- 1Department of Burn and Plastic Surgery, Affiliated Hospital of North Sichuan Medical College, Nanchong, China
- 2Biotechnology Innovation Drug Application and Transformation Key Laboratory of Sichuan Province, North Sichuan Medical College, Nanchong, Sichuan, China
Bone and soft tissue injuries resulting from trauma, metabolic disorders, and tumors pose a serious threat to public health, and their treatment faces numerous challenges, including infection, chronic inflammation, and impaired vascularization. Photothermal hydrogels, a new class of biomaterials, can sterilize tissues via photothermal therapy (PTT) and, through intelligent material design, exhibit multiple biological functions such as modulating the pathological microenvironment in bone and soft tissues. These properties have earned them a reputation as a “star material” in tissue engineering. However, excessive heating (above 50 °C) can cause irreversible thermal damage to tissues. Therefore, functional hydrogels that generate a mild photothermal effect (approximately 40 °C–45 °C) have recently become a research focus. This review provides a comprehensive overview of the types and fabrication strategies of photothermal agents used in mild photothermal hydrogels, systematically summarizes recent progress in their applications for bone and soft tissue injury repair, and delves into the underlying mechanisms by which they promote tissue regeneration. By summarizing current findings and outlining future perspectives on the use of mild photothermal hydrogels in modern regenerative medicine, we aim to advance the development of tissue engineering.
1 Introduction
Bone and soft tissue injuries caused by trauma, burns, tumors, and sports-related incidents have become increasingly common, representing a major global public health challenge (Jin et al., 2024). Functional hydrogels, one of the most promising therapeutic strategies, have been successfully applied in various tissue repair contexts (Enayati et al., 2024). Among these, photothermal hydrogels have emerged as an innovative material that has attracted extensive research attention in recent years due to their unique biological advantages (Xiao et al., 2025; Zhang R. et al., 2025).
Photothermal hydrogels primarily work by converting light energy into heat in response to near-infrared (NIR) irradiation (e.g., wavelengths of 808 nm or 1,064 nm), thereby triggering biological effects in local tissues. Based on the temperature range achieved, photothermal hydrogels can be categorized into mild photothermal, moderate photothermal, and high-temperature photothermal types (He et al., 2024). Compared to moderate and high-temperature photothermal hydrogels, mild photothermal hydrogels (operating at 40 °C–45 °C) have gained attention for causing less thermal damage to tissues and providing a broader therapeutic window. They are considered to have the following biological advantages: 1) enhanced blood circulation: mild heating can dilate microvasculature, improving local blood flow (Wu A. L. et al., 2025). 2) Activation of heat shock proteins (HSPs): For example, upregulation of HSP70 and HSP27 enhances cellular stress resistance and promotes tissue repair (Cheng et al., 2023; Jiang et al., 2024). 3) Regulation of cell behavior: Studies have shown that temperatures around 42 °C can promote fibroblast migration, osteoblast mineralization activity, and the angiogenic potential of endothelial cells (Gao et al., 2025; Zhang et al., 2022). 4) Simplified fabrication: Mild photothermal hydrogels can achieve stable temperature control with simpler processes, while also reducing the required concentration of photothermal agents and light intensity (He et al., 2024).
Given their broad application prospects in tissue engineering, this review delves into the construction strategies and fabrication methods of mild photothermal hydrogel systems. It provides a comprehensive analysis of their biomedical application scenarios and underlying mechanisms, summarizes the therapeutic limitations of mild photothermal effects, and outlines future development trajectories in the field of bone and soft tissue repair.
2 Construction strategies of mild photothermal hydrogels
2.1 Inorganic photothermal materials for mild photothermal hydrogels
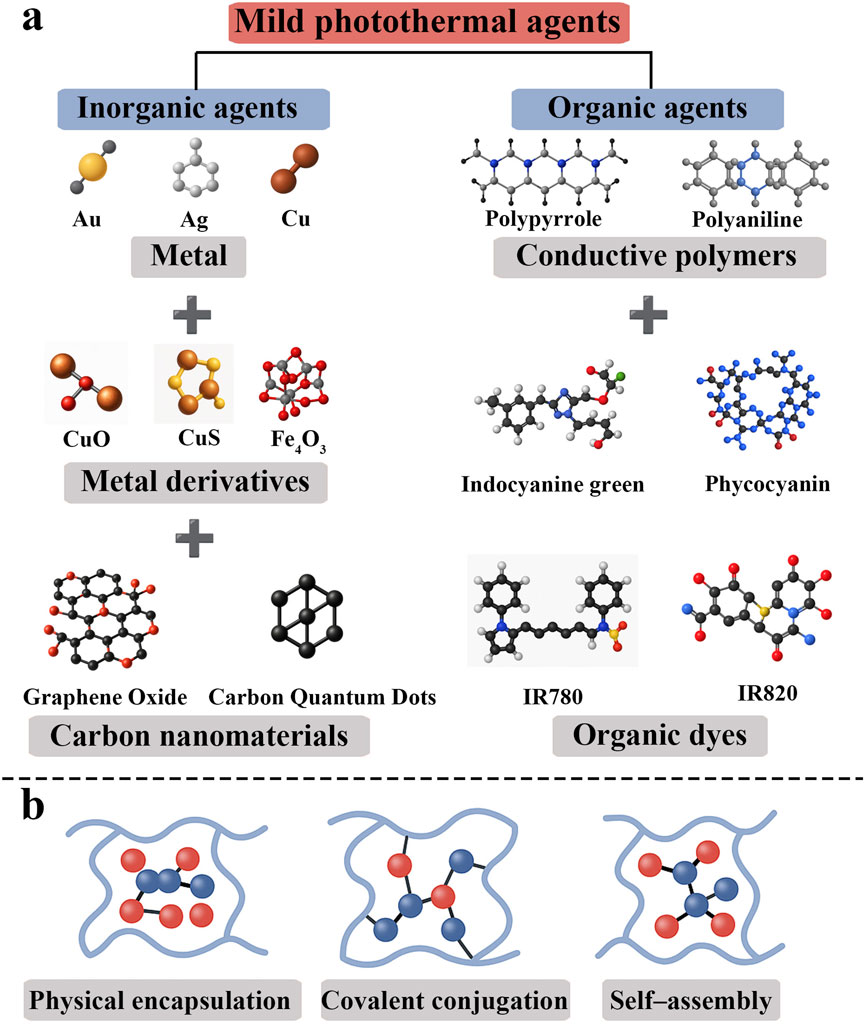
Mild photothermal hydrogels generate a controlled photothermal effect by incorporating functional materials with high photothermal conversion efficiency; thus, the choice of photothermal agent is crucial for system design. The main photothermal agents currently in use can be classified into inorganic and organic materials (Li W. et al., 2023) (Figure 1a). Common inorganic photothermal materials include metallic nanomaterials and carbon-based materials.
2.1.1 Metal-based inorganic photothermal agents
Noble-metal nanoparticles (e.g., Au, Ag, Cu) are well known to exhibit excellent photothermal performance owing to their localized surface plasmon resonance (LSPR) effects (Hu et al., 2024). By contrast, semiconductor-based photothermal agents operate via bandgap excitation rather than plasmonic effects. Absorbed photons in a semiconductor promote electrons across the bandgap, generating electron-hole pairs. Only semiconductors that are heavily doped or intrinsically defect-rich can approach the free-carrier densities of metals and thereby exhibit LSPR-like resonances (Liu et al., 2024). For this reason, metal-based nanoparticles are widely used as photothermal agents in applications such as cancer therapy, optical imaging, and solar-driven heat generation, where effective light-to-heat conversion is critical. In addition, these metal nanomaterials (especially noble metals like Au or Ag) are readily surface-functionalized that permit easy ligand attachment. For example, thiol-bearing molecules bind strongly to Au or Ag surfaces (forming metal–S bonds) (Cho et al., 2024), and original capping agents (e.g., citrate or CTAB) can be replaced by thiolated polymers, peptides or antibodies (ligand-exchange) (Duman et al., 2024). Likewise, charged ligands or biomolecules can adsorb electrostatically onto oppositely charged NP surfaces. These covalent (e.g., Au-S or amine-metal bonds) and noncovalent (electrostatic, hydrophobic) strategies allow stable conjugation of targeting ligands, drugs or imaging dyes to the nanoparticle surface (Cho et al., 2024).
Gold nanomaterials are among the most extensively studied photothermal agents. In recent studies, gold-based photothermal agents are often formed into photothermal composite hydrogels either by in situ generation or by pre-synthesis followed by incorporation into the hydrogel matrix. For example, one study directly mixed pre-made gold nanoparticles (AuNPs) into a calcium alginate hydrogel, upon irradiation with 808 nm near-infrared light (0.1–1 W/cm2) for several minutes, this system’s temperature rapidly rose to about 40 °C (Wu Y. et al., 2025). Silver nanoparticles (AgNPs) exhibit a similar LSPR photothermal effect to that of gold and also have inherent broad-spectrum antibacterial activity. Silver-loaded photothermal hydrogels are typically constructed by embedding or in situ generation of silver nanomaterials (Wang K. et al., 2025). Hao et al. reported a “carrier-free” silver nanoparticle hydrogel in which evenly dispersed AgNPs were generated in situ by the reduction of the natural polyphenol puerarin. Under 808 nm irradiation, the AgNPs produced a mild photothermal effect (∼45 °C) that quickly killed multidrug-resistant bacteria and continuously released silver ions to inhibit the regrowth of residual bacteria (Hao et al., 2025). Copper nanomaterials are cost-effective, have a broad antibacterial spectrum, and possess certain biological activities. Similar to gold and silver, copper nanomaterials also exhibit an LSPR effect. Lin and colleagues incorporated copper sulfide (CuS) nanoparticles into a hyaluronic acid hydrogel; under 808 nm irradiation, this material maintained a temperature of about 43 °C (Lin et al., 2021).
In summary, metallic nanomaterials (e.g., Au, Ag, Cu nanoparticles) offer strong NIR light absorption and highly efficient photothermal conversion due to their own chemical structural characteristics, and they can be readily functionalized for imaging or targeted delivery. However, these noble metal agents are non-biodegradable and tend to accumulate in vivo, raising concerns about long-term toxicity and clearance, which limits their clinical translation (Chen et al., 2024).
2.1.2 Carbon-based inorganic photothermal agents
Carbon nanomaterials have high thermal stability and excellent thermal conductivity, and they are less prone to photothermal degradation. These materials also have a very large specific surface area, which allows them to carry growth factors, drugs, etc., and they can be functionalized through abundant surface groups (Cui et al., 2023; Liu et al., 2023).
Graphene and its derivatives (including graphene oxide [GO] and reduced graphene oxide [rGO]) have been widely studied due to their two-dimensional sheet structure and outstanding photothermal properties. A typical construction method is to introduce functionalized graphene into a hydrogel network to form a nanocomposite scaffold. For example, by grafting branched polyethyleneimine (BPEI) onto GO and then forming dynamic Schiff-base crosslinks with aldehyde-bearing polymers, a stable three-dimensional network was constructed. In vitro experiments showed that an osteogenic scaffold loaded with this functionalized could reach 43 °C after 3 min of 808 nm laser irradiation (Zhang et al., 2021). Carbon quantum dots (CDs) are zero-dimensional carbon nanoparticles smaller than 10 nm. In constructing mild photothermal hydrogels, a common strategy is to incorporate CDs into the hydrogel network. On one hand, the carboxyl and amino groups on the surface of CDs can undergo Schiff-base reactions with multi-aldehyde polymers, achieving dynamic covalent cross-linking (Sharma et al., 2022); on the other hand, CDs can be simply dispersed in a polymer matrix and held inside the gel through hydrogen bonding or electrostatic interactions (Lu et al., 2025). Carbon nanotubes (CNTs) are one-dimensional hollow tubular structures made of sp2-hybridized carbon atoms. They possess excellent mechanical, electrical, and thermal properties. Common construction methods include physical dispersion and surface functionalization. Physical dispersion involves mixing CNTs uniformly in a polymer via non-covalent interactions (Ding et al., 2024). However, CNTs tend to aggregate, so dispersants or structure-directing agents (such as clay nanosheets or emulsifiers) are often needed. Surface functionalization improves the CNTs’ hydrophilicity and biocompatibility through covalent or non-covalent modifications (Deng et al., 2022; Ravanbakhsh et al., 2020). For example, oxidative treatment can introduce hydroxyl or carboxyl groups at the ends of CNTs, enabling them to form cross-links or hydrogen bonds with the hydrogel matrix (Forero-Doria et al., 2020).
Carbon nanomaterials are photothermally stable with excellent thermal conductivity and large surface areas for drug loading, and they can be surface-tailored to improve dispersibility and biocompatibility. On the other hand, pristine carbon agents are intrinsically hydrophobic and not readily biodegradable-they tend to aggregate in biological media and may persist in tissues, so appropriate functionalization or nanoscale design is required to mitigate potential chronic toxicity (Wang P. et al., 2025).
2.2 Organic photothermal materials for mild photothermal hydrogels
Compared to traditional inorganic materials, organic photothermal agents generally have better biocompatibility, making them suitable for long-term implantation in vivo. Their molecular structures are highly tunable, allowing researchers to adjust their optical absorption peaks via molecular design and functional modification to match the “NIR biological window” (∼650–950 nm for NIR-I; ∼1,000–1,350 nm for NIR-II), where absorption by hemoglobin and water is minimal, allowing deeper tissue penetration and thus improving the efficacy of photothermal tumor ablation (Guo S. et al., 2023). Typical organic photothermal agents include conductive polymers, and organic dyes (Makabenta et al., 2021; Wu et al., 2023).
2.2.1 Conductive polymer photothermal agents
Conjugated conductive polymers such as polypyrrole (PPy), polyaniline (PANI), and poly (3,4-ethylenedioxythiophene) (PEDOT) usually have broad absorption in the near-infrared region. These materials also possess electrical conductivity and thermal stability, and they easily integrate with hydrogel networks to form composites. Polypyrrole (PPy), due to its dark conjugated structure, exhibits excellent photothermal performance. One study constructed a three-dimensional porous hydrogel containing PPy and loaded with a heat-sensitive nitric oxide donor (BNN6). After 10 min of 808 nm laser irradiation (1.0 W/cm2), the PPy component achieved an approximately 80% photothermal conversion efficiency and triggered the release of NO from the donor, combining mild photothermal effects with chemical antimicrobial action (Guo W. et al., 2023). Polyaniline (PANI) is another typical conductive polymer photothermal agent. Researchers have prepared methacrylate-terminated polyaniline nanoparticles (Me-PANI NPs) and used the vinyl groups as chemical crosslinking points to construct a PANI-crosslinked conductive photothermal hydrogel. In vitro experiments showed that the introduction of Me-PANI NPs enabled the hydrogel to exhibit mild photothermal antibacterial activity under NIR irradiation, while also endowing the hydrogel with excellent mechanical properties (Pang et al., 2022).
Conjugated polymers exhibit broad NIR absorption and can achieve high photothermal efficiencies while generally showing good biocompatibility, importantly, many can be engineered to be biodegradable, addressing long-term safety to a degree. A key limitation, however, is that maintaining optimal photothermal performance and assured biodegradability can be challenging-polymer nanostructure and doping chemistry influence stability and heat conversion, and incomplete degradation or byproducts could still pose biocompatibility issues that require careful design (Bao et al., 2025).
2.2.2 Organic dye photothermal agents
Organic dye photothermal agents include both naturally derived pigments and synthetic small-molecule dyes that strongly absorb NIR light. Compared to inorganic nanomaterials, these organic agents generally exhibit superior biocompatibility and avoid heavy-metal-associated toxicities (Zhou et al., 2025). Natural pigments (e.g., phycocyanin, a blue phycobiliprotein from Spirulina) offer excellent biocompatibility and even possess inherent bioactive properties (immune-regulatory, antioxidant, anti-inflammatory) (Bai et al., 2023). However, as proteinaceous pigments they are prone to degradation under heat or light, which can reduce photothermal stability. This limits their standalone photothermal efficacy, and thus they are often combined with nanomaterials to enhance light-to-heat conversion. Synthetic dyes (e.g., indocyanine green (ICG) and other indocyanines like IR780/IR820) provide high molar absorption in the NIR and effective photothermal conversion. Indeed, ICG is an FDA-approved imaging agent that has also been widely explored as a photothermal agent (Cai et al., 2025). Their key drawback is instability in physiological conditions - ICG and its analogues tend to photobleach, aggregate, and clear rapidly from the body. Moreover, certain dyes, such as IR780 and IR820, are highly hydrophobic, necessitating encapsulation in carriers (polymers, liposomes, proteins, etc.) to improve water dispersibility and stability (Fialho et al., 2025). Such methods have been shown to prevent dye aggregation/photobleaching and prolong circulation, thereby significantly improving the photothermal performance of these organic dye agents.
Generally speaking, organic dye photothermal agents are typically low-cost, biocompatible, and readily excreted, with strong NIR absorption profiles, but they generally have lower photothermal conversion efficiency and limited photostability (e.g., photobleaching and rapid clearance), constraining their effectiveness in sustained photothermal therapy (Teng et al., 2024).
2.3 Photothermal agent loading strategies in hydrogels
Constructing a stable, efficient, and responsive mild photothermal hydrogel system is closely related to the method of incorporating photothermal agents into the hydrogel. Common loading strategies include: 1) Physical encapsulation: this method directly embeds photothermal agents into the hydrogel network through electrostatic adsorption, hydrophobic interactions, or van der Waals forces (Hu et al., 2024). The construction process is simple and requires mild reaction conditions. For example, GO or CuS nanoparticles can be mixed with sodium alginate, gelatin, etc., and crosslinked with Ca2+ or glutaraldehyde to form composite hydrogels (Huang et al., 2023). Physical encapsulation is straightforward, but lacking covalent bonds, the photothermal agents may “leak” or migrate over time with prolonged use. 2) Covalent conjugation: specific chemical reactions are used to covalently bind photothermal agents to the hydrogel network, enhancing system stability and sustained responsiveness. For instance, GO bearing surface carboxyl groups can be grafted onto an amino–functionalized gelatin backbone via EDC/NHS coupling, or polymer–photothermal agent covalent networks can be built through thiol–ene “click” chemistry (Degirmenci et al., 2024; Zhang W. et al., 2025). 3) In situ self–assembly: a newer strategy is to form a three-dimensional network via the self–assembly of the photothermal agent itself or its non-covalent interactions with polymers. For example, ICG and a gelatin/PEG matrix can be co–assembled into a self-healing photothermal hydrogel via a freeze–dry–rehydration approach, yielding a material with responsiveness, moldability, and biodegradability (Li et al., 2022). In addition, certain natural small molecules (such as tannic acid) have dual abilities to chelate metal ions and stack π–π bonds, allowing them to induce gelation in situ under mild conditions, which is an important direction for green self-assembly strategies (Zhou et al., 2024) (Figure 1b).
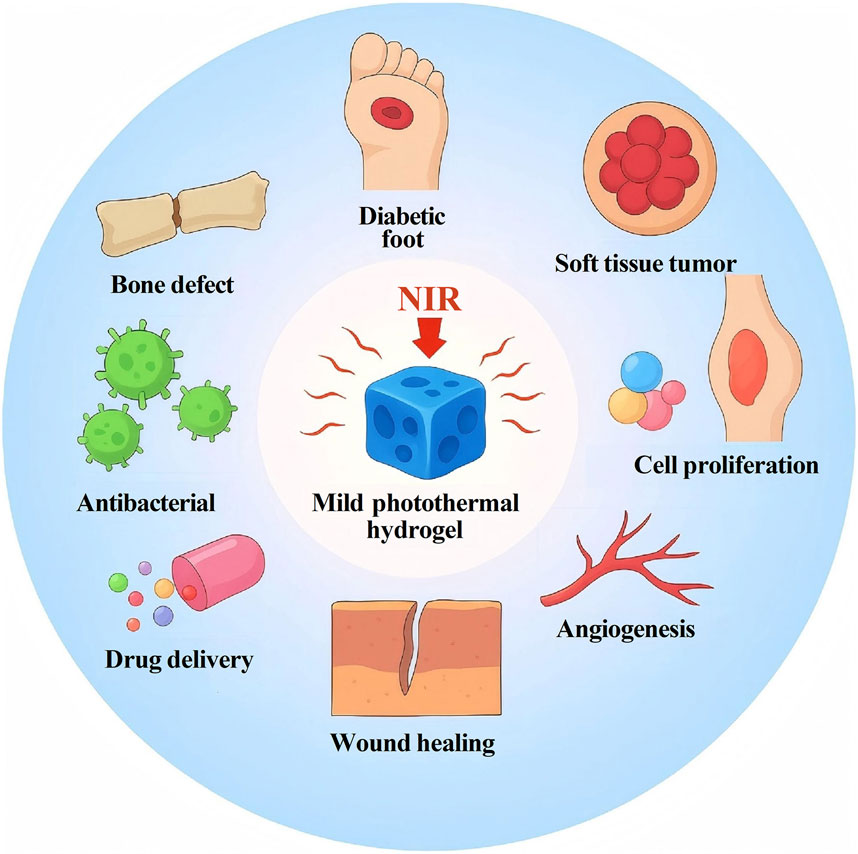
3 Biomedical applications of mild photothermal hydrogels
3.1 Applications in wound healing
Both chronic and acute wounds are often accompanied by pathological changes such as disruption of the skin barrier, persistent infection, high oxidative stress, and chronic inflammation, which make healing difficult. There is an urgent need for novel multifunctional therapeutic strategies to address these multiple challenges simultaneously (Byun et al., 2024). In recent years, many researchers have achieved significant results in combating infection and controlling pathogens in wounds by combining mild photothermal effects with multimodal antibacterial strategies (Figure 2). For example, Gao et al. prepared a chitosan hydrogel containing ZIF–8 nanoparticles coated with polydopamine (PDA). Under 808 nm laser irradiation, the temperature of this hydrogel was maintained at 40 °C–45 °C. This system, through Zn2+-mediated bacterial membrane rupture combined with local mild heating, was highly effective against methicillin-resistant S. aureus (MRSA), achieving a 99.5% kill rate (Gao et al., 2025). In a burn infection model, Yu et al. prepared a gelatin–oxidized dandelion hydrogel containing natural black currant extract. Under 808 nm irradiation (2.5 W/cm2, temperature controlled at 45 °C), 10 min of treatment could kill 99% of S. aureus, 98% of E. coli, and 82% of P. aeruginosa (Yu et al., 2024). Mild photothermal therapy (PTT <45 °C) exerts broad antibacterial effects through multiple mechanisms. First, sub-lethal photothermal heating damages bacterial membranes, increasing their permeability and causing leakage of cytoplasmic contents, which leads to cell lysis (Zhang et al., 2022). Second, heat stress can denature bacterial proteins (including enzymes and structural proteins), impairing essential cellular functions. Third, mild PTT may elevate bacterial reactive oxygen species (ROS) levels, inducing oxidative damage to lipids, proteins, and DNA within the microbes (Chen et al., 2025). Fourth, localized photothermal heating can disrupt biofilm structures by weakening the extracellular polymeric matrix and enhancing the susceptibility of biofilm-encased bacteria to treatment. Finally, mild hyperthermia can modulate the host immune response at the wound site–for example, by promoting immune cell recruitment and activation, which aids in clearing the infection (Zhao et al., 2023). Collectively, these mechanisms enable mild photothermal therapy to effectively reduce bacterial burden and facilitate wound healing while minimizing damage to healthy tissue.
Beyond efficient antibacterial activity, using the inherent biological activities of materials to correct the wound microenvironment’s oxidative stress and chronic inflammation is equally crucial. Ma et al. reported a composite hydrogel containing black phosphorus (BP) nanosheets and quaternized chitosan (QCS). Upon NIR irradiation, this hydrogel significantly reduced the expression of pro-inflammatory cytokine IL-6 and increased the expression of anti-inflammatory IL-10. This anti-inflammatory effect is attributed to the synergistic action of QCS itself and the mild photothermal stimulation mediated by BP (Ma et al., 2025). Li et al. used dopamine-modified hyaluronic acid and PDA-coated Ti3C2 MXene nanosheets to prepare an injectable, self-catalyzing cross-linked hydrogel. Leveraging the non-enzymatic antioxidant properties of the MXene photothermal agent, this system efficiently scavenges reactive oxygen species (ROS) and maintains cellular redox homeostasis (Li et al., 2022). Simultaneously, the HA-DA (hyaluronic acid-dopamine) scaffold induces macrophages to polarize toward the anti-inflammatory M2 phenotype. Together, these effects allow the hydrogel to effectively improve the pathological microenvironment of infected diabetic wounds.
Adequate angiogenesis is also essential for wound healing. Huang et al. reported a copper/Zn-MOF composite hydrogel with photothermal functionality. This hydrogel precisely modulated the M1/M2 balance of macrophages at the wound site, skewing macrophages toward the M2 phenotype, thereby promoting neural tissue and blood vessel regeneration and accelerating chronic wound healing (Huang K. et al., 2022). Gao et al. constructed a tri-component crosslinked hydrogel made of carboxymethyl chitosan (CMCS), gelatin, and oxidized sodium alginate (OSA), into which PDA-modified ZIF-8 nanoparticles were embedded. Under mild NIR irradiation, this material promoted endothelial cell proliferation and the expression of angiogenic markers (VEGF and CD31), enhancing blood vessel formation and collagen alignment, which together accelerated wound closure (Gao et al., 2025). In addition, other studies have incorporated growth factors (such as VEGF and bFGF) or nanoparticles (such as Au nanoparticles or PbS quantum dots) into mild photothermal hydrogels (Guo et al., 2024; Xia et al., 2021; Zhu et al., 2023). The mild heating from NIR irradiation stimulates controlled release of these factors, and combining the system with immunomodulatory molecules (such as IL-10 or TGF-β) can further enhance angiogenesis and immune-mediated healing.
3.2 Applications in bone and soft tissue tumor therapy
Conventional tumor treatments (including surgical resection and radiotherapy) often face issues such as residual tumor cells post-surgery, local recurrence, cancer cell drug resistance, and systemic toxicity from high-dose chemotherapy. High-temperature photothermal therapy has been shown to directly lyse cancer cells, but excessively high temperatures (>50 °C) can cause damage to normal tissues and elicit inflammatory responses (Xia et al., 2021). Therefore, a series of recent studies have focused on designing and applying mild photothermal systems (Figure 2). Luo et al. reported an HTA hydrogel constructed from hydroxypropyl chitosan, tannic acid, and Fe3+ complexes. Under 808 nm laser irradiation (1 W/cm2), the hydrogel temperature stabilized at 42 °C–43 °C, significantly inducing apoptosis in osteosarcoma cells and causing tumor-associated macrophages to shift from the M2-type to the M1-type phenotype (Luo et al., 2023). This confirmed that a mild photothermal effect can achieve both immune microenvironment remodeling and antitumor effects. Chen et al. developed a composite hydrogel of silk fibroin, sericin-dopamine, tannic acid, and Cu2+. Under 808 nm laser irradiation (0.75 W/cm2, 20 min), the hydrogel temperature was maintained at approximately 44 °C; it was able to inhibit the proliferation of osteosarcoma cells and induce apoptosis, while the Cu2+ and polyphenols exerted antioxidant effects that improved the tissue repair environment (Chen et al., 2025). In another study, Zhang et al. developed an injectable gelatin-dopamine hydrogel composite with magnesium peroxide (MgO2) nanoparticles. Under 808 nm laser irradiation (0.5 W/cm2, 10 min), the hydrogel temperature reached about 43 °C. This hydrogel not only suppressed osteosarcoma cell activity through the mild photothermal effect but also released Mg2+ to promote bone regeneration, demonstrating a dual “antitumor and osteogenic repair” function (Zhang R. et al., 2025). These results indicate that by precisely adjusting the concentration of photothermal agents and the irradiation conditions, hydrogels can stably generate a mild heating effect. This enables dual therapeutic effects of antitumor activity and tissue repair without damaging normal tissues.
Mild photothermal therapy (heating tissues to <45 °C) can itself impede tumor growth via multiple mechanisms. Even moderate hyperthermia (≈42 °C–45 °C) induces partial protein denaturation, membrane disruption, and oxidative stress in cancer cells. These sub-lethal injuries trigger mitochondrial dysfunction-elevated temperature increases mitochondrial membrane permeability and ROS generation-which in turn activates intrinsic apoptosis pathways (Cai et al., 2025). Consequently, mild photothermal heating can promote tumor cell apoptosis without reaching ablative temperatures (Huang Q. et al., 2022). On the other hand, many hydrogel-based systems combine mild photothermal effects with additional therapies to achieve synergistic antitumor activity. The photothermal agents (e.g., polydopamine, gold nanomaterials) embedded in the hydrogel convert NIR into heat, raising the local temperature above a critical transition. This can induce a volume phase change in thermo-responsive polymers or break thermosensitive linkages, freeing the payload. For example, Liu et al. reported a chitosan-based hydrogel that remains stable at 37 °C but, upon NIR irradiation, undergoes rapid network collapse into a porous state, thereby releasing its drug load on-demand (Liu et al., 2021). Likewise, Kong et al. designed an injectable liposomal hydrogel in which an NIR photothermal dye generates mild heat to rupture encapsulated thermosensitive liposomes, instantly releasing gemcitabine at the target site. Immune checkpoint inhibitors (such as anti-PD-1/PD-L1 antibodies) or cytokines (e.g., IFN-γ, IL-12) can be co-encapsulated in injectable hydrogels for sustained, localized delivery to the tumor (Kong et al., 2021). The hydrogels protect these biomolecules and concentrate them in the tumor microenvironment, improving therapeutic index (Mohammadzadeh et al., 2025). Mild photothermal therapy (sub-ablative hyperthermia) further enhances their efficacy by stimulating immunogenic tumor cell death and promoting immune cell infiltration. For instance, a recent alginate hydrogel loaded with anti-PD-L1 antibodies and Fe3O4 nanoparticles showed that NIR irradiation induced tumor cell apoptosis and the release of tumor antigens, while simultaneously releasing anti-PD-L1 in situ; this led to robust T-cell activation and tumor regression in vivo compared to antibody or PTT alone (Mohammadzadeh et al., 2025). In general, the heat from mild PTT can “prime” tumors for immunotherapy-increasing dendritic cell maturation and cytotoxic T-lymphocyte activity and upregulating checkpoint ligand expression on cancer cells-thereby transforming an immunosuppressive tumor into an “immunologically hot” state more responsive to checkpoint blockade therapy.
3.3 Applications in bone defect repair
Like wound healing, the treatment of bone defects faces many challenges. Firstly, due to limited donor availability, autograft bone transplantation is constrained and can lead to immune rejection and complications. In addition, bone infection—especially that caused by drug-resistant bacteria secondary to trauma or surgery—often complicates treatment. Bone defect and infection sites also often suffer from poor circulation, hypoxia, and an excessive local immune response (Wu A. L. et al., 2025).
Studies have shown that mild heat stimulation (in the range of 37 °C–45 °C) can upregulate heat shock proteins (e.g., HSP70, HSP90) and indirectly or directly activate osteogenic signaling pathways such as Wnt/β-catenin, PI3K/AKT/mTOR, BMP/Smad, and MAPK (ERK1/2), thereby promoting the expression of bone markers (ALP, OCN, COL-I) (Yao et al., 2024) (Figure 2). Wang et al. reported a photothermal hydrogel system based on silk and calcium phosphate composites. By near-infrared (NIR) irradiation (808 nm, 1 W/cm2 for 5 min), the local temperature was raised to 42 °C. The mild photothermal effect activated heat shock proteins and osteogenesis-related genes (Runx2 and ALP), promoting the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs) (Wang et al., 2023). Li et al. designed a photothermal hydrogel system containing gold nanoparticles; using 808 nm NIR light (1 W/cm2 for 6 min), they generated a mild photothermal effect (∼40 °C) in the hydrogel. This promoted the osteogenic differentiation of BMSCs and also enhanced bone mineralization by releasing dissolved calcium ions (Li X. et al., 2023).
In addition, antibacterial activity, immune regulation, and pro-angiogenic effects are also considered crucial in bone defect treatment. Wei et al. combined copper nanoparticles with a photothermal hydrogel; under NIR irradiation (808 nm, 1.5 W/cm2, 5 min), the copper nanomaterials and the mild heat effectively killed bacteria in a bone infection model, accelerating the healing of the bone defect (Wei et al., 2024). Sun et al. designed a photothermal hydrogel system using 808 nm NIR laser irradiation (1 W/cm2, 5 min) to achieve a mild photothermal effect that raised the local temperature to 42 °C. This thermal stimulation not only promoted polarization of macrophages to the M2 anti-inflammatory phenotype, reducing inflammation, but also increased the expression of angiogenic factors such as VEGF and bFGF, promoting new blood vessel formation (Sun et al., 2025).
4 Concluding and perspectives
To date, researchers have successfully constructed various composite hydrogels with mild photothermal functionality and have achieved precise control over temperature elevation in space and time. However, several challenges and bottlenecks remain. Technically, the shallow penetration of NIR light in tissue (typically only a few millimeters to ∼1 cm) limits the treatment of deep lesions. Furthermore, current platforms lack real-time thermal feedback mechanisms, making it difficult to precisely control the local temperature during therapy; the long-term safety of repeated NIR irradiations also remains to be fully evaluated. A delicate balance must be struck between photothermal efficacy and biocompatibility-high laser powers or photothermal agent doses can produce effective heating but may injure surrounding healthy tissue. Translationally, there are substantial hurdles in scaling up the production of photothermal hydrogel systems while maintaining reproducibility and regulatory compliance, which complicates their path to clinical use. Indeed, despite encouraging preclinical outcomes, most reported mild photothermal hydrogel therapies have yet to progress into clinical trials, highlighting the gap between laboratory research and clinical translation (Sun et al., 2024).
To address these issues, future improvements could include: 1) Developing new NIR-II responsive materials: enhancing tissue penetration by using materials activated by the second near-infrared window. 2) Introducing self-regulating thermal elements or degradable thermal buffering layers: Achieving a smoother and more stable heat output. 3) Exploring combinations with non-optical stimulation methods: Incorporating stimuli such as ultrasound or electrical stimulation, which can penetrate deeper into tissues, to complement photothermal therapy and overcome the limitations of light penetration (Xie et al., 2022; Zhu et al., 2024).
In summary, as a biomedical material with immense potential, mild photothermal hydrogels are poised to play an increasingly important role in future tissue repair and regeneration. Through interdisciplinary collaboration and continuous innovation, we can anticipate that mild photothermal hydrogels will offer patients more effective, safer, and more personalized treatment options.
Author contributions
PN: Data curation, Software, Writing – original draft, Writing – review and editing. J-LJ: Formal Analysis, Methodology, Software, Writing – review and editing. R-PL: Formal Analysis, Investigation, Project administration, Resources, Writing – original draft. FY: Investigation, Methodology, Validation, Writing – review and editing. S-FL: Data curation, Project administration, Supervision, Writing – review and editing. X-ZC: Funding acquisition, Project administration, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Sichuan Medical Science and Technology Innovation Research Association (Grant No. YCH-KY-YCZD2024-169).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bai, Y., Li, X., Xie, Y., Wang, Y., Dong, X., and Qi, H. (2023). Ultrasound treatment enhanced the functional properties of phycocyanin with phlorotannin from Ascophyllum nodosum. Front. Nutr. 10, 1181262. doi:10.3389/fnut.2023.1181262
Bao, B., Li, L., Li, M., Long, Y., Zhu, Y., Yin, J., et al. (2025). Biomimetic composite nanoparticles with immune modulation and CRISPR gene editing for enhancing mild photothermal therapy-based synergistic antitumor therapy. Biomacromolecules 26, 5245–5257. doi:10.1021/acs.biomac.5c00735
Byun, H., Han, Y., Kim, E., Jun, I., Lee, J., Jeong, H., et al. (2024). Cell-homing and immunomodulatory composite hydrogels for effective wound healing with neovascularization. Bioact. Mat. 36, 185–202. doi:10.1016/j.bioactmat.2024.02.029
Cai, Y., Chai, T., Nguyen, W., Liu, J., Xiao, E., Ran, X., et al. (2025). Phototherapy in cancer treatment: strategies and challenges. Signal Transduct. Target. Ther. 10, 115. doi:10.1038/s41392-025-02140-y
Chen, Z., Li, Y., Xiang, Q., Wu, Y., Ran, H., and Cao, Y. (2024). Metallic copper-based dual-enzyme biomimetic nanoplatform for mild photothermal enhancement of anticancer catalytic activity. Biomater. Res. 28, 0034. doi:10.34133/bmr.0034
Chen, S., Hassan, N., Kopp, A., Eufrásio-da-Silva, T., Arfaoui, J., Isella, B., et al. (2025). Theragenerative injectable bone-adhesive hydrogels for combined photothermal osteosarcoma therapy and bone repair. Biomater. Sci. 13, 3544–3560. doi:10.1039/D5BM00559K
Cheng, J., Zhu, Y., Dai, Y., Li, L., Zhang, M., Jin, D., et al. (2023). Gas-mediated tumor energy remodeling for sensitizing mild photothermal therapy. Angew. Chem. Int. Ed. 62, e202304312. doi:10.1002/anie.202304312
Cho, Y. H., Won, T. K., and Ahn, D. J. (2024). Energy transfer-based recognition of membrane cholesterol by controlling intradistance oflinker. Sensors-Basel 24, 2315. doi:10.3390/s24072315
Cui, X., Ruan, Q., Zhuo, X., Xia, X., Hu, J., Fu, R., et al. (2023). Photothermal nanomaterials: a powerful light-to-heat converter. Chem. Rev. 123, 6891–6952. doi:10.1021/acs.chemrev.3c00159
Degirmenci, A., Sanyal, R., and Sanyal, A. (2024). Metal-free click-chemistry: a powerful tool for fabricating hydrogels for biomedical applications. Bioconjugate Chem. 35, 433–452. doi:10.1021/acs.bioconjchem.4c00003
Deng, X., Xie, S., Wang, W., Luo, C., and Luo, F. (2022). From carbon nanotubes to ultra-sensitive, extremely-stretchable and self-healable hydrogels. Eur. Polym. J. 178, 111485. doi:10.1016/j.eurpolymj.2022.111485
Ding, X., Yu, Y., Li, W., Bian, F., Gu, H., and Zhao, Y. (2024). Multifunctional carbon nanotube hydrogels with on-demand removability for wearable electronics. Nano Today 54, 102124. doi:10.1016/j.nantod.2023.102124
Duman, H., Akdaşçi, E., Eker, F., Bechelany, M., and Karav, S. (2024). Gold nanoparticles: multifunctional properties, synthesis, and future prospects. Nanomaterials-Basel 14, 1805. doi:10.3390/nano14221805
Enayati, M., Liu, W., Madry, H., Neisiany, R. E., and Cucchiarini, M. (2024). Functionalized hydrogels as smart gene delivery systems to treat musculoskeletal disorders. Adv. Colloid Interface Sci. 331, 103232. doi:10.1016/j.cis.2024.103232
Fialho, M. C. P., de Oliveira, M. A., Machado, M. G. C., Lacerda, C. M., and Mosqueira, V. C. F. (2025). IR780-based nanotheranostics and in vivo effects: a review. J. Nanotheranostics 6, 8. doi:10.3390/jnt6010008
Forero-Doria, O., Polo, E., Marican, A., Guzmán, L., Venegas, B., Vijayakumar, S., et al. (2020). Supramolecular hydrogels based on cellulose for sustained release of therapeutic substances with antimicrobial and wound healing properties. Carbohydr. Polym. 242, 116383. doi:10.1016/j.carbpol.2020.116383
Gao, Q., Hu, F., Chai, Z., Zheng, C., Zhang, W., Pu, K., et al. (2025). Multifunctional hydrogel with mild photothermal properties enhances diabetic wound repair by targeting MRSA energy metabolism. J. Nanobiotechnol 23, 380. doi:10.1186/s12951-025-03451-6
Guo, S., Gu, D., Yang, Y., Tian, J., and Chen, X. (2023a). Near-infrared photodynamic and photothermal co-therapy based on organic small molecular dyes. J. Nanobiotechnol. 21, 348. doi:10.1186/s12951-023-02111-x
Guo, W., Hao, J., Wang, M., Huang, T., Miao, C., Yin, L., et al. (2023b). Polypyrrole hydrogel with near-infrared light-driven nitric oxide release and photothermal activities for rapid synergistic sterilization and infected wound therapy. Colloid Surf. A 677, 132411. doi:10.1016/j.colsurfa.2023.132411
Guo, Q., Yin, T., Huang, W., Nan, R., Xiang, T., and Zhou, S. (2024). Hybrid hydrogels for immunoregulation and proangiogenesis through mild heat stimulation to accelerate whole-process diabetic wound healing. Adv. Healthc. Mat. 13, 2304536. doi:10.1002/adhm.202304536
Hao, M., Wang, Y., Wang, Y., Su, S., Zhou, Y., and Wei, S. (2025). A carrier-free hydrogel loading silver nanoparticles with photothermal effect to heal refractory diabetic wound by robust bacteria elimination and repair-promotion. Chem. Eng. J. 519, 165250. doi:10.1016/j.cej.2025.165250
He, C., Bi, S., Zhang, R., Chen, C., Liu, R., Zhao, X., et al. (2024). A hyaluronic acid hydrogel as a mild photothermal antibacterial, antioxidant, and nitric oxide release platform for diabetic wound healing. J. Control. Release 370, 543–555. doi:10.1016/j.jconrel.2024.05.011
Hu, Z., Zhang, H., Li, Z., Zhao, T., Gu, Z., Yuan, Q., et al. (2024). Multifunctional photothermal hydrogels: design principles, various functions, and promising biological applications. Chin. Chem. Lett. 35, 109527. doi:10.1016/j.cclet.2024.109527
Huang, K., Liu, W., Wei, W., Zhao, Y., Zhuang, P., Wang, X., et al. (2022a). Photothermal hydrogel encapsulating intelligently bacteria-capturing bio-MOF for infectious wound healing. ACS Nano 16, 19491–19508. doi:10.1021/acsnano.2c09593
Huang, Q., Lyu, M., Tang, W., Qi, P., and Hu, H. (2022b). Hydrogel co-loading SO2 prodrug and FeGA nanoparticles for enhancing chemodynamic therapy by photothermal-triggered SO2 gas therapy. Front. Bioeng. Biotechnol. 10, 1024089. doi:10.3389/fbioe.2022.1024089
Huang, B., Guan, W., Wang, C., Wu, S., Cui, Z., Zheng, Y., et al. (2023). S–Cu–FC/CuS modified GO carboxymethyl cellulose hydrogel for enhanced photocatalytic sterilization through homo-heterojunction interface accelerated charge transfer. Biomater. Sci. 11, 3589–3602. doi:10.1039/D3BM00260H
Jiang, Q., Li, J., Du, Z., Li, M., Chen, L., Zhang, X., et al. (2024). High-performance NIR-II fluorescent type I/II photosensitizer enabling augmented mild photothermal therapy of tumors by disrupting heat shock proteins. Adv. Healthc. Mat. 13, 2400962. doi:10.1002/adhm.202400962
Jin, S., Luo, Z., Cai, Y., Wen, J., Lu, P., Fu, X., et al. (2024). Exosome-functionalized heterogeneous nanofibrous scaffolds repair bone defects accompanied by muscle injury. Chem. Eng. J. 485, 149681. doi:10.1016/j.cej.2024.149681
Kong, Y., Dai, Y., Qi, D., Du, W., Ni, H., Zhang, F., et al. (2021). Injectable and thermosensitive liposomal hydrogels for NIR-II light-triggered photothermal-chemo therapy of pancreatic cancer. ACS Appl. Bio Mater 4, 7595–7604. doi:10.1021/acsabm.1c00864
Li, Y., Fu, R., Duan, Z., Zhu, C., and Fan, D. (2022). Artificial nonenzymatic antioxidant MXene nanosheet-anchored injectable hydrogel as a mild photothermal-controlled oxygen release platform for diabetic wound healing. ACS Nano 16, 7486–7502. doi:10.1021/acsnano.1c10575
Li, W., Wu, S., Ren, L., Feng, B., Chen, Z., Li, Z., et al. (2023a). Development of an antiswelling hydrogel system incorporating M2-exosomes and photothermal effect for diabetic wound healing. ACS Nano 17, 22106–22120. doi:10.1021/acsnano.3c09220
Li, X., Huang, X., Li, L., Wu, J., Yi, W., Lai, Y., et al. (2023b). LL-37-coupled porous composite scaffold for the treatment of infected segmental bone defect. Pharmaceutics 15, 88. doi:10.3390/pharmaceutics15010088
Lin, X., Fang, Y., Hao, Z., Wu, H., Zhao, M., Wang, S., et al. (2021). Bacteria-triggered multifunctional hydrogel for localized chemodynamic and low-temperature photothermal sterilization. SMALL 17, 2103303. doi:10.1002/smll.202103303
Liu, S., Liu, Z., Wu, M., Xu, X., Huang, F., Zhang, L., et al. (2021). NIR as a “trigger switch” for rapid phase change, on-demand release, and photothermal synergistic antibacterial treatment with chitosan-based temperature-sensitive hydrogel. Int. J. Biol. Macromol. 191, 344–358. doi:10.1016/j.ijbiomac.2021.09.093
Liu, G., Li, B., Li, J., Dong, J., Baulin, V. E., Feng, Y., et al. (2023). Photothermal carbon dots chelated hydroxyapatite filler: high photothermal conversion efficiency and enhancing adhesion of hydrogel. ACS Appl. Mat. Interfaces 15, 55335–55345. doi:10.1021/acsami.3c11957
Liu, X., Huang, B., Li, J., Li, B., and Lou, Z. (2024). Full-spectrum plasmonic semiconductors for photocatalysis. Mat. Horiz. 11, 5470–5498. doi:10.1039/D4MH00515E
Lu, T., Chen, Y., Sun, M., Chen, Y., Tu, W., Zhou, Y., et al. (2025). Multifunctional carbon-based nanocomposite hydrogels for wound healing and health management. Gels 11, 345. doi:10.3390/gels11050345
Luo, G., Xu, Z., Zhong, H., Shao, H., Liao, H., Liu, N., et al. (2023). Biodegradable photothermal thermosensitive hydrogels treat osteosarcoma by reprogramming macrophages. Biomater. Sci. 11, 2818–2827. doi:10.1039/D2BM01900K
Ma, S., Zhang, C., Ren, X., Song, L., Shan, J., Liu, Y., et al. (2025). Photothermally responsive hydrogel releases basic fibroblast growth factor to promote the healing of infected wounds. Biomater. Res. 29, 0156. doi:10.34133/bmr.0156
Makabenta, J. M. V., Nabawy, A., Li, C.-H., Schmidt-Malan, S., Patel, R., and Rotello, V. M. (2021). Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 19, 23–36. doi:10.1038/s41579-020-0420-1
Mohammadzadeh, V., Atapour-Mashhad, H., Shahvali, S., Salehi, B., Shaban, M., Shirzad, M., et al. (2025). Hydrogels as advanced drug delivery platforms for cancer immunotherapy: promising innovations and future outlook. J. Nanobiotechnol. 23, 545. doi:10.1186/s12951-025-03613-6
Pang, Q., Wu, K., Jiang, Z., Shi, Z., Si, Z., Wang, Q., et al. (2022). A polyaniline nanoparticles crosslinked hydrogel with excellent photothermal antibacterial and mechanical properties for wound dressing. Macromol. Biosci. 22, 2100386. doi:10.1002/mabi.202100386
Ravanbakhsh, H., Bao, G., and Mongeau, L. (2020). Carbon nanotubes promote cell migration in hydrogels. Sci. Rep. 10, 2543. doi:10.1038/s41598-020-59463-9
Sharma, A., Panwar, V., Salaria, N., and Ghosh, D. (2022). Protease-responsive hydrogel, cross-linked with bioactive curcumin-derived carbon dots, encourage faster wound closure. Mat. Sci. Eng. C-Mater 139, 212978. doi:10.1016/j.bioadv.2022.212978
Sun, S., Jiang, G., Dong, J., Xie, X., Liao, J., and Tian, Y. (2024). Photothermal hydrogels for infection control and tissue regeneration. Front. Bioeng. Biotechnol. 12, 1389327. doi:10.3389/fbioe.2024.1389327
Sun, Y., Yao, X., Zhang, Y., Zhang, W., Zhu, C., Shen, C., et al. (2025). Zinc oxide-copper sulfide nanozyme hydrogels for bone defect repair by modulating the bone immune microenvironment and promoting osteogenesis/Angiogenesis. ACS Appl. Mat. Interfaces 17, 29100–29118. doi:10.1021/acsami.4c23069
Teng, C., Xu, Y., Wang, Y., Chen, D., Yin, D., and Yan, L. (2024). J-aggregates of multi-groups cyanine dye for NIR-IIa fluorescence-guided mild photothermal therapy under 1064 nm irradiation. J. Colloid Interface Sci. 670, 751–761. doi:10.1016/j.jcis.2024.05.149
Wang, W., Zhang, G., Wang, Y., Ran, J., Chen, L., Wei, Z., et al. (2023). An injectable and thermosensitive hydrogel with nano-aided NIR-II phototherapeutic and chemical effects for periodontal antibacteria and bone regeneration. J. Nanobiotechnol. 21, 367. doi:10.1186/s12951-023-02124-6
Wang, K., Jia, Y., Liu, X., Pan, L., Shi, M., Pan, W., et al. (2025a). Fenton reaction-enhanced mild photothermal therapy for cancer suppression with a multifunctional platform. J. Photoch Photobio B 272, 113250. doi:10.1016/j.jphotobiol.2025.113250
Wang, P., Wang, L., Liu, Y., Mu, Y., Deng, Y., Jia, A., et al. (2025b). Core–satellite nanocomposites synchronize mild photothermal disruption and ROS scavenging for periodontal microenvironment rebalancing. ACS Appl. Mat. Interfaces 17, 47969–47984. doi:10.1021/acsami.5c09922
Wei, X., Wan, C., Peng, X., Luo, Y., Hu, M., Cheng, C., et al. (2024). Copper-based carbon dots modified hydrogel with osteoimmunomodulatory and osteogenesis for bone regeneration. J. Mat. Chem. B 12, 5734–5748. doi:10.1039/D4TB00526K
Wu, Y., Xiao, D., Liu, P., Liao, Q., Ruan, Q., Huang, C., et al. (2023). Nanostructured conductive polypyrrole for antibacterial components in flexible wearable devices. Res 6, 0074. doi:10.34133/research.0074
Wu, A. L., Wu, A. F., Chen, C.-Y., Moreno, R. L., Wu, J.-L., and Wong, P.-C. (2025a). Laser-induced photothermal hydrogels promote the proliferation of MC3T3-E1 preosteoblasts for enhanced bone healing. J. Funct. Biomater. 16, 63. doi:10.3390/jfb16020063
Wu, Y., Xie, X., Luo, G., Xie, J., Ye, X., Gu, W., et al. (2025b). Photothermal sensitive nanocomposite hydrogel for infectious bone defects. Bone Res. 13, 22. doi:10.1038/s41413-024-00377-x
Xia, J., Qing, X., Shen, J., Ding, M., Wang, Y., Yu, N., et al. (2021). Enzyme-loaded pH-sensitive photothermal hydrogels for mild-temperature-mediated combinational cancer therapy. Front. Chem. 9, 736468. doi:10.3389/fchem.2021.736468
Xiao, T., Dai, H., Wu, Y., Liu, Z., Yeow, J., Xing, X., et al. (2025). Tailoring physicochemical properties of photothermal hydrogels toward intrinsically regenerative therapies. Adv. Funct. Mat. 35, 2414419. doi:10.1002/adfm.202414419
Xie, G., Zhou, N., Du, S., Gao, Y., Suo, H., Yang, J., et al. (2022). Transparent photothermal hydrogels for wound visualization and accelerated healing. Fundam. Res. 2, 268–275. doi:10.1016/j.fmre.2021.10.001
Yao, J., He, Q., Zheng, X., Shen, S., Hui, J., and Fan, D. (2024). An injectable hydrogel system with mild photothermal effects combined with ion release for osteosarcoma-related bone defect repair. Adv. Funct. Mat. 34, 2315217. doi:10.1002/adfm.202315217
Yu, Y., Yang, M., Zhao, H., Zhang, C., Liu, K., Liu, J., et al. (2024). Natural blackcurrant extract contained gelatin hydrogel with photothermal and antioxidant properties for infected burn wound healing. Mat. Today Bio 26, 101113. doi:10.1016/j.mtbio.2024.101113
Zhang, X., Tan, B., Wu, Y., Zhang, M., and Liao, J. (2021). A review on hydrogels with photothermal effect in wound healing and bone tissue engineering. Polymers-Basel 13, 2100. doi:10.3390/polym13132100
Zhang, X., Tan, B., Wu, Y., Zhang, M., Xie, X., and Liao, J. (2022). An injectable, self-healing carboxymethylated chitosan hydrogel with mild photothermal stimulation for wound healing. Carbohydr. Polym. 293, 119722. doi:10.1016/j.carbpol.2022.119722
Zhang, R., Feng, J., Chen, H., Zhang, G., Liang, X., Xu, C., et al. (2025a). Hybrid hydrogel with photothermal stimulation elicits immunomodulation-mediated wound healing. Adv. Funct. Mat. 35, 2419170. doi:10.1002/adfm.202419170
Zhang, W., Li, L., Wang, Z., Nie, Y., Yang, Y., Li, C., et al. (2025b). Injectable and adhesive MgO2-potentiated hydrogel with sequential tumor synergistic therapy and osteogenesis for challenging postsurgical osteosarcoma treatment. Biomaterials 315, 122959. doi:10.1016/j.biomaterials.2024.122959
Zhao, P., Zhang, Y., Chen, X., Xu, C., Guo, J., Deng, M., et al. (2023). Versatile hydrogel dressing with skin adaptiveness and mild photothermal antibacterial activity for methicillin-resistant staphylococcus aureus-infected dynamic wound healing. Adv. Sci. 10, 2206585. doi:10.1002/advs.202206585
Zhou, L., Shi, W., Zhang, X., Liu, M., Zhang, L., Jiang, X., et al. (2024). Injectable tannin-containing hydroxypropyl chitin hydrogel as novel bioactive pulp capping material accelerates repair of inflamed dental pulp. Biomolecules 14, 1129. doi:10.3390/biom14091129
Zhou, R., Chen, Y., Yao, S., Zhang, W., and Ye, D. (2025). Advances in second near-infrared window photothermal agents and photothermal therapy for tumors in interdisciplinary medical research. Pharmaceutics 17, 1178. doi:10.3390/pharmaceutics17091178
Zhu, S., Zhao, B., Li, M., Wang, H., Zhu, J., Li, Q., et al. (2023). Microenvironment responsive nanocomposite hydrogel with NIR photothermal therapy, vascularization and anti-inflammation for diabetic infected wound healing. Bioact. Mat. 26, 306–320. doi:10.1016/j.bioactmat.2023.03.005
Zhu, Y., Liu, H., Wu, P., Chen, Y., Deng, Z., Cai, L., et al. (2024). Multifunctional injectable hydrogel system as a mild photothermal-assisted therapeutic platform for programmed regulation of inflammation and osteo-microenvironment for enhanced healing of diabetic bone defects in situ. Theranostics 14, 7140–7198. doi:10.7150/thno.102779
Keywords: hydrogels, mild photothermal effect, tissue repair, bone regeneration, multifunctional materials
Citation: Na P, Jiang J-L, Lv R-P, Yang F, Li S-F and Chen X-Z (2025) Advances in mild photothermal hydrogel-based therapies for bone and soft tissue injuries. Front. Cell Dev. Biol. 13:1696209. doi: 10.3389/fcell.2025.1696209
Received: 31 August 2025; Accepted: 19 September 2025;
Published: 26 September 2025.
Edited by:
Hui Zhang, West China Hospital, Sichuan University, ChinaReviewed by:
Zhiguo Bi, First Affiliated Hospital of Jilin University, ChinaCopyright © 2025 Na, Jiang, Lv, Yang, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xian-Zhuo Chen, Y2hlbi54LnpAaG90bWFpbC5jb20=
 Peng Na1,2
Peng Na1,2 Xian-Zhuo Chen
Xian-Zhuo Chen