- 1Nutrition, Food Science and Technology Programme, School of Life Sciences, The Chinese University of Hong Kong, Hong KongSAR, China
- 2West China Clinical Medical College of Sichuan University, Chengdu, China
- 3Department of Orthopedics surgery, Trauma medical center, West China Hospital, Sichuan University, Chengdu, China
Stem cell adhesion and migration are fundamental processes in tissue regeneration and repair; however, their efficiency in vivo is often limited by the complexity of the microenvironment. Endogenous bioelectrical cues, such as electric fields present during development and wound healing, play a critical role in guiding these cellular behaviors. Piezoelectric biomaterials, which can convert mechanical stimuli into electrical signals, have recently emerged as promising platforms for recapitulating these bioelectric cues without the need for external power sources. In this mini-review, we summarize the recent advances in the use of piezoelectric scaffolds to modulate stem cell adhesion and migration. We highlight the underlying mechanisms, including integrin/focal adhesion kinase activation, calcium signaling, and electrotaxis, which mediate enhanced adhesion, focal adhesion maturation, and directed cell migration. Representative applications in bone, cartilage, nerve, and muscle tissue engineering are discussed, with an emphasis on how piezoelectric scaffolds improve regeneration by providing dynamic and self-sustained electrical stimulation. Finally, we outline the major challenges, such as balancing piezoelectric output with biocompatibility, controlling in vivo stimulation parameters, and elucidating precise sensing mechanisms, and propose future directions for clinical translation. By integrating insights from materials science, mechanobiology, and regenerative medicine, piezoelectric biomaterials hold strong potential as next-generation smart scaffolds for orchestrating stem cell behavior and accelerating functional tissue repair.
1 Introduction
Cell adhesion and migration are crucial biological processes for tissue regeneration and form the basis of cellular activities necessary for healing and repair. These processes are dynamic and coordinated, initiating the engraftment, survival, and differentiation of stem cells (Ridley et al., 2003; Devreotes and Horwitz, 2015). The regulation of these phenomena is complex, influenced by numerous factors, including biochemical signals such as growth factors and cytokines (Brizzi et al., 2012; Devreotes and Horwitz, 2015), as well as physical stimuli like mechanical stress, substrate stiffness (Engler et al., 2006; Wozniak and Chen, 2008), and bioelectric fields (Levin, 2021). Among these, endogenous electric fields are particularly noteworthy for their significant role in directing cell migration during critical biological events, including embryonic development, wound healing, and bone repair (Zhao et al., 2006; Zhao, 2008; Griffin and Bayat, 2025). Strategically harnessing these bioelectrical cues offers an innovative approach to enhance regenerative outcomes across a wide range of therapeutic applications (Levin, 2021; Kim et al., 2024).
Piezoelectric biomaterials represent a fascinating class of materials with the unique capability to convert mechanical deformations into localized electric potentials (Mokhtari et al., 2021; Deng et al., 2025). This property effectively simulates the bioelectric stimuli that cells encounter in their natural microenvironments (Vinikoor et al., 2023; Huang et al., 2025). Traditional piezoelectric materials, such as inorganic crystals like barium titanate (BaTiO3) and zinc oxide (ZnO), are known for their high piezoelectric coefficients and are commonly used in applications requiring precise electrical responses (Liu et al., 2025). In contrast, organic polymers, including polyvinylidene fluoride (PVDF) and poly (L-lactic acid) (PLLA), provide enhanced flexibility and processability, making them particularly suitable for biomedical applications (Mohammadpourfazeli et al., 2023; Ahbab et al., 2025; Schönlein et al., 2025). When engineered into scaffolds, these piezoelectric materials can generate localized and dynamic electrical signals in response to mechanical loading, cell traction forces, or external ultrasound activation (Cafarelli et al., 2021; Alvarez-Lorenzo et al., 2023). This self-powered electromechanical stimulation offers distinct advantages over conventional inert scaffolds, as it more accurately replicates the dynamic extracellular matrix (ECM) microenvironment crucial for supporting various cellular activities (Ricotti et al., 2024; Zaszczyńska et al., 2024).
This mini-review aims to elucidate the complex mechanisms by which piezoelectric biomaterials influence stem cell adhesion and migration, and explore the implications of these effects within the broader context of tissue regeneration. We will begin with an overview of the fundamental principles underlying piezoelectric biomaterials, detailing their structural characteristics, functional properties, and the mechanisms by which they generate electrical signals in response to mechanical stimuli (Bai et al., 2024; Ni et al., 2025; Wang et al., 2025). A comprehensive understanding of these principles is vital for the rational design and effective application of these materials in regenerative medicine applications. Building on this foundational knowledge, we will investigate the cellular mechanisms governing adhesion and migration, summarizing recent research findings that demonstrate how piezoelectric stimulation can enhance adhesion strength, accelerate focal adhesion turnover, and guide the directional migration of stem cells (Zhao, 2008; Bai et al., 2024; Shlapakova et al., 2024).
Furthermore, we will discuss representative applications of piezoelectric biomaterials in tissue engineering, highlighting their potential to improve outcomes in various clinical scenarios. The prospects for the clinical translation of these innovative materials are also examined, emphasizing the necessity for continued research and development to fully realize their therapeutic potential. By bridging the gap between materials science and cellular biology, this review aims to provide insights that could pave the way for novel strategies in regenerative medicine, ultimately contributing to enhanced healing and recovery processes across a range of tissue types.
2 Fundamental principles and properties of piezoelectric biomaterials
The principles underlying piezoelectric biomaterials are based on the phenomenon of piezoelectricity, which refers to the capability of certain materials to produce an electric charge when subjected to mechanical stress (Smith and Kar-Narayan, 2021). This effect arises from mechanical deformation that causes the reorientation of dipoles within a non-centrosymmetric lattice structure or along polymer chains, leading to charge separation and the creation of an electric potential difference across the material (Kamel, 2022; Ahbab et al., 2025). This distinctive characteristic has profound implications for various biomedical applications, particularly in the creation of advanced biomaterials that can dynamically interact with biological systems (Ni et al., 2025).
Inorganic ceramics, such as barium Titanate (BaTiO3) and zinc Oxide (ZnO), are recognized for their strong piezoelectric responses. These materials possess high piezoelectric coefficients, making them efficient in converting mechanical energy into electrical energy (Lay et al., 2021; Wu et al., 2024). However, their advantageous properties are often tempered by inherent brittleness, which can result in mechanical failure under physiological conditions (Kapat et al., 2020). Furthermore, their limited biodegradability presents challenges for long-term use in implantable devices, as they may not integrate effectively with surrounding biological tissues or degrade appropriately over time (Li et al., 2020; Zheng et al., 2022; Bai et al., 2024).
Conversely, piezoelectric polymers such as Polyvinylidene fluoride (PVDF) and Poly (L-lactic acid) (PLLA) offer a promising alternative (Li et al., 2019; Purushothaman et al., 2023). Although these polymers generally exhibit lower piezoelectric coefficients than their inorganic counterparts, they provide significant benefits in terms of flexibility, biocompatibility, and ease of processing (Li et al., 2019; Zaszczyńska et al., 2024). Their inherent flexibility facilitates better integration with soft biological tissues, making them suitable for applications in tissue engineering and regenerative medicine. Additionally, these polymers can be readily fabricated into nanofibers or hydrogels, enhancing their surface area and promoting cellular interactions, which ultimately aids in tissue regeneration (Dai et al., 2022; Fathollahzadeh et al., 2025).
Recent advancements in biomaterials science have led to the creation of hybrid scaffolds that merge the advantageous properties of both polymers and piezoelectric nanoparticles or aligned nanofibers (Ren et al., 2023). These hybrid materials have shown improved electromechanical conversion capabilities, which can be utilized to stimulate cellular activities and enhance tissue healing (Ricotti et al., 2024; Wu et al., 2024). The incorporation of piezoelectric nanoparticles into polymer matrices not only boosts the piezoelectric response but also preserves the flexibility and biocompatibility of the scaffold, establishing a versatile platform for various biomedical applications (Fathollahzadeh et al., 2025).
One of the most intriguing features of piezoelectric biomaterials is their ability to be activated by physiological movements, such as joint loading or the rhythmic contractions of the heart (Liu et al., 2022). This capability enables the generation of bioelectrical cues in response to the body’s natural movements, which can be essential for promoting cellular responses and facilitating tissue regeneration (Alvarez-Lorenzo et al., 2023). Moreover, these materials can also be stimulated by non-invasive external stimuli, such as ultrasound, allowing researchers and clinicians to control the spatiotemporal delivery of electrical signals in vivo (Cafarelli et al., 2021; Ricotti et al., 2024). This characteristic paves the way for new therapeutic interventions, enabling targeted treatments that can adapt to the dynamic nature of the biological systems.
In summary, the principles of piezoelectric biomaterials encompass a wide array of materials, each with distinct properties and potential applications. Ongoing research and development in this field are poised to yield innovative solutions to challenges in tissue engineering, regenerative medicine, and beyond, ultimately advancing healthcare technologies (Chen et al., 2024).
3 Mechanisms of stem cell adhesion and migration on piezoelectric scaffolds
The mechanisms of stem cell adhesion are crucial for understanding how stem cells interact with their microenvironment, particularly in tissue engineering and regenerative medicine. Adhesion begins with the integrin-mediated recognition of extracellular matrix (ECM) ligands, which is a fundamental process that allows stem cells to anchor themselves to their surroundings (Huttenlocher and Horwitz, 2011; Iwamoto and Calderwood, 2015; Yamaguchi and Knaut, 2022). This initial interaction is followed by the assembly of focal adhesions, which serve as critical junctions linking the cytoskeleton of the cell to the ECM. The formation and maturation of these focal adhesions are essential for maintaining cellular stability and facilitating communication between the cell and its environment (Iwamoto and Calderwood, 2015; Yamaguchi and Knaut, 2022).
Recent advancements in materials science have introduced piezoelectric surfaces, which can significantly modulate the adhesion process in various ways. One of the primary effects of these surfaces is the enhancement of the adhesion strength. For instance, polarized polyvinylidene fluoride (PVDF) or poly (L-lactic acid) (PLLA) fibers have been shown to increase the adsorption of adhesion proteins on their surface. This leads to the formation of larger and more mature focal adhesions, which are vital for effective cell anchorage and signaling (Ribeiro et al., 2012; Low et al., 2013; Yamaguchi and Knaut, 2022). The increased surface area and the unique electrical properties of these materials create an environment conducive to robust cell adhesion.
Another critical mechanism by which piezoelectric surfaces influence stem cell adhesion is by modulating calcium influx. The electrical potentials generated at the scaffold surface can activate voltage-gated calcium channels, resulting in intracellular calcium transients (Zaszczyńska et al., 2024; Zhang et al., 2024). These calcium signals play a pivotal role in promoting actin polymerization, which is essential for stabilizing adhesion complexes. The dynamic interplay between calcium signaling and cytoskeletal rearrangement underscores the importance of electrical cues in regulating stem cell behavior (Tsai et al., 2015; Lehne et al., 2022).
Furthermore, the activation of focal adhesion kinase (FAK) and the subsequent phosphorylation events are significantly enhanced when stem cells are cultured on piezoelectric scaffolds. This elevated phosphorylation of FAK leads to the activation of downstream signaling pathways, including the mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K)/Akt pathways. These signaling cascades are crucial for mediating various cellular responses, including proliferation, survival, and differentiation (Velling et al., 2004; Heng et al., 2021; Tan et al., 2023; Katoh, 2024). Through these interconnected mechanisms, piezoelectric stimulation can establish a positive feedback loop. Specifically, stronger cell adhesion leads to greater cellular traction forces that further deform the piezoelectric scaffold. This deformation generates additional electrical cues, which in turn reinforce cellular adhesion (Liu et al., 2021; Xia et al., 2022; Shlapakova et al., 2024; Zaszczyńska et al., 2024).
In addition to adhesion, the mechanisms of directed migration are vital for stem cell functionality. Cell migration is a complex process that requires the establishment of polarity, extension of lamellipodia, formation of new adhesions, and detachment at the rear end (Ridley et al., 2003; Devreotes and Horwitz, 2015). Piezoelectric scaffolds significantly influence this migratory behavior by providing localized electric fields that mimic the phenomenon of electrotaxis (Xia et al., 2022; Alvarez-Lorenzo et al., 2023; Zaszczyńska et al., 2024). One of the key mechanisms involved is the directional bias induced by the voltage gradients along the aligned piezoelectric fibers. This gradient guides mesenchymal stem cells (MSCs) to preferentially migrate toward the cathode, thereby enhancing their migratory efficiency (Banks et al., 2015; Tai et al., 2018; Shlapakova et al., 2024).
Moreover, piezoelectric stimulation increases the migration speed by accelerating adhesion turnover. This results in shorter migration cycles and enhanced net displacement, allowing stem cells to reach target sites more effectively (Xiang et al., 2023). The polarization of signaling molecules, such as PI3K, calcium channels, and integrins, to the cathodal side of the cell further strengthens the front-rear polarity necessary for directed movement (Zhao et al., 2006; Lin et al., 2017; Tsai et al., 2020). Additionally, piezoelectric stimulation can induce cells to secrete chemokines, such as transforming growth factor-beta 1 (TGF-β1), which indirectly amplifies the recruitment of neighboring cells, enhancing the overall regenerative response (Vinikoor et al., 2023; Ricotti et al., 2024).
In summary, piezoelectric scaffolds provide a multifaceted approach to enhance stem cell adhesion and migration, ultimately improving regenerative outcomes in tissue engineering applications (Table 1). The interplay between electrical cues, biochemical signals, and mechanical forces creates a conducive environment for stem cell behavior, paving the way for innovative therapeutic strategies in regenerative medicine.
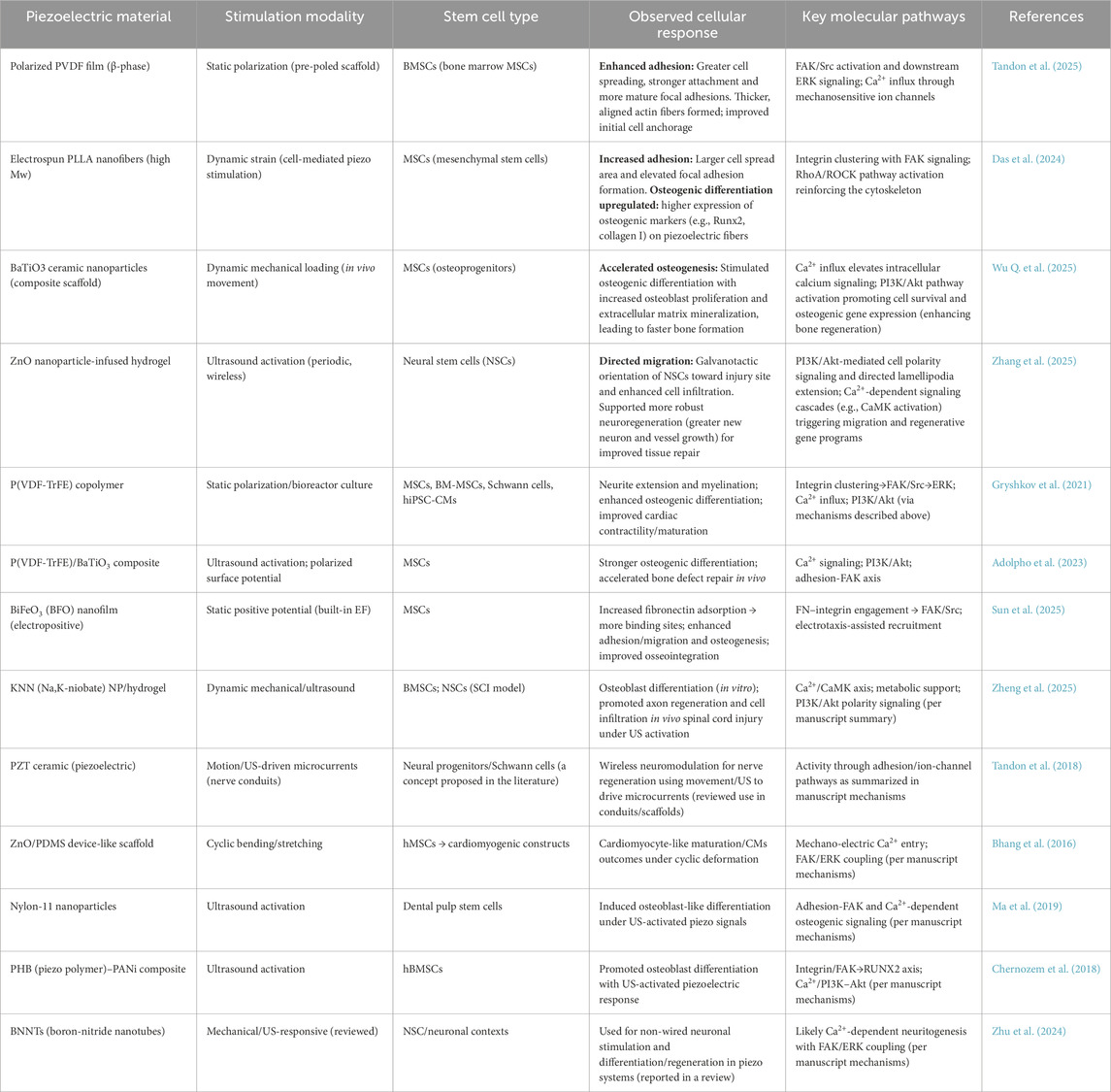
Table 1. Representative piezoelectric materials, stimulation modalities, stem cell types, cellular responses, and key signaling pathways are summarized in this review.
4 Applications of piezoelectric biomaterials in regenerative medicine
Regenerative medicine has emerged as a transformative field, harnessing the body’s innate healing capabilities to restore function and repair damaged tissues (Mao and Mooney, 2015). Among the various tissues that can be regenerated, bone, cartilage, tendon, neural tissue, skeletal muscle, and cardiac tissue have garnered significant attention due to their complex structures and vital roles in overall health (Alvarez-Lorenzo et al., 2023). The integration of piezoelectric materials into regenerative strategies has opened new avenues for enhancing tissue repair and regeneration (Ahn and Grodzinsky, 2009; Ni et al., 2025).
Bone is a dynamic tissue characterized by its ability to remodel and heal itself, a process that is significantly influenced by mechanical stress (Carter et al., 2021). The inherent piezoelectric properties of bone, primarily attributed to collagen polarization under mechanical load, have inspired the development of piezoelectric scaffolds for bone regeneration. These scaffolds aim to recreate the electrical microenvironments that are often disrupted in bone defects (Kwon and Cho, 2022; Zaszczyńska et al., 2024; Ni et al., 2025). Notably, polarized polyvinylidene fluoride (PVDF) membranes and barium titanate/poly (L-lactic acid) (BaTiO3/PLLA) composites have demonstrated remarkable efficacy in accelerating bone healing. The application of these piezoelectric scaffolds has been demonstrated to stimulate osteogenic differentiation and enhance matrix deposition, resulting in improved bone regeneration (Zhou et al., 2016; Dai et al., 2022; Zuchuat et al., 2023). In various animal studies, the implantation of these scaffolds has led to complete closure of calvarial defects and significant regeneration of the osteochondral interface, highlighting their potential for clinical applications in orthopedic surgery (Wu et al., 2022).
Cartilage, being avascular and lacking intrinsic electrical activity, presents unique challenges in regeneration. However, mechanical loading can induce streaming potentials that may be harnessed for therapeutic purposes (Becher et al., 2015; Farooqi et al., 2018). Recent advancements in injectable piezoelectric hydrogels, activated by ultrasound, have shown promise in promoting mesenchymal stem cell (MSC) chondrogenesis and the secretion of transforming growth factor-beta 1 (TGF-β1). This innovative approach has led to the regeneration of hyaline-like cartilage in osteoarthritis models, demonstrating the potential of piezoelectric materials in cartilage repair (Kwon et al., 2016; Vinikoor et al., 2023). Similarly, tendon injuries, which often result in impaired function and pain, can benefit from piezoelectric scaffolds that incorporate these fibers. These scaffolds have been shown to improve collagen alignment and tensile strength, thereby enhancing the repair process and restoring the mechanical integrity of the tendon (Fernandez-Yague et al., 2021; Wu W. et al., 2025).
The intricate architecture of neural tissues necessitates a careful approach to regeneration, particularly because of their sensitivity to electrical cues. Piezoelectric hydrogels containing potassium sodium niobate (KNN) nanoparticles or PVDF nanofibers have been utilized in the context of spinal cord injuries and peripheral nerve repair. Upon ultrasound activation, these scaffolds generate microcurrents that facilitate neural progenitor cell migration, promote axonal outgrowth, and support remyelination. Such enhancements have been linked to functional recovery in rodent models, underscoring the potential of piezoelectric materials in neural tissue engineering (Gryshkov et al., 2021; Tai et al., 2023; Shi et al., 2025).
The regeneration of skeletal and cardiac muscle tissues is critically dependent on rhythmic electrical stimulation, which is essential for their maturation and functional recovery (Ronaldson-Bouchard et al., 2018; Mueller et al., 2020). Piezoelectric scaffolds have been investigated as innovative solutions for providing wireless electrical stimulation (Cafarelli et al., 2021; Ricotti et al., 2024). For example, PVDF-TrFE membranes, when coupled with ultrasound, can generate pulsed electrical signals that synchronize cardiomyocyte contraction in vitro (Bryan et al., 2023; Westphal et al., 2023). This concept extends to the development of biodegradable piezoelectric films that could be applied to infarcted myocardium, harnessing thoracic movements to deliver therapeutic stimulation without the need for external leads or batteries (Li et al., 2020; Bai et al., 2024; Chen et al., 2024; Schönlein et al., 2025). Such advancements could revolutionize cardiac repair strategies, offering a more integrated and less invasive approach to treatment than the current strategies.
In conclusion, the integration of piezoelectric materials into regenerative medicine represents a promising frontier with applications spanning bone, cartilage, tendon, neural, skeletal muscle, and cardiac tissues. The ability to harness electrical cues for tissue regeneration not only enhances the healing process but also paves the way for innovative therapeutic strategies that could significantly improve patient outcomes in the future.
5 Challenges and future perspectives
Material Design: A high piezoelectric output often leads to a reduction in biocompatibility, particularly in ceramic materials. Future research should prioritize the development of biodegradable composites and bio-derived piezoelectric molecules that effectively balance performance and safety.
In Vivo Control: Currently, the electrical output relies on patient activity or external stimuli. Therefore, it is crucial to develop responsive systems that enable precise spatiotemporal control of stimulation.
Mechanistic Understanding: The mechanisms by which cells detect piezoelectric signals remain poorly understood. Potential mediators, such as integrins, calcium channels, and mechanosensitive Piezo ion channels, require further investigation.
Integration with Multimodal Therapies: Future scaffolds should integrate piezoelectric stimulation with drug delivery, magnetothermal regulation, or optogenetics to replicate the complex regenerative environment more accurately.
Clinical Translation: Before piezoelectric scaffolds can be used in clinical trials, long-term safety, degradation products, and regulatory approval must be thoroughly addressed.
6 Conclusion
Piezoelectric biomaterials offer a unique strategy for delivering dynamic, self-sustained electrical stimulation to influence stem cell adhesion and migration. By replicating the bioelectric signals inherent in natural regeneration, these advanced scaffolds enhance cellular interactions, facilitate directional migration, and expedite tissue repair across various organ systems. Ongoing advancements in material design, mechanistic understanding, and multimodal integration are expected to address current limitations and propel piezoelectric scaffolds towards clinical application. As research converges at the intersection of materials science, mechanobiology, and regenerative medicine, piezoelectric biomaterials are poised to transform the paradigm of stem cell–based therapies.
Author contributions
NC: Writing – original draft, Writing – review and editing. YS: Writing – original draft, Writing – review and editing. RZ: Writing – original draft, Writing – review and editing. XD: Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Development and Clinical Demonstration of an Innovative Internal Fixation System for Femoral Neck Fractures (Grant: 2023YFC2508804), Natural Science Foundation of Sichuan Province (Nos 2025ZNSFSC0342, 2024NSFSC0196 and 2024NSFSC1507), National Key R&D Program of China (No. 2023YFE0105600), Science and Technology Projects of Xizang Autonomous Region (XZ202301ZY0046G and XZ202501ZY0129), Sichuan Science and Technology Program (2023NSFSC1753), Commission of Sichuan Province MedicalScience and Technology Program (24QNMP005), 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (2023-309), and Project of Engineering Characteristic Team of Sichuan University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adolpho, L. F., Ribeiro, L. M. S., Freitas, G. P., Lopes, H. B., Gomes, M. P. O., Ferraz, E. P., et al. (2023). Mesenchymal stem cells combined with a P(VDF-TrFE)/BaTiO3 scaffold and photobiomodulation therapy enhance bone repair in rat calvarial defects. J. Funct. Biomater. 14, 306. doi:10.3390/jfb14060306
Ahbab, N., Naz, S., Xu, T.-B., and Zhang, S. (2025). A comprehensive review of piezoelectric PVDF polymer fabrications and characteristics. Micromachines 16, 386. doi:10.3390/mi16040386
Ahn, A. C., and Grodzinsky, A. J. (2009). Relevance of collagen piezoelectricity to “Wolff’s law”: a critical review. Med. Eng. Phys. 31, 733–741. doi:10.1016/j.medengphy.2009.02.006
Alvarez-Lorenzo, C., Zarur, M., Seijo-Rabina, A., Blanco-Fernandez, B., Rodríguez-Moldes, I., and Concheiro, A. (2023). Physical stimuli-emitting scaffolds: the role of piezoelectricity in tissue regeneration. Mater Today Bio 22, 100740. doi:10.1016/j.mtbio.2023.100740
Bai, Y., Meng, H., Li, Z., and Wang, Z. L. (2024). Degradable piezoelectric biomaterials for medical applications. MedMat 1, 40–49. doi:10.1097/mm9.0000000000000002
Banks, T. A., Luckman, P. S. B., Frith, J. E., and Cooper-White, J. J. (2015). Effects of electric fields on human mesenchymal stem cell behaviour and morphology using a novel multichannel device. Integr. Biol. 7, 693–712. doi:10.1039/c4ib00297k
Becher, C., Ricklefs, M., Willbold, E., Hurschler, C., and Abedian, R. (2015). Electromechanical assessment of human knee articular cartilage with compression-induced streaming potentials. Cartilage 7, 62–69. doi:10.1177/1947603515599191
Bhang, S. H., Jang, W. S., Han, J., Yoon, J., La, W., Lee, E., et al. (2016). Zinc oxide nanorod-based piezoelectric dermal patch for wound healing. Adv. Funct. Mater. 27, 1603497. doi:10.1002/adfm.201603497
Brizzi, M. F., Tarone, G., and Defilippi, P. (2012). Extracellular matrix, integrins, and growth factors as tailors of the stem cell niche. Curr. Opin. Cell Biol. 24, 645–651. doi:10.1016/j.ceb.2012.07.001
Bryan, A. E., Krutko, M., Westphal, J., Sheth, M., Esfandiari, L., and Harris, G. M. (2023). Ultrasound-activated piezoelectric polyvinylidene fluoride–trifluoroethylene scaffolds for tissue engineering applications. Mil. Med. 188, 61–66. doi:10.1093/milmed/usad018
Cafarelli, A., Marino, A., Vannozzi, L., Puigmartí-Luis, J., Pané, S., Ciofani, G., et al. (2021). Piezoelectric nanomaterials activated by ultrasound: the pathway from discovery to future clinical adoption. ACS Nano 15, 11066–11086. doi:10.1021/acsnano.1c03087
Carter, A., Popowski, K., Cheng, K., Greenbaum, A., Ligler, F. S., and Moatti, A. (2021). Enhancement of bone regeneration through the converse piezoelectric effect, A novel approach for applying mechanical stimulation. Bioelectricity 3, 255–271. doi:10.1089/bioe.2021.0019
Chen, S., Tong, X., Huo, Y., Liu, S., Yin, Y., Tan, M., et al. (2024). Piezoelectric biomaterials inspired by nature for applications in biomedicine and nanotechnology. Adv. Mater. 36, e2406192. doi:10.1002/adma.202406192
Chernozem, R. V., Surmeneva, M. A., and Surmenev, R. A. (2018). Hybrid biodegradable scaffolds of piezoelectric polyhydroxybutyrate and conductive polyaniline: piezocharge constants and electric potential study. Mater. Lett. 220, 257–260. doi:10.1016/j.matlet.2018.03.022
Dai, X., Yao, X., Zhang, W., Cui, H., Ren, Y., Deng, J., et al. (2022). The osteogenic role of barium titanate/polylactic acid piezoelectric composite membranes as guiding membranes for bone tissue regeneration. Int. J. Nanomed. 17, 4339–4353. doi:10.2147/ijn.s378422
Das, R., Le, D., Kan, H.-M., Le, T. T., Park, J., Nguyen, T. D., et al. (2024). Osteo-inductive effect of piezoelectric stimulation from the poly(l-lactic acid) scaffolds. PLoS One 19, e0299579. doi:10.1371/journal.pone.0299579
Deng, X., Zhuang, Y., Cui, J., Wang, L., Zhan, H., Wang, X., et al. (2025). Open challenges and opportunities in piezoelectricity for tissue regeneration. Adv. Sci. 12, e10349. doi:10.1002/advs.202510349
Devreotes, P., and Horwitz, A. R. (2015). Signaling networks that regulate cell migration. Cold Spring Harb. Perspect. Biol. 7, a005959. doi:10.1101/cshperspect.a005959
Engler, A. J., Sen, S., Sweeney, H. L., and Discher, D. E. (2006). Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689. doi:10.1016/j.cell.2006.06.044
Farooqi, A. R., Bader, R., and van Rienen, U. (2018). Numerical study on electromechanics in cartilage tissue with respect to its electrical properties. Tissue Eng. Part B Rev. 25, 152–166. doi:10.1089/ten.teb.2018.0214
Fathollahzadeh, V., Khodaei, M., Emadi, S., and Hajisharifi, K. (2025). Plasma activated PVDF-BaTiO3 composite nanofiber scaffolds loaded with vancomycin for enhancing biocompatibility and piezoelectric response. Sci. Rep. 15, 28515. doi:10.1038/s41598-025-14391-4
Fernandez-Yague, M. A., Trotier, A., Demir, S., Abbah, S. A., Larrañaga, A., Thirumaran, A., et al. (2021). A self-powered piezo-bioelectric device regulates tendon repair-associated signaling pathways through modulation of mechanosensitive ion channels. Adv. Mater. 33, 2008788. doi:10.1002/adma.202008788
Griffin, M., and Bayat, A. (2025). Electrical stimulation in bone healing: critical analysis by evaluating levels of evidence.
Gryshkov, O., AL Halabi, F., Kuhn, A. I., Leal-Marin, S., Freund, L. J., Förthmann, M., et al. (2021). PVDF and P(VDF-TrFE) electrospun scaffolds for nerve graft engineering: a comparative study on piezoelectric and structural properties, and in vitro biocompatibility. IJMS 22, 11373. doi:10.3390/ijms222111373
Heng, B. C., Bai, Y., Li, X., Meng, Y., Zhang, X., and Deng, X. (2021). Signaling pathways implicated in enhanced stem/progenitor cell differentiation on electroactive scaffolds. Smart. Mater. Med. 3, 4–11. doi:10.1016/j.smaim.2021.11.003
Huang, H., Wang, K., Liu, X., Liu, X., Wang, J., Suo, M., et al. (2025). Piezoelectric biomaterials for providing electrical stimulation in bone tissue engineering: barium titanate. J. Orthop. Transl. 51, 94–107. doi:10.1016/j.jot.2024.12.011
Huttenlocher, A., and Horwitz, A. R. (2011). Integrins in cell migration. Cold Spring Harb. Perspect. Biol. 3, a005074. doi:10.1101/cshperspect.a005074
Iwamoto, D. V., and Calderwood, D. A. (2015). Regulation of integrin-mediated adhesions. Curr. Opin. Cell Biol. 36, 41–47. doi:10.1016/j.ceb.2015.06.009
Kamel, N. A. (2022). Bio-piezoelectricity: fundamentals and applications in tissue engineering and regenerative medicine. Biophys. Rev. 14, 717–733. doi:10.1007/s12551-022-00969-z
Kapat, K., Shubhra, Q. T. H., Zhou, M., and Leeuwenburgh, S. (2020). Piezoelectric nano-biomaterials for biomedicine and tissue regeneration. Adv. Funct. Mater. 30, 1909045. doi:10.1002/adfm.201909045
Katoh, K. (2024). Signal transduction mechanisms of focal adhesions: src and FAK-mediated cell response. Front. Biosci. Landmark 29, 392. doi:10.31083/j.fbl2911392
Kim, H.-S., Baby, T., Lee, J.-H., Shin, U. S., and Kim, H.-W. (2024). Biomaterials-enabled electrical stimulation for tissue healing and regeneration. Med-X 2, 7. doi:10.1007/s44258-024-00020-8
Kwon, J., and Cho, H. (2022). Collagen piezoelectricity in osteogenesis imperfecta and its role in intrafibrillar mineralization. Commun. Biol. 5, 1229. doi:10.1038/s42003-022-04204-z
Kwon, H. J., Lee, G. S., and Chun, H. (2016). Electrical stimulation drives chondrogenesis of mesenchymal stem cells in the absence of exogenous growth factors. Sci. Rep. 6, 39302. doi:10.1038/srep39302
Lay, R., Deijs, G. S., and Malmström, J. (2021). The intrinsic piezoelectric properties of materials – a review with a focus on biological materials. RSC Adv. 11, 30657–30673. doi:10.1039/d1ra03557f
Lehne, F., Pokrant, T., Parbin, S., Salinas, G., Großhans, J., Rust, K., et al. (2022). Calcium bursts allow rapid reorganization of EFhD2/Swip-1 cross-linked actin networks in epithelial wound closure. Nat. Commun. 13, 2492. doi:10.1038/s41467-022-30167-0
Levin, M. (2021). Bioelectric signaling: reprogrammable circuits underlying embryogenesis, regeneration, and cancer. Cell 184, 1971–1989. doi:10.1016/j.cell.2021.02.034
Li, Y., Liao, C., and Tjong, S. C. (2019). Electrospun polyvinylidene fluoride-based fibrous scaffolds with piezoelectric characteristics for bone and neural tissue engineering. Nanomater. (Basel). 9, 952. doi:10.3390/nano9070952
Li, J., Long, Y., Yang, F., and Wang, X. (2020). Degradable piezoelectric biomaterials for wearable and implantable bioelectronics. Curr. Opin. Solid. State. Mater. Sci. 24, 100806. doi:10.1016/j.cossms.2020.100806
Lin, B., Tsao, S., Chen, A., Hu, S.-K., Chao, L., and Chao, P. G. (2017). Lipid rafts sense and direct electric field-induced migration. Proc. Natl. Acad. Sci. U. S. A. 114, 8568–8573. doi:10.1073/pnas.1702526114
Liu, Z., Cai, M., Zhang, X., Yu, X., Wang, S., Wan, X., et al. (2021). Cell-traction-triggered on-demand electrical stimulation for neuron-like differentiation. Adv. Mater. 33, e2106317. doi:10.1002/adma.202106317
Liu, Y., Dzidotor, G., Le, T. T., Vinikoor, T., Morgan, K., Curry, E. J., et al. (2022). Exercise-induced piezoelectric stimulation for cartilage regeneration in rabbits. Sci. Transl. Med. 14, eabi7282. doi:10.1126/scitranslmed.abi7282
Liu, L., Li, Z., Zhu, T., Sun, Y., and Xu, J. (2025). Advances in applications of low-dimensional piezoelectric materials in musculoskeletal system. Mater. Today. bio. 33, 102065. doi:10.1016/j.mtbio.2025.102065
Low, Y. K. A., Zou, X., Fang, Y. M., Wang, J. L., Lin, W. S., Boey, F. Y. C., et al. (2013). β-Phase poly(vinylidene fluoride) films encouraged more homogeneous cell distribution and more significant deposition of fibronectin towards the cell–material interface compared to α-phase poly(vinylidene fluoride) films. Mater. Sci. Eng. C 34, 345–353. doi:10.1016/j.msec.2013.09.029
Ma, B., Liu, F., Li, Z., Duan, J., Kong, Y., Hao, M., et al. (2019). Piezoelectric nylon-11 nanoparticles with ultrasound assistance for high-efficiency promotion of stem cell osteogenic differentiation. J. Mater. Chem. B 7, 1847–1854. doi:10.1039/c8tb03321h
Mao, A. S., and Mooney, D. J. (2015). Regenerative medicine: current therapies and future directions. Proc. Natl. Acad. Sci. U.S.A. 112, 14452–14459. doi:10.1073/pnas.1508520112
Mohammadpourfazeli, S., Arash, S., Ansari, A., Yang, S., Mallick, K., and Bagherzadeh, R. (2023). Future prospects and recent developments of polyvinylidene fluoride (PVDF) piezoelectric polymer; fabrication methods, structure, and electro-mechanical properties. RSC Adv. 13, 370–387. doi:10.1039/d2ra06774a
Mokhtari, F., Azimi, B., Salehi, M., Hashemikia, S., and Danti, S. (2021). Recent advances of polymer-based piezoelectric composites for biomedical applications. J. Mech. Behav. Biomed. Mater 122, 104669. doi:10.1016/j.jmbbm.2021.104669
Mueller, C., Trujillo-Miranda, M., Maier, M., Heath, D. E., O’Connor, A. J., and Salehi, S. (2020). Effects of external stimulators on engineered skeletal muscle tissue maturation. Adv. Mater. Interfaces 8, 2001167. doi:10.1002/admi.202001167
Ni, X., Cui, Y., Salehi, M., Nai, M. L. S., Zhou, K., Vyas, C., et al. (2025). Piezoelectric biomaterials for bone regeneration: roadmap from dipole to osteogenesis. Adv. Sci. 12, e14969. doi:10.1002/advs.202414969
Purushothaman, S. M., Tronco, M. F., Kottathodi, B., Royaud, I., Ponçot, M., Kalarikkal, N., et al. (2023). A review on electrospun PVDF-Based nanocomposites: recent trends and developments in energy harvesting and sensing applications. Polymer 283, 126179. doi:10.1016/j.polymer.2023.126179
Ren, K., Shen, Y., and Wang, Z. L. (2023). Piezoelectric properties of electrospun polymer nanofibers and related energy harvesting applications. Macromol. Mater. Eng. 309, 2300307. doi:10.1002/mame.202300307
Ribeiro, C., Panadero, J. A., Sencadas, V., Lanceros-Méndez, S., Tamaño, M. N., Moratal, D., et al. (2012). Fibronectin adsorption and cell response on electroactive poly(vinylidene fluoride) films. Biomed. Mater. 7, 035004. doi:10.1088/1748-6041/7/3/035004
Ricotti, L., Cafarelli, A., Manferdini, C., Trucco, D., Vannozzi, L., Gabusi, E., et al. (2024). Ultrasound stimulation of piezoelectric nanocomposite hydrogels boosts chondrogenic differentiation in vitro,, in both a normal and inflammatory milieu. ACS Nano 18, 2047–2065. doi:10.1021/acsnano.3c08738
Ridley, A. J., Schwartz, M. A., Burridge, K., Firtel, R. A., Ginsberg, M. H., Borisy, G., et al. (2003). Cell migration: integrating signals from front to back. Science 302, 1704–1709. doi:10.1126/science.1092053
Ronaldson-Bouchard, K., Ma, S. P., Yeager, K., Chen, T., Song, L., Sirabella, D., et al. (2018). Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 556, 239–243. doi:10.1038/s41586-018-0016-3
Schönlein, R., Bhattarai, P., Raef, M., Larrañaga, X., Poudel, A., Biggs, M., et al. (2025). Piezoelectric polylactic acid-based biomaterials: fundamentals, challenges and opportunities in medical device design. Biomaterials 324, 123522. doi:10.1016/j.biomaterials.2025.123522
Shi, G., Su, T., Li, J., Wang, A., Gao, G., Tao, B., et al. (2025). Biomimetic piezoelectric hydrogel system for energy metabolism reprogramming in spinal cord injury repair. Theranostics 15, 4955–4969. doi:10.7150/thno.108329
Shlapakova, L. E., Surmeneva, M. A., Kholkin, A. L., and Surmenev, R. A. (2024). Revealing an important role of piezoelectric polymers in nervous-tissue regeneration: a review. Mater. Today. Bio 25, 100950. doi:10.1016/j.mtbio.2024.100950
Smith, M., and Kar-Narayan, S. (2021). Piezoelectric polymers: theory, challenges and opportunities. Int. Mater. Rev. 67, 65–88. doi:10.1080/09506608.2021.1915935
Sun, L., Yang, W., Xie, S., Xi, X., Song, A., Li, G., et al. (2025). Piezoelectric iridium-doped bismuth ferrite/sodium alginate hydrogel for antibiosis and stimulating osteoblastic differentiation. ACS Appl. Nano Mater. 8, 6782–6796. doi:10.1021/acsanm.5c01503
Tai, G., Tai, M., and Zhao, M. (2018). Electrically stimulated cell migration and its contribution to wound healing. Burns Trauma 6, 20. doi:10.1186/s41038-018-0123-2
Tai, Y., Tonmoy, T. I., Win, S., Brinkley, N. T., Park, B. H., and Nam, J. (2023). Enhanced peripheral nerve regeneration by mechano-electrical stimulation. npj Regen. Med. 8, 57. doi:10.1038/s41536-023-00334-y
Tan, X., Yan, Y., Song, B., Zhu, S., Mei, Q., and Wu, K. (2023). Focal adhesion kinase: from biological functions to therapeutic strategies. Exp. Hematol. Oncol. 12, 83. doi:10.1186/s40164-023-00446-7
Tandon, B., Blaker, J. J., and Cartmell, S. H. (2018). Piezoelectric materials as stimulatory biomedical materials and scaffolds for bone repair. Acta Biomater. 73, 1–20. doi:10.1016/j.actbio.2018.04.026
Tandon, B., Cosme, J. R. A., Xue, R., Srirussamee, K., Aguilar-Tadeo, J., Ballestrem, C., et al. (2025). Co-stimulation with piezoelectric PVDF films and low intensity pulsed ultrasound enhances osteogenic differentiation. Biomater. Adv. 173, 214283. doi:10.1016/j.bioadv.2025.214283
Tsai, F.-C., Kuo, G.-H., Chang, S.-W., and Tsai, P.-J. (2015). Ca2+Signaling in cytoskeletal reorganization, cell migration, and cancer metastasis. Biomed. Res. Int. 2015, 409245–13. doi:10.1155/2015/409245
Tsai, H.-F., Ijspeert, C., and Shen, A. Q. (2020). Voltage-gated ion channels mediate the electrotaxis of glioblastoma cells in a hybrid PMMA/PDMS microdevice. Apl. Bioeng. 4, 036102. doi:10.1063/5.0004893
Velling, T., Nilsson, S., Stefansson, A., and Johansson, S. (2004). beta1-Integrins induce phosphorylation of akt on serine 473 independently of focal adhesion kinase and src family kinases. EMBO Rep. 5, 901–905. doi:10.1038/sj.embor.7400234
Vinikoor, T., Dzidotor, G. K., Le, T. T., Liu, Y., Kan, H.-M., Barui, S., et al. (2023). Injectable and biodegradable piezoelectric hydrogel for osteoarthritis treatment. Nat. Commun. 14, 6257. doi:10.1038/s41467-023-41594-y
Wang, X., Stefanello, S. T., Shahin, V., and Qian, Y. (2025). From mechanoelectric conversion to tissue regeneration: translational progress in piezoelectric materials. Adv. Mater. 37, e2417564. doi:10.1002/adma.202417564
Westphal, J. A., Bryan, A. E., Krutko, M., Esfandiari, L., Schutte, S. C., and Harris, G. M. (2023). Innervation of an ultrasound-mediated PVDF-TrFE scaffold for skin-tissue engineering. Biomimetics 9, 2. doi:10.3390/biomimetics9010002
Wozniak, M. A., and Chen, C. S. (2008). Mechanotransduction in development: a growing role for contractility. Nat. Rev. Mol. Cell Biol. 10, 34–43. doi:10.1038/nrm2592
Wu, J., Chen, T., Wang, Y., Bai, J., Lao, C., Luo, M., et al. (2022). Piezoelectric effect of antibacterial biomimetic hydrogel promotes osteochondral defect repair. Biomedicines 10, 1165. doi:10.3390/biomedicines10051165
Wu, Y., Zou, J., Tang, K., Xia, Y., Wang, X., Song, L., et al. (2024). From electricity to vitality: the emerging use of piezoelectric materials in tissue regeneration. Burns Trauma 12, tkae013. doi:10.1093/burnst/tkae013
Wu, Q., Chen, H., Huang, B., Hu, W., Wang, Y., Li, S., et al. (2025). Copper-doped barium titanate coating: a piezoelectric match to natural bone for enhanced osteogenesis. ACS Appl. Mater. Interfaces 17, 37548–37561. doi:10.1021/acsami.5c04997
Wu W., W., Yang, H., Li, T., Xie, Y., Huang, G., and Zhang, W. (2025). Conductive and piezoelectric biomaterials: a comprehensive review of load-bearing soft tissue repair. Biomater. Sci. 13, 3755–3771. doi:10.1039/d5bm00368g
Xia, G., Song, B., and Fang, J. (2022). Electrical stimulation enabled via electrospun piezoelectric polymeric nanofibers for tissue regeneration. 9896274. doi:10.34133/2022/9896274
Xiang, X.-W., Liu, H.-T., Liu, W., Yan, Z.-Y., Zeng, Y.-L., Wang, Y.-J., et al. (2023). Revolutionizing wound healing: ultrashort pulse electric fields in seconds for highly aligned extracellular matrix and efficient cell migration. Chem. Eng. J. 471, 144267. doi:10.1016/j.cej.2023.144267
Yamaguchi, N., and Knaut, H. (2022). Focal adhesion-mediated cell anchoring and migration: fromin vitrotoin vivo. Development 149, dev200647. doi:10.1242/dev.200647
Zaszczyńska, A., Zabielski, K., Gradys, A., Kowalczyk, T., and Sajkiewicz, P. (2024). Piezoelectric scaffolds as smart materials for bone tissue engineering. Polymers 16, 2797. doi:10.3390/polym16192797
Zhang, J., Liu, C., Li, J., Yu, T., Ruan, J., and Yang, F. (2024). Advanced piezoelectric materials, devices, and systems for orthopedic medicine. Adv. Sci. 12, e2410400. doi:10.1002/advs.202410400
Zhang, S., Huang, L., Chen, W., Chen, Q., Liu, X., Su, D., et al. (2025). Piezoelectric hydrogel with self-powered biomechanical stimulation enhances bone regeneration. Acta Biomater. 195, 117–133. doi:10.1016/j.actbio.2025.02.016
Zhao, M. (2008). Electrical fields in wound healing-An overriding signal that directs cell migration. Semin. Cell Dev. Biol. 20, 674–682. doi:10.1016/j.semcdb.2008.12.009
Zhao, M., Song, B., Pu, J., Wada, T., Reid, B., Tai, G., et al. (2006). Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-γ and PTEN. Nature 442, 457–460. doi:10.1038/nature04925
Zheng, T., Yu, Y., Pang, Y., Zhang, D., Wang, Y., Zhao, H., et al. (2022). Improving bone regeneration with composites consisting of piezoelectric poly(l-lactide) and piezoelectric calcium/manganese co-doped barium titanate nanofibers. Compos. Part B Eng. 234, 109734. doi:10.1016/j.compositesb.2022.109734
Zheng, Y., Wang, S., Jin, W., Li, Z., Yang, G., Li, X., et al. (2025). An ultrasound-driven PLGA/Zn-KNN hybrid piezoelectric scaffold with direct and immunoregulatory antibacterial activity for bone infection. Bioact. Mater. 47, 295–312. doi:10.1016/j.bioactmat.2025.01.026
Zhou, Z., Li, W., He, T., Qian, L., Tan, G., and Ning, C. (2016). Polarization of an electroactive functional film on titanium for inducing osteogenic differentiation. Sci. Rep. 6, 35512. doi:10.1038/srep35512
Zhu, K., Li, R., Yin, S., Yang, F., Sun, Y., Xing, Y., et al. (2024). A novel ultrasound-driven piezoelectric GBR membrane dispersed with boron nitride nanotubes promotes bone regeneration and anti-bacterial properties. Mater. Today. Bio 30, 101418. doi:10.1016/j.mtbio.2024.101418
Keywords: piezoelectric biomaterials, stem cells, cell adhesion, cell migration, tissue regeneration, bioelectric stimulation
Citation: Chen N, Su Y, Zhao R and Deng X (2025) Bioelectric cues from piezoelectric materials in stem-cell adhesion and migration. Front. Cell Dev. Biol. 13:1707436. doi: 10.3389/fcell.2025.1707436
Received: 17 September 2025; Accepted: 20 October 2025;
Published: 06 November 2025.
Edited by:
Bo Zhang, University of California, Los Angeles, United StatesReviewed by:
Devin Wang, Wuhan University, ChinaCopyright © 2025 Chen, Su, Zhao and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Renliang Zhao, enJsZGNkQDE2My5jb20=; Xiangtian Deng, d2QxNTgwMjgyODgxMEBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Ning Chen1†
Ning Chen1† Yangyang Su
Yangyang Su Renliang Zhao
Renliang Zhao Xiangtian Deng
Xiangtian Deng