- 1Department of Plastic and Burn Surgery, West China Hospital, West China School of Medicine, Sichuan University, Chengdu, China
- 2Program of Traditional Chinese Medicine Health Preservation, Second Clinical Medical College, Henan University of Chinese Medicine, Zhengzhou, China
- 3Technology and Media University of Henan Kaifeng, Kaifeng, China
Skin injuries, including acute wounds, burns, and chronic ulcers, pose significant clinical challenges due to their potential to cause delayed healing and functional impairment. Exosome-like nanovesicles (ELNVs) derived from traditional Chinese medicinal (TCM) herbs have recently emerged as promising natural agents for skin repair and regeneration. These nanoscale vesicles combine the structural advantages of plant-derived delivery systems with the inherent pharmacological activities of TCM phytochemicals, offering dual roles as both bioactive agents and therapeutic carriers. Accumulating evidence indicates that TCM-derived ELNVs modulate key processes in wound healing, including inflammation resolution, fibroblast and keratinocyte activation, angiogenesis, and oxidative stress reduction. Moreover, certain vesicles have demonstrated potential in promoting hair follicle regeneration and protecting against photoaging, further highlighting their relevance in functional skin restoration. Compared with vesicles from common edible plants, TCM-ELNVs benefit from standardized cultivation, well-established traceable sourcing systems, and consistent phytochemical profiles, enhancing their suitability for therapeutic development. This review summarizes recent progress in the characterization, biological functions, and preclinical applications of TCM-derived ELNVs in cutaneous healing. Special attention is given to their mechanisms of action and their potential to serve as platforms for drug delivery and regenerative therapies. Overall, TCM-ELNVs represent a promising class of bioactive nanovesicles with broad translational potential in wound repair and skin regenerative medicine.
1 Introduction
Injuries to the skin present major clinical challenges by leading to impaired healing, infection, or scarring (Peña and Martin, 2024; Jeschke et al., 2023). The process of cutaneous wound repair is orchestrated by a dynamic and tightly regulated cascade, involving oxidative stress, inflammatory responses, extracellular matrix (ECM) remodeling, fibroblast activation, and angiogenesis (Wang K. et al., 2023). Disruption of this sequence often results in pathological outcomes such as chronic non-healing wounds or excessive scar formation, both of which severely compromise patients’ quality of life. As illustrated in Figure 1A, oxidative stress and immune cell infiltration create a pro-inflammatory microenvironment that promotes fibroblast-to-myofibroblast transition and excessive ECM deposition, ultimately leading to tissue stiffness and fibrosis (Wang K. et al., 2023). Figure 1B highlights the feedback loops between pathological mechanical and immune microenvironments that perpetuate skin fibrosis and hinder regenerative outcomes (Wang K. et al., 2023).
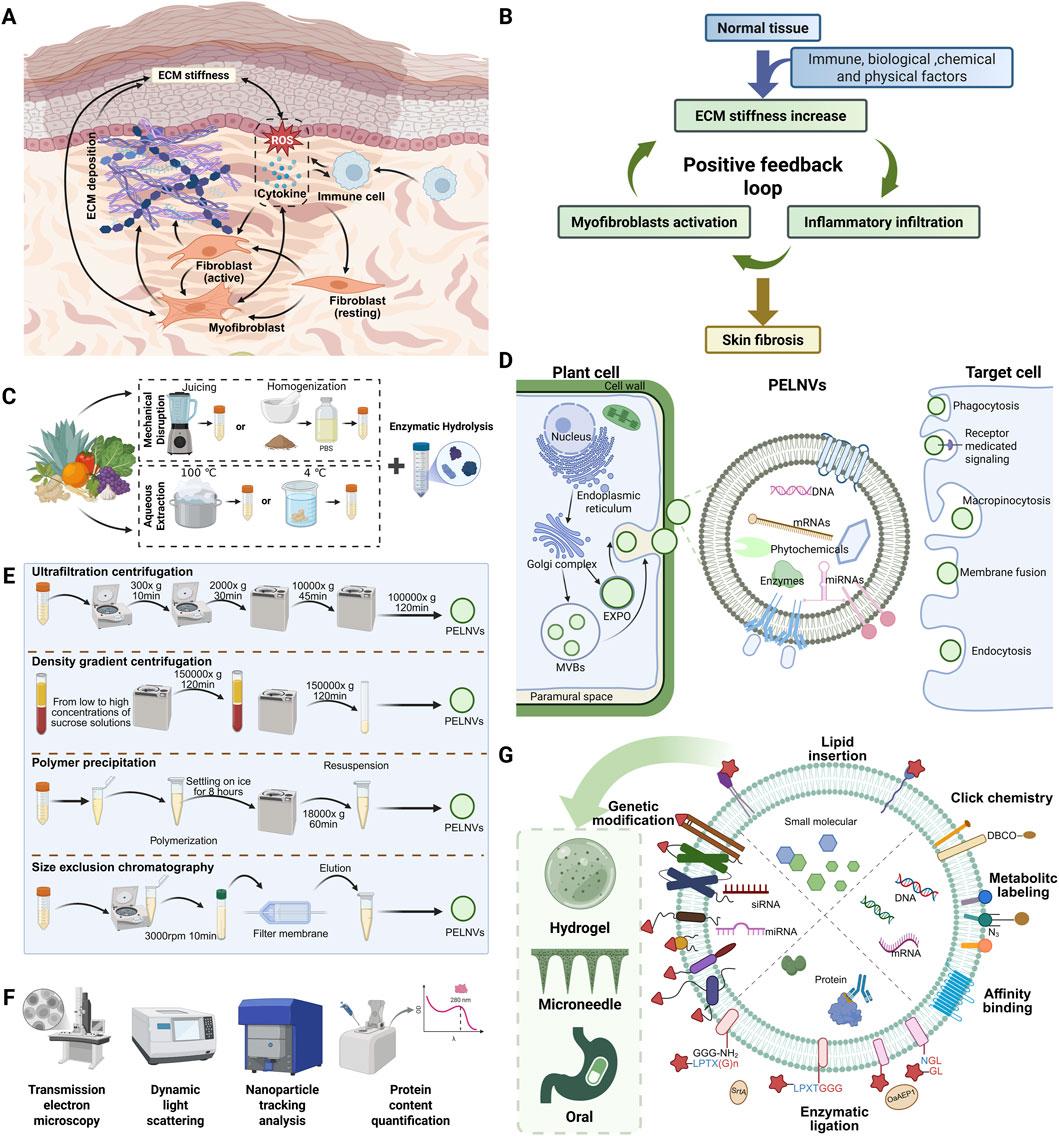
Figure 1. Mechanisms of skin scarring and the preparation, characterization, modification, and engineering of PELNVs. (A) Cellular interactions during the process of skin fibrosis. (B) Positive feedback between mechanical and inflammatory signals leading to ECM deposition and scar formation. (C) Preprocessing steps prior to PELNVs isolation. (D) Biogenesis, uptake, and composition of PELNVs. PELNVs are secreted by plant cells through two major pathways: the multivesicular body (MVB) pathway and the exocyst-positive organelle (EXPO) pathway. These nanovesicles carry diverse bioactive cargos, including proteins, nucleic acids (DNA, mRNA, miRNA), and plant-derived secondary metabolites. Once released, they can be internalized by recipient cells via multiple uptake mechanisms, thereby mediating a wide range of biological effects. (E) Commonly used isolation techniques for PELNVs, typically based on differences in physical properties such as size and density. (F) Representative approaches for the characterization of PELNVs. (G) Potential strategies for drug loading, membrane modification, and vesicle delivery using PELNVs (Liu Q. et al., 2023).
Over the past decade, exosomes (membrane-bound extracellular vesicles typically 30–200 nm in diameter) have gained considerable attention for their regenerative capabilities (Kalluri et al., 2020). Exosomes derived from mammalian cells have demonstrated promising effects in promoting wound healing through intercellular communication and the delivery of bioactive molecules such as proteins and nucleic acids (Pegtel and Gould, 2019; Yu et al., 2024). However, the clinical application of mammalian exosomes is constrained by several limitations, including immunogenicity, potential pathogen contamination, high production costs, and variability between batches (Zheng M. et al., 2024; Chu et al., 2024). As an alternative, plant-derived exosome-like nanovesicles (PELNVs) have emerged as a natural and biocompatible vesicle system with regenerative potential. PELNVs have been shown to possess anti-inflammatory, antioxidant, and wound-healing properties (Cao et al., 2023; Wang F. et al., 2025). Compared to mammalian exosomes, plant-derived exosomes offer superior oral bioavailability, scalable production, and eliminated zoonotic transmission risks (Chu et al., 2024).
Among the diverse sources of PELNVs, exosome-like vesicles derived from traditional Chinese medicinal herbs (TCM-ELNVs) represent a particularly promising subclass. Unlike many edible plants that primarily offer nutritional benefits, TCM herbs have well-documented pharmacological activities rooted in centuries of clinical practice and modern pharmacological studies (Yang et al., 2016). Furthermore, the production of TCM-ELNVs is often more consistent and reproducible due to the long-established sourcing and quality control systems inherent to traditional Chinese medicine (Zhang R. et al., 2021; Bilia et al., 2025). Unlike cell-based systems that are sensitive to culture conditions, TCM plant materials are often derived from well-characterized strains cultivated under defined agronomic practices. As a result, the composition and bioactivity of TCM-ELNVs can be more stable across batches, which is a critical factor for their potential clinical application. Despite their therapeutic promise, the use of TCM-ELNVs in cutaneous wound healing is still in its early stages. This mini review provides an overview of the current researches on the biogenesis, composition, and functional mechanisms of TCM-ELNVs. It also summarizes recent advances in their extraction, modification, and therapeutic delivery, while highlighting the challenges and opportunities for their application in skin repair and regenerative dermatology.
2 Composition of TCM-ELNVs
PELNVs have been successfully isolated from a broad range of plant sources, including fruits, vegetables, roots, and seeds (Figure 1C) (Sha et al., 2024). As illustrated in Figure 1D, PELNVs are believed to originate from the multivesicular bodies within plant cells (Cui et al., 2016; Wang Y. et al., 2023). During vesicle formation, various intracellular components such as small RNAs, secondary metabolites, enzymes, and lipids are selectively loaded into the nanovesicles (Yi et al., 2023; Ly et al., 2023).
Despite the diversity in botanical origin and molecular composition, these vesicles generally share key structural characteristics, such as a lipid bilayer membrane, spherical morphology, and the capacity to encapsulate and deliver bioactive cargos (Shinge et al., 2022). It is important to note, however, that research specifically focused on TCM-ELNVs remains limited. Therefore, considering that TCM-ELNVs are essentially a subset of PELNVs, the following section summarizes the general characteristics of PELNVs, which can be intended to serve as a foundational reference for understanding and advancing research on TCM-ELNVs.
2.1 Lipid and membrane composition
The membrane of PELNVs is rich in plant-specific lipids such as phosphatidic acid (PA), phosphatidylcholine (PC), digalactosyldiacylglycerol (DGDG), and monogalactosyldiacylglycerol (MGDG) (Subha et al., 2023). These lipids play essential roles in vesicle stability, membrane fusion, and cellular uptake. PA in particular has been shown to enhance gastrointestinal absorption and modulate immune interactions (Subha et al., 2023), while DGDG and MGDG contribute to the fluidity and structural resilience of the lipid bilayer (Che et al., 2025). The lipid profile also supports vesicle stability under acidic and enzymatic conditions, making oral or topical application feasible (Che et al., 2025).
2.2 Protein and enzymatic content
Although the proteomic profile of PELNVs is less thoroughly characterized compared to their mammalian counterparts, recent mass spectrometry analyses have revealed a diverse array of protein classes within PELNVs (Che et al., 2025). These proteins can generally be categorized into transmembrane proteins and other membrane-associated proteins. The specific composition varies significantly depending on the plant species, but commonly identified proteins include aquaporins, heat shock proteins, metabolic enzymes, signaling molecules, and adhesion-related factors. These proteins are believed to play critical roles in mediating the biological and pharmacological activities of PELNVs (Dad et al., 2021).
2.3 Nucleic acids and small RNAs
Small RNAs, particularly microRNAs, are important functional cargos within ELNVs. These RNAs can modulate gene expression in mammalian cells, demonstrating cross-kingdom regulatory effects. Studies have identified plant-derived miRNAs involved in suppressing inflammatory pathways, modulating macrophage polarization, and regulating collagen metabolism (Wei et al., 2023; Yang et al., 2025; Li et al., 2025). The encapsulation of microRNAs within vesicles protects them from degradation, allowing functional delivery to recipient skin cells or immune cells in the wound microenvironment.
2.4 Phytochemical enrichment
A key distinguishing feature of TCM-ELNVs is their high content of endogenous phytochemicals. Exosome-like nanovesicles derived from Camellia sinensis flowers are rich in polyphenols and flavonoids, including epigallocatechin gallate (EGCG), epicatechin gallate (ECG), and epicatechin (EC) (Chen et al., 2022). Vesicles isolated from Zingiber officinale contain abundant 6-gingerol and 6-shogaol (Zhang et al., 2016), while Panax ginseng-derived vesicles have been shown to encapsulate bioactive ginsenosides (Cao et al., 2019). These compounds are known for their well-established pharmacological activities, such as anti-inflammatory.
3 Isolation, modification, and delivery strategies of TCM-ELNVs
The successful therapeutic application of TCM-ELNVs depends not only on their intrinsic biological activities but also on the ability to efficiently isolate, engineer, and deliver these vesicles to target tissues. Due to their plant origin, the extraction and formulation of TCM-ELNVs present unique advantages and challenges compared to mammalian vesicle systems. This section summarizes current approaches to isolation and purification, strategies for vesicle engineering, and methods of delivery with relevance to cutaneous applications.
3.1 Isolation and purification techniques
The most widely used methods for isolating PELNVs (including TCM-ELNVs) are adapted from protocols used for mammalian extracellular vesicles. These primarily include differential centrifugation, ultracentrifugation, and density gradient centrifugation. In brief, plant materials such as root juice, decoction extract, or apoplastic fluids are first clarified by low-speed centrifugation, followed by high-speed spins to concentrate vesicles as illustrated in Figures 1C,E (Huang et al., 2021; Liu et al., 2024). Final purification is often achieved using sucrose or iodixanol density gradients to remove residual debris and co-precipitated proteins (Huang et al., 2021). When isolating ELNVs from TCM herbs, several practical considerations can influence yield and purity. The presence of abundant polysaccharides, secondary metabolites, and essential oils in certain herbal materials may interfere with sedimentation efficiency or cause vesicle aggregation. To address this, enzymatic pretreatments (e.g., pectinase or cellulase) have been introduced as complementary or alternative strategies (Figure 1C) (Zhao et al., 2023). Additional processing methods can be further referenced in the review by Jung et al. (2025).
3.2 Characterization of vesicle properties
Once isolated, TCM-ELNVs are typically characterized by transmission electron microscopy (TEM), dynamic light scattering (DLS), and nanoparticle tracking analysis (NTA) to assess their morphology, size distribution, and concentration (Figure 1F) (Matloob et al., 2024). Additional characterization involves zeta potential measurement, protein content quantification, and RNA analysis (Matloob et al., 2024; Gao et al., 2025). Surface markers are more variable and less defined compared to mammalian exosomes; thus, functional characterization (e.g., cellular uptake, bioactivity assays) often substitutes for strict molecular marker identification (Rashidi et al., 2025; Huang Z. et al., 2024).
3.3 Surface modification and vesicle engineering
To enhance the therapeutic performance of TCM-ELNVs, various strategies have been developed to modify their surface properties or to encapsulate functional cargos. One common approach involves chemical conjugation of specific ligands onto the vesicle membrane to improve cellular uptake or tissue specificity (Figure 1G). For instance, vesicles may be functionalized with folic acid, peptides, or antibodies that recognize markers on keratinocytes or activated fibroblasts (Matloob et al., 2024; Liu and Su, 2019). Another strategy focuses on cargo loading, whereby therapeutic agents such as small-molecule drugs, phytochemicals, or RNA molecules are introduced into the vesicles. This can be achieved through passive incubation, membrane permeabilization techniques such as sonication or electroporation, or lipid fusion (Liu and Su, 2019). Additionally, lipid components extracted from PELNVs can be reassembled into synthetic nanovesicles using classical liposome preparation methods such as thin-film hydration and membrane extrusion (Loureiro et al., 2017; Zhuang et al., 2016; Wang et al., 2013).
3.4 Delivery strategies for skin repair
Effective delivery of PELNVs to injured skin tissue is critical for their therapeutic efficacy (Figure 1G). Among the available approaches, direct topical application is the most practical and noninvasive route for treating surface wounds. PELNVs can be incorporated into hydrogel matrices, thermosensitive creams, or polymer-based wound dressings to improve their adhesion, stability, and bioavailability on the skin surface (Wang Z. et al., 2024; Zheng Y. et al., 2024; Weng et al., 2025). Enhancements in skin penetration may be achieved through adjunctive technologies such as microneedle-assisted delivery (Li et al., 2024; Zheng et al., 2025). Oral administration has also been investigated in the broader context of plant-derived vesicles (Gong et al., 2024). The structural resilience of PELNVs enables them to survive gastrointestinal transit and deliver bioactive compounds to systemic circulation or distant tissues (Di et al., 2023). Oral delivery may be advantageous in conditions where skin pathology is linked to systemic inflammation or immune dysregulation (Jin et al., 2024).
4 Biological functions and preclinical applications of TCM-derived ELNVs in skin repair
The process of skin repair is a complex and highly coordinated biological sequence involving hemostasis, inflammation, cellular proliferation, angiogenesis, ECM remodeling, and re-epithelialization (Takeo et al., 2015; Werner and Grose, 2003). TCM-ELNVs have shown diverse therapeutic effects throughout these stages, including immunomodulation, promotion of angiogenesis, oxidative stress reduction, and stimulation of skin cell proliferation (Wu et al., 2024). Table 1 summarizes the functions and preclinical applications of TCM-ELNVs in wound repair and skin regeneration.
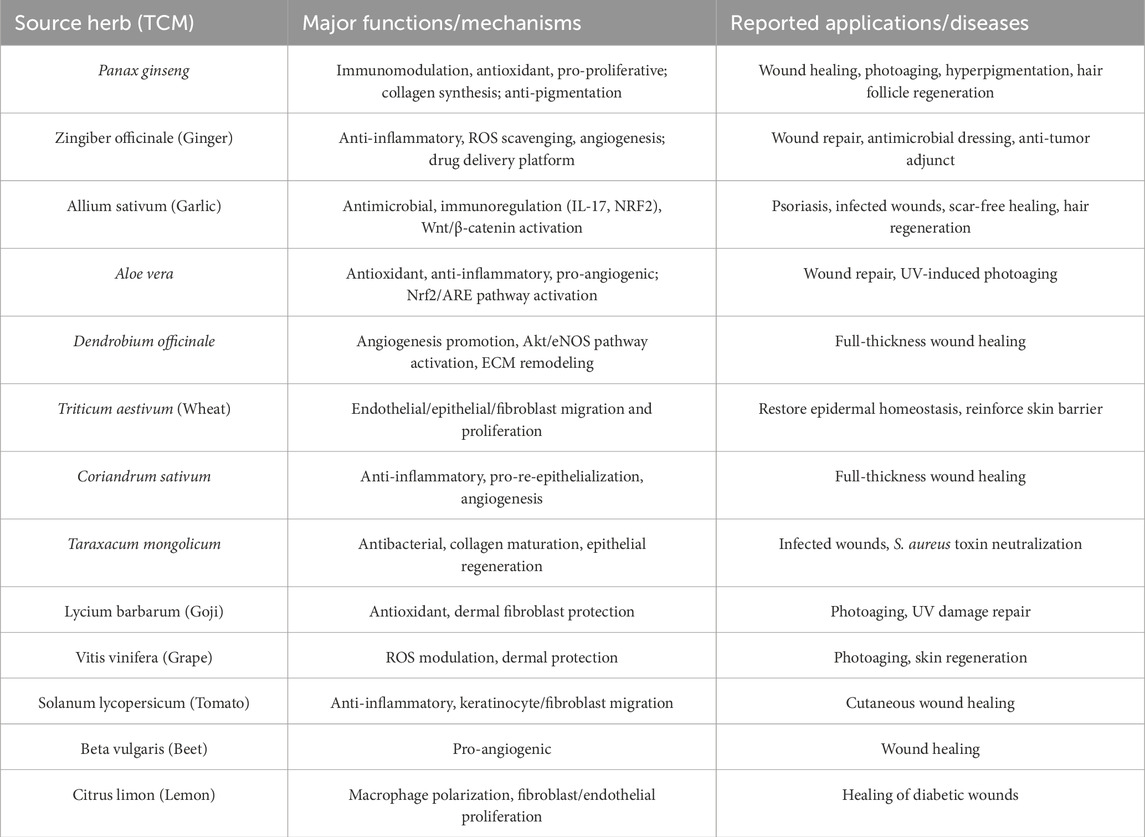
Table 1. Selected TCM-ELNVs and their demonstrated functions and applications in Wound Repair and Skin Regeneration. This table summarizes the primary biological functional mechanisms and reported therapeutic applications of some exosome-like nanovesicles derived from Traditional Chinese Medicine. These nanovesicles predominantly promote wound repair and regeneration, with some also exhibiting protection against photoaging.
4.1 Panax ginseng
Panax ginseng, a cornerstone herb in traditional Chinese medicine, has been used for centuries to restore vitality, strengthen the immune system, and promote tissue recovery (Qi et al., 2011; Zhou et al., 2023). In recent years, its pharmacological effects on skin have gained increasing attention (Zhou et al., 2023; Kim et al., 2024). Experimental studies have demonstrated that ginseng enhances fibroblast proliferation, stimulates collagen synthesis, improves microcirculation, and protects skin cells from oxidative stress and ultraviolet radiation (You and Cho, 2021; Cong et al., 2023). These properties make it a promising candidate for skin regeneration therapies.
Building on this foundation, researchers have recently focused on ELNVs derived from P. ginseng as natural, nanoscale therapeutic systems. Ginseng-derived ELNVs have been widely studied in various disease models and are known for their strong immunomodulatory properties (Gu et al., 2024; Kim et al., 2023; Yang et al., 2024; Ma et al., 2024). In the treatment of post-inflammatory hyperpigmentation (PIH), microneedling combined with topical application of ginseng-derived exosomes significantly reduced local inflammatory cytokines and improved pigmentation in patients with acne-related PIH. In a clinical study, three sessions of microneedling followed by topical ginseng exosome application led to visibly reduced pigmentation and a more even skin tone, with no adverse effects reported (Wan et al., 2025). Additionally, ginseng-derived ELNVs have demonstrated protective effects against skin photoaging (Cho et al., 2021). These vesicles exert three primary functions (immunomodulation, anti-aging, and anti-pigmentation) by simultaneously enhancing skin immunity and improving aging phenotypes, whether intrinsic or extrinsic.
In the context of wound healing, ginseng-derived ELNVs promote cell migration and proliferation, reduce oxidative stress, suppress pro-inflammatory mediators, and stimulate collagen synthesis. Several in vivo and in vitro studies support their efficacy in accelerating skin regeneration and enhancing tissue repair (Tan M. et al., 2024; Yang et al., 2023; Choi et al., 2024; Park et al., 2025). Beyond cutaneous healing, ginseng ELNVs also support neural stem cell differentiation and peripheral nerve regeneration by modulating growth factor signaling and oxidative defense pathways (Xu et al., 2021). Moreover, ginseng-derived vesicles contribute to hair follicle regeneration, which is essential for functional skin repair. In a recent pilot intervention, daily scalp application of ginseng ELNVs over 16 weeks significantly increased the number of newly formed hairs (Aberdam et al., 2022). Taken together, ginseng-derived ELNVs not only promote wound healing but also contribute to hair follicle regeneration. These dual actions highlight their potential in functional skin regeneration. With further development, ginseng ELNVs may serve as a versatile platform for drug delivery and the restoration of skin integrity and function.
4.2 Zingiber officinale (ginger)
Zingiber officinale, commonly known as ginger, is a widely used medicinal herb in both traditional Chinese medicine and modern phytotherapy (Crichton et al., 2022). Its bioactive constituents have been reported to suppress pro-inflammatory cytokines, scavenge ROS, and promote skin wound repair (Zhang M. et al., 2021; Shaukat et al., 2023; Al-Samydai et al., 2022; Chen et al., 2012; Mokhtare and Saglam, 2025). Building upon this pharmacological foundation, ELNVs derived from ginger have emerged as a novel natural platform for disease treatment and as natural nanocarriers for drug delivery, including chemotherapy agents and RNA-based therapeutics (Pan et al., 2025; Teng et al., 2025; Huang S. et al., 2024; Guo et al., 2025; Teng et al., 2018).
In skin-related applications, ginger ELNVs have shown promise in promoting wound healing and tissue regeneration. For instance, thermosensitive hydrogels loaded with ginger-derived vesicles significantly promoted dermal fibroblast proliferation and migration and attenuated UVA-induced inflammatory damage in keratinocytes (Wang J. et al., 2024). A composite nanomembrane integrating vesicles from Zingiber officinale, Aloe barbadensis, and Azadirachta indica, when applied to diabetic wound models, enhanced healing outcomes and demonstrated intrinsic antibacterial effects (Miya et al., 2025). In a separate study involving osteosarcoma patients undergoing surgical excision, ginger vesicles were incorporated into a wound dressing composed of carboxymethyl chitosan methacrylate (CMCSMA) and tannic acid (TA), co-delivering doxorubicin (DOX). This therapeutic complex finally eliminate residual tumor cells, reduce reactive oxygen species (ROS), and mitigate inflammation, thereby accelerating postoperative wound closure and reducing tumor recurrence (Zhang Q. et al., 2025).
4.3 Allium sativum (garlic)
Allium sativum, commonly known as garlic, has long been valued in traditional medicine for its broad-spectrum antimicrobial, anti-inflammatory, and immunomodulatory properties (Rose et al., 2018; El-Saadony et al., 2024). It contains bioactive compounds that have demonstrated efficacy in wound healing, infection control, and tissue repair (El-Saadony et al., 2024). More recently, ELNVs derived from garlic have been investigated as a natural delivery platform capable of enhancing and concentrating these therapeutic effects. Like ginger, garlic-derived vesicles exhibit strong immunoregulatory and anti-inflammatory effects (Liu J. et al., 2023; Huang X. Z. et al., 2024; Wang X. et al., 2024). In dermatological research, garlic ELNVs have shown efficacy in treating psoriasis via dual modulation of IL-17 and NRF2 pathways, leading to reduced epidermal hyperplasia and cytokine overproduction (Kalarikkal et al., 2025).
In wound care, garlic-derived ELNVs have demonstrated intrinsic antibacterial activity against common skin pathogens. When incorporated into a hydrogel-based delivery system along with vancomycin, these vesicles effectively eradicated Staphylococcus aureus from infected wounds, offering a promising approach for managing antibiotic-resistant skin infections (Zhou et al., 2024). Notably, garlic vesicles have also been linked to hair follicle regeneration through activation of the Wnt/β-catenin signaling pathway and the upregulation of growth factors. These combined properties, which include antimicrobial, anti-inflammatory, and regenerative potential, suggest that garlic-derived ELNVs hold significant promise for scar-free wound healing and hair follicle restoration, warranting further investigation (Inan et al., 2023).
4.4 Aloe vera
Aloe vera is a well-known medicinal plant with a long history of therapeutic use, particularly in dermatology. Numerous studies have demonstrated that Aloe vera plays an important role in accelerating wound healing by promoting epithelial regeneration, modulating inflammation, and reducing oxidative stress (Liang et al., 2021). Its extracts have been formulated into various topical preparations, including gels, hydrogels, and microneedle patches, and have shown significant clinical benefits in treating burns, abrasions, and other skin injuries (Luan et al., 2024; Tehrani et al., 2022; Zhang et al., 2022; Nascimento Júnior et al., 2025).
Recent studies have identified ELNVs derived from Aloe vera as active components contributing to its therapeutic efficacy. These vesicles have been shown to promote the migration of human keratinocytes and dermal fibroblasts, while dose-dependently reducing intracellular reactive oxygen species (ROS) levels in keratinocytes (Kim et al., 2021). In addition, Aloe ELNVs suppress the expression of pro-inflammatory cytokines, and promote angiogenesis, suggesting their role in both early and late phases of wound healing (Kim and Park, 2022). Furthermore, in UV-induced skin aging models, topical application of Aloe ELNVs significantly reduced levels of malondialdehyde (MDA) and modulated antioxidant enzymes, including superoxide dismutase (SOD), in mouse skin tissues. These effects were mediated via activation of the Nrf2/ARE signaling pathway, highlighting their potential to protect against photoaging and oxidative damage (Sun et al., 2025). Collectively, Aloe ELNVs demonstrate multifunctional activity in skin repair, including antioxidant, anti-inflammatory, and pro-regenerative effects. Their integration into wound dressings or delivery systems may offer a promising strategy for enhancing cutaneous regeneration and delaying skin aging.
4.5 Other TCM-ELNVs
In addition to well-characterized herbs such as ginseng, ginger, garlic, and aloe, a number of other TCM plants have been identified as promising sources of ELNVs with potential applications in cutaneous wound healing and skin regeneration. Among them, Dendrobium officinale, a prized herb in Chinese pharmacopeia, is rich in polysaccharides and flavonoids that contribute to its anti-inflammatory and antioxidant effects (Zhang et al., 2023). Recent studies have shown that Dendrobium-derived nanovesicles significantly accelerate wound healing in full-thickness skin models by promoting angiogenesis, reducing IL-1β expression, activating the Akt/eNOS pathway, and enhancing ECM remodeling (Tu et al., 2024; Tu et al., 2025). Similarly, wheat-derived nanovesicles have been shown to promote the proliferation and migration of endothelial cells, epithelial cells, and dermal fibroblasts, indicating their potential in restoring epidermal homeostasis and reinforcing skin barrier function (Şahin et al., 2019).
Other edible or medicinal plants used in TCM have also demonstrated skin-healing potential. For example, ELNVs from Coriandrum sativum (coriander) enhanced re-epithelialization and neovascularization in whole-layer skin wound model of mice, with concurrent downregulation of inflammatory cytokines (Wang T. et al., 2025). A gel formulation incorporating Taraxacum mongolicum (dandelion)-derived nanovesicles was shown to neutralize Staphylococcus aureus exotoxins, accelerate epithelial regeneration, promote collagen maturation, and alleviate inflammation (Tan S. et al., 2024). Both grape-derived and Lycium barbarum (goji berry)-derived ELNVs have demonstrated potential in mitigating photoaging and promoting the repair of UV-induced skin damage (Zhang Y. et al., 2025; Wang et al., 2022; M et al., 2025). These effects are attributed particularly to their antioxidant and protective effects on dermal fibroblasts. In addition, tomato-derived ELNVs exhibit anti-inflammatory activity and accelerate wound healing by promoting the migration of keratinocytes and fibroblasts (Mammadova et al., 2023; Daniello et al., 2024). Beta vulgaris (beet) vesicles demonstrated pro-angiogenic effects (Mahdipour, 2022). Lemon-derived vesicles modulate macrophage polarization reprogramming and promote the proliferation and migration of endothelial cells and fibroblasts, thereby facilitating the healing of diabetic wounds (Jin et al., 2025).
5 Conclusion and perspective
ELNVs derived from TCM herbs hold great therapeutic potential for wound repair and skin regeneration. The nano vesicles constructed by combining the structural advantages of plant-based vesicles with the inherent biological activity of traditional Chinese medicine phytochemicals can not only serve as delivery carriers for wound treatment, but also as functional agents to promote skin regeneration. Current studies suggest that TCM-ELNVs can promote skin regeneration through anti-inflammatory, pro-proliferative, angiogenic, and antioxidant mechanisms. Compared with vesicles from common edible plants, TCM-ELNVs benefit from advantages such as standardized cultivation of source plants, well-established traceable sourcing systems, and reproducible pharmacological properties, all of which significantly enhance their translational potential. Looking ahead, several challenges and opportunities remain. First, deeper mechanistic studies are needed to link specific phytochemicals within ELNVs to their observed bioactivities, which will facilitate rational design of vesicle-based therapeutics. Second, scaling up production under good manufacturing practice (GMP) conditions and evaluating long-term safety in preclinical and clinical settings are essential for translational success. Finally, the incorporation of advanced biomaterials such as hydrogels enables the modification, loading, and targeted delivery of TCM-ELNVs, thereby enhancing their stability, bioavailability, and therapeutic efficacy in wound repair and skin regeneration. Collectively, ongoing pharmacological and technological advances are poised to transform TCM-ELNVs into promising candidates for targeted drug delivery and functional skin regeneration systems.
Author contributions
KW: Writing – original draft, Writing – review and editing. Z-TY: Writing – original draft, Writing – review and editing. FW: Writing – original draft, Writing – review and editing. Y-QM: Writing – original draft, Writing – review and editing. YQ: Writing – original draft, Writing – review and editing. Z-YZ: Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by grants from Project of Chengdu Medical Research (No. 2023009), Health Commission of Sichuan Province Medical Science and Technology Program (No. 24QNMP029), Key research and development project of Sichuan (No. 2024YFFK0054).
Acknowledgments
Figures were created with the authorization of Biorender.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aberdam, E., Le Riche, A., Bordes, S., Closs, B., Park, B. S., and Aberdam, D. (2022). “Extracellular vesicles including exosomes for hair follicle regeneration,” in Hair follicle regeneration. Editors F. Jimenez, and C. Higgins (Cham: Springer International Publishing), 205–218. Available online at:. doi:10.1007/978-3-030-98331-4_9
Al-Samydai, A., Qaraleh, M. A., Alshaer, W., Al-Halaseh, L. K., Issa, R., Alshaikh, F., et al. (2022). Preparation, characterization, wound healing, and cytotoxicity assay of PEGylated nanophytosomes loaded with 6-gingerol. Nutrients 14 (23), 5170. doi:10.3390/nu14235170
Bilia, A. R., Ballerini, R., Qu, L., and Wang, M. (2025). Traditional chinese herbal medicine in european union: state of art, challenges, and future perspectives focusing on Italian market. Chin. Herb. Med. 17 (1), 3–18. doi:10.1016/j.chmed.2024.11.008
Cao, M., Yan, H., Han, X., Weng, L., Wei, Q., Sun, X., et al. (2019). Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J. Immunother. Cancer 7, 326. doi:10.1186/s40425-019-0817-4
Cao, M., Diao, N., Cai, X., Chen, X., Xiao, Y., Guo, C., et al. (2023). Plant exosome nanovesicles (PENs): green delivery platforms. Mater Horiz. 10 (10), 3879–3894. doi:10.1039/d3mh01030a
Che, K., Wang, C., and Chen, H. (2025). Advancing functional foods: a systematic analysis of plant-derived exosome-like nanoparticles and their health-promoting properties. Front. Nutr. 12, 1544746. doi:10.3389/fnut.2025.1544746
Chen, C. Y., Cheng, K. C., Chang, A. Y., Lin, Y. T., Hseu, Y. C., and Wang, H. M. (2012). 10-shogaol, an antioxidant from zingiber officinale for skin cell proliferation and migration enhancer. Int. J. Mol. Sci. 13 (2), 1762–1777. doi:10.3390/ijms13021762
Chen, Q., Li, Q., Liang, Y., Zu, M., Chen, N., Canup, B. S. B., et al. (2022). Natural exosome-like nanovesicles from edible tea flowers suppress metastatic breast cancer via ROS generation and microbiota modulation. Acta Pharm. Sin. B 12 (2), 907–923. doi:10.1016/j.apsb.2021.08.016
Cho, E. G., Choi, S. Y., Kim, H., Choi, E. J., Lee, E. J., Park, P. J., et al. (2021). Panax ginseng-derived extracellular vesicles facilitate anti-senescence effects in human skin cells: an eco-friendly and sustainable way to use ginseng substances. Cells 10 (3), 486. doi:10.3390/cells10030486
Choi, W., Cho, J. H., Park, S. H., Kim, D. S., Lee, H. P., Kim, D., et al. (2024). Ginseng root-derived exosome-like nanoparticles protect skin from UV irradiation and oxidative stress by suppressing activator protein-1 signaling and limiting the generation of reactive oxygen species. J. Ginseng Res. 48 (2), 211–219. doi:10.1016/j.jgr.2024.01.001
Chu, K., Liu, J., Zhang, X., Wang, M., Yu, W., Chen, Y., et al. (2024). Herbal medicine-derived exosome-like nanovesicles: a rising star in cancer therapy. Int. J. Nanomed 19, 7585–7603. doi:10.2147/IJN.S477270
Cong, L., Ma, J., Zhang, Y., Zhou, Y., Cong, X., and Hao, M. (2023). Effect of anti-skin disorders of ginsenosides-a systematic review. J. Ginseng Res. 47 (5), 605–614. doi:10.1016/j.jgr.2023.04.005
Crichton, M., Davidson, A. R., Innerarity, C., Marx, W., Lohning, A., Isenring, E., et al. (2022). Orally consumed ginger and human health: an umbrella review. Am. J. Clin. Nutr. 115 (6), 1511–1527. doi:10.1093/ajcn/nqac035
Cui, Y., Shen, J., Gao, C., Zhuang, X., Wang, J., and Jiang, L. (2016). Biogenesis of plant prevacuolar multivesicular bodies. Mol. Plant 9 (6), 774–786. doi:10.1016/j.molp.2016.01.011
Dad, H. A., Gu, T. W., Zhu, A. Q., Huang, L. Q., and Peng, L. H. (2021). Plant exosome-like nanovesicles: emerging therapeutics and drug delivery nanoplatforms. Mol. Ther. J. Am. Soc. Gene Ther. 29 (1), 13–31. doi:10.1016/j.ymthe.2020.11.030
Daniello, V., De Leo, V., Lasalvia, M., Hossain, M. N., Carbone, A., Catucci, L., et al. (2024). Solanum lycopersicum (Tomato)-derived nanovesicles accelerate wound healing by eliciting the migration of keratinocytes and fibroblasts. Int. J. Mol. Sci. 25 (5), 2452. doi:10.3390/ijms25052452
Di, R. R., Mizzoni, D., Spada, M., Dolo, V., Fais, S., and Logozzi, M. (2023). Oral treatment with plant-derived exosomes restores redox balance in H2O2-treated mice. Antioxid. basel Switz. 12 (6), 1169. doi:10.3390/antiox12061169
El-Saadony, M. T., Saad, A. M., Korma, S. A., Salem, H. M., Abd El-Mageed, T. A., Alkafaas, S. S., et al. (2024). Garlic bioactive substances and their therapeutic applications for improving human health: a comprehensive review. Front. Immunol. 15, 1277074. doi:10.3389/fimmu.2024.1277074
Gao, C., Chen, Y., Wen, X., Han, R., Qin, Y., Li, S., et al. (2025). Plant-derived exosome-like nanoparticles in tissue repair and regeneration. J. Mater Chem. B 13 (7), 2254–2271. doi:10.1039/d4tb02394c
Gong, Q., Sun, Y., Liu, L., Pu, C., and Guo, Y. (2024). Oral administration of tea-derived exosome-like nanoparticles protects epithelial and immune barrier of intestine from psychological stress. Heliyon 10 (17), e36812. doi:10.1016/j.heliyon.2024.e36812
Gu, W., Guo, W., Ren, Z., Zhang, Y., Han, M., Zhao, Q., et al. (2024). A bioactive nanocomposite integrated specific TAMs target and synergistic TAMs repolarization for effective cancer immunotherapy. Bioact. Mater 38, 472–485. doi:10.1016/j.bioactmat.2024.04.029
Guo, Z., Li, G., Shen, L., Pan, J., Dou, D., Gong, Y., et al. (2025). Ginger-derived exosome-like nanoparticles loaded with indocyanine green enhances phototherapy efficacy for breast cancer. Int. J. Nanomed 20, 1147–1169. doi:10.2147/IJN.S478435
Huang, Y., Wang, S., Cai, Q., and Jin, H. (2021). Effective methods for isolation and purification of extracellular vesicles from plants. J. Integr. Plant Biol. 63 (12), 2020–2030. doi:10.1111/jipb.13181
Huang, Z., Nielsen, S. D. H., Whitehead, B., Nejsum, P., Corredig, M., and Rasmussen, M. K. (2024). Importance of isolation method on characteristics and bioactivity of extracellular vesicles from tomatoes. J. Food Compos Anal. 129, 106064. doi:10.1016/j.jfca.2024.106064
Huang, S., Zhang, M., Li, X., Pei, J., Zhou, Z., Lei, P., et al. (2024). Formulation, characterization, and evaluation of curcumin-loaded ginger-derived nanovesicles for anti-colitis activity. J. Pharm. Anal. 14 (12), 101014. doi:10.1016/j.jpha.2024.101014
Huang X. Z., X. Z., Yii, C. Y., Yong, S. B., and Li, C. J. (2024). peu-MIR2916-p3-enriched garlic exosomes ameliorate murine colitis by reshaping gut microbiota, especially by boosting the anti-colitic bacteroides thetaiotaomicron - correspondence. Pharmacol. Res. 202, 107131. doi:10.1016/j.phrs.2024.107131
Inan, Y. E., Cicek, D., Demir, B., Sahin, K., Tuzcu, M., Orhan, C., et al. (2023). Garlic exosomes promote hair growth through the wnt/β-catenin pathway and growth factors. Cureus 15 (7), e42142. doi:10.7759/cureus.42142
Jeschke, M. G., Wood, F. M., Middelkoop, E., Bayat, A., Teot, L., Ogawa, R., et al. (2023). Scars. Nat. Rev. Dis. Prim. 9 (1), 64. doi:10.1038/s41572-023-00474-x
Jin, Z., Na, J., Lin, X., Jiao, R., Liu, X., and Huang, Y. (2024). Plant-derived exosome-like nanovesicles: a novel nanotool for disease therapy. Heliyon 10 (9), e30630. doi:10.1016/j.heliyon.2024.e30630
Jin, E., Yang, Y., Cong, S., Chen, D., Chen, R., Zhang, J., et al. (2025). Lemon-derived nanoparticle-functionalized hydrogels regulate macrophage reprogramming to promote diabetic wound healing. J. Nanobiotechnol 23 (1), 68. doi:10.1186/s12951-025-03138-y
Jung, D., Kim, N. E., Kim, S., Bae, J. H., Jung, I. Y., Doh, K. W., et al. (2025). Plant-derived nanovesicles and therapeutic application. Pharmacol. Ther. 269, 108832. doi:10.1016/j.pharmthera.2025.108832
Kalarikkal, S. P., Kumar, M. N., Rajendran, S., Bethi, C. M. S., Ravilla, J., Narayanan, J., et al. (2025). Natural plant-derived nanovesicles for effective psoriasis therapy via dual modulation of IL-17 and NRF2 pathway. Iscience 28 (6), 112556. doi:10.1016/j.isci.2025.112556
Kalluri, R., and LeBleu, V. S. (2020). The biology, function, and biomedical applications of exosomes. Sci. (n Y NY) 367 (6478), eaau6977. doi:10.1126/science.aau6977
Kim, M., and Park, J. H. (2022). Isolation of aloe saponaria-derived extracellular vesicles and investigation of their potential for chronic wound healing. Pharmaceutics 14 (9), 1905. doi:10.3390/pharmaceutics14091905
Kim, M. K., Choi, Y. C., Cho, S. H., Choi, J. S., and Cho, Y. W. (2021). The antioxidant effect of small extracellular vesicles derived from aloe vera peels for wound healing. Tissue Eng. Regen. Med. 18 (4), 561–571. doi:10.1007/s13770-021-00367-8
Kim, J., Zhu, Y., Chen, S., Wang, D., Zhang, S., Xia, J., et al. (2023). Anti-glioma effect of ginseng-derived exosomes-like nanoparticles by active blood-brain-barrier penetration and tumor microenvironment modulation. J. Nanobiotechnol 21 (1), 253. doi:10.1186/s12951-023-02006-x
Kim, J. H., Lee, R., Hwang, S. H., Choi, S. H., Kim, J. H., Cho, I. H., et al. (2024). Ginseng and ginseng byproducts for skincare and skin health. J. Ginseng Res. 48 (6), 525–534. doi:10.1016/j.jgr.2024.09.006
Li, Y., Wang, Y., Zhao, H., Pan, Q., and Chen, G. (2024). Engineering strategies of plant-derived exosome-like nanovesicles: current knowledge and future perspectives. Int. J. Nanomed 19, 12793–12815. doi:10.2147/IJN.S496664
Li, S., Liu, F., Zhang, S., Sun, X., Li, X., Yue, Q., et al. (2025). Lavender exosome-like nanoparticles attenuate UVB-induced photoaging via miR166-mediated inflammation and collagen regulation. Sci. Rep. 15 (1), 21286. doi:10.1038/s41598-025-08817-2
Liang, J., Cui, L., Li, J., Guan, S., Zhang, K., and Li, J. (2021). Aloe vera: a medicinal plant used in skin wound healing. B Rev. 27 (5), 455–474. doi:10.1089/ten.TEB.2020.0236
Liu, C., and Su, C. (2019). Design strategies and application progress of therapeutic exosomes. Theranostics 9 (4), 1015–1028. doi:10.7150/thno.30853
Liu, J., Li, W., Bian, Y., Jiang, X., Zhu, F., Yin, F., et al. (2023). Garlic-derived exosomes regulate PFKFB3 expression to relieve liver dysfunction in high-fat diet-fed mice via macrophage-hepatocyte crosstalk. Phytomed Int. J. Phytother. Phytopharm. 112, 154679. doi:10.1016/j.phymed.2023.154679
Liu, Q., Li, D., Pan, X., and Liang, Y. (2023). Targeted therapy using engineered extracellular vesicles: principles and strategies for membrane modification. J. Nanobiotechnol 21 (1), 334. doi:10.1186/s12951-023-02081-0
Liu, Y., Xiao, S., Wang, D., Qin, C., Wei, H., and Li, D. (2024). A review on separation and application of plant-derived exosome-like nanoparticles. J. Sep. Sci. 47 (8), 2300669. doi:10.1002/jssc.202300669
Loureiro, J. A., Andrade, S., Duarte, A., Neves, A. R., Queiroz, J. F., Nunes, C., et al. (2017). Resveratrol and grape extract-loaded solid lipid nanoparticles for the treatment of alzheimer’s disease. Molecules 22 (2), 277. doi:10.3390/molecules22020277
Luan, Q., Qiao, R., Wu, X., Shan, J., Song, C., Zhao, X., et al. (2024). Plant-derived chinese herbal hydrogel microneedle patches for wound healing. Small weinh Bergstr Ger. 20 (45), e2404850. doi:10.1002/smll.202404850
Ly, N. P., Han, H. S., Kim, M., Park, J. H., and Choi, K. Y. (2023). Plant-derived nanovesicles: current understanding and applications for cancer therapy. Bioact. Mater 22, 365–383. doi:10.1016/j.bioactmat.2022.10.005
M, W., J, C., W, C., Y, M., J, G., Q, W., et al. (2025). Grape-derived exosome-like nanovesicles effectively ameliorate skin photoaging by protecting epithelial cells. J. Food Sci. 90 (6), e70309. doi:10.1111/1750-3841.70309
Ma, C., Liu, K., Wang, F., Fei, X., Niu, C., Li, T., et al. (2024). Neutrophil membrane-engineered panax ginseng root-derived exosomes loaded miRNA 182-5p targets NOX4/drp-1/NLRP3 signal pathway to alleviate acute lung injury in sepsis: experimental studies. Int. J. Surg. lond Engl. 110 (1), 72–86. doi:10.1097/JS9.0000000000000789
Mahdipour, E. (2022). Beta vulgaris juice contains biologically active exosome-like nanoparticles. Tissue Cell 76, 101800. doi:10.1016/j.tice.2022.101800
Mammadova, R., Maggio, S., Fiume, I., Bokka, R., Moubarak, M., Gellén, G., et al. (2023). Protein biocargo and anti-inflammatory effect of tomato fruit-derived nanovesicles separated by density gradient ultracentrifugation and loaded with curcumin. Pharmaceutics 15 (2), 333. doi:10.3390/pharmaceutics15020333
Matloob, A., Gu, X., Rehman Sheikh, A., Javed, M., Fang, Z., and Luo, Z. (2024). Plant exosomes-like nano-vesicles: characterization, functional food potential, and emerging therapeutic applications as a nano medicine. Food Saf. Health 2 (4), 429–450. doi:10.1002/fsh3.12060
Miya, M. B., Ashutosh, M., Dey, D., Pathak, V., Khare, E., Kalani, K., et al. (2025). Accelerated diabetic wound healing using a chitosan-based nanomembrane incorporating nanovesicles from aloe barbadensis, azadirachta indica, and zingiber officinale. Int. J. Biol. Macromol. 310, 143169. doi:10.1016/j.ijbiomac.2025.143169
Mokhtare, B., and Saglam, Y. S. (2025). Investigation of the zingerone’s effects on wound healing in induced diabetic rats model. Arch. Dermatol Res. 317 (1), 484. doi:10.1007/s00403-025-03924-6
Nascimento Júnior, J. A. C., Oliveira, A. M. S., Porras, K. D. L., Menezes, P. D. P., Araujo, A. A. de S., Nunes, P. S., et al. (2025). Exploring trends in natural product-based treatments to skin burn: a comprehensive review. Phytomed Int. J. Phytother. Phytopharm. 139, 156481. doi:10.1016/j.phymed.2025.156481
Pan, C., Jiang, X., Wei, J., Liu, C., Zhang, M., Gao, C., et al. (2025). Ameba-inspired strategy enhances probiotic efficacy via prebound nutrient supply. Nat. Commun. 16 (1), 1827. doi:10.1038/s41467-025-57071-7
Park, H. Y., Kang, M. H., Lee, G., and Kim, J. W. (2025). Enhancement of skin regeneration through activation of TGF-β/SMAD signaling pathway by panax ginseng meyer non-edible callus-derived extracellular vesicles. J. Ginseng Res. 49 (1), 34–41. doi:10.1016/j.jgr.2024.08.002
Pegtel, D. M., and Gould, S. J. (2019). Exosomes. Annu. Rev. Biochem. 88, 487–514. doi:10.1146/annurev-biochem-013118-111902
Peña, O. A., and Martin, P. (2024). Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. 25 (8), 599–616. doi:10.1038/s41580-024-00715-1
Qi, L. W., Wang, C. Z., and Yuan, C. S. (2011). Isolation and analysis of ginseng: advances and challenges. Nat. Prod. Rep. 28 (3), 467–495. doi:10.1039/c0np00057d
Rashidi, N., Liu, C., Guillot, P. V., and Tamaddon, M. (2025). Isolation, characterization, and in vitro cell studies of plant-based exosome-like nanovesicles for treatment of early osteoarthritis. Int. J. Mol. Sci. 26 (5), 2211. doi:10.3390/ijms26052211
Rose, P., Moore, P. K., and Zhu, Y. Z. (2018). Garlic and gaseous mediators. Trends Pharmacol. Sci. 39 (7), 624–634. doi:10.1016/j.tips.2018.03.009
Şahin, F., Koçak, P., Güneş, M. Y., Özkan, İ., Yıldırım, E., and Kala, E. Y. (2019). In vitro wound healing activity of wheat-derived nanovesicles. Appl. Biochem. Biotechnol. 188 (2), 381–394. doi:10.1007/s12010-018-2913-1
Sha, A., Luo, Y., Xiao, W., He, J., Chen, X., Xiong, Z., et al. (2024). Plant-derived exosome-like nanoparticles: a comprehensive overview of their composition, biogenesis, isolation, and biological applications. Int. J. Mol. Sci. 25 (22), 12092. doi:10.3390/ijms252212092
Shaukat, M. N., Nazir, A., and Fallico, B. (2023). Ginger bioactives: a comprehensive review of health benefits and potential food applications. Antioxid. basel Switz. 12 (11), 2015. doi:10.3390/antiox12112015
Shinge, S. A. U., Xiao, Y., Xia, J., Liang, Y., and Duan, L. (2022). New insights of engineering plant exosome-like nanovesicles as a nanoplatform for therapeutics and drug delivery. Extracell. Vesicles Circ. Nucl. Acids 3 (2), 150–162. doi:10.20517/evcna.2021.25
Subha, D., Harshnii, K., Madhikiruba, K. G., Nandhini, M., and Tamilselvi, K. S. (2023). Plant derived exosome-like nanovesicles: an updated overview. Plant Nano Biol. 3, 100022. doi:10.1016/j.plana.2022.100022
Sun, Z., Zheng, Y., Wang, T., Zhang, J., Li, J., Wu, Z., et al. (2025). Aloe vera gel and rind-derived nanoparticles mitigate skin photoaging via activation of Nrf2/ARE pathway. Int. J. Nanomed 20, 4051–4067. doi:10.2147/IJN.S510352
Takeo, M., Lee, W., and Ito, M. (2015). Wound healing and skin regeneration. Cold Spring Harb. Perspect. Med. 5 (1), a023267. doi:10.1101/cshperspect.a023267
Tan, M., Liu, Y., Xu, Y., Yan, G., Zhou, N., Chen, H., et al. (2024). Plant-derived exosomes as novel nanotherapeutics contrive glycolysis reprogramming-mediated angiogenesis for diabetic ulcer healing. Biomater. Res. 28, 0035. doi:10.34133/bmr.0035
Tan, S., Liu, Z., Cong, M., Zhong, X., Mao, Y., Fan, M., et al. (2024). Dandelion-derived vesicles-laden hydrogel dressings capable of neutralizing staphylococcus aureus exotoxins for the care of invasive wounds. J. Control Release Off. J. Control Release Soc. 368, 355–371. doi:10.1016/j.jconrel.2024.02.045
Tehrani, F. K., Sheikhi, M., Rafiemanzelat, F., Esmaeili, F., Ghodsi, S., Koohmareh, G. A., et al. (2022). Protein and polysaccharide-based asymmetric mat with tuned bilayer configuration for enhanced wound healing efficiency. Carbohydr. Polym. 15 (292), 119666. doi:10.1016/j.carbpol.2022.119666
Teng, Y., Ren, Y., Sayed, M., Hu, X., Lei, C., Kumar, A., et al. (2018). Plant-derived exosomal MicroRNAs shape the gut microbiota. Cell Host Microbe 24 (5), 637–652. doi:10.1016/j.chom.2018.10.001
Teng, Y., Luo, C., Qiu, X., Mu, J., Sriwastva, M. K., Xu, Q., et al. (2025). Plant-nanoparticles enhance anti-PD-L1 efficacy by shaping human commensal microbiota metabolites. Nat. Commun. 16 (1), 1295. doi:10.1038/s41467-025-56498-2
Tu, J., Jiang, F., Fang, J., Xu, L., Zeng, Z., Zhang, X., et al. (2024). Anticipation and verification of dendrobium-derived nanovesicles for skin wound healing targets, predicated upon immune infiltration and senescence. Int. J. Nanomed 19, 1629–1644. doi:10.2147/IJN.S438398
Tu, J., Xu, L., Guo, Y., Zhang, M., Gan, M., Bao, X., et al. (2025). Dendrobium officinale-derived nanovesicles: a natural therapy for comprehensive regulation of angiogenesis, inflammation, and tissue repair to enhance skin wound healing. Bioresour. Bioprocess 12 (1), 74. doi:10.1186/s40643-025-00915-3
Wan, J., Kim, S. B., Yoon, S. E., Cartier, H., Garson, S., and Yi, K. H. (2025). Treatment of postinflammatory hyperpigmentation following acne with microneedling and panax ginseng–derived exosomes. Plast. Reconstr. Surg. Glob. Open 13 (7), e6975. doi:10.1097/GOX.0000000000006975
Wang, Q., Zhuang, X., Mu, J., Deng, Z. B., Jiang, H., Zhang, L., et al. (2013). Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat. Commun. 4, 1867. doi:10.1038/ncomms2886
Wang, L., Wan, G., Wang, G., Zhang, M., Li, N., Zhang, Q., et al. (2022). Anthocyanin from lycium ruthenicum murr. in the qaidam basin alleviates ultraviolet-induced apoptosis of human skin fibroblasts by regulating the death receptor pathway. Clin. Cosmet. Investig. Dermatol 15, 2925–2932. doi:10.2147/CCID.S388418
Wang, K., Wen, D., Xu, X., Zhao, R., Jiang, F., Yuan, S., et al. (2023). Extracellular matrix stiffness-the central cue for skin fibrosis. Front. Mol. Biosci. 10, 1132353. doi:10.3389/fmolb.2023.1132353
Wang, Y., Wei, Y., Liao, H., Fu, H., Yang, X., Xiang, Q., et al. (2023). Plant exosome-like nanoparticles as biological shuttles for transdermal drug delivery. Bioengineering 10 (1), 104. doi:10.3390/bioengineering10010104
Wang, Z., Yuan, J., Xu, Y., Shi, N., Lin, L., Wang, R., et al. (2024). Olea europaea leaf exosome-like nanovesicles encapsulated in a hyaluronic acid/tannic acid hydrogel dressing with dual “defense-repair” effects for treating skin photoaging. Mater Today 26, 101103. doi:10.1016/j.mtbio.2024.101103
Wang, J., Ran, B., Ma, W., Teng, Y., Bello, M. G., Chen, L., et al. (2024). Development of ginger-derived extracellular vesicles thermosensitive gel for UVA-induced photodamage of skin. J. Drug Deliv. Sci. Technol. 96, 105649. doi:10.1016/j.jddst.2024.105649
Wang, X., Liu, Y., Dong, X., Duan, T., Wang, C., Wang, L., et al. (2024). peu-MIR2916-p3-enriched garlic exosomes ameliorate murine colitis by reshaping gut microbiota, especially by boosting the anti-colitic bacteroides thetaiotaomicron. Pharmacol. Res. 200, 107071. doi:10.1016/j.phrs.2024.107071
Wang, F., Yao, J., Zuo, H., Jiao, Y., Wu, J., and Meng, Z. (2025). Diverse-origin exosomes therapeutic strategies for diabetic wound healing. Int. J. Nanomed 20, 7375–7402. doi:10.2147/IJN.S519379
Wang, T., Li, Y., Hao, L., Liu, Y., Liu, D., Zhang, C., et al. (2025). Coriander-derived exosome-like nanovesicles laden hydrogel with antioxidant property accelerates wound healing. Macromol. Biosci. 25 (7), 2400640. doi:10.1002/mabi.202400640
Wei, X., Li, X., Zhang, Y., Wang, J., and Shen, S. (2023). Advances in the therapeutic applications of plant-derived exosomes in the treatment of inflammatory diseases. Biomedicines 11 (6), 1554. doi:10.3390/biomedicines11061554
Weng, J., Chen, Y., Zeng, Y., Jin, W., Ji, Y., Zhang, W., et al. (2025). A novel hydrogel loaded with plant exosomes and stem cell exosomes as a new strategy for treating diabetic wounds. Mater Today Bio 32, 101810. doi:10.1016/j.mtbio.2025.101810
Werner, S., and Grose, R. (2003). Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 83 (3), 835–870. doi:10.1152/physrev.2003.83.3.835
Wu, W., Zhang, B., Wang, W., Bu, Q., Li, Y., Zhang, P., et al. (2024). Plant-derived exosome-like nanovesicles in chronic wound healing. Int. J. Nanomed 19, 11293–11303. doi:10.2147/IJN.S485441
Xu, X. H., Yuan, T. J., Dad, H. A., Shi, M. Y., Huang, Y. Y., Jiang, Z. H., et al. (2021). Plant exosomes as novel nanoplatforms for MicroRNA transfer stimulate neural differentiation of stem cells in vitro and in vivo. Nano Lett. 21 (19), 8151–8159. doi:10.1021/acs.nanolett.1c02530
Yang, L., Yang, C., Li, C., Zhao, Q., Liu, L., Fang, X., et al. (2016). Recent advances in biosynthesis of bioactive compounds in traditional chinese medicinal plants. Sci. Bull. 61, 3–17. doi:10.1007/s11434-015-0929-2
Yang, S., Lu, S., Ren, L., Bian, S., Zhao, D., Liu, M., et al. (2023). Ginseng-derived nanoparticles induce skin cell proliferation and promote wound healing. J. Ginseng Res. 47 (1), 133–143. doi:10.1016/j.jgr.2022.07.005
Yang, S., Li, W., Bai, X., Di Nunzio, G., Fan, L., Zhao, Y., et al. (2024). Ginseng-derived nanoparticles alleviate inflammatory bowel disease via the TLR4/MAPK and p62/Nrf2/Keap1 pathways. J. Nanobiotechnol 22 (1), 48. doi:10.1186/s12951-024-02313-x
Yang, S., Fan, L., Yin, L., Zhao, Y., Li, W., Zhao, R., et al. (2025). Ginseng exosomes modulate M1/M2 polarisation by activating autophagy and target IKK/IкB/NF-кB to alleviate inflammatory bowel disease. J. Nanobiotechnol 23 (1), 198. doi:10.1186/s12951-025-03292-3
Yi, Q., Xu, Z., Thakur, A., Zhang, K., Liang, Q., Liu, Y., et al. (2023). Current understanding of plant-derived exosome-like nanoparticles in regulating the inflammatory response and immune system microenvironment. Pharmacol. Res. 190, 106733. doi:10.1016/j.phrs.2023.106733
You, L., and Cho, J. Y. (2021). The regulatory role of korean ginseng in skin cells. J. Ginseng Res. 45 (3), 363–370. doi:10.1016/j.jgr.2020.08.004
Yu, H., Feng, H., Zeng, H., Wu, Y., Zhang, Q., Yu, J., et al. (2024). Exosomes: the emerging mechanisms and potential clinical applications in dermatology. Int. J. Biol. Sci. 20 (5), 1778–1795. doi:10.7150/ijbs.92897
Zhang, M., Viennois, E., Prasad, M., Zhang, Y., Wang, L., Zhang, Z., et al. (2016). Edible ginger-derived nanoparticles: a novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 101, 321–340. doi:10.1016/j.biomaterials.2016.06.018
Zhang, R., Zhang, M. X., Chen, Y., Wang, C. C., Zhang, C. H., Heuberger, H., et al. (2021). Future development of good agricultural practice in China under globalization of traditional herbal medicine trade. Chin. Herb. Med. 13 (4), 472–479. doi:10.1016/j.chmed.2021.09.010
Zhang, M., Zhao, R., Wang, D., Wang, L., Zhang, Q., Wei, S., et al. (2021). Ginger (zingiber officinale rosc.) and its bioactive components are potential resources for health beneficial agents. Phytother. Res. PTR 35 (2), 711–742. doi:10.1002/ptr.6858
Zhang, Q., Zhang, M., Wang, T., Chen, X., Li, Q., and Zhao, X. (2022). Preparation of aloe polysaccharide/honey/PVA composite hydrogel: antibacterial activity and promoting wound healing. Int. J. Biol. Macromol. 211, 249–258. doi:10.1016/j.ijbiomac.2022.05.072
Zhang, P., Zhang, X., Zhu, X., and Hua, Y. (2023). Chemical constituents, bioactivities, and pharmacological mechanisms of dendrobium officinale: a review of the past decade. J. Agric. Food Chem. 71 (41), 14870–14889. doi:10.1021/acs.jafc.3c04154
Zhang, Q., Zhang, Y., Chen, H., Sun, L. N., Zhang, B., Yue, D. S., et al. (2025). Dual-functional injectable adhesive hydrogel delivering ginger-derived doxorubicin vesicles for osteosarcoma recurrence suppression and post-resection wound healing. Front. Bioeng. Biotechnol. 13, 1609673. doi:10.3389/fbioe.2025.1609673
Zhang, Y., Zhao, B., Wang, J., Zhang, Z., Shen, M., Ren, C., et al. (2025). Therapeutic potential of lycium barbarum-derived exosome-like nanovesicles in combating photodamage and enhancing skin barrier repair. Extracell. Vesicle 5, 100072. doi:10.1016/j.vesic.2025.100072
Zhao, Q., Liu, G., Liu, F., Xie, M., Zou, Y., Wang, S., et al. (2023). An enzyme-based system for extraction of small extracellular vesicles from plants. Sci. Rep. 13 (1), 13931. doi:10.1038/s41598-023-41224-z
Zheng, M., Chavda, V. P., Vaghela, D. A., Bezbaruah, R., Gogoi, N. R., Patel, K., et al. (2024). Plant-derived exosomes in therapeutic nanomedicine, paving the path toward precision medicine. Phytomedicine 135, 156087. doi:10.1016/j.phymed.2024.156087
Zheng, Y., Pan, C., Xu, P., and Liu, K. (2024). Hydrogel-mediated extracellular vesicles for enhanced wound healing: the latest progress, and their prospects for 3D bioprinting. J. Nanobiotechnol 22 (1), 57. doi:10.1186/s12951-024-02315-9
Zheng, L., Sun, J., Wang, L., Ding, Z., Tang, Y., Dai, M., et al. (2025). Construction and applications of exosome-microneedle integrated systems. Int. J. Pharm. 10, 100360. doi:10.1016/j.ijpx.2025.100360
Zhou, G., Wang, C. Z., Mohammadi, S., Sawadogo, W. R., Ma, Q., and Yuan, C. S. (2023). Pharmacological effects of ginseng: multiple constituents and multiple actions on humans. Am. J. Chin. Med. 51 (5), 1085–1104. doi:10.1142/S0192415X23500507
Zhou, S., Huang, P., Cao, Y., Hua, X., Yang, Y., and Liu, S. (2024). Garlic-derived exosome-like nanovesicles-based wound dressing for staphylococcus aureus infection visualization and treatment. ACS Appl. Bio Mater 7 (3), 1888–1898. doi:10.1021/acsabm.3c01256
Keywords: traditional Chinese medicine, exosome-like nanovesicles, skin regeneration, wound healing, anti-inflammatory nanocarriers, angiogenesis, oxidative stress modulation
Citation: Wang K, Yang Z-T, Wang F, Ma Y-Q, Qing Y and Zhang Z-Y (2025) Traditional Chinese medicine derived exosome-like nanovesicles in wound repair and skin regeneration. Front. Cell Dev. Biol. 13:1680757. doi: 10.3389/fcell.2025.1680757
Received: 06 August 2025; Accepted: 04 September 2025;
Published: 17 September 2025.
Edited by:
Bo Zhang, University of California, Los Angeles, United StatesReviewed by:
Youbai Chen, People’s Liberation Army General Hospital, ChinaXianrui Yang, University of Florida, United States
Copyright © 2025 Wang, Yang, Wang, Ma, Qing and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Qing, aHhxaW5neW9uZ0AxNjMuY29t; Zhen-Yu Zhang, emhhbmd6aGVueXVAc2N1LmVkdS5jbg==
 Kang Wang
Kang Wang Zi-Ting Yang
Zi-Ting Yang Fei Wang2
Fei Wang2