- 1Department of Gastroenterology, The First Hospital of Hunan University of Chinese Medicine, Changsha, Hunan, China
- 2First Clinical College, Guizhou University of Traditional Chinese Medicine, Guiyang, Guizhou, China
Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD) characterized primarily by immune dysregulation. Its pathogenesis involves multiple factors, including dysregulation of T-cell subsets, hypersecretion of pro-inflammatory cytokines, imbalance in the gut microbiota, and disruption of the intestinal barrier. Among T-cell subsets, abnormal activation of Th1 and Th17 cells, in conjunction with Treg dysfunction, significantly amplifies local pro-inflammatory signals. Pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-17, exacerbate apoptosis and disrupt tight junctions (TJs) in intestinal epithelial cells (IECs), thereby creating favorable conditions for invasion by pathogenic bacteria and their metabolites. Intestinal microecological imbalance not only leads to significant alterations in the structure of the bacterial flora but also involves abnormal fluctuations in its metabolites that directly regulate intestinal immune homeostasis, a factor closely associated with the severity of inflammation and prognosis of ulcerative colitis. Recent studies have demonstrated that in the treatment of UC, traditional Chinese medicine (TCM) achieves a multi-target, multi-pathway integrated intervention by regulating immune cell differentiation, balancing inflammatory factor levels, repairing the intestinal epithelial barrier, and remodeling the structure of the bacterial flora. This article reviews the pathogenic mechanisms underlying immune dysregulation in UC and the advances in research on TCM’s role in immune regulation, anti-inflammatory repair, and flora modulation, encompassing the mechanisms of action of individual active ingredients and classic TCM compound formulas. Although some studies have preliminarily confirmed TCM’s potential to modulate immunity and repair the intestinal barrier, breakthroughs in mechanism analysis, herb standardization, and large-scale validation remain forthcoming. It is anticipated that the unique advantages of TCM will be translated into a more precise therapeutic strategy for UC through modern molecular and systems biology approaches.
1 Introduction
Ulcerative colitis (UC) is a chronic, progressive, and recurrent inflammatory bowel disease (IBD) characterized by persistent, nonspecific inflammation of the rectum or distal colon and superficial mucosal ulceration (Liu X. et al., 2024). The typical clinical symptoms of UC include abdominal pain, diarrhea, hematochezia, weight loss, and fatigue, and in severe cases, these symptoms can lead to complications such as intestinal perforation and colorectal cancer (Iyengar et al., 2024; Liang et al., 2024). Globally, the incidence of UC is on the rise, particularly in North America, Western Europe, and several Asian regions (e.g., Japan and Korea) (Le Berre et al., 2023). Epidemiologic data indicate that the annual incidence of UC ranges from approximately 8.8–23.1 cases per 100,000 person-years in North America and from 0.6 to 24.3 cases per 100,000 person-years in Europe (Du and Ha, 2020). In newly industrialized countries, including China, India, and regions in Latin America, both the incidence and hospitalization rates of UC are rapidly increasing (Buie et al., 2023). UC poses not only a significant threat to patients’ physical health but also imposes substantial social and economic burdens. The direct medical costs and indirect economic losses associated with UC have been estimated to amount to billions of dollars per year worldwide (Constantin et al., 2019). Despite the availability of various treatment options, including immunosuppressive agents, biologics, and intestinal surgery, the therapeutic efficacy remains limited, and the side effects of these drugs cannot be overlooked (Biedermann et al., 2024; Dai et al., 2023). For example, immunosuppressive treatments may cause adverse effects, including infections and an increased risk of tumors, whereas biologics are challenged by high treatment costs and drug resistance (Song and Wu, 2022).
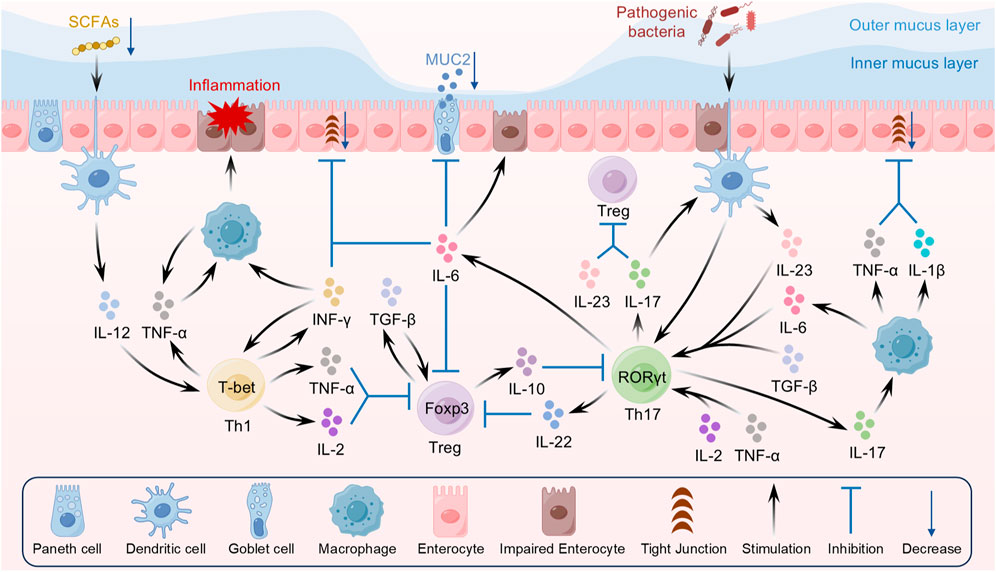
The pathogenesis of UC remains incompletely understood; however, studies have demonstrated that multifactorial interactions—including genetic susceptibility, immune abnormalities, dietary factors, and environmental influences—are the primary causative factors in its development (Chen J. et al., 2024; Ananthakrishnan, 2015). In recent years, immune dysregulation has garnered significant attention as a central element in the pathogenesis of UC. As a disease primarily characterized by immune system dysfunction, the pathological progression of UC is closely associated with abnormal immune cell activation and dysregulated immune responses (Li et al., 2020; Cheng H. et al., 2024). Specifically, immune dysregulation in UC is manifested by dysregulation of T-cell subsets, hypersecretion of pro-inflammatory cytokines, imbalance in the gut microbiota, and disruption of the intestinal barrier (Figure 1). In patients with UC, the excessive differentiation of Th1 and Th17 cells and their secretion of pro-inflammatory cytokines (e.g., IFN-γ, IL-17, and TNF-α) are considered primary triggers of chronic intestinal inflammation (Cao et al., 2025; Hu et al., 2024). Furthermore, imbalances in the gut microbiota play a crucial role in immune regulation (Guo S. et al., 2021). The reduction of beneficial flora and the overgrowth of pathogenic microorganisms in the gut not only disrupt the integrity of the intestinal barrier but also exacerbate the inflammatory response by activating the immune system and promoting the recruitment of immune cells to the gut (Zhang Y-J. et al., 2015). Therefore, immune dysregulation is not only the central pathological process of UC but also a key determinant of disease progression and prognosis. An in-depth exploration of the mechanisms underlying immune dysregulation will offer valuable insights for the precise treatment of UC.
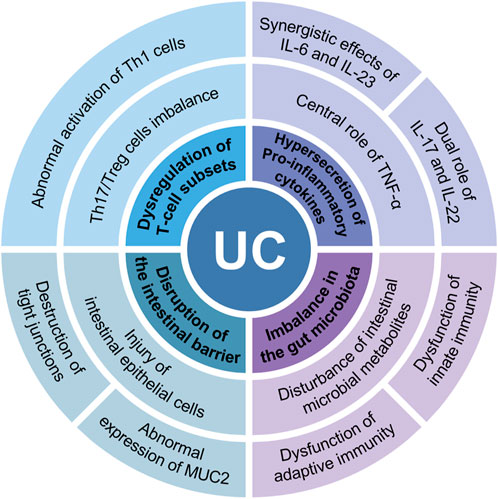
Figure 1. Immune dysregulation in UC is manifested in 4 main areas: dysregulation of T-cell subsets, hypersecretion of proinflammatory cytokines, imbalance in the gut microbiota, and disruption of the intestinal barrier.
As a traditional healing system with a history of thousands of years, traditional Chinese medicine (TCM) has shown particular strengths in the treatment of UC. A large body of studies have shown that TCM exhibits a multi-component, multi-target, and multi-pathway approach in the treatment of UC. TCM can modulate overall immune function, restore intestinal immune tolerance, attenuate inflammatory responses, and exert therapeutic effects by re-establishing the balance of intestinal microecology (Li et al., 2025; Huang Y. et al., 2024). In recent years, an increasing number of clinical studies and experimental data have confirmed that TCM is efficacious in alleviating UC symptoms, prolonging remission periods, and reducing drug side effects. Notably, in regulating the immune system and enhancing intestinal microecology, certain TCM compounds and herbal formulas have shown the potential to inhibit hyperactive immune responses, repair the intestinal barrier, and restore the balance of the gut microbiota (Zhu et al., 2023; Zhou et al., 2024a). Therefore, the aim of this paper is to review the mechanisms underlying immune dysregulation in UC and to highlight the potential of active TCM ingredients and herbal formulas in its treatment.
2 Immune dysregulation and UC
2.1 Dysregulation of T-cell subsets
The maintenance of immune homeostasis relies on a delicate balance among various immune cells, with T cell subsets playing a pivotal regulatory role. Recent studies have shown that the abnormal activation and dysfunction of T cell subsets represent a key pathogenic factor in UC (Schardey et al., 2019). Under the stimulation of inflammatory cytokines, naïve T cells differentiate into multiple lineages, including Th1, Th2, Th17, and Treg. Under normal conditions, these cells maintain mutual checks and balances through complex cytokine networks and signaling pathways, thereby preserving immune tolerance and defense. However, in UC patients, dysfunction of Th1, Th17, and Treg cells leads to abnormal local immune activation, ultimately triggering chronic inflammation in the intestinal tract (Figure 2).
2.1.1 Abnormal activation of Th1 cells
Th1 cells are an important subpopulation of CD4+ T cells, with their differentiation driven by cytokines such as IL-12 and IFN-γ, and key functional gene expression regulated by the transcription factor T-bet (Ruan et al., 2023; Monteleone et al., 2012). IL-12 is a key cytokine regulating the differentiation of Th1 cells, as it promotes the expression of signal transducer and activator of transcription 4 (STAT4), which in turn induces the expression of the transcription factor T-bet to drive the transformation of CD4+ T cells into the Th1 subpopulation (Hsieh et al., 1993; Baumann et al., 2015). Under physiological conditions, Th1 cells, together with cytotoxic T lymphocytes (CTLs) and macrophages, constitute a crucial line of defense against pathogens, as their secretion of IFN-γ enhances macrophage phagocytosis and boosts antigen presentation efficiency, thereby promoting cellular immune responses (Shen et al., 2021; Thuner and Coutant, 2023). However, when Th1 cells are abnormally activated, their secretion of IFN-γ and TNF-α markedly increases, directly activating intestinal epithelial cells (IECs) and lamina propria macrophages, inducing high chemokine expression (e.g., CXCL9, CXCL10), and recruiting additional Th1 cells and neutrophils to the inflammatory site, thereby exacerbating the local inflammatory response (Elia and Guglielmi, 2018). Clinical data have shown that IFN-γ levels are elevated in the colonic mucosa of UC patients compared to healthy controls, with its expression significantly and positively correlated with disease activity (Morvaridi et al., 2025). As a key cytokine secreted by Th1 cells, IFN-γ inhibits the expression of intestinal epithelial tight junctions (TJs), resulting in impaired barrier function and increased permeability, thereby facilitating bacterial invasion and further inflammation (Boivin et al., 2009). Recent experimental results have shown that IFN-γ synergizes with TNF-α to kill IECs via the CASP8-JAK1/2-STAT1 module, thereby further disrupting intestinal barrier function (Woznicki et al., 2021). In addition to the pro-inflammatory cytokine cascade, the interaction between Th1 and Th17 cells, along with the suppression of Treg function, serves as an important driver of chronic inflammation in UC. IL-2 and TNF-α secreted by aberrantly activated Th1 cells not only inhibit Foxp3 expression in Treg cells, thereby disrupting immune tolerance, but also promote the differentiation of pathogenic Th17 cells through enhancement of the STAT4 signaling pathway (Hu et al., 2024; Xu et al., 2017). In addition, an imbalance in the gut microbiota has recently been implicated as a significant external factor contributing to Th1 cell polarization. Reduced levels of gut microbial metabolites, such as short chain fatty acids (SCFAs), in patients with UC result in decreased histone deacetylase (HDAC) inhibition, prompting dendritic cells (DCs) to secrete increased levels of IL-12 that drive the over-differentiation of Th1 cells (Shen et al., 2018). Animal model experiments have shown that probiotic intervention can effectively reduce the proportion of Th1 cells in the colon and ameliorate the symptoms of DSS-induced colitis (Guo and Lv, 2023).
2.1.2 Th17/Treg cells imbalance
Th17 cells are a subset of helper T cells differentiated from CD4+ T cells, with their differentiation dependent on the activation of the transcription factor RORγt and synergistically regulated by cytokines such as IL-6, TGF-β, and IL-23 (Korn et al., 2009; Mickael et al., 2020). Early in the process, initial differentiation signals are provided by IL-6 and TGF-β, followed by IL-23—which enhances RORγt-mediated differentiation and pathogenicity of Th17 cells via activation of the STAT3 signaling pathway (Gomez-Bris et al., 2023). In the physiological state, Th17 cells participate in the intrinsic immune defense of the mucosal barrier through secretion of IL-17 family cytokines, playing a central role in clearing extracellular pathogens (e.g., bacteria and fungi) (Letizia et al., 2022). In addition, Th17 cells regulate neutrophil recruitment and macrophage activation, orchestrating the intensity and duration of local inflammatory responses (Fu et al., 2020). However, when Th17 cells are excessively proliferated and activated, they can induce aberrant immune responses that lead to various autoimmune diseases, including UC (Ueno et al., 2018). In the intestinal mucosa of patients with active UC, elevated numbers of Th17 cells and increased levels of associated cytokines (IL-17 and IL-23) suggest that Th17 cells play a significant role in disease activity and mucosal damage (Cao et al., 2025; Jia R. et al., 2024).
In contrast to pro-inflammatory Th17 cells, Treg cells primarily exert immunosuppressive functions, with their differentiation dependent on Foxp3 expression and activation of TGF-β and IL-2 signaling pathways (Zhong et al., 2022a; Jia et al., 2020). Under normal physiological conditions, Treg cells maintain immune tolerance by inhibiting effector T cell overactivation via cell-contact-dependent mechanisms (e.g., CTLA-4 and PD-1) and secretion of inhibitory cytokines such as IL-10 and TGF-β (Mayne and Williams, 2013). IL-10, a major inhibitory factor, effectively suppresses effector T cell proliferation and pro-inflammatory cytokine secretion, with its signaling involving activation of JAK1 and Tyk2 to mediate negative feedback regulation of the inflammatory response (Zhu et al., 2017). TGF-β, on the other hand, promotes proliferation and repair of IECs through activation of MEK1/2 signaling, thereby restoring intestinal barrier integrity and reducing immune stimulation by intestinal contents (Chen et al., 2018). In the intestinal microenvironment, Treg cells inhibit Th17 cell overproliferation and limit antigen presentation by modulating DC maturation. In addition, Treg cells adapt to the hypoxic intestinal environment through metabolic reprogramming, such as increased fatty acid oxidation, and their functional stability is closely linked to levels of gut microbiota metabolites (e.g., SCFAs) (Trapecar et al., 2020). Furthermore, a reduction or functional deficit of Treg cells leads to uncontrolled immune responses against the intestinal microbiota, thereby exacerbating chronic gut inflammation (Wang et al., 2024a).
In the pathogenesis of UC, an imbalance between Th17 and Treg cells is considered a key molecular basis. Significantly elevated levels of IL-6 and IL-23 in the intestines of UC mouse models not only promote RORγt-mediated Th17 differentiation but also inhibit TGF-β-driven Foxp3 expression, resulting in a severe Th17/Treg imbalance (Rana et al., 2021). At the transcriptional level, competitive binding of RORγt to Foxp3 within CD4+ T cells shifts cellular differentiation—characterized by upregulated RORγt expression in Th17 cells and Foxp3 suppression via DNA methylation modifications (e.g., SMARCA5-mediated epigenetic silencing)—thereby exacerbating immune imbalance (Wang et al., 2024b). In addition, disturbances in the gut microbiota–immunity axis play a significant role in UC pathogenesis. Pathogenic bacteria (e.g., E. coli LF82) activate DCs via the TLR4/MyD88 pathway, promoting Th17 cell polarization and inhibiting Treg cell differentiation; conversely, probiotics (e.g., Lactobacillus casei R3) help reverse the Th17/Treg imbalance by restoring SCFA levels through their metabolites (Trapecar et al., 2020; Sha et al., 2023; Huang et al., 2021). Regarding intracellular metabolic regulation, overactivation of the mTOR signaling pathway promotes Th17 cell differentiation while suppressing Treg cell immunosuppressive function; conversely, SIRT1 deacetylase enhances Treg function by modulating Foxp3 stability, although its expression is typically reduced in UC patients (Wang Y. et al., 2024; Zhu et al., 2024; Xu Y. et al., 2021). Further studies have revealed that dysregulation of the AhR-PPARγ signaling axis is critical for maintaining Th17/Treg homeostasis. AhR agonists (e.g., dimethylindole) ameliorate colitis symptoms by inhibiting Th17 differentiation and promoting Treg expansion, whereas defective PPARγ signaling disrupts the balance between RORγt and Foxp3, exacerbating the inflammatory response (Liu S. et al., 2023; Cheng et al., 2021). Meanwhile, the expression of the immune co-inhibitory molecule TIGIT is reduced in memory Th17 (mTh17) cells, weakening its negative regulatory effect on DCs and thereby exacerbating intestinal inflammation. Studies have shown that Astragalus polysaccharides can partially restore the balance between mTh17 and mTreg cells by upregulating TIGIT expression (Wan Q. et al., 2024).
2.2 Hypersecretion of pro-inflammatory cytokines
Abnormal and excessive secretion of pro-inflammatory cytokines plays a pivotal role in UC pathogenesis. Under pathological conditions, the immune system remains continuously activated, resulting in an amplified local and systemic inflammatory response in the intestine that ultimately leads to disruption of the intestinal mucosal barrier and subsequent tissue damage. Numerous studies have demonstrated that pro-inflammatory cytokines, such as TNF-α, IL-6, IL-23, IL-17, and IL-22, not only play a crucial role in localized inflammatory responses in UC but also exacerbate the immune response via systemic circulation (Figure 2).
2.2.1 Central role of TNF-α
TNF-α, as a key pro-inflammatory cytokine, plays a pivotal role in regulating immune responses and inflammation. It is primarily secreted by monocyte-derived macrophages, T lymphocytes, and IECs, and regulates the immune-inflammatory response by binding to transmembrane receptors TNFR1 and TNFR2, thereby initiating a cascade of downstream signaling events (Kaur and Goggolidou, 2020; Veerasubramanian et al., 2024). Under physiological conditions, TNF-α mediates immune defense functions—including pathogen clearance and tissue repair—primarily through activation of the NF-κB and MAPK signaling pathways, while also regulating the balance between proliferation and apoptosis of IECs to maintain mucosal barrier integrity (Veerasubramanian et al., 2024; Nakase et al., 2022). In addition, TNF-α mediates the directional migration of immune cells by regulating chemokine expression, thereby functioning as a “double-edged sword” in local immune homeostasis.
However, under pathological conditions—particularly during UC development—TNF-α emerges as a significant pathological factor. First, TNF-α induces overexpression of pro-inflammatory factors such as IL-6 and IL-1β by activating the NF-κB signaling pathway, thereby establishing a positive inflammatory feedback loop that leads to persistent injury of the intestinal mucosa. Secondly, TNF-α and IL-1β contribute to the degradation of intestinal epithelial TJ proteins (e.g., occludin, claudin, ZO-1), resulting in impaired barrier function and increased mucosal permeability, which facilitates the translocation of intestinal microbiota and their metabolites, thereby activating the innate immune system and exacerbating inflammatory responses (Yu et al., 2022; Kaminsky et al., 2021). Experiments have shown that colonic TJ proteins, such as ZO-1 and Claudin-1, can be restored and colitis symptoms ameliorated by reducing TNF-α levels and inhibiting the NF-κB pathway (Song et al., 2024). In addition, TNF-α promotes the homing of α4β7 integrin-positive lymphocytes to the intestines by upregulating the expression of the vascular adhesion molecule MAdCAM-1, thereby disturbing the Th17/Treg balance and contributing to mucosal immune dysregulation (Wang et al., 2024b; Nakase et al., 2022). It was found that upregulation of colonic endothelial cell adhesion molecules can be significantly inhibited by blocking TNF-α-mediated NF-κB activation in a DSS-induced UC mouse model, thereby alleviating inflammation (Xue et al., 2023). Meanwhile, high concentrations of TNF-α can activate the caspase-8-dependent apoptotic pathway, inducing excessive apoptosis of colonic epithelial cells and downregulating the expression of the anti-apoptotic protein Bcl-2, thereby significantly reducing the repair capacity of the intestinal mucosa (Liu W. et al., 2025; Ye and Lu, 2022).
2.2.2 Synergistic effects of IL-6 and IL-23
IL-6 is a pleiotropic cytokine secreted by a variety of cells, including T cells, B cells, macrophages, and endothelial cells, and its expression is markedly upregulated during inflammatory and infectious states, playing a critical role in immune regulation and tissue repair (Gong et al., 2025). Under normal physiological conditions, IL-6 regulates acute-phase inflammatory responses, promotes hematopoietic stem cell differentiation, and facilitates tissue repair primarily through activation of the JAK/STAT3 signaling pathway (Jia R. et al., 2024). In addition, IL-6 maintains immune tolerance in intestinal homeostasis by regulating the Treg/Th17 balance and enhances mucin secretion by goblet cells to protect the intestinal mucosal barrier (Leng et al., 2024; Wei et al., 2021). Unlike IL-6, IL-23, which belongs to the IL-12 family and is predominantly secreted by antigen-presenting cells (e.g., DCs and macrophages), plays a unique role in immune regulation by virtue of its heterodimeric structure composed of p19 and p40 subunits. IL-23 promotes the differentiation of Th17 cells and enhances host defense against extracellular pathogens by binding to the IL-23R/gp130 receptor complex (Aebisher et al., 2024). Meanwhile, IL-23 induces IECs to express antimicrobial peptides (AMPs) and helps maintain the dynamic balance between the gut microbiota and the host (Korta et al., 2023).
During UC pathology, IL-6 and IL-23 act synergistically via multiple signaling pathways to establish a complex, mutually reinforcing pro-inflammatory network. Specifically, IL-6 binds to its receptor IL-6R/gp130 and activates STAT3 phosphorylation, inducing junctional IECs to secrete high levels of pro-inflammatory cytokines (e.g., TNF-α, IL-1β) and chemokines (e.g., MCP-1), thereby triggering neutrophil recruitment and exacerbating local tissue inflammation (Gong et al., 2025; Zhou et al., 2020). Meanwhile, IL-6 synergizes with TGF-β to upregulate the expression of the RORγt transcription factor, prompting the differentiation of naïve T cells into Th17 cells that subsequently secrete IL-17A and IL-22, further amplifying the intestinal inflammatory response (Jia R. et al., 2024; Wei et al., 2021). In addition, IL-6 impairs the intestinal mucosal barrier and increases permeability by inhibiting MUC2 mucin synthesis and disrupting the XBP1s-mediated endoplasmic reticulum stress pathway, which results in a reduction of goblet cells and degradation of tight junction proteins (Leng et al., 2024; Zhou et al., 2020). Correspondingly, IL-23 sustains the proliferation and survival of Th17 cells by activating the STAT3 and RORγt signaling pathways, prompting them to secrete IL-17A, IL-21, and IL-22 and thereby establishing a sustained pro-inflammatory microenvironment (Wang et al., 2022; Zhong et al., 2022b). In addition, IL-23 induces Th17 cells to secrete IL-6, which further activates STAT3 and upregulates IL-23R expression, thereby establishing a positive feedback loop between IL-6 and IL-23 that exacerbates local inflammation (Wei et al., 2021; Aebisher et al., 2024). IL-23 also inhibits Treg cell differentiation by down-regulating Foxp3 expression while upregulating IL-6 and TNF-α levels, thereby disrupting the Treg/Th17 balance, leading to loss of immune tolerance and chronic inflammation (Wei et al., 2021; Zhong et al., 2022b).
2.2.3 Dual role of IL-17 and IL-22
In recent years, IL-17 and IL-22 have garnered extensive attention as pivotal regulators of mucosal immune and inflammatory responses. IL-17 is a class of pro-inflammatory cytokines primarily secreted by activated Th17 cells, whereas IL-22 is produced by Th17 cells, Th22 cells, and Group 3 Innate Lymphoid Cells (ILC3), with its function tightly regulated by the local tissue microenvironment.
Under normal physiological conditions, IL-17 upholds the innate immune defense of the mucosal barrier by inducing epithelial cells to secrete AMPs (e.g., β-defensin) and chemokines (e.g., CXCL1, CXCL8) (Swedik et al., 2022; Pernomian et al., 2020). IL-17 facilitates neutrophil recruitment via activation of the STAT3 signaling pathway and augments host pathogen clearance (Lv et al., 2023). In addition, it attenuates intestinal inflammation by upregulating atypical M2 macrophage subpopulations (Nishikawa et al., 2014). IL-22, on the other hand, activates the STAT3 and MAPK pathways by binding to its receptor, thereby promoting IECs proliferation, mucus secretion, and the expression of TJ proteins to safeguard intestinal barrier integrity (Huang et al., 2022). IL-22 also induces the expression of Reg3β and Reg3γ, thereby accelerating mucosal repair following injury. In models of chronic inflammation or infection, neutralization of IL-22 has been shown to compromise intestinal barrier integrity and exacerbate tissue damage (Lo et al., 2019). Notably, a functional synergism exists between IL-17 and IL-22 under homeostatic conditions; for example, IL-22 can further potentiate the barrier immune response by enhancing IL-17-mediated AMPs expression (Swedik et al., 2022).
However, in UC, the aberrant expression and dysregulated signaling of IL-17 and IL-22 convert their roles from protective to pro-inflammatory and tissue-damaging. IL-17 stimulates the release of IL-6, TNF-α, and IL-1β from IECs and lamina propria macrophages via activation of the NF-κB and MAPK pathways, thereby creating a chronic inflammatory microenvironment (Yu et al., 2023; Yin et al., 2021). Clinical studies have shown that IL-17 expression is markedly elevated in the serum and inflamed bowel mucosa of active UC patients and positively correlates with disease activity (Fujino et al., 2003). Second, IL-23 continuously promotes IL-17 secretion by upregulating RORγt expression in Th17 cells, while IL-17 in turn augments IL-23 production by antigen-presenting cells (e.g., DCs), thereby establishing a self-amplifying inflammatory loop. IL-23 inhibitors have been shown to significantly reduce mucosal IL-17 levels in UC patients by interrupting this loop (Gottlieb and Sands, 2022). In addition, IL-22 induces IL-17C overexpression in epithelial cells via activation of the AP-1 transcription factor and p38 MAPK signaling, which further stimulates Th17 cells to secrete IL-17A, thereby creating an inflammatory amplification loop between the epithelium and immune cells (Swedik et al., 2022). Abnormal elevation of IL-22 also leads to excessive IECs proliferation and goblet cells depletion, resulting in mucus layer thinning and increased barrier permeability (Huang et al., 2022). In addition, IL-17 and IL-22 together disrupt immune homeostasis and exacerbate the Th17/Treg imbalance by inhibiting Treg cell differentiation (via Foxp3 downregulation) and suppressing Th1 cell function (by reducing IFN-γ secretion), respectively (Lv et al., 2023). It is worth mentioning that under co-stimulation by TNF-α and IL-17A, IECs secrete IL-17C, which activates STAT3 signaling in Th17 cells via the IL-17RE receptor, thereby further driving the release of IL-17A and IL-22 and forming a vicious cycle (Swedik et al., 2022). Meanwhile, during the active phase of UC, the pro-repair isoform of IL-22 (e.g., IL-22/STAT3/Reg3γ axis) shifts to a pro-inflammatory isoform (e.g., IL-22/AP-1/IL-17C axis), a functional polarization closely linked to intestinal microbiota dysbiosis and Dectin-1 signaling activation (Azizollah et al., 2024).
2.3 Imbalance in the gut microbiota
As the largest microecosystem in the human body, the intestinal microbiota plays an indispensable role in maintaining host physiological homeostasis and regulating immune function (Ma et al., 2019). This community predominantly comprises Bacteroidetes, Firmicutes, Proteobacteria, Actinobacteria, and Verrucomicrobia, with Bacteroidetes and Firmicutes constituting over 90% of the total flora and primarily colonizing the colon and rectum (Schroeder and Bäckhed, 2016; Rajilić-Stojanović et al., 2007). In recent years, a large body of experimental evidence has demonstrated that the intestinal microbiota is not only involved in energy metabolism but also serves as a crucial modulator of innate and adaptive immune responses, thereby contributing to the maintenance of intestinal barrier integrity and overall host health (Ansaldo et al., 2021; Wang et al., 2024d). In addition, metabolites derived from the microbiota—such as short-chain fatty acids (SCFAs), bile acids (BAs), and tryptophan (Trp)—are vital for maintaining the gut barrier and regulating immune homeostasis (Franzosa et al., 2019; Lloyd-Price et al., 2019) (Figure 3).
2.3.1 Imbalance of gut microbiota and dysfunction of innate immunity
The intestinal innate immune response primarily depends on IECs and resident immune cells (e.g., macrophages, DCs, and neutrophils) to mount rapid responses to external stimuli. In the pathogenesis of UC, an imbalance in the gut microbiota is closely associated with aberrant activation of pattern recognition receptors (PRRs). As two core families of PRRs, Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain-like receptors (NLRs) are activated by recognizing microbe-associated molecular patterns (MAMPs) and play pivotal roles in regulating innate immunity.
TLR4 is particularly important in UC pathology as a key receptor for recognizing Gram-negative bacterial lipopolysaccharides (LPS). When the intestinal microbiota is dysbiotic, Gram-negative bacteria such as Proteobacteria proliferate excessively and release copious amounts of LPS, which binds to TLR4 on IECs and lamina propria macrophages, thereby activating the MyD88-dependent NF-κB signaling pathway and inducing the expression of pro-inflammatory cytokines (e.g., TNF-α, IL-6, and IL-1β) and chemokines (e.g., CXCL1 and CXCL8) (Chen et al., 2020; Mirpuri et al., 2014; Miao et al., 2021). Clinical observations have shown that TLR4 expression is markedly upregulated in the colonic mucosa of UC patients and positively correlates with disease activity (Xu N. et al., 2021). TLR2 mainly recognizes lipoproteins from Gram-positive bacteria and exerts a bidirectional regulatory role in maintaining intestinal homeostasis. When the microbiota is dysbiotic, the abnormal colonization of pathogenic bacteria (e.g., Klebsiella pneumoniae) and alterations in their lipoprotein structures may lead to overactivation of TLR2, prompting dendritic cells to secrete abundant IL-23 that induces Th17 cell differentiation and IL-17A release, thereby compromising epithelial barrier function (Atarashi et al., 2017). Conversely, probiotics (e.g., Lactobacillus) can enhance the suppressive function of Treg cells via a TLR2-dependent mechanism (Al-Sadi et al., 2021).
NOD2 is a critical cytoplasmic receptor for recognizing bacterial muramyl dipeptide (MDP), and its loss of function or mutation is recognized as a significant genetic risk factor for UC. NOD2 deficiency not only reduces the capacity of Paneth cells to secrete AMPs such as α-defensins—thereby facilitating commensal bacterial penetration and subsequent TLR4 activation—but is also closely associated with a diminished response to probiotic therapy in UC patients, suggesting a complex interplay between genetic factors and bacterial dysbiosis (Wehkamp et al., 2008; Liu et al., 2022). Meanwhile, NOD2 clears intracellular invading pathogens (e.g., adherent-invasive Escherichia coli [AIEC]) by regulating the autophagy-related gene ATG16L1, and its functional defects can lead to abnormal AIEC proliferation and subsequent activation of NLRP3 inflammasomes (Zheng et al., 2022; Tran et al., 2023). Imbalances in the gut microbiota also generate various metabolites—such as reactive oxygen species (ROS), hydrogen sulfide (H2S), and pathogen-associated molecules (e.g., bacterial flagellin epitopes)—which activate the NLRP3 inflammasome via a two-step signaling mechanism: the first signal, induced by the TLR4/NF-κB pathway, upregulates NLRP3 and pro-IL-1β expression, while the second signal—mediated by potassium efflux or mitochondrial ROS—promotes inflammasome assembly and caspase-1 activation, leading to the maturation of IL-1β and IL-18 (Zhou et al., 2011; He et al., 2016; Swanson et al., 2019). In colon biopsies of UC patients, levels of both NLRP3 and IL-1β are markedly elevated; moreover, application of the NLRP3 inhibitor MCC950 in animal models significantly alleviates colonic inflammatory damage (Liu et al., 2017; Perera et al., 2018).
2.3.2 Imbalance of gut microbiota and dysfunction of adaptive immunity
Intestinal adaptive immunity is a specific response mediated by T and B lymphocytes that recognize gut microbiota and their metabolites via antigen-presenting cells, thereby establishing a protective mechanism characterized by antigen specificity and immune memory crucial for maintaining host homeostasis (Owen and Mohamadzadeh, 2013). Gut microbiota imbalance triggers mucosal immune abnormalities and inflammatory injury by disrupting the activation, differentiation, and function of T and B cells.
Dysregulation of T cell subsets constitutes a central feature of UC immunopathology. Specifically, the symbiotic bacterium Faecalibacterium prausnitzii, whose abundance is markedly reduced in UC patients, secretes a microbial anti-inflammatory molecule (MAM) that curtails Th17 cell differentiation by inhibiting the IL-6/STAT3 signaling pathway (Zhao et al., 2021; Ratajczak et al., 2019; Qiu et al., 2013). In contrast, pathogenic bacteria (e.g., AIEC) stabilize and expand the Th17 cell population by activating dendritic cells and promoting IL-23 secretion, which in turn triggers robust IL-17A production and exacerbates mucosal inflammation (Leccese et al., 2024). In addition, the gut microbiota further promotes Th17 cell polarization by secreting exosomes that carry 16S rRNA fragments capable of directly activating lamina propria CD4+ T cells via TLR7 (Zhou et al., 2021; Harrell et al., 2019). Meanwhile, the recent discovery of IL-17-producing Treg cells suggests a delicate dynamic balance between Th17 and Treg cell populations. Metabolites derived from Clostridiales enhance Treg cell IL-10 secretion, thereby suppressing inflammatory responses and helping to maintain immune homeostasis (Cui et al., 2024). However, clostridial abundance is generally reduced in UC patients compared with healthy individuals, thereby weakening Treg cell regulatory functions and predisposing to uncontrolled inflammatory cytokine release (Rossen et al., 2015).
B cells also play a crucial role in maintaining gut microbiota homeostasis. In healthy individuals, intestinal IgA+ B cells maintain microbial balance by secreting polyclonal IgA to neutralize pathogens, whereas in UC patients, aberrant IgA class switching leads to increased secretion of pathogenic IgG antibodies (e.g., anticommensal bacterial antibodies), which disrupt the intestinal epithelial barrier and facilitate translocation of bacterial antigens to the lamina propria, thereby activating T cells and triggering an autoimmune response (Fleming et al., 2022; Sengupta and Sen, 2024). In addition, IL-6 and B-cell activating factor (BAFF) secreted by B cells promote Th17 cell differentiation while concurrently suppressing Treg cell function, thereby exacerbating mucosal inflammation (Kim, 2021). Some B cells also directly disrupt tight junction proteins in the intestinal epithelium by secreting matrix metalloproteinases (MMPs) and other pro-inflammatory factors, thereby increasing mucosal permeability and creating a vicious inflammatory cycle (Kim, 2021; Inciuraite et al., 2024). Notably, the marked reduction in phage abundance in UC patients impairs the lytic capacity against pathogenic bacteria (e.g., E. coli), indirectly promoting aberrant B-cell-mediated autoantibody production (Bai et al., 2022; Özdirik et al., 2021).
2.3.3 Disturbance of intestinal microbial metabolites
The gut microbiota not only regulates immune responses through direct cell-to-cell interactions, but its metabolites also play multifaceted roles in UC development. Under normal conditions, gut microbial metabolites—such as SCFAs, BAs, and Trp—help maintain luminal pH balance, stabilize the microecological barrier, and modulate inflammatory responses by providing energy to epithelial cells, regulating TJ proteins, and supporting goblet cells differentiation, respectively. However, in UC, deficiencies or metabolic disorders of these metabolites can lead to impaired intestinal barrier function, aberrant release of pro-inflammatory factors, and overgrowth of pathogenic bacteria, collectively driving disease onset and progression.
Dietary fiber and other indigestible carbohydrates are fermented by intestinal commensal bacteria to produce SCFAs (Blaak et al., 2020). SCFAs include acetate, propionate, and butyrate; acetate and propionate are predominantly produced by Bacteroidetes, whereas butyrate is largely derived from the metabolic activities of Firmicutes (Vogt et al., 2015). Butyrate, a major energy source for colonocytes, is crucial for maintaining the repair and barrier integrity of IECs (Hodgkinson et al., 2023). High concentrations of butyrate not only inhibit the adhesion and colonization of pathogenic bacteria, thereby reducing infection risk, but also exert potent anti-inflammatory effects by preserving Th17/Treg cell homeostasis through HDAC inhibition, promotion of Foxp3 expression, and blockade of the IL-6/STAT3/IL-17 signaling pathway (Akhtar et al., 2022; Zhou et al., 2018). However, in UC patients, butyrate levels are markedly reduced, resulting in insufficient HDAC inhibition, decreased MUC2 mucin secretion and tight junction protein expression, and consequently increased intestinal mucosal permeability (Langhorst et al., 2020; Yin et al., 2024). Notably, butyrate, a ligand for SCFAs-sensing G-protein-coupled receptors (GPCRs), exerts anti-inflammatory signaling by binding to GPR41, GPR43, and GPR109A (Siddiqui et al., 2024; Kalkan et al., 2025). Studies have demonstrated that butyrate activates the GPR109A signaling pathway to enhance the anti-inflammatory functions of colonic macrophages and dendritic cells and to promote the differentiation of Treg cells (Xiao et al., 2022; Song et al., 2025). Furthermore, butyrate alleviates intestinal inflammation by activating GPR43, which upregulates granzyme B expression in CD4+ T cells, particularly IL-10-producing Th1 cells (Yang W. et al., 2024). Clinical studies have shown that the abundance of butyrate-producing bacteria (e.g., Clostridium globosum and Clostridium fascicularis) in the feces of UC patients is reduced, with lower butyrate concentrations correlating negatively with disease activity and inflammation severity (Kumari et al., 2013). In addition, SCFAs deficiency impairs Treg cell differentiation and fails to inhibit Th17 cell polarization, leading to an abnormal increase in pro-inflammatory factors (e.g., IL-17 and IFN-γ) that exacerbate intestinal inflammation (Bajaj et al., 2024; Kovtonyuk and McCoy, 2023).
BAs are amphiphilic molecules synthesized by the liver from cholesterol and play a key role in regulating glucolipid metabolism and immune responses (Wang et al., 2021). Upon entering the intestine, primary BAs are metabolized by the intestinal flora—first undergoing deconjugation by bile salt hydrolase (BSH) and subsequently 7α-dehydroxylation—to generate secondary BAs (Cai et al., 2022). Under physiological conditions, secondary BAs induce the expression of antimicrobial factors (e.g., nitric oxide synthase and IL-18), thereby maintaining the intestinal microecological barrier (Inagaki et al., 2006). Secondary BAs also regulate intestinal metabolism and immune responses through activation of signaling pathways such as the G protein-coupled bile acid receptor 1 (TGR5) and farnesoid X receptor (FXR), and inhibit pathogenic bacterial proliferation to maintain microbiota homeostasis (Hang et al., 2019). In UC patients, reduced secondary BA levels lead to insufficient FXR activation, thereby impairing its anti-inflammatory effects through NF-κB pathway inhibition (Sun C. et al., 2024; Liu Y. et al., 2025). Conversely, excessive accumulation of primary BAs induces ROS generation and caspase-3 activation in mitochondria, thereby promoting apoptosis of colonic epithelial cells (Liu Y. et al., 2025; Chen and Ho, 2023). In addition, an altered BAs composition disrupts the microbiota-host balance by promoting the overgrowth of pathogenic bacteria (e.g., Proteobacteria) while inhibiting butyrate-producing bacteria (e.g., Faecalibacterium), thereby creating a pro-inflammatory microenvironment (Yin et al., 2024; Sun C. et al., 2024).
Trp, an essential amino acid, is metabolized by gut microbiota into various bioactive compounds—such as indole and its derivatives (e.g., indole propionic acid, indole acetic acid), 5-hydroxytryptamine (5-HT), and kynurenine—following dietary intake (Agus et al., 2018). Normally, Trp metabolites regulate the intestinal immune response and maintain barrier integrity by activating the aryl hydrocarbon receptor (AhR), which promotes goblet cell differentiation and mucus secretion. However, UC patients often exhibit a marked reduction in indole metabolites, resulting in impaired AhR signaling, decreased goblet cell numbers, and mucus layer thinning, thereby increasing the risk of pathogen colonization (Deng et al., 2024; Wang et al., 2024e). A significant decrease in indole and indole propionic acid levels has also been observed in a DSS-induced colitis mouse model (Cheng W. et al., 2024). Meanwhile, enhanced indoleamine 2,3-dioxygenase 1 (IDO1) activity converts Trp to kynurenine, promotes Th17 cell differentiation and IL-22 secretion, and drives chronic inflammation (Kovtonyuk and McCoy, 2023; Alexeev et al., 2018). Clinically, a negative correlation between serum Trp levels and disease activity in IBD patients has been observed, suggesting that Trp deficiency is closely linked to inflammation onset and progression (Nikolaus et al., 2017).
2.4 Disruption of the intestinal barrier
Intestinal barrier primarily composed of synergistic physical and chemical components: the physical barrier—including IECs and their TJs—and the chemical barrier, represented by extracellular mucus (Zhou et al., 2024a; Zong et al., 2023). An intact intestinal barrier blocks exogenous pathogens while limiting the penetration of endogenous toxins and bacterial products. When barrier function is impaired, bacteria, toxins, and other harmful substances readily penetrate the epithelium into the submucosa, triggering abnormal local immune activation and a chronic inflammatory response that plays a central role in ulcer pathogenesis (Turner, 2009). (Figure 3).
2.4.1 Injury of intestinal epithelial cells
IECs, the core functional units of the physical barrier, comprise various cell types—including enterocytes, goblet cells, Paneth cells, enteroendocrine cells (EECs), and LGR5+ intestinal stem cells (ISCs)—which are organized in an orderly and synergistic manner (Zheng and Duan, 2023). Their apical surface forms a continuous physical barrier via TJ proteins, while the basolateral surface establishes a dynamic interface with immune cells through adhesion molecules, collectively maintaining intestinal homeostasis (Li H. et al., 2023). Under normal conditions, enterocytes are primarily responsible for nutrient uptake and metabolism (Pan X. et al., 2024); goblet cells secrete the mucin MUC2 to form a uniform mucus layer that blocks direct pathogen contact and provides an ecological niche for commensals (Gustafsson and Hansson, 2025); Paneth cells regulate gut microbiota balance and enhance antimicrobial defense by secreting AMPs such as α-defensins (Wang et al., 2018); and LGR5+ ISCs continuously replenish and repair the epithelium via the Wnt/β-catenin signaling pathway (Ayansola et al., 2024). Moreover, IECs secrete cytokines and chemokines that alert the mucosal immune system to local damage and coordinate inflammatory as well as repair processes (Kuhn et al., 2014).
In pathological states, injury to IECs triggers UC onset and progression via multiple mechanisms. First, oxidative stress plays a critical role. Activation of the myeloperoxidase (MPO) system leads to excessive ROS production, which directly damages mitochondrial DNA in IECs and induces endoplasmic reticulum stress (ERS) and apoptosis via the mitochondrial pathway (Wan et al., 2022). Single-cell sequencing has revealed that oxidative stress-related genes (e.g., GPX4 and SOD2) are significantly downregulated in IECs from UC patients (Hsu et al., 2023). Second, an imbalance in cell death patterns in IECs is another key mechanism in UC development. Recent studies have found that the P2Y14 receptor (P2Y14R) activates RIPK1 via the cAMP/PKA/CREB axis, inducing necroptosis in IECs and triggering the release of damage-associated molecular patterns (DAMPs) that further activate macrophages and neutrophils, exacerbating inflammation (Liu C. et al., 2024; Wang et al., 2024f). Regenerative dysfunction of ISCs is also critical; the inflammatory microenvironment reduces LGR5+ ISC numbers by inhibiting Wnt/β-catenin signaling and impairs intestinal regeneration by skewing differentiation toward goblet cells via Notch pathway dysregulation (Ou et al., 2024). Cytokine imbalance further contributes to IECs damage: IL-1β secretion by myeloid cells inhibits neuregulin 1 (NRG1) expression in fibroblasts, thereby blocking EGFR-mediated repair signaling (Qiu et al., 2024), whereas IL-22 promotes MUC2 synthesis via STAT3 activation, although its protective effect is modulated by autophagy levels (Nong et al., 2024).
2.4.2 Destruction of tight junctions
TJs of IECs are an essential multiprotein complex located on the apical surface, comprising transmembrane proteins (e.g., occludin, claudin family proteins, and junctional adhesion molecules [JAMs]) and cytoplasmic scaffolding proteins (e.g., ZO-1, ZO-2, ZO-3), which together form a dynamic structure functioning as both a “molecular fence” and a “molecular sieve” (Kaminsky et al., 2021; Suzuki, 2020). Among these, occludin—the first identified TJ transmembrane protein—is primarily located at cell–cell contact sites, where it “seals” intercellular gaps and regulates the permeability of ions and small molecules (Furuse et al., 1993). The claudin family comprises 27 isoforms with specific roles in intercellular junctions; for example, claudin-1 enhances intercellular sealing and modulates Notch signaling to regulate epithelial homeostasis (Chen et al., 2024b; Pope et al., 2014), whereas claudin-2 functions as a cation-selective channel, and its overexpression is positively correlated with increased permeability and inflammatory activity in UC (Van Itallie and Anderson, 2006; Raju et al., 2020). In addition, JAMs, particularly JAM-A, play a key role in regulating inter-epithelial permeability; their high expression under normal conditions helps maintain barrier function, whereas their levels are significantly decreased in UC patients and DSS-induced colitis models (Vetrano et al., 2008). As a scaffolding protein with multiple domains, ZO-1 interacts with transmembrane proteins and the cytoskeleton via its PDZ domain, providing a platform for TJ assembly and stabilization. It not only participates in TJ reconstruction but also regulates cell proliferation and polarity, thereby promoting mucosal repair (Kuo et al., 2021; Yoseph et al., 2016).
In UC patients, the TJ structure is disrupted by multiple factors, constituting an important mechanism for disease exacerbation. First, pro-inflammatory cytokines mediate abnormal TJ protein expression. TNF-α, IL-6, and IFN-γ significantly downregulate TJ proteins (e.g., occludin and ZO-1) via activation of STAT3/NF-κB signaling, while concurrently upregulating pore-forming proteins such as claudin-2, thereby increasing barrier permeability abnormally (Rawat et al., 2020; Li et al., 2021). In addition, IL-1β inhibits claudin-1 expression by modulating microRNAs (e.g., miR-195-5p), further disrupting barrier integrity (Scalavino et al., 2022). Second, dysregulation of the interactions between the gut microbiota and TJs also plays a key role in UC pathogenesis. UC patients often exhibit mucus layer defects (e.g., reduced MUC2 expression), allowing commensal bacteria direct access to IECs that activate the TLR4/MyD88 pathway and suppress occludin transcription (Zhang et al., 2024a; Jin et al., 2024). Meanwhile, serine proteases secreted by pathogenic bacteria (e.g., Clostridium nucleatum) degrade ZO-1, whereas probiotics enhance claudin-5 expression and facilitate barrier repair by upregulating IL-34 (Jin et al., 2024; Chen et al., 2025). Furthermore, gut microbiota metabolites such as SCFAs regulate claudin-4 and occludin expression via activation of the AhR pathway; however, AhR ligand levels (e.g., Trp metabolites) are markedly decreased in UC (Wang X. et al., 2024; Li Y-Y. et al., 2022). In addition, aberrant mTOR pathway activation inhibits autophagy-related proteins (e.g., LC3-II), impairing clearance of damaged TJ proteins and further exacerbating barrier dysfunction. Rapamycin can restore autophagy and promote TJ protein regeneration by inhibiting mTOR, a mechanism involving complex regulation via the AMPK/mTOR/ULK1 pathway (Xu et al., 2024; Pan S-M. et al., 2024). Oxidative stress is also a critical factor in TJ disruption; excess ROS and reactive nitrogen species (RNS) can modify proteins such as occludin via nitration or chlorination, destabilizing their conformation and causing ZO-1 to dissociate from the cytoskeleton (Zhang Q. et al., 2025; Cartwright et al., 2024). At sites of UC inflammation, abundant MPO catalyzes chlorination modifications of proteins (e.g., claudin-3), inducing TJ mislocalization (Cartwright et al., 2024). Once the TJ structure is compromised, intestinal luminal antigens (e.g., LPS) can traverse the paracellular pathway into the lamina propria, activate dendritic cells, promote Th17 differentiation, and trigger IL-17 secretion that further inhibits claudin-1 expression (Rawat et al., 2020; Mansouri et al., 2025).
2.4.3 Abnormal expression of MUC2
MUC2 is a high–molecular-weight mucin specifically secreted by intestinal goblet cells and constitutes a major structural component of the colonic mucus layer. Its core function depends on a highly glycosylated structure that forms a gel-like matrix to maintain the integrity of the intestinal chemical barrier. Under normal conditions, the double-layered mucus—comprising an outer loose layer and an inner dense layer—formed by MUC2 secretion not only prevents direct contact between pathogens and IECs but also enhances innate immune defense by trapping secretory IgA and AMPs (Yao D. et al., 2021; Wei et al., 2023). Moreover, its complex glycosylation provides abundant metabolic substrates for commensal flora, thereby maintaining intestinal microbial balance, and it participates in regulating goblet cell autophagy to promote epithelial repair and preserve dynamic barrier function (Zhang et al., 2024a).
Abnormalities in MUC2 expression and function have emerged as a key molecular mechanism in UC development. Defective O-glycosylation of MUC2 reduces mucus viscoelasticity, facilitating pathogenic bacteria (e.g., E. coli) penetration and direct invasion of IECs, while inducing NF-κB signaling that contributes to the release of pro-inflammatory factors (e.g., TNF-α, IL-6) (He et al., 2023). Meanwhile, structural defects in glycan chains weaken interactions with commensal bacteria and contribute to a shift toward a pro-inflammatory microbial phenotype; clinical data indicate that decreased MUC2 O-glycosylation in early UC is negatively correlated with virulence gene expression (Wei et al., 2023; Wang T. et al., 2024). Furthermore, MUC2 missense mutations (e.g., as observed in the Winnie mouse model) trigger protein misfolding, leading to persistent ERS and activation of the unfolded protein response (Wilson et al., 2020; Talà et al., 2022). ERS induces goblet cell apoptosis via the PERK-eIF2α pathway and inhibits normal MUC2 secretion, resulting in mucus layer disruption and the release of allergens (e.g., IL-33) that drive a Th2-type inflammatory response (Wilson et al., 2020; Singh et al., 2020). Microbiota dysbiosis (e.g., enrichment of Proteobacteria) further accelerates MUC2 degradation, establishing a vicious cycle of barrier disruption, bacterial translocation, and inflammation amplification (Wei et al., 2023; Cheng et al., 2022). Abnormal MUC2 accumulation inhibits autophagic flux in goblet cells, leading to lysosomal dysfunction and ROS buildup; autophagy defects then inhibit MUC2 transcription via mTORC1 feedback and reduce AMPK phosphorylation, impairing cellular energy stress responses (Zhang et al., 2024a; Cai et al., 2021). Notably, interactions among receptor signaling pathways also regulate MUC2 secretion. Activation of the adenosine A3 receptor (ADORA3) stimulates MUC2 secretion via the cAMP/PKA pathway; however, its expression is significantly downregulated in UC patients, resulting in insufficient mucus production. Conversely, nicotinic receptor agonists enhance MUC2 secretion by inhibiting ERK phosphorylation, suggesting a potential role for cholinergic neuromodulation in mucus barrier repair (Singh et al., 2020; Zeng et al., 2024).
3 Treatment of UC with TCM
3.1 TCM active ingredients
3.1.1 Polysaccharides
Polygonatum cyrtonema polysaccharides (PCPs), primarily derived from the dried rhizomes of the Liliaceae plant P. cyrtonema, exhibit various biological activities, including immunomodulatory, antioxidant, anti-inflammatory, and intestinal microbiota–modulating effects (Yuan et al., 2025; Shan et al., 2024). PCPs have been found to alleviate DSS-induced colitis by inhibiting pro-inflammatory factor expression, enhancing antioxidant enzyme activity, and restoring the integrity of TJ proteins in the intestinal mucosal barrier. Moreover, macrogenomic and metabolomic analyses have confirmed that PCPs enrich beneficial bacteria (e.g., Akkermansia muciniphila and Muribaculum), inhibit pathogenic bacteria (e.g., CAG-873), and significantly reverse DSS-induced microbiota dysbiosis. The efficacy of PCPs depends on the integrity of the gut microbiota; indeed, antibiotic treatment abolishes their therapeutic effects, whereas fecal transplantation restores them (Lin et al., 2024).
Ginseng polysaccharides (GPs) are primarily derived from the roots of Panax ginseng and are extracted via aqueous or enzymatic methods (Hua et al., 2020). Modern pharmacological studies have demonstrated that GPs possess immunomodulatory, antitumor, antioxidant, and anti-inflammatory effects, including enhanced immune responses via activation of macrophages and T cells (Lee et al., 2021; Li et al., 2016). Studies have shown that GPs significantly reduce intestinal 5-HT levels and inhibit HTR3A receptor signaling by remodeling the gut microbiota—specifically, by enriching Lactobacillus and inhibiting pathogenic bacterial proliferation—and subsequently regulating the Trp metabolic pathway. Additionally, GPs effectively maintain intestinal barrier integrity by restoring TJ protein expression and reducing intestinal permeability, while inhibiting the TLR4/MyD88/NF-κB pathway and pro-inflammatory cytokine secretion, ultimately reprogramming the intestinal inflammatory microenvironment. This effect is closely associated with the core pathways through which GPs regulate microbial metabolism and immune homeostasis via multiple targets (Wan L. et al., 2024).
Panax quinquefolius polysaccharides (WQP) are the primary active ingredient in the dried roots of P. quinquefolius (Guo M. et al., 2021). Modern pharmacological studies have demonstrated that WQP exhibit a range of biological activities, including antitumor, anti-inflammatory, antioxidant, and immunomodulatory effects (Ghosh et al., 2019). WQP have been shown to elevate fecal SCFAs levels by enriching SCFAs-producing genera, and these metabolites exhibit a strong positive correlation with upregulation of TJ protein expression, suggesting that SCFAs—especially butyric acid—may directly promote intestinal barrier repair via activation of the PPAR-γ signaling pathway. Meanwhile, WQP significantly reduce pro-inflammatory factors (IL-1β, TNF-α, IL-6) and induce anti-inflammatory IL-10 secretion by inhibiting the NF-κB pathway, demonstrating a dual immunomodulatory function (Ren et al., 2023).
Astragalus polysaccharides (APS), primarily derived from the dried roots of Astragalus membranaceus, possess antiviral, anti-inflammatory, and immunomodulatory effects, and enhance macrophage activity by activating the JNK/MAPK, Erk/MAPK, and NF-κB signaling pathways (Li et al., 2015; Aleebrahim-Dehkordi et al., 2022; Lu et al., 2024). APS have been found to significantly increase the abundance of SCFAs-producing bacteria by modulating gut microbiota structure, thereby restoring SCFAs levels and enhancing intestinal barrier function. Meanwhile, SCFAs reduce Th17 differentiation and promote Treg expansion by activating free fatty acid receptor 2/3 (FFAR2/3) and concurrently inhibiting both the TLR4/MyD88/NF-κB pathway and HDAC3-mediated epigenetic regulation (Zhang Y. et al., 2025).
Poria cocos polysaccharide (PCP), primarily derived from the dried mycelium of the Polyporaceae fungus Poria cocos, exhibits a wide range of biological activities, including antioxidant, immunomodulatory, anti-inflammatory, anticancer, hepatoprotective, and intestinal microbiota–modulating effects (Ng et al., 2024; Lee et al., 2017). In DSS-induced colitis models, PCP improves intestinal barrier function by upregulating TJ proteins such as occludin, claudin-1, and ZO-1. At the molecular level, PCP alleviates intestinal inflammation by inhibiting the NF-κB pathway, reducing pro-inflammatory factor expression (e.g., IL-1β, IL-12, TNF-α), and elevating anti-inflammatory IL-10 levels. Furthermore, PCP modulates intestinal microbiota structure by restoring the abundance of beneficial bacteria and SCFAs production, and its beneficial effects have been validated through fecal microbial transplantation experiments (Wan J. et al., 2024).
Codonopsis pilosula polysaccharide (CPP), primarily derived from the root of the traditional Chinese medicine C. pilosula, exhibits various pharmacological effects—including immunomodulatory, anti-inflammatory, antioxidant, anti-fatigue, and hepatoprotective activities—and its structural characteristics are closely linked to its biological functions (Ma et al., 2024; Fu et al., 2025; Meng et al., 2020). Recent studies have found that CPP significantly enriches SCFAs-producing probiotic flora (e.g., Ligilactobacillus and Akkermansia), promotes acetic acid and butyric acid production, and inhibits the NLRP3 inflammasome signaling pathway via activation of its receptor, GPR43/GPR109A, thereby reducing pro-inflammatory factors (e.g., IL-1β and IL-18). Fecal transplantation experiments further validated that the gut microbiota regulated by CPP can effectively alleviate colonic inflammation via the SCFAs-mediated GPR/NLRP3 signaling axis (Zhou et al., 2025).
3.1.2 Alkaloids
Berberine (BBR) is an isoquinoline alkaloid widely distributed in Berberidaceae plants such as Coptis chinensis and Phellodendron chinense (Wang et al., 2019). In UC, BBR may exert therapeutic effects via immune modulation, anti-inflammatory and mucosal repair mechanisms, and regulation of the gut microbiota. Multi-omics data have demonstrated that BBR significantly ameliorates gut microbiota dysbiosis in UC models by restoring metabolic homeostasis and attenuating inflammatory responses—achieved through increasing beneficial bacteria (e.g., Bacteroides and Akkermansia) and inhibiting arachidonic acid (AA) metabolic pathways (Yang T. et al., 2024). Another study focusing on intestinal neural–immune regulation found that BBR effectively rebuilt the mucosal barrier and reduced immune cell overactivation by preserving intestinal neuroglia function and enhancing interactions between IECs and immune cells, thereby alleviating local inflammation (Li et al., 2020).
Matrine (MT) is a natural quinolizidine alkaloid primarily derived from the legume Sophora flavescens, traditionally used to treat skin inflammation, diarrhea, and vaginal itching (Gao et al., 2024). Modern pharmacological studies have demonstrated that MT exhibits multiple biological activities, including anti-inflammatory, antioxidant, immunomodulatory, and gut microbiota–modulating effects (Yao H. et al., 2021; Sun J. et al., 2024). Recent studies have found that MT restores goblet cell numbers and TJ protein expression, suggesting that it alleviates disease progression by repairing intestinal barrier integrity. In addition, MT effectively alleviates oxidative stress injury by upregulating SOD and CAT activities and reducing MDA levels. Notably, MT significantly corrects intestinal immune imbalance, as evidenced by an increased Treg and decreased Th17 proportion in the spleen and mesenteric lymph nodes, suggesting that it inhibits excessive immune responses by regulating the Treg/Th17 balance (Mao et al., 2024).
Evodiamine (EVO) is a quinolone alkaloid isolated from Evodia rutaecarpa that exhibits a wide range of biological activities, including anticancer, anti-hypoxia, antithrombotic, anti-inflammatory, and analgesic effects (Yu et al., 2013; Jiang and Hu, 2009). EVO has been found to activate intestinal epithelial GPR43 receptors by promoting acetate production, which in turn inhibits the NF-κB pathway and downregulates pro-inflammatory factor expression. Meanwhile, EVO repairs intestinal barrier function by restoring goblet cell mucin (MUC2) secretion, upregulating Reg3γ/β antimicrobial peptides, and enhancing TJ protein Claudin-1 expression. Fecal microbiota transplantation experiments further confirmed that EVO-modulated microbiota exert independent therapeutic effects, while Lactobacillus supplementation partially mimics EVO’s anti-inflammatory effects, highlighting the central role of microbiota metabolites (Wang et al., 2020).
Palmatine (PAL) is a natural isoquinoline alkaloid primarily derived from traditional Chinese medicinal plants such as C. chinensis and P. chinense (Ji et al., 2024a). Studies have shown that PAL ameliorates clinical symptoms and histopathological changes in DSS-induced UC models by inhibiting pro-inflammatory factor secretion (e.g., TNF-α, IL-1β, IL-6, IL-18), reducing oxidative stress and iron loading, and modulating the expression of GPX4, ACSL4, COX-2, and Nrf2/HO-1 (Ji et al., 2024b). Another study found that 8-Oxypalmatine (OPAL), a metabolite of PAL in vivo, exhibits superior therapeutic effects. OPAL not only repairs the intestinal mucus barrier and restores TJ protein expression but also effectively alleviates UC inflammatory responses by activating AMPK, inhibiting the NF-κB pathway, and promoting macrophage polarization from M1 to M2 (Huang et al., 2025).
Sinomenine, an isoquinoline alkaloid, is primarily extracted from the dried vine stems of Sinomenii Caulis. Modern pharmacological studies have demonstrated that sinomenine possesses significant anti-inflammatory, immunosuppressive, and antitumor activities, achieving its effects by inhibiting inflammatory factor release and modulating immune cell function, particularly in UC treatment (Zhang et al., 2024b; Tian et al., 2024). Further experiments showed that sinomenine activates the HO-1/Nrf2 pathway, upregulates SOD and CAT activities, and simultaneously inhibits RIP1 and Caspase-1 mRNA expression—key components of the NLRP3 inflammasome—to achieve a dual blockade of oxidative stress and inflammatory cascades. Particularly, 16S rRNA sequencing confirmed that sinomenine can reconfigure the gut microbiota, resulting in an increased Firmicutes/Bacteroidetes (F/B) ratio and higher abundance of probiotics (e.g., Lactobacillus spp), thereby enhancing intestinal barrier function via colony–immunity interactions (Niu et al., 2024).
3.1.3 Flavonoids and Polyphenols
Galangin, a natural flavonoid primarily derived from Alpinia officinarum, exhibits anti-inflammatory, antioxidant, and immunomodulatory effects. Galangin has been found to activate the autophagy pathway, significantly upregulate ATG5, ATG7, and the LC3B-II/I ratio, promote clearance of damaged cells, and inhibit activation of NLRP3 inflammasomes. In addition, Galangin remodels gut microbiota structure, enhances α-diversity, and enriches butyric acid–producing bacteria—thereby driving butyric acid production that enhances intestinal barrier function and inhibits NF-κB–mediated release of TNF-α and IL-1β via PPAR-γ signaling (Xuan et al., 2020). Another study found that Galangin targets the TLR4/NF-κB pathway, downregulates TLR4 mRNA and HMGB1 expression, and reduces NF-κB nuclear translocation, thereby decreasing pro-inflammatory factor levels. Its phenolic hydroxyl groups directly scavenge ROS and enhance antioxidant enzyme activities, synergistically mitigating oxidative damage. However, the long-term efficacy, human bioavailability, and capacity to modulate intestinal barrier proteins of Galangin remain to be thoroughly verified (Gerges et al., 2020).
Baicalin, a flavonoid extracted from Scutellaria baicalensis, exhibits a wide range of activities, including anti-inflammatory, antioxidant, immunomodulatory, and anti-apoptotic effects (Jia Y. et al., 2024; Zou et al., 2015; Hu et al., 2021). Studies have shown that Baicalin significantly corrects Th17/Treg imbalance and repairs the intestinal barrier by upregulating tight junction proteins and mucin MUC2. Meanwhile, Baicalin elevates fecal butyric acid levels by enriching butyric acid–producing bacteria and activates the butyric acid–GPR43/HDAC inhibitory axis to enhance anti-inflammatory effects (Zhu et al., 2020). Zhang et al. developed water-soluble baicalin magnesium (BA-Mg), which significantly increases secondary bile acid levels, activates FXR/GPBAR1 receptors, and inhibits the NF-κB inflammatory pathway. Moreover, BA-Mg promotes the proliferation of bile acid–converting bacteria (e.g., Oscillibacter) by remodeling the microbiota structure, thereby forming a positive feedback loop among flora, BAs, and immunity (Zhang L. et al., 2024).
Alpinetin, a flavonoid extracted from the seeds of Alpinia katsumadai, exhibits a wide range of biological activities (Lv et al., 2018). In a DSS-induced colitis model, Alpinetin significantly attenuates intestinal inflammatory injury by restoring colonic Th17/Treg balance. Further studies revealed that Alpinetin promotes miR-302 expression in CD4+ T cells via activation of the AhR/ARNT/XRE pathway while inhibiting DNMT-1 activity, thereby reducing Foxp3 promoter methylation and enhancing Treg cell differentiation and function. In addition, Alpinetin specifically enhances CREB binding to the Foxp3 promoter without affecting CREB nuclear translocation or DNA binding, suggesting that it strengthens Treg cell immunosuppressive function via epigenetic reprogramming (Lv et al., 2018).
Curcumin (Cur) is a natural polyphenolic compound extracted from the rhizome of Curcuma longa, a ginger family plant, and is one of the active ingredients of TCM. Modern pharmacological studies have shown that Cur exhibits significant anti-inflammatory, antioxidant, antitumor, and immunomodulatory effects (Nelson et al., 2017). In UC treatment, Cur exerts protective effects via multiple pathways. At the immunomodulatory level, Cur bidirectionally regulates the adaptive immune system: on the one hand, it promotes Treg cell differentiation via TGF-β1/Smad3 pathway activation and inhibits Th17 cell polarization to restore Treg/Th17 homeostasis (Guo et al., 2022); on the other hand, its inhibition of the TLR4/MyD88/NF-κB axis through epigenetic regulation reduces PD-L1+ inhibitory B cells while promoting IL-10 secretion in CD19+CD1d+CD5+ B10 cells, thereby enabling cross-talk among T cells, B cells, and epithelial cells (Huang et al., 2023). In terms of intrinsic immune defense, Cur directly targets RIP3—a key regulator of necroptosis in IECs—and blocks formation of the RIP1-RIP3-MLKL complex by occupying its RHIM domain, thereby reducing phosphorylated MLKL levels and the release of intestinal barrier DAMPs (Y et al., 2023).
3.1.4 Terpenoids and glycosides
Andrographolide (AND) is a diterpenoid isolated from Andrographis paniculata, a plant in the Jurassicaceae family, and exhibits a wide range of biological effects as its main active ingredient (Jing et al., 2019). Zhu et al. found, based on in vitro experiments with peripheral blood mononuclear cells (PBMCs) from UC patients, that AND remodels T cell subset balance by downregulating the Th1-specific transcription factor T-bet and the Th17 key regulator ROR-γt, while upregulating the Th2 marker GATA-3. AND is particularly effective at reversing IL-23-induced hyperpolarization of Th1/Th17, suggesting that its targeted inhibition of the IL-23/Th17 inflammatory axis represents a central mechanism for ameliorating immune imbalance in UC (Zhu et al., 2018). Further studies revealed that AND significantly alleviates oxazolone-induced intestinal inflammation in a rat UC model by modulating the IL-4R/STAT6 pathway—specifically inhibiting STAT6 phosphorylation while enhancing IL-4 receptor expression—suggesting that it exerts anti-inflammatory effects through dual modulation of the Th2-related pathway (Zhang et al., 2019).
Triptolide is a diterpene lactone active compound isolated from Tripterygium wilfordii, a traditional Chinese medicine, and exhibits remarkable anti-inflammatory, immunomodulatory, and antitumor properties (Tang et al., 2020; Moudgil and Venkatesha, 2022). Triptolide has been found to inhibit macrophage polarization toward the pro-inflammatory M1 phenotype and reduce ROS production via activation of the NRF2/HO-1 antioxidant pathway, thereby attenuating oxidative stress damage. In addition, Triptolide inhibits the PDE4B-dependent AKT/NF-κB signaling cascade, thereby downregulating pro-inflammatory cytokine expression (e.g., TNF-α, IL-6) and blocking the amplification of intestinal inflammation (Tang et al., 2020). Another study addressed the toxicity bottleneck of Triptolide by designing a novel derivative, ZT01, to reduce its reproductive toxicity via structural modification. Experiments showed that ZT01 treatment significantly improves intestinal epithelial barrier function, reduces Th1 and Th17 cell differentiation, and regulates JAK-STAT signaling activity, suggesting its therapeutic role by inhibiting T-cell-mediated immune abnormalities and macrophage-driven inflammatory responses (Fu et al., 2021).
Glycyrrhizin is a triterpenoid saponin compound primarily derived from the rhizomes of Glycyrrhiza glabra, a leguminous plant, and is one of its most abundant active ingredients. Modern pharmacological studies have shown that Glycyrrhizin exhibits significant anti-inflammatory, antioxidant, and immunomodulatory effects. Animal experiments revealed that Glycyrrhizin significantly alleviates DSS-induced histopathological damage in the mouse colon, reduces overproduction of IL-6 and IL-17 in the colonic mucosa, and demonstrates high-affinity binding to inflammation-related targets (e.g., TLR4/MyD88 pathway proteins) as confirmed by molecular docking analysis. Meanwhile, Glycyrrhizin inhibits LPS-induced inflammatory injury in IECs in cellular models, suggesting its therapeutic role in maintaining intestinal barrier integrity (Kong et al., 2024).
Tanshinone IIA (Tan IIA) is a major fat-soluble diterpene quinone extracted from the roots and rhizomes of Salvia miltiorrhiza, a traditional Chinese medicinal herb, and exhibits a wide range of modern pharmacological activities (Zhang X. et al., 2015). In UC treatment, Tan IIA significantly ameliorates histopathological damage in the colon of experimental animals, reduces the disease activity index score, and modulates serum inflammatory factor levels—lowering pro-inflammatory factors (TNF-α, IL-1β, and IL-6) while elevating anti-inflammatory IL-10 (Zhu et al., 2022). In addition, its mechanism may involve inhibition of the TLR4/PI3K/AKT/mTOR pathway, thereby reducing apoptosis and inflammatory cell infiltration in IECs (Peng et al., 2021). Clinical studies have shown that Tan IIA combined with mesalazine significantly increases overall treatment efficacy and decreases TNF-α and CRP levels in UC patients, suggesting its potential as an adjuvant therapy (Chen et al., 2024c). However, its long-term efficacy and safety remain to be further validated in multicenter clinical studies.
Salidroside (SAL) is a glycoside compound extracted from the perennial herb Rhodiola rosea and exhibits anticancer, anti-inflammatory, and antioxidant activities (Zhang et al., 2021). Recent studies have found that SAL inhibits NLRP3 inflammasome activation by targeting the TREM1/SYK signaling axis, as evidenced by reduced expression of key proteins (NLRP3, caspase-1 p20, and GSDMD p30) in colonic tissues, and that TREM1 knockdown completely blocks its regulatory effect on NLRP3. Notably, SAL significantly remodels gut microbiota structure, resulting in an elevated Firmicutes/Bacteroidetes (F/B) ratio and specific enrichment of SCFAs-producing genera. The microbiota-dependent restoration of Th17/Treg balance was confirmed by antibiotic clearance and fecal transplantation experiments (Liu X. et al., 2023).
Paeoniflorin (PF) is a monoterpene glycoside primarily derived from the dried roots of Paeonia lactiflora and Paeonia veitchii, exhibiting anti-inflammatory, antioxidant, immunomodulatory, and neuroprotective effects (Li Q. et al., 2024). PF has been found to reduce the abnormal accumulation of the Trp metabolite indole-3-lactic acid (ILA) by modulating intestinal microbiota structure. As a key effector, ILA inhibits autophagy in junctional IECs by activating mTOR signaling, thereby enhancing NLRP3 inflammasome activation. In contrast, PF treatment significantly restores autophagic flux and blocks the release of pro-inflammatory factors (e.g., IL-1β), thereby alleviating DSS-induced colitis (Fan et al., 2020). Another in vitro experiment showed that PF-pretreated dendritic cells significantly promote Treg (Foxp3+IL-10+) differentiation and inhibit Th17 (IL-17A+) polarization, reducing the Th17/Treg ratio by over 80%. In a TNBS colitis model, PF restores intestinal immune tolerance by regulating T cell differentiation through inhibition of dendritic cell maturation (Zheng et al., 2020). Further studies have found that PF also exhibits significant mucosal regenerative and reparative functions; it promotes the proliferation and directed differentiation of ISCs by activating the PI3K-AKT-mTOR pathway, thereby driving regeneration of goblet and Paneth cells and accelerating epithelial barrier repair (Ma et al., 2023).
Ginsenosides are a class of triterpenoid saponins extracted from P. ginseng, a member of the Araliaceae family, and constitute the main active ingredients of ginseng (Yuan et al., 2024; Zhou et al., 2024b). Based on structural differences, ginsenosides can be categorized into proto-ginseng diol types (e.g., Rb1, Rd, Rh2) and proto-ginseng triol types (e.g., Rg1, RT4), with their biosynthesis involving glycosyltransferase-mediated modifications (Li Y. et al., 2023; Guan and Qi, 2023). In UC treatment, several ginsenosides exert therapeutic effects through multi-target mechanisms. Ginsenoside Rb1 (GRb1) attenuates DSS-induced ulcerative colitis in mice by synergistically modulating the VDR/PPARγ/NF-κB pathway—inhibiting TNF-α and IL-6 while upregulating IL-10 expression—and repairs the intestinal barrier by enhancing tight junction proteins (e.g., ZO-1, occludin, and E-cadherin), as validated by multi-omics analysis and molecular docking (Zhou et al., 2024b). Ginsenoside Rd (GRd) induces p62-mediated mitochondrial autophagy by activating the AMPK/ULK1 pathway, removes damaged mitochondria, and inhibits NLRP3 inflammasome activation, thereby attenuating DSS-induced colitis in mice (Liu et al., 2018). In addition, in a rat TNBS model of recurrent colitis, GRd effectively reduces levels of the oxidative stress marker MDA and pro-inflammatory mediators (iNOS/NO) by inhibiting neutrophil infiltration (decreasing MPO activity), modulating Ca2+ signaling, and augmenting SOD/GSH-Px enzyme activity (Yang et al., 2012). Ginsenoside Rh2 (GRh2) significantly enhances SMAD2/3 phosphorylation and inhibits pro-inflammatory factors (IL-6, TNFα, IFNγ) via activation of the TGFβ signaling pathway, with its anti-inflammatory effects reversed by the TGFβ receptor I inhibitor SB431542 (Ye et al., 2014). Ginsenoside Rg1 (GRg1) repairs the intestinal barrier by upregulating the TJ protein ZO-1, reducing pro-inflammatory factors (TNF-α and IFN-γ), and elevating anti-inflammatory IL-4 levels, with efficacy comparable to the first-line drug 5-ASA (He et al., 2022). In a DSS-induced mouse model of colitis, Ginsenoside RT4 (GRT4) inhibits its target gene SLC7A11 by upregulating miR-144-3p expression, reduces pro-inflammatory factor expression (e.g., TNF-α and IL-1β), and elevates anti-inflammatory IL-10 levels. In addition, GRT4 promotes macrophage polarization from the pro-inflammatory M1 to the anti-inflammatory M2 phenotype by regulating the miR-144-3p/SLC7A11 pathway, thereby accelerating colonic tissue repair (Li Y. et al., 2023) (Table 1).
3.2 TCM herbal formulas
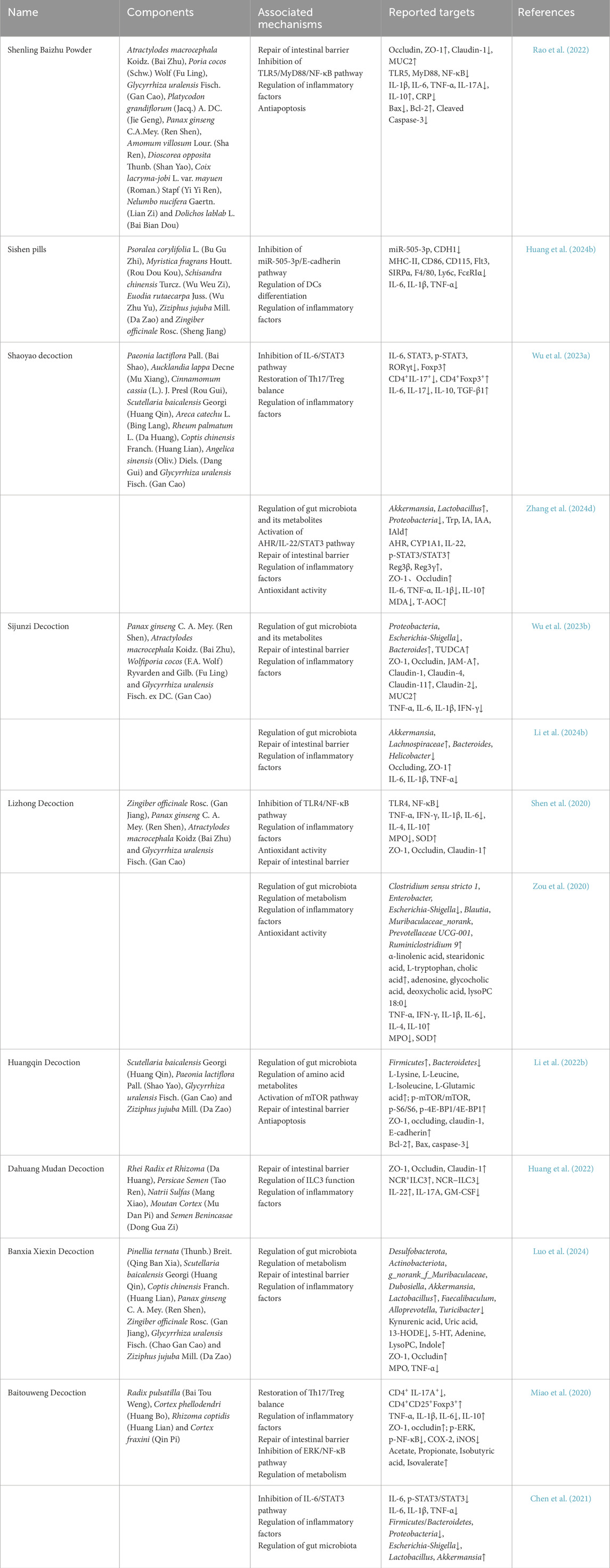
Shenling Baizhu Powder (SBP) is a traditional Chinese herbal formula known for strengthening the spleen, benefiting qi, resolving dampness, and stopping diarrhea, and is commonly used to treat digestive disorders of the spleen-deficiency and dampness-abundance type (Chen et al., 2022). SBP has been found to repair the damaged intestinal barrier in TNBS-induced rat colitis by restoring normal expression and distribution of intestinal epithelial TJ proteins and promoting production of the key mucus component, MUC2. In addition, SBP significantly inhibits apoptosis, as evidenced by a reduced Bax/Bcl-2 ratio and lower caspase-3 activity. Further mechanistic studies have shown that SBP regulates the TLR5/MyD88/NF-κB pathway and reduces inflammatory signaling, thereby slowing the inflammatory response (Rao et al., 2022).
Sishen Pills (SSP) is a classic Chinese compound used for treating chronic diarrhea and other digestive disorders associated with spleen and kidney yang deficiency (Tao et al., 2022). By integrating transcriptome sequencing and network pharmacology analysis, SSP was found to significantly downregulate miR-505-3p in colon tissues, thereby inhibiting CDH1-encoded E-cadherin expression and blocking dendritic cell differentiation into a pro-inflammatory phenotype. In vitro and in vivo experiments confirmed that this mechanism effectively reduces secretion of pro-inflammatory factors (IL-6, IL-1β, TNF-α) and ameliorates DSS-induced colonic injury. Furthermore, SSP studies identified EVO as a key active component via molecular docking and functional validation, showing that it exerts anti-inflammatory effects through the same pathway (Huang J. et al., 2024).
Shaoyao Decoction (SYD) is a traditional Chinese formula known for clearing heat, resolving dampness and dryness, and regulating qi and blood, and is commonly used to treat damp-heat diarrhea. In terms of immunomodulation, SYD significantly reduces the proportion of Th17 cells and RORγt expression by inhibiting the IL-6/STAT3 axis, while upregulating Treg cell numbers and Foxp3 levels to restore the Th17/Treg balance. This immune remodeling effect is accompanied by a decrease in pro-inflammatory factors (IL-17, IL-6) and an increase in anti-inflammatory factors (IL-10, TGF-β1) expression (Wu D. et al., 2023). In addition, SYD remodels gut microbiota by increasing the abundance of butyric acid–producing bacteria and promoting SCFAs production, while regulating Trp metabolism and restoring indole metabolite levels (IA, IAA, IAld) to normal. These metabolites induce IL-22 secretion via AHR receptor activation, which in turn activates STAT3 phosphorylation and promotes expression of intestinal epithelial Reg3β/γ antimicrobial peptides and repair of TJ proteins ZO-1 and occludin (Zhang Y. et al., 2024).
Sijunzi Decoction (SJZD) is a classic tonic formula of TCM, originating from the “Taiping Huimin Heji Prescription” of the Song Dynasty and serving as a core treatment for Spleen-Qi deficiency. SJZD restores gut microbiota homeostasis by reducing the abundance of Proteobacteria and Escherichia-Shigella. In addition, SJZD promotes BAs synthesis—especially taurodeoxycholic acid (TUDCA), a metabolite that improves intestinal barrier function and inhibits inflammatory responses via the TGR5-cAMP pathway—thus exerting anti-UC effects (Wu Y. et al., 2023). Another study further supported the anti-inflammatory and barrier-protective effects of SJZD, finding that it significantly reduces inflammatory factor levels (e.g., IL-6, IL-1β, TNF-α) and upregulates key TJ proteins, such as occludin and ZO-1, while demonstrating that SJZD alleviates UC by remodeling gut microbiota (e.g., modulating levels of Alistipes, Akkermansia, and Lachnospiraceae) and revealing correlations among gut microbiota, inflammatory factors, and TJ proteins (Li H. et al., 2024).
Lizhong Decoction (LZD) is a classic warming formula in TCM, originating from Zhang Zhongjing’s “Treatise on Miscellaneous Diseases of Typhoid” during the Eastern Han Dynasty, and represents a classic prescription for treating cold deficiency of the spleen and stomach. Studies have demonstrated that LZD inhibits the secretion of pro-inflammatory factors (TNF-α, IL-6, IFN-γ), enhances the expression of anti-inflammatory factors (IL-4, IL-10), and modulates the inflammatory cascade by downregulating the TLR4/NF-κB signaling pathway. Notably, LZD also significantly upregulates the expression of TJ proteins (ZO-1, occludin, and claudin-1) and repairs the intestinal mechanical barrier function (Shen et al., 2020). Gut microbiota-based studies further revealed that LZD modifies the gut microbiota structure in UC mice by reducing the abundance of conditionally pathogenic bacteria (e.g., Clostridium sensu stricto 1, Escherichia-Shigella) and promoting the proliferation of SCFAs-producing flora (e.g., Blautia, Prevotellaceae UCG-001), thereby improving intestinal microenvironmental homeostasis through the regulation of purine metabolism, BAs synthesis, and α-linolenic acid metabolism, among other pathways (Zou et al., 2020).
Huangqin Decoction (HQD) is a TCM compound primarily used to treat diarrhea, dysentery, and damp-heat conditions, with demonstrated efficacy in clearing heat, removing toxins, harmonizing the middle, and relieving pain. In a mouse model of DSS-induced colitis, HQD enhanced the proliferative capacity of IECs by remodeling gut microbiota homeostasis (evidenced by an increased F/B ratio), elevating the levels of 12 amino acid metabolites (including L-leucine), and activating the mTOR pathway to promote S6/4E-BP1 phosphorylation. Additionally, HQD upregulated the expression of TJ proteins (ZO-1, occludin, and claudin-1) and the adhesion junction protein E-cadherin, thereby repairing intestinal mucosal barrier function. In vitro experiments further confirmed that microbiota metabolites and key amino acids regulated by HQD inhibited DSS-induced apoptosis in FHC cells and reduced the expression of pro-apoptotic proteins Bax and cleaved caspase-3, while preserving the integrity of intercellular junctions (Li M-X. et al., 2022).
Dahuang Mudan Decoction (DMD), derived from Zhang Zhongjing’s “The Essentials of the Golden Chamber” during the Eastern Han Dynasty, is a classic TCM formula for treating intestinal carbuncle, exhibiting effects such as relieving diarrhea and heat, breaking up stasis, dispersing knots, and eliminating swelling. Studies have shown that DMD effectively reduces intestinal permeability and serum endotoxin levels by restoring the expression of colonic epithelial TJ proteins (ZO-1, occludin, and claudin-1), thereby limiting the translocation of pathogenic bacteria from the intestinal mucosa to peripheral immune organs. Further immunological analyses revealed that DMD modulates the subpopulation ratio of ILC3 cells in the intestine by increasing NCR+ ILC3 cells, which secrete the repair factor IL-22, and by inhibiting NCR− ILC3 cells, which secrete inflammatory factors such as IL-17A and GM-CSF. In vitro experiments also confirmed that conditioned medium from DMD-treated cells promoted Caco-2 cell migration and enhanced TJ protein expression, thereby strengthening epithelial barrier function (Huang et al., 2022).
Banxia Xiexin Decoction (BXD) originates from Zhang Zhongjing’s “Treatise on Typhoid Miscellaneous Diseases” during the Eastern Han Dynasty, and modern studies have confirmed its multi-targeted effects on regulating gastrointestinal function. A recent study found that BXD upregulates the expression of TJ proteins (ZO-1 and occludin) in the colon, thereby repairing the damaged intestinal barrier. In addition, BXD modulates the composition and diversity of the intestinal microbiota by significantly increasing the abundance of beneficial bacteria (e.g., Muribaculaceae, Akkermansia, and Lactobacillus) and inhibiting inflammation-associated flora (e.g., Faecalibaculum and Alloprevotella), thereby improving key metabolic pathways, including those involving SCFAs. Fecal transplantation experiments further verified that microbiota remodeling plays a key role in its anti-UC effects. Overall, BXD achieves combined anti-inflammatory and mucosal repair effects by restoring intestinal microecological balance and regulating the metabolic network (Luo et al., 2024).
Baitouweng Decoction (BD) is a TCM compound with a long history of clinical application, and modern studies have confirmed its favorable therapeutic effect in treating UC (Li X. et al., 2023). Studies have shown that BD reduces pro-inflammatory factors (e.g., IL-1β, IL-6, and TNF-α) while elevating the expression of the anti-inflammatory factor IL-10 by inhibiting Th17 cell differentiation and promoting Treg cell expansion. Regarding intestinal barrier protection, BD significantly reduces intestinal permeability by inhibiting the phosphorylation of the ERK/NF-κB signaling pathway and promoting the synthesis of TJ proteins, including occludin and ZO-1 (Miao et al., 2020). Further studies found that BD remodels the intestinal microbiota, resulting in a decreased abundance of Proteobacteria, elevated production of SCFAs, and modulated mucosal immunity via inhibition of the IL-6/STAT3 pathway (Chen et al., 2021) (Table 2).
4 Conclusion and perspectives
In this paper, we systematically review the key mechanisms of immune dysregulation in the pathogenesis of UC, encompassing pathological aspects such as dysregulation of T-cell subsets, hypersecretion of pro-inflammatory cytokines, imbalance in the gut microbiota, and disruption of the intestinal barrier that synergistically contribute to the onset and progression of UC. Current studies have shown that immune dysregulation in UC is not confined solely to local inflammatory responses but also entails complex alterations in systemic immune regulation and metabolic networks. The overactivation of Th1 and Th17 cells and the insufficiency of Treg function amplify local pro-inflammatory signals; simultaneously, pro-inflammatory factors (e.g., TNF-α, IL-6, IL-17) activate signaling pathways such as NF-κB and MAPK, thereby aggravating epithelial cell apoptosis and TJ disruption, ultimately leading to a ‘leaky gut’ that facilitates the invasion of pathogenic bacteria and their products. In addition, gut microbiota imbalance not only alters bacterial composition but also modulates intestinal immune homeostasis via metabolites such as SCFAs, BAs, and Trp derivatives, whose dysregulation is closely associated with UC inflammation and prognosis. These findings offer novel insights into the pathomechanisms of UC and pave the way for precision treatment. As a traditional medical system, TCM has demonstrated unique advantages in the prevention and treatment of UC through multi-target and multi-pathway mechanisms, including immune regulation, anti-inflammatory repair, and flora remodeling. Both single compounds (e.g., berberine, matrine, curcumin) and compound formulations (e.g., Shenling Baizhu Powder, Sishen pills, Shaoyao decoction) ameliorate the inflammatory state and pathological alterations of UC by synergistically modulating the immune network, repairing intestinal barriers, and remodeling bacterial flora structure. This multilevel, multidimensional regulatory mechanism not only effectively alleviates local inflammation but also facilitates comprehensive disease intervention through systemic immune modulation.
Although clinical and experimental studies on TCM for UC treatment have yielded promising results, many urgent challenges remain. First, the mechanisms underlying TCM herbal formulas and their active ingredients are relatively complex, and the precise elucidation of their multi-target effects at the molecular level requires further investigation using systems biology, network pharmacology, and multi-omics technologies. Secondly, existing clinical trials are relatively small in scale, and there is a lack of large-scale, multicenter randomized controlled studies to validate the long-term efficacy and safety of TCM. In addition, the quality of Chinese herbal medicines varies across different origins and batches, necessitating the development of standardized formulations. Therefore, there is an urgent need to establish stringent quality control systems and standardized evaluation indices to enhance the reproducibility and reliability of Chinese medicine research. Modern molecular biology and systems biology can facilitate an in-depth analysis of the multi-component, multi-target regulation of the immune network by TCM, thereby revealing the scientific connotation. Simultaneously, integrating clinical data with multicenter randomized trials can further elucidate the synergistic effects of Chinese medicine when combined with conventional treatments, thereby providing a basis for developing individualized and precise treatment plans. Given the unique advantages of TCM in modulating intestinal flora, repairing intestinal barriers, and balancing immune cells, there is significant potential for exploring combination therapies. For example, TCM could be developed in conjunction with monoclonal antibodies targeting various cytokines or with inhibitors of STAT or JAK pathways. This approach could enhance the therapeutic efficacy of UC treatment by leveraging the distinct mechanisms of action of TCM alongside conventional therapies. In summary, the combined use of TCM with probiotics, metabolic modulators, and other novel therapeutic approaches warrants future exploration. Such multimodal interventions could advance UC treatment toward comprehensive regulation and improved patient outcomes.
In conclusion, this article has comprehensively explored the roles of dysregulation of T-cell subsets, hypersecretion of pro-inflammatory cytokines, imbalance in the gut microbiota, and disruption of the intestinal barrier in UC pathogenesis, thereby providing a solid theoretical foundation for elucidating its pathophysiological mechanisms. Moreover, Chinese medicine, with its unique multi-component, multi-target, and holistic regulatory advantages, has demonstrated irreplaceable efficacy in preventing and treating ulcerative colitis by modulating the immune network, repairing the intestinal barrier, and reshaping intestinal flora ecology. In the future, systems biology, multi-omics technologies, and large-scale clinical trials will further elucidate the multilevel regulatory mechanisms of TCM and promote the translation of ulcerative colitis treatment toward comprehensive intervention and individualized precision therapy.
Author contributions
XT: Conceptualization, Investigation, Supervision, Visualization, Writing – original draft. YH: Conceptualization, Formal Analysis, Investigation, Writing – original draft. YZ: Funding acquisition, Supervision, Writing – review and editing. YX: Funding acquisition, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the National Natural Science Foundation of China (82374426), the Domestic First-class Construction Discipline of Chinese Medicine in Hunan University of Chinese Medicine, the Construction Project of Inheritance Studio of National Famous Traditional Chinese Medicine Experts of National Administration of Traditional Chinese Medicine ((2022) No. 75), and the Scientific Research Project of Education Department of Hunan Province (23C0170).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aebisher, D., Bartusik-Aebisher, D., Przygórzewska, A., Oleś, P., Woźnicki, P., and Kawczyk-Krupka, A. (2024). Key interleukins in inflammatory bowel disease-A review of recent studies. Int. J. Mol. Sci. 26, 121. doi:10.3390/ijms26010121
Agus, A., Planchais, J., and Sokol, H. (2018). Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 23, 716–724. doi:10.1016/j.chom.2018.05.003
Akhtar, M., Chen, Y., Ma, Z., Zhang, X., Shi, D., Khan, J. A., et al. (2022). Gut microbiota-derived short chain fatty acids are potential mediators in gut inflammation. Anim. Nutr. 8, 350–360. doi:10.1016/j.aninu.2021.11.005
Aleebrahim-Dehkordi, E., Heidari-Soureshjani, E., Aryan, A., Ganjirad, Z., Soveyzi, F., Hoseinsalari, A., et al. (2022). Antiviral compounds based on natural Astragalus polysaccharides (APS): research and foresight in the strategies for combating SARS-CoV-2 (COVID-19). Mini Rev. Med. Chem. 22, 2299–2307. doi:10.2174/1389557522666220301143113
Alexeev, E. E., Lanis, J. M., Kao, D. J., Campbell, E. L., Kelly, C. J., Battista, K. D., et al. (2018). Microbiota-derived indole metabolites promote human and murine intestinal homeostasis through regulation of interleukin-10 receptor. Am. J. Pathol. 188, 1183–1194. doi:10.1016/j.ajpath.2018.01.011
Al-Sadi, R., Nighot, P., Nighot, M., Haque, M., Rawat, M., and Ma, T. Y. (2021). Lactobacillus acidophilus induces a strain-specific and toll-like receptor 2-dependent enhancement of intestinal epithelial tight junction barrier and protection against intestinal inflammation. Am. J. Pathol. 191, 872–884. doi:10.1016/j.ajpath.2021.02.003
Ananthakrishnan, A. N. (2015). Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 12, 205–217. doi:10.1038/nrgastro.2015.34
Ansaldo, E., Farley, T. K., and Belkaid, Y. (2021). Control of immunity by the microbiota. Annu. Rev. Immunol. 39, 449–479. doi:10.1146/annurev-immunol-093019-112348
Atarashi, K., Suda, W., Luo, C., Kawaguchi, T., Motoo, I., Narushima, S., et al. (2017). Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science 358, 359–365. doi:10.1126/science.aan4526
Ayansola, H., Mayorga, E. J., and Jin, Y. (2024). Subepithelial stromal cells: their roles and interactions with intestinal epithelial cells during gut mucosal homeostasis and regeneration. Biomedicines 12, 668. doi:10.3390/biomedicines12030668
Azizollah, N., Sharifinejad, N., Mozhgani, S.-H., Mousavian, S. M., Bakhtiyari, M., and Mahmoudi, E. (2024). Possible role of intestinal fungal dysbiosis in dectin-1 and cytokines expression in patients with ulcerative colitis. Indian J. Gastroenterol. 43, 832–840. doi:10.1007/s12664-024-01605-2
Bai, G.-H., Lin, S.-C., Hsu, Y.-H., and Chen, S.-Y. (2022). The human virome: viral metagenomics, relations with human diseases, and therapeutic applications. Viruses 14, 278. doi:10.3390/v14020278
Bajaj, A., Markandey, M., Kedia, S., and Ahuja, V. (2024). Gut bacteriome in inflammatory bowel disease: an update on recent advances. Indian J. Gastroenterol. 43, 103–111. doi:10.1007/s12664-024-01541-1
Baumann, C., Bonilla, W. V., Fröhlich, A., Helmstetter, C., Peine, M., Hegazy, A. N., et al. (2015). T-bet- and STAT4-dependent IL-33 receptor expression directly promotes antiviral Th1 cell responses. Proc. Natl. Acad. Sci. U. S. A. 112, 4056–4061. doi:10.1073/pnas.1418549112
Biedermann, L., Doulberis, M., Schreiner, P., Nielsen, O. H., The, F. O., Brand, S., et al. (2024). Efficacy and safety of anthocyanin-rich extract in patients with ulcerative colitis: a randomized controlled trial. Nutrients 16, 4197. doi:10.3390/nu16234197
Blaak, E. E., Canfora, E. E., Theis, S., Frost, G., Groen, A. K., Mithieux, G., et al. (2020). Short chain fatty acids in human gut and metabolic health. Benef. Microbes 11, 411–455. doi:10.3920/BM2020.0057
Boivin, M. A., Roy, P. K., Bradley, A., Kennedy, J. C., Rihani, T., and Ma, T. Y. (2009). Mechanism of interferon-gamma-induced increase in T84 intestinal epithelial tight junction. J. Interferon Cytokine Res. 29, 45–54. doi:10.1089/jir.2008.0128
Buie, M. J., Quan, J., Windsor, J. W., Coward, S., Hansen, T. M., King, J. A., et al. (2023). Global hospitalization trends for crohn’s disease and ulcerative colitis in the 21st century: a systematic review with temporal analyses. Clin. Gastroenterol. Hepatol. 21, 2211–2221. doi:10.1016/j.cgh.2022.06.030
Cai, J., Liu, J., Fan, P., Dong, X., Zhu, K., Liu, X., et al. (2021). Dioscin prevents DSS-induced colitis in mice with enhancing intestinal barrier function and reducing colon inflammation. Int. Immunopharmacol. 99, 108015. doi:10.1016/j.intimp.2021.108015
Cai, J., Sun, L., and Gonzalez, F. J. (2022). Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe 30, 289–300. doi:10.1016/j.chom.2022.02.004
Cao, H., Liu, H., Dai, X., Shi, B., Yuan, J., Shan, J., et al. (2025). Qingchang suppository ameliorates mucosal inflammation in ulcerative colitis by inhibiting the differentiation and effector functions of Th1 and Th17 cells. J. Ethnopharmacol. 337, 118865. doi:10.1016/j.jep.2024.118865
Cartwright, I. M., Zhou, L., Koch, S. D., Welch, N., Zakharov, D., Callahan, R., et al. (2024). Chlorination of epithelial tight junction proteins by neutrophil myeloperoxidase promotes barrier dysfunction and mucosal inflammation. JCI Insight 9, e178525. doi:10.1172/jci.insight.178525
Chen, C.-Y., and Ho, H.-C. (2023). Roles of gut microbes in metabolic-associated fatty liver disease. Tzu Chi Med. J. 35, 279–289. doi:10.4103/tcmj.tcmj_86_23
Chen, F., Yang, W., Huang, X., Cao, A. T., Bilotta, A. J., Xiao, Y., et al. (2018). Neutrophils promote amphiregulin production in intestinal epithelial cells through TGF-β and contribute to intestinal homeostasis. J. Immunol. 201, 2492–2501. doi:10.4049/jimmunol.1800003
Chen, J., Shen, B., and Jiang, Z. (2022). Traditional Chinese medicine prescription Shenling BaiZhu powder to treat ulcerative colitis: clinical evidence and potential mechanisms. Front. Pharmacol. 13, 978558. doi:10.3389/fphar.2022.978558
Chen, J., Zhang, H., Fu, T., Zhao, J., Nowak, J. K., Kalla, R., et al. (2024a). Exposure to air pollution increases susceptibility to ulcerative colitis through epigenetic alterations in CXCR2 and MHC class III region. EBioMedicine 110, 105443. doi:10.1016/j.ebiom.2024.105443
Chen, W., Zhou, T., Liu, Y., Luo, L., Ye, Y., Wei, L., et al. (2025). Genetically engineered bacteria expressing IL-34 alleviate DSS-induced experimental colitis by promoting tight junction protein expression in intestinal mucosal epithelial cells. Mol. Immunol. 178, 64–75. doi:10.1016/j.molimm.2025.01.008
Chen, X., Li, P., Liu, M., Zheng, H., He, Y., Chen, M.-X., et al. (2020). Gut dysbiosis induces the development of pre-eclampsia through bacterial translocation. Gut 69, 513–522. doi:10.1136/gutjnl-2019-319101
Chen, X., Zhang, Y., Huang, W., Zhang, Y., Kong, W., and Zhou, Z. (2024b). Effects of moxibustion on intestinal barrier function and TLR4/NF-κB p65 signaling pathway in obese rats. Zhongguo Zhen Jiu 44, 449–454. doi:10.13703/j.0255-2930.20230529-k0003
Chen, X., Zhou, Q., Wang, B., Feng, D., Jiang, R., and Wang, X. (2024c). Efficacy and safety of tanshinone IIA in combination with mesalazine in the treatment of ulcerative colitis: a Systematic review and meta-analysis. BMC Gastroenterol. 24, 410. doi:10.1186/s12876-024-03496-1
Chen, X.-Q., Xiang-Yu, L. V., and Shi-Jia, L. I. U. (2021). Baitouweng decoction alleviates dextran sulfate sodium-induced ulcerative colitis by regulating intestinal microbiota and the IL-6/STAT3 signaling pathway. J. Ethnopharmacol. 265, 113357. doi:10.1016/j.jep.2020.113357
Cheng, C., Zhang, W., Zhang, C., Ji, P., Wu, X., Sha, Z., et al. (2021). Hyperoside ameliorates DSS-induced colitis through MKRN1-mediated regulation of PPARγ signaling and Th17/treg balance. J. Agric. Food Chem. 69, 15240–15251. doi:10.1021/acs.jafc.1c06292
Cheng, H., Yang, Y., Hu, J., Chen, L., Yuan, M., Du, H., et al. (2024a). Cyclic adenosine 3’, 5’-monophosphate (cAMP) signaling is a crucial therapeutic target for ulcerative colitis. Life Sci. 353, 122901. doi:10.1016/j.lfs.2024.122901
Cheng, S., Li, H., Huang, Y., Su, Y., Li, Y., Jia, A., et al. (2022). Lactobacillus gasseri JM1 isolated from infant feces alleviates colitis in mice via protecting the intestinal barrier. Nutrients 15, 139. doi:10.3390/nu15010139
Cheng, W., Zhu, N., Wang, J., and Yang, R. (2024b). A role of gut microbiota metabolites in HLA-E and NKG2 blockage immunotherapy against tumors: new insights for clinical application. Front. Immunol. 15, 1331518. doi:10.3389/fimmu.2024.1331518
Constantin, J., Atanasov, P., Wirth, D., and Borsi, A. (2019). Indirect costs associated with ulcerative colitis: a systematic literature review of real-world data. BMC Gastroenterol. 19, 179. doi:10.1186/s12876-019-1095-9
Cui, H., Wang, N., Li, H., Bian, Y., Wen, W., Kong, X., et al. (2024). The dynamic shifts of IL-10-producing Th17 and IL-17-producing Treg in health and disease: a crosstalk between ancient “Yin-Yang” theory and modern immunology. Cell Commun. Signal 22, 99. doi:10.1186/s12964-024-01505-0
Dai, N., Haidar, O., Askari, A., and Segal, J. P. (2023). Colectomy rates in ulcerative colitis: a systematic review and meta-analysis. Dig. Liver Dis. 55, 13–20. doi:10.1016/j.dld.2022.08.039
Deng, W., Yi, P., Xiong, Y., Ying, J., Lin, Y., Dong, Y., et al. (2024). Gut metabolites acting on the gut-brain Axis: regulating the functional state of microglia. Aging Dis. 15, 480–502. doi:10.14336/AD.2023.0727
Du, L., and Ha, C. (2020). Epidemiology and pathogenesis of ulcerative colitis. Gastroenterol. Clin. North Am. 49, 643–654. doi:10.1016/j.gtc.2020.07.005
Elia, G., and Guglielmi, G. (2018). CXCL9 chemokine in ulcerative colitis. Clin. Ter. 169, e235–e241. doi:10.7417/CT.2018.2085
Fan, Q., Guan, X., Hou, Y., Liu, Y., Wei, W., Cai, X., et al. (2020). Paeoniflorin modulates gut microbial production of indole-3-lactate and epithelial autophagy to alleviate colitis in mice. Phytomedicine 79, 153345. doi:10.1016/j.phymed.2020.153345
Fleming, A., Castro-Dopico, T., and Clatworthy, M. R. (2022). B cell class switching in intestinal immunity in health and disease. Scand. J. Immunol. 95, e13139. doi:10.1111/sji.13139
Franzosa, E. A., Sirota-Madi, A., Avila-Pacheco, J., Fornelos, N., Haiser, H. J., Reinker, S., et al. (2019). Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 4, 293–305. doi:10.1038/s41564-018-0306-4
Fu, J., Chang, C., Ren, J., Cheng, J., Gao, Z., and Meng, Y. (2025). The structure characterization of polysaccharides from Codonopsis pilosula and the structure-activity relationship with immune-regulation on THP-1 cells. Chem. Biodivers. 22, e202401167. doi:10.1002/cbdv.202401167
Fu, J., Zang, Y., Zhou, Y., Chen, C., Shao, S., Shi, G., et al. (2021). Exploring a novel triptolide derivative possess anti-colitis effect via regulating T cell differentiation. Int. Immunopharmacol. 94, 107472. doi:10.1016/j.intimp.2021.107472
Fu, S.-H., Chien, M.-W., Hsu, C.-Y., Liu, Y.-W., and Sytwu, H.-K. (2020). Interplay between cytokine circuitry and transcriptional regulation shaping helper T cell pathogenicity and plasticity in inflammatory bowel disease. Int. J. Mol. Sci. 21, 3379. doi:10.3390/ijms21093379
Fujino, S., Andoh, A., Bamba, S., Ogawa, A., Hata, K., Araki, Y., et al. (2003). Increased expression of interleukin 17 in inflammatory bowel disease. Gut 52, 65–70. doi:10.1136/gut.52.1.65
Furuse, M., Hirase, T., Itoh, M., Nagafuchi, A., Yonemura, S., Tsukita, S., et al. (1993). Occludin: a novel integral membrane protein localizing at tight junctions. J. Cell Biol. 123, 1777–1788. doi:10.1083/jcb.123.6.1777
Gao, B. B., Wang, L., Li, L. Z., Fei, Z. Q., Wang, Y. Y., Zou, X. M., et al. (2024). Beneficial effects of oxymatrine from Sophora flavescens on alleviating Ulcerative colitis by improving inflammation and ferroptosis. J. Ethnopharmacol. 332, 118385. doi:10.1016/j.jep.2024.118385
Gerges, S. H., Tolba, M. F., Elsherbiny, D. A., and El-Demerdash, E. (2020). The natural flavonoid galangin ameliorates dextran sulphate sodium-induced ulcerative colitis in mice: effect on Toll-like receptor 4, inflammation and oxidative stress. Basic Clin. Pharmacol. Toxicol. 127, 10–20. doi:10.1111/bcpt.13388
Ghosh, R., Smith, S. A., Nwangwa, E. E., Arivett, B. A., Bryant, D. L., Fuller, M. L., et al. (2019). Panax quinquefolius (North American ginseng) cell suspension culture as a source of bioactive polysaccharides: immunostimulatory activity and characterization of a neutral polysaccharide AGC1. Int. J. Biol. Macromol. 139, 221–232. doi:10.1016/j.ijbiomac.2019.07.215
Gomez-Bris, R., Saez, A., Herrero-Fernandez, B., Rius, C., Sanchez-Martinez, H., and Gonzalez-Granado, J. M. (2023). CD4 T-cell subsets and the pathophysiology of inflammatory bowel disease. Int. J. Mol. Sci. 24, 2696. doi:10.3390/ijms24032696
Gong, S., Xu, R., Wang, Y., Mao, S., Zhang, Y., Bu, Q., et al. (2025). Danggui beimu kushen pill alleviates colitis-induced inflammation in mice by regulating the IL-6/IL-6R and IL-17a/IL-17ra signaling pathways. Pharm. (Basel) 18, 141. doi:10.3390/ph18020141
Gottlieb, Z. S., and Sands, B. E. (2022). Personalised medicine with IL-23 blockers: myth or reality? J. Crohns Colitis 16, ii73–ii94. doi:10.1093/ecco-jcc/jjab190
Guan, W., and Qi, W. (2023). Ginsenoside Rh2: a shining and potential natural product in the treatment of human nonmalignant and malignant diseases in the near future. Phytomedicine 118, 154938. doi:10.1016/j.phymed.2023.154938
Guo, J., Zhang, Y.-Y., Sun, M., and Xu, L.-F. (2022). Therapeutic potential of curcumin in a rat model of dextran sulfate sodium-induced ulcerative colitis by regulating the balance of Treg/Th17 cells. Inflammation 45, 2163–2171. doi:10.1007/s10753-022-01678-1
Guo, M., Shao, S., Wang, D., Zhao, D., and Wang, M. (2021b). Recent progress in polysaccharides from Panax ginseng C. A. Meyer. Food Funct. 12, 494–518. doi:10.1039/d0fo01896a
Guo, N., and Lv, L.-L. (2023). Mechanistic insights into the role of probiotics in modulating immune cells in ulcerative colitis. Immun. Inflamm. Dis. 11, e1045. doi:10.1002/iid3.1045
Guo, S., Geng, W., Chen, S., Wang, L., Rong, X., Wang, S., et al. (2021a). Ginger alleviates DSS-induced ulcerative colitis severity by improving the diversity and function of gut microbiota. Front. Pharmacol. 12, 632569. doi:10.3389/fphar.2021.632569
Gustafsson, J. K., and Hansson, G. C. (2025). Immune regulation of goblet cell and mucus functions in health and disease. Annu. Rev. Immunol. 43, 169–189. doi:10.1146/annurev-immunol-101721-065224
Hang, S., Paik, D., Yao, L., Kim, E., Trinath, J., Lu, J., et al. (2019). Bile acid metabolites control TH17 and Treg cell differentiation. Nature 576, 143–148. doi:10.1038/s41586-019-1785-z
Harrell, C. R., Jovicic, N., Djonov, V., Arsenijevic, N., and Volarevic, V. (2019). Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells 8, 1605. doi:10.3390/cells8121605
He, C., Gao, H., Xin, S., Hua, R., Guo, X., Han, Y., et al. (2023). View from the biological property: insight into the functional diversity and complexity of the gut mucus. Int. J. Mol. Sci. 24, 4227. doi:10.3390/ijms24044227
He, X.-Y., Gao, Z.-Y., Liang, W., and Sun, Y.-C. (2022). Ameliorative effect of ginsenoside Rg1 on dextran sulfate sodium-induced colitis: involvement of intestinal barrier remodeling in mice. Ann. Transl. Med. 10, 1328. doi:10.21037/atm-22-5467
He, Y., Hara, H., and Núñez, G. (2016). Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem. Sci. 41, 1012–1021. doi:10.1016/j.tibs.2016.09.002
Hodgkinson, K., El Abbar, F., Dobranowski, P., Manoogian, J., Butcher, J., Figeys, D., et al. (2023). Butyrate’s role in human health and the current progress towards its clinical application to treat gastrointestinal disease. Clin. Nutr. 42, 61–75. doi:10.1016/j.clnu.2022.10.024
Hsieh, C. S., Macatonia, S. E., Tripp, C. S., Wolf, S. F., O’Garra, A., and Murphy, K. M. (1993). Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 260, 547–549. doi:10.1126/science.8097338
Hsu, N.-Y., Nayar, S., Gettler, K., Talware, S., Giri, M., Alter, I., et al. (2023). NOX1 is essential for TNFα-induced intestinal epithelial ROS secretion and inhibits M cell signatures. Gut 72, 654–662. doi:10.1136/gutjnl-2021-326305
Hu, Q., Zhang, W., Wu, Z., Tian, X., Xiang, J., Li, L., et al. (2021). Baicalin and the liver-gut system: pharmacological bases explaining its therapeutic effects. Pharmacol. Res. 165, 105444. doi:10.1016/j.phrs.2021.105444
Hu, Y., Tang, J., Xie, Y., Xu, W., Zhu, W., Xia, L., et al. (2024). Gegen Qinlian decoction ameliorates TNBS-induced ulcerative colitis by regulating Th2/Th1 and Tregs/Th17 cells balance, inhibiting NLRP3 inflammasome activation and reshaping gut microbiota. J. Ethnopharmacol. 328, 117956. doi:10.1016/j.jep.2024.117956
Hua, M., Sun, Y., Shao, Z., Lu, J., Lu, Y., and Liu, Z. (2020). Functional soluble dietary fiber from ginseng residue: polysaccharide characterization, structure, antioxidant, and enzyme inhibitory activity. J. Food Biochem. 44, e13524. doi:10.1111/jfbc.13524
Huang, J., Wu, T., Zhong, Y., Huang, J., Kang, Z., Zhou, B., et al. (2023). Effect of curcumin on regulatory B cells in chronic colitis mice involving TLR/MyD88 signaling pathway. Phytother. Res. 37, 731–742. doi:10.1002/ptr.7656
Huang, J., Yang, Z., Li, Y., Chai, X., Liang, Y., Lin, B., et al. (2021). Lactobacillus paracasei R3 protects against dextran sulfate sodium (DSS)-induced colitis in mice via regulating Th17/Treg cell balance. J. Transl. Med. 19, 356. doi:10.1186/s12967-021-02943-x
Huang, J., Zhong, Y., Cheng, N., Zhang, Z., Huang, L., Song, L., et al. (2024b). Sishen pills inhibit inflammatory dendritic cell differentiation via miR-505-3p mediated E-cadherin downregulation in ulcerative colitis. Phytomedicine 135, 156035. doi:10.1016/j.phymed.2024.156035
Huang, Q.-T., Ma, X.-D., Zhang, J.-N., Lin, W.-X., Shen, X.-X., Huang, Z.-W., et al. (2025). A hepatic oxidative metabolite of palmatine ameliorates DSS-induced ulcerative colitis by regulating macrophage polarization through AMPK/NF-κB pathway. Am. J. Chin. Med. 53, 285–307. doi:10.1142/S0192415X25500119
Huang, S., Wang, X., Xie, X., Su, Y., Pan, Z., Li, Y., et al. (2022). Dahuang Mudan decoction repairs intestinal barrier in chronic colitic mice by regulating the function of ILC3. J. Ethnopharmacol. 299, 115652. doi:10.1016/j.jep.2022.115652
Huang, Y., Wu, Q., Li, S., Lin, X., Yang, S., Zhu, R., et al. (2024a). Harnessing nature’s pharmacy: investigating natural compounds as novel therapeutics for ulcerative colitis. Front. Pharmacol. 15, 1394124. doi:10.3389/fphar.2024.1394124
Inagaki, T., Moschetta, A., Lee, Y.-K., Peng, L., Zhao, G., Downes, M., et al. (2006). Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc. Natl. Acad. Sci. U. S. A. 103, 3920–3925. doi:10.1073/pnas.0509592103
Inciuraite, R., Gedgaudas, R., Lukosevicius, R., Tilinde, D., Ramonaite, R., Link, A., et al. (2024). Constituents of stable commensal microbiota imply diverse colonic epithelial cell reactivity in patients with ulcerative colitis. Gut Pathog. 16, 16. doi:10.1186/s13099-024-00612-0
Iyengar, P., Godoy-Brewer, G., Maniyar, I., White, J., Maas, L., Parian, A. M., et al. (2024). Herbal medicines for the treatment of active ulcerative colitis: a systematic review and meta-analysis. Nutrients 16, 934. doi:10.3390/nu16070934
Ji, W., Huo, Y., Zhang, Y., Qian, X., Ren, Y., Hu, C., et al. (2024a). Palmatine inhibits expression fat mass and obesity associated protein (FTO) and exhibits a curative effect in dextran sulfate sodium (DSS)-induced experimental colitis. Int. Immunopharmacol. 132, 111968. doi:10.1016/j.intimp.2024.111968
Ji, W., Zhang, Y., Qian, X., Hu, C., and Huo, Y. (2024b). Palmatine alleviates inflammation and modulates ferroptosis against dextran sulfate sodium (DSS)-induced ulcerative colitis. Int. Immunopharmacol. 143, 113396. doi:10.1016/j.intimp.2024.113396
Jia, J., Zheng, K., Shen, H., Yu, J., Zhu, P., Yan, S., et al. (2020). Qingchang Huashi granule ameliorates experimental colitis via restoring the dendritic cell-mediated Th17/Treg balance. BMC Complement. Med. Ther. 20, 291. doi:10.1186/s12906-020-03088-y
Jia, R., Zheng, H., Li, S., Chen, W., Yang, Y., Wu, H., et al. (2024a). QingChang-XiaoPi decoction ameliorates intestinal inflammation of ulcerative colitis by regulating the pathogenicity of Th17 cells. Phytomedicine 132, 155779. doi:10.1016/j.phymed.2024.155779
Jia, Y., Gengji, J., Gong, T., Zhang, Z., and Deng, L. (2024b). An amorphous solid dispersion of baicalin and its oral therapeutic effect on ulcerative colitis. Pharm. Res. 41, 2377–2389. doi:10.1007/s11095-024-03804-0
Jiang, J., and Hu, C. (2009). Evodiamine: a novel anti-cancer alkaloid from Evodia rutaecarpa. Molecules 14, 1852–1859. doi:10.3390/molecules14051852
Jin, Y., Wu, J., Huang, K., and Liang, Z. (2024). Heat-killed Saccharomyces boulardii alleviates dextran sulfate sodium-induced ulcerative colitis by restoring the intestinal barrier, reducing inflammation, and modulating the gut microbiota. Nutrients 16, 702. doi:10.3390/nu16050702
Jing, M., Wang, Y., and Xu, L. (2019). Andrographolide derivative AL-1 ameliorates dextran sodium sulfate-induced murine colitis by inhibiting NF-κB and MAPK signaling pathways. Oxid. Med. Cell Longev. 2019, 6138723. doi:10.1155/2019/6138723
Kalkan, A. E., BinMowyna, M. N., Raposo, A., Ahmad, M. F., Ahmed, F., Otayf, A. Y., et al. (2025). Beyond the gut: unveiling butyrate’s global health impact through gut health and dysbiosis-related conditions: a narrative review. Nutrients 17, 1305. doi:10.3390/nu17081305
Kaminsky, L. W., Al-Sadi, R., and Ma, T. Y. (2021). IL-1β and the intestinal epithelial tight junction barrier. Front. Immunol. 12, 767456. doi:10.3389/fimmu.2021.767456
Kaur, A., and Goggolidou, P. (2020). Ulcerative colitis: understanding its cellular pathology could provide insights into novel therapies. J. Inflamm. (Lond) 17, 15. doi:10.1186/s12950-020-00246-4
Kim, C. H. (2021). Control of lymphocyte functions by gut microbiota-derived short-chain fatty acids. Cell Mol. Immunol. 18, 1161–1171. doi:10.1038/s41423-020-00625-0
Kong, J., Xiang, Q., Ge, W., Wang, Y., Xu, F., and Shi, G. (2024). Network pharmacology mechanisms and experimental verification of licorice in the treatment of ulcerative colitis. J. Ethnopharmacol. 324, 117691. doi:10.1016/j.jep.2023.117691
Korn, T., Bettelli, E., Oukka, M., and Kuchroo, V. K. (2009). IL-17 and Th17 cells. Annu. Rev. Immunol. 27, 485–517. doi:10.1146/annurev.immunol.021908.132710
Korta, A., Kula, J., and Gomułka, K. (2023). The role of IL-23 in the pathogenesis and therapy of inflammatory bowel disease. Int. J. Mol. Sci. 24, 10172. doi:10.3390/ijms241210172
Kovtonyuk, L. V., and McCoy, K. D. (2023). Microbial metabolites and immunotherapy: Basic rationale and clinical indications. Semin. Immunol. 67, 101755. doi:10.1016/j.smim.2023.101755
Kuhn, K. A., Pedraza, I., and Demoruelle, M. K. (2014). Mucosal immune responses to microbiota in the development of autoimmune disease. Rheum. Dis. Clin. North Am. 40, 711–725. doi:10.1016/j.rdc.2014.07.013
Kumari, R., Ahuja, V., and Paul, J. (2013). Fluctuations in butyrate-producing bacteria in ulcerative colitis patients of North India. World J. Gastroenterol. 19, 3404–3414. doi:10.3748/wjg.v19.i22.3404
Kuo, W.-T., Zuo, L., Odenwald, M. A., Madha, S., Singh, G., Gurniak, C. B., et al. (2021). The tight junction protein ZO-1 is dispensable for barrier function but critical for effective mucosal repair. Gastroenterology 161, 1924–1939. doi:10.1053/j.gastro.2021.08.047
Langhorst, J., Koch, A. K., Voiss, P., Dobos, G. J., and Rueffer, A. (2020). Distinct patterns of short-chain fatty acids during flare in patients with ulcerative colitis under treatment with mesalamine or a herbal combination of myrrh, chamomile flowers, and coffee charcoal: secondary analysis of a randomized controlled trial. Eur. J. Gastroenterol. Hepatol. 32, 175–180. doi:10.1097/MEG.0000000000001582
Le Berre, C., Honap, S., and Peyrin-Biroulet, L. (2023). Ulcerative colitis. Lancet 402, 571–584. doi:10.1016/S0140-6736(23)00966-2
Leccese, G., Chiara, M., Dusetti, I., Noviello, D., Billard, E., Bibi, A., et al. (2024). AIEC-dependent pathogenic Th17 cell transdifferentiation in Crohn’s disease is suppressed by rfaP and ybaT deletion. Gut Microbes 16, 2380064. doi:10.1080/19490976.2024.2380064
Lee, S. J., In, G., Lee, J.-W., and Shin, K.-S. (2021). Elucidation of the microstructure of an immuno-stimulatory polysaccharide purified from Korean red ginseng using sequential hydrolysis. Int. J. Biol. Macromol. 186, 13–22. doi:10.1016/j.ijbiomac.2021.06.202
Lee, S. R., Lee, S., Moon, E., Park, H.-J., Park, H. B., and Kim, K. H. (2017). Bioactivity-guided isolation of anti-inflammatory triterpenoids from the sclerotia of Poria cocos using LPS-stimulated Raw264.7 cells. Bioorg Chem. 70, 94–99. doi:10.1016/j.bioorg.2016.11.012
Leng, Y., Zhang, X., Zhang, Q., Xia, J., Zhang, Y., Ma, C., et al. (2024). Gallic acid attenuates murine ulcerative colitis by promoting group 3 innate lymphocytes, affecting gut microbiota, and bile acid metabolism. J. Nutr. Biochem. 131, 109677. doi:10.1016/j.jnutbio.2024.109677
Letizia, M., Wang, Y.-H., Kaufmann, U., Gerbeth, L., Sand, A., Brunkhorst, M., et al. (2022). Store-operated calcium entry controls innate and adaptive immune cell function in inflammatory bowel disease. EMBO Mol. Med. 14, e15687. doi:10.15252/emmm.202215687
Li, A.-P., Li, Z.-Y., Sun, H.-F., Li, K., Qin, X.-M., and Du, G.-H. (2015). Comparison of two different astragali radix by a 1H NMR-based metabolomic approach. J. Proteome Res. 14, 2005–2016. doi:10.1021/pr501167u
Li, H., Fan, C., Lu, H., Feng, C., He, P., Yang, X., et al. (2020). Protective role of berberine on ulcerative colitis through modulating enteric glial cells-intestinal epithelial cells-immune cells interactions. Acta Pharm. Sin. B 10, 447–461. doi:10.1016/j.apsb.2019.08.006
Li, H., Gu, L., Zhong, Y., Chen, Y., Zhang, L., Zhang, A. R., et al. (2016). Administration of polysaccharide from Panax notoginseng prolonged the survival of H22 tumor-bearing mice. Onco Targets Ther. 9, 3433–3441. doi:10.2147/OTT.S79427
Li, H., Pu, X., Lin, Y., Yu, X., Li, J., Bo, L., et al. (2024b). Sijunzi decoction alleviates inflammation and intestinal epithelial barrier damage and modulates the gut microbiota in ulcerative colitis mice. Front. Pharmacol. 15, 1360972. doi:10.3389/fphar.2024.1360972
Li, H., Ye, X.-F., Su, Y.-S., He, W., Zhang, J.-B., Zhang, Q., et al. (2023a). Mechanism of acupuncture and moxibustion on promoting mucosal healing in ulcerative colitis. Chin. J. Integr. Med. 29, 847–856. doi:10.1007/s11655-022-3531-x
Li, M.-X., Li, M.-Y., Lei, J.-X., Wu, Y.-Z., Li, Z.-H., Chen, L.-M., et al. (2022b). Huangqin decoction ameliorates DSS-induced ulcerative colitis: role of gut microbiota and amino acid metabolism, mTOR pathway and intestinal epithelial barrier. Phytomedicine 100, 154052. doi:10.1016/j.phymed.2022.154052
Li, Q., Zheng, S., Niu, K., Qiao, Y., Liu, Y., Zhang, Y., et al. (2024a). Paeoniflorin improves ulcerative colitis via regulation of PI3K-AKT based on network pharmacology analysis. Exp. Ther. Med. 27, 125. doi:10.3892/etm.2024.12414
Li, X., Wang, Z., Gao, H., Xiao, Y., Li, M., Huang, Y., et al. (2023c). Pulsatillae radix extract alleviates DSS-induced colitis via modulating gut microbiota and inflammatory signaling pathway in mice. Heliyon 9, e21869. doi:10.1016/j.heliyon.2023.e21869
Li, Y., Han, L., Hou, B., and Ji, Y. (2023b). Novel ginsenoside monomer RT4 promotes colitis repair in mice by regulating miR-144-3p/SLC7A11 signaling pathway. Fundam. Clin. Pharmacol. 37, 1129–1138. doi:10.1111/fcp.12934
Li, Y., Jia, Y., Cui, T., and Zhang, J. (2021). IL-6/STAT3 signaling pathway regulates the proliferation and damage of intestinal epithelial cells in patients with ulcerative colitis via H3K27ac. Exp. Ther. Med. 22, 890. doi:10.3892/etm.2021.10322
Li, Y.-Y., Wang, X.-J., Su, Y.-L., Wang, Q., Huang, S.-W., Pan, Z.-F., et al. (2022a). Baicalein ameliorates ulcerative colitis by improving intestinal epithelial barrier via AhR/IL-22 pathway in ILC3s. Acta Pharmacol. Sin. 43, 1495–1507. doi:10.1038/s41401-021-00781-7
Li, Z., Chu, T., Sun, X., Zhuang, S., Hou, D., Zhang, Z., et al. (2025). Polyphenols-rich Portulaca oleracea L. (purslane) alleviates ulcerative colitis through restiring the intestinal barrier, gut microbiota and metabolites. Food Chem. 468, 142391. doi:10.1016/j.foodchem.2024.142391
Liang, J., Dai, W., Liu, C., Wen, Y., Chen, C., Xu, Y., et al. (2024). Gingerenone A attenuates ulcerative colitis via targeting IL-17ra to inhibit inflammation and restore intestinal barrier function. Adv. Sci. (Weinh) 11, e2400206. doi:10.1002/advs.202400206
Lin, C., Song, D., Wang, S., Chu, Y., Chi, C., Jia, S., et al. (2024). Polygonatum cyrtonema polysaccharides reshape the gut microbiota to ameliorate dextran sodium sulfate-induced ulcerative colitis in mice. Front. Pharmacol. 15, 1424328. doi:10.3389/fphar.2024.1424328
Liu, C., Wang, H., Han, L., Zhu, Y., Ni, S., Zhi, J., et al. (2024b). Targeting P2Y14R protects against necroptosis of intestinal epithelial cells through PKA/CREB/RIPK1 axis in ulcerative colitis. Nat. Commun. 15, 2083. doi:10.1038/s41467-024-46365-x
Liu, C., Wang, J., Yang, Y., Liu, X., Zhu, Y., Zou, J., et al. (2018). Ginsenoside Rd ameliorates colitis by inducing p62-driven mitophagy-mediated NLRP3 inflammasome inactivation in mice. Biochem. Pharmacol. 155, 366–379. doi:10.1016/j.bcp.2018.07.010
Liu, L., Dong, Y., Ye, M., Jin, S., Yang, J., Joosse, M. E., et al. (2017). The pathogenic role of NLRP3 inflammasome activation in inflammatory bowel diseases of both mice and humans. J. Crohns Colitis 11, 737–750. doi:10.1093/ecco-jcc/jjw219
Liu, S., Yan, W., Lv, Q., Yang, L., Miao, Y., Hu, Y., et al. (2023a). 3, 3’-diindolylmethane, a natural aryl hydrocarbon receptor agonist, alleviates ulcerative colitis by enhancing “glycolysis-lactate-STAT3” and TIP60 signals-mediated Treg differentiation. Mol. Immunol. 163, 147–162. doi:10.1016/j.molimm.2023.09.009
Liu, W., Yan, X., An, J., Wang, X., Mi, H., and Liu, F. (2025a). Modified Jiaoqi Powder enhances epithelial autophagy against TNF-triggered apoptosis in chronic ulcerative colitis. Phytomedicine 136, 155996. doi:10.1016/j.phymed.2024.155996
Liu, X., Zhang, M., Chen, S., Liu, H., Ma, H., Hu, T., et al. (2024a). Grifola frondosa polysaccharide’s therapeutic potential in oxazolone-induced ulcerative colitis. Carbohydr. Polym. 344, 122517. doi:10.1016/j.carbpol.2024.122517
Liu, X., Zhou, M., Dai, Z., Luo, S., Shi, Y., He, Z., et al. (2023b). Salidroside alleviates ulcerative colitis via inhibiting macrophage pyroptosis and repairing the dysbacteriosis-associated Th17/Treg imbalance. Phytother. Res. 37, 367–382. doi:10.1002/ptr.7636
Liu, Y., Xiao, H., Wang, Z., Pan, Q., Zhao, X., and Lu, B. (2025b). Interactions between dietary cholesterol and intestinal flora and their effects on host health. Crit. Rev. Food Sci. Nutr. 65, 494–506. doi:10.1080/10408398.2023.2276883
Liu, Z., Zhang, Y., Jin, T., Yi, C., Ocansey, D. K. W., and Mao, F. (2022). The role of NOD2 in intestinal immune response and microbiota modulation: a therapeutic target in inflammatory bowel disease. Int. Immunopharmacol. 113, 109466. doi:10.1016/j.intimp.2022.109466
Lloyd-Price, J., Arze, C., Ananthakrishnan, A. N., Schirmer, M., Avila-Pacheco, J., Poon, T. W., et al. (2019). Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 569, 655–662. doi:10.1038/s41586-019-1237-9
Lo, B. C., Shin, S. B., Canals Hernaez, D., Refaeli, I., Yu, H. B., Goebeler, V., et al. (2019). IL-22 preserves gut epithelial integrity and promotes disease remission during chronic Salmonella infection. J. Immunol. 202, 956–965. doi:10.4049/jimmunol.1801308
Lu, Y., Wu, Y., Sun, L., Yang, S., Kuang, H., Li, R., et al. (2024). Identifying the anti-inflammatory effects of Astragalus polysaccharides in anti-N-Methyl-D-Aspartate receptor encephalitis: network pharmacology and experimental validation. Comb. Chem. High. Throughput Screen 27, 1022–1032. doi:10.2174/1386207326666230816162113
Luo, Y., Fu, S., Liu, Y., Kong, S., Liao, Q., Lin, L., et al. (2024). Banxia Xiexin decoction modulates gut microbiota and gut microbiota metabolism to alleviate DSS-induced ulcerative colitis. J. Ethnopharmacol. 326, 117990. doi:10.1016/j.jep.2024.117990
Lv, L., Chen, Z., Bai, W., Hao, J., Heng, Z., Meng, C., et al. (2023). Taurohyodeoxycholic acid alleviates trinitrobenzene sulfonic acid induced ulcerative colitis via regulating Th1/Th2 and Th17/Treg cells balance. Life Sci. 318, 121501. doi:10.1016/j.lfs.2023.121501
Lv, Q., Shi, C., Qiao, S., Cao, N., Guan, C., Dai, Y., et al. (2018). Alpinetin exerts anti-colitis efficacy by activating AhR, regulating miR-302/DNMT-1/CREB signals, and therefore promoting Treg differentiation. Cell Death Dis. 9, 890. doi:10.1038/s41419-018-0814-4
Ma, K., Yi, X., Yang, S.-T., Zhu, H., Liu, T.-Y., Jia, S.-S., et al. (2024). Isolation, purification, and structural characterization of polysaccharides from Codonopsis pilosula and its therapeutic effects on non-alcoholic fatty liver disease in vitro and in vivo. Int. J. Biol. Macromol. 265, 130988. doi:10.1016/j.ijbiomac.2024.130988
Ma, Q., Li, Y., Li, P., Wang, M., Wang, J., Tang, Z., et al. (2019). Research progress in the relationship between type 2 diabetes mellitus and intestinal flora. Biomed. Pharmacother. 117, 109138. doi:10.1016/j.biopha.2019.109138
Ma, Y., Lang, X., Yang, Q., Han, Y., Kang, X., Long, R., et al. (2023). Paeoniflorin promotes intestinal stem cell-mediated epithelial regeneration and repair via PI3K-AKT-mTOR signalling in ulcerative colitis. Int. Immunopharmacol. 119, 110247. doi:10.1016/j.intimp.2023.110247
Mansouri, P., Mansouri, P., Behmard, E., Najafipour, S., Kouhpayeh, A., and Farjadfar, A. (2025). Novel targets for mucosal healing in inflammatory bowel disease therapy. Int. Immunopharmacol. 144, 113544. doi:10.1016/j.intimp.2024.113544
Mao, N., Yu, Y., He, J., Yang, Y., Liu, Z., Lu, Y., et al. (2024). Matrine ameliorates DSS-induced colitis by suppressing inflammation, modulating oxidative stress and remodeling the gut microbiota. Int. J. Mol. Sci. 25, 6613. doi:10.3390/ijms25126613
Mayne, C. G., and Williams, C. B. (2013). Induced and natural regulatory T cells in the development of inflammatory bowel disease. Inflamm. Bowel Dis. 19, 1772–1788. doi:10.1097/MIB.0b013e318281f5a3
Meng, Y., Xu, Y., Chang, C., Qiu, Z., Hu, J., Wu, Y., et al. (2020). Extraction, characterization and anti-inflammatory activities of an inulin-type fructan from Codonopsis pilosula. Int. J. Biol. Macromol. 163, 1677–1686. doi:10.1016/j.ijbiomac.2020.09.117
Miao, F., Shan, C., and Ning, D. (2021). Walnut oil alleviates LPS-induced intestinal epithelial cells injury by inhibiting TLR4/MyD88/NF-κB pathway activation. J. Food Biochem. 45, e13955. doi:10.1111/jfbc.13955
Miao, Z., Chen, L., Feng, H., Gu, M., Yan, J., Xu, Y., et al. (2020). Baitouweng decoction ameliorates ulcerative colitis in mice partially attributed to regulating Th17/treg balance and restoring intestinal epithelial barrier. Front. Pharmacol. 11, 531117. doi:10.3389/fphar.2020.531117
Mickael, M. E., Bhaumik, S., and Basu, R. (2020). Retinoid-related orphan receptor RORγt in CD4+ T-cell-mediated intestinal homeostasis and inflammation. Am. J. Pathol. 190, 1984–1999. doi:10.1016/j.ajpath.2020.07.010
Mirpuri, J., Raetz, M., Sturge, C. R., Wilhelm, C. L., Benson, A., Savani, R. C., et al. (2014). Proteobacteria-specific IgA regulates maturation of the intestinal microbiota. Gut Microbes 5, 28–39. doi:10.4161/gmic.26489
Monteleone, I., Sarra, M., Pallone, F., and Monteleone, G. (2012). Th17-related cytokines in inflammatory bowel diseases: friends or foes? Curr. Mol. Med. 12, 592–597. doi:10.2174/156652412800620066
Morvaridi, M., Aryaeian, N., Alavinejad, P., Seyedian, S. S., Ghafourian, M., Bakhtiari, N., et al. (2025). Zatariamultiflora hydroalcoholic extract: a triple-blind randomized controlled trial on immune genes, inflammation, and ulcerative colitis symptoms. J. Ethnopharmacol. 344, 119527. doi:10.1016/j.jep.2025.119527
Moudgil, K. D., and Venkatesha, S. H. (2022). The anti-inflammatory and immunomodulatory activities of natural products to control autoimmune inflammation. Int. J. Mol. Sci. 24, 95. doi:10.3390/ijms24010095
Nakase, H., Sato, N., Mizuno, N., and Ikawa, Y. (2022). The influence of cytokines on the complex pathology of ulcerative colitis. Autoimmun. Rev. 21, 103017. doi:10.1016/j.autrev.2021.103017
Nelson, K. M., Dahlin, J. L., Bisson, J., Graham, J., Pauli, G. F., and Walters, M. A. (2017). The essential medicinal chemistry of curcumin. J. Med. Chem. 60, 1620–1637. doi:10.1021/acs.jmedchem.6b00975
Ng, C. Y. J., Lai, N. P. Y., Ng, W. M., Siah, K. T. H., Gan, R.-Y., and Zhong, L. L. D. (2024). Chemical structures, extraction and analysis technologies, and bioactivities of edible fungal polysaccharides from Poria cocos: an updated review. Int. J. Biol. Macromol. 261, 129555. doi:10.1016/j.ijbiomac.2024.129555
Nikolaus, S., Schulte, B., Al-Massad, N., Thieme, F., Schulte, D. M., Bethge, J., et al. (2017). Increased tryptophan metabolism is associated with activity of inflammatory bowel diseases. Gastroenterology 153, 1504–1516. doi:10.1053/j.gastro.2017.08.028
Nishikawa, K., Seo, N., Torii, M., Ma, N., Muraoka, D., Tawara, I., et al. (2014). Interleukin-17 induces an atypical M2-like macrophage subpopulation that regulates intestinal inflammation. PLoS One 9, e108494. doi:10.1371/journal.pone.0108494
Niu, Z., Li, X., Yang, X., and Sun, Z. (2024). Protective effects of sinomenine against dextran sulfate sodium-induced ulcerative colitis in rats via alteration of HO-1/Nrf2 and inflammatory pathway. Inflammopharmacology 32, 2007–2022. doi:10.1007/s10787-024-01455-6
Nong, H., Yuan, H., Lin, Y., Chen, S., Li, Y., Luo, Z., et al. (2024). IL-22 promotes occludin expression by activating autophagy and treats ulcerative colitis. Mol. Cell Biochem. 479, 1443–1450. doi:10.1007/s11010-023-04806-z
Ou, W., Xu, W., Wang, Y., Hua, Z., Ding, W., Cui, L., et al. (2024). Cooperation of Wnt/β-catenin and Dll1-mediated Notch pathway in Lgr5-positive intestinal stem cells regulates the mucosal injury and repair in DSS-induced colitis mice model. Gastroenterol. Rep. (Oxf) 12, goae090. doi:10.1093/gastro/goae090
Owen, J. L., and Mohamadzadeh, M. (2013). Microbial activation of gut dendritic cells and the control of mucosal immunity. J. Interferon Cytokine Res. 33, 619–631. doi:10.1089/jir.2013.0046
Özdirik, B., Müller, T., Wree, A., Tacke, F., and Sigal, M. (2021). The role of microbiota in primary sclerosing cholangitis and related biliary malignancies. Int. J. Mol. Sci. 22, 6975. doi:10.3390/ijms22136975
Pan, S.-M., Wang, C.-L., Hu, Z.-F., Zhang, M.-L., Pan, Z.-F., Zhou, R.-Y., et al. (2024b). Baitouweng decoction repairs the intestinal barrier in DSS-induced colitis mice via regulation of AMPK/mTOR-mediated autophagy. J. Ethnopharmacol. 318, 116888. doi:10.1016/j.jep.2023.116888
Pan, X., Köberle, M., and Ghashghaeinia, M. (2024a). Vitamin C-dependent uptake of non-heme iron by enterocytes, its impact on erythropoiesis and redox capacity of human erythrocytes. Antioxidants (Basel) 13, 968. doi:10.3390/antiox13080968
Peng, K.-Y., Gu, J.-F., Su, S.-L., Zhu, Y., Guo, J.-M., Qian, D.-W., et al. (2021). Salvia miltiorrhiza stems and leaves total phenolic acids combination with tanshinone protect against DSS-induced ulcerative colitis through inhibiting TLR4/PI3K/AKT/mTOR signaling pathway in mice. J. Ethnopharmacol. 264, 113052. doi:10.1016/j.jep.2020.113052
Perera, A. P., Fernando, R., Shinde, T., Gundamaraju, R., Southam, B., Sohal, S. S., et al. (2018). MCC950, a specific small molecule inhibitor of NLRP3 inflammasome attenuates colonic inflammation in spontaneous colitis mice. Sci. Rep. 8, 8618. doi:10.1038/s41598-018-26775-w
Pernomian, L., Duarte-Silva, M., and de Barros Cardoso, C. R. (2020). The aryl hydrocarbon receptor (AHR) as a potential target for the control of intestinal inflammation: insights from an immune and bacteria sensor receptor. Clin. Rev. Allergy Immunol. 59, 382–390. doi:10.1007/s12016-020-08789-3
Pope, J. L., Bhat, A. A., Sharma, A., Ahmad, R., Krishnan, M., Washington, M. K., et al. (2014). Claudin-1 regulates intestinal epithelial homeostasis through the modulation of Notch-signalling. Gut 63, 622–634. doi:10.1136/gutjnl-2012-304241
Qiu, D., Xu, S., Ji, K., and Tang, C. (2024). Myeloid cell-derived IL-1 signaling damps neuregulin-1 from fibroblasts to suppress colitis-induced early repair of the intestinal epithelium. Int. J. Mol. Sci. 25, 4469. doi:10.3390/ijms25084469
Qiu, X., Zhang, M., Yang, X., Hong, N., and Yu, C. (2013). Faecalibacterium prausnitzii upregulates regulatory T cells and anti-inflammatory cytokines in treating TNBS-induced colitis. J. Crohns Colitis 7, e558–e568. doi:10.1016/j.crohns.2013.04.002
Rajilić-Stojanović, M., Smidt, H., and de Vos, W. M. (2007). Diversity of the human gastrointestinal tract microbiota revisited. Environ. Microbiol. 9, 2125–2136. doi:10.1111/j.1462-2920.2007.01369.x
Raju, P., Shashikanth, N., Tsai, P.-Y., Pongkorpsakol, P., Chanez-Paredes, S., Steinhagen, P. R., et al. (2020). Inactivation of paracellular cation-selective claudin-2 channels attenuates immune-mediated experimental colitis in mice. J. Clin. Invest 130, 5197–5208. doi:10.1172/JCI138697
Rana, M. N., Lu, J., Xue, E., Ruan, J., Liu, Y., Zhang, L., et al. (2021). PDE9 inhibitor PF-04447943 attenuates DSS-induced colitis by suppressing oxidative stress, inflammation, and regulating T-cell polarization. Front. Pharmacol. 12, 643215. doi:10.3389/fphar.2021.643215
Rao, K., Qin, S., Yang, Y., Zhan, K., Wu, H., Zheng, H., et al. (2022). Shenling Baizhu powder alleviates TNBS-induced colitis in rats by improving intestinal epithelial permeability and inhibiting inflammation through the TLR5/MyD88/NF-κB pathway. Front. Pharmacol. 13, 883918. doi:10.3389/fphar.2022.883918
Ratajczak, W., Rył, A., Mizerski, A., Walczakiewicz, K., Sipak, O., and Laszczyńska, M. (2019). Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs). Acta Biochim. Pol. 66, 1–12. doi:10.18388/abp.2018_2648
Rawat, M., Nighot, M., Al-Sadi, R., Gupta, Y., Viszwapriya, D., Yochum, G., et al. (2020). IL1B increases intestinal tight junction permeability by up-regulation of MIR200C-3p, which degrades occludin mRNA. Gastroenterology 159, 1375–1389. doi:10.1053/j.gastro.2020.06.038
Ren, D.-D., Chen, K.-C., Li, S.-S., Zhang, Y.-T., Li, Z.-M., Liu, S., et al. (2023). Panax quinquefolius polysaccharides ameliorate ulcerative colitis in mice induced by dextran sulfate sodium. Front. Immunol. 14, 1161625. doi:10.3389/fimmu.2023.1161625
Rossen, N. G., Fuentes, S., Boonstra, K., D’Haens, G. R., Heilig, H. G., Zoetendal, E. G., et al. (2015). The mucosa-associated microbiota of PSC patients is characterized by low diversity and low abundance of uncultured Clostridiales II. J. Crohns Colitis 9, 342–348. doi:10.1093/ecco-jcc/jju023
Ruan, S., Xu, L., Sheng, Y., Wang, J., Zhou, X., Zhang, C., et al. (2023). Th1 promotes M1 polarization of intestinal macrophages to regulate colitis-related mucosal barrier damage. Aging (Albany NY) 15, 6721–6735. doi:10.18632/aging.204629
Scalavino, V., Piccinno, E., Bianco, G., Schena, N., Armentano, R., Giannelli, G., et al. (2022). The increase of miR-195-5p reduces intestinal permeability in ulcerative colitis, modulating tight junctions’ expression. Int. J. Mol. Sci. 23, 5840. doi:10.3390/ijms23105840
Schardey, J., Globig, A.-M., Janssen, C., Hofmann, M., Manegold, P., Thimme, R., et al. (2019). Vitamin D inhibits pro-inflammatory T cell function in patients with inflammatory bowel disease. J. Crohns Colitis 13, 1546–1557. doi:10.1093/ecco-jcc/jjz090
Schroeder, B. O., and Bäckhed, F. (2016). Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 22, 1079–1089. doi:10.1038/nm.4185
Sengupta, S., and Sen, M. (2024). Requirement of a Wnt5A-microbiota axis in the maintenance of gut B-cell repertoire and protection from infection. mSphere 9, e0020424. doi:10.1128/msphere.00204-24
Sha, S., Zeng, H., Gao, H., Shi, H., Quan, X., Chen, F., et al. (2023). Adherent-invasive Escherichia coli LF82 aggravated intestinal inflammation in colitis mice by affecting the gut microbiota and Th17/Treg cell differentiation balance. Arch. Microbiol. 205, 218. doi:10.1007/s00203-023-03570-4
Shan, J., Ma, W., Guo, Y., Chang, X., Xie, J., Chen, Y., et al. (2024). Unveiling the immunomodulatory mechanism of polysaccharides from Polygonum cyrtonema based on RNA-seq. Food Res. Int. 175, 113755. doi:10.1016/j.foodres.2023.113755
Shen, L., Zhou, Y., He, H., Chen, W., Lenahan, C., Li, X., et al. (2021). Crosstalk between macrophages, T cells, and iron metabolism in tumor microenvironment. Oxid. Med. Cell Longev. 2021, 8865791. doi:10.1155/2021/8865791
Shen, Y., Zou, J., Chen, M., Zhang, Z., Liu, C., Jiang, S., et al. (2020). Protective effects of Lizhong decoction on ulcerative colitis in mice by suppressing inflammation and ameliorating gut barrier. J. Ethnopharmacol. 259, 112919. doi:10.1016/j.jep.2020.112919
Shen, Z.-H., Zhu, C.-X., Quan, Y.-S., Yang, Z.-Y., Wu, S., Luo, W.-W., et al. (2018). Relationship between intestinal microbiota and ulcerative colitis: mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J. Gastroenterol. 24, 5–14. doi:10.3748/wjg.v24.i1.5
Siddiqui, M. T., Han, Y., Shapiro, D., West, G., Fiocchi, C., and Cresci, G. A. M. (2024). The postbiotic butyrate mitigates gut mucosal disruption caused by acute ethanol exposure. Int. J. Mol. Sci. 25, 1665. doi:10.3390/ijms25031665
Singh, S. P., Chand, H. S., Banerjee, S., Agarwal, H., Raizada, V., Roy, S., et al. (2020). Acetylcholinesterase inhibitor pyridostigmine bromide attenuates gut pathology and bacterial dysbiosis in a murine model of ulcerative colitis. Dig. Dis. Sci. 65, 141–149. doi:10.1007/s10620-019-05838-6
Song, K., and Wu, D. (2022). Shared decision-making in the management of patients with inflammatory bowel disease. World J. Gastroenterol. 28, 3092–3100. doi:10.3748/wjg.v28.i26.3092
Song, X., Wang, W., Liu, L., Zhao, Z., Shen, X., Zhou, L., et al. (2024). Poria cocos attenuated DSS-induced ulcerative colitis via NF-κB signaling pathway and regulating gut microbiota. Molecules 29, 2154. doi:10.3390/molecules29092154
Song, Z., Qiao, Z., Liu, J., Han, L., Chen, X., and Wang, Y. (2025). Sea buckthorn berries alleviate ulcerative colitis via regulating gut Faecalibaculum rodentium-mediated butyrate biosynthesis. Phytomedicine 139, 156490. doi:10.1016/j.phymed.2025.156490
Sun, C., Zhu, D., Zhu, Q., He, Z., Lou, Y., and Chen, D. (2024a). The significance of gut microbiota in the etiology of autoimmune hepatitis: a narrative review. Front. Cell Infect. Microbiol. 14, 1337223. doi:10.3389/fcimb.2024.1337223
Sun, J., Wang, S., Zhao, Z., Lu, J., Zhang, Y., An, W., et al. (2024b). Oxymatrine attenuates ulcerative colitis through inhibiting pyroptosis mediated by the NLRP3 inflammasome. Molecules 29, 2897. doi:10.3390/molecules29122897
Suzuki, T. (2020). Regulation of the intestinal barrier by nutrients: the role of tight junctions. Anim. Sci. J. 91, e13357. doi:10.1111/asj.13357
Swanson, K. V., Deng, M., and Ting, J. P.-Y. (2019). The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 19, 477–489. doi:10.1038/s41577-019-0165-0
Swedik, S. M., Madola, A., Cruz, M. A., Llorens-Bonilla, B. J., and Levine, A. D. (2022). Th17-Derived cytokines synergistically enhance IL-17C production by the colonic epithelium. J. Immunol. 209, 1768–1777. doi:10.4049/jimmunol.2200125
Talà, A., Guerra, F., Resta, S. C., Calcagnile, M., Barca, A., Tredici, S. M., et al. (2022). Phenotyping of fecal microbiota of Winnie, a rodent model of spontaneous chronic colitis, reveals specific metabolic, genotoxic, and pro-inflammatory properties. Inflammation 45, 2477–2497. doi:10.1007/s10753-022-01706-0
Tang, B., Zhu, J., Zhang, B., Wu, F., Wang, Y., Weng, Q., et al. (2020). Therapeutic potential of triptolide as an anti-inflammatory agent in dextran sulfate sodium-induced murine experimental colitis. Front. Immunol. 11, 592084. doi:10.3389/fimmu.2020.592084
Tao, Y., Li, C., Gao, T., and Huo, J. (2022). Molecular mechanism of Sishen pills in the treatment of diarrheal diabetic enteropathy based on network pharmacology. Med. Baltim. 101, e30096. doi:10.1097/MD.0000000000030096
Thuner, J., and Coutant, F. (2023). IFN-γ: an overlooked cytokine in dermatomyositis with anti-MDA5 antibodies. Autoimmun. Rev. 22, 103420. doi:10.1016/j.autrev.2023.103420
Tian, J., Yang, C., Wang, Y., and Zhou, C. (2024). Evaluation of the mechanism of Sinomenii caulis in treating ulcerative colitis based on network pharmacology and molecular docking. Curr. Comput. Aided Drug Des. 20, 195–207. doi:10.2174/1573409919666230420083102
Tran, S., Juliani, J., Fairlie, W. D., and Lee, E. F. (2023). The emerging roles of autophagy in intestinal epithelial cells and its links to inflammatory bowel disease. Biochem. Soc. Trans. 51, 811–826. doi:10.1042/BST20221300
Trapecar, M., Communal, C., Velazquez, J., Maass, C. A., Huang, Y.-J., Schneider, K., et al. (2020). Gut-liver physiomimetics reveal paradoxical modulation of IBD-related inflammation by short-chain fatty acids. Cell Syst. 10, 223–239. doi:10.1016/j.cels.2020.02.008
Turner, J. R. (2009). Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809. doi:10.1038/nri2653
Ueno, A., Jeffery, L., Kobayashi, T., Hibi, T., Ghosh, S., and Jijon, H. (2018). Th17 plasticity and its relevance to inflammatory bowel disease. J. Autoimmun. 87, 38–49. doi:10.1016/j.jaut.2017.12.004
Van Itallie, C. M., and Anderson, J. M. (2006). Claudins and epithelial paracellular transport. Annu. Rev. Physiol. 68, 403–429. doi:10.1146/annurev.physiol.68.040104.131404
Veerasubramanian, P. K., Wynn, T. A., Quan, J., and Karlsson, F. J. (2024). Targeting TNF/TNFR superfamilies in immune-mediated inflammatory diseases. J. Exp. Med. 221, e20240806. doi:10.1084/jem.20240806
Vetrano, S., Rescigno, M., Cera, M. R., Correale, C., Rumio, C., Doni, A., et al. (2008). Unique role of junctional adhesion molecule-a in maintaining mucosal homeostasis in inflammatory bowel disease. Gastroenterology 135, 173–184. doi:10.1053/j.gastro.2008.04.002
Vogt, S. L., Peña-Díaz, J., and Finlay, B. B. (2015). Chemical communication in the gut: effects of microbiota-generated metabolites on gastrointestinal bacterial pathogens. Anaerobe 34, 106–115. doi:10.1016/j.anaerobe.2015.05.002
Wan, J., Wang, F., Xiao, Y., Cheng, Y., Zheng, S., Jiang, Q., et al. (2024c). Poria cocos polysaccharide alleviates dextran sulphate sodium-induced ulcerative colitis in mice by modulating intestinal inflammatory responses and microbial dysbiosis. Int. J. Biol. Macromol. 283, 137450. doi:10.1016/j.ijbiomac.2024.137450
Wan, L., Qian, C., Yang, C., Peng, S., Dong, G., Cheng, P., et al. (2024b). Ginseng polysaccharides ameliorate ulcerative colitis via regulating gut microbiota and tryptophan metabolism. Int. J. Biol. Macromol. 265, 130822. doi:10.1016/j.ijbiomac.2024.130822
Wan, Q., Huang, J., Xiao, Q., Zhang, Z., Zhang, Z., Huang, L., et al. (2024a). Astragalus polysaccharide alleviates ulcerative colitis by regulating the balance of mTh17/mTreg cells through TIGIT/CD155 signaling. Molecules 29, 241. doi:10.3390/molecules29010241
Wan, Y., Yang, L., Jiang, S., Qian, D., and Duan, J. (2022). Excessive apoptosis in ulcerative colitis: crosstalk between apoptosis, ROS, ER stress, and intestinal homeostasis. Inflamm. Bowel Dis. 28, 639–648. doi:10.1093/ibd/izab277
Wang, D., Doestzada, M., Chen, L., Andreu-Sánchez, S., van den Munckhof, I. C. L., Augustijn, H. E., et al. (2021). Characterization of gut microbial structural variations as determinants of human bile acid metabolism. Cell Host Microbe 29, 1802–1814.e5. doi:10.1016/j.chom.2021.11.003
Wang, H.-Y., Ge, W., Liu, S.-Q., Long, J., Jiang, Q.-Q., Zhou, W., et al. (2022). Curcumin inhibits T follicular helper cell differentiation in mice with dextran sulfate sodium (DSS)-Induced colitis. Am. J. Chin. Med. 50, 275–293. doi:10.1142/S0192415X22500100
Wang, J., He, M., Yang, M., and Ai, X. (2024d). Gut microbiota as a key regulator of intestinal mucosal immunity. Life Sci. 345, 122612. doi:10.1016/j.lfs.2024.122612
Wang, J., Hou, Y., Mu, L., Yang, M., and Ai, X. (2024a). Gut microbiota contributes to the intestinal and extraintestinal immune homeostasis by balancing Th17/Treg cells. Int. Immunopharmacol. 143, 113570. doi:10.1016/j.intimp.2024.113570
Wang, J., Jiang, Y., Wang, B., and Zhang, N. (2019). A review on analytical methods for natural berberine alkaloids. J. Sep. Sci. 42, 1794–1815. doi:10.1002/jssc.201800952
Wang, J., Zhu, N., Su, X., and Yang, R. (2024e). Gut microbiota: a double-edged sword in immune checkpoint blockade immunotherapy against tumors. Cancer Lett. 582, 216582. doi:10.1016/j.canlet.2023.216582
Wang, K., Liu, F., Muchu, B., Deng, J., Peng, J., Xu, Y., et al. (2024b). E3 ubiquitin ligase RNF180 mediates the ALKBH5/SMARCA5 axis to promote colon inflammation and Th17/Treg imbalance in ulcerative colitis mice. Arch. Pharm. Res. 47, 645–658. doi:10.1007/s12272-024-01507-z
Wang, K., Zhong, F., Zhang, Z.-D., Li, H.-Q., and Tian, S. (2024f). Recent advances in the development of P2Y14R inhibitors: a patent and literature review (2018-present). Expert Opin. Ther. Pat. 34, 611–625. doi:10.1080/13543776.2024.2369634
Wang, M.-X., Lin, L., Chen, Y.-D., Zhong, Y.-P., Lin, Y.-X., Li, P., et al. (2020). Evodiamine has therapeutic efficacy in ulcerative colitis by increasing Lactobacillus acidophilus levels and acetate production. Pharmacol. Res. 159, 104978. doi:10.1016/j.phrs.2020.104978
Wang, S.-L., Shao, B.-Z., Zhao, S.-B., Fang, J., Gu, L., Miao, C.-Y., et al. (2018). Impact of Paneth cell autophagy on inflammatory bowel disease. Front. Immunol. 9, 693. doi:10.3389/fimmu.2018.00693
Wang, T., Sun, H., Zhang, M., Shen, P., and Li, Y. (2024h). B3GNT7 regulates mucin O-glycosylation to alleviate colonic inflammation. BMC Gastroenterol. 24, 202. doi:10.1186/s12876-024-03287-8
Wang, X., Xie, X., Li, Y., Xie, X., Huang, S., Pan, S., et al. (2024g). Quercetin ameliorates ulcerative colitis by activating aryl hydrocarbon receptor to improve intestinal barrier integrity. Phytother. Res. 38, 253–264. doi:10.1002/ptr.8027
Wang, Y., Li, M., and Zha, A. (2024c). mTOR promotes an inflammatory response through the HIF1 signaling pathway in ulcerative colitis. Int. Immunopharmacol. 134, 112217. doi:10.1016/j.intimp.2024.112217
Wehkamp, J., Koslowski, M., Wang, G., and Stange, E. F. (2008). Barrier dysfunction due to distinct defensin deficiencies in small intestinal and colonic Crohn’s disease. Mucosal Immunol. 1 (Suppl. 1), S67–S74. doi:10.1038/mi.2008.48
Wei, C., Wang, J.-Y., Xiong, F., Wu, B.-H., Luo, M.-H., Yu, Z.-C., et al. (2021). Curcumin ameliorates DSS-induced colitis in mice by regulating the Treg/Th17 signaling pathway. Mol. Med. Rep. 23, 34. doi:10.3892/mmr.2020.11672
Wei, J., Chen, C., Feng, J., Zhou, S., Feng, X., Yang, Z., et al. (2023). Muc2 mucin O-glycosylation interacts with enteropathogenic Escherichia coli to influence the development of ulcerative colitis based on the NF-kB signaling pathway. J. Transl. Med. 21, 793. doi:10.1186/s12967-023-04687-2
Wilson, R., Gundamaraju, R., Vemuri, R., Angelucci, C., Geraghty, D., Gueven, N., et al. (2020). Identification of key pro-survival proteins in isolated colonic goblet cells of Winnie, a murine model of spontaneous colitis. Inflamm. Bowel Dis. 26, 80–92. doi:10.1093/ibd/izz179
Woznicki, J. A., Saini, N., Flood, P., Rajaram, S., Lee, C. M., Stamou, P., et al. (2021). TNF-α synergises with IFN-γ to induce caspase-8-JAK1/2-STAT1-dependent death of intestinal epithelial cells. Cell Death Dis. 12, 864. doi:10.1038/s41419-021-04151-3
Wu, D., Zhang, Y., Zou, B., Lu, Y., and Cao, H. (2023a). Shaoyao decoction alleviates TNBS-induced ulcerative colitis by decreasing inflammation and balancing the homeostasis of Th17/Treg cells. BMC Complement. Med. Ther. 23, 424. doi:10.1186/s12906-023-04237-9
Wu, Y., Zheng, Y., Wang, X., Tang, P., Guo, W., Ma, H., et al. (2023b). Ginseng-containing sijunzi decoction ameliorates ulcerative colitis by orchestrating gut homeostasis in microbial modulation and intestinal barrier integrity. Am. J. Chin. Med. 51, 677–699. doi:10.1142/S0192415X23500325
Xiao, S., Jing, S., Jiakui, S., Lei, Z., Ying, L., Han, L., et al. (2022). Butyrate ameliorates intestinal epithelial barrier injury via enhancing Foxp3+ regulatory T-cell function in severe acute pancreatitis model. Turk J. Gastroenterol. 33, 710–719. doi:10.5152/tjg.2022.21307
Xu, N., Bai, X., Cao, X., Yue, W., Jiang, W., and Yu, Z. (2021b). Changes in intestinal microbiota and correlation with TLRs in ulcerative colitis in the coastal area of northern China. Microb. Pathog. 150, 104707. doi:10.1016/j.micpath.2020.104707
Xu, X., Wang, Y., Wei, Z., Wei, W., Zhao, P., Tong, B., et al. (2017). Madecassic acid, the contributor to the anti-colitis effect of madecassoside, enhances the shift of Th17 toward Treg cells via the PPARγ/AMPK/ACC1 pathway. Cell Death Dis. 8, e2723. doi:10.1038/cddis.2017.150
Xu, Y., Cai, R., Zhao, Z., Zhou, L., Zhou, Q., Hassan, S., et al. (2021a). Thiomyristoyl ameliorates colitis by blocking the differentiation of Th17 cells and inhibiting SIRT2-induced metabolic reprogramming. Int. Immunopharmacol. 90, 107212. doi:10.1016/j.intimp.2020.107212
Xu, Y., Ou, J., Zhang, C., Chen, J., Chen, J., Li, A., et al. (2024). Rapamycin promotes the intestinal barrier repair in ulcerative colitis via the mTOR/PBLD/AMOT signaling pathway. Biochim. Biophys. Acta Mol. Basis Dis. 1870, 167287. doi:10.1016/j.bbadis.2024.167287
Xuan, H., Ou, A., Hao, S., Shi, J., and Jin, X. (2020). Galangin protects against symptoms of dextran sodium sulfate-induced acute colitis by activating autophagy and modulating the gut microbiota. Nutrients 12, 347. doi:10.3390/nu12020347
Xue, C., Zhang, X., Ge, H., Tang, Q., Jeon, J., Zhao, F., et al. (2023). Total flavone of flowers of Abelmoschus manihot (L.) Medic inhibits the expression of adhesion molecules in primary mesenteric arterial endothelial cells and ameliorates dextran sodium sulphate-induced ulcerative colitis in mice. Phytomedicine 112, 154713. doi:10.1016/j.phymed.2023.154713
Yang, T., Qin, N., Liu, F., Zhao, Y., Liu, W., and Fan, D. (2024b). Berberine regulates intestinal microbiome and metabolism homeostasis to treat ulcerative colitis. Life Sci. 338, 122385. doi:10.1016/j.lfs.2023.122385
Yang, W., Yu, T., Liu, X., Yao, S., Khanipov, K., Golovko, G., et al. (2024a). Microbial metabolite butyrate modulates granzyme B in tolerogenic IL-10 producing Th1 cells to regulate intestinal inflammation. Gut Microbes 16, 2363020. doi:10.1080/19490976.2024.2363020
Yang, X.-L., Guo, T.-K., Wang, Y.-H., Gao, M.-T., Qin, H., and Wu, Y.-J. (2012). Therapeutic effect of ginsenoside Rd in rats with TNBS-induced recurrent ulcerative colitis. Arch. Pharm. Res. 35, 1231–1239. doi:10.1007/s12272-012-0714-6
Yao, D., Dai, W., Dong, M., Dai, C., and Wu, S. (2021a). MUC2 and related bacterial factors: therapeutic targets for ulcerative colitis. EBioMedicine 74, 103751. doi:10.1016/j.ebiom.2021.103751
Yao, H., Shi, Y., Yuan, J., Sa, R., Chen, W., and Wan, X. (2021b). Matrine protects against DSS-induced murine colitis by improving gut barrier integrity, inhibiting the PPAR-α signaling pathway, and modulating gut microbiota. Int. Immunopharmacol. 100, 108091. doi:10.1016/j.intimp.2021.108091
Ye, B., and Lu, Z. (2022). Role of TRIM22 in ulcerative colitis and its underlying mechanisms. Mol. Med. Rep. 26, 249. doi:10.3892/mmr.2022.12765
Ye, H., Wu, Q., Zhu, Y., Guo, C., and Zheng, X. (2014). Ginsenoside Rh2 alleviates dextran sulfate sodium-induced colitis via augmenting TGFβ signaling. Mol. Biol. Rep. 41, 5485–5490. doi:10.1007/s11033-014-3422-0
Yin, Q., Pi, X., Jiang, Y., Ren, G., Liu, Z., Liu, H., et al. (2021). An immuno-blocking agent targeting IL-1β and IL-17A reduces the lesion of DSS-induced ulcerative colitis in mice. Inflammation 44, 1724–1736. doi:10.1007/s10753-021-01449-4
Yin, T., Zhang, X., Xiong, Y., Li, B., Guo, D., Sha, Z., et al. (2024). Exploring gut microbial metabolites as key players in inhibition of cancer progression: mechanisms and therapeutic implications. Microbiol. Res. 288, 127871. doi:10.1016/j.micres.2024.127871
Yoseph, B. P., Klingensmith, N. J., Liang, Z., Breed, E. R., Burd, E. M., Mittal, R., et al. (2016). Mechanisms of intestinal barrier dysfunction in sepsis. Shock 46, 52–59. doi:10.1097/SHK.0000000000000565
Yu, H., Jin, H., Gong, W., Wang, Z., and Liang, H. (2013). Pharmacological actions of multi-target-directed evodiamine. Molecules 18, 1826–1843. doi:10.3390/molecules18021826
Yu, S., Sun, Y., Shao, X., Zhou, Y., Yu, Y., Kuai, X., et al. (2022). Leaky gut in IBD: intestinal barrier-gut microbiota interaction. J. Microbiol. Biotechnol. 32, 825–834. doi:10.4014/jmb.2203.03022
Yu, Z.-Y., Xu, Y.-S., Tang, M., and Xin, W.-F. (2023). The effect of olsalazine of Chinese generic drugs on ulcerative colitis induced by dextran sulfate sodium salt in BALB/c mice. Acta Cir. Bras. 38, e382923. doi:10.1590/acb382923
Yuan, L., Li, W., Hu, S., Wang, Y., Wang, S., Tian, H., et al. (2024). Protective effects of ginsenosides on ulcerative colitis: a meta-analysis and systematic review to reveal the mechanisms of action. Inflammopharmacology 32, 3079–3098. doi:10.1007/s10787-024-01516-w
Yuan, Q., Liu, W., Wu, H., Yang, X., Li, H., Chen, Y., et al. (2025). Fructans with various molecular weights from Polygonatum cyrtonema Hua differentially ameliorate intestinal inflammation by regulating the gut microbiota and maintaining intestinal barrier. Int. J. Biol. Macromol. 285, 138359. doi:10.1016/j.ijbiomac.2024.138359
Y, Z., Y, T., Q, M., L, C., W, Z., X, L., et al. (2023). Curcumin alleviates experimental colitis in mice by suppressing necroptosis of intestinal epithelial cells. Front. Pharmacol. 14, 14. doi:10.3389/fphar.2023.1170637
Zeng, X., Hu, Y., Qiao, S., Cao, X., Dai, Y., Wu, F., et al. (2024). ADORA3 activation promotes goblet cell differentiation via enhancing HMGCS2-mediated ketogenesis in ulcerative colitis. Int. Immunopharmacol. 140, 112729. doi:10.1016/j.intimp.2024.112729
Zhang, H., Wang, X., Zhao, L., Zhang, K., Cui, J., and Xu, G. (2024a). Biochanin a ameliorates DSS-induced ulcerative colitis by improving colonic barrier function and protects against the development of spontaneous colitis in the Muc2 deficient mice. Chem. Biol. Interact. 395, 111014. doi:10.1016/j.cbi.2024.111014
Zhang, H., Zheng, H., Wang, Q., Ma, Z., Liu, W., Xu, L., et al. (2024b). Sinomenine hydrochloride improves DSS-induced colitis in mice through inhibition of the TLR2/NF-κB signaling pathway. Clin. Res. Hepatol. Gastroenterol. 48, 102411. doi:10.1016/j.clinre.2024.102411
Zhang, L., Cao, N., Wang, Y., Wang, Y., Wu, C., Cheng, X., et al. (2019). Improvement of oxazolone-induced ulcerative colitis in rats using andrographolide. Molecules 25, 76. doi:10.3390/molecules25010076
Zhang, L., Miao, C., Wang, Z., Guan, X., Ma, Y., Song, J., et al. (2024c). Preparation and characterisation of baicalin magnesium and its protective effect in ulcerative colitis via gut microbiota-bile acid axis modulation. Phytomedicine 126, 155416. doi:10.1016/j.phymed.2024.155416
Zhang, Q., Peng, L., Zhang, Q., Guo, J., Yu, N., Yang, J., et al. (2025a). Oral Akkermansia muciniphila biomimetic nanotherapeutics for ulcerative colitis targeted treatment by repairing intestinal epithelial barrier and restoring redox homeostasis. ACS Appl. Mater Interfaces 17, 5942–5954. doi:10.1021/acsami.4c18301
Zhang, X., Wang, Y., Ma, Z., Liang, Q., Tang, X., Hu, D., et al. (2015b). Tanshinone IIA ameliorates dextran sulfate sodium-induced inflammatory bowel disease via the pregnane X receptor. Drug Des. Devel Ther. 9, 6343–6362. doi:10.2147/DDDT.S79388
Zhang, X., Xie, L., Long, J., Xie, Q., Zheng, Y., Liu, K., et al. (2021). Salidroside: a review of its recent advances in synthetic pathways and pharmacological properties. Chem. Biol. Interact. 339, 109268. doi:10.1016/j.cbi.2020.109268
Zhang, Y., Han, L., Dong, J., Yuan, Z., Yao, W., Ji, P., et al. (2024d). Shaoyao decoction improves damp-heat colitis by activating the AHR/IL-22/STAT3 pathway through tryptophan metabolism driven by gut microbiota. J. Ethnopharmacol. 326, 117874. doi:10.1016/j.jep.2024.117874
Zhang, Y., Ji, W., Qin, H., Chen, Z., Zhou, Y., Zhou, Z., et al. (2025b). Astragalus polysaccharides alleviate DSS-induced ulcerative colitis in mice by restoring SCFA production and regulating Th17/Treg cell homeostasis in a microbiota-dependent manner. Carbohydr. Polym. 349, 122829. doi:10.1016/j.carbpol.2024.122829
Zhang, Y.-J., Li, S., Gan, R.-Y., Zhou, T., Xu, D.-P., and Li, H.-B. (2015a). Impacts of gut bacteria on human health and diseases. Int. J. Mol. Sci. 16, 7493–7519. doi:10.3390/ijms16047493
Zhao, H., Xu, H., Chen, S., He, J., Zhou, Y., and Nie, Y. (2021). Systematic review and meta-analysis of the role of Faecalibacterium prausnitzii alteration in inflammatory bowel disease. J. Gastroenterol. Hepatol. 36, 320–328. doi:10.1111/jgh.15222
Zheng, K., Jia, J., Yan, S., Shen, H., Zhu, P., and Yu, J. (2020). Paeoniflorin ameliorates ulcerative colitis by modulating the dendritic cell-mediated TH17/Treg balance. Inflammopharmacology 28, 1705–1716. doi:10.1007/s10787-020-00722-6
Zheng, L., and Duan, S.-L. (2023). Molecular regulation mechanism of intestinal stem cells in mucosal injury and repair in ulcerative colitis. World J. Gastroenterol. 29, 2380–2396. doi:10.3748/wjg.v29.i16.2380
Zheng, L., Duan, S.-L., Dai, Y.-C., and Wu, S.-C. (2022). Role of adherent invasive Escherichia coli in pathogenesis of inflammatory bowel disease. World J. Clin. Cases 10, 11671–11689. doi:10.12998/wjcc.v10.i32.11671
Zhong, Y., Liu, W., Xiong, Y., Li, Y., Wan, Q., Zhou, W., et al. (2022a). Astragaloside Ⅳ alleviates ulcerative colitis by regulating the balance of Th17/Treg cells. Phytomedicine 104, 154287. doi:10.1016/j.phymed.2022.154287
Zhong, Y., Xiao, Q., Kang, Z., Huang, J., Ge, W., Wan, Q., et al. (2022b). Astragalus polysaccharide alleviates ulcerative colitis by regulating the balance of Tfh/Treg cells. Int. Immunopharmacol. 111, 109108. doi:10.1016/j.intimp.2022.109108
Zhou, J., Liu, J., Gao, Y., Shen, L., Li, S., and Chen, S. (2021). miRNA-based potential biomarkers and new molecular insights in ulcerative colitis. Front. Pharmacol. 12, 707776. doi:10.3389/fphar.2021.707776
Zhou, J., Yang, Q., Wei, W., Huo, J., and Wang, W. (2025). Codonopsis pilosula polysaccharide alleviates ulcerative colitis by modulating gut microbiota and SCFA/GPR/NLRP3 pathway. J. Ethnopharmacol. 337, 118928. doi:10.1016/j.jep.2024.118928
Zhou, L., Zhang, M., Wang, Y., Dorfman, R. G., Liu, H., Yu, T., et al. (2018). Faecalibacterium prausnitzii produces butyrate to maintain Th17/treg balance and to ameliorate colorectal colitis by inhibiting histone deacetylase 1. Inflamm. Bowel Dis. 24, 1926–1940. doi:10.1093/ibd/izy182
Zhou, Q., Zhang, W.-X., He, Z.-Q., Wu, B.-S., Shen, Z.-F., Shang, H.-T., et al. (2020). The possible anti-inflammatory effect of dehydrocostus lactone on DSS-induced colitis in mice. Evid. Based Complement. Altern. Med. 2020, 5659738. doi:10.1155/2020/5659738
Zhou, R., Yazdi, A. S., Menu, P., and Tschopp, J. (2011). A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225. doi:10.1038/nature09663
Zhou, Y., Xiong, X., Cheng, Z., Chen, Z., Wu, S., Yu, Y., et al. (2024b). Ginsenoside Rb1 alleviates DSS-induced ulcerative colitis by protecting the intestinal barrier through the signal network of VDR, PPARγ and NF-κB. Drug Des. Devel Ther. 18, 4825–4838. doi:10.2147/DDDT.S481769
Zhou, Y., Zhang, D., Cheng, H., Wu, J., Liu, J., Feng, W., et al. (2024a). Repairing gut barrier by traditional Chinese medicine: roles of gut microbiota. Front Cell Infect. Microbiol. 14, 1389925. doi:10.3389/fcimb.2024.1389925
Zhu, G., Wu, X., Jiang, S., Wang, Y., Kong, D., Zhao, Y., et al. (2022). The application of omics techniques to evaluate the effects of Tanshinone IIA on dextran sodium sulfate induced ulcerative colitis. Mol. Omics 18, 666–676. doi:10.1039/d2mo00074a
Zhu, L., Shi, T., Zhong, C., Wang, Y., Chang, M., and Liu, X. (2017). IL-10 and IL-10 receptor mutations in very early onset inflammatory bowel disease. Gastroenterol. Res. 10, 65–69. doi:10.14740/gr740w
Zhu, L., Xu, L.-Z., Zhao, S., Shen, Z.-F., Shen, H., and Zhan, L.-B. (2020). Protective effect of baicalin on the regulation of Treg/Th17 balance, gut microbiota and short-chain fatty acids in rats with ulcerative colitis. Appl. Microbiol. Biotechnol. 104, 5449–5460. doi:10.1007/s00253-020-10527-w
Zhu, M., Song, Y., Xu, Y., and Xu, H. (2023). Manipulating microbiota in inflammatory bowel disease treatment: clinical and natural product interventions explored. Int. J. Mol. Sci. 24, 11004. doi:10.3390/ijms241311004
Zhu, Q., Zheng, P., Zhou, J., Chen, X., Feng, Y., Wang, W., et al. (2018). Andrographolide affects Th1/Th2/Th17 responses of peripheral blood mononuclear cells from ulcerative colitis patients. Mol. Med. Rep. 18, 622–626. doi:10.3892/mmr.2018.8992
Zhu, Y., Wang, Y., Zuo, X., Liu, S., Cao, L., Wang, J., et al. (2024). Inhibition SIRT1 to regulate FOXP3 or RORγt can restore the balance of Treg/Th17 axis in ulcerative colitis and enhance the anti-inflammatory effect of moxibustion. Front. Immunol. 15, 1525469. doi:10.3389/fimmu.2024.1525469
Zong, Y., Meng, J., Mao, T., Han, Q., Zhang, P., and Shi, L. (2023). Repairing the intestinal mucosal barrier of traditional Chinese medicine for ulcerative colitis: a review. Front. Pharmacol. 14, 1273407. doi:10.3389/fphar.2023.1273407
Zou, J., Shen, Y., Chen, M., Zhang, Z., Xiao, S., Liu, C., et al. (2020). Lizhong decoction ameliorates ulcerative colitis in mice via modulating gut microbiota and its metabolites. Appl. Microbiol. Biotechnol. 104, 5999–6012. doi:10.1007/s00253-020-10665-1
Keywords: ulcerative colitis, immune dysregulation, traditional Chinese medicine, T-cell subsets, gut microbiota, gut barrier repair
Citation: Tang X, Huang Y, Zhu Y and Xu Y (2025) Immune dysregulation in ulcerative colitis: pathogenic mechanisms and therapeutic strategies of traditional Chinese medicine. Front. Cell Dev. Biol. 13:1610435. doi: 10.3389/fcell.2025.1610435
Received: 12 April 2025; Accepted: 26 May 2025;
Published: 05 June 2025.
Edited by:
Xinhua Shu, Glasgow Caledonian University, United KingdomReviewed by:
Yunhua Chang Marchand, INSERM U1151 Institut Necker Enfants Malades, FranceJianping Li, Guangdong Jiangmen Chinese Medicine College, China
Xing Li, School of Basic Medicine, Shaoyang University, China
Copyright © 2025 Tang, Huang, Zhu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Zhu, emh1eWluZzA4OUAxMjYuY29t; Yin Xu, MzExMTE4QGhudWNtLmVkdS5jbg==
 Xudong Tang
Xudong Tang Yilin Huang2
Yilin Huang2 Yin Xu
Yin Xu