- Department of Ophthalmology, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, National Clinical Research Center for Eye Diseases, Shanghai Key Laboratory of Ocular Fundus Diseases, Shanghai Engineering Center for Visual Science and Photomedicine, Shanghai engineering center for precise diagnosis and treatment of eye diseases, Shanghai, China
Background: To examine the association between estimated pulse wave velocity (ePWV), a marker of arterial stiffness, and glaucoma incidence in Chinese cohort, highlighting ePWV’s potential as a scalable population-level risk factor for glaucoma screening.
Materials and methods: Data were obtained from the China Health and Retirement Longitudinal Study (CHARLS). A total of 11,968 adults aged ≥45 years without glaucoma at baseline (2011–2018) were followed for up to 7 years ePWV was calculated from age and blood pressure and divided into quartiles. Cox proportional hazards models assessed risk, while restricted cubic spline and two-piecewise models explored dose–response patterns. Subgroup analyses tested effect modification by demographic and lifestyle factors.
Results: During a 7-year follow-up, participants in the highest ePWV quartile (≥10.58 m/s) had a higher risk of glaucoma compared with the lowest quartile (<8.01 m/s) (HR [hazard ratio] 1.39, 95% CI [confidence interval] 1.00–1.93). Each 1 m/s increase in ePWV was associated with a 7% higher glaucoma risk (HR 1.07, 95% CI 1.01–1.13). The dose response relationship was linear without evidence of a threshold. Associations were consistent across most subgroups. Sensitivity analyses showed that ePWV was a stronger predictor of glaucoma than age or blood pressure alone.
Conclusion: Higher ePWV independently links to greater glaucoma risk in middle-aged and older Chinese. This observed association indicates that ePWV provides incremental predictive value beyond traditional demographic or clinical factors. Building on this characteristic, incorporating ePWV into future artificial intelligence (AI)-enabled glaucoma screening models may potentially contribute to improving risk-stratification accuracy and facilitating early identification of high-risk individuals.
Introduction
Glaucoma is one of the world’s leading causes of irreversible blindness. In 2020 an estimated 80 million people were living with glaucoma, making it the second leading cause of blindness worldwide (Tham et al., 2014; Jayaram et al., 2023).Projections from a recent meta-analysis estimate that the number of people aged 40–80 years with glaucoma will rise from 76.0 million in 2020 to 111.8 million by 2040, with Asia and Africa bearing the largest share of cases (Tham et al., 2014; Song et al., 2017). In China, rapid population ageing and urbanization are likely to amplify this trend, posing a major public health challenge (Tang et al., 2019). Glaucoma is asymptomatic in its early stage and silently impairs visual function, typically being identified only when it progresses to an advanced stage. Given that glaucomatous damage is irreversible, early screening and detection of glaucoma are of critical importance. Against the backdrop of China’s substantial population base4, the number of glaucoma specialists is vastly inadequate to support large-scale population-wide screening. Thus, the development of artificial intelligence (AI)-enabled systems for glaucoma screening holds vital significance for both clinical practice and public health.
Recent advances in AI have greatly enhanced glaucoma screening, particularly via fundus photography and optical coherence tomography (OCT) scans, enabling automated detection of structural optic nerve changes with high sensitivity and specificity (Dong et al., 2022; Ran et al., 2022; Chen and Bai, 2025; Djulbegovic et al., 2025).However, AI methods based solely on ocular images face limitations: they may underperform in cases with ambiguous disc appearance, myopia, or early vascular pathology.AI models trained on multimodal data, including fundus photographs, OCT scans, and physiological parameters, have demonstrated performance comparable to, or even superior to, human ophthalmologists in detecting early glaucomatous damage (Dong et al., 2022; Wang et al., 2024; Djulbegovic et al., 2025; Koornwinder et al., 2025), and providing appropriate physiological parameters within such AI systems is the foundation for these systems to exert their maximum effectiveness. Therefore, our research focus is precisely on identifying quantifiable, objective, and most importantly glaucoma related physiological parameters that can be effectively integrated into the development and optimization of AI models to enhance their performance in early glaucoma detection and risk stratification (Chen and Bai, 2025; Djulbegovic et al., 2025).The combination of imaging and easily obtained systemic markers presents a promising path toward more robust and scalable AI-based glaucoma screening, particularly in settings where access to ophthalmic specialists or advanced imaging is limited (Chen and Bai, 2025).
In glaucoma pathogenesis, increasing interest lies in the role of vascular dysfunction (Shoshani et al., 2012; Shin et al., 2022), according to the vascular theory of glaucoma that fluctuations in ocular blood flow and impaired autoregulation may damage the optic nerve head (Shoshani et al., 2012). Emerging evidence supports an association between increased arterial stiffness, a prominent indicator of vascular dysfunction, and glaucoma (Lee et al., 2021; Beros et al., 2024). Arterial stiffness is commonly measured by pulse wave velocity (PWV), while in large-scale studies, an estimated PWV (ePWV) derived from age and blood pressure has been introduced as a convenient surrogate for direct PWV measurement, and it has shown strong predictive value for cardiovascular events and mortality (Ohkuma et al., 2017; Lee et al., 2021; Cheng et al., 2024). In a large prospective study conducted in Western populations, higher estimated pulse wave velocity (ePWV) was associated with a significantly increased risk of incident glaucoma (Beros et al., 2024; Yang et al., 2024). These findings suggest that arterial stiffness could serve as a novel marker of glaucoma risk and a potential target for preventive strategies.
Despite this emerging evidence, the relationship between arterial stiffness and glaucoma remains underexplored in Asian populations. We therefore used longitudinal data from the China Health and Retirement Longitudinal Study (CHARLS) (Li et al., 2025), a nationally representative cohort of adults aged 45 years and older, to examine the association between ePWV and incident glaucoma-a parameter whose quantifiable, accessible nature also makes it a promising input for AI-driven glaucoma screening models. Leveraging the large sample size and comprehensive follow-up of CHARLS, we aimed to determine whether higher ePWV predicts future glaucoma beyond established demographic, lifestyle and clinical risk factors, with findings that could further validate ePWV’s utility for integrating into AI-based risk stratification tools. The results of this study may provide new insights into the vascular contribution to glaucoma and inform risk stratification and prevention strategies in ageing populations, including the development of AI screening systems tailored to Asian populations, where such tools are urgently needed to address specialist shortages.
Materials and methods
Study design
This was a prospective, population-based cohort study using data from the China Health and Retirement Longitudinal Study (CHARLS), a nationally representative survey of adults aged ≥45 years, conducted from 2011 to 2018. Participants without glaucoma at baseline were followed for up to 7 years to assess the association between estimated pulse wave velocity (ePWV) and incident glaucoma.Patients and the public were not involved in the design, conduct, reporting or dissemination plans of our research.
Participant selection process and study setting
The CHARLS study employed a stratified, multistage probability sampling design covering 150 counties/districts and 450 villages/urban communities across 28 provinces, thereby ensuring broad demographic and geographic representation. Data were collected using structured household questionnaires, clinical examinations, and blood-based biomarkers. Participants with BMI ≥40 kg/m2 were excluded due to the rarity of such cases in our cohort (n = 49, <0.2%) and the increased likelihood of measurement error, which could bias covariate adjustment.
A total of 25,586 participants were recruited. Exclusion criteria included: (1) only one glaucoma survey available (n = 3,398), (2) missing age or blood pressure information (n = 9,495), (3) age <45 years (n = 425), (4) missing key covariates (n = 194) including sex, education, marital status, location, or BMI ≥40, and (5) self-reported glaucoma at baseline (n = 106). After exclusions, 11,968 participants with complete data were included for analysis of the association between ePWV and incident glaucoma.
Ethical statement
The CHARLS protocol was approved by the Biomedical Ethics Review Committee of Peking University (IRB00001052-11015) and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants. This study adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Assessment of ePWV and covariates
ePWV, a validated non-invasive marker of arterial stiffness, was calculated using the formula developed by Greve et al. (2016), based on age and blood pressure parameters (Greve et al., 2016; Liu et al., 2025). Blood pressure was measured by trained staff using an Omron HEM-7200 device, with participants seated, left arm supported at heart level, and three consecutive readings averaged for accuracy. ePWV was calculated from mean blood pressure (MBP) and age using the following formula: ePWV = 9.587–0.402×age+4.560 × 10−3×age2–2.621 × 10−5×age2×MBP +3.176 × 10−3×age × MBP –1.832 × 10−2×MBP. MBP was derived from systolic (SBP) and diastolic (DBP) blood pressure as: MBP = DBP + 0.4 × (SBP–DBP) (Greve et al., 2016). In addition, ePWV was divided into quartiles to form a ‘quartile categorical variable’ for analysing the risk of developing glaucoma after follow-up (Shi et al., 2024; Ke et al., 2025a; Liu et al., 2025).
Covariates included demographic factors (sex [male/female], marital status [married vs non-married], education [primary school or below, high school, college or above], and location [village vs city/town]); lifestyle factors (smoking status [never/current/former], drinking frequency [never/less than once per month/more than once per month], and sleep duration [hours/day]); and clinical factors (BMI, prior stroke, heart disease, glycemic control, lipid-lowering therapy, and antihypertensive therapy).Glycemic control was defined as the use of hypoglycemic medications or insulin injections, while antihypertensive and lipid-lowering therapies were defined by self-reported medication use.Covariates for the multivariable models were selected a priori on the basis of established clinical relevance and prior epidemiological evidence, following the approach described from previous studies (Bozic et al., 2025; Ke et al., 2025a; Ke et al., 2025b).
Assessment of glaucoma
Glaucoma was assessed through self-reported responses to the CHARLS questionnaire. Participants were classified as having glaucoma if they answered “yes” to the question: “Has a doctor, nurse, or paramedic ever treated you for glaucoma?” Incident glaucoma was determined based on new positive responses during follow-up among participants who were free of glaucoma at baseline (Li et al., 2025).
Follow-up
The follow-up period was defined as the time elapsing from baseline to either the first self-reported glaucoma diagnosis or the last available follow-up assessment, whichever occurred first. Participants who remained free of glaucoma were censored at the time of their last follow-up interview. Data collection spanned from 2011 (baseline) to 2018 (final follow-up), with a mean follow-up duration of 6.4 ± 1.3 years.
Statistical analysis
Baseline characteristics across ePWV quartiles were compared using chi-square tests for categorical variables and the Kruskal–Wallis test for continuous variables. Complete case analysis was performed after applying exclusion criteria to remove missing values.
Survival analyses were used to investigate the association between ePWV and glaucoma incidence. Kaplan–Meier survival curves were constructed, and differences across quartiles were tested with the log-rank test. Cox proportional hazards regression models estimated hazard ratios (HRs) and 95% confidence intervals (CIs). Three models were developed: Model 1 (unadjusted); Model 2 (adjusted for sex, marital status, education, location, smoking status, drinking frequency, and sleep duration); and Model 3 (further adjusted for BMI, prior stroke, heart disease, glycemic control, dyslipidemia treatment, and antihypertensive treatment). The proportional hazards assumption was assessed using Schoenfeld residuals, with no significant violations observed.
Restricted cubic spline (RCS) and two-piecewise linear regression models were applied to examine potential nonlinear or threshold relationships between ePWV and glaucoma risk. Subgroup analyses were performed according to demographic and lifestyle factors, with interaction terms included to test for effect modification. Sensitivity analyses were conducted to compare the predictive strength of ePWV against its individual components (age, systolic blood pressure, and diastolic blood pressure).
All analyses were conducted using R software (version 4.3.3). The “survival” package was used for survival analyses, the “rms” package for RCS models, the “segmented” package for threshold analysis, and the “mice” package for multiple imputation when appropriate. A two-sided p-value <0.05 was considered statistically significant. The q-values represent p-values adjusted for multiple comparisons using the false discovery rate (FDR) method.
Results
Patient enrollment and follow-ups
The systematic selection process of study participants from the CHARLS cohort were recruited between 2011 and 2018 (Figure 1). Starting with 25,586 recruited participants, sequential exclusions were applied based on predefined criteria: participants with only one glaucoma survey (n = 3,398), those lacking age and blood pressure information (n = 9,495), participants younger than 45 years (n = 425), those missing key covariates including sex, education, marriage status, location, and participants with BMI≥40 (n = 194), and participants who had glaucoma at baseline (n = 106). The final analytical sample comprised 11,968 participants who met all inclusion criteria and had complete data for the analysis of the association between estimated pulse wave velocity and glaucoma incidence.
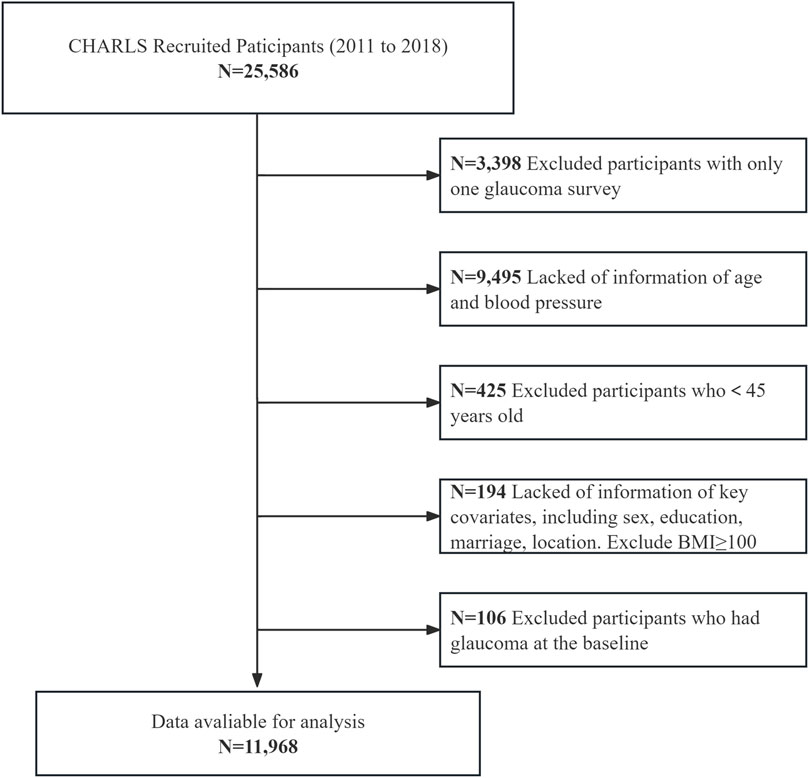
Figure 1. Participant selection flowchart of participant recruitment and exclusion. This resulted in a final analytic cohort of 11,968 participants from CHARLS (2011–2018).
Baseline characteristics of study participants
During 7 years of follow-up, a total of 11,968 participants were stratified into quartiles of estimated pulse wave velocity (ePWV): Q1 (<8.01 m/s), Q2 (8.01–9.14 m/s), Q3 (9.14–10.58 m/s), and Q4 (≥10.58 m/s; n = 2,992 each) (Table 1). Baseline characteristics differed significantly across quartiles (all q < 0.05, FDR-adjusted). Participants in higher ePWV quartiles were older (median age 49 vs 69 years from Q1 to Q4) with progressively higher SBP and DBP, shorter sleep duration, and higher obesity prevalence. The prevalence of glaucoma rose from 2.3% in Q1 to 3.8% in Q4, paralleled by higher rates of stroke (1.1%–3.9%) and heart problems (6.9%–15%). Sociodemographic differences included more males, lower education, and more non-married individuals in higher quartiles. Most participants lived in villages (82%), though city/town residence increased modestly with ePWV. Lifestyle and treatment patterns also reflected greater comorbidity at higher ePWV: ex-smoking was more frequent, regular drinking less common, and use of antihypertensive, lipid-lowering, and diabetes medications substantially higher (all p < 0.001).
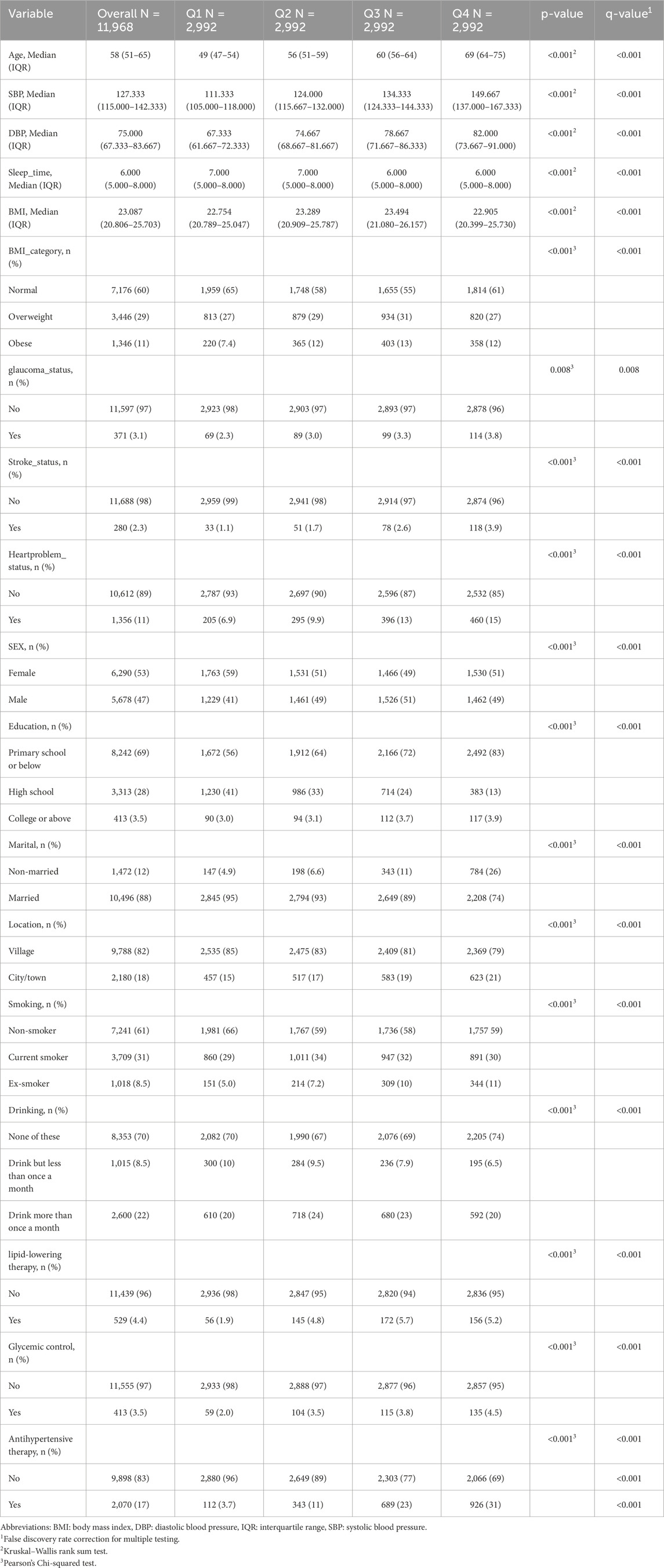
Table 1. Demographics and Baseline characteristics of participants by quartiles of estimated pulse wave velocity (ePWV).
Glaucoma risk across quartiles of estimated pulse wave velocity
As shown in Figure 2, Kaplan–Meier curves revealed a stepwise decline in glaucoma-free survival with higher ePWV quartiles, with Q1 showing the most favorable outcomes (log-rank p < 0.05). Cox regression analyses confirmed these findings (Table 2): each 1 m/s increase in ePWV was associated with a 13% higher glaucoma risk in the unadjusted model (HR 1.13, 95% CI 1.07–1.19), and the association remained significant after adjustment for sociodemographic, lifestyle, and clinical factors (HR 1.07, 95% CI 1.01–1.13). Per interquartile increase, risk rose by 36%, 22%, and 19% across models. Quartile analyses showed a dose–response pattern, with Q4 participants having a 39% higher risk compared with Q1 after full adjustment (HR 1.39, 95% CI 1.00–1.93), though the linear trend was attenuated in the fully adjusted model (p for trend = 0.065).
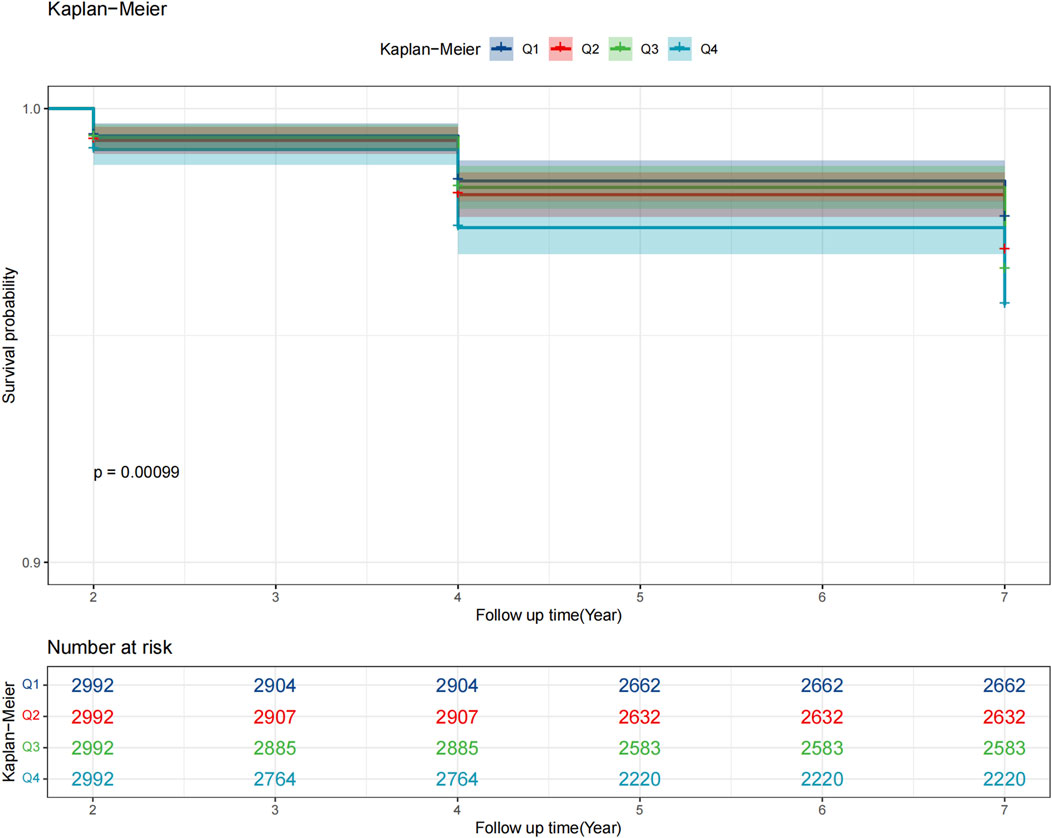
Figure 2. Kaplan–Meier survival curves of glaucoma incidence across quartiles of estimated pulse wave velocity (ePWV). ePWV quartiles (Q1 <8.01 m/s; Q2 8.01–9.14 m/s; Q3 9.14–10.58 m/s; Q4 ≥10.58 m/s).
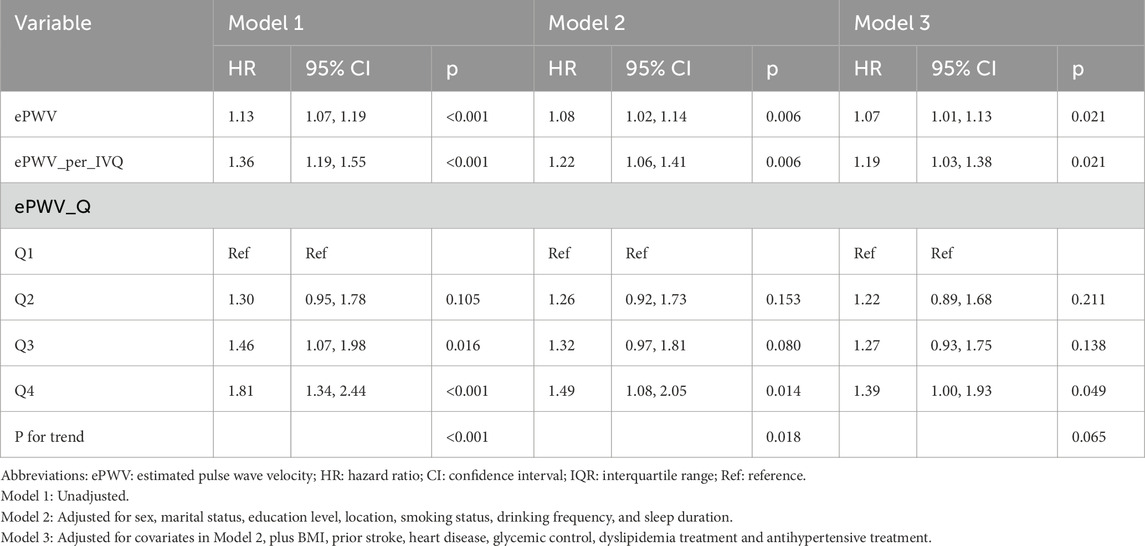
Table 2. Cox proportional hazards models for glaucoma risk associated with estimated pulse wave velocity (ePWV).
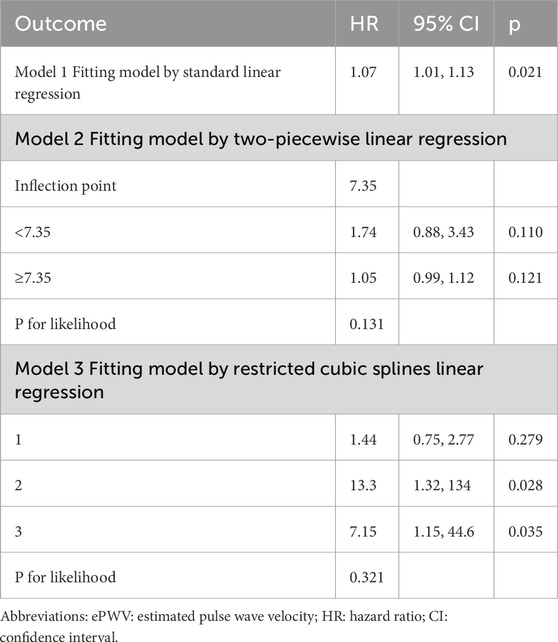
Threshold effect and dose-response relationship
Threshold effect analysis was used to assess the dose–response relationship between ePWV and glaucoma risk (Table 3). In the linear model, each 1 m/s increase in ePWV was associated with a 7% higher glaucoma risk (HR 1.07, 95% CI 1.01–1.13, p = 0.021; Figure 3a). A two-piecewise model identified an inflection point at 7.35 m/s, but risks below and above this point were nonsignificant, and model fit was not improved (p = 0.131; Figure 3b). Restricted cubic spline regression showed localized increases at certain knots but no overall evidence of nonlinearity (p = 0.321; Figures 3c,d). Collectively, these results indicate a predominantly linear relationship between higher ePWV and glaucoma risk, without evidence of a threshold effect.
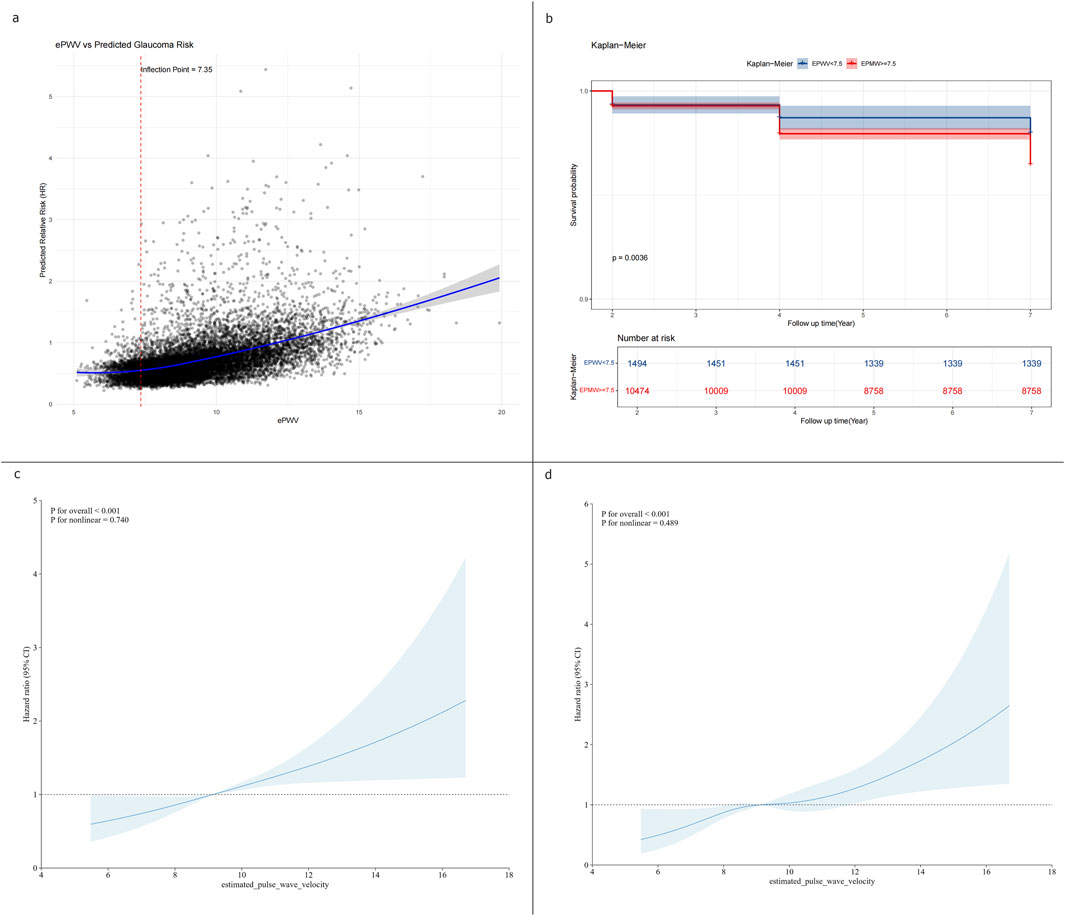
Figure 3. Threshold effect and dose–response relationship between ePWV and glaucoma risk. (a) Linear regression model showing a 7% increase in glaucoma risk per 1 m/s increment in ePWV (HR 1.07, 95% CI 1.01–1.13). (b) Two-piecewise linear regression model identifying an inflection point at 7.35 m/s (c) Restricted cubic spline models showing a steady linear increase in glaucoma risk with higher ePWV. (d) Restricted quartic spline models showing a steady linear increase in glaucoma risk with higher ePWV.
Subgroup analysis
Subgroup analyses showed that the association between ePWV and glaucoma was consistent across sex, marital status, education, residence, smoking, and BMI, with no significant interactions (all P > 0.05; Figure 4). Drinking status was the only significant modifier (P for interaction = 0.047): ePWV was more strongly linked to glaucoma in non-drinkers (HR 1.15, 95% CI 1.09–1.22), whereas no significant association was found in drinkers. Baseline characteristics (Supplementary Table 1) suggested that non-drinkers had higher ePWV, more glaucoma events, and were older, which may partly explain their greater susceptibility. To further clarify whether drinking status independently modified the ePWV-glaucoma association, we performed an age-stratified analysis at the cohort median age of 58 years. In participants aged <58 years, the association between ePWV and glaucoma was not statistically significant (HR 0.95, 95% CI 0.79–1.16,p = 0.628),whereas in those aged ≥58 years the association remained significant (HR 1.07, 95% CI 1.00–1.14,p = 0.049). Importantly, the drinking-status interaction was no longer evident in either age stratum (P for interaction >0.05), indicating that the previously observed stronger association in non-drinkers was not independent of age.These findings indicate that arterial stiffness has a stronger impact on glaucoma among non-drinkers, while the weaker association in drinkers may reflect their younger age profile.
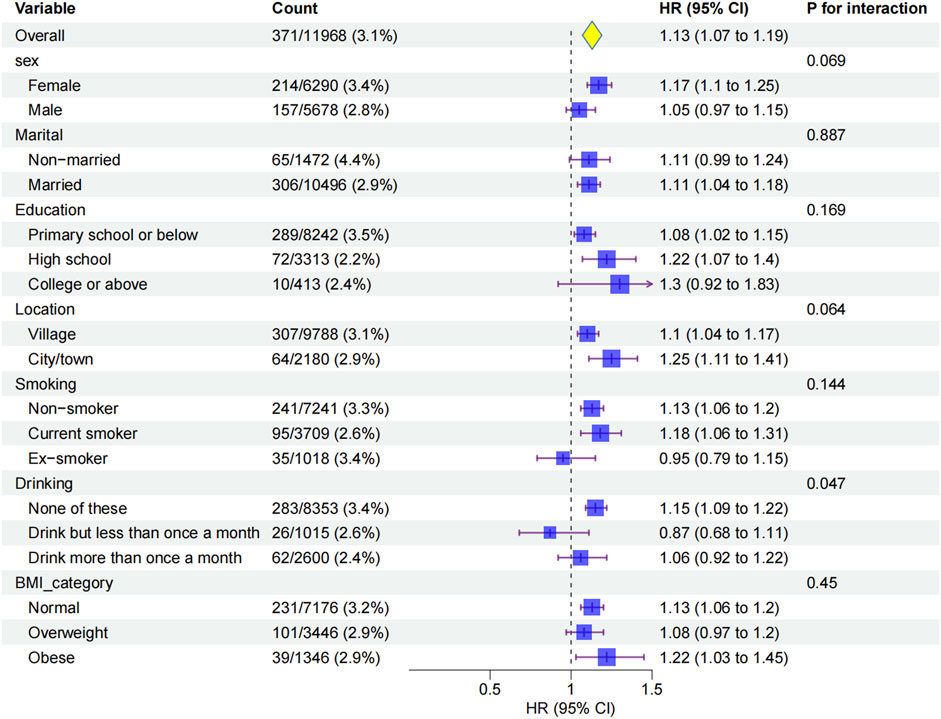
Figure 4. Subgroup analyses of the association between ePWV and glaucoma incidence.Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using Cox proportional hazards models adjusted for sociodemographic, lifestyle, and clinical factors (Model 3). Subgroups were stratified by sex, marital status, education, location, smoking status, drinking status, and BMI.
Sensitive analyses
To validate the independent predictive value of ePWV for glaucoma incidence, we compared its constituent parameters (age and blood pressure) with glaucoma risk (Supplementary Table 2). Age was significantly associated with glaucoma, with each 1-year increase conferring a 4% higher risk (HR 1.04, 95% CI 1.03–1.05, p < 0.001). However, this effect size was smaller than that observed for ePWV (HR 1.13, 95% CI 1.07–1.19), indicating that ePWV exerts a stronger influence on glaucoma incidence than age alone. In contrast, systolic blood pressure (SBP) and diastolic blood pressure (DBP) were not significantly associated with glaucoma risk (SBP: HR 1.00, 95% CI 1.00–1.00, p = 0.977; DBP: HR 0.99, 95% CI 0.98–1.00, p = 0.062), suggesting that blood pressure itself is not an independent risk factor. Overall, these findings underscore that ePWV better captures vascular stiffness status and provides stronger predictive value for glaucoma risk than age or blood pressure alone.
Discussion
This large prospective cohort study investigated the association between ePWV and incident glaucoma in 11,968 middle-aged and older adults from CHARLS over a 7-year follow-up. We observed a clear stepwise increase in glaucoma incidence across ePWV quartiles, with participants in the highest quartile (≥10.58 m/s) having a 39% higher risk compared with the lowest (<8.01 m/s) after full adjustment. Each 1 m/s increase in ePWV was associated with a 7% increase in glaucoma risk, independent of demographic, lifestyle, and clinical factors. Subgroup analyses showed consistent associations across sex, marital status, education, residence, smoking, and BMI, with drinking status emerging as the only significant modifier: non-drinkers demonstrated a stronger positive association, while no significant relationship was observed among drinkers. Sensitivity analyses further revealed that ePWV had a stronger predictive effect on glaucoma than age or blood pressure alone, underscoring its value as an integrative marker of vascular aging, one with notable potential for AI model development.
Our findings align with recent evidence suggesting that arterial stiffness contributes to glaucoma risk. In the ViDA study, higher carotid-femoral PWV was significantly associated with glaucoma incidence over a decade of follow-up (HR 1.36 per SD increase) (Beros et al., 2024). Cross-sectional studies have also reported that glaucoma patients exhibit higher PWV values and lower macular vessel density, suggesting compromised ocular microcirculation (Lee et al., 2021). Moreover, UK Biobank data demonstrated that elevated pulse pressure, a surrogate of arterial stiffness, was an independent predictor of glaucoma (Macri et al., 2022). Our study extends these findings to a large Chinese cohort, using ePWV as a practical, non-invasive stiffness marker. Notably, while an earlier smaller cross-sectional study found no association, these discrepancies may be explained by limited sample size, design, or population characteristics. Our findings differ from the small cross-sectional study by Chiba’s study in 2008 (Chiba et al., 2008), which reported no association between directly measured Brachial-ankle PWV and prevalent glaucoma in 51 Japanese patients. The difference likely reflects our substantially larger, nationally representative Chinese cohort (n = 11,968), the prospective design capturing incident glaucoma, and the use of validated ePWV derived from age and blood pressure, which together provide greater statistical power and broader generalisability. Taken together, the growing body of evidence supports arterial stiffness as a robust vascular risk factor for glaucoma across populations, reinforcing ePWV’s relevance for data-driven AI applications.
The biological plausibility of our findings rests on the vascular theory of glaucoma. Arterial stiffening impairs the Windkessel effect of large arteries, increasing pulse pressure and reducing diastolic perfusion, thereby destabilizing ocular blood flow and compromising optic nerve head autoregulation (Choi and Kook, 2015). This hemodynamic stress may predispose to ischemia-reperfusion injury of retinal ganglion cells. Additionally, arterial stiffness reflects cumulative vascular aging, endothelial dysfunction, and microvascular remodeling, all of which can reduce optic nerve perfusion reserve (Beros et al., 2024). The stronger association in non-drinkers could be partly explained by their older age and higher baseline stiffness, while younger drinkers may not yet manifest stiffness-related glaucoma risk (Del Giorno et al., 2022). The stronger association originally observed in non-drinkers is largely explained by their older age and higher baseline stiffness. Age-stratified analyses confirmed that the ePWV-glaucoma association remained significant only in participants aged ≥58 years, and the drinking-status interaction disappeared (P for interaction >0.05). Chronic alcohol consumption has complex vascular effects—moderate intake may transiently enhance endothelial function, whereas heavy or long-term use is associated with increased arterial stiffness and impaired vascular compliance (Del Giorno et al., 2022; Schutte et al., 2024; Gusti et al., 2025). These mechanisms could partly influence the observed interaction; however, this result should be considered hypothesis-generating and requires confirmation in cohorts with detailed alcohol-consumption data.
In normal tension glaucoma, higher carotid femoral pulse wave velocity (PWV) has been correlated with lower macular vessel density measured by OCTA(Lee et al., 2021).Systemic blood pressure profiles related to arterial stiffness also influence ocular perfusion pressure (OPP), a recognized risk factor for open angle glaucoma (Kim et al., 2020). Because estimated PWV (ePWV) is a validated surrogate for carotid femoral PWV(Greve et al., 2016), elevated ePWV likely reflects the same hemodynamic burden that lowers OPP and compromises retinal capillary density, providing a biologically plausible pathway from systemic stiffness to glaucomatous neurodegeneration (Wang et al., 2023). Overall, these mechanisms highlight the role of systemic vascular health in glaucomatous neurodegeneration beyond intraocular pressure-strengthening the rationale for incorporating ePWV into AI models that aim to capture multi-dimensional glaucoma risk.
Our study provides evidence that ePWV, a simple and non-invasive marker derived from age and blood pressure, may serve as an independent predictor of glaucoma risk.
The marginal p for trend (0.065) after full adjustment likely reflects confounding by antihypertensive therapy (included in Model 3).These medications reduce arterial stiffness while potentially modifying glaucoma risk. Nevertheless, the significant HR for Q4 vs Q1 (1.39, 95% CI 1.00–1.93) still support a meaningful dose-response relationship, with the marginal trend attributed to residual variability from covariate adjustment. As glaucoma often remains asymptomatic until advanced stages, incorporating vascular markers such as ePWV into risk stratification could improve early identification of high-risk individuals (Beros et al., 2024). From a preventive perspective, interventions to mitigate arterial stiffness, which can be conducted through lifestyle modifications or pharmacologic therapies, may hold promise in reducing glaucoma burden, particularly in aging societies like China. Our observation that each 1 m/s increase in ePWV was associated with a 7% higher risk of glaucoma, supporting biological plausibility for a stiffness-related pathway (Lee et al., 2022; Beros et al., 2024). Moreover, incident-glaucoma analyses using arterial stiffness measures show prospective associations (Lee et al., 2022; Beros et al., 2024).ePWV reflects cumulative arterial stiffening (Greve et al., 2016), supplying additional systemic information beyond ocular imaging. Integrating vascular health monitoring into ophthalmic practice could thus represent a novel approach to comprehensive glaucoma prevention and management.
Several limitations should be acknowledged. First, glaucoma was self-reported without confirmatory optic-nerve imaging (fundus photography or OCT) or IOP and misclassification cannot be excluded (Paul et al., 2023; Higgins et al., 2024). Large epidemiologic and Mendelian-randomization analyses indicate only small positive effects of systemic blood pressure on IOP(Plotnikov et al., 2022). In our models, adjustment for blood pressure and antihypertensive therapy should reduce confounding through IOP. Second, intraocular pressure and detailed ophthalmic examinations were unavailable, precluding subtype analyses, e.g., normal-tension vs. high-tension glaucoma that could refine AI model stratification.Future studies should incorporate these indicators to enable more precise risk stratification in AI-based screening models.Third, although ePWV is validated, it is an indirect surrogate and not a direct measure of carotid-femoral PWV, which may affect its predictive precision in AI systems. This indirect derivation from age and blood pressure may introduce nondifferential measurement error that could attenuate effect sizes and slightly reduce the incremental value of ePWV in multimodal AI models (Alansare et al., 2022; Heffernan et al., 2023). Future studies comparing AI models that incorporate ePWV with those using directly measured cfPWV will be valuable to confirm robustness and reliability.Fourth, residual confounding from unmeasured factors, e.g., family history, ocular perfusion pressure, cannot be ruled out. Finally, our findings are based on Chinese adults and may not be generalizable to other populations, emphasizing the need for diverse datasets to optimize AI model generalizability. Our cohort consisted of older Chinese adults, and 18% of the analytic sample lived in urban areas.Future studies in younger cohorts and in more highly urbanised populations are needed. Future studies integrating direct vascular measures, comprehensive ophthalmic data, and interventional designs are warranted to confirm causality and explore clinical translation, including the development of ePWV-integrated AI screening tools.
Notably, unlike other vascular markers such as carotid intima-media thickness, which requires ultrasound or pulse pressure, which is more sensitive to short-term blood pressure fluctuations, ePWV only needs age and routine blood pressure for calculation (Greve et al., 2016; Bozic et al., 2025). Both are readily available in primary care at low cost, avoiding specialized equipment, reducing screening costs, and enabling seamless integration into AI models using common health records (Cheng et al., 2024). Beyond single-modality AI, ePWV could also be combined with ocular imaging data such as fundus photographs or OCT in multimodal AI systems to enhance glaucoma risk prediction and provide complementary systemic vascular information, adding it may help in cases where ocular imaging is equivocal, e.g., optic disc margin ambiguity and high myopia (Huang et al., 2023; Heckel et al., 2025; Koornwinder et al., 2025; Sivakumar and Penkova, 2025). This enables ePWV to be computed automatically from routine blood-pressure data and to flag high-risk individuals for targeted glaucoma screening in community and primary-care settings.
Conclusion
In summary, this nationally representative cohort study demonstrates that higher ePWV is independently associated with increased glaucoma risk in middle-aged and older Chinese adults. The relationship was linear, consistent across most subgroups, and stronger in non-drinkers. Compared with age or blood pressure alone, ePWV provided superior predictive value, suggesting it captures cumulative vascular aging relevant to glaucomatous neurodegeneration. These results suggest that ePWV could serve as a promising candidate marker for incorporation into future AI-assisted glaucoma screening strategies in middle-aged and older Chinese populations.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://charls.charlsdata.com/users/profile/index/zh-cn.html. The CHARLS protocol was approved by the Biomedical Ethics Review Committee of Peking University (IRB00001052-11015) and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants. This study adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Ethics statement
The studies involving humans were approved by The CHARLS protocol was approved by the Biomedical Ethics Review Committee of Peking University (IRB00001052-11015) and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants. This study adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YX: Data curation, Writing – original draft. ZY: Investigation, Writing – original draft, Data curation, Methodology, Formal analysis. QG: Formal analysis, Data curation, Investigation, Writing – original draft. MZ: Writing – review and editing. CL: Writing – review and editing. HW: Conceptualization, Project administration, Supervision, Writing – review and editing, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (Nos. 82571227, 82201246 and 81870666) and Lumitin Vision to Brightness Research Funding for the Young and middle-aged Ophthalmologists (No. BCF-KH-YK-20230803–05) and National Clinical Key Specialty Construction Project (10000015Z155080000004). The sponsors and funding organizations had no role in the study design; collection, analysis and interpretation of data; writing of this report or decision to submit this manuscript for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2025.1700378/full#supplementary-material
Abbreviations
CHARLS, China Health and Retirement Longitudinal Study; ePWV, estimated pulse wave velocity; IOP, intraocular pressure measurement; OCT, optical coherence tomography; OCTA, optical coherence tomography angiography; OPP, ocular perfusion pressure; PWV, pulse wave velocity.
References
Alansare, A. B., Stoner, L., Aljuhani, O. E., and Gibbs, B. B. (2022). Utility of estimated pulse wave velocity for tracking the arterial response to prolonged sitting. J. Cardiovasc Dev. Dis. 9 (12), 411. doi:10.3390/jcdd9120411
Beros, A. L., Sluyter, J. D., Hughes, A. D., Hametner, B., Wassertheurer, S., and Scragg, R. K. R. (2024). Arterial stiffness and incident glaucoma: a large population-based cohort study. Am. J. Ophthalmol. 266, 68–76. doi:10.1016/j.ajo.2024.05.015
Bozic, M., Maric, V., Milutinovic, V., Lucic, M., and Vasilijevic, J. (2025). Estimated pulse wave velocity (ePWV) in different glaucoma types. Biomedicines 13 (8), 2033. doi:10.3390/biomedicines13082033
Chen, S., and Bai, W. (2025). Artificial intelligence technology in ophthalmology public health: current applications and future directions. Front. Cell Dev. Biol. 13, 1576465. doi:10.3389/fcell.2025.1576465
Cheng, W., Kong, F., Pan, H., Luan, S., Yang, S., and Chen, S. (2024). Superior predictive value of estimated pulse wave velocity for all-cause and cardiovascular disease mortality risk in U.S. general adults. BMC Public Health 24 (1), 600. doi:10.1186/s12889-024-18071-2
Chiba, T., Chiba, N., and Kashiwagi, K. (2008). Systemic arterial stiffness in glaucoma patients. J. Glaucoma 17 (1), 15–18. doi:10.1097/IJG.0b013e318098737a
Choi, J., and Kook, M. S. (2015). Systemic and ocular hemodynamic risk factors in glaucoma. Biomed. Res. Int. 2015, 141905. doi:10.1155/2015/141905
Del Giorno, R., Maddalena, A., Bassetti, S., and Gabutti, L. (2022). Association between alcohol intake and arterial stiffness in healthy adults: a systematic review. Nutrients 14 (6), 1207. doi:10.3390/nu14061207
Djulbegovic, M. B., Bair, H., Gonzalez, D. J. T., Ishikawa, H., Wollstein, G., and Schuman, J. S. (2025). Artificial intelligence for optical coherence tomography in glaucoma. Transl. Vis. Sci. Technol. 14 (1), 27. doi:10.1167/tvst.14.1.27
Dong, L., He, W., Zhang, R., Ge, Z., Wang, Y. X., Zhou, J., et al. (2022). Artificial intelligence for screening of multiple retinal and optic nerve diseases. JAMA Netw. Open 5 (5), e229960. doi:10.1001/jamanetworkopen.2022.9960
Greve, S. V., Blicher, M. K., Kruger, R., Sehestedt, T., Gram-Kampmann, E., Rasmussen, S., et al. (2016). Estimated carotid-femoral pulse wave velocity has similar predictive value as measured carotid-femoral pulse wave velocity. J. Hypertens. 34 (7), 1279–1289. doi:10.1097/hjh.0000000000000935
Gusti, Y., Liu, W., Athar, F., Cahill, P. A., and Redmond, E. M. (2025). Endothelial homeostasis under the influence of alcohol-relevance to atherosclerotic cardiovascular disease. Nutrients 17 (5), 802. doi:10.3390/nu17050802
Heckel, A. R., Glasgow, A. C., Cho, W., and Kim, J. Y. (2025). The association between estimated pulse wave velocity and balance function in U.S. adults. J. Hum. Hypertens. doi:10.1038/s41371-025-01062-0
Heffernan, K. S., Stoner, L., London, A. S., Augustine, J. A., and Lefferts, W. K. (2023). Estimated pulse wave velocity as a measure of vascular aging. PLoS One 18 (1), e0280896. doi:10.1371/journal.pone.0280896
Higgins, B. E., Leonard-Hawkhead, B., and Azuara-Blanco, A. (2024). Quality of reporting electronic health record data in glaucoma: a systematic literature review. Ophthalmol. Glaucoma 7 (5), 422–430. doi:10.1016/j.ogla.2024.04.002
Huang, X., Islam, M. R., Akter, S., Ahmed, F., Kazami, E., Serhan, H. A., et al. (2023). Artificial intelligence in glaucoma: opportunities, challenges, and future directions. Biomed. Eng. Online 22 (1), 126. doi:10.1186/s12938-023-01187-8
Jayaram, H., Kolko, M., Friedman, D. S., and Gazzard, G. (2023). Glaucoma: now and beyond. Lancet 402 (10414), 1788–1801. doi:10.1016/s0140-6736(23)01289-8
Ke, W. K., Cheng, J. P., and Xu, L. L. (2025a). Association between estimated pulse wave velocity and hip fracture in middle-aged and older adults: a prospective cohort study in China. Bone 197, 117499. doi:10.1016/j.bone.2025.117499
Ke, W. K., Xu, L. L., and Luo, N. (2025b). Predictive value of insulin resistance metabolic score for cardiovascular disease in Chinese arthritis patients: a prospective cohort study. Rheumatol. Oxf. 64 (6), 3388–3395. doi:10.1093/rheumatology/keaf048
Kim, K. E., Oh, S., Baek, S. U., Ahn, S. J., Park, K. H., and Jeoung, J. W. (2020). Ocular perfusion pressure and the risk of open-angle glaucoma: systematic review and meta-analysis. Sci. Rep. 10 (1), 10056. doi:10.1038/s41598-020-66914-w
Koornwinder, A., Zhang, Y., Ravindranath, R., Chang, R. T., Bernstein, I. A., and Wang, S. Y. (2025). Multimodal artificial intelligence models predicting glaucoma progression using electronic health records and retinal nerve fiber layer scans. Transl. Vis. Sci. Technol. 14 (3), 27. doi:10.1167/tvst.14.3.27
Lee, T., Bae, H. W., Seong, G. J., Kim, C. Y., and Lee, S. Y. (2021). High pulse wave velocity is associated with decreased macular vessel density in normal-tension glaucoma. Invest Ophthalmol. Vis. Sci. 62 (10), 12. doi:10.1167/iovs.62.10.12
Lee, J. S., Bae, H. W., Park, S., Kim, C. Y., and Lee, S. Y. (2022). Systemic arterial stiffness is associated with structural progression in early open-angle glaucoma. Invest Ophthalmol. Vis. Sci. 63 (3), 28. doi:10.1167/iovs.63.3.28
Li, X., Luo, Z. Y., Lei, S., Zhang, Z. J., Tang, J. F., and Sun, Y. Q. (2025). Exploring association between ambient air pollution and glaucoma in China: a nationwide analysis with predictive modeling based on the China health and retirement longitudinal study. Front. Public Health 13, 1541803. doi:10.3389/fpubh.2025.1541803
Liu, G., Sha, W., Wu, Y., Luo, J., Cai, Y., Zhang, T., et al. (2025). The association between estimated pulse wave velocity and cardio-cerebrovascular disease risk: a cohort study. Eur. J. Med. Res. 30 (1), 16. doi:10.1186/s40001-024-02217-4
Macri, C., Wong, C. X., Tu, S. J., Casson, R., Singh, K., Wang, S. Y., et al. (2022). Blood pressure measures and incident primary open-angle glaucoma. Invest Ophthalmol. Vis. Sci. 63 (13), 3. doi:10.1167/iovs.63.13.3
Ohkuma, T., Ninomiya, T., Tomiyama, H., Kario, K., Hoshide, S., Kita, Y., et al. (2017). Brachial-ankle pulse wave velocity and the risk prediction of cardiovascular disease: an individual participant data meta-analysis. Hypertension 69 (6), 1045–1052. doi:10.1161/hypertensionaha.117.09097
Paul, M. E., Tseng, V. L., Kitayama, K., Yu, F., and Coleman, A. L. (2023). Evaluating discrepancies in self-reported glaucoma and electronic health records in the national institutes of health all of us database. Ophthalmol. Glaucoma 6 (5), 521–529. doi:10.1016/j.ogla.2023.03.003
Plotnikov, D., Huang, Y., Khawaja, A. P., Foster, P. J., Zhu, Z., Guggenheim, J. A., et al. (2022). High blood pressure and intraocular pressure: a mendelian randomization Study. Invest Ophthalmol. Vis. Sci. 63 (6), 29. doi:10.1167/iovs.63.6.29
Ran, A. R., Wang, X., Chan, P. P., Chan, N. C., Yip, W., Young, A. L., et al. (2022). Three-dimensional multi-task deep learning model to detect glaucomatous optic neuropathy and myopic features from optical coherence tomography scans: a retrospective multi-centre study. Front. Med. (Lausanne) 9, 860574. doi:10.3389/fmed.2022.860574
Schutte, R., Zhang, J., Kiran, M., and Ball, G. (2024). Alcohol and arterial stiffness in middle-aged and older adults: cross-sectional evidence from the UK Biobank study. Alcohol Clin. Exp. Res. Hob. 48 (10), 1915–1922. doi:10.1111/acer.15426
Shi, Y., Yu, C., Zhou, W., Wang, T., Zhu, L., Bao, H., et al. (2024). Estimated pulse wave velocity as a predictor of all-cause and cardiovascular mortality in patients with hypertension in China: a prospective cohort study. Front. Cardiovasc Med. 11, 1365344. doi:10.3389/fcvm.2024.1365344
Shin, D. Y., Hong, K. E., Lee, N. Y., Park, C. K., and Park, H. Y. L. (2022). Association of choroidal blood flow with autonomic dysfunction in patients with normal tension glaucoma. Sci. Rep. 12 (1), 5136. doi:10.1038/s41598-022-09162-4
Shoshani, Y., Harris, A., Shoja, M. M., Arieli, Y., Ehrlich, R., Primus, S., et al. (2012). Impaired ocular blood flow regulation in patients with open-angle glaucoma and diabetes. Clin. Exp. Ophthalmol. 40 (7), 697–705. doi:10.1111/j.1442-9071.2012.02778.x
Sivakumar, R., and Penkova, A. (2025). Enhancing glaucoma detection through multi-modal integration of retinal images and clinical biomarkers. Eng. Appl. Artif. Intell. 143, 110010. doi:10.1016/j.engappai.2025.110010
Song, P., Wang, J., Bucan, K., Theodoratou, E., Rudan, I., Chan, K. Y., et al. (2017). National and subnational prevalence and burden of glaucoma in China: a systematic analysis. J. Glob. Health 7 (2), 020705. doi:10.7189/jogh.07.020705
Tang, J., Liang, Y., O'Neill, C., Kee, F., Jiang, J., and Congdon, N. (2019). Cost-effectiveness and cost-utility of population-based glaucoma screening in China: a decision-analytic Markov model. Lancet Glob. Health 7 (7), e968–e978. doi:10.1016/s2214-109x(19)30201-3
Tham, Y. C., Li, X., Wong, T. Y., Quigley, H. A., Aung, T., and Cheng, C. Y. (2014). Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 121 (11), 2081–2090. doi:10.1016/j.ophtha.2014.05.013
Wang, X., Wang, M., Liu, H., Mercieca, K., Prinz, J., Feng, Y., et al. (2023). The Association between vascular abnormalities and glaucoma-what comes first? Int. J. Mol. Sci. 24 (17), 13211. doi:10.3390/ijms241713211
Wang, S., He, X., Jian, Z., Li, J., Xu, C., Chen, Y., et al. (2024). Advances and prospects of multi-modal ophthalmic artificial intelligence based on deep learning: a review. Eye Vis. 11 (1), 38. doi:10.1186/s40662-024-00405-1
Keywords: glaucoma, AI, estimated pulse wave velocity, incidence, prospective cohort study
Citation: Xu Y, Yin Z, Gong Q, Zhu M, Lu C and Wang H (2025) ePWV as a scalable risk factor for large-scale glaucoma screening: evidence from a national Chinese cohort. Front. Cell Dev. Biol. 13:1700378. doi: 10.3389/fcell.2025.1700378
Received: 06 September 2025; Accepted: 29 September 2025;
Published: 27 October 2025.
Edited by:
Guang-Yu Li, Jilin University, ChinaReviewed by:
Weihua Yang, Southern Medical University, ChinaXuri Li, Sun Yat-sen University, China
Ji Dan, Other, China
Lei Wang, Wenzhou Medical University, China
Copyright © 2025 Xu, Yin, Gong, Zhu, Lu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiyan Wang, aHl3YW5nQHNqdHUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Yupeng Xu
Yupeng Xu Zihan Yin†
Zihan Yin† Qiaoyun Gong
Qiaoyun Gong