- 1School of Basic Medicine, Institute for Pathogenesis and Clinical Translational Research of Esophageal Cancer, Shanxi Medical University, Taiyuan, Shanxi, China
- 2Translational Medicine Institute, The First People’s Hospital of Chenzhou, Hengyang Medical School, University of South China, Chenzhou, China
- 3Department of Basic Medical Sciences, Qinghai University Medical College, Xining, Qinghai, China
Metabolic reprogramming of Branched-chain amino acids (BCAAs)-leucine, isoleucine, and valine-has emerged as a constitutive feature of cancer, extending far beyond their canonical roles in protein synthesis and energy provision. In malignancy, these essential amino acids function as pivotal signaling mediators and epigenetic modulators, thereby propelling tumor progression, facilitating immune evasion, and conferring resistance to therapeutic agents. This review delineates how cancer cells subvert branched-chain amino acid metabolism to fuel anabolic processes, activate oncogenic signaling cascades including mTOR and PI3K/AKT, and remodel the tumor microenvironment. A framework is presented to categorize the differential reliance of various cancers on key catabolic enzymes-BCAT1, BCAT2 and BCKDK-underscoring their therapeutic vulnerability. The paradoxical role of BCAAs in modulating anti-tumor immunity is examined alongside the potential of dietary modulation and the development of pharmacological inhibitors targeting this pathway. Concluding perspectives highlight the trajectory for translating these insights into precision oncology, advocating for biomarker-guided and context-specific therapeutic strategies.
1 Introduction
Among numerous diseases, cancer stands as one of the most critical challenges to human health, affecting millions of people worldwide each year and leading to countless fatalities (Siegel et al., 2023). According to the latest IARC/WHO estimates, approximately 20 million new cancer cases and 9.7 million cancer-related deaths occurred globally in 2025, highlighting the immense and growing burden of cancer worldwide (Bray et al., 2024; Liu and Zheng, 2024; Zhang et al., 2025a). Tumor initiation and progression are multifactorial processes involving genetic mutations, environmental exposures, immune evasion and metabolic dysregulation (Yang et al., 2019; Zhou et al., 2017). Metabolism constitutes a foundational pillar of cellular life. Core biological processes-including growth, differentiation, death, and stress adaptation-are intricately governed by metabolites and metabolic enzymes (Pavlova and Thompson, 2016; Xu et al., 2021; O'Sullivan et al., 2019). Emerging evidence further positions metabolites as bona fide signaling molecules, capable of modulating cellular signal transduction and participating in diverse processes such as intercellular communication and epigenetic regulation (Bergers and Fendt, 2021; Bayoumi et al., 2020; Li and Wang, 2020).
The aberrant metabolic phenotype of tumors was first described in the 1920s by the German physiologist and physician Otto Warburg, who identified a fundamental difference in glucose utilization between tumor and normal cells (Warburg, 1956). In contrast to normal physiology, tumor metabolism represents a highly complex and dynamically rewired state, characterized by imbalances in multiple metabolites and a comprehensive restructuring of metabolic networks (Xia et al., 2021). Critically, the metabolic activities of both tumor cells and infiltrating immune cells within the tumor microenvironment (TME) play decisive roles in cancer progression and anti-tumor immunity (Chen and Li, 2016). Metabolic reprogramming is established as a hallmark of cancer, conferring upon tumor cells the plasticity to adjust their metabolic flux in response to diverse microenvironmental cues and stresses, thereby promoting survival and proliferation (Xia et al., 2021; Martínez-Reyes and Chandel, 2021). This reprogramming frequently originates from the activation of oncogenes or the inactivation of tumor suppressors, genetic alterations that ultimately drive metabolic remodeling and tumor evolution by dysregulating the expression and activity of key metabolic enzymes (Guo et al., 2025). To fuel their relentless expansion, tumor cells exhibit a pronounced dependency on macromolecular substrates-including glucose, amino acids, and fatty acids-which are indispensable for energy generation and biomass synthesis (Altman et al., 2016; Cheng et al., 2018; An et al., 2022).
In recent years, research in metabolomics and tumor biology has revealed the pivotal roles of amino acid metabolism in cancer development, progression, and treatment response (Vettore et al., 2020; Safrhansova et al., 2022). Amino acids not only serve as building blocks for proteins but also function as critical precursors for energy metabolism and biosynthetic pathways. Dysregulated amino acid metabolism is intimately linked to tumor cell proliferation, invasion, metastasis, and immune escape (Shi et al., 2024). Reprogramming of amino acid metabolism in tumors fuels tumor growth by providing both energy and biosynthetic precursors, while also functioning as signaling molecules to regulate tumor development. This metabolic rewiring plays pivotal roles in maintaining cellular redox homeostasis, driving nucleotide synthesis, and supporting bioenergetics. Notably, restricting the metabolism of specific amino acids presents a promising strategy to therapeutically target the progression of particular tumor types. Among amino acids, Branched-chain amino acids (BCAAs)-leucine, isoleucine, and valine-are essential and cannot be synthesized de novo in humans. Beyond serving as fundamental substrates for protein synthesis and muscle maintenance-by supporting anabolic signaling and attenuating proteolysis to promote nitrogen balance and metabolic homeostasis (Kawaguchi et al., 2011; Bifari and Nisoli, 2017; Bo and Fujii, 2024). Branched-chain amino acids (BCAAs) are also actively oxidized in peripheral tissues such as skeletal muscle, adipose tissue, and the liver. This catabolic pathway channels intermediates into the tricarboxylic acid (TCA) cycle, thereby contributing to cellular ATP production (Choi et al., 2024; Mann et al., 2021). Importantly, BCAAs metabolism extends beyond mere fuel supply; it is intimately linked to nutrient-sensitive signaling networks. Leucine, in particular, functions as a potent activator of the mechanistic target of rapamycin complex 1 (mTORC1), a central regulator that integrates nutrient availability with cellular growth and autophagic flux (Choi et al., 2024; Di Malta and Ballabio, 2018). Furthermore, the transamination of BCAAs yields metabolites such as glutamate and branched-chain ketoacids, which participate in glial-neuronal metabolic communication and thereby influence neurotransmitter synthesis (Dimou et al., 2022; Kadowaki and Knox, 1982).
Thus, through their dual roles in both bioenergetics and signal transduction, BCAAs emerge as critical players in maintaining systemic metabolic and functional equilibrium. Intriguingly, BCAAs have been shown to inhibit tumor cell migration and metastasis by downregulating N-cadherin expression (Chi et al., 2022). Nonetheless, the role of BCAAs in cancer remains controversial, as some studies demonstrate that their catabolism can promote tumor growth and progression in a dose-dependent manner (Li et al., 2020), while other studies demonstrate that BCAAs can suppress tumor development by enhancing anti-tumor immune responses, as observed in models of pancreatic cancer and triple-negative breast cancer (Chi et al., 2022; Lei et al., 2020; Yao et al., 2023). Consequently, targeting enzymes involved in BCAAs catabolism or adopting BCAAs-enriched dietary regimens has emerged as a potential therapeutic avenue (Lei et al., 2020). However, given the dual-faced role of BCAAs in cancer, translating these strategies into clinical practice may encounter substantial challenges and limitation (Dimou et al., 2022).
This review synthesizes the role of branched-chain amino acid (BCAAs) metabolism in cancer. We detail how BCAAs metabolic reprogramming promotes tumor progression and immune evasion, examine its diagnostic potential, and evaluate therapeutic strategies-including enzyme inhibition and dietary modulation-that target this pathway to suppress tumor growth and enhance therapy response. We also discuss the clinical prospects and challenges of these approaches.
2 BCAAs metabolism in physiology
Branched-chain amino acids (BCAAs)-leucine, isoleucine, and valine-are essential nutrients that support protein synthesis, energy homeostasis, and metabolic signaling (Figure 1) (Bo and Fujii, 2024; Choi et al., 2024). Leucine is abundantly present in proteins, and its breakdown-whether from exogenous dietary sources or endogenous sources such as muscles-releases a substantial amount of this amino acid, such as during metabolic turnover (Kamphorst et al., 2015). Their transport across both the intestinal epithelium and the plasma membrane is facilitated by specific transporters, namely, members of the L-type amino acid transporter (LATs) (Verkerke et al., 2024). Intracellular BCAAs are catabolized via two compartmentalized pathways: one involves mitochondrial import via Solute Carrier Family 25 Member 44 (SLC25A44) followed by transamination and degradation, and the other involves cytosolic transamination. These initial transamination steps are catalyzed by the respective branched-chain amino acid transaminase (BCAT) isoforms-BCAT1 in the cytosol and BCAT2 in mitochondria (Kadowaki and Knox, 1982; Ichihara, 1975). BCAT1 is predominantly expressed in neuronal tissues and immune cells, such as activated T lymphocytes and macrophages. In contrast, BCAT2 has a broader tissue distribution and is expressed in most tissues, with the highest levels found in skeletal muscle, followed by the kidney, and the lowest in the liver (Bledsoe et al., 1997; Suryawan et al., 1998). The BCAT1 and BCAT2 transfer the amino group from BCAAs to α-ketoglutarate (α-KG), yielding glutamate and branched-chain α-keto acids (BCKAs). Leucine, valine, and isoleucine are catalyzed to form α-ketoisocaproate (KIC), α-ketoisovalerate (KIV), and α-keto-β-methylvalerate (KMV), respectively. Notably, to replenish α-ketoglutarate and Branched-chain amino acids (BCAAs), the transaminases BCAT1 and BCAT2 transfer the nitrogen from glutamate back to a branched-chain α-keto acid (Dimou et al., 2022). This latter reaction is catalyzed by the branched-chain α-ketoacid dehydrogenase (BCKDH) complex, which comprises three functional components: the thiamine-dependent decarboxylase (E1), consisting of α- and β-subunits encoded by the BCKDHA and BCKDHB, respectively; the dihydrolipoyl transacylase (E2), encoded by the DBT; and the dihydrolipoamide dehydrogenase (E3), encoded by the DLD (Neinast et al., 2019; Li Z. et al., 2024). BCKDH has been reported as the rate-limiting enzyme in BCAAs catabolism. Its overall activity is dynamically regulated by BCKDK-mediated phosphorylation and Protein Phosphatase, Mg2+/Mn2+ Dependent 1K (PPM1K)-mediated dephosphorylation through reversible phosphorylation (Paxton and Harris, 1982; Pettit et al., 1978; Patel et al., 2014). BCKDH yielding corresponding branched-chain acyl-CoA derivatives. Specifically, leucine catabolism generates acetyl-CoA and acetoacetate, valine yields propionyl-CoA, and isoleucine produces both acetyl-CoA and propionyl-CoA. Propionyl-CoA is subsequently converted to succinyl-CoA (Mann et al., 2021; Du et al., 2022). These intermediates are ultimately converted into acetyl-CoA or succinyl-CoA, which enter the tricarboxylic acid (TCA) cycle to support adenosine triphosphate (ATP) production (Figure 2) (Du et al., 2022).

Figure 1. Chemical structures of Branched-chain amino acids. Ball - and - stick models illustrating the chemical structures of branched - chain amino acids: valine, leucine, and isoleucine.

Figure 2. Schematic representation of branched-chain amino acid (BCAAs) metabolism and regulation. This schematic illustrates the compartmentalized BCAA catabolic pathway across the cytosol and mitochondria. In the extracellular space, the L-type amino acid transporter (LATs) imports leucine (Leu), isoleucine (Ile), and valine (Val) into the cytosol. In the cytosol, BCAT1 catalyzes the transamination of BCAAs to generate their corresponding α-keto acids (α-KIC, α-KMV, α-KIV) and glutamate (Glu). These α-keto acids are then transported into the mitochondrial matrix via the SLC25A44 transporter. Within the mitochondria, BCAT2 completes the transamination of BCAAs, producing α-keto acids that are further oxidized by the branched-chain α-keto acid dehydrogenase (BCKDH) complex. The BCKDH complex, regulated by phosphorylation (via PPM1K) and feedback inhibition, generates acyl-CoA intermediates: isovaleryl-CoA, 2-methylbutyryl-CoA, and isobutyryl-CoA. These intermediates are channeled into downstream metabolic pathways, including the production of ketone bodies and acetyl-CoA/propionyl-CoA, which feed into the tricarboxylic acid (TCA) cycle to support cellular energy metabolism and biosynthesis.
3 BCAAs metabolism in cancer
3.1 Reprogramming of BCAAs metabolism from catabolism to anabolic signaling
In cancer, BCAAs metabolism is rewired to drive anabolic growth. Tumors frequently upregulate BCAT1, enhancing the conversion of BCAAs to BCKAs in the cytosol (Qian et al., 2023). Concurrently, BCKDH activity is often suppressed through dysregulation of its regulatory enzymes-typically via enhanced BCKDK activity or loss of PPM1K function-leading to marked accumulation of BCKAs (Lu et al., 2024; Du et al., 2018). These metabolites function as biosynthetic precursors for protein and lipid synthesis, directly facilitating biomass accumulation in proliferating cells. This metabolic shift is frequently accompanied by aberrant overexpression of transporters such as LAT1 and SLC25A44, thereby supplying ample substrate to fuel BCAT-driven metabolic flux (Schroeder et al., 2024; Yoneshiro et al., 2019).
Notably, accumulated BCKAs contribute to sustained activation of the mechanistic target of rapamycin complex 1 (mTORC1), further stimulating cell growth and establishing a feed-forward loop that reinforces metabolic reprogramming and malignant progression (Di Malta and Ballabio, 2018). Thus, tumor cells divert BCAAs catabolism from energy generation toward biosynthesis and oncogenic signaling, unveiling this pathway as a key metabolic vulnerability in cancer.
3.2 Classification of tumor dependency on BCAAs metabolism
The reliance of various cancers on BCAAs metabolism can be stratified into three distinct archetypes, providing a clarifying framework that moves beyond a simple listing.
3.2.1 Tumors with strong (obligate) dependency
Cancers such as glioblastoma (GBM) and acute myeloid leukemia (AML) exhibit a non-redundant, cell-autonomous reliance on BCAAs metabolic reprogramming, primarily driven by BCAT1 overexpression (Hattori et al., 2017). In these malignancies, BCAT1 activity is a critical engine for sustaining proliferation, stemness, and survival (Hattori et al., 2017; Zhang et al., 2022). Inhibition of this pathway presents a clear and direct therapeutic vulnerability, as evidenced by synthetic lethality upon BCAT1 knockdown or pharmacological inhibition.
3.2.2 Tumors with tissue-of-origin dependency
This category highlights how the tissue microenvironment dictates specific BCAAs utilization strategies. For instance, lung adenocarcinomas actively scavenge extracellular BCAAs as a nitrogen source to support anabolic needs (Mayers et al., 2016). In contrast, pancreatic ductal adenocarcinomas (PDAC) exhibit a more complex, parasitic relationship with their stroma, relying on BCAT2-mediated metabolism and potentially on BCAAs-derived metabolites from the tumor microenvironment (Li et al., 2020; Lei et al., 2020). This fundamental difference underscores that therapeutic targeting must consider the ecological context of the tumor (Figure 3).

Figure 3. BCAAs metabolism-related enzyme (BCAT1/2, BCKDK, BCKDHA) regulatory networks and functional outcomes across human cancers. This schematic integrates tissue-specific (color-coded by cancer type: e.g., CRC, PDAC, GBM, BRCA, GC, HCC, LUAD, CAML) regulatory circuits of branched-chain amino acid (BCAAs) metabolism enzymes and their downstream effects in tumorigenesis. Arrows (↑/↓) denote upregulation/downregulation of the enzyme; dashed lines link enzymatic activity to functional consequences (e.g., signaling, metabolism, cell death, therapy resistance).
3.2.3 Tumors with contextual duality or opposing roles
In cancers such as breast cancer (particularly triple-negative) and colorectal cancer (CRC), BCAAs metabolism plays a complex, double-edged role. Within tumor cells, it fuels anabolic growth and proliferation, with BCAT1 being essential in TNBC models (Huang et al., 2025; Thewes et al., 2017). Systemically, however, it simultaneously modulates anti-tumor immunity-potentially enhancing CD8+ T cell function (Chi et al., 2022; Yao et al., 2023), while also supporting immunosuppressive regulatory T cells (Tregs) (Ikeda et al., 2017). This paradox creates a therapeutic dilemma where inhibiting tumor-intrinsic BCAAs catabolism may have unintended consequences on the immune response (Dimou et al., 2022), necessitating highly context-aware strategies.
This stratification clarifies that BCAAs metabolism is not a uniformly similar pathway across cancers but a context-dependent vulnerability. It enhances the review’s guidance by emphasizing that future therapeutic strategies-whether involving enzyme inhibitors, dietary modulation, or their combination with immunotherapy-must be precisely tailored according to these dependency archetypes, integrating tumor lineage, genetic drivers, and the specific immune and metabolic landscape of the tumor microenvironment.
3.3 BCAAs metabolism as an integrative node in tumor progression: a coordinating framework
Reprogramming of BCAAs metabolism operates as a central signaling hub. This hub dynamically coordinates tumor growth, immune evasion, and therapy resistance through a network of interlocking feedback loops (Figure 4). Initiation of this circuit commonly stems from tumor-intrinsic metabolic rewiring, typified by upregulation of BCAT1 and/or suppression of the BCKDH complex. This diversion of BCAAs from oxidative catabolism toward biosynthetic pathways serves a dual role: it supplies precursors for macromolecule synthesis (e.g., branched-chain α-keto acids for protein and lipid production) and generates metabolite-derived oncogenic signals. A pivotal consequence of elevated BCAT1 activity is the depletion of cytosolic α-ketoglutarate (α-KG). This reduction impairs the function of α-KG-dependent dioxygenases, such as the TET family of DNA demethylases, leading to epigenetic dysregulation (e.g., DNA hypermethylation) and stabilization of hypoxia-inducible factors (HIFs). Consequently, a pro-growth transcriptional program becomes entrenched (Hattori et al., 2017; Meurs and Nagrath, 2022; Raffel et al., 2017). In parallel, accumulating BCKAs and leucine provide sustained activation of mTORC1, establishing a feed-forward loop that further induces the expression of BCAAs transporters like LAT1, thereby amplifying substrate uptake and reinforcing the metabolic shift (Di Malta and Ballabio, 2018; Mayers et al., 2016).

Figure 4. Core metabolic rewiring in the tumor microenvironment drives immunosuppression, therapy resistance, and tumor survival. This schematic outlines the multifaceted roles of BCAT1 in tumor pathophysiology. In the core panel, BCAT1 catabolizes branched-chain amino acids (BCAAs) to fuel biosynthesis, elevate intracellular leucine, and activate mTORC1-LAT1 signaling, creating a feed-forward BCAA uptake cycle. Concurrently, BCAT1-mediated α-ketoglutarate (α-KG) depletion stabilizes HIF-1α in hypoxic/acidic tumor microenvironments (TMEs), driving a pro-growth transcriptional program. In the immunosuppressive TME (bottom left), BCAT1-dependent metabolic competition restricts nutrient availability for effector T cells, polarizes macrophages to an M2 phenotype, expands regulatory T cells, and impairs CD8+ T-cell function. In therapy resistance (bottom right), BCAT1 activates adaptive signaling and DNA repair pathways to counteract chemotherapy, targeted therapy, and radiation, with therapy-induced selective pressure further upregulating BCAT1 to reinforce a pro-survival, therapy-driven loop. Hypoxia and acidosis in the TME (top left) initiate this cascade by stabilizing HIF-1α and inducing BCAT1 expression.
The altered metabolic state of the cancer cell extends its influence to remodel the tumor microenvironment (TME). Nutrient competition and the release of specific metabolites, including BCKAs, enact context-dependent immunomodulatory effects. These changes can potentiate the immunosuppressive activity of regulatory T cells (Tregs) and drive the polarization of tumor-associated macrophages toward a pro-tumorigenic M2 phenotype. Simultaneously, they contribute to a metabolically restrictive niche that compromises the effector functions of CD8+ T cells and natural killer (NK) cells (Ikeda et al., 2017; Zhang et al., 2024; Zhang et al., 2023). Collectively, these actions reshape the TME into an immunosuppressive ecosystem conducive to immune escape.
This integrated metabolic network also constitutes a direct engine of therapy resistance. The very enzymes that propel tumor growth and immune suppression simultaneously confer resilience against therapeutic agents. For example, BCAT1 has been shown to mediate resistance to conventional chemotherapeutics like cisplatin and to targeted agents such as EGFR tyrosine kinase inhibitors, achieved through adaptive signaling and epigenetic modifications (Zhang et al., 2024; Sjögren, 2021). Correspondingly, activation of BCKDK promotes tumor progression via mTORC1 and MAPK signaling pathways and has been linked to radioresistance by enhancing DNA damage repair mechanisms. This connection implicates BCAAs metabolism in treatment failure across diverse therapeutic modalities (Bo et al., 2025; Wang Y. et al., 2021; Liu et al., 2025).
These processes are not linear but are interconnected through reinforcing feedback loops. The hypoxic and acidic conditions of the TME, frequently a product of rapid tumor expansion, can themselves induce BCAT1 expression via HIF signaling (Meurs and Nagrath, 2022). The subsequent BCAT1-mediated depletion of α-KG then further stabilizes HIFs, creating a vicious cycle that perpetuates both metabolic and epigenetic reprogramming. In a similar vein, therapeutic pressure can select for cell populations with upregulated BCAAs pathway enzymes or directly induce their expression, thereby driving acquired resistance.
In summary, BCAAs metabolic reprogramming not as a peripheral supportive feature, but as a core orchestrator of malignant progression. It functions as a self-amplifying network that synergistically augments tumor cell fitness, dismantles anti-tumor immunity, and subverts therapeutic efficacy. Disruption of this network-via targeted enzyme inhibition, dietary modulation, or combination strategies informed by specific tumor dependency archetypes-represents a promising multidimensional therapeutic approach. Such strategies aim to simultaneously intercept multiple hallmark capabilities of cancer. Future research and clinical translation must rigorously account for the interconnected nature of this pathway to design effective and context-specific interventions.
4 The role of BCAT1 and BCAT2 in cancer
4.1 Integrated role of BCAT1 and BCAT2 in BCAAs metabolism and signaling
BCAT1 and BCAT2 constitute a spatially compartmentalized regulatory circuit that fundamentally dictates BCAAs metabolic fate. Their distinct subcellular localization-cytosolic versus mitochondrial-establishes a metabolic bifurcation point (Figure 5) (Ananieva and Wilkinson, 2018). BCAT1 primarily channels BCAAs toward anabolic and signaling outputs: its transamination reaction depletes α-ketoglutarate (α-KG), influencing epigenetics and hypoxia responses, while elevating leucine to directly activate mTORC1. The resultant branched-chain α-keto acids (BCKAs) may accumulate or be secreted, acting as paracrine signals (Wegermann et al., 2018). In contrast, BCAT2 commits BCKAs to oxidative catabolism in the mitochondria, thereby controlling their clearance rate and preventing aberrant accumulation that can lead to sustained, low-grade mTORC1 activation (Sjögren, 2021). This dynamic interplay between the two enzymes regulates the balance between cytosolic α-KG availability, BCKA pools, and downstream anabolic signaling. Functionally, BCAT1 often drives pro-inflammatory immune modulation and confers resistance to chemotherapy and targeted therapies (Mayers et al., 2016; Zhang et al., 2024; Zheng et al., 2016). Conversely, BCAT2 frequently supports an immunosuppressive tumor microenvironment and its modulation can synergize with specific agents to induce metabolic stress (Cai et al., 2023; Wang K. et al., 2021). Thus, the BCAT1/BCAT2 axis acts as a metabolic rheostat, where the relative activity of each enzyme determines whether BCAAs fuel growth signals or are catabolized for homeostasis-a balance frequently disrupted in cancer to support progression and therapy resistance.
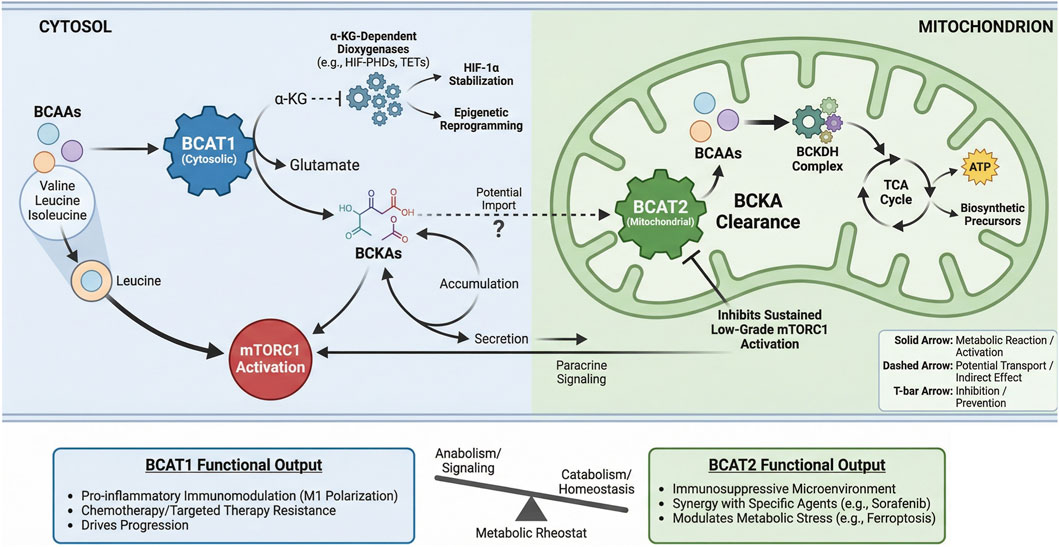
Figure 5. Spatial regulation of BCAAs metabolism by BCAT1 and BCAT2 dictates functional Output. BCAT1 (cytosolic) and BCAT2 (mitochondrial) compartmentalize branched-chain amino acid (BCAAs) metabolism, leading to distinct functional consequences. Cytosolic BCAT1 activity consumes α- KG to produce glutamate, supporting α-KG-dependent dioxygenases and generating BCKAs that can activate mTORC1 or signal in a paracrine manner. Its functional output promotes inflammation, therapy resistance, and disease progression. Mitochondrial BCAT2 initiates BCKA catabolism via the BCKDH complex, feeding the TCA cycle and preventing sustained mTORC1 activation. BCAT2 function fosters an immunosuppressive microenvironment and modulates metabolic stress responses.
4.2 The role of BCAT1 in cancer
Branched-chain amino acid transaminase 1 (BCAT1) is frequently overexpressed across multiple cancer types (Table 1), where it has been functionally linked to enhanced tumor proliferation, therapy resistance, and immune evasion. In gastric cancer, elevated BCAT1 activates PI3K/AKT/mTOR pathway, drives tumor proliferation, invasion, and angiogenesis, correlating with unfavorable clinical outcomes (Shu et al., 2021; Xu et al., 2018). Similarly, in oral carcinomas, BCAT1 promotes cell proliferation and migration through activation of the PI3K/Akt signaling axis (Yuan et al., 2025). Within triple-negative breast cancer models, BCAT1 appears indispensable for sustaining tumor growth; its inhibition induces apoptosis via suppression of the SHOC2–RAS–ERK pathway induce apoptosis, highlighting a potential therapeutic vulnerability (Huang et al., 2025). Consistent with these findings, BCAT1 expression is markedly higher in breast tumor tissues compared to adjacent normal tissue, where it supports tumor cell proliferation by promoting mitochondrial biogenesis through an mTOR-dependent mechanism (Thewes et al., 2017). Further evidence indicates that in chronic myeloid leukemia (CML), BCAT1 catalyzes the conversion of branched-chain α-keto acids (BCKAs) to BCAAs, leading to mTOR pathway activation independent of AKT signaling and thereby accelerating disease progression (Hattori et al., 2017). Experimental inhibition of BCAT1 with gabapentin significantly curbs proliferation in human colorectal cancer cells (Jedi et al., 2018). In glioblastoma, knockdown of BCAT1 compromises oxidative phosphorylation (OXPHOS), mTORC1 activity, and nucleotide synthesis. Supplementation with α-ketoglutarate synergistically potentiates these impairments, leading to tumor cell death (Zhang et al., 2022; Meurs and Nagrath, 2022). Under hypoxic conditions, hypoxia-inducible factor (HIF) regulates BCAAs metabolic reprogramming via BCAT1, and gabapentin treatment substantially impairs colony-forming capacity in glioma cell lines (Zhang et al., 2021). It should be noted, however, that BCAT1 is not universally overexpressed; in certain non-small cell lung cancer (NSCLC) subtypes, its expression remains relatively low, and inhibition exerts no significant effect on tumor growth (Mayers et al., 2016).
Beyond promoting tumor progression, BCAT1 also mediates resistance to chemotherapy. In hepatocellular carcinoma (HCC), it confers resistance to cisplatin (Zheng et al., 2016) while in lung adenocarcinoma, BCAT1 drives resistance to EGFR tyrosine kinase inhibitors (TKI) through epigenetic modulation of glycolytic pathways (Zhang et al., 2024). A parallel role is observed in CML, where BCAT1 enhances TKI resistance (Jiang et al., 2025). Collectively, these observations position BCAT1-driven metabolic reprogramming as a recurrent mechanism underlying acquired therapy resistance in diverse malignancies.
4.3 The role of BCAT2 in cancer
BCAT2, another key enzyme in BCAAs metabolism, is essential for the development and progression of various cancers (Table 2). BCAT2 enhances cellular BCAAs uptake, maintains metabolic homeostasis, and supports mitochondrial respiration. Notably, in mouse models of pancreatic cancer, exogenous BCAAs promote ductal organoid growth, whereas supplementation with BCKAs and nucleobases reverses the antitumor effects of BCAT2 inhibition (Li et al., 2020). In PDAC patient specimens and murine models, BCAT2 is consistently upregulated; its targeted knockdown in pancreatic tissue effectively suppresses the formation of pancreatic intraepithelial neoplasia (Lei et al., 2020). Post-translational regulation of BCAT2 involves acetylation at lysine 44 (K44), which promotes degradation via the ubiquitin-proteasome system, thereby attenuating BCAAs catabolism and restraining pancreatic cancer cell growth (Lei et al., 2020). In melanoma, BCAT2 fosters lipogenesis by depleting acetyl-CoA and inhibiting histone acetylation at promoter regions of lipogenic enzymes such as fatty acid synthase and ATP-citrate lyase (Tian et al., 2023a). In colorectal cancer, loss of BCAT2 leads to sustained mTORC1 activation and accelerates tumor progression (Kang et al., 2024). In prostate cancer, BCAT2 interacts with poly(C)-binding protein 1 through leucine 239, suppressing autophagy-dependent apoptosis and ferroptosis via the PI3K/AKT pathway (Mei et al., 2025). Furthermore, BCAT2 contributes to an immunosuppressive tumor microenvironment by downregulating pro-inflammatory chemokines and limiting cytotoxic lymphocyte infiltration, which in turn drives resistance to anti-PD-1/PD-L1 therapies (Cai et al., 2023). In HCC, BCAT2 modulates ferroptosis and exhibits synergistic antitumor activity when combined with sorafenib and sulfasalazine (Wang K. et al., 2021).
In summary, BCAT1 upregulation is observed in a wide spectrum of cancers-including gastric, oral, nasopharyngeal, breast, gliomas, certain NSCLC subtypes, and AML-where it promotes progression through PI3K/Akt, SHOC2–RAS–ERK, and mTOR signaling pathways. BCAT2, by contrast, has been implicated in NSCLC, PDAC, melanoma, HCC, and prostate cancer, where it accelerates tumorigenesis via mechanisms such as K44 acetylation-mediated degradation, ferroptosis regulation, and activation of mTORC1 and PI3K/AKT signaling. Although both enzymes generally exert pro-tumor effects, their roles can diverge depending on context. In AML, co-upregulation of BCAT1 and BCAT2 triggers BCAAs metabolic reprogramming and fuels disease progression. Interestingly, in colorectal cancer, the two enzymes appear to function antagonistically: BCAT1 inhibition impairs proliferation, whereas BCAT2 loss activates mTORC1 and accelerates disease, suggesting tissue-specific regulatory networks that remain to be fully elucidated. The differential involvement of BCAT1 and BCAT2 in therapy resistance further highlights their clinical relevance. While BCAT1 confers resistance to cisplatin in HCC and to TKIs in lung adenocarcinoma and CML, BCAT2 synergizes with sorafenib and sulfasalazine in HCC. These contrasting profiles imply that tailored therapeutic strategies-combining specific chemotherapy agents with selective inhibition or modulation of BCAT1 or BCAT2-could offer novel avenues for overcoming treatment resistance, particularly in malignancies such as HCC.
5 The role of BCKDH–BCKDK–PPM1K complex in cancer
The branched-chain α-ketoacid dehydrogenase complex (BCKDH) is the core enzyme complex for BCAAs catabolism. It is composed of three catalytic components: a heterotetrametric (α2β2) branched-chain α-ketoacid decarboxylase (E1), dihydrolipoyl transacylase (E2), and dihydrolipoyl dehydrogenase (E3). BCKDH directly regulates the oxidative metabolism of BCAAs and energy supply. The BCKDH complex catalyzes the rate-limiting step in BCAAs oxidation and is regulated by phosphorylation via BCKDK (inhibiting) and dephosphorylation via PPM1K (activating) (White et al., 2018; Sivanand and Vander Heiden, 2020; Peng et al., 2020). Reduced BCKDHA expression in pancreatic ductal adenocarcinoma (PDAC) suppresses proliferation by inhibiting fatty acid synthesis, suggesting a tumor-suppressive role (Lee et al., 2019). BCKDHA expression is markedly upregulated in melanoma tissues and cell lines, where it facilitates tumor progression by enhancing the expression of key lipogenic enzymes, FASN and ACLY (Tian et al., 2023b). Furthermore, X-ray irradiation induces BCKDHA dephosphorylation, activating branched-chain amino acid (BCAAs) catabolism. The resulting accumulation of BCAAs confers radio resistance by mitigating DNA damage and promoting cancer cell survival (Bo et al., 2025). PPM1K modulates BCAAs levels to regulate CDC20-mediated ubiquitination of MEIS1 and p21, affecting stem cell function in hematological malignancies (Liu et al., 2018). PPM1K is downregulated in pancreatic adenocarcinoma (PAAD) and correlates inversely with EMT and poor prognosis (Zhuang et al., 2023). BCKDH, the core enzymatic complex in BCAAs catabolism regulated by phosphorylation (BCKDK/PPM1K), plays a context-dependent role in cancer. It suppresses tumor growth in pancreatic cancer by inhibiting fatty acid synthesis but promotes progression in melanoma via enhanced lipogenesis. Additionally, its activation confers radio resistance through BCAAs accumulation-mediated DNA damage reduction, and its regulator PPM1K is associated with poor cancer prognosis.
BCKDK is overexpressed in many cancers and promotes growth and proliferation (Maguolo et al., 2022). Its inhibition with BT2 activates BCKDH, lowering BCAAs and BCKA levels (East et al., 2021). In NSCLC, BCKDK activates mTORC1, promoting proliferation and suppressing apoptosis (Wang Y. et al., 2021), potentially through enhancement of Rab1A–mTORC1 signaling (Xue et al., 2023). BCKDK also promotes EMT in CRC via Src-mediated phosphorylation at Y246, increasing its stability and activity (Tian et al., 2020). In HCC, APN mediates BCKDK phosphorylation at S31, activating ERK signaling and promoting metastasis (Zhai et al., 2020). In breast cancer, BCKDK stabilizes Talin1 by interfering with TRIM21-mediated ubiquitination, activating FAK/MAPK signaling and driving metastasis (Xu et al., 2023). Similarly, in ovarian cancer, BCKDK promotes proliferation and migration via MEK/ERK activation (Li H. et al., 2022). Targeting BCKDK may thus offer novel therapeutic opportunities. BCKDK is overexpressed in multiple cancers and promotes tumor growth, proliferation, and metastasis by activating key oncogenic pathways such as mTORC1, ERK, and FAK/MAPK signaling. Its inhibition, for example, with BT2, presents a promising therapeutic strategy by reactivating BCAAs catabolism (Table 3).
6 The role of BCAAs in modulating anti-tumor immunity
Branched-chain amino acids (BCAAs) and their metabolic reprogramming regulate immune system function by influencing the activity of key immune cells-including T cells, macrophages, and natural killer (NK) cells (Figure 6).

Figure 6. The dual role of branched-chain amino acids (BCAAs) in modulating anti-tumor immunity. This schematic illustrates the differential effects of branched-chain amino acids (BCAAs) on immune cell modulation within the tumor microenvironment. Left Panel: T Cell Modulation – BCAAs promote glucose metabolism reprogramming in CD8+ T cells via TCR and mTORC1 signaling, enhancing effector function and antitumor immunity. Middle Panel: Macrophage Modulation – Accumulation of BCAAs in macrophages suppresses nitric oxide (NO) production and mitigates H2O2-induced DNA damage, but also promotes M2-like polarization, supporting tumor growth and poor prognosis. Right Panel: NK Cell Modulation – BCAAs enhance the functional state of natural killer (NK) cells, contributing to antitumor immunity.
6.1 BCAAs metabolism in T cell-mediated tumor immunity
BCAAs play a dual and context-dependent role in tumor progression by modulating T cell function. On one hand, BCAAs-particularly leucine-are essential for immune regulation through metabolic reprogramming and can contribute to antitumor responses, though their precise mechanisms remain incompletely elucidated (Zhou et al., 2025). On the other hand, BCAAs depletion may compromise T cell effector functions, dampening the anti-tumor immune response (Xia et al., 2021). Specific ablation of Slc3a2 in Foxp3+ Tregs impairs their in vivo expansion by disrupting isoleucine-dependent mTORC1 activation and cellular metabolism; conversely, enhanced BCAA metabolism sustains Treg survival and immunosuppressive function to promote tumor immune evasion (Ikeda et al., 2017). BCAAs accumulation enhances CD8+ T cell antitumor immunity by reprogramming glucose metabolism. This mechanism strengthens CD8+ T cell effector function, improving responses in lung cancer (Yao et al., 2023). Correspondingly, high BCAAs levels inhibit breast cancer progression and metastasis, likely through boosting CD8+ T cell activity (Chi et al., 2022). SLC3A2 and SLC7A5 serve as primary transporters for essential amino acids (O'Sullivan et al., 2019). Within activated T cells, SLC3A2 promotes T cell expansion, whereas SLC7A5 is involved in T cell differentiation, mTORC1 signaling activation, and c-Myc expression (Ikeda et al., 2017; Nachef et al., 2021; Kanai, 2022). Notably, inhibition of SLC7A5 compromises the capacity of T cells to eliminate tumor cells. Following T cell receptor activation, human CD4+ T cells upregulate both BCAT1 and SLC7A5, thereby enhancing leucine influx and catabolism-a process particularly critical for T helper 17 (Th17) cell responses (Najumudeen et al., 2021).
6.2 BCAAs metabolism in macrophage-mediated tumor immunity
Branched-chain amino acids (BCAAs) are implicated in the metabolism and genetics of macrophages. BCAAs significantly suppress nitric oxide production in macrophages, with leucine exhibiting the most pronounced inhibitory effect. Additionally, BCAAs exert a protective role by mitigating hydrogen peroxide-induced DNA damage in macrophages (Lee et al., 2017). BCAAs metabolism also regulates macrophage polarization. In the pancreatic tumor microenvironment, infiltration of M2-type macrophages is markedly elevated which promotes pancreatic tumor growth and is associated with poor disease prognosis (Zhang et al., 2023). BCAT1 collaborates with bone marrow stromal antigen 2 (BST2) and the tyrosine kinase MERTK to facilitate cancer progression by regulating the type 2 polarization of tumor-associated macrophages, offering a potential therapeutic target for pancreatic cancer (Peng et al., 2023). Furthermore, BCAAs accumulation may promote M2-type macrophages, which support tumor growth and immunosuppression, while suppressing M1-type macrophages that possess anti-tumor functions.
6.3 BCAAs metabolism in NK cell-mediated tumor immunity
In acute myeloid leukemia (AML), elevated expression of SLC7A5 enables natural killer (NK) cells to sustain a proliferative and activated state even under arginine-depleted conditions, promoting apoptosis of AML cells (Stavrou et al., 2023). Beyond leukemic contexts, increased levels of Branched-chain amino acids (BCAAs) enhance NK cell function, thereby restraining breast cancer progression and metastasis (Chi et al., 2022). Conversely, in cytokine-activated NK cells, pharmacological inhibition of SLC7A5 lowers c-Myc protein abundance and suppresses mTORC1 signaling, which unexpectedly amplifies their antitumor efficacy (Loftus et al., 2018). Together, these observations highlight the context-dependent role of SLC7A5 and BCAAs metabolism in shaping NK cell activity, offering potential therapeutic avenues for modulating antitumor immunity.
BCAAs metabolism orchestrates a context-dependent immunomodulatory network that differentially regulates the function of major immune cell populations. In T cells, BCAAs exhibit a dual role: while required for maintaining CD8+ T cell effector capacity and antitumor immunity, they concurrently reinforce the immunosuppressive activity of regulatory T cells (Tregs), thereby facilitating immune escape. In macrophages, BCAAs-driven metabolic reprogramming promotes polarization toward a pro-tumor M2 phenotype while suppressing the antitumor functions associated with M1 macrophages. With respect to NK cells, although BCAAs availability generally supports cytotoxic function, pharmacological inhibition of the BCAAs transporter SLC7A5 has been observed to unexpectedly enhance antitumor efficacy, revealing a targetable metabolic checkpoint in these cells. Central to this regulatory network, SLC7A5 integrates extracellular BCAAs levels with intracellular metabolic and signaling rewiring across immune cell types, positioning it as a promising therapeutic target for reprogramming antitumor immunity.
7 BCAAs and epigenetic regulation in cancer
Epigenetic dysregulation-including DNA methylation, histone modifications, and RNA methylation-plays a key role in cancer (Esteller, 2008; Goldberg et al., 2007). METTL16 regulates BCAT1/2 expression via m6A modification, reprogramming BCAAs metabolism to support tumorigenesis (Zou et al., 2019). In AML, BCAT1 overexpression or IDH mutation reduces α-KG levels, inhibiting TET enzymes and causing DNA hypermethylation (Hattori et al., 2017; Raffel et al., 2017). In HCC, BCAT1 upregulation correlates with promoter hypomethylation (Zou et al., 2019). In lung cancer and leukemia, BCAT1 expression is suppressed by H3K9me2/3 (via G9a/SUV39H1) and H3K27me3 (via EZH2) (Wang et al., 2019; Gu et al., 2019). BCAAs metabolism fuels tumorigenesis through epigenetic mechanisms: METTL16 upregulates BCAT1/2 via m6A modification; BCAT1 or IDH mutations deplete α-KG, causing DNA hypermethylation; and repressive histone marks suppress BCAT1 in lung cancer.
BCAAs-derived acetyl-CoA contributes to histone acetylation, influencing gene expression. Leucine-derived acetyl-CoA promotes tumor growth, while lysine-derived acetyl-CoA supports self-renewal in CRC cells (La Vecchia and Sebastián, 2020). Intriguingly, a low-BCAAs diet reduces histone acetylation and delays aging in Drosophila, reversible by isoleucine supplementation (Weaver et al., 2023). Given the shared epigenetic mechanisms between aging and cancer, BCAAs-mediated epigenetic regulation represents a promising research avenue. BCAAs-derived acetyl-CoA drives histone acetylation and tumor growth. Leucine and lysine support cancer progression, while a low-BCAAs diet delays aging through reduced histone acetylation, suggesting shared epigenetic pathways in cancer and aging.
8 Dietary BCAAs interventions in cancer therapy
The potential of branched-chain amino acid (BCAAs) supplementation as an adjunct to cancer therapy is characterized by conflicting evidence, revealing a context-dependent relationship that varies by tumor type and clinical setting.
In colorectal cancer (CRC), findings appear divergent. While one investigation reported a positive association between higher dietary BCAAs intake and all-cause mortality in CRC patients (Long et al., 2021), this contrasts with data from large-scale case-control and major US cohort studies, which have indicated an inverse association between BCAAs intake and the risk of sigmoid colon cancer specifically (Rossi et al., 2021; Katagiri et al., 2020). This discrepancy highlights the need for subtype-specific analyses.
A more consistent pro-tumorigenic role for BCAAs has been observed in pancreatic cancer models. Both clinical epidemiology and preclinical experimentation align in this context. An Italian multicenter case-control study identified a positive correlation between dietary BCAAs intake and pancreatic cancer risk in humans (Rossi et al., 2023). Correspondingly, in genetically engineered LSL-KrasG12D/+; Pdx1-Cre (KC) mice-a model of pancreatic carcinogenesis--a BCAAs-enriched diet was shown to accelerate the progression of pancreatic intraepithelial neoplasia (PanIN) (Li J. T. et al., 2022).
Conversely, in breast cancer, evidence points toward a potential protective or therapeutic effect of BCAAs. Epidemiological data suggest an inverse correlation between dietary BCAAs intake and the risk of postmenopausal breast cancer (Nouri-Majd et al., 2022). This observation is supported by mechanistic studies demonstrating that elevated BCAAs concentrations in vitro impair the migration and invasion capacities of breast cancer cells, an effect potentially mediated by N-cadherin downregulation. Furthermore, in vivo, a high-BCAAs diet was found to suppress both primary tumor growth and lung metastasis in mouse models, suggesting a role for BCAAs supplementation in inhibiting breast cancer progression (Chi et al., 2022). However, Assessment of long-term dietary BCAAs intake did not support an association with invasive breast cancer risk, overall or for any individual BCAAs (Tobias et al., 2021).
Beyond direct antitumor effects, BCAAs supplementation demonstrates considerable utility in supportive oncology care, particularly in hepatic malignancies. Perioperative administration of BCAAs has been associated with a reduced incidence of postoperative complications, including ascites and infections, in patients undergoing cancer surgery (Cogo et al., 2021). More specifically, in hepatocellular carcinoma (HCC) patients receiving locoregional therapies (e.g., radiofrequency ablation, transarterial chemoembolization), BCAAs supplementation has been shown to improve nutritional and metabolic parameters-such as serum albumin levels and the non-protein respiratory quotient (npRQ)-while also enhancing quality of life. Importantly, this supportive intervention is linked to improved clinical outcomes, including a reduction in Child-Pugh score, lower recurrence rates, and prolonged overall survival (Sideris et al., 2023). These benefits suggest that BCAAs may effectively mitigate adverse effects associated with conventional anticancer treatments, including surgery, chemotherapy, and radiotherapy.
In summary, the impact of BCAAs supplementation is not uniform across cancers, exhibiting pro-tumor effects in pancreatic models, potential inhibitory effects in breast cancer, and significant supportive benefits in the management of HCC. This tissue- and context-specificity underscores the necessity for precise, indication-driven application in clinical practice.
9 Targeted BCAAs inhibitors in cancer
The accumulation and dietary intake of Branched-chain amino acids (BCAAs) have been shown to modulate tumor cell proliferation, overall tumor burden, and patient survival. Consequently, key components of BCAAs handling-including specific transporters and catabolic enzymes such as BCAT1, BCAT2, and BCKDK-are increasingly regarded as potential therapeutic targets in oncology. Several classes of inhibitors directed against these enzymes have subsequently been identified and characterized (Table 4).

Table 4. Pharmacological targeting of the BCAAs metabolic network: inhibitors of BCAT1, BCAT2, and BCKDK.
9.1 BCAT1 inhibitors
BCAT1, which is highly expressed in neuronal tissues and contributes to glutamate synthesis can be inhibited by several compound classes N-Arylcarbonylarylsulfonylhydrazides function as BCAT1 inhibitors and have been explored as neuroprotective agents in neurodegenerative contexts. Their mechanism extends to the preservation of mitochondrial function, achieved by mitigating oleic acid–induced ROS generation and membrane potential loss, thereby reducing autophagic flux and suppressing associated airway inflammation and remodeling (Hu et al., 2006). Thiazolones represent another class of BCAT1 inhibitors primarily investigated for preventing neuronal loss, though their potential application in cancer therapy remains to be elucidated (Li et al., 2004). Among compounds evaluated in oncology, 4-methyl-5-oxohexanoic acid (MOHA) has been identified as a BCAT1 inhibitor with antitumor activity. In MDA-MB-231 breast cancer cells, MOHA downregulates surface CD147 expression, attenuates immune reactivity, and inhibits proliferation. It also suppresses the growth of other cancer lines, including MCF-7, MDA-MB-435, and HT-29, while reducing levels of matrix metalloproteinase-2 (MMP2) (Papatthanassiu, 2012). The widely used neuroactive agent gabapentin-along with its analogs-acts as a selective, competitive inhibitor of BCAT1 and has been proposed for the treatment of glioblastoma and astrocytoma (Kukkar et al., 2013; Chen et al., 2022; Radlwimmer et al., 2013). Interestingly, however, studies in HCT116 colorectal cancer cells revealed that gabapentin-mediated growth suppression occurs independently of BCAAs transamination, pointing to an off-target effect possibly involving mitochondrial BCKA catabolism (Grankvist et al., 2018). This suggests that gabapentin may exert context-dependent antitumor effects through both BCAT1-dependent and -independent pathways. Beyond conventional small molecules, several other agents modulate BCAT1 activity in cancer settings. Bufalin, a broad-spectrum anticancer compound known to regulate PI3K/Akt, Wnt/β-catenin, and NF-κB signaling, has recently been shown to bind directly to BCAT1, thereby sensitizing pancreatic cancer cells to gemcitabine and 5-fluorouracil (Zhang W. et al., 2025). The GABA-derived molecule WQQ-345, featuring a unique bridged bicyclic scaffold, exhibits in vitro and in vivo activity against TKI-resistant lung cancer cells with high BCAT1 expression (Luo et al., 2025). Epigenetic regulation also plays a role: low circulating levels of miR-320a in patients with growth hormone–secreting pituitary neuroendocrine tumors correlate with BCAT1 upregulation, and restoration of this microRNA suppresses tumor progression via BCAT1 targeting (Zhao et al., 2024). Furthermore, curcumin induces apoptosis in cytarabine-resistant AML cells partly through downregulation of BCAT1 and inhibition of mTOR signaling (Tseng et al., 2021). The AT1R antagonist candesartan dose-dependently inhibits both wild-type and mutant BCAT1 (E61A), impairs migration and Ras Homolog Gene Family, Member C (RhoC) activity in esophageal and gastric cancer cells, and suppresses peritoneal metastasis in a RhoC-dependent manner (Qian et al., 2023).
9.2 BCAT2 inhibitors
Inhibition of BCAT2 has been pursued using structurally diverse compounds. Fused imidazoles containing cis-1,3-cycloalkanedi-amine scaffolds-such as benzimidazoles, pyridinoimidazoles, and pyrimidinoimidazoles-effectively reduce BCAT2 levels in engineered A549 cells (Deng et al., 2015). Through screening of DNA-encoded libraries encompassing 14 billion compounds, Deng et al. identified biphenyl-tetrahydropyrrole (12-15e) as a potent BCAT2 inhibitor active after DNA cleavage (Deng et al., 2015). Another orally available agent, dihydropyrazolopyrimidine with benzylamine (13–61), elevates plasma BCAAs concentrations in a dose-dependent manner via BCAT2 inhibition. Additional BCAT2-directed molecules include thiophenopyrimidine carboxamides, which show micromolar inhibition, detectable cellular activity, and favorable solubility. The clinically used angiotensin receptor blocker telmisartan concentration-dependently suppresses BCAT2 enzymatic activity in biochemical and cellular assays and may promote lipid metabolism through BCAT2-mediated PPAR activation, suggesting potential utility in metabolic disorders (Zhang et al., 2025b). Finally, Ray and colleagues designed a series of pyrazoline derivatives, among which compounds 16-4a, 16-4e, and 16-4f demonstrated effective binding to the BCAT2 active site in molecular docking studies (Ray et al., 2023). Together, these findings underscore the pharmacologic tractability of BCAAs catabolic enzymes and highlight a growing repertoire of chemical tools and drug candidates for potentially modulating BCAT1 and BCAT2 in cancer and related pathologies.
9.3 BCKDK inhibitors
Branched-chain α-ketoacid dehydrogenase kinase (BCKDK) plays a multifaceted role in tumorigenesis, notably through phosphorylation of MEK to activate the RAS/RAF/MEK/ERK signaling axis, thereby promoting cellular proliferation (Xue et al., 2017). Given its central regulatory function, BCKDK has emerged as a compelling therapeutic target in oncology. Pharmacological inhibition of BCKDK, achieved via small-molecule allosteric modulators such as 3,6-dichloro-1-benzothiophene-2-carboxylic acid (BT2) and (S)-α-chlorophenylpropionate, disrupts its activity by inducing conformational shifts within the N-terminal domain helix (Tso et al., 2014). This structural alteration triggers the dissociation of BCKDK from the BCKDH complex and facilitates its subsequent degradation, ultimately suppressing tumor growth (Du et al., 2022; East et al., 2021). Comparative analyses indicate that BT2 possesses superior inhibitory potency and metabolic stability relative to (S)-α-chlorophenylpropionate, alongside an ability to enhance BCKDH activity in primary hepatocytes (East et al., 2021).
The therapeutic potential of BCKDK inhibition has been validated across diverse malignancies. In triple-negative breast cancer models, intervention targeting BCKDK lowers branched-chain ketoacid levels, remodels BCAAs metabolic flux, and concurrently upregulates Sestrin2 while suppressing mTORC1 signaling-a combination that attenuates proliferation and induces apoptotic cell death. Synergy with doxorubicin has further been documented (Biswas et al., 2021). Similarly, in ovarian cancer and non-small cell lung cancer, BCKDK expression correlates with disease progression, and its genetic or pharmacological inhibition curbs cancer cell proliferation, migration, and cell cycle progression (Li H. et al., 2022; Bagheri et al., 2022). Continuous optimization of inhibitory strategies is underway. Leveraging virtual screening and structural biology, novel allosteric compounds including PPHN and POAB have been identified, demonstrating potent BCKDK inhibition coupled with marked anti-proliferative and pro-apoptotic activities both in vitro and in vivo (Li C. et al., 2024). One particularly optimized derivative, PF-07328948, was developed through strategic modification of the BT2 core via 3-aryl substitution, capitalizing on a cryptic binding pocket. This agent effectively degrades BCKDK in situ, markedly reduces circulating BCAAs and BCKA concentrations, exhibits favorable pharmacokinetics, and improves both metabolic parameters and cardiac function endpoints in rodent models of heart failure (Filipski et al., 2025).
Beyond its metabolic roles, BCKDK participates in DNA damage response pathways, including homologous recombination repair. Co-administration of BCKDK inhibitors such as GSK180736A with DNA-damaging chemotherapeutics can therefore overcome treatment resistance in breast cancer models (Liu et al., 2025). In parallel, fragment-based screening employing NMR and computational methods has identified tetrazole-containing scaffolds that bind BCKDK at multiple sites. Subsequent structure-based virtual screening revealed that certain angiotensin receptor blocker antihypertensives and related compounds also act as potent BCKDK inhibitors, suggesting a novel therapeutic avenue that merges BCKDK inhibition with cardiovascular management in conditions like heart failure (Liu et al., 2023). Reports of bioactive bicyclic carboxyamide inhibitors further underscore the expanding chemical space for targeting this kinase (Liang et al., 2024). Collectively, these advances highlight BCKDK and associated pathways-including the USP1/BCAT2 axis-as precision oncology targets with translational promise across several disease contexts.
It should be noted, however, that current inhibitors present certain pharmacological challenges. BT2, for instance, acts as a lipophilic weak acid capable of mitochondrial uncoupling, which may lead to off-target effects. Its high affinity for plasma albumin also potently reduces systemic tryptophan levels, a phenomenon that warrants consideration in therapeutic development.
9.4 Therapeutic promise and translational hurdles
Building upon prior mechanistic insights, the translational targeting of branched-chain amino acid (BCAAs) metabolism-especially through inhibition of BCAT1, BCAT2, and BCKDK-reveals considerable therapeutic potential alongside substantial complexity.
Concerning inhibitor development, although multiple chemical scaffolds have been explored-from gabapentin and its analogs against BCAT1 to allosteric modulators such as BT2 for BCKDK-most remain in preclinical or early discovery stages (Kukkar et al., 2013; Tso et al., 2014; Li C. et al., 2024). Exceptions include repurposed clinically approved agents like the AT1R antagonist candesartan and the neuroactive drug gabapentin, which exhibit direct BCAT1 inhibitory activity in cancer models and may allow accelerated clinical evaluation (Qian et al., 2023; Kukkar et al., 2013). Nevertheless, a dedicated, clinical-grade inhibitor designed specifically for oncology applications has not yet been developed, underscoring a persistent disconnect between target validation and drug discovery.
A central translational concern involves potential effects on normal tissues. BCAAS metabolism is integral to physiological homeostasis, particularly in the brain and skeletal muscle. BCAT1, for example, supports glutamate synthesis in neuronal tissue, raising the possibility of neurotoxicity with systemic inhibition (Dimou et al., 2022). Likewise, BCAT2 plays a key role in nitrogen and energy balance in muscle. Consequently, establishing a therapeutic window will likely require tumor-selective delivery approaches-such as nanoparticle conjugates or protease-activated prodrugs-or the identification of isoform- or context-selective inhibitors that preserve essential physiological functions.
Several key challenges hinder clinical translation. First, metabolic plasticity within tumors and a dynamic tumor microenvironment may enable compensatory pathways or alternative nutrient acquisition, leading to inherent or adaptive resistance. Second, the absence of validated predictive biomarkers represents a major obstacle to identifying patients whose tumors display genuine dependence on BCAAs pathways. Putative biomarkers, including BCAT1 promoter methylation, circulating levels of branched-chain ketoacids (BCKAs), or specific transcriptional signatures, require rigorous clinical validation (Wegermann et al., 2018; Zou et al., 2019). Third, achieving favorable pharmacokinetics and target selectivity with current lead compounds, while limiting on-target toxicity in normal tissues, remains a demanding pharmacological challenge.
Pharmacological modulation may also provoke unintended off-target or compensatory responses. For instance, gabapentin’s anti-proliferative effects in certain settings appear independent of BCAT1 inhibition and may involve off-target mitochondrial actions (Grankvist et al., 2018). The BCKDK inhibitor BT2, though effective, functions as a lipophilic weak acid capable of mitochondrial uncoupling-a property that could influence both its efficacy and adverse effect profile (East et al., 2021). Moreover, systemic BCAT2 inhibition would be expected to increase circulating BCAAs, which might inadvertently support tumor growth in contexts where BCAAs uptake is not limiting, or induce unanticipated systemic metabolic alterations.
In summary, while BCAAs-catabolizing enzymes remain attractive therapeutic targets, their successful incorporation into oncology demands a multifaceted approach. This includes the rational design of next-generation inhibitors with improved selectivity, the parallel development of reliable companion diagnostics, and the proactive addressing of metabolic adaptation through rationally designed combination regimens. Navigating these translational subtleties will be critical to transforming the compelling preclinical data on BCAAs metabolism into precise and clinically viable cancer therapies.
10 Conclusion and future perspectives
Branched-chain amino acid (BCAAs) metabolism, once viewed primarily through the lens of energy production and protein synthesis, is now recognized as a central signaling node and a critical driver of metabolic reprogramming in cancer. Its role in tumor progression is multifaceted, encompassing not only the provision of biosynthetic precursors but also the regulation of key oncogenic pathways-including mTOR and PI3K/AKT-the modulation of epigenetic programs, and the active contribution to an immunosuppressive tumor microenvironment (TME). Enzymes such as BCAT1, BCAT2, and BCKDK, which govern this metabolic network, thus represent promising albeit complex therapeutic targets. Translating this mechanistic understanding into clinical advancement necessitates a coordinated strategy addressing several interdependent fronts.
A primary challenge lies in the development of inhibitors with high isoform selectivity and tumor-specific delivery. Research efforts must prioritize the creation of clinical-grade compounds targeting BCAT1 and BCKDK, coupled with refined delivery mechanisms designed to minimize on-target toxicity in healthy tissues-a hurdle that has historically limited the therapeutic window of metabolism-targeting agents.
Closely linked to this therapeutic aim is the urgent need to identify predictive biomarkers. The deployment of comprehensive multi-omics profiling across diverse malignancies will be essential to define reliable indicators-for instance, BCAT1 promoter methylation status, circulating branched-chain ketoacid (BCKA) concentrations, or specific transcriptional subtypes-that can stratify patient populations whose tumors are truly dependent on BCAAs metabolism and are therefore more likely to respond to its inhibition.
The physiological relevance of preclinical models also requires critical reassessment. Traditional cell lines and xenograft systems often fail to recapitulate the metabolic interplay and immune context of human tumors. A shift toward more integrated models, such as patient-derived organoids, immunocompetent genetically engineered mouse models (GEMMs), and humanized mouse models, would provide a more accurate platform for evaluating drug efficacy and understanding TME-specific metabolic crosstalk.
Moreover, the systemic consequences of prolonged BCAAs pathway inhibition remain poorly characterized. Potential impacts on whole-organism physiology-including muscle homeostasis, systemic glucose regulation, and immune function-must be rigorously investigated to anticipate and manage possible side effects in future clinical applications.
Ultimately, the clinical evaluation of these agents will depend on innovative trial design. Well-controlled, early-phase studies that incorporate biomarker-driven patient stratification are imperative to assess the safety and preliminary efficacy of BCAAs pathway inhibitors, both as monotherapies and in rational combination with existing modalities like chemotherapy, radiotherapy, or immunotherapy.
By deciphering the context-dependent roles of BCAAs metabolism in cancer, fundamental insights into tumor biology will be gained, while simultaneously paving the way for novel, metabolism-directed therapeutic strategies with the potential to improve patient outcomes.
Author contributions
BH: Writing – original draft, Writing – review and editing. LL: Writing – original draft, Writing – review and editing. YL: Writing – original draft, Writing – review and editing. MH: Writing – original draft, Writing – review and editing. LZ: Writing – original draft, Writing – review and editing, Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration. RH: Writing – original draft, Writing – review and editing, Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This work was supported by the Health Research Project of Hunan Provincial Health Commission (W20242017) and the Science and Technology Innovation Team of Shanxi Province (No. 202204051001024).
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Altman, B. J., Stine, Z. E., and Dang, C. V. (2016). From krebs to clinic: glutamine metabolism to cancer therapy. Nat. Rev. Cancer 16 (10), 619–634. doi:10.1038/nrc.2016.114
An, Q., Lin, R., Wang, D., and Wang, C. (2022). Emerging roles of fatty acid metabolism in cancer and their targeted drug development. Eur. J. Med. Chem. 240, 114613. doi:10.1016/j.ejmech.2022.114613
Ananieva, E. A., and Wilkinson, A. C. (2018). Branched-chain amino acid metabolism in cancer. Curr. Opin. Clin. Nutr. Metab. Care 21 (1), 64–70. doi:10.1097/MCO.0000000000000430
Bagheri, Y., Saeidi, M., Yazdani, R., Babaha, F., Falak, R., Azizi, G., et al. (2022). Evaluation of effective factors on IL-10 signaling in B cells in patients with selective IgA deficiency. Eur. Cytokine Netw. 33 (1), 1–12. doi:10.1684/ecn.2021.0464
Bayoumi, A., Grønbæk, H., George, J., and Eslam, M. (2020). The epigenetic drug discovery landscape for metabolic-associated fatty liver disease. Trends Genet. 36 (6), 429–441. doi:10.1016/j.tig.2020.03.003
Bergers, G., and Fendt, S. M. (2021). The metabolism of cancer cells during metastasis. Nat. Rev. Cancer 21 (3), 162–180. doi:10.1038/s41568-020-00320-2
Bifari, F., and Nisoli, E. (2017). Branched-chain amino acids differently modulate catabolic and anabolic states in mammals: a pharmacological point of view. Br. J. Pharmacol. 174 (11), 1366–1377. doi:10.1111/bph.13624
Biswas, D., Slade, L., Duffley, L., Mueller, N., Dao, K. T., Mercer, A., et al. (2021). Inhibiting BCKDK in triple negative breast cancer suppresses protein translation, impairs mitochondrial function, and potentiates doxorubicin cytotoxicity. Cell Death Discov. 7 (1), 241. doi:10.1038/s41420-021-00602-0
Bledsoe, R. K., Dawson, P. A., and Hutson, S. M. (1997). Cloning of the rat and human mitochondrial branched chain aminotransferases (BCATm). Biochim. Biophys. Acta 1339 (1), 9–13. doi:10.1016/s0167-4838(97)00044-7
Bo, T., and Fujii, J. (2024). Primary roles of branched chain amino acids (BCAAs) and their metabolism in physiology and metabolic disorders. Molecules 30 (1), 56. doi:10.3390/molecules30010056
Bo, T., Osaki, T., and Fujii, J. (2025). Dephosphorylation of branched-chain α-keto acid dehydrogenase E1α (BCKDHA) promotes branched-chain amino acid catabolism and renders cancer cells resistant to X-rays by mitigating DNA damage. Biochem. Biophys. Res. Commun. 742, 151154. doi:10.1016/j.bbrc.2024.151154
Bray, F., Laversanne, M., Sung, H., Ferlay, J., Siegel, R. L., Soerjomataram, I., et al. (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74 (3), 229–263. doi:10.3322/caac.21834
Cai, Z., Chen, J., Yu, Z., Li, H., Liu, Z., Deng, D., et al. (2023). BCAT2 shapes a noninflamed tumor microenvironment and induces resistance to Anti-PD-1/PD-L1 immunotherapy by negatively regulating proinflammatory chemokines and anticancer immunity. Adv. Sci. (Weinh) 10 (8), e2207155. doi:10.1002/advs.202207155
Chen, Y., and Li, P. (2016). Fatty acid metabolism and cancer development. Sci. Bull. 61 (19), 1473–1479. doi:10.1007/s11434-016-1129-4
Chen, Y., Wu, Q., Jin, Z., Qin, Y., Meng, F., and Zhao, G. (2022). Review of voltage-gated calcium channel α2δ subunit ligands for the treatment of chronic neuropathic pain and insight into structure-activity relationship (SAR) by pharmacophore modeling. Curr. Med. Chem. 29 (30), 5097–5112. doi:10.2174/0929867329666220407093727
Cheng, C., Geng, F., Cheng, X., and Guo, D. (2018). Lipid metabolism reprogramming and its potential targets in cancer. Cancer Commun. (Lond) 38 (1), 27. doi:10.1186/s40880-018-0301-4
Chi, R., Yao, C., Chen, S., Liu, Y., He, Y., Zhang, J., et al. (2022). Elevated BCAA suppresses the development and metastasis of breast cancer. Front. Oncol. 12, 887257. doi:10.3389/fonc.2022.887257
Choi, B. H., Hyun, S., and Koo, S. H. (2024). The role of BCAA metabolism in metabolic health and disease. Exp. Mol. Med. 56 (7), 1552–1559. doi:10.1038/s12276-024-01263-6
Cogo, E., Elsayed, M., Liang, V., Cooley, K., Guerin, C., Psihogios, A., et al. (2021). Are supplemental branched-chain amino acids beneficial during the oncological peri-operative period: a systematic review and meta-analysis. Integr. Cancer Ther. 20, 1534735421997551. doi:10.1177/1534735421997551
Deng, H., Zhou, J., Sundersingh, F. S., Summerfield, J., Somers, D., Messer, J. A., et al. (2015). X-ray binding mode study of BCATm inhibitors from a novel DNA-encoded library. ACS Med. Chem. Lett. 6 (8), 919–924. doi:10.1021/acsmedchemlett.5b00179
Di Malta, C., and Ballabio, A. (2018). Transcriptional regulation of mTORC1 in cancer. Oncotarget 9 (95), 36734–36735. doi:10.18632/oncotarget.26229
Dimou, A., Tsimihodimos, V., and Bairaktari, E. (2022). The critical role of the branched chain amino acids (BCAAs) catabolism-regulating enzymes, branched-chain aminotransferase (BCAT) and branched-chain α-Keto acid dehydrogenase (BCKD), in human pathophysiology. Int. J. Mol. Sci. 23 (7), 67–174. doi:10.3390/ijms23074022
Du, X., You, H., Li, Y., Wang, Y., Hui, P., Qiao, B., et al. (2018). Relationships between circulating branched chain amino acid concentrations and risk of adverse cardiovascular events in patients with STEMI treated with PCI. Sci. Rep. 8 (1), 15809. doi:10.1038/s41598-018-34245-6
Du, C., Liu, W. J., Yang, J., Zhao, S. S., and Liu, H. X. (2022). The role of branched-chain amino acids and branched-chain α-Keto acid dehydrogenase kinase in metabolic disorders. Front. Nutr. 9, 932670. doi:10.3389/fnut.2022.932670
East, M. P., Laitinen, T., and Asquith, C. R. M. (2021). BCKDK: an emerging kinase target for metabolic diseases and cancer. Nat. Rev. Drug Discov. 20 (7), 498. doi:10.1038/d41573-021-00107-6
Esteller, M. (2008). Epigenetics in cancer. N. Engl. J. Med. 358 (11), 1148–1159. doi:10.1056/NEJMra072067
Filipski, K. J., Martinez-Alsina, L. A., Reese, M. R., Evrard, E., Buzon, L. M., Cameron, K. O., et al. (2025). Discovery of first branched-chain ketoacid dehydrogenase kinase (BDK) inhibitor clinical candidate PF-07328948. J. Med. Chem. 68 (3), 2466–2482. doi:10.1021/acs.jmedchem.4c02230
Goldberg, A. D., Allis, C. D., and Bernstein, E. (2007). Epigenetics: a landscape takes shape. Cell 128 (4), 635–638. doi:10.1016/j.cell.2007.02.006
Grankvist, N., Lagerborg, K. A., Jain, M., and Nilsson, R. (2018). Gabapentin can suppress cell proliferation independent of the cytosolic branched-chain amino acid transferase 1 (BCAT1). Biochemistry 57 (49), 6762–6766. doi:10.1021/acs.biochem.8b01031
Gu, Z., Liu, Y., Cai, F., Patrick, M., Zmajkovic, J., Cao, H., et al. (2019). Loss of EZH2 reprograms BCAA metabolism to drive leukemic transformation. Cancer Discov. 9 (9), 1228–1247. doi:10.1158/2159-8290.CD-19-0152
Guo, D., Meng, Y., Zhao, G., Wu, Q., and Lu, Z. (2025). Moonlighting functions of glucose metabolic enzymes and metabolites in cancer. Nat. Rev. Cancer 25 (6), 426–446. doi:10.1038/s41568-025-00800-3
Hattori, A., Tsunoda, M., Konuma, T., Kobayashi, M., Nagy, T., Glushka, J., et al. (2017). Cancer progression by reprogrammed BCAA metabolism in myeloid leukaemia. Nature 545 (7655), 500–504. doi:10.1038/nature22314
Hu, L.-Y., Boxer, P. A., Kesten, S. R., Lei, H. J., Wustrow, D. J., Moreland, D. W., et al. (2006). The design and synthesis of human branched-chain amino acid aminotransferase inhibitors for treatment of neurodegenerative diseases. Bioorg. Med. Chem. Lett. 16 (9), 2337–2340. doi:10.1016/j.bmcl.2005.07.058
Huang, L., Li, G., Zhang, Y., Zhuge, R., Qin, S., Qian, J., et al. (2025). Small-molecule targeting BCAT1-mediated BCAA metabolism inhibits the activation of SHOC2-RAS-ERK to induce apoptosis of triple-negative breast cancer cells. J. Adv. Res. 75, 723–738. doi:10.1016/j.jare.2024.10.021
Ichihara, A. (1975). Isozyme patterns of branched-chain amino acid transaminase during cellular differentiation and carcinogenesis. Ann. N. Y. Acad. Sci. 259, 347–354. doi:10.1111/j.1749-6632.1975.tb25431.x
Ikeda, K., Kinoshita, M., Kayama, H., Nagamori, S., Kongpracha, P., Umemoto, E., et al. (2017). Slc3a2 mediates branched-chain amino-acid-dependent maintenance of regulatory T cells. Cell Rep. 21 (7), 1824–1838. doi:10.1016/j.celrep.2017.10.082
Jedi, M., Young, G. P., Pedersen, S. K., and Symonds, E. L. (2018). Methylation and gene expression of BCAT1 and IKZF1 in colorectal cancer tissues. Clin. Med. Insights Oncol. 12, 1179554918775064. doi:10.1177/1179554918775064
Jiang, Y., Zhang, D., He, X., Chen, C., Xie, L., Liu, L., et al. (2025). BCAT1 contributes to the development of TKI-resistant CML. Cell Oncol. (Dordr) 48 (2), 411–424. doi:10.1007/s13402-024-01003-y
Kadowaki, H., and Knox, W. E. (1982). Cytosolic and mitochondrial isoenzymes of branched-chain amino acid aminotransferase during development of the rat. Biochem. J. 202 (3), 777–783. doi:10.1042/bj2020777
Kamphorst, J. J., Nofal, M., Commisso, C., Hackett, S. R., Lu, W., Grabocka, E., et al. (2015). Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 75 (3), 544–553. doi:10.1158/0008-5472.CAN-14-2211
Kanai, Y. (2022). Amino acid transporter LAT1 (SLC7A5) as a molecular target for cancer diagnosis and therapeutics. Pharmacol. Ther. 230, 107964. doi:10.1016/j.pharmthera.2021.107964
Kang, Z. R., Jiang, S., Han, J. X., Gao, Y., Xie, Y., Chen, J., et al. (2024). Deficiency of BCAT2-mediated branched-chain amino acid catabolism promotes colorectal cancer development. Biochim. Biophys. Acta Mol. Basis Dis. 1870 (2), 166941. doi:10.1016/j.bbadis.2023.166941
Katagiri, R., Song, M., Zhang, X., Lee, D. H., Tabung, F. K., Fuchs, C. S., et al. (2020). Dietary intake of branched-chain amino acids and risk of colorectal cancer. Cancer Prev. Res. (Phila) 13 (1), 65–72. doi:10.1158/1940-6207.CAPR-19-0297
Kawaguchi, T., Izumi, N., Charlton, M. R., and Sata, M. (2011). Branched-chain amino acids as pharmacological nutrients in chronic liver disease. Hepatology 54 (3), 1063–1070. doi:10.1002/hep.24412
Kukkar, A., Bali, A., Singh, N., and Jaggi, A. S. (2013). Implications and mechanism of action of gabapentin in neuropathic pain. Arch. Pharm. Res. 36 (3), 237–251. doi:10.1007/s12272-013-0057-y
La Vecchia, S., and Sebastián, C. (2020). Metabolic pathways regulating colorectal cancer initiation and progression. Semin. Cell Dev. Biol. 98, 63–70. doi:10.1016/j.semcdb.2019.05.018
Lee, J. H., Park, E., Jin, H. J., Lee, Y., Choi, S. J., Lee, G. W., et al. (2017). Anti-inflammatory and anti-genotoxic activity of branched chain amino acids (BCAA) in lipopolysaccharide (LPS) stimulated RAW 264.7 macrophages. Food Sci. Biotechnol. 26 (5), 1371–1377. doi:10.1007/s10068-017-0165-4
Lee, J. H., Cho, Y. R., Kim, J. H., Kim, J., Nam, H. Y., Kim, S. W., et al. (2019). Branched-chain amino acids sustain pancreatic cancer growth by regulating lipid metabolism. Exp. Mol. Med. 51 (11), 1–11. doi:10.1038/s12276-019-0350-z
Lei, M. Z., Li, X. X., Zhang, Y., Li, J. T., Zhang, F., Wang, Y. P., et al. (2020). Acetylation promotes BCAT2 degradation to suppress BCAA catabolism and pancreatic cancer growth. Signal Transduct. Target Ther. 5 (1), 70. doi:10.1038/s41392-020-0168-0
Li, X., and Wang, J. (2020). Mechanical tumor microenvironment and transduction: Cytoskeleton mediates cancer cell invasion and metastasis. Int. J. Biol. Sci. 16 (12), 2014–2028. doi:10.7150/ijbs.44943
Li, L., Sengupta, A., Haque, N., Grundke-Iqbal, I., and Iqbal, K. (2004). Memantine inhibits and reverses the alzheimer type abnormal hyperphosphorylation of tau and associated neurodegeneration. FEBS Lett. 566 (1-3), 261–269. doi:10.1016/j.febslet.2004.04.047
Li, J. T., Yin, M., Wang, D., Wang, J., Lei, M. Z., Zhang, Y., et al. (2020). BCAT2-mediated BCAA catabolism is critical for development of pancreatic ductal adenocarcinoma. Nat. Cell Biol. 22 (2), 167–174. doi:10.1038/s41556-019-0455-6
Li, H., Yu, D., Li, L., Xiao, J., Zhu, Y., Liu, Y., et al. (2022). BCKDK promotes ovarian cancer proliferation and migration by activating the MEK/ERK signaling pathway. J. Oncol. 2022, 3691635. doi:10.1155/2022/3691635
Li, J. T., Li, K. Y., Su, Y., Shen, Y., Lei, M. Z., Zhang, F., et al. (2022). Diet high in branched-chain amino acid promotes PDAC development by USP1-mediated BCAT2 stabilization. Natl. Sci. Rev. 9 (5), nwab212. doi:10.1093/nsr/nwab212
Li, Z., Wang, Y., and Sun, H. (2024). The role of branched-chain amino acids and their metabolism in cardiovascular diseases. J. Cardiovasc Transl. Res. 17 (1), 85–90. doi:10.1007/s12265-024-10479-w
Li, C., Yang, Q., and Zhang, L. (2024). Identification of putative allosteric inhibitors of BCKDK via virtual screening and biological evaluation. J. Enzyme Inhib. Med. Chem. 39 (1), 2290458. doi:10.1080/14756366.2023.2290458
Liang, J., Wang, X., Ortiz, F., Shishodia, G., Liu, T., Gao, C., et al. (2024). Bicyclic inhibitors of branched-chain α-Keto acid dehydrogenase kinase (BDK) with in vivo activity. ACS Med. Chem. Lett. 15 (11), 1899–1906. doi:10.1021/acsmedchemlett.4c00362
Liu, Y., and Zheng, Z. (2024). Understanding the global cancer statistics 2022: growing cancer burden. Sci. China Life Sci. 67 (10), 2274–2276. doi:10.1007/s11427-024-2657-y
Liu, X., Zhang, F., Zhang, Y., Li, X., Chen, C., Zhou, M., et al. (2018). PPM1K regulates hematopoiesis and leukemogenesis through CDC20-Mediated ubiquitination of MEIS1 and p21. Cell Rep. 23 (5), 1461–1475. doi:10.1016/j.celrep.2018.03.140
Liu, S., Kormos, B. L., Knafels, J. D., Sahasrabudhe, P. V., Rosado, A., Sommese, R. F., et al. (2023). Structural studies identify angiotensin II receptor blocker-like compounds as branched-chain ketoacid dehydrogenase kinase inhibitors. J. Biol. Chem. 299 (3), 102959. doi:10.1016/j.jbc.2023.102959
Liu, H., Feng, J., Pan, T., Zhang, P., Ye, L., Jiang, Z., et al. (2025). Nuclear-localized BCKDK facilitates homologous recombination repair to support breast cancer progression and therapy resistance. Adv. Sci. (Weinh) 12 (22), e2416590. doi:10.1002/advs.202416590
Loftus, R. M., Assmann, N., Kedia-Mehta, N., O'Brien, K. L., Garcia, A., Gillespie, C., et al. (2018). Amino acid-dependent cMyc expression is essential for NK cell metabolic and functional responses in mice. Nat. Commun. 9 (1), 2341. doi:10.1038/s41467-018-04719-2
Long, L., Yang, W., Liu, L., Tobias, D. K., Katagiri, R., Wu, K., et al. (2021). Dietary intake of branched-chain amino acids and survival after colorectal cancer diagnosis. Int. J. Cancer 148 (10), 2471–2480. doi:10.1002/ijc.33449
Lu, X., Yang, R., Chen, Y., and Chen, D. (2024). NAD metabolic therapy in metabolic dysfunction-associated steatotic liver disease: possible roles of gut microbiota. iScience 27 (3), 109174. doi:10.1016/j.isci.2024.109174
Luo, W., Pan, Z., Zhu, X., Li, Y., Li, Y., Zhang, Y., et al. (2025). Design, synthesis and biological activity study of γ-Aminobutyric acid (GABA) derivatives containing bridged bicyclic skeletons as BCAT1 inhibitors. Molecules 30 (4), 904. doi:10.3390/molecules30040904
Maguolo, A., Rodella, G., Giorgetti, A., Nicolodi, M., Ribeiro, R., Dianin, A., et al. (2022). A gain-of-function mutation on BCKDK gene and its possible pathogenic role in branched-chain amino acid metabolism. Genes (Basel) 13 (2), 233. doi:10.3390/genes13020233
Mann, G., Mora, S., Madu, G., and Adegoke, O. A. J. (2021). Branched-chain amino acids: catabolism in skeletal muscle and implications for muscle and whole-body metabolism. Front. Physiol. 12, 702826. doi:10.3389/fphys.2021.702826
Martínez-Reyes, I., and Chandel, N. S. (2021). Cancer metabolism: looking forward. Nat. Rev. Cancer 21 (10), 669–680. doi:10.1038/s41568-021-00378-6
Mayers, J. R., Torrence, M. E., Danai, L. V., Papagiannakopoulos, T., Davidson, S. M., Bauer, M. R., et al. (2016). Tissue of origin dictates branched-chain amino acid metabolism in mutant Kras-driven cancers. Science 353 (6304), 1161–1165. doi:10.1126/science.aaf5171
Mei, W., Wei, M., Tang, C., Li, W., Ye, B., Xin, S., et al. (2025). BCAT2 binding to PCBP1 regulates the PI3K/AKT signaling pathway to inhibit autophagy-related apoptosis and ferroptosis in prostate cancer. Cell Death Dis. 16 (1), 337. doi:10.1038/s41419-025-07559-3
Meurs, N., and Nagrath, D. (2022). Driving with both feet: supplementing AKG while inhibiting BCAT1 leads to synthetic lethality in GBM. Cancer Res. 82 (13), 2354–2356. doi:10.1158/0008-5472.CAN-22-1619
Nachef, M., Ali, A. K., Almutairi, S. M., and Lee, S. H. (2021). Targeting SLC1A5 and SLC3A2/SLC7A5 as a potential strategy to strengthen anti-tumor immunity in the tumor microenvironment. Front. Immunol. 12, 624324. doi:10.3389/fimmu.2021.624324
Najumudeen, A. K., Ceteci, F., Fey, S. K., Hamm, G., Steven, R. T., Hall, H., et al. (2021). The amino acid transporter SLC7A5 is required for efficient growth of KRAS-mutant colorectal cancer. Nat. Genet. 53 (1), 16–26. doi:10.1038/s41588-020-00753-3
Neinast, M., Murashige, D., and Arany, Z. (2019). Branched chain amino acids. Annu. Rev. Physiol. 81, 139–164. doi:10.1146/annurev-physiol-020518-114455
Nouri-Majd, S., Salari-Moghaddam, A., Benisi-Kohansal, S., Azadbakht, L., and Esmaillzadeh, A. (2022). Dietary intake of branched-chain amino acids in relation to the risk of breast cancer. Breast Cancer 29 (6), 993–1000. doi:10.1007/s12282-022-01379-5
O'Sullivan, D., Sanin, D. E., Pearce, E. J., and Pearce, E. L. (2019). Metabolic interventions in the immune response to cancer. Nat. Rev. Immunol. 19 (5), 324–335. doi:10.1038/s41577-019-0140-9
Patel, M. S., Nemeria, N. S., Furey, W., and Jordan, F. (2014). The pyruvate dehydrogenase complexes: structure-based function and regulation. J. Biol. Chem. 289 (24), 16615–16623. doi:10.1074/jbc.R114.563148
Pavlova, N. N., and Thompson, C. B. (2016). The emerging hallmarks of cancer metabolism. Cell Metab. 23 (1), 27–47. doi:10.1016/j.cmet.2015.12.006
Paxton, R., and Harris, R. A. (1982). Isolation of rabbit liver branched chain alpha-ketoacid dehydrogenase and regulation by phosphorylation. J. Biol. Chem. 257 (23), 14433–14439.
Peng, H., Wang, Y., and Luo, W. (2020). Multifaceted role of branched-chain amino acid metabolism in cancer. Oncogene 39 (44), 6747–6756. doi:10.1038/s41388-020-01480-z
Peng, Y., Yin, D., Li, X., Wang, K., Li, W., Huang, Y., et al. (2023). Integration of transcriptomics and metabolomics reveals a novel gene signature guided by FN1 associated with immune response in oral squamous cell carcinoma tumorigenesis. J. Cancer Res. Clin. Oncol. 149 (9), 6097–6113. doi:10.1007/s00432-023-04572-x
Pettit, F. H., Yeaman, S. J., and Reed, L. J. (1978). Purification and characterization of branched chain alpha-keto acid dehydrogenase complex of bovine kidney. Proc. Natl. Acad. Sci. U. S. A. 75 (10), 4881–4885. doi:10.1073/pnas.75.10.4881
Qian, L., Li, N., Lu, X. C., Xu, M., Liu, Y., Li, K., et al. (2023). Enhanced BCAT1 activity and BCAA metabolism promotes RhoC activity in cancer progression. Nat. Metab. 5 (7), 1159–1173. doi:10.1038/s42255-023-00818-7
Radlwimmer, B., Barbus, S., Tonjes, M., Todt, G., Lichter, P., and Reifenberger, G. (2013). Methods for the diagnosis and prognosis of a tumor using bcat1 protein. Google Patents.
Raffel, S., Falcone, M., Kneisel, N., Hansson, J., Wang, W., Lutz, C., et al. (2017). BCAT1 restricts αKG levels in AML stem cells leading to IDHmut-like DNA hypermethylation. Nature 551 (7680), 384–388. doi:10.1038/nature24294
Ray, P. K., Salahuddin, S., Mazumder, A., Kumar, R., Ahsan, M. J., and Shahar Yar, M. (2023). Synthesis, anticonvulsant, and molecular docking studies of (3, 5-disubstituted-4, 5-dihydro-1H-pyrazol-1-yl)(4-chlorophenyl) methanone derivatives. Ind. J. Pharm. Edu. Res. 57 (1), 202–209. doi:10.5530/001954641727
Rossi, M., Mascaretti, F., Parpinel, M., Serraino, D., Crispo, A., Celentano, E., et al. (2021). Dietary intake of branched-chain amino acids and colorectal cancer risk. Br. J. Nutr. 126 (1), 22–27. doi:10.1017/S0007114520003724
Rossi, M., Turati, F., Strikoudi, P., Ferraroni, M., Parpinel, M., Serraino, D., et al. (2023). Dietary intake of branched-chain amino acids and pancreatic cancer risk in a case-control study from Italy. Br. J. Nutr. 129 (9), 1574–1580. doi:10.1017/S0007114522000939
Safrhansova, L., Hlozkova, K., and Starkova, J. (2022). Targeting amino acid metabolism in cancer. Int. Rev. Cell Mol. Biol. 373, 37–79. doi:10.1016/bs.ircmb.2022.08.001
Schroeder, M., Fuenzalida, B., Yi, N., Shahnawaz, S., Gertsch, J., Pellegata, D., et al. (2024). LAT1-dependent placental methionine uptake is a key player in fetal programming of metabolic disease. Metabolism 153, 155793. doi:10.1016/j.metabol.2024.155793
Shi, X., Wang, X., Yao, W., Shi, D., Shao, X., Lu, Z., et al. (2024). Mechanism insights and therapeutic intervention of tumor metastasis: latest developments and perspectives. Signal Transduct. Target Ther. 9 (1), 192. doi:10.1038/s41392-024-01885-2
Shu, X., Zhan, P. P., Sun, L. X., Yu, L., Liu, J., Sun, L. C., et al. (2021). BCAT1 activates PI3K/AKT/mTOR pathway and contributes to the angiogenesis and tumorigenicity of gastric cancer. Front. Cell Dev. Biol. 9, 659260. doi:10.3389/fcell.2021.659260
Sideris, G. A., Tsaramanidis, S., Vyllioti, A. T., and Njuguna, N. (2023). The role of branched-chain amino acid supplementation in combination with locoregional treatments for hepatocellular carcinoma: systematic review and meta-analysis. Cancers (Basel) 15 (3), 926. doi:10.3390/cancers15030926
Siegel, R. L., Miller, K. D., Wagle, N. S., and Jemal, A. (2023). Cancer statistics, 2023. CA Cancer J. Clin. 73 (1), 17–48. doi:10.3322/caac.21763
Sivanand, S., and Vander Heiden, M. G. (2020). Emerging roles for branched-chain amino acid metabolism in cancer. Cancer Cell 37 (2), 147–156. doi:10.1016/j.ccell.2019.12.011
Sjögren, R. J. O. (2021). Branched-chain amino acid metabolism is regulated by ERRα in primary human myotubes and is further impaired by glucose loading in type 2 diabetes. Diabetologia 64 (9), 2077–2091. doi:10.1007/s00125-021-05481-9
Stavrou, V., Fultang, L., Booth, S., De Simone, D., Bartnik, A., Scarpa, U., et al. (2023). Invariant NKT cells metabolically adapt to the acute myeloid leukaemia environment. Cancer Immunol. Immunother. 72 (3), 543–560. doi:10.1007/s00262-022-03268-4
Suryawan, A., Hawes, J. W., Harris, R. A., Shimomura, Y., Jenkins, A. E., and Hutson, S. M. (1998). A molecular model of human branched-chain amino acid metabolism. Am. J. Clin. Nutr. 68 (1), 72–81. doi:10.1093/ajcn/68.1.72
Thewes, V., Simon, R., Hlevnjak, M., Schlotter, M., Schroeter, P., Schmidt, K., et al. (2017). The branched-chain amino acid transaminase 1 sustains growth of antiestrogen-resistant and ERα-negative breast cancer. Oncogene 36 (29), 4124–4134. doi:10.1038/onc.2017.32
Tian, Q., Yuan, P., Quan, C., Li, M., Xiao, J., Zhang, L., et al. (2020). Phosphorylation of BCKDK of BCAA catabolism at Y246 by src promotes metastasis of colorectal cancer. Oncogene 39 (20), 3980–3996. doi:10.1038/s41388-020-1262-z
Tian, Y., Ma, J., Wang, H., Yi, X., Wang, H., Zhang, H., et al. (2023a). BCAT2 promotes melanoma progression by activating lipogenesis via the epigenetic regulation of FASN and ACLY expressions. Cell Mol. Life Sci. 80 (11), 315. doi:10.1007/s00018-023-04965-8
Tian, Y., Ma, J., Wang, M., Yi, X., Guo, S., Wang, H., et al. (2023b). BCKDHA contributes to melanoma progression by promoting the expressions of lipogenic enzymes FASN and ACLY. Exp. Dermatol 32 (10), 1633–1643. doi:10.1111/exd.14865
Tobias, D. K., Chai, B., Tamimi, R. M., Manson, J. E., Hu, F. B., Willett, W. C., et al. (2021). Dietary intake of branched chain amino acids and breast cancer risk in the NHS and NHS II prospective cohorts. JNCI Cancer Spectr. 5 (3), pkab032. doi:10.1093/jncics/pkab032
Tseng, Y. H., Yang, R. C., Chiou, S. S., Shieh, T. M., Shih, Y. H., and Lin, P. C. (2021). Curcumin induces apoptosis by inhibiting BCAT1 expression and mTOR signaling in cytarabine-resistant myeloid leukemia cells. Mol. Med. Rep. 24 (2), 19–24. doi:10.3892/mmr.2021.12204
Tso, S. C., Gui, W. J., Wu, C. Y., Chuang, J. L., Qi, X., Skvora, K. J., et al. (2014). Benzothiophene carboxylate derivatives as novel allosteric inhibitors of branched-chain α-ketoacid dehydrogenase kinase. J. Biol. Chem. 289 (30), 20583–20593. doi:10.1074/jbc.M114.569251
Verkerke, A. R. P., Wang, D., Yoshida, N., Taxin, Z. H., Shi, X., Zheng, S., et al. (2024). BCAA-nitrogen flux in brown fat controls metabolic health independent of thermogenesis. Cell 187 (10), 2359–2374.e18. doi:10.1016/j.cell.2024.03.030
Vettore, L., Westbrook, R. L., and Tennant, D. A. (2020). New aspects of amino acid metabolism in cancer. Br. J. Cancer 122 (2), 150–156. doi:10.1038/s41416-019-0620-5
Wang, Y., Zhang, J., Ren, S., Sun, D., Huang, H. Y., Wang, H., et al. (2019). Branched-chain amino acid metabolic reprogramming orchestrates drug resistance to EGFR tyrosine kinase inhibitors. Cell Rep. 28 (2), 512–525.e6. doi:10.1016/j.celrep.2019.06.026
Wang, Y., Xiao, J., Jiang, W., Zuo, D., Wang, X., Jin, Y., et al. (2021). BCKDK alters the metabolism of non-small cell lung cancer. Transl. Lung Cancer Res. 10 (12), 4459–4476. doi:10.21037/tlcr-21-885
Wang, K., Zhang, Z., Tsai, H. I., Liu, Y., Gao, J., Wang, M., et al. (2021). Branched-chain amino acid aminotransferase 2 regulates ferroptotic cell death in cancer cells. Cell Death Differ. 28 (4), 1222–1236. doi:10.1038/s41418-020-00644-4
Warburg, O. (1956). On the origin of cancer cells. Science 123 (3191), 309–314. doi:10.1126/science.123.3191.309
Weaver, K. J., Holt, R. A., Henry, E., Lyu, Y., and Pletcher, S. D. (2023). Effects of hunger on neuronal histone modifications slow aging in drosophila. Science 380 (6645), 625–632. doi:10.1126/science.ade1662
Wegermann, K., Henao, R., Diehl, A. M., Murphy, S. K., Abdelmalek, M. F., and Moylan, C. A. (2018). Branched chain amino acid transaminase 1 (BCAT1) is overexpressed and hypomethylated in patients with non-alcoholic fatty liver disease who experience adverse clinical events: a pilot study. PLoS One 13 (9), e0204308. doi:10.1371/journal.pone.0204308
White, P. J., McGarrah, R. W., Grimsrud, P. A., Tso, S. C., Yang, W. H., Haldeman, J. M., et al. (2018). The BCKDH kinase and phosphatase integrate BCAA and lipid metabolism via regulation of ATP-citrate lyase. Cell Metab. 27 (6), 1281–1293.e7. doi:10.1016/j.cmet.2018.04.015
Xia, L., Oyang, L., Lin, J., Tan, S., Han, Y., Wu, N., et al. (2021). The cancer metabolic reprogramming and immune response. Mol. Cancer 20 (1), 28. doi:10.1186/s12943-021-01316-8
Xu, Y., Yu, W., Yang, T., Zhang, M., Liang, C., Cai, X., et al. (2018). Overexpression of BCAT1 is a prognostic marker in gastric cancer. Hum. Pathol. 75, 41–46. doi:10.1016/j.humpath.2018.02.003
Xu, D., Shao, F., Bian, X., Meng, Y., Liang, T., and Lu, Z. (2021). The evolving landscape of noncanonical functions of metabolic enzymes in cancer and other pathologies. Cell Metab. 33 (1), 33–50. doi:10.1016/j.cmet.2020.12.015
Xu, C., Yang, K., Xuan, Z., Li, J., Liu, Y., Zhao, Y., et al. (2023). BCKDK regulates breast cancer cell adhesion and tumor metastasis by inhibiting TRIM21 ubiquitinate talin1. Cell Death Dis. 14 (7), 445. doi:10.1038/s41419-023-05944-4
Xue, P., Zeng, F., Duan, Q., Xiao, J., Liu, L., Yuan, P., et al. (2017). BCKDK of BCAA catabolism cross-talking with the MAPK pathway promotes tumorigenesis of colorectal cancer. EBioMedicine 20, 50–60. doi:10.1016/j.ebiom.2017.05.001
Xue, M., Xiao, J., Jiang, W., Wang, Y., Zuo, D., An, H., et al. (2023). Loss of BCAA catabolism enhances Rab1A-mTORC1 signaling activity and promotes tumor proliferation in NSCLC. Transl. Oncol. 34, 101696. doi:10.1016/j.tranon.2023.101696
Yang, Y. H., Deng, Q., Yang, T. B., Gui, Y., Zhang, Y. X., Liu, J. B., et al. (2019). A case report of cholangiocarcinoma combined with moderately differentiated gastric adenocarcinoma. Med. Baltim. 98 (30), e16332. doi:10.1097/MD.0000000000016332
Yao, C. C., Sun, R. M., Yang, Y., Zhou, H. Y., Meng, Z. W., Chi, R., et al. (2023). Accumulation of branched-chain amino acids reprograms glucose metabolism in CD8(+) T cells with enhanced effector function and anti-tumor response. Cell Rep. 42 (3), 112186. doi:10.1016/j.celrep.2023.112186
Yoneshiro, T., Wang, Q., Tajima, K., Matsushita, M., Maki, H., Igarashi, K., et al. (2019). BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 572 (7771), 614–619. doi:10.1038/s41586-019-1503-x
Yuan, Z., Li, M., and Tang, Z. (2025). BCAT1 promotes cell proliferation, migration, and invasion via the PI3K-Akt signaling pathway in oral squamous cell carcinoma. Oral Dis. 31 (2), 364–375. doi:10.1111/odi.15084
Zhai, M., Yang, Z., Zhang, C., Li, J., Jia, J., Zhou, L., et al. (2020). APN-mediated phosphorylation of BCKDK promotes hepatocellular carcinoma metastasis and proliferation via the ERK signaling pathway. Cell Death Dis. 11 (5), 396. doi:10.1038/s41419-020-2610-1
Zhang, B., Chen, Y., Shi, X., Zhou, M., Bao, L., Hatanpaa, K. J., et al. (2021). Regulation of branched-chain amino acid metabolism by hypoxia-inducible factor in glioblastoma. Cell Mol. Life Sci. 78 (1), 195–206. doi:10.1007/s00018-020-03483-1
Zhang, B., Peng, H., Zhou, M., Bao, L., Wang, C., Cai, F., et al. (2022). Targeting BCAT1 combined with α-Ketoglutarate triggers metabolic synthetic lethality in glioblastoma. Cancer Res. 82 (13), 2388–2402. doi:10.1158/0008-5472.CAN-21-3868
Zhang, X., Sun, Y., Ma, Y., Gao, C., Zhang, Y., Yang, X., et al. (2023). Tumor-associated M2 macrophages in the immune microenvironment influence the progression of renal clear cell carcinoma by regulating M2 macrophage-associated genes. Front. Oncol. 13, 1157861. doi:10.3389/fonc.2023.1157861
Zhang, T., Pan, Z., Gao, J., Wu, Q., Bai, G., Li, Y., et al. (2024). Branched-chain amino acid transaminase 1 confers EGFR-TKI resistance through epigenetic glycolytic activation. Signal Transduct. Target Ther. 9 (1), 216. doi:10.1038/s41392-024-01928-8
Zhang, X., Gao, B., and Wang, W. (2025a). Early-onset gastric cancer global burden profile, trends, and contributors. Cancer Biol. Med. 22 (10), 1240–1254. doi:10.20892/j.issn.2095-3941.2025.0320
Zhang, W., Fan, Y., Ashrafizadeh, M., Shi, D., Sethi, G., Ertas, Y. N., et al. (2025). A novel BCAT1 inhibitor bufalin sensitizes pancreatic cancer cells to chemotherapy. Genes Dis. 12 (3), 101503. doi:10.1016/j.gendis.2024.101503
Zhang, X., Zhu, X., Li, Y., Li, Y., Luo, W., Khan, M., et al. (2025b). A review on branched-chain amino acid aminotransferase (BCAT) inhibitors: current status, challenges and perspectives. Curr. Med. Chem. 32 (42), 9533–9554. doi:10.2174/0109298673320136241024054435
Zhao, S., Li, B., Gao, H., and Zhang, Y. (2024). MiR-320a acts as a tumor suppressor in somatotroph pituitary neuroendocrine tumors by targeting BCAT1. Neuroendocrinology 114 (1), 14–24. doi:10.1159/000533549
Zheng, Y. H., Hu, W. J., Chen, B. C., Grahn, T. H. M., Zhao, Y. R., Bao, H. L., et al. (2016). BCAT1, a key prognostic predictor of hepatocellular carcinoma, promotes cell proliferation and induces chemoresistance to cisplatin. Liver Int. 36 (12), 1836–1847. doi:10.1111/liv.13178
Zhou, S., Lu, Z., Wu, H., Gu, C. Y., Zhang, D. Y., Sun, W. L., et al. (2017). Synchronous multiple primary gallbladder and gastric malignancies: report of two cases and review of the literature. Mol. Clin. Oncol. 7 (5), 869–873. doi:10.3892/mco.2017.1397
Zhou, Y., Kou, J., Li, W., Wang, Y., Su, X., and Zhang, H. (2025). BCAA metabolism in cancer progression and therapy resistance: the balance between fuel and cell signaling. Front. Pharmacol. 16, 1595176. doi:10.3389/fphar.2025.1595176
Zhuang, Y., Lan, S., Zhong, W., Huang, F., Peng, J., and Zhang, S. (2023). Comprehensive analysis of PPMs in pancreatic adenocarcinoma indicates the value of PPM1K in the tumor microenvironment. Cancers (Basel) 15 (2), 474. doi:10.3390/cancers15020474
Keywords: BCAAs, dietary therapy, immunity, metabolism, targeted inhibitors
Citation: He B, Li L, Liu Y, Hao M, Zhang L and He R (2026) BCAAs and related metabolic enzymes: partners in crime driving tumor development. Front. Cell Dev. Biol. 14:1748587. doi: 10.3389/fcell.2026.1748587
Received: 18 November 2025; Accepted: 21 January 2026;
Published: 13 February 2026.
Edited by:
Liangbin Lin, Southwest Jiaotong University, ChinaReviewed by:
Wenhao Ouyang, Sun Yat-sen University, ChinaBurkitkan Akbay, Nazarbayev University, Kazakhstan
Copyright © 2026 He, Li, Liu, Hao, Zhang and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rongzhang He, cm9uZ3poYW5nNDEyQDE2My5jb20=; Ling Zhang, emhhbmdsaW5ndHlAc3htdS5lZHUuY24=
†These authors have contributed equally to this work
 Binfan He1†
Binfan He1† Ling Zhang
Ling Zhang Rongzhang He
Rongzhang He

