- 1The First Affiliated Hospital of Shandong First Medical University, Jinan, China
- 2Yale School of Medicine, New Haven, CT, United States
- 3Shandong University of Traditional Chinese Medicine, Jinan, China
- 4The Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, China
The skeleton functions as an endocrine organ. Osteocytes maintenance of skeletal strength and energy balance by sensing mechanical stress and communicating with surrounding cells. They are currently considered key regulators of bone remodeling, mineral metabolism, and systemic homeostasis. Osteocytes originate from osteoblasts and are embedded in the lacunar-tubular network. They express proteins such as DMP1, sclerostin, and FGF23, and influence Wnt signaling, the RANKL/OPG axis, and phosphate metabolism. We review the latest studies in the field of osteocyte biology, focusing on their mechanotransduction through Piezo1 and integrins, regulation of osteoclastogenesis and osteogenesis, and their interactions with the bone marrow microenvironment, including immune and vascular cells. In osteoporosis, osteocyte dysfunction is manifested by apoptosis, ferroptosis, and pyroptosis. These changes, together with altered secretion, lead to uncoupled remodeling, disruption of the lacuno-canalicular network and metabolic imbalances that are intertwined with inflammation and bone marrow fat deposition. Osteocytes play an important role in fracture healing and adaptive remodeling under mechanical stimulation, promoting angiogenesis and stem cell recruitment. A growing number of emerging approaches, including stem cell therapy, CRISPR editing, and AI-driven multi-omics for precision medicine, are accelerating osteocyte-related research and the development of therapeutic strategies. These studies reveal the clinical potential of osteocyte-targeted therapies to prevent osteoporosis, improve bone strength, and enhance regeneration. By integrating molecular, cellular, and systems knowledge, we highlight osteocytes as a key therapeutic target to combat bone diseases and promote bone regeneration.
1 Introduction
Bone is far more than just a rigid support for the body. In reality, it is an active and adaptable tissue—functioning as an endocrine organ, a mineral ion reservoir, and a site of ongoing renewal that responds to mechanical forces, hormones, and changing metabolic needs (Szeliga et al., 2024). This constant process of turnover—where bone is built, broken down, and maintained—preserves skeletal strength and also helps regulate broader physiological systems, such as calcium and phosphate homeostasis and even aspects of energy balance (Zhou et al., 2021). When the balance between these processes is disturbed, serious disorders can result. Osteoporosis, for example, is marked by weak, fracture-prone bones and affects millions of people worldwide. In recent years, researchers have started to view the bone microenvironment as a highly interactive system, one that brings together a variety of cell types and complex signaling networks, opening up new possibilities for therapy (Zaidi et al., 2018).
There is great diversity in cells and signals in bone. Regions interact. Rather than existing as isolated compartments, regions like the endosteum, periosteum, marrow stroma, and the vascular network are in constant interaction, each contributing distinct cellular residents: hematopoietic stem cells, various mesenchymal and immune cells (including macrophages and T cells), endothelial cells, and adipocytes, all within an extracellular matrix rich in collagen and growth factors (Busch et al., 2024). In recent years, spatial transcriptomics and imaging have helped researchers pick apart these niches. For example, endosteal zones are generally linked to hematopoietic stem cell quiescence (with CXCL12+ stromal cells), while areas around blood vessels appear to foster osteoprogenitor differentiation through angiocrine factors (Dalle Carbonare et al., 2025). But osteocytes—so often underappreciated—seem to interact with nearly all of these other players, using gap junctions or secreted vesicles to influence both inflammation and stem cell fate. It is worth mentioning that, especially with age or in certain diseases, osteocytes can enter a senescent state, pumping out more pro-inflammatory cytokines and in turn tilting the immune environment and driving osteoclast activity (Chen S. et al., 2024). Emerging insights from recent studies highlight microbial influences, where gut-derived metabolites shape bone cell diversity, linking the microbiome to skeletal health (Dalle Carbonare et al., 2025). This multifaceted microenvironment not only sustains bone remodeling but also contributes to pathologies like osteoporosis, where dysregulated cell interactions exacerbate bone loss.
In this review, we synthesize recent advances in our understanding of osteocyte biology, with a particular focus on their roles in bone metabolism, osteoporosis, and skeletal remodeling. We begin by summarizing the developmental origins and molecular features of osteocytes, followed by an exploration of their central regulatory functions and signaling networks. We then highlight mechanisms of osteocyte dysfunction in osteoporosis and their contributions to pathological bone loss. Finally, we discuss the involvement of osteocytes in bone regeneration and remodeling, emerging regulatory pathways, and the translational potential of targeting osteocyte signaling in therapeutic strategies for metabolic bone diseases. We aim to provide a comprehensive perspective on osteocytes as master regulators of bone health and disease, and highlight osteocytes are key therapeutic targets that can help treat bone diseases and promote bone regeneration (Lu et al., 2025; Wu M. et al., 2024).
2 Osteocyte biology: origin, morphology, and molecular characteristics
Our understanding of osteocytes has changed a lot over time. Early on, these cells were largely regarded as quiet, “buried” components of bone, with little thought given to any active role they might play. This view was based on early histological studies from the 19th century, which described osteocytes as little more than passive managers of local mineral exchange. In truth, osteocytes are the most long-lived cells found in bone. They originate from osteoblasts as bones are laid down, eventually becoming encased within the mineralized matrix. At the same time, they develop an extensive network of dendritic processes, forming the lacuno-canalicular network (LCN) that reaches throughout the bone tissue (Dallas et al., 2013). Based on this network, osteocytes can sense mechanical changes, transport nutrients, and send paracrine signals. They are also sensitive to microdamage, variations in fluid flow, and hormonal signals. Research breakthroughs in the 1990s—driven by advances in genetic manipulation and imaging—demonstrated that osteocytes actively secrete regulatory molecules such as sclerostin (which inhibits Wnt signaling) and RANKL (which promotes osteoclast formation), confirming their central role in bone regulation (Bonewald, 2011; Delgado-Calle and Bellido, 2022). Single-cell omics and in vivo lineage tracing studies reveal the central role of osteocytes in processes ranging from periosteum remodeling to immune regulation (Tresguerres et al., 2020; Creecy et al., 2020).
Originating from osteoblasts, once situated within their lacunae, osteocytes take on a variety of responsibilities—such as sensing mechanical forces, managing mineral metabolism, and maintaining communication with other cells—all of which are vital for bone health. For a long time, osteocytes were considered passive “bystanders” in the skeleton. However, with the development of technologies such as single-cell omics and high-resolution imaging, researchers have gradually revealed the central role of osteocytes in bone biology (Palumbo and Ferretti, 2021).
2.1 Osteocyte developmental lineage and differentiation
Mesenchymal stem cells (MSCs) differentiate into the osteoblast lineage and drive maturation under the influence of key transcription factors such as Runx2 and Osterix (Sp7) (Zhu S. et al., 2024; Ponzetti and Rucci, 2021). During bone formation, osteoblasts secrete extracellular matrix (ECM), and some of these cells eventually become osteocytes as they undergo both morphological and functional changes. The transition from osteoblasts to osteocytes is characterized by a significant decrease in anabolic activity, the growth of dendritic processes, and the gradual encapsulation of the cells into pits formed in the mineralized matrix (Dallas and Bonewald, 2010; Mullen et al., 2013). This embedding is further shaped by perilacunar remodeling—a process in which osteocytes themselves take on an active role, modifying the surrounding matrix by means of osteocytic osteolysis, enabling localized bone resorption and remodeling, and also altering lacunar morphology, thereby influencing both mineral balance and the fine structure of bone (Franz-Odenda et al., 2006). Notably, this transition is coupled with changes in cellular metabolism. Glycolysis is especially important in the early stages of differentiation. Mature osteocytes, however, become more versatile in the types of fuel they use, which helps them survive in the relatively low-oxygen conditions found within the bone matrix (Prideaux et al., 2025).
Osteocytes gain biomarkers as they mature. Each biomarker links to a specific function. In differentiation, dentin matrix protein 1 (DMP1) takes on a central role in maintaining phosphate balance and facilitating matrix mineralization. It is well established that mutations in DMP1 can result in hypophosphatemic rickets as well as defects in osteocyte function (Dussold et al., 2019; Li et al., 2022). As osteocytes reach maturity, they begin to express sclerostin—known to inhibit the Wnt signaling pathway and thus limit bone formation—as well as fibroblast growth factor 23 (FGF23), which acts as a hormone regulating phosphate and vitamin D metabolism (Knowles et al., 2023; Ratsma et al., 2023; Ratsma et al., 2024). The synthesis of FGF23 in osteocytes is affected by local phosphate levels and FGFR1-mediated signaling, and studies involving targeted deletions in osteocytes have highlighted the importance of this pathway in guarding against hyperphosphatemia (Courbon et al., 2023; Xiao et al., 2014). Additionally, molecules such as Phex and Mepe serve to further characterize osteocyte identity, and their co-expression in mature osteocytes has been confirmed through single cell approaches (Prideaux et al., 2016; Hanai et al., 2023). These biomarkers do more than identify cells. For example, anti-sclerostin antibodies can increase bone mass in osteoporosis models. Other key markers include E11/gp38, which serves as an early osteocyte marker and helps with dendrite formation (Prideaux et al., 2012; Zhang et al., 2006). MT1-MMP aids in canaliculi formation during osteocyte development (Holmbeck et al., 2005; Kul et al., 2012; Karsdal et al., 2004). CapG and destrin regulate the cytoskeleton and control cytoplasmic processes in dendrites (Delgado-Calle and Bellido, 2015). Osteocytes are also richer in molecules like PHEX that control phosphate homeostasis compared to osteoblasts (Donmez et al., 2022; Rowe, 2012).
Epigenetics guide osteocyte development and function. In the context of bone, changes such as histone modification and DNA methylation are widely accepted as important drivers of lineage commitment (Park-Min, 2017; Dashti et al., 2024). The transition from osteoblasts to osteocytes is largely dependent on chromatin remodeling; enzymes such as histone deacetylases (HDACs) and the methyltransferase EZH2 (which modifies H3K27) repress genes involved in proliferation while regulating the expression of genes associated with bone formation (Zhu S. et al., 2024; Husain and Jeffries, 2017; Zhang et al., 2024). Furthermore, methylation of CpG sites within the SOST promoter has been associated with reduced sclerostin production following mechanical stimulation (Delgado-Calle et al., 2012). In osteocytes, miR-29b-3p responds to mechanical strain and regulates osteoblast differentiation by controlling IGF-1 secretion (Zeng et al., 2019). The non-coding RNA miR-218 expressed in osteocytes inhibits osteoblast differentiation and regulates its function through the Wnt pathway (Hassan et al., 2012). Meanwhile, technological advances like single-cell RNA sequencing (scRNA-seq) and spatial transcriptomics have dramatically expanded our perspective on osteocyte heterogeneity (Wang et al., 2025; Agoro et al., 2023; Feng et al., 2023). ScRNA-seq has revealed previously unrecognized, transcriptionally distinct subpopulations within the broader bone cell milieu—including not only osteocytes, but also mesenchymal stem cells, osteoblasts, chondrocytes, fibroblasts, osteoclasts, and vascular cells (Chai, 2022). Spatial transcriptomics, by mapping gene expression within intact tissue, helps clarify how these cell types interact within their microenvironments (Matsushita et al., 2023; Mathavan et al., 2025). Key transcription factors like ATF4 and HIF-1α integrate signaling pathways, with senescence-associated epigenetic changes in driving age-related dysfunction (Nusrat et al., 2025). These omics methods show the diversity of osteocytes. They help in better treatments for bone diseases.
2.2 Osteocyte ultrastructure and network architecture
Osteocytes are characterized by their stellate shape and extend numerous cytoplasmic processes (dendrites), which together form a complex LCN (Tiede-Lewis and Dallas, 2019; Moriishi and Komori, 2022). Within the LCN, the lacunae accommodate the osteocyte cell bodies, while the canaliculi—narrow, branching channels—permeate the mineralized matrix, thus permitting the exchange of nutrients and waste as well as fluid movement required for mechanotransduction (van Tol et al., 2020; Schemenz et al., 2020). Modern imaging methods, notably synchrotron X-ray tomography, have made it possible to appreciate how the LCN varies: for example, denser canalicular networks are generally observed in cortical bone, while the trabecular regions are less interconnected. Notably, age-related changes, such as occlusion of canaliculi, are linked with increased bone fragility (Moriishi and Komori, 2022). Shear stress generated by fluid movement within these channels can activate integrins and specific ion channels like Piezo1, leading to calcium influx and subsequent activation of pathways such as Wnt/β-catenin (Qin et al., 2020). Communication between osteocytes relies heavily on gap junctions formed by connexin 43 (Cx43), which assemble into hemichannels that permit the passage of small molecules, including ATP, prostaglandins, and cyclic nucleotides (Zhang et al., 2025). When osteocytes are subjected to mechanical loading, Cx43 hemichannels facilitate the release of prostaglandin E2 (PGE2), which can modulate the activity of nearby osteoblasts. In addition to these classic structures, extracellular vesicles and tunneling nanotubes also play a part, allowing for communication over longer distances (Delgado-Calle and Bellido, 2022). Loss of LCN connectivity—whether due to age or disease—tends to impair mechanosensation and promote cellular senescence, further emphasizing the significance of this system in preserving bone strength and integrity (Tiede-Lewis and Dallas, 2019).
3 Osteocyte as the master regulator of bone metabolism
Osteocytes are found deep within the bone matrix, where they play a vital role in keeping the skeleton balanced. These cells constantly process a mix of signals—mechanical loads, biochemical fluctuations, and hormonal inputs—allowing them to coordinate the complex process of bone metabolism. They are sensitive to changes in their local environment and can release various factors that act on osteoblasts and osteoclasts, ensuring that bone tissue can adjust to different physiological demands as needed (Qin et al., 2020; Choi et al., 2024). If osteocyte communication goes awry, issues like abnormal phosphate levels or a breakdown in the coupling of bone formation and resorption may develop, highlighting why these cells have become promising therapeutic targets in diseases such as osteoporosis (Michigami, 2022; Han et al., 2018).
3.1 Mechanosensation in osteocytes
Osteocytes sense mechanical loading and convert it into biochemical signals (Qin et al., 2020; Choi et al., 2021). Fluid shear stress stimulate mechanosensitive pathways. Among these, Piezo1 acts as a cation channel that drives calcium entry when the membrane is deformed (Li et al., 2019; Wang et al., 2023; Sun et al., 2019). Piezo1 activation in osteocytes triggers a downstream cascade, including Wnt/β-catenin signaling, which enhances osteogenesis and inhibits bone resorption, as evidenced by impaired bone formation and increased osteoclast activity in conditional knockout models (Li X. et al., 2025; Nottmeier et al., 2023). Integrins mediate mechanotransduction by linking cytoskeletal tension to focal adhesions, facilitating force transmission to the nucleus (Qin et al., 2022; Qin et al., 2023). YAP/TAZ, co-activators shuttled to the nucleus under mechanical cues, amplify this process by transcribing genes for matrix remodeling, promote perilacunar resorption to adapt to loading (Qin et al., 2020).
The osteocyte mechanosensory network is critical for adapting bone structure to mechanical load (Figure 1). Cyclic loading of the skeleton triggers changes in osteocyte function, including cortical bone thickening, whereas cyclical reduction in bone use often leads to bone loss (Iandolo et al., 2021; Ma et al., 2023). In microgravity or immobilization, diminished mechanosignaling exacerbates bone loss, underscoring osteocytes’ role in Wolff’s law—bone adapts to the loads it endures (Ma et al., 2023). Recent studies in single-cell analysis have identified diverse osteocyte subpopulations (Youlten et al., 2021). Notably, some subpopulations display high expression genes linked to neuronal network assembly. This finding broadens current insight into how bone responds to shifts in its local environment.
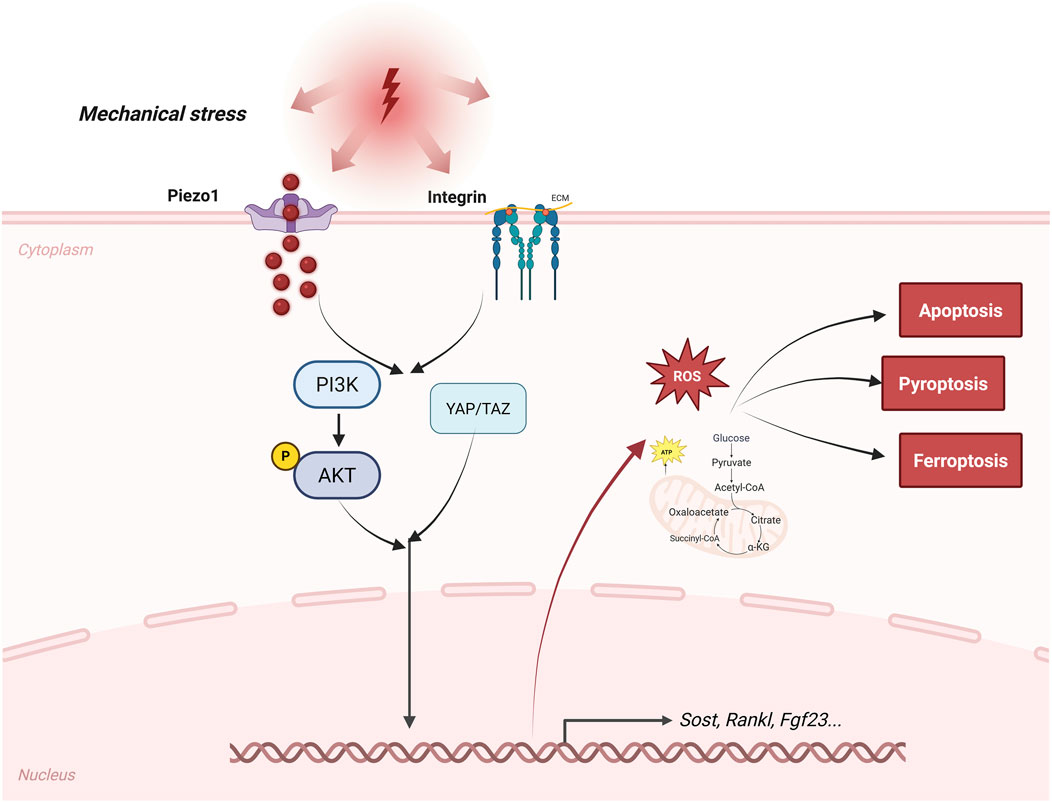
Figure 1. Pathway of bone cells sensing mechanical stress. This figure outlines the signaling pathways by which osteocytes sense mechanical stress. Mechanotransduction pathways, such as those involving Piezo1, integrins, YAP/TAZ, and Wnt/β-catenin, as well as paracrine and endocrine signals, such as sclerostin, RANKL, and FGF23, are depicted. The figure also labels important regulated forms of cell death, including apoptosis, ferroptosis, and pyroptosis, as well as metabolic changes, such as a shift toward glycolysis and increased production of reactive oxygen species, that contribute to osteocyte dysfunction during osteoporosis. Arrows indicate the main signaling pathways and feedback mechanisms in these processes.
3.2 Regulation of bone remodeling signals
Osteocytes govern remodeling through a sophisticated secretome, balancing formation and resorption (Bolamperti et al., 2022). SOST, a Wnt antagonist, is downregulated by mechanical loading to permit osteoblast activation, while its upregulation in unloading inhibits bone accrual; anti-sclerostin therapies exploit this for anabolic effects (Knowles et al., 2023). RANKL, secreted by osteocytes, drives osteoclastogenesis by binding RANK on precursors, with OPG acting as a decoy receptor to temper this; the RANKL/OPG ratio thus fine-tunes resorption, as seen in osteocyte-specific RANKL deletions attenuating bone loss (Udagawa et al., 2021; Yoshimoto et al., 2022). FGF23, an endocrine regulator, maintains phosphate homeostasis by suppressing renal reabsorption and vitamin D activation, linking skeletal metabolism to systemic mineral balance (Michigami, 2022; Han et al., 2018). These molecules help coordinate the activities of osteoblasts and osteoclasts, making sure that areas of bone resorption are properly restored. Signals released from osteocytes keep this process synchronized by means of paracrine feedback loops. If these regulatory loops are disturbed, either with aging or under disease conditions, bone remodeling can become abnormal (Ru and Wang, 2020) (Figure 1).
3.3 Osteocyte interactions with the bone marrow microenvironment
Osteocytes extend beyond the matrix, engaging in “crosstalk” with immune, and stromal elements in the marrow niche (Elango et al., 2018; Cao H. et al., 2020). Perivascular osteoblasts secrete angiogenic factors, such as VEGF, which affect endothelial cells to maintain nutrient supply or cancer metastasis (Chen et al., 2017; Mulcrone et al., 2020). Through MYD88-dependent signaling in response to PAMPs, these cells are able to attract macrophages and T cells, which can intensify inflammation during infection or in arthritis (Yoshimoto et al., 2022). Their engagement with the surrounding matrix includes perilacunar remodeling; in this process, osteocytes use enzymes like MMP13 to break down the local extracellular matrix, allowing them to adjust to metabolic changes (Mazur et al., 2019). Mitochondrial transfer from osteolineage cells to myeloid populations further regulates immune-mediated bone turnover (Ding et al., 2024). Transfer of osteocyte mitochondria to transcortical vascular endothelial cells accelerates angiogenesis and promotes the repair of cortical bone defects (Liao et al., 2024). Mechanically strained osteocyte-derived exosomes containing miR-3110-5p and miR-3058-3p were transported to osteoblasts, accompanied by increased PGE2, IGF-1 and NOS activities, thereby promoting the osteoblastic differentiation (Zhu Y. et al., 2024). Exosomes secreted by osteocytes under mechanical stress disrupt chondrocyte mitochondrial autophagy through miR-23b-3p, promote cartilage breakdown and inhibit synthesis, thereby accelerating the progression of osteoarthritis (Liu et al., 2025). Downregulation of miR-494-3p in extracellular vesicles derived from senescent osteocytes inhibits osteogenic differentiation and accelerates age-related bone loss via the PTEN/PI3K/AKT pathway (Yao et al., 2024). These interactions highlight the role of osteocytes in microenvironmental homeostasis (Figure 2) and have important implications for therapies targeting microenvironmental dysregulation in osteoporosis or metastasis.
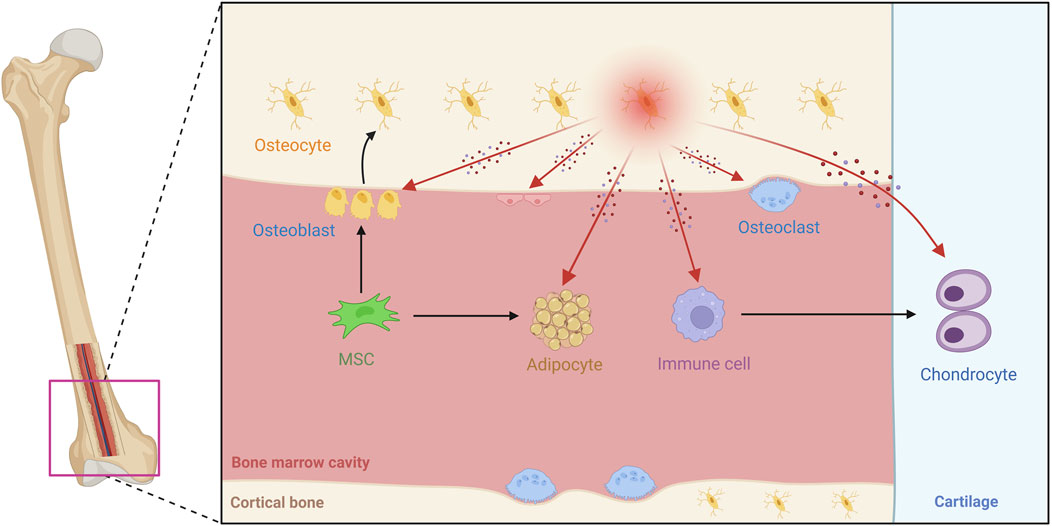
Figure 2. Overview of osteocyte biology and microenvironmental interactions in bone tissue. This schematic diagram shows the bone microenvironment, illustrating how mesenchymal stem cells (MSCs) differentiate into osteoblasts and mature into osteocytes. The image shows the widespread distribution of osteocyte dendritic processes. The image covers a variety of cell types, with osteocytes communicating with osteoblasts, osteoclasts, endothelial cells, bone marrow adipocytes, and immune cells, among others. These interactions occur through paracrine factors such as Sost, RANKL, and FGF23, as well as direct physical contact. Different anatomical regions are labeled, such as cortical bone, articular cartilage, and the bone marrow cavity. The image reveals the diversity of cell populations within the bone and the organization of the specialized microenvironment, with arrows marking the pathways of interaction between osteocytes and other cells.
4 Osteocyte dysfunction in osteoporosis
Osteoporosis is characterized by reduced bone mass and deterioration of bone microarchitecture, resulting from an imbalance in bone remodeling whereby bone resorption exceeds bone formation. Recent studies suggests that osteocyte dysfunction is the important link connecting cell death, signaling abnormalities, metabolic alterations, and systemic factors such as estrogen deficiency and inflammation.
4.1 Osteocyte dysfunction in osteoporosis
In osteoporosis, several forms of regulated cell death (RCD) impair osteocyte survival, including apoptosis, ferroptosis, and pyroptosis, each of which perturbs bone homeostasis and promotes bone loss (Ru and Wang, 2020; Zhao et al., 2025). Dying osteocytes release RANKL, which stimulates osteoclast activity (Zhao et al., 2025; Cheng et al., 2022; Sfei et al., 2022). Ferroptosis, an iron-dependent form of cell death driven by lipid peroxidation, occurs more frequently in osteoporosis linked to diabetes or aging. Loss of glutathione peroxidase 4 (GPX4) weakens cellular antioxidant capacity, leaving osteocytes vulnerable to oxidative injury. GPX4 prevents ferroptosis by enzymatically reducing lipid hydroperoxides, while ferrostatin-1 is a pharmacological antioxidant that mimics this protective effect by chemically blocking lipid peroxidation. The protective effect of ferrostatin-1 further supports the contribution of ferroptosis to bone loss (Wu X. et al., 2024; Yang et al., 2022). Pyroptosis, inflammasome-mediated lysis via NLRP3 and gasdermin D, is implicated in inflammatory osteoporosis, releasing IL-1β to exacerbate resorption (Zhao et al., 2022). Together, these RCD pathways form a “death-to-resorption” axis, in which apoptotic bodies and DAMPs derived from ferroptotic or pyroptotic cells promote osteoclastogenesis (Zhao et al., 2025; Li C. et al., 2025).
Disruption of the LCN further weakens osteocyte function. Reduced connectivity limits mechanosensation and molecular transport (Tiede-Lewis and Dallas, 2019; Schurman et al., 2021). Aging or glucocorticoid exposure reduces canalicular density and connectivity, impairing fluid shear stress transmission (Yılmaz et al., 2025; Rodriguez et al., 2025). LCN fragmentation is associated with lacunar mineralization and greater fracture susceptibility (Yu et al., 2020). Importantly, alterations in the LCN appear before cortical porosity develops in osteoporotic bone, underscoring the essential role of network integrity in skeletal adaptation (van Tol et al., 2020; Vahidi et al., 2021).
4.2 Osteocyte-derived signaling molecules in osteoporosis
Osteocytes regulate bone metabolism through secreted factors, which show marked dysregulation in osteoporosis. Sclerostin, a Wnt/β-catenin inhibitor from mature osteocytes, suppresses osteoblast differentiation and bone formation (Zhang et al., 2023). Estrogen deficiency, as seen in postmenopausal osteoporosis, increases sclerostin expression, suppressing Wnt-driven bone formation and inducing osteocyte apoptosis (Reppe et al., 2015). Epigenetic changes, such as SOST promoter methylation, further reduce osteogenic transcriptional activity in postmenopausal osteoporosis (Shan et al., 2019). FGF23, a phosphatonin, rises in osteoporotic bone, disturbing phosphate balance and impairing mineralization (Dallas et al., 2013; Michigami, 2023). RANKL expression in osteocytes also escalates, driving osteoclast maturation and activity via the RANK/RANKL/OPG axis. These shifts are amplified by aging, where senescent osteocytes accumulate, releasing pro-inflammatory cytokines like IL-6 and TNF-α that further upregulate RANKL and sclerostin (Wang et al., 2019; Florencio-Silva et al., 2015). Inflammatory conditions and oxidative stress enhance this dysregulation by inducing apoptosis and releasing DAMPs, which sustain resorption signals (Yu et al., 2017; Yan et al., 2023). Endocrine imbalance, particularly low estrogen, also links directly to ferroptosis and bone fragility (Jiang et al., 2024).
4.3 Osteocyte metabolic abnormalities and interactions with bone marrow fat and inflammation
Beyond signaling perturbations, osteocyte metabolic abnormalities in osteoporosis intertwine with bone marrow adipose tissue (BMAT) expansion and an inflammatory microenvironment, fostering a vicious cycle of metabolic reprogramming, oxidative stress, and inflammatory factor release. Osteocytes experience notable changes in their metabolism, often shifting toward glycolysis instead of relying mainly on oxidative phosphorylation. This metabolic adaptation helps these cells survive in low-oxygen environments, but it can also result in increased production of ROS, which in turn impairs mitochondrial function and encourages cellular aging (Srivastava et al., 2022; Bertels et al., 2024). Such reprogramming has been associated with the buildup of BMAT; lipids released from adipocytes can accumulate in the marrow, where they inhibit the formation of new osteoblasts and stimulate osteoclast activity, partly through adipokines such as leptin and adiponectin (Xiao et al., 2024). Oxidative stress further complicates this relationship: higher ROS levels in osteocytes can drive lipid peroxidation and ferroptosis-like cell death, releasing factors that attract macrophages and sustain a bone-resorbing environment (Zhang et al., 2023). In both aging and estrogen deficiency, these metabolic changes in osteocytes have also been linked to systemic consequences like sarcopenia, largely due to interactions between bone and muscle (Choi et al., 2024; He et al., 2020).
Together, these mechanisms place osteocytes at the center of osteoporosis development, where they integrate hormonal, inflammatory, and age-related cues with various metabolic disturbances. Malfunctioning osteocytes not only disturb bone remodeling locally but also exert influence on other organs through endocrine signaling, demonstrating their broader role in physiology. Nonetheless, there are still many uncertainties regarding how these changes unfold over time and across different bone regions, highlighting the need for improved experimental models to clarify osteocyte involvement in osteoporosis progression and guide targeted therapies.
5 Osteocyte in bone remodeling and regeneration
Embedded in the mineralized matrix, osteocytes are central to skeletal homeostasis. They integrate mechanical, biochemical, and hormonal cues to guide remodeling and repair, ensuring structural integrity and adaptation. Dysregulated signaling in these cells contributes to disorders such as osteEmbedded in the mineralized matrix, osteocytes are central to skeletal homeostasis.
5.1 Role of osteocytes in the bone remodeling cycle
Bone remodeling is a continuous process maintaining skeletal mass and architecture, involving coordinated resorption by osteoclasts and formation by osteoblasts within basic multicellular unit (BMU). Osteocytes initiate and regulate this cycle by sensing microenvironmental changes and mediating coupling between resorption and formation phases (Bolamperti et al., 2022; Niedźwiedzki and Filipowska, 2015). As mechanosensory, osteocytes detect fluid shear stress and matrix deformation via their dendritic processes and primary cilia, transducing these into biochemical signals that modulate remodeling (Qin et al., 2020; Choi et al., 2021). In the initiation phase, osteocyte apoptosis—triggered by microdamage, unloading, or glucocorticoid exposure—releases DAMPs and cytokines like IL-6, attracting osteoclast precursors and activating resorption (Bellido, 2014). Osteocytes help maintain the health of their local environment by releasing matrix metalloproteinases (MMPs), which play a part in perilacunar remodeling. Through this process, they keep canaliculi open and support the diffusion of nutrients (Li et al., 2021). Coupling mechanisms ensure formation follows resorption; osteocytes release factors like TGF-β from resorbed matrix, recruiting osteoblast progenitors, while downregulating sclerostin to activate Wnt signaling and promote osteogenesis (Cao W. et al., 2020). When these activities are disrupted—for example, as a result of aging—remodeling becomes uncoordinated, further demonstrating the central role osteocytes play in sustaining the microenvironment. Building on this foundation, osteocytes also direct adaptive responses to external conditions such as mechanical loading or unloading, further shaping skeletal integrity.
5.2 Key functions of osteocytes in adaptive bone remodeling
Bone adapts to mechanical demands through Wolff’s law, with osteocytes as primary sensors translating physical stimuli into molecular responses that adjust mass and geometry (Hughes et al., 2020; Wang et al., 2022). Under loading (e.g., exercise), fluid flow activates integrin-αvβ3 and Piezo1 channels, triggering Ca2+ influx and downstream pathways like ERK/MAPK, which downregulate sclerostin and upregulate Wnt ligands for enhanced osteogenesis (Wang et al., 2022). Concurrently, loading suppresses RANKL, inhibiting resorption and promoting perilacunar matrix mineralization via DMP1 and MEPE (Choi et al., 2021; Klein-Nulend et al., 2012). In contrast, unloading—as in microgravity or bed rest—induces osteocyte senescence and apoptosis, elevating sclerostin and RANKL, leading to uncoupled resorption and bone loss (Hu et al., 2014; Man et al., 2022). Molecularly, reduced mechanotransduction disrupts cytoskeleton-integrin linkages, activating NF-κB and oxidative stress pathways, with upregulated FGF23 exacerbating phosphate waste (Sonawane et al., 2025). Spaceflight studies show that microgravity alters the LCN, impairing fluid and nutrient transport (Man et al., 2022). Osteocytes adapt to environmental change through several mechanisms (Hughes et al., 2020): first, they participate in random (stochastic) remodeling to help maintain bone; second, they are involved in repairing small areas of damage; third, they respond to inactivity by encouraging bone resorption; and finally, they contribute to bone formation in response to mechanical loading. Osteocytes use their cytoskeleton, composed of actin filaments and microtubules, to integrate signals and maintain skeletal health. Looking ahead, new technologies such as organoids and in vivo imaging may help clarify how these processes change over time and space, which could eventually support the design of therapies that mimic healthy mechanical signaling.
5.3 Osteocytes in fracture healing and bone regeneration: regulation of bone repair, angiogenesis, and stem cell recruitment
Fracture healing takes place in several phases, starting with inflammation, followed by soft callus formation, hard callus ossification, and ultimately remodeling. Osteocytes, both at the injury site and surrounding area, are not passive during these events; instead, they influence each phase by adjusting local inflammation, guiding the entry of new blood vessels, and affecting how progenitor cells behave (Choy et al., 2020). Upon fracture, mechanical disruption induces osteocyte apoptosis, releasing pro-inflammatory signals, which recruit macrophages and initiate hematoma formation (Bahney et al., 2019). Surviving osteocytes upregulate hypoxia-inducible factor-1α (HIF-1α) in response to local hypoxia, promoting VEGF expression to drive angiogenesis essential for nutrient supply and progenitor influx (Bixel et al., 2024; van Brakel et al., 2024). The connection between blood vessel growth and new bone formation is especially important. Osteocytes produce signals such as PDGF-BB and endothelin-1, which promote the growth and maturation of endothelial cells, while type H vessels present in the callus region foster the development of osteoprogenitors (Grosso et al., 2017). In the later stages, as the bone remodels, osteocytes detect how mechanical forces shift within the callus. In response, they alter RANKL and OPG levels, which helps shape the newly formed bone and restore the cortex (Iaquinta et al., 2019). Recently, experimental approaches using exosomes derived from mesenchymal stem cells to target osteocyte pathways have shown promise in boosting bone repair, reflecting the far-reaching, hormone-like roles that osteocytes take on during healing.
6 Therapeutic targeting of osteocytes in osteoporosis and bone disease
Through a combination of paracrine signaling, detection of mechanical forces, and control of both osteoclast and osteoblast activity, osteocytes direct the ongoing remodeling of bone. When the regulatory role of osteocytes is disrupted, it leads to osteoporosis and other bone diseases characterized by decreased bone strength and increased risk of fractures. Traditionally, most treatments for osteoporosis have targeted osteoclasts (to reduce bone resorption) or osteoblasts (to enhance bone formation). However, osteocytes stand at the center of this balance, as they coordinate both sides by secreting sclerostin, RANKL, and other mediators. This places osteocytes not as isolated players, but as master regulators of the osteoblast–osteoclast axis. Progress in this field has therefore brought increasing attention to osteocytes as therapeutic targets, with new treatments focusing on blocking osteocyte-derived molecules, exploring tissue regeneration, and moving toward precision medicine. The following sections highlight representative clinical and preclinical advances.
6.1 Sclerostin inhibitors and novel bone anabolic agents
Sclerostin, secreted by osteocytes, inhibits Wnt/β-catenin signaling and suppresses bone formation. Neutralizing sclerostin has emerged as a powerful anabolic strategy for osteoporosis (Ke et al., 2012). Romosozumab, a monoclonal antibody targeting sclerostin, promotes bone accrual by enhancing osteoblast activity while transiently reducing resorption. Phase 3 trials, including FRAME and ARCH, demonstrated significant increases in bone mineral density (BMD) at the spine and hip with fracture risk reductions for vertebral fractures over 12 months (Lewiecki, 2020). Recent real-world studies from 2024 to 2025 further confirmed its efficacy in postmenopausal women, reporting BMD increases of 6.58%–14.65% at lumbar spine and femoral neck after 12 months, especially when sequenced after denosumab to prevent rebound bone loss (Park et al., 2025; Piasentier et al., 2025). Moreover, microarchitectural improvements, assessed via high-resolution peripheral quantitative CT, reveal enhanced trabecular connectivity and cortical thickness (McClung et al., 2025). Beyond romosozumab, bispecific antibodies combining sclerostin inhibition with RANKL blockade are under development, aiming for dual anabolic–antiresorptive effects in preclinical studies (Xu et al., 2023; Elahmer et al., 2024). It is worth noting that other clinical-stage therapies, including the PTH analogs teriparatide and abaloparatide, as well as the anti-RANKL antibody denosumab, have already shown proven efficacy in reducing fracture risk and improving bone mass. These agents primarily act by stimulating osteoblast activity (teriparatide/abaloparatide) or suppressing osteoclast function (denosumab) (Ebina et al., 2025; Bone et al., 2017). In this therapeutic landscape, sclerostin inhibitors like romosozumab are distinctive in directly targeting osteocyte-derived signals, thereby complementing existing approaches and broadening options for individualized osteoporosis management.
6.2 Stem cell and gene editing therapies: prospects for osteocyte-targeted regenerative medicine
Regenerative strategies focus on rebuilding osteocyte networks and restoring bone balance, with approaches that use stem cells as well as gene-editing technologies. MSCs, sourced from either bone marrow or fat tissue, can differentiate into osteocytes and also release growth factors such as BMPs, which contribute to bone repair (Dalle Carbonare et al., 2025; Chu et al., 2024). In some models of osteoporosis, MSCs engineered to produce more PDGFB have been shown to boost the formation of trabecular bone and increase bone strength, with reports of up to 45% greater bone volume (Chen et al., 2015). Researchers have also used CRISPR-Cas9 gene editing to specifically alter genes expressed in osteocytes, including those involved in sclerostin regulation and mechanosensing pathways (He et al., 2017; Michalski and Williams, 2023). Some preclinical work has transplanted iPSCs modified by CRISPR into bone defects, resulting in the generation of mature, functional osteocytes that enhance both mineralization and blood vessel growth within the repaired tissue (Iaquinta et al., 2019). Delivering edited MSCs via exosomes has also been explored as a way to strengthen paracrine signaling, lower inflammation, and support new bone formation in osteoporosis models (Chen Y. et al., 2024). One study showed that mesenchymal stem cells combined with strontium-containing scaffolds enhanced cell attachment and promoted bone growth in osteoporotic rats (Wu et al., 2020). In a key study, AAV gene therapy targeting SHN3 in bone reversed bone loss in osteoporosis models (Lin et al., 2024). Genetically modified stem cell therapy is a safe and effective method that can significantly improve BMD and BV/TV in animal models of osteoporosis (Huang et al., 2025). While these approaches are promising, they face obstacles such as immune rejection of transplanted cells, off-target gene editing effects, and scalability for human applications.
6.3 Multi-omics, AI, and personalized precision therapy
Multi-omics integration—encompassing genomics, proteomics, and metabolomics—unveils osteocyte-driven biomarkers for osteoporosis. Genome-wide association studies identify variants in osteocyte genes like SOST, influencing BMD and fracture susceptibility (Li et al., 2024). Proteomic studies have identified higher levels of sclerostin and DKK1 as markers that may help predict bone health, while analyses of cellular metabolites have pointed to abnormal lipid patterns in aging osteocytes (Wang et al., 2024; Yuan et al., 2024). Recently, machine learning and other AI-based tools have begun to combine these biological datasets for risk prediction, often providing a more precise estimate of fracture risk than what is possible with standard DXA imaging (Saleem and January 2024; Mis et al., 2025). Deep learning on multi-modal datasets stratifies patients for personalized therapies.
7 Perspectives and future directions
Recent studies highlight how osteocytes employ diverse molecular sensors—including the cytoskeleton, primary cilia, integrins, and ion channels such as Piezo1—to convert mechanical forces into biochemical signals that regulate remodeling and structural adaptation. This complexity explains their central role not only in osteoporosis but also in conditions such as osteoarthritis and bone metastasis, where impaired mechanotransduction aggravates disease. Currently, a growing number of studies are employing systems biology approaches to elucidate the functions of osteoblasts. Single-cell RNA sequencing, spatial transcriptomics, and artificial intelligence technologies are helping us to more precisely map osteoblast heterogeneity and the LCN (Tong et al., 2024). Improvements in live imaging and organ-on-a-chip platforms also provide opportunities to study osteocyte–osteoblast–osteoclast interactions under mechanical load. There is increasing recognition of osteocyte-endocrine effects on muscle and kidney—which could open new perspectives on aging and whole-body disease. However, major gaps remain in human in vivo research, as most data are derived from animal and preclinical models. High-resolution imaging, single-cell, and spatial omics are essential for characterizing osteocyte biology in the human skeleton. Moving forward, teamwork across disciplines, especially between bioengineering and pharmacology, will be essential for turning basic discoveries about osteocytes into tailored therapies that boost regeneration and bone health.
Author contributions
YW: Formal Analysis, Investigation, Writing – original draft. DG: Investigation, Writing – review and editing. ZL: Formal Analysis, Investigation, Writing – original draft. DQ: Formal Analysis, Investigation, Writing – original draft. GT: Formal Analysis, Funding acquisition, Investigation, Writing – original draft. ZX: Formal Analysis, Funding acquisition, Supervision, Writing – review and editing. HX: Formal Analysis, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82174410 and 82474541) and the Shandong Province Medical Staff Science and Technology Program (SDYWZGKCJH2022039).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Grammar correction and language polishing.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agoro, R., Nookaew, I., Noonan, M. L., Marambio, Y. G., Liu, S., Chang, W., et al. (2023). Single cell cortical bone transcriptomics define novel osteolineage gene sets altered in chronic kidney disease. Front. Endocrinol. (Lausanne) 14, 1063083. doi:10.3389/fendo.2023.1063083
Bahney, C. S., Zondervan, R. L., Allison, P., Theologis, A., Ashley, J. W., Ahn, J., et al. (2019). Cellular biology of fracture healing. J. Orthop. Res. 37, 35–50. doi:10.1002/jor.24170
Bellido, T. (2014). Osteocyte-driven bone remodeling. Calcif. Tissue Int. 94, 25–34. doi:10.1007/s00223-013-9774-y
Bertels, J. C., He, G., and Long, F. (2024). Metabolic reprogramming in skeletal cell differentiation. Bone Res. 12, 57. doi:10.1038/s41413-024-00374-0
Bixel, M. G., Sivaraj, K. K., Timmen, M., Mohanakrishnan, V., Aravamudhan, A., Adams, S., et al. (2024). Angiogenesis is uncoupled from osteogenesis during calvarial bone regeneration. Nat. Commun. 15, 4575. doi:10.1038/s41467-024-48579-5
Bolamperti, S., Villa, I., and Rubinacci, A. (2022). Bone remodeling: an operational process ensuring survival and bone mechanical competence. Bone Res. 10, 48. doi:10.1038/s41413-022-00219-8
Bone, H. G., Wagman, R. B., Brandi, M. L., Brown, J. P., Chapurlat, R., Cummings, S. R., et al. (2017). 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 5, 513–523. doi:10.1016/s2213-8587(17)30138-9
Busch, C., Nyamondo, K., and Wheadon, H. (2024). Complexities of modeling the bone marrow microenvironment to facilitate hematopoietic research. Exp. Hematol. 135, 104233. doi:10.1016/j.exphem.2024.104233
Cao, H., Yan, Q., Wang, D., Lai, Y., Zhou, B., Zhang, Q., et al. (2020a). Focal adhesion protein Kindlin-2 regulates bone homeostasis in mice. Bone Res. 8 (2), 2. doi:10.1038/s41413-019-0073-8
Cao, W., Helder, M. N., Bravenboer, N., Wu, G., Jin, J., Ten Bruggenkate, C. M., et al. (2020b). Is there a governing role of osteocytes in bone tissue regeneration? Curr. Osteoporos. Rep. 18, 541–550. doi:10.1007/s11914-020-00610-6
Dalle Carbonare, L., Cominacini, M., Trabetti, E., Bombieri, C., Pessoa, J., Romanelli, M. G., et al. (2025). The bone microenvironment: new insights into the role of stem cells and cell communication in bone regeneration. Stem Cell Res. Ther. 16, 169. doi:10.1186/s13287-025-04288-4
Chai, R. C. (2022). Single-cell RNA sequencing: unravelling the bone one cell at a time. Curr. Osteoporos. Rep. 20, 356–362. doi:10.1007/s11914-022-00735-w
Chen, W., Baylink, D. J., Brier-Jones, J., Neises, A., Kiroyan, J. B., Rundle, C. H., et al. (2015). PDGFB-Based stem cell gene therapy increases bone strength in the mouse. Proc. Natl. Acad. Sci. U. S. A. 112, E3893–E3900. doi:10.1073/pnas.1501759112
Chen, H., Liu, W., Wu, X., Gou, M., Shen, J., and Wang, H. (2017). Advanced glycation end products induced IL-6 and VEGF-A production and apoptosis in osteocyte-like MLO-Y4 cells by activating RAGE and ERK1/2, P38 and STAT3 signalling pathways. Int. Immunopharmacol. 52, 143–149. doi:10.1016/j.intimp.2017.09.004
Chen, S., Lei, J., Mou, H., Zhang, W., Jin, L., Lu, S., et al. (2024a). Multiple influence of immune cells in the bone metastatic cancer microenvironment on tumors. Front. Immunol. 15, 1335366. doi:10.3389/fimmu.2024.1335366
Chen, Y., Huang, Y., Li, J., Jiao, T., and Yang, L. (2024b). Enhancing osteoporosis treatment with engineered mesenchymal stem cell-derived extracellular vesicles: mechanisms and advances. Cell Death Dis. 15, 119. doi:10.1038/s41419-024-06508-w
Cheng, C. H., Chen, L. R., and Chen, K. H. (2022). Osteoporosis due to hormone imbalance: an overview of the effects of estrogen deficiency and glucocorticoid overuse on bone turnover. Int. J. Mol. Sci. 23, 1376. doi:10.3390/ijms23031376
Choi, J. U. A., Kijas, A. W., Lauko, J., and Rowan, A. E. (2021). The mechanosensory role of osteocytes and implications for bone health and disease states. Front. Cell Dev. Biol. 9, 770143. doi:10.3389/fcell.2021.770143
Choi, I. A., Umemoto, A., Mizuno, M., and Park-Min, K. H. (2024). Bone metabolism - an underappreciated player. NPJ Metab. Health Dis. 2, 12. doi:10.1038/s44324-024-00010-9
Choy, M. H. V., Wong, R. M. Y., Chow, S. K. H., Li, M. C., Chim, Y. N., Li, T. K., et al. (2020). How much do we know about the role of osteocytes in different phases of fracture healing? A systematic review. J. Orthop. Transl. 21, 111–121. doi:10.1016/j.jot.2019.07.005
Chu, X., Xiong, Y., Lu, L., Wang, Y., Wang, J., Zeng, R., et al. (2024). Research progress of gene therapy combined with tissue engineering to promote bone regeneration. Apl. Bioeng. 8, 031502. doi:10.1063/5.0200551
Courbon, G., Kentrup, D., Thomas, J. J., Wang, X., Tsai, H. H., Spindler, J., et al. (2023). FGF23 directly inhibits osteoprogenitor differentiation in Dmp1-knockout mice. JCI Insight 8, e156850. doi:10.1172/jci.insight.156850
Creecy, A., Damrath, J. G., and Wallace, J. M. (2020). Control of bone matrix properties by osteocytes. Front. Endocrinol. (Lausanne) 11, 578477. doi:10.3389/fendo.2020.578477
Dallas, S. L., and Bonewald, L. F. (2010). Dynamics of the transition from osteoblast to osteocyte. Ann. N. Y. Acad. Sci. 1192, 437–443. doi:10.1111/j.1749-6632.2009.05246.x
Dallas, S. L., Prideaux, M., and Bonewald, L. F. (2013). The osteocyte: an endocrine cell. and more. More. Endocr. Rev. 34, 658–690. doi:10.1210/er.2012-1026
Dashti, P., Lewallen, E. A., Gordon, J. A. R., Montecino, M. A., Davie, J. R., Stein, G. S., et al. (2024). Epigenetic regulators controlling osteogenic lineage commitment and bone formation. Bone 181, 117043. doi:10.1016/j.bone.2024.117043
Delgado-Calle, J., and Bellido, T. (2015). Osteocytes and skeletal pathophysiology. Curr. Mol. Biol. Rep. 1, 157–167. doi:10.1007/s40610-015-0026-y
Delgado-Calle, J., and Bellido, T. (2022). The osteocyte as a signaling cell. Physiol. Rev. 102, 379–410. doi:10.1152/physrev.00043.2020
Delgado-Calle, J., Sañudo, C., Bolado, A., Fernández, A. F., Arozamena, J., Pascual-Carra, M. A., et al. (2012). DNA methylation contributes to the regulation of sclerostin expression in human osteocytes. J. Bone Min. Res. 27, 926–937. doi:10.1002/jbmr.1491
Ding, P., Gao, C., Zhou, J., Mei, J., Li, G., Liu, D., et al. (2024). Mitochondria from osteolineage cells regulate myeloid cell-mediated bone resorption. Nat. Commun. 15, 5094. doi:10.1038/s41467-024-49159-3
Donmez, B. O., Karagur, E. R., Donmez, A. C., Choi, J., and Akkus, O. (2022). Calcium-dependent activation of PHEX, MEPE and DMP1 in osteocytes. Mol. Med. Rep. 26, 359. doi:10.3892/mmr.2022.12876
Dussold, C., Gerber, C., White, S., Wang, X., Qi, L., Francis, C., et al. (2019). DMP1 prevents osteocyte alterations, FGF23 elevation and left ventricular hypertrophy in mice with chronic kidney disease. Bone Res. 7, 12. doi:10.1038/s41413-019-0051-1
Ebina, K., Etani, Y., Noguchi, T., Nakata, K., and Okada, S. (2025). Clinical effects of teriparatide, abaloparatide, and romosozumab in postmenopausal osteoporosis. J. Bone Min. Metab. 43, 3–9. doi:10.1007/s00774-024-01536-0
Elahmer, N. R., Wong, S. K., Mohamed, N., Alias, E., Chin, K. Y., and Muhammad, N. (2024). Mechanistic insights and therapeutic strategies in osteoporosis: a comprehensive review. Biomedicines 12, 1635. doi:10.3390/biomedicines12081635
Elango, J., Sanchez, C., de Val, J. E. M. S., Henrotin, Y., Wang, S., Motaung, K. S. C. M., et al. (2018). Cross-talk between primary osteocytes and bone marrow macrophages for osteoclastogenesis upon collagen treatment. Sci. Rep. 8, 5318. doi:10.1038/s41598-018-23532-x
Feng, S., Li, J., Tian, J., Lu, S., and Zhao, Y. (2023). Application of single-cell and spatial omics in musculoskeletal disorder research. Int. J. Mol. Sci. 24, 2271. doi:10.3390/ijms24032271
Florencio-Silva, R., Sasso, G. R., Sasso-Cerri, E., Simões, M. J., and Cerri, P. S. (2015). Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed. Res. Int. 2015, 421746. doi:10.1155/2015/421746
Franz-Odendaal, T. A., Hall, B. K., and Witten, P. E. (2006). Buried alive: how osteoblasts become osteocytes. Dev. Dyn. 235, 176–190. doi:10.1002/dvdy.20603
Grosso, A., Burger, M. G., Lunger, A., Schaefer, D. J., Banfi, A., and Di Maggio, N. (2017). It takes two to Tango: coupling of angiogenesis and osteogenesis for bone regeneration. Front. Bioeng. Biotechnol. 5, 68. doi:10.3389/fbioe.2017.00068
Han, Y., You, X., Xing, W., Zhang, Z., and Zou, W. (2018). Paracrine and endocrine actions of bone-the functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts. Bone Res. 6, 16. doi:10.1038/s41413-018-0019-6
Hanai, A., Kawabata, A., Nakajima, K., Masuda, K., Urakawa, I., Abe, M., et al. (2023). Single-cell RNA sequencing identifies Fgf23-expressing osteocytes in response to 1,25-dihydroxyvitamin D(3) treatment. Front. Physiol. 14, 1102751. doi:10.3389/fphys.2023.1102751
Hassan, M. Q., Maeda, Y., Taipaleenmaki, H., Zhang, W., Jafferji, M., Gordon, J. A. R., et al. (2012). miR-218 directs a Wnt signaling circuit to promote differentiation of osteoblasts and osteomimicry of metastatic cancer cells. J. Biol. Chem. 287, 42084–42092. doi:10.1074/jbc.M112.377515
He, Q., Bouley, R., Liu, Z., Wein, M. N., Zhu, Y., Spatz, J. M., et al. (2017). Large G protein α-subunit XLαs limits clathrin-mediated endocytosis and regulates tissue iron levels in vivo. Proc. Natl. Acad. Sci. U. S. A. 114, e9559–e9568. doi:10.1073/pnas.1712670114
He, C., He, W., Hou, J., Chen, K., Huang, M., Yang, M., et al. (2020). Bone and muscle crosstalk in aging. Front. Cell Dev. Biol. 8, 585644. doi:10.3389/fcell.2020.585644
Holmbeck, K., Bianco, P., Pidoux, I., Inoue, S., Billinghurst, R. C., Wu, W., et al. (2005). The metalloproteinase MT1-MMP is required for normal development and maintenance of osteocyte processes in bone. J. Cell Sci. 118, 147–156. doi:10.1242/jcs.01581
Hu, L., Li, R., Su, P., Arfat, Y., Zhang, G., Shang, P., et al. (2014). Response and adaptation of bone cells to simulated microgravity. Acta Astronaut. 104, 396–408. doi:10.1016/j.actaastro.2014.05.008
Huang, M., Wu, X. S., Xiao, N., Huang, X., and Lin, P. F. (2025). Genetically modified stem cells for osteoporosis: a systematic review and meta-analysis of preclinical studies. BMC Musculoskelet. Disord. 26, 259. doi:10.1186/s12891-025-08507-0
Hughes, J. M., Castellani, C. M., Popp, K. L., Guerriere, K. I., Matheny, R. W., Nindl, B. C., et al. (2020). The central role of osteocytes in the four adaptive pathways of bone's mechanostat. Exerc Sport Sci. Rev. 48, 140–148. doi:10.1249/jes.0000000000000225
Husain, A., and Jeffries, M. A. (2017). Epigenetics and bone remodeling. Curr. Osteoporos. Rep. 15, 450–458. doi:10.1007/s11914-017-0391-y
Iandolo, D., Strigini, M., Guignandon, A., and Vico, L. (2021). Osteocytes and weightlessness. Curr. Osteoporos. Rep. 19, 626–636. doi:10.1007/s11914-021-00713-8
Iaquinta, M. R., Mazzoni, E., Bononi, I., Rotondo, J. C., Mazziotta, C., Montesi, M., et al. (2019). Adult stem cells for bone regeneration and repair. Front. Cell Dev. Biol. 7, 268. doi:10.3389/fcell.2019.00268
Jiang, Z., Qi, G., He, X., Yu, Y., Cao, Y., Zhang, C., et al. (2024). Ferroptosis in osteocytes as a target for protection against postmenopausal osteoporosis. Adv. Sci. (Weinh) 11, e2307388. doi:10.1002/advs.202307388
Karsdal, M. A., Andersen, T. A., Bonewald, L., and Christiansen, C. (2004). Matrix metalloproteinases (MMPs) safeguard osteoblasts from apoptosis during transdifferentiation into osteocytes: MT1-MMP maintains osteocyte viability. DNA Cell Biol. 23, 155–165. doi:10.1089/104454904322964751
Ke, H. Z., Richards, W. G., Li, X., and Ominsky, M. S. (2012). Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases. Endocr. Rev. 33, 747–783. doi:10.1210/er.2011-1060
Klein-Nulend, J., Bacabac, R. G., and Bakker, A. D. (2012). Mechanical loading and how it affects bone cells: the role of the osteocyte cytoskeleton in maintaining our skeleton. Eur. Cell Mater 24, 278–291. doi:10.22203/ecm.v024a20
Knowles, H. J., Chanalaris, A., Koutsikouni, A., Cribbs, A. P., Grover, L. M., and Hulley, P. A. (2023). Mature primary human osteocytes in mini organotypic cultures secrete FGF23 and PTH1-34-regulated sclerostin. Front. Endocrinol. (Lausanne) 14, 1167734. doi:10.3389/fendo.2023.1167734
Kulkarni, R. N., Bakker, A. D., Gruber, E. V., Chae, T. D., Veldkamp, J. B. B., Klein-Nulend, J., et al. (2012). MT1-MMP modulates the mechanosensitivity of osteocytes. Biochem. Biophys. Res. Commun. 417, 824–829. doi:10.1016/j.bbrc.2011.12.045
Lewiecki, E. M. (2020). Romosozumab, clinical trials, and real-world care of patients with osteoporosis. Ann. Transl. Med. 8, 974. doi:10.21037/atm.2020.03.196
Li, X., Han, L., Nookaew, I., Mannen, E., Silva, M. J., Almeida, M., et al. (2019). Stimulation of Piezo1 by mechanical signals promotes bone anabolism. Elife 8, e49631. doi:10.7554/eLife.49631
Li, M. C. M., Chow, S. K. H., Wong, R. M. Y., Qin, L., and Cheung, W. H. (2021). The role of osteocytes-specific molecular mechanism in regulation of mechanotransduction - a systematic review. J. Orthop. Transl. 29, 1–9. doi:10.1016/j.jot.2021.04.005
Li, M. C. M., Chow, S. K. H., Wong, R. M. Y., Chen, B., Cheng, J. C. Y., Qin, L., et al. (2022). Osteocyte-specific dentin matrix protein 1: the role of mineralization regulation in low-magnitude high-frequency vibration enhanced osteoporotic fracture healing. Bone Jt. Res. 11, 465–476. doi:10.1302/2046-3758.117.Bjr-2021-0476.R2
Li, Q., Wang, J., and Zhao, C. (2024). From genomics to metabolomics: molecular insights into osteoporosis for enhanced diagnostic and therapeutic approaches. Biomedicines 12, 2389. doi:10.3390/biomedicines12102389
Li, X., Zhang, C., Vail, C. E., Sherrill, J. T., and Xiong, J. (2025a). Piezo1 expression in mature osteocytes is dispensable for the skeletal response to mechanical loading. Bone 190, 117276. doi:10.1016/j.bone.2024.117276
Li, C., Gong, H., Shi, P., Liu, S., and Zhang, Q. (2025b). Different forms of regulated cell death in type-2-diabetes-mellitus-related osteoporosis: a focus on mechanisms and therapeutic strategies. Int. J. Mol. Sci. 26, 4417. doi:10.3390/ijms26094417
Liao, P., Chen, L., Zhou, H., Mei, J., Chen, Z., Wang, B., et al. (2024). Osteocyte mitochondria regulate angiogenesis of transcortical vessels. Nat. Commun. 15, 2529. doi:10.1038/s41467-024-46095-0
Lin, C., Yang, Y. S., Ma, H., Chen, Z., Chen, D., John, A. A., et al. (2024). Engineering a targeted and safe bone anabolic gene therapy to treat osteoporosis in alveolar bone loss. Mol. Ther. 32, 3080–3100. doi:10.1016/j.ymthe.2024.06.036
Liu, N., Ma, Y., Gong, W., Shao, X., Shi, T., Li, L., et al. (2025). Osteocyte-derived extracellular vesicles mediate the bone-to-cartilage crosstalk and promote osteoarthritis progression. Nat. Commun. 16, 4746. doi:10.1038/s41467-025-59861-5
Lu, J., He, Q., Wang, H., Yao, L., Duffy, M., Guo, H., et al. (2025). Bone marrow adipogenic lineage precursors are the major regulator of bone resorption in adult mice. Bone Res. 13, 39. doi:10.1038/s41413-025-00405-4
Ma, Q., Miri, Z., Haugen, H. J., Moghanian, A., and Loca, D. (2023). Significance of mechanical loading in bone fracture healing, bone regeneration, and vascularization. J. Tissue Eng. 14, 20417314231172573. doi:10.1177/20417314231172573
Man, J., Graham, T., Squires-Donelly, G., and Laslett, A. L. (2022). The effects of microgravity on bone structure and function. NPJ Microgravity 8 (9), 9. doi:10.1038/s41526-022-00194-8
Mathavan, N., Singh, A., Marques, F. C., Günther, D., Kuhn, G. A., Wehrle, E., et al. (2025). Spatial transcriptomics in bone mechanomics: exploring the mechanoregulation of fracture healing in the era of spatial omics. Sci. Adv. 11, eadp8496. doi:10.1126/sciadv.adp8496
Matsushita, Y., Noguchi, A., Ono, W., and Ono, N. (2023). Multi-omics analysis in developmental bone biology. Jpn. Dent. Sci. Rev. 59, 412–420. doi:10.1016/j.jdsr.2023.10.006
Mazur, C. M., Woo, J. J., Yee, C. S., Fields, A. J., Acevedo, C., Bailey, K. N., et al. (2019). Osteocyte dysfunction promotes osteoarthritis through MMP13-dependent suppression of subchondral bone homeostasis. Bone Res. 7, 34. doi:10.1038/s41413-019-0070-y
McClung, M. R., Betah, D., Leder, B. Z., Kendler, D. L., Oates, M., Timoshanko, J., et al. (2025). Romosozumab improves microarchitecture as assessed by tissue thickness-adjusted trabecular bone score in postmenopausal women with osteoporosis. J. Bone Min. Res. 40, 193–200. doi:10.1093/jbmr/zjae194
Michalski, M. N., and Williams, B. O. (2023). The past, present, and future of genetically engineered mouse models for skeletal biology. Biomolecules 13, 1311. doi:10.3390/biom13091311
Michigami, T. (2022). Roles of osteocytes in phosphate metabolism. Front. Endocrinol. (Lausanne) 13, 967774. doi:10.3389/fendo.2022.967774
Michigami, T. (2023). Paracrine and endocrine functions of osteocytes. Clin. Pediatr. Endocrinol. 32, 1–10. doi:10.1297/cpe.2022-0053
Misir, A., and Yuce, A. (2025). AI in orthopedic research: a comprehensive review. J. Orthop. Res. 43, 1508–1527. doi:10.1002/jor.26109
Moriishi, T., and Komori, T. (2022). Osteocytes: their lacunocanalicular structure and mechanoresponses. Int. J. Mol. Sci. 23, 4373. doi:10.3390/ijms23084373
Mulcrone, P. L., Edwards, S. K. E., Petrusca, D. N., Haneline, L. S., Delgado-Calle, J., and Roodman, G. D. (2020). Osteocyte Vegf-a contributes to myeloma-associated angiogenesis and is regulated by Fgf23. Sci. Rep. 10, 17319. doi:10.1038/s41598-020-74352-x
Mullen, C. A., Haugh, M. G., Schaffler, M. B., Majeska, R. J., and McNamara, L. M. (2013). Osteocyte differentiation is regulated by extracellular matrix stiffness and intercellular separation. J. Mech. Behav. Biomed. Mater 28, 183–194. doi:10.1016/j.jmbbm.2013.06.013
Niedźwiedzki, T., and Filipowska, J. (2015). Bone remodeling in the context of cellular and systemic regulation: the role of osteocytes and the nervous system. J. Mol. Endocrinol. 55, R23–R36. doi:10.1530/jme-15-0067
Nottmeier, C., Lavicky, J., Gonzalez Lopez, M., Knauth, S., Kahl-Nieke, B., Amling, M., et al. (2023). Mechanical-induced bone remodeling does not depend on Piezo1 in dentoalveolar hard tissue. Sci. Rep. 13, 9563. doi:10.1038/s41598-023-36699-9
Nusrat, S., Din, R. U., Tariq, M. A., and Yang, H. (2025). Epigenetic dysregulation and osteocyte senescence: convergent drivers of osteosarcopenia in aging bone and muscle. Aging Dis. doi:10.14336/ad.2025.0370
Palumbo, C., and Ferretti, M. (2021). The osteocyte: from “Prisoner” to “Orchestrator”. J. Funct. Morphol. Kinesiol 6, 28. doi:10.3390/jfmk6010028
Park, J. Y., Park, H. M., Song, J. Y., Hwang, K. J., Kim, M. R., and Chung, Y. J. (2025). Real-world evaluation of 12-Month romosozumab treatment in Korean women with severe osteoporosis: potential synergy with hormone therapy. J. Clin. Med. 14, 2958. doi:10.3390/jcm14092958
Park-Min, K. H. (2017). Epigenetic regulation of bone cells. Connect. Tissue Res. 58, 76–89. doi:10.1080/03008207.2016.1177037
Piasentier, A., Fanti, A., Birtolo, M. F., Vena, W., Colle, R., Gentile, L. M. S., et al. (2025). Early administration of romosozumab prevents rebound of bone resorption related to denosumab withdrawal in fractured post-menopausal women: a real-world prospective study. J. Endocrinol. Invest. 48, 1249–1256. doi:10.1007/s40618-025-02542-3
Ponzetti, M., and Rucci, N. (2021). Osteoblast differentiation and signaling: established concepts and emerging topics. Int. J. Mol. Sci. 22, 6651. doi:10.3390/ijms22136651
Prideaux, M., Loveridge, N., Pitsillides, A. A., and Farquharson, C. (2012). Extracellular matrix mineralization promotes E11/gp38 glycoprotein expression and drives osteocytic differentiation. PLoS One 7, e36786. doi:10.1371/journal.pone.0036786
Prideaux, M., Schutz, C., Wijenayaka, A. R., Findlay, D. M., Campbell, D. G., Solomon, L. B., et al. (2016). Isolation of osteocytes from human trabecular bone. Bone 88, 64–72. doi:10.1016/j.bone.2016.04.017
Prideaux, M., Palmier, M., Kitase, Y., Bonewald, L. F., and O'Connell, T. (2025). Osteocyte differentiation requires glycolysis, but mature osteocytes display metabolic flexibility. bioRxiv, 2025.05.09.652291. doi:10.1101/2025.05.09.652291
Qin, L., Liu, W., Cao, H., and Xiao, G. (2020). Molecular mechanosensors in osteocytes. Bone Res. 8, 23. doi:10.1038/s41413-020-0099-y
Qin, L., He, T., Yang, D., Wang, Y., Li, Z., Yan, Q., et al. (2022). Osteocyte β1 integrin loss causes low bone mass and impairs bone mechanotransduction in mice. J. Orthop. Transl. 34, 60–72. doi:10.1016/j.jot.2022.03.008
Qin, L., Chen, Z., Yang, D., He, T., Xu, Z., Zhang, P., et al. (2023). Osteocyte β3 integrin promotes bone mass accrual and force-induced bone formation in mice. J. Orthop. Transl. 40, 58–71. doi:10.1016/j.jot.2023.05.001
Ratsma, D. M. A., Muller, M., Koedam, M., Zillikens, M. C., and van der Eerden, B. C. J. (2023). In vitro regulation of fibroblast growth factor 23 by 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D synthesized by osteocyte-like MC3T3-E1 cells. Eur. J. Endocrinol. 189, 448–459. doi:10.1093/ejendo/lvad131
Ratsma, D. M. A., Muller, M., Koedam, M., van Leeuwen, J. P. T. M., Zillikens, M. C., and van der Eerden, B. C. J. (2024). Organic phosphate but not inorganic phosphate regulates Fgf23 expression through MAPK and TGF-β signaling. iScience 27, 109625. doi:10.1016/j.isci.2024.109625
Reppe, S., Noer, A., Grimholt, R. M., Halldórsson, B. V., Medina-Gomez, C., Gautvik, V. T., et al. (2015). Methylation of bone SOST, its mRNA, and serum sclerostin levels correlate strongly with fracture risk in postmenopausal women. J. Bone Min. Res. 30, 249–256. doi:10.1002/jbmr.2342
Rodriguez, J., Lacave, M., Sánchez, L. M., De Lucca, R. C., Tasat, D. R., and Bozal, C. B. (2025). Impact of glucocorticoids on osteocytes and on the lacuno-canalicular system of maxillary and mandibular alveolar bone. Eur. J. Oral Sci. 133, e70000. doi:10.1111/eos.70000
Rowe, P. S. (2012). Regulation of bone-renal mineral and energy metabolism: the PHEX, FGF23, DMP1, MEPE ASARM pathway. Crit. Rev. Eukaryot. Gene Expr. 22, 61–86. doi:10.1615/critreveukargeneexpr.v22.i1.50
Ru, J. Y., and Wang, Y. F. (2020). Osteocyte apoptosis: the roles and key molecular mechanisms in resorption-related bone diseases. Cell Death Dis. 11, 846. doi:10.1038/s41419-020-03059-8
Saleem, S. M., and Jan, S. S. (2024). Integrating machine learning for personalized fracture risk assessment: a multimodal approach. Korean J. Fam. Med. 45, 356–358. doi:10.4082/kjfm.24.0134
Schemenz, V., Gjardy, A., Chamasemani, F. F., Roschger, A., Roschger, P., Zaslansky, P., et al. (2020). Heterogeneity of the osteocyte lacuno-canalicular network architecture and material characteristics across different tissue types in healing bone. J. Struct. Biol. 212, 107616. doi:10.1016/j.jsb.2020.107616
Schurman, C. A., Verbruggen, S. W., and Alliston, T. (2021). Disrupted osteocyte connectivity and pericellular fluid flow in bone with aging and defective TGF-β signaling. Proc. Natl. Acad. Sci. U. S. A. 118, e2023999118. doi:10.1073/pnas.2023999118
Sfeir, J. G., Drake, M. T., Khosla, S., and Farr, J. N. (2022). Skeletal aging. Mayo Clin. Proc. 97, 1194–1208. doi:10.1016/j.mayocp.2022.03.011
Shan, Y., Wang, L., Li, G., Shen, G., Zhang, P., and Xu, Y. (2019). Methylation of bone SOST impairs SP7, RUNX2, and ERα transactivation in patients with postmenopausal osteoporosis. Biochem. Cell Biol. 97, 369–374. doi:10.1139/bcb-2018-0170
Sonawane, R., Patil, S., Rahaman, J., and Mukherjee, D. (2025). Effect of microgravity on bone tissue: mechanisms of osteodegeneration and advanced treatment modalities. Biochem. Biophys. Res. Commun. 771, 152055. doi:10.1016/j.bbrc.2025.152055
Srivastava, R. K., Sapra, L., and Mishra, P. K. O. (2022). Osteometabolism: metabolic alterations in bone pathologies. Cells 11, 3943. doi:10.3390/cells11233943
Sun, W., Chi, S., Li, Y., Ling, S., Tan, Y., Xu, Y., et al. (2019). The mechanosensitive Piezo1 channel is required for bone formation. Elife 8, e47454. doi:10.7554/eLife.47454
Szeliga, A., Grymowicz, M., Kostrzak, A., Smolarczyk, R., Bala, G., Smolarczyk, K., et al. (2024). Bone: a neglected endocrine organ? J. Clin. Med. 13, 3889. doi:10.3390/jcm13133889
Tiede-Lewis, L. M., and Dallas, S. L. (2019). Changes in the osteocyte lacunocanalicular network with aging. Bone 122, 101–113. doi:10.1016/j.bone.2019.01.025
Tong, L., Wijnen, A. J. v., Wang, H., and Chen, D. (2024). Advancing bone biology: the mutual promotion of biology and pioneering technologies. Innovation Life 2, 100078. doi:10.59717/j.xinn-life.2024.100078
Tresguerres, F. G. F., Torres, J., López-Quiles, J., Hernández, G., Vega, J. A., and Tresguerres, I. F. (2020). The osteocyte: a multifunctional cell within the bone. Ann. Anat. 227, 151422. doi:10.1016/j.aanat.2019.151422
Udagawa, N., Koide, M., Nakamura, M., Nakamichi, Y., Yamashita, T., Uehara, S., et al. (2021). Osteoclast differentiation by RANKL and OPG signaling pathways. J. Bone Min. Metab. 39, 19–26. doi:10.1007/s00774-020-01162-6
Vahidi, G., Rux, C., Sherk, V. D., and Heveran, C. M. (2021). Lacunar-canalicular bone remodeling: impacts on bone quality and tools for assessment. Bone 143, 115663. doi:10.1016/j.bone.2020.115663
van Brakel, F., Zhao, Y., and van der Eerden, B. C. J.(2024). Fueling recovery: the importance of energy coupling between angiogenesis and osteogenesis during fracture healing. Bone Rep. 21, 101757. doi:10.1016/j.bonr.2024.101757
van Tol, A. F., Schemenz, V., Wagermaier, W., Roschger, A., Razi, H., Vitienes, I., et al. (2020). The mechanoresponse of bone is closely related to the osteocyte lacunocanalicular network architecture. Proc. Natl. Acad. Sci. U. S. A. 117, 32251–32259. doi:10.1073/pnas.2011504117
Wang, T., Yu, X., and He, C. (2019). Pro-inflammatory cytokines: cellular and molecular drug targets for glucocorticoid-induced-osteoporosis via osteocyte. Curr. Drug Targets 20, 1–15. doi:10.2174/1389450119666180405094046
Wang, L., You, X., Zhang, L., Zhang, C., and Zou, W. (2022). Mechanical regulation of bone remodeling. Bone Res. 10, 16. doi:10.1038/s41413-022-00190-4
Wang, J., Sun, Y. X., and Li, J. (2023). The role of mechanosensor Piezo1 in bone homeostasis and mechanobiology. Dev. Biol. 493, 80–88. doi:10.1016/j.ydbio.2022.11.002
Wang, X. Y., Zhang, R. Z., Wang, Y. K., Pan, S., Yun, S. M., Li, J. J., et al. (2024). An updated overview of the search for biomarkers of osteoporosis based on human proteomics. J. Orthop. Transl. 49, 37–48. doi:10.1016/j.jot.2024.08.015
Wang, H., He, X., Ma, M., Dou, T., Wei, Y., Rux, D., et al. (2025). Integrating spatial and single-cell transcriptomics to characterize mouse long bone fracture healing process. Commun. Biol. 8, 887. doi:10.1038/s42003-025-08316-0
Wu, Q., Wang, X., Jiang, F., Zhu, Z., Wen, J., and Jiang, X. (2020). Study of sr-ca-si-based scaffolds for bone regeneration in osteoporotic models. Int. J. Oral Sci. 12, 25. doi:10.1038/s41368-020-00094-1
Wu, M., Wu, S., Chen, W., and Li, Y. P. (2024a). The roles and regulatory mechanisms of TGF-β and BMP signaling in bone and cartilage development, homeostasis and disease. Cell Res. 34, 101–123. doi:10.1038/s41422-023-00918-9
Wu, X., Fang, X., Lu, F., Chen, Q., Liu, J., and Zheng, L. (2024b). An update on the role of ferroptosis in the pathogenesis of osteoporosis. EFORT Open Rev. 9, 712–722. doi:10.1530/eor-23-0148
Xiao, Z., Huang, J., Cao, L., Liang, Y., Han, X., and Quarles, L. D. (2014). Osteocyte-specific deletion of Fgfr1 suppresses FGF23. PLoS One 9, e104154. doi:10.1371/journal.pone.0104154
Xiao, H., Li, W., Qin, Y., Lin, Z., Qian, C., Wu, M., et al. (2024). Crosstalk between lipid metabolism and bone homeostasis: exploring intricate signaling relationships. Research (Wash D C) 7, 0447. doi:10.34133/research.0447
Xu, H., Wang, W., Liu, X., Huang, W., Zhu, C., Xu, Y., et al. (2023). Targeting strategies for bone diseases: signaling pathways and clinical studies. Signal Transduct. Target Ther. 8, 202. doi:10.1038/s41392-023-01467-8
Yan, M., Zhang, Y., Niu, W., Liu, K., Xue, L., and Zhou, K. (2023). Reactive oxygen species-mediated endoplasmic reticulum stress contributes to osteocyte death induced by orthodontic compressive force. Microsc. Res. Tech. 86, 1529–1541. doi:10.1002/jemt.24382
Yang, Y., Lin, Y., Wang, M., Yuan, K., Wang, Q., Mu, P., et al. (2022). Targeting ferroptosis suppresses osteocyte glucolipotoxicity and alleviates diabetic osteoporosis. Bone Res. 10, 26. doi:10.1038/s41413-022-00198-w
Yao, C., Sun, J., Luo, W., Chen, H., Chen, T., Chen, C., et al. (2024). Down-expression of miR-494-3p in senescent osteocyte-derived exosomes inhibits osteogenesis and accelerates age-related bone loss via PTEN/PI3K/AKT pathway. Bone Jt. Res. 13, 52–65. doi:10.1302/2046-3758.132.Bjr-2023-0146.R2
Yılmaz, D., Marques, F. C., Gregorio, L., Schlatter, J., Gehre, C., Pararajasingam, T., et al. (2025). Age- and sex-specific deterioration on bone and osteocyte lacuno-canalicular network in a mouse model of premature aging. Bone Res. 13, 55. doi:10.1038/s41413-025-00428-x
Yoshimoto, T., Kittaka, M., Doan, A. A. P., Urata, R., Prideaux, M., Rojas, R. E., et al. (2022). Osteocytes directly regulate osteolysis via MYD88 signaling in bacterial bone infection. Nat. Commun. 13, 6648. doi:10.1038/s41467-022-34352-z
Youlten, S. E., Kemp, J. P., Logan, J. G., Ghirardello, E. J., Sergio, C. M., Dack, M. R. G., et al. (2021). Osteocyte transcriptome mapping identifies a molecular landscape controlling skeletal homeostasis and susceptibility to skeletal disease. Nat. Commun. 12, 2444. doi:10.1038/s41467-021-22517-1
Yu, C., Huang, D., Wang, K., Lin, B., Liu, Y., Liu, S., et al. (2017). Advanced oxidation protein products induce apoptosis, and upregulate sclerostin and RANKL expression, in osteocytic MLO-Y4 cells via JNK/p38 MAPK activation. Mol. Med. Rep. 15, 543–550. doi:10.3892/mmr.2016.6047
Yu, B., Pacureanu, A., Olivier, C., Cloetens, P., and Peyrin, F. (2020). Assessment of the human bone lacuno-canalicular network at the nanoscale and impact of spatial resolution. Sci. Rep. 10, 4567. doi:10.1038/s41598-020-61269-8
Yuan, C., Yu, X. T., Wang, J., Shu, B., Wang, X. Y., Huang, C., et al. (2024). Multi-modal molecular determinants of clinically relevant osteoporosis subtypes. Cell Discov. 10, 28. doi:10.1038/s41421-024-00652-5
Zaidi, M., Yuen, T., Sun, L., and Rosen, C. J. (2018). Regulation of skeletal homeostasis. Endocr. Rev. 39, 701–718. doi:10.1210/er.2018-00050
Zeng, Q., Wang, Y., Gao, J., Yan, Z., Li, Z., Zou, X., et al. (2019). miR-29b-3p regulated osteoblast differentiation via regulating IGF-1 secretion of mechanically stimulated osteocytes. Cell Mol. Biol. Lett. 24, 11. doi:10.1186/s11658-019-0136-2
Zhang, K., Barragan-Adjemian, C., Ye, L., Kotha, S., Dallas, M., Lu, Y., et al. (2006). E11/gp38 selective expression in osteocytes: regulation by mechanical strain and role in dendrite elongation. Mol. Cell Biol. 26, 4539–4552. doi:10.1128/mcb.02120-05
Zhang, C., Li, H., Li, J., Hu, J., Yang, K., and Tao, L. (2023). Oxidative stress: a common pathological state in a high-risk population for osteoporosis. Biomed. Pharmacother. 163, 114834. doi:10.1016/j.biopha.2023.114834
Zhang, Y., Wang, Q., Xue, H., Guo, Y., Wei, S., Li, F., et al. (2024). Epigenetic regulation of autophagy in bone metabolism. Funct. (Oxf) 5, zqae004. doi:10.1093/function/zqae004
Zhang, J., Acosta, F. M., Wang, X., Zhao, D., Zhang, L., Hua, R., et al. (2025). Osteocyte connexin hemichannels and prostaglandin E(2) release dictate bone marrow mesenchymal stromal cell commitment. Proc. Natl. Acad. Sci. U. S. A. 122, e2412144122. doi:10.1073/pnas.2412144122
Zhao, S., Ge, C., Li, Y., Chang, L., Dan, Z., Tu, Y., et al. (2022). Desferrioxamine alleviates UHMWPE particle-induced osteoclastic osteolysis by inhibiting caspase-1-dependent pyroptosis in osteocytes. J. Biol. Eng. 16, 34. doi:10.1186/s13036-022-00314-8
Zhao, W., Qian, J., Li, J., Su, T., Deng, X., Fu, Y., et al. (2025). From death to birth: how osteocyte death promotes osteoclast formation. Front. Immunol. 16, 1551542. doi:10.3389/fimmu.2025.1551542
Zhou, R., Guo, Q., Xiao, Y., Guo, Q., Huang, Y., Li, C., et al. (2021). Endocrine role of bone in the regulation of energy metabolism. Bone Res. 9, 25. doi:10.1038/s41413-021-00142-4
Zhu, S., Chen, W., Masson, A., and Li, Y. P. (2024a). Cell signaling and transcriptional regulation of osteoblast lineage commitment, differentiation, bone formation, and homeostasis. Cell Discov. 10, 71. doi:10.1038/s41421-024-00689-6
Keywords: osteocyte, bone, microenvironment, osteoporosis, fracture
Citation: Wu Y, Gan D, Liu Z, Qiu D, Tan G, Xu Z and Xue H (2025) Osteocytes: master orchestrators of skeletal homeostasis, remodeling, and osteoporosis pathogenesis. Front. Cell Dev. Biol. 13:1670716. doi: 10.3389/fcell.2025.1670716
Received: 22 July 2025; Accepted: 09 September 2025;
Published: 25 September 2025.
Edited by:
Ye Chun Ruan, Hong Kong Polytechnic University, Hong Kong, SAR ChinaReviewed by:
Yang Liu, Shanghai Jiao Tong University, ChinaHaihui Han, Shanghai University of Traditional Chinese Medicine, China
Xiao-Na Xiang, West China Hospital, Sichuan University, China
Copyright © 2025 Wu, Gan, Liu, Qiu, Tan, Xu and Xue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhanwang Xu, WFpXNjAwMUAxNjMuY29t; Haipeng Xue, MTMyODc3MTE1MTFAMTYzLmNvbQ==
†These authors share first authorship
 Yan Wu1†
Yan Wu1† Donghao Gan
Donghao Gan Guoqing Tan
Guoqing Tan Haipeng Xue
Haipeng Xue