- 1Ministry of Education Key Laboratory of Molecular and Cellular Biology, Hebei Anti-Tumour Molecular Target Technology Innovation Center, College of Life Science, Hebei Normal University, Shijiazhuang, China
- 2Jianyuan Precision Medicines (Zhangjiakou) Co., Ltd., Zhangjiakou, China
- 3Shijiazhuang Zhuohan Biological Technology Co., Ltd., Shijiazhuang, China
- 4Guangxi Academy of Sciences, Nanning, China
Extracellular vesicles (EVs), nanoscale vesicles released by various cell types, have garnered significant attention in regenerative medicine. Mesenchymal stem cell-derived EVs (MSC-EVs) exhibit unique advantages, including their compact size, ability to traverse the blood-brain barrier (BBB), low immunogenicity, and high biosafety profile. However, challenges such as standardization of isolation protocols, establishment of quality control criteria, and scalability of production remain unresolved. This review critically examines the methodologies for preparation, characterization, and pharmacokinetic profiling of MSC-EVs, alongside their therapeutic potential in neurological disorders. By synthesizing current advancements, this work aims to elucidate the translational value of EVs in clinical practice. Additionally, it seeks to accelerate their transition from preclinical research to therapeutic applications, and provide a robust theoretical foundation for novel strategies in treating neurological diseases.
1 Introduction
Extracellular vesicles (EVs), nanoscale membrane-bound particles released by diverse cell types including fibroblasts, immune cells (e.g., T cells, B cells, dendritic cells), adipocytes, stem cells, and tumor cells, have emerged as critical mediators of intercellular communication. These vesicles are ubiquitously present in all bodily fluids, such as blood, urine, breast milk, amniotic fluid, and bronchoalveolar lavage fluid (Kimiz-Gebologlu and Oncel, 2022). EVs hold immense potential in clinical diagnostics and therapeutics, with mesenchymal stem cell-derived EVs (MSC-EVs) representing a novel paradigm for disease intervention.
Stem cells encompass multiple subtypes, notably mesenchymal stem cells (MSCs), induced pluripotent stem cells (iPSCs), hematopoietic stem cells (HSCs), and others. Unlike other stem cell types, MSCs exhibit broader tissue distribution, superior multipotency, and enhanced self-renewal capacity (Li J. et al., 2023). MSCs secrete heterogeneous EV populations, primarily classified into EVs (30–150 nm in diameter), microvesicles (150–500 nm in diameter), and apoptotic bodies (500–800 nm in diameter) based on biogenesis pathways and size (Patel et al., 2024). MSC-EVs are regarded as important paracrine mediators for information transfer between MSCs and their target cells. Initially, MSC-EVs were underappreciated due to limited understanding of their biological relevance (Guo et al., 2020; Johnstone et al., 1987). However, accumulating research has demonstrated that MSC-EVs possess distinct functional properties. For instance, they can accurately reflect the specific state of their parental cells (Rhim et al., 2023). They also play roles in maintaining cellular homeostasis, anti-inflammation, tissue repair, immunomodulation, and other aspects (Drobiova et al., 2023). Additionaslly, MSC-EVs can efficiently deliver exogenous chemicals and biomolecules, serving as a novel and promising drug delivery platform and opening up new avenues for regenerative medicine (Miron et al., 2024).
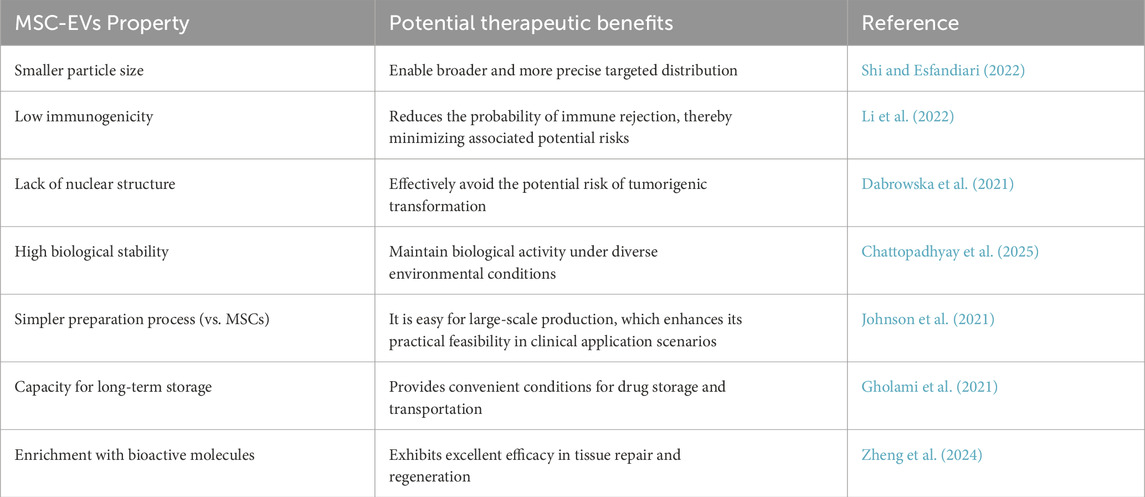
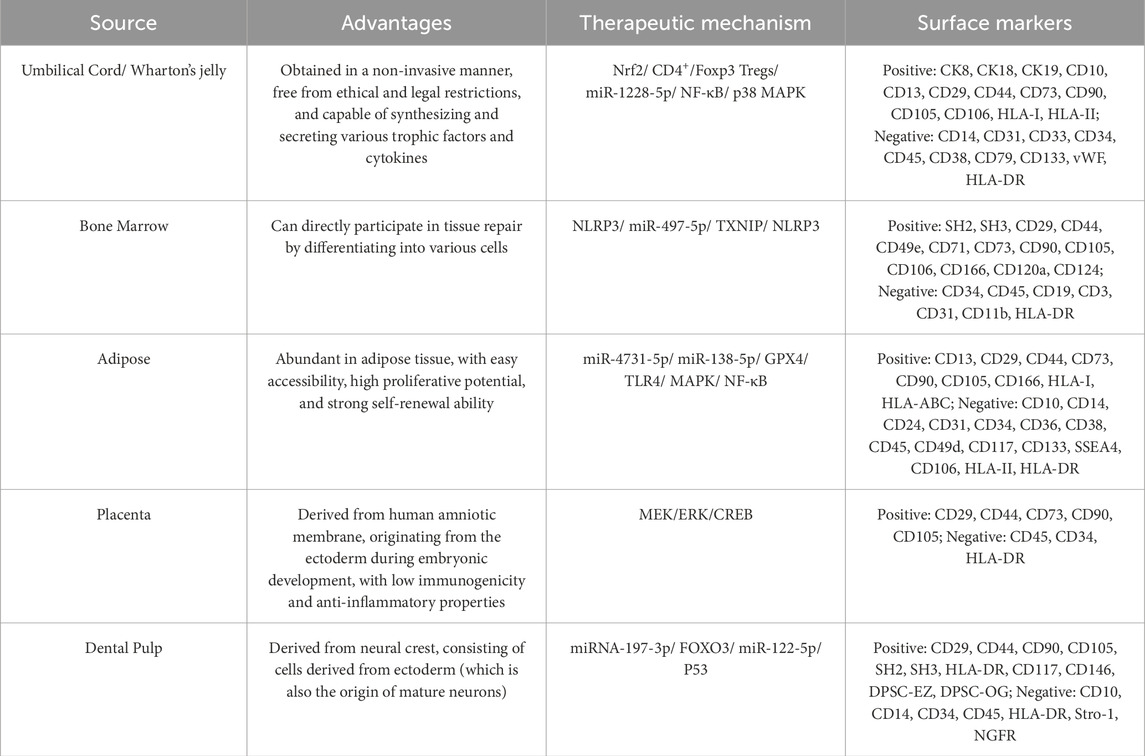
Compared with EVs from other sources, EVs derived from MSCs have several potential therapeutic advantages. This is mainly due to a series of novel and beneficial properties (Table 1). For example, they are smaller in size, enabling a more extensive and precise distribution in vivo. They have low immunogenicity, which makes them less likely to cause rejection reactions and reduces potential risks. They lack a cell nucleus, effectively preventing the risk of tumor transformation. They have higher stability and can maintain their activity under different environmental conditions (Chattopadhyay et al., 2025). They are easier to produce, improving the feasibility of their clinical application. They can be preserved for a long time, providing convenience for the storage and transportation of drugs. Moreover, they also have great potential to load proteins, small molecules, or RNAs to deliver biomolecules (Xie et al., 2023; Lotfy et al., 2023; Muskan et al., 2024). In addition to other bioactive molecules, more than 304 proteins and 150 microRNAs have been found in MSC-EVs (Lotfy et al., 2023). All these bioactive molecules have a good therapeutic effect on tissue recovery by maintaining and recruiting endogenous stem cells, inhibiting apoptosis, regulating the immune system, and stimulating angiogenesis (Hassanzadeh et al., 2021). Over the past decades, preclinical and clinical studies have validated the therapeutic efficacy of MSC-EVs across diverse pathologies, including neurological disorders, respiratory diseases, kidney diseases, heart diseases, liver diseases, bone defects, and malignancies (Figure 1). Here, we focus on the applications of MSC-EVs derived from different sources in the content of neurological disorders.
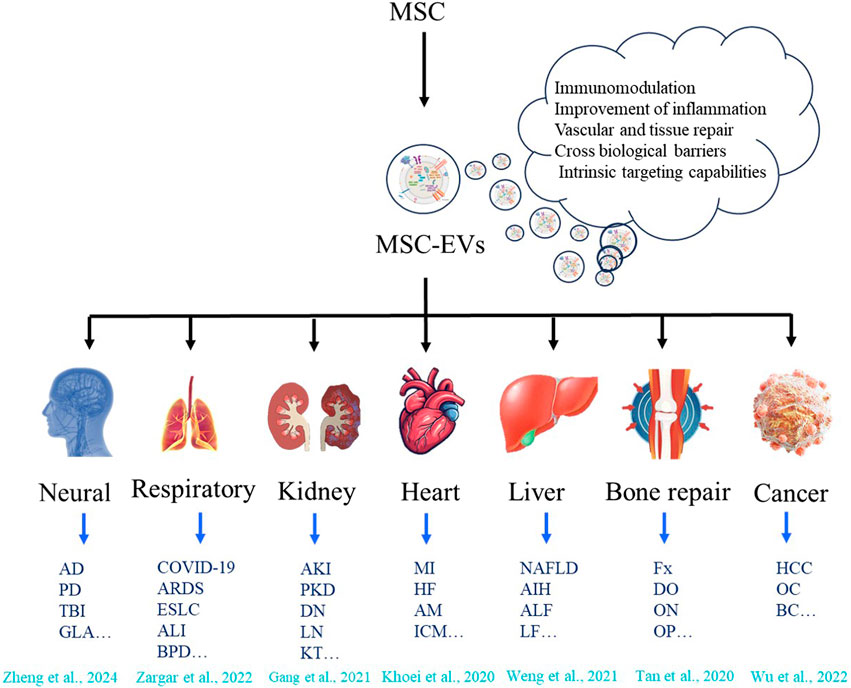
Figure 1. Therapeutic applications of MSC-EVs across diverse disease types. MSC can release MSC - EVs with capabilities of immunomodulation, improvement of inflammation, vascular and tissue repair, crossing biological barriers, and intrinsic targeting (Hade et al., 2021; Zheng et al., 2024; Zargar et al., 2022; Gang et al., 2021; Khoei et al., 2020; Weng et al., 2021; Tan et al., 2020; Wu et al., 2022). These vesicles show therapeutic application potential in various disease types, including neurological diseases (such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Traumatic brain injury (TBI), Glaucoma (GLA) etc.); respiratory diseases (such as COVID-19, Acute respiratory distress syndrome (ARDS), Early-staged lung cancer (ESLC), Acute lung injury (ALI), Broncho-pulmonary dysplasia (VPD) etc.); kidney diseases (such as Acute kidney injury (AKI), Polycystic kidney disease (PKD), Diabetic nephropathy (DN), Lupus nephritis (LN), Kidney transplant (KT) etc.); heart diseases (such as Myocardial infarction (MI), Heart failure (HF), Acute myocarditis (AM), Ischemic cardiomyopathy (ICM) etc.); liver diseases (such as non-alcoholic fatty liver disease (NAFLD), autoimmune hepatitis (AIH), Acute liver failure (ALF), Liver Fibrosis (LF) etc.); bone repair (such as Fracture (Fx), Distraction osteogenesis (DO), Osteonecrosis (ON), Osteoporosis (OP) etc.), and cancers (such as Hepatocellular carcinoma (HCC), Ovarian cancer (OC), Ovarian cancer (OC), Breast cancer (BC) etc.).
2 Preparation of MSC-EVs
MSC-EVs exhibit heterogeneous diameters ranging from tens to hundreds of nanometers, with their size distribution being method-dependent during isolation (Patel et al., 2019). This section summarizes common preparation techniques, compares their merits and limitations, and provides guidance for selecting optimal protocols under specific experimental conditions.
2.1 Centrifugation-based methods
2.1.1 Differential ultracentrifugation
Differential ultracentrifugation remains a gold standard for EVs isolation. The protocol involves sequential centrifugation steps with increasing centrifugal forces to remove cellular debris and subcellular components, followed by ultracentrifugation (≥100,000 × g) to pellet EVs. Initial low-speed centrifugation eliminates intact cells and large debris, while subsequent high-speed steps clear smaller contaminants. Final ultracentrifugation precipitates EVs, with optional repeat cycles to enhance purity (Aliakbari et al., 2024; Tiszbein et al., 2025).
The EVs isolated by the differential ultracentrifugation method have a relatively high purity. This method requires expensive specialized equipment such as an ultracentrifuge, with the centrifugal speed needing to reach 100,000 × g. EVs can be damaged due to multiple centrifugations at excessively high speeds. During the extraction process, experimenters may perform multiple ultracentrifugations, which may cause certain damage to the structure of EVs.
2.1.2 Density gradient centrifugation
Density gradient centrifugation is an improved method of traditional differential ultracentrifugation. EVs are separated based on buoyant density differences using continuous or discontinuous gradients of sucrose, iodixanol, or cesium chloride. Particles migrate to equilibrium positions matching their densities during centrifugation.
The density gradient centrifugation method is an important separation technique. The number of ultracentrifugations can be reduced, thereby maintaining the integrity of vesicles, enabling the separation of different types of extracellular vesicles, and it has been widely applied in fields such as biology and medicine (Auquière et al., 2025; Chen et al., 2019; D'Acunzo et al., 2022). This method can improve the purity of the separated EVs (Langevin et al., 2019). However, this method has a complex operation process, further washing is required to remove the density gradient medium from the EVs suspension, resulting in a low yield of EVs. It requires a high level of technical skills from the operators, is time-consuming, and cannot effectively remove lipoproteins and chylomicrons from blood samples (Wang C. C. et al., 2024).
2.2 Ultrafiltration
Ultrafiltration employs size-exclusion membranes under pressure gradients to concentrate EVs. Variants include sequential filtration, centrifugal ultrafiltration, and tangential flow filtration (Lai et al., 2022; Busatto et al., 2018; Xu et al., 2015). These methods use membrane filters with specific molecular weight or size exclusion limits to separate suspended particles or polymers according to their size or molecular weight.
Ultrafiltration achieves separation mainly through the pore size sieving effect of the membrane, eliminating the need for introducing chemical substances such as precipitants, organic solvents, and chelating agents, thus reducing chemical contamination and its impact on the contents of EVs (Yang et al., 2020). The disadvantage is that membrane adhesion reduces the yield of EVs. The pressure and shear force during filtration may damage the morphology of EVs. Under the premise of reasonably controlling the pressure, the ultrafiltration method can minimize damage to the EVs’ morphology. Additionally, the filter membrane is prone to damage or blockage, which affects the separation efficiency.
2.3 Anion exchange chromatography (AEC)
AEC is a reliable method for preparing EVs, which utilizes the interaction between the inherent negative charges of EVs and the charged packing materials inside the chromatographic column. This technique involves several key steps: preparation of reagents, materials, and instrumental equipment; equilibration of the chromatographic column; sample loading, elution of EVs, and collection (Wang S. et al., 2024). Since this method can concentrate samples, it is easy to be combined with other purification methods (such as ultrafiltration and tangential flow filtration) to construct a two-dimensional purification strategy based on differences in surface charge and size (Saari et al., 2023). It was found that the combination of AEC with size exclusion chromatography (SEC) can improve the sample purity of EVs compared with the use of SEC alone (Wang H. et al., 2023). Seo et al. concentrated and deproteinized the culture supernatants by ultrafiltration, then performed AEC, which enabled the acquisition of a large quantity of high-purity EVs without reducing their biological activity (Seo et al., 2022). Pirolli et al. successfully combined tangential flow filtration with AEC to purify EVs, thus improving the purity (Pirolli et al., 2023).
2.4 Size exclusion chromatography (SEC)
SEC is a well-established method for separating macromolecules based on their molecular size or hydrodynamic volume. A standard SEC configuration consists of a porous stationary phase for chromatographic separation, which can optionally be coupled with a pump for elution. According to the size and shape of EVs, separation is achieved using the porous packing material in the chromatographic column. Molecules of different sizes have different retention times in the column, and EVs are eluted within a specific time frame (Al-Madhagi, 2024). SEC has been widely used to separate EVs from different sample matrices of prokaryotes and eukaryotes, including specimens derived from cell cultures, blood samples (Liangsupree et al., 2021), urine (Gan et al., 2022), saliva (Han et al., 2023), and tears (Aqrawi et al., 2019).
SEC has several advantages, such as simple operation, good reproducibility, the ability to handle large-volume samples, and the relatively uniform size of the separated EVs. However, its drawback is that it may separate particles with similar sizes to other particles, leading to a decrease in purity. Currently, commercial chromatographic columns for separating and purifying EVs have been developed based on the SEC principle. For example, the qEV chromatographic column, a commercial product from iZON based on SEC, can extract high concentration EVs from an initial sample volume ranging from 150 μL to 10 mL within 15 min. It ensures the stability of the biochemical components and morphological structure of EVs while achieving efficient extraction (Reseco et al., 2024).
2.5 Polymer precipitation
Polymer-based coprecipitation is a commonly used strategy in commercial EVs separation kits, such as ExoQuick™ (System Biosciences, United States), ExoPrep (HansaBioMed, Estonia), and Total Exosome Isolation™ (Invitrogen, United States). Among various hydrophilic polymers, Polyethylene glycol (PEG) is widely used as a precipitation reagent. Hydrophilic PEG can interact with the water molecules surrounding EVs, thus forming a hydrophobic microenvironment. During this process, the solubility of EVs decreases, and they will precipitate under low-speed centrifugation (Staubach et al., 2021). These commercial kits have a high yield and are easy to adapt to different studies. At the same time, this method avoids expensive ultracentrifugation and reduces damage to EVs. However, it is worth noting that the polymer precipitation process may coprecipitate proteins, nucleic acids, and lipids (Xu et al., 2022).
Due to the simplicity of this method, commercially available kits for separating EVs by PEG precipitation have also been developed. The processing time of PEG precipitation is relatively short, but it faces challenges such as low purity and recovery rate, and it is difficult to remove PEG in the last step. Therefore, this method is rarely used alone nowadays and is usually an auxiliary method, and is not recommended for clinical use due to the presence of non-EVs proteins, immunoglobulins, viral particles, immune complexes, and other contaminants in addition to EVs in the final pellet obtained from the PEG-based EVs isolation process (Welsh et al., 2024; Sidhom et al., 2020).
2.6 Immunomagnetic beads
This method utilizes specific markers on the surface of EVs. By interacting with magnetic beads conjugated with specific antibodies, and under the action of an external magnetic field, the magnetic beads carrying EVs are separated from other components, thus achieving the separation and purification of EVs. Magnetic particles such as iron, nickel, neodymium, or magnetite can be easily functionalized with biomolecules (such as antibodies). This modification enables the magnetic particles to specifically attach to EVs, facilitating the extraction of EVs from complex matrices through magnetic drive. This method helps to eliminate the interference caused by the biological fluid matrix, achieve the preconcentration of EVs, and improve the sensitivity of the detection process (Lima Moura et al., 2020).
The immunomagnetic bead method is a commonly used approach for preparing EVs and has the following advantages. High specificity: By selecting antibodies against specific markers of EVs, highly specific separation of EVs can be achieved. For instance, antibodies agianst CD9, CD 63 and CD81 can be used for purification of EVs (Cheng et al., 2025; Allelein et al., 2022; Li D. et al., 2024). High purity: Through multiple steps of washing and magnetic separation, most of the impurities can be removed, obtaining EVs with a relatively high purity. Relatively simple operation: The operation steps of the immunomagnetic bead method are relatively simple, and it does not require complex equipment and techniques. However, overall, it has a high cost: The cost of antibodies and magnetic beads is relatively high, increasing the experimental expenses. There may be non-specific binding: In some cases, the magnetic beads may have non-specific binding with other components in the sample, affecting the purity of EVs. High requirements for antibody selection: EVs from different sources may have different marker expressions, and appropriate antibodies or a combination of several antibodies need to be selected to effectively separate EVs (Lane et al., 2017; Peng et al., 2025; Liu C. et al., 2024; Zhang et al., 2025; He et al., 2017).
2.7 Microfluidic technologies
Traditional methods for the separation and purification of EVs often require a large amount of samples, and the operation is complex and time-consuming. Microfluidic technology, with its unique advantages such as adjustability, diverse material options, low cost, high processing efficiency, and minimal sample requirement, provides an ideal platform for precision medicine applications (Lan et al., 2025). Microfluidics is an EVs extraction technology based on signal detection, which specifically targets major protein markers on EVs for EVs purification. This innovative method can effectively separate and allow visualization of EVs enriched on the surface of microspheres, and is capable of manipulating tiny fluids in microtubes (with volumes ranging from a few microliters to several hundred microliters) (Wang W. et al., 2024; Theel and Schwaminger, 2022). Woo et al. developed a procedure consisting of two filtration chambers (with pore sizes of 600 nm and 20 nm respectively) for the automatic enrichment and separation of EVs and other vesicles from biological samples, which requires only low centrifugal force (<500 g) and can complete the enrichment process quickly (Woo et al., 2017). The microfluidic chip developed by Hassanpour Tamrin et al. has unique advantages such as fast separation speed, high throughput, and low sample requirement, making it very suitable for separating EVs from small amounts of precious biological sample (Hassanpour Tamrin et al., 2021). Suwatthanarak et al. modified antibodies, aptamers, or peptides in microfluidic channels to improve the specificity of EVs separation (Suwatthanarak et al., 2021). Yu et al. reported a highly integrated EVs separation and detection chip (EVs SD). This chip, modified with anti-CD63 antibodies, can effectively separate EVs from the cell culture supernatants and clinical sera of gastric cancer patients (stages I and II) (Yu et al., 2021). Despite the continuous development and expanding application scope of microfluidic separation technologies, their popularity in standardized laboratories remains relatively limited, and the application of these technologies requires specific professional knowledge and dedicated preparation equipment as support (Wu et al., 2023).
2.8 Ultrafast-isolation system: EXODUS
The EXODUS employs a dual-frequency harmonic oscillator integrated with negative pressure oscillation (NPO) to isolate EVs from biofluids (e.g., urine, plasma, tears) (Chen et al., 2021). This system utilizes nanoporous membranes subjected to high-frequency (5–8 kHz) piezoelectric vibration and low-frequency (∼200 Hz) mechanical oscillation, coupled with alternating pressure gradients. The synergistic effect minimizes membrane fouling while enhancing particle resuspension, enabling rapid, clog-free processing of samples (Chen et al., 2021; Hu et al., 2022). Key advantages include exceptional EVs recovery (>90%), high purity (>98% contaminant removal). Additionally, different models of EXODUS can cover processing volumes ranging from 10 µL to 10 L, while still maintaining the integrity of EVs, enabling downstream molecular analysis (Hu et al., 2023; Jiang S. et al., 2024).
EXODUS facilitates high-yield EV isolation for biomarker discovery across multiple disease contexts. It enables non-invasive detection of Alzheimer’s-associated proteins (Aβ, tau) in blood EVs at zeptomolar sensitivity (Zheng et al., 2025), stratifies prostate cancer risk via urinary EV RNA signatures (EPS model: FOXA1/PCA3/KLK3), reducing unnecessary biopsies by 26% (Jiang W. et al., 2024), and identifies ocular disorder biomarkers in tear EVs (Hu et al., 2022). However, its adoption faces limitations, including dependency on specialized equipment, variable performance with viscous samples requiring pre-filtration (Chen et al., 2021). Cost constraints of disposable cartridges further challenge large-scale implementation (Hu et al., 2023).
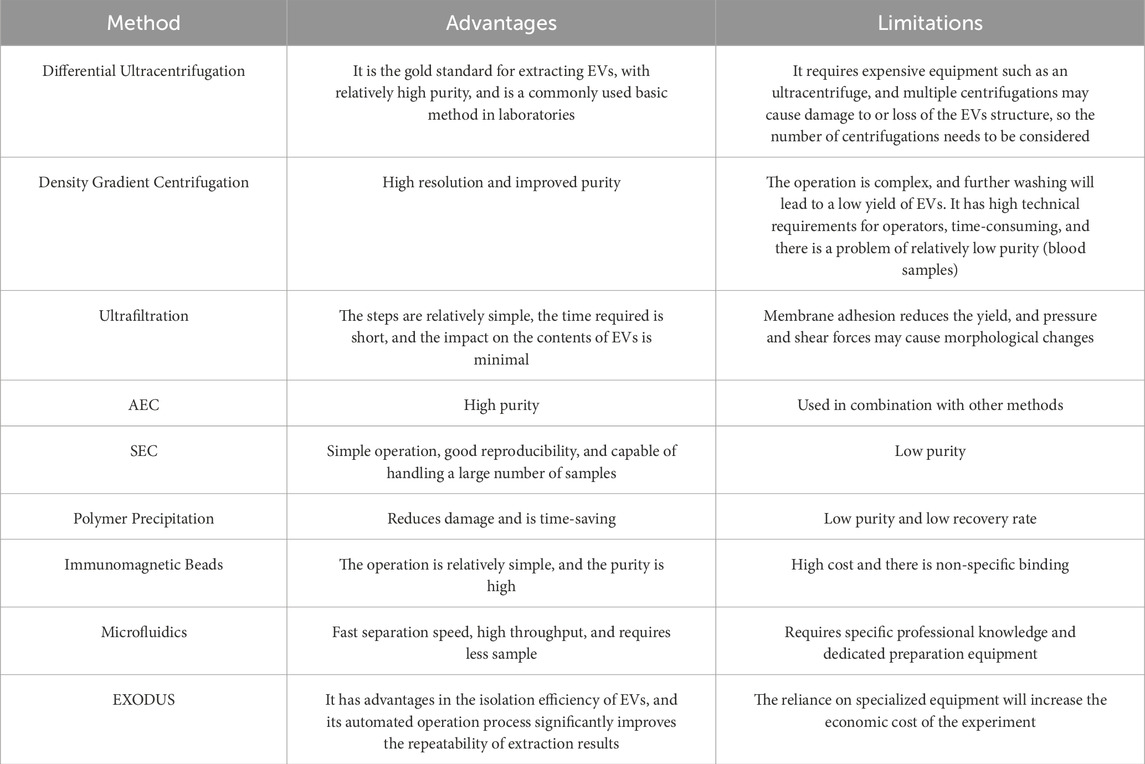
In summary, EVs isolation initially relied on ultracentrifugation, which remains the current gold standard. To address its limitations, alternative techniques have been developed, based on EVs biophysical and biochemical properties such as size or surface markers (Jia et al., 2022). However, these methods still have issues with specific isolation, yield, and purity. This is partly due to EVs heterogeneity and the co-isolation of non-EV contaminants like lipoproteins and uromodulin (Doyle and Wang, 2019; Théry et al., 2018) (Table 2). Method selection depends on research or clinical needs. For example, SEC, despite being prone to serum protein contamination, offers high yield and is suitable for large-sample studies (An et al., 2018). Isolation techniques also affect EVs function: SEC-isolated EVs, for instance, show a stronger effect in promoting endothelial migration than those isolated by ultracentrifugation, though this difference disappears when normalized by particle number (Takov et al., 2018). Thus, choosing optimal isolation methods, combined with multi-dimensional detection, is critical for the clinical translation of EVs. While combining multiple methods can improve purity and yield, it increases costs and complexity, which hinders clinical application. Developing rapid, efficient, and reproducible techniques remains a key challenge (Kimiz-Gebologlu and Oncel, 2022).
3 Characterization of EVs
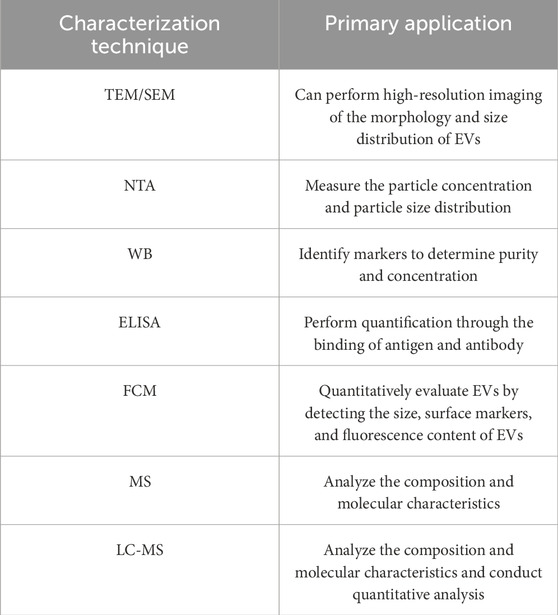
Obtaining high-quality EVs poses certain challenges. The identification of EVs is critical for EVs-related research and applications. This step is mainly aimed at determining whether the extracted particles are EVs, so downstream analysis becomes particularly crucial. To date, the main downstream analysis methods include electron microscopy imaging, Nanoparticle Tracer Analysis (NTA), Western blotting (WB), Enzyme-Linked Immunosorbent Assay (ELISA), Flow Cytometry (FCM), and proteomics techniques (Ströhle et al., 2022; Lai et al., 2024; Welsh et al., 2024). By applying these analytical techniques, the molecular components of EVs can be discovered, which provides a basis for the management of EVs (Szatanek et al., 2015).
3.1 Electron microscopy
Techniques such as Transmission Electron Microscopy (TEM) and Scanning Electron Microscope (SEM) in electron microscopy can perform high-resolution imaging of the morphology and size distribution of EVs. TEM is a widely used technique and is crucial for evaluating the quality and purity of samples containing EVs. This technique can distinguish between individual EVs and particles of a similar size to EVs (Klaihmon et al., 2023). TEM can provide a detailed view of the ultrastructure of EVs, revealing their lipid bilayer membranes and internal cargo. SEM offers 3D imaging capabilities, which helps in visualizing the surface morphology of EVs (Ju et al., 2022). Electron microscopy is invaluable for confirming the identity of EVs and assessing their structural integrity (Colombo et al., 2021).
3.2 NTA
NTA is another key technology used for quantifying and determining the size of EVs. This technique demonstrates good reproducibility and accuracy when measuring the particle concentration and determining the particle size distribution of EVs isolated from different sources through various methods (Comfort et al., 2021). NTA employs laser scattering to monitor the movement of EVs, which helps in the immediate examination of their size changes and diversity. NTA is a rapid and sensitive method for quantifying EVs, but it may be affected by factors such as particle aggregation and sample viscosity (Colombo et al., 2021). Therefore, in order to further improve the reproducibility of EVs research, the standardization of the NTA analysis method is essential.
3.3 WB
WB is a technique used to identify the presence of protein markers on EVs. It requires the analyte to have a relatively high purity and concentration, and it can quantitatively and qualitatively analyze specific proteins (Torres et al., 2024). Researchers typically use transmembrane protein families (CD9, CD63, CD81), heterotrimeric G protein subunit β-like proteins (Alix), tumor susceptibility gene 101 (TSG101), and syntenin-1, etc. as positive markers; endoplasmic reticulum chaperone protein Calnexin, Golgi matrix protein GM130, etc. as negative markers to identify the presence of EVs (Li P. et al., 2024; Khanabdali et al., 2024).
3.4 ELISA
ELISA has high detection sensitivity, convenient operation, and a fast detection speed, and it can be scaled up for higher-throughput measurements (Karachaliou et al., 2023). ELISA is a well-known quantitative analysis technique. It achieves colorimetric changes to indicate antigen-antibody interactions by incorporating enzyme-linked conjugates and enzyme substrates (Islam et al., 2023). Therefore, this method requires a pair of non-interacting antibodies, and it has higher requirements for detection specificity. It can only analyze EVs at the individual level. The determination can quantify EVs derived from specific cells based on the selection of the primary antibody, and this detection method is usually single.
3.5 FCM
FCM is an instrument used to measure the fluorescence and light scattering signals of individual particles flowing through a focused light source (most commonly a laser beam) in a liquid suspension (Welsh et al., 2023), capable of detecting and characterizing individual particles at a flux of thousands of particles per second. FCM allows for the quantitative assessment of EVs by detecting their size, surface markers, and fluorescence content. Fluorescent dyes or antibodies targeting specific surface antigens label the EVs, which are then analyzed using a flow cytometer equipped with appropriate detectors (Bettin et al., 2023). FCM is capable of performing multiparametric analysis of EVs subpopulations and is compatible with high-throughput screening applications (Kuiper et al., 2021).
3.6 Proteomic profiling
Compared with the small-scale analysis carried out by WB and ELISA, proteomics technology has obvious advantages. The term “proteomics” is used to describe the study of a large number of proteins in biological complexes, including cells, tissues, organelles, vesicles, or protein complexes (Abyadeh et al., 2024). Through proteomics determination, the protein composition of EVs can be comprehensively reflected. In the era of omics science, many researchers have utilized these methods to identify reliable tissue biomarkers. Particularly in the field of proteomics, studies on the protein content or quality of EVs have attracted attention (Aparicio et al., 2024). Through proteomic analysis, the protein composition of EVs can be comprehensively reflected, and their roles under different physiological and pathological conditions can be understood (Abyadeh et al., 2024). It is also possible to explore the differences in various biomolecules encapsulated or carried within EVs, and their associations with differences in sources of MSCs, in vitro aging caused by passaging, and differences in in vitro functional assays (Liu Y. et al., 2024). Giuliani et al. demonstrated that proteomic analysis can provide proteomic profiles to study differences in EVs proteins, while enabling the identification of new biomarkers (Giuliani et al., 2024). Martinez-Zalbidea identified 35 proteins through proteomic analysis of EVs and detected a variety of cargo components with potential therapeutic relevance (Martinez-Zalbidea et al., 2025).
3.6.1 Mass spectrometry (MS)
MS is an effective method for detecting and measuring the molecules (such as proteins, lipids, and nucleic acids) carried in EVs. MS-based proteomics, lipidomics, and nucleic acid sequencing provide comprehensive insights into the composition and molecular characteristics of EVs (Burton et al., 2023). Therefore, it enables higher-throughput quantitative analysis of EVs and also allows for the qualitative analysis of the molecular components of EVs. However, it should be noted that MS analysis requires samples to have a relatively high purity, because the salts present in biological fluids and other proteins such as lipoproteins can cause deviations in the analysis results (Pocsfalvi et al., 2016).
3.6.2 Liquid chromatography-MS (LC-MS)
LC-MS has become an effective method for a meticulous and rapid proteomic examination of EVs (Pei et al., 2024). LC separates proteins according to their hydrophobicity or size and is combined with MS detection for identification and quantitative analysis. LC-MS helps to identify a large number of proteins in EVs samples, including low-abundance proteins and proteins with post-translational modifications. This technique is highly suitable for discovery-based proteomics and the quantitative analysis of protein expression changes in AD (Chang et al., 2024).
In practice, multiple methods are required for accurate EVs identification. This is to ensure that the quality of EVs meet the desired requirements (Table 3).
4 Pharmacokinetics of EVs
Emerging studies have highlighted the unique therapeutic properties of EVs, including anti-inflammatory, anti-apoptotic, and regenerative capabilities, which offer offering immense therapeutic potential in clinical applications (Jahanbani et al., 2023). The biogenesis of EVs is a highly orchestrated process involving diverse molecular machinery that governs their formation, trafficking, and secretion (Akbari et al., 2020). To elucidate their biological functions and facilitate the development of EV-based therapies, a thorough understanding of EVs pharmacokinetics encompassing biodistribution, cellular uptake, retention, and clearance is imperative (Cheng and Kalluri, 2023).
4.1 Administration routes
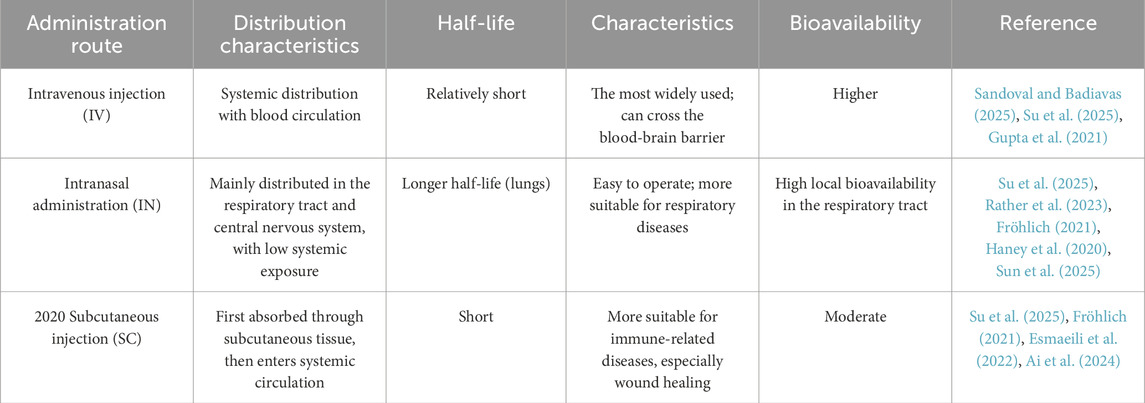
EVs are naturally distributed through biological fluids including blood, urine, breast milk, cerebrospinal fluid, amniotic fluid, ascites, saliva, bile, and bronchoalveolar lavage fluid enabling targeted delivery to specific cells and tissues (Kowal et al., 2014; Li et al., 2025). Researchers have explored diverse administration routes to optimize therapeutic efficacy across disease models (Table 4).
Intravenous (IV) Injection: The most widely used route, offering rapid systemic circulation. However, EVs exhibit a relatively short plasma half-life due to hepatic and renal clearance (Liu et al., 2017). However, in vivo neuroimaging has demonstrated that, compared with intravenous injection, EVs can cross the blood-brain barrier (BBB) more effectively after intranasal administration (Guy and Offen, 2020). Near-infrared imaging further supports this, as the imaging results show that DiR-labeled EVs can be delivered to the brain after intranasal administration, while tail vein injection mainly leads to their accumulation in the liver and kidneys (Liang et al., 2020). However, Hong et al. showed through in vivo imaging after intravenous administration that EVs can effectively cross the BBB and have a higher fluorescence density in the mouse brain (Hong et al., 2023). Preclinical data indicate that in acute respiratory distress syndrome, the delivery of EVs via the intravenous and intratracheal routes has similar efficacy (Zhu et al., 2014). The study by Fröhlich et al. shows that MSC-EVs are a promising option for both intravenous injection and inhalation therapy. Because they can cross the epithelial barrier better, have stronger stability, and have less procoagulant effect, but the advantages of the inhalation administration route are greatly reduced (Fröhlich, 2021). After subcutaneous injection of EVs, they will first diffuse in the local tissues and interact with surrounding cells. Then, some of the EVs can enter the circulatory system through local capillaries or lymphatic vessels, accumulate in the connected lymph nodes, and then be distributed to other parts (Sakurai et al., 2023). Therefore, subcutaneous injection of EVs can be used for research in immune-related disease models. Yang et al. subcutaneously injected EVs into the skin wounds of mice, which can effectively promote the healing of skin injuries (Yang J. et al., 2024). Currently, most EVs can be used for local administration, but the use of subcutaneous injection as an administration route has not been approved (Tawanwongsri and Vachiramon, 2024).
In recent years, significant progress has been made in biomaterials. Drug delivery research shows that hydrogels have attracted interest due to their excellent biocompatibility, degradability, and processability. In the study of various diseases, the encapsulation of EVs in hydrogels may help regulate the activity of EVs in vivo, thereby enhancing the therapeutic effect of EVs. Hydrogel-encapsulated EVs provide an alternative to traditional intravenous, arterial, or intramuscular injection methods (Farahzadi et al., 2024). For example, EVs are encapsulated in gelatin methacryloyl that covers the surface of the heart, and local administration may greatly increase the retention rate of EVs (Tang et al., 2022).
In conclusion, due to the many differences between studies, it is difficult to determine the advantages of the administration routes of EVs. These differences include variations in the production and preparation of EVs, uncertainties in issues related to administration, and differences between disease stages.
4.2 Biodistribution
EVs exhibit tissue-specific tropism in vivo, influenced by their cellular origin and surface modifications (Wang Y. et al., 2023). Advanced labeling techniques enable precise tracking of EVs biodistribution and spatial-temporal dynamics.
4.2.1 Lipophilic fluorescent dye labeling
Carbonaceous dyes are a class of lipophilic fluorescent dyes that can be used for staining cell membranes and other lipid-soluble biological structures. When they bind to the lipid membrane, the fluorescence intensity is greatly enhanced, and this type of dye has a high quenching constant and a long excited state lifetime. Once the lipid bilayer is stained, these dyes will diffuse across the entire membrane, and at the optimal concentration, they can be applied to the entire membrane structure of EVs. Common carbocyanine dyes include DiIC18(5) (DiD), DiIC18(3) (DiI), DiOC18(3) (DiO), and DiIC18(7) (DiR) (Bao et al., 2023). After Zhang et al. labeled EVs with DiR dye and injected them into mice via the tail vein, they could observe and measure the distribution of EVs in the organs of the mice, most EVs are first distributed to the lungs and liver through the circulatory system, and can achieve retention in lung tissues (Zhang et al., 2024). DIO staining can be used to examine the uptake of EVs by human lung microvascular endothelial cells (Sun et al., 2023). PKH67 and PKH26 are also lipophilic fluorescent dyes. PKH67 and PKH26 tend to form more aggregated micelles than DiI, but an excessive amount of PKH26 may damage the structure of EVs (Chen et al., 2023). Jiang et al. isolated EVs from the representative epidermal cells of psoriasis-like mice, labeled them with the green fluorescent dye PKH67, and injected them into the back of psoriasis-like mice through microneedles to observe the effect of EVs on the pathogenesis of psoriasis (It localizes in the epidermis approximately 48 h after in situ injection) (Jiang S. et al., 2024).
4.2.2 Radioisotope labeling
Radioisotope-labeled compounds can be used for in vivo tracing of EVs by introducing radioisotopes into EVs and utilizing nuclear medicine imaging techniques (such as positron emission tomography; single-photon emission computed tomography). This method has high sensitivity and specificity, but it requires professional nuclear medicine equipment and protective measures (Jiang W. et al., 2024). The main advantages of this method include the wide availability of radioisotopes and the preservation of the morphology of EVs after labeling (Almeida et al., 2020). Chung directly labeled EVs with the radioisotope Tc-99m and evaluated the in vivo tracking of EVs through single-photon emission computed tomography. The EVs were mainly absorbed by the liver and spleen (Chung et al., 2024). Lazaro-Ibáñez et al. covalently linked diethylenetriaminepentaacetic dianhydride to the surface of EVs and labeled EVs with indium-111 (111In3+). This labeling method showed a high radioactive labeling efficiency for EVs and enabled the observation of the specific situation in mice. EVs rapidly accumulate in the peri-abdominal area including the liver, spleen, and kidneys, and the accumulation in these organs is retained at the subsequent time points of 4 h and 24 h (Lázaro-Ibáñez et al., 2021).
4.2.3 Bioluminescence imaging (BLI)
BLI is a technique used to visualize the physiological change processes in animals. Based on the bioluminescence phenomenon, researchers discovered the mechanism by which luciferase reacts with luciferin to generate light and developed BLI. BLI allows for multiple imaging sessions without euthanizing the animals to assess the changes in physiological processes over time. After injecting the luciferin substrate, the luciferase in the body interacts with the luciferin to produce light of a specific wavelength, enabling observation (Li S. et al., 2023). Villa et al. used BLI to confirm the targeting and delivery capabilities of EVs, in the absence of tumors, canine glioma-derived vesicles are capable of crossing the BBB in healthy mice, with a propensity for accumulation within the brain tissue (Villa et al., 2024). Bioluminescent reporter genes are derived from light-producing organisms, including Gaussia luciferase (GLuc) or Renilla reniformis (RLuc), etc. They have been used for the tracking of EVs. BLI has a wide range of applications and high sensitivity (Liu et al., 2022).
4.2.4 Magnetic resonance imaging (MRI)
MRI is mainly a medical imaging technique, suitable for the non-invasive visualization of the body’s anatomy and physiology in diseases and health conditions. An MRI scanner uses magnetic fields, electric fields, and radio waves to generate images of organs and body structures. By using different MRI pulse sequences, MRI can provide detailed anatomical images that reflect the characteristics of tissues (Hussain et al., 2022). In addition to its use in detailed tissue imaging, MRI can also be applied to track and monitor EVs in vivo, thereby enhancing their visibility in biological systems. However, MRI is not the best choice for imaging the heart and gastrointestinal tract (Arifin et al., 2022).
EVs have been labeled with paramagnetic gadolinium (Gd)-based contrast agents. Gd has been bound to phospholipids and integrated into EVs derived from macrophages or MSCs (Rayamajhi et al., 2020; Abello et al., 2019). The process of inserting the Gd contrast agent into the EVs membrane occurs during extrusion, and there will be slight changes in its surface charge, size distribution, and morphology. Ultrasmall superparamagnetic iron oxide nanoparticles (USPIOs) are currently the most widely used negative contrast agents for labeling EVs and can be used for histological verification in vitro or ex vivo. In rodent models, EVs derived from MSCs, macrophages, or other stem cells labeled with USPIOs can be detected in vivo and exhibit signals, MRI revealed that intravenously administered magneto-EVs possess the homing function to injury sites, including the kidneys and heart (Han et al., 2021).
4.2.5 Computed tomography (CT)
The imaging principle of CT is to use an X-ray beam to perform continuous cross-sectional scans around a certain part of the human body. It has the characteristics of fast scanning speed and clear images, and can be used for the examination of various diseases (Albers and Kinnaird, 2024). Gold nanoparticles have become the most widely used CT probes due to their excellent X-ray absorption and biological inertia. Researchers used gold nanoparticles to label EVs and conducted longitudinal and quantitative tracking of EVs in A431 tumor-bearing mice through CT, demonstrating that MSC-EVs derived from the umbilical cord may have superior tumor-targeting therapeutic capabilities (Cohen et al., 2021). The CT imaging technique was used to obtain the biodistribution curves of EVs in the major organs of adult rhesus monkeys, thereby describing the biodistribution and in vivo kinetics of EVs. They are able to cross the biological barriers and appear in larger quantities in the brain (Haney et al., 2022). CT imaging has been used to study the effect of EVs on tumor targeting, but CT examination has a certain level of radiation (Huang P. et al., 2024).
4.3 Clearance mechanisms
The clearance of EVs is a finely regulated process critical for maintaining intercellular communication, disease progression, and physiological homeostasis.
4.3.1 Macrophage mediated phagocytosis
As an important part of the body’s immune system, macrophages have a powerful phagocytic function. They can recognize and phagocytize EVs (Yang et al., 2022), and then, through digestive and degradation mechanisms such as lysosomes, they bind to lysosomes and decompose and remove EVs (Buratta et al., 2020). Methyl-β-cyclodextrin may promote the decomposition of the vesicle contents, thus leading to a reduction in EVs. Previous studies have shown that in the AD model, Aβ-related EVs are sorted into lysosomes and subsequently degraded in microglia (Pantazopoulou et al., 2023).
4.3.2 Renal filtration
Small EVs undergo glomerular filtration due to their size and negative surface charge, while larger EVs remain in systemic circulation. Charge-selective glomerular pores preferentially clear anionic EVs, whereas neutral or cationic EVs exhibit prolonged circulation times (Alli, 2023).
4.4 Safety profile
EVs retain the bioactive properties of parental cells while circumventing risks associated with cell-based therapies, such as tumorigenicity and vascular occlusion, owing to their non-replicative nature (Xia et al., 2024). A prospective, single-arm, open-label Phase I trial conducted at the State Key Laboratory of Ophthalmology (Guangzhou, China) demonstrated the safety and efficacy of umbilical cord-derived MSC-EVs in treating refractory graft-versus-host disease (GvHD)-associated dry eye disease (DED). Topical administration (4× daily for 14 days) significantly reduced ocular surface inflammation, accelerated corneal epithelial regeneration, and showed no systemic adverse effects (Zhou et al., 2022). In addition, MSC-EVs cannot self-replicate, which avoids many of the risks associated with stem cell therapy. At the same time, they exhibit beneficial effects such as activating the immune system and prolonging the therapeutic effect (Yin et al., 2023).
By understanding the processes such as the distribution and metabolism of EVs in vivo, it is necessary to determine the appropriate dosage, administration frequency, and administration route to improve the efficacy of EV-based drugs and reduce adverse reactions. There are still many aspects that need in-depth exploration in the study of the pharmacokinetics of EVs. With the continuous advancement of technology, a clearer and more accurate understanding of their dynamic changes in vivo will be achieved, thereby better promoting the clinical applications related to EVs.
5 Clinical investigations of MSC-EVs
A growing body of evidence shows that MSC-EVs have cell therapeutic bioactivities similar to their parental cells, while avoiding the safety risks of live stem cell administration (Yang R. et al., 2024). ClinicalTrials.gov data reveals a rising number of registered clinical trials using MSC-EVs as interventions. As of 25 April 2025, a search for “MSC-exosomes” and “MSC-EVs” on the platform identified 28 clinical studies in total (Table 5).
These studies cover various conditions: 5 focus on pneumonia, 3 on diabetes-related diseases, and 2 on knee osteoarthritis. Single studies address AD, meniscus injury, multiple organ failure, decompensated liver cirrhosis, macular hole, psoriasis, perianal fistula (with two separate studies), dry eye syndrome, myocardial infarction, mucositis, cerebrovascular diseases, ALS, dystrophic epidermolysis bullosa, sepsis, metastatic pancreatic adenocarcinoma, lung diseases, and endometrial injury.
Notably, neurological diseases such as AD, ALS, and cerebrovascular diseases are included. These trials not only highlight the potential of MSC-EVs in treating neurological diseases but also provide preliminary evidence for their safety in clinical settings.
6 Therapeutic applications of MSC-EVs in neurological disorders
Neurological diseases are a group of diseases characterized by the breakdown of the structure and function of neural networks, as well as impaired memory, cognitive, behavioral, sensory, and motor functions. They include various conditions such as AD, PD, Multiple sclerosis, etc. Neurological diseases are the leading cause of disability and the second leading cause of death, and they also impose a heavy economic burden (Cheng et al., 2024). Globally, approximately 30% of people may experience neurological problems at some point in their lives (Salehpour et al., 2024). Stroke, migraine, AD, and other dementias are the most common neurological causes of disability. In the past few years, the number of deaths has climbed by 30%, and the number of years of life lost due to disability has decreased by 15% (Feigin et al., 2020). MSC-EVs mimic certain properties of MSCs, including their ability to suppress the immune system and promote tissue repair. MSC-EVs carry a large number of biomolecules, such as proteins (extracellular, intracellular, enzymes, receptors), lipids, and nucleic acids (Hamidi et al., 2024). MSC-EVs exhibit low immunogenicity, high heterogeneity, and the ability to promote intercellular signaling and regulate the extracellular matrix, significantly enhancing their involvement in various physiological and pathological processes. Compared with MSCs, MSC-EVs offer several advantages for clinical applications (Karnas et al., 2023). MSC-EVs have a wide range of therapeutic applications in the treatment of neurological diseases. MSC-EVs have been found to be beneficial for neurological diseases caused by AD, PD, Amyotrophic Lateral Sclerosis (ALS), Peripheral Nerve Injury (PNI), Spinal Cord Injury (SCI), and hearing loss.
6.1 Umbilical cord derived MSC-EVs (UCMSC-EVs)
AD is a progressive neurodegenerative disease characterized by mitochondrial dysfunction, the accumulation of β-amyloid plaques, and hyperphosphorylated tau tangles in the brain, leading to memory loss and cognitive deficits. EVs contain proteins, lipids, microRNAs, and other molecules that can affect the functions of neighboring cells and promote regeneration (Belousova et al., 2024). EVs derived from UCMSCs have been well demonstrated to cross the BBB and target their active components (such as proteins, miRNAs, etc.) to the damaged areas of the central nervous system, becoming a research hotspot in the field of neural therapy (Hu et al., 2024).
UCMSC-EVs can improve the occurrence of Aβ accumulation and neuroinflammation in AD animals by regulating the activity of microglia (Fallahi et al., 2024). PD is a common neurodegenerative disease that has received extensive attention. However, the current clinical treatments can only alleviate its symptoms and cannot effectively protect dopaminergic neurons. UCMSC-EVs enhance the cellular antioxidant defense mechanism by activating the nuclear factor erythroid 2-related factor 2 signaling pathway, protecting neurons from damage (Wang Z. et al., 2024). Compared with EVs derived from bone marrow mesenchymal stem cells (BMSC-EVs), UCMSC-EVs are richer in the regulatory potential related to the cell cycle, DNA replication, and repair, which can improve the motor ability of PD model mice and enhance the recovery of the olfactory and motor functions of the mice (Huang W. et al., 2024). Depression is characterized by neuroinflammation and neurodegeneration. UCMSC-EVs exhibit antidepressant effects by inhibiting the neuroinflammation of M1 microglia (Li S. et al, 2024). Ischemic stroke is one of the cardiovascular diseases globally, characterized by a high incidence and mortality rate. After a period of long-term ischemia caused by a stroke, the restoration of blood flow will trigger inflammation and the excessive production of reactive oxygen species (ROS), leading to neuronal cell death and brain damage. This phenomenon is called cerebral ischemia/reperfusion injury (CIRI) (Datta et al., 2020). UCMSC-EVs have certain therapeutic potential in alleviating CIRI in various cell types, including neuronal cells (Wang C. C. et al., 2024). TBI is a major cause of neurological dysfunction, and the current treatment methods have limited effects. UCMSC-EVs can improve the damaged microenvironment by inhibiting the injury-induced excessive activation of microglia and the neuroinflammatory response, effectively alleviating TBI (Cui et al., 2024). The results of a first-in-human, single-arm, open-label, phase I clinical trial indicate that the intrathecal administration of UCMSC-EVs is safe for patients with subacute SCI, and there are significant improvements in the subscales of respiratory and sphincter management (Akhlaghpasand et al., 2024). Multiple sclerosis is an immune-mediated central nervous system disease characterized by causing inflammation, demyelination, and neuronal degeneration. Common symptoms include motor and sensory disorders, fatigue, pain, visual impairment, and cognitive dysfunction (Kråkenes et al., 2024). UCMSC-EVs can regulate the immune response of mice with experimental autoimmune encephalomyelitis (an Multiple sclerosis model) by promoting the expression of Lag-3 on CD4+/Foxp3 Tregs and reducing the infiltration of immune cells in the hypothalamus (Mohammadzadeh et al., 2024). Wharton’s jelly (WJ) is a major source of MSCs from the umbilical cord. WJ-MSCs are isolated from umbilical cord tissue through a non-invasive and painless procedure, and their EVs are also referred to as UCMSC-EVs (Patel et al., 2024; Drobiova et al., 2023). WJ-MSC-EVs can protect hippocampal neurons from oxidative stress and damage induced by Aβ oligomers, an effect associated with the transfer of enzymatically active catalase contained in these EVs (Bodart-Santos et al., 2019). After intranasal administration of WJ-MSC-EVs to mice, Zhdanova et al. found that the spatial memory of olfactory bulbectomized mice was improved, with labeled EVs detected in the hippocampus and cortex (Zhdanova et al., 2021).
6.2 Bone marrow derived MSC-EVs (BMSC-EVs)
SCI severely impairs the quality of life of patients, manifesting as complications such as motor dysfunction, neuropathic pain, disorders of urination and defecation, and sexual dysfunction (Yang S. et al., 2024). BMSC-EVs show promising potential for the repair of SCI, a neurological disease. The research results of Xu indicate that BMSC-EVs have improved SCI in rats, manifested as the recovery of motor function, the alleviation of pathological conditions, and the reduction of apoptosis, inflammatory response, and oxidative stress. BMSC-EVs promote SCI repair through miR-497-5p/TXNIP/NLRP3, which may be a target for alleviating SCI-related nerve damage (Xu et al., 2024). Ischemic stroke, caused by a clot disrupting the blood supply to the brain, is an important cause of long-term neurological disability and death among adults worldwide (Fan et al., 2023). When intranasal BMSC-EVs treatment is combined with drug treatment, adult rats with transient middle cerebral artery occlusion stroke exhibit significant functional recovery in the neurological severity score and the beam balance test, with the striatum and cortex being greatly affected (Gomez-Galvez et al., 2024). ALS is a fatal neurodegenerative disease of motor neurons, and it is estimated that 6.6 out of every 100,000 people in the United States suffer from this disease. The study by Crose et al. shows that BMSC-EVs treatment has no serious side effects, has a certain degree of safety, and may have the potential to delay the progression of ALS (Crose et al., 2024). Sensorineural Hearing Loss (SNHL) is one of the most prevalent sensory deficits, having a severe negative impact on patients. SNHL usually involves damage to the central auditory pathway. Intratympanic injection of BMSC-EVs is a specific and effective strategy for the treatment of neurological hearing loss and can minimize surgical trauma and systemic side effects (Chen et al., 2024).
6.3 Adipose derived MSC-EVs (ADSC-EVs)
As outlined by the World Health Organization (WHO), Glioblastoma Multiforme (GBM) is a grade 4 central nervous system tumor and a fatal cancer with a high incidence and mortality rate. EVs derived from ADSCs contain microRNA-4731-5p (miR-4731-5p), and its expression has good antitumor properties against GBM (Babaei et al., 2024). Intranasal administration of ADSCs-EVs can improve the motor performance of ALS mice and inhibit muscle atrophy (Turano et al., 2024). ADSCs, especially those from hypoxic-preconditioned ADSCs-MSCs, possess therapeutic properties that can promote spinal cord repair in SCI (Shao et al., 2024). Spinal Muscular Atrophy (SMA) is an autosomal recessive neuromuscular disease. Its typical symptoms include hypomyotonia, muscle atrophy, and weakness caused by denervation, until the muscles of the upper and lower limbs as well as the trunk muscles are eventually paralyzed. Relevant studies have shown that when treating SMNΔ7 mice (a model of SMA type II), ADSCs-EVs may play a neuroprotective role at the central level and in terms of motor function (Virla et al., 2024). Glaucoma is a progressive neurodegenerative disease and one of the most common causes of irreversible blindness. Increasing evidence indicates that neuroinflammation is associated with the pathological process of glaucoma (Ji et al., 2024). Some researchers believe that compared with MSC-EVs from other sources, ADSCs-EVs have a better effect in immune inflammation regulation (Tham et al., 2014). Ji et al. believe that ADSCs-EVs can reduce the expression of microglia-related CD68, CCL2, and TLR4 in the retina and optic nerve of mice, and they are potential therapeutic candidates or adjuvant treatment options for glaucoma (Ji et al., 2024).
6.4 Placental derived MSC-EVs (PMSC-EVs)
EVs derived from PMSCs contain proteins with proliferative-promoting properties (FGF-2, PDGF, and HGF), anti-catabolic properties (TIMP-1 and TIMP-2), and anti-inflammatory properties (sICAM-1) (Shipman et al., 2024). Intrathecal injection of PMSC-EVs during the acute phase of SCI in female rats can improve functional recovery, reduce injury-related pathological changes, and prevent chronic diseases after SCI. Therefore, PMSC-EVs have certain neuroprotective and anti-apoptotic potentials (Soleimani et al., 2024). Intravenous injection of PMSC-EVs can promote the activation of endogenous neural progenitor cells and the ability of neurogenesis, and facilitate the recovery of motor and autonomic nerve functions damaged by SCI (Zhou et al., 2021). Duan et al. found that in-situ injection of PMSC-EVs can inhibit the occurrence of inflammation and reduce oxidative stress (Duan et al., 2020). High-dose PMSC-EVs can improve motor function in EAE, a mouse model of Multiple sclerosis, and this may be linked to neuroprotective mechanisms (Clark et al., 2019). The placenta, like the umbilical cord, comes from mesenchymal tissues in embryonic development. But because of its complex structure, isolating and purifying PMSCs from it needs more refined procedures (Wang et al., 2025). There is less research on PMSC-EVs compared with UCMSC-EVs. Going forward, it is important to dig deeper into their neuroprotective mechanisms and clinical application value.
6.5 Dental pulp derived MSC-EVs (DPSC-EVs)
Subarachnoid Hemorrhage (SAH) caused by an aneurysm is a severe subtype of stroke, accounting for approximately 5% of all stroke cases. Compared with other types of strokes, SAH is characterized by a younger onset age, higher morbidity and mortality rates (Liang et al., 2024). EVs derived from DPSCs can be extracted from human wisdom teeth or deciduous teeth. The extraction method is minimally invasive, safe, and free of ethical issues, and they exhibit immunomodulatory properties (Vu et al., 2022). DPSCs-EVs can inhibit the activation of microglia and the secretion of pro-inflammatory cytokines after SAH, and significantly alleviate the overall brain edema and nerve damage in rats (Liang et al., 2024). PNI is a common and destructive neurodegenerative disease, which easily leads to motor and sensory dysfunctions in patients. DPSCs-EVs can promote axonal regeneration and remyelination after PNI by increasing the proliferation, migration, and secretion of neurotrophic factors (Chai et al., 2024). Recent studies show DPSC-EVs promote axonal regeneration and functional recovery in injured mouse sciatic nerves by enhancing Schwann cell proliferation/migration and upregulating c-JUN, Notch1, GFAP, and SRY-box 2 (Mao et al., 2019). In ischemic stroke-related CIRI, DPSC-EVs reduce cerebral edema, infarct volume, and neurological deficits by alleviating neuronal apoptosis—likely via miR-877-3p interacting with Bclaf1 (Miao et al., 2024). A single systemic administration also lessens infarct size, neuroinflammation, and impairment (Ji et al., 2019). DPSC-EVs, rich in VEGF and MCP-1, mitigate Aβ cytotoxicity, boost cell viability, and regulate Bcl-2/Bax to inhibit apoptosis (Ahmed et al., 2016). Their parent DPSCs’ high proliferation and neurogenic capacity support potential in craniofacial/neural regeneration (Ahmad et al., 2025). However, DPSC extraction (requiring tooth removal) is impractical and unethical except for wisdom/orthodontic teeth.
This article summarizes MSC-EVs from five different sources (Table 6), which provide abundant resources and broad application prospects for the treatment and research of numerous neurological diseases (Figure 2) (Li Y. et al., 2024; Rather et al., 2023; Santilli et al., 2024; Mushahary et al., 2018).
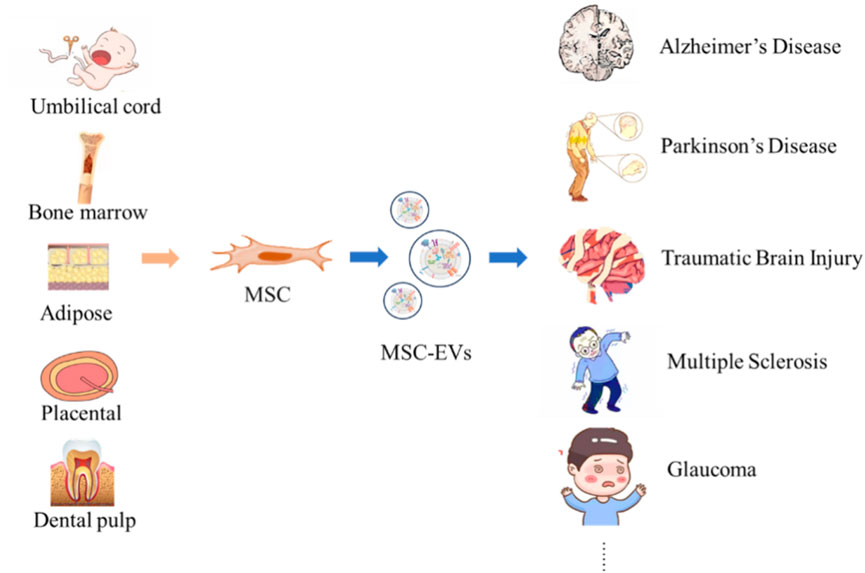
Figure 2. Neurological Disorders Targeted by MSC-EVs from Diverse Origins. MSC can be obtained from tissues such as umbilical cord, bone marrow, adipose tissue, placenta, and dental pulp. MSC secrete MSC-EVs, and these vesicles show potential in the therapeutic application for neurological diseases such as AD, PD, TBI, Multiple sclerosis, and GLA.
7 Conclusion
MSC-EVs have emerged as promising alternatives to whole-cell therapies, leveraging their unique advantages such as BBB penetrance, low immunogenicity, and high biosafety. These attributes position MSC-EVs at the forefront of biomedical and clinical research, offering novel therapeutic strategies for refractory diseases (Rashidi et al., 2022; Zanirati et al., 2024). Despite their transformative potential, the clinical translation of MSC-EVs faces significant challenges. A critical hurdle lies in the standardization and validation of isolation protocols to ensure inter-batch consistency and reproducibility (Ma et al., 2025). Current methods including ultracentrifugation, differential centrifugation, density gradient centrifugation, ultrafiltration, AEC, SEC, polymer precipitation, immunomagnetic beads, and microfluidics each present trade-offs in purity, yield, and scalability. No single technique universally addresses all research needs, necessitating integrated approaches for optimal EVs recovery and functionality.
Secondly, this article analyzes various identification techniques and pharmacokinetics of MSC-EVs. In clinical applications, although MSC-EVs do not pose the same ethical and immunogenic dilemmas as stem cells, their administration methods still need to be further standardized. Different diseases models usually require different administration methods. Before clinical application, an appropriate administration method of EVs must be selected to evaluate its therapeutic effect and potential toxic and side effects, thus promoting subsequent scientific research.
Finally, we summarize the relevant research on the therapeutic applications of MSC-EVs in various neurological diseases. Up to now, the Food and Drug Administration (FDA) of the United States has not approved any MSC-EVs-based therapies for clinical use. MSC-EVs from different parental sources are being studied in various preclinical and clinical research to understand their potential in treating a range of neurological diseases (such as AD, PD, SCI, ALS, etc.), with the hope of providing guidance for future clinical research.
In conclusion, MSC-EVs have good therapeutic potential in treating nerve injuries and neurological diseases, but several challenges must still be addressed before clinical application. In the future, continuous research is crucial for fully unleashing the therapeutic potential of MSC-EVs and overcoming the technical and regulatory barriers they face for effective use in clinical practice.
Author contributions
SL: Conceptualization, Writing – original draft. JZ: Conceptualization, Writing – original draft. LS: Writing – review and editing. ZY: Writing – review and editing. XnL: Methodology, Writing – review and editing. JL: Supervision, Writing – review and editing. XfL: Project administration, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from the HNU Startup Grant (Grant No.: HNU20180101), the Sciences reform and Development special project of Guangxi Academy (2024YGFZ505-908).
Conflict of interest
Authors SL, JZ, ZY, and XL were employed by Jianyuan Precision Medicines (Zhangjiakou) Co., Ltd. Author JZ was employed by Shijiazhuang Zhuohan Biological Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abello, J., Nguyen, T. D. T., Marasini, R., Aryal, S., and Weiss, M. L. (2019). Biodistribution of gadolinium-and near infrared-labeled human umbilical cord mesenchymal stromal cell-derived exosomes in tumor bearing mice. Theranostics 9, 2325–2345. doi:10.7150/thno.30030
Abyadeh, M., Alikhani, M., Mirzaei, M., Gupta, V., Shekari, F., and Salekdeh, G. H. (2024). Proteomics provides insights into the theranostic potential of extracellular vesicles. Adv. Protein Chem. Struct. Biol. 138, 101–133. doi:10.1016/bs.apcsb.2023.08.001
Ahmad, P., Estrin, N., Farshidfar, N., Zhang, Y., and Miron, R. J. (2025). Isolation methods of exosomes derived from dental stem cells. Int. J. Oral Sci. 17, 50. doi:10.1038/s41368-025-00370-y
Ahmed, N. el-M., Murakami, M., Hirose, Y., and Nakashima, M. (2016). Therapeutic potential of dental pulp stem cell secretome for alzheimer's disease treatment: an in vitro study. Stem Cells Int. 2016, 8102478. doi:10.1155/2016/8102478
Ai, X., Yang, J., Liu, Z., Guo, T., and Feng, N. (2024). Recent progress of microneedles in transdermal immunotherapy: a review. Int. J. Pharm. 662, 124481. doi:10.1016/j.ijpharm.2024.124481
Akbari, A., Jabbari, N., Sharifi, R., Ahmadi, M., Vahhabi, A., Seyedzadeh, S. J., et al. (2020). Free and hydrogel encapsulated exosome-based therapies in regenerative medicine. Life Sci. 249, 117447. doi:10.1016/j.lfs.2020.117447
Akhlaghpasand, M., Tavanaei, R., Hosseinpoor, M., Yazdani, K. O., Soleimani, A., Zoshk, M. Y., et al. (2024). Safety and potential effects of intrathecal injection of allogeneic human umbilical cord mesenchymal stem cell-derived exosomes in complete subacute spinal cord injury: a first-in-human, single-arm, open-label, phase I clinical trial. Stem Cell Res. Ther. 15, 264. doi:10.1186/s13287-024-03868-0
Al-Madhagi, H. (2024). The landscape of exosomes biogenesis to clinical applications. Int. J. Nanomedicine 19, 3657–3675. doi:10.2147/IJN.S463296
Albers, P., and Kinnaird, A. (2024). Advanced imaging for localized prostate cancer. Cancers 16, 3490. doi:10.3390/cancers16203490
Aliakbari, F., Stocek, N. B., Cole-André, M., Gomes, J., Fanchini, G., Pasternak, S. H., et al. (2024). A methodological primer of extracellular vesicles isolation and characterization via different techniques. Biol. Methods Protoc. 9, bpae009. doi:10.1093/biomethods/bpae009
Allelein, S., Aerchlimann, K., Rösch, G., Khajehamiri, R., Kölsch, A., Freese, C., et al. (2022). Prostate-specific membrane antigen (PSMA)-positive extracellular vesicles in urine-a potential liquid biopsy strategy for prostate cancer diagnosis? Cancers 14, 2987. doi:10.3390/cancers14122987
Alli, A. A. (2023). Extracellular Vesicles: investigating the Pathophysiology of diabetes-associated hypertension and diabetic nephropathy. Biology 12, 1138. doi:10.3390/biology12081138
Almeida, S., Santos, L., Falcão, A., Gomes, C., and Abrunhosa, A. (2020). In vivo tracking of extracellular vesicles by nuclear imaging: advances in radiolabeling strategies. Int. J. Mol. Sci. 21, 9443. doi:10.3390/ijms21249443
An, M., Wu, J., Zhu, J., and Lubman, D. M. (2018). Comparison of an optimized ultracentrifugation method versus size-exclusion chromatography for isolation of exosomes from human serum. J. Proteome Res. 17, 3599–3605. doi:10.1021/acs.jproteome.8b00479
Aparicio, P., Navarrete-Villanueva, D., Gómez-Cabello, A., López-Royo, T., Santamaría, E., Fernández-Irigoyen, J., et al. (2024). Proteomic profiling of human plasma extracellular vesicles identifies PF4 and C1R as novel biomarker in sarcopenia. J. Cachexia, Sarcopenia Muscle 15, 1883–1897. doi:10.1002/jcsm.13539
Aqrawi, L. A., Galtung, H. K., Guerreiro, E. M., Øvstebø, R., Thiede, B., Utheim, T. P., et al. (2019). Proteomic and histopathological characterisation of sicca subjects and primary Sjögren’s syndrome patients reveals promising tear, saliva and extracellular vesicle disease biomarkers. Arthritis Res. Ther. 21, 181. doi:10.1186/s13075-019-1961-4
Arifin, D. R., Witwer, K. W., and Bulte, J. W. M. (2022). Non-Invasive imaging of extracellular vesicles: quo vaditis in vivo? J. Extracell. Vesicles 11, e12241. doi:10.1002/jev2.12241
Auquière, M., Muccioli, G. G., and des Rieux, A. (2025). Methods and challenges in purifying drug-loaded extracellular vesicles. J. Extracell. Vesicles 14, e70097. doi:10.1002/jev2.70097
Babaei, A., Yazdi, A. T., Ranji, R., Bahadoran, E., Taheri, S., Nikkhahi, F., et al. (2024). Therapeutic effects of exosomal mirna-4731-5p from adipose tissue-derived stem cells on human glioblastoma cells. Archives Med. Res. 55, 103061. doi:10.1016/j.arcmed.2024.103061
Bao, C., Xiang, H., Chen, Q., Zhao, Y., Gao, Q., Huang, F., et al. (2023). A review of labeling approaches used in small extracellular vesicles tracing and imaging. Int. J. Nanomedicine 18, 4567–4588. doi:10.2147/IJN.S416131
Belousova, E., Salikhova, D., Maksimov, Y., Nebogatikov, V., Sudina, A., Goldshtein, D., et al. (2024). Proposed mechanisms of cell therapy for alzheimer's disease. Int. J. Mol. Sci. 25, 12378. doi:10.3390/ijms252212378
Bettin, B. A., Varga, Z., Nieuwland, R., and van der Pol, E. (2023). Standardization of extracellular vesicle concentration measurements by flow cytometry: the past, present, and future. J. Thrombosis Haemostasis JTH 21, 2032–2044. doi:10.1016/j.jtha.2023.04.042
Bodart-Santos, V., de Carvalho, L. R. P., de Godoy, M. A., Batista, A. F., Saraiva, L. M., Lima, L. G., et al. (2019). Extracellular vesicles derived from human Wharton's jelly mesenchymal stem cells protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-β oligomers. Stem Cell Res. Ther. 10, 332. doi:10.1186/s13287-019-1432-5
Buratta, S., Tancini, B., Sagini, K., Delo, F., Chiaradia, E., Urbanelli, L., et al. (2020). Lysosomal exocytosis, exosome release and secretory autophagy: the autophagic- and endo-lysosomal systems go extracellular. Int. J. Mol. Sci. 21, 2576. doi:10.3390/ijms21072576
Burton, J. B., Carruthers, N. J., and Stemmer, P. M. (2023). Enriching extracellular vesicles for mass spectrometry. Mass Spectrom. Rev. 42, 779–795. doi:10.1002/mas.21738
Busatto, S., Vilanilam, G., Ticer, T., Lin, W. L., Dickson, D. W., Shapiro, S., et al. (2018). Tangential flow filtration for highly efficient concentration of extracellular vesicles from large volumes of fluid. Cells 7, 273. doi:10.3390/cells7120273
Chai, Y., Liu, Y., Liu, Z., Wei, W., Dong, Y., Yang, C., et al. (2024). Study on the role and mechanism of exosomes derived from dental pulp stem cells in promoting regeneration of myelin sheath in rats with sciatic nerve injury. Mol. Neurobiol. 61, 6175–6188. doi:10.1007/s12035-024-03960-9
Chang, C. J., Huang, Y. N., Lu, Y. B., Zhang, Y., Wu, P. H., Huang, J. S., et al. (2024). Proteomic analysis of serum extracellular vesicles from biliary tract infection patients to identify novel biomarkers. Sci. Rep. 14, 5707. doi:10.1038/s41598-024-56036-y
Chattopadhyay, S., Rajendran, R. L., Chatterjee, G., Reyaz, D., Prakash, K., Hong, C. M., et al. (2025). Mesenchymal stem cell-derived exosomes: a paradigm shift in clinical therapeutics. Exp. Cell Res. 450, 114616. doi:10.1016/j.yexcr.2025.114616
Chen, B. Y., Sung, C. W., Chen, C., Cheng, C. M., Lin, D. P., Huang, C. T., et al. (2019). Advances in exosomes technology. Clin. Chimica Acta; Int. J. Clin. Chem. 493, 14–19. doi:10.1016/j.cca.2019.02.021
Chen, Y., Zhu, Q., Cheng, L., Wang, Y., Li, M., Yang, Q., et al. (2021). Exosome detection via the ultrafast-isolation system: EXODUS. Nat. Methods 18, 212–218. doi:10.1038/s41592-020-01034-x
Chen, C., Cai, N., Niu, Q., Tian, Y., Hu, Y., and Yan, X. (2023). Quantitative assessment of lipophilic membrane dye-based labelling of extracellular vesicles by nano-flow cytometry. J. Extracell. Vesicles 12, e12351. doi:10.1002/jev2.12351
Chen, A., Qu, J., You, Y., Pan, J., Scheper, V., Lin, Y., et al. (2024). Intratympanic injection of MSC-derived small extracellular vesicles protects spiral ganglion neurons from degeneration. Biomed. Pharmacother. 179, 117392. doi:10.1016/j.biopha.2024.117392
Cheng, K., and Kalluri, R. (2023). Guidelines for clinical translation and commercialization of extracellular vesicles and exosomes based therapeutics. Extracell. Vesicle 2, 100029. doi:10.1016/j.vesic.2023.100029
Cheng, H., Liu, J., Zhang, D., Wu, J., Wu, J., Zhou, Y., et al. (2024). Natural products: harnessing the power of gut microbiota for neurological health. Phytomedicine Int. J. Phytotherapy Phytopharm. 135, 156019. doi:10.1016/j.phymed.2024.156019
Cheng, J., Zhu, N., Zhang, Y., Yu, Y., Kang, K., Yi, Q., et al. (2025). Correction: hedgehog-inspired immunomagnetic beads for high-efficient capture and release of exosomes. J. Mater. Chem. B 13, 1118–1119. doi:10.1039/d4tb90208d
Chung, Y. H., Ho, Y. P., Farn, S. S., Tsai, W. C., Li, Z. X., Lin, T. Y., et al. (2024). In vivo SPECT imaging of Tc-99m radiolabeled exosomes from human umbilical-cord derived mesenchymal stem cells in small animals. Biomed. J. 47, 100721. doi:10.1016/j.bj.2024.100721
Clark, K., Zhang, S., Barthe, S., Kumar, P., Pivetti, C., Kreutzberg, N., et al. (2019). Placental mesenchymal stem cell-derived extracellular vesicles promote myelin regeneration in an animal model of multiple sclerosis. Cells 8, 1497. doi:10.3390/cells8121497
Cohen, O., Betzer, O., Elmaliach-Pnini, N., Motiei, M., Sadan, T., Cohen-Berkman, M., et al. (2021). “Golden” exosomes as delivery vehicles to target tumors and overcome intratumoral barriers: in vivo tracking in a model for head and neck cancer. Biomaterials Sci. 9, 2103–2114. doi:10.1039/d0bm01735c
Colombo, F., Norton, E. G., and Cocucci, E. (2021). Microscopy approaches to study extracellular vesicles. Biochimica Biophysica Acta. 1865, 129752. doi:10.1016/j.bbagen.2020.129752
Comfort, N., Cai, K., Bloomquist, T. R., Strait, M. D., Ferrante, A. W., and Baccarelli, A. A. (2021). Nanoparticle tracking analysis for the quantification and size determination of extracellular vesicles. J. Vis. Exp. JoVE 169. doi:10.3791/62447
Crose, J. J., Crose, A., Ransom, J. T., and Lightner, A. L. (2024). Bone marrow mesenchymal stem cell-derived extracellular vesicle infusion for amyotrophic lateral sclerosis. Neurodegener. Dis. Manag. 14, 111–117. doi:10.1080/17582024.2024.2344396
Cui, L., Li, D., Xu, J., Li, H., Pan, Y., Qiu, J., et al. (2024). Exosomal miRNA-21 derived from umbilical cord mesenchymal stem cells inhibits microglial overactivation to counteract nerve damage. Mol. Biol. Rep. 51, 941. doi:10.1007/s11033-024-09878-8
D'Acunzo, P., Kim, Y., Ungania, J. M., Pérez-González, R., Goulbourne, C. N., and Levy, E. (2022). Isolation of mitochondria-derived mitovesicles and subpopulations of microvesicles and exosomes from brain tissues. Nat. Protoc. 17, 2517–2549. doi:10.1038/s41596-022-00719-1
Dabrowska, S., Andrzejewska, A., Janowski, M., and Lukomska, B. (2021). Immunomodulatory and regenerative effects of mesenchymal stem cells and extracellular vesicles: therapeutic outlook for inflammatory and degenerative diseases. Front. Immunol. 11, 591065. doi:10.3389/fimmu.2020.591065
Datta, A., Sarmah, D., Mounica, L., Kaur, H., Kesharwani, R., Verma, G., et al. (2020). Cell death pathways in ischemic stroke and targeted pharmacotherapy. Transl. Stroke Res. 11, 1185–1202. doi:10.1007/s12975-020-00806-z
Doyle, L. M., and Wang, M. Z. (2019). Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 8, 727. doi:10.3390/cells8070727
Drobiova, H., Sindhu, S., Ahmad, R., Haddad, D., Al-Mulla, F., and Al Madhoun, A. (2023). Wharton’s jelly mesenchymal stem cells: a concise review of their secretome and prospective clinical applications. Front. Cell Dev. Biol. 11, 1211217. doi:10.3389/fcell.2023.1211217
Duan, L., Huang, H., Zhao, X., Zhou, M., Chen, S., Wang, C., et al. (2020). Extracellular vesicles derived from human placental mesenchymal stem cells alleviate experimental colitis in mice by inhibiting inflammation and oxidative stress. Int. J. Mol. Med. 46, 1551–1561. doi:10.3892/ijmm.2020.4679
Esmaeili, A., Alini, M., Baghaban Eslaminejad, M., and Hosseini, S. (2022). Engineering strategies for customizing extracellular vesicle uptake in a therapeutic context. Stem Cell Res. Ther. 13, 129. doi:10.1186/s13287-022-02806-2
Fallahi, S., Zangbar, H. S., Farajdokht, F., Rahbarghazi, R., Mohaddes, G., and Ghiasi, F. (2024). Exosomes as a therapeutic tool to promote neurorestoration and cognitive function in neurological conditions: achieve two ends with a single effort. CNS Neurosci. Ther. 30, e14752. doi:10.1111/cns.14752
Fan, J., Li, X., Yu, X., Liu, Z., Jiang, Y., Fang, Y., et al. (2023). Global burden, risk factor analysis, and prediction study of ischemic stroke, 1990-2030. Neurology 101, e137–e150. doi:10.1212/WNL.0000000000207387
Farahzadi, R., Fathi, E., Vandghanooni, S., and Valipour, B. (2024). Hydrogel encapsulation of mesenchymal stem cells-derived extracellular vesicles as a novel therapeutic approach in cancer therapy. Biochimica Biophysica Acta 1879, 189177. doi:10.1016/j.bbcan.2024.189177
Feigin, V. L., Vos, T., Nichols, E., Owolabi, M. O., Carroll, W. M., Dichgans, M., et al. (2020). The global burden of neurological disorders: translating evidence into policy. Lancet. Neurology 19, 255–265. doi:10.1016/S1474-4422(19)30411-9
Fröhlich, E. (2021). Therapeutic potential of mesenchymal stem cells and their products in lung diseases-intravenous administration versus inhalation. Pharmaceutics 13, 232. doi:10.3390/pharmaceutics13020232
Gan, J., Zeng, X., Wang, X., Wu, Y., Lei, P., Wang, Z., et al. (2022). Effective diagnosis of prostate cancer based on mrnas from urinary exosomes. Front. Med. 9, 736110. doi:10.3389/fmed.2022.736110
Gang, D., Yu, C. J., Zhu, S., Zhu, P., and Nasser, M. I. (2021). Application of mesenchymal stem cell-derived exosomes in kidney diseases. Cell. Immunol. 364, 104358. doi:10.1016/j.cellimm.2021.104358
Gholami, L., Nooshabadi, V. T., Shahabi, S., Jazayeri, M., Tarzemany, R., Afsartala, Z., et al. (2021). Extracellular vesicles in bone and periodontal regeneration: current and potential therapeutic applications. Cell Biosci. 11, 16. doi:10.1186/s13578-020-00527-8
Giuliani, P., De Simone, C., Febo, G., Bellasame, A., Tupone, N., Di Virglio, V., et al. (2024). Proteomics studies on extracellular vesicles derived from glioblastoma: where do we stand? Int. J. Mol. Sci. 25, 9778. doi:10.3390/ijms25189778
Gomez-Galvez, Y., Gupta, M., Kaur, M., Fusco, S., Podda, M. V., Grassi, C., et al. (2024). Recovery after human bone marrow mesenchymal stem cells (hBM-MSCs)-derived extracellular vesicles (EVs) treatment in post-MCAO rats requires repeated handling. PloS One 19, e0312298. doi:10.1371/journal.pone.0312298
Guo, M., Yin, Z., Chen, F., and Lei, P. (2020). Mesenchymal stem cell-derived exosome: a promising alternative in the therapy of Alzheimer's disease. Alzheimer’s Res. Ther. 12, 109. doi:10.1186/s13195-020-00670-x
Gupta, D., Zickler, A. M., and El Andaloussi, S. (2021). Dosing extracellular vesicles. Adv. Drug Deliv. Rev. 178, 113961. doi:10.1016/j.addr.2021.113961
Guy, R., and Offen, D. (2020). Promising opportunities for treating neurodegenerative diseases with mesenchymal stem cell-derived exosomes. Biomolecules 10, 1320. doi:10.3390/biom10091320
Hade, M. D., Suire, C. N., and Suo, Z. (2021). Mesenchymal stem cell-derived exosomes: applications in regenerative medicine. Cells 10, 1959. doi:10.3390/cells10081959
Hamidi, S. H., Etebar, N., Rahimzadegan, M., Zali, A., Roodsari, S. R., and Niknazar, S. (2024). Mesenchymal stem cells and their derived exosomes in multiple sclerosis disease: from paper to practice. Mol. Cell. Biochem. 479, 1643–1671. doi:10.1007/s11010-024-05051-8
Han, Z., Liu, S., Pei, Y., Ding, Z., Li, Y., Wang, X., et al. (2021). Highly efficient magnetic labelling allows MRI tracking of the homing of stem cell-derived extracellular vesicles following systemic delivery. J. Extracell. Vesicles 10, e12054. doi:10.1002/jev2.12054
Han, P., Jiao, K., Moran, C. S., Liaw, A., Zhou, Y., Salomon, C., et al. (2023). TNF-α and OSX mRNA of salivary small extracellular vesicles in periodontitis: a pilot study. Tissue Eng. Part C. Methods 29, 298–306. doi:10.1089/ten.TEC.2023.0051
Haney, M. J., Zhao, Y., Jin, Y. S., and Batrakova, E. V. (2020). Extracellular vesicles as drug carriers for enzyme replacement therapy to treat CLN2 batten disease: optimization of drug administration routes. Cells 9, 1273. doi:10.3390/cells9051273
Haney, M. J., Yuan, H., Shipley, S. T., Wu, Z., Zhao, Y., Pate, K., et al. (2022). Biodistribution of biomimetic drug carriers, mononuclear cells, and extracellular vesicles, in nonhuman primates. Adv. Biol. 6, e2101293. doi:10.1002/adbi.202101293
Hassanpour Tamrin, S., Sanati Nezhad, A., and Sen, A. (2021). Label-Free isolation of exosomes using microfluidic technologies. ACS Nano 15, 17047–17079. doi:10.1021/acsnano.1c03469
Hassanzadeh, A., Rahman, H. S., Markov, A., Endjun, J. J., Zekiy, A. O., Chartrand, M. S., et al. (2021). Mesenchymal stem/stromal cell-derived exosomes in regenerative medicine and cancer; overview of development, challenges, and opportunities. Stem Cell Res. Ther. 12, 297. doi:10.1186/s13287-021-02378-7
He, F., Liu, H., Guo, X., Yin, B. C., and Ye, B. C. (2017). Direct exosome quantification via bivalent-cholesterol-labeled dna anchor for signal amplification. Anal. Chem. 89, 12968–12975. doi:10.1021/acs.analchem.7b03919
Hong, R., Luo, L., Wang, L., Hu, Z. L., Yin, Q. R., Li, M., et al. (2023). Lepidium meyenii Walp (Maca)-derived extracellular vesicles ameliorate depression by promoting 5-HT synthesis via the modulation of gut-brain axis. iMeta 2, e116. doi:10.1002/imt2.116
Hu, L., Zhang, T., Ma, H., Pan, Y., Wang, S., Liu, X., et al. (2022). Discovering the secret of diseases by incorporated tear exosomes analysis via rapid-isolation system: iTEARS. ACS Nano 16, 11720–11732. doi:10.1021/acsnano.2c02531
Hu, L., Liu, X., Zheng, Q., Chen, W., Xu, H., Li, H., et al. (2023). Interaction network of extracellular vesicles building universal analysis via eye tears: INEBULA. Sci. Adv. 9, eadg1137. doi:10.1126/sciadv.adg1137
Hu, W. J., Wei, H., Cai, L. L., Xu, Y. H., Du, R., Zhou, Q., et al. (2024). Magnetic targeting enhances the neuroprotective function of human mesenchymal stem cell-derived iron oxide exosomes by delivering miR-1228-5p. J. Nanobiotechnology 22, 665. doi:10.1186/s12951-024-02941-3
Huang, P., Yan, H., Shang, J., and Xie, X. (2024a). Prior information guided deep-learning model for tumor bed segmentation in breast cancer radiotherapy. BMC Med. Imaging 24, 312. doi:10.1186/s12880-024-01469-0
Huang, W., Zhang, T., Li, X., Gong, L., Zhang, Y., Luan, C., et al. (2024b). Intranasal administration of umbilical cord mesenchymal stem cell exosomes alleviates Parkinson's disease. Neuroscience 549, 1–12. doi:10.1016/j.neuroscience.2024.04.010
Hussain, S., Mubeen, I., Ullah, N., Shah, S. S. U. D., Khan, B. A., Zahoor, M., et al. (2022). Modern diagnostic imaging technique applications and risk factors in the medical field: a review. BioMed Res. Int. 2022, 5164970. doi:10.1155/2022/5164970
Islam, M. S., Gopalan, V., Lam, A. K., and Shiddiky, M. J. A. (2023). Current advances in detecting genetic and epigenetic biomarkers of colorectal cancer. Biosens. Bioelectron. 239, 115611. doi:10.1016/j.bios.2023.115611
Jahanbani, Y., Beiranvand, T., Mamaghani, P. Y., Aghebati-Maleki, L., and Yousefi, M. (2023). Exosome-based technologies as a platform for diagnosis and treatment of male and female infertility-related diseases. J. Reproductive Immunol. 156, 103833. doi:10.1016/j.jri.2023.103833
Ji, L., Bao, L., Gu, Z., Zhou, Q., Liang, Y., Zheng, Y., et al. (2019). Comparison of immunomodulatory properties of exosomes derived from bone marrow mesenchymal stem cells and dental pulp stem cells. Immunol. Res. 67, 432–442. doi:10.1007/s12026-019-09088-6
Ji, S., Peng, Y., Liu, J., Xu, P., and Tang, S. (2024). Human adipose tissue-derived stem cell extracellular vesicles attenuate ocular hypertension-induced retinal ganglion cell damage by inhibiting microglia- TLR4/MAPK/NF-κB proinflammatory cascade signaling. Acta Neuropathol. Commun. 12, 44. doi:10.1186/s40478-024-01753-8
Jia, Y., Yu, L., Ma, T., Xu, W., Qian, H., Sun, Y., et al. (2022). Small extracellular vesicles isolation and separation: current techniques, pending questions and clinical applications. Theranostics 12, 6548–6575. doi:10.7150/thno.74305
Jiang, S., Lu, F., Chen, J., Jiao, Y., Qiu, Q., Nian, X., et al. (2024a). UPCARE: urinary extracellular vesicles-derived prostate cancer assessment for risk evaluation. J. Extracell. Vesicles 13, e12491. doi:10.1002/jev2.12491
Jiang, W., Zhang, T., Qiu, Y., Liu, Q., Chen, X., Wang, Q., et al. (2024b). Keratinocyte-to-macrophage communication exacerbate psoriasiform dermatitis via LRG1-enriched extracellular vesicles. Theranostics 14, 1049–1064. doi:10.7150/thno.89180
Johnson, J., Shojaee, M., Mitchell Crow, J., and Khanabdali, R. (2021). From mesenchymal stromal cells to engineered extracellular vesicles: a new therapeutic paradigm. Front. Cell Dev. Biol. 9, 705676. doi:10.3389/fcell.2021.705676
Johnstone, R. M., Adam, M., Hammond, J. R., Orr, L., and Turbide, C. (1987). Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 262, 9412–9420. doi:10.1016/s0021-9258(18)48095-7
Ju, Y., Hu, Y., Yang, P., Xie, X., and Fang, B. (2022). Extracellular vesicle-loaded hydrogels for tissue repair and regeneration. Mater. Today. Bio. 18, 100522. doi:10.1016/j.mtbio.2022.100522
Karachaliou, C. E., Koukouvinos, G., Goustouridis, D., Raptis, I., Kakabakos, S., Petrou, P., et al. (2023). Cortisol immunosensors: a literature review. Biosensors 13, 285. doi:10.3390/bios13020285
Karnas, E., Dudek, P., and Zuba-Surma, E. K. (2023). Stem cell-derived extracellular vesicles as new tools in regenerative medicine - immunomodulatory role and future perspectives. Front. Immunol. 14, 1120175. doi:10.3389/fimmu.2023.1120175
Khanabdali, R., Mandrekar, M., Grygiel, R., Vo, P. A., Palma, C., Nikseresht, S., et al. (2024). High-throughput surface epitope immunoaffinity isolation of extracellular vesicles and downstream analysis. Biol. Methods Protoc. 9, bpae032. doi:10.1093/biomethods/bpae032
Khoei, S. G., Dermani, F. K., Malih, S., Fayazi, N., and Sheykhhasan, M. (2020). The use of mesenchymal stem cells and their derived extracellular vesicles in cardiovascular disease treatment. Curr. Stem Cell Res. Ther. 15, 623–638. doi:10.2174/1574888X15666200501235201
Kimiz-Gebologlu, I., and Oncel, S. S. (2022). Exosomes: large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J. Control. Release 347, 533–543. doi:10.1016/j.jconrel.2022.05.027
Klaihmon, P., Pattanapanyasat, K., and Phannasil, P. (2023). An update on recent studies of extracellular vesicles and their role in hypercoagulability in thalassemia (Review). Biomed. Rep. 20, 31. doi:10.3892/br.2023.1719
Kowal, J., Tkach, M., and Théry, C. (2014). Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 29, 116–125. doi:10.1016/j.ceb.2014.05.004
Kråkenes, T., Sandvik, C. E., Ytterdal, M., Gavasso, S., Evjenth, E. C., Bø, L., et al. (2024). The therapeutic potential of exosomes from mesenchymal stem cells in multiple sclerosis. Int. J. Mol. Sci. 25, 10292. doi:10.3390/ijms251910292
Kuiper, M., van de Nes, A., Nieuwland, R., Varga, Z., and van der Pol, E. (2021). Reliable measurements of extracellular vesicles by clinical flow cytometry. Am. J. Reproductive Immunol. 85, e13350. doi:10.1111/aji.13350
Lai, J. J., Chau, Z. L., Chen, S. Y., Hill, J. J., Korpany, K. V., Liang, N. W., et al. (2022). Exosome processing and characterization approaches for research and technology development. Adv. Sci. Weinheim, Baden-Wurttemberg, Ger. 9, e2103222. doi:10.1002/advs.202103222
Lai, J. J., Hill, J. J., Huang, C. Y., Lee, G. C., Mai, K. W., Shen, M. Y., et al. (2024). Unveiling the complex world of extracellular vesicles: novel characterization techniques and manufacturing considerations. Chonnam Med. J. 60, 1–12. doi:10.4068/cmj.2024.60.1.1
Lan, Z., Chen, R., Zou, D., and Zhao, C. X. (2025). Microfluidic nanoparticle separation for precision medicine. Adv. Sci. Weinheim, Baden-Wurttemberg, Ger. 12, e2411278. doi:10.1002/advs.202411278
Lane, R. E., Korbie, D., Trau, M., and Hill, M. M. (2017). Purification protocols for extracellular vesicles. Methods Mol. Biol. Clift. N.J. 1660, 111–130. doi:10.1007/978-1-4939-7253-1_10
Langevin, S. M., Kuhnell, D., Orr-Asman, M. A., Biesiada, J., Zhang, X., Medvedovic, M., et al. (2019). Balancing yield, purity and practicality: a modified differential ultracentrifugation protocol for efficient isolation of small extracellular vesicles from human serum. RNA Biol. 16, 5–12. doi:10.1080/15476286.2018.1564465
Lázaro-Ibáñez, E., Faruqu, F. N., Saleh, A. F., Silva, A. M., Tzu-Wen Wang, J., Rak, J., et al. (2021). Selection of fluorescent, bioluminescent, and radioactive tracers to accurately reflect extracellular vesicle biodistribution in vivo. ACS Nano 15, 3212–3227. doi:10.1021/acsnano.0c09873
Li, K., Yan, G., Huang, H., Zheng, M., Ma, K., Cui, X., et al. (2022). Anti-inflammatory and immunomodulatory effects of the extracellular vesicles derived from human umbilical cord mesenchymal stem cells on osteoarthritis via M2 macrophages. J. Nanobiotechnology 20, 38. doi:10.1186/s12951-021-01236-1
Li, J., Huang, Y., Sun, H., and Yang, L. (2023a). Mechanism of mesenchymal stem cells and exosomes in the treatment of age-related diseases. Front. Immunol. 14, 1181308. doi:10.3389/fimmu.2023.1181308
Li, S., Wang, K., Wang, Z., Zhang, W., Liu, Z., Cheng, Y., et al. (2023b). Application and trend of bioluminescence imaging in metabolic syndrome research. Front. Chem. 10, 1113546. doi:10.3389/fchem.2022.1113546
Li, D., Zou, S., Huang, Z., Sun, C., and Liu, G. (2024a). Isolation and quantification of L1CAM-positive extracellular vesicles on a chip as a potential biomarker for Parkinson's Disease. J. Extracell. Vesicles 13, e12467. doi:10.1002/jev2.12467
Li, P., Zhang, F., Huang, C., Zhang, C., Yang, Z., Zhang, Y., et al. (2024b). Exosomes derived from dpa-treated ucmscs attenuated depression-like behaviors and neuroinflammation in a model of depression induced by chronic stress. J. Neuroimmune Pharmacol. 19 (1), 55. doi:10.1007/s11481-024-10154-6
Li, S., Zhang, J., Liu, X., Wang, N., Sun, L., Liu, J., et al. (2024c). Proteomic characterization of hUC-MSC extracellular vesicles and evaluation of its therapeutic potential to treat Alzheimer's disease. Sci. Rep. 14, 5959. doi:10.1038/s41598-024-56549-6
Li, Y., Zhu, Z., Li, S., Xie, X., Qin, L., Zhang, Q., et al. (2024d). Exosomes: compositions, biogenesis, and mechanisms in diabetic wound healing. J. Nanobiotechnology 22, 398. doi:10.1186/s12951-024-02684-1
Li, K., Lin, S., Zhou, P., Guo, Y., Lin, S., and Ji, C. (2025). The role of exosomal lncRNAs in mediating apoptosis and inflammation in UV-induced skin photoaging. Front. Cell Dev. Biol. 13, 1538197. doi:10.3389/fcell.2025.1538197
Liang, Y., Duan, L., Xu, X., Li, X., Liu, M., Chen, H., et al. (2020). Mesenchymal stem cell-derived exosomes for treatment of autism spectrum disorder. ACS Appl. Bio Mater. 3, 6384–6393. doi:10.1021/acsabm.0c00831
Liang, X., Miao, Y., Tong, X., Chen, J., Liu, H., He, Z., et al. (2024). Dental pulp mesenchymal stem cell-derived exosomes inhibit neuroinflammation and microglial pyroptosis in subarachnoid hemorrhage via the miRNA-197-3p/FOXO3 axis. J. Nanobiotechnology 22, 426. doi:10.1186/s12951-024-02708-w
Liangsupree, T., Multia, E., and Riekkola, M. L. (2021). Modern isolation and separation techniques for extracellular vesicles. J. Chromatogr. A 1636, 461773. doi:10.1016/j.chroma.2020.461773
Lima Moura, S., Martì, M., and Pividori, M. I. (2020). Matrix effect in the isolation of breast cancer-derived nanovesicles by immunomagnetic separation and electrochemical immunosensing-a comparative study. Sensors Basel, Switz. 20, 965. doi:10.3390/s20040965
Liu, X., Yang, Y., Li, Y., Niu, X., Zhao, B., Wang, Y., et al. (2017). Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale 9, 4430–4438. doi:10.1039/c7nr00352h
Liu, Q., Huang, J., Xia, J., Liang, Y., and Li, G. (2022). Tracking tools of extracellular vesicles for biomedical research. Front. Bioeng. Biotechnol. 10, 943712. doi:10.3389/fbioe.2022.943712
Liu, C., Lin, H., Yu, H., Mai, X., Pan, W., Guo, J., et al. (2024a). Isolation and enrichment of extracellular vesicles with double-positive membrane protein for subsequent biological studies. Adv. Healthc. Mater. 13, e2303430. doi:10.1002/adhm.202303430
Liu, Y., Sun, L., Li, Y., and Holmes, C. (2024b). Mesenchymal stromal/stem cell tissue source and in vitro expansion impact extracellular vesicle protein and miRNA compositions as well as angiogenic and immunomodulatory capacities. J. Extracell. Vesicles 13, e12472. doi:10.1002/jev2.12472
Lotfy, A., AboQuella, N. M., and Wang, H. (2023). Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res. Ther. 14, 66. doi:10.1186/s13287-023-03287-7
Ma, X., Peng, L., Zhu, X., Chu, T., Yang, C., Zhou, B., et al. (2025). Isolation, identification, and challenges of extracellular vesicles: emerging players in clinical applications. Apoptosis Int. J. Program. Cell Death 30, 422–445. doi:10.1007/s10495-024-02036-2
Mao, Q., Nguyen, P. D., Shanti, R. M., Shi, S., Shakoori, P., Zhang, Q., et al. (2019). Gingiva-Derived mesenchymal stem cell-extracellular vesicles activate schwann cell repair phenotype and promote nerve regeneration. Tissue Eng. Part A 25, 887–900. doi:10.1089/ten.TEA.2018.0176
Martinez-Zalbidea, I., Wagner, G., Bergendahl, N., Mesfin, A., Puvanesarajah, V., Hitzl, W., et al. (2025). CRISPR-dCas9 activation of tsg-6 in mscs modulates the cargo of msc-derived extracellular vesicles and attenuates inflammatory responses in human intervertebral disc cells in vitro. Cell. Mol. Bioeng. 18, 83–98. doi:10.1007/s12195-025-00843-4
Miao, Y., Liang, X., Chen, J., Liu, H., He, Z., Qin, Y., et al. (2024). Transfer of miR-877-3p via extracellular vesicles derived from dental pulp stem cells attenuates neuronal apoptosis and facilitates early neurological functional recovery after cerebral ischemia-reperfusion injury through the Bclaf1/P53 signaling pathway. Pharmacol. Res. 206, 107266. doi:10.1016/j.phrs.2024.107266
Miron, R. J., Estrin, N. E., Sculean, A., and Zhang, Y. (2024). Understanding exosomes: Part 2-Emerging leaders in regenerative medicine. Periodontology 94, 257–414. doi:10.1111/prd.12561
Mohammadzadeh, A., Lahouty, M., Charkhian, H., Ghafour, A. A., Moazzendizaji, S., Rezaei, J., et al. (2024). Human umbilical cord mesenchymal stem cell-derived exosomes alleviate the severity of experimental autoimmune encephalomyelitis and enhance lag-3 expression on foxp3 + CD4 + T cells. Mol. Biol. Rep. 51, 522. doi:10.1007/s11033-024-09433-5
Mushahary, D., Spittler, A., Kasper, C., Weber, V., and Charwat, V. (2018). Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytom. Part A 93, 19–31. doi:10.1002/cyto.a.23242
Muskan, M., Abeysinghe, P., Cecchin, R., Branscome, H., Morris, K. V., and Kashanchi, F. (2024). Therapeutic potential of RNA-enriched extracellular vesicles: the next generation in RNA delivery via biogenic nanoparticles. Mol. Ther. J. Am. Soc. Gene Ther. 32, 2939–2949. doi:10.1016/j.ymthe.2024.02.025
Pantazopoulou, M., Lamprokostopoulou, A., Karampela, D. S., Alexaki, A., Delis, A., Coens, A., et al. (2023). Differential intracellular trafficking of extracellular vesicles in microglia and astrocytes. Cell. Mol. Life Sci. CMLS 80, 193. doi:10.1007/s00018-023-04841-5
Patel, G. K., Khan, M. A., Zubair, H., Srivastava, S. K., Khushman, M., Singh, S., et al. (2019). Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci. Rep. 9, 5335. doi:10.1038/s41598-019-41800-2
Patel, A. A., Mohamed, A. H., Rizaev, J., Mallick, A. K., Qasim, M. T., Abdulmonem, W. A., et al. (2024). Application of mesenchymal stem cells derived from the umbilical cord or Wharton's jelly and their extracellular vesicles in the treatment of various diseases. Tissue Cell 89, 102415. doi:10.1016/j.tice.2024.102415
Pei, J., Palanisamy, C. P., Jayaraman, S., Natarajan, P. M., Umapathy, V. R., Roy, J. R., et al. (2024). Proteomics profiling of extracellular vesicle for identification of potential biomarkers in Alzheimer's disease: a comprehensive review. Ageing Res. Rev. 99, 102359. doi:10.1016/j.arr.2024.102359
Peng, N., Gao, X., Yong, Z., Zhang, Y., Guo, X., Wang, Q., et al. (2025). “Sample-in, result-out” liquid biopsy chip based on immunomagnetic separation and CRISPR detection for multiplex analysis of exosomal microRNAs. Biosens. Bioelectron. 280, 117460. doi:10.1016/j.bios.2025.117460
Pirolli, N. H., Reus, L. S. C., Mamczarz, Z., Khan, S., Bentley, W. E., and Jay, S. M. (2023). High performance anion exchange chromatography purification of probiotic bacterial extracellular vesicles enhances purity and anti-inflammatory efficacy. Biotechnol. Bioeng. 120, 3368–3380. doi:10.1002/bit.28522
Pocsfalvi, G., Stanly, C., Vilasi, A., Fiume, I., Capasso, G., Turiák, L., et al. (2016). Mass spectrometry of extracellular vesicles. Mass Spectrom. Rev. 35, 3–21. doi:10.1002/mas.21457
Rashidi, M., Bijari, S., Khazaei, A. H., Shojaei-Ghahrizjani, F., and Rezakhani, L. (2022). The role of milk-derived exosomes in the treatment of diseases. Front. Genet. 13, 1009338. doi:10.3389/fgene.2022.1009338
Rather, H. A., Almousa, S., Craft, S., and Deep, G. (2023). Therapeutic efficacy and promise of stem cell-derived extracellular vesicles in Alzheimer's disease and other aging-related disorders. Ageing Res. Rev. 92, 102088. doi:10.1016/j.arr.2023.102088
Rayamajhi, S., Marasini, R., Nguyen, T. D. T., Plattner, B. L., Biller, D., and Aryal, S. (2020). Strategic reconstruction of macrophage-derived extracellular vesicles as a magnetic resonance imaging contrast agent. Biomaterials Sci. 8, 2887–2904. doi:10.1039/d0bm00128g
Reseco, L., Molina-Crespo, A., Atienza, M., Gonzalez, E., Falcon-Perez, J. M., and Cantero, J. L. (2024). Characterization of extracellular vesicles from human saliva: effects of age and isolation techniques. Cells 13, 95. doi:10.3390/cells13010095
Rhim, W. K., Kim, J. Y., Lee, S. Y., Cha, S. G., Park, J. M., Park, H. J., et al. (2023). Recent advances in extracellular vesicle engineering and its applications to regenerative medicine. Biomaterials Res. 27, 130. doi:10.1186/s40824-023-00468-6
Saari, H., Pusa, R., Marttila, H., Yliperttula, M., and Laitinen, S. (2023). Development of tandem cation exchange chromatography for high purity extracellular vesicle isolation: the effect of ligand steric availability. J. Chromatogr. A 1707, 464293. doi:10.1016/j.chroma.2023.464293
Sakurai, Y., Ohtani, A., Nakayama, Y., Gomi, M., Masuda, T., Ohtsuki, S., et al. (2023). Logistics and distribution of small extracellular vesicles from the subcutaneous space to the lymphatic system. J. Control. Release 361, 77–86. doi:10.1016/j.jconrel.2023.07.043
Salehpour, A., Karimi, Z., Ghasemi Zadeh, M., Afshar, M., Kameli, A., Mooseli, F., et al. (2024). Therapeutic potential of mesenchymal stem cell-derived exosomes and miRNAs in neuronal regeneration and rejuvenation in neurological disorders: a mini review. Front. Cell. Neurosci. 18, 1427525. doi:10.3389/fncel.2024.1427525
Sandoval, A. G. W., and Badiavas, E. V. (2025). Towards extracellular vesicles in the treatment of epidermolysis bullosa. Bioeng. Basel, Switz. 12, 574. doi:10.3390/bioengineering12060574
Santilli, F., Fabrizi, J., Santacroce, C., Caissutti, D., Spinello, Z., Candelise, N., et al. (2024). Analogies and differences between dental stem cells: focus on secretome in combination with scaffolds in neurological disorders. Stem Cell Rev. Rep. 20, 159–174. doi:10.1007/s12015-023-10652-9
Seo, N., Nakamura, J., Kaneda, T., Tateno, H., Shimoda, A., Ichiki, T., et al. (2022). Distinguishing functional exosomes and other extracellular vesicles as a nucleic acid cargo by the anion-exchange method. J. Extracell. Vesicles 11, e12205. doi:10.1002/jev2.12205
Shao, M., Ye, S., Chen, Y., Yu, C., and Zhu, W. (2024). Exosomes from hypoxic ADSCs ameliorate neuronal damage post spinal cord injury through circ-Wdfy3 delivery and inhibition of ferroptosis. Neurochem. Int. 177, 105759. doi:10.1016/j.neuint.2024.105759
Shi, L., and Esfandiari, L. (2022). A label-free and low-power microelectronic impedance spectroscopy for characterization of exosomes. PLoS One 17, e0270844. doi:10.1371/journal.pone.0270844
Shipman, W. D., Fonseca, R., Dominguez, M., Bhayani, S., Gilligan, C., Diwan, S., et al. (2024). An update on emerging regenerative medicine applications: the use of extracellular vesicles and exosomes for the management of chronic pain. Curr. Pain Headache Rep. 28, 1289–1297. doi:10.1007/s11916-024-01309-4
Sidhom, K., Obi, P. O., and Saleem, A. (2020). A review of exosomal isolation methods: is size exclusion chromatography the best option? Int. J. Mol. Sci. 21, 6466. doi:10.3390/ijms21186466
Soleimani, A., Oraee Yazdani, S., Pedram, M., Saadinam, F., Rasaee, M. J., and Soleimani, M. (2024). Intrathecal injection of human placental mesenchymal stem cells derived exosomes significantly improves functional recovery in spinal cord injured rats. Mol. Biol. Rep. 51, 193. doi:10.1007/s11033-023-08972-7
Staubach, S., Bauer, F. N., Tertel, T., Börger, V., Stambouli, O., Salzig, D., et al. (2021). Scaled preparation of extracellular vesicles from conditioned media. Adv. Drug Deliv. Rev. 177, 113940. doi:10.1016/j.addr.2021.113940
Ströhle, G., Gan, J., and Li, H. (2022). Affinity-based isolation of extracellular vesicles and the effects on downstream molecular analysis. Anal. Bioanal. Chem. 414, 7051–7067. doi:10.1007/s00216-022-04178-1
Su, X., Wang, H., Li, Q., and Chen, Z. (2025). Extracellular vesicles: a review of their therapeutic potentials, sources, biodistribution, and administration routes. Int. J. Nanomedicine 20, 3175–3199. doi:10.2147/IJN.S502591
Sun, H., Lu, S., Qu, G., Li, J., and Song, B. (2023). Mesenchymal stem cells-derived exosomes ameliorate high glucose and lipopolysaccharide-induced HPMECs injury through the Nrf2/HO-1 pathway. Autoimmunity 56, 2290357. doi:10.1080/08916934.2023.2290357
Sun, C., Sha, S., Shan, Y., Gao, X., Li, L., Xing, C., et al. (2025). Intranasal delivery of BACE1 siRNA and berberine via engineered stem cell exosomes for the treatment of Alzheimer's Disease. Int. J. Nanomedicine 20, 5873–5891. doi:10.2147/IJN.S506793
Suwatthanarak, T., Tanaka, M., Miyamoto, Y., Miyado, K., and Okochi, M. (2021). Inhibition of cancer-cell migration by tetraspanin CD9-binding peptide. Chem. Commun. Camb. Engl. 57, 4906–4909. doi:10.1039/d1cc01295a
Szatanek, R., Baran, J., Siedlar, M., and Baj-Krzyworzeka, M. (2015). Isolation of extracellular vesicles: determining the correct approach (Review). Int. J. Mol. Med. 36, 11–17. doi:10.3892/ijmm.2015.2194
Takov, K., Yellon, D. M., and Davidson, S. M. (2018). Comparison of small extracellular vesicles isolated from plasma by ultracentrifugation or size-exclusion chromatography: yield, purity and functional potential. J. Extracell. Vesicles 8, 1560809. doi:10.1080/20013078.2018.1560809
Tan, S. H. S., Wong, J. R. Y., Sim, S. J. Y., Tjio, C. K. E., Wong, K. L., Chew, J. R. J., et al. (2020). Mesenchymal stem cell exosomes in bone regenerative strategies-a systematic review of preclinical studies. Mater. Today. Bio. 7, 100067. doi:10.1016/j.mtbio.2020.100067
Tang, J., Cui, X., Zhang, Z., Xu, Y., Guo, J., Soliman, B. G., et al. (2022). Injection-free delivery of msc-derived extracellular vesicles for myocardial infarction therapeutics. Adv. Healthc. Mater. 11, e2100312. doi:10.1002/adhm.202100312
Tawanwongsri, W., and Vachiramon, V. (2024). Skin necrosis after intradermal injection of lyophilized exosome: a case report and a review of the literature. J. Cosmet. Dermatology 23, 1597–1603. doi:10.1111/jocd.16206
Tham, Y. C., Li, X., Wong, T. Y., Quigley, H. A., Aung, T., and Cheng, C. Y. (2014). Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 121, 2081–2090. doi:10.1016/j.ophtha.2014.05.013
Theel, E. K., and Schwaminger, S. P. (2022). Microfluidic approaches for affinity-based exosome separation. Int. J. Mol. Sci. 23, 9004. doi:10.3390/ijms23169004
Théry, C., Witwer, K. W., Aikawa, E., Alcaraz, M. J., Anderson, J. D., Andriantsitohaina, R., et al. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7, 1535750. doi:10.1080/20013078.2018.1535750
Tiszbein, K., Koss-Mikołajczyk, I., and Martysiak-Żurowska, D. (2025). Unlocking the secrets of human milk: isolation and characterization of extracellular vesicles. Adv. Nutr. (Bethesda, Md.) 16, 100430. doi:10.1016/j.advnut.2025.100430
Torres, A., Michea, M. A., Végvári, Á., Arce, M., Pérez, V., Alcota, M., et al. (2024). A multi-platform analysis of human gingival crevicular fluid reveals ferroptosis as a relevant regulated cell death mechanism during the clinical progression of periodontitis. Int. J. Oral Sci. 16, 43. doi:10.1038/s41368-024-00306-y
Turano, E., Virla, F., Scambi, I., Dabrowska, S., Bankole, O., and Mariotti, R. (2024). Adipose mesenchymal stem cells-derived extracellular vesicles exert their preferential action in damaged central sites of SOD1 mice rather than peripherally. Eur. J. Histochem. EJH. 68, 4040. doi:10.4081/ejh.2024.4040
Villa, A., De Mitri, Z., Vincenti, S., Crippa, E., Castiglioni, L., Gelosa, P., et al. (2024). Canine glioblastoma-derived extracellular vesicles as precise carriers for glioblastoma imaging: targeting across the blood-brain barrier. Biomed. Pharmacother. Biomedecine Pharmacother. 172, 116201. doi:10.1016/j.biopha.2024.116201
Virla, F., Turano, E., Scambi, I., Schiaffino, L., Boido, M., and Mariotti, R. (2024). Correction: administration of adipose-derived stem cells extracellular vesicles in a murine model of spinal muscular atrophy: effects of a new potential therapeutic strategy. Stem Cell Res. Ther. 15, 126. doi:10.1186/s13287-024-03744-x
Vu, H. T., Han, M. R., Lee, J. H., Kim, J. S., Shin, J. S., Yoon, J. Y., et al. (2022). Investigating the effects of conditioned media from stem cells of human exfoliated deciduous teeth on dental pulp stem cells. Biomedicines 10, 906. doi:10.3390/biomedicines10040906
Wang, H., Bian, K., Guo, L., Dai, Y., and Yu, Y. (2023a). Progress on exosomes in the diagnosis and treatment of disease and drug delivery system. J. Pharm. Pract. Serv. 41, 265–272. doi:10.12206/j.issn.2097-2024.202207022
Wang, Y., Zhang, Y., Li, Z., Wei, S., Chi, X., Yan, X., et al. (2023b). Combination of size-exclusion chromatography and ion exchange adsorption for improving the proteomic analysis of plasma-derived extracellular vesicles. Proteomics 23, e2200364. doi:10.1002/pmic.202200364
Wang, C. C., Hu, X. M., Long, Y. F., Huang, H. R., He, Y., Xu, Z. R., et al. (2024a). Treatment of Parkinson's disease model with human umbilical cord mesenchymal stem cell-derived exosomes loaded with BDNF. Life Sci. 356, 123014. doi:10.1016/j.lfs.2024.123014
Wang, S., Li, X., Wang, T., Sun, Z., Feng, E., and Jin, Y. (2024b). Overexpression of USP35 enhances the protective effect of hUC-MSCs and their extracellular vesicles in oxygen-glucose deprivation/reperfusion-induced SH-SY5Y cells via stabilizing FUNDC1. Commun. Biol. 7, 1330. doi:10.1038/s42003-024-07024-5
Wang, W., Sun, H., Duan, H., Sheng, G., Tian, N., Liu, D., et al. (2024c). Isolation and usage of exosomes in central nervous system diseases. CNS Neurosci. Ther. 30, e14677. doi:10.1111/cns.14677
Wang, Z., Zhou, X., Kong, Q., He, H., Sun, J., Qiu, W., et al. (2024d). Extracellular vesicle preparation and analysis: a state-of-the-art review. Adv. Sci. Weinheim, Baden-Wurttemberg, Ger. 11, e2401069. doi:10.1002/advs.202401069
Wang, Y., Wang, H., Tan, J., Cao, Z., Wang, Q., Wang, H., et al. (2025). Therapeutic effect of mesenchymal stem cells and their derived exosomes in diseases. Mol. Biomed. 6, 34. doi:10.1186/s43556-025-00277-4
Welsh, J. A., Arkesteijn, G. J. A., Bremer, M., Cimorelli, M., Dignat-George, F., Giebel, B., et al. (2023). A compendium of single extracellular vesicle flow cytometry. J. Extracell. Vesicles 12, e12299. doi:10.1002/jev2.12299
Welsh, J. A., Goberdhan, D. C. I., O'Driscoll, L., Buzas, E. I., Blenkiron, C., Bussolati, B., et al. (2024). Minimal information for studies of extracellular vesicles (MISEV2023): from basic to advanced approaches. J. Extracell. Vesicles 13, e12404. doi:10.1002/jev2.12404
Weng, Z., Zhang, B., Wu, C., Yu, F., Han, B., Li, B., et al. (2021). Therapeutic roles of mesenchymal stem cell-derived extracellular vesicles in cancer. J. Hematol. Oncol. 14, 136. doi:10.1186/s13045-021-01141-y
Woo, H. K., Sunkara, V., Park, J., Kim, T. H., Han, J. R., Kim, C. J., et al. (2017). Exodisc for rapid, size-selective, and efficient isolation and analysis of nanoscale extracellular vesicles from biological samples. ACS Nano 11, 1360–1370. doi:10.1021/acsnano.6b06131
Wu, R., Fan, X., Wang, Y., Shen, M., Zheng, Y., Zhao, S., et al. (2022). Mesenchymal stem cell-derived extracellular vesicles in liver immunity and therapy. Front. Immunol. 13, 833878. doi:10.3389/fimmu.2022.833878
Wu, J., Fang, H., Zhang, J., and Yan, S. (2023). Modular microfluidics for life sciences. J. Nanobiotechnology 21, 85. doi:10.1186/s12951-023-01846-x
Xia, Y., Zhang, J., Liu, G., and Wolfram, J. (2024). Immunogenicity of extracellular vesicles. Adv. Mater. Deerf. Beach, Fla. 36, e2403199. doi:10.1002/adma.202403199
Xie, F., Huang, Y., Zhan, Y., and Bao, L. (2023). Exosomes as drug delivery system in gastrointestinal cancer. Front. Oncol. 12, 1101823. doi:10.3389/fonc.2022.1101823
Xu, R., Greening, D. W., Rai, A., Ji, H., and Simpson, R. J. (2015). Highly-purified exosomes and shed microvesicles isolated from the human colon cancer cell line LIM1863 by sequential centrifugal ultrafiltration are biochemically and functionally distinct. Methods (San Diego, Calif.) 87, 11–25. doi:10.1016/j.ymeth.2015.04.008
Xu, K., Jin, Y., Li, Y., Huang, Y., and Zhao, R. (2022). Recent progress of exosome isolation and peptide recognition-guided strategies for exosome research. Front. Chem. 10, 844124. doi:10.3389/fchem.2022.844124
Xu, J., Zhang, J., Liu, Q., and Wang, B. (2024). Bone marrow mesenchymal stem cells-derived exosomes promote spinal cord injury repair through the miR-497-5p/TXNIP/NLRP3 axis. J. Mol. Histology. 56, 16. doi:10.1007/s10735-024-10289-z
Yang, D., Zhang, W., Zhang, H., Zhang, F., Chen, L., Ma, L., et al. (2020). Progress, opportunity, and perspective on exosome isolation-efforts for efficient exosome-based theranostics. Theranostics 10, 3684–3707. doi:10.7150/thno.41580
Yang, P., Qin, H., Li, Y., Xiao, A., Zheng, E., Zeng, H., et al. (2022). CD36-mediated metabolic crosstalk between tumor cells and macrophages affects liver metastasis. Nat. Commun. 13, 5782. doi:10.1038/s41467-022-33349-y
Yang, J., Xie, Y., Xia, Z., Ji, S., Yang, X., Yue, D., et al. (2024a). HucMSC-Exo induced n2 polarization of neutrophils: implications for angiogenesis and tissue restoration in wound healing. Int. J. Nanomedicine 19, 3555–3575. doi:10.2147/IJN.S458295
Yang, R., Guo, Y., and Yin, H. (2024b). From apoptosis to pyroptosis: a two-decade analysis of spinal cord injury systematic review. Medicine 103 (40), e39951. doi:10.1097/MD.0000000000039951
Yang, S., Sun, Y., and Yan, C. (2024c). Recent advances in the use of extracellular vesicles from adipose-derived stem cells for regenerative medical therapeutics. J. Nanobiotechnology 22, 316. doi:10.1186/s12951-024-02603-4
Yin, T., Liu, Y., Ji, W., Zhuang, J., Chen, X., Gong, B., et al. (2023). Engineered mesenchymal stem cell-derived extracellular vesicles: a state-of-the-art multifunctional weapon against Alzheimer's disease. Theranostics 13, 1264–1285. doi:10.7150/thno.81860
Yu, Z., Lin, S., Xia, F., Liu, Y., Zhang, D., Wang, F., et al. (2021). ExoSD chips for high-purity immunomagnetic separation and high-sensitivity detection of gastric cancer cell-derived exosomes. Biosens. Bioelectron. 194, 113594. doi:10.1016/j.bios.2021.113594
Zanirati, G., Dos Santos, P. G., Alcará, A. M., Bruzzo, F., Ghilardi, I. M., Wietholter, V., et al. (2024). Extracellular vesicles: the next generation of biomarkers and treatment for central nervous system diseases. Int. J. Mol. Sci. 25, 7371. doi:10.3390/ijms25137371
Zargar, M. J., Kaviani, S., Vasei, M., Soufi Zomorrod, M., Heidari Keshel, S., and Soleimani, M. (2022). Therapeutic role of mesenchymal stem cell-derived exosomes in respiratory disease. Stem Cell Res. Ther. 13, 194. doi:10.1186/s13287-022-02866-4
Zhang, W. Y., Wen, L., Du, L., Liu, T. T., Sun, Y., Chen, Y. Z., et al. (2024). S-RBD-modified and miR-486-5p-engineered exosomes derived from mesenchymal stem cells suppress ferroptosis and alleviate radiation-induced lung injury and long-term pulmonary fibrosis. J. Nanobiotechnology 22, 662. doi:10.1186/s12951-024-02830-9
Zhang, J., Yu, L., Wu, X., and Pan, W. (2025). Small extracellular vesicles promote hbv replication via mettl3-igf2bp2-mediated m6a modification. Front. Biosci. Landmark Ed. 30, 36291. doi:10.31083/FBL36291
Zhdanova, D. Y., Poltavtseva, R. A., Svirshchevskaya, E. V., and Bobkova, N. V. (2021). Effect of intranasal administration of multipotent mesenchymal stromal cell exosomes on memory of mice in Alzheimer's Disease model. Bull. Exp. Biol. Med. 170, 575–582. doi:10.1007/s10517-021-05109-3
Zheng, J., Yang, B., Liu, S., Xu, Z., Ding, Z., and Mo, M. (2024). Applications of exosomal mirnas from mesenchymal stem cells as skin boosters. Biomolecules 14, 459. doi:10.3390/biom14040459
Zheng, J., Jiang, X., Bai, S., Lai, M., Yu, J., Chen, M., et al. (2025). Clinically accurate diagnosis of alzheimer's disease via single-molecule bioelectronic label-free profiling of multiple blood extracellular vesicle biomarkers. Adv. Mater. Deerf. Beach, Fla. 27, e2505262. doi:10.1002/adma.202505262
Zhou, W., Silva, M., Feng, C., Zhao, S., Liu, L., Li, S., et al. (2021). Exosomes derived from human placental mesenchymal stem cells enhanced the recovery of spinal cord injury by activating endogenous neurogenesis. Stem Cell Res. Ther. 12, 174. doi:10.1186/s13287-021-02248-2
Zhou, T., He, C., Lai, P., Yang, Z., Liu, Y., Xu, H., et al. (2022). miR-204-containing exosomes ameliorate GVHD-associated dry eye disease. Sci. Adv. 8, eabj9617. doi:10.1126/sciadv.abj9617
Keywords: mesenchymal stem cell-derived extracellular vesicles (MSC-EVs), preparation, characterization, pharmacokinetics, neurological disorders
Citation: Li S, Zhang J, Sun L, Yang Z, Liu X, Liu J and Liu X (2025) Mesenchymal stem cell-derived extracellular vesicles: current advances in preparation and therapeutic applications for neurological disorders. Front. Cell Dev. Biol. 13:1626996. doi: 10.3389/fcell.2025.1626996
Received: 12 May 2025; Accepted: 28 July 2025;
Published: 18 August 2025.
Edited by:
Khalid Ahmed Al-Anazi, King Fahad Specialist Hospital, Saudi ArabiaReviewed by:
Anula Singh, Sapien Bioscience Pvt. Ltd, IndiaBurcak Yavuz, Altınbaş University, Türkiye
Copyright © 2025 Li, Zhang, Sun, Yang, Liu, Liu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianling Liu, NjAyNTcwODY0QHFxLmNvbQ==; Xifu Liu, eGZsaXVAaGVidHUuZWR1LmNu
†These authors have contributed equally to this work
 Shuang Li
Shuang Li Jiayi Zhang2,3†
Jiayi Zhang2,3† Xifu Liu
Xifu Liu