- Department of Gynecology and Obstetrics, The Second Affiliated Hospital of Fujian Medical University, Quanzhou, China
Epithelial Cell Transformation Factor 2 (ECT2) is highly expressed in a variety of cancers, including gynecological tumors. The mislocalization of ECT2 can abnormally activate Ras homolog family member A (RhoA) in the Ras homolog gene family (Rho) Guanine nucleotide Exchange Factor (GEF) family. Activated RhoA binds to Rho-associated protein kinase (ROCK), phosphorylates various target proteins, triggers a cascade reaction, regulates the functions of downstream proteins, and thereby plays an important role in the occurrence and development of tumors. This article reviews the roles of ECT2 and RhoA/ROCK signaling pathways in ovarian cancer, cervical cancer, and endometrial cancer, and summarizes and discusses the research progress of downstream molecules, transduction pathways, and mechanisms related to them. Through comprehensive analysis and summary of the current research results, it is revealed that the ECT2/RhoA/ROCK signaling pathway and related crosstalk pathways play an important role in the occurrence, development, and metastasis of gynecological tumors. This article aims to provide a basis for related research and offer relevant references for the treatment of gynecological tumors in the future.
1 Introduction
ECT2 is closely related to cell proliferation-related pathways. As a guanine prenucleotide change factor of Rho GTPase, high ECT2 is a factor for poor prognosis in cancer patients (Cook et al., 2014). ECT2 is overexpressed in various cancers such as non-small lung cell carcinoma, glioblastoma, and prostate cancer (Sano et al., 2006; Hirata et al., 2009; Guo et al., 2017). Generally speaking, a high ECT2 level is an unfavorable factor for the prognosis of patients. Recent studies have shown that ECT2 can abnormally activate RhoA, and then the activated RhoA binds to ROCK, participating in the abnormal proliferation and migration of various tumor cells by mediating various cascade reactions (Zubor et al., 2020; Saito et al., 2004; Huff et al., 2013). Furthermore, multiple crosstalk pathways interact between ECT and the RhoA/ROCK signaling pathway, jointly participating in the occurrence and development of tumors. However, few studies have explored the role of the ECT2 and RhoA/ROCK signaling pathways in gynecological malignancies. This article aims to summarize the research status and related mechanisms of ECT2 and RhoA/ROCK in gynecological tumor tissues and to analyze and explore the interactions of related crosstalk signal molecules and signal pathways in the growth, invasion, metastasis, and diffusion of gynecological malignant tumor cells. Based on these mechanisms, future research can combine clinicopathological data to identify and validate new markers in gynecological cancers. This may improve the early detection of gynecological malignancies and provide potential value for future research on therapeutic targets.
2 ECT2 and RhoA/ROCK signaling pathways
2.1 Structure and role of ECT2
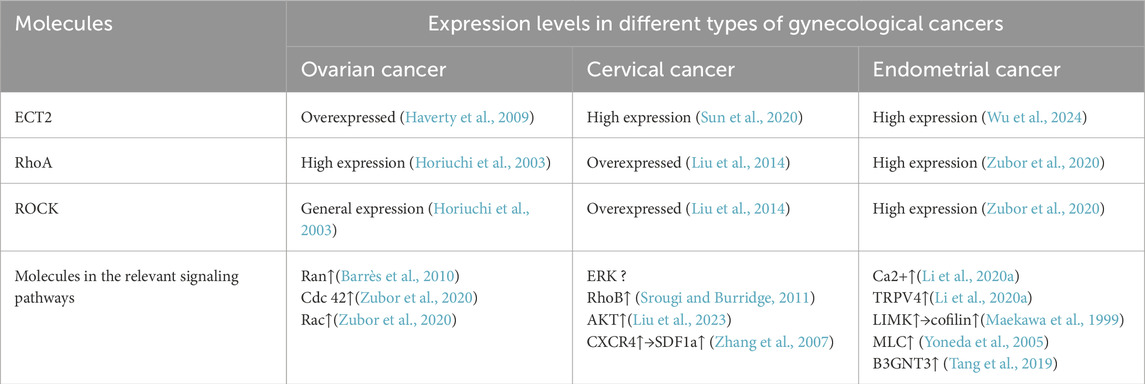
ECT2 is a GEF that plays a crucial role in cytokinesis (Tsuji et al., 2012). ECT2 contains multiple domains, the N-terminal domain is related to cell cycle regulators and is involved in cell cycle regulation. The N-terminal domain includes three tandem breast oncogene-1 carboxy terminus (BRCTs), namely, BRCT0, BRCT1, and BRCT2 (Sheng et al., 2011). Previous studies have suggested that the BRCT domain may be related to DNA damage response, cell cycle checkpoints, and DNA transcription. Therefore, if the structural changes in the BRCT domain and then cause ECT2 function mutations, it may lead to tumors or cancer (Huyton et al., 2000). Its N-terminal sequence also includes the X-ray repair cross complementing 1 (XRCC1) domain, a protein associated with repairing defective DNA strands (Thompson et al., 1990). Between the XRCC1 and BRCT domains is the Cyclin B6(Clb6) domain. In eukaryotic cells, the S phase is responsible for DNA damage repair and DNA replication. The occurrence of this cell cycle depends on cyclin-dependent kinase (CDK), and Clb6 is an important molecule that activates CDK (Palou et al., 2010). The other end of ECT2 is the C-terminal domain. There is a small domain connection between the N-terminal and C-terminal domains, also known as the S-loop, which contains a nuclear localization sequence (NLS) that regulates the intracellular localization of ECT2 (Solski et al., 2004). The C-terminus consists of Dbl homology (DH) and pleckstrin homology (PH) domains is the catalytic center of ECT2 and can activate RhoA GTPase (Solski et al., 2004) (Figure 1A). In the M phase, ECT2 is recruited to the central spindle and activates the Rho signaling pathway, which subsequently induces the formation and contraction of contractile rings (Niiya et al., 2006). Deletion of ECT2 interferes with cytokinesis but has no significant effect on mitosis. In addition to being involved in normal cell activity, ECT2 is also involved in cell malignant transformation, tumorigenesis, and metastasis (Saito et al., 2004). ECT2 contains the nuclear localization signal (NLS) and is mainly distributed in the nucleus (Justilien and Fields, 2009), it is independent of cytokinesis regulation, and nuclear ECT2 promotes cellular malignant transformation in other ways (Chen et al., 2015). In addition, ECT2 is a multifunctional oncogenic protein that upregulates Rho-extracellular signal-regulated kinase (Erk) signaling, promotes cell proliferation, inhibits apoptosis, and induces distant cell metastasis (Hall, 2012).
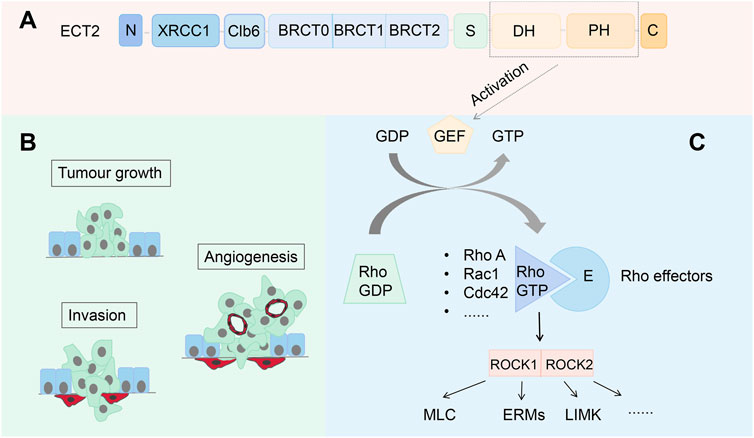
Figure 1. (A) Model of ECT2 structure; (B) Rho GTPase is involved in cell proliferation, invasion, and induction of tumor angiogenesis; (C) ECT2 catalyzes the conversion of GDP to GTP, which then binds to and activates the Rho protein; When RhoA binds to ROCK, it binds to or phosphorylates various target proteins to regulate the function of downstream proteins.
2.2 The function of Rho protein
Rho GTPases are molecular switches that act as signaling nodes to integrate extracellular signals and propagate intracellular signals. They control many normal cellular processes, including actomyosin remodeling, cell polarity, gene expression, and cell cycle progression (Ellenbroek and Collard, 2007). The most prominent members of this family are RhoA, ras-related C3 botulinum toxin substrate 1(Rac1), and cell division cycle 42(Cdc42). These Rho GTPases are often overexpressed or dysregulated in tumors (Ellenbroek and Collard, 2007). Rho GTPases regulate the cytoskeleton involved in the regulation of actin by regulating downstream effector protein activity (Aspenström et al., 2004; Ridley, 2006), thereby mediating vesicle trafficking to polarize cells (Halbleib and Nelson, 2006)and causing them to undergo a series of events such as cell shape change, cell adhesion, cell proliferation, and cell migration (Yoshioka et al., 1999). In addition, RhoA molecules localized to the cytoplasmic membrane are activated to signal ROCK and actomyosin to promote cell invasion (van Golen et al., 2002).In addition to this, Rho GTPase can also increase the production of angiogenesis, especially vascular endothelial growth factor (VEGF), which induces tumor angiogenesis and further promotes tumor cell metastasis (Saito et al., 2004). In conclusion, current studies have shown that Rho GTPase is involved in various processes of tumorigenesis and development in a variety of ways through multiple pathways (Figure 1B).
2.3 ECT2 can activate the RhoA/ROCK signaling pathway
The mislocalization of ECT2 can anomalously activate RhoA, Rac1, and Cdc42 in the Rho GTP family (Saito et al., 2004). GEF catalyzes the conversion of GDP to GTP and binds to Rho proteins. GDP-bound Rho proteins are in an off state, while GTP-bound proteins are in an open state, where they bind to their effector targets to exert effects and transmit downstream signals (Julian and Olson, 2014). Activated RhoA binds to ROCK, which then phosphorylates various target proteins or binds to certain target proteins to regulate the function and role of downstream proteins, which in turn leads to cell proliferation and migration (Huff et al., 2013) (Figure 1C). The NLS in the ECT2 domain locate it in the cell nucleus. At this stage, the N-terminal domain in ECT2 interacts with the catalytic domain to inhibit its exchange activity and isolate it from Rho GTPases. However, in tumor cells, the NLS structure of ECT2 is missing, resulting in its mislocalization into the cytoplasm, the disappearance of its auto-inhibitory effect, and then the activation of Rho family GTPase to drive transformation (Saito et al., 2004).
3 Molecules and signaling pathways involved in ovarian cancer
The mortality rate of ovarian cancer ranks first among gynecological malignant tumors, and its poor prognosis is due to the insidious onset of the disease, no obvious specific symptoms in the early stage, and no specific screening method has been found at present, and about 70% of patients are already at an advanced stage when they are found. Therefore, a deeper understanding of the molecular process of early ovarian cancer and the identification of specific biomarkers can help predict and diagnose its occurrence earlier.
By retrieving and obtaining transcriptome sequencing data from 427 ovarian cancer samples in the TCGA database (https://cancergenome.nih.gov/), significant differences were found in the expression level of ECT2 between ovarian cancer and normal ovary (Figure 2A). Survival analysis revealed that the expression level of ECT2 was associated with patient prognosis, and the survival time of patients in the high ECT2 expression group was shorter than that in the low ECT2 expression group (Figure 2B). And through the correlation analysis of the expressions of ECT2 and RHOA, ROCK1, and ROCK2 in ovarian cancer samples in TCGA database, it was found that the expression of ECT2 was significantly positively correlated with RhoA, ROCK1, and ROCK2 (Figure 2C).
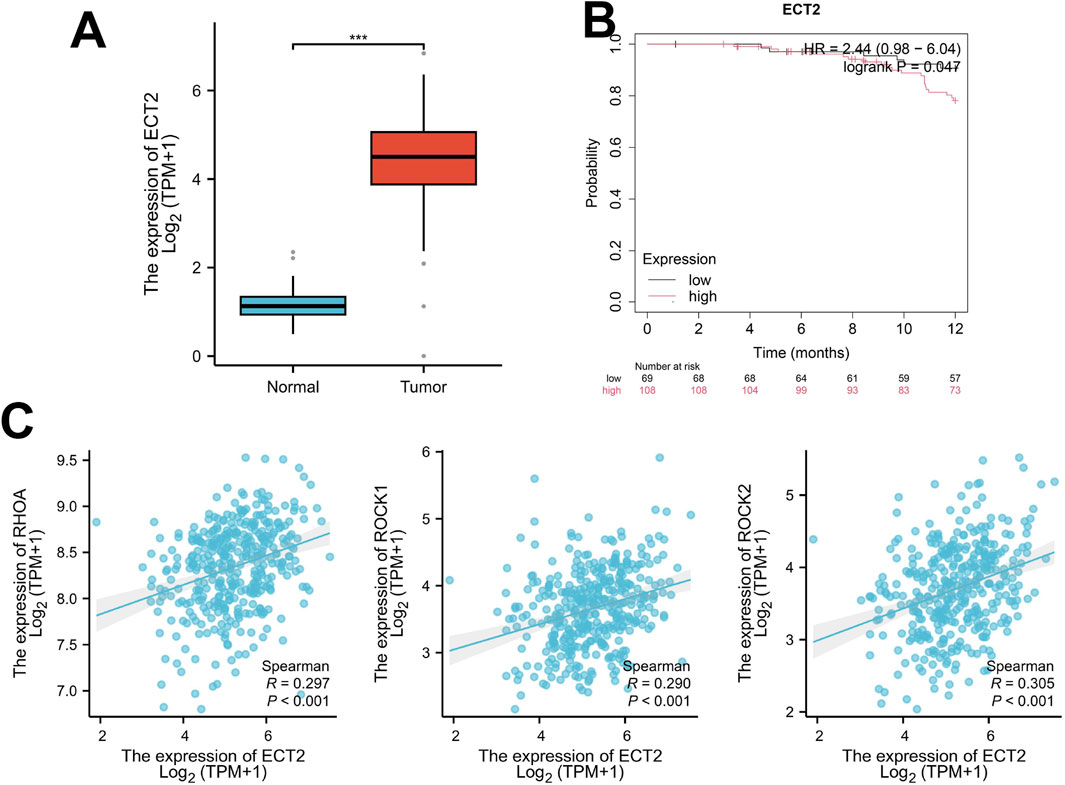
Figure 2. (A) The expression of ECT2 in ovarian cancer tissues is significantly higher than that in normal ovarian tissues; (B) The survival time of the ECT2 high-expression group was shorter than that of the ECT2 low-expression group; (C) The expression of ECT2 in ovarian cancer was significantly positively correlated with RhoA, ROCK1, and ROCK2.
3.1 ECT2 is highly expressed in ovarian cancer
Ovarian cancer is a malignancy with the second-highest frequency of ECT2 amplification, and ECT2 is also overexpressed at the mRNA level (Haverty et al., 2009). Bioinformatics analysis showed that ECT2, as a part of the 3q26 amplicon, co-amplified and overexpressed with (protein kinase C iota) PRKCI, and other molecules in ovarian serous cancer (Callebaut and Mornon, 1997; Wang et al., 2013). Genetic mutations in BRCA1 are known to predispose to breast and ovarian cancer (Mota et al., 2023), as an important tumor suppressor gene, BRCA1 plays a key role in the occurrence and development of breast cancer and ovarian cancer, and BRCA1 protein is composed of multiple domains, among which the BRCT domain is its core component (Ismail et al., 2024). Functionally, BRCA1 maintains genome integrity through homology-directed repair (HDR) and stall fork protection (SFP), while the recognition of phosphoric acid by the BRCT domain is a key link in the HDR and SFP pathways. Once there is a mutation in the BRCT domain, HDR and SFP will fail at the same time, resulting in impaired mechanisms for maintaining genomic stability (Zubor et al., 2020). As mentioned above, the ECT2 domain includes three tandem BRCTs, which may be one of the important mechanisms by which ECT2 is involved in the occurrence and development of ovarian cancer. An experimental result showed that ECT2 can act as GEF to mediate the transformation and growth of tumor cells by activating Rac in the nucleus and RhoA in the cytoplasm; In cells, nuclear-localized ECT2 and its Rho GEF activity can promote the growth and transformation of ovarian cancer cells; However, cytoplasmically localized ECT2 does not promote the transformation of cancer cells, but may counteract its role in the nucleus (Haverty et al., 2009).
3.2 The occurrence and progression of ovarian cancer are related to the RhoA/ROCK signaling pathway
In one study, the expression level of RhoA mRNA was quantified using real-time polymerase chain reaction (RT-PCR) and Western blotting (WB). It found a strong response band of RhoA mRNA was observed after reverse transcription(RT) response from ovarian malignant tumors, while weak response bands were observed in benign or borderline tumors, indicating that the expression level of RhoA mRNA in ovarian malignant tumors was significantly higher than benign and borderline tumors (Horiuchi et al., 2003; Zaoui et al., 2019). In the histological subtypes of ovarian cancer, the expression level of RhoA mRNA in serous carcinoma is significantly higher than that of endometrial or clear cell carcinoma (Barrès et al., 2010). One case study found that in the FIGO stages of ovarian cancer (Shepherd, 1989), the expression levels of RhoA mRNA were significantly higher in stage III-IV tumors than in stage I-II (Ellenbroek and Collard, 2007). In addition, the response band of ROCK was observed in all ovarian tumors, but there was no significant difference in benign, borderline, or malignant tumors (Barrès et al., 2010).
RhoA GTPase can form a complex with the small GTPase nucleocytoplasmic shuttle protein Ras-associated nucleoprotein (Ran), which is closely related to the occurrence, progression, survival, and recurrence of epithelial ovarian cancer (EOC) (Horiuchi et al., 2003). The comparative analysis of primary and metastatic lesions of ovarian cancer showed that the expression level of RhoA mRNA in metastatic lesions was significantly higher than that of primary lesions (Barrès et al., 2010). Ran regulates cell proliferation and migration or invasion through RhoA recruitment (Horiuchi et al., 2003), This may be the mechanism for its higher expression in metastatic lesions. It has been reported that the depletion of Ran can reduce tumor cell migration, prevent EOC cell proliferation, and inhibit their growth (McGrail et al., 2014; Liu et al., 2023). These suggest that the Ran-RhoA signaling complex may be a molecular target to control ovarian cancer metastasis and may be a potential therapeutic pathway. Previous studies have shown that ovarian cancer cells are more likely to metastasize through peritoneal implantation, preferentially proliferating and metastasizing in soft tissues, such as fat cells, which have an abundant source of energy. Further studies have shown that this phenomenon may be due to its chemical and mechanical sensitivities (Sharma et al., 2012). The mechanistic sensitivity is related to the RhoA-ROCK signaling pathway (Horiuchi et al., 2003; Sharma et al., 2012). Fat cells secrete more leptin, which functions through the leptin receptor (Ob-Rb) (Ghasemi et al., 2017). After leptin binds to OB-Rb on the surface of ovarian cancer cells, it can increase the expression of RhoA and activate the RhoA-ROCK pathway. On the other hand, studies have found that soft matrices can alter the local mechanical properties of the cellular environment and the density of binding sites, thereby optimizing cell adhesion in local areas and further enhancing the generation of intracellular internal forces (Sun et al., 2022; Maheshwari et al., 2000). Enhanced intracellular internal forces can activate the RhoA-ROCK pathway (Sun et al., 2022). These two pathways activate the RhoA-ROCK pathway and induce the activation of myosin phosphatase targeting subunit 1(MYPT1) and urokinase (uPA) (Ghasemi et al., 2017; Leve et al., 2011; Kato et al., 2015). MMP7 and uPA can reorganize the cytoskeleton, stimulate the formation of stress fibers and focal adhesion complexes, and regulate cell movement to affect the invasion and metastasis of tumor cells (Kato et al., 2015). In this study, the occurrence of metastasis was reduced by inhibiting the RhoA-ROCK signaling pathway to reduce the mechanosensitivity of tumor cells (Sharma et al., 2012). And, cisplatin resistance in ovarian cancer has been reported to be related to its mechanistic nature (Solski et al., 2004). These studies have shown that the Rho-ROCK signaling pathway is a potential therapeutic pathway for ovarian cancer, and it is expected that new drugs will be developed as a target (Figure 3A).
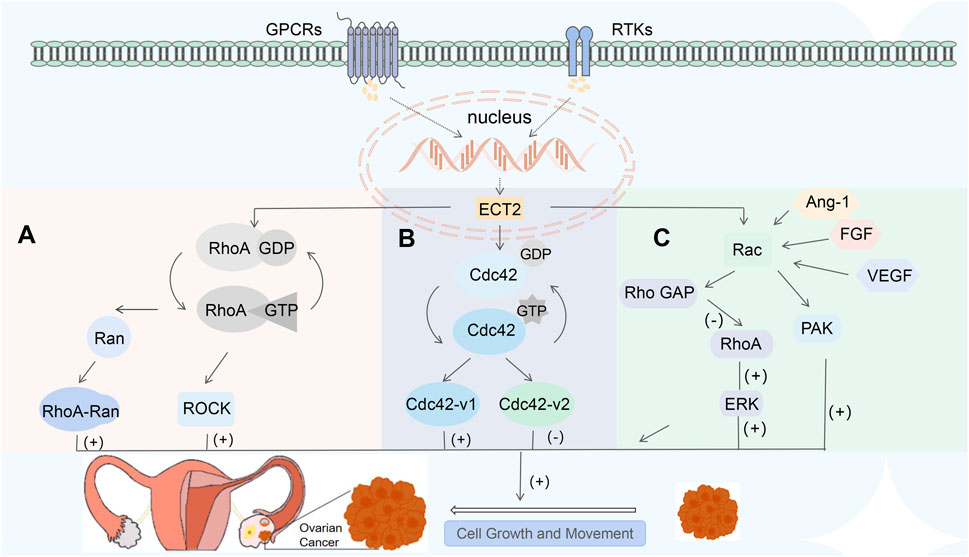
Figure 3. (A) RhoA is involved in cell metastasis by forming complexes with RAN and activating ROCK to regulate the mechanosensitivity of tumor cells; (B) ECT2 can bind Cdc42 and GTP to activate various downstream effectors; Cdc42-v1 and Cdc42-v2 have the opposite effect; (C) Rac is activated by VEGF, Ang-1, FGF, etc., which then mediates cascades of downstream molecules; Rac and RhoA are antagonistic to each other.
3.3 Other crosstalk pathways mediated by ECT2
Sequences outside the DH and PH domains in ECT2 may affect the GTPase specificity of ECT2, causing it to activate not only RhoA but also Rac and Cdc42 in vivo (Cerione, 2004). CDC42 gene is involved in the regulation of various cellular processes such as cytoskeletal remodeling, cell migration, proliferation, and adhesion (Sinha and Yang, 2008). GEF converts Cdc42 from the inactive GDP-binding form to the active GTP-binding form. Conversely, the activated Cdc42 mediates the signal cascade reaction, leading to the activation of various downstream effectors and thereby participating in the tumorigenesis process (He et al., 2015). Previous studies have found that on the one hand, Cdc42 promotes tumor transformation, tumor invasion, and metastasis (Yang et al., 2007); on the other hand, the knockdown of the CDC42 gene leads to dysregulated expression of key transcription factors that promote tumorigenesis or partial transformation (Hudson et al., 2018). This suggests that the CDC42 gene is also a tumor suppressor. It is not clear what mechanism causes this contradiction. The human Cdc42 gene encodes two distinct Cdc42 variants, Cdc42-v1 and Cdc42-v2. Previous studies have hypothesized that: the tumor-promoting activity of the CDC42 gene is derived from CDC42-V1, while the tumor-suppressive activity is derived from CDC42-V2 (Yang et al., 2007), but there is a lack of research on its related mechanisms. (Figure 3B).
The small GTPase Ras-related C3 botulinum toxin substrate (Rac) is the intersection of multiple signaling pathways, which is involved in cell proliferation, apoptosis, differentiation, tumor metastasis, and regulates cell-environment interactions (Wang et al., 2018). Existing studies have confirmed that Rac is highly expressed in EOC (Leng et al., 2015). Excessive activation of Rac is an important driving factor for ovarian cancer. Inhibiting Rac activity or knocking down Rac expression can suppress the migration and invasion of ovarian cancer cells (Hudson et al., 2018). Previous studies have shown that nuclear Rac activity can rescue defects in ECT2-mediated transformation growth (Haverty et al., 2009). Angiogenesis promotes tumor growth by providing essential nutrients to tumor cells (Scott et al., 2010), and Rac is an essential part of the angiogenesis process (Zhu et al., 2004). Rac is activated by vascular endothelial growth factor (VEGF), angiogenin 1 (Ang-1), and fibroblast growth factor (FGF) (Bid et al., 2013), thereby regulating cell adhesion, cell proliferation and migration, and participating in tumor vascular invasion (Brantley-Sieders et al., 2009). Extracellular signaling mediated by various cell surface receptors, such as integrins, cytokine receptors, G protein-coupled receptors (GPCRs), cadherins, and receptor tyrosine kinases (RTKs), can enable ECT2 to function to recruit Rac from the cytoplasm to the plasma membrane or other cellular locations (Chan et al., 2005; Bensen and Brognard, 2021); Rac then activates downstream effector molecules, including various proteins, kinases, and adaptor proteins that mediate subsequent cascade reactions, such as p21-activated kinase (PAK), etc. (Brantley-Sieders et al., 2009). The activity of Rho family GTPases is determined by three different types of regulatory molecules: specific GEF, GTPase-activating proteins (GAP), and guanine nucleotide dissociation inhibitors (GDI). GEF activates Rho GTPase by catalyzing the exchange between the bound GDP molecules and GTP, while GAP stimulates the intrinsic GTPase activity of Rho proteins, thereby restoring them to an inactive GDP-binding state (Bos et al., 2007). GDI is responsible for maintaining a stable and inactive cytoplasmic bank of Rho GTP enzymes (Garcia-Mata et al., 2011). Rac GAP can inactivate Rac by reducing the level of GPT-RAC. Interestingly, the level of RhoA was also upregulated in EOC cells overexpressing Rac GAP; Similarly, Rac GAP is positively correlated with the activity and expression of the downstream effector Erk protein of RhoA. Conversely, knocking down the Rac GAP inhibited the level of RhoA (Wang et al., 2018). As the activity of Rac gradually increases, the Rho GAP mediated by Rac will be activated, and then the activity of RhoA will decrease (Lawson and Burridge, 2014). This indicates that there is a negative crosstalk between Rac and RhoA. Current researchers are still unable to explain why Rac and RhoA antagonize each other but simultaneously promote the occurrence and development of ovarian cancer. The mechanism therein awaits further study (Figure 3C).
4 Molecules and signaling pathways involved in cervical cancer and human papillomavirus (HPV)
Cervical cancer is still the fourth most common cancer in women worldwide, and the occurrence of cervical cancer is associated with persistent HPV virus infection. Therefore, it is particularly important to elucidate the mechanism of the occurrence and progression of cervical cancer and explore new treatments.
By retrieving and obtaining transcriptome sequencing data from 306 cervical cancer samples in the TCGA database (https://cancergenome.nih.gov/), significant differences were found in the expression level of ECT2 between cervical cancer and normal cervix (Figure 4A). Moreover, survival analysis revealed that the expression level of ECT2 was related to the prognosis of patients. The survival time of patients in the high expression group of ECT2 was shorter than that in the low expression group of ECT2 (Figure 4B). And through the correlation analysis of the expressions of ECT2 and RHOA, ROCK1, and ROCK2 in cervical cancer samples in TCGA database, it was found that the expression of ECT2 was significantly positively correlated with RhoA, ROCK1, and ROCK2 (Figure 4C).
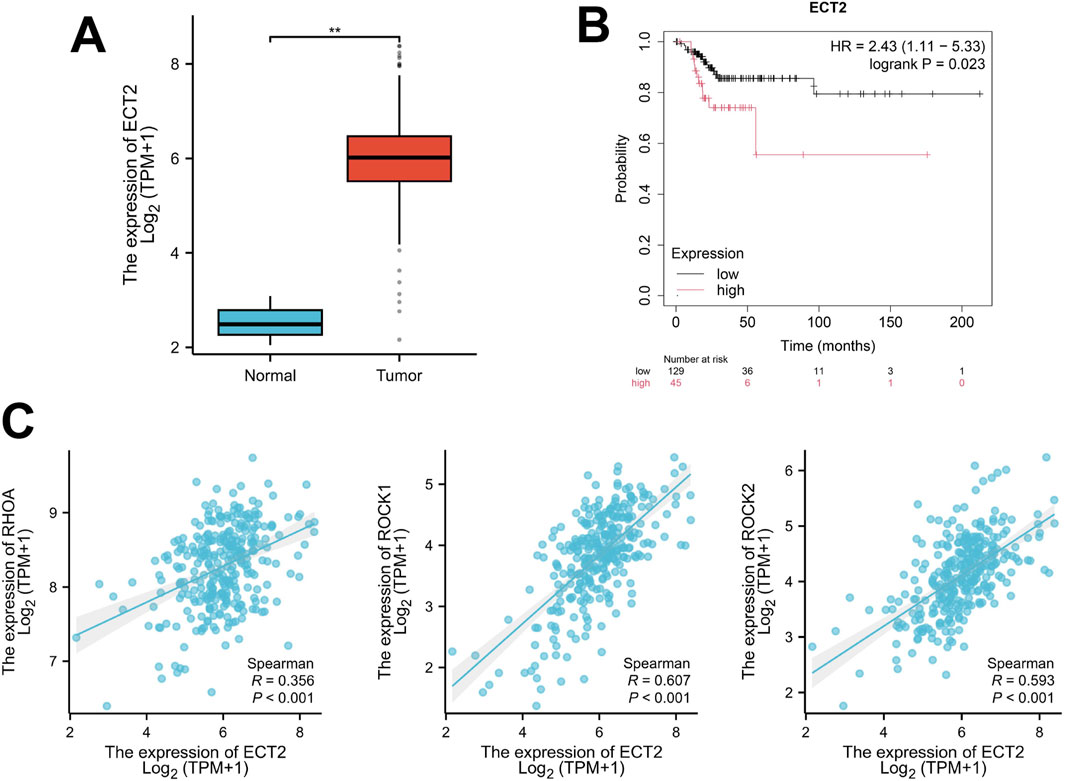
Figure 4. (A) The expression of ECT2 in cervical cancer tissues is significantly higher than that in normal cervical tissues; (B) The survival time of the ECT2 high-expression group was shorter than that of the ECT2 low-expression group; (C) The expression of ECT2 in cervical cancer was significantly positively correlated with RhoA, ROCK1, and ROCK2.
4.1 ECT2 is highly expressed in cervical cancer
Squamous cell carcinoma of the cervix accounts for 75%–80% of cervical cancers. The effect of ECT2 on cervical cancer is currently unknown. Previous studies have shown that chromosome 3q contains many oncogenes that determine squamous cell differentiation, including ECT2, and its amplification is the most common chromosomal aberration in most squamous cell carcinomas, and it is also a key gene that leads to squamous cell differentiation (Hudson et al., 2018; Sun et al., 2020). ECT2 regulates apoptosis after DNA damage in cervical cell lines (Chen et al., 2022), and the expression of this gene increases with the onset of DNA synthesis (Chen et al., 2015). ECT2 is the gene with the highest -fold upregulation in chromosome 3q, making it a good candidate for a driver oncogene in cervical cancer cells (Chen et al., 2022). Previous studies downloaded gene expression data from Gene Expression Omnibus (GEO) normal cervical tissues and cervical cancer tissues and applied bioinformatics analysis to show that the expression level of ECT2 mRNA in cervical cancer tissues was higher than that in normal cervical tissues based on GSE9750, GSE29570, and GSE52903 datasets (Sharma et al., 2012). Some studies have found that after the knockdown of ECT2, the proliferation rate of cervical cancer cells decreases; However, after overexpression of ECT2, the proliferation rate of cervical cancer cells increases (Amaya et al., 2017). In addition, one study significantly improved the migration and invasion ability of ECT2 after increasing its expression. After injecting cancer cells with high ECT2 expression into mice, the volume and weight of tumors in mice increased significantly compared to the control group (Sharma et al., 2012). These results suggest that ECT2 may act as a pro-tumor gene to promote cervical cancer cell proliferation.
4.2 ECT promotes the proliferation and migration of cervical cancer cells through the RhoA pathway
Previous reports have shown that ECT2 has Rho GTPase activity, which may promote the occurrence and progression of squamous cell carcinoma by mediating the overexpression of Rho A (Mendez and Ramirez, 2013; Chen et al., 2015; Sun et al., 2020). Previous studies have shown that the expression of RhoA, ROCK-1, and ROCK-2 is significantly elevated in cervical cancer cells. Also, the expression of RhoA was higher in patient specimens of vascular invasion or lymph node metastasis. In this study, the methods of functional gain and loss of function were used to further verify the promoting effect of RhoA in the proliferation of cervical cancer cells: The proliferation of Hela cells overexpressing RhoA in the experimental group was significantly higher than that in the control group. RhoA overexpression in Hela (RhoA+) cells was then blocked with RhoA-specific siRNA, and the proliferation of Hela (RhoA+) cells was significantly lower than that of the control group, and it was determined that siRNA transfection reversed the promotion of HeLa cell proliferation by overexpressing RhoA (Mendez and Ramirez, 2013). Previous studies have confirmed that ECT2 promotes the development and metastasis of hepatocellular carcinoma and esophageal squamous cells by modulating the RhoA/ERK signaling axis (Xu et al., 2013). ECT2 may be also involved in the occurrence and progression of cervical cancer through this signaling pathway, but no further studies have confirmed this so far. Rho GTPases are thought to be able to modulate the release of pro-angiogenic factors to promote neovascularization (Sun et al., 2020), and induction of tumor vascularization plays a key role in promoting the progression and metastasis of malignant tumors. Matrix metalloproteinases (MMPs) and VEGF are involved in angiogenesis, metastasis, and tumor cell proliferation (Tanaka et al., 2020). Overexpression of ECT2 can activate RhoA binding to GTP, which can then activate ERK to produce p-ERK (Sun et al., 2020; Cao et al., 2021). Activation of p-ERK can greatly increase the expression levels of MMP and VEGF in cervical cancer cell lines (Tanaka et al., 2020). This suggests that inducing tumor vascularization may act through a signaling pathway composed of ECT2, RhoA, RhoA-GTP, ERK, and VEGF/MMP. (Figure 5A1).
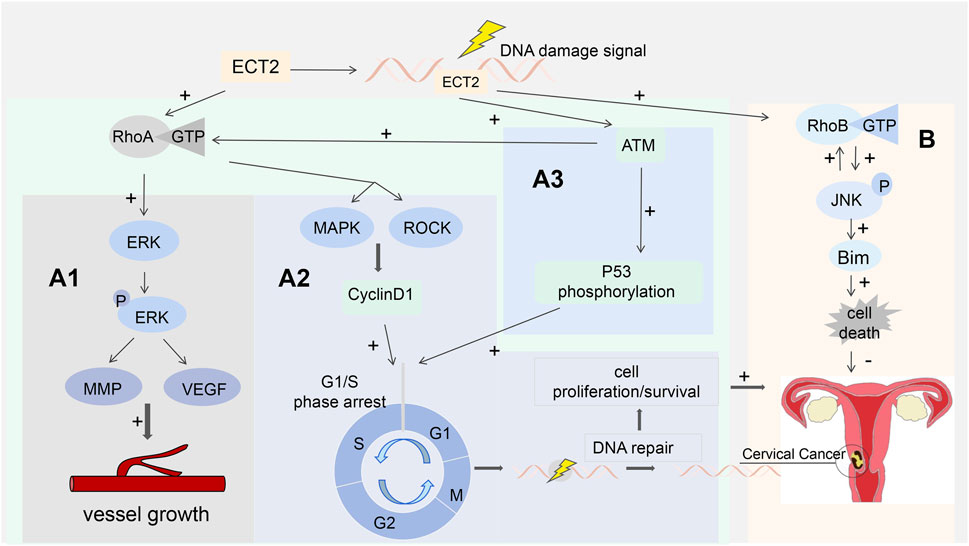
Figure 5. (A1) ECT2 binds RhoA to GTP, which then activates ERK to produce p-ERK, which in turn increases the expression levels of MMP and VEGF; (A2) ATM activates RhoA and mediates the MAPK and ROCK pathways to regulate the expression level of cyclin D1 and participate in the DDR process; (A3) p53 is the substrate of ATM participating in DDR; ECT2 assists in ATM-mediated phosphorylation of p53; (B) The ECT2-RhoB-JNK pathway leads to cell death by triggering the pro-apoptotic protein Bim.
In terms of treatment, previous studies have shown that ECT2 in cancer cells overcomes endogenous DNA damage by promoting double-strand break (DSB) repair to maintain their survival and reduce the effect of radiotherapy or chemotherapy (Liu et al., 2014). In patients with FIGO stage III, RhoA overexpression was associated with significantly worse disease-free survival (DFS) and long-distance metastasis-free survival (DMFS); It is also associated with distant metastases after concurrent chemoradiotherapy (CCRT); These may be related to the ECT-RhoA-ROCK signaling pathway (Liu et al., 2014; Osaki et al., 2016; Sun et al., 2020). Ionizing radiation can inhibit cell metastasis in which RhoA activity decreases, and RhoA overactivation causes cells to metastasize after radiation therapy (Ridley, 2001). This suggests that the regulation of RhoA activity affects the sensitivity of cervical cancer cells to radiation therapy (Mordes et al., 2008). ECT2 and RhoA can be used as potential therapeutic targets for the reversal of tolerance to radiotherapy and chemotherapy for cervical cancer. The N-terminal BRCT domain of ECT2 is highly similar to the sequence of DNA topoisomerase 2-binding protein 1 (Sheng et al., 2011). Topoisomerase 2-binding protein 1 is an important regulator of DNA damage response (DDR) (Shiloh, 2001). As a result, ECT2 recruits damaged chromatin to participate in DSB repair and DDR processes. Ataxia - Telangiectasia Mutated (ATM) kinases are key proteins responsible for detecting DNA damage and activating cellular responses to avoid genetic instability (Cheng et al., 2021). ATM-dependent activation of RhoA is involved in the DDR process by regulating the expression level of cyclin D1 through its downstream mitogen-activated protein kinase (MAPK) and ROCK (Paull, 2015) (Figure 5A2). p53 is a substrate in which ATM participates in DDR (He et al., 2016). ECT2 acts as an adaptor protein to assist ATM-mediated phosphorylation of p53 (Srougi and Burridge, 2011) (Figure 5A3).
4.3 ECT mediates other crosstalk pathways and cervical cancer
Preliminary studies have shown that DNA damage-mediated activation of ATM can trigger ECT2 localization to the cytoplasm, and ECT2 in the cytoplasm can activate RhoB (Chen et al., 2000). Many studies have shown that RhoB may have antitumor activity and can inhibit the malignant transformation of tumor cells (Mazieres et al., 2004). Re-expression of RhoB reduces proliferation and tumor growth in vivo (Xie et al., 2019). Cyclin B1 plays a key role in inhibiting apoptosis in tumor cells (Kamasani et al., 2004). RhoB can inhibit the activity of the cyclin B1 promoter to reduce its expression level, which in turn leads to cervical cancer cell death (Sun et al., 2002). C-Jun N-terminal kinase (JNK) is a downstream signal of RhoB, which can directly upregulate the expression level of RhoB and/or increase the levels of GEF and ECT2 to increase the activity of RhoB, thereby phosphorylating JNK, triggering the pro-apoptotic protein Bim and leading to cell death; it has been speculated that there is a positive feedback relationship between them, with RhoB activity stimulating sustained JNK phosphorylation, thereby enhancing cervical cancer cell death through upregulation of RhoB (Chen et al., 2000) (Figure 5B). It has been proven that ECT2 also promotes cervical cancer progression by regulating the AKT/mTOR pathway, and the expression levels of p-AKT and p-mTOR are also significantly increased in cells overexpressing ECT2, while the opposite is true for underexpressing ECT2. ECT2 can directly bind to AKT to form the ECT2/AKT complex, which in turn promotes the phosphorylation of AKT, thereby regulating the downstream mTOR pathway. To further validate the relationship between them, the accelerated proliferation and reduced apoptosis induced by ECT2 overexpression were negated by the transfection of si-AKT into ECT2-overexpressing cervical cancer cells; These results suggest that the downregulation of AKT can reverse the anti-apoptosis, proliferation and activation of the AKT/mTOR pathway induced by ECT2 overexpression, and further reveal that ECT2 promotes the malignant progression of cervical cancer by regulating the AKT/mTOR pathway (Sharma et al., 2012). ECT2 can mediate a variety of signaling pathways involved in the progression of cervical cancer, and other pathways are still under investigation.
4.4 RhoA is associated with high-risk HPV infection
Previous reports have shown no correlation between RhoA expression and age, sex, tumor size, and HPV infection, but a positive correlation with FIGO stage, vascular invasion, and lymphatic metastasis (Mendez and Ramirez, 2013; Osaki et al., 2016). In another report, RhoGTPase activity was increased in SiHa, a high-risk human papillomavirus (hr-HPV)-infected cervical cell line. The oncogenic potential of hr-HPV is attributed to its E6 and E7 tumor proteins, and the presence of E6 tumor proteins is associated with increased RhoGTPase activity and RhoA expression. RhoA GTPase is negatively regulated by ARHGAP35 (p190GAP family) and is ARHGAP35 one of the targets of E7, and HPV E7 is involved in cell migration by regulating RhoA and Rac1 (Zaoui et al., 2019). High expression of some chemokines such as SDF-1α and its migration-promoting effect on HeLa cells have been identified early in cervical cancer cells (Zhang et al., 2007). C-X-C chemokine receptor type 4 (CXCR4) was also found to be highly expressed in HPV-positive cervical cancer cells (Bleul et al., 1996). CXCR4 is a chemokine receptor, whereas stromal cell-derived factor-1α (SDF-1α) is the only chemokine of CXCR4 (Peng et al., 2005). The binding of SDF-1α to CXCR4 results in calcium mobilization, reduction of intracellular cyclic AMP/AMP proteins, and activation of multiple signal transduction pathways (Bartolomé et al., 2004). Among them, SDF-1α incubation stimulates CXCR4, which in turn activates the RhoA/ROCK-mediated cascade in cancer cells (Kaibuchi et al., 1999; Amine et al., 2009). On the one hand, the interaction between SDF-1α and CXCR4 can remodel the basement membrane and degrade the basement membrane (Amine et al., 2009); On the other hand, in harmony with the degradation of the basement membrane, the RhoA/ROCK-mediated cascade can reorganize the actin cytoskeleton and activate the motility mechanism of tumor cells (Baiden-Amissah et al., 2021). The combination of these two aspects may be the mechanism that leads to the trans-basement membrane invasion of HPV-positive cervical cancer cells (Figure 6). The mechanism by which Rho GTPase signaling interacts with human papillomavirus proteins is still under investigation.
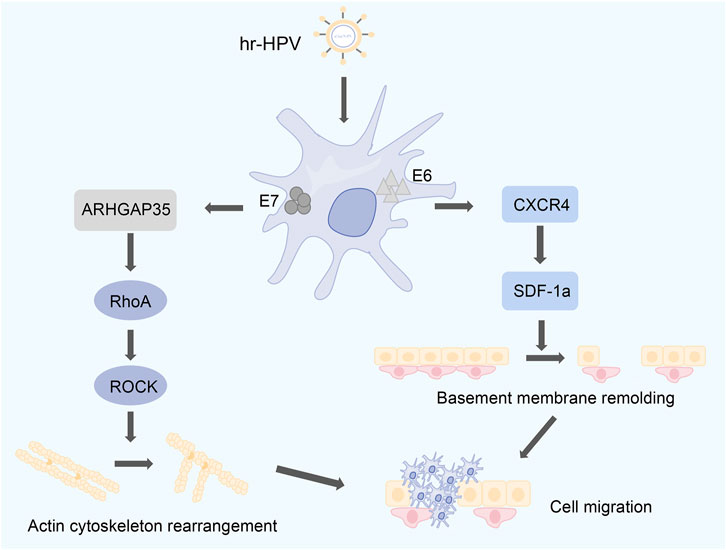
Figure 6. The carcinogenic potential of hr-HPV is associated with E6 and E7. E6 is associated with high expression of RhoA. E7 regulates the expression levels of RhoA and Rac1 through ARHGAP35. SDF-1α stimulates CXCR4, which in turn activates the RhoA/ROCK-mediated cascade. On the one hand, the interaction between SDF-1α and CXCR4 can remodel the basement membrane of cells. On the other hand, the RhoA/ROCK-mediated cascade can reconstitute the actin cytoskeleton.
5 Role and mechanism of RhoA/ROCK in endometrial cancer
The overall 5-year survival rate for endometrial cancer (EC) in the early stages ranges from 74% to 91%, but the survival rate for advanced-stage cancer is only 20%–26% because there is no effective treatment in advanced and metastatic cases. ECs are divided into two groups by histopathological features, type I includes estrogen-dependent tumors, type II is hormone-independent tumors, and type I is more common, accounting for 80% of all tumors (Hao et al., 2015). Distant metastases are the most common cause of death from endometrial cancer (Izdebska et al., 2018). Therefore, it is important to study the biomarkers and molecular mechanisms related to endometrial cancer cell migration.
By retrieving and obtaining transcriptome sequencing data from 554 endometrial cancer samples in the TCGA database (https://cancergenome.nih.gov/), significant differences were found in the expression level of ECT2 between endometrial cancer and normal endometrium (Figure 7A), Moreover, survival analysis revealed that the expression level of ECT2 was related to the prognosis of patients. The survival time of patients in the high expression group of ECT2 was shorter than that in the low expression group of ECT2 (Figure 7B). And through the correlation analysis of the expressions of ECT2 and RhoA, ROCK1, and ROCK2 in endometrial cancer samples in TCGA database, it was found that the expression of ECT2 was significantly positively correlated with RHOA, ROCK1, and ROCK2 (Figure 7C).
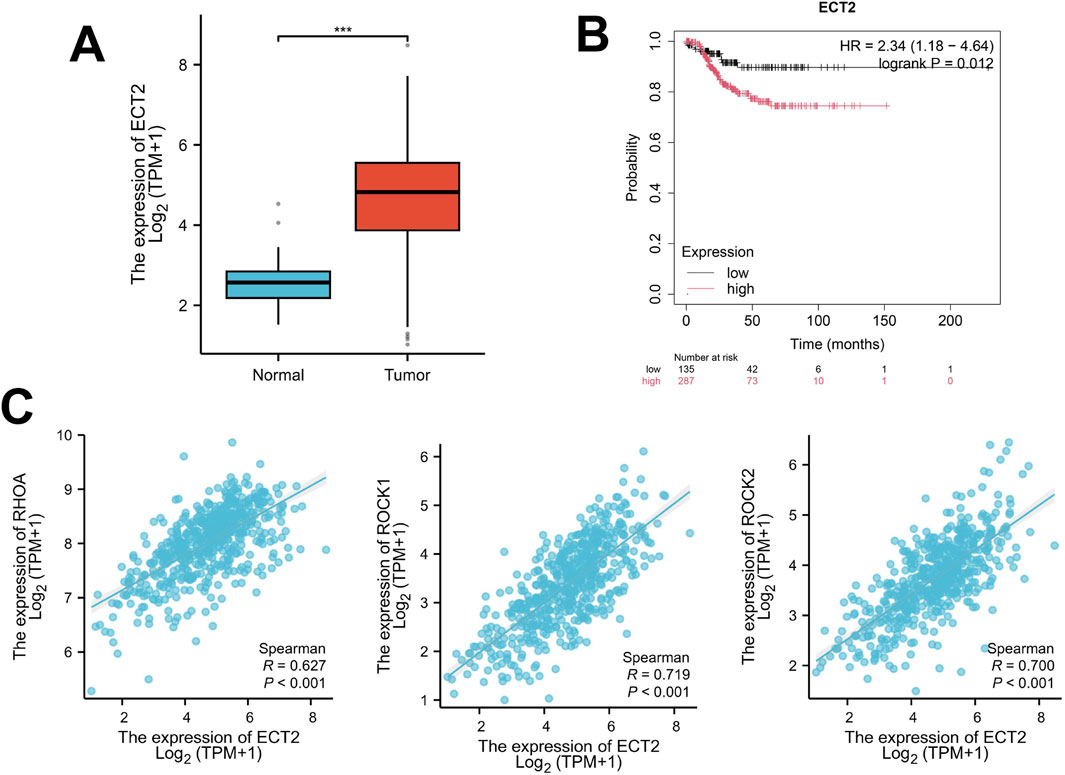
Figure 7. (A) The expression of ECT2 in endometrial cancer tissues is significantly higher than that in normal endometrial tissues; (B) The survival time of the ECT2 high-expression group was shorter than that of the ECT2 low-expression group; (C) The expression of ECT2 in endometrial cancer was significantly positively correlated with RhoA, ROCK1, and ROCK2.
5.1 The expression of ECT2 is elevated in endometrial cancer
A study found through the analysis of differentially expressed gene (DEGs) data that ECT2 was highly expressed in atypical endometrial hyperplasia (AH) and endometrial cancer. ECT2 has high accuracy in differentiating benign and malignant endometrium and is expected to become a promising biomarker for the diagnosis of endometrial cancer and a prognostic indicator for endometrial cancer (Wu et al., 2024).
5.2 RhoA/ROCK is involved in endometrial cancer by mediating calcium concentrations
Previous studies have shown that aberrant activity of actin cytoskeletal regulatory proteins may have an impact on cell adhesion and migration, leading to increased cancer cell mobility, promoting accelerated disease progression, and subsequently accelerated metastasis formation (Horiuchi et al., 2003; Yamazaki et al., 2005; Fiorio Pla and Gkika, 2013). The calcium channel Transient Receptor Potential Vanilloid 4 (TRPV4) is a member of the transient receptor potential (TRP) family that regulates calcium homeostasis in living organisms (Stewart et al., 2015). Calcium ions play an important role in the changes in the actin cytoskeleton. It has been reported that the concentration of cytoplasmic free calcium ions regulates cancer cell function (Li et al., 2019). The increase in calcium ionization promotes the metastasis of abdominal tumor cells and the metastasis of lymph nodes in endometrial cancer (Schwartz and Skinner, 2012; Li et al., 2020b). In estrogen-dependent endometrial cancer, estrogen may work synergistically with calcium (Mo and Yang, 2018). Stimulated by estrogen, calcium ions flow through L-type calcium channels (LTCC) and promote the proliferation and migration of endometrial cancer cells (Hao et al., 2015). Overactive calcium channels may promote cancer cell metastasis and colonization by reorganizing the actin cytoskeleton, degrading the extracellular matrix, and remodeling the tumor microenvironment (Li et al., 2020a). The levels of free calcium and TRPV4 in the cancer cells of endometrial cancer FIGO stage II-IV were significantly higher than those in stage I (Déliot and Constantin, 2015). In most cases, increased expression and/or activity of calcium channels maintain high levels of calcium ions, promoting cancer cell proliferation and migration (Fife et al., 2014). To confirm the role of TRPV4-mediated calcium ions in endometrial cancer, in a 2020 experiment, after inhibiting the expression of TRPV4 in vitro, cell motility decreased, and conversely, overexpression of TRPV4 enhanced the motility of endometrial cancer cells; in this study, EC cells with high expression of TRPV4 and antagonistic TRPV4 were constructed and injected into nude mice, and it was found that the number of metastatic peritoneal nodules in the high-expression group increased, while the number of metastatic peritoneal nodules in the antagonistic group decreased significantly (Déliot and Constantin, 2015). This study verified that TRPV4 is closely related to the occurrence and development of endometrial cancer both in vitro and in vivo.
One of the most common mechanisms that regulate actin is through the Rho GTPase pathway, which may be triggered by calcium influx (Li and Wang, 2020). In endometrial cancer cells, the expression of RhoA and its downstream effector molecule ROCK was caused by a high concentration of free calcium ions, and its expression level increased with the increase of calcium ion concentration. However, the expression level of calcium ions decreased after the function of calcium ions was hindered by calcium chelators (Déliot and Constantin, 2015). RhoA-ROCK signaling is a key regulator of actomyosin contractility, which regulates cell shape, cytoskeletal arrangement, and thus cell functions such as cell proliferation, differentiation, motility, and adhesion (Maekawa et al., 1999). These studies suggest that the influx of TRPV4 and calcium ions activates the RhoA/ROCK pathway in EC cells, triggering the mechanism of cytoskeletal changes, which in turn mediates tumor cell migration. Western blotting showed that the expression levels of LIM domain kinase (LIMK) and cofilin were altered by the activity of TRPV4, and the levels of ROCK were also increased after ROCK overexpression. This suggests that LIMK and cofilin are key downstream components of ROCK in regulating the actin cytoskeleton. RhoA activation increases the expression of ROCK1 and further activates LIMK, which in turn phosphorylates cofilin, inhibits actin depolymerization activity, and stabilizes agonist stress fibers (Maekawa et al., 1999). These findings demonstrate that cytoskeletal regulation initiated by TRPV4 and calcium ion influx may act through the RhoA/ROCK/LIMK/cofilin signaling pathway (Figure 8A).
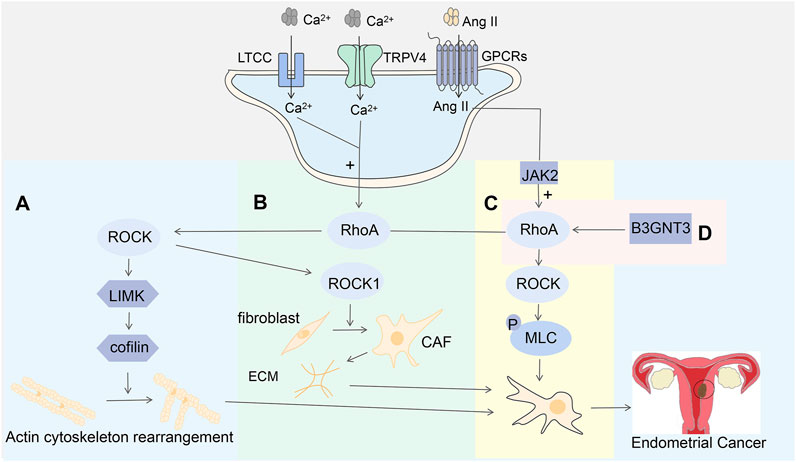
Figure 8. (A) Calcium influx from TRPV4 and L-type calcium channels activates the RhoA-ROCK-LIMK-cofilin pathway involved in cytoskeletal regulation; (B) The RhoA-ROCK1 signaling pathway promotes the transformation of fibroblasts to CAF, which in turn leads to physical remodeling of the pro-aggressive ECM; (C) Ang II enhances cell contraction by activating the JAK2-RhoA-ROCK-MLC signaling pathway through G protein-coupled receptors; (D) B3GNT3 is expressed at high levels in EC cells, where it mediates the regulation of the RhoA-RAC1 signaling pathway.
5.3 RhoA/ROCK is involved in endometrial cancer through other mechanisms
ROCK is one of the most important effector molecules downstream of RhoA, and it has two isoforms in cells: ROCK1 and ROCK2.ROCK1 is mainly involved in the formation of stress fibers, while ROCK2 is mainly involved in the process of cell phagocytosis and cell contraction (Wang et al., 2009; Bussard et al., 2016). Cancer-associated fibroblasts (CAFs) have a significant impact on cancer progression by remodeling extracellular matrix proteins (ECMs) and secreting various cytokines to stimulate cancer cell proliferation and cell migration (Subramaniam et al., 2013). Previous studies have demonstrated that CAF has pro-cancer activity (Bussard et al., 2016), it promotes the growth, migration, and invasion of EC tumors by secreting pro-tumor factors, including IL-6, IL-8, matrix-derived factor-1 α, VEGF, etc., (Orimo et al., 2001; Matsubara and Bissell, 2016). RhoA activation of ROCK1 is involved in the formation of stress fibers in CAF (Sanz-Moreno et al., 2011). RhoA/ROCK1 signaling-activated actin contractility promotes the conversion of fibroblasts to CAFs, which in turn leads to physical remodeling of the pro-invasive ECM, favoring tumor aggressiveness and dissemination (Mierke et al., 2008) (Figure 8B).
In addition, in smooth muscle and non-muscle cells, contraction is driven by the myosin II motor protein complex. When myosin II regulates myosin II regulatory light chain(MLC) phosphorylation, the myosin II motor protein complex is activated, which in turn activates myosin ATPase, thereby enhancing cell contractility (Fields and Justilien, 2010). Active ROCK can also affect cell migration by affecting MLC phosphorylation and activity (Yoneda et al., 2005).ROCK increases MLC phosphorylation, which contributes to actin recombination and stress fiber formation (Guilluy et al., 2010). Angiotensin II (Ang II) activates Janus kinase 2 (JAK2) through G protein-coupled receptors, which can phosphorylate Rho GTPase, which in turn activates RhoA, leading to the activation of ROCK and MLC phosphorylation (Zhang et al., 2015) (Figure 8C). Current studies have shown that β-1,3-N-acetylglucosamine transferase-3 (B3GNT3) is an oncogene that affects the energy metabolism of tumors by regulating galactosyltransferase, thereby affecting the survival and metastasis of tumor cells (Tang et al., 2019). High levels of B3GNT3 expression were monitored in endometrial cancer cells (Wang et al., 2021). In previous studies, it was observed that downregulation of B3GNT3 expression levels significantly reduced the expression levels of RhoA and RAC1 (Kim et al., 2005). These suggest that B3GNT3 may promote the migration and invasion of EC cells by modulating the RhoA/RAC1 signaling pathway (Figure 8D), but the upstream and downstream signaling molecules involved in this study remain to be studied.
6 Conclusion
This review comprehensively elucidates the intricate roles and contemporary research advancements of the ECT2 and RhoA/ROCK signaling pathways in the pathogenesis and progression of gynecological malignancies. ECT2, firmly established as an oncogene, exhibits consistent overexpression across ovarian, cervical, and endometrial cancers, a phenomenon intricately intertwined with tumor initiation, malignant progression, and adverse patient prognosis. Through the activation of the RhoA/ROCK signaling cascade, ECT2 orchestrates a series of oncogenic processes, including tumorigenesis, unrestrained proliferation, invasion, and metastasis. The RhoA/ROCK pathway, in turn, propels the development of gynecological tumors via a complex network of multi-tiered cascades and extensive molecular crosstalk. These signaling axes converge on pivotal aspects of tumor biology, encompassing angiogenesis, mechanical sensing, cytoskeletal remodeling, and DNA damage response, where they engage in dynamic synergistic or antagonistic interactions that govern cancer cell growth, dissemination, and colonization (Table 1).
Notwithstanding these significant strides, substantial knowledge gaps remain in the exploration of the ECT2 and RhoA/ROCK pathways and their intricate crosstalk mechanisms within gynecological tumors. The precise identification of upstream regulators and downstream effectors, as well as the molecular intricacies of their interactions, remain incompletely characterized. Notably, the role and underlying mechanisms of ECT2 in endometrial cancer represent a critical area of uncertainty, necessitating focused and in-depth investigation.
Looking forward, the genes and molecules within these signaling pathways hold substantial promise as potential therapeutic targets and prognostic biomarkers for gynecological malignancies. Future research endeavors should prioritize the development of innovative targeted therapies that precisely modulate the ECT2 and RhoA/ROCK pathways. Integration of these targeted interventions with emerging immunotherapeutic strategies offers a compelling opportunity to revolutionize the management of gynecological cancers, potentially enhancing early detection rates, improving treatment efficacy, and ultimately improving patient outcomes. Addressing the current research limitations through collaborative, interdisciplinary efforts will be instrumental in translating these findings into clinical practice and advancing the field of gynecological oncology.
Author contributions
LS: Writing – original draft, Writing – review and editing. ML: Writing – original draft, Writing – review and editing. YW: Writing – review and editing. ZZ: Writing – review and editing. YX: Writing – original draft. YK: Funding acquisition, Supervision, Writing – review and editing. ZW: Funding acquisition, Resources, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research leading to these results received funding from the Department of Science and Technology of Fujian Province under Grant Agreement No (2022J01769) and The Second Affiliated Hospital of Fujian Medical University Doctoral Miaopu Project (BS202401).
Acknowledgments
The authors thank the Department of Science and Technology of Fujian Province and the Second Affiliated Hospital of Fujian Medical University for supporting this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Amaya, C. N., Mitchell, D. C., and Bryan, B. A. (2017). Rho kinase proteins display aberrant upregulation in vascular tumors and contribute to vascular tumor growth. BMC Cancer 17, 485. doi:10.1186/s12885-017-3470-7
Amine, A., Rivera, S., Opolon, P., Dekkal, M., Biard, D. S., Bouamar, H., et al. (2009). Novel anti-metastatic action of cidofovir mediated by inhibition of E6/E7, CXCR4 and Rho/ROCK signaling in HPV tumor cells. PLoS One 4, e5018. doi:10.1371/journal.pone.0005018
AspenströM, P., Fransson, A., and Saras, J. (2004). Rho GTPases have diverse effects on the organization of the actin filament system. Biochem. J. 377, 327–337. doi:10.1042/BJ20031041
Baiden-Amissah, R. E. M., Annibali, D., Tuyaerts, S., and Amant, F. (2021). Endometrial cancer molecular characterization: the key to identifying high-risk patients and defining guidelines for clinical decision-making? Cancers (Basel) 13, 3988. doi:10.3390/cancers13163988
BarrèS, V., Ouellet, V., Lafontaine, J., Tonin, P. N., Provencher, D. M., and Mes-Masson, A. M. (2010). An essential role for Ran GTPase in epithelial ovarian cancer cell survival. Mol. Cancer 9, 272. doi:10.1186/1476-4598-9-272
Bartolomé, R. A., GáLVEZ, B. G., Longo, N., Baleux, F., Van Muijen, G. N., SáNCHEZ-Mateos, P., et al. (2004). Stromal cell-derived factor-1alpha promotes melanoma cell invasion across basement membranes involving stimulation of membrane-type 1 matrix metalloproteinase and Rho GTPase activities. Cancer Res. 64, 2534–2543. doi:10.1158/0008-5472.can-03-3398
Bensen, R., and Brognard, J. (2021). New therapeutic opportunities for the treatment of squamous cell carcinomas: a focus on novel driver kinases. Int. J. Mol. Sci. 22, 2831. doi:10.3390/ijms22062831
Bid, H. K., Roberts, R. D., Manchanda, P. K., and Houghton, P. J. (2013). RAC1: an emerging therapeutic option for targeting cancer angiogenesis and metastasis. Mol. Cancer Ther. 12, 1925–1934. doi:10.1158/1535-7163.MCT-13-0164
Bleul, C. C., Farzan, M., Choe, H., Parolin, C., Clark-Lewis, I., Sodroski, J., et al. (1996). The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature 382, 829–833. doi:10.1038/382829a0
Bos, J. L., Rehmann, H., and Wittinghofer, A. (2007). GEFs and GAPs: critical elements in the control of small G proteins. Cell 129, 865–877. doi:10.1016/j.cell.2007.05.018
Brantley-Sieders, D. M., Zhuang, G., Vaught, D., Freeman, T., Hwang, Y., Hicks, D., et al. (2009). Host deficiency in Vav2/3 guanine nucleotide exchange factors impairs tumor growth, survival, and angiogenesis in vivo. Mol. Cancer Res. 7, 615–623. doi:10.1158/1541-7786.MCR-08-0401
Bussard, K. M., Mutkus, L., Stumpf, K., Gomez-Manzano, C., and Marini, F. C. (2016). Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res. 18, 84. doi:10.1186/s13058-016-0740-2
Callebaut, I., and Mornon, J. P. (1997). From BRCA1 to RAP1: a widespread BRCT module closely associated with DNA repair. FEBS Lett. 400, 25–30. doi:10.1016/s0014-5793(96)01312-9
Cao, C., Han, P., Liu, L., Tang, Y., Tian, S., Zhang, K., et al. (2021). Epithelial cell transforming factor ECT2 is an important regulator of DNA double-strand break repair and genome stability. J. Biol. Chem. 297, 101036. doi:10.1016/j.jbc.2021.101036
Cerione, R. A. (2004). Cdc42: new roads to travel. Trends Cell Biol. 14, 127–132. doi:10.1016/j.tcb.2004.01.008
Chan, A. Y., Coniglio, S. J., Chuang, Y. Y., Michaelson, D., Knaus, U. G., Philips, M. R., et al. (2005). Roles of the Rac1 and Rac3 GTPases in human tumor cell invasion. Oncogene 24, 7821–7829. doi:10.1038/sj.onc.1208909
Cheng, C., Seen, D., Zheng, C., Zeng, R., and Li, E. (2021). Role of small GTPase RhoA in DNA damage response. Biomolecules 11, 212. doi:10.3390/biom11020212
Chen, J., Xia, H., Zhang, X., Karthik, S., Pratap, S. V., Ooi, L. L., et al. (2015). ECT2 regulates the Rho/ERK signalling axis to promote early recurrence in human hepatocellular carcinoma. J. Hepatol. 62, 1287–1295. doi:10.1016/j.jhep.2015.01.014
Chen, Y., Song, Z., Gao, Y., and Cai, H. (2022). Effect of ECT2 gene on proliferation of cervical cancer cells and its mechanism. Cancer Res. Prev. Treat. 49, 1015–1020.
Chen, Z., Sun, J., Pradines, A., Favre, G., Adnane, J., and Sebti, S. M. (2000). Both farnesylated and geranylgeranylated RhoB inhibit malignant transformation and suppress human tumor growth in nude mice. J. Biol. Chem. 275, 17974–17978. doi:10.1074/jbc.C000145200
Cook, D. R., Rossman, K. L., and Der, C. J. (2014). Rho guanine nucleotide exchange factors: regulators of rho GTPase activity in development and disease. Oncogene 33, 4021–4035. doi:10.1038/onc.2013.362
Déliot, N., and Constantin, B. (2015). Plasma membrane calcium channels in cancer: alterations and consequences for cell proliferation and migration. Biochim. Biophys. Acta 1848, 2512–2522. doi:10.1016/j.bbamem.2015.06.009
Ellenbroek, S. I., and Collard, J. G. (2007). Rho GTPases: functions and association with cancer. Clin. Exp. Metastasis 24, 657–672. doi:10.1007/s10585-007-9119-1
Fields, A. P., and Justilien, V. (2010). The guanine nucleotide exchange factor (GEF) Ect2 is an oncogene in human cancer. Adv. Enzyme Regul. 50, 190–200. doi:10.1016/j.advenzreg.2009.10.010
Fife, C. M., Mccarroll, J. A., and Kavallaris, M. (2014). Movers and shakers: cell cytoskeleton in cancer metastasis. Br. J. Pharmacol. 171, 5507–5523. doi:10.1111/bph.12704
Fiorio Pla, A., and Gkika, D. (2013). Emerging role of TRP channels in cell migration: from tumor vascularization to metastasis. Front. Physiol. 4, 311. doi:10.3389/fphys.2013.00311
Garcia-Mata, R., Boulter, E., and Burridge, K. (2011). The 'invisible hand': regulation of RHO GTPases by RHOGDIs. Nat. Rev. Mol. Cell Biol. 12, 493–504. doi:10.1038/nrm3153
Ghasemi, A., Hashemy, S. I., Aghaei, M., and Panjehpour, M. (2017). RhoA/ROCK pathway mediates leptin-induced uPA expression to promote cell invasion in ovarian cancer cells. Cell Signal 32, 104–114. doi:10.1016/j.cellsig.2017.01.020
Guilluy, C., BréGEON, J., Toumaniantz, G., Rolli-Derkinderen, M., Retailleau, K., Loufrani, L., et al. (2010). The Rho exchange factor Arhgef1 mediates the effects of angiotensin II on vascular tone and blood pressure. Nat. Med. 16, 183–190. doi:10.1038/nm.2079
Guo, Z., Chen, X., Du, T., Zhu, D., Lai, Y., Dong, W., et al. (2017). Elevated levels of epithelial cell transforming sequence 2 predicts poor prognosis for prostate cancer. Med. Oncol. 34, 13. doi:10.1007/s12032-016-0872-3
Halbleib, J. M., and Nelson, W. J. (2006). Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 20, 3199–3214. doi:10.1101/gad.1486806
Hao, J., Bao, X., Jin, B., Wang, X., Mao, Z., Li, X., et al. (2015). Ca2+ channel subunit α 1D promotes proliferation and migration of endometrial cancer cells mediated by 17β-estradiol via the G protein-coupled estrogen receptor. Faseb J. 29, 2883–2893. doi:10.1096/fj.14-265603
Haverty, P. M., Hon, L. S., Kaminker, J. S., Chant, J., and Zhang, Z. (2009). High-resolution analysis of copy number alterations and associated expression changes in ovarian tumors. BMC Med. Genomics 2, 21. doi:10.1186/1755-8794-2-21
He, D., Xiang, J., Li, B., and Liu, H. (2016). The dynamic behavior of Ect2 in response to DNA damage. Sci. Rep. 6, 24504. doi:10.1038/srep24504
He, X., Yuan, C., and Yang, J. (2015). Regulation and functional significance of CDC42 alternative splicing in ovarian cancer. Oncotarget 6, 29651–29663. doi:10.18632/oncotarget.4865
Hirata, D., Yamabuki, T., Miki, D., Ito, T., Tsuchiya, E., Fujita, M., et al. (2009). Involvement of epithelial cell transforming sequence-2 oncoantigen in lung and esophageal cancer progression. Clin. Cancer Res. 15, 256–266. doi:10.1158/1078-0432.CCR-08-1672
Horiuchi, A., Imai, T., Wang, C., Ohira, S., Feng, Y., Nikaido, T., et al. (2003). Up-regulation of small GTPases, RhoA and RhoC, is associated with tumor progression in ovarian carcinoma. Lab. Invest 83, 861–870. doi:10.1097/01.lab.0000073128.16098.31
Hudson, L. G., Gillette, J. M., Kang, H., Rivera, M. R., and Wandinger-Ness, A. (2018). Ovarian tumor microenvironment signaling: convergence on the Rac1 GTPase. Cancers (Basel) 10, 358. doi:10.3390/cancers10100358
Huff, L. P., Decristo, M. J., Trembath, D., Kuan, P. F., Yim, M., Liu, J., et al. (2013). The role of Ect2 nuclear RhoGEF activity in ovarian cancer cell transformation. Genes Cancer 4, 460–475. doi:10.1177/1947601913514851
Huyton, T., Bates, P. A., Zhang, X., Sternberg, M. J., and Freemont, P. S. (2000). The BRCA1 C-terminal domain: structure and function. Mutat. Res. 460, 319–332. doi:10.1016/s0921-8777(00)00034-3
Ismail, T., Alzneika, S., Riguene, E., Al-Maraghi, S., Alabdulrazzak, A., Al-Khal, N., et al. (2024). BRCA1 and its vulnerable C-Terminal BRCT domain: structure, function, genetic mutations and links to diagnosis and treatment of breast and ovarian cancer. Pharm. (Basel) 17, 333. doi:10.3390/ph17030333
Izdebska, M., Zielińska, W., Grzanka, D., and Gagat, M. (2018). The role of actin dynamics and actin-binding proteins expression in epithelial-to-mesenchymal transition and its association with cancer progression and evaluation of possible therapeutic targets. Biomed. Res. Int. 2018, 4578373. doi:10.1155/2018/4578373
Julian, L., and Olson, M. F. (2014). Rho-associated coiled-coil containing kinases (ROCK): structure, regulation, and functions. Small GTPases 5, e29846. doi:10.4161/sgtp.29846
Justilien, V., and Fields, A. P. (2009). Ect2 links the PKCiota-Par6alpha complex to Rac1 activation and cellular transformation. Oncogene 28, 3597–3607. doi:10.1038/onc.2009.217
Kaibuchi, K., Kuroda, S., and Amano, M. (1999). Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu. Rev. Biochem. 68, 459–486. doi:10.1146/annurev.biochem.68.1.459
Kamasani, U., Huang, M., Duhadaway, J. B., Prochownik, E. V., Donover, P. S., and Prendergast, G. C. (2004). Cyclin B1 is a critical target of RhoB in the cell suicide program triggered by farnesyl transferase inhibition. Cancer Res. 64, 8389–8396. doi:10.1158/0008-5472.CAN-04-2437
Kato, S., Abarzua-Catalan, L., Trigo, C., Delpiano, A., Sanhueza, C., GarcíA, K., et al. (2015). Leptin stimulates migration and invasion and maintains cancer stem-like properties in ovarian cancer cells: an explanation for poor outcomes in obese women. Oncotarget 6, 21100–21119. doi:10.18632/oncotarget.4228
Kim, J. E., Billadeau, D. D., and Chen, J. (2005). The tandem BRCT domains of Ect2 are required for both negative and positive regulation of Ect2 in cytokinesis. J. Biol. Chem. 280, 5733–5739. doi:10.1074/jbc.M409298200
Lawson, C. D., and Burridge, K. (2014). The on-off relationship of Rho and Rac during integrin-mediated adhesion and cell migration. Small GTPases 5, e27958. doi:10.4161/sgtp.27958
Leng, R., Liao, G., Wang, H., Kuang, J., and Tang, L. (2015). Rac1 expression in epithelial ovarian cancer: effect on cell EMT and clinical outcome. Med. Oncol. 32, 329. doi:10.1007/s12032-014-0329-5
Leve, F., Marcondes, T. G., Bastos, L. G., Rabello, S. V., Tanaka, M. N., and Morgado-DíAZ, J. A. (2011). Lysophosphatidic acid induces a migratory phenotype through a crosstalk between RhoA-Rock and Src-FAK signalling in colon cancer cells. Eur. J. Pharmacol. 671, 7–17. doi:10.1016/j.ejphar.2011.09.006
Li, X. C., Dong, Y. Y., Cheng, Y., Zhou, J. Y., Yang, X., Shen, B. Q., et al. (2020b). Increased serum calcium level promotes the risk of lymph node metastasis in endometrial cancer. Cancer Manag. Res. 12, 5023–5030. doi:10.2147/CMAR.S253914
Liu, X., Chen, D., and Liu, G. (2014). Overexpression of RhoA promotes the proliferation and migration of cervical cancer cells. Biosci. Biotechnol. Biochem. 78, 1895–1901. doi:10.1080/09168451.2014.943650
Liu, X., Zhang, J., Ju, S., Liu, L., Sun, Y., Guo, L., et al. (2023). ECT2 promotes malignant phenotypes through the activation of the AKT/mTOR pathway and cisplatin resistance in cervical cancer. Cancer Gene Ther. 30, 62–73. doi:10.1038/s41417-022-00525-7
Li, X., Cheng, Y., Dong, Y., Shen, B., Yang, X., Wang, J., et al. (2019). An elevated preoperative serum calcium level is a significant predictor for positive peritoneal cytology in endometrial carcinoma. Chin. J. Cancer Res. 31, 965–973. doi:10.21147/j.issn.1000-9604.2019.06.12
Li, X., Cheng, Y., Wang, Z., Zhou, J., Jia, Y., He, X., et al. (2020a). Calcium and TRPV4 promote metastasis by regulating cytoskeleton through the RhoA/ROCK1 pathway in endometrial cancer. Cell Death Dis. 11, 1009. doi:10.1038/s41419-020-03181-7
Li, X., and Wang, J. (2020). Mechanical tumor microenvironment and transduction: Cytoskeleton mediates cancer cell invasion and metastasis. Int. J. Biol. Sci. 16, 2014–2028. doi:10.7150/ijbs.44943
Maekawa, M., Ishizaki, T., Boku, S., Watanabe, N., Fujita, A., Iwamatsu, A., et al. (1999). Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science 285, 895–898. doi:10.1126/science.285.5429.895
Maheshwari, G., Brown, G., Lauffenburger, D. A., Wells, A., and Griffith, L. G. (2000). Cell adhesion and motility depend on nanoscale RGD clustering. J. Cell Sci. 113 (Pt 10), 1677–1686. doi:10.1242/jcs.113.10.1677
Matsubara, M., and Bissell, M. J. (2016). Inhibitors of Rho kinase (ROCK) signaling revert the malignant phenotype of breast cancer cells in 3D context. Oncotarget 7, 31602–31622. doi:10.18632/oncotarget.9395
Mazieres, J., Antonia, T., Daste, G., Muro-Cacho, C., Berchery, D., Tillement, V., et al. (2004). Loss of RhoB expression in human lung cancer progression. Clin. Cancer Res. 10, 2742–2750. doi:10.1158/1078-0432.ccr-03-0149
Mcgrail, D. J., Kieu, Q. M., and Dawson, M. R. (2014). The malignancy of metastatic ovarian cancer cells is increased on soft matrices through a mechanosensitive Rho-ROCK pathway. J. Cell Sci. 127, 2621–2626. doi:10.1242/jcs.144378
Mendez, P., and Ramirez, J. L. (2013). Copy number gains of FGFR1 and 3q chromosome in squamous cell carcinoma of the lung. Transl. Lung Cancer Res. 2, 101–111. doi:10.3978/j.issn.2218-6751.2013.03.05
Mierke, C. T., Rösel, D., Fabry, B., and Brábek, J. (2008). Contractile forces in tumor cell migration. Eur. J. Cell Biol. 87, 669–676. doi:10.1016/j.ejcb.2008.01.002
Mo, P., and Yang, S. (2018). The store-operated calcium channels in cancer metastasis: from cell migration, invasion to metastatic colonization. Front. Biosci. (Landmark Ed) 23, 1241–1256. doi:10.2741/4641
Mordes, D. A., Glick, G. G., Zhao, R., and Cortez, D. (2008). TopBP1 activates ATR through ATRIP and a PIKK regulatory domain. Genes Dev. 22, 1478–1489. doi:10.1101/gad.1666208
Mota, M. B. S., Woods, N. T., Carvalho, M. A., Monteiro, A. N. A., and Mesquita, R. D. (2023). Evolution of the triplet BRCT domain. DNA Repair (Amst) 129, 103532. doi:10.1016/j.dnarep.2023.103532
Niiya, F., Tatsumoto, T., Lee, K. S., and Miki, T. (2006). Phosphorylation of the cytokinesis regulator ECT2 at G2/M phase stimulates association of the mitotic kinase Plk1 and accumulation of GTP-bound RhoA. Oncogene 25, 827–837. doi:10.1038/sj.onc.1209124
Orimo, A., Tomioka, Y., Shimizu, Y., Sato, M., Oigawa, S., Kamata, K., et al. (2001). Cancer-associated myofibroblasts possess various factors to promote endometrial tumor progression. Clin. Cancer Res. 7, 3097–3105.
Osaki, J. H., Espinha, G., Magalhaes, Y. T., and Forti, F. L. (2016). Modulation of RhoA GTPase activity sensitizes human cervix carcinoma cells to γ-radiation by attenuating DNA repair pathways. Oxid. Med. Cell Longev. 2016, 6012642. doi:10.1155/2016/6012642
Palou, G., Palou, R., Guerra-Moreno, A., Duch, A., Travesa, A., and Quintana, D. G. (2010). Cyclin regulation by the s phase checkpoint. J. Biol. Chem. 285, 26431–26440. doi:10.1074/jbc.M110.138669
Paull, T. T. (2015). Mechanisms of ATM activation. Annu. Rev. Biochem. 84, 711–738. doi:10.1146/annurev-biochem-060614-034335
Peng, S. B., Peek, V., Zhai, Y., Paul, D. C., Lou, Q., Xia, X., et al. (2005). Akt activation, but not extracellular signal-regulated kinase activation, is required for SDF-1alpha/CXCR4-mediated migration of epitheloid carcinoma cells. Mol. Cancer Res. 3, 227–236. doi:10.1158/1541-7786.MCR-04-0193
Ridley, A. J. (2001). Rho GTPases and cell migration. J. Cell Sci. 114, 2713–2722. doi:10.1242/jcs.114.15.2713
Ridley, A. J. (2006). Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 16, 522–529. doi:10.1016/j.tcb.2006.08.006
Saito, S., Liu, X. F., Kamijo, K., Raziuddin, R., Tatsumoto, T., Okamoto, I., et al. (2004). Deregulation and mislocalization of the cytokinesis regulator ECT2 activate the rho signaling pathways leading to malignant transformation. J. Biol. Chem. 279, 7169–7179. doi:10.1074/jbc.M306725200
Sano, M., Genkai, N., Yajima, N., Tsuchiya, N., Homma, J., Tanaka, R., et al. (2006). Expression level of ECT2 proto-oncogene correlates with prognosis in glioma patients. Oncol. Rep. 16, 1093–1098. doi:10.3892/or.16.5.1093
Sanz-Moreno, V., Gaggioli, C., Yeo, M., Albrengues, J., Wallberg, F., Viros, A., et al. (2011). ROCK and JAK1 signaling cooperate to control actomyosin contractility in tumor cells and stroma. Cancer Cell 20, 229–245. doi:10.1016/j.ccr.2011.06.018
Schwartz, G. G., and Skinner, H. G. (2012). A prospective study of total and ionized serum calcium and time to fatal prostate cancer. Cancer Epidemiol. Biomarkers Prev. 21, 1768–1773. doi:10.1158/1055-9965.EPI-12-0585
Scott, R. W., Hooper, S., Crighton, D., Li, A., KöNIG, I., Munro, J., et al. (2010). LIM kinases are required for invasive path generation by tumor and tumor-associated stromal cells. J. Cell Biol. 191, 169–185. doi:10.1083/jcb.201002041
Sharma, S., Santiskulvong, C., Bentolila, L. A., Rao, J., Dorigo, O., and Gimzewski, J. K. (2012). Correlative nanomechanical profiling with super-resolution F-actin imaging reveals novel insights into mechanisms of cisplatin resistance in ovarian cancer cells. Nanomedicine 8, 757–766. doi:10.1016/j.nano.2011.09.015
Sheng, Z. Z., Zhao, Y. Q., and Huang, J. F. (2011). Functional evolution of BRCT domains from binding DNA to protein. Evol. Bioinform Online 7, 87–97. doi:10.4137/EBO.S7084
Shepherd, J. H. (1989). Revised FIGO staging for gynaecological cancer. Br. J. Obstet. Gynaecol. 96, 889–892. doi:10.1111/j.1471-0528.1989.tb03341.x
Shiloh, Y. (2001). ATM and ATR: networking cellular responses to DNA damage. Curr. Opin. Genet. Dev. 11, 71–77. doi:10.1016/s0959-437x(00)00159-3
Sinha, S., and Yang, W. (2008). Cellular signaling for activation of Rho GTPase Cdc42. Cell Signal 20, 1927–1934. doi:10.1016/j.cellsig.2008.05.002
Solski, P. A., Wilder, R. S., Rossman, K. L., Sondek, J., Cox, A. D., Campbell, S. L., et al. (2004). Requirement for C-terminal sequences in regulation of Ect2 guanine nucleotide exchange specificity and transformation. J. Biol. Chem. 279, 25226–25233. doi:10.1074/jbc.M313792200
Srougi, M. C., and Burridge, K. (2011). The nuclear guanine nucleotide exchange factors Ect2 and Net1 regulate RhoB-mediated cell death after DNA damage. PLoS One 6, e17108. doi:10.1371/journal.pone.0017108
Stewart, T. A., Yapa, K. T., and Monteith, G. R. (2015). Altered calcium signaling in cancer cells. Biochim. Biophys. Acta 1848, 2502–2511. doi:10.1016/j.bbamem.2014.08.016
Subramaniam, K. S., Tham, S. T., Mohamed, Z., Woo, Y. L., Mat Adenan, N. A., and Chung, I. (2013). Cancer-associated fibroblasts promote proliferation of endometrial cancer cells. PLoS One 8, e68923. doi:10.1371/journal.pone.0068923
Sun, B. Y., Wei, Q. Q., Liu, C. X., Zhang, L., Luo, G., Li, T., et al. (2020). ECT2 promotes proliferation and metastasis of esophageal squamous cell carcinoma via the RhoA-ERK signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 24, 7991–8000. doi:10.26355/eurrev_202008_22482
Sun, Q., Hou, Y., Chu, Z., and Wei, Q. (2022). Soft overcomes the hard: flexible materials adapt to cell adhesion to promote cell mechanotransduction. Bioact. Mater 10, 397–404. doi:10.1016/j.bioactmat.2021.08.026
Sun, Y., Cheng, Z., Ma, L., and Pei, G. (2002). Beta-arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J. Biol. Chem. 277, 49212–49219. doi:10.1074/jbc.M207294200
Tanaka, K., Matsumoto, Y., Ishikawa, H., Fukumitsu, N., Numajiri, H., Murofushi, K., et al. (2020). Impact of RhoA overexpression on clinical outcomes in cervical squamous cell carcinoma treated with concurrent chemoradiotherapy. J. Radiat. Res. 61, 221–230. doi:10.1093/jrr/rrz093
Tang, F. H., Chang, W. A., Tsai, E. M., Tsai, M. J., and Kuo, P. L. (2019). Investigating novel genes potentially involved in endometrial adenocarcinoma using next-generation sequencing and bioinformatic approaches. Int. J. Med. Sci. 16, 1338–1348. doi:10.7150/ijms.38219
Thompson, L. H., Brookman, K. W., Jones, N. J., Allen, S. A., and Carrano, A. V. (1990). Molecular cloning of the human XRCC1 gene, which corrects defective DNA strand break repair and sister chromatid exchange. Mol. Cell Biol. 10, 6160–6171. doi:10.1128/mcb.10.12.6160-6171.1990
Tsuji, T., Higashida, C., Aoki, Y., Islam, M. S., Dohmoto, M., and Higashida, H. (2012). Ect2, an ortholog of Drosophila pebble, regulates formation of growth cones in primary cortical neurons. Neurochem. Int. 61, 854–858. doi:10.1016/j.neuint.2012.02.011
Van Golen, K. L., Bao, L. W., Pan, Q., Miller, F. R., Wu, Z. F., and Merajver, S. D. (2002). Mitogen activated protein kinase pathway is involved in RhoC GTPase induced motility, invasion and angiogenesis in inflammatory breast cancer. Clin. Exp. Metastasis 19, 301–311. doi:10.1023/a:1015518114931
Wang, J. S., Ruan, F., Guo, L. Z., Wang, F. G., Wang, F. L., and An, H. M. (2021). B3GNT3 acts as a carcinogenic factor in endometrial cancer via facilitating cell growth, invasion and migration through regulating RhoA/RAC1 pathway-associated markers. Genes Genomics 43, 447–457. doi:10.1007/s13258-021-01072-5
Wang, C., Wang, W., Liu, Y., Yong, M., Yang, Y., and Zhou, H. (2018). Rac GTPase activating protein 1 promotes oncogenic progression of epithelial ovarian cancer. Cancer Sci. 109, 84–93. doi:10.1111/cas.13434
Wang, Y., Hill, K. S., and Fields, A. P. (2013). PKCι maintains a tumor-initiating cell phenotype that is required for ovarian tumorigenesis. Mol. Cancer Res. 11, 1624–1635. doi:10.1158/1541-7786.MCR-13-0371-T
Wang, Y., Zheng, X. R., Riddick, N., Bryden, M., Baur, W., Zhang, X., et al. (2009). ROCK isoform regulation of myosin phosphatase and contractility in vascular smooth muscle cells. Circ. Res. 104, 531–540. doi:10.1161/CIRCRESAHA.108.188524
Wu, X. G., Wu, Y., Pan, Y. H., Chen, J. J., Huang, S. Y., Zhou, X. X., et al. (2024). Elevated expression of ECT2 as a diagnostic marker and prognostic indicator in endometrial cancer. Gene 927, 148756. doi:10.1016/j.gene.2024.148756
Xie, B., Wang, S., Jiang, N., and Li, J. J. (2019). Cyclin B1/CDK1-regulated mitochondrial bioenergetics in cell cycle progression and tumor resistance. Cancer Lett. 443, 56–66. doi:10.1016/j.canlet.2018.11.019
Xu, Y. B., Du, Q. H., Zhang, M. Y., Yun, P., and He, C. Y. (2013). Propofol suppresses proliferation, invasion and angiogenesis by down-regulating ERK-VEGF/MMP-9 signaling in Eca-109 esophageal squamous cell carcinoma cells. Eur. Rev. Med. Pharmacol. Sci. 17, 2486–2494.
Yamazaki, D., Kurisu, S., and Takenawa, T. (2005). Regulation of cancer cell motility through actin reorganization. Cancer Sci. 96, 379–386. doi:10.1111/j.1349-7006.2005.00062.x
Yang, L., Wang, L., Kalfa, T. A., Cancelas, J. A., Shang, X., Pushkaran, S., et al. (2007). Cdc42 critically regulates the balance between myelopoiesis and erythropoiesis. Blood 110, 3853–3861. doi:10.1182/blood-2007-03-079582
Yoneda, A., Multhaupt, H. A., and Couchman, J. R. (2005). The Rho kinases I and II regulate different aspects of myosin II activity. J. Cell Biol. 170, 443–453. doi:10.1083/jcb.200412043
Yoshioka, K., Nakamori, S., and Itoh, K. (1999). Overexpression of small GTP-binding protein RhoA promotes invasion of tumor cells. Cancer Res. 59, 2004–2010.
Zaoui, K., Boudhraa, Z., Khalifé, P., Carmona, E., Provencher, D., and Mes-Masson, A. M. (2019). Ran promotes membrane targeting and stabilization of RhoA to orchestrate ovarian cancer cell invasion. Nat. Commun. 10, 2666. doi:10.1038/s41467-019-10570-w
Zhang, J. P., Lu, W. G., Ye, F., Chen, H. Z., Zhou, C. Y., and Xie, X. (2007). Study on CXCR4/SDF-1alpha axis in lymph node metastasis of cervical squamous cell carcinoma. Int. J. Gynecol. Cancer 17, 478–483. doi:10.1111/j.1525-1438.2007.00786.x
Zhang, W., Hou, T., Niu, C., Song, L., and Zhang, Y. (2015). B3GNT3 expression is a novel marker correlated with pelvic lymph node metastasis and poor clinical outcome in early-stage cervical cancer. PLoS One 10, e0144360. doi:10.1371/journal.pone.0144360
Zhu, J., Huang, J. W., Tseng, P. H., Yang, Y. T., Fowble, J., Shiau, C. W., et al. (2004). From the cyclooxygenase-2 inhibitor celecoxib to a novel class of 3-phosphoinositide-dependent protein kinase-1 inhibitors. Cancer Res. 64, 4309–4318. doi:10.1158/0008-5472.CAN-03-4063
Zubor, P., Dankova, Z., Kolkova, Z., Holubekova, V., Brany, D., Mersakova, S., et al. (2020). Rho GTPases in gynecologic cancers: in-depth analysis toward the paradigm change from reactive to predictive, preventive, and personalized medical approach benefiting the patient and healthcare. Cancers (Basel) 12, 1292. doi:10.3390/cancers12051292
Keywords: Ect2, RhoA, ROCK, ovarian cancer, cervical cancer, endometrial cancer
Citation: Sheng L, Liang M, Wang Y, Zhou Z, Xie Y, Ke Y and Wu Z (2025) Research progress of ECT2 and RhoA-related signaling pathways in gynecological tumors. Front. Cell Dev. Biol. 13:1602649. doi: 10.3389/fcell.2025.1602649
Received: 30 March 2025; Accepted: 18 June 2025;
Published: 27 June 2025.
Edited by:
Girijesh Kumar Patel, Motilal Nehru National Institute of Technology, IndiaReviewed by:
Sandhya Annamaneni, Osmania University, IndiaVinita Yadav, University of Michigan, United States
Saamarthy Karunakar, Thomas Jefferson University, United States
Copyright © 2025 Sheng, Liang, Wang, Zhou, Xie, Ke and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhuna Wu, d3V6aHVuYUBhbGl5dW4uY29t; Yumin Ke, Mzk4MDMxMzEzQHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Liying Sheng
Liying Sheng Meili Liang†
Meili Liang† Yumin Ke
Yumin Ke Zhuna Wu
Zhuna Wu