Abstract
Hypoxic culture (1–5% O2) significantly enhances the biological activity and therapeutic potential of mesenchymal stromal cells (MSCs) by activating the HIF-1α signaling pathway. This activation promotes stemness maintenance, enhances proliferative capacity, and improves immunomodulatory functions, such as upregulating the secretion of indoleamine 2,3‒dioxygenase (IDO) and prostaglandin E2 (PGE2). Furthermore, hypoxia optimizes paracrine effects through modulating the release of vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF), while also improving cell homing and post-transplantation survival rates. Under hypoxic conditions, MSCs primarily rely on glycolytic metabolism, resulting in lactate accumulation. This lactate serves not only as a metabolic byproduct but also as a precursor for lactylation, a novel form of epigenetic modification. Given the limited research on MSC-specific metabolic mechanisms driven by lactylation, investigating lactylation modifications‒such as histone H3 lysine 18 lactylation (H3K18la)‒and their impact on MSCs function is crucial. We propose that the ‘hypoxia-lactate-lactylation’ axis represents a key metabolic-epigenetic mechanism that may further enhance immunomodulatory and tissue‒repair capabilities via epigenetic regulation, offering novel targets for metabolic intervention in clinical cell therapy. This approach could maximize the therapeutic potential of MSCs in clinical applications, with a high safety profile that avoids risks such as tumorigenicity, donor-dependent variability, and senescence.
1 Introduction
Mesenchymal stromal cells (MSCs) hold significant therapeutic value in immunomodulatory functions and cell-based therapies due to their remarkable paracrine capacity, multipotent differentiation potential, and immunomodulatory properties. However, conventional in vitro expansion under ambient oxygen tension (21% O2) often leads to loss of stemness and functional impairment, limiting clinical efficacy. Studies demonstrate that hypoxic culture (1–5% O2) recapitulates the physiological microenvironment by activating hypoxia‒inducible factor‒1α (HIF‒1α). This activation enhances key MSCs properties, including stemness maintenance, proliferative capacity, differentiation potential, migratory ability, and paracrine activity, while simultaneously optimizing their immunomodulatory functions (Sun et al., 2020; Kanichai et al., 2008; Xu et al., 2019; Lan et al., 2015; Noronha et al., 2019; Zhu et al., 2023). While the tumor-promoting roles of MSCs had also been reported previously (Melze et al., 2016). However, hypoxia triggers metabolic reprogramming in MSCs, shifting from oxidative phosphorylation to glycolysis and results in substantial lactate accumulation. Established evidence indicates that lactate serves as a key mediator in the immunomodulatory effects of human umbilical cord MSCs (huc-MSCs) (Selleri et al., 2016). A novel immunosuppressive pathway in MSCs was first described in 2023, which functions independently from the classical immunomodulatory mechanism that relies on glycolysis-derived lactate metabolites (Pradenas et al., 2023). This phenomenon not only impacts the cellular microenvironment but may also regulate MSCs functionality through a novel post‒translational modification: lactylation.
Lactylation is a recently discovered epigenetic regulatory mechanism wherein lactate acts as a substrate to covalently modify histones (e.g., H3K18la) or non‒histone proteins, thereby modulating gene expression (Zhang et al., 2019). In tumor and immune cells, lactylation regulates inflammatory responses, metabolic adaptation, and cell fate determination. However, research on lactylation’s modulation of MSCs biological functions under hypoxic remains nascent. Current evidence suggests that lactylation may enhance MSCs therapeutic potential by upregulating immunomodulatory molecules, promoting tissue‒repair factor secretion, and improving homing and engraftment efficiency (Xie et al., 2023). Furthermore, aberrant lactylation accumulation may induce metabolic stress and compromise MSCs safety. Precise regulation of lactylation levels is therefore critical for optimizing cell‒based therapeutic strategies. This review aims to explore the emerging role of the ‘hypoxia-lactate-lactylation’ axis in modulating MSCs biology.
1.1 Background introduction
1.1.1 Definition, origin, and primary functions of MSCs
In 2025, Yan et al. revealed the difference for the first time between MSCs and stem cells through single‒cell transcriptomic analysis (Yan et al., 2025). This study redefined biomarkers to discriminate these populations and laid the foundation for updating MSCs standards. Subsequently, the Delphi study was used to reformulate the definition of MSCs, resulting in the retention of nine items as core criteria after multiple deliberation rounds (Renesme et al., 2025). At its 2025 annual meeting, the International Society for Cellular Therapy (ISCT) published revised MSC identification criteria, explicitly defining MSCs as mesenchymal stromal cells (Renesme et al., 2025). The defining markers now must include positive markers (CD73+, CD90+, CD105+) and negative markers (CD45‒), while eliminating the 2006 criteria for demonstrating trilineage differentiation potential and adherent growth under standard culture conditions (Renesme et al., 2025; Dominici et al., 2006). The ability to differentiate is a key part of their functional identity, even if it's not used solely for definition anymore. Furthermore, the updated standards emphasizes the need to indicate the source of the organization. Critically, use of the term “stem” (i.e., mesenchymal stem cells) requires experimental evidence demonstrating stemness (Renesme et al., 2025).
MSCs can be isolated from diverse tissue sources, including bone marrow, adipose tissue, umbilical cord blood, fetal blood, placenta, dental pulp, Wharton’s jelly, skeletal muscle, dermis, and menstrual blood‒derived endometrial tissue, etc (Thirumala et al., 2009; Zuk et al., 2001; Erices et al., 2000; Campagnoli et al., 2001; In 't Anker et al., 2004; Gronthos et al., 2000; Wang et al., 2004; Kita et al., 2010; Fernández-Santos et al., 2022). The Delphi study further identifies dental follicle as an MSCs source (Renesme et al., 2025). These cells exhibit robust and critical functions in tissue homeostasis, injury repair, and immunomodulation (Miao et al., 2006). Their therapeutic efficacy in tissue injury repair, homeostasis maintenance, anti‒inflammatory responses, immunomodulation, and regenerative medicine stem from their multi-potent differentiation capacity and paracrine activity. Recent studies reveal their trans‒lineage differentiation potential: under specific in vitro culture conditions, MSCs undergo ectodermal differentiation (e.g., neural lineage cells expressing βIII‒tubulin and microtubule-Associated Protein 2 [MAP2]) and endodermal differentiation (e.g., hepatocyte‒like cells exhibiting albumin secretion and cytochrome P450 family three subfamily a polypeptide 4 [CYP3A4] activity) (Ababneh et al., 2022). Capitalizing on these distinctive properties, MSCs have emerged as a leading cellular therapeutic strategy in clinical applications (Alwohoush et al., 2024).
1.1.2 Functional properties and mechanisms of MSCs
The functional properties of MSCs are attributed to their paracrine effect, immunomodulatory properties, and tissue regenerative functions. These cells exert their therapeutic effects through multiple mechanisms, including: paracrine signaling, cell‒cell interactions, mitochondrial transfer and epigenetic regulation.
MSCs’ classical capacity for trilineage differentiation–encompassing ostegenic, chondrogenic, and adipogenic lineages (mesodermal derivatives)–is pivotal for tissue regeneration. Experimental evidence demonstrates that directed in vitro differentiation for 1–3 weeks induced MSCs commitment to chondrocytes, osteoblasts, and adipocytes. Notably, extended culture (three to four weeks) reveals trans‒lineage plasticity through differentiation into functional cardiomyocyte‒like cells, which express troponin T and contract spontaneously (Zhidu et al., 2024; Pittenger et al., 1999). A seminal 2008 study identified key signaling pathways governing MSCs differentiation, identifying activin‒mediated transforming growth factor–β (TGF–β) signaling, platelet‒derived growth factor (PDGF) receptor cascades, and fibroblast growth factor (FGF) mitogenic pathways as critical regulators of lineage commitment (Ng et al., 2008).
In addition, extracellular vesicles and exosomes are now recognized as key mediators of the regenerative and immunomodulatory functions of MSCs. MSCs mediate repair and regeneration of damaged cells and tissues through two principal mechanisms (Sun et al., 2020): lineage‒specific differentiation into tissue‒resident cell types to replace damaged cells (Kanichai et al., 2008). Membrane fusion‒mediated cytoprotection via direct cell‒cell contact, facilitating organelle/cytoplasmic transfer to rescue compromised or apoptotic cells (Tao et al., 2016; Watt et al., 2013; Spees et al., 2016). Furthermore, MSCs paracrine activity orchestrates regeneration through bioactive molecules that promote: (i) angiogenesis (e.g., VEGF, angiopoietin-1 [ANG‒1]), (ii) extracellular matrix remodeling (e.g., HGF, insulin-like growth factor-1 [IGF‒1]), (iii) anti‒fibrotic responses (e.g., tumor necrosis factor-inducible gene six protein [TSG‒6]), and (iv) immunoregulation (e.g., stromal cell-derived factor-1/C-X-C motif chemokine ligand 12 [SDF‒1/CXCL12]) (Ong and Dilley, 2018; Mihaylova et al., 2018; Hamid et al., 2022; Zacharek et al., 2007; Keshavarz et al., 2019; Meng et al., 2021; Katagiri et al., 2017). Beyond direct differentiation, MSCs facilitate repair via: (i) intercellular organelle transfer (notably mitochondria) and (ii) tunneling nanotube (TNT)‒mediated molecular trafficking (Figeac et al., 2014; Feng et al., 2019). Additionally, their intrinsic homing capacity enables targeted migration to injury through chemokine receptor‒dependent sensing of inflammatory mediators (Song and Li, 2011; Imitola et al., 2004).
Under pathological conditions, MSCs regulate immunity through dual mechanisms: (Sun et al., 2020): paracrine signaling mediated by extracellular vesicles and soluble factors, which suppresses pro‒inflammatory T cells/natural killer (NK) cells while promoting regulatory T cells (Tregs) expansion; (Kanichai et al., 2008); direct cell‒contact interactions that modulate B cell maturation and drive macrophage polarization toward anti‒inflammatory (alternatively activated macrophage [M2]) phenotypes (Li et al., 2021). Mechanistically, MSC-derived extracellular vesicles, particularly exosomes, together with secreted immunomodulatory factors, collectively establish an immunosuppressive niche. These vesicles transfer a variety of bioactive molecules, including: interleukin-10 (IL‒10) (anti‒inflammatory cytokine), interleukin-11 (IL‒11) (tissue‒protective signaling), PGE2 (myeloid suppression), TGF‒β (Treg induction), programmed cell death ligand 1/2 (PD‒L1/2) (T cell exhaustion induction), and IDO (tryptophan catabolism‒mediated suppression). These collectively dampen excessive immune activation (Li et al., 2021; Cho et al., 2024; Kulesza et al., 2023; Huang et al., 2024; Putra et al., 2018; Davies et al., 2017; Su et al., 2014).
The therapeutic value of MSCs stems from their dual capabilities: robust proliferative capacity in vitro and multipotent differentiation into clinically relevant cell lineages, enabling tissue maintenance and regeneration. Furthermore, their potent immunomodulatory properties make them as promising therapeutic agents for treating various diseases.
In hepatic disorders, huc‒MSCs exert therapeutic effect by suppressing hepatic stellate cell (HSC) activation, delivering cytoprotective factors via exosome, and counteracting oxidative stress‒induced hepatocyte injury (Shi et al., 2024; Xie et al., 2020). For systemic lupus erythematosus (SLE), huc-MSCs immunotherapy demonstrate clinical efficacy through immune tolerance restoration, reduced auto-antibody production, and attenuated end‒organ damage, with reported survival rates exceeding 80% (Wang et al., 2018). In the treatment of inflammatory arthritis, huc‒MSCs mediate disease‒modifying effects via osteochondral differentiation, micro-environment reprogramming through anti‒inflammatory cytokines secreting. (Ma et al., 2019; Gu et al., 2015; Dao et al., 2019). For the treatment of cerebrovascular diseases (stroke, traumatic brain injury), huc‒MSCs mitigate injury through VEGF‒mediated revascularization, trophic factor induction (glial cell line-derived neurotrophic factor [GDNF], brain-derived neurotrophic factor [BDNF]) reducing neuronal apoptosis, synaptic plasticity support, pro‒inflammatory cytokines suppression to restore neural circuitry and motor function (Peng et al., 2015; Wang et al., 2013; Qi et al., 2018). In cardiovascular diseases, huc‒MSCs drive cardiac repair by promoting cardiomyocyte regeneration, enhancing neovascularization, modulating cytokine storm (Lim et al., 2018).
Variability in MSCs clinical efficacy is attributed to donor‒related factors, including tissue source and intrinsic biological differences. Proteomic analysis of equine MSCs secretomes (314 identified proteins) revealed that donor age and tissue origin significantly influence protein composition, potentially impacting therapeutic outcomes (Turlo et al., 2023). Bone marrow‒derived MSCs (BMSCs) and adipose‒derived MSCs (ADSCs) are extensively utilized in cell‒based therapies due to their compatibility with both autologous and allogeneic applications (Liu Y. et al., 2024). Key clinical advantages include accessibility, therapeutic versatility, and regulatory progress evidenced by > 500 registered clinical trials. BMSCs were the first MSCs population used clinically. However, donor age significantly reduces BMSC yield, cellular quality, and multipotent differentiation capacity (Kern et al., 2006; Bagge et al., 2022). Furthermore, the highly invasive nature of bone marrow aspiration often causes significant patient morbidity (Thirumala et al., 2009). Suboptimal therapeutic efficacy has contributed to BMSC failures in several Phase III clinical trials (Liao et al., 2017). While tissue origin drives MSCs heterogeneity, donor‒specific factors (age, metabolic status) and ex vivo manipulations (prolonged culture‒induced senescence, oxygen tension shifts) further amplify their therapeutic potential diversity (Turlo et al., 2023; Liu Y. et al., 2024). Thus, fine‒tuning MSCs phenotypes through preconditioning strategies, epigenetic modulation, or biomechanical priming represents a promising approach to overcome current limitations in cell‒based therapies.
1.2 Reasearch meaning
Hypoxic preconditioning (1–5% O2) recapitulates physiological O2 tension in native stem cell niches (e.g., bone marrow, umbilical cord), inducing HIF‒1α‒mediated transcriptional reprogramming that enhances MSCs therapeutic efficacy. Mechanistically, hypoxia stabilizes HIF‒1α by inhibiting prolyl hydroxylase (PHD)‒dependent degradation. This stabilization upregulates pluripotency markers (Oct4, Nanog), suppresses differentiation‒related genes, maintains MSCs in an undifferentiated state. HIF‒1α further enhances proliferative capacity through dual regulation of metabolic reprogramming and cell cycle progression. Regarding immunomodulation, HIF‒1α potentiates MSC‒mediated immunosuppression by activating immune checkpoints and enhancing paracrine activity. Specifically, HIF‒1α upregulates IDO and PGE2 via the cyclooxygenase-2/prostaglandin E2 (COX‒2/PGE2) pathway, depleting local tryptophan while increasing immunosuppressive kynurenines. This inhibits T‒cell proliferation and polarizes macrophages toward regulatory phenotypes. Besides, HIF‒1α drives the secretion of VEGF and HGF to facilitate tissue repair. Hypoxic preconditioning also upregulates homing receptors (e.g., C-X-C motif chemokine receptor 4 [CXCR4]), and modulates apoptotic pathways to enhance the therapeutic efficacy of MSCs. Specifically, Hypoxia induces CXCR4 expression throutgh HIF‒1α binding to the CXCR4 promoter, potentiating SDF‒1/CXCR4‒mediated chemotaxis. Anti‒apoptotic programming occurs via upregulating the expression of B‒cell lymphoma‒2 (Bcl‒2) and downregulating the expression of pro‒apoptotic Bcl‒2 Associated X‒protein (Bax) (Feng and Wang, 2017). This dual regulation significantly improves post‒transplantation cell survival. However, severe hypoxia can impair the in vitro therapeutic efficacy of MSCs and promote their senescence and apoptosis (Jaraba-Álvarez et al., 2025; Khasawneh and Abu-El-Rub, 2022).
2 Hypoxic culture: principles and impacts
2.1 Description of hypoxic culture conditions
Hypoxia is defined as a pathophysiological state and characterized by inadequate oxygen supply to tissues/cells or excessive oxygen consumption, resulting in subphysiological oxygen tension (typically <5% O2 in most tissues) (MacIntyre, 2014; Wang X. et al., 2022). Hypoxia can be classified into three principal categories: systemic hypoxia, localized hypoxia, and functional hypoxia. The underlying mechanisms involve (Sun et al., 2020): hypoxemia (reduced arterial oxygen saturation); (Kanichai et al., 2008); impaired tissue oxygen delivery (due to circulatory insufficiency or hemoglobin dysfunction) (Xu et al., 2019); defective cellular oxygen utilization (Hypoxemia vs. hypoxia, 1966; Mallat et al., 2022; Østergaard et al., 2015). In stem cell biology, oxygen concentration critically regulates biological properties through hypoxia‒inducible factor (HIF)‒mediated pathways, influencing pluripotency maintenance, differentiation potential, and metabolic reprogramming (glycolytic shift) (Xin et al., 2023).
2.2 Hypoxic conditioning of MSCs: Mechanisms and therapeutic implications
The hypoxic environment shifts the metabolic process of MSCs toward glycolysis, leading to excessive lactate production. It is well established that lactate derived from MSCs serves as a key mediator in regulating immune function. Furthermore, hypoxic preconditioning modulates MSCs proliferation, differentiation, migration, and angiogenesis while also enhancing homing potential, suppressing apoptosis and inflammation, improving post‒transplantation survival, increasing stress tolerance, and augmenting therapeutic efficacy (Pouikli et al., 2022; Rosová et al., 2008; Gupta et al., 2022; Yang Y. et al., 2022; Tang et al., 2005). Furthermore, hypoxia reduces reactive oxygen species (ROS) generation, mitigating cellular damage and necrosis in MSCs (Bertram and Hass, 2008). Mechanistically, whether lactate acts as a primary mediator influencing these functional alterations remains debatable. What is certain, however, is that hypoxia (1–5% O2) upregulates key angiogenesis and vasoactivity regulators including: angiopoietin (ANG), VEGF, basic fibroblast growth factor (bFGF), transforming growth factor‒β1 (TGF‒β1), monocyte chemoattractant protein‒1 (MCP‒1), tissue inhibitor of metalloproteinase‒1 (TIMP‒1), matrix metallopeptidase‒9 (MMP‒9), and chemokine ligand 20 (CCL20) (Chen et al., 2014; Xia et al., 2018; Quade et al., 2020). Hypoxic preconditioning further elevates the expression of VEGF, TGF‒β1, IGF‒1, fibroblast growth factor 10 (FGF10), and epidermal growth factor (EGF). These factors synergize with exsome-contained miRNA to activate (Sun et al., 2020): the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) pathway to promote cell survival, proliferation, and migration (Kanichai et al., 2008); the transforming growth factor-beta/mothers against decapentaplegic homolog 2 (TGF‒β/SMAD2) pathway to regulate anti‒apoptotic and pro‒regenerative responses (Vizoso et al., 2017; Jung et al., 2007; Chang et al., 2013). Critically, hypoxic’s modulation of MSCs function is a double‒edged sword, with effects determined by oxygen concentration (hypoxia severity) and exposure duration.
Extensive studies document hypoxia‒induced alterations in MSCs biological functions across varying oxygen tensions.Hypoxia‒inducible miR‒486 enhances PI3K/AKT signaling activity via targeted phosphatase and tensin homolog (PTEN) suppression, promoting BMSC proliferation and survival (Shi et al., 2016). Normoxia (21% O2) irreversibly impairs MSCs functionality and osteogenic differentiation capacity, promoting exploration of hypoxic conditioning (Pouikli et al., 2022). Acute hypoxia (1% O2) significantly enhances BMSC migration and angiogenesis, while hypoxic preconditioning robustly stimulates proliferation and multilineage differentiation (Annabi et al., 2003; Ren et al., 2006; Xie et al., 2006). Moderate hypoxia (5% O2) elicts a biphasic proliferative response: reduced cell numbers in primary cultures but enhanced expansion in passaged cells (Ejtehadifar et al., 2015). hMSCs under moderate hypoxia (2% O2) exhibit prolonged lag phases but sustained proliferation, with elevated colony‒forming unit (CFU) capacity and stemness‒related gene expression (Grayson et al., 2006). Mechanistically, hypoxia induces MSCs proliferation via activation of the PI3K/AKT signaling (evidenced by elevated p‒AKT levels) (Sheng et al., 2017), further potentiated by: angiotensin II type 1 (AT1) receptor‒mediated PI3K activation in murine MSCs under hypoxia (3% O2) (Zhang et al., 2015) and SNHG16 lncRNA modulation in human placenta‒derived MSCs (hP‒MSCs) (Feng et al., 2022). However, contrasting findings were reported that severe hypoxia (1% O2) transiently reduces induced MSCs (iMSCs) proliferation/viability, yet prolonged exposure (50 h) yields superior iMSC growth compared to normoxic cultures (Alwohoush et al., 2024). These collective results suggest that the proliferative response of MSCs to hypoxia is multifactorial, governed by oxygen concentration gradient, exposure duration and cell type‒specific adaptations.
The multipotent differentiation potential of MSCs is significantly modulated by hypoxic conditions. Substantial evidence indicates that hypoxia consistently promotes chondrogenic differentiation in MSCs (Yang Y. et al., 2022). In contrast, its effects on adipogenic and osteogenic differentiation demonstrate context‒dependent regulation, with either stimulatory or inhibitory outcomes depending on specific experimental conditions. Mechanistically, hypoxic treatment of BMSCs enhances citrate carrier (CiC) activity, which prevents acetyl‒Coenzyme A (CoA) accumulation in mitochondria and subsequently promotes histone acetylation. Additionally, hypoxia reduces chromatin condensation at osteogenic gene promoter and enhancer. These coordinated epigenetic modifications collectively enhance osteogenic differentiation capacity under hypoxic conditions (Pouikli et al., 2022).
Hypoxic conditioning significantly enhances the paracrine activity of MSCs. Moreover, under hypoxic conditions, MSCs extensively use exosomes for intercellular communication. These vesicles deliver miRNAs and proteins to modulate the relevant cytokines expression (Yuan et al., 2025; Pulido-Escribano et al., 2022), thereby orchestrates long-distance cellular responses that promote angiogenesis, cell survival, and tissue regeneration. The hypoxia‒induced MSCs secretome plays crucial roles in promoting angiogenesis, suppressing inflammatory responses, and providing cytoprotective effects against apoptosis. Regarding angiogenic mechanisms, MSCs contribute to neovascularization through two principal pathways: (Sun et al., 2020): direct differentiation into vascular smooth muscle cells (SMCs) and endothelial cells (ECs), (Kanichai et al., 2008), paracrine regulation via intercellular communication with ECs and secretion of pro‒angiogenic factors (Hegde et al., 2024). At the molecular level, hypoxia triggers HIF‒1α stabilization and nuclear accumulation in endothelial cells. Activated HIF‒1α binds to VEGF promoters, up-regulating its transcription and subsequent pro‒angiogenic activity (Ahluwalia and Tarnawski, 2012). 24‒hour hypoxic preconditioning (1.5% O2) significantly increases the expression of erythropoietin receptor (EPOR) and VEGF in mouse-derived MSCs (mdMSCs) compared to versus controls (Lan et al., 2015). Current research demonstrate that hypoxic preconditioning activates HIF‒α signaling, coordinates the expression of VEGF and its cognate receptors vascular endothelial growth factor receptor 1/2 (VEGFR1/2), EPOR, and ANG-1 (Hu et al., 2008). This HIF‒mediated transcriptional program represents the fundamental mechanism underlyig hypoxia‒induced angiogenic potentiation in MSCs.
Li et al. (2023) demonstrated that 24‒hour hypoxic preconditioning (2% O2) significantly enhances the immunosuppressive properties of huc-MSCs, particularly their anti‒inflammatory capacity (Li et al., 2023). Regarding cytoprotective mechanisms, Li et al. reported that hypoxia‒treated MSCs (1% O2) elevated the expression of pro‒survival factors (AKT kinase and p‒AKT, hypoxia-inducible factor-alpha [HIF‒α]) and activated key cytoprotective mediators in target cells (anti‒apoptotic proteinsBcl‒2, Caspase‒3 inhibitors, metallothionein-2 [MTP‒2], TGF‒β1) (Li et al., 2017). As noted, lactate produced by MSCs has been reported to regulate the immunosuppressive functions via a novel alternative pathway (Pradenas et al., 2023). Furthermore, hypoxia‒preconditioned MSCs exhibit enhanced antioxidant capacity. These findings collectively establish hypoxic preconditioning as an effective strategy to enhance MSC‒mediated cytoprotection against various stressors.
3 Concept and biological significance of lactylation modification
3.1 Definition of lactylation modification
Post‒translational modifications (PTMs) critically regulate protein conformation, activity, and function, participating nearly all cellular pathways. These modifications drive diverse physiological and pathological processes while maintaining cellular homeostasis. Histones‒core structural components of nucleosomes‒consist of five major classes (H1, H2A, H2B, H3, and H4). Characterized by structured globular domains and flexible N‒terminal tails, histones are particularly susceptible to modifications at their tail regions, with the N‒terminus serving as the primary modification site. Enzymatic PTMs of histones are essential regulators of gene expression, chromatin architecture, and cellular functions. Common histone PTMs include methylation, acetylation, phosphorylation, ubiquitination, lactylation, and carboxylation.
Lactylation is a recently discovered, functionally significant PTMs. In 2019, Yingming Zhao’s research group (University of Chicago) first identified lysine lactylation (Kla) as a novel histone mark induced by lactate (Zhang et al., 2019). Their seminal work mapped 28 lactylation sites on core histones in both human and murine cells. Histone lactylation predominantly involves L‒lactate covalently modifying lysine residues through lactyl group addition, thereby regulating gene transcription (Wang N. et al., 2022; Zhan et al., 2021). Emerging evidence indicates histone acetyltransferase p300 participates in H3 lactylation modification (Hu et al., 2024). Both knockdown and overexpression experiments in mouse bone marrow‒derived macrophages and germinal vesicle (GV) oocytes demonstrate P300’s regulatory role in histone lactylation dynamics (Cui et al., 2021; Lin et al., 2022). This PTMs establishes a molecular link between lactate metabolism, transcriptional regulation, and epigenetics (Yu et al., 2024). Current research reveals that protein lactylation plays crucial roles in metabolic regulation, cell cycle control, protein function and stability, signal transduction, cellular stress responses, and tumor microenvironment (TME) modulation (Liu et al., 2023; Niu et al., 2021; Izzo and Wellen, 2019). Furthermore, lactylation modifications are implicated in various pathological conditions, including malignancies, inflammatory disorders, psychiatric diseases, infectious diseases, neurodegenerative conditions, and metabolic dysregulation (Sun et al., 2023; Yao et al., 2024; Li et al., 2022). Therefore, investigating lactylation deepens our understanding of fundermental cellular regulatory mechanisms. Under hypoxic or hyperglycemic, cells adapt to hypoxia through glycolytic reprogramming, increasing lactate production. Subsequent histone lactation then links metabolic states to gene regulation.
Lactate, a key metabolic intermediate, functions both as a post‒translational modification mediator and a metabolic regulator. In mammalian systems, lactate transport is primarily mediated by monocarboxylate transporters (MCTs), with distinct functional properties. monocarboxylate transporter 1 (MCT1) exhibits the highest affinity for lactate and functions as a bidirectional transporter dependent on substrate concentration gradients. In contrast, monocarboxylate transporter 4 (MCT4) is predominantly expressed in highly glycolytic tissues (e.g., tumors), specialized for lactate efflux despite its bidirectional transport capability (Beloueche-Babari et al., 2020). Intracellular lactate accumulation in lysosomes, mitochondria, and nuclei regulates multiple cellular processes through transcriptional modulation, signal transduction regulation, and metabolic reprogramming (Ivashkiv, 2020). Elevated lactate levels exert pleiotropic effect on cellular metabolism and immune responses via orchestrating inflammatory progression, modulating tumor immune tolerance, and activating critical signaling cascades (Zha et al., 2024), as summarized in Figure 1. While acute inflammation serves as a protective host response, its dysregulation may progress to tissue necrosis and chronic pathologies.
FIGURE 1
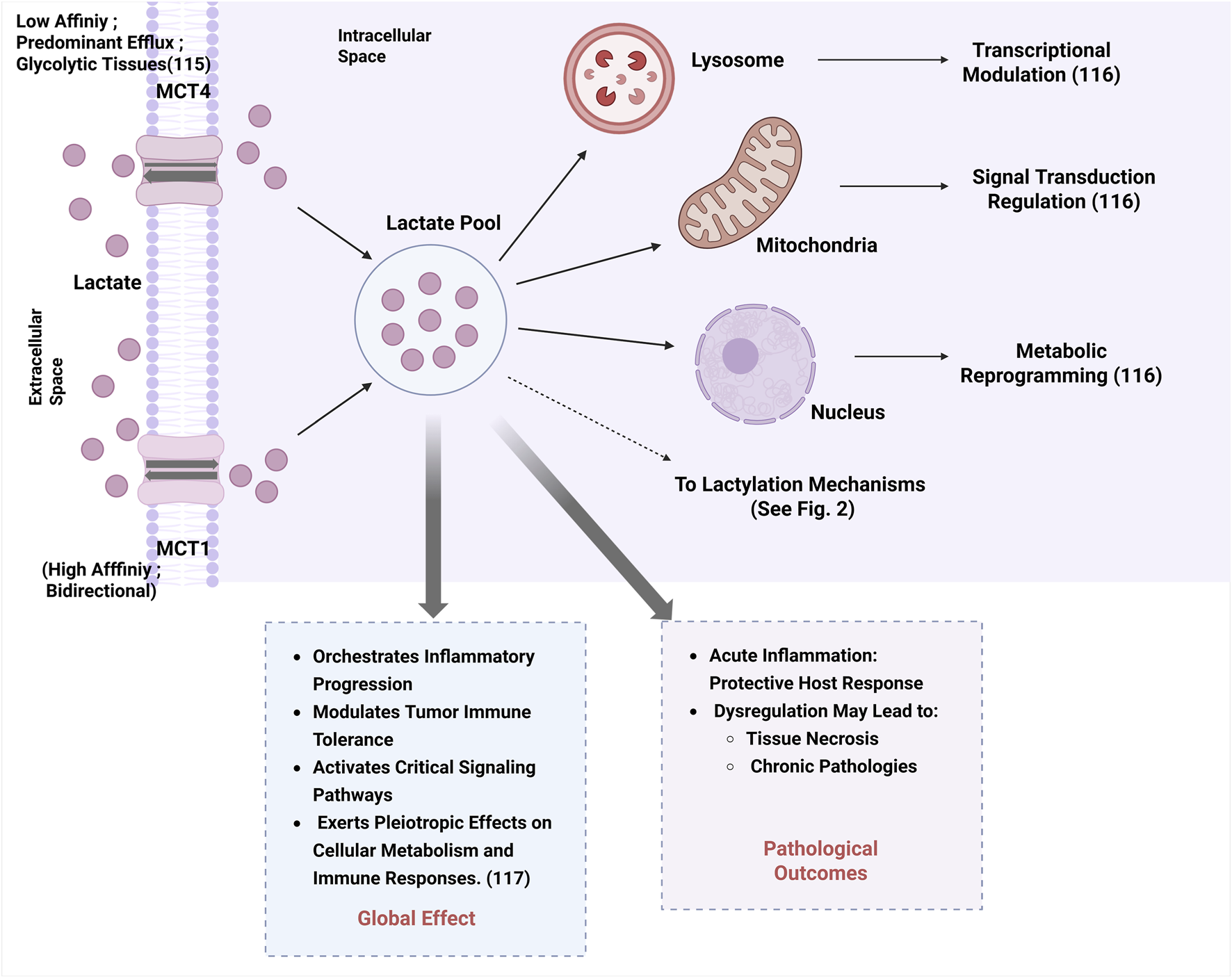
Lactate transport, intracellular accumulation, and global cellular effects.
As shown in Figure 2, protein lactylation are mediated by two distinct mechanisms: enzymatic and non‒enzymatic pathways. While both utilize lactate as a common substrate, they differ in chiral specificity and biochemical requirements (He et al., 2024). Enzymatic lactylation primarily utilizes L‒lactate and occurs via two distinct pathways. The first pathway converts L‒lactate into L‒lactyl‒CoA, which serves as the direct substrate for lactylation. This activated intermediate facilitates lactyl group transfer to lysine residues on target proteins (Zhang et al., 2019). The second pathway is mediated by aminoacyl‒tRNA synthetases (alanyl-tRNA synthetase 1 [AARS1] and alanyl-tRNA synthetase 2 [AARS2]), which directly couple L‒lactate with adenosine triphosphate (ATP) to generate lactyl‒adenosine monophosphate (AMP). This high‒energy intermediate subsequently donates the lactyl group to lysine residues, yielding lactylation (Mao et al., 2024; Ju et al., 2024; Zong et al., 2024). In contrast, non‒enzymatic lactylation is mediated by D‒lactate, a intermediate of glycolysis. This process utilizes methylglyoxal (MGO) that reacts with glutathione to produce lactylglutathione, the direct substrate for non‒enzymatic lactylation. Unlike enzymatic lactylation, this mechanism operates independently of specific transferases, depending instead on spontaneous chemical modifications. Several enzymes regulate histone lactylation, including EP300 and its homolog CREB‒binding protein (CBP), lysine acetyltransferases (lysine acetyltransferase 7 [KAT7] and lysine acetyltransferase 8 [KAT8]), histone deacetylases (histone deacetylase 1, 2, 3 [HDAC1–3] and histone deacetylase 8 [HDAC8]), and sirtuins (sirtuin 1, 2, 3 [SIRT1–3]) (Zhang et al., 2019; He et al., 2024; Yang K. et al., 2022), which collectively link metabolic flux to epigenetic regulation through lactylation control.
FIGURE 2
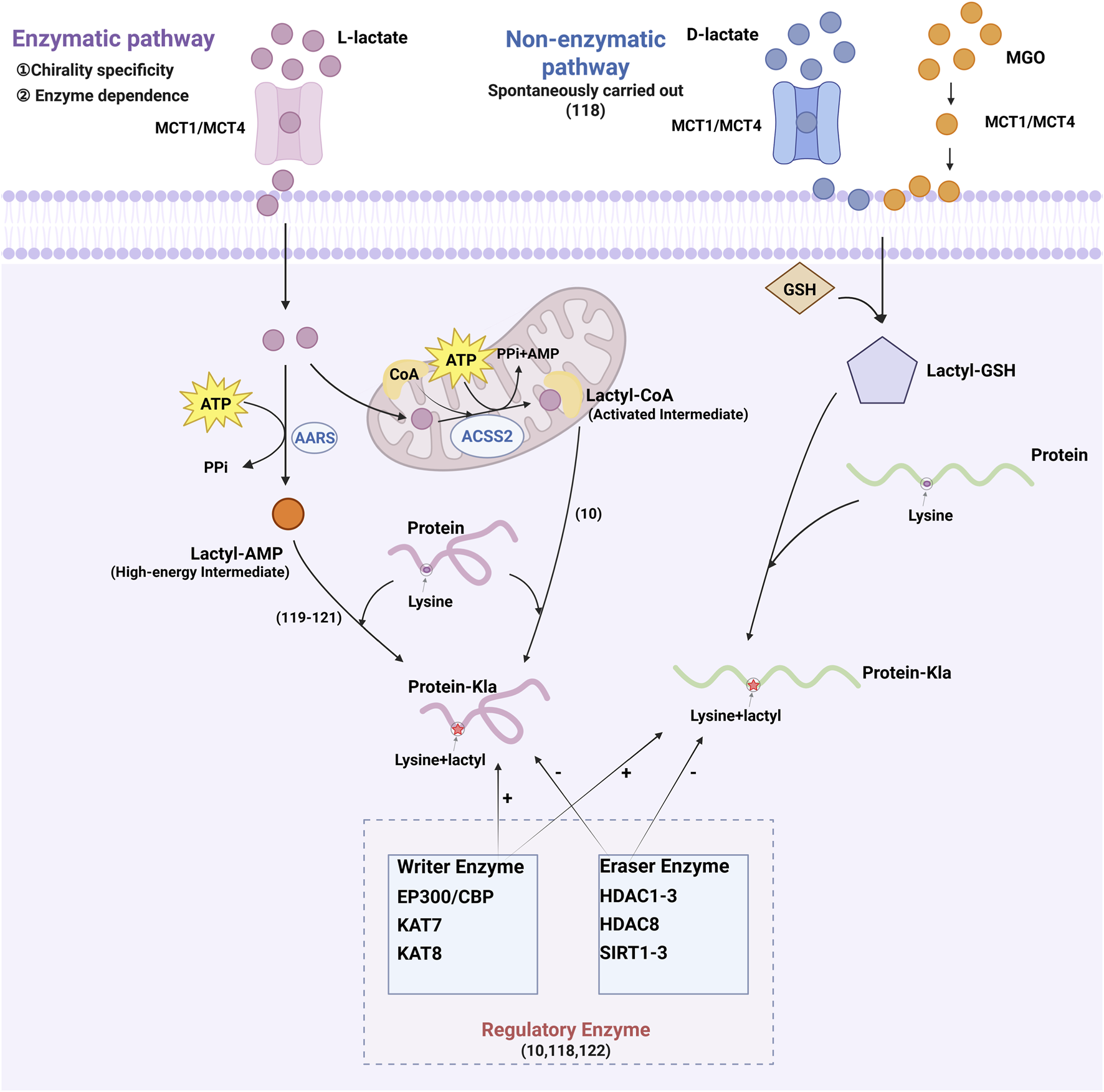
Lactic acid transport and protein lactylation modification mechanism.
3.2 Lactylation mechanisms in physiological contexts
As described in Figure 3, emerging evidence establishes lactylation as a key regulator of physiological processes including embryonic development, cell division, cellular differentiation, angiogenesis, and memory formation under normal physiological conditions (Li et al., 2022; Wang J. et al., 2024; Yang et al., 2021). Yang et al. (2021) first characterized the dynamic pattens of histone lactylation (histone H3 lysine 23 lactylation [H3K23la], H3K18la, and pan‒histone lactylation) during mouse oocyte maturation and preimplantation embryonic development (Yang et al., 2021). Their works revealed that these modifications were enriched in germinal vesicle (GV)‒stage oocytes but declined post‒fertilization, with oxygen tension identified as a critical modulator (Yang et al., 2021). In embryonic stem cells (ESCs), lactate supplementation upregulates germline and zygotic genome activation (ZGA)‒related genes (particularly Zscan4) through H3K18la accumulation at these loci, where lactylated co-factors promote transcriptional elongation (Tian and Zhou, 2022; Xie et al., 2022). Conversely, Lin et al. demonstrated that in mouse oocytes, Tfap2α over-expression elevates p300 expression, increasing global histone lactylation levels‒such as H3K18la, histone H4 lysine 12 lactylation (H4K12la) and pan‒Kla‒and impairing spindle assembly and chromosomal alignment (Lin et al., 2022). Beyond histones, lactylation regulates non‒histone proteins like Yin Yang‒1 (YY1). Wang et al. reported hypoxia‒induced YY1‒lysine 183 lactylation (K183la) activates fibroblast growth factor 2 (FGF2) transcription to drive retinal neovascularization (Wang X. et al., 2023). Additionally, Descalzi’s et al. (2019) revealed that astrocyte‒derived lactate mediates memory consolidation by enhancing neuronal mRNA translation and Arc/Arg3.1 expression (Descalzi et al., 2019). It should be noted that the molecular mechanisms by which hypoxia-induced histone lactylation influences MSCs function remain insufficiently explored. A study by Chen et al. reported that chronic intermittent hypoxia (CIH) enhances glycolysis and lactate production in mouse BMSCs, leading to increased H3K18la levels at the proliferator‒activated receptor gamma (PPARγ) promoter region. This epigenetic modification promotes PPARγ transcription and subsequently impairs osteogenic differentiation (Chen et al., 2025). In contrast, another study demonstrated that a 3D-printed polycaprolactone/nano-hydroxyapatite (PCL/nHA) scaffold enabling sustained lactate release (mimicking hypoxia conditions) promotes signal transducer and transcription 1, lysine 193 (STAT1-K193) lactylation, which in turn releases runt-related transcription factor 2 (Runx2) and enhances osteogenic transcription in human BMSCs (Zeng et al., 2025).
FIGURE 3
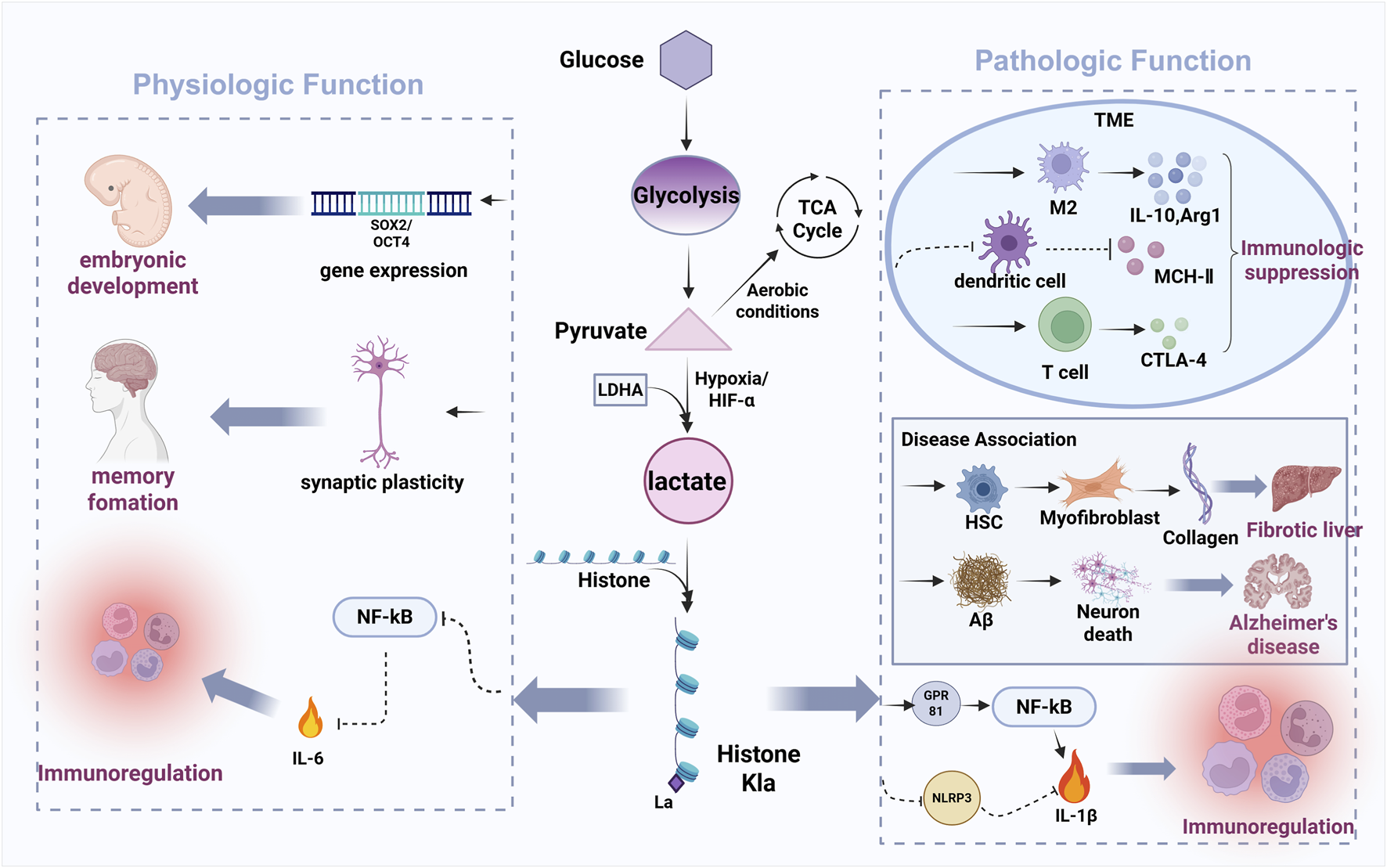
The bidirectional regulatory role of lactylation in physiological and pathological processes.
3.3 Lactylation mechanisms in cancer cells and the TME
Figure 3 has shown that the Warburg effect, a hallmark of cancer metabolism, describes tumor cells’ preferential use of glycolysis over oxidative phosphorylation for energy production, even under oxygen‒rich conditions (Hanahan and Weinberg, 2011; Koppenol et al., 2011; Yu et al., 2021). This metabolic reprogramming results in substantial lactate accumulation, which functions as both a key metabolic intermediate and a signaling molecule within the TME. Throuth its regulation of gene transcription and protein function, lactylation drives metabolic reprogramming that enables tumor adaption to nutrient deprivation and sustains proliferative capacity (He et al., 2024). This suggests histone lactylation is frequently dysregulated in cancer, representing a promising therapeutic target. Within the TME, abundant lactate serves as the substrate for lactylation modifications in both tumor and infiltrating immune cells. Lactate is known to modulate immune cell behavior, including cytotoxic T-lymphocyte-associated protein 4 (CTLA‒4) upregulation in T cells, macrophage polarization, and dendritic cell immunosuppression. Pharmacologically, sodium dichloroacetate (DCA) and oxamate suppress lactate production by inhibiting the activity of pyruvate dehydrogenase (PDH) and lactate dehydrogenase (LDH), thereby reducing intracellular lactate and Kla (lysine lactylation) modifications (Zhang et al., 2019). In contrast, rotenone enhances glycolysis by blocking mitochondrial respiration, increasing both lactate and Kla levels (Zhang et al., 2019). Zhang et al. first elucidated the impact of histone lactylation on macrophage polarization, showing lactate dehydrogenase A (LDHA)‒knockout reduces lactate production, histone Kla levels, and M2 marker Arg1, while lactate supplementation increases Arg1 and Vegfa (both M2‒like macrophage associated genes) (Zhang et al., 2019). These findings indicate histone lactylation promotes Arg‒1 and wound‒healing gene expression, facilitating the pro‒inflammatory classically activated macrophage (M1) to immunosuppressive M2 macrophage phenotypic switch. In malignancy, lactate and the TME critically promote tumorigenisis throuth angiogenesis, invasion, metastasis, and immune evasion (Chen et al., 2022). Importantly, histone Kla contributes to immune suppression by reinforcing M2‒like macrophage polarization, thereby inhibiting anti‒tumor immune responses. Together, these findings highlight the lactate‒lactylation axis as a critical regulator of TME immunosuppression and a viable target for anti‒cancer therapies (Chen et al., 2022; Ngwa et al., 2019).
Current research on lactylation modification primarily focuses on TME and immune cells. These findings provide a critical conceptual framework and mechanistic insights for understanding the potential role of lactylation in MSCs biology. However, it is crucial to emphasize the differences arising from cell type and metabolic status in this process.
3.4 Impact of protein lactylation on disease pathogenesis
Recent studies have delineated how Kla contributes to disease pathogenesis, demonstrating its capacity to either directly alter cellular signaling pathways or indirectly regulate downstream effects through upstream signaling cascades. These findings provide promising novel therapeutic targets and offer innovative approaches for modulating disease‒relevant pathways.
A 2023 study using liver biopsies from cirrhosis patients revealed that huc‒MSCs therapy significantly alters protein lactylation profiles, particularly affecting glucose metabolic enzymes, suggesting glucometabolic pathways may mediate huc‒MSCs’ therapeutic effects in cirrhosis (Xie et al., 2023). This finding demonstrates that the immunomodulatory and regenerative functions of MSCs are closely associated with metabolic reprogramming and lactylation modification.
3.5 Biological significance of lactylation
3.5.1 Histone lactylation in gene expression regulation
Histone lactylation regulates gene expression through altering chromatin architecture, controlling transcription factor/cofactor recruitment, and directly modulating specific target gene expression. As chromatin’s fundamental structural units, histones coordinate genomic organization and transcriptional regulation through interactions with deoxyribonucleic acid (DNA) and non‒coding ribonucleic acid (nc RNA). At the molecular level, lactylation influences gene expression by altering histone charge state, interfering with transcription factor binding, and modulating transcriptional initiation and elongation. MSCs may utilize these mechanisms to regulate the expression of key functional genes. Additionally, growing evidence indicates lactylation may indirectly modulate gene expression by influencing other PTMs status, particularly histone acetylation, forming a multi‒layered regulatory system.
3.5.2 Lactylation couples cellular metabolism with gene expression
Lactylation represents a crucial epigenetic mechanism that bridges cellular metabolic states (e.g., hypoxia, enhanced glycolysis) with transcriptional regulation, effectively coupling metabolic flux with gene expression reprogramming. Furthermore, lactylation regulates the expression of metabolic pathway genes (including glycolysis and oxidative phosphorylation components), enabling cellular adaptation to metabolic stress. Hypoxic preconditioning enhances glycolytic activity in MSCs, leading to lactate accumulation and subsequent lactylation that regulates gene expression. Conversely, lactylation may further potentiate the glycolytic pathway in MSCs, thereby forming a metabolic–epigenetic cycle that promotes their adaptation to the surrounding microenvironment. Fei Li et al. found that histone lactylation promotes glycolysis by activating the transcription and expresssion of metabolic regulators. Their work in pancreatic ductal adenocarcinoma (PDAC) also revealed that H3K18la enrichment at promoter regions enhances the transcription of TTK protein kinase (TTK) and BUB1 mitotic checkpoint serine/threonine kinase B (BUB1B), which upregulates the histone acetyltransferase p300 and subsequently enhances glycolytic upregulation (Li et al., 2024a). Within the TME, this histone lactylation‒driven metabolic reprogramming further promotes oncogenesis and cancer progression. Such epigenetic regulation enables cancer cells to maintain their proliferative capacity and survival advantage under metabolic constraints.
3.5.3 Lactylation in immunomodulation
Growing evidence highlights the pivotal role of lactylation in immune regulation, particularly in controlling macrophage polarization and T‒cell function. In microglia and macrophages, histone lactylation serves as a key modulator of the M1/M2 polarization balance, thereby influencing inflammatory responses and immune homeostasis. Mechanistically, lactate‒induced histone lactylation simultaneously suppresses pro‒inflammatory M1‒associated signaling pathways while promoting the transcriptional activation of anti‒inflammatory M2 phenotype genes (Ivashkiv, 2020; Xin et al., 2022). These immunomodulatory effects are further confirmed in the TME, where lactate exposure upregulates M2 markers while downregulates M1 markers in microglia (Longhitano et al., 2023). Beyond macrophages, lactylation exerts broad immunosuppressive effects by impairing cytotoxic immune cell function, which compromises both CD8+ T cell and natural killer T (NKT) cell anti‒tumor activity (Hao et al., 2024). Wang et al. demonstrated that in malignant pleural effusion (MPE), H3K18la promotes forkhead box protein P3 (FOXP3) expression in peripheral blood mononuclear cells (PBMCs), simultaneously enhancing the immunosuppressive function of Tregs while inhibiting NKT cell‒mediated anti‒tumor responses (Wang ZH. et al., 2023). Similarly, in glioblastoma (GBM) stem cells, histone lactylation drives immunosuppression through two coordiated mechanisms: CD47 upregulation to attenuate phagocytic activity and signal transducer and transcription 3 (STAT3) activation to reduce microglial/macrophage infiltration and impair immune surveillance (Wang S. et al., 2024). Together, these findings position lactylation as a critical epigenetic regulator that reprograms immune responses in pathological conditions.
Growing evidence demonstrates histone lactylation as a key epigenetic regulator of inflammatory gene expression that critically modulates immune cell activation and function. In GBM, lactate‒induced histone lactylation in monocyte‒derived macrophages upregulates IL‒10 expression, leading to T‒cell suppression and impaired anti-tumor immune responses (De Leo et al., 2024). Lactylation plays a critical role in M2 polarization and Treg-induced immunosuppression, providing valuable insights into its role in mediating the immunomodulatory functions of MSCs and supporting the safety of anticancer therapies.
3.5.4 Lactylation dictates cellular fate
Lactylation functions as a crucial metabolic‒epigenetic regulator that governs cell lineage specification and reprogramming pathways. The transition between cellular states, particularly direct reprogramming (transdifferentiation) that bypasses pluripotent intermediates, is precisely regulated by coordinated metabolic remodeling and chromatin plasticity (Wang et al., 2021). This sophisticated process is regulated by an integrated network of transcription factors, RNA‒binding proteins, and chromatin remodelers that interact with metabolic pathways to determine cell fate (Ryall et al., 2015). CIH impairs osteogenesis and long bone growth in mouse BMSCs by modulating histone lactylation (Chen et al., 2025). In contrast, both exercise-mediated mechanical stress and sustained lactate release via 3D-printed PCL/nHA scaffolds (mimicking hypoxia condition) enhance lactylation levels, thereby promoting osteogenic differentiation in both mouse and human BMSCs (Zeng et al., 2025; Dai et al., 2025). These findings highlight the dual and the context-specific regulatory functions of lactylation in celluar reprogramming and self-renewal ability.
To provide a clear distinction between direct evidence from MSCs studies and indirect inferences from other cell systems, Table 1 summarizes the key findings on lactylation in MSCs, including study type, MSC source, lactylation target, and observed biological effects.
TABLE 1
| References | Study type | MSCs source | Lactylation target | Biological effect |
|---|---|---|---|---|
| Chen et al. (2025) | Experimental study (in vitro) | Mouse BMSCs | H3K18la at PPARγ promoter region | Impairs osteogenic differentiation |
| Zeng et al. (2025) | Experimental study (in vitro) | Human BMSCs | STAT1-K193 lactylation | Enhances osteogenic differentiation |
| Xie et al. (2023) | Clinical study (patient samples) | Huc-MSCs | Protein lactylation on metabolic enzymes | Alters lactylation profiles; mediates therapeutic effects in cirrhosis |
| Kastner et al. (2020) | Experimental study (in vitro) | Human BMSCs | Lactylation-mediated signaling | Enhances proliferation capacity |
| Selleri et al. (2016) | Experimental study (in vitro) | Huc-MSCs | Lactate secretion | Enhances M2-macrophage differentiation |
| Wu et al. (2023) | Experimental study | Mouse BMSCs | H3K18la | Promotes osteogenic differentiation |
| Kolodziej et al. (2019) | Experimental study (in vitro) | Human ADSCs | Lactylation activates PPARγ | Drives adipocyte differentiation |
Summary of direct evidence on lactylation modification in MSCs.
4 Hypoxia‒Driven lactylation modulates MSC functionality
Hypoxic conditioning triggers HIF‒α‒mediated transcriptional activation of glycolytic enzymes and hypoxia‒responsive genes, thereby enhancing lactate production and lactylation modification (Li Y. et al., 2024). This process profoundly influences MSCs morphology, functional adaptability, and therapeutic potential, particularly in tissue regeneration applications.
4.1 Morphological adaptations
Lactate‒mediated PTMs coordinate cytoskeletal remodeling through three distinct but interconnected mechanisms: (1) Direct lactylation of cytoskeletal components (including actin filaments and microtubule‒related proteins) alters their polymerization kinetics, promoting morphological transition in hypoxia‒primed MSCs from spindle‒shaped to flattened/stellate configurations that facilitate enhanced MSCs migration capacity; (2) Histone Kla at pro‒inflammatory and differentiation‒related gene loci activates epithelial-mesenchymal transition (EMT)‒related transcriptional programs, resulting in secondary cytoskeletal reorganization; and (3) Lactate‒induced lactylation of membrane surface receptors disrupts focal adhesion kinase (FAK) signaling pathways, thereby reducing substrate adhesion and modulating microenvironmental navigation.
4.2 Functional regulation of lactylation in hypoxic microenvironments
Hypoxia induces the Warburg effect, resulting in lactate accumulation that subsequently promotes histone lactylation through increased substrate availability. This metabolic‒epigenetic coupling regulates cellular proliferation via modulating the expression of proliferation‒associated genes and integration of key signaling pathways, including HIF‒1α and mechanistic target of rapamycin (mTOR) pathways. In various cell types, including glioma, non-small cell lung cancer (NSCLC), and esophageal cancer cells, lactylation has been shown to promote cell proliferation by regulating signaling axes such as HIF-1α and YTHDF2-BNIP3, implying a potentially similar role in MSCs (Dong et al., 2025; Chen et al., 2023; Zang et al., 2024; Yan et al., 2024).
Hypoxia‒induced lactylation differentially regulates MSCs proliferation in a time‒ and dose‒dependent manner. Acute hypoxia (≤48 h) enhances proliferation capacity via lactylation‒mediated Ki‒67 upregulation (Kastner et al., 2020). On the contrary, sustained hypoxia (>72 h) promotes ROS accumulation, leading to DNA damage and cell cycle arrest. This biphasic regulation is mirrored by lactate concentration effects: low‒dose lactate promotes mitotic activity, while high‒dose lactate induces gap 2/mitosis (G2/M) phase arrest via extracellular acidification. Therefore, elevating lactylation levels in MSCs via hypoxic preconditioning may represent a potential strategy to optimize their homing efficiency to injury sites.
Hypoxia‒induced lactylation coordinates MSCs migration through three complementary mechanisms (Selleri et al., 2016). First, lactylated transcription factors directly promote cell motility. As demonstrated by Yan et al. (2024), hypoxia‒mediated SRY-box transcription factor 9 (SOX9) lactylation enhanced stemness, migratory capacity, and invasiveness in NSCLC cells by activating EMT pathways (Yan et al., 2024). Secondly, lactylation dynamically modulates the SDF‒1/CXCR4 signaling axis, thereby amplifying chemotactic responses to injury‒associated chemokine gradients. Third, lactate increases matrix metalloproteinase (matrix metalloproteinase-2 [MMP‒2] and MMP‒9 activity), promoting extracellular matrix degradation and tissue barrier penetration (Wang CY. et al., 2022; Meng et al., 2024; Lin et al., 2016).
Hypoxic‒induced lactylation precisely modulates MSCs secretome via exosomal cargo modification and cytokine profile polarization. Specifically, lactylated proteins (including heat shock protein 90 [HSP90] and miR‒21‒5p) within exosomes significantly enhance their anti‒inflammatory and pro‒angiogenic capacities. Additionally, this metabolic‒epigenetic regulation promotes increased secretion of regenerative factors (including VEGF and interleukin-6 [IL‒6]) while suppressing pro‒inflammatory mediators expression at the transcriptional level (Selleri et al., 2016; Liu X. et al., 2024; Lopez et al., 2022).
Growing evidence demonstrates that lactylation exhibits distinct lineage‒specific regulatory effects in MSCs. Under hypoxic conditions, lactylation promotes osteogenic differentiation through wingless/integrated - beta-catenin (Wnt/β‒catenin) signaling potentiation and direct modification of osteogenesis‒related genes (Wu et al., 2023; Wu et al., 2024). Conversely, in adipogenic commitment, hyperglycemia‒induced lactate accumulation activates PPARγ via lactylation, driving lipid droplet formation and adipocyte differentiation (Kolodziej et al., 2019).
Futhermore, lactate metabolites function as key immunometabolic regulators that orchestrate immune cell polarization through distinct mechanisms: (1) promoting macrophage polarization via signal transducer and transcription 6/arginase 1 (STAT6/ARG1) pathway activation, and (2) suppressing T cell function via coordinated PD‒L1 upregulation and tryptophan depletion, collectively creating an immunosuppressive microenvironment (Pradenas et al., 2023; Chen et al., 2022).
5 Translation perspectives: targeting lactylation to enhance MSCs therapy
Accumulating evidence establishes lactylation as a pivotal regulator in disease pathogenesis, highlighting its dual potential as both a diagnostic biomarker and therapeutic target, particularly for cancer and metabolic disorders. Current therapy strategies focus on two primary approaches: (1) modulation of lactate metabolism through MCTs inhibition, and (2) direct targeting of histone lactylation. The MCT1 inhibitor AZD3965, currently in Phase I/II trials, demonstrates synergistic effects with immune checkpoint inhibitors (ICIs) by reducing TME lactate levels and potentiating anti‒tumor immunity (Beloueche-Babari et al., 2020). Similarly, MCT4 inhibition improves programmed cell death protein 1 (PD‒1) blockade efficacy in hepatocellular carcinoma (HCC) models, suggesting a potential therapeutic strategy for ICI‒resistant HCC patients (Chen et al., 2022). Studies have demonstrated that targeting lactylation-related pathways can effectively reverse therapeutic resistance in multiple malignancies, including colorectal cancer and bladder cancer (Li W. et al., 2024; Li et al., 2024d). Therefore, leveraging strategies from oncology that modulate lactate metabolism or directly inhibit lactylation could be applied in the MSCs field to enhance specific therapeutic functions.
Following strategies have revealed promising translational avenues for targeting lactylation in clinical applications, including personalized immunotherapy, chemoresistance reversal, anti‒angiogenic therapy, and cancer stem cell (CSC) targeting (He et al., 2024). By leveraging therapeutic strategies from the field of oncology, novel tools may be developed to optimize MSC-based therapies.
6 Discussion and future perspectives
6.1 Critial challenges and current research focus
Recent studies define a hypoxia‒MSC‒lactylation regulatory axis, revealing a coordinated metabolic‒epigenetic‒functional cascade that critically governs MSCs therapeutic efficacy. Several critical challenges remain unsolved in lactylation research. First, it is essential to acknowledge the current limitations in the MSCs field, particularly the scarcity of studies directly investigating lactylation in MSCs themselves. Many current mechanistic insights are extrapolated from cancer or immune cell models. Second, the technological challenges in detecting lactylation, such as the lack of highly specific, site-specific anti-lactylation antibodies, it hampers the precise mapping and validation of lactylation events. Third, the potential crosstalk among lactylation, acetylation, and methylation in disease progression demands systematic investigation. Finally, it remains exceptionally difficult to distinguish whether observed lactylation modifications are drivers of functional changes or merely correlative epiphenomena, necessitating the development of more sophisticated genetic and pharmacological tools for causal inference. Addressing these gaps will help elucidate lactylation’s role in pathogenesis and facilitate therapeutic development. Current research efforts primarily focused on two key areas: (1) mechanistic characterization through comprehensive lactylation site mapping via integrated metabolomic and epigenomic analyses, coupled with functional validation using gene editing or targeted pharmacological inhibition; and (2) preclinical development, involving systematic assessment of lactylation‒modulated MSCs functional modulation in established murine disease models.
Future research should focus on three key priorities to advance MSC‒based therapies: (1) network elucidation‒comprehensive mapping of tissue‒specific lactylation interactomes in MSCs to identify origin‒dependent regulatory networks; (2) clinical‒translation‒establishment of standardized lactylation levels as a critical quality attribute (CQA) during MSCs production to ensure batch consistency; and (3) combinatorial approaches‒strategic integration of lactylation modulation with biomaterial scaffolds or cytokine priming to synergistically enhance therapeutic efficacy.
6.2 Future perspectives: bridging discovery to therapy
Research on lactylation has reached a critical preclinical‒to‒clinical transition phase, with four transformative research directions emerging: (1) Mechanistic elucidation: Employing single‒cell multi‒omics to delineate spatio-temporal lactylation dynamics accross MSCs subpopulations and conducting clustered regularly interspaced short palindromic repeats (CRISPR)‒based functional genomics screens to identify lactylation‒modifying enzymes (“writers” and “erasers”); (2) Technological innovation: Developing lactylation‒specific fluorescent biosensors for real‒time visualization in living MSCs and creating artificial intelligence (AI)‒powered predictive models of lactylation‒mediated gene regulatory networks; (3) Clinical standardization: establishing quantitative lactylation thresholds as critical release criteria for MSC‒based products and implementing longitudinal lactylation biomarker tracking in clinical trial protocols; and (4) Epigenetic crosstalk: systematically investigating the interplay between lactylation, acetylation, and methionine metabolism in MSCs fate determination and engineering next‒generation “smart MSCs” with lactylation‒responsive genetic circuits for microenvironment‒adaptive tissue repair.
Outstanding Questions and Future Directions.
Technology and Specificity: How can we develop next-generation tools to overcome current detection limitations?
Causality and Correlation: What innovative experimental approaches can establish lactylation as a functional driver rather than a passive correlate in MSCs biology?
Epigenetic crosstalk: How can we deconvolute the interconnected regulation of lactylation and other PTMs?
Therapy window: How can we achieve cell-type or context-specific lactylation targets to ensure safety for MSC-based products?
7 Conclusion
Hypoxic preconditioning has emerged as an effective approach to enhance the therapeutic potential of MSCs, primarily through glycolytic reprogramming and subsequent lactate accumulation. Beyond its conventional role as a metabolic byproduct, lactate is now recognized as a key signaling molecule that regulates cellular functions via lactylation‒mediated PTMs‒a novel metabolic‒epigenetic regulatory axis. This hypoxia‒lactylation crosstalk critically regulates MSCs functionality through two key dimensions: mechanistic regulation and therapeutic application. At the mechanistic level, hypoxia‒lactylation crosstalk: (1) directs immunomodulatory polarization via lactylation‒dependent PD‒L1/IL‒10 upregulation, (2) enhances tissue repair capacity by activating pro‒angiogenic factors and extracellular matrix remodeling pathways, and (3) maintains stemness properties via SRY-box transcription factor 2/octamer-binding transcription factor 4 (SOX2/OCT4) lactylation‒mediated pluripotency network stabilization. Furthermore, the metabolic‒epigenetic synergy plays a pivotal role in modulating these mechanisms. Clinically, current therapeutic strategies targeting this axis encompass: (1) lactylation‒specific agents (e.g., small‒molecule inhibitors against LDH or MCTs to modulate lactylation dynamics), (2) combination therapies (e.g., integrating hypoxic preconditioning with biomaterial scaffolds), and (3) safety evaluation parameters (e.g., establishing lactylation thresholds as CQA).
Statements
Author contributions
FZ: Writing – original draft, Software, Conceptualization, Writing – review and editing. QT: Supervision, Writing – review and editing, Conceptualization. JL: Writing – review and editing, Funding acquisition, Supervision, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the National Natural Science Foundation of China (82060242).
Acknowledgments
The authors would like to thank the Regenerative Medicine Research Center of The First People’s Hospital of Yunnan Province and the Medicine School, Kunming University of Science and Technology, for their support during the preparation of this review. We especially thank Professor Xiaoyu Yang from our research group for valuable guidance and assistance throughout the conception and writing process.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Ababneh N. A. Al-Kurdi B. Jamali F. Awidi A. (2022). A comparative study of the capability of MSCs isolated from different human tissue sources to differentiate into neuronal stem cells and dopaminergic-like cells. PeerJ10, e13003. 10.7717/peerj.13003
2
Ahluwalia A. Tarnawski A. S. (2012). Critical role of hypoxia sensor--HIF-1α in VEGF gene activation. Implications for angiogenesis and tissue injury healing. Curr. Med. Chem.19 (1), 90–97. 10.2174/092986712803413944
3
Alwohoush E. Ismail M. A. Al-Kurdi B. Barham R. Al Hadidi S. Awidi A. et al (2024). Effect of hypoxia on proliferation and differentiation of induced pluripotent stem cell-derived mesenchymal stem cells. Heliyon10 (19), e38857. 10.1016/j.heliyon.2024.e38857
4
Annabi B. Lee Y. T. Turcotte S. Naud E. Desrosiers R. R. Champagne M. et al (2003). Hypoxia promotes murine bone-marrow-derived stromal cell migration and tube formation. Stem Cells21 (3), 337–347. 10.1634/stemcells.21-3-337
5
Bagge J. Berg L. C. Janes J. MacLeod J. N. (2022). Donor age effects on in vitro chondrogenic and osteogenic differentiation performance of equine bone marrow- and adipose tissue-derived mesenchymal stromal cells. BMC Vet. Res.18 (1), 388. 10.1186/s12917-022-03475-2
6
Beloueche-Babari M. Casals Galobart T. Delgado-Goni T. Wantuch S. Parkes H. G. Tandy D. et al (2020). Monocarboxylate transporter 1 blockade with AZD3965 inhibits lipid biosynthesis and increases tumour immune cell infiltration. Br. J. Cancer122 (6), 895–903. 10.1038/s41416-019-0717-x
7
Bertram C. Hass R. (2008). Cellular responses to reactive oxygen species-induced DNA damage and aging. Biol. Chem.389 (3), 211–220. 10.1515/BC.2008.031
8
Campagnoli C. Roberts I. A. Kumar S. Bennett P. R. Bellantuono I. Fisk N. M. (2001). Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood98 (8), 2396–2402. 10.1182/blood.v98.8.2396
9
Chang C. P. Chio C. C. Cheong C. U. Chao C. M. Cheng B. C. Lin M. T. (2013). Hypoxic preconditioning enhances the therapeutic potential of the secretome from cultured human mesenchymal stem cells in experimental traumatic brain injury. Clin. Sci. (Lond).124 (3), 165–176. 10.1042/CS20120226
10
Chen L. Xu Y. Zhao J. Zhang Z. Yang R. Xie J. et al (2014). Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PLoS One9 (4), e96161. 10.1371/journal.pone.0096161
11
Chen L. Huang L. Gu Y. Cang W. Sun P. Xiang Y. (2022). Lactate-lactylation hands between metabolic reprogramming and immunosuppression. Int. J. Mol. Sci.23 (19), 11943. 10.3390/ijms231911943
12
Chen J. Zhang M. Liu Y. Zhao S. Wang Y. Wang M. et al (2023). Histone lactylation driven by mROS-mediated glycolytic shift promotes hypoxic pulmonary hypertension. J. Mol. Cell Biol.14 (12), mjac073. 10.1093/jmcb/mjac073
13
Chen F. Gu M. Xu H. Zhou S. Shen Z. Li X. et al (2025). Chronic intermittent hypoxia impairs BM-MSC osteogenesis and long bone growth through regulating histone lactylation. J. Transl. Med.23 (1), 845. 10.1186/s12967-025-06849-w
14
Cho W. J. Pulimamidi V. K. Mittal S. K. Chauhan S. K. (2024). Mesenchymal stromal cells protect tissues from Th1 immune responses via IL-11 secretion. Faseb J.38 (10), e23683. 10.1096/fj.202400078R
15
Cui H. Xie N. Banerjee S. Ge J. Jiang D. Dey T. et al (2021). Lung myofibroblasts promote macrophage profibrotic activity through lactate-induced histone lactylation. Am. J. Respir. Cell Mol. Biol.64 (1), 115–125. 10.1165/rcmb.2020-0360OC
16
Dai S. Chen Z. Liu X. Dong J. Zhang Z. Yang Z. et al (2025). Exercise-mediated mechanical stress promotes osteogenic differentiation of BMSCs through upregulation of lactylation via the IER3/LDHB axis. Faseb J.39 (8), e70537. 10.1096/fj.202403157R
17
Dao T. T. Nguyen C. T. Vu N. B. Le H. T. Nguyen P. D. Van Pham P. (2019). Evaluation of proliferation and osteogenic differentiation of human umbilical cord-derived mesenchymal stem cells in porous scaffolds. Adv. Exp. Med. Biol.1084, 207–220. 10.1007/5584_2019_343
18
Davies L. C. Heldring N. Kadri N. Le Blanc K. (2017). Mesenchymal stromal cell secretion of programmed Death-1 ligands regulates T cell mediated immunosuppression. Stem Cells35 (3), 766–776. 10.1002/stem.2509
19
De Leo A. Ugolini A. Yu X. Scirocchi F. Scocozza D. Peixoto B. et al (2024). Glucose-driven histone lactylation promotes the immunosuppressive activity of monocyte-derived macrophages in glioblastoma. Immunity57 (5), 1105–23.e8. 10.1016/j.immuni.2024.04.006
20
Descalzi G. Gao V. Steinman M. Q. Suzuki A. Alberini C. M. (2019). Lactate from astrocytes fuels learning-induced mRNA translation in excitatory and inhibitory neurons. Commun. Biol.2, 247. 10.1038/s42003-019-0495-2
21
Dominici M. Le Blanc K. Mueller I. Slaper-Cortenbach I. Marini F. Krause D. et al (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The international Society for Cellular Therapy position statement. Cytotherapy8 (4), 315–317. 10.1080/14653240600855905
22
Dong F. Yin H. Zheng Z. (2025). Hypoxia-Inducible Factor-1α regulates BNIP3-Dependent mitophagy and mediates metabolic reprogramming through Histone Lysine lactylation modification to affect glioma proliferation and invasion. J. Biochem. Mol. Toxicol.39 (2), e70069. 10.1002/jbt.70069
23
Ejtehadifar M. Shamsasenjan K. Movassaghpour A. Akbarzadehlaleh P. Dehdilani N. Abbasi P. et al (2015). The effect of hypoxia on mesenchymal stem cell biology. Adv. Pharm. Bull.5 (2), 141–149. 10.15171/apb.2015.021
24
Erices A. Conget P. Minguell J. J. (2000). Mesenchymal progenitor cells in human umbilical cord blood. Br. J. Haematol.109 (1), 235–242. 10.1046/j.1365-2141.2000.01986.x
25
Feng J. Wang W. (2017). Hypoxia pretreatment and EPO-modification enhance the protective effects of MSC on neuron-like PC12 cells in a similar way. Biochem. Biophys. Res. Commun.482 (2), 232–238. 10.1016/j.bbrc.2016.11.046
26
Feng Y. Zhu R. Shen J. Wu J. Lu W. Zhang J. et al (2019). Human bone marrow mesenchymal stem cells rescue endothelial cells experiencing chemotherapy stress by mitochondrial transfer via tunneling nanotubes. Stem Cells Dev.28 (10), 674–682. 10.1089/scd.2018.0248
27
Feng X. D. Zhou J. H. Chen J. Y. Feng B. Hu R. T. Wu J. et al (2022). Long non-coding RNA SNHG16 promotes human placenta-derived mesenchymal stem cell proliferation capacity through the PI3K/AKT pathway under hypoxia. World J. Stem Cells14 (9), 714–728. 10.4252/wjsc.v14.i9.714
28
Fernández-Santos M. E. Garcia-Arranz M. Andreu E. J. García-Hernández A. M. López-Parra M. Villarón E. et al (2022). Optimization of Mesenchymal Stromal cell (MSC) manufacturing processes for a better therapeutic outcome. Front. Immunol.13, 918565. 10.3389/fimmu.2022.918565
29
Figeac F. Lesault P. F. Le Coz O. Damy T. Souktani R. Trébeau C. et al (2014). Nanotubular crosstalk with distressed cardiomyocytes stimulates the paracrine repair function of mesenchymal stem cells. Stem Cells32 (1), 216–230. 10.1002/stem.1560
30
Grayson W. L. Zhao F. Izadpanah R. Bunnell B. Ma T. (2006). Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. J. Cell Physiol.207 (2), 331–339. 10.1002/jcp.20571
31
Gronthos S. Mankani M. Brahim J. Robey P. G. Shi S. (2000). Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A.97 (25), 13625–13630. 10.1073/pnas.240309797
32
Gu J. Gu W. Lin C. Gu H. Wu W. Yin J. et al (2015). Human umbilical cord mesenchymal stem cells improve the immune-associated inflammatory and prothrombotic state in collagen type-Ⅱ-induced arthritic rats. Mol. Med. Rep.12 (5), 7463–7470. 10.3892/mmr.2015.4394
33
Gupta S. Rawat S. Krishnakumar V. Rao E. P. Mohanty S. (2022). Hypoxia preconditioning elicit differential response in tissue-specific MSCs via immunomodulation and exosomal secretion. Cell Tissue Res.388 (3), 535–548. 10.1007/s00441-022-03615-y
34
Hamid T. Xu Y. Ismahil M. A. Rokosh G. Jinno M. Zhou G. et al (2022). Cardiac mesenchymal stem cells promote fibrosis and remodeling in heart failure: role of PDGF signaling. JACC Basic Transl. Sci.7 (5), 465–483. 10.1016/j.jacbts.2022.01.004
35
Hanahan D. Weinberg R. A. (2011). Hallmarks of cancer: the next generation. Cell144 (5), 646–674. 10.1016/j.cell.2011.02.013
36
Hao Z. N. Tan X. P. Zhang Q. Li J. Xia R. Ma Z. (2024). Lactate and lactylation: dual regulators of T-Cell-Mediated tumor immunity and immunotherapy. Biomolecules14 (12), 1646. 10.3390/biom14121646
37
He Y. Song T. Ning J. Wang Z. Yin Z. Jiang P. et al (2024). Lactylation in cancer: mechanisms in tumour biology and therapeutic potentials. Clin. Transl. Med.14 (11), e70070. 10.1002/ctm2.70070
38
Hegde M. Singh A. K. Kannan S. Kolkundkar U. Seetharam R. N. (2024). Therapeutic applications of engineered mesenchymal stromal cells for enhanced angiogenesis in cardiac and cerebral ischemia. Stem Cell Rev. Rep.20 (8), 2138–2154. 10.1007/s12015-024-10787-3
39
Hu X. Yu S. P. Fraser J. L. Lu Z. Ogle M. E. Wang J. A. et al (2008). Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J. Thorac. Cardiovasc Surg.135 (4), 799–808. 10.1016/j.jtcvs.2007.07.071
40
Hu Y. He Z. Li Z. Wang Y. Wu N. Sun H. et al (2024). Lactylation: the novel histone modification influence on gene expression, protein function, and disease. Clin. Epigenetics16 (1), 72. 10.1186/s13148-024-01682-2
41
Huang L. L. Yang J. Hou Y. Y. Bai Y. H. Jiang H. Y. (2024). Bone marrow mesenchymal stem cells ameliorate diabetes and diabetic renal fibrosis by modulating the inflammatory factor IL-11. Curr. Stem Cell Res. Ther.20, 978–989. 10.2174/011574888X348254241216171655
42
Hypoxemia vs. hypoxia (1966). N. Engl. J. Med.274 (16), 908–909. 10.1056/NEJM196604212741613
43
Imitola J. Raddassi K. Park K. I. Mueller F. J. Nieto M. Teng Y. D. et al (2004). Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc. Natl. Acad. Sci. U. S. A.101 (52), 18117–18122. 10.1073/pnas.0408258102
44
In 't Anker P. S. Scherjon S. A. Kleijburg-van der Keur C. de Groot-Swings G. M. Claas F. H. Fibbe W. E. et al (2004). Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells22 (7), 1338–1345. 10.1634/stemcells.2004-0058
45
Ivashkiv L. B. (2020). The hypoxia-lactate axis tempers inflammation. Nat. Rev. Immunol.20 (2), 85–86. 10.1038/s41577-019-0259-8
46
Izzo L. T. Wellen K. E. (2019). Histone lactylation links metabolism and gene regulation. Nature574 (7779), 492–493. 10.1038/d41586-019-03122-1
47
Jaraba-Álvarez W. V. Uscanga-Palomeque A. C. Sanchez-Giraldo V. Madrid C. Ortega-Arellano H. Halpert K. et al (2025). Hypoxia-induced metabolic reprogramming in mesenchymal stem cells: unlocking the regenerative potential of secreted factors. Front. Cell Dev. Biol.13, 1609082. 10.3389/fcell.2025.1609082
48
Ju J. Zhang H. Lin M. Yan Z. An L. Cao Z. et al (2024). The alanyl-tRNA synthetase AARS1 moonlights as a lactyltransferase to promote YAP signaling in gastric cancer. J. Clin. Invest134 (10), e174587. 10.1172/JCI174587
49
Jung S. A. Lee H. K. Yoon J. S. Kim S. J. Kim C. Y. Song H. et al (2007). Upregulation of TGF-beta-induced tissue transglutaminase expression by PI3K-Akt pathway activation in human subconjunctival fibroblasts. Invest Ophthalmol. Vis. Sci.48 (5), 1952–1958. 10.1167/iovs.06-1164
50
Kanichai M. Ferguson D. Prendergast P. J. Campbell V. A. (2008). Hypoxia promotes chondrogenesis in rat mesenchymal stem cells: a role for AKT and hypoxia-inducible factor (HIF)-1alpha. J. Cell Physiol.216 (3), 708–715. 10.1002/jcp.21446
51
Kastner N. Mester-Tonczar J. Winkler J. Traxler D. Spannbauer A. Rüger B. M. et al (2020). Comparative effect of MSC secretome to MSC Co-culture on cardiomyocyte gene expression under hypoxic conditions in vitro. Front. Bioeng. Biotechnol.8, 502213. 10.3389/fbioe.2020.502213
52
Katagiri W. Kawai T. Osugi M. Sugimura-Wakayama Y. Sakaguchi K. Kojima T. et al (2017). Angiogenesis in newly regenerated bone by secretomes of human mesenchymal stem cells. Maxillofac. Plast. Reconstr. Surg.39 (1), 8. 10.1186/s40902-017-0106-4
53
Kern S. Eichler H. Stoeve J. Klüter H. Bieback K. (2006). Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells24 (5), 1294–1301. 10.1634/stemcells.2005-0342
54
Keshavarz S. Nassiri S. M. Siavashi V. Alimi N. S. (2019). Regulation of plasticity and biological features of endothelial progenitor cells by MSC-derived SDF-1. Biochim. Biophys. Acta Mol. Cell Res.1866 (2), 296–304. 10.1016/j.bbamcr.2018.11.013
55
Khasawneh R. R. Abu-El-Rub E. (2022). Hypoxia disturbs the Migration and Adhesion characteristics of Mesenchymal Stem Cells. Cell Mol. Biol. (Noisy-le-grand)68 (11), 28–32. 10.14715/cmb/2022.68.11.5
56
Kita K. Gauglitz G. G. Phan T. T. Herndon D. N. Jeschke M. G. (2010). Isolation and characterization of mesenchymal stem cells from the sub-amniotic human umbilical cord lining membrane. Stem Cells Dev.19 (4), 491–502. 10.1089/scd.2009.0192
57
Kolodziej M. Strauss S. Lazaridis A. Bucan V. Kuhbier J. W. Vogt P. M. et al (2019). Influence of glucose and insulin in human adipogenic differentiation models with adipose-derived stem cells. Adipocyte8 (1), 254–264. 10.1080/21623945.2019.1636626
58
Koppenol W. H. Bounds P. L. Dang C. V. (2011). Otto Warburg's contributions to current concepts of cancer metabolism. Nat. Rev. Cancer11 (5), 325–337. 10.1038/nrc3038
59
Kulesza A. Paczek L. Burdzinska A. (2023). The role of COX-2 and PGE2 in the regulation of immunomodulation and other functions of mesenchymal stromal cells. Biomedicines11 (2), 445. 10.3390/biomedicines11020445
60
Lan Y. W. Choo K. B. Chen C. M. Hung T. H. Chen Y. B. Hsieh C. H. et al (2015). Hypoxia-preconditioned mesenchymal stem cells attenuate bleomycin-induced pulmonary fibrosis. Stem Cell Res. Ther.6 (1), 97. 10.1186/s13287-015-0081-6
61
Li B. Li C. Zhu M. Zhang Y. Du J. Xu Y. et al (2017). Hypoxia-Induced mesenchymal stromal cells exhibit an enhanced therapeutic effect on radiation-induced lung injury in mice due to an increased proliferation potential and enhanced antioxidant ability. Cell Physiol. Biochem.44 (4), 1295–1310. 10.1159/000485490
62
Li A. Guo F. Pan Q. Chen S. Chen J. Liu H. F. et al (2021). Mesenchymal stem cell therapy: hope for patients with systemic lupus erythematosus. Front. Immunol.12, 728190. 10.3389/fimmu.2021.728190
63
Li X. Yang Y. Zhang B. Lin X. Fu X. An Y. et al (2022). Lactate metabolism in human health and disease. Signal Transduct. Target Ther.7 (1), 305. 10.1038/s41392-022-01151-3
64
Li H. Ji X. Q. Zhang S. M. Bi R. H. (2023). Hypoxia and inflammatory factor preconditioning enhances the immunosuppressive properties of human umbilical cord mesenchymal stem cells. World J. Stem Cells15 (11), 999–1016. 10.4252/wjsc.v15.i11.999
65
Li F. Si W. Xia L. Yin D. Wei T. Tao M. et al (2024a). Positive feedback regulation between glycolysis and histone lactylation drives oncogenesis in pancreatic ductal adenocarcinoma. Mol. Cancer23 (1), 90. 10.1186/s12943-024-02008-9
66
Li Y. Cao Q. Hu Y. He B. Cao T. Tang Y. et al (2024b). Advances in the interaction of glycolytic reprogramming with lactylation. Biomed. Pharmacother.177, 116982. 10.1016/j.biopha.2024.116982
67
Li W. Zhou C. Yu L. Hou Z. Liu H. Kong L. et al (2024c). Tumor-derived lactate promotes resistance to bevacizumab treatment by facilitating autophagy enhancer protein RUBCNL expression through histone H3 lysine 18 lactylation (H3K18la) in colorectal cancer. Autophagy20 (1), 114–130. 10.1080/15548627.2023.2249762
68
Li F. Zhang H. Huang Y. Li D. Zheng Z. Xie K. et al (2024d). Single-cell transcriptome analysis reveals the association between histone lactylation and cisplatin resistance in bladder cancer. Drug Resist Updat73, 101059. 10.1016/j.drup.2024.101059
69
Liao L. Shi B. Chang H. Su X. Zhang L. Bi C. et al (2017). Heparin improves BMSC cell therapy: anticoagulant treatment by heparin improves the safety and therapeutic effect of bone marrow-derived mesenchymal stem cell cytotherapy. Theranostics7 (1), 106–116. 10.7150/thno.16911
70
Lim M. Wang W. Liang L. Han Z. B. Li Z. Geng J. et al (2018). Intravenous injection of allogeneic umbilical cord-derived multipotent mesenchymal stromal cells reduces the infarct area and ameliorates cardiac function in a porcine model of acute myocardial infarction. Stem Cell Res. Ther.9 (1), 129. 10.1186/s13287-018-0888-z
71
Lin H. Angeli M. Chung K. J. Ejimadu C. Rosa A. R. Lee T. (2016). sFRP2 activates Wnt/β-catenin signaling in cardiac fibroblasts: differential roles in cell growth, energy metabolism, and extracellular matrix remodeling. Am. J. Physiol. Cell Physiol.311 (5), C710–C719. 10.1152/ajpcell.00137.2016
72
Lin J. Ji Z. Di Z. Zhang Y. Yan C. Zeng S. (2022). Overexpression of Tfap2a in Mouse oocytes impaired spindle and chromosome Organization. Int. J. Mol. Sci.23 (22), 14376. 10.3390/ijms232214376
73
Liu W. Wang Y. Bozi L. H. M. Fischer P. D. Jedrychowski M. P. Xiao H. et al (2023). Lactate regulates cell cycle by remodelling the anaphase promoting complex. Nature616 (7958), 790–797. 10.1038/s41586-023-05939-3
74
Liu Y. Sun L. Li Y. Holmes C. (2024a). Mesenchymal stromal/stem cell tissue source and in vitro expansion impact extracellular vesicle protein and miRNA compositions as well as angiogenic and immunomodulatory capacities. J. Extracell. Vesicles13 (8), e12472. 10.1002/jev2.12472
75
Liu X. Wang J. Lao M. Liu F. Zhu H. Man K. et al (2024b). Study on the effect of protein lysine lactylation modification in macrophages on inhibiting periodontitis in rats. J. Periodontol.95 (1), 50–63. 10.1002/jper.23-0241
76
Longhitano L. Vicario N. Forte S. Giallongo C. Broggi G. Caltabiano R. et al (2023). Lactate modulates microglia polarization via IGFBP6 expression and remodels tumor microenvironment in glioblastoma. Cancer Immunol. Immunother.72 (1), 1–20. 10.1007/s00262-022-03215-3
77
Lopez K. A. Nehring H. P. Krause F. F. Wempe A. Raifer H. Nist A. et al (2022). Lactate induces metabolic and epigenetic reprogramming of pro-inflammatory Th17 cells. EMBO Rep.23 (12), e54685. 10.15252/embr.202254685
78
Ma D. Xu K. Zhang G. Liu Y. Gao J. Tian M. et al (2019). Immunomodulatory effect of human umbilical cord mesenchymal stem cells on T lymphocytes in rheumatoid arthritis. Int. Immunopharmacol.74, 105687. 10.1016/j.intimp.2019.105687
79
MacIntyre N. R. (2014). Tissue hypoxia: implications for the respiratory clinician. Respir. Care59 (10), 1590–1596. 10.4187/respcare.03357
80
Mallat J. Rahman N. Hamed F. Hernandez G. Fischer M. O. (2022). Pathophysiology, mechanisms, and managements of tissue hypoxia. Anaesth. Crit. Care Pain Med.41 (4), 101087. 10.1016/j.accpm.2022.101087
81
Mao Y. Zhang J. Zhou Q. He X. Zheng Z. Wei Y. et al (2024). Hypoxia induces mitochondrial protein lactylation to limit oxidative phosphorylation. Cell Res.34 (1), 13–30. 10.1038/s41422-023-00864-6
82
Melzer C. Yang Y. Hass R. (2016). Interaction of MSC with tumor cells. Cell Commun. Signal14 (1), 20. 10.1186/s12964-016-0143-0
83
Meng S. S. Guo F. M. Huang L. L. Huang Y. Z. Xie J. F. Yang C. S. et al (2021). mTORC2 activation mediated by mesenchymal stem cell-secreted hepatocyte growth factors for the recovery of lipopolysaccharide-induced vascular endothelial barrier. Stem Cells Int.2021, 9981589. 10.1155/2021/9981589
84
Meng S. Sørensen E. E. Ponniah M. Thorlacius-Ussing J. Crouigneau R. Larsen T. et al (2024). MCT4 and CD147 colocalize with MMP14 in invadopodia and support matrix degradation and invasion by breast cancer cells. J. Cell Sci.137 (8), jcs261608. 10.1242/jcs.261608
85
Miao Z. Jin J. Chen L. Zhu J. Huang W. Zhao J. et al (2006). Isolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cells. Cell Biol. Int.30 (9), 681–687. 10.1016/j.cellbi.2006.03.009
86
Mihaylova Z. Tsikandelova R. Sanimirov P. Gateva N. Mitev V. Ishkitiev N. (2018). Role of PDGF-BB in proliferation, differentiation and maintaining stem cell properties of PDL cells in vitro. Arch. Oral Biol.85, 1–9. 10.1016/j.archoralbio.2017.09.019
87
Ng F. Boucher S. Koh S. Sastry K. S. Chase L. Lakshmipathy U. et al (2008). PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood112 (2), 295–307. 10.1182/blood-2007-07-103697
88
Ngwa V. M. Edwards D. N. Philip M. Chen J. (2019). Microenvironmental metabolism regulates Antitumor immunity. Cancer Res.79 (16), 4003–4008. 10.1158/0008-5472.CAN-19-0617
89
Niu D. Luo T. Wang H. Xia Y. Xie Z. (2021). Lactic acid in tumor invasion. Clin. Chim. Acta.522, 61–69. 10.1016/j.cca.2021.08.011
90
Noronha N. C. Mizukami A. Caliári-Oliveira C. Cominal J. G. Rocha J. L. M. Covas D. T. et al (2019). Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res. Ther.10 (1), 131. 10.1186/s13287-019-1224-y
91
Ong H. T. Dilley R. J. (2018). Novel non-angiogenic role for mesenchymal stem cell-derived vascular endothelial growth factor on keratinocytes during wound healing. Cytokine Growth Factor Rev.44, 69–79. 10.1016/j.cytogfr.2018.11.002
92
Østergaard L. Finnerup N. B. Terkelsen A. J. Olesen R. A. Drasbek K. R. Knudsen L. et al (2015). The effects of capillary dysfunction on oxygen and glucose extraction in diabetic neuropathy. Diabetologia58 (4), 666–677. 10.1007/s00125-014-3461-z
93
Peng W. Sun J. Sheng C. Wang Z. Wang Y. Zhang C. et al (2015). Systematic review and meta-analysis of efficacy of mesenchymal stem cells on locomotor recovery in animal models of traumatic brain injury. Stem Cell Res. Ther.6 (1), 47. 10.1186/s13287-015-0034-0
94
Pittenger M. F. Mackay A. M. Beck S. C. Jaiswal R. K. Douglas R. Mosca J. D. et al (1999). Multilineage potential of adult human mesenchymal stem cells. Science284 (5411), 143–147. 10.1126/science.284.5411.143
95
Pouikli A. Maleszewska M. Parekh S. Yang M. Nikopoulou C. Bonfiglio J. J. et al (2022). Hypoxia promotes osteogenesis by facilitating acetyl-coa-mediated mitochondrial-nuclear communication. Embo J.41 (23), e111239. 10.15252/embj.2022111239
96
Pradenas C. Luque-Campos N. Oyarce K. Contreras-Lopez R. Bustamante-Barrientos F. A. Bustos A. et al (2023). Lactate: an alternative pathway for the immunosuppressive properties of mesenchymal stem/stromal cells. Stem Cell Res. Ther.14 (1), 335. 10.1186/s13287-023-03549-4
97
Pulido-Escribano V. Torrecillas-Baena B. Camacho-Cardenosa M. Dorado G. Gálvez-Moreno M. Casado-Díaz A. (2022). Role of hypoxia preconditioning in therapeutic potential of mesenchymal stem-cell-derived extracellular vesicles. World J. Stem Cells14 (7), 453–472. 10.4252/wjsc.v14.i7.453
98
Putra A. Ridwan F. B. Putridewi A. I. Kustiyah A. R. Wirastuti K. Sadyah N. A. C. et al (2018). The role of TNF-α induced MSCs on suppressive inflammation by increasing TGF-β and IL-10. Open Access Maced. J. Med. Sci.6 (10), 1779–1783. 10.3889/oamjms.2018.404
99
Qi L. Xue X. Sun J. Wu Q. Wang H. Guo Y. et al (2018). The promising effects of transplanted umbilical cord mesenchymal stem cells on the treatment in traumatic brain injury. J. Craniofac Surg.29 (7), 1689–1692. 10.1097/SCS.0000000000005042
100
Quade M. Münch P. Lode A. Duin S. Vater C. Gabrielyan A. et al (2020). The secretome of hypoxia conditioned hMSC loaded in a central depot induces chemotaxis and angiogenesis in a biomimetic mineralized collagen bone replacement material. Adv. Healthc. Mater9 (2), e1901426. 10.1002/adhm.201901426
101
Ren H. Cao Y. Zhao Q. Li J. Zhou C. Liao L. et al (2006). Proliferation and differentiation of bone marrow stromal cells under hypoxic conditions. Biochem. Biophys. Res. Commun.347 (1), 12–21. 10.1016/j.bbrc.2006.05.169
102
Renesme L. Cobey K. D. Lalu M. M. Bubela T. Chinnadurai R. De Vos J. et al (2025). Delphi-driven consensus definition for mesenchymal stromal cells and clinical reporting guidelines for mesenchymal stromal cell-based therapeutics. Cytotherapy27 (2), 146–168. 10.1016/j.jcyt.2024.10.008
103
Rosová I. Dao M. Capoccia B. Link D. Nolta J. A. (2008). Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells26 (8), 2173–2182. 10.1634/stemcells.2007-1104
104
Ryall J. G. Cliff T. Dalton S. Sartorelli V. (2015). Metabolic reprogramming of stem cell epigenetics. Cell Stem Cell17 (6), 651–662. 10.1016/j.stem.2015.11.012
105
Selleri S. Bifsha P. Civini S. Pacelli C. Dieng M. M. Lemieux W. et al (2016). Human mesenchymal stromal cell-secreted lactate induces M2-macrophage differentiation by metabolic reprogramming. Oncotarget7 (21), 30193–30210. 10.18632/oncotarget.8623
106
Sheng L. Mao X. Yu Q. Yu D. (2017). Effect of the PI3K/AKT signaling pathway on hypoxia-induced proliferation and differentiation of bone marrow-derived mesenchymal stem cells. Exp. Ther. Med.13 (1), 55–62. 10.3892/etm.2016.3917
107
Shi X. F. Wang H. Xiao F. J. Yin Y. Xu Q. Q. Ge R. L. et al (2016). MiRNA-486 regulates angiogenic activity and survival of mesenchymal stem cells under hypoxia through modulating Akt signal. Biochem. Biophys. Res. Commun.470 (3), 670–677. 10.1016/j.bbrc.2016.01.084
108
Shi X. Zhang K. Qi Q. Zhou W. Yu F. Zhang Y. (2024). Human umbilical cord-derived mesenchymal stem cells attenuate hepatic stellate cells activation and liver fibrosis. Mol. Biol. Rep.51 (1), 734. 10.1007/s11033-024-09664-6
109
Song C. Li G. (2011). CXCR4 and matrix metalloproteinase-2 are involved in mesenchymal stromal cell homing and engraftment to tumors. Cytotherapy13 (5), 549–561. 10.3109/14653249.2010.542457
110
Spees J. L. Lee R. H. Gregory C. A. (2016). Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res. Ther.7 (1), 125. 10.1186/s13287-016-0363-7
111
Su J. Chen X. Huang Y. Li W. Li J. Cao K. et al (2014). Phylogenetic distinction of iNOS and IDO function in mesenchymal stem cell-mediated immunosuppression in mammalian species. Cell Death Differ.21 (3), 388–396. 10.1038/cdd.2013.149
112
Sun J. Shen H. Shao L. Teng X. Chen Y. Liu X. et al (2020). HIF-1α overexpression in mesenchymal stem cell-derived exosomes mediates cardioprotection in myocardial infarction by enhanced angiogenesis. Stem Cell Res. Ther.11 (1), 373. 10.1186/s13287-020-01881-7
113
Sun W. Jia M. Feng Y. Cheng X. (2023). Lactate is a bridge linking glycolysis and autophagy through lactylation. Autophagy19 (12), 3240–3241. 10.1080/15548627.2023.2246356
114
Tang Y. L. Tang Y. Zhang Y. C. Qian K. Shen L. Phillips M. I. (2005). Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J. Am. Coll. Cardiol.46 (7), 1339–1350. 10.1016/j.jacc.2005.05.079
115
Tao H. Han Z. Han Z. C. Li Z. (2016). Proangiogenic features of mesenchymal stem cells and their therapeutic applications. Stem Cells Int.2016, 1314709. 10.1155/2016/1314709
116
Thirumala S. Goebel W. S. Woods E. J. (2009). Clinical grade adult stem cell banking. Organogenesis5 (3), 143–154. 10.4161/org.5.3.9811
117
Tian Q. Zhou L. Q. (2022). Lactate activates germline and cleavage embryo genes in Mouse embryonic stem cells. Cells11 (3), 548. 10.3390/cells11030548
118
Turlo A. J. Hammond D. E. Ramsbottom K. A. Soul J. Gillen A. McDonald K. et al (2023). Mesenchymal stromal cell secretome is affected by tissue source and donor age. Stem Cells41 (11), 1047–1059. 10.1093/stmcls/sxad060
119
Vizoso F. J. Eiro N. Cid S. Schneider J. Perez-Fernandez R. (2017). Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int. J. Mol. Sci.18 (9), 1852. 10.3390/ijms18091852
120
Wang H. S. Hung S. C. Peng S. T. Huang C. C. Wei H. M. Guo Y. J. et al (2004). Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells22 (7), 1330–1337. 10.1634/stemcells.2004-0013
121
Wang S. Cheng H. Dai G. Wang X. Hua R. Liu X. et al (2013). Umbilical cord mesenchymal stem cell transplantation significantly improves neurological function in patients with sequelae of traumatic brain injury. Brain Res.1532, 76–84. 10.1016/j.brainres.2013.08.001
122
Wang D. Zhang H. Liang J. Wang H. Hua B. Feng X. et al (2018). A long-term Follow-Up study of allogeneic mesenchymal stem/stromal cell transplantation in patients with drug-resistant systemic lupus erythematosus. Stem Cell Rep.10 (3), 933–941. 10.1016/j.stemcr.2018.01.029
123
Wang H. Yang Y. Liu J. Qian L. (2021). Direct cell reprogramming: approaches, mechanisms and progress. Nat. Rev. Mol. Cell Biol.22 (6), 410–424. 10.1038/s41580-021-00335-z
124
Wang X. Cui L. Ji X. (2022a). Cognitive impairment caused by hypoxia: from clinical evidences to molecular mechanisms. Metab. Brain Dis.37 (1), 51–66. 10.1007/s11011-021-00796-3
125
Wang N. Wang W. Wang X. Mang G. Chen J. Yan X. et al (2022b). Histone lactylation boosts reparative gene activation post-myocardial infarction. Circ. Res.131 (11), 893–908. 10.1161/CIRCRESAHA.122.320488
126
Wang C. Y. Hsieh M. K. Hu Y. J. Bit A. Lai P. L. (2022c). Monocarboxylate transporter 1-mediated lactate accumulation promotes nucleus pulposus degeneration under hypoxia in a 3D multilayered nucleus pulposus degeneration model. Eur. Cell Mater43, 53–65. 10.22203/eCM.v043a06
127
Wang X. Fan W. Li N. Ma Y. Yao M. Wang G. et al (2023a). YY1 lactylation in microglia promotes angiogenesis through transcription activation-mediated upregulation of FGF2. Genome Biol.24 (1), 87. 10.1186/s13059-023-02931-y
128
Wang Z. H. Zhang P. Peng W. B. Ye L. L. Xiang X. Wei X. S. et al (2023b). Altered phenotypic and metabolic characteristics of FOXP3(+)CD3(+)CD56(+) natural killer T (NKT)-Like cells in human malignant pleural effusion. Oncoimmunology12 (1), 2160558. 10.1080/2162402X.2022.2160558
129
Wang J. Wang Z. Wang Q. Li X. Guo Y. (2024a). Ubiquitous protein lactylation in health and diseases. Cell Mol. Biol. Lett.29 (1), 23. 10.1186/s11658-024-00541-5
130
Wang S. Huang T. Wu Q. Yuan H. Wu X. Yuan F. et al (2024b). Lactate reprograms glioblastoma immunity through CBX3-regulated histone lactylation. J. Clin. Invest134 (22), e176851. 10.1172/JCI176851
131
Watt S. M. Gullo F. van der Garde M. Markeson D. Camicia R. Khoo C. P. et al (2013). The angiogenic properties of mesenchymal stem/stromal cells and their therapeutic potential. Br. Med. Bull.108 (1), 25–53. 10.1093/bmb/ldt031
132
Wu J. Hu M. Jiang H. Ma J. Xie C. Zhang Z. et al (2023). Endothelial cell-derived lactate triggers bone mesenchymal stem cell histone lactylation to attenuate osteoporosis. Adv. Sci. (Weinh)10 (31), e2301300. 10.1002/advs.202301300
133
Wu Y. Wang X. Zhang Y. Wen Z. Li Y. Zhang K. et al (2024). Proanthocyanidins ameliorate LPS-Inhibited osteogenesis of PDLSCs by restoring lysine lactylation. Int. J. Mol. Sci.25 (5), 2947. 10.3390/ijms25052947
134
Xia X. Chiu P. W. Y. Lam P. K. Chin W. C. Ng E. K. W. Lau J. Y. W. (2018). Secretome from hypoxia-conditioned adipose-derived mesenchymal stem cells promotes the healing of gastric mucosal injury in a rodent model. Biochim. Biophys. Acta Mol. Basis Dis.1864 (1), 178–188. 10.1016/j.bbadis.2017.10.009
135
Xie X. J. Wang J. A. Cao J. Zhang X. (2006). Differentiation of bone marrow mesenchymal stem cells induced by myocardial medium under hypoxic conditions. Acta Pharmacol. Sin.27 (9), 1153–1158. 10.1111/j.1745-7254.2006.00436.x
136
Xie Q. Liu R. Jiang J. Peng J. Yang C. Zhang W. et al (2020). What is the impact of human umbilical cord mesenchymal stem cell transplantation on clinical treatment?Stem Cell Res. Ther.11 (1), 519. 10.1186/s13287-020-02011-z
137
Xie Y. Hu H. Liu M. Zhou T. Cheng X. Huang W. et al (2022). The role and mechanism of histone lactylation in health and diseases. Front. Genet.13, 949252. 10.3389/fgene.2022.949252
138
Xie Y. Li Y. Yao J. Song X. Wang H. Zhang J. et al (2023). Protein lactylation modification and proteomics features in cirrhosis patients after UC-MSC treatment. Curr. Issues Mol. Biol.45 (10), 8444–8460. 10.3390/cimb45100532
139
Xin Q. Wang H. Li Q. Liu S. Qu K. Liu C. et al (2022). Lactylation: a passing fad or the future of posttranslational modification. Inflammation45 (4), 1419–1429. 10.1007/s10753-022-01637-w
140
Xin Y. Zhao L. Peng R. (2023). HIF-1 signaling: an emerging mechanism for mitochondrial dynamics. J. Physiol. Biochem.79 (3), 489–500. 10.1007/s13105-023-00966-0
141
Xu W. Xu R. Li Z. Wang Y. Hu R. (2019). Hypoxia changes chemotaxis behaviour of mesenchymal stem cells via HIF-1α signalling. J. Cell Mol. Med.23 (3), 1899–1907. 10.1111/jcmm.14091
142
Yan F. Teng Y. Li X. Zhong Y. Li C. Yan F. et al (2024). Hypoxia promotes non-small cell lung cancer cell stemness, migration, and invasion via promoting glycolysis by lactylation of SOX9. Cancer Biol. Ther.25 (1), 2304161. 10.1080/15384047.2024.2304161
143
Yan K. Ma F. Song X. Wang H. Liu P. Zhang J. et al (2025). Unveiling distinctions between mesenchymal stromal cells and stem cells by single-cell transcriptomic analysis. Heliyon11 (4), e42311. 10.1016/j.heliyon.2025.e42311
144
Yang W. Wang P. Cao P. Wang S. Yang Y. Su H. et al (2021). Hypoxic in vitro culture reduces histone lactylation and impairs pre-implantation embryonic development in mice. Epigenetics Chromatin14 (1), 57. 10.1186/s13072-021-00431-6
145
Yang Y. Lee E. H. Yang Z. (2022a). Hypoxia-Conditioned mesenchymal stem cells in tissue regeneration application. Tissue Eng. Part B Rev.28 (5), 966–977. 10.1089/ten.TEB.2021.0145
146
Yang K. Fan M. Wang X. Xu J. Wang Y. Tu F. et al (2022b). Lactate promotes macrophage HMGB1 lactylation, acetylation, and exosomal release in polymicrobial sepsis. Cell Death Differ.29 (1), 133–146. 10.1038/s41418-021-00841-9
147
Yao W. Hu X. Wang X. (2024). Crossing epigenetic frontiers: the intersection of novel histone modifications and diseases. Signal Transduct. Target Ther.9 (1), 232. 10.1038/s41392-024-01918-w
148
Yu J. Chai P. Xie M. Ge S. Ruan J. Fan X. et al (2021). Histone lactylation drives oncogenesis by facilitating m(6)A reader protein YTHDF2 expression in ocular melanoma. Genome Biol.22 (1), 85. 10.1186/s13059-021-02308-z
149
Yu H. Zhu T. Ma D. Cheng X. Wang S. Yao Y. (2024). The role of nonhistone lactylation in disease. Heliyon10 (18), e36296. 10.1016/j.heliyon.2024.e36296
150
Yuan F. Liu J. Zhong L. Liu P. Li T. Yang K. et al (2025). Enhanced therapeutic effects of hypoxia-preconditioned mesenchymal stromal cell-derived extracellular vesicles in renal ischemic injury. Stem Cell Res. Ther.16 (1), 39. 10.1186/s13287-025-04166-z
151
Zacharek A. Chen J. Cui X. Li A. Li Y. Roberts C. et al (2007). Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J. Cereb. Blood Flow. Metab.27 (10), 1684–1691. 10.1038/sj.jcbfm.9600475
152
Zang Y. Wang A. Zhang J. Xia M. Jiang Z. Jia B. et al (2024). Hypoxia promotes histone H3K9 lactylation to enhance LAMC2 transcription in esophageal squamous cell carcinoma. iScience27 (7), 110188. 10.1016/j.isci.2024.110188
153
Zeng M. Liu H. Lu W. Chen C. Lin Z. Zhao R. (2025). Lactate-functionalized 3D-printed PCL/nHA scaffold drives BMSC osteogenesis via metabolic-epigenetic crosstalk. Mater Today Bio33, 102101. 10.1016/j.mtbio.2025.102101
154
Zha J. Zhang J. Lu J. Zhang G. Hua M. Guo W. et al (2024). A review of lactate-lactylation in malignancy: its potential in immunotherapy. Front. Immunol.15, 1384948. 10.3389/fimmu.2024.1384948
155
Zhang Q. Cao X. (2021). Epigenetic remodeling in innate immunity and inflammation. Annu. Rev. Immunol.39, 279–311. 10.1146/annurev-immunol-093019-123619
156
Zhang Y. Lv J. Guo H. Wei X. Li W. Xu Z. (2015). Hypoxia-induced proliferation in mesenchymal stem cells and angiotensin II-mediated PI3K/AKT pathway. Cell Biochem. Funct.33 (2), 51–58. 10.1002/cbf.3080
157
Zhang D. Tang Z. Huang H. Zhou G. Cui C. Weng Y. et al (2019). Metabolic regulation of gene expression by histone lactylation. Nature574 (7779), 575–580. 10.1038/s41586-019-1678-1
158
Zhidu S. Ying T. Rui J. Chao Z. (2024). Translational potential of mesenchymal stem cells in regenerative therapies for human diseases: challenges and opportunities. Stem Cell Res. Ther.15 (1), 266. 10.1186/s13287-024-03885-z
159
Zhu W. Chen Q. Li Y. Wan J. Li J. Tang S. (2023). HIF-1α-Overexpressing mesenchymal stem cells attenuate colitis by regulating M1-like macrophages polarization toward M2-like macrophages. Biomedicines11 (3), 825. 10.3390/biomedicines11030825
160
Zong Z. Xie F. Wang S. Wu X. Zhang Z. Yang B. et al (2024). Alanyl-tRNA synthetase, AARS1, is a lactate sensor and lactyltransferase that lactylates p53 and contributes to tumorigenesis. Cell187 (10), 2375–92.e33. 10.1016/j.cell.2024.04.002
161
Zuk P. A. Zhu M. Mizuno H. Huang J. Futrell J. W. Katz A. J. et al (2001). Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng.7 (2), 211–228. 10.1089/107632701300062859
Summary
Keywords
mesenchymal stromal cells, hypoxic microenvironment, lactylation, regenerative medicine, metabolic reprogramming
Citation
Zhao F, Tang Q and Liu J (2025) Functional impacts of lactylation in Hypoxia‒primed mesenchymal stromal cells. Front. Cell Dev. Biol. 13:1678282. doi: 10.3389/fcell.2025.1678282
Received
02 August 2025
Accepted
15 October 2025
Published
24 October 2025
Volume
13 - 2025
Edited by
Valentina Audrito, Università del Piemonte Orientale, Italy
Reviewed by
Anam Ahsan, University of South Australia, Australia
Cyrille Girard, Curexsys Gmbh, Germany
Updates
Copyright
© 2025 Zhao, Tang and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Liu, 9y140499@kust.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.