- 1Department of Gastroenterology, Taizhou Second People’s Hospital Affiliated to Yangzhou University, Taizhou, Jiangsu, China
- 2Department of General Surgery, Liyang People’s Hospital, Liyang Branch Hospital of Jiangsu Province Hospital, Liyang, Jiangsu, China
- 3Department of General Surgery, the First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu, China
- 4The First Clinical Medical College of Guizhou University of Traditional Chinese Medicine, Guiyang, Guizhou, China
- 5Department of Radiation Oncology, The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou Municipal Hospital, Suzhou, Jiangsu, China
Colorectal cancer (CRC), as a highly prevalent malignant tumor worldwide, has a persistently high incidence and mortality rate. In recent years, metabolic reprogramming and immunosenescence have received extensive attention as key mechanisms for tumorigenesis, development and treatment resistance. Metabolic reprogramming not only provides energy and biosynthetic precursors for tumor cells, but also regulates immune responses by reconstructing the tumor microenvironment (TME). Immunosenescence is characterized by the depletion of effector immune cell function and the increase in the proportion of immunosuppressive cells. The two jointly promote the immune escape and therapeutic resistance of CRC. This article systematically reviews the research progress of metabolic reprogramming and immunosenescence in colorectal cancer and explores the related targeted therapeutic strategies, aiming to provide a new theoretical perspective for the precise treatment of CRC.
1 Introduction
Colorectal cancer (CRC), as a highly prevalent malignant tumor in the global digestive system, has a persistently high incidence and mortality rate. According to GLOBOCAN 2022 data, there are approximately 1.926 million new cases and 904,000 deaths worldwide each year. The incidence rate ranks third among malignant tumors, and the mortality rate ranks second (Bray et al., 2024).
While early-onset CRC is rising, older adults still represent the majority of CRC cases and deaths. Given the global trend of population aging, the burden of CRC is projected to grow further (He et al., 2025). Age-associated changes such as immunosenescence, chronic low-grade inflammation, gut microbiota dysbiosis, and metabolic dysfunction collectively contribute to tumorigenesis and immune evasion (Jin et al., 2022). These challenges require more precise, personalized, and elderly-friendly screening and treatment strategies. In recent years, the rise of immunotherapy and metabolic-targeted therapies has drawn significant attention to the interaction between metabolic reprogramming and the immune microenvironment in CRC. Immunosenescence—characterized by impaired adaptive immunity and chronic inflammation—weakens antitumor immune surveillance (Yu et al., 2024). Simultaneously, metabolic reprogramming enhances tumor cell survival and immune evasion through modulation of glucose, lipid, and amino acid metabolism (Nenkov et al., 2021; Sun et al., 2024). Together, these processes synergistically shape an immunosuppressive tumor microenvironment, playing a key role in CRC progression and therapy resistance.
Thus, an in-depth exploration of the interplay between immunosenescence and metabolic reprogramming, especially in elderly CRC populations, may offer novel insights for precision oncology. This review aims to comprehensively summarize recent advances in this field and discuss their potential applications in personalized treatment approaches.
2 Mechanisms and roles of metabolic reprogramming in colorectal cancer
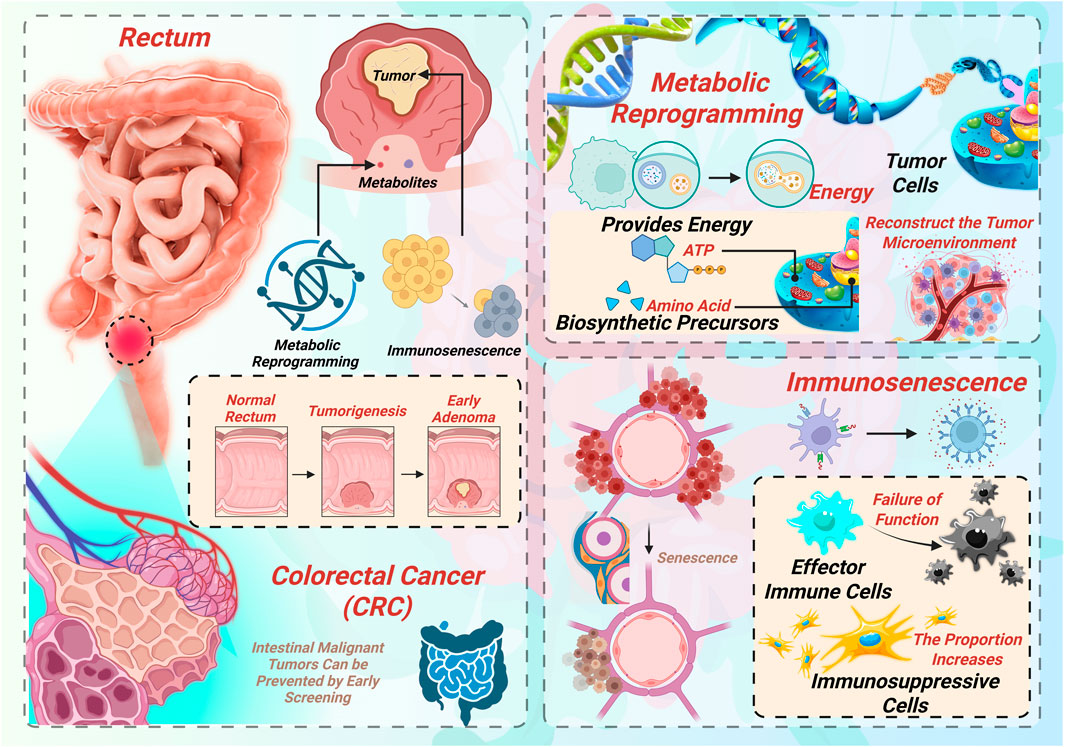
To better understand CRC progression and therapeutic resistance, it is essential to first elucidate the specific metabolic adaptations of CRC cells and how these alterations shape the tumor microenvironment. Tumor metabolic reprogramming refers to the adaptive shift in cellular metabolism that cancer cells undergo in response to specific microenvironmental stresses—such as hypoxia and nutrient deprivation—to support rapid proliferation, migration, and immune evasion (Qin et al., 2024). CRC cells exhibit a variety of such metabolic adaptations, primarily involving alterations in glucose metabolism, lipid metabolism, amino acid metabolism, and metabolic coupling with the tumor microenvironment. A schematic summary of these processes is illustrated in Figure 1, showing the intertwined roles of metabolic reprogramming and immunosenescence in CRC development.
2.1 Aerobic glycolysis (Warburg effect)
CRC cells tend to rely on glycolysis for energy production even in the presence of oxygen—a phenomenon known as aerobic glycolysis or the Warburg effect (Qin et al., 2024). This metabolic shift involves the upregulation of key enzymes such as glucose transporter 1 (GLUT1), hexokinase 2 (HK2), and lactate dehydrogenase A (LDHA), which collectively enhance glucose uptake and lactate production. These processes generate metabolic intermediates that fuel rapid cell proliferation (Hu et al., 2023). Research by Shen et al. demonstrated that elevated expression of HK2 and GLUT1 is associated with poor prognosis in CRC. METTL3 promotes this process by stabilizing HK2 and GLUT1 mRNA through IGF2BPs-mediated m6A modification, thereby enhancing glycolytic activity and CRC cell proliferation, ultimately driving tumor progression (Shen et al., 2020).
2.2 Reprogramming of amino acid metabolism
Glutamine is a critical nitrogen and carbon source for CRC cells. Glutamine deficiency has been shown to enhance EMT, promoting CRC recurrence and metastasis (Sun H. et al., 2022). Under high nitrogen demand, CRC cells upregulate glutamine transporters (e.g., SLC1A5) and glutaminase (GLS) to increase glutamine uptake and catabolism. This results in the production of α-ketoglutarate (α-KG) for the TCA cycle, and glutathione for maintaining redox balance and resisting oxidative stress and therapy-induced apoptosis. In addition, the tryptophan–kynurenine pathway (via IDO/TDO-AhR) is frequently activated in CRC (Chang et al., 2024). Its metabolite, kynurenine, exerts potent immunosuppressive effects by inducing T cell exhaustion and expanding regulatory T cells (Tregs), thereby facilitating immune evasion and enhancing tumor cell survival (Zhang et al., 2021).
2.3 Metabolic coupling between tumor cells and the microenvironment
CRC cells engage in complex metabolic interactions with components of the tumor microenvironment, including cancer-associated fibroblasts (CAFs), gut microbiota, and immune cells. For instance, CAFs can support tumor metabolism through the “reverse Warburg effect,” providing lactate as an energy source. Additionally, CRC cells absorb fatty acids and phospholipids secreted by CAFs, promoting their migration (Lyu et al., 2024; Gong et al., 2020). Metabolites from the gut microbiota, such as butyrate and secondary bile acids, also act as metabolic bridges linking microbial activity with tumor metabolism and immune regulation. Butyrate, for example, induces apoptosis in CRC cells by inhibiting histone deacetylases (HDACs), whereas an imbalance in secondary bile acids can activate inflammatory pathways like NF-κB, driving tumor proliferation and immune evasion (Qu et al., 2023).
3 Characteristics and roles of immunosenescence in colorectal cancer
While tumor metabolic reprogramming significantly influences CRC progression, age-related immune decline—immunosenescence—also profoundly impacts tumor immunity. Thus, understanding the dynamics of immunosenescence provides additional insight into why CRC remains resistant to conventional therapies, especially among elderly patients. Immunosenescence refers to the structural and functional decline of the immune system during physiological aging. It is characterized by reduced immune cell numbers, impaired function, and chronic activation of inflammatory mediators (Wang Y. et al., 2024). As the CRC patient population continues to age, immunosenescence has emerged as a key factor influencing tumor development, progression, and therapeutic response.
3.1 Functional decline of T Cell lineages
CD8+ cytotoxic T lymphocytes (CTLs) play a central role in antitumor immunity by directly killing malignant cells. High levels of CD8+ T cell infiltration—within the tumor core or invasive margin (known as the “Immunoscore”)—have been identified as strong prognostic indicators for recurrence and overall survival in CRC (Mlecnik et al., 2020). With age, the thymus undergoes progressive involution, a hallmark of T cell aging, reducing the output of naïve T cells (Kientega et al., 2024). In CD8+ T cells, expression of CD28 is downregulated, while aging markers such as CD57 and KLRG1 are upregulated (Pangrazzi and Weinberger, 2020), leading to terminal differentiation into phenotypes with diminished proliferative and cytotoxic capacities. These senescent T cells exhibit poor responsiveness and are ineffective at clearing tumor cells in CRC. Thymic involution reduces the production of naïve T cells, significantly narrowing the peripheral T cell receptor (TCR) repertoire (Cao et al., 2023). This reduction compromises antigen recognition, limits responses to neoantigens, and weakens immune surveillance against tumors.
In the CRC tumor microenvironment—particularly in elderly patients—there is marked enrichment of regulatory T cells (Tregs). Colonic adenocarcinoma patients show accumulation of PD-1+ Tregs, with elevated levels of suppressive molecules such as CTLA-4 and IL-10, which impair the local effector function of CD8+ T cells (Mokhta et al., 2023). Meanwhile, markers of exhaustion such as PD-1 and TIM-3 are increasingly expressed on CD8+ T cells; blockade of these pathways can partially restore their function (Deng et al., 2010). The expansion of Tregs and accumulation of exhausted CD8+ T cells collectively establish an immunosuppressive microenvironment that facilitates CRC immune evasion.
3.2 Innate immune decline and inflammaging in CRC
With advancing age, innate immunity in CRC patients undergoes marked functional decline: NK cells from individuals over 70 years exhibit sharply reduced perforin and granzyme B, while downregulated NKG2D expression further weakens their tumour-lytic capacity and predicts poorer postoperative outcomes; dendritic-cell maturation and co-stimulatory signalling (CD80/CD86) likewise diminish, blunting antigen presentation and T-cell priming (Maciejewski et al., 2013). This immunodeficiency is exacerbated by “inflammaging,” a chronic, low-grade inflammatory milieu typified by raised IL-6, TNF-α, C-reactive protein and HMGB1, which drive STAT3-and NF-κB-mediated proliferation, angiogenesis, epithelial–mesenchymal transition and recruitment of suppressive myeloid and regulatory cells, thereby re-shaping the tumour microenvironment toward immune evasion (Li et al., 2024). Consequently, immunosenescent CRC patients respond less favourably and for shorter durations to PD-1/PD-L1 blockade, underscoring the need to stratify individuals by immunosenescence status when optimising immunotherapy regimens (Kim et al., 2022; Verma et al., 2022).
4 Interplay between metabolic reprogramming and immunosenescence in colorectal cancer
Although metabolic reprogramming and immunosenescence have traditionally been studied independently, recent evidence highlights significant interactions between these two mechanisms. Clarifying their mutual influence is critical to fully grasp the complexity of CRC development and resistance mechanisms. In the development and progression of CRC, metabolic reprogramming of tumor cells and host immunosenescence do not occur independently. Rather, they interact through multiple levels and pathways, synergistically shaping a tumor microenvironment that is both highly immunosuppressive and metabolically supportive. This interplay not only enhances tumor adaptability and invasiveness but also presents a major obstacle to effective therapeutic intervention. The complex feedback loop between tumor metabolism and immune aging is conceptually illustrated in Figure 2, highlighting key regulatory molecules and therapeutic intervention points.
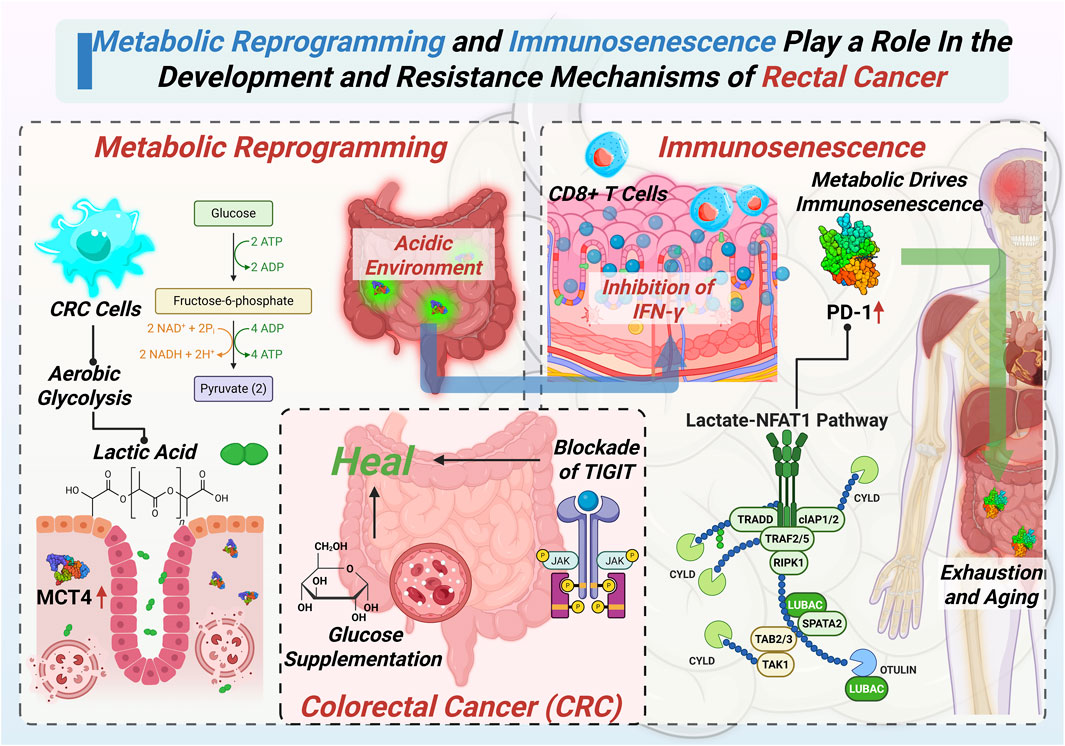
Figure 2. Detailed mechanisms highlighting how metabolic reprogramming and immunosenescence synergistically promote the progression and therapeutic resistance of CRC.
4.1 Metabolic reprogramming drives immune dysfunction
Metabolic abnormalities in tumor cells produce byproducts that suppress immune cell function and accelerate immunosenescence through several mechanisms:
CRC cells engage in aerobic glycolysis, producing large amounts of lactate (Siemińska and Lenart, 2025). Monocarboxylate transporter 4 (MCT4), the main transporter for lactate export, is upregulated in various tumors and is associated with poor prognosis in CRC, especially in left-sided tumors (Abe et al., 2019). Lactate accumulation and its export into the TME via MCT4 lead to a drop in local pH (Sun Q. et al., 2022). This acidified environment inhibits CD8+ T cell proliferation and effector cytokine (e.g., IFN-γ) production (Brand et al., 2016), and induces PD-1 upregulation via the lactate–NFAT1 pathway, pushing T cells toward an exhausted or senescent phenotype (Kumagai et al., 2022).
In CRC, tumor and immunosuppressive cells (e.g., MDSCs) often overexpress IDO1 (Shi et al., 2022), accelerating tryptophan degradation into kynurenine (Kyn). Kyn, acting as a ligand for the aryl hydrocarbon receptor (AhR), promotes Foxp3+ Treg expansion and suppresses CD8+ T cell function (Aristin Revilla et al., 2022). AhR also upregulates PD-1 and induces mitochondrial inactivation, driving T cell apoptosis/exhaustion—a hallmark of “metabolically driven immunosenescence” (Zhai et al., 2018).
CRC cells consume glucose and glutamine (Gln) aggressively via GLUT1-mediated glycolysis and glutamine addiction, depleting TME resources (Zhong et al., 2022). This competition suppresses mTOR signaling and IFN-γ production in tumor-infiltrating CD8+ T cells (Chang et al., 2015), and Gln deficiency further impairs T cell proliferation and memory formation (Wang B. et al., 2024). Glucose supplementation or TIGIT blockade can partially reverse this (Shao et al., 2021). Thus, CRC cells create a nutrient-deprived TME that induces energy exhaustion and senescence in T cells through “glucose-glutamine dual deprivation.”
4.2 Immunosenescence feeds back to enhance tumor metabolism
Aging immune cells also promote tumor metabolic reprogramming via cytokines and secreted factors: Senescent immune or stromal cells secrete pro-inflammatory SASP factors (e.g., IL-6, IL-8, TNF-α, CXCL1), activating STAT3 and NF-κB pathways in tumor cells (Fitsiou et al., 2022). This upregulates metabolic enzymes such as GLUT1, FASN, and GLS, enhancing glycolysis, lipogenesis, and glutamine dependency (De Simone et al., 2015; Han et al., 2016). SASP factors also stabilize hypoxia-inducible factor HIF-1α, amplifying transcriptional programs for metabolic reprogramming and deepening TME immunosuppression (Camporeale et al., 2014).
Senescent cells—including therapy-induced tumor cells, CAFs, and immune cells—secrete increased exosomes into serum and the TME. These vesicles are rich in metabolic regulatory miRNAs/lncRNAs and enzymes (Zhang et al., 2024). Upon uptake by CRC cells, they trigger nuclear translocation of PKM2 and activate STAT3-dependent glycolytic gene transcription, upregulating GLUT1 and LDHA (Wang et al., 2020; Xie et al., 2023).
4.3 A self-reinforcing feedback loop
The “metabolism → immunosenescence” and “immunosenescence → metabolic adaptation” processes form a positive feedback loop that perpetuates the hostile tumor microenvironment:
High lactate/kynurenine → T cell exhaustion/senescence → SASP secretion↑ → Enhanced tumor metabolic reprogramming → Stronger immune evasion → Sustained immunosenescence.
This vicious cycle is a key mechanism behind treatment failure and rapid disease progression in advanced CRC. Disrupting this metabolism-immunity feedback loop may represent a critical breakthrough to improve immunotherapy outcomes.
5 Therapeutic strategies targeting the metabolism–immunity axis in colorectal cancer
Given this intertwined relationship between tumor metabolism and immunosenescence, developing therapeutic approaches that simultaneously target both pathways represents an innovative and potentially more effective strategy against CRC. CRC progression is increasingly recognized as being fueled by a reciprocal interaction between tumor metabolic reprogramming and immune dysfunction, particularly immunosenescence. These intertwined processes create a TME that favors immune escape and therapeutic resistance. Consequently, there is growing interest in integrated strategies that disrupt aberrant metabolism while revitalizing anti-tumor immunity. Below, we highlight key therapeutic approaches under investigation that aim to remodel the TME, overcome resistance, and enhance treatment outcomes.
5.1 Targeting metabolic reprogramming
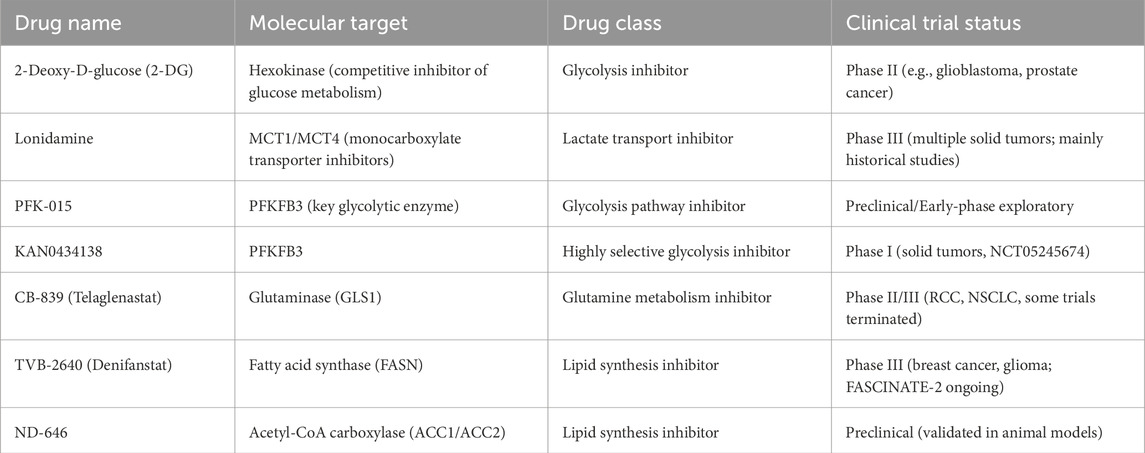
Metabolic adaptations enable CRC cells to thrive in hypoxic, nutrient-deprived environments while evading immune detection. Glycolysis inhibitors such as 2-deoxyglucose (2-DG), Lonidamine, and PFKFB3 inhibitors have demonstrated the ability to limit glucose utilization, reduce lactate production, and mitigate acidosis in the TME. These effects not only impair tumor proliferation but also enhance T cell viability and activity, thereby improving the response to PD-1/PD-L1 checkpoint blockade. Glutamine metabolism represents another therapeutic vulnerability. The glutaminase inhibitor CB-839 (Telaglenastat), currently in clinical trials for solid tumors, disrupts glutamine-dependent biosynthesis and immune evasion (Varghese et al., 2021). Preclinical studies suggest it can suppress M2 macrophage polarization in the TME, fostering an immunostimulatory milieu (Oyarce et al., 2021).
Glutaminase inhibitors commonly cause nausea, vomiting, fatigue, and appetite loss in clinical trials. By disrupting core metabolic pathways, they may pose long-term risks to organs like the liver and intestines, warranting caution in patients with poor nutrition or metabolic disorders. Additionally, targeting lipid metabolism has shown promise. Inhibitors of FASN and ACC1 block de novo lipogenesis, which is critical for membrane formation and oncogenic signaling. Agents such as TVB-2640 (Denifanstat) promote ferroptosis by increasing polyunsaturated fatty acid (PUFA) content, while ND-646 inhibits ACC1/ACC2 dimerization, effectively curbing fatty acid synthesis and tumor growth Inhibitors of FASN and ACC1 suppress fatty acid synthesis, disrupt membrane formation and signaling, and impair metastasis and immune evasion. TVB-2640 (Denifanstat) increases PUFA levels in CRC cells, inducing ferroptosis. ND-646 inhibits ACC1/ACC2 by preventing dimerization, blocking FA synthesis in vitro and in vivo (Yu et al., 2023). Table 1 provides a systematic overview of the principal targets, mechanisms of action, and latest pre-clinical and clinical advances of the metabolism-targeted therapeutics discussed in this study.
5.2 Reversing immunosenescence
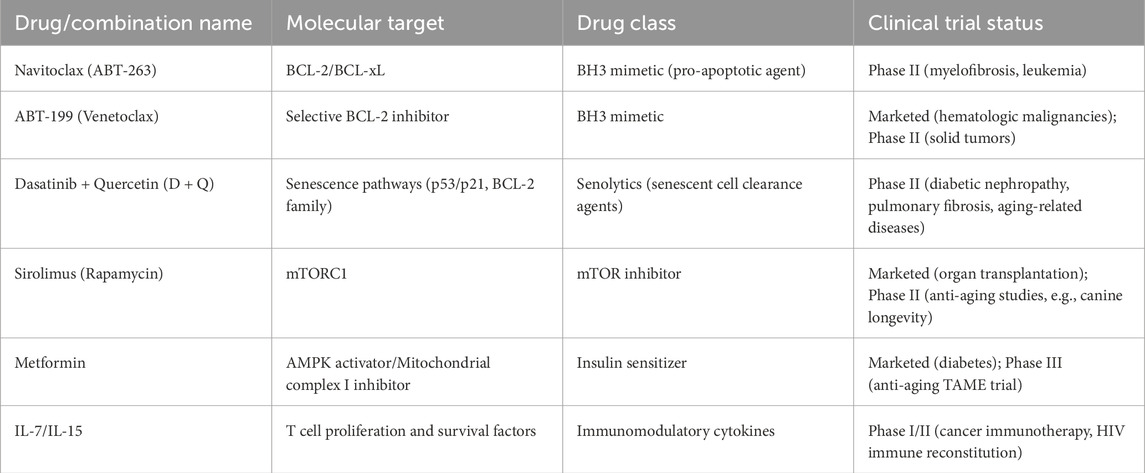
Immune aging is a major barrier to durable anti-tumor immunity in CRC, particularly among older patients. Senolytic agents, such as navitoclax and the dasatinib–quercetin (D + Q) combination, selectively eliminate senescent cells that contribute to immunosuppression (Bogdanova et al., 2024). Navitoclax (ABT-263) induces apoptosis in BCL-2-dependent senescent cells and activates STING signaling, leading to enhanced type I interferon production and T cell recruitment (Zhang et al., 2025). As a typical senolytic drug, its dose-limiting toxicity is mainly manifested as thrombocytopenia, because BCL-xL inhibition affects platelet survival. This side effect is more risky for elderly patients (those with impaired bone marrow hematopoietic function) and patients who need combined chemotherapy or radiotherapy. In contrast, senomorphic agents aim to suppress the pro-inflammatory SASP without killing senescent cells. mTOR inhibitors like rapamycin and metabolic regulators such as metformin reduce SASP production, preserve T cell homeostasis, and delay immunosenescence (Zhou et al., 2025). Efforts to rejuvenate T cell function directly are also underway. Cytokines such as IL-7 and IL-15 enhance the function of aged T cells, while adoptive cell therapies—including CAR-T, TCR-engineered T cells, and young donor-derived T cells—offer strategies to restore effective cytotoxicity in immunosenescent patients (Hombach et al., 2020). Table 2 presents a concise summary of the key strategies, potential clinical value, and recent research progress of the immunological and senescence-modulating therapies explored in this work.
5.3 Synergistic combination therapies
Combination strategies that integrate metabolic inhibition with immune checkpoint blockade and senescence-targeted therapies are showing synergistic effects in preclinical models. For instance, combining 2-DG with anti-PD-1 antibodies under a fasting-mimicking diet in CT26 CRC mouse models reduced intratumoral lactate levels and enhanced CD8+ T cell activation, resulting in significant tumor regression and prolonged survival. Although promising, these results await clinical validation (Peng et al., 2025). Similarly, CB-839 in combination with nivolumab has entered early-phase trials for solid tumors (Raczka and Reynolds, 2019). While CRC-specific data are limited, studies in melanoma models suggest this combination promotes T cell infiltration and augments checkpoint inhibitor efficacy (Varghese et al., 2021). In models of therapy-induced senescence (TIS), immune checkpoint inhibitors alone have limited efficacy due to the accumulation of immunosuppressive senescent cells. The addition of senolytics like ABT-263 restored immune competence by clearing these cells and enhancing the anti-tumor effects of PD-L1 blockade (Maggiorani et al., 2024).
5.4 Prospects for personalized therapy
The future of CRC therapy lies in personalized, multi-dimensional interventions that account for both metabolic and immune states. Advances in single-cell RNA sequencing, spatial transcriptomics, and artificial intelligence are enabling refined patient stratification based on tumor-intrinsic and TME features. Emerging biomarker panels now allow stratification by metabolic and immune profiles. Together, these approaches represent a shift from monotherapy to rational, biomarker-driven combination therapies that modulate both tumor metabolism and immune function, offering new avenues to overcome resistance and improve survival in CRC patients.
Despite these exciting developments, several real-world limitations may hinder widespread clinical adoption. Technologies such as single-cell RNA sequencing and spatial transcriptomics remain costly and require access to specialized platforms and significant bioinformatics expertise—resources that may not be available in many clinical settings. Additionally, variability in sample quality, processing standards, and data interpretation presents further challenges to integration into routine workflows. Addressing these barriers through continued technological innovation, training, and infrastructure development will be essential to fully realize the potential of personalized CRC therapy.
6 Conclusion and outlook
CRC, as one of the most prevalent and lethal malignancies globally, presents formidable challenges due to its intricate pathogenesis and high therapeutic resistance. Emerging evidence highlights the central roles of metabolic reprogramming and immunosenescence not only as independent contributors to tumor progression but also as interactive forces that establish a profoundly immunosuppressive and metabolically adaptive TME. This dynamic “metabolism–immunity feedback loop” is now increasingly recognized as a key driver of CRC progression and a critical barrier to effective immunotherapy, especially in elderly patients.
In this pathological loop, aberrant tumor metabolism ensures a steady supply of energy and biosynthetic substrates while generating immunosuppressive byproducts—such as lactate and kynurenine—that impair T cell function and accelerate immune aging. Simultaneously, senescent immune cells amplify tumor metabolic adaptation through the secretion of SASP factors, which promote tumor plasticity and immune evasion. This mutually reinforcing mechanism underpins the limited efficacy of immunotherapy and targeted treatments in aging CRC populations and suggests that both metabolic and immune dysregulation must be addressed in tandem.
Looking forward, future research should focus on constructing a comprehensive map of the CRC metabolism–immunity landscape using single-cell and spatial multi-omics tools, enabling the identification of actionable regulatory nodes. Parallel efforts should aim to develop multi-targeted interventions that simultaneously address key mechanisms such as lactate accumulation, glutamine addiction, and T cell exhaustion. Personalized therapeutic strategies—tailored to patient age, immunosenescence profiles, and metabolic states—may offer greater precision and efficacy. Importantly, real-world clinical trials that incorporate diverse patient populations are needed to evaluate the safety and effectiveness of these integrated approaches.
In conclusion, deeper mechanistic insight into the interplay between metabolic reprogramming and immunosenescence offers an opportunity to overcome longstanding barriers in CRC therapy. Disrupting this pathological feedback loop through precise, personalized, and age-adapted interventions holds promise for transforming CRC management and improving outcomes in patient populations most vulnerable to treatment failure.
Author contributions
JZ: Writing – original draft. KS: Writing – original draft. QS: Writing – original draft. GY: Writing – original draft, Writing – review and editing. XC: Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abe, Y., Nakayama, Y., Katsuki, T., Inoue, Y., Minagawa, N., Torigoe, T., et al. (2019). The prognostic significance of the expression of monocarboxylate transporter 4 in patients with right- or left-sided colorectal cancer. Asia Pac J. Clin. Oncol. 15, e49–e55. doi:10.1111/ajco.13077
Aristin Revilla, S., Kranenburg, O., and Coffer, P. J. (2022). Colorectal cancer-infiltrating regulatory T cells: functional heterogeneity, metabolic adaptation, and therapeutic targeting. Front. Immunol. 13, 903564. doi:10.3389/fimmu.2022.903564
Bogdanova, D. A., Kolosova, E. D., Pukhalskaia, T. V., Levchuk, K. A., Demidov, O. N., and Belotserkovskaya, E. V. (2024). The differential effect of senolytics on SASP cytokine secretion and regulation of EMT by CAFs. Int. J. Mol. Sci. 25, 4031. doi:10.3390/ijms25074031
Brand, A., Singer, K., Koehl, G. E., Kolitzus, M., Schoenhammer, G., Thiel, A., et al. (2016). LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell metab. 24, 657–671. doi:10.1016/j.cmet.2016.08.011
Bray, F., Laversanne, M., Sung, H., Ferlay, J., Siegel, R. L., Soerjomataram, I., et al. (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA a cancer J. Clin. 74, 229–263. doi:10.3322/caac.21834
Camporeale, A., Demaria, M., Monteleone, E., Giorgi, C., Wieckowski, M. R., Pinton, P., et al. (2014). STAT3 activities and energy metabolism: dangerous liaisons. Cancers (Basel) 6, 1579–1596. doi:10.3390/cancers6031579
Cao, Y., Wang, J., Hou, W., Ding, Y., Zhu, Y., Zheng, J., et al. (2023). Colorectal cancer-associated T cell receptor repertoire abnormalities are linked to gut microbiome shifts and somatic cell mutations. Gut Microbes 15, 2263934. doi:10.1080/19490976.2023.2263934
Chang, C. H., Qiu, J., O'Sullivan, D., Buck, M. D., Noguchi, T., Curtis, J. D., et al. (2015). Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162, 1229–1241. doi:10.1016/j.cell.2015.08.016
Chang, Y., Ou, Q., Zhou, X., Nie, K., Zheng, P., Liu, J., et al. (2024). Jianpi jiedu decoction suppresses colorectal cancer growth by inhibiting M2 polarization of TAMs through the tryptophan metabolism-AhR pathway. Int. Immunopharmacol. 138, 112610. doi:10.1016/j.intimp.2024.112610
De Simone, V., Franze, E., Ronchetti, G., Colantoni, A., Fantini, M. C., Di Fusco, D., et al. (2015). Th17-type cytokines, IL-6 and TNF-Alpha synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene 34, 3493–3503. doi:10.1038/onc.2014.286
Deng, L., Zhang, H., Luan, Y., Zhang, J., Xing, Q., Dong, S., et al. (2010). Accumulation of foxp3+ T regulatory cells in draining lymph nodes correlates with disease progression and immune suppression in colorectal cancer patients. Clin. cancer Res. official J. Am. Assoc. Cancer Res. 16, 4105–4112. doi:10.1158/1078-0432.CCR-10-1073
Fitsiou, E., Soto-Gamez, A., and Demaria, M. (2022). Biological functions of therapy-induced senescence in cancer. Seminars cancer Biol. 81, 5–13. doi:10.1016/j.semcancer.2021.03.021
Gong, J., Lin, Y., Zhang, H., Liu, C., Cheng, Z., Yang, X., et al. (2020). Reprogramming of lipid metabolism in cancer-associated fibroblasts potentiates migration of colorectal cancer cells. Cell death & Dis. 11, 267. doi:10.1038/s41419-020-2434-z
Han, J., Meng, Q., Xi, Q., Zhang, Y., Zhuang, Q., Han, Y., et al. (2016). Interleukin-6 stimulates aerobic glycolysis by regulating PFKFB3 at early stage of colorectal cancer. Int. J. Oncol. 48, 215–224. doi:10.3892/ijo.2015.3225
He, K. J., Liu, Z., and Gong, G. (2025). Addressing the rising colorectal cancer burden in the older adult: examining modifiable risk and protective factors for comprehensive prevention strategies. Front. Oncol. 15, 1487103. doi:10.3389/fonc.2025.1487103
Hombach, A. A., Geumann, U., Gunther, C., Hermann, F. G., and Abken, H. (2020). IL7-IL12 engineered mesenchymal stem cells (MSCs) improve A CAR T cell attack against colorectal cancer cells. Cells 9, 873. doi:10.3390/cells9040873
Hu, J., Tan, P., Ishihara, M., Bayley, N. A., Schokrpur, S., Reynoso, J. G., et al. (2023). Tumor heterogeneity in VHL drives metastasis in clear cell renal cell carcinoma. Signal Transduct. Target. Ther. 8, 155. doi:10.1038/s41392-023-01362-2
Jin, E. H., Han, K., Lee, D. H., Shin, C. M., Lim, J. H., Choi, Y. J., et al. (2022). Association between metabolic syndrome and the risk of colorectal cancer diagnosed before age 50 years according to tumor location. Gastroenterology 163, 637–648 e2. doi:10.1053/j.gastro.2022.05.032
Kientega, T., Marcoux, S., Bourbonnais, J., Montpetit, J., Caru, M., Cardin, G. B., et al. (2024). Premature thymic functional senescence is a hallmark of childhood acute lymphoblastic leukemia survivorship. Blood Cancer J. 14, 96. doi:10.1038/s41408-024-01071-1
Kim, C. M., Lee, J. B., Shin, S. J., Ahn, J. B., Lee, M., and Kim, H. S. (2022). The efficacy of immune checkpoint inhibitors in elderly patients: a meta-analysis and meta-regression. ESMO Open 7, 100577. doi:10.1016/j.esmoop.2022.100577
Kumagai, S., Koyama, S., Itahashi, K., Tanegashima, T., Lin, Y. T., Togashi, Y., et al. (2022). Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer cell 40, 201–218 e9. doi:10.1016/j.ccell.2022.01.001
Li, X. M., Yang, Y., Jiang, F. Q., Hu, G., Wan, S., Yan, W. Y., et al. (2024). Histone lactylation inhibits RARγ expression in macrophages to promote colorectal tumorigenesis through activation of TRAF6-IL-6-STAT3 signaling. Cell Rep. 43, 113688. doi:10.1016/j.celrep.2024.113688
Lyu, P., Gu, X., Wang, F., Sun, H., Zhou, Q., Yang, S., et al. (2024). Advances in targeting cancer-associated fibroblasts through single-cell spatial transcriptomic sequencing. Biomark. Res. 12, 73. doi:10.1186/s40364-024-00622-9
Maciejewski, R., Radej, S., Furmaga, J., Chroscicki, A., Rudzki, S., Rolinski, J., et al. (2013). Evaluation of immature monocyte-derived dendritic cells generated from patients with colorectal cancer. Pol. Przegl Chir. 85, 714–720. doi:10.2478/pjs-2013-0109
Maggiorani, D., Le, O., Lisi, V., Landais, S., Moquin-Beaudry, G., Lavallee, V. P., et al. (2024). Senescence drives immunotherapy resistance by inducing an immunosuppressive tumor microenvironment. Nat. Commun. 15, 2435. doi:10.1038/s41467-024-46769-9
Mlecnik, B., Bifulco, C., Bindea, G., Marliot, F., Lugli, A., Lee, J. J., et al. (2020). Multicenter international society for immunotherapy of cancer study of the consensus immunoscore for the prediction of survival and response to chemotherapy in stage III Colon cancer. J. Clin. Oncol. 38, 3638–3651. doi:10.1200/JCO.19.03205
Mokhtari, Z., Rezaei, M., Sanei, M. H., Dehghanian, A., Faghih, Z., Heidari, Z., et al. (2023). Tim3 and PD-1 as a therapeutic and prognostic targets in colorectal cancer: relationship with sidedness, clinicopathological parameters, and survival. Front. Oncol. 13, 1069696. doi:10.3389/fonc.2023.1069696
Nenkov, M., Ma, Y., Gassler, N., and Chen, Y. (2021). Metabolic reprogramming of colorectal cancer cells and the microenvironment: implication for therapy. Int. J. Mol. Sci. 22, 6262. doi:10.3390/ijms22126262
Oyarce, C., Vizcaino-Castro, A., Chen, S., Boerma, A., and Daemen, T. (2021). Re-polarization of immunosuppressive macrophages to tumor-cytotoxic macrophages by repurposed metabolic drugs. Oncoimmunology 10, 1898753. doi:10.1080/2162402X.2021.1898753
Pangrazzi, L., and Weinberger, B. (2020). T cells, aging and senescence. Exp. Gerontol. 134, 110887. doi:10.1016/j.exger.2020.110887
Peng, S., Wu, M., Yan, Q., Xu, G., Xie, Y., Tang, G., et al. (2025). Disrupting EDEM3-induced M2-like macrophage trafficking by glucose restriction overcomes resistance to PD-1/PD-L1 blockade. Clin. Transl. Med. 15, e70161. doi:10.1002/ctm2.70161
Qin, R., Fan, X., Huang, Y., Chen, S., Ding, R., Yao, Y., et al. (2024). Role of glucose metabolic reprogramming in colorectal cancer progression and drug resistance. Transl. Oncol. 50, 102156. doi:10.1016/j.tranon.2024.102156
Qu, R., Zhang, Y., Ma, Y., Zhou, X., Sun, L., Jiang, C., et al. (2023). Role of the gut microbiota and its metabolites in tumorigenesis or development of colorectal cancer. Adv. Sci. (Weinh) 10, e2205563. doi:10.1002/advs.202205563
Raczka, A. M., and Reynolds, P. A. (2019). Glutaminase inhibition in renal cell carcinoma therapy. Cancer Drug Resist 2, 356–364. doi:10.20517/cdr.2018.004
Shao, Q., Wang, L., Yuan, M., Jin, X., Chen, Z., and Wu, C. (2021). TIGIT induces (CD3+) T cell dysfunction in colorectal cancer by inhibiting glucose metabolism. Front. Immunol. 12, 688961. doi:10.3389/fimmu.2021.688961
Shen, C., Xuan, B., Yan, T., Ma, Y., Xu, P., Tian, X., et al. (2020). m(6)A-dependent glycolysis enhances colorectal cancer progression. Mol. cancer 19, 72. doi:10.1186/s12943-020-01190-w
Shi, D., Wu, X., Jian, Y., Wang, J., Huang, C., Mo, S., et al. (2022). USP14 promotes tryptophan metabolism and immune suppression by stabilizing IDO1 in colorectal cancer. Nat. Commun. 13, 5644. doi:10.1038/s41467-022-33285-x
Siemińska, I., and Lenart, M. (2025). Immunometabolism of innate immune cells in gastrointestinal cancer. Cancers 17, 1467. doi:10.3390/cancers17091467
Sun, H., Zhang, C., Zheng, Y., Liu, C., Wang, X., and Cong, X. (2022). Glutamine deficiency promotes recurrence and metastasis in colorectal cancer through enhancing epithelial-mesenchymal transition. J. Transl. Med. 20, 330. doi:10.1186/s12967-022-03523-3
Sun, Q., Wu, J., Zhu, G., Li, T., Zhu, X., Ni, B., et al. (2022). Lactate-related metabolic reprogramming and immune regulation in colorectal cancer. Front. Endocrinol. 13, 1089918. doi:10.3389/fendo.2022.1089918
Sun, M., Yue, Y., Wang, X., Feng, H., Qin, Y., Chen, M., et al. (2024). ALKBH5-mediated upregulation of CPT1A promotes macrophage fatty acid metabolism and M2 macrophage polarization, facilitating malignant progression of colorectal cancer. Exp. cell Res. 437, 113994. doi:10.1016/j.yexcr.2024.113994
Varghese, S., Pramanik, S., Williams, L. J., Hodges, H. R., Hudgens, C. W., Fischer, G. M., et al. (2021). The glutaminase inhibitor CB-839 (telaglenastat) enhances the Antimelanoma activity of T-Cell-Mediated immunotherapies. Mol. Cancer Ther. 20, 500–511. doi:10.1158/1535-7163.MCT-20-0430
Verma, A., Aberg-Zingmark, E., Sparrman, T., Mushtaq, A. U., Rogne, P., Grundstrom, C., et al. (2022). Insights into the evolution of enzymatic specificity and catalysis: from asgard archaea to human adenylate kinases. Sci. Adv. 8, eabm4089. doi:10.1126/sciadv.abm4089
Wang, X., Zhang, H., Yang, H., Bai, M., Ning, T., Deng, T., et al. (2020). Exosome-delivered circRNA promotes glycolysis to induce chemoresistance through the miR-122-PKM2 axis in colorectal cancer. Mol. Oncol. 14, 539–555. doi:10.1002/1878-0261.12629
Wang, Y., Cao, X., Yang, C., Fan, J., Zhang, X., Wu, X., et al. (2024). Ferroptosis and immunosenescence in colorectal cancer. Seminars cancer Biol. 106-107, 156–165. doi:10.1016/j.semcancer.2024.10.003
Wang, B., Pei, J., Xu, S., Liu, J., and Yu, J. (2024). A glutamine tug-of-war between cancer and immune cells: recent advances in unraveling the ongoing battle. J. Exp. Clin. Cancer Res. 43, 74. doi:10.1186/s13046-024-02994-0
Xie, Z., Xia, J., Jiao, M., Zhao, P., Wang, Z., Lin, S., et al. (2023). Exosomal lncRNA HOTAIR induces PDL1(+) B cells to impede anti-tumor immunity in colorectal cancer. Biochem. Biophys. Res. Commun. 644, 112–121. doi:10.1016/j.bbrc.2023.01.005
Yu, Y., Nie, Q., Wang, Z., Di, Y., Chen, X., and Ren, K. (2023). Targeting acetyl-CoA carboxylase 1 for cancer therapy. Front. Pharmacol. 14, 1129010. doi:10.3389/fphar.2023.1129010
Yu, W., Yu, Y., Sun, S., Lu, C., Zhai, J., Lei, Y., et al. (2024). Immune alterations with aging: mechanisms and intervention strategies. Nutrients 16, 3830. doi:10.3390/nu16223830
Zhai, L., Ladomersky, E., Lenzen, A., Nguyen, B., Patel, R., Lauing, K. L., et al. (2018). IDO1 in cancer: a gemini of immune checkpoints. Cell. & Mol. Immunol. 15, 447–457. doi:10.1038/cmi.2017.143
Zhang, X., Liu, X., Zhou, W., Du, Q., Yang, M., Ding, Y., et al. (2021). Blockade of IDO-Kynurenine-AhR axis ameliorated colitis-associated Colon cancer via inhibiting immune tolerance. Cell Mol. Gastroenterol. Hepatol. 12, 1179–1199. doi:10.1016/j.jcmgh.2021.05.018
Zhang, D., Zhang, J. W., Xu, H., Chen, X., Gao, Y., Jiang, H. G., et al. (2024). Therapy-induced senescent tumor cell-derived extracellular vesicles promote colorectal cancer progression through SERPINE1-mediated NF-κB p65 nuclear translocation. Mol. cancer 23, 70. doi:10.1186/s12943-024-01985-1
Zhang, W., Pan, X., Wang, L., Li, W., Dai, X., Zheng, M., et al. (2025). Selective BCL-2 inhibitor triggers STING-Dependent antitumor immunity via inducing mtDNA release. J. Immunother. cancer 13, e010889. doi:10.1136/jitc-2024-010889
Zhong, X., He, X., Wang, Y., Hu, Z., Huang, H., Zhao, S., et al. (2022). Warburg effect in colorectal cancer: the emerging roles in tumor microenvironment and therapeutic implications. J. Hematol. & Oncol. 15, 160. doi:10.1186/s13045-022-01358-5
Keywords: colorectal cancer, immunosenescence, metabolic reprogramming, tumor microenvironment, immunotherapy
Citation: Zhu J, Shen K, Su Q, Yao G and Chen X (2025) Metabolic reprogramming and immunosenescence in colorectal cancer: mechanisms and therapeutic implications. Front. Cell Dev. Biol. 13:1662464. doi: 10.3389/fcell.2025.1662464
Received: 09 July 2025; Accepted: 31 July 2025;
Published: 13 August 2025.
Edited by:
Qiong Lu, Central South University, ChinaReviewed by:
Izabela Siemińska, Jagiellonian University Medical College, PolandCopyright © 2025 Zhu, Shen, Su, Yao and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaochen Chen, Y2hlbnhpYW9jaGVuc3pAMTYzLmNvbQ==; Guozhong Yao, eWFvZ3VvemhvbmcxMzNAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Jun Zhu1†
Jun Zhu1† Kuan Shen
Kuan Shen