Abstract
Taste perception is crucial for animals to assess food’s nutritional value while avoiding toxic substances. Recent decades have unveiled the presence of taste receptors beyond the oral cavity, expressed in diverse non-gustatory tissues including gastrointestinal, cardiovascular, reproductive, and neural tissues. These ectopically expressed taste receptors are implicated in a multitude of physiological processes such as the regulation of hormone secretion, nutrient sensing and digestive processes, pathogen defense, and modulation of locomotor abilities. Moreover, these receptors present potential pharmacological targets for therapeutic interventions in diseases related to the respiratory, digestive, and cardiovascular systems. In this review, we summarize the recent advances in understanding the distribution and functions of extraoral taste receptors in mammals, teleosts, insects, and nematodes, emphasizing the commonalities and variations among different species. The emerging paradigm positions taste receptors as polymodal sensors integrating environmental cues with physiological homeostasis beyond their canonical gustatory functions, offering new perspectives on sensory system evolution and organismal adaptation.
1 Introduction
Taste perception is an important component of animal adaptation to the environment. Traditionally, it is understood that human can perceive five basic taste modalities: sour, sweet, bitter, salty and umami (Chen et al., 2011; Hoon et al., 1999; Lindemann, 2001). However, certain animals, such as insects, may possess a more diverse array of taste sensations (Chen and Dahanukar, 2020). In mammals, taste is primarily sensed through taste buds confined to the oral cavity (Yarmolinsky, Zuker, and Ryba, 2009), whereas in fruit flies, gustatory organs are more broadly distributed, appearing not only in the mouthparts but also on the legs, wings, and female genitalia (Liman et al., 2014). This broad distribution of taste-related organs is not unique to insects; Similarly, fish have taste organs on their tentacles, fins, and even skin (Ishimaru et al., 2005). While human taste organs are classically considered confined to the oral cavity, the distribution of taste receptors extends into many non-gustatory tissues, contributing to physiological functions beyond taste perception (Behrens and Lang, 2022; Kurumi et al., 2013; Jonas et al., 2019), such as energy homeostasis and immune regulation. These findings have expanded the conceptual framework regarding the roles of taste receptors.
Fruit flies have long served as an important model in taste neurophysiology research, and more recently, studies on piscine taste perception have also emerged as a significant branch of vertebrate taste studies. Despite the extensive distribution of taste organs in these species, the specific roles and distribution of taste receptors in non-gustatory tissues remain inadequately explored. The comparison of ectopically expressed taste receptors’ functions and regulatory mechanisms in non-gustatory tissues across species enables deeper understanding of taste perception’s evolutionary trajectories and provides further insights into the fundamental nature of gustatory mechanisms.
2 Taste perception in mammals
Human and most other mammals rely on taste buds situated in the oral cavity to discern flavors. These taste buds are embedded within papillae on the tongue’s surface and the palate. They form the basic structure for taste perception. The tongue hosts three types of taste papillae: fungiform, foliate, and circumvallate, while the soft palate contains numerous isolated taste buds (Martin and Martin, 2019; Melis et al., 2020; Doyle et al., 2023; Yarmolinsky et al., 2009) (Figure 1A). It is generally accepted that there are five primary taste modalities:sour, sweet, bitter, salty and umami (Chen et al., 2011; Hoon et al., 1999; Lindemann, 2001; Beauchamp, 2019). However, noncanonical taste qualities such as fat (Brondel et al., 2022; Liu, D, et al., 2018) and alkaline taste have also been identified (Mi et al., 2023). Additionally, other sensory inputs, such as olfactory cues and temperature, can significantly affect taste perception (Talavera et al., 2007; He et al. 2023; Stevenson, 2014). These observations imply the complex combinational logic underlying human taste perception (Fontanini, 2023). In general, various taste stimulations signal the presence of diverse nutrients or toxic substances in the environment, enabling animals to respond. Many foods can trigger multiple taste sensations; for instance, salt can evoke both salty and bitter tastes, depending on its concentration. Moreover, certain bitter foods, like coffee and tea, might ordinarily be expected to elicit aversion, yet they are widely relished (Vecchio et al., 2019; Harwood et al., 2012). This suggests that feeding behavior associated with taste perception, as well as taste perception itself, may be influenced by physiological conditions and even emotional states (Jones et al., 2006; Vincis and Fontanini, 2019).
FIGURE 1
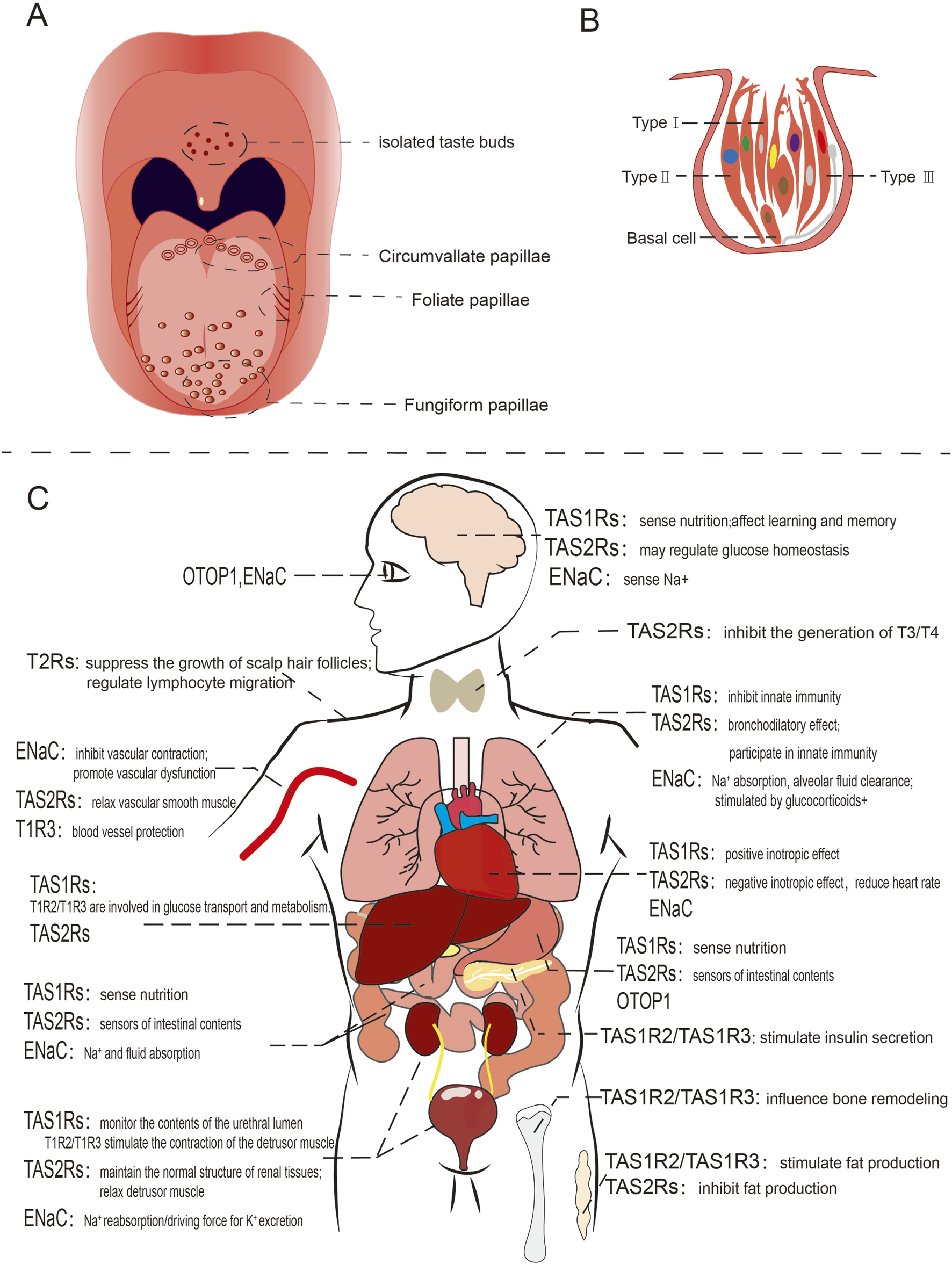
Schematic diagram of human taste organ and ectopically expressed taste receptors. (A) The distribution of taste buds on the human tongue; (B) Taste bud in humans, direct synaptic connection with afferent nerve fibers showed with gray line; (C) Ectopic expression and functions of taste receptors in various human organs.
A human taste bud comprises approximately 50–100 taste receptor cells, which are categorized into four distinct types. Type I cells resembling astrocytes, enclose other cells types, whereas type II and type III cells are elongated and spindle-shaped, featuring taste receptors on their surface. Type III cells have direct synaptic connections with afferent nerve fibers within taste buds. Type IV cells are referred to as basial cells (Doyle et al., 2023) (Figure 1B). The receptors responsible for detecting sweet, umami, and bitter tastes are G-protein-coupled receptors (GPCR), including TAS1R2+TAS1R3 for umami, TAS1R1+TAS1R3 for sweet, and TAS2Rs for bitter (Chen et al., 2011; Ki and Jeong, 2022; Chandrashekar et al., 2000; Grace et al., 2003). In contrast, salty and sour taste are mediated through ion-selective membrane channels (Huang et al., 2006; Chandrashekar et al., 2006; Ishimaru et al., 2006). Each taste is uniquely sensed by its corresponding type of taste receptor cells (Yarmolinsky et al., 2009; Melis et al., 2020; Tomchik et al., 2007).
2.1 The taste receptors in mammals
GPCRs (G protein-coupled receptors) are one of the largest families of membrane proteins and are widely involved in various physiological signal transductions. These receptors share a characteristic structure comprising seven transmembrane (TM) helices and interact with heterotrimeric guanine nucleotide-binding proteins (G proteins) (Hu et al., 2017). Human GPCRs can be categorized into several classes: Class A (Rhodopsin) encompasses olfactory receptors, a variety of neurotransmitters receptors, and photoreceptors, comprising approximately 80% of GPCRs. Class B (Secretin) is further subdivided into the secretin family (B1) and the adhesion family (B2) within the GRAFS classification system. Class C (Glutamate) includes metabotropic glutamate (mGlu) receptors, gamma-aminobutyric acid (GABA) receptors, calcium-sensing receptors, and taste receptors. Class F (Frizzled) constitutes another category, while Class T (T2R) is characterized by its low sequence similarity to other classes and complex classification (Herrera et al., 2025; Di Pizio and Niv, 2015). Additionally, there exist other subgroups beyond these primary classes.
The human taste receptor TAS1Rs are members of the Class C GPCRs. TAS1Rs have the typical structural features of this family, consisting of an N-terminal extracellular domain and a seven-transmembrane domain (7-TMD). The N-terminus is further subdivided into a venus flytrap domain (VFTD) and a cysteine-rich domain (CRD). TAS1Rs have a larger bilobed N-terminus and can form a heterodimeric structure (Kim S. K. et al., 2017), for example, the TAS1R2 and TAS1R3 subunits combine to detect sweetness (Charles et al., 2001), whereas the TAS1R1 and TAS1R3 subunits are responsible for umami perception (Grace et al., 2003). Besides, umami taste may also be sensed by the metabotropic glutamate receptor (mGLUR) (Chaudhari et al., 2000; San Gabriel et al., 2005). Bitter taste detection is mediated by the Class T TAS2R family, which similarly contains a seven-transmembrane helix region (7-TMD) (Singh et al., 2011a). Sour taste has been found to be associated with Otopetrin protein (OTOPs) and proteins in the TRP family, specifically PKD2L1/PKD1L3 (Ishimaru et al., 2006). OTOP receptors are homodimers comprising 12 transmembrane helices (TM1–TM12), with TM1–TM6 forming the N-domain and TM7–TM12 forming the C-domain (Saotome et al., 2019). The Epithelial sodium channel (ENaC), responsible for salt taste perception, is a heterotrimeric complex of three distinct subunits: α, β, and γ. Each ENaC subunit consists of two transmembrane domains (TM1 and TM2), with intracellular N- and C-termini and a large extracellular domain (ECD) (Figure 2).
FIGURE 2
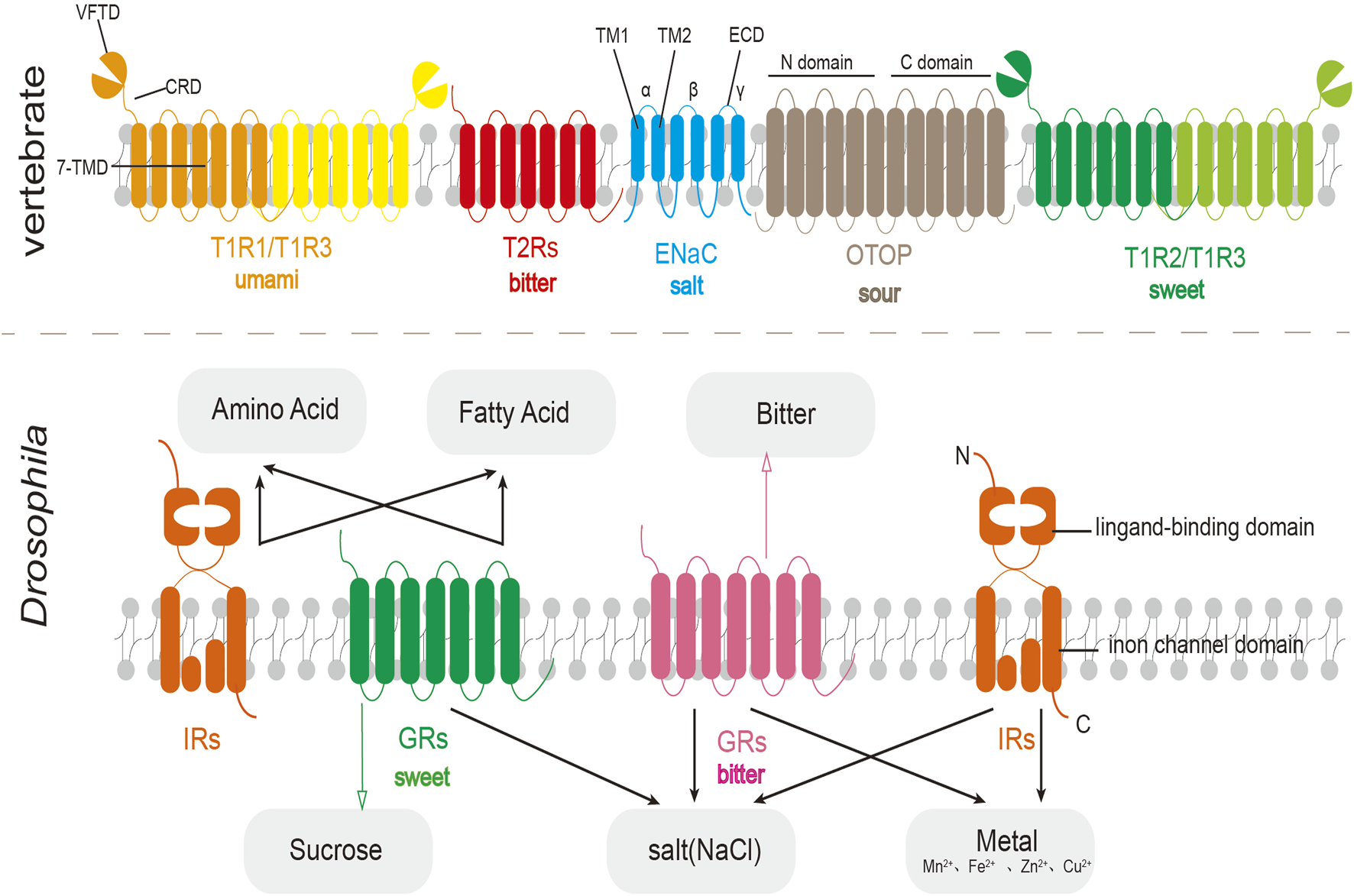
Schematic diagram of the structure of vertebrate taste receptors (top) and fruit fly taste receptors (bottom). In fruit flies, IRs often work together with GRs to perceive taste substances.
2.2 Distribution and function of extraoral taste receptors in mammals
Taste receptors are present in various tissues and organs, including the brain, respiratory tract, lungs, stomach, intestines, pancreas, liver, kidneys, reproductive organs, skin, and muscles, and they are involved in numerous physiological functions (Figure 1C). In this section, we summarize previous research on extraoral taste receptors in mammals. Additionally, we recommend consulting several earlier reviews on this topic for further reading (Behrens and Lang, 2022; Kurumi et al., 2013; Jonas et al., 2019; Li et al., 2024).
2.2.1 Gastrointestinal tract
The anatomical complexity of gastrointestinal tract (GIT) prompts extensive investigation into the differential spatial expression of taste receptors across its segments (Simone et al., 2018; Kok et al., 2018). The distribution and expression levels of bitter taste receptors (TAS2Rs) in the intestine vary among different segments, and different species possess unique expression profiles of these receptors. Studies based on detecting the mRNA expression levels of taste receptors in humans reveals that among the 26 TAS2Rs, TAS2R4 and TAS2R14 are the most ubiquitously expressed subtypes in the GIT (Jalševac et al., 2024). In contrast, certain receptors exhibit strict tissue specificity. TAS2R1, TAS2R42, TAS2R45, TAS2R46, TAS2R50, and TAS2R60 are exclusively detected in the colon, whereas TAS2R7 and TAS2R8 are restricted to the jejunum (Descamps-Solà et al., 2023; Simone et al., 2018).
Rodents (mouse and rat) and human bitter taste receptors do not follow the same expression pattern. Only a few receptors are expressed both in human and rodents’ GIT, including the widely expressed TAS2R4 (homologue of rodent TAS2r118), TAS2R38 (homologue of rodent TAS2r138) expressed in the duodenum, and TAS2R1 specifically expressed in human colon, whereas its rodent ortholog, TAS2r119, is expressed throughout all gastrointestinal tract (GIT) tissues. In addition, two receptors, TAS2R16 and TAS2R41, are not expressed in humans but are expressed in rodents (Descamps-Solà et al., 2023). In mice, sweet taste receptor expression was higher in the ileum than in the jejunum and duodenum (Dyer et al., 2005), whereas umami taste receptors show no significant regional variation in proximal and distal parts of the small intestine (Daly et al., 2013). Despite these findings, the expression landscape of human sweet and umami taste receptors in the GIT remains poorly characterized, highlighting a critical knowledge gap in this field.
Taste receptors are also expressed in functionally distinct gastrointestinal cells (Figure 3). For example, bitter taste receptors are distributed across various cellular types, including gastric parietal cells, endocrine cells, goblet cells, tuft cells, and paneth cells (Tuzim and Korolczuk, 2021). In gastric parietal cells, TAS2Rs mediate the production of gastric acid (Liszt et al., 2017); within endocrine cells (Kidd et al., 2008; Janssen et al., 2011; Kidd et al., 2009; Kok et al., 2018), these receptors facilitate the release of intestinal hormones such as glucagon-like peptide 1 (GLP1), which play an important role in metabolic regulation (Kok et al., 2018); goblet cells rely on TAS2Rs to promote protective mucus secretion (Prandi et al., 2013), while in paneth cells, these receptors sense bacterial secretions (Simone et al., 2018); and in tuft cells, they are involved in parasite-induced type 2 immunity (Gerbe et al., 2016). In general, TAS2Rs serve as sensors of intestinal contents (Sternini and Rozengurt, 2025). Additionally, in both human and mice gastrointestinal smooth muscle cells, activation of bitter taste receptors also results in inhibition of gastric emptying (Wu et al., 2025; Avau et al., 2015b).
FIGURE 3
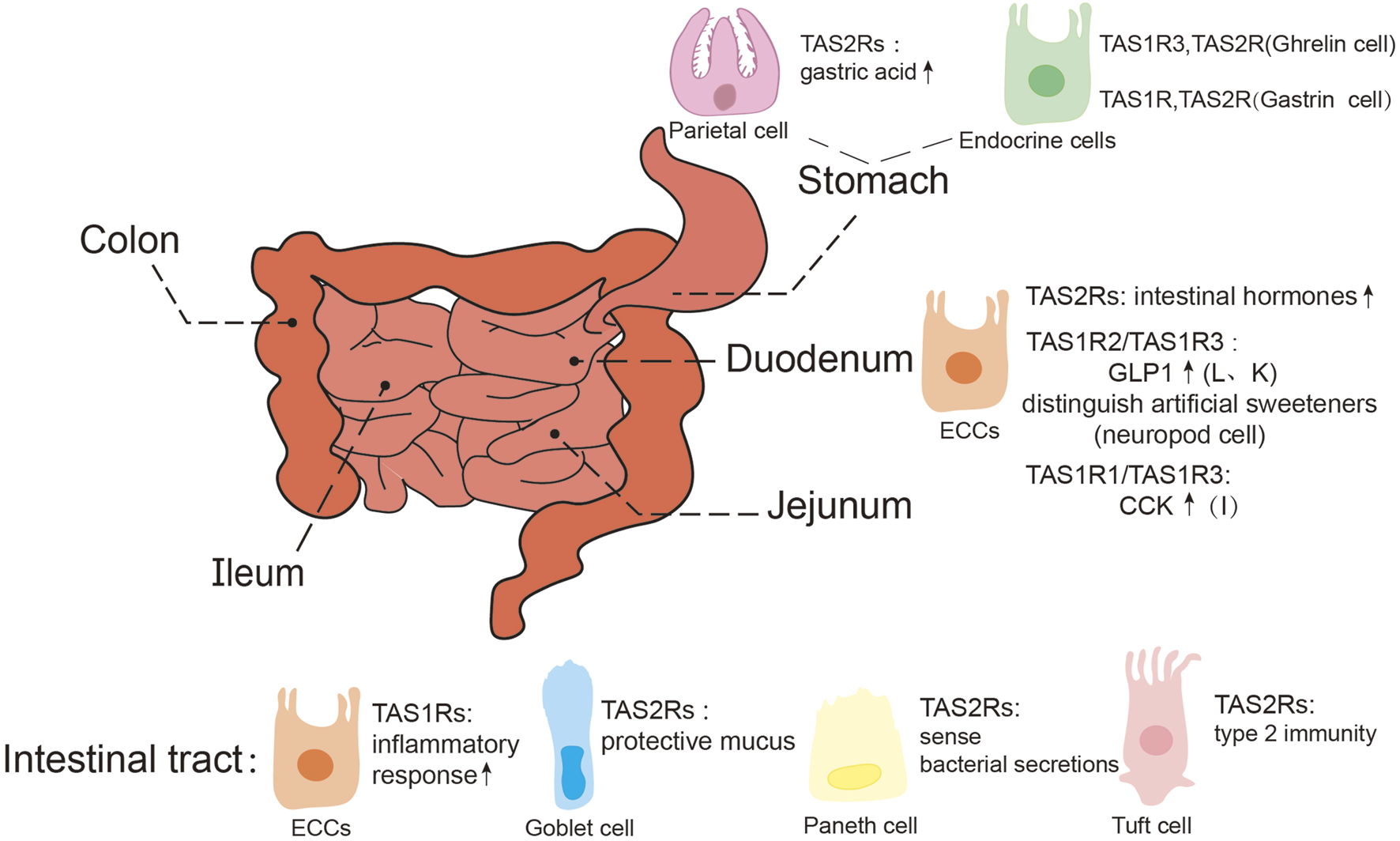
Expression of taste receptors in the gastrointestinal tract. GLP1, glucagon-like peptide 1; CCK, cholecy-stokinin; ECCs, enteroendocrine cells, Including I, K, L cells, etc., are mainly concentrated in the duodenum.
Ectopically expressed sweet taste receptors are primarily located in enteroendocrine cells (Li et al., 2024), particularly L cells and K cells, where the TAS1R2/TAS1R3 receptor complex regulates glucose absorption and GLP-1 secretion. However, it was also reported that the homodimer of TAS1R3 alone is capable of sugar detection (Ohtsu et al., 2014). Studies on mice have revealed that the intestine can distinguish between sugar and artificial sweeteners, with this function predominantly localized to neuropod cells (a type of enteroendocrine cells) in the proximal small intestine rather than the stomach or ileum. Single-cell RT-qPCR analyses have revealed the absence of TAS1R2 expression in neuropod cells, indicating that TAS1R3 is solely responsible for sensing sugars and artificial sweeteners in small intestine (Buchanan et al., 2022). Recent studies have expanded on the role of sweet taste receptors, demonstrating that enteroendocrine cells, through TAS1R3, can detect nutritional factors, triggering inflammatory responses in mice consuming a sugar-rich western diet (Shon et al., 2023). The umami receptors have also been identified in the GIT (Oya et al., 2011; Wang et al., 2011; Daly et al., 2013; Nunez-Salces et al., 2020), contributing to nutritional sensing function (Mace et al., 2009). These findings indicate the multifaceted roles of sweet and umami taste receptors in gastrointestinal physiology.
2.2.2 Cardiovascular system
The expression of bitter taste receptors has been identified in human and mouse cardiomyocytes, as well as in mouse fibroblasts. Activation of TAS2Rs induces a negative inotropic effect (Foster et al., 2014; Foster et al., 2013). Furthermore, these receptors are also present in the sinoatrial (SA) node and left ventricle of rat hearts. Langendorff perfusion of isolated hearts and isolated sinoatrial nodes demonstrated that the activation of bitter taste receptors can reduce heart rate by prolonging ventricular depolarization and attenuating SA node pacing through a phosphodiesterase (PDE)-dependent mechanism (Yuan G, et al., 2020). Notably, each TAS2R encompasses a range of polymorphisms and is highly expressed in cardiac tissue (Foster et al., 2013). Comparative analyses of receptors respond to specific ligands have shown that these polymorphisms can alter receptor signaling. Some result in moderate functional impairment (e.g., TAS2R31 and TAS2R50), while others abolish detectable ligand-dependent signaling (e.g., TAS2R14, TAS2R30, and TAS2R46); conversely, certain variants (e.g., TAS2R10, TAS2R14, and TAS2R31) exhibit slight enhancements in signaling (Bloxham et al., 2024). Receptors with functional alterations are prevalent in the heart and are associated with various physiological and diseases conditions (Foster et al., 2015). These polymorphic variants may influence the feasibility of targeting bitter taste receptors as potential therapeutic interventions for cardiovascular diseases.
TAS1Rs are also expressed in the cardiac tissue, with TAS1R1, TAS1R2, and TAS1R3 localized to the plasma membranes of cardiomyocytes in both mice and humans (Yoder et al., 2025; Papadaki, Osorio- Valencia, and Kirk, 2021). Activation of sweet taste receptors enhances cardiac contractility by exerting positive inotropic effects and accelerating calcium handling (Yoder et al., 2025). Similarly, stimulation of rat ventricular myocytes with umami agonists has been shown to induce comparable inotropic responses (Papadaki et al., 2021). These findings suggest that sweet and umami taste receptors function as nutrient sensors, modulating cardiac contractility in response to metabolic cues. Given their regulatory role, TAS1Rs may hold therapeutic potential for conditions such as heart failure, warranting further investigation into their clinical applications.
There are also taste receptors on blood vessels. Activation of bitter taste receptors induces relaxation of vascular smooth muscle cells (Manson et al., 2014). These receptors have been identified in various vascular beds, including the aorta of mice (Manson et al., 2014), pulmonary arteries (Manson et al., 2014) and omentum arteries of humans, as well as the mesenteric and cerebral arteries of rats (Chen et al., 2017). The sweet receptor TAS1R3 is expressed in the endothelial cells of human primary glomerular microvessels (Enuwosa et al., 2021), human retinal microvessels (Lizunkova et al., 2019), and rat pulmonary microvessels, where it exerts a protective effect on blood vessels. In contrast, The ENaC is expressed in endothelial cells, where exposure to high salt concentrations promotes vascular dysfunction, characterized by reduced nitric oxide production, increased cell stiffness, and pressure-independent vascular remodeling (Mutchler et al., 2021; Zheng et al., 2016). ENaC is also found in smooth muscle cells, inhibition of this channel effectively nullifies pressure-induced vascular contraction in the renal arteries of mice (Jernigan and Drummond, 2005) and cerebral vessels of rats (Kim et al., 2012). It is plausible that taste receptors are present in additional regions of the cardiovascular system, such as the coronary arteries, which supply blood to the heart and whose dysfunction may result in angina pectoris (Savulescu-Fiedler et al., 2025). Investigating the roles and expression patterns of taste receptors in these arteries could provide valuable insights into developing novel therapeutic strategies for angina pectoris.
2.2.3 Reproductive system
Taste receptors are also present in the reproductive system and play critical roles in maintaining male and female fertility as well as regulating immune function within this system (Lu et al., 2024) (Figure 4).
FIGURE 4
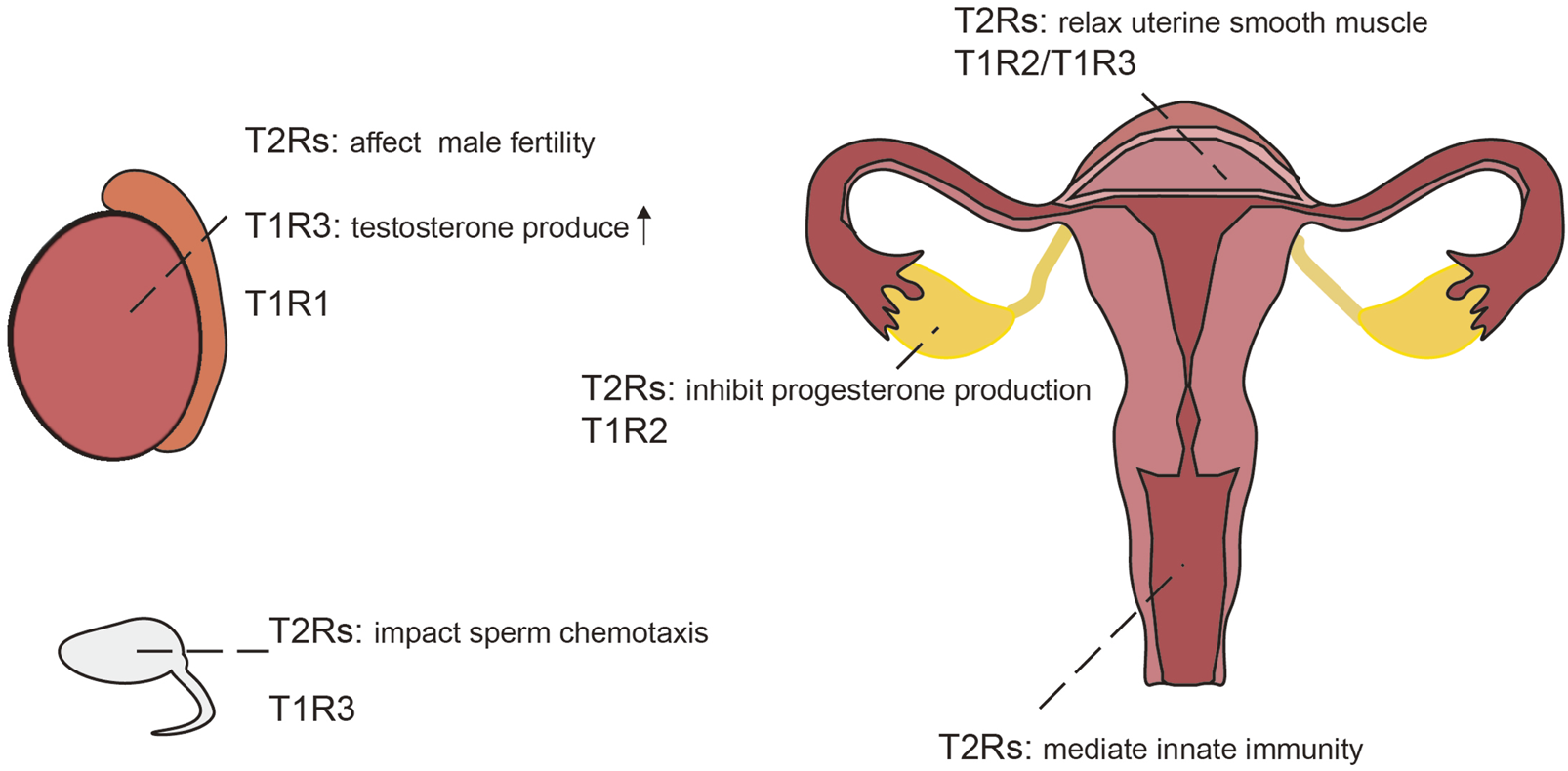
Expression of taste receptors in the reproductive system,the testicles and sperm of the male (left); the reproductive system of the female (right).
2.2.3.1 Testis and sperm
TAS2r105 is expressed in early to mid-stage round spermatids in mice, but not present in spermatocytes or spermatogonia (Li and Zhou, 2012). In contrast, TAS2r131 is distributed throughout the lumen of seminiferous tubules of adult testes and small tubules in the epididymis in 3-day-old mice (Voigt et al., 2015; Voigt et al., 2012). Recent studies have further corroborated the presence of TAS2Rs subtypes and their downstream signaling components in human testes and sperm, suggesting a conserved role across species (Governini et al., 2020). Bitter taste receptors are increasingly recognized as significant contributors to physiological processes such as sperm chemotaxis and male fertility (Lu et al., 2024), similarly, some olfactory receptors also involved in the sperm chemotaxis process (Spehr et al., 2003). Besides bitter taste receptors, sweet and umami taste receptor subunits TAS1R1 and TAS1R3 have also been identified in both human and murine testes (Frolikova et al., 2020; Meyer et al., 2012), with TAS1R3 specifically located on the head of sperm in mice (Frolikova et al., 2020). Stimulation of TAS1R3 enhances testosterone production in Leydig cells via the cAMP-PKA-SP1 pathway (Liu et al., 2025; Shon et al., 2024; Liu et al., 2022), where TAS1R3 knockout results in reduced male fertility (Seong et al., 2024; Shon et al., 2024). In addition, abnormal spermatogenesis was found in mice deficient in the umami receptor subunit TAS1R1 (Meyer et al., 2012).
2.2.3.2 Ovaries, uterus, placenta and vagina
Bitter taste receptors are also present in the female reproductive system. Specifically, TAS2Rs and their downstream signaling components been identified in granulosa cells and cumulus cells within the human ovary (Semplici et al., 2021). Experiments using high concentrations of saccharin to activate TAS2Rs in the corpus luteum of rats have demonstrated an inhibition of progesterone production in pseudopregnant rats, mediated through NO/cGMP signaling and apoptosis pathways (Jiang et al., 2021). The sweet taste receptor TAS1R2 is similarly expressed in the corpus luteum of the rat ovary (Jiang et al., 2021). Administration of sweet taste agonists has been shown to modulate ovarian function, with saccharin increasing and stevia decreasing progesterone levels in young rats (Jiang et al., 2018); However, dietary effects cannot be entirely ruled out as contributing factors. Similar to saccharin, certain steviosides can also activate bitter taste receptors in gustatory organs (Tian et al., 2022); but whether ectopically expressed bitter taste receptors in the reproductive system can likewise be activated by steviosides remains to be further investigated.
Transcripts of bitter taste receptors and their associated signal transduction components have been detected in the myometrium of both human and mice. Activation of bitter taste receptors with chloroquine induces relaxation of precontracted uterine smooth muscle strips, outperforming currently used tocolytics (Zheng et al., 2017). Moreover, activation of TAS2R5 with phenanthroline leads to extensive uterine relaxation (Delpapa et al., 2023), suggesting potential as novel uterine relaxants. Sweet taste receptor, TAS1R2/TAS1R3, have also been identified in the uterus of guinea pigs (Li et al., 2020), although their precise role within the uterine context warrants further investigation.
The TAS2R14 gene has been detected in human and mouse placentas, as well as in human trophoblast cell lines (Taher et al., 2019). TAS2R38 is expressed in human placental tissue and JEG-3 cells, where it demonstrates functionality by facilitating calcium influx and potentially mediating innate immune response to placental infection (Wölfle et al., 2016). Furthermore, TAS2r143 was found in epithelial cells of the vagina and cervix in mice and may also be involved in innate immunity (Liu et al., 2017).
2.2.4 Respiratory system
The expression of TAS2R38, TAS2R4 and TAS2R16 proteins has been identified in ciliated cells of human sinus epithelium. Notably, TAS2R4 and TAS2R16 mediate NO-dependent immune defenses, leading to an increase in ciliary beat frequency, which accelerates the clearance of nasal mucosa cilia (Yan et al., 2017).
Tuft cells, also known as solitary chemosensory cells (SCC), are found in epithelial tissues at various sites, including the nasal mucosa of the upper airway and lower airway (Ye and Bankova, 2022). In rat and mouse sinonasal SCC, both mRNA and protein levels of TAS2Rs and TAS1R3 expressions can be detected (Tizzano et al., 2011). Another study using immunofluorescence techniques has demonstrated the presence of TAS2Rs and TAS1R2/TAS1R3 in human sinonasal SCCs, indicating that TAS2Rs are widely distributed throughout the respiratory epithelium and may play a role in innate immunity (Lee R. J. et al., 2014). In contrast, sweet and umami taste receptors within the respiratory epithelium have been observed to inhibit the innate immunity mediated by bitter taste receptors under both healthy and inflammatory conditions (Lee R. J. et al., 2014; McMahon et al., 2023a, 2023b).
In both mice and humans, the activation of TAS2R receptors induces relaxation of bronchial smooth muscle (Deshpande et al., 2010; Grassin-Delyle et al., 2013). Importantly, the relaxation effect induced by bitter taste receptors is similar to that of the β2 agonist isoproterenol (Deshpande et al., 2011), without functional downregulation during airway inflammation (Robinett et al., 2014), and maintaining full efficacy during β2 agonist tolerance (An et al., 2012). These findings suggest that TAS2R receptor agonists could serve as potent bronchodilators.
2.2.5 Brain
Taste receptors are widely distributed in the mammalian brain. Transcripts and proteins of TAS2Rs have been detected in the brainstem, cerebellum, cerebral cortex and nucleus accumbens of rats (Singh et al., 2011b), as well as in the epithelial cells of the human choroid plexus (CP), with in vitro studies demonstrating responsiveness of human CP epithelial cells to bitter tastants (Duarte et al., 2020). While numerous exogenous bitter compounds are blocked by the blood-brain barrier (BBB), certain food-derived dipeptides and tripeptides have been shown to cross the BBB, though their physiological effects remain unclear (Upadhyaya et al., 2010). As an active interface between blood and cerebrospinal fluid (CSF), the choroid plexus may utilize taste receptors to mediate the sensing of blood-borne substances. Notably, TAS2R14 has been implicated in modulating the activity of ABC efflux transporters in the CP, thereby regulating the transcellular transport of resveratrol across the human blood-CSF barrier (Duarte et al., 2020). Recent studies have identified a subset of functional tanycytes (neuroglia-like cells) in the median process at the base of the brain, which do not express sweet taste receptors and whose ablation leads to impaired glucose tolerance, suggesting that bitter taste receptors may be involved in the regulation of glucose homeostasis (Yu et al., 2023). In addition, psychotropic drugs exposure alters cerebral bitter taste receptors expression, indicating these receptors as potential pharmacological targets (Ansoleaga et al., 2015).
The sweet taste receptor TAS1R2 is ubiquitously present across various neuronal populations in mice, with localization in glial cells of periventricular organs and vascular structures within the cortex, thalamus, and striatum (Jang et al., 2021). Intriguingly, while a subset of tanycytes express bitter taste receptors, the majority exhibit TAS1R2/TAS1R3-mediated glucose sensing (Benford et al., 2017; Kohno et al., 2016). Genetic ablation of TAS1R3 in mice leads to changes in learning and memory functions and deficits in social ability, underscoring its critical role in central nervous system neurotrophic functions (Martin et al., 2017).
2.2.6 Other organs and systems
In the immune system, bitter taste receptors are expressed in various human immune cells, including macrophages, lymphocytes, monocytes and neutrophils (Gopallawa et al., 2021; Tran et al., 2018; Maurer et al., 2015; Malki et al., 2015),regulating innate and adaptive immunity and suppressing the production of inflammatory factors. Umami taste receptors (TAS1R1/TAS1R3) in mouse neutrophils mediate chemotaxis and attenuate lipopolysaccharide-induced inflammatory responses upon activation (Lee N et al., 2014).
The urinary system demonstrates taste receptor-specific modulation. TAS2Rs in murine kidneys (Rajkumar et al., 2014; Liu et al., 2015) and human bladders (Zhai et al., 2016) maintain glomerular structure and promote detrusor relaxation, whereas sweet taste receptors (TAS1R2/TAS1R3) induce detrusor contraction in rat bladders (Elliott et al., 2011). Adipose tissue expresses both receptor families across species (Cancello et al., 2020; Avau et al., 2015a; Simon et al., 2013), and exhibiting significant roles in adipogenesis regulation (Avau et al., 2015a; Ning et al., 2016), but the underlying mechanisms are controversial (Ki and Jeong, 2022; Tuzim and Korolczuk, 2021). Expression of TAS1Rs and TAS2Rs has also been found in mouse liver (Kurtz et al., 2020; Liu S, et al., 2018), where TAS1R2/TAS1R3 are involved in glucose transport and metabolism (Luo et al., 2022). Moreover, pancreatic β-cell TAS1R2/TAS1R3 mediate sweetener-induced insulin secretion (Nakagawa et al., 2009), while influencing bone remodeling processes (Simon et al., 2014). Notably, TAS2Rs exhibit pleiotropic effects, modulating inflammation in mouse gingival SCC(Zheng et al., 2019), inhibiting thyroid hormone (T3/T4) secretion in thyrocytes (Clark et al., 2015), and suppressing hair follicle growth in human scalp tissue (Sakakibara et al., 2022; Gherardini et al., 2025).
Beyond classical taste receptors, ENaC regulate fluid homeostasis in pulmonary, colonic, renal, and ocular tissues (Rotin and Staub, 2021). Free fatty acids receptors (FFARs) demonstrate broad tissue distribution including CNS and pancreatic expression (Roy et al., 2022; Kimura et al., 2019), while OTOP1 localizes to brown adipose tissue and reproductive organs (Tu et al., 2018), though their precise physiological mechanisms remain incompletely characterized.
Collectively, ectopically expressed taste receptors across various tissues and organs have been implicated in numerous non-gustatory functions. Unlike canonical taste perception, these functions mediated by ectopic taste receptors, such as the chemotaxis of immune cells and sperm, can operate independently of complex neural circuitry. Consequently, the conventional boundaries demarcating the operational paradigms of taste receptors from other chemosensory receptors, as well as their respective physiological roles, have become increasingly blurred. This phenomenon finds parallel observations in studies of teleosts and invertebrates (see subsequent sections).
3 Taste perception in teleosts
Teleost fishes represent a diverse group of vertebrate species, with zebrafish (Danio rerio), medaka (Oryzias latipes), and pufferfish (Tetraodon nigroviridis) serving as predominant model organisms in scientific research (Yasuoka and Abe, 2009). The gustatory system in these species exhibits a broad anatomical distribution, with taste buds localized in the lips, gill rakers, pharynx, oral cavity, and also on integumentary surface, while some species additionally possess these sensory structures on barbels and fins (Ishimaru et al., 2005; Hardy and Hale, 2022; Ovalle and Shinn, 1977; Yacoob et al., 2001) (Figure 5). Morphologically analogous to mammals, piscine taste buds display elongated, onion-shaped or pear-shaped configurations, embedded within sensory epithelium richly innervated by afferent nerve fibers. These chemosensory organs comprise three distinct cell populations: taste receptor cells (TRCs), supporting cells, and basal cells (Morais, 2017; Giaquinto et al., 2024). Despite the extensive utilization of teleost models in biological research, investigations of gustatory mechanisms remain relatively underdeveloped (Okada, 2015). Systematic studies of taste perception pathways in fish have only emerged in recent years. Comparative analyses across multiple species reveal subtle interspecific variations in both the spatial distribution of taste buds and the expression patterns of taste receptors (Yuan X. C. et al., 2020; Hardy and Hale, 2022; Bhatia et al., 2022; John et al., 1993; Maik, Tatjana, and Sigrun, 2023).
FIGURE 5
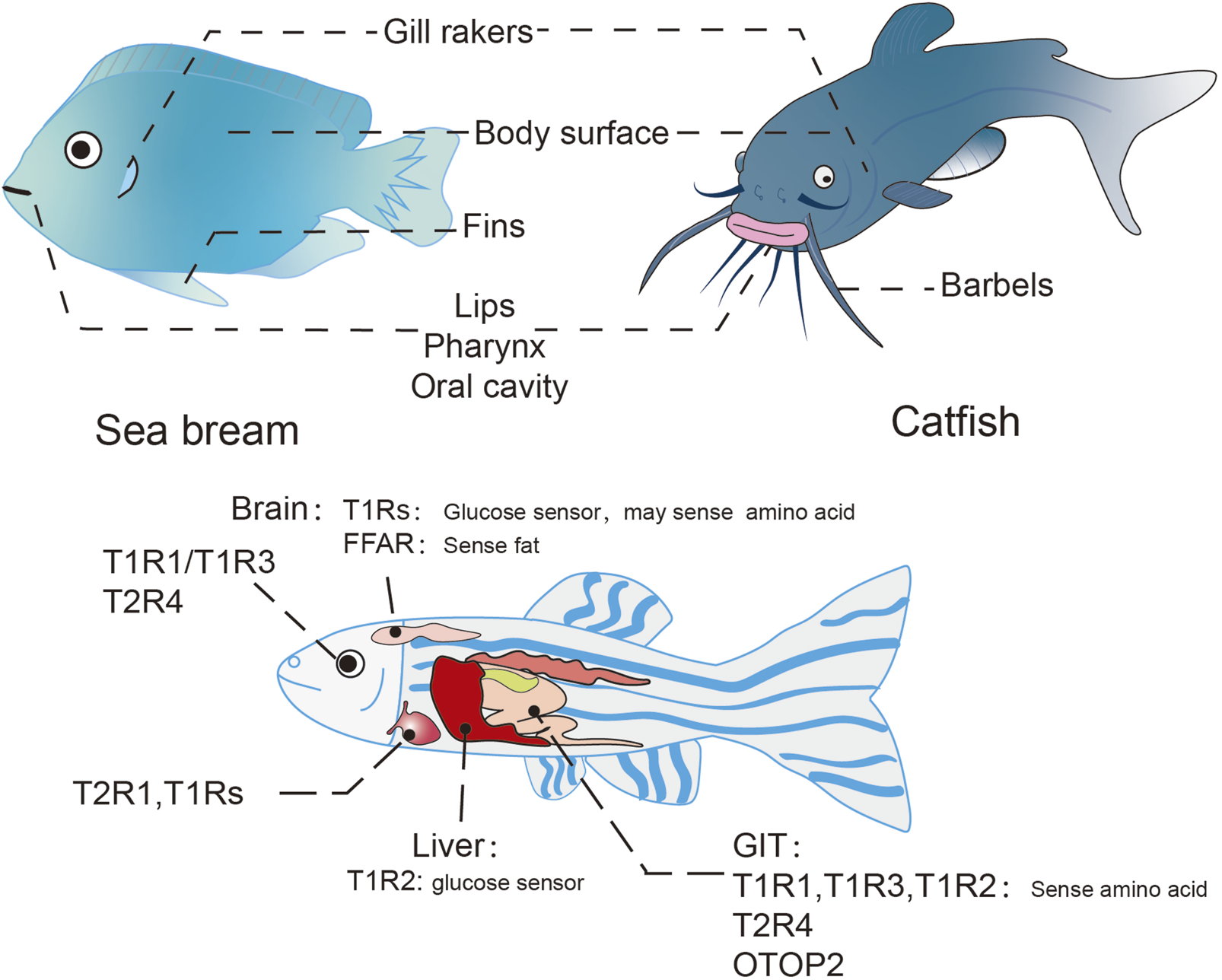
Taste organ and ectopically expressed taste receptors in teleosts. Distribution of taste organs in teleost (above), taking the sea bream and catfish for example; Ectopic taste receptors in teleost (below), taking the zebrafish for example.
Marine animals and terrestrial animals differ in the mechanism of the “tasting” behavior. For terrestrial animals, tasting typically involves the direct contact with substances like sugar or amino acids which are non-volatile and water-soluble. In contrast, marine animals, immersed in an aqueous environment, can perceive those substances from a distance, in a manner functionally analogous to olfaction in terrestrial animals. Conversely, certain volatile, water-insoluble substances that are perceived through smell on land can be perceived by marine animals through direct contact, similar to the “taste” mechanism in terrestrials (Mollo et al., 2014; Giordano et al., 2017; Mollo et al., 2022). Therefore, when studying the “taste” receptors of animals (especially marine animals), one should not be confined to the traditional binary classification framework of “smell-taste”. An open research perspective should be maintained to avoid ignoring potential ligand substances.
3.1 The taste receptors in teleosts
Comparative genomic analyses reveal that piscine T1R and T2R taste receptors exhibit sequence homology with their mammalian orthologs. T1R1 and T1R3 demonstrate high evolutionary conservation across fishes and mammals, whereas fish T1R2 shows only weak homology to the mammalian T1R2 genes (Okada, 2015; Ishimaru et al., 2005). While vertebrates T1R1/T1R3 heterodimer typically function as umami receptors and T1R2/T1R3 as sweet receptors (Yarmolinsky et al., 2009; Yuan X. C. et al., 2020), teleost fishes exhibit a distinctive adaptation: both T1R1/T1R3 and multiple T1R2/T1R3 variants serve as amino acid chemoreceptors. The evolutionary advantage of maintaining multiple amino acid taste receptors remains unclear, though it may reflect an adaptive strategy to diverse dietary niches, given the critical role of amino acids in energy metabolism for both carnivorous and herbivorous species (Calo et al., 2021).
The number of T2R genes among vertebrates exhibits significant variability; for instance, most fish possess merely 2 ∼ 5 T2Rs, whereas mammals typically have approximately 20 ∼ 50 T2R genes (Dong et al., 2009). This diversity arises through dynamic birth-and-death evolutionary processes that generate both lineage-specific functional subtypes and nonfunctional pseudogenes (Nei and Rooney, 2005). The bitter taste system is widely hypothesized to have evolved as a chemosensory defense mechanism, with T2R gene family expansion patterns showing strong correlation with ecological niche specialization and dietary adaptation. The remarkable expansion observed in certain lineages likely reflects the selective pressures imposed by complex phytochemical environments (Dong et al., 2009; Shi and Zhang, 2006). The substantial interspecies variation in the repertoire and diversity of T2R receptors results in marked differences in both the recognition spectrum and sensitivity to bitter compounds among species. A substance perceived as bitter by humans does not necessarily elicit similar responses in other species, as this depends on the presence of specific T2R receptors capable of detecting the compound. For instance, certain substances classified as ‘extremely bitter’ by human standards fail to evoke significant avoidance behaviors in fish, indicating that bitter substances have specificity for different species (Shi and Zhang, 2006; Oike et al., 2007; Meyerhof et al., 2010; Kasumyan and Døving, 2003).
Fish also possess specialized sensory mechanisms including acid-sensing ion channels (ASICs) and transient receptor potential (TRP) channels (Montalbano et al., 2021; Levanti et al., 2016). As aquatic organisms, osmoregulation presents unique challenges, particularly for freshwater species. Intriguingly, while mammals utilize ENaC for salt detection, zebrafish and other teleosts appear to have evolved alternative salt-sensing pathways, potentially involving olfactory integration, given their apparent lack of ENaC-mediated salt taste transduction (Herrera et al., 2021).
Phylogenetic comparison of taste receptor evolution provides valuable insights into the adaptive logic of sensory system diversification. However, significant gaps remain in our understanding of ectopic taste receptor evolution, particularly regarding the conservation of non-gustatory functions across species. Further investigation of these receptors may uncover fundamental principles governing the functional diversification of chemosensory systems during vertebrate evolution.
3.2 Distribution and function of ectopic taste receptors in teleosts
Emerging evidence demonstrates widespread expression of taste receptors in non-gustatory tissues of teleost fishes, including the gastrointestinal tract, visual system, cardiovascular organs, and hepatic tissues (Yuan X. C. et al., 2020; Xia et al., 2020; Yanan et al., 2017) (Figure 5). However, these ectopic chemosensory receptors remain poorly characterized across the phylogenetic diversity of fish species, warranting systematic investigation of their spatiotemporal expression patterns and functional divergence.
3.2.1 Gastrointestinal tract
Comparative analyses reveal distinct organizational principles of taste receptor distribution in the teleost gastrointestinal tract compared to mammalian systems. In rainbow trout, T1R1 exhibits pan-enteric expression, while two T1R2 paralogs show segment-specific enrichment, demonstrating elevated expression in gastric mucosa and distal intestinal regions. Conversely, T1R3 maintains constitutive but low-level expression throughout the gastrointestinal epithelium (Calo et al., 2021). Another study in snapper reflected a significantly higher distribution of T1R3 in the midgut and hindgut segments (Angotzi et al., 2022), suggesting conserved yet species-specific patterns of amino acid chemoreception along the enterohepatic axis. These observations support a model of compartmentalized nutrient sensing, where persistent luminal amino acid detection in distal segments may compensate for incomplete digestive processes. In situ hybridization analyses reveal both autonomous and enteroendocrine cell (EEC)-associated localization of T1Rs in snapper, suggesting that T1Rs-mediated chemosensing may occur in these tissues through an endocrine dependent and independent mechanism (Angotzi et al., 2022).
Other receptors, such as the acid-sensing receptor OTOP2 also express in myenteric neuronal subsets of the enteric nervous system as well as in EECs (Levanti et al., 2011), suggesting integrated chemosensory-neuroendocrine circuits in teleost gut physiology.
3.2.2 Brain
Multiple T1R genes have been identified as being expressed in the brain of the snapper, with T1R2d showing significantly high levels of expression in both the forebrain and hindbrain compared to other T1R genes, and T1R2b being markedly elevated in the midbrain (Angotzi et al., 2022). T1Rs have been found to be involved in nutrient perception in the brain, in which the hypothalamus plays an important role (Soengas et al., 2024). In rainbow trout (Otero-Rodiño et al., 2015) and mammals (Ren et al., 2009), the exposure of the hypothalamus to glucose resulted in decreased expression of T1R2/T1R3, suggesting a potential role in glucose sensing. Contrastingly, another study revealed that glucose stimulation in the telencephalon, a part of the forebrain in snapper, resulted in an upregulation of T1R2/T1R3 expression. This discrepancy implies that taste receptors in the telencephalon could be engaged in glucose-dependent processes that function as a reward mechanism (Cristina et al., 2018). Studies into amino acid sensing pathways in rainbow trout (Comesaña et al., 2017; Comesaña et al. 2020; Comesaña et al. 2024) indicates an active amino acid sensing mechanism within the hypothalamus, potentially involving umami receptors, and demonstrates that amino acid stimulation in the ventricle can affect both central and peripheral metabolic processes. Furthermore, adipose receptors FFA1 and FFA4, also expressed in the hypothalamus, are implicated in fat sensing and participating in the regulation of food intake (Velasco et al., 2020). Additional chemosensory components, including OTOP channels, are broadly expressed throughout the central nervous system, though their precise functional contributions remain to be elucidated (Paukert et al., 2004).
3.2.3 Other organs and systems
In rainbow trout, T1Rs and T2R4 have been identified in the mucosal tissues of various regions, including the eye mucosa; however, the function of taste receptors in ocular tissue remains undetermined (Xia et al., 2020). T1Rs have also been detected in the liver of grass carp (Yuan X. C. et al., 2020), with in vitro studies on rainbow trout liver suggesting that the sweet taste receptor may function as glucose sensor (Otero-Rodiño et al., 2016). Cardiac tissues likewise exhibit T1R expression, indicating possible chemosensory functions in cardiovascular regulation that await mechanistic characterization (Roy et al., 2022).
Research on the common carp (Toshiki et al., 2021) identified three non-gustatory T2R isoforms, ccT2R200-2, ccT2R201, and ccT2R203-1, that respond to bitter compounds, implying novel chemosensory roles in systemic physiology. Nonetheless, the exact in vivo distribution and functions of these genes require further investigation. In zebrafish, RL-TGR, an aversive taste co-receptor, has been detected in the mechanosensory cells of neuromasts within the lateral line. Disruption of RL-TGR activity adversely affects normal lateral line development (Mojib et al., 2017). Previously, it has been shown that the chemokine receptor Cxcr4, which is mediated by GPCR signaling, plays a crucial role in the primordial cell migration in the lateral line (Yu et al., 2012; Dambly-Chaudière et al., 2007). Therefore, RL-TGR, alongside unidentified GPCRs, may significantly impacts the development of the lateral line system.
4 Taste perception in Drosophila
Drosophila melanogaster exhibits a taste perception spectrum comparable to that of mammals, capable of detecting the five canonical taste modalities (Yarmolinsky et al., 2009; Liman et al., 2014) alongside alkaline (Mi et al., 2023), lipid (Tauber et al., 2017), and heavy metal taste sensations (Li X, et al., 2023). Notably, Drosophila demonstrates exceptional gustatory sensitivity, with behavior studies revealing sucrose detection thresholds as low as 0.5 mM - an order of magnitude more sensitive than human perception (5 mM threshold). Furthermore, flies can detect B vitamins at nanomolar concentrations (10 nM) (Wu et al., 2021; Weiffenbach et al., 1982). Despite the similarities in detectable taste types between flies and mammals, their peripheral neural architectures differ fundamentally: mammalian taste buds contain gustatory receptor neurons (GRNs) capable of responding to all taste categories (Liman et al., 2014), whereas Drosophila employs distributed sensory processing through distinct GRN combinations across spatially segregated taste bristles (Makoto et al., 2002).
4.1 An overview of taste organs in Drosophila
The taste organs of Drosophila are distributed across multiple body parts, with external organs located on the proboscis, the anterior margins of the wings, the distal five segments of the legs, and the female ovipositor (Figure 6A). Complementing these external structures, three internal pharyngeal taste organs are present: the labral sense organ (LSO), the ventral cibarial sense organ (VCSO), and the dorsal cibarial sense organ (DCSO) (Chen and Dahanukar, 2020; Liman et al., 2014; Stocker, 1994; Chen and Dahanukar, 2017). In adult Drosophila, the proboscis, analogous to the mammalian tongue, terminates in two fused labella serving as an external tasting apparatus. Each labellum hosts 31 taste bristles, categorized by morphology into S-type (short), I-type (intermediate), and L-type (long). L-type bristles, distinguished by their length, predominantly sense attractive flavors, lacking neurons responsive to bitter taste. In contrast, I-type bristles comprise neurons for sweet and bitter tastes, while S-type encompass four neuronal types: A: responsive to sugar and low sodium concentrations; B, reacting to aversive chemicals such as bitter compounds; C, detecting water; and D, responding to calcium and elevated cation concentrations. Approximately 30 taste pegs are situated on the inner surfaces of each labial palp (Figure 6B), with about six dedicated to sugar detection and the remainder responsive to CO2(Jaeger et al., 2018; Singh, 1997; Meunier et al., 2003; Yarmolinsky et al., 2009).
FIGURE 6
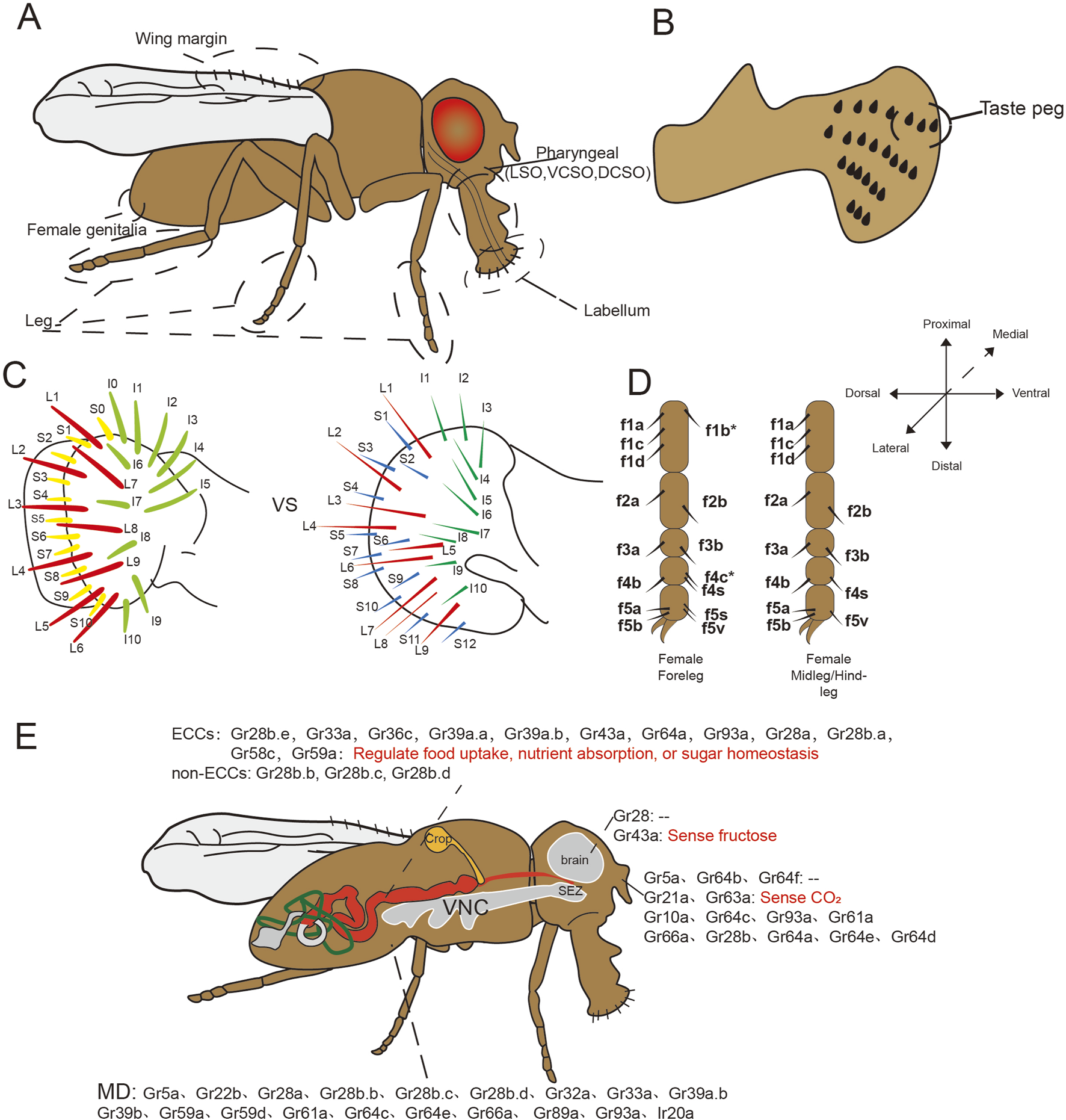
Schematic diagram of taste organs and ectopic expression of taste receptors in Drosophila. (A–D) The taste organs of Drosophila include the labellum, legs, wing margins, and even the female genitalia as external taste organs, the pharynx as an internal taste organ; (C) Comparison of the two distribution pattern of Drosophila labellar bristles in Canton S strain, the left side (Linnea et al., 2011) has one less S-type bristle and one more L-type bristle compared with the right side (Makoto et al., 2002); (E) Ectopic expression and functions of taste receptors in fruit flies. SEZ, subesophageal zone; VNC, ventral nerve cord; MD, multidendritic neurons.
Notably, interstrain variation exists in labellar bristle number, with the Canton S strain consistently exhibiting 31 bristles per labellum. However, two distinct bristle distribution patterns have been documented even within this strain, one pattern displays an additional S-type bristle and one less I-type bristle compared to the other (Figure 6C) (Isono and Kikuchi, 1974; Raphael et al., 1976; Krishanu et al., 1993; Shubha et al., 2001; Makoto et al., 2002; Linnea et al., 2011).
In neurophysiological analyses of Drosophila leg taste bristles, the female foreleg is typically used as a representative model due to its heightened density of sensilla, including unique f5s sensilla absent from other legs (Shubha et al., 1983; Ling et al., 2014; Kopp and Barmina, 2022) (Figure 6D). These sensilla facilitate gustatory engagement as fruit flies often utilize their forelegs for tasting. Relative to other legs, more foreleg sensilla respond to sugars, and these sensilla exhibit heightened sensitivity to bitter compounds (Ling et al., 2014). Despite these findings, comparative analyses of receptor expression across different legs remain limited, with available data primarily derived from taxa such as nymphalid butterflies and the blowfly Phormia regina (Ômura et al., 2011; Dethier and Vincent, 1976). The anatomical positioning of mid and hindlegs presents challenges to electrophysiological analysis. This knowledge gap presents intriguing questions regarding potential leg-specific taste processing, including differential sensitivity to identical stimuli across appendages or integration of conflicting foreleg/midleg inputs. Furthermore, the functional characterization of other gustatory structures including wing, genital, and pharyngeal organs remains incomplete, representing promising avenues for future research (Chen and Dahanukar, 2020).
4.2 The taste receptor in Drosophila
The taste receptors of Drosophila primarily consist of the gustatory receptor (GR) family, which comprises 68 known seven-transmembrane proteins (Arntsen et al., 2024). Initially believed to be exclusive to invertebrates, recent evidence suggests that these receptors originated from a common eukaryotic ancestor. Moreover, two additional lineages have been identified, demonstrating that its superfamily has not been completely lost in chordates (Benton and Himmel, 2023). Sweet taste receptors, such as Gr64f, are primarily responsible for detecting attractive substances like sucrose, while bitter taste receptors, exemplified by Gr66a, perceive aversive substances including caffeine. Other key taste receptor families include the Ionotropic receptor (Irs), which detect Na+, fatty acid, amino acid, and heavy metal ions; the pickpocket (ppk) receptors, which respond to water (ppk28) and pheromone (ppk23); and Transient receptor potential (Trp) receptors, implicated in sensing noxious or pungent compounds (Chen and Dahanukar, 2020; Shrestha and Lee, 2023; Freeman and Dahanukar, 2015). Irs, which are critical and ubiquitously expressed in fruit flies, share structural characteristics with synaptic, glutamate-gated ion channels, featuring three transmembrane domains and a bilobed extracellular binding domain (Arntsen et al., 2024) (Figure 2).
In recent years, investigations into the neurophysiological mechanisms underlying gustatory perception in Drosophila have intensified (Freeman and Dahanukar, 2015; Shrestha and Lee, 2023; Chen and Dahanukar, 2020; Montell, 2009). However, the majority of these studies are focused on macronutrients, while limited exploration of taste perception of micronutrients, such as vitamins (Shrestha, Aryal, and Lee, 2023; Wu et al., 2021). Additionally, receptor expression exhibits spatial diversity, providing insights into specific feeding behaviors of fruit flies. Although some receptors are ubiquitously expressed, their functional deployment requires tissue-specific co-receptors, resulting in localized perception (McDowell, Stanley, and Gordon, 2022; Shrestha, Aryal, and Lee, 2023). This compartmentalization likely underlies observed feeding behaviors, with receptor localization patterns potentially reflecting specialized physiological roles (Kim H. et al., 2017).
4.2.1 Ectopic taste receptors in gastrointestinal tract
Taste receptors have been identified in the GIT of insects (Toyam et al., 2023; Mang et al., 2023). Specifically, 12 Grs have been observed in the midgut endocrine cells (ECCs), organized into two distinct subpopulations with differential receptor profiles (Figure 6E). Notably, the sweet taste receptors Gr64a and Gr43a, along with several bitter taste receptors, were found in the ECCs, except for Gr66a and Gr32a, despite their wide expression in bitter GRNs. Furthermore, the co-localization of multiple taste receptors with regulatory peptides such as neuropeptide F (NPF), locustatachykinin (LTK), and diuretic hormone 31 (DH31) suggests that these receptors may play roles in sensing intestinal contents, regulating feeding behavior and gastrointestinal motility, and modulating intestinal osmotic pressure. Beyond ECCs, three taste receptors (Gr28b.b, Gr28b.c, and Gr28b.d) were expressed in non-ECCs of the midgut (Park and Kwon, 2011a). Gr28b.c is implicated in responding to various noxious stimuli in gustatory organs (Ahn and Amrein, 2023; Sang, Rimal, and Lee, 2019), while Gr28b.d is associated with heat sensing in the antennae (Capek et al., 2025), indicating potential non-nutritional sensory functions in the gut analogous to those in other organs.
4.2.2 Ectopic taste receptors in nervous system
In Drosophila, several Gr28 genes are expressed in multidendritic neurons located in the abdomen, the putative hygroreceptor neurons of the arista, neurons linked with Johnston’s organ, peripheral proprioceptive neurons in the legs, and neurons within the brain of both larval and adult stages, as well as oenocytes. These GR genes are implicated in non-gustatory roles, including proprioception and hygroreception (Thorne and Amrein, 2008). Additionally, other studies further identified 18 GR genes and Ionotropic receptor gene Ir20a expressed in multidendritic neurons in the abdomen (Park and Kwon, 2011b; Koh et al., 2014).
Gr64a and Gr43a are expressed in the brain (Fujii et al., 2015), with Gr43a, as a fructose receptor, facilitating post-ingestion fructose perception in the brain (Miyamoto et al., 2012). Gr43a was also located in larval foregut neurons (Mishra et al., 2013), and its central activation may be mediated by the neuropeptide Corazonin (Miyamoto and Amrein, 2014). Importantly, synaptic connections also exist between enteric sNPF neurons and Gr43a neurons within the hypocerebral ganglion (HCG) in the foregut. Enteric sNPF neurons regulates the balance of sugar and yeast intake by modulating the neuronal activity of HCG Gr43a neurons (Yoshinari et al., 2024).
4.2.3 Ectopic taste receptors in other organs and systems
Fourteen GRs exhibit olfactory system expression, including three sugar receptors (Gr5a, Gr64b, Gr64f), which potentially forming sugar receptors for gustatory functions or interacting with olfactory receptors to manifest novel functions (Fujii et al., 2015; Menuz et al., 2014). Most IRs also function as olfactory receptors within the fruit fly’s olfactory system, indicating the blurred boundaries of the insect chemosensation. Within the reproductive system, Gr43a expression has been observed in the uterus of Drosophila (Miyamoto and Amrein, 2014), while another study did not observe the expression of taste receptors in reproductive organs, but found the expression on the neurons innervating both male and female reproductive organs (Park and Kwon, 2011b).Additionally, flies with Gr28b mutations exhibit immunodeficiency, possibly due to altered feeding behavior impacting immune function in vitro, or a direct role in vivo immune responses (Ayres et al., 2008). In other Lepidopteran insects, taste receptors are expressed in the fat body of Manduca sexta (Koenig et al., 2015) and the Malpighian tubules of silkworm (Mang et al., 2016), though research on these expressions in Drosophila remains insufficient. Finally, it is crucial to note that current expression maps primarily derive from GAL4/UAS reporter systems, which may underestimate weakly expressing populations due to sensitivity thresholds and nonspecific background signals.
5 Taste and taste receptors in C. elegans
The nematode Caenorhabditis elegans possesses a fixed somatic cell count that differentiates into diverse tissues. The majority of its neurons are clustered near the pharynx, where their axonal projections coalesce into a circumpharyngeal nerve ring, functioning as the primary integrative neuropil (Hukema, 2006). Through laser ablation studies, five classes of gustatory neurons have been identified, each characterized by bilaterally symmetric pairs, with ASE neurons exhibiting the highest correlation (Etchberger et al., 2007) (Figure 7). Earlier studies have found that C. elegans can sense many water-soluble attractants, including salt, cAMP, amino acids and serotonin, mainly through ASE (Bargmann and Horvitz, 1991; Ward, 1973).
FIGURE 7
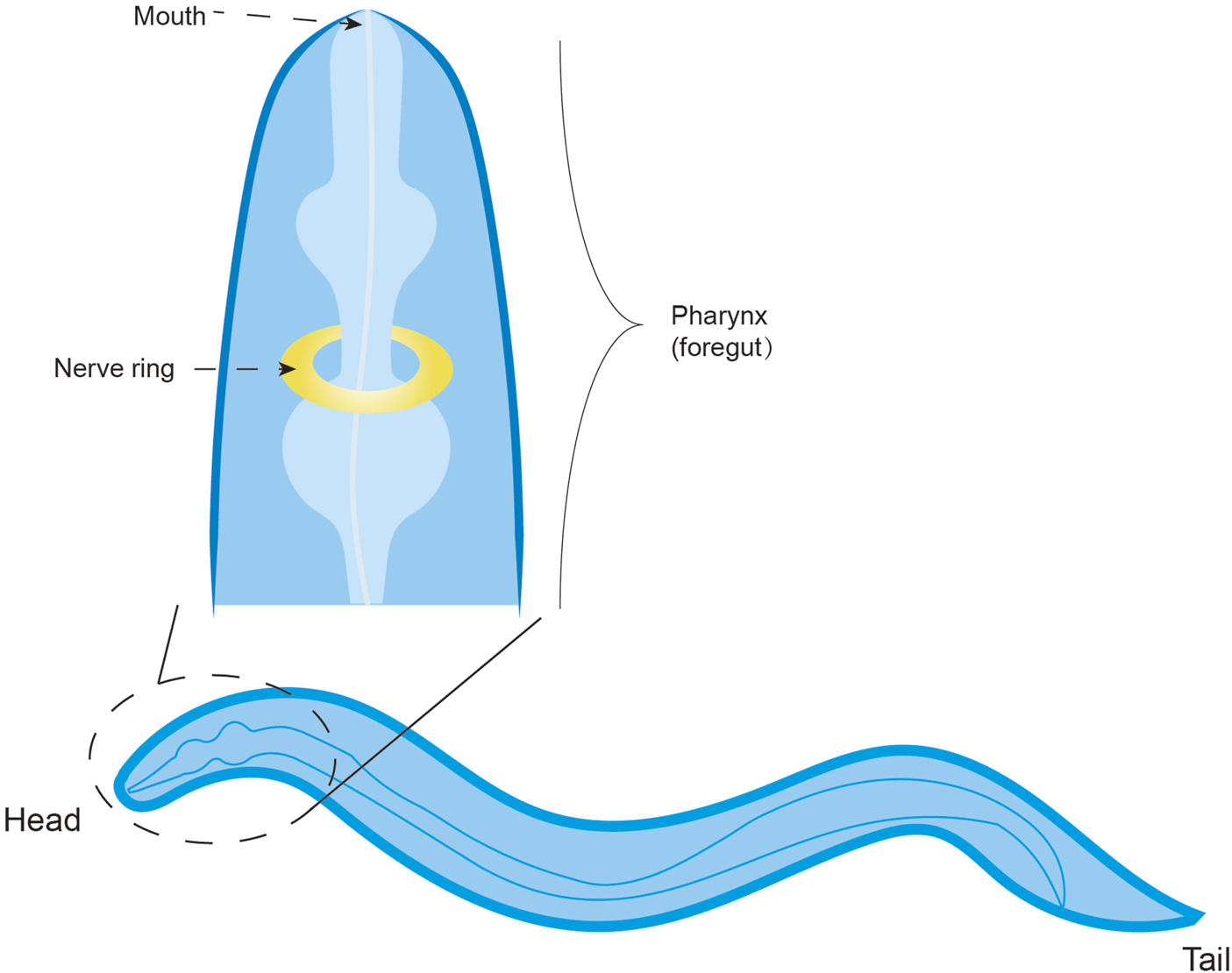
The taste system of C. elegans.
C. elegans expresses a repertoire of 1,341 genes encoding for chemosensory GPCRs, which display a partially overlapping and intersecting expression pattern within the sensory neurons responsible for gustatory perception (detection of water-soluble cues) and olfactory perception (detection of volatile cues) (Thomas and Robertson, 2008; Ferkey et al., 2021; Bargmann, 2006). Notably, these putative receptors are distributed not only within chemosensory organs but also across diverse interneurons, motor neurons, and non-neuronal cell types, suggesting their potential roles in detecting both environmental and internal cues (Vidal et al., 2018). However, only 244 of these genes have been functionally characterized. Given that some chemosensory receptors in C. elegans are not fully functionally differentiated between taste and smell, certain receptors may participate in the detection of both volatile odorants and soluble compounds. Moreover, some molecules, such as the receptor-type guanylyl cyclases, also act as taste receptors (Ortiz et al., 2009). Of particular interest is the plasticity of GPCR expression, especially during the dauer diapause stage induced by environmental cues, indicating that GPCR expression patterns may serve as a molecular signatures of the life history.
Additionally, C. elegans expresses the receptor LITE-1, a homolog of the insect seven-transmembrane receptor GR, distinguished by its inverted membrane topology with an intracellular N-terminus. Intriguingly, LITE-1 mediates phototransduction (Ghosh et al., 2021; Gong et al., 2016), expanding the sensory repertoire of this organism. Other chemosensory receptors include OTOP1, implicated in acid detection. C. elegans possesses eight OTOP genes, and single-gene mutants retain acid sensitivity, indicating functional redundancy (Li S, et al., 2023). While OTOP expression is primarily cephalic, transcripts of otpl-1, otpl-2, and otpl-5 have been detected in coelomocytes, warranting further investigation into their tissue-specific roles.
6 Conclusion and prospect
The emerging picture of ectopic taste receptors reveals an intricate sensory network extending far beyond canonical gustation. These molecular sentinels have evolved to serve important roles as systemic regulators of physiological homeostasis. Comparative analyses across species demonstrate both conserved functions (like nutrient sensing) and lineage-specific adaptations (such as osmoregulation in teleosts). The widespread distribution of taste receptors in non-gustatory tissues underscores their importance in fundamental biological processes including metabolism, immunity, and reproduction.
The functional roles of ectopic taste receptors, which deviate from canonical taste functions, in fact challenge the traditional notion of taste receptors as highly specialized, and instead reframe them within the broader context of chemosensation. Indeed, taste receptors in both gustatory and non-gustatory tissues, as well as other classes of chemoreceptors throughout the body, appear to operate via fundamentally similar mechanisms—all mediated physiological processes initiate from ligand-receptor interactions. Consequently, these studies provide strong experimental support for the recently proposed unified chemosensory theory, which elegantly integrates classical taste, olfaction, and chemical senses mediated by various receptors (including taste and olfactory receptors) under a single chemosensory framework without requiring further categorical distinctions (Mollo et al., 2022). This perspective may facilitate moving beyond the classical gustatory paradigm to develop research strategies for ectopic taste receptors that better reflect their fundamental physiological nature.
Beyond redefining the nature of taste receptors within the framework of a unified chemosensory theory, the key challenges in this field remain in elucidating the precise signaling mechanisms and functional consequences of extra-oral taste receptor activation. Future research should prioritize: (1) comprehensive receptor localization mapping using single-cell technologies, (2) structural characterization of receptor-ligand interactions, and (3) development of genetic tools for tissue-specific receptor manipulation. The therapeutic potential of targeting these receptors warrants exploration, particularly for metabolic disorders and immune modulation. Evolutionary studies comparing receptor repertoires across taxa could reveal adaptive patterns in sensory system diversification.
Technological advances in biosensors, organoids, and in vivo imaging will be crucial for probing receptor dynamics in physiological contexts. Integrating these approaches with systems biology may uncover novel receptor networks coordinating whole-body homeostasis. As the field moves beyond descriptive studies, mechanistic investigations should address how receptor signals are integrated with other sensory inputs and endocrine pathways. Ultimately, decoding the “taste receptome” may reshape our understanding of interoception and organism-environment interactions.
Statements
Author contributions
KC: Writing – review and editing, Writing – original draft, Conceptualization. XL: Writing – review and editing. HY: Writing – review and editing. GY: Supervision, Writing – review and editing. QW: Funding acquisition, Conceptualization, Writing – review and editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by National Natural Science Foundation of China (32200383) and Luzhou Science and Technology Program (2024JYJ001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Ahn J. E. Amrein H. (2023). Opposing chemosensory functions of closely related gustatory receptors. Elife12. 10.7554/eLife.89795
2
An S. S. Wang W. C. Koziol-White C. J. Ahn K. Lee D. Y. Kurten R. C. et al (2012). TAS2R activation promotes airway smooth muscle relaxation despite β(2)-adrenergic receptor tachyphylaxis. Am. J. Physiol. Lung Cell Mol. Physiol.303, L304–L311. 10.1152/ajplung.00126.2012
3
Angotzi A. R. Leal E. Puchol S. Cerdá-Reverter J. M. Morais S. (2022). Exploring the potential for an evolutionarily conserved role of the taste 1 receptor gene family in gut sensing mechanisms of fish. Anim. Nutr.11, 293–308. 10.1016/j.aninu.2022.08.010
4
Ansoleaga B. Garcia-Esparcia P. Pinacho R. Haro J. M. Ramos B. Ferrer I. (2015). Decrease in olfactory and taste receptor expression in the dorsolateral prefrontal cortex in chronic schizophrenia. J. Psychiatric Res.60, 109–116. 10.1016/j.jpsychires.2014.09.012
5
Arntsen C. Guillemin J. Audette K. Stanley M. (2024). Tastant-receptor interactions: insights from the fruit fly. Front. Nutr.11, 1394697. 10.3389/fnut.2024.1394697
6
Avau B. Bauters D. Steensels S. Vancleef L. Laermans J. Lesuisse J. et al (2015a). The gustatory signaling pathway and bitter taste receptors affect the development of obesity and adipocyte metabolism in mice. PLoS One10, e0145538. 10.1371/journal.pone.0145538
7
Avau B. Rotondo A. Thijs T. Andrews C. N. Janssen P. Tack J. et al (2015b). Targeting extra-oral bitter taste receptors modulates gastrointestinal motility with effects on satiation. Sci. Rep.5, 15985. 10.1038/srep15985
8
Ayres J. S. Freitag N. Schneider D. S. (2008). Identification of drosophila mutants altering defense of and endurance to Listeria monocytogenes infection. Genetics178, 1807–1815. 10.1534/genetics.107.083782
9
Bargmann C. I. (2006). Chemosensation in C. elegans. WormBook, 1–29.
10
Bargmann C. I. Horvitz H. R. (1991). Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron7, 729–742. 10.1016/0896-6273(91)90276-6
11
Beauchamp G. K. (2019). Basic taste: a perceptual concept. J. Agric. Food Chem.67, 13860–13869. 10.1021/acs.jafc.9b03542
12
Behrens M. Lang T. (2022). Extra-oral taste receptors-function, disease, and perspectives. Front. Nutr.9, 881177. 10.3389/fnut.2022.881177
13
Benford H. Bolborea M. Pollatzek E. Lossow K. Hermans-Borgmeyer I. Liu B. et al (2017). A sweet taste receptor-dependent mechanism of glucosensing in hypothalamic tanycytes. Glia65, 773–789. 10.1002/glia.23125
14
Benton R. Himmel N. J. (2023). Structural screens identify candidate human homologs of insect chemoreceptors and cryptic drosophila gustatory receptor-like proteins. Elife12, e85537. 10.7554/eLife.85537
15
Bhatia V. de Jesus V. C. Shaik F. A. Jaggupilli A. Singh N. Chelikani P. et al (2022). Extraoral expression and characterization of bitter taste receptors in Astyanax mexicanus (Mexican tetra fish). FASEB Bioadv4, 574–584. 10.1096/fba.2022-00032
16
Bloxham C. J. Hulme K. D. Fierro F. Fercher C. Pegg C. L. O'Brien S. L. et al (2024). Cardiac human bitter taste receptors contain naturally occurring variants that alter function. Biochem. Pharmacol.219, 115932. 10.1016/j.bcp.2023.115932
17
Brondel L. Quilliot D. Mouillot T. Khan N. A. Bastable P. Boggio V. et al (2022). Taste of fat and obesity: different hypotheses and our point of view. Nutrients14, 555. 10.3390/nu14030555
18
Buchanan K. L. Rupprecht L. E. Kaelberer M. M. Sahasrabudhe A. Klein M. E. Villalobos J. A. et al (2022). The preference for sugar over sweetener depends on a gut sensor cell. Nat. Neurosci.25, 191–200. 10.1038/s41593-021-00982-7
19
Calo J. Blanco A. M. Comesaña S. Conde-Sieira M. Morais S. Soengas J. L. (2021). First evidence for the presence of amino acid sensing mechanisms in the fish gastrointestinal tract. Sci. Rep.11, 4933. 10.1038/s41598-021-84303-9
20
Cancello R. Micheletto G. Meta D. Lavagno R. Bevilacqua E. Panizzo V. et al (2020). Expanding the role of bitter taste receptor in extra oral tissues: TAS2R38 is expressed in human adipocytes. Adipocyte9, 7–15. 10.1080/21623945.2019.1709253
21
Capek M. Arenas O. M. Alpert M. H. Zaharieva E. E. Méndez-González I. D. Simões J. M. et al (2025). Evolution of temperature preference in flies of the genus drosophila. Nature641, 447–455. 10.1038/s41586-025-08682-z
22
Chandrashekar J. Mueller K. L. Hoon M. A. Adler E. Feng L. Guo W. et al (2000). T2Rs function as bitter taste receptors. Cell100, 703–711. 10.1016/s0092-8674(00)80706-0
23
Chandrashekar J. Hoon M. A. Ryba N. J. Zuker C. S. (2006). The receptors and cells for mammalian taste. Nature444, 288–294. 10.1038/nature05401
24
Charles S. Z. Nicholas J. P. R. Gregory A. N. Gregory A. N. Mark A. H. Mark A. H. et al (2001). Mammalian sweet taste receptors. Cell106, 381–390. 10.1016/s0092-8674(01)00451-2
25
Chaudhari N. Landin A. M. Roper S. D. (2000). A metabotropic glutamate receptor variant functions as a taste receptor. Nat. Neurosci.3, 113–119. 10.1038/72053
26
Chen Y. D. Dahanukar A. (2017). Molecular and cellular organization of taste neurons in adult drosophila pharynx. Cell Rep.21, 2978–2991. 10.1016/j.celrep.2017.11.041
27
Chen Y. D. Dahanukar A. (2020). Recent advances in the genetic basis of taste detection in drosophila. Cell Mol. Life Sci.77, 1087–1101. 10.1007/s00018-019-03320-0
28
Chen X. Gabitto M. Peng Y. Ryba N. J. Zuker C. S. (2011). A gustotopic map of taste qualities in the mammalian brain. Science333, 1262–1266. 10.1126/science.1204076
29
Chen J.-G. Ping N.-N. Liang D. Li M.-Y. Mi Y.-N. Li S. et al (2017). The expression of bitter taste receptors in mesenteric, cerebral and omental arteries. Life Sci.170, 16–24. 10.1016/j.lfs.2016.11.010
30
Clark A. A. Dotson C. D. Elson A. E. Voigt A. Boehm U. Meyerhof W. et al (2015). TAS2R bitter taste receptors regulate thyroid function. Faseb J.29, 164–172. 10.1096/fj.14-262246
31
Comesaña S. Velasco C. Ceinos R. M. López-Patiño M. A. Míguez J. M. Morais S. et al (2017). Evidence for the presence in rainbow trout brain of amino acid-sensing systems involved in the control of food intake. Am. J. Physiology-Regulatory, Integr. Comp. Physiology314, R201–R215. 10.1152/ajpregu.00283.2017
32
Comesaña S. Conde-Sieira M. Velasco C. Soengas J. L. Morais S. (2020). Oral and pre-absorptive sensing of amino acids relates to hypothalamic control of food intake in rainbow trout. J. Exp. Biol.223, jeb221721. 10.1242/jeb.221721
33
Comesaña S. Antomagesh F. Soengas J. L. Blanco A. M. Vijayan M. M. (2024). Valine administration in the hypothalamus alters the brain and plasma metabolome in rainbow trout. Am. J. Physiology-Regulatory, Integr. Comp. Physiology327, R261–R273. 10.1152/ajpregu.00056.2024
34
Cristina O.-R. Cristina O.-R. Ana R. Ana R. Rosa Á.-O. Rosa Á.-O. et al (2018). Glucosensing capacity of rainbow trout telencephalon. J. Neuroendocrinol. 10.1111/jne.12583
35
Daly K. Al-Rammahi M. Moran A. Marcello M. Ninomiya Y. Shirazi-Beechey S. P. (2013). Sensing of amino acids by the gut-expressed taste receptor T1R1-T1R3 stimulates CCK secretion. Am. J. Physiol. Gastrointest. Liver Physiol.304, G271–G282. 10.1152/ajpgi.00074.2012
36
Dambly-Chaudière C. Cubedo N. Ghysen A. (2007). Control of cell migration in the development of the posterior lateral line: antagonistic interactions between the chemokine receptors CXCR4 and CXCR7/RDC1. BMC Dev. Biol.7, 23. 10.1186/1471-213X-7-23
37
Delpapa E. H. Simas T. M. ZhuGe R. Qu M. (2023). Bitter taste receptor TAS2R5 agonist phenanthroline as a broad-spectrum uterine relaxant. Am. J. Obstetrics and Gynecol.228, S25–S26. 10.1016/j.ajog.2022.11.036
38
Descamps-Solà M. Vilalta A. Jalsevac F. Blay M. T. Rodríguez-Gallego E. Pinent M. et al (2023). Bitter taste receptors along the gastrointestinal tract: comparison between humans and rodents. Front. Nutr.10, 1215889. 10.3389/fnut.2023.1215889
39
Deshpande D. A. Wang W. C. McIlmoyle E. L. Robinett K. S. Schillinger R. M. An S. S. et al (2010). Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat. Med.16, 1299–1304. 10.1038/nm.2237
40
Deshpande D. A. Robinett K. S. Wang W. C. H. Sham J. S. K. An S. S. Liggett S. B. (2011). Bronchodilator activity of bitter tastants in human tissue. Nat. Med.17, 776–778. 10.1038/nm0711-776a
41
Dethier V. G. Vincent G. D. (1976). The hungry fly: a physiological study of the behavior associated with feeding.
42
Di Pizio A. Niv M. Y. (2015). Promiscuity and selectivity of bitter molecules and their receptors. Bioorg. and Med. Chem.23, 4082–4091. 10.1016/j.bmc.2015.04.025
43
Dong D. Jones G. Zhang S. (2009). Dynamic evolution of bitter taste receptor genes in vertebrates. BMC Evol. Biol.9, 12. 10.1186/1471-2148-9-12
44
Doyle M. E. Premathilake H. U. Yao Q. Mazucanti C. H. Egan J. M. (2023). Physiology of the tongue with emphasis on taste transduction. Physiol. Rev.103, 1193–1246. 10.1152/physrev.00012.2022
45
Duarte A. C. Santos J. Costa A. R. Ferreira C. L. Tomás J. Quintela T. et al (2020). Bitter taste receptors profiling in the human blood-cerebrospinal fluid-barrier. Biochem. Pharmacol.177, 113954. 10.1016/j.bcp.2020.113954
46
Dyer J. Salmon K. S. H. Zibrik L. Shirazi-Beechey S. P. (2005). Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem. Soc. Trans.33, 302–305. 10.1042/BST0330302
47
Elliott R. A. Kapoor S. Tincello D. G. (2011). Expression and distribution of the sweet taste receptor isoforms T1R2 and T1R3 in human and rat bladders. J. Urol.186, 2455–2462. 10.1016/j.juro.2011.07.083
48
Enuwosa E. Gautam L. King L. Chichger H. (2021). Saccharin and sucralose protect the glomerular microvasculature in vitro against VEGF-induced permeability. Nutrients13, 2746. 10.3390/nu13082746
49
Etchberger J. F. Lorch A. Sleumer M. C. Zapf R. Jones S. J. Marra M. A. et al (2007). The molecular signature and cis-regulatory architecture of a C. elegans gustatory neuron. Genes Dev.21, 1653–1674. 10.1101/gad.1560107
50
Ferkey D. M. Sengupta P. L'Etoile N. D. (2021). Chemosensory signal transduction in Caenorhabditis elegans. Genetics217, iyab004. 10.1093/genetics/iyab004
51
Fontanini A. (2023). Taste. Curr. Biol.33, R130–R135. 10.1016/j.cub.2023.01.005
52
Foster S. R. Porrello E. R. Purdue B. Chan H. W. Voigt A. Frenzel S. et al (2013). Expression, regulation and putative nutrient-sensing function of taste GPCRs in the heart. PLoS One8, e64579. 10.1371/journal.pone.0064579
53
Foster S. R. Blank K. Hoe L. E. S. Behrens M. Meyerhof W. Peart J. N. et al (2014). Bitter taste receptor agonists elicit G-protein-dependent negative inotropy in the murine heart. FASEB J.28, 4497–4508. 10.1096/fj.14-256305
54
Foster S. R. Roura E. Molenaar P. Thomas W. G. (2015). G protein-coupled receptors in cardiac biology: old and new receptors. Biophys. Rev.7, 77–89. 10.1007/s12551-014-0154-2
55
Freeman E. G. Dahanukar A. (2015). Molecular neurobiology of Drosophila taste. Curr. Opin. Neurobiol.34, 140–148. 10.1016/j.conb.2015.06.001
56
Frolikova M. Otcenaskova T. Valasková E. Postlerova P. Stopkova R. Stopka P. et al (2020). The role of taste receptor mTAS1R3 in chemical communication of gametes. Int. J. Mol. Sci.21, 2651. 10.3390/ijms21072651
57
Fujii S. Yavuz A. Slone J. Jagge C. Song X. Amrein H. (2015). Drosophila sugar receptors in sweet taste perception, olfaction, and internal nutrient sensing. Curr. Biol.25, 621–627. 10.1016/j.cub.2014.12.058
58
Gerbe F. Sidot E. Smyth D. J. Ohmoto M. Matsumoto I. Dardalhon V. et al (2016). Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature529, 226–230. 10.1038/nature16527
59
Gherardini J. Rouillé T. Stone R. C. Fehrholz M. Funk W. Rodríguez-Feliz J. et al (2025). Human scalp hair follicles can ‘taste’: chemosensory signalling via the bitter taste receptor TAS2R4 inhibits hair growth ex vivo. Br. J. Dermatology, ljaf060. 10.1093/bjd/ljaf060
60
Ghosh D. D. Lee D. Jin X. Horvitz H. R. Nitabach M. N. (2021). C. elegans discriminates colors to guide foraging. Science371, 1059–1063. 10.1126/science.abd3010
61
Giaquinto D. Fonsatti E. Bortoletti M. Radaelli G. De Felice E. de Girolamo P. et al (2024). Olfactory and gustatory chemical sensor systems in the African turquoise killifish: insights from morphology. Cell Tissue Res.398, 239–252. 10.1007/s00441-024-03923-5
62
Giordano G. Carbone M. Ciavatta M. L. Silvano E. Gavagnin M. Garson M. J. et al (2017). Volatile secondary metabolites as aposematic olfactory signals and defensive weapons in aquatic environments. Proc. Natl. Acad. Sci.114, 3451–3456. 10.1073/pnas.1614655114
63
Gong J. Yuan Y. Ward A. Kang L. Zhang B. Wu Z. et al (2016). The C. elegans taste receptor homolog LITE-1 is a photoreceptor. Cell167, 1252–1263. 10.1016/j.cell.2016.10.053
64
Gopallawa I. Freund J. R. Lee R. J. (2021). Bitter taste receptors stimulate phagocytosis in human macrophages through calcium, nitric oxide, and cyclic-GMP signaling. Cell Mol. Life Sci.78, 271–286. 10.1007/s00018-020-03494-y
65
Governini L. Semplici B. Pavone V. Crifasi L. Marrocco C. De Leo V. et al (2020). Expression of taste receptor 2 subtypes in human testis and sperm. J. Clin. Med.9, 264. 10.3390/jcm9010264
66
Grace Z. Grace Q. Z. Yifeng Z. Yifeng Z. Mrinalini H. Mark A. H. et al (2003). The receptors for mammalian sweet and umami taste. Cell115, 255–266. 10.1016/s0092-8674(03)00844-4
67
Grassin-Delyle S. Abrial C. Fayad-Kobeissi S. Brollo M. Faisy C. Alvarez J. C. et al (2013). The expression and relaxant effect of bitter taste receptors in human bronchi. Respir. Res.14, 134. 10.1186/1465-9921-14-134
68
Hardy A. R. Hale M. E. (2022). Extraoral taste buds on the paired fins of damselfishes. Integr. Org. Biol.4, obac035. 10.1093/iob/obac035
69
Harwood M. L. Ziegler G. R. Hayes J. E. (2012). Rejection thresholds in chocolate milk: evidence for segmentation. Food Qual. Prefer26, 128–133. 10.1016/j.foodqual.2012.04.009
70
He W. Liang L. Zhang Y. (2023). Pungency perception and the interaction with basic taste sensations: an overview. Foods12, 2317. 10.3390/foods12122317
71
Herrera K. J. Panier T. Guggiana-Nilo D. Engert F. (2021). Larval zebrafish use olfactory detection of sodium and chloride to avoid salt water. Curr. Biol.31, 782–793.e3. 10.1016/j.cub.2020.11.051
72
Herrera L. P. T. Andreassen S. N. Caroli J. Rodríguez-Espigares I. Kermani A. A. Keserű G. M. et al (2025). GPCRdb in 2025: adding odorant receptors, data mapper, structure similarity search and models of physiological ligand complexes. Nucleic Acids Res.53, D425–D435. 10.1093/nar/gkae1065
73
Hoon M. A. Adler E. Lindemeier J. Battey J. F. Ryba N. J. P. Zuker C. S. (1999). Putative mammalian taste receptors: a class of taste-specific GPCRs with distinct topographic selectivity. Cell96, 541–551. 10.1016/s0092-8674(00)80658-3
74
Hu G.-M. Mai T.-L. Chen C.-M. (2017). Visualizing the GPCR network: classification and evolution. Sci. Rep.7, 15495. 10.1038/s41598-017-15707-9
75
Huang A. L. Chen X. Hoon M. A. Chandrashekar J. Guo W. Tränkner D. et al (2006). The cells and logic for mammalian sour taste detection. Nature442, 934–938. 10.1038/nature05084
76
Hukema R. K. (2006). Gustatory behaviour in Caenorhabditis elegans. erasmus university rotterdam.
77
Ishimaru Y. Okada S. Naito H. Nagai T. Yasuoka A. Matsumoto I. et al (2005). Two families of candidate taste receptors in fishes. Mech. Dev.122, 1310–1321. 10.1016/j.mod.2005.07.005
78
Ishimaru Y. Inada H. Kubota M. Zhuang H. Tominaga M. Matsunami H. (2006). Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc. Natl. Acad. Sci. U. S. A.103, 12569–12574. 10.1073/pnas.0602702103
79
Isono K. Kikuchi T. (1974). Behavioral and electrical responses to sugars in Drosophila melanogaster. Jpn. J. Genet.49, 113–124. 10.1266/jjg.49.113
80
Jaeger A. H. Stanley M. Weiss Z. F. Musso P. Y. Chan R. C. Zhang H. et al (2018). A complex peripheral code for salt taste in drosophila. Elife7, e37167. 10.7554/eLife.37167
81
Jalševac F. Descamps-Solà M. Grau-Bové C. Segú H. Auguet T. Avilés-Jurado F. X. et al (2024). Profiling bitter taste receptors (TAS2R) along the gastrointestinal tract and their influence on enterohormone secretion. Gender- and age-related effects in the colon. Front. Endocrinol. (Lausanne)15, 1436580. 10.3389/fendo.2024.1436580
82
Jang J. H. Kim H. K. Seo D. W. Ki S. Y. Park S. Choi S. H. et al (2021). Whole-brain mapping of the expression pattern of T1R2, a subunit specific to the sweet taste receptor. Front. Neuroanat.15, 751839. 10.3389/fnana.2021.751839
83
Janssen S. Laermans J. Verhulst P.-J. Thijs T. Tack J. Depoortere I. (2011). Bitter taste receptors and α-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc. Natl. Acad. Sci.108, 2094–2099. 10.1073/pnas.1011508108
84
Jernigan N. L. Drummond H. A. (2005). Vascular ENaC proteins are required for renal myogenic constriction. Am. J. Physiol. Ren. Physiol.289, F891–F901. 10.1152/ajprenal.00019.2005
85
Jiang J. Qi L. Wei Q. Shi F. (2018). Effects of daily exposure to saccharin sodium and rebaudioside A on the ovarian cycle and steroidogenesis in rats. Reprod. Toxicol.76, 35–45. 10.1016/j.reprotox.2017.12.006
86
Jiang J. Liu S. Qi L. Wei Q. Shi F. (2021). Activation of ovarian taste receptors inhibits progesterone production potentially via NO/cGMP and apoptotic signaling. Endocrinology162, bqaa240. 10.1210/endocr/bqaa240
87
John C. John C. Joseph G. B. Joseph G. B. Joseph G. B. John H. T. et al (1993). The taste system of the channel catfish: from biophysics to behavior. Trends Neurosci.16, 192–197. 10.1016/0166-2236(93)90152-c
88
Jonas T. Jonas C. T. Maik B. Maik B. Wolfgang M. Wolfgang M. (2019). Taste receptor function. Handb. Clin. Neurology164, 173–185. 10.1016/B978-0-444-63855-7.00011-3
89
Jones L. M. Fontanini A. Katz D. B. (2006). Gustatory processing: a dynamic systems approach. Curr. Opin. Neurobiol.16, 420–428. 10.1016/j.conb.2006.06.011
90
Kasumyan A. O. Døving K. B. (2003). Taste preferences in fishes. Fish Fish.4, 289–347. 10.1046/j.1467-2979.2003.00121.x
91
Ki S. Y. Jeong Y. T. (2022). Taste receptors beyond taste buds. Int. J. Mol. Sci.23, 9677. 10.3390/ijms23179677
92
Kidd M. Modlin I. M. Gustafsson B. I. Drozdov I. Hauso O. Pfragner R. (2008). Luminal regulation of normal and neoplastic human EC cell serotonin release is mediated by bile salts, amines, tastants, and olfactants. Am. J. Physiology-Gastrointestinal Liver Physiology295, G260–G272. 10.1152/ajpgi.00056.2008
93
Kidd M. Hauso Ø. Drozdov I. Gustafsson B. I. Modlin I. M. (2009). Delineation of the chemomechanosensory regulation of gastrin secretion using pure rodent G cells. Gastroenterology137, 231–241. 10.1053/j.gastro.2009.01.005
94
Kim E. C. Ahn D. S. Yeon S. I. Lim M. Lee Y. H. (2012). Epithelial Na+ channel proteins are mechanotransducers of myogenic constriction in rat posterior cerebral arteries. Exp. Physiol.97, 544–555. 10.1113/expphysiol.2011.062232
95
Kim H. Jeong Y. T. Choi M. S. Choi J. Moon S. J. Kwon J. Y. (2017). Involvement of a Gr2a-Expressing drosophila pharyngeal gustatory receptor neuron in regulation of aversion to high-salt foods. Mol. Cells40, 331–338. 10.14348/molcells.2017.0028
96
Kim S. K. Chen Y. Abrol R. Goddard W. A. 3rd Guthrie B. (2017). Activation mechanism of the G protein-coupled sweet receptor heterodimer with sweeteners and allosteric agonists. Proc. Natl. Acad. Sci. U. S. A.114, 2568–2573. 10.1073/pnas.1700001114
97
Kimura I. Ichimura A. Ohue-Kitano R. Igarashi M. (2019). Free fatty acid receptors in health and disease. Physiol. Rev.100, 171–210. 10.1152/physrev.00041.2018
98
Koenig C. Hirsh A. Bucks S. Klinner C. Vogel H. Shukla A. et al (2015). A reference gene set for chemosensory receptor genes of Manduca sexta. Insect Biochem. Mol. Biol.66, 51–63. 10.1016/j.ibmb.2015.09.007
99
Koh T. W. He Z. Gorur-Shandilya S. Menuz K. Larter N. K. Stewart S. et al (2014). The drosophila IR20a clade of ionotropic receptors are candidate taste and pheromone receptors. Neuron83, 850–865. 10.1016/j.neuron.2014.07.012
100
Kohno D. Koike M. Ninomiya Y. Kojima I. Kitamura T. Yada T. (2016). Sweet taste receptor serves to activate Glucose- and leptin-responsive neurons in the hypothalamic arcuate nucleus and participates in glucose responsiveness. Front. Neurosci.10, 502. 10.3389/fnins.2016.00502
101
Kok B. P. Galmozzi A. Littlejohn N. K. Albert V. Godio C. Kim W. et al (2018). Intestinal bitter taste receptor activation alters hormone secretion and imparts metabolic benefits. Mol. Metab.16, 76–87. 10.1016/j.molmet.2018.07.013
102
Kopp A. Barmina O. (2022). Interspecific variation in sex-specific gustatory organs in drosophila. J. Comp. Neurol.530, 2439–2450. 10.1002/cne.25340
103
Krishanu R. Krishanu R. Volker H. Volker H. Veronica R. Veronica R. (1993). Development of the taste bristles on the labellum of Drosophila melanogaster. Dev. Biol.155, 26–37. 10.1006/dbio.1993.1003
104
Kurtz R. Steinberg L. G. Betcher M. Fowler D. Shepard B. D. (2020). The sensing liver: localization and ligands for hepatic murine olfactory and taste receptors. Front. Physiology11, 574082. 10.3389/fphys.2020.574082
105
Kurumi Y. Kurumi Y. Yoshiro I. Yoshiro I. (2013). Oral and extra-oral taste perception. Seminars Cell and Dev. Biol.24, 240–246. 10.1016/j.semcdb.2012.08.005
106
Lee N. Jung Y. S. Lee H. Y. Kang N. Park Y. J. Hwang J. S. et al (2014). Mouse neutrophils express functional umami taste receptor T1R1/T1R3. BMB Rep.47, 649–654. 10.5483/bmbrep.2014.47.11.185
107
Lee R. J. Kofonow J. M. Rosen P. L. Siebert A. P. Chen B. Doghramji L. et al (2014). Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J. Clin. Investigation124, 1393–1405. 10.1172/JCI72094
108
Levanti M. B. Guerrera M. C. Calavia M. G. Ciriaco E. Montalbano G. Cobo J. et al (2011). Acid-sensing ion channel 2 (ASIC2) in the intestine of adult zebrafish. Neurosci. Lett.494, 24–28. 10.1016/j.neulet.2011.02.046
109
Levanti M. Randazzo B. Viña E. Montalbano G. Garcia-Suarez O. Germanà A. et al (2016). Acid-sensing ion channels and transient-receptor potential ion channels in zebrafish taste buds. Ann. Anat. - Anatomischer Anzeiger207, 32–37. 10.1016/j.aanat.2016.06.006
110
Li F. Zhou M. (2012). Depletion of bitter taste transduction leads to massive spermatid loss in transgenic mice. Mol. Hum. Reprod.18, 289–297. 10.1093/molehr/gas005
111
Li J. Shen T. Shi F. Fu Y. (2020). Influences of non-nutritive sweeteners on ovarian and uterine expression of T1R2 and T1R3 in peripubertal female Guinea pigs. Animal Sci. J.91, e13348. 10.1111/asj.13348
112
Li C. Li Y. Sun Q. Abdurehim A. Xu J. Xie J. et al (2024). Taste and its receptors in human physiology: a comprehensive look. Food Front.5, 1512–1533. 10.1002/fft2.407
113
Li S. Al-Sheikh U. Chen Y. Kang L. (2023). Nematode homologs of the sour taste receptor Otopetrin1 are evolutionarily conserved acid-sensitive proton channels. Front. Cell Dev. Biol.11, 1133890. 10.3389/fcell.2023.1133890
114
Li X. Sun Y. Gao S. Li Y. Liu L. Zhu Y. (2023). Taste coding of heavy metal ion-induced avoidance in drosophila. iScience26, 106607. 10.1016/j.isci.2023.106607
115
Liman E. R. Zhang Y. V. Montell C. (2014). Peripheral coding of taste. Neuron81, 984–1000. 10.1016/j.neuron.2014.02.022
116
Lindemann B. (2001). Receptors and transduction in taste. Nature413, 219–225. 10.1038/35093032
117
Ling F. Dahanukar A. Weiss L. A. Kwon J. Y. Carlson J. R. (2014). The molecular and cellular basis of taste coding in the legs of drosophila. J. Neurosci.34, 7148–7164. 10.1523/JNEUROSCI.0649-14.2014
118
Linnea A. W. Linnea A. W. Anupama D. Anupama D. Anupama D. Jae Young K. et al (2011). The molecular and cellular basis of bitter taste in drosophila. Neuron69, 258–272. 10.1016/j.neuron.2011.01.001
119
Liszt K. I. Ley J. P. Lieder B. Behrens M. Stöger V. Reiner A. et al (2017). Caffeine induces gastric acid secretion via bitter taste signaling in gastric parietal cells. Proc. Natl. Acad. Sci. U. S. A.114, E6260–e6269. 10.1073/pnas.1703728114
120
Liu D. Costanzo A. Evans M. D. M. Archer N. S. Nowson C. Duesing K. et al (2018). Expression of the candidate fat taste receptors in human fungiform papillae and the association with fat taste function. Br. J. Nutr.120, 64–73. 10.1017/S0007114518001265
121
Liu X. Gu F. Jiang L. Chen F. Li F. (2015). Expression of bitter taste receptor Tas2r105 in mouse kidney. Biochem. Biophys. Res. Commun.458, 733–738. 10.1016/j.bbrc.2015.01.089
122
Liu S. Lu S. Xu R. Atzberger A. Günther S. Wettschureck N. et al (2017). Members of bitter taste receptor cluster Tas2r143/Tas2r135/Tas2r126 are expressed in the epithelium of murine airways and other non-gustatory tissues. Front. Physiol.8, 849. 10.3389/fphys.2017.00849
123
Liu W. Gong T. Shi F. Xu H. Chen X. (2022). Taste receptors affect male reproduction by influencing steroid synthesis. Front. Cell Dev. Biol.10, 956981. 10.3389/fcell.2022.956981
124
Liu W. wang H. Mu Q. Gong T. (2025). Taste receptor T1R3 regulates testosterone synthesis via the cAMP-PKA-SP1 pathway in testicular leydig cells. Theriogenology231, 210–221. 10.1016/j.theriogenology.2024.10.019
125
Liu S. Xu M. Zhu C. Zhao Q. Zhou F. (2018). Taste receptor T1R1/T1R3 promotes the tumoricidal activity of hepatic CD49a+CD49b− natural killer cells. Eur. J. Immunol.48, 2031–2041. 10.1002/eji.201847688
126
Lizunkova P. Enuwosa E. Chichger H. (2019). Activation of the sweet taste receptor T1R3 by sucralose attenuates VEGF-induced vasculogenesis in a cell model of the retinal microvascular endothelium. Graefe's Archive Clin. Exp. Ophthalmol.257, 71–81. 10.1007/s00417-018-4157-8
127
Lu P. Moore Simas T. A. Delpapa E. ZhuGe R. (2024). Bitter taste receptors in the reproductive system: function and therapeutic implications. J. Cell Physiol.239, e31179. 10.1002/jcp.31179
128
Luo M. J. Wang Y. Chen S. Y. Yang Z. M. (2022). Astragalus polysaccharides alleviate type 2 diabetic rats by reversing the expressions of sweet taste receptors and genes related to glycolipid metabolism in liver. Front. Pharmacol.13, 916603. 10.3389/fphar.2022.916603
129
Mace O. J. Lister N. Morgan E. Shepherd E. Affleck J. Helliwell P. et al (2009). An energy supply network of nutrient absorption coordinated by calcium and T1R taste receptors in rat small intestine. J. Physiol.587, 195–210. 10.1113/jphysiol.2008.159616
130
Maik B. Tatjana L. Sigrun I. K. (2023). A singular shark bitter taste receptor provides insights into the evolution of bitter taste perception. Proc. Natl. Acad. Sci. U. S. A.120, e2310347120. 10.1073/pnas.2310347120
131
Makoto H. Makoto H. Frédéric M. P. Frédéric M.-P. Teiichi T. Teiichi T. (2002). Differentiated response to sugars among labellar chemosensilla in Drosophila. Zoological Sci.19, 1009–1018. 10.2108/zsj.19.1009
132
Malki A. Fiedler J. Fricke K. Ballweg I. Pfaffl M. W. Krautwurst D. (2015). Class I odorant receptors, TAS1R and TAS2R taste receptors, are markers for subpopulations of circulating leukocytes. J. Leukoc. Biol.97, 533–545. 10.1189/jlb.2A0714-331RR
133
Mang D. Shu M. Endo H. Yoshizawa Y. Nagata S. Kikuta S. et al (2016). Expression of a sugar clade gustatory receptor, BmGr6, in the oral sensory organs, midgut, and central nervous system of larvae of the silkworm Bombyx mori. Insect Biochem. Mol. Biol.70, 85–98. 10.1016/j.ibmb.2015.12.008
134
Mang D. Toyama T. Yamagishi T. Sun J. Purba E. R. Endo H. et al (2023). Dietary compounds activate an insect gustatory receptor on enteroendocrine cells to elicit myosuppressin secretion. Insect Biochem. Mol. Biol.155, 103927. 10.1016/j.ibmb.2023.103927
135
Manson M. L. Säfholm J. Al-Ameri M. Bergman P. Orre A.-C. Swärd K. et al (2014). Bitter taste receptor agonists mediate relaxation of human and rodent vascular smooth muscle. Eur. J. Pharmacol.740, 302–311. 10.1016/j.ejphar.2014.07.005
136
Martin W. Martin W. (2019). Anatomy and development of the human taste system. Handb. Clin. Neurology164, 147–171. 10.1016/B978-0-444-63855-7.00010-1
137
Martin B. Wang R. Cong W. N. Daimon C. M. Wu W. W. Ni B. et al (2017). Altered learning, memory, and social behavior in type 1 taste receptor subunit 3 knock-out mice are associated with neuronal dysfunction. J. Biol. Chem.292, 11508–11530. 10.1074/jbc.M116.773820
138
Maurer S. Wabnitz G. H. Kahle N. A. Stegmaier S. Prior B. Giese T. et al (2015). Tasting Pseudomonas aeruginosa biofilms: human neutrophils express the bitter receptor T2R38 as sensor for the quorum sensing molecule N-(3-Oxododecanoyl)-l-Homoserine lactone. Front. Immunol.6, 369. 10.3389/fimmu.2015.00369
139
McDowell S. A. T. Stanley M. Gordon M. D. (2022). A molecular mechanism for high salt taste in drosophila. Curr. Biol.32, 3070–3081.e5. 10.1016/j.cub.2022.06.012
140
McMahon D. B. Jolivert J. F. Kuek L. E. Adappa N. D. Palmer J. N. Lee R. J. (2023a). Savory signaling: T1R umami receptor modulates endoplasmic reticulum calcium Store content and release dynamics in airway epithelial cells. Nutrients15, 493. 10.3390/nu15030493
141
McMahon D. B. Jolivert J. F. Kuek L. E. Adappa N. D. Palmer J. N. Lee R. J. (2023b). Utilizing the off-target effects of T1R3 antagonist lactisole to enhance nitric oxide production in basal airway epithelial cells. Nutrients15, 517. 10.3390/nu15030517
142
Melis M. Sollai G. Mastinu M. Pani D. Cosseddu P. Bonfiglio A. et al (2020). Electrophysiological responses from the human tongue to the six taste qualities and their relationships with PROP taster status. Nutrients12, 2017. 10.3390/nu12072017
143
Menuz K. Larter N. K. Park J. Carlson J. R. (2014). An RNA-seq screen of the Drosophila antenna identifies a transporter necessary for ammonia detection. PLoS Genet.10, e1004810. 10.1371/journal.pgen.1004810
144
Meunier N. Marion-Poll F. Rospars J. P. Tanimura T. (2003). Peripheral coding of bitter taste in drosophila. J. Neurobiol.56, 139–152. 10.1002/neu.10235
145
Meyer D. Voigt A. Widmayer P. Borth H. Huebner S. Breit A. et al (2012). Expression of Tas1 taste receptors in mammalian spermatozoa: functional role of Tas1r1 in regulating basal Ca2+ and cAMP concentrations in spermatozoa. PLoS One7, e32354. 10.1371/journal.pone.0032354
146
Meyerhof W. Batram C. Kuhn C. Brockhoff A. Chudoba E. Bufe B. et al (2010). The molecular receptive ranges of human TAS2R bitter taste receptors. Chem. Senses35, 157–170. 10.1093/chemse/bjp092
147
Mi T. Mack J. O. Koolmees W. Lyon Q. Yochimowitz L. Teng Z. Q. et al (2023). Alkaline taste sensation through the alkaliphile chloride channel in drosophila. Nat. Metab.5, 466–480. 10.1038/s42255-023-00765-3
148
Mishra D. Miyamoto T. Rezenom Y. H. Broussard A. Yavuz A. Slone J. et al (2013). The molecular basis of sugar sensing in Drosophila larvae. Curr. Biol.23, 1466–1471. 10.1016/j.cub.2013.06.028
149
Miyamoto T. Amrein H. (2014). Diverse roles for the drosophila fructose sensor Gr43a. Fly. (Austin)8, 19–25. 10.4161/fly.27241
150
Miyamoto T. Slone J. Song X. Amrein H. (2012). A fructose receptor functions as a nutrient sensor in the drosophila brain. Cell151, 1113–1125. 10.1016/j.cell.2012.10.024
151
Mojib N. Xu J. Bartolek Z. Imhoff B. McCarty N. A. Shin C. H. et al (2017). Zebrafish aversive taste co-receptor is expressed in both chemo- and mechanosensory cells and plays a role in lateral line development. Sci. Rep.7, 13475. 10.1038/s41598-017-14042-3
152
Mollo E. Fontana A. Roussis V. Polese G. Amodeo P. Ghiselin M. T. (2014). Sensing marine biomolecules: smell, taste, and the evolutionary transition from aquatic to terrestrial life. Front. Chem.2, 92. 10.3389/fchem.2014.00092
153
Mollo E. Boero F. Peñuelas J. Fontana A. Garson M. J. Roussis V. et al (2022). Taste and smell: a unifying chemosensory theory. Q. Rev. Biol.97, 69–94. 10.1086/720097
154
Montalbano G. Levanti M. Mhalhel K. Abbate F. Laurà R. Guerrera M. C. et al (2021). Acid-sensing ion channels in zebrafish. Anim. (Basel)11, 2471. 10.3390/ani11082471
155
Montell C. (2009). A taste of the drosophila gustatory receptors. Curr. Opin. Neurobiol.19, 345–353. 10.1016/j.conb.2009.07.001
156
Morais S. (2017). The physiology of taste in fish: potential implications for feeding stimulation and gut chemical sensing. Rev. Fish. Sci. and Aquac.25, 133–149. 10.1080/23308249.2016.1249279
157
Mutchler S. M. Kirabo A. Kleyman T. R. (2021). Epithelial sodium channel and salt-sensitive hypertension. Hypertension77, 759–767. 10.1161/HYPERTENSIONAHA.120.14481
158
Nakagawa Y. Nagasawa M. Yamada S. Hara A. Mogami H. Nikolaev V. O. et al (2009). Sweet taste receptor expressed in pancreatic beta-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLoS One4, e5106. 10.1371/journal.pone.0005106
159
Nei M. Rooney A. P. (2005). Concerted and birth-and-death evolution of multigene families. Annu. Rev. Genet.39, 121–152. 10.1146/annurev.genet.39.073003.112240
160
Ning X. He J. Shi X. Yang G. (2016). Regulation of adipogenesis by quinine through the ERK/S6 pathway. Int. J. Mol. Sci.17, 504. 10.3390/ijms17040504
161
Nunez-Salces M. Li H. Feinle-Bisset C. Young R. L. Page A. J. (2020). Nutrient-sensing components of the mouse stomach and the gastric ghrelin cell. Neurogastroenterol. and Motil.32, e13944. 10.1111/nmo.13944
162
Ohtsu Y. Nakagawa Y. Nagasawa M. Takeda S. Arakawa H. Kojima I. (2014). Diverse signaling systems activated by the sweet taste receptor in human GLP-1-secreting cells. Mol. Cell. Endocrinol.394, 70–79. 10.1016/j.mce.2014.07.004
163
Oike H. Nagai T. Furuyama A. Okada S. Aihara Y. Ishimaru Y. et al (2007). Characterization of ligands for fish taste receptors. J. Neurosci.27, 5584–5592. 10.1523/JNEUROSCI.0651-07.2007
164
Okada S. (2015). The taste system of small fish species. Biosci. Biotechnol. Biochem.79, 1039–1043. 10.1080/09168451.2015.1023251
165
Ômura H. Honda K. Asaoka K. Inoue T. A. (2011). Divergent behavioral and electrophysiological taste responses in the mid-legs of adult butterflies, Vanessa indica and argyreus hyperbius. J. Insect Physiology57, 118–126. 10.1016/j.jinsphys.2010.09.012
166
Ortiz C. O. Faumont S. Takayama J. Ahmed H. K. Goldsmith A. D. Pocock R. et al (2009). Lateralized gustatory behavior of C. elegans is controlled by specific receptor-type guanylyl cyclases. Curr. Biol.19, 996–1004. 10.1016/j.cub.2009.05.043
167
Otero-Rodiño C. Librán-Pérez M. Velasco C. López-Patiño M. A. Míguez J. M. Soengas J. L. (2015). Evidence for the presence of glucosensor mechanisms not dependent on glucokinase in hypothalamus and hindbrain of rainbow trout (Oncorhynchus mykiss). PLoS One10, e0128603. 10.1371/journal.pone.0128603
168
Otero-Rodiño C. Velasco C. Álvarez-Otero R. López-Patiño M. A. Míguez J. M. Soengas J. L. (2016). In vitro evidence in rainbow trout supporting glucosensing mediated by sweet taste receptor, LXR, and mitochondrial activity in brockmann bodies, and sweet taste receptor in liver. Comp. Biochem. Physiology Part B Biochem. Mol. Biol.200, 6–16. 10.1016/j.cbpb.2016.04.010
169
Ovalle W. K. Shinn S. L. (1977). Surface morphology of taste buds in catfish barbels. Cell Tissue Res.178, 375–384. 10.1007/BF00218701
170
Oya M. Suzuki H. Watanabe Y. Sato M. Tsuboi T. (2011). Amino acid taste receptor regulates insulin secretion in pancreatic β-cell line MIN6 cells. Genes cells.16, 608–616. 10.1111/j.1365-2443.2011.01509.x
171
Papadaki M. Osorio- Valencia S. Kirk J. A. (2021). Abstract P514: the role of sweet and umami taste receptors in the heart. Circulation Res.129, AP514. 10.1161/res.129.suppl_1.p514
172
Park J. H. Kwon J. Y. (2011a). Heterogeneous expression of drosophila gustatory receptors in enteroendocrine cells. PLoS One6, e29022. 10.1371/journal.pone.0029022
173
Park J. H. Kwon J. Y. (2011b). A systematic analysis of drosophila gustatory receptor gene expression in abdominal neurons which project to the central nervous system. Mol. Cells32, 375–381. 10.1007/s10059-011-0128-1
174
Paukert M. Sidi S. Russell C. Siba M. Wilson S. W. Nicolson T. et al (2004). A family of acid-sensing ion channels from the zebrafish: widespread expression in the central nervous system suggests a conserved role in neuronal communication. J. Biol. Chem.279, 18783–18791. 10.1074/jbc.M401477200
175
Prandi S. Bromke M. Hübner S. Voigt A. Boehm U. Meyerhof W. et al (2013). A subset of mouse colonic goblet cells expresses the bitter taste receptor Tas2r131. PLoS One8, e82820. 10.1371/journal.pone.0082820
176
Rajkumar P. Aisenberg W. H. Acres O. W. Protzko R. J. Pluznick J. L. (2014). Identification and characterization of novel renal sensory receptors. PLoS One9, e111053. 10.1371/journal.pone.0111053
177
Raphael F. Raphael F. Naomi B.-A. Naomi B. A. Judith A. Judith A. (1976). Labellar taste organs of Drosophila melanogaster. J. Morphol.150, 327–341. 10.1002/jmor.1051500206
178
Ren X. Zhou L. Terwilliger R. Newton S. S. de Araujo I. E. (2009). Sweet taste signaling functions as a hypothalamic glucose sensor. Front. Integr. Neurosci.3, 12. 10.3389/neuro.07.012.2009
179
Robinett K. S. Koziol-White C. J. Akoluk A. An S. S. Panettieri R. A. Jr. Liggett S. B. (2014). Bitter taste receptor function in asthmatic and nonasthmatic human airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol.50, 678–683. 10.1165/rcmb.2013-0439RC
180
Rotin D. Staub O. (2021). Function and regulation of the epithelial Na(+) channel ENaC. Compr. Physiol.11, 2017–2045. 10.1002/cphy.c200012
181
Roy J. Baranek E. Mercier Y. Larroquet L. Surget A. Ganot A. et al (2022). Involvement of taste receptors in the Oro-sensory perception of nutrients in rainbow trout (Oncorhynchus mykiss) fed diets with different fatty acid profiles. Aquac. Nutr.2022, 1–20. 10.1155/2022/1152463
182
Sakakibara M. Sumida H. Yanagida K. Miyasato S. Nakamura M. Sato S. (2022). Bitter taste receptor T2R38 is expressed on skin-infiltrating lymphocytes and regulates lymphocyte migration. Sci. Rep.12, 11790. 10.1038/s41598-022-15999-6
183
San Gabriel A. Uneyama H. Yoshie S. Torii K. (2005). Cloning and characterization of a novel mGluR1 variant from vallate papillae that functions as a receptor for L-glutamate stimuli. Chem. Senses30 (Suppl. 1), i25–i26. 10.1093/chemse/bjh095
184
Sang J. Rimal S. Lee Y. (2019). Gustatory receptor 28b is necessary for avoiding saponin in Drosophila melanogaster. EMBO Rep.20, e47328. 10.15252/embr.201847328
185
Saotome K. Teng B. Tsui C. C. A. Lee W. H. Tu Y. H. Kaplan J. P. et al (2019). Structures of the otopetrin proton channels Otop1 and Otop3. Nat. Struct. Mol. Biol.26, 518–525. 10.1038/s41594-019-0235-9
186
Savulescu-Fiedler I. Baz R. O. Baz R. A. Scheau C. Gegiu A. (2025). Coronary artery spasm: from physiopathology to diagnosis. Life (Basel)15, 597. 10.3390/life15040597
187
Semplici B. Luongo F. P. Passaponti S. Landi C. Governini L. Morgante G. et al (2021). Bitter taste receptors expression in human granulosa and cumulus cells: new perspectives in female fertility. Cells10, 3127. 10.3390/cells10113127
188
Seong H. Song J. W. Lee K.-H. Jang G. Shin D.-M. Shon W.-J. (2024). Taste receptor type 1 member 3 regulates Western diet-induced male infertility. Biochimica Biophysica Acta (BBA) - Mol. Cell Biol. Lipids1869, 159433. 10.1016/j.bbalip.2023.159433
189
Shi P. Zhang J. (2006). Contrasting modes of evolution between vertebrate sweet/umami receptor genes and bitter receptor genes. Mol. Biol. Evol.23, 292–300. 10.1093/molbev/msj028
190
Shon W. J. Song J. W. Oh S. H. Lee K. H. Seong H. You H. J. et al (2023). Gut taste receptor type 1 member 3 is an intrinsic regulator of Western diet-induced intestinal inflammation. BMC Med.21, 165. 10.1186/s12916-023-02848-0
191
Shon W.-J. Seong H. Song J. W. Shin D.-M. (2024). Taste receptor type 1 member 3 is required for the fertility of male mice. Heliyon10, e24577. 10.1016/j.heliyon.2024.e24577
192
Shrestha B. Lee Y. (2023). Molecular sensors in the taste system of drosophila. Genes and Genomics45, 693–707. 10.1007/s13258-023-01370-0
193
Shrestha B. Aryal B. Lee Y. (2023). The taste of vitamin C in drosophila. EMBO Rep.24, e56319. 10.15252/embr.202256319
194
Shubha V. N. Shubha V. N. Ranjana S. Singh R. N. (1983). Sensilla on the tarsal segments and mouthparts of adult Drosophila melanogaster meigen (diptera: drosophilidae). International Journal of Insect Morphology and Embryology.
195
Shubha R. S. Shanbhag S. R. Park S. K. Park S. K. Claudio W. P. Pikielny C. W. et al (2001). Gustatory organs of drosophila melanogaster: fine structure and expression of the putative odorant-binding protein PBPRP2. Cell Tissue Res.304, 423–437. 10.1007/s004410100388
196
Simon B. R. Parlee S. D. Learman B. S. Mori H. Scheller E. L. Cawthorn W. P. et al (2013). Artificial sweeteners stimulate adipogenesis and suppress lipolysis independently of sweet taste receptors. J. Biol. Chem.288, 32475–32489. 10.1074/jbc.M113.514034
197
Simon B. R. Learman B. S. Parlee S. D. Scheller E. L. Mori H. Cawthorn W. P. et al (2014). Sweet taste receptor deficient mice have decreased adiposity and increased bone mass. PLoS One9, e86454. 10.1371/journal.pone.0086454
198
Simone P. Simone P. Anja V. Anja V. Wolfgang M. Wolfgang M. et al (2018). Expression profiling of Tas2r genes reveals a complex pattern along the mouse GI tract and the presence of Tas2r131 in a subset of intestinal paneth cells. Cell. Mol. Life Sci.75, 49–65. 10.1007/s00018-017-2621-y
199
Singh R. N. (1997). Neurobiology of the gustatory systems of drosophila and some terrestrial insects. Microsc. Res. Tech.39, 547–563. 10.1002/(SICI)1097-0029(19971215)39:6<547::AID-JEMT7>3.0.CO;2-A
200
Singh N. Pydi S. P. Upadhyaya J. Chelikani P. (2011a). Structural basis of activation of bitter taste receptor T2R1 and comparison with class A G-protein-coupled receptors (GPCRs). J. Biol. Chem.286, 36032–36041. 10.1074/jbc.M111.246983
201
Singh N. Vrontakis M. Parkinson F. Chelikani P. (2011b). Functional bitter taste receptors are expressed in brain cells. Biochem. Biophys. Res. Commun.406, 146–151. 10.1016/j.bbrc.2011.02.016
202
Soengas J. L. Comesaña S. Conde-Sieira M. Blanco A. M. (2024). Hypothalamic integration of nutrient sensing in fish. J. Exp. Biol.227, jeb247410. 10.1242/jeb.247410
203
Spehr M. Gisselmann G. Poplawski A. Riffell J. A. Wetzel C. H. Zimmer R. K. et al (2003). Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science299, 2054–2058. 10.1126/science.1080376
204
Sternini C. Rozengurt E. (2025). Bitter taste receptors as sensors of gut luminal contents. Nat. Rev. Gastroenterol. Hepatol.22, 39–53. 10.1038/s41575-024-01005-z
205
Stevenson R. J. (2014). Flavor binding: its nature and cause. Psychol. Bull.140, 487–510. 10.1037/a0033473
206
Stocker R. F. (1994). The organization of the chemosensory system in drosophila melanogaster: a review. Cell Tissue Res.275, 3–26. 10.1007/BF00305372
207
Taher S. Borja Y. Cabanela L. Costers V. J. Carson-Marino M. Bailes J. C. et al (2019). Cholecystokinin, gastrin, cholecystokinin/gastrin receptors, and bitter taste receptor TAS2R14: trophoblast expression and signaling. Am. J. Physiol. Regul. Integr. Comp. Physiol.316, R628-R639–r639. 10.1152/ajpregu.00153.2018
208
Talavera K. Ninomiya Y. Winkel C. Voets T. Nilius B. (2007). Influence of temperature on taste perception. Cell Mol. Life Sci.64, 377–381. 10.1007/s00018-006-6384-0
209
Tauber J. M. Brown E. B. Li Y. Yurgel M. E. Masek P. Keene A. C. (2017). A subset of sweet-sensing neurons identified by IR56d are necessary and sufficient for fatty acid taste. PLoS Genet.13, e1007059. 10.1371/journal.pgen.1007059
210
Thomas J. H. Robertson H. M. (2008). The caenorhabditis chemoreceptor gene families. BMC Biol.6, 42. 10.1186/1741-7007-6-42
211
Thorne N. Amrein H. (2008). Atypical expression of drosophila gustatory receptor genes in sensory and central neurons. J. Comp. Neurology506, 548–568. 10.1002/cne.21547
212
Tian X. Zhong F. Xia Y. (2022). Dynamic characteristics of sweetness and bitterness and their correlation with chemical structures for six steviol glycosides. Food Res. Int.151, 110848. 10.1016/j.foodres.2021.110848
213
Tizzano M. Cristofoletti M. Sbarbati A. Finger T. E. (2011). Expression of taste receptors in solitary chemosensory cells of rodent airways. BMC Pulm. Med.11, 3. 10.1186/1471-2466-11-3
214
Tomchik S. M. Berg S. Kim J. W. Chaudhari N. Roper S. D. (2007). Breadth of tuning and taste coding in mammalian taste buds. J. Neurosci.27, 10840–10848. 10.1523/JNEUROSCI.1863-07.2007
215
Toshiki S. Toshiki S. Takashi K. Takashi K. Ryota A. Ryota A. et al (2021). Expression profiles and functional characterization of common carp (Cyprinus carpio) T2Rs. Biochem. biophysics Rep.28, 101123. 10.1016/j.bbrep.2021.101123
216
Toyam T. Yamagishi T. Sato R. (2023). The roles of enteroendocrine cell distribution and gustatory receptor expression in regulating peptide hormone secretion in the midgut of Bombyx mori larvae. Archives Insect Biochem. Physiology114, e22032. 10.1002/arch.22032
217
Tran H. T. T. Herz C. Ruf P. Stetter R. Lamy E. (2018). Human T2R38 bitter taste receptor expression in resting and activated lymphocytes. Front. Immunol.9, 2949. 10.3389/fimmu.2018.02949
218
Tu Y. H. Cooper A. J. Teng B. Chang R. B. Artiga D. J. Turner H. N. et al (2018). An evolutionarily conserved gene family encodes proton-selective ion channels. Science359, 1047–1050. 10.1126/science.aao3264
219
Tuzim K. Korolczuk A. (2021). An update on extra-oral bitter taste receptors. J. Transl. Med.19, 440. 10.1186/s12967-021-03067-y
220
Upadhyaya J. Pydi S. P. Singh N. Aluko R. E. Chelikani P. (2010). Bitter taste receptor T2R1 is activated by dipeptides and tripeptides. Biochem. Biophysical Res. Commun.398, 331–335. 10.1016/j.bbrc.2010.06.097
221
Vecchio R. Cavallo C. Cicia G. Del Giudice T. (2019). Are (all) consumers averse to bitter taste?Nutrients11, 323. 10.3390/nu11020323
222
Velasco C. Conde-Sieira M. Comesaña S. Chivite M. Díaz-Rúa A. Míguez J. M. et al (2020). The long-chain fatty acid receptors FFA1 and FFA4 are involved in food intake regulation in fish brain. J. Exp. Biol.223, jeb227330. 10.1242/jeb.227330
223
Vidal B. Aghayeva U. Sun H. Wang C. Glenwinkel L. Bayer E. A. et al (2018). An atlas of Caenorhabditis elegans chemoreceptor expression. PLoS Biol.16, e2004218. 10.1371/journal.pbio.2004218
224
Vincis R. Fontanini A. (2019). Central taste anatomy and physiology. Handb. Clin. Neurol.164, 187–204. 10.1016/B978-0-444-63855-7.00012-5
225
Voigt A. Hübner S. Lossow K. Hermans-Borgmeyer I. Boehm U. Meyerhof W. (2012). Genetic labeling of Tas1r1 and Tas2r131 taste receptor cells in mice. Chem. Senses37, 897–911. 10.1093/chemse/bjs082
226
Voigt A. Hübner S. Döring L. Perlach N. Hermans-Borgmeyer I. Boehm U. et al (2015). Cre-mediated recombination in Tas2r131 Cells-A unique way to explore bitter taste receptor function inside and outside of the taste system. Chem. Senses40, 627–639. 10.1093/chemse/bjv049
227
Wang J. H. Inoue T. Higashiyama M. Guth P. H. Engel E. Kaunitz J. D. et al (2011). Umami receptor activation increases duodenal bicarbonate secretion via glucagon-like peptide-2 release in rats. J. Pharmacol. Exp. Ther.339, 464–473. 10.1124/jpet.111.184788
228
Ward S. (1973). Chemotaxis by the nematode caenorhabditis elegans: identification of attractants and analysis of the response by use of mutants. Proc. Natl. Acad. Sci. U. S. A.70, 817–821. 10.1073/pnas.70.3.817
229
Weiffenbach J. M. Baum B. J. Burghauser R. (1982). Taste thresholds: quality specific variation with human aging. J. Gerontology37, 372–377. 10.1093/geronj/37.3.372
230
Wölfle U. Elsholz F. A. Kersten A. Haarhaus B. Schumacher U. Schempp C. M. (2016). Expression and functional activity of the human bitter taste receptor TAS2R38 in human placental tissues and JEG-3 cells. Molecules21, 306. 10.3390/molecules21030306
231
Wu Q. Park S. J. Yang M. Ja W. W. (2021). Vitamin preference in drosophila. Curr. Biol.31, R946–r947. 10.1016/j.cub.2021.06.046
232
Wu Z. Yang W. Wu T. Liu Y. Pu Y. Hu W. et al (2025). Long term coptidis rhizoma intake induce gastrointestinal emptying inhibition and colon barrier weaken via bitter taste receptors activation in mice. Phytomedicine136, 156292. 10.1016/j.phymed.2024.156292
233
Xia L. Xia L. Yongyao Y. Yongyao Y. Da-Cheng Q. Dacheng Q. et al (2020). Expression analysis of taste receptor genes (T1R1, T1R3, and T2R4) in response to bacterial, viral and parasitic infection in rainbow trout, Oncorhynchus mykiss. Fish and Shellfish Immunol.101, 176–185. 10.1016/j.fsi.2020.03.055
234
Yacoob S. Y. Anraku K. Marui T. Matsuoka T. Kawamura G. Archdale M. V. (2001). Gustatory sensitivity of the external taste buds of Oreochromis niloticus L. to amino acids. Aquac. Res.32, 217–222. 10.1046/j.1365-2109.2001.00550.x
235
Yan C. H. Hahn S. McMahon D. Bonislawski D. Kennedy D. W. Adappa N. D. et al (2017). Nitric oxide production is stimulated by bitter taste receptors ubiquitously expressed in the sinonasal cavity. Am. J. Rhinol. Allergy31, 85–92. 10.2500/ajra.2017.31.4424
236
Yanan H. Yijin H. Song M. Song M. Bo L. Bo L. et al (2017). Molecular cloning and expression of taste receptor gene T2R1 of obscure puffer, Takifugu fasciatus. Pak. J. Zoology. 10.17582/journal.pjz/2017.49.5.1683.1691
237
Yarmolinsky D. A. Zuker C. S. Ryba N. J. (2009). Common sense about taste: from mammals to insects. Cell139, 234–244. 10.1016/j.cell.2009.10.001
238
Yasuoka A. Abe K. (2009). Gustation in fish: search for prototype of taste perception. Results Probl. Cell Differ.47, 239–255. 10.1007/400_2008_6
239
Ye Q. Bankova L. G. (2022). Brush cells fine-tune neurogenic inflammation in the airways. J. Clin. Investigation132, e161439. 10.1172/JCI161439
240
Yoder M. Papadaki M. Zied A. Kelly E. Markle B. Minh D. D. L. et al (2025). BPS2025 - sweet taste receptor activation regulates calcium handling and contractility in cardiomyocytes. Biophysical J.124, 529a–530a. 10.1016/j.bpj.2024.11.2774
241
Yoshinari Y. Nishimura T. Yoshii T. Kondo S. Tanimoto H. Kobayashi T. et al (2024). A high-protein diet-responsive gut hormone regulates behavioral and metabolic optimization in Drosophila melanogaster. Nat. Commun.15, 10819. 10.1038/s41467-024-55050-y
242
Yu F. X. Zhao B. Panupinthu N. Jewell J. L. Lian I. Wang L. H. et al (2012). Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell150, 780–791. 10.1016/j.cell.2012.06.037
243
Yu Q. Gamayun I. Wartenberg P. Zhang Q. Qiao S. Kusumakshi S. et al (2023). Bitter taste cells in the ventricular walls of the murine brain regulate glucose homeostasis. Nat. Commun.14, 1588. 10.1038/s41467-023-37099-3
244
Yuan G. Jing Y. Wang T. Fernandes V. S. Xin W. (2020). The bitter taste receptor agonist-induced negative chronotropic effects on the Langendorff-perfused isolated rat hearts. Eur. J. Pharmacol.876, 173063. 10.1016/j.ejphar.2020.173063
245
Yuan X. C. Liang X. F. Cai W. J. He S. Guo W. J. Mai K. S. (2020). Expansion of sweet taste receptor genes in grass carp (Ctenopharyngodon idellus) coincided with vegetarian adaptation. BMC Evol. Biol.20, 25. 10.1186/s12862-020-1590-1
246
Zhai K. Yang Z. Zhu X. Nyirimigabo E. Mi Y. Wang Y. et al (2016). Activation of bitter taste receptors (tas2rs) relaxes detrusor smooth muscle and suppresses overactive bladder symptoms. Oncotarget7, 21156–21167. 10.18632/oncotarget.8549
247
Zheng W. W. Li X. Y. Liu H. B. Wang Z. R. Hu Q. Q. Li Y. X. et al (2016). AMP-activated protein kinase attenuates high salt-induced activation of epithelial sodium channels (ENaC) in human umbilical vein endothelial cells. Oxid. Med. Cell Longev.2016, 1531392. 10.1155/2016/1531392
248
Zheng K. Lu P. Delpapa E. Bellve K. Deng R. Condon J. C. et al (2017). Bitter taste receptors as targets for tocolytics in preterm labor therapy. Faseb J.31, 4037–4052. 10.1096/fj.201601323RR
249
Zheng X. Tizzano M. Redding K. He J. Peng X. Jiang P. et al (2019). Gingival solitary chemosensory cells are immune sentinels for periodontitis. Nat. Commun.10, 4496. 10.1038/s41467-019-12505-x
Summary
Keywords
ectopic taste receptor, mammal, teleost, Drosophila , nematode
Citation
Chen K, Liang X, Yi H, Yu G and Wu Q (2025) Ectopic taste receptors in animal physiology: evolutionary conservation and functional diversification. Front. Cell Dev. Biol. 13:1660529. doi: 10.3389/fcell.2025.1660529
Received
06 July 2025
Accepted
02 September 2025
Published
10 September 2025
Volume
13 - 2025
Edited by
Gianluca Polese, University of Naples Federico II, Italy
Reviewed by
Giovanni Battista Appendino, University of Eastern Piedmont, Italy
Ernesto Mollo, National Research Council (CNR), Italy
Updates
Copyright
© 2025 Chen, Liang, Yi, Yu and Wu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guixiang Yu, ygx@swmu.edu.cn; Qi Wu, wuqi@swmu.edu.cn
†Lead contact
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.