- 1Departments of Pharmacy and Biotechnology, University of Bologna, Bologna, Italy
- 2IRCCS Istituto delle Scienze Neurologiche di Bologna, Programma di Neurogenetica, Bologna, Italy
- 3Neuroscience Institute, Italian Research Council (CNR), Padova, Italy
- 4Division of Ophthalmology, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL, United States
- 5Department of Ophthalmology, Northwestern University Feinberg School of Medicine, Chicago, IL, United States
- 6Department of Biology, University of Padova, Padua, Italy
Editorial on the Research Topic
Zebrafish: bona fide pathophysiological models of human diseases
The zebrafish (Danio rerio) has emerged as a pivotal vertebrate model in biomedical research, occupying a unique position at the intersection of developmental biology, disease modeling, and translational medicine. With remarkable genetic conservation, optical transparency, rapid embryonic development, and a rich repertoire of genetic tools, zebrafish are now extensively employed to unravel the molecular and cellular mechanisms underlying a broad range of human diseases. This Research Topic gathers a compelling set of eight studies that illustrate the versatility and power of zebrafish in modeling human pathophysiology across diverse organ systems (Figure 1).
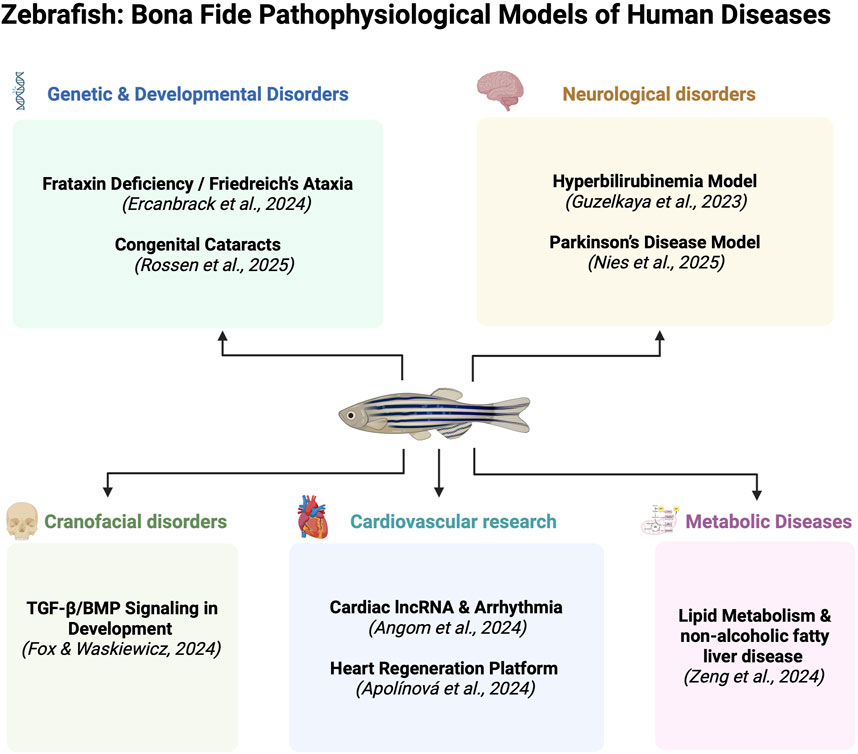
Figure 1. Zebrafish as Bona Fide Pathophysiological Models of Human Diseases. The figure summarizes examples of human diseases modeled in zebrafish. The references correspond to the contributions included in the current Research Topic as discussed in this Editorial. The figure was created with BioRender.com.
Neurodevelopmental and neurodegenerative diseases are well represented in this Research Topic. Guzelkaya et al. present a zebrafish model of neonatal hyperbilirubinemia to investigate bilirubin-induced neurological dysfunction (BIND), a condition still poorly understood in human neonates. Their study shows that bilirubin exposure impairs zebrafish locomotion and brain morphology, providing a vertebrate platform for dissecting bilirubin toxicity and testing neuroprotective interventions (Guzelkaya et al.).
In a related exploration of nervous system pathology, Nies et al. employ a rotenone-induced Parkinson’s disease model in zebrafish to assess the therapeutic potential of human metallothionein II (hMT2). Their findings reveal that hMT2 not only mitigates dopaminergic neurodegeneration and oxidative stress but also restores locomotor function, highlighting zebrafish as a translational platform for screening neuroprotective compounds (Nies et al.).
The vertebrate cardiac system is another focal point addressed in this Research Topic. Angom et al. use a forward genetic screen with gene-breaking trap lines to uncover grin2bbART, a novel long non-coding RNA that regulates calcium handling in the zebrafish heart. This study underscores the capacity of zebrafish as a model for uncovering regulators of heart physiology that might otherwise remain elusive in mammalian systems (Angom et al.). In a related study, Apolínová et al. present ZebraReg, an innovative automated platform that leverages the innate ability of zebrafish to regenerate heart tissue after injury. This tool allows for high-throughput screening of genes and compounds that influence cardiac regeneration, with clear translational implications for regenerative medicine in humans (Angom et al.).
In the domain of metabolic disease, Zeng et al. investigate cobll1a, a gene encoding a putative cytoskeletal protein involved in lipid metabolism. Their work shows that cobll1a-deficient zebrafish exhibit hepatic lipid accumulation due to disrupted retinoic acid signaling, linking this pathway to metabolic disorders, such as fatty liver disease and obesity. These findings reinforce the relevance of zebrafish for modeling and delineating the pathophysiological origins of complex metabolic syndromes (Zeng et al.).
Zebrafish also continue to provide crucial insights into developmental genetics and organogenesis. Ercanbrack et al. study the effects of frataxin knockdown in zebrafish embryos, modeling Friedreich’s Ataxia—a neurodegenerative disorder with mitochondrial dysfunction. These authors report defects in pronephros (early kidney) formation and embryonic development, expanding our understanding of the systemic roles of frataxin and its links to renal phenotypes in human disease (Ercanbrack et al.).
Zebrafish are immensely useful and popular genetic models for studying craniofacial development. In the present Research Topic, Fox and Waskiewicz review the contributions of zebrafish to understanding the roles of TGF-β and BMP signaling pathways in craniofacial morphogenesis. These authors highlight how zebrafish models replicate key features of congenital anomalies, such as cleft palate and craniosynostosis, offering a tractable system to study gene-environment interactions that influence facial structure (Fox and Waskiewicz).
Finally, Rossen et al. offer a comprehensive review of how zebrafish are used to model congenital cataracts, particularly those involving crystallin gene mutations. Given the high degree of conservation in lens development between zebrafish and humans, these models allow for real-time imaging of lens transparency and the testing of gene variants implicated in pediatric vision loss. This study highlights the zebrafish as a valuable model for both basic ocular research and therapeutic screening (Rossen et al.).
Together, these contributions exemplify the breadth and depth of zebrafish disease modeling. Across these studies, a variety of methodological approaches are employed, including CRISPR-based gene editing, morpholino knockdowns, transgenic reporters, forward genetic screens, and high-throughput imaging and behavioral assays. These tools enable fine-scale dissection of cellular and molecular processes in vivo and at scale—an asset particularly valuable for preclinical discovery pipelines.
Importantly, several studies in this Research Topic employ advancements beyond modeling toward therapeutic exploration. Nies et al. demonstrate the efficacy of hMT2 in ameliorating Parkinsonian phenotypes (Nies et al.), while ZebraReg offers a pipeline for regenerative compound discovery (Apolínová et al.). Such efforts underscore the translational potential of zebrafish not just as disease models but also as platforms for therapeutic innovation.
This Research Topic of studies also reflects the ability of zebrafish modeling to integrate systems-level insights. For example, links between developmental signaling and metabolic disease (Zeng et al.), or mitochondrial dysfunction and renal development (Ercanbrack et al.), illustrate the capacity of zebrafish models to reveal inter-organ and cross-pathway relationships that mirror human physiology and pathology.
Altogether, these studies affirm the zebrafish as a bona fide model of human disease, revealing the application of these animals in elucidating disease mechanisms, uncovering new gene functions, and screening therapeutics for a multitude of diseases ranging from monogenic conditions to complex multifactorial disorders. As research advances toward more patient-specific and systems-based approaches, zebrafish remain a cornerstone of translational research, bridging molecular discoveries to practical applications for the prevention of diseases and improvement of human health outcomes.
Author contributions
NF: Writing – review and editing, Writing – original draft. AW: Writing – original draft, Writing – review and editing. NT: Writing – review and editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. AI was used for grammar check of the text.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: zebrafish, danio rerio, model, mutant, human disease
Citation: Facchinello N, Williams AL and Tiso N (2025) Editorial: Zebrafish: bona fide pathophysiological models of human diseases. Front. Cell Dev. Biol. 13:1624614. doi: 10.3389/fcell.2025.1624614
Received: 07 May 2025; Accepted: 16 June 2025;
Published: 27 June 2025.
Edited by:
James Alan Marrs, Indiana University–Purdue University Indianapolis, United StatesReviewed by:
Muthukumar Karuppasamy, University of Alabama at Birmingham, United StatesCopyright © 2025 Facchinello, Williams and Tiso. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicola Facchinello, bmljb2xhLmZhY2NoaW5lbGxvQHVuaWJvLml0; Antionette L. Williams, YW53aWxsaWFtc0BsdXJpZWNoaWxkcmVucy5vcmc=; Natascia Tiso, bmF0YXNjaWEudGlzb0B1bmlwZC5pdA==
 Nicola Facchinello
Nicola Facchinello Antionette L. Williams
Antionette L. Williams Natascia Tiso
Natascia Tiso