- Seattle Children’s Research Institute, Center for Integrative Brain Research, University of Washington, School of Medicine, Seattle, WA, USA
For patients with a craniopharyngioma (CP), treatment of hypothalamic obesity (HO) and hyperphagia following resection and/or radiotherapy is extremely difficult and few reports have been published on potential drug therapies. Psychomotor stimulant methylphenidate (MPH) has been reported to inhibit food intake (FI). In this paper, we report reduction of body mass index (BMI) and appetite in an adolescent CP patient suffering from HO. We then tested the ability of MPH to attenuate the FI and body weight (BW) gain in a rat model consistent with the neuroanatomical and metabolic disturbances commonly observed in obese CP patients. Specifically, we used a novel electrolytically generated combined medial hypothalamic lesion (CMHL) affecting the arcuate nucleus, ventromedial hypothalamic nucleus, and dorsomedial hypothalamic nucleus to induce hyperphagia, rapid weight gain, and adiposity. Both CMHL and control animals (n = 7 per group) were administered either methylphenidate HCl (MPH; 20 mg kg−1 day−1) or saline for 4 days in a crossover design experiment 28 weeks post-surgery. A significant decrease in percent baseline FI (CMHL −23%, p = 0.008; control −20%, p = 0.002) and percent change in BW (CMHL −1.97%/4 days, p = 0.011; control −1.75%/4 days, p = 0.003) was observed during MPH treatment as compared to saline. Conclusion: This study shows MPH treatment of severely obese CMHL rats resulted in significantly reduced FI and BW loss.
Introduction
Severe hypothalamic obesity (HO) and hyperphagia are common sequelae following tumor resection in child and adolescent craniopharyngioma (CP) patients, ranging in rate from 22 to 62% (Brauner et al., 1987; Sorva, 1988; Muller, 2008). Radical tumor resection and subtotal tumor resection with or without radiotherapy are the current therapeutic standard. Post-resection and/or treatment, a distinct trend in weight gain has been observed in children who developed HO. Specifically, a rapid gain in body mass index (BMI) SDS for the first 6 months followed by long-term stabilization with little appreciable decline (Ahmet et al., 2006). Even more distressing, attempts at weight loss through caloric restriction and exercise are largely unsuccessful (Lustig et al., 2003; Eyal et al., 2006). Pharmacological treatment of HO and hyperphagia has shown success in a few clinical studies and case reports (Mason et al., 2002; Lustig et al., 2003; Danielsson et al., 2007; Hamilton et al., 2011) though weaker weight reductions were observed compared to uncomplicated obesity (Danielsson et al., 2007). A recent study of both normal weight and obese CP patients showed that insulin resistance and altered gut hormone secretion are not exclusively a result of obesity but hypothalamic disruption caused by the tumor and/or its treatment (Roth et al., 2011b).
Animal models utilizing electrolytically and chemically generated lesions produce similar effects to those observed clinically and are thereby an excellent model in which to study therapeutic interventions for HO (Dawson et al., 1989; Tokunaga et al., 1991, 1993; Schoelch et al., 2002; Leitner and Bartness, 2008). Recently we developed a novel combined medial hypothalamic lesion (CMHL) model that utilizes electrolytic lesions in the arcuate nucleus (ARC), ventromedial hypothalamic nucleus (VMN), and dorsomedial hypothalamic nucleus (DMN) to model the sequelae commonly observed in HO resulting from resection and/or treatment of CP (Roth et al., 2011a). Similar effects were observed when combining chemically induced ARC lesion with electrolytically induced VMN lesions (Elfers et al., 2011). Specifically, the CMHL model exhibits rapid weight gain, hyperphagia, hyperinsulinemia, and hyperleptinemia.
One potential therapeutic target for the treatment of hyperphagia in HO is the dopaminergic system. Methylphenidate HCl (MPH), a psychomotor stimulant used in the treatment of attention deficit hyperactivity disorder in children, produces an increase in brain synaptic dopamine (DA) signaling with the side effect of anorexia (Leddy et al., 2009). However, there is variance in the reported long-term ability of MPH to sustain weight loss ranging from 3 months to the duration of administration of a clinically effective dose (Leddy et al., 2009).
The aim of our study was to test the inhibitory effect of MPH treatment on food intake (FI) in our novel CMHL rat model of HO. We hypothesized that administration of MPH to severely obese CMHL rats will result in reduction of FI and body weight (BW) gain.
Case Report
A 5.9-year-old male presented with short stature, headaches, nausea, and vomiting. Neuroimaging (CT, MRI) indicated a large sellar and suprasellar mass causing obstructive hydrocephalus. Following gross total resection of a large CP, the patient was diagnosed with panhypopituitarism. Two years later the patient underwent cranial radiation therapy due to tumor relapse. At the time of tumor diagnosis, height was 110 cm (25th percentile), weight 23.4 kg (75th percentile), and BMI 19.3 kg/m2 (+1.9 z-score). Following resection, the patient noted a very low level of energy, increased hunger, and difficulty becoming satiated. One year post-tumor resection, height was 117 cm (25th percentile), weight 34.1 kg (3 kg >97th percentile), and BMI 24.5 kg/m2 (+2.5 z-score). Despite optimal endocrine management, the patient continued to experience significant weight gain that was unable to be mediated by lifestyle changes. As part of hormone replacement therapy desmopressin acetate (DDAPV), hydrocortisone, growth hormone, and levothyroxine were prescribed. At age 15 years, height was 184 cm (>90th percentile), weight 154 kg (70 kg >97th percentile), and BMI 45.5 kg/m2 (+2.9 z-score).
MPH was identified as a potential therapeutic to attenuate hunger due to its frequently observed anorexic side effect. MPH treatment began at age 15 years with a 20 mg day−1 dose (morning: 10 mg; evening: 10 mg; 30–45 min prior to meals) and was gradually increased to 50 mg day−1 dose (morning: 30 mg; noon: 10 mg; evening: 10 mg; 30–45 min prior to meals) over the course of 10 weeks. Following start of MPH treatment, overall weight gain ceased (Figure 1) and patient noted a dose dependent decrease in hunger. After 31 weeks treatment, the MPH dose was adjusted to 60 mg day−1 dose (morning: 30 mg; noon: 10 mg; evening: 20 mg; 30–45 min prior to meals) due to a slight increase in BMI. After this last dose adjustment, the patient has had continued decrease in BMI for over 56 weeks. After 87 weeks of MPH treatment, height was 186 cm (>90th percentile), weight 142 kg (47 kg >97th percentile), and BMI 41.4 kg m−2 (+2.8 z-score). It is important to note that this patient was not under any exercise regimen during this time and therefore any change in BMI is independent of exercise.
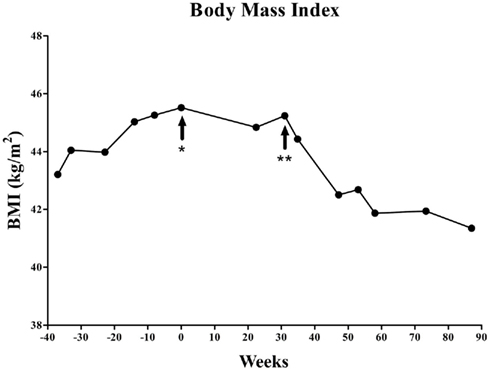
Figure 1. Changes of BMI in the patient course of methylphenidate treatment. At the start of the methylphenidate treatment the patient was 76 kg over ideal weight given height and age. After 87 weeks of treatment the patient lost 12 kg of excess weight. *Start of methylphenidate treatment (20 mg day−1 dose; morning: 10 mg; evening: 10 mg). **Start of final dose (60 mg day−1 dose; morning: 30 mg; noon: 10 mg; evening: 20 mg).
Materials and Methods for Rat Study
Animals
Young adult male Sprague Dawley rats, weighing 250–260 g, were purchased from Charles River Laboratory (Wilmington, MA, USA). Animals were individually housed on a 12/12-h light/dark cycle (lights on at 07:00 h) in a temperature (23°C) and humidity (50 ± 10%) controlled room. Ad libitum access to regular chow (5053 Pico Lab Rodent Diet, Purina LabDiet, Richmond, CA, USA) and water was provided. Following surgery, the animals’ BW and FI were recorded. All procedures performed were approved by the Institutional Animal Care and Use Committee at the Seattle Children’s Research Institute and were in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Electrolytic Lesion
To electrolytically induce the CMHL, animals were placed under a surgical-plane of anesthesia (isoflurane/oxygen mix, 5% induction, 2–4% throughout the procedure) and were mounted in a Kopf stereotaxic frame (Tujunga, CA, USA). The upper incisor bar was 3.5 mm above the interaural line. Lesions were targeted to the ARC, VMN, and DMN through the placement of an insulated stainless steel electrode at stereotaxic coordinates based on our previous study (Roth et al., 2011a). Specifically, an anodal electric current (110 V, 1.7 mA for 15 s) was passed through the tip of the electrode while placed 2.6 mm posterior to the bregma, 0.5 mm lateral to the midsagittal line, and both 10 mm (ARC) and 8.6 mm (VMN/DMN) ventral from the skull according to the method described in our recent paper (Roth et al., 2011a).
Injection of Methylphenidate and Data Collection
A crossover experimental design was utilized to determine the effects of daily MPH injections (20 mg kg−1 BW) on FI and BW. For 6 days prior to the start of the injections, FI was recorded and averaged to determine baseline values (n = 7 per group). Four animals from each group received daily i.p. injections of 20 mg kg−1 MPH (Letco Medical, Decatur, AL, USA) while three animals from each group received i.p. saline injections (0.9% sodium chloride inj., USP, Hospira) for a period of 4 days, after which, the treatments were switched for an additional 4 days of testing. Administration of all injections as well as measurement of FI and BW occurred in the evening (18:30 h, 30 min prior to lights out). MPH has been show to have a half-life of approximately 1 h in rats (Kuczenski and Segal, 2002), therefore we did not provide a washout period between treatments. Lee index [LI; BW−1/3/snout to anus length (mm)] measures were taken pre- and post-treatment as a indicator of total adiposity.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism Software (La Jolla, CA, USA). Outcome variables between study groups were compared using Student’s t-tests for continuous variables. Two-way ANOVA modeling with a Bonferroni post hoc was used for contrasts of two factors between study groups. In all instances, a two-sided p < 0.05 was considered significant unless otherwise stated.
Results for Rat Study
General Results
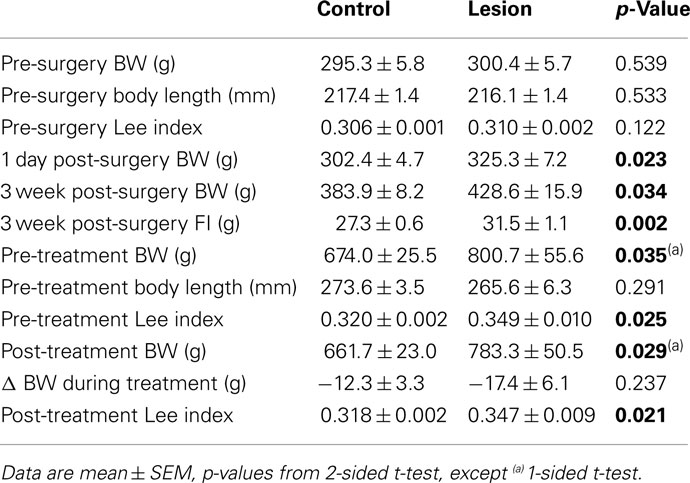
Pre-surgery BW, body length (BL), and LI were comparable in lesion and control groups (p = 0.539, p = 0.533, p = 0.122, respectively). An immediate increase in FI and BW (1 day post-surgery) was observed in the lesion group and significant changes in BW were observed 3 weeks post-surgery (p = 0.034). At the time of MPH treatment (28 weeks post-surgery), there was a significant difference in pre-treatment LI (p = 0.025) indicating a greater degree of obesity in the lesion group (Table 1).
Body Weight
Both control and lesion groups exhibited a slight trend of weight gain during the 4 days of saline injection (control: 0.155 ± 0.213% BW, lesion: 0.340 ± 0.388% BW). Significant weight loss was experienced by both groups when administered MPH with respect to the saline (control: −1.752 ± 0.410% BW, p = 0.003; lesion: −1.968 ± 0.628% BW, p = 0.011; Figures 2A,B). No significant difference was observed in either the absolute or percentage change in BW between the lesion and control groups within one treatment.
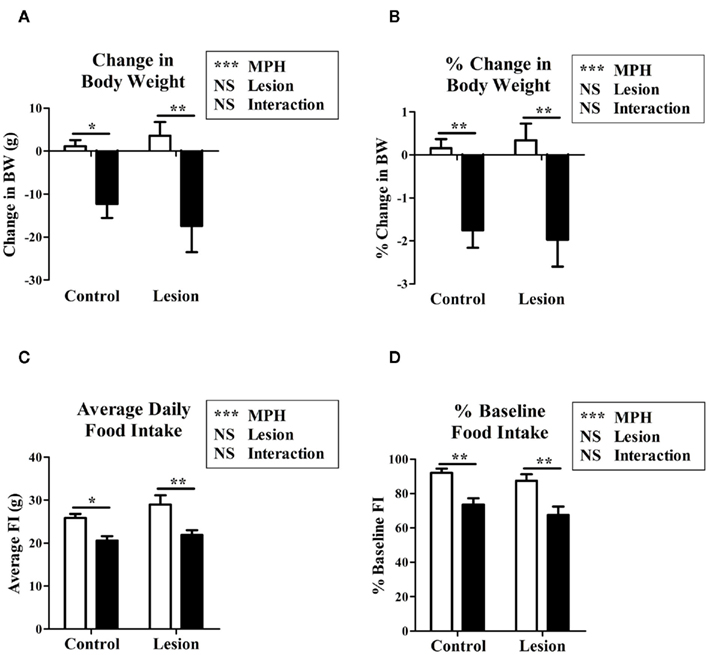
Figure 2. The effects of methylphenidate on either lesion or control animals on change in body weight during treatment (A), percentage change in body weight (B), average daily food intake (C), percentage baseline (6 day average prior to treatment) food intake (D), in methylphenidate injected (black bars) vs. saline injected (white bars) rats. Two-way ANOVA modeling with a Bonferroni post hoc was used for contrasts of two factors between study groups. *p < 0.05, **p < 0.01, ***p < 0.001.
Food Intake
Lesion vs. control animals displayed no significant difference in baseline FI (p = 0.183). A reduction in FI compared to baseline was observed as a trend in both control (92.0 ± 2.4% of baseline, p = 0.131) and lesion (87.4 ± 3.9% of baseline, p = 0.288) groups when receiving injections of saline. When treated with MPH, a significant reduction in FI relative to saline injection was seen in the control (80.1 ± 4.1% of saline, p = 0.002) and lesion (76.9 ± 3.6% of saline, p = 0.008) groups (Figures 2C,D).
Discussion
Our current study examined the inhibitory effect of MPH on FI through daily evening i.p. injections (MPH, 20 mg kg−1 in saline) on our CMHL rat model of HO. MPH treatment significantly reduced FI in both the control and lesion group when compared with saline treatments. Significant weight loss was observed in both groups during MPH treatment whereas no change occurred during saline treatment. Given that drugs might be less effective in reducing BW in HO (Danielsson et al., 2007), these results are remarkable in that lesion group experienced similar weight loss to the control group while being administered MPH. This experiment also illustrates the dualism of hedonic and homeostatic regulation of FI. The CMHL model of HO by nature results in severe dysregulation of homeostatic regulatory pathways through partial destruction of the hypothalamus. The ability of MPH to attenuate homeostatic dysfunction reflects the ability of hedonic regulation to override homeostatic pathways.
The observed weight loss is likely a result of more than just a reduction in FI. It has been shown that i.p. injections of MPH lead to a significant increase in locomotor activity when compared to both i.p. vehicle and intragastrical MPH administration (Gerasimov et al., 2000). Additionally, as a psychomotor stimulant, MPH has been used clinically to treat narcolepsy (Roth, 2007). While locomotor activity and duration of sleep cycles were not quantified in this experiment, they are likely confounding factors responsible for the weight loss and should not be omitted from this discussion.
It should be noted that the MPH dose administered to rats is significantly higher than dosages ordinarily administered to humans; this is to account for metabolic differences between rats and humans. An i.p. dose of 2.5–10 mg kg−1 MPH in a rat is comparable to the clinical dosage administered to children and adults for the treatment of ADHD (Yang et al., 2006) although a 20 mg kg−1 dose is commonly used in rat studies (Crawford et al., 1998; McDougall et al., 1999; Meririnne et al., 2001; Teo et al., 2003). In a study by Teo et al. (2002) examining long-term (90 day) oral gavage toxicity levels for MPH, the no-observed- effect-level was determined to be 40 mg kg−1 MPH. Given that this was a short exploratory study, the higher 20 mg kg−1 dose was used to determine if any change in FI or BW could be observed.
We are aware of three clinical studies that have examined the anorexic effect of MPH with differing results. One study showed a 34% reduction in FI in obese males after administration of the lowest effective dose of MPH (0.5 mg kg−1 in seven of nine subjects; Leddy et al., 2004); where a similar study reported an 11% reduction in energy intake following MPH administration in both obese and non-obese adults (0.5 mg kg−1; Goldfield et al., 2007). In contrast, a third study in obese and non-obese adults found a reduction in FI along with lower appetite and food craving ratings following MPH (0.5 mg kg−1) administration in everyone except obese males. The obese males experienced increases in all three categories after MPH administration (Davis et al., 2011). The results in our model are comparable to the Leddy et al. (2004) and Goldfield et al. (2007) studies with a 19.9 and 23.1% decrease in FI in control and lesion animals respectively following MPH administration when compared to saline treatment.
In our presented case report, the patient experienced significant weight gain and hyperphagia following resection of a CP that was unable to be mediated by lifestyle modification. MPH dosage was gradually increased to a dose that resulted in sustained decrease in BMI. These findings are significant in that MPH treatment has resulted in 87 weeks of nearly consistent reduction of BMI in a patient with HO independent of an exercise regimen. Moreover, the last 56 weeks after final dosage adjustment have resulted in even more dramatic reduction in BMI. These results indicate that MPH may be a potential therapeutic for weight loss in HO.
The mechanism by which MPH induces anorexia is not completely known, although it is attributed to being an effect of MPH action on the dopaminergic system to elicit a reward response. It has been shown that MPH increases DA signaling through actions at the synapse, specifically, through blockade of DA reuptake into the presynaptic terminal, increasing availability of pre-synaptic DA D2 autoreceptors and activation of D1 receptors on the postsynaptic neuron (Wilens, 2008). It is possible that MPH elicits a reward response normally induced by FI, therefore suppressing the normal drive to eat. Interestingly, imaging studies have shown that striatal DA D2 receptors are reduced in obese subjects compared to non-obese controls and in proportion to their BMI (Wang et al., 2001). Furthermore, in morbidly obese subjects, DA D2 receptor availability was associated with metabolic activity in prefrontal cortical regions implicated in the regulation of FI and hyperphagia (Volkow et al., 2009). This suggests that obese individuals have a dysfunction in their DA systems and therefore do not have the same capacity to elicit reward and thus compensate with increased FI.
Several limitations exist in this exploratory experimental design; most notable is the relatively short duration of treatment. Clinically, the anorexic side effect of MPH has been observed to decrease over time, therefore a longer exposure is needed in follow up experiments. In addition, the current study did not focus on the hormone changes associated with this model and treatment. Additionally, in humans the therapeutic effect of MPH requires sustained brain levels, a condition that is not easily met in rats due to their high rate of clearance of MPH as indicated by the relatively short half-life. In the current study this was addressed by administering a single large dose once per day that would effectively saturate DA transporters for a longer time. In future experiments this will be addressed through the use of several smaller doses administered over the course of the day or implantation of a micro infusion pump. Other factors this experiment did not directly address are any potential effects due to changes in psychomotor stimulation or alterations in the animal’s sleep cycle. Finally, given the potential variability of the lesions, the study size (n = 7 per group) is rather small and will be expanded in future studies.
In conclusion, the results of this exploratory study indicate that MPH significantly attenuates FI and elicits BW loss in our novel CMHL rat model. These results correlate well with our clinical observations in patients with HO as well as previous clinical studies reported by others on the anorexic effect of MPH in normal weight and obese subjects. The presented results are significant in that they present a model, which may be used to elucidate the mechanisms by which MPH improves some of the most distressing sequelae of HO. A better understanding of these mechanisms could illuminate new targets for future drug therapies for the treatment of HO. This reinforces the need for future hypothesis driven studies focused on the underlying mechanisms that address changes in hormones (leptin, gut hormones), insulin resistance and body composition.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Michael W. Schwartz, and Gregory Morton, Diabetes and Obesity Center of Excellence, Seattle, as well as James Blevins, Department of Veterans Affairs Medical Center, Seattle, WA, USA, for their constructive ideas and comments while developing the CMHL rat model. We also thank S. R. Ojeda, Oregon National Primate Research Center, Oregon, Portland, for providing us with equipment and advice while establishing this rat model. The authors are grateful to John Welsh, Seattle Children’s Research Institute, for supporting this study.
References
Ahmet, A., Blaser, S., Stephens, D., Guger, S., Rutkas, J. T., and Hamilton, J. (2006). Weight gain in craniopharyngioma – a model for hypothalamic obesity. J. Pediatr. Endocrinol. Metab. 19, 121–127.
Brauner, R., Malandry, F., Rappaport, R., Pierre-Kahn, A., and Hirsch, J. F. (1987). Craniopharyngioma in children. Endocrine evaluation and treatment. Apropos of 37 cases. Arch. Fr. Pediatr. 44, 765–769.
Crawford, C. A., McDougall, S. A., Meier, T. L., Collins, R. L., and Watson, J. B. (1998). Repeated methylphenidate treatment induces behavioral sensitization and decreases protein kinase A and dopamine-stimulated adenylyl cyclase activity in the dorsal striatum. Psychopharmacology (Berl.) 136, 34–43.
Danielsson, P., Janson, A., Norgren, S., and Marcus, C. (2007). Impact sibutramine therapy in children with hypothalamic obesity or obesity with aggravating syndromes. J. Clin. Endocrinol. Metab. 92, 4101–4106.
Davis, C., Fattore, L., Kaplan, A. S., Carter, J. C., Levitan, R. D., and Kennedy, J. L. (2011). The suppression of appetite and food consumption by methylphenidate: the moderating effects of gender and weight status in healthy adults. Int. J. Neuropsychopharmacol. 7, 1–7.
Dawson, R. Jr., Simpkins, J. W., and Wallace, D. R. (1989). Age- and dose-dependent effects of neonatal monosodium glutamate (MSG) administration to female rats. Neurotoxicol. Teratol. 11, 331–337.
Elfers, C., Ralston, M., and Roth, C. L. (2011). Studies of different female rat models of hypothalamic obesity. J. Pediatr. Endocrinol. Metab. 24, 131–137.
Eyal, O., Sundararajan, S., Inge, T. H., and Rose, S. R. (2006). Obesity in patients with craniopharyngioma. Endocrinologist 16, 286–293.
Gerasimov, M. R., Franceschi, M., Volkow, N. D., Gifford, A., Gatley, S. J., Marsteller, D., Molina, P. E., and Dewey, S. L. (2000). Comparison between intraperitoneal and oral methylphenidate administration: a microdialysis and locomotor activity study. J. Pharmacol. Exp. Ther. 295, 51–57.
Goldfield, G. S., Lorello, C., and Doucet, E. (2007). Methylphenidate reduces energy intake and dietary fat intake in adults: a mechanism of reduced reinforcing value of food? Am. J. Clin. Nutr. 86, 308–315.
Hamilton, J. K., Conwell, L. S., Syme, C., Ahmet, A., Jeffery, A., and Daneman, D. (2011). Hypothalamic obesity following craniopharyngioma surgery: results of a pilot trial of combined diazoxide and metformin therapy. Int. J. Pediatr. Endocrinol. 2011, 417949.
Kuczenski, R., and Segal, D. S. (2002). Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J. Neurosci. 22, 7264–7271.
Leddy, J. J., Epstein, L. H., Jaroni, J. L., Roemmich, J. N., Paluch, R. A., Goldfield, G. S., and Lerman, C. (2004). Influence of methylphenidate on eating in obese men. Obes. Res. 12, 224–232.
Leddy, J. J., Waxmonsky, J. G., Salis, R. J., Paluch, R. A., Gnagy, E. M., Mahaney, P., Erbe, R., Pelham, W. E., and Epstein, L. H. (2009). Dopamine-related genotypes and the dose-response effect of methylphenidate on eating in attention-deficit/hyperactivity disorder youths. J. Child Adolesc. Psychopharmacol. 19, 127–136.
Leitner, C., and Bartness, T. J. (2008). Food deprivation-induced changes in body fat mobilization after neonatal monosodium glutamate treatment. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R775–R783.
Lustig, R. H., Hinds, P. S., Ringwald-Smith, K., Christensen, R. K., Kaste, S. C., Schreiber, R. E., Rai, S. N., Lensing, S. Y., Wu, S., and Xiong, X. (2003). Octreotide therapy of pediatric hypothalamic obesity: a double-blind, placebo-controlled trial. J. Clin. Endocrinol. Metab. 88, 2586–2592.
Mason, P. W., Krawiecki, N., and Meacham, L. R. (2002). The use of dextroamphetamine to treat obesity and hyperphagia in children treated for craniopharyngioma. Arch. Pediatr. Adolesc. Med. 156, 887–892.
McDougall, S. A., Collins, R. L., Karper, P. E., Watson, J. B., and Crawford, C. A. (1999). Effects of repeated methylphenidate treatment in the young rat: sensitization of both locomotor activity and stereotyped sniffing. Exp. Clin. Psychopharmacol. 7, 208–218.
Meririnne, E., Kankaanpaa, A., and Seppala, T. (2001). Rewarding properties of methylphenidate: sensitization by prior exposure to the drug and effects of dopamine D1- and D2-receptor antagonists. J. Pharmacol. Exp. Ther. 298, 539–550.
Muller, H. L. (2008). Childhood craniopharyngioma. Recent advances in diagnosis, treatment and follow-up. Horm. Res. 69, 193–202.
Roth, C. L., Blevins, J. E., Ralston, M., Elfers, C., Ogimoto, K., Kaiyala, K. J., and Morton, G. J. (2011a). A novel rodent model that mimics the metabolic sequelae of obese craniopharyngioma patients. Pediatr. Res. 69, 230–236.
Roth, C. L., Gebhardt, U., and Muller, H. L. (2011b). Appetite-regulating hormone changes in patients with craniopharyngioma. Obesity (Silver Spring) 19, 36–42.
Schoelch, C., Hubschle, T., Schmidt, I., and Nuesslein-Hildesheim, B. (2002). MSG lesions decrease body mass of suckling-age rats by attenuating circadian decreases of energy expenditure. Am. J. Physiol. Endocrinol. Metab. 283, E604–E611.
Sorva, R. (1988). Children with craniopharyngioma. Early growth failure and rapid postoperative weight gain. Acta Paediatr. Scand. 77, 587–592.
Teo, S., Stirling, D., Thomas, S., Hoberman, A., Kiorpes, A., and Khetani, V. (2002). A 90-day oral gavage toxicity study of D-methylphenidate and D,L-methylphenidate in Sprague-Dawley rats. Toxicology 179, 183–196.
Teo, S. K., Stirling, D. I., Hoberman, A. M., Christian, M. S., Thomas, S. D., and Khetani, V. D. (2003). D-methylphenidate and D,L-methylphenidate are not developmental toxicants in rats and rabbits. Birth Defects Res. B Dev. Reprod. Toxicol. 68, 162–171.
Tokunaga, K., Bray, G. A., and Matsuzawa, Y. (1993). Improved yield of obese rats using a double coordinate system to locate the ventromedial or paraventricular nucleus. Brain Res. Bull. 32, 191–194.
Tokunaga, K., Matsuzawa, Y., Fujioka, S., Kobatake, T., Keno, Y., Odaka, H., Matsuo, T., and Tarui, S. (1991). PVN-lesioned obese rats maintain ambulatory activity and its circadian rhythm. Brain Res. Bull. 26, 393–396.
Volkow, N. D., Tomasi, D., Wang, G. J., Telang, F., Fowler, J. S., Wang, R. L., Logan, J., Wong, C., Jayne, M., and Swanson, J. M. (2009). Hyperstimulation of striatal D2 receptors with sleep deprivation: implications for cognitive impairment. Neuroimage 45, 1232–1240.
Wang, G. J., Volkow, N. D., Logan, J., Pappas, N. R., Wong, C. T., Zhu, W., Netusil, N., and Fowler, J. S. (2001). Brain dopamine and obesity. Lancet 357, 354–357.
Wilens, T. E. (2008). Effects of methylphenidate on the catecholaminergic system in attention-deficit/hyperactivity disorder. J. Clin. Psychopharmacol. 28, S46-53.
Keywords: hypothalamic lesion, hyperphagia, methylphenidate, weight gain, food intake
Citation: Elfers CT and Roth CL (2011) Effects of methylphenidate on weight gain and food intake in hypothalamic obesity. Front. Endocrin. 2:78. doi: 10.3389/fendo.2011.00078
Received: 25 August 2011;
Paper pending published: 13 September 2011;
Accepted: 06 November 2011;
Published online: 14 December 2011.
Edited by:
Hermann Lothar Mueller, Klinikum Oldenburg gGmbH, GermanyReviewed by:
Günter K. Karl Stalla, Max-Planck-Institute of Psychiatry, GermanyCarlos Dieguez, University of Santiago de Compostela, Spain
Copyright: © 2011 Elfers and Roth. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Christian Ludwig Roth, Division of Endocrinology, Seattle Children’s Hospital Research Institute, 1900 Ninth Avenue, Seattle, WA 98101, USA. e-mail:Y2hyaXN0aWFuLnJvdGhAc2VhdHRsZWNoaWxkcmVucy5vcmc=