- 1INSERM, Laboratory of Development and Plasticity of the Postnatal Brain, Jean-Pierre Aubert Research Center, Unité 837, Lille, France
- 2School of Medicine, UDSL, Lille, France
The semaphorin proteins are among the best-studied families of guidance cues, contributing to morphogenesis and homeostasis in a wide range of tissue types. The major semaphorin receptors are plexins and neuropilins, however other receptors and co-receptors are capable to mediate signaling by semaphorins. These guidance proteins were originally identified as growth cone “collapsing factors” or as inhibitory signals, crucial for nervous system development. Since those seminal discoveries, the list of functions of semaphorins has rapidly grown. Over the past few years, a growing body of data indicates that semaphorins are involved in the regulation of the immune and vascular systems, in tumor growth/cancer cell metastasis and in neural circuit formation. Recently there has been increasing emphasis on research to determine the potential influence of semaphorins on the development and homeostasis of hormone systems and how circulating reproductive hormones regulate their expression and functions. Here, we focus on the emerging role of semaphorins in the development, differentiation and plasticity of unique neurons that secrete gonadotropin-releasing hormone (GnRH), which are essential for the acquisition and maintenance of reproductive competence in all vertebrates. Genetic evidence is also provided showing that insufficient semaphorin signaling contributes to some forms of reproductive disorders in humans, characterized by the reduction or failure of sexual competence. Finally, we will review some studies with the goal of highlighting how the expression of semaphorins and their receptors might be regulated by gonadal hormones in physiological and pathological conditions.
Semaphorins and Their Receptors
Semaphorins are one of the largest protein families of phylogenetically conserved guidance cues and have been extensively studied in a variety of species, including Caenorhabditis elegans, Drosophila, zebrafish, rodents, and humans. More than 20 semaphorin coding genes have been identified named and grouped into eight classes according to their structural homologies and phylogenetic relationships, including invertebrates (Classes 1 and 2), vertebrates (Classes 3–7), and viral semaphorins (Class V) [Figure 1; (1, 2)].
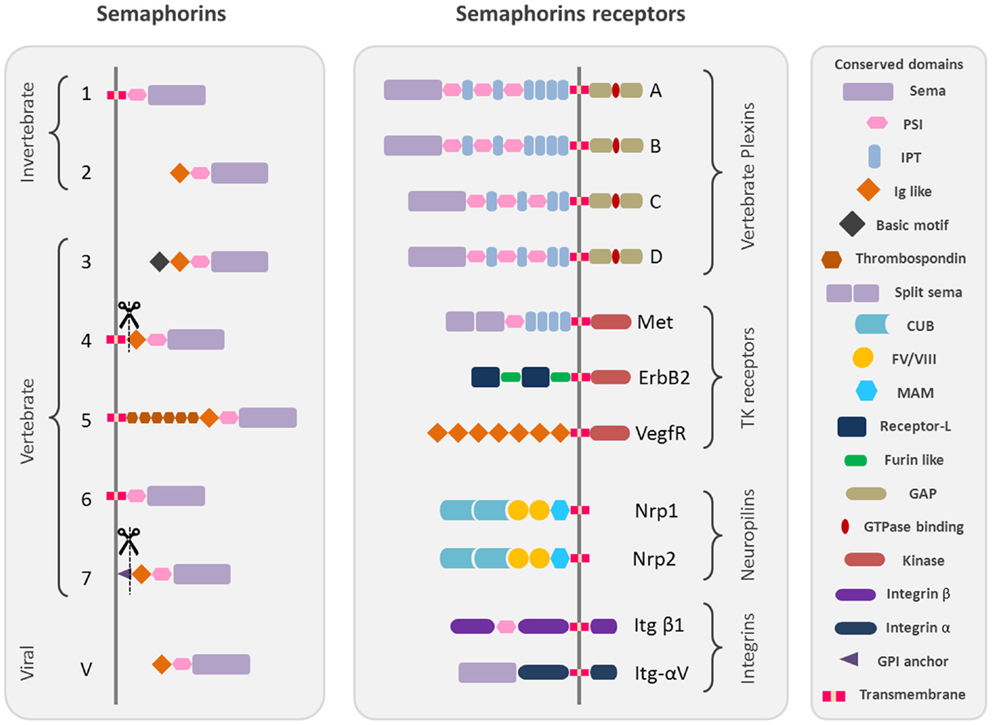
Figure 1. Schematic representation of the protein structure of semaphorins and their receptors. Semaphorins are represented in their classification into eighth classes. Class 1 and 2 semaphorins are found in invertebrates. Class 3–7 semaphorins are found in vertebrates. Both semaphorins and plexins are characterized by Sema domains. Additional domains present in semaphorins and plexins include PSI domains (plexin, semaphorin, and integrin) and immunoglobulin (Ig)-like domains. The structural conserved domains are drawn in different shapes and colors as indicated in the figure. Domains abbreviations: PSI, plexin semaphorin integrin; IPT, Ig-like Plexin Transcription factors; Ig-like, immunoglobulin like; CUB, complement C1r/C1s, Uegf, Bmp1; FV/VIII, coagulation factor V/VIII homology like; MAM, meprin like; GPI, glycosylphosphatidylinositol.
All semaphorins are characterized by the presence of a sema domain, important for dimerization and binding specificity with the receptors, followed by a PSI (plexins, semaphorins, and integrins) domain and a C terminus domain, which confers class specific features (3–5).
The main receptors of semaphorins are the plexins, which are their high affinity receptors. Nine vertebrate plexins have been identified so far, which have been grouped into four subfamilies and show high structural homology with semaphorins as they are characterized by the presence of a sema domain (Figure 1) [reviewed by (6)]. Some secreted semaphorins also require the presence of obligate co-receptors, neuropilins (Nrp-1/Nrp-2), which function as the ligand-binding partner in co-receptor complexes for both plexins and vascular endothelial growth factor receptors (VEGFRs) (7, 8) (Figure 1) [reviewed by (9–11)].
Moreover, some semaphorins exert their effect by binding to various holoreceptor complexes associated to plexins. It is the case for different tyrosine kinase receptors like Met (12–14), which also possess a sema homologous domain, ErbB2 (15), and the VEGFR2 (16) but also extracellular matrix receptors like alpha and beta integrins (17, 18).
Although the function of semaphorins and their receptors was first acknowledged in the development of the nervous system, these molecules are widely expressed both inside and outside the nervous system [reviewed by (9, 19–22)]. The involvement of semaphorins and plexins in diverse biological processes, such as the development of the nervous and cardiovascular systems, the function of the immune system, and pathological processes such as tumor progression, has also been extensively reviewed (10, 11, 21, 23, 24). Here, we will highlight recent advances in our understanding of the molecular mechanisms underlying the effects of semaphorins on the motility, survival, and axonal plasticity of neurons that secrete gonadotropin-releasing hormone (GnRH).
Semaphorins in Guidance of GnRH Neuronal Migration
In vertebrates, the GnRH decapeptide regulates the secretion of luteinizing hormone and follicle-stimulating hormone, which govern the onset of puberty, gametogenesis, and estrous cycling, from anterior pituitary gonadotropes (25). During postnatal life, GnRH – secreting neurons are integral members of the hypothalamic-pituitary-gonadal axis. However, during embryonic development, these cells originate from an extracerebral region, namely the nasal placode (26), and migrate to the hypothalamus apposed to olfactory-vomeronasal nerves (VNNs) (27, 28). Alterations in the development of this system or the secretion of GnRH are associated with hypogonadotropic hypogonadism (HH) in humans, a condition characterized by the reduction or failure of sexual competence (29). Unraveling the genetic pathways involved in the regulation of GnRH system development is crucial to understanding the basis of its pathogenesis in human reproductive disorders and formulating novel therapies.
Cell migration plays an essential role in tissue formation during development. The forebrain is one of the most intricate regions of the mammalian brain, and complex migratory movements are required to generate the extraordinary degree of organization observed in this neural structure. Defects in neuronal migration during the development of the forebrain lead to severe learning and cognitive deficits. Understanding how cell migration occurs in the forebrain is therefore essential to discerning the mechanisms underlying its normal and pathological development (30). Abnormal neuronal migration also occurs in diseases that ultimately affect reproduction. For example, in humans, several monogenic disorders leading to idiopathic hypogonadotropic hypogonadism (IHH) are a consequence of the disruption of GnRH neuronal ontogeny/migration (29). The migration of GnRH neurons from the nasal placode, where they are born (27, 28), to the postnatal preoptic area and hypothalamus constitutes one of the best-characterized examples of axonophilic migration in the forebrain (31). To reach their final destination, these neurons migrate along the nasal septum, cross the cribriform plate under the olfactory bulb, and traverse the forebrain, migrating along vomeronasal (VMN) axons (Figure 2A).
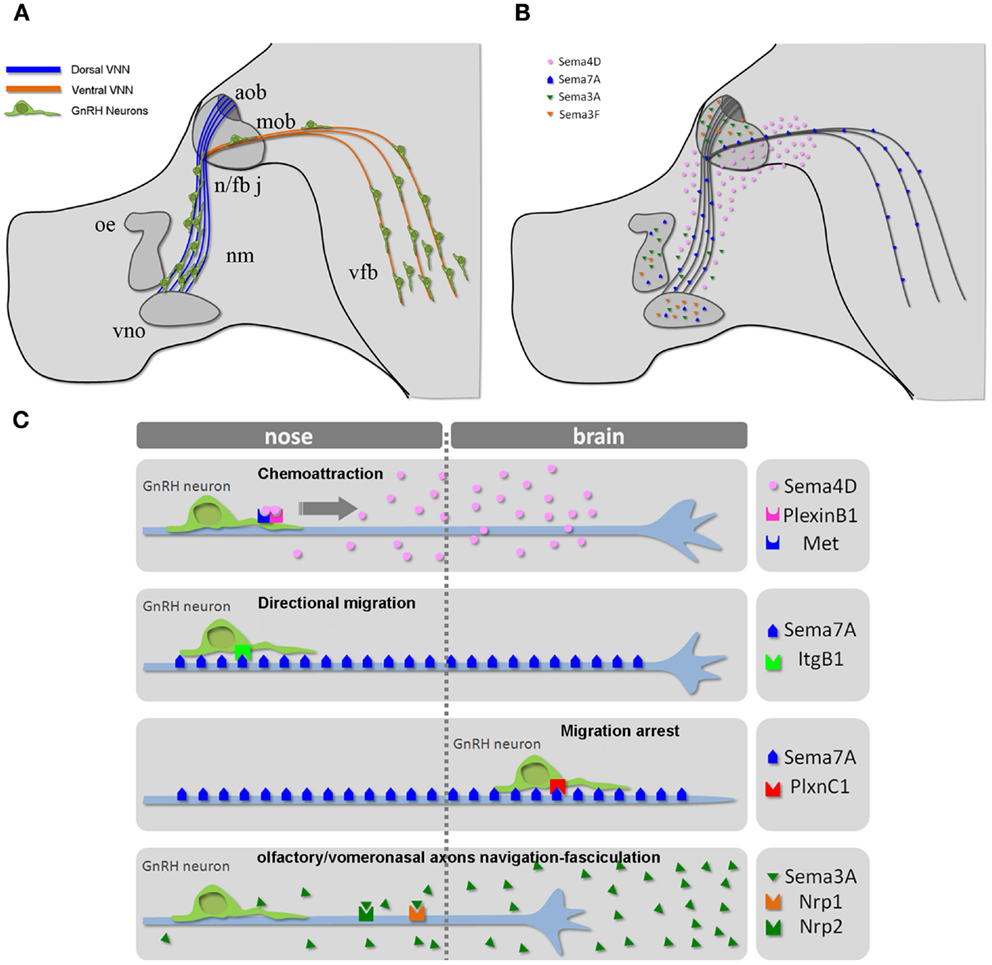
Figure 2. The GnRH neuronal migratory route. (A) Schematic representation of the head of a mouse embryo at E14.5, depicting the scaffold of vomeronasal/terminal nerve fibers along which GnRH cells migrate from the nose to the ventral forebrain region. (B) Schematic depicting the expression pattern of different semaphorins across the GnRH migratory pathway. (C) Mechanisms of action of the indicated semaphorins on GnRH cell motility and/or vomeronasal axonal navigation. Abbreviations: VNN, vomeronasal nerve; cx, cerebral cortex; ob, olfactory bulb; nm, nasal mesenchyme; oe, olfactory epithelium; vno, vomeronasal organ; n/fb j, nasal/forebrain junction; aob, accessory olfactory bulb; mob, main olfactory bulb; vfb, ventral forebrain.
The full repertoire of molecular cues regulating the migratory process and precise targeting of GnRH neurons to the hypothalamus has not been completely elucidated. The putative underlying mechanisms involve different classes of signaling molecules, and the list of potential candidates has lengthened during the last decade (26, 32, 33). Another emergent theme from work on various axonal and cell migration guidance cues is that the expression and function of their receptors is under tight molecular control. Such control allows the spatiotemporal regulation of the responsiveness of growth cones to guidance cues. However, even the large number of molecules already identified likely underestimates the complexity of the potential interactions involved. Indeed, GnRH neurons spatially and temporally travel across areas (the nasal region, nasal/forebrain junction, and forebrain; Figure 2) that contain a variety of guidance molecules and factors. In addition, many molecules defy anatomical boundaries by being expressed in multiple areas and may produce different responses depending on the receptor complexes expressed by GnRH neurons as a function of time (embryonic stage) and space (anatomical localization).
During embryonic development, several semaphorins are expressed all along the GnRH migratory route (Figure 2B) (34–39, 14, 40, 41), prompting several investigators to study the potential role of semaphorins in GnRH neuronal migration. Although the regulation of semaphorin receptors remains incompletely understood, recent studies have begun to provide more insight into the sophisticated molecular mechanisms that allow for the diversification and spatiotemporal control of semaphorin responsiveness in developing GnRH neurons and olfactory/vomeronasal axons.
Semaphorin 4D
Semaphorin 4D (Sema4D) is a membrane-bound semaphorin that can also be proteolytically released (“shed”) into the extracellular space in an active form (42, 43). An intriguing aspect of neural circuit development is that a relatively small number of proteins set up the wiring of a vast number of neuronal connections. Accumulating evidence indicates that mechanisms exist to diversify the effects of guidance proteins, allowing them to mediate this large number of wiring events. How do neurons diversify their responses to semaphorin signaling? One way this goal is achieved is through the ability of individual semaphorins to function as attractive or repulsive guidance cues by activating different receptor complexes on the target cell. One such mechanism has been elucidated for Sema4D, which, in addition to functioning as a collapsing signal for axonal growth cones (44), has previously been shown to induce chemotaxis in epithelial and endothelial cells, and functions as a proangiogenic factor through the coupling its receptor PlexinB1 with the hepatocyte growth factor (HGF) receptor Met tyrosine kinase (12, 13, 45).
PlexinB1 is highly expressed in the developing nasal placode and on olfactory axons and GnRH cells during prenatal development (14), whereas Sema4D is present in the nasal mesenchyme, although its expression is higher in the nasal/forebrain junction and in the developing forebrain. Besides those regions, a recent study showed that olfactory ensheathing cells, which surround GnRH neurons and provide them with an important microenvironment for their migration during development, express Sema4D transcript as well (46).
PlexinB1-deficient mice exhibit a defect in the migration of GnRH neurons that leads to a reduction of GnRH neurons in the adult brain. Postnatal day-3 tissues show a decrease in GnRH neurons in the caudal region of the forebrain and an increase in GnRH neurons in rostral brain regions as a consequence of this migratory defect, which affects the normal embryonic development of these neuroendocrine cells. Interestingly, it has been recently shown that reproduction is also impaired in PlexinB1-ligand, Sema4D knock-out mice as a consequence of a significant decrease in GnRH hypothalamic expression and/or reduced ovarian follicle maturation observed in these mutants (47).
In addition, Sema4D promotes directional migration in immortalized GnRH cells by coupling PlexinB1 with the activation of the Met tyrosine kinase [Figure 3A, reprinted with permission from (14)]. In that study, no abnormalities were observed in the development or organization of olfactory axons, which suggests that the observed migratory defect may be cell-autonomous.
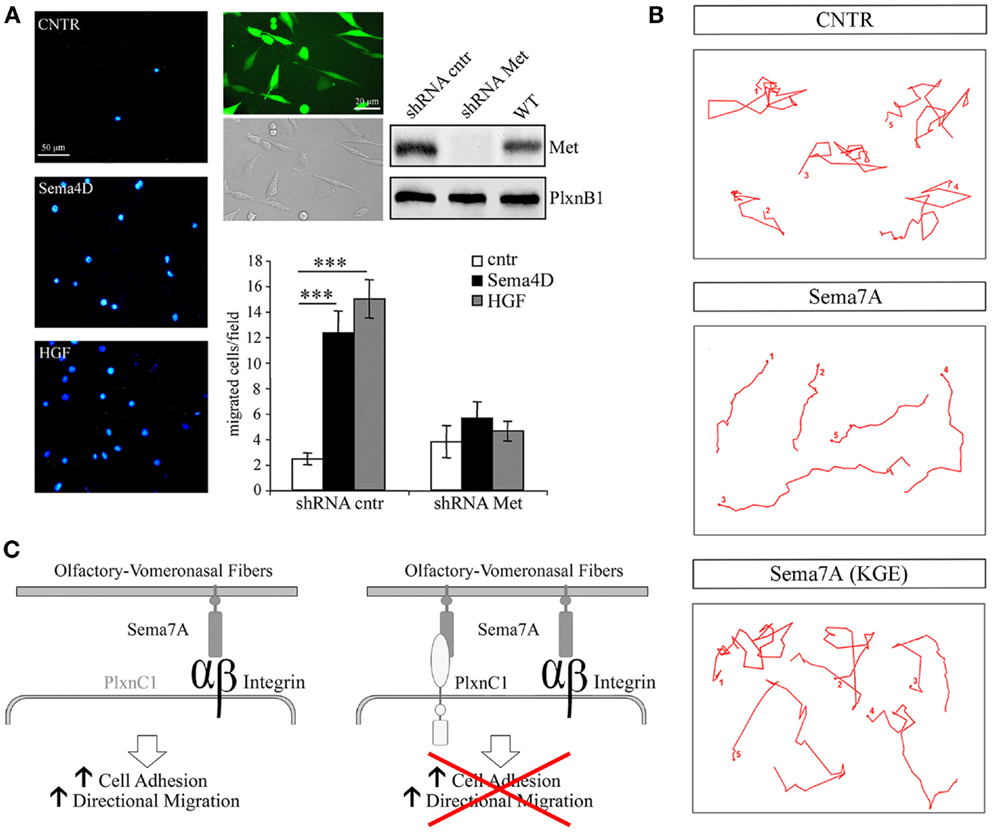
Figure 3. Semaphorin 4D and 7A directly regulate the motility of GnRH cells. (A) Sema4D promotes directional migration in immortalized GnRH cells, GN11, by coupling Plexin B1 with the activation of the hepatocyte growth factor (HGF) receptor Met tyrosine kinase. Left panel, representative images of a Boyden’s chamber assay showing that GN11 cells migrated through 8 μm membrane pores, attracted by 5 nM Sema4D or 0.5 nM HGF added to the lower chamber. Cells that migrated to the other side of the filter were stained with the nuclear marker DAPI; scale bar, 50 μm. Right panel, GN11 cells were infected with a lentiviral construct encoding for a Met shRNA sequence (shRNA Met) or for a mismatched Met shRNA (shRNA cntr), and for the GFP sequence. Virtually all GN11 cells were infected and expressed GFP. Scale bar, 10 μm. Western blot analysis of Met protein levels in total cell lysates demonstrated that, in Met shRNA – infected cells, Met was silenced. The graph represents the quantitative data of Boyden’s chamber experiments. shRNA cntr cells or shRNA Met cells were treated with 5 nM Sema4D or 0.5 nM HGF. GN11 cells in which Met was silenced were unable to migrate toward a Sema4D gradient. Results are expressed as mean ± SEM. ***P < 0.0001 from two-way ANOVA. (B) Representative images from time-lapse videos at different time points show GN11 neurons migrating farther onto Sema7A-coated stripes than onto Sema7A(KGE)-coated stripes as a consequence of increased persistence. The movement of individual cells was recorded during 6 h and plotted. (C) Schematic representation of Sema7A function on the GnRH migratory behavior: Sema7A is expressed along the developing olfactory/vomeronasal axons and acts on GnRH neurons increasing adhesion and persistent migration through β1-integrin binding and activation. Expression of PlexinC1 in GnRH neurons switches off their migratory response to Sema7A. Reprinted with permission from (14, 40).
Semaphorin 7A
The pleiotropic nature of semaphorins is particularly evident for Semaphorin7A (Sema7A), the only glycophosphatidylinositol (GPI)-linked member of the semaphorin family [reviewed in (48)]. Sema7A has been extensively studied in the context of immune function (18) and cancer biology (49–51), and a few reports have addressed its role in neuronal development as well (17, 52–54). Sema7A binds to PlexinC1 to decrease integrin-mediated cell attachment and spreading (49), and its interaction with β1-integrin induces integrin clustering and the activation of MAPK pathways (17). We have also recently reported a role for Sema7A in the regulation of GnRH cell motility (40). Sema7A is expressed along the GnRH migratory route during mouse embryonic development, with high levels of expression observed in the nasal pit, where these cells begin their migratory process, and along the olfactory/vomeronasal axonal scaffold. In addition GnRH cells differentially express the Sema7A receptors β1-integrin and PlexinC1 as a function of their migratory stage: at early stages of their migratory route, GnRH neurons only express β1-integrin, whereas they begin to express PlexinC1 during subsequent developmental stages and at anatomical sites at which these cells terminate their migration (i.e., in the ventral forebrain). Semaphorin signaling is multifaceted, and a subset of these ligands (e.g., Sema4D, Sema6D, and Sema7A) has been shown to elicit integrin activation/cell-substrate adhesion, axonal outgrowth, or cell chemotaxis under distinct conditions (19). Although the molecular mechanisms responsible for these antagonistic activities are not completely understood, they seem to involve distinct signaling pathways that depend on the targeted cells and the different components of semaphorin receptor complexes. For example, Sema7A stimulates the rapid phosphorylation of FAK and ERK1/2 in immortalized GnRH cells and increases the directional migration of these cells in a β1-integrin-dependent manner [Figures 3B,C, reprinted with permission from (40)]. In contrast, the overexpression of PlexinC1 in GnRH neurons leads to reduced migration in the presence of Sema7A (40). This switch may be essential for proper guidance of migrating neurons into the hypothalamus. It is unknown how PlexinC1 expression is induced in migratory GnRH neurons. One possibility is that molecular cues presented by intermediate targets such as the cribriform plate regulate this receptor switch.
In vivo, the loss of Sema7A impacts the migration of GnRH neurons, resulting in a significant reduction in the GnRH neuronal population in the brains of adult Sema7A-deficient mice, as well as reduced gonadal size and altered fertility (40). Consistent with these findings, the conditional inactivation of the Sema7A receptor, β1-integrin, in GnRH neurons results in a phenotype that largely resembles that of Sema7A knockdown animals (55). A very interesting aspect of this study is the finding that male reproductive function is not affected by the disruption of β1-integrin signaling in GnRH neurons, in spite of the comparable decrease in the size of the GnRH neuronal population of both male and female GnRH:Cre;Itgb1loxP/loxP mice when compared with controls (30% reduction). However, the extent of the reduction in GnRH neuronal projections is sexually dimorphic, being milder in male than in female mutant mice (33% reduction in males versus 70% reduction in females). Several reports indicate that gonadal steroids are capable of modulating the expression of different classes of guidance molecules, and thus directing the formation of sexually dimorphic circuits by influencing axonal guidance and synaptogenesis in addition to neurogenesis and cell migration, differentiation, and death (56, 57).
Semaphorin-3A and -3F
Semaphorins either bind to plexins directly or, in the case of class 3 semaphorins (Sema3s), bind to neuropilins (Nrps), which act as ligand-binding semaphorin co-receptors. The inclusion of additional modulatory co-receptors in the semaphorin receptor complex could provide these receptors with unique signaling properties (21). Two class 3 semaphorins, Sema3A, and Sema3F, are expressed in and around the developing olfactory system (34, 38). The specific co-receptors of these class 3 semaphorins, Nrp-1, and Nrp-2, are expressed on sensory neurons in the main and accessory olfactory epithelia of rodents and zebrafish (34, 36, 38, 58). Members of the PlexinA subfamily are concomitantly expressed in the olfactory system, with the robust expression of PlexinA1 in the vomeronasal organ (VNO) and VNNs (59).
The abnormal accumulation of GnRH neurons has also been observed in the nasal compartment of Nrp-2 knock-out mice, and Nrp-2 has been characterized as the receptor for secreted Sema3F (60). This migratory defect potentially reflects defasciculation problems that affect the olfactory/vomeronasal axons (36, 60) that guide GnRH neurons along their migratory path. Interestingly, consistent with these findings, it has been reported that Nrp-2-deficient mice are typically infertile (36, 61).
Sema3A is a secretory protein with repulsive effects on primary olfactory axons expressing the co-receptor Nrp-1 (37, 62, 63). The role of semaphorins in the navigation of vomeronasal axons and embryonic GnRH cells remains unclear, but previous studies in rodents have shown that migratory GnRH cells are morphologically associated with Nrp-1-immunoreactive axons and are themselves immunoreactive (64, 65). We have recently confirmed these findings in E14.5 mouse embryos and extended them to a 9-week-old human fetus using immunohistofluorescence experiments (66). Notably, the caudal branch of the VNN, which accompanies GnRH cells along their intracerebral path, also expresses Nrp-1.
In a series of genetic mouse models and in vitro experiments, it has recently been shown that the development of the GnRH system relies on Sema3A signaling through Nrp-1 and Nrp-2 (64–66). Mice lacking Sema3A or semaphorin signaling through both Nrps show phenotypical features associated with fetal X-linked Kallmann’s Syndrome (KS) (67), i.e., the accumulation of GnRH neurons and vomeronasal axons at the dorsal surface of the cribriform plate (64–66). Additionally, DiI axonal labeling at E14.5 shows the abnormal projection of the VNN into the ventral forebrain in mutant embryos lacking a functional semaphorin-binding domain in Nrp-1 [Nrp-1sema/sema; Figures 4A–D, Figures 4C,D are reprinted with permission from (66)]. Considerable abnormal cell migration is seen in these mutants as a consequence of the aberrant projections of the VNN (66). Incidentally, in conditional mutant mice lacking Nrp-1 in only GnRH neurons (GnRH:cre; Nrp-1loxP/loxP mice), the distribution of these cells between the nose and the brain at E14.5 is normal, as is the number of these cells in the adult brain (66), thus confirming that the defective migration seen in Nrp-1sema/sema embryos is not a cell-autonomous trait but rather a consequence of the misrouting of the VNNs into the ventral forebrain (Figures 4A–D).
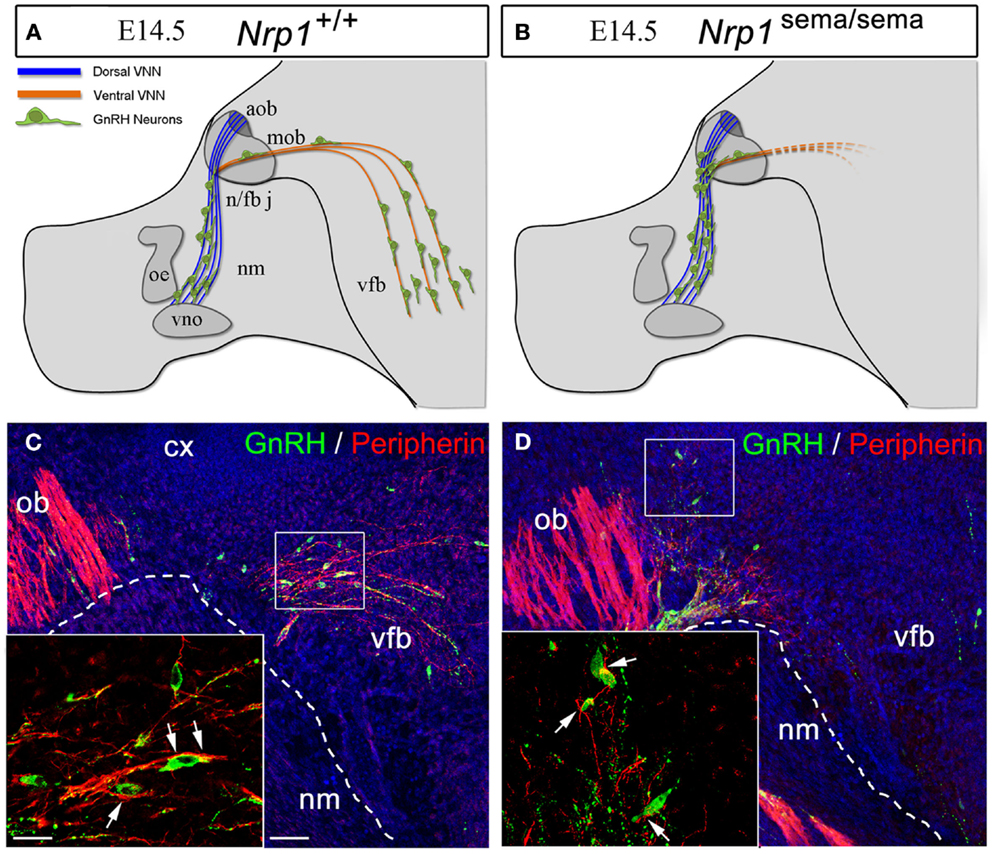
Figure 4. The defective migration of Nrp-1sema/sema embryos is not a cell-autonomous trait. (A,B) Defects in olfactory and vomeronasal axons as well as GnRH cell migration in Nrp-1sema/sema mutant mice. The vomeronasal nerve extends across the medial aspect of the olfactory bulb and projects both dorsally to the accessory olfactory bulb and caudally to the ventral forebrain (vfb). In mutant mice, fibers in the caudal branch are scarce when compared with wild-type mice. (C,D) Sagittal sections of the rostral and ventral forebrain regions at E14.5, immunostained for GnRH (green, arrowheads) and the olfactory/vomeronasal marker, peripherin (red). Note the abnormal distribution of GnRH-immunoreactive cells in the Nrp-1sema/sema mouse [(D), arrowheads]. Other abbreviations: VNN, vomeronasal nerve; cx, cerebral cortex; ob, olfactory bulb; nm, nasal mesenchyme; oe, olfactory epithelium; vno, vomeronasal organ; n/fb j, nasal/forebrain junction; aob, accessory olfactory bulb; mob, main olfactory bulb; vfb, ventral forebrain. Scale bars: (C,D), 50 μm; insets, 20 μm. Adapted from Ref. (66), with permission.
In addition to binding secreted semaphorins, the Nrps are recognized co-receptors for vascular endothelial growth factor (VEGF) (68–70), which is a key regulator of angiogenesis in health and disease (71, 72) and a critical molecule for vascular development (73, 74). It has recently been shown that Sema3A-mediated axonal guidance takes place in cooperation with the alternative Nrp-1 ligand, VEGF164, which mediates neuronal survival through neuronal, but not endothelial, Nrp-1 expression, to ensure that migrating GnRH neurons reach the brain (64). This study challenges two widely held models, which suggest that (1) the lack of a catalytic intracellular domain forces Nrp-1 to use KDR, the main VEGF receptor in blood vessels, as a co-receptor for the transduction of VEGF signals (75) and (2) KDR is the main VEGF receptor involved in the promotion of neuronal survival (76–78). In contrast to these models, Cariboni and colleagues have observed that VEGF164 signaling in GnRH neurons does not require KDR, which instead promotes the survival of migrating GnRH neurons via the co-activation of ERK and AKT signaling pathways through Nrp-1.
It is widely accepted that the vascular role of Nrp-1 reflects the loss of VEGF rather than Sema3A signaling, as supported by the fact that mice expressing a mutant Nrp-1 that is only capable of binding VEGF, but not semaphorins, do not exhibit vascular defects (79). Whether neuronal VEGF acts through unconventional and previously unsuspected signaling pathways is still a matter of investigation.
Semaphorin Mutations in Human Forms of Reproductive Insufficiency
The use of animal models has been tremendously helpful in showing that semaphorin signaling is crucial for the development, migration, survival, and maturation of the GnRH system, and that various forms of reproductive insufficiency involve mutations in several genes of the semaphorin family.
Isolated GnRH deficiency, termed central hypogonadism, is an inheritable yet underestimated disorder characterized by absent or incomplete sexual maturation and low circulating gonadotropin and sex steroid levels but otherwise normal pituitary function/imaging (80, 81). The disease can be associated with either a normal sense of smell (normosmic idiopathic central hypogonadism, nICH) or with anosmia/hyposmia (KS), and in both cases, anomalies in the embryonic development of the olfactory and GnRH neuronal system have been described.
Mutations affecting at least 17 disease-genes have been associated with the onset of nICH/KS (82): anosmin-1 (or KAL1), fibroblast growth factor receptor-1 (FGFR1), fibroblast growth factor 8 (FGF8), GnRH-1 and GnRH receptor (GnRHR), nasal embryonic LHRH Factor (NELF), G-protein-coupled receptor 54 (GPR54)/kisspeptin receptor (KISS-R) and kisspeptin1 (KISS-1), prokineticin-2 (PROK2) and prokineticin receptor-2 (PROKR2), chromodomain helicase DNA-binding protein-7 (CHD7), neurokinin-B (TAC3) and neurokinin-B receptor (TAC3R), heparan sulfate 6-O-sulfotransferase 1 (HS6ST1), WD protein interacting with EMX1 transcription factor (WDR11), SEMA3A and more recently SOX10 (83). However, these mutations account for only 30% of nICH/KS patients (84), and current efforts thus concentrate on the identification of other genes that could contribute to these disorders.
One research strategy is based on the histopathological examination of targeted mutant mice that could reproduce the human KS phenotype. Using this strategy, we have recently shown that Nrp-1sema/sema mutant mice, which lack the semaphorin-binding domain in Nrp-1, exhibit a KS-like phenotype, and genetic evidence shows that insufficient semaphorin-3A signaling contributes to the KS phenotype in humans (66). We identified eight different mutations in the Sema3A gene in 24 of the 386 KS patients studied (i.e., approximately 6%). Interestingly, the mutations were consistently observed in the heterozygous state, and five patients carried additional heterozygous mutations in PROKR2, PROK2, KAL1, or FGFR1. These results thus identify Sema3A as a novel contributory gene in KS and further substantiate the oligogenic pattern of inheritance in this developmental disorder (85, 86).
Young and colleagues have also reported a large deletion in Sema3A associated with KS in the heterozygous state in two siblings and their clinically affected father (87). However, these findings do not support autosomal dominant Mendelian inheritance in the analyzed family, and suggest that another, as yet unidentified, genetic hit is associated with the Sema3A haploinsufficiency that results in this disease phenotype (66).
A mutation in another class 3 semaphorin, Sema3E, has also been reported in an individual affected by CHARGE syndrome (88). CHARGE syndrome, which includes eye coloboma, heart malformations, atresia of the choanae, retardation of growth/development, genital anomalies, and ear abnormalities, is caused by mutations in CHD7 (89). CHARGE patients may display anosmia and/or hypogonadism, which are features that overlap with IHH and KS. Similarly, some IHH/KS patients also display partial CHARGE features. Therefore, it has been hypothesized that IHH/KS represents a milder allelic variant of CHARGE syndrome, which has been supported by the identification of heterozygous CHD7 mutations in nICH/KS individuals (89, 90).
Semaphorins and the Plasticity of Adult Hormone Systems
Blood vessels and axons employ similar mechanisms and follow common guidance cues for growth and navigation during embryonic development (91, 92). Blood vessels influence axonal trajectories to permit them to reach the appropriate destinations (93). In the adult brain, blood vessels communicate with neurons and glia to meet physiological demands (94). Endothelial cells are ideally positioned to sense peripheral inputs and to convey signals that could influence neuronal structure and synaptic plasticity. However, whether these cells are capable of influencing axonal plasticity in the mature central nervous system remains largely unknown. Recent evidence suggests that semaphorins are constitutively expressed in the postnatal brain and may be involved in neuronal plasticity and nervous system physiology (95). Semaphorins are also expressed in endothelial cells during vascular development (96). Interestingly, as elaborated in the previous sections, semaphorins act as guidance factors during the migration of GnRH neurons (14, 40, 60, 65, 66), which retain a high degree of plasticity in the mature brain (97). As the final common element in the central control of gonadotropin secretion, GnRH neurons, which project into the hypothalamic median eminence and release the neurohormone into a specialized capillary network for delivery to the anterior pituitary (98), are affected by numerous regulatory homeostatic and external factors to provide levels of fertility appropriate to the organism. For instance, GnRH neurons undergo extensive axonal growth toward the vascular wall during critical time windows in adulthood, such as at the onset of the preovulatory surge, when massive GnRH release occurs to induce ovulation (97) (Figure 5). They are thus an ideal system for analyzing the complex relationships involved in neurosecretion and morphological plasticity as well as the function of specific molecules in the homeostasis of the adult nervous system (97, 99, 100). Emerging data from our group shows that this periodic sprouting of GnRH axon terminals is under the control of specific semaphorins, which thus play a pivotal role in orchestrating the central control of reproduction (101, 102). Altogether, these findings raise the intriguing possibility that semaphorins may play important and unexpected roles in the adult neural plasticity that underlies key physiological processes, such as reproduction.
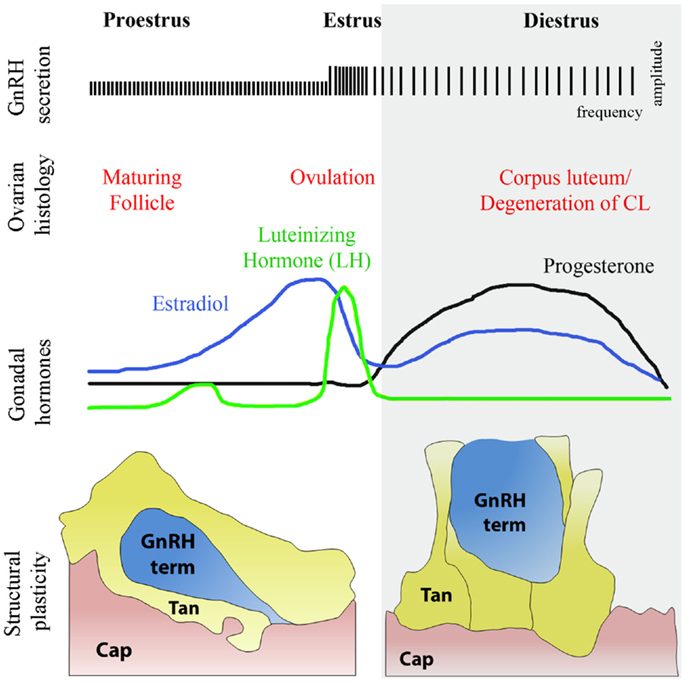
Figure 5. Structural plasticity of the GnRH nerve terminals and tanycytic end-feet in the median eminence of the hypothalamus during diestrus and proestrus. The schematic highlights the causal relationships during the different phases of the ovulatory cycle with changes respectively in GnRH secretion, ovarian histology, and circulating sex hormones levels. Changes in circulating gonadal steroids are responsible for the neuro/glial structural plasticity of the median eminence. During proestrus, GnRH nerve endings (blue) sprout toward the basal lamina delineating the pericapillary space (pink, Cap), with which they eventually make direct contact, while tanycytes retract. In diestrus, under conditions of low gonadotropin output, GnRH-secreting axon terminals are distant from the pericapillary space and tanycytes enwrap GnRH nerve endings, thus impairing access of the neurohormone to the pituitary portal circulation.
Regulation of Semaphorins Expression in the Pituitary-Gonadal Axis
The expression of semaphorins, plexins, and neuropilins is consistent with a prominent role for these molecules in the development and function of the hypothalamic-pituitary-gonadal system. The pituitary consists of three lobes: the neural lobe, the intermediate lobe, and the anterior lobe. The neural lobe is derived from diencephalic tissue, and the anterior and intermediate lobes from an invagination of the oral ectoderm called Rathke’s pouch. During pituitary development, the expression of Sema7A is most pronounced in Rathke’s pouch, whereas it is strongly expressed in the intermediate and anterior lobes at later developmental stages (39). In contrast, PlexinC1 is largely confined to the neural area of the pituitary, with only weak expression observed in the intermediate lobe at the late embryonic stage and not at all at the postnatal or adult stages.
Outside the central nervous system, fluctuations in reproductive hormones play a well-established role in the maintenance of body homeostasis. For instance, ex vivo data have shown that Sema4D is involved in the maturation of ovarian follicles in mice (103). Sema4D and its receptor PlexinB1 are both expressed in the mouse ovary in vivo mostly in the granulosa cells. The finding that Sema4D increases after hormonal treatment suggests follicular auto-regulation of the plexin/semaphorin system. However, the mechanism by which Sema4D/PlexinB1 system affects follicular growth and steroidogenesis requires further clarification.
An analysis of Sema4D−/− and PlexinB1−/− mice and of mice expressing a dominant-negative form of RhoA specifically in osteoblasts has revealed an osteosclerotic phenotype in these mutants (104). Interestingly, this phenotype is strictly dependent on ovarian function (circulating estrogens) as an ovariectomy suppresses the bone resorption phenotype in Sema4D−/− mice (47), thus suggesting that Sema4D expression might be under the control of circulating gonadal hormones. This hypothesis is supported by previous studies, which have demonstrated that semaphorins and their receptors expressions are regulated by steroid hormones in physiological and pathological conditions. Pavelock and coworkers provided direct evidence for the expressions of Nrp-1 and Nrp-2 transcripts in uterine tissue and they have shown that the levels of these neuropilins mRNAs are respectively progesterone- and estrogen-dependent (105). The regulated expression and differential localization of Nrp-1 and Nrp-2 in the rat uterus suggest that these receptors may participate in hormonally regulated changes occurring throughout the female reproductive cycle (105).
Class 3 semaphorins, Sema3B and Sema3F, are secreted proteins that regulate angiogenesis, tumor growth, and metastasis by binding to their transmembrane receptor complex consisting of plexins and neuropilins (23). Interestingly, a significantly reduced expression of Sema3B (83 kDa), Sema3F (90 kDa), and plexin-A3 was observed in human ovarian cancer cell lines when compared with normal human ovarian surface epithelial cells (106). The treatment of tumor cells with luteinizing hormone, follicle-stimulating hormone, estrogen, and progesterone induced significant changes in Sema3B and Sema3F expression (106), implying that gonadotropin- and/or estrogen-mediated maintenance of Sema3 expression could control ovarian cancer angiogenesis and metastasis. These findings have important clinical ramifications as they may explain why some of these tumors become more prevalent around the perimenopausal and postmenopausal periods of women. The same research group has also provided compelling evidence that Sema3B, Sema3F, and plexin-A3 were expressed strongly in normal endometrial tissues, whereas grade-dependent decreases were found in endometrial carcinomas (107). Moreover, treatment of cancer cells with progesterone and 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] for a period of 72 h induced a significant upregulation of these semaphorins as well as inhibited growth of cancer cells by increasing caspase-3 activity (107).
Besides hormonal signaling, recent studies have demonstrated that different classes of growth factors might also regulate semaphorins’ expression. Fibroblast growth factor 8 (FGF8) has been shown to repel midbrain dopaminergic neuron (mDAN) axons that extend through the diencephalon (108). This repulsion is mediated by Sema3F, since the expression of Sema3F is up-regulated by ectopic expression of FGF8 and Sema3F repels mDAN axonal growth (108).
Another study showed that Sema7A expression is stimulated by transforming growth factor-beta1 (TGF-β1) in the murine lung, with Sema7A being a critical regulator of tissue remodeling in TGF-β1-induced pulmonary fibrosis (109).
It is likely that the some growth factors might also be under the control of hormonal signaling. Whether these hormones regulate semaphorins expression directly and/or indirectly through growth factors modulation in neuronal and non-neuronal adult tissues will require future investigation.
Conclusion
Semaphorin signaling plays a pivotal role in nervous system development and neural network assembly, and has been shown to influence cellular morphology in a large variety of systems. In this review, we have focused on factors and receptors belonging to this diverse family of guidance cues and their influence on the development and function of multifaceted hormonal tissues, with an emphasis on neuroendocrine systems. These activities underlie complex developmental processes, such as the migration of neurons that control fertility from the nose to the brain, the wiring of neuroendocrine networks and the genetic bases of some forms of reproductive disorders. The recent identification of mutations in semaphorin genes in patients with developmental neuroendocrine deficiencies associated with infertility illustrates the importance of semaphorins in this process. Furthermore, semaphorin expression persists in adulthood, and it has been proposed that these signals play an important role in the plasticity of the neuroendocrine systems that defend homeostatic set points to enable the survival of individuals and species. The identification of semaphorins and their associated receptors as participants in both the development and functional plasticity of hormone systems creates new avenues of investigation in endocrinology and neuroendocrinology.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the Agence Nationale de la Recherche, ANR, France, (grant number: ANR-2010-JCJC-1404-01); the Institut National de la Santé et de la Recherche Médicale, INSERM, France, (grant number: U837); and the University of Lille 2, Lille, France (grant: Appel á Projets du Conseil Scientifique de l’Université Lille 2). We thank Daniele Avanzato (PhD student in Complex Systems in Life Sciences, University of Torino) for graphical assistance.
References
1. Kolodkin AL, Matthes DJ, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell (1993) 75:1389–99. doi: 10.1016/0092-8674(93)90625-Z
2. Semaphorin Nomenclature Committee. Unified nomenclature for the semaphorins/collapsins. Cell (1999) 97:551–2. doi:10.1016/S0092-8674(00)80766-7
3. Liu H, Juo ZS, Shim AH, Focia PJ, Chen X, Garcia KC, et al. Structural basis of semaphorin-plexin recognition and viral mimicry from Sema7A and A39R complexes with PlexinC1. Cell (2010) 142:749–61. doi:10.1016/j.cell.2010.07.040
4. Nogi T, Yasui N, Mihara E, Matsunaga Y, Noda M, Yamashita N, et al. Structural basis for semaphorin signalling through the plexin receptor. Nature (2010) 467:1123–7. doi:10.1038/nature09473
5. Janssen BJ, Robinson RA, Pérez-Brangulí F, Bell CH, Mitchell KJ, Siebold C, et al. Structural basis of semaphorin-plexin signalling. Nature (2010) 467:1118–22.
6. Tamagnone L, Comoglio PM. Signalling by semaphorin receptors: cell guidance and beyond. Trends Cell Biol (2000) 10:377–83. doi:10.1016/S0962-8924(00)01816-X
7. Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell (1999) 99:71–80. doi:10.1016/S0092-8674(00)80063-X
8. Takahashi T, Fournier A, Nakamura F, Wang LH, Murakami Y, Kalb RG, et al. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell (1999) 99:59–69. doi:10.1016/S0092-8674(00)80062-8
9. Kruger RP, Aurandt J, Guan KL. Semaphorins command cells to move. Nat Rev Mol Cell Biol (2005) 6:789–800. doi:10.1038/nrm1740
10. Zhou Y, Gunput RA, Pasterkamp RJ. Semaphorin signaling: progress made and promises ahead. Trends Biochem Sci (2008) 33:161–70. doi:10.1016/j.tibs.2008.01.006
11. Neufeld G, Kessler O. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nat Rev Cancer (2008) 8:632–45. doi:10.1038/nrc2404
12. Giordano S, Corso S, Conrotto P, Artigiani S, Gilestro G, Barberis D, et al. The semaphorin 4D receptor controls invasive growth by coupling with Met. Nat Cell Biol (2002) 4:720–4. doi:10.1038/ncb843
13. Conrotto P, Valdembri D, Corso S, Serini G, Tamagnone L, Comoglio PM, et al. Sema4D induces angiogenesis through Met recruitment by Plexin B1. Blood (2005) 105:4321–9. doi:10.1182/blood-2004-07-2885
14. Giacobini P, Messina A, Morello F, Ferraris N, Corso S, Penachioni J, et al. Semaphorin 4D regulates gonadotropin hormone-releasing hormone-1 neuronal migration through PlexinB1-Met complex. J Cell Biol (2008) 183:555–66. doi:10.1083/jcb.200806160
15. Swiercz JM, Kuner R, Offermanns S. Plexin-B1/RhoGEF-mediated RhoA activation involves the receptor tyrosine kinase ErbB-2. J Cell Biol (2004) 165:869–80. doi:10.1083/jcb.200312094
16. Toyofuku T, Zhang H, Kumanogoh A, Takegahara N, Suto F, Kamei J, et al. Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev (2004) 18:435–47. doi:10.1101/gad.1167304
17. Pasterkamp RJ, Peschon JJ, Spriggs MK, Kolodkin AL. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature (2003) 424:398–405. doi:10.1038/nature01790
18. Suzuki K, Okuno T, Yamamoto M, Pasterkamp RJ, Takegahara N, Takamatsu H, et al. Semaphorin 7A initiates T-cell-mediated inflammatory responses through alpha1beta1 integrin. Nature (2007) 446:680–4. doi:10.1038/nature05652
19. Casazza A, Fazzari P, Tamagnone L. Semaphorin signals in cell adhesion and cell migration: functional role and molecular mechanisms. Adv Exp Med Biol (2007) 600:90–108. doi:10.1007/978-0-387-70956-7_8
20. Tran TS, Kolodkin AL, Bharadwaj R. Semaphorin regulation of cellular morphology. Annu Rev Cell Dev Biol (2007) 23:263–92. doi:10.1146/annurev.cellbio.22.010605.093554
21. Pasterkamp RJ. Getting neural circuits into shape with semaphorins. Nat Rev Neurosci (2012) 13:605–18. doi:10.1038/nrn3302
22. Perala N, Sariola H, Immonen T. More than nervous: the emerging roles of plexins. Differentiation (2012) 83:77–91. doi:10.1016/j.diff.2011.08.001
23. Capparuccia L, Tamagnone L. Semaphorin signaling in cancer cells and in cells of the tumor microenvironment – two sides of a coin. J Cell Sci (2009) 122:1723–36. doi:10.1242/jcs.030197
24. Ch’ng ES, Kumanogoh A. Roles of Sema4D and Plexin-B1 in tumor progression. Mol Cancer (2010) 9:251. doi:10.1186/1476-4598-9-251
25. Ojeda SR, Skinner MK. Physiology of the gonadotropin-releasing hormone neuronal network. In: Knobil E, Neill JD, editors. Physiology of Reproduction. New York: Raven Press (2006). p. 2061–126.
26. Wray S. From nose to brain: development of gonadotrophin-releasing hormone-1 neurones. J Neuroendocrinol (2010) 22:743–53. doi:10.1111/j.1365-2826.2010.02034.x
27. Schwanzel-Fukuda M, Pfaff DW. Origin of luteinizing hormone-releasing hormone neurons. Nature (1989) 338:161–4. doi:10.1038/338161a0
28. Wray S, Grant P, Gainer H. Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci U S A (1989) 86:8132–6. doi:10.1073/pnas.86.20.8132
29. Gonzalez-Martinez D, Hu Y, Bouloux PM. Ontogeny of GnRH and olfactory neuronal systems in man: novel insights from the investigation of inherited forms of Kallmann’s syndrome. Front Neuroendocrinol (2004) 25:108–30. doi:10.1016/j.yfrne.2004.06.001
30. Marin O, Rubenstein JL. Cell migration in the forebrain. Annu Rev Neurosci (2003) 26:441–83. doi:10.1146/annurev.neuro.26.041002.131058
31. Wray S. Development of gonadotropin-releasing hormone-1 neurons. Front Neuroendocrinol (2002) 23:292–316. doi:10.1016/S0091-3022(02)00001-8
32. Schwarting GA, Wierman ME, Tobet SA. Gonadotropin-releasing hormone neuronal migration. Semin Reprod Med (2007) 25:305–12. doi:10.1055/s-2007-984736
33. Wierman ME, Kiseljak-Vassiliades K, Tobet S. Gonadotropin-releasing hormone (GnRH) neuron migration: initiation, maintenance and cessation as critical steps to ensure normal reproductive function. Front Neuroendocrinol (2011) 32:43–52. doi:10.1016/j.yfrne.2010.07.005
34. Giger RJ, Wolfer DP, De Wit GM, Verhaagen J. Anatomy of rat semaphorin III/collapsin-1 mRNA expression and relationship to developing nerve tracts during neuroembryogenesis. J Comp Neurol (1996) 375:378–92.
35. de Castro F, Hu L, Drabkin H, Sotelo C, Chedotal A. Chemoattraction and chemorepulsion of olfactory bulb axons by different secreted semaphorins. J Neurosci (1999) 19:4428–36.
36. Giger RJ, Cloutier JF, Sahay A, Prinjha RK, Levengood DV, Moore SE, et al. Neuropilin-2 is required in vivo for selective axon guidance responses to secreted semaphorins. Neuron (2000) 25:29–41. doi:10.1016/S0896-6273(00)80869-7
37. Schwarting GA, Kostek C, Ahmad N, Dibble C, Pays L, Puschel AW. Semaphorin 3A is required for guidance of olfactory axons in mice. J Neurosci (2000) 20:7691–7.
38. Cloutier JF, Giger RJ, Koentges G, Dulac C, Kolodkin AL, Ginty DD. Neuropilin-2 mediates axonal fasciculation, zonal segregation, but not axonal convergence, of primary accessory olfactory neurons. Neuron (2002) 33:877–92. doi:10.1016/S0896-6273(02)00635-9
39. Pasterkamp RJ, Kolk SM, Hellemons AJ, Kolodkin AL. Expression patterns of semaphorin7A and plexinC1 during rat neural development suggest roles in axon guidance and neuronal migration. BMC Dev Biol (2007) 7:98. doi:10.1186/1471-213X-7-98
40. Messina A, Ferraris N, Wray S, Cagnoni G, Donohue DE, Casoni F, et al. Dysregulation of Semaphorin7A/beta1-integrin signaling leads to defective GnRH-1 cell migration, abnormal gonadal development and altered fertility. Hum Mol Genet (2011) 20:4759–74. doi:10.1093/hmg/ddr403
41. Ebert AM, Lamont RE, Childs SJ, McFarlane S. Neuronal expression of class 6 semaphorins in zebrafish. Gene Expr Patterns (2012) 12:117–22. doi:10.1016/j.gep.2012.01.007
42. Elhabazi A, Delaire S, Bensussan A, Boumsell L, Bismuth G. Biological activity of soluble CD100. I. The extracellular region of CD100 is released from the surface of T lymphocytes by regulated proteolysis. J Immunol (2001) 166:4341–7.
43. Wang X, Kumanogoh A, Watanabe C, Shi W, Yoshida K, Kikutani H. Functional soluble CD100/Sema4D released from activated lymphocytes: possible role in normal and pathologic immune responses. Blood (2001) 97:3498–504. doi:10.1182/blood.V97.11.3498
44. Swiercz JM, Kuner R, Behrens J, Offermanns S. Plexin-B1 directly interacts with PDZ-RhoGEF/LARG to regulate RhoA and growth cone morphology. Neuron (2002) 35:51–63. doi:10.1016/S0896-6273(02)00750-X
45. Conrotto P, Corso S, Gamberini S, Comoglio PM, Giordano S. Interplay between scatter factor receptors and B plexins controls invasive growth. Oncogene (2004) 23:5131–7. doi:10.1038/sj.onc.1207650
46. Geller S, Kolasa E, Tillet Y, Duittoz A, Vaudin P. Olfactory ensheathing cells form the microenvironment of migrating GnRH-1 neurons during mouse development. Glia (2013) 61:550–66. doi:10.1002/glia.22455
47. Dacquin R, Domenget C, Kumanogoh A, Kikutani H, Jurdic P, Machuca-Gayet I. Control of bone resorption by semaphorin 4D is dependent on ovarian function. PLoS ONE (2011) 6:e26627. doi:10.1371/journal.pone.0026627
48. Jongbloets BC, Ramakers GM, Pasterkamp RJ. Semaphorin7A and its receptors: pleiotropic regulators of immune cell function, bone homeostasis, and neural development. Semin Cell Dev Biol (2013) 24:129–38. doi:10.1016/j.semcdb.2013.01.002
49. Scott GA, McClelland LA, Fricke AF. Semaphorin 7a promotes spreading and dendricity in human melanocytes through beta1-integrins. J Invest Dermatol (2008) 128:151–61. doi:10.1038/sj.jid.5700974
50. Lazova R, Gould Rothberg BE, Rimm D, Scott G. The semaphorin 7A receptor Plexin C1 is lost during melanoma metastasis. Am J Dermatopathol (2009) 31:177–81. doi:10.1097/DAD.0b013e318196672d
51. Scott GA, McClelland LA, Fricke AF, Fender A. Plexin C1, a receptor for semaphorin 7a, inactivates cofilin and is a potential tumor suppressor for melanoma progression. J Invest Dermatol (2009) 129:954–63. doi:10.1038/jid.2008.329
52. Ohsawa S, Hamada S, Asou H, Kuida K, Uchiyama Y, Yoshida H, et al. Caspase-9 activation revealed by semaphorin 7A cleavage is independent of apoptosis in the aged olfactory bulb. J Neurosci (2009) 29:11385–92. doi:10.1523/JNEUROSCI.4780-08.2009
53. Ohsawa S, Hamada S, Kuida K, Yoshida H, Igaki T, Miura M. Maturation of the olfactory sensory neurons by Apaf-1/caspase-9-mediated caspase activity. Proc Natl Acad Sci U S A (2010) 107:13366–71. doi:10.1073/pnas.0910488107
54. Fukunishi A, Maruyama T, Zhao H, Tiwari M, Kang S, Kumanogoh A, et al. The action of Semaphorin7A on thalamocortical axon branching. J Neurochem (2011) 118:1008–15. doi:10.1111/j.1471-4159.2011.07390.x
55. Parkash J, Cimino I, Ferraris N, Casoni F, Wray S, Cappy H, et al. Suppression of beta1-integrin in gonadotropin-releasing hormone cells disrupts migration and axonal extension resulting in severe reproductive alterations. J Neurosci (2012) 32:16992–7002. doi:10.1523/JNEUROSCI.3057-12.2012
56. Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci (2002) 25:507–36. doi:10.1146/annurev.neuro.25.112701.142745
57. Tobet S, Knoll JG, Hartshorn C, Aurand E, Stratton M, Kumar P, et al. Brain sex differences and hormone influences: a moving experience? J Neuroendocrinol (2009) 21:387–92. doi:10.1111/j.1365-2826.2009.01834.x
58. Yu HH, Houart C, Moens CB. Cloning and embryonic expression of zebrafish neuropilin genes. Gene Expr Patterns (2004) 4:371–8. doi:10.1016/j.modgep.2004.01.011
59. Murakami Y, Suto F, Shimizu M, Shinoda T, Kameyama T, Fujisawa H. Differential expression of plexin-A subfamily members in the mouse nervous system. Dev Dyn (2001) 220:246–58. doi:10.1002/1097-0177(20010301)220:3<246::AID-DVDY1112>3.0.CO;2-2
60. Cariboni A, Hickok J, Rakic S, Andrews W, Maggi R, Tischkau S, et al. Neuropilins and their ligands are important in the migration of gonadotropin-releasing hormone neurons. J Neurosci (2007) 27:2387–95. doi:10.1523/JNEUROSCI.5075-06.2007
61. Walz A, Rodriguez I, Mombaerts P. Aberrant sensory innervation of the olfactory bulb in neuropilin-2 mutant mice. J Neurosci (2002) 22:4025–35.
62. Pasterkamp RJ, De Winter F, Holtmaat AJ, Verhaagen J. Evidence for a role of the chemorepellent semaphorin III and its receptor neuropilin-1 in the regeneration of primary olfactory axons. J Neurosci (1998) 18:9962–76.
63. Imai T, Yamazaki T, Kobayakawa R, Kobayakawa K, Abe T, Suzuki M, et al. Pre-target axon sorting establishes the neural map topography. Science (2009) 325:585–90. doi:10.1126/science.1173596
64. Cariboni A, Davidson K, Dozio E, Memi F, Schwarz Q, Stossi F, et al. VEGF signalling controls GnRH neuron survival via NRP1 independently of KDR and blood vessels. Development (2011) 138:3723–33. doi:10.1242/dev.063362
65. Cariboni A, Davidson K, Rakic S, Maggi R, Parnavelas JG, Ruhrberg C. Defective gonadotropin-releasing hormone neuron migration in mice lacking SEMA3A signalling through NRP1 and NRP2: implications for the aetiology of hypogonadotropic hypogonadism. Hum Mol Genet (2011) 20:336–44. doi:10.1093/hmg/ddq468
66. Hanchate NK, Giacobini P, Lhuillier P, Parkash J, Espy C, Fouveaut C, et al. SEMA3A, a gene involved in axonal pathfinding, is mutated in patients with Kallmann syndrome. PLoS Genet (2012) 8:e1002896. doi:10.1371/journal.pgen.1002896
67. Schwanzel-Fukuda M, Bick D, Pfaff DW. Luteinizing hormone-releasing hormone (LHRH)-expressing cells do not migrate normally in an inherited hypogonadal (Kallmann) syndrome. Brain Res Mol Brain Res (1989) 6:311–26. doi:10.1016/0169-328X(89)90076-4
68. Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell (1998) 92:735–45. doi:10.1016/S0092-8674(00)81402-6
69. Gluzman-Poltorak Z, Cohen T, Herzog Y, Neufeld G. Neuropilin-2 is a receptor for the vascular endothelial growth factor (VEGF) forms VEGF-145 and VEGF-165. J Biol Chem (2000) 275:18040–5. doi:10.1074/jbc.M909259199
70. Karpanen T, Heckman CA, Keskitalo S, Jeltsch M, Ollila H, Neufeld G, et al. Functional interaction of VEGF-C and VEGF-D with neuropilin receptors. FASEB J (2006) 20:1462–72. doi:10.1096/fj.05-5646com
71. Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science (1983) 219:983–5. doi:10.1126/science.6823562
72. Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science (1989) 246:1306–9. doi:10.1126/science.2479986
73. Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature (1996) 380:435–9. doi:10.1038/380435a0
74. Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature (1996) 380:439–42. doi:10.1038/380439a0
75. Fantin A, Maden CH, Ruhrberg C. Neuropilin ligands in vascular and neuronal patterning. Biochem Soc Trans (2009) 37:1228–32. doi:10.1042/BST0371228
76. Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci (1999) 19:5731–40.
77. Oosthuyse B, Moons L, Storkebaum E, Beck H, Nuyens D, Brusselmans K, et al. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet (2001) 28:131–8. doi:10.1038/88842
78. Ogunshola OO, Antic A, Donoghue MJ, Fan SY, Kim H, Stewart WB, et al. Paracrine and autocrine functions of neuronal vascular endothelial growth factor (VEGF) in the central nervous system. J Biol Chem (2002) 277:11410–5. doi:10.1074/jbc.M111085200
79. Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, et al. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell (2003) 5:45–57. doi:10.1016/S1534-5807(03)00169-2
80. de Roux N, Young J, Misrahi M, Genet R, Chanson P, Schaison G, et al. A family with hypogonadotropic hypogonadism and mutations in the gonadotropin-releasing hormone receptor. N Engl J Med (1997) 337:1597–602.
81. Mitchell AL, Dwyer A, Pitteloud N, Quinton R. Genetic basis and variable phenotypic expression of Kallmann syndrome: towards a unifying theory. Trends Endocrinol Metab (2011) 22:249–58. doi:10.1016/j.tem.2011.03.002
82. Bonomi M, Libri DV, Guizzardi F, Guarducci E, Maiolo E, Pignatti E, et al. New understandings of the genetic basis of isolated idiopathic central hypogonadism. Asian J Androl (2012) 14:49–56. doi:10.1038/aja.2011.68
83. Pingault V, Bodereau V, Baral V, Marcos S, Watanabe Y, Chaoui A. Loss-of-function mutations in SOX10 cause Kallmann syndrome with deafness. Am J Hum Genet (2013) 92:707–24. doi:10.1016/j.ajhg.2013.03.024
84. Balasubramanian R, Crowley WF Jr. Isolated GnRH deficiency: a disease model serving as a unique prism into the systems biology of the GnRH neuronal network. Mol Cell Endocrinol (2011) 346:4–12. doi:10.1016/j.mce.2011.07.012
85. Dode C, Hardelin JP. Kallmann syndrome. Eur J Hum Genet (2009) 17:139–46. doi:10.1038/ejhg.2008.206
86. Sykiotis GP, Plummer L, Hughes VA, Au M, Durrani S, Nayak-Young S, et al. Oligogenic basis of isolated gonadotropin-releasing hormone deficiency. Proc Natl Acad Sci U S A (2010) 107:15140–4. doi:10.1073/pnas.1009622107
87. Young J, Metay C, Bouligand J, Tou B, Francou B, Maione L, et al. SEMA3A deletion in a family with Kallmann syndrome validates the role of semaphorin 3A in human puberty and olfactory system development. Hum Reprod (2012) 27:1460–5. doi:10.1093/humrep/des022
88. Lalani SR, Safiullah AM, Molinari LM, Fernbach SD, Martin DM, Belmont JW. SEMA3E mutation in a patient with CHARGE syndrome. J Med Genet (2004) 41:e94. doi:10.1136/jmg.2003.017640
89. Kim HG, Layman LC. The role of CHD7 and the newly identified WDR11 gene in patients with idiopathic hypogonadotropic hypogonadism and Kallmann syndrome. Mol Cell Endocrinol (2011) 346:74–83. doi:10.1016/j.mce.2011.07.013
90. Kim HG, Kurth I, Lan F, Meliciani I, Wenzel W, Eom SH, et al. Mutations in CHD7, encoding a chromatin-remodeling protein, cause idiopathic hypogonadotropic hypogonadism and Kallmann syndrome. Am J Hum Genet (2008) 83:511–9. doi:10.1016/j.ajhg.2008.09.005
91. Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature (2005) 436:193–200. doi:10.1038/nature03875
92. Larrivee B, Freitas C, Suchting S, Brunet I, Eichmann A. Guidance of vascular development: lessons from the nervous system. Circ Res (2009) 104:428–41. doi:10.1161/CIRCRESAHA.108.188144
93. Makita T, Sucov HM, Gariepy CE, Yanagisawa M, Ginty DD. Endothelins are vascular-derived axonal guidance cues for developing sympathetic neurons. Nature (2008) 452:759–63. doi:10.1038/nature06859
94. Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci (2004) 5:347–60. doi:10.1038/nrn1387
95. Pasterkamp RJ, Giger RJ. Semaphorin function in neural plasticity and disease. Curr Opin Neurobiol (2009) 19:263–74. doi:10.1016/j.conb.2009.06.001
96. Serini G, Valdembri D, Zanivan S, Morterra G, Burkhardt C, Caccavari F, et al. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature (2003) 424:391–7. doi:10.1038/nature01946
97. Prevot V, Hanchate NK, Bellefontaine N, Sharif A, Parkash J, Estrella C, et al. Function-related structural plasticity of the GnRH system: a role for neuronal-glial-endothelial interactions. Front Neuroendocrinol (2010) 31:241–58. doi:10.1016/j.yfrne.2010.05.003
98. Page R. The anatomy of the hypothalamo-hypophysial complex. In: Knobil E, Neill JD, editors. Physiology of Reproduction. New York: Raven Press (1994). p. 1527–619.
99. Prevot V, Bellefontaine N, Baroncini M, Sharif A, Hanchate NK, Parkash J, et al. Gonadotrophin-releasing hormone nerve terminals, tanycytes and neurohaemal junction remodelling in the adult median eminence: functional consequences for reproduction and dynamic role of vascular endothelial cells. J Neuroendocrinol (2010) 22:639–49. doi:10.1111/j.1365-2826.2010.02033.x
100. Bellefontaine N, Hanchate NK, Parkash J, Campagne C, de Seranno S, Clasadonte J, et al. Nitric oxide as key mediator of neuron-to-neuron and endothelia-to-glia communication involved in the neuroendocrine control of reproduction. Neuroendocrinology (2011) 93:74–89. doi:10.1159/000324147
101. Campagne C, Bouret SG, Leroy D, Beauvillain J-C, Prevot V. Semaphorin3A may be a chemotropic factor used by endothelial cells of the median eminence to regulate GnRH axon plasticity during the rat estrous cycle. In: Abstracts of the 38th Annual Meeting of the Society for Neuroscience (No. 618.3). Washington, DC (2008).
102. Parkash J, Loyens A, Gallet S, Balland E, Pralong F, Pasterkamp RJ, et al. Semaphorin 7A expression in tanycytes is regulated by sex-steroid hormones and controls gonadotropin-releasing hormone-1 (GnRH-1) cell plasticity. In: Abstracts of the 41st Annual Meeting of the Society for Neuroscience (No. 500.09), Washington, DC (2011).
103. Regev A, Goldman S, Shalev E. Semaphorin-4D (Sema-4D), the Plexin-B1 ligand, is involved in mouse ovary follicular development. Reprod Biol Endocrinol (2007) 5:12. doi:10.1186/1477-7827-5-12
104. Negishi-Koga T, Shinohara M, Komatsu N, Bito H, Kodama T, Friedel RH, et al. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat Med (2011) 17:1473–80. doi:10.1038/nm.2489
105. Pavelock K, Braas K, Ouafik L, Osol G, May V. Differential expression and regulation of the vascular endothelial growth factor receptors neuropilin-1 and neuropilin-2 in rat uterus. Endocrinology (2001) 142:613–22. doi:10.1210/en.142.2.613
106. Joseph D, Ho SM, Syed V. Hormonal regulation and distinct functions of semaphorin-3B and semaphorin-3F in ovarian cancer. Mol Cancer (2010) 9:499–509. doi:10.1158/1535-7163.MCT-09-0664
107. Nguyen H, Ivanova VS, Kavandi L, Rodriguez GC, Maxwell GL, Syed V. Progesterone and 1,25-dihydroxyvitamin D inhibit endometrial cancer cell growth by upregulating semaphorin 3B and semaphorin 3F. Mol Cancer Res (2011) 9:1479–92. doi:10.1158/1541-7786.MCR-11-0213
108. Yamauchi K, Mizushima S, Tamada A, Yamamoto N, Takashima S, Murakami F. FGF8 signaling regulates growth of midbrain dopaminergic axons by inducing semaphorin 3F. J Neurosci (2009) 29:4044–55. doi:10.1523/JNEUROSCI.4794-08.2009
Keywords: gonadotropin-releasing hormone, reproduction, neuronal plasticity, cell migration, development
Citation: Messina A and Giacobini P (2013) Semaphorin signaling in the development and function of the gonadotropin hormone-releasing hormone system. Front. Endocrinol. 4:133. doi: 10.3389/fendo.2013.00133
Received: 13 July 2013; Paper pending published: 13 August 2013;
Accepted: 09 September 2013; Published online: 23 September 2013.
Edited by:
Susan Wray, National Institutes of Health, USAReviewed by:
Elisabeth Eppler, University of Zürich, SwitzerlandSuraj Unniappan, York University, Canada
Copyright: © 2013 Messina and Giacobini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paolo Giacobini, INSERM, Laboratory of Development and Plasticity of the Postnatal Brain, Jean-Pierre Aubert Research Center, Unit 837, Place de Verdun, 59045 Lille Cedex, France e-mail:cGFvbG8uZ2lhY29iaW5pQGluc2VybS5mcg==

