- 1The First Clinical College, Shanxi Medical University, Taiyuan, Shanxi, China
- 2The Reproductive Medicine Center, The First Hospital of Shanxi Medical University, Taiyuan, Shanxi, China
Menstrual blood (MB), a biofluid rich in diverse cell types and biomolecules, has emerged as a vital resource for investigating female reproductive health and diseases because of its unique composition and noninvasive accessibility. This review explores the potential of MB in medical research and clinical applications, focusing on its diagnostic and therapeutic prospects. For disease diagnosis, MB offers a noninvasive sampling method for identifying biomarkers in endometriosis, cervical cancer, and other gynecological conditions. Therapeutically, stem cells derived from MB (menstrual blood-derived stem cells, MenSCs) exhibit pluripotency, high proliferative capacity, and low immunogenicity, positioning them as promising candidates in regenerative medicine. Preclinical and clinical studies have demonstrated the efficacy of MenSCs in treating infertility, premature ovarian insufficiency, intrauterine adhesions, hepatic disorders, cutaneous injuries, and neurological diseases. MenSCs also exert therapeutic effects through paracrine mechanisms by releasing cytokines and exosomes that modulate immunity, attenuate inflammation, and promote tissue repair. Despite existing challenges, MenSCs hold substantial promise for developing novel therapeutic strategies across multiple disease domains.
1 Background
Menstruation is a central physiological phenomenon of the female reproductive system characterized by cyclic shedding and bleeding of the endometrium triggered by ovarian cyclical changes. Throughout the reproductive lifespan, women typically undergo over 400 cycles of endometrial regeneration, differentiation, and shedding (Lv et al., 2018). These cyclic processes are regulated primarily by the hypothalamus‒pituitary‒ovary axis.
Menstrual blood (MB) is a complex biofluid containing blood from endometrial spiral arteries, vaginal secretions, and endometrial cells (van der Molen et al., 2014; Yang et al., 2012). Notably, MB results in significant differences in the concentrations of specific biomolecules and cellular compositions compared with those of peripheral blood (PB) (Iribarne-Durán et al., 2020; Hosseini et al., 2019). The shed endometrial cells encompass diverse types, including stromal cells, epithelial cells, vascular cells, and immune cells. These components not only reflect endometrial homeostasis but also provide critical insights for investigating reproductive health and disease mechanisms.
Given the heterogeneous and multifaceted composition of MB, research interest in this field has expanded substantially in recent years. This review systematically examines the applications of MB over the past decade in disease diagnostics, therapeutic development, pathogenesis elucidation, and genetic research. Furthermore, it evaluates the current challenges in clinical translation and outlines future directions to advance MB-based biomedical innovations.
2 Disease diagnosis
As a diagnostic specimen, MB offers multiple advantages, including ease of sampling, noninvasiveness, self-collection capability, periodic availability, and fewer ethical concerns (Hosoya et al., 2023). In terms of population acceptability, Wong et al. evaluated the willingness of 5,000 women to use MB as a diagnostic sample, with the results indicating that 87% of participants supported its utilization for testing (Wong et al., 2018). Another study by Budukh further validated the feasibility of MB as a screening specimen for cervical cancer (Budukh et al., 2018). This noninvasive collection method not only eliminates the discomfort associated with traditional sampling procedures but also empowers women to flexibly manage their daily activities. Consequently, MB has significant advantages as a diagnostic specimen applicable to all menstruating women.
2.1 Cervical cancer
Cervical cancer remains a leading cause of cancer-related mortality among women globally, accounting for approximately 25% of all female malignancies (Harro et al., 2001). With a mortality rate second only to that of breast cancer, it is a critical public health concern (Jin et al., 1999). Human papillomavirus (HPV) is the primary etiological agent, driving lesion progression from low-grade cervical intraepithelial neoplasia (CIN1) to high-grade neoplasia (CIN2/3) and microinvasive lesions, ultimately leading to invasive cervical cancer (Holowaty et al., 1999). Understanding this pathophysiological trajectory is essential for developing diagnostic, therapeutic, and preventive strategies. Early detection and intervention during the precancerous phase are pivotal to halting disease progression (Spitzer, 1998).
Current screening and diagnostic methods for cervical cancer rely primarily on cytology and histology. From the conventional Papanicolaou smear to improved liquid-based cytology and automated processes, these approaches have significantly reduced cervical cancer mortality in developed countries. However, owing to the high false-positive rates of cytology, colposcopy with directed biopsy is often required for further evaluation. While colposcopy can detect low- and high-grade dysplasia, its sensitivity for identifying microinvasive lesions remains limited. In cases with inconclusive findings or incomplete visualization of the squamocolumnar junction, cone biopsy is necessary to confirm diagnoses through histopathological identification of HPV-associated features. Additionally, molecular detection of high-risk HPV DNA sequences has been introduced in recent years, enhancing diagnostic sensitivity and specificity. Emerging HPV testing technologies, such as next-generation sequencing (NGS), enable hypothesis-free comprehensive genetic analysis, offering novel strategies for cervical cancer screening.
Currently, cervical cancer screening relies on cytology and histology. These methods, ranging from conventional Pap smears to advanced liquid-based cytology and automated systems, have significantly reduced mortality in developed nations (Hu and Ma, 2018). However, the high false-positive rates of cytology necessitate confirmatory colposcopy with directed biopsy. While colposcopy identifies dysplasia, its sensitivity for detecting microinvasive lesions is limited, particularly in cases with incomplete visualization of the squamocolumnar junction, which requires cone biopsy for histopathological confirmation (Burd, 2003). Molecular HPV DNA testing has emerged as a complementary tool, enhancing diagnostic accuracy (Burd, 2003). Next-generation sequencing (NGS) represents a novel frontier, enabling comprehensive genetic analysis without prior hypotheses (Tsang et al., 2022; Pei et al., 2023).
Despite these advancements, all current methods require invasive sampling, which can cause patient discomfort and psychological stress. Emerging studies on HPV detection in MB propose a noninvasive alternative. Zhang et al. (2021) collected MB via sanitary pads from premenopausal women with high-risk HPV positivity, extracted DNA, and performed next-generation sequencing (NGS)-based HPV genotyping. Compared with conventional cervical smears, MB-based testing achieved 97.7% sensitivity, identifying additional HPV genotypes, multiple infections, and true-negative cases. It also accurately detected high-risk HPV in routine false-negative test results.
Similarly, Tsang et al. (2024) used NGS to analyze MB samples from CIN/HPV-positive patients and reported a 66.7% sensitivity for high-risk HPV detection. Wong et al. (2018) further explored HPV DNA and genetic polymorphisms in MB from CIN/HPV patients and healthy controls. They reported that 83% of the participants tested HPV-positive and successfully genotyped, whereas 4% of the controls tested HPV-positive. Notably, TAP1 gene polymorphisms (I333V and D637G) in MB were linked to a reduced risk of high-grade intraepithelial neoplasia, offering actionable insights for clinical management and resource allocation.
2.2 Endometriosis
Endometriosis is a chronic disease characterized by the ectopic growth of endometrial-like tissue outside the uterine cavity, resulting in inflammatory, hormone dependent, immunological, systemic, and heterogeneous pathophysiological features, predominantly affecting reproductive-aged women (Sinaii et al., 2008). Its primary symptoms include pelvic pain, which may manifest as dysmenorrhea, dyspareunia, or chronic pelvic pain, often accompanied by overlapping symptoms (e.g., urinary or gastrointestinal manifestations), complicating clinical diagnosis (Parasar et al., 2017). Owing to symptom overlap with other gynecological conditions (e.g., ovarian cysts, uterine fibroids, or pelvic inflammatory disease sequelae) or chronic pain syndromes, a definitive diagnosis requires the integration of patient history, clinical examination, and imaging (Sinaii et al., 2008; Ballweg, 2004). However, the gold-standard diagnostic method—laparoscopy—is limited by its invasiveness (Parasar et al., 2017). Current research on noninvasive biomarker-based diagnostic approaches remains underdeveloped (Falcone and Flyckt, 2018).
The discovery of aromatase has opened new avenues for noninvasive endometriosis diagnosis. Noble et al. (1996) demonstrated aromatase expression in both the eutopic endometria and ectopic lesions of endometriosis patients, whereas normal endometrial and nondiseased peritoneal tissues lacked detectable aromatase. Malik et al. (2018) performed immunohistochemical analysis of menstrual blood samples from endometriosis patients and nonendometriosis patients. They reported significantly increased P450 aromatase (CYP19A1) expression in endometriosis patients: 32.4% of the endometriosis patients presented moderate expression, and 67.6% presented strong expression, with no cases of negative or weak expression observed. These findings suggest that elevated aromatase levels in menstrual blood may serve as a potential noninvasive biomarker for endometriosis, offering insights for early diagnosis and disease management.
Ji et al. (2023) utilized data-independent acquisition coupled with mass spectrometry and bioinformatics to quantify differentially expressed proteins in menstrual blood. They reported significantly upregulated expression of Chemokine Ligand 5 and Interleukin-1 Receptor Antagonist in endometriosis patients. These findings highlight Chemokine Ligand 5 and Interleukin-1 Receptor Antagonist as promising candidates for endometriosis diagnosis, advancing biomarker research in this field.
2.3 Genital tuberculosis
Genital tuberculosis typically arises as a complication of pulmonary or extrapulmonary tuberculosis and spreads via blood or lymphatic pathways (Aliyu et al., 2004). Female genital tuberculosis (FGTB) is a clinically silent chronic disease that primarily involves the fallopian tubes in nearly all cases, leading to infertility, dyspareunia, menstrual irregularities, and chronic pelvic inflammation (Namavar et al., 2001). Owing to its atypical presentation, such as infertility or mild pelvic pain, which often overlaps with symptoms of other gynecological conditions, such as pelvic inflammatory disease or endometriosis, diagnosis requires the integration of patient history, imaging (e.g., ultrasound or MRI), and mycobacterial testing (Bose, 2011). Currently, no single diagnostic test is sufficient for FGTB confirmation, necessitating a multidisciplinary approach.
Paine et al. (2018) demonstrated that MB analysis via multiplex polymerase chain reaction offers a noninvasive alternative for FGTB diagnosis. The method achieved 90.2% sensitivity and 86.1% specificity, significantly outperforming traditional endometrial-based approaches. By analyzing MB samples without requiring invasive procedures such as dilation and curettage (D&C) or laparoscopy and delivering results within hours, this approach addresses the critical limitations of conventional diagnostics. Clinically, rapid, noninvasive MB testing enables early FGTB detection, allowing timely antitubercular therapy initiation to improve fertility outcomes and quality of life. In resource-limited settings, it circumvents costly and painful procedures such as D&C, reducing healthcare costs and patient discomfort. Additionally, the high sensitivity and specificity of multiplex polymerase chain reaction provide a reliable screening tool for asymptomatic or early-stage FGTB, particularly in reproductive-aged women, minimizing diagnostic delays and misdiagnosis risks while advancing clinical practice.
3 Disease treatment
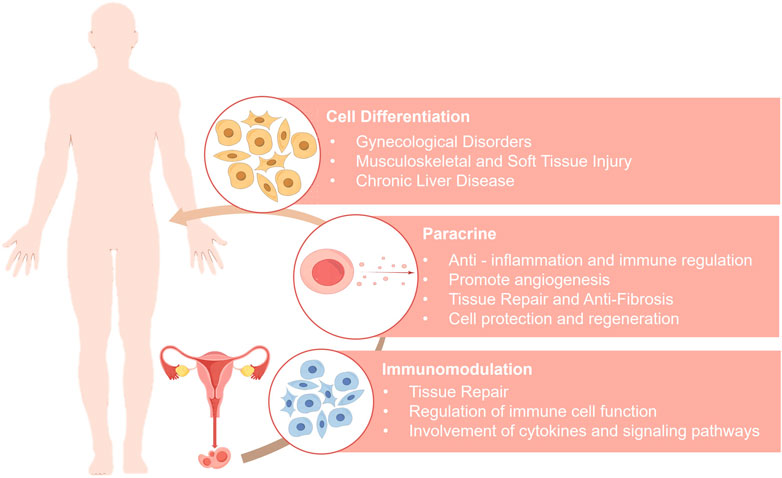
MenSCs hold significant promise in the field of regenerative medicine (Figure 1). Clinically, MenSCs exhibit low immunogenicity and can be expanded for more than 20 passages in vitro. This immune privilege is attributed to their low expression of major histocompatibility complex class II molecules (MHC-II, specifically HLA-DR) and the absence of co-stimulatory molecules (CD80/CD86), supporting their capacity for immune evasion (Khoury et al., 2014). Both preclinical and clinical studies have demonstrated that MenSC transplantation does not elicit immune rejection or severe adverse effects (Khanjani et al., 2014; Zhong et al., 2009; Bockeria et al., 2013).
In addition to MenSCs, other cell types derived from menstrual blood—such as endometrial regenerative cells (ERCs) and endometrial stromal cells (ESCs)—can also be easily obtained and have been widely applied in the research and treatment of various diseases. Overall, menstrual blood-derived cells represent a valuable source for stem cell-based therapies, with broad therapeutic potential across a range of medical conditions.
3.1 Therapeutic potential of cell differentiation
MenSCs harness their pluripotency and self-renewal capacity to differentiate into various functional cell types under specific in vitro induction conditions, thereby restoring or replacing damaged tissues and achieving tissue regeneration. Their differentiation potential spans multiple tissue and cell types, demonstrating notable therapeutic potential in the treatment of a variety of diseases.
3.1.1 Treatment of gynecological disorders
3.1.1.1 Infertility
For women with unexplained infertility, endometrial functional abnormalities may contribute to the underlying pathology. Cell-based therapies, particularly those that leverage the capacity of stem cells for decidualization or epithelial differentiation, offer a potential solution to improve endometrial function. Such therapies promote endometrial tissue remodeling, creating optimal conditions for embryo implantation. Piersma et al. (2015) successfully induced decidualization in MenSCs, observing the upregulation of characteristic decidualization markers such as prolactin, progesterone receptor, estrogen receptor, and insulin-like growth factor-binding protein. Additionally, key genes involved in decidualization including FOXO1, NOTCH1, NANOG, WNT4, KLF4, OCT4, SOX2, and LIN28A, as well as angiogenesis-related genes such as HIF1A, VEGFR-2, and VEGFR-3, were significantly upregulated. This coordinated gene expression enhances endometrial functionality, providing a novel direction for cellular replacement therapies in the treatment of infertility.
3.1.1.2 Ovarian disorders
Premature ovarian insufficiency (POI) is characterized by menstrual irregularities (amenorrhea, oligomenorrhea, or polymenorrhea) in women under 40 years of age and is accompanied by elevated follicle-stimulating hormone (FSH) levels (>25 U/L) and fluctuating estrogen decline (Feng et al., 2019). Studies have demonstrated that the transplantation of MenSCs improves ovarian function in POI models through multiple mechanisms. When transplanted via the tail vein into cyclophosphamide-induced POI model mice, MenSCs regulate follicular development, restore estrous cyclicity, reduce ovarian apoptosis, and maintain microenvironmental homeostasis via ECM-dependent FAK/AKT signaling activation (Feng et al., 2019). MenSCs also significantly improve physiological parameters in mice with ovarian damage, including serum hormone levels (e.g., FSH and estradiol (E2)), body weight, estrous cyclicity, ovarian reserve markers (e.g., anti-Müllerian hormone and FSH receptor (FSHR)), and follicle counts (Lai et al., 2015; Liu et al., 2014). Gene expression analysis revealed that posttransplantation ovarian gene expression profiles in mice closely resemble those of human ovarian tissue, suggesting that the POI microenvironment may induce MenSCss differentiation into ovarian-like cells (Liu et al., 2014).
Furthermore, the ability of MenSCs to promote germ cell differentiation has increased their therapeutic utility in treating ovarian disorders. Lai et al. (2016) demonstrated that MenSCs exposed to follicular fluid in vitro differentiate into oocyte-like cells and theca-like cells expressing FSHR and luteinizing hormone receptor, with steroidogenic capacity. Under coculture conditions, MenSCs further generate multinucleated embryoid structures, indicating their potential for oocyte development.
3.1.1.3 Intrauterine adhesions
Intrauterine adhesion (IUA), an acquired disorder following endometrial injury, is characterized by inadequate endometrial thickness, resulting in infertility and pregnancy loss even after assisted reproduction. Malik et al. (2018) demonstrated that MenSCs differentiated toward the endometrial lineage via PDGF, TGF-β, EGF, and 17β-estradiol expressed endometrial markers (cytokeratin (CK), vimentin, estrogen receptor, and progesterone receptor (PR)) in NOD-SCID mice posttransplantation, suggesting their capacity to reconstruct the endometrial architecture and restore fertility.
Fibrosis in IUA involves TGF-β-induced myofibroblast differentiation of endometrial stromal cells and the upregulation of α-smooth muscle actin (αSMA), type I collagen, CTGF, and fibronectin while impairing ESC migration (Deans and Abbott, 2010; Piersma et al., 2015; Zhu et al., 2019). Zhu et al. (Deans and Abbott, 2010) revealed that MenSCs-endometrial stromal cells coculture suppressed TGF-β-driven fibrosis by activating Hippo signaling (via TAZ phosphorylation), restoring ESC migratory capacity and promoting endometrial repair.
Clinical research has further verified the therapeutic potential of MenSCs. MenSCs isolated from the menstrual effluent of seven severe IUA patients were injected into the uterine cavity with hormone therapy, resulting in increased endometrial thickness in all patients (Malik et al., 2018). Among these, three achieved pregnancy with one live birth, demonstrating the efficacy of MenSCs in improving endometrial function and fertility outcomes (Malik et al., 2018; Chen et al., 2016).
3.1.2 Treatment of musculoskeletal and soft tissue injury
MenSCs exhibit remarkable potential in the treatment of musculoskeletal disorders and the repair of soft tissue injuries. Through directed differentiation, these cells can be induced to transform into osteoblasts, chondrocytes, and adipocytes, offering new therapeutic avenues for the repair of bone injuries, degenerative diseases, and congenital defects.
In the context of bone and cartilage regeneration, MenSCs cultured in osteogenic induction medium for 21 days presented marked morphological changes, along with significantly upregulated expression of key osteogenic markers such as alkaline phosphatase (ALP), secreted phosphoprotein 1 (SPP1), and bone gamma-carboxyglutamic acid-containing protein (BGLAP) (Skliutė et al., 2021). During chondrogenic differentiation, increased expression of the collagen type II alpha 1 (COL2A1) gene further supports its regenerative potential in skeletal and cartilage tissues.
With respect to muscle regeneration and pelvic organ prolapse (POP), the ability of MenSCs to differentiate into smooth muscle cells provides new insights for POP repair. The pathological basis of POP involves degeneration of vaginal smooth muscle, leading to loss of pelvic floor support (Chen et al., 2016). In a study by Chen et al. (2016), MenSCs were successfully induced to differentiate into smooth muscle cells under TGF-β1 stimulation via the TGFBR2/ALK5/Smad2/3 signaling pathway, laying the groundwork for cell-based therapies. Furthermore, in a clinical study of Duchenne muscular dystrophy (DMD), a patient who received an intramuscular injection of 116 million MenSCs presented significantly improved muscle strength and restored dystrophin expression (Ichim et al., 2010), highlighting the therapeutic potential of MenSCs in muscle repair.
In the context of soft tissue regeneration, the adipogenic potential of MenSCs is critical. Under rosiglitazone induction, the mRNA expression of adipogenic markers—including leptin receptor (LEPR), peroxisome proliferator-activated receptor gamma (PPAR-γ), and lipoprotein lipase (LPL)—was significantly upregulated (Khanmohammadi et al., 2014). Compared with BM-MSCs, MenSCs offer advantages in terms of accessibility and ethical acceptability, making them ideal candidates for repairing soft tissue defects resulting from burns or tumor resection.
The application of MenSCs also extends to cutaneous wound healing. These cells can differentiate into mature keratinocytes and express epidermal-specific markers in vitro, including keratin 14 (K14), p63, and involucrin (IVL) (Fard et al., 2018; Akhavan-Tavakoli et al., 2017). This epithelial differentiation capacity positions MenSCs as promising cell sources for the treatment of skin injuries such as burns and ulcers.
3.1.3 Treatment of chronic liver disease
MenSCs demonstrate significant potential for hepatocyte differentiation in the treatment of chronic liver diseases, suggesting a novel direction for regenerative medicine. Studies indicate that under the induction of factors such as hepatocyte growth factor (HGF) and Oncostatin M (OSM), MenSCs differentiate into hepatocyte-like cells, with the degree of differentiation showing a positive correlation with the concentrations of these factors (Malik et al., 2018). Differentiated cells express key hepatocyte markers—including albumin, tyrosine aminotransferase (TAT), and cytokeratin-18 (CK18)—at both the mRNA and protein levels and exhibit essential hepatocyte functions, such as albumin secretion, glycogen storage, and cytochrome P450 7A1 expression, thereby effectively mimicking the metabolic and detoxification functions of mature hepatocytes (Malik et al., 2018). Additionally, induced ERCs have been shown to differentiate into functional hepatocyte-like cells (Khademi et al., 2014). These findings suggest that menstrual blood-derived cells offer promising new avenues for cell-based therapies in chronic liver diseases, particularly in the regenerative treatment of hepatic fibrosis and cirrhosis.
3.1.4 Expanded therapeutic potential of MenSCs through multilineage differentiation
Multilineage-differentiating stress-enduring (Muse) cells, derived from mesenchymal stem cells (MSCs), are pluripotent cells with the capacity to differentiate into all three germ layers and exhibit enhanced resistance to environmental stress (Heneidi et al., 2013). Muse cells can evade immunological barriers, such as the pulmonary capillary network (Li et al., 2013a), and preferentially home to sites of tissue injury (Kushida et al., 2018), thereby overcoming the limitations associated with conventional MSCs in clinical applications. Li et al. (2024) successfully isolated Muse cells from MenSCs via prolonged trypsin digestion and demonstrated their significant therapeutic efficacy in animal models of acute liver injury and intracerebral hemorrhage, with superior homing ability and treatment outcomes compared with those of conventional MSCs.
Induced pluripotent stem cells (iPSCs) are pluripotent cells reprogrammed from somatic cells without relying on embryonic sources (Li et al., 2013b). iPSCs exhibit gene expression, pluripotency, and epigenetic profiles highly similar to those of embryonic stem cells (Park et al., 2008a; Ratajczak et al., 2008; Ye et al., 2009; Lengerke and Daley, 2010), providing an ethically acceptable cell resource for disease modeling, drug development, and regenerative medicine. Although iPSCs can be generated from various somatic cell sources (Takahashi et al., 2007; Yu et al., 2007; Hockemeyer et al., 2008; Huangfu et al., 2008; Lowry et al., 2008; Park et al., 2008b; Aasen et al., 2008; Kim et al., 2009; Sun et al., 2009; Li et al., 2010; Zhao et al., 2010; Zhou et al., 2011), traditional sources such as human dermal fibroblasts (HDFs) require invasive skin biopsies and prolonged in vitro expansion, limiting their practical utility. In contrast, MenSCs, owing to their noninvasive sourcing, ease of acquisition, robust proliferation, and stem cell-like phenotype (Malik et al., 2018; Patel et al., 2008), represent ideal candidates for iPSC generation.
3.2 Therapeutic potential via paracrine mechanisms
The therapeutic effects of MSCs are primarily mediated through their paracrine activity, which involves the secretion of bioactive factors—such as growth factors, chemokines, cytokines, and EVs—to modulate the local microenvironment (Pittenger et al., 2019; Fan et al., 2020; Wang et al., 2015; Seo et al., 2021; Liang et al., 2014; Chang et al., 2021; Razavi et al., 2020). With increasing research on MenSCs, the therapeutic potential of their paracrine mechanisms has been validated in various diseases. Factors secreted by MenSCs can modulate immune responses, promote tissue regeneration, inhibit inflammation and fibrosis, and enhance angiogenesis, thereby offering novel therapeutic strategies for chronic disease management and regenerative medicine.
3.2.1 Treatment of gynecological diseases
In the treatment of gynecological diseases, the paracrine activity of MenSCs has multiple therapeutic potential. The low success rate of in vitro fertilization (IVF) in older women is closely associated with abnormal reactive oxygen species (ROS) metabolism in oocytes (Mihalas et al., 2017). Studies have shown that EVs secreted by MenSCs can function as exogenous ROS scavengers, thereby reducing the dependence of embryos from aged female mice on endogenous antioxidant proteins such as superoxide dismutase 1 (SOD1) and glutathione peroxidase 1 (GPX1), ultimately improving IVF outcomes (Marinaro et al., 2018). Proteomic analyses further suggest that gene expression changes related to oxidative stress (GPX1, superoxide dismutase 1), metabolism (ACACA, GAPDH), placentation (PGF, VEGF-A), and stem cell differentiation (POU5F1, SOX2) may underlie the observed improvement in embryo quality (Marinaro et al., 2018; Marinaro et al., 2019).
In intrauterine adhesion (IUA) repair, MenSCs transplantation has been shown to accelerate endometrial regeneration. Zhang et al. (2016) reported that after being transplanted into a mouse model of endometrial injury, MenSCs activated the AKT and MAPK signaling pathways and upregulated angiogenesis-related proteins such as eNOS, VEGFA, VEGFR1, VEGFR2, and Tie2. These effects significantly shortened the repair time (complete regeneration within 7 days) and improved pregnancy rates and fetal counts (Huang et al., 2015; Lv et al., 2016). Additionally, fibroblast growth factor 2 secreted by MenSCs further promotes angiogenesis and cellular proliferation, thereby attenuating ovarian fibrosis and restoring sex hormone levels (Seo et al., 2013).
In the treatment of poor ovarian response (POR), paracrine factors from MenSCs modulate the local microenvironment to promote follicular development, as well as the differentiation of embryonic-like and ovarian stem cells. This leads to significant improvements in oocyte yield and quality, fertilization rates, embryo development rates, pregnancy rates, and live birth rates (Zafardoust et al., 2020; Rajabi et al., 2018; Bhartiya, 2018).
Moreover, the paracrine effects of MenSCs have shown their ability to inhibit epithelial ovarian cancer (EOC). Bu et al. (2016) demonstrated that MenSCs increased the proapoptotic Bax/Bcl-2 and Bad/Bcl-xL ratios, reduced the mitochondrial membrane potential, and activated the intrinsic apoptotic pathway to suppress EOC cell proliferation. Furthermore, they inhibited EOC cell cycle progression by blocking AKT phosphorylation. These findings suggest a promising strategy for the use of MenSCs in EOC therapy.
3.2.2 Treatment of liver diseases
The paracrine effects of MenSCs have significant potential in the treatment of liver diseases. In nonalcoholic fatty liver disease, the progression of which is closely associated with metabolic dysregulation—such as insulin resistance and lipid accumulation (Zhao et al., 2017)—MenSCs have been shown to modulate the expression of the key gene Rnf186 by secreting HGF. Rnf186 interferes with insulin signaling and lipid synthesis, and its abnormal expression is suppressed by the paracrine action of MenSCs, thereby alleviating metabolic disturbances (Du et al., 2023).
In models of cholestatic liver injury, MenSCs repair tissue damage via paracrine mechanisms. Yang et al. (2022) demonstrated that MenSCs significantly enhanced survival in mice while reducing the serum levels of liver injury markers (AST, ALT, ALP, DBIL). This effect is attributed to the upregulation of hepatic β-catenin, which helps to restore the integrity of tight junctions and normalize the expression of bile transport proteins (OATP2, BSEP, and NTCP1), ultimately inhibiting intrahepatic bile duct dilation, cholestasis, and the progression of fibrosis.
With respect to liver fibrosis, MenSCs secrete an array of factors that collectively form a synergistic paracrine network. For example, monocyte chemoattractant protein-1 recruits immune cells to the injured area for targeted inflammation regulation (Chen et al., 2017a), whereas Interleukin (IL)-6 and IL-8 stimulate proliferative pathways in hepatic cells and promote the recruitment of neutrophils and macrophages, thereby enhancing tissue repair (Qiu et al., 2012; Cai et al., 2008). Moreover, HGF secreted by MenSCs inhibits the activation of hepatic stellate cells (e.g., the LX-2 cell line), directly impeding fibrogenesis (Chen et al., 2017a). In addition, angiopoietin stimulates cell proliferation and survival (Li and Hu, 2012) and, together with Axl, coregulates apoptotic and proliferative signaling (D’Ar et al., 2002). IGFBP-6 prevents fibroblast apoptosis (Qiu et al., 2012; Micutkova et al., 2011), further mitigating the chronic inflammation that drives fibrosis. Collectively, these paracrine factors contribute to a therapeutic network that counteracts the progression of liver fibrosis.
For hepatocellular carcinoma (HCC) treatment, MenSCs exert their therapeutic effects through epigenetic modulation. In a time-dependent manner, their paracrine secretions can restore the levels of 5-hydroxymethylcytosine (5-hmC) and TET1 in HCC cells (Wu et al., 2019). TET1 and TET2 regulate DNA demethylation to suppress aberrant demethylation in HCC cells (Chen Q. et al., 2017; Ko et al., 2010). Upon activation by MenSCs, increased enrichment of 5-hmC and 5-mC in key enhancer regions leads to downregulation of the PI3K/AKT and RAF/ERK pathways, thereby inhibiting HCC cell proliferation and promoting apoptosis. Moreover, MenSCs modulate the DNA modification of chemoresistance-related genes, such as ID4 and HMGA1, enhancing their therapeutic efficacy against HCC(96). These findings suggest that the paracrine mechanisms of MenSCs, which involve the targeting of oncogenic gene expression through epigenetic regulation, constitute a novel therapeutic strategy for HCC.
3.2.3 Treatment of pulmonary diseases
Idiopathic pulmonary fibrosis is a chronic and progressive interstitial lung disease characterized by excessive fibrosis of the lung tissue and is associated with high morbidity and mortality rates (Blackwell et al., 2014). Although lung transplantation remains the most effective treatment, its clinical application is limited by donor scarcity, and existing pharmacological interventions provide only modest antifibrotic efficacy. Chen et al. (2020a) demonstrated that MenSCs transplantation alleviated fibrosis in bleomycin-induced Idiopathic pulmonary fibrosis models by homing to injured sites, suppressing epithelial cell apoptosis and proinflammatory cytokine (e.g., IL-1β and TNF-α) secretion, and inhibiting myofibroblast activation and collagen deposition.
ERCs also exhibit multipotent therapeutic potential in Idiopathic pulmonary fibrosis treatment (Zhao et al., 2018a). On the one hand, ERCs suppress TGF-β signaling and downregulate the proinflammatory cytokines IL-1β and TNF-α by upregulating IL-10 (Sziksz et al., 2015). They also restore total superoxide dismutase (T-SOD) activity to reduce oxidative stress and modulate apoptotic signaling via downregulation of the Bax/Bcl-2 ratio, contributing to alveolar epithelial cell protection. On the other hand, ERCs upregulate antifibrotic genes such as HGF and matrix metalloproteinase-9 (MMP-9), further inhibiting fibrosis progression.
In acute lung injury animal models, MenSCs also exhibit therapeutic efficacy (Ren et al., 2018; Xiang et al., 2017). Through paracrine signaling, MenSCs increase proliferating cell nuclear antigen (PCNA) expression to promote cell proliferation, inhibit caspase-3-mediated apoptosis to reduce cell death, increase the secretion of anti-inflammatory factors such as IL-10 and keratinocyte growth factor (KGF), and simultaneously suppress proinflammatory cytokines such as IL-1β, neutrophil infiltration, and myeloperoxidase (MPO) activity, thereby attenuating pulmonary inflammation and restoring lung function.
3.2.4 Treatment of diabetic skin wounds
Under diabetic conditions, wound healing is disrupted through multiple mechanisms, including persistent inflammation, hypoxia, cellular dysfunction, impaired angiogenesis, and neuropathy (Guo and Dipietro, 2010). The chronic nature of diabetic wounds is closely associated with dysregulated inflammatory phase transitions. While inflammation is essential for initiating tissue repair (Aller et al., 2004), excessive activation of proinflammatory macrophages (M1) and the resulting prolonged inflammatory state significantly impede healing (Landén et al., 2016). The phenotypic switch from M1 to reparative macrophages (M2) is critical for tissue regeneration (Finley et al., 2016; Zhang et al., 2010; Emin et al., 2007; Murray et al., 2014); however, in the diabetic microenvironment, downregulation of M2-associated genes (e.g., Arg-1) leads to impaired polarization (Finley et al., 2016; Schwab et al., 2007). MenSC-Exos promote M2 polarization by modulating inducible nitric oxide synthase activity and the ARG: inducible nitric oxide synthase ratio, with a sustained increase in the M2/M1 ratio observed between days 7–14 posttreatment (Dalirfardouei et al., 2019; Mirzadegan et al., 2022).
Macrophage function is also closely linked to neural regeneration. As the primary source of neuroprotectin D1 (NPD1), M2 macrophages may facilitate axonal regeneration and contribute to wound repair (C et al., 2013; Hong et al., 2014). Mirzadegan et al. (Mirzadegan et al., 2022) demonstrated that MenSC intervention significantly increased the density of protein-gene product 9.5 (PGP9.5)-positive nerve fibers in diabetic wounds, suggesting the potential for reversing cutaneous neuropathy. At the molecular level, MenSC-Exos activate the NF-κB signaling pathway to increase keratinocyte proliferation and differentiation (Dalirfardouei et al., 2019; Brantley et al., 2001). Additionally, through interactions with the Notch pathway (Na et al., 2017; Shi et al., 2015) and Jumonji domain-containing protein D3, they may regulate epigenetic remodeling in keratinocytes (Na et al., 2016), thereby promoting migration and re-epithelialization.
The balance of collagen metabolism is crucial for optimal wound healing. MenSC-Exos induce the expression of type I collagen and type III collagen mRNAs, initially promoting type III collagen synthesis to support granulation tissue formation (Volk et al., 2011). At later stages, they reduce the type I collagen/type III collagen ratio to suppress scar hyperplasia (Yates et al., 2012; Cuttle et al., 2005; Larson et al., 2010; Zhang et al., 2015).
In terms of angiogenesis, MenSC-Exos dose-dependently upregulated key angiogenic markers, including vascular endothelial growth factor A (VEGFA), CD31, and von Willebrand factor (vWF), with 10 μg of MenSC-Exos demonstrating tenfold greater efficacy than exosomes from other stem cell sources (Dalirfardouei et al., 2019; Mirzadegan et al., 2022; Hu et al., 2016; Liang et al., 2016).
Importantly, although MenSCs exhibit strong migratory capacity due to high CXCR4 expression (Luz-Crawford et al., 2016), dysfunctional fibroblasts in diabetic wounds often fail to produce stromal cell-derived factor-1, thereby compromising MenSC homing efficiency (Mirzadegan et al., 2022; Brem et al., 2007). This paradox underscores the critical role of the local microenvironment in modulating stem cell-based therapies.
3.2.5 Treatment of central nervous system disorders
Spinal cord injury (SCI), characterized by sensory and motor dysfunction, continues to pose major clinical challenges because of the limited therapeutic efficacy of current treatment paradigms (Kunte et al., 2015). Recent studies have demonstrated that MenSCs transplantation promotes neural repair via multiple mechanisms. Wu et al. (2018) confirmed that MenSCs increase the expression of brain-derived neurotrophic factor, thereby supporting neuronal survival and axonal regeneration (Uchida et al., 2016). Additionally, MenSCs upregulate mature neuronal markers such as neurofilament-200 (NF-200) and microtubule-associated protein-2 (MAP-2), facilitating structural reconstruction of neural tissue (Li et al., 2013a). MenSCs also contribute to neural repair by attenuating glial scar formation, as evidenced by the reduced expression of inhibitory molecules such as chondroitin sulfate proteoglycans (CSPGs) (Anderson et al., 2016; Fan et al., 2017). Concurrently, MenSCs suppress the production of proinflammatory cytokines, including TNF-α and IL-1β, thereby alleviating neuroinflammation. In animal models, local injection of MenSCs significantly improved hindlimb motor function in rats with SCI(131).
Hjazi and colleagues (Hjazi et al., 2024) further advanced this therapeutic approach by combining MenSC-Exos with hyperbaric oxygen therapy (HBOT) in a model of traumatic SCI (TSCI). While MenSC-Exos exert anti-inflammatory and antiapoptotic effects (Dalirfardouei et al., 2019), HBOT reduces neuronal apoptosis by downregulating the expression of proinflammatory cytokines (e.g., IL-1β and TNF-α) (Ghaemi et al., 2023; Zhao et al., 2021). The combined therapy synergistically regulated apoptosis, oxidative stress, and inflammation, resulting in improved histopathological outcomes and functional recovery.
Alzheimer’s disease, the most common type of dementia, is pathologically defined by amyloid-beta (Aβ) plaques and neurofibrillary tangles (NFTs). Aβ originates from proteolytic cleavage of the transmembrane amyloid precursor protein (APP), whereas NFTs consist of hyperphosphorylated and misfolded tau proteins (Ballard et al., 2011). Zhao et al. (2018b) reported that MenSCs transplantation reduced Aβ deposition in the hippocampus and cortex, potentially by regulating aberrant APP processing, enhancing Aβ clearance by microglia, and suppressing tau hyperphosphorylation. Moreover, MenSCs promoted the transformation of microglia into a neuroprotective phenotype, facilitating anti-inflammatory cytokine secretion and markedly improving cognitive deficits in APP/PS1 transgenic mice.
Notably, Lopez-Verrilli et al. (2016) reported a biphasic effect of MenSCs on neurite outgrowth. While direct contact between MenSCs and neurons inhibited neurite elongation, their secreted factors—including VEGF, HGF, and brain-derived neurotrophic factor—promoted axonal extension. Further investigation revealed that the exosomal fractions of MenSC-EVs promoted neurite outgrowth, whereas the microvesicle fractions had inhibitory effects. This functional heterogeneity provides new insights into the application of MenSCs in treating neurodegenerative diseases.
3.2.6 Treatment of other systemic diseases
Myocardial infarction (MI), an acute cardiovascular emergency, is primarily treated by curtailing the progression of myocardial necrosis and promoting functional recovery (Lu et al., 2015). Wang et al. (2017) reported that extracellular vesicles derived from MenSC-EVs regulate the PTEN/Akt pathway through high expression of miR-21. This mechanism both inhibits cardiomyocyte apoptosis and promotes angiogenesis, thereby significantly improving cardiac function in infarcted rat models. This discovery provides a novel strategy for myocardial regeneration therapy.
In the field of prostate cancer (PC) treatment, Alcayaga-Miranda et al. (2016) revealed that MenSC-Exos reduce tumor cell ROS levels and downregulate the expression of vascular endothelial growth factor (VEGF) and hypoxia-inducible factor-1α (HIF-1α). This disrupts the tumor’s hypoxic adaptation, thereby inhibiting tumor hemoglobin biosynthesis and neovascularization, which contributes to an antitumor effect. Such microenvironmental modulation offers a new avenue for targeted therapy in solid tumors.
With respect to ulcerative colitis (UC), (Malik et al., 2018) demonstrated that ERCs exert anti-inflammatory effects by rebalancing cytokine profiles—reducing IL-2 and TNF-α while increasing IL-4 and IL-10 levels. Further research has shown that preconditioning with stromal cell-derived factor-1 enhances CXCR4 expression in ERCs, promoting M2 macrophage polarization and Treg cell generation (Li et al., 2019); prestimulation with IL-1β inhibits the DKK1 protein and activates the Wnt/β-catenin pathway, thereby strengthening their immunomodulatory function (Yu et al., 2021); and melatonin preconditioning enhances ERC efficacy via antioxidant protection, significantly lowering both the disease activity index and tissue damage scores (Hao et al., 2024).
In the treatment of type 1 diabetes mellitus (T1DM), (Malik et al., 2018) confirmed that intravenous infusion of MenSCs results in targeted migration to the injured pancreas. This migration activates Ngn3-positive endocrine progenitor cells, facilitating β-cell regeneration, effectively reversing hyperglycemia, increasing insulin secretion, and restoring islet architecture. This finding provides a potential solution to the shortage of islet donors.
In sepsis treatment research, (Alcayaga-Miranda et al., 2015a) reported that MenSCs enhance host defense by increasing the expression of antimicrobial peptides and hepcidin. When combined with antibiotics, this approach significantly improves bacterial clearance and survival. Notably, MenSC-Exos exert a protective effect against LPS-induced liver injury by modulating abnormal natural killer (NK) cell activation (Chen et al., 2017c), thereby reducing inflammatory organ damage and demonstrating multitarget therapeutic potential.
3.2.7 Molecular mediators and signal transduction cascades
MenSCs exert reparative effects via the paracrine release of small extracellular vesicles (sEVs), which mediate multiple signaling pathways. miRNA profiling of exosomes has identified the enrichment of key microRNAs (miRNAs), such as miR-21 and miR-lethal-7 (let-7), in MenSC-derived sEVs. miR-21 enhances cell survival and improves cardiac function by targeting PTEN and activating the AKT/PKB signaling cascade (Wang et al., 2017). Let-7 modulates the NLRP3 pathway, enhancing the inhibition of ROS production and mitochondrial DNA damage via suppression of lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1), thereby facilitating the repair of alveolar epithelial cells (Sun et al., 2019). In pancreatic regeneration, sEVs activate the PDX-1 signaling axis to promote β-cell regeneration and insulin secretion (Mahdipour et al., 2019).
In terms of immune regulation, MenSC-Exo induce phosphorylation of STAT3/STAT6, driving M2 macrophage polarization (Liu et al., 2017). Similarly, CD73 present in exosomes from endometrial regenerative cells promotes M2 polarization through a MAPK-dependent hydrolysis of ATP, thereby contributing to renal protection (Shao et al., 2025).
Regarding metabolic regulation, hepatocyte growth factor (HGF) secreted by MenSCs downregulates hepatic Rnf186 expression and modulates the AMPK-mTOR signaling pathway, improving glucose and lipid metabolism. In addition, HGF upregulates β-catenin, contributing to the repair of tight junctions and the expression of bile transporters (OATP2, BSEP, NTCP1), thereby attenuating the progression of hepatic fibrosis (Du et al., 2023; Yang et al., 2022).
At the epigenetic level, MenSCs restore the expression of TET1/2 and 5-hmC levels in hepatocellular carcinoma cells in a time-dependent manner. By modulating the 5-hmC/5-methylcytosine enrichment at enhancer regions of PI3K/AKT and RAF/ERK pathway target genes, MenSCs relieve repression of FOXO3, subsequently promoting downstream apoptotic processes (Wu et al., 2019).
3.3 Therapeutic effects mediated by immunomodulation
MenSCs exhibit unique advantages in the field of immunotherapy. Like BM-MSCs, MenSCs express low levels of HLA-B and HLA-C and lack HLA-DR expression, indicating potential immune privilege properties (Luz-Crawford et al., 2016). However, further investigations revealed distinct immunoregulatory features compared with those of BM-MSCs. Notably, MenSCs express lower levels of the Toll-like receptors TLR3 and TLR4 and exhibit weaker suppression of T cell subsets under low-concentration conditions (Luz-Crawford et al., 2016). In specific microenvironments, they may even stimulate lymphocyte proliferation (Nikoo et al., 2012). These distinctive immunomodulatory properties offer an important theoretical basis for the development of novel immunotherapeutic strategies.
3.3.1 Effects on tissue repair
ERCs demonstrate multifaceted immunomodulatory capabilities during tissue repair. Studies have shown that ERCs can induce the polarization of M2 macrophages, tolerogenic dendritic cells, and regulatory T and B cells both in vivo and in vitro. They also significantly suppress IgG and IgM antibody deposition and infiltration of CD4+ and CD8+ T cells. These immunoregulatory effects are closely associated with stromal cell-derived factor-1–mediated immunosuppressive mechanisms (Xu et al., 2017).
3.3.2 Regulation of immune cell function
MenSCs exert notable regulatory effects on various immune cell populations. In reproductive immunology, uterine NK cells play essential roles in early pregnancy tolerance through killer cell immunoglobulin-like receptors (Male and Moffett, 2023). Shokri et al. reported that IFN-γ- and IL-1β-pretreated MenSCs inhibited NK cell cytotoxicity via the IL-6 and TGF-β pathways, thereby reducing perforin and granzyme production and promoting an immune-tolerant microenvironment conducive to pregnancy (de Pedro et al., 2021). Additionally, Hosseini et al. reported a significant decrease in TCRγδ cell proportions in the menstrual blood of patients with recurrent spontaneous abortion, suggesting a possible link to pregnancy-related immune dysregulation (Hosseini et al., 2016). With respect to dendritic cells, Bozorgmehr and colleagues demonstrated that MenSCs dose-dependently suppress monocyte differentiation into immature dendritic cells, possibly through high secretion levels of IL-6 and IL-10 (Bozorgmehr et al., 2014). In antitumor immunity, menstrual blood-derived immune cells also show substantial potential. Qin et al. isolated DC-CIK cells from the menstrual blood of ovarian cancer patients and reported that these cells inhibited ovarian cancer stem cells by activating the TNFR1–ASK1–AIP1–JNK signaling pathway (Qin et al., 2018).
3.3.3 Involvement of cytokines and signaling pathways
Although MenSCs lack HLA-DR expression, limiting their antigen-presenting capacity, they exert immunoregulatory effects through the secretion of various paracrine molecules, including IL-6, TGF-β, COX-2, PGE2, indoleamine 2,3-dioxygenase, and programmed death ligand-1 (Malik et al., 2018; Luz-Crawford et al., 2016; Cuenca et al., 2018). Proteomic analysis revealed high expression levels of adhesion molecules such as activated leukocyte cell adhesion molecule and intercellular adhesion molecule-1 in MenSCs (Liu et al., 2018). These molecules interact with receptors such as LFA-1, directly participating in immune cell activation and regulation, and play critical roles in the immunomodulatory functions of MenSCs. Notably, MenSCs also secrete multiple angiogenic factors, including VEGF, HGF, angiogenin, and MMP-1, which contribute to both tissue regeneration and immune regulation.
4 Investigation of disease pathogenesis and therapeutic mechanisms
The pathogenesis of endometriosis remains incompletely understood; however, analysis of MenSCs from affected individuals has provided novel insights into its underlying mechanisms. Transcriptomic studies have shown significantly elevated levels of miR-200b-3p in MenSCs from patients with endometriosis (de Oliveira et al., 2022). This microRNA, which is commonly associated with tumor progression, regulates key cellular processes such as motility, proliferation, migration, and differentiation (Humphries and Yang, 2015). By suppressing the transcription factor ZEB1, miR-200b-3p reverses epithelial–mesenchymal transition, induces mesenchymal–epithelial transition, and modulates angiogenesis by targeting VEGF and epidermal growth factor receptor 2 (Wellner et al., 2009; Yang et al., 2016). These mechanisms may contribute to the proliferation, maintenance of stemness, and mesenchymal–epithelial transition of endometrial cells transported by retrograde menstruation, thereby promoting lesion formation and persistence.
The application of high-throughput technologies has further advanced our understanding of the molecular pathophysiology of endometriosis (Meola et al., 2010). However, these approaches require stable reference genes for accurate data interpretation. Zucherato et al. (2021) identified EIF2B1 and POP4 as the most stable reference genes in MenSCs derived from menstrual blood, whereas GAPDH and ACTB were found to be less reliable, providing an essential foundation for future experimental designs. Moreover, MenSCs from patients presented higher expression levels of the surface markers CD9, CD10, and CD29 than those from healthy individuals did, along with increased proliferative and invasive capacities (Nikoo et al., 2014). When cocultured with PB mononuclear cells, these MenSCs presented increased expression of indoleamine 2,3-dioxygenase-1 and COX-2, as well as elevated secretion of proinflammatory cytokines such as IFN-γ, IL-10, and monocyte chemoattractant protein-1, suggesting that MenSCs may contribute to disease progression through immunomodulation and altered cellular behavior (Nikoo et al., 2014).
High mobility group box-1 (HMGB1) also plays a critical role in endometriosis. HMGB1, a nonhistone nuclear protein abundant in mammalian chromatin, is involved in DNA transcription, repair, replication, and remodeling (Liu et al., 2010). Elevated HMGB1 levels have been detected in the blood of patients, where it interacts with receptors such as RAGE and TLR4, forming complexes with IL-1β or LPS to activate proinflammatory signaling pathways (Shimizu et al., 2017). Additionally, HMGB1 promotes endothelial cell proliferation and the release of angiogenic factors such as VEGF, thereby facilitating the implantation and survival of ectopic endometrial cells (Shimizu et al., 2017). Integrative omics analyses by Penariol et al. (2022) identified key genes (e.g., ATF3, ID1, ID3, and FOSB) and proteins (e.g., MT2A and COL1A1) involved in disease development. The chronic inflammatory microenvironment may disrupt MenSCs function, leading to dysregulation of gene and protein expression and contributing to the progression of endometriosis (Penariol et al., 2022).
In therapeutic investigations, the natural flavonoid components of Citrus latifolia have been utilized for their anti-inflammatory properties to alleviate dysmenorrhea and menorrhagia. Robeldo et al. (2020) demonstrated that Citrus latifolia increases the production of prostaglandin F2α, which acts on prostaglandin receptors to reduce the menstrual volume and inhibits the release of proinflammatory cytokines such as TNF-α (Ekström et al., 1991). This mechanism effectively blocks inflammatory signaling and nociceptive sensitization. Compared with nonsteroidal anti-inflammatory drugs, this approach is particularly effective in patients with menstrual disorders associated with insufficient prostaglandin F2α secretion (Robeldo et al., 2020).
5 Comparison with other mesenchymal stem cells
Phenotypically, MenSCs share similar surface markers with other mesenchymal stem cells (MSCs), such as CD10, CD29, CD44, CD73, CD90, and CD105 (Cordeiro et al., 2022; Margiana et al., 2022), and possess multilineage differentiation potential. However, MenSCs display a significantly faster proliferation rate, with a population doubling time of approximately 19.4 hours—roughly twice as fast as that of bone marrow-derived MSCs (BM-MSCs) (Skliutė et al., 2021). Functionally, their colony-forming unit-fibroblast frequency is 2–4 times higher than that of BM-MSCs, and their in vitro migratory ability is also superior.
In terms of paracrine activity, MenSCs secrete higher levels of VEGF and basic fibroblast growth factor, leading to enhanced angiogenesis. Notably, MenSCs induce hemoglobin production at levels 8-fold greater than BM-MSCs. They also exhibit superior feeder layer characteristics, supporting the expansion and maintenance of CD34+CD133+ hepatic stellate cells. MenSCs promote a 3-fold increase in cell expansion and generate more CFU-GEMM colonies (Alcayaga-Miranda et al., 2015b). By secreting growth factors and extracellular matrix proteins, MenSCs contribute to reconstructing the bone marrow microenvironment and support the ex vivo expansion of hematopoietic stem cells (Khoury et al., 2011).
Comparative studies in therapeutic applications also highlight MenSCs’ advantages (Wang et al., 2017). evaluated the effects of intramyocardial injection of BM-MSCs, adipose-derived MSCs, and MenSCs in a murine MI model. MenSCs exhibited superior cardioprotective effects and increased microvascular density, attributed to high miR-21 levels in their exosomes that activate the PTEN/Akt signaling pathway (Lopez-Verrilli et al., 2016). compared the effects of MenSCs, BM-MSCs, umbilical cord, and chorionic MSCs on cortical neurite outgrowth. MenSC-derived exosomes showed the strongest neurite-promoting effect, whereas their microvesicles inhibited neurite extension, suggesting functional specificity among extracellular vesicle subtypes. In liver regeneration, Fathi-Kazerooni et al. (Malik et al., 2018) demonstrated that MenSC-derived hepatocyte progenitor-like cells more effectively improved liver injury markers compared to BM-MSCs, with greater reductions in inflammation, necrosis, and collagen deposition. Mechanistically, this was linked to stronger anti-inflammatory effects exerted by MenSCs.
6 Comparison of menstrual blood and peripheral blood
In studies directly comparing MB and PB, (Iribarne-Durán et al., 2020) reported that MB samples contain substantial levels of endocrine-disrupting chemicals, notably parabens and benzophenones. These endocrine-disrupting chemicals concentrations are correlated with specific sociodemographic and lifestyle factors, such as the use of cosmetics, skin oils/creams, and hair dyes (Jiménez-Díaz et al., 2016). Importantly, MB levels of parabens and benzophenones were found to be lower than those measured in PB serum (Matsumoto et al., 2007), providing evidence for a blood–uterine barrier: a physical and/or metabolic partition that may restrict certain compounds from reaching the endometrium, potentially via glucuronidation-mediated metabolism and increased excretion of endocrine-disrupting chemicals. Further research revealed marked differences in immune cell composition between MB and PB. Hosseini et al. (2019) demonstrated that the frequencies of NKT cell subsets and their associated cytokine profiles differ significantly between MB and PB in fertile women, infertile women, and those with unexplained recurrent spontaneous abortion. This finding underscores the profound influence of the endometrial immune microenvironment on the cellular makeup of MB. Together, these studies not only validate MB as a promising biomarker for assessing endocrine-disrupting chemicals exposure and its link to gynecological conditions but also highlight its unique utility in probing local uterine immune mechanisms.
7 Current status of clinical trial research
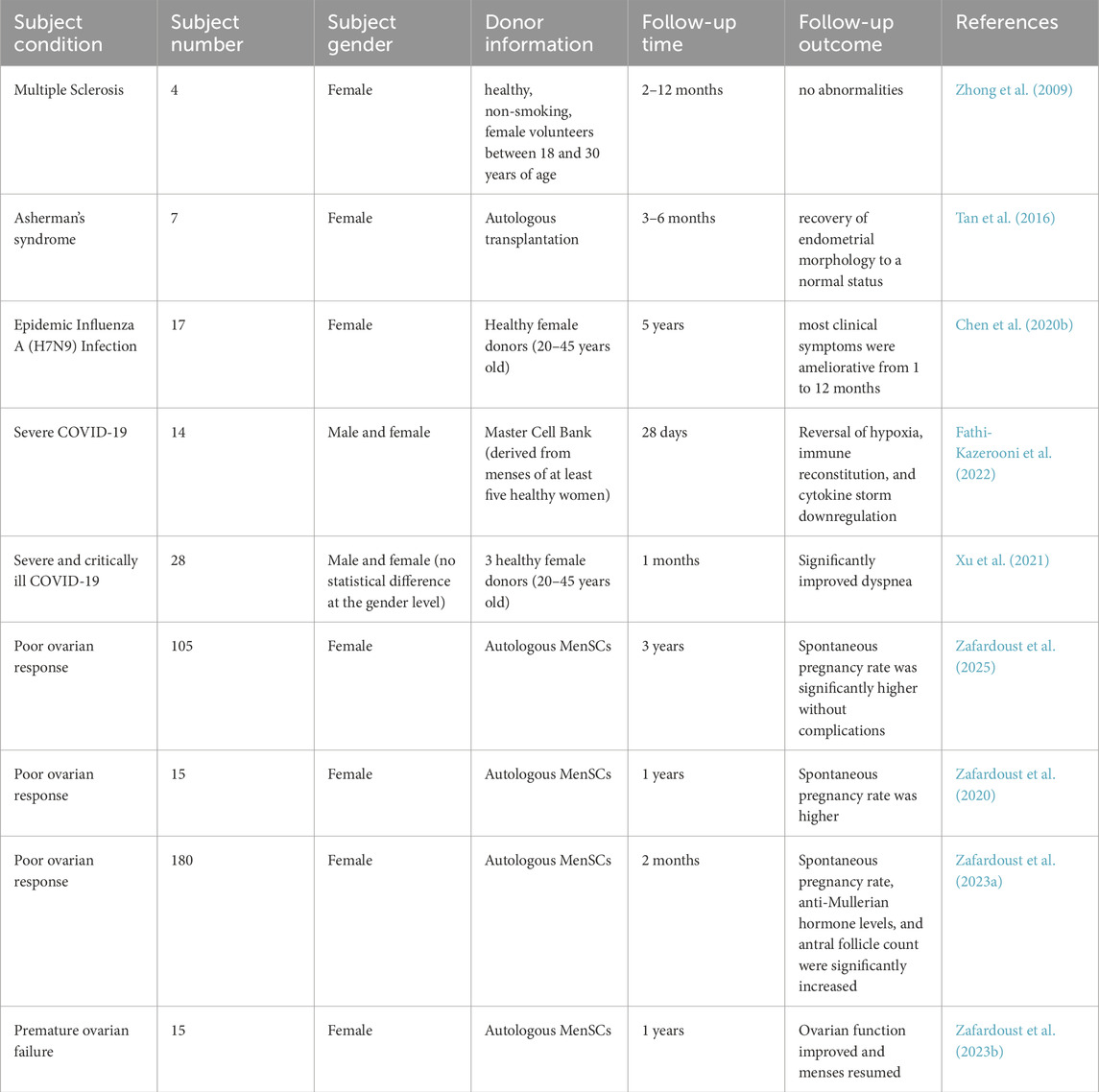
Although preclinical studies in cellular and animal models have confirmed the safety and efficacy of MenSC transplantation, the clinical application of MenSCs remains limited compared to other stem cell sources. This is primarily due to the restricted availability of menstrual blood donors and the lack of standardized in vitro culture protocols. To date, clinical trials involving MenSCs have primarily evaluated their therapeutic effects in diseases such as multiple sclerosis, H7N9 influenza virus infection, severe COVID-19, Asherman’s syndrome, and ovarian insufficiency (Zhong et al., 2009; Zafardoust et al., 2020; Tan et al., 2016; Chen J. et al., 2020; Fathi-Kazerooni et al., 2022; Xu et al., 2021; Zafardoust et al., 2025; Zafardoust et al., 2023a; Zafardoust et al., 2023b). The results indicate that MenSC transplantation leads to marked clinical improvement in these conditions, with no serious adverse events or complications observed, underscoring the potential value of MenSCs in clinical applications.
In the context of ovarian insufficiency, the team led by Zafardoust has conducted long-term follow-up studies to assess the efficacy and potential complications of autologous MenSC therapy. Their clinical trial demonstrated that autologous MenSC transplantation can increase the rate of natural conception, improve ovarian function, and restore regular menstrual cycles. Importantly, no serious complications—such as endometriosis, ovarian malignancies, or autoimmune diseases—were reported, suggesting that autologous MenSC transplantation represents a safe and promising approach for improving reproductive outcomes in women with ovarian insufficiency (Table 1).
However, current clinical research on MenSCs still faces limitations. Most studies involve small sample sizes and short follow-up durations, which weakens the statistical power for evaluating long-term safety despite supporting evidence from basic research. Therefore, large-scale, multicenter clinical trials are necessary to comprehensively assess the efficacy and safety of MenSC-based therapies. In addition, the majority of enrolled participants have been female, and only the study by Xu et al. (2021) has conducted sex-stratified analyses. Given the female-specific origin of MenSCs, sex-based stratification should be considered an essential component of future clinical investigations.
8 Conclusion
This review provides a comprehensive overview of the potential of MB in both basic research and clinical applications, with a particular emphasis on its innovative roles in disease diagnosis and therapy. As a biologically rich fluid containing diverse cellular and molecular constituents, MB offers a non-invasive, highly acceptable platform for investigating women’s reproductive health and pathology. In the therapeutic arena, MenSCs have emerged as a key resource in regenerative medicine owing to their pluripotency, low immunogenicity, and ease of procurement. Through mechanisms of direct differentiation, paracrine signaling, and immune modulation, MenSCs have demonstrated promising efficacy in preclinical models and early clinical studies.
9 Limitations
Nonetheless, the clinical translation of MenSC-based therapies faces several critical challenges—including in vivo cell tracking post transplantation, donor-related variability in therapeutic outcomes, the establishment of robust quality control and standardization protocols, and the need for long-term safety data. In addition, unresolved ethical and sociocultural considerations remain. Although the use of menstrual blood (MB) as a research sample raises fewer ethical concerns compared to embryonic or fetal tissues, ethical guidelines for MB collection are still evolving.
Moreover, cultural norms, religious beliefs, and disparities in education continue to pose barriers to MB donation, especially in low- and middle-income countries. In Islamic jurisprudence, menstruation is considered a state of ritual impurity, which may discourage donation. Similarly, in Hindu traditions, menstrual blood is sometimes perceived as carrying “negative energy,” potentially reducing donor willingness. In patriarchal societies, it is not uncommon for women to require spousal consent to donate biological materials, thereby limiting female autonomy. Trust may also be compromised when research teams are male-dominated, as some women may feel uncomfortable discussing menstrual health issues with male investigators.
10 Future directions
Despite these hurdles, ongoing technological and scientific advances are steadily unveiling the unique bioactive components and mechanisms of action of MB. To better preserve the three-dimensional architecture of the native extracellular matrix (ECM) and replicate the microenvironment required for cell proliferation and differentiation, scaffold-based technologies have been employed to facilitate the directed differentiation of MenSCs. Polylactic acid and multi-walled carbon nanotubes, as promising biomaterials, have been incorporated into scaffold designs due to their favorable mechanical properties and biocompatibility, enhancing MenSC differentiation toward germ cell lineages (Eyni et al., 2017).
A bilayer scaffold composed of amniotic membrane and silk fibroin has shown therapeutic potential in wound healing and skin regeneration. This scaffold mimics the skin ECM by providing optimized mechanical strength and 3D structure, significantly improving the regenerative capacity of MenSCs. On an immunomodulatory level, anti-inflammatory factors secreted by the amniotic membrane (e.g., SLPI/elafin) synergize with MenSC-derived paracrine cytokines such as IDO, PGE2, and TGF-β, promoting the polarization of macrophages from the pro-inflammatory M1 phenotype to the regenerative M2 phenotype. This scaffold system has also been shown to induce MenSC differentiation into keratinocyte-like cells (Mirzadegan et al., 2022), while upregulating VEGFA to activate CD34+ endothelial cells and stimulate angiogenesis. Concurrently, restoration of the SDF-1/CXCR4 signaling axis improves endothelial progenitor cell homing (Mirzadegan et al., 2020). In vivo studies in diabetic mouse models have demonstrated enhanced epidermal thickness and type I collagen synthesis following treatment, resulting in effective wound regeneration and healing (Araste et al., 2020).
Further translational progress has been made through large animal studies. For instance, (Emmerson et al., 2019) investigated the integration of endometrial MSCs with synthetic meshes in a sheep model of POP. Their findings showed that autologous eMSCs improved mesh biocompatibility and restored vaginal tissue strength, while exhibiting long-term survival in vivo and contributing to immune modulation, ECM remodeling, and fibrosis regulation.In parallel, organoid technologies are emerging as essential tools to study the menstrual cycle and endometrial diseases. Wiwatpanit et al. (2020) developed a scaffold-free endometrial organoid model consisting of epithelial and stromal components that closely mimics the physiology of native endometrium. This model provides a versatile 3D system to investigate hormone-induced pathological changes in the endometrium. Such insights are poised to drive the broader application of MB in regenerative and immune-based therapies, offering novel strategies to address persistent clinical needs.
Overall, MB represents a novel and promising tool for both disease diagnosis and therapeutic intervention. Its emerging applications in regenerative medicine and immunotherapy hold significant potential to address unresolved clinical challenges and advance the development of precision medicine.
Author contributions
YF: Writing – original draft, Conceptualization, Writing – review and editing. YH: Writing – review and editing, Supervision, Formal Analysis.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
MB, Menstrual Blood; MenSCs, Menstrual blood-derived stem cells; PB, Peripheral Blood; HPV, Human papillomavirus; CIN, Cervical intraepithelial neoplasia; NGS, Next-generation sequencing; TGF-β, Transforming growth factor-beta; BM-MSCs, Bone marrow-derived mesenchymal stem cells; POI, Premature ovarian insufficiency; FSH, Follicle-stimulating hormone; IUA, Intrauterine adhesion; VEGF, Vascular endothelial growth factor; HGF, Hepatocyte growth factor; EVs, Extracellular vesicles; ROS, Reactive oxygen species; IL, Interleukin; HCC, Hepatocellular carcinoma; MenSC-Exos, Menstrual blood-derived stem cell exosomes; Aβ, Amyloid-beta; NK, Natural killer; TNF-α, Tumor necrosis factor alpha; HMGB1, High mobility group box-1.
References
Aasen, T., Raya, A., Barrero, M. J., Garreta, E., Consiglio, A., Gonzalez, F., et al. (2008). Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat. Biotechnol. 26 (11), 1276–1284. doi:10.1038/nbt.1503
Akhavan-Tavakoli, M., Fard, M., Khanjani, S., Zare, S., Edalatkhah, H., Mehrabani, D., et al. (2017). In vitro differentiation of menstrual blood stem cells into keratinocytes: a potential approach for management of wound healing. Biol. J. Int. Assoc. Biol. Stand 48, 66–73. doi:10.1016/j.biologicals.2017.05.005
Alcayaga-Miranda, F., Cuenca, J., Martin, A., Contreras, L., Figueroa, F. E., and Khoury, M. (2015a). Combination therapy of menstrual derived mesenchymal stem cells and antibiotics ameliorates survival in sepsis. Stem Cell. Res. Ther. 6, 199. doi:10.1186/s13287-015-0192-0
Alcayaga-Miranda, F., Cuenca, J., Luz-Crawford, P., Aguila-Díaz, C., Fernandez, A., Figueroa, F. E., et al. (2015b). Characterization of menstrual stem cells: angiogenic effect, migration and hematopoietic stem cell support in comparison with bone marrow mesenchymal stem cells. Stem Cell. Res. Ther. 6 (1), 32. doi:10.1186/s13287-015-0013-5
Alcayaga-Miranda, F., González, P. L., Lopez-Verrilli, A., Varas-Godoy, M., Aguila-Díaz, C., Contreras, L., et al. (2016). Prostate tumor-induced angiogenesis is blocked by exosomes derived from menstrual stem cells through the inhibition of reactive oxygen species. Oncotarget 7 (28), 44462–44477. doi:10.18632/oncotarget.9852
Aliyu, M. H., Aliyu, S. H., and Salihu, H. M. (2004). Female genital tuberculosis: a global review. Int. J. Fertil. Womens Med. 49 (3), 123–136. doi:10.1093/humupd/dmh018
Aller, M. A., Arias, J. L., Nava, M. P., and Arias, J. (2004). Posttraumatic inflammation is a complex response based on the pathological expression of the nervous, immune, and endocrine functional systems. Exp. Biol. Med. Maywood N. J. 229 (2), 170–181. doi:10.1177/153537020422900206
Anderson, M. A., Burda, J. E., Ren, Y., Ao, Y., O’Shea, T. M., Kawaguchi, R., et al. (2016). Astrocyte scar formation aids central nervous system axon regeneration. Nature 532 (7598), 195–200. doi:10.1038/nature17623
Arasteh, S., Khanjani, S., Golshahi, H., Mobini, S., Jahed, M. T., Heidari-Vala, H., et al. (2020). Efficient wound healing using a synthetic nanofibrous bilayer skin substitute in murine model. J. Surg. Res. 245, 31–44. doi:10.1016/j.jss.2019.07.017
Ballard, C., Gauthier, S., Corbett, A., Brayne, C., Aarsland, D., and Jones, E. (2011). Alzheimer’s disease. Lancet Lond Engl. 377 (9770), 1019–1031. doi:10.1016/S0140-6736(10)61349-9
Ballweg, M. L. (2004). Impact of endometriosis on women’s health: comparative historical data show that the earlier the onset, the more severe the disease. Best. Pract. Res. Clin. Obstet. Gynaecol. 18 (2), 201–218. doi:10.1016/j.bpobgyn.2004.01.003
Bhartiya, D. (2018). Stem cells survive oncotherapy and can regenerate non-functional gonads: a paradigm shift for oncofertility. Indian J. Med. Res. 148 (Suppl. l), S38-S49–49. doi:10.4103/ijmr.IJMR_2065_17
Blackwell, T. S., Tager, A. M., Borok, Z., Moore, B. B., Schwartz, D. A., Anstrom, K. J., et al. (2014). Future directions in idiopathic pulmonary fibrosis research. An NHLBI workshop report. Am. J. Respir. Crit. Care Med. 189 (2), 214–222. doi:10.1164/rccm.201306-1141WS
Bockeria, L., Bogin, V., Bockeria, O., Le, T., Alekyan, B., Woods, E. J., et al. (2013). Endometrial regenerative cells for treatment of heart failure: a new stem cell enters the clinic. J. Transl. Med. 11, 56. doi:10.1186/1479-5876-11-56
Bose, M. (2011). Female genital tract tuberculosis: how long will it elude diagnosis? Indian J. Med. Res. 134 (1), 13–14. doi:10.2217/IMT.11.80
Bozorgmehr, M., Moazzeni, S. M., Salehnia, M., Sheikhian, A., Nikoo, S., and Zarnani, A. H. (2014). Menstrual blood-derived stromal stem cells inhibit optimal generation and maturation of human monocyte-derived dendritic cells. Immunol. Lett. 162 (2 Pt B), 239–246. doi:10.1016/j.imlet.2014.10.005
Brantley, D. M., Chen, C. L., Muraoka, R. S., Bushdid, P. B., Bradberry, J. L., Kittrell, F., et al. (2001). Nuclear factor-kappaB (NF-kappaB) regulates proliferation and branching in mouse mammary epithelium. Mol. Biol. Cell. 12 (5), 1445–1455. doi:10.1091/mbc.12.5.1445
Brem, H., Stojadinovic, O., Diegelmann, R. F., Entero, H., Lee, B., Pastar, I., et al. (2007). Molecular markers in patients with chronic wounds to guide surgical debridement. Mol. Med. Camb Mass 13 (1–2), 30–39. doi:10.2119/2006-00054.Brem
Bu, S., Wang, Q., Zhang, Q., Sun, J., He, B., Xiang, C., et al. (2016). Human endometrial mesenchymal stem cells exhibit intrinsic anti-tumor properties on human epithelial ovarian cancer cells. Sci. Rep. 6, 37019. doi:10.1038/srep37019
Budukh, A., Palayekar, V., Maheshwari, A., Deodhar, K., Purwar, P., Bagal, S., et al. (2018). Menstrual pad, a cervical cancer screening tool, a population-based study in rural India. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ ECP 27 (6), 546–552. doi:10.1097/CEJ.0000000000000387
Burd, E. M. (2003). Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 16 (1), 1–17. doi:10.1128/cmr.16.1.1-17.2003
Cheng, C., Singh, V., Krishnan, A., Kan, M., Martinez, J. A., and Zochodne, D. W. (2013). Loss of innervation and axon plasticity accompanies impaired diabetic wound healing. PloS One 8 (9), e75877. doi:10.1371/journal.pone.0075877
Cai, J., Kehoe, O., Smith, G. M., Hykin, P., and Boulton, M. E. (2008). The angiopoietin/Tie-2 system regulates pericyte survival and recruitment in diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 49 (5), 2163–2171. doi:10.1167/iovs.07-1206
Chang, C., Yan, J., Yao, Z., Zhang, C., Li, X., and Mao, H. Q. (2021). Effects of mesenchymal stem cell-derived paracrine signals and their delivery strategies. Adv. Healthc. Mater 10 (7), e2001689. doi:10.1002/adhm.202001689
Chen, X., Kong, X., Liu, D., Gao, P., Zhang, Y., Li, P., et al. (2016). In vitro differentiation of endometrial regenerative cells into smooth muscle cells: Α potential approach for the management of pelvic organ prolapse. Int. J. Mol. Med. 38 (1), 95–104. doi:10.3892/ijmm.2016.2593
Chen, L., Zhang, C., Chen, L., Wang, X., Xiang, B., Wu, X., et al. (2017a). Human menstrual blood-derived stem cells ameliorate liver fibrosis in mice by targeting hepatic stellate cells via paracrine mediators. Stem Cells Transl. Med. 6 (1), 272–284. doi:10.5966/sctm.2015-0265
Chen, Q., Yin, D., Zhang, Y., Yu, L., Li, X. D., Zhou, Z. J., et al. (2017b). MicroRNA-29a induces loss of 5-hydroxymethylcytosine and promotes metastasis of hepatocellular carcinoma through a TET-SOCS1-MMP9 signaling axis. Cell. Death Dis. 8 (6), e2906. doi:10.1038/cddis.2017.142
Chen, L., Xiang, B., Wang, X., and Xiang, C. (2017c). Exosomes derived from human menstrual blood-derived stem cells alleviate fulminant hepatic failure. Stem Cell. Res. Ther. 8 (1), 9. doi:10.1186/s13287-016-0453-6
Chen, X., Wu, Y., Wang, Y., Chen, L., Zheng, W., Zhou, S., et al. (2020a). Human menstrual blood-derived stem cells mitigate bleomycin-induced pulmonary fibrosis through anti-apoptosis and anti-inflammatory effects. Stem Cell. Res. Ther. 11 (1), 477. doi:10.1186/s13287-020-01926-x
Chen, J., Hu, C., Chen, L., Tang, L., Zhu, Y., Xu, X., et al. (2020b). Clinical study of mesenchymal stem cell treatment for acute Respiratory Distress syndrome induced by Epidemic influenza A (H7N9) infection: a Hint for COVID-19 treatment. Eng. Beijing China 6 (10), 1153–1161. doi:10.1016/j.eng.2020.02.006
Cordeiro, M. R., Carvalhos, C. A., and Figueiredo-Dias, M. (2022). The emerging role of menstrual-blood-derived stem cells in endometriosis. Biomedicines 11 (1), 39. doi:10.3390/biomedicines11010039
Cuenca, J., Le-Gatt, A., Castillo, V., Belletti, J., Díaz, M., Kurte, G. M., et al. (2018). The reparative Abilities of menstrual stem cells modulate the wound matrix Signals and improve cutaneous regeneration. Front. Physiol. 9, 464. doi:10.3389/fphys.2018.00464
Cuttle, L., Nataatmadja, M., Fraser, J. F., Kempf, M., Kimble, R. M., and Hayes, M. T. (2005). Collagen in the scarless fetal skin wound: detection with picrosirius-polarization. Wound Repair Regen. Off. Publ. Wound Heal Soc. Eur. Tissue Repair Soc. 13 (2), 198–204. doi:10.1111/j.1067-1927.2005.130211.x
Dalirfardouei, R., Jamialahmadi, K., Jafarian, A. H., and Mahdipour, E. (2019). Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J. Tissue Eng. Regen. Med. 13 (4), 555–568. doi:10.1002/term.2799
de Oliveira, R. Z., de Oliveira Buono, F., Cressoni, A. C. L., Penariol, L. B. C., Padovan, C. C., Tozetti, P. A., et al. (2022). Overexpression of miR-200b-3p in menstrual blood-derived mesenchymal stem cells from endometriosis women. Reprod. Sci. Thousand Oaks Calif. 29 (3), 734–742. doi:10.1007/s43032-022-00860-y
de Pedro, M. Á., Gómez-Serrano, M., Marinaro, F., López, E., Pulido, M., Preußer, C., et al. (2021). IFN-gamma and TNF-alpha as a priming strategy to enhance the immunomodulatory capacity of Secretomes from menstrual blood-derived stromal cells. Int. J. Mol. Sci. 22 (22), 12177. doi:10.3390/ijms222212177
Deans, R., and Abbott, J. (2010). Review of intrauterine adhesions. J. Minim. Invasive Gynecol. 17 (5), 555–569. doi:10.1016/j.jmig.2010.04.016
Du, J., Jiang, Y., Liu, X., Ji, X., Xu, B., Zhang, Y., et al. (2023). HGF secreted by menstrual blood-derived endometrial stem cells ameliorates non-alcoholic fatty liver disease through downregulation of hepatic Rnf186. Stem Cells Dayt Ohio 41 (2), 153–168. doi:10.1093/stmcls/sxac091
D’Arcangelo, D., Gaetano, C., and Capogrossi, M. C. (2002). Acidification prevents endothelial cell apoptosis by Axl activation. Circ. Res. 91 (7), e4–e12. doi:10.1161/01.res.0000036753.50601.e9
Ekström, P., Alm, P., and Akerlund, M. (1991). Differences in vasomotor responses between main stem and smaller branches of the human uterine artery. Acta Obstet. Gynecol. Scand. 70 (6), 429–433. doi:10.3109/00016349109007155
Eming, S. A., Krieg, T., and Davidson, J. M. (2007). Inflammation in wound repair: molecular and cellular mechanisms. J. Investig. Dermatol. 127 (3), 514–525. doi:10.1038/sj.jid.5700701
Emmerson, S., Mukherjee, S., Melendez-Munoz, J., Cousins, F., Edwards, S. L., Karjalainen, P., et al. (2019). Composite mesh design for delivery of autologous mesenchymal stem cells influences mesh integration, exposure and biocompatibility in an ovine model of pelvic organ prolapse. Biomaterials 225, 119495. doi:10.1016/j.biomaterials.2019.119495
Eyni, H., Ghorbani, S., Shirazi, R., Salari Asl, L., P Beiranvand, S., and Soleimani, M. (2017). Three-dimensional wet-electrospun poly(lactic acid)/multi-wall carbon nanotubes scaffold induces differentiation of human menstrual blood-derived stem cells into germ-like cells. J. Biomater. Appl. 32 (3), 373–383. doi:10.1177/0885328217723179
Falcone, T., and Flyckt, R. (2018). Clinical management of endometriosis. Obstet. Gynecol. 131 (3), 557–571. doi:10.1097/AOG.0000000000002469
Fan, C., Li, X., Xiao, Z., Zhao, Y., Liang, H., Wang, B., et al. (2017). A modified collagen scaffold facilitates endogenous neurogenesis for acute spinal cord injury repair. Acta Biomater. 51, 304–316. doi:10.1016/j.actbio.2017.01.009
Fan, X. L., Zhang, Y., Li, X., and Fu, Q. L. (2020). Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell. Mol. Life Sci. CMLS 77 (14), 2771–2794. doi:10.1007/s00018-020-03454-6
Fard, M., Akhavan-Tavakoli, M., Khanjani, S., Zare, S., Edalatkhah, H., Arasteh, S., et al. (2018). Bilayer amniotic membrane/Nano-fibrous fibroin scaffold promotes differentiation capability of menstrual blood stem cells into keratinocyte-like cells. Mol. Biotechnol. 60 (2), 100–110. doi:10.1007/s12033-017-0049-0
Fathi-Kazerooni, M., Fattah-Ghazi, S., Darzi, M., Makarem, J., Nasiri, R., Salahshour, F., et al. (2022). Safety and efficacy study of allogeneic human menstrual blood stromal cells secretome to treat severe COVID-19 patients: clinical trial phase I and II. Stem Cell. Res. Ther. 13 (1), 96. doi:10.1186/s13287-022-02771-w
Feng, P., Li, P., and Tan, J. (2019). Human menstrual blood-derived stromal cells promote recovery of premature ovarian insufficiency via regulating the ECM-dependent FAK/AKT signaling. Stem Cell. Rev. Rep. 15 (2), 241–255. doi:10.1007/s12015-018-9867-0
Finley, P. J., DeClue, C. E., Sell, S. A., DeBartolo, J. M., and Shornick, L. P. (2016). Diabetic wounds exhibit decreased Ym1 and Arginase expression with increased expression of IL-17 and IL-20. Adv. Wound Care 5 (11), 486–494. doi:10.1089/wound.2015.0676
Ghaemi, A., Ghiasvand, M., Omraninava, M., Merza, M. Y., Alkhafaji, A. T., Raoofi, A., et al. (2023). Hyperbaric oxygen therapy and coenzyme Q10 synergistically attenuates damage progression in spinal cord injury in a rat model. J. Chem. Neuroanat. 132, 102322. doi:10.1016/j.jchemneu.2023.102322
Guo, S., and Dipietro, L. A. (2010). Factors affecting wound healing. J. Dent. Res. 89 (3), 219–229. doi:10.1177/0022034509359125
Hao, J., Ma, A., Sun, C., Qin, H., Zhu, Y., Li, G., et al. (2024). Melatonin pretreatment improves endometrial regenerative cell-mediated therapeutic effects in experimental colitis. Int. Immunopharmacol. 133, 112092. doi:10.1016/j.intimp.2024.112092
Harro, C. D., Pang, Y. Y., Roden, R. B., Hildesheim, A., Wang, Z., Reynolds, M. J., et al. (2001). Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J. Natl. Cancer Inst. 93 (4), 284–292. doi:10.1093/jnci/93.4.284
Heneidi, S., Simerman, A. A., Keller, E., Singh, P., Li, X., Dumesic, D. A., et al. (2013). Awakened by cellular stress: isolation and characterization of a novel population of pluripotent stem cells derived from human adipose tissue. PloS One 8 (6), e64752. doi:10.1371/journal.pone.0064752
Hjazi, A., Alghamdi, A., Aloraini, G. S., Alshehri, M. A., Alsuwat, M. A., Albelasi, A., et al. (2024). Combination use of human menstrual blood stem cell-derived exosomes and hyperbaric oxygen therapy, synergistically promote recovery after spinal cord injury in rats. Tissue Cell. 88, 102378. doi:10.1016/j.tice.2024.102378
Hockemeyer, D., Soldner, F., Cook, E. G., Gao, Q., Mitalipova, M., and Jaenisch, R. (2008). A drug-inducible system for direct reprogramming of human somatic cells to pluripotency. Cell. Stem Cell. 3 (3), 346–353. doi:10.1016/j.stem.2008.08.014
Holowaty, P., Miller, A. B., Rohan, T., and To, T. (1999). Natural history of dysplasia of the uterine cervix. J. Natl. Cancer Inst. 91 (3), 252–258. doi:10.1093/jnci/91.3.252
Hong, S., Tian, H., Lu, Y., Laborde, J. M., Muhale, F. A., Wang, Q., et al. (2014). Neuroprotectin/protectin D1: endogenous biosynthesis and actions on diabetic macrophages in promoting wound healing and innervation impaired by diabetes. Am. J. Physiol. Cell. Physiol. 307 (11), C1058–C1067. doi:10.1152/ajpcell.00270.2014
Hosoya, S., Yokomizo, R., Kishigami, H., Fujiki, Y., Kaneko, E., Amita, M., et al. (2023). Novel therapeutic strategies for injured endometrium: intrauterine transplantation of menstrual blood-derived cells from infertile patients. Stem Cell. Res. Ther. 14 (1), 297. doi:10.1186/s13287-023-03524-z
Hosseini, S., Shokri, F., Ansari Pour, S., Jeddi-Tehrani, M., Nikoo, S., Yousefi, M., et al. (2016). A shift in the balance of T17 and Treg cells in menstrual blood of women with unexplained recurrent spontaneous abortion. J. Reprod. Immunol. 116, 13–22. doi:10.1016/j.jri.2016.03.001
Hosseini, S., Shokri, F., Pour, S. A., Khoshnoodi, J., Jeddi-Tehrani, M., and Zarnani, A. H. (2019). Diminished frequency of menstrual and peripheral blood NKT-like cells in patients with unexplained recurrent spontaneous abortion and infertile women. Reprod. Sci. Thousand Oaks Calif. 26 (1), 97–108. doi:10.1177/1933719118766261
Hu, Z., and Ma, D. (2018). The precision prevention and therapy of HPV-related cervical cancer: new concepts and clinical implications. Cancer Med. 7 (10), 5217–5236. doi:10.1002/cam4.1501
Hu, L., Wang, J., Zhou, X., Xiong, Z., Zhao, J., Yu, R., et al. (2016). Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci. Rep. 6, 32993. doi:10.1038/srep32993
Huang, J. J., Shi, Y. Q., Li, R. L., Hu, A., Lu, Z. Y., Weng, L., et al. (2015). Angiogenesis effect of therapeutic ultrasound on HUVECs through activation of the PI3K-Akt-eNOS signal pathway. Am. J. Transl. Res. 7 (6), 1106–1115.
Huangfu, D., Osafune, K., Maehr, R., Guo, W., Eijkelenboom, A., Chen, S., et al. (2008). Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat. Biotechnol. 26 (11), 1269–1275. doi:10.1038/nbt.1502
Humphries, B., and Yang, C. (2015). The microRNA-200 family: small molecules with novel roles in cancer development, progression and therapy. Oncotarget 6 (9), 6472–6498. doi:10.18632/oncotarget.3052
Ichim, T. E., Alexandrescu, D. T., Solano, F., Lara, F., Campion, R. D. N., Paris, E., et al. (2010). Mesenchymal stem cells as anti-inflammatories: implications for treatment of Duchenne muscular dystrophy. Cell. Immunol. 260 (2), 75–82. doi:10.1016/j.cellimm.2009.10.006
Iribarne-Durán, L. M., Domingo-Piñar, S., Peinado, F. M., Vela-Soria, F., Jiménez-Díaz, I., Barranco, E., et al. (2020). Menstrual blood concentrations of parabens and benzophenones and related factors in a sample of Spanish women: an exploratory study. Environ. Res. 183, 109228. doi:10.1016/j.envres.2020.109228
Ji, S., Liu, Y., Yan, L., Zhang, Y., Li, Y., Zhu, Q., et al. (2023). DIA-based analysis of the menstrual blood proteome identifies association between CXCL5 and IL1RN and endometriosis. J. Proteomics 289, 104995. doi:10.1016/j.jprot.2023.104995
Jiménez-Díaz, I., Iribarne-Durán, L. M., Ocón, O., Salamanca, E., Fernández, M. F., Olea, N., et al. (2016). Determination of personal care products -benzophenones and parabens-in human menstrual blood. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1035, 57–66. doi:10.1016/j.jchromb.2016.09.035
Jin, X. W., Cash, J., and Kennedy, A. W. (1999). Human papillomavirus typing and the reduction of cervical cancer risk. Cleve Clin. J. Med. 66 (9), 533–539. doi:10.3949/ccjm.66.9.533
Khademi, F., Soleimani, M., Verdi, J., Tavangar, S. M., Sadroddiny, E., Massumi, M., et al. (2014). Human endometrial stem cells differentiation into functional hepatocyte-like cells. Cell. Biol. Int. 38 (7), 825–834. doi:10.1002/cbin.10278
Khanjani, S., Khanmohammadi, M., Zarnani, A. H., Akhondi, M. M., Ahani, A., Ghaempanah, Z., et al. (2014). Comparative evaluation of differentiation potential of menstrual blood-versus bone marrow-derived stem cells into hepatocyte-like cells. PloS One 9 (2), e86075. doi:10.1371/journal.pone.0086075
Khanmohammadi, M., Khanjani, S., Edalatkhah, H., Zarnani, A. H., Heidari-Vala, H., Soleimani, M., et al. (2014). Modified protocol for improvement of differentiation potential of menstrual blood-derived stem cells into adipogenic lineage. Cell. Prolif. 47 (6), 615–623. doi:10.1111/cpr.12133
Khoury, M., Drake, A., Chen, Q., Dong, D., Leskov, I., Fragoso, M. F., et al. (2011). Mesenchymal stem cells secreting angiopoietin-like-5 support efficient expansion of human hematopoietic stem cells without compromising their repopulating potential. Stem Cells Dev. 20 (8), 1371–1381. doi:10.1089/scd.2010.0456
Khoury, M., Alcayaga-Miranda, F., Illanes, S. E., and Figueroa, F. E. (2014). The promising potential of menstrual stem cells for antenatal diagnosis and cell therapy. Front. Immunol. 5, 205. doi:10.3389/fimmu.2014.00205
Kim, J. B., Greber, B., Araúzo-Bravo, M. J., Meyer, J., Park, K. I., Zaehres, H., et al. (2009). Direct reprogramming of human neural stem cells by OCT4. Nature 461 (7264), 649–643. doi:10.1038/nature08436
Ko, M., Huang, Y., Jankowska, A. M., Pape, U. J., Tahiliani, M., Bandukwala, H. S., et al. (2010). Impaired hydroxylation of 5-methylcytosine in myeloid cancers with mutant TET2. Nature 468 (7325), 839–843. doi:10.1038/nature09586
Kunte, H., Farhadi, H. F., Sheth, K. N., Simard, J. M., and Kronenberg, G. (2015). Sulfonylureas--a novel treatment to reduce tissue damage after acute spinal cord injury? Lancet Neurol. 14 (4), 352. doi:10.1016/S1474-4422(15)70013-X
Kushida, Y., Wakao, S., and Dezawa, M. (2018). Muse cells are endogenous reparative stem cells. Adv. Exp. Med. Biol. 1103, 43–68. doi:10.1007/978-4-431-56847-6_3
Li, Y., Li, X., Zhao, H., Feng, R., Zhang, X., Tai, D., et al. (2013a). Efficient induction of pluripotent stem cells from menstrual blood. Stem Cells Dev. 22 (7), 1147–1158. doi:10.1089/scd.2012.0428
Lai, D., Wang, F., Yao, X., Zhang, Q., Wu, X., and Xiang, C. (2015). Human endometrial mesenchymal stem cells restore ovarian function through improving the renewal of germline stem cells in a mouse model of premature ovarian failure. J. Transl. Med. 13, 155. doi:10.1186/s12967-015-0516-y
Lai, D., Guo, Y., Zhang, Q., Chen, Y., and Xiang, C. (2016). Differentiation of human menstrual blood-derived endometrial mesenchymal stem cells into oocyte-like cells. Acta Biochim. Biophys. Sin. 48 (11), 998–1005. doi:10.1093/abbs/gmw090
Landén, N. X., Li, D., and Ståhle, M. (2016). Transition from inflammation to proliferation: a critical step during wound healing. Cell. Mol. Life Sci. CMLS 73 (20), 3861–3885. doi:10.1007/s00018-016-2268-0
Larson, B. J., Longaker, M. T., and Lorenz, H. P. (2010). Scarless fetal wound healing: a basic science review. Plast. Reconstr. Surg. 126 (4), 1172–1180. doi:10.1097/PRS.0b013e3181eae781
Lengerke, C., and Daley, G. Q. (2010). Autologous blood cell therapies from pluripotent stem cells. Blood Rev. 24 (1), 27–37. doi:10.1016/j.blre.2009.10.001
Li, S., and Hu, G. F. (2012). Emerging role of angiogenin in stress response and cell survival under adverse conditions. J. Cell. Physiol. 227 (7), 2822–2826. doi:10.1002/jcp.23051
Li, Y., Zhao, H., Lan, F., Lee, A., Chen, L., Lin, C., et al. (2010). Generation of human-induced pluripotent stem cells from gut mesentery-derived cells by ectopic expression of OCT4/SOX2/NANOG. Cell. Reprogr. 12 (3), 237–247. doi:10.1089/cell.2009.0103
Li, X., Xiao, Z., Han, J., Chen, L., Xiao, H., Ma, F., et al. (2013b). Promotion of neuronal differentiation of neural progenitor cells by using EGFR antibody functionalized collagen scaffolds for spinal cord injury repair. Biomaterials 34 (21), 5107–5116. doi:10.1016/j.biomaterials.2013.03.062
Li, X., Lan, X., Zhao, Y., Wang, G., Shi, G., Li, H., et al. (2019). SDF-1/CXCR4 axis enhances the immunomodulation of human endometrial regenerative cells in alleviating experimental colitis. Stem Cell. Res. Ther. 10 (1), 204. doi:10.1186/s13287-019-1298-6
Li, H., Wei, J., Li, M., Li, Y., Zhang, T., Tian, J., et al. (2024). Biological characteristics of Muse cells derived from MenSCs and their application in acute liver injury and intracerebral hemorrhage diseases. Regen. Ther. 27, 48–62. doi:10.1016/j.reth.2024.03.003
Liang, X., Ding, Y., Zhang, Y., Tse, H. F., and Lian, Q. (2014). Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell. Transpl. 23 (9), 1045–1059. doi:10.3727/096368913X667709
Liang, X., Zhang, L., Wang, S., Han, Q., and Zhao, R. C. (2016). Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J. Cell. Sci. 129 (11), 2182–2189. doi:10.1242/jcs.170373
Liu, Y., Prasad, R., and Wilson, S. H. (2010). HMGB1: roles in base excision repair and related function. Biochim. Biophys. Acta 1799 (1–2), 119–130. doi:10.1016/j.bbagrm.2009.11.008
Liu, T., Huang, Y., Zhang, J., Qin, W., Chi, H., Chen, J., et al. (2014). Transplantation of human menstrual blood stem cells to treat premature ovarian failure in mouse model. Stem Cells Dev. 23 (13), 1548–1557. doi:10.1089/scd.2013.0371
Liu, T., Zhang, L., Joo, D., and Sun, S. C. (2017). NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2, 17023-. doi:10.1038/sigtrans.2017.23
Liu, Y., Niu, R., Yang, F., Yan, Y., Liang, S., Sun, Y., et al. (2018). Biological characteristics of human menstrual blood-derived endometrial stem cells. J. Cell. Mol. Med. 22 (3), 1627–1639. doi:10.1111/jcmm.13437
Lopez-Verrilli, M. A., Caviedes, A., Cabrera, A., Sandoval, S., Wyneken, U., and Khoury, M. (2016). Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience 320, 129–139. doi:10.1016/j.neuroscience.2016.01.061
Lowry, W. E., Richter, L., Yachechko, R., Pyle, A. D., Tchieu, J., Sridharan, R., et al. (2008). Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc. Natl. Acad. Sci. U. S. A. 105 (8), 2883–2888. doi:10.1073/pnas.0711983105
Lu, L., Liu, M., Sun, R., Zheng, Y., and Zhang, P. (2015). Myocardial infarction: symptoms and treatments. Cell. Biochem. Biophys. 72 (3), 865–867. doi:10.1007/s12013-015-0553-4
Luz-Crawford, P., Torres, M. J., Noël, D., Fernandez, A., Toupet, K., Alcayaga-Miranda, F., et al. (2016). The immunosuppressive signature of menstrual blood mesenchymal stem cells entails opposite effects on experimental arthritis and graft versus host diseases. Stem Cells Dayt Ohio 34 (2), 456–469. doi:10.1002/stem.2244
Lv, M., Xia, Y., Li, B., Liu, H., Pan, J., bei, L., et al. (2016). Serum amyloid A stimulates vascular endothelial growth factor receptor 2 expression and angiogenesis. J. Physiol. Biochem. 72 (1), 71–81. doi:10.1007/s13105-015-0462-4
Lv, H., Hu, Y., Cui, Z., and Jia, H. (2018). Human menstrual blood: a renewable and sustainable source of stem cells for regenerative medicine. Stem Cell. Res. Ther. 9 (1), 325. doi:10.1186/s13287-018-1067-y
Mahdipour, E., Salmasi, Z., and Sabeti, N. (2019). Potential of stem cell-derived exosomes to regenerate β islets through Pdx-1 dependent mechanism in a rat model of type 1 diabetes. J. Cell. Physiol. 234 (11), 20310–20321. doi:10.1002/jcp.28631
Male, V., and Moffett, A. (2023). Natural killer cells in the human uterine Mucosa. Annu. Rev. Immunol. 41, 127–151. doi:10.1146/annurev-immunol-102119-075119
Malik, R., Chauhan, G., Traylor, M., Sargurupremraj, M., Okada, Y., Mishra, A., et al. (2018). Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat. Genet. 50 (4), 524–537. doi:10.1038/s41588-018-0058-3
Margiana, R., Markov, A., Zekiy, A. O., Hamza, M. U., Al-Dabbagh, K. A., Al-Zubaidi, S. H., et al. (2022). Clinical application of mesenchymal stem cell in regenerative medicine: a narrative review. Stem Cell. Res. Ther. 13 (1), 366. doi:10.1186/s13287-022-03054-0
Marinaro, F., Pericuesta, E., Sánchez-Margallo, F. M., Casado, J. G., Álvarez, V., Matilla, E., et al. (2018). Extracellular vesicles derived from endometrial human mesenchymal stem cells improve IVF outcome in an aged murine model. Reprod. Domest. Anim. Zuchthyg 53 (Suppl. 2), 46–49. doi:10.1111/rda.13314
Marinaro, F., Macías-García, B., Sánchez-Margallo, F. M., Blázquez, R., Álvarez, V., Matilla, E., et al. (2019). Extracellular vesicles derived from endometrial human mesenchymal stem cells enhance embryo yield and quality in an aged murine model. Biol. Reprod. 100 (5), 1180–1192. doi:10.1093/biolre/ioy263
Matsumoto, J., Iwano, H., Inoue, H., Iwano, N., Yamashiki, N., and Yokota, H. (2007). Metabolic barrier against bisphenol A in rat uterine endometrium. Toxicol. Sci. Off. J. Soc. Toxicol. 99 (1), 118–125. doi:10.1093/toxsci/kfm148
Meola, J., Rosa, E., Silva, J. C., Dentillo, D. B., da Silva, W. A., Veiga-Castelli, L. C., et al. (2010). Differentially expressed genes in eutopic and ectopic endometrium of women with endometriosis. Fertil. Steril. 93 (6), 1750–1773. doi:10.1016/j.fertnstert.2008.12.058
Micutkova, L., Diener, T., Li, C., Rogowska-Wrzesinska, A., Mueck, C., Huetter, E., et al. (2011). Insulin-like growth factor binding protein-6 delays replicative senescence of human fibroblasts. Mech. Ageing Dev. 132 (10), 468–479. doi:10.1016/j.mad.2011.07.005
Mihalas, B. P., Redgrove, K. A., McLaughlin, E. A., and Nixon, B. (2017). Molecular mechanisms responsible for increased Vulnerability of the ageing oocyte to oxidative damage. Oxid. Med. Cell. Longev. 2017, 4015874. doi:10.1155/2017/4015874
Mirzadegan, E., Golshahi, H., and Kazemnejad, S. (2020). Current evidence on immunological and regenerative effects of menstrual blood stem cells seeded on scaffold consisting of amniotic membrane and silk fibroin in chronic wound. Int. Immunopharmacol. 85, 106595. doi:10.1016/j.intimp.2020.106595
Mirzadegan, E., Golshahi, H., Saffarian, Z., Darzi, M., Khorasani, S., Edalatkhah, H., et al. (2022). The remarkable effect of menstrual blood stem cells seeded on bilayer scaffold composed of amniotic membrane and silk fibroin aiming to promote wound healing in diabetic mice. Int. Immunopharmacol. 102, 108404. doi:10.1016/j.intimp.2021.108404
Murray, P. J., Allen, J. E., Biswas, S. K., Fisher, E. A., Gilroy, D. W., Goerdt, S., et al. (2014). Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41 (1), 14–20. doi:10.1016/j.immuni.2014.06.008
Na, J., Lee, K., Na, W., Shin, J. Y., Lee, M. J., Yune, T. Y., et al. (2016). Histone H3K27 Demethylase JMJD3 in Cooperation with NF-κB regulates keratinocyte wound healing. J. Investig. Dermatol 136 (4), 847–858. doi:10.1016/j.jid.2015.11.029
Na, J., Shin, J. Y., Jeong, H., Lee, J. Y., Kim, B. J., Kim, W. S., et al. (2017). JMJD3 and NF-κB-dependent activation of Notch1 gene is required for keratinocyte migration during skin wound healing. Sci. Rep. 7 (1), 6494. doi:10.1038/s41598-017-06750-7
Namavar, J. B., Parsanezhad, M. E., and Ghane-Shirazi, R. (2001). Female genital tuberculosis and infertility. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 75 (3), 269–272. doi:10.1016/S0020-7292(01)00494-5
Nikoo, S., Ebtekar, M., Jeddi-Tehrani, M., Shervin, A., Bozorgmehr, M., Kazemnejad, S., et al. (2012). Effect of menstrual blood-derived stromal stem cells on proliferative capacity of peripheral blood mononuclear cells in allogeneic mixed lymphocyte reaction. J. Obstet. Gynaecol. Res. 38 (5), 804–809. doi:10.1111/j.1447-0756.2011.01800.x
Nikoo, S., Ebtekar, M., Jeddi-Tehrani, M., Shervin, A., Bozorgmehr, M., Vafaei, S., et al. (2014). Menstrual blood-derived stromal stem cells from women with and without endometriosis reveal different phenotypic and functional characteristics. Mol. Hum. Reprod. 20 (9), 905–918. doi:10.1093/molehr/gau044
Noble, L. S., Simpson, E. R., Johns, A., and Bulun, S. E. (1996). Aromatase expression in endometriosis. J. Clin. Endocrinol. Metab. 81 (1), 174–179. doi:10.1210/jcem.81.1.8550748
Paine, S. K., Basu, A., Choudhury, R. G., Bhattacharya, B., Chatterjee, S., and Bhattacharya, C. (2018). Multiplex PCR from menstrual blood: a non-invasive Cost-effective approach to reduce diagnostic Dilemma for genital tuberculosis. Mol. Diagn Ther. 22 (3), 391–396. doi:10.1007/s40291-018-0322-3
Parasar, P., Ozcan, P., and Terry, K. L. (2017). Endometriosis: Epidemiology, diagnosis and clinical management. Curr. Obstet. Gynecol. Rep. 6 (1), 34–41. doi:10.1007/s13669-017-0187-1
Park, I. H., Arora, N., Huo, H., Maherali, N., Ahfeldt, T., Shimamura, A., et al. (2008a). Disease-specific induced pluripotent stem cells. Cell. 134 (5), 877–886. doi:10.1016/j.cell.2008.07.041
Park, I. H., Zhao, R., West, J. A., Yabuuchi, A., Huo, H., Ince, T. A., et al. (2008b). Reprogramming of human somatic cells to pluripotency with defined factors. Nature 451 (7175), 141–146. doi:10.1038/nature06534
Patel, A. N., Park, E., Kuzman, M., Benetti, F., Silva, F. J., and Allickson, J. G. (2008). Multipotent menstrual blood stromal stem cells: isolation, characterization, and differentiation. Cell. Transpl. 17 (3), 303–311. doi:10.3727/096368908784153922
Pei, X. M., Yeung, M. H. Y., Wong, A. N. N., Tsang, H. F., Yu, A. C. S., Yim, A. K. Y., et al. (2023). Targeted sequencing approach and its clinical applications for the molecular diagnosis of human diseases. Cells 12 (3), 493. doi:10.3390/cells12030493
Penariol, L. B. C., Thomé, C. H., Tozetti, P. A., Paier, C. R. K., Buono, F. O., Peronni, K. C., et al. (2022). What do the transcriptome and proteome of menstrual blood-derived mesenchymal stem cells Tell Us about endometriosis? Int. J. Mol. Sci. 23 (19), 11515. doi:10.3390/ijms231911515
Piersma, B., Bank, R. A., and Boersema, M. (2015). Signaling in fibrosis: TGF-β, WNT, and YAP/TAZ Converge. Front. Med. 2, 59. doi:10.3389/fmed.2015.00059
Pittenger, M. F., Discher, D. E., Péault, B. M., Phinney, D. G., Hare, J. M., and Caplan, A. I. (2019). Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen. Med. 4, 22. doi:10.1038/s41536-019-0083-6
Qin, W., Xiong, Y., Chen, J., Huang, Y., and Liu, T. (2018). DC-CIK cells derived from ovarian cancer patient menstrual blood activate the TNFR1-ASK1-AIP1 pathway to kill autologous ovarian cancer stem cells. J. Cell. Mol. Med. 22 (7), 3364–3376. doi:10.1111/jcmm.13611
Qiu, J., Ma, X. L., Wang, X., Chen, H., and Huang, B. R. (2012). Insulin-like growth factor binding protein-6 interacts with the thyroid hormone receptor α1 and modulates the thyroid hormone-response in osteoblastic differentiation. Mol. Cell. Biochem. 361 (1–2), 197–208. doi:10.1007/s11010-011-1104-y
Rajabi, Z., Yazdekhasti, H., Noori Mugahi, S. M. H., Abbasi, M., Kazemnejad, S., Shirazi, A., et al. (2018). Mouse preantral follicle growth in 3D co-culture system using human menstrual blood mesenchymal stem cell. Reprod. Biol. 18 (1), 122–131. doi:10.1016/j.repbio.2018.02.001
Ratajczak, M. Z., Zuba-Surma, E. K., Wysoczynski, M., Wan, W., Ratajczak, J., Wojakowski, W., et al. (2008). Hunt for pluripotent stem cell -- regenerative medicine search for almighty cell. J. Autoimmun. 30 (3), 151–162. doi:10.1016/j.jaut.2007.12.003
Razavi, M., Rezaee, M., Telichko, A., Inan, H., Dahl, J., Demirci, U., et al. (2020). The paracrine function of mesenchymal stem cells in response to Pulsed focused ultrasound. Cell. Transpl. 29, 963689720965478. doi:10.1177/0963689720965478
Ren, H., Zhang, Q., Wang, J., and Pan, R. (2018). Comparative effects of umbilical cord- and menstrual blood-derived MSCs in repairing acute lung injury. Stem Cells Int. 2018, 7873625. doi:10.1155/2018/7873625
Robeldo, T., Canzi, E. F., de Andrade, P. M., Santana, J. P. P., Teixeira, F. R., Spagnol, V., et al. (2020). Effect of Tahiti lime (Citrus latifolia) juice on the production of the PGF2α/PGE2 and pro-inflammatory cytokines involved in menstruation. Sci. Rep. 10 (1), 7063. doi:10.1038/s41598-020-63477-8
Schwab, J. M., Chiang, N., Arita, M., and Serhan, C. N. (2007). Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 447 (7146), 869–874. doi:10.1038/nature05877
Seo, J. H., Yu, J. H., Suh, H., Kim, M. S., and Cho, S. R. (2013). Fibroblast growth factor-2 induced by enriched environment enhances angiogenesis and motor function in chronic hypoxic-ischemic brain injury. PloS One 8 (9), e74405. doi:10.1371/journal.pone.0074405
Seo, Y., Kang, M. J., and Kim, H. S. (2021). Strategies to potentiate paracrine therapeutic efficacy of mesenchymal stem cells in inflammatory diseases. Int. J. Mol. Sci. 22 (7), 3397. doi:10.3390/ijms22073397
Shao, B., Wang, H., Ren, S. H., Chen, Q., Wang, Z. B., Xu, Y. N., et al. (2025). Exosomes derived from a mesenchymal-like endometrial regenerative cells ameliorate renal ischemia reperfusion injury through delivery of CD73. Stem Cell. Res. Ther. 16 (1), 148. doi:10.1186/s13287-025-04275-9
Shi, Y., Shu, B., Yang, R., Xu, Y., Xing, B., Liu, J., et al. (2015). Wnt and Notch signaling pathway involved in wound healing by targeting c-Myc and Hes1 separately. Stem Cell. Res. Ther. 6 (1), 120. doi:10.1186/s13287-015-0103-4
Shimizu, K., Kamada, Y., Sakamoto, A., Matsuda, M., Nakatsuka, M., and Hiramatsu, Y. (2017). High expression of high-mobility group box 1 in menstrual blood: implications for endometriosis. Reprod. Sci. Thousand Oaks Calif. 24 (11), 1532–1537. doi:10.1177/1933719117692042
Sinaii, N., Plumb, K., Cotton, L., Lambert, A., Kennedy, S., Zondervan, K., et al. (2008). Differences in characteristics among 1,000 women with endometriosis based on extent of disease. Fertil. Steril. 89 (3), 538–545. doi:10.1016/j.fertnstert.2007.03.069
Skliutė, G., Baušytė, R., Borutinskaitė, V., Valiulienė, G., Kaupinis, A., Valius, M., et al. (2021). Menstrual blood-derived endometrial stem cells’ Impact for the treatment perspective of female infertility. Int. J. Mol. Sci. 22 (13), 6774. doi:10.3390/ijms22136774
Spitzer, M. (1998). Cervical screening adjuncts: recent advances. Am. J. Obstet. Gynecol. 179 (2), 544–556. doi:10.1016/s0002-9378(98)70393-x
Sun, N., Panetta, N. J., Gupta, D. M., Wilson, K. D., Lee, A., Jia, F., et al. (2009). Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. Proc. Natl. Acad. Sci. U. S. A. 106 (37), 15720–15725. doi:10.1073/pnas.0908450106
Sun, L., Zhu, M., Feng, W., Lin, Y., Yin, J., Jin, J., et al. (2019). Exosomal miRNA let-7 from menstrual blood-derived endometrial stem cells alleviates pulmonary fibrosis through regulating mitochondrial DNA damage. Oxid. Med. Cell. Longev. 2019, 4506303. doi:10.1155/2019/4506303
Sziksz, E., Pap, D., Lippai, R., Béres, N. J., Fekete, A., Szabó, A. J., et al. (2015). Fibrosis related inflammatory Mediators: role of the IL-10 cytokine family. Mediat. Inflamm. 2015, 764641. doi:10.1155/2015/764641
Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K., et al. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 131 (5), 861–872. doi:10.1016/j.cell.2007.11.019
Tan, J., Li, P., Wang, Q., Li, Y., Li, X., Zhao, D., et al. (2016). Autologous menstrual blood-derived stromal cells transplantation for severe Asherman’s syndrome. Hum. Reprod. Oxf Engl. 31 (12), 2723–2729. doi:10.1093/humrep/dew235
Tsang, H. F., Yu, A. C. S., Jin, N., Yim, A. K. Y., Leung, W. M. S., Lam, K. W., et al. (2022). The clinical application of metagenomic next-generation sequencing for detecting pathogens in bronchoalveolar lavage fluid: case reports and literature review. Expert Rev. Mol. Diagn 22 (5), 575–582. doi:10.1080/14737159.2022.2071607
Tsang, H. F., Cheung, Y. S., Yu, C. S. A., Chan, C. S. S., Wong, C. B. T., Yim, K. Y. A., et al. (2024). Menstrual blood as a diagnostic specimen for human papillomavirus genotyping and genital tract infection using next-generation sequencing as a novel diagnostic tool. Diagn Basel Switz. 14 (7), 686. doi:10.3390/diagnostics14070686
Uchida, S., Hayakawa, K., Ogata, T., Tanaka, S., Kataoka, K., and Itaka, K. (2016). Treatment of spinal cord injury by an advanced cell transplantation technology using brain-derived neurotrophic factor-transfected mesenchymal stem cell spheroids. Biomaterials 109, 1–11. doi:10.1016/j.biomaterials.2016.09.007
van der Molen, R. G., Schutten, J. H. F., van Cranenbroek, B., ter Meer, M., Donckers, J., Scholten, R. R., et al. (2014). Menstrual blood closely resembles the uterine immune micro-environment and is clearly distinct from peripheral blood. Hum. Reprod. Oxf Engl. 29 (2), 303–314. doi:10.1093/humrep/det398
Volk, S. W., Wang, Y., Mauldin, E. A., Liechty, K. W., and Adams, S. L. (2011). Diminished type III collagen promotes myofibroblast differentiation and increases scar deposition in cutaneous wound healing. Cells Tissues Organs 194 (1), 25–37. doi:10.1159/000322399
Wang, Z., Wang, Y., Wang, Z., Gutkind, J. S., Wang, Z., Wang, F., et al. (2015). Engineered mesenchymal stem cells with enhanced tropism and paracrine secretion of cytokines and growth factors to treat traumatic brain injury. Stem Cells Dayt Ohio 33 (2), 456–467. doi:10.1002/stem.1878
Wang, K., Jiang, Z., Webster, K. A., Chen, J., Hu, H., Zhou, Y., et al. (2017). Enhanced cardioprotection by human endometrium mesenchymal stem cells driven by exosomal MicroRNA-21. Stem Cells Transl. Med. 6 (1), 209–222. doi:10.5966/sctm.2015-0386
Wellner, U., Schubert, J., Burk, U. C., Schmalhofer, O., Zhu, F., Sonntag, A., et al. (2009). The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat. Cell. Biol. 11 (12), 1487–1495. doi:10.1038/ncb1998
Wiwatpanit, T., Murphy, A. R., Lu, Z., Urbanek, M., Burdette, J. E., Woodruff, T. K., et al. (2020). Scaffold-free endometrial organoids Respond to excess Androgens associated with Polycystic ovarian syndrome. J. Clin. Endocrinol. Metab. 105 (3), 769–780. doi:10.1210/clinem/dgz100
Wong, S. C. C., Au, T. C. C., Chan, S. C. S., Ng, L. P. W., and Tsang, H. F. (2018). Menstrual blood human papillomavirus DNA and TAP1 gene polymorphisms as potential biomarkers for screening and Monitoring of cervical squamous intraepithelial lesion. J. Infect. Dis. 218 (11), 1739–1745. doi:10.1093/infdis/jiy369
Wu, Q., Wang, Q., Li, Z., Li, X., Zang, J., Wang, Z., et al. (2018). Human menstrual blood-derived stem cells promote functional recovery in a rat spinal cord hemisection model. Cell. Death Dis. 9 (9), 882. doi:10.1038/s41419-018-0847-8
Wu, Y., Chen, X., Zhao, Y., Wang, Y., Li, Y., and Xiang, C. (2019). Genome-wide DNA methylation and hydroxymethylation analysis reveal human menstrual blood-derived stem cells inhibit hepatocellular carcinoma growth through oncogenic pathway suppression via regulating 5-hmC in enhancer elements. Stem Cell. Res. Ther. 10 (1), 151. doi:10.1186/s13287-019-1243-8
Xiang, B., Chen, L., Wang, X., Zhao, Y., Wang, Y., and Xiang, C. (2017). Transplantation of menstrual blood-derived mesenchymal stem cells promotes the repair of LPS-induced acute lung injury. Int. J. Mol. Sci. 18 (4), 689. doi:10.3390/ijms18040689
Xu, X., Li, X., Gu, X., Zhang, B., Tian, W., Han, H., et al. (2017). Prolongation of cardiac Allograft survival by endometrial regenerative cells: focusing on B-cell responses. Stem Cells Transl. Med. 6 (3), 778–787. doi:10.5966/sctm.2016-0206
Xu, X., Jiang, W., Chen, L., Xu, Z., Zhang, Q., Zhu, M., et al. (2021). Evaluation of the safety and efficacy of using human menstrual blood-derived mesenchymal stromal cells in treating severe and critically ill COVID-19 patients: an exploratory clinical trial. Clin. Transl. Med. 11 (2), e297. doi:10.1002/ctm2.297
Yang, H., Zhou, B., Prinz, M., and Siegel, D. (2012). Proteomic analysis of menstrual blood. Mol. Cell. Proteomics MCP 11 (10), 1024–1035. doi:10.1074/mcp.M112.018390
Yang, R. Q., Teng, H., Xu, X. H., Liu, S. Y., Wang, Y. H., Guo, F. J., et al. (2016). Microarray analysis of microRNA deregulation and angiogenesis-related proteins in endometriosis. Genet. Mol. Res. GMR 15 (2). doi:10.4238/gmr.15027826
Yang, Y., Chen, Y., Zhao, Y., Ji, F., Zhang, L., Tang, S., et al. (2022). Human menstrual blood-derived stem cell transplantation suppresses liver injury in DDC-induced chronic cholestasis. Stem Cell. Res. Ther. 13 (1), 57. doi:10.1186/s13287-022-02734-1
Yates, C. C., Hebda, P., and Wells, A. (2012). Skin wound healing and scarring: fetal wounds and regenerative restitution. Birth Defects Res. Part C Embryo Today Rev. 96 (4), 325–333. doi:10.1002/bdrc.21024
Ye, L., Chang, J. C., Lin, C., Sun, X., Yu, J., and Kan, Y. W. (2009). Induced pluripotent stem cells offer new approach to therapy in thalassemia and sickle cell anemia and option in prenatal diagnosis in genetic diseases. Proc. Natl. Acad. Sci. U. S. A. 106 (24), 9826–9830. doi:10.1073/pnas.0904689106
Yu, J., Vodyanik, M. A., Smuga-Otto, K., Antosiewicz-Bourget, J., Frane, J. L., Tian, S., et al. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science 318 (5858), 1917–1920. doi:10.1126/science.1151526
Yu, D., Zhao, Y., Wang, H., Kong, D., Jin, W., Hu, Y., et al. (2021). IL-1β pre-stimulation enhances the therapeutic effects of endometrial regenerative cells on experimental colitis. Stem Cell. Res. Ther. 12 (1), 324. doi:10.1186/s13287-021-02392-9
Zafardoust, S., Kazemnejad, S., Darzi, M., Fathi-Kazerooni, M., Rastegari, H., and Mohammadzadeh, A. (2020). Improvement of pregnancy rate and live birth rate in poor ovarian responders by intraovarian Administration of autologous menstrual blood derived- mesenchymal stromal cells: phase I/II clinical trial. Stem Cell. Rev. Rep. 16 (4), 755–763. doi:10.1007/s12015-020-09969-6
Zafardoust, S., Kazemnejad, S., Fathi-Kazerooni, M., Darzi, M., Sadeghi, M. R., Sadeghi Tabar, A., et al. (2023a). The effects of intraovarian injection of autologous menstrual blood-derived mesenchymal stromal cells on pregnancy outcomes in women with poor ovarian response. Stem Cell. Res. Ther. 14 (1), 332. doi:10.1186/s13287-023-03568-1
Zafardoust, S., Kazemnejad, S., Darzi, M., Fathi-Kazerooni, M., Saffarian, Z., Khalili, N., et al. (2023b). Intraovarian Administration of autologous menstrual blood derived-mesenchymal stromal cells in women with premature ovarian failure. Arch. Med. Res. 54 (2), 135–144. doi:10.1016/j.arcmed.2022.12.015
Zafardoust, S., Didar, H., Ganagard, M. G., Tavakoli, M., Arefi, S., Arjmandinaloo, S., et al. (2025). Intra-ovarian injection of autologous menstrual blood-derived-mesenchymal stromal cells: a safe and promising method to improve pregnancy rate in poor ovarian responders. Stem Cell. Res. Ther. 16 (1), 171. doi:10.1186/s13287-025-04278-6
Zhang, Q. Z., Su, W. R., Shi, S. H., Wilder-Smith, P., Xiang, A. P., Wong, A., et al. (2010). Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells Dayt Ohio 28 (10), 1856–1868. doi:10.1002/stem.503
Zhang, J., Guan, J., Niu, X., Hu, G., Guo, S., Li, Q., et al. (2015). Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J. Transl. Med. 13, 49. doi:10.1186/s12967-015-0417-0
Zhang, Y., Lin, X., Dai, Y., Hu, X., Zhu, H., Jiang, Y., et al. (2016). Endometrial stem cells repair injured endometrium and induce angiogenesis via AKT and ERK pathways. Reprod. Camb Engl. 152 (5), 389–402. doi:10.1530/REP-16-0286
Zhang, J., Tian, X., Chen, Y., Huang, S., Cui, Z., Tian, R., et al. (2021). Feasibility and accuracy of menstrual blood testing for high-risk human papillomavirus detection with Capture sequencing. JAMA Netw. Open 4 (12), e2140644. doi:10.1001/jamanetworkopen.2021.40644
Zhao, H., Li, Y., Jin, H., Xie, L., Liu, C., Jiang, F., et al. (2010). Rapid and efficient reprogramming of human amnion-derived cells into pluripotency by three factors OCT4/SOX2/NANOG. Differ. Res. Biol. Divers. 80 (2–3), 123–129. doi:10.1016/j.diff.2010.03.002
Zhao, G. N., Zhang, P., Gong, J., Zhang, X. J., Wang, P. X., Yin, M., et al. (2017). Tmbim1 is a multivesicular body regulator that protects against non-alcoholic fatty liver disease in mice and monkeys by targeting the lysosomal degradation of Tlr4. Nat. Med. 23 (6), 742–752. doi:10.1038/nm.4334
Zhao, Y., Lan, X., Wang, Y., Xu, X., Lu, S., Li, X., et al. (2018a). Human endometrial regenerative cells attenuate bleomycin-induced pulmonary fibrosis in mice. Stem Cells Int. 2018, 3475137. doi:10.1155/2018/3475137
Zhao, Y., Chen, X., Wu, Y., Wang, Y., Li, Y., and Xiang, C. (2018b). Transplantation of human menstrual blood-derived mesenchymal stem cells alleviates Alzheimer’s disease-like pathology in APP/PS1 transgenic mice. Front. Mol. Neurosci. 11, 140. doi:10.3389/fnmol.2018.00140
Zhao, X., Zhao, X., and Wang, Z. (2021). Synergistic neuroprotective effects of hyperbaric oxygen and N-acetylcysteine against traumatic spinal cord injury in rat. J. Chem. Neuroanat. 118, 102037. doi:10.1016/j.jchemneu.2021.102037
Zhong, Z., Patel, A. N., Ichim, T. E., Riordan, N. H., Wang, H., Min, W. P., et al. (2009). Feasibility investigation of allogeneic endometrial regenerative cells. J. Transl. Med. 7, 15. doi:10.1186/1479-5876-7-15
Zhou, T., Benda, C., Duzinger, S., Huang, Y., Li, X., Li, Y., et al. (2011). Generation of induced pluripotent stem cells from urine. J. Am. Soc. Nephrol. JASN 22 (7), 1221–1228. doi:10.1681/ASN.2011010106
Zhu, H., Pan, Y., Jiang, Y., Li, J., Zhang, Y., and Zhang, S. (2019). Activation of the Hippo/TAZ pathway is required for menstrual stem cells to suppress myofibroblast and inhibit transforming growth factor β signaling in human endometrial stromal cells. Hum. Reprod. Oxf Engl. 34 (4), 635–645. doi:10.1093/humrep/dez001
Keywords: menstruation, diagnosis, therapy, stem cells, research progress
Citation: Feng Y and He Y (2025) The secrets of menstrual blood: emerging frontiers from diagnostic tools to stem cell therapies. Front. Cell Dev. Biol. 13:1623959. doi: 10.3389/fcell.2025.1623959
Received: 06 May 2025; Accepted: 04 August 2025;
Published: 20 August 2025.
Edited by:
Valerie Kouskoff, The University of Manchester, United KingdomReviewed by:
Soumya Ranjan Jena, Ravenshaw University, IndiaVinod Reddy Lekkala, University of California, San Francisco, United States
Copyright © 2025 Feng and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yujie He, MzI3MjE2NjEyNEBxcS5jb20=
 Yige Feng1,2
Yige Feng1,2 Yujie He
Yujie He