- 1Clinica Valle Giulia, G.en.e.r.a. Centers for Reproductive Medicine, Rome, Italy
- 2Clinica Ruesch, G.en.e.r.a. Centers for Reproductive Medicine, Naples, Italy
- 3G.en.e.r.a. Veneto, G.en.e.r.a. Centers for Reproductive Medicine, Marostica, Italy
- 4G.en.e.r.a. Umbria, G.en.e.r.a. Centers for Reproductive Medicine, Umbertide, Italy
- 5University of Targu Mures, Targu Mures, Romania
A panel of experts known as the POSEIDON group has recently redefined the spectrum of poor responder patients and introduced the concept of suboptimal response. Since an ideal management for these patients is still missing, they highlighted the importance of tailoring the ovarian stimulation based on the chance of each woman to obtain an euploid blastocyst. Interestingly, a novel pattern of follicle recruitment has been defined: multiple waves may arise during a single ovarian cycle. This evidence opened important clinical implications for the treatment of poor responders. For instance, double stimulation in the follicular (FPS) and luteal phase (LPS) of the same ovarian cycle (DuoStim) is an intriguing option to perform two oocyte retrievals in the shortest possible time. Here, we reported our 2-year experience of DuoStim application in four private IVF centers. To date, 310 poor prognosis patients completed a DuoStim protocol and underwent IVF with blastocyst-stage preimplantation-genetic-testing. LPS resulted into a higher mean number of oocytes collected than FPS; however, their competence (i.e., fertilization, blastocyst, euploidy rates, and clinical outcomes after euploid single-embryo-transfer) was comparable. Importantly, the rate of patients obtaining at least one euploid blastocyst increased from 42.3% (n = 131/310) after FPS to 65.5% (n = 203/310) with the contribution of LPS. A summary of the putative advantages and disadvantages of DuoStim was reported here through a Strengths–Weaknesses–Opportunities–Threats analysis. The strengths of this approach make it very promising. However, more studies are needed in the future to limit its weaknesses, shed light on its putative threats, and realize its opportunities.
Introduction
In IVF, poor response to controlled ovarian stimulation (COS) represents an important issue, which may affect 9–24% of the infertile women (1). Such a wide range is indeed indicative of a heterogeneous population of patients. Hence, several definitions have been proposed to classify “poor responders,” namely up to 41 according to the systematic review by Polyzos and Devroey (2), and numerous protocols have been adopted to treat these women. The Bologna criteria (3) represented the first successful attempt to outline some guidelines in the definition of poor ovarian response. At least two of the following characteristics must be present to define “a poor responder patient”: advanced maternal age (>40 years) and/or scarce response to a previous conventional stimulation (≤3 oocytes) and/or reduced ovarian reserve (antral follicle count, AFC < 5–7 follicles, and/or AMH < 1.1 ng/ml).
Yet, some criticism arose, since oocyte competence may be severely affected from numerous factors, among which maternal age is the most important (4, 5), to point out that the classification should be more patient-oriented and match the putative number of retrievable oocytes with their putative chance to develop as an euploid blastocyst. Hard evidence support that both the number of retrieved oocytes and woman age are indeed the most important parameters to predict the chance to conceive after IVF for all patients, including poor prognosis ones (6–9). An efficient prediction of the ovarian response is, therefore, pivotal to define a tailored-COS for each patient, especially poor responders, which should be based upon AFC and AMH, namely, the most widely used biomarkers at present (10).
A new classification by a panel of experts, known as the POSEIDON (Patient-Oriented Strategies Encompassing IndividualizeD Oocyte Number) group (11), has been introduced to better categorize the spectrum of poor responder patients. Currently, the treatment for this heterogeneous group is not evidence-based, yet, and the prognosis is highly dependent upon patients’ specific characteristics, rather than upon the COS protocol chosen (9). The POSEIDON group highlighted instead the importance of tailoring the stimulation based on the chance of each woman to obtain an euploid blastocyst, proposed as novel main goal of COS. Indeed, blastocyst transfer (12), especially of euploid embryos (13), showed to date the most promising results per transfer achievable in IVF. The POSEIDON group then introduced the concept of “sub-optimal response.” In this group of patients collecting 4–9 oocytes, 4 sub-clusters were outlined according to both the ovarian reserve and the maternal age. Specifically, groups 3 and 4 are represented from women younger than 35 or older than 35, respectively, with a compromised ovarian reserve (AFC < 5 and AMH < 1.2 ng/ml), an issue, which cannot be resolved pharmacologically, as already reported in several studies (14–21).
The aim of this paper is to provide an update about the IVF management of poor responders, as well as to describe and encourage the use of novel strategies, especially for the patients of POSEIDON groups 3 and 4, to increase the cumulative live birth per IVF treatment.
Theories of Follicle Recruitment
Follicular development is an extremely dynamic process. According to the classic theory (single recruitment episode theory), a single cohort of antral follicles grows during the follicular phase of the ovarian cycle after luteal regression. However, this theory has been overtaken by the evidence of multiple waves arising during an ovarian cycle in many mammals. Such evidence, at first reported in large animal models (22–27), was confirmed also in humans leading to the definition of two further theories of follicle recruitment (28): the continuous recruitment theory, according to which the follicles start growing and regress continuously during the ovarian cycle; and the waves theory, according to which 2–3 cohorts of antral follicles are recruited per ovarian cycle. However, the mechanisms underlying follicular recruitment have not been fully elucidated yet. Several intraovarian regulators, FSH and progesterone levels, inflammatory markers (e.g., serum C reactive protein) were all proposed as modulators of the dynamics behind the origin of follicular waves (28–30). From a clinical perspective, the growing knowledge of human ovarian follicular waves, opened new options for COS to improve the efficiency and possibly the efficacy of IVF.
DuoStim: Considerations, Indications, and Framework
Currently, there is insufficient evidence to recommend an ideal management of poor responders as defined through the Bologna Criteria. Indeed, regardless the COS protocol adopted, consistently low live birth rates were achieved in this population of patients (31–33). The choice of COS for patients with poor ovarian reserve markers and/or of advanced maternal age can be challenging. Yet, the number as well as the quality of the oocytes retrieved are important factors to increase the cumulative live birth rate. Moreover, these women have a limited time left to attempt to conceive with their own eggs: their “follicular heritage” suffers from a dramatic physiological decline of oocyte quantity and quality. The gonadotrophins can only support the growth of cohorts of follicles already present in the ovaries, but they cannot induce the de novo production of follicles. Therefore, increasing the dose of gonadotrophins administered or even adopting more powerful drugs will never compensate a reduced ovarian reserve.
In this scenario, a novel COS strategy has been proposed: double stimulation in the same ovarian cycle (DuoStim). Such protocol particularly suits poor prognosis and oncological patients, who require maximizing the exploitation of their ovarian reserve in a limited time (34–36). DuoStim, by combining conventional follicular phase stimulation (FPS) with luteal phase stimulation (LPS), can be considered a valuable option in patients with reduced ovarian reserve and/or advanced maternal age to maximize the number of oocytes retrieved in a single ovarian cycle, and for patients who did not collect oocytes or did not produce competent embryos after conventional FPS (37).
The very first experience with double stimulation has been reported by Kuang and colleagues (36) who showed that COS conducted in both the FPS and LPS of the same ovarian cycle results in the collection of oocytes with similar developmental competence (36). The drugs used for COS in the Shanghai protocol, as it was called in the paper, were clomiphene citrate 25 mg/day, letrozole 2.5 mg/day, and mild dose of human menopausal gonadotrophin 150–225 IU/day. Moreover, the final oocytes maturation was induced with triptorelin followed by ibuprofen 0.6 g the day of trigger and the day after, in both FPS and LPS. In 2016, we published our proof-of-concept study where a DuoStim protocol was adopted together with a pre-implantation genetic testing (PGT-A) program in poor prognosis patients (34). The most important outcome outlined by this study was that the application of DuoStim in this thorny patient population increased the chance of obtaining at least one euploid blastocyst in a single ovarian cycle from 40 to 70%. Contrary to the Shanghai protocol, the DuoStim protocol consists in a co-treatment with maximal dose of FSH plus LH and GnRh antagonist to prevent ovulation in both FPS and LPS. The rationale of administrating FSH 300 IU/day plus LH 75 IU/day in an antagonist protocol, instead of adopting a mild stimulation, is to limit the risk for cycle cancelation and possibly decrease time-to-pregnancy by maximizing the number of oocytes collected per stimulation. To this regard, mild stimulation has been associated with a reduced number of oocytes retrievable per COS cycle (38). Therefore, even if no randomized controlled trial (RCT) has been performed to compare mild versus conventional COS in a DuoStim protocol, it is reasonable to hypothesize that while the cost of the former COS approach might involve lower expense than the latter (39), effectiveness is questionable. This is especially true if we account cumulative live birth rate per started cycle as the measure of success in IVF (40, 41).
The patient drop-out is then another very important issue in the treatment of poor prognosis patients. It has been reported largely variable (20–60%) among couples undergoing IVF worldwide (42–44). Still, a generally valid information cannot be produced due to heterogeneity in terms of cost, reimbursement policies, accessibility to IVF, indication for PGT-A, etc., among the different countries (45, 46). Importantly, the most significant drop-out rate involves the second attempt after a first failed IVF cycle. Furthermore, when a second attempt is performed, ~10 months often pass from the former retrieval, while the time is crucial especially for poor prognosis patients (47). These cases might be rescued via the application of a DuoStim approach, which would at least allow to conduct two retrievals in a single ovarian cycle. A future RCT comparing double FPS versus DuoStim and entailing also the drop-out rate among the outcomes under investigation might provide an answer to this issue.
Indications to Duostim
Since October 2015, DuoStim has been proposed at our four centers, after extensive counseling, to all patients matching at least two of the following criteria: AMH < 1.5 ng/mg, AFC ≤ 6 follicles, ≤5 metaphase II (MII) oocytes retrieved in a previous cycle, advanced maternal age (≥35 years). Importantly, a single parameter is insufficient to outline an indication to DuoStim, since AFC evaluation per se might be limited from large inter-operator variability and AMH measurement per se might be affected from sample handling, storage, and low inter-laboratory reproducibility (48–50).
Another possible application of this strategy is urgent fertility preservation, in case few mature oocytes are collected after conventional COS and the time left before starting cancer therapy allows it (51).
Framework of a Duostim Protocol
To all patients undergoing Duostim, luteal estradiol priming (4 mg/day of estradiol valerate) was started in day 21 of the previous menstrual cycle to promote the synchronization and coordination of follicular growth (52, 53). After the transvaginal ultrasound and basal assessment of the ovaries, on day 2 to day 3 of the menstrual cycle, luteal estradiol priming was stopped, and FPS was started with fixed dose of rec-FSH 300 IU/day plus LH 75 IU/day for 4 days. Follicular growth was monitored on day 5 and then every 2–3 days. GnRh antagonist was administered daily after the identification of a leading follicle with a diameter ≥ 13–14 mm in FPS and LPS until the day of ovulation trigger. The final maturation of oocytes was triggered by a subcutaneous bolus of buserelin (dose 0.5 ml) to reduce the time of luteolysis. Egg retrieval was performed 35 h after the trigger. ICSI, blastocyst culture, trophectoderm biopsy, and vitrification, were performed as described in detail elsewhere (8, 54–56). Five days after the first retrieval, namely, the time needed to complete luteolysis (57), LPS was started with the same protocol and daily dose regardless of the number of antral follicles visible through ultrasound scan in the anovulatory wave. A freeze-all approach was adopted and the biopsy fragments from both stimulations were shipped together and analyzed in the same run at an external genetic lab (Igenomix, Italy). In presence of euploid blastocyst(s), frozen single embryo transfers were performed in a modified-natural or artificial cycle (58).
Multicenter Experience at G.EN.E.R.A. Centers for Reproductive Medicine (Rome, Naples, Umbertide, and Marostica, Italy) to Date
DuoStim was suggested to 353 consecutive couples approaching G.EN.E.R.A. centers for reproductive medicine (Rome, Naples, Marostica, and Umbertide, Italy) between October 2015 and December 2017. All the related data were prospectively recorded in a relational database [Fertilab Manager (FLM), Italy]. Among them, 17 did not respond to FPS and were excluded from this analysis (4.8%). Then, 336 patients underwent LPS and 26 (7.7%) did not respond. The 43 patients who did not respond to either FPS or LPS were stopped after 8–9 days of gonadotrophins administration. Overall, 310 patients completed the DuoStim approach with two oocyte retrievals of at least one cumulus-oocyte-complex in a single menstrual cycle and were included in this analysis (Figure S1A in Supplementary Material). The maternal age of the patients included in the analysis was 40.0 ± 3.0 years (33.0–44.0), the AFC was 5.3 ± 2.5 (3–13), the AMH was 1.0 ± 1.0 (0.1–2), and they already underwent 1.0 ± 1.3 (0–6) previous IVF cycles collecting 4.0 ± 2.6 (0–14) MII oocytes. LPS was on average 1 day longer than FPS. No increase of the post-oocyte retrieval complications has been reported so far compared to FPS-only cycles.
299 FPS- (96.5%) and 298 LPS-derived (96.1%) oocyte retrievals resulted in at least one MII oocyte collected (Figure S1B in Supplementary Material). Figure 1A displays the number of MII oocytes, fertilized oocytes, and blastocysts obtained on average after each LPS and FPS, respectively. Interestingly, a higher number of oocytes was collected after LPS, which involved also a higher number of fertilized oocytes and blastocysts (Wilcoxon Signed Rank test: p < 0.01). No difference was reported to date in terms of mean number of euploid blastocysts obtained from this cohort. The mean fertilization, blastocyst, and euploid blastocyst rates calculated per number of MII oocytes collected from each cycle (FPS- and LPS-derived ones, respectively) are reported in Figure 1B and were similar in the two groups. The overall fertilization, blastocyst, and euploid blastocyst rates of the 1,229 and 1,442 MII oocytes obtained after FPS and LPS, respectively, were also similar (Figure 1C).
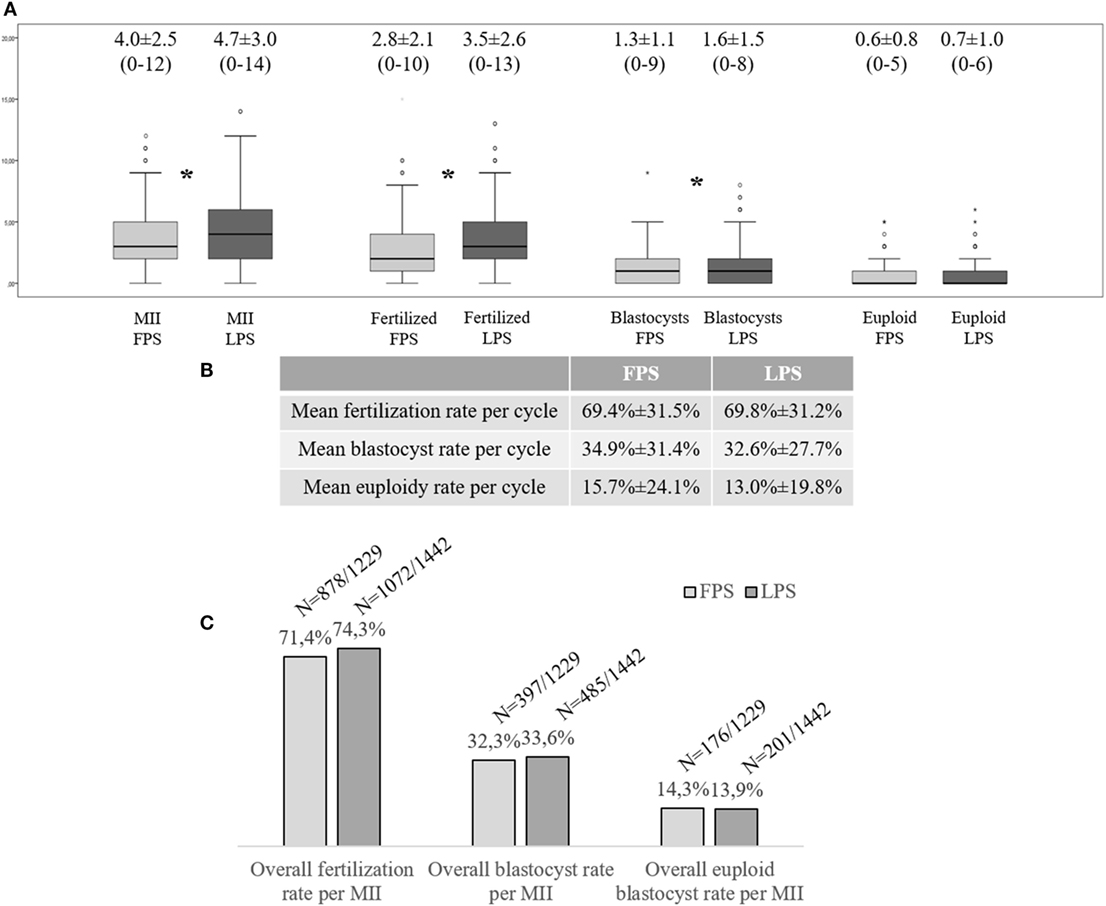
Figure 1. Multicenter clinical experience at the G.EN.E.R.A. centers for reproductive medicine (Rome, Naples, Marostica, and Umbertide) with the application of a DuoStim approach. (A) Mean number of metaphase (MII) oocytes, fertilized embryos, blastocysts, and euploid blastocysts obtained per cycle after follicular phase stimulation (FPS) and luteal phase one (LPS); (B) Mean embryological results calculated per MII oocyte retrieved and inseminated in FPS- and LPS-derived cycles; (C) Overall embryological results of the MII oocytes collected after FPS and LPS, respectively. The stars identify statistically significant differences. The non-Gaussian distribution of the data was assessed through the Shapiro–Wilk test. Wilcoxon signed-rank test and Fisher’s exact test were used to test for significant differences between FPS- and LPS-derived data.
229 (73.9%) and 230 (74.2%) patients obtained at least one blastocyst after FPS and LPS, respectively. This resulted in 280 patients who obtained at least one blastocyst in a single menstrual cycle due to DuoStim (90.3%). 131 (42.3%) and 129 (41.6%) patients obtained at least one euploid blastocyst after FPS and LPS, respectively. This resulted in 203 (65.5%) patients who obtained at least one euploid blastocyst in a single menstrual cycle due to DuoStim (Figure S1B in Supplementary Material).
81 and 83 FPS-derived and LPS-derived single euploid blastocyst transfers have been performed, respectively. In presence of euploid blastocysts from both FPS and LPS, the embryo to transfer was randomly chosen. The positive pregnancy rates were 48.1% (n = 39/81) and 59.0% (n = 49/83; Fisher’s exact test: p = NS). The biochemical pregnancy loss rates were 7.7% (n = 3/39) and 8.2% (n = 4/49; p = NS). The miscarriage rates were 11.1% (n = 4/36) and 8.9% (n = 4/45; p = NS). Therefore, the ongoing pregnancy rates were 39.5% (n = 32/81) and 49.4% (n = 41/83; p = NS) (Table S1 in Supplementary Material).
Discussion
This perspective paper dealing with the definition and implementation of DuoStim highlights the value of this strategy in treating poor prognosis patients. Importantly, the competence of the oocytes collected after both stimulations conducted in the FP and LP is similar in terms of fertilization, blastulation, and euploidy rates, as well as clinical outcomes after single euploid blastocyst transfer. However, the LPS seems to induce a better exploitation of the ovarian reserve with almost one more oocyte on average collected with respect to the FPS. Interestingly, these data further support the exploitation of anovulatory waves of follicle recruitment to obtain competent oocytes (34, 59–64). This practice is in countertendency with respect to the ovarian physiological behavior, but apparently it may be very successful. However, more stimulation cycles were canceled in the LP due to no response to the stimulation with respect to the FP.
The idea of DuoStim has been initially proposed to manage patients with poor ovarian reserve. However, the POSEIDON group highlighted the importance of obtaining at least one euploid embryo after COS as novel primary outcome in IVF. Therefore, based on this new concept, DuoStim in the future could be proposed not only a priori according to the inclusion criteria previously defined in this paper but also post hoc according to the number of blastocysts obtained after FPS. Clearly, the decision to perform also LPS (i.e., DuoStim) should depend on the expected euploidy rate of those FPS-derived blastocysts. To this end, the combination between the maternal age at oocyte retrieval and the number of embryos obtained after FPS represent the most predictive scheme to make a more appropriate choice (6, 7). Instead, in case of unexpectedly positive outcomes after FPS only (i.e., higher blastocyst rate than expected), we can consider avoiding LPS. Future studies should be properly designed to validate this putative strategy.
The data reported in this paper represent a further evidence to support the use of DuoStim to increase the number of poor prognosis patients obtaining an euploid blastocyst in a single menstrual cycle. No embryological, gynecological, or clinical issue has been reported to date. Yet, more biological, obstetrical, and neonatal evidence of safety is required, as well as an analysis of its cost-effectiveness.
SWOT Analysis of DuoStim
To summarize the putative advantages and disadvantages of DuoStim, we conducted a SWOT analysis (Figure 2), namely an efficient analytical framework useful to summarize the Strengths, Weaknesses, Opportunities, and Threats of a technology. The strengths are: a higher number of oocyte (and embryos) might be obtained per ovarian cycle; more patients obtaining a (chromosomally normal) blastocyst per ovarian cycle; no difference has been reported to date in terms of competence between oocytes obtained after FPS and LPS. The weaknesses are: a higher number of stimulations seems to be canceled in the LP than in the FP; no RCT or cost-effectiveness analysis has been performed to date investigating the use of DuoStim; a freeze-all approach is mandatory; it has been applied only to poor prognosis patients. The opportunities are: a decrease in the time and increase in the chance to obtain at least one competent embryo in a single menstrual cycle; the DuoStim protocol might be better-tolerated from the patients than consecutive FPS cycles; the drop-out rate might be reduced; the knowledge regarding the mechanisms of follicular recruitment and ovarian physiology might be increased. The threats are: an analysis of the cost-effectiveness is yet eagerly needed; the total dose of gonadotrophins to be administrated is substantial; few biological, gynecological, obstetrical, and neonatal evidence of safety have been produced to date. The strengths of this approach make it very promising. However, more studies are needed in the future to limit its weaknesses, shed light on its putative threats, and realize its opportunities.
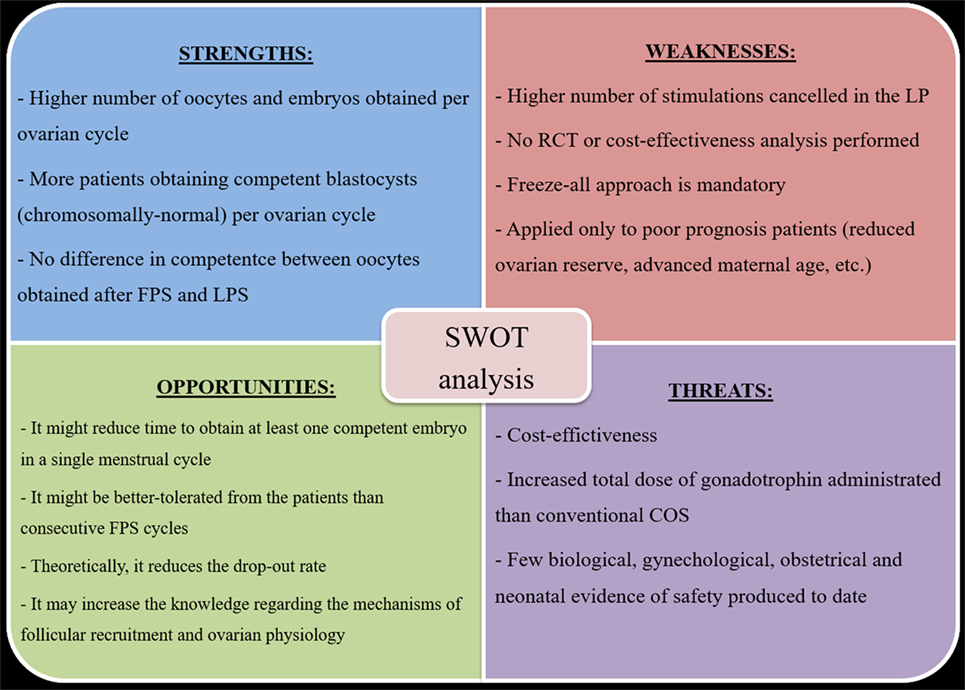
Figure 2. DuoStim SWOT analysis. Abbreviations: FPS, follicular phase stimulation; LPS, luteal phase stimulation; RCT, randomized controlled trial; COS, controlled ovarian stimulation.
Conclusion
The evidence that multiple waves of follicle recruitment may arise during a single ovarian cycle in women opened important clinical implications for the treatment of poor prognosis patients. LPS in general has become a promising protocol for patients who need to collect the highest number of oocytes in the shortest possible time (e.g., oncological patients). DuoStim approach conjugates FPS to LPS with very successful results reported to date. Still, any stimulation protocol, which exploits anovulatory waves of follicle recruitment should undergo a thorough biological and clinical investigation before it can be generally implemented. To this regard, DuoStim still needs a more extensive and wider validation to testify its safety. Interesting future perspectives to investigate its clinical efficacy/efficiency would entail (i) a RCT comparing double-FPS versus DuoStim; (ii) the application of DuoStim in cancer patients for fertility preservation; (iii) as well as in prospective analyses focused on patients clustered according to either the Bologna criteria or the Poseidon stratification. Until such evidence would be produced, DuoStim should be clinically applied only to a population of patients of poor prognosis and/or to whom time represents a critical issue.
Ethics Statement
The institutional review board of the involved clinics approved this study. The project was reviewed by two different members of each committee, none directly involved in the study to exclude any potential conflict of interest. All members approved the study. The study was performed in accordance with the local regulation and all patients gave written informed consent to it in accordance with the Declaration of Helsinki. This study was considered in line with the clinics’ protocols and standard procedures.
Author Contributions
AV and DC analyzed the data and drafted the manuscript. All authors contributed to the interpretation and discussion of the data.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fendo.2018.00317/full#supplementary-material.
Figure S1. (A) Flowchart and (B) cycle outcomes of 2-year multicenter application of DuoStim at G.EN.E.R.A. centers for reproductive medicine (Rome, Naples, Marostica, and Umbertide). FPS, follicular phase stimulation; LPS, luteal phase stimulation; MII, metaphase II oocyte.
References
1. Ubaldi F, Vaiarelli A, D’Anna R, Rienzi L. Management of poor responders in IVF: is there anything new? Biomed Res Int (2014) 2014:352098. doi:10.1155/2014/352098
2. Polyzos NP, Devroey P. A systematic review of randomized trials for the treatment of poor ovarian responders: is there any light at the end of the tunnel? Fertil Steril (2011) 96:1058–61.e7. doi:10.1016/j.fertnstert.2011.09.048
3. Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L, et al. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod (2011) 26:1616–24. doi:10.1093/humrep/der092
4. Ferraretti AP, Gianaroli L. The Bologna criteria for the definition of poor ovarian responders: is there a need for revision? Hum Reprod (2014) 29:1842–5. doi:10.1093/humrep/deu139
5. Younis JS, Ben-Ami M, Ben-Shlomo I. The Bologna criteria for poor ovarian response: a contemporary critical appraisal. J Ovarian Res (2015) 8:76. doi:10.1186/s13048-015-0204-9
6. Ata B, Kaplan B, Danzer H, Glassner M, Opsahl M, Tan SL, et al. Array CGH analysis shows that aneuploidy is not related to the number of embryos generated. Reprod Biomed Online (2012) 24:614–20. doi:10.1016/j.rbmo.2012.02.009
7. Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, et al. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril (2014) 101(3): 656–663.e1. doi:10.1016/j.fertnstert.2013.11.004
8. Ubaldi FM, Capalbo A, Colamaria S, Ferrero S, Maggiulli R, Vajta G, et al. Reduction of multiple pregnancies in the advanced maternal age population after implementation of an elective single embryo transfer policy coupled with enhanced embryo selection: pre- and post-intervention study. Hum Reprod (2015) 30:2097–106. doi:10.1093/humrep/dev159
9. Patrizio P, Vaiarelli A, Levi Setti PE, Tobler KJ, Shoham G, Leong M, et al. How to define, diagnose and treat poor responders? Responses from a worldwide survey of IVF clinics. Reprod Biomed Online (2015) 30:581–92. doi:10.1016/j.rbmo.2015.03.002
10. Iliodromiti S, Anderson RA, Nelson SM. Technical and performance characteristics of anti-Mullerian hormone and antral follicle count as biomarkers of ovarian response. Hum Reprod Update (2015) 21:698–710. doi:10.1093/humupd/dmu062
11. Poseidon Group (Patient-Oriented Strategies Encompassing IndividualizeD Oocyte Number), Alviggi C, Andersen CY, Buehler K, Conforti A, De Placido G, et al. A new more detailed stratification of low responders to ovarian stimulation: from a poor ovarian response to a low prognosis concept. Fertil Steril (2016) 105:1452–3. doi:10.1016/j.fertnstert.2016.02.005
12. Glujovsky D, Farquhar C, Quinteiro Retamar AM, Alvarez Sedo CR, Blake D. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev (2016) (6):CD002118. doi:10.1002/14651858.CD002118.pub5
13. Dahdouh EM, Balayla J, Garcia-Velasco JA. Comprehensive chromosome screening improves embryo selection: a meta-analysis. Fertil Steril (2015) 104:1503–12. doi:10.1016/j.fertnstert.2015.08.038
14. Duffy JM, Ahmad G, Mohiyiddeen L, Nardo LG, Watson A. Growth hormone for in vitro fertilization. Cochrane Database Syst Rev (2010) (1):CD000099. doi:10.1002/14651858.CD000099.pub3
15. Yeung T, Chai J, Li R, Lee V, Ho PC, Ng E. A double-blind randomised controlled trial on the effect of dehydroepiandrosterone on ovarian reserve markers, ovarian response and number of oocytes in anticipated normal ovarian responders. BJOG (2016) 123:1097–105. doi:10.1111/1471-0528.13808
16. Yeung TW, Chai J, Li RH, Lee VC, Ho PC, Ng EH. A randomized, controlled, pilot trial on the effect of dehydroepiandrosterone on ovarian response markers, ovarian response, and in vitro fertilization outcomes in poor responders. Fertil Steril (2014) 102:108–115.e1. doi:10.1016/j.fertnstert.2014.03.044
17. Yeung TW, Li RH, Lee VC, Ho PC, Ng EH. A randomized double-blinded placebo-controlled trial on the effect of dehydroepiandrosterone for 16 weeks on ovarian response markers in women with primary ovarian insufficiency. J Clin Endocrinol Metab (2013) 98:380–8. doi:10.1210/jc.2012-3071
18. Balasch J, Fabregues F, Penarrubia J, Carmona F, Casamitjana R, Creus M, et al. Pretreatment with transdermal testosterone may improve ovarian response to gonadotrophins in poor-responder IVF patients with normal basal concentrations of FSH. Hum Reprod (2006) 21:1884–93. doi:10.1093/humrep/del052
19. Fabregues F, Penarrubia J, Creus M, Manau D, Casals G, Carmona F, et al. Transdermal testosterone may improve ovarian response to gonadotrophins in low-responder IVF patients: a randomized, clinical trial. Hum Reprod (2009) 24:349–59. doi:10.1093/humrep/den428
20. Bosdou JK, Venetis CA, Dafopoulos K, Zepiridis L, Chatzimeletiou K, Anifandis G, et al. Transdermal testosterone pretreatment in poor responders undergoing ICSI: a randomized clinical trial. Hum Reprod (2016) 31:977–85. doi:10.1093/humrep/dew028
21. Polyzos NP, Davis SR, Drakopoulos P, Humaidan P, De Geyter C, Vega AG, et al. Testosterone for poor ovarian responders: lessons from ovarian physiology. Reprod Sci (2016). doi:10.1177/1933719116660849
22. Adams GP, Singh J, Baerwald AR. Large animal models for the study of ovarian follicular dynamics in women. Theriogenology (2012) 78:1733–48. doi:10.1016/j.theriogenology.2012.04.010
23. Jacob JC, Gastal EL, Gastal MO, Carvalho GR, Beg MA, Ginther OJ. Follicle deviation in ovulatory follicular waves with one or two dominant follicles in mares. Reprod Domest Anim (2009) 44:248–54. doi:10.1111/j.1439-0531.2007.01048.x
24. Jacob JC, Gastal EL, Gastal MO, Carvalho GR, Beg MA, Ginther OJ. Temporal relationships and repeatability of follicle diameters and hormone concentrations within individuals in mares. Reprod Domest Anim (2009) 44:92–9. doi:10.1111/j.1439-0531.2007.01003.x
25. Ginther OJ, Knopf L, Kastelic JP. Temporal associations among ovarian events in cattle during oestrous cycles with two and three follicular waves. J Reprod Fertil (1989) 87:223–30. doi:10.1530/jrf.0.0870223
26. Ginther OJ, Jacob JC, Gastal MO, Gastal EL, Beg MA. Development of one vs multiple ovulatory follicles and associated systemic hormone concentrations in mares. Reprod Domest Anim (2009) 44:441–9. doi:10.1111/j.1439-0531.2008.01109.x
27. Ginther OJ. The mare: a 1000-pound guinea pig for study of the ovulatory follicular wave in women. Theriogenology (2012) 77:818–28. doi:10.1016/j.theriogenology.2011.09.025
28. Baerwald AR, Adams GP, Pierson RA. Ovarian antral folliculogenesis during the human menstrual cycle: a review. Hum Reprod Update (2012) 18:73–91. doi:10.1093/humupd/dmr039
29. Baerwald AR, Adams GP, Pierson RA. Characterization of ovarian follicular wave dynamics in women. Biol Reprod (2003) 69:1023–31. doi:10.1095/biolreprod.103.017772
30. Clancy KB, Baerwald AR, Pierson RA. Systemic inflammation is associated with ovarian follicular dynamics during the human menstrual cycle. PLoS One (2013) 8:e64807. doi:10.1371/journal.pone.0064807
31. Polyzos NP, Nwoye M, Corona R, Blockeel C, Stoop D, Haentjens P, et al. Live birth rates in Bologna poor responders treated with ovarian stimulation for IVF/ICSI. Reprod Biomed Online (2014) 28:469–74. doi:10.1016/j.rbmo.2013.11.010
32. La Marca A, Grisendi V, Giulini S, Sighinolfi G, Tirelli A, Argento C, et al. Live birth rates in the different combinations of the Bologna criteria poor ovarian responders: a validation study. J Assist Reprod Genet (2015) 32:931–7. doi:10.1007/s10815-015-0476-4
33. Busnelli A, Papaleo E, Del Prato D, La Vecchia I, Iachini E, Paffoni A, et al. A retrospective evaluation of prognosis and cost-effectiveness of IVF in poor responders according to the Bologna criteria. Hum Reprod (2015) 30:315–22. doi:10.1093/humrep/deu319
34. Ubaldi FM, Capalbo A, Vaiarelli A, Cimadomo D, Colamaria S, Alviggi C, et al. Follicular versus luteal phase ovarian stimulation during the same menstrual cycle (DuoStim) in a reduced ovarian reserve population results in a similar euploid blastocyst formation rate: new insight in ovarian reserve exploitation. Fertil Steril (2016) 105:1488–95.e1. doi:10.1016/j.fertnstert.2016.03.002
35. Vaiarelli A, Venturella R, Vizziello D, Bulletti F, Ubaldi FM. Dual ovarian stimulation and random start in assisted reproductive technologies: from ovarian biology to clinical application. Curr Opin Obstet Gynecol (2017) 29(3):153–9. doi:10.1097/GCO.0000000000000365
36. Kuang Y, Chen Q, Hong Q, Lyu Q, Ai A, Fu Y, et al. Double stimulations during the follicular and luteal phases of poor responders in IVF/ICSI programmes (Shanghai protocol). Reprod Biomed Online (2014) 29:684–91. doi:10.1016/j.rbmo.2014.08.009
37. Vaiarelli A, Cimadomo D, Ubaldi N, Rienzi L, Ubaldi FM. What is new in the management of poor ovarian response in IVF? Curr Opin Obstet Gynecol (2018) 30(3):155–62. doi:10.1097/GCO.0000000000000452
38. Kamath MS, Maheshwari A, Bhattacharya S, Lor KY, Gibreel A. Oral medications including clomiphene citrate or aromatase inhibitors with gonadotropins for controlled ovarian stimulation in women undergoing in vitro fertilisation. Cochrane Database Syst Rev (2017) 11:CD008528. doi:10.1002/14651858.CD008528.pub3
39. Heijnen EM, Eijkemans MJ, De Klerk C, Polinder S, Beckers NG, Klinkert ER, et al. A mild treatment strategy for in-vitro fertilisation: a randomised non-inferiority trial. Lancet (2007) 369:743–9. doi:10.1016/S0140-6736(07)60360-2
40. Revelli A, Casano S, Salvagno F, Delle Piane L. Milder is better? Advantages and disadvantages of “mild” ovarian stimulation for human in vitro fertilization. Reprod Biol Endocrinol (2011) 9:25. doi:10.1186/1477-7827-9-25
41. Maheshwari A, McLernon D, Bhattacharya S. Cumulative live birth rate: time for a consensus? Hum Reprod (2015) 30:2703–7. doi:10.1093/humrep/dev263
42. Domar AD, Smith K, Conboy L, Iannone M, Alper M. A prospective investigation into the reasons why insured United States patients drop out of in vitro fertilization treatment. Fertil Steril (2010) 94:1457–9. doi:10.1016/j.fertnstert.2009.06.020
43. Van den Broeck U, Holvoet L, Enzlin P, Bakelants E, Demyttenaere K, D’Hooghe T. Reasons for dropout in infertility treatment. Gynecol Obstet Invest (2009) 68:58–64. doi:10.1159/000214839
44. Bodri D, Kawachiya S, De Brucker M, Tournaye H, Kondo M, Kato R, et al. Cumulative success rates following mild IVF in unselected infertile patients: a 3-year, single-centre cohort study. Reprod Biomed Online (2014) 28:572–81. doi:10.1016/j.rbmo.2014.01.002
45. Brandes M, van der Steen JO, Bokdam SB, Hamilton CJ, de Bruin JP, Nelen WL, et al. When and why do subfertile couples discontinue their fertility care? A longitudinal cohort study in a secondary care subfertility population. Hum Reprod (2009) 24:3127–35. doi:10.1093/humrep/dep340
46. Roest J, van Heusden AM, Zeilmaker GH, Verhoeff A. Cumulative pregnancy rates and selective drop-out of patients in in-vitro fertilization treatment. Hum Reprod (1998) 13:339–41. doi:10.1093/humrep/13.2.339
47. Goswami M, Hyslop LA, Murdoch AP. NHS-funded IVF: consequences of NICE implementation. Hum Fertil (Camb) (2013) 16:121–7. doi:10.3109/14647273.2013.786840
48. Rustamov O, Smith A, Roberts SA, Yates AP, Fitzgerald C, Krishnan M, et al. Anti-Mullerian hormone: poor assay reproducibility in a large cohort of subjects suggests sample instability. Hum Reprod (2012) 27:3085–91. doi:10.1093/humrep/des260
49. Rustamov O, Smith A, Roberts SA, Yates AP, Fitzgerald C, Krishnan M, et al. The measurement of anti-Mullerian hormone: a critical appraisal. J Clin Endocrinol Metab (2014) 99:723–32. doi:10.1210/jc.2013-3476
50. Nelson SM. Biomarkers of ovarian response: current and future applications. Fertil Steril (2013) 99:963–9. doi:10.1016/j.fertnstert.2012.11.051
51. Tsampras N, Gould D, Fitzgerald CT. Double ovarian stimulation (DuoStim) protocol for fertility preservation in female oncology patients. Hum Fertil (Camb) (2017) 20:248–53. doi:10.1080/14647273.2017.1287433
52. Reynolds KA, Omurtag KR, Jimenez PT, Rhee JS, Tuuli MG, Jungheim ES. Cycle cancellation and pregnancy after luteal estradiol priming in women defined as poor responders: a systematic review and meta-analysis. Hum Reprod (2013) 28:2981–9. doi:10.1093/humrep/det306
53. Fanchin R, Salomon L, Castelo-Branco A, Olivennes F, Frydman N, Frydman R. Luteal estradiol pre-treatment coordinates follicular growth during controlled ovarian hyperstimulation with GnRH antagonists. Hum Reprod (2003) 18:2698–703. doi:10.1093/humrep/deg516
54. Rienzi L, Capalbo A, Stoppa M, Romano S, Maggiulli R, Albricci L, et al. No evidence of association between blastocyst aneuploidy and morphokinetic assessment in a selected population of poor-prognosis patients: a longitudinal cohort study. Reprod Biomed Online (2015) 30:57–66. doi:10.1016/j.rbmo.2014.09.012
55. Capalbo A, Rienzi L, Cimadomo D, Maggiulli R, Elliott T, Wright G, et al. Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod (2014) 29:1173–81. doi:10.1093/humrep/deu033
56. Cobo A, Bellver J, Domingo J, Perez S, Crespo J, Pellicer A, et al. New options in assisted reproduction technology: the Cryotop method of oocyte vitrification. Reprod Biomed Online (2008) 17:68–72. doi:10.1016/S1472-6483(10)60295-7
57. Fatemi HM, Polyzos NP, van Vaerenbergh I, Bourgain C, Blockeel C, Alsbjerg B, et al. Early luteal phase endocrine profile is affected by the mode of triggering final oocyte maturation and the luteal phase support used in recombinant follicle-stimulating hormone-gonadotropin-releasing hormone antagonist in vitro fertilization cycles. Fertil Steril (2013) 100:742–7. doi:10.1016/j.fertnstert.2013.05.028
58. Yarali H, Polat M, Mumusoglu S, Yarali I, Bozdag G. Preparation of endometrium for frozen embryo replacement cycles: a systematic review and meta-analysis. J Assist Reprod Genet (2016) 33:1287–304. doi:10.1007/s10815-016-0787-0
59. Massin N. New stimulation regimens: endogenous and exogenous progesterone use to block the LH surge during ovarian stimulation for IVF. Hum Reprod Update (2017) 23:211–20. doi:10.1093/humupd/dmw047
60. Martinez F, Clua E, Devesa M, Rodriguez I, Arroyo G, Gonzalez C, et al. Comparison of starting ovarian stimulation on day 2 versus day 15 of the menstrual cycle in the same oocyte donor and pregnancy rates among the corresponding recipients of vitrified oocytes. Fertil Steril (2014) 102:1307–11. doi:10.1016/j.fertnstert.2014.07.741
61. Kuang Y, Hong Q, Chen Q, Lyu Q, Ai A, Fu Y, et al. Luteal-phase ovarian stimulation is feasible for producing competent oocytes in women undergoing in vitro fertilization/intracytoplasmic sperm injection treatment, with optimal pregnancy outcomes in frozen-thawed embryo transfer cycles. Fertil Steril (2014) 101:105–11. doi:10.1016/j.fertnstert.2013.09.007
62. Wang N, Wang Y, Chen Q, Dong J, Tian H, Fu Y, et al. Luteal-phase ovarian stimulation vs conventional ovarian stimulation in patients with normal ovarian reserve treated for IVF: a large retrospective cohort study. Clin Endocrinol (Oxf) (2016) 84:720–8. doi:10.1111/cen.12983
63. Lin LT, Wang PH, Tsui KH. The use of luteal-phase ovarian stimulation for poor ovarian responders undergoing in vitro fertilization/intracytoplasmic sperm injection-embryo transfer treatment. Taiwan J Obstet Gynecol (2016) 55:307–8. doi:10.1016/j.tjog.2016.04.002
Keywords: duostim, double stimulation, dual-stimulation, low prognosis patients, poor responder, IVF, euploid blastocyst, Poseidon
Citation: Vaiarelli A, Cimadomo D, Trabucco E, Vallefuoco R, Buffo L, Dusi L, Fiorini F, Barnocchi N, Bulletti FM, Rienzi L and Ubaldi FM (2018) Double Stimulation in the Same Ovarian Cycle (DuoStim) to Maximize the Number of Oocytes Retrieved From Poor Prognosis Patients: A Multicenter Experience and SWOT Analysis. Front. Endocrinol. 9:317. doi: 10.3389/fendo.2018.00317
Received: 04 April 2018; Accepted: 28 May 2018;
Published: 14 June 2018
Edited by:
Sandro C. Esteves, Androfert, Andrology and Human Reproduction Clinic, BrazilReviewed by:
Hakan Yarali, Anatolia IVF, TurkeyGiuliano Marchetti Bedoschi, University of São Paulo, Brazil
Copyright: © 2018 Vaiarelli, Cimadomo, Trabucco, Vallefuoco, Buffo, Dusi, Fiorini, Barnocchi, Bulletti, Rienzi and Ubaldi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alberto Vaiarelli, YWxiZXJ0by52YWlhcmVsbGlAZ21haWwuY29t
 Alberto Vaiarelli
Alberto Vaiarelli Danilo Cimadomo
Danilo Cimadomo Elisabetta Trabucco
Elisabetta Trabucco Roberta Vallefuoco2
Roberta Vallefuoco2 Fabrizio Fiorini
Fabrizio Fiorini Filippo Maria Ubaldi
Filippo Maria Ubaldi