- 1Department of Vascular Surgery, Taizhou Second People’s Hospital Affiliated to Yangzhou University, Taizhou, Jiangsu, China
- 2Department of Cardiology, Taizhou Second People’s Hospital Affiliated to Yangzhou University, Taizhou, Jiangsu, China
- 3Operating room, Suzhou BenQ Medical Center, Suzhou, Jiangsu, China
- 4Department of Vascular Surgery, The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou, Jiangsu, China
Abdominal aortic aneurysm (AAA) is often asymptomatic in its early stages, and rupture poses a life threatening risk. Currently, no effective pharmacological therapies are available, underscoring the importance of mechanistic research. Metabolic reprogramming—an adaptive process encompassing glucose, lipid, and amino acid metabolism—has increasingly gained attention in the context of AAA. These metabolic shifts, which coordinate cellular energy supply, biosynthesis, and signaling, critically shape vascular smooth muscle cell (VSMC) behavior, macrophage polarization, extracellular matrix remodeling, oxidative stress responses, and immune activation. Importantly, growing evidence suggests that crosstalk among these metabolic pathways orchestrates complex pathophysiological networks driving AAA initiation and progression. Exploring AAA pathogenesis from an integrated metabolic perspective not only helps elucidate underlying mechanisms but also provides new insights and potential therapeutic targets.
1 Introduction
Aneurysms, particularly abdominal aortic aneurysms (AAA), are common cardiovascular disorders characterized by localized dilation of the aortic wall. When persistent, they may progress to rupture, posing a life-threatening risk (Wanhainen et al., 2019). AAAs are typically asymptomatic, yet rupture is frequently fatal if surgical repair cannot be achieved in time. It has been reported that, in 2017, AAA accounted for approximately 167,200 deaths worldwide, with an estimated 3 million disability-adjusted life years (DALYs) lost (Wei et al., 2021). Each year, rupture of AAA leads to about 8,000 deaths in the United Kingdom and approximately 15,000 deaths in the United States. The disease is more prevalent in men, with an estimated prevalence of 1.3%–8.9%, compared to 1.0%–2.2% in women. The overall mortality of ruptured AAA ranges from 65% to 85%, with nearly half of the deaths occurring before patients reach the operating room (Sakalihasan et al., 2005). Most AAAs are nonspecific, with no clearly defined cause (Johnston et al., 1991). A minority of aneurysms have established etiologies, secondary to conditions such as atherosclerotic disease, trauma, connective tissue disorders (e.g., Marfan syndrome, Ehlers–Danlos syndrome type IV), infectious diseases (e.g., tuberculosis, syphilis, bacterial, or fungal infections), and inflammatory diseases (Sakalihasan et al., 2005). Although the mechanisms underlying aneurysm formation have been extensively investigated over the past decades, their exact pathophysiological processes remain incompletely understood.
Accumulating evidence suggests that metabolic reprogramming contributes to the onset and progression of cardiovascular diseases (Chiong et al., 2014; Hall et al., 2001). Metabolic reprogramming refers to the process by which cells alter their metabolic patterns to meet bioenergetic and biosynthetic demands, thereby promoting survival, proliferation, and growth. This encompasses glucose, lipid, and amino acid metabolism. Intracellular metabolic regulation of vascular smooth muscle cells (VSMCs) has been implicated in the pathogenesis of atherosclerosis, systemic hypertension, diabetes, pulmonary hypertension, vascular calcification, and aneurysm formation (Shi et al., 2020). In response to distinct stimuli, macrophages undergo a spectrum of transcriptional and proteomic changes that correspond to different phenotypic states. Classically activated macrophages (M1) exhibit a pro-inflammatory phenotype that accelerates AAA development by inducing inflammatory responses, secreting matrix metalloproteinases (MMPs) that promote extracellular matrix (ECM) degradation, upregulating peroxidase expression, and enhancing oxidative stress (Raffort et al., 2017). Collectively, emerging studies indicate that these metabolic alterations, acting through multicellular and multipathway mechanisms, critically modulate AAA progression. This provides multiple potential therapeutic targets and novel strategies for clinical intervention. Accordingly, this review focuses on recent advances regarding the pathological roles of metabolic reprogramming in the initiation and progression of AAA.
2 Pathophysiological basis of abdominal aortic aneurysm
The development of abdominal aortic aneurysm (AAA) is closely associated with alterations in the connective tissue of the aortic wall, in which elastic fibers and fibrillar collagens are the principal determinants of aortic mechanical properties (Melrose et al., 1998). Elastin and its associated proteins form an elastic fiber network that imparts viscoelasticity to the arterial wall; while intermolecular cross-links maintain structural stability, these fibers are simultaneously susceptible to degradation by elastolytic proteases. Elastic fibers are predominantly localized within the medial layer of the aorta, closely integrated with vascular smooth muscle cells (VSMCs), whereas collagens are abundantly distributed in both the medial and adventitial layers. Type I and type III collagens constitute the major components, conferring tensile strength and preserving vascular structural integrity. A hallmark pathological feature of AAA tissue is the fragmentation of elastic fibers and the depletion of elastin, a process that typically arises during the early stages of aneurysm expansion and continues up to rupture (Baxter et al., 1992; Sakalihasan et al., 1993). Loss of elastin represents an early event in aneurysm formation, while collagen degradation is a critical factor contributing to rupture. Matrix metalloproteinases (MMPs) play a central role in AAA progression; their excessive activation, combined with an imbalance against antiproteases, markedly accelerates vascular wall degradation, thereby promoting aneurysmal enlargement and rupture (Rao et al., 1996; Eriksson et al., 2004). In parallel, VSMCs exert dual functions during vascular remodeling: they not only synthesize various extracellular matrix proteins but also secrete proteases, thereby participating in the dynamic regulation of aortic structure (Lopez-Candales et al., 1997). Moreover, aneurysm progression is closely linked to intraluminal thrombus (ILT) formation. The processes of thrombus development and resolution can induce local hypoxia and trigger inflammatory responses, further driving aneurysm progression (Wang et al., 2014). Collectively, degradation of elastin and collagen, alterations in VSMC function, and the formation and remodeling of intraluminal thrombus represent the core mechanisms underlying the initiation and progression of abdominal aortic aneurysm.
3 Overview of metabolic reprogramming
Metabolic reprogramming refers to the process by which cells adapt to varying physiological or pathological conditions by altering their metabolic pathways to meet new demands. For example, in tumor cells, glucose metabolism is often shifted toward aerobic glycolysis—known as the Warburg effect—to support rapid proliferation (Wang et al., 2014). Similarly, immune cells, endothelial cells, and vascular smooth muscle cells undergo comparable metabolic reprogramming in response to different environmental stresses. Metabolic reprogramming not only influences cellular energy supply but also regulates cell function. Alterations in fatty acid metabolism, amino acid metabolism, and redox balance are closely associated with biological processes such as proliferation, migration, inflammatory responses, and apoptosis (Chen et al., 2024). Increasing evidence indicates that metabolic reprogramming plays a critical role in cardiovascular diseases, including atherosclerosis and heart failure.
4 Role of metabolic reprogramming in abdominal aortic aneurysm
4.1 Glucose metabolism
Genomic analyses have revealed that a prominent feature in patients with abdominal aortic aneurysm (AAA) as well as in the angiotensin II (Ang II) experimental model is metabolic reprogramming, characterized by enhanced glycolysis and suppressed glucose oxidative phosphorylation. Imaging studies have further demonstrated increased GLUT-mediated ^18F-fluorodeoxyglucose (^18F-FDG) uptake in AAA tissues, indicating elevated glucose metabolic activity within the lesions. Subsequent investigations showed that the glycolytic inhibitor 2-deoxy-D-glucose (2-DG) attenuates CaCl2-induced aortic dilation and reduces aneurysm formation in the Ang II model. This metabolic shift promotes the initiation and progression of AAA primarily by modulating the physiological and pathological functions of vascular smooth muscle cells and macrophages (Tsuruda et al., 2012).
4.1.1 Vascular smooth muscle cells
In the pathogenesis and progression of abdominal aortic aneurysm (AAA), glucose metabolic reprogramming is recognized as one of the key molecular features. Vascular smooth muscle cells (VSMCs) represent the principal effector cells of this process, with glucose uptake primarily dependent on GLUT1 (Hruz and Mueckler, 2001) and regulated by signaling pathways such as Akt/mTOR (Lin et al., 2013). By promoting glycolysis and the tricarboxylic acid (TCA) cycle, this reprogramming accelerates glucose flux, thereby enhancing cellular proliferation and resistance to apoptosis. A phenomenon analogous to the Warburg effect in cancer cells has also been observed in VSMCs, wherein aerobic glycolysis is favored even under normoxic conditions (Warburg, 1956; Chen et al., 2018). Although energetically less efficient, this metabolic mode provides rapid ATP generation and abundant biosynthetic intermediates, thereby supporting cell proliferation, migration, and phenotypic switching (Yang et al., 2010; Pfeiffer et al., 2001). Within this context, key glycolytic enzymes (Jia et al., 2022; Jain et al., 2021) and lactate metabolism (Perez et al., 2010; Zhao et al., 2020) play central roles. Enhanced glycolysis mediated by pyruvate kinase isoform M2 (PKM2), together with lactate accumulation and altered transport, reshapes the intra- and extracellular metabolic milieu and further modulates VSMC phenotypic transformation and matrix remodeling via signaling pathways (Zhou et al., 2019; Zhang et al., 2022). Simultaneously, alterations in the pyruvate dehydrogenase kinase (PDK)/pyruvate dehydrogenase (PDH) balance redistribute glucose utilization between oxidative phosphorylation and glycolysis (Roche et al., 2001). In particular, upregulation of PDK4 has been linked not only to metabolic dysregulation (Zhu et al., 2018) but also to vascular calcification and impaired autophagy. Moreover, TCA cycle intermediates such as α-ketoglutarate (α-KG) have been proposed to exert protective effects through their antioxidant (Tian et al., 2020) and anti-inflammatory (Asadi Shahmirzadi et al., 2020) properties, mitigating AAA progression by reducing reactive oxygen species generation (Liu et al., 2022). In addition, diversion of glucose into the pentose phosphate pathway (PPP) supplies NADPH and nucleotides that support anabolic biosynthesis and antioxidative defense, thereby contributing to the survival and functional stability of VSMCs (Alamri et al., 2018; Ruiz-Ramírez et al., 2014; Dong et al., 2015). Overall, from glucose uptake to glycolysis, lactate metabolism, PDK/PDH regulation, PPP flux, and TCA cycle intermediates, glucose metabolic reprogramming shapes the functional state of VSMCs through multilayered mechanisms. These metabolic alterations not only drive the pathology of AAA but also highlight new directions for metabolic interventions and potential therapeutic targets.
4.1.2 Macrophages
In the pathophysiology of abdominal aortic aneurysm (AAA), macrophage infiltration and polarization are considered pivotal events (Song et al., 2022; Dale et al., 2016; Batra et al., 2018). Classically activated M1 macrophages predominantly rely on glycolytic metabolism (Odegaard and Chawla, 2011). By enhancing glucose uptake, increasing lactate production, and elevating reactive oxygen species (ROS) generation (Fukuzumi et al., 1996), they drive inflammatory responses (Freemerman et al., 2014), extracellular matrix degradation, and oxidative stress, thereby accelerating structural injury of the aortic wall. In contrast, alternatively activated M2 macrophages preferentially utilize oxidative metabolism and exhibit anti-inflammatory and tissue-reparative properties. Their upregulation is regarded as a compensatory mechanism that helps restrain AAA expansion and rupture (Rateri et al., 2011). Metabolic reprogramming plays a central role in macrophage polarization. Upregulation of GLUT1 and regulation of the glycolytic enzyme pyruvate kinase isoform M2 (PKM2) (Gao et al., 2012) not only fuel proinflammatory cytokine production but also sustain the M1 phenotype through signaling pathways such as HIF-1 and STAT3. Conversely, downregulation of lactate dehydrogenase A (LDHA) can reduce lactate levels and attenuate inflammatory responses (Song et al., 2019). Tricarboxylic acid (TCA) cycle intermediates also exert bidirectional regulatory effects: for instance, succinate promotes proinflammatory cytokine expression, whereas α-ketoglutarate (α-KG) supports M2 polarization and anti-inflammatory gene expression through epigenetic remodeling (Liu et al., 2017). Although current pharmacological studies targeting glucose metabolism are primarily focused on oncology (Yang et al., 2021; Pelicano et al., 2006; Anderson et al., 2018), accumulating preclinical evidence suggests that targeting glycolysis, the TCA cycle, and oxidative phosphorylation may represent promising strategies for AAA intervention. Advancing this area of research will not only help elucidate the crosstalk between metabolism and immunity but also provide novel therapeutic avenues and potential targets for the prevention and treatment of AAA.
Recent studies further emphasize that macrophage polarization is not solely determined by single metabolic cues but by the integrated influence of glucose, fatty acid, and amino acid metabolism. For instance, succinate and α-ketoglutarate jointly regulate pro- and anti-inflammatory transcriptional programs, while fatty acid oxidation modulates HIF-1α activity and ROS production, creating a metabolic–immune feedback loop that sustains inflammatory microenvironments.
4.2 Lipid metabolism
Recent studies have demonstrated that long-chain polyunsaturated fatty acids (LCPUFAs), particularly ω-3 fatty acids such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are closely associated with the development and progression of abdominal aortic aneurysm (AAA) (Meital et al., 2019). Since humans cannot synthesize polyunsaturated fatty acids, their levels primarily depend on dietary intake. Clinical and randomized controlled trials have reported reduced EPA levels in patients with AAA, with both absolute EPA concentration and the EPA/arachidonic acid (ARA) ratio showing significant inverse correlations with aneurysm diameter and growth rate (Aikawa et al., 2017). Mechanistically, dietary supplementation with EPA and DHA enriches cell membrane phospholipids with ω-3 fatty acids, reduces the generation of ARA and its pro-inflammatory metabolites (e.g., PGE2, TXA2, LTB4), and suppresses macrophage-mediated inflammatory responses, thereby exerting protective effects against AAA progression (Yoshihara et al., 2015). Conversely, ARA, as an ω-6 fatty acid, aggravates disease through its pro-inflammatory actions (Ricciotti and FitzGerald, 2011; Soto et al., 2018). Animal studies further show that inhibition of the COX pathway improves the structural integrity of vascular elastin and downregulates matrix metalloproteinase (MMP) expression (Guo et al., 2013). Observational studies suggest that low-dose aspirin may slow the growth of medium-sized AAAs; however, no randomized controlled trials have yet confirmed the efficacy of any pharmacological agent in stabilizing or halting AAA expansion (Lindholt et al., 2008). Overall, the anti-inflammatory and immunomodulatory properties of LCPUFAs provide novel insights into the prevention and treatment of AAA, though further high-quality evidence is required to support clinical application. At present, the role of fatty acid metabolism in AAA pathogenesis remains incompletely understood. Metabolomic analyses indicate aberrant lipid metabolism in AAA tissues (Xie et al., 2023; Zhang et al., 2023), and elevated serum platelet-derived growth factor (PDGF) levels in patients can promote fatty acid oxidation in vascular smooth muscle cells (VSMCs) by upregulating carnitine palmitoyl transferase 1 (CPT1). This enhances cell proliferation and inhibits apoptosis-related pathways, suggesting that fatty acid oxidation may contribute to vascular remodeling (Yuwen et al., 2019). In macrophages, however, the role of fatty acid oxidation is controversial: some studies suggest that it suppresses inflammation and lipid accumulation (Malandrino et al., 2015; Namgaladze et al., 2014), while others indicate that CPT deficiency may confer anti-inflammatory and anti-atherogenic effects (Nomura et al., 2019). In addition, fatty acid biosynthesis has attracted attention in the context of VSMC phenotypic switching. In synthetic VSMCs, fatty acid synthase is upregulated, and altered activity of stearoyl-CoA desaturase 1 (SCD1) affects lipid composition and vascular metabolic homeostasis.
4.3 Amino acid metabolism
4.3.1 Sulfur-containing amino acids
Cysteine (Cys), methionine (Met), and their metabolic derivative homocysteine (Hcy) play important roles in the context of abdominal aortic aneurysm (AAA) (Zaric et al., 2019). Hyperhomocysteinemia accelerates vascular wall degradation and aneurysmal expansion by increasing reactive oxygen species (ROS) generation, depleting nitric oxide (NO), upregulating matrix metalloproteinase (MMP) activity, and promoting the phenotypic transition of vascular smooth muscle cells (VSMCs) from a contractile to a synthetic state (Steed and Tyagi, 2011). Clinically, folate, vitamins B6/B12, and methionine-restricted diets have been shown to reduce Hcy levels, suggesting their potential for therapeutic intervention (Warsi et al., 2004). In addition, novel agents such as cystathionine β-synthase (CBS) modifiers (e.g., OT-58) hold promise as future targeted strategies (Bublil and Majtan, 2020).
4.3.2 Tryptophan
Tryptophan (Trp) metabolism through the kynurenine pathway (KP) is upregulated in abdominal aortic aneurysm (AAA) tissues, with increased expression of key enzymes and metabolites (Nishimura et al., 2021). Indoleamine 2,3-dioxygenase (IDO)-mediated Trp metabolism promotes inflammation, matrix metalloproteinase (MMP) expression (Wang et al., 2017), and apoptosis, whereas its metabolite 5-methoxytryptophan (5-MTP) exhibits vasoprotective and anti-inflammatory effects (Wu et al., 2020; Yang et al., 2015). Animal studies have demonstrated that inhibition of the KP can delay AAA formation, and IDO inhibitors have already been applied in other diseases (Watanabe et al., 2018); however, clinical validation in vascular disorders remains lacking (Song et al., 2017; Ramprasath et al., 2021). Notably, KP metabolism is closely associated with the aging process, which aligns with the strong age dependence of AAA, underscoring its potential significance for future research.
4.3.3 Taurine
Taurine (Tau) exerts significant vasoprotective effects through its antioxidant activity, scavenging of oxidants such as hypochlorous acid (HClO), reduction of inflammatory cell infiltration, and inhibition of matrix metalloproteinase (MMP) activity (Bkaily et al., 2020; Kim et al., 2017; Chao de la Barca et al., 2022). Animal studies have demonstrated that taurine supplementation effectively suppresses angiotensin II (Ang II)-induced AAA formation (Brethel et al., 2023). Moreover, its regulatory roles in VSMC migration, anti-apoptotic responses, and anti-calcification suggest its involvement in maintaining vascular wall homeostasis (Korshunov et al., 2006). However, clinical data in patients with AAA are currently lacking, and its protective effects remain to be further validated.
4.3.4 Glycine
Glycine (Gly) plays an important role in antioxidant and anti-inflammatory processes by restoring glutathione synthesis, inhibiting reactive oxygen species (ROS) production, and suppressing NF-κB activation, thereby alleviating vascular inflammation (Ruiz-Ramírez et al., 2014; Chao de la Barca et al., 2022). Metabolomic analyses have revealed decreased serum glycine levels in AAA models, which may diminish its protective effects. In addition, glycine has been shown to reduce blood lipid levels (Rom et al., 2017), thereby mitigating the adverse impact of hyperlipidemia on AAA. However, its protective role has not yet been clinically validated.
4.3.5 Glutamine and glutamate
Glutamine (Gln) serves as a critical substrate for cellular proliferation. It is transported into cells via the high-affinity L-Gln transporter solute carrier family 1 member 5 (SLC1A5), where it activates the mTORC1 signaling pathway and promotes the proliferation of vascular smooth muscle cells (VSMCs) (Osman et al., 2019). Both glutamate and glutamine also contribute to nitric oxide (NO) synthesis, with NO exerting protective effects by maintaining extracellular matrix homeostasis and promoting vasodilation (Zhang et al., 2003; Zhou et al., 2023). However, under conditions of metabolic dysregulation, excessive NO may induce vascular injury, suggesting a bidirectional role. Clinical studies have shown that glutamine supplementation can improve nitrogen balance and immune status following aortic surgery; nevertheless, its potential role in the prevention or treatment of AAA requires further investigation (Brinkmann et al., 2016).
4.3.6 Branched-chain amino acids
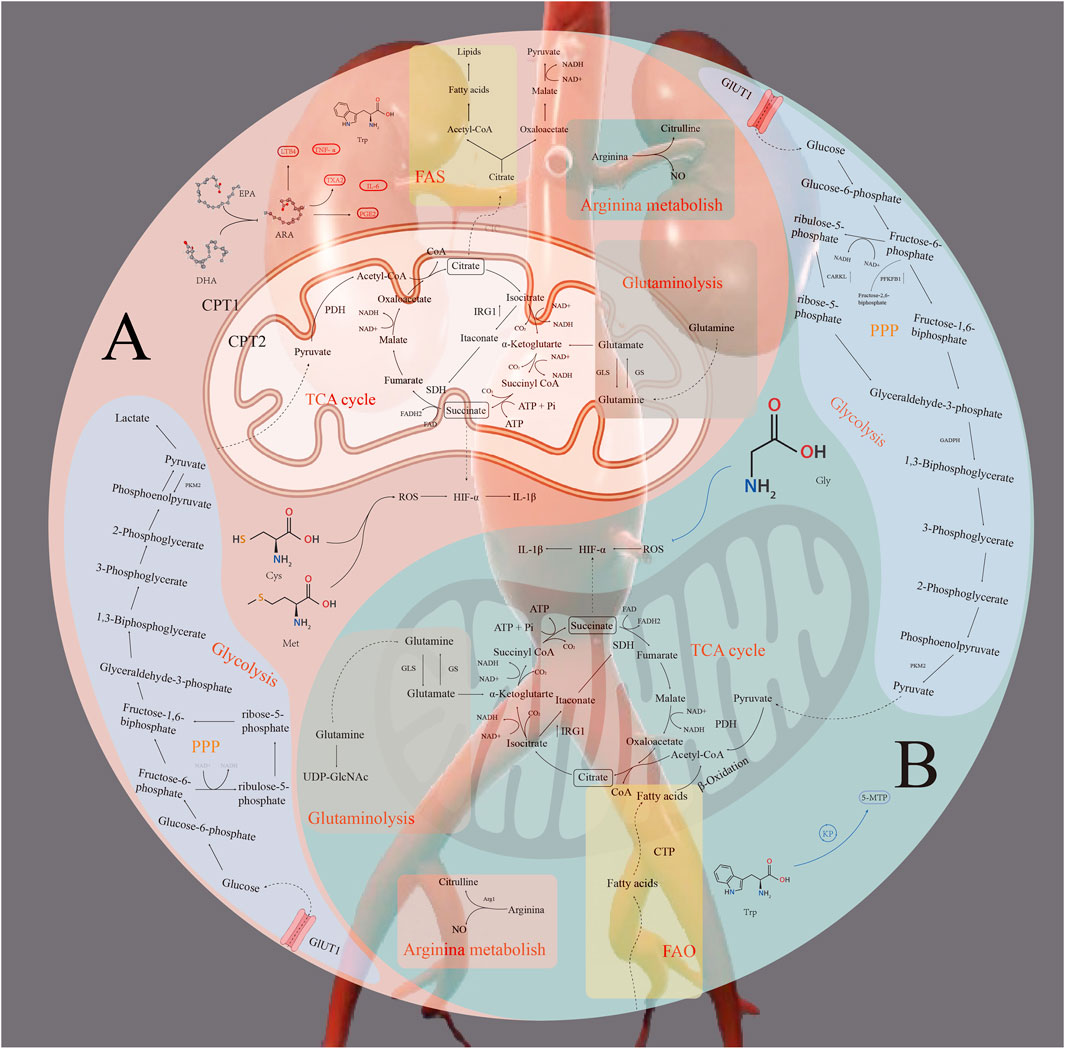
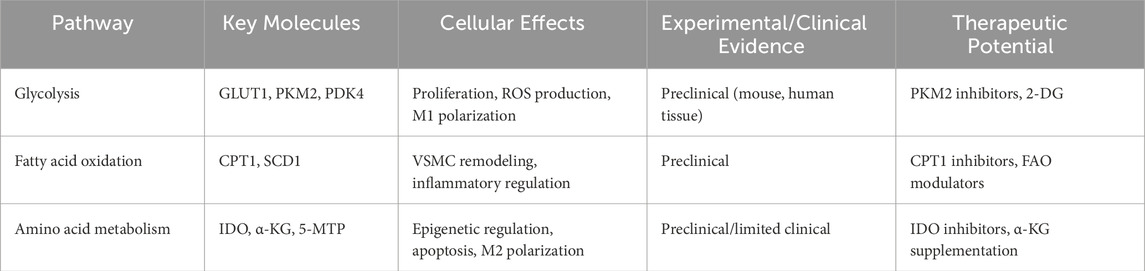
Branched-chain amino acid (BCAA) levels, as well as their ratios with Gly and Gln, have been identified through metabolomic analyses as potential biomarkers of AAA (Zaric et al., 2020). Leucine supplementation has been shown to improve macrophage lipid metabolism, enhance mitochondrial function, attenuate inflammation, and improve vascular elasticity. Clinical studies indicate that leucine-rich diets enhance cardiometabolic health in older adults; however, direct evidence supporting a protective role in AAA remains lacking (Kirk et al., 2021). All of the above-described mechanisms of metabolic reprogramming are summarized in Figure 1. Table 1 provides an overview summarizing the main metabolic pathways involved in abdominal aortic aneurysm (AAA) as well as the key molecules and targets.
Figure 1 provides an overview of metabolic reprogramming in abdominal aortic aneurysm (AAA), illustrating glucose, lipid, and amino acid metabolism on the basis of M1 and M2 macrophage phenotypes. A: M1 macrophage; B: M2 macrophage; FAS: fatty acid synthesis; FAO: fatty acid oxidation.
4.4 Integrative perspective on metabolic reprogramming
Metabolic reprogramming represents a dynamic and interconnected network that orchestrates cellular adaptation to pathological stimuli. Rather than functioning as isolated processes, glucose, lipid, and amino acid metabolism are tightly integrated: for example, glycolytic intermediates fuel fatty acid synthesis, lipid oxidation regulates inflammatory tone and mitochondrial function, and amino acid–derived metabolites modulate epigenetic states that influence macrophage polarization. This interdependence suggests that AAA progression results from the synergistic actions of metabolic pathways, highlighting the importance of a systems-level view when considering therapeutic interventions.
5 Therapy
Given the complex interplay between metabolism and inflammation, targeting immune metabolism holds significant therapeutic potential, though relevant strategies remain in their early stages. The metabolic reprogramming of artery-resident macrophages provides a promising avenue for further investigation, opening new strategies for cardiovascular disease treatment. We propose that inhibiting glycolysis and the fatty acid synthesis (FAS) pathway in M1 macrophages, or enhancing fatty acid oxidation (FAO) in M2 macrophages, may effectively reduce foam cell formation, mitigate inflammation, and slow the progression of atherosclerosis (Liu et al., 2021; Hou et al., 2023). Future studies should further elucidate the mechanisms of macrophage metabolic reprogramming in atherosclerosis, as they may yield highly specific therapeutic targets capable of improving plaque stability, reducing inflammation, and significantly enhancing clinical outcomes. This approach also provides a solid theoretical and practical foundation for the treatment of abdominal aortic aneurysm (An et al., 2025). In addition, dimethyl fumarate (DMF), a derivative of the tricarboxylic acid cycle, suppresses aerobic glycolysis in immune cells by modifying cysteine residues (e.g., GAPDH), thereby inducing macrophage polarization toward an anti-inflammatory phenotype. This results in improved ventricular remodeling, reduced collagen deposition, and enhanced angiogenesis following myocardial infarction, while also alleviating myocardial injury in diabetic models, demonstrating cardiovascular protective effects (Mouton et al., 2021; Bresciani et al., 2023). Similarly, the immunometabolite itaconate, generated from cis-aconitate via CAD (encoded by IRG), competitively inhibits succinate dehydrogenase (SDH), decreases mitochondrial ROS and pro-inflammatory gene expression (downregulating IL-1β and IL-6; upregulating IL-1RA and IL-10), and modifies glycolytic enzymes such as GAPDH, ALDOA, and LDHA to inhibit glycolysis, thereby exerting protective effects in murine models of myocardial infarction and doxorubicin-induced cardiotoxicity. Moreover, rapamycin inhibits mTORC1, thereby reducing glycolysis and inflammatory polarization, limiting post-MI macrophage infiltration, and improving outcomes (Shan et al., 2024; Shan et al., 2019; Sciarretta et al., 2014). Collectively, these findings indicate that multiple metabolism-targeting agents hold promise for the treatment and prevention of cardiovascular diseases, yet further studies are needed to elucidate underlying mechanisms, identify precise targets, and advance innovative therapeutic strategies into clinical practice.
6 Clinical translation and therapeutic perspectives
While numerous metabolic targets (e.g., PKM2, PDK4, IDO, CPT1) and modulators (e.g., DMF, itaconate, rapamycin) show promise in preclinical models, significant barriers remain in translating these findings into clinical therapies. Challenges include metabolic heterogeneity across patient populations, off-target effects of systemic metabolic modulators, and a lack of reliable biomarkers to monitor metabolic changes in vivo. To bridge this gap, future studies should: (1) develop cell-specific metabolic interventions, (2) integrate metabolomics with single-cell and spatial omics to stratify patients by metabolic phenotype, and (3) conduct prospective clinical trials assessing safety, efficacy, and biomarker-guided treatment responses.
7 Future directions
Despite major advances, several critical questions remain unresolved. Future research should: Characterize metabolic heterogeneity across VSMC subpopulations and macrophage subsets using spatial transcriptomics and metabolomics. Investigate how aging reshapes metabolic networks, given the age dependence of AAA. Explore metabolic–immune crosstalk in the context of systemic comorbidities (e.g., diabetes, dyslipidemia). Design combinatorial therapies targeting multiple metabolic pathways simultaneously to maximize therapeutic efficacy.
8 Conclusion
Abdominal aortic aneurysm (AAA) is associated with extremely high mortality due to its insidious onset and the lack of effective interventions. Metabolic reprogramming plays a pivotal role in AAA initiation and progression, influencing vascular wall homeostasis through intertwined metabolic networks that regulate inflammation, oxidative stress, apoptosis, and phenotypic switching. Integrating these insights into clinical strategies requires deeper mechanistic understanding, robust biomarker development, and innovative therapeutic design. Ultimately, viewing AAA through the lens of metabolic systems biology may unlock transformative avenues for individualized prevention and treatment.
Author contributions
XW: Conceptualization, Visualization, Writing – original draft. JL: Investigation, Writing – original draft. QJ: Writing – review and editing. YZ: Conceptualization, Writing – review and editing. JC: Conceptualization, Investigation, Supervision, Visualization, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aikawa, T., Miyazaki, T., Shimada, K., Sugita, Y., Shimizu, M., Ouchi, S., et al. (2017). Low serum levels of EPA are associated with the size and growth rate of abdominal aortic aneurysm. J. Atheroscler. Thromb. 24 (9), 912–920. doi:10.5551/jat.38315
Alamri, A., Burzangi, A. S., Coats, P., and Watson, D. G. (2018). Untargeted metabolic profiling cell-based approach of pulmonary artery smooth muscle cells in response to high glucose and the effect of the antioxidant vitamins D and E. Metabolites 8 (4), 87. doi:10.3390/metabo8040087
An, T., Guo, M., Wang, Z., and Liu, K. (2025). Tissue-resident macrophages in cardiovascular diseases: heterogeneity and therapeutic potential. Int. J. Mol. Sci. 26 (10), 4524. doi:10.3390/ijms26104524
Anderson, N. M., Mucka, P., Kern, J. G., and Feng, H. (2018). The emerging role and targetability of the TCA cycle in cancer metabolism. Protein Cell 9 (2), 216–237. doi:10.1007/s13238-017-0451-1
Asadi Shahmirzadi, A., Edgar, D., Liao, C. -Y., Hsu, Y. -M., Lucanic, M., Asadi Shahmirzadi, A., et al. (2020). Alpha-ketoglutarate, an endogenous metabolite, extends lifespan and compresses morbidity in aging mice. Cell Metab. 32 (3), 447–456.e446. doi:10.1016/j.cmet.2020.08.004
Batra, R., Suh, M. K., Carson, J. S., Dale, M. A., Meisinger, T. M., Fitzgerald, M., et al. (2018). IL-1β (Interleukin-1β) and TNF-α (tumor necrosis Factor-α) impact abdominal aortic aneurysm formation by differential effects on macrophage polarization. Arterioscler. Thromb. Vasc. Biol. 38 (2), 457–463. doi:10.1161/ATVBAHA.117.310333
Baxter, B. T., McGee, G. S., Shively, V. P., Drummond, I. A., Dixit, S. N., Yamauchi, M., et al. (1992). Elastin content, cross-links, and mRNA in normal and aneurysmal human aorta. J. Vasc. Surg. 16 (2), 192–200. doi:10.1016/0741-5214(92)90107-j
Bkaily, G., Jazzar, A., Normand, A., Simon, Y., Al-Khoury, J., and Jacques, D. (2020). Taurine and cardiac disease: state of the art and perspectives. Can. J. Physiol. Pharmacol. 98 (2), 67–73. doi:10.1139/cjpp-2019-0313
Bresciani, G., Manai, F., Davinelli, S., Tucci, P., Saso, L., and Amadio, M. (2023). Novel potential pharmacological applications of dimethyl fumarate-an overview and update. Front. Pharmacol. 14, 1264842. doi:10.3389/fphar.2023.1264842
Brethel, S., Locker, S., Girens, R., Rivera, P., Meurs, K., and Adin, D. (2023). The effect of taurine supplementation on the renin-angiotensin-aldosterone system of dogs with congestive heart failure. Sci. Rep. 13 (1), 10700. doi:10.1038/s41598-023-37978-1
Brinkmann, S. J., Buijs, N., Vermeulen, M. A., Oosterink, E., Schierbeek, H., Beishuizen, A., et al. (2016). Perioperative glutamine supplementation restores disturbed renal arginine synthesis after open aortic surgery: a randomized controlled clinical trial. Am. J. Physiol. Ren. Physiol. 311 (3), F567–F575. doi:10.1152/ajprenal.00340.2015
Bublil, E. M., and Majtan, T. (2020). Classical homocystinuria: from cystathionine beta-synthase deficiency to novel enzyme therapies. Biochimie 173, 48–56. doi:10.1016/j.biochi.2019.12.007
Chao de la Barca, J. M., Richard, A., Robert, P., Eid, M., Fouquet, O., Tessier, L., et al. (2022). Metabolomic profiling of Angiotensin-II-Induced abdominal aortic aneurysm in Ldlr(-/-) mice points to alteration of nitric oxide, lipid, and energy metabolisms. Int. J. Mol. Sci. 23 (12), 6387. doi:10.3390/ijms23126387
Chen, Z., Liu, M., Li, L., and Chen, L. (2018). Involvement of the warburg effect in non-tumor diseases processes. J. Cell Physiol. 233 (4), 2839–2849. doi:10.1002/jcp.25998
Chen, X., Wu, H., Liu, Y., Liu, L., Houser, S. R., and Wang, W. E. (2024). Metabolic reprogramming: a byproduct or a driver of cardiomyocyte proliferation? Circulation 149 (20), 1598–1610. doi:10.1161/CIRCULATIONAHA.123.065880
Chiong, M., Cartes-Saavedra, B., Norambuena-Soto, I., Mondaca-Ruff, D., Morales, P. E., García-Miguel, M., et al. (2014). Mitochondrial metabolism and the control of vascular smooth muscle cell proliferation. Front. Cell Dev. Biol. 2, 72. doi:10.3389/fcell.2014.00072
Dale, M. A., Xiong, W., Carson, J. S., Suh, M. K., Karpisek, A. D., Meisinger, T. M., et al. (2016). Elastin-derived peptides promote abdominal aortic aneurysm formation by modulating M1/M2 macrophage polarization. J. Immunol. 196 (11), 4536–4543. doi:10.4049/jimmunol.1502454
Dong, L. H., Li, L., Song, Y., Duan, Z. L., Sun, S. G., Lin, Y. L., et al. (2015). TRAF6-Mediated SM22α K21 ubiquitination promotes G6PD activation and NADPH production, contributing to GSH homeostasis and VSMC survival in vitro and in vivo. Circ. Res. 117 (8), 684–694. doi:10.1161/CIRCRESAHA.115.306233
Eriksson, P., Jones, K. G., Brown, L. C., Greenhalgh, R. M., Hamsten, A., and Powell, J. T. (2004). Genetic approach to the role of cysteine proteases in the expansion of abdominal aortic aneurysms. Br. J. Surg. 91 (1), 86–89. doi:10.1002/bjs.4364
Freemerman, A. J., Johnson, A. R., Sacks, G. N., Milner, J. J., Kirk, E. L., Troester, M. A., et al. (2014). Metabolic reprogramming of macrophages: glucose transporter 1 (GLUT1)-Mediated glucose metabolism drives a proinflammatory phenotype. J. Biol. Chem. 289 (11), 7884–7896. doi:10.1074/jbc.M113.522037
Fukuzumi, M., Shinomiya, H., Shimizu, Y., Ohishi, K., and Utsumi, S. (1996). Endotoxin-induced enhancement of glucose influx into murine peritoneal macrophages via GLUT1. Infect. Immun. 64 (1), 108–112. doi:10.1128/IAI.64.1.108-112.1996
Gao, X., Wang, H., Yang, J. J., Liu, X., and Liu, Z. R. (2012). Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Mol. Cell 45 (5), 598–609. doi:10.1016/j.molcel.2012.01.001
Guo, G., Ott, C. E., Grünhagen, J., Muñoz-García, B., Pletschacher, A., Kallenbach, K., et al. (2013). Indomethacin prevents the progression of thoracic aortic aneurysm in Marfan syndrome mice. Aorta (Stamford). 1 (1), 5–12. doi:10.12945/j.aorta.2013.13.007
Hall, J. L., Chatham, J. C., Eldar-Finkelman, H., and Gibbons, G. H. (2001). Upregulation of glucose metabolism during intimal lesion formation is coupled to the inhibition of vascular smooth muscle cell apoptosis. Role of GSK3beta. Diabetes 50 (5), 1171–1179. doi:10.2337/diabetes.50.5.1171
Hou, P., Fang, J., Liu, Z., Shi, Y., Agostini, M., Bernassola, F., et al. (2023). Macrophage polarization and metabolism in atherosclerosis. Cell Death Dis. 14 (10), 691. doi:10.1038/s41419-023-06206-z
Hruz, P. W., and Mueckler, M. M. (2001). Structural analysis of the GLUT1 facilitative glucose transporter (review). Mol. Membr. Biol. 18 (3), 183–193. doi:10.1080/09687680110072140
Jain, M., Dhanesha, N., Doddapattar, P., Nayak, M. K., Guo, L., Cornelissen, A., et al. (2021). Smooth muscle cell-specific PKM2 (pyruvate kinase muscle 2) promotes smooth muscle cell phenotypic switching and neointimal hyperplasia. Arterioscler. Thromb. Vasc. Biol. 41 (5), 1724–1737. doi:10.1161/ATVBAHA.121.316021
Jia, Y., Mao, C., Ma, Z., Huang, J., Li, W., Ma, X., et al. (2022). PHB2 maintains the contractile phenotype of VSMCs by counteracting PKM2 splicing. Circ. Res. 131 (10), 807–824. doi:10.1161/CIRCRESAHA.122.321005
Johnston, K. W., Rutherford, R. B., Tilson, M. D., Shah, D. M., Hollier, L., and Stanley, J. C. (1991). Suggested standards for reporting on arterial aneurysms. Subcommittee on reporting standards for arterial aneurysms, ad hoc committee on reporting standards, society for vascular surgery and North American chapter, international society for cardiovascular surgery. J. Vasc. Surg. 13 (3), 452–458. doi:10.1067/mva.1991.26737
Kim, H. W., Blomkalns, A. L., Ogbi, M., Thomas, M., Gavrila, D., Neltner, B. S., et al. (2017). Role of myeloperoxidase in abdominal aortic aneurysm formation: mitigation by taurine. Am. J. Physiol. Heart Circ. Physiol. 313 (6), H1168–H1179. doi:10.1152/ajpheart.00296.2017
Kirk, B., Mooney, K., Vogrin, S., Jackson, M., Duque, G., Khaiyat, O., et al. (2021). Leucine-enriched whey protein supplementation, resistance-based exercise, and cardiometabolic health in older adults: a randomized controlled trial. J. Cachexia Sarcopenia Muscle 12 (6), 2022–2033. doi:10.1002/jcsm.12805
Korshunov, V. A., Mohan, A. M., Georger, M. A., and Berk, B. C. (2006). Axl, a receptor tyrosine kinase, mediates flow-induced vascular remodeling. Circ. Res. 98 (11), 1446–1452. doi:10.1161/01.RES.0000223322.16149.9a
Lin, C. Y., Hsu, S. C., Lee, H. S., Lin, S. H., Tsai, C. S., Huang, S. M., et al. (2013). Enhanced expression of glucose transporter-1 in vascular smooth muscle cells via the Akt/tuberous sclerosis complex subunit 2 (TSC2)/mammalian target of rapamycin (mTOR)/ribosomal S6 protein kinase (S6K) pathway in experimental renal failure. J. Vasc. Surg. 57 (2), 475–485. doi:10.1016/j.jvs.2012.07.037
Lindholt, J. S., Sorensen, H. T., Michel, J. B., Thomsen, H. F., and Henneberg, E. W. (2008). Low-dose aspirin may prevent growth and later surgical repair of medium-sized abdominal aortic aneurysms. Vasc. Endovasc. Surg. 42 (4), 329–334. doi:10.1177/1538574408315205
Liu, P. S., Wang, H., Li, X., Chao, T., Teav, T., Christen, S., et al. (2017). α-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat. Immunol. 18 (9), 985–994. doi:10.1038/ni.3796
Liu, Y., Xu, R., Gu, H., Zhang, E., Qu, J., Cao, W., et al. (2021). Metabolic reprogramming in macrophage responses. Biomark. Res. 9 (1), 1. doi:10.1186/s40364-020-00251-y
Liu, J., Liu, M., Feng, J., Zhu, H., Wu, J., Zhang, H., et al. (2022). Alpha-ketoglutarate ameliorates abdominal aortic aneurysm via inhibiting PXDN/HOCL/ERK signaling pathways. J. Transl. Med. 20 (1), 461. doi:10.1186/s12967-022-03659-2
Lopez-Candales, A., Holmes, D. R., Liao, S., Scott, M. J., Wickline, S. A., and Thompson, R. W. (1997). Decreased vascular smooth muscle cell density in medial degeneration of human abdominal aortic aneurysms. Am. J. Pathology 150 (3), 993–1007. doi:10.1161/01.cir.99.1.96
Malandrino, M. I., Fucho, R., Weber, M., Calderon-Dominguez, M., Mir, J. F., Valcarcel, L., et al. (2015). Enhanced fatty acid oxidation in adipocytes and macrophages reduces lipid-induced triglyceride accumulation and inflammation. Am. J. Physiol. Endocrinol. Metab. 308 (9), E756–E769. doi:10.1152/ajpendo.00362.2014
Meital, L. T., Windsor, M. T., Ramirez Jewell, R. M. L., Young, P., Schulze, K., Magee, R., et al. (2019). n-3 PUFAs improve erythrocyte fatty acid profile in patients with small AAA: a randomized controlled trial. J. Lipid Res. 60 (6), 1154–1163. doi:10.1194/jlr.P093013
Melrose, J., Whitelock, J., Xu, Q., and Ghosh, P. (1998). Pathogenesis of abdominal aortic aneurysms: possible role of differential production of proteoglycans by smooth muscle cells. J. Vasc. Surg. 28 (4), 676–686. doi:10.1016/s0741-5214(98)70094-1
Mouton, A. J., Flynn, E. R., Moak, S. P., Aitken, N. M., Omoto, A. C. M., Li, X., et al. (2021). Dimethyl fumarate preserves left ventricular infarct integrity following myocardial infarction via modulation of cardiac macrophage and fibroblast oxidative metabolism. J. Mol. Cell Cardiol. 158, 38–48. doi:10.1016/j.yjmcc.2021.05.008
Namgaladze, D., Lips, S., Leiker, T. J., Murphy, R. C., Ekroos, K., Ferreiros, N., et al. (2014). Inhibition of macrophage fatty acid β-oxidation exacerbates palmitate-induced inflammatory and endoplasmic reticulum stress responses. Diabetologia 57 (5), 1067–1077. doi:10.1007/s00125-014-3173-4
Nishimura, M., Yamashita, A., Matsuura, Y., Okutsu, J., Fukahori, A., Hirata, T., et al. (2021). Upregulated kynurenine pathway enzymes in aortic atherosclerotic aneurysm: macrophage kynureninase downregulates inflammation. J. Atheroscler. Thromb. 28 (11), 1214–1240. doi:10.5551/jat.58248
Nomura, M., Liu, J., Yu, Z. X., Yamazaki, T., Yan, Y., Kawagishi, H., et al. (2019). Macrophage fatty acid oxidation inhibits atherosclerosis progression. J. Mol. Cell Cardiol. 127, 270–276. doi:10.1016/j.yjmcc.2019.01.003
Odegaard, J. I., and Chawla, A. (2011). Alternative macrophage activation and metabolism. Annu. Rev. Pathol. 6, 275–297. doi:10.1146/annurev-pathol-011110-130138
Osman, I., He, X., Liu, J., Dong, K., Wen, T., Zhang, F., et al. (2019). TEAD1 (TEA domain transcription factor 1) promotes smooth muscle cell proliferation through upregulating SLC1A5 (solute carrier family 1 member 5)-Mediated glutamine uptake. Circ. Res. 124 (9), 1309–1322. doi:10.1161/CIRCRESAHA.118.314187
Pelicano, H., Martin, D. S., Xu, R. H., and Huang, P. (2006). Glycolysis inhibition for anticancer treatment. Oncogene 25 (34), 4633–4646. doi:10.1038/sj.onc.1209597
Perez, J., Hill, B. G., Benavides, G. A., Dranka, B. P., and Darley-Usmar, V. M. (2010). Role of cellular bioenergetics in smooth muscle cell proliferation induced by platelet-derived growth factor. Biochem. J. 428 (2), 255–267. doi:10.1042/BJ20100090
Pfeiffer, T., Schuster, S., and Bonhoeffer, S. (2001). Cooperation and competition in the evolution of ATP-producing pathways. Science 292 (5516), 504–507. doi:10.1126/science.1058079
Raffort, J., Lareyre, F., Clément, M., Hassen-Khodja, R., Chinetti, G., and Mallat, Z. (2017). Monocytes and macrophages in abdominal aortic aneurysm. Nat. Rev. Cardiol. 14 (8), 457–471. doi:10.1038/nrcardio.2017.52
Ramprasath, T., Han, Y. M., Zhang, D., Yu, C. J., and Zou, M. H. (2021). Tryptophan catabolism and inflammation: a novel therapeutic target for aortic diseases. Front. Immunol. 12, 731701. doi:10.3389/fimmu.2021.731701
Rao, S. K., Reddy, K. V., and Cohen, J. R. (1996). Role of serine proteases in aneurysm development. Ann. N. Y. Acad. Sci. 800, 131–137. doi:10.1111/j.1749-6632.1996.tb33304.x
Rateri, D. L., Howatt, D. A., Moorleghen, J. J., Charnigo, R., Cassis, L. A., and Daugherty, A. (2011). Prolonged infusion of angiotensin II in apoE(-/-) mice promotes macrophage recruitment with continued expansion of abdominal aortic aneurysm. Am. J. Pathol. 179 (3), 1542–1548. doi:10.1016/j.ajpath.2011.05.049
Ricciotti, E., and FitzGerald, G. A. (2011). Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 31 (5), 986–1000. doi:10.1161/ATVBAHA.110.207449
Roche, T. E., Baker, J. C., Yan, X., Hiromasa, Y., Gong, X., Peng, T., et al. (2001). Distinct regulatory properties of pyruvate dehydrogenase kinase and phosphatase isoforms. Prog. Nucleic Acid. Res. Mol. Biol. 70, 33–75. doi:10.1016/s0079-6603(01)70013-x
Rom, O., Grajeda-Iglesias, C., Najjar, M., Abu-Saleh, N., Volkova, N., Dar, D. E., et al. (2017). Atherogenicity of amino acids in the lipid-laden macrophage model system in vitro and in atherosclerotic mice: a key role for triglyceride metabolism. J. Nutr. Biochem. 45, 24–38. doi:10.1016/j.jnutbio.2017.02.023
Ruiz-Ramírez, A., Ortiz-Balderas, E., Cardozo-Saldaña, G., Diaz-Diaz, E., and El-Hafidi, M. (2014). Glycine restores glutathione and protects against oxidative stress in vascular tissue from sucrose-fed rats. Clin. Sci. (Lond). 126 (1), 19–29. doi:10.1042/CS20130164
Sakalihasan, N., Heyeres, A., Nusgens, B. V., Limet, R., and Lapière, C. M. (1993). Modifications of the extracellular matrix of aneurysmal abdominal aortas as a function of their size. Eur. J. Vasc. Surg. 7 (6), 633–637. doi:10.1016/s0950-821x(05)80708-x
Sakalihasan, N., Limet, R., and Defawe, O. D. (2005). Abdominal aortic aneurysm. Lancet 365 (9470), 1577–1589. doi:10.1016/S0140-6736(05)66459-8
Sciarretta, S., Volpe, M., and Sadoshima, J. (2014). Mammalian target of rapamycin signaling in cardiac physiology and disease. Circ. Res. 114 (3), 549–564. doi:10.1161/CIRCRESAHA.114.302022
Shan, Q., Li, X., Zheng, M., Lin, X., Lu, G., Su, D., et al. (2019). Protective effects of dimethyl itaconate in mice acute cardiotoxicity induced by doxorubicin. Biochem. Biophys. Res. Commun. 517 (3), 538–544. doi:10.1016/j.bbrc.2019.07.046
Shan, W., Cui, J., Song, Y., Yan, D., Feng, L., Jian, Y., et al. (2024). Itaconate as a key player in cardiovascular immunometabolism. Free Radic. Biol. Med. 219, 64–75. doi:10.1016/j.freeradbiomed.2024.04.218
Shi, J., Yang, Y., Cheng, A., Xu, G., and He, F. (2020). Metabolism of vascular smooth muscle cells in vascular diseases. Am. J. Physiol. Heart Circ. Physiol. 319 (3), H613–H631. doi:10.1152/ajpheart.00220.2020
Song, P., Ramprasath, T., Wang, H., and Zou, M. H. (2017). Abnormal kynurenine pathway of tryptophan catabolism in cardiovascular diseases. Cell Mol. Life Sci. 74 (16), 2899–2916. doi:10.1007/s00018-017-2504-2
Song, Y. J., Kim, A., Kim, G. T., Yu, H. Y., Lee, E. S., Park, M. J., et al. (2019). Inhibition of lactate dehydrogenase A suppresses inflammatory response in RAW 264.7 macrophages. Mol. Med. Rep. 19 (1), 629–637. doi:10.3892/mmr.2018.9678
Song, H., Yang, Y., Sun, Y., Wei, G., Zheng, H., Chen, Y., et al. (2022). Circular RNA Cdyl promotes abdominal aortic aneurysm formation by inducing M1 macrophage polarization and M1-type inflammation. Mol. Ther. 30 (2), 915–931. doi:10.1016/j.ymthe.2021.09.017
Soto, M. E., Guarner-Lans, V., Herrera-Morales, K. Y., and Pérez-Torres, I. (2018). Participation of arachidonic acid metabolism in the aortic aneurysm formation in patients with Marfan syndrome. Front. Physiol. 9, 77. doi:10.3389/fphys.2018.00077
Steed, M. M., and Tyagi, S. C. (2011). Mechanisms of cardiovascular remodeling in hyperhomocysteinemia. Antioxid. Redox Signal 15 (7), 1927–1943. doi:10.1089/ars.2010.3721
Tian, Q., Zhao, J., Yang, Q., Wang, B., Deavila, J. M., Zhu, M. J., et al. (2020). Dietary alpha-ketoglutarate promotes beige adipogenesis and prevents obesity in middle-aged mice. Aging Cell 19 (1), e13059. doi:10.1111/acel.13059
Tsuruda, T., Hatakeyama, K., Nagamachi, S., Sekita, Y., Sakamoto, S., Endo, G. J., et al. (2012). Inhibition of development of abdominal aortic aneurysm by glycolysis restriction. Arterioscler. Thromb. Vasc. Biol. 32 (6), 1410–1417. doi:10.1161/ATVBAHA.111.237065
Wang, J. H., Eguchi, K., Matsumoto, S., Fujiu, K., Komuro, I., Nagai, R., et al. (2014). The ω-3 polyunsaturated fatty acid, eicosapentaenoic acid, attenuates abdominal aortic aneurysm development via suppression of tissue remodeling. PLoS One 9 (5), e96286. doi:10.1371/journal.pone.0096286
Wang, Q., Ding, Y., Song, P., Zhu, H., Okon, I., Ding, Y. N., et al. (2017). Tryptophan-derived 3-Hydroxyanthranilic acid contributes to angiotensin II-Induced abdominal aortic aneurysm Formation in mice in vivo. Circulation 136 (23), 2271–2283. doi:10.1161/CIRCULATIONAHA.117.030972
Wanhainen, A., Verzini, F., Van Herzeele, I., Allaire, E., Bown, M., Cohnert, T., et al. (2019). Editor's choice - European society for vascular surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur. J. Vasc. Endovasc. Surg. 57 (1), 8–93. doi:10.1016/j.ejvs.2018.09.020
Warburg, O. (1956). On respiratory impairment in cancer cells. Science 124 (3215), 269–270. doi:10.1126/science.124.3215.269
Warsi, A. A., Davies, B., Morris-Stiff, G., Hullin, D., and Lewis, M. H. (2004). Abdominal aortic aneurysm and its correlation to plasma homocysteine, and vitamins. Eur. J. Vasc. Endovasc. Surg. 27 (1), 75–79. doi:10.1016/j.ejvs.2003.09.001
Watanabe, Y., Koyama, S., Yamashita, A., Matsuura, Y., Nishihira, K., Kitamura, K., et al. (2018). Indoleamine 2,3-dioxygenase 1 in coronary atherosclerotic plaque enhances tissue factor expression in activated macrophages. Res. Pract. Thromb. Haemost. 2 (4), 726–735. doi:10.1002/rth2.12128
Wei, L., Bu, X., Wang, X., Liu, J., Ma, A., and Wang, T. (2021). Global burden of aortic aneurysm and attributable risk factors from 1990 to 2017. Glob. Heart 16 (1), 35. doi:10.5334/gh.920
Wu, K. K., Kuo, C. C., Yet, S. F., Lee, C. M., and Liou, J. Y. (2020). 5-methoxytryptophan: an arsenal against vascular injury and inflammation. J. Biomed. Sci. 27 (1), 79. doi:10.1186/s12929-020-00671-w
Xie, T., Lei, C., Song, W., Wu, X., Wu, J., Li, F., et al. (2023). Plasma lipidomics analysis reveals the potential role of lysophosphatidylcholines in abdominal aortic aneurysm progression and formation. Int. J. Mol. Sci. 24 (12), 10253. doi:10.3390/ijms241210253
Yang, D., Wang, M. T., Tang, Y., Chen, Y., Jiang, H., Jones, T. T., et al. (2010). Impairment of mitochondrial respiration in mouse fibroblasts by oncogenic H-RAS(Q61L). Cancer Biol. Ther. 9 (2), 122–133. doi:10.4161/cbt.9.2.10379
Yang, T. H., Hsu, P. Y., Meng, M., and Su, C. C. (2015). Supplement of 5-hydroxytryptophan before induction suppresses inflammation and collagen-induced arthritis. Arthritis Res. Ther. 17, 364. doi:10.1186/s13075-015-0884-y
Yang, Y. F., Chuang, H. W., Kuo, W. T., Lin, B. S., and Chang, Y. C. (2021). Current development and application of anaerobic glycolytic enzymes in urothelial cancer. Int. J. Mol. Sci. 22 (19), 10612. doi:10.3390/ijms221910612
Yoshihara, T., Shimada, K., Fukao, K., Sai, E., Sato-Okabayashi, Y., Matsumori, R., et al. (2015). Omega 3 polyunsaturated fatty acids suppress the development of aortic aneurysms through the inhibition of macrophage-mediated inflammation. Circ. J. 79 (7), 1470–1478. doi:10.1253/circj.CJ-14-0471
Yuwen, L., Ciqiu, Y., Yi, S., Ruilei, L., Yuanhui, L., Bo, L., et al. (2019). A pilot study of protein microarray for simultaneous analysis of 274 cytokines between abdominal aortic aneurysm and normal aorta. Angiology 70 (9), 830–837. doi:10.1177/0003319719844678
Zaric, B. L., Obradovic, M., Bajic, V., Haidara, M. A., Jovanovic, M., and Isenovic, E. R. (2019). Homocysteine and hyperhomocysteinaemia. Curr. Med. Chem. 26 (16), 2948–2961. doi:10.2174/0929867325666180313105949
Zaric, B. L., Radovanovic, J. N., Gluvic, Z., Stewart, A. J., Essack, M., Motwalli, O., et al. (2020). Atherosclerosis linked to aberrant amino acid metabolism and immunosuppressive amino acid catabolizing enzymes. Front. Immunol. 11, 551758. doi:10.3389/fimmu.2020.551758
Zhang, J., Schmidt, J., Ryschich, E., Mueller-Schilling, M., Schumacher, H., and Allenberg, J. R. (2003). Inducible nitric oxide synthase is present in human abdominal aortic aneurysm and promotes oxidative vascular injury. J. Vasc. Surg. 38 (2), 360–367. doi:10.1016/s0741-5214(03)00148-4
Zhang, Y., Li, L., and Ma, L. (2022). Integrative analysis of transcriptome-wide association study and mRNA expression profile identified candidate genes and pathways associated with aortic aneurysm and dissection. Gene 808, 145993. doi:10.1016/j.gene.2021.145993
Zhang, H., Zhang, Y., Wang, H., Yang, P., Lu, C., Liu, Y., et al. (2023). Global proteomic analysis reveals lysine succinylation contributes to the pathogenesis of aortic aneurysm and dissection. J. Proteomics 280, 104889. doi:10.1016/j.jprot.2023.104889
Zhao, F., Chen, T., and Jiang, N. (2020). CDR1as/miR-7/CKAP4 axis contributes to the pathogenesis of abdominal aortic aneurysm by regulating the proliferation and apoptosis of primary vascular smooth muscle cells. Exp. Ther. Med. 19 (6), 3760–3766. doi:10.3892/etm.2020.8622
Zhou, Q., Xu, J., Liu, M., He, L., Zhang, K., Yang, Y., et al. (2019). Warburg effect is involved in apelin-13-induced human aortic vascular smooth muscle cells proliferation. J. Cell Physiol. 234 (9), 14413–14421. doi:10.1002/jcp.28218
Zhou, M., Zha, Z., Zheng, Z., and Pan, Y. (2023). Cordycepin suppresses vascular inflammation, apoptosis and oxidative stress of arterial smooth muscle cell in thoracic aortic aneurysm with VEGF inhibition. Int. Immunopharmacol. 116, 109759. doi:10.1016/j.intimp.2023.109759
Keywords: glucose metabolism, lipid metabolism, amino acid metabolism, abdominal aortic aneurysm, vascular smooth muscle cells
Citation: Wu X, Li J, Ju Q, Zeng Y and Cao J (2025) Mechanisms of metabolic reprogramming in abdominal aortic aneurysm. Front. Cell Dev. Biol. 13:1718220. doi: 10.3389/fcell.2025.1718220
Received: 03 October 2025; Accepted: 27 October 2025;
Published: 13 November 2025.
Edited by:
Qiong Lu, Central South University, ChinaReviewed by:
Yangyang Zhang, Shanghai Jiao Tong University, ChinaCopyright © 2025 Wu, Li, Ju, Zeng and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junjie Cao, NTk2MTA1NzM4QHFxLmNvbQ==; Yuqi Zeng, MTg3MTYxNjk5N0BxcS5jb20=
 Xiaodong Wu1
Xiaodong Wu1 Yuqi Zeng
Yuqi Zeng Junjie Cao
Junjie Cao