Abstract
Background:
Radiation-induced muscle fibrosis (RIF) is a severe late-stage side effect of radiotherapy in adjacent normal tissues, significantly affecting anticancer therapeutic efficacy and potentially being life-threatening. Previous studies have shown that satellite cells (SCs) become activated after ionizing radiation to facilitate muscle tissue repair. However, the acceleration and strengthening of this process have received little attention until recently. Adipose-derived stem cells (ADSCs), a type of mesenchymal stem cell, have emerged as a promising therapeutic option in regenerative medicine due to their accessibility, abundance, and plasticity in adult organisms. In this study, we explored whether ADSCs could enhance SC proliferation and differentiation after radiation therapy.
Methods:
ADSCs were harvested, cultured, and passaged from male Sprague–Dawley rats and characterized in vitro. In vivo, rats were randomly assigned to control and ADSC-treated groups (n = 6). ADSCs were transplanted into RIF rat models at different time points (4, 12, and 24 w). The therapeutic effects of transplanted ADSCs were assessed via Masson’s trichrome staining, electron microscopy, and hematoxylin–eosin (H&E) staining. SC activation, proliferation, and central nuclear immigration following ADSC transplantation therapy were evaluated via real-time polymerase chain reaction and H&E staining.
Results:
In vivo, fibrosis was markedly alleviated over time following ADSC treatment. In the RIF rat model, ultrastructural histopathological changes, including mitochondrial edema and vacuolization, myofilament dissolution, and vascular endothelial swelling, were notably attenuated by ADSC transplantation. Additionally, SCs exhibited a significant increase in activation and proliferation in the ADSC-treated groups, accompanied by a decrease in fibrotic symptoms.
Conclusion:
Our study provides evidence that ADSCs protect against RIF by promoting SC activation, proliferation, and differentiation in vivo. ADSCs may represent a promising therapeutic candidate for restoring muscle dysfunction and abnormalities caused by RIF.
Introduction
Over the past several decades, the survival rates of cancer patients have significantly improved (Siegel et al., 2023; Lu et al., 2023). Radiation therapy remains a mainstay of cancer treatment (Chandra et al., 2021). However, exposure of the surrounding normal tissues to radiation often leads to serious side effects, resulting in functional impairments in cancer survivors (Palmer et al., 2021; Stokkevåg et al., 2024; Wang et al., 2024). Radiation-induced fibrosis (RIF) is a severe adverse effect of radiation therapy, especially in patients with head and neck malignancies (Lennox et al., 2002) or breast cancer (Van Geel et al., 2011). RIF primarily manifests as skin induration and thickening (Dancey and Waters, 2006; Yanaba et al., 2015), muscle weakness (Ghosh and Milone, 2015), atrophy (Jit et al., 2021), restricted mouth opening (Abboud et al., 2020), difficulty in eating and swallowing (Kawashita et al., 2022), and even respiratory failure (Lei et al., 2021). Several treatment options for RIF are currently available in clinical practice, including pentoxifylline (Okunieff et al., 2004; Binatti et al., 2021), sulforaphane (Wang et al., 2022), vitamin E (Krejbich and Birringer, 2022), glucocorticoids (Agha-Hosseini et al., 2021), and hyaluronic acid (Agha-Hosseini et al., 2021). However, the efficacy of these therapies remains limited, prompting the need for alternatives to mitigate this secondary injury. The underlying biological mechanisms of RIF are traditionally associated with DNA single- and double-strand breaks, inflammation (Chen et al., 2023; Vallée et al., 2017; Kiang et al., 2010), apoptosis, pyroptosis (Yu et al., 2021), and autophagy (Chaurasia et al., 2019). These processes directly or indirectly impair skeletal muscle function. Simultaneously, fibrosis reduces the regenerative capacity of skeletal muscle in vivo, resulting in sarcopenia and muscle contractile dysfunction (Gionet-Gonzales et al., 2023; Garg et al., 2015).
Adipose-derived stem cells (ADSCs) are multipotent mesenchymal adult stem cells derived from adipose tissue, and they are a promising therapeutic option for various diseases (Al-Ghadban and Bunnell, 2020; Alió Del Barrio et al., 2022). ADSCs are thought to possess robust biological potency of exocrine and paracrine functions (Harasymiak-Krzyżanowska et al., 2013), comprising factors such as the fibroblast growth factor (FGF) (Guo et al., 2022), vascular endothelial growth factor (VEGF) (Alió Del Barrio et al., 2022), transforming growth factor beta 1 (TGF-β1) (Ademi et al., 2023), and insulin-like growth factor 1 (IGF-1) (Li et al., 2019). The majority of these factors contribute to cell proliferation and neovascularization (Hu et al., 2020; Liao et al., 2024), especially preventing cell apoptosis (Song et al., 2024). Previous research revealed that ADSC-conditioned medium decreased the expression of apoptosis-related proteins in mice with myocardial infarction through the miR-221/222/p38/NF-κB pathway (Lee et al., 2021). Interestingly, Ai et al. found that ADSC transplantation could mitigate granulosa cell apoptosis in a rat model of premature ovarian failure (Ai et al., 2023). In addition, exosomes secreted from ADSCs ameliorated diabetic nephropathy complications by inhibiting podocyte apoptosis, thus improving outcomes in vivo (Jin et al., 2019). Moreover, ADSC transplantation promoted a wide range of anti-inflammatory cytokines in a multiple sclerosis murine model, including IL-6, IL-10, and TGF-β, thus preventing astrocyte activation and promoting the macrophage M2 phenotype (Al-Ghadban and Bunnell, 2020). Numerous studies showed that exosomes secreted by ADSCs consist of lipids, proteins, and miRNAs and are similar to their parental cells, whose antioxidant, anti-apoptotic, anti-inflammatory, and anti-fibrotic capabilities are evidently elevated in cardiovascular disease (Ren et al., 2024). In addition, cultured ADSCs isolated from adipose tissue have been shown to influence myofibroblast differentiation and can alleviate collagen accumulation, a process partly mediated by their paracrine functions in hypertrophic dermal scarring (Higginbotham et al., 2024). Furthermore, human platelet lysate-cultured ADSC sheets could significantly accelerate wound healing and mitigate macrophage recruitment while reducing subsequent wound tissue fibrosis in a burn wound Wistar rat model in vivo (Chen et al., 2024). Taken together, therapeutic intervention with ADSCs reduces cell apoptosis and promotes cell proliferation to ameliorate the damage caused to various tissues in early disease and fibrosis in the advanced state.
Satellite cells (SCs), which are unipotent stem cells, are responsible for postnatal skeletal muscle repair following various injuries (Zhang et al., 2024). They are generally present in a state of quiescence and are located in a specialized compartment (niche) between the basal lamina and myofiber sarcolemma (Zeng et al., 2022). SCs are activated in response to injury or altered muscle homeostasis and then divide asymmetrically in order to maintain self-renewal or form a proliferative population of myoblasts (Price et al., 2024). These committed precursors proliferate and differentiate into new myofibers, fusing with each other or with damaged fibers (Sampath et al., 2018). Paired gene 7 (Pax7) is expressed in the quiescent SC state as a unique marker in skeletal muscles (Diao et al., 2012). In contrast, the myogenic determination factor (MyoD) is not expressed in detectable levels in quiescent SCs, but it is upregulated early after activation (Yoshimoto et al., 2020). In the late 1980s and early 1990s, researchers discovered that myogenic factor 5 (Myf5) coordinates with numerous other myogenic regulatory factors, including myogenin (MyoG), participating in the regulation of the myogenic process (Brack and Rando, 2012). Although the nuclei are located at the center in most cells, the nuclei of normal muscle fibers lie peripherally near the cell membrane (Snijders et al., 2020). Nuclei centripetal migration in skeletal muscle cells can be observed only under certain specific conditions, such as during growth, regeneration, and pathophysiological processes (Snijders et al., 2020). According to Srikuea and Hirunsai, the central nuclei migration originating from SCs is a hallmark of muscle regeneration (Srikuea and Hirunsai, 2016).
In our previous research, we found that apoptosis plays an important role in radiation-induced dermatitis and fibrosis using an established dermatitis rat model (Sheng et al., 2019). Furthermore, we revealed that ADSCs alleviated radiation-induced dermatitis by suppressing apoptosis in a cathepsin F-dependent manner (Yao et al., 2021). More importantly, SCs are activated from the dormant state and undergo myogenic differentiation after ionizing radiation stimulation, but this is insufficient to combat fibrosis formation due to the low rate of muscle regeneration (Zeng et al., 2022). Based on this research, we inferred that ADSCs may similarly promote regeneration and inhibit muscle cell apoptosis in RIF. In this study, we first extracted ADSCs from adipose tissue and characterized them. Then, we treated an RIF rat model with ADSCs to evaluate the proliferation and regeneration of SCs in vivo and evaluated the inhibitory effect of ADSCs on apoptosis in irradiated muscle. In summary, we explored the potential therapeutic capability of ADSCs in RIF and its underlying mechanism preliminarily.
Materials and methods
Experimental animals
Two-month-old Sprague–Dawley (SD) rats procured from Hunan SJA Laboratory Animal Co., Ltd. (Hunan, China) were utilized in this study. All research members were formally trained in technologies and obtained relevant certification at Central South University before the operation. All the rats had free access to an irradiated chow diet and tap water for 7 days to acclimate to the new environment. Ethical approval and consent were obtained from the Animal Ethics Committee of Hunan Cancer Hospital, in accordance with the institutional guidelines for animal protocols.
Establishment of RIF rat models and ADSC treatments
All 42 female rats were divided randomly into seven groups (n = 6), namely, normal, untreated control 90 Gy-4 w, 90 Gy-12 w, and 90 Gy-24 w, and ADSCs-treated 90 Gy-4w, ADSCs-90Gy-12 w, and ADSCs-90Gy-24 w groups. The normal group received only phosphate-buffered saline (PBS) injections. The radiation sites were the medial left thigh of the rat hind limb, which was clipped free of hair. After rats were anesthetized using 5% pentobarbital sodium, the untreated control groups received a single dose of 90 Gy irradiation and PBS injection, while the treated groups received 90 Gy irradiation and 107 ADSC injections within 24 h, as a previous study reported (Yao et al., 2021). The detailed transplantation steps were as follows: first, the ADSCs were resuspended in 100 μL of PBS, divided into 25 μL per injection, and injected at a dose of 25 μL at each of the four points, i.e., above, below, to the left, and to the right of the radiotherapy site. At 4, 12, and 24 weeks after radiotherapy, all rats from each group were sacrificed under deep anesthesia.
Isolation, culture, differentiation, and characterization of ADSCs
Subcutaneous adipose tissue was aseptically harvested from the groin region of male SD rats under anesthesia. After carefully removing large blood vessels, the tissue was rinsed three times with PBS (Gibco, Carlsbad, CA, United States) and then sheared into small fragments of 1 mm3. These fragments were digested with type I collagenase (1 μg/mL; Gibco) in a centrifuge tube and incubated in a thermostatic water bath at 37 °C for 1 h. Cell culture medium was added to the centrifuge tube to terminate the collagenase activity, and the disaggregated tissues were collected for centrifugation at 1,000 g at 25 °C for 10 min. The supernatant was aspirated and discarded using a pipette, and the cell pellets were filtered through a 70-μm stainless steel mesh to remove excess tissue clumps. The filtrate was then centrifuged again at 1,000 × g at 25 °C for 10 min, the supernatant was discarded, and the cell pellet was resuspended and cultured in pre-warmed Dulbecco’s modified Eagle’s medium (Gibco, Grand Island, NY, United States) supplemented with 10% fetal bovine serum (Gibco, Gaithersburg, MD, United States) and 1% penicillin–streptomycin (Gibco, Grand Island, NY, United States). Cultures were maintained at 37 °C in a humidified atmosphere with 5% CO2. Third-passage ADSCs were used for characterization and tri-lineage differentiation. Adipogenic, osteogenic, and chondrogenic differentiation were induced using specific conditional differentiation media, as previously described (Yao et al., 2021). To examine adipogenic differentiation, ADSCs were cultured in the adipogenic induction medium for 20 days, followed by Oil Red O-staining to visualize lipid droplets. To assess osteogenic differentiation, ADSCs were cultured in the osteogenic or chondrogenic induction medium for 21 days. Calcium deposition in bone nodules was assessed using alizarin red dye and an alkaline phosphatase staining kit. For chondrogenic differentiation analysis, ADSCs were cultured in the chondrogenic induction medium for 21 days. Chondrogenic lineage differentiation was confirmed by toluidine blue-staining. All positively stained areas were examined under a microscope with 200× magnification (Carl Zeiss, Oberkochen, Germany). The expression levels of various ADSCs’ surface markers, including CD34, CD45, CD90, CD105, and CD10 (Abcam, Cambridge, United Kingdom), were assessed via flow cytometry, and the purity of the ADSCs was consistently >90%.
In vivo tracing of ADSCs
For in vivo tracing experiments, rat ADSCs were transduced with specially designed lentiviral vectors containing both green fluorescence protein (GFP) and luciferase reporter genes. ADSCs transfected with this vector could be detected in vitro using fluorescence microscopy via GFP expression and in vivo using the In Vivo Imaging System (IVIS) via luciferase expression (GFP/luciferase-ADSCs). Next, 107 GFP/luciferase-ADSCs were transplanted into female SD rats and tracked in vivo using IVIS. The detailed transplantation procedure was as follows: the surface of the medial rectus femoris on the left thigh (irradiation site) was marked using a marker. Then, GFP/luciferase-ADSCs were resuspended in 100 μL of PBS and divided into 25 μL per injection. A volume of 25 μL was injected at four points surrounding the marked site: upper, lower, left, and right.
Histological examinations of hematoxylin–eosin and Masson’s trichrome staining
Skeletal muscle tissues from each group were fixed in 4% paraformaldehyde for 24 h, embedded in paraffin, and sectioned at 4 μm thickness. The sectioned lamellae were stained with H&E (ServiceBio, GP1031) and Masson’s trichrome (ServiceBio, GP1032) following the manufacturer’s protocols. The tissues were analyzed, and images were acquired using an Axio Scope A1 Inverted Microscope (Carl Zeiss, Oberkochen, Germany). The percentage of skeletal muscle fibrosis was quantified using Image-Pro Plus 6.0 software. All visual fields in each tissue section were analyzed. First, the areas of blue-stained collagen fibers and the total tissue area were measured. Then, the percentage of fibrosis was calculated as the ratio of the collagen fiber area to the total area.
Electron microscopy
Samples prepared for transmission electron microscopy analysis were fixed in 2.5% glutaraldehyde and washed with PBS several times to remove impurities. Post-fixation was carried out for 2 h using 1% osmium tetroxide. Following fixation, the samples were dehydrated through a graded ethanol series followed by propylene oxide treatment, and then they were gradually embedded in Epon 812 resin. Ultrathin sections (50-nm thickness) were obtained and double-stained with 3% uranyl acetate and lead citrate for 15 min. The ultrastructure of skeletal muscle cells, including organelles and vascular structures, was observed and recorded using a transmission electron microscope (FEI, Hillsboro, OR, United States).
Real-time polymerase chain reaction
Total RNA was extracted from muscle tissues using the SteadyPure RNA Extraction Kit (Accurate Biology, Changsha, China). For each sample, 1 µg of total RNA was reverse-transcribed into cDNA using the Evo M-MLV Reverse Transcription Kit (Accurate Biology, Changsha, China). RT-PCR was performed on the LightCycler 96 system (Roche, Basel, Switzerland) using the Hieff® qPCR SYBR Green Master Mix (No Rox) (Yeasen, Shanghai, China). Gene expression levels were calculated using the comparative cycle threshold (Ct) method, with normalization to B2M. The housekeeping gene Gapdh was selected as an internal reference, and relative Ct values were calculated using the average data of triplicate experiments. The primer sequences used in this study are listed in Table 1.
TABLE 1
| Gene name | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|
| GAPDH | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
| PAX7 | GAGTATAAGAGGGAGAACCCCG | TTGATTCTGAGCACTCGGCTAA |
| MyoD | GCTCTGATGGCATGATGGATTAC | CTATGCTGGACAGGCAGTCG |
| MyoG | ACTACCTTCCTGTCCACCTTCA | AGGCCTCATTCACTTTCTTGAG |
| Mrf4 | ACAGCTACAAACCCAAGCAAGA | CTTGCTCCTCCTTCCTTAGCAG |
| Myf5 | TCTGATGGCATGCCTGAATGTAA | AAGGAGCTCTTATCTGAAGCACA |
Primer sequence of the marker genes.
Terminal deoxynucleotidyl transferase dUTP nick end labeling staining
Apoptotic cells in irradiated muscle tissues were detected using the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) apoptosis assay kit (G1501; ServiceBio, Wuhan, China), as described in our previous study (Sheng et al., 2019). TUNEL-positive cells were counted under an inverted fluorescence microscope (Carl Zeiss, Oberkochen, Germany), and the percentage of TUNEL-positive cells was calculated.
Statistical analysis
All data are presented as the mean ± standard error of the mean. Statistical analyses were performed using SPSS (version 28.0; SPSS, Chicago, Illinois, United States). Differences between the two groups were analyzed using the Student’s t-test. For comparisons among multiple groups, one-way analysis of variance was applied. A p-value <0.05 was considered statistically significant.
Results
Extraction and characterization of primary ADSCs
ADSCs were extracted and isolated from the superficial subcutaneous adipose tissue of male rats. The cultured primary ADSCs exhibited a relatively homogeneous, spindle-shaped morphology and demonstrated robust growth (Figure 1A). Notably, ADSCs displayed strong adipogenic, osteogenic, and chondrogenic differentiation potential when cultured in the specific induction differentiation medium (Figures 1B–F). Subsequently, a flow cytometer was used to confirm the surface markers of ADSCs in vitro, and the results showed high expression levels of CD90 (90.16% ± 0.16%), CD105 (97.53% ± 0.72%), and CD10 (86.36% ± 0.26%), along with low expression levels of CD34 (0.92% ± 0.10%) and CD45 (1.29% ± 0.005%) (Figure 1G).
FIGURE 1
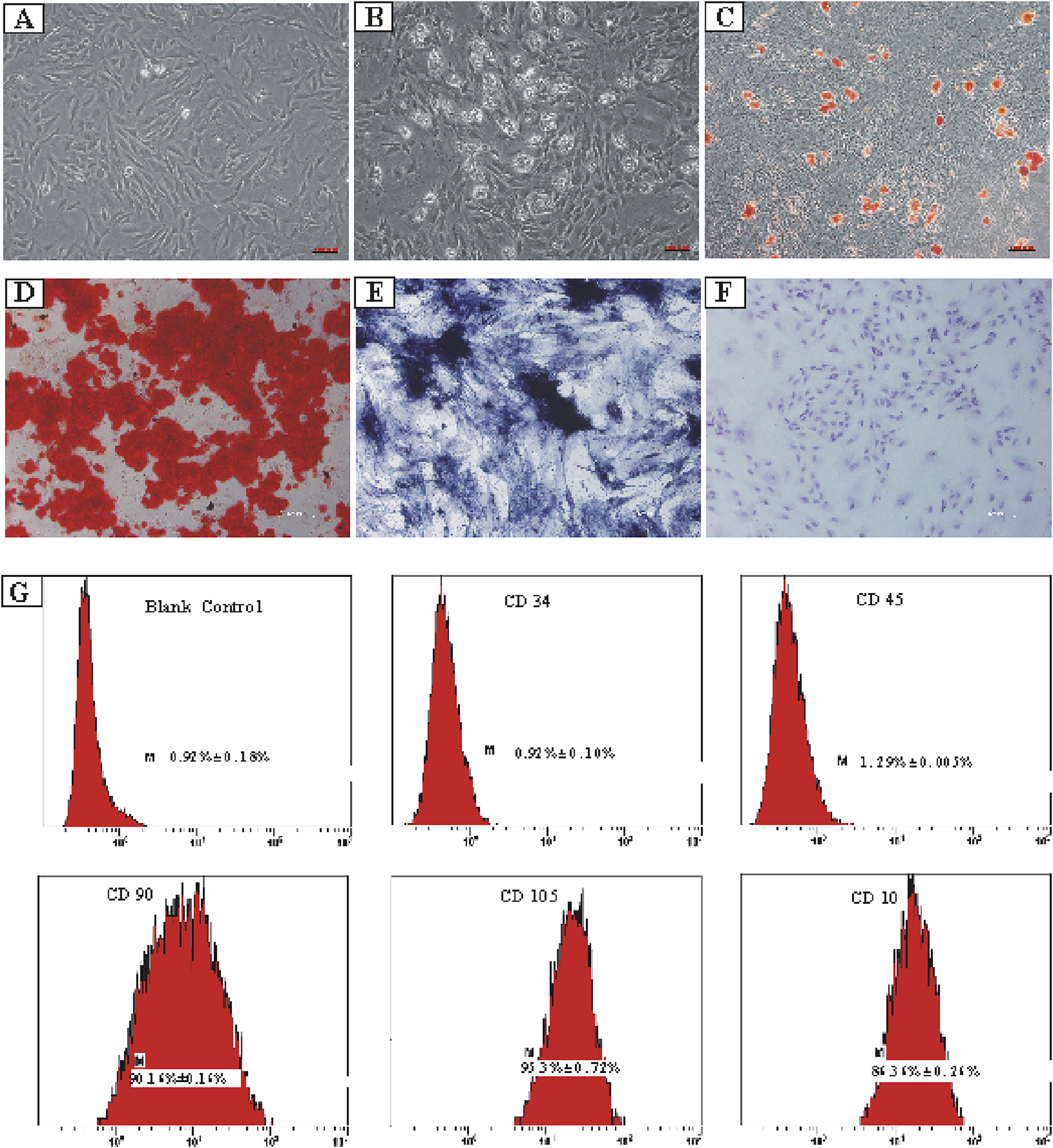
Isolation and lineage tracing of ADSCs, including tri-lineage differentiation and flow cytometry analysis. (A) ADSCs separated from superficial subcutaneous adipose tissue of male rats. (B) Differentiation of the ADSCs into adipocytes. (C) Precipitation of oil droplets in the differentiated ADSCs detected using oil red O-staining. (D) Osteogenic differentiation of ADSCs confirmed by calcium deposition using alizarin red-staining. (E) Osteogenic differentiation of ADSCs verified using alkaline phosphatase-staining. (F) Chondrogenic differentiation of ADSCs visualized using toluidine blue-staining. (G) Expression percentages of various ADSCs’ surface markers. CD34: 0.92% ± 0.10%, CD45: 1.29% ± 0.005%, CD90: 90.16% ± 0.16%, CD105: 97.53% ± 0.72%, and CD10: 86.36% ± 0.26%.
Cell tracing of ADSCs
To assess the post-transplant survival of ADSCs in vivo, GFP/luciferase-ADSCs were harvested. First, the expression of GFP in the transduced ADSCs was confirmed in vitro using fluorescence microscopy, where most cells exhibited green fluorescence (Figure 2A). We also observed cells expressing firefly fluorescence in vitro using IVIS, and intense fluorescence was detected (Figure 2B). For in vivo tracking, GFP/luciferase-ADSCs were intramuscularly injected into female rats, and the luciferase signal was monitored via IVIS at 2 h post-injection; an intense, localized fluorescence signal was detected within the injection site (Figure 2C).
FIGURE 2
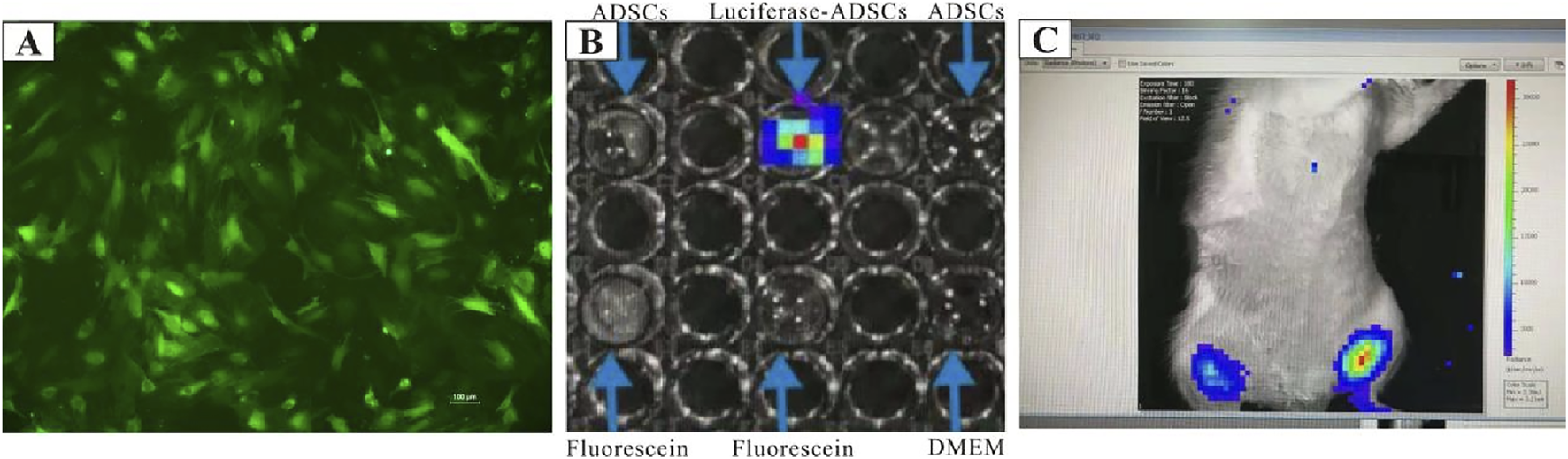
Cell tracing of ADSCs in vitro and in vivo. (A) GFP/luciferase-ADSCs observed under fluorescence microscopy in vitro.(B)In vitro bioluminescence imaging of GFP/luciferase-ADSCs using the IVIS system. (C)In vivo tracking of GFP/luciferase-ADSCs following injection into rats. High-intensity fluorescence signals were detected with IVIS at 2 h post-injection, compared with the contralateral trials.
ADSC treatment attenuates muscle fibrosis induced by radiation
To ascertain the extent of muscle fibrosis in RIF rats, Masson’s trichrome staining method was carried out in the control and ADSC-treated groups. Increased collagen deposition in muscle tissues was observed over time (Figures 3A, C, E); however, this collagen accumulation could be reversed by ADSC treatment (Figures 3B, D, F). Although the collagen fibers did not significantly decrease by ADSC treatment at the acute injury stage, the percentage of skeletal muscle collagenous fibers showed a pronounced reduction after treatment at the advanced stage (Figure 3G).
FIGURE 3
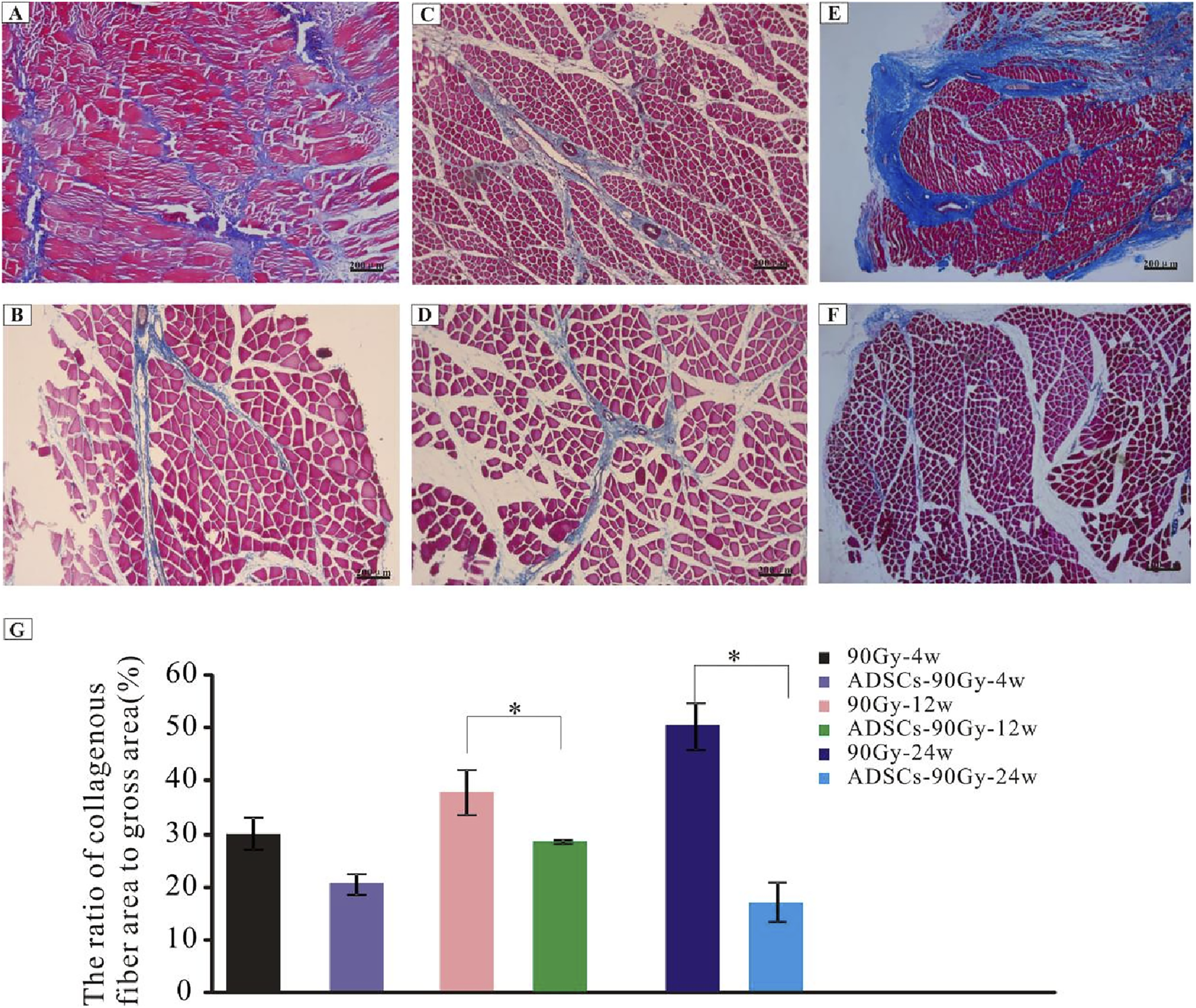
Degree of fibrosis in the control and ADSC-treated groups. Masson’s trichrome-staining of irradiated muscle tissues from rats in the control group: (A) 90 Gy-4 w, (C) 90 Gy-12 w, and (E) 90 Gy-24 w and from rats in the ADSC-treated group: (B) ADSCs-90 Gy-4 w, (D) ADSCs-90 Gy-12 w, and (F) ADSCs-90 Gy-24 w. (G) Quantification of collagen content shows a reduction in the percentage of skeletal muscle collagenous fibers in the ADSC-treated groups than that in the control group. *p < 0.05.
ADSCs alleviate radiation-induced injuries in muscles
To investigate the intricate architecture damage in vivo, the morphological and ultrastructural features of muscle tissues obtained from each group were characterized using transmission electron microscopy. Vacuolization and edema in the mitochondria, dissolved myofilaments, and vascular endothelial swelling were observed in the RIF model cohort at 4 weeks post-radiation (Figures 4A–C). However, these disorders were partially or completely rescued by ADSC transplantation (Figures 4D–F).
FIGURE 4
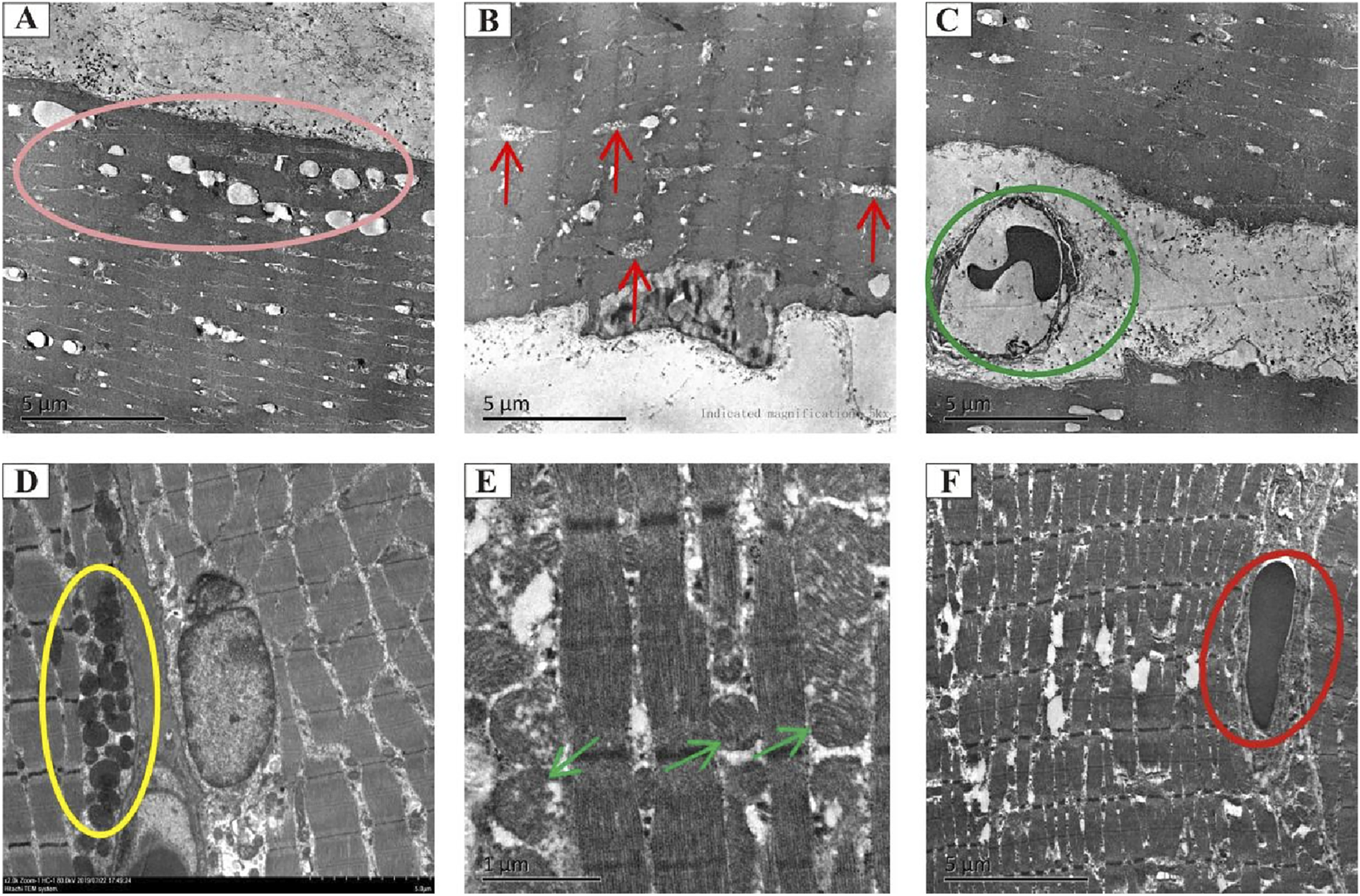
Transmission electron microscopy analysis of the morphology and microstructure of rat muscle tissues. Representative electron micrographs showing the ultrastructure of muscle tissues in the model cohort (90 Gy-4 w) (A–C) and the ADSC-treated cohort (ADSCs-90 Gy-4 w) (D–F). The pink circle indicates vacuolization and edema in mitochondria (A). Red arrows indicate mitochondrial cristae loss and vacuolization (B). Green circles indicate irregular vasculature (C). Yellow circles show mitochondria with no apparent structural abnormalities (D). Green arrows indicate mitochondria with no obvious vacuolization (E). Blue circle marks vascular endothelium with no obvious swelling (F).
ADSCs promote activation, proliferation, and differentiation of SCs in vivo
To examine the mechanism of ADSC-induced SC stabilization, activation, and myogenic processing, the relative gene expression levels were quantified using RT-PCR. Myogenic-associated transcription factors, including Pax7, Myf5, MyoD, and MyoG, were analyzed as markers of SC activation and myogenesis. Our results revealed that Pax7 was significantly upregulated at 12 weeks post-irradiation in the ADSC group compared with that in the control group (Figure 5A). Although the gene expression of MyoD did not differ in the irradiated muscles of each group across the whole trial epochs (Figure 5C), Myf5 expression elevated drastically at 4 weeks post-irradiation in the ADSC group (Figure 5B). Moreover, the expression of the myogenic marker MyoG was increased in the ADSC-treated group at 24 weeks post-irradiation (Figure 5D). Together, our results provide new insights regarding ADSC therapy targeting radiation-induced muscle injury.
FIGURE 5
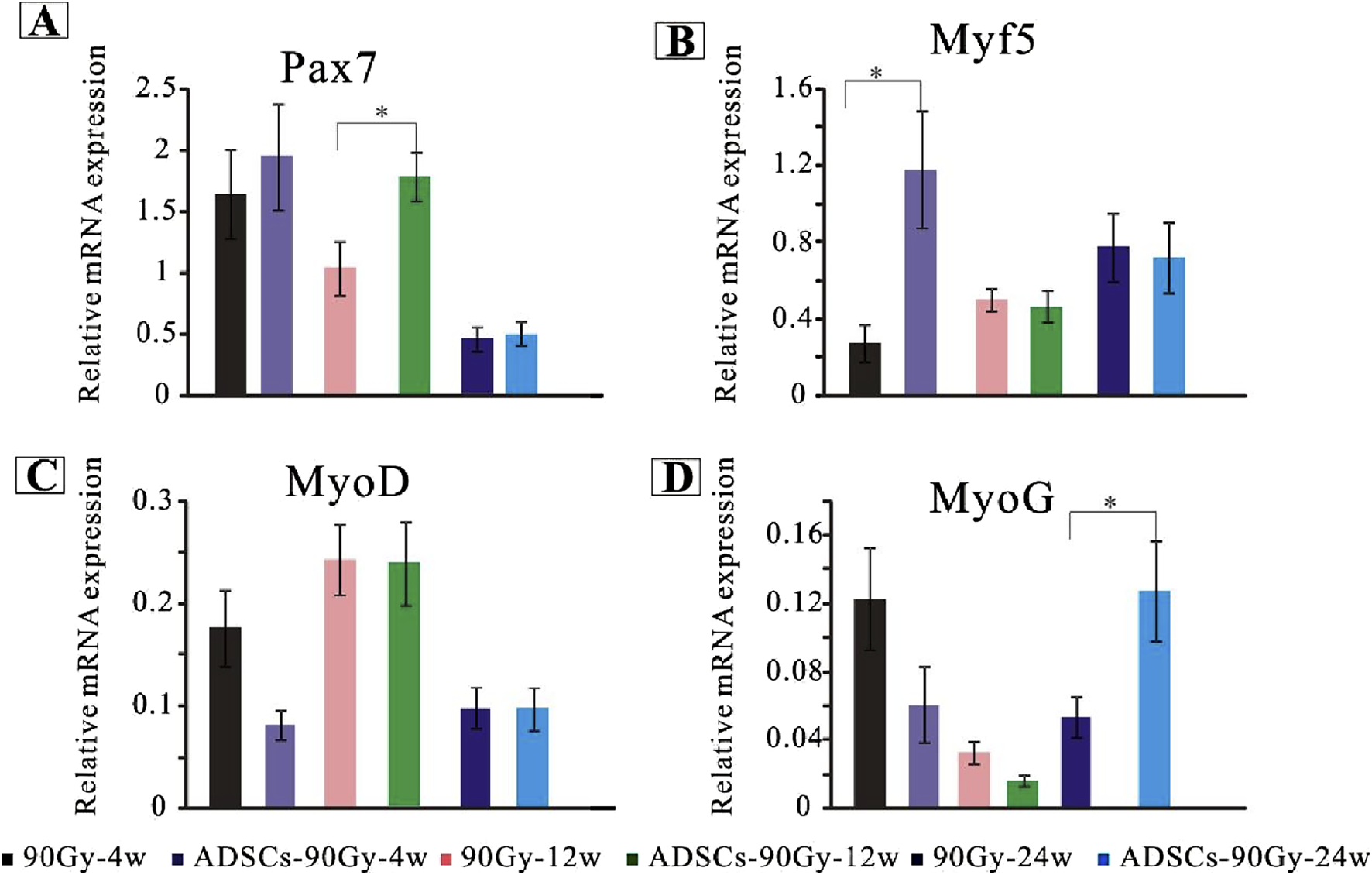
ADSCs enhance SC activation in response to radiation-induced muscle damage. Quantitative polymerase chain reaction analysis of gene expression levels for key markers of SC activation, proliferation, and differentiation in the control and ADSC-treated groups, including Pax7(A), Myf5(B), MyoD(C), and MyoG(D).*p < 0.05.
ADSCs facilitate central nuclear translocation in RIF rat muscle fibers
Considering the important role of central nuclear migration in muscle regeneration, we performed H&E-staining to confirm nuclear positioning in vivo and quantify it. We transplanted ADSCs into the femoris muscle of normal rats and found that the muscle structure remained normal, and no central nuclei appeared (data not shown). Comparison to the control littermates, irradiated rats appeared susceptible to central nuclear translocation in muscle fibers, ranging from limited to intermediate levels in the ADSC groups (Figure 6). At 4 weeks post-irradiation, skeletal muscle experienced a progressive deterioration in fiber alignment, with no apparent centralized nuclei; instead, increased proportions of myofibers with central nucleation were determined within the ADSC group (Figures 6A, B). Notably, limited centrally nucleated myofibers appeared at 12 and 24 weeks post-irradiation in the mammalian models (Figures 6C, E), and the centrally located nuclei exhibited high susceptibility to ADSC treatment with the extent of time (Figures 6B, D, F). Combined, these results provide strong support for our hypothesis that ADSCs may promote muscle regeneration in the RIF model rats.
FIGURE 6
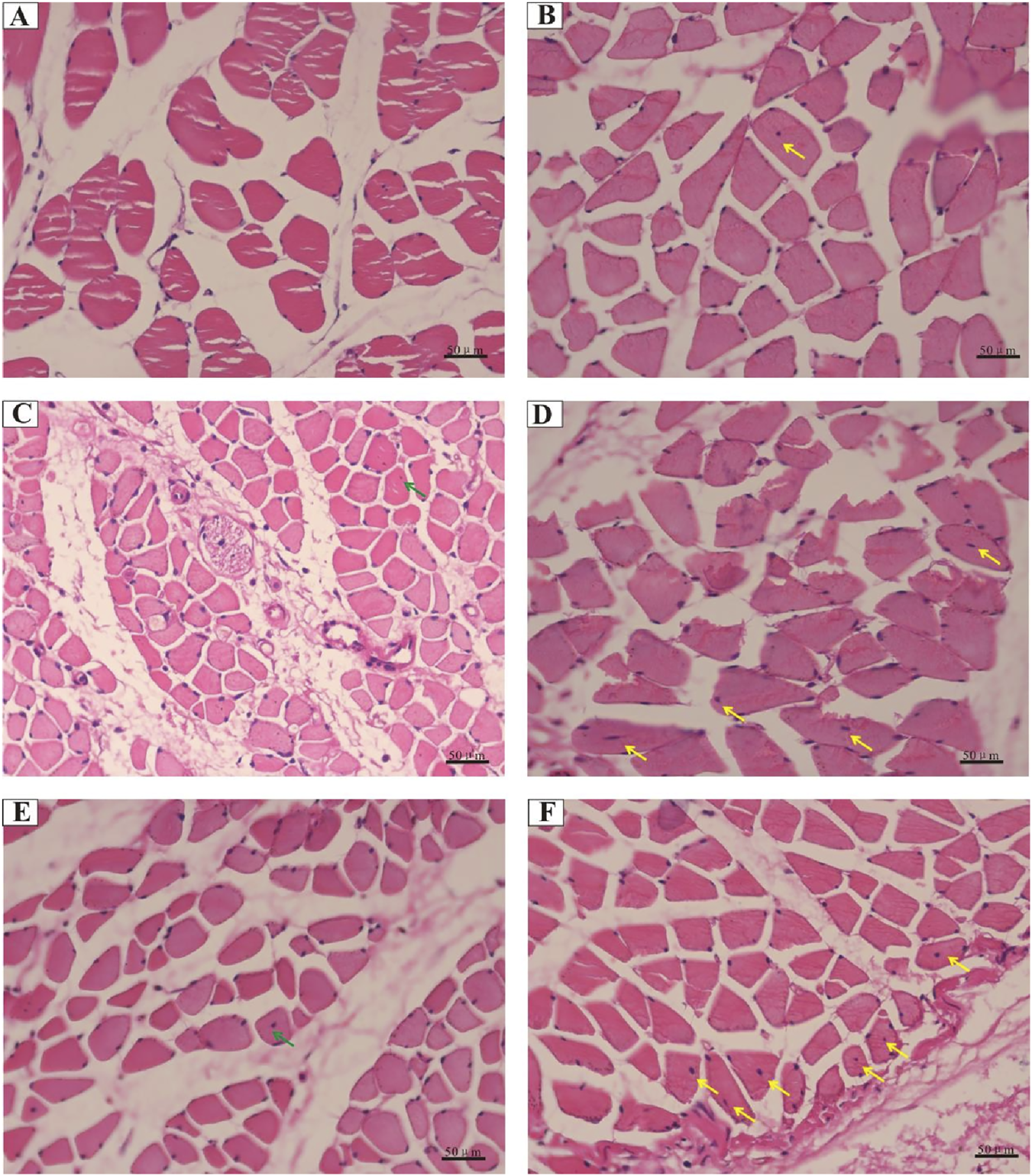
Central nuclear translocation in muscle fibers following radiation exposure. Representative histology images of hematoxylin and eosin-stained muscle sections from rats of the control groups: (A) 90 Gy-4 w, (C) 90 Gy-12 w, and (E) 90 Gy-24 w and from rats of ADSC-treated experimental groups: (B) ADSCs-90 Gy-4 w, (D) ADSCs-90 Gy-12 w, and (F) ADSCs-90Gy-24 w. Green arrows indicate a limited number of centrally nucleated myofibers in the control groups, while yellow arrows indicate highly centrally nucleated myofibers in the ADSC-treated groups.
ADSCs inhibit apoptosis in irradiated muscle tissue
Representative images of TUNEL‐positive apoptotic cells from each group are shown in Figures 7A–F. A large number of apoptotic cells were found in the control 90 Gy-4 w (A), 90 Gy-12 w (B), and 90 Gy-24 w (C) groups, but only a few apoptotic cells were found in the ADSCs-90 Gy-4 w (D), ADSCs-90 Gy-12 w(E), and ADSCs-90 Gy-24 w(F) groups. In contrast to the control group, although the number of apoptotic cells slightly decreased in the ADSC-treated group at 4 and 12 weeks, the differences were not statistically significant, but the difference at 24 weeks was statistically significant (Figure 7G). These findings revealed that ADSCs inhibit apoptosis in irradiated muscle in vivo.
FIGURE 7
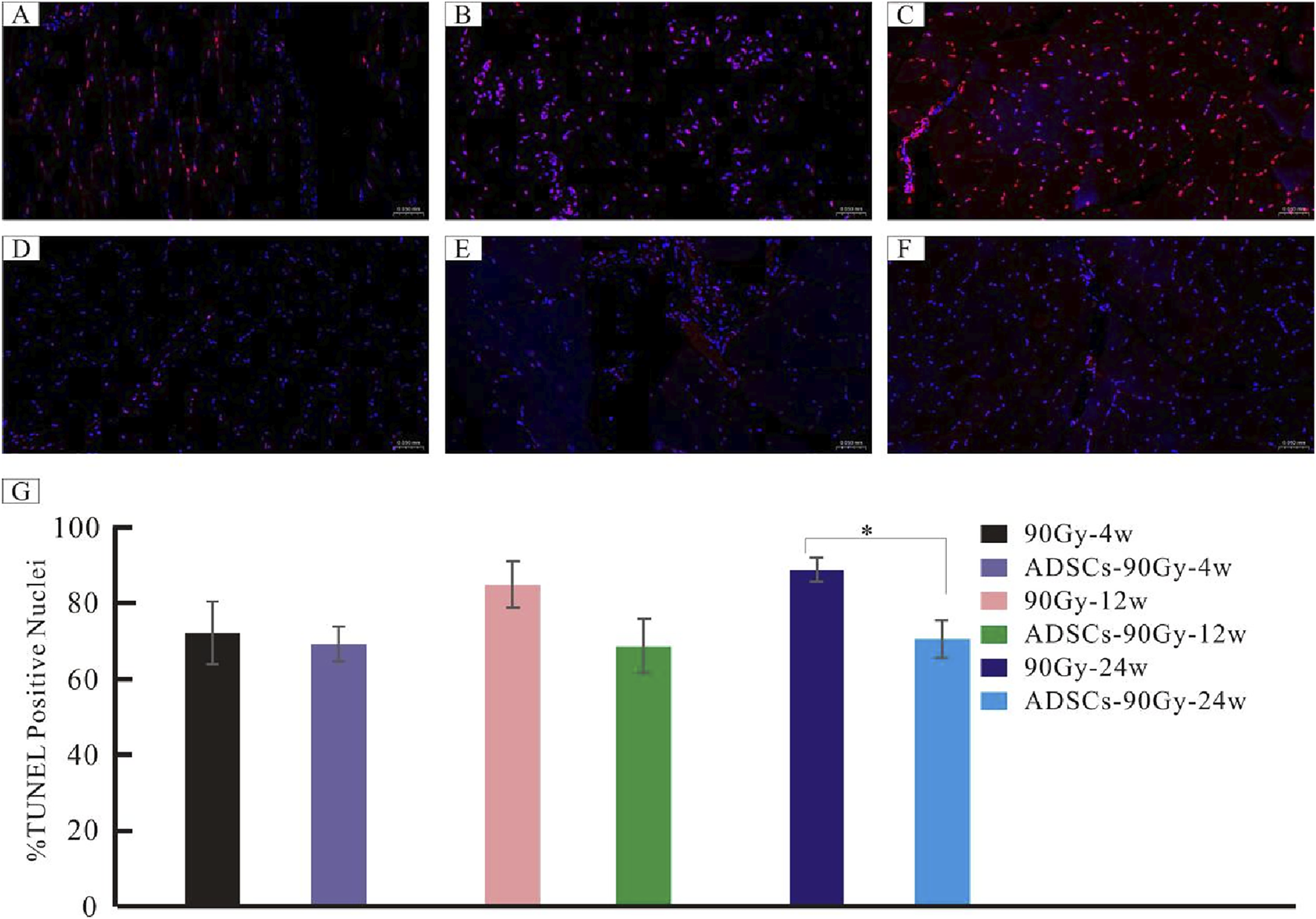
Detection of apoptotic cells in irradiated muscle tissues. Representative TUNEL-stained images are shown for the control groups: (A) 90 Gy-4 w, (B) 90 Gy-12 w, and (C) 90 Gy-24 w and the ADSC-treated experimental groups: (D) ADSCs-90Gy-4w, (E) ADSCs-90Gy-12 w, and (F) ADSCs-90Gy-24 w. TUNEL-positive nuclei are stained red. The percentage of apoptotic cells was significantly higher in the 90 Gy-24 w control group than that in the ADSCs-90Gy-24 w group. Data are presented as the mean ± SEM. *p < 0.05 compared with the respective control group. (G) The Percentage of apoptotic cells was significantly higher in the 90Gy-24 w control group than that in the ADSCs-90Gy-24 w group.
Discussion
RIF is a severe advanced complication of radiotherapy, especially in patients with head-and-neck tumors or breast cancer subtypes (Lennox et al., 2002; van Geel et al., 2011). Although various treatment strategies are available to manage RIF, the therapeutic efficacy of existing modalities remains limited (Okunieff et al., 2004; Wang et al., 2022; Krejbich and Birringer, 2022), providing renewed impetus for the exploring novel therapeutic approaches. In this study, we demonstrated that ADSCs ameliorated muscle tissue injuries by reducing collagen fibrillogenesis; inhibiting apoptosis; and promoting SC activation, proliferation, and differentiation in vivo. These findings provide fundamental evidence for the clinical therapeutic potential of ADSCs in RIF.
ADSCs are highly promising for multipotent stem cell-based therapies due to their easy accessibility, cost-effectiveness, and high proliferation. Although no unique single-cell surface marker characterizes ADSCs, they exhibit functional characteristics similar to mesenchymal stem cells, such as adipogenic, osteogenic, and chondrogenic differentiation capability in vitro. In addition to tri-lineage differentiation, ADSCs express surface markers such as CD90, CD105, and CD10, which is consistent with our previous findings (Yao et al., 2021). In this study, we characterized ADSCs using previously described ways of representation.
ADSCs represent a new therapeutic strategy in musculoskeletal diseases. Over the past several decades, numerous growth factors secreted by ADSCs, including IGF-1, TGF-β1, bFGF, VEGF, and hepatocyte growth factor, have been shown to be associated with growth in vivo, providing robust evidence for their role in muscle repair (Rivera-Izquierdo et al., 2019). Radiation exposure induces long-term muscle atrophy and fibrosis (Collao et al., 2023). In our previous study, we established an RIF rat model (Zhou et al., 2018) and observed SC activation; however, this activation-related muscle regeneration was not sufficient to counteract fibrosis formation (Zeng et al., 2022). Ni et al. (2014) reported that ADSC transplantation repaired radiation-induced skeletal muscle injury in New Zealand white rabbit models, which was associated with the upregulation of VEGF and bFGF. Wang et al. (2014) demonstrated that transplantation of bone marrow stromal cells overexpressing VEGF enhanced muscle repair in radiation-injured rat models. In addition, Rybalko et al. (2017) reported that co-culturing ADSCs with macrophages ameliorated the functional decline and reperfusion injury in typical peripheral artery disease, resulting in enhanced skeletal muscle regeneration. Gastrocnemius muscular atrophy caused by irretrievable resection and retraction of the sciatic nerve in a programmed process could be rescued through diffuse intramuscular injection of human ADSCs in mice (Qu et al., 2022; Schilling et al., 2019). Numerous clinical trials utilized ADSCs to treat various diseases (Lee et al., 2019; Li et al., 2023; Fujita et al., 2023; Iglesias et al., 2023), and we anticipate future clinical applications targeting muscle fibrosis and atrophy. In our study, ADSCs alleviated collagen fiber formation, inhibited apoptosis, promoted SC proliferation, and enhanced myoblast differentiation and muscle regeneration in irradiated muscle tissue in vivo. However, the mechanisms underlying these therapeutic effects remain to be elucidated. In the future, our findings may serve as a foundation for exploring the underlying cellular mechanisms of ADSC-based treatment.
Under physiological conditions, muscle regeneration and degradation are maintained in a dynamic equilibrium. However, under pathological conditions, this homeostasis is disrupted, leading to excessive deposition of fibrillar collagen in damaged muscle tissue (Zeng et al., 2022). The balance between collagen deposition and muscle fiber regeneration is complex and poorly regulated. Recent advances in biogenetics and cellular biology have highlighted that mitochondrial metabolism plays a crucial role in apoptosis, a form of programmed cell death (Schapira, 2012). Apoptosis of oligodendrocytes was significantly increased in a rat model of radiation-induced diffuse brain injury (Sano et al., 2000). In our previous work, we identified apoptosis as a key factor in a radiation-induced dermatitis model (Yao et al., 2021). Our current results showed a high level of apoptotic cells and mitochondrial abnormalities in irradiated muscle tissue, which is inconsistent with prior findings. However, this could be alleviated following ADSC treatment. Traditionally, central nuclear migration in nascent muscle fibers and SC activation are both regarded as sources of muscle regeneration (Srikuea and Hirunsai, 2016). In our study, although ADSCs demonstrated promising potential for treating RIF, their therapeutic effects require further improvement. Future work will focus on improving the regenerative capacity of ADSCs to more effectively promote muscle repair and treat RIF.
Conclusion
Collectively, our findings provide evidence of the therapeutic potential of ADSCs in treating chronic RIF in vivo. Collagen deposition showed an apparent decrease in the ADSC-treated group compared with the control group. Additionally, ADSCs promoted SC activation, proliferation, differentiation, and central nuclear formation in muscle cells. These results suggest that ADSCs are a promising candidate for repairing radiation-impaired muscle in tissue engineering.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by the Institutional Animal Care and Use Committees of Hunan Cancer Hospital. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
SL: Writing – original draft, Investigation. MP: Writing – review and editing, Validation, Writing – original draft. XO: Data curation, Writing – review and editing. ZZ: Data curation, Formal Analysis, Writing – review and editing. LX: Investigation, Methodology, Writing – review and editing. YG: Formal Analysis, Methodology, Writing – review and editing. ZS: Data curation, Writing – review and editing. XZ: Funding acquisition, Writing – review and editing. CS: Visualization, Writing – review and editing. XS: Conceptualization, Funding acquisition, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (82030056), the Changsha Municipal Natural Science Foundation (kq2403126, kq2502328, and 23A0334), and the Foundation of Hunan Province (2024JK2139, 2023ZJ1120, and 2023ZJ1125), high level talent support program of Hunan Cancer Hospital, 20250731-1022.
Acknowledgments
The authors acknowledge the support from the 14th Five-Year Plan “Application Characteristic Discipline of Hunan Province (Clinical Medicine)” and the Aid Program for Science and Technology Innovative Research Team in Higher Educational Institutions of Hunan Province, China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abboud W. A. Hassin-Baer S. Alon E. E. Gluck I. Dobriyan A. Amit U. et al (2020). Restricted mouth opening in head and neck cancer: etiology, prevention, and treatment. JCO Oncol. Pract.16, 643–653. 10.1200/OP.20.00266
2
Ademi H. Michalak-Micka K. Moehrlen U. Biedermann T. Klar A. S. (2023). Effects of an adipose mesenchymal stem cell-derived conditioned medium and TGF-β1 on human keratinocytes in vitro. Int. J. Mol. Sci.24, 14726. 10.3390/ijms241914726
3
Agha-Hosseini F. Pourpasha M. Amanlou M. Moosavi M.-S. (2021). Mouthwash containing vitamin E, triamcinolon, and hyaluronic acid compared to triamcinolone mouthwash alone in patients with radiotherapy-induced oral mucositis: randomized clinical trial. Front. Oncol.11, 614877. 10.3389/fonc.2021.614877
4
Ai G. Meng M. Guo J. Li C. Zhu J. Liu L. et al (2023). Adipose-derived stem cells promote the repair of chemotherapy-induced premature ovarian failure by inhibiting granulosa cells apoptosis and senescence. Stem Cell Res. Ther.14, 75. 10.1186/s13287-023-03297-5
5
Al-Ghadban S. Bunnell B. A. (2020). Adipose tissue-derived stem cells: immunomodulatory effects and therapeutic potential. Physiol. (Bethesda)35, 125–133. 10.1152/physiol.00021.2019
6
Alió Del Barrio J. L. De la Mata A. De Miguel M. P. Arnalich-Montiel F. Nieto-Miguel T. El Zarif M. et al (2022). Corneal regeneration using adipose-derived mesenchymal stem cells. Cells11, 2549. 10.3390/cells11162549
7
Binatti E. Zoccatelli G. Zanoni F. Donà G. Mainente F. Chignola R. (2021). Effects of combination treatments with astaxanthin-loaded microparticles and pentoxifylline on intracellular ROS and radiosensitivity of J774A.1 macrophages. Molecules26, 5152. 10.3390/molecules26175152
8
Brack A. S. Rando T. A. (2012). Tissue-specific stem cells: lessons from the skeletal muscle satellite cell. Cell Stem Cell10, 504–514. 10.1016/j.stem.2012.04.001
9
Chandra R. A. Keane F. K. Voncken F. E. M. Thomas C. R. (2021). Contemporary radiotherapy: present and future. Lancet398, 171–184. 10.1016/S0140-6736(21)00233-6
10
Chaurasia M. Gupta S. Das A. Dwarakanath B. S. Simonsen A. Sharma K. (2019). Radiation induces EIF2AK3/PERK and ERN1/IRE1 mediated pro-survival autophagy. Autophagy15, 1391–1406. 10.1080/15548627.2019.1582973
11
Chen P. Liu H. Xin H. Cheng B. Sun C. Liu Y. et al (2023). Inhibiting the cytosolic phospholipase A2-Arachidonic acid pathway with arachidonyl trifluoromethyl ketone attenuates radiation-induced lung fibrosis. Int. J. Radiat. Oncol. Biol. Phys.115, 476–489. 10.1016/j.ijrobp.2022.03.008
12
Chen Y.-C. Chuang E.-Y. Tu Y.-K. Hsu C.-L. Cheng N.-C. (2024). Human platelet lysate-cultured adipose-derived stem cell sheets promote angiogenesis and accelerate wound healing via CCL5 modulation. Stem Cell Res. Ther.15, 163. 10.1186/s13287-024-03762-9
13
Collao N. D'Souza D. Messeiller L. Pilon E. Lloyd J. Larkin J. et al (2023). Radiation induces long-term muscle fibrosis and promotes a fibrotic phenotype in fibro-adipogenic progenitors. J. cachexia, sarcopenia muscle14, 2335–2349. 10.1002/jcsm.13320
14
Dancey A. L. Waters R. A. (2006). Morphea of the breast. Two case reports and discussion of the literature. J. Plast. Reconstr. Aesthet. Surg.59, 1114–1117. 10.1016/j.bjps.2006.01.018
15
Diao Y. Guo X. Li Y. Sun K. Lu L. Jiang L. et al (2012). Pax3/7BP is a Pax7-and Pax3-binding protein that regulates the proliferation of muscle precursor cells by an epigenetic mechanism. Cell Stem Cell11, 231–241. 10.1016/j.stem.2012.05.022
16
Fujita M. Matsumoto T. Sobajima S. Tsubosaka M. Matsushita T. Iwaguro H. et al (2023). Clinical and radiological comparison of single and double intra-articular injection of adipose-derived stromal vascular fraction for knee osteoarthritis. Cell Transplant.32, 9636897231190175. 10.1177/09636897231190175
17
Garg K. Corona B. T. Walters T. J. (2015). Therapeutic strategies for preventing skeletal muscle fibrosis after injury. Front. Pharmacol.6, 87. 10.3389/fphar.2015.00087
18
Ghosh P. S. Milone M. (2015). Clinical and laboratory findings of 21 patients with radiation-induced myopathy. J. Neurol. Neurosurg. Psychiatry86, 152–158. 10.1136/jnnp-2013-307447
19
Gionet-Gonzales M. A. Gresham R. C. H. Griffin K. H. Casella A. Wohlgemuth R. P. Ramos-Rodriguez D. H. et al (2023). Mesenchymal stromal cell spheroids in sulfated alginate enhance muscle regeneration. Acta Biomater.155, 271–281. 10.1016/j.actbio.2022.10.054
20
Guo X. Schaudinn C. Blume-Peytavi U. Vogt A. Rancan F. (2022). Effects of adipose-derived stem cells and their conditioned medium in a human Ex Vivo wound model. Cells11, 1198. 10.3390/cells11071198
21
Harasymiak-Krzyżanowska I. Niedojadło A. Karwat J. Kotuła L. Gil-Kulik P. Sawiuk M. et al (2013). Adipose tissue-derived stem cells show considerable promise for regenerative medicine applications. Cell Mol. Biol. Lett.18, 479–493. 10.2478/s11658-013-0101-4
22
Higginbotham S. Workman V. L. Giblin A. V. Green N. H. Lambert D. W. Hearnden V. (2024). Inhibition and reversal of a TGF-β1 induced myofibroblast phenotype by adipose tissue-derived paracrine factors. Stem Cell Res. Ther.15, 166. 10.1186/s13287-024-03776-3
23
Hu Z. Zhao G. Gou W. Cheng H. (2020). Myricitrin inhibits vascular endothelial growth factor-induced angiogenesis of human umbilical vein endothelial cells and mice. Biomed. Pharmacother.130, 110726. 10.1016/j.biopha.2020.110726
24
Iglesias M. Torre-Villalvazo I. Butrón-Gandarillas P. Rodríguez-Reyna T. S. Torre-Anaya E. A. Guevara-Cruz M. et al (2023). Adipose derived stromal vascular fraction and fat graft for treating the hands of patients with systemic sclerosis. A randomized clinical trial. PloS one18, e0289594. 10.1371/journal.pone.0289594
25
Jin J. Shi Y. Gong J. Zhao L. Li Y. He Q. et al (2019). Exosome secreted from adipose-derived stem cells attenuates diabetic nephropathy by promoting autophagy flux and inhibiting apoptosis in podocyte. Stem Cell Res. Ther.10, 95. 10.1186/s13287-019-1177-1
26
Jit B. P. Pradhan B. Dash R. Bhuyan P. P. Behera C. Behera R. K. et al (2021). Phytochemicals: potential therapeutic modulators of radiation induced signaling pathways. Antioxidants (Basel)11, 49. 10.3390/antiox11010049
27
Kawashita Y. Soutome S. Umeda M. Saito T. (2022). Predictive risk factors associated with severe radiation-induced mucositis in nasopharyngeal or oropharyngeal cancer patients: a retrospective study. Biomedicines10, 2661. 10.3390/biomedicines10102661
28
Kiang J. G. Garrison B. R. Gorbunov N. V. (2010). Radiation combined injury: DNA damage, apoptosis, and autophagy. Adapt Med.2, 1–10. 10.4247/AM.2010.ABA004
29
Krejbich P. Birringer M. (2022). The self-administered use of complementary and alternative medicine (CAM) supplements and antioxidants in cancer therapy and the critical role of Nrf-2-A systematic review. Antioxidants (Basel)11, 2149. 10.3390/antiox11112149
30
Lee W. S. Kim H. J. Kim K. I. Kim G. B. Jin W. (2019). Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: a phase IIb, randomized, placebo-controlled clinical trial. Stem cells Transl. Med.8, 504–511. 10.1002/sctm.18-0122
31
Lee T.-L. Lai T. C. Lin S. R. Lin S. W. Chen Y. C. Pu C. M. et al (2021). Conditioned medium from adipose-derived stem cells attenuates ischemia/reperfusion-induced cardiac injury through the microRNA-221/222/PUMA/ETS-1 pathway. Theranostics11, 3131–3149. 10.7150/thno.52677
32
Lei X. He N. Zhu L. Zhou M. Zhang K. Wang C. et al (2021). Mesenchymal stem cell-derived extracellular vesicles attenuate radiation-induced lung injury via miRNA-214-3p. Antioxid. Redox Signal35, 849–862. 10.1089/ars.2019.7965
33
Lennox A. J. Shafer J. P. Hatcher M. Beil J. Funder S. J. (2002). Pilot study of impedance-controlled microcurrent therapy for managing radiation-induced fibrosis in head-and-neck cancer patients. Int. J. Radiat. Oncol. Biol. Phys.54, 23–34. 10.1016/s0360-3016(02)02898-5
34
Li X. Ma T. Sun J. Shen M. Xue X. Chen Y. et al (2019). Harnessing the secretome of adipose-derived stem cells in the treatment of ischemic heart diseases. Stem Cell Res. Ther.10, 196. 10.1186/s13287-019-1289-7
35
Li F. Lu J. Shi X. Li D. Zhou T. Jiang T. et al (2023). Effect of adipose tissue-derived stem cells therapy on clinical response in patients with primary sjogren's syndrome. Sci. Rep.13, 13521. 10.1038/s41598-023-40802-5
36
Liao W. Chen X. Zhang S. Chen J. Liu C. Yu K. et al (2024). Megakaryocytic IGF1 coordinates activation and ferroptosis to safeguard hematopoietic stem cell regeneration after radiation injury. Cell Commun. Signal22, 292. 10.1186/s12964-024-01651-5
37
Lu Z. Chen Y. Liu D. Jiao X. Liu C. Wang Y. et al (2023). The landscape of cancer research and cancer care in China. Nat. Med.29, 3022–3032. 10.1038/s41591-023-02655-3
38
Ni X. Sun W. Sun S. Yu J. Wang J. Nie B. et al (2014). Therapeutic potential of adipose stem cells in tissue repair of irradiated skeletal muscle in a rabbit model. Cell. Reprogr.16, 140–150. 10.1089/cell.2013.0056
39
Okunieff P. Augustine E. Hicks J. E. Cornelison T. L. Altemus R. M. Naydich B. G. et al (2004). Pentoxifylline in the treatment of radiation-induced fibrosis. J. Clin. Oncol.22, 2207–2213. 10.1200/JCO.2004.09.101
40
Palmer J. D. Tsang D. S. Tinkle C. L. Olch A. J. Kremer L. C. M. Ronckers C. M. et al (2021). Late effects of radiation therapy in pediatric patients and survivorship. Pediatr. Blood Cancer68 (Suppl. 2), e28349. 10.1002/pbc.28349
41
Price F. D. Matyas M. N. Gehrke A. R. Chen W. Wolin E. A. Holton K. M. et al (2024). Organoid culture promotes dedifferentiation of mouse myoblasts into stem cells capable of complete muscle regeneration. Nat. Biotechnol.43, 889–903. 10.1038/s41587-024-02344-7
42
Qu S. Ma N. Wang W. Chen S. Wu Q. Li Y. et al (2022). Human adipose-derived stem cells delay muscular atrophy after peripheral nerve injury in rats. Cell Biochem. biophysics80, 555–562. 10.1007/s12013-022-01082-4
43
Ren Y. Wang W. Yu C. Wang Y. Qiu Y. Yue Z. et al (2024). An injectable exosome-loaded hyaluronic acid-polylysine hydrogel for cardiac repair via modulating oxidative stress and the inflammatory microenvironment. Int. J. Biol. Macromol.275, 133622. 10.1016/j.ijbiomac.2024.133622
44
Rivera-Izquierdo M. Cabeza L. Láinez-Ramos-Bossini A. Quesada R. Perazzoli G. Alvarez P. et al (2019). An updated review of adipose derived-mesenchymal stem cells and their applications in musculoskeletal disorders. Expert Opin. Biol. Ther.19, 233–248. 10.1080/14712598.2019.1563069
45
Rybalko V. Hsieh P. L. Ricles L. M. Chung E. Farrar R. P. Suggs L. J. (2017). Therapeutic potential of adipose-derived stem cells and macrophages for ischemic skeletal muscle repair. Regen. Med.12, 153–167. 10.2217/rme-2016-0094
46
Sampath S. C. Sampath S. C. Ho A. T. V. Corbel S. Y. Millstone J. D. Lamb J. et al (2018). Induction of muscle stem cell quiescence by the secreted niche factor oncostatin M. Nat. Commun.9, 1531. 10.1038/s41467-018-03876-8
47
Sano K. Morii K. Sato M. Mori H. Tanaka R. (2000). Radiation-induced diffuse brain injury in the neonatal rat model--radiation-induced apoptosis of oligodendrocytes. Neurol. Med. Chir. (Tokyo)40, 495–499. 10.2176/nmc.40.495
48
Schapira A. H. V. (2012). Mitochondrial diseases. Lancet379, 1825–1834. 10.1016/S0140-6736(11)61305-6
49
Schilling B. K. Schusterman M. A. Kim D. Y. Repko A. J. Klett K. C. Christ G. J. et al (2019). Adipose-derived stem cells delay muscle atrophy after peripheral nerve injury in the rodent model. Muscle and nerve59, 603–610. 10.1002/mus.26432
50
Sheng X. Zhou Y. Wang H. Shen Y. Liao Q. Rao Z. et al (2019). Establishment and characterization of a radiation-induced dermatitis rat model. J. Cell Mol. Med.23, 3178–3189. 10.1111/jcmm.14174
51
Siegel R. L. Miller K. D. Wagle N. S. Jemal A. (2023). Cancer statistics, 2023. CA Cancer J. Clin.73, 17–48. 10.3322/caac.21763
52
Snijders T. Aussieker T. Holwerda A. Parise G. van Loon L. J. C. Verdijk L. B. (2020). The concept of skeletal muscle memory: evidence from animal and human studies. Acta Physiol. (Oxf)229, e13465. 10.1111/apha.13465
53
Song P. Han T. Wu Z. Fang H. Liu Y. Ying W. et al (2024). Transplantation of neural stem cells loaded in an IGF-1 bioactive supramolecular nanofiber hydrogel for the effective treatment of spinal cord injury. Adv. Sci. (Weinh)11, e2306577. 10.1002/advs.202306577
54
Srikuea R. Hirunsai M. (2016). Effects of intramuscular administration of 1α,25(OH)2D3 during skeletal muscle regeneration on regenerative capacity, muscular fibrosis, and angiogenesis. J. Appl. Physiol. (1985)120, 1381–1393. 10.1152/japplphysiol.01018.2015
55
Stokkevåg C. H. Journy N. Vogelius I. R. Howell R. M. Hodgson D. Bentzen S. M. (2024). Radiation therapy technology advances and mitigation of subsequent neoplasms in childhood cancer survivors. Int. J. Radiat. Oncol. Biol. Phys.119, 681–696. 10.1016/j.ijrobp.2024.01.206
56
Vallée A. Lecarpentier Y. Guillevin R. Vallée J.-N. (2017). Interactions between TGF-β1, canonical WNT/β-catenin pathway and PPAR γ in radiation-induced fibrosis. Oncotarget8, 90579–90604. 10.18632/oncotarget.21234
57
van Geel A. N. Lans T. E. Haen R. Tjong Joe Wai R. Menke-Pluijmers M. B. E. (2011). Partial mastectomy and M. Latissimus dorsi reconstruction for radiation-induced fibrosis after breast-conserving cancer therapy. World J. Surg.35, 568–572. 10.1007/s00268-010-0911-8
58
Wang T. Liao T. Wang H. Deng W. Yu D. (2014). Transplantation of bone marrow stromal cells overexpressing human vascular endothelial growth factor 165 enhances tissue repair in a rat model of radiation-induced injury. Chin. Med. J.127, 1093–1099. 10.3760/cma.j.issn.0366-6999.20132337
59
Wang H. Wang B. Wei J. Zheng Z. Su J. Bian C. et al (2022). Sulforaphane regulates Nrf2-mediated antioxidant activity and downregulates TGF-β1/Smad pathways to prevent radiation-induced muscle fibrosis. Life Sci.311, 121197. 10.1016/j.lfs.2022.121197
60
Wang J. Qiu D. Dong X. Liu Y. Chen J. (2024). Efficacy and safety of QingReJieDu therapy in preventing acute radiation esophagitis: a systematic review and meta-analysis. Medicine103, e40779. 10.1097/MD.0000000000040779
61
Yanaba K. Umezawa Y. Nakagawa H. (2015). A case of radiation-induced generalized morphea with prominent mucin deposition and tenderness. Am. J. Case Rep.16, 279–282. 10.12659/AJCR.893481
62
Yao C. Zhou Y. Wang H. Deng F. Chen Y. Zhu X. et al (2021). Adipose-derived stem cells alleviate radiation-induced dermatitis by suppressing apoptosis and downregulating cathepsin F expression. Stem Cell Res. Ther.12, 447. 10.1186/s13287-021-02516-1
63
Yoshimoto Y. Ikemoto-Uezumi M. Hitachi K. Fukada S.-I. Uezumi A. (2020). Methods for accurate assessment of myofiber maturity during skeletal muscle regeneration. Front. Cell Dev. Biol.8, 267. 10.3389/fcell.2020.00267
64
Yu P. Zhang X. Liu N. Tang L. Peng C. Chen X. (2021). Pyroptosis: mechanisms and diseases. Signal Transduct. Target Ther.6, 128. 10.1038/s41392-021-00507-5
65
Zeng X. Xie L. Ge Y. Zhou Y. Wang H. Chen Y. et al (2022). Satellite cells are activated in a rat model of radiation-induced muscle fibrosis. Radiat. Res.197, 638–649. 10.1667/RADE-21-00183.1
66
Zhang Q. Han W. Wu R. Deng S. Meng J. Yang Y. et al (2024). Spermidine-eIF5A axis is essential for muscle stem cell activation via translational control. Cell Discov.10, 94. 10.1038/s41421-024-00712-w
67
Zhou Y. Sheng X. Deng F. Wang H. Shen L. Zeng Y. et al (2018). Radiation-induced muscle fibrosis rat model: establishment and valuation. Radiat. Oncol. Lond. Engl.13, 160. 10.1186/s13014-018-1104-0
Summary
Keywords
radiation-induced muscle fibrosis, radiotherapy, adipose-derived stem cell, satellite cells, Apoptosis
Citation
Li S, Peng M, Ou X, Zhou Z, Xie L, Ge Y, Song Z, Zhou X, Shi C and Sheng X (2025) Adipose-derived stem cells alleviate radiation-induced muscle fibrosis by promoting muscle regeneration. Front. Cell Dev. Biol. 13:1620998. doi: 10.3389/fcell.2025.1620998
Received
30 April 2025
Accepted
10 September 2025
Published
24 October 2025
Volume
13 - 2025
Edited by
Finosh Thankam, Western University of Health Sciences, United States
Reviewed by
Sithara Thomas, University of Texas Health Science Center at Houston, United States
Resmi Rajalekshmi, Western University of Health Sciences, United States
Updates
Copyright
© 2025 Li, Peng, Ou, Zhou, Xie, Ge, Song, Zhou, Shi and Sheng.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaowu Sheng, shengxiaowu789@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.