Abstract
Cancer stem cells (CSCs) drive tumor progression, therapy resistance, and metastasis through unique membrane biology, glycosylation patterns, and metabolic adaptations. CSCs exhibit a distinct glycocalyx profile enriched in hyaluronan, heparan sulfate, and sialylated glycans, facilitating immune evasion, adhesion, and survival. Key signaling pathways—Wnt/β-catenin, Hedgehog, Notch, JAK/STAT, TGF/SMAD, and PI3K/AKT/mTOR—regulate CSC stemness and therapeutic resistance. Emerging biomarkers (CD44, CD133, ALDH1, EpCAM) and targeted therapies (CAR-T cells, miRNA modulation, lipid metabolism inhibitors) show promise in disrupting CSC resilience. Advances in single-cell omics, CRISPR screening, and patient-derived organoids (PDOs) enhance CSC characterization and precision medicine applications. However, challenges remain in standardizing organoid cultures, replicating tumor microenvironments, and overcoming CSC plasticity. Integrating CSC-targeted strategies with conventional therapies may improve clinical outcomes by eradicating therapy-resistant populations and preventing relapse. This review underscores the need for innovative combination therapies to eradicate CSCs and improve clinical outcomes, while addressing challenges in biomarker validation, therapeutic resistance, and translational applications.
Introduction
Cancer, “a wound that never heals”, is a leading cause of death worldwide, which is characterized by the uncontrolled proliferation of abnormal cells and aberrant recognition of immune system (Ushijima et al., 2021; Hanahan, 2022). The global World Health Organization (WHO) survey on universal health coverage (UHC) and cancer show that only 39% of participating countries covered the basics of cancer management as part of their financed core health services for all citizens, while only 28% of participating countries additionally covered care for people who require palliative care (WHO, 2024).
Cancer stem cells (CSCs) represent a subpopulation of tumor cells with self-renewal capacity, differentiation potential, and resistance to conventional therapies, driving tumor initiation, progression, metastasis, and recurrence (Kreso and Dick, 2014; Clarke, 2019). Unlike differentiated cancer cells, CSCs exhibit unique membrane biology and glycosylation patterns that contribute to their malignant behavior. A distinctive glycocalyx profile, characterized by the overexpression of specific glycans, proteoglycans, and glycosylation enzymes, plays a pivotal role in CSC interactions with the tumor microenvironment (TME), immune evasion, and adhesion-mediated metastasis (Kang et al., 2018; Zheng et al., 2022). Notably, glycosaminoglycans (GAGs) such as hyaluronan and heparan sulfate facilitate pro-survival signaling (e.g., CD44-HA and Wnt/β-catenin), while proteoglycans like syndecan-1 and glypican-3 enhance epithelial-mesenchymal transition (EMT) and growth factor signaling (Wang et al., 2025; Teixeira et al., 2020). Furthermore, CSC glycocalyx components recruit immunosuppressive cells (e.g., Tregs, MDSCs, TAMs) and engage immune checkpoints (e.g., PD-L1, Siglec receptors), promoting an immune-privileged niche (Ju et al., 2022; Oshimori, 2020).
Beyond membrane dynamics, CSCs rely on exosome-mediated crosstalk with the TME to foster angiogenesis, stromal activation, and drug resistance (da Costa et al., 2021). CSC-derived exosomes transfer EMT-inducing factors (e.g., Twist, Snail, TGF-β) and oncogenic miRNAs, reinforcing stemness and metastatic potential (Babaei et al., 2021). Metabolic adaptations, particularly in lipid metabolism (e.g., FAO, lipogenesis), further sustain CSC resilience under stress. Key pathways such as Wnt/β-catenin, Hedgehog, Notch, JAK/STAT3, and PI3K/AKT/mTOR orchestrate CSC maintenance (Su et al., 2015; Wang et al., 2012; Sari et al., 2018; Wang T. et al., 2018; Karami et al., 2022), while biomarkers like CD44, CD133, and ALDH1 aid in their identification and targeting (Martinez-Gamero et al., 2021; Azzoni et al., 2022). Recently, three-dimensional (3D) organoids have bridged the gap between in vitro two-dimensional (2D) cell lines and in vivo mouse models for cancer research, which are established from adult stem cells (ASCs) and pluripotent stem cells (PSCs) (Sato et al., 2009; McCauley and Wells, 2017).
Emerging technologies—single-cell omics, CRISPR screens, and AI—are refining CSC characterization and therapeutic strategies. Patient-derived organoids (PDOs) now enable precision medicine by modeling tumor heterogeneity and therapy responses. However, clinical translation faces challenges, including CSC plasticity, niche-mediated resistance, and toxicity concerns. This review synthesizes advances in CSC biology, therapeutic targeting, and innovative models, offering insights into overcoming CSC-driven therapy resistance and improving cancer outcomes.
Methodological overview of CSC identification and validation
To provide a robust foundation for our discussion of cancer stem cells (CSCs) and their role in personalized therapy, it is essential to first establish the experimental framework used to identify and validate these elusive cell populations. The characterization of CSCs relies on a combination of surface marker analysis, functional assays, and in vivo validation, each contributing unique insights into their biological properties.
Surface marker-based isolation represents one of the most widely adopted approaches, with markers such as CD44, CD133, and ALDH1 serving as key indicators of CSC populations (Rugg-Gunn, 2022). Flow cytometry enables the precise enrichment of these subpopulations, with specific combinations—such as CD44+CD24−/low cells in breast cancer—providing clinically relevant signatures (Chen et al., 2020). Complementing this, the Aldefluor assay detects elevated aldehyde dehydrogenase (ALDH) activity, an enzyme frequently overexpressed in CSCs, allowing for fluorescence-based separation of ALDH-high cells (Duan et al., 2023; Del Vecchio et al., 2024).
Functional assays further validate CSC properties, with sphere formation being a hallmark of self-renewal capacity (Bahmad et al., 2018; Perry and Li, 2010). When cultured in serum-free, non-adherent conditions, CSCs generate three-dimensional spheres, reflecting their ability to proliferate and maintain stemness over multiple passages. This assay is particularly valuable for assessing the hierarchical organization of tumors (Ma et al., 2019).
The gold standard for CSC validation remains in vivo tumorigenicity assays, wherein sorted cells are injected into immunocompromised mice to evaluate their tumor-initiating potential (Skidan and Steiniger, 2014; Sato et al., 2019). Notably, even a minimal cell population can suffice to generate tumors, underscoring the profound biological potency of CSCs. This approach not only confirms stemness but also provides critical insights into therapeutic resistance mechanisms (Makena et al., 2020).
Collectively, these methodologies establish a framework for identifying and characterizing CSCs, bridging molecular observations with clinically relevant phenotypes. By integrating these techniques, researchers can better delineate the molecular pathways driving CSC maintenance and develop targeted therapies to disrupt their survival and proliferation.
Membrane biology and glycosylation of CSCs
The unique membrane biology and glycosylation patterns of CSCs play a pivotal role in their interactions with the TME and their resistance to therapies, which is essential for developing targeted therapeutic strategies. CSCs possess a unique glycocalyx profile characterized by the overexpression of specific glycans, proteoglycans, and glycosylation enzymes, which serve as critical mediators of their malignant behavior (Park et al., 2024). CSC glycocalyx enriches in certain glycosaminoglycans (GAGs), such as hyaluronan and heparan sulfate, which facilitate interactions with the tumor microenvironment and activate pro-survival signaling pathways. CSC glycocalyx also displays an upregulation of proteoglycans like syndecan-1 and glypican-3, which modulate growth factor signaling and enhance epithelial-mesenchymal transition (Wei et al., 2024).
Glycocalyx components, particularly hyaluronan and chondroitin sulfate proteoglycans, create a pro-tumorigenic microenvironment by recruiting immunosuppressive cells such as regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs), which further inhibit effector immune responses and foster CSC survival. Glycocalyx components, such as mucins and sialylated glycans, interact with inhibitory immune checkpoints like PD-L1 and Siglec receptors (Buffone and Weaver, 2020). Overexpression of sialic acid residues (e.g., polysialic acid) engages Siglecs on immune cells, transmitting “do not eat me” signals that suppress phagocytosis by macrophages and dendritic cells. Similarly, mucins can directly bind to immune receptors, dampening cytotoxic responses (Ayyalasomayajula and Cudic, 2024). Consequently, targeting glycocalyx components represents a promising therapeutic strategy to overcome CSC-mediated immune escape and improve cancer immunotherapy efficacy.
The glycocalyx facilitates CSC adhesion through specific ligand-receptor binding, such as CD44-Hyaluronan (HA) interaction, selectin-mediated adhesion, and integrin activation (Kanyo et al., 2020). The glycocalyx acts as a physical barrier and shock absorber, allowing CSCs to withstand hemodynamic shear forces during circulation, which facilitates transient adhesion and firm attachment to vascular endothelia (Lipowsky, 2018). Enzymatic modifications alter glycocalyx density, which affect adhesion strength and migratory capacity. The strategies for disrupting glycocalyx-mediated adhesion include enzymatic degradation of glycocalyx components (e.g., hyaluronidase), inhibition of glycan-binding receptors (e.g., anti-CD44 antibodies, selectin antagonists), nanotechnology-based approaches (nanoparticles).
CSCs exhibit distinct glycosylation patterns compared to differentiated cancer cells and normal stem cells, which include truncated O-Glycans (e.g., Tn and Sialyl-Tn antigens), sialylation (e.g., α2,6- and α2,3-linked sialic acids), fucosylation (e.g., Lewis antigens, Core fucosylation), heparan sulfate proteoglycans (HSPGs), N-Glycan branching (e.g., β1,6-GlcNAc branching by MGAT5) (Rømer et al., 2021; Mun and kley, 2022; Shan et al., 2019; Onyeisi et al., 2020; Taniguchi et al., 2022). Glycan-based biomarkers hold great promise for CSC detection and therapeutic targeting, which include CD44 variants (CD44v) with unique glycosylation, CD133 (Prominin-1) glycosylation, podocalyxin (PODXL), integrins with altered glycosylation, and glycosphingolipids (GSLs) (Hu et al., 2019).
Understanding the unique glycocalyx profile of CSCs provides a foundation for exploring their interactions with the TME and their role in therapy resistance, which leads us to examine the mechanisms by which CSCs communicate with their surroundings and maintain their stemness, including exosome-mediated crosstalk and EMT.
Exosomes, EMT, and CSC crosstalk
Exosomes, a subset of extracellular vesicles (EVs) ranging from 30 to 150 nm in diameter, mediate bidirectional crosstalk between CSC and TME, which influence tumor initiation, progression, metastasis, therapeutic resistance, and immune evasion (Li et al., 2024). CSC-derived exosomes can reprogram neighboring non-CSC tumor cells into a stem-like phenotype, promoting tumor heterogeneity. CSC-secreted exosomes contribute to the formation of a supportive niche by inducing angiogenesis, activating fibroblasts, and remodeling the extracellular matrix (Zabeti et al., 2024). CSC-secreted exosomes can inhibit T-cell and NK-cell activity while promoting regulatory T-cell (Treg) expansion, which establish an immune-privileged niche conducive to CSC survival (Yang and Teng, 2023).
CSC-secreted exosomes contribute to metastatic dissemination by priming pre-metastatic niches and enhancing the invasiveness of cancer cells, which transfer pro-metastatic factors to neighboring stromal and tumor cells, promoting angiogenesis, extracellular matrix (ECM) remodeling, and immune evasion (Li X. et al., 2022; Rashid et al., 2023). CSC-exosomes confer chemoresistance by transferring drug-efflux pumps, anti-apoptotic proteins, and resistance-associated miRNAs to sensitive cancer cells, which modulate the TME by inducing a cancer-associated fibroblast phenotype to secrete protective cytokines and shield CSCs from therapy (Chung et al., 2021; Zhuang et al., 2023).
Epithelial-mesenchymal transition (EMT), a critical process in cancer metastasis and recurrence, could involve the transformation of epithelial cells into a mesenchymal phenotype, which enhances migratory capacity, invasiveness, and resistance to apoptosis (Mortezaee et al., 2022). EMT transcription factors serve as molecular bridges between cellular plasticity and stemness, which enable CSCs to evade therapy and drive metastasis (Lu and Kang, 2019). CSC-derived exosomes carry a repertoire of EMT-promoting molecules, including transcription factors (e.g., Twist, Snail, Slug, Zeb1/2), miRNAs (e.g., miR-21, miR-10b, miR-155), and cytokines (e.g., TGF-β, IL-6), which modulate signaling pathways such as Wnt/β-catenin, NF-κB, and PI3K/Akt, leading to the downregulation of epithelial markers (E-cadherin) and upregulation of mesenchymal markers (N-cadherin, vimentin, fibronectin) (Debnath et al., 2022; Lin et al., 2014; Ray et al., 2023; Kitazawa et al., 2024; Guo et al., 2024).
EMT-induced stemness confers tumor cells with increased self-renewal capacity, plasticity, and adaptability, contributing to tumor heterogeneity and therapy evasion, which could promote resistance to conventional therapies (chemotherapy, radiotherapy, and targeted therapies) through multiple mechanisms, such as enhanced DNA repair and survival pathways, drug efflux pumps, metabolic adaptations, and immune evasion (Lei et al., 2024; Roy et al., 2021; Voon et al., 2013). Given the role of EMT-induced stemness in therapeutic resistance, novel strategies are needed to target this aggressive cell population, which include epigenetic modulators (reverse EMT-associated transcriptional reprogramming), immune-based strategies (CSC-targeted vaccines or CAR-T cells engineered to recognize EMT/CSC markers), combination therapies (target both EMT and stemness pathways) (Peixoto et al., 2019; Qian et al., 2020; Sadrkhanloo et al., 2022).
The role of exosomes and EMT in CSC biology highlights the importance of understanding the metabolic adaptations that sustain CSC resilience. The metabolic adaptations of CSCs, particularly in lipid metabolism, are crucial for their survival and stemness maintenance. These metabolic pathways provide energy and biosynthetic precursors, enabling CSCs to thrive in challenging microenvironments.
Lipid metabolism in CSCs
Lipid metabolism, including fatty acid oxidation (FAO) and de novo lipogenesis, could not only fulfill the bioenergetic and biosynthetic demands of CSCs but also regulate redox homeostasis, signaling pathways, and epigenetic modifications, thereby maintaining stemness and survival under stress conditions (Singh et al., 2024). CSCs exhibit heightened reliance on mitochondrial FAO to generate adenosine triphosphate (ATP), especially in nutrient-deprived or hypoxic microenvironments, which is mediated by upregulation of key enzymes such as carnitine palmitoyltransferase 1 (CPT1) and is linked to chemoresistance via enhanced oxidative stress mitigation (Mascaraque et al., 2024). Lipogenesis, de novo fatty acid synthesis, driven by acetyl-CoA carboxylase (ACC) and fatty acid synthase (FASN), supports CSC membrane biogenesis, lipid raft formation, and oncogenic signaling (Liu Z. et al., 2023). Targeting lipid metabolism in CSCs presents a promising strategy to overcome therapy resistance, which include FAO inhibitors (e.g., etomoxir, ranolazine) and lipogenesis inhibitors (e.g., orlistat, TVB-3166) (Ahn et al., 2024; Batchuluun et al., 2022).
Key enzymes in lipid metabolism, such as carnitine palmitoyltransferase 1A (CPT1A) and ATP-citrate lyase (ACLY), have been implicated in CSC maintenance. CPT1A, the rate-limiting enzyme in mitochondrial FAO, facilitates the transport of long-chain fatty acids for β-oxidation as well as supports energy production under metabolic stress (e.g., hypoxia or nutrient deprivation) (Zhu et al., 2025). Due to the upregulation of CPTIA in CSCs promoting chemoresistance and metastasis, CPT1A inhibitors (e.g., etomoxir or novel analogs) are promising candidates to disrupt CSC resilience (Zha et al., 2025). ACLY, a central enzyme linking glycolysis to lipogenesis, converts citrate to acetyl-CoA for fatty acid synthesis, fueling membrane production and oncogenic signaling (e.g., via histone acetylation) (Bort et al., 2020). Due to the overexpression of ACLY in CSCs correlating with poor prognosis, ACLY inhibitors (e.g., SB-204990 or BMS-303141) show efficacy in suppressing CSC populations, tumor growth, and metastasis (Sola-García et al., 2023; Puertas-Umbert et al., 2025).
Pathways and cancer immunotherapy
This section focuses on the signaling pathways that regulate CSC maintenance and survival, highlighting potential therapeutic targets. We will discuss emerging strategies for disrupting these pathways to improve treatment outcomes. Many highly complex and evolutionarily conserved signaling pathways are interwoven networks of signaling mediators to regulate CSC growth, which also induces the expression of downstream genes (apoptosis, anti-apoptotic, proliferation, and metastasis) (Zeng et al., 2023). At present, the main signaling pathways are the WNT/β-Catenin, hedgehog, Notch, JAK/STAT, TGF/SMAD, and PI3K/AKT/mTOR signaling pathways (Figure 1).
FIGURE 1

Core signaling pathways regulating cancer stem cell (CSC) properties. Illustrated pathways (WNT/β-catenin, Hedgehog, Notch, JAK-STAT, TGF-β/SMAD, PI3K/AKT/mTOR) govern CSC self-renewal, survival, metastasis, and therapy resistance. Each pathway modulates: Proliferation (WNT/β-catenin, Hedgehog, Notch), Survival/Apoptosis evasion (PI3K/AKT/mTOR, JAK-STAT), Metastasis (TGF-β/SMAD, JAK-STAT), Stemness maintenance (Cross-talk between all pathways).
The WNT/β-Catenin signaling pathway in CSCs, consisting of 19 Wnt ligands and more than 15 receptors, could regulate stemness, tumorigenesis, metastasis, apoptosis, and differentiation across diverse cancer types, including lung, liver, thyroid, colorectal, cervical, and glioblastoma (Liu et al., 2021; Feng et al., 2021; He et al., 2021). The hedgehog signaling pathway in CSCs, consisting of extracellular Hh ligands, transmembrane protein PTCH and SMO, intermediate transduction molecules, as well as downstream molecule GLI, plays a pivotal role in maintaining stemness, promoting initiation, and enhancing tumorigenicity in liver cancer, bladder cancer, breast cancer, and glioblastoma (Mok et al., 2022; Li et al., 2016; Qin et al., 2018). The Notch signaling pathway in CSCs, consisting of the Notch receptor, Notch ligand, CBF-1, hairless suppressor, Lag (CSL), DNA binding protein, and downstream target genes, is associated with metastasis, stemness maintenance, tumorigenesis, differentiation, and immune regulation of CSCs in various tumors such as glioma, breast cancer, renal cancer, and ovarian cancer (Chen et al., 2018; Rajakulendran et al., 2019; Cao et al., 2023). The JAK/STAT signaling pathway in CSCs, consisting of tyrosine kinase-related receptors that receive signals, tyrosine kinase JAK that transmits signals, and transcription factors STAT, is intricately linked to stemness, tumorigenesis, metastasis, and metabolic reprogramming of CSCs in glioblastoma, osteosarcoma, liposarcoma, liver cancer, prostate cancer, gastric cancer, thyroid cancer, and breast cancer (Garner et al., 2019; Xiong et al., 2024; Shiraiwa et al., 2019). The TGF/SMAD signaling pathway in CSCs, consisting of two ligand groups (TGF-β/activin and BMP/GDF), plays a pivotal role in metastasis, tumorigenesis, and stemness of glioma, breast cancer, prostate cancer, pancreatic cancer, and oral/esophageal squamous cell carcinoma (Zhao et al., 2019; Nong et al., 2022; You et al., 2020). The PI3K/AKT/mTOR signaling pathway in CSCs, consisting of intracellular phosphatidylinositol kinase PI3K, serine/threonine kinase AKT, downstream target mTOR, and negative regulator PTEN, is intricately linked to metastasis, stemness, and tumorigenicity in gastric cancer, endometrial cancer, ovarian cancer, lung cancer, osteosarcoma, as well as head and neck squamous cell carcinoma (Wang et al., 2021; Li et al., 2021; Wang JH. et al., 2020).
The intricate crosstalk among multiple signaling pathways regulates the tumorigenicity, differentiation, and metastasis capabilities of CSCs. WNT/β-Catenin, hedgehog, Notch, and TGF-β pathways influence the differentiation of colorectal CSCs (Regan et al., 2017). The interaction between IL-6/JAK/STAT3 and TGF-β/Smad signaling induces the proliferation and metastasis of lung CSCs (Liu et al., 2014). TGF-β1 activating induces lncRNA NKILA expression to block NF-κB signaling in breast CSCs (Wu et al., 2018). PI3K/AKT/mTOR signaling upregulates STAT3 expression to promote the survival and proliferation of breast CSCs (Zhou et al., 2007). The collaboration of PI3K/AKT/mTOR with Sonic Hedgehog pathways could inhibit the growth of pancreatic CSCs (Zuo et al., 2015).
The signaling pathways regulating the maintenance and survival of CSCs have become oncology targets, with early clinical trials for Notch and hedgehog pathway inhibitors. There are three major clinical methods for inhibiting Notch signaling, such as secretase inhibition (γ-secretase inhibitor, GSI), Notch receptor or ligand antibodies, and combination therapy. There are several GSIs entering the clinical trial stage, such as MK-0752 (NCT00100152), RO4929097 (NCT01154452), Nirogacestat/PF-03084014 (NCT01981551), BMS-906024 (NCT01292655), BMS-986115 (NCT01986218), CB-103 (NCT03422679), Crenigacestat/LY3039478 (NCT02836600), and LY900009 (NCT01158404) (Yang L. et al., 2020). The hedgehog signaling pathway regulates target gene expression through smoothened (SMO)-mediated nuclear transfer of transcription factors. Three oral SMO antagonists, Vismodegib (GDC-0449), Sonidegib (LDE225), and Glasdegib (PF-04449913), have been approved by the Food and Drug Administration (FDA), which show significant activity in locally advanced and metastatic basal cell carcinoma, as well as in acute myeloid leukemia (Sekulic et al., 2012; Dummer et al., 2016; Norsworthy et al., 2019).
Biomarkers and therapeutic approaches
Cancer biomarkers are molecular, cellular, tissue, and process-based alterations providing indications of current and future cancer behaviors, which are applied in pharmaceutical discovery and preclinical development, clinical trials, and patient care (Hayes et al., 1996; Xiao et al., 2021). Cancer biomarkers could be clarified into two categories: clinical trials (chemoprevention, screening, diagnosis, prognosis, prediction, treatment stratification, therapy monitoring, posttreatment surveillance, risk stratification, and risk management) (Borrebaeck, 2017; Le-Rademacher et al., 2018; Gonzalez-Ericsson et al., 2020; Jordan and Thomas, 2023) and drug development (target validation, early compound screening, pharmacodynamic assays, patient selection, and surrogate endpoint) (Pors and Moreb, 2014; Twomey et al., 2017; Lara et al., 2020; Wang E. et al., 2024). The identification of CSC biomarkers has facilitated the development of targeted therapies aimed at disrupting CSC maintenance and survival. These biomarkers serve as diagnostic, prognostic, and therapeutic targets, providing insights into CSC biology and potential therapeutic vulnerabilities.
CSCs biomarkers, such as cell surface markers, signaling pathways, transcription factors, and drug transporters, are involved in self-renewal, immune evasion, tumor metastasis, tumor re-growth, tumor relapse, and therapy resistance (Yehya et al., 2023). CSC biomarkers could serve as diagnostic (resistance), therapeutic (metastasis and tumor stage/size/resistance), and prognostic (survival and resistance) approaches in multiple deadliest cancers, such as lung cancer, liver cancer, breast cancer, gastric cancer, prostate cancer, bladder cancer, and colon cancer (Table 1).
TABLE 1
| Biomarker | Primary clinical role | Key molecular function | Prostate cancer | Colon cancer | Bladder cancer | Breast cancer | Lung cancer | Liver cancer | Gastric cancer |
|---|---|---|---|---|---|---|---|---|---|
| CD24 | Diagnostic (Resistance) | WNT/β-Catenin, MAPK, Notch regulation | Metastasis | Metastasis association | CSC niche maintenance | Therapy resistance | Prognostic marker | Chemoresistance | |
| CD44 | Therapeutic (Metastasis) | VEGFR/PI3K/Akt activation; c-Met coreceptor | Therapy resistance | Metastasis | Prognostic marker | Metastasis | Tumor initiation | Stemness maintenance | EMT induction |
| CD49f | Therapeutic (Niche) | Tumor-microenvironment crosstalk | Tumor initiation | CSC isolation marker | CSC niche maintenance | Self-renewal regulator | Stemness marker | ||
| CD133 | Diagnostic (Relapse) | WNT/Ras/Notch pathway modulation | Chemoresistance; Prognostic | Recurrence predictor | Recurrence predictor | Self-renewal regulator | Prognostic marker | Prognostic marker | Stemness marker |
| EpCAM | Therapeutic (CTC target) | Circulating tumor cell anchor; EMT regulator | Stemness maintenance | Wnt signaling activator | Tumorigenicity driver | Prognostic marker | Metastasis driver | Stemness maintenance | Tumorigenicity driver |
| CXCR4 | Therapeutic (Metastasis) | WNT/NF-κB mediated metastasis | Metastasis driver | Metastasis driver | Metastasis driver | Metastasis driver | Prognostic marker | ||
| LGR5 | Therapeutic (Self-renewal) | Wnt/β-catenin signaling receptor | Wnt signaling activator | Self-renewal regulator | Prognostic marker | Stemness maintenance | |||
| ALDH | Diagnostic (Resistance) | Tumor dedifferentiation/EMT driver | Chemoresistance; Prognostic | Chemoresistance marker | CSC activity marker | Chemoresistance marker | Prognostic marker | Prognostic marker | |
| ABCG2 | Therapeutic (Resistance) | Drug efflux pump | Drug efflux mediator | Chemoresistance marker | Drug efflux mediator | Chemoresistance marker | Drug efflux mediator | ||
| EGFR | Therapeutic (Target) | Stemness/dormancy regulator | Therapy target | Metastasis driver | Stemness regulator | Survival regulator | Tumorigenicity driver |
Clinically validated cancer stem cell biomarkers in solid tumors: Functional mechanisms and diagnostic utility.
CD24, also known as Heat Stable Antigen (HSA), functions as a cell-cell adhesion molecule to mediate WNT/β-Catenin, MAPK, PI3K/AKT/mTOR, Notch, and hedgehog pathways (Zhang et al., 2017a; Kapeleris et al., 2020; Ooki et al., 2018; Al-Hajj et al., 2003; Wang et al., 2018b; Zhang et al., 2011). CD44, also known as Homing Cell Adhesion Molecule (HCAM) and Phagocytic Glycoprotein-1 (Pgp-1), recruits ezrin/radixin/moesin (ERM) proteins to interact with VEGFR and to activate the PI3K/Akt and Src/MAPK pathways, which also serves as a c-Met co-receptor (Hurt et al., 2008; Du et al., 2008; Chan et al., 2009; Koh et al., 2021; Leung et al., 2010; Asai et al., 2019). CD24 has been investigated in combination with CD44 in breast cancer, prostate cancer, and gastric cancer (Zhang et al., 2011; Hurt et al., 2008; Koh et al., 2021). CD49f, also known as integrin α6, is the only conserved biomarker in more than 30 different stem cell populations, which mediates the stem cell niche through interactions with the extracellular matrix as well as communication between tumor cells and tumor microenvironment (Marcinkiewicz et al., 2012; Bo et al., 2009; Zhang et al., 2020; Zhang et al., 2017b). CD90, also known as THY1, is glycophosphatidylinositol anchored cell surface protein in T cell adhesion and signal transduction, which marks hematopoietic cells and fibroblasts in mice as well as mesenchymal cells and stromal cells in humans (Huynh et al., 2016; Abugomaa et al., 2020; Yan et al., 2013; Zhu et al., 2015; Shu et al., 2019). CD133, also known as prominin-1, is a five-transmembrane glycoprotein in stem and progenitor cells, which mediate WNT/β-Catenin, Ras/ERK, Src/FAK, PI3K/AKT, Notch, and hedgehog signaling pathways (Kanwal et al., 2018; O'Brien et al., 2007; Huang et al., 2013; Wang et al., 2013; Li D. et al., 2022; Lu et al., 2016).
Epithelial Cell Adhesion Molecule (EpCAM), a homophilic cell-cell adhesion glycoprotein, serves as a prognostic marker, therapeutic target, and anchor molecule on circulating and disseminated tumor cells, which regulates tumor cell adhesion, proliferation, migration, stemness, and epithelial-to-mesenchymal transition (Ni et al., 2013; Leng et al., 2018; Bryan, 2015; Wang et al., 2018c; Alibolandi et al., 2015; Noh et al., 2018; Dai et al., 2017). C-X-C chemokine receptor type 4 (CXCR4, CD184), is involved in WNT/β-Catenin, Notch, hedgehog, PI3K/AKT, JAK/STAT, NF-κB, MAPK, and EGFR/HER2-neu signaling pathways for tumor cell survival, proliferation, migration, and metastasis (El-Benhawy et al., 2021; Serna et al., 2020; Yi et al., 2014; Testa et al., 2018; Xue et al., 2017). Leucine-rich repeat-containing G protein-coupled receptor 5 (LGR5), also known as G protein-coupled receptor 49 (GPR49), belongs to the G protein-coupled receptor family, serves as cell surface-expressed Wnt target gene for cancer stem cell proliferation and self-renewal by regulating Wnt/β-catenin signaling pathway (Takahashi et al., 2011; Green et al., 2019; Nakajima et al., 2016). Aldehyde dehydrogenases (ALDH), consisting of 19 putative members, are involved in tumor cell differentiation, proliferation, invasion, metastasis, and epithelial-mesenchymal transition (Gorodetska et al., 2024; Holah et al., 2017; Namekawa et al., 2020; Louhichi et al., 2018; Gao et al., 2015; Senel et al., 2017). ATP-binding cassette superfamily G member 2 (ABCG2), a cell membrane pump encoded by the ABCG2 gene, could protect cells against compounds initiating and/or intensifying neoplasia, which is responsible for cancer growth, drug resistance, and recurrence (Wang L. et al., 2020; Xie et al., 2014; Das et al., 2019). Epidermal Growth Factor Receptor (EGFR), activated by receptor overexpression and ligand-dependent/independent mechanisms, serves as an essential receptor tyrosine kinase regulator for cancer stem cell functions, which include stemness, metabolism, immunomodulatory activity, dormancy, and therapy-resistance (Rossini et al., 2020; Mouillet-Richard, 2022; Steelman et al., 2016; Si et al., 2019; Liu Y. et al., 2023).
Targets and drug discovery
CSC microenvironment is involved in tumor invasion, differentiation, metastasis, angiogenesis, genotoxicity, and self-renewal, which includes vascular niches, hypoxia, extracellular matrix, tumor-associated macrophages, as well as cancer-associated fibroblasts and mesenchymal stem cells (Xue et al., 2015). Plerixafor (AMD3100), the most well-characterized CXCR4-targeted drug, has been applied for the phase I/II study of non-Hodgkin’s lymphoma (NHL), acute myeloid leukemia (AML), and myelodysplastic syndrome (MDS) (Cashen et al., 2008). LY2510924, a potent and selective CXCR4 antagonist, has been investigated for the phase II study of renal cell carcinoma and small-cell lung cancer in the combination of sunitinib and carboplatin/etoposide respectively (Hainsworth et al., 2016). The combined therapy of LY2510924 with other drugs is under clinical trials for gliomas (NCT03746080, NCT01977677, and NCT01288573) and multiple myeloma (NCT00103662, NCT01220375, and NCT00903968) (Yang L. et al., 2020). Understanding the therapeutic targets in CSCs is essential for developing novel therapies. Emerging technologies are revolutionizing our ability to study CSC biology and identify new targets.
Chimeric antigen receptor (CAR)-T cells are engineered T cells expressing an artificial receptor specific to tumor associated antigens (TAAs), which could induce the release of cytotoxic cytokines, perforin and granzyme (Benmebarek et al., 2019). CSC-targeted CAR-T cell monotherapy has been applied to many clinical trials, which could be clarified into phase I study (CD22 CAR-T, CD33 CAR-T, EGFR IL-12 CAR-T, MESO-19 CAR-T, MOv19-BBz CAR -T, LeY CAR-T, and EpCAM CAR-T), phase II study (CD19 CAR-T, CD123 CAR-T, CD38 CAR-T, CD138 CAR-T, and BCMA CAR-T), and phase I/II study (CD22 CAR-T, CD33 CAR-T, MUC1 CAR-T/PD-1 KO, and MESO CAR-T) (Yang L. et al., 2020). The combined therapy of CSCs-specific CAR-T cells with chemotherapy and radiotherapy could completely eradicate tumors without recurrence risk (Figure 2A), which has been applied in the treatment of glioma (NKG2D CAR-T cells with regional radiotherapy), ovarian cancer (CD133 CAR-NK92 cells with cisplatin), colorectal cancer (EpCAM CAR-NK92 cells with regorafenib) (Masoumi et al., 2021; Weiss et al., 2018; Klapdor et al., 2017; Zhang et al., 2018).
FIGURE 2
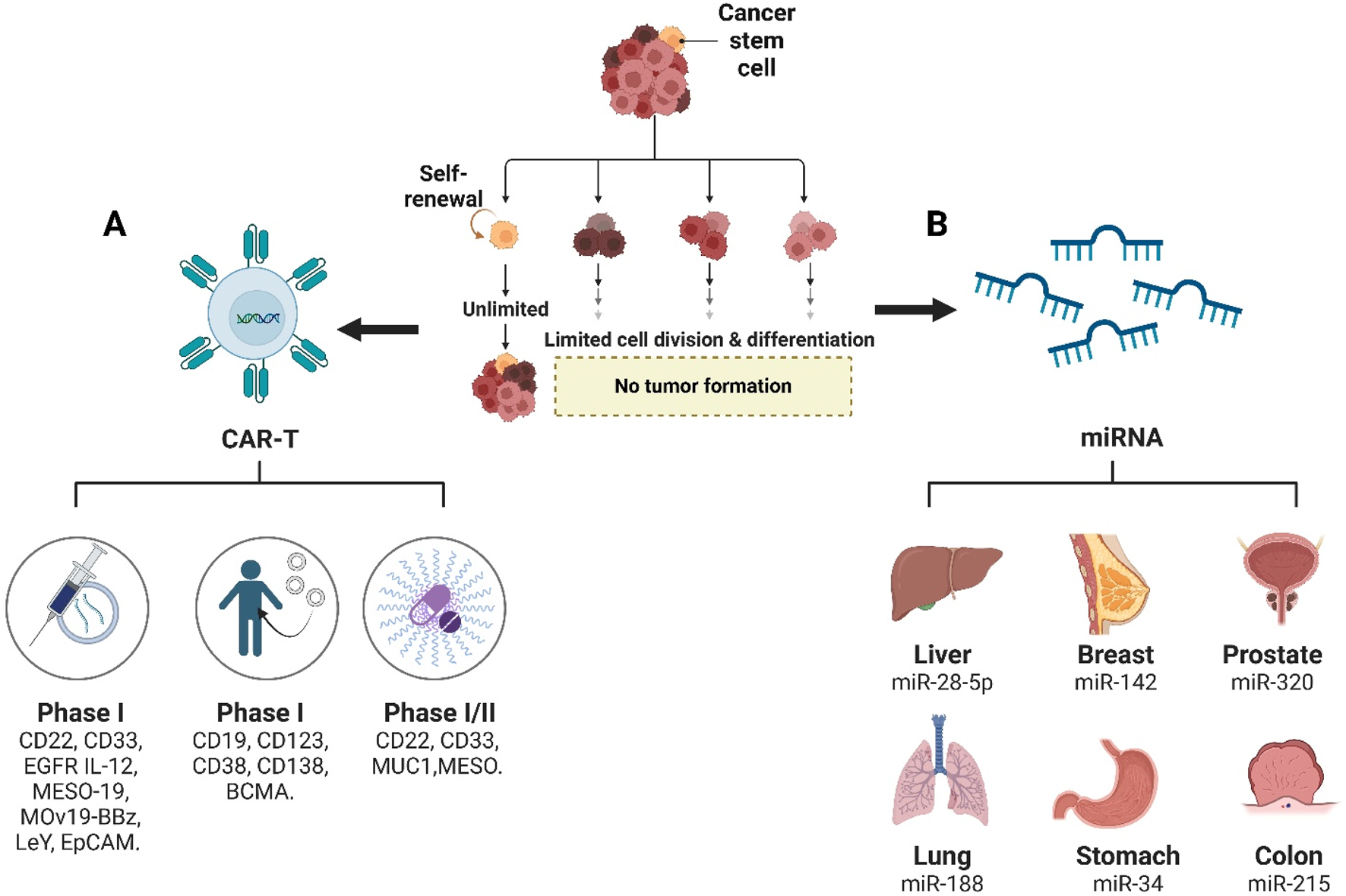
Therapeutic strategies targeting cancer stem cells (CSCs). (A) CAR-T cell therapies against CSC surface markers are currently in clinical development, with Phase I trials targeting CD19, CD22, CD33, CD38, CD123, CD138, BCMA, EGFR, IL-12, MESO, and EpCAM, and Phase I/II trials evaluating CD22, MUC1, and MESO. (B) Organ-specific miRNA therapeutics for CSC suppression include miR-28-5p in liver cancer, miR-142 in breast cancer, miR-320 in prostate cancer, miR-188 in lung cancer, miR-34a in gastric cancer, and miR-215 in colon cancer.
MicroRNAs (miRNAs), small single-stranded 22 nucleotides length RNA molecules, serves as new promising diagnostic and therapeutic tools for CSCs control (Figure 2B), such as proliferation, migration, invasion, differentiation, apoptosis, differentiation, tumourigenesis, and recurrence, which was awarded jointly to Victor Ambros and Gary Ruvkun by the Nobel Prize in Physiology or Medicine 2024 (Nobel Prize, 2024). miR-28-5p overexpression inhibited self-renewal and tumorigenesis of liver CSCs, which depends on the direct target insulin-like growth factor-1 (IGF-1) (Xia et al., 2019). miR-188 inhibited the biological activity of lung CSCs by targeting midkine (MDK) and mediating the Hippo pathway (Yang X. et al., 2020). miR-142-3p overexpression could result in reduced mammosphere formation and irradiation survival, with the decrease of CD44, CD133, ALDH1, Bod1, and BRCA2 in breast CSCs (Troschel et al., 2018). miR-34 is involved in self-renewal/differentiation decision-making of gastric CSCs (Jafari and Abediankenari, 2017). miR-320 could to suppress prostate cancer stem-like properties, such as tumorsphere formation, chemoresistance and tumorigenic abilities (Hsieh et al., 2013). miR-497 overexpression could suppress gemcitabine resistance, migration, invasion, and metastasis of pancreatic CSCs by directly targeting nuclear factor kappa B 1 (NFκB1) (Yu Q. et al., 2022). miR-215 serves as CDX1 effector and BMI1 repressor to regulate self-renewal and multipotency of colorectal CSCs (Jones et al., 2015).
Mechanisms of drug resistance in CSCs
CSCs exhibit both intrinsic and extrinsic drug resistance mechanisms, allowing them to evade conventional therapies and contribute to tumor recurrence. The intrinsic drug resistance mechanisms make CSCs a major challenge in cancer treatment, which include quiescence, enhanced DNA repair, and adaptive signaling pathways (Choi et al., 2020). The extrinsic drug resistance mechanisms involve interactions between CSCs and their surrounding tumor microenvironment or niche, which include niche-mediated protection, immune evasion, and metabolic adaptation (Najafi et al., 2019).
CSC quiescence is a key survival strategy contributing to tumor recurrence, metastasis, and therapy resistance, which is modulated by intrinsic factors (cell cycle regulation, epigenetic modifications, and metabolic adaptation) and extrinsic factors (hypoxia, stromal interactions, and immune surveillance) (Gulaia et al., 2018). Targeting CSC quiescence requires a multi-pronged approach combining epigenetic, metabolic, and niche-disrupting therapies alongside immunotherapy, which include forcing quiescent CSCs into cell cycle/“Wake-Up and Kill” (dormancy pathway inhibitors, epigenetic modulators, and pro-oxidant therapies), inducing permanent dormancy or senescence (p38 MAPK activators, mTOR inhibitors, and senescence-inducing drugs), applying immune-based approaches (checkpoint inhibitors and CAR-T/NK cells), as well as targeting the Niche (CXCR4 inhibitors and anti-angiogenic therapies) (Chen et al., 2016; Cho et al., 2019). CSCs exhibit upregulation of multiple DNA repair mechanisms compared to non-CSC tumor cells, contributing to their therapy resistance, which include homologous recombination (HR), non-homologous end joining (NHEJ), base excision repair (BER), nucleotide excision repair (NER), and mismatch repair (MMR) (Lu and Zhang, 2022). Targeting the enhanced DNA repair capacity of CSCs through protein inhibitors (PARP inhibitors, ATM/ATR inhibitors, and DNA-PKcs inhibitors), epigenetic modulators (HDAC/DNMT inhibitors), ROS-induced DNA damage amplification, and synthetic lethality strategies holds significant potential to improve cancer treatment outcomes (Skvortsov et al., 2015). The adaptive signaling networks of CSCs underscore their role as a “persister” population in tumors (Thakur and Ray, 2017; Vazquez-Santillan et al., 2015; Chi et al., 2022). The adaptive signaling networks of CSCs underscore the need for multimodal therapies that simultaneously disrupt survival pathways, target the TME, and exploit metabolic dependencies (Mengistu et al., 2024). Advances in single-cell sequencing and CRISPR screening could refine CSC-targeted interventions and offer hope for durable remission.
CSC niche provides physical protection, biochemical signaling, and metabolic support, which enable CSCs to evade conventional therapies (Plaks et al., 2015). The niche-mediated drug resistance mechanism involves multiple intricate interactions, including stromal cell crosstalk, extracellular matrix remodeling, and hypoxia-induced adaptations (Kalavska et al., 2020). While niche-targeted strategies are promising, their success hinges on personalized approaches and overcoming the plasticity of CSCs and their microenvironment, which include niche disruption, stromal targeting, and dual-targeting approaches. The key feature of CSCs is their ability to evade immune surveillance, enabling tumor progression, metastasis, and recurrence (Su et al., 2020). The immune evasion mechanisms employed by CSCs are multifaceted, involving alterations in antigen presentation, immunosuppressive microenvironment modulation, and resistance to immune effector functions (Tsuchiya and Shiota, 2021). Personalized, combinatorial approaches, such as immune checkpoint blockade, CSC-directed vaccines, and CAR-T/NK cell therapy, could pave the way for improving clinical outcomes in refractory cancers. Unlike most cancer cells that rely predominantly on glycolysis (the Warburg effect), CSCs exhibit dynamic metabolic adaptations that allow them to thrive in harsh microenvironments, evade immune surveillance, and resist chemo- and radiotherapy (El-Sahli and Wang, 2020). Their unique metabolic adaptations—such as reliance on glycolysis, oxidative phosphorylation (OXPHOS), or fatty acid oxidation (FAO)—have emerged as promising therapeutic targets (Nimmakayala et al., 2021). Metabolic adaptation therapy aims to disrupt these pathways, selectively eradicating CSCs while sparing normal cells, which include glycolysis inhibitors, OXPHOS targeting, and FAO/glutaminase inhibition.
Emerging technologies
This section explores the impact of emerging technologies such as single-cell omics and CRISPR screening on our understanding of CSC biology. We will discuss how these tools are revolutionizing cancer research and enabling the development of precision medicine approaches. Since 2020, several emerging technologies, such as single-cell omics and CRISPR screens, have significantly advanced CSC research, enabling deeper understanding of their biology and the development of novel therapeutic strategies.
Single-cell technologies have revolutionized CSC research by enabling high-resolution analysis of cellular heterogeneity, plasticity, and functional states within tumors, which provide unprecedented insights into CSC biology, including their origin, regulatory mechanisms, and role in therapy resistance (Zheng et al., 2018). Single-cell RNA sequencing (scRNA-seq) could identify rare CSC populations within heterogeneous tumors, characterize CSC transcriptional programs at unprecedented resolution, discover novel CSC markers and signaling pathways, and analyze CSC plasticity and transitions between stem and non-stem states (Zheng et al., 2024). Single-cell Assay for Transposase-Accessible Chromatin using sequencing (scATAC-seq), a powerful tool to investigate chromatin accessibility at single-cell resolution, could identify CSC-specific regulatory landscapes, transcription factor networks, and potential therapeutic targets (Liu et al., 2024). Single-cell spatial omics/Multi-omics integration, powerful tools to dissect the heterogeneity, microenvironment interactions, and molecular mechanisms of CSCs at unprecedented resolution, could track CSC subpopulations and their spatial expansion over time, link hypoxia-driven metabolic shifts to CSC survival, as well as identify spatially resolved drug-resistance mechanisms (Wang Y. et al., 2024).
CRISPR-based screens enable genome-wide or targeted interrogation of gene function, facilitating the discovery of novel therapeutic targets and mechanistic insights into CSC biology, which pave the way for targeted therapies aimed at eradicating CSCs, potentially improving cancer treatment outcomes (Li et al., 2023). Pooled CRISPR knockout (KO) screens utilize sgRNA libraries to disrupt gene function genome-wide or in focused sets (Joung et al., 2017). CRISPR interference (CRISPRi) and activation (CRISPRa) modulate gene expression without DNA cleavage (Pandey et al., 2024). In vivo CRISPR screens are conducted in animal models to study CSC maintenance and metastasis in a physiological context (Su et al., 2024).
The integration of artificial intelligence (AI) into CSC research has revolutionized the understanding, diagnosis, and treatment of cancer, which enhanced CSC identification by analyzing multi-omics data (genomics, transcriptomics, proteomics, and epigenomics). AI could accelerate the discovery of CSC-targeting drugs by screening large chemical libraries and predicting drug-CSC interactions (Shende and Devlekar, 2021; Ramović Hamzagić et al., 2024a). AI models integrating drug sensitivity data with CSC gene expression profiles could identify potential therapeutic vulnerabilities. Deep learning models, such as convolutional neural networks (CNNs), have been applied to histopathological images to detect CSC-rich regions in tumors, improving diagnostic accuracy (Chen et al., 2023; Aida et al., 2020). AI-powered platforms could analyze patient-derived data (e.g., liquid biopsies, circulating tumor cells) to stratify patients based on CSC prevalence and predict individualized treatment responses. Machine learning models combining clinical, genomic, and imaging data have guided precision oncology decisions, optimizing therapy regimens to eliminate CSCs and prevent relapse (Chambost et al., 2022; Ramović Hamzagić et al., 2024b).
Organoids and precision medicine
Cancer is a genetic disease with inherent genetic instability that generates abnormal proteins, driven by the accumulation of mutations. In 2024, a bleak milestone for the first time with 2 million daily cancer cases in the United States (US), over 611,000 deaths from cancer are projected for the whole year, while more than 1,600 deaths are projected for each day (Cancer.org, 2024). By 2029, there will be around 2.2 million new cancer cases diagnosed in the United Kingdom (UK), with more than 900,000 cancer deaths (Cancer Research UK, 2024).
Organoids, three-dimensional (3D) multicellular cultures, are generated from adult stem cells (ASCs) and pluripotent stem cells (PSCs) in many tissues (brain, liver, skin, eye, kidney, lung, stomach, intestine, thyroid, and inner ear), which are widely applied in tissue development, tumorigenesis and cancer therapy due to their capabilities of self-renewal and self-proliferation (Zou et al., 2024). Adult stem cell-derived organoids retain organ identity and genome stability, which could serve as an unlimited source for replacing damaged tissues due to their prospectivities of differentiating into virtually all present organ cellular lineages (Drost and Clevers, 2017). Pluripotent stem cell-derived organoids are established from embryonic stem cells (ESC) and induced PSC (iPSC), which could differentiate into three germ layers (endoderm, mesoderm, and ectoderm) as well as transfect with four transcription factors (Klf4, Oct3/4, Sox2, and c-Myc) (Takahashi et al., 2007). Cancer organoids have been successfully established from both ASCs and PSCs for the maintenance of interpatient and intratumor variation as well as the capture of tumor heterogeneity of individual patients (Figure 3), which are urgently needed for precision medicine, therapy resistance, and cancer progression.
FIGURE 3
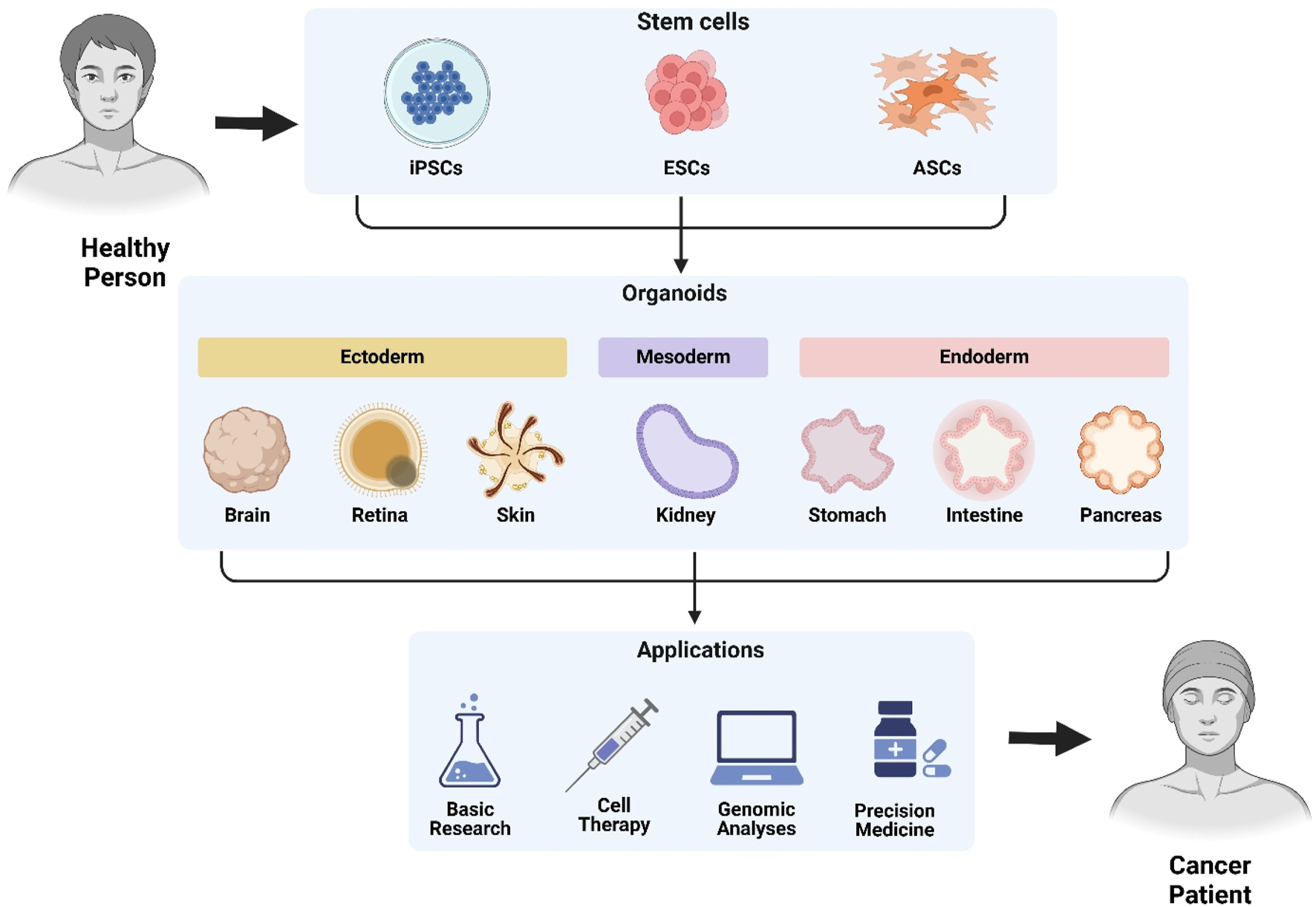
Stem cell-derived organoids: Generation and translational applications. Patient-derived organoids are generated from induced pluripotent stem cells (iPSCs), embryonic stem cells (ESCs), or adult stem cells (ASCs). These differentiate through germ layers (ectoderm, mesoderm, endoderm) into functional organoids including brain, retina, skin, kidney, gastric, intestinal, and pancreatic models. Applications span basic research, cell therapy, genomic analyses, precision medicine, and cancer modeling.
Patient-derived organoids (PDOs) generate ‘living’ organoid biobanks to retain the heterogeneous genetic composition in various cancers, such as breast cancer (Sachs et al., 2018), esophageal adenocarcinoma (Li et al., 2018), gastric cancer (Yan et al., 2018), which is applied in drug efficacy screenings and drug discovery validations. Two complementary strategies have been applied in PDOs for understanding cancer molecular genetics. The first strategy is PDO mutational analysis through whole-genome sequencing (WGS), whole-exome sequencing (WES), and targeted sequencing, which could confirm PDO functions on representing inter-tumor heterogeneity and forming the basis of the inter- and intra-tumor diversification (Michels et al., 2020). The second strategy is probing tumorigenesis mutations through gene editing, which could replicate key features of cancer progression (niche factors independence, chromosome instability, metastasis ability, aneuploidy, and invasiveness), as well as investigate mutations roles in DNA mismatch repair genes (MLH1, BRAF, TP53, and BAP1) (Drost et al., 2015; Fessler et al., 2016).
Patient-derived cells (PDCs) are generated from tumor specimens (Li et al., 2025). PDC can reflect patient tumor characteristics and clinical responses, but lack diversity in terms of cell type, spatial organization, and tumor microenvironment. Patient-derived xenograft (PDX) model can effectively replicate tumor growth and preserve tumor heterogeneity, which requires long culture time, high procedure cost, and low transplantation rate (Invrea et al., 2020). The major obstacle of personalized medicine is the lack of effective preclinical models for accurately predicting drug response, sensitivity, and resistance (Voest and Bernards, 2016). Compared with PDCs and PDX, PDOs can better capture and retain the molecular, cellular, genetic, histological, and heterogeneous phenotypes of patient‐specific tumors, which are much more suitable for cancer-personalized therapies to identify key targets and signaling pathways as well as develop incorporate cells of the tumor microenvironment (stromal cells and immune cells). The ovarian cancer PDOs could show accuracy in reflecting clinical responses of patients to platinum-based chemotherapy (Tao et al., 2022). The colorectal cancer PDOs could predict cytoreductive surgery responses followed by hyperthermic intraperitoneal chemotherapy (Ubink et al., 2019). Pancreatic cancer PDOs could accurately predict chemotherapy drug resistance as well as advance personalized medicine (Tiriac et al., 2018). The gastrointestinal cancer PDOs could be used for rapid and actionable drug detection (Gao et al., 2021).
The establishment of PDO biobanks, large-scale collections of organoids from diverse patients, could enable high-throughput drug screening (HTS), which facilitate the identification of novel therapeutics and personalized treatment strategies (Vlachogiannis et al., 2018). PDO biobanks could enable “clinical trial in a dish” approaches by testing drug responses across diverse patient populations, which bridge the gap between preclinical research and clinical application (Herpers et al., 2022). By capturing patient diversity and enabling large-scale pharmacological testing, PDO biobanks hold immense promise for accelerating drug development and advancing precision medicine (Yu YY. et al., 2022; Cui et al., 2024). Future advancements, such as microfluidics, AI-driven image analysis, and organ-on-chip technologies, could enhance the utility of organoid biobanks in drug screening.
While PDOs offer valuable insights into tumor biology and therapeutic responses, it is important to recognize their limitations. Organoids often lack a complete immune component, which can restrict their utility in studying immune responses and the effectiveness of immunotherapies. Additionally, the absence of a vascular system in organoids hinders the accurate modeling of tumor angiogenesis and drug delivery mechanisms. Inter-laboratory variability and challenges in standardizing culture conditions also affect the reproducibility of results, emphasizing the need for improved protocols and quality control measures. Regarding iPSCs, the process of generating these cells through reprogramming somatic cells involves the use of transcription factors delivered by viral vectors, which can lead to insertional mutagenesis and genomic instability. Furthermore, iPSCs may retain epigenetic memory from the original cell type, potentially influencing their differentiation potential and phenotype. Despite these challenges, iPSCs hold significant promise for disease modeling, drug screening, and regenerative medicine, particularly when used to generate organoids that closely resemble patient-specific tissues. To address these limitations, ongoing research is focused on improving organoid models by incorporating immune cells and developing vascularized structures. Additionally, advancements in iPSC reprogramming techniques aim to minimize genomic and epigenetic issues, enhancing the safety and reliability of these cells for clinical applications. By acknowledging and addressing these challenges, we can improve the utility and applicability of organoids and iPSCs in cancer research and personalized medicine.
From bench to bedside: challenges and opportunities
CSC research is driving precision oncology trials by uncovering vulnerabilities in therapy-resistant populations. Successful translation requires combination strategies, biomarker validation, and innovative trial designs to eradicate CSCs and improve long-term survival. Integrating CSC research into clinical practice offers a paradigm shift from symptom-focused to rhythm-focused medicine, improving outcomes across multiple disciplines.
While preclinical studies have identified promising CSC-targeting approaches, their clinical translation remains difficult due to biological, technical, and practical hurdles. Many CSC-associated pathways are crucial for normal stem cell function, which may cause severe side effects (compensatory mechanisms, gut toxicity, and hematopoietic suppression) by pathway inhibition. Many CSC-targeting agents show efficacy in animal xenograft models but fail in human clinical trials due to CSC biology differences. CSCs exhibit substantial heterogeneity across cancer types and even within individual tumors, which make universal targeting difficult. Non-CSCs can dedifferentiate into CSCs under stress (e.g., chemotherapy or radiation), which lead to therapeutic escape. Available solution could focus on precision medicine approaches to selectively eliminate CSCs while sparing normal stem cells, ultimately improving cancer treatment outcomes, which include epigenetic modulation (DNA methylation and histone modification), drug delivery (nanoparticles), signature identification (single-cell RNA sequencing and liquid biopsies), and long-term outcomes (progression-free survival and recurrence rates).
Discussion
Our comprehensive review of cancer stem cells (CSCs) in personalized therapy underscores the intricate role these cells play in tumor progression, therapeutic resistance, and metastasis. While our primary focus has been on the molecular pathways and regulatory mechanisms governing CSC behavior, it is essential to broaden our perspective to include the ecological and evolutionary aspects of cancer.
Variability in CSC markers
The identification and targeting of CSCs rely heavily on specific biomarkers such as CD44 and CD133. However, the expression levels and functional relevance of these markers can vary significantly across different tumor types and even within the same tumor (Zhou et al., 2021; Liu and Wang, 2024). This heterogeneity raises important questions about the robustness of these markers as universal indicators of CSCs. The clinical translation of CSC-targeted therapies must account for this variability to ensure accurate identification and effective targeting of CSC populations. Future research should focus on identifying more reliable and universal CSC markers, possibly through the integration of multi-omics approaches and functional assays.
Multifactorial nature of therapeutic resistance
CSC-mediated therapeutic resistance is a complex phenomenon involving multiple signaling pathways and cellular mechanisms. Key pathways such as Wnt/β-catenin, Notch, and Hedgehog play pivotal roles in maintaining CSC stemness and mediating resistance to conventional therapies (Steinbichler et al., 2018; Phi et al., 2018). However, the interplay between these pathways and their contribution to resistance is not fully understood. Conflicting evidence regarding the activation status of these pathways in different CSC populations highlights the need for further investigation. Ethical considerations are paramount when manipulating these pathways, as unintended consequences could impact patient safety. Future studies should aim to elucidate the precise mechanisms by which these pathways interact and contribute to therapeutic resistance, paving the way for the development of more effective therapeutic strategies.
Dynamic interactions within the tumor microenvironment (TME)
The TME is a dynamic and heterogeneous milieu composed of various cell types, including immune cells, fibroblasts, endothelial cells, and the extracellular matrix (ECM). This complex environment plays a crucial role in tumor progression, metastasis, and therapeutic resistance (Bilotta et al., 2022; Wang L. et al., 2024). CSCs do not exist in isolation; they interact extensively with the TME, and these interactions are bidirectional. CSCs can secrete factors that recruit and modulate immune cells, creating an immunosuppressive environment that fosters their survival. Conversely, the TME provides physical and biochemical cues that support CSC self-renewal and maintenance. For instance, hypoxic conditions can enhance CSC stemness, while stromal cells can secrete growth factors and ECM components that promote CSC survival and therapeutic resistance. Exosome-mediated communication is another critical aspect of TME-CSC interactions. CSCs release exosomes containing microRNAs and proteins that can reprogram neighboring cells and modify the TME, further supporting CSC maintenance and therapeutic resistance. Understanding these complex interactions is essential for developing targeted therapies that disrupt the supportive niche of CSCs and sensitize them to conventional treatments.
Tumor ecosystem and CSC behavior
Viewing the TME as a complex ecosystem that includes immune cells, fibroblasts, and the extracellular matrix provides a broader perspective on CSC behavior. This ecosystem perspective highlights the role of “stemness” as a survival strategy employed by CSCs, allowing them to mimic normal stem cell characteristics to thrive in challenging microenvironments (Luo, 2023; Luo, 2025). Recent research suggests that understanding the tumor ecosystem is crucial for addressing critical clinical issues such as recurrence and metastasis. The tumor ecosystem can be seen as a dynamic system where CSCs, along with other cell types, engage in reciprocal interactions that influence tumor growth and treatment response. This ecological viewpoint emphasizes the importance of considering the tumor as a whole, rather than focusing solely on CSCs. By understanding the complex web of interactions within the tumor ecosystem, researchers can develop strategies that target not only CSCs but also the supportive microenvironment that sustains them. This approach is essential for addressing the challenges of recurrence and metastasis, which are often driven by the adaptive capabilities of the tumor ecosystem. Integrating insights from the tumor ecosystem and ecological pathology allows for a more holistic understanding of CSC behavior and therapy resistance. This broader perspective is crucial for developing effective therapeutic strategies that target both the molecular pathways and the ecological dynamics of the tumor.
Metabolic adaptations of CSCs
CSCs exhibit distinct metabolic adaptations that support their survival and stemness under stressful conditions (Chen et al., 2021; Masoudi et al., 2024). While lipid metabolism has been a focus, glycolysis and oxidative phosphorylation also play significant roles in CSC metabolism. The Warburg effect, characterized by increased glycolysis even in the presence of oxygen, provides CSCs with rapid energy production and intermediates for biosynthetic pathways. This metabolic shift supports rapid proliferation and biomass production, contributing to CSC survival and therapeutic resistance. Oxidative phosphorylation, although less prominent in CSCs, can be utilized under certain conditions, providing flexibility in energy metabolism. Lipid metabolism, through fatty acid oxidation and lipogenesis, supports membrane biogenesis and signaling, further enhancing CSC maintenance. The integration of these metabolic pathways highlights the metabolic flexibility of CSCs, which must be considered when designing targeted therapies. Inhibiting multiple metabolic pathways simultaneously could enhance the efficacy of therapies by disrupting the metabolic plasticity of CSCs.
Ethical, safety, and regulatory considerations
The clinical application of advanced experimental strategies, such as CRISPR, CAR-T, and PDOs, raises critical ethical, safety, and regulatory considerations. CRISPR-based therapies involve precise genetic modifications that could have unintended consequences, necessitating rigorous preclinical testing and ethical oversight (Laurent et al., 2024). CAR-T cell therapies, while promising, can elicit severe immune reactions, highlighting the need for careful patient selection and monitoring (Sterner and Sterner, 2021). PDOs, as models for personalized medicine, must be validated to ensure they accurately represent patient tumors and respond appropriately to therapies (Tong et al., 2024). Regulatory frameworks must evolve to address the complexities and challenges associated with these advanced strategies. Ensuring the safety and efficacy of novel therapies while balancing innovation and patient benefit is a delicate task that requires ongoing dialogue among researchers, clinicians, ethicists, and policymakers.
In summary, while our review highlights the potential of CSC-targeted therapies, it is imperative to address the broader ecological and evolutionary aspects of cancer to improve clinical outcomes. Future research should focus on understanding the dynamic interactions within the tumor ecosystem and leveraging this knowledge to develop innovative combination therapies.
Conclusion and future prospectives
In this review, we summarize valuable insights regarding suitable cancer stem cells (CSCs)-associated signaling pathways, important biomarkers, and therapeutic approaches, which is essential to elucidate CSC functions in tumor metastasis, therapy resistance, and immune escape. Advances in stem cell cultures provide the foundation to powerful three-dimensional organoid technology, which could accurately recapitulate molecular characteristics, genomic alterations, expression profiles, organ structures, specific functions, and tumor microenvironment. The application of patient-derived organoids (PDOs) is beneficial for the comprehensive development of high-throughput drug screening, therapy response, and personalized medicine, which can undoubtedly alleviate serious drug attrition for drug development as well as guide optimized therapeutic strategies for individual patients.
Although CSCs, especially PDOs, show great potential in cancer research, clinical applications, and personalized therapies, there are still many challenges, bottlenecks, and difficulties remaining to be solved in further research. Firstly, establish standardized and optimized organoid culture conditions for improving large-scale reproducibility, reducing culture time, reserving genetic information, and avoiding new mutations. Secondly, build a PDO co-culturing system with immune cells and fibroblasts to replicate and mimic patient-specific immune environments among different tumor types and subject groups. Thirdly, apply genetic manipulation tools in organoid testing platforms to facilitate high-throughput drug screening, clinical practice, therapy guidance, and regenerative medicine.
By reshaping the narrative flow and providing a clear structure, this review aims to deepen the reader’s understanding of CSC biology and its implications for personalized cancer therapy.
Statements
Author contributions
LY: Writing – original draft, Investigation, Software, Conceptualization, Writing – review and editing. SZ: Writing – review and editing. HZ: Writing – review and editing. YS: Writing – review and editing. SW: Writing – review and editing. TJ: Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. T.J. is supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB0490000, XDB0940301), the National Key Research and Development Program of China (Grants No. 2022YFC2304102 and 2022YFC2303300), the National Natural Science Foundation of China (Grant No.: 82272301), Anhui Provincial Key Research and Development Project (Grant No. 2022i01020025), USTC Research Funds of the Double First-Class Initiative (YD9100002056). H.Z. is supported by the National Natural Science Foundation of China (Grant No.: 82402072).
Conflict of interest
Authors YS and TJ were employed by Anhui Genebiol Biotech. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abugomaa A. Elbadawy M. Yamawaki H. Usui T. Sasaki K. (2020). Emerging roles of cancer stem cells in bladder cancer progression, tumorigenesis, and resistance to chemotherapy: a potential therapeutic target for bladder cancer. Cells9 (1), 235. 10.3390/cells9010235
2
Ahn S. Park J. H. Grimm S. L. Piyarathna D. W. B. Samanta T. Putluri V. et al (2024). Metabolomic rewiring promotes endocrine therapy resistance in breast cancer. Cancer Res.84 (2), 291–304. 10.1158/0008-5472.CAN-23-0184
3
Aida S. Okugawa J. Fujisaka S. Kasai T. Kameda H. Sugiyama T. (2020). Deep learning of cancer stem cell morphology using conditional generative adversarial networks. Biomolecules10 (6), 931. 10.3390/biom10060931
4
Al-Hajj M. Wicha M. S. Benito-Hernandez A. Morrison S. J. Clarke M. F. (2003). Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. U. S. A.100 (7), 3983–3988. 10.1073/pnas.0530291100
5
Alibolandi M. Ramezani M. Abnous K. Sadeghi F. Atyabi F. Asouri M. et al (2015). In vitro and in vivo evaluation of therapy targeting epithelial-cell adhesion-molecule aptamers for non-small cell lung cancer. J. Control Release209, 88–100. 10.1016/j.jconrel.2015.04.026
6
Asai R. Tsuchiya H. Amisaki M. Makimoto K. Takenaga A. Sakabe T. et al (2019). CD44 standard isoform is involved in maintenance of cancer stem cells of a hepatocellular carcinoma cell line. Cancer Med.8 (2), 773–782. 10.1002/cam4.1968
7
Ayyalasomayajula R. Cudic M. (2024). Targeting siglec-sialylated MUC1 immune axis in cancer. Cancers (Basel)16 (7), 1334. 10.3390/cancers16071334
8
Azzoni V. Wicinski J. Macario M. Castagné M. Finetti P. Ambrosova K. et al (2022). BMI1 nuclear location is critical for RAD51-dependent response to replication stress and drives chemoresistance in breast cancer stem cells. Cell Death Dis.13 (2), 96. 10.1038/s41419-022-04538-w
9
Babaei G. Aziz S. G. Jaghi N. Z. Z. (2021). EMT, cancer stem cells and autophagy; the three main axes of metastasis. Biomed. Pharmacother.133, 110909. 10.1016/j.biopha.2020.110909
10
Bahmad H. F. Cheaito K. Chalhoub R. M. Hadadeh O. Monzer A. Ballout F. et al (2018). Sphere-formation assay: three-dimensional in vitro culturing of prostate cancer stem/progenitor sphere-forming cells. Front. Oncol.8, 347. 10.3389/fonc.2018.00347
11
Batchuluun B. Pinkosky S. L. Steinberg G. R. (2022). Lipogenesis inhibitors: therapeutic opportunities and challenges. Nat. Rev. Drug Discov.21 (4), 283–305. 10.1038/s41573-021-00367-2
12
Benmebarek M. R. Karches C. H. Cadilha B. L. Lesch S. Endres S. Kobold S. (2019). Killing mechanisms of chimeric antigen receptor (CAR) T cells. Int. J. Mol. Sci.20 (6), 1283. 10.3390/ijms20061283
13
Bilotta M. T. Antignani A. Fitzgerald D. J. (2022). Managing the TME to improve the efficacy of cancer therapy. Front. Immunol.13, 954992. 10.3389/fimmu.2022.954992
14
Borrebaeck C. A. (2017). Precision diagnostics: moving towards protein biomarker signatures of clinical utility in cancer. Nat. Rev. Cancer17 (3), 199–204. 10.1038/nrc.2016.153
15
Bort A. Sánchez B. G. de Miguel I. Mateos-Gómez P. A. Diaz-Laviada I. (2020). Dysregulated lipid metabolism in hepatocellular carcinoma cancer stem cells. Mol. Biol. Rep.47 (4), 2635–2647. 10.1007/s11033-020-05352-3
16
Botchkina I. L. Rowehl R. A. Rivadeneira D. E. Karpeh M. S. Jr Crawford H. Dufour A. et al (2009). Phenotypic subpopulations of metastatic Colon cancer stem cells: genomic analysis. Cancer Genomics Proteomics6 (1), 19–29.
17
Bryan R. T. (2015). Cell adhesion and urothelial bladder cancer: the role of cadherin switching and related phenomena. Philos. Trans. R. Soc. Lond B Biol. Sci.370 (1661), 20140042. 10.1098/rstb.2014.0042
18
Buffone A. Weaver V. M. (2020). Don't sugarcoat it: how glycocalyx composition influences cancer progression. J. Cell Biol.219 (1), e201910070. 10.1083/jcb.201910070
19
Cancer.org (2024). Cancer facts and figures. Available online at: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2024-cancer-facts-figures.html.
20
Cancer Research UK (2024). More than 900,000 cancer deaths predicted in the next 5 years. Available online at: https://news.cancerresearchuk.org/2024/09/09/more-than-900000-cancer-deaths-predicted-in-the-next-5-years/.
21
Cao Y. Liu B. Cai L. Li Y. Huang Y. Zhou Y. et al (2023). G9a promotes immune suppression by targeting the Fbxw7/Notch pathway in glioma stem cells. CNS Neurosci. Ther.29 (9), 2508–2521. 10.1111/cns.14191
22
Cashen A. Lopez S. Gao F. Calandra G. MacFarland R. Badel K. et al (2008). A phase II study of plerixafor (AMD3100) plus G-CSF for autologous hematopoietic progenitor cell mobilization in patients with hodgkin lymphoma. Biol. Blood Marrow Transpl.14 (11), 1253–1261. 10.1016/j.bbmt.2008.08.011
23
Chambost A. J. Berabez N. Cochet-Escartin O. Ducray F. Gabut M. Isaac C. et al (2022). Machine learning-based detection of label-free cancer stem-like cell fate. Sci. Rep.12 (1), 19066. 10.1038/s41598-022-21822-z
24
Chan K. S. Espinosa I. Chao M. Wong D. Ailles L. Diehn M. et al (2009). Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc. Natl. Acad. Sci. U. S. A.106 (33), 14016–14021. 10.1073/pnas.0906549106
25
Chen J. Liu S. Su Y. Zhang X. (2020). ALDH1+ stem cells demonstrate more stem cell-like characteristics than CD44+/CD24-/low stem cells in different molecular subtypes of breast cancer. Transl. Cancer Res.9 (3), 1652–1659. 10.21037/tcr.2020.01.53
26
Chen J. Xu L. Li X. Park S. (2023). Deep learning models for cancer stem cell detection: a brief review. Front. Immunol.14, 1214425. 10.3389/fimmu.2023.1214425
27
Chen J. H. Kuo K. T. Bamodu O. A. Lin Y. C. Yang R. B. Yeh C. T. et al (2018). Upregulated SCUBE2 expression in breast cancer stem cells enhances triple negative breast cancer aggression through modulation of notch signaling and epithelial-to-mesenchymal transition. Exp. Cell Res.370 (2), 444–453. 10.1016/j.yexcr.2018.07.008
28
Chen K. Zhang C. Ling S. Wei R. Wang J. Xu X. (2021). The metabolic flexibility of quiescent CSC: implications for chemotherapy resistance. Cell Death Dis.12 (9), 835. 10.1038/s41419-021-04116-6
29
Chen W. Dong J. Haiech J. Kilhoffer M. C. Zeniou M. (2016). Cancer stem cell quiescence and plasticity as major challenges in cancer therapy. Stem Cells Int.2016, 1740936. 10.1155/2016/1740936
30
Chi F. Jin X. Chen L. He G. Han S. (2022). TRG16, targeted by miR-765, inhibits breast cancer stem cell-like properties via regulating the NF-κB pathway. Mol. Cell Biochem.477 (12), 2801–2816. 10.1007/s11010-022-04480-7
31
Cho I. J. Lui P. P. Obajdin J. Riccio F. Stroukov W. Willis T. L. et al (2019). Mechanisms, hallmarks, and implications of stem cell quiescence. Stem Cell Rep.12 (6), 1190–1200. 10.1016/j.stemcr.2019.05.012
32
Choi H. J. Jhe Y. L. Kim J. Lim J. Y. Lee J. E. Shin M. K. et al (2020). FoxM1-dependent and fatty acid oxidation-mediated ROS modulation is a cell-intrinsic drug resistance mechanism in cancer stem-like cells. Redox Biol.36, 101589. 10.1016/j.redox.2020.101589
33
Chung W. M. Molony R. D. Lee Y. F. (2021). Non-stem bladder cancer cell-derived extracellular vesicles promote cancer stem cell survival in response to chemotherapy. Stem Cell Res. Ther.12 (1), 533. 10.1186/s13287-021-02600-6
34
Clarke M. F. (2019). Clinical and therapeutic implications of cancer stem cells. N. Engl. J. Med.380 (23), 2237–2245. 10.1056/NEJMra1804280
35
Cui Y. Ran R. Da Y. Zhang H. Jiang M. Qi X. et al (2024). The combination of breast cancer PDO and mini-PDX platform for drug screening and individualized treatment. J. Cell Mol. Med.28 (9), e18374. 10.1111/jcmm.18374
36
da Costa V. R. Araldi R. P. Vigerelli H. D'Ámelio F. Mendes T. B. Gonzaga V. et al (2021). Exosomes in the tumor microenvironment: from biology to clinical applications. Cells10 (10), 2617. 10.3390/cells10102617
37
Dai M. Yuan F. Fu C. Shen G. Hu S. Shen G. (2017). Relationship between epithelial cell adhesion molecule (EpCAM) overexpression and gastric cancer patients: a systematic review and meta-analysis. PLoS One12 (4), e0175357. 10.1371/journal.pone.0175357
38
Das S. Mukherjee P. Chatterjee R. Jamal Z. Chatterji U. (2019). Enhancing chemosensitivity of breast cancer stem cells by downregulating SOX2 and ABCG2 using wedelolactone-encapsulated nanoparticles. Mol. Cancer Ther.18 (3), 680–692. 10.1158/1535-7163.MCT-18-0409
39
Debnath P. Huirem R. S. Dutta P. Palchaudhuri S. (2022). Epithelial-mesenchymal transition and its transcription factors. Biosci. Rep.42 (1), BSR20211754. 10.1042/BSR20211754
40
Del Vecchio V. La Noce M. Tirino V. (2024). ALDH activity assay: a method for cancer stem cell (CSC) identification and isolation. Methods Mol. Biol.2777, 83–89. 10.1007/978-1-0716-3730-2_6
41
Drost J. Clevers H. (2017). Translational applications of adult stem cell-derived organoids. Development144 (6), 968–975. 10.1242/dev.140566
42
Drost J. van Jaarsveld R. H. Ponsioen B. Zimberlin C. van Boxtel R. Buijs A. et al (2015). Sequential cancer mutations in cultured human intestinal stem cells. Nature521 (7550), 43–47. 10.1038/nature14415
43
Du L. Wang H. He L. Zhang J. Ni B. Wang X. et al (2008). CD44 is of functional importance for colorectal cancer stem cells. Clin. Cancer Res.14 (21), 6751–6760. 10.1158/1078-0432.CCR-08-1034
44
Duan J. J. Cai J. Gao L. Yu S. C. (2023). ALDEFLUOR activity, ALDH isoforms, and their clinical significance in cancers. J. Enzyme Inhib. Med. Chem.38 (1), 2166035. 10.1080/14756366.2023.2166035
45
Dummer R. Guminski A. Gutzmer R. Dirix L. Lewis K. D. Combemale P. et al (2016). The 12-month analysis from basal cell carcinoma outcomes with LDE225 treatment (BOLT): a phase II, randomized, double-blind study of sonidegib in patients with advanced basal cell carcinoma. J. Am. Acad. Dermatol75 (1), 113–125. 10.1016/j.jaad.2016.02.1226
46
El-Benhawy S. A. Morsi M. I. Fahmy E. I. Soula M. A. Khalil FAZF Arab A. R. (2021). Role of resveratrol as radiosensitizer by targeting cancer stem cells in radioresistant prostate cancer cells (PC-3). Asian Pac J. Cancer Prev.22 (12), 3823–3837. 10.31557/APJCP.2021.22.12.3823
47
El-Sahli S. Wang L. (2020). Cancer stem cell-associated pathways in the metabolic reprogramming of breast cancer. Int. J. Mol. Sci.21 (23), 9125. 10.3390/ijms21239125
48
Feng D. Yan K. Liang H. Liang J. Wang W. Yu H. et al (2021). CBP-Mediated Wnt3a/β-catenin signaling promotes cervical oncogenesis initiated by Piwil2. Neoplasia23 (1), 1–11. 10.1016/j.neo.2020.10.013
49
Fessler E. Drost J. van Hooff S. R. Linnekamp J. F. Wang X. Jansen M. et al (2016). TGFβ signaling directs serrated adenomas to the mesenchymal colorectal cancer subtype. EMBO Mol. Med.8 (7), 745–760. 10.15252/emmm.201606184
50
Gao F. Zhou B. Xu J. C. Gao X. Li S. X. Zhu G. C. et al (2015). The role of LGR5 and ALDH1A1 in non-small cell lung cancer: cancer progression and prognosis. Biochem. Biophys. Res. Commun.462 (2), 91–98. 10.1016/j.bbrc.2015.04.029
51
Gao M. Harper M. M. Lin M. Qasem S. A. Patel R. A. Mardini S. H. et al (2021). Development of a single-cell technique to increase yield and use of gastrointestinal cancer organoids for personalized medicine application. J. Am. Coll. Surg.232 (4), 504–514. 10.1016/j.jamcollsurg.2020.11.009
52
Garner K. E. L. Hull N. J. Sims A. H. Lamb R. Clarke R. B. (2019). The milk protein alpha-casein suppresses triple negative breast cancer stem cell activity via STAT and HIF-1alpha signalling pathways in breast cancer cells and fibroblasts. J. Mammary Gland. Biol. Neoplasia24 (3), 245–256. 10.1007/s10911-019-09435-1
53
Gonzalez-Ericsson P. I. Stovgaard E. S. Sua L. F. Reisenbichler E. Kos Z. Carter J. M. et al (2020). The path to a better biomarker: application of a risk management framework for the implementation of PD-L1 and TILs as immuno-oncology biomarkers in breast cancer clinical trials and daily practice. J. Pathol.250 (5), 667–684. 10.1002/path.5406
54
Gorodetska I. Offermann A. Püschel J. Lukiyanchuk V. Gaete D. Kurzyukova A. et al (2024). ALDH1A1 drives prostate cancer metastases and radioresistance by interplay with AR- and RAR-Dependent transcription. Theranostics14 (2), 714–737. 10.7150/thno.88057
55
Green R. Howell M. Khalil R. Nair R. Yan J. Foran E. et al (2019). Actinomycin D and Telmisartan combination targets lung cancer stem cells through the wnt/beta catenin pathway. Sci. Rep.9 (1), 18177. 10.1038/s41598-019-54266-z
56
Gulaia V. Kumeiko V. Shved N. Cicinskas E. Rybtsov S. Ruzov A. et al (2018). Molecular mechanisms governing the stem cell's fate in brain cancer: factors of stemness and quiescence. Front. Cell Neurosci.12, 388. 10.3389/fncel.2018.00388
57
Guo D. Sheng K. Zhang Q. Li P. Sun H. Wang Y. et al (2024). Single-cell transcriptomic analysis reveals the landscape of epithelial-mesenchymal transition molecular heterogeneity in esophageal squamous cell carcinoma. Cancer Lett.587, 216723. 10.1016/j.canlet.2024.216723
58
Hainsworth J. D. Reeves J. A. Mace J. R. Crane E. J. Hamid O. Stille J. R. et al (2016). A randomized, open-label phase 2 study of the CXCR4 inhibitor LY2510924 in combination with sunitinib versus sunitinib alone in patients with metastatic renal cell carcinoma (RCC). Target Oncol.11 (5), 643–653. 10.1007/s11523-016-0434-9
59
Hanahan D. (2022). Hallmarks of cancer: new dimensions. Cancer Discov.12 (1), 31–46. 10.1158/2159-8290.CD-21-1059
60
Hayes D. F. Bast R. C. Desch C. E. Fritsche H. Jr Kemeny N. E. Jessup J. M. et al (1996). Tumor marker utility grading system: a framework to evaluate clinical utility of tumor markers. J. Natl. Cancer Inst.88 (20), 1456–1466. 10.1093/jnci/88.20.1456
61
He Y. Jiang X. Duan L. Xiong Q. Yuan Y. Liu P. et al (2021). LncRNA PKMYT1AR promotes cancer stem cell maintenance in non-small cell lung cancer via activating wnt signaling pathway. Mol. Cancer20 (1), 156. 10.1186/s12943-021-01469-6
62
Herpers B. Eppink B. James M. I. Cortina C. Cañellas-Socias A. Boj S. F. et al (2022). Functional patient-derived organoid screenings identify MCLA-158 as a therapeutic EGFR × LGR5 bispecific antibody with efficacy in epithelial tumors. Nat. Cancer3 (4), 418–436. 10.1038/s43018-022-00359-0
63
Holah N. S. Aiad H. A. Asaad N. Y. Elkhouly E. A. Lasheen A. G. (2017). Evaluation of the role of ALDH1 as cancer stem cell marker in colorectal carcinoma: an immunohistochemical study. J. Clin. Diagn Res.11 (1), EC17–EC23. 10.7860/JCDR/2017/22671.9291
64
Hsieh I. S. Chang K. C. Tsai Y. T. Ke J. Y. Lu P. J. Lee K. H. et al (2013). MicroRNA-320 suppresses the stem cell-like characteristics of prostate cancer cells by downregulating the wnt/Beta-Catenin signaling pathway. Carcinogenesis34 (3), 530–538. 10.1093/carcin/bgs371
65
Hu M. Lan Y. Lu A. Ma X. Zhang L. (2019). Glycan-based biomarkers for diagnosis of cancers and other diseases: past, present, and future. Prog. Mol. Biol. Transl. Sci.162, 1–24. 10.1016/bs.pmbts.2018.12.002
66
Huang P. Watanabe M. Kaku H. Ueki H. Noguchi H. Sugimoto M. et al (2013). Cancer stem cell-like characteristics of a CD133+ subpopulation in the J82 human bladder cancer cell line. Mol. Clin. Oncol.1 (1), 180–184. 10.3892/mco.2012.29
67
Hurt E. M. Kawasaki B. T. Klarmann G. J. Thomas S. B. Farrar W. L. (2008). CD44+ CD24(-) prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br. J. Cancer98 (4), 756–765. 10.1038/sj.bjc.6604242
68
Huynh P. T. Beswick E. J. Coronado Y. A. Johnson P. O'Connell M. R. Watts T. et al (2016). CD90(+) stromal cells are the major source of IL-6, which supports cancer stem-like cells and inflammation in colorectal cancer. Int. J. Cancer138 (8), 1971–1981. 10.1002/ijc.29939
69
Invrea F. Rovito R. Torchiaro E. Petti C. Isella C. Medico E. (2020). Patient-derived xenografts (PDXs) as model systems for human cancer. Curr. Opin. Biotechnol.63, 151–156. 10.1016/j.copbio.2020.01.003
70
Jafari N. Abediankenari S. (2017). MicroRNA-34 dysregulation in gastric cancer and gastric cancer stem cell. Tumour Biol.39 (5), 1010428317701652. 10.1177/1010428317701652
71
Jones M. F. Hara T. Francis P. Li X. L. Bilke S. Zhu Y. et al (2015). The CDX1-microRNA-215 axis regulates colorectal cancer stem cell differentiation. Proc. Natl. Acad. Sci. U. S. A.112 (13), E1550–E1558. 10.1073/pnas.1503370112
72
Jordan H. A. Thomas S. N. (2023). Novel proteomic technologies to address gaps in pre-clinical ovarian cancer biomarker discovery efforts. Expert Rev. Proteomics20 (12), 439–450. 10.1080/14789450.2023.2295861
73
Joung J. Konermann S. Gootenberg J. S. Abudayyeh O. O. Platt R. J. Brigham M. D. et al (2017). Genome-scale CRISPR-Cas9 knockout and transcriptional activation screening. Nat. Protoc.12 (4), 828–863. 10.1038/nprot.2017.016
74
Ju F. Atyah M. M. Horstmann N. Gul S. Vago R. Bruns C. J. et al (2022). Characteristics of the cancer stem cell niche and therapeutic strategies. Stem Cell Res. Ther.13 (1), 233. 10.1186/s13287-022-02904-1
75
Kalavska K. Kucerova L. Schmidtova S. Chovanec M. Mego M. (2020). Cancer stem cell niche and immune-active tumor microenvironment in testicular germ cell tumors. Adv. Exp. Med. Biol.1226, 111–121. 10.1007/978-3-030-36214-0_9
76
Kang H. Wu Q. Sun A. Liu X. Fan Y. Deng X. (2018). Cancer cell glycocalyx and its significance in cancer progression. Int. J. Mol. Sci.19 (9), 2484. 10.3390/ijms19092484
77
Kanwal R. Shukla S. Walker E. Gupta S. (2018). Acquisition of tumorigenic potential and therapeutic resistance in CD133+ subpopulation of prostate cancer cells exhibiting stem-cell like characteristics. Cancer Lett.430, 25–33. 10.1016/j.canlet.2018.05.014
78
Kanyo N. Kovacs K. D. Saftics A. Szekacs I. Peter B. Santa-Maria A. R. et al (2020). Glycocalyx regulates the strength and kinetics of cancer cell adhesion revealed by biophysical models based on high resolution label-free optical data. Sci. Rep.10 (1), 22422. 10.1038/s41598-020-80033-6
79
Kapeleris J. Zou H. Qi Y. Gu Y. Li J. Schoning J. et al (2020). Cancer stemness contributes to cluster formation of Colon cancer cells and high metastatic potentials. Clin. Exp. Pharmacol. Physiol.47 (5), 838–847. 10.1111/1440-1681.13247
80
Karami F. M. Ebrahimi M. Nourbakhsh E. Zia Hazara A. Mirzaei A. Shafieyari S. et al (2022). PI3K/Akt/mTOR signaling pathway in cancer stem cells. Pathol. Res. Pract.237, 154010. 10.1016/j.prp.2022.154010
81
Kitazawa K. Tanaka K. Kubota Y. Musashi M. Higashi K. Nagasawa T. et al (2024). Expression of epithelial-mesenchymal transition markers in epidermal layer of atopic dermatitis. Biol. Pharm. Bull.47 (1), 49–59. 10.1248/bpb.b23-00291
82
Klapdor R. Wang S. Hacker U. Büning H. Morgan M. Dörk T. et al (2017). Improved killing of ovarian cancer stem cells by combining a novel chimeric antigen receptor-based immunotherapy and chemotherapy. Hum. Gene Ther.28 (10), 886–896. 10.1089/hum.2017.168
83
Koh M. Z. Ho W. Y. Yeap S. K. Ali N. M. Boo L. Alitheen N. B. (2021). Regulation of cellular and cancer stem cell-related putative gene expression of parental and CD44+CD24- sorted MDA-MB-231 cells by cisplatin. Pharm. (Basel).14 (5), 391. 10.3390/ph14050391
84
Kreso A. Dick J. E. (2014). Evolution of the cancer stem cell model. Cell Stem Cell14 (3), 275–291. 10.1016/j.stem.2014.02.006
85
Lara G. A. B. Carvalho Oliveira L. J. Jardim D. L. (2020). Impact of the biomarker enrichment strategy in drug development. Expert Rev. Mol. Diagn20 (6), 611–618. 10.1080/14737159.2020.1711734
86
Laurent M. Geoffroy M. Pavani G. Guiraud S. (2024). CRISPR-based gene therapies: from preclinical to clinical treatments. Cells13 (10), 800. 10.3390/cells13100800
87
Lei Z. N. Teng Q. X. Koya J. Liu Y. Chen Z. Zeng L. et al (2024). The correlation between cancer stem cells and epithelial-mesenchymal transition: molecular mechanisms and significance in cancer theragnosis. Front. Immunol.15, 1417201. 10.3389/fimmu.2024.1417201
88
Leng Z. Xia Q. Chen J. Li Y. Xu J. Zhao E. et al (2018). Lgr5+CD44+EpCAM+ strictly defines cancer stem cells in human colorectal cancer. Cell Physiol. biochem.46 (2), 860–872. 10.1159/000488743
89
Le-Rademacher J. Dahlberg S. Lee J. J. Adjei A. A. Mandrekar S. J. (2018). Biomarker clinical trials in lung cancer: design, logistics, challenges, and practical considerations. J. Thorac. Oncol.13 (11), 1625–1637. 10.1016/j.jtho.2018.08.2019
90
Leung E. L. Fiscus R. R. Tung J. W. Tin V. P. Cheng L. C. Sihoe A. D. et al (2010). Non-small cell lung cancer cells expressing CD44 are enriched for stem cell-like properties. PLoS One5 (11), e14062. 10.1371/journal.pone.0014062
91
Li C. Du Y. Yang Z. He L. Wang Y. Hao L. et al (2016). GALNT1-Mediated glycosylation and activation of sonic hedgehog signaling maintains the self-renewal and tumor-initiating capacity of bladder cancer stem cells. Cancer Res.76 (5), 1273–1283. 10.1158/0008-5472.CAN-15-2309
92
Li C. Pan J. Shi Z. Zeng X. Xia X. He X. et al (2025). Engineered endometrial clear cell cancer on-a-chip reveals early invasion-metastasis Cascade of cancer cells. Biomaterials Res.29, 0177. 10.34133/bmr.0177
93
Li D. Zhang Q. Zhou Y. Zhu H. Li T. Du F. (2022b). A novel nitidine chloride nanoparticle overcomes the stemness of CD133+EPCAM+ Huh7 hepatocellular carcinoma cells for liver cancer therapy. BMC Pharmacol. Toxicol.23 (1), 48. 10.1186/s40360-022-00589-z
94
Li J. Wang J. Xie D. Pei Q. Wan X. Xing H. R. et al (2021). Characteristics of the PI3K/AKT and MAPK/ERK pathways involved in the maintenance of self-renewal in lung cancer stem-like cells. Int. J. Biol. Sci.17 (5), 1191–1202. 10.7150/ijbs.57871
95
Li L. Z. Yang K. Jing Y. Fan Y. Jiang X. Wang S. et al (2023). CRISPR-Based screening identifies XPO7 as a positive regulator of senescence. Protein Cell14 (8), 623–628. 10.1093/procel/pwad012
96
Li Q. He G. Yu Y. Li X. Peng X. Yang L. (2024). Exosome crosstalk between cancer stem cells and tumor microenvironment: cancer progression and therapeutic strategies. Stem Cell Res. Ther.15 (1), 449. 10.1186/s13287-024-04061-z
97
Li X. Francies H. E. Secrier M. Perner J. Miremadi A. Galeano-Dalmau N. et al (2018). Organoid cultures recapitulate esophageal adenocarcinoma heterogeneity providing a model for clonality studies and precision therapeutics. Nat. Commun.9 (1), 2983. 10.1038/s41467-018-05190-9
98
Li X. Li X. Zhang B. He B. (2022a). The role of cancer stem cell-derived exosomes in cancer progression. Stem Cells Int.2022, 9133658. 10.1155/2022/9133658
99
Lin C. W. Kao S. H. Yang P. C. (2014). The miRNAs and epithelial-mesenchymal transition in cancers. Curr. Pharm. Des.20 (33), 5309–5318. 10.2174/1381612820666140128204508
100
Lipowsky H. H. (2018). Role of the glycocalyx as a barrier to leukocyte-endothelium adhesion. Adv. Exp. Med. Biol.1097, 51–68. 10.1007/978-3-319-96445-4_3
101
Liu R. Y. Zeng Y. Lei Z. Wang L. Yang H. Liu Z. et al (2014). JAK/STAT3 signaling is required for TGF-β-induced epithelial-mesenchymal transition in lung cancer cells. Int. J. Oncol.44 (5), 1643–1651. 10.3892/ijo.2014.2310
102
Liu X. Su K. Sun X. Jiang Y. Wang L. Hu C. et al (2021). Sec62 promotes stemness and chemoresistance of human colorectal cancer through activating Wnt/β-catenin pathway. J. Exp. Clin. Cancer Res.40 (1), 132. 10.1186/s13046-021-01934-6
103
Liu X. W. Wang P. Zhang L. Zhu Y. Zhai J. Y. Wang C. N. et al (2024). Single-cell RNA sequencing and ATAC sequencing identify novel biomarkers for bicuspid aortic valve-associated thoracic aortic aneurysm. Front. Cardiovasc Med.11, 1265378. 10.3389/fcvm.2024.1265378
104
Liu Y. Wang H. (2024). Biomarkers and targeted therapy for cancer stem cells. Trends Pharmacol. Sci.45 (1), 56–66. 10.1016/j.tips.2023.11.006
105
Liu Y. Zhang Y. Chen S. Zhong X. Liu Q. (2023b). Effect of LGR4/EGFR signaling on cell growth and cancer stem cell-like characteristics in liver cancer. Cytokine165, 156185. 10.1016/j.cyto.2023.156185
106
Liu Z. Lei J. Wu T. Hu W. Zheng M. Wang Y. et al (2023a). Lipogenesis promotes mitochondrial fusion and maintains cancer stemness in human NSCLC. JCI Insight8 (6), e158429. 10.1172/jci.insight.158429
107
Louhichi T. Ziadi S. Saad H. Dhiab M. B. Mestiri S. Trimeche M. (2018). Clinicopathological significance of cancer stem cell markers CD44 and ALDH1 expression in breast cancer. Breast Cancer25 (6), 698–705. 10.1007/s12282-018-0875-3
108
Lu L. Wu M. Sun L. Li W. Fu W. Zhang X. et al (2016). Clinicopathological and prognostic significance of cancer stem cell markers CD44 and CD133 in patients with gastric cancer: a comprehensive meta-analysis with 4729 patients involved. Med. Baltim.95 (42), e5163. 10.1097/MD.0000000000005163
109
Lu W. Kang Y. (2019). Epithelial-mesenchymal plasticity in cancer progression and metastasis. Dev. Cell49 (3), 361–374. 10.1016/j.devcel.2019.04.010
110
Lu Y. Zhang X. (2022). Radiochemotherapy-induced DNA repair promotes the biogenesis of gastric cancer stem cells. Stem Cell Res. Ther.13 (1), 481. 10.1186/s13287-022-03165-8
111
Luo W. (2023). Nasopharyngeal carcinoma ecology theory: cancer as multidimensional spatiotemporal “unity of ecology and evolution” pathological ecosystem. Theranostics13 (5), 1607–1631. 10.7150/thno.82690
112
Luo W. R. (2025). Rethinking cancer. Zhonghua Zhong Liu Za Zhi47 (6), 463–467. 10.3760/cma.j.cn112152-20250401-00145
113
Ma X. L. Sun Y. F. Wang B. L. Shen M. N. Zhou Y. Chen J. W. et al (2019). Sphere-forming culture enriches liver cancer stem cells and reveals Stearoyl-CoA desaturase 1 as a potential therapeutic target. BMC Cancer19 (1), 760. 10.1186/s12885-019-5963-z
114
Makena M. R. Ranjan A. Thirumala V. Reddy A. P. (2020). Cancer stem cells: road to therapeutic resistance and strategies to overcome resistance. Biochim. Biophys. Acta Mol. Basis Dis.1866 (4), 165339. 10.1016/j.bbadis.2018.11.015
115
Marcinkiewicz K. Scotland K. B. Boorjian S. A. Nilsson E. M. Persson J. L. Abrahamsson P. A. et al (2012). The androgen receptor and stem cell pathways in prostate and bladder cancers (review). Int. J. Oncol.40 (1), 5–12. 10.3892/ijo.2011.1212
116
Martinez-Gamero C. Malla S. Aguilo F. (2021). LSD1: expanding functions in stem cells and differentiation. Cells10 (11), 3252. 10.3390/cells10113252
117
Mascaraque M. Courtois S. Royo-García A. Barneda D. Stoian A. M. Villaoslada I. et al (2024). Fatty acid oxidation is critical for the tumorigenic potential and chemoresistance of pancreatic cancer stem cells. J. Transl. Med.22 (1), 797. 10.1186/s12967-024-05598-6
118
Masoudi M. Moti D. Masoudi R. Auwal A. Hossain M. M. Pronoy T. U. H. et al (2024). Metabolic adaptations in cancer stem cells: a key to therapy resistance. Biochim. Biophys. Acta Mol. Basis Dis.1870 (5), 167164. 10.1016/j.bbadis.2024.167164
119
Masoumi J. Jafarzadeh A. Abdolalizadeh J. Khan H. Philippe J. Mirzaei H. et al (2021). Cancer stem cell-targeted chimeric antigen receptor (CAR)-T cell therapy: challenges and prospects. Acta Pharm. Sin. B11 (7), 1721–1739. 10.1016/j.apsb.2020.12.015
120
McCauley H. A. Wells J. M. (2017). Pluripotent stem cell-derived organoids: using principles of developmental biology to grow human tissues in a dish. Development144 (6), 958–962. 10.1242/dev.140731
121
Mengistu B. A. Tsegaw T. Demessie Y. Getnet K. Bitew A. B. Kinde M. Z. et al (2024). Comprehensive review of drug resistance in Mammalian cancer stem cells: implications for cancer therapy. Cancer Cell Int.24 (1), 406. 10.1186/s12935-024-03558-0
122
Michels B. E. Mosa M. H. Streibl B. I. Zhan T. Menche C. Abou-El-Ardat K. et al (2020). Pooled in vitro and in vivo CRISPR-Cas9 screening identifies tumor suppressors in human Colon organoids. Cell Stem Cell26 (5), 782–792. 10.1016/j.stem.2020.04.003
123
Mok E. H. K. Con L. Zhou L. Lei M. M. L. Leung H. W. Tong M. et al (2022). Caspase-3-Induced activation of SREBP2 drives drug resistance via promotion of cholesterol biosynthesis in hepatocellular carcinoma. Cancer Res.82 (17), 3102–3115. 10.1158/0008-5472.CAN-21-2934
124
Mortezaee K. Majidpoor J. Kharazinejad E. (2022). Epithelial-mesenchymal transition in cancer stemness and heterogeneity: updated. Med. Oncol.39 (12), 193. 10.1007/s12032-022-01801-0
125
Mouillet-Richard S. (2022). ERBB2 in anti-EGFR-resistant colorectal cancer: cancer stem cells come into play. Gut71 (1), 4–5. 10.1136/gutjnl-2020-323924
126
Munkley J. (2022). Aberrant sialylation in cancer: therapeutic opportunities. Cancers (Basel)14 (17), 4248. 10.3390/cancers14174248
127
Najafi M. Mortezaee K. Majidpoor J. (2019). Cancer stem cell (CSC) resistance drivers. Life Sci.234, 116781. 10.1016/j.lfs.2019.116781
128
Nakajima T. Uehara T. Maruyama Y. Iwaya M. Kobayashi Y. Ota H. (2016). Distribution of Lgr5-positive cancer cells in intramucosal gastric signet-ring cell carcinoma. Pathol. Int.66 (9), 518–523. 10.1111/pin.12451
129
Namekawa T. Ikeda K. Horie-Inoue K. Suzuki T. Okamoto K. Ichikawa T. et al (2020). ALDH1A1 in patient-derived bladder cancer spheroids activates retinoic acid signaling leading to TUBB3 overexpression and tumor progression. Int. J. Cancer146 (4), 1099–1113. 10.1002/ijc.32505
130
Ni J. Cozzi P. Hao J. Beretov J. Chang L. Duan W. et al (2013). Epithelial cell adhesion molecule (EpCAM) is associated with prostate cancer metastasis and chemo/radioresistance via the PI3K/Akt/mTOR signaling pathway. Int. J. Biochem. Cell Biol.45 (12), 2736–2748. 10.1016/j.biocel.2013.09.008
131
Nimmakayala R. K. Leon F. Rachagani S. Rauth S. Nallasamy P. Marimuthu S. et al (2021). Metabolic programming of distinct cancer stem cells promotes metastasis of pancreatic ductal adenocarcinoma. Oncogene40 (1), 215–231. 10.1038/s41388-020-01518-2
132
Nobel Prize (2024). The nobel prize in physiology or medicine. Available online at: https://www.nobelprize.org/prizes/medicine/2024/summary/.
133
Noh C. K. Wang H. J. Kim C. M. Kim J. Yoon S. Y. Lee G. H. et al (2018). EpCAM as a predictive marker of tumor recurrence and survival in patients who underwent surgical resection for hepatocellular carcinoma. Anticancer Res.38 (7), 4101–4109. 10.21873/anticanres.12700
134
Nong S. Wang Z. Wei Z. Ma L. Guan Y. Ni J. (2022). HN1L promotes stem cell-like properties by regulating TGF-β signaling pathway through targeting FOXP2 in prostate cancer. Cell Biol. Int.46 (1), 83–95. 10.1002/cbin.11701
135
Norsworthy K. J. By K. Subramaniam S. Zhuang L. Del Valle P. L. Przepiorka D. et al (2019). FDA approval summary: Glasdegib for newly diagnosed acute myeloid leukemia. Clin. Cancer Res.25 (20), 6021–6025. 10.1158/1078-0432.CCR-19-0365
136
O'Brien C. A. Pollett A. Gallinger S. Dick J. E. (2007). A human Colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature445 (7123), 106–110. 10.1038/nature05372
137
Onyeisi J. O. S. Ferreira B. Z. F. Nader H. B. Lopes C. C. (2020). Heparan sulfate proteoglycans as targets for cancer therapy: a review. Cancer Biol. Ther.21 (12), 1087–1094. 10.1080/15384047.2020.1838034
138
Ooki A. VandenBussche C. J. Kates M. Hahn N. M. Matoso A. McConkey D. J. et al (2018). CD24 regulates cancer stem cell (CSC)-Like traits and a panel of CSC-Related molecules serves as a non-invasive urinary biomarker for the detection of bladder cancer. Br. J. Cancer119 (8), 961–970. 10.1038/s41416-018-0291-7
139
Oshimori N. (2020). Cancer stem cells and their niche in the progression of squamous cell carcinoma. Cancer Sci.111 (11), 3985–3992. 10.1111/cas.14639
140
Pandey H. Yadav B. Shah K. Kaur R. Choudhary D. Sharma N. et al (2024). A new method for the robust expression and single-step purification of dCas9 for CRISPR interference/activation (CRISPRi/a) applications. Protein Expr. Purif.220, 106500. 10.1016/j.pep.2024.106500
141
Park S. Colville M. J. Paek J. H. Shurer C. R. Singh A. Secor E. J. et al (2024). Immunoengineering can overcome the glycocalyx armour of cancer cells. Nat. Mater23 (3), 429–438. 10.1038/s41563-024-01808-0
142
Peixoto P. Etcheverry A. Aubry M. Missey A. Lachat C. Perrard J. et al (2019). EMT is associated with an epigenetic signature of ECM remodeling genes. Cell Death Dis.10 (3), 205. 10.1038/s41419-019-1397-4
143
Perry J. M. Li L. (2010). Functional assays for hematopoietic stem cell self-renewal. Methods Mol. Biol.636, 45–54. 10.1007/978-1-60761-691-7_3
144
Phi L. T. H. Sari I. N. Yang Y. G. Lee S. H. Jun N. Kim K. S. et al (2018). Cancer stem cells (CSCs) in drug resistance and their therapeutic implications in cancer treatment. Stem Cells Int.2018, 5416923. 10.1155/2018/5416923
145
Plaks V. Kong N. Werb Z. (2015). The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells?Cell Stem Cell16 (3), 225–238. 10.1016/j.stem.2015.02.015
146
Pors K. Moreb J. S. (2014). Aldehyde dehydrogenases in cancer: an opportunity for biomarker and drug development?Drug Discov. Today19 (12), 1953–1963. 10.1016/j.drudis.2014.09.009
147
Puertas-Umbert L. Alonso J. Blanco-Casoliva L. Almendra-Pegueros R. Camacho M. Rodríguez-Sinovas A. et al (2025). Inhibition of ATP-Citrate lyase by bempedoic acid protects against abdominal aortic aneurysm formation in mice. Biomed. Pharmacother.184, 117876. 10.1016/j.biopha.2025.117876
148
Qian X. Leonard F. Wenhao Y. Sudhoff H. Hoffmann T. K. Ferrone S. et al (2020). Immunotherapeutics for head and neck squamous cell carcinoma stem cells. HNO68 (2), 94–99. 10.1007/s00106-020-00819-y
149
Qin T. Li B. Feng X. Fan S. Liu L. Liu D. et al (2018). Abnormally elevated USP37 expression in breast cancer stem cells regulates stemness, epithelial-mesenchymal transition and cisplatin sensitivity. J. Exp. Clin. Cancer Res.37 (1), 287. 10.1186/s13046-018-0934-9
150
Rajakulendran N. Rowland K. J. Selvadurai H. J. Ahmadi M. Park N. I. Naumenko S. et al (2019). Wnt and notch signaling govern self-renewal and differentiation in a subset of human glioblastoma stem cells. Genes Dev.33 (9-10), 498–510. 10.1101/gad.321968.118
151
Ramović Hamzagić A. Cvetković D. Gazdić Janković M. Milivojević Dimitrijević N. Nikolić D. Živanović M. et al (2024a). Modeling 5-FU-Induced chemotherapy selection of a drug-resistant cancer stem cell subpopulation. Curr. Oncol.31 (3), 1221–1234. 10.3390/curroncol31030091
152
Ramović Hamzagić A. Gazdić Janković M. Cvetković D. Nikolić D. Nikolić S. Milivojević Dimitrijević N. et al (2024b). Machine learning model for prediction of development of cancer stem cell subpopulation in tumurs subjected to polystyrene nanoparticles. Toxics12 (5), 354. 10.3390/toxics12050354
153
Rashid K. Ahmad A. Meerasa S. S. Khan A. Q. Wu X. Liang L. et al (2023). Cancer stem cell-derived exosome-induced metastatic cancer: an orchestra within the tumor microenvironment. Biochimie212, 1–11. 10.1016/j.biochi.2023.03.014
154
Ray I. Michael A. Meira L. B. Ellis P. E. (2023). The role of cytokines in epithelial-mesenchymal transition in gynaecological cancers: a systematic review. Cells12 (3), 416. 10.3390/cells12030416
155
Regan J. L. Schumacher D. Staudte S. Steffen A. Haybaeck J. Keilholz U. et al (2017). Non-canonical hedgehog signaling is a positive regulator of the WNT pathway and is required for the survival of Colon cancer stem cells. Cell Rep.21 (10), 2813–2828. 10.1016/j.celrep.2017.11.025
156
Rømer T. B. Aasted M. K. M. Dabelsteen S. Groen A. Schnabel J. Tan E. et al (2021). Mapping of truncated O-glycans in cancers of epithelial and non-epithelial origin. Br. J. Cancer125 (9), 1239–1250. 10.1038/s41416-021-01530-7
157
Rossini A. Giussani M. Ripamonti F. Aiello P. Regondi V. Balsari A. et al (2020). Combined targeting of EGFR and HER2 against prostate cancer stem cells. Cancer Biol. Ther.21 (5), 463–475. 10.1080/15384047.2020.1727702
158
Roy S. Sunkara R. R. Parmar M. Y. Shaikh S. Waghmare S. K. (2021). EMT imparts cancer stemness and plasticity: new perspectives and therapeutic potential. Front. Biosci. Landmark Ed.26 (2), 238–265. 10.2741/4893
159
Rugg-Gunn P. J. (2022). Flow cytometry analysis of cell-surface markers to identify human naïve pluripotent stem cells. Methods Mol. Biol.2416, 257–265. 10.1007/978-1-0716-1908-7_16
160
Sachs N. de Ligt J. Kopper O. Gogola E. Bounova G. Weeber F. et al (2018). A living biobank of breast cancer organoids captures disease heterogeneity. Cell172 (1-2), 373–386. 10.1016/j.cell.2017.11.010
161
Sadrkhanloo M. Entezari M. Orouei S. Ghollasi M. Fathi N. Rezaei S. et al (2022). STAT3-EMT axis in tumors: modulation of cancer metastasis, stemness and therapy response. Pharmacol. Res.182, 106311. 10.1016/j.phrs.2022.106311
162
Sari I. N. Phi L. T. H. Jun N. Wijaya Y. T. Lee S. Kwon H. Y. (2018). Hedgehog signaling in cancer: a prospective therapeutic target for eradicating cancer stem cells. Cells7 (11), 208. 10.3390/cells7110208
163
Sato T. Vries R. G. Snippert H. J. van de Wetering M. Barker N. Stange D. E. et al (2009). Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature459 (7244), 262–265. 10.1038/nature07935
164
Sato Y. Bando H. Di Piazza M. Gowing G. Herberts C. Jackman S. et al (2019). Tumorigenicity assessment of cell therapy products: the need for global consensus and points to consider. Cytotherapy21 (11), 1095–1111. 10.1016/j.jcyt.2019.10.001
165
Sekulic A. Migden M. R. Oro A. E. Dirix L. Lewis K. D. Hainsworth J. D. et al (2012). Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N. Engl. J. Med.366 (23), 2171–2179. 10.1056/NEJMoa1113713
166
Senel F. Kökenek Unal T. D. Karaman H. Inanç M. Aytekin A. (2017). Prognostic value of cancer stem cell markers CD44 and ALDH1/2 in gastric cancer cases. Asian Pac J. Cancer Prev.18 (9), 2527–2531. 10.22034/APJCP.2017.18.9.2527
167
Serna N. Álamo P. Ramesh P. Vinokurova D. Sánchez-García L. Unzueta U. et al (2020). Nanostructured toxins for the selective destruction of drug-resistant human CXCR4+ colorectal cancer stem cells. J. Control Release320, 96–104. 10.1016/j.jconrel.2020.01.019
168
Shan M. Yang D. Dou H. Zhang L. (2019). Fucosylation in cancer biology and its clinical applications. Prog. Mol. Biol. Transl. Sci.162, 93–119. 10.1016/bs.pmbts.2019.01.002
169
Shende P. Devlekar N. P. (2021). A review on the role of artificial intelligence in stem cell therapy: an initiative for modern medicines. Curr. Pharm. Biotechnol.22 (9), 1156–1163. 10.2174/1389201021666201007122524
170
Shiraiwa K. Matsuse M. Nakazawa Y. Ogi T. Suzuki K. Saenko V. et al (2019). JAK/STAT3 and NF-κB signaling pathways regulate cancer stem-cell properties in Anaplastic thyroid cancer cells. Thyroid29 (5), 674–682. 10.1089/thy.2018.0212
171
Shu X. Liu H. Pan Y. Sun L. Yu L. Sun L. et al (2019). Distinct biological characterization of the CD44 and CD90 phenotypes of cancer stem cells in gastric cancer cell lines. Mol. Cell Biochem.459 (1-2), 35–47. 10.1007/s11010-019-03548-1
172
Si J. Ma Y. Bi J. W. Xiong Y. Lv C. Li S. et al (2019). Shisa3 brakes resistance to EGFR-TKIs in lung adenocarcinoma by suppressing cancer stem cell properties. J. Exp. Clin. Cancer Res.38 (1), 481. 10.1186/s13046-019-1486-3
173
Singh M. K. Han S. Kim S. Kang I. (2024). Targeting lipid metabolism in cancer stem cells for anticancer treatment. Int. J. Mol. Sci.25 (20), 11185. 10.3390/ijms252011185
174
Skidan I. Steiniger S. C. (2014). In vivo models for cancer stem cell research: a practical guide for frequently used animal models and available biomarkers. J. Physiol. Pharmacol.65 (2), 157–169.
175
Skvortsov S. Debbage P. Lukas P. Skvortsova I. (2015). Crosstalk between DNA repair and cancer stem cell (CSC) associated intracellular pathways. Semin. Cancer Biol.31, 36–42. 10.1016/j.semcancer.2014.06.002
176
Sola-García A. Cáliz-Molina M. Á. Espadas I. Petr M. Panadero-Morón C. González-Morán D. et al (2023). Metabolic reprogramming by acly inhibition using SB-204990 alters glucoregulation and modulates molecular mechanisms associated with aging. Commun. Biol.6 (1), 250. 10.1038/s42003-023-04625-4
177
Steelman L. S. Fitzgerald T. Lertpiriyapong K. Cocco L. Follo M. Y. Martelli A. M. et al (2016). Critical roles of EGFR family members in breast cancer and breast cancer stem cells: targets for therapy. Curr. Pharm. Des.22 (16), 2358–2388. 10.2174/1381612822666160304151011
178
Steinbichler T. B. Dudás J. Skvortsov S. Ganswindt U. Riechelmann H. Skvortsova I. I. (2018). Therapy resistance mediated by cancer stem cells. Semin. Cancer Biol.53, 156–167. 10.1016/j.semcancer.2018.11.006
179
Sterner R. C. Sterner R. M. (2021). CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J.11 (4), 69. 10.1038/s41408-021-00459-7
180
Su P. Liu Y. Chen T. Xue Y. Zeng Y. Zhu G. et al (2024). In vivo CRISPR screens identify a dual function of MEN1 in regulating tumor-microenvironment interactions. Nat. Genet.56 (9), 1890–1902. 10.1038/s41588-024-01874-9
181
Su R. Dong L. Li Y. Gao M. Han L. Wunderlich M. et al (2020). Targeting FTO suppresses cancer stem cell maintenance and immune evasion. Cancer Cell38 (1), 79–96. 10.1016/j.ccell.2020.04.017
182
Su Y. J. Chang Y. W. Lin W. H. Liang C. L. Lee J. L. (2015). An aberrant nuclear localization of E-cadherin is a potent inhibitor of Wnt/β-catenin-elicited promotion of the cancer stem cell phenotype. Oncogenesis4 (6), e157. 10.1038/oncsis.2015.17
183
Takahashi H. Ishii H. Nishida N. Takemasa I. Mizushima T. Ikeda M. et al (2011). Significance of Lgr5(+ve) cancer stem cells in the Colon and rectum. Ann. Surg. Oncol.18 (4), 1166–1174. 10.1245/s10434-010-1373-9
184
Takahashi K. Tanabe K. Ohnuki M. Narita M. Ichisaka T. Tomoda K. et al (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell131 (5), 861–872. 10.1016/j.cell.2007.11.019
185
Taniguchi N. Ohkawa Y. Maeda K. Kanto N. Johnson E. L. Harada Y. (2022). N-glycan branching enzymes involved in cancer, alzheimer's disease and COPD and future perspectives. Biochem. Biophys. Res. Commun.633, 68–71. 10.1016/j.bbrc.2022.09.027
186
Tao M. Sun F. Wang J. Wang Y. Zhu H. Chen M. et al (2022). Developing patient-derived organoids to predict PARP inhibitor response and explore resistance overcoming strategies in ovarian cancer. Pharmacol. Res.179, 106232. 10.1016/j.phrs.2022.106232
187
Teixeira FCOB Vijaya Kumar A. Kumar Katakam S. Cocola C. Pelucchi P. Graf M. et al (2020). The heparan sulfate sulfotransferases HS2ST1 and HS3ST2 are novel regulators of breast cancer stem-cell properties. Front. Cell Dev. Biol.8, 559554. 10.3389/fcell.2020.559554
188
Testa U. Castelli G. Pelosi E. (2018). Lung cancers: molecular characterization, clonal heterogeneity and evolution, and cancer stem cells. Cancers (Basel)10 (8), 248. 10.3390/cancers10080248
189
Thakur B. Ray P. (2017). Cisplatin triggers cancer stem cell enrichment in platinum-resistant cells through NF-κB-TNFα-PIK3CA loop. J. Exp. Clin. Cancer Res.36 (1), 164. 10.1186/s13046-017-0636-8
190
Tiriac H. Belleau P. Engle D. D. Plenker D. Deschênes A. Somerville T. D. D. et al (2018). Organoid profiling identifies common responders to chemotherapy in pancreatic cancer. Cancer Discov.8 (9), 1112–1129. 10.1158/2159-8290.CD-18-0349
191
Tong L. Cui W. Zhang B. Fonseca P. Zhao Q. Zhang P. et al (2024). Patient-derived organoids in precision cancer medicine. Med5 (11), 1351–1377. 10.1016/j.medj.2024.08.010
192
Troschel F. M. Böhly N. Borrmann K. Braun T. Schwickert A. Kiesel L. et al (2018). miR-142-3p attenuates breast cancer stem cell characteristics and decreases radioresistance in vitro. Tumour Biol.40 (8), 1010428318791887. 10.1177/1010428318791887
193
Tsuchiya H. Shiota G. (2021). Immune evasion by cancer stem cells. Regen. Ther.17, 20–33. 10.1016/j.reth.2021.02.006
194
Twomey J. D. Brahme N. N. Zhang B. (2017). Drug-biomarker co-development in oncology - 20 years and counting. Drug Resist Updat30, 48–62. 10.1016/j.drup.2017.02.002
195
Ubink I. Bolhaqueiro A. C. F. Elias S. G. Raats D. A. E. Constantinides A. Peters N. A. et al (2019). Organoids from colorectal peritoneal metastases as a platform for improving hyperthermic intraperitoneal chemotherapy. Br. J. Surg.106 (10), 1404–1414. 10.1002/bjs.11206
196
Ushijima T. Clark S. J. Tan P. (2021). Mapping genomic and epigenomic evolution in cancer ecosystems. Science373 (6562), 1474–1479. 10.1126/science.abh1645
197
Vazquez-Santillan K. Melendez-Zajgla J. Jimenez-Hernandez L. Martínez-Ruiz G. Maldonado V. (2015). NF-κB signaling in cancer stem cells: a promising therapeutic target?Cell Oncol. (Dordr)38 (5), 327–339. 10.1007/s13402-015-0236-6
198
Vlachogiannis G. Hedayat S. Vatsiou A. Jamin Y. Fernández-Mateos J. Khan K. et al (2018). Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science359 (6378), 920–926. 10.1126/science.aao2774
199
Voest E. E. Bernards R. (2016). DNA-guided precision medicine for cancer: a case of irrational exuberance?Cancer Discov.6 (2), 130–132. 10.1158/2159-8290.CD-15-1321
200
Voon D. C. Wang H. Koo J. K. Chai J. H. Hor Y. T. Tan T. Z. et al (2013). EMT-Induced stemness and tumorigenicity are fueled by the EGFR/ras pathway. PLoS One8 (8), e70427. 10.1371/journal.pone.0070427
201
Wang E. Henderson M. Yalamanchili P. Cueto J. Islam Z. Dharmani C. et al (2024a). Potential biomarkers in breast cancer drug development: application of the biomarker qualification evidentiary framework. Biomark. Med.18 (6), 265–277. 10.2217/bmm-2023-0048
202
Wang H. Zhang G. Liu Y. He Y. Guo Q. Du Y. et al (2025). Glycocalyx hyaluronan removal-induced increasing of cell stiffness delays breast cancer cells progression. Cell Mol. Life Sci.82 (1), 96. 10.1007/s00018-025-05577-0
203
Wang J. Sullenger B. A. Rich J. N. (2012). Notch signaling in cancer stem cells. Adv. Exp. Med. Biol.727, 174–185. 10.1007/978-1-4614-0899-4_13
204
Wang J. H. Gong C. Guo F. J. Zhou X. Zhang M. S. Qiu H. et al (2020a). Knockdown of STIP1 inhibits the invasion of CD133-positive cancer stem-like cells of the osteosarcoma MG63 cell line via the PI3K/Akt and ERK1/2 pathways. Int. J. Mol. Med.46 (6), 2251–2259. 10.3892/ijmm.2020.4764
205
Wang L. Stadlbauer B. Lyu C. Buchner A. Pohla H. (2020b). Shikonin enhances the antitumor effect of cabazitaxel in prostate cancer stem cells and reverses cabazitaxel resistance by inhibiting ABCG2 and ALDH3A1. Am. J. Cancer Res.10 (11), 3784–3800.
206
Wang L. Zhang L. Zhang Z. Wu P. Zhang Y. Chen X. (2024c). Advances in targeting tumor microenvironment for immunotherapy. Front. Immunol.15, 1472772. 10.3389/fimmu.2024.1472772
207
Wang R. Li Y. Tsung A. Huang H. Du Q. Yang M. et al (2018b). iNOS promotes CD24+CD133+ liver cancer stem cell phenotype through a TACE/ADAM17-dependent notch signaling pathway. Proc. Natl. Acad. Sci. U. S. A.115 (43), E10127–E10136. 10.1073/pnas.1722100115
208
Wang R. Yang L. Li S. Ye D. Yang L. Liu Q. et al (2018c). Quercetin inhibits breast cancer stem cells via downregulation of aldehyde dehydrogenase 1A1 (ALDH1A1), chemokine receptor type 4 (CXCR4), mucin 1 (MUC1), and epithelial cell adhesion molecule (EpCAM). Med. Sci. Monit.24, 412–420. 10.12659/msm.908022
209
Wang S. Li Z. Zhu G. Hong L. Hu C. Wang K. et al (2021). RNA-Binding protein IGF2BP2 enhances circ_0000745 abundancy and promotes aggressiveness and stemness of ovarian cancer cells via the microRNA-3187-3p/ERBB4/PI3K/AKT axis. J. Ovarian Res.14 (1), 154. 10.1186/s13048-021-00917-7
210
Wang S. Xu Z. Y. Wang L. F. Su W. (2013). CD133+ cancer stem cells in lung cancer. Front. Biosci. Landmark Ed.18 (2), 447–453. 10.2741/4113
211
Wang T. Fahrmann J. F. Lee H. Li Y. J. Tripathi S. C. Yue C. et al (2018a). JAK/STAT3-Regulated fatty acid β-Oxidation is critical for breast cancer stem cell self-renewal and chemoresistance. Cell Metab.27 (1), 1357–150.e5. 10.1016/j.cmet.2018.04.018
212
Wang Y. Song W. Feng C. Wu S. Qin Z. Liu T. et al (2024b). Multi-omics analysis unveils the predictive value of IGF2BP3/SPHK1 signaling in cancer stem cells for prognosis and immunotherapeutic response in muscle-invasive bladder cancer. J. Transl. Med.22 (1), 900. 10.1186/s12967-024-05685-8
213
Wei Y. Wei A. Li Y. Yang Y. Si Y. Li Y. et al (2024). Deciphering the cell surface glyco-code: a promising perspective on unveiling the vulnerability of cancer stem cells. Cancer Biol. Med.21 (11), 963–969. 10.20892/j.issn.2095-3941.2024.0408
214
Weiss T. Weller M. Guckenberger M. Sentman C. L. Roth P. (2018). NKG2D-Based CAR T cells and radiotherapy exert synergistic efficacy in glioblastoma. Cancer Res.78 (4), 1031–1043. 10.1158/0008-5472.CAN-17-1788
215
WHO (2024). Global cancer burden growing, amidst mounting need for services. Available online at: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services.
216
Wu W. Chen F. Cui X. Yang L. Chen J. Zhao J. et al (2018). LncRNA NKILA suppresses TGF-β-induced epithelial-mesenchymal transition by blocking NF-κB signaling in breast cancer. Int. J. Cancer143 (9), 2213–2224. 10.1002/ijc.31605
217
Xia Q. Han T. Yang P. Wang R. Li H. Zhang J. et al (2019). MicroRNA-28-5p regulates liver cancer stem cell expansion via IGF-1 pathway. Stem Cells Int.2019, 8734362. 10.1155/2019/8734362
218
Xiao Q. Zhang F. Xu L. Yue L. Kon O. L. Zhu Y. et al (2021). High-throughput proteomics and AI for cancer biomarker discovery. Adv. Drug Deliv. Rev.176, 113844. 10.1016/j.addr.2021.113844
219
Xie Z. Y. Lv K. Xiong Y. Guo W. H. (2014). ABCG2-meditated multidrug resistance and tumor-initiating capacity of side population cells from Colon cancer. Oncol. Res. Treat.37 (11), 666–668. 10.1159/000368842
220
Xiong Z. Xu X. Zhang Y. Ma C. Hou C. You Z. et al (2024). IFITM3 promotes glioblastoma stem cell-mediated angiogenesis via regulating JAK/STAT3/bFGF signaling pathway. Cell Death Dis.15 (1), 45. 10.1038/s41419-023-06416-5
221
Xue J. Zhu Y. Sun Z. Ji R. Zhang X. Xu W. et al (2015). Tumorigenic hybrids between mesenchymal stem cells and gastric cancer cells enhanced cancer proliferation, migration and stemness. BMC Cancer15, 793. 10.1186/s12885-015-1780-1
222
Xue L. J. Mao X. B. Ren L. L. Chu X. Y. (2017). Inhibition of CXCL12/CXCR4 axis as a potential targeted therapy of advanced gastric carcinoma. Cancer Med.6 (6), 1424–1436. 10.1002/cam4.1085
223
Yan H. H. N. Siu H. C. Law S. Ho S. L. Yue S. S. K. Tsui W. Y. et al (2018). A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell23 (6), 882–897. 10.1016/j.stem.2018.09.016
224
Yan X. Luo H. Zhou X. Zhu B. Wang Y. Bian X. (2013). Identification of CD90 as a marker for lung cancer stem cells in A549 and H446 cell lines. Oncol. Rep.30 (6), 2733–2740. 10.3892/or.2013.2784
225
Yang J. Teng Y. (2023). Harnessing cancer stem cell-derived exosomes to improve cancer therapy. J. Exp. Clin. Cancer Res.42 (1), 131. 10.1186/s13046-023-02717-x
226
Yang L. Shi P. Zhao G. Xu J. Peng W. Zhang J. et al (2020a). Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target Ther.5 (1), 8. 10.1038/s41392-020-0110-5
227
Yang X. Wang B. Chen W. Man X. (2020b). MicroRNA-188 inhibits biological activity of lung cancer stem cells through targeting MDK and mediating the hippo pathway. Exp. Physiol.105 (8), 1360–1372. 10.1113/EP088704
228
Yehya A. Youssef J. Hachem S. Ismael J. Abou-Kheir W. (2023). Tissue-specific cancer stem/progenitor cells: therapeutic implications. World J. Stem Cells15 (5), 323–341. 10.4252/wjsc.v15.i5.323
229
Yi T. Zhai B. Yu Y. Kiyotsugu Y. Raschle T. Etzkorn M. et al (2014). Quantitative phosphoproteomic analysis reveals system-wide signaling pathways downstream of SDF-1/CXCR4 in breast cancer stem cells. Proc. Natl. Acad. Sci. U. S. A.111 (21), E2182–E2190. 10.1073/pnas.1404943111
230
You X. Zhou Z. Chen W. Wei X. Zhou H. Luo W. (2020). MicroRNA-495 confers inhibitory effects on cancer stem cells in oral squamous cell carcinoma through the HOXC6-mediated TGF-β signaling pathway. Stem Cell Res. Ther.11 (1), 117. 10.1186/s13287-020-1576-3
231
Yu Q. Xiu Z. Jian Y. Zhou J. Chen X. Chen X. et al (2022a). microRNA-497 prevents pancreatic cancer stem cell gemcitabine resistance, migration, and invasion by directly targeting nuclear factor kappa B 1. Aging (Albany NY)14 (14), 5908–5924. 10.18632/aging.204193
232
Yu Y. Y. Zhu Y. J. Xiao Z. Z. Chen Y. D. Chang X. S. Liu Y. H. et al (2022b). The pivotal application of patient-derived organoid biobanks for personalized treatment of gastrointestinal cancers. Biomark. Res.10 (1), 73. 10.1186/s40364-022-00421-0
233
Zabeti T. A. Norollahi S. E. Najafizadeh A. Babaei K. Bakhshalipour E. Vahidi S. et al (2024). Therapeutic combinations of exosomes alongside cancer stem cells (CSCs) and of CSC-Derived exosomes (CSCEXs) in cancer therapy. Cancer Cell Int.24 (1), 334. 10.1186/s12935-024-03514-y
234
Zeng Z. Fu M. Hu Y. Wei Y. Wei X. Luo M. (2023). Regulation and signaling pathways in cancer stem cells: implications for targeted therapy for cancer. Mol. Cancer22 (1), 172. 10.1186/s12943-023-01877-w
235
Zha H. Lv S. Hu Y. Xie Y. Wang L. Yang C. et al (2025). Isorhapontigenin alleviates acetaminophen-induced liver injury by promoting fatty acid oxidation. Biochim. Biophys. Acta Mol. Basis Dis.1871 (2), 167575. 10.1016/j.bbadis.2024.167575
236
Zhang C. Li C. He F. Cai Y. Yang H. (2011). Identification of CD44+CD24+ gastric cancer stem cells. J. Cancer Res. Clin. Oncol.137 (11), 1679–1686. 10.1007/s00432-011-1038-5
237
Zhang Q. Zhang H. Ding J. Liu H. Li H. Li H. et al (2018). Combination therapy with EpCAM-CAR-NK-92 cells and regorafenib against human colorectal cancer models. J. Immunol. Res.2018, 4263520. 10.1155/2018/4263520
238
Zhang X. Powell K. Li L. (2020). Breast cancer stem cells: biomarkers, identification and isolation methods, regulating mechanisms, cellular origin, and beyond. Cancers (Basel)12 (12), 3765. 10.3390/cancers12123765
239
Zhang Y. Li B. Zhang X. Sonpavde G. P. Jiao K. Zhang A. et al (2017a). CD24 is a genetic modifier for risk and progression of prostate cancer. Mol. Carcinog.56 (2), 641–650. 10.1002/mc.22522
240
Zhang Y. Xu W. Guo H. Zhang Y. He Y. Lee S. H. et al (2017b). NOTCH1 signaling regulates self-renewal and platinum chemoresistance of cancer stem-like cells in human non-small cell lung cancer. Cancer Res.77 (11), 3082–3091. 10.1158/0008-5472.CAN-16-1633
241
Zhao Y. Zhu J. Shi B. Wang X. Lu Q. Li C. et al (2019). The transcription factor LEF1 promotes tumorigenicity and activates the TGF-β signaling pathway in esophageal squamous cell carcinoma. J. Exp. Clin. Cancer Res.38 (1), 304. 10.1186/s13046-019-1296-7
242
Zheng H. Pomyen Y. Hernandez M. O. Li C. Livak F. Tang W. et al (2018). Single-cell analysis reveals cancer stem cell heterogeneity in hepatocellular carcinoma. Hepatology68 (1), 127–140. 10.1002/hep.29778
243
Zheng L. Lu J. Kong D. Zhan Y. (2024). Single-cell sequencing analysis revealed that WDR72 was a novel cancer stem cells related gene in gastric cancer. Heliyon10 (15), e35549. 10.1016/j.heliyon.2024.e35549
244
Zheng W. He R. Liang X. Roudi S. Bost J. Coly P. M. et al (2022). Cell-specific targeting of extracellular vesicles through engineering the glycocalyx. J. Extracell. Vesicles11 (12), e12290. 10.1002/jev2.12290
245
Zhou H. M. Zhang J. G. Zhang X. Li Q. (2021). Targeting cancer stem cells for reversing therapy resistance: mechanism, signaling, and prospective agents. Signal Transduct. Target Ther.6 (1), 62. 10.1038/s41392-020-00430-1
246
Zhou J. Wulfkuhle J. Zhang H. Gu P. Yang Y. Deng J. et al (2007). Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc. Natl. Acad. Sci. U. S. A.104 (41), 16158–16163. 10.1073/pnas.0702596104
247
Zhu L. Zhang W. Wang J. Liu R. (2015). Evidence of CD90+CXCR4+ cells as circulating tumor stem cells in hepatocellular carcinoma. Tumour Biol.36 (7), 5353–5360. 10.1007/s13277-015-3196-6
248
Zhu Y. Chen S. Su H. Meng Y. Zang C. Ning P. et al (2025). CPT1A-mediated MFF succinylation promotes stemness maintenance in ovarian cancer stem cells. Commun. Biol.8 (1), 250. 10.1038/s42003-025-07720-w
249
Zhuang J. Shen L. Li M. Sun J. Hao J. Li J. et al (2023). Cancer-associated fibroblast-derived miR-146a-5p generates a niche that promotes bladder cancer stemness and chemoresistance. Cancer Res.83 (10), 1611–1627. 10.1158/0008-5472.CAN-22-2213
250
Zou R. Shi Z. Li C. Xu F. Zheng X. Du X. et al (2024). Engineered inflammation-induced neurodevelopmental disorders using a neurovascular-unit-on-a-chip. Bio-Design Manuf.
251
Zuo M. Rashid A. Churi C. Vauthey J. N. Chang P. Li Y. et al (2015). Novel therapeutic strategy targeting the hedgehog signalling and mTOR pathways in biliary tract cancer. Br. J. Cancer112 (6), 1042–1051. 10.1038/bjc.2014.625
Summary
Keywords
cancer stem cells, metabolic reprogramming, immune evasion, organoids, personalized therapy
Citation
Yin L, Zhou S, Zhang H, Shang Y, Wu S and Jin T (2025) Cancer stem cells in personalized therapy: mechanisms, microenvironment crosstalk, and therapeutic vulnerabilities. Front. Cell Dev. Biol. 13:1619597. doi: 10.3389/fcell.2025.1619597
Received
28 April 2025
Accepted
18 July 2025
Published
30 July 2025
Volume
13 - 2025
Edited by
Monia Orciani, Marche Polytechnic University, Italy
Reviewed by
Weiren Luo, The Second Affiliated hospital of Southern University of Science and Technology, China
Adolfo Lopez, Hospital Juárez de México, Mexico
Updates
Copyright
© 2025 Yin, Zhou, Zhang, Shang, Wu and Jin.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tengchuan Jin, jint@ustc.edu.cn; Songquan Wu, lswsq163@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.