- 1School of Traditional Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
- 2Dongfang Hospital, Beijing University of Chinese Medicine, Beijing, China
- 3Department of Oncology, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
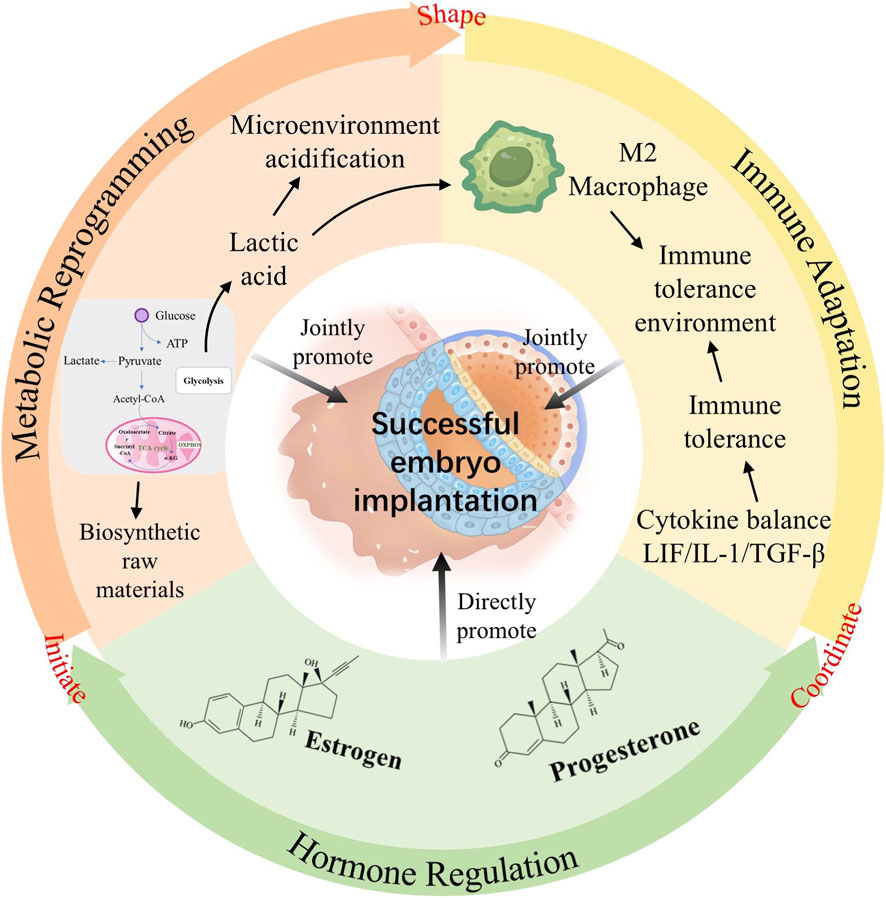
Introduction: Endometrial receptivity (ER), critical for successful embryo implantation and a major limiting factor in infertility affecting ∼1 in 6 couples globally, remains poorly understood, with few effective interventions targeting the embryo-endometrium interaction. Intriguingly, similarities exist between the implantation microenvironment and the Warburg effect, a metabolic hallmark of cancer characterized by aerobic glycolysis, lactate production, and low pH.
Methods: We conducted a comprehensive review (PubMed search up to April 2025) using keywords related to the Warburg effect (aerobic glycolysis, lactate, mitophagy), infertility (IVF, embryo implantation, TCM), cancer, cytokines (IL-1, LIF, TGF-β), and hormones (estrogen, progesterone).
Results: The review identified significant mechanistic parallels: 1) Blastocysts and trophoblasts establish a pro-receptive, high-lactate/low-pH microenvironment via Warburg-like glycolysis; 2) Shared immune modulation occurs (e.g., PI3K-AKT-FOXO1 pathway), balancing inflammatory attachment and immune tolerance; 3) Glycolysis regulates key ER-associated genes (e.g., MRAP2, BCL2L15) and cytokines (IL-1, LIF, TGF-β); 4) Invasive trophoblast behavior mirrors cancer cell invasion, potentially fueled by Warburg metabolism; 5) Hormones (estrogen, progesterone) critically orchestrate glycolytic enzyme expression (e.g., GLUT1, PFKFB3), substrate availability, and lactate-mediated immune suppression to establish this metabolic state.
Discussion: While direct experimental evidence linking the Warburg effect to ER is currently limited, the compelling mechanistic overlap offers a novel paradigm for understanding implantation failure. Targeting this shared metabolic-immune-hormonal axis holds immense potential for developing innovative strategies (e.g., metabolic modulators, refined TCM approaches) to improve ER, enhance embryo implantation rates in infertility (including IVF) and recurrent miscarriage, ultimately advancing global reproductive health. Further research is needed to validate core mechanisms.
1 Background
Infertility is a reproductive challenge currently faced by both Eastern and Western societies. Statistics indicate that approximately one in six couples may experience fertility issues (Bueno-Sánchez et al., 2024). As research progresses and the technologies advance nowadays, investigators have found that the successful implantation of embryos into the endometrium is crucial for achieving and sustaining pregnancy. This process is closely linked to “endometrial receptivity” (Miravet-Valenciano et al., 2015). Endometrial receptivity refers to the uterus’s capacity to receive and accommodate embryos during the 6–10 days following ovulation. The synchrony between high-quality fertilized oocytes and the endometrium is critical for successful conception and pregnancy maintenance, highlighting embryo invasiveness and endometrial receptivity as decisive factors for successful implantation (Lessey and Young, 2019). Investigations indicate that in natural cycles, around 30% of pregnancies fail before embryo implantation (Simón et al., 1998), underscoring that many natural conceptions fail to initiate or complete implantation to achieve ongoing pregnancy (Macklon et al., 2002). Even with assisted reproductive technologies, embryo implantation remains a pivotal determinant of success rates.
Modern clinical approaches primarily aim to improve endometrial receptivity through various interventions to enhance embryo implantation rates and maintain pregnancy. In terms of pharmacological treatment, hormone replacement therapy (such as estrogen and progesterone) is widely used to regulate the endometrial cycle (Paulson, 2019), while traditional Chinese medicine (TCM) (Xin et al., 2018) and Chinese patent medicines (Paulson, 2019; Pan et al., 2023) are also commonly applied.
In TCM research focused on improving endometrial receptivity, several active components have shown potential therapeutic value. Paeoniflorin, the main active compound in Paeonia lactiflora, significantly improves embryo implantation rates in an RU486-induced implantation failure mouse model, and enhances the adhesion ability of human trophoblasts and endometrial cells by upregulating leukemia inhibitory factor (LIF) expression. The mechanism of this effect may be related to the regulation of the LIF signaling pathway (Park et al., 2021). Ginsenosides from Panax ginseng exert their effects through multiple pathways: Rg3 inhibits the VEGFR-2-mediated PI3K/Akt/mTOR signaling pathway, reducing ectopic endometrial angiogenesis and inducing cell apoptosis (Cao et al., 2017); Rg1 alleviates endometrial fibrosis by interfering with the ROS/NLRP3 inflammasome signaling pathway (Song et al., 2023); and Rh3 combats oxidative damage to endometrial cells by activating the Nrf2 signaling pathway (Wang XM. et al., 2020). In compound studies, the PRP formula composed of Paeonia lactiflora, Rehmannia glutinosa, and Perilla frutescens, treated by polysaccharide removal, showed enhanced endometrial receptivity (Eun-Yeong et al., 2019), while the Bushen Cuyun Recipe can improve receptivity by mitigating RU486-induced endometrial damage (Jiang et al., 2023). WSYXD regulates PI3K, HIF-1α signaling, and VEGF expression to assist in the recovery of endometrial receptivity (Xin et al., 2018), promoting endometrial angiogenesis. KLP administration increases endometrial thickness and the number of endometrial glands and pinopodes. In the endometrium, KLP supplementation upregulates the expression of estrogen receptor α, progesterone receptor, endothelial nitric oxide synthase, and integrin αVβ3 in mouse uteri (Pan et al., 2023). These studies provide a robust theoretical foundation for the use of TCM in treating endometrial receptivity disorders.
In assisted reproductive technology, embryo selection (such as PGT-A) and endometrial synchronization are critical steps; however, even with the transplantation of chromosomally normal blastocysts, the ongoing pregnancy rate remains around 50%, highlighting the importance of endometrial receptivity. Other auxiliary methods include acupuncture (Tian et al., 2024; Zhong et al., 2019; Shuai et al., 2015) (possibly through the regulation of uterine blood flow), immunomodulatory treatments (Seles et al., 2024) (targeting Th1/Th2 balance), and emerging intrauterine platelet-rich plasma (PRP) infusion (Li et al., 2023) (possibly promoting endometrial repair via growth factors). It is noteworthy that mechanical endometrial injury (scratching) has been proposed to enhance receptivity, but its clinical benefits remain controversial (Santos-Ribeiro et al., 2017). Currently, tools for endometrial receptivity testing (such as RNA-Seq-based ERT) have been developed to more accurately define individualized implantation windows through transcriptomic analysis (He et al., 2021; Ben Rafael, 2021). However, existing treatments mainly focus on the endometrial microenvironment rather than directly intervening in the “embryo-endometrium dialogue.” Future research needs to further elucidate the roles of pathways such as BMP/ACVR2A7 (Monsivais et al., 2021), LIF/STAT38 (Liang et al., 2021), and HOXA109 (Mishra and Modi, 2025) in the establishment of receptivity, to develop more precise intervention strategies.
Research suggests that the high lactic acid and low pH environment created by blastocysts can improve endometrial receptivity (Gurner et al., 2022). This specific microenvironment is achieved through elevated glucose flux facilitated by aerobic glycolysis, supporting heightened biosynthesis in rapidly proliferating cells such as blastocysts, trophoblast cells, and cancers (Gou and Zhang, 2023). This phenomenon, which is widely acknowledged as the “Warburg effect,” is utilized by cancers to support their aggressive growth (Liberti and Locasale, 2016a). Given the functional similarities between blastocyst invasion of the endometrium and cancer invasion of surrounding tissues, it is plausible that cancers employ similar cellular processes and signaling pathways as blastocysts.
Based on this hypothesis, this study conducted a literature review using the keywords “endometrial receptivity,” “Warburg effect,” and “endometrial receptivity and Warburg effect” to explore their correlation, aiming to provide novel insights for clinical management of infertility.
2 The concept and process of the Warburg effect
Cells in various tissues of the human body can take up glucose from the blood. Under conditions of sufficient oxygen supply, glucose undergoes complete oxidation, primarily via glycolysis in non-proliferative tissues. In this pathway, glucose is metabolized to pyruvate, which enters the mitochondria and is completely oxidized to CO2 and H2O, at the same time releasing substantial energy to drive ATP synthesis. However, when oxygen supply is limited, glucose in the cytoplasm proceeds through glycolysis to generate pyruvate, which is then converted to lactate. This process yields noticeably less ATP per glucose molecule compared to oxidative phosphorylation under aerobic conditions. Lactate production helps regenerate NAD+ to sustain glycolysis, but each glucose molecule produces approximately 28–30 fewer ATP molecules than through oxidative phosphorylation (Berg et al., 2002).
In the 1920s, Otto Warburg observed that tumor cells produce large amounts of lactate even in Adequate oxygen supply (Warburg and Negelein, 1927). Most tumor cells primarily generate ATP through anaerobic glycolysis, leading to lactic acid accumulation, a phenomenon known as the “Warburg effect”. This phenomenon is not limited to tumor cells but also occurs under physiological conditions such as during neural crest migration (Bhattacharya et al., 2020), lymphocyte proliferation (Wang et al., 1976), and macrophage proliferation (Hard, 1970). The “Warburg effect” in tumor cells forms the basis of FDG-PET imaging for tumors and is now widely used in clinical practice (Gallamini et al., 2014).
Why do tumor cells and some proliferating cells alternative a less efficient metabolic mode even when the supply is sufficient? While Warburg and others initially attributed this phenomenon to mitochondrial defects impairing the respiratory chain (WARBURG O, 1956), recent research has shown that mitochondria in tumor cells are functional dynamic and operational (Xu et al., 2015). Current understanding suggests that this metabolic preference may be driven by oncogenic signaling pathways involving kinases and transcription factors (Hsu and Sabatini, 2008; Kroemer and Pouyssegur, 2008). Studies have also demonstrated that estrogen-related receptor (ERR), acting as a coactivator of hypoxia-inducible factor (HIF), interacts with HIF to enhance the expression of glycolytic genes under hypoxic conditions (Ao et al., 2008).
3 Embryo implantation and the Warburg effect
3.1 Embryo implantation process
Figure 1 the process of embryo implantation involves the initial interaction between an implantation-competent blastocyst and a receptive uterine endometrium, marking the first intimate dialogue between the two. This process, known as embryo implantation, is artificially divided into three stages: apposition, adhesion, and invasion (Enders and Schlafke, 1967). By the fourth day after ovulation, the blastocyst enters the uterine cavity through the tubal ostium and freely rolls within the uterine cavity. In mice, by the fourth or fifth day post-fertilization (pd4/pd5), the trophoblast differentiates into the polar trophectoderm (PTE), which contacts the inner cell mass, and the mural trophectoderm (MTE), which adheres to the uterine wall and initiates implantation (Bondarenko et al., 2023). Several factors, such as E-cadherin (Fleming et al., 2001), dynamic interactions between uterine epithelial cell actin cytoskeleton and integrins (Thie and Denker, 2002), facilitate the trophectoderm cells to adhere to the receptive uterine epithelial cells in appropriate positions and orientations. Subsequently, the invasive blastocyst penetrates the uterine epithelial cells, infiltrates into the stroma, and becomes enveloped by the uterine endometrium. Within the stroma, the embryo undergoes vascular wall remodeling and luminal widening (Pijnenborg et al., 2006) to accommodate high-speed blood flow (Carter et al., 2015), forming an extensive vascular network (Achache and Revel, 2006; Bhurke et al., 2020). This network supports embryo growth and ensures successful implantation.
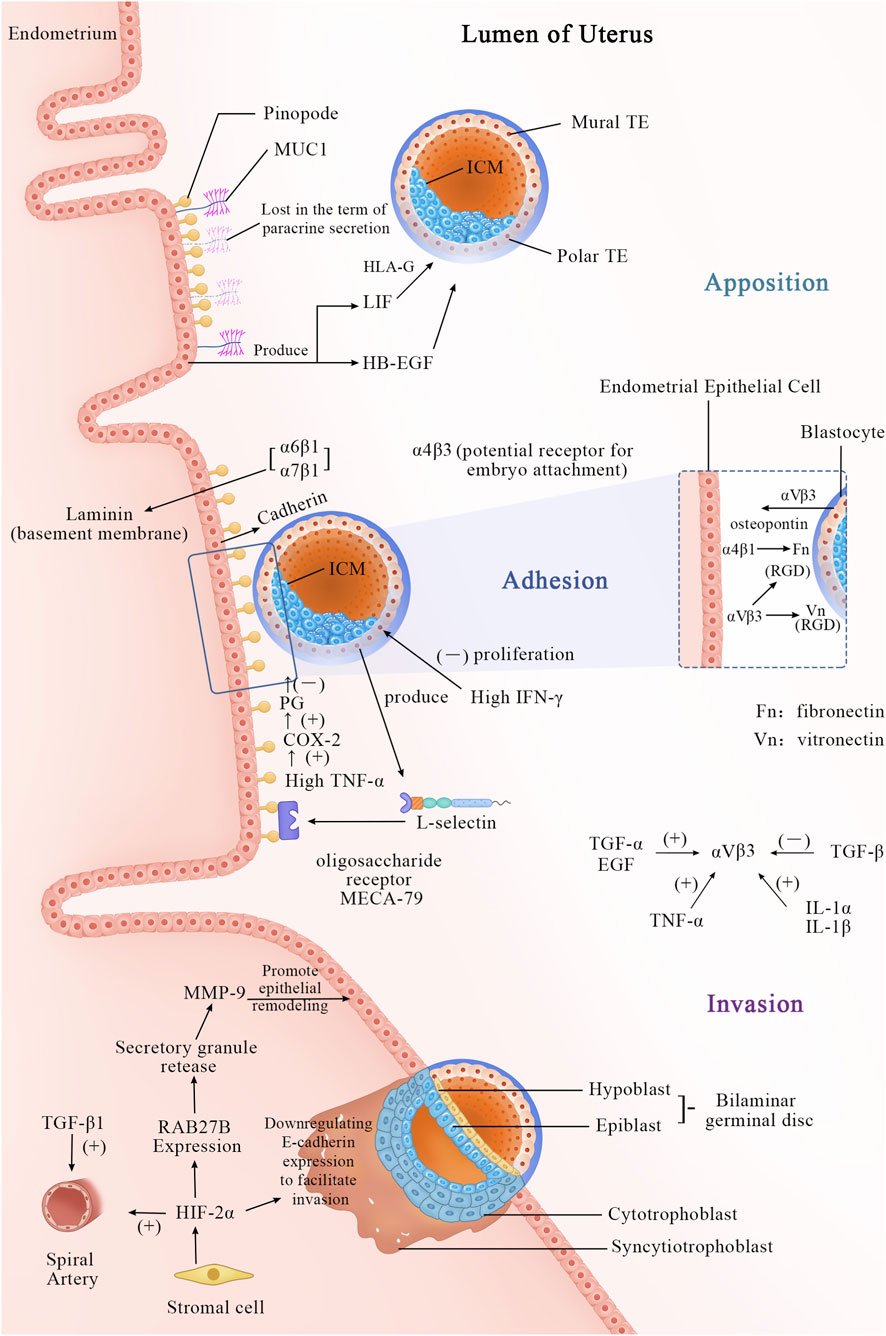
Figure 1. Apposition Phase (Day 5–6 Post-Fertilization): Cellular Events: The blastocyst completes hatching and is released into the uterine cavity. Through rotation and movement, the blastocyst makes initial contact with the uterine epithelial surface, where microvilli interact, but no firm adhesion is yet formed. The blastocyst adjusts its position and orientation, preparing for subsequent attachment. Molecular Events: A. The morphological feature of a receptive endometrium is the presence of apical protrusions on the luminal epithelial cells, described as “mushroom-shaped” or “sea anemone-like,” known as “pinopodes.” B. MUC1, a mucin, is expressed in the endometrium and may initially play a repulsive role to prevent non-implantation during the non-receptive period. At the end of the apposition phase, it is locally lost via paracrine secretion, assisting in positioning. C. Other molecules, such as LIF and HB-EGF, begin to participate as well. Adhesion phase (Day 6–7 post-fertilization): Cellular Events: The blastocyst establishes a stable, irreversible connection with the uterine epithelial surface via adhesion molecules. This stage anchors the blastocyst to the endometrium, preventing detachment. Molecular Events: A. MUC1 is completely cleaved, permitting direct attachment. B. Adhesion molecules, such as cadherins, integrins, and L-selectins, mediate intercellular connections. L-selectin is expressed on the surface of trophoblast cells and binds to L-selectin ligands (e.g., MECA-79) on the uterine epithelial surface, facilitating initial contact and orientation. C. The integrin subunit α4β3 is a hallmark of the implantation window. αVβ3 may serve as a potential receptor for embryo attachment, binding to the RGD sequence and initiating cell-cell interactions for trophoblast adhesion to the uterine epithelium. The αVβ3 dimer is present in both the uterine epithelium and trophoblast cells. This reciprocal interaction facilitates embryo anchorage: trophoblast αVβ3 recognizes uterine extracellular matrix (ECM) proteins, while uterine αVβ3 interacts with vitronectin and fibronectin expressed by trophoblast cells. The expression of αVβ3 is closely associated with several growth factors: TGF-α, EGF, TNF-α, IL-1α, and IL-1β, all of which can promote its expression under appropriate conditions, whereas TGF-β suppresses it. During embryo attachment to the endometrium, newly expressed integrin subunits α2, α6, and α7 indicate the blastocyst’s capability for implantation. Among these, integrins α6β1 and α7β1 bind to laminin, which is highly distributed in the basal membrane of the endometrial epithelium during the implantation window. Additionally, integrin α4β1, which is also expressed in the endometrium, interferes with fibronectin function in the trophoblast. D. During the adhesion process, the high expression of IFN-γ regulates the inflammatory microenvironment, decreasing the proliferation and invasion capacity of trophoblast cells. E. The increased secretion of TNF-α by the embryo is associated with implantation failure. TNF-α disrupts embryo-endometrium adhesion directly or indirectly by upregulating the COX-2/PGs pathway. Invasion phase (Day 7–12 post-fertilization): Cellular Events: The trophoblast differentiates into cytotrophoblast and syncytiotrophoblast. Trophoblast cells of the blastocyst invade the uterine epithelium, penetrate the stromal layer, and eventually embed deep into the uterine wall. This phase involves extracellular matrix degradation, vascular invasion, and decidualization, laying the foundation for placenta formation. Molecular Events: A. Stromal cells secrete HIF-2α, which reshapes the maternal epithelial barrier by activating the RAB27B-MMP-9 pathway, while also directly inhibiting E-cadherin to reduce cell adhesion. B. Meanwhile, TGF-β1 regulates the functional adaptation of the spiral arteries. The cytotrophoblast proliferates and differentiates into syncytiotrophoblasts, which, with the cooperation of these molecules, penetrate maternal tissues, completing embryo implantation.
The initial step of the implantation process relates to the interaction between L-selectin expressed on trophoblast cells and oligosaccharide ligands expressed on the endometrium. L-selectin, a cell adhesion molecule, plays a fundamental role during implantation (Heemann et al., 1994). Similar to its role in leukocyte-mediated early inflammation, L-selectin interacts with ligands on vascular endothelium, facilitating leukocyte rolling, adhesion, and migration. The rolling phenomenon of leukocytes parallels the attachment of blastocysts to the endometrial epithelium.
During invasion, trophoblasts, tasked with bridging the gap between placenta and uterus, are believed to utilize L-selectin to mediate interstitial migration (Prakobphol et al., 2006; Fazleabas and Kim, 2003). Studies indicate that L-selectin ligands such as MECA-79 and HECA-452 are upregulated during the implantation window. MECA-79 localizes to the endometrial epithelium throughout the menstrual cycle, aiding blastocysts in locating the optimal implantation site within the uterine cavity. Additionally, chemotactic and cytokine factors attract blastocysts to the optimal implantation site within the uterine cavity (Achache and Revel, 2006).
Most epithelial cells in the apex of the upper surface are protected by a glycocalyx, and endometrial epithelial cells are undoubtedly no exception (Aplin et al., 1994; Devine and McKenzie, 1992). Analysis shows that mucin MUC1 extends beyond the glycocalyx, potentially serving as the first molecule encountered by the blastocyst during implantation. MUC1 and MUC16 may have a negative influence on the implantation process, acting as an endometrial anti-adhesive molecule (Meseguer et al., 2001). During the receptive phase in humans, the endometrial epithelium is influenced by progesterone (Horne et al., 2006) and the interaction of the blastocyst (Meseguer et al., 2001), with MUC1 persistently elevated. When the blastocyst allows adhesion to the EEC (Endometrial Epithelial Cells) monolayer, MUC1 is locally lost only at the implantation site in a paracrine manner (Meseguer et al., 2001). The morphological characteristics of receptive endometrium include apical protrusions on luminal epithelial cells that can be described as “mushroom-like” or “sea-anemonae-like” (Nilsson, 1972; Psychoyos and Mandon, 1971). Originally termed “pinopods) (“drinking foot” in Greek) The term “uterodome” has also been used to dissociate these protrusions from an implied function. Most existing literature refers to these protrusions as pinopodes. Pinopodes are generally considered to be 5–10 μm cell protrusions at the apical surface of uterine epithelial cells, often associated with successful implantation (NILSSON, 1958; Usadi et al., 2003) and strongly regulated by ovarian steroid hormones (Quinn et al., 2020). Pinopodes serve as implantation sites, crucially providing an area unaffected by MUC1-mediated inhibition of embryo-endometrial interaction (Aplin, 1997). However, there remains controversy in the academic community regarding whether it is MUC1 or MUC16 that plays a role. Experimental evidence has detected the presence of MUC1 on pinopodes, while short interfering RNA (siRNA) knockdown of MUC1 does not affect the adhesion of trophoblast cells; conversely, in parallel experiments, knockdown of MUC16 using siRNA increases trophoblast cell adhesion (Gipson et al., 2008) Moreover, MUC1 has been implicated in potentially exerting significant influence on guiding blastocysts to appropriate implantation sites within the uterine cavity (Achache and Revel, 2006; Ashary et al., 2018). Simultaneously, MUC1 may facilitate blastocyst implantation; recent analyses have identified L-selectin on the surface of human blastocysts and complementary selectin ligands (such as sialylated oligosaccharides) on the receptive-phase uterine surface, supporting early blastocyst adhesion. However, it remains unclear which specific MUC1 molecules carry selectin ligands due to the glycan diversity of MUC1. MUC1 may participate in interactions with selectins, thus potentially facilitating blastocyst implantation (Carson et al., 2006).
Adhesion molecules such as integrins and calcium-binding proteins firmly attach the blastocyst to the implantation site of the endometrium to ensure successful implantation. The implantation process involves the blastocyst invading the superficial layers of the decidua, uterine myometrium, and spiral arteries of the uterus, while transitioning to an interstitial cell phenotype (Burrows et al., 1996; Vićovac and Aplin, 1996). This deep penetration of the uterine wall by trophoblast cells enables the placenta to securely affix itself and establish vascular connections between the two tissues (Merviel et al., 2001). Current exploration has identified various integrins present in the luminal and glandular epithelium of the human endometrium. During days 20–24 of the human menstrual cycle, three cycle-specific integrins—α1β1, α4β1, and αVβ3, as defined histologically—are co-expressed in the human endometrium, with only β3 mRNA expression showing an increase after day 20 (Merviel et al., 2001). The presence of both α1 and α4 subunits coincides with embryonic implantation during this window (days 20–24). Blockade of integrin β3 notably affects endometrial receptivity (Liu et al., 2013). The α4ß3 heterodimer serves as an excellent marker for the implantation window (Merviel et al., 2001), while αVβ3 may act as a potential receptor for embryo attachment. Experimental evidence has demonstrated the binding of αVβ3 to the RGD sequence, initiating cell-cell interactions necessary for attachment of trophoblast integrins to the uterine epithelium (Merviel et al., 2001). E-cadherin may possess a dual role in this process. Initially, its surface expression on cells is required for adhesion, whereas subsequently, E-cadherin may be downregulated to facilitate epithelial cell detachment and trophoblast invasion (Achache and Revel, 2006).
Research endeavor on the mechanism of embryo invasion into the endometrium is limited. Existing studies illustrate that during implantation, hypoxia-inducible factor 2α (HIF-2α), which is induced in stromal cells beneath the endometrium, plays a vital role early in pregnancy. HIF-2α is essential for the generation, survival, and/or maintenance of PGCs (Covello et al., 2006). As the embryo implants, rapid growth at the implantation site exceeds the blood supply, creating a relatively hypoxic environment (Covello et al., 2006). Under low oxygen conditions, hypoxia activates factors such as HIF-1α and HIF-2α. HIF-2α during implantation regulates the expression of RAB27B (Ma et al., 2022), a member of the Rab GTPase family that controls secretion granule release. These granules participate in the transport of MMP-9 from stroma to epithelium, promoting luminal epithelial remodeling during embryo invasion. Additionally, HIF-2α can directly or indirectly activate Oct-4 (Covello et al., 2006), which in turn activates Fgf-4, crucial pre-implantation growth factors necessary for embryo viability (Feldman et al., 1995). Evaluation also demonstrates that exposure of pre-implantation endometrial stromal cells to hypoxic conditions in vitro significantly enhances HIF2α expression. Thus, HIFs likely regulate the implantation process by modulating the uterine environment to a hypoxic state (Prakobphol et al., 2006). Furthermore, research project indicates that HIF-1α drives mouse embryonic stem cells to rely entirely on glycolysis for energy acquisition (Zhou et al., 2012). According to the study by (Maybin et al., 2018) et al., HIF-1α is essential for normal endometrial repair during menstruation, including genes associated with glucose metabolism (Wu et al., 2023) and angiogenesis (Meo and de Nigris, 2024). Due to the disappearance and remodeling of the spiral arteries during the menstrual cycle and pre-implantation endometrial preparation, the endometrium frequently experiences localized hypoxia and cellular oxidative stress (OS). This highlights the importance of the precise regulatory pattern of HIF-1α in the endometrium (Vaux et al., 2001; Gashaw et al., 2008). Under physiological conditions, the levels of HIF-1α fluctuate due to localized hypoxia, which is essential for tissue healing in the physiological regenerative process of endometrial receptivity preparation (Greenhill, 2018). This process has significant implications for endometrial decidualization (Dai et al., 2024).
3.2 The Warburg effect during embryo implantation
Figure 2 during the process of embryo implantation, physiological processes analogous to the Warburg effect may be observed. First, in early pregnancy, trophoblast cells proliferate rapidly and exhibit invasive properties, akin to cancer cells (Kohlrausch and Keefe, 2020). Secondly, embryos generate lactate-specific metabolism, creating a microenvironment at the implantation site characterized by high lactate levels and low pH (Gurner et al., 2022), similar to the microenvironment observed in the Warburg effect of cancer cells, where lactate is produced via glycolysis. Moreover, following implantation into the endometrium, embryos undergo angiogenesis within the stroma, remodeling blood vessel walls and widening lumens (Pijnenborg et al., 2006), to accommodate high-speed blood flow, thereby forming an extensive vascular network (Carter et al., 2015). Furthermore, studies reveal that during embryo implantation, trophectoderm cells undergo epithelial-mesenchymal transition to acquire appropriate adhesion and invasive abilities, which if uncontrolled, may contribute to cancer progression. This supports the notion of similarities between embryo implantation and the Warburg effect in cancer cells (Hantak et al., 2014). In vitro research proves that embryos appear to transition from oxidative to glycolytic metabolism pre-implantation, followed by a re-induction of oxidative metabolism post-implantation (Johnson et al., 2003). Thi T Truong et al. demonstrated that antioxidants can improve pre-implantation embryo development and viability in mice (Truong et al., 2016), further emphasizing the association between embryo implantation and the Warburg effect.

Figure 2. Within the embryonic cells, aerobic glycolysis is active, generating pyruvate and a limited yield of ATP enzymatically. Concurrently, acetyl-CoA facilitates the transition of embryonic cells into a growth phase by promoting histone acetylation. T helper 1 (Th1) cells modulate macrophage function through the secretion of interferon-gamma (IFN-γ), inducing alterations in the surrounding macrophages characterized by enhanced glucose uptake and increased expression of glycolytic enzymes. The interleukin-1 (IL-1) secreted by these cells, which shows a positive correlation with estrogen levels, upregulates the expression of adhesion factors in the endometrial epithelial cells, thereby promoting blastocyst implantation. Furthermore, leukemia inhibitory factor (LIF) secreted by macrophages stimulates the growth of embryonic trophoblast cells, accelerating embryonic development. Macrophages, Th1 cells, and natural killer (NK) cells collectively secrete tumor necrosis factor-alpha (TNF-α), which can induce aerobic glycolysis by provoking an inflammatory response. Simultaneously, the endometrial epithelial cells upregulate monocarboxylate transporter 4 (MCT4) under hypoxic conditions, further promoting aerobic glycolysis.
There is substantial glycolytic data supporting this hypothesis. The study by Jie Zheng (Zheng et al., 2023) et al. provides robust support for our hypothesis. In their investigation of HRT-FET cycles—that is, frozen embryo transfer (FET) performed during hormone replacement therapy (HRT) cycles—they observed a marked decrease in serum levels of TCA cycle substrates around the time of endometrial transformation. This suggests a potential reduction in oxidative metabolism in vivo, which closely resembles the metabolic features of the Warburg effect Weng et al. (2025). Found that impaired glycolysis can lead to defects in endometrial decidualization and contribute to infertility associated with endometriosis, indirectly indicating the importance of the Warburg effect for successful pregnancy. Most importantly, recent evidence clearly demonstrates that decidualization of human endometrial stromal cells is driven by Warburg-like glycolysis and regulated through mechanisms such as lactylation-mediated gene expression (Huang et al., 2025).
3.3 The role of mitochondrial dynamics in the process of embryo implantation
Mitochondrial dynamics play a crucial role in both blastocyst and endometrial tissue during the process of embryo implantation. Studies have shown that the balance between mitochondrial fusion and fission is essential for blastocyst formation, and disruption of this dynamic equilibrium significantly impairs blastocyst developmental quality, leading to abnormal cell lineage allocation, impaired energy metabolism, and the regulation of gene expression through epigenetic modifications (Sun et al., 2025). During the blastocyst stage, mitochondrial DNA (mtDNA) content significantly increases, and its replication, uncoupled from mitochondrial abundance, serves as a critical safeguard for overcoming developmental bottlenecks post-implantation (Winstanley et al., 2024; Shavit et al., 2025). Simultaneously, the functional status of the endometrial tissue directly influences implantation success, with premature senescence of endometrial stromal cells impairing decidualization through the accumulation of reactive oxygen species (ROS), thereby disrupting the interaction between the endometrium and the blastocyst (Deryabin et al., 2022). Changes in mitochondrial oxidative phosphorylation levels in endometrial stromal cells manifest as the downregulation of energy metabolism-related genes, such as PGC-1α, which may be associated with mitochondrial dysfunction in patients with repeated implantation failure (RIF) (Han et al., 2021; Han et al., 2023). These findings collectively reveal the pivotal role of mitochondrial dynamics in the coordinated interplay between blastocyst developmental potential and endometrial receptivity through the regulation of energy metabolism, gene expression, and intercellular communication.
4 How the Warburg effect affects endometrial receptivity
4.1 Immune modulation
Investigations imply that (Xu et al., 2021) nutrient metabolism and growth factor signaling are highly integrated processes. Glycolytic ATP serves as a variable resistor to measure the role of PI3K-AKT-FOXO1 signaling in T cell immune regulation, thereby elucidating as the Warburg effect. During implantation (Fujiwara et al., 2020), embryonic signals can induce immune tolerance and a robust local inflammatory response. The former prevents immune rejection reactions, while the latter promotes endometrial proliferation and maternal tissue remodeling crucial for embryo implantation and placental formation. It can be inferred that the immunomodulatory function of the Warburg effect is related to immune responses during embryo implantation.
Studies have shown that there exists a bidirectional regulatory relationship between energy metabolism and immune responses (Weerasinghe et al., 2024; Turner et al., 2016). In postpartum dairy cows, the defense of the uterine endometrium against bacterial infections primarily relies on innate immune mechanisms (Nash and Giles, 2025; Turner et al., 2012). However, when the organism is in a state of metabolic energy stress, this defensive capacity is significantly impaired, leading to reproductive disorders such as endometritis and infertility (Ji et al., 2025; Zhao et al., 2018). Specifically, inflammation significantly increases glucose consumption in the endometrium and induces metabolic reprogramming similar to the Warburg effect. This aberrant metabolic state may further exacerbate the decline in tissue glucose utilization. More importantly, energy metabolism stress significantly impacts uterine receptivity by interfering with glucose utilization and the AMPK signaling pathway (Zhang et al., 2022)—two core regulatory factors of cellular energy metabolism.
4.2 Microenvironment acidification
Embryos possess a unique metabolism that results in the establishment of a microenvironment characterized by elevated lactate levels and decreased pH (Gurner et al., 2022). Similarly, cancer exhibits aerobic glycolysis, thereby creating a comparable microenvironment (Apostolova and Pearce, 2022) to facilitate invasion of surrounding tissues.
Research indicates that lactate alters the endometrial receptivity remodeling process (Gurner et al., 2022), where lactate and reduced pH present in the microenvironment at implantation serve as early embryo signals to modify the function of endometrial epithelial cells, thereby enhancing endometrial receptivity and implantation initiation. Meanwhile, lactate and the associated decreased extracellular pH have been proven to convert the expression of several lactate-responsive genes and the epigenetic “lactylation” (Chen et al., 2021), further supporting this concept. During the establishment of uterine receptivity, the progesterone-dependent decidualization process significantly activates glycolysis and lactate synthesis. The produced lactate regulates redox homeostasis and apoptotic balance by inducing histone lactylation, a novel epigenetic modification, thereby ensuring successful embryo implantation. Inhibition of histone lactylation impairs the decidualization process (Zhao et al., 2023; Yang et al., 2022). Studies in ovine uterine tissue have revealed the crucial role of lactate in inducing H3K18 lactylation of the uterine endometrium and regulating redox balance and apoptotic equilibrium to ensure successful implantation. Concurrently, under pathological conditions, higher levels of lactate and lactate dehydrogenase A in ectopic endometrial tissue and ectopic endometrial stromal cells enhance histone H3 lysine 18 lactylation (H3K18lac), which can promote cell proliferation, migration, and invasion in endometriosis (Chen et al., 2023). This phenomenon was also observed in endometrial carcinoma cells, where histone lactylation was significantly elevated. Furthermore, histone lactylation regulates the expression of USP39, which, through interaction with PGK1, activates the PI3K/AKT/HIF-1α signaling pathway (Wei et al., 2024). Finally, the stimulation of glycolysis leads to the production of more lactate, further increasing histone lactylation. This reinforces the intimate connection between the Warburg effect in cancer and uterine receptivity.
Similarly, lactate can promote morphological and functional changes in other areas of the endometrium, manifested through modulation of stromal cell migration and promotion of endothelial vessel formation (Guido et al., 2012). Another aspect of lactate’s impact on endometrial receptivity remodeling, aside from changes in the luminal epithelium, is the differentiation of endometrial stromal cells, termed decidualization. Decidualization supports ongoing pregnancy through regulation of trophoblast invasion, support of vascular development, and formation of the placenta (Zuo et al., 2015), which are essential processes supported by maternal endometrial stromal cells for pregnancy (Lindsay et al., 2023). Lactate can trigger M2 or M1 macrophage polarization through oxidative phosphorylation and glycolytic regulation to exert decidual macrophage function (Gao et al., 2022). Warburg-like glycolysis and local lactate shuttle are activated during decidualization and play critical roles in supporting early pregnancy. Observations suggest (Young et al., 2014) that increased lactate levels may contribute to promoting the survival of ectopic endometrial cells and the establishment of lesions, akin to cancer cell metastasis. Endometriotic lesions and adjacent peritoneal tissues exhibit remarkably elevated glycolytic markers, suggesting metabolic phenotypic changes resembling the Warburg effect in tumorigenesis. Patients with endometriosis and cancer patients (Guido et al., 2012) are similarly influenced by TGF-β1 and demonstrate a significant lactate increase in peritoneal fluid (Young et al., 2014). This could potentially “feed” ectopic endometrial cells, enabling their survival, implantation, and invasion into the peritoneum, leading to endometriotic lesions.
4.3 Gene expression regulation
4.3.1 Mechanism speculation
4.3.1.1 Regulation of cell metabolism and growth genes
Recent research has demonstrated that the chromatin structure plays a crucial role in regulating various cellular functions, including DNA repair and gene transcription (Liberti and Locasale, 2016b). Furthermore, it has been found that glycolytic metabolism can influence chromatin structure (Liu et al., 2015). Now it is acknowledged that there is a direct link between cellular metabolism and the regulation of growth genes, with intracellular levels of acetyl-CoA potentially representing a widely conserved mechanism that promotes this important connection (Cai et al., 2011). It has been established that the substrate for histone acetylation, acetyl-CoA, can be regulated through glucose flux (Evertts et al., 2013). Elevated levels of acetyl-CoA may be sufficient to drive cells into a growth phase through histone acetylation (Lu and Thompson, 2012). Therefore, we hypothesize that the Warburg effect may regulate growth genes by altering cellular metabolism processes.
4.3.1.2 The Warburg effect enhances glycolysis and glutamine breakdown to maintain cell proliferation
In normal functional cells, glucose is consumed via the tricarboxylic acid cycle to provide ATP for normal cellular physiological activities. However, in rapidly proliferating cells, aerobic glycolysis consumes large amounts of glucose to generate components essential for cell proliferation. The intersection between anabolic and catabolic pathways is primarily regulated by pyruvate kinase, which is re-expressed in its embryonic form as PKM2 (Mazurek et al., 2007). When PKM2 is inactive, it promotes anabolic metabolism (and branch pathways of glycolysis) (Lepleux et al., 2012), whereas active PKM2 catalyzes the conversion of phosphoenolpyruvate (PEP) to pyruvate, thereby generating one molecule of ATP.
Meanwhile, the inactivation of pyruvate dehydrogenase due to the Warburg effect blocks pyruvate from entering the tricarboxylic acid (TCA) cycle, forcing tumor cells to rely on glutamine as an alternative carbon and nitrogen source to sustain TCA cycle function (Delgir et al., 2021; Altman et al., 2016). This metabolic shift has been described as the “second Warburg effect,” where glutamine metabolism transitions from traditional catabolic pathways to nucleotide biosynthesis to meet the biosynthetic demands of malignant progression (Kodama and Nakayama, 2020). Mechanistically, the Warburg effect and glutamine catabolism are co-regulated in a synergistic manner: the increased glycolytic flux promotes lactate secretion through NAD+ consumption, while α-ketoglutarate (α-KG) produced from glutamine catabolism replenishes TCA cycle intermediates, together maintaining the acidic tumor microenvironment and providing biosynthetic precursors (Vallée et al., 2021; Chiarella et al., 2022). Computational biological models further confirm that the coupling of the Warburg effect with glutamine catabolism optimizes energy metabolism efficiency through linear programming, wherein glutamine not only serves as an energy substrate but also supports nucleotide synthesis via nitrogen metabolism reprogramming (Zhang and Boley, 2022; Ewald et al., 2024). Therapeutically, the glutamine metabolic reprogramming driven by the Warburg effect induces a “nutrient addiction” in tumor cells, providing a theoretical foundation for targeting the glycolysis-glutaminolysis axis as a therapeutic strategy (Lee and Kim, 2016). Recent studies also reveal that oncogenic pathways such as the Wnt signaling pathway can simultaneously activate both the Warburg effect and glutamine catabolism, further enhancing tumor metabolic plasticity through the promotion of macropinocytosis (Flores-Hernández et al., 2025).
4.3.2 Genes and proteins may be involved
4.3.2.1 MRAP2
Immunohistochemical evaluation revealed significant differences in MRAP2-positive staining among patients with idiopathic infertility. Compared to the control group, protein imprinting analysis of specific bands showed increased expression of MRAP2 protein in patients with idiopathic infertility (D'Aurora et al., 2019).
4.3.2.2 BCL2L15
An animal experiment demonstrated upregulation of BCL2L15 in endometrial epithelial cells (EECs) of goats under treatment with progesterone (P4), estradiol (E2), and interferon-tau (IFN-τ). Yang et al. observed a prominent endometrial receptivity effect by specifically knocking down BCL2L15 using shRNA (Yang et al., 2020).
4.3.2.3 PGC1α
As a central regulator of oxidative metabolism, PGC1α protects cells from oxidative damage by activating antioxidant genes while concurrently reversing the Warburg effect. During embryonic development, this metabolic regulation is critical for pre-implantation embryos in responding to oxidative stress, as the implantation stage necessitates a precise balance between aerobic glycolysis (Warburg effect) and oxidative phosphorylation to support rapid cell proliferation and differentiation (Lu, 2019).
4.3.2.4 LDHA
This protein is a key enzyme in glycolysis, and its expression exhibits a decreasing trend from the cell stage to the blastocyst stage during embryonic development (Pu et al., 2020), which correlates with variations in the intensity of the Warburg effect. LDHA, as a hub gene in the gene co-expression network, significantly influences the gene expression pattern of embryos, and its mediation of lactate production plays a crucial role in regulating the acidic microenvironment during blastocyst formation and implantation (Li et al., 2025).
4.3.2.5 ZC3H11A
Proteomic analysis reveals that this protein forms a close interactive network with mRNA export proteins in embryonic stem cells. Transcriptomic data confirm that the deletion of ZC3H11A leads to dysregulation of glycolysis and fatty acid metabolism pathways in embryos, directly affecting developmental competence at the blastocyst implantation stage. This indicates that ZC3H11A participates in the regulation of the implantation process by coordinating metabolic reprogramming (Younis et al., 2023).
4.3.2.6 BCAT2
As a regulator of branched-chain amino acid metabolism, RNA-Seq data demonstrate that its expression during the blastocyst stage is influenced by the status of mitochondrial DNA. This protein, by linking amino acid metabolism to energy supply, may participate in regulating the intensity of the Warburg effect in pre-implantation embryos, while also impacting subsequent developmental potential (Tsai et al., 2018).
4.4 Regulation of cytokines
Endometrial receptivity refers to the comprehensive state that allows adhesion, invasion, and eventual implantation of the fertilized egg during the implantation process. This is regulated by various cytokines such as IL-1, IFN-γ, LIF, TNF-α, and TGF, among others. Abnormal endometrial receptivity or delayed establishment thereof can lead to implantation failure and subsequent infertility (Quinn et al., 2020).
Related studies highlight that cytokines such as IL-1, IFN-γ, LIF, TNF-α, and TGF (Guido et al., 2012) may promote glycolysis and the production of growth factors to participate in the Warburg effect. Macrophages, a functionally heterogeneous group of cells, are primarily formed by various microenvironmental stimuli. Zhu et al. propose that macrophages activated by interleukin-1β (IL-1β) and interferon-γ (IFN-γ) exhibit a Warburg effect similar to that in tumor cells (Zhu et al., 2015). Appelberg et al. recommend that IFN-γ activation of macrophages increases glucose uptake, enhances lactate production, and upregulates key glycolytic enzymes, thereby participating in the Warburg effect (Appelberg et al., 2015). Vaughan et al. propound that aerobic glycolysis in the Warburg effect can be induced directly by the inflammatory microenvironment (TNF-α), independent of additional genetic mutations and signals from adjacent cells (Vaughan et al., 2013). Jena et al. introduce to the public that TGF induces autophagy in cancer-associated fibroblasts under hypoxic conditions, promoting glycolysis in the Warburg effect through the upregulation of MCT4 (Jena et al., 2022).
4.4.1 Interleukin-1(IL-1)
IL-1 is mainly derived from macrophages and can promote thymocyte proliferation. Hence it is referred to as lymphocyte activation factor (Dinarello, 1988). The IL-1 family consists of three members: IL-1α, IL-1β, and IL-1Ra. In recent years, the role of IL-1β in pregnancy has garnered increasing attention (Hantoushzadeh et al., 2020) and is believed to be associated with endometrial receptivity. Serum IL-1β levels are positively correlated with estrogen (Sunder and Lenton, 2000), and experimental evidence has shown that IL-1β levels are substantially elevated in patients with endometriosis primarily induced by estrogen (Montagna et al., 2008). Estrogen plays a key role in follicular growth and development as well as in endometrial growth. It can be inferred that IL-1β may participate in follicular growth, development, maturation, and induction of ovulation, potentially enhancing fertilization rates and embryo quality, thereby improving embryo implantation rates. IL-1 regulates endometrial receptivity by increasing the expression of epithelial cell adhesion molecules, thereby enhancing epithelial adherence to the blastocyst (Wang H. et al., 2018).
4.4.2 Leukemia inhibitory factor (LIF)
LIF, a member of the IL-6 family, is a multifunctional cytokine with broad biological activities impacting various aspects of reproductive function (Onishi and Zandstra, 2015). These include the growth and development of oocytes, as well as the growth, differentiation, and implantation of embryos, making it a specific molecular marker for endometrial receptivity (Sharkey, 1998). LIF can promote the growth and proliferation of trophoblast cells, facilitating embryo adhesion and implantation. Additionally, it takes part in regulating trophoblast invasiveness by controlling the expression of human leukocyte antigen-G (HLA-G) in trophoblast cells (Chung et al., 2019). Therefore, increasing the expression levels of LIF in the endometrium may enhance endometrial receptivity and improve embryo implantation rates.
4.4.3 Interferon-γ(IFN-γ)
IFN-γ is a hallmark cytokine of Th1 cells, capable of regulating the functions of other immune cells and promoting endometrial angiogenesis and vascular remodeling (Tayade et al., 2006), ensuring endothelial integrity (Jia and Li, 2020), thinning vascular walls, and increasing vascular permeability. Liu et al. (2017) present that elevated IFN-γ levels may induce excessive apoptosis of trophoblast outer layer cells, inhibit trophoblast cell line proliferation, hinder trophoblast invasion, and affect early embryo cytoskeletal formation. Zhang et al. (2015) proposed that IFN-γ can enhance the anti-apoptotic capability of in situ endometrial cells and stimulate cell adhesion molecule expression, thereby considerably influencing reduced endometrial receptivity (Qiu et al., 2020). Hence, excessive IFN-γ levels may decrease endometrial receptivity, exerting detrimental effects on pregnancy and embryo implantation.
4.4.4 Tumor necrosis factor-α (TNF-α)
TNF-α is primarily produced by macrophages, Th1 cells, and NK cells, and it can stimulate the production of other pro-inflammatory cytokines (such as IL-1β and IL-6). It plays a paramount role during embryo implantation by promoting VEGF production, angiogenesis, and controlling trophoblast invasion, proliferation, and differentiation. Adequate TNF-α is necessary for maintaining pregnancy in women, as low concentrations can effectively defend against pathogenic microbial infections. While elevated levels of TNF-α can downregulate endothelial nitric oxide synthase (eNOS) and MMP-2 expression, promote prostaglandin production by inducing COX-2 overexpression, trigger inflammatory responses, and simultaneously inhibit trophoblast invasion, thereby hindering embryo implantation (Xu et al., 2017). Consequently, high levels of TNF-α can stimulate excessive proliferation of endometrial cells (Wang XM. et al., 2018) and reduce endometrial receptivity.
4.4.5 Transforming growth factor -β (TGF -β)
TGF -β is a multifunctional peptide growth factor that stimulates proliferation of stromal cells, inhibits growth of epithelial-like cells, promotes extracellular matrix formation, suppresses immune function, and participates in angiogenesis. TGF-β1 exhibits differential expression in the endometrium at different stages: it is minimally expressed in the early and mid-proliferative phases in endometrial glands or stromal cells, whereas its expression notably increases in the late proliferative phase, predominantly in the stroma over glands. This suggests that TGF-β1 may promote endometrial growth in vivo and exert local estrogenic effects. TGF-β1 is minimally expressed during the early secretion phase but shows abundant cytoplasmic expression in glands and stromal cells during the mid-secretory phase, indicating its involvement in the formation of secretory endometrium (Erickson et al., 2004). TGF-β1 plays a role in both proliferative and secretory phases of endometrial development, promotes endometrial angiogenesis, enhances endometrial receptivity, and is one of the critical factors contributing to successful embryo implantation.
4.5 Hormone
4.5.1 Estrogen
The placenta is the primary source of estrogen during pregnancy, particularly in humans and higher primates, where the biosynthesis of placental estrogen is distinctive. After the ninth week of gestation, the placenta gradually supplants the ovaries as the major organ for estrogen secretion, leading to a significant increase in circulating estrogen levels during the later stages of pregnancy (Albrecht and Pepe, 2010). Placental estrogen not only supports pregnancy by regulating progesterone biosynthesis and the maturation of the fetal hypothalamic-pituitary-adrenal axis, but also plays a pivotal role in the formation of the placental villous vasculature and the development of fetal ovarian follicles (Albrecht and Pepe, 2010).
In terms of endometrial receptivity, estrogen promotes decidualization and embryo implantation by optimizing the uterine microenvironment (Gellersen and Brosens, 2014; Lessey, 1998; Carson et al., 2000). Studies indicate that the transient estrogen surge occurring 7–10 days post-ovulation serves as a critical signal for the “window of implantation,” which regulates epithelial cell proliferation and stromal decidualization through the activation of estrogen receptor α (ERα) in the uterine epithelial and stromal cells (Simon et al., 2003; Pawar et al., 2015). Additionally, estrogen enhances uteroplacental blood flow and promotes angiogenesis (Reynolds and Redmer, 2001), thereby providing nutritional support for embryo implantation.
4.5.1.1 Estrogen and metabolic reprogramming
Metabolomic analysis reveals that estrogen significantly affects the expression of genes associated with the mitochondrial electron transport chain, glycolysis, and the pentose phosphate pathway. This regulation may influence PARP-1 activity by altering the NAD/NADH ratio, thereby coordinating the relationship between the Warburg effect and estrogen synthesis (Riera Leal et al., 2020; Kaiser et al., 2020). In vitro experiments further confirm that estrogen exposure alters the metabolic phenotype of embryos, resulting in diminished mitochondrial respiration and increased lactate production in blastocysts generated via in vitro fertilization (IVF) (Lee et al., 2022; Chang et al., 2022). However, excessive estrogen stimulation may impair implantation potential by disrupting uterine receptivity (Liu et al., 2023). These findings reveal the dual role of the estrogen-Warburg effct axis in embryo implantation: moderate activation of Warburg effect benefits embryonic energy supply (Pu et al., 2020; Cagnone and Sirard, 2016).
4.5.1.2 Estrogen receptors and their signaling mechanisms
The biological effects of estrogen are primarily mediated by two nuclear receptor isoforms (ERα and ERβ), which regulate gene expression in a tissue-specific manner (Arnal et al., 2017; Winuthayanon et al., 2014; Mata et al., 2015; Kuiper et al., 1996; Paech et al., 1997). ERα predominantly mediates the proliferative effects of estrogen in uterine epithelial cells, while the ERα signaling dialogue between epithelial and stromal cells regulates the process of decidualization (Winuthayanon et al., 2014). In contrast, ERβ is more involved in the relaxation of vascular smooth muscle, such as the ERβ in the mesenteric arteries during pregnancy, which mediates vasodilation to accommodate blood flow demands.
Recent studies have revealed that estrogen receptors not only exert their effects through the classical nuclear transcription mechanism but also trigger rapid non-genomic effects via membrane-initiated steroid signaling (MISS). For instance, ERα is localized to the cell membrane due to palmitoylation modification, and it rapidly regulates gene expression by activating kinase signaling pathways such as MAPK and PI3K (Arnal et al., 2017). This dual mechanism enables estrogen to coordinate short-term vasodilation with long-term vascular remodeling, as orphan nuclear receptors, synergistically enhance the expression of glycolytic genes in conjunction with HIF-1α during energy metabolism and angiogenesis (Cai et al., 2013), further expanding the complexity of estrogen signaling.
4.5.1.3 Regulation of angiogenesis by estrogen
Angiogenesis is a critical process in endometrial receptivity and placental formation, involving endothelial cell proliferation, migration, and vascular maturation (Folkman, 1995). Estrogen directly promotes angiogenesis by upregulating the expression of vascular endothelial growth factor VEGF (Ferrara, 2004). For instance, during primate placental development, VEGF and its receptors drive the formation of the early pregnancy villous capillary bed by activating tyrosine kinase signaling (Reynolds and Redmer, 2001; Carmeliet and Jain, 2000). Estrogen also enhances uterine vascular permeability and endothelial cell proliferation (Johns et al., 1996), and promotes vascular smooth muscle cell hypertrophy through an ERα-dependent mechanism (Osol and Moore, 2014).
It is noteworthy that the angiogenic effects of estrogen are dual in nature (Laschke and Menger, 2016; Carmeliet, 2005). Under physiological conditions (such as pregnancy or endometrial repair), estrogen activates glycolytic enzyme PFKFB3 via G protein-coupled estrogen receptor (GPER1), promoting vascular regeneration in hypoxic tissues (Trenti et al., 2017; Prossnitz and Arterburn, 2015). However, in pathological conditions (such as endometriosis or tumors), estrogen-mediated aberrant angiogenesis may exacerbate inflammation and lesion progression (Laschke and Menger, 2016). For instance, tamoxifen, as a selective estrogen receptor modulator (SERM), can inhibit endothelial cell metabolic reprogramming and block tumor angiogenesis.
Additionally, ERRα, as a central regulator of energy metabolism, stimulates the expression of VEGF via a PGC-1α-dependent pathway, thereby promoting vascular repair following spinal cord injury (Hu et al., 2015; Puigserver and Spiegelman, 2003). This finding unveils the synergistic interaction between estrogen signaling and the energy metabolism network in angiogenesis.
Estrogen integrates the angiogenesis, energy metabolism, and cellular remodeling processes required for endometrial receptivity through a multi-receptor, multi-pathway mechanism (Simon et al., 2003; Cheng et al., 2023). The synergistic interaction between its classical nuclear receptors (ERα/ERβ) and membrane receptors (e.g., GPER1), along with the regulation of the metabolic-vascular network by estrogen-related receptors (ERRs), collectively ensures the establishment and maintenance of pregnancy (Cai et al., 2013). However, the complexity of estrogen signaling also leads to its dual role in pathological conditions, suggesting that future studies need to further elucidate its spatiotemporal-specific mechanisms in order to develop targeted therapeutic strategies (Laschke and Menger, 2016).
4.5.2 Progestogen
Progesterone (PROG) is one of the earliest identified hormones, often referred to as a female steroid along with estradiol (Kolatorova et al., 2022). As a core hormone regulating female reproductive physiology, progesterone (P4) plays a pivotal role, particularly in the establishment of endometrial receptivity, embryo implantation, and the maintenance of pregnancy (Carson et al., 2000; Lessey et al., 1994).
4.5.2.1 Progestogen and metabolic reprogramming
Progesterone plays a pivotal role in the process of embryo implantation, with its association to the Warburg effect primarily reflected in the regulation of metabolic reprogramming and embryonic development. Progesterone directly regulates endometrial receptivity through its receptor (PR) signaling pathway, for example, by downregulating miR-152 expression to inhibit GLUT3-mediated glucose uptake, thereby maintaining appropriate glucose concentrations in the uterine cavity to support embryonic development and implantation (Nie et al., 2019). Furthermore, progesterone enhances mitochondrial membrane potential in oocytes and early embryos, which may provide metabolic support for pre-implantation embryos (Dai et al., 2020). At the molecular level, the progesterone-PR signaling pathway modulates the deposition of hyaluronan (Hadas et al., 2020), cell cycle-related factors (such as IHH, BMP2), and key enzymes in glycolysis (HK2, PKM2, LDHA) (Chen et al., 2016), coordinating endometrial decidualization and embryo implantation. Notably, the intensity of the Warburg effect during embryo implantation dynamically changes, with glycolytic rates gradually decreasing from the morula to the blastocyst stage (Pu et al., 2020). Progesterone may ensure the synchronization of the embryo with the endometrium by regulating uterine receptivity (Hirota, 2019).
4.5.2.2 Molecular basis of endometrial receptivity regulation
Progesterone induces the transformation of the endometrium from the proliferative phase to the secretory phase, establishing the “window of implantation (WOI)” (Gellersen and Brosens, 2014). Studies have shown that elevated levels of progesterone during the luteal phase significantly upregulate the expression of endometrial receptivity markers such as integrin αvβ3 and osteopontin (Carson et al., 2000; Lessey et al., 1994), providing a molecular foundation for embryo adhesion (Günther et al., 2023). Notably, local progesterone levels in the endometrium show no significant correlation with serum concentrations, which may be attributed to differences in the activity of steroid-metabolizing enzymes (e.g., 17β-hydroxysteroid dehydrogenase) within the endometrium (Huhtinen et al., 2012). Clinical research further confirms that in endometrial receptivity analysis (ERA™), the synergistic effect of local progesterone and 17α-hydroxyprogesterone has a statistically significant association with receptivity status (Labarta et al., 2021).
4.5.2.3 Molecular synchronization and spatiotemporal regulation of embryo implantation
Embryo implantation consists of three stages: apposition, adhesion, and invasion of the blastocyst (Carson et al., 2000). Progesterone drives the proliferative-differentiation switch (PDS) by regulating molecular communication between endometrial epithelial and stromal cells (Gellersen and Brosens, 2014; Massimiani et al., 2019). For example, progesterone activates the Wnt/β-catenin pathway in stromal cells, promoting the secretion of leukemia inhibitory factor (LIF), which enhances the epithelial cell’s adhesion capacity to the embryo (Massimiani et al., 2019). Simultaneously, progesterone suppresses the expression of epithelial E-cadherin, reducing intercellular junction tightness and facilitating blastocyst invasion (Hirota, 2019). This spatiotemporal precision in regulation ensures the synchronization of endometrial and embryo development.
4.5.2.4 Metabolic reprogramming supporting embryo implantation
Embryo implantation requires a substantial energy supply. Progesterone enhances glucose uptake and metabolic efficiency in the endometrium by upregulating the expression of glucose transporter 1 (GLUT1) (Zhang et al., 2020). Animal experiments have shown that progesterone treatment in mice significantly increases GLUT1 protein levels in the endometrium by 2–3 times, and glucose consumption rate correlates positively with the embryo implantation success rate (Zhang et al., 2020). This mechanism not only provides energy support for the embryo but also optimizes the local microenvironment’s pH balance through the regulation of metabolic byproducts like lactate, further promoting implantation.
4.5.2.5 miRNA-mediated epigenetic regulation
Progesterone indirectly influences endometrial function by regulating the expression of microRNAs (miRNAs) (Shekibi et al., 2022). For example, progesterone upregulates the expression of miR-145 and miR-199 in human endometrial epithelial cells (HEECs), thereby inhibiting the expression of podocalyxin (PODXL). PODXL is a transmembrane protein that negatively regulates endometrial receptivity, and its downregulation significantly enhances embryo adhesion. In vitro experiments have shown that in endometrial cells transfected with miR-145 or miR-199, both the mRNA and protein levels of PODXL were reduced by 60% and 45%, respectively, and the embryo adhesion rate increased by more than 30% (Shekibi et al., 2022). This finding uncovers a novel pathway by which progesterone finely regulates endometrial function through epigenetic mechanisms.
4.5.2.6 Dynamic balance between angiogenesis and decidualization
Angiogenesis is a crucial characteristic of endometrial receptivity (Gellersen and Brosens, 2014; Salmasi et al., 2021). Progesterone, in synergy with ovarian stimulation, significantly increases endometrial microvascular density by upregulating the expression of vascular endothelial growth factor (VEGF) (Zhang et al., 2020; Salmasi et al., 2021). Clinical studies have shown that exogenous progesterone treatment can elevate VEGF protein levels in the endometrium by 1.5 times and increase the density of CD31-positive endothelial cells by 40% (Salmasi et al., 2021). Furthermore, progesterone drives the transition of decidualization from the pro-inflammatory to the anti-inflammatory phase by secreting factors such as prolactin (PRL) and insulin-like growth factor binding protein 1 (IGFBP1), thereby limiting excessive trophoblast invasion and guiding its directional migration (Gellersen and Brosens, 2014). Abnormal decidualization, such as PR-A subtype defects, is closely associated with recurrent implantation failure (RIF) (Lydon et al., 1995).
4.5.2.7 Diversity of receptor-mediated signal transduction
The functions of progesterone are mediated by its two receptor isoforms, PR-A and PR-B (Lydon et al., 1995). PR-A predominantly governs embryo implantation and pregnancy maintenance, with PR-A knockout mice exhibiting decidualization defects and implantation failure. In contrast, aberrant activation of PR-B leads to endometrial hyperplasia and chronic inflammation (Lydon et al., 1995). Moreover, the activity of PR is regulated by the estrogen receptor (ERα): estrogen during the proliferative phase induces PR expression through ERα, whereas progesterone during the luteal phase suppresses ERα via a negative feedback mechanism, preventing excessive endometrial proliferation (Chen et al., 2009). This dynamic balance between receptors is crucial for maintaining stable reproductive function.
4.5.2.8 Synergistic regulation with estrogen
The synergistic interaction between progesterone and estrogen is essential throughout the reproductive cycle (Gellersen and Brosens, 2014). During the implantation window, progesterone promotes the differentiation of stromal cells and inhibits the proliferation of epithelial cells induced by estrogen, thus establishing a “proliferation-secretion” balance (Gellersen and Brosens, 2014). ERα knockout mice, which are unable to respond to estrogen signals, exhibit the absence of PR expression and arrested endometrial development (Chen et al., 2009). Clinical studies have confirmed that improper timing of progesterone supplementation during assisted reproductive technology (ART) may disrupt hormonal balance, leading to a shift in the receptive window and subsequent implantation failure (Paulson, 2011).
Progesterone precisely coordinates endometrial function through multiple mechanisms, including metabolic reprogramming, angiogenesis, epigenetic modifications, and receptor signaling pathways, thereby creating an optimal microenvironment for embryo implantation. Its effects are not only dependent on its concentration but also closely linked to the activity of receptor isoforms, local metabolic conditions, and synergistic hormonal interactions (Table 1).
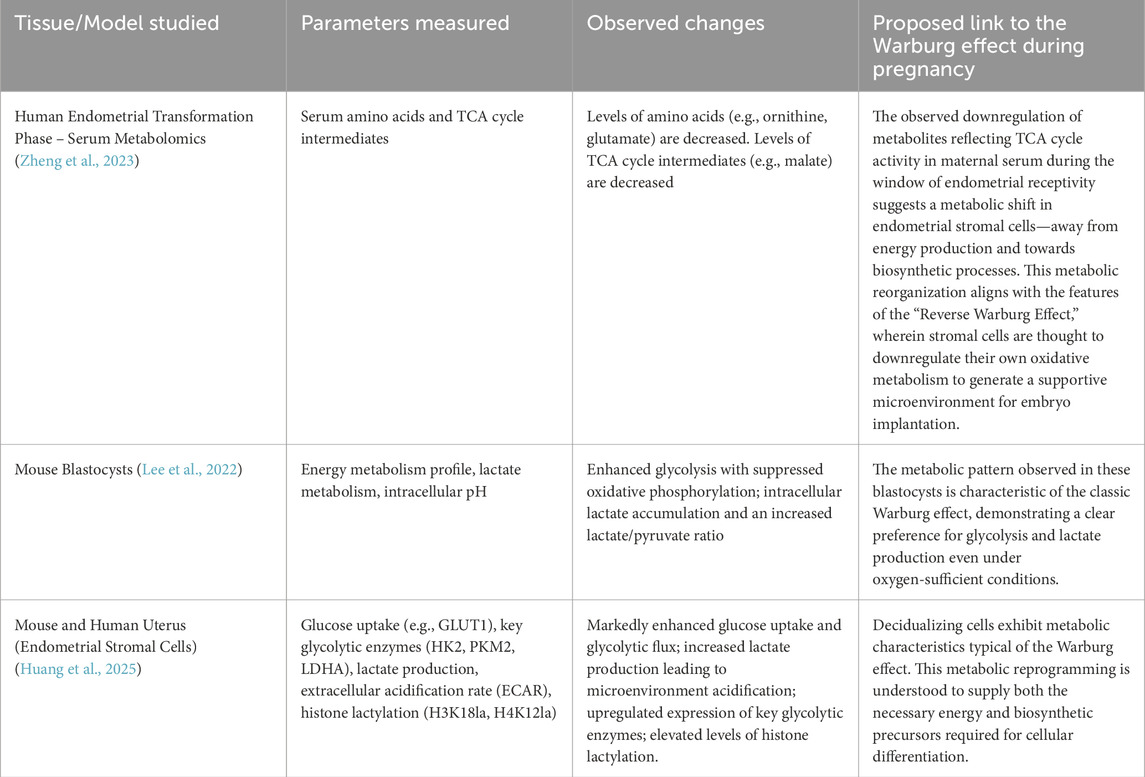
Table 1. This table summarizes data related to the Warburg effect in both humans and mice during pregnancy.
4.6 Age-dependent regulation
Existing research indicates that the effect of age on endometrial receptivity remains controversial. Some studies have found no significant differences in the expression of endometrial receptivity markers CD146 and PDGF-Rβ between the advanced age group and younger groups (p > 0.05), suggesting that patient age may not influence endometrial receptivity (Guo et al., 2023). Similarly, Abdalla et al. conducted a retrospective analysis of cases in which two different age groups of recipients shared oocytes from the same donor and received the same number of embryos. They evaluated the significance of recipient age (uterine age) on pregnancy rates, delivery rates, and miscarriage rates following egg donation, and concluded that the decline in fertility with age cannot be solely explained by uterine factors (Abdalla et al., 1997). However, other studies have reported significant decreases in the endometrial vascularization index (VI), flow index (FI), and vascular flow comprehensive index (VFI) in older women, along with markedly lower clinical pregnancy rates compared to younger women. These findings suggest that age may impact receptivity by altering the endometrial microenvironment (Wang L. et al., 2020). Furthermore, the prolonged duration of infertility associated with advanced age is significantly correlated with abnormal endometrial receptivity, manifesting as a higher tendency for older women to exhibit “early-receptive” or “pre-receptive” abnormalities (Opuchlik et al., 2025).
In assisted reproductive technology (ART), endometrial aging in older women may lead to a pro-inflammatory state and tissue fibrosis, and may alter the biological age of the endometrium through epigenetic regulation (Pathare et al., 2023). Research on the effect of age on the Warburg effect during the embryo implantation phase is currently lacking, highlighting the urgent need for further investigation by researchers in this area.
5 Current evidence gaps and potential future research directions
Due to the limited current evidence, further studies are required to validate this hypothesis. ① Lack of in vivo dynamic metabolic flux evidence: Current approaches, such as measuring lactate levels in culture medium or analyzing gene expression in tissues, cannot accurately determine the ratio of glycolysis to mitochondrial oxidative phosphorylation in endometrial energy metabolism during pregnancy. Consequently, it remains unclear whether the endometrium preferentially directs glucose toward lactate production via glycolysis or toward complete oxidation. Future research could employ isotope tracing techniques for metabolic flux analysis and fluxomics-driven determination of reaction free energy (Xu et al., 2020). ② Lack of functional validation of key metabolic enzymes and transporters: Molecules such as GLUT1, PFKFB3, and PKM2 have been highlighted in the literature as potentially critical in the metabolic process, but this assumption is based primarily on expression levels. Whether these molecules functionally contribute to establishing endometrial receptivity still needs verification. A promising future direction involves using CRISPR-Cas9 gene editing to knock out the corresponding genes, following the approach of Zou et al. (2025) in treating head and neck squamous cell carcinoma. Subsequent observation of glucose uptake, lactate production, and receptivity-related markers could then directly test if these nodal points drive receptivity. ③Lack of metabolic heterogeneity information at the endometrial cell subpopulation level: The endometrium is a complex system composed of epithelial, stromal, and immune cells, among others. Conventional bulk detection methods likely mask the unique metabolic patterns of distinct cell types. Therefore, during the window of implantation, it is unknown whether all epithelial cells undergo metabolic reprogramming or only specific subpopulations do. Single-cell RNA sequencing (scRNA-seq) could be applied to analyze the expression profiles of glycolytic pathway genes in individual cells, similar to how Chen et al. (2025) used scRNA-seq to identify transcription factors highly expressed in different cellular subpopulations.
By integrating these multi-level, complementary cutting-edge technologies, future research will be well-positioned to systematically elucidate the central role of the Warburg effect in endometrial receptivity. This work holds the potential to ultimately yield novel metabolic diagnostic biomarkers and therapeutic targets for infertility and recurrent pregnancy loss.
6 Conclusion and prospects
In summary, the processes of embryo implantation and the Warburg effect observed in cancer cells exhibit notable similarities. By integrating and analyzing clinical reports, connections can be identified across several dimensions: ① Immunomodulation (the inflammatory response induced by the uterus during the implantation process increases glucose consumption, thereby triggering the Warburg effect); ② Microenvironment alterations (the glycolytic activity of the blastocyst generates a high lactate and low pH environment, enhancing endometrial receptivity and angiogenesis); ③ Gene expression (glycolytic influences on genes such as MRAP2 and BCL2L15 may induce infertility); ④ Cytokine regulation (both cancer progression and embryo implantation demonstrate a requirement for and activation of multiple cytokines, including IL-1, IFN-γ, LIF, TNF-α, and TGF). Furthermore, during the implantation process, following the adhesion of the blastocyst to the uterus, trophoblast cells undergo epithelial-to-mesenchymal transition to acquire the necessary adhesive and invasive capabilities. If this process is uncontrolled, it may lead to carcinogenesis, indirectly highlighting the parallels between embryo implantation and the Warburg effect. However, current clinical and experimental research that clarifies the relationship between the two remains limited, and the underlying mechanisms await further exploration. This research could provide novel insights and directions for clinical studies and inform treatments for conditions associated with altered endometrial receptivity, such as infertility and recurrent miscarriage. Additionally, it presents a potential focus for traditional Chinese medicine approaches in treating infertility, representing significant research and translational implications. In the future, it may demonstrate remarkable efficacy in clinical settings, contributing profoundly to the field of maternal and child health globally.
Author contributions
XZ: Investigation, Writing – review and editing, Writing – original draft. QZ: Writing – review and editing, Conceptualization, Writing – original draft, Investigation. WN: Writing – review and editing, Investigation, Visualization, Writing – original draft. XY: Investigation, Writing – review and editing, Writing – original draft. ZY: Writing – original draft, Writing – review and editing, Investigation. LT: Supervision, Writing – review and editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. We acknowledge the support from the National Administration of Traditional Chinese Medicine High-level Key Discipline Construction Project (Grant No. zyyzdxk-2023262).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2025.1683790/full#supplementary-material
References
Abdalla, H. I., Wren, M. E., Thomas, A., and Korea, L. (1997). Age of the uterus does not affect pregnancy or implantation rates; a study of egg donation in women of different ages sharing oocytes from the same donor. Hum. Reprod. 12 (4), 827–829. doi:10.1093/humrep/12.4.827
Achache, H., and Revel, A. (2006). Endometrial receptivity markers, the journey to successful embryo implantation. Hum. Reprod. Update 12 (6), 731–746. doi:10.1093/humupd/dml004
Albrecht, E. D., and Pepe, G. J. (2010). Estrogen regulation of placental angiogenesis and fetal ovarian development during primate pregnancy. Int. J. Dev. Biol. 54 (2-3), 397–408. doi:10.1387/ijdb.082758ea
Altman, B. J., Stine, Z. E., and Dang, C. V. (2016). From Krebs to clinic: glutamine metabolism to cancer therapy. Nat. Rev. Cancer 16 (12), 749. doi:10.1038/nrc.2016.114
Ao, A., Wang, H., Kamarajugadda, S., and Lu, J. (2008). Involvement of estrogen-related receptors in transcriptional response to hypoxia and growth of solid tumors. Proc. Natl. Acad. Sci. U. S. A. 105 (22), 7821–7826. doi:10.1073/pnas.0711677105
Aplin, J. D. (1997). Adhesion molecules in implantation. Rev. Reprod. 2 (2), 84–93. doi:10.1530/ror.0.0020084
Aplin, J. D. S. M., Graham, R. A., Hey, N. A., Behzad, F., and Campbell, S. (1994). The endometrial cell surface and implantation. Expression of the polymorphic mucin MUC-1 and adhesion molecules during the endometrial cycle. Ann. N. Y. Acad. Sci. 734, 103–121. doi:10.1111/j.1749-6632.1994.tb21739.x
Apostolova, P., and Pearce, E. L. (2022). Lactic acid and lactate: revisiting the physiological roles in the tumor microenvironment. Trends Immunol. 43 (12), 969–977. doi:10.1016/j.it.2022.10.005
Appelberg, R., Moreira, D., Barreira-Silva, P., Borges, M., Silva, L., Dinis-Oliveira, R. J., et al. (2015). The Warburg effect in mycobacterial granulomas is dependent on the recruitment and activation of macrophages by interferon-γ. Immunology 145 (4), 498–507. doi:10.1111/imm.12464
Arnal, J. F., Lenfant, F., Metivier, R., Flouriot, G., Henrion, D., Adlanmerini, M., et al. (2017). Membrane and nuclear estrogen receptor alpha actions: from tissue specificity to medical implications. Physiol. Rev. 97 (3), 1045–1087. doi:10.1152/physrev.00024.2016
Ashary, N., Tiwari, A., and Modi, D. (2018). Embryo implantation: war in times of love. Endocrinology 159 (2), 1188–1198. doi:10.1210/en.2017-03082
Ben Rafael, Z. (2021). Endometrial receptivity analysis (ERA) test: an unproven technology. Hum. Reprod. Open 2021 (2), hoab010. doi:10.1093/hropen/hoab010
Bhattacharya, D., Azambuja, A. P., and Simoes-Costa, M. (2020). Metabolic reprogramming promotes neural crest migration via Yap/Tead signaling. Dev. Cell 53 (2), 199–211.e6. doi:10.1016/j.devcel.2020.03.005
Bhurke, A., Kannan, A., Neff, A., Ma, Q., Laws, M. J., Taylor, R. N., et al. (2020). A hypoxia-induced Rab pathway regulates embryo implantation by controlled trafficking of secretory granules. Proc. Natl. Acad. Sci. U. S. A. 117 (25), 14532–14542. doi:10.1073/pnas.2000810117
Bondarenko, V., Nikolaev, M., Kromm, D., Belousov, R., Wolny, A., Blotenburg, M., et al. (2023). Embryo-uterine interaction coordinates mouse embryogenesis during implantation. EMBO J. 42 (17), e113280. doi:10.15252/embj.2022113280
Bueno-Sánchez, L., Alhambra-Borrás, T., Gallego-Valadés, A., and Garcés-Ferrer, J. (2024). Psychosocial impact of infertility diagnosis and conformity to gender norms on the quality of life of infertile Spanish couples. Int. J. Environ. Res. Public Health 21 (2), 158. doi:10.3390/ijerph21020158
Burrows, T. D., King, A., and Loke, Y. W. (1996). Trophoblast migration during human placental implantation. Hum. Reprod. Update 2 (4), 307–321. doi:10.1093/humupd/2.4.307
Cagnone, G., and Sirard, M. A. (2016). The embryonic stress response to in vitro culture: insight from genomic analysis. Reproduction 152 (6), R247–R261. doi:10.1530/REP-16-0391
Cai, L., Sutter, B. M., Li, B., and Tu, B. P. (2011). Acetyl-CoA induces cell growth and proliferation by promoting the acetylation of histones at growth genes. Mol. Cell 42 (4), 426–437. doi:10.1016/j.molcel.2011.05.004
Cai, Q., Lin, T., Kamarajugadda, S., and Lu, J. (2013). Regulation of glycolysis and the Warburg effect by estrogen-related receptors. Oncogene 32 (16), 2079–2086. doi:10.1038/onc.2012.221
Cao, Y., Ye, Q., Zhuang, M., Xie, S., Zhong, R., Cui, J., et al. (2017). Ginsenoside Rg3 inhibits angiogenesis in a rat model of endometriosis through the VEGFR-2-mediated PI3K/Akt/mTOR signaling pathway. PLoS One 12 (11), e0186520. doi:10.1371/journal.pone.0186520
Carmeliet, P. (2005). Angiogenesis in life, disease and medicine. Nature 438 (7070), 932–936. doi:10.1038/nature04478
Carmeliet, P., and Jain, R. K. (2000). Angiogenesis in cancer and other diseases. Nature 407 (6801), 249–257. doi:10.1038/35025220
Carson, D. D., Bagchi, I., Dey, S. K., Enders, A. C., Fazleabas, A. T., Lessey, B. A., et al. (2000). Embryo implantation. Dev. Biol. 223 (2), 217–237. doi:10.1006/dbio.2000.9767
Carson, D. D., Julian, J., Lessey, B. A., Prakobphol, A., and Fisher, S. J. (2006). MUC1 is a scaffold for selectin ligands in the human uterus. Front. Biosci. 11, 2903–2908. doi:10.2741/2018
Carter, A. M., Enders, A. C., and Pijnenborg, R. (2015). The role of invasive trophoblast in implantation and placentation of primates. Philos. Trans. R. Soc. Lond B Biol. Sci. 370 (1663), 20140070. doi:10.1098/rstb.2014.0070
Chang, K. T., Su, Y. T., Tsai, Y. R., Lan, K. C., Hsuuw, Y. D., Kang, H. Y., et al. (2022). High levels estradiol affect blastocyst implantation and post-implantation development directly in mice. Biomed. J. 45 (1), 179–189. doi:10.1016/j.bj.2021.01.004
Chen, M., Wolfe, A., Wang, X., Chang, C., Yeh, S., and Radovick, S. (2009). Generation and characterization of a complete null estrogen receptor alpha mouse using Cre/LoxP technology. Mol. Cell Biochem. 321 (1-2), 145–153. doi:10.1007/s11010-008-9928-9
Chen, X., Fu, J., and Wang, A. (2016). Expression of genes involved in progesterone receptor paracrine signaling and their effect on litter size in pigs. J. Anim. Sci. Biotechnol. 7, 31. doi:10.1186/s40104-016-0090-z
Chen, A. N., Luo, Y., Yang, Y. H., Fu, J. T., Geng, X. M., Shi, J. P., et al. (2021). Lactylation, a novel metabolic reprogramming code: current status and prospects. Front. Immunol. 12, 688910. doi:10.3389/fimmu.2021.688910
Chen, J., Qin, P., Sun, Y., and Han, S. (2023). Histone lactylation promotes cell proliferation, migration and invasion through targeting HMGB1 in endometriosis. J. Biomed. Res. 37 (6), 470–478. doi:10.7555/JBR.37.20230095
Chen, B., Liu, J., Zhang, Y., Shi, C., Zhu, D., Zhang, G., et al. (2025). Enhancer extrachromosomal circular DNA ANKRD28 elicits drug resistance via POU2F2-Mediated transcriptional network in multiple myeloma. Adv. Sci. (Weinh) 12 (21), e2415695. doi:10.1002/advs.202415695
Cheng, J., Sha, Z., Li, J., Li, B., Luo, X., Zhang, Z., et al. (2023). Progress on the role of estrogen and progesterone signaling in mouse embryo implantation and decidualization. Reprod. Sci. 30 (6), 1746–1757. doi:10.1007/s43032-023-01169-0
Chiarella, E., Nisticò, C., Di Vito, A., Morrone, H. L., and Mesuraca, M. (2022). Targeting of mevalonate-isoprenoid pathway in acute myeloid leukemia cells by bisphosphonate drugs. Biomedicines 10 (5), 1146. doi:10.3390/biomedicines10051146
Chung, T. W., Park, M. J., Lee, H., Kim, K. J., Kim, C. H., Choi, H. J., et al. (2019). Enhancement of endometrial receptivity by Cnidium officinale through expressing LIF and integrins. Evid. Based Complement. Altern. Med. 2019, 7560631. doi:10.1155/2019/7560631
Covello, K. L., Kehler, J., Yu, H., Gordan, J. D., Arsham, A. M., Hu, C. J., et al. (2006). HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 20 (5), 557–570. doi:10.1101/gad.1399906
D’Aurora, M., Romani, F., Franchi, S., Diomede, F., Merciaro, I., Impicciatore, G. G., et al. (2019). MRAP2 regulates endometrial receptivity and function. Gene 703, 7–12. doi:10.1016/j.gene.2019.04.001
Dai, Q., Provost, M. P., Raburn, D. J., and Price, T. M. (2020). Progesterone increases mitochondria membrane potential in non-human primate oocytes and embryos. Reprod. Sci. 27 (5), 1206–1214. doi:10.1007/s43032-019-00132-2
Dai, W., Guo, R., Na, X., Jiang, S., Liang, J., Guo, C., et al. (2024). Hypoxia and the endometrium: an indispensable role for HIF-1α as therapeutic strategies. Redox Biol. 73, 103205. doi:10.1016/j.redox.2024.103205
Delgir, S., Bastami, M., Ilkhani, K., Safi, A., Seif, F., and Alivand, M. R. (2021). The pathways related to glutamine metabolism, glutamine inhibitors and their implication for improving the efficiency of chemotherapy in triple-negative breast cancer. Mutat. Res. Rev. Mutat. Res. 787, 108366. doi:10.1016/j.mrrev.2021.108366
Deryabin, P. I., Ivanova, J. S., and Borodkina, A. V. (2022). Senescent endometrial stromal cells transmit reactive oxygen species to the trophoblast-like cells and impair spreading of blastocyst-like spheroids. Mol. Hum. Reprod. 28 (12), gaac039. doi:10.1093/molehr/gaac039
Devine, P. L., and McKenzie, I. F. (1992). Mucins: structure, function, and associations with malignancy. Bioessays 14 (9), 619–625. doi:10.1002/bies.950140909
Dinarello, C. A. (1988). Interleukin-1. Ann. N. Y. Acad. Sci. 546, 122–132. doi:10.1111/j.1749-6632.1988.tb21627.x
Enders, A., and Schlafke, S. (1967). A morphological analysis of the early implantation stages in the rat. Am. J. Anat. 120 (2), 185–225. doi:10.1002/aja.1001200202
Erickson, G. F., Fuqua, L., and Shimasaki, S. (2004). Analysis of spatial and temporal expression patterns of bone morphogenetic protein family members in the rat uterus over the estrous cycle. J. Endocrinol. 182 (2), 203–217. doi:10.1677/joe.0.1820203
Eun-Yeong, K., Tae-Wook, C., Hee-Jung, C., Ki-Tae, H., Yeon-Seop, J., Syng-Ook, L., et al. (2019). Extracts from Paeonia lactiflora Pallas, Rehmannia glutinosa var. Purpurea makino, Perilla frutescens var. Acuta kudo may increase the endometrial receptivity through expression of leukemia inhibitory factor and adhesion molecules. J. Tradit. Chin. Med. 39 (1), 15–25. doi:10.1016/j.jphs.2016.07.004
Evertts, A. G., Zee, B. M., Dimaggio, P. A., Gonzales-Cope, M., Coller, H. A., and Garcia, B. A. (2013). Quantitative dynamics of the link between cellular metabolism and histone acetylation. J. Biol. Chem. 288 (17), 12142–12151. doi:10.1074/jbc.M112.428318
Ewald, J., He, Z., Dimitriew, W., and Schuster, S. (2024). Including glutamine in a resource allocation model of energy metabolism in cancer and yeast cells. NPJ Syst. Biol. Appl. 10 (1), 77. doi:10.1038/s41540-024-00393-x
Fazleabas, A. T., and Kim, J. J. (2003). Development. What makes an embryo stick? Science 299 (5605), 355–356. doi:10.1126/science.1081277
Feldman, B., Poueymirou, W., Papaioannou, V. E., DeChiara, T. M., and Goldfarb, M. (1995). Requirement of FGF-4 for postimplantation mouse development. Science. 267 (5195), 246–249. doi:10.1126/science.7809630
Ferrara, N. (2004). Vascular endothelial growth factor: basic science and clinical progress. Endocr. Rev. 25 (4), 581–611. doi:10.1210/er.2003-0027
Fleming, T. P., Sheth, B., and Fesenko, I. (2001). Cell adhesion in the preimplantation mammalian embryo and its role in trophectoderm differentiation and blastocyst morphogenesis. Front. Biosci. 6, D1000–D1007. doi:10.2741/fleming
Flores-Hernández, E., Binder, G., Mei, K. C., and Tejeda-Muñoz, N. (2025). Targeting Wnt-driven metabolic adaptations in cancer: integrating glycolysis, glutaminolysis, IDO1-mediated immune evasion, and therapeutic delivery strategies. Front. Cell Dev. Biol. 13, 1622218. doi:10.3389/fcell.2025.1622218
Folkman, J. (1995). Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1 (1), 27–31. doi:10.1038/nm0195-27
Fujiwara, H., Ono, M., Sato, Y., Imakawa, K., Iizuka, T., Kagami, K., et al. (2020). Promoting roles of embryonic signals in embryo implantation and placentation in cooperation with endocrine and immune systems. Int. J. Mol. Sci. 21 (5), 1885. doi:10.3390/ijms21051885
Gallamini, A., Zwarthoed, C., and Borra, A. (2014). Positron emission tomography (PET) in oncology. Cancers (Basel) 6 (4), 1821–1889. doi:10.3390/cancers6041821
Gao, L., Xu, Q. H., Ma, L. N., Luo, J., Muyayalo, K. P., Wang, L. L., et al. (2022). Trophoblast-derived lactic acid orchestrates decidual macrophage differentiation via SRC/LDHA signaling in early pregnancy. Int. J. Biol. Sci. 18 (2), 599–616. doi:10.7150/ijbs.67816
Gashaw, I., Stiller, S., Böing, C., Kimmig, R., and Winterhager, E. (2008). Premenstrual regulation of the pro-angiogenic factor CYR61 in human endometrium. Endocrinology 149 (5), 2261–2269. doi:10.1210/en.2007-1568
Gellersen, B., and Brosens, J. J. (2014). Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr. Rev. 35 (6), 851–905. doi:10.1210/er.2014-1045
Gipson, I. K., Blalock, T., Tisdale, A., Spurr-Michaud, S., Allcorn, S., Stavreus-Evers, A., et al. (2008). MUC16 is lost from the uterodome (pinopode) surface of the receptive human endometrium: in vitro evidence that MUC16 is a barrier to trophoblast adherence. Biol. Reprod. 78 (1), 134–142. doi:10.1095/biolreprod.106.058347
Gou, R. Z. X., and Zhang, X. (2023). Glycolysis: a fork in the path of normal and pathological pregnancy. FASEB J. 37 (12), e23263. doi:10.1096/fj.202301230R
Greenhill, C. (2018). Reproductive endocrinology: hypoxia in endometrial repair. Nat. Rev. Endocrinol. 14 (3), 130. doi:10.1038/nrendo.2018.12
Guido, C., Whitaker-Menezes, D., Capparelli, C., Balliet, R., Lin, Z., Pestell, R. G., et al. (2012). Metabolic reprogramming of cancer-associated fibroblasts by TGF-β drives tumor growth: connecting TGF-β signaling with “Warburg-like” cancer metabolism and L-lactate production. Cell Cycle 11 (16), 3019–3035. doi:10.4161/cc.21384
Günther, V., Allahqoli, L., Deenadayal-Mettler, A., Maass, N., Mettler, L., Gitas, G., et al. (2023). Molecular determinants of uterine receptivity: Comparison of successful implantation, recurrent miscarriage, and recurrent implantation failure. Int. J. Mol. Sci. 24 (24), 17616. doi:10.3390/ijms242417616
Guo, S., Zhang, D., Zhao, S., Zhang, H., Sun, Y., and Yan, L. (2023). A preliminary study on the correlation between age and endometrial receptivity. Pharmgenomics Pers. Med. 16, 425–432. doi:10.2147/PGPM.S406257
Gurner, K. H., Evans, J., Hutchison, J. C., Harvey, A. J., and Gardner, D. K. (2022). A microenvironment of high lactate and low pH created by the blastocyst promotes endometrial receptivity and implantation. Reprod. Biomed. Online 44 (1), 14–26. doi:10.1016/j.rbmo.2021.09.012
Hadas, R., Gershon, E., Cohen, A., Atrakchi, O., Lazar, S., Golani, O., et al. (2020). Hyaluronan control of the primary vascular barrier during early mouse pregnancy is mediated by uterine NK cells. JCI Insight 5 (22), e135775. doi:10.1172/jci.insight.135775
Han, S., Liu, M., Liu, S., and Li, Y. (2021). Transcriptomic analysis of human endometrial stromal cells during early embryo invasion. Ann. Med. 53 (1), 1758–1771. doi:10.1080/07853890.2021.1988139
Han, S., Liu, S., Jin, N. Q., Lv, Y. S., Yang, M., Ma, S., et al. (2023). Mitochondrial energy metabolism is downregulated in repeated implantation failure patients related to alteration of PGC-1α acetylation level. Mol. Reprod. Dev. 90 (6), 397–405. doi:10.1002/mrd.23691
Hantak, A. M., Bagchi, I. C., and Bagchi, M. K. (2014). Role of uterine stromal-epithelial crosstalk in embryo implantation. Int. J. Dev. Biol. 58 (2-4), 139–146. doi:10.1387/ijdb.130348mb
Hantoushzadeh, S., Anvari Aliabad, R., and Norooznezhad, A. H. (2020). Antibiotics, inflammation, and preterm labor: a missed conclusion. J. Inflamm. Res. 13, 245–254. doi:10.2147/JIR.S248382
Hard, G. C. (1970). Some biochemical aspects of the immune macrophage. Br. J. Exp. Pathol. 51 (1), 97–105.
He, A., Zou, Y., Wan, C., Zhao, J., Zhang, Q., Yao, Z., et al. (2021). The role of transcriptomic biomarkers of endometrial receptivity in personalized embryo transfer for patients with repeated implantation failure. J. Transl. Med. 19 (1), 176. doi:10.1186/s12967-021-02837-y
Heemann, U. W., Tullius, S. G., Azuma, H., Kupiec-Weglinsky, J., and Tilney, N. L. (1994). Adhesion molecules and transplantation. Ann. Surg. 219 (1), 4–12. doi:10.1097/00000658-199401000-00002
Hirota, Y. (2019). Progesterone governs endometrial proliferation-differentiation switching and blastocyst implantation. Endocr. J. 66 (3), 199–206. doi:10.1507/endocrj.EJ18-0431
Horne, A. W., Lalani, E. N., Margara, R. A., and White, J. O. (2006). The effects of sex steroid hormones and interleukin-1-beta on MUC1 expression in endometrial epithelial cell lines. Reproduction 131 (4), 733–742. doi:10.1530/rep.1.00883
Hsu, P. P., and Sabatini, D. M. (2008). Cancer cell metabolism: Warburg and beyond. Cell 134 (5), 703–707. doi:10.1016/j.cell.2008.08.021
Hu, J. Z., Long, H., Wu, T. D., Zhou, Y., and Lu, H. B. (2015). The effect of estrogen-related receptor α on the regulation of angiogenesis after spinal cord injury. Neuroscience 290, 570–580. doi:10.1016/j.neuroscience.2015.01.067
Huang, Y., Zhu, Q., and Sun, Y. (2025). Glucose metabolism and endometrium decidualization. Front. Endocrinol. (Lausanne) 16, 1546335. doi:10.3389/fendo.2025.1546335
Huhtinen, K., Desai, R., Ståhle, M., Salminen, A., Handelsman, D. J., Perheentupa, A., et al. (2012). Endometrial and endometriotic concentrations of estrone and estradiol are determined by local metabolism rather than circulating levels. J. Clin. Endocrinol. Metab. 97 (11), 4228–4235. doi:10.1210/jc.2012-1154
Jena, B. C., Das, C. K., Banerjee, I., Bharadwaj, D., Majumder, R., Das, S., et al. (2022). TGF-β1 induced autophagy in cancer associated fibroblasts during hypoxia contributes EMT and glycolysis via MCT4 upregulation. Exp. Cell Res. 417 (1), 113195. doi:10.1016/j.yexcr.2022.113195
Ji, G., Feng, X., Hu, C., Zhang, J., Sheng, H., Na, R., et al. (2025). HADHA promotes apoptosis and inflammatory response in bovine endometrial epithelial cells by regulating transcription and metabolism. Int. J. Biol. Macromol. 304 (Pt 2), 140980. doi:10.1016/j.ijbiomac.2025.140980
Jia, N., and Li, J. (2020). Human uterine decidual NK cells in women with a history of early pregnancy enhance angiogenesis and trophoblast invasion. Biomed. Res. Int. 2020, 6247526. doi:10.1155/2020/6247526
Jiang, M., Huang, L., Wang, Y., Wang, Y., Kang, Q., Chen, C., et al. (2023). Yueliang yin ameliorates endometrial receptivity in mice with embryo implantation failure by reducing pyroptosis and activating BDNF/TrkB pathway. Mol. Nutr. Food Res. 67 (23), e2300339. doi:10.1002/mnfr.202300339
Johns, A., Freay, A. D., Fraser, W., Korach, K. S., and Rubanyi, G. M. (1996). Disruption of estrogen receptor gene prevents 17 beta estradiol-induced angiogenesis in transgenic mice. Endocrinology 137 (10), 4511–4513. doi:10.1210/endo.137.10.8828515
Johnson, M. T., Mahmood, S., and Patel, M. S. (2003). Intermediary metabolism and energetics during murine early embryogenesis. J. Biol. Chem. 278 (34), 31457–31460. doi:10.1074/jbc.R300002200
Kaiser, A., Krüger, T., Eiselt, G., Bechler, J., Kniemeyer, O., Huber, O., et al. (2020). Identification of PARP-1, histone H1 and SIRT-1 as new regulators of breast cancer-related aromatase promoter I.3/II. Cells 9 (2), 427. doi:10.3390/cells9020427
Kodama, M., and Nakayama, K. I. (2020). A second Warburg-like effect in cancer metabolism: the metabolic shift of glutamine-derived nitrogen: a shift in glutamine-derived nitrogen metabolism from glutaminolysis to de novo nucleotide biosynthesis contributes to malignant evolution of cancer. Bioessays 42 (12), e2000169. doi:10.1002/bies.202000169
Kohlrausch, F. B., and Keefe, D. L. (2020). Telomere erosion as a placental clock: from placental pathologies to adverse pregnancy outcomes. Placenta 97, 101–107. doi:10.1016/j.placenta.2020.06.022
Kolatorova, L., Vitku, J., Suchopar, J., Hill, M., and Parizek, A. (2022). Progesterone: a steroid with wide range of effects in physiology as well as human medicine. Int. J. Mol. Sci. 23 (14), 7989. doi:10.3390/ijms23147989
Kroemer, G., and Pouyssegur, J. (2008). Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell 13 (6), 472–482. doi:10.1016/j.ccr.2008.05.005
Kuiper, G. G., Enmark, E., Pelto-Huikko, M., Nilsson, S., and Gustafsson, J. A. (1996). Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. U. S. A. 93 (12), 5925–5930. doi:10.1073/pnas.93.12.5925
Labarta, E., Sebastian-Leon, P., Devesa-Peiro, A., Celada, P., Vidal, C., Giles, J., et al. (2021). Analysis of serum and endometrial progesterone in determining endometrial receptivity. Hum. Reprod. 36 (11), 2861–2870. doi:10.1093/humrep/deab184
Laschke, M. W., and Menger, M. D. (2016). The gut microbiota: a puppet master in the pathogenesis of endometriosis? Am. J. Obstet. Gynecol. 215 (1), 68.e1–68.e684. doi:10.1016/j.ajog.2016.02.036
Lee, N., and Kim, D. (2016). Cancer metabolism: fueling more than just growth. Mol. Cells 39 (12), 847–854. doi:10.14348/molcells.2016.0310
Lee, S. H., Liu, X., Jimenez-Morales, D., and Rinaudo, P. F. (2022). Murine blastocysts generated by in vitro fertilization show increased Warburg metabolism and altered lactate production. Elife 11, e79153. doi:10.7554/eLife.79153
Lepleux, C., Abeilard-Lemoisson, E., Duval, M., Icard, P., and Lincet, H. (2012). siPGK1 sensitizes chemoresistant human ovarian cancer cell lines to cisplatin. Anticancer Res. 32 (10), 4277–4286.
Lessey, B. A. (1998). Endometrial integrins and the establishment of uterine receptivity. Hum. Reprod. 13 (Suppl. 3), 247–261; discussion 59-61. doi:10.1093/humrep/13.suppl_3.247
Lessey, B. A., and Young, S. L. (2019). What exactly is endometrial receptivity? Fertil. Steril. 111 (4), 611–617. doi:10.1016/j.fertnstert.2019.02.009
Lessey, B. A., Castelbaum, A. J., Sawin, S. W., Buck, C. A., Schinnar, R., Bilker, W., et al. (1994). Aberrant integrin expression in the endometrium of women with endometriosis. J. Clin. Endocrinol. Metab. 79 (2), 643–649. doi:10.1210/jcem.79.2.7519194
Li, C. J., Zhan, Y. D., Zhou, X. L., Yang, J., Deng, L., Li, X. L., et al. (2023). Value of intrauterine autologous platelet-rich plasma therapy on endometrial receptivity: a literature review. Curr. Med. Sci. 43 (6), 1075–1083. doi:10.1007/s11596-023-2816-4
Li, N., Chen, H., Zheng, H., Yang, C., Li, M., Xiao, P., et al. (2025). Revealing the effects of lactate on bovine SCNT embryo development through transcriptome sequencing analyses. Theriogenology 236, 137–146. doi:10.1016/j.theriogenology.2025.02.007
Liang, Y., Shuai, Q., Wang, Y., Jin, S., Feng, Z., Chen, B., et al. (2021). 1-Nitropyrene exposure impairs embryo implantation through disrupting endometrial receptivity genes expression and producing excessive ROS. Ecotoxicol. Environ. Saf. 227, 112939. doi:10.1016/j.ecoenv.2021.112939
Liberti, M. V., and Locasale, J. W. (2016a). The warburg effect: how does it benefit cancer cells? Trends Biochem. Sci. 41 (3), 211–218. doi:10.1016/j.tibs.2015.12.001
Liberti, M. V., and Locasale, J. W. (2016b). The Warburg effect: how does it benefit cancer cells? Trends Biochem. Sci. 41 (3), 287. doi:10.1016/j.tibs.2016.01.004
Lindsay, C. V., Potter, J. A., Grimshaw, A. A., Abrahams, V. M., and Tong, M. (2023). Endometrial responses to bacterial and viral infection: a scoping review. Hum. Reprod. Update 29 (5), 675–693. doi:10.1093/humupd/dmad013
Liu, N., Zhou, C., Chen, Y., and Zhao, J. (2013). The involvement of osteopontin and β3 integrin in implantation and endometrial receptivity in an early mouse pregnancy model. Eur. J. Obstet. Gynecol. Reprod. Biol. 170 (1), 171–176. doi:10.1016/j.ejogrb.2013.06.019
Liu, X. S., Little, J. B., and Yuan, Z. M. (2015). Glycolytic metabolism influences global chromatin structure. Oncotarget 6 (6), 4214–4225. doi:10.18632/oncotarget.2929
Liu, W., Liu, X., Luo, M., Luo, Q., Tao, H., et al. (2017). dNK derived IFN-γ mediates VSMC migration and apoptosis via the induction of LncRNA MEG3: a role in uterovascular transformation. Placenta 50, 32–39. doi:10.1016/j.placenta.2016.12.023
Liu, L., Zhuo, Y., Zhang, H., Li, J., Jiang, X., Han, X., et al. (2023). Time-restricted feeding ameliorates uterine epithelial estrogen receptor α transcriptional activity at the time of embryo implantation in mice fed a high-fat diet. J. Nutr. 153 (6), 1753–1761. doi:10.1016/j.tjnut.2023.04.012
Lu, J. (2019). The Warburg metabolism fuels tumor metastasis. Cancer Metastasis Rev. 38 (1-2), 157–164. doi:10.1007/s10555-019-09794-5
Lu, C., and Thompson, C. B. (2012). Metabolic regulation of epigenetics. CELL Metab. 16, 9–17. doi:10.1016/j.cmet.2012.06.001
Lydon, J. P., DeMayo, F. J., Funk, C. R., Mani, S. K., Hughes, A. R., Montgomery, C. A., et al. (1995). Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 9 (18), 2266–2278. doi:10.1101/gad.9.18.2266
Ma, Q., Beal, J. R., Song, X., Bhurke, A., Bagchi, I. C., and Bagchi, M. K. (2022). Extracellular vesicles secreted by mouse decidual cells carry critical information for the establishment of pregnancy. Endocrinology 163 (12), bqac165. doi:10.1210/endocr/bqac165
Macklon, N. S., Geraedts, J. P., and Fauser, B. C. (2002). Conception to ongoing pregnancy: the ‘Black box’ of early pregnancy loss. Hum. Reprod. Update 8 (4), 333–343. doi:10.1093/humupd/8.4.333
Massimiani, M., Lacconi, V., La Civita, F., Ticconi, C., Rago, R., and Campagnolo, L. (2019). Molecular signaling regulating endometrium-blastocyst crosstalk. Int. J. Mol. Sci. 21 (1), 23. doi:10.3390/ijms21010023
Mata, K. M., Li, W., Reslan, O. M., Siddiqui, W. T., Opsasnick, L. A., and Khalil, R. A. (2015). Adaptive increases in expression and vasodilator activity of estrogen receptor subtypes in a blood vessel-specific pattern during pregnancy. Am. J. Physiol. Heart Circ. Physiol. 309 (10), H1679–H1696. doi:10.1152/ajpheart.00532.2015
Maybin, J. A., Murray, A. A., Saunders, P. T. K., Hirani, N., Carmeliet, P., and Critchley, H. O. D. (2018). Hypoxia and hypoxia inducible factor-1α are required for normal endometrial repair during menstruation. Nat. Commun. 9 (1), 295. doi:10.1038/s41467-017-02375-6
Mazurek, S., Grimm, H., Boschek, C. B., Vaupel, P., and Eigenbrodt, E. (2007). Pyruvate kinase type M2: a crossroad in the tumor metabolome. Br. J. Nutr. 87 (S1), S23–S29. doi:10.1079/bjn2001454
Meo, C., and de Nigris, F. (2024). Clinical potential of YY1-Hypoxia axis for vascular normalization and to improve immunotherapy. Cancers (Basel) 16 (3), 491. doi:10.3390/cancers16030491
Merviel, P., Challier, J. C., Carbillon, L., Foidart, J. M., and Uzan, S. (2001). The role of integrins in human embryo implantation. Fetal Diagn Ther. 16 (6), 364–371. doi:10.1159/000053942
Meseguer, M., Aplin, J. D., Caballero-Campo, P., O'Connor, J. E., Martín, J. C., Remohí, J., et al. (2001). Human endometrial mucin MUC1 is up-regulated by progesterone and down-regulated in vitro by the human blastocyst. Biol. Reprod. 64 (2), 590–601. doi:10.1095/biolreprod64.2.590
Miravet-Valenciano, J. A., Rincon-Bertolin, A., Vilella, F., and Simon, C. (2015). Understanding and improving endometrial receptivity. Curr. Opin. Obstet. Gynecol. 27, 187–192. doi:10.1097/GCO.0000000000000173
Mishra, A., and Modi, D. (2025). Role of HOXA10 in pathologies of the endometrium. Rev. Endocr. Metab. Disord. 26 (1), 81–96. doi:10.1007/s11154-024-09923-8
Monsivais, D., Nagashima, T., Prunskaite-Hyyryläinen, R., Nozawa, K., Shimada, K., Tang, S., et al. (2021). Endometrial receptivity and implantation require uterine BMP signaling through an ACVR2A-SMAD1/SMAD5 axis. Nat. Commun. 12 (1), 3386. doi:10.1038/s41467-021-23571-5
Montagna, P., Capellino, S., Villaggio, B., Remorgida, V., Ragni, N., Cutolo, M., et al. (2008). Peritoneal fluid macrophages in endometriosis: correlation between the expression of estrogen receptors and inflammation. Fertil. Steril. 90 (1), 156–164. doi:10.1016/j.fertnstert.2006.11.200
Nash, D. M., and Giles, J. L. (2025). Uterine inflammation and lessons from large animal models of endometritis. Nat. Rev. Immunol. doi:10.1038/s41577-025-01200-2
Nie, L., Zhao, Y. B., Zhao, D., Long, Y., Lei, Y., Liu, M., et al. (2019). Progesterone-induced miR-152 interferes with embryonic implantation by downregulating GLUT3 in endometrial epithelium. Am. J. Physiol. Endocrinol. Metab. 316 (4), E557–E567. doi:10.1152/ajpendo.00245.2018
Nilsson, O. (1958). Ultrastructure of mouse uterine surface epithelium under different estrogenic influences. 3. Late effect of estrogen administered to spayed animals. J. Ultrastruct. Res. 2 (2), 185–199. doi:10.1016/s0022-5320(58)90017-0
Nilsson, O. (1972). Ultrastructure of the process of secretion in the rat uterine epithelium at preimplantation. J. Ultrastruct. Res. 40 (5), 572–580. doi:10.1016/s0022-5320(72)80044-3
Onishi, K., and Zandstra, P. W. (2015). LIF signaling in stem cells and development. Development 142 (13), 2230–2236. doi:10.1242/dev.117598
Opuchlik, K., Pankiewicz, K., Pierzyński, P., Sierdziński, J., Aleksejeva, E., Salumets, A., et al. (2025). Factors influencing endometrial receptivity in women with recurrent implantation failure. BMC Womens Health 25 (1), 15. doi:10.1186/s12905-024-03531-z
Osol, G., and Moore, L. G. (2014). Maternal uterine vascular remodeling during pregnancy. Microcirculation 21 (1), 38–47. doi:10.1111/micc.12080
Paech, K., Webb, P., Kuiper, G. G., Nilsson, S., Gustafsson, J., Kushner, P. J., et al. (1997). Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science 277 (5331), 1508–1510. doi:10.1126/science.277.5331.1508
Pan, X., Qing, Q., Zhou, J., Sun, H., Li, L., Cao, W., et al. (2023). Effect of Chinese patent medicine Kunling Pill on endometrial receptivity: a clinical trial, network pharmacology, and animal-based study. Drug Discov. Ther. 17 (4), 257–269. doi:10.5582/ddt.2023.01016
Park, H. R., Choi, H. J., Kim, B. S., Chung, T. W., Kim, K. J., Joo, J. K., et al. (2021). Paeoniflorin enhances endometrial receptivity through leukemia inhibitory factor. Biomolecules 11 (3), 439. doi:10.3390/biom11030439
Pathare, A. D. S., Loid, M., Saare, M., Gidlöf, S. B., Zamani Esteki, M., Acharya, G., et al. (2023). Endometrial receptivity in women of advanced age: an underrated factor in infertility. Hum. Reprod. Update 29 (6), 773–793. doi:10.1093/humupd/dmad019
Paulson, R. J. (2011). Hormonal induction of endometrial receptivity. Fertil. Steril. 96 (3), 530–535. doi:10.1016/j.fertnstert.2011.07.1097
Paulson, R. J. (2019). Introduction: endometrial receptivity: evaluation, induction and inhibition. Fertil. Steril. 111 (4), 609–610. doi:10.1016/j.fertnstert.2019.02.029
Pawar, S., Laws, M. J., Bagchi, I. C., and Bagchi, M. K. (2015). Uterine epithelial estrogen receptor-α controls decidualization via a paracrine mechanism. Mol. Endocrinol. 29 (9), 1362–1374. doi:10.1210/me.2015-1142
Pijnenborg, R., Vercruysse, L., and Hanssens, M. (2006). The uterine spiral arteries in human pregnancy: facts and controversies. Placenta 27 (9-10), 939–958. doi:10.1016/j.placenta.2005.12.006
Prakobphol, A., Genbacev, O., Gormley, M., Kapidzic, M., and Fisher, S. J. (2006). A role for the L-selectin adhesion system in mediating cytotrophoblast emigration from the placenta. Dev. Biol. 298 (1), 107–117. doi:10.1016/j.ydbio.2006.06.020
Prossnitz, E. R., and Arterburn, J. B. (2015). International union of basic and clinical pharmacology. XCVII. G protein-coupled estrogen receptor and its pharmacologic modulators. Pharmacol. Rev. 67 (3), 505–540. doi:10.1124/pr.114.009712
Psychoyos, A., and Mandon, P. (1971). Scanning electron microscopy of the surface of the rat uterine epithelium during delayed implantation. J. Reprod. Fertil. 26 (1), 137–138. doi:10.1530/jrf.0.0260137
Pu, L., Shahzad, Q., Chen, F., Yao, S., Tang, Y., Chen, D., et al. (2020). Proteomic analysis demonstrates that parthenogenetically activated swamp buffalo embryos have dysregulated energy metabolism. Reprod. Domest. Anim. 55 (12), 1764–1773. doi:10.1111/rda.13838
Puigserver, P., and Spiegelman, B. M. (2003). Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr. Rev. 24 (1), 78–90. doi:10.1210/er.2002-0012
Qiu, X. M., Lai, Z. Z., Ha, S. Y., Yang, H. L., Liu, L. B., Wang, Y., et al. (2020). IL-2 and IL-27 synergistically promote growth and invasion of endometriotic stromal cells by maintaining the balance of IFN-γ and IL-10 in endometriosis. Reproduction 159 (3), 251–260. doi:10.1530/REP-19-0411
Quinn, K. E., Matson, B. C., Wetendorf, M., and Caron, K. M. (2020). Pinopodes: recent advancements, current perspectives, and future directions. Mol. Cell Endocrinol. 501, 110644. doi:10.1016/j.mce.2019.110644
Reynolds, L. P., and Redmer, D. A. (2001). Angiogenesis in the placenta. Biol. Reprod. 64 (4), 1033–1040. doi:10.1095/biolreprod64.4.1033
Riera Leal, A., Ortiz-Lazareno, P. C., Jave-Suárez, L. F., Ramírez De Arellano, A., Aguilar-Lemarroy, A., Ortiz-García, Y. M., et al. (2020). 17β-estradiol-induced mitochondrial dysfunction and Warburg effect in cervical cancer cells allow cell survival under metabolic stress. Int. J. Oncol. 56 (1), 33–46. doi:10.3892/ijo.2019.4912
Salmasi, S., Sharifi, M., and Rashidi, B. (2021). Ovarian stimulation and exogenous progesterone affect the endometrial miR-16-5p, VEGF protein expression, and angiogenesis. Microvasc. Res. 133, 104074. doi:10.1016/j.mvr.2020.104074
Santos-Ribeiro, S., Mackens, S., Tournaye, H., Blockeel, C., and Stoop, D. (2017). Endometrial receptivity enhancement through induced injury and repair during ovarian stimulation: the receptivity enhancement by follicular-phase renewal after endometrial ScratcHing (REFRESH) trial protocol. Hum. Reprod. Open 2017 (3), hox022. doi:10.1093/hropen/hox022
Seles, L., Zaha, I. A., Luncan, M., Bodog, A., Sachelarie, L., Sandor, M., et al. (2024). Immunomodulatory treatment impact on IVF outcomes in KIR AA genotype: personalized fertility insights. Med. Kaunas. 60 (6), 948. doi:10.3390/medicina60060948
Sharkey, A. (1998). Cytokines and implantation. Rev. Reprod. 3 (1), 52–61. doi:10.1530/ror.0.0030052
Shavit, M., Vancová, M., Jedlicka, J., Bílý, T., Mahrouk, M., Cendelin, J., et al. (2025). Abundance of maternal mitochondrial genome is dispensable up to the mitochondrial genome activation in post-implantation embryos. Faseb J. 39 (17), e70986. doi:10.1096/fj.202501179R
Shekibi, M., Heng, S., Wang, Y., Samarajeewa, N., Rombauts, L., and Nie, G. (2022). Progesterone suppresses podocalyxin partly by up-regulating miR-145 and miR-199 in human endometrial epithelial cells to enhance receptivity in in vitro models. Mol. Hum. Reprod. 28 (11), gaac034. doi:10.1093/molehr/gaac034
Shuai, Z., Lian, F., Li, P., and Yang, W. (2015). Effect of transcutaneous electrical acupuncture point stimulation on endometrial receptivity in women undergoing frozen-thawed embryo transfer: a single-blind prospective randomised controlled trial. Acupunct. Med. 33 (1), 9–15. doi:10.1136/acupmed-2014-010572
Simón, C. M. C., Remohí, J., and Pellicer, A. (1998). Cytokines and embryo implantation. J. Reprod. Immunol. 39 (1-2), 117–131. doi:10.1016/s0165-0378(98)00017-5
Simon, C., Domínguez, F., Valbuena, D., and Pellicer, A. (2003). The role of estrogen in uterine receptivity and blastocyst implantation. Trends Endocrinol. Metab. 14 (5), 197–199. doi:10.1016/s1043-2760(03)00084-5
Song, L., Wang, L., Li, X., and Xiao, L. (2023). Ginsenoside Rg1 alleviates lipopolysaccharide-induced fibrosis of endometrial epithelial cells in dairy cows by inhibiting reactive oxygen species-activated NLRP3. Anim. (Basel) 13 (23), 3723. doi:10.3390/ani13233723
Sun, X., Li, Q., and Chen, B. (2025). Role of mitochondrial dynamics in mouse preimplantation embryonic development and its molecular mechanisms. Sci. Rep. 15 (1), 21751. doi:10.1038/s41598-025-05622-9
Sunder, S., and Lenton, E. A. (2000). Endocrinology of the peri-implantation period. Baillieres Best. Pract. Res. Clin. Obstet. Gynaecol. 14 (5), 789–800. doi:10.1053/beog.2000.0119
Tayade, C., Black, G. P., Fang, Y., and Croy, B. A. (2006). Differential gene expression in endometrium, endometrial lymphocytes, and trophoblasts during successful and abortive embryo implantation. J. Immunol. 176 (1), 148–156. doi:10.4049/jimmunol.176.1.148
Thie, M., and Denker, H. W. (2002). In vitro studies on endometrial adhesiveness for trophoblast: cellular dynamics in uterine epithelial cells. Cells Tissues Organs 172 (3), 237–252. doi:10.1159/000066963
Tian, Z., Zhang, C., Liao, X., Yang, S., Hong, Y., Shi, A., et al. (2024). Trends in acupuncture for infertility: a scoping review with bibliometric and visual analysis. Front. Endocrinol. (Lausanne) 15, 1351281. doi:10.3389/fendo.2024.1351281
Trenti, A., Tedesco, S., Boscaro, C., Ferri, N., Cignarella, A., Trevisi, L., et al. (2017). The glycolytic enzyme PFKFB3 is involved in estrogen-mediated angiogenesis via GPER1. J. Pharmacol. Exp. Ther. 361 (3), 398–407. doi:10.1124/jpet.116.238212
Truong, T. T., Soh, Y. M., and Gardner, D. K. (2016). Antioxidants improve mouse preimplantation embryo development and viability. Hum. Reprod. 31 (7), 1445–1454. doi:10.1093/humrep/dew098
Tsai, T. S., Tyagi, S., and St John, J. C. (2018). The molecular characterisation of mitochondrial DNA deficient oocytes using a pig model. Hum. Reprod. 33 (5), 942–953. doi:10.1093/humrep/dey052
Turner, M. L., Healey, G. D., and Sheldon, I. M. (2012). Immunity and inflammation in the uterus. Reprod. Domest. Anim. 47 (Suppl. 4), 402–409. doi:10.1111/j.1439-0531.2012.02104.x
Turner, M. L., Cronin, J. G., Noleto, P. G., and Sheldon, I. M. (2016). Glucose availability and AMP-activated protein kinase link energy metabolism and innate immunity in the Bovine endometrium. PLoS One 11 (3), e0151416. doi:10.1371/journal.pone.0151416
Usadi, R. S., Murray, M. J., Bagnell, R. C., Fritz, M. A., Kowalik, A. I., Meyer, W. R., et al. (2003). Temporal and morphologic characteristics of pinopod expression across the secretory phase of the endometrial cycle in normally cycling women with proven fertility. Fertil. Steril. 79 (4), 970–974. doi:10.1016/s0015-0282(02)04929-4
Vallée, A., Lecarpentier, Y., and Vallée, J. N. (2021). The key role of the WNT/β-Catenin pathway in metabolic reprogramming in cancers under normoxic conditions. Cancers (Basel) 13 (21), 5557. doi:10.3390/cancers13215557
Vaughan, R. A., Garcia-Smith, R., Dorsey, J., Griffith, J. K., Bisoffi, M., and Trujillo, K. A. (2013). Tumor necrosis factor alpha induces Warburg-like metabolism and is reversed by anti-inflammatory curcumin in breast epithelial cells. Int. J. Cancer 133 (10), 2504–2510. doi:10.1002/ijc.28264
Vaux, E. C., Metzen, E., Yeates, K. M., and Ratcliffe, P. J. (2001). Regulation of hypoxia-inducible factor is preserved in the absence of a functioning mitochondrial respiratory chain. Blood 98 (2), 296–302. doi:10.1182/blood.v98.2.296
Vićovac, L., and Aplin, J. D. (1996). Epithelial-mesenchymal transition during trophoblast differentiation. Acta Anat. (Basel) 156 (3), 202–216. doi:10.1159/000147847
Wang, T., Marquardt, C., and Foker, J. (1976). Aerobic glycolysis during lymphocyte proliferation. Nature 261 (5562), 702–705. doi:10.1038/261702a0
Wang, H., Shi, G., Li, M., Fan, H., Ma, H., and Sheng, L. (2018a). Correlation of IL-1 and HB-EGF with endometrial receptivity. Exp. Ther. Med. 16 (6), 5130–5136. doi:10.3892/etm.2018.6840
Wang, X. M., Ma, Z. Y., and Song, N. (2018b). Inflammatory cytokines IL-6, IL-10, IL-13, TNF-α and peritoneal fluid flora were associated with infertility in patients with endometriosis. Eur. Rev. Med. Pharmacol. Sci. 22 (9), 2513–2518. doi:10.26355/eurrev_201805_14899
Wang, X. M., She, C., Li, Q., Zhang, D., Xu, J. X., Li, M. H., et al. (2020a). Ginsenoside Rh3 activates Nrf2 signaling and protects endometrial cells from oxygen and glucose deprivation-reoxygenation. Aging (Albany NY) 12 (7), 6109–6119. doi:10.18632/aging.103009
Wang, L., Lv, S., Mao, W., Bai, E., and Yang, X. (2020b). Fecundity disorders in older women: declines in follicular development and endometrial receptivity. BMC Womens Health 20 (1), 115. doi:10.1186/s12905-020-00979-7
Warburg, O. W. F., and Negelein, E. (1927). The metabolism of tumors in the body. J. Gen. Physiol. 8 (6), 519–530. doi:10.1085/jgp.8.6.519
Warburg O, O. (1956). On the origin of cancer cells. Science 123 (3191), 309–314. doi:10.1126/science.123.3191.309
Weerasinghe, H., Stölting, H., Rose, A. J., and Traven, A. (2024). Metabolic homeostasis in fungal infections from the perspective of pathogens, immune cells, and whole-body systems. Microbiol. Mol. Biol. Rev. 88 (3), e0017122. doi:10.1128/mmbr.00171-22
Wei, S., Zhang, J., Zhao, R., Shi, R., An, L., Yu, Z., et al. (2024). Histone lactylation promotes malignant progression by facilitating USP39 expression to target PI3K/AKT/HIF-1α signal pathway in endometrial carcinoma. Cell Death Discov. 10 (1), 121. doi:10.1038/s41420-024-01898-4
Weng, R., Liu, Y., and Xiong, W. (2025). Decreased glycolysis due to LDHA m6A methylation in endometrium of endometriosis impairs its decidualization and contributes to related infertility. Am. J. Pathol. 26.
Winstanley, Y. E., Liu, J., Adhikari, D., Gonzalez, M. B., Russell, D. L., Carroll, J., et al. (2024). Dynamics of mitochondrial DNA copy number and membrane potential in mouse pre-implantation embryos: responses to diverse types of oxidative stress. Genes (Basel) 15 (3), 367. doi:10.3390/genes15030367
Winuthayanon, W., Hewitt, S. C., and Korach, K. S. (2014). Uterine epithelial cell estrogen receptor alpha-dependent and -independent genomic profiles that underlie estrogen responses in mice. Biol. Reprod. 91 (5), 110. doi:10.1095/biolreprod.114.120170
Wu, H., Zhao, X., Hochrein, S. M., Eckstein, M., Gubert, G. F., Knöpper, K., et al. (2023). Mitochondrial dysfunction promotes the transition of precursor to terminally exhausted T cells through HIF-1α-mediated glycolytic reprogramming. Nat. Commun. 14 (1), 6858. doi:10.1038/s41467-023-42634-3
Xin, M., He, J., Yang, W., Yin, X., and Wang, J. (2018). Wenshen Yangxue decoction improves endometrial receptivity recovery and promotes endometrial angiogenesis in a rat model. Pharm. Biol. 56 (1), 573–579. doi:10.1080/13880209.2018.1510973
Xu, X. D., Shao, S. X., Jiang, H. P., Cao, Y. W., Wang, Y. H., Yang, X. C., et al. (2015). Warburg effect or reverse Warburg effect? A review of cancer metabolism. Oncol. Res. Treat. 38 (3), 117–122. doi:10.1159/000375435
Xu, B., Shanmugalingam, R., Chau, K., Pears, S., Hennessy, A., and Makris, A. (2017). The effect of acetyl salicylic acid (Aspirin) on trophoblast-endothelial interaction in vitro. J. Reprod. Immunol. 124, 54–61. doi:10.1016/j.jri.2017.10.044
Xu, J., Martien, J., Gilbertson, C., Ma, J., Amador-Noguez, D., and Park, J. O. (2020). Metabolic flux analysis and fluxomics-driven determination of reaction free energy using multiple isotopes. Curr. Opin. Biotechnol. 64, 151–160. doi:10.1016/j.copbio.2020.02.018
Xu, K., Yin, N., Peng, M., Stamatiades, E. G., Shyu, A., Li, P., et al. (2021). Glycolysis fuels phosphoinositide 3-kinase signaling to bolster T cell immunity. Science 371 (6527), 405–410. doi:10.1126/science.abb2683
Yang, D., Liu, A., Wu, Y., Nan, S., Yin, R., et al. (2020). BCL2L15 depletion inhibits endometrial receptivity via the STAT1 signaling pathway. Genes (Basel) 11 (7), 816. doi:10.3390/genes11070816
Yang, Q., Liu, J., Wang, Y., Zhao, W., Wang, W., Cui, J., et al. (2022). A proteomic atlas of ligand-receptor interactions at the ovine maternal-fetal interface reveals the role of histone lactylation in uterine remodeling. J. Biol. Chem. 298 (1), 101456. doi:10.1016/j.jbc.2021.101456
Young, V. J., Brown, J. K., Maybin, J., Saunders, P. T., Duncan, W. C., and Horne, A. W. (2014). Transforming growth factor-β induced Warburg-like metabolic reprogramming may underpin the development of peritoneal endometriosis. J. Clin. Endocrinol. Metab. 99 (9), 3450–3459. doi:10.1210/jc.2014-1026
Younis, S., Jouneau, A., Larsson, M., Oudin, J. F., Adenot, P., Omar, J., et al. (2023). Ablation of ZC3H11A causes early embryonic lethality and dysregulation of metabolic processes. Proc. Natl. Acad. Sci. U. S. A. 120 (23), e2216799120. doi:10.1073/pnas.2216799120
Zhang, Y., and Boley, D. (2022). Nonlinear multi-objective flux balance analysis of the Warburg effect. J. Theor. Biol. 550, 111223. doi:10.1016/j.jtbi.2022.111223
Zhang, L., Zhao, M., Jiao, F., Xu, X., Liu, X., Jiang, Y., et al. (2015). Interferon gamma is involved in apoptosis of trophoblast cells at the maternal-fetal interface following Toxoplasma gondii infection. Int. J. Infect. Dis. 30, 10–16. doi:10.1016/j.ijid.2014.10.027
Zhang, H., Qi, J., Wang, Y., Sun, J., Li, Z., Sui, L., et al. (2020). Progesterone regulates glucose metabolism through glucose transporter 1 to promote endometrial receptivity. Front. Physiol. 11, 543148. doi:10.3389/fphys.2020.543148
Zhang, Q., Liu, S., Zhang, C. S., Wu, Q., Yu, X., Zhou, R., et al. (2022). AMPK directly phosphorylates TBK1 to integrate glucose sensing into innate immunity. Mol. Cell 82 (23), 4519–36.e7. doi:10.1016/j.molcel.2022.10.026
Zhao, G., Jiang, K., Yang, Y., Zhang, T., Wu, H., Shaukat, A., et al. (2018). The potential therapeutic role of miR-223 in bovine endometritis by targeting the NLRP3 inflammasome. Front. Immunol. 9, 1916. doi:10.3389/fimmu.2018.01916
Zhao, W., Wang, Y., Liu, J., Yang, Q., Zhang, S., Hu, X., et al. (2023). Progesterone activates the histone lactylation-Hif1α-glycolysis feedback loop to promote decidualization. Endocrinology 165 (1), bqad169. doi:10.1210/endocr/bqad169
Zheng, J., Tang, X., Han, T. L., Zhang, C., and Zhang, S. (2023). Metabolomics analysis of serum metabolites during endometrial transformation: association with recurrent implantation failure in hormonal replacement therapy-frozen embryo transfers cycles. J. Assist. Reprod. Genet. 40 (10), 2473–2483. doi:10.1007/s10815-023-02904-x
Zhong, Y., Zeng, F., Liu, W., Ma, J., Guan, Y., and Song, Y. (2019). Acupuncture in improving endometrial receptivity: a systematic review and meta-analysis. BMC Complement. Altern. Med. 19 (1), 61. doi:10.1186/s12906-019-2472-1
Zhou, W., Choi, M., Margineantu, D., Margaretha, L., Hesson, J., Cavanaugh, C., et al. (2012). HIF1α induced switch from bivalent to exclusively glycolytic metabolism during ESC-to-EpiSC/hESC transition. EMBO J. 31 (9), 2103–2116. doi:10.1038/emboj.2012.71
Zhu, L., Zhao, Q., Yang, T., Ding, W., and Zhao, Y. (2015). Cellular metabolism and macrophage functional polarization. Int. Rev. Immunol. 34 (1), 82–100. doi:10.3109/08830185.2014.969421
Zou, W., Huo, B., Tu, Y., Zhu, Y., Hu, Y., Li, Q., et al. (2025). Metabolic reprogramming by chemo-gene co-delivery nanoparticles for chemo-immunotherapy in head and neck squamous cell carcinoma. Acta Biomater. 199, 361–373. doi:10.1016/j.actbio.2025.04.031
Keywords: Warburg effect, endometrial receptivity, embryo implantation, infertility, glycolysis
Citation: Zhang X, Zhu Q, Nie W, Yan X, Yuan Z and Tian L (2025) Linking the Warburg effect to endometrial receptivity: metabolic parallels in embryo implantation. Front. Cell Dev. Biol. 13:1683790. doi: 10.3389/fcell.2025.1683790
Received: 11 August 2025; Accepted: 29 October 2025;
Published: 17 November 2025.
Edited by:
Beate Emmi Margarete Brand-Saberi, Ruhr University Bochum, GermanyReviewed by:
Soumya Ranjan Jena, Ravenshaw University, IndiaSatya Srirama Karthik Divvela, Ruhr University Bochum, Germany
Copyright © 2025 Zhang, Zhu, Nie, Yan, Yuan and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingwen Zhu, YnVjbUBxcS5jb20=
†These authors have contributed equally to this work and share first authorship
 Xiaoyang Zhang
Xiaoyang Zhang Qingwen Zhu1*
Qingwen Zhu1*