- The State Key Laboratory of Oral Diseases & National Clinical Research Center for Oral Diseases, Department of Prosthodontics, West China Hospital of Stomatology, Sichuan University, Chengdu, Sichuan, China
Bone and dental tissues are highly mineralized and mechanically sensitive hard tissues. They detect and respond to mechanical forces via mechanosensitive Piezo channels, modulating physiological and pathological processes. While Piezo mechanobiology has been explored, systematic comparison of their roles across bone and dental tissues, particularly their potential crosstalk in adaptation and disease, remains underexamined in existing reviews. This review consolidates recent advances in Piezo channel biology, clarifying their structural properties, tissue-specific distribution, and functional roles in mineralized tissues. Emerging evidence highlights Piezo channels as key mechanotransducers ubiquitously expressed in skeletal and dental cellular populations. By mediating distinct mechanotransduction pathways, Piezo1 and Piezo2 modulate diverse processes, including bone remodeling, osteoblast-osteoclast communication, dental stem cell differentiation, dental hard tissue mineralization, and orthodontic tooth movement. Furthermore, their dysregulation is implicated in pathologies such as osteoporosis, pulpitis, and dentin hypersensitivity. The elucidated mechanisms establish a theoretical framework for Piezo-mediated mechanotransduction in cellular adaptation and disease progression. By integrating molecular mechanisms with regenerative applications across both osseous and dental contexts, this review advances understanding of shared mechanobiological principles in mineralized tissues and highlights translational relevance for skeletal and dental therapies. These insights align with mechanobiology and tissue engineering research, supporting future development of mechanosensitive interventions.
1 Introduction
Mammalian physiological hard tissues, comprising bone and dental tissues, are highly mineralized tissues with sophisticated structures that render them extremely sensitive to mechanical stimuli (Haelterman and Lim, 2019; Pei et al., 2021). This mechanosensitivity underpins a range of clinical interventions (Li et al., 2021; Shah et al., 2021)—such as distraction osteogenesis and orthodontic tooth movement—as well as pathological conditions, including osteoarthritis and traumatic periodontitis (Herrera et al., 2014; Glyn-Jones et al., 2015). Mechanotransduction denotes the cellular mechanism transducing biomechanical stimuli into intracellular biochemical signaling events (Jin et al., 2020; Jiang et al., 2021b), typically mediated through membrane depolarization or the influx of cations via mechanosensitive channels (Douguet and Honoré, 2019; Jiang et al., 2021b). These channels, which are expressed in mechanosensory organs, directly mediate cellular responses to mechanical stimuli through alterations in their physical properties (Jin et al., 2020). Existing defined mechanosensitive channels comprise the Piezo family, epithelial sodium channel/degenerin (ENaC/DEG)-superfamily proteins, TWIK-Related K+ Channel (TREK) subfamily proteins, transient receptor potential (TRP) polymodal receptors, transmembrane protein (TMEM) 16 superfamily, and reduced hyperosmolality-induced [Ca2+]i increase (OSCA)/TMEM63 (Jin et al., 2020).
Notably, Piezo channels were initially characterized as non-selective cation mechanosensitive ion channels (Murthy et al., 2017), and their remarkable sensitivity is attributable to their elaborate structure. Functioning as mechanosensory receptors, Piezo channels play key roles in proprioception (Woo et al., 2015), touch (Ranade et al., 2014; Earley et al., 2022), and mechanical pain (Szczot et al., 2018; Zhang et al., 2019; Della Pietra et al., 2020), as well as in a variety of pathophysiological processes, such as inflammatory response (Solis et al., 2019), tumorigenesis and cancer progression (Jiang et al., 2022), musculoskeletal development, cardiovascular hemodynamics, renal filtration, and pulmonary homeostasis (Douguet et al., 2019; Qin et al., 2021; Xiong et al., 2022). Piezo1 and Piezo2 are the exclusive paralogs found within the mammalian Piezo channel family (Coste et al., 2010; Coste et al., 2012; Zhao et al., 2016; Murthy et al., 2017). They display no detectable sequence homology to other ion channel classes (Xiao, 2024). Predominantly localized in non-excitable cells, Piezo1 mediates intracellular Ca2+ signaling and activates subordinate downstream cascades to regulate diverse physiological processes (Jiang et al., 2021b). In contrast, Piezo2 is mainly distributed in excitable cells, including Merkel cells, Schwann cells, and sensory neurons (Acheta et al., 2022; Feng et al., 2022; Han et al., 2022; Sonkodi, 2022), and is indispensable for itch and pain-sensing (Feng et al., 2022), myelin formation (Acheta et al., 2022), and proprioception (Assaraf et al., 2020).
Recent studies have established Piezo channels as bona fide mechanotransducers, with growing evidence highlighting their crucial role in the mechanosensing of hard tissues. Specifically, Piezo-mediated mechanotransduction is critical for bone development and repair, stress-induced bone remodeling, toothache occurrence, and orthodontic tooth movement (OTM) (Lee et al., 2019; Jiang et al., 2021a; Qin et al., 2021; Xu et al., 2022). This review systematically introduces how Piezo1 and Piezo2 channels sense mechanical forces in bone and dental tissues, detailing their structure, activation mechanisms, distribution, and functions, thereby providing a reference framework for further investigation.
2 Properties of the Piezo family
2.1 Structural conformation
Piezo1 and Piezo2 exhibit a high degree of structural homology (Jiang et al., 2021b). Both channels adopt a distinctive triskelion-like architecture consisting of a central ion-conducting pore domain, an apical extracellular dome, and tripartite curved blade subunits connected to helical beams gating three lateral portals (Ge et al., 2015; Wang et al., 2019; Yang et al., 2022) (Figure 1A).
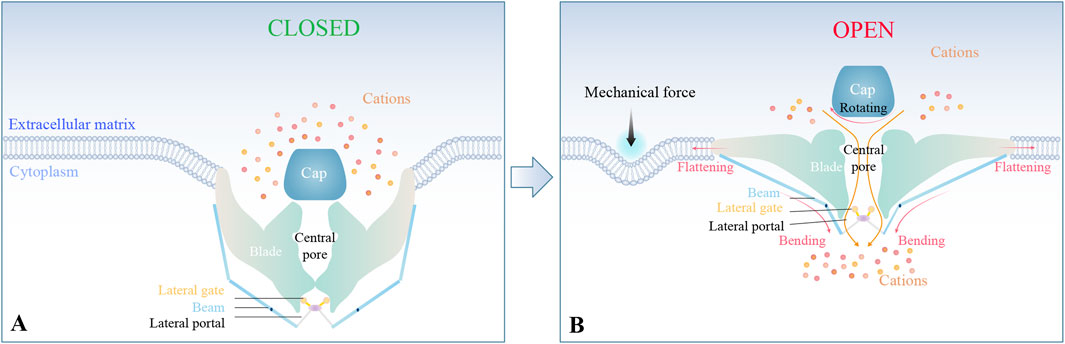
Figure 1. Structural basis of Piezo-mediated mechanotransduction. (A) Piezo1/2 adopt a propeller-shaped structure, including a central pore, an apical extracellular domain, and tripartite curved blade subunits connected to helical beams gating three lateral portals. (B) Under mechanical stimulation, the nanobowl-like conformation undergoes flattening, thereby mediating cation influx through the central pore and lateral portals.
2.2 Activation and inhibition mechanisms
Mechanical stimulation triggers conformational changes in Piezo channels. Specifically, the triskelion configuration distorts the bound lipid membranes into a nanobowl-like structure complex. This nanobowl-like complex flattens upon mechanical stimulation (Ge et al., 2015; Wang et al., 2019; Yang et al., 2022). The resulting flattening of the blades, coupled with the rotation of the cap and bending of the beams, facilitates cation signal transduction through the central pore and lateral portals (Ge et al., 2015; Wang et al., 2019; Yang et al., 2022). This finely tuned conformation underlies the high sensitivity of Piezo channels to mechanical forces, enabling them to modulate pathophysiological processes via cation-influx-triggered molecular signaling cascades (Figure 1B).
Piezo1 channels are gated by diverse mechanical perturbations such as poking, mechanical stretch, fluid shear stress (FSS), and hydrostatic pressure (HP), whereas Piezo2 is primarily responsive to poking and exhibits insensitivity to stretching (Sugimoto et al., 2017; Jiang et al., 2021b). Two primary paradigms govern the mechanoactivation of ion channels: the “force-from-lipids” model and the “force-from-filaments” model (Murthy et al., 2017). The former hypothesis emphasizes the mechanical energy transfer through lipid bilayer deformation under membrane interfacial tension (Arnadóttir and Chalfie, 2010). The latter hypothesis attributes stimulus transduction to molecular tethers coupling to extracellular matrix (ECM) proteins or cytoskeletal elements (Arnadóttir and Chalfie, 2010). Gating of Piezo is intimately linked to the deformations of the lipid (Cox et al., 2016; Cox et al., 2017), and cytoskeletal regulation of membrane tension may further modulate channel activity (Jin et al., 2020). Notably, the presence of the ECM enhances Piezo sensitivity, while its absence renders the channels less responsive to mechanical forces (Gaub and Müller, 2017).
Several synthetic agonists selective for Piezo1 have been identified, such as Yoda1 and Jedi1/2 (Syeda et al., 2015; Lacroix et al., 2018; Wang et al., 2018; Botello-Smith et al., 2019; Xiao, 2020). Yoda1, which is hydrophobic, activates Piezo1 possibly through a “molecular wedge mechanism,” inserting between two domains of the Piezo1 blade to promote blade extension and channel opening under subthreshold stimulation (Botello-Smith et al., 2019). However, mutations in the Piezo1 beam that abrogate Yoda1 activation suggest the involvement of additional, yet unidentified, mechanotransduction pathways (Botello-Smith et al., 2019). Moreover, Dooku1, a derivative of Yoda1, competitively blocks Yoda1-activated Piezo1-dependent Ca2+ signaling (Evans et al., 2018). In contrast, Piezo1 activation can be elicited by Jedi1/2, which are hydrophilic, through binding to the extracellular region of its blade structure and utilizing key mechanotransduction points in the beam (Wang et al., 2018; Xiao, 2020). Interestingly, Yoda1 and Jedi1/2 exert minimal effects on Piezo2. Nonspecific inhibitors, including GsMTx-4, FM1-43, polycationic ruthenium red (RR), streptomycin, and gadolinium, can block Piezo1/2-mediated ion signaling (Coste et al., 2010; Bae et al., 2011; Eijkelkamp et al., 2013; Xu et al., 2021). Among them, GsMTx-4 is the only known selective cation mechanical ion channel inhibitor by altering the surrounding membrane curvature (Bowman et al., 2007; Bae et al., 2011; Alcaino et al., 2017).
3 The position-specific function of the Piezo family in bone
Bone tissues are highly mechanosensitive and undergo adaptive remodeling in response to mechanical stimuli such as exercise and gravity (Morgan et al., 2018). Piezo1 and Piezo2 are both detected in osseous tissues, although Piezo1 exhibits a more extensive expression pattern compared to Piezo2 (Nie and Chung, 2022). Piezo1 is required to sense the biomechanical load and modulate bone formation, thereby influencing human bone mineral density (Haelterman and Lim, 2019; Bai et al., 2020). Besides, Piezo1 responds to oscillatory cortical forces by orienting and driving 3-D cell intercalations, which are important for shaping the mandibular arch in mice (Tao et al., 2019). Piezo2-mediated proprioception is essential to prevent the occurrence of skeletal deformities (Assaraf et al., 2020). Piezo channels exhibit widespread distribution across bone tissues (Figure 2).
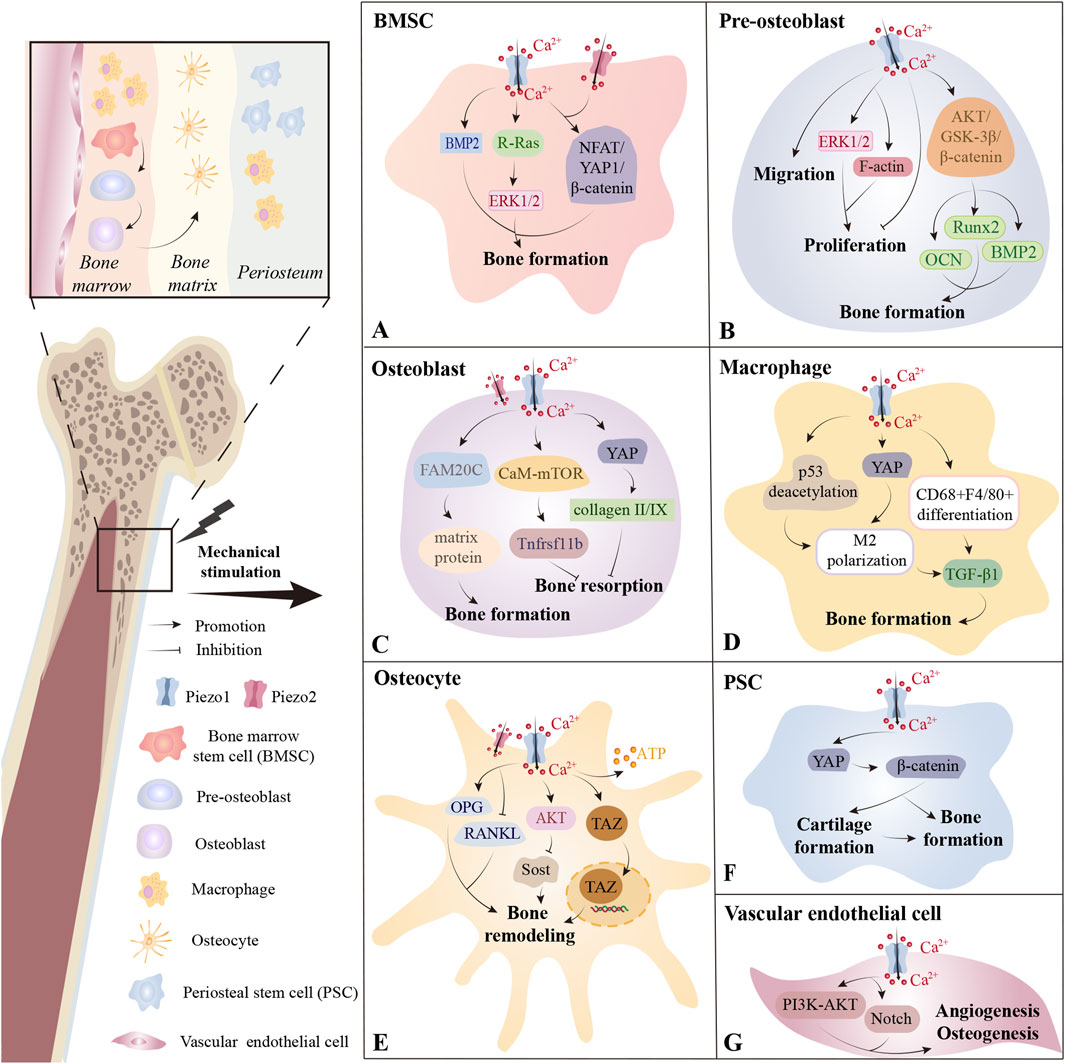
Figure 2. The distribution of Piezo channels across bone tissues. (A) In bone marrow, Piezo1 on bone marrow stem cells (BMSCs) plays a critical role in sensing mechanical stimulation and promoting bone formation through various pathways. Piezo2 may also contribute in some contexts. (B) Activation of the Piezo1 channel on pre-osteoblasts by LIPUS promotes cell proliferation, whereas its activation by Yoda1 inhibits proliferation. Piezo1 is also closely associated with pre-osteoblast migration and osteogenic differentiation. (C) In osteoblasts, Piezo1 helps maintain bone homeostasis by regulating bone formation and bone resorption. (D) Piezo1 on macrophages residing in both bone marrow and periosteum promotes bone formation by facilitating M2 polarization or CD68+F4/80+ differentiation and the secretion of TGF-β1. (E) Within the mineralized bone matrix, Piezo1 is primarily distributed on osteocytes and regulates bone remodeling. (F) In the periosteum, Piezo1 participates in PSC-mediated cartilage formation, bone formation, and the transition from cartilage to bone. (G) Piezo1 on vascular endothelial cells in bone marrow promotes angiogenesis and osteogenesis primarily through the PI3K-AKT and Notch pathways. *BMP2, Bone Morphogenetic Protein 2; ERK1/2, Extracellular Signal-regulated Kinase 1/2; NFAT, Nuclear Factor of Activated T cells; YAP, Yes-associated Protein; AKT, Protein Kinase B; GSK-3β, Glycogen Synthase Kinase 3 Beta; Runx2, Runt-related Transcription Factor 2; OCN, Osteocalcin; BMP2, Bone Morphogenetic Protein 2; FAM20C, Family with Sequence Similarity 20; Member C, CaM-mTOR; Calmodulin-Mammalian Target of Rapamycin; TGF-β1, Transforming Growth Factor Beta 1; OPG, Osteoprotegerin; RANKL, Receptor Activator of Nuclear Factor Kappa-Β Ligand; Sost, Sclerostin; TAZ, Transcriptional Coactivator with PDZ-binding Motif; PI3K, Phosphatidylinositol 3-Kinase.
3.1 In bone marrow, Piezo affects hematopoiesis and bone regeneration by mediating the mechanosensing of mesenchymal lineage cells, immune cells, and endothelial cells
Bone marrow serves as the primary site for lifelong hematopoiesis and bone regeneration, processes that are governed by interactions between bone marrow resident cells including mesenchymal lineage cells, immune cells, hematopoietic stem/progenitor cells, endothelial cells, and neuronal cells (Baccin et al., 2020). The Piezo family, particularly Piezo1, is widely distributed across mesenchymal lineage cells, immunocytes, and endothelial cells within bone marrow niches, mediating physiological functions including bone regeneration and hematopoiesis.
3.1.1 Piezo family in mesenchymal lineage cells affects bone regeneration
In neonatal mice, specific deletion of Piezo1 in osteoblastic mesenchymal progenitor cells causes impaired osteoblast function and increased bone resorption, resulting in multiple spontaneous fractures (Zhou et al., 2020). Piezo1-mediated mechanotransduction is vital for the anti-aging maintenance of peri-arteriolar osteogenic progenitors and the bone morphogenetic protein 2 (BMP2) upregulation-related osteoblastic differentiation of bone marrow mesenchymal stem cells (BMSCs) (Sugimoto et al., 2017; Shen et al., 2021) (Figure 2A). In BMSCs, Piezo1/2 mediate mechanotransduction by synergistically activating nuclear factor of activated T cells/Yes-associated protein/beta catenin (NFAT/YAP1/β-catenin) (Zhou et al., 2020), and the mechanosensing function of Piezo1 has even been exploited in wearable pulsed triboelectric nanogenerator designed to facilitate bone repair (Wang et al., 2022). Recent evidence also implicates Piezo1 in activating the extracellular signal-regulated kinase 1/2 (ERK1/2) signaling cascade within BMSCs, with Piezo1’s C-terminal R-Ras binding domain critically regulating osteoblastic differentiation (Sugimoto et al., 2023).
Piezo1 deficiency in pre-osteoblasts and osteoblasts also causes reduced bone mass and spontaneous fractures (Zhou et al., 2020; Hendrickx et al., 2021). Mechanical loading parameters critically influence Piezo1-dependent responses: exposure to low-intensity pulsed ultrasound (LIPUS) enhances MC3T3-E1 pre-osteoblast proliferation via Piezo1 activation, which promotes phosphorylation of ERK1/2 and polymerization of F-actin around the nucleus—effects reversed by genetic silencing of Piezo1 (Zhang et al., 2021) (Figure 2B). In contrast, Yoda1-induced Piezo1 activation inhibits the proliferative capability of MC3T3-E1 (Yoneda et al., 2019), indicating that different modes of Piezo1 activation can produce divergent cellular outcomes. Besides, Piezo1 silencing independently inhibited the migratory capacity of MC3T3-E1 (Yan et al., 2019). Furthermore, Piezo1 is closely linked to osteogenic differentiation and matrix protein secretion (Song et al., 2020; Dzamukova et al., 2022; Kong et al., 2022), as is demonstrated by its role in nanotube-stimulated osteogenesis in MC3T3-E1 cells (Kong et al., 2022) and in upregulating osteogenic genes (e.g., runt-related transcription factor 2 (Runx2) (Song et al., 2020), BMP2, and osteocalcin (OCN) (Kang et al., 2024)) via pathways such as protein kinase B/glycogen synthase kinase 3 beta/β-catenin (AKT/GSK-3β/β-catenin) (Song et al., 2020). Besides, centrifugation force upregulates family with sequence similarity 20, member C (FAM20C) production in osteoblasts via Piezo1, leading to matrix protein secretion that modulates vascular conversion and enhances bone mineralization (Dzamukova et al., 2022) (Figure 2C).
Analogous to its role in osteogenesis, Piezo1 also critically regulates bone resorption dynamics. Piezo1-deficient mice also exhibit elevated bone resorption and resistance to unloading-induced resorption, a phenomenon that may stem from Piezo1-mediated regulation of osteoclastic differentiation through YAP-driven secretion of collagen II/IX from osteoblasts (Wang et al., 2020). However, selective deletion of Piezo1 in osteoclasts does not impact murine skeletal mass, implying that Piezo1-mediated regulation of bone homeostasis is likely independent of osteoclasts (Wang et al., 2020). Moreover, Piezo1-mediated calcium ion/calmodulin/mammalian target of rapamycin (Ca2+/CaM/mTOR) signaling in osteoblasts and osteocytes suppresses osteoclast formation via regulation of Tnfrsf11b expression, underscoring its protective role against age-related bone loss (Li et al., 2023). Besides, the increase of Piezo1 levels in hematopoietic progenitor cells is irrelevant to the property of applied wall shear stresses (osteoprotective or osteodestructive) (Bratengeier et al., 2020). The above studies suggest that Piezo1 affects osteoclastic activity primarily by osteoblast-osteoclast crosstalk rather than direct actions on osteoclasts and hematopoietic progenitor cells.
Osteoporosis, clinically defined as reduced bone mineral density, microstructural degradation, and elevated fracture susceptibility (Rachner et al., 2011), is alleviated by weight-bearing exercise linked to bone mechanosensitivity (Pagnotti et al., 2019). Osteoporotic patients exhibit markedly decreased Piezo1 protein levels, which correlate positively with key osteogenic differentiation biomarkers (alkaline phosphatase (ALP), OCN, and collagen type I alpha 1 (COL1A)) (Sun et al., 2019). Conditional knockout of Piezo1 within osteochondral lineages further demonstrates that loss of Piezo1 results in impaired skeletal microarchitecture, diminished mechanical integrity, and increased susceptibility to spontaneous fractures, highlighting its importance in trabecular bone formation (Sun et al., 2019). Besides, piezoelectric micro-vibration mitigates osteoporosis induced by estrogen loss via Piezo1 promotion in osteoblasts (Wu et al., 2021).
3.1.2 Piezo1 in immune cells and endothelial cells affects hematopoiesis and bone regeneration
Macrophage Piezo1-YAP signaling axis activation promotes angiogenesis and osteogenesis by inducing M2 polarization (Tang et al., 2021) (Figure 2D). Changes in the physical microenvironment are caused by irradiation exerting mechanical stretch stimulation on residual bone marrow macrophages (BM-Mφs), thereby upregulating Piezo1 and activating the calcineurin/NFAT/hypoxia-inducible factor-1 alpha (HIF-1α) pathway (Zhang et al., 2022). This cascade enhances the expression of Vascular growth factor A (VEGF-A), which is a key factor for hematopoiesis (Zhang et al., 2022). Piezo1 also influences the function and glucose metabolism of bone-marrow-derived dendritic cells under tension (Chakraborty et al., 2021). Deletion of Piezo1 in bone vasculature impairs angiogenesis and osteogenesis via phosphatidylinositol 3-kinase (PI3K)/AKT and Notch signaling pathways (Chen et al., 2021) (Figure 2G).
3.2 In mineralized bone matrix, Piezo1 is involved in osteocyte-mediated bone remodeling
Osteocytes encased in mineralized matrix serve as primary mechanosensory cells in bone tissues (Haelterman and Lim, 2019). Piezo1 vitally functions in osteocyte sensation of FSS, supported by in vivo evidence showing that modulation of Piezo1 alters the load-dependent bone formation (Li et al., 2019; Sun et al., 2019) (Figure 2E). Under mechanical loading, osteocytes balance bone remodeling by adjusting the receptor activator of nuclear factor kappa-Β ligand (RANKL)/osteoprotegerin (OPG) ratio and sclerostin/dickkopf-related protein 1 (Sost/Dkk1)-mediated Wnt pathway (Nakashima et al., 2011; Robling and Bonewald, 2020; Karthik and Guntur, 2021). In vitro, MLO-Y4 osteocytes sense FSS through Piezo1, accompanied by upregulation of the bone formation factor OPG and downregulation of the bone resorption factor RANKL (Liu et al., 2022b). The mechanically induced Sost expression suppression in osteocytic cell line IDG-SW3 is abrogated by Piezo1 deficiency or inhibition and AKT inhibitors, suggesting that the Piezo1/AKT pathway may mediate this regulatory process (Sasaki et al., 2020). Collectively, Piezo1 regulates the transcription of critical osteogenic and osteoclastic markers of osteocytes and is fundamental to mechanosensitive osteocyte-mediated bone remodeling. Additionally, MLO-Y4 cells can detect stretching forces via Piezo1, which triggers calcium influx, transcriptional coactivator with PDZ-binding motif (TAZ) nuclear translocation, and ATP production—events that amplify BMSCs’ osteogenic capacity and may offer novel intervention strategies for mechanical bone remodeling (Ru et al., 2024).
3.3 In periosteum, Piezo1 coordinates bone formation through stem cell migration/differentiation and macrophage-mediated osteoprogenitor recruitment
High levels of Piezo1 expression are detected in periosteal stem cells (PSCs) as well as macrophages in the periosteum (Deng et al., 2022; Liu et al., 2022a) (Figure 2F). Studies have consistently demonstrated that Piezo1 is indispensable for PSC-mediated chondrogenesis, bone formation, and cartilaginous bone transformation during fracture repair (Liu et al., 2022a). This role may be attributed to Piezo1’s ability to enhance the migratory, osteogenic, and pro-angiogenic capacities of PSCs, primarily via the YAP/β-catenin pathway activation (Liu et al., 2022a). During the meniscal regeneration process, biomechanical stimulation triggers Piezo1-mediated Ca2+ influx, subsequently activating calcium/calmodulin-dependent protein kinase (CaMK) and nuclear factor of activated T-cells, cytoplasmic 1 (NFATc1), thus promoting the YAP/phosphorylated Smad2/3 (pSmad2/3)/SRY-related HMG-box 9 (SOX9) pathway (Yan et al., 2023). However, the mechanisms by which Piezo1 modulates cell migration and pro-angiogenic secretion of VEGF-A remain to be fully elucidated.
CD68+ macrophages are the most mechanosensitive type of macrophage in the periosteum (Deng et al., 2022) (Figure 2D). Mechanical loading activates Piezo1 in CD68+F4/80- macrophage subsets, driving their differentiation into the CD68+F4/80+ phenotypes (Deng et al., 2022). These differentiated macrophages secrete transforming growth factor beta 1 (TGF-β1) and thrombospondin-1 (Thbs1, a cytokine activating TGF-β1 by phosphorylating Smad2/3) to recruit osteoprogenitor cells (Deng et al., 2022). Additionally, mechanical strain-induced Ca2+ influx triggers p53 post-translational modification through coordinated acetylation/deacetylation dynamics (Cai et al., 2023). These epigenetic reprogrammings drive macrophage polarization toward an M2 reparative phenotype, enabling TGF-β1 secretion that stimulates BMSCs recruitment, clonal expansion, and osteogenic differentiation (Cai et al., 2023).
3.4 In periosteal nerve, Piezo2 mediates proprioception to maintain the normal development of bone tissue
In mammals, Piezo2 serves as the primary mechanotransducer for proprioception (Woo et al., 2015). Loss of Piezo2 function in humans leads to prenatal proprioceptive impairment, triggering abnormalities in joint positioning and ultimately leading to bone disorders like hip dysplasia, scoliosis, and distal arthrogryposis (Coste et al., 2013; McMillin et al., 2014; Chesler et al., 2016; Delle Vedove et al., 2016; Haliloglu et al., 2017; Mahmud et al., 2017; Uehara et al., 2020). A study in mice further confirmed that selective Piezo2 deficiency in proprioceptive neurons—but not in chondro-osteoprogenitor lineages—resulted in skeletal malformations such as aberrant hip and spinal structure (Assaraf et al., 2020).
4 Piezo family in tooth tissue
Similar to bone tissue, Piezo channels in dental tissue regulate cell differentiation and pathological processes by sensing mechanical forces, but their distribution and function are tissue-specific. Tooth tissues consist of the inner pulp and the outer hard tissues including dentin, enamel, and cementum. The cementum is connected to the periodontal tissues to support the tooth. While prior research has delineated Piezo channel localization within the pulp, dentin, and periodontal tissues, their distribution in other dental compartments and involvement in various dental-related pathophysiological activities remain to be further investigated. Figure 3 shows the distribution of Piezo in dental tissues and the related downstream pathways (Figure 3).
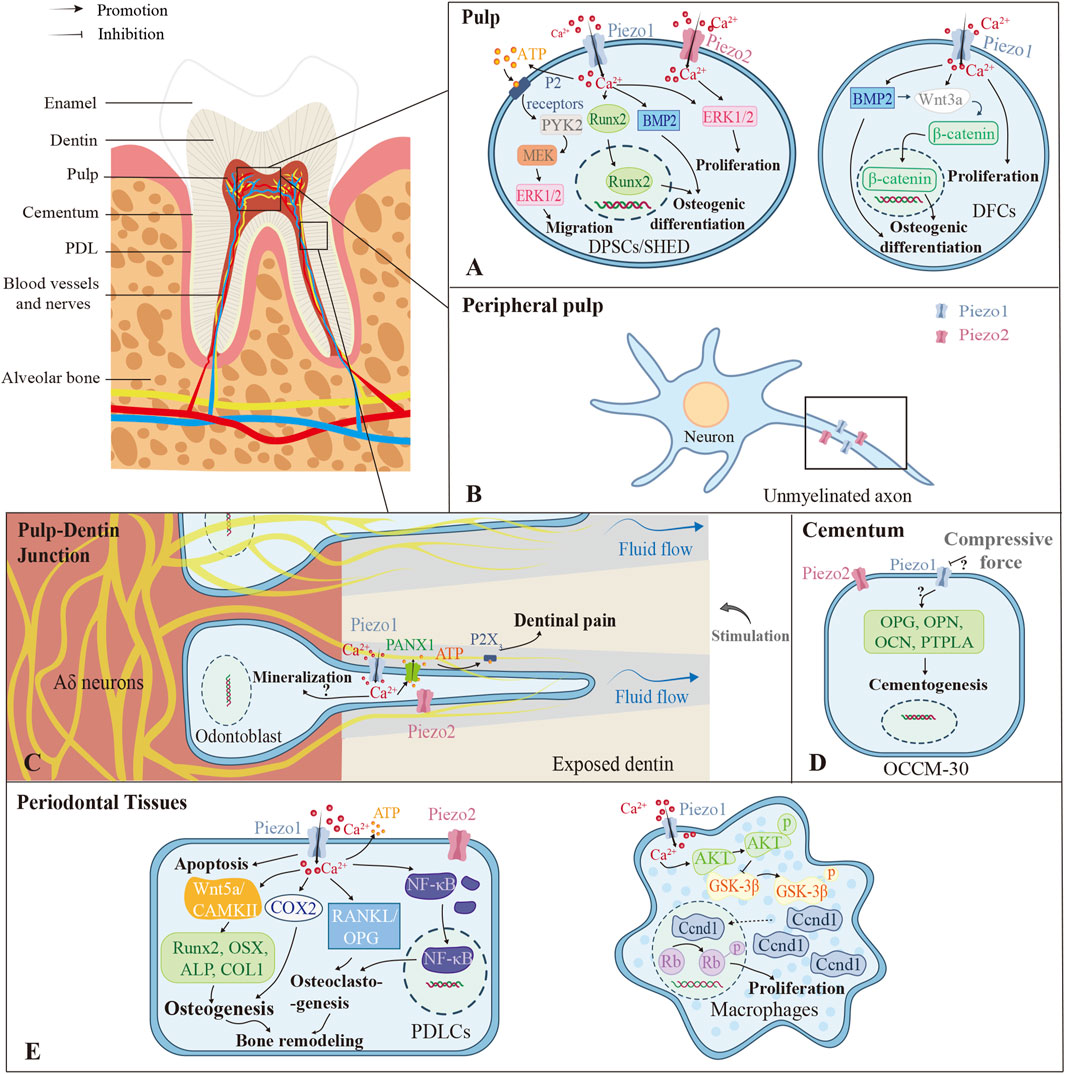
Figure 3. Different roles the Piezo channels play in different parts of the tooth. (A) The pulp contains stem cells regularly. Piezo channels in DPSCs and SHED can promote migration, osteogenic differentiation, and proliferation through various pathways with mechanical stimulation. DFC-expressed Piezo1 promotes cell proliferation and osteogenic differentiation capacity primarily through β-catenin-mediated Wnt3a signaling. (B) In peripheral pulp, Piezo1/2 are mainly localized in unmyelinated axons. (C) The Piezos are closely related to dentin sensitivity, mainly via the Piezo1-PANX1-P2X3 axis. But whether Piezo1/2 promote dentin mineralization still remains controversial. (D) In cementum, compressive force downregulates Piezo1 expression and cementogenic markers in OCCM-30, while some studies show opposite results. (E) In periodontal tissues, Piezo1/2 in PDLCs affect cell apoptosis, osteogenesis, osteoclastogenesis, and bone remodeling. Piezo1 also exerts positive effects on the proliferation of macrophages through the Piezo1-AKT-Ccnd1 pathway. *PDL, Periodontal Ligament; DPSCs, Dental Pulp Stem Cells; SHED, Human Exfoliated Deciduous Teeth; DFCs, Dental Follicle Cells; PTPLA, Protein Tyrosine Phosphatase-like Protein-A; PDLCs, Periodontal Ligament Cells; PYK2, Protein Tyrosine Kinase 2; Runx2, Runt-related Transcription Factor 2; MEK, Mitogen-activated Protein Kinase; ERK1/2, Extracellular Signal-regulated Kinase 1/2; BMP2, Bone Morphogenetic Protein 2; PANX1, Pannexin-1; OPG, Osteoprotegerin; OPN, Osteopontin; OCN, Osteocalcin; CAMKII, Calcium/Calmodulin-dependent Protein Kinase II; COX2, Cyclooxygenase-2; OSX, Osterix; ALP, Alkaline Phosphatase; COLI, Collagen Type I; RANKL, Receptor Activator of Nuclear Factor Kappa-Β Ligand; NF-κB, Nuclear Factor Kappa-Β; AKT, Protein Kinase B; GSK-3β, Glycogen Synthase Kinase 3 Beta; Ccnd, Cyclin D.
4.1 In dental pulp, Piezo affects the proliferative activity and differentiation capacity of pulp-derived stem cells and is involved in pulpal pain perception
Dental tissues harbor various stem cell populations, exemplified by dental pulp stem cells (DPSCs) and stem cells from human exfoliated deciduous teeth (SHED), both demonstrating self-renewal and multipotency—attributes that make them promising for tissue engineering (Zhai et al., 2019) (Figure 3A). DPSCs are sensitive to mechanical stimulations, which promote their proliferation while reducing the viability and adhesive properties (Gaite et al., 2024). Yoda1-induced activation of Piezo1 enhances migration of human DPSCs (hDPSCs) via an ATP-dependent protein tyrosine kinase 2 (PYK2)/mitogen-activated protein kinase (MEK)/ERK signaling cascade (Mousawi et al., 2020), while mechanical or chemical activation of Piezo1 promotes osteogenic differentiation by modulating BMP2 expression (Sugimoto et al., 2017). Static pressure can increase the expression of Piezo2 in hDPSCs as well (Kang et al., 2024). In SHED, Piezo1 regulates the nuclear translocation of Runx2, which is essential for osteoblast and odontoblast differentiation (Miyazaki et al., 2019). Additionally, in dental follicle cells (DFCs), which are contributors to cementogenesis, periodontal ligament formation, and alveolar bone development (Zhou et al., 2019), Piezo1 activation via Wnt3a/β-catenin signaling promotes proliferation and osteoblastic lineage commitment (Xing et al., 2022) (Figure 3A).
Pulp pain is mediated by slow-conducted unmyelinated C-fibers and fast-conducted myelinated A-fibers (Bender, 2000). In pulpitis, inflammatory mediators lower the nociceptor thresholds, activating pain-associated ion channels (Yang et al., 2024). Piezo channels may contribute to the initial hyperemic response, potentially via Piezo2-mediated vascular mechanotransduction, given its detection in the blood vessel walls of human dental pulp (Gaite et al., 2024).
Piezo1 predominantly drives inflammatory progression, localizing to small myelinated Aδ fibers (60.2%), large myelinated Aβ fibers (24.3%), and unmyelinated C fibers (15.5%) (Bryniarska-Kubiak et al., 2024). Its expression increases progressively during irreversible pulpitis and correlates significantly with pro-inflammatory cytokines (interleukin-1beta (IL-1β), IL-6, tumor necrosis factor-alpha (TNF-α)) (Yang et al., 2024).
Piezo2 serves as the primary mechanonociception transducer, functioning as a low-threshold mechano-detector (Wan et al., 2024) enriched in Merkel cells and myelinated afferents (Ohyama et al., 2022). Critically, in peripheral pulp, both channels localize to unmyelinated axons ascending toward dentin, indicating their roles in mediating acute mechanical pain (Figure 3B) (Cho et al., 2022; Han et al., 2022). Piezo2 specifically facilitates glutamate release via vesicular transporters (Cho et al., 2022; Han et al., 2022). During irreversible pulpitis, Piezo2 downregulation occurs despite its strong association with pain mediators (neuropeptide Y (NPY), substance P, Tachykinin 1 (TAC1)) and overall pain intensity (Yang et al., 2024). Mechanistically, the cAMP signaling pathway potentiates Piezo2 mechanosensitivity in inflammation (Yang et al., 2024), consistent with its role in inflammatory mechanical hyperalgesia (Wan et al., 2024).
Collectively, although both Piezo1/2 are expressed on pulp nerve fibers and function as mechanosensitive channels directly involved in mediating pulp pain, they exhibit functional divergence: Piezo1 amplifies inflammatory responses while Piezo2 directly mediates nociception and may participate in vascular hyperemic responses. Their inverse expression dynamics highlight distinct pathophysiological roles.
4.2 In dentin, Piezo mediates the perception of pain and is associated with dentin formation
In rodents, Piezo2 is predominantly expressed in mature odontoblasts (Khatibi Shahidi et al., 2015), while Piezo1 is primarily localized to the cell membrane and cytoplasm of human and murine odontoblasts (Huang et al., 2024). However, Gaite et al. (2024) proposed an alternative expression pattern in human teeth, demonstrating that Piezo1/2 are mainly present in pre-odontoblasts rather than mature odontoblasts and are absent in dentinal tubules. In contrast, murine odontoblasts exhibit widespread Piezo1/2 immunoreactivity, particularly at the basal pole (Gaite et al., 2024). These discrepancies are likely attributed to technical variations, highlighting the need for further investigation into Piezo channel localization in human odontoblasts.
Dentin sensitivity (DS) refers to pain arising from exposed dentin, not attributable to other dental diseases, and is best explained by hydrodynamic theory (Mantzourani and Sharma, 2013). According to this theoretical framework, external stimuli increase dentinal tubular fluid efflux, generating hydrodynamic shear stress on mechanosensory nerves in the tubules and then activating the Aδ nerve at the pulp-dentin junction, eventually leading to pain (Mantzourani and Sharma, 2013). Researchers demonstrate that Piezo1 and TRPV1/2/3/4 channels function as mechanosensors in this process, facilitating pannexin-1 (PANX1)-dependent ATP secretion, thereby establishing a communication pathway between odontoblasts and sensory neurons (Ohyama et al., 2022) (Figure 3C). Dental pain is triggered by P2X3 receptor activation due to extracellular ATP release (Sato et al., 2018). Pharmacological blockade of the Piezo1/TRPA1-PANX1-P2X3 axis in odontoblasts significantly reduces cold-induced pain responses in exposed dentin (Ohyama et al., 2022).
Additionally, Huang et al. (2024) revealed that Piezo1 promotes odontoblast mineralization in vitro via Ca2+/PI3K-AKT/semaphorin 3A (SEMA3A) by way of inducing hDPSCs to differentiate into odontoblasts, and they further confirmed its involvement in reactive dentin formation in vivo. Conversely, Matsunaga et al. (2021) identified that chemically activated Piezo1 inhibits the mineralization of odontoblasts, whereas knockdown of Piezo1 promotes the mineralization, and that Piezo1 is also vital for the suppression of dentinogenesis after cellular deformation within dentin tubules. These findings suggest that Piezo1 may exert context-dependent effects on odontoblast mineralization, warranting further investigation.
4.3 In cementum, Piezo1 affects the cementogenic activity of cementoblasts
The cementum, which covers the root dentin and provides the anchor for the periodontal ligament (PDL) (Foster, 2017), is primarily composed of a mineralized matrix secreted by cementoblasts and collagen fibers derived from the PDL (Nuñez et al., 2019). The ability of cementoblasts to secrete mineralized matrix makes them pivotal in the formation of restorative cementum and the reconstruction of periodontal function (Nuñez et al., 2019). An in vitro study using the murine cementoblast model OCCM-30 confirmed the Piezo1 expression and found that the knockdown of Piezo1 exacerbated the decrease in the expression of cementogenic activity markers caused by static mechanical force (Zhang et al., 2017) (Figure 3D). Additionally, micro-CT imaging of Piezo1-knockout mice revealed marked reductions in cellular cementum, alveolar bone volume, and cementum ECM mass (Zhang et al., 2023), but this may result from diminished periodontal ligament stem cells (PDLSCs) differentiation into cementoblasts. In contrast, HP has been shown to increase Piezo1 expression in cementoblasts while suppressing cell migration and OPG expression, indicating that compressive forces impair cementoblast function by enhancing Piezo1 activity (Wang et al., 2023). Therefore, the precise role of Piezo1 in human cementogenesis and the underlying mechanisms involved remain to be further investigated.
4.4 In periodontal tissues, Piezo mediates periodontal tissue remodeling due to orthodontic tooth movement
OTM denotes the therapeutic application of controlled biomechanical forces to reposition misaligned dental units through coordinated periodontal remodeling (Jiang et al., 2021a). This biological process specifically involves structural adaptation of the periodontium components: alveolar bone, periodontal ligament (PDL), and gingiva (Jiang et al., 2021a). During OTM, the remodeling of tissues is triggered by the mechanical signal transduction of periodontal ligament cells (PDLCs) and osteocytes (Li et al., 2021). PDLCs exhibit dual expression of Piezo1 and Piezo2, with Piezo1 being more abundant (Horie et al., 2023). However, RR-mediated Piezo suppression has no effect on the proliferation of PDLCs (Gao et al., 2017). Activation of Piezo1 by Yoda1 promotes periodontal tissue regeneration through the stimulation of Lepr+ periodontal ligament stem cells (PDLSCs) (Zhang et al., 2023) and converts mechanical stimuli into intracellular calcium influx that modulates downstream signaling cascades such as Notch, ERK, nuclear factor kappa-Β (NF-κB), and so on (Jiang et al., 2024).
In a rat OTM model, Piezo1 activation on the tension side boosts osteogenic markers (Runx2, osterix (OSX), ALP, and collagen type I (COL1)) and elevates osteoclastic activity, both fundamental for alveolar bone remodeling (Jiang et al., 2021a) (Figure 3E). The non-canonical Wnt/Ca2+ pathway could be associated with this process, as indicated by correlations between Wnt5a/CAMKII expression and bone-related molecules on the tension side (Du and Yang, 2023). An in vitro study also showed that Piezo1 expression was upregulated in PDLCs after stretch loading, and this upregulation was closely related to stress-stimulated transcriptional activation of cyclooxygenase-2 (COX2) coupled with modulation of RANKL/OPG signaling axis in PDLCs (Shen et al., 2020). Furthermore, Piezo1 expression can be enhanced by mechanical tensile force in human PDLSCs (hPDLSCs), thus activating the Notch1 pathway and facilitating their osteogenic differentiation capacity (Lin et al., 2020).
Recent studies manifest that Piezo1 has an impact on pressure-triggered PDLC apoptosis (Jiang et al., 2024) and inflammatory gene expression (Schröder et al., 2023), while also promoting osteoclast differentiation (Zheng et al., 2024). Under mechanical pressure, Piezo1 activation in PDLCs elevates the expression of pro-inflammatory genes (TNF, IL-6, prostaglandin-endoperoxide synthase 2 (PTGS2)) (Schröder et al., 2023), upregulates pro-apoptotic proteins (Bax and caspase-3), and inhibits anti-apoptotic proteins, thereby promoting apoptosis via the p38/ERK1/2 pathway (Shen et al., 2023). Importantly, in the compression areas of OTM models, upregulated Piezo1 and β-catenin can be detected (Jiang et al., 2024), accompanied by increased RANKL/OPG ratios (Zheng et al., 2024). Inhibition of Piezo1 reduces the distance of tooth movement (Horie et al., 2023; Jiang et al., 2024). Interestingly, Piezo1 appears to mediate osteoclastogenesis in a context-dependent manner—upregulating RANKL under pressure while downregulating OPG in the absence of mechanical strain (Schröder et al., 2023). This may involve distinct intracellular signaling pathways, calling for further exploration. Additionally, Piezo1 on the PDLC membrane facilitates extracellular ATP release under compressive force, a mechanism that is critical for both bone remodeling and pain perception during orthodontic treatment (Horie et al., 2023).
Furthermore, immune cells including macrophages are recruited and play an integral regulatory role during OTM (Li et al., 2021). Early recruitment of M1 macrophages initiates osteoclastogenesis, whereas later recruitment of M2 macrophages suppresses osteoclastic activity and promotes bone deposition (Li et al., 2021). The Piezo1/AKT/cyclin D (Ccnd1) axis is essential for the proliferation and infiltration of macrophages in periodontal tissues during OTM (Xu et al., 2022) (Figure 3E). GsMTx4 also indirectly encourages the osteolytic differentiation of RAW264.7 by affecting the NF-κB pathway in periodontal ligament cells (Jin et al., 2015). Nevertheless, it remains enigmatic whether Piezo1 mediates macrophage polarization during OTM.
5 Discussion
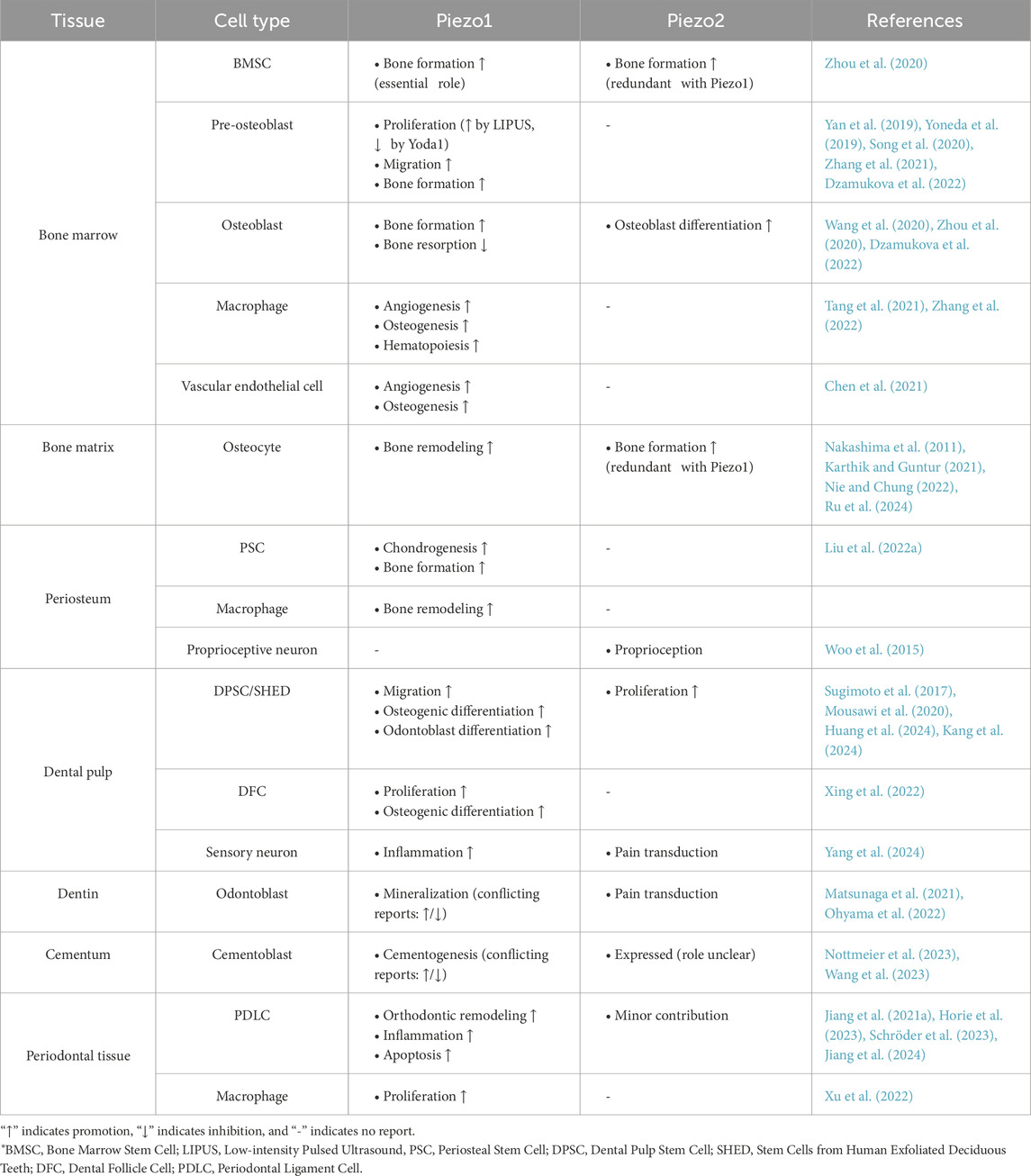
As mechanotransducers, Piezo1/2 channels convert mechanical stress into cation influx, activating downstream signaling pathways that regulate diverse pathophysiological processes. Piezo1 and Piezo2, activated by different mechanical and chemical stimuli, are widely distributed in bone and teeth tissues. The cell-type-specific expression patterns and functional roles of Piezo channels across these mineralized tissues are systematically summarized in Table 1.
Building upon this comprehensive synthesis, in bone, Piezo channels are localized to mesenchymal cells, immune cells, and osteocytes within osteo-microenvironments, mediating hematopoiesis and skeletal regeneration/remodeling. In dental tissues, Piezo channels in the pulp, dentin, cementum, and periodontal tissues influence cell differentiation, proliferation, and migration and are closely associated with pulpitis and DS-induced pain, dentin/cementum mineralization, and periodontal adaptation during OTM.
Despite the progress, several unresolved questions still persist. For example, most current studies are largely confined to cellular or animal models, leaving the precise localization of Piezo channels in human hard tissues contentious. Beyond that, conflicting conclusions exist regarding Piezo-regulated dentin/cementum formation under identical mechanical force, potentially due to experimental techniques and conditions limitations, calling for the necessity for advanced techniques and standardized experimental models to reconcile context-dependent outcomes. Also, variations in force magnitude, duration, or other subtle factors may lead to the completely opposite effect that Piezo channels have on the same objects, which needs deeper investigations.
Mechanobiological understanding of Piezo channels in osseous and dental tissues could pave the way for innovative approaches in tissue engineering and disease treatment. Future studies should be based on the existing studies to clarify Piezo channels’ precise functional roles and relative mechanisms in the physiopathological processes of bone and teeth.
Author contributions
JD: Writing – original draft, Data curation. RL: Writing – original draft, Data curation. YC: Writing – original draft. GZ: Writing – review and editing, Project administration, Conceptualization, Supervision. XL: Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Acheta, J., Bhatia, U., Haley, J., Hong, J., Rich, K., Close, R., et al. (2022). Piezo channels contribute to the regulation of myelination in schwann cells. Glia 70 (12), 2276–2289. doi:10.1002/glia.24251
Alcaino, C., Knutson, K., Gottlieb, P. A., Farrugia, G., and Beyder, A. (2017). Mechanosensitive ion channel Piezo2 is inhibited by D-GsMTx4. Channels 11 (3), 245–253. doi:10.1080/19336950.2017.1279370
Arnadóttir, J., and Chalfie, M. (2010). Eukaryotic mechanosensitive channels. Annu. Rev. Biophys. 39, 111–137. doi:10.1146/annurev.biophys.37.032807.125836
Assaraf, E., Blecher, R., Heinemann-Yerushalmi, L., Krief, S., Carmel Vinestock, R., Biton, I. E., et al. (2020). Piezo2 expressed in proprioceptive neurons is essential for skeletal integrity. Nat. Commun. 11 (1), 3168. doi:10.1038/s41467-020-16971-6
Baccin, C., Al-Sabah, J., Velten, L., Helbling, P. M., Grünschläger, F., Hernández-Malmierca, P., et al. (2020). Combined single-cell and spatial transcriptomics reveal the molecular, cellular and spatial bone marrow niche organization. Nat. Cell Biol. 22 (1), 38–48. doi:10.1038/s41556-019-0439-6
Bae, C., Sachs, F., and Gottlieb, P. A. (2011). The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry 50 (29), 6295–6300. doi:10.1021/bi200770q
Bai, W.-Y., Wang, L., Ying, Z.-M., Hu, B., Xu, L., Zhang, G.-Q., et al. (2020). Identification of PIEZO1 polymorphisms for human bone mineral density. Bone 133, 115247. doi:10.1016/j.bone.2020.115247
Bender, I. B. (2000). Pulpal pain diagnosis--a review. J. Endod. 26 (3), 175–179. doi:10.1097/00004770-200003000-00012
Botello-Smith, W. M., Jiang, W., Zhang, H., Ozkan, A. D., Lin, Y. C., Pham, C. N., et al. (2019). A mechanism for the activation of the mechanosensitive Piezo1 channel by the small molecule Yoda1. Nat. Commun. 10 (1), 4503. doi:10.1038/s41467-019-12501-1
Bowman, C. L., Gottlieb, P. A., Suchyna, T. M., Murphy, Y. K., and Sachs, F. (2007). Mechanosensitive ion channels and the peptide inhibitor GsMTx-4: history, properties, mechanisms and pharmacology. Toxicon 49 (2), 249–270. doi:10.1016/j.toxicon.2006.09.030
Bratengeier, C., Liszka, A., Hoffman, J., Bakker, A. D., and Fahlgren, A. (2020). High shear stress amplitude in combination with prolonged stimulus duration determine induction of osteoclast formation by hematopoietic progenitor cells. FASEB J. 34 (3), 3755–3772. doi:10.1096/fj.201901458R
Bryniarska-Kubiak, N., Basta-Kaim, A., and Kubiak, A. (2024). Mechanobiology of dental pulp cells. Cells 13 (5), 375. doi:10.3390/cells13050375
Cai, G., Lu, Y., Zhong, W., Wang, T., Li, Y., Ruan, X., et al. (2023). Piezo1-mediated M2 macrophage mechanotransduction enhances bone formation through secretion and activation of transforming growth factor-β1. Cell Prolif. 56 (9), e13440. doi:10.1111/cpr.13440
Chakraborty, M., Chu, K., Shrestha, A., Revelo, X. S., Zhang, X., Gold, M. J., et al. (2021). Mechanical stiffness controls dendritic cell metabolism and function. Cell Rep. 34 (2), 108609. doi:10.1016/j.celrep.2020.108609
Chen, P., Zhang, G., Jiang, S., Ning, Y., Deng, B., Pan, X., et al. (2021). Mechanosensitive Piezo1 in endothelial cells promotes angiogenesis to support bone fracture repair. Cell Calcium 97, 102431. doi:10.1016/j.ceca.2021.102431
Chesler, A. T., Szczot, M., Bharucha-Goebel, D., Čeko, M., Donkervoort, S., Laubacher, C., et al. (2016). The role of PIEZO2 in human mechanosensation. N. Engl. J. Med. 375 (14), 1355–1364. doi:10.1056/NEJMoa1602812
Cho, Y. S., Han, H. M., Jeong, S. Y., Kim, T. H., Choi, S. Y., Kim, Y. S., et al. (2022). Expression of Piezo1 in the trigeminal neurons and in the axons that innervate the dental pulp. Front. Cell Neurosci. 16, 945948. doi:10.3389/fncel.2022.945948
Coste, B., Houge, G., Murray, M. F., Stitziel, N., Bandell, M., Giovanni, M. A., et al. (2013). Gain-of-function mutations in the mechanically activated ion channel PIEZO2 cause a subtype of distal arthrogryposis. Proc. Natl. Acad. Sci. U. S. A. 110 (12), 4667–4672. doi:10.1073/pnas.1221400110
Coste, B., Mathur, J., Schmidt, M., Earley, T. J., Ranade, S., Petrus, M. J., et al. (2010). Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330(6000), 55–60. doi:10.1126/science.1193270
Coste, B., Xiao, B., Santos, J. S., Syeda, R., Grandl, J., Spencer, K. S., et al. (2012). Piezo proteins are pore-forming subunits of mechanically activated channels. Nat 483 (7388), 176–181. doi:10.1038/nature10812
Cox, C. D., Bae, C., Ziegler, L., Hartley, S., Nikolova-Krstevski, V., Rohde, P. R., et al. (2016). Removal of the mechanoprotective influence of the cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nat. Commun. 7, 10366. doi:10.1038/ncomms10366
Cox, C. D., Bavi, N., and Martinac, B. (2017). Origin of the force: the force-from-lipids principle applied to piezo channels. Curr. Top. Membr. 79, 59–96. doi:10.1016/bs.ctm.2016.09.001
Della Pietra, A., Mikhailov, N., and Giniatullin, R. (2020). The emerging role of mechanosensitive piezo channels in migraine pain. Int. J. Mol. Sci. 21 (3), 696. doi:10.3390/ijms21030696
Delle Vedove, A., Storbeck, M., Heller, R., Hölker, I., Hebbar, M., Shukla, A., et al. (2016). Biallelic loss of proprioception-related PIEZO2 causes muscular atrophy with perinatal respiratory distress, arthrogryposis, and scoliosis. Am. J. Hum. Genet. 99 (5), 1406–1408. doi:10.1016/j.ajhg.2016.11.009
Deng, R., Li, C., Wang, X., Chang, L., Ni, S., Zhang, W., et al. (2022). Periosteal CD68(+) F4/80(+) macrophages are mechanosensitive for cortical bone formation by secretion and activation of TGF-β1. Adv. Sci. (Weinh) 9 (3), e2103343. doi:10.1002/advs.202103343
Douguet, D., and Honoré, E. (2019). Mammalian mechanoelectrical transduction: structure and function of force-gated ion channels. Cell 179 (2), 340–354. doi:10.1016/j.cell.2019.08.049
Douguet, D., Patel, A., Xu, A., Vanhoutte, P. M., and Honoré, E. (2019). Piezo ion channels in cardiovascular mechanobiology. Trends Pharmacol. Sci. 40 (12), 956–970. doi:10.1016/j.tips.2019.10.002
Du, Y., and Yang, K. (2023). Role of mechanosensitive ion channel Piezo1 in tension-side orthodontic alveolar bone remodeling in rats. Arch. Oral Biol. 155, 105798. doi:10.1016/j.archoralbio.2023.105798
Dzamukova, M., Brunner, T. M., Miotla-Zarebska, J., Heinrich, F., Brylka, L., Mashreghi, M. F., et al. (2022). Mechanical forces couple bone matrix mineralization with inhibition of angiogenesis to limit adolescent bone growth. Nat. Commun. 13 (1), 3059. doi:10.1038/s41467-022-30618-8
Earley, S., Santana, L. F., and Lederer, W. J. (2022). The physiological sensor channels TRP and piezo: nobel prize in physiology or medicine 2021. Physiol. Rev. 102 (2), 1153–1158. doi:10.1152/physrev.00057.2021
Eijkelkamp, N., Linley, J. E., Torres, J. M., Bee, L., Dickenson, A. H., Gringhuis, M., et al. (2013). A role for Piezo2 in EPAC1-dependent mechanical allodynia. Nat. Commun. 4, 1682. doi:10.1038/ncomms2673
Evans, E. L., Cuthbertson, K., Endesh, N., Rode, B., Blythe, N. M., Hyman, A. J., et al. (2018). Yoda1 analogue (Dooku1) which antagonizes Yoda1-evoked activation of Piezo1 and aortic relaxation. Br. J. Pharmacol. 175 (10), 1744–1759. doi:10.1111/bph.14188
Feng, J., Zhao, Y., Xie, Z., Zang, K., Sviben, S., Hu, X., et al. (2022). Miswiring of merkel cell and pruriceptive C fiber drives the itch-scratch cycle. Sci. Transl. Med. 14 (653), eabn4819. doi:10.1126/scitranslmed.abn4819
Foster, B. L. (2017). On the discovery of cementum. J. Periodontal Res. 52 (4), 666–685. doi:10.1111/jre.12444
Gaite, J. J., Solé-Magdalena, A., García-Mesa, Y., Cuendias, P., Martin-Cruces, J., García-Suárez, O., et al. (2024). Immunolocalization of the mechanogated ion channels PIEZO1 and PIEZO2 in human and mouse dental pulp and periodontal ligament. Anat. Rec. Hob. 307 (5), 1960–1968. doi:10.1002/ar.25351
Gao, Q., Cooper, P. R., Walmsley, A. D., and Scheven, B. A. (2017). Role of piezo channels in ultrasound-stimulated dental stem cells. J. Endod. 43 (7), 1130–1136. doi:10.1016/j.joen.2017.02.022
Gaub, B. M., and Müller, D. J. (2017). Mechanical stimulation of Piezo1 receptors depends on extracellular matrix proteins and directionality of force. Nano Lett. 17 (3), 2064–2072. doi:10.1021/acs.nanolett.7b00177
Ge, J., Li, W., Zhao, Q., Li, N., Chen, M., Zhi, P., et al. (2015). Architecture of the Mammalian mechanosensitive Piezo1 channel. Nat 527 (7576), 64–69. doi:10.1038/nature15247
Glyn-Jones, S., Palmer, A. J., Agricola, R., Price, A. J., Vincent, T. L., Weinans, H., et al. (2015). Osteoarthr. Lancet 386 (9991), 376–387. doi:10.1016/s0140-6736(14)60802-3
Haliloglu, G., Becker, K., Temucin, C., Talim, B., Küçükşahin, N., Pergande, M., et al. (2017). Recessive PIEZO2 stop mutation causes distal arthrogryposis with distal muscle weakness, scoliosis and proprioception defects. J. Hum. Genet. 62 (4), 497–501. doi:10.1038/jhg.2016.153
Han, H. M., Jeong, S. Y., Cho, Y. S., Choi, S. Y., and Bae, Y. C. (2022). Expression of Piezo2 in the dental pulp, sensory root, and trigeminal ganglion and its coexpression with vesicular glutamate transporters. J. Endod. 48 (11), 1407–1413. doi:10.1016/j.joen.2022.07.012
Hendrickx, G., Fischer, V., Liedert, A., von Kroge, S., Haffner-Luntzer, M., Brylka, L., et al. (2021). Piezo1 inactivation in chondrocytes impairs trabecular bone formation. J. Bone Min. Res. 36 (2), 369–384. doi:10.1002/jbmr.4198
Herrera, D., Alonso, B., de Arriba, L., Santa Cruz, I., Serrano, C., and Sanz, M. (2014). Acute periodontal lesions. Periodontol 65 (1), 149–177. doi:10.1111/prd.12022
Horie, S., Nakatomi, C., Ito-Sago, M., Morii, A., Orimoto, A., Ikeda, H., et al. (2023). PIEZO1 promotes ATP release from periodontal ligament cells following compression force. Eur. J. Orthod. 45 (5), 565–574. doi:10.1093/ejo/cjad052
Huang, P., Jiang, R. X., Wang, F., Qiao, W. W., Ji, Y. T., Meng, L. Y., et al. (2024). PIEZO1 promotes odontoblast-mediated reactionary dentinogenesis via SEMA3A. J. Dent. Res. 103 (9), 889–898. doi:10.1177/00220345241257866
Jiang, Y., Guan, Y., Lan, Y., Chen, S., Li, T., Zou, S., et al. (2021a). Mechanosensitive Piezo1 in periodontal ligament cells promotes alveolar bone remodeling during orthodontic tooth movement. Front. Physiol. 12, 767136. doi:10.3389/fphys.2021.767136
Jiang, Y., Lin, H., Chen, Y., Lan, Y., Wang, H., Li, T., et al. (2024). Piezo1 contributes to alveolar bone remodeling by activating β-catenin under compressive stress. Am. J. Orthod. Dentofac. Orthop. 165 (4), 458–470. doi:10.1016/j.ajodo.2023.10.020
Jiang, Y., Yang, X., Jiang, J., and Xiao, B. (2021b). Structural designs and mechanogating mechanisms of the mechanosensitive piezo channels. Trends Biochem. Sci. 46 (6), 472–488. doi:10.1016/j.tibs.2021.01.008
Jiang, Y., Zhang, H., Wang, J., Liu, Y., Luo, T., and Hua, H. (2022). Targeting extracellular matrix stiffness and mechanotransducers to improve cancer therapy. J. Hematol. Oncol. 15 (1), 34. doi:10.1186/s13045-022-01252-0
Jin, P., Jan, L. Y., and Jan, Y.-N. (2020). Mechanosensitive ion channels: structural features relevant to mechanotransduction mechanisms. Annu. Rev. Neurosci. 43 (1), 207–229. doi:10.1146/annurev-neuro-070918-050509
Jin, Y., Li, J., Wang, Y., Ye, R., Feng, X., Jing, Z., et al. (2015). Functional role of mechanosensitive ion channel Piezo1 in human periodontal ligament cells. Angle Orthod. 85 (1), 87–94. doi:10.2319/123113-955.1
Kang, T., Yang, Z., Zhou, M., Lan, Y., Hong, Y., Gong, X., et al. (2024). The role of the Piezo1 channel in osteoblasts under cyclic stretching: a study on osteogenic and osteoclast factors. Arch. Oral Biol. 163, 105963. doi:10.1016/j.archoralbio.2024.105963
Karthik, V., and Guntur, A. R. (2021). Energy metabolism of osteocytes. Curr. Osteoporos. Rep. 19 (4), 444–451. doi:10.1007/s11914-021-00688-6
Khatibi Shahidi, M., Krivanek, J., Kaukua, N., Ernfors, P., Hladik, L., Kostal, V., et al. (2015). Three-dimensional imaging reveals new compartments and structural adaptations in odontoblasts. J. Dent. Res. 94 (7), 945–954. doi:10.1177/0022034515580796
Kong, K., Chang, Y., Hu, Y., Qiao, H., Zhao, C., Rong, K., et al. (2022). TiO(2) nanotubes promote osteogenic differentiation through regulation of Yap and Piezo1. Front. Bioeng. Biotechnol. 10, 872088. doi:10.3389/fbioe.2022.872088
Lacroix, J. J., Botello-Smith, W. M., and Luo, Y. (2018). Probing the gating mechanism of the mechanosensitive channel Piezo1 with the small molecule Yoda1. Nat. Commun. 9 (1), 2029. doi:10.1038/s41467-018-04405-3
Lee, K., Lee, B. M., Park, C. K., Kim, Y. H., and Chung, G. (2019). Ion channels involved in tooth pain. Int. J. Mol. Sci. 20 (9), 2266. doi:10.3390/ijms20092266
Li, X., Han, L., Nookaew, I., Mannen, E., Silva, M. J., Almeida, M., et al. (2019). Stimulation of Piezo1 by mechanical signals promotes bone anabolism. Elife 8, e49631. doi:10.7554/eLife.49631
Li, X., Zhang, C., Bowman, H. H., Stambough, J. B., Stronach, B. M., Mears, S. C., et al. (2023). Piezo1 opposes age-associated cortical bone loss. Aging Cell 22 (6), e13846. doi:10.1111/acel.13846
Li, Y., Zhan, Q., Bao, M., Yi, J., and Li, Y. (2021). Biomechanical and biological responses of periodontium in orthodontic tooth movement: up-date in a new decade. Int. J. Oral Sci. 13 (1), 20. doi:10.1038/s41368-021-00125-5
Lin, W., Xi, W., Nan, J., Haimei, L., and Shixin, C. (2020). Mechanisms of the mechanically activated ion channel Piezo1 protein in mediating osteogenic differentiation of perio-dontal ligament stem cells via the notch signaling pathway. West China J. Stomatol. 38 (6), 628–636. doi:10.7518/hxkq.2020.06.004
Liu, Y., Tian, H., Hu, Y., Cao, Y., Song, H., Lan, S., et al. (2022a). Mechanosensitive Piezo1 is crucial for periosteal stem cell-mediated fracture healing. Int. J. Biol. Sci. 18 (10), 3961–3980. doi:10.7150/ijbs.71390
Liu, Z., Tang, Y., He, L., Geng, B., Lu, F., He, J., et al. (2022b). Piezo1-mediated fluid shear stress promotes OPG and inhibits RANKL via NOTCH3 in MLO-Y4 osteocytes. Channels (Austin) 16 (1), 127–136. doi:10.1080/19336950.2022.2085379
Mahmud, A. A., Nahid, N. A., Nassif, C., Sayeed, M. S. B., Ahmed, M. U., Parveen, M., et al. (2017). Loss of the proprioception and touch sensation channel PIEZO2 in siblings with a progressive form of contractures. Clin. Genet. 91 (3), 470–475. doi:10.1111/cge.12850
Mantzourani, M., and Sharma, D. (2013). Dentine sensitivity: past, present and future. J. Dent. 41, S3–S17. doi:10.1016/S0300-5712(13)70002-2
Matsunaga, M., Kimura, M., Ouchi, T., Nakamura, T., Ohyama, S., Ando, M., et al. (2021). Mechanical stimulation-induced calcium signaling by Piezo1 channel activation in human odontoblast reduces dentin mineralization. Front. Physiol. 12, 704518. doi:10.3389/fphys.2021.704518
McMillin, M. J., Beck, A. E., Chong, J. X., Shively, K. M., Buckingham, K. J., Gildersleeve, H. I., et al. (2014). Mutations in PIEZO2 cause gordon syndrome, marden-walker syndrome, and distal arthrogryposis type 5. Am. J. Hum. Genet. 94 (5), 734–744. doi:10.1016/j.ajhg.2014.03.015
Miyazaki, A., Sugimoto, A., Yoshizaki, K., Kawarabayashi, K., Iwata, K., Kurogoushi, R., et al. (2019). Coordination of WNT signaling and ciliogenesis during odontogenesis by piezo type mechanosensitive ion channel component 1. Sci. Rep. 9 (1), 14762. doi:10.1038/s41598-019-51381-9
Morgan, E. F., Unnikrisnan, G. U., and Hussein, A. I. (2018). Bone mechanical properties in healthy and diseased states. Annu. Rev. Biomed. Eng. 20, 119–143. doi:10.1146/annurev-bioeng-062117-121139
Mousawi, F., Peng, H., Li, J., Ponnambalam, S., Roger, S., Zhao, H., et al. (2020). Chemical activation of the Piezo1 channel drives mesenchymal stem cell migration via inducing ATP release and activation of P2 receptor purinergic signaling. Stem Cells 38 (3), 410–421. doi:10.1002/stem.3114
Murthy, S. E., Dubin, A. E., and Patapoutian, A. (2017). Piezos thrive under pressure: mechanically activated ion channels in health and disease. Nat. Rev. Mol. Cell Biol. 18 (12), 771–783. doi:10.1038/nrm.2017.92
Nakashima, T., Hayashi, M., Fukunaga, T., Kurata, K., Oh-Hora, M., Feng, J. Q., et al. (2011). Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 17 (10), 1231–1234. doi:10.1038/nm.2452
Nie, X., and Chung, M.-K. (2022). Piezo channels for skeletal development and homeostasis: insights from mouse genetic models. Differentiation 126, 10–15. doi:10.1016/j.diff.2022.06.001
Nottmeier, C., Lavicky, J., Gonzalez Lopez, M., Knauth, S., Kahl-Nieke, B., Amling, M., et al. (2023). Mechanical-induced bone remodeling does not depend on Piezo1 in dentoalveolar hard tissue. Sci. Rep. 13 (1), 9563. doi:10.1038/s41598-023-36699-9
Nuñez, J., Vignoletti, F., Caffesse, R. G., and Sanz, M. (2019). Cellular therapy in periodontal regeneration. Periodontol 79 (1), 107–116. doi:10.1111/prd.12250
Ohyama, S., Ouchi, T., Kimura, M., Kurashima, R., Yasumatsu, K., Nishida, D., et al. (2022). Piezo1-pannexin-1-P2X3 axis in odontoblasts and neurons mediates sensory transduction in dentinal sensitivity. Front. Physiol. 13, 891759. doi:10.3389/fphys.2022.891759
Pagnotti, G. M., Styner, M., Uzer, G., Patel, V. S., Wright, L. E., Ness, K. K., et al. (2019). Combating osteoporosis and obesity with exercise: leveraging cell mechanosensitivity. Nat. Rev. Endocrinol. 15 (6), 339–355. doi:10.1038/s41574-019-0170-1
Pei, F., Liu, J., Zhang, L., Pan, X., Huang, W., Cen, X., et al. (2021). The functions of mechanosensitive ion channels in tooth and bone tissues. Cell Signal 78, 109877. doi:10.1016/j.cellsig.2020.109877
Qin, L., He, T., Chen, S., Yang, D., Yi, W., Cao, H., et al. (2021). Roles of mechanosensitive channel Piezo1/2 proteins in skeleton and other tissues. Bone Res. 9 (1), 44. doi:10.1038/s41413-021-00168-8
Rachner, T. D., Khosla, S., and Hofbauer, L. C. (2011). Osteoporosis: now and the future. Lancet 377 (9773), 1276–1287. doi:10.1016/s0140-6736(10)62349-5
Ranade, S. S., Woo, S. H., Dubin, A. E., Moshourab, R. A., Wetzel, C., Petrus, M., et al. (2014). Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nat 516 (7529), 121–125. doi:10.1038/nature13980
Robling, A. G., and Bonewald, L. F. (2020). The osteocyte: new insights. Annu. Rev. Physiol. 82, 485–506. doi:10.1146/annurev-physiol-021119-034332
Ru, Y., Gu, H., Sun, L., Zhang, W., and Wang, L. (2024). Mechanical stretch-induced ATP release from osteocytes promotes osteogenesis of bone marrow mesenchymal stem cells. Discov. Med. 36 (182), 494–508. doi:10.24976/Discov.Med.202436182.46
Sasaki, F., Hayashi, M., Mouri, Y., Nakamura, S., Adachi, T., and Nakashima, T. (2020). Mechanotransduction via the Piezo1-Akt pathway underlies sost suppression in osteocytes. Biochem. Biophys. Res. Commun. 521 (3), 806–813. doi:10.1016/j.bbrc.2019.10.174
Sato, M., Ogura, K., Kimura, M., Nishi, K., Ando, M., Tazaki, M., et al. (2018). Activation of mechanosensitive transient receptor potential/piezo channels in odontoblasts generates action potentials in cocultured isolectin B(4)-negative medium-sized trigeminal ganglion neurons. J. Endod. 44 (6), 984–991. doi:10.1016/j.joen.2018.02.020
Schröder, A., Neher, K., Krenmayr, B., Paddenberg, E., Spanier, G., Proff, P., et al. (2023). Impact of PIEZO1-channel on inflammation and osteoclastogenesis mediated via periodontal ligament fibroblasts during mechanical loading. Eur. J. Oral Sci. 131 (1), e12913. doi:10.1111/eos.12913
Shah, H. N., Jones, R. E., Borrelli, M. R., Robertson, K., Salhotra, A., Wan, D. C., et al. (2021). Craniofacial and long bone development in the context of distraction osteogenesis. Plast. Reconstr. Surg. 147 (1), 54e–65e. doi:10.1097/prs.0000000000007451
Shen, B., Tasdogan, A., Ubellacker, J. M., Zhang, J., Nosyreva, E. D., Du, L., et al. (2021). A mechanosensitive peri-arteriolar niche for osteogenesis and lymphopoiesis. Nat 591 (7850), 438–444. doi:10.1038/s41586-021-03298-5
Shen, X., Wu, W., Ying, Y., Zhou, L., and Zhu, H. (2023). A regulatory role of Piezo1 in apoptosis of periodontal tissue and periodontal ligament fibroblasts during orthodontic tooth movement. Aust. Endod. J. 49 (S1), 228–237. doi:10.1111/aej.12721
Shen, Y., Pan, Y., Guo, S., Sun, L., Zhang, C., and Wang, L. (2020). The roles of mechanosensitive ion channels and associated downstream MAPK signaling pathways in PDLC mechanotransduction. Mol. Med. Rep. 21 (5), 2113–2122. doi:10.3892/mmr.2020.11006
Solis, A. G., Bielecki, P., Steach, H. R., Sharma, L., Harman, C. C. D., Yun, S., et al. (2019). Mechanosensation of cyclical force by PIEZO1 is essential for innate immunity. Nat 573 (7772), 69–74. doi:10.1038/s41586-019-1485-8
Song, J., Liu, L., Lv, L., Hu, S., Tariq, A., Wang, W., et al. (2020). Fluid shear stress induces Runx-2 expression via upregulation of PIEZO1 in MC3T3-E1 cells. Cell Biol. Int. 44 (7), 1491–1502. doi:10.1002/cbin.11344
Sonkodi, B. (2022). Delayed onset muscle soreness and critical neural microdamage-derived neuroinflammation. Biomolecules 12 (9), 1207. doi:10.3390/biom12091207
Sugimoto, A., Iwata, K., Kurogoushi, R., Tanaka, M., Nakashima, Y., Yamakawa, Y., et al. (2023). C-terminus of PIEZO1 governs Ca(2+) influx and intracellular ERK1/2 signaling pathway in mechanotransduction. Biochem. Biophys. Res. Commun. 682, 39–45. doi:10.1016/j.bbrc.2023.09.080
Sugimoto, A., Miyazaki, A., Kawarabayashi, K., Shono, M., Akazawa, Y., Hasegawa, T., et al. (2017). Piezo type mechanosensitive ion channel component 1 functions as a regulator of the cell fate determination of mesenchymal stem cells. Sci. Rep. 7 (1), 17696. doi:10.1038/s41598-017-18089-0
Sun, W., Chi, S., Li, Y., Ling, S., Tan, Y., Xu, Y., et al. (2019). The mechanosensitive Piezo1 channel is required for bone formation. Elife 8, e47454. doi:10.7554/eLife.47454
Syeda, R., Xu, J., Dubin, A. E., Coste, B., Mathur, J., Huynh, T., et al. (2015). Chemical activation of the mechanotransduction channel Piezo1. Elife 4, e07369. doi:10.7554/eLife.07369
Szczot, M., Liljencrantz, J., Ghitani, N., Barik, A., Lam, R., Thompson, J. H., et al. (2018). PIEZO2 mediates injury-induced tactile pain in mice and humans. Sci. Transl. Med. 10 (462), eaat9892. doi:10.1126/scitranslmed.aat9892
Tang, Z., Wei, X., Li, T., Wu, H., Xiao, X., Hao, Y., et al. (2021). Three-dimensionally printed Ti2448 with low stiffness enhanced angiogenesis and osteogenesis by regulating macrophage polarization via Piezo1/YAP signaling axis. Front. Cell Dev. Biol. 9, 750948. doi:10.3389/fcell.2021.750948
Tao, H., Zhu, M., Lau, K., Whitley, O. K. W., Samani, M., Xiao, X., et al. (2019). Oscillatory cortical forces promote three dimensional cell intercalations that shape the murine mandibular arch. Nat. Commun. 10 (1), 1703. doi:10.1038/s41467-019-09540-z
Uehara, M., Kosho, T., Takano, K., Inaba, Y., Kuraishi, S., Ikegami, S., et al. (2020). Proximal junctional kyphosis after posterior spinal fusion for severe kyphoscoliosis in a patient with PIEZO2-deficient arthrogryposis syndrome. Spine (Phila Pa 1976) 45 (10), E600–e604. doi:10.1097/brs.0000000000003347
Wan, Y., Zhou, J., and Li, H. (2024). The role of mechanosensitive piezo channels in chronic pain. J. Pain Res. 17, 4199–4212. doi:10.2147/JPR.S490459
Wang, B., Li, G., Zhu, Q., Liu, W., Ke, W., Hua, W., et al. (2022). Bone repairment via mechanosensation of Piezo1 using wearable pulsed triboelectric nanogenerator. Small 18 (30), e2201056. doi:10.1002/smll.202201056
Wang, L., You, X., Lotinun, S., Zhang, L., Wu, N., and Zou, W. (2020). Mechanical sensing protein PIEZO1 regulates bone homeostasis via osteoblast-osteoclast crosstalk. Nat. Commun. 11 (1), 282. doi:10.1038/s41467-019-14146-6
Wang, L., Zhou, H., Zhang, M., Liu, W., Deng, T., Zhao, Q., et al. (2019). Structure and mechanogating of the Mammalian tactile channel PIEZO2. Nat 573 (7773), 225–229. doi:10.1038/s41586-019-1505-8
Wang, Y., Chi, S., Guo, H., Li, G., Wang, L., Zhao, Q., et al. (2018). A lever-like transduction pathway for long-distance chemical- and mechano-gating of the mechanosensitive Piezo1 channel. Nat. Commun. 9 (1), 1300. doi:10.1038/s41467-018-03570-9
Wang, Y., Groeger, S., Yong, J., and Ruf, S. (2023). Orthodontic compression enhances macrophage M2 polarization via histone H3 hyperacetylation. Int. J. Mol. Sci. 24 (4), 3117. doi:10.3390/ijms24043117
Woo, S.-H., Lukacs, V., de Nooij, J. C., Zaytseva, D., Criddle, C. R., Francisco, A., et al. (2015). Piezo2 is the principal mechanotransduction channel for proprioception. Nat. Neurosci. 18 (12), 1756–1762. doi:10.1038/nn.4162
Wu, R. W., Lian, W. S., Chen, Y. S., Ko, J. Y., Wang, S. Y., Jahr, H., et al. (2021). Piezoelectric microvibration mitigates estrogen loss-induced osteoporosis and promotes Piezo1, MicroRNA-29a, and Wnt3a signaling in osteoblasts. Int. J. Mol. Sci. 22 (17), 9476. doi:10.3390/ijms22179476
Xiao, B. (2020). Levering mechanically activated piezo channels for potential pharmacological intervention. Annu. Rev. Pharmacol. Toxicol. 60 (1), 195–218. doi:10.1146/annurev-pharmtox-010919-023703
Xiao, B. (2024). Mechanisms of mechanotransduction and physiological roles of PIEZO channels. Nat. Rev. Mol. Cell Biol. 25 (11), 886–903. doi:10.1038/s41580-024-00773-5
Xing, Y., Yang, B., He, Y., Xie, B., Zhao, T., and Chen, J. (2022). Effects of mechanosensitive ion channel Piezo1 on proliferation and osteogenic differentiation of human dental follicle cells. Ann. Anat. 239, 151847. doi:10.1016/j.aanat.2021.151847
Xiong, H., Yang, J., Guo, J., Ma, A., Wang, B., and Kang, Y. (2022). Mechanosensitive piezo channels mediate the physiological and pathophysiological changes in the respiratory system. Respir. Res. 23 (1), 196. doi:10.1186/s12931-022-02122-6
Xu, H., Guan, J., Jin, Z., Yin, C., Wu, S., Sun, W., et al. (2022). Mechanical force modulates macrophage proliferation via Piezo1-AKT-Cyclin D1 axis. Faseb J. 36 (8), e22423. doi:10.1096/fj.202200314R
Xu, X., Liu, S., Liu, H., Ru, K., Jia, Y., Wu, Z., et al. (2021). Piezo channels: Awesome mechanosensitive structures in cellular mechanotransduction and their role in bone. Int. J. Mol. Sci. 22 (12), 6429. doi:10.3390/ijms22126429
Yan, L., Jiang, J., Ma, C., Li, R., and Xia, Y. (2019). Effect of knocking Down Piezo1 mechanically sensitive protein on migration of MC3T3-E1 osteoblast cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 33 (1), 28–34. doi:10.7507/1002-1892.201806121
Yan, W., Maimaitimin, M., Wu, Y., Fan, Y., Ren, S., Zhao, F., et al. (2023). Meniscal fibrocartilage regeneration inspired by meniscal maturational and regenerative process. Sci. Adv. 9 (45), eadg8138. doi:10.1126/sciadv.adg8138
Yang, W., Lin, L., Hu, S., Jiang, B., Yang, R., Yu, W., et al. (2024). Expression patterns of mechanosensitive ion channel PIEZOs in irreversible pulpitis. BMC Oral Health 24 (1), 465. doi:10.1186/s12903-024-04209-6
Yang, X., Lin, C., Chen, X., Li, S., Li, X., and Xiao, B. (2022). Structure deformation and curvature sensing of PIEZO1 in lipid membranes. Nat 604 (7905), 377–383. doi:10.1038/s41586-022-04574-8
Yoneda, M., Suzuki, H., Hatano, N., Nakano, S., Muraki, Y., Miyazawa, K., et al. (2019). PIEZO1 and TRPV4, which are distinct mechano-sensors in the osteoblastic MC3T3-E1 cells, modify cell-proliferation. Int. J. Mol. Sci. 20 (19), 4960. doi:10.3390/ijms20194960
Zhai, Q., Dong, Z., Wang, W., Li, B., and Jin, Y. (2019). Dental stem cell and dental tissue regeneration. Front. Med. 13 (2), 152–159. doi:10.1007/s11684-018-0628-x
Zhang, D., Lin, W., Jiang, S., Deng, P., Liu, L., Wang, Q., et al. (2023). Lepr-expressing PDLSCs contribute to periodontal homeostasis and respond to mechanical force by Piezo1. Adv. Sci. (Weinh) 10 (29), 2303291. doi:10.1002/advs.202303291
Zhang, G., Li, X., Wu, L., and Qin, Y. X. (2021). Piezo1 channel activation in response to mechanobiological acoustic radiation force in osteoblastic cells. Bone Res. 9 (1), 16. doi:10.1038/s41413-020-00124-y
Zhang, M., Wang, Y., Geng, J., Zhou, S., and Xiao, B. (2019). Mechanically activated piezo channels mediate touch and suppress acute mechanical pain response in mice. Cell Rep. 26 (6), 1419–1431. doi:10.1016/j.celrep.2019.01.056
Zhang, X., Hou, L., Li, F., Zhang, W., Wu, C., Xiang, L., et al. (2022). Piezo1-mediated mechanosensation in bone marrow macrophages promotes vascular niche regeneration after irradiation injury. Theranostics 12 (4), 1621–1638. doi:10.7150/thno.64963
Zhang, Y. Y., Huang, Y. P., Zhao, H. X., Zhang, T., Chen, F., and Liu, Y. (2017). Cementogenesis is inhibited under a mechanical static compressive force via Piezo1. Angle Orthod. 87 (4), 618–624. doi:10.2319/110616-799.1
Zhao, Q., Wu, K., Geng, J., Chi, S., Wang, Y., Zhi, P., et al. (2016). Ion permeation and mechanotransduction mechanisms of mechanosensitive piezo channels. Neuron 89 (6), 1248–1263. doi:10.1016/j.neuron.2016.01.046
Zheng, F., Wu, T., Wang, F., Li, H., Tang, H., Cui, X., et al. (2024). Low-intensity pulsed ultrasound promotes the osteogenesis of mechanical force-treated periodontal ligament cells via Piezo1. Front. Bioeng. Biotechnol. 12, 1347406. doi:10.3389/fbioe.2024.1347406
Zhou, T., Gao, B., Fan, Y., Liu, Y., Feng, S., Cong, Q., et al. (2020). Piezo1/2 mediate mechanotransduction essential for bone formation through concerted activation of NFAT-YAP1-ß-catenin. Elife 9, e52779. doi:10.7554/eLife.52779
Keywords: Piezo protein, cellular mechanotransduction, bone, tooth, ion channels
Citation: Dong J, Li R, Chen Y, Zhu G and Liang X (2025) Mechanosensitive Piezo channels in mineralized tissues: emerging roles in osteodental adaptation and disease. Front. Cell Dev. Biol. 13:1607337. doi: 10.3389/fcell.2025.1607337
Received: 07 April 2025; Accepted: 24 June 2025;
Published: 10 July 2025.
Edited by:
Weimin Gao, Barrow Neurological Institute (BNI), United StatesCopyright © 2025 Dong, Li, Chen, Zhu and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xing Liang, bGlhbmd4aW5nQHNjdS5lZHUuY24=; Guixin Zhu, emh1Z3VpeGluMTk5N0AxNjMuY29t
†Guixin Zhu, Shaoxing Stomatological Hospital, Shaoxing, Zhejiang, China
‡These authors share senior authorship
 Junchi Dong
Junchi Dong Ran Li
Ran Li Yuhuang Chen
Yuhuang Chen Guixin Zhu
Guixin Zhu Xing Liang
Xing Liang