Abstract
Ferroptosis, an iron-dependent form of regulated cell death characterized by lipid peroxidation, has emerged as a pivotal mechanism in bone disorders including osteoporosis and osteonecrosis. The nuclear factor erythroid 2–related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) signaling axis plays a paradoxical role—contributing to cytoprotection under oxidative stress, yet potentially promoting ferroptosis through excessive iron accumulation. This review summarizes how the Nrf2/HO-1 pathway modulates ferroptosis across osteoblasts, osteoclasts, and osteocytes, and its impact on bone homeostasis. We explore the pathway’s involvement in the shift from physiological bone remodeling to pathological bone loss. Given its dual role, the Nrf2/HO-1 axis represents both a challenge and an opportunity for therapeutic intervention. Understanding its context-specific functions is essential for developing precise, ferroptosis-targeted strategies in bone disease treatment.
1 Introduction
Bone homeostasis is a fundamental physiological process that preserves the structural integrity and mechanical function of bone tissue. This process is primarily governed by the dynamic balance between osteoblasts and osteoclasts (Zhu et al., 2024; Zhang et al., 2021). Osteoblasts are responsible for the synthesis and mineralization of new bone, while osteoclasts facilitate bone remodeling by resorbing aged or damaged bone tissue (Wu et al., 2024). Under normal physiological conditions, bone formation and resorption are tightly coupled to maintain bone mass and microarchitectural stability (Ze et al., 2025). However, this equilibrium is highly sensitive to the bone microenvironment and is regulated by various factors, including growth factors, cytokines, mechanical loading, oxidative stress, and metabolic byproducts (Gheorghe et al., 2024). Once the regulatory network is disordered, it can easily lead to abnormal bone metabolism, manifested as bone loss, bone microstructure destruction and decreased biomechanical properties.
In clinical settings, bone homeostasis imbalance is closely associated with several metabolic bone disorders, most notably osteoporosis. The core pathological mechanism of osteoporosis involves an increased rate of bone resorption relative to bone formation, leading to trabecular microfracture, decreased bone mineral density (BMD), and a heightened risk of fracture (Sun et al., 2023; Tao et al., 2020). In recent years, osteonecrosis, particularly in the context of vertebral compression fractures, has drawn increasing attention. Studies suggest that impaired local blood supply, elevated oxidative stress, and osteocyte dysfunction post-fracture may contribute to osteocyte death and subsequent bone tissue necrosis, thereby impeding bone regeneration and repair (Cabrera et al., 2022; Li et al., 2023a). Furthermore, conditions such as stress-related bone injury, hormone-induced osteonecrosis, and chemotherapy-associated osteotoxicity are frequently accompanied by varying degrees of bone homeostasis disruption. However, the underlying mechanisms remain poorly understood and warrant systematic investigation at the cellular and molecular levels.
Cell death plays a pivotal role in both maintaining bone homeostasis and contributing to bone pathology. While earlier research has focused on classical cell death pathways such as apoptosis (Yao et al., 2023) and autophagy (Montaseri et al., 2020; Laha et al., 2022), recent attention has turned toward ferroptosis (Jiang et al., 2024)—a distinct, non-apoptotic form of programmed cell death characterized by iron-dependent lipid peroxidation. Ferroptosis is primarily driven by the inactivation of glutathione peroxidase 4 (GPX4), dysregulation of intracellular iron homeostasis, and excessive accumulation of reactive oxygen species (ROS), ultimately resulting in lipid membrane rupture and loss of cellular function (Wang et al., 2021). Although ferroptosis has been implicated in tumorigenesis, neurodegeneration, and cardiovascular diseases, its role in bone homeostasis, particularly in regulating osteoblast survival and function, remains inadequately understood.
Among the various signaling pathways that regulate ferroptosis, the Nrf2 and its downstream effector HO-1 constitute a key axis for oxidative stress defense, iron metabolism, and ROS detoxification (Wang et al., 2022). Under oxidative stress, Nrf2 dissociates from Keap1 repression, translocates to the nucleus, and induces the expression of a suite of antioxidant and iron-handling genes, including HO-1 (Guo et al., 2021). HO-1 catabolizes heme into ferrous iron (Fe2+), carbon monoxide (CO), and biliverdin (BV), thereby exerting cytoprotective effects under certain physiological conditions (Laporte et al., 2019). However, the Fe2+ released in this process may also exacerbate lipid peroxidation and trigger ferroptosis under pathological conditions, suggesting that the Nrf2/HO-1 pathway may play a dual regulatory role in determining osteocyte fate. On one hand, this pathway can suppress ferroptosis and protect osteoblast function by mitigating lipid ROS accumulation and preserving iron homeostasis (Iseda et al., 2022). On the other hand, persistent activation of this axis under certain stimuli may promote ferroptosis due to iron overload, impair bone formation, and ultimately disrupt bone homeostasis (Liu G. Z. et al., 2021).
Therefore, elucidating the precise regulatory mechanisms of the Nrf2/HO-1 pathway in osteocyte ferroptosis has significant implications for understanding the pathogenesis of bone metabolic diseases and identifying novel therapeutic targets. This review aims to comprehensively examine the role of ferroptosis in bone homeostasis, with a particular focus on the bidirectional effects of the Nrf2/HO-1 signaling pathway in osteocyte ferroptosis. Furthermore, it explores the current research progress and therapeutic prospects of targeting this pathway in conditions such as osteoporosis and vertebral osteonecrosis, thereby providing a theoretical foundation for future studies and precision medicine approaches in bone metabolic disorders.
2 The Nrf2/HO-1 pathway: structure and function
2.1 Structure and function of Nrf2
Nuclear factor erythroid 2–related factor 2 (Nrf2) is a pivotal member of the Cap ‘n’ Collar (CNC) family of transcription factors. As a key regulator of the cellular antioxidant defense system, Nrf2 exerts its biological functions through the coordinated action of multiple functional domains (Karunatilleke et al., 2021). Among these, the Neh2 domain, located at the N-terminus, is critically involved in the interaction with Kelch-like ECH-associated protein 1 (Keap1), which facilitates the ubiquitination and proteasomal degradation of Nrf2 under basal conditions (Wang M. et al., 2019). In contrast, the C-terminal basic leucine zipper (bZIP) domain is essential for DNA binding and transcriptional activation (Zhang S. et al., 2022).
Under physiological (non-stressed) conditions, Keap1—primarily localized in the cytoplasm—acts as a major negative regulator of Nrf2. It forms a part of an E3 ubiquitin ligase complex, with Cullin3 (Cul3) serving as the scaffold protein, promoting continuous ubiquitin-dependent degradation of Nrf2 and thereby maintaining its low basal expression (Alonso-Piñeiro et al., 2021). However, upon exposure to oxidative stress, electrophilic agents, or metabolic perturbations, key cysteine residues in Keap1 (notably Cys151, Cys273, and Cys288) undergo oxidation or covalent modification. These modifications induce conformational changes in Keap1, impairing its ability to bind and target Nrf2 for degradation (Pribil Pardun et al., 2024).
As a result, stabilized Nrf2 accumulates in the cytoplasm and translocates into the nucleus, where it forms heterodimers with small Maf proteins. This complex specifically binds to the antioxidant response element (ARE) within the promoter regions of target genes, thereby initiating the transcriptional activation of a wide array of cytoprotective genes, including heme oxygenase-1 (HO-1), NAD(P)H quinone dehydrogenase 1 (NQO1), glutamate–cysteine ligase modifier subunit (GCLM), and ferritin heavy chain 1 (FTH1), among others (Zhai et al., 2022). Taken together, Nrf2 is widely regarded as the master regulator of intracellular redox homeostasis. Its activation plays a central role in orchestrating antioxidant responses, regulating metal ion metabolism, and suppressing various forms of regulated cell death—including ferroptosis—thereby maintaining cellular integrity under stress conditions.
2.2 HO-1: Nrf2 downstream target genes and their metabolites function
Heme oxygenase-1 (HO-1) is one of the most prominent target genes in the Nrf2 transcriptional regulatory network (Song and Long, 2020). It encodes a rate-limiting enzyme responsible for the degradation of heme into three key metabolites: carbon monoxide (CO), ferrous iron (Fe2+), and biliverdin (BV). Biliverdin can subsequently be converted into bilirubin (BR) by the enzyme biliverdin reductase (Li et al., 2023b).
These metabolites—CO, Fe2+, and BV—exert significant pleiotropic effects in maintaining cellular homeostasis and modulating stress responses. As an intracellular signaling molecule, CO has anti-apoptotic, anti-inflammatory, and vasoregulatory properties (Wang et al., 2020). Meanwhile, BV and its derivative BR are fat-soluble antioxidants that can efficiently scavenge peroxides within the cell membrane’s phospholipid bilayer, thus mitigating oxidative damage (Xie F. et al., 2023). In terms of iron metabolism, the proteins ferritin, ferroportin (FPN), and transferrin receptor 1 (TfR1) constitute the iron homeostasis system, ensuring the safe transport, storage, and regulation of intracellular iron levels (Bogdan et al., 2016).
However, under conditions of sustained high expression of HO-1 or when iron regulation is impaired, excessive accumulation of Fe2+ can exacerbate oxidative stress. This is due to the Fenton reaction, where Fe2+ catalyzes the generation of highly reactive hydroxyl radicals (•OH), promoting lipid peroxidation and acting as a key driver of ferroptosis (Chen B. et al., 2024).
Thus, HO-1 exhibits a dose-dependent or context-dependent dual regulatory role in cellular stress defense and ferroptosis induction. Moderate activation of HO-1 confers protective effects against cellular damage, while excessive activation or dysregulation of downstream pathways can transform HO-1 into a pathogenic factor, contributing to cellular dysfunction and ferroptotic cell death.
2.3 Multiple biological functions of Nrf2/HO-1 pathway
The Nrf2/HO-1 signaling pathway is not only a central axis of the cellular antioxidant response but also plays a pivotal role in the regulation of several fundamental physiological processes. It orchestrates a tightly coordinated cytoprotective network that includes the following core functions: (1) Maintenance of Redox Homeostasis: Nrf2 mitigates the accumulation of ROS by upregulating a range of antioxidant enzymes, including HO-1, glutathione synthetase, superoxide dismutase (SOD), and catalase (CAT). Through this regulation, Nrf2 prevents mitochondrial dysfunction, DNA fragmentation, and protein oxidation, serving as the first line of defense against oxidative stress-induced cellular injury (Bu et al., 2023). (2) Iron Metabolism Remodeling and Homeostatic Regulation: Nrf2 modulates the expression of multiple genes involved in iron metabolism, such as ferritin, ferroportin (FPN), transferrin receptor 1 (TfR1), and hepcidin. These genes work in concert with Fe2+ released via HO-1-mediated heme degradation to maintain iron homeostasis (Kajarabille and Latunde-Dada, 2019). This regulatory mechanism is essential in protecting cells from iron overload-induced cytotoxicity, particularly under conditions of high metabolic activity or pathological iron accumulation. (3) Lipid Peroxidation Defense and Ferroptosis Suppression: In addition to its role in redox and iron balance, Nrf2 also regulates the expression of lipid peroxidation defense genes, such as glutathione peroxidase 4 (GPX4), ferritin heavy chain 1 (FTH1), and SLC7A11. These factors are essential for suppressing lipid ROS accumulation, preserving membrane integrity, and thereby inhibiting ferroptosis (Zhang et al., 2025). Notably, Nrf2 deficiency is often associated with GPX4 downregulation and increased phospholipid-derived ROS, which are critical initiating events in the ferroptotic cascade (Liao et al., 2024) (Figure 1).
FIGURE 1
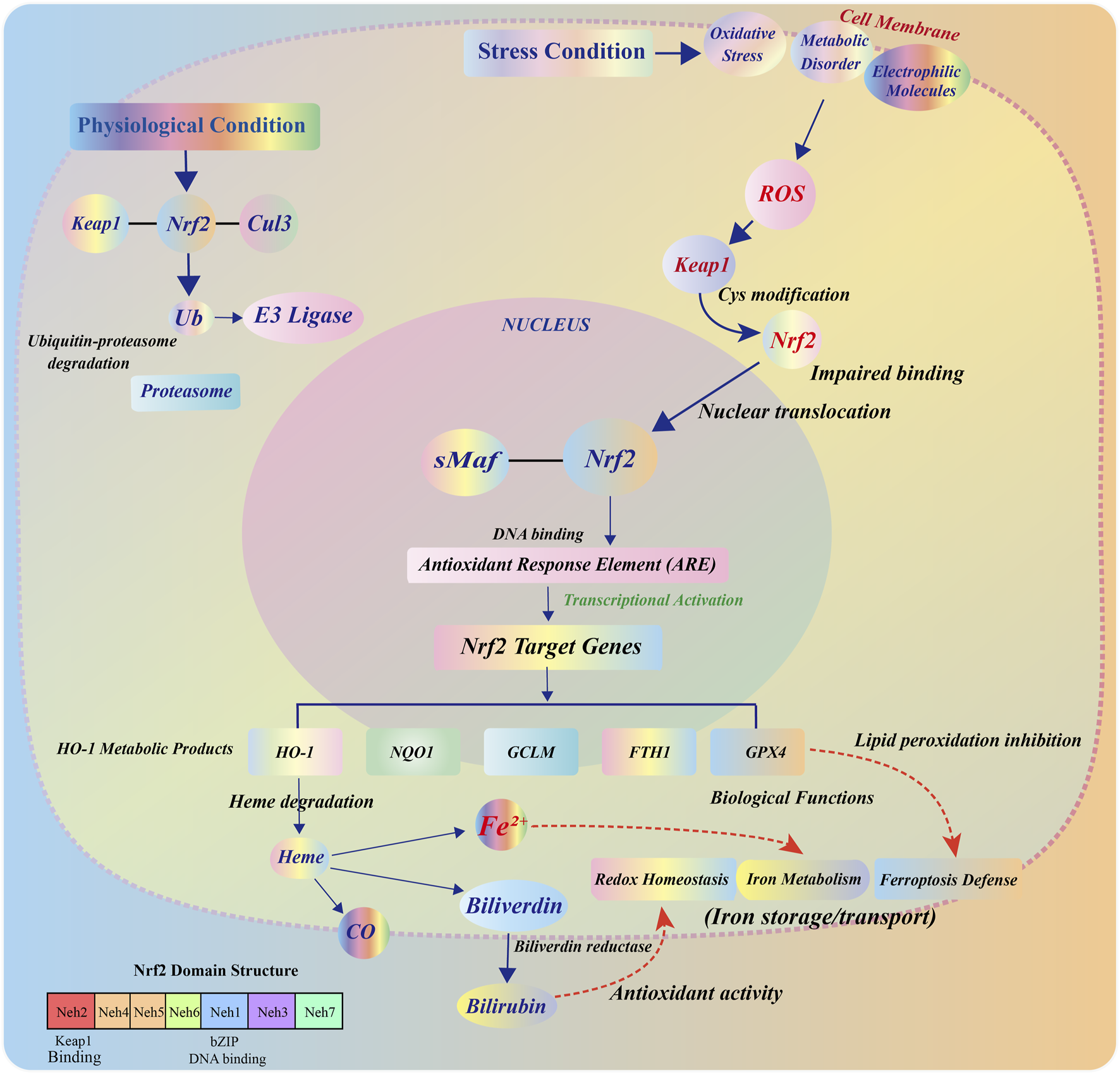
Structural composition, regulatory mechanism, and functional effects of the Nrf2/HO-1 pathway. Under steady-state conditions, Nrf2 is localized in the cytoplasm and forms a complex with Keap1, which binds to the Cul3-E3 ubiquitin ligase complex through the BTB, IVR, and DC domains. This complex mediates the ubiquitination and degradation of Nrf2 in the proteasome, maintaining low levels of its expression. Upon oxidative stress, cysteine residues in Keap1 undergo oxidative modification, allowing Nrf2 to escape degradation and accumulate in the cytoplasm. Nrf2 then translocates to the nucleus, forms a heterodimer with small Maf proteins, binds to the ARE, and induces the transcription of downstream target genes. As an important target gene of Nrf2, HO-1 encodes a heme-degrading enzyme that catalyzes the cleavage of heme into CO, Fe2+, and BV, which is converted to BR by biliverdin reductase. The released Fe2+ participates in the regulation of cellular iron homeostasis through FTH/FTL, FPN, and TfR1, and collectively contributes to antioxidant defense and ferroptosis inhibition.
Therefore, the Nrf2/HO-1 pathway serves as an integrated protective mechanism in response to various cellular stressors, regulating redox homeostasis, iron balance, and lipid peroxidation networks in a synergistic manner. However, its biological function exhibits significant tissue-specific and pathology-dependent variations. Some studies indicate that, under certain stimuli, this pathway may shift from a protective to a disease-promoting role, highlighting the complexity of its regulatory mechanisms.
3 Ferroptosis and its emerging role in bone homeostasis
This section comprehensively examines ferroptosis from two perspectives: first, we elucidate the molecular mechanisms underlying this unique form of regulated cell death; second, we explore how ferroptosis differentially affects various bone cell types and its implications for bone homeostasis.
3.1 Molecular mechanisms of ferroptosis
3.1.1 The concept and molecular characteristics of ferroptosis
Ferroptosis is a distinct form of programmed cell death characterized by the iron-dependent accumulation of lipid peroxides, and it exhibits molecular and morphological features that are fundamentally different from those of apoptosis, autophagy, and necrosis (Ji et al., 2022; Sun et al., 2024). The hallmark of ferroptosis is the oxidation of polyunsaturated fatty acid (PUFA) residues in the phospholipid bilayer of the cell membrane. Morphologically, it is typically accompanied by reduced mitochondrial volume, loss of cristae, and diminished membrane potential, while the overall integrity of the plasma membrane remains largely unaffected (Xie Y. et al., 2023; Kim et al., 2023). Unlike apoptosis, ferroptosis does not involve the activation of caspase family proteins, but is instead driven by lipid ROS accumulation and GPX4 dysfunction (Zhong et al., 2022).
3.1.2 The main molecular mechanisms of ferroptosis
At the molecular level, ferroptosis is critically dependent on the activity of GPX4, the only known antioxidant enzyme capable of directly reducing phospholipid hydroperoxides within cellular membranes (Dos Santos and Friedmann-Angeli, 2024; Tao et al., 2025). The enzymatic function of GPX4 relies on glutathione (GSH) as an electron donor (Zhang Y. et al., 2022). When GPX4 is inactivated or when intracellular GSH is depleted, lipid peroxides accumulate within the membrane, resulting in irreversible oxidative damage (Wang et al., 2023). In parallel, free Fe2+ within the cytoplasm contributes to the Fenton reaction, generating hydroxyl radicals (•OH) that further propagate lipid peroxidation cascades, acting as a key driving force of ferroptosis (Liu et al., 2023; Song et al., 2024). Ferroptosis is co-regulated by multiple signaling networks, and its core regulatory mechanism can be summarized into the following functional modules (Figures 2A,B).
FIGURE 2
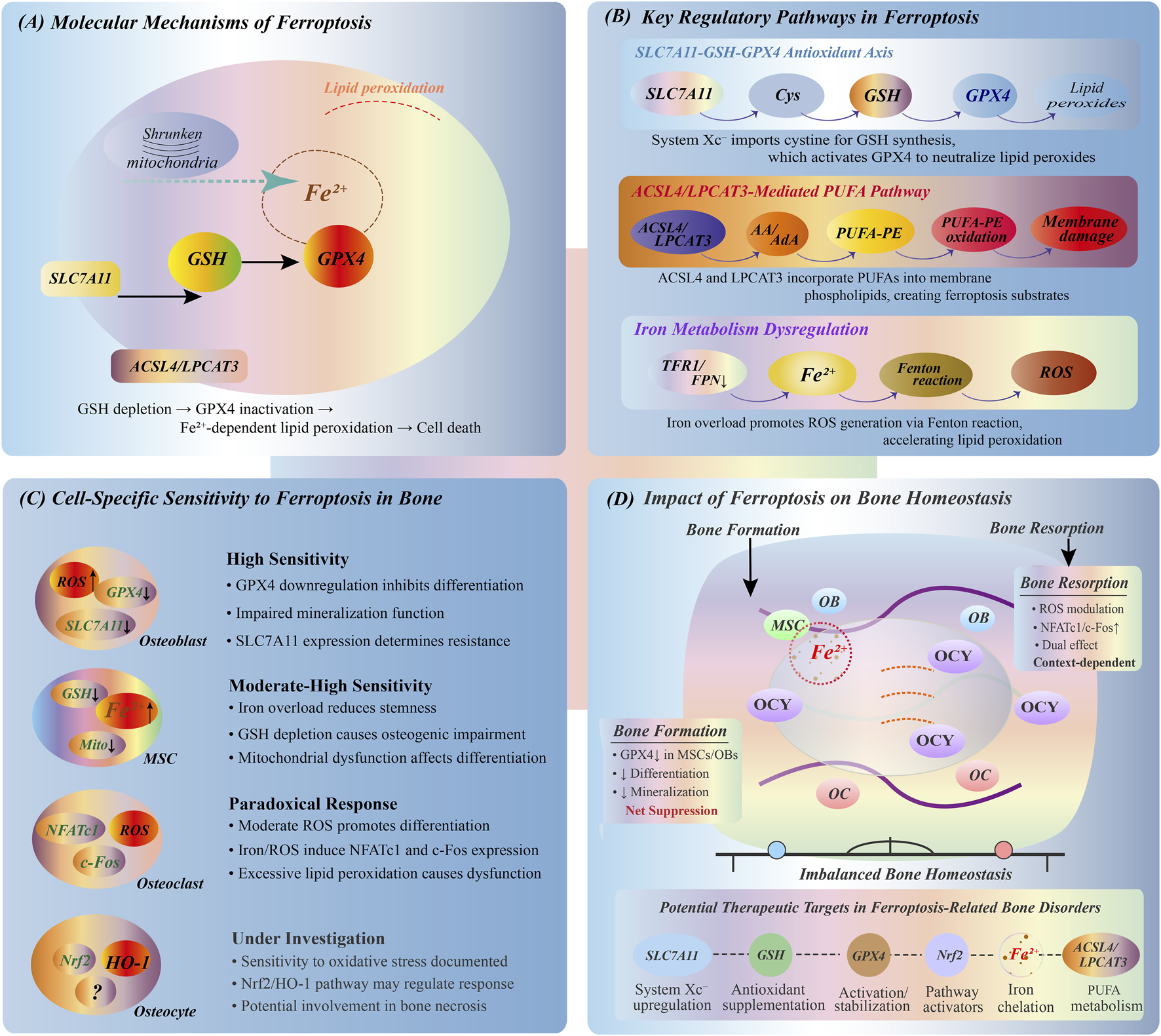
Ferroptosis mechanisms and bone cell responses. (A) Core ferroptosis mechanism showing Fe2+-dependent lipid peroxidation, mitochondrial dysfunction, and GPX4 inactivation leading to cell death. (B) Three key regulatory pathways: SLC7A11-GSH-GPX4 antioxidant axis for lipid peroxide removal; ACSL4/LPCAT3-mediated PUFA incorporation into membranes; iron metabolism via TFR1/FPN1 regulating Fenton reaction and ROS production. (C) Differential ferroptosis sensitivity in bone cells: osteoblasts (OBs) and MSCs show high susceptibility with impaired differentiation; osteoclasts (OCs) exhibit bidirectional ROS response affecting NFATc1/c-Fos expression; osteocytes (OCYs) demonstrate sensitivity through Nrf2/HO-1 pathway. (D) Ferroptosis impact on bone homeostasis showing disrupted balance between bone formation and resorption, with potential intervention targets including SLC7A11 regulation, GPX4 stabilization, and iron chelation.
3.1.2.1 SLC7A11-GSH-GPX4 antioxidant axis
System Xc−, composed of SLC7A11/SLC3A2 heterodimers, mediates the exchange of extracellular cystine for intracellular glutamate and serves as the upstream pathway for intracellular GSH synthesis (Jin et al., 2024). Downregulation of SLC7A11 expression or its functional inhibition markedly reduces intracellular GSH levels, resulting in GPX4 inactivation. This process ultimately impairs the cellular capacity to neutralize lipid peroxides, representing a critical step in the induction of ferroptosis (Yan et al., 2023).
3.1.2.2 Iron metabolism and transport mechanisms
Dysregulation of iron metabolism is a crucial prerequisite for ferroptosis (Jia et al., 2024). Transferrin receptor 1 (TFR1) facilitates cellular iron uptake, ferritin heavy chain (FTH1) mediates intracellular Fe2+ storage, and FPN governs iron export (Bogdan et al., 2016; Deng et al., 2023). The balance between these iron regulatory proteins determines the labile iron pool within cells, which directly influences ferroptosis susceptibility.
3.1.2.3 ACSL4/LPCAT3-mediated PUFA acylation and peroxidation
Long-chain acyl-CoA synthetase 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3) cooperatively catalyze the acylation and incorporation of polyunsaturated fatty acids (PUFAs) into membrane phospholipids, generating molecules such as phosphatidylethanolamine-adrenic acid (PE-AdA) and phosphatidylethanolamine-arachidonic acid (PE-AA). These PUFA-containing phospholipids serve as direct substrates for iron-catalyzed lipid peroxidation (Wei et al., 2023; Dang et al., 2022). Upon GPX4 inactivation, these phospholipid PUFAs become prime targets for oxidative damage, leading to cell membrane dysfunction and the subsequent activation of ferroptotic cell death signaling.
3.1.2.4 Fenton reaction-mediated oxidative toxicity
Excessive iron influx, inadequate storage capacity, or impaired export leads to accumulation of labile Fe2+, which enhances Fenton reaction activity, amplifies ROS production, and triggers lipid peroxidation-induced cellular damage (Chen and Chen, 2022). The Fenton reaction (Fe2+ + H2O2 → Fe3+ + •OH + OH−) generates highly reactive hydroxyl radicals that initiate and propagate lipid peroxidation cascades, ultimately leading to ferroptotic cell death.
3.2 Ferroptosis in bone homeostasis
Bone homeostasis depends on the precise coupling between osteogenesis and bone resorption (Lin et al., 2022; Huo G. et al., 2024). As a regulated way of cell death, ferroptosis shows significant heterogeneity in the response patterns of different bone-related cells (Figures 2 C,D).
3.2.1 Osteoblasts
Osteoblasts dominate the synthesis and mineralization of bone matrix and are highly sensitive to iron metabolism and ROS levels (Shou et al., 2024). Studies have shown that elevated iron load or activation of ferroptosis pathway can downregulate the expression of GPX4 and SLC7A11, resulting in accumulation of lipid peroxidation and inhibition of osteoblast differentiation and mineralization (Iantomasi et al., 2023; Huo K. et al., 2024). This phenomenon suggests that the regulation of ferroptosis axis can be a new strategy for the intervention of bone formation disorders.
3.2.2 Osteoclasts
Osteoclasts achieve bone renewal by absorbing bone, and are functionally opposite to osteoblasts (Liu N. et al., 2021). Different from osteoblasts, osteoclasts have a certain tolerance to ROS, and their differentiation and activity depend on the activation of ROS signaling pathway to a certain extent (Qi et al., 2024; Feng et al., 2023). It has been found that the increase of Fe2+ level can upregulate the expression of NFATc1 and c-Fos in osteoclast precursors and enhance their differentiation ability (Kim et al., 2019; Wang et al., 2018). However, excessive lipid peroxidation may still cause osteoclast dysfunction, suggesting that its response to ferroptosis may be bidirectional and worthy of further exploration.
3.2.3 Mesenchymal stem cells (MSCs)
MSCs are precursor cells of osteoblasts, and maintaining their “stemness” is of decisive significance for bone regeneration (Zheng et al., 2022). Iron overload can induce the decrease of mitochondrial membrane potential and the increase of lipid ROS in MSCs, resulting in the decrease of pluripotency (Li M. et al., 2023; An et al., 2023). The GPX4 knockout model suggests that impaired antioxidant capacity accelerates MSCs senescence and osteogenic differentiation disorder, which is an important mechanism basis for ferroptosis-mediated bone regeneration defects (Yang et al., 2014; Bersuker et al., 2019).
3.2.4 Osteocytes
As the terminal differentiation product of osteoblasts, osteocytes are the key executive units of stress perception and remodeling regulation of bone tissue (Samsa et al., 2016; Abd et al., 2018). Although the current research on ferroptosis in bone cells is limited, preliminary evidence has shown that it is highly sensitive to oxidative stress and changes in iron homeostasis, and may mediate ferroptosis response in pathological processes such as osteonecrosis and fracture repair disorders (Xu et al., 2022). The role of Nrf2/HO-1 axis in this process remains to be systematically studied and has important research potential.
In summary, ferroptosis, as a new type of programmed cell death mode, is characterized by lipid peroxidation accumulation and iron-dependent ROS burst caused by GPX4 inactivation. This process is synergistically driven by SLC7A11-GSH-GPX4 antioxidant axis, PUFA lipid acylation pathway and iron metabolism disorder. There are significant differences in the sensitivity of different types of bone-associated cells to ferroptosis: osteoblasts and MSCs are highly susceptible to ferroptosis, and their impaired function directly inhibits bone formation. Osteoclasts have a positive response to early oxidative stress signals, and may also lose bone resorption capacity when oxidative damage is excessive; as the center of bone homeostasis regulation, the role of osteocytes in ferroptosis needs to be systematically elucidated. These heterogeneous reactions not only reveal the complexity of ferroptosis in the regulation of bone homeostasis, but also provide a new perspective for understanding its bidirectional regulation in metabolic bone diseases such as osteoporosis and osteonecrosis. Future research should focus on ferroptosis threshold recognition of different osteocyte subtypes, specific molecular regulatory networks, and precise definition of intervention window period, so as to develop new anti-bone loss drugs.
4 Modulation of ferroptosis by Nrf2/HO-1 in bone physiology and pathophysiology
Ferroptosis is a type of programmed cell death characterized by iron-dependent lipid peroxidation accumulation. In recent years, it has been considered to play an important role in the regulation of bone homeostasis (Xiong et al., 2022; Gao et al., 2019). Nrf2 and its downstream effector HO-1 together constitute the key signal axis of cellular anti-oxidative stress and iron metabolism regulation, which not only participates in the defense of oxidative damage, but also plays a significant regulatory role in ferroptosis during the maintenance of bone metabolic balance (Montoya et al., 2021; Ma et al., 2022). Studies have shown that Nrf2/HO-1 signaling pathway is involved in the fate determination of osteoblasts and osteoclasts by regulating antioxidant defense, iron ion homeostasis and lipid metabolism, thus affecting the dynamic balance between bone formation and bone resorption (Wang N. et al., 2019; Malakoti et al., 2022).
4.1 Nrf2/HO-1 pathway regulates osteoblast function through ferroptosis modulation
In osteoblasts, the Nrf2/HO-1 signaling axis, as a core pathway regulating cellular antioxidant capacity and iron metabolism, is critical for inhibiting ferroptosis and protecting osteogenic function (Wu and Huang, 2024; Tonelli et al., 2018). Nrf2 transcriptionally activates a series of antioxidant enzymes, including GPX4, to effectively remove lipid peroxides in cell membrane phospholipids, thereby limiting the occurrence of ferroptosis (Fan et al., 2017; Yang et al., 2016). As the core enzyme of ferroptosis defense system, GPX4 can reduce phospholipid peroxides by GSH, which is a key factor to maintain the membrane integrity and functional stability of osteoblasts (Fang et al., 2020; Huang et al., 2024).
In addition to lipid oxidation, Nrf2 can also upregulate the expression of ferritin heavy chain 1 (FTH1), enhance the intracellular iron storage capacity, reduce the level of free Fe2+, and inhibit the production of hydroxyl radicals (•OH) caused by Fenton reaction, thus blocking the iron-catalyzed lipid oxidation reaction chain (Zeng et al., 2023; Liu et al., 2020). This mechanism fundamentally curbs the risk of activation of ferroptosis in osteoblasts.
In addition, HO-1, as a classical target gene of Nrf2, catalyzes the degradation of heme to produce products-CO, BV and Fe2+, which can participate in anti-apoptosis and anti-inflammatory processes under certain conditions. At the same time, it cooperates with the iron homeostasis system to regulate the Fe2+ load level (Martínez-Casales et al., 2021; Lv et al., 2024). Although the activation of HO-1 has ferroptosis potential in some contexts, its mild induction in osteoblasts is more likely to be biased towards inhibiting lipid peroxidation and ROS accumulation, thereby exerting a protective effect (Figure 3).
FIGURE 3
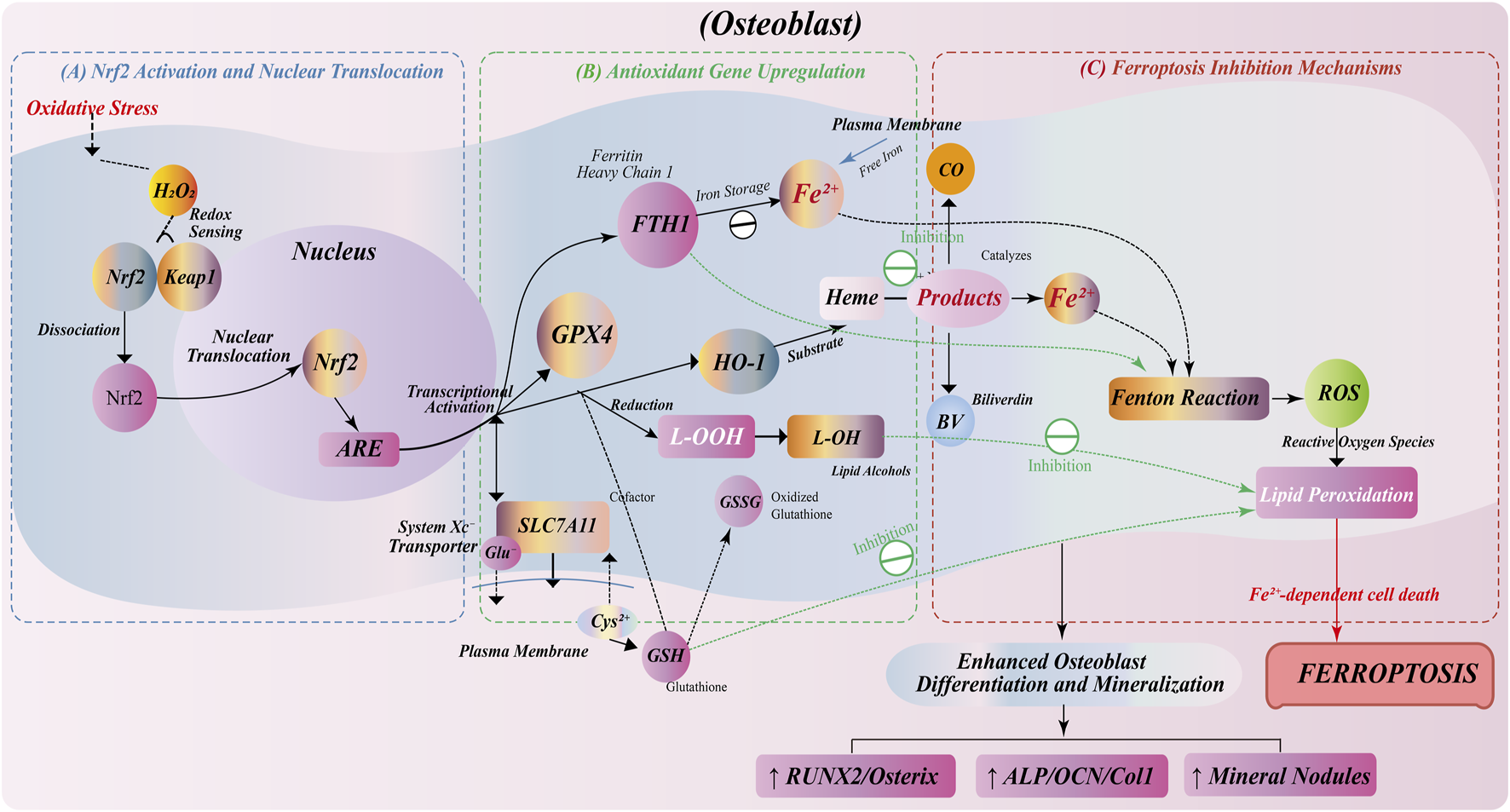
Anti-ferroptosis regulation by Nrf2/HO-1 pathway in osteoblasts. (A) Oxidative stress induces Nrf2 dissociation from Keap1, nuclear translocation, and ARE binding for antioxidant gene transcription. (B) Nrf2 activation upregulates antioxidant genes: GPX4 for lipid peroxide reduction; FTH1 for iron storage; SLC7A11 for GSH synthesis; HO-1 for heme degradation into CO, Fe2+, and BV. (C) Ferroptosis inhibition through multiple mechanisms: FTH1-mediated iron sequestration prevents Fenton reaction; GPX4/GSH system eliminates lipid peroxides; HO-1 metabolites (BV/CO) suppress ROS. This protective pathway enhances osteoblast survival, differentiation, and mineralization, with increased expression of osteogenic markers (RUNX2, Osterix, ALP, OCN, Col1) and mineralized nodule formation.
In summary, the Nrf2/HO-1 pathway constructs a multi-level barrier of osteoblasts to ferroptosis stress by integrating and regulating redox homeostasis, lipid antioxidant system and iron metabolism pathway. Its activation can not only slow down cell damage and apoptosis during bone formation, but also enhance osteogenic ability and promote bone matrix deposition and mineralization, which is expected to become a potential target for maintaining bone homeostasis and preventing bone loss.
4.2 Bidirectional control of osteoclast activity by Nrf2/HO-1-mediated ferroptosis
The role of Nrf2/HO-1 pathway in osteoclasts shows a certain bidirectionality. Moderate Nrf2 activation has a protective effect on osteoclasts, but excessive HO-1 metabolites may promote osteoclast activity and lead to excessive bone resorption (Pan et al., 2021; Fang et al., 2024).
Moderate activation of Nrf2 can upregulate the expression of antioxidant enzymes and reduce the accumulation of ROS, thereby reducing the damage of osteoclasts caused by oxidative stress (Li et al., 2018). Oxidative stress is an important regulator of osteoclast function. Excessive ROS can cause osteoclast damage and aggravate bone resorption (Ji et al., 2023; XiaHumulus lupulus et al., 2023). Therefore, Nrf2 plays a protective role in bone metabolism by maintaining the antioxidant capacity of osteoclasts (Figure 4A).
FIGURE 4
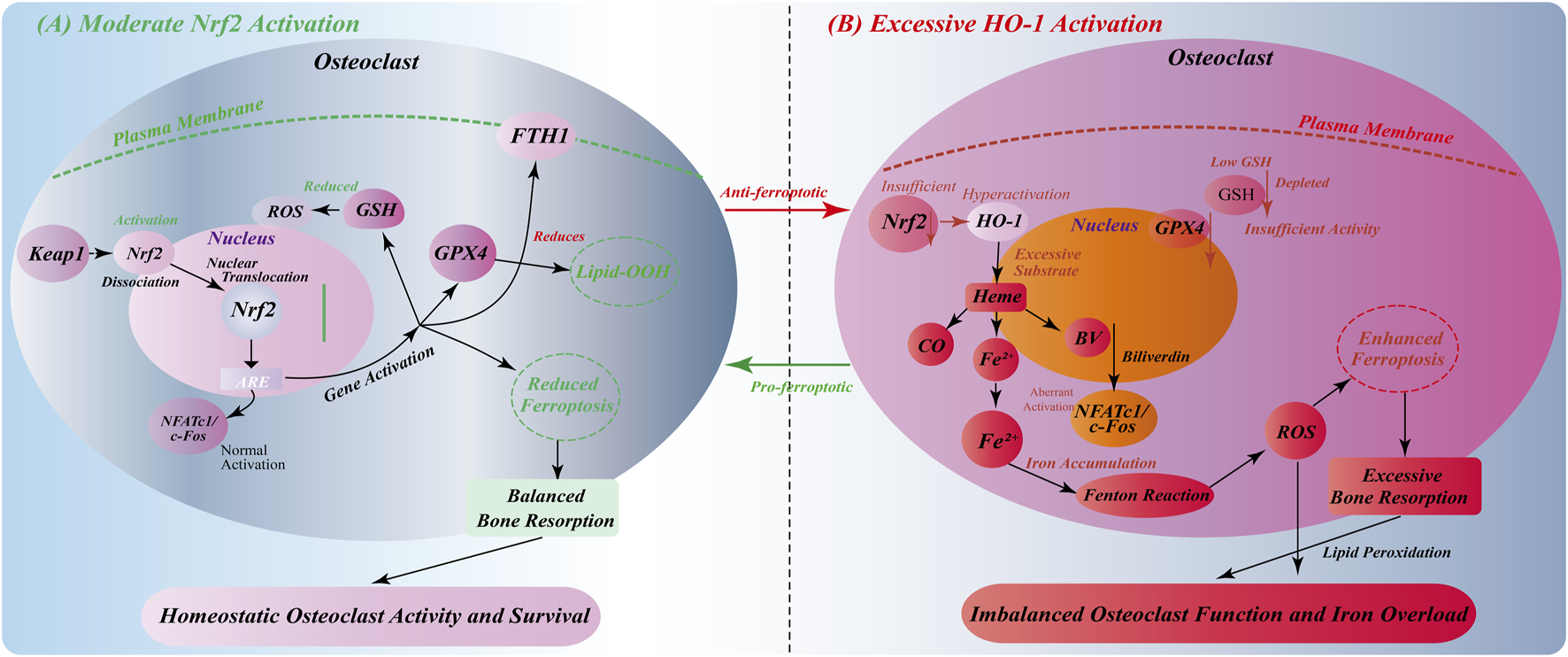
Environment-dependent bidirectional regulation of Nrf2/HO-1 signaling in osteoclasts. (A) Moderate Nrf2 activation: Oxidative stress induces Keap1-Nrf2 dissociation, nuclear translocation, and ARE-mediated gene expression. This upregulates GSH synthesis, GPX4 activity, and FTH1 expression, reducing ROS/lipid peroxidation and inhibiting ferroptosis. Normal NFATc1/c-Fos activation maintains balanced osteoclast function and bone resorption. (B) Excessive HO-1 activation: Insufficient Nrf2 function with abnormally high HO-1 expression causes excessive heme degradation and Fe2+ accumulation. GSH depletion and inadequate GPX4 activity, combined with Fenton reaction-generated ROS, promote ferroptosis. Iron overload triggers aberrant NFATc1/c-Fos signaling, leading to osteoclast hyperfunction and excessive bone resorption, disrupting bone homeostasis.
However, excessive HO-1 metabolites, such as Fe2+, may have adverse effects. Ferroptosis is triggered by free radicals generated by Fenton reaction, which promote lipid peroxidation (Cai et al., 2024; Chen et al., 2023). Ferroptosis is a form of osteoclast death, and excessive Fe2+ can lead to excessive bone resorption (Chen Y. et al., 2024; Qu et al., 2024). Therefore, the role of Nrf2/HO-1 pathway in osteoclasts requires fine regulation. Moderate HO-1 activity has a protective effect on bone resorption, while excessive HO-1 activation may promote bone resorption and affect bone homeostasis (Figure 4B).
Taken together, this two-way regulatory mechanism reveals the importance of the precise balance of the Nrf2/HO-1 signaling axis in osteoclasts. Moderate activation has anti-ferroptosis and homeostasis maintenance effects, while excessive activation promotes ferroptosis and osteoclast dysfunction. This finding provides a new perspective for understanding the molecular mechanism of abnormal osteoclast activity in various bone metabolic diseases, and also lays a foundation for the development of precise treatment strategies for ferroptosis and bone resorption imbalance.
4.3 HO-1 metabolites determine osteocyte survival via ferroptosis regulation
HO-1 produces a series of metabolites by degrading heme, including CO, Fe2+ and BV (Tun et al., 2020). These metabolites play an important role in the regulation of bone cell fate.
As one of the products of HO-1, CO has antioxidant properties (Zhang et al., 2020). At low concentrations, CO can protect bone cells by reducing the accumulation of ROS, improve mitochondrial function, and enhance the antioxidant capacity of cells (Wang et al., 2024). The inhibitory effect of CO on oxidative stress in osteocytes helps maintain bone homeostasis (Van Phan et al., 2013). Fe2+ is another important product of HO-1. Although it is essential for the physiological function of bone cells, excessive Fe2+ will generate free radicals through Fenton reaction, induce lipid peroxidation, and eventually lead to ferroptosis (Yang and Shang, 2022). As a form of iron-dependent cell death, ferroptosis poses a threat to the survival of bone cells. Therefore, the excessive accumulation of Fe2+ may have a negative impact on the health of bone cells. BV is another product produced during the degradation of heme by HO-1, which has a strong antioxidant effect (Wu and Hsieh, 2022). BV can remove excessive ROS and reduce the damage of oxidative stress to bone cells, thereby maintaining bone homeostasis (Zhou et al., 2021). Its antioxidant properties make it a key factor in protecting bone cells in HO-1 metabolites (Figure 5).
FIGURE 5
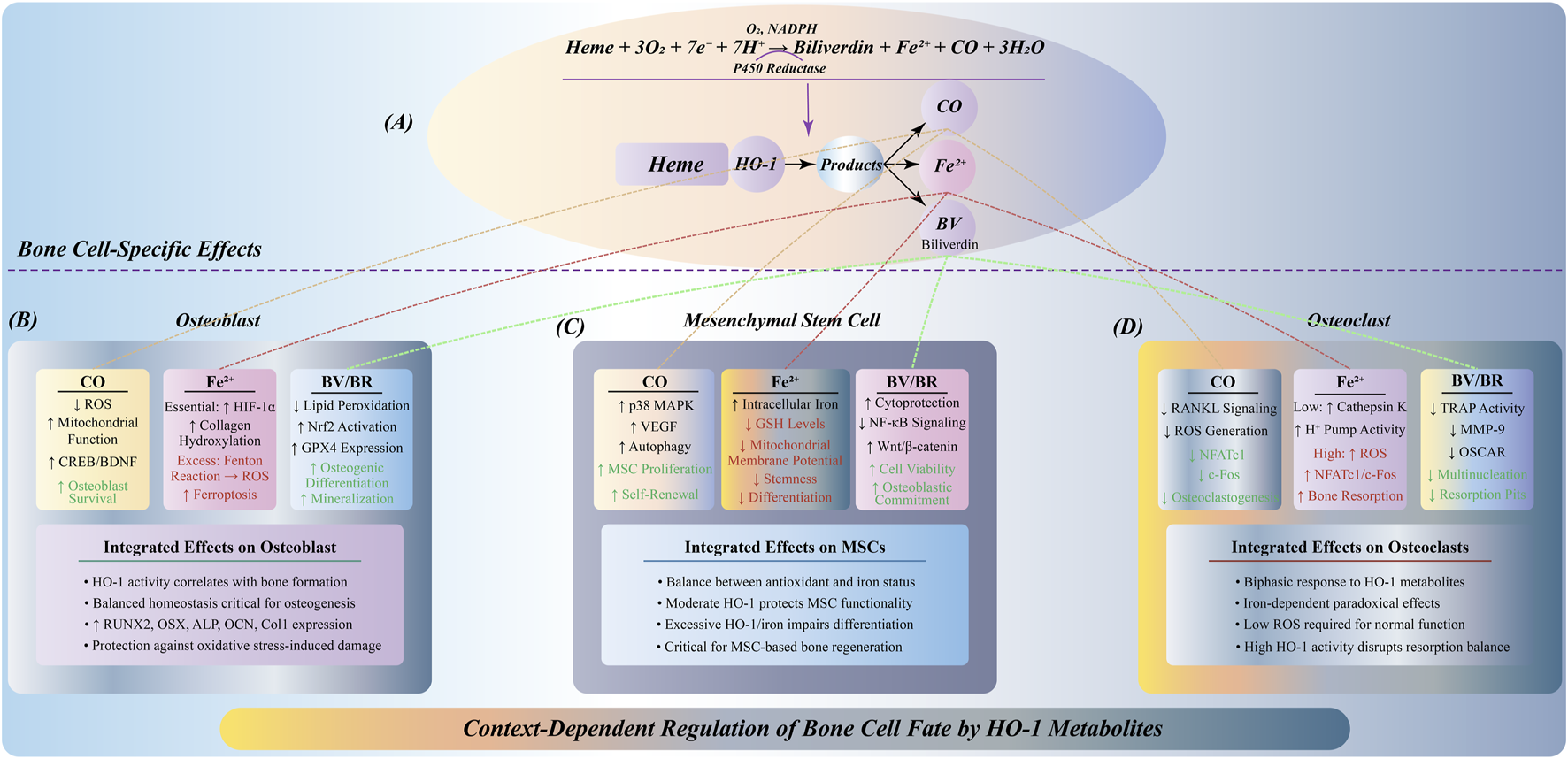
Multidimensional regulation of HO-1 metabolites on bone cell fate. (A) HO-1 catalyzes heme degradation to produce CO, Fe2+, and BV, differentially affecting three bone cell types. (B) In osteoblasts: CO reduces ROS and activates CREB/BDNF signaling; Fe2+ shows dual effects—moderate levels stabilize HIF-1α supporting bone formation, while excess induces ferroptosis; BV/BR inhibits lipid peroxidation and activates Nrf2/GPX4, promoting osteogenic differentiation. (C) In MSCs: CO activates p38 MAPK/VEGF promoting proliferation; Fe2+ accumulation depletes GSH impairing stemness; BV/BR enhances viability via NF-κB inhibition and Wnt/β-catenin activation. Dose-dependent responses are critical for MSC-based therapies. (D) In osteoclasts: CO inhibits RANKL signaling reducing osteoclastogenesis; Fe2+ effects are concentration-dependent—low levels maintain function, high levels promote excessive resorption; BV/BR suppresses TRAP/MMP-9 expression inhibiting bone resorption.
In summary, the regulation of HO-1 metabolites on the fate of osteocytes is highly environmentally dependent, and produces distinct biological effects under different cell types and different concentrations, which constitutes the molecular basis for the fine regulation of bone homeostasis. This environment-specific and cell-specific mode of action provides a new theoretical framework for understanding the pathological mechanism of bone metabolic diseases and developing targeted treatment strategies.
4.4 Nrf2/HO-1-ferroptosis axis in pathogenesis of bone metabolic diseases
The role of Nrf2/HO-1 pathway in bone metabolic diseases, especially in osteoporosis and osteonecrosis, has received extensive attention (Li et al., 2019; Hu et al., 2022). In these diseases, decreased expression or functional inactivation of Nrf2 is often accompanied by increased ferroptosis, excessive bone resorption, and bone loss.
In the animal model of estrogen deficiency-induced osteoporosis, the expression of Nrf2 is significantly decreased, which is closely related to the inhibition of osteogenesis and the decrease of bone mineralization function (Liu et al., 2024). Studies have shown that Nrf2 activation can upregulate the expression of antioxidant enzymes, reduce oxidative stress and ferroptosis, thereby inhibiting bone resorption, promoting bone formation, improving bone structure, and slowing the process of osteoporosis and osteonecrosis (Xue et al., 2019). In addition, HO-1, as a downstream effector molecule of the Nrf2 pathway, its inducer can promote bone repair by reducing ROS accumulation and inhibiting bone resorption in the early stage (Han et al., 2022; Liu et al., 2025). Therefore, the regulation of Nrf2/HO-1 pathway has potential application prospects in the treatment of bone metabolic diseases such as osteoporosis and osteonecrosis (Figure 6).
FIGURE 6
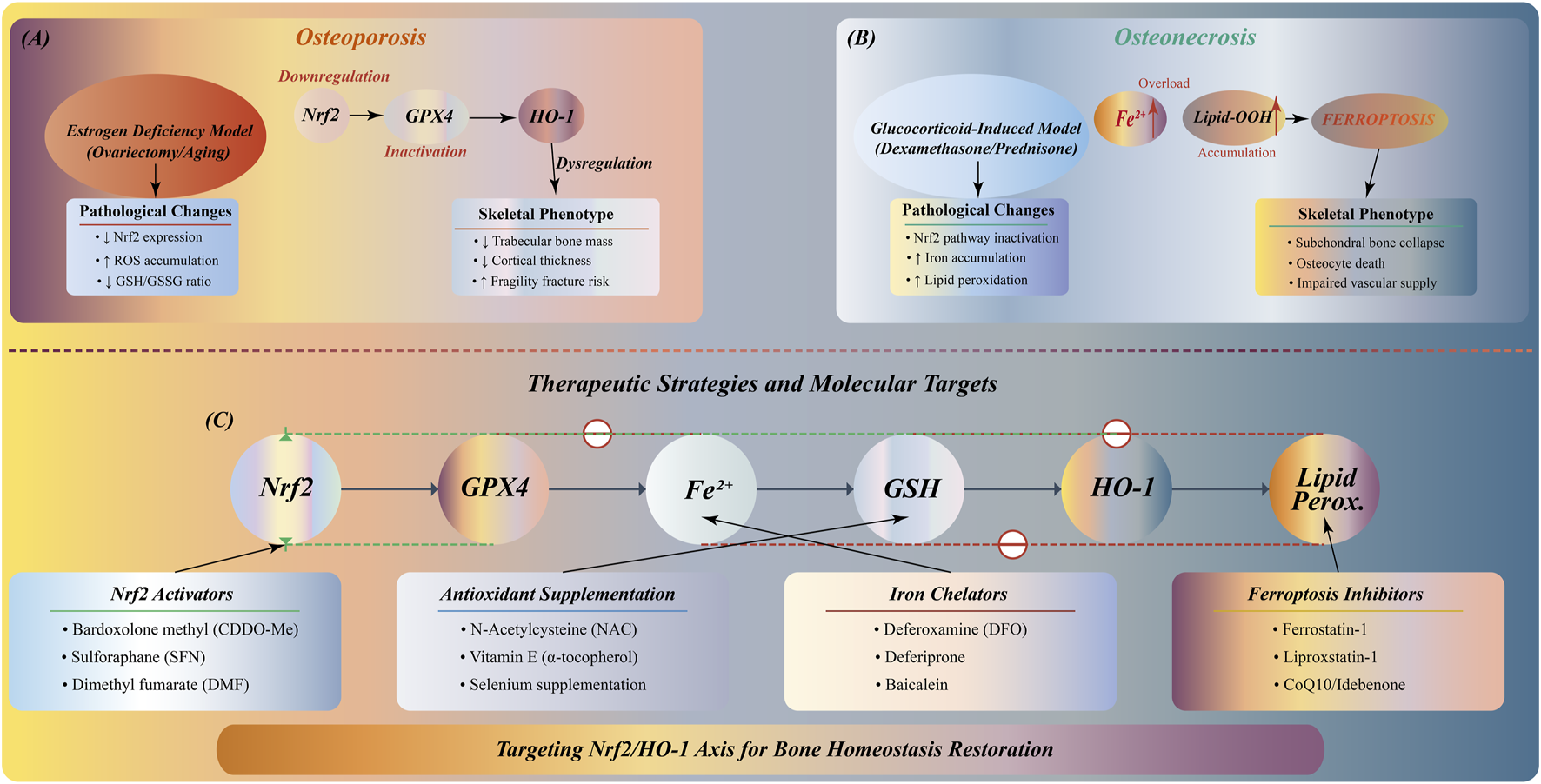
Nrf2/HO-1 pathway in bone metabolic diseases: pathology and therapeutic strategies. (A) Osteoporosis: Estrogen deficiency downregulates Nrf2/GPX4/HO-1, increasing ROS accumulation and decreasing GSH/GSSG ratio, resulting in trabecular bone loss, reduced cortical thickness, and fracture risk. (B) Osteonecrosis: Glucocorticoid treatment inactivates Nrf2 pathway, causing Fe2+ accumulation and lipid peroxidation, triggering ferroptosis. (C) Multi-target therapeutic network: Nrf2 activators (sulforaphane, melatonin), antioxidants (vitamin E, quercetin), iron chelators (DFO, deferiprone), and ferroptosis inhibitors (ferrostatin-1) targeting different nodes of the pathway.
Therefore, Nrf2 dysfunction caused by estrogen deficiency or glucocorticoid treatment leads to increased oxidative stress, iron metabolism disorders and lipid peroxidation, and ultimately damages bone structure. Based on these findings, multi-target treatment strategies from Nrf2 activators to ferroptosis inhibitors have shown clinical application prospects. However, there are still many challenges in this field. The regulatory mechanism of Nrf2/HO-1 pathway in different bone cells has not been fully elucidated. The targeted delivery and long-term safety of therapeutic drugs need to be verified, and the interaction with other bone metabolic networks needs further study.
5 Therapeutic targeting of the Nrf2/HO-1-ferroptosis axis in bone diseases
The Nrf2/HO-1 pathway plays a pivotal role in regulating bone homeostasis through modulation of ferroptosis, providing novel therapeutic strategies for various bone diseases (Zhang et al., 2023). This section discusses targeted interventions based on specific bone pathologies, highlighting how manipulation of the Nrf2/HO-1-ferroptosis axis can effectively improve disease outcomes (Table 1).
TABLE 1
| Disease category | Compound | Main mechanism | Clinical application | References | |
|---|---|---|---|---|---|
| Osteoporosis | Postmenopausal | Sulforaphane | Alkylation of Keap1 Cys151→Nrf2/ARE activation↑→↑HO-1, NQO1 expression | OVX rats: ↑BMD and improved trabecular architecture | Thaler et al. (2016) |
| Diabetic | Melatonin | MT2→ERK/AKT→↑Nrf2/HO-1→enhanced ROS clearance | Improved bone mineral density | Hu et al. (2022) | |
| General | α-Tocopherol (Vitamin E) | Lipid-chain termination→radical clearance→reinforced membrane antioxidant barrier | Prevention: improved implant biocompatibility | Lovati et al. (2018) | |
| General | Quercetin | PI3K/AKT→↑Nrf2+↓IKK/NF-κB→dual antioxidant and anti-inflammatory effects | Enhanced osteoblast mineralization | Mao et al. (2024) | |
| Osteonecrosis | Glucocorticoid-induced | Deferoxamine | Fe2+ chelation→Fenton reaction blockade→↑HIF-1α stabilization→↑VEGF release | Bone defect scaffolds: ↑vascularization and osteogenesis | Shen et al. (2024) |
| Bone defects | Curcumin | p62-mediated Keap1 degradation→↑Nrf2↓JAK/STAT→anti-inflammatory | Nanoparticle delivery: enhanced bone defect repair | Astaneh et al. (2024) | |
| General | Ferrostatin-1 | Inhibition of ACSL4-mediated PUFA peroxidation→mitochondrial membrane protection | In vitro MSCs: ↑viability | Valanezhad et al. (2021) | |
| Inflammatory Bone Diseases | Osteolytic conditions | Dimethyl fumarate | Keap1 alkylation→ARE activation↑→↑GSH synthesis→ferroptosis inhibition | Osteolytic models: ↓osteoclast activity, ↑Runx2 | Yamaguchi et al. (2018) |
| Rheumatoid arthritis | Tin protoporphyrin IX | Competitive HO-1 inhibition→↓CO production→suppress excess HO-1-mediated bone resorption | Inhibited bone loss | Ibáñez et al. (2011) | |
| Osteoarthritis | Quercetin | PI3K/AKT→↑Nrf2+↓IKK/NF-κB→dual antioxidant and anti-inflammatory effects | Cartilage protection | Mao et al. (2024) | |
| Radiation-induced injury | Tanshinone IIA | SIRT1-mediated Nrf2 deacetylation→↑ARE-driven antioxidants↓p38/MAPK→inflammation inhibition | Restoration of marrow microenvironment | Cheng et al. (2024) | |
| Iron Overload Disorders | Transfusion-induced | Deferiprone | Small-molecule chelation of labile iron→↓ROS | Bone density preservation | Shen et al. (2024) |
| Chronic overload | Deferasirox | Bidentate Fe3+ coordination→↓bone-matrix iron accumulation | Reduced fracture risk | Casale et al. (2014) | |
| Emerging Therapies | Bardoxolone methyl (CDDO-Me) | Keap1 Cys151 binding→ultra-potent Nrf2 activation↓NF-κB→potent antioxidant/anti-inflammatory | Phase I trial: potential trabecular bone protection | Yang et al. (2023) | |
Therapeutic interventions targeting the Nrf2/HO-1-ferroptosis axis in bone diseases: Mechanisms and clinical applications.
5.1 Therapeutic strategies for osteoporosis
Osteoporosis, characterized by decreased bone density and increased fracture risk, has emerged as a primary target for Nrf2/HO-1 pathway modulation. Nrf2 agonists have shown remarkable efficacy in preclinical osteoporosis models. Sulforaphane, through alkylation of Keap1 Cys151 residues, promotes Nrf2 nuclear translocation and ARE activation, leading to increased HO-1 and NQO1 expression (Lin et al., 2014). In ovariectomized rat models, sulforaphane treatment significantly improved BMD and trabecular architecture (Thaler et al., 2016).
Melatonin represents another promising therapeutic agent for diabetic osteoporosis. By activating the MT2-ERK/AKT-Nrf2/HO-1 cascade, melatonin reduces ROS levels, upregulates SLC7A11 expression, and increases GPX4 activity, thereby protecting osteoblasts from ferroptosis and improving bone mineral density (Hu et al., 2022) (Yan et al., 2022). Similarly, traditional Chinese medicine components have demonstrated efficacy: quercetin enhances osteoblast mineralization through PI3K/AKT-mediated Nrf2 activation, while simultaneously suppressing NF-κB inflammatory signaling (Xiao et al., 2023; Mao et al., 2024).
For postmenopausal osteoporosis, α-tocopherol (Vitamin E) functions as a lipid-chain termination agent, reinforcing membrane antioxidant barriers and improving implant biocompatibility (Lovati et al., 2018). These diverse approaches underscore the multifaceted therapeutic potential of targeting the Nrf2/HO-1 pathway in osteoporosis management.
5.2 Interventions for osteonecrosis
Osteonecrosis, particularly glucocorticoid-induced osteonecrosis, involves excessive ferroptosis and compromised bone vascularization. Iron chelators have emerged as crucial therapeutic agents in this context. Deferoxamine (DFO) blocks the Fenton reaction by chelating Fe2+, leading to HIF-1α stabilization and enhanced VEGF release, thereby promoting vascularization and osteogenesis in bone defect models (Guo et al., 2023; Shen et al., 2024).
The nano-delivery system has shown particular promise for osteonecrosis treatment. Curcumin-loaded nanoparticles enhance bone defect repair through p62-mediated Keap1 degradation, resulting in sustained Nrf2 activation and dual antioxidant/anti-inflammatory effects (Wang, 2024; Astaneh et al., 2024). This targeted delivery approach improves bioavailability while minimizing systemic side effects (Cheng et al., 2017; Chen Y. J. et al., 2024).
Combined therapeutic strategies have proven especially effective. The synergistic use of DFO with lipid antioxidants like Ferrostatin-1 addresses both iron overload and lipid peroxidation, providing comprehensive protection against ferroptotic cell death in osteonecrosis (Guo et al., 2023; Valanezhad et al., 2021).
5.3 Applications in inflammatory bone diseases
Inflammatory bone diseases, including rheumatoid arthritis and osteoarthritis, present unique therapeutic challenges due to the interplay between inflammation and oxidative stress. Dimethyl fumarate targets this dual pathology through Keap1 alkylation, promoting GSH synthesis while inhibiting ferroptosis. In osteolytic models, this approach reduces osteoclast activity and upregulates Runx2 expression (Sánchez-de-Diego et al., 2021; Yamaguchi et al., 2018).
For rheumatoid arthritis, HO-1 modulation requires careful balance. Tin protoporphyrin IX, a competitive HO-1 inhibitor, reduces CO production and suppresses excess HO-1-mediated bone resorption, effectively inhibiting bone loss in arthritis models (Ibáñez et al., 2011). This highlights the importance of context-dependent HO-1 regulation (Kajarabille and Latunde-Dada, 2019; Li et al., 2020; Yang et al., 2022; Castany et al., 2016).
Tanshinone IIA offers a multifaceted approach for radiation-induced bone injury through SIRT1-mediated Nrf2 deacetylation, driving ARE-dependent antioxidant expression while suppressing p38/MAPK inflammatory signaling. This dual action helps restore bone marrow microenvironment (Cao et al., 2024; Cheng et al., 2024).
5.4 Treatment of iron overload-related bone disorders
Chronic iron overload conditions, such as those seen in transfusion-dependent thalassemia, require specialized therapeutic approaches. Deferiprone provides small-molecule chelation of labile iron, effectively preserving bone density in transfusion-induced iron overload (Shen et al., 2024). Similarly, deferasirox coordinates bidentate Fe3+ binding to reduce bone-matrix iron accumulation, significantly decreasing fracture risk in chronic iron overload patients (Casale et al., 2014).
These iron-specific interventions demonstrate the critical importance of maintaining iron homeostasis in bone health, particularly in patients with systemic iron metabolism disorders.
5.5 Emerging therapeutic modalities and future directions
Novel therapeutic agents continue to emerge. Bardoxolone methyl (CDDO-Me), currently in Phase I trials, represents an ultra-potent Nrf2 activator through Keap1 Cys151 binding, offering potential trabecular bone protection with combined antioxidant and anti-inflammatory properties (Yang et al., 2023).
Advanced delivery systems are revolutionizing treatment approaches. Bone-targeted nanoparticle formulations enable precise delivery of Nrf2 agonists or traditional medicine components directly to affected bone tissue, maximizing therapeutic efficacy while minimizing off-target effects (Astaneh et al., 2024; Cheng et al., 2017; Chen Y. J. et al., 2024).
The integration of combination therapies—utilizing Nrf2 activators (Xiao et al., 2023; Cao et al., 2024), iron chelators (Guo et al., 2023), and ferroptosis inhibitors (Valanezhad et al., 2021)—represents the future of personalized bone disease treatment, allowing clinicians to address the specific pathological mechanisms underlying each patient’s condition.
6 Conclusion and prospects
Whether ferroptosis constitutes an indispensable driver of osteonecrosis or osteoporosis remains an open question. Although Fe2+ accumulation, lipid peroxidation, and the ensuing oxidative stress have been consistently observed in diverse bone‐pathology models—most notably in glucocorticoid‐induced osteoporosis and osteonecrosis—definitive proof that ferroptosis is a “necessary condition” for disease initiation is still lacking. It is increasingly apparent that ferroptosis may represent a critical facet of disease progression rather than the singular pathogenic mechanism. Adding further complexity, the Nrf2 signaling axis exerts dichotomous effects: on one hand, activation of Nrf2 promotes the transcription of antioxidant enzymes, mitigates oxidative damage, and suppresses ferroptosis, thereby safeguarding osteocytic integrity; on the other hand, chronic or excessive Nrf2 activation can drive overproduction of HO-1 metabolites (including labile Fe2+), thereby potentiating ferroptotic cell death and skeletal deterioration. Dissecting these “protective” versus “pathogenic” roles of Nrf2—particularly in a cell‐type–and time‐dependent manner (e.g., osteoblasts versus osteoclasts, early versus late disease stages)—remains a formidable challenge. Moreover, the translational gap between animal models and human clinical specimens is still wide: while preclinical data are abundant, robust validation in patient cohorts is insufficient. Future research must therefore integrate sophisticated in vivo and ex vivo platforms, and prioritize the development of bone‐targeted, tissue‐specific Nrf2 modulators capable of finely tuning redox homeostasis without eliciting systemic iron overload.
Overall, the Nrf2/HO-1 signaling cascade emerges as a pivotal regulator of bone homeostasis, intricately linking oxidative stress, iron metabolism, and regulated cell death pathways. Investigations into ferroptosis have unveiled novel insights into the mechanistic interplay between iron dysregulation and osteocellular injury, offering a fresh conceptual framework for osteoporosis and osteonecrosis pathogenesis. Looking ahead, the precise manipulation of the Nrf2/HO-1 axis—particularly through bone‐targeted, cell‐contextual therapeutics—holds promise for transformative interventions in skeletal disease. Realizing this potential will require surmounting key obstacles, including delineation of cell‐type specificity, temporal window optimization, and the refinement of delivery systems to ensure localized efficacy while minimizing off‐target effects. With continued multidisciplinary advances, targeted modulation of the Nrf2/HO-1 pathway is poised to inaugurate a new era of precision medicine in bone pathology.
Statements
Author contributions
WN: Writing – review and editing, Methodology, Writing – original draft, Investigation, Funding acquisition, Formal Analysis, Project administration. W-MZ: Data curation, Writing – review and editing, Formal Analysis. J-LZ: Data curation, Writing – review and editing. Y-QS: Supervision, Writing – review and editing. Y-BD: Data curation, Writing – review and editing. WS: Software, Writing – review and editing. Y-CM: Visualization, Validation, Writing – review and editing. H-HZ: .
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 82360435); Lanzhou University Second Hospital Cuiying Youth Fund Project (No. CY2022-QN-A03); Cuiying Science and Technology Innovation Program Project (No. 2022-M-A10); Lanzhou Youth Science and technology Talents Innovation Project (2023-2-39); Natural Science Foundation of Gansu Province (No. 24JRRA1101).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abdallah D. Jourdain M. L. Braux J. Guillaume C. Gangloff S. C. Jacquot J. et al (2018). An optimized method to generate human active osteoclasts from peripheral blood monocytes. Front. Immunol.9, 632. 10.3389/fimmu.2018.00632
2
Alonso-Piñeiro J. A. Gonzalez-Rovira A. Sánchez-Gomar I. Moreno J. A. Durán-Ruiz M. C. (2021). Nrf2 and heme Oxygenase-1 involvement in atherosclerosis related oxidative stress. Antioxidants (Basel)10 (9), 1463. 10.3390/antiox10091463
3
An F. Zhang J. Gao P. Xiao Z. Chang W. Song J. et al (2023). New insight of the pathogenesis in osteoarthritis: the intricate interplay of ferroptosis and autophagy mediated by mitophagy/chaperone-mediated autophagy. Front. Cell Dev. Biol.11, 1297024. 10.3389/fcell.2023.1297024
4
Astaneh M. E. Noori F. Fereydouni N. (2024). Curcumin-loaded scaffolds in bone regeneration. Heliyon10 (11), e32566. 10.1016/j.heliyon.2024.e32566
5
Bersuker K. Hendricks J. M. Li Z. Magtanong L. Ford B. Tang P. H. et al (2019). The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature575 (7784), 688–692. 10.1038/s41586-019-1705-2
6
Bogdan A. R. Miyazawa M. Hashimoto K. Tsuji Y. (2016). Regulators of iron homeostasis: new players in metabolism, cell death, and disease. Trends Biochem. Sci.41 (3), 274–286. 10.1016/j.tibs.2015.11.012
7
Bu T. Huang J. Yu Y. Sun P. Yang K. (2023). Whey protein hydrolysate ameliorated high-fat-diet induced bone loss via suppressing oxidative stress and regulating GSK-3β/Nrf2 signaling pathway. Nutrients15 (13), 2863. 10.3390/nu15132863
8
Cabrera J. P. Camino-Willhuber G. Guiroy A. Carazzo C. A. Gagliardi M. Joaquim A. F. (2022). Vertebral augmentation plus short-segment fixation versus vertebral augmentation alone in Kümmell's disease: a systematic review and meta-analysis. Neurosurg. Rev.45 (2), 1009–1018. 10.1007/s10143-021-01661-8
9
Cai W. Wu S. Ming X. Li Z. Pan D. Yang X. et al (2024). IL6 derived from macrophages under intermittent hypoxia exacerbates NAFLD by promoting ferroptosis via MARCH3-Led ubiquitylation of GPX4. Adv. Sci. (Weinh)11 (41), e2402241. 10.1002/advs.202402241
10
Cao G. Hu S. Ning Y. Dou X. Ding C. Wang L. et al (2024). Traditional Chinese medicine in osteoporosis: from pathogenesis to potential activity. Front. Pharmacol.15, 1370900. 10.3389/fphar.2024.1370900
11
Casale M. Citarella S. Filosa A. De Michele E. Palmieri F. Ragozzino A. et al (2014). Endocrine function and bone disease during long-term chelation therapy with deferasirox in patients with β-thalassemia major. Am. J. Hematol.89 (12), 1102–1106. 10.1002/ajh.23844
12
Castany S. Carcolé M. Leánez S. Pol O. (2016). The induction of heme oxygenase 1 decreases painful diabetic neuropathy and enhances the antinociceptive effects of morphine in diabetic mice. PLoS One11 (1), e0146427. 10.1371/journal.pone.0146427
13
Chen J. Chen X. (2022). Editorial: ferroptosis as new therapeutic targets in cancer: from molecular mechanisms to therapeutic opportunities. Front. Pharmacol.13, 1019395. 10.3389/fphar.2022.1019395
14
Chen Y. Fang Z. M. Yi X. Wei X. Jiang D. S. (2023). The interaction between ferroptosis and inflammatory signaling pathways. Cell Death Dis.14 (3), 205. 10.1038/s41419-023-05716-0
15
Chen B. Dong X. Zhang J. L. Sun X. Zhou L. Zhao K. et al (2024a). Natural compounds target programmed cell death (PCD) signaling mechanism to treat ulcerative colitis: a review. Front. Pharmacol.15, 1333657. 10.3389/fphar.2024.1333657
16
Chen Y. Zhao W. Hu A. Lin S. Chen P. Yang B. et al (2024b). Type 2 diabetic mellitus related osteoporosis: focusing on ferroptosis. J. Transl. Med.22 (1), 409. 10.1186/s12967-024-05191-x
17
Chen Y. J. Jia L. H. Han T. H. Zhao Z. H. Yang J. Xiao J. P. et al (2024c). Osteoporosis treatment: current drugs and future developments. Front. Pharmacol.15, 1456796. 10.3389/fphar.2024.1456796
18
Cheng H. Chawla A. Yang Y. Li Y. Zhang J. Jang H. L. et al (2017). Development of nanomaterials for bone-targeted drug delivery. Drug Discov. Today22 (9), 1336–1350. 10.1016/j.drudis.2017.04.021
19
Cheng S. Hu X. Sun K. Huang Z. Zhao Y. Sun Y. et al (2024). Local application of tanshinone IIA protects mesenchymal stem cells from apoptosis and promotes fracture healing in ovariectomized mice. J. Orthop. Surg. Res.19 (1), 309. 10.1186/s13018-024-04793-x
20
Dang D. Zhang C. Meng Z. Lv X. Li Z. Wei J. et al (2022). Integrative analysis links ferroptosis to necrotizing enterocolitis and reveals the role of ACSL4 in immune disorders. iScience25 (11), 105406. 10.1016/j.isci.2022.105406
21
Deng L. He S. Guo N. Tian W. Zhang W. Luo L. (2023). Molecular mechanisms of ferroptosis and relevance to inflammation. Inflamm. Res.72 (2), 281–299. 10.1007/s00011-022-01672-1
22
Dos Santos A. F. Friedmann-Angeli J. P. (2024). Troubling bonds: lipid unsaturation promotes selenium dependency and sensitivity to ferroptosis. EMBO Mol. Med.16 (11), 2657–2659. 10.1038/s44321-024-00150-x
23
Fan Z. Wirth A. K. Chen D. Wruck C. J. Rauh M. Buchfelder M. et al (2017). Nrf2-Keap1 pathway promotes cell proliferation and diminishes ferroptosis. Oncogenesis6 (8), e371. 10.1038/oncsis.2017.65
24
Fang X. Cai Z. Wang H. Han D. Cheng Q. Zhang P. et al (2020). Loss of cardiac ferritin H facilitates cardiomyopathy via Slc7a11-Mediated ferroptosis. Circ. Res.127 (4), 486–501. 10.1161/CIRCRESAHA.120.316509
25
Fang Y. W. Wang C. K. Lin C. Y. (2024). The relationship between serum monoterpene levels and bone health: a retrospective cross-sectional analysis from the national health and nutrition examination survey (NHANES) data. Front. Public Health12, 1436415. 10.3389/fpubh.2024.1436415
26
Feng M. Liu L. Qu Z. Zhang B. Wang Y. Yan L. et al (2023). CRISPR/Cas9 knockout of MTA1 enhanced RANKL-Induced osteoclastogenesis in RAW264.7 cells partly via increasing ROS activities. J. Cell Mol. Med.27 (5), 701–713. 10.1111/jcmm.17692
27
Gao M. Yi J. Zhu J. Minikes A. M. Monian P. Thompson C. B. et al (2019). Role of mitochondria in ferroptosis. Mol. Cell73 (2), 354–363. 10.1016/j.molcel.2018.10.042
28
Gheorghe S. R. Crăciun A. M. Ilyés T. Tisa I. B. Sur L. Lupan I. et al (2024). Converging mechanisms of vascular and cartilaginous calcification. Biol. (Basel)13 (8), 565. 10.3390/biology13080565
29
Guo L. Huang Z. Huang L. Liang J. Wang P. Zhao L. et al (2021). Surface-modified engineered exosomes attenuated cerebral ischemia/reperfusion injury by targeting the delivery of Quercetin towards impaired neurons. J. Nanobiotechnology19 (1), 141. 10.1186/s12951-021-00879-4
30
Guo Z. Lin J. Sun K. Guo J. Yao X. Wang G. et al (2023). Corrigendum: deferoxamine alleviates osteoarthritis by inhibiting chondrocyte ferroptosis and activating the Nrf2 pathway. Front. Pharmacol.14, 1199951. 10.3389/fphar.2023.1199951
31
Han J. Yang K. Jiang N. Fu S. Tang X. (2022). The role of NRF2 in bone metabolism - friend or foe?Front. Endocrinol. (Lausanne)13, 813057. 10.3389/fendo.2022.813057
32
Hu W. Liang K. Zhu H. Zhao C. Hu H. Yin S. (2022). Ferroptosis and its role in chronic diseases. Cells11 (13), 2040. 10.3390/cells11132040
33
Huang L. Zhang S. Bian M. Xiang X. Xiao L. Wang J. et al (2024). Injectable, anti-collapse, adhesive, plastic and bioactive bone graft substitute promotes bone regeneration by moderating oxidative stress in osteoporotic bone defect. Acta Biomater.180, 82–103. 10.1016/j.actbio.2024.04.016
34
Huo G. Lin Y. Liu L. He Y. Qu Y. Liu Y. et al (2024a). Decoding ferroptosis: transforming orthopedic disease management. Front. Pharmacol.15, 1509172. 10.3389/fphar.2024.1509172
35
Huo K. Yang Y. Yang T. Zhang W. Shao J. (2024b). Identification of drug targets and agents associated with ferroptosis-related osteoporosis through integrated network pharmacology and molecular docking technology. Curr. Pharm. Des.30 (14), 1103–1114. 10.2174/0113816128288225240318045050
36
Iantomasi T. Romagnoli C. Palmini G. Donati S. Falsetti I. Miglietta F. et al (2023). Oxidative stress and inflammation in osteoporosis: molecular mechanisms involved and the relationship with microRNAs. Int. J. Mol. Sci.24 (4), 3772. 10.3390/ijms24043772
37
Ibáñez L. Alcaraz M. J. Maicas N. Guede D. Caeiro J. R. Koenders M. I. et al (2011). Up-regulation of the inflammatory response by ovariectomy in collagen-induced arthritis. Effects of tin protoporphyrin IX. Inflammation34 (6), 585–596. 10.1007/s10753-010-9266-4
38
Iseda N. Itoh S. Toshida K. Tomiyama T. Morinaga A. Shimokawa M. et al (2022). Ferroptosis is induced by lenvatinib through fibroblast growth factor receptor-4 inhibition in hepatocellular carcinoma. Cancer Sci.113 (7), 2272–2287. 10.1111/cas.15378
39
Ji Y. Zheng K. Li S. Ren C. Shen Y. Tian L. et al (2022). Insight into the potential role of ferroptosis in neurodegenerative diseases. Front. Cell Neurosci.16, 1005182. 10.3389/fncel.2022.1005182
40
Ji J. Wu S. Bao X. Liu S. Ye Y. Liu J. et al (2023). Mediating oxidative stress through the Palbociclib/miR-141-3p/STAT4 axis in osteoporosis: a bioinformatics and experimental validation study. Sci. Rep.13 (1), 19560. 10.1038/s41598-023-46813-6
41
Jia X. Zhang G. Yu D. (2024). Application of extracellular vesicles in diabetic osteoporosis. Front. Endocrinol. (Lausanne)15, 1466775. 10.3389/fendo.2024.1466775
42
Jiang Z. Qi G. He X. Yu Y. Cao Y. Zhang C. et al (2024). Ferroptosis in osteocytes as a target for protection against postmenopausal osteoporosis. Adv. Sci. (Weinh)11 (12), e2307388. 10.1002/advs.202307388
43
Jin B. Zhang Z. Zhang Y. Yang M. Wang C. Xu J. et al (2024). Ferroptosis and myocardial ischemia-reperfusion: mechanistic insights and new therapeutic perspectives. Front. Pharmacol.15, 1482986. 10.3389/fphar.2024.1482986
44
Kajarabille N. Latunde-Dada G. O. (2019). Programmed cell-death by ferroptosis: antioxidants as mitigators. Int. J. Mol. Sci.20 (19), 4968. 10.3390/ijms20194968
45
Karunatilleke N. C. Fast C. S. Ngo V. Brickenden A. Duennwald M. L. Konermann L. et al (2021). Nrf2, the major regulator of the cellular oxidative stress response, is partially disordered. Int. J. Mol. Sci.22 (14), 7434. 10.3390/ijms22147434
46
Kim I. Kim J. H. Kim K. Seong S. Kim N. (2019). The IRF2BP2-KLF2 axis regulates osteoclast and osteoblast differentiation. BMB Rep.52 (7), 469–474. 10.5483/BMBRep.2019.52.7.104
47
Kim J. W. Lee J. Y. Oh M. Lee E. W. (2023). An integrated view of lipid metabolism in ferroptosis revisited via lipidomic analysis. Exp. Mol. Med.55 (8), 1620–1631. 10.1038/s12276-023-01077-y
48
Laha D. Sarkar J. Maity J. Pramanik A. Howlader M. S. I. Barthels D. et al (2022). Polyphenolic compounds inhibit osteoclast differentiation while reducing autophagy through limiting ROS and the mitochondrial membrane potential. Biomolecules12 (9), 1220. 10.3390/biom12091220
49
Laporte C. Tubbs E. Cristante J. Gauchez A. S. Pesenti S. Lamarche F. et al (2019). Human mesenchymal stem cells improve rat islet functionality under cytokine stress with combined upregulation of heme oxygenase-1 and ferritin. Stem Cell Res. Ther.10 (1), 85. 10.1186/s13287-019-1190-4
50
Li H. Huang C. Zhu J. Gao K. Fang J. (2018). Lutein suppresses oxidative stress and inflammation by Nrf2 activation in an osteoporosis rat model. Med. Sci. Monit.24, 5071–5075. 10.12659/MSM.908699
51
Li Y. Zhu Z. Zhang T. Zhou Y. (2019). Ligustrazine attenuates inflammation and oxidative stress in a rat model of arthritis via the Sirt1/NF-κB and Nrf-2/HO-1 pathways. Arch. Pharm. Res.42 (9), 824–831. 10.1007/s12272-018-1089-0
52
Li J. Cao F. Yin H. L. Huang Z. J. Lin Z. T. Mao N. et al (2020). Ferroptosis: past, present and future. Cell Death and Dis.11 (2), 88. 10.1038/s41419-020-2298-2
53
Li Y. Qian Y. Shen G. Tang C. Zhong X. He S. (2023a). Percutaneous mesh-container-plasty versus percutaneous kyphoplasty for the treatment of Kümmell's disease: a retrospective cohort study. J. Orthop. Surg. Res.18 (1), 260. 10.1186/s13018-023-03753-1
54
Li Y. Yang Y. Guo T. Weng C. Yang Y. Wang Z. et al (2023b). Heme oxygenase-1 determines the cell fate of ferroptotic death of alveolar macrophages in COPD. Front. Immunol.14, 1162087. 10.3389/fimmu.2023.1162087
55
Li M. Pan Z. He Q. Xiao J. Chen B. Wang F. et al (2023c). Arctiin attenuates iron overload-induced osteoporosis by regulating the PI3K/Akt pathway. Int. J. Mol. Med.52 (5), 108. 10.3892/ijmm.2023.5311
56
Liao W. Zhang R. Chen G. Zhu X. Wu W. Chen Z. et al (2024). Berberine synergises with ferroptosis inducer sensitizing NSCLC to ferroptosis in p53-dependent SLC7A11-GPX4 pathway. Biomed. Pharmacother.176, 116832. 10.1016/j.biopha.2024.116832
57
Lin H. Wei B. Li G. Zheng J. Sun J. Chu J. et al (2014). Sulforaphane reverses glucocorticoid-induced apoptosis in osteoblastic cells through regulation of the Nrf2 pathway. Drug Des. Devel Ther.8, 973–982. 10.2147/DDDT.S65410
58
Lin L. He E. Wang H. Guo W. Wu Z. Huang K. et al (2022). Intravenous transplantation of human hair follicle-derived mesenchymal stem cells ameliorates trabecular bone loss in osteoporotic mice. Front. Cell Dev. Biol.10, 814949. 10.3389/fcell.2022.814949
59
Liu Z. Lv X. Song E. Song Y. (2020). Fostered Nrf2 expression antagonizes iron overload and glutathione depletion to promote resistance of neuron-like cells to ferroptosis. Toxicol. Appl. Pharmacol.407, 115241. 10.1016/j.taap.2020.115241
60
Liu G. Z. Xu X. W. Tao S. H. Gao M. J. Hou Z. H. (2021). HBx facilitates ferroptosis in acute liver failure via EZH2 mediated SLC7A11 suppression. J. Biomed. Sci.28 (1), 67. 10.1186/s12929-021-00762-2
61
Liu N. Sun S. Wang P. Sun Y. Hu Q. Wang X. (2021). The mechanism of secretion and metabolism of gut-derived 5-Hydroxytryptamine. Int. J. Mol. Sci.22 (15), 7931. 10.3390/ijms22157931
62
Liu J. Han X. Zhou J. Leng Y. (2023). Molecular mechanisms of ferroptosis and their involvement in acute kidney injury. J. Inflamm. Res.16, 4941–4951. 10.2147/JIR.S427505
63
Liu X. F. Liao Y. T. Shao J. H. He D. D. Fan Z. H. Xu Y. N. et al (2024). Angelicin improves osteoporosis in ovariectomized rats by reducing ROS production in osteoclasts through regulation of the KAT6A/Nrf2 signalling pathway. Chin. Med.19 (1), 91. 10.1186/s13020-024-00961-7
64
Liu Y. Li J. Zhang Z. Li Q. Tian Y. Wang S. et al (2025). Echinococcus granulosus promotes MAPK pathway-mediated osteoclast differentiation by inhibiting Nrf2 in osseous echinococcosis. Veterinary Res.56 (1), 81. 10.1186/s13567-025-01510-2
65
Lovati A. B. Bottagisio M. Maraldi S. Violatto M. B. Bortolin M. De Vecchi E. et al (2018). Vitamin E phosphate coating stimulates bone deposition in implant-related infections in a rat model. Clin. Orthop. Relat. Res.476 (6), 1324–1338. 10.1097/01.blo.0000534692.41467.02
66
Lv W. Hu S. Yang F. Lin D. Zou H. Zhang W. et al (2024). Heme oxygenase-1: potential therapeutic targets for periodontitis. PeerJ12, e18237. 10.7717/peerj.18237
67
Ma T. Chen H. Ruan H. Lv L. Yu Y. Jia L. et al (2022). Natural product, bilobalide, improves joint health in rabbits with osteoarthritis by anti-matrix degradation and antioxidant activities. Front. Vet. Sci.9, 1034623. 10.3389/fvets.2022.1034623
68
Malakoti F. Zare F. Zarezadeh R. Raei Sadigh A. Sadeghpour A. Majidinia M. et al (2022). The role of melatonin in bone regeneration: a review of involved signaling pathways. Biochimie202, 56–70. 10.1016/j.biochi.2022.08.008
69
Mao H. Feng Y. Feng J. Yusufu Y. Sun M. Yang L. et al (2024). Quercetin-3-O-β-D-glucuronide attenuates osteoarthritis by inhibiting cartilage extracellular matrix degradation and inflammation. J. Orthop. Transl.45, 236–246. 10.1016/j.jot.2024.01.007
70
Martínez-Casales M. Hernanz R. Alonso M. J. (2021). Vascular and macrophage heme Oxygenase-1 in hypertension: a mini-review. Front. Physiol.12, 643435. 10.3389/fphys.2021.643435
71
Montaseri A. Giampietri C. Rossi M. Riccioli A. Del Fattore A. Filippini A. (2020). The role of autophagy in osteoclast differentiation and bone resorption function. Biomolecules10 (10), 1398. 10.3390/biom10101398
72
Montoya T. Sánchez-Hidalgo M. Castejón M. L. Rosillo M. Á. González-Benjumea A. Alarcón-de-la-Lastra C. (2021). Dietary oleocanthal supplementation prevents inflammation and oxidative stress in collagen-induced arthritis in mice. Antioxidants (Basel)10 (5), 650. 10.3390/antiox10050650
73
Pan B. Zheng L. Fang J. Lin Y. Lai H. Gao J. et al (2021). Azilsartan suppresses osteoclastogenesis and ameliorates ovariectomy-induced osteoporosis by inhibiting reactive oxygen species production and activating Nrf2 signaling. Front. Pharmacol.12, 774709. 10.3389/fphar.2021.774709
74
Pribil Pardun S. Bhat A. Anderson C. P. Allen M. F. Bruening W. Jacob J. et al (2024). Electrical pulse stimulation protects C2C12 myotubes against hydrogen peroxide-induced cytotoxicity via Nrf2/Antioxidant pathway. Antioxidants (Basel)13 (6), 716. 10.3390/antiox13060716
75
Qi S. Peng B. Xu Z. Qiu D. Tan G. (2024). The relationship between non-HDL-C/HDL-C ratio and bone mineral density: an NHANES study. Front. Nutr.11, 1486370. 10.3389/fnut.2024.1486370
76
Qu Z. Zhang B. Kong L. Zhang Y. Zhao Y. Gong Y. et al (2024). Myeloid zinc finger 1 knockdown promotes osteoclastogenesis and bone loss in part by regulating RANKL-Induced ferroptosis of osteoclasts through Nrf2/GPX4 signaling pathway. J. Leukoc. Biol.115 (5), 946–957. 10.1093/jleuko/qiae011
77
Samsa W. E. Vasanji A. Midura R. J. Kondratov R. V. (2016). Deficiency of circadian clock protein BMAL1 in mice results in a low bone mass phenotype. Bone84, 194–203. 10.1016/j.bone.2016.01.006
78
Sánchez-de-Diego C. Pedrazza L. Pimenta-Lopes C. Martinez-Martinez A. Dahdah N. Valer J. A. et al (2021). NRF2 function in osteocytes is required for bone homeostasis and drives osteocytic gene expression. Redox Biol.40, 101845. 10.1016/j.redox.2020.101845
79
Shen H. Ma Y. Qiao Y. Zhang C. Chen J. Zhang R. (2024). Application of deferoxamine in tissue regeneration attributed to promoted angiogenesis. Molecules29 (9), 2050. 10.3390/molecules29092050
80
Shou Z. Bai Z. Zhou H. Shen Y. Huang X. Meng H. et al (2024). Engineering tunable dual peptide hybrid coatings promote osseointegration of implants. Mater Today Bio24, 100921. 10.1016/j.mtbio.2023.100921
81
Song X. Long D. (2020). Nrf2 and ferroptosis: a new research direction for neurodegenerative diseases. Front. Neurosci.14, 267. 10.3389/fnins.2020.00267
82
Song Q. Zhang Y. Hu H. Yang X. Xing X. Wu J. et al (2024). Augment of ferroptosis with photothermal enhanced fenton reaction and glutathione inhibition for tumor synergistic nano-catalytic therapy. Int. J. Nanomedicine19, 11923–11940. 10.2147/IJN.S480586
83
Sun A. Hu J. Wang S. Yin F. Liu Z. (2023). Association of the visceral adiposity index with femur bone mineral density and osteoporosis among the U.S. older adults from NHANES 2005-2020: a cross-sectional study. Front. Endocrinol. (Lausanne)14, 1231527. 10.3389/fendo.2023.1231527
84
Sun K. Gao L. Li S. Zheng J. Zhu Z. Zhi K. et al (2024). Circ-CDK8 regulates SLC7A11-mediated ferroptosis by inhibiting miR-615-5p to promote progression in oral squamous cell carcinomas. Front. Pharmacol.15, 1432520. 10.3389/fphar.2024.1432520
85
Tao Z. Wang J. Wen K. Yao R. Da W. Zhou S. et al (2020). Pyroptosis in osteoblasts: a novel hypothesis underlying the pathogenesis of osteoporosis. Front. Endocrinol. (Lausanne)11, 548812. 10.3389/fendo.2020.548812
86
Tao L. Xu J. Jiang L. Hu J. Tang Z. (2025). Investigation into the influence of mild hypothermia on regulating ferroptosis through the P53-SLC7A11/GPX4 signaling pathway in sepsis-induced acute lung injury. Intensive Care Med. Exp.13 (1), 4. 10.1186/s40635-025-00713-3
87
Thaler R. Maurizi A. Roschger P. Sturmlechner I. Khani F. Spitzer S. et al (2016). Anabolic and antiresorptive modulation of bone homeostasis by the epigenetic modulator sulforaphane, a naturally occurring isothiocyanate. J. Biol. Chem.291 (13), 6754–6771. 10.1074/jbc.M115.678235
88
Tonelli C. Chio I. I. C. Tuveson D. A. (2018). Transcriptional regulation by Nrf2. Antioxid. Redox Signal29 (17), 1727–1745. 10.1089/ars.2017.7342
89
Tun S. Spainhower C. J. Cottrill C. L. Lakhani H. V. Pillai S. S. Dilip A. et al (2020). Therapeutic efficacy of antioxidants in ameliorating obesity phenotype and associated comorbidities. Front. Pharmacol.11, 1234. 10.3389/fphar.2020.01234
90
Valanezhad A. Odatsu T. Abe S. Watanabe I. (2021). Bone formation ability and cell viability enhancement of MC3T3-E1 cells by Ferrostatin-1 a ferroptosis inhibitor of cancer cells. Int. J. Mol. Sci.22 (22), 12259. 10.3390/ijms222212259
91
Van Phan T. Sul O. J. Ke K. Lee M. H. Kim W. K. Cho Y. S. et al (2013). Carbon monoxide protects against ovariectomy-induced bone loss by inhibiting osteoclastogenesis. Biochem. Pharmacol.85 (8), 1145–1152. 10.1016/j.bcp.2013.01.014
92
Wang K. (2024). The potential therapeutic role of curcumin in osteoporosis treatment: based on multiple signaling pathways. Front. Pharmacol.15, 1446536. 10.3389/fphar.2024.1446536
93
Wang H. H. Hsu Y. H. Chang M. S. (2018). IL-20 bone diseases involvement and therapeutic target potential. J. Biomed. Sci.25 (1), 38. 10.1186/s12929-018-0439-z
94
Wang M. Qiu L. Ru X. Song Y. Zhang Y. (2019). Distinct isoforms of Nrf1 diversely regulate different subsets of its cognate target genes. Sci. Rep.9 (1), 2960. 10.1038/s41598-019-39536-0
95
Wang N. Xin H. Xu P. Yu Z. Shou D. (2019). Erxian decoction attenuates TNF-α induced osteoblast apoptosis by modulating the Akt/Nrf2/HO-1 signaling pathway. Front. Pharmacol.10, 988. 10.3389/fphar.2019.00988
96
Wang B. Huang C. Chen L. Xu D. Zheng G. Zhou Y. et al (2020). The emerging roles of the gaseous signaling molecules NO, H(2)S, and CO in the regulation of stem cells. ACS Biomater. Sci. Eng.6 (2), 798–812. 10.1021/acsbiomaterials.9b01681
97
Wang R. Xing R. Su Q. Yin H. Wu D. Lv C. et al (2021). Knockdown of SFRS9 inhibits progression of colorectal cancer through triggering ferroptosis mediated by GPX4 reduction. Front. Oncol.11, 683589. 10.3389/fonc.2021.683589
98
Wang D. Tang L. Zhang Y. Ge G. Jiang X. Mo Y. et al (2022). Regulatory pathways and drugs associated with ferroptosis in tumors. Cell Death Dis.13 (6), 544. 10.1038/s41419-022-04927-1
99
Wang C. S. Xue H. B. Zhuang L. Sun H. P. Zheng H. Wang S. et al (2023). Developing single-atomic manganese nanozymes for synergistic mild photothermal/multienzymatic therapy. ACS Omega8 (51), 49289–49301. 10.1021/acsomega.3c07714
100
Wang X. Fan F. Hou Y. Meng X. (2024). Tile: construction of a specific nanoprobe for scavenging ROS in hypobaric hypoxia induced brain injury of mice. Heliyon10 (20), e38958. 10.1016/j.heliyon.2024.e38958
101
Wei X. Li X. Hu S. Cheng J. Cai R. (2023). Regulation of ferroptosis in lung adenocarcinoma. Int. J. Mol. Sci.24 (19), 14614. 10.3390/ijms241914614
102
Wu Y. H. Hsieh H. L. (2022). Roles of heme Oxygenase-1 in neuroinflammation and brain disorders. Antioxidants (Basel)11 (5), 923. 10.3390/antiox11050923
103
Wu Q. Huang F. (2024). Targeting ferroptosis as a prospective therapeutic approach for diabetic nephropathy. Ann. Med.56 (1), 2346543. 10.1080/07853890.2024.2346543
104
Wu Y. Song P. Wang M. Liu H. Jing Y. Su J. (2024). Extracellular derivatives for bone metabolism. J. Adv. Res.66, 329–347. 10.1016/j.jare.2024.01.011
105
XiaHumulus lupulus T. L. Guo Y. Jiang Y. Qiao F. Li K. et al (2023). Humulus lupulus L. extract protects against senior osteoporosis through inhibiting amyloid β deposition and oxidative stress in APP/PS1 mutated transgenic mice and osteoblasts. Molecules28 (2), 583. 10.3390/molecules28020583
106
Xiao J. Zhang G. Chen B. He Q. Mai J. Chen W. et al (2023). Quercetin protects against iron overload-induced osteoporosis through activating the Nrf2/HO-1 pathway. Life Sci.322, 121326. 10.1016/j.lfs.2022.121326
107
Xie F. Song Y. Yi Y. Jiang X. Ma S. et al (2023a). Therapeutic potential of molecular hydrogen in metabolic diseases from bench to bedside. Pharm. (Basel)16 (4), 541. 10.3390/ph16040541
108
Xie Y. Kang R. Klionsky D. J. Tang D. (2023b). GPX4 in cell death, autophagy, and disease. Autophagy19 (10), 2621–2638. 10.1080/15548627.2023.2218764
109
Xiong Y. Chen L. Lin Z. Hu Y. Panayi A. C. Zhou W. et al (2022). The regulatory role of ferroptosis in bone homeostasis. Stem Cells Int.2022, 3568597. 10.1155/2022/3568597
110
Xu P. Lin B. Deng X. Huang K. Zhang Y. Wang N. (2022). VDR activation attenuates osteoblastic ferroptosis and senescence by stimulating the Nrf2/GPX4 pathway in age-related osteoporosis. Free Radic. Biol. Med.193 (Pt 2), 720–735. 10.1016/j.freeradbiomed.2022.11.013
111
Xue P. Hu X. Powers J. Nay N. Chang E. Kwon J. et al (2019). CDDO-Me, sulforaphane and tBHQ attenuate the RANKL-Induced osteoclast differentiation via activating the NRF2-mediated antioxidant response. Biochem. Biophys. Res. Commun.511 (3), 637–643. 10.1016/j.bbrc.2019.02.095
112
Yamaguchi Y. Kanzaki H. Katsumata Y. Itohiya K. Fukaya S. Miyamoto Y. et al (2018). Dimethyl fumarate inhibits osteoclasts via attenuation of reactive oxygen species signalling by augmented antioxidation. J. Cell Mol. Med.22 (2), 1138–1147. 10.1111/jcmm.13367
113
Yan C. Zhang J. An F. Wang J. Shi Y. Yuan L. et al (2022). Research progress of ferroptosis regulatory network and bone remodeling in osteoporosis. Front. Public Health10, 910675. 10.3389/fpubh.2022.910675
114
Yan X. Liu Y. Mao X. Xu T. Hu Z. et al (2023). Pien-tze-huang prevents hepatocellular carcinoma by inducing ferroptosis via inhibiting SLC7A11-GSH-GPX4 axis. Cancer Cell Int.23 (1), 109. 10.1186/s12935-023-02946-2
115
Yang N. Shang Y. (2022). Ferrostatin-1 and 3-Methyladenine ameliorate ferroptosis in OVA-induced asthma model and in IL-13-Challenged BEAS-2B cells. Oxid. Med. Cell Longev.2022, 9657933. 10.1155/2022/9657933
116
Yang W. S. SriRamaratnam R. Welsch M. E. Shimada K. Skouta R. Viswanathan V. S. et al (2014). Regulation of ferroptotic cancer cell death by GPX4. Cell156 (1-2), 317–331. 10.1016/j.cell.2013.12.010
117
Yang W. S. Kim K. J. Gaschler M. M. Patel M. Shchepinov M. S. Stockwell B. R. (2016). Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. U. S. A.113 (34), E4966–E4975. 10.1073/pnas.1603244113
118
Yang Y. Lin Y. Wang M. Yuan K. Wang Q. Mu P. et al (2022). Targeting ferroptosis suppresses osteocyte glucolipotoxicity and alleviates diabetic osteoporosis. Bone Res.10 (1), 26. 10.1038/s41413-022-00198-w
119
Yang R. Guo Y. Zong S. Ma Z. Wang Z. Zhao J. et al (2023). Bardoxolone methyl ameliorates osteoarthritis by inhibiting osteoclastogenesis and protecting the extracellular matrix against degradation. Heliyon9 (2), e13080. 10.1016/j.heliyon.2023.e13080
120
Yao Z. Y. Fan S. Y. Song Z. F. Li Z. C. (2023). Network pharmacology-based and molecular docking-based analysis of you-gui-yin for the treatment of osteonecrosis of the femoral head. Med. Baltim.102 (43), e35581. 10.1097/MD.0000000000035581
121
Ze Y. Wu Y. Tan Z. Li R. Li R. Gao W. et al (2025). Signaling pathway mechanisms of circadian clock gene Bmal1 regulating bone and cartilage metabolism: a review. Bone Res.13 (1), 19. 10.1038/s41413-025-00403-6
122
Zeng J. Guo J. Huang S. Cheng Y. Luo F. Xu X. et al (2023). The roles of sirtuins in ferroptosis. Front. Physiol.14, 1131201. 10.3389/fphys.2023.1131201
123
Zhai Z. Huang Y. Zhang Y. Zhao L. Li W. (2022). Clinical research progress of small molecule compounds targeting Nrf2 for treating inflammation-related diseases. Antioxidants (Basel)11 (8), 1564. 10.3390/antiox11081564
124
Zhang H. Jin Y. Wang M. Loor J. J. Wang H. (2020). N-Carbamylglutamate and l-arginine supplementation improve hepatic antioxidant status in intrauterine growth-retarded suckling lambs. RSC Adv.10 (19), 11173–11181. 10.1039/c9ra09316h
125
Zhang Y. Liu X. Li Y. Song M. Li Y. Yang A. et al (2021). Aucubin slows the development of osteoporosis by inhibiting osteoclast differentiation via the nuclear factor erythroid 2-related factor 2-mediated antioxidation pathway. Pharm. Biol.59 (1), 1556–1565. 10.1080/13880209.2021.1996614
126
Zhang S. Duan S. Xie Z. Bao W. Xu B. Yang W. et al (2022a). Epigenetic therapeutics targeting NRF2/KEAP1 signaling in cancer oxidative stress. Front. Pharmacol.13, 924817. 10.3389/fphar.2022.924817
127
Zhang Y. Li L. Li Y. Fei Y. Xue C. Yao X. et al (2022b). An ROS-activatable nanoassembly remodulates tumor cell metabolism for enhanced ferroptosis therapy. Adv. Healthc. Mater11 (2), e2101702. 10.1002/adhm.202101702
128
Zhang J. Zhang L. Yao G. Zhao H. Wu S. (2023). NRF2 is essential for iron-overload stimulated osteoclast differentiation through regulation of redox and iron homeostasis. Cell Biol. Toxicol.39 (6), 3305–3321. 10.1007/s10565-023-09834-5
129
Zhang Y. Kong F. Tao L. Zhai J. Ma J. et al (2025). Potential role of SIRT1 in cell ferroptosis. Front. Cell Dev. Biol.13, 1525294. 10.3389/fcell.2025.1525294
130
Zheng Y. Gao A. Bai J. Liao Q. Wu Y. Zhang W. et al (2022). A programmed surface on polyetheretherketone for sequentially dictating osteoimmunomodulation and bone regeneration to achieve ameliorative osseointegration under osteoporotic conditions. Bioact. Mater14, 364–376. 10.1016/j.bioactmat.2022.01.042
131
Zhong Y. Li Z. N. Jiang X. Y. Tian X. Deng M. H. Cheng M. S. et al (2022). Identification of novel artemisinin hybrids induce apoptosis and ferroptosis in MCF-7 cells. Int. J. Mol. Sci.23 (24), 15768. 10.3390/ijms232415768
132
Zhou X. Yuan W. Xiong X. Zhang Z. Liu J. Zheng Y. et al (2021). HO-1 in bone biology: potential therapeutic strategies for osteoporosis. Front. Cell Dev. Biol.9, 791585. 10.3389/fcell.2021.791585
133
Zhu S. Chen W. Masson A. Li Y. P. (2024). Cell signaling and transcriptional regulation of osteoblast lineage commitment, differentiation, bone formation, and homeostasis. Cell Discov.10 (1), 71. 10.1038/s41421-024-00689-6
Summary
Keywords
Nrf2, HO-1, ferroptosis, bone homeostasis, oxidative stress
Citation
Nan W, Zhou W-M, Zi J-L, Shi Y-Q, Dong Y-B, Song W, Ma Y-C and Zhang H-H (2025) Ferroptosis and bone metabolic diseases: the dual regulatory role of the Nrf2/HO-1 signaling axis. Front. Cell Dev. Biol. 13:1615197. doi: 10.3389/fcell.2025.1615197
Received
20 April 2025
Accepted
26 August 2025
Published
05 September 2025
Volume
13 - 2025
Edited by
Xianwei Wang, Xinxiang Medical University, China
Reviewed by
Farah Deba, University of Texas at Tyler, United States
Hongbing Yin, The Third Affiliated Clinical Hospital of Changchun University of Traditional Chinese Medicine, China
Bo Zhang, Nanjing medical university, China
Updates
Copyright
© 2025 Nan, Zhou, Zi, Shi, Dong, Song, Ma and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hai-Hong Zhang, Zhanghh22@foxmail.com; Yan-Chao Ma, mayc2024@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.