- 1Program in Developmental and Stem Cell Biology, The Hospital for Sick Children, Toronto, ON, Canada
- 2Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, ON, Canada
The respiratory system relies on a diverse repertoire of epithelial cell types to ensure efficient air conduction and gas exchange. This cellular heterogeneity arises through tightly coordinated intercellular signaling events that extend from embryonic development into the postnatal period. Among the key regulatory pathways, Notch signaling plays an integral role in guiding cell fate determination, proliferation, and differentiation. It is indispensable for the proper formation, maintenance, and repair of the airway epithelium. This review examines the broad influence of Notch signaling on mammalian airway epithelial biology and highlights unresolved questions—particularly those specific to human lung development—where human induced pluripotent stem cell–derived models offer promising tools to bridge existing knowledge gaps.
Introduction
The mammalian lung is the primary site of gas exchange, comprising anatomically and functionally distinct compartments. The upper airway includes the nasal and oral cavities, pharynx, and larynx, whereas the lower airway consists of the trachea, bronchioles, and alveolar sacs. Within the lower airway, proximal regions (trachea and bronchi) function chiefly in air conduction, while distal regions (respiratory bronchioles and alveoli) are specialized for gas exchange, each harboring unique epithelial niches (Weibel, 2017; Van Scott et al., 2013; Pohunek, 2004; Banov, 1989). Here, we review the role of canonical Notch signaling—a conserved cell–cell communication pathway—in airway progenitor specification, differentiation, and regeneration.
Human and mouse lung morphogenesis
Lung morphogenesis proceeds through five stages in both humans and mice: embryonic, pseudoglandular, canalicular, saccular, and alveolar. The first four stages occur in utero; alveologenesis extends postnatally (up to ∼2 years in humans; until postnatal day 28 in mice) (Nikolić et al., 2018; Swarr and Morrisey, 2015; Cardoso and Lü, 2006). During the embryonic phase (mouse E9–E12; human 3–5 gestational weeks [GW]), the NKX2.1+ endodermal buds evaginate from the ventral foregut, forming the trachea and primary lung buds. These buds branch into secondary (three on the right, two on the left) and tertiary bronchi, yielding ten branch tips per lung by stage end (Nikolić et al., 2018; Swarr and Morrisey, 2015; Cardoso and Lü, 2006). In the pseudoglandular stage (mouse E12–15; human 5–16 GW), iterative branching establishes the conducting airway tree; distal tips remain progenitor-rich, while stalk cells begin fate specification (Nikolić et al., 2018; Swarr and Morrisey, 2015; Cardoso and Lü, 2006). The canalicular (mouse E15–17; human 17–24 GW) and saccular stages (mouse E17–postnatal day [P]0; human 24–38 GW) generate respiratory bronchioles and saccules, which mature into alveoli during alveologenesis (mouse P0–20; human 38 GW–2 years) (Nikolić et al., 2018; Swarr and Morrisey, 2015; Cardoso and Lü, 2006).
Progenitor behavior is patterned along the proximal–distal axis, with region-specific niches guiding proliferation, migration, and differentiation (Eenjes et al., 2022; Zhang et al., 2024). In mice, pulmonary neuroendocrine cells (PNECs), expressing CGRP and ASCL1, originate proximally and coalesce into clusters at airway branch points before dispersing into terminal bronchioles (Noguchi et al., 2020; Candeli and Dayton, 2024). They are important during early fetal lung development as signaling hubs, and play roles in oxygen sensing, niche signaling to modulate nearby stem cells, and immune responses (Branchfield et al., 2016; Xu et al., 2020). Proximal–distal patterning underpins adult lung homeostasis and repair through WNT, BMP/TGFβ, and Notch signaling interplays (Hussain et al., 2017; Frum et al., 2023; Rey et al., 2022; Kiyokawa and Morimoto, 2020; Cumplido-Laso et al., 2023).
The proximal pseudostratified epithelium contains basal stem cells (BSC), expressing P63 and KRT5, club cells (SCGB1A1+, CCSP+), secretory cell subset (SCGB3A1+, SCGB3A2+) (Reynolds et al., 2002), goblet cells (MUC5AC+), deuterosomal cells (FOXJ1+, DEUP1+), ciliated cells (FOXJ1+, acetylated α-tubulin+), tuft cells (DCLK1+), and ionocytes (FOXI1+, CFTR+) (Montoro et al., 2018; Plasschaert et al., 2018) (Figure 1A). Basal cells maintain epithelial turnover and mediate repair post-injury (Rock et al., 2009); deuterosomal cells are ciliated progenitor cells (Deprez et al., 2020); ciliated and secretory cells drive mucociliary clearance and barrier function (Bustamante-Marin and Cilia, 2017); tuft cells sense luminal stimuli; ionocytes regulate ionic homeostasis and mucus hydration (Yuan et al., 2023). Distally, epithelium transitions to simple columnar and cuboidal bronchiole cells, culminating in squamous alveolar type 1 cells (AT1), expressing AGER and AQP5 and alveolar type 2 cells (AT2), expressing SFTPC and ABCA3 (Weibel, 2017; Van Scott et al., 2013; Banov, 1989).
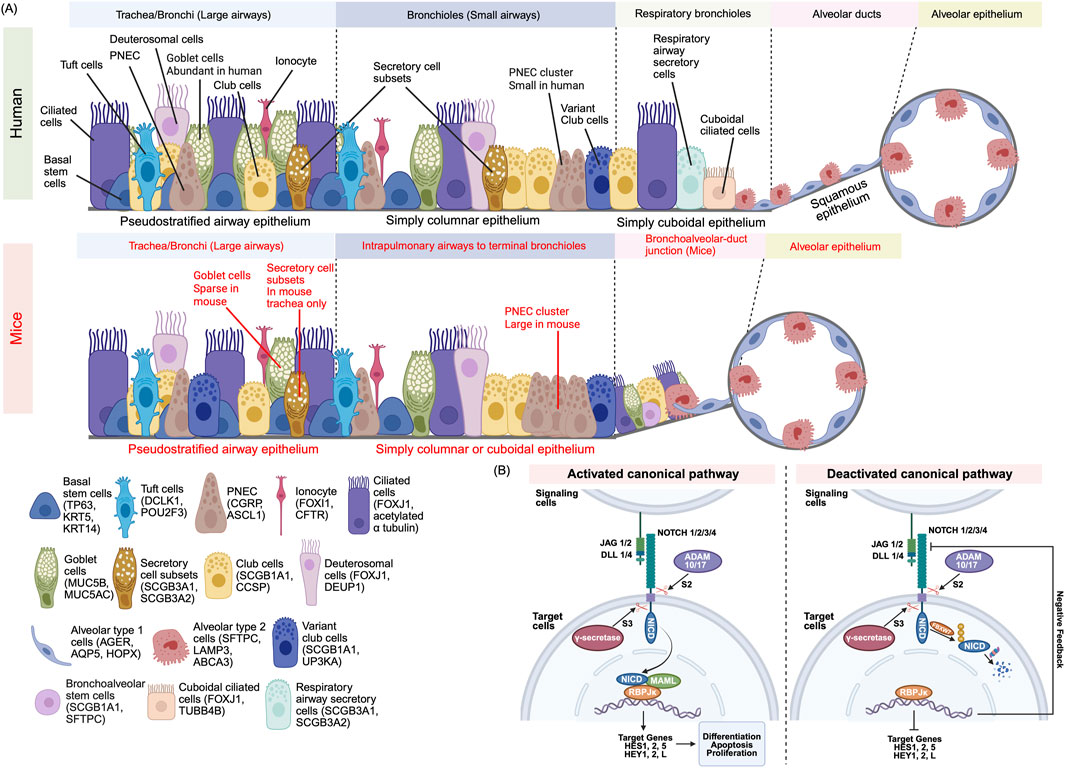
Figure 1. (A) Cellular Composition of the Mammalian Airway Epithelium. The mammalian airway epithelium exhibits region-specific cellular diversity. The proximal airway epithelium is composed of multiple specialized cell types, each marked by distinct lineage-defining genes: Basal stem cells (TP63+KRT5+) are resident stem cells that play a role in regenerating the epithelium, Club cells (SCGB1A1+) secrete protective proteins, Goblet cells (MUC5AC+) produce mucus, Deuterosomal cells (DEUP1+) are ciliated cell precursors, Ciliated cells (FOXJ1+) drive mucociliary clearance, Ionocyte (FOXI1+) regulate ion transport, Tuft cells (DCLK1+) function in chemo sensation, and Pulmonary neuroendocrine cells (PNEC) (CGRP+) mediate endocrine signaling. In human respiratory bronchioles, respiratory secretory cells (SCGB3A1+) and cuboidal ciliated cells (TUBB4B+) protects the airways through mucociliary transport, whereas in mice, the distal airway epithelium is connected by a bronchioalveolar duct junction. The distal airway epithelium is involved in respiratory gas exchange and surfactant production and consists of Alveolar type 1 (AGER+) which facilitates gas exchange and Alveolar type 2 cells (SFTPC+) which secrete surfactants. Mouse-specific markers are highlighted in red, while human’s features are shown in black. (B) Canonical Notch Signaling: Activation and Termination. Mammalian cells utilize four Notch receptors (NOTCH1–4) and four canonical ligands (JAG1, JAG2, DLL1, DLL4) to regulate cell fate decisions. In the activation process, ligand binding initiates cleavage at the S2 site via ADAM10/17. A second cleavage at the S3 site by γ-secretase releases the Notch intracellular domain (NICD). The NICD translocates to the nucleus and forms a transcriptional complex with RBPJκ (also known as CSL) and MAML1/2. This complex activates target genes such as HES and HEY, switching RBPJκ from a transcriptional repressor to an activator. During the signal termination process, NICD is tagged for degradation by FBXW7-mediated ubiquitination. Proteasomal degradation of NICD halts signaling. HES/HEY proteins may also provide negative feedback by repressing Notch pathway components.
Notably, humans and mice differ in epithelial distribution. In humans, the pseudostratified epithelium spans the entire human conducting airway from the trachea to the bronchioles but in mice, this is confined to the trachea and main bronchi (Nikolić et al., 2018). Human airways contain abundant goblet cells in the proximal airways and abundant club cells in the distal regions, whereas mouse distal lungs contain sparse goblet but abundant club cells (Danopoulos et al., 2019). The mouse trachea contains a subset of secretory cells, marked by SCGB3A1 and SCGB3A2 expression, which are found near club and goblet cells; however, in human airways, these cells are confined to the trachea and main the bronchi (Reynolds et al., 2002). PNECs form clusters at mouse bronchiolar neuroepithelial bodies but are solitary in humans (Danopoulos et al., 2019). Distally, human terminal bronchiole cells transition to respiratory bronchiole cells including respiratory airway secretory cells (SCGB3A1+, SCGB3A2+), club cells, and cuboidal ciliated cells (FOXJ1+, TUBB4B+) (Basil et al., 2022; Dodd et al., 2024). In mice, the terminal bronchiole cells directly transition to alveolar ducts through a bronchoalveolar duct junction, containing bronchoalveolar stem cells alongside club, ciliated, and alveolar cells (Kim et al., 2005). This cellular distribution reflects species-specific adaptations and has implications for how airway diseases like asthma or COPD manifest and respond to treatment in humans versus mice. Understanding these species-specific differences in airway architecture and cell distribution provides essential context for exploring the molecular mechanisms, such as Notch signaling, that govern epithelial cell fate and differentiation.
Canonical notch signaling
Mammals express four Notch receptors (NOTCH1–4, single pass transmembrane protein) and five ligands (JAG1/2, DLL1/3/4), four of them are canonical ligands (JAG1/2, DLL1/4) (Gomi et al., 2015; Stupnikov et al., 2019). Canonical activation (Figure 1B) ensues when ligand binding induces ADAM10/17-mediated S2 cleavage at extracellular juxtamembrane site, and γ-secretase–mediated S3 cleavage, liberating the Notch intracellular domain (NICD). NICD translocates to the nucleus, where it associates with RBPJκ also known as CSL and MAML1/2 cofactors, to drive HES/HEY transcriptional programs as the RBPJ κ (CSL) converts from co-repressor to co-activator (Alabi et al., 2018; D’Souza et al., 2010). To terminate the signaling pathway, NICD is ubiquitinated by FBXW7 and degraded in the proteasome while HES/HEY proteins can auto-regulate and repress Notch ligand/receptor expression as a negative feedback loop (Kar et al., 2021). Non-canonical Notch pathways—independent of CSL or γ-secretase—also contribute to context-specific outcomes but are less well characterized in the lung (D’Souza et al., 2010).
Notch signaling in mouse airway development
Notch pathway components are expressed from the earliest lung bud stages (mouse E9–12), orchestrating proximal–distal fate decisions (Tsao et al., 2008; Kong et al., 2004). Pharmacological γ-secretase inhibition (DAPT) in E8.5 CD1 mouse embryos foregut explants expand distal Nkx2.1+ tip progenitors while depleting Sox2+ proximal progenitors; similarly, DAPT during branching morphogenesis yields enlarged distal buds, dysregulated distal markers (Nkx2.1, Bmp4, Sftpc), and reduced Sox2 (Tsao et al., 2008). Pan-epithelial deletion of Pofut1 (Pofut1F/–;ShhCre/+), an enzyme required for Notch activation, nearly abolishes Sox2 expression in E18.5 lungs, underscoring Notch’s necessity for proximal lineage initiation (Tsao et al., 2009).
Spatial and temporal expression patterns of individual Notch receptors and ligands hint at specialized roles. NOTCH1 is enriched in the distal endoderm from E11.5–E13.5, whereas NOTCH2/3 localizes to mesenchyme, as shown by in situ hybridization (Post et al., 2000); NOTCH4 is restricted to vasculature (Ito et al., 2000; Uyttendaele et al., 1996). Notch1/2/3 genes are expressed from E11– P14, with Notch1 detectable as early as E10 and continuing postnatally, based on semi-quantitative RT-PCR (Kong et al., 2004). Notch ligands also exhibit dynamic expression patterns. Jag1 and Jag2 are dynamically expressed from E11–P14, with Jag2 peaking at E16.0 and preceding Jag1 onset (Stupnikov et al., 2019; Kong et al., 2004; Zhang et al., 2013); Dll1 emerges in secondary bronchi at E13.5 and intensifies in bronchioles and branch points until birth (Post et al., 2000). These patterns align with Notch’s central role in branching morphogenesis and regionalization.
Within the proximal epithelium, Notch directs the emergence of cellular diversity (Figure 2A). Loss of Notch signaling—via receptor knockouts or downstream effectors ablation (HES1)—enhances NE bodies (NEB) size and PNEC differentiation (Ito et al., 2000; Morimoto et al., 2012), with DLL1/4 acting as dominant ligands; Dll1/Dll4 double knockouts show marked increases in Ascl1+ progenitors that co-express Cgrp upon PNEC maturation (Stupnikov et al., 2019). Post-PNEC specification, Notch2 activation in Sox2+ proximal airway progenitors fosters Scgb3a2+ intermediate progenitors (Kiyokawa et al., 2021), which adopt secretory or ciliated fates depending on subsequent Notch2 signaling levels (Kiyokawa et al., 2021). Notch1/2 alleles dose-dependently regulate secretory cell differentiation (Morimoto et al., 2012; Guseh et al., 2009; Boucherat et al., 2012), with Notch2 playing a dominant role in generating Cc10+ secretory cells (Morimoto et al., 2012). In contrast, Sox2+ progenitors lacking Notch2 activation differentiate into Krt17+ basal progenitors (Kiyokawa et al., 2021). Collectively, these studies suggest an important temporal and possibly spatial role of Notch signaling in regulating cell fate during airway morphogenesis.
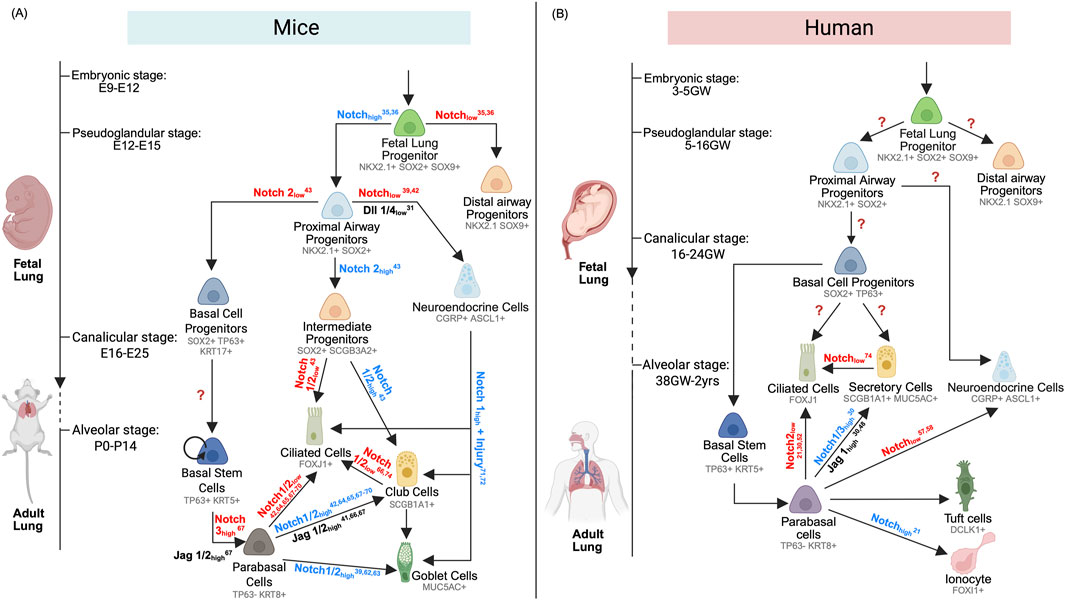
Figure 2. (A) Mouse Proximal Airway Development and Regeneration. Dynamic Notch signaling orchestrates cell fate specification throughout mouse airway development and repair. From embryonic stages (E9–12) to alveolar maturation (P0–14), Notch activity directs NKX2.1+SOX2+SOX9+ fetal lung progenitors toward either distal (NKX2.1+SOX9+) or proximal (NKX2.1+SOX2+) lineages. Proximal progenitors give rise to basal cell progenitors (SOX2+TP63+), pulmonary neuroendocrine cells (CGRP+), and intermediate progenitors (SOX2+SCGB3A2+). Basal cell progenitors mature into basal stem cells (TP63+KRT5+), which, during development or regeneration, generate parabasal cells (TP63-KRT8+) that differentiate into ciliated (FOXJ1+), club (SCGB1A1+), and goblet (MUC5AC+) cells. Intermediate progenitors also contribute to ciliated and club cell lineages during development. During regeneration, PNEC can give rise to all three secretory and ciliated cell types. Notch inhibition enables club cells to transdifferentiate into ciliated cells. (B) Human Proximal Airway Development and Regeneration. In humans, Notch signaling similarly governs airway lineage specification from embryonic (3–5 gestational weeks) to alveolar stages (38 GW–2 years). NKX2.1+SOX2+SOX9+ fetal lung progenitors are directed toward distal (NKX2.1+SOX9+) or proximal (NKX2.1+SOX2+) fates. Proximal progenitors differentiate into basal cell progenitors (SOX2+TP63+) and pulmonary neuroendocrine cells (CGRP+). During fetal development, basal progenitors can directly form ciliated (FOXJ1+) and secretory cells (SCGB1A1+, MUC5AC+) or mature into basal stem cells (TP63+KRT5+) that regenerate the airway epithelium via a parabasal intermediate. In regeneration, Notch signaling also promotes differentiation of ionocytes (FOXI1+) and PNEC (CGRP+). Goblet cells (MUC5AC+) can transdifferentiate into ciliated cells upon Notch inhibition.
Limited studies on NOTCH regulation during human fetal airway development
Although limited, current studies on Notch regulation during human fetal airway development highlight a significant knowledge gap. Most of our understanding of Notch receptor–ligand interactions during fetal airway development derives from murine models, which have elucidated roles for Notch1–3 and ligands Dll1/4 and Jag1/2 in proximal–distal patterning and epithelial differentiation (Stupnikov et al., 2019; Tsao et al., 2008; Tsao et al., 2009; Ito et al., 2000; Zhang et al., 2013; Morimoto et al., 2012; Kiyokawa et al., 2021; Guseh et al., 2009; Boucherat et al., 2012) (Figure 2). In contrast, direct investigation of Notch-dependent lineage specification in the human fetal lung remains virtually unexplored. Using human embryonic stem cell (hESC) differentiation models, Chen et al. demonstrated that Notch pathway inhibition via DAPT enhances the differentiation of PNECs (Chen et al., 2019). However, to date, human studies have largely been confined to postnatal primary airway cultures, where Notch modulation alters basal, secretory, ciliated, and neuroendocrine cell proportions (Rey et al., 2022; Gomi et al., 2015; Giuranno et al., 2020; Gomi et al., 2016; Kang et al., 2011; Vladar et al., 2023; Bodas et al., 2021; Danahay et al., 2015; Marcet et al., 2011; Bou Saab et al., 2016; Paul et al., 2014). Restricted access to human fetal tissue across developmental stages, coupled with limitations of conventional in vitro airway epithelial models, has impeded dissection of ligand–receptor dynamics in utero.
Human induced pluripotent stem cell (hiPSC)–based platforms now offer a scalable, stage-specific system for modeling airway morphogenesis. Directed differentiation of hiPSCs recapitulates successive milestones from definitive endoderm to mature, pseudostratified airway epithelium, enabling temporal control of Notch pathway perturbation (Wang et al., 2023). Two independent hiPSC studies demonstrate that γ-secretase inhibitors (DAPT or DBZ) expand BSC, ciliated, and pulmonary neuroendocrine cell (PNEC) populations while suppressing club cell differentiation—phenocopying murine embryonic lung responses to Notch blockade (de Carvalho et al., 2019; Hor et al., 2020). Although promising, these initial reports rely on pan-Notch inhibition; future work must leverage ligand- and receptor-specific manipulations, coupled with single-cell and organoid assays, to resolve how distinct Notch inputs sculpt human airway lineage hierarchies.
Overall, hiPSC-derived airways, human lung organoids, and ex vivo fetal explants represent complementary systems for untangling the multifaceted roles of Notch signaling in human lung development. Strategic application of these models will be essential to define the temporal and spatial rules by which Notch ligand–receptor codes drive epithelial patterning in the human fetus.
Notch signaling in mouse airway regeneration
The adult airway epithelium undergoes continual turnover—estimated to be every 30–50 days in rodents and there are limited studies in humans (Bowden, 1983)—and relies on BSCs for homeostatic replenishment and repair after injury (Rock et al., 2009; Travaglini et al., 2020; Mou et al., 2021; Wu et al., 2022). In both mouse and human adult airways, BSCs act as resident stem cells capable of self-renewal and regenerating various proximal airway epithelial cell types. Notch signaling plays a critical role in maintaining mature airway epithelial cells homeostasis and promoting injury-induced repair by orchestrating BSC differentiation, directly progenitors toward secretory or ciliated lineages (Eenjes et al., 2022; Wu et al., 2022; Sriu et al., 2002) (Figures 2A,B).
In mouse conducting airway, Notch1/2 activation is both necessary and sufficient to promote club and goblet cell differentiation (Morimoto et al., 2012; Morimoto et al., 2010; Lingamallu et al., 2024). Conversely, inhibition of Notch1/2 promotes ciliated cell differentiation in adult mouse airway epithelial cells grown in air-liquid interface (ALI) cultures (Lafkas et al., 2015). JAG1/2 also plays a critical role in balancing distinct cell populations in adult mice conducting airways (Zhang et al., 2013; Lafkas et al., 2015; Mori et al., 2015) (Figure 2A). Ligands JAG1/2 maintain the secretory compartment—neutralizing JAG1 or JAG2 selectively using antibodies depletes club cells while expanding ciliated cells in adult mice airways with a more prominent effect observed with JAG1 inhibition than JAG2, and combined JAG1/2 blockade yields an almost exclusively ciliated epithelium (Lafkas et al., 2015). Therefore, JAG1/2-activated Notch signaling is crucial for the development of secretory cells, while its inhibition favors ciliated cell development, as confirmed in several studies (Guseh et al., 2009; Boucherat et al., 2012; Morimoto et al., 2010; Ou-Yang et al., 2013) (Figure 2A). Notch3, by contrast, activated by JAG1/2 drives Krt8+, Tp63– parabasal progenitor formation (Mori et al., 2015), a distinct intermediate state that later activates Notch1/2 for secretory and ciliated cell fate decision (Mori et al., 2015; Rock et al., 2011; Pardo-Saganta et al., 2015). Single-cell RNA sequencing in adult mouse airways further implicates Notch in the specification of rare epithelial types: Ascl1 in PNECs, Ascl2 in tuft cells, and Ascl3 in ionocytes, and identifies Nfia as a club cell–enriched modulator of Notch-dependent homeostasis (Noguchi et al., 2020; Montoro et al., 2018).
Following chemical injuries such as naphthalene, sulfur dioxide, chlorine gas, or polidocanol treatment, Notch signaling is a key pathway for efficient regeneration in mouse airways (Paul et al., 2014; Morimoto et al., 2010; Rock et al., 2011; Pardo-Saganta et al., 2015; Ouadah et al., 2019; Yao et al., 2018; Xing et al., 2012). In naphthalene models, absence of Notch signaling in PNECs impairs their conversion into club, ciliated, and goblet cells (Ouadah et al., 2019; Yao et al., 2018), while Notch inhibition in goblet cells accelerates ciliated transdifferentiation (Ruiz Ga et al., 2019). Sulfur dioxide and chlorine injuries activate Notch + parabasal cells (Tp63–, Krt8+) to replenish luminal progenitors (Rock et al., 2011; Pardo-Saganta et al., 2015). Within BSCs, NICD2 marks cells fated for secretory lineages, whereas c-myb-expressing BSCs yield ciliated progeny; Rbpjκ deletion shifts the balance toward ciliated cell lineages (Pardo-Saganta et al., 2015). In polidocanol-injured airways, elevated reactive oxygen species engage NRF2, a key transcription factor that regulates oxidative stress and inflammation via the NRF2-antioxidant response element pathway, to activate NOTCH1 in Notch pathway, driving BSC proliferation and self-renewal (Paul et al., 2014). Overall, Notch signalling is important in the regeneration of the airways post-injury in mice.
Notch signaling in human airway regeneration
In human primary ALI and 3D bronchosphere models, broad inhibition of NOTCH signaling using small molecules like DAPT or DBZ reduces the number of secretory cells and ionocytes, while promoting expansion of basal and ciliated cell populations (Rey et al., 2022; Plasschaert et al., 2018; Gomi et al., 2015; Gomi et al., 2016; Danahay et al., 2015; Paul et al., 2014). Conversely, constitutive activation of NOTCH1 and NOTCH3, achieved through lentiviral overexpression of their active intracellular domains (NICD1/3), in human primary BSCs cultured in ALI conditions increases secretory cells (e.g., MUC5AC, SCGB1A1) at the expense of basal (KRT5, TP63) and ciliated (DNAI1, TEKT1) cells (Gomi et al., 2015). These findings highlight the role of NOTCH1/3 receptor activation in modulating differentiation among secretory, basal, and ciliated cell types.
Further evidence from a doxycycline-inducible NICD1 system in iPSC-derived BSC-like cells showed that NOTCH1 activation does not promote ionocyte differentiation (Wang et al., 2023). In contrast, using neutralizing antibodies against NOTCH1 in a 3D human BSC-derived bronchosphere model led to increased expression of BSC markers, without affecting goblet or ciliated cell differentiation (Danahay et al., 2015). Inhibition of NOTCH3 slightly elevated markers associated with both BSCs and goblet cells, but did not alter ciliated cell differentiation (Danahay et al., 2015). Meanwhile, neutralizing NOTCH2 reduced secretory cell populations and increased basal and ciliated cell markers (Danahay et al., 2015).
Additional support for Notch signaling’s role in human airway cell development comes from studies that manipulate NOTCH ligands or downstream transcriptional regulators. For instance, lentiviral overexpression or knockdown of the ligand JAG1 selectively influences the expansion of secretory cell lineages, while leaving ciliated cell populations unaffected (Gomi et al., 2015; Gomi et al., 2016). Furthermore, knockdown of DLL1/NOTCH1 via miR-449 microRNA in deuterosomal cells leads to increased expression of HES6—a Notch pathway inhibitor—and decreased levels of HEY1 and HES4, which are Notch activators, along with reduced expression of Notch receptors 1, 2, and 3. This shift suppresses secretory cell differentiation and enhances multiciliated cell formation in human airway epithelial ALI cultures (Marcet et al., 2011; Ruiz Ga et al., 2019).
Altogether, these findings emphasize the intricate specificity of the NOTCH pathway in directing cell lineage outcomes, which is essential for refining differentiation protocols aimed at generating specialized cell types from human iPSCs.
Disease-associated analyses implicate aberrant Notch activity in chronic airway disorders. In cystic fibrosis (CF), increased basal proliferation and ciliated cell loss coincides with heightened Notch signaling; DBZ/DAPT treatment restores ciliated abundance in CF epithelium (Vladar et al., 2023; Bou Saab et al., 2016; Barbry et al., 2020). Airway epithelial cells from patients with smoking-induced chronic obstructive pulmonary disease (COPD) display elevated secretory and reduced basal cell proportions when compared to non-smokers and downregulated expression of Notch pathway genes compared to non-smokers (Tilley et al., 2009). This data highlight Notch pathway modulation as a promising avenue for regenerative therapies aimed at reestablishing balanced epithelial architecture.
Discrepant outcomes in notch–mediated regulation in airway lineages
While most murine studies consistently show that Notch activation inhibits ciliated cell differentiation and that its suppression promotes multiciliogenesis (Stupnikov et al., 2019; Tsao et al., 2008; Tsao et al., 2009; Morimoto et al., 2012; Guseh et al., 2009; Vladar et al., 2023; Danahay et al., 2015; Lafkas et al., 2015), a few investigations challenge this paradigm. In embryonic mouse lungs, Kiyokawa et al. observed a slight increase in ciliated cells following Notch stimulation, indicating that multiciliogenesis may be influenced by receptor dosage or the timing of activation (Kiyokawa et al., 2021). Similar inconsistencies appear in adult lung injury models. For example, in naphthalene-treated mice, Ouadah and colleagues reported that NOTCH1 activation in NICD1-overexpressing mice suppressed PNEC differentiation (Ouadah et al., 2019), and other studies have shown PNEC expansion following Notch1 knockout mice (Xing et al., 2012). Contrastingly, Morimoto’s group found no significant change in PNEC numbers after manipulating NOTCH 1 signaling (Morimoto et al., 2012).
Human airway studies also reveal conflicting data. In primary human cell cultures, most reports show that Notch inhibition promotes ciliated cell formation (Plasschaert et al., 2018; Giuranno et al., 2020; Vladar et al., 2023; Danahay et al., 2015; Marcet et al., 2011; Bou Saab et al., 2016; de Carvalho et al., 2019), yet others describe decreased multiciliogenesis under similar conditions or after HEYL knockdown (Gomi et al., 2016; Bodas et al., 2021; Hor et al., 2020). Targeted NOTCH3 activation likewise yields mixed outcomes: Gomi et al. documented reduced ciliated markers in NICD3-overexpressing epithelia (Gomi et al., 2015), but Bodas et al. observed no alteration in ciliated cell numbers despite robust NICD3 induction (Bodas et al., 2021).
NOTCH1/3 activation and NOTCH2 inhibition are key in human mature airway cell fate decisions (Gomi et al., 2015; Bodas et al., 2021; Danahay et al., 2015; Hor et al., 2020; Ou-Yang et al., 2013). In mice, NOTCH1/2 is crucial for mature airway cell differentiation, but NOTCH3 is not involved in either activation or inhibition (Morimoto et al., 2012; Kiyokawa et al., 2021; Danahay et al., 2015; Morimoto et al., 2010; Lafkas et al., 2015; Mori et al., 2015). These findings suggest discrepancies between human and mouse Notch signaling activation and inhibition.
Together, these inconsistencies underscore the need to dissect receptor- and ligand-specific contributions, as well as the influence of developmental stage, injury context, and species differences, on Notch-driven airway lineage allocation.
Harnessing hiPSC-Based models and cutting-edge genomic technologies to elucidate notch signaling dynamics
Many hiPSC-derived airway epithelium differentiation protocols have leveraged Notch signaling regulation, highlighting its importance and potential on lineage regulation during human lung development and homeostasis. Particularly, DAPT had been applied to induce the differentiation of CFTR-expressing ciliated cells in fetal airway organoids (Wong et al., 2015; Ngan et al., 2022; Konishi et al., 2016) and in ALI cultures of mature airway epithelium (Firth et al., 2014). Notch inhibition via DAPT/DBZ has also been shown to induce the differentiation of BSCs, ciliated cells, and PNECs, while reducing club cells differentiation (de Carvalho et al., 2019; Hor et al., 2020). Therefore, the role of Notch signaling in the pulmonary disease—such as lung cancer, CF, and COPD—as well as in airway epithelial repair/regeneration, can be further investigated using hiPSC-derived fetal and adult airway epithelium (Wong et al., 2015; Konishi et al., 2016; Firth et al., 2014; Ahmed et al., 2022; Marcoux et al., 2023).
Human iPSC-derived models of airway epithelium, when integrated with powerful gene-editing platforms such as CRISPR/Cas9 and prime editing, enable the creation of epithelial cell lines with targeted knock-in or knock-out modifications to Notch receptors and transcription factors. These cell lines facilitate receptor-specific investigations across various stages of lung development (Huang and Loewer, 2022). Additionally, cellular barcoding, a technique that reverse-transcriptionally labels individual cells with unique molecular identifiers via lentiviral delivery (Shakiba et al., 2019), can be coupled with high resolution single-cell RNA sequencing technologies to trace airway epithelial lineages and uncover patterns of cellular competition modulated by Notch signaling throughout human lung morphogenesis, using hiPSC-derived cells at defined developmental intervals. These advanced in-vitro platforms offer a powerful and versatile system for dissecting Notch-driven mechanisms of airway development, mapping cellular trajectories, and benchmarking engineered cell states against native human lung tissue (Quach et al., 2024).
Despite advances, studies exploring the dynamic interactions between specific Notch receptor-ligand pairs during lung development remain limited. Emerging single-cell spatial transcriptomic platforms (Garcia-Alonso et al., 2021), offer promising avenues for mapping Notch signaling gene expression within hiPSC-derived lung tissues. These technologies may illuminate how Notch signaling influences the spatial organization and fate of epithelial cell lineages in discrete regions of the developing lung, and support inferences regarding canonical Notch-mediated cell-cell communication (Armingol et al., 2022).
Discussion
In this review, we have highlighted the complex role of Notch signaling in lung development and regeneration. There are extensive studies in mouse models demonstrating the importance of the Notch signaling pathway in development and regeneration, and particularly its role in cellular differentiation. Understandably, there is less research on the role of this pathway in human airways, especially in the context of lung development and disease.
Two studies highlight the essential role of Notch signaling in regulating epithelial basal cell function using hiPSCs and mouse genetic models (Huang et al., 2024; Zhang et al., 2018). These investigations were conducted in the esophagus, an organ that is anatomically and developmentally contiguous with the ventral lung, underscoring their relevance to airway biology and support the broader context of Notch signaling in epithelial maintenance.
Despite the physiological parallels between mice and humans, murine models fall short in fully replicating the entirety of human physiology. In addition, the effects of Notch signaling in early human lung development remain ambiguous. With advancements in human iPSC-derived models combined with effective gene targeting tools and powerful platforms such as cellular barcoding and single cell spatial transcriptomics technologies, these will become important tools to uncover the role of Notch signaling in human lung models. The experimentally tractable models of iPSC differentiation enable temporal studies of the effects of Notch (and other pathways) in lung development and repair.
Overall, future studies leveraging human iPSC-derived airway epithelium models may reveal the complex dynamic role of Notch signaling across development from fetal to “adulthood” (maturation), informing how cells emerge to how they are regenerated and the impact on disease.
Author contributions
YS: Conceptualization, Visualization, Writing – original draft, Writing – review and editing, Data curation. SY: Visualization, Writing – original draft, Data curation, Conceptualization, Writing – review and editing. TL: Visualization, Writing – review and editing. HQ: Visualization, Writing – review and editing. JY: Data curation, Writing – review and editing. RS: Writing – review and editing, Visualization. AW: Supervision, Writing – review and editing, Writing – original draft, Conceptualization, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. 2021 Stem Cell Network Innovation Award awarded to APW; 2020 SickKids/CIHR IHDCYH New Investigator grant awarded to APW and the 2023 Canadian Lung Associate (CLA) studentship awarded to YS.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmed, E., Fieldes, M., Bourguignon, C., Mianné, J., Petit, A., Jory, M., et al. (2022). Differentiation of human induced pluripotent stem cells from patients with severe COPD into functional airway epithelium. Cells 11 (15), 2422. doi:10.3390/cells11152422
Alabi, R. O., Farber, G., and Blobel, C. P. (2018). Intriguing roles for endothelial ADAM10/Notch signaling in the development of organ-specific vascular beds. Physiol. Rev. 98 (4), 2025–2061. doi:10.1152/physrev.00029.2017
Armingol, E., Ghaddar, A., Joshi, C. J., Baghdassarian, H., Shamie, I., Chan, J., et al. (2022). Inferring a spatial code of cell-cell interactions across a whole animal body. PLoS Comput. Biol. 18 (11), e1010715. doi:10.1371/journal.pcbi.1010715
Banov, C. H. (1989). Anatomy and physiology of the lower and upper airway. J. Allergy Clin. Immunol. 84 (6), 1044–1046. doi:10.1016/0091-6749(89)90149-8
Barbry, P., Cavard, A., Chanson, M., Jaffe, A. B., and Plasschaert, L. W. (2020). Regeneration of airway epithelial cells to study rare cell states in cystic fibrosis. J. Cyst. Fibros. 19 (1), S42-S46–6. doi:10.1016/j.jcf.2019.09.010
Basil, M. C., Cardenas-Diaz, F. L., Kathiriya, J. J., Morley, M. P., Carl, J., Brumwell, A. N., et al. (2022). Human distal airways contain a multipotent secretory cell that can regenerate alveoli. Nature 604 (7904), 120–126. doi:10.1038/s41586-022-04552-0
Bodas, M., Subramaniyan, B., Moore, A. R., Metcalf, J. P., Ocañas, S. R., Freeman, W. M., et al. (2021). The NOTCH3 downstream target HEYL is required for efficient human airway basal cell differentiation. Cells 10 (11), 3215. doi:10.3390/cells10113215
Bou Saab, J., Bacchetta, M., and Chanson, M. (2016). Ineffective correction of PPARγ signaling in cystic fibrosis airway epithelial cells undergoing repair. Int. J. Biochem. Cell Biol. 78, 361–369. doi:10.1016/j.biocel.2016.07.035
Boucherat, O., Chakir, J., and Jeannotte, L. (2012). The loss of Hoxa5 function promotes notch-dependent goblet cell metaplasia in lung airways. Biol. Open 1 (7), 677–691. doi:10.1242/bio.20121701
Bowden, D. H. (1983). Cell turnover in the lung. Am. Rev. Respir. Dis. 128 (2 Pt 2), S46–S48. doi:10.1164/arrd.1983.128.2P2.S46
Branchfield, K., Nantie, L., Verheyden, J. M., Sui, P., Wienhold, M. D., and Sun, X. (2016). Pulmonary neuroendocrine cells function as airway sensors to control lung immune response. Science 351 (6274), 707–710. doi:10.1126/science.aad7969
Bustamante-Marin, X. M., and Cilia, O. L. E. (2017). Cilia and mucociliary clearance. Cold Spring Harb. Perspect. Biol. 9 (4), a028241. doi:10.1101/cshperspect.a028241
Candeli, N., and Dayton, T. (2024). Investigating pulmonary neuroendocrine cells in human respiratory diseases with airway models. Dis. Models and Mech. 17 (5), dmm050620. doi:10.1242/dmm.050620
Cardoso, W. V., and Lü, J. (2006). Regulation of early lung morphogenesis: questions, facts and controversies. Development 133 (9), 1611–1624. doi:10.1242/dev.02310
Chen, H. J., Poran, A., Unni, A. M., Huang, S. X., Elemento, O., Snoeck, H. W., et al. (2019). Generation of pulmonary neuroendocrine cells and SCLC-Like tumors from human embryonic stem cells. J. Exp. Med. 216 (3), 674–687. doi:10.1084/jem.20181155
Cumplido-Laso, G., Benitez, D. A., Mulero-Navarro, S., and Carvajal-Gonzalez, J. M. (2023). Transcriptional regulation of airway epithelial cell differentiation: insights into the notch pathway and beyond. Int. J. Mol. Sci. 24 (19), 14789. doi:10.3390/ijms241914789
Danahay, H., Pessotti, A. D., Coote, J., Montgomery, B. E., Xia, D., Wilson, A., et al. (2015). Notch2 is required for inflammatory cytokine-driven goblet cell metaplasia in the lung. Cell Rep. 10 (2), 239–252. doi:10.1016/j.celrep.2014.12.017
Danopoulos, S., Shiosaki, J., and Al Alam, D. (2019). FGF signaling in lung development and disease: human versus mouse. Front. Genet. 10, 170. doi:10.3389/fgene.2019.00170
de Carvalho, ALRT, Strikoudis, A., Liu, H. Y., Chen, Y. W., Dantas, T. J., Vallee, R. B., et al. (2019). Glycogen synthase kinase 3 induces multilineage maturation of human pluripotent stem cell-derived lung progenitors in 3D culture. Development 146 (2), dev171652. doi:10.1242/dev.171652
Deprez, M., Zaragosi, L. E., Truchi, M., Becavin, C., Ruiz García, S., Arguel, M. J., et al. (2020). A single-cell atlas of the human healthy airways. Am. J. Respir. Crit. Care Med. 202 (12), 1636–1645. doi:10.1164/rccm.201911-2199OC
Dodd, D. O., Mechaussier, S., Yeyati, P. L., McPhie, F., Anderson, J. R., Khoo, C. J., et al. (2024). Ciliopathy patient variants reveal organelle-specific functions for TUBB4B in axonemal microtubules. Science 384 (6694), eadf5489. doi:10.1126/science.adf5489
D’Souza, B., Meloty-Kapella, L., and Weinmaster, G. (2010). Canonical and non-canonical notch ligands. Curr. Top. Dev. Biol. 92, 73–129. doi:10.1016/S0070-2153(10)92003-6
Eenjes, E., Tibboel, D., Wijnen, R. M. H., and Rottier, R. J. (2022). Lung epithelium development and airway regeneration. Front. Cell Dev. Biol. 10, 1022457. doi:10.3389/fcell.2022.1022457
Firth, A. L., Dargitz, C. T., Qualls, S. J., Menon, T., Wright, R., Singer, O., et al. (2014). Generation of multiciliated cells in functional airway epithelia from human induced pluripotent stem cells. Proc. Natl. Acad. Sci. U. S. A. 111 (17), E1723–E1730. doi:10.1073/pnas.1403470111
Frum, T., Hsu, P. P., Hein, R. F. C., Conchola, A. S., Zhang, C. J., Utter, O. R., et al. (2023). Opposing roles for TGFβ- and BMP-Signaling during nascent alveolar differentiation in the developing human lung. npj Regen. Med. 8 (1), 48–19. doi:10.1038/s41536-023-00325-z
Garcia-Alonso, L., Handfield, L. F., Roberts, K., Nikolakopoulou, K., Fernando, R. C., Gardner, L., et al. (2021). Mapping the temporal and spatial dynamics of the human endometrium in vivo and in vitro. Nat. Genet. 53 (12), 1698–1711. doi:10.1038/s41588-021-00972-2
Giuranno, L., Roig, E. M., Wansleeben, C., van den Berg, A., Groot, A. J., Dubois, L., et al. (2020). NOTCH inhibition promotes bronchial stem cell renewal and epithelial barrier integrity after irradiation. Stem Cells Transl. Med. 9 (7), 799–812. doi:10.1002/sctm.19-0278
Gomi, K., Arbelaez, V., Crystal, R. G., and Walters, M. S. (2015). Activation of NOTCH1 or NOTCH3 signaling skews human airway basal cell differentiation toward a secretory pathway. PLoS One 10 (2), e0116507. doi:10.1371/journal.pone.0116507
Gomi, K., Staudt, M. R., Salit, J., Kaner, R. J., Heldrich, J., Rogalski, A. M., et al. (2016). JAG1-Mediated notch signaling regulates secretory cell differentiation of the human airway epithelium. Stem Cell Rev. Rep. 12 (4), 454–463. doi:10.1007/s12015-016-9656-6
Guseh, J. S., Bores, S. A., Stanger, B. Z., Zhou, Q., Anderson, W. J., Melton, D. A., et al. (2009). Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development 136 (10), 1751–1759. doi:10.1242/dev.029249
Hor, P., Punj, V., Calvert, B. A., Castaldi, A., Miller, A. J., Carraro, G., et al. (2020). Efficient generation and transcriptomic profiling of human iPSC-Derived pulmonary neuroendocrine cells. iScience 23 (5), 101083. doi:10.1016/j.isci.2020.101083
Huang, Z., and Loewer, A. (2022). Generating somatic knockout cell lines with CRISPR-Cas9 technology to investigate SMAD signaling. Methods Mol. Biol. 2488, 81–97. doi:10.1007/978-1-0716-2277-3_7
Huang, H., Jiang, Y., Liu, J., Luo, D., Yuan, J., Mu, R., et al. (2024). Jag1/2 maintain esophageal homeostasis and suppress foregut tumorigenesis by restricting the basal progenitor cell pool. Nat. Commun. 15, 4124. doi:10.1038/s41467-024-48347-5
Hussain, M., Xu, C., Lu, M., Wu, X., Tang, L., and Wu, X. (2017). Wnt/β-catenin signaling links embryonic lung development and asthmatic airway remodeling. Biochim. Biophys. Acta Mol. Basis Dis. 1863 (12), 3226–3242. doi:10.1016/j.bbadis.2017.08.031
Ito, T., Udaka, N., Yazawa, T., Okudela, K., Hayashi, H., Sudo, T., et al. (2000). Basic helix-loop-helix transcription factors regulate the neuroendocrine differentiation of fetal mouse pulmonary epithelium. Development 127 (18), 3913–3921. doi:10.1242/dev.127.18.3913
Kang, J. H., Lee, E. H., Park, S. W., and Chung, I. Y. (2011). MUC5AC expression through bidirectional communication of notch and epidermal growth factor receptor pathways. J. Immunol. 187 (1), 222–229. doi:10.4049/jimmunol.1003606
Kar, R., Jha, S. K., Ojha, S., Sharma, A., Dholpuria, S., Raju, V. S. R., et al. (2021). The FBXW7-NOTCH interactome: a ubiquitin proteasomal system-induced crosstalk modulating oncogenic transformation in human tissues. Cancer Rep. 4 (4), e1369. doi:10.1002/cnr2.1369
Kim, C. F. B., Jackson, E. L., Woolfenden, A. E., Lawrence, S., Babar, I., Vogel, S., et al. (2005). Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 121 (6), 823–835. doi:10.1016/j.cell.2005.03.032
Kiyokawa, H., and Morimoto, M. (2020). Notch signaling in the Mammalian respiratory system, specifically the trachea and lungs, in development, homeostasis, regeneration, and disease. Dev. Growth Differ. 62 (1), 67–79. doi:10.1111/dgd.12628
Kiyokawa, H., Yamaoka, A., Matsuoka, C., Tokuhara, T., Abe, T., and Morimoto, M. (2021). Airway basal stem cells reutilize the embryonic proliferation regulator, Tgfβ-Id2 axis, for tissue regeneration. Dev. Cell 56 (13), 1917–1929.e9. doi:10.1016/j.devcel.2021.05.016
Kong, Y., Glickman, J., Subramaniam, M., Shahsafaei, A., Allamneni, K. P., Aster, J. C., et al. (2004). Functional diversity of notch family genes in fetal lung development. Am. J. Physiol. Lung Cell Mol. Physiol. 286 (5), L1075–L1083. doi:10.1152/ajplung.00438.2002
Konishi, S., Gotoh, S., Tateishi, K., Yamamoto, Y., Korogi, Y., Nagasaki, T., et al. (2016). Directed induction of functional multi-ciliated cells in proximal airway epithelial spheroids from human pluripotent stem cells. Stem Cell Rep. 6 (1), 18–25. doi:10.1016/j.stemcr.2015.11.010
Lafkas, D., Shelton, A., Chiu, C., de Leon Boenig, G., Chen, Y., Stawicki, S. S., et al. (2015). Therapeutic antibodies reveal notch control of transdifferentiation in the adult lung. Nature 528 (7580), 127–131. doi:10.1038/nature15715
Lingamallu, S. M., Deshpande, A., Joy, N., Ganeshan, K., Ray, N., Ladher, R. K., et al. (2024). Neuroepithelial bodies and terminal bronchioles are niches for distinctive club cells that repair the airways following acute notch inhibition. Cell Rep. 43 (9), 114654. doi:10.1016/j.celrep.2024.114654
Marcet, B., Chevalier, B., Luxardi, G., Coraux, C., Zaragosi, L. E., Cibois, M., et al. (2011). Control of vertebrate multiciliogenesis by miR-449 through direct repression of the delta/notch pathway. Nat. Cell Biol. 13 (6), 693–699. doi:10.1038/ncb2241
Marcoux, P., Hwang, J. W., Desterke, C., Imeri, J., Bennaceur-Griscelli, A., and Turhan, A. G. (2023). Modeling RET-rearranged non-small cell lung cancer (NSCLC): generation of lung progenitor cells (LPCs) from patient-derived induced pluripotent stem cells (iPSCs). Cells 12 (24), 2847. doi:10.3390/cells12242847
Montoro, D. T., Haber, A. L., Biton, M., Vinarsky, V., Lin, B., Birket, S. E., et al. (2018). A revised airway epithelial hierarchy includes CFTR-Expressing ionocytes. Nature 560 (7718), 319–324. doi:10.1038/s41586-018-0393-7
Mori, M., Mahoney, J. E., Stupnikov, M. R., Paez-Cortez, J. R., Szymaniak, A. D., Varelas, X., et al. (2015). Notch3-Jagged signaling controls the pool of undifferentiated airway progenitors. Development 142 (2), 258–267. doi:10.1242/dev.116855
Morimoto, M., Liu, Z., Cheng, H. T., Winters, N., Bader, D., and Kopan, R. (2010). Canonical notch signaling in the developing lung is required for determination of arterial smooth muscle cells and selection of clara versus ciliated cell fate. J. Cell Sci. 123 (2), 213–224. doi:10.1242/jcs.058669
Morimoto, M., Nishinakamura, R., Saga, Y., and Kopan, R. (2012). Different assemblies of notch receptors coordinate the distribution of the major bronchial clara, ciliated and neuroendocrine cells. Development 139 (23), 4365–4373. doi:10.1242/dev.083840
Mou, H., Yang, Y., Riehs, M. A., Barrios, J., Shivaraju, M., Haber, A. L., et al. (2021). Airway basal stem cells generate distinct subpopulations of PNECs. Cell Rep. 35 (3), 109011. doi:10.1016/j.celrep.2021.109011
Ngan, S. Y., Quach, H. T., Laselva, O., Huang, E. N., Mangos, M., Xia, S., et al. (2022). Stage-specific generation of human pluripotent stem cell derived lung models to measure CFTR function. Curr. Protoc. 2 (1), e341. doi:10.1002/cpz1.341
Nikolić, M. Z., Sun, D., and Rawlins, E. L. (2018). Human lung development: recent progress and new challenges. Development 145 (16), dev163485. doi:10.1242/dev.163485
Noguchi, M., Furukawa, K. T., and Morimoto, M. (2020). Pulmonary neuroendocrine cells: physiology, tissue homeostasis and disease. Dis. Model Mech. 13 (12), dmm046920. doi:10.1242/dmm.046920
Ou-Yang, H. F., Wu, C. G., Qu, S. Y., and Li, Z. K. (2013). Notch signaling downregulates MUC5AC expression in airway epithelial cells through Hes1-dependent mechanisms. Respiration 86 (4), 341–346. doi:10.1159/000350647
Ouadah, Y., Rojas, E. R., Riordan, D. P., Capostagno, S., Kuo, C. S., and Krasnow, M. A. (2019). Rare pulmonary neuroendocrine cells are stem cells regulated by Rb, p53, and notch. Cell 179 (2), 403–416. doi:10.1016/j.cell.2019.09.010
Pardo-Saganta, A., Law, B. M., Tata, P. R., Villoria, J., Saez, B., Mou, H., et al. (2015). Injury induces direct lineage segregation of functionally distinct airway basal stem/progenitor cell subpopulations. Cell Stem Cell 16 (2), 184–197. doi:10.1016/j.stem.2015.01.002
Paul, M., Bisht, B., Darmawan, D., Chiou, R., Ha, V., Wallace, W., et al. (2014). Dynamic changes in intracellular ROS levels regulate airway basal stem cell homeostasis through Nrf2-dependent notch signaling. Cell Stem Cell 15 (2), 199–214. doi:10.1016/j.stem.2014.05.009
Plasschaert, L. W., Žilionis, R., Choo-Wing, R., Savova, V., Knehr, J., Roma, G., et al. (2018). A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature 560 (7718), 377–381. doi:10.1038/s41586-018-0394-6
Pohunek, P. (2004). Development, structure and function of the upper airways. Paediatr. Respir. Rev. 5 (1), 2–8. doi:10.1016/j.prrv.2003.09.002
Post, L. C., Ternet, M., and Hogan, B. L. M. (2000). Notch/delta expression in the developing mouse lung. Mech. Dev. 98 (1), 95–98. doi:10.1016/s0925-4773(00)00432-9
Quach, H., Farrell, S., Wu, M. J. M., Kanagarajah, K., Leung, J. W. H., Xu, X., et al. (2024). Early human fetal lung atlas reveals the temporal dynamics of epithelial cell plasticity. Nat. Commun. 15 (1), 5898. doi:10.1038/s41467-024-50281-5
Reynolds, S. D., Hill, C. L., Alsudayri, A., Lallier, S. W., Wijeratne, S., Tan, Z. H., et al. (2022). Assemblies of JAG1 and JAG2 determine tracheobronchial cell fate in mucosecretory lung disease. JCI Insight 7 (15), e157380. doi:10.1172/jci.insight.157380
Reynolds, S. D., Reynolds, P. R., Pryhuber, G. S., Finder, J. D., and Stripp, B. R. (2002). Secretoglobins SCGB3A1 and SCGB3A2 define secretory cell subsets in mouse and human airways. Am. J. Respir. Crit. Care Med. 166 (11), 1498–1509. doi:10.1164/rccm.200204-285OC
Rock, J. R., Onaitis, M. W., Rawlins, E. L., Lu, Y., Clark, C. P., Xue, Y., et al. (2009). Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl. Acad. Sci. 106 (31), 12771–12775. doi:10.1073/pnas.0906850106
Rock, J. R., Gao, X., Xue, Y., Randell, S. H., Kong, Y. Y., and Hogan, B. L. (2011). Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell 8 (6), 639–648. doi:10.1016/j.stem.2011.04.003
Ruiz García, S., Deprez, M., Lebrigand, K., Cavard, A., Paquet, A., Arguel, M. J., et al. (2019). Novel dynamics of human mucociliary differentiation revealed by single-cell RNA sequencing of nasal epithelial cultures. Development 146 (20), dev177428. doi:10.1242/dev.177428
Shakiba, N., Fahmy, A., Jayakumaran, G., McGibbon, S., David, L., Trcka, D., et al. (2019). Cell competition during reprogramming gives rise to dominant clones. Science 364 (6438), eaan0925. doi:10.1126/science.aan0925
Sriuranpong, V., Borges, M. W., Strock, C. L., Nakakura, E. K., Watkins, D. N., Blaumueller, C. M., et al. (2002). Notch signaling induces rapid degradation of achaete-scute homolog 1. Mol. Cell Biol. 22 (9), 3129–3139. doi:10.1128/mcb.22.9.3129-3139.2002
Stupnikov, M. R., Yang, Y., Mori, M., Lu, J., and Cardoso, W. V. (2019). “Jagged and Delta-like ligands control distinct events during airway progenitor cell differentiation,”. Editors E. E. Morrisey, and D. Y. Stainier, 8. e50487. doi:10.7554/eLife.50487
Swarr, D. T., and Morrisey, E. E. (2015). Lung endoderm morphogenesis: gasping for form and function. Annu. Rev. Cell Dev. Biol. 31, 553–573. doi:10.1146/annurev-cellbio-100814-125249
Tilley, A. E., Harvey, B. G., Heguy, A., Hackett, N. R., Wang, R., O’Connor, T. P., et al. (2009). Down-regulation of the notch pathway in human airway epithelium in association with smoking and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 179 (6), 457–466. doi:10.1164/rccm.200705-795OC
Travaglini, K. J., Nabhan, A. N., Penland, L., Sinha, R., Gillich, A., Sit, R. V., et al. (2020). A molecular cell atlas of the human lung from single-cell RNA sequencing. Nature 587 (7835), 619–625. doi:10.1038/s41586-020-2922-4
Tsao, P. N., Chen, F., Izvolsky, K. I., Walker, J., Kukuruzinska, M. A., Lu, J., et al. (2008). Gamma-secretase activation of notch signaling regulates the balance of proximal and distal fates in progenitor cells of the developing lung. J. Biol. Chem. 283 (43), 29532–29544. doi:10.1074/jbc.M801565200
Tsao, P. N., Vasconcelos, M., Izvolsky, K. I., Qian, J., Lu, J., and Cardoso, W. V. (2009). Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development 136 (13), 2297–2307. doi:10.1242/dev.034884
Uyttendaele, H., Marazzi, G., Wu, G., Yan, Q., Sassoon, D., and Kitajewski, J. (1996). Notch4/int-3, a mammary proto-oncogene, is an endothelial cell-specific Mammalian notch gene. Development 122 (7), 2251–2259. doi:10.1242/dev.122.7.2251
Van Scott, M. R., Chandler, J., Olmstead, S., Brown, J. M., and Mannie, M. (2013). “Airway anatomy, physiology, and inflammation,” in The toxicant induction of irritant asthma, rhinitis, and related conditions. Editor W. J. Meggs (Boston, MA: Springer US), 19–61. doi:10.1007/978-1-4614-9044-9_2
Vladar, E. K., Kunimoto, K., Rojas-Hernandez, L. S., Spano, J. M., Sellers, Z. M., Joo, N. S., et al. (2023). Notch signaling inactivation by small molecule γ-secretase inhibitors restores the multiciliated cell population in the airway epithelium. Am. J. Physiol. Lung Cell Mol. Physiol. 324 (6), L771–L782. doi:10.1152/ajplung.00382.2022
Wang, R., Simone-Roach, C., Lindstrom-Vautrin, J., Wang, F., Rollins, S., Bawa, P. S., et al. (2023). De novo Generation of Pulmonary Ionocytes from Normal and Cystic Fibrosis Human Induced Pluripotent Stem Cells. Am. J. Respir. Crit. Care Med. 207 (9), 1249–1253. doi:10.1164/rccm.202205-1010LE
Weibel, E. R. (2017). Lung morphometry: the link between structure and function. Cell Tissue Res. 367 (3), 413–426. doi:10.1007/s00441-016-2541-4
Wong, A. P., Chin, S., Xia, S., Garner, J., Bear, C. E., and Rossant, J. (2015). Efficient generation of functional CFTR-Expressing airway epithelial cells from human pluripotent stem cells. Nat. Protoc. 10 (3), 363–381. doi:10.1038/nprot.2015.021
Wu, M., Zhang, X., Lin, Y., and Zeng, Y. (2022). Roles of airway basal stem cells in lung homeostasis and regenerative medicine. Respir. Res. 23 (1), 122. doi:10.1186/s12931-022-02042-5
Xing, Y., Li, A., Borok, Z., Li, C., and Minoo, P. (2012). NOTCH1 is required for regeneration of clara cells during repair of airway injury. Stem Cells 30 (5), 946–955. doi:10.1002/stem.1059
Xu, J., Yu, H., and Sun, X. (2020). Less is more: rare pulmonary neuroendocrine cells function as critical sensors in lung. Dev. Cell 55 (2), 123–132. doi:10.1016/j.devcel.2020.09.024
Yao, E., Lin, C., Wu, Q., Zhang, K., Song, H., and Chuang, P. T. (2018). Notch signaling controls transdifferentiation of pulmonary neuroendocrine cells in response to lung injury. Stem Cells 36 (3), 377–391. doi:10.1002/stem.2744
Yuan, F., Gasser, G. N., Lemire, E., Montoro, D. T., Jagadeesh, K., Zhang, Y., et al. (2023). Transgenic ferret models define pulmonary ionocyte diversity and function. Nature 621 (7980), 857–867. doi:10.1038/s41586-023-06549-9
Zhang, S., Loch, A. J., Radtke, F., Egan, S. E., and Xu, K. (2013). Jagged1 is the major regulator of notch-dependent cell fate in proximal airways. Dev. Dyn. 242 (6), 678–686. doi:10.1002/dvdy.23965
Zhang, Y., Yang, Y., Jiang, M., Huang, S. X., Zhang, W., Al Alam, D., et al. (2018). 3D modeling of esophageal development using human PSC-derived basal progenitors reveals a critical role for notch signaling. Cell Stem Cell 23 (4), 516–529. doi:10.1016/j.stem.2018.08.009
Zhang, K., Aung, T., Yao, E., and Chuang, P. T. (2024). Lung patterning: is a distal-to-proximal gradient of cell allocation and fate decision a general paradigm? a gradient of distal-to-proximal distribution and differentiation of tip progenitors produces distinct compartments in the lung. Bioessays 46 (1), e2300083. doi:10.1002/bies.202300083
Glossary
AT1 Alveolar type 1
AT2 Alveolar type 2
ASCL1/2/3 Achaete-scute homolog 1/2/3
BSCs Basal stem cells
BADJ Bronchoalveolar duct junctions
CSL CBF1/Su(H)/Lag-1
CALCA Calcitonin related polypeptide alpha
CFTR Cystic fibrosis transmembrane conductance regulator
c-myb C-MYB proto-oncogene
CRISPR Clustered Regularly Interspaced Short Palindromic Repeats
DLL1/3/4 Delta-like 1/3/4
E Embryonic day
FOXI1 Forkhead box I1
FOXJ1 Forkhead. box J1
GW Gestational week
DAPT/DBZ γ-secreatase inhibitor
HAEC Human airway epithelial cells
hiPSC Human induced pluripotent stem cell
ISH In situ hybridization
JAG1/2 Jagged1/2
KRT5 Keratin five
KRT8 Keratin8
Lfng Lunatic Fringe proteins
MAML Mastermind-like proteins
MUC5ac/5b Mucin 5ac/5b
NE Neuroendocrine
NICD Notch intracellular domain
NEB NE bodies
NFIA Nuclear factor IA
NRF2 Nuclear factor erythroid-2-related factor 2
PNEC Pulmonary neuroendocrine cells
RBPJκ Recombination Signal Binding Protein For Immunoglobulin Kappa J Region κ
Rfng Radical Fringe proteins
SO2 Sulfur dioxide
P63 Tumor protein 63 (Protein)
Trp63 Transformation related protein 63
Keywords: notch signaling, lung development, airway epithelial cells, lung differentiation, induced pluripotent stem cells, regeneration and repair
Citation: Song Y, Yang S, Lam T, Quach HT, Yang J, Salih R and Wong AP (2025) The next frontier in lung development and regeneration research: harnessing iPSC models to illuminate notch signaling pathways. Front. Cell Dev. Biol. 13:1672074. doi: 10.3389/fcell.2025.1672074
Received: 23 July 2025; Accepted: 27 August 2025;
Published: 15 September 2025.
Edited by:
Munemasa Mori, Mayo Clinic, United StatesReviewed by:
Yongchun Zhang, Shanghai Jiao Tong University, ChinaYizhuo Zhou, Columbia University Medical Center, United States
Copyright © 2025 Song, Yang, Lam, Quach, Yang, Salih and Wong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amy P. Wong, YXB3b25nQHNpY2traWRzLmNh
†These authors have contributed equally to this work
 Yuetong Song
Yuetong Song Sha Yang
Sha Yang Timothy Lam
Timothy Lam Henry T. Quach
Henry T. Quach Jielin Yang
Jielin Yang Rasha Salih
Rasha Salih Amy P. Wong
Amy P. Wong