- 1Human Physiology Department, Medical Research Institute, Alexandria University, Alexandria, Egypt
- 2Zoology Department, Faculty of Science, Alexandria University, Alexandria, Egypt
Oxytocin (OT), a hormone most commonly associated with labor and lactation, may have a wide variety of physiological and pathological functions, which makes OT and its receptor potential targets for drug therapy. In this review, we highlight the newly discovered metabolic role of OT in diabetes and its complication, such as diabetic osteopathy. OT may have positive metabolic effects; this is based on the change in glucose metabolism, lipid profile, and insulin sensitivity. It may modify glucose uptake and insulin sensitivity both through direct and indirect effects. It may also cause regenerative changes in diabetic pancreatic islet cells. Moreover, it has an anabolic effect on the bone biology. So, the activation of the OT receptor pathway by infusion of OT, OT analogs, or OT agonists may represent a promising approach for the treatment of diabetes and some of its complications, including diabetic osteopathy.
Introduction
The story of oxytocin (OT) begins right before labor, continues during birth, and later during the early period of lactation. Several physiological and pathological functions are governed directly or indirectly by OT (1, 2). This makes OT, and its receptor, potential targets for drug therapy. The therapeutic use of the OT is limited to, initiate labor and milk ejection (1, 2). However, there are indications that show a significant role of OT away from pregnancy (1, 2). OT is considered as a cardiovascular hormone with cardio-protective effects under experimental conditions (3). It has been shown that cardiovascular dysfunctions are related to metabolic disorders, such as obesity, insulin resistance, and diabetes (4).
Diabetes is one of the metabolic disorders considered as one of the major causes of mortality worldwide. Its incidence is expected to increase dramatically over the coming decades (5). It is characterized by a chain of complications that affect many organs. Osteoporosis (diabetic osteopathy) is one of these complications (6). Many endogenous and exogenous factors, more or less dependent on each other, are known to influence energy metabolism and bone mass accumulation. OT is one of these endogenous factors. Several lines of evidence have largely demonstrated that OT has many positive metabolic effects; this is based on a remarkable change in glucose metabolism, lipid profile, and insulin sensitivity after OT administration (7–10). In addition, it has also an anabolic effect on bone metabolism (11–13).
Metabolic Effect of OT
Feeding behavior, body weight, and energy balance are crucially regulated by the hypothalamus in the CNS (14). Several studies have linked OT to the hypothalamus–brainstem circuits that negatively regulate feeding (15–17). However, the physiological relevance and pathological relevance of OT were unexplored until researchers found that obesity linked to OT release defect, and OT treatment was able to effectively correct overeating and obesity (8–10). Deficits in OT or its receptor developed hyperleptinemia and late-onset obesity with increases in abdominal fats and fasting plasma triglycerides (TGs) (18, 19).
Oxytocin has a role in affecting glucose and insulin homeostasis, as well as regulating body weight balance. It has been reported that OT promotes glucose uptake (20, 21) and stimulates insulin secretion (22–26). It stimulated insulin secretion in pancreatic islets independently of plasma glucose changes while it also stimulated pancreatic glucagon secretion (25). This might suggest the involvement of OT in pathophysiology of diabetes. Recent research demonstrated that OT administration reduced obesity-related diabetic changes, such as glucose intolerance, insulin resistance, and pancreatic islet hypertrophy (7–10, 27, 28). Two weeks treatment with OT decreased adiposity and food intake in obese mice lacking leptin, although it worsen glucose metabolism, most likely due to an increase in corticosterone levels and enhanced hepatic glucose production. It could be suggested that the effect of OT in decreasing fat mass is independent of leptin, while the beneficial impact on glucose metabolism requires the presence of leptin (29). Whereas, OT treatment for longer period, notably reduced body fat accumulation, fasting blood glucose levels, and improved insulin sensitivity and glucose tolerance in leptin receptor deficient mice (30). The hypoglycemic effect, stimulatory effect on insulin secretion and sensitivity, and improvement of pancreatic islet cells after OT administration, strongly suggested that OT might be a therapeutic target for treating diabetes.
Role of Oxytocin Signaling on Glucose and Lipid Metabolism
Coupling of Oxytocin Receptors to Intracellular Cascades
Oxytocin receptor (OTR) is known to be present in neurons. This receptor is also present in other tissues like adipose tissue and pancreas (31). OT receptor is a G protein-coupled receptor (GPCR), which linked through Gαq/11 to phospholipase C (PLC) (32). Activation of PLC causes an increase in inositol trisphosphate (IP3) and diacyl glycerol (DAG). IP3 activates specific receptors in the sarcoplasmic reticulum in order to release Ca2+ into the cytosol to form a complex with calmodulin. This activates myosin light chain (MCL) kinase to phosphorylate MLCs causing myocyte contraction (33). DAG induces protein kinase C (PKC) activation, which has been implicated in uterine contraction. OT receptor activation leads to an increase in prostaglandin production through phospholipase A2 and cyclooxygenase-2. The MAP-kinase (MAPK) cascade is activated by different pathways like trans-activation of receptor tyrosine kinases and also by possibly different G protein-linked pathways (34). In fact, the coupling of the OTR to Gs and Gi proteins is also occurring. The trophic effect of OT is occurring via a PKC-mediated activation of eukaryotic elongation factor-2 (35). In different cell systems, the multiple signaling pathways are activated by OTRs, which may act synergistically like contraction of myometrial cells that induced by OTR coupling to Gαq-11 and to the small G proteins of the Rho pathway (36). However, they may also have an opposite effect on the same cell function, such as the proliferative effects of OT, which appear to be Gq-linked and involve MAPK activation which leads to c-fos and c-jun induction. On the other hand, inhibition of cell growth has been reported to be Gi mediated (37). Due to this heterogeneity in the final outcome, functional selective ligands would be of great importance in identifying the roles of the different OTR-elicited pathways in physiological functions.
Arginine-vasopressin (AVP) is another posterior pituitary hormone, which differ from OT only by two amino acids. AVP and OT trigger their effects through at least four subtypes of receptors (V1a, V1b, V2, and OT receptors). V1a receptors mediate glycogenolysis and vasoconstriction, Vlb receptors mediate the release of adrenocorticotropic hormone (ACTH), catecholamine, insulin, and glucagon, and V2 receptors mediate antidiuresis (38). Because OT and AVP share similar structures, it is not unexpected that they cross-react with each other’s receptor. For instance, AVP stimulates myometrial contraction via OTR (39) and OT stimulates the release of ACTH through V1b (40). OT interacts with AVP receptors with a lower affinity. It has been reported that both OT and AVP induce insulin and glucagon release from the pancreas by activating V1b receptors (41, 42).
Glucose and Insulin Homeostasis
Oxytocin can be a good choice to decrease the blood glucose level and increase the insulin level. The hypoglycemic effect of OT can be explained by increasing glucose uptake via insulin-like signaling pathway (20, 21). The glucose uptake in cardiomyocytes (CMs) is increased by OT through stimulation of intracellular release of Ca2+, and also activation of phosphoinositide-3-kinase (PI3K), calcium-calmodulin kinase kinase (Ca-CAMKK), and AMP-activated protein kinase (AMPK) pathways (Figure 1) (20, 21). The hypoglycemic actions of OT are also believed to be mediated indirectly through changes in pancreatic functions (25) as OT modulates insulin secretion centrally via vagal cholinergic neurons innervating β-cells and peripherally via phosphoinositide turnover and activation of protein kinase-C in pancreatic β-cells (Figure 1) (22–26).
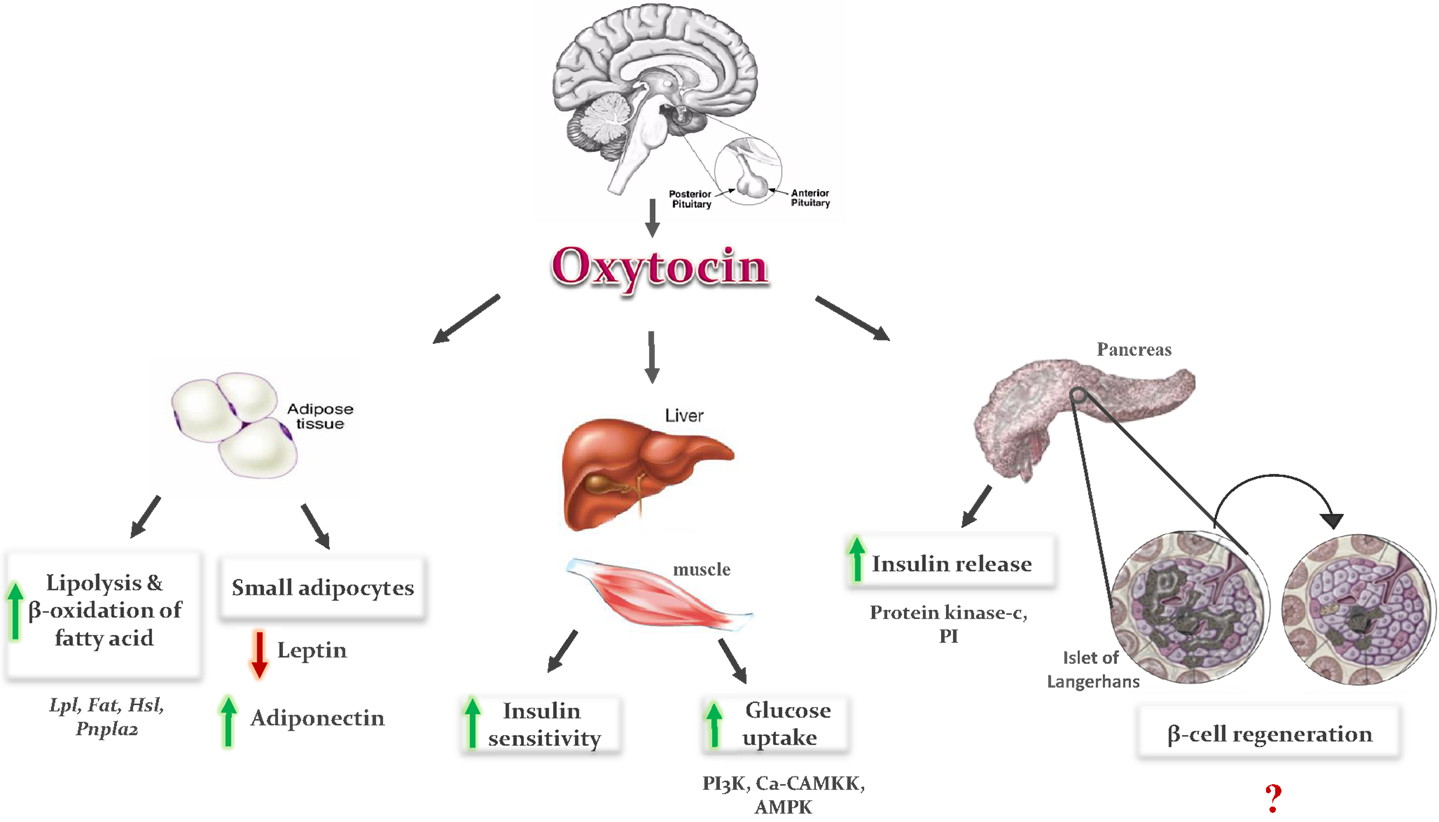
Figure 1. Metabolic effects of oxytocin: OT is secreted from the posterior lobe of the pituitary gland and binds to its receptor in peripheral tissues. In adipose tissue, it induces fatty acid oxidation and lipolysis, and formation of small adipocytes. Small adipocytes increase secretion of adiponectin and decrease leptin secretion, which improve insulin sensitivity in adipose tissue, liver, and muscles. In pancreas, it induces insulin secretion via phosphoinositide (PI) turnover and activation of protein kinase C, and regeneration of pancreatic β-cells. In liver and muscles, it enhances glucose uptake by stimulation of intracellular release of calcium, and activation of phosphoinositid-3-kinase (PI3K), calcium-calmodulin kinase kinase (Ca-CAMKK), and AMP-activated protein kinase (AMPK).
Pancreatic islet inflammation is an important factor in the pathogenesis of diabetes. The protection of β-cells from death is considered as a new therapeutic target. This inflammatory process is probably a combined consequence of dyslipidemia, hyperglycemia, and increased circulating proinflammatory cytokines [leptin, interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) (43)]. OT decreases pancreatic islet hypertrophy (7, 28). Yet, the remaining question is whether OT directly promotes β-cell regeneration or it acts indirectly. Unfortunately, the exact mechanism is still unknown and further studies are needed. Whereas, the indirect effect(s) may be related to its action as insulin sensitizers, with a reduction in gluco- and lipotoxicity (7–10). As well as, OT has antioxidant and anti-inflammatory effects (44–46).
In addition to increasing insulin secretion, OT also improves insulin sensitivity by both reducing gluco- and lipotoxicity (7–10) and regulating cytokines like leptin and adiponectin (7, 9). OT decreased fat mass, resulting in reduction in leptin level, which could also be one of the reasons underlying the OT-induced improvement in insulin sensitivity (9). However, the results of adiponectin after OT administration are contradictory. In some studies, adiponectin concentration was not affected by OT administration (30, 47), and in others, OT treatment induces an increase in adiponectin level (7). OT treatment induced formation of small adipocytes in treated rats as compared to control (47). It could be the smaller adipocytes, which are more sensitive to insulin produce a lower amounts of leptin and higher amounts of adiponectin (Figure 1). The balance of leptin and adiponectin in diabetic patients can be used as a predictor of insulin resistance and a useful indicator for the choice of drug to treat diabetes mellitus (48).
Lipid Metabolism
Oxytocin improves lipid profile of diabetic rodents as well by lowering serum low-density lipoprotein (LDL), TG, and cholesterol levels, and a propensity for high-density lipoprotein (HDL) level improvements (7). OT induces lipolysis and β-oxidation of fatty acids (9, 10). The effects of OT on adipocytes are mediated either directly by a mechanism via its peripheral effects on adipocytes, or indirectly through the central anorectic action and centrally mediated activation of sympathetic nerve innervating fat tissue. Peripheral actions of the OT are mediated by OTR. OT receptor mRNA is highly expressed in mouse adipocytes and increased during the differentiation of adipocyte (49). The stimulatory effect of OT on lipid metabolism is mediated by an increase in the mRNA expression of lipoprotein lipase (Lpl) and fatty acid transporter (Fat, also known as CD36). These two enzymes are responsible for the uptake of circulating TG and fatty acids, respectively. On the other hand, OT does not modify the mRNA level of enzymes involved in lipogenesis and TG storage, such as acetyl-coenzyme A carboxylase α (Acaca, also known as ACC-α), fatty acid synthase (Fasn), and diacylglycerol O-acyltransferase homolog 1 (Dgat1). OT also increases the mRNA levels of the enzymes involved in the process of lipolysis, such as hormone-sensitive lipase (Hsl) and patatin-like phospholipase domain containing 2 (Pnpla2). The increase in lipolysis process and TG uptake without a change in lipogenesis and TG storage under the effect of OT shows that OT promotes the use of fat as energy substrate (9). OT treatment induces formation of small adipocytes in treated rats as compared to control (47). This adipogenic effect of OT could be explained by the activation of peroxisome proliferator-activated receptors-γ (PPARγ), a key regulator of adipocyte differentiation (50).
Anabolic Actions of OT on the Skeleton
One of the most important bone-research results has revealed that the pituitary hormones have an intense outcome on bone (51), so that the pituitary–bone axis turned into one of the most important topics in skeletal physiology. Based on the fact that calcium is mobilized from the maternal skeleton during lactation and late pregnancy, it might be expected that the same hormone that regulates parturition and lactation might also controls skeletal homeostasis. Our research group and other scholars have developed studies to find new bone formation therapies. As a primitive neurohypophyseal hormone, OT has been reported to be an anabolic bone mass regulator (11–13).
A specific and accurate method for measuring OT is urgently needed for a better understanding of OT role, because the methods that are currently used to measure the peripheral OT level lack reliability (52). Previous study reported that plasma levels of OT are notably lower in postmenopausal women who develop osteoporosis than in healthy peers (12). Changes in bone remodeling and osteopenia in ovariectomized (OVX) rodents resulted in a significantly decreased OT level when it is compared with sham-operated controls (53). Moreover, in genetically modified animals, it was confirmed that deletion of OT or OTR causes a remarkable reduction in bone formation and this suggested that OT is crucial for basal skeletal homeostasis (13). Interestingly, it has been found that OT treatment in mice increased bone mineral density as well as osteoblast formation (11), it also reversed bone loss in 8-week-old OVX mice, and reduced marrow adiposity (12, 13). Its positive bone balance suggests that OT could be used therapeutically as an ally in the recovery of osteopathy resulting from diabetes.
Role of Oxytocin Signaling on Bone Metabolism
At the cellular level, OT stimulates differentiation of osteoblasts to a mineralizing phenotype by up-regulating bone morphogenic protein-2 (13). The effect of OT on osteoclasts was found to be more complex as osteoclast formation was stimulated, while bone resorption by mature osteoclasts was inhibited (7, 54). The effect of OT on bone could be resulted from the direct interaction of the hormone with its receptors in bone cells (54, 55). The signaling cascades that mediate the OT action on bone cells eventually release of Ca2+ from intracellular stores and trigger MAPK signaling (13). The increase in intracellular Ca2+ in osteoblasts provokes cellular cascades, such as JNK, P38, extracellular signal regulated kinase (ERK), PKA, and PI3K (56). This leads to an increase in prostaglandin E2 synthesis, with subsequent positive bone balance. OT and OTR interactions in osteoblast may also provoke other intracellular events, such as MAP-kinase phosphorylation and induce c-Fos expression (Figure 2) (55). It has been reported that OTR, on stimulation by its ligand OT, translocates into the nucleus of osteoblasts (57). The passage of OTR into the nucleus is facilitated by successive interactions with β-arrestins (ARRBS), the small GTPase Rab5, importin-β (KPNB1), and transportin-1 (TNPO1). This mechanism for OTR translocation will open several possibilities for direct transcriptional modulation by nuclear GPCRs (57). While in osteoclast, OT has dual effects. It increases osteoclast formation both directly, by activating nuclear factor-kβ and MAPK signaling, and indirectly via the up-regulation of receptor activator of nuclear factor kappa-B ligand (RANK-L) (Figure 2). The increase in osteoclastogenesis is coupled to the decrease in bone resorption of mature osteoclasts and this can be explained by triggering the cytosolic Ca2+ release and nitric oxide synthesis (13, 54). This might explain the significant decrease in the levels of TRAP, a bone-resorption marker that observed after OT administration (7). In addition to OTR, both AVP receptors, V1a and V2, are expressed in osteoblasts and osteoclasts, and their stimulation induces ERK activation, which in turn inhibits bone formation and stimulates bone resorption. This coupling would lead to bone loss (58). So, it could be concluded that the anabolic effects of OT on bone are not occurring through activation of vasopressin receptor signaling.
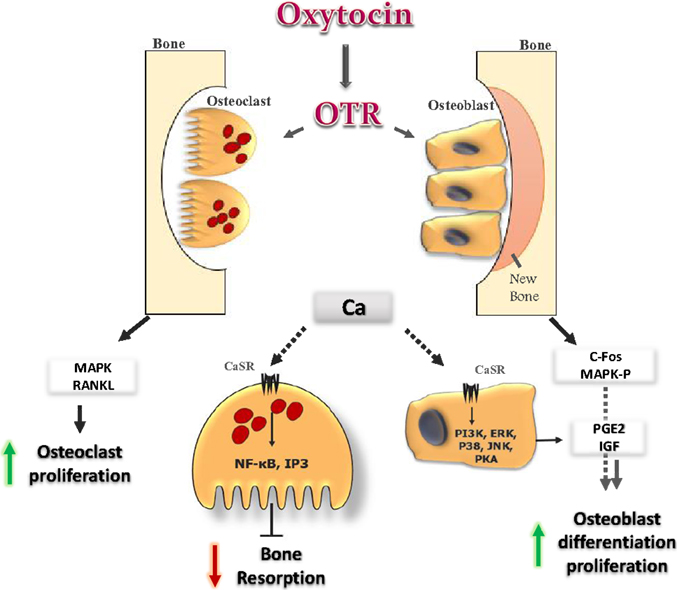
Figure 2. Oxytocin signaling in bone cells: OT binds to its receptor in osteoclast (OC) and osteoblast (OB), and initiates several cellular cascades. It induces OB differentiation by inducing c-fos expression and MAP-kinase phosphorylation. OT induces release of Ca2+ from intracellular stores. In OB, the increase in Ca2+ provokes several cellular cascades (JNK, P38, ERK, PKA, and PI3K), which lead to an increase in prostaglandin E2 synthesis, with positive effects on OB. In OC, OT increases OC formation directly, by activating MAPK signaling, and indirectly through the up-regulation of RANK-L from OB. The increase in Ca2+ induces NF-kb and IP3 which inhibit bone resorption of mature OC.
In addition to its direct effect, OT was found to have an indirect effect on bone through its action on cytokines (leptin and adiponectin) (7, 9) that have a considerable role in bone biology. Leptin has two opposite effects on bone. Centrally, it suppresses bone formation by restraining osteoblast proliferation. Peripherally, it stimulates osteoblastic differentiation and inhibits osteoclastogenesis. The two pathways could compensate each other, with the peripheral and positive effects being the major when leptin central resistance occurs with obesity onset (59). Adiponectin stimulates the differentiation and mineralization of osteoblasts and inhibits the macrophage colony-stimulating factor (M-CSF)- and RANKL-induced osteoclast differentiation, as well as the bone-resorption activity of mature osteoclasts (60).
Exogenous Oxytocin Administration
Besides its use in obstetrics, it has been reported that OT could play an important role in treating psychiatric disorders, such as autism and schizophrenia (61, 62). The most precise and reliable mode of delivering OT is through infusing it directly into the blood. Orally administered OT, due to its peptide structure, is rapidly degraded by proteolytic enzymes in the gut. Plasma-delivered OT, such as an intravenous injection, does not cross the blood–brain barrier. Intranasal OT is currently the favored administration method for neurobehavioral research because of its absorption through the nasal mucosa. Efficient brain penetration and activation of OTR are potentially an important limitation to the current OT therapies. Yet, the next generation of therapeutics may bypass this problem. Pharmacologically enhancing endogenous OT release, or developing small-molecule agonists and positive allosteric modulators, may be more effective (2). Regarding therapeutic consequences, the coupling of OTR to different G proteins exhibiting opposite effects, renders the definition of “agonist” and “antagonist” rather questionable. The ability to design compounds that can discriminate between many pathways, and predominantly activate a specific intracellular reaction, will be at the heart of future OTR-based drug strategies (2). Taking into consideration a potential use of OT as a therapeutic agent, an important issue to be addressed is the possible side effects of this treatment. OT’s side effect profile appears relatively benign according to recent studies focused on its effects on children and adolescents. No severe side effects of any kind, metabolic, or otherwise were reported (61, 62), but larger studies are still needed.
Conclusion
These beneficial observations, which are discussed above, clearly showed that OT and its analogs are involved in regulation of metabolic and bone balance and are opening up a new window for drug development in diabetes and osteoporosis therapeutics. The potential therapeutic uses of OT and more long-acting and specific analogs of OT are huge. As a short nine amino acid peptide, OT is considered to be ideal for the design of agonists and antagonists of its receptor. Continued studies are still needed to develop new drugs including the use of OT, OT agonists, and antagonists for various human disorders including diabetes and osteoporosis.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Lippert TH, Mueck AO, Seeger H, Pfaff A. Effects of oxytocin outside pregnancy. Horm Res (2003) 60(6):262–71. doi: 10.1159/000074243
2. Viero C, Shibuya I, Kitamura N, Verkhratsky A, Fujihara H, Katoh A, et al. Oxytocin: crossing the bridge between basic science and pharmacotherapy. CNS Neurosci Ther (2010) 16(5):e138–56. doi:10.1111/j.1755-5949.2010.00185.x
3. Ondrejcakova M, Ravingerova T, Bakos J, Pancza D, Jezova D. Oxytocin exerts protective effects on in vitro myocardial injury induced by ischemia and reperfusion. Can J Physiol Pharmacol (2009) 87(2):137–42. doi:10.1139/Y08-108
4. Kendall DM, Harmel AP. The metabolic syndrome, type 2 diabetes, and cardiovascular disease: understanding the role of insulin resistance. Am J Manag Care (2002) 8(20):S635–53.
5. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care (2004) 27(5):1047–53. doi:10.2337/diacare.27.5.1047
6. Yamaguchi T. Diabetes mellitus and osteoporosis. How to start drug therapy for osteoporosis in patients with diabetes mellitus. Clin Calcium (2012) 22(9):1403–9. doi:CliCa120914031409
7. Elabd S, Sabry I, Mohasseb M, Algendy A. Oxytocin as a novel therapeutic option for type I diabetes and diabetic osteopathy. Endocr Regul (2014) 48(2):87–102. doi:10.4149/endo_2014_02_87
8. Zhang H, Wu C, Chen Q, Chen X, Xu Z, Wu J, et al. Treatment of obesity and diabetes using oxytocin or analogs in patients and mouse models. PLoS One (2013) 8(5):e61477. doi:10.1371/journal.pone.0061477
9. Deblon N, Veyrat-Durebex C, Bourgoin L, Caillon A, Bussier AL, Petrosino S, et al. Mechanisms of the anti-obesity effects of oxytocin in diet-induced obese rats. PLoS One (2011) 6(9):e25565. doi:10.1371/journal.pone.0025565
10. Maejima Y, Iwasaki Y, Yamahara Y, Kodaira M, Sedbazar U, Yada T. Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging (Albany NY) (2011) 3(12):1169–77.
11. Elabd SK, Sabry I, Hassan WB, Nour H, Zaky K. Possible neuroendocrine role for oxytocin in bone remodeling. Endocr Regul (2007) 41(4):131–41.
12. Elabd C, Basillais A, Beaupied H, Breuil V, Wagner N, Scheideler M, et al. Oxytocin controls differentiation of human mesenchymal stem cells and reverses osteoporosis. Stem Cells (2008) 26(9):2399–407. doi:10.1634/stemcells.2008-0127
13. Tamma R, Colaianni G, Zhu LL, DiBenedetto A, Greco G, Montemurro G, et al. Oxytocin is an anabolic bone hormone. Proc Natl Acad Sci U S A (2009) 106(17):7149–54. doi:10.1073/pnas.0901890106
14. Myers MG Jr, Olson DP. Central nervous system control of metabolism. Nature (2012) 491(7424):357–63. doi:10.1038/nature11705
15. Arletti R, Benelli A, Bertolini A. Oxytocin inhibits food and fluid intake in rats. Physiol Behav (1990) 48(6):825–30. doi:10.1016/0031-9384(90)90234-U
16. Douglas AJ, Johnstone LE, Leng G. Neuroendocrine mechanisms of change in food intake during pregnancy: a potential role for brain oxytocin. Physiol Behav (2007) 91(4):352–65. doi:10.1016/j.physbeh.2007.04.012
17. Olszewski PK, Klockars A, Schiöth HB, Levine AS. Oxytocin as feeding inhibitor: maintaining homeostasis in consummatory behavior. Pharmacol Biochem Behav (2010) 97(1):47–54. doi:10.1016/j.pbb.2010.05.026
18. Takayanagi Y, Kasahara Y, Onaka T, Takahashi N, Kawada T, Nishimori K. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport (2008) 19(9):951–5. doi:10.1097/WNR.0b013e3283021ca9
19. Camerino C. Low sympathetic tone and obese phenotype in oxytocin-deficient mice. Obesity (Silver Spring) (2009) 17(5):980–4. doi:10.1038/oby.2009.12
20. Florian M, Jankowski M, Gutkowska J. Oxytocin increases glucose uptake in neonatal rat cardiomyocytes. Endocrinology (2010) 151(2):482–91. doi:10.1210/en.2009-0624
21. Lee ES, Uhm KO, Lee YM, Kwon J, Park SH, Soo KH. Oxytocin stimulates glucose uptake in skeletal muscle cells through the calcium-CaMKK-AMPK pathway. Regul Pept (2008) 151(1–3):71–4. doi:10.1016/j.regpep.2008.05.001
22. Knudtzon J. Acute effects of oxytocin and vasopressin on plasma levels of glucagon, insulin and glucose in rabbits. Horm Metab Res (1983) 15:103–4. doi:10.1055/s-2007-1018641
23. Chiodera P, Coiro V, Camellini L, Rossi G, Pignatti D, Volpi R, et al. Effect of pharmacological doses of oxytocin on insulin response to glucose in normal man. Horm Res (1984) 20(2):150–4. doi:10.1159/000179988
24. Paolisso G, Sgambato S, Giugliano D, Pizza G, Tesauro P, Varricchio M, et al. Effects of oxytocin delivery on counter-regulatory hormone response in insulin-dependent (type 1) diabetic subjects. Horm Res (1989) 31:250–5. doi:10.1159/000181126
25. Altszuler N, Winkler B, Rosenberg CR, Pi-Sunyer FX, Fuchs AR. Role of insulin and glucagon in oxytocin effects on glucose metabolism. Proc Soc Exp Biol Med (1992) 199(2):236–42. doi:10.3181/00379727-199-43353
26. Björkstrand E, Eriksson M, Uvnäs-Moberg K. Evidence of a peripheral and a central effect of oxytocin on pancreatic hormone release in rats. Neuroendocrinology (1996) 63(4):377–83. doi:10.1159/000126978
27. Zhang G, Cai D. Circadian intervention of obesity development via resting-stage feeding manipulation or oxytocin treatment. Am J Physiol Endocrinol Metab (2011) 301:E1004–12. doi:10.1152/ajpendo.00196.2011
28. Zhang G, Bai H, Zhang H, Dean C, Wu Q, Li J, et al. Neuropeptide exocytosis involving synaptotagmin-4 and oxytocin in hypothalamic programming of body weight and energy balance. Neuron (2011) 69:523–35. doi:10.1016/j.neuron.2010.12.036
29. Altirriba J, Poher AL, Caillon A, Arsenijevic D, Veyrat-Durebex C, Lyautey J, et al. Divergent effects of oxytocin treatment of obese diabetic mice on adiposity and diabetes. Endocrinology (2014) 155(11):4189–201. doi:10.1210/en.2014-1466
30. Plante E, Menaouar A, Danalache BA, Yip D, Broderick TL, Chiasson JL, et al. Oxytocin treatment prevents the cardiomyopathy observed in obese diabetic male db/db mice. Endocrinology (2015) 156(4):1416–28. doi:10.1210/en.2014-1718
31. Zingg HH, Laporte SA. The oxytocin receptor. Trends Endocrinol Metab (2003) 14(5):222–7. doi:10.1016/S1043-2760(03)00080-8
32. Ku CY, Qian A, Wen Y, Anwer K, Sanborn BM. Oxytocin stimulates myometrial guanosine triphosphatase and phospholipase-C activities via coupling to G alpha q/11. Endocrinology (1995) 136(4):1509–15. doi:10.1210/en.136.4.1509
33. Phaneuf S, Europe-Finner GN, Varney M, MacKenzie IZ, Watson SP, López Bernal A. Oxytocin-stimulated phosphoinositide hydrolysis in human myometrial cells: involvement of pertussis toxin-sensitive and -insensitive G-proteins. J Endocrinol (1993) 136(3):497–509. doi:10.1677/joe.0.1360497
34. Burns PD, Mendes JO Jr, Yemm RS, Clay CM, Nelson SE, Hayes SH, et al. Cellular mechanisms by which oxytocin mediates ovine endometrial prostaglandin F2alpha synthesis: role of G(i) proteins and mitogen-activated protein kinases. Biol Reprod (2001) 65(4):1150–5. doi:10.1095/biolreprod65.4.1150
35. Devost D, Carrier M, Zingg H. Oxytocin-induced activation of eukaryotic elongation factor 2 in myometrial cells is mediated by protein kinase C. Endocrinology (2008) 149:131–8. doi:10.1210/en.2007-0548
36. Sanborn BM. Hormones and calcium: mechanisms controlling uterine smooth muscle contractile activity. The Litchfield Lecture. Exp Physiol (2001) 86(2):223–37. doi:10.1113/eph8602179
37. Guzzi F, Zanchetta D, Cassoni P, Guzzi V, Francolini M, Parenti M, et al. Localization of the human oxytocin receptor in caveolin-1 enriched domains turns the receptor-mediated inhibition of cell growth into a proliferative response. Oncogene (2002) 21(11):1658–67. doi:10.1038/sj.onc.1205219
38. Koshimizu TA, Nakamura K, Egashira N, Hiroyama M, Nonoguchi H, Tanoue A. Vasopressin V1a and V1b receptors: from molecules to physiological systems. Physiol Rev (2012) 92(4):1813–64. doi:10.1152/physrev.00035.2011
39. Yu H, Zhuge R, Hsu WH. Lysine vasopressin-induced increases in porcine myometrial contractility and intracellular Ca2+ concentrations of myometrial cells: involvement of oxytocin receptors. Biol Reprod (1995) 52(3):584–90. doi:10.1095/biolreprod52.3.584
40. Schlosser SF, Almeida OF, Patchev VK, Yassouridis A, Elands J. Oxytocin-stimulated release of adrenocorticotropin from the rat pituitary is mediated by arginine vasopressin receptors of the V1b type. Endocrinology (1994) 135(5):2058–63. doi:10.1210/endo.135.5.7956927
41. Lee B, Yang C, Chen TH, al-Azawi N, Hsu WH. Effect of AVP and oxytocin on insulin release: involvement of V1b receptors. Am J Physiol (1995) 269(6 Pt 1):E1095–100.
42. Yibchok-anun S, Hsu WH. Effects of arginine vasopressin and oxytocin on glucagon release from clonal alpha-cell line In-R1-G9: involvement of V1b receptors. Life Sci (1998) 63(21):1871–8. doi:10.1016/S0024-3205(98)00463-9
43. Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes (2005) 54(2):S97–107. doi:10.2337/diabetes.54.suppl_2.S97
44. Clark SL, Simpson KR, Knox GE, Garite TJ. Oxytocin: new perspectives on an old drug. Am J Obstet Gynecol (2009) 200(1):.e1–6. doi:10.1016/j.ajog.2008.06.010
45. Jankowski M, Bissonauth V, Gao L, Gangal M, Wang D, Danalache B, et al. Anti-inflammatory effect of oxytocin in rat myocardial infarction. Basic Res Cardiol (2010) 105(2):205–18. doi:10.1007/s00395-009-0076-5
46. Szeto A, Nation DA, Mendez AJ, Dominguez-Bendala J, Brooks LG, Schneiderman N, et al. Oxytocin attenuates NADPH-dependent superoxide activity and IL-6 secretion in macrophages and vascular cells. Am J Physiol Endocrinol Metab (2008) 295(6):E1495–501. doi:10.1152/ajpendo.90718.2008
47. Eckertova M, Ondrejcakova M, Krskova K, Zorad S, Jezova D. Subchronic treatment of rats with oxytocin results in improved adipocyte differentiation and increased gene expression of factors involved in adipogenesis. Br J Pharmacol (2011) 162(2):452–63. doi:10.1111/j.1476-5381.2010.01037.x
48. Oda N, Imamura S, Fujita T, Uchida Y, Inagaki K, Kakizawa H, et al. The ratio of leptin to adiponectin can be used as an index of insulin resistance. Metabolism (2008) 57(2):268–73. doi:10.1016/j.metabol.2007.09.011
49. Yi KJ, So KH, Hata Y, Suzuki Y, Kato D, Watanabe K, et al. The regulation of oxytocin receptor gene expression during adipogenesis. J Neuroendocrinol (2015) 27(5):335–42. doi:10.1111/jne.12268
50. Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell (1994) 79(7):1147–56. doi:10.1016/0092-8674(94)90006-X
51. Bolanowski M, Halupczok J, Jawiarczyk-Przybyłowska A. Pituitary disorders and osteoporosis. Int J Endocrinol (2015) 2015:206853. doi:10.1155/2015/206853
52. Mc-Cullough ME, Churchland PS, Mendez AJ. Problems with measuring peripheral oxytocin: can the data on oxytocin and human behavior be trusted? Neurosci Biobehav Rev (2013) 37(8):1485–92. doi:10.1016/j.neubiorev.2013.04.018
53. Rouach V, Katzburg S, Koch Y, Stern N, Somjen D. Bone loss in ovariectomized rats: dominant role for estrogen but apparently not for FSH. J Cell Biochem (2011) 112(1):128–37. doi:10.1002/jcb.22908
54. Colucci S, Colaianni G, Mori G, Grano M, Zallone A. Human osteoclasts express oxytocin receptor. Biochem Biophys Res Commun (2002) 297(3):442–5. doi:10.1016/S0006-291X(02)02009-0
55. Copland JA, Ives KL, Simmons DJ, Soloff MS. Functional oxytocin receptors discovered in human osteoblasts. Endocrinology (1999) 140(9):4371–4. doi:10.1210/endo.140.9.7130
56. Danciu TE, Adam RM, Naruse K, Freeman MR, Hauschka PV. Calcium regulates the PI3K-Akt pathway in stretched osteoblasts. FEBS Lett (2003) 536(1–3):193–7. doi:10.1016/S0014-5793(03)00055-3
57. Di Benedetto A, Sun L, Zambonin CG, Tamma R, Nico B, Calvano CD, et al. Osteoblast regulation via ligand-activated nuclear trafficking of the oxytocin receptor. Proc Natl Acad Sci U S A (2014) 111(46):16502–7. doi:10.1073/pnas.1419349111
58. Tamma R, Sun L, Cuscito C, Lu P, Corcelli M, Li J, et al. Regulation of bone remodeling by vasopressin explains the bone loss in hyponatremia. Proc Natl Acad Sci U S A (2013) 110(46):18644–9. doi:10.1073/pnas.1318257110
59. Thomas T. The complex effects of leptin on bone metabolism through multiple pathways. Curr Opin Pharmacol (2004) 4(3):295–300. doi:10.1016/j.coph.2004.01.009
60. Oshima K, Nampei A, Matsuda M, Iwaki M, Fukuhara A, Hashimoto J, et al. Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochem Biophys Res Commun (2005) 331(2):520–6. doi:10.1016/j.bbrc.2005.03.210
61. Tachibana M, Kagitani-Shimono K, Mohri I, Yamamoto T, Sanefuji W, Nakamura A, et al. Long-term administration of intranasal oxytocin is a safe and promising therapy for early adolescent boys with autism spectrum disorders. J Child Adolesc Psychopharmacol (2013) 23(2):123–7. doi:10.1089/cap.2012.0048
Keywords: oxytocin, diabetes, insulin sensitivity, glucose uptake, osteoporosis, bone formation, bone resorption
Citation: Elabd S and Sabry I (2015) Two birds with one stone: possible dual-role of oxytocin in the treatment of diabetes and osteoporosis. Front. Endocrinol. 6:121. doi: 10.3389/fendo.2015.00121
Received: 24 February 2015; Accepted: 23 July 2015;
Published: 10 August 2015
Edited by:
Ez-Zoubir Amri, CNRS University of Nice-Sophia Antipolis, FranceReviewed by:
Peter J. Toth, University of Oklahoma Health Sciences Center, USAFrançoise Rohner-Jeanrenaud, University of Geneva, Switzerland
Copyright: © 2015 Elabd and Sabry. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seham Elabd, Department of Human Physiology, Medical Research Institute, Alexandria University, 165, Horreya Avenue, Hadara, Alexandria, Egypt,c2VoYW0uZWxhYmRAYWxleC1tcmkuZWR1LmVn
 Seham Elabd
Seham Elabd Ismail Sabry
Ismail Sabry