- 1Regenerative Medicine, Department of Health Science and Technology, Aalborg University, Aalborg, Denmark
- 2Department of Ophthalmology, Aarhus University Hospital, Aarhus, Denmark
Introduction: The corneal homeostasis is maintained by limbal epithelial stem cells (LESCs), which reside in the limbal niche. This microenvironment comprises the cells, the extracellular matrix (ECM), and their interactions that balance the quiescent and proliferative states of LESCs. The stress caused by removing the cells from their niche triggers the quiescent stem cells to enter the proliferative state, which is beneficial for in vitro expansion, but reduces their self-renewal capability, making them less suitable for transplantation. Fibronectin (FN), a key ECM component, widely used in tissue engineering and scaffold structure, has been shown to preserve the self-renewal ability of LESCs in vitro. In parallel, paracrine growth factors are crucial for maintaining limbal niche homeostasis and promoting corneal epithelial regeneration. Limbal-niche-cells-conditioned media is a potential reservoir of limbal niche paracrine growth factors. However, whether utilizing fibronectin and limbal-niche-cells-conditioned media can sustain or enhance the stemness and proliferation ability of LESCs in vitro has not yet been investigated.
Methods: Primary cultures of limbal niche cells, including LESCs, limbal mesenchymal stromal cells (LMSCs), and limbal melanocytes (LM), were established from remnant human corneal transplant specimens, and human epidermal melanocytes (HEMn) were included as a negative control. The proliferation ability (doubling time) and self-renewal potential (as assessed by PEDF and HES1 gene expressions) of LESCs were evaluated after culture in LM-, LMSC-, and HEMn-conditioned media, as well as coating with 3, 5, and 8 µg/cm2 concentrations of FN.
Results: Compared to the control group, the LMSC- and LM-conditioned media showed a clear trend towards upregulated PEDF and HES1 gene expressions. FN coating generally upregulated the expression of PEDF and HES1 genes, with this effect being most prominent at 3 µg/cm2.
Conclusion: These findings illustrate the potential of utilizing niche-cell-conditioned media and direct contact with FN on the self-renewal of LESCs in vitro. Further research is required to provide a more comprehensive understanding of these effects and to elucidate the underlying mechanisms of action.
1 Introduction
Corneal transparency is essential for vision and is maintained by the continuous regeneration of the corneal epithelium, a process sustained by local adult stem cells, the limbal epithelial stem cells (LESCs) (Ehlers and Hjortdal, 2005). Trauma, radiation, inflammation, autoimmune disorders, or prolonged contact lens use (Gonzalez et al., 2018; Le et al., 2018) can compromise the regenerative capacity of the LESCs, resulting in limbal stem cell deficiency (LSCD) and subsequent visual impairment (Le et al., 2018). Complications with current therapeutic approaches for LSCD, such as autologous serum administration (Azari and Rapuano, 2015), and allograft or autograft tissue transplantation (Cheung and Holland, 2017; Bilge, 2018), have led to increasing interest in in-vitro cultured cell transplantation as a promising alternative (Dobrowolski et al., 2015; Casaroli-Marano et al., 2015; Sacchetti et al., 2018). Cultivated epithelial stem cell transplantation (CLET) has emerged as a promising strategy for LSCD treatment (Sacchetti et al., 2018).
Similar to many other adult stem cells, LESCs typically reside in a quiescent, non-proliferative state, becoming activated only when required to restore tissue homeostasis (de Morree and Rando, 2023). Their ability to proliferate, self-renew, differentiate into mature cell types, and be expanded in vitro (Li and Clevers, 2010) makes them a great candidate for regenerative medicine (Li and Clevers, 2010; Ramalho-Santos and Willenbring, 2007). However, this advantage can quickly diminish when quiescent stem cells are cultured in vitro, presenting a significant challenge limiting the effectiveness of autologous transplantation therapies (Sacchetti et al., 2018; Marqués-Torrejón et al., 2021; Kobayashi et al., 2019; Quarta et al., 2016).
The concept of the stem cell niche, introduced by Schoefield et al., in 1978, highlighted the theory that the surrounding microenvironment regulates stemness and self-renewal, and removing stem cells from their niche leads to differentiation (Schofield, 1978). The quiescent state, enabling stem cells to support tissue regeneration in response to environmental signals (Urbán et al., 2019), is regulated by a combination of intrinsic and extrinsic mechanisms, including cell cycle and transcriptional regulators, metabolic factors, local and systemic signals, and interactions with the extracellular matrix (ECM) (Urbaìn and Cheung, 2021; Cho et al., 2019). In particular, cell-cell interactions regulate quiescence, self-renewal, differentiation, and survival (Farahzadi et al., 2023; Peerani and Zandstra, 2010; Pennings et al., 2018), while ECM proteins provide both mechanical scaffolding and biochemical signalling (Ferraro et al., 2010).
LESCs express various molecular markers, including P63, ABCG2, N-cadherin, NGF/Trk, integrin α9, integrin α6/CD71, HES1, nectin 3, and importin 13. PEDF is also recognized as a regulator of stemness, enhancing LESC self-renewal and proliferation. Moreover, HES1, as a key target gene of the Notch signalling pathway, is crucial for maintaining the LESC phenotype and quiescence (González et al., 2019; Kulkarni et al., 2010; Robertson et al., 2021).
Sacchetti et al. reported that less than 3% of isolated, cultivated, and transplanted LESCs are quiescent stem cells (P63+) capable of proliferation and renewal, necessitating repeated treatments (Sacchetti et al., 2018). This underscores the urgent need to develop strategies that enhance LESC self-renewal while maintaining the desirable transplantation characteristics.
The limbal niche includes both cellular and non-cellular components, including the extracellular matrix (ECM) and the niche cells (Mei et al., 2012; Polisetti et al., 2016), which provide regulatory signals crucial for LESC function (Mei et al., 2012; Polisetti et al., 2016; Mikhailova et al., 2015; Moreno et al., 2023).
The ECM contributes structural support and biochemical regulation, components like laminin (Polisetti et al., 2017), hyaluronan (HA) (Gesteira et al., 2017), and Fibronectin (FN) (Zheng et al., 2019), known to enhance LESC stemness. Niche-resident cells, including limbal melanocytes (LM), immune cells, LMSCs, vascular endothelial cells, and nerve cells, interact with LESCs either directly or through paracrine factors (Polisetti et al., 2022; Aghazadeh et al., 2024; Notara et al., 2010; Yazdanpanah et al., 2019; Polisetti et al., 2016).
LMs play a protective role against UV radiation, promote LESC stemness (Liu et al., 2018; Dziasko et al., 2015; Polisetti et al., 2021), and improve corneal regeneration (Yazdanpanah et al., 2019; Li et al., 2012; Polisetty et al., 2008; Dziasko and Daniels, 2016). Similar to other mesenchymal stem/stromal cells (MSCs), LMSCs secrete several growth factors, such as keratinocyte growth factor (KGF) (Gonzalez et al., 2018), nerve growth factor (NGF) (Amin et al., 2021), pigment epithelium-derived factor (PEDF) (Aghazadeh et al., 2024; Amin et al., 2021; Ho et al., 2013), insulin-like growth factor 1(IGF-1) (Trosan et al., 2012), fibroblast growth factor (FGF), ciliary neurotrophic factor, interleukin (IL)-1, and hepatocyte growth factor (HGF) (Amin et al., 2021), which are critical for preserving the limbal stem cell niche. While direct contact of LESCs with ECM components can further promote stemness (Polisetti et al., 2017; Zheng et al., 2019), the paracrine growth factor signalling also plays a key role in regulating LESCs’ stemness and niche homeostasis (Amin et al., 2021).
The interaction between cellular and non-cellular components in the limbal niche is essential for maintaining the stemness and self-renewal ability of limbal epithelial stem cells (LESCs). This importance is highlighted by the loss of these properties when quiescent LESCs are removed from their natural in vivo environment and cultured in vitro (Robertson et al., 2021). Nevertheless, several studies have indicated that ECM components and paracrine signalling can partially preserve the stemness and quiescent properties of LESCs (Urbaìn and Cheung, 2021; Robertson et al., 2021; Bonnet et al., 2021). Conditioned media (CM), which includes factors secreted by niche cells, has shown promise as a source of vital signals, although its role in supporting the self-renewal of LESCs in vitro is not yet fully explored (Jabbehdari et al., 2020a; Osugi et al., 2012; Smolinská et al., 2023). Therefore, to improve the potential of in vitro LESC culture for transplantation applications, this study aimed to systematically examine and compare the effects of fibronectin at concentrations of 3, 5, and 8 µg/cm2, along with conditioned media derived from LM, LMSC, and HEMn, on the proliferation (measured as doubling time) and stemness (assessed via PEDF and HES1 expression) of LESCs in vitro.
2 Materials and methods
2.1 Cell isolation and cultivation
Under the relevant Danish legislation, remnants of anonymized corneal transplant specimens used for posterior lamellar keratoplasty from donors (aged 30–70) without any corneal disease were obtained from the Danish Cornea Bank (Aarhus University Hospital, Aarhus, Denmark). The specimens were stored in a specific organ-culture storage medium to preserve viability.
The limbus tissues were collected by removing the cornea using a trephine and trimming any remaining tissue from the outer edge. Each limbus was divided in half, dissected into 1–2 mm pieces, and incubated for 1 h at 37 °C in 1 mL of 2 mg/mL collagenase (Roche Diagnostics, United States), for LMSC isolation. The pieces from the second half of the limbus were suspended in dispase (Roche Diagnostics, United States) for an hour at 37 °C to isolate LESC and LM. The resultant cell clusters were collected using reversible cell strainers with a 37 µm pore size. The collected clusters were broken up into single cells by further digestion in 1 mL of 0.25% trypsin and 0.02% EDTA (Gibco, Taastrup, Denmark) at 37 °C for 15 min. For primary cultures, single-cell suspensions were seeded into T25 flasks (Greiner Bio-one, Frickenhausen, Germany) and cultured in a “complete medium” comprising DMEM/F12 (Gibco, Taastrup, Denmark) containing 10% FCS (Gibco, Taastrup, Germany) and 1% penicillin/Streptomycin (Gibco, Taastrup, Denmark) to support LMSCs. Complete media supplemented with 1% Human corneal epithelial supplement (Gibco, Taastrup, Denmark) was used to support LESCs, while complete media supplemented with 1% melanocyte Growth supplement (Sigma Aldrich, Germany) was used to support LM and HEMn (ATCC, Denmark) culture.
The media was changed every other day until the cells reached 80% confluency. Sub-culture was carried out by rinsing the cells twice with 1X sterile PBS (phosphate-buffered saline) (Gibco, Taastrup, Denmark) to remove dead cells and debris before being treated for 90 s with an appropriate amount of TrypLE (Gibco, Taastrup, Denmark) based on the flask size, to detach the cells. The enzyme activity was neutralized by adding media twice the volume of TrypLE, the cell suspension was centrifuged at 500 g for 5 min, and the supernatant was removed. The cells were resuspended in the relevant media and transferred to three T75 flasks (Greiner Bio-one, Frickenhausen, Germany). In the second passage, the image of the cells was taken by an inverted microscope (Zeiss, Germany), and their morphology was studied. To remove the contamination with LMSCs, a low concentration of geneticin (0.2 mg/mL) was added to the LM-specific medium for 48 h from passages 1 to 2.
2.2 Identification and characterization of isolated cells
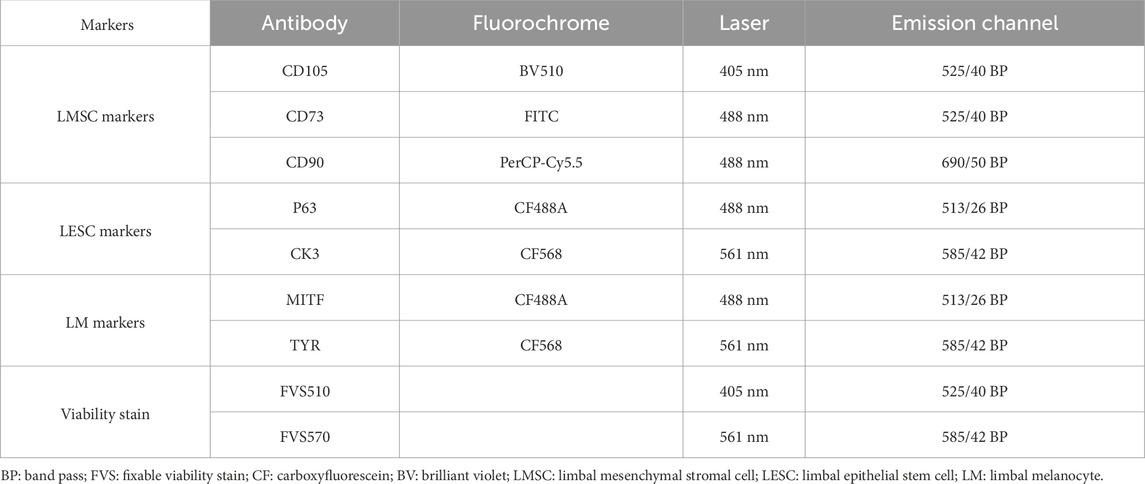
Confirmation of the isolated cell types was carried out by flow cytometric characterization of surface and intracellular markers, optimized using the directly labelled antibodies (Table 1). All staining buffers were based on sterile PBS containing 50% Accumax (Sigma-Aldrich) and 25 nM HEPES (Life Technologies) to maintain the appropriate PH range and prevent cell clumping.
Dead cells were first eliminated from the analysis following incubation with Fixable Viability Stains 570 (FVS570) and 510 (FVS510) (BD Bioscience, Lyngby, Denmark) (Table 1) at room temperature for 15 min. Positivity thresholds were determined by fluorescence minus one (FMO).
To confirm the presence of LMSCs, the cells were stained with CD90, CD73, and CD105 antibodies (BD Bioscience, Lyngby, Denmark) (LMSC markers) diluted in PBS supplemented with 2% FCS and 0.1% sodium azide (Merck Schuchardt, Hohenbrunn, Germany) at 4 °C for 30 min in the dark.
Cells were fixed and permeabilized to detect intracellular antigens in Fix/Perm buffer (BD Pharmingen, Denmark) containing 5% formaldehyde and 1.76% methanol for 50 min at 4 °C. LESCs were confirmed by staining with P63 (1:100; Biotium, Denmark)) and CK3 (1:200; Biotium, Denmark) antibodies, while LMs were identified using MITF (1:100) (Biotium, Denmark) and Tyrosinase (1:200; Novusbio, USA). All staining steps were incubated for 50 min at 4 °C.
The stained cells were then rinsed and transferred into a 5 mL round-bottom glass FACS tube (BD Falcon, Albertslund, Denmark) for surface epitope analysis using the CytoFLEX (Beckman Colter, Copenhagen, Denmark) flow cytometer. Before analysis, compensation values were established using the BD CompBeads Plus Set Anti-Mouse Ig, κ, and Anti-rat Ig, κ (BD Biosciences, New Jersey, USA). The data were analyzed using Kaluza 2.1 software (Beckman Coulter, Indianapolis, IN, USA), and basic gating was applied to target live singlets, while the top 2.5 percentile of unstained cells (fluorescence minus one (FMO) control) was regarded as positive.
2.3 Conditioned media preparation
In the second passage, LESC, LMSC, LM, and HEM cells were first cultured in media containing the respective supplements until they reached 80% confluency. The supplemented medium was then replaced with DMEM/F12 lacking FCS and any other supplements, and were allowed to incubate for 48 h before the supplement-free media was collected and used as CM.
2.4 Fibronectin coating
Fibronectin (FN) (Sigma Aldrich, Germany) was diluted in PBS to prepare coating solutions at concentrations of 3, 5, and 8 µg/cm2. Three separate 6-well plates were assigned to each concentration. The wells were coated with the respective FN solution, incubated for 1 h at room temperature, and then the coating solution was removed. Wells were subsequently washed once with PBS to remove any unbound material.
A fourth plate, left uncoated, served as a control. All four plates were seeded with LESCs (5 × 103 cells/cm2) cultured in complete media containing 1% corneal epithelial growth supplement for 7 days, while the media was refreshed every other day.
2.5 Cell proliferation assay
LESCs were seeded at a concentration of 5 × 103 cell/cm2 in two sets of 6-well plates, one set containing different concentrations of FN coating (3, 5, and 8 µg/cm2) and called FN3, FN5, and FN8 groups, the other set supplemented with LMSC, LM, and HEMn-CM Their proliferation rate was calculated based on the doubling times on days three, five, and seven after the cultivation, as the cells were washed three times with sterile PBS to remove the dead cells or debris and detached using 500 µL TrypLE (Gibco, Taastrup, Denmark). Following a 5-min centrifuge at 500 g, the cell suspension was counted using a hemacytometer (Bürker-Türk, Assistant, Germany) under a light microscope (Zeiss, Germany). Doubling time was calculated according to the formula below, where NT was the number of the cells at the end of passage, N0 was the starting number of the cells, T was time in any unit, and doubling time was represented as days:
2.6 Real time-qPCR
This part was performed in two steps, as in the first step, we had two sets of four six-well plates. One set was seeded with second passage LESCs (5 × 103/cm2) treated with 1:1 complete media containing 1% corneal epithelial growth supplement, and LESC, LMSC-, LM-, or HEM- CM, and one plate was treated with complete media as a control. The second set was coated with previously described concentrations of FN, as FN3, FN5, FN8, and non-coated as a control, and treated with complete media. The cells were cultured for 7 days, and the media was replaced every other day. Based on the results from the initial FN coating and CM supplementation tests, LESC cultures were prepared that combined 3 µg/cm2 FN coating and supplementation (1:1) with LM-, LMSC-, and HEMn-CM for 7 days. The cells were then collected and assessed for the expression of PEDF and HES1 markers. In brief, the Aurum Total RNA Mini Kit (Bio-Rad, USA) was utilized for RNA isolation from LESCs at the second passage. The purity and concentration of RNA were determined using a nanodrop spectrophotometer (NanoDrop; Thermo Fisher Scientific, Massachusetts, USA), and first-strand cDNA synthesis was performed using RNA from lysed cultured cells and iScript™ reverse transcriptase kit (Bio-Rad, California, USA). The qPCR reactions were carried out using a CFX Connect Real-Time PCR instrument (Bio-Rad, California, USA), with target-specific primers (TAG Copenhagen A/S, Denmark) (Table 2), IQ SYBR Green Supermix (Bio-Rad, California, USA), and cDNA according to the manufacturer’s instructions. A housekeeping gene, PPIA (Peptidylprolyl isomerase A), was used, and PEDF (pigment epithelial-derived factor) and HES1(Hairy and Enhancer of Split 1) were considered as genes of interest (GOI) to evaluate the role of treatments on proliferative and stemness ability of LESCs. Normalized to PPIA, gene expression levels and ratios were compared using the Livak (2−ΔΔCq) method, and the Pfaffl method would be accurate when PCR efficiencies are not optimal or differ between target and reference genes.
2.7 Statistical analysis
The data were assessed for normal distribution using the Shapiro-Wilks test. The relative expression ratios are reported as the mean fold-change ± standard deviation. Doubling time and changes in fold-regulation of the assessed genes in the different treatment sub-groups were compared using the Kruskal Wallis non-parametric test. All statistical analyses were carried out using the SPSS statistical software (Ver.29; IBM, New York, USA). p < 0.05 was considered as significant and adjusted by Bonferroni correction for multiple tests.
3 Results
3.1 Cell culture and morphology
From passage 1 to 2, the isolated LMSC, LESC, and LM groups demonstrated a mean ± SD cell doubling time of 1.85 ± 0.06, 1.96 ± 0.03, and 2.23 ± 0.25, respectively. The LMSCs presented an elongated or spindle shape with a single nucleus, typical of fibroblasts. LESCs showed a relatively large nucleus compared to the amount of cytoplasm, and LM demonstrated a dendritic morphology characterized by a small cell body with long, branching processes (dendrites) extending outward (Figure 1).
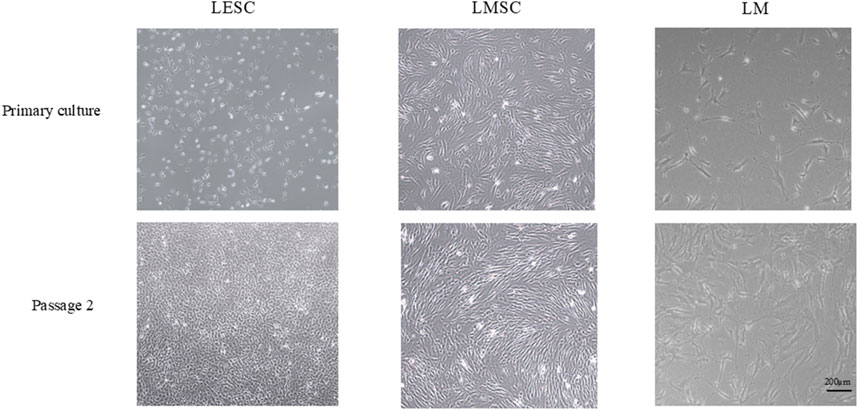
Figure 1. Morphology of limbal niche cell populations in primary culture and at passage 2 (original magnification, 4×). LMSC, limbal mesenchymal stromal cell; LESC, limbal epithelial stem cell; LM, limbal melanocyte.
3.2 Immunophenotypical characterization of isolated cells
Flow cytometry analysis was conducted to distinguish the various isolated cell populations within the limbal niche (Table 3), demonstrating high expression levels of CD90, CD73, and CD105, confirming the presence of LMSCs. The expression of P63 and CK3, as well as the limbal epithelial cell markers, indicated the presence of LESCs, while the expression of TYR and MITF confirmed the LM population.
In the LESC group, 82.12% of the cells presented the CK3 epithelial cell marker, while 64.71% exhibited the P63 stem cell marker, indicating the presence of limbal epithelial stem cells (LESCs). Furthermore, 62.79% of the cells co-expressed both P63 and CK3, indicating that the stemness potency of the limbal epithelial cells is in different stages (Figure 2).

Figure 2. The prevalence of the immunophenotype of limbal niche cells. LESC: limbal epithelial stem cell, LM: limbal melanocyte, LMSC: limbal mesenchymal stromal cell. The data are presented as mean ± standard deviations (SDs).
The purity of the isolated cell populations was validated using negative controls; LESCs were confirmed to be negative for CD90 and CD117 expression, LMSCs for CD117 and TYR expression, and LM cells for CD90 and P63.
3.3 The impact of conditioned media on LESCs
Treatment of LESCs with LMSC-derived conditioned media showed a lower doubling time than all other groups, although this difference was only significant (P < 0.05) compared to the HEMn-derived group on day three, and was not pronounced after five and 7 days (Figure 3).

Figure 3. Changes in the doubling time of limbal epithelial stem cells (LESCs) following three, five, and 7 days of treatment with limbal mesenchymal stromal cell (LMSC), limbal melanocyte (LM), and human epidermal melanocyte (HEMn)-derived conditioned media. The data is presented as mean ± standard deviations (SDs). The Pairwise significant differences (p < 0.05) were adjusted for multiple tests using the Bonferroni correction.
Supplementation with LMSC, LM, and HEMn-conditioned media did not result in statistically significant changes in PEDF or HES1 expression overall. Meanwhile, conditioned media from LMSC and LM exhibited a trend toward increased expression of PEDF (1.5-fold) and HES1 (1.29-fold), respectively (Figure 4).

Figure 4. PEDF and HES1 gene expression ratio in limbal epithelial stem cells (LESCs) following treatment of limbal mesenchymal stromal cell (LMSC)-, limbal melanocyte (LM)-, and human epidermal melanocyte (HEMn)-derived conditioned media, normalized to non-treated LESCs. The data is presented as mean ± standard deviations (SDs).
3.4 Effect of fibronectin coating on LESCs
Coating with different concentrations of FN did not show any significant effect on the proliferation ability of LESCs (Figure 5). However, FN coating at a concentration of 3 µg/cm2 resulted in a 5.09 (±0.685)-fold upregulation of PEDF gene relative to the control (p < 0.05). FN coating at concentrations of 5 and 8 µg/cm2 exhibited lower PEDF expression than 3 µg/cm2, while still higher than the control, although non-significant (Figure 6).

Figure 5. Effect of coating with various fibronectin (FN) concentrations on the proliferation ability of limbal epithelial stem cells (LESCs). The data is presented as mean ± standard deviations (SDs). The Pairwise significant differences (p < 0.05) were adjusted for multiple tests using the Bonferroni correction.

Figure 6. Effect of coating with different fibronectin (FN) concentrations on the stemness and self-renewal ability of limbal epithelial stem cells (LESCs). The data is presented as mean ± standard deviations (SDs). Pairwise significant differences (P < 0.05) in each row are indicated with * and † and adjusted by the Bonferroni correction for multiple tests.
FN coatings at 3 µg/cm2 and 5 µg/cm2 presented a 13.5 (±1.370)- and 12.4 (±1.061)- fold higher HES1 gene expression, respectively, compared with the control group (p < 0.05). FN coating at 8 µg/cm2 concentrations showed a lower HES1 expression than 3, and 5 µg/cm2, while still higher than the control, although non-significant (Figure 6).
3.5 Effect of fibronectin and conditioned media combination on LESCs
While coating with 3 µg/cm2 FN resulted in a significant upregulation of PEDF and HES1, adding limbal niche cell-derived conditioned media alongside this FN concentration did not further enhance PEDF upregulation compared to the control group (p < 0.05). However, the results showed that FN coating alone led to a 3.28 (±0.283)-fold increase in PEDF gene expression; this improvement was higher compared to the 1.03 (±0.283)-fold change observed with FN coating supplemented with HEMn-CM (p < 0.05). The only FN-coated and combining FN-coating supplemented with LM-CM groups indicated 9.67 (±0.247) and 7.59 (±1,584)-fold upregulation of HES1, respectively, compared to the control group (p < 0.05). At the same time, HEMn-CM demonstrated results similar to those of the non-treated control group (Figure 7).

Figure 7. Expression of PEDF and HES1 genes in limbal epithelial stem cells (LESCs) after 7 days of culture on the 3 µg/cm2 fibronectin (FN) alone or combined with conditioned media from limbal mesenchymal stromal cell (LMSC), limbal melanocyte (LM), and human epidermal melanocyte (HEMn) culture. The data is presented as mean ± standard deviations (SDs). The Pairwise significant differences (p < 0.05) were adjusted for multiple tests using the Bonferroni correction.
4 Discussion
Corneal transparency depends on the normal function of the LESCs and their interactions with limbal niche cells and extracellular matrix (ECM) (Robertson et al., 2021; Bonnet et al., 2021). While proliferating is a primary and essential characteristic of stem cells (Kulkarni et al., 2010; Robertson et al., 2021), remaining quiescent, characterized by a non-proliferative state, is also vital for the long-term maintenance of adult stem cells and tissue homeostasis (Urbán et al., 2019). The limbal niche regulates the balance between proliferation and differentiation, preserving quiescence while maintaining stemness potential (Urbán et al., 2019; Robertson et al., 2021; Bonnet et al., 2021). This allows LESCs to remain dormant until paracrine factors trigger proliferation and regeneration when repair is needed (de Morree and Rando, 2023; Urbaìn and Cheung, 2021). This study aimed to independently assess the potential effects of limbal niche cells’ conditioned media, which may provide limbal paracrine factors, and Fibronectin (FN) coating as one of the ECM components, on the proliferation, quiescence, and stemness of LESCs in vitro. Considering the suggested influence of pigmentation on the LESCs’ stemness (Liu et al., 2018), human epidermal melanocytes (HEMn) were included as a comparator to limbal niche melanocytes, to investigate the potential niche-specific interaction of these cells (Upadhyay et al., 2021; Li et al., 2006). None of the conditioned media showed a considerable difference in the proliferation ability or stemness of LESCs compared to the control group, while FN coating significantly enhanced stemness and self-renewal ability without impairing their proliferation ability.
Previous studies have demonstrated that different limbal niche cells, including LESCs, could be isolated from limbal tissue using enzymatic digestion followed by culture in cell-type-specific supplemented media (Polisetti et al., 2022; Dziasko et al., 2015). In this study, the identity of LMSC, LESC, and LM isolated from the limbal tissue was confirmed by their morphological characteristics (Polisetti et al., 2022; Dziasko and Daniels, 2016; Dziasko et al., 2014; Polisetti et al., 2020) and expression of cell-type-specific immunophenotypic markers (Dziasko and Daniels, 2016; Dziasko et al., 2014; Polisetti et al., 2020; Cui and Man, 2023).
Conditioned medium from in vitro culture contains the cell secretions and can act as a reservoir of growth factors, facilitating regeneration (Jabbehdari et al., 2020a; Osugi et al., 2012; Smolinská et al., 2023; Jabbehdari et al., 2020b). Several animal studies have shown that conditioned media, whether from uterine cervical stem cells (Bermudez et al., 2015), corneal mesenchymal stromal cells (Jabbehdari et al., 2020a), or LMSCs (Amirjamshidi et al., 2011), can accelerate corneal wound healing in vivo by delivering growth factors and modulating inflammation. This effect may involve IL-1–induced upregulation of hepatocyte growth factors (HGF) and keratinocyte growth factor (KGF) in stromal cells, promoting proliferation, migration, and transition from the inflammatory to the proliferative phase (Wilson, 2020).
LMSCs are mesenchymal stromal cells that can produce and release various growth factors that increase cell proliferation (Zhuang et al., 2021; Hefka Blahnova et al., 2020). An in vivo study on an animal model of limbal stem cell deficiency (LSCD) showed that topical treatment with conditioned media obtained from limbal fibroblasts (mesenchymal stromal cells) enhanced the growth of corneal epithelium, while skin fibroblast-derived conditioned media supported the growth of conjunctival type epithelium in the same model, suggesting that this proliferative effect may be niche-specific (Amirjamshidi et al., 2011). Another previous study demonstrated the effectiveness of HEMn-derived conditioned media in promoting keratinocyte proliferation in vitro (Deveci et al., 2001).
In the present study, LMSC-conditioned media showed a significantly enhanced proliferation rate of LESCs compared to HEMn-derived conditioned media, supporting the niche-specific nature of paracrine signaling. These effects appear dependent on the cellular origin and local microenvironment, where LMSC-secreted factors promote proliferation, while other niche components may usually help preserve quiescence (Urbán et al., 2019; Polisetti et al., 2021; Li et al., 2006; Bermudez et al., 2015).
However, LMSC-conditioned media did not significantly impact LESC proliferation rates, which could be attributed to the in-vitro model lacking the cascade of proinflammatory cytokines released during cell damage in vivo (Weng et al., 1997; Xiao et al., 2020). Furthermore, preparing conditioned media under serum-free conditions may induce stress-related alterations in the secretum, and potentially affecting its content (Jin et al., 2022). This represents a limitation of the current study, as serum-free conditions may not fully replicate the native paracrine environment.
The conditioned media failed to create an optimal environment to maintain quiescence, characterized by a non-proliferative state (de Morree and Rando, 2023; Urbán et al., 2019), as they could not significantly enhance the upregulation of PEDF and HES1 relative to controls. However, LMSC-derived growth factors and LM relatively increased PEDF and HES1gene expressions, respectively. Liu et al. (2018) suggested a link between pigmentation and the stemness potential of LESCs. This pigmentation was attributed to melanocytes dispersed within the basal epithelium of the limbus, which could potentially enhance the stemness of LESCs (Liu et al., 2018; Polisetti et al., 2021). In our study, however, HEMn-derived conditioned media did not improve stemness. Whereas LM-conditioned media upregulated HES1 expression, suggesting that limbal melanocytes promote self-renewal through niche-specific mechanisms beyond pigmentation. This aligns with evidence that melanocytes from different niches exhibit distinct properties shaped by their developmental origins and microenvironments (Liu et al., 2018; Polisetti et al., 2021; Zocco and Blanpain, 2017). Thus, limbal melanocytes appear more effective than epidermal melanocytes in supporting the quiescent state of LESCs.
The direct interaction between stem cells and the ECM plays a significant role in their proliferative or quiescent state (de Morree and Rando, 2023). As a primary component of the limbal niche ECM, FN closely interacts with LESCs and can improve their self-renewal ability through the Wnt non-canonical pathway (Robertson et al., 2021; Zheng et al., 2019). The findings of the current investigation align with previous studies, indicating that FN can promote LESC stemness and self-renewal ability by upregulating PEDF and HES1 gene expression. However, despite varying FN concentrations, this glycoprotein did not enhance the proliferation rate of LESCs based on their doubling time.
Different signalling pathways, including canonical and non-canonical Wnt and Notch, regulate LESC fate and maintenance (Robertson et al., 2021). Notch signalling, via its downstream effector HES1, is strongly expressed in the limbal epithelium and is central to maintaining a reserve of quiescent stem cells for corneal regeneration (Kulkarni et al., 2010; Mikhailova et al., 2015; Ahmadi and Jakobiec, 2002; Djalilian et al., 2008). In this study, FN upregulated HES1 expression without altering proliferation, suggesting that FN may promote self-renewal by engaging Notch-related mechanisms, though the downstream interactions remain to be clarified and validated in future mechanistic studies. The upregulation of HES1 in vitro may therefore enhance the self-renewal capacity of LESCs in their quiescent state, even in the absence of a proliferative response, as observed when LESCs were in direct contact with FN in the present study (Yu et al., 2010; Giannasi et al., 2023; Cichorek et al., 2013; Nakamura et al., 2008).
PEDF has been shown to enhance the regeneration of the cornea and limbus in animal models (Cichorek et al., 2013; Yeh et al., 2015) through activating signalling pathways, such as MAPK and STAT, which are essential for cell proliferation (Yu et al., 2010; Giannasi et al., 2023; Fan et al., 2019). The preliminary findings are consistent with previous reports, suggesting that FN may promote LESC stemness and self-renewal by upregulating PEDF and HES1, without increasing proliferation.
5 Conclusion
This preliminary study suggests that FN coating generally upregulated the expression of PEDF and HES1 genes, with this effect being most prominent at 3 µg/cm2. It significantly increased PEDF expression, but this effect was diminished by conditioned media, highlighting FN’s primary role over paracrine factors in promoting LESC self-renewal. Conversely, FN coating enhanced HES1 expression, further improved with LM-derived conditioned media, indicating a combined role of FN and paracrine factors in regulating LESC self-renewal via HES1. These exploratory findings raise the possibility of utilizing niche-cell-conditioned media and direct contact with FN on the self-renewal ability of LESCs in vitro. Further mechanistic and functional studies are required to validate and expand upon these preliminary observations.
Data availability statement
The data that support the findings of this study are available from the corresponding author (SA), upon reasonable request.
Ethics statement
Ethical considerations were addressed in accordance with Danish healthcare legislation and following guidance from the local ethical committee of the Central Denmark Region prior to collection in 2021. The study used anonymized remnant tissues from human corneal grafts, specifically Descemet’s membranes removed during endothelial keratoplasty, which are normally discarded. All donors had provided prior consent for corneal donation through registration in the Danish Donor Registry, permitting clinical use of their tissues. The committee determined that the secondary use of remnant donor tissue for research fell within the scope of ethically permissible practice under applicable regulations; accordingly, formal approval from an Institutional Review Board (IRB) was not required.
Author contributions
SA: Investigation, Conceptualization, Validation, Writing – original draft, Methodology, Formal Analysis, Data curation. QP: Methodology, Writing – review and editing. FD: Validation, Writing – review and editing. JØ: Resources, Validation, Writing – review and editing. VZ: Supervision, Resources, Conceptualization, Validation, Writing – review and editing, Methodology, Funding acquisition. HA: Project administration, Methodology, Conceptualization, Resources, Formal Analysis, Data curation, Writing – review and editing, Validation, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was financially supported through internal grants by the Regenerative Medicine group, Department of Health Science and Technology, Aalborg University, Aalborg, Denmark.
Acknowledgments
The authors would like to thank their colleagues in the Regenerative Medicine group at Aalborg University for their scientific input and the technician team for their technical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aghazadeh, S., Peng, Q., Dardmeh, F., Hjortdal, J. Ø., Zachar, V., and Alipour, H. (2024). Immunophenotypical characterization of limbal mesenchymal stromal cell subsets during in vitro expansion. Int. J. Mol. Sci. 25 (16), 8684. doi:10.3390/ijms25168684
Ahmadi, A. J., and Jakobiec, F. A. (2002). Corneal wound healing: cytokines and extracellular matrix proteins. Int. Ophthalmol. Clin. 42, 13–22. doi:10.1097/00004397-200207000-00004
Amin, S., Jalilian, E., Katz, E., Frank, C., Yazdanpanah, G., Guaiquil, V. H., et al. (2021). The limbal niche and regenerative strategies. Vis. Switz. 5 (4), 43. doi:10.3390/vision5040043
Amirjamshidi, H., Milani, B. Y., Sagha, H. M., Movahedan, A., Shafiq, M. A., Lavker, R. M., et al. (2011). Limbal fibroblast conditioned media: a non-invasive treatment for limbal stem cell deficiency. Mol. Vis. 17, 658–666. Available online at: https://pmc.ncbi.nlm.nih.gov/articles/PMC3056128/.
Azari, A. A., and Rapuano, C. J. (2015). Autologous serum eye drops for the treatment of ocular surface disease. Eye Contact Lens 41 (3), 133–140. doi:10.1097/ICL.0000000000000104
Bermudez, M. A., Sendon-Lago, J., Eiro, N., Treviño, M., Gonzalez, F., Yebra-Pimentel, E., et al. (2015). Corneal epithelial wound healing and bactericidal effect of conditioned medium from human uterine cervical stem cells. Invest Ophthalmol. Vis. Sci. 56 (2), 983–992. doi:10.1167/iovs.14-15859
Bilge, A. D. (2018). Comparison of conjunctival autograft and conjunctival transposition flap techniques in primary pterygium surgery. Saudi J. Ophthalmol. 32 (2), 110–113. doi:10.1016/j.sjopt.2017.11.002
Bonnet, C., González, S., Roberts, J. A. S., Robertson, S. Y. T., Ruiz, M., Zheng, J., et al. (2021). Human limbal epithelial stem cell regulation, bioengineering and function. Prog. Retin Eye Res. 85, 100956. doi:10.1016/j.preteyeres.2021.100956
Casaroli-Marano, R. P., Casaroli-Marano, R. P., Casaroli-Marano, R. P., Nieto-Nicolau, N., Martínez-Conesa, E. M., Edel, M., et al. (2015). Potential role of induced pluripotent stem cells (Ipscs) for cell-based therapy of the ocular surface. J. Clin. Med. 4 (2), 318–342. doi:10.3390/jcm4020318
Cheung, A. Y., and Holland, E. J. (2017). Keratolimbal allograft. Curr. Opin. Ophthalmol. 28 (4), 377–381. doi:10.1097/ICU.0000000000000374
Cho, I. J., Lui, P. P. W., Obajdin, J., Riccio, F., Stroukov, W., Willis, T. L., et al. (2019). Mechanisms, hallmarks, and implications of stem cell quiescence. Stem Cell Rep. 12 (6), 1190–1200. doi:10.1016/j.stemcr.2019.05.012
Cichorek, M., Wachulska, M., Stasiewicz, A., and Tymińska, A. (2013). Skin melanocytes: biology and development. Postepy Dermatol Alergol. 30 (1), 30–41. doi:10.5114/pdia.2013.33376
Cui, Y. Z., and Man, X. Y. (2023). Biology of melanocytes in mammals. Front. Cell Dev. Biol. 11, 1309557. doi:10.3389/fcell.2023.1309557
de Morree, A., and Rando, T. A. (2023). Regulation of adult stem cell quiescence and its functions in the maintenance of tissue integrity. Nat. Rev. Mol. Cell Biol. 24 (5), 334–354. doi:10.1038/s41580-022-00568-6
Deveci, M., Gilmont, R. R., Terashi, H., Ahmed, A. H., Smith, D. J., and Marcelo, C. (2001). Melanocyte-conditioned medium stimulates while melanocyte/keratinocyte contact inhibits keratinocyte proliferation. J. Burn Care and Rehabilitation 22, 9–14. doi:10.1097/00004630-200101000-00004
Djalilian, A. R., Namavari, A., Ito, A., Balali, S., Afshar, A., Lavker, R. M., et al. (2008). Down-regulation of Notch signaling during corneal epithelial proliferation. Mol. Vis. 14, 1041–1049.
Dobrowolski, D., Orzechowska-Wylegala, B., Wowra, B., Wroblewska-Czajka, E., Grolik, M., Szczubialka, K., et al. (2015). Cultivated oral mucosa epithelium in ocular surface reconstruction in aniridia patients. Biomed. Res. Int. 2015, 281870. doi:10.1155/2015/281870
Dziasko, M. A., and Daniels, J. T. (2016). Anatomical features and cell-cell interactions in the human limbal epithelial stem cell niche. Ocul. Surf. 14 (3), 322–330. doi:10.1016/j.jtos.2016.04.002
Dziasko, M. A., Armer, H. E., Levis, H. J., Shortt, A. J., Tuft, S., and Daniels, J. T. (2014). Localisation of epithelial cells capable of holoclone formation in vitro and direct interaction with stromal cells in the native human limbal crypt. PLoS One 9 (4), e94283. doi:10.1371/journal.pone.0094283
Dziasko, M. A., Tuft, S. J., and Daniels, J. T. (2015). Limbal melanocytes support limbal epithelial stem cells in 2D and 3D microenvironments. Exp. Eye Res. 138, 70–79. doi:10.1016/j.exer.2015.06.026
Ehlers, N., and Hjortdal, J. (2005). The cornea. Adv. Organ Biol., 83–111. doi:10.1016/s1569-2590(05)10003-2
Fan, N. W., Ho, T. C., Wu, C. W., and Tsao, Y. P. (2019). Pigment epithelium-derived factor peptide promotes limbal stem cell proliferation through hedgehog pathway. J. Cell Mol. Med. 23 (7), 4759–4769. doi:10.1111/jcmm.14364
Farahzadi, R., Valipour, B., Montazersaheb, S., and Fathi, E. (2023). Targeting the stem cell niche micro-environment as therapeutic strategies in aging. Front. Cell Dev. Biol. 11, 1162136. doi:10.3389/fcell.2023.1162136
Ferraro, F., Lo Celso, C., and Scadden, D. (2010). Adult stem cels and their niches. Adv. Exp. Med. Biol. 695, 155–168. doi:10.1007/978-1-4419-7037-4_11
Gesteira, T. F., Sun, M., Coulson-Thomas, Y. M., Yamaguchi, Y., Yeh, L. K., Hascall, V., et al. (2017). Hyaluronan rich microenvironment in the limbal stem cell niche regulates limbal stem cell differentiation. Invest Ophthalmol. Vis. Sci. 58 (11), 4407–4421. doi:10.1167/iovs.17-22326
Giannasi, C., Niada, S., Della Morte, E., Casati, S. R., De Palma, C., and Brini, A. T. (2023). Serum starvation affects mitochondrial metabolism of adipose-derived stem/stromal cells. Cytotherapy 25 (7), 704–711. doi:10.1016/j.jcyt.2023.03.004
Gonzalez, G., Sasamoto, Y., Ksander, B. R., Frank, M. H., and Frank, N. Y. (2018). Limbal stem cells: identity, developmental origin, and therapeutic potential. Wiley Interdiscip. Rev. Dev. Biol. 7 (2), e303. doi:10.1002/wdev.303
González, S., Uhm, H., and Deng, S. X. (2019). Notch inhibition prevents differentiation of human limbal stem/progenitor cells in vitro. Sci. Rep. 9 (1), 10373. doi:10.1038/s41598-019-46793-6
Hefka Blahnova, V., Dankova, J., Rampichova, M., Filova, E., and Hefka Blahnova, V. (2020). Combinations of growth factors for human mesenchymal stem cell proliferation and osteogenic differentiation. Bone Jt. Res. 9 (7), 412–420. doi:10.1302/2046-3758.97.BJR-2019-0183.R2
Ho, T. C., Chen, S. L., Wu, J. Y., Ho, M. Y., Chen, L. J., Hsieh, J. W., et al. (2013). PEDF promotes self-renewal of limbal stem cell and accelerates corneal epithelial wound healing. Stem Cells 31 (9), 1775–1784. doi:10.1002/stem.1393
Jabbehdari, S., Yazdanpanah, G., Kanu, L. N., Chen, E., Kang, K., Anwar, K. N., et al. (2020a). Therapeutic effects of lyophilized conditioned-medium derived from corneal mesenchymal stromal cells on corneal epithelial wound healing. Curr. Eye Res. 45 (12), 1490–1496. doi:10.1080/02713683.2020.1762227
Jabbehdari, S., Yazdanpanah, G., Kanu, L. N., Anwar, K. N., Shen, X., Rabiee, B., et al. (2020b). Reproducible derivation and expansion of corneal mesenchymal stromal cells for therapeutic applications. Transl. Vis. Sci. Technol. 9 (3), 26. doi:10.1167/tvst.9.3.26
Jin, Q. H., Kim, H. K., Na, J. Y., Jin, C., and Seon, J. K. (2022). Anti-inflammatory effects of mesenchymal stem cell-conditioned media inhibited macrophages activation in vitro. Sci. Rep. 12 (1), 4754. doi:10.1038/s41598-022-08398-4
Kobayashi, H., Morikawa, T., Okinaga, A., Hamano, F., Hashidate-Yoshida, T., Watanuki, S., et al. (2019). Environmental optimization enables maintenance of quiescent hematopoietic stem cells ex vivo. Cell Rep. 28 (1), 145–158. doi:10.1016/j.celrep.2019.06.008
Kulkarni, B. B., Tighe, P. J., Mohammed, I., Yeung, A. M., Powe, D. G., Hopkinson, A., et al. (2010). Comparative transcriptional profiling of the limbal epithelial crypt demonstrates its putative stem cell niche characteristics. BMC Genomics 11 (1), 526. doi:10.1186/1471-2164-11-526
Le, Q., Xu, J., and Deng, S. X. (2018). The diagnosis of limbal stem cell deficiency. Ocul. Surf. 16 (1), 58–69. doi:10.1016/j.jtos.2017.11.002
Li, N., and Clevers, H. (2010). Coexistence of quiescent and active adult stem cells in mammals. Science 327 (5965), 542–545. doi:10.1126/science.1180794
Li, L., Hu, D. N., Zhao, H., McCormick, S. A., Nordlund, J. J., and Boissy, R. E. (2006). Uveal melanocytes do not respond to or express receptors for α-melanocyte-stimulating hormone. Invest Ophthalmol. Vis. Sci. 47 (10), 4507–4512. doi:10.1167/iovs.06-0391
Li, G. G., Zhu, Y. T., Xie, H. T., Chen, S. Y., and Tseng, S. C. G. (2012). Mesenchymal stem cells derived from human limbal niche cells. Invest Ophthalmol. Vis. Sci. 53 (9), 5686–5697. doi:10.1167/iovs.12-10300
Liu, L., Nielsen, F. M., Emmersen, J., Bath, C., Østergaard Hjortdal, J., Riis, S., et al. (2018). Pigmentation is associated with stemness hierarchy of progenitor cells within cultured limbal epithelial cells. Stem Cells 36 (9), 1411–1420. doi:10.1002/stem.2857
Marqués-Torrejón, M. Á., Williams, C. A. C., Southgate, B., Alfazema, N., Clements, M. P., Garcia-Diaz, C., et al. (2021). LRIG1 is a gatekeeper to exit from quiescence in adult neural stem cells. Nat. Commun. 12 (1), 2594. doi:10.1038/s41467-021-22813-w
Mei, H., Gonzalez, S., and Deng, S. (2012). Extracellular matrix is an important component of limbal stem cell niche. J. Funct. Biomater. 3 (4), 879–894. doi:10.3390/jfb3040879
Mikhailova, A., Jylhä, A., Rieck, J., Nättinen, J., Ilmarinen, T., Veréb, Z., et al. (2015). Comparative proteomics reveals human pluripotent stem cell-derived limbal epithelial stem cells are similar to native ocular surface epithelial cells. Sci. Rep. 5, 14684. doi:10.1038/srep14684
Moreno, I. Y., Parsaie, A., Gesteira, T. F., and Coulson-Thomas, V. J. (2023). Characterization of the limbal epithelial stem cell niche. Invest Ophthalmol. Vis. Sci. 64 (13), 48. doi:10.1167/iovs.64.13.48
Nakamura, T., Ohtsuka, T., Sekiyama, E., Cooper, L. J., Kokubu, H., Fullwood, N. J., et al. (2008). Hes1 regulates corneal development and the function of corneal epithelial stem/progenitor cells. Stem Cells 26 (5), 1265–1274. doi:10.1634/stemcells.2007-1067
Notara, M., Shortt, A. J., Galatowicz, G., Calder, V., and Daniels, J. T. (2010). IL6 and the human limbal stem cell niche: a mediator of epithelial-stromal interaction. Stem Cell Res. 5 (3), 188–200. doi:10.1016/j.scr.2010.07.002
Osugi, M., Katagiri, W., Yoshimi, R., Inukai, T., Hibi, H., and Ueda, M. (2012). Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng. Part A 18 (13–14), 1479–1489. doi:10.1089/ten.TEA.2011.0325
Peerani, R., and Zandstra, P. W. (2010). Enabling stem cell therapies through synthetic stem cell-niche engineering. J. Clin. Investigation 120 (1), 60–70. doi:10.1172/JCI41158
Pennings, S., Liu, K. J., and Qian, H. (2018). The stem cell niche: interactions between stem cells and their environment. Stem Cells Int. 2018, 4879379. doi:10.1155/2018/4879379
Polisetti, N., Zenkel, M., Menzel-Severing, J., Kruse, F. E., and Schlötzer-Schrehardt, U. (2016). Cell adhesion molecules and stem cell-niche-interactions in the limbal stem cell niche. Stem Cells 34 (1), 203–219. doi:10.1002/stem.2191
Polisetti, N., Sorokin, L., Okumura, N., Koizumi, N., Kinoshita, S., Kruse, F. E., et al. (2017). Laminin-511 and -521-based matrices for efficient ex vivo-expansion of human limbal epithelial progenitor cells. Sci. Rep. 7 (1), 5152. doi:10.1038/s41598-017-04916-x
Polisetti, N., Schlötzer-Schrehardt, U., Reinhard, T., and Schlunck, G. (2020). Isolation and enrichment of melanocytes from human corneal limbus using CD117 (c-Kit) as selection marker. Sci. Rep. 10 (1), 17588. doi:10.1038/s41598-020-74869-1
Polisetti, N., Gießl, A., Zenkel, M., Heger, L., Dudziak, D., Naschberger, E., et al. (2021). Melanocytes as emerging key players in niche regulation of limbal epithelial stem cells. Ocul. Surf. 22, 172–189. doi:10.1016/j.jtos.2021.08.006
Polisetti, N., Sharaf, L., Schlötzer-Schrehardt, U., Schlunck, G., and Reinhard, T. (2022). Efficient isolation and functional characterization of niche cells from human corneal limbus. Int. J. Mol. Sci. 23 (5), 2750. doi:10.3390/ijms23052750
Polisetty, N., Fatima, A., Madhira, S. L., Sangwan, V. S., and Vemuganti, G. K. (2008). Mesenchymal cells from limbal stroma of human eye. Mol. Vis. 14, 431–442.
Quarta, M., Brett, J. O., DiMarco, R., De Morree, A., Boutet, S. C., Chacon, R., et al. (2016). An artificial niche preserves the quiescence of muscle stem cells and enhances their therapeutic efficacy. Nat. Biotechnol. 34 (7), 752–759. doi:10.1038/nbt.3576
Ramalho-Santos, M., and Willenbring, H. (2007). On the origin of the term “stem cell.”. Cell Stem Cell 1 (1), 35–38. doi:10.1016/j.stem.2007.05.013
Robertson, S. Y. T., Roberts, J. S., and Deng, S. X. (2021). Regulation of limbal epithelial stem cells: importance of the niche. Int. J. Mol. Sci. 22 (21), 11975. doi:10.3390/ijms222111975
Sacchetti, M., Rama, P., Bruscolini, A., and Lambiase, A. (2018). Limbal stem cell transplantation: clinical results, limits, and perspectives. Stem Cells Int. 2018, 8086269. doi:10.1155/2018/8086269
Schofield, R. (1978). The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 4 (1–2), 7–25.
Smolinská, V., Boháč, M., and Danišovič, Ľ. (2023). Current status of the applications of conditioned media derived from mesenchymal stem cells for regenerative medicine. Physiol. Res. 72, S233–S245. doi:10.33549/physiolres.935186
Trosan, P., Svobodova, E., Chudickova, M., Krulova, M., Zajicova, A., and Holan, V. (2012). The key role of insulin-like growth factor i in limbal stem cell differentiation and the corneal wound-healing process. Stem Cells Dev. 21 (18), 3341–3350. doi:10.1089/scd.2012.0180
Upadhyay, P. R., Ho, T., and Abdel-Malek, Z. A. (2021). Participation of keratinocyte- and fibroblast-derived factors in melanocyte homeostasis, the response to UV, and pigmentary disorders. Pigment. Cell Melanoma Res. 34 (4), 762–776. doi:10.1111/pcmr.12985
Urbaìn, N., and Cheung, T. H. (2021). Stem cell quiescence: the challenging path to activation. Dev. Camb. 148 (3), dev165084. doi:10.1242/dev.165084
Urbán, N., Blomfield, I. M., and Guillemot, F. (2019). Quiescence of adult mammalian neural stem cells: a highly regulated rest. Neuron 104 (5), 834–848. doi:10.1016/j.neuron.2019.09.026
Weng, J., Mohan, R. R., Qian, L., and Wilson Steven, E. (1997). IL-1 upregulates keratinocyte growth factor and hepatocyte growth factor mRNA and protein production by cultured stromal fibroblast cells. Cornea 15.
Wilson, S. E. (2020). Welcome to the first corneal special issue. Exp. Eye Res. 197, 108143. doi:10.1016/j.exer.2020.108143
Xiao, T., Yan, Z., Xiao, S., and Xia, Y. (2020). Proinflammatory cytokines regulate epidermal stem cells in wound epithelialization. Stem Cell Res. Ther. 11 (1), 232. doi:10.1186/s13287-020-01755-y
Yazdanpanah, G., Haq, Z., Kang, K., Jabbehdari, S., Rosenblatt, M. L., and Djalilian, A. R. (2019). Strategies for reconstructing the limbal stem cell niche. Ocul. Surf. 17 (2), 230–240. doi:10.1016/j.jtos.2019.01.002
Yeh, S. I., Ho, T. C., Chen, S. L., Chen, C. P., Cheng, H. C., Lan, Y. W., et al. (2015). Pigment epithelial-derived factor peptide facilitates the regeneration of a functional limbus in rabbit partial limbal deficiency. Invest Ophthalmol. Vis. Sci. 56 (4), 2126–2134. doi:10.1167/iovs.14-15983
Yu, F. S. X., Yin, J., Xu, K., and Huang, J. (2010). Growth factors and corneal epithelial wound healing. Brain Res. Bull. 81 (2–3), 229–235. doi:10.1016/j.brainresbull.2009.08.024
Zheng, M., Tian, C., Fan, T., and Xu, B. (2019). Fibronectin regulates the self-renewal of rabbit limbal epithelial stem cells by stimulating the Wnt11/Fzd7/ROCK non-canonical Wnt pathway. Exp. Eye Res. 185, 107681. doi:10.1016/j.exer.2019.05.021
Zhuang, W. Z., Lin, Y. H., Su, L. J., Wu, M. S., Jeng, H. Y., Chang, H. C., et al. (2021). Mesenchymal stem/stromal cell-based therapy: mechanism, systemic safety and biodistribution for precision clinical applications. J. Biomed. Sci. 28 (1), 28. doi:10.1186/s12929-021-00725-7
Keywords: limbal stem cells, conditioned media, fibronectin, stemness, PEDF, HES1
Citation: Aghazadeh S, Peng Q, Dardmeh F, Østergaard Hjortdal J, Zachar V and Alipour H (2025) The impact of the limbal niche interactions on the self-renewal capability of limbal epithelial stem cells. Front. Cell Dev. Biol. 13:1667309. doi: 10.3389/fcell.2025.1667309
Received: 16 July 2025; Accepted: 19 September 2025;
Published: 29 October 2025.
Edited by:
Zhangheng Huang, Sichuan University, ChinaReviewed by:
Aastha Garg, Dr. Shroff Charity Eye Hospital, IndiaFatemeh Tavakoli, National Eye Institute Neurobiology Neurodegeneration and Repair Laboratory, United States
Copyright © 2025 Aghazadeh, Peng, Dardmeh, Østergaard Hjortdal, Zachar and Alipour. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara Aghazadeh, c2FyYWFnQGhzdC5hYXUuZGs=; Hiva Alipour, aGl2YUBoc3QuYWF1LmRr
 Sara Aghazadeh
Sara Aghazadeh Qiuyue Peng
Qiuyue Peng Fereshteh Dardmeh
Fereshteh Dardmeh Jesper Østergaard Hjortdal2
Jesper Østergaard Hjortdal2 Vladimir Zachar
Vladimir Zachar Hiva Alipour
Hiva Alipour