- 1Department of Ophthalmology, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Department of Ophthalmology and Research Laboratory of Ophthalmology, West China Hospital, Sichuan University, Chengdu, Sichuan, China
MYCN amplification defines a highly aggressive subtype of neuroblastoma and is strongly associated with poor clinical outcomes. Due to the intrinsically disordered structure of the N-Myc protein, it remains largely undruggable. This review provides a comprehensive summary of the molecular regulatory network surrounding MYCN, including upstream pathways, key cofactors, and downstream effectors involved in cell cycle control, metabolic reprogramming, and ferroptosis. We further discuss the roles of epigenetic modulators, noncoding RNAs, and positive feedback loops in sustaining MYCN-driven oncogenic programs. Emerging therapeutic strategies such as PROTACs, metabolic inhibitors, immune-based approaches, and RNA-targeting technologies offer promising alternatives to direct MYCN inhibition. This review aims to provide a theoretical foundation for future development of precise and effective therapies targeting MYCN-amplified neuroblastoma.
1 Introduction
The V-myc avian myelocytomatosis viral oncogene neuroblastoma-derived homolog (MYCN) gene is a member of the MYC oncogene family and is located in the short arm of the human chromosome 2, 2p24.3 region (Nishio et al., 2024). MYCN is highly expressed in many cancers, including neuroblastoma (NB) (Huang and Weiss, 2013), medulloblastoma (Korshunov et al., 2012), cervical cancer (García et al., 2024), and non-small cell lung cancer (Dietzsch et al., 1994). Approximately 20% of NBs exhibit MYCN amplification (MYCN-amplified neuroblastoma, MNA-NB) (Huang and Weiss, 2013). MYCN promotes NB growth mainly by forming a complex with MDM2 and inhibiting the action of the p53 protein (Slack et al., 2005). MYCN amplification is positively correlated with poor prognosis in NB patients and negatively correlated with overall survival (OS) (Valentijn et al., 2012; Campbell et al., 2019). MYCN encodes the N-Myc protein that lacks the clear hydrophobic pocket that small molecules usually target and is considered undruggable. Currently, there are still no drugs that directly target N-Myc (Bushweller, 2019; Wolpaw et al., 2021).
This review systematically discusses the molecular regulatory network related to MYCN in NB and summarizes the indirect intervention strategies targeting the MYCN gene and its products from the current research on MNA-NB. Through a comprehensive analysis of the biological functions and pathogenic mechanisms of MYCN, this review aims to provide a theoretical basis for the development of new therapeutic strategies with MYCN amplification as a therapeutic target, especially for neuroblastoma.
2 Current treatment options for MNA-NB
NB shows characteristic molecular alterations that shape its biology and clinical outcome. The most frequent events include MYCN amplification, ALK mutations or amplification, and alterations of ATRX and TERT (Pugh et al., 2013; Pinto et al., 2015). Beyond these drivers, NB cells are increasingly recognized to depend on aberrant RNA splicing, with dysregulated splicing regulators (e.g., SF3B1, HNRNPA1, PTBP1) supporting proliferation and survival (Zhang et al., 2016; Bonnal et al., 2020; Shi et al., 2021). Notably, MYCN amplification strengthens this reliance, as N-Myc cooperates with spliceosomal machinery and sensitizes NB cells to spliceosome inhibition (Hsu et al., 2015; Kress et al., 2015). These findings indicate that NB pathogenesis involves both canonical genetic alterations and post-transcriptional vulnerabilities that may be therapeutically exploitable. Given its pivotal role in tumor progression, the subsequent discussion will primarily focus on MYCN and its associated molecular mechanisms.
All NBs with MYCN amplification are considered high risk, regardless of the stage or other risk factors (Irwin et al., 2021). There is currently no treatment targeting MYCN. Patients with MNA-NB are treated according to the treatment of high-risk NB, on the basis of the results of phase III trials and open clinical trials. The initial induction therapies include cisplatin, alkylators (Kushner et al., 2004), autologous stem cell collection and transplantation (Park et al., 2019). The efficacy of new regimens such as 131I-MIBG or anti-GD2 (Disialoganglioside GD2) monoclonal antibody therapy is currently being researched in clinical trials (McCluskey et al., 2005; Del Bufalo et al., 2023). The efficacy of induction therapy is limited, and patients with a poor response to treatment receive consolidation therapy (Pinto et al., 2019), using high-dose chemotherapy with autologous stem cell rescue, followed by radiotherapy of primary and metastatic lesions (Zhao et al., 2020). Patients without disease progression after consolidation therapy receive postconsolidation therapy containing the anti-GD2 antibody Dinutuximab and isotretinoin (Yu A. L. et al., 2021). Patients who still progress receive chemoimmunotherapy or participate in clinical trials. Eflornithine [2,5-diamino-2-(difluoromethyl) pentanoic acid hydrochloride hydrate] is an inhibitor of ornithine decarboxylase that has been approved by the FDA for patients who have responded to anti-GD2 immunotherapy (Oesterheld et al., 2024; Nazir et al., 2024; Bagatell et al., 2024).
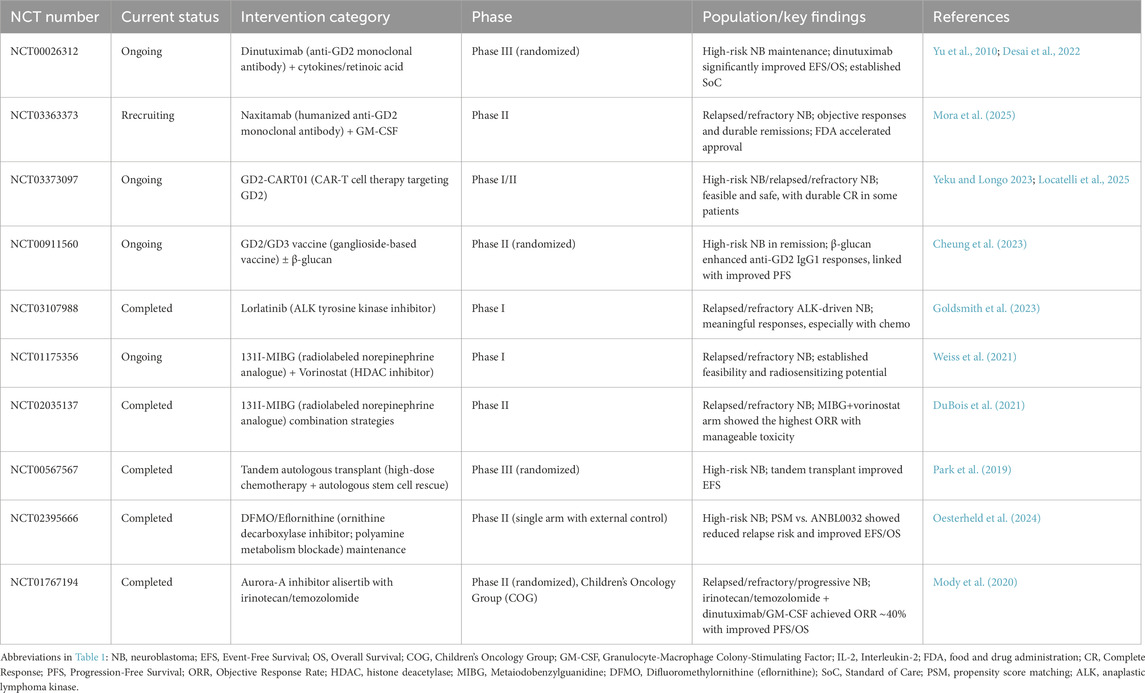
To highlight translational relevance, Table 1 summarizes representative clinical trials in neuroblastoma, including ongoing and completed studies with different treatment strategies. Figure 1 presents the treatment algorithm for MNA-NB (Figure 1; Table 1).
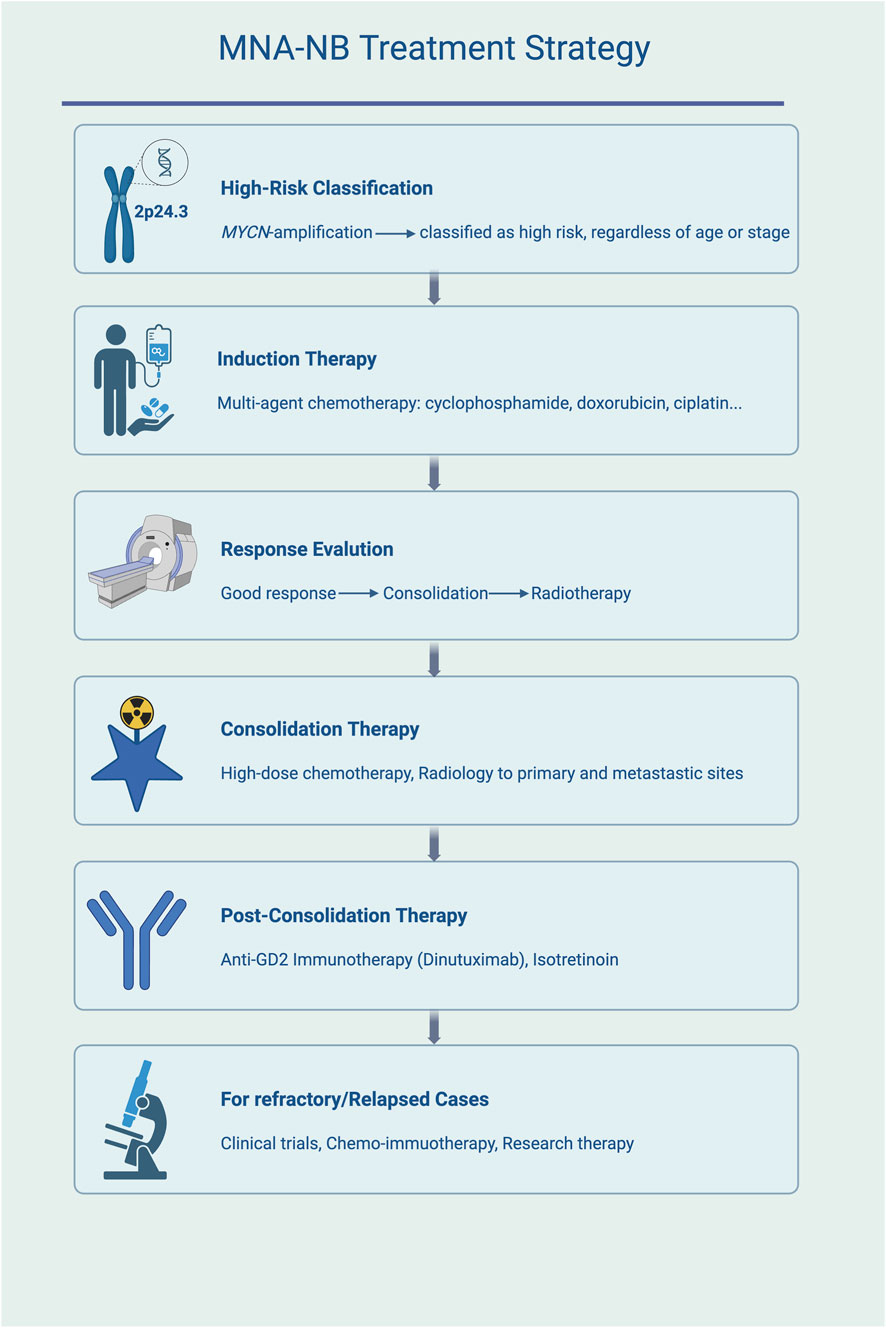
Figure 1. Treatment algorithm for MNA-NB. High-risk classification is assigned to all cases with MYCN amplification. Standard management includes induction chemotherapy, consolidation with high-dose chemotherapy and radiotherapy, post-consolidation anti-GD2 immunotherapy and isotretinoin, and experimental approaches for refractory or relapsed disease.
3 Therapeutic approaches that indirectly target MYCN
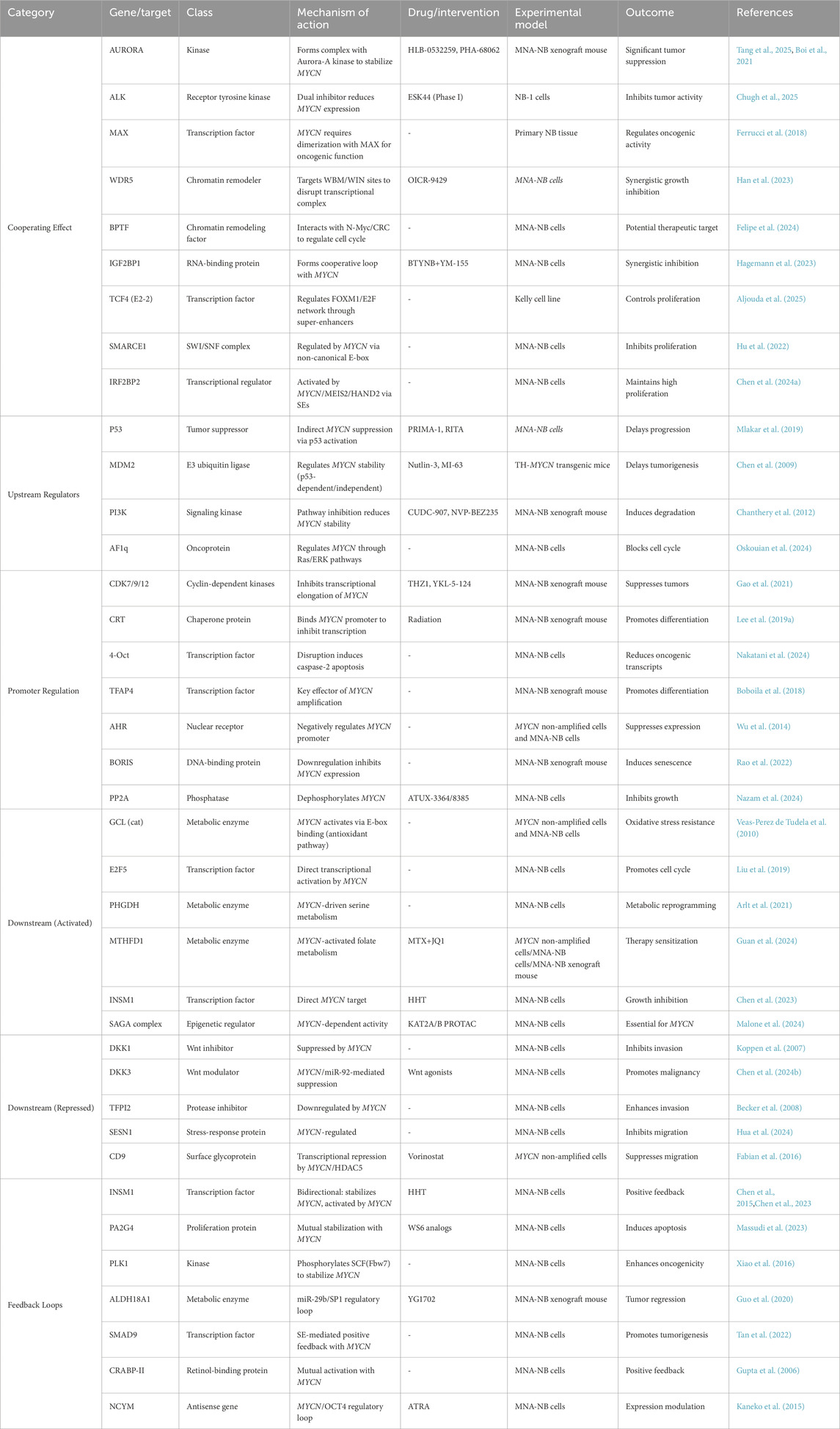
While all MNA-NB are classified as high-risk neuroblastomas, there is still a lack of treatment options that directly target MYCN due to an insufficient understanding of the structure of the N-Myc protein (Bell et al., 2010; Wolpaw et al., 2021). Therefore, many studies have focused on the MYCN gene and its transcriptional regulatory products, as well as the interactions between MYCN and other genes and proteins, to identify new strategies for the treatment of MNA-NB. This review summarizes the current research progress of therapeutics that indirectly target MYCN for the treatment of neuroblastomato provide new ideas for the treatment of MNA-NB and other MYCN-amplified tumors. Figure 2 and Table 2 provide an overview of the MYCN regulatory network, including its upstream regulators, cooperating partners, downstream positive and negative targets, and mutual adjustment loops (Figure 2; Table 2).
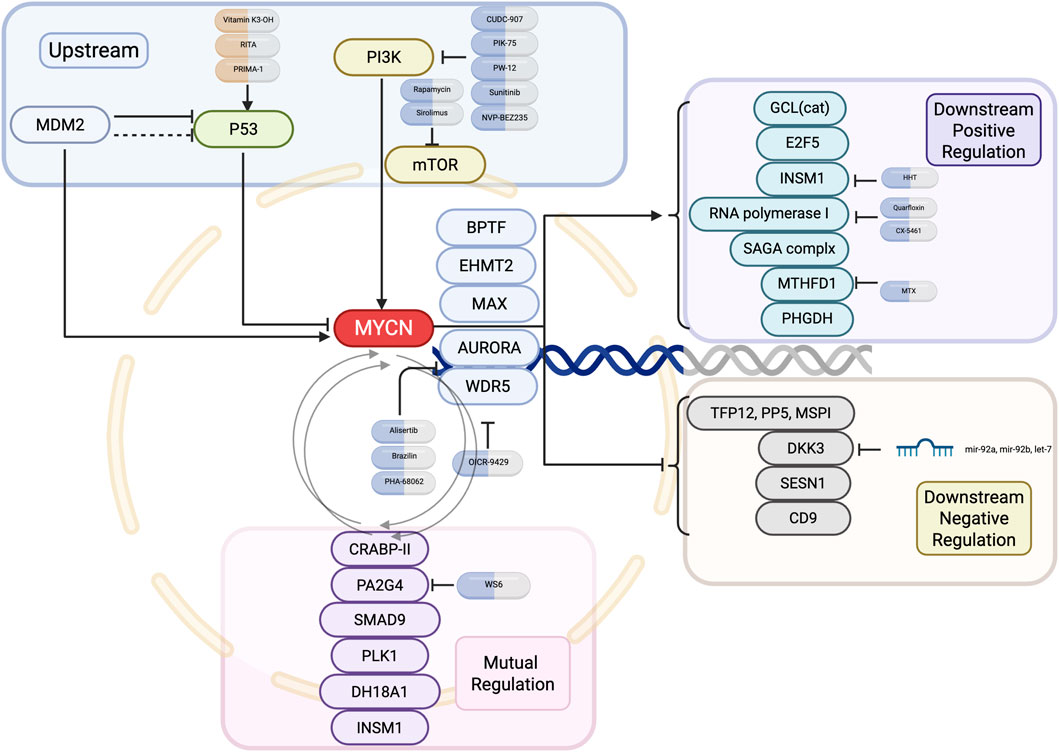
Figure 2. Upstream and downstream regulatory network of MYCN in neuroblastoma. Key upstream pathways, mutual regulators, and downstream positive and negative effectors of MYCN are shown, along with representative small-molecule inhibitors targeting each node.
3.1 Cooperating effect
MYCN cooperates with multiple oncogenic partners, and these synergistic interactions amplify tumorigenic signaling in NB. A series of studies have shown that MYCN has synergistic effects with genes such as AURORA, ALK, and MDM2.
3.1.1 AURORA
Aurora-A kinase (AURKA) stabilizes N-Myc by preventing its proteasomal degradation, thereby sustaining oncogenic signaling in MNA-NB (Otto et al., 2009). Since N-Myc is undruggable, AURKA inhibition provides an indirect therapeutic strategy. The AURKA inhibitor alisertib (MLN8237) disrupts this interaction and promotes N-Myc degradation (Manfredi et al., 2011; Cervantes et al., 2012). Beyond single-agent activity, alisertib synergizes with BET inhibitors such as JQ1 to enhance N-Myc degradation and suppress tumor growth in preclinical models (Brockmann et al., 2013; Yi et al., 2021). Clinically, alisertib has been tested in combination regimens. In the Children’s Oncology Group phase I/II study (NCT01154816) (Mossé et al., 2019), alisertib with irinotecan and temozolomide was well tolerated and demonstrated clinical responses in relapsed or refractory NB in an international phase II trial (NCT01601535) (DuBois et al., 2018). These findings support AURKA inhibition as a promising indirect MYCN-targeting approach, particularly in combination with epigenetic or DNA-damaging agents currently under investigation.
3.1.2 ALK
Activating ALK mutations, especially F1174L, frequently co-occur with MYCN amplification, synergistically accelerating neuroblastoma progression (Schönherr et al., 2012; Zhu et al., 2012; Hasan et al., 2013). Even without ALK mutations, activation of ALKAL2, a physiological ligand of ALK, enhances MYCN-driven tumorigenesis (Borenäs et al., 2021). ALK inhibitors provide a rational strategy. Clinically, NCT00939770 defined ALK inhibitor crizotinib dosing with partial responses (Foster et al., 2021), while NCT03107988 established the phase 2 dose of another ALK inhibitor lorlatinib (Goldsmith et al., 2023). An ongoing phase III trial (NCT03126916, ANBL1531) is testing lorlatinib in frontline high-risk NB (Weiss et al., 2021).
3.1.3 MAX
N-Myc binds to E-box sequences in DNA by forming a heterodimer with MAX, thereby activating downstream oncogenic transcriptional programs, including genes involved in the cell cycle, metabolism, and proliferation (Wenzel and Schwab, 1995). The relative expression ratio of MAX to MYCN is a key factor in disease progression and clinical prognosis: a higher MAX/MYCN ratio can inhibit the oncogenic effects of MYCN and improve neuroblastoma prognosis (Lu et al., 2003). Therefore, MAX is not only a necessary binding partner for MYCN function, but may also play a balancing or antagonistic role in the MYCN regulatory network (Ferrucci et al., 2018).
3.1.4 WDR5
As a key component of the N-Myc transcription complex, WDR5 participates in transcriptional regulation through its WBM site and mediates chromosome interactions through the WIN site. Han et al. screened 6 compounds targeting the WBM site, which were able to significantly inhibit the growth of the IMR32 and LAN5 cell lines with MYCN amplification and had a synergistic effect when combined with the WIN site inhibitor OICR-9429 (Han et al., 2023). Moreover, WDR5 promotes the recruitment of N-Myc to its conserved binding sites while recruiting DNA repair- and cell cycle-related genes to the N-Myc-WDR5 complex (Sun et al., 2015; Bumpous et al., 2023). WDR5 promotes the binding of N-Myc to the promoter region, while N-Myc recruits the histone methyltransferase G9a to the enhancer region to repress neuronal differentiation genes, including GAP43 and NRXN1. These two cofactors thus cooperate to reinforce MYCN-driven transcriptional repression. Consistently, combined targeting of WDR5 and G9a has been shown to synergistically inhibit N-Myc activity and effectively block neuroblastoma growth (Liu et al., 2024).
3.1.5 Other related cooperating genes
N-Myc acts synergistically with the core regulatory circuitry (CRC). Felipe et al. reported that the nucleosome remodeling factor BPTF interacts with N-Myc/CRC and colocalizes with the promoters of cell cycle genes, suggesting that BPTF is a potential therapeutic target for MNA-NB (Felipe et al., 2024). In MNA-NB, IGF2BP1 (17q) and MYCN form a synergistic loop, promoting 2p/17q chromosome amplification and upregulating BIRC5 expression. The BIRC5/survivin inhibitor YM155 can inhibit USP7 deubiquitinase activity, promote the degradation of N-Myc in MNA cells, and inhibit the growth of MNA-NB in vivo (Li et al., 2023). The combined use of BTYNB (an IGF2BP1 inhibitor), a BRD inhibitor, and YM-155 (a BIRC5 inhibitor) can synergistically inhibit the growth of MNA-NB cells, supporting its therapeutic potential (Hagemann et al., 2023). Studies by Aljouda et al. have shown that the class I basic helix-loop-helix transcription factor TCF4 (also known as E2-2), a component of the super enhancer (SE)-associated transcription factor network, regulates the FOXM1/E2F-mediated gene network and synergizes with the N-Myc protein to regulate the cell cycle progression and proliferation of neuroblastoma, playing a key role in the progression of NB (Aljouda et al., 2025). Chen et al. reported that MYCN, MEIS2, and HAND2 activated IRF2BP2 through SEs and that the AP-1 family was enriched at its binding sites, synergistically promoting ALK expression to maintain high proliferation of NB (Chen X. et al., 2024).
SMARCE1, as the coding gene of the SWI/SNF chromatin remodeling complex, regulates the expression of MYCN target genes such as PLK1, ODC1, and E2F2. In MNA-NB cells, SMARCE1 knockdown inhibits cell proliferation and colony formation. Mechanistically, N-Myc directly regulates its transcriptional activity by binding to the nonclassical E-box in the promoter region of SMARCE1 (Hu et al., 2022).
3.2 Upstream genes
Genes upstream of MYCN such as TP53 and PI3K encoding genes, critically regulate its expression, thereby indirectly shaping tumor growth and therapeutic response in NB.
3.2.1 TP53
The p53 activator PRIMA-1 rapidly induces oxidative stress and cell death in MNA-NB cells, which is caused by modulation of the methionine/cysteine/glutathione axis rather than induction of apoptosis (Mlakar et al., 2019). RITA, a p53 activator, activates p53 and triggers apoptosis, inhibiting the expression of MYCN (Burmakin et al., 2013). However, MDM2 promotes neuroblastoma development by inhibiting p53. For example, in MDM2 haploinsufficient MYCN transgenic mice, tumor onset is delayed and survival is prolonged, and in human xenograft models, MDM2 knockdown significantly inhibits tumor growth through a p53-dependent mechanism (Chen et al., 2009). In MNA-NB cells, MDM2 can also regulate MYCN expression in a p53-independent manner, thereby affecting NB tumor growth (He et al., 2011). Gu et al. revealed that MDM2 binds to the ARE element of the MYCN mRNA 3′UTR through the C-terminal RING domain, enhancing mRNA stability and translation. In NB cells, MDM2 overexpression can upregulate MYCN expression in a p53-independent manner (Gu et al., 2012). Agarwal et al. reported that N-Myc directly binds to the C-terminal domain of the p53 tetramer and that this effect is independent of the formation of the N-Myc/MAX heterodimer. The N-Myc-p53 complex targets genes involved in DNA repair and exacerbates carcinogenesis (Agarwal et al., 2018). Notably, vitamin K3-OH, a derivative of vitamin K3, can enhance the action of p53 in MNA-NB cells and inhibit the expression of MYCN, accompanied by a decrease in miRNA LIN28 (Yoda et al., 2019). Using the SHEP Tet21N MYCN-regulatable system, Gamble et al. reported that MNA-NB cells were more sensitive to apoptosis induced by the MDM2-p53 antagonists Nutlin-3 and MI-63 than those without high MYCN expression. These compounds may be particularly effective in treating high-risk MYCN amplification diseases (Gamble et al., 2012).
3.2.2 PI3K encoding genes
High expression of PI3K and histone deacetylase (HDAC) is associated with poor OS in NB patients. CUDC-907, a dual inhibitor of PI3K and HDAC, can reduce MYCN levels in NB cells and increase H3K9Ac levels, promoting apoptosis (Chilamakuri and Agarwal, 2022). In MYCN-driven neuroblastoma and medulloblastoma mouse models, the PI3K inhibitors PIK-75 and PW-12 have been shown to induce tumor apoptosis by disrupting N-Myc stability (Cage et al., 2015). The receptor tyrosine kinase (RTK) inhibitor sunitinib decreases N-Myc protein levels by inhibiting PI3K/AKT signaling and GSK3 beta (Calero et al., 2014). The clinical PI3K inhibitor NVP-BEZ235 induces N-Myc degradation, inhibits angiogenesis, and prolongs survival in MNA-NB, including in humans and transgenic mouse models (Chanthery et al., 2012; Vaughan et al., 2016). In MNA-NB cells, PI3K inhibition downregulates N-Myc, while MYCN siRNA combined with rapamycin synergistically inhibits VEGF expression (Kang et al., 2008). The expression of the mTOR substrate-encoding gene eukaryotic translation initiation factor 4E-binding protein 1 (EIF4EBP1) is positively correlated with MYCN expression (Voeltzke et al., 2022). Moreover, the combination of the mTOR inhibitor temsirolimus and BET inhibitors (JQ1 or OTX-015) has a significant synergistic inhibitory effect on MNA-NB (Kling et al., 2021). AF1q is an oncoprotein, and AF1q gene knockdown can degrade N-Myc protein through the Ras/ERK and AKT/GSK3β pathways while activating p53 to block cell cycle progression. AF1q is a newly identified type of N-Myc regulatory factor (Oskouian et al., 2024).
3.2.3 Regulation of the MYCN promoter
Delehouzé et al. demonstrated that the CDK inhibitors (R)-roscovitine and (S)-CR8 significantly reduce MYCN expression by blocking CDK7/9/12 and verified this effect in MNA-NB cells and a mouse transplant model (Delehouzé et al., 2014). THZ1, a CDK7 and SE inhibitor, inhibits MYCN gene transcription (Chipumuro et al., 2014). When combined with the tyrosine kinase inhibitors ponatinib and lapatinib, it reduces N-Myc protein expression by downregulating PNUTS (Tee et al., 2020). YKL-5-124 is a CDK7-specific inhibitor that is different from the multitarget inhibitor THZ1. In MNA-NB, YKL-5-124 induces cell cycle abnormalities and significantly inhibits tumor cell growth and the development of patient-derived xenografts when combined with the BRD4 inhibitor JQ1 (Gao et al., 2021).
Calreticulin (CRT) binds to the MYCN 5′ proximal promoter, which may overlap with the binding site of the transcription factor E2F1 and is an inhibitor of MYCN. CRT-mediated MYCN inhibition leads to increased differentiation of MNA-NB. Ionizing radiation increases CRT expression in NBs in a dose-dependent manner. The combined effect of these two factors can slow tumor growth in xenograft MNA-NB models (Lee et al., 2019). OCT4 is a transcription factor that regulates MYCN expression, and inhibiting its binding can induce caspase-2-dependent apoptosis in MNA-NB and reduce the high translation of HNRNPA1/PTBP1 transcripts maintained by MYCN. Targeting OCT4-MYCN binding may be an effective therapeutic strategy for MNA-NB (Nakatani et al., 2024). Transcription factor activator protein 4 (TFAP4) is a key effector of MYCN amplification in neuroblastoma. Knockdown of TFAP4 slows tumor growth and promotes tumor differentiation in a mouse xenograft model of MNA-NB (Boboila et al., 2018). Aryl hydrocarbon receptor (AHR) expression is negatively correlated with MYCN expression. AHR overexpression can inhibit MYCN promoter activity, whereas E2F1 overexpression can reverse this effect (Wu et al., 2014). As a nucleic acid-binding protein, the downregulation of Brother of Regulator of Imprinted Sites (BORIS) can inhibit the expression of B-cell-specific Moloney murine leukemia virus integration site (Bmi1), Akt, and MYCN and destroy telomere stability, leading to cell senescence. MNA-NB cells lacking BORIS lose their tumorigenic ability in xenograft models (Rao et al., 2022). Protein phosphatase 2A (PP2A) is a tumor suppressor protein. The PP2A activators ATUX-3364 and ATUX-8385 can lead to the dephosphorylation of the N-Myc protein at Serine 62 (S62) and reduce MYCN expression, resulting in slower growth of MYCN-amplified SK-N-BE (2) tumors (Nazam et al., 2024).
3.3 Downstream genes
Downstream effectors of MYCN govern proliferation, metabolism, and differentiation, making them key determinants of neuroblastoma progression. Many MYCN-related genes, such as ACSL4, E2F5, and SKA3, have been identified and described (Valentijn et al., 2012). The regulation of downstream genes by MYCN can be divided into two types: positive and negative.
3.3.1 Positive regulation
MYCN regulates cell proliferation, oxidative stress, and metabolic reprogramming by directly binding to target gene promoters. Veas-Perez de Tudela et al. reported that MYCN regulates the expression of the gene encoding glutamate-cysteine ligase (GCL (cat)) by binding to the promoter E-box. Knockdown of MYCN increases the sensitivity of NB cells to oxidative damage, indicating that MYCN promotes tumor survival through an antioxidant mechanism (Veas-Perez de Tudela et al., 2010). N-Myc directly binds to the Myc E-Box within the E2F5 gene promoter to induce its transcription. E2F5 knockdown can inhibit cell cycle progression by regulating CDK2 and CDK6, thereby inhibiting the proliferation of MNA-NB cells (Liu et al., 2019). Chen et al. screened a plant alkaloid, homoharringtonine (HHT), which can effectively inhibit the INSM1 promoter. Mechanistic studies have shown that HHT interferes with the binding of N-Myc to the INSM1 promoter, inhibits PI3K/AKT-mediated N-Myc stability, and helps inhibit the growth of NB tumor cells (Chen et al., 2023).
In addition, MYCN maintains its oncogenic function by activating ribosome biogenesis and epigenetic regulation. MYCN upregulates ribosomal RNA and protein synthesis through transcription, whereas the RNA polymerase I inhibitors quarfloxin and CX-5461 can induce cell cycle arrest and apoptosis by inhibiting MYCN and activating p53. Among them, CX-5461 significantly inhibits tumor growth in the MNA-NB mouse model (Hald et al., 2019). Malone et al. identified through functional genomic screening that the Spt-Ada-Gcn5-acetyltransferase (SAGA) complex is a downstream target of MYCN. SAGA activity is positively correlated with MYCN expression and stability and can be used as a target for the proteolysis-targeting chimera (PROTAC) (Malone et al., 2024).
Targeting MYCN downstream metabolic enzymes or combining them with epigenetic drugs may increase their efficacy. PHGDH encodes the protein phosphoglycerate dehydrogenase and is associated with MYCN that is continuously expressed. MYCN binds to its two promoter regions. Inhibition of PHGDH can slow the proliferation of NB cells in vitro, but in vivo experiments have shown that it can also induce NB resistance to cisplatin (Arlt et al., 2021). Methylenetetrahydrofolate dehydrogenase 1 (MTHFD1) is positively correlated with MYCN expression, and ChIP‒qPCR and dual-luciferase reporter assays have revealed that MYCN directly activates MTHFD1. Knockdown of MTHFD1 reduces the NADPH/NADP (+) and GSH/GSSG ratios, increases reactive oxygen spieces (ROS), and inhibits NB cell proliferation and migration. Knockdown of MTHFD1 or the use of methotrexate (MTX) can enhance the antitumor effect of JQ1 both in vitro and in vivo, and combined treatment has potential for clinical translation (Guan et al., 2024).
3.3.2 Negative regulation
In NB cell lines, Koppen et al. reported that the upregulation of MYCN can lead to the inhibition of Dickkopf-1 (DKK1) protein expression. The IMR32 cell line exhibited impaired proliferation when DKK1 was overexpressed, which may be related to the regulation of DKK1 by genes that inhibit cell proliferation and invasion, including SYNPO2 (Koppen et al., 2007). J. Becker, S. et al. reported that in MNA-NB, the level of tissue factor pathway inhibitor 2 (TFP12, PP5, MSPI), which inhibits matrix metalloproteinases, was reduced, which may be related to the increased invasiveness of MNA-NB (Becker et al., 2008). Dickkopf 3 (DKK3) is a target site of basic helix-loop-helix (BHLH) transcription of the N-Myc. In neuroblastoma, the expression levels of MYCN and DKK3 are negatively correlated. Validation via Wnt agonists and the p53 inhibitor PFT-alpha revealed that MYCN activates the Wnt/beta-catenin/Fra-1 pathway by inhibiting DKK3 expression, ultimately inhibiting p53 activity and leading to the malignant progression of NB (Chen Y. et al., 2024). Haug et al. reported that MYCN-regulated miR-92a/b and let-7e can target the 3′ UTR of DKK3 and confirmed their ability to inhibit DKK3 transcription through luciferase reporter experiments; in NB cells, miR-92 also regulates the secretion of DKK3 (Haug et al., 2011). Hua et al. regulated the expression of MYCN and SESN1 (a member of the Sestrin family and a target gene of p53) in NB cells by small interfering RNA (siRNA) or overexpression plasmids and confirmed that SESN1 is regulated by MYCN. The overexpression of SESN1 inhibited NB cell proliferation, migration, and invasion through the Toll-like receptor (TLR) signaling pathway (Hua et al., 2024). N-Myc colocalizes with HDAC5 at the promotor of the gene that encodes cell surface glycoprotein CD9, and represses its transcription. In SH-EP cells, CD9 expression inhibits migration and invasion, whereas the HDAC inhibitor vorinostat induces CD9 expression (Fabian et al., 2016).
3.4 Mutual adjustment or loop formation
Mutual adjustment refers to reciprocal regulation, where MYCN and a partner gene influence each other’s expression or stability. Loop formation, in contrast, represents a stable positive feedback circuit in which MYCN directly upregulates a factor that in turn reinforces MYCN, creating sustained oncogenic signaling.
Studies have shown that MYCN directly binds to the CRABP-II promoter to induce its transcription, while CRABP-II overexpression can increase N-Myc protein levels, forming a positive feedback regulatory loop (Gupta et al., 2006).
N-Myc can directly bind to the proliferation-associated protein 2G4 (PA2G4) gene promoter and bind to the PA2G4 protein through a 14-amino acid sequence. Inhibiting N-Myc-PA2G4 binding effectively inhibits MNA-NB tumor growth and reduces the levels of both proteins (Koach et al., 2019). Moreover, PA2G4 can bind to N-Myc and increase its stability. WS6 and its low-toxicity analogs can bind to PA2G4, reduce N-Myc levels in MNA-NB cells, and induce apoptosis (Massudi et al., 2023).
SMAD9 is an independent prognostic factor for high-risk neuroblastoma, and its expression is directly regulated by MYCN through SEs. Studies have confirmed that SMAD9 also binds to the MYCN promoter to form a positive feedback loop, and gene silencing experiments have shown that SMAD9 knockout can significantly inhibit MNA-NB cell proliferation and tumor formation ability (Tan et al., 2022). Polo-like kinase-1 (PLK1) phosphorylates and ubiquitinates SCF (Fbw7), promoting its degradation and blocking the decomposition of N-Myc. Stable N-Myc can directly activate PLK1 transcription, forming a positive feedback loop and enhancing the oncogenic activity of N-Myc (Xiao et al., 2016). Aldehyde dehydrogenase family 18 member A1 (ALDH18A1) can posttranscriptionally regulate MYCN expression through the miR-29b/SP1 autoregulatory loop, and MYCN can transactivate ALDH18A1. The ALDH18A1 inhibitor YG1702 causes tumor regression and prolongs survival in a patient-derived xenograft (PDX) model of NB (Guo et al., 2020). INSM1 inhibits N-myc phosphorylation (Thr58) and degradation through the PI3K/AKT/GSK3β pathway, while N-myc binds to the INSM1 promoter and activates its expression (Chen et al., 2015). MYCN and its cis-antisense gene NCYM form a positive feedback loop with OCT4, and all-trans retinoic acid (RA) can reduce the expression of MYCN, NCYM, and OCT4 in MNA-NB (Kaneko et al., 2015).
3.5 Immunotherapy
Immunotherapy has emerged as a critical strategy in MNA-NB, aiming to overcome immune evasion and improve clinical outcomes. Immunotherapy targeting GD2 is the current first-line treatment for MNA-NB. However, MNA-NB utilizes an immune escape mechanism involing epigenetic regulation and immune microenvironment remodeling. These findings provide a theoretical basis and translational ideas for optimizing the clinical treatment of MNA-NB.
3.5.1 Targeting GD2
The inclusion of dinutuximab (a monoclonal antibody against GD2) in first-line therapy significantly improves survival, and its combination with chemotherapy is effective in recurrent disease. However, GD2 expression levels in TH-MYCN (a transgenic neuroblastoma model driven by tyrosine hydroxylase promoter–driven MYCN overexpression) NBs were previously considered too low for targeting, resulting in the inadequate use of this model for GD2-targeted therapy studies (McNerney et al., 2022). The HDAC inhibitor vorinostat induces the upregulation of GD2 on the surface of MNA-NB cells and has a synergistic effect on the inhibition of MNA-NB by an anti-GD2 mAb (Kroesen et al., 2016). Indoleamine-pyrrole 2,3-dioxygenase 1 (IDO1) inhibits immune cell activity through tryptophan metabolism, interferes with IFNγ production, and thus weakens the killing effect of GD2. Chimeric antigen receptor T cell (CAR-T)/NK (natural killer) cells on NBs. MYCN transcriptionally inhibits the IDO1 promoter, suggesting that the IDO1 inhibitor BMS-986205 combined with GD2. CAR-T/NK cells have the potential to treat MNA-NB (Caforio et al., 2021). The multifunctional nanomedicine (ANM) constructed by Zhang et al. contains a GD2 aptamer, MYCN siRNA, and doxorubicin, which can target GD2 (+) IMR32 tumors and inhibit their growth in vivo (Zhang et al., 2021).
3.5.2 Immune escape in MNA-NB
Elisa Brandetti et al. reported that in the MYCN-inducible Tet-21/N-cell line, downregulation of MYCN expression can lead to the upregulation of ligands such as MICA, ULBPs, and PVR, making NB more easily recognized by NK cells, thereby avoiding their own immune defense (Brandetti et al., 2017). MYCN mediates immune suppression by increasing chemokine-like factor (CKLF) secretion to recruit CCR4+CD4(+) T cells. Targeting the CKLF pathway can inhibit MNA-NB immune escape (Qin et al., 2024). Seier et al. reported that the H3K9 euchromatic histone-lysine methyltransferases (EHTM) 1 and EHMT2 inhibited the transcriptional response to IFN-γ in MNA-NB, whereas EHMT inhibitors increased IFN-γ-induced CXCL9 and CXCL10 expression and promoted T-cell infiltration. The combined use of EHMT and EZH2 inhibitors tazemetostat (EPZ-6438) or GSK126 promoted MNA-NB regression by increasing Th1 chemokine expression (Seier et al., 2021).
3.5.3 Harnessing the immune system to kill MNA-NB
Himoudi et al. used the MYCN-derived HLA-A2-restricted peptide VILKKATEYV to stimulate T cells and obtained two CTL clones that could kill MNA-NB (Himoudi et al., 2008). Sussman et al. identified the target gene calmodulin kinase-like vesicle-associated gene (CAMKV) regulated by N-Myc in MNA-NB cells through ChIP-seq. This calcium-binding transmembrane protein is specifically expressed in the central nervous system and MNA-NB and is a potential nonblood–brain barrier-penetrating immunotherapy target (Sussman et al., 2020). Boboila et al. transplanted the neuroblastoma cell line 9464D derived from TH-MYCN transgenic mice into the kidneys of C57BL/6 mice to construct an MNA-NB metastasis model and confirmed that anti-PD1 antibodies combined with high-dose radiotherapy can inhibit tumor growth and enhance CD3(+) CD8(+) T-cell infiltration (Boboila et al., 2023). Grunewald et al. cocultured L1CAM-CAR-T cells with MYCN-induced neuroblastoma models and MNA-NB cell lines, performed RNA sequencing and public omics data analysis, and reported that high expression of MYCN inhibits L1CAM. The indirect MYCN inhibitor MLN8237 combined with L1CAM-CAR-T-cell therapy can enhance the killing effect on MYCN-overexpressing tumors (Grunewald et al., 2025).
CRISPR screening has been used to identify H2AFY as a PD-1 agent nivolumab resistance gene, and knocking out H2AFY reverses PD-1 blockade resistance in MNA-NB and promotes mesenchymal transformation (Nagarajan et al., 2024). The BET inhibitor JQ-1 promotes the infiltration of PD-1(+) CD8(+) T cells, CD4(+) T cells, and regulatory T (Treg) cells into TH-MYCN mouse neuroblastoma cells, and its combination with anti-PD-1 therapy can synergistically enhance the tumor inhibitory effect (Sauvage et al., 2022).
3.6 Epigenetic regulation
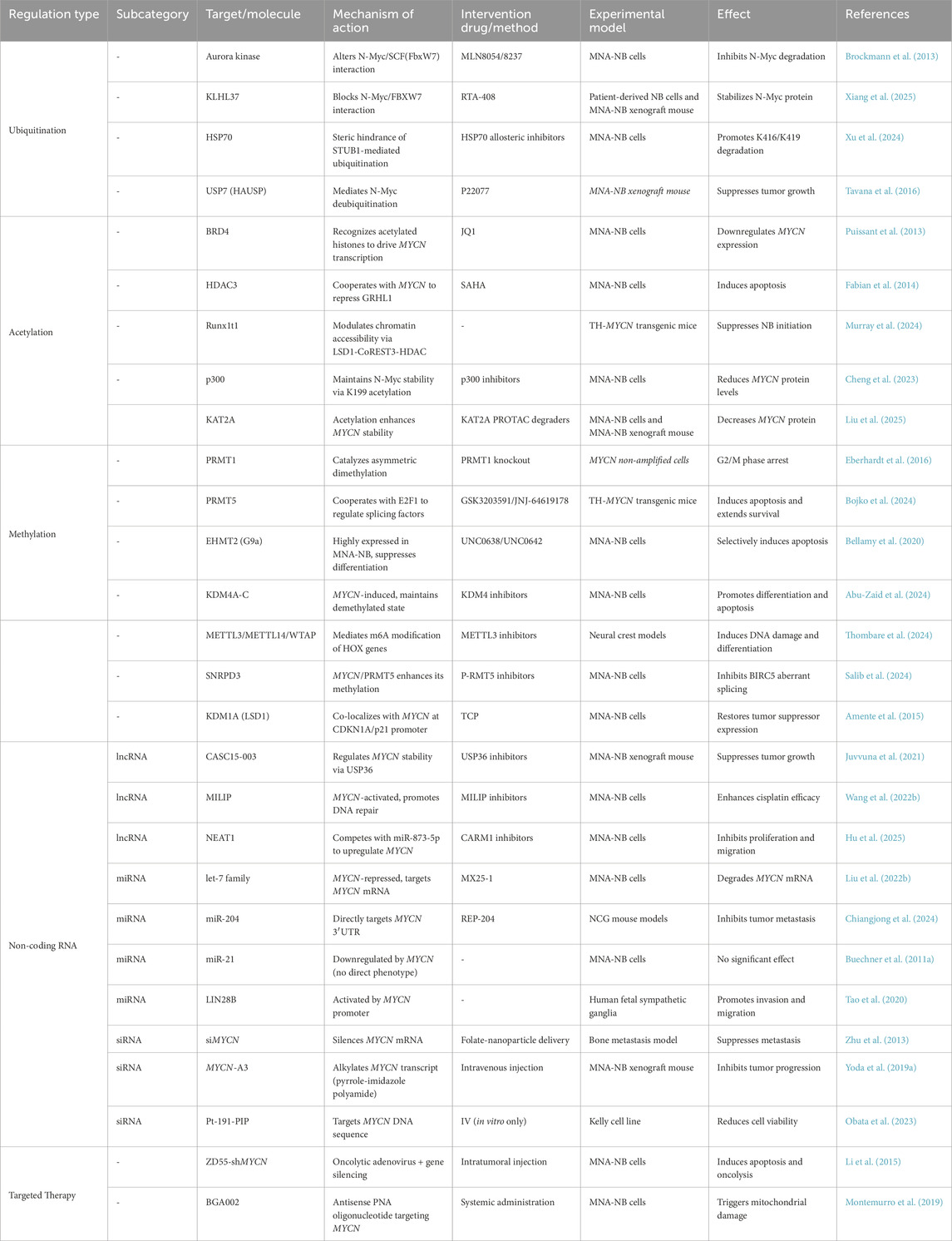
Epigenetic regulation controls the stability and activity of MYCN, shaping NB progression. This section highlights how ubiquitination, acetylation, methylation, and noncoding RNAs fine-tune MYCN expression. Figures 3, 4 and Table 3 summarize the major epigenetic mechanisms regulating MYCN stability, including ubiquitination, acetylation, methylation, and RNA-based regulation (Figures 3, 4; Table 3).
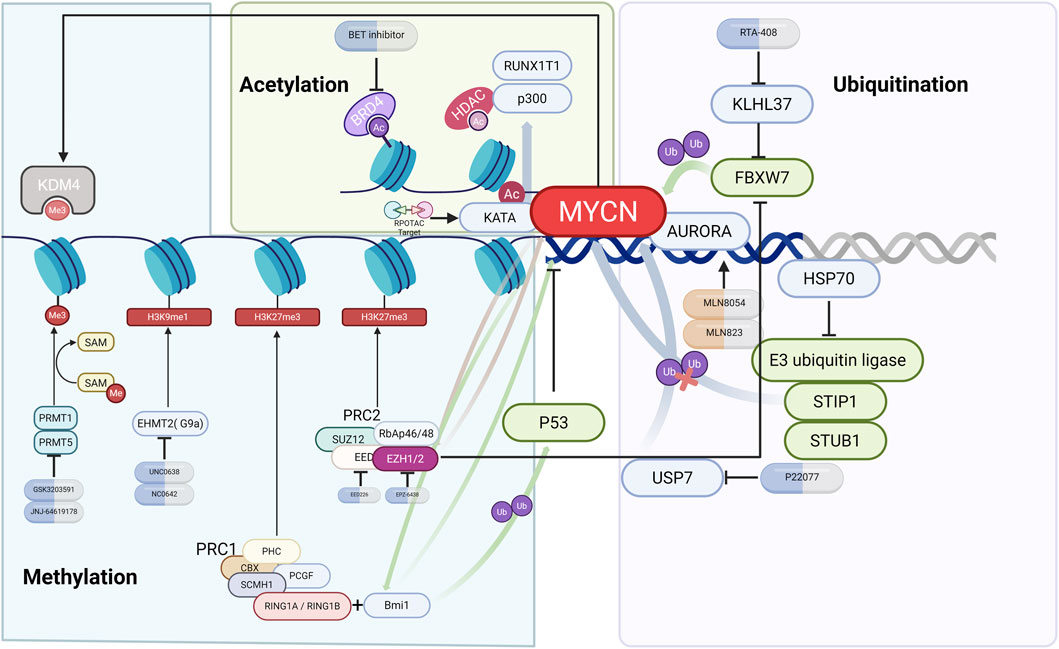
Figure 3. Epigenetic regulation of MYCN in NB. Schematic illustration of MYCN regulation by ubiquitination, acetylation, and methylation. Key modifiers (such as AURKA, USP7, BRD4, EZH2, PRMT5, and G9a) are shown together with representative inhibitors (e.g., alisertib, P22077, JQ1, tazemetostat, and UNC0638) and their effects on MYCN stability or transcriptional activity.
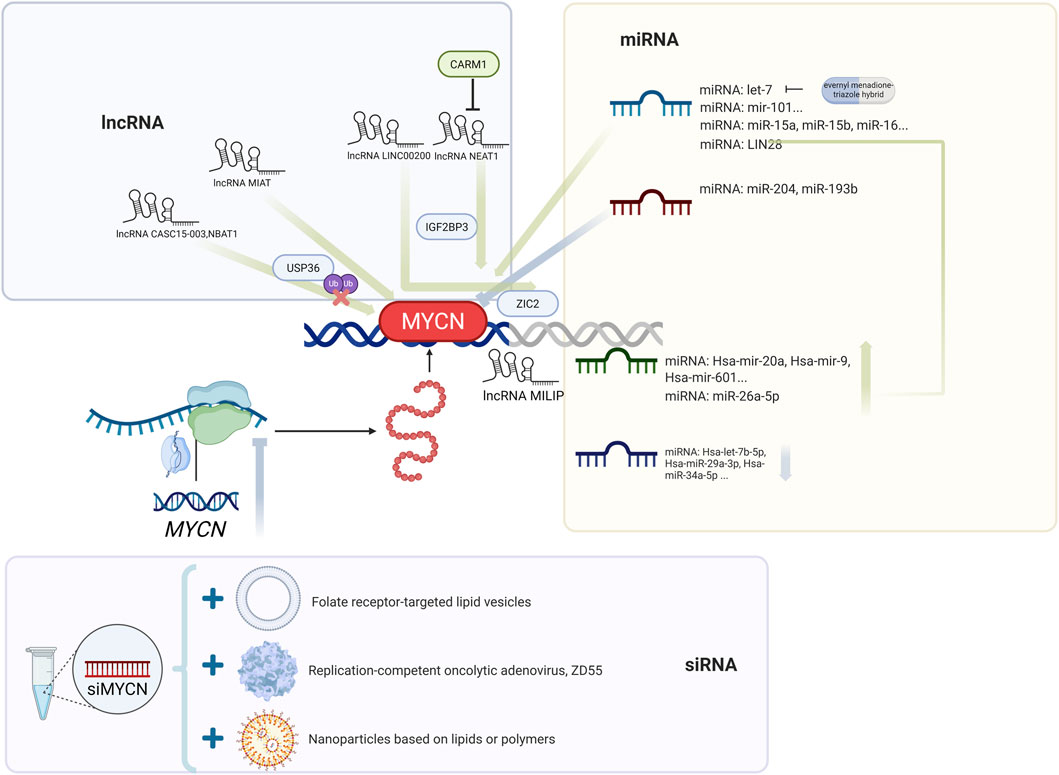
Figure 4. Post-transcriptional regulation of MYCN by noncoding RNAs. Schematic overview of MYCN regulation through lncRNAs (e.g., CASC15-003, NBAT1, MIAT, LINC00200, NEAT1, MILIP), miRNAs (e.g., let-7 family, miR-204, miR-193b), and siRNA-based strategies. Representative approaches such as miRNA mimics, siMYCN, antisense oligonucleotides (BGA002), and nanoparticle-mediated delivery are highlighted.
3.6.1 Ubiquitination
The protein kinase Aurora inhibits the stability of N-Myc by altering the way N-Myc interacts with SCF (FbxW7) and preventing its ubiquitination (Richards et al., 2016). The AURKA inhibitor MLN8054/8237 disrupts the AURKA/N-Myc interaction and promotes Fbxw7-mediated N-Myc degradation (Brockmann et al., 2013).
Kelch-like protein 37 (KLHL37) stabilizes N-Myc-promoting cancer by blocking the N-Myc/FbxW7 interaction, and its inhibitor RTA-408 can inhibit neuroblastoma both in vivo and in vitro (Xiang et al., 2025). Shock protein 70 (HSP70), a key chaperone protein of N-Myc, maintains the stability of N-Myc by blocking STUB1-mediated ubiquitination through steric hindrance; its allosteric inhibitor can promote the ubiquitination degradation of N-Myc at the K416 and K419 sites (Xu et al., 2024). Ubiquitin-specific protease 7 (USP7, HAUSP) can bind to N-Myc and induce N-Myc deubiquitination. The HAUSP small molecule inhibitor P22077 has been shown to significantly inhibit the growth of the MNA-NB xenograft mouse model (Tavana et al., 2016).
3.6.2 Acetylation
BRD4, a member of the BET family, recognizes and binds to acetylated histones and drives MYCN transcription, whereas inhibitors such as JQ1 can displace BRD4 from the MYCN promoter region and downregulate MYCN expression (Puissant et al., 2013).
Cortés et al. reported that the HDAC inhibitor suberoylanilide hydroxamic acid (SAHA) induced apoptosis in all NB cell lines (regardless of MYCN status) and reduced MYCN expression, but changes in MYCN expression levels did not affect the efficacy of SAHA (Cortés et al., 2015). HDAC3 synergizes with MYCN to repress Grainyhead-like 1 (GRHL1) gene transcription, and GRHL1 overexpression inhibits the proliferation and in vivo growth of MNA-NB, suggesting that GRHL1 is a potential therapeutic target for HDAC inhibitors vorinostat (SAHA) or panobinostat (LBH589) (Fabian et al., 2014). Through large-scale mutagenesis screening, Murray et al. reported that knocking out Runx1t1 can prevent the occurrence of neuroblastoma in transgenic mice. Although no direct interaction between Runx1t1 and MYCN has been detected, the RUNX1T1 protein, a component of the LSD1-CoREST3-HDAC inhibitory complex, may inhibit the growth of NB with high MYCN expression by regulating chromatin accessibility through the inhibitory effect of the complex in the enhancer region (Murray et al., 2024). Cheng et al. identified 16 posttranslational modification sites and 114 interacting proteins through N-Myc coimmunoprecipitation and mass spectrometry analysis and reported that p300 maintains its stability by regulating the acetylation/ubiquitination of the N-Myc K199 site, suggesting that p300 is a potential target for N-Myc-targeted therapy (Cheng et al., 2023). Whole-genome sequencing of IMR32 cells revealed that N-Myc recruits the transcriptional coactivator KAT2A to bind to DNA, promoting the expression of genes related to ribosome biogenesis and RNA processing, whereas KAT2A increases the stability of N-Myc through acetylation. Moreover, PROTAC-mediated KAT2A degradation can reduce N-Myc levels (Liu et al., 2025).
3.6.3 Methylation
Protein arginine methyltransferase 1 (PRMT1) catalyzes the formation of asymmetric dimethylarginine. Knockdown of the gene encoding PRMT1 can arrest the cell cycle at G (2)/M and increase the p53 protein level in the MNB-NB cell line SK-N-SH (Eberhardt et al., 2016; Lee et al., 2019). Bate-Eya et al. reported that protein arginine methyltransferase 5 (PRMT5) and the transcriptional regulator E2F1 cooperate with MYCN to regulate splicing factor genes, disrupt RNA splicing programs, and inhibit NB apoptosis. The PRMT5 inhibitor T1-44 or E2F1 inactivation promotes NB apoptosis (Park et al., 2015; Bate-Eya et al., 2025). The PRMT5 inhibitor GSK3203591 inhibits growth and induces apoptosis in neuroblastoma, accompanied by changes in the DNA damage response, epitranscriptome and metabolism, and reduces GLS expression. GSK3203591 also prolongs the survival of TH-MYCN mice and has potential for use in MNA-NB therapy (Bojko et al., 2024).
Bmi1 is one of the core proteins of polycomb repressive complex 1 (PRC1) (Lin et al., 2015; Fitieh et al., 2021). Ochiai et al. confirmed the transcriptional regulation of Bmi1 by targeting the N-Myc binding site in the Bmi1 promoter. The study revealed that MYCN was significantly positively correlated with Bmi1 expression. Mechanistic studies have shown that Bmi1 promotes the proliferation of neuroblastoma cells and inhibits their differentiation by binding to the promoter regions of the tumor suppressor genes TSLC1 and KIF1Bβ (Ochiai et al., 2010). In NB, Bmi1 also mediates the ubiquitination and degradation of p53 by binding to the polycomb complex protein Ring1A/Ring1B, leading to abnormal overexpression of the MYCN protein during embryonic development (Calao et al., 2013).
Embryonic ectoderm development (EED) is a component of polycomb repressive complex 2 (PRC2). EED knockout by shRNA, sgRNA, and the EED small-molecule inhibitor EED226 can significantly inhibit NB cell proliferation (Shaliman et al., 2022). Using CRISPR-Cas9 screening and ChIP analysis, Chen et al. reported that MYCN promotes EZH2 expression by binding to the EZH2 promoter, inhibiting neuronal differentiation in a PRC2-dependent manner, suggesting that EZH2 inhibitors tazemetostat (EPZ-6438) or GSK126 may be used to treat MNA-NB (Chen et al., 2018). Moreover, Wang et al. reported that EZH2 can compete with the SCF (Fbw7) ubiquitin ligase for N-Myc binding, counteracting Fbw7-mediated N-Myc polyubiquitination and proteasomal degradation. Knockdown of EZH2 can directly reduce the level of MN-Myc (Wang L. et al., 2022). Tsubota et al. reported that EZH2 and N-Myc are physically associated and that H3K27me3 expression is increased in the TH-MYCN mouse NB model. Moreover, the inhibitor EPZ-6438 can inhibit orthotopic tumor growth (Tsubota et al., 2017).
G9a is highly expressed in MNA-NB cells, and its knockout or inhibitors UNC0638 or UNC0642 selectively induce the apoptosis of MNA-NB cells. MYCN amplification enhances this effect, indicating that G9a is a potential therapeutic target for MNA-NB (Bellamy et al., 2020). EZH1 knockout or UNC1999 inhibition have been shown to downregulate E2F target genes (TYMS, POLA2, CCNA1) by reducing H3K27me1 levels, and a reduction in TYMS (encoding thymidylate synthetase) significantly increased the sensitivity of MNA-NB to 5-FU (Shinno et al., 2022). Abu-Zaid et al. reported that MYCN can induce the expression of histone lysine demethylase 4 family members (KDM4A-C), and pharmacological inhibition of KDM4 can block MYCN expression and H3K9me3, inducing neuroblast differentiation and apoptosis (Abu-Zaid et al., 2024). METTL3/METTL14/WTAP mediate HOX gene m6A modification in neural crest cells, and HOX gene m6A modification in MNA-NB has been shown to lead to downregulation of HOX expression. METTL3 inhibition can induce DNA damage and differentiation in MNA-NB cells (Thombare et al., 2024). Salib et al. revealed that MYCN enhances the methylation of the core spliceosomal protein SNRPD3 by binding to SNRPD3 and PRMT5, thereby increasing its expression level and promoting the differential splicing of cell cycle factors such as BIRC5, forming a bidirectional regulatory loop. The PRMT5 inhibitor JNJ-64619178 can effectively inhibit the activity of MNA-NB cells (Salib et al., 2024). The chromatin-modifying enzyme lysine-specific demethylase 1 (KDM1A, LSD1) colocalizes with N-Myc in the promoter regions of CDKN1A/p21 and CLU, and the use of the KDM1A inhibitor TCP can restore the expression of these two genes in MNA-NB cells (Amente et al., 2015).
3.6.4 Long noncoding RNA (lncRNA)
Li et al. developed a new computational process, LncFusion. By analyzing the transcriptome data of three major childhood cancer cohorts, TARGET, Gabriella Miller Kids First, and St. Jude Cloud, they reported that MNA-NBs presented subtype-specific enrichment of lnc-fusion genes, suggesting that these genes may be involved in the pathogenesis of NB and providing a new target for MNA treatment (Li N. et al., 2025). Juvvuna et al. reported that the lncRNAs CASC15-003 and NBAT1 affect the stability of the N-Myc by regulating the deubiquitinase USP36. Downregulation of USP36 can significantly inhibit the growth of NB cells and transplanted tumors (Juvvuna et al., 2021). Downregulation of the lncRNA myocardial infarction-associated transcript (MIAT) reduces N-Myc expression and the MYCN target gene ODC1 in MNA-NB and inhibits glycolysis, supporting its potential as a therapeutic target (Feriancikova et al., 2021). Chen et al. reported that the lncRNA LINC00200 is highly expressed in MNA-NB tissues and that its overexpression promotes the expression of the MYCN target gene ZIC2 by binding to IGF2BP3, thereby increasing the proliferation, invasion, and migration ability of NB cells (Chen et al., 2022). Hu et al. confirmed through a luciferase reporter gene that the lncRNA NEAT1 acts as a competitive sponge for miR-873-5p, increasing MYCN and GalNAcT-I expression, whereas the inhibition of CARM1 can reduce NEAT1 activity (Hu et al., 2025). N-Myc promotes DNA repair by transcriptionally activating the lncRNA MILIP. MILIP knockout leads to the accumulation of DNA double-strand breaks (DSBs), induces apoptosis, and inhibits the proliferation of MNA-NB cells. Targeted inhibition of MILIP enhances the in vivo growth inhibitory effect of cisplatin on neuroblastoma (Wang P. L. et al., 2022).
3.6.5 microRNA (miRNA)
Mestdagh et al. investigated the miRNA signature of MYCN using primary NB tumor data and reported that MYCN causes extensive miRNA repression by binding to the promoter region of targeted miRNAs and that these miRNA pathways are conserved in multiple tumors (Mestdagh et al., 2010). Through genome-wide miRNA target screening combined with patient data, 12 miRNAs that target and inhibit MYCN were identified. In the TH-MYCN transgenic mouse model, 9 of these miRNAs, including hsa-let-7a-5p, hsa-let-7b-5p, and hsa-miR-29a-3p, were expressed at low levels in tumor tissues, suggesting that MYCN maintains its high expression by inhibiting these miRNAs (Beckers et al., 2015a).
J. Buechner et al. predicted the miRNA targets of the MYCN 3′UTR through bioinformatics and verified them in neuroblastoma cells with MYCN amplification. They reported that the overexpression of let-7 and miR-101 significantly inhibited tumor cell proliferation and colony formation ability (Buechner et al., 2011). Liu et al. reported that the small molecule MX25-1 promoted the degradation of MYCN mRNA by binding to its 3′UTR, and this process was dependent on let-7 regulation (Liu F. et al., 2022). Szewczyk et al. constructed a tTR-KRAB repressor protein-regulated GFP reporter system and reported that kenpaullone and BIO inhibited N-Myc expression by enhancing let-7 miRNA expression (Szewczyk et al., 2024). Mendonza et al. constructed six hybrid compounds by simulating the classic structure of HDAC inhibitors, connecting evernyl groups with menadione via triazole linkers. Among them, Compound 6b significantly inhibited the proliferation of neuroblastoma by a mechanism involving the induction of the expression of microRNAs, including let-7, and thus downregulating N-Myc. In addition, Compound 6b enhanced the differentiation effect of RA combined therapy (Mendonza et al., 2023).
Chava et al. predicted and verified that miR-15a/15b/16 targets MYCN mRNA through databases such as TargetScan and that its overexpression significantly inhibits neuroblastoma cell proliferation, migration, invasion, and tumorigenesis in NCG mice (Chava et al., 2020).
J. Buechner, J. R et al. reported that knocking down MYCN in SK-N-BE cells led to the downregulation of 12 miRNAs (including mir-21), but artificial regulation of mir-21 levels (overexpression or inhibition) did not significantly affect the differentiation or proliferation of neuroblastoma cells with high MYCN levels (Buechner et al., 2011). LIN28 can increase the invasion and migration of MNA-NBs (Tao et al., 2020). In MYCN-amplified NB, MYCN can also increase the expression of the oncogene LIN28B by directly acting on the promoter and indirectly regulating miR-26a-5p (Beckers et al., 2015b). The MYCN-induced miRNA signature of NB cells revealed that miR-18a and miR-19a promoted NB development by downregulating estrogen receptor α (ESR1) expression and inhibiting the differentiation of human fetal sympathetic ganglia (Lovén et al., 2010). Interfering with miR-18a or overexpressing ERα can induce nerve growth factor (NGF) signaling and promote the differentiation of MNA-NB cells into neurons (Dzieran et al., 2018). miR-204 can directly bind to MYCN mRNA and inhibit MYCN expression (Ooi et al., 2018). Chiangjong et al. synthesized erythrocyte extracellular vesicles (REP-204) loaded with miR-204. SWATH proteomics revealed that REP-204 inhibited mRNA splicing and protein synthesis through the spliceosome, thereby inhibiting the growth and migration of neuroblastoma (Chiangjong et al., 2024). Introduction of miR-193b into different NB cell lines significantly inhibited cell growth by reducing the expression of MYCN, Cyclin D1, and MCL-1. Therefore, miR-193b may be a new candidate drug for MNA-NB (Roth et al., 2018).
3.6.6 Small interfering RNA (siRNA)
In MNA-NB cell lines, silencing MYCN with siRNA molecules can promote cell differentiation, and this effect is better than that of RA. siMYCN may therefore become a new treatment for RA-resistant MNA-NB (Maeshima et al., 2020). The efficiency of folate receptor-targeted liposome delivery of MYCN siRNA to LA-N-5 cells reached 92%, which effectively silenced MYCN expression (Feng et al., 2010). The MYCN-specific antigene PNA oligonucleotide BGA002 can reduce the level of N-Myc protein in MNA-NB in vitro and in vivo, causing severe mitochondrial damage, thereby increasing ROS and inducing NB apoptosis (Montemurro et al., 2019). Folate-nanoliposome-entrapped MYCN siRNA can cause decreased MYCN expression and apoptosis of metastatic NBs in the bone marrow and bone metastasis xenograft mouse MNA-NB model (Zhu et al., 2013). Tagalakis et al. developed lipid-based and polymer-based nanoparticles to deliver siRNA targeting MYCN intravenously, effectively inhibiting the growth of neuroblastoma xenografts and prolonging the survival of mice (Tagalakis et al., 2021). The ZD55-shMYCN oncolytic adenovirus blocks the cell cycle and induces apoptosis in MNA-NB cells by inhibiting MYCN expression and directly promoting cancer cell lysis (Li et al., 2013; Li et al., 2015).
Except for the interfering small RNA, the MYCN-targeting pyrrole-imidazole polyamide (PIP) MYCN-A3 can directly bind to and alkylate MYCN transcripts, downregulating MYCN expression and inhibiting tumor progression in the MNA-NB mouse xenograft suppressor model (Yoda et al., 2019). Obata et al. developed a new platinum-191-labeled compound based on pyrrole-imidazole polyamide (PIP), which can specifically bind to DNA containing the MYCN gene target sequence and significantly inhibit the viability of the neuroblastoma cell line Kelly of with high MYCN expression. However, animal experiments have shown that the uptake rate of this compound in tumor tissue after intravenous injection is low, limiting its clinical application value (Obata et al., 2023).
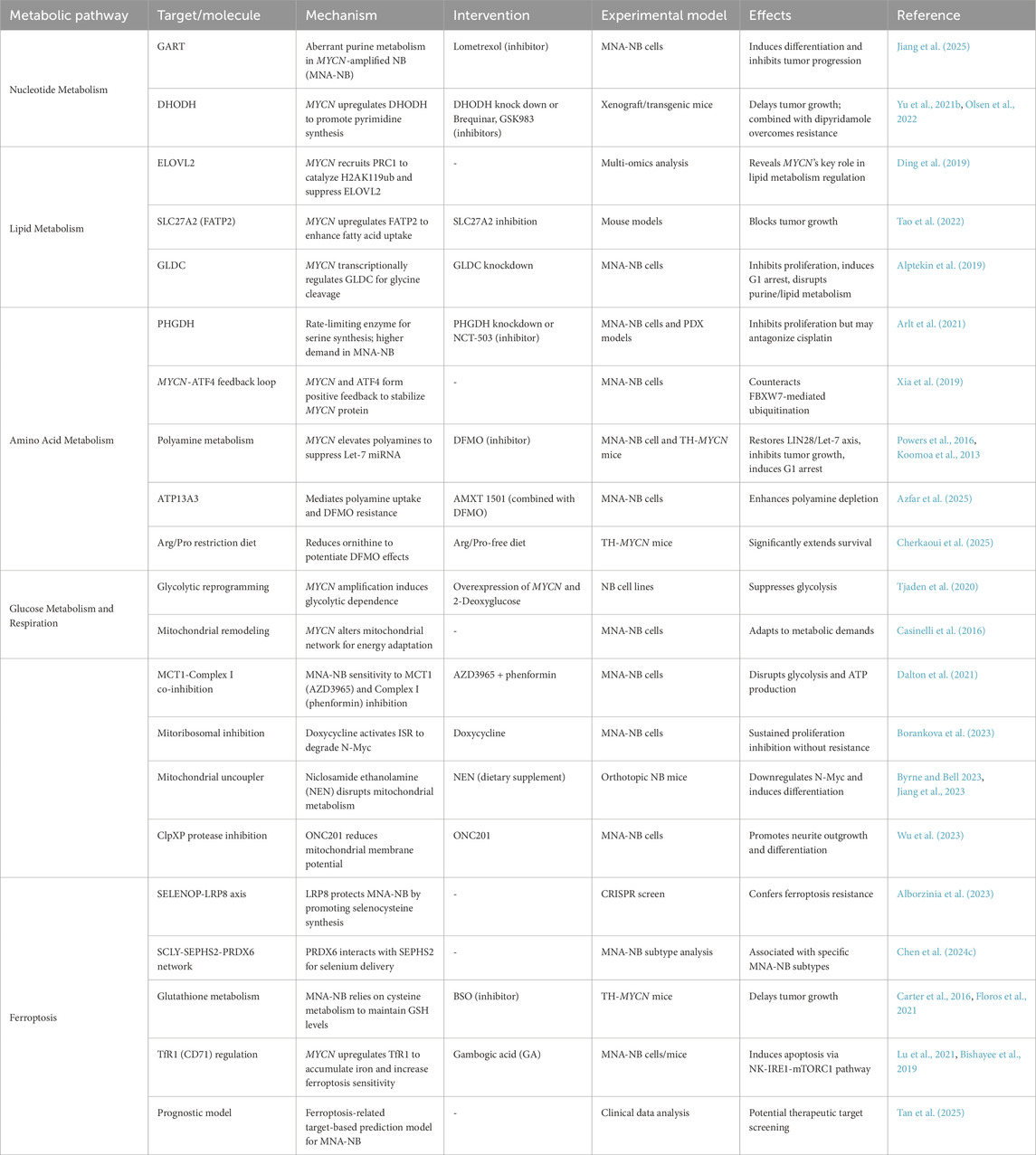
3.7 Metabolism-related targets
MYCN amplification drives profound metabolic reprogramming in NB, creating vulnerabilities across nucleotide, lipid, amino acid, glucose, and ferroptosis pathways. Through combined metabolome‒transcriptome analysis, Du et al. reported that, compared with non-MNA-NBs, MNA- NBs presented three abnormal pathways related to glycerolipid metabolism, purine metabolism, and lysine degradation, which can serve as potential therapeutic targets (Du et al., 2024). The following section summarizes MYCN-related metabolic targets (Table 4).
3.7.1 Nucleotide metabolism
Using mass spectrometry metabolomics and public RNA sequencing data, Jiang et al. reported that MNA-NBs purine metabolism enzyme-encoding genes were abnormal compared with non-MNA-NBs. Lometrexol, an inhibitor of the purine biosynthesis enzyme phosphoribosylglycinamide formyltransferase (GART), can induce differentiation in MNA-NB and inhibit tumor progression (Jiang et al., 2025).
The mitochondrial membrane protein dihydroorotate dehydrogenase (DHODH) is a key enzyme in pyrimidine synthesis, and MYCN promotes the production of pyrimidine nucleotides by upregulating the expression of DHODH. Knocking down DHODH or using inhibitors such as brequinar and GSK983 can delay the growth of MNA-NB tumors. Blocking uridine transport with dipyridamole can overcome the serum uridine-dependent resistance of DHODH inhibitors (Yu Y. et al., 2021). DHODH is regulated by MYCN in MNA-NB, and its high expression is significantly associated with poor patient prognosis. Brequinar effectively inhibits the growth of neuroblastoma in both transplanted tumors and transgenic mouse models, supporting its therapeutic potential (Olsen et al., 2022).
3.7.2 Lipid metabolism
Through multiomics analysis, Ding et al. reported that MYCN inhibits ELOVL fatty acid elongase 2 gene (ELOVL2) expression by recruiting PRC1 to catalyze H2AK119ub, thereby reducing docosahexaenoic acid synthesis, revealing the key role of MYCN in the regulation of lipid metabolism in neuroblastoma (Ding et al., 2019). Through multidimensional metabolic analysis, it was found that MNA-NB is highly dependent on fatty acid metabolism. MYCN promotes fatty acid uptake by upregulating fatty acid transport protein 2 (FATP2), which is encoded by SLC27A2. Inhibition of SLC27A2 can block tumor growth in mice, supporting the therapeutic potential of targeting fatty acid metabolism (Tao et al., 2022). Glycine decarboxylase (GLDC) is a key enzyme for glycine decomposition and is directly regulated by MYCN at the transcriptional level; its knockdown can inhibit MNA-NB cell proliferation, induce G1 phase arrest, disrupt purine metabolism, and reduce cholesterol and fatty acid levels, indicating that GLDC can be used as a therapeutic target for MNA-NB (Alptekin et al., 2019).
3.7.3 Amino acid metabolism
Phosphoglycerate dehydrogenase (PHGDH) is the rate-limiting enzyme for serine synthesis. Isotope metabolomics have indicated that MNA-NB cells have a greater demand for serine synthesis than non-MNA-NB cells do. Serine/glycine starvation impairs only the nucleotide pool and proliferation of diploid MYCN cells and is ineffective against MYCN-amplified cells. PHGDH knockdown or the inhibitor NCT-503 inhibits MNA-NB cell proliferation. However, in the MNA-NB mouse PDX model, PHGDH inhibition antagonized the efficacy of cisplatin, suggesting that its therapeutic value is limited (Arlt et al., 2021). Moreover, the transcriptional activation of the serine metabolism pathway in MNA-NB cells requires MYCN and ATF4 to form a positive feedback loop, antagonizing Fbxw7-mediated N-Myc ubiquitination to stabilize the N-Myc (Xia et al., 2019).
Spermidine and spermine are the major polyamines whose levels are elevated in MNA-NBs, leading to the downregulation of the tumor suppressor Let-7 miRNA via the upregulation of LIN28 (Powers et al., 2016). Difluoromethylornithine (DFMO), an ornithine decarboxylase inhibitor, has been shown to reduce polyamine levels, restore the balance of the LIN28/Let-7 axis, and inhibit tumor growth (Jiang and Yu, 2024). DFMO also induces p27(Kip1)/Rb-dependent G1 arrest in MNA-NB cells through accumulation of the p27(Kip1) protein (Koomoa et al., 2013). Azfar et al. reported that ATPase 13A3 (ATP13A3) promoted polyamine uptake under the action of the polyamine inhibitor DFMO and that AMXT 1501 inhibited this effect. The combination of these two drugs may become a new treatment for NB (Azfar et al., 2025). Cherkaoui et al. reported that a diet without arginine/proline reduced the content of the polyamine precursor ornithine and enhanced the depletion of tumor polyamines by DFMO, leading to ribosome stalling at adenylate-terminal codons. Dietary restriction of upstream amino acid substrates has been shown to significantly improve survival in the TH-MYCN mouse model (Cherkaoui et al., 2025).
3.7.4 Glucose metabolism and cellular respiration
Exogenous overexpression of MYCN in NB cell lines can cause metabolic reprogramming of NB cells and sensitivity to the glycolysis inhibitor 2-deoxyglucose (Tjaden et al., 2020).
MYCN amplification in neuroblastoma induces structural changes in the mitochondrial network to adapt to altered energy demands (Casinelli et al., 2016). The antiprotozoal drug nifurtimox can increase oxidative stress in NB cell lines, reduce the activity of lactate dehydrogenase in anaerobic respiration, and reduce the expression of MYCN (Cabanillas Stanchi et al., 2015). Monocarboxylate transporters (MCTs) consist of four members (MCTs 1–4). Through metabolic targeted screening, Dalton et al. reported that MNA-NB cells are highly sensitive to the combined effects of the lactate transporter MCT1 inhibitor AZD3965 and complex I of the mitochondrial inhibitor phenformin, which can induce a sharp interruption in glycolysis and ATP production (Dalton et al., 2021).
The mitoribosomal inhibitor doxycycline can activate the mitochondrial stress response (MSR), inhibit protein synthesis, and promote N-Myc protein degradation. In MNA-NB cells, doxycycline can continuously reduce N-Myc levels and inhibit cell proliferation, and repeated administration does not induce drug resistance, indicating that it has a sustained therapeutic effect on MNA-NB (Borankova et al., 2023). Similarly, the mitochondrial uncoupler niclosamide ethanolamine (NEN) inhibits the growth of MNA-NB. NEN induces differentiation by disrupting mitochondrial metabolism and downregulating N-Myc, but its pharmacokinetic properties are poor and require further optimization (Byrne and Bell, 2023). The inclusion of NEN in the diet reduced the tumor growth rate and the expression of N-Myc and β-catenin in tumors in an orthotopic neuroblastoma mouse model (Jiang et al., 2023). ONC201, an inhibitor of the mitochondrial ClpXP proteases ClpP and ClpX, reduces the mitochondrial membrane potential of MNA-NB, and knocking down the ClpP and ClpX genes promotes neurite outgrowth. ONC201 treatment significantly reduces the expression of NYCN, promotes the differentiation of MNA-NB, and is a potential drug for the treatment of MNA-NB (Wu et al., 2023).
3.7.5 Ferroptosis
Ferroptosis is an iron dependent, lipid peroxidation-driven form of programmed cell death that is closely related to tumorigenesis, development, and treatment resistance (Liu et al., 2020; Su et al., 2020).
Cheng et al. combined data from the ArrayExpress database with the Gene Expression Omnibus and FerroptosisDB websites and reported that MYCN is a key gene in NB regulation (Cheng et al., 2024). The predictive model developed by Tan et al.can predict the prognosis of MNA-NB using ferroptosis-related intervention targets and is expected to become a new therapeutic target for MNA-NB (Tan et al., 2025). Using CRISPR screening, Alborzinia et al. reported that the selenoprotein P (SELENOP) receptor LRP8 protects MNA-NB cells by promoting selenocysteine synthesis and helping to produce the anti-ferroptotic GPX4 (Alborzinia et al., 2023). Selenocysteine lyase (SCLY) uses SELENOP to synthesize selenide as a substrate for selenophosphate synthase 2 (SEPHS2). The (H(2)SePO(3) (−)) synthesized by SEPHS2 is used as a substrate for the synthesis of Sec-tRNA. Chen et al. reported that peroxidase 6 (PRDX6) can interact with SEPHS2 independently of SCLY, providing a selenium delivery system. Moreover, increased RDX6 expression is significantly associated with a subtype of MNA-NB (Chen Z. et al., 2024).
Neuroblastomas in TH-MYCN mice show stronger glutathione anabolism than those in wild-type mice, and preventive treatment with the glutathione biosynthesis inhibitor buthionine sulfoximine (BSO) delays tumor growth (Carter et al., 2016). Alborzinia et al. reported that MNA-NB depends on cysteine metabolism and that its deficiency can induce ferroptosis. MNA-NB maintains glutathione (GSH) levels by regulating cysteine metabolism and transsulfurization. In a mouse model, inhibiting cysteine uptake, transsulfurization, and glutathione peroxidase 4 (GPX4) activity can inhibit NB growth (Floros et al., 2021). MYCN can also increase the expression of transferrin receptor 1 (TfR1, CD71), leading to the accumulation of intracellular divalent iron ions and increasing the sensitivity of MNA-NB to ferroptosis induction (Lu et al., 2021; Alborzinia et al., 2022). Gambogic acid (GA), the natural ligand of TfR1, can also induce MNA-NB apoptosis through the NK-IRE1-mTORC1 pathway (Bishayee et al., 2019).
3.8 Adjunctive therapy
Adjunctive approaches aim to enhance the efficacy of existing therapies for MNA-NB, particularly by overcoming resistance to retinoic acid (RA) and other conventional agents. For existing MNA-NB or existing broad-spectrum tumor chemotherapy drugs, several studies have aimed to increase the effectiveness of these agents.
3.8.1 Retinoic acid
Retinoic acid (RA) can bind to NB cells, reduce the level of N-Myc in NBs, induce NB differentiation, and inhibit proliferation (Haussler et al., 1983; Thiele et al., 1985). However, NB can be resistant to RA (Bates et al., 1989; Matsumoto et al., 1994).
Vasoactive intestinal peptide (VIP) significantly reduces MYCN expression in neuroblastoma cells (SH-SY5Y/IMR-32) and exhibits a synergistic inhibitory effect when combined with RA (Chevrier et al., 2008). Inhibition of transforming growth factor β (TGF-β) signaling is one of the mechanisms of MNA-NB resistance to retinoids. The combination of RA and the TGF-β activator kartogenin can inactivate RA-resistant MNA-NB cells (Duffy et al., 2017). Otsuka et al. reported that the tenascin-C-derived peptide TNIIIA2 combined with RA reduced neuroblastoma N-Myc protein levels, promoted differentiation, and inhibited tumor growth in a mouse model (Otsuka et al., 2019). BGA002 is a MYCN-specific antisense oligonucleotide that can synergistically inhibit MYCN when combined with RA. This combination therapy promotes autophagy by inhibiting the mTOR pathway and effectively inhibits tumor angiogenesis in the MNA-NB mouse model, significantly prolonging survival (Lampis et al., 2022). Broso et al. reported that isorhamnetin (ISR) synergistically inhibited MNA-NB cell viability by upregulating ADRA1B expression and retinoid isotretinoin. The α1 antagonist doxazosin also enhanced the antitumor effect of RA in a transplanted tumor model, indicating that inhibition of α-adrenaline receptors can enhance the growth inhibition and differentiation-promoting effects of RA (Broso et al., 2023). The aryl hydrocarbon receptor (AhR) inhibitor clofazimine can synergize with RA both in vivo and in vitro to induce MNA-NB differentiation (Chaudhry et al., 2023). RA induces the expression of the RA-metabolizing enzymes CYP26A1 and CYP26B1 in both MYCN-amplified Kelly and MYCN-nonamplified SH-SY5Y cells. RA combined with the selective CYP26 inhibitor talarozole or the RA-degrading enzyme CYP3A inhibitor ketoconazole can significantly reduce cell viability. The combination of RA with metabolic or HGF signaling pathway inhibitors may prevent the development of RA-resistant neuroblastoma (Issa et al., 2024).
4 Conclusion and prospects
MYCN plays a very important role in NB as a proto-oncogene. For clinicians and basic researchers, the undruggable nature of MYCN is a difficult problem to address. Fortunately, some research progress has been made on molecules directly related to MYCN.
Pandher et al. reported that the MYCN selective inhibitor M606 reduced N-Myc levels by binding to its promoter, upregulated hypoxia-inducible factor 1 alpha (HIF1A), and delayed the progression of NB in TH-MYCN mice (Pandher et al., 2025). The small molecule inhibitor 10058-F4 and its analogs developed by Müller et al. can bind to the C-Myc bHLHZip dimerization domain and inhibit the C-Myc/MAX interaction, inducing the apoptosis of MNA-NB cells (Müller et al., 2014). MYCMI-7, developed by Castel et al., can specifically block Myc/MAX and N-Myc/MAX interactions. In the MNA-NB mouse model, the compound can downregulate MYC/MYCN expression, induce apoptosis, and inhibit tumor growth with low toxicity (Castell et al., 2022). Yang et al. used small-molecule microarrays (SMMs) to identify a hairpin-containing G4 structure that targets the n-myc protein MY-8. MY-8 can reduce the level of N-Myc in MNA-NB cells (Yang et al., 2021). Quarfloxin and CX-5461 downregulate MYCN and activate p53 in NB cells through the inhibition of RNA polymerase I, leading to cell cycle arrest and apoptosis (Hald et al., 2019). Affinity proteomics and molecular docking-based studies have revealed that ceftriaxone inhibits N-Myc translation by targeting DEAD-box helicase 3 X-linked (DDX3X), thereby inducing apoptosis in MYCN-amplified retinoblastoma and neuroblastoma cells (Chittavanich et al., 2024). Indisulam, a splicing modulator that induces RBM39 degradation, shows strong activity in MNA-NB, including TH-MYCN models, and may enhance anti-GD2 immunotherapy in preclinical studies (Singh et al., 2021; Nijhuis et al., 2022).
PROTAC technology was first proposed by Sakamoto et al., in 2001 (Sakamoto et al., 2001). PROTAC usually consists of a ligand (mostly a small-molecule inhibitor) of the protein of interest and a covalently linked ligand of an E3 ubiquitin ligase (E3). E3 ubiquitin ligases recruit E3 to ubiquitinate and degrade the target protein (Zou et al., 2019). Currently, the PROTAC drug Vepdegestrant, which targets estrogen receptors in breast cancer, has completed phase III clinical trials (Campone et al., 2025), and PROTAC drugs such as BGB-16673, which target BTK in chronic lymphocytic leukemia, have entered phase III clinical trials (Li Z. et al., 2025). PROTAC drugs have become a research hotspot for targeting cancer-related proteins that cannot be drugged by traditional drug development methods (Li and Song, 2020; Wang et al., 2020). There are currently no PROTAC small molecules that directly target MYC family proteins. However, PROTAC molecules for BRD4 and Aurora A, which are closely related to MYCN, have been studied.
In addition to conventional small-molecule inhibitors, PROTAC technology offers a promising approach for targeting the AURKA–N-Myc axis. Recently developed AURKA-directed PROTACs have shown potent ability to degrade AURKA (Adhikari et al., 2020; Liu F. et al., 2022), disrupt its interaction with N-Myc, and reduced Aurora-A and N-Myc levels in MNA-NB cells and xenograft NB model (Nelson et al., 2025; Tang et al., 2025). These findings highlight the translational potential of AURKA PROTACs as next-generation therapeutics for MNA-NB, warranting further preclinical development and early-phase clinical trials.
The PROTAC molecule dBET57, which targets BRD4, degrades the BET protein family and MYCN through CRBN-mediated ubiquitination, effectively inhibiting the MYCN SE regulatory genes TBX3 and ZMYND8 in the MNA-NB xenograft model and showing therapeutic potential (Jia et al., 2022). Zhang et al. reported that MZ1 is also a PROTAC inducer that targets BET. MZ1 can inhibit MNA-NB cell proliferation and the normal cell cycle and promote apoptosis (Zhang et al., 2022). Another BET-targeting PROTAC inducer, ARV-825, effectively reduces the expression of the BET protein, thereby inhibiting the expression of MYCN and suppressing tumor growth in PDX mice (Li et al., 2020).
Although significant progress has been made in the fields of ubiquitination, epigenetic regulation, and small-molecule inhibitors that target MYCN, the treatment of MNA-NB still faces challenges in terms of drug resistance and targeting efficiency. Small-molecule inhibitors targeting the N-Myc complex provide new ideas at the level of transcriptional regulation, and the emerging PROTAC technology has shown breakthrough therapeutic potential through the selective degradation of key MYCN cofactors. Future research should focus on the following directions: optimizing the tumor-targeting ability of PROTACs to reduce off-target effects; combining the targeting of MYCN upstream and downstream pathways to overcome drug resistance; and exploring specific binding sites of the dynamic structure of the N-Myc and developing more selective inhibitors. The integration of multimodal strategies may provide new directions for the precision treatment of MNA-NB.
Author contributions
YC: Methodology, Writing – review and editing, Writing – original draft. HY: Writing – original draft, Data curation. LX: Writing – original draft, Data curation. NY: Writing – review and editing, Supervision. MZ: Writing – original draft, Writing – review and editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Key Research and Development Project of Sichuan Science and Technology Department (Grant No. 024YFFK0379).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abu-Zaid, A., Fang, J., Jin, H., Singh, S., Pichavaram, P., Wu, Q., et al. (2024). Histone lysine demethylase 4 family proteins maintain the transcriptional program and adrenergic cellular state of MYCN-amplified neuroblastoma. Cell Rep. Med. 5, 101468. doi:10.1016/j.xcrm.2024.101468
Adhikari, B., Bozilovic, J., Diebold, M., Schwarz, J. D., Hofstetter, J., Schröder, M., et al. (2020). PROTAC-mediated degradation reveals a non-catalytic function of AURORA-A kinase. Nat. Chem. Biol. 16, 1179–1188. doi:10.1038/s41589-020-00652-y
Agarwal, S., Milazzo, G., Rajapakshe, K., Bernardi, R., Chen, Z., Barbieri, E., et al. (2018). MYCN acts as a direct co-regulator of p53 in MYCN amplified neuroblastoma. Oncotarget 9, 20323–20338. doi:10.18632/oncotarget.24859
Alborzinia, H., Flórez, A. F., Kreth, S., Brückner, L. M., Yildiz, U., Gartlgruber, M., et al. (2022). MYCN mediates cysteine addiction and sensitizes neuroblastoma to ferroptosis. Nat. Cancer 3, 471–485. doi:10.1038/s43018-022-00355-4
Alborzinia, H., Chen, Z., Yildiz, U., Freitas, F. P., Vogel, F. C. E., Varga, J. P., et al. (2023). LRP8-mediated selenocysteine uptake is a targetable vulnerability in MYCN-amplified neuroblastoma. EMBO Mol. Med. 15, e18014. doi:10.15252/emmm.202318014
Aljouda, N. A., Shrestha, D., DeVaux, C., Olsen, R. R., Alleboina, S., Walker, M., et al. (2025). Transcription factor 4 is a key mediator of oncogenesis in neuroblastoma by promoting MYC activity. Mol. Oncol. 19, 808–824. doi:10.1002/1878-0261.13714
Alptekin, A., Ye, B., Yu, Y., Poole, C. J., van Riggelen, J., Zha, Y., et al. (2019). Glycine decarboxylase is a transcriptional target of MYCN required for neuroblastoma cell proliferation and tumorigenicity. Oncogene 38, 7504–7520. doi:10.1038/s41388-019-0967-3
Amente, S., Milazzo, G., Sorrentino, M. C., Ambrosio, S., Di Palo, G., Lania, L., et al. (2015). Lysine-specific demethylase (LSD1/KDM1A) and MYCN cooperatively repress tumor suppressor genes in neuroblastoma. Oncotarget 6, 14572–14583. doi:10.18632/oncotarget.3990
Arlt, B., Zasada, C., Baum, K., Wuenschel, J., Mastrobuoni, G., Lodrini, M., et al. (2021). Inhibiting phosphoglycerate dehydrogenase counteracts chemotherapeutic efficacy against MYCN-amplified neuroblastoma. Int. J. Cancer 148, 1219–1232. doi:10.1002/ijc.33423
Azfar, M., Gao, W., Van den Haute, C., Xiao, L., Karsa, M., Pandher, R., et al. (2025). The polyamine transporter ATP13A3 mediates difluoromethylornithine-induced polyamine uptake in neuroblastoma. Mol. Oncol. 19, 913–936. doi:10.1002/1878-0261.13789
Bagatell, R., Park, J. R., Acharya, S., Aldrink, J., Allison, J., Alva, E., et al. (2024). Neuroblastoma, version 2.2024, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 22, 413–433. doi:10.6004/jnccn.2024.0040
Bate-Eya, L. T., Albayrak, G., Carr, S. M., Shrestha, A., Kanapin, A., Samsonova, A., et al. (2025). Sustained cancer-relevant alternative RNA splicing events driven by PRMT5 in high-risk neuroblastoma. Mol. Oncol. 19, 741–763. doi:10.1002/1878-0261.13702
Bates, S. E., Mickley, L. A., Chen, Y. N., Richert, N., Rudick, J., Biedler, J. L., et al. (1989). Expression of a drug resistance gene in human neuroblastoma cell lines: modulation by retinoic acid-induced differentiation. Mol. Cell. Biol. 9, 4337–4344. doi:10.1128/mcb.9.10.4337-4344.1989
Becker, J., Volland, S., Noskova, I., Schramm, A., Schweigerer, L. L., and Wilting, J. (2008). Keratoepithelin reverts the suppression of tissue factor pathway inhibitor 2 by MYCN in human neuroblastoma: a mechanism to inhibit invasion. Int. J. Oncol. 32, 235–240. doi:10.3892/ijo.32.1.235
Beckers, A., Van Peer, G., Carter, D. R., Gartlgruber, M., Herrmann, C., Agarwal, S., et al. (2015a). MYCN-driven regulatory mechanisms controlling LIN28B in neuroblastoma. Cancer Lett. 366, 123–132. doi:10.1016/j.canlet.2015.06.015
Beckers, A., Van Peer, G., Carter, D. R., Mets, E., Althoff, K., Cheung, B. B., et al. (2015b). MYCN-targeting miRNAs are predominantly downregulated during MYCN-driven neuroblastoma tumor formation. Oncotarget 6, 5204–5216. doi:10.18632/oncotarget.2477
Bell, E., Chen, L., Liu, T., Marshall, G. M., Lunec, J., and Tweddle, D. A. (2010). MYCN oncoprotein targets and their therapeutic potential. Cancer Lett. 293, 144–157. doi:10.1016/j.canlet.2010.01.015
Bellamy, J., Szemes, M., Melegh, Z., Dallosso, A., Kollareddy, M., Catchpoole, D., et al. (2020). Increased efficacy of histone methyltransferase G9a inhibitors against MYCN-amplified neuroblastoma. Front. Oncol. 10, 818. doi:10.3389/fonc.2020.00818
Bishayee, K., Habib, K., Sadra, A., and Huh, S.-O. (2019). Targeting the difficult-to-drug CD71 and MYCN with gambogic acid and vorinostat in a class of neuroblastomas. Cell. Physiol. Biochem. 53, 258–280. doi:10.33594/000000134
Boboila, S., Lopez, G., Yu, J., Banerjee, D., Kadenhe-Chiweshe, A., Connolly, E. P., et al. (2018). Transcription factor activating protein 4 is synthetically lethal and a master regulator of MYCN-amplified neuroblastoma. Oncogene 37, 5451–5465. doi:10.1038/s41388-018-0326-9
Boboila, S., Okochi, S., Banerjee, D., Barton, S., Street, C., Zenilman, A. L., et al. (2023). Combining immunotherapy with high-dose radiation therapy (HDRT) significantly inhibits tumor growth in a syngeneic mouse model of high-risk neuroblastoma. Heliyon 9, e17399. doi:10.1016/j.heliyon.2023.e17399
Boi, D., Souvalidou, F., Capelli, D., Polverino, F., Marini, G., Montanari, R., et al. (2021). PHA-680626 is an effective inhibitor of the interaction between Aurora-A and N-Myc. Int. J. Mol. Sci. 22, 13122. doi:10.3390/ijms222313122
Bojko, J., Kollareddy, M., Szemes, M., Bellamy, J., Poon, E., Moukachar, A., et al. (2024). Spliceosomal vulnerability of MYCN-amplified neuroblastoma is contingent on PRMT5-mediated regulation of epitranscriptomic and metabolomic pathways. Cancer Lett. 604, 217263. doi:10.1016/j.canlet.2024.217263
Bonnal, S. C., López-Oreja, I., and Valcárcel, J. (2020). Roles and mechanisms of alternative splicing in cancer - implications for care. Nat. Rev. Clin. Oncol. 17, 457–474. doi:10.1038/s41571-020-0350-x
Borankova, K., Krchniakova, M., Leck, L. Y. W., Kubistova, A., Neradil, J., Jansson, P. J., et al. (2023). Mitoribosomal synthetic lethality overcomes multidrug resistance in MYC-driven neuroblastoma. Cell Death Dis. 14, 747. doi:10.1038/s41419-023-06278-x
Borenäs, M., Umapathy, G., Lai, W.-Y., Lind, D. E., Witek, B., Guan, J., et al. (2021). ALK ligand ALKAL2 potentiates MYCN-driven neuroblastoma in the absence of ALK mutation. EMBO J. 40, e105784. doi:10.15252/embj.2020105784
Brandetti, E., Veneziani, I., Melaiu, O., Pezzolo, A., Castellano, A., Boldrini, R., et al. (2017). MYCN is an immunosuppressive oncogene dampening the expression of ligands for NK-cell-activating receptors in human high-risk neuroblastoma. Oncoimmunology 6, e1316439. doi:10.1080/2162402X.2017.1316439
Brockmann, M., Poon, E., Berry, T., Carstensen, A., Deubzer, H. E., Rycak, L., et al. (2013). Small molecule inhibitors of aurora-a induce proteasomal degradation of N-myc in childhood neuroblastoma. Cancer Cell 24, 75–89. doi:10.1016/j.ccr.2013.05.005
Broso, F., Gatto, P., Sidarovich, V., Ambrosini, C., De Sanctis, V., Bertorelli, R., et al. (2023). Alpha-1 adrenergic antagonists sensitize neuroblastoma to therapeutic differentiation. Cancer Res. 83, 2733–2749. doi:10.1158/0008-5472.CAN-22-1913
Buechner, J., Henriksen, J. R., Haug, B. H., Tømte, E., Flaegstad, T., and Einvik, C. (2011a). Inhibition of mir-21, which is up-regulated during MYCN knockdown-mediated differentiation, does not prevent differentiation of neuroblastoma cells. Differentiation 81, 25–34. doi:10.1016/j.diff.2010.09.184
Buechner, J., Tømte, E., Haug, B. H., Henriksen, J. R., Løkke, C., Flægstad, T., et al. (2011b). Tumour-suppressor microRNAs let-7 and mir-101 target the proto-oncogene MYCN and inhibit cell proliferation in MYCN-amplified neuroblastoma. Br. J. Cancer 105, 296–303. doi:10.1038/bjc.2011.220
Bumpous, L. A., Moe, K. C., Wang, J., Carver, L. A., Williams, A. G., Romer, A. S., et al. (2023). WDR5 facilitates recruitment of N-MYC to conserved WDR5 gene targets in neuroblastoma cell lines. Oncogenesis 12, 32. doi:10.1038/s41389-023-00477-z
Burmakin, M., Shi, Y., Hedström, E., Kogner, P., and Selivanova, G. (2013). Dual targeting of wild-type and mutant p53 by small molecule RITA results in the inhibition of N-Myc and key survival oncogenes and kills neuroblastoma cells in vivo and in vitro. Clin. Cancer Res. 19, 5092–5103. doi:10.1158/1078-0432.CCR-12-2211
Bushweller, J. H. (2019). Targeting transcription factors in cancer - from undruggable to reality. Nat. Rev. Cancer 19, 611–624. doi:10.1038/s41568-019-0196-7
Byrne, F. L., and Bell, J. L. (2023). Neuroblastoma differentiation: the untapped potential of mitochondrial uncouplers. Cancer Res. 83, 167–169. doi:10.1158/0008-5472.CAN-22-3350
Cabanillas Stanchi, K. M., Bruchelt, G., Handgretinger, R., and Holzer, U. (2015). Nifurtimox reduces N-Myc expression and aerobic glycolysis in neuroblastoma. Cancer Biol. Ther. 16, 1353–1363. doi:10.1080/15384047.2015.1070987
Caforio, M., Sorino, C., Caruana, I., Weber, G., Camera, A., Cifaldi, L., et al. (2021). GD2 redirected CAR T and activated NK-cell-mediated secretion of IFNγ overcomes MYCN-dependent IDO1 inhibition, contributing to neuroblastoma cell immune escape. J. Immunother. Cancer 9, e001502. doi:10.1136/jitc-2020-001502
Cage, T. A., Chanthery, Y., Chesler, L., Grimmer, M., Knight, Z., Shokat, K., et al. (2015). Downregulation of MYCN through PI3K inhibition in mouse models of pediatric neural cancer. Front. Oncol. 5, 111. doi:10.3389/fonc.2015.00111
Calao, M., Sekyere, E. O., Cui, H. J., Cheung, B. B., Thomas, W. D., Keating, J., et al. (2013). Direct effects of Bmi1 on p53 protein stability inactivates oncoprotein stress responses in embryonal cancer precursor cells at tumor initiation. Oncogene 32, 3616–3626. doi:10.1038/onc.2012.368
Calero, R., Morchon, E., Johnsen, J. I., and Serrano, R. (2014). Sunitinib suppress neuroblastoma growth through degradation of MYCN and inhibition of angiogenesis. PLoS One 9, e95628. doi:10.1371/journal.pone.0095628
Campbell, K., Shyr, D., Bagatell, R., Fischer, M., Nakagawara, A., Nieto, A. C., et al. (2019). Comprehensive evaluation of context dependence of the prognostic impact of MYCN amplification in neuroblastoma: a report from the international neuroblastoma risk group (INRG) project. Pediatr. Blood Cancer 66, e27819. doi:10.1002/pbc.27819
Campone, M., De Laurentiis, M., Jhaveri, K., Hu, X., Ladoire, S., Patsouris, A., et al. (2025). Vepdegestrant, a PROTAC estrogen receptor degrader, in advanced breast cancer. N. Engl. J. Med. 393, 556–568. doi:10.1056/NEJMoa2505725
Carter, D. R., Sutton, S. K., Pajic, M., Murray, J., Sekyere, E. O., Fletcher, J., et al. (2016). Glutathione biosynthesis is upregulated at the initiation of MYCN-driven neuroblastoma tumorigenesis. Mol. Oncol. 10, 866–878. doi:10.1016/j.molonc.2016.02.004
Casinelli, G., LaRosa, J., Sharma, M., Cherok, E., Banerjee, S., Branca, M., et al. (2016). N-Myc overexpression increases cisplatin resistance in neuroblastoma via deregulation of mitochondrial dynamics. Cell Death Discov. 2, 16082. doi:10.1038/cddiscovery.2016.82
Castell, A., Yan, Q., Fawkner, K., Bazzar, W., Zhang, F., Wickström, M., et al. (2022). MYCMI-7: a small MYC-binding compound that inhibits MYC: MAX interaction and tumor growth in a MYC-dependent manner. Cancer Res. Commun. 2, 182–201. doi:10.1158/2767-9764.CRC-21-0019
Cervantes, A., Elez, E., Roda, D., Ecsedy, J., Macarulla, T., Venkatakrishnan, K., et al. (2012). Phase I pharmacokinetic/pharmacodynamic study of MLN8237, an investigational, oral, selective aurora a kinase inhibitor, in patients with advanced solid tumors. Clin. Cancer Res. 18, 4764–4774. doi:10.1158/1078-0432.CCR-12-0571
Chanthery, Y. H., Gustafson, W. C., Itsara, M., Persson, A., Hackett, C. S., Grimmer, M., et al. (2012). Paracrine signaling through MYCN enhances tumor-vascular interactions in neuroblastoma. Sci. Transl. Med. 4, 115ra3. doi:10.1126/scitranslmed.3002977
Chaudhry, K. A., Jacobi, J. J., Gillard, B. M., Karasik, E., Martin, J. C., da Silva Fernandes, T., et al. (2023). Aryl hydrocarbon receptor is a tumor promoter in MYCN-amplified neuroblastoma cells through suppression of differentiation. iScience 26, 108303. doi:10.1016/j.isci.2023.108303
Chava, S., Reynolds, C. P., Pathania, A. S., Gorantla, S., Poluektova, L. Y., Coulter, D. W., et al. (2020). miR-15a-5p, miR-15b-5p, and miR-16-5p inhibit tumor progression by directly targeting MYCN in neuroblastoma. Mol. Oncol. 14, 180–196. doi:10.1002/1878-0261.12588
Chugh, S., Tien, J. C., Hon, J., Kenum, C., Mannan, R., Cheng, Y., et al. (2025). Therapeutic benefit of the dual ALK/FAK inhibitor ESK440 in ALK-driven neuroblastoma. Neoplasia 60, 100964. doi:10.1016/j.neo.2024.100964
Chen, Z., Lin, Y., Barbieri, E., Burlingame, S., Hicks, J., Ludwig, A., et al. (2009). Mdm2 deficiency suppresses MYCN-driven neuroblastoma tumorigenesis in vivo. Neoplasia 11, 753–762. doi:10.1593/neo.09466
Chen, C., Breslin, M. B., and Lan, M. S. (2015). INSM1 increases N-myc stability and oncogenesis via a positive-feedback loop in neuroblastoma. Oncotarget 6, 36700–36712. doi:10.18632/oncotarget.5485
Chen, L., Alexe, G., Dharia, N. V., Ross, L., Iniguez, A. B., Conway, A. S., et al. (2018). CRISPR-Cas9 screen reveals a MYCN-amplified neuroblastoma dependency on EZH2. J. Clin. Invest. 128, 446–462. doi:10.1172/JCI90793
Chen, J., Sun, M., Huang, L., and Fang, Y. (2022). The long noncoding RNA LINC00200 promotes the malignant progression of MYCN-amplified neuroblastoma via binding to insulin like growth factor 2 mRNA binding protein 3 (IGF2BP3) to enhance the stability of Zic family member 2 (ZIC2) mRNA. Pathol. Res. Pract. 237, 154059. doi:10.1016/j.prp.2022.154059
Chen, C., Wu, J., Hicks, C., and Lan, M. S. (2023). Repurposing a plant alkaloid homoharringtonine targets insulinoma associated-1 in N-Myc-activated neuroblastoma. Cell. Signal. 109, 110753. doi:10.1016/j.cellsig.2023.110753
Chen, X., Ouyang, L., Ke, N., Pi, L., and Zhou, X. (2024a). Study on the role of MYCN in retinoblastoma by inhibiting p53 and activating wnt/βcatenin/Fra-1 signaling pathway by reducing DKK3. Drug Dev. Res. 85, e22222. doi:10.1002/ddr.22222
Chen, Y., Zhuo, R., Sun, L., Tao, Y., Li, G., Zhu, F., et al. (2024b). Super-enhancer-driven IRF2BP2 enhances ALK activity and promotes neuroblastoma cell proliferation. Neuro. Oncol. 26, 1878–1894. doi:10.1093/neuonc/noae109
Chen, Z., Inague, A., Kaushal, K., Fazeli, G., Schilling, D., Xavier da Silva, T. N., et al. (2024c). PRDX6 contributes to selenocysteine metabolism and ferroptosis resistance. Mol. Cell 84, 4645–4659.e9. doi:10.1016/j.molcel.2024.10.027
Cheng, C., He, T., Chen, K., Cai, Y., Gu, Y., Pan, L., et al. (2023). P300 interacted with N-Myc and regulated its protein stability via altering its post-translational modifications in neuroblastoma. Mol. Cell. Proteomics 22, 100504. doi:10.1016/j.mcpro.2023.100504
Cheng, J., Dong, X., Yang, Y., Qin, X., Zhou, X., and Zhang, D. (2024). Synergistic machine learning models utilizing ferroptosis-related genes for improved neuroblastoma outcome prediction. Transl. Pediatr. 13, 2164–2182. doi:10.21037/tp-24-323
Cherkaoui, S., Turn, C. S., Yuan, Y., Lu, W., Yang, L., McBride, M. J., et al. (2025). Reprogramming neuroblastoma by diet-enhanced polyamine depletion. Nature. doi:10.1038/s41586-025-09564-0
Cheung, I. Y., Mauguen, A., Modak, S., Ragupathi, G., Basu, E. M., Roberts, S. S., et al. (2023). Effect of oral β-glucan on antibody response to ganglioside vaccine in patients with high-risk neuroblastoma: a phase 2 randomized clinical trial. JAMA Oncol. 9, 242–250. doi:10.1001/jamaoncol.2022.5999
Chevrier, L., Meunier, A.-C., Cochaud, S., Muller, J.-M., and Chadéneau, C. (2008). Vasoactive intestinal peptide decreases MYCN expression and synergizes with retinoic acid in a human MYCN-amplified neuroblastoma cell line. Int. J. Oncol. 33, 1081–1089. doi:10.3892/ijo_00000097
Chiangjong, W., Panachan, J., Keadsanti, S., Newburg, D. S., Morrow, A. L., Hongeng, S., et al. (2024). Development of red blood cell-derived extracellular particles as a biocompatible nanocarrier of microRNA-204 (REP-204) to harness anti-neuroblastoma effect. Nanomedicine 60, 102760. doi:10.1016/j.nano.2024.102760
Chilamakuri, R., and Agarwal, S. (2022). Dual targeting of PI3K and HDAC by CUDC-907 inhibits pediatric neuroblastoma growth. Cancers (Basel) 14, 1067. doi:10.3390/cancers14041067
Chipumuro, E., Marco, E., Christensen, C. L., Kwiatkowski, N., Zhang, T., Hatheway, C. M., et al. (2014). CDK7 inhibition suppresses super-enhancer-linked oncogenic transcription in MYCN-driven cancer. Cell 159, 1126–1139. doi:10.1016/j.cell.2014.10.024
Chittavanich, P., Saengwimol, D., Roytrakul, S., Rojanaporn, D., Chaitankar, V., Srimongkol, A., et al. (2024). Ceftriaxone exerts antitumor effects in MYCN-driven retinoblastoma and neuroblastoma by targeting DDX3X for translation repression. Mol. Oncol. 18, 918–938. doi:10.1002/1878-0261.13553
Cortés, C., Kozma, S. C., Tauler, A., and Ambrosio, S. (2015). MYCN concurrence with SAHA-induced cell death in human neuroblastoma cells. Cell. Oncol. 38, 341–352. doi:10.1007/s13402-015-0233-9
Dalton, K. M., Lochmann, T. L., Floros, K. V., Calbert, M. L., Kurupi, R., Stein, G. T., et al. (2021). Catastrophic ATP loss underlies a metabolic combination therapy tailored for MYCN-amplified neuroblastoma. Proc. Natl. Acad. Sci. U. S. A. 118, e2009620118. doi:10.1073/pnas.2009620118
Del Bufalo, F., De Angelis, B., Caruana, I., Del Baldo, G., De Ioris, M. A., Serra, A., et al. (2023). GD2-CART01 for relapsed or refractory high-risk neuroblastoma. N. Engl. J. Med. 388, 1284–1295. doi:10.1056/NEJMoa2210859
Delehouzé, C., Godl, K., Loaëc, N., Bruyère, C., Desban, N., Oumata, N., et al. (2014). CDK/CK1 inhibitors roscovitine and CR8 downregulate amplified MYCN in neuroblastoma cells. Oncogene 33, 5675–5687. doi:10.1038/onc.2013.513
Desai, A. V., Gilman, A. L., Ozkaynak, M. F., Naranjo, A., London, W. B., Tenney, S. C., et al. (2022). Outcomes following GD2-directed postconsolidation therapy for neuroblastoma after cessation of random assignment on ANBL0032: a report from the children’s oncology group. J. Clin. Oncol. 40, 4107–4118. doi:10.1200/JCO.21.02478
Dietzsch, E., Lukeis, R. E., Vrazas, V., Hasthorpe, S., and Garson, O. M. (1994). Characterization of homogeneously staining regions in a small cell lung cancer cell line, using in situ hybridization with an MYCN probe. Genes Chromosom. Cancer 10, 213–216. doi:10.1002/gcc.2870100312
Ding, Y., Yang, J., Ma, Y., Yao, T., Chen, X., Ge, S., et al. (2019). MYCN and PRC1 cooperatively repress docosahexaenoic acid synthesis in neuroblastoma via ELOVL2. J. Exp. Clin. Cancer Res. 38, 498. doi:10.1186/s13046-019-1492-5
Du, B., Zhang, Y., Zhang, P., Zhang, M., Yu, Z., Li, L., et al. (2024). Joint metabolomics and transcriptomics analysis systematically reveal the impact of MYCN in neuroblastoma. Sci. Rep. 14, 20155. doi:10.1038/s41598-024-71211-x
DuBois, S. G., Mosse, Y. P., Fox, E., Kudgus, R. A., Reid, J. M., McGovern, R., et al. (2018). Phase II trial of alisertib in combination with irinotecan and temozolomide for patients with relapsed or refractory neuroblastoma. Clin. Cancer Res. 24, 6142–6149. doi:10.1158/1078-0432.CCR-18-1381
DuBois, S. G., Granger, M. M., Groshen, S., Tsao-Wei, D., Ji, L., Shamirian, A., et al. (2021). Randomized phase II trial of MIBG versus MIBG, vincristine, and irinotecan versus MIBG and vorinostat for patients with relapsed or refractory neuroblastoma: a report from NANT consortium. J. Clin. Oncol. 39, 3506–3514. doi:10.1200/JCO.21.00703
Duffy, D. J., Krstic, A., Halasz, M., Schwarzl, T., Konietzny, A., Iljin, K., et al. (2017). Retinoic acid and TGF-β signalling cooperate to overcome MYCN-induced retinoid resistance. Genome Med. 9, 15. doi:10.1186/s13073-017-0407-3
Dzieran, J., Rodriguez Garcia, A., Westermark, U. K., Henley, A. B., Eyre Sánchez, E., Träger, C., et al. (2018). MYCN-amplified neuroblastoma maintains an aggressive and undifferentiated phenotype by deregulation of estrogen and NGF signaling. Proc. Natl. Acad. Sci. U. S. A. 115, E1229–E1238. doi:10.1073/pnas.1710901115
Eberhardt, A., Hansen, J. N., Koster, J., Lotta, L. T., Wang, S., Livingstone, E., et al. (2016). Protein arginine methyltransferase 1 is a novel regulator of MYCN in neuroblastoma. Oncotarget 7, 63629–63639. doi:10.18632/oncotarget.11556
Fabian, J., Lodrini, M., Oehme, I., Schier, M. C., Thole, T. M., Hielscher, T., et al. (2014). GRHL1 acts as tumor suppressor in neuroblastoma and is negatively regulated by MYCN and HDAC3. Cancer Res. 74, 2604–2616. doi:10.1158/0008-5472.CAN-13-1904
Fabian, J., Opitz, D., Althoff, K., Lodrini, M., Hero, B., Volland, R., et al. (2016). MYCN and HDAC5 transcriptionally repress CD9 to trigger invasion and metastasis in neuroblastoma. Oncotarget 7, 66344–66359. doi:10.18632/oncotarget.11662
Felipe, I., Martínez-de-Villarreal, J., Patel, K., Martínez-Torrecuadrada, J., Grossmann, L. D., Roncador, G., et al. (2024). BPTF cooperates with MYCN and MYC to link neuroblastoma cell cycle control to epigenetic cellular states. bioRxiv, 2024.02.11.579816. doi:10.1101/2024.02.11.579816
Feng, C., Wang, T., Tang, R., Wang, J., Long, H., Gao, X., et al. (2010). Silencing of the MYCN gene by siRNA delivered by folate receptor-targeted liposomes in LA-N-5 cells. Pediatr. Surg. Int. 26, 1185–1191. doi:10.1007/s00383-010-2703-5
Feriancikova, B., Feglarova, T., Krskova, L., Eckschlager, T., Vicha, A., and Hrabeta, J. (2021). MIAT is an upstream regulator of NMYC and the disruption of the MIAT/NMYC axis induces cell death in NMYC amplified neuroblastoma cell lines. Int. J. Mol. Sci. 22, 3393. doi:10.3390/ijms22073393
Ferrucci, F., Ciaccio, R., Monticelli, S., Pigini, P., di Giacomo, S., Purgato, S., et al. (2018). MAX to MYCN intracellular ratio drives the aggressive phenotype and clinical outcome of high risk neuroblastoma. Biochim. Biophys. Acta Gene Regul. Mech. 1861, 235–245. doi:10.1016/j.bbagrm.2018.01.007
Fitieh, A., Locke, A. J., Motamedi, M., and Ismail, I. H. (2021). The role of polycomb group protein BMI1 in DNA repair and genomic stability. Int. J. Mol. Sci. 22, 2976. doi:10.3390/ijms22062976
Floros, K. V., Cai, J., Jacob, S., Kurupi, R., Fairchild, C. K., Shende, M., et al. (2021). MYCN-amplified neuroblastoma is addicted to iron and vulnerable to inhibition of the system Xc-/glutathione axis. Cancer Res. 81, 1896–1908. doi:10.1158/0008-5472.CAN-20-1641
Foster, J. H., Voss, S. D., Hall, D. C., Minard, C. G., Balis, F. M., Wilner, K., et al. (2021). Activity of crizotinib in patients with ALK-aberrant relapsed/refractory neuroblastoma: a children’s oncology group study (ADVL0912). Clin. Cancer Res. 27, 3543–3548. doi:10.1158/1078-0432.CCR-20-4224
Gamble, L. D., Kees, U. R., Tweddle, D. A., and Lunec, J. (2012). MYCN sensitizes neuroblastoma to the MDM2-p53 antagonists Nutlin-3 and MI-63. Oncogene 31, 752–763. doi:10.1038/onc.2011.270
Gao, Y., Volegova, M., Nasholm, N., Das, S., Kwiatkowski, N., Abraham, B. J., et al. (2021). Synergistic anti-tumor effect of combining selective CDK7 and BRD4 inhibition in neuroblastoma. Front. Oncol. 11, 773186. doi:10.3389/fonc.2021.773186
García, D. A., Briceño, I., Castillo, M., and Aristizábal, F. A. (2024). Detection of gene amplification in MYCN, C-MYC, MYCL1, ERBB2, EGFR, AKT2, and human papilloma virus in samples from cervical smear normal cytology, intraepithelial cervical neoplasia (CIN I, II, III), and cervical cancer. Colomb. Med. 42, 144–153. doi:10.25100/cm.v42i2.765
Goldsmith, K. C., Park, J. R., Kayser, K., Malvar, J., Chi, Y.-Y., Groshen, S. G., et al. (2023). Lorlatinib with or without chemotherapy in ALK-driven refractory/relapsed neuroblastoma: phase 1 trial results. Nat. Med. 29, 1092–1102. doi:10.1038/s41591-023-02297-5
Grunewald, L., Andersch, L., Helmsauer, K., Schwiebert, S., Klaus, A., Henssen, A. G., et al. (2025). Targeting MYCN upregulates L1CAM tumor antigen in MYCN-dysregulated neuroblastoma to increase CAR T cell efficacy. Pharmacol. Res. 212, 107608. doi:10.1016/j.phrs.2025.107608
Gu, L., Zhang, H., He, J., Li, J., Huang, M., and Zhou, M. (2012). MDM2 regulates MYCN mRNA stabilization and translation in human neuroblastoma cells. Oncogene 31, 1342–1353. doi:10.1038/onc.2011.343
Guan, J., Li, M., Wang, Y., Zhang, Y., Que, Y., Lu, S., et al. (2024). MTHFD1 regulates the NADPH redox homeostasis in MYCN-amplified neuroblastoma. Cell Death Dis. 15, 124. doi:10.1038/s41419-024-06490-3
Guo, Y.-F., Duan, J.-J., Wang, J., Li, L., Wang, D., Liu, X.-Z., et al. (2020). Inhibition of the ALDH18A1-MYCN positive feedback loop attenuates MYCN-amplified neuroblastoma growth. Sci. Transl. Med. 12, eaax8694. doi:10.1126/scitranslmed.aax8694
Gupta, A., Williams, B. R. G., Hanash, S. M., and Rawwas, J. (2006). Cellular retinoic acid-binding protein II is a direct transcriptional target of MycN in neuroblastoma. Cancer Res. 66, 8100–8108. doi:10.1158/0008-5472.CAN-05-4519
Hagemann, S., Misiak, D., Bell, J. L., Fuchs, T., Lederer, M. I., Bley, N., et al. (2023). IGF2BP1 induces neuroblastoma via a druggable feedforward loop with MYCN promoting 17q oncogene expression. Mol. Cancer 22, 88. doi:10.1186/s12943-023-01792-0
Hald, Ø. H., Olsen, L., Gallo-Oller, G., Elfman, L. H. M., Løkke, C., Kogner, P., et al. (2019). Inhibitors of ribosome biogenesis repress the growth of MYCN-amplified neuroblastoma. Oncogene 38, 2800–2813. doi:10.1038/s41388-018-0611-7
Han, Q.-L., Zhang, X.-L., Ren, P.-X., Mei, L.-H., Lin, W.-H., Wang, L., et al. (2023). Discovery, evaluation and mechanism study of WDR5-targeted small molecular inhibitors for neuroblastoma. Acta Pharmacol. Sin. 44, 877–887. doi:10.1038/s41401-022-00999-z
Hasan, M. K., Nafady, A., Takatori, A., Kishida, S., Ohira, M., Suenaga, Y., et al. (2013). ALK is a MYCN target gene and regulates cell migration and invasion in neuroblastoma. Sci. Rep. 3, 3450. doi:10.1038/srep03450
Haug, B. H., Henriksen, J. R., Buechner, J., Geerts, D., Tømte, E., Kogner, P., et al. (2011). MYCN-regulated miRNA-92 inhibits secretion of the tumor suppressor DICKKOPF-3 (DKK3) in neuroblastoma. Carcinogenesis 32, 1005–1012. doi:10.1093/carcin/bgr073
Haussler, M., Sidell, N., Kelly, M., Donaldson, C., Altman, A., and Mangelsdorf, D. (1983). Specific high-affinity binding and biologic action of retinoic acid in human neuroblastoma cell lines. Proc. Natl. Acad. Sci. U. S. A. 80, 5525–5529. doi:10.1073/pnas.80.18.5525
He, J., Gu, L., Zhang, H., and Zhou, M. (2011). Crosstalk between MYCN and MDM2-p53 signal pathways regulates tumor cell growth and apoptosis in neuroblastoma. Cell Cycle 10, 2994–3002. doi:10.4161/cc.10.17.17118
Himoudi, N., Yan, M., Papanastasiou, A., and Anderson, J. (2008). MYCN as a target for cancer immunotherapy. Cancer Immunol. Immunother. 57, 693–700. doi:10.1007/s00262-007-0409-x
Hsu, T. Y.-T., Simon, L. M., Neill, N. J., Marcotte, R., Sayad, A., Bland, C. S., et al. (2015). The spliceosome is a therapeutic vulnerability in MYC-driven cancer. Nature 525, 384–388. doi:10.1038/nature14985
Hu, X., Liu, R., Hou, J., Peng, W., Wan, S., Xu, M., et al. (2022). SMARCE1 promotes neuroblastoma tumorigenesis through assisting MYCN-mediated transcriptional activation. Oncogene 41, 4295–4306. doi:10.1038/s41388-022-02428-1
Hu, Z., Xu, W., Wang, H., Li, M., Wang, J., Sun, C., et al. (2025). CARM1-induced lncRNA NEAT1 synchronously activates MYCN and GalNAcT-I to accelerate the progression of neuroblastoma. Gene 938, 149164. doi:10.1016/j.gene.2024.149164
Hua, Z., Chen, B., Gong, B., Lin, M., Ma, Y., and Li, Z. (2024). SESN1 functions as a new tumor suppressor gene via toll-like receptor signaling pathway in neuroblastoma. CNS Neurosci. Ther. 30, e14664. doi:10.1111/cns.14664
Huang, M., and Weiss, W. A. (2013). Neuroblastoma and MYCN. Cold Spring Harb. Perspect. Med. 3, a014415. doi:10.1101/cshperspect.a014415
Irwin, M. S., Naranjo, A., Zhang, F. F., Cohn, S. L., London, W. B., Gastier-Foster, J. M., et al. (2021). Revised neuroblastoma risk classification system: a report from the children’s oncology group. J. Clin. Oncol. 39, 3229–3241. doi:10.1200/JCO.21.00278
Issa, R. S., Kaehler, M., Pommert, N. S., Cascorbi, I., and Waetzig, V. (2024). Enhancing retinoic acid-mediated effects through inhibition of CYP26A1, CYP26B1 and HGF signaling in neuroblastoma cells. Anticancer Res. 44, 4189–4202. doi:10.21873/anticanres.17249
Jia, S.-Q., Zhuo, R., Zhang, Z.-M., Yang, Y., Tao, Y.-F., Wang, J.-W., et al. (2022). The BRD4 inhibitor dBET57 exerts anticancer effects by targeting superenhancer-related genes in neuroblastoma. J. Immunol. Res. 2022, 7945884. doi:10.1155/2022/7945884
Jiang, J., and Yu, Y. (2024). Eflornithine for treatment of high-risk neuroblastoma. Trends Pharmacol. Sci. 45, 577–578. doi:10.1016/j.tips.2024.04.005
Jiang, H., Greathouse, R. L., Tiche, S. J., Zhao, M., He, B., Li, Y., et al. (2023). Mitochondrial uncoupling induces epigenome remodeling and promotes differentiation in neuroblastoma. Cancer Res. 83, 181–194. doi:10.1158/0008-5472.CAN-22-1029
Jiang, Y., Xiao, H., Yang, Y., Chen, G., Zhang, Y., Wu, X., et al. (2025). Inhibition of purine metabolism promotes the differentiation of neuroblastoma driven by MYCN. Cancer Med. 14, e70953. doi:10.1002/cam4.70953
Juvvuna, P. K., Mondal, T., Di Marco, M., Kosalai, S. T., Kanduri, M., and Kanduri, C. (2021). NBAT1/CASC15-003/USP36 control MYCN expression and its downstream pathway genes in neuroblastoma. Neurooncol. Adv. 3, vdab056. doi:10.1093/noajnl/vdab056
Kaneko, Y., Suenaga, Y., Islam, S. M. R., Matsumoto, D., Nakamura, Y., Ohira, M., et al. (2015). Functional interplay between MYCN, NCYM, and OCT4 promotes aggressiveness of human neuroblastomas. Cancer Sci. 106, 840–847. doi:10.1111/cas.12677
Kang, J., Rychahou, P. G., Ishola, T. A., Mourot, J. M., Evers, B. M., and Chung, D. H. (2008). N-myc is a novel regulator of PI3K-mediated VEGF expression in neuroblastoma. Oncogene 27, 3999–4007. doi:10.1038/onc.2008.15
Kling, M. J., Griggs, C. N., McIntyre, E. M., Alexander, G., Ray, S., Challagundla, K. B., et al. (2021). Synergistic efficacy of inhibiting MYCN and mTOR signaling against neuroblastoma. BMC Cancer 21, 1061. doi:10.1186/s12885-021-08782-9
Koach, J., Holien, J. K., Massudi, H., Carter, D. R., Ciampa, O. C., Herath, M., et al. (2019). Drugging MYCN oncogenic signaling through the MYCN-PA2G4 binding interface. Cancer Res. 79, 5652–5667. doi:10.1158/0008-5472.CAN-19-1112
Koomoa, D.-L. T., Geerts, D., Lange, I., Koster, J., Pegg, A. E., Feith, D. J., et al. (2013). DFMO/eflornithine inhibits migration and invasion downstream of MYCN and involves p27Kip1 activity in neuroblastoma. Int. J. Oncol. 42, 1219–1228. doi:10.3892/ijo.2013.1835
Koppen, A., Ait-Aissa, R., Hopman, S., Koster, J., Haneveld, F., Versteeg, R., et al. (2007). Dickkopf-1 is down-regulated by MYCN and inhibits neuroblastoma cell proliferation. Cancer Lett. 256, 218–228. doi:10.1016/j.canlet.2007.06.011
Korshunov, A., Remke, M., Kool, M., Hielscher, T., Northcott, P. A., Williamson, D., et al. (2012). Biological and clinical heterogeneity of MYCN-amplified medulloblastoma. Acta Neuropathol. 123, 515–527. doi:10.1007/s00401-011-0918-8
Kress, T. R., Sabò, A., and Amati, B. (2015). MYC: connecting selective transcriptional control to global RNA production. Nat. Rev. Cancer 15, 593–607. doi:10.1038/nrc3984
Kroesen, M., Büll, C., Gielen, P. R., Brok, I. C., Armandari, I., Wassink, M., et al. (2016). Anti-GD2 mAb and vorinostat synergize in the treatment of neuroblastoma. Oncoimmunology 5, e1164919. doi:10.1080/2162402X.2016.1164919
Kushner, B. H., Kramer, K., LaQuaglia, M. P., Modak, S., Yataghene, K., and Cheung, N.-K. V. (2004). Reduction from seven to five cycles of intensive induction chemotherapy in children with high-risk neuroblastoma. J. Clin. Oncol. 22, 4888–4892. doi:10.1200/JCO.2004.02.101
Lampis, S., Raieli, S., Montemurro, L., Bartolucci, D., Amadesi, C., Bortolotti, S., et al. (2022). The MYCN inhibitor BGA002 restores the retinoic acid response leading to differentiation or apoptosis by the mTOR block in MYCN-amplified neuroblastoma. J. Exp. Clin. Cancer Res. 41, 160. doi:10.1186/s13046-022-02367-5
Lee, A. C.-L., Shih, Y.-Y., Zhou, F., Chao, T.-C., Lee, H., Liao, Y.-F., et al. (2019a). Calreticulin regulates MYCN expression to control neuronal differentiation and stemness of neuroblastoma. J. Mol. Med. 97, 325–339. doi:10.1007/s00109-018-1730-x
Lee, Y.-J., Chang, W.-W., Chang, C.-P., Liu, T.-Y., Chuang, C.-Y., Qian, K., et al. (2019b). Downregulation of PRMT1 promotes the senescence and migration of a non-MYCN amplified neuroblastoma SK-N-SH cells. Sci. Rep. 9, 1771. doi:10.1038/s41598-018-38394-6
Li, X., and Song, Y. (2020). Proteolysis-targeting chimera (PROTAC) for targeted protein degradation and cancer therapy. J. Hematol. Oncol. 13, 50. doi:10.1186/s13045-020-00885-3
Li, Y., Zhang, B., Zhang, H., Zhu, X., Feng, D., Zhang, D., et al. (2013). Oncolytic adenovirus armed with shRNA targeting MYCN gene inhibits neuroblastoma cell proliferation and in vivo xenograft tumor growth. J. Cancer Res. Clin. Oncol. 139, 933–941. doi:10.1007/s00432-013-1406-4
Li, Y., Zhang, H., Zhu, X., Feng, D., Zhang, D., Zhuo, B., et al. (2015). Oncolytic adenovirus-mediated short hairpin RNA targeting MYCN gene induces apoptosis by upregulating RKIP in neuroblastoma. Tumour Biol. 36, 6037–6043. doi:10.1007/s13277-015-3280-y
Li, Z., Lim, S. L., Tao, Y., Li, X., Xie, Y., Yang, C., et al. (2020). PROTAC bromodomain inhibitor ARV-825 displays anti-tumor activity in neuroblastoma by repressing expression of MYCN or c-Myc. Front. Oncol. 10, 574525. doi:10.3389/fonc.2020.574525
Li, X., Yang, F., He, N., Zhang, M., Lv, Y., Yu, Y., et al. (2023). YM155 inhibits neuroblastoma growth through degradation of MYCN: a new role as a USP7 inhibitor. Eur. J. Pharm. Sci. 181, 106343. doi:10.1016/j.ejps.2022.106343
Li, N., Sheng, J., and Zhu, H.-H. (2025a). Breakthroughs in treatment for hematological malignancies: latest updates on molecular glue, PROTACs and RNA degraders from ASH 2024. J. Hematol. Oncol. 18, 26. doi:10.1186/s13045-025-01674-6
Li, Z., Wu, J., Zhou, P., and Zhou, C. (2025b). Frequent lncRNA-derived fusions in pediatric neuroblastoma identified by LncFusion: potential biomarker and therapeutic implications. medRxiv., 2025.01.16.25320696. medRxiv. doi:10.1101/2025.01.16.25320696
Lin, X., Ojo, D., Wei, F., Wong, N., Gu, Y., and Tang, D. (2015). A novel aspect of tumorigenesis-BMI1 functions in regulating DNA damage response. Biomolecules 5, 3396–3415. doi:10.3390/biom5043396
Liu, Y., Liu, D., and Wan, W. (2019). MYCN-induced E2F5 promotes neuroblastoma cell proliferation through regulating cell cycle progression. Biochem. Biophys. Res. Commun. 511, 35–40. doi:10.1016/j.bbrc.2019.01.087
Liu, Z., Zhao, Q., Zuo, Z.-X., Yuan, S.-Q., Yu, K., Zhang, Q., et al. (2020). Systematic analysis of the aberrances and functional implications of ferroptosis in cancer. iScience 23, 101302. doi:10.1016/j.isci.2020.101302
Liu, F., Wang, X., Duan, J., Hou, Z., Wu, Z., Liu, L., et al. (2022a). A temporal PROTAC cocktail-mediated sequential degradation of AURKA abrogates acute myeloid leukemia stem cells. Adv. Sci. (Weinh.) 9, e2104823. doi:10.1002/advs.202104823
Liu, T., Gu, L., Wu, Z., Albadari, N., Li, W., and Zhou, M. (2022b). MYCN mRNA degradation and cancer suppression by a selective small-molecule inhibitor in MYCN-amplified neuroblastoma. Front. Oncol. 12, 1058726. doi:10.3389/fonc.2022.1058726
Liu, Z., Zhang, X., Xu, M., Hong, J. J., Ciardiello, A., Lei, H., et al. (2024). MYCN drives oncogenesis by cooperating with the histone methyltransferase G9a and the WDR5 adaptor to orchestrate global gene transcription. PLoS Biol. 22, e3002240. doi:10.1371/journal.pbio.3002240
Liu, Z., Hong, J. J., Zhang, X., Sayers, C. M., Fang, W., Xu, M., et al. (2025). MYCN and KAT2A form a feedforward loop to drive an oncogenic transcriptional program in neuroblastoma. Oncogenesis 14, 13. doi:10.1038/s41389-025-00557-2
Locatelli, F., Pagliara, D., De Ioris, M. A., Becilli, M., Del Baldo, G., Serra, A., et al. (2025). GD2-targeting CAR T cells in high-risk neuroblastoma: a phase 1/2 trial. Nat. Med. doi:10.1038/s41591-025-03874-6
Lovén, J., Zinin, N., Wahlström, T., Müller, I., Brodin, P., Fredlund, E., et al. (2010). MYCN-regulated microRNAs repress estrogen receptor-alpha (ESR1) expression and neuronal differentiation in human neuroblastoma. Proc. Natl. Acad. Sci. U. S. A. 107, 1553–1558. doi:10.1073/pnas.0913517107
Lu, X., Pearson, A., and Lunec, J. (2003). The MYCN oncoprotein as a drug development target. Cancer Lett. 197, 125–130. doi:10.1016/s0304-3835(03)00096-x
Lu, Y., Yang, Q., Su, Y., Ji, Y., Li, G., Yang, X., et al. (2021). MYCN mediates TFRC-dependent ferroptosis and reveals vulnerabilities in neuroblastoma. Cell Death Dis. 12, 511. doi:10.1038/s41419-021-03790-w
Maeshima, R., Moulding, D., Stoker, A. W., and Hart, S. L. (2020). MYCN silencing by RNAi induces neurogenesis and suppresses proliferation in models of neuroblastoma with resistance to retinoic acid. Nucleic Acid. Ther. 30, 237–248. doi:10.1089/nat.2019.0831
Malone, C. F., Mabe, N. W., Forman, A. B., Alexe, G., Engel, K. L., Chen, Y.-J. C., et al. (2024). The KAT module of the SAGA complex maintains the oncogenic gene expression program in MYCN-amplified neuroblastoma. Sci. Adv. 10, eadm9449. doi:10.1126/sciadv.adm9449
Manfredi, M. G., Ecsedy, J. A., Chakravarty, A., Silverman, L., Zhang, M., Hoar, K. M., et al. (2011). Characterization of alisertib (MLN8237), an investigational small-molecule inhibitor of aurora A kinase using novel in vivo pharmacodynamic assays. Clin. Cancer Res. 17, 7614–7624. doi:10.1158/1078-0432.CCR-11-1536
Massudi, H., Luo, J.-S., Holien, J. K., Gadde, S., Krishan, S., Herath, M., et al. (2023). Inhibitors of the oncogenic PA2G4-MYCN protein-protein interface. Cancers (Basel) 15, 1822. doi:10.3390/cancers15061822
Matsumoto, K., Lucarelli, E., Minniti, C., Gaetano, C., and Thiele, C. J. (1994). Signals transduced via insulin-like growth factor I receptor (IGF(R)) mediate resistance to retinoic acid-induced cell growth arrest in a human neuroblastoma cell line. Cell Death Differ. 1, 49–58.
McCluskey, A. G., Boyd, M., Gaze, M. N., and Mairs, R. J. (2005). [131I]MIBG and topotecan: a rationale for combination therapy for neuroblastoma. Cancer Lett. 228, 221–227. doi:10.1016/j.canlet.2004.11.062
McNerney, K. O., Karageorgos, S., Ferry, G. M., Wolpaw, A. J., Burudpakdee, C., Khurana, P., et al. (2022). TH-MYCN tumors, but not tumor-derived cell lines, are adrenergic lineage, GD2+, and responsive to anti-GD2 antibody therapy. Oncoimmunology 11, 2075204. doi:10.1080/2162402X.2022.2075204
Mendonza, J. J., Reddy, S. T., Dutta, H., Makani, V. K. K., Uppuluri, V. M., Jain, N., et al. (2023). Retinoic acid and evernyl-based menadione-triazole hybrid cooperate to induce differentiation of neuroblastoma cells. Naunyn. Schmiedeb. Arch. Pharmacol. 396, 2651–2665. doi:10.1007/s00210-023-02489-3
Mestdagh, P., Fredlund, E., Pattyn, F., Schulte, J. H., Muth, D., Vermeulen, J., et al. (2010). MYCN/c-MYC-induced microRNAs repress coding gene networks associated with poor outcome in MYCN/c-MYC-activated tumors. Oncogene 29, 1394–1404. doi:10.1038/onc.2009.429
Mlakar, V., Jurkovic Mlakar, S., Lesne, L., Marino, D., Rathi, K. S., Maris, J. M., et al. (2019). PRIMA-1MET-induced neuroblastoma cell death is modulated by p53 and mycn through glutathione level. J. Exp. Clin. Cancer Res. 38, 69. doi:10.1186/s13046-019-1066-6
Mody, R., Yu, A. L., Naranjo, A., Zhang, F. F., London, W. B., Shulkin, B. L., et al. (2020). Irinotecan, temozolomide, and dinutuximab with GM-CSF in children with refractory or relapsed neuroblastoma: a report from the children’s oncology group. J. Clin. Oncol. 38, 2160–2169. doi:10.1200/JCO.20.00203
Montemurro, L., Raieli, S., Angelucci, S., Bartolucci, D., Amadesi, C., Lampis, S., et al. (2019). A novel MYCN-specific antigene oligonucleotide deregulates mitochondria and inhibits tumor growth in MYCN-amplified neuroblastoma. Cancer Res. 79, 6166–6177. doi:10.1158/0008-5472.CAN-19-0008
Mora, J., Chan, G. C. F., Morgenstern, D. A., Amoroso, L., Nysom, K., Faber, J., et al. (2025). The anti-GD2 monoclonal antibody naxitamab plus GM-CSF for relapsed or refractory high-risk neuroblastoma: a phase 2 clinical trial. Nat. Commun. 16, 1636. doi:10.1038/s41467-025-56619-x
Mossé, Y. P., Fox, E., Teachey, D. T., Reid, J. M., Safgren, S. L., Carol, H., et al. (2019). A phase II study of alisertib in children with recurrent/refractory solid tumors or leukemia: children’s oncology group phase I and pilot consortium (ADVL0921). Clin. Cancer Res. 25, 3229–3238. doi:10.1158/1078-0432.CCR-18-2675
Müller, I., Larsson, K., Frenzel, A., Oliynyk, G., Zirath, H., Prochownik, E. V., et al. (2014). Targeting of the MYCN protein with small molecule c-MYC inhibitors. PLoS One 9, e97285. doi:10.1371/journal.pone.0097285
Murray, J. E., Valli, E., Milazzo, G., Mayoh, C., Gifford, A. J., Fletcher, J. I., et al. (2024). The transcriptional co-repressor Runx1t1 is essential for MYCN-driven neuroblastoma tumorigenesis. Nat. Commun. 15, 5585. doi:10.1038/s41467-024-49871-0
Nagarajan, D., Parracho, R. T., Corujo, D., Xie, M., Kutkaite, G., Olsen, T. K., et al. (2024). Epigenetic regulation of cell state by H2AFY governs immunogenicity in high-risk neuroblastoma. J. Clin. Invest. 134, e175310. doi:10.1172/JCI175310
Nakatani, K., Kogashi, H., Miyamoto, T., Setoguchi, T., Sakuma, T., Kugou, K., et al. (2024). Inhibition of OCT4 binding at the MYCN locus induces neuroblastoma cell death accompanied by downregulation of transcripts with high-open reading frame dominance. Front. Oncol. 14, 1237378. doi:10.3389/fonc.2024.1237378
Nazam, N., Bownes, L. V., Julson, J. R., Quinn, C. H., Erwin, M. H., Marayati, R., et al. (2024). Novel PP2A-activating compounds in neuroblastoma. Cancers (Basel) 16, 3836. doi:10.3390/cancers16223836
Nazir, A., Nazir, A., and Kandel, K. (2024). Advancing neuroblastoma care: future horizons after approval of eflornithine by FDA. Int. J. Surg. 110, 2511–2512. doi:10.1097/JS9.0000000000001182
Nelson, S. E., Tucker, J. R., Prado, M. G., Tierney, L. C., Quigley, S. L., Lumpkin, A. T., et al. (2025). Development of dual Aurora-A and Aurora-B degrading PROTACs for MCYN-amplified neuroblastoma. ChemMedChem 20, e202400703. doi:10.1002/cmdc.202400703
Nijhuis, A., Sikka, A., Yogev, O., Herendi, L., Balcells, C., Ma, Y., et al. (2022). Indisulam targets RNA splicing and metabolism to serve as a therapeutic strategy for high-risk neuroblastoma. Nat. Commun. 13, 1380. doi:10.1038/s41467-022-28907-3
Nishio, Y., Kato, K., Oishi, H., Takahashi, Y., and Saitoh, S. (2024). MYCN in human development and diseases. Front. Oncol. 14, 1417607. doi:10.3389/fonc.2024.1417607
Obata, H., Tsuji, A. B., Sudo, H., Sugyo, A., Hashiya, K., Ikeda, H., et al. (2023). Novel auger-electron-emitting 191Pt-labeled pyrrole-imidazole polyamide targeting MYCN increases cytotoxicity and cytosolic dsDNA granules in MYCN-amplified neuroblastoma. Pharm. (Basel) 16, 1526. doi:10.3390/ph16111526
Ochiai, H., Takenobu, H., Nakagawa, A., Yamaguchi, Y., Kimura, M., Ohira, M., et al. (2010). Bmi1 is a MYCN target gene that regulates tumorigenesis through repression of KIF1Bbeta and TSLC1 in neuroblastoma. Oncogene 29, 2681–2690. doi:10.1038/onc.2010.22
Oesterheld, J., Ferguson, W., Kraveka, J. M., Bergendahl, G., Clinch, T., Lorenzi, E., et al. (2024). Eflornithine as postimmunotherapy maintenance in high-risk neuroblastoma: externally controlled, propensity score-matched survival outcome comparisons. J. Clin. Oncol. 42, 90–102. doi:10.1200/JCO.22.02875
Olsen, T. K., Dyberg, C., Embaie, B. T., Alchahin, A., Milosevic, J., Ding, J., et al. (2022). DHODH is an independent prognostic marker and potent therapeutic target in neuroblastoma. JCI Insight 7, e153836. doi:10.1172/jci.insight.153836
Ooi, C. Y., Carter, D. R., Liu, B., Mayoh, C., Beckers, A., Lalwani, A., et al. (2018). Network modeling of microRNA-mRNA interactions in neuroblastoma tumorigenesis identifies miR-204 as a direct inhibitor of MYCN. Cancer Res. 78, 3122–3134. doi:10.1158/0008-5472.CAN-17-3034
Oskouian, B., Lee, J. Y., Asgharzadeh, S., Khan, R., Zhang, M., Weisbrod, J. R., et al. (2024). AF1q is a universal marker of neuroblastoma that sustains N-Myc expression and drives tumorigenesis. Oncogene 43, 1203–1213. doi:10.1038/s41388-024-02980-y
Otsuka, K., Sasada, M., Iyoda, T., Nohara, Y., Sakai, S., Asayama, T., et al. (2019). Combining peptide TNIIIA2 with all-trans retinoic acid accelerates N-Myc protein degradation and neuronal differentiation in MYCN-amplified neuroblastoma cells. Am. J. Cancer Res. 9, 434–448.
Otto, T., Horn, S., Brockmann, M., Eilers, U., Schüttrumpf, L., Popov, N., et al. (2009). Stabilization of N-Myc is a critical function of Aurora A in human neuroblastoma. Cancer Cell 15, 67–78. doi:10.1016/j.ccr.2008.12.005
Pandher, R., Xue, C., Gamble, L. D., Milazzo, G., Di Giacomo, S., Murray, J., et al. (2025). The cell-permeable iron chelator M606 inhibits MYCN-driven neuroblastoma via an E2F3-mediated response. Proc. Natl. Acad. Sci. U. S. A. 122, e2420011122. doi:10.1073/pnas.2420011122
Park, J. H., Szemes, M., Vieira, G. C., Melegh, Z., Malik, S., Heesom, K. J., et al. (2015). Protein arginine methyltransferase 5 is a key regulator of the MYCN oncoprotein in neuroblastoma cells. Mol. Oncol. 9, 617–627. doi:10.1016/j.molonc.2014.10.015
Park, J. R., Kreissman, S. G., London, W. B., Naranjo, A., Cohn, S. L., Hogarty, M. D., et al. (2019). Effect of tandem autologous stem cell transplant vs single transplant on event-free survival in patients with high-risk neuroblastoma: a randomized clinical trial. JAMA 322, 746–755. doi:10.1001/jama.2019.11642
Pinto, N. R., Applebaum, M. A., Volchenboum, S. L., Matthay, K. K., London, W. B., Ambros, P. F., et al. (2015). Advances in risk classification and treatment strategies for neuroblastoma. J. Clin. Oncol. 33, 3008–3017. doi:10.1200/JCO.2014.59.4648
Pinto, N., Naranjo, A., Hibbitts, E., Kreissman, S. G., Granger, M. M., Irwin, M. S., et al. (2019). Predictors of differential response to induction therapy in high-risk neuroblastoma: a report from the Children’s oncology group (COG). Eur. J. Cancer 112, 66–79. doi:10.1016/j.ejca.2019.02.003
Powers, J. T., Tsanov, K. M., Pearson, D. S., Roels, F., Spina, C. S., Ebright, R., et al. (2016). Multiple mechanisms disrupt the let-7 microRNA family in neuroblastoma. Nature 535, 246–251. doi:10.1038/nature18632
Pugh, T. J., Morozova, O., Attiyeh, E. F., Asgharzadeh, S., Wei, J. S., Auclair, D., et al. (2013). The genetic landscape of high-risk neuroblastoma. Nat. Genet. 45, 279–284. doi:10.1038/ng.2529
Puissant, A., Frumm, S. M., Alexe, G., Bassil, C. F., Qi, J., Chanthery, Y. H., et al. (2013). Targeting MYCN in neuroblastoma by BET bromodomain inhibition. Cancer Discov. 3, 308–323. doi:10.1158/2159-8290.CD-12-0418
Qin, X., Lam, A., Zhang, X., Sengupta, S., Iorgulescu, J. B., Ni, H., et al. (2024). CKLF instigates a “cold” microenvironment to promote MYCN-mediated tumor aggressiveness. Sci. Adv. 10, eadh9547. doi:10.1126/sciadv.adh9547
Rao, G. K., Makani, V. K. K., Mendonza, J. J., Edathara, P. M., Patel, N., Ramakrishna, M., et al. (2022). Downregulation of BORIS/CTCFL leads to ROS-dependent cellular senescence and drug sensitivity in MYCN-amplified neuroblastoma. FEBS J. 289, 2915–2934. doi:10.1111/febs.16309
Richards, M. W., Burgess, S. G., Poon, E., Carstensen, A., Eilers, M., Chesler, L., et al. (2016). Structural basis of N-Myc binding by Aurora-A and its destabilization by kinase inhibitors. Proc. Natl. Acad. Sci. U. S. A. 113, 13726–13731. doi:10.1073/pnas.1610626113
Roth, S. A., Hald, Ø. H., Fuchs, S., Løkke, C., Mikkola, I., Flægstad, T., et al. (2018). MicroRNA-193b-3p represses neuroblastoma cell growth via downregulation of Cyclin D1, MCL-1 and MYCN. Oncotarget 9, 18160–18179. doi:10.18632/oncotarget.24793
Sakamoto, K. M., Kim, K. B., Kumagai, A., Mercurio, F., Crews, C. M., and Deshaies, R. J. (2001). Protacs: chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc. Natl. Acad. Sci. U. S. A. 98, 8554–8559. doi:10.1073/pnas.141230798
Salib, A., Jayatilleke, N., Seneviratne, J. A., Mayoh, C., De Preter, K., Speleman, F., et al. (2024). MYCN and SNRPD3 cooperate to maintain a balance of alternative splicing events that drives neuroblastoma progression. Oncogene 43, 363–377. doi:10.1038/s41388-023-02897-y
Sauvage, D., Bosseler, M., Viry, E., Kanli, G., Oudin, A., Berchem, G., et al. (2022). The BET protein inhibitor JQ1 decreases hypoxia and improves the therapeutic benefit of anti-PD-1 in a high-risk neuroblastoma mouse model. Cells 11, 2783. doi:10.3390/cells11182783
Schönherr, C., Ruuth, K., Kamaraj, S., Wang, C.-L., Yang, H.-L., Combaret, V., et al. (2012). Anaplastic lymphoma kinase (ALK) regulates initiation of transcription of MYCN in neuroblastoma cells. Oncogene 31, 5193–5200. doi:10.1038/onc.2012.12
Seier, J. A., Reinhardt, J., Saraf, K., Ng, S. S., Layer, J. P., Corvino, D., et al. (2021). Druggable epigenetic suppression of interferon-induced chemokine expression linked to MYCN amplification in neuroblastoma. J. Immunother. Cancer 9, e001335. doi:10.1136/jitc-2020-001335
Shaliman, D., Takenobu, H., Sugino, R. P., Ohira, M., and Kamijo, T. (2022). The PRC2 molecule EED is a target of epigenetic therapy for neuroblastoma. Eur. J. Cell Biol. 101, 151238. doi:10.1016/j.ejcb.2022.151238
Shi, Y., Yuan, J., Rraklli, V., Maxymovitz, E., Cipullo, M., Liu, M., et al. (2021). Aberrant splicing in neuroblastoma generates RNA-fusion transcripts and provides vulnerability to spliceosome inhibitors. Nucleic Acids Res. 49, 2509–2521. doi:10.1093/nar/gkab054
Shinno, Y., Takenobu, H., Sugino, R. P., Endo, Y., Okada, R., Haruta, M., et al. (2022). Polycomb EZH1 regulates cell cycle/5-fluorouracil sensitivity of neuroblastoma cells in concert with MYCN. Cancer Sci. 113, 4193–4206. doi:10.1111/cas.15555
Singh, S., Quarni, W., Goralski, M., Wan, S., Jin, H., Van de Velde, L.-A., et al. (2021). Targeting the spliceosome through RBM39 degradation results in exceptional responses in high-risk neuroblastoma models. Sci. Adv. 7, eabj5405. doi:10.1126/sciadv.abj5405
Slack, A., Chen, Z., Tonelli, R., Pule, M., Hunt, L., Pession, A., et al. (2005). The p53 regulatory gene MDM2 is a direct transcriptional target of MYCN in neuroblastoma. Proc. Natl. Acad. Sci. U. S. A. 102, 731–736. doi:10.1073/pnas.0405495102
Su, Y., Zhao, B., Zhou, L., Zhang, Z., Shen, Y., Lv, H., et al. (2020). Ferroptosis, a novel pharmacological mechanism of anti-cancer drugs. Cancer Lett. 483, 127–136. doi:10.1016/j.canlet.2020.02.015
Sun, Y., Bell, J. L., Carter, D., Gherardi, S., Poulos, R. C., Milazzo, G., et al. (2015). WDR5 supports an N-Myc transcriptional complex that drives a protumorigenic gene expression signature in neuroblastoma. Cancer Res. 75, 5143–5154. doi:10.1158/0008-5472.CAN-15-0423
Sussman, R. T., Rokita, J. L., Huang, K., Raman, P., Rathi, K. S., Martinez, D., et al. (2020). CAMKV is a candidate immunotherapeutic target in MYCN amplified neuroblastoma. Front. Oncol. 10, 302. doi:10.3389/fonc.2020.00302
Szewczyk, S., Buckley, B., Chernov, M., Wang, X., Pathak, S., Yeger, H., et al. (2024). Cell-based assay to detect small molecules restoring levels of let-7 miRNAs. Am. J. Cancer Res. 14, 4772–4787. doi:10.62347/MBLD9480
Tagalakis, A. D., Jayarajan, V., Maeshima, R., Ho, K. H., Syed, F., Wu, L.-P., et al. (2021). Integrin-targeted, short interfering RNA nanocomplexes for neuroblastoma tumor-specific delivery achieve MYCN silencing with improved survival. Adv. Funct. Mater. 31, 2104843. doi:10.1002/adfm.202104843
Tan, K., Mo, J., Li, M., Dong, Y., Han, Y., Sun, X., et al. (2022). SMAD9-MYCN positive feedback loop represents a unique dependency for MYCN-amplified neuroblastoma. J. Exp. Clin. Cancer Res. 41, 352. doi:10.1186/s13046-022-02563-3
Tan, L., He, G., Shen, C., He, S., Chen, Y., and Guo, X. (2025). Construction of a ferroptosis-based prediction model for the prognosis of MYCN-amplified neuroblastoma and screening and verification of target sites. Hereditas 162, 41. doi:10.1186/s41065-025-00413-8
Tang, J., Moorthy, R., Hirsch, L. E., Demir, Ö., Baker, Z. D., Naumann, J. A., et al. (2025). Targeting N-Myc in neuroblastoma with selective Aurora kinase A degraders. Cell Chem. Biol. 32, 352–362.e10. doi:10.1016/j.chembiol.2024.12.006
Tao, T., Shi, H., Mariani, L., Abraham, B. J., Durbin, A. D., Zimmerman, M. W., et al. (2020). LIN28B regulates transcription and potentiates MYCN-induced neuroblastoma through binding to ZNF143 at target gene promotors. Proc. Natl. Acad. Sci. U. S. A. 117, 16516–16526. doi:10.1073/pnas.1922692117
Tao, L., Mohammad, M. A., Milazzo, G., Moreno-Smith, M., Patel, T. D., Zorman, B., et al. (2022). MYCN-driven fatty acid uptake is a metabolic vulnerability in neuroblastoma. Nat. Commun. 13, 3728. doi:10.1038/s41467-022-31331-2
Tavana, O., Li, D., Dai, C., Lopez, G., Banerjee, D., Kon, N., et al. (2016). HAUSP deubiquitinates and stabilizes N-Myc in neuroblastoma. Nat. Med. 22, 1180–1186. doi:10.1038/nm.4180
Tee, A. E., Ciampa, O. C., Wong, M., Fletcher, J. I., Kamili, A., Chen, J., et al. (2020). Combination therapy with the CDK7 inhibitor and the tyrosine kinase inhibitor exerts synergistic anticancer effects against MYCN-amplified neuroblastoma. Int. J. Cancer 147, 1928–1938. doi:10.1002/ijc.32936
Thiele, C. J., Reynolds, C. P., and Israel, M. A. (1985). Decreased expression of N-myc precedes retinoic acid-induced morphological differentiation of human neuroblastoma. Nature 313, 404–406. doi:10.1038/313404a0
Thombare, K., Vaid, R., Pucci, P., Ihrmark Lundberg, K., Ayyalusamy, R., Baig, M. H., et al. (2024). METTL3/MYCN cooperation drives neural crest differentiation and provides therapeutic vulnerability in neuroblastoma. EMBO J. 43, 6310–6335. doi:10.1038/s44318-024-00299-8
Tjaden, B., Baum, K., Marquardt, V., Simon, M., Trajkovic-Arsic, M., Kouril, T., et al. (2020). N-Myc-induced metabolic rewiring creates novel therapeutic vulnerabilities in neuroblastoma. Sci. Rep. 10, 7157. doi:10.1038/s41598-020-64040-1
Tsubota, S., Kishida, S., Shimamura, T., Ohira, M., Yamashita, S., Cao, D., et al. (2017). PRC2-Mediated transcriptomic alterations at the embryonic stage govern tumorigenesis and clinical outcome in MYCN-driven neuroblastoma. Cancer Res. 77, 5259–5271. doi:10.1158/0008-5472.CAN-16-3144
Valentijn, L. J., Koster, J., Haneveld, F., Aissa, R. A., van Sluis, P., Broekmans, M. E. C., et al. (2012). Functional MYCN signature predicts outcome of neuroblastoma irrespective of MYCN amplification. Proc. Natl. Acad. Sci. U. S. A. 109, 19190–19195. doi:10.1073/pnas.1208215109
Vaughan, L., Clarke, P. A., Barker, K., Chanthery, Y., Gustafson, C. W., Tucker, E., et al. (2016). Inhibition of mTOR-kinase destabilizes MYCN and is a potential therapy for MYCN-dependent tumors. Oncotarget 7, 57525–57544. doi:10.18632/oncotarget.10544
Veas-Perez de Tudela, M., Delgado-Esteban, M., Cuende, J., Bolaños, J. P., and Almeida, A. (2010). Human neuroblastoma cells with MYCN amplification are selectively resistant to oxidative stress by transcriptionally up-regulating glutamate cysteine ligase. J. Neurochem. 113, 819–825. doi:10.1111/j.1471-4159.2010.06648.x
Voeltzke, K., Scharov, K., Funk, C. M., Kahler, A., Picard, D., Hauffe, L., et al. (2022). EIF4EBP1 is transcriptionally upregulated by MYCN and associates with poor prognosis in neuroblastoma. Cell Death Discov. 8, 157. doi:10.1038/s41420-022-00963-0
Wang, Y., Jiang, X., Feng, F., Liu, W., and Sun, H. (2020). Degradation of proteins by PROTACs and other strategies. Acta Pharm. Sin. B 10, 207–238. doi:10.1016/j.apsb.2019.08.001
Wang, L., Chen, C., Song, Z., Wang, H., Ye, M., Wang, D., et al. (2022a). EZH2 depletion potentiates MYC degradation inhibiting neuroblastoma and small cell carcinoma tumor formation. Nat. Commun. 13, 12. doi:10.1038/s41467-021-27609-6
Wang, P. L., Teng, L., Feng, Y. C., Yue, Y. M., Han, M. M., Yan, Q., et al. (2022b). The N-Myc-responsive lncRNA MILIP promotes DNA double-strand break repair through non-homologous end joining. Proc. Natl. Acad. Sci. U. S. A. 119, e2208904119. doi:10.1073/pnas.2208904119
Weiss, B. D., Yanik, G., Naranjo, A., Zhang, F. F., Fitzgerald, W., Shulkin, B. L., et al. (2021). A safety and feasibility trial of 131 I-MIBG in newly diagnosed high-risk neuroblastoma: a children’s oncology group study. Pediatr. Blood Cancer 68, e29117. doi:10.1002/pbc.29117
Wenzel, A., and Schwab, M. (1995). The mycN/max protein complex in neuroblastoma. Short review. Eur. J. Cancer 31A, 516–519. doi:10.1016/0959-8049(95)00060-v
Wolpaw, A. J., Bayliss, R., Büchel, G., Dang, C. V., Eilers, M., Gustafson, W. C., et al. (2021). Drugging the “undruggable” MYCN oncogenic transcription factor: overcoming previous obstacles to impact childhood cancers. Cancer Res. 81, 1627–1632. doi:10.1158/0008-5472.CAN-20-3108
Wu, P.-Y., Liao, Y.-F., Juan, H.-F., Huang, H.-C., Wang, B.-J., Lu, Y.-L., et al. (2014). Aryl hydrocarbon receptor downregulates MYCN expression and promotes cell differentiation of neuroblastoma. PLoS One 9, e88795. doi:10.1371/journal.pone.0088795
Wu, J.-C., Huang, C.-C., Wang, P.-W., Chen, T.-Y., Hsu, W.-M., Chuang, J.-H., et al. (2023). ONC201 suppresses neuroblastoma growth by interrupting mitochondrial function and reactivating nuclear ATRX expression while decreasing MYCN. Int. J. Mol. Sci. 24, 1649. doi:10.3390/ijms24021649
Xia, Y., Ye, B., Ding, J., Yu, Y., Alptekin, A., Thangaraju, M., et al. (2019). Metabolic reprogramming by MYCN confers dependence on the serine-glycine-one-carbon biosynthetic pathway. Cancer Res. 79, 3837–3850. doi:10.1158/0008-5472.CAN-18-3541
Xiang, S., Chen, P., Shi, X., Cai, H., Shen, Z., Liu, L., et al. (2025). Disruption of the KLHL37-N-Myc complex restores N-Myc degradation and arrests neuroblastoma growth in mouse models. J. Clin. Invest. 135, e176655. doi:10.1172/JCI176655
Xiao, D., Yue, M., Su, H., Ren, P., Jiang, J., Li, F., et al. (2016). Polo-like kinase-1 regulates Myc stabilization and activates a feedforward circuit promoting tumor cell survival. Mol. Cell 64, 493–506. doi:10.1016/j.molcel.2016.09.016
Xu, P., Yang, J. C., Chen, B., Ning, S., Zhang, X., Wang, L., et al. (2024). Proteostasis perturbation of N-Myc leveraging HSP70 mediated protein turnover improves treatment of neuroendocrine prostate cancer. Nat. Commun. 15, 6626. doi:10.1038/s41467-024-50459-x
Yang, M., Carter, S., Parmar, S., Bume, D. D., Calabrese, D. R., Liang, X., et al. (2021). Targeting a noncanonical, hairpin-containing G-quadruplex structure from the MYCN gene. Nucleic Acids Res. 49, 7856–7869. doi:10.1093/nar/gkab594
Yeku, O. O., and Longo, D. L. (2023). CAR T cells for neuroblastoma. N. Engl. J. Med. 388, 1328–1331. doi:10.1056/NEJMe2300317
Yi, J. S., Sias-Garcia, O., Nasholm, N., Hu, X., Iniguez, A. B., Hall, M. D., et al. (2021). The synergy of BET inhibitors with aurora A kinase inhibitors in MYCN-amplified neuroblastoma is heightened with functional TP53. Neoplasia 23, 624–633. doi:10.1016/j.neo.2021.05.003
Yoda, H., Inoue, T., Shinozaki, Y., Lin, J., Watanabe, T., Koshikawa, N., et al. (2019a). Direct targeting of MYCN gene amplification by site-specific DNA alkylation in neuroblastoma. Cancer Res. 79, 830–840. doi:10.1158/0008-5472.CAN-18-1198
Yoda, H., Nakayama, T., Miura, M., Toriyama, M., Motohashi, S., and Suzuki, T. (2019b). Vitamin K3 derivative induces apoptotic cell death in neuroblastoma via downregulation of MYCN expression. Biochem. Biophys. Rep. 20, 100701. doi:10.1016/j.bbrep.2019.100701
Yu, A. L., Gilman, A. L., Ozkaynak, M. F., London, W. B., Kreissman, S. G., Chen, H. X., et al. (2010). Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 363, 1324–1334. doi:10.1056/NEJMoa0911123
Yu, A. L., Gilman, A. L., Ozkaynak, M. F., Naranjo, A., Diccianni, M. B., Gan, J., et al. (2021a). Long-term follow-up of a phase III study of ch14.18 (dinutuximab) + cytokine immunotherapy in children with high-risk neuroblastoma: COG study ANBL0032. Clin. Cancer Res. 27, 2179–2189. doi:10.1158/1078-0432.CCR-20-3909
Yu, Y., Ding, J., Zhu, S., Alptekin, A., Dong, Z., Yan, C., et al. (2021b). Therapeutic targeting of both dihydroorotate dehydrogenase and nucleoside transport in MYCN-amplified neuroblastoma. Cell Death Dis. 12, 821. doi:10.1038/s41419-021-04120-w
Zhang, S., Wei, J. S., Li, S. Q., Badgett, T. C., Song, Y. K., Agarwal, S., et al. (2016). MYCN controls an alternative RNA splicing program in high-risk metastatic neuroblastoma. Cancer Lett. 371, 214–224. doi:10.1016/j.canlet.2015.11.045
Zhang, L., Wang, M., Zhu, Z., Chen, S., Wu, H., Yang, Y., et al. (2021). A GD2-aptamer-mediated, self-assembling nanomedicine for targeted multiple treatments in neuroblastoma theranostics. Mol. Ther. Nucleic Acids 26, 732–748. doi:10.1016/j.omtn.2021.08.021
Zhang, X., Guo, X., Zhuo, R., Tao, Y., Liang, W., Yang, R., et al. (2022). BRD4 inhibitor MZ1 exerts anti-cancer effects by targeting MYCN and MAPK signaling in neuroblastoma. Biochem. Biophys. Res. Commun. 604, 63–69. doi:10.1016/j.bbrc.2022.03.039
Zhao, Q., Liu, Y., Zhang, Y., Meng, L., Wei, J., Wang, B., et al. (2020). Role and toxicity of radiation therapy in neuroblastoma patients: a literature review. Crit. Rev. Oncol. Hematol. 149, 102924. doi:10.1016/j.critrevonc.2020.102924
Zhu, S., Lee, J.-S., Guo, F., Shin, J., Perez-Atayde, A. R., Kutok, J. L., et al. (2012). Activated ALK collaborates with MYCN in neuroblastoma pathogenesis. Cancer Cell 21, 362–373. doi:10.1016/j.ccr.2012.02.010
Zhu, Q., Feng, C., Liao, W., Zhang, Y., and Tang, S. (2013). Target delivery of MYCN siRNA by folate-nanoliposomes delivery system in a metastatic neuroblastoma model. Cancer Cell Int. 13, 65. doi:10.1186/1475-2867-13-65
Zou, Y., Ma, D., and Wang, Y. (2019). The PROTAC technology in drug development. Cell Biochem. Funct. 37, 21–30. doi:10.1002/cbf.3369
Glossary
AhR Aryl hydrocarbon receptor
ALDH18A1 Aldehyde dehydrogenase family 18 member A1
ANM Multifunctional nanomedicine
ATP13A3 ATPase 13A3
BET Bromodomain and Extra-Terminal
BHLH Basic helix–loop–helix
BORIS Brother of Regulator of Imprinted Sites
BPTF Bromodomain PHD Finger Transcription Factor
BSO Buthionine sulfoximine
CAMKV Calmodulin kinase-like vesicle-associated gene
CAR-T cell Chimeric antigen receptor T cell
CRC Core regulatory circuitry
CRT Calreticulin
DDX3X DEAD-box helicase 3 X-linked
DFMO Difluoromethylornithine
DHODH Dihydroorotate dehydrogenase
DKK1 Dickkopf-1
DKK3 Dickkopf-3
DSBs DNA double-strand breaks
EED Embryonic ectoderm development
EHMT Euchromatic histone-lysine methyltransferases
EIF4EBP1 Eukaryotic translation initiation factor 4E-binding protein 1
ELOVL2 ELOVL fatty acid elongase 2 gene
ESR1 Estrogen receptor α
FATP2 Fatty acid transport protein 2
GA Gambogic acid
GD2 Disialoganglioside GD2
GLDC Glycine decarboxylase
GPX4 Glutathione peroxidase 4
GRHL1 Grainyhead-like 1 gene
GSH Glutathione
HDAC Histone deacetylase
HHT Homoharringtonine
HIF1A Hypoxia-inducible factor 1 alpha
HSP70 Heat shock protein 70
IDO1 Indoleamine 2,3-dioxygenase 1
KLHL37 Kelch-like protein 37
lncRNA Long noncoding RNA
MCTs Monocarboxylate transporters
MIAT Myocardial infarction-associated transcript
miRNA MicroRNA
MNA-NB MYCN-amplified neuroblastoma
MSR Mitochondrial stress response
MTHFD1 Methylenetetrahydrofolate dehydrogenase 1
MTX Methotrexate
MYCN V-myc avian myelocytomatosis viral oncogene neuroblastoma-derived homolog
NB Neuroblastoma
NEN Niclosamide ethanolamine
NGF Nerve growth factor
NK cell Natural killer cell
OS Overall survival
PA2G4 Proliferation-associated protein 2G4
PDX model Patient-derived xenograft model
PHGDH Phosphoglycerate dehydrogenase
PIP Pyrrole–imidazole polyamide
PLK1 Polo-like kinase-1
PP2A Protein phosphatase 2A
PRC1 Polycomb repressive complex 1
PRC2 Polycomb repressive complex 2
PRDX6 Peroxiredoxin 6
PRMT1 Protein arginine methyltransferase 1
PRMT5 Protein arginine methyltransferase 5
RA Retinoic acid
REP-204 Erythrocyte extracellular vesicles
ROS Reactive oxygen species
RTK Receptor tyrosine kinase
SAGA complex Spt–Ada–Gcn5-acetyltransferase complex
SAHA Suberoylanilide hydroxamic acid
SCLY Selenocysteine lyase
SE Super enhancer
SELENOP Selenoprotein P
SEPHS2 Selenophosphate synthase 2
siRNA Small interfering RNA
SMMs Small-molecule microarrays
TAD Transcription activation domain
TFAP4 Transcription factor activator protein 4
TfR1/CD71 Transferrin receptor 1
TGF-β Transforming growth factor β
TLR Toll-like receptor
USP7/HAUSP Ubiquitin-specific protease 7
VIP Vasoactive intestinal peptide
Keywords: MYCN amplification, N-myc, MYCN protein, neuroblastoma, epigenetic regulation, PROTAC, noncoding RNA, metabolic reprogramming
Citation: Chen Y, Yang H, Xiao L, Yan N and Zhang M (2025) Molecular regulation and therapeutic targeting of MYCN in neuroblastoma: a comprehensive review. Front. Cell Dev. Biol. 13:1683331. doi: 10.3389/fcell.2025.1683331
Received: 10 August 2025; Accepted: 13 October 2025;
Published: 30 October 2025.
Edited by:
Barbara Illi, National Research Council (CNR), ItalyReviewed by:
Vina Lestari Putra, University of New South Wales, AustraliaJodie Bojko, University of Bristol, United Kingdom
Copyright © 2025 Chen, Yang, Xiao, Yan and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Zhang, bWluZ3poYW5nc2N1QDE2My5jb20=; Naihong Yan, eWFubmFpaG9uZ0B3Y2hzY3UuY24=
†These authors share first authorship
 Yi Chen
Yi Chen Huixian Yang1†
Huixian Yang1† Ming Zhang
Ming Zhang