 sessions | Improvement of ETDRS BCVA and HFA VF |
| Jolly et al. (2020) | RP (n = 105) | Single-arm open label interventional safety study | TES | DTL electrode | Biphasic < 1.0 mA, 5 ms/phase, 20 Hz, 30 min, once a week for 6 mo | Transient dry eye symptoms, no serious adverse events, no improvement in visual function |
| Demir et al. (2022) | RP (n = 15) | Prospective, randomized, controlled study | TES | DTL electrode | Biphasic 200% of EPT, 2 ms/phase, 20 Hz, 30 min, once a week for 12 wk | Improvement in BCVA, color vision, mERG(ring1) |
| Sinim Kahraman and Oner (2020) | RP (n = 101) | Prospective, randomized, controlled study | TES | DTL electrode | Biphasic 150% of EPT, 5 ms/phase, 20 Hz, 30 min, once a week for 8 wk | Improvement in VA or VF at 1 mo after TES |
| Kurimoto et al. (2020) | LHON (n = 10) | Prospective, non-randomized, open-label, uncontrolled study | TdES | Skin electrode(lower eye lid) | Biphasic 1.0 mA, 10 ms/phase, 20 Hz, 30 min, every 2 wk for 6 sessions | Improvement in VA |
| Dizdar Yigit et al. (2022) | RP (n = 15) | Prospective, randomized, fellow-eye–controlled study | TES | DTL electrode | Biphasic 200% of EPT, 5 ms/phase, 20 Hz, 30 min, once a week for 6 mo | Stabilization of retinal function (mERG) |
| Stett et al. (2023) | RP (n = 31), RP (sham, n = 20) | Prospective, randomized, partially masked study | TES | DTL electrode | Biphasic intensity 150% or 200% of EPT, 5 ms/phase, 20 Hz, 30 min, once a week for 1 yr | Reduction of loss of VF |
| Miura et al. (2023) | ION (n = 5) | Prospective, non-randomized, open-label, uncontrolled study | TdES | Skin electrode (lower eye lid) | Biphasic 1.0 mA, 10 ms/phase, 20 Hz, 30 min, every 2 wk for 6 sessions | Improvement in VA or VF |
Summary of clinical studies of TES and similar ES therapies.
BRAO, branch retinal artery occlusion; BVMD, Best vitelliform macular dystrophy; CRAO, central retinal artery occlusion; DTL, Dawson–Trick–Litzkow; ERG, electroretinogram; ETDRS, Early Treatment Diabetic Retinopathy Study; EPT, electrical phosphene threshold ; GVF, Goldmann visual field; HFA, Humphrey field analyzer; ION, ischemic optic neuropathy; LHON, Leber hereditary optic neuropathy; mERG, multifocal electroretinogram; NTG, normal tension glaucoma; OND, optic nerve damage; RP, retinitis pigmentosa; rtACS, transorbital alternating current stimulation; TdES, transdermal electrical stimulation; TES, transcorneal electrical stimulation; TON, traumatic optic neuropathy; qCSF, quick contrast sensitivity function; VA, Visual acuity; VF, visual field.
Clinical studies on the neuroprotective effects of ES, involving many patients, have been conducted for both RP and optic neuropathies. Among these, Schatz et al. (2011) conducted the first randomized controlled trial (RCT) of TES in patients with RP. The study reported the safety of TES in RP patients. and enhancements in the VF area (VFA) and scotopic b-wave amplitude. A continuation of this study revealed a trend toward improved safety and function (specifically scotopic b-wave amplitude) with 1 year of continued treatment (Schatz et al., 2017). Furthermore, regular and dose-dependent use of TES significantly reduced the loss of VFA (V4e) in treated eyes compared with untreated eyes in patients with RP (Stett et al., 2023).
Since then, more RCTs on TES for patients with RP have been conducted. Wagner et al. (2017) demonstrated that TES was safe and well-tolerated in patients with RP. However, visual function measurements at 6 months were not significantly different between the control and treated eyes.
With TES, some patients experienced a significant improvement in VA and VF (Bittner and Seger, 2018b). A single-arm open-label interventional trial involving 105 patients with RP reported an excellent safety profile for TES; however, it did not observe significant improvements in visual function (Jolly et al., 2020). Another single-arm open-label interventional trial with 101 patients with RP found that the mean BCVA and VF test scores improved significantly 1 month after TES initiation. However, these improvements were transient and disappeared after the treatment was discontinued (Sinim Kahraman and Oner, 2020). In addition, single-arm open-label interventional trials have suggested that TES may slow deterioration in multifocal electroretinography (Demir et al., 2022; Dizdar Yigit et al., 2022). Furthermore, RCTs investigating transdermal ES (TdES), a technique similar to TES, in patients with RP demonstrated both safety and significant improvements in VA and VF for up to 3 months (Miura et al., 2019).
In clinical applications of TES and TdES for RP, some patients demonstrate notable improvements in visual function, whereas others do not exhibit significant changes. Such heterogeneity in outcomes is likely attributable to multiple factors, including disease stage and severity, inter-individual anatomical and physiological differences, and variations in stimulation parameters (e.g., current intensity, frequency, duration, and interval of treatment sessions).”
TES has shown potential in improving VA and VF in patients with RP. However, as the underlying disease continues to progress, these improvements may be temporary, eventually giving way to further deterioration in visual acuity and visual field. Therefore, a critical challenge in clinical practice is to develop strategies that can sustain the therapeutic effects of TES and help slow the progression of retinal degeneration over time.
TES has been widely performed for patients with various optic neuropathies. An RCT of repetitive transorbital alternating current stimulation (rtACS), which is similar to TES, for patients with ON damage, such as traumatic optic neuropathy, revealed that rtACS facilitated vision restoration in VA and VF size (Sabel et al., 2011; Gall et al., 2011; Fedorov et al., 2011; Gall et al., 2016).
Preliminary studies have investigated the potential of TdES as a treatment option for optic neuropathies. In a study on Leber hereditary optic neuropathy, 10 patients received TdES over 10 weeks. Significant improvements in VA were observed at all follow-up points, with half of the patients demonstrating notable enhancements in VF sensitivity (Kurimoto et al., 2020). Another study evaluated TdES for nonarteritic anterior ischemic optic neuropathy in five patients treated over 12 weeks. Some cases showed improvements in VA and VF sensitivity without adverse events (Miura et al., 2023).
Despite the relatively few treatment reports on the effects of TES on optic neuropathies (Fujikado et al., 2006), results of previous animal experiments and clinical trials of rtACS suggest the significant potential efficacy of TES for these conditions. Therefore, RCTs are needed to further investigate the therapeutic effects of TES on optic neuropathies.
Finally, regarding the safety of TES treatment, numerous studies to date have reported no serious complications, with only transient dry eye symptoms and punctate superficial keratitis being observed (Jolly et al., 2020; Sinim Kahraman and Oner, 2020). TES induces corneal epithelial damage in mice by disrupting mucin homeostasis (Yang et al., 2022). However, these were mild and all resolved without sequelae (Jolly et al., 2020; Sinim Kahraman and Oner, 2020).
Although potential side effects of TdES treatment—such as keratitis, dermatitis, facial or trigeminal nerve disorders, and nasal abnormalities—were anticipated due to the use of skin electrodes, none of these adverse events were observed during treatment. The skin sensory irritation and discomfort caused by the electrical stimulation were well tolerated by the patients (Kurimoto et al., 2020; Miura et al., 2023).
7 Clinical potential of TES for treating brain disorders
As mentioned earlier, TES stimulates the retina, resulting in phosphene generation in the visual cortex of the brain. Thus, ES of the eye affects the CNS. Because the eyes are an extension of the brain, examining ocular symptoms is gradually becoming a common practice in diagnosing brain pathologies.
Ophthalmological evaluations have revealed that neurodegenerative and neurological diseases, such as AD, PD, and multiple sclerosis, manifest retinal symptoms (Majeed et al., 2021; Chang et al., 2022; Bostan et al., 2023).
To treat these diseases, interest is growing in leveraging the connection between the eyes and the brain for therapeutic interventions. Most forms of ES of the brain are invasive, such as deep brain stimulation and motor cortex stimulation, and often involve postoperative complications. Conversely, noninvasive forms, such as tES and VNS, exhibit significant variability in response to the stimulation (Reed and Cohen Kadosh, 2018).
TES is considered a novel approach for noninvasively stimulating the eye to modulate brain networks in neurodegenerative diseases. TES to the retina modulates the brain coherence and connectivity of the visual and nonvisual cortices, and the observed alterations are largely maintained. TES holds a strong potential to modulate higher cortical functions, including cognition, awareness, emotion, and memory (Agadagba et al., 2022).
In rat models of retinal degeneration and chronic unpredictable stress, TES has shown promising effects, such as promoting antidepressant-like actions and recovering cognitive impairments (Yu et al., 2021; Yu et al., 2022a; Yu et al., 2022b). Despite the lack of basic or clinical research on the therapeutic effects of TES on AD or PD, noninvasive TES shows potential as a tool for modulating brain function in the treatment of brain diseases. This approach offers hope for the future treatment of patients with neurodegenerative diseases.
8 Concluding remarks and future directions
This review summarizes the results of studies on the role of TES as an assessment method of inner retinal layer function in patients with photoreceptor degeneration and as a neuroprotective treatment for retinal and ON diseases. Furthermore, it explores foundational research investigating the potential of TES as a neuroprotective therapy for brain disorders. TES not only influences retinal function by promoting neurotrophic factor production and immunosuppression and increasing blood flow but also affects other brain regions, including the visual cortex and hippocampus. With further clinical advancements, TES shows promise as a therapeutic approach for degenerative conditions of the retina and ON and neurological disorders.
Statements
Author contributions
TM: Conceptualization, Funding acquisition, Resources, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Japan Agency for Medical Research and Development (AMED; No. JP25gm1510010, JP24gm1510010, JP23gm1510010, JP22gm1510010), and the Japan Society for the Promotion of Science (KAKENHI; Nos. 22H00539, 19K09969, 18H04116, 22K19796, and T22K19796).
Acknowledgments
The author would like to thank Enago (www.enago.jp) for the English language review.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Agadagba S. K. Eldaly A. B. M. Chan L. L. H. (2022). Transcorneal electrical stimulation induces long-lasting enhancement of brain functional and directional connectivity in retinal degeneration mice. Front. Cell. Neurosci.16, 785199. 10.3389/fncel.2022.785199
2
Alarcon-Martinez L. Shiga Y. Villafranca-Baughman D. Cueva Vargas J. L. Vidal Paredes I. A. Quintero H. et al (2023). Neurovascular dysfunction in glaucoma. Prog. Retin Eye Res.97, 101217. 10.1016/j.preteyeres.2023.101217
3
Austelle C. W. Cox S. S. Wills K. E. Badran B. W. (2024). Vagus nerve stimulation (VNS): recent advances and future directions. Clin. Auton. Res.34, 529–547. 10.1007/s10286-024-01065-w
4
Bittner A. K. Seger K. (2018b). Longevity of visual improvements following transcorneal electrical stimulation and efficacy of retreatment in three individuals with retinitis pigmentosa. Graefes. Arch. Clin. Exp. Ophthalmol.256, 299–306. 10.1007/s00417-017-3858-8
5
Bittner A. K. Seger K. Salveson R. Kayser S. Morrison N. Vargas P. et al (2018a). Randomized controlled trial of electro-stimulation therapies to modulate retinal blood flow and visual function in retinitis pigmentosa. Acta. Ophthalmol.96, e366–e376. 10.1111/aos.13581
6
Bostan M. Pîrvulescu R. Tiu C. Bujor I. Popa-Cherecheanu A. (2023). OCT and OCT-A biomarkers in multiple sclerosis - review. Rom. J. Ophthalmol.67, 107–110. 10.22336/rjo.2023.20
7
Brindley G. S. (1960). Physiology of the retina and visual pathway. Edward Arnold, 155–160.
8
Budohoski K. P. Guilfoyle M. Helmy A. Huuskonen T. Czosnyka M. Kirollos R. et al (2014). The pathophysiology and treatment of delayed cerebral ischaemia following subarachnoid haemorrhage. J. Neurol. Neurosurg. Psychiatry.85, 1343–1353. 10.1136/jnnp-2014-307711
9
Castoldi V. Rossi E. Marenna S. Comi G. Leocani L. (2025). Transcorneal electrical stimulation modulates visual pathway function in mice. J. Neurosci. Res.103, e70026. 10.1002/jnr.70026
10
Chader G. J. Weiland J. Humayun M. S. (2009). Artificial vision: needs, functioning, and testing of a retinal electronic prosthesis. Prog. Brain Res.175, 317–332. 10.1016/S0079-6123(09)17522-2
11
Chang Z. Xie F. Li H. Yuan F. Zeng L. Shi L. et al (2022). Retinal nerve fiber layer thickness and associations with cognitive impairment in Parkinson’s disease. Front. Aging. Neurosci.14, 832768. 10.3389/fnagi.2022.832768
12
Cheng K. Wang Z. Bai J. Xiong J. Chen J. Ni J. (2022). Research advances in the application of vagus nerve electrical stimulation in ischemic stroke. Front. Neurosci.16, 1043446. 10.3389/fnins.2022.1043446
13
Demir M. N. Acar U. Sobacı G. Göksülük D. (2022). Outcomes of transcorneal electrical stimulation therapy in the early stages of retinitis pigmentosa. Turk. J. Med. Sci.52, 741–746. 10.55730/1300-0144.5368
14
Dizdar Yigit D. Sevik M. O. Şahin Ö. (2022). Transcorneal electrical stimulation therapy may have a stabilization effect on multifocal electroretinography for patients with retinitis pigmentosa. Retina42, 923–933. 10.1097/IAE.0000000000003386
15
Dorfman L. J. Gaynon M. Ceranski J. Louis A. A. Howard J. E. (1987). Visual electrical evoked potentials: evaluation of ocular injuries. Neurology37, 123–128. 10.1212/wnl.37.1.123
16
Enayati S. Chang K. Achour H. Cho K. S. Xu F. Guo S. et al (2020). Electrical stimulation induces retinal müller cell proliferation and their progenitor cell potential. Cells9, 781. 10.3390/cells9030781
17
Enayati S. Chang K. Lennikov A. Yang M. Lee C. Ashok A. et al (2024). Optimal transcorneal electrical stimulation parameters for preserving photoreceptors in a mouse model of retinitis pigmentosa. Neural Regen. Res.19, 2543–2552. 10.4103/1673-5374.392888
18
Fedorov A. Jobke S. Bersnev V. Chibisova A. Chibisova Y. Gall C. et al (2011). Restoration of vision after optic nerve lesions with noninvasive transorbital alternating current stimulation: a clinical observational study. Brain Stimul.4, 189–201. 10.1016/j.brs.2011.07.007
19
Fu L. Fung F. K. Lo A. C. Chan Y. K. So K. F. Wong I. Y. et al (2018). Transcorneal electrical stimulation inhibits retinal microglial activation and enhances retinal ganglion cell survival after acute ocular hypertensive injury. Transl. Vis. Sci. Technol.7, 7. 10.1167/tvst.7.3.7
20
Fujikado T. Morimoto T. Kanda H. Kusaka S. Nakauchi K. Ozawa M. et al (2007). Evaluation of phosphenes elicited by extraocular stimulation in normals and by suprachoroidal-transretinal stimulation in patients with retinitis pigmentosa. Graefes. Arch. Clin. Exp. Ophthalmol.245, 1411–1419. 10.1007/s00417-007-0563-z
21
Fujikado T. Morimoto T. Matsushita K. Shimojo H. Okawa Y. Tano Y. (2006). Effect of transcorneal electrical stimulation in patients with nonarteritic ischemic optic neuropathy or traumatic optic neuropathy. Jpn. J. Ophthalmol.50, 266–273. 10.1007/s10384-005-0304-y
22
Gall C. Fedorov A. B. Ernst L. Borrmann A. Sabel B. A. (2010). Repetitive transorbital alternating current stimulation in optic neuropathy. NeuroRehabilitation27, 335–341. 10.3233/NRE-2010-0617
23
Gall C. Schmidt S. Schittkowski M. P. Antal A. Ambrus G. G. Paulus W. et al (2016). Alternating current stimulation for vision restoration after optic nerve damage: a randomized clinical trial. PloS One11, e0156134. 10.1371/journal.pone.0156134
24
Gall C. Sgorzaly S. Schmidt S. Brandt S. Fedorov A. Sabel B. A. (2011). Noninvasive transorbital alternating current stimulation improves subjective visual functioning and vision-related quality of life in optic neuropathy. Brain Stimul.4, 175–188. 10.1016/j.brs.2011.07.003
25
Gekeler F. Messias A. Ottinger M. Bartz-Schmidt K. U. Zrenner E. (2006). Phosphenes electrically evoked with DTL electrodes: a study in patients with retinitis pigmentosa, glaucoma, and homonymous visual field loss and normal subjects. Invest. Ophthalmol. Vis. Sci.47, 4966–4974. 10.1167/iovs.06-0459
26
Goldberg J. L. Espinosa J. S. Xu Y. Davidson N. Kovacs G. T. Barres B. A. (2002). Retinal ganglion cells do not extend axons by default: promotion by neurotrophic signaling and electrical activity. Neuron33, 689–702. 10.1016/s0896-6273(02)00602-5
27
Gonzalez Calle A. Paknahad J. Pollalis D. Kosta P. Thomas B. Tew B. Y. et al (2023). An extraocular electrical stimulation approach to slow down the progression of retinal degeneration in an animal model. Sci. Rep.13, 15924. 10.1038/s41598-023-40547-1
28
Haider A. A. Rex T. S. Wareham L. K. (2022). cGMP signaling in the neurovascular unit-implications for retinal ganglion cell survival in glaucoma. Biomolecules12, 1671. 10.3390/biom12111671
29
Hartshorn D. O. Miller J. M. Altschuler R. A. (1991). Protective effect of electrical stimulation in the deafened Guinea pig cochlea. Otolaryngol. Head. Neck. Surg.104, 311–319. 10.1177/019459989110400305
30
Huang Q. Chowdhury V. Coroneo M. T. (2009). Evaluation of patient suitability for a retinal prosthesis using structural and functional tests of inner retinal integrity. J. Neural. Eng.6, 035010. 10.1088/1741-2560/6/3/035010
31
Inomata K. Shinoda K. Ohde H. Tsunoda K. Hanazono G. Kimura I. et al (2007). Transcorneal electrical stimulation of retina to treat longstanding retinal artery occlusion. Graefes. Arch. Clin. Exp. Ophthalmol.245, 1773–1780. 10.1007/s00417-007-0610-9
32
Jarvik M. E. Kopp R. (1967). Transcorneal electroconvulsive shock and retrograde amnesia in mice. J. Comp. Physiol. Psychol.64, 431–433. 10.1037/h0025199
33
Jassim A. H. Cavanaugh M. Shah J. S. Willits R. Inman D. M. (2021). Transcorneal electrical stimulation reduces neurodegenerative process in a mouse model of glaucoma. Ann. Biomed. Eng.49, 858–870. 10.1007/s10439-020-02608-8
34
Jolly J. K. Wagner S. K. Martus P. MacLaren R. E. Wilhelm B. Webster A. R. et al (2020). Transcorneal electrical stimulation for the treatment of retinitis pigmentosa: a multicenter safety study of the OkuStim® System (TESOLA-Study). Ophthalmic Res.63, 234–243. 10.1159/000505001
35
Kaplan F. S. Maguire T. G. Lee V. M. Selzer M. E. Spindler K. Black J. et al (1988). Direct extracellular electrical stimulation influences density dependent aggregation of fetal rat cerebrocortical neurons in vitro. Neurosci. Lett.94, 33–38. 10.1016/0304-3940(88)90266-2
36
Kelbsch C. Jalligampala A. Strasser T. Richter P. Stingl K. Braun C. et al (2018). Phosphene perception and pupillary responses to sinusoidal electrostimulation - for an objective measurement of retinal function. Exp. Eye Res.176, 210–218. 10.1016/j.exer.2018.07.010
37
Kelbsch C. Maeda F. Lisowska J. Lisowski L. Strasser T. Stingl K. et al (2017). Analysis of retinal function using chromatic pupillography in retinitis pigmentosa and the relationship to electrically evoked phosphene thresholds. Acta. Ophthalmol.95, e261–e269. 10.1111/aos.13259
38
Kurimoto T. Oono S. Oku H. Tagami Y. Kashimoto R. Takata M. et al (2010). Transcorneal electrical stimulation increases chorioretinal blood flow in normal human subjects. Clin. Ophthalmol.4, 1441–1446. 10.2147/OPTH.S14573
39
Kurimoto T. Ueda K. Mori S. Kamada S. Sakamoto M. Yamada-Nakanishi Y. et al (2020). A single-arm, prospective, exploratory study to preliminarily test effectiveness and safety of skin electrical stimulation for leber hereditary optic neuropathy. J.Clin. Med.9, 1359. 10.3390/jcm9051359
40
Li J. Zhou W. Liang L. Li Y. Xu K. Li X. et al (2024). Noninvasive electrical stimulation as a neuroprotective strategy in retinal diseases: a systematic review of preclinical studies. J. Transl. Med.22, 28. 10.1186/s12967-023-04766-4
41
Liu F. Zhang M. Xiong G. Han X. Lee V. W. H. So K. F. et al (2022). Trans-sclera electrical stimulation improves retinal function in a mouse model of retinitis pigmentosa. Life12, 1917. 10.3390/life12111917
42
Liu J. Tong K. Lin Y. Lee V. W. H. So K. F. Shih K. C. et al (2021). Effectiveness of microcurrent stimulation in preserving retinal function of blind leading retinal degeneration and optic neuropathy: a systematic review. Neuromodulation24, 992–1002. 10.1111/ner.13414
43
Lousteau R. J. (1987). Increased spiral ganglion cell survival in electrically stimulated, deafened Guinea pig cochleae. Laryngoscope97, 836–842. 10.1288/00005537-198707000-00012
44
Majeed A. Marwick B. Yu H. Fadavi H. Tavakoli M. (2021). Ophthalmic biomarkers for alzheimer's disease: a review. Front. Aging. Neurosci.13, 720167. 10.3389/fnagi.2021.720167
45
Mihashi T. Okawa Y. Miyoshi T. Kitaguchi Y. Hirohara Y. Fujikado T. (2011). Comparing retinal reflectance changes elicited by transcorneal electrical retinal stimulation with those of optic chiasma stimulation in cats. Jpn. J. Ophthalmol.55, 49–56. 10.1007/s10384-010-0906-x
46
Ming G. Henley J. Tessier-Lavigne M. Song H. Poo M. (2001). Electrical activity modulates growth cone guidance by diffusible factors. Neuron29, 441–452. 10.1016/s0896-6273(01)00217-3
47
Miura G. Fujiwara T. Ozawa Y. Shiko Y. Kawasaki Y. Nizawa T. et al (2023). Efficacy and safety of transdermal electrical stimulation in patients with nonarteritic anterior ischemic optic neuropathy. Bioelectron. Med.9, 22. 10.1186/s42234-023-00125-2
48
Miura G. Sugawara T. Kawasaki Y. Tatsumi T. Nizawa T. Baba T. et al (2019). Clinical trial to evaluate safety and efficacy of transdermal electrical stimulation on visual functions of patients with retinitis pigmentosa. Sci. Rep.9, 11668. 10.1038/s41598-019-48158-5
49
Miyake K. Yoshida M. Inoue Y. Hata Y. (2007). Neuroprotective effect of transcorneal electrical stimulation on the acute phase of optic nerve injury. Invest. Ophthalmol. Vis. Sci.48, 2356–2361. 10.1167/iovs.06-1329
50
Miyake Y. Yanagida K. Yagasaki K. (1980a). Clinical application of EER (electrically evoked response). (1) Analysis of EER in normal subjects (author's transl). Nippon. Ganka. Gakkai. Zasshi.84, 354–360. Japanese.
51
Miyake Y. Yanagida K. Yagasaki K. (1980b). Clinical application of EER (electrically evoked response) (2) Analysis of EER in patients with dysfunctional rod or cone visual pathway (author's transl). Nippon. Ganka. Gakkai. Zasshi.84, 502–509. Japanese.
52
Miyake Y. Yanagida K. Yagasaki K. (1980c). Clinical application of EER (electrically evoked response). (3) Analysis of EER in patients with central retinal arterial occlusion (author's transl). Nippon. Ganka. Gakkai. Zasshi.84, 587–593. Japanese.
53
Morimoto T. (2012). Role of electrical activity of neurons for neuroprotection. Int. Rev. Neurobiol.105, 19–38. 10.1016/B978-0-12-398309-1.00003-2
54
Morimoto T. Fujikado T. Choi J. S. Kanda H. Miyoshi T. Fukuda Y. et al (2007). Transcorneal electrical stimulation promotes the survival of photoreceptors and preserves retinal function in royal college of surgeons rats. Invest. Ophthalmol. Vis. Sci.48, 4725–4732. 10.1167/iovs.06-1404
55
Morimoto T. Fukui T. Matsushita K. Okawa Y. Shimojyo H. Kusaka S. et al (2006). Evaluation of residual retinal function by pupillary constrictions and phosphenes using transcorneal electrical stimulation in patients with retinal degeneration. Graefes. Arch. Clin. Exp. Ophthalmol.244, 1283–1292. 10.1007/s00417-006-0260-3
56
Morimoto T. Kanda H. Kondo M. Terasaki H. Nishida K. Fujikado T. (2012). Transcorneal electrical stimulation promotes survival of photoreceptors and improves retinal function in rhodopsin P347L transgenic rabbits. Invest. Ophthalmol. Vis. Sci.53, 4254–4261. 10.1167/iovs.11-9067
57
Morimoto T. Kanda H. Miyoshi T. Hirohara Y. Mihashi T. Kitaguchi Y. et al (2014). Characteristics of retinal reflectance changes induced by transcorneal electrical stimulation in cat eyes. PloS one9, e92186. 10.1371/journal.pone.0092186
58
Morimoto T. Miyoshi T. Fujikado T. Tano Y. Fukuda Y. (2002). Electrical stimulation enhances the survival of axotomized retinal ganglion cells in vivo. Neuroreport13, 227–230. 10.1097/00001756-200202110-00011
59
Morimoto T. Miyoshi T. Matsuda S. Tano Y. Fujikado T. Fukuda Y. (2005). Transcorneal electrical stimulation rescues axotomized retinal ganglion cells by activating endogenous retinal IGF-1 system. Invest. Ophthalmol. Vis. Sci.46, 2147–2155. 10.1167/iovs.04-1339
60
Morimoto T. Miyoshi T. Sawai H. Fujikado T. (2010). Optimal parameters of transcorneal electrical stimulation (TES) to be neuroprotective of axotomized RGCs in adult rats. Exp. Eye Res.90, 285–291. 10.1016/j.exer.2009.11.002
61
Motokawa K. Ebe M. Arakawa Y. Oikawa T. (1951). Retinal colour responses to microstimulation. Nature167, 729–730. 10.1038/167729a0
62
Motolese F. Capone F. Di Lazzaro V. (2022). New tools for shaping plasticity to enhance recovery after stroke. Handb. Clin. Neurol.184, 299–315. 10.1016/B978-0-12-819410-2.00016-3
63
Naycheva L. Schatz A. Röck T. Willmann G. Messias A. Bartz-Schmidt K. U. et al (2012). Phosphene thresholds elicited by transcorneal electrical stimulation in healthy subjects and patients with retinal diseases. Invest. Ophthalmol. Vis. Sci.53, 7440–7448. 10.1167/iovs.12-9612
64
Naycheva L. Schatz A. Willmann G. Bartz-Schmidt K. U. Zrenner E. Röck T. et al (2013). Transcorneal electrical stimulation in patients with retinal artery occlusion: a prospective, randomized, sham-controlled pilot study. Ophthalmol. Ther.2, 25–39. 10.1007/s40123-013-0012-5
65
Ni Y. Q. Gan D. K. Xu H. D. Xu G. Z. Da C. D. (2009). Neuroprotective effect of transcorneal electrical stimulation on light-induced photoreceptor degeneration. Exp. Neurol.219, 439–452. 10.1016/j.expneurol.2009.06.016
66
Okazaki Y. Morimoto T. Sawai H. (2008). Parameters of optic nerve electrical stimulation affecting neuroprotection of axotomized retinal ganglion cells in adult rats. Neurosci. Res.61, 129–135. 10.1016/j.neures.2008.01.016
67
Oono S. Kurimoto T. Kashimoto R. Tagami Y. Okamoto N. Mimura O. (2011). Transcorneal electrical stimulation improves visual function in eyes with branch retinal artery occlusion. Clin. Ophthalmol.5, 397–402. 10.2147/OPTH.S17751
68
Ota Y. Ozeki N. Yuki K. Shiba D. Kimura I. Tsunoda K. et al (2018). The Efficacy of transcorneal electrical stimulation for the treatment of primary open-angle glaucoma: a pilot study. Keio. J. Med.67, 45–53. 10.2302/kjm.2017-0015-OA
69
Ozeki N. Shinoda K. Ohde H. Ishida S. Tsubota K. (2013). Improvement of visual acuity after transcorneal electrical stimulation in case of Best vitelliform macular dystrophy. Graefes. Arch. Clin. Exp. Ophthalmol.251, 1867–1870. 10.1007/s00417-013-2341-4
70
Pardue M. T. Ciavatta V. T. Hetling J. R. (2014). Neuroprotective effects of low level electrical stimulation therapy on retinal degeneration. Adv. Exp. Med. Biol.801, 845–851. 10.1007/978-1-4614-3209-8_106
71
Pilloni G. Charvet L. E. Bikson M. Palekar N. Kim M. J. (2022). Potential of transcranial direct current stimulation in Alzheimer's disease: optimizing trials toward clinical use. J. Clin. Neurol.18, 391–400. 10.3988/jcn.2022.18.4.391
72
Potts A. M. Inoue J. (1969). The electrically evoked response (EER) of the visual system. II. Effect of adaptation and retinitis pigmentosa. Invest. Ophthalmol.8, 605–612.
73
Potts A. M. Inoue J. (1970). The electrically evoked response of the visual system (EER). 3. Further contribution to the origin of the EER. Invest. Ophthalmol.9, 814–819.
74
Potts A. M. Inoue J. Buffum D. (1968). The electrically evoked response of the visual system (EER). Invest. Ophthalmol.7, 269–278.
75
Powell K. White T. G. Nash C. Rebeiz T. Woo H. H. Narayan R. K. et al (2022). The potential role of neuromodulation in subarachnoid hemorrhage. Neuromodulation25, 1215–1226. 10.1016/j.neurom.2021.12.002
76
Rahmani S. Bogdanowicz L. Thomas J. Hetling J. R. (2013). Chronic delivery of low-level exogenous current preserves retinal function in pigmented P23H rat. Vis. Res.76, 105–113. 10.1016/j.visres.2012.10.016
77
Ranjan R. Chourey A. Kabir Y. García Mata H. D. Tiepolo E. Fiallos Vinueza I. L. et al (2024). Role of neurosurgical interventions in the treatment of movement disorders like Parkinson's disease, dystonia, and tourette syndrome. Cureus16, e72613. 10.7759/cureus.72613
78
Reed T. Cohen Kadosh R. (2018). Transcranial electrical stimulation (tES) mechanisms and its effects on cortical excitability and connectivity. J. Inherit. Metab. Dis.41, 1123–1130. 10.1007/s10545-018-0181-4
79
Sabel B. A. Fedorov A. B. Naue N. Borrmann A. Herrmann C. Gall C. (2011). Non-invasive alternating current stimulation improves vision in optic neuropathy. Restor. Neurol. Neurosci.29, 493–505. 10.3233/RNN-2011-0624
80
Sato T. Fujikado T. Lee T. S. Tano Y. (2008a). Direct effect of electrical stimulation on induction of brain-derived neurotrophic factor from cultured retinal Müller cells. Invest. Ophthalmol. Vis. Sci.49, 4641–4646. 10.1167/iovs.08-2049
81
Sato T. Fujikado T. Morimoto T. Matsushita K. Harada T. Tano Y. (2008b). Effect of electrical stimulation on IGF-1 transcription by L-type calcium channels in cultured retinal Müller cells. Jpn. J.Ophthalmol.52, 217–223. 10.1007/s10384-008-0533-y
82
Sato T. Lee T. S. Takamatsu F. Fujikado T. (2008c). Induction of fibroblast growth factor-2 by electrical stimulation in cultured retinal Mueller cells. Neuroreport19, 1617–1621. 10.1097/WNR.0b013e3283140f25
83
Schatz A. Pach J. Gosheva M. Naycheva L. Willmann G. Wilhelm B. et al (2017). Transcorneal electrical stimulation for patients with retinitis pigmentosa: a prospective, randomized, sham-controlled follow-up study over 1 year. Invest. Ophthalmol. Vis. Sci.58, 257–269. 10.1167/iovs.16-19906
84
Schatz A. Röck T. Naycheva L. Willmann G. Wilhelm B. Peters T. et al (2011). Transcorneal electrical stimulation for patients with retinitis pigmentosa: a prospective, randomized, sham-controlled exploratory study. Invest. Ophthalmol. Vis. Sci.52, 4485–4496. 10.1167/iovs.10-6932
85
Shimazu K. Miyake Y. Fukatsu Y. Watanabe S. (1996). Striate cortical contribution to the transcorneal electrically evoked response of the visual system. Jpn. J.Ophthalmol.40, 469–479.
86
Shimazu K. Miyake Y. Watanabe S. (1999). Retinal ganglion cell response properties in the transcorneal electrically evoked response of the visual system. Vis. Res.39, 2251–2260. 10.1016/s0042-6989(98)00331-9
87
Sinim Kahraman N. Oner A. (2020). Effect of transcorneal electrical stimulation on patients with retinitis pigmentosa. J. Ocul. Pharmacol. Ther.36, 609–617. 10.1089/jop.2020.0017
88
Stett A. Schatz A. Gekeler F. Franklin J. (2023). Transcorneal electrical stimulation dose-dependently slows the visual field loss in retinitis pigmentosa. Transl. Vis. Sci. Technol.12, 29. 10.1167/tvst.12.2.29
89
Su X. Zheng H. Li Q. Sun P. Zhou M. Li H. et al (2020). Retinal neurovascular responses to transcorneal electrical stimulation measured with optical coherence tomography. Exp. Biol. Med.245, 289–300. 10.1177/1535370219900495
90
Tagami Y. Kurimoto T. Miyoshi T. Morimoto T. Sawai H. Mimura O. (2009). Axonal regeneration induced by repetitive electrical stimulation of crushed optic nerve in adult rats. Jpn. J.Ophthalmol.53, 257–266. 10.1007/s10384-009-0657-8
91
Takei K. Nakano H. Hommura S. Iketani N. (1993). Analysis of the components of electrically evoked response using a monopolar recording technique. Invest. Ophthalmol. Vis. Sci.34, 1923–1929.
92
Tao Y. Chen T. Liu Z. Y. Wang L. Q. Xu W. W. Qin L. M. et al (2016). Topographic quantification of the transcorneal electrical stimulation (TES)-Induced protective effects on N-methyl-N-Nitrosourea-Treated retinas. Invest. Ophthalmol. Vis. Sci.57, 4614–4624. 10.1167/iovs.16-19305
93
Tew B. Y. Gooden G. C. Lo P. A. Pollalis D. Ebright B. Kalfa A. J. et al (2024). Transcorneal electrical stimulation restores DNA methylation changes in retinal degeneration. Front. Mol. Neurosci.17, 1484964. 10.3389/fnmol.2024.1484964
94
Wagner S. K. Jolly J. K. Pefkianaki M. Gekeler F. Webster A. R. Downes S. M. et al (2017). Transcorneal electrical stimulation for the treatment of retinitis pigmentosa: results from the TESOLAUK trial. BMJ. Ophthalmol.2, e000096. 10.1136/bmjophth-2017-000096
95
Wang X. Mo X. Li D. Wang Y. Fang Y. Rong X. et al (2011). Neuroprotective effect of transcorneal electrical stimulation on ischemic damage in the rat retina. Exp. Eye Res.93, 753–760. 10.1016/j.exer.2011.09.022
96
Willmann G. Schäferhoff K. Fischer M. D. Arango-Gonzalez B. Bolz S. Naycheva L. et al (2011). Gene expression profiling of the retina after transcorneal electrical stimulation in wild-type Brown Norway rats. Invest. Ophthalmol. Vis. Sci.52, 7529–7537. 10.1167/iovs.11-7838
97
Yang M. Lennikov A. Chang K. Ashok A. Lee C. Cho K. S. et al (2022). Transcorneal but not transpalpebral electrical stimulation disrupts mucin homeostasis of the ocular surface. BMC Ophthalmol.22, 490. 10.1186/s12886-022-02717-z
98
Yu H. Enayati S. Chang K. Cho K. Lee S. W. Talib M. et al (2020). Noninvasive electrical stimulation improves photoreceptor survival and retinal function in mice with inherited photoreceptor degeneration. Invest. Ophthalmol. Vis. Sci.61, 5. 10.1167/iovs.61.4.5
99
Yu W. S. Aquili L. Wong K. H. Lo A. C. Y. Chan L. L. H. Chan Y. S. et al (2022b). Transcorneal electrical stimulation enhances cognitive functions in aged and 5XFAD mouse models. Ann. N. Y. Acad. Sci.1515, 249–265. 10.1111/nyas.14850
100
Yu W. S. Kwon S. H. Agadagba S. K. Chan L. L. H. Wong K. H. Lim L. W. (2021). Neuroprotective effects and therapeutic potential of transcorneal electrical stimulation for depression. Cells10, 2492. 10.3390/cells10092492
101
Yu W. S. Tse A. C. K. Guan L. Chiu J. L. Y. Tan S. Z. K. Khairuddin S. et al (2022a). Antidepressant-like effects of transcorneal electrical stimulation in rat models. Brain Stimul.15, 843–856. 10.1016/j.brs.2022.05.018
Summary
Keywords
transcorneal electrical stimulation, neuroprotection, photoreceptor, retinal ganglion cell, neuromodulation
Citation
Morimoto T (2025) Transcorneal electrical stimulation: impact on healthcare and future potential. Front. Cell Dev. Biol. 13:1569759. doi: 10.3389/fcell.2025.1569759
Received
01 February 2025
Accepted
09 April 2025
Published
16 May 2025
Volume
13 - 2025
Edited by
Daisy Y. Shu, University of New South Wales, Australia
Reviewed by
Wen Wen, Fudan University, China
Chenna Kesavulu Sugali, Indiana University–Purdue University Indianapolis, United States
Anton Lennikov, Schepens Eye Research Institute and Harvard Medical School, United States
Updates
Copyright
© 2025 Morimoto.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takeshi Morimoto, takeshi.morimoto@ophthal.med.osaka-u.ac.jp
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.