- 1Department of Periodontics and Oral Medicine, School of Dentistry, University of Michigan, Ann Arbor, MI, United States
- 2Department of Periodontics, College of Dentistry, University of Illinois Chicago, Chicago, IL, United States
- 3Department of Radiology, Michigan Medicine, University of Michigan, Ann Arbor, MI, United States
- 4Department of Surgery, Stanford School of Medicine, Stanford University, Stanford, CA, United States
- 5Private Practice, Fenton, MI, United States
- 6Division of Periodontology, College of Dentistry, The Ohio State University, Columbus, OH, United States
Objectives: Understanding the available methods to study blood flow in the oral cavity can enhance knowledge of research methodology on periodontal circulation related to disease initiation and progression as well as wound healing. This study aims to systematically review non-invasive techniques that allow for the assessment of oral tissue perfusion in clinical and pre-clinical studies.
Methods: A complete electronic literature search in 5 databases (NLM PubMed, Embase, EBSCOhost CINAHL, EBSCOhost Dentistry and Oral Sciences Source, and Wiley Cochrane Central Register of Controlled Trials) was conducted by two reviewers. The search terms included gingival blood flow, tissue perfusion, imaging perfusion, soft tissue perfusion, diagnostic, vascularization, soft tissue, and microvascularization. The focused question is: What are the available non-invasive and quantitative imaging techniques used to evaluate oral and periodontal tissue perfusion?
Results: A total of 79 articles were included for qualitative analysis. Various methods were identified, including Laser Doppler Flowmetry (LDF), Laser Speckle Contrast Imaging (LSCI), Spectral Imaging Methods (such as Diffuse Reflectance Spectroscopy), Ultrasound (US), Intravital Video Microscopy, and Oral Videocapillaroscopy. LDF is the most applied to estimate blood flow in a small focal area for the study of periodontal diseases and oral wound healing, among other indications. LSCI, providing surrogate superficial blood flow values in a 2-dimensional, larger field-of-view, has been used for similar reasons. The use of cross-sectional ultrasound is on a rise to record blood velocity and blood volume using color flow and color power modes, respectively. Comparisons of the available technologies revealed their strengths and limitations related to their spatial resolution, sensitivity, reliability, accuracy, invasiveness, dependence of (image) data in the field of view relative to probe positioning and angulation, and safety. The ideal features of such a device pertinent to probe geometry, data acquisition, recording, and infection control needs were also discussed.
Conclusions: A few imaging technologies have been identified in the literature to study blood flow in the oral cavity. These methods could potentially augment our ability to diagnose oral diseases and monitor wound healing objectively and timely. In combination, these could potentially enhance treatment outcomes significantly.
1 Introduction
Anatomical variability of oral and periodontal microcirculation and its reaction to the outside stimuli, such as bacterial pathogens and surgical trauma have been a topic of interest for the diagnosis of oral diseases and outcome evaluation of dental treatment. Anatomical studies in human cadavers and animals have provided a profound insight into oral soft tissue perfusion (1–5). Recent studies have reiterated the apico-coronal orientation of the microvascular plexus and its intricately interconnected network of vessels as the key supplier to the mucogingival complex (1, 6, 7). More specifically, these principal micro-vessels, the supraperiosteal, intra-ligamental, and intra-osseous plexuses, have their own territories yet extensively interconnected. However, the spatial distribution of the supraperiosteal plexus is not well-defined. A recent study suggested the distribution is random and varies individually (7). As far as surgical wound healing is concerned, vascular distribution and inclusion in the flap, and the degree to which microvasculature is traumatized can influence the speed of tissue re-perfusion postoperatively, wound stability, and ultimately outcome predictability (7–10). This knowledge could further assist the clinician in identifying strategies to preserve vascularization and consequently achieve optimal wound healing (7, 11).
In addition to anatomical evaluation of microvasculature, vascular circulation, such as the amount and speed of the blood flow can also provide valuable insight into oral disease diagnosis and tissue healing capabilities, potentially influencing clinical outcomes. Under normal healing events of a periodontal access flap, there is a short period of significant ischemia following local anesthesia and immediately post-operatively (12). A hyperemic response follows and persists for a few days (13). In general, the flow returns to the baseline between the 1st and 2nd week. Deviation from this normal pattern may suggest altered healing that warrants attention. For example, delayed reperfusion could indicate compromised and inferior healing. Prolonged tissue hyper-perfusion may be related to the occurrence of infection and sustained inflammation. This dynamic shift in the amount of periodontal tissue perfusion may also be influenced by incision tracing and flap designs (13, 14). The simplified papilla preservation flap may be associated with faster recovery of the gingival blood flow post-operatively compared to the modified Widman flap (15). Other factors, such as a minimally invasive approach, could also affect tissue perfusion and wound healing. Microsurgery for treating gingival recession with the use of the operating microscope has been shown to encourage faster revascularization and better root coverage outcomes (16). Clinical methods for quantitative evaluation of the baseline as well as postoperative tissue perfusion have not been established.
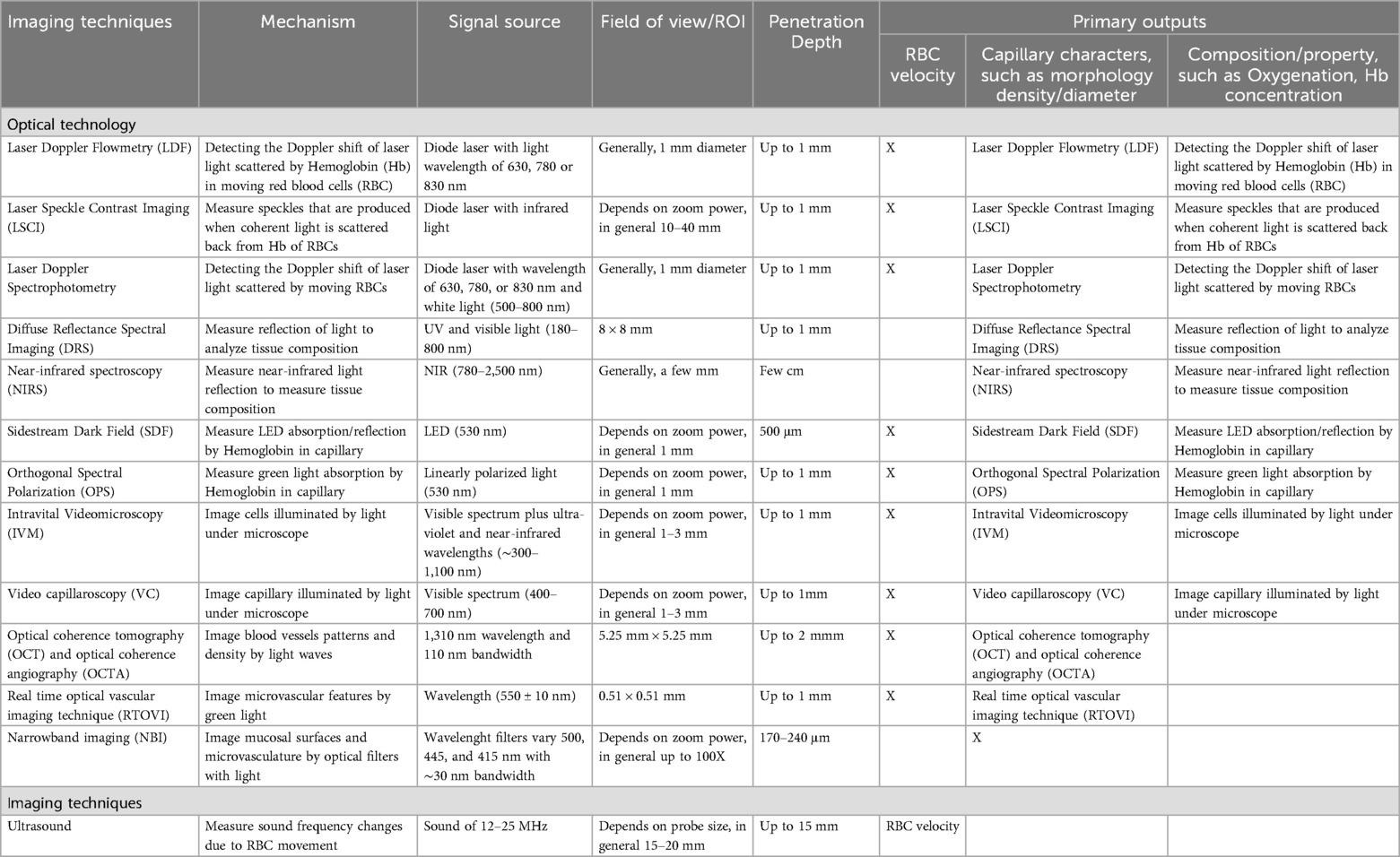
Several techniques have been used to estimate and study tissue perfusion in the past few decades (1, 2, 6, 9–12, 15, 17–34). Laser Doppler Flowmetry (LDF) for example, can provide relative blood flow at a focal area of a few millimeters in diameter. Laser Speckle Contrast Imaging (LSCI) also offers relative superficial blood flow and image display showing the perfusion intensity on a scale, with the region of interest covering a few teeth. Recently, the use of ultrasound to estimate tissue perfusion has been increasing. The two main flow imaging modes are color flow and power mode, quantifying flow velocity and blood volume, respectively. The literature suggests that monitoring tissue perfusion at the individual level could prove to be more valuable instead of differentiating the mean perfusion amount of the cohorts (18, 28). Various techniques offer valuable information in different aspects, depending on their uses and indications. Realizing and contrasting these available modalities could lay the foundation for studying oral and periodontal tissue perfusion, disease pathogenesis and progression, as well as wound healing because of different surgical concepts, designs, or conditions. Therefore, the present study aims to systematically review non-invasive imaging techniques that quantitatively assess oral and tissue perfusion.
2 Materials and methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was consulted for the present systematic review process, which complies with the appropriate guidelines/checklist (35) (Supplementary File).
Focused question:
What are the available non-invasive and quantitative imaging techniques used to evaluate oral and periodontal tissue perfusion?
2.1 Eligibility criteria
2.1.1 Inclusion criteria
• Human as well as pre-clinical in vivo studies that used imaging technology to quantify oral and periodontal tissue circulation.
2.1.2 Exclusion criteria
• Ex vivo studies.
• Review articles, including workshop consensuses.
• Retrospective studies.
• Articles not written in English.
2.2 Search strategy
An electronic search was conducted by two reviewers (A.R. and F.A.) from January 2022 to October 2023 in the following databases: NLM PubMed, Embase, EBSCOhost CINAHL, EBSCOhost Dentistry and Oral Sciences Source, and Wiley Cochrane Central Register of Controlled Trials. The working PubMed search was as follows: (((gingival blood flow) AND (tissue perfusion)) OR (imaging perfusion)) OR (soft tissue perfusion) AND ((diagnostic) OR (vascularization)) OR (soft tissue)) OR (microvascularization))). A second PubMed search was performed to include the following search terms: ((soft tissue) OR (tissue)) AND ((blood flow) OR (perfusion) OR (vascularization)) AND (diagnostic) AND (imaging) AND (oral) AND (gingival).
2.3 Data extraction and analysis
Data were manually extracted by the same examiners (A.R. and F.A.) independently. When they disagreed, a third (D.V.), fourth (H.L.C.), fifth (A.B.), and sixth examiner (O.D.K.) checked the variable and a consensus agreement was reached. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines/checklist were followed during the present systematic review. Two researchers (A.R., F.A.) independently assessed the quality of the included studies. In cases of disagreement, a third, fourth, fifth, and sixth researcher (D.V., H.L.C., A.B., O.D.K.) were consulted. Due to the heterogeneity of the evaluated parameters, time points, and clinical or preclinical scenarios, a meta-analysis could not be performed.
2.4 Risk of bias assessment
The quality analysis process was based on the updated version of the Handbook for Systematic Reviews of Interventions and the CONSORT (Consolidated Standards of Reporting Trials) statement (36). Study characteristics, quality, and the risk-of-bias tool (ROBINS-I) for non-randomized studies of interventions (non-RCTs) (37), and RoB2 for randomized clinical trials (RCT) (38). These assessments were conducted independently by two authors (A.R.B. and F.A.), with final evaluations reached through discussion. The degree of bias was categorized as follows: (1) low risk if one criterion was missing; (2) moderate risk if two or three criteria were missing; (3) serious risk if three or more criteria were missing, and (4) critical if four or more criteria were missing.
3 Results
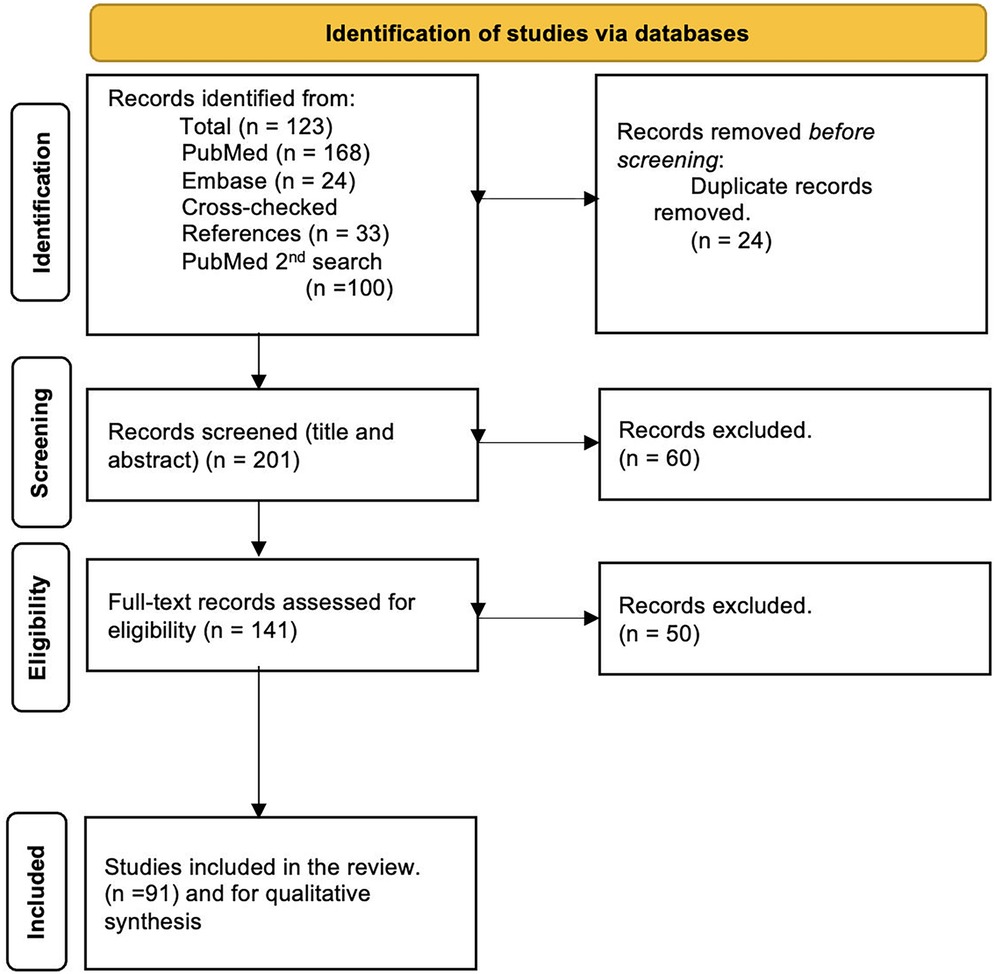
3.1 Study selection
The literature selection process is illustrated by a PRISMA flowchart (Figure 1). Initial screening yielded a total of 223 articles, 68 in PubMed, 24 in the EMBASE database search, 33 cross-checking references, and a second PubMed search yielded 100 articles. References from these searches were combined, and after removing 24 duplicates, 201 articles were available for title and abstract review. Of these articles, 60 did not meet the inclusion criteria and were excluded. Following a full-text review of the remaining 141 articles, 50 articles were further excluded, resulting in 91 included articles. An overview of the identified techniques is given in Table 1. For each imaging mechanism, a summary table is provided with technique-specific details: Laser Doppler Flowmetry (LDF) (Table 2), Laser Speckle Contrast Imaging (LSCI) (Table 3), Spectral Imaging Methods (Table 4), Ultrasound (US) (Table 5), and other imaging methods (Videocapillaroscopy, Videomicroscopy, Optical coherence tomography (OCT) with OCT angiography (OCTA), real-time optical vascular imaging (RTOVI), Narrowband imaging (NBI) (Table 6). Considerable heterogeneity was found in the evaluated parameters, which precluded quantitative data analysis.
3.2 Risk of bias assessment
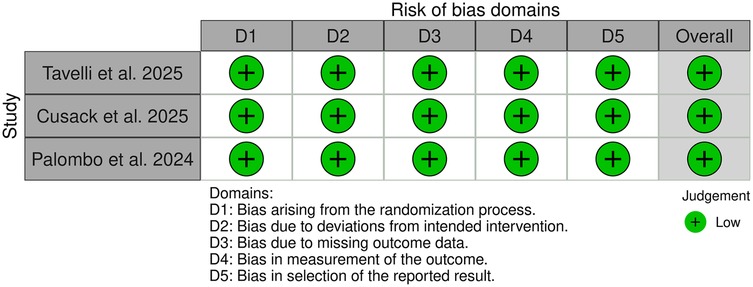
Among the 91 included studies, 11 were preclinical, 77 were clinical non-RCT studies (Figure 2), and 3 were clinical RCTs (Figure 3). Of the non-RCT studies, 40 were assessed as having a “low” overall risk of bias, 24 had a “moderate” risk, 12 were rated as “serious”, and 10 were deemed to have a “critical” risk of bias (Supplementary Table S1). All three RCTs were evaluated as having a “low” risk of bias. These findings are visualized in Figures 2, 3 (39).
3.3 Laser Doppler flowmetry (LDF)
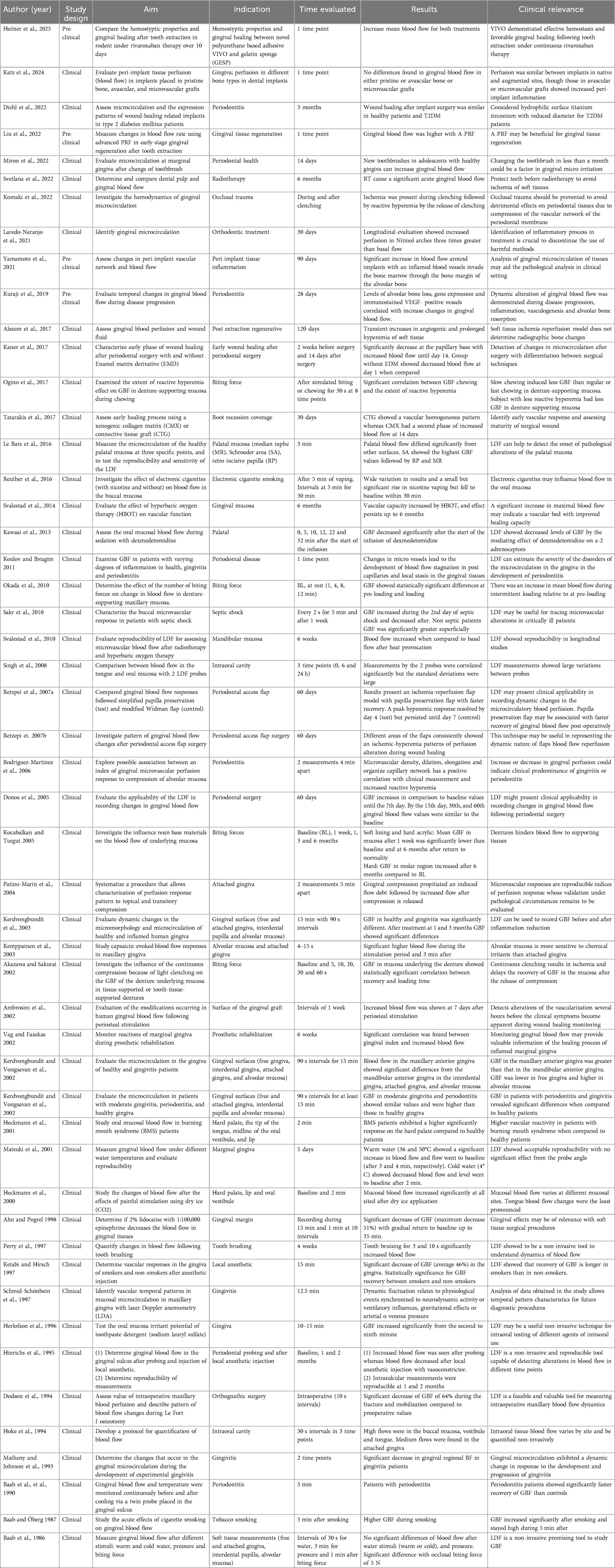
Laser Doppler Flowmetry is based on the frequency shift of the backscattered laser light from moving objects (Doppler shift principle) (40–43). When an infrared laser is reflected by or scattered off of red blood cells (RBC), a frequency change is seen (12, 25, 40, 42, 44). The system uses a probe with a focal distance of less than or equal to 3 mm. Probe placement is in a perpendicular orientation to the area of interest (43, 44) (Table 2). A total of 49 studies were identified related to LDF. It was used to assess microvascular dynamics of the gingiva (45–54), several bone types (55), periodontal and peri-implant disease (44, 56–60), flap design (12, 15, 25, 61), periodontal tissues exposed to occlusal trauma (40), occlusal loading in denture-supported tissues (62–65), orthodontic treatment (42), orthognathic surgery (24), the effect of hyperbaric oxygen therapy (HBOT) (43, 66), burning mouth syndrome (BMS) (67), septic shock (68), periodontal tissues reaction during prosthetic rehabilitation (69), tooth brushing (70, 71), the effect of toothpaste detergent (72), the effect of temperature stimuli (52, 73, 74), perfusion characterization after transient compression (6, 75), hemostyptic properties and gingival healing (76) the effect of local anesthetic (77–79), the effect of dexmedetomidine (80), early vascular response (12, 15, 17, 25, 29, 81), smoking (82, 83), wound healing in type 2 diabetes patients (84), and gingival tissue regeneration (85) (Table 2). LDF is easy to perform, non-contact, and economical. The main disadvantages of LDF are its sensitivity to light, artificial motion, pressure, probe angle. Other drawbacks include (1) arbitrary perfusion unit presented, (2) lack of anatomical images, and (3) superficial penetration depth (up to 1 mm) (40, 41).
3.4 Laser speckle contrast imaging (LSCI)
LSCI quantifies the magnitude of the superficial tissue perfusion by analyzing the speckle pattern of scattered light using an invisible laser that is scanned over a 2D region of interest (wavelengths close to 785 nm) (10, 27). This technique estimates the velocity of the moving targets, in this case RBCs, across a 2D surface and therefore also incorporates the gingival vascular density and thus its fractional blood volume. Blood flow velocity distributions are shown as relative color encoded perfusion units (LSPU): blue-cyan (low perfusion), green-yellow (moderate perfusion), and orange-red (high perfusion) (Figure 4) (11, 26, 31, 32).
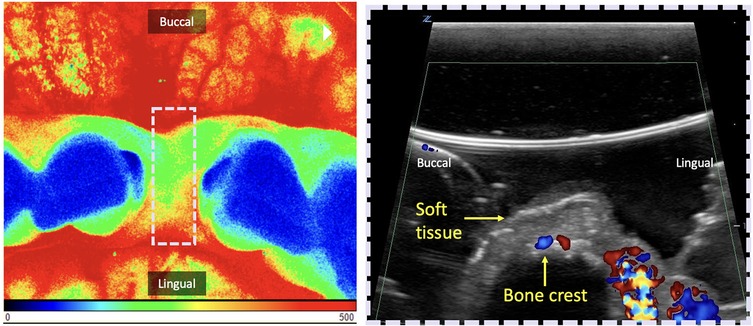
Figure 4. Demonstration of two perfusion imaging techniques of the edentulous crest. Both can be used in the occlusal view for pre-assessment of future implant placement in the anterior mandible. Left: Laser Speckle Contrast Imaging (LCSI). Here, blood flow is expressed through Laser Speckle Perfusion Units (LSPU) and depicted as false color images: blue-cyan (low perfusion), green-yellow (moderate perfusion), orange-red (high perfusion). The region of interest (ROI) delimited by the dashed white rectangle is the location of the ultrasound shown on the right. Right: Cross-sectional B-mode ultrasound image with color flow in velocity mode. Color velocity images display hues of blue to red, which correspond to the detected blood flow velocity. Changes in this velocity potentially serve as a quantitative indicator for the degree of inflammation. In this case, in both techniques, the presence of high tissue perfusion can be seen on the lingual surface and low to medium perfusion mid-occlusal.
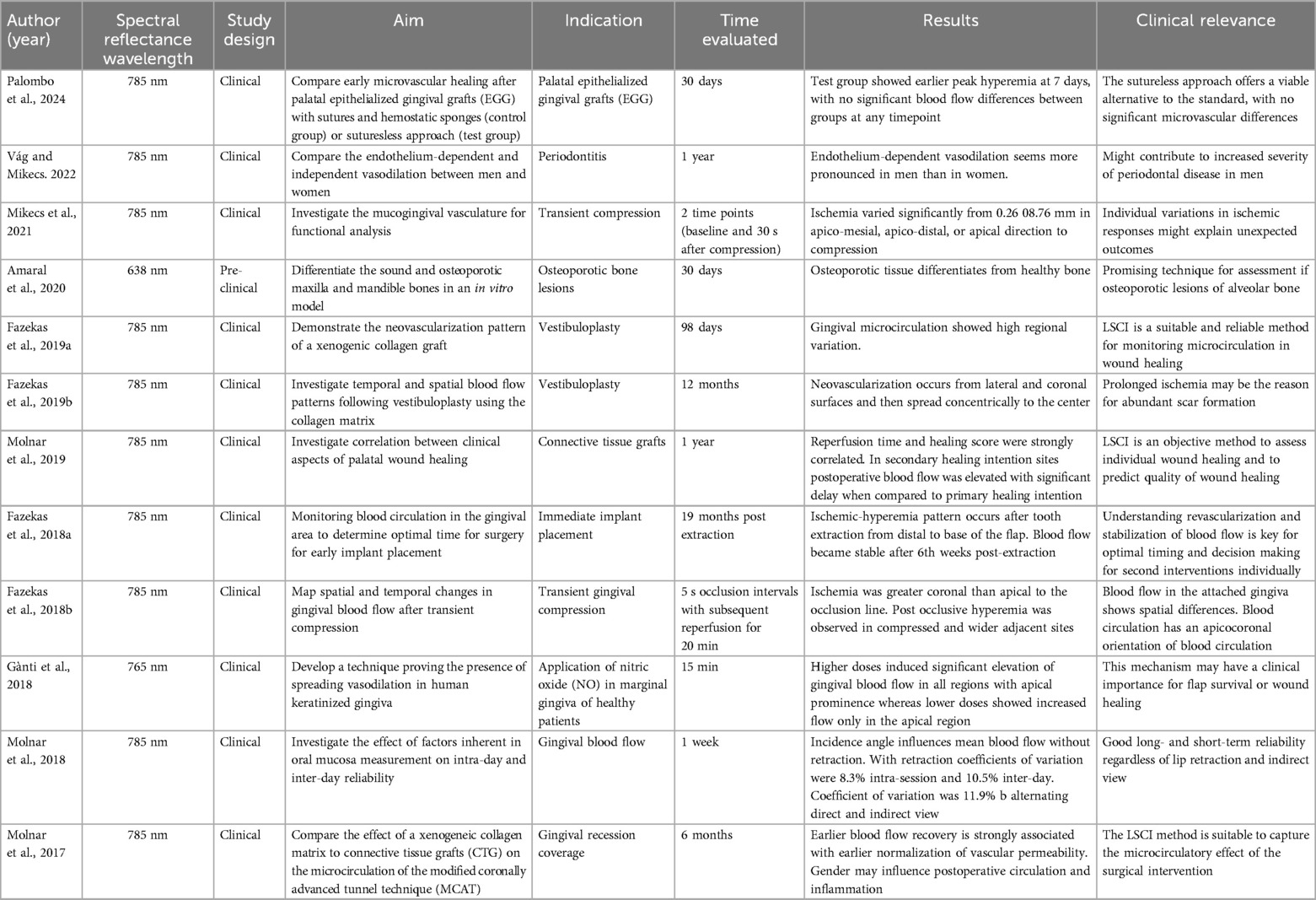
A total of 12 studies were identified related to LSCI (6, 10, 11, 18, 26, 28, 31–33, 69, 86, 87). In them, LSCI was used for evaluating gingival perfusion after transient compression of osteoporotic lesions, after vestibuloplasty, after immediate implant placement, in response to nitric oxide, factors influencing the readings, compare vasodilation between men and women to evaluate the increase in the severity of periodontitis (10, 86) and after soft tissue augmentation procedures (18) (Table 3). Two studies validated the reproducibility of this technique (10, 32, 33). A recent randomized clinical trial (87) evaluated early microvascular healing after palatal epithelialized gingival grafts (ECG) harvesting using traditional hemostasis vs. a sutureless approach, assessed by LSCI at 7, 14, and 30 days. The sutureless group showed faster hyperemia resolution and a 13-min shorter surgical time, with no significant differences in perfusion, bleeding, or analgesic use, supporting it as a viable alternative. Major advantages are: (1) large field of view that can cover few teeth and (2) anatomical images included, allowing knowledge of the spatial distribution of the circulation. LSCI is limited by the need for direct line-of-sight, restricting access to areas like the molars. It also has shallow penetration (∼1 mm), is sensitive to working distance, patient movement, gingival temperature, and measurement angle—especially in curved regions like the maxilla and mandible, which can affect blood flow readings (28) (Table 3).
3.5 Spectral imaging methods
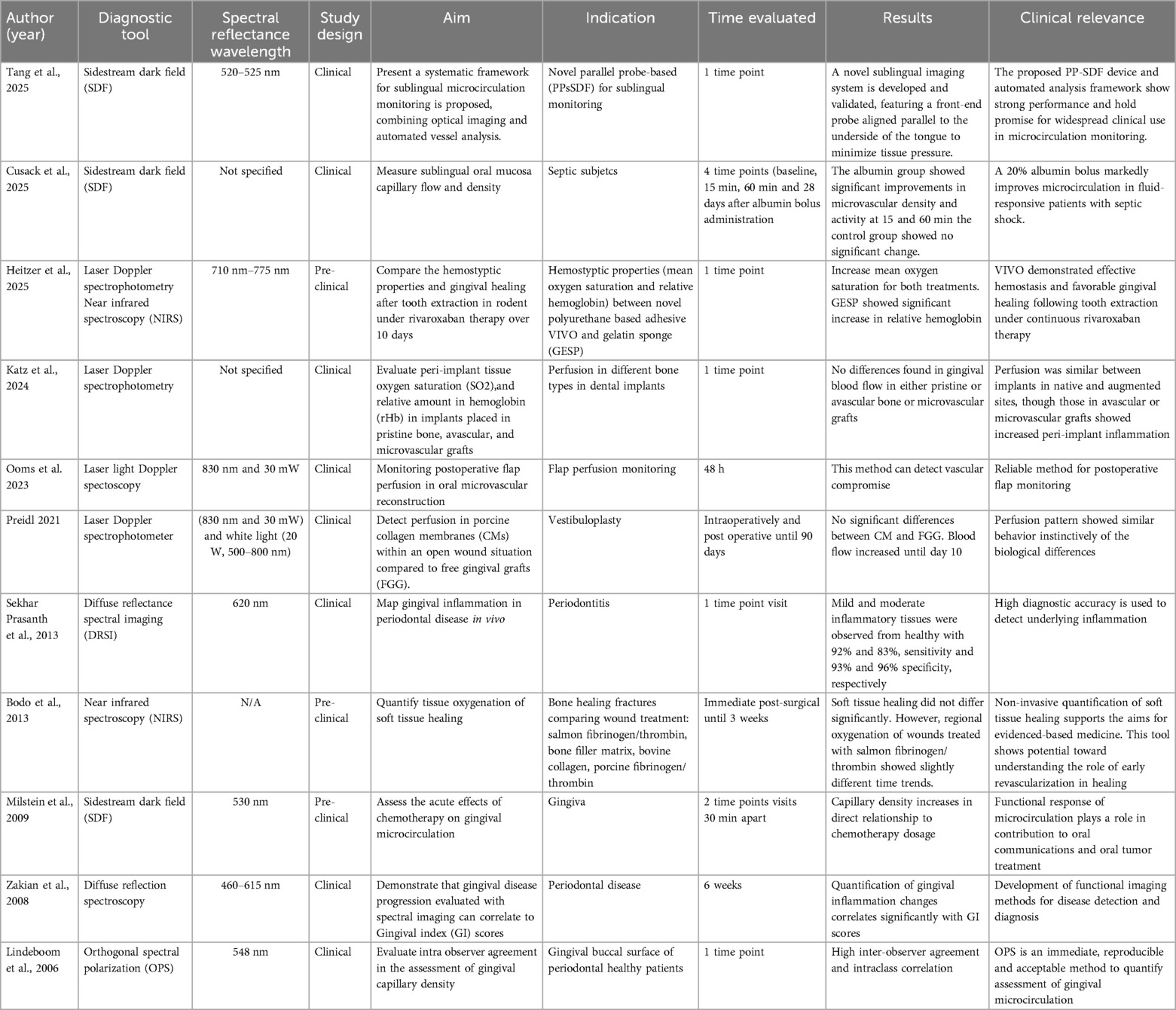
Spectral methods have been identified for evaluating periodontal tissue perfusion in 7 articles. The light source can be either ultraviolet-visible (UV-VIS), Diffuse Reflectance Spectroscopy, DRS) or near-infrared (Near Infrared Spectroscopy, NIRS). The mechanisms by which the interaction of light and matter can be quantified include the well-established Diffuse Reflectance Spectroscopy (DRS), Orthogonal Spectral Polarization (OPS), and most recently Sidestream Dark Field (SDF) (30, 88–91) (Table 4). The reflected light in at different wavelengths after it interacts with the sample is quantified and reported, in our case, oxygen saturation, and hemoglobin concentration. Laser Doppler Spectroscopy (LDS) integrates laser Doppler and visible light spectroscopy to assess soft tissue grafts after vestibuloplasty at a 2 mm tissue depth (90). It uses continuous-wave laser light (830 nm, 30 nW) and white light (20 W, 500–800 nm), with real-time signal detection via a single optical probe connected to a computer. Measurements include blood flow velocity and flow (AU), derived from Doppler shifts, tissue oxygen saturation (SO₂%) based on light absorption/reflection, and relative hemoglobin concentration [rHb], independent of vessel density or size (90). Laser Doppler flowmetry and tissue spectrophotometry (LDF-TS) (55, 76) have also been used to monitor microsurgical flaps at extraction sites (76) and peri-implant tissues (55), providing quantified perfusion data including SO₂ (%), rHb (AU), and blood flow (AU). This system's intraoral use is made possible by a compact 5 × 2 mm probe with 1 mm depth, specifically designed for such applications (55).
Near Infrared Spectroscopy (NIRS) offers significantly deeper soft tissue penetration (∼25 mm) (20). In contrast, UV/Vis spectroscopy assesses corneocyte levels at a much shallower depth (∼430 µm) in skin within preclinical models (92). Both, NIRS and UV/Vis spectroscopy quantify corneocyte amounts but differ in wavelength ranges and measurement approaches (92). NIRS in the near-infrared and UV/Vis in the visible spectrum. As a result, pseudo-absorption values are not directly comparable due to differing interactions with skin components (92). Changes in tissue oxygenation may be an indicator of tissue inflammation (20). Tissue hypoxia was defined when oxygen tension is <40 mmHg. DRS has shown a significant correlation between the Gingival Index and the level of gingival inflammation (89, 91). Additionally, in vivo near-infrared (NIR) fluorescent imaging scan can be acquired with ultrafocus magnification via 360° rotation at 0.75° increments (0.3 s/°) and requires fluorescent dyes (76). Fluorescence imaging was performed first using a laser and filter system (excitation: 710 nm, emission: 775 nm), with data captured by a cooled CCD camera and reconstructed into 2D images (76). Some limitations of this technique are the lack of laterality (limited field of view) and the fact that NIRS does not distinguish between hemoglobin and myoglobin (20). Other limitations include the possibility of cross-contamination, optical probe size, sensitivity to optical probe position, and pressure (91) (Table 4). Due to the promising capabilities of spectral imaging in quantifying physiological tissue components, a recent study introduced an RGBIR (Red, Green, Blue, and Infrared) image sensor designed to capture spectral information from both oral and skin tissues (93). Tissue optical including chromophore concentrations and scattering characteristics, is essential for modeling light behavior and optimizing device design. Due to variability between individuals and tissue sites, real-time measurement is often necessary for personalized care (94). Red wavelengths typically range from approximately 620 to 740 nm, green from 520 to 570 nm, and blue from 440 to 470 nm. The objective was to estimate chromophore concentrations. Raw pixel values were calibrated to reflectance and then mapped to a diffusion model-based lookup table, linking tissue reflectance to melanin, oxygen saturation, and water content (93). Initial benchtop experiments demonstrated chromophore changes corresponding to an applied finger occlusion. The technique was later applied to intraoral imaging, where reflectance changes were associated with areas of erythema (93). Additionally, a color polarization camera (RGB) was utilized to quantify blood volume and tissue oxygen saturation in both superficial and deep layers of the skin (95). This system captures co- and cross-polarized RGB images within a single acquisition frame. Polarization data enables the differentiation of skin layers, while RGB color information serves as input for a neural network model that predicts tissue blood volume and oxygen saturation. Findings demonstrated in vivo differentiation based on age and skin tone among human subjects (95).
Orthogonal Spectral Polarization (OPS) uses linearly polarized green light (548 µm) to illuminate the sample. The reflected depolarized light is then received by a sensor in orthogonal direction to the emitter. It can measure capillary blood flow, vessel diameter, and vessel density (96). The system measures tissue perfusion using two types of optical probes: an attached surface probe and an unattached surface probe. The attached probe, which assesses perfusion at a depth of 3 mm, was secured with four sutures at the center of the radial forearm free flap (96) and about 1 cm from the perforator vessel in the anterolateral thigh flap (ALTF). The unattached probe, capable of measuring at depths of 2 mm and 8 mm, was manually positioned parallel to the attached probe during each reading (96). The advantage of OPS over other spectral techniques its sensitivity to capillary blood flow, vessel diameter, and vessel density. The latter has been used to estimate tissue perfusion (96). Limitations include optical probe positioning, patient movement, and lack of access to the interdental gingiva (88).
Insufficient image sharpness in OPS led to the development of Sidestream Dark Field (SDF) imaging, a non-invasive technique used to quantify capillary density in the mucosal microcirculation (97). SDF specifically visualizes the flow of red blood cells through small vessels such as capillaries, using green light at a wavelength of 530 nm, which is absorbed by hemoglobin to enhance contrast and enable clear visualization of microvascular flow (30). Milstein et al. described a handheld video microscope based on SDF technology, which employs concentrically arranged light-emitting diodes (LEDs) around a central light guide. These LEDs emit light at wavelengths that are absorbed by both oxy- and deoxyhemoglobin, allowing detailed imaging of red blood cell movement (30). To overcome limitations of traditional vertical-capture SDF systems, Tang et al. introduced a novel parallel probe-based SDF (PP-SDF) device, offering improved portability and usability (98). Clinical studies have demonstrated this device's strong performance and its potential for future applications in real-time microcirculation monitoring (98). Furthermore, a recent randomized controlled trial by Cusack et al. assessed the microcirculatory impact of fluid resuscitation in septic shock and found that a 20% albumin bolus significantly improved microvascular parameters compared to crystalloids, despite similar macro hemodynamic measures, highlighting the concept of hemodynamic incoherence (99). The advantages of SDF include reduced image contamination from tissue surface reflections and a wider imaging field; however, limitations such as low image resolution and contrast still remain (30).
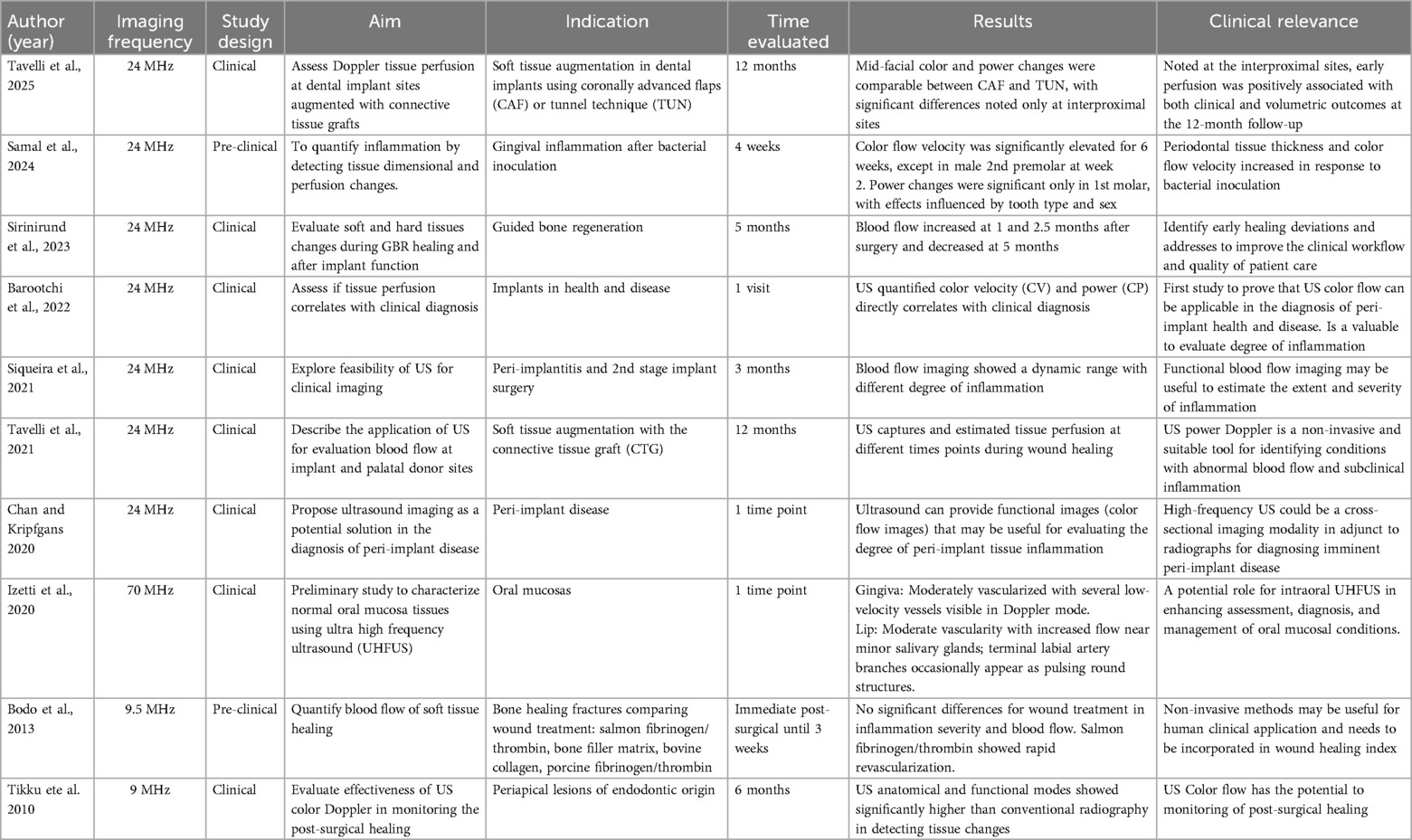
3.6 Ultrasound (US)
Ultrasound is a cross-sectional imaging modality based on the reflection and scattering of sound by the interaction of emitted acoustic waves with tissues (19, 34, 100). For tissue perfusion evaluation, flow modalities, e.g., Color Velocity (CV), and Color Power (CP) have been used. The grayscale (B-mode) display is overlaid with color pixels in which blood flow velocities are displayed that exceed the (noise/wall) filter threshold. Flowing red blood cells scatter the incident acoustic wave and produce a scattered signal that changes in the radio frequency phase if the flow direction is non-perpendicular to the ultrasound beam. CV images display red and blue colors, which correspond to the orientation of the detected blood flow velocity (speed) with respect to the ultrasound beam direction. CP is displayed as a single hue of red, that is quantitatively or qualitatively proportional to the amount of moving blood within the ultrasound resolution cell associated with a given pixel. Additionally, CV and CP can, as a surrogate, describe dynamic blood flow, and thus tissue perfusion, variation as an indicator of the degree of inflammation, correlation with clinical disease diagnoses, and healing monitoring (19, 20, 22, 34, 100–104) (Table 5, Figure 4). A recent RCT (103), compared tissue perfusion at implant sites treated with connective tissue grafts using either a coronally advanced flap (CAF) or tunnel technique (TUN). While mid-facial perfusion showed no significant difference, TUN demonstrated higher interproximal perfusion and early graft vascularization. Early perfusion was associated with improved soft-tissue outcomes at 12 months, highlighting Doppler ultrasonography as a valuable tool for monitoring healing. In addition, a preclinical study (105), assessed inflammation by measuring tissue thickness and perfusion changes in mini-pigs following bacterial inoculation. Soft tissue thickness and color flow velocity significantly increased post-inoculation across most teeth and time points, with variations by tooth type and sex. These changes suggest a correlation between bacterial challenge and periodontal inflammation, though tooth eruption may have influenced results. Limitations of this imaging technology are the reliance on a (gel) coupling medium, potential user dependency, sensitivity to probe pressure over tissues, scanning angulation, and access (Table 5).
3.7 Other imaging techniques
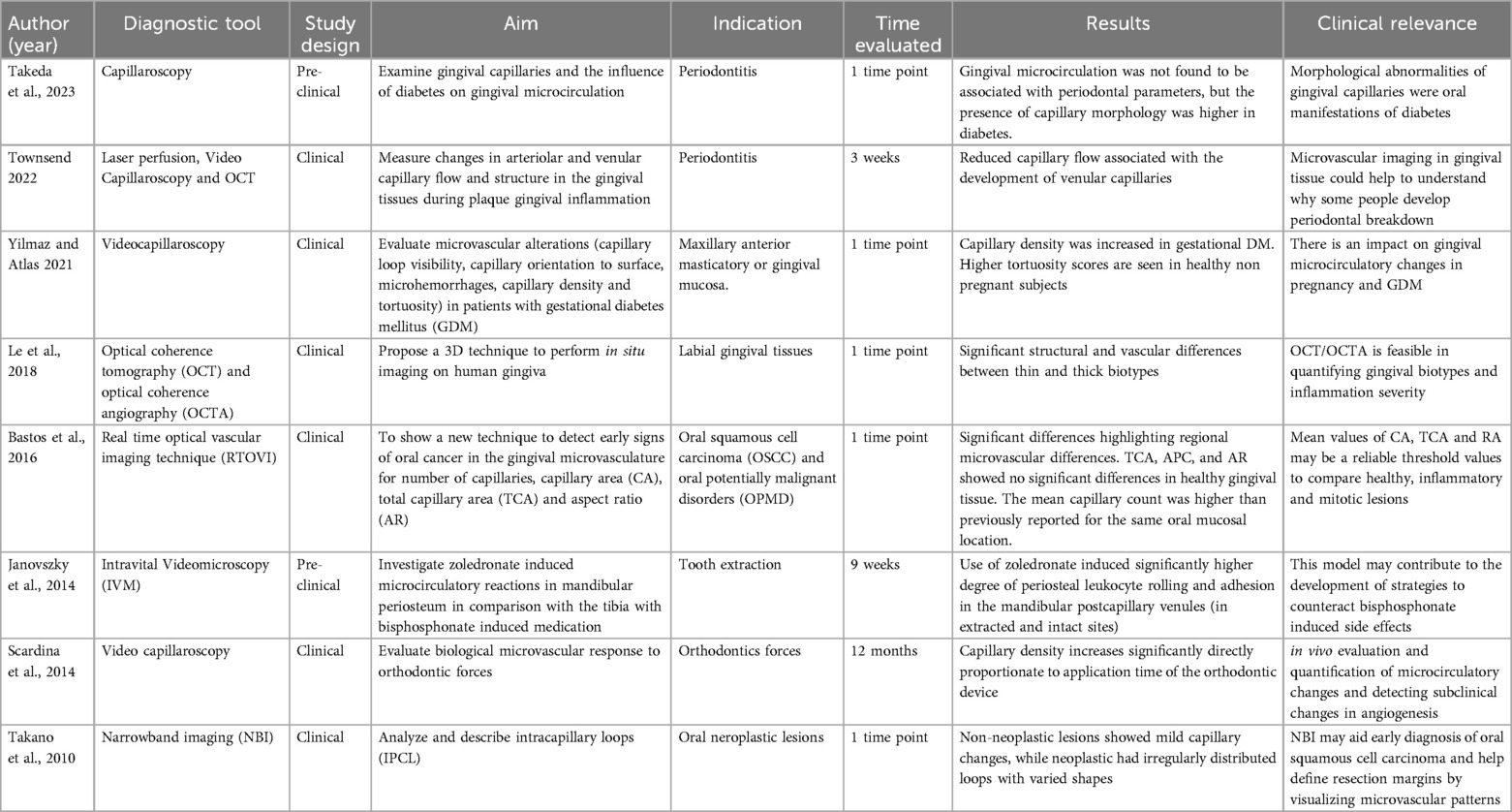
Intravital Video Microscopy (IVM) (106) is mainly used in preclinical studies because of the use of fluorescence dye and and maximal sample stability. A study demonstrated how zoledronic acid may induce microcirculatory inflammatory reactions in the mandibular periosteum in contrast to long bones (tibia). In addition, it was used to examine gingival capillaries during plaque-induced gingival inflammation and to evaluate for the influence of diabetes on gingival microcirculation in pre-clinical and clinical studies (107, 108) (Table 6). The advantage of IVM is that it quantifies microcirculatory parameters of red blood cell velocity (RBCV, μm/s). This takes place offline by inter frame analysis of the recorded images. Limitations of this technique include the need of a fluorescent dye and only superficial penetration (106) (Table 6). Another example is Oral Videocapillaroscopy using a fiber optic probe with high color resolution, a focal spot size of 1.8 mm, and variable magnification (10–1,000×) (109) (Table 6). On the other hand, Videocapillaroscopy was conducted using a computerized video microscope system with a 200× optical probe, and a varying focal spot of (0–2 mm). All assessments were performed by the same operator in the morning under consistent lighting conditions (110). Limitations of this technique is that it only visualizes capillary apices in gingiva, limiting assessment of full loop structure, and potential microvascular changes from overweight status (110).
Optical coherence tomography (OCT) with OCT angiography (OCTA) is a noninvasive 3D imaging method used to assess gingival tissue in situ (111). OCT/OCTA provides detailed structural and vascular information up to 2 mm deep for improved periodontal evaluation. The hand-held OCT probe allows movement in three translational and one rotational direction for precise positioning, with stabilization provided by a bite bar (111). The system uses a swept-source OCT with a 1,310 nm wavelength and 110 nm bandwidth, offering 7 μm axial resolution. This study employed a lightweight probe mounted on a kinetic arm for accurate gingival scanning. Lee et al. highlighted significant structural and vascular differences between thick and thin gingival biotypes, as well as distinct vascular patterns in gingival inflammation. OCT/OCTA is effective for quantifying these biotypic attributes and inflammation severity (111). Limitations of OCT/OCTA include issues with vascular leakage, slow-flow lesions, artifacts, patient stillness, and device variability (111).
Additionally, Bastos and Cook 2016 introduced real-time optical vascular imaging (RTOVI), which detects early signs of oral cancer by observing the gingival microvasculature with green light (112). RTOVI visualizes tissue function and behavior, assessing microvascular features like capillary count, area, and aspect ratio (112). The system uses a specific wavelength (550 ± 10 nm) to optimize red blood cell imaging, combining transmission and reflection techniques. RTOVI enables detection of individual red blood cells (RBCs) in the microvasculature, clearly delineating its structure. RTOVI utilizes a field of view (FOV) of 0.51 × 0.51 mm, with a pixel resolution of 0.35 × 0.35 µm. The study found a higher mean capillary count (45.06 per mm2) compared to previous studies, suggesting that RTOVI, alongside image analysis methods, could serve as a reliable tool for monitoring microvascular changes and exploring inflammatory or precancerous lesions (112). Limitations for this technique include low contrast and not enough resolution. Takano et al. (113) investigated intrapapillary capillary loops (IPCLs), key indicators of early oral neoplastic changes, using a narrowband imaging (NBI) system. NBI enhances visualization of mucosal surfaces and microvasculature by employing optical filters that narrow light bandwidths. When combined with magnification, it improves early cancer detection by highlighting vascular features such as IPCLs. Originally developed for the pharynx and esophagus, NBI has since been adapted for oral cavity assessment. The system uses rotating RGB filters (500, 445, and 415 nm, each with ∼30 nm bandwidth), enabling superficial tissue penetration (170–240 μm) for detailed vascular imaging. However, its effectiveness is limited in areas of hyperplasia (e.g., leukoplakia) and mucosal erosion, where IPCL structures may be obscured or destroyed.
4 Discussion
4.1 General discussion
A few technologies have been identified to study oral vasculature non-invasively in this review, including optical as well as ultrasound based methods (Tables 1–6). They were used to detect and quantify microcirculatory changes resulting from oral diseases, exterior stimuli, and surgical interventions. IVM is among the first developed imaging technology for evaluating microcirculation. It is primarily used for preclinical studies. VC was then developed for easily accessible locations. Technology advancement leads to OPS and soon later SDF. Compared to VC, OPS has higher contrast, is user-friendly, and costs less. SDF enjoys higher image sharpness and contrast than OPS. These 4 methods can provide morphologic (capillary size/diameter) as well as functional (blood flow velocity in mm/s) evaluation of capillaries. DRS and NIRS are another category of imaging technology, generally used for estimating oxygenation and Hb concentration. Last, LDF provides arbitrary perfusion units based on Doppler phase shift without spatial/anatomical information. LSCI also provides arbitrary perfusion units based on speckle pattern changes with a larger field of view and spatial/anatomical information.
4.2 Clinical impact of studying oral microcirculation
Previous studies observed an ischemic-reperfusion pattern of gingival blood flow with faster recovery rates in minimally invasive flaps when compared to extensive incisions and flap design (12, 15, 17, 25, 29, 81). Faster blood flow recovery is strongly associated with earlier normalization of vascular permeability, and periosteal perfusion significantly influences bone healing and determines the prognosis of soft tissue survival (109). In addition, gingival perfusion changes is suggested as an indicator of disease severity (17, 19, 33, 34, 44, 58–60). Imaging technologies that objectively monitor healing in real time, using non-invasive methods, are urgently needed for diagnostic purposes and to inform management strategies. This could potentially eliminate human bias and facilitate integration of artificial intelligence (AI) in perfusion analysis. Their relevant clinical applications span from pre-surgical to intraoperative and post-surgical assessments. Pre-surgically, it is beneficial to obtain three-dimensional maps that visualize the distribution of microcirculation for preventing vascular complications. Perfusion evaluation can document changes in gingival blood flow and assess response to treatment. The integrity of collateral microcirculation in the gingiva is vital for surgical predictability, healing speed, patient morbidity, and wound closure probability (1). Vascular flow alterations may provide insight into a better understanding of tissue healing capacities, which would translate into better clinical performance (10, 12, 15, 19, 20, 34, 40, 42). Therefore, understanding the dynamics of oral microvasculature during incision tracing and flap design may result in optimal vascular reorganization and wound healing (1, 10, 11, 26).
Reliability is key for use of these technologies. Temporal fluctuation of flow does not necessarily reflect myogenic vasomotion; in fact, it can have multiple causes (6, 11, 18). Flow sensitivity may be influenced by systemic conditions such as diabetes, arterial hypertension, and behavioral factors, such as smoking (17, 26, 27, 41, 42, 106). Changes in gingival microcirculation can be related to physiological events synchronized to neurodynamic activity, ventilatory influences, gravitational effects, and arterial and venous pressure (31, 32, 42). In addition, other studies have investigated the high temporal variation of gingival blood flow (Tables 2–5) that may be related to physiological factors such as blood pressure (59), the effect of temperature (52, 73, 74), mechanical stimuli (10), gingival inflammation (17, 19, 22, 31–34, 44, 60), circadian rhythm, occlusion forces (40, 69), orthodontic forces (42), and tooth brushing (71). These factors should be carefully considered and evaluated to improve the reliability of using these technologies as a research tool.
4.3 Features of a desirable device for quantifying microcirculation
An ideal device for microcirculation quantification would possess features that relate to probe/device geometry, the relationship to actual physiological blood flow/perfusion, measurement accuracy and precision, data recording time and data presentation, as well as infection control requirements. In addition, microcirculation maps (surface and/or cross-sectional) should be generated with high spatial resolution (∼100 µm) for better definition. Furthermore, the device should be portable, affordable, and have minimal environmental constraints.
4.4 Available technologies comparison
A summary on the advantages and limitations of the included technologies is included in Table 7. Single-point LDF was developed in 1970s and is most frequently studied for in vivo periodontal tissue perfusion. It provides arbitrary unit for tissue perfusion in a focal region of ∼1 mm3. LSCI quantifies the magnitude of tissue perfusion with a color scale superimposed on the anatomic structure in a larger field of view when, compared to single-point non-anatomical LDF (28, 31–33). Common limitations of LDF and LSCI include limited depth of penetration, making both only suitable for superficial circulation evaluation and provision of arbitrary perfusion units that require a reference for comparison, such as the baseline or contralateral measurements (6, 10, 11, 18, 26–28, 31–33, 41) (Table 3). The field of view provides mesio-distal frame that can vary from the recording of a wider area of interest provides advantages of LSCI over LDF, DRS, OPS, and to US because it gives insight into adjacent tissues. This compensates for some of the limitations compared to other techniques as it offers additional anatomical information.
OSP and SDF provide morphologic (diameter, density) and functional (true blood flow velocity in mm/s) evaluation of capillaries. Only superficial circulation is measured due to limited depth of penetration. Limited field of view can be another disadvantage, making large area evaluation time-comsuming, if not impossible. DRSI and NIRS can offer oxygen and hemoglobin concentration of superficial soft tissues. Lower sensitivity and requirement of complicated computing methods are major disadvantages (88).
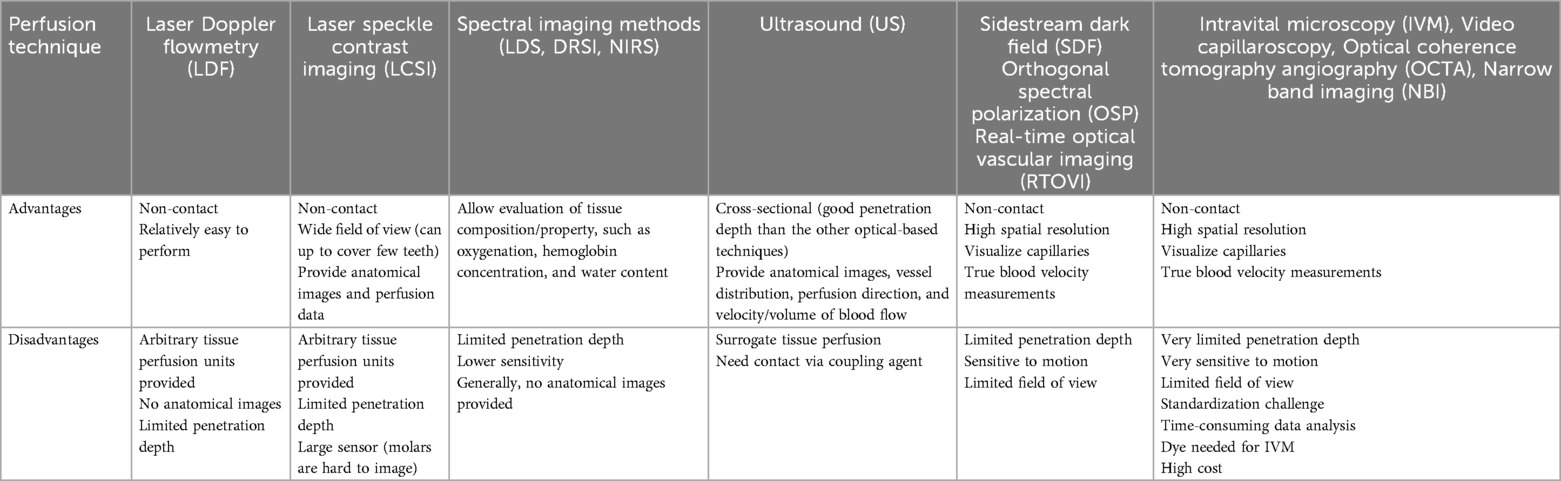
Ultrasound visualizes and estimate blood flow with anatomical reference. In contrast to the other optical methods, this method provides cross-sectional imaging with acceptable depth of penetration for oral mucosa. US is sensitive to the motion of RBCs and obtains velocity information that is a projection of the true velocity vector onto the acoustic beam. For over 15 years US emerges as a reliable and valuable tool for longitudinal assessment of soft and hard tissue healing and has proven to aid clinicians in decision-making at an individual level (19, 20, 100, 102). As with the other methods, there are limitations, such as provision of surrogate values for velocity and volume, reliance on coupling medium, and lower spatial resolution than optical images in general (Tables 5, 7, Figure 3). Previous limitations, may have been lifted with the introduction of a small form factor (toothbrush size) and high-frequency (25 MHz) ultrasound transducer suitable for practical intra-oral scanning (21–23, 34, 105). The costs of these technologies can be categorized as low, moderate, or high. Low-cost techniques are generally priced up to $20,000, moderate-cost technologies range up to $60,000, and high-cost systems are priced at $60,000 and above. High-cost methods, such as LSCI, and Spectral Imaging Methods are expensive due to the complexity of the technology and equipment. In contrast, moderate-cost options like LDF and Ultrasound systems are more affordable, with costs varying based on their features and specific applications. Low-cost techniques, such as Videocapillaroscopy and Videomicroscopy, are typically more budget-friendly but may have limitations in resolution and functionality compared to the more advanced methods. The total cost of each technique includes training, maintenance, and consumables. The cost-benefit ratio depends on the clinical or research needs, frequency of use, and required image resolution (Table 1).
4.5 Future directions
Imaging techniques for assessing wound healing have evolved significantly in recent years and have become important tools in understanding complex oral disease progression and healing processes. Early diagnosis of infection and inflammation as well as quantification of soft tissue healing could significantly improve future patient care and surgical outcome. Longitudinal assessment of wound healing in a range of surgical techniques, compromised patients, graft maturity, and complication detection may soon aid clinicians in establishing evidence-based (treatment) procedures. Bias and limitations of each technique evaluated, are inherently connected. For example, having 3D images instead of 2D images in US would make the field of view more robust. For LCSI, increasing the penetration depth, would allow for better comparison with ultrasound. For LDF, standardization of the applied pressure (from the probe) to the tissue could improve the repeatability of the image. Additionally, factors such as patient movement, the overall depth of the field, and variability in probe positioning would benefit from reduced sensitivity in relation to the estimated gingival blood flow, leading to more consistent and reliable results.
4.6 Limitations of the study
The limitations of this study include a variety of parameters used for estimating circulation, as well as the heterogeneity of the techniques employed. Additionally, the assessment of blood flow was conducted across different clinical scenarios, including both non-surgical/surgical, pre-clinical/clinical settings, and at varying time points, and clinical and preclinical. These factors introduce variability and impact the consistency and comparability of the results. As such, further research is essential to establish more consistent methodologies for more robust comparisons between the different techniques used for measuring gingival blood flow.
5 Conclusions
This systematic review highlights currently available in vivo imaging techniques for non-invasive quantification of periodontal blood flow for the monitoring oral of wound healing healing and classifying diseases. Understanding the mechanisms, readouts, advantages, and limitations of each technique allows for the selection of an appropriate imaging method. Even though having been used extensively in medicine, these techniques are only used for research without much penetration into clinical care. Future investigations should focus on the longitudinal assessment of tissue perfusion after surgical procedures of differing incisions and flap designs to understand the implications of their use. Mapping and quantifying microcirculatory gingival pattern variations and their modifying factors could provide evidence-based treatment planning procedures. Using these tools could result in more predictable surgical outcomes and therefore enhance tissue healing and patient-centered outcomes. Cross-sectional US offers sufficient depth of penetration for oral soft tissue, can complement other optical methods that primarily evaluate superficial perfusion.
Author contributions
AR: Conceptualization, Formal analysis, Validation, Writing – original draft, Writing – review & editing, Investigation, Methodology, Project administration, Software, Visualization. OK: Conceptualization, Validation, Writing – original draft, Writing – review & editing, Supervision. FA: Validation, Writing – original draft, Writing – review & editing, Formal analysis, Investigation, Methodology. DV: Supervision, Validation, Writing – original draft, Writing – review & editing. AB: Writing – original draft, Writing – review & editing. H-LC: Writing – original draft, Writing – review & editing, Conceptualization, Formal analysis, Funding acquisition, Resources, Supervision, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the National Institutes of Health (NIH) with grant number 1-R56-DE-030872-01.
Acknowledgments
All human subject studies were carried out at the University of Michigan School of Dentistry as approved by the University Institutional Review Board (HUM00203875). This assertion pertains to Figure 4, illustrating the application of two perfusion imaging methods on the edentulous crest of a human subject. Informed consent was obtained from all human subjects. The privacy rights of human subjects were always observed. This assertion pertains to Figure 3, illustrating the application of two perfusion imaging methods on the edentulous crest.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdmed.2025.1587821/full#supplementary-material
Supplementary Table S1 | Quality assessment: Assessment for non-RCT (ROBINS-I).
Supplementary Table S2 | Quality assessment: Assessment for RCT (ROB2).
References
1. Kleinheinz J, Büchter A, Kruse-Lösler B, Weingart D, Joos U. Incision design in implant dentistry based on vascularization of the mucosa. Clin Oral Implants Res. (2005) 16(5):518–23. doi: 10.1111/j.1600-0501.2005.01158.x
2. Kuwabara A, Uemura M, Toda I, Suwa F, Takemura A. Microvascular architecture of the buccal periosteum of the mandibular body in the dog. J Osaka Dent Univ. (2018) 52(2):129–37.
3. Pilsl U, Anderhuber F, Neugebauer S. The facial artery-the main blood vessel for the anterior face? Dermatol Surg. (2016) 42(2):203–8. doi: 10.1097/DSS.0000000000000599
4. Shahbazi A, Feigl G, Sculean A, Grimm A, Palkovics D, Molnár B, et al. Vascular survey of the maxillary vestibule and gingiva-clinical impact on incision and flap design in periodontal and implant surgeries. Clin Oral Investig. (2021) 25(2):539–46. doi: 10.1007/s00784-020-03419-w
5. Shahbazi A, Grimm A, Feigl G, Gerber G, Székely AD, Molnár B, et al. Analysis of blood supply in the hard palate and maxillary tuberosity-clinical implications for flap design and soft tissue graft harvesting (a human cadaver study). Clin Oral Investig. (2019) 23(3):1153–60. doi: 10.1007/s00784-018-2538-3
6. Fazekas R, Molnár E, Lohinai Z, Dinya E, Tóth Z, Windisch P, et al. Functional characterization of collaterals in the human gingiva by laser speckle contrast imaging. Microcirculation. (2018) 25(3):e12446. doi: 10.1111/micc.12446
7. Rodriguez A, Velasquez D, Chan HL. Review of intraoral vasculature and implications on incision designs of periodontal and implant surgeries. Int J Periodontics Restorative Dent. (2023) 43(6):753–61. doi: 10.11607/prd.6213
8. Burkhardt R, Preiss A, Joss A, Lang NP. Influence of suture tension to the tearing characteristics of the soft tissues: an in vitro experiment. Clin Oral Implants Res. (2008) 19(3):314–9. doi: 10.1111/j.1600-0501.2007.01352.x
9. Marini L, Rojas MA, Sahrmann P, Aghazada R, Pilloni A. Early wound healing score: a system to evaluate the early healing of periodontal soft tissue wounds. J Periodontal Implant Sci. (2018) 48(5):274–83. doi: 10.5051/jpis.2018.48.5.274
10. Mikecs B, Vág J, Gerber G, Molnár B, Feigl G, Shahbazi A. Revisiting the vascularity of the keratinized gingiva in the maxillary esthetic zone. BMC Oral Health. (2021) 21(1):1–10. doi: 10.1186/s12903-021-01445-y
11. Fazekas R, Molnár B, Kőhidai L, Láng O, Molnár E, Gánti B, et al. Blood flow kinetics of a xenogeneic collagen matrix following a vestibuloplasty procedure in the human gingiva—an explorative study. Oral Dis. (2019) 25(7):1780–8. doi: 10.1111/odi.13163
12. Retzepi M, Tonetti M, Donos N. Gingival blood flow changes following periodontal access flap surgery using laser Doppler flowmetry. J Clin Periodontol. (2007) 34(5):437–43. doi: 10.1111/j.1600-051X.2007.01062.x
13. Dias AT, de Menezes CC, Kahn S, Fischer RG, da Silva Figueredo CM, Fernandes G. Gingival recession treatment with enamel matrix derivative associated with coronally advanced flap and subepithelial connective tissue graft: a split-mouth randomized controlled clinical trial with molecular evaluation. Clin Oral Investig. (2022) 26:1453–63. doi: 10.1007/s00784-021-04119-9
14. Marques T, Santos N, Sousa M, Fernandes JCH, Fernandes GVO. Mixed-thickness tunnel access (MiTT) through a linear vertical mucosal incision for a minimally invasive approach for root coverage procedures in anterior and posterior sites: technical description and case series with 1-year follow-up. Dent J. (2023) 11(10):235. doi: 10.3390/dj11100235
15. Retzepi M, Tonetti M, Donos N. Comparison of gingival blood flow during healing of simplified papilla preservation and modified widman flap surgery: a clinical trial using laser Doppler flowmetry. J Clin Periodontol. (2007) 34(10):903–11. doi: 10.1111/j.1600-051X.2007.01119.x
16. Burkhardt R, Lang NP. Coverage of localized gingival recessions: comparison of micro- and macrosurgical techniques. J Clin Periodontol. (2005) 32(3):287–93. doi: 10.1111/j.1600-051X.2005.00660.x
17. Alssum L, Eubank TD, Roy S, Erdal BS, Yildiz VO, Tatakis DN, et al. Gingival perfusion and tissue biomarkers during early healing of postextraction regenerative procedures: a prospective case series. J Periodontol. (2017) 88(11):1163–72. doi: 10.1902/jop.2017.170117
18. Amaral MM, del-Valle M, Raele MP, De Pretto LR, Ana PA. Osteoporosis evaluation through full developed speckle imaging. J Biophotonics. (2020) 13(7):e202000025. doi: 10.1002/jbio.202000025
19. Barootchi S, Tavelli L, Majzoub J, Chan H, Wang H, Kripfgans O. Ultrasonographic tissue perfusion in peri-implant health and disease. J Dent Res. (2022) 101(3):278–85. doi: 10.1177/00220345211035684
20. Bodo M, Settle T, Royal J, Lombardini E, Sawyer E, Rothwell SW. Multimodal noninvasive monitoring of soft tissue wound healing. J Clin Monit Comput. (2013) 27(6):677–88. doi: 10.1007/s10877-013-9492-z
21. Chan H-LA, Kripfgans OD. Dental Ultrasound in Periodontology and Implantology. Cham: Springer (2020). p. 161–75.
22. Chan HL, Kripfgans OD. Ultrasonography for diagnosis of peri-implant diseases and conditions: a detailed scanning protocol and case demonstration. Dentomaxillofac Radiol. (2020) 49(7):20190445. doi: 10.1259/dmfr.20190445
23. Chan HL, Sinjab K, Li J, Chen Z, Wang HL, Kripfgans OD. Ultrasonography for noninvasive and real-time evaluation of peri-implant tissue dimensions. J Clin Periodontol. (2018) 45(8):986–95. doi: 10.1111/jcpe.12918
24. Dodson TB, Neuenschwander MC, Bays RA. Intraoperative assessment of maxillary perfusion during Le fort I osteotomy. J Oral Maxillofac Surg. (1994) 52(8):827–31. doi: 10.1016/0278-2391(94)90228-3
25. Donos N, D'Aiuto F, Retzepi M, Tonetti M. Evaluation of gingival blood flow by the use of laser Doppler flowmetry following periodontal surgery. A pilot study. J Periodontal Res. (2005) 40(2):129–37. doi: 10.1111/j.1600-0765.2005.00777.x
26. Fazekas R, Molnár E, Mikecs B, Lohinai Z, Vág J. A novel approach to monitoring graft neovascularization in the human gingiva. J Vis Exp. (2019) (143):e58535. doi: 10.3791/58535
27. Fazekas R, Molnár E, Nagy P, Mikecs B, Windisch P, Vág J. A proposed method for assessing the appropriate timing of early implant placements: a case report. J Oral Implantol. (2018) 44(5):378–83. doi: 10.1563/aaid-joi-D-17-00295
28. Gánti B, Molnár E, Fazekas R, Mikecs B, Lohinai Z, Mikó S, et al. Evidence of spreading vasodilation in the human gingiva evoked by nitric oxide. J Periodontal Res. (2019) 54(5):499–505. doi: 10.1111/jre.12650
29. Kaner D, Soudan M, Zhao H, Gaßmann G, Schönhauser A, Friedmann A. Early healing events after periodontal surgery: observations on soft tissue healing, microcirculation, and wound fluid cytokine levels. Int J Mol Sci. (2017) 18(2):283. doi: 10.3390/ijms18020283
30. Milstein DM, Bezemer R, Lindeboom JA, Ince C. The acute effects of CMF-based chemotherapy on maxillary periodontal microcirculation. Cancer Chemother Pharmacol. (2009) 64(5):1047–52. doi: 10.1007/s00280-009-1082-x
31. Molnár B, Molnar E, Fazekas R, Gánti B, Mikecs B, Vag J. Assessment of palatal mucosal wound healing following connective-tissue harvesting by laser speckle contrast imaging: an observational case series study. Int J Periodontics Restorative Dent. (2019) 39(2):e64–70. doi: 10.11607/prd.3878
32. Molnár E, Fazekas R, Lohinai Z, Tóth Z, Vág J. Assessment of the test-retest reliability of human gingival blood flow measurements by Laser speckle contrast imaging in a healthy cohort. Microcirculation. (2018) 25(2):e12420. doi: 10.1111/micc.12420
33. Molnár E, Molnár B, Lohinai Z, Tóth Z, Benyó Z, Hricisák L, et al. Evaluation of llaser speckle contrast imaging for the assessment of oral mucosal blood flow following periodontal plastic surgery: an exploratory study. Biomed Res Int. (2017) 2017:4042902. doi: 10.1155/2017/4042902
34. Siqueira R, Sinjab K, Pan YC, Soki F, Chan HL, Kripfgans O. Comprehensive peri-implant tissue evaluation with ultrasonography and cone-beam computed tomography: a pilot study. Clin Oral Implants Res. (2021) 32(7):777–85. doi: 10.1111/clr.13758
35. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
36. Schulz KF, Altman DG, Moher D, Group* C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. (2010) 152(11):726–32. doi: 10.7326/0003-4819-152-11-201006010-00232
37. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Br Med J. (2016) 355:i4919. doi: 10.1136/bmj.i4919
38. Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. (2019) 366:l4898. doi: 10.1136/bmj.l4898
39. McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. (2020) 12(1):55–61. doi: 10.1002/jrsm.1411
40. Komaki S, Ozaki H, Takahashi S-s, Wada-Takahashi S, Fushima K. Gingival blood flow before, during, and after clenching, measured by laser Doppler blood flowmeter: a pilot study. Am J Orthod Dentofacial Orthop. (2022) 161(1):46–52. doi: 10.1016/j.ajodo.2020.06.045
41. Kouadio AA, Jordana F, Koffi NJ, Le Bars P, Soueidan A. The use of laser Doppler flowmetry to evaluate oral soft tissue blood flow in humans: a review. Arch Oral Biol. (2018) 86:58–71. doi: 10.1016/j.archoralbio.2017.11.009
42. Laredo-Naranjo MA, Patiño-Marín N, Martínez-Castañón GA, Medina-Solís CE, Velázquez-Hernández C, Niño-Martínez N, et al. Identification of gingival microcirculation using Laser Doppler flowmetry in patients with orthodontic treatment—a longitudinal pilot study. Medicina (B Aires). (2021) 57(10):1081. doi: 10.3390/medicina57101081
43. Svalestad J, Hellem S, Vaagbø G, Irgens Å, Thorsen E. Reproducibility of transcutaneous oximetry and laser Doppler flowmetry in facial skin and gingival tissue. Microvasc Res. (2010) 79(1):29–33. doi: 10.1016/j.mvr.2009.10.003
44. Kuraji R, Wu YH, Hashimoto S, Mishiro S, Maeda Y, Miyashita Y, et al. Temporal and dynamic changes in gingival blood flow during progression of ligature-induced periodontitis. Oral Dis. (2020) 26(6):1292–301. doi: 10.1111/odi.13328
45. Ambrosini P, Cherene S, Miller N, Weissenbach M, Penaud J. A laser Doppler study of gingival blood flow variations following periosteal stimulation. J Clin Periodontol. (2002) 29(2):103–7. doi: 10.1034/j.1600-051x.2002.290203.x
46. Hoke JA, Burkes EJ, White JT, Duffy MB, Klitzman B. Blood-flow mapping of oral tissues by laser Doppler flowmetry. Int J Oral Maxillofac Surg. (1994) 23(5):312–5. doi: 10.1016/S0901-5027(05)80117-1
47. Kemppainen P, Avellan NL, Handwerker HO, Forster C. Differences between tooth stimulation and capsaicin-induced neurogenic vasodilatation in human gingiva. J Dent Res. (2003) 82(4):303–7. doi: 10.1177/154405910308200412
48. Kerdvongbundit V, Sirirat M, Sirikulsathean A, Kasetsuwan J, Hasegawa A. Blood flow and human periodontal status. Odontology. (2002) 90(1):52–6. doi: 10.1007/s102660200008
49. Kerdvongbundit V, Vongsavan N, Soo-Ampon S, Hasegawa A. Microcirculation and micromorphology of healthy and inflamed gingivae. Odontology. (2003) 91(1):19–25. doi: 10.1007/s10266-003-0024-z
50. Kerdvongbundit V, Vongsavan N, Soo-Ampon S, Phankosol P, Hasegawa A. Microcirculation of the healthy human gingiva. Odontology. (2002) 90(1):48–51. doi: 10.1007/s102660200007
51. Le Bars P, Niagha G, Kouadio AA, Demoersman J, Roy E, Armengol V, et al. Pilot study of laser Doppler measurement of flow variability in the microcirculation of the palatal Mucosa. Biomed Res Int. (2016) 2016:5749150. doi: 10.1155/2016/5749150
52. Matsuki M, Xu YB, Nagasawa T. Gingival blood flow measurement with a non-contact laser flowmeter. J Oral Rehabil. (2001) 28(7):630–3. doi: 10.1046/j.1365-2842.2001.00729.x
53. Singh DB, Stansby G, Harrison DK. Assessment of oxygenation and perfusion in the tongue and oral mucosa by visible spectrophotometry and laser Doppler flowmetry in healthy subjects. Adv Exp Med Biol. (2008) 614:227–33. doi: 10.1007/978-0-387-74911-2_26
54. Antic S, Markovic-Vasiljkovic B, Dzeletovic B, Jelovac DB, Kuzmanovic-Pficer J. Assesment of radiotherapy effects on the blood flow in gingiva and dental pulp—a laser Doppler flowmetry study. J Appl Oral Sci. (2022) 30:e20220329. doi: 10.1590/1678-7757-2022-0329
55. Katz MS, Ooms M, Winnand P, Heitzer M, Peters F, Kniha K, et al. Evaluation of peri-implant perfusion in patients who underwent avascular augmentation or microvascular reconstruction using laser Doppler flowmetry and tissue spectrophotometry: a prospective comparative clinical study. Clin Oral Investig. (2024) 28(8):431. doi: 10.1007/s00784-024-05825-w
56. Baab DA, Oberg A, Lundström A. Gingival blood flow and temperature changes in young humans with a history of periodontitis. Arch Oral Biol. (1990) 35(2):95–101. doi: 10.1016/0003-9969(90)90169-B
57. Kozlov V, Ibragim R. Determination of tissue blood flow in the gums and microcirculatory disorders in chronic periodontitis. J Vasc Res. (2011) 48:265.
58. Rodríguez-Martínez M, Patiño-Marín N, Loyola-Rodríguez JP, Brito-Orta MD. Gingivitis and periodontitis as antagonistic modulators of gingival perfusion. J Periodontol. (2006) 77(10):1643–50. doi: 10.1902/jop.2006.050311
59. Schmid-Schönbein H, Ziege S, Grebe R, Blazek V, Spielmann R, Linzenich F. Synergetic interpretation of patterned vasomotor activity in microvascuiar perfusion: discrete effects of myogenic and neurogenic vasoconstriction as well as arterial and venous pressure fluctuations. Int J Microcirc. (1997) 17(6):346–59. doi: 10.1159/000179251
60. Yamamoto R, Amano K, Takahashi S-W, To M, Takahashi S, Matsuo M. Changes in the microcirculation in periodontal tissue due to experimental peri-implantitis. J Oral Biosci. (2021) 63(2):153–60. doi: 10.1016/j.job.2021.03.002
61. Matheny JL, Abrams H, Johnson DT, Roth GI. Microcirculatory dynamics in experimental human gingivitis. J Clin Periodontol. (1993) 20(8):578–83. doi: 10.1111/j.1600-051X.1993.tb00774.x
62. Akazawa H, Sakurai K. Changes of blood flow in the mucosa underlying a mandibular denture following pressure assumed as a result of light clenching. J Oral Rehabil. (2002) 29(4):336–40. doi: 10.1046/j.1365-2842.2002.00912.x
63. Kocabalkan E, Turgut M. Variation in blood flow of supporting tissue during use of mandibular complete dentures with hard acrylic resin base and soft relining: a preliminary study. Int J Prosthodont. (2005) 18(3):210–3.15945307
64. Ogino T, Ueda T, Ogami K, Koike T, Sakurai K. Effects of chewing rate and reactive hyperemia on blood flow in denture-supporting mucosa during simulated chewing. J Prosthodont Res. (2017) 61(1):54–60. doi: 10.1016/j.jpor.2016.04.002
65. Okada C, Ueda T, Sakurai K. Blood flow in denture-supporting maxillary mucosa in response to simulated mastication by loading. J Prosthodont Res. (2010) 54(4):159–63. doi: 10.1016/j.jpor.2010.03.001
66. Svalestad J, Thorsen E, Vaagbø G, Hellem S. Effect of hyperbaric oxygen treatment on oxygen tension and vascular capacity in irradiated skin and mucosa. Int J Oral Maxillofac Surg. (2014) 43(1):107–12. doi: 10.1016/j.ijom.2013.07.006
67. Heckmann SM, Heckmann JG, HiIz MJ, Popp M, Marthol H, Neundörfer B, et al. Oral mucosal blood flow in patients with burning mouth syndrome. Pain. (2001) 90(3):281–6. doi: 10.1016/S0304-3959(00)00410-3
68. Sakr Y, Gath V, Oishi J, Klinzing S, Simon TP, Reinhart K, et al. Characterization of buccal microvascular response in patients with septic shock. Eur J Anaesthesiol. (2010) 27(4):388–94. doi: 10.1097/EJA.0b013e3283349db3
69. Vag J, Fazekas A. Influence of restorative manipulations on the blood perfusion of human marginal gingiva as measured by laser Doppler flowmetry. J Oral Rehabil. (2002) 29(1):52–7. doi: 10.1046/j.1365-2842.2002.00818.x
70. Miron MI, Barcutean M, Luca RE, Todea CD, Tudor A, Ogodescu E. The effect of changing the toothbrush on the marginal gingiva microcirculation in the adolescent population—a Laser Doppler flowmetry assessment. Diagnostics. (2022) 12(8):1830. doi: 10.3390/diagnostics12081830
71. Perry DA, McDowell J, Goodis HE. Gingival microcirculation response to tooth brushing measured by laser Doppler flowmetry. J Periodontol. (1997) 68(10):990–5. doi: 10.1902/jop.1997.68.10.990
72. Herlofson BB, Brodin P, Aars H. Increased human gingival blood flow induced by sodium lauryl sulfate. J Clin Periodontol. (1996) 23(11):1004–7. doi: 10.1111/j.1600-051X.1996.tb00528.x
73. Heckmann JG, Hilz MJ, Hummel T, Popp M, Marthol H, Neundörfer B, et al. Oral mucosal blood flow following dry ice stimulation in humans. Clin Auton Res. (2000) 10(5):317–21. doi: 10.1007/BF02281116
74. Baab DA, Oberg PA, Holloway GA. Gingival blood flow measured with a laser Doppler flowmeter. J Periodontal Res. (1986) 21(1):73–85. doi: 10.1111/j.1600-0765.1986.tb01440.x
75. Patiño-Marín N, Martínez F, Loyola-Rodríguez JP, Tenorio-Govea E, Brito-Orta MD, Rodríguez-Martínez M. A novel procedure for evaluating gingival perfusion status using laser-Doppler flowmetry. J Clin Periodontol. (2005) 32(3):231–7. doi: 10.1111/j.1600-051X.2005.00655.x
76. Heitzer M, Winnand P, Katz MS, Grottke O, Magnuska Z, Kiessling F, et al. Hemostasis and gingival healing—polyurethane adhesive postextraction under rivaroxaban therapy in a rodent model. Int J Dent. (2025) 2025(1):3384210. doi: 10.1155/ijod/3384210
77. Ahn J, Pogrel MA. The effects of 2% lidocaine with 1:100,000 epinephrine on pulpal and gingival blood flow. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (1998) 85(2):197–202. doi: 10.1016/S1079-2104(98)90426-7
78. Hinrichs JE, LaBelle LL, Aeppli D. An evaluation of laser Doppler readings obtained from human gingival sulci. J Periodontol. (1995) 66(3):171–6. doi: 10.1902/jop.1995.66.3.171
79. Ketabi M, Hirsch SR. The effects of local anesthetic containing adrenaline on gingival blood flow in smokers and non smokers. J Clin Periodontol. (1997) 24(12):888–92. doi: 10.1111/j.1600-051X.1997.tb01207.x
80. Kawaai H, Yoshida K, Tanaka E, Togami K, Tada H, Ganzberg S, et al. Dexmedetomidine decreases the oral mucosal blood flow. Br J Oral Maxillofac Surg. (2013) 51(8):928–31. doi: 10.1016/j.bjoms.2013.07.007
81. Tatarakis N, Gkranias N, Darbar U, Donos N. Blood flow changes using a 3D xenogeneic collagen matrix or a subepithelial connective tissue graft for root coverage procedures: a pilot study. Clin Oral Investig. (2018) 22(4):1697–705. doi: 10.1007/s00784-017-2261-5
82. Baab DA, Oberg PA. The effect of cigarette smoking on gingival blood flow in humans. J Clin Periodontol. (1987) 14(7):418–24. doi: 10.1111/j.1600-051X.1987.tb01547.x
83. Reuther WJ, Hale B, Matharu J, Blythe JN, Brennan PA. Do you mind if I vape? Immediate effects of electronic cigarettes on perfusion in buccal mucosal tissue–a pilot study. Br J Oral Maxillofac Surg. (2016) 54(3):338–41. doi: 10.1016/j.bjoms.2015.12.001
84. Diehl D, Kaner D, Bockholt A, Bilhan H, Friedmann A. Microcirculation and neutrophil-related cytokine concentrations are not altered around narrow diameter implants in T2DM patients during wound healing. Clin Oral Investig. (2023) 27(3):1167–75. doi: 10.1007/s00784-022-04731-3
85. Liu Y-H, To M, Okudera T, Wada-Takahashi S, Takahashi S-S, Su C-y, et al. Advanced platelet-rich fibrin (A-PRF) has an impact on the initial healing of gingival regeneration after tooth extraction. J Oral Biosci. (2022) 64(1):141–7. doi: 10.1016/j.job.2021.11.001
86. Vág J, Nagy TL, Mikecs B. Sex-related differences in endothelium-dependent vasodilation of human gingiva. BMC Oral Health. (2022) 22(1):1–13. doi: 10.1186/s12903-022-02186-2
87. Palombo D, Dobos A, Duran ML, Esporrin JS, Sanz M. Harvest of epithelialized gingival grafts without application of hemostatic sutures: a randomized clinical trial using laser speckle contrast imaging. J Periodontol. (2024) 95(12):1160–70. doi: 10.1002/JPER.23-0620
88. Lindeboom J, Mathura K, Ramsoekh D, Harkisoen S, Aartman I, Van den Akker H, et al. The assessment of the gingival capillary density with orthogonal spectral polarization (OPS) imaging. Arch Oral Biol. (2006) 51(8):697–702. doi: 10.1016/j.archoralbio.2006.02.009
89. Prasanth CS, Betsy J, Jayanthi JL, Nisha UG, Prasantila J, Subhash N. In vivo inflammation mapping of periodontal disease based on diffuse reflectance spectral imaging: a clinical study. J Biomed Opt. (2013) 18(2):026019. doi: 10.1117/1.JBO.18.2.026019
90. Preidl RH, Reichert S, Coronel TV, Kesting M, Wehrhan F, Schmitt CM. Free gingival graft and collagen matrix revascularization in an enoral open wound situation. J Oral Maxillofac Surg. (2021) 79(5):1027–37. doi: 10.1016/j.joms.2020.12.019
91. Zakian CM, Pretty IA, Ellwood R, Hamlin D. In vivo quantification of gingival inflammation using spectral imaging. J Biomed Opt. (2008) 13(5):054045. doi: 10.1117/1.2982536
92. Schwarz J, Klang V, Hoppel M, Wolzt M, Valenta C. Corneocyte quantification by NIR densitometry and UV/vis spectroscopy for human and porcine skin and the role of skin cleaning procedures. Skin Pharmacol Physiol. (2012) 25(3):142–9. doi: 10.1159/000336787
93. Jain V, Livecchi TT, Oliveira AH, Jacques SL, Pierce MC, Subhash HM. Comprehensive characterization and multimodal image processing with the polaroid P31 intraoral camera. Multimodal Biomedical Imaging XX (2025).
94. Jacques SL. Optical properties of biological tissues: a review. Phys Med Biol. (2013) 58(11):R37. doi: 10.1088/0031-9155/58/11/R37
95. Livecchi TT, Thomas A, Oliveira A, Jain V, Jacques SL, Subhash HM, et al. Prediction of blood volume and oxygen saturation in superficial and deep skin layers using a color polarization camera. In: Zeng H, Rajadhyaksha M, editors. Proc. SPIE PC13292, Photonics in Dermatology and Plastic Surgery 2025, PC132920E. San Francisco: SPIE Dental Library (2025). doi: 10.1117/12.3044091
96. Ooms M, Winnand P, Heitzer M, Peters F, Bock A, Katz M, et al. Flap perfusion monitoring with an attached surface probe in microvascular reconstruction of the oral cavity. Clin Oral Investig. (2023) 27:5577–85. doi: 10.1007/s00784-023-05177-x
97. Sha M, Griffin M, Denton CP, Butler PE. Sidestream dark field (SDF) imaging of oral microcirculation in the assessment of systemic sclerosis. Microvasc Res. (2019) 126:103890. doi: 10.1016/j.mvr.2019.103890
98. Tang P, Wu C, Chi X, Xu B, Chen B, Tian Y, et al. Tongue-parallel probe-based microcirculation monitoring and automated sublingual vessel estimation. Opt Laser Technol. (2025) 187:112803. doi: 10.1016/j.optlastec.2025.112803
99. Cusack RA, Rodríguez A, Cantan B, Garduno A, Connolly E, Zilahi G, et al. Microcirculation properties of 20% albumin in sepsis; a randomised controlled trial. J Crit Care. (2025) 87:155039. doi: 10.1016/j.jcrc.2025.155039
100. Tavelli L, Barootchi S, Majzoub J, Chan HL, Giannobile WV, Wang HL, et al. Ultrasonographic tissue perfusion analysis at implant and palatal donor sites following soft tissue augmentation: a clinical pilot study. J Clin Periodontol. (2021) 48(4):602–14. doi: 10.1111/jcpe.13424
101. Sirinirund B, Wang IC, Ramadan G, Kripfgans OD, Chan HL. Ridge augmentation planning, wound healing evaluation, and peri-implant tissue phenotype assessment with ultrasonography: a case report. Clinic Adv Periodontics. (2023) 1:30–7. doi: 10.1002/cap.10234
102. Tikku AP, Kumar S, Loomba K, Chandra A, Verma P, Aggarwal R. Use of ultrasound, color Doppler imaging and radiography to monitor periapical healing after endodontic surgery. J Oral Sci. (2010) 52(3):411–6. doi: 10.2334/josnusd.52.411
103. Tavelli L, Kripfgans OD, Chan HL, Vera Rodriguez M, Sabri H, Mancini L, et al. Doppler ultrasonographic evaluation of tissue revascularization following connective tissue graft at implant sites. J Clin Periodontol. (2025) 52(1):68–79. doi: 10.1111/jcpe.13889
104. Izzetti R, Vitali S, Aringhieri G, Oranges T, Dini V, Nisi M, et al. Discovering a new anatomy: exploration of oral mucosa with ultra-high frequency ultrasound. Dentomaxillofac Radiol. (2020) 49(7):20190318. doi: 10.1259/dmfr.20190318
105. Samal A, Kripfgans OD, Wang I-C, Betancourt ABR, Webber L, Quesada C, et al. High-frequency ultrasound characterization of periodontal soft tissues pre-and post-bacterial inoculation. Ultrasound Med Biol. (2025) 51(5):860–9. doi: 10.1016/j.ultrasmedbio.2025.01.014
106. Janovszky Á, Szabó A, Varga R, Garab D, Boros M, Mester C, et al. Periosteal microcirculatory reactions in a zoledronate-induced osteonecrosis model of the jaw in rats. Clin Oral Investig. (2015) 19(6):1279–88. doi: 10.1007/s00784-014-1347-6
107. Takeda K, Mizutani K, Mikami R, Fujino A, Ito Y, Takeuchi S, et al. Morphological analysis of the impact of diabetes on gingival capillaries with non-invasive blood flow scope–a preliminary study. J Dent Sci. (2023) 18(3):1134–40. doi: 10.1016/j.jds.2022.10.024
108. Townsend D. Identification of venular capillary remodelling: a possible link to the development of periodontitis? J Periodontal Implant Sci. (2022) 52(1):65. doi: 10.5051/jpis.2101160058
109. Scardina GA, Cacioppo A, Seidita F, Garofalo G, Lotti M, Messina P. Evaluation of gingival microcirculation in patients undergoing fixed orthodontic treatment: a pilot study. Eur J Paediatr Dent. (2014) 15(2):143–6.25102464
110. Yilmaz D, Altas A. Evaluation of gingival microcirculation in patients with gestational diabetes mellitus. Microvasc Res. (2021) 138:104222. doi: 10.1016/j.mvr.2021.104222
111. Le NM, Song S, Zhou H, Xu J, Li Y, Sung CE, et al. A noninvasive imaging and measurement using optical coherence tomography angiography for the assessment of gingiva: an in vivo study. J Biophotonics. (2018) 11(12):e201800242. doi: 10.1002/jbio.201800242
112. Bastos P, Cook R. Real time optical vascular imaging: a potential technique for the diagnosis of mucosal disease including early oral cancer. Prim Dent J. (2016) 5(1):86–91. doi: 10.1177/205016841600500112
Keywords: ultrasound, tissue perfusion, microvasculature, laser speckle, laser Doppler flowmetry, non-invasive
Citation: Rodriguez A, Kripfgans O, Aellos F, Velasquez D, Baltazar A and Chan H-L (2025) Non-invasive and quantitative methods for assessment of blood flow in periodontal and oral soft tissues: a systematic review. Front. Dent. Med. 6:1587821. doi: 10.3389/fdmed.2025.1587821
Received: 5 March 2025; Accepted: 5 May 2025;
Published: 22 May 2025.
Edited by:
Gustavo Fernandes, A.T. Still University, United StatesReviewed by:
Ben Urban, Shimane University, JapanVivianne Chappuis, University of Bern, Switzerland
Hiroki Kodama, Jikei University School of Medicine, Japan
Copyright: © 2025 Rodriguez, Kripfgans, Aellos, Velasquez, Baltazar and Chan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hsun-Liang Chan, Y2hhbi4xMDY5QG9zdS5lZHU=
 Amanda Rodriguez
Amanda Rodriguez Oliver Kripfgans3
Oliver Kripfgans3 Hsun-Liang Chan
Hsun-Liang Chan