Abstract
Skin aging manifests as structural degradation, functional decline, and heightened disease susceptibility. Central to this process is the overactivation of the mitogen-activated protein kinase (MAPK) signaling pathway triggered by reactive oxygen species (ROS). Autophagy, a lysosomal degradation mechanism essential for maintaining cellular homeostasis, demonstrates context-dependent duality in skin aging by mediating cytoprotective effects and stress-induced dysfunction. Emerging evidence highlights that the interplay between MAPK signaling and autophagy critically modulates skin aging progression. Despite its therapeutic potential, the lack of effective targeting strategies severely hinders clinical translation. Therefore, this review synthesizes current evidence on MAPK–autophagy interplay across key cutaneous cell populations, namely, keratinocytes, fibroblasts, and melanocytes (including melanoma), revealing cell-type-specific regulatory networks that influence skin aging. Subsequently, we explore the therapeutic potential of natural bioactive compounds targeting this interplay to accelerate the translation of evidence into the progression of strategies for combating skin aging.
1 Introduction
Skin is the barrier that separates the body from the external environment and is responsible for protecting internal organs from external stimuli. As the most voluminous body organ, skin changes are the most recognizable signs of aging. Skin aging is typically characterized by thinning, dryness, reduced elasticity, and abnormal pigmentation, which is determined by the combined influence of intrinsic factors (such as gene mutations, cellular metabolism, or hormonal agents) and extrinsic factors (such as ultraviolet (UV) light, air pollution, smoking, and unhealthy diet) (Krutmann et al., 2021; Mora Huertas et al., 2016). Skin aging affects appearance and triggers health issues, including increased fragility, diminished immune function, impaired vascular support, delayed wound healing, and a heightened risk of skin malignancies. These changes ultimately lead to a decline in the structural integrity of the skin and a loss of its protective barrier function (Quan, 2023; Russell-Goldman and Murphy, 2020). With the acceleration of global aging, skin aging, particularly photoaging, has emerged as an increasingly significant health and societal concern. According to a 15-year longitudinal study, the incidence of photoaging has significantly increased from 42% to 88% (Hughes et al., 2021). Therefore, exploring effective treatments for combating skin aging and photodamage is crucial to address the health challenges of an aging society.
Multiple signaling pathways are involved in the regulation of skin aging. For instance, the transforming growth factor-β (TGF-β)/Smad pathway modulates collagen synthesis, the nuclear factor kappa B (NF-κB) pathway drives inflammatory progression, and the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway mediates antioxidant responses (Chaiprasongsuk and Panich, 2022). Among these, the mitogen-activated protein kinase (MAPK) pathway has received widespread attention because of its key role in skin aging progression and related signaling pathways (Gu et al., 2020). MAPK is a serine/threonine protein kinase that converts extracellular stimuli into a wide range of cellular responses (Sun et al., 1999). Endogenous aging processes (e.g., mitochondrial dysfunction) and environmental assaults (notably UV irradiation) converge to amplify reactive oxygen species (ROS) generation, leading to oxidative stress and an overactivated MAPK signaling pathway (Giorgi et al., 2018; Zhang and Duan, 2018). The activation of this pathway results in collagen degradation, extracellular matrix (ECM) disruption, and excessive inflammatory factor release, thereby accelerating skin aging and establishing it as a key therapeutic target (Bae et al., 2008; Mantena and Katiyar, 2006; Muthusamy and Piva, 2010; Afnan et al., 2016).
Autophagy serves as a pivotal mechanism to maintain cellular homeostasis by mediating the catabolism of cytoplasmic components and the degradation and recycling of damaged proteins and organelles (Levine and Kroemer, 2019). Although autophagy activation is generally associated with delayed aging, emerging evidence suggests that it can also act as a double-edged sword in skin aging (Eckhart et al., 2019; Jeong et al., 2020b). Autophagy is closely linked to the MAPK signaling pathway, which critically regulates autophagy processes through phosphorylation events (Gu et al., 2020). However, the role of the interplay between MAPK signaling and autophagy in aging skin remains incompletely elucidated, particularly regarding cell type-specific outcomes and their therapeutic implications.
In this review, 28 core articles published in recent years were collected from PubMed, Web of Science, Embase, and other databases using keywords such as “MAPK”, “autophagy”, and “skin aging” (Figure 1). The main selection criteria focused on the interplay between MAPK signaling and autophagy in regulating skin aging. This review examines the role and regulatory mechanism of the MAPK–autophagy interplay in the aging of key cutaneous cell populations, such as keratinocytes, fibroblasts, and melanocytes (including melanoma). We also explore emerging strategies targeting this interplay for anti-aging interventions, thereby providing new insights for developing effective skin anti-aging therapies.
FIGURE 1
Literature search flowchart (using PubMed as an example).
2 Skin aging
Skin aging is a complex biological process driven by both intrinsic and extrinsic factors, ultimately leading to the progressive deterioration of skin structure and function (Figure 2). The details of these processes are elucidated in the following sections.
FIGURE 2
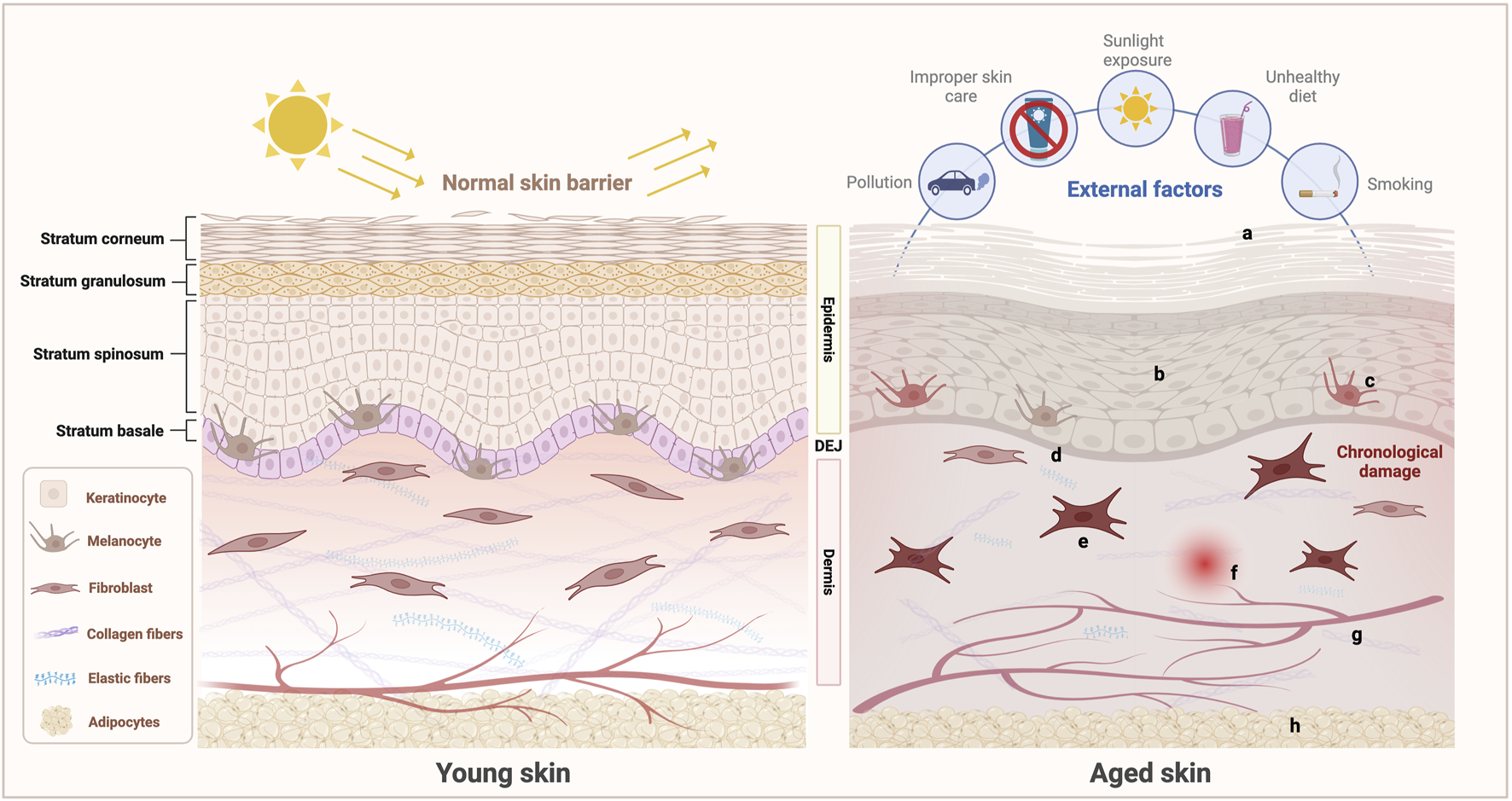
Schematic of the characteristics of young and aged skin. Under the combined influence of intrinsic and extrinsic factors, aging skin undergoes a series of complex structural and functional changes: (a) Epidermal dehydration and impaired barrier function; (b) Proliferation, differentiation, and migration abilities of keratinocytes weaken; (c) Decreased number and declined function of melanocytes, leading to abnormal melanin production and increased risk of melanoma; (d) Flattened DEJ with weakened structural integrity; (e) Senescent changes in the fibroblasts in the dermis, characterized by a reduction in number and functional decline, leading to the decreased synthesis and increased degradation of ECM components such as collagen and elastic fibers and thereby disrupting the dermal reticular structure; (f) Induced inflammation; (g) Compromised integrity of the microvascular network, resulting in vascular fragmentation; (h) Atrophied subcutaneous adipose tissue, accompanied by a reduction in adipocyte numbers. These changes collectively contribute to the characteristic manifestations of skin aging. (Created with BioRender.com).
2.1 Skin structure and function
The skin, the body’s largest organ (approximately 1.8 m2 surface area), serves as a physical barrier against environmental pathogens/insults and a critical regulator of systemic homeostasis through water retention and thermoregulation. Its multilayered architecture and specialized cellular components collectively provide robust defense against microbial invasion, mechanical stress, and chemical exposure (Chambers and Vukmanovic-Stejic, 2020; Nestle et al., 2009). From an anatomical point of view, the skin comprises the following three distinct layers from superficial to deep: epidermis, dermis, and subcutaneous tissue. The epidermis (50–100 μm) consists of the stratum basale, stratum spinosum, stratum granulosum, and outermost stratum corneum. Keratinocytes represent the predominant cell type, constituting over 90% of epidermal cells. Other specialized cell populations include melanocytes, Langerhans cells, dendritic cells, and Merkel cells (Dahabra et al., 2021; Eckhart and Zeeuwen, 2018). Keratinocyte differentiation is initiated by proliferative activity in the stratum basale, followed by progressive migration through epidermal layers. Terminal differentiation culminates during corneocyte formation within the stratum corneum. These organelle-free dead cells establish a formidable physical barrier that effectively prevents pathogen invasion and minimizes transepidermal water loss (Nestle et al., 2009). Melanocytes, residing in the stratum basale, produce pigment-containing melanosomes that determine skin pigmentation and provide photoprotective functions through UV radiation absorption (Rittié and Fisher, 2015).
The dermis (3–5 mm), the principal structural component of the skin, lies beneath the epidermis and interfaces with it through a wavelike dermal–epidermal junction (DEJ) (Lavker et al., 1989). Histologically divided into two regions, the superficial papillary layer consists of loose connective tissues containing extensive vascular networks and sensory nerve endings that facilitate epidermal nourishment and sensory transduction. The deeper reticular layer, comprising dense irregular connective tissues rich in collagen and elastic fibers, constitutes the bulk of the dermis and confers mechanical resilience and elasticity to the skin (Brown and Krishnamurthy, 2024). The metabolically active dermis serves as the primary site for wound healing (Dahabra et al., 2021). Cellular constituents primarily include fibroblasts, macrophages, and other immune cells. Fibroblasts, the principal resident cells, synthesize and maintain collagen/elastic fibers, proteoglycans, and glycosaminoglycans, thereby establishing a 3D ECM network essential for dermal structural integrity (Gerasymchuk et al., 2020; Shin et al., 2019). These cells also play pivotal roles in wound repair and tissue remodeling (Ren et al., 2022). The dermis further harbors numerous skin appendages, including hair follicles, sebaceous glands, and sweat glands (Brown and Krishnamurthy, 2024). Beneath the dermis lies the subcutaneous tissue (hypodermis), which is predominantly composed of adipocytes, bursae, vasculature, and connective tissues. This layer provides thermal insulation, serves as an energy reservoir, and mechanically anchors the skin to underlying musculoskeletal structures while cushioning mechanical impacts (Fore, 2006).
2.2 Intrinsic skin aging
Intrinsic skin aging is a series of chronologically physiological changes primarily driven by genetic and hormonal factors. This process involves a cascade of molecular dysregulations, including DNA damage, telomere shortening, excessive ROS production, and mitochondrial dysfunction, which collectively drive the accumulation of senescent cells in tissues and thereby contribute to progressive senescence (Salminen et al., 2022). Age-dependent functional deterioration occurs across all skin strata—epidermis, DEJ, and dermis. Epidermal aging is characterized by diminished keratinocyte proliferative capacity, resulting in progressive thinning of both the viable epidermis and stratum corneum (Low et al., 2021). Impaired keratinocyte differentiation characterized by the reduced synthesis of keratins and structural proteins compromises the epidermal barrier integrity and hydration capacity (Wang et al., 2020). Age-related declines in keratinocyte migratory potential further disrupt reepithelialization during wound healing, resulting in delayed tissue repair (Ross et al., 2011). In addition, cellular attrition exacerbates epidermal atrophy, with melanocyte populations reportedly decreasing by 8%–20% per decade (Gilchrest et al., 1979). Intrinsic skin aging also manifests through structural alterations in the dermal papillae and basal layer, characterized by progressive thinning. This anatomical remodeling induces DEJ flattening, thereby reducing the interfacial contact area between dermal and epidermal compartments. As a consequence, the diminished exchange surface impairs nutrient diffusion to the epidermis while suppressing basal keratinocyte proliferative capacity (Russell-Goldman and Murphy, 2020; Sauermann et al., 2002).
During intrinsic aging, the dermis exhibits a progressive decline in the number and functional capacity of fibroblasts. This cellular deterioration leads to the diminished synthesis of collagen and elastic fibers, thereby contributing to cutaneous laxity and wrinkle formation (Freitas-Rodríguez et al., 2017; Kohl et al., 2011). Fibroblast senescence also contributes to skin aging by secreting a senescence-associated secretory phenotype, which decreases proliferation by impeding the release of essential growth factors and enhances the degradation of the ECM by activating matrix metalloproteinases (MMPs) (Ghosh and Capell, 2016). The ROS produced during aging are the main stimuli increasing MMP levels in aging skin, leading to collagen degradation and fibroblast senescence. Aged fibroblasts also produce additional ROS, further promoting MMP expression and forming a vicious cycle that accelerates dermal aging. This process severely hinders mechanical interactions between fibroblasts and ECM, resulting in reduced fibroblast volume (Freitas-Rodríguez et al., 2017; Shin et al., 2019). The functional decline and volume reduction of dermal fibroblasts are important reasons for the slowed wound healing in aged skin (Boismal et al., 2020).
2.3 Extrinsic skin aging (photoaging)
Skin aging is not solely driven by intrinsic factors; long-term exposure to solar UV radiation is the primary cause of extrinsic skin aging, also known as photoaging, which accounts for approximately 80% of total skin aging (Friedman, 2005). Photoaging and intrinsic aging overlap, causing areas frequently exposed to sunlight, such as the face, neck, forearms, and dorsal hands, to exhibit premature aging phenotypes compared with photoprotected skin (Rittié and Fisher, 2015). Photoaged skin is clinically characterized by distinct pathological features, including leathery texture, deep rhytides, telangiectasia (“broken” capillaries), mottled hyperpigmentation, and lentigines, and is in stark contrast to skin that predominantly undergoes intrinsic aging (Brooke et al., 2001). UV radiation is classified into the three subtypes based on wavelength, biological activity, and cutaneous penetration: long-wave UVA (320–400 nm), medium-wave UVB (280–320 nm), and short-wave UVC (200–280 nm) (Dorf and Maciejczyk, 2024). Despite being a weak mutagen, UVA penetrates deeply into the dermal and subcutaneous layers due to its long wavelength, acting as the principal driver of photoaging through sustained oxidative damage (Battie et al., 2014). By contrast, UVB radiation, though limited to epidermal absorption due to its short wavelengths, exhibits potent genotoxicity by directly inducing thymine dimer photoproducts that cause DNA damage, thereby initiating mutagenic processes and carcinogenesis (Guan et al., 2021). UVC demonstrates the strongest mutagenic potential but is effectively filtered by the stratospheric ozone under normal atmospheric conditions and thus fails to reach the Earth’s surface (Dorf and Maciejczyk, 2024).
UV radiation initiates a cascade of molecular and cellular events that drive accelerated skin aging. It induces ROS generation, resulting in oxidative stress and DNA damage. Concurrent inflammatory pathway activation, MMP upregulation, and disrupted collagen/elastin synthesis further exacerbate cutaneous degeneration and elasticity loss (Cai et al., 2023). A hallmark histological feature of photoaging is the accumulation of abnormally thickened and fragmented elastic fibers, termed “solar elastosis” (Tsuji, 1980). Extrinsic aging also induces a drastic reduction in type I collagen and an increase in type III collagen deposition. This imbalance in the I/III collagen ratio renders aged skin fragile and inelastic (Dorf and Maciejczyk, 2024). The epidermal rete of photoaged skin is thicker than that of chronologically aged skin, and epidermal atrophy is observed in severely photoaged skin (Bhawan et al., 1995). UV radiation-induced keratinocyte damage reduces cellular activity and decelerates epidermal turnover, leading to compromised barrier function. This effect manifests clinically as severe xerosis (dryness) and desquamation, with UV-driven effects often surpassing the severity of intrinsic aging phenotypes (Farage et al., 2013). As a photoprotective mechanism, melanogenesis is upregulated to counteract UV-generated ROS (Eller et al., 1994). However, chronic sun exposure promotes uneven pigmentation in aged skin that frequently progresses to solar lentigines, which are characteristic hyperpigmented lesions pathognomonic of photoaging (Black, 2016; Rittié and Fisher, 2015). Furthermore, excessive UV exposure increases melanoma risk through melanocytic malignant transformation, while age-related remodeling of the tumor microenvironment during physiological senescence further enhances susceptibility, establishing melanoma as a critical event in cutaneous aging (Tsai and Chien, 2022; Urban et al., 2021). Finally, photoaging accelerates the loss of subcutaneous fat, weakening the skin’s supportive structure and leading to increased sagging and hollowing of the skin (Scharffetter-Kochanek et al., 2000).
3 Autophagy: key event in skin aging
3.1 Molecular mechanisms of autophagy
Autophagy is a highly conserved cellular degradation and recycling process present in all eukaryotic organisms. In mammalian cells, autophagy primarily includes three types: chaperone-mediated autophagy (CMA), microautophagy, and macroautophagy. These types all facilitate the proteolytic degradation of cytoplasmic components within lysosomes, but their transport mechanisms differ (Mizushima and Komatsu, 2011; Yang and Klionsky, 2010). In CMA, targeted proteins are translocated across the lysosomal membrane by complexing them with chaperone proteins (such as HSC-70), which are recognized by the lysosomal membrane receptor lysosomal-associated membrane protein 2A, resulting in their unfolding and degradation (Saftig et al., 2008). In microautophagy, invaginations or protrusions of the lysosomal membrane are used to capture cargo, and the uptake occurs directly at the limiting membrane of the lysosome (Parzych and Klionsky, 2014). Macroautophagy (hereafter referred to as autophagy) is the most extensively studied type to date. It is the engulfment of large structures through selective and nonselective mechanisms and is characterized by the formation of autophagosomes. This process involves three key steps: initiation, nucleation, and elongation, each of which is regulated by multiple critical genes and signaling pathways (Cao et al., 2021). Stress signals that trigger autophagy typically encompass starvation, hypoxia, oxidative stress, protein aggregation, and endoplasmic reticulum (ER) stress (Glick et al., 2010). A classic example is starvation-induced autophagy, where a reduction in nutrient supply leads to bioenergetic stress and elevated AMP levels. This phenomenon activates AMP-activated protein kinase (AMPK), which subsequently inhibits the mammalian target of rapamycin complex 1 (mTORC1). mTORC1 inhibition promotes the assembly of the UNC-51 like kinase 1 (ULK1) complex, which comprises ULK1 (homologous to yeast autophagy-related gene 1 (ATG1)), ATG13, ATG101, and focal adhesion kinase family interacting protein of 200 kDa (FIP200). In response to stress signaling, the ULK1 complex is recruited to the phagophore assembly site on the ER, initiating autophagosome formation (Xue et al., 2024). Conversely, the presence of nutrients and growth factors activates mTORC1, leading to the phosphorylation of autophagy-related proteins and the suppression of autophagy (Kim and Guan, 2015; Wang and Zhang, 2019). In the second step, the activated ULK1 complex induces phagophore nucleation by promoting the production of phosphatidylinositol 3-phosphate (PI3P) through the formation of a supramolecular complex with class III phosphoinositide 3-kinase (PI3K) activity. This complex consists of VPS34, VPS15, ATG14, Beclin-1, autophagy and Beclin-1 regulator 1 (AMBRA1), and/or UV radiation resistance-associated gene (UVRAG). It accompanies the recruitment of ATG9-containing vesicles, and its activity is subjected to tonic inhibition by B-cell lymphoma-2 (Bcl-2). Finally, in the phagophore elongation step, ATG7 and ATG10 catalyze the formation of the ATG12–ATG5–ATG16L1 complex. At the same time, ATG4, ATG7, and ATG3 cooperate to cleave the precursor of microtubule-associated protein light chain 3 (LC3)-like proteins into their mature forms, which are then conjugated with phosphatidylethanolamine (PE) and recruited to the autophagosome with the support of WD-repeat protein interacting with phosphoinositide (WIPI) proteins. LC3 and LC3 homologs enable the autophagosome to bind to autophagy substrates and/or mediate cargo-selective proteins, including p62 (Galluzzi and Green, 2019; Xue et al., 2024). Upon completion, the phagophore is sealed to form the characteristic double-membrane autophagosome. As autophagy progresses, autophagosomes fuse with lysosomes to form autolysosomes. This fusion enables progressive acidification, consequently activating lysosomal hydrolases that mediate substrate degradation. The resulting breakdown products are released back into the cytoplasm for reuse or to provide energy (Choi et al., 2013).
3.2 Dual role of autophagy in skin aging
Autophagy serves as a pivotal regulator of cutaneous homeostasis, functioning as a critical mechanism of intracellular quality control for keratinocytes, fibroblasts, and melanocytes (Sukseree et al., 2013). Under physiological and stress conditions, autophagy facilitates the clearance of senescent organelles and misfolded proteins, thereby modulating skin cell functionality and delaying aging progression (Vikram et al., 2024; Wang et al., 2019b; Zhong et al., 2024). For instance, the autophagy inducer heptasodium hexacarboxymethyl dipeptide-12 (Aquatide™) enhances oxidative stress resistance in normal human epidermal keratinocytes in vitro while clinically improving skin elasticity and texture (Lim et al., 2019). Similarly, rapamycin (mTOR inhibitor) attenuates UVB-induced fibroblast photoaging by activating autophagy to suppress ROS accumulation (Qin et al., 2018). Preclinical studies revealed that a variety of naturally derived bioactive compounds exert significant anti-aging effects on the skin through a multitarget regulation of the autophagy pathway (Li et al., 2018; Lin et al., 2024; Yang et al., 2023). Moreover, skin aging is closely linked to and partially driven by autophagy defects (Eckhart et al., 2019). ATG7-deficient keratinocytes have exhibited increased DNA damage and senescence markers following oxidative stress induced by paraquat, a cellular senescence-inducing oxidant (Song et al., 2017). Autophagy deficiency is strongly associated with premature senescence and oxidative damage accumulation in melanocytes (Ni et al., 2016; Setaluri, 2015; Zhang et al., 2015). Furthermore, chronic and repeated UVA exposure disrupts lysosomal function in skin fibroblasts, impairing intracellular degradation mechanisms and contributing significantly to photoaging (Huang et al., 2019).
Despite these compelling data, some studies took the opposite view and suggested that autophagy activation may promote skin aging. A significant increase in autophagy vesicles was observed in senescent fibroblasts (Gerland et al., 2003), and an increase in autophagic activity was reported in senescent human keratinocytes induced by ROS (Gosselin et al., 2009). These findings suggest that cells may enhance autophagic activity in response to stress to cope with damage and restore survival (protective autophagy). However, this activity may lead to the accumulation of damaged cells, thereby promoting aging. Several studies supported this view. Young et al. (2009) demonstrated that oncogene-induced fibroblast senescence depends on prior autophagic activity, and reducing autophagic expression levels through pharmacological or genetic approaches can inhibit the onset of senescence. Similarly, ATG5 overexpression has been shown to reduce the proliferation of melanoma cells and induce senescence, while autophagy inhibition delays oncogene-induced senescence (Liu et al., 2013). These results indicate that the role of autophagy in skin aging may exhibit a context-dependent duality. If autophagy is excessively activated or prolonged, it may lead to cell death due to the excessive elimination of essential cellular proteins or organelles. This phenomenon explains why autophagy is also referred to as type II programmed cell death (type I being apoptosis) (Lin et al., 2024; Tsujimoto and Shimizu, 2005). Autophagic flux-dependent outcomes have been observed in cutaneous oncology. Studies found that in senescent human keratinocytes, excessive autophagy activation can induce senescent cell death, thereby inhibiting tumors; whereas moderate autophagy activation facilitates the escape of senescent cells from death and promotes the growth of tumor cells (Deruy et al., 2014). This phenomenon has also been widely observed in melanoma (Pangilinan et al., 2024) (Figure 3). In summary, these findings position autophagy regulation as an essential therapeutic target for skin anti-aging interventions.
FIGURE 3
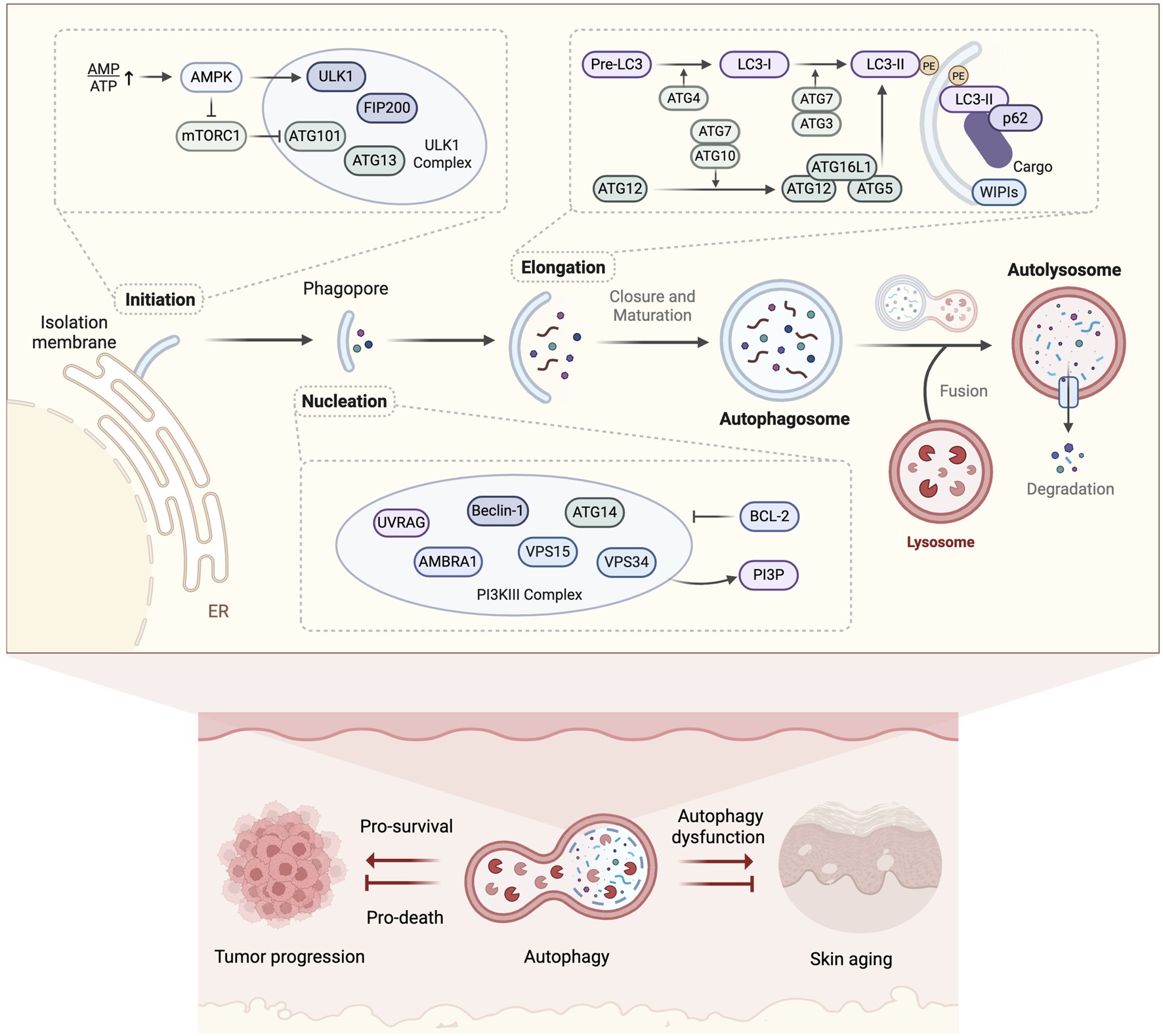
Molecular mechanisms of autophagy and its dual role in skin aging. Autophagy mediates cytosolic cargo clearance through double-membrane autophagosome formation, lysosomal degradation, and metabolite recycling, exhibiting dual regulatory roles in skin aging and associated tumor progression. (Created with BioRender.com).
4 MAPK signaling pathway: potential bridge between skin aging and autophagy
4.1 Role of MAPK signaling pathway in skin aging
The signaling proteins of the MAPK pathway mainly belong to the family of serine/threonine protein kinases; this pathway is a key cellular signal transduction mechanism widely involved in the regulation of cell proliferation, differentiation, stress response, and apoptosis and other biological processes (Pearson et al., 2001). It comprises three main classes of kinases: mitogen-activated protein 3 kinase (e.g., Raf), mitogen-activated protein 2 kinase (e.g., MEK) and MAPK. Among the pathways of MAPK family, extracellular signal-regulated kinases 1 and 2 (ERK1/2), c-Jun amino-terminal kinases 1 to 3 (JNK1 to 3), and p38 MAPK (α, β, γ, and δ) are the most widely studied. By activating upstream signals through external stimuli (such as growth factors, cytokines, neurotransmitters, hormones, and cellular stress), the MAPK signaling pathway can regulate the expression of downstream target genes and thereby influence cellular physiological states (Cargnello and Roux, 2011; Gaestel, 2015).
MAPK is important in skin aging and is primarily activated by the ROS generated in response to age-related mitochondrial dysfunction and external factors, such as UV radiation (Chaiprasongsuk and Panich, 2022; Shin et al., 2019) (Figure 4). The heterodimer activator protein 1 (AP-1) composed of c-Fos and c-Jun is a key transcription factor that regulates the expression of MMP-1, MMP-3, and MMP-9, resulting in collagen degradation. The expression of c-Jun and c-Fos is regulated by the MAPK signaling pathways, with ERK stimulating c-Fos expression; meanwhile, the activation of p38 and JNK is essential for c-Jun expression (Chiang et al., 2013; McBride and Nemer, 1998; Pramanik et al., 2003). The MAPK-induced activation of AP-1 suppresses TGF-β signaling, a key regulator of ECM biosynthesis (Gao et al., 2018; Pittayapruek et al., 2016). TGF-β enhances collagen synthesis and inhibits its degradation by downregulating MMPs through the Smad pathway and upregulating the tissue inhibitors of metalloproteinases (TIMPs) (Shin et al., 2019; Verrecchia and Mauviel, 2002). NF-κB is another important MMP transcription factor activated by the MAPK signaling (Baek et al., 2024). NF-κB activity upregulates MMPs such as MMP-1 and MMP-3 in dermal fibroblasts (Lee et al., 2012; Park et al., 2014). In addition, ROS and activated MAPK signaling pathways facilitate the dissociation of the NF-κB inhibitor protein IκB in the cytoplasm, leading to the translocation of NF-κB into the nucleus where it induces the expression of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), IL-1β, and cyclooxygenase-2 (COX-2), contributing to skin damage and inflammatory responses (Bell et al., 2003; Han et al., 2022; Wang et al., 2019a). These pro-inflammatory cytokines could further induce the expression of MMPs (Nisar et al., 2022). Activated NF-κB upregulates the expression of heme oxygenase-1 (HO-1), indirectly increasing the levels of free iron in the cells and thereby promoting ROS production through the Fenton reaction (Kammeyer and Luiten, 2015; Li et al., 2019). The MAPK signaling can also mediate UVB-induced melanogenesis (Hu et al., 2019; Zhou et al., 2022). Its abnormal activation is a major cause of melanoma progression. For instance, UV radiation triggers the sequential activation of Ras, Raf, MEK, and ERK in cells, thereby regulating various carcinogenic biological activities (Guo et al., 2021).
FIGURE 4
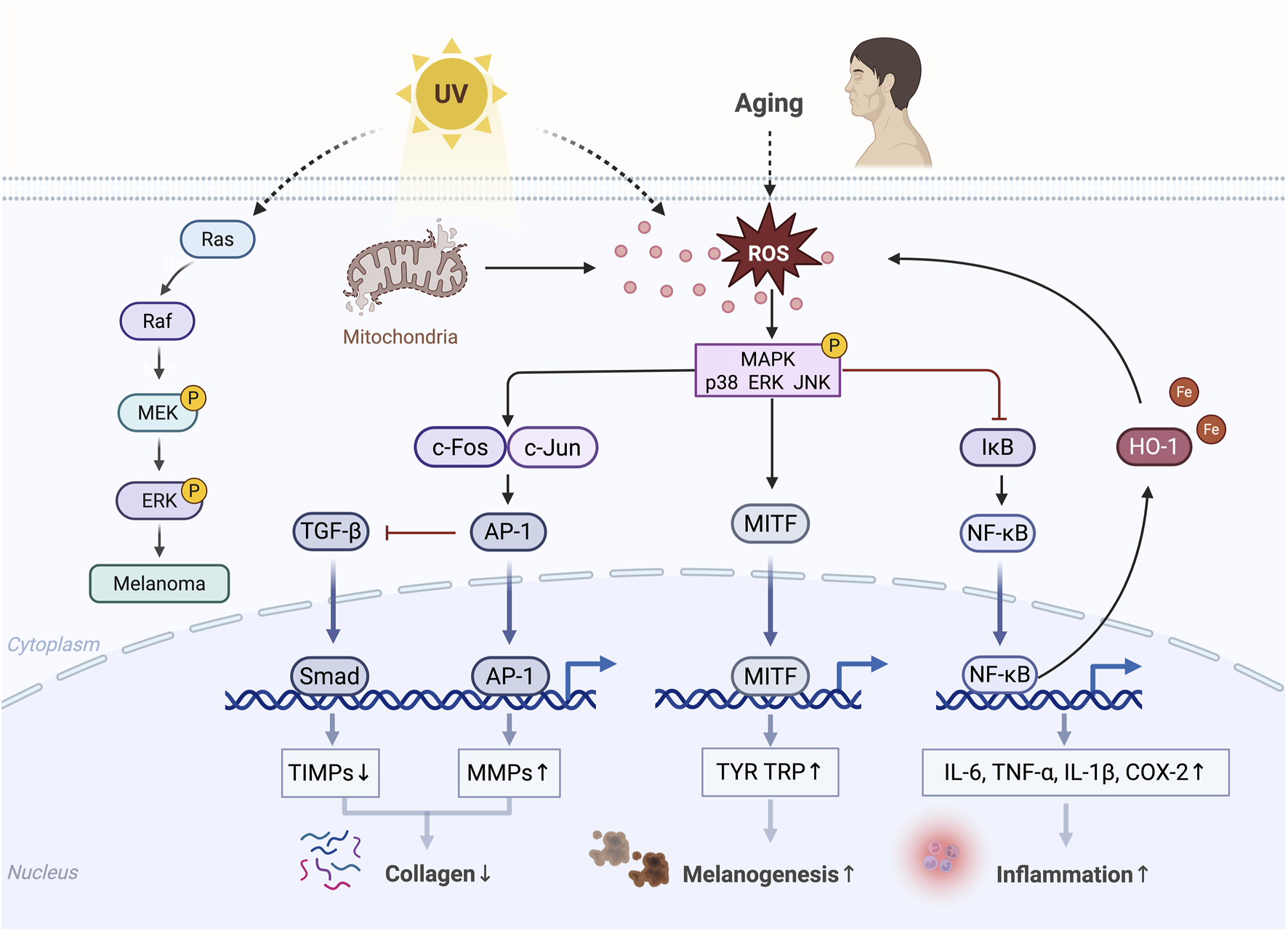
Diagram of MAPK-mediated skin aging. Phosphorylated MAPK, activated by ROS, inhibits collagen production; promotes inflammatory responses, melanogenesis, and melanoma progression; and ultimately accelerates skin aging.↑, upregulation; ↓, downregulation (Created with BioRender.com).
4.2 Role of MAPK and autophagy in skin aging
Increasing evidence highlights a mechanistic interplay between the MAPK signaling pathway and autophagy that modulates the aging of key skin cell populations, such as keratinocytes, fibroblasts, and melanocytes (including melanoma) (Figure 5; Table 1). The specific mechanisms involved are detailed in the following subsections.
FIGURE 5
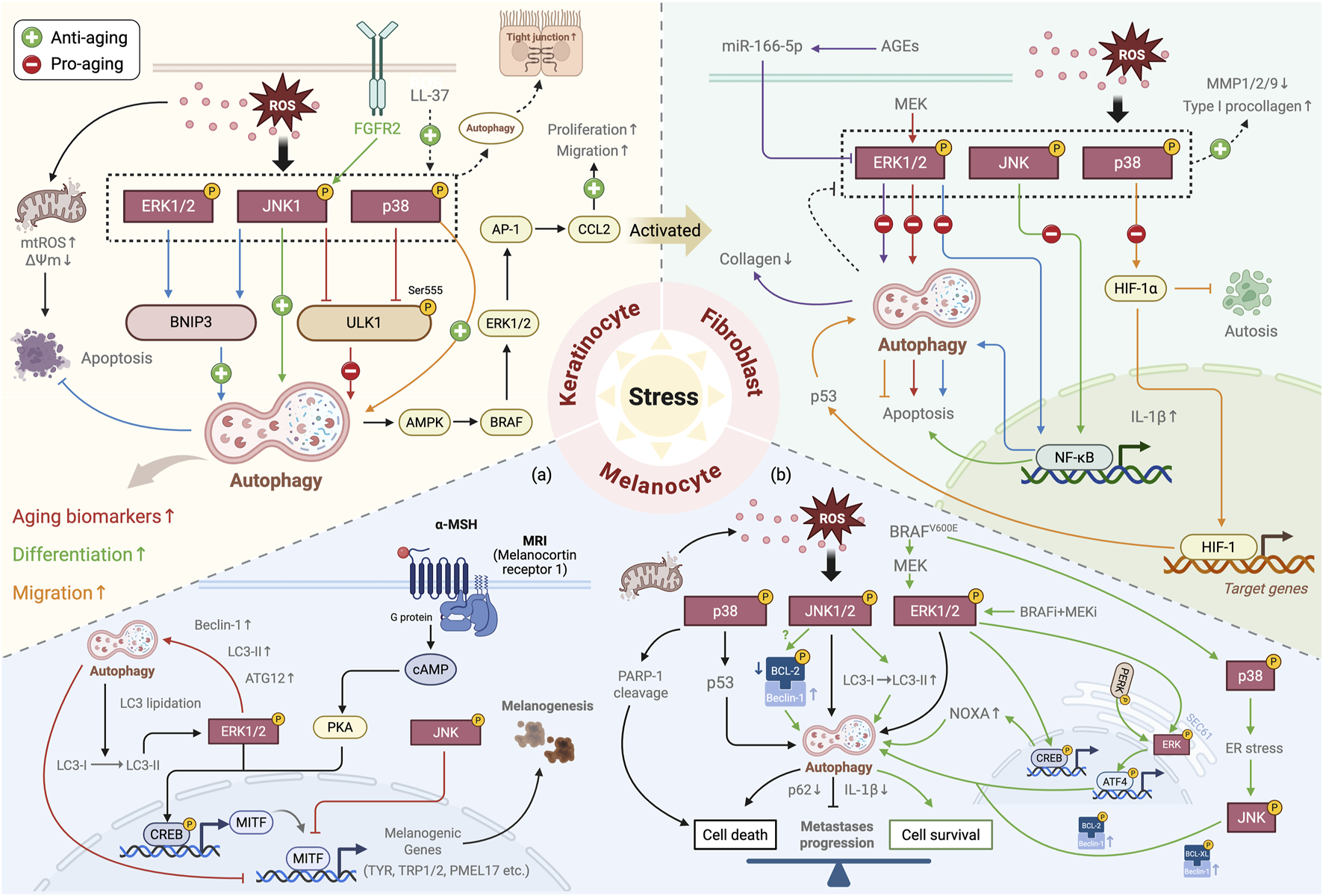
Summary of the role of the MAPK–autophagy interplay in skin aging. Under stress conditions, the interplay between the MAPK signaling and autophagy plays a crucial regulatory role in key skin cell populations: in keratinocytes, it regulates cell proliferation, differentiation, and migration, influencing epidermal barrier function and the expression of aging-related biomarkers; in skin fibroblasts, it primarily modulates the crosstalk between autophagy and apoptosis and collagen synthesis and degradation, participating in skin aging; in melanocytes (a) it regulates melanogenesis (black pathway) and degradation (red pathway); and in melanoma (b) it exhibits pro-tumor (green pathway) and anti-tumor (black pathway) effects by modulating the balance between cell death and survival. In the diagram, the differently colored lines illustrate the regulatory mechanisms mediated by the interplay of specific MAPK subtypes and autophagy, and the dotted lines represent the overall activation/inhibition of the MAPK pathway.↑, upregulation; ↓, downregulation (Created with BioRender.com).
TABLE 1
| Skin cell types | Functional module | Mechanisms and involved molecules | References |
|---|---|---|---|
| Keratinocyte |
|
|
Gu et al. (2022b), Gu et al. (2022a) and Moriyama et al. (2017) |
|
|
Ikutama et al. (2023), Li et al. (2019), Nanni et al. (2018), Qiang et al. (2020) and Zhang et al. (2019) | |
| Fibroblast |
|
|
Lim et al. (2020), Qiang et al. (2020) and Zeng et al. (2019) |
|
|
Lim et al. (2021), Bravo-San Pedro et al. (2013) and Zuo and Ma (2024) | |
| Melanocyte |
|
|
Cho et al. (2017) and Yun et al. (2016) |
|
|
Liu et al. (2009), Cho et al. (2017) and Tan et al. (2018) | |
|
|
Liu et al. (2014), Jeon et al. (2023), Hseu et al. (2019), Valli et al. (2020), Corazzari et al. (2015) and Ojha et al. (2019) |
The regulatory role of MAPK–autophagy interplay in skin aging.
4.2.1 Role of MAPK–autophagy interplay in keratinocytes
Keratinocytes are central to skin aging due to their functional decline and diminished regenerative capacity, which compromise epidermal barrier integrity and accelerate aging-related pathologies (Jeong et al., 2020b). The MAPK signaling pathway serves as a crucial regulator of autophagy and aging mechanisms in these cells. Pharmacological inhibition of the p38 MAPK pathway activates autophagy, effectively counteracting oxidative stress-induced senescence in HaCaT keratinocytes and mitigating UVB-induced photoaging in murine models. This phenomenon is exemplified by the p38 MAPK activator dehydrocorydaline (DE), which suppresses autophagy by elevating p62 expression and reducing Beclin-1 activation alongside diminished LC3II/I conversion. Conversely, the p38 MAPK inhibitor SB203580 amplifies autophagic activity (Gu et al., 2022b). Parallel research revealed that the dual inhibition of p38 MAPK and JNK pathways enhances autophagy in HaCaT cells exposed to the free radical generator AAPH, thereby diminishing the expression of senescence markers p21, p16, and K9-trimethylated histone 3 (K9M-H3) and concurrently ameliorating UVB-induced photodamage in vivo. These effects are mediated by the phosphorylation of ULK1 at Ser555, with the MAPK modulation directly influencing ULK1 phosphorylation status and subsequent autophagic activity (Gu et al., 2022a). This result reveals that autophagy regulation in keratinocytes depends on MAPK signaling, with ULK1 serving as its essential downstream mediator. Previous studies in other cell types have demonstrated that the MAPK/ULK1 axis critically modulates autophagy-related inflammatory responses and aging (Jin et al., 2022; She et al., 2018; Slobodnyuk et al., 2019). All these findings highlight MAPK pathway inhibition as a promising therapeutic strategy against keratinocyte senescence through autophagy activation. Further exploration of MAPK/ULK1 axis regulatory mechanisms remains essential for advancing cutaneous anti-aging interventions.
Contrary to the above findings, MAPK inactivation suppresses autophagic flux in psoriasiform keratinocytes (Wang et al., 2021), suggesting the context-dependent pro-autophagic roles of MAPK activation. UVB irradiation upregulates BCL2 and adenovirus E1B 19-kDa interacting protein 3 (BNIP3), a pro-autophagy mitochondrial protein, protecting human primary epidermal keratinocytes from apoptosis (Moriyama et al., 2014). Further studies revealed that BNIP3-induced autophagy occurs through the UVB-generated ROS-mediated activation of JNK and ERK, wherein JNK regulates and synergizes with ERK1/2 to enhance BNIP3 expression. This autophagic process increases mitochondrial membrane potential, reduces mitochondrial ROS production, and degrades dysfunctional mitochondria, ultimately protecting keratinocytes from apoptosis (Moriyama et al., 2017). These findings suggest that MAPK-mediated autophagy is crucial for shielding keratinocytes from UV-induced photodamage. Furthermore, the interplay between MAPK signaling and autophagy critically regulates keratinocyte migration, differentiation, and proliferation. During wound healing, the ROS accumulation induced by the hypoxic microenvironment enhances the phosphorylation of p38 MAPK and JNK, which in turn upregulates BNIP3-mediated autophagy and facilitates HaCaT cell migration (Zhang et al., 2019). However, a high-glucose environment inhibits the p38 MAPK pathway, leading to autophagy inactivation and blocked HaCaT cell migration; the p38 MAPK activator MKK6(Glu) can restore migratory capacity in an autophagy-dependent manner. Immunoprecipitation experiments showed that p38 MAPK–ATG5 interaction is strengthened by MKK6(Glu) overexpression but weakened upon ATG5 silencing, suggesting that the p38 MAPK activates autophagy through the transcriptional regulation of ATGs to promote cell migration (Li et al., 2019). Specifically expressed in epithelial cells, fibroblast growth factor receptor 2b (FGFR2b) can induce the transcriptional activation of autophagy by triggering the downstream JNK1 pathway, thereby promoting early differentiation in HaCaT cells (Nanni et al., 2018). Recent studies in human primary epidermal keratinocytes and their engineered 3D skin equivalent models showed that cathelicidin LL-37, a multifunctional antimicrobial peptide expressed in keratinocytes, induces the phosphorylation of ERK, JNK, and p38 MAPK, triggering autophagy. This process enhances cellular differentiation, promotes the membrane distribution of tight junction proteins such as Claudin-1 and ZO-1, and ultimately improves epidermal barrier function (Ikutama et al., 2023). All these results suggest that the modulation of autophagy by the MAPK pathway (either activation or inhibition) and its subsequent effects on keratinocyte senescence are context-dependent, varying with stress conditions and cell model. For example, in the same cell model, oxidative stress activates p38 MAPK to suppress autophagy and accelerate senescence (Gu et al., 2022b), whereas a high-glucose environment inhibits p38 MAPK, impairing autophagy and cell migration (Li et al., 2019). Under oxidative stress, JNK suppresses autophagy in immortalized HaCaT cells but promotes it in primary keratinocytes (Gu et al., 2022a; Moriyama et al., 2017). These cellular context differences may explain the subtype-specific effects of MAPKs. Notably, the interplay between MAPK signaling and autophagy is binary, and the autophagy can regulate MAPK activity reversely. Qiang et al. (2020) demonstrated that autophagic processes activate AMPK/B-Raf proto-oncogene (BRAF)/ERK1/2/AP-1 signaling cascade, thereby enhancing chemokine C-C motif ligand 2 (CCL2)-mediated keratinocyte migration and proliferation. This intricate bidirectional regulatory network underscores the critical role of MAPK–autophagy interplay in keratinocyte senescence. Thus, the mechanistic link between MAPK signaling pathway and autophagy in keratinocytes deserves further exploration.
4.2.2 Role of MAPK–autophagy interplay in skin fibroblasts
The interplay between MAPK signaling and autophagy plays a pivotal role in skin fibroblast senescence. Although early investigations revealed comparable autophagic flux between aged and young fibroblasts, autophagy may not be sufficient to maintain cellular cleanliness due to the increased accumulation of waste products resulting from the increased metabolic rate of senescent cells, which can lead to skin aging (Kim et al., 2018). Recent studies have positioned fibroblast autophagy as a critical determinant in skin rejuvenation (Lu et al., 2024), with the combined regulation of MAPK and autophagy demonstrating protective efficacy against UVB-induced photoaging (Kim et al., 2024; Wen et al., 2018; Zhang et al., 2022). Primarily produced in the dermis, advanced glycation end products (AGEs) are critical mediators of skin aging (Chen et al., 2020). MiR-106b-5p in endothelium-derived exosomes induced by AGEs targets and downregulates ERK1/2, which in turn activates autophagy in human primary foreskin fibroblasts, leading to the reduction of collagen and finally resulted in the delayed wound healing (Zeng et al., 2019). However, in a full-thickness excisional wound mouse model, autophagy upregulates CCL2 expression via the AMPK/BRAF/ERK1/2/AP1 pathway, enhancing fibroblast activation and coordinating keratinocyte-fibroblast interactions to facilitate wound healing (Qiang et al., 2020). Furthermore, activating autophagy in HDFs reduces ROS-mediated MAPK activation under UVB irradiation, leading to a decreased expression of MMP-1, MMP-2, and MMP-9 and a promoted production of type I procollagen (Lim et al., 2020). These results further highlight the context-dependent interplay between MAPK signaling and autophagy in skin aging regulation. Interestingly, an increase in BNIP3 levels in dermal fibroblasts was also observed during wound healing under hypoxic conditions, further activating the autophagic process and promoting cell migration and proliferation (Zhang et al., 2024). Whether BNIP3 upregulation in skin fibroblasts is regulated by MAPK signaling pathways, as observed in keratinocytes, requires further confirmation in future studies.
The crosstalk between autophagy and apoptosis in fibroblasts, governed by MAPK signaling, represents a critical regulatory axis in cutaneous aging. Apoptosis plays a pivotal role in aging, and the accumulation of apoptotic cells accelerates skin aging (Guerrero-Navarro et al., 2024; Victorelli et al., 2023). Mechanistic studies revealed that ROS/ERK/NF-κB pathway drives autophagy-dependent apoptosis in HDFs exposed to bisphenol A (a chemical known to accelerate skin aging), significantly compromising cellular viability. Pharmacological inhibition of ERK signaling attenuates autophagic gene expression and delays autophagic cell death (Lim et al., 2021). Despite parallel investigations demonstrating the ROS/JNK/NF-κB-mediated induction of apoptosis and IL-1β secretion in HDFs, the involvement of autophagy in this pathway requires further validation (Park et al., 2021). In dermal fibroblasts derived from patients with Parkinson’s disease, the autophagy induced by the MEK/ERK1/2 pathway enhances cellular sensitivity to damage and leads to increased apoptosis, which can be reversed through MAPK pathway inhibition (Bravo-San Pedro et al., 2013). These findings suggest that inhibition of MAPK-mediated pro-apoptotic autophagy may delay skin aging under different pathological conditions. However, in cases of autophagy dysregulation (such as excessive protective autophagy), inducing apoptosis is crucial for eliminating the accumulation of senescent or damaged cells. Previous work showed that autophagy is required for UVB-induced aging in HDFs, with its inhibition redirecting cell fate from senescence to apoptosis clearance (Cavinato et al., 2017). Lu et al. (2024) further proposed that prolonged autophagy in fibroblasts impedes harmful substance clearance. In this situation, inhibiting autophagy and promoting fibroblast apoptosis can help alleviate the negative state of the skin, leading to the generation of fibroblasts that secrete fibrous and amorphous ECM proteins and thereby maintaining the integrity of the skin structure. Recent studies have found that regulating the MAPK signaling pathway can trigger a shift from protective autophagy to apoptosis in human skin hypertrophic scar fibroblasts. Specifically, inhibiting ROS-mediated p38 MAPK phosphorylation under hypoxic and ischemic conditions can decrease the expression levels of hypoxia-inducible factor-1α (HIF-1α) and p53, leading to a reduction in LC3-II and Beclin-1. Meanwhile, the expression levels of cleaved caspase 3 and Bax increases, and autosis (a form of autophagy-dependent non-apoptotic cell death) is observed (Zuo and Ma, 2024). All these findings suggest that modulating the MAPK signaling pathway could be a promising strategy to balance autophagy and apoptosis for anti-aging interventions. Therefore, exploring the modulation of the MAPK signaling pathway under different conditions to effectively manage the transition between autophagy and apoptosis in skin fibroblasts and achieve anti-aging effects deserves further investigation.
4.2.3 Role of MAPK–autophagy interplay in melanocytes and melanoma
4.2.3.1 Melanocytes
Pigmentation changes during aging; hyperpigmentation, which is characterized by increased melanin production, affects the skin’s appearance, impairs its barrier function, and exacerbates skin aging (Kovacs et al., 2022; Lee, 2021). Emerging evidence positions autophagy-related proteins and MAPK signaling as coregulators of melanogenic pathways (Chen et al., 2021; Jeong et al., 2020a; Jeong et al., 2019; Lee et al., 2022; Yu et al., 2024; Zhu et al., 2020), though their mechanistic interplay remains elusive. Central to this process is microphthalmia transcription factor (MITF), the master regulator of melanocyte differentiation (E, 2017). In Melan-a melanocytes, knockdown of LC3 (but not Beclin-1/ATG5) significantly reduces MITF expression and melanogenesis. Specifically, LC3 knockdown suppresses α-melanocyte-stimulating hormone (α-MSH, a representative stimulator of MITF expression)-mediated melanogenesis by attenuating cAMP response element-binding protein (CREB) phosphorylation and MITF expression via decreased ERK activity, thereby downregulating melanogenesis-related genes such as tyrosinase (TYR) and premelanosome protein (PMEL) 17. ERK overexpression reverses the effect of LC3 knockdown on CREB phosphorylation and MITF expression (Yun et al., 2016). In contrast to normal melanocytes, ERK1/2 activation in melanoma cells induces the autophagic degradation of MITF via Beclin-1/ATG12/LC3-II upregulation, leading to the downregulation of TYR and tyrosinase-related proteins (TRP) 1/2 and ultimately suppressing melanin production (Cho et al., 2017). Moreover, MAPK and autophagy may regulate melanin homeostasis in different cellular contexts. A study found that in normal melanocytes, the activation of the JNK pathway inhibits MITF expression, exerting an antimelanogenic effect; under H2O2-induced oxidative stress, autophagy activation sustains MITF expression to promote cell survival (Cho, 2021). Unfortunately, this work did not explore the specific relationship between the MAPK signaling pathway and autophagy. These results suggest that the interplay between MAPK signaling and autophagy bidirectionally regulates melanogenesis, with the direction of regulation dependent on the cellular context (physiological or pathological conditions). Considering the important therapeutic potential of the MAPK signaling pathway and autophagy in melanogenesis and age-related hyperpigmentation, further research is needed to elucidate their interplay mechanisms.
4.2.3.2 Melanoma
Melanoma, a highly invasive malignant tumor resulting from melanocyte transformation, represents a key pathological event associated with skin aging. Approximately 60% of melanomas contain BRAF proto-oncogene mutations, with the most common being the valine-to-glutamic acid substitution at codon 600 (BRAFV600E), which leads to the abnormal activation of MAPK signaling (Davies et al., 2002; Holderfield et al., 2014). Autophagy is pivotal in melanoma progression through its intricate interplay with MAPK signaling. For instance, mitochondrial-derived ROS engages p38 MAPK/p53 signaling to induce pro-apoptotic autophagy in human melanoma cells (Liu et al., 2009). ERK1/2-mediated autophagic cell death, induced by immune modulators, enhances radiotherapy sensitivity and anti-tumor immunity in preclinical mouse models (Cho et al., 2017). In addition, inducing autophagy through the simultaneous inhibition of p38 MAPK and activation of JNK1/2 reduces p62 levels and IL-1β secretion, effectively inhibiting metastatic growth in melanoma cells (Tan et al., 2018). These results indicate that activating autophagy via MAPK signaling may positively influence melanoma treatment outcomes. However, accumulating evidence suggests that autophagy may also contribute to the progression of drug resistance in melanoma (Bahar et al., 2023; Fratta et al., 2023; Pangilinan et al., 2024). BRAFV600E-mediated MEK/ERK/CREB signaling upregulates NOXA to trigger autophagy, enabling melanoma cells to acquire anti-apoptotic capabilities under nutrient starvation conditions (Liu et al., 2014). Photodynamic therapy-mediated MAPK activation exemplifies pathway-specific outcomes: p38 MAPK promotes apoptosis by upregulating cleaved poly (ADP-ribose) polymerase (PARP) 1, and JNK1/2 facilitates autophagosome formation through LC3 conversion, thereby protecting melanoma cells from death. JNK1/2 may also regulate Bcl-2 phosphorylation to release Beclin-1 for autophagy induction, as indicated by the observed increase in Beclin-1 levels and decrease in Bcl-2 levels (Valli et al., 2020). ER-mediated protective autophagy represents another critical resistance mechanism in BRAF-mutant melanoma (Ma et al., 2014). BRAFV600E induces chronic ER stress by activating the p38 MAPK signaling pathway, which in turn activates the JNK signaling pathway. Activated JNK releases Beclin-1 by phosphorylating Bcl-2 and B-cell lymphoma-extra large (Bcl-XL), thereby activating autophagy and enhancing cellular resistance to apoptosis in melanoma cells (Corazzari et al., 2015). Following BRAF and MEK inhibition (BRAFi + MEKi) treatment in BRAF mutant melanoma, the MAPK signaling pathway translocates into the ER via an SEC61-dependent mechanism. ER-localized ERK is rephosphorylated by protein kinase R-like endoplasmic reticulum kinase (PERK), leading to the reactivation of ERK. This reactivated ERK further phosphorylates activating transcription factor 4 (ATF4), thereby triggering cytoprotective autophagy (Ojha et al., 2019). These results collectively indicate that the regulation of melanoma cell death depends on the balance between pro-death and pro-survival mechanisms. The dual role of MAPK-mediated autophagy could be attributed to autophagic flux regulation. Although autophagy regulates multiple cell death modalities, it is typically initiated as a cytoprotective mechanism under conditions of low basal autophagy, such as nutrient starvation or ER stress. However, excessive autophagic flux, usually induced by pharmacological treatment or specific therapies, can trigger autophagic cell death and exert anti-tumor effects (Liu et al., 2023). Therefore, targeting the MAPK pathway to modulate autophagic flux from pro-survival to pro-death offers a promising strategy against melanoma drug resistance, warranting further investigation.
5 MAPK–autophagy axis modulation by natural bioactive compounds in skin aging
Natural bioactive compounds from plants and other natural sources have been widely used to combat skin aging due to their excellent photoprotective, antioxidant, and low-risk (or no-risk) properties (He et al., 2024; Hu et al., 2024; Tanveer et al., 2023). These compounds influence skin aging through multifaceted mechanisms, with growing evidence highlighting their capacity to regulate autophagy via MAPK pathway manipulation (Table 2). Bamboo leaf flavonoids can activate autophagy by inhibiting the p38 MAPK signaling pathway, thereby reducing oxidative stress-induced keratinocyte senescence and ultimately alleviating UVB-induced photoaging in mice (Gu et al., 2022b). Another natural flavonoid compound found in Angelica sinensis, 4,4ʹ-dimethoxychalcone, can also activate autophagy by inhibiting the p38 MAPK and JNK signaling pathways, showing photoprotective effects in vitro and in vivo (Gu et al., 2022a). Novel drug delivery systems may allow for the improved absorption and skin anti-aging activity of these compounds (Tavakoli and Klar, 2020). In murine full-thickness skin defect models, a temperature-sensitive hydrogel loaded with taxifolin (a flavonoid derived from Siberian larch) accelerates wound healing by activating the MAPK signaling pathway, which downregulates p62 and upregulates the expression of autophagy-related proteins (LC3-II, Beclin-1, ATG5, and ATG7) (Ding et al., 2023). However, in the same delivery system and in vivo model, ginsenoside Rg3 (an important components from the traditional Chinese medicine Panax ginseng) promotes wound healing by increasing the expression of autophagy proteins through the inhibition of the MAPK and NF-kB pathways (Peng et al., 2022). Another traditional Chinese medicine, resveratrol-loaded mesoporous silica nanoparticles transition skin hypertrophic scar fibroblasts from protective autophagy to apoptosis by suppressing the ROS/p38 MAPK/HIF-1α/p53 signaling axis, thereby inhibiting hypertrophic scar formation (Zuo and Ma, 2024). Furthermore, dietary exogenous nucleotides improve mitochondrial function and reduce senescence marker p16 expression in senescence-accelerated mouse prone-8 mice by activating autophagy via MAPK pathway inhibition and AMPK pathway activation (Fan et al., 2024). The natural carotenoid astaxanthin reverses bisphenol A-induced autophagic cell death in HDFs by suppressing the ROS-ERK-NF-κB signaling axis (Lim et al., 2021). These findings reveal that natural bioactive compounds exhibit multi-target and multi-pathway mechanisms against skin aging through their diverse classes or optimized delivery system-based combinatorial strategies. Anticancer applications include Polygonatum cyrtonema lectin, extracted from the Polygonatum cyrtonema plant, which induces autophagic cell death in human melanoma A375 cells through the mitochondria-mediated ROS/p38/p53 pathway (Liu et al., 2009). In a lung metastasis model established through the tail vein injection of B16F10 melanoma cells in C57BL/6 mice showed that algal oil rich in n-3 polyunsaturated fatty acids effectively inhibits the metastatic outgrowth of melanoma cells by inactivating p38 MAPK and activating JNK1/2 to induce autophagy (Tan et al., 2018). However, several natural bioactive compounds, such as amide alkaloid piperlongumine and bufadienolide derivative kalantuboside B, simultaneously trigger ERK-dependent apoptosis and protective autophagy in human melanoma cells (Hseu et al., 2019; Jeon et al., 2023). This dual effect may arise from differences among natural compound classes and the roles of specific MAPK subtypes, wherein sustained ERK activation and its interplay with autophagy established as a key mechanism underlying acquired resistance in multiple tumor types (Bahar et al., 2023; Huang et al., 2023). Notably, inhibiting ERK-mediated autophagy enhances anticancer efficacy (Jeon et al., 2023), indicating that combining dual-effect natural compounds with autophagy inhibitors holds great potential for melanoma therapy. Collectively, these findings highlight the potential of natural bioactive compounds to regulate the MAPK–autophagy axis, presenting a promising therapeutic strategy to combat skin aging and skin cancer. Further studies are needed to identify additional natural bioactive compounds targeting the MAPK–autophagy axis and to elucidate their precise mechanisms, thereby accelerating clinical translation in this field.
TABLE 2
| Classification | Bioactive compounds | Effect on MAPK | Mechanism | Consequence | References |
|---|---|---|---|---|---|
| Flavonoids | Bamboo leaf flavonoids | Inhibition p38 | p-p38↓, LC3-II/I, Beclin-1↑, p21, p16, K9M-H3↓ | Suppressed oxidative stress-induced senescence of HaCaT keratinocytes and UVB-induced photoaging of mice | Gu et al. (2022b) |
| 4,4′-Dimethoxychalcone | Inhibition p38, JNK | p-p38, p-JNK↓, p-ULK1, LC3-II/I, Beclin-1↑, p21, p16, K9M-H3↓ | Gu et al. (2022a) | ||
| Taxifolin | Activation p38, JNK, ERK1/2 | p-p38, p-JNK, p-ERK1/2↑, LC3 II/I, Beclin-1, ATG5, ATG7↑, p62↓ | Promoted traumatic skin repair in mice | Ding et al. (2023) | |
| Traditional Chinese medicine | Ginsenoside Rg3 | Inhibition p38, JNK, ERK | p-p38, p-JNK, p-ERK, NF-κB↓, p62↓, LC3-II/I, Beclin-1↑ | Promoted skin healing in full-thickness skin defect models in mice | Peng et al. (2022) |
| Resveratrol | Inhibition p38 | p-p38, HIF-1α, p53↓, LC3-II, Beclin-1↓, cleaved caspase3, Bax↑ | Induced transition from protective autophagy to apoptosis in human skin hypertrophic scar fibroblasts | Zuo and Ma (2024) | |
| Nucleic acid | Exogenous nucleotides | Inhibition p38, JNK, ERK1/2 | p-p38, p-JNK, p-ERK1/2↓, p-AMPK↑, p62↓, LC3-II/I↑, p16↓ | Improved skin aging in senescence-accelerated mouse prone-8 mice | Fan et al. (2024) |
| Carotenoids | Astaxanthin | Inhibition ERK |
p-ERK, p-NF-κB↓, LC3-II, Beclin-1, ATG12, ATG14↓ | Inhibited Bisphenol A-induced autophagic cell death in HDFs | Lim et al. (2021) |
| Plant lectins | Polygonatum cyrtonema lectin | Activation p38 | p-p38, p-p53↑, LC3-II/I, Beclin-1↑, Bax, Cytochrome c, Caspase-3, Caspase-9, Cleaved PARP↑, Bcl-2, Bcl-XL↓ | Induced pro-apoptotic autophagy in human melanoma A375 cells | Liu et al. (2009) |
| Lipid Extracts | Algal oil-derived n-3 polyunsaturated fatty acids | Activation JNK1/2 Inhibition p38 |
p-JNK1/2, LC3-II↑, p-p38, p62, IL-1β↓ | Inhibited the metastatic outgrowth of melanoma cells | Tan et al. (2018) |
| Amide alkaloids | Piperlongumine | Activation ERK |
p-ERK↑, LC3-II↑, Cleaved PARP, Bax↑, Bcl-2↓ | Induced apoptosis and protective autophagy in human melanoma A375P cells | Jeon et al. (2023) |
| Bufadienolides | Kalantuboside B | Activation ERK |
p-ERK↑, LC3-II/I↑, Cleaved PARP↓ | Induced apoptosis and protective autophagy in human melanoma A2058 cells | Hseu et al. (2019) |
MAPK–autophagy axis modulation by natural bioactive compounds in skin aging.
6 Conclusion
As described in this review, the MAPK–autophagy interplay critically regulates senescence in keratinocytes, fibroblasts, and melanocytes, and influences skin aging-related processes such as epidermal barrier function, wound healing, and melanoma progression. MAPKs are important mediators of autophagy upon various stresses, such as oxidative stress, UV radiation, and hypoxia. Notably, MAPK activation does not always accelerate skin aging but can instead attenuate it through autophagy induction. However, MAPK-mediated autophagy exhibits dual effects that can either inhibit senescence or contribute to senescent cell accumulation and tumor drug resistance progression. Autophagy can also affect MAPK signaling activity to modulate skin aging. Therefore, the regulatory role of the MAPK–autophagy interplay is highly context-dependent, with its functional outcomes influenced by the intervention strategy, stress condition, cell model (e.g., cell type or origin), and the specific MAPK subtype involved. Future studies should precisely target the MAPK–autophagy interplay and elucidate the exact mechanisms underlying its multifaceted roles by strictly controlling these influencing factors. Such efforts will bridge the current gap in clinical translation and provide innovative strategies for developing effective skin anti-aging therapies.
7 Future perspectives
Although natural bioactive compounds demonstrate anti-aging effects via MAPK–autophagy axis modulation in preclinical studies, clinical applications targeting this axis remain unexplored. Several natural compounds, such as astaxanthin (Ito et al., 2018; Phetcharat et al., 2015; Tominaga et al., 2012; Yoon et al., 2014), resveratrol (Maruki-Uchida et al., 2018; Shi et al., 2024), taxifolin (Micek et al., 2021), and polyunsaturated fatty acids (McDaniel et al., 2008; Pilkington et al., 2013), have shown clinically measurable anti-aging effects, providing strong evidence for the feasibility of this therapeutic strategy. To bridge the preclinical-clinical divide, the implementation of skin organoid models emerges as a promising translational strategy (Hong et al., 2023; Wang et al., 2024). By integrating advanced 3D culture techniques (e.g., 3D bioprinting) (Cubo et al., 2016), genetic engineering tools (e.g., CRISPR-Cas9) (Hendriks et al., 2020), and artificial intelligence and machine learning approaches (Flament et al., 2022; Gantenbein et al., 2025; Xiao et al., 2023), these models can better recapitulate skin aging and age-related skin cancer progression. This integrated approach will not only elucidate the anti-aging regulatory mechanisms of natural compounds targeting the MAPK–autophagy axis across diverse physiological and pathological contexts but also accelerate the discovery and screening of novel bioactive compounds from natural sources. Accurate and efficient delivery system is also a challenge that cannot be ignored. Nanocarriers and hydrogels have shown therapeutic potential (Ding et al., 2023; Peng et al., 2022; Zuo and Ma, 2024). An innovative approach is to integrate them with physical drug delivery enhancement techniques, such as electrical (e.g., iontophoresis), mechanical (e.g., microneedles), sound (e.g., sonophoresis) and thermal (e.g., fractional laser) methods (Yamada and Prow, 2020), to further improve the bioavailability and therapeutic efficacy of natural active compounds.
Future studies should prioritize elucidating the dynamic interplay between MAPK and autophagy across diverse aging microenvironments, along with the precise molecular mechanisms modulating skin aging progression, to comprehensively decipher their context-dependent regulatory networks. Furthermore, in melanoma treatment, regulating autophagic flux through the MAPK pathway to mediate the switch from pro-survival to pro-death autophagy has significant therapeutic implications. Therefore, investigating the molecular markers of autophagy-associated death and defining the “tipping point” between autophagy-mediated cell protection and killing may provide novel therapeutic strategies for melanoma. In summary, the MAPK–autophagy interplay represents a promising therapeutic target for anti-skin aging interventions and melanoma treatment. Multidisciplinary collaboration is urgently needed to elucidate these mechanisms and accelerate clinical translation. Further well-designed pharmacological and clinical studies are warranted to target this dual-pathway approach and develop effective skin anti-aging strategies.
Statements
Author contributions
XL (1st author): Conceptualization, Writing – original draft, Writing – review and editing. BC: Conceptualization, Visualization, Writing – review and editing. XL (3rd author): Writing – review and editing. XZ: Funding acquisition, Supervision, Writing – review and editing. JW: Supervision, Validation, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Applied Basic Research Program for the Department of Science and Technology of Liaoning Province (No. 2023JH2) and the Key Laboratory Construction Project of Acupuncture Biology of the Liaoning Provincial Department of Education (No. LJ232410162029).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
- UV
Ultraviolet
- TGF-β
Transforming growth factor-β
- NF-κB
Nuclear factor kappa B
- NRF2
nuclear factor erythroid 2-related factor 2
- MAPK
Mitogen-activated protein kinase
- ROS
Reactive oxygen species
- ECM
Extracellular matrix
- DEJ
Dermal–epidermal junction
- MMPs
Matrix metalloproteinases
- CMA
Chaperone-mediated autophagy
- ER
Endoplasmic reticulum
- AMPK
AMP-activated protein kinase
- mTORC1
Mammalian target of rapamycin complex 1
- ULK1
UNC-51 like kinase 1
- ATG
Autophagy-related gene
- FIP200
Focal adhesion kinase family interacting protein of 200 kDa
- PI3P
Phosphatidylinositol 3-phosphate
- PI3K
Phosphoinositide 3-kinase
- AMBRA1
Autophagy and Beclin-1 regulator 1
- UVRAG
UV radiation resistance-associated gene
- Bcl-2
B-cell lymphoma-2
- LC3
Microtubule-associated protein light chain 3
- PE
Phosphatidylethanolamine
- WIPI
WD-repeat protein interacting with phosphoinositide
- HDFs
Human dermal fibroblasts
- ERK
Extracellular signal-regulated kinases
- JNK
c-Jun amino-terminal kinases
- AP-1
Activator protein 1
- TIMPs
Tissue inhibitors of metalloproteinases
- TNF-α
Tumor necrosis factor-α
- COX-2
Cyclooxygenase-2
- HO-1
Heme oxygenase-1
- K9M-H3
K9 trimethylated histone 3
- BNIP3
BCL2 and adenovirus E1B 19-kDa interacting protein 3
- FGFR2b
Fibroblast growth factor receptor 2b
- BRAF
B-Raf proto-oncogene
- CCL2
C-C motif chemokine ligand 2
- AGEs
Advanced glycation end products
- HIF-1α
Hypoxia-inducible factor-1α
- MITF
Microphthalmia transcription factor
- α-MSH
α-melanocyte-stimulating hormone
- CREB
cAMP response element-binding protein
- TYR
Tyrosinase
- PMEL 17
Premelanosome protein 17
- TRP
Tyrosinase-related proteins
- Bcl-XL
B-cell lymphoma-extra large
- PERK
Protein kinase R-like endoplasmic reticulum kinase
- ATF4
Activating transcription factor 4
References
1
Afnan Q. Kaiser P. J. Rafiq R. A. Nazir L. A. Bhushan S. Bhardwaj S. C. et al (2016). Glycyrrhizic acid prevents ultraviolet-B-induced photodamage: a role for mitogen-activated protein kinases, nuclear factor kappa B and mitochondrial apoptotic pathway. Exp. Dermatol.25, 440–446. 10.1111/exd.12964
2
Bae J.-Y. Choi J.-S. Choi Y.-J. Shin S.-Y. Kang S.-W. Han S. J. et al (2008). (−)epigallocatechin gallate hampers collagen destruction and collagenase activation in ultraviolet-B-irradiated human dermal fibroblasts: involvement of mitogen-activated protein kinase. Food Chem. Toxicol.46, 1298–1307. 10.1016/j.fct.2007.09.112
3
Baek J. Kim J.-H. Park J. Kim D. H. Sa S. Han J.-S. et al (2024). 1-Kestose blocks UVB-induced skin inflammation and promotes type I procollagen synthesis via regulating MAPK/AP-1, NF-κB and TGF-β/Smad pathway. J. Microbiol. Biotechnol.34, 911–919. 10.4014/jmb.2311.11020
4
Bahar M. E. Kim H. J. Kim D. R. (2023). Targeting the RAS/RAF/MAPK pathway for cancer therapy: from mechanism to clinical studies. Signal Transduct. Target. Ther.8, 455. 10.1038/s41392-023-01705-z
5
Battie C. Jitsukawa S. Bernerd F. Del Bino S. Marionnet C. Verschoore M. (2014). New insights in photoaging, UVA induced damage and skin types. Exp. Dermatol.23 (Suppl. 1), 7–12. 10.1111/exd.12388
6
Bell S. Degitz K. Quirling M. Jilg N. Page S. Brand K. (2003). Involvement of NF-kappaB signalling in skin physiology and disease. Cell. Signal.15, 1–7. 10.1016/s0898-6568(02)00080-3
7
Bhawan J. Andersen W. Lee J. Labadie R. Solares G. (1995). Photoaging versus intrinsic aging: a morphologic assessment of facial skin. J. Cutan. Pathol.22, 154–159. 10.1111/j.1600-0560.1995.tb01399.x
8
Black J. O. (2016). Xeroderma pigmentosum. Head. Neck Pathol.10, 139–144. 10.1007/s12105-016-0707-8
9
Boismal F. Serror K. Dobos G. Zuelgaray E. Bensussan A. Michel L. (2020). Skin aging: pathophysiology and innovative therapies. Med. Sci. MS36, 1163–1172. 10.1051/medsci/2020232
10
Bravo-San Pedro J. M. Niso-Santano M. Gómez-Sánchez R. Pizarro-Estrella E. Aiastui-Pujana A. Gorostidi A. et al (2013). The LRRK2 G2019S mutant exacerbates basal autophagy through activation of the MEK/ERK pathway. Cell. Mol. Life Sci. CMLS70, 121–136. 10.1007/s00018-012-1061-y
11
Brooke R. C. Newbold S. A. Telfer N. R. Griffiths C. E. (2001). Discordance between facial wrinkling and the presence of basal cell carcinoma. Arch. Dermatol.137, 751–754.
12
Brown T. M. Krishnamurthy K. (2024). “Histology, dermis,” in StatPearls (Treasure Island FL: StatPearls Publishing).
13
Cai C.-S. He G.-J. Xu F.-W. (2023). Advances in the applications of extracellular vesicle for the treatment of skin photoaging: a comprehensive review. Int. J. Nanomedicine.18, 6411–6423. 10.2147/IJN.S433611
14
Cao W. Li J. Yang K. Cao D. (2021). An overview of autophagy: mechanism, regulation and research progress. Bull. Cancer (Paris)108, 304–322. 10.1016/j.bulcan.2020.11.004
15
Cargnello M. Roux P. P. (2011). Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. MMBR.75, 50–83. 10.1128/MMBR.00031-10
16
Cavinato M. Koziel R. Romani N. Weinmüllner R. Jenewein B. Hermann M. et al (2017). UVB-induced senescence of human dermal fibroblasts involves impairment of proteasome and enhanced autophagic activity. J. Gerontol. A. Biol. Sci. Med. Sci.72, 632–639. 10.1093/gerona/glw150
17
Chaiprasongsuk A. Panich U. (2022). Role of phytochemicals in skin photoprotection via regulation of Nrf2. Front. Pharmacol.13, 823881. 10.3389/fphar.2022.823881
18
Chambers E. S. Vukmanovic‐Stejic M. (2020). Skin barrier immunity and ageing. Immunology160, 116–125. 10.1111/imm.13152
19
Chen J. Waqas K. Tan R. C. Voortman T. Ikram M. A. Nijsten T. E. C. et al (2020). The association between dietary and skin advanced glycation end products: the rotterdam study. Am. J. Clin. Nutr.112, 129–137. 10.1093/ajcn/nqaa117
20
Chen S.-J. Hseu Y.-C. Gowrisankar Y. V. Chung Y.-T. Zhang Y.-Z. Way T.-D. et al (2021). The anti-melanogenic effects of 3-O-ethyl ascorbic acid via Nrf2-mediated α-MSH inhibition in UVA-Irradiated keratinocytes and autophagy induction in melanocytes. Free Radic. Biol. Med.173, 151–169. 10.1016/j.freeradbiomed.2021.07.030
21
Chiang H.-M. Chen H.-C. Chiu H.-H. Chen C.-W. Wang S.-M. Wen K.-C. (2013). Neonauclea reticulata (havil.) merr stimulates skin regeneration after UVB exposure via ROS scavenging and modulation of the MAPK/MMPs/Collagen pathway. Evid.-Based Complement. Altern. Med. ECAM.2013, 324864. 10.1155/2013/324864
22
Cho J. H. Lee H.-J. Ko H.-J. Yoon B.-I. Choe J. Kim K.-C. et al (2017). The TLR7 agonist imiquimod induces anti-cancer effects via autophagic cell death and enhances anti-tumoral and systemic immunity during radiotherapy for melanoma. Oncotarget8, 24932–24948. 10.18632/oncotarget.15326
23
Cho Y. H. (2021). Codonopsis pilosula extract protects melanocytes against H2O2-Induced oxidative stress by activating autophagy. Cosmetics8, 67. 10.3390/cosmetics8030067
24
Cho Y. H. Park J. E. Lim D. S. Lee J. S. (2017). Tranexamic acid inhibits melanogenesis by activating the autophagy system in cultured melanoma cells. J. Dermatol. Sci.88, 96–102. 10.1016/j.jdermsci.2017.05.019
25
Choi A. M. K. Ryter S. W. Levine B. (2013). Autophagy in human health and disease. N. Engl. J. Med.368, 651–662. 10.1056/NEJMra1205406
26
Corazzari M. Rapino F. Ciccosanti F. Giglio P. Antonioli M. Conti B. et al (2015). Oncogenic BRAF induces chronic ER stress condition resulting in increased basal autophagy and apoptotic resistance of cutaneous melanoma. Cell Death Differ.22, 946–958. 10.1038/cdd.2014.183
27
Cubo N. Garcia M. Del Cañizo J. F. Velasco D. Jorcano J. L. (2016). 3D bioprinting of functional human skin: production and in vivo analysis. Biofabrication9, 015006. 10.1088/1758-5090/9/1/015006
28
Dahabra L. Broadberry G. Le Gresley A. Najlah M. Khoder M. (2021). Sunscreens containing Cyclodextrin inclusion complexes for enhanced efficiency: a strategy for skin cancer prevention. Molecules26, 1698. 10.3390/molecules26061698
29
Davies H. Bignell G. R. Cox C. Stephens P. Edkins S. Clegg S. et al (2002). Mutations of the BRAF gene in human cancer. Nature417, 949–954. 10.1038/nature00766
30
Deruy E. Nassour J. Martin N. Vercamer C. Malaquin N. Bertout J. et al (2014). Level of macroautophagy drives senescent keratinocytes into cell death or neoplastic evasion. Cell Death Dis.5, e1577. 10.1038/cddis.2014.533
31
Ding C. Liu Z. Zhao T. Sun S. Liu X. Zhang J. et al (2023). A temperature-sensitive hydrogel loaded with taxifolin promotes skin repair by modulating MAPK-Mediated autophagic pathway. J. Mater. Sci.58, 14831–14845. 10.1007/s10853-023-08951-0
32
Dorf N. Maciejczyk M. (2024). Skin Senescence—From basic research to clinical practice. Front. Med.11, 1484345. 10.3389/fmed.2024.1484345
33
Eckhart L. Tschachler E. Gruber F. (2019). Autophagic control of skin aging. Front. Cell Dev. Biol.7, 143. 10.3389/fcell.2019.00143
34
Eckhart L. Zeeuwen P. L. J. M. (2018). The skin barrier: epidermis vs environment. Exp. Dermatol.27, 805–806. 10.1111/exd.13731
35
Eller M. S. Yaar M. Gilchrest B. A. (1994). DNA damage and melanogenesis. Nature372, 413–414. 10.1038/372413a0
36
Fan R. Zhang Y. Liu R. Wei C. Wang X. Wu X. et al (2024). Exogenous nucleotides improve the skin aging of SAMP8 mice by modulating autophagy through MAPKs and AMPK pathways. Nutrients16, 1907. 10.3390/nu16121907
37
Farage M. A. Miller K. W. Elsner P. Maibach H. I. (2013). Characteristics of the aging skin. Adv. Wound Care2, 5–10. 10.1089/wound.2011.0356
38
Flament F. Jacquet L. Ye C. Amar D. Kerob D. Jiang R. et al (2022). Artificial intelligence analysis of over half a million European and Chinese women reveals striking differences in the facial skin ageing process. J. Eur. Acad. Dermatol. Venereol. JEADV36, 1136–1142. 10.1111/jdv.18073
39
Fore J. (2006). A review of skin and the effects of aging on skin structure and function. Ostomy. Wound manage.52, 24–37.
40
Fratta E. Giurato G. Guerrieri R. Colizzi F. Col J. D. Weisz A. et al (2023). Autophagy in BRAF-Mutant cutaneous melanoma: recent advances and therapeutic perspective. Cell Death Discov.9, 202. 10.1038/s41420-023-01496-w
41
Freitas-Rodríguez S. Folgueras A. R. López-Otín C. (2017). The role of matrix metalloproteinases in aging: tissue remodeling and beyond. Biochim. Biophys. Acta Mol. Cell Res.1864, 2015–2025. 10.1016/j.bbamcr.2017.05.007
42
Friedman O. (2005). Changes associated with the aging face. Facial Plast. Surg. Clin. N. Am.13, 371–380. 10.1016/j.fsc.2005.04.004
43
Gaestel M. (2015). MAPK-activated protein kinases (MKs): Novel insights and challenges. Front. Cell Dev. Biol.3, 88. 10.3389/fcell.2015.00088
44
Galluzzi L. Green D. R. (2019). Autophagy-independent functions of the autophagy machinery. Cell177, 1682–1699. 10.1016/j.cell.2019.05.026
45
Gantenbein L. Cerminara S. E. Maul J.-T. Navarini A. A. Maul L. V. (2025). Artificial intelligence-driven skin aging simulation as a novel skin cancer prevention. Dermatol. Basel Switz.241, 59–71. 10.1159/000541943
46
Gao W. Lin P. Hwang E. Wang Y. Yan Z. Ngo H. T. T. et al (2018). Pterocarpus santalinus L. regulated ultraviolet B irradiation-induced procollagen reduction and matrix metalloproteinases expression through activation of TGF-β/Smad and inhibition of the MAPK/AP-1 pathway in normal human dermal fibroblasts. Photochem. Photobiol.94, 139–149. 10.1111/php.12835
47
Gerasymchuk M. Cherkasova V. Kovalchuk O. Kovalchuk I. (2020). The role of microRNAs in organismal and skin aging. Int. J. Mol. Sci.21, 5281. 10.3390/ijms21155281
48
Gerland L.-M. Peyrol S. Lallemand C. Branche R. Magaud J.-P. Ffrench M. (2003). Association of increased autophagic inclusions labeled for beta-galactosidase with fibroblastic aging. Exp. Gerontol.38, 887–895. 10.1016/s0531-5565(03)00132-3
49
Ghosh K. Capell B. C. (2016). The senescence-associated secretory phenotype: critical effector in skin cancer and aging. J. Invest. Dermatol.136, 2133–2139. 10.1016/j.jid.2016.06.621
50
Gilchrest B. A. Blog F. B. Szabo G. (1979). Effects of aging and chronic sun exposure on melanocytes in human skin. J. Invest. Dermatol.73, 141–143. 10.1111/1523-1747.ep12581580
51
Giorgi C. Marchi S. Simoes I. C. M. Ren Z. Morciano G. Perrone M. et al (2018). Mitochondria and reactive oxygen species in aging and age-related diseases. Int. Rev. Cell Mol. Biol.340, 209–344. 10.1016/bs.ircmb.2018.05.006
52
Glick D. Barth S. Macleod K. F. (2010). Autophagy: cellular and molecular mechanisms. J. Pathol.221, 3–12. 10.1002/path.2697
53
Gosselin K. Deruy E. Martien S. Vercamer C. Bouali F. Dujardin T. et al (2009). Senescent keratinocytes die by autophagic programmed cell death. Am. J. Pathol.174, 423–435. 10.2353/ajpath.2009.080332
54
Gu Y. Han J. Jiang C. Zhang Y. (2020). Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev.59, 101036. 10.1016/j.arr.2020.101036
55
Gu Y. Han J. Xue F. Xiao H. Chen L. Zhao Z. et al (2022a). 4,4’-Dimethoxychalcone protects the skin from AAPH-Induced senescence and UVB-Induced photoaging by activating autophagy. Food Funct.13, 4114–4129. 10.1039/d1fo04130d
56
Gu Y. Xue F. Xiao H. Chen L. Zhang Y. (2022b). Bamboo leaf flavonoids suppress oxidative stress-induced senescence of HaCaT cells and UVB-induced photoaging of mice through p38 MAPK and autophagy signaling. Nutrients14, 793. 10.3390/nu14040793
57
Guan L. L. Lim H. W. Mohammad T. F. (2021). Sunscreens and photoaging: a review of current literature. Am. J. Clin. Dermatol.22, 819–828. 10.1007/s40257-021-00632-5
58
Guerrero‐Navarro L. Jansen‐Dürr P. Cavinato M. (2024). Synergistic interplay of UV radiation and urban particulate matter induces impairment of autophagy and alters cellular fate in senescence‐prone human dermal fibroblasts. Aging Cell23, e14086. 10.1111/acel.14086
59
Guo W. Wang H. Li C. (2021). Signal pathways of melanoma and targeted therapy. Signal Transduct. Target. Ther.6, 424. 10.1038/s41392-021-00827-6
60
Han S. H. Ballinger E. Choung S.-Y. Kwon J. Y. (2022). Anti-photoaging effect of hydrolysates from Pacific whiting skin via MAPK/AP-1, NF-κB, TGF-β/Smad, and Nrf-2/HO-1 signaling pathway in UVB-induced human dermal fibroblasts. Mar. Drugs.20, 308. 10.3390/md20050308
61
He X. Gao X. Guo Y. Xie W. (2024). Research progress on bioactive factors against skin aging. Int. J. Mol. Sci.25, 3797. 10.3390/ijms25073797
62
Hendriks D. Clevers H. Artegiani B. (2020). CRISPR-cas tools and their application in genetic engineering of human stem cells and organoids. Cell Stem Cell27, 705–731. 10.1016/j.stem.2020.10.014
63
Holderfield M. Deuker M. M. McCormick F. McMahon M. (2014). Targeting RAF kinases for cancer therapy: BRAF mutated melanoma and beyond. Nat. Rev. Cancer.14, 455–467. 10.1038/nrc3760
64
Hong Z.-X. Zhu S.-T. Li H. Luo J.-Z. Yang Y. An Y. et al (2023). Bioengineered skin organoids: from development to applications. Mil. Med. Res.10, 40. 10.1186/s40779-023-00475-7
65
Hseu Y.-C. Cho H.-J. Gowrisankar Y. V. Thiyagarajan V. Chen X.-Z. Lin K.-Y. et al (2019). Kalantuboside B induced apoptosis and cytoprotective autophagy in human melanoma A2058 cells: an in vitro and in vivo study. Free Radic. Biol. Med.143, 397–411. 10.1016/j.freeradbiomed.2019.08.015
66
Hu S. Huang J. Pei S. Ouyang Y. Ding Y. Jiang L. et al (2019). Ganoderma lucidum polysaccharide inhibits UVB-Induced melanogenesis by antagonizing cAMP/PKA and ROS/MAPK signaling pathways. J. Cell. Physiol.234, 7330–7340. 10.1002/jcp.27492
67
Hu X. Chen M. Nawaz J. Duan X. (2024). Regulatory mechanisms of natural active ingredients and compounds on keratinocytes and fibroblasts in mitigating skin photoaging. Clin. Cosmet. Investig. Dermatol.17, 1943–1962. 10.2147/CCID.S478666
68
Huang Y. Li Y. Qu Y. Zheng Y. Ouyang M. Zhang Y. et al (2019). UVA-Induced photoaging inhibits autophagic degradation by impairing lysosomal function in dermal fibroblasts. Biochem. Biophys. Res. Commun.518, 611–618. 10.1016/j.bbrc.2019.08.103
69
Huang Y. Zhen Y. Chen Y. Sui S. Zhang L. (2023). Unraveling the interplay between RAS/RAF/MEK/ERK signaling pathway and autophagy in cancer: from molecular mechanisms to targeted therapy. Biochem. Pharmacol.217, 115842. 10.1016/j.bcp.2023.115842
70
Hughes M. C. B. Williams G. M. Pageon H. Fourtanier A. Green A. C. (2021). Dietary antioxidant capacity and skin photoaging: a 15-Year longitudinal study. J. Invest. Dermatol.141, 1111–1118.e2. 10.1016/j.jid.2020.06.026
71
Ikutama R. Peng G. Tsukamoto S. Umehara Y. Trujillo-Paez J. V. Yue H. et al (2023). Cathelicidin LL-37 activates human keratinocyte autophagy through the P2X7, mechanistic target of rapamycin, and MAPK pathways. J. Invest. Dermatol.143, 751–761.e7. 10.1016/j.jid.2022.10.020
72
Ito N. Seki S. Ueda F. (2018). The protective role of astaxanthin for UV-Induced skin deterioration in healthy People-A randomized, double-blind, placebo-controlled trial. Nutrients10, 817. 10.3390/nu10070817
73
Jeon S.-J. Choi E.-Y. Han E.-J. Lee S.-W. Moon J.-M. Jung S.-H. et al (2023). Piperlongumine induces apoptosis via the MAPK pathway and ERK-Mediated autophagy in human melanoma cells. Int. J. Mol. Med.52, 115. 10.3892/ijmm.2023.5318
74
Jeong D. Lee J. Park S. H. Kim Y. A. Park B. J. Oh J. et al (2019). Antiphotoaging and antimelanogenic effects of Penthorum chinense pursh ethanol extract due to Antioxidant- and autophagy-inducing properties. Oxid. Med. Cell. Longev.2019, 9679731. 10.1155/2019/9679731
75
Jeong D. Park S. H. Kim M.-H. Lee S. Cho Y. K. Kim Y. A. et al (2020a). Anti-melanogenic effects of ethanol extracts of the leaves and roots of Patrinia villosa (thunb.) juss through their inhibition of CREB and induction of ERK and autophagy. Molecules25, 5375. 10.3390/molecules25225375
76
Jeong D. Qomaladewi N. P. Lee J. Park S. H. Cho J. Y. (2020b). The role of autophagy in skin fibroblasts, keratinocytes, melanocytes, and epidermal stem cells. J. Invest. Dermatol.140, 1691–1697. 10.1016/j.jid.2019.11.023
77
Jin L. Li J. Yang S. Zhang R. Hu C. Chen Y. et al (2022). MAPK p38/Ulk1 pathway inhibits autophagy and induces IL-1β expression in hepatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol.322, G360–G367. 10.1152/ajpgi.00230.2021
78
Kammeyer A. Luiten R. M. (2015). Oxidation events and skin aging. Ageing Res. Rev.21, 16–29. 10.1016/j.arr.2015.01.001
79
Kim C.-W. Alam M. B. Song B.-R. Lee C. H. Kim S. L. Lee S.-H. (2024). γ-Mangosteen, an autophagy enhancer, prevents skin-aging via activating KEAP1/NRF2 signaling and downregulating MAPKs/AP-1/NF-κB-mediated MMPs. Phytomedicine Int. J. Phytother. Phytopharm.132, 155815. 10.1016/j.phymed.2024.155815
80
Kim H. S. Park S.-Y. Moon S. H. Lee J. D. Kim S. (2018). Autophagy in human skin fibroblasts: impact of age. Int. J. Mol. Sci.19, 2254. 10.3390/ijms19082254
81
Kim Y. C. Guan K.-L. (2015). mTOR: a pharmacologic target for autophagy regulation. J. Clin. Invest.125, 25–32. 10.1172/JCI73939
82
Kohl E. Steinbauer J. Landthaler M. Szeimies R.-M. (2011). Skin ageing. J. Eur. Acad. Dermatol. Venereol. JEADV25, 873–884. 10.1111/j.1468-3083.2010.03963.x
83
Kovacs D. Cardinali G. Picardo M. Bastonini E. (2022). Shining light on autophagy in skin pigmentation and pigmentary disorders. Cells11, 2999. 10.3390/cells11192999
84
Krutmann J. Schikowski T. Morita A. Berneburg M. (2021). Environmentally-induced (extrinsic) skin aging: exposomal factors and underlying mechanisms. J. Invest. Dermatol.141, 1096–1103. 10.1016/j.jid.2020.12.011
85
Lavker R. M. Zheng P. S. Dong G. (1989). Morphology of aged skin. Clin. Geriatr. Med.5, 53–67. 10.1016/s0749-0690(18)30695-5
86
Lee A.-Y. (2021). Skin pigmentation abnormalities and their possible relationship with skin aging. Int. J. Mol. Sci.22, 3727. 10.3390/ijms22073727
87
Lee K. W. Kim M. Lee S. H. Kim K. D. (2022). The function of autophagy as a regulator of melanin homeostasis. Cells11, 2085. 10.3390/cells11132085
88
Lee Y.-R. Noh E.-M. Han J.-H. Kim J.-M. Hwang J.-K. Hwang B.-M. et al (2012). Brazilin inhibits UVB-Induced MMP-1/3 expressions and secretions by suppressing the NF-κB pathway in human dermal fibroblasts. Eur. J. Pharmacol.674, 80–86. 10.1016/j.ejphar.2011.10.016
89
Levine B. Kroemer G. (2019). Biological functions of autophagy genes: a disease perspective. Cell176, 11–42. 10.1016/j.cell.2018.09.048
90
Li L. Ngo H. T. T. Hwang E. Wei X. Liu Y. Liu J. et al (2019). Conditioned medium from human adipose-derived mesenchymal stem cell culture prevents UVB-induced skin aging in human keratinocytes and dermal fibroblasts. Int. J. Mol. Sci.21, 49. 10.3390/ijms21010049
91
Li L.-F. Zhang J. Zhang Q. Zhang D. Xiang F. Jia J. et al (2019). High glucose suppresses keratinocyte migration through the inhibition of p38 MAPK/autophagy pathway. Front. Physiol.10, 24. 10.3389/fphys.2019.00024
92
Li Y.-F. Ouyang S.-H. Tu L.-F. Wang X. Yuan W.-L. Wang G.-E. et al (2018). Caffeine protects skin from oxidative stress-induced senescence through the activation of autophagy. Theranostics8, 5713–5730. 10.7150/thno.28778
93
Lim G.-E. Park J. E. Cho Y. H. Lim D. S. Kim A.-J. Moh S. H. et al (2020). Alpha-neoendorphin can reduce UVB-Induced skin photoaging by activating cellular autophagy. Arch. Biochem. Biophys.689, 108437. 10.1016/j.abb.2020.108437
94
Lim J. Lim C. J. Kim S. Nam G. Chang M. Park K. et al (2019). Antiaging and antioxidant effects of topical autophagy activator: a randomized, placebo‐controlled, double‐blinded study. J. Cosmet. Dermatol.18, 197–203. 10.1111/jocd.12530
95
Lim S.-R. Kim D.-W. Sung J. Kim T. H. Choi C.-H. Lee S.-J. (2021). Astaxanthin inhibits autophagic cell death induced by bisphenol A in human dermal fibroblasts. Antioxid. Basel Switz.10, 1273. 10.3390/antiox10081273
96
Lin X. Deng N. Li H. Duan J. Chen W. Liu T. et al (2024). The skin photoprotective effect of trilinolein: induction of cellular autophagy via the AMPK-mTOR signaling pathway. Toxicol. Appl. Pharmacol.483, 116836. 10.1016/j.taap.2024.116836
97
Lin Y. Wu X. Yang Y. Wu Y. Xiang L. Zhang C. (2024). The multifaceted role of autophagy in skin autoimmune disorders: a guardian or culprit?Front. Immunol.15, 1343987. 10.3389/fimmu.2024.1343987
98
Liu B. Cheng Y. Zhang B. Bian H. Bao J. (2009). Polygonatum cyrtonema lectin induces apoptosis and autophagy in human melanoma A375 cells through a mitochondria-mediated ROS-p38-p53 pathway. Cancer Lett.275, 54–60. 10.1016/j.canlet.2008.09.042
99
Liu H. He Z. von Rütte T. Yousefi S. Hunger R. E. Simon H.-U. (2013). Down-regulation of autophagy-related protein 5 (ATG5) contributes to the pathogenesis of early-stage cutaneous melanoma. Sci. Transl. Med.5, 202ra123. 10.1126/scitranslmed.3005864
100
Liu S. Yao S. Yang H. Liu S. Wang Y. (2023). Autophagy: regulator of cell death. Cell Death Dis.14, 648. 10.1038/s41419-023-06154-8
101
Liu Y. L. Lai F. Wilmott J. S. Yan X. G. Liu X. Y. Luan Q. et al (2014). Noxa upregulation by oncogenic activation of MEK/ERK through CREB promotes autophagy in human melanoma cells. Oncotarget5, 11237–11251. 10.18632/oncotarget.2616
102
Low E. Alimohammadiha G. Smith L. A. Costello L. F. Przyborski S. A. von Zglinicki T. et al (2021). How good is the evidence that cellular senescence causes skin ageing?Ageing Res. Rev.71, 101456. 10.1016/j.arr.2021.101456
103
Lu Y. Pan G. Wei Z. Li Y. Pan X. (2024). Role of fibroblast autophagy and proliferation in skin anti-aging. Exp. Gerontol.196, 112559. 10.1016/j.exger.2024.112559
104
Ma X.-H. Piao S.-F. Dey S. Mcafee Q. Karakousis G. Villanueva J. et al (2014). Targeting ER stress–induced autophagy overcomes BRAF inhibitor resistance in melanoma. J. Clin. Invest.124, 1406–1417. 10.1172/JCI70454
105
Magnúsdóttir E. (2017). Melanocyte differentiation-beyond the master regulator. Pigment. Cell Melanoma Res.30, 449–451. 10.1111/pcmr.12604
106
Mantena S. K. Katiyar S. K. (2006). Grape seed proanthocyanidins inhibit UV-radiation-induced oxidative stress and activation of MAPK and NF-kappaB signaling in human epidermal keratinocytes. Free Radic. Biol. Med.40, 1603–1614. 10.1016/j.freeradbiomed.2005.12.032
107
Maruki-Uchida H. Morita M. Yonei Y. Sai M. (2018). Effect of passion fruit seed extract rich in piceatannol on the skin of women: a randomized, placebo-controlled, double-blind trial. J. Nutr. Sci. Vitaminol. (Tokyo).64, 75–80. 10.3177/jnsv.64.75
108
McBride K. Nemer M. (1998). The C-terminal domain of c-fos is required for activation of an AP-1 site specific for jun-fos heterodimers. Mol. Cell. Biol.18, 5073–5081. 10.1128/MCB.18.9.5073
109
McDaniel J. C. Belury M. Ahijevych K. Blakely W. (2008). Omega-3 fatty acids effect on wound healing. Wound Repair Regen. Off. Publ. Wound heal. Soc. Eur. Tissue Repair Soc.16, 337–345. 10.1111/j.1524-475X.2008.00388.x
110
Micek I. Nawrot J. Seraszek-Jaros A. Jenerowicz D. Schroeder G. Spiżewski T. et al (2021). Taxifolin as a promising ingredient of cosmetics for adult skin. Antioxidants10, 1625. 10.3390/antiox10101625
111
Mizushima N. Komatsu M. (2011). Autophagy: renovation of cells and tissues. Cell147, 728–741. 10.1016/j.cell.2011.10.026
112
Mora Huertas A. C. Schmelzer C. E. H. Hoehenwarter W. Heyroth F. Heinz A. (2016). Molecular-level insights into aging processes of skin elastin. Biochimie128–129, 163–173. 10.1016/j.biochi.2016.08.010
113
Moriyama M. Moriyama H. Uda J. Kubo H. Nakajima Y. Goto A. et al (2017). BNIP3 upregulation via stimulation of ERK and JNK activity is required for the protection of keratinocytes from UVB-Induced apoptosis. Cell Death Dis.8, e2576. 10.1038/cddis.2017.4
114
Moriyama M. Moriyama H. Uda J. Matsuyama A. Osawa M. Hayakawa T. (2014). BNIP3 plays crucial roles in the differentiation and maintenance of epidermal keratinocytes. J. Invest. Dermatol.134, 1627–1635. 10.1038/jid.2014.11
115
Muthusamy V. Piva T. J. (2010). The UV response of the skin: a review of the MAPK, NFkappaB and TNFalpha signal transduction pathways. Arch. Dermatol. Res.302, 5–17. 10.1007/s00403-009-0994-y
116
Nanni M. Ranieri D. Rosato B. Torrisi M. R. Belleudi F. (2018). Role of fibroblast growth factor receptor 2b in the cross talk between autophagy and differentiation: involvement of Jun N-Terminal protein kinase signaling. Mol. Cell. Biol.38, e00119-18–18. 10.1128/MCB.00119-18
117
Nestle F. O. Di Meglio P. Qin J.-Z. Nickoloff B. J. (2009). Skin immune sentinels in health and disease. Nat. Rev. Immunol.9, 679–691. 10.1038/nri2622
118
Ni C. Narzt M.-S. Nagelreiter I.-M. Zhang C. F. Larue L. Rossiter H. et al (2016). Autophagy deficient melanocytes display a senescence associated secretory phenotype that includes oxidized lipid mediators. Int. J. Biochem. Cell Biol.81, 375–382. 10.1016/j.biocel.2016.10.006
119
Nisar M. F. Liu T. Wang M. Chen S. Chang L. Karisma V. W. et al (2022). Eriodictyol protects skin cells from UVA irradiation-induced photodamage by inhibition of the MAPK signaling pathway. J. Photochem. Photobiol. B226, 112350. 10.1016/j.jphotobiol.2021.112350
120
Ojha R. Leli N. M. Onorati A. Piao S. Verginadis I. I. Tameire F. et al (2019). ER translocation of the MAPK pathway drives therapy resistance in BRAF-mutant melanoma. Cancer Discov.9, 396–415. 10.1158/2159-8290.CD-18-0348
121
Pangilinan C. Klionsky D. J. Liang C. (2024). Emerging dimensions of autophagy in melanoma. Autophagy20, 1700–1711. 10.1080/15548627.2024.2330261
122
Park J.-E. Pyun H.-B. Woo S. W. Jeong J.-H. Hwang J.-K. (2014). The protective effect of Kaempferia parviflora extract on UVB-Induced skin photoaging in hairless mice. Photodermatol. Photoimmunol. Photomed.30, 237–245. 10.1111/phpp.12097
123
Park S.-J. Kim D.-W. Lim S.-R. Sung J. Kim T. H. Min I. S. et al (2021). Kaempferol blocks the skin fibroblastic interleukin 1β expression and cytotoxicity induced by 12-O-tetradecanoylphorbol-13-acetate by suppressing c-Jun N-terminal kinase. Nutrients13, 3079. 10.3390/nu13093079
124
Parzych K. R. Klionsky D. J. (2014). An overview of autophagy: morphology, mechanism, and regulation. Antioxid. Redox Signal.20, 460–473. 10.1089/ars.2013.5371
125
Pearson G. Robinson F. Beers Gibson T. Xu B. E. Karandikar M. Berman K. et al (2001). Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev.22, 153–183. 10.1210/edrv.22.2.0428
126
Peng X. Ding C. Zhao Y. Hao M. Liu W. Yang M. et al (2022). Poloxamer 407 and hyaluronic acid thermosensitive hydrogel-encapsulated ginsenoside Rg3 to promote skin wound healing. Front. Bioeng. Biotechnol.10, 831007. 10.3389/fbioe.2022.831007
127
Phetcharat L. Wongsuphasawat K. Winther K. (2015). The effectiveness of a standardized rose hip powder, containing seeds and shells of Rosa canina, on cell longevity, skin wrinkles, moisture, and elasticity. Clin. Interv. Aging10, 1849–1856. 10.2147/CIA.S90092
128
Pilkington S. M. Massey K. A. Bennett S. P. Al-Aasswad N. M. Roshdy K. Gibbs N. K. et al (2013). Randomized controlled trial of oral omega-3 PUFA in solar-simulated radiation-induced suppression of human cutaneous immune responses. Am. J. Clin. Nutr.97, 646–652. 10.3945/ajcn.112.049494
129
Pittayapruek P. Meephansan J. Prapapan O. Komine M. Ohtsuki M. (2016). Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci.17, 868. 10.3390/ijms17060868
130
Pramanik R. Qi X. Borowicz S. Choubey D. Schultz R. M. Han J. et al (2003). p38 isoforms have opposite effects on AP-1-dependent transcription through regulation of c-Jun. The determinant roles of the isoforms in the p38 MAPK signal specificity. J. Biol. Chem.278, 4831–4839. 10.1074/jbc.M207732200
131
Qiang L. Yang S. Cui Y.-H. He Y.-Y. (2020). Keratinocyte autophagy enables the activation of keratinocytes and fibroblastsand facilitates wound healing. Autophagy17, 2128–2143. 10.1080/15548627.2020.1816342
132
Qin D. Ren R. Jia C. Lu Y. Yang Q. Chen L. et al (2018). Rapamycin protects skin fibroblasts from ultraviolet B-Induced photoaging by suppressing the production of reactive oxygen species. Cell. Physiol. biochem. Int. J. Exp. Cell. Physiol. biochem. Pharmacol.46, 1849–1860. 10.1159/000489369
133
Quan T. (2023). Human skin aging and the anti-aging properties of retinol. Biomolecules13, 1614. 10.3390/biom13111614
134
Ren H. Zhao F. Zhang Q. Huang X. Wang Z. (2022). Autophagy and skin wound healing. Burns Trauma10, tkac003. 10.1093/burnst/tkac003
135
Rittié L. Fisher G. J. (2015). Natural and sun-induced aging of human skin. Cold Spring Harb. Perspect. Med.5, a015370. 10.1101/cshperspect.a015370
136
Ross C. Alston M. Bickenbach J. R. Aykin-Burns N. (2011). Oxygen tension changes the rate of migration of human skin keratinocytes in an age-related manner. Exp. Dermatol.20, 58–63. 10.1111/j.1600-0625.2010.01190.x
137
Russell-Goldman E. Murphy G. F. (2020). The pathobiology of skin aging: new insights into an old dilemma. Am. J. Pathol.190, 1356–1369. 10.1016/j.ajpath.2020.03.007
138
Saftig P. Beertsen W. Eskelinen E.-L. (2008). LAMP-2: a control step for phagosome and autophagosome maturation. Autophagy4, 510–512. 10.4161/auto.5724
139
Salminen A. Kaarniranta K. Kauppinen A. (2022). Photoaging: UV radiation-induced inflammation and immunosuppression accelerate the aging process in the skin. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. Al.71, 817–831. 10.1007/s00011-022-01598-8
140
Sauermann K. Clemann S. Jaspers S. Gambichler T. Altmeyer P. Hoffmann K. et al (2002). Age related changes of human skin investigated with histometric measurements by confocal laser scanning microscopy in vivo. Skin. Res. Technol. Off. J. Int. Soc. Bioeng. Skin. ISBS Int. Soc. Digit. Imaging Skin. ISDIS Int. Soc. Skin. Imaging ISSI8, 52–56. 10.1046/j.0909-752x.2001.10297.x
141
Scharffetter-Kochanek K. Brenneisen P. Wenk J. Herrmann G. Ma W. Kuhr L. et al (2000). Photoaging of the skin from phenotype to mechanisms. Exp. Gerontol.35, 307–316. 10.1016/s0531-5565(00)00098-x
142
Setaluri V. (2015). Autophagy as a melanocytic self-defense mechanism. J. Invest. Dermatol.135, 1215–1217. 10.1038/jid.2015.19
143
She H. He Y. Zhao Y. Mao Z. (2018). Autophagy in inflammation: the p38α MAPK-ULK1 axis. Macrophage5, e1629.
144
Shi Y. Shen C. Zhang W. (2024). Efficacy and safety of a topical skincare regimen containing CE ferulic serum and resveratrol BE serum following ablative fractional CO2 laser treatment: a prospective, randomized, split-face, controlled trial. J. Eur. Acad. Dermatol. Venereol. JEADV38 (Suppl. 6), 17–25. 10.1111/jdv.20070
145
Shin J.-W. Kwon S.-H. Choi J.-Y. Na J.-I. Huh C.-H. Choi H.-R. et al (2019). Molecular mechanisms of dermal aging and antiaging approaches. Int. J. Mol. Sci.20, 2126. 10.3390/ijms20092126
146
Slobodnyuk K. Radic N. Ivanova S. Llado A. Trempolec N. Zorzano A. et al (2019). Autophagy-induced senescence is regulated by p38α signaling. Cell Death Dis.10, 376. 10.1038/s41419-019-1607-0
147
Song X. Narzt M. S. Nagelreiter I. M. Hohensinner P. Terlecki-Zaniewicz L. Tschachler E. et al (2017). Autophagy deficient keratinocytes display increased DNA damage, senescence and aberrant lipid composition after oxidative stress in vitro and in vivo. Redox Biol.11, 219–230. 10.1016/j.redox.2016.12.015
148
Sukseree S. Eckhart L. Tschachler E. Watanapokasin R. (2013). Autophagy in epithelial homeostasis and defense. Front. Biosci. Elite, 5, 1000–1010. 10.2741/e679
149
Sun Q. Y. Breitbart H. Schatten H. (1999). Role of the MAPK Cascade in Mammalian germ cells. Reprod. Fertil. Dev.11, 443–450. 10.1071/rd00014
150
Tan R.-H. Wang F. Fan C.-L. Zhang X.-H. Zhao J.-S. Zhang J.-J. et al (2018). Algal oil rich in n-3 polyunsaturated fatty acids suppresses B16F10 melanoma lung metastasis by autophagy induction. Food Funct.9, 6179–6186. 10.1039/c8fo01617h
151
Tanveer M. A. Rashid H. Tasduq S. A. (2023). Molecular basis of skin photoaging and therapeutic interventions by plant-derived natural product ingredients: a comprehensive review. Heliyon9, e13580. 10.1016/j.heliyon.2023.e13580
152
Tavakoli S. Klar A. S. (2020). Advanced hydrogels as wound dressings. Biomolecules10, 1169. 10.3390/biom10081169
153
Tominaga K. Hongo N. Karato M. Yamashita E. (2012). Cosmetic benefits of astaxanthin on humans subjects. Acta Biochim. Pol.59, 43–47. 10.18388/abp.2012_2168
154
Tsai J. Chien A. L. (2022). Photoprotection for skin of color. Am. J. Clin. Dermatol.23, 195–205. 10.1007/s40257-021-00670-z
155
Tsuji T. (1980). Loss of dermal elastic tissue in solar elastosis. Arch. Dermatol.116, 474–475. 10.1001/archderm.1980.01640280110031
156
Tsujimoto Y. Shimizu S. (2005). Another way to die: Autophagic programmed cell death. Cell Death Differ.12 (Suppl. 2), 1528–1534. 10.1038/sj.cdd.4401777
157
Urban K. Mehrmal S. Uppal P. Giesey R. L. Delost G. R. (2021). The global burden of skin cancer: a longitudinal analysis from the global burden of disease study, 1990–2017. JAAD Int.2, 98–108. 10.1016/j.jdin.2020.10.013
158
Valli F. García Vior M. C. Roguin L. P. Marino J. (2020). Crosstalk between oxidative stress-induced apoptotic and autophagic signaling pathways in Zn(II) phthalocyanine photodynamic therapy of melanoma. Free Radic. Biol. Med.152, 743–754. 10.1016/j.freeradbiomed.2020.01.018
159
Verrecchia F. Mauviel A. (2002). Transforming growth factor-beta signaling through the smad pathway: role in extracellular matrix gene expression and regulation. J. Invest. Dermatol.118, 211–215. 10.1046/j.1523-1747.2002.01641.x
160
Victorelli S. Salmonowicz H. Chapman J. Martini H. Vizioli M. G. Riley J. S. et al (2023). Apoptotic stress causes mtDNA release during senescence and drives the SASP. Nature622, 627–636. 10.1038/s41586-023-06621-4
161
Vikram A. Patel S. K. Singh A. Pathania D. Ray R. S. Upadhyay A. K. et al (2024). Natural autophagy activators: a promising strategy for combating photoaging. Phytomedicine Int. J. Phytother. Phytopharm.132, 155508. 10.1016/j.phymed.2024.155508
162
Wang X.-Y. Jia Q.-N. Li J. Zheng H.-Y. (2024). Organoids as tools for investigating skin aging: mechanisms, applications, and insights. Biomolecules14, 1436. 10.3390/biom14111436
163
Wang Y. Wang L. Wen X. Hao D. Zhang N. He G. et al (2019a). NF-κB signaling in skin aging. Mech. Ageing Dev.184, 111160. 10.1016/j.mad.2019.111160
164
Wang Y. Wen X. Hao D. Zhou M. Li X. He G. et al (2019b). Insights into autophagy machinery in cells related to skin diseases and strategies for therapeutic modulation. Biomed. Pharmacother. Biomedecine Pharmacother.113, 108775. 10.1016/j.biopha.2019.108775
165
Wang Y. Zhang H. (2019). Regulation of autophagy by mTOR signaling pathway. Adv. Exp. Med. Biol.1206, 67–83. 10.1007/978-981-15-0602-4_3
166
Wang Z. Man M.-Q. Li T. Elias P. M. Mauro T. M. (2020). Aging-associated alterations in epidermal function and their clinical significance. Aging12, 5551–5565. 10.18632/aging.102946
167
Wang Z. Zhou H. Zheng H. Zhou X. Shen G. Teng X. et al (2021). Autophagy-based unconventional secretion of HMGB1 by keratinocytes plays a pivotal role in psoriatic skin inflammation. Autophagy17, 529–552. 10.1080/15548627.2020.1725381
168
Wen W. Chen J. Ding L. Luo X. Zheng X. Dai Q. et al (2018). Astragaloside exerts anti-photoaging effects in UVB-Induced premature senescence of rat dermal fibroblasts through enhanced autophagy. Arch. Biochem. Biophys.657, 31–40. 10.1016/j.abb.2018.09.007
169
Xiao X. Feng H. Liao Y. Tang H. Li L. Li K. et al (2023). Identification of key circadian rhythm genes in skin aging based on bioinformatics and machine learning. Aging15, 11672–11689. 10.18632/aging.205155
170
Xue S. Lin Y. Chen H. Yang Z. Zha J. Jiang X. et al (2024). Mechanisms of autophagy and their implications in dermatological disorders. Front. Immunol.15, 1486627. 10.3389/fimmu.2024.1486627
171
Yamada M. Prow T. W. (2020). Physical drug delivery enhancement for aged skin, UV damaged skin and skin cancer: translation and commercialization. Adv. Drug Deliv. Rev.153, 2–17. 10.1016/j.addr.2020.04.008
172
Yang K. E. Nam S.-B. Jang M. Park J. Lee G.-E. Cho Y.-Y. et al (2023). Ginsenoside Rb2 suppresses cellular senescence of human dermal fibroblasts by inducing autophagy. J. Ginseng Res.47, 337–346. 10.1016/j.jgr.2022.11.004
173
Yang Z. Klionsky D. J. (2010). Mammalian autophagy: core molecular machinery and signaling regulation. Curr. Opin. Cell Biol.22, 124–131. 10.1016/j.ceb.2009.11.014
174
Yoon H.-S. Cho H. H. Cho S. Lee S.-R. Shin M.-H. Chung J. H. (2014). Supplementating with dietary astaxanthin combined with collagen hydrolysate improves facial elasticity and decreases matrix metalloproteinase-1 and -12 expression: a comparative study with placebo. J. Med. Food.17, 810–816. 10.1089/jmf.2013.3060
175
Young A. R. J. Narita M. Ferreira M. Kirschner K. Sadaie M. Darot J. F. J. et al (2009). Autophagy mediates the mitotic senescence transition. Genes Dev.23, 798–803. 10.1101/gad.519709
176
Yu E. Oh S. W. Park S.-H. Kwon K. Han S. B. Kang S. H. et al (2024). The pigmentation of blue light is mediated by both melanogenesis activation and autophagy inhibition through OPN3-TRPV1. J. Invest. Dermatol.S0022-202X (24), 908–918.e6. 10.1016/j.jid.2024.07.034
177
Yun W. J. Kim E.-Y. Park J.-E. Jo S. Y. Bang S. H. Chang E.-J. et al (2016). Microtubule-associated protein light chain 3 is involved in melanogenesis via regulation of MITF expression in melanocytes. Sci. Rep.6, 19914. 10.1038/srep19914
178
Zeng T. Wang X. Wang W. Feng Q. Lao G. Liang Y. et al (2019). Endothelial cell-derived small extracellular vesicles suppress cutaneous wound healing through regulating fibroblasts autophagy. Clin. Sci. Lond. Engl. 1979.133, CS20190008. 10.1042/CS20190008
179
Zhang C. Li H. Jiang M. Zhang Q. Chen J. Jia J. et al (2024). Hypoxic microenvironment promotes dermal fibroblast migration and proliferation via a BNIP3-autophagy pathway. FEBS J.291, 358–375. 10.1111/febs.16985
180
Zhang C.-F. Gruber F. Ni C. Mildner M. Koenig U. Karner S. et al (2015). Suppression of autophagy dysregulates the antioxidant response and causes premature senescence of melanocytes. J. Invest. Dermatol.135, 1348–1357. 10.1038/jid.2014.439
181
Zhang J. Zhang C. Jiang X. Li L. Zhang D. Tang D. et al (2019). Involvement of autophagy in hypoxia-BNIP3 signaling to promote epidermal keratinocyte migration. Cell Death Dis.10, 234. 10.1038/s41419-019-1473-9
182
Zhang S. Duan E. (2018). Fighting against skin aging: the way from bench to bedside. Cell Transpl.27, 729–738. 10.1177/0963689717725755
183
Zhang Y. Liu P. Fu H. Wang D. Zhao D. Zhang J. et al (2022). Effects of Lactobacillus kefiri fermentation supernatant on skin aging caused by oxidative stress. J. Funct. Foods.96, 105222. 10.1016/j.jff.2022.105222
184
Zhong X. Deng Y. Yang H. Du X. Liu P. Du Y. (2024). Role of autophagy in skin photoaging: a narrative review. Med. Baltim.103, e37178. 10.1097/MD.0000000000037178
185
Zhou Y. Zeng H.-L. Wen X.-Y. Jiang L. Fu C.-H. Hu Y.-B. et al (2022). Selaginellin inhibits melanogenesis via the MAPK signaling pathway. J. Nat. Prod.85, 838–845. 10.1021/acs.jnatprod.1c00971
186
Zhu W. Zhao Z. Cheng B. (2020). The role of autophagy in skin pigmentation. Eur. J. Dermatol. EJD.30, 655–662. 10.1684/ejd.2020.3930
187
Zuo J. Ma S. (2024). Resveratrol-laden mesoporous silica nanoparticles regulate the autophagy and apoptosis via ROS-Mediated p38-MAPK/HIF-1a/p53 signaling in hypertrophic scar fibroblasts. Heliyon10, e24985. 10.1016/j.heliyon.2024.e24985
Summary
Keywords
skin aging, MAPK, autophagy, interplay, natural bioactive compounds
Citation
Liu X, Chen B, Liu X, Zhang X and Wu J (2025) Interplay between MAPK signaling pathway and autophagy in skin aging: mechanistic insights and therapeutic implications. Front. Cell Dev. Biol. 13:1625357. doi: 10.3389/fcell.2025.1625357
Received
08 May 2025
Accepted
20 June 2025
Published
27 June 2025
Volume
13 - 2025
Edited by
Ivan Conte, University of Naples Federico II, Italy
Reviewed by
Giuliana Giamundo, University of Naples Federico II, Italy
Maria Celeste Ruete, National Scientific and Technical Research Council (CONICET), Argentina
Updates
Copyright
© 2025 Liu, Chen, Liu, Zhang and Wu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoqing Zhang, zjtn-zhangxq@lnutcm.edu.cn; Jingdong Wu, lnzywjd0719@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.