- 1Department of Health Sciences, University “Magna Græcia” of Catanzaro, Catanzaro, Italy
- 2Department of Life Sciences, University of Trieste, Trieste, Italy
- 3Department of Medical and Surgical Sciences, University “Magna Græcia” of Catanzaro, Catanzaro, Italy
- 4Department of Clinical and Experimental Medicine, University “Magna Græcia” of Catanzaro, Catanzaro, Italy
HMGA1 (high mobility group A1) is a nonhistone architectural chromosomal protein that functions mainly as a dynamic regulator of chromatin structure and gene transcription. As such, HMGA1 is involved in a variety of fundamental cellular processes, including gene expression, epigenetic regulation, cell differentiation and proliferation, as well as DNA repair. In the last years, many reports have demonstrated a role of HMGA1 in the transcriptional regulation of several genes implicated in glucose homeostasis. Initially, it was proved that HMGA1 is essential for normal expression of the insulin receptor (INSR), a critical link in insulin action and glucose homeostasis. Later, it was demonstrated that HMGA1 is also a downstream nuclear target of the INSR signaling pathway, representing a novel mediator of insulin action and function at this level. Moreover, other observations have indicated the role of HMGA1 as a positive modulator of the Forkhead box protein O1 (FoxO1), a master regulatory factor for gluconeogenesis and glycogenolysis, as well as a positive regulator of the expression of insulin and of a series of circulating proteins that are involved in glucose counterregulation, such as the insulin growth factor binding protein 1 (IGFBP1), and the retinol binding protein 4 (RBP4). Thus, several lines of evidence underscore the importance of HMGA1 in the regulation of glucose production and disposal. Consistently, lack of HMGA1 causes insulin resistance and diabetes in humans and mice, while variations in the HMGA1 gene are associated with the risk of type 2 diabetes and metabolic syndrome, two highly prevalent diseases that share insulin resistance as a common pathogenetic mechanism. This review intends to give an overview about our current knowledge on the role of HMGA1 in glucose metabolism. Although research in this field is ongoing, many aspects still remain elusive. Future directions to improve our insights into the pathophysiology of glucose homeostasis may include epigenetic studies and the use of “omics” strategies. We believe that a more comprehensive understanding of HMGA1 and its networks may reveal interesting molecular links between glucose metabolism and other biological processes, such as cell proliferation and differentiation.
Introduction
Glucose homeostasis is essential for life, and its maintenance is ensured through evolutionarily conserved regulatory mechanisms, that implicate complex and fine-tuned interplays between a variety of organs, tissues, hormones, receptors, nutrients, sensors, enzymes, and other molecules that may act locally and systemically (1, 2). In a physiological setting, the earliest mechanisms regulating postprandial hyperglycemia involve: (i) the readily releasable pool of insulin granules; (ii) the membrane translocation of glucose transporters in insulin-target tissues; (iii) post-translational regulatory mechanisms, mostly based upon post-translational modifications (i.e., phosphorylation), which affect enzyme functions that are implicated in carbohydrate metabolism, glucose homeostasis and disposal. Instead, during fasting conditions, when blood glucose levels are low, glucagon secretion increases to activate glycogenolysis and gluconeogenesis, thereby promoting hepatic glucose production to maintain fasting euglycemia. On a longer time-scale, instead, other effective mechanisms take place, which entail the transcriptional activation of genes and gene networks that function to control glucose homeostasis. In this context, glucose and insulin may regulate several “metabolic” genes by modulating the activity of nuclear factors toward their target genes (3). For example, it has been shown that glucose influences insulin gene transcription by inducing the phosphorylation of the glucose-sensitive PDX-1 transcription factor in pancreatic beta cells (4), while insulin can inhibit genes by triggering the phosphorylation of the forkhead box protein O1 (FoxO1), and its consequent relocation from the nucleus to the cytoplasm (5, 6). However, although in the last decades many studies have contributed to a better understanding of the transcriptional regulation of glucose metabolism, the role and interplay of several nuclear transcription factors in this scenario need further elucidation.
The high mobility group A1 (HMGA1) protein (also formerly known as HMGI/Y) is an architectural transcription factor involved in global chromatin remodeling. By interacting with both DNA and transcription factors, it regulates many fundamental biological processes, ranging from embryonic development to cell proliferation and differentiation, apoptosis, senescence and repair of DNA (7–13). Before the last two decades, HMGA1 has been mainly studied for its role in oncology, and to a lesser extent, in inflammation (9, 10, 14). Later, as part of investigations aimed at understanding the molecular basis of regulation of insulin receptor (INSR) gene expression, HMGA1 has emerged as a crucial factor in the transcriptional regulation of the INSR gene, and other genes relevant to glucose metabolism (15–18). Within this metabolic context, novel HMGA1 molecular partners have been identified, and their functional interplay investigated, while, in the meantime, HMGA1 gene variants have been identified as reliably linked to both type 2 diabetes mellitus, and the metabolic syndrome (19–21).
The purpose of this review is to summarize current information on the structural and functional characteristics of HMGA1, and its role in the transcriptional regulation of the metabolic genes so far investigated. In this scenario, HMGA1 emerges as a crucial factor in the regulation of glucose production and disposal. Also, a recently recognized role of the HMGA1 gene locus as a favored locus for susceptibility to insulin resistance and metabolic diseases is discussed, while future research directions are proposed to gain further insights into the links between HMGA1 and the pathophysiology of glucose metabolism and homeostasis.
General Characteristics of HMGA1
Gene Structure, Transcriptional Regulation, and Protein Synthesis
In humans, the HMGA1 gene is located on chromosome 6p21 (NC_000006.12), and it is well conserved among species (22, 23). Cloning and characterization of this gene reveal a very complex genomic organization, which includes a 5′ untranslated region (UTR) that undergoes alternative splicing, a coding region distributed on four exons, and a large 3′UTR (Figure 1). As for other genes, it is plausible that the size of the 5′UTR may influence the stability and translation efficacy of HMGA1 transcripts (24). The regulatory region of the HMGA1 gene is highly GC-rich as a whole and lacks TATA and CAAT box sequences; also, it includes at least two transcription start sites with different promoter/enhancer regions (22, 25, 26), as it occurs for genes that are regulated without the preferential selection of any specific start site. However, a privileged utilization of start site 2 has been demonstrated in certain cell types and under certain experimental conditions (26), thus indicating a tight gene regulation that results in the transcription of specific mRNAs in response to different stimuli. In addition, it has been reported that the human HMGA1 gene displays a basal transcriptional activity mainly controlled by the specificity protein 1 (Sp1) and the activator protein 1 (AP1) transcription factors, both of which stimulate HMGA1 gene expression from the transcription start site 1 and the transcription start site 2, respectively (27).
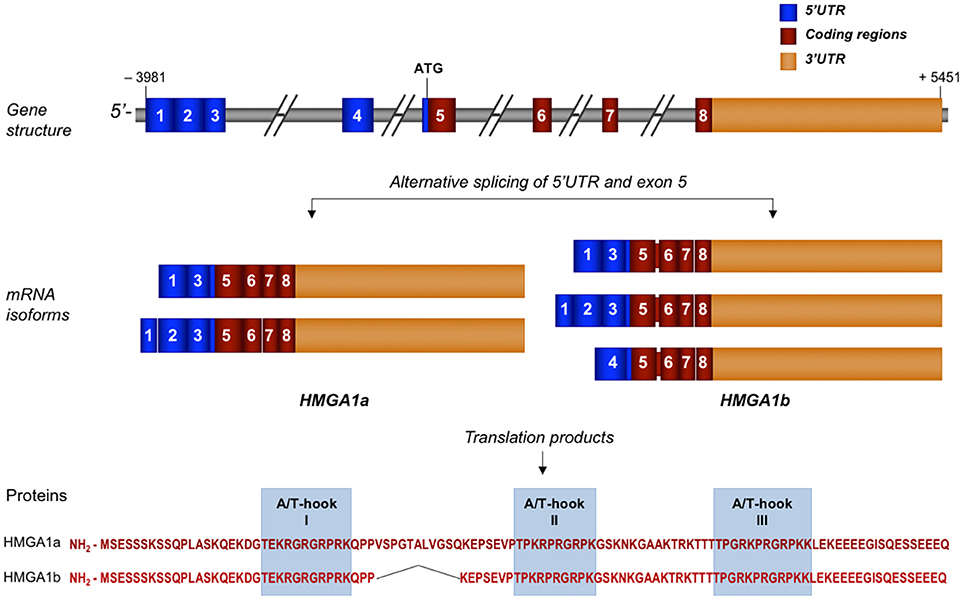
Figure 1. Schematic representation of human HMGA1 gene, transcripts, and protein isoforms. Exons are indicated by colored, numbered boxes. Main mRNA isoforms for both HMGA1a and HMGA1b are illustrated. Aminoacid sequence with the three functional AT-hook domains of both isoform proteins are reported.
A functional c-Myc-Max consensus DNA binding site was identified in the HMGA1 promoter and, consistent with this, the oncoprotein c-Myc and its protein partner Max bind to this site and activate HMGA1 gene transcription (28). Also, the HMGA1 promoter is activated by the transforming growth factor-β1 (TGF-β1) (29). Recently, we identified an octamer motif ATGCAAAT at the beginning of exon 1, where the octamer transcription factors Oct-1 and Oct-2 exert a differential regulation of HMGA1 gene transcription, and demonstrated that, by binding its own promoter, HMGA1 can contribute to its transactivation by Oct-2 (30), thus supporting a previous observation about the role of HMGA1 in an auto-regulatory circuit (31). More recently, it has been reported that G9a, an activator of gene transcription and a histone methyltransferase, positively regulates the expression of the HMGA1 gene in hepatic cells (32).
The complexity of the HMGA1 gene structure and transcriptional regulation results in the generation of a series of mRNA isoforms that are largely derived from an extensive alternative splicing in the 5′UTR (Figure 1). Instead, the coding sequence undergoes only one differential splicing that produces the two protein isoforms, HMGA1a, consisting of 107 aminoacids, and HMGA1b, which lacks 11 aminoacids at the end of exon 5 (33) (Figure 1). Both protein isoforms contain three AT-hook DNA binding domains, a protein-protein interaction domain, which overlaps with the second AT-hook and includes the aminoacid sequence up to the third AT- hook, and a highly negative and constitutively phosphorylated C-terminal tail (11). Each DNA binding domain includes the core peptide motif Pro-Arg-Gly-Arg-Pro (P-R-G-R-P) (Figure 1), through which HMGA1 preferentially interacts with the minor groove of AT-rich DNA sequences (34, 35). Although all three AT-hook motifs synergize during target recognition, the first two AT-hooks contribute to the majority of HMGA1 affinity for DNA (36). The two different HMGA1 isoforms may have different biological functions, as indicated by studies in MCF-7 breast epithelial cells, where HMGA1b forced expression confers a more aggressive neoplastic phenotype than HMGA1a (37). However, in the context of other cell lines or of other biological processes, including metabolism, more investigations are needed to deepen this issue.
Being among the most abundant non-histone, chromatin-associated protein, HMGA1 has been shown to cooperate with other nuclear proteins, including the chaperone nucleophosmin (38), and to play a role in the chromatin organization by an interplay with histones (39, 40). Interactions of HMGA1 with transcription factors will be later discussed in the “DNA, protein and RNA interactions” paragraph.
Post-Transcriptional Regulation
The functional activity of HMGA1 relies on a complex and fine regulation of its own expression. This includes a series of regulatory elements that act within the 3′UTR HMGA1 mRNA (41). In addition, recent studies indicate that many microRNAs could bind the HMGA1 3′UTR mRNA, causing its degradation or inhibiting its translation (42). Down-regulation of some of these microRNAs—miR15, miR-16, miR26, miR-196a-2, and let-7—have been described to cause increased levels of HMGA1 in pituitary adenomas (42). Interestingly, some of the same miRNAs involved in tumorigenesis also play a role in metabolism. For example, miR-26a has been shown to target key regulators of insulin signaling and glucose metabolism in the liver, while its impairment is associated with hepatic oncogenesis and metabolic disorders (43).
Another peculiar mechanism of HMGA1 post-transcriptional regulation refers to the role of processed pseudogenes as potential regulators of mRNA stability/degradation (44). Processed pseudogenes are non-functional copies of normal genes generated by a process of mRNA retrotransposition. Compared with homologous normal genes, they lack introns and contain single nucleotide substitutions, deletions, insertions, and residues of poly (A) tails (45, 46). Human genome includes thousands of pseudogenes, accumulated during evolution (45, 46). However, although our actual knowledge about the real biological role of pseudogenes is still limited, increasing evidences exist, supporting a functional significance for these macromolecules (47). So far, eight HMGA1 pseudogenes have been described (48). Some of them act on the stability of HMGA1 mRNA or prevent miRNAs from targeting HMGA1 mRNA, thereby behaving as competing endogenous RNAs (ceRNAs). The RNA encoded by one of them, the HMGA1-p pseudogene, by effectively competing for the trans-acting cytoplasmic protein αCP1, accelerates the degradation of mRNA from the homolog normal gene, thereby reducing the longevity of HMGA1 mRNA transcript (44). Some pseudogenes display aminoacid sobstitutions at the level of specific aminoacid residues that in the native HMGA1 are subjected to post-translational modifications involved in the modulation of HMGA1's activities. An intriguing possibility is that, if expressed, these proteins could compete with the native HMGA1, escaping the modulatory effects of these post-translational modifications that strongly impact on HMGA1 ability for chromatin remodeling and protein-protein interactions (48).
Post-Translational Modifications (PTMs)
A variety of extracellular and intracellular signals can induce different PTMs on HMGA1 protein, which influence its ability to interact with either DNA or proteins, thereby affecting HMGA1 nuclear function (8, 49). PTMs include phosphorylation, methylation, and acetylation (8, 12, 49, 50). Generally, increased level of HMGA1 phosphorylation reduces DNA-binding affinity and transcriptional activation, and this status correlates with an elevated residence time of the HMGA1a isoform in the repressed inactive heterochromatin, rather than in the active euchromatin (35). In detail, phosphorylation can affect different serine (Ser) and threonine (Thr) residues. The phosphorylation at Thr-52 and Thr-77 by the cell-cycle dependent kinase cdc2 results in decreased binding of HMGA1a to DNA (51, 52). The same effect is produced following the phosphorylation of HMGA1a at Ser-43 and Ser-63 by the protein kinase C (PKC) pathway (53). The acidic C-terminal tail of HMGA1 is constitutively phosphorylated. At this level, the protein kinase CK2 catalyzes the phosphorylation of Ser-98, Ser-101, and Ser-102 of HMGA1a (54), while it has been demonstrated that phosphorylation of the C-terminal tail has structural consequences on HMGA1 compactness (55). HMGA1 is also susceptible to acetylation on several lysine residues (49). It has been reported that acetylation by the histone acetyltransferases CBP (CREB-binding protein) and p300/CBP associated factor (PCAF)/GCN5 have a role in the kinetics of enhanceosomes assembly/disassembly. Acetylation at Lys-64, by CBP, destabilizes the enhanceosome formation on the human interferon beta (IFN-beta) gene, leading to transcriptional inhibition of this gene; on the contrary, PCAF-induced acetylation at Lys-70 increased the transcription of the IFN-beta gene through enhanceosome stabilization (56). HMGA1 is also methylated at several residues located exclusively within the AT-hook motifs. Although the significance of this type of PTM is still largely unknown, methylation at the AT-hook motifs indicate a potential role for methylation in regulating HMGA1-DNA binding activity (12, 49, 57–59).
Tissue Expression of HMGA1
HMGA1 is highly expressed during embryogenesis, suggesting its critical role during the embryonic development (60). It is also highly expressed in adult stem cells, including intestinal and hematopoietic stem cells (61). The important role of HMGA1 at these levels is supported by phenotypic studies in Hmga1-knockout mice, indicating that mice lacking HMGA1 develop cardiomyopathy, aberrant hematopoiesis, and defective pancreatic beta cell development (19, 62). Also, HMGA1 plays a role in adipogenesis, and myogenesis, although its levels decrease before terminal cell differentiation (63, 64). Vice versa, overexpression of HMGA1 is found in a wide range of human cancers, including prostate, thyroid, colon, breast, lung, bladder, pancreas, stomach, kidney, uterus, and hepatocellular carcinomas, as well as non-melanoma skin cancers, and hematopoietic malignancies (10, 14, 65, 66). In some of these cancers, HMGA1 expression strongly correlates with an advanced stage, the metastatic potential and reduced survival. Poorer prognosis is due to the fact that HMGA1 promotes the transcription of many genes involved in tumor growth, invasion, migration, neoangiogenesis, epithelial-mesenchymal transition and cancer metastasis (8, 10, 67–71). Nevertheless, it has been also reported that HMGA1 can have anti-oncogenic effects, depending on the cellular context (72). This bivalent function proves the relevance of HMGA1 in both physiological and pathological conditions and explains the reason why HMGA1 requires a fine-tuned spatio-temporal expression and activity modulation.
DNA, Protein and RNA Interactions
HMGA1 regulates cell cycle-related chromosomal changes, DNA replication and repair, and molecular chaperoning (11, 38, 73). Also, by inducing a more open chromatin state, HMGA1 assists gene transcription (8, 74). By itself, HMGA1 has no intrinsic transcriptional activity; rather, it can participate in the transactivation of gene promoters through mechanisms that facilitate the assembly and stability of stereospecific DNA-protein complexes, termed “enhanceosomes,” that drive gene transcription (Figure 2) (75–77). HMGA1 performs this task by interacting with a large variety of activating or inhibiting transcription factors and orchestrating their assembly on promoter regions. The enhanceosome model provides one of the best-understood examples of how HMGA1 can interact with other transcription factors, leading to a highly specific activation of gene transcription in higher eukaryotes (76, 77). In this sense, enhanceosome formation over the IFN-beta promoter (75, 77) or the INSR promoter (15) is of paradigmatic importance. In relation to protein-protein interactions, the HMGA1 network has been reported to be very wide (78, 79), as HMGA1 has been shown to physically and functionally interact with many ubiquitous and tissue-specific transcription factors. Although the repertoire is far for being complete, the interplays of HMGA1 with the transcription factors p53, NF-κB and ATF-2/c-Jun, C/EBP beta and Sp1, as well as PDX-1 and MafA have been studied in depth, being crucial, for example, in the transcriptional regulation of Bcl 2 (80), IFN-beta (75), INSR (15), and insulin (18) genes, respectively. Besides C/EBPbeta and PDX-1, among the nuclear factors known to be involved in glucose metabolism, HNF-1alpha has been shown to bind and cooperate with HMGA1 in the regulation of the IGFBP1 and IGFBP3 promoters (16), whereas the peroxisome proliferator-activated receptor-gamma (PPARgamma), which reduces INSR gene transcription, failed to show any direct interaction with HMGA1 in this context (81). The potential interplay of HMGA1 with other nuclear metabolic sensors, such as ChREBP, SREBP1-c, FoxO family members, and PGC-1, instead, has not yet been investigated.
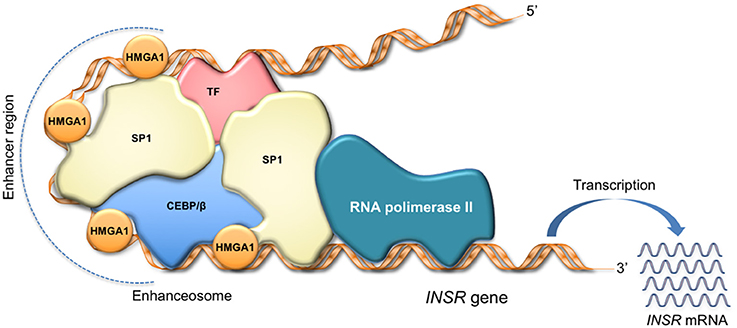
Figure 2. HMGA1 as facilitator of enhanceosome formation. HMGA1 binds to the enhancer region through its DNA-binding domain, while interacting with other transcription factors in the promoter, forming a multiprotein-DNA complex that enhances gene transcription. The scheme refers to activation of the INSR gene.
Emerging lines of evidence indicate that HMGA1 interacts also with different RNAs. The first evidence supporting a specific RNA affinity of HMGA1 has been reported in studies pointing to a role of HMGA1 during the exon skipping of presenilin-2 pre-mRNA, which resulted in the production of a deleterious protein isoform causing sporadic Alzheimer's disease (82). More recently, a new interaction has been reported between HMGA1 and the nuclear non-coding 7SK RNA (83), a factor which negatively affects Polymerase II transcription elongation and influences HMGA1 biological functions by competing for the first AT-hook binding with DNA (84). Also, through the first AT-hook, HMGA1 has been shown to interact with the origin recognition complex, thus playing a key role in DNA replication (85).
HMGA1 and Glucose Homeostasis
HMGA1 in the Regulation of the INSR Gene
The peptide hormone insulin exerts its biological effects by binding to the INSR, a specific tyrosine kinase receptor glycoprotein located in the plasma membrane of insulin target cells. As a key regulator of insulin action, many studies have explored the INSR gene (86, 87). Nuclear binding proteins that recognized the INSR promoter were initially identified during muscle and adipose cell differentiation in the context of two AT-rich sequences of the regulatory region of the INSR gene (88). Using conventional chromatographic purification methods, combined with electrophoretic mobility shift assays and immunoblots, these proteins were identified as HMGA1, while reporter gene analysis findings showed that HMGA1 is required for proper transcription of the INSR gene (89). Further studies demonstrated that transcriptional activation of the human INSR gene required the assembly of a transcriptionally active multiprotein-DNA complex, including the ubiquitously expressed transcription factor Sp1 and C/EBPbeta, in addition to HMGA1 (15). As HMGA1 physically interacts with these proteins and facilitates their binding to DNA, functional integrity of this protein-DNA complex is necessary for full transactivation of the INSR gene promoter by Sp1 and C/EBPbeta (15). In support of the role of HMGA1 in INSR gene transcription, in vitro investigations in beta-pancreatic cells demonstrated that sustained hyperglycemia impaired HMGA1 expression, a condition affecting INSR content in beta cells and, thus, insulin secretion (90).
These observations, which were based mainly on in vitro analyses, were substantiated by studies in vivo, in Hmga1-knockout mice, in which a marked decrease in INSR gene and protein expression was observed in the major targets of insulin action, contributing to a phenotype characteristic of human type 2 diabetes (19). Studies in patients with low INSR as a consequence of defects in HMGA1 will be discussed in the “HMGA1 in insulin resistant diseases” section, while discrepancies between human and mouse phenotypes will be discussed later.
Subsequent investigations revealed that other transcription factors, such as the activating protein 2 (AP2) and PPARgamma, can influence INSR gene transcription in a variety of cell types (81, 91), while studies in cultured myocytes aimed at deciphering the mechanisms by which free fatty acid (FFA) contribute to the development of insulin resistance and type 2 diabetes, showed that FFA can impair INSR expression and insulin signaling and sensitivity by affecting HMGA1 (92–95). In particular, FFA induce phosphorylation and nuclear translocation of the protein kinase C epsilon type (PKCepsilon). In the nucleus, PKCepsilon phosphorylates HMGA1 and downregulates its expression by deactivating the transcription factor Sp1, thereby attenuating INSR gene expression by direct and indirect mechanisms, which in turn compromise insulin action and sensitivity (92–95). Furthermore, recent studies addressed the mechanisms linking the downregulation of the histone methyltransferase G9a/EHMT2 with insulin resistance in murine models and in cultured human hepatic cells. G9a/EHMT2 upregulates HMGA1 and G9a knockdown hepatic cells showed reduced INSRs, whose expression was restored by overexpressing HMGA1 (32). Importantly, restoration of G9a levels in db/db mice improved hepatic insulin signaling and ameliorated hyperglycemia and hyperinsulinemia at least in part by upregulating HMGA1 (32). Altogether, these findings clearly indicate that HMGA1 is a crucial component of the insulin signaling pathway, and plays an important role in INSR gene expression in insulin target tissues.
HMGA1 in the Transcription of the Insulin (INS) Gene
A direct role of HMGA1 in insulin production and pancreatic islet development and beta cell function has been postulated starting from the observation that, compared to wild-type littermates, Hmga1-knockout mice showed decreased insulin secretion and reduced beta cell mass (19). On the other hand, a functional interplay between HMGA1 and the homeodomain transcription factor PDX-1 (a key regulator of pancreatic islet development and beta cell function) has been shown previously in the context of the INS gene and other pancreatic islet-specific genes (96). The possibility for HMGA1 to play a role also in this context, was substantiated by the fact that binding of PDX-1 to the INS gene promoter was reduced in Hmga1-knockout mice (19). Also, a protein-protein interplay between PDX-1, the neurogenic differentiation 1 (NeuroD1), and HMGA1 had been previously described at the level of the rat insulin mini-enhancer element E2A3/4 (96). Subsequent studies added more details in our understanding of the INS gene regulation. In the insulin-secreting beta-cell line INS-1, as demonstrated by chromatin immunoprecipitation experiments, glucose stimulated binding of HMGA1 to the INS promoter, resulting in a significant increase in insulin production and secretion (18). Coherently, when INS-1 cells were treated with HMGA1 siRNA, a significant reduction in glucose-induced insulin secretion was observed, thereby confirming the importance of HMGA1 in this scenario (18). Even in the absence of HMGA1-DNA binding sites on the INS gene promoter, the assembly of a transcriptionally active multiprotein-DNA complex involving HMGA1, PDX-1 and the transcription factor MafA, was required for proper transcription of both human and mouse INS genes (18). In line with this observation, the deficit in HMGA1 compromised binding of PDX-1 and MafA to the INS promoter, thereby imparing INS gene transcription and glucose-induced insulin secretion (18). However, given that substantial interspecies differences exist in pancreatic islet development and function (19, 97, 98), any parallelism between human and mouse at this level must be considered carefully and further details on this should be provided. For example, based on our recent observations highlighting a novel relationship between HMGA1 and FoxO1 (99), further investigation in this field could deliver deeper information on the possibility that an interplay among HMGA1 and FoxO1 can be a component of this regulation, as an overarching role of FoxO1 in pancreatic beta cell function has been already outlined (6, 100–103).
HMGA1 as a Downstream Target of the INSR Signaling Pathway
Besides being required for both insulin and INSR gene transcription, HMGA1 plays an important role in the regulation of the insulin signaling cascade (98). The gluconeogenic genes phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase), as well as the IGFBP1 gene (which plays a glucose counterregulatory role by preventing the potential hypoglycemic effects of IGF1) are known to be inhibited by insulin (for example, after a meal). As reported in vitro, in hepatic cells, insulin, via the phosphatidylinositol 3-kinase (PI-3K)/Akt pathway, and the Akt/protein kinase CK2 signaling, exerts its transcriptional repression of these genes by inducing HMGA1 phosphorylation (104). In fact, by triggering the phosphorylation of HMGA1 at the level of the three serine residues, Ser-98, Ser-101, and Ser-102, insulin promotes the detachment of HMGA1 from promoter target genes and its corresponding nuclear localization in the inactive heterochromatin. Thus, HMGA1 acts as a downstream modulator of insulin action, and is an important key player in insulin and nutritionally-regulated transcription of genes involved in glucose metabolism and homeostasis. Also, as phosphorylation/dephosphorylation of HMGA1 can act as a molecular switch for deactivating or activating INSR protein expression during fed and fasting conditions, respectively (104), HMGA1 can function as a key feedback regulator of insulin signaling during the fasting and refeeding periods.
Given that the role of the transcription factor FoxO1 in the control of gluconeogenesis is well established (6, 105), as for the regulation of pancreatic beta cell function, a cross-talk between HMGA1 and FoxO1 can be hypothesized also in this case and investigated in future studies.
HMGA1 and Insulin-Independent Metabolic Signaling
Data from the Hmga1-knockout mouse model evidenced a complex metabolic phenotype, in which peripheral insulin hypersensitivity paradoxically coexisted with a condition of impaired glucose tolerance and overt diabetes (19), thus supporting the existence of alternative insulin signaling pathways ensuring peripheral glucose utilization and disposal by insulin-independent mechanisms. Coexistence of insulin hypersensitivity in peripheral tissues with insulin resistance has been observed before in liver of ob/ob mice (106) and in Cdk4 knockout mice with defects in pancreatic beta cell development and insulin secretion (107). The possibility that the activation of insulin-independent mechanisms aimed at ameliorating glucose disposal under disadvantageous metabolic conditions, like those affecting Hmga1-knockout animals, is underlined by the identification of novel biochemical pathways involving the cAMP-HMGA1-RBP4 system (17, 108) and the HMGA1-IGF1/IGFBP system (16), whose activation may play a role in glucose homeostasis in both rodents and humans. Further studies in vitro confirmed that HMGA1 has a role in the activation of both IGFBP1 and IGFBP3 gene transcription (16, 109). Therefore, it is plausible that under physiological circumstances (e.g., during fasting), in which HMGA1 increases (17, 108), the increment of both IGFBP1 and IGFBP3 helps in limiting IGF1 bioavailability, thereby preventing peripheral glucose uptake by insulin-independent mechanisms.
Role of HMGA1 During Fasting
The counter-regulatory hormone glucagon, which acts in opposition to insulin, binds its cognate G-protein coupled receptor on liver cell membrane and stimulates the transmembrane adenylyl cyclase to produce cyclic AMP (cAMP) as second messenger. This, in turn, leads to the activation of protein kinase A (PKA), which, among many other proteins, phosphorylates the Cyclic AMP Responsive Elements Binding Protein (CREB) transcription factor (110, 111). The final event is the assembly of a functional transcriptional machinery on the promoter regions of gluconeogenic genes (112). Some observations in cultured hepatic cells indicate that cAMP also increases HMGA1 protein expression (17, 108). Consistently, Hmga1 RNA levels were significantly increased in liver of mice following systemic administration of glucagon.
In agreement with the observations mentioned above, upregulation of FoxO1 expression via the glucagon-cAMP-PKA signaling has been reported in liver of fasting mice to maintain fasting euglycemia (113). Thus, upregulation of HMGA1 during fasting (when glucagon peaks) may contribute to the mechanisms necessary to prevent hypoglycemia, through activation of FoxO1 (99) and gluconeogenic gene expression. The opposite occurs after a meal, when insulin peaks, and glucagon declines (Figure 3). In this metabolic scenario, inactivation of HMGA1 by insulin-induced HMGA1 phosphorylation, by causing the detachment of FoxO1 from DNA and its nuclear exclusion, inhibits gluconeogenesis and contributes to restoration of postprandial euglycemia (Figure 3).
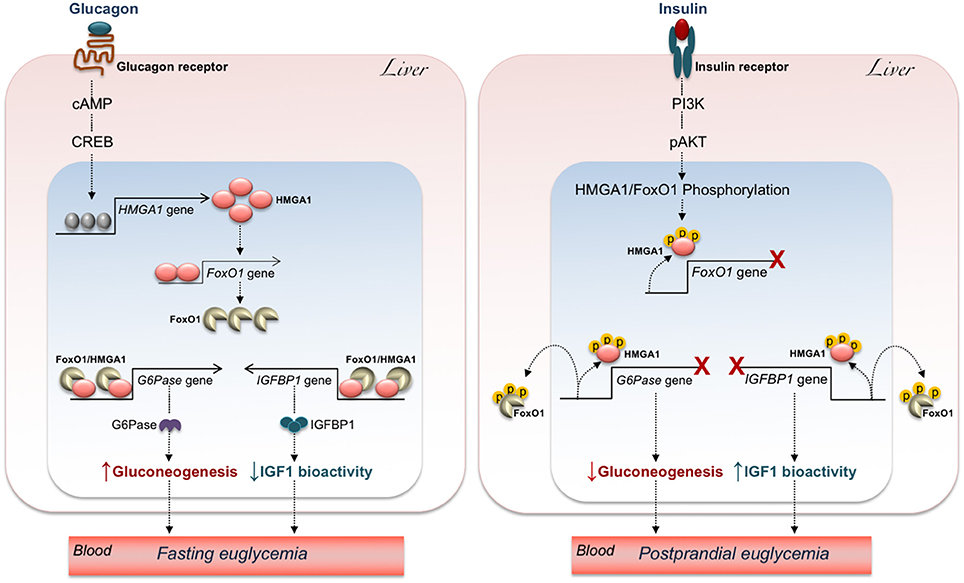
Figure 3. Hypothetical mechanisms underlying the effects of HMGA1/FoxO1 on glucose homeostasis. The increase of glucagon during fasting (Left) turns on the cAMP-PKA-CREB pathway, allowing HMGA1 gene activation and protein expression. In turn, HMGA1 activates the FoxO1 gene and promotes transactivation of G6Pase and IGFBP1 promoters by FoxO1, thereby maintaining fasting euglycemia through elevation of hepatic gluconeogenesis and attenuation of IGF1 bioactivity. Under feeding conditions (Right), binding of insulin to its receptor initiates a series of events culminating in the sequential phosphorylation (p) of HMGA1 and FoxO1, which reduces FoxO1 gene expression, promotes the detachment of FoxO1 from G6Pase and IGFBP1 gene promoters, and leads to FoxO1 nuclear exclusion, thereby ensuring postprandial euglycemia through inhibition of hepatic gluconeogenesis and augmentation of IGF1 bioactivity.
Kidney exerts an important role in gluconeogenesis, being responsible of approximately 15% of glucose production (1). In a recent paper, after 3-day fasting or restriction diet in mice, renal gene expression, assayed by microarray, demonstrated, among other transcription factors, an increment in HMGA1 expression (114). These findings are coherent with previous findings in the liver, in which an effect of HMGA1 on gluconeogenic genes has been described (104).
Another glucose metabolism-related gene, which has been shown to be regulated by HMGA1, is the one encoding for the retinol binding protein 4 (RBP-4). RBP-4 is mostly produced by the liver, although adipose tissue also contributes, and plays a role in systemic insulin resistance. RBP-4 expression in fat and its levels in blood inversely correlated with the adipose-specific glucose transporter GLUT-4 in obesity and type 2 diabetes (115). In vitro studies with human HepG2 and murine Hepa 1 hepatoma cells have demonstrated that HMGA1 binds to and increases transcription of the RBP-4 gene promoter both in basal and in cAMP-induced conditions (17, 108), while in vivo, in whole mice, injection of glucagon, by inducing increased intracellular cAMP, activates both HMGA1 and RBP-4 expression in liver and fat. Consequently, under physiological circumstances, this loop has an important relapse in conditions of low glucose availability, in which intracellular cAMP increases. In this scenario, HMGA1, by inducing FoxO1-dependent gluconeogenic genes, and by upregulating RBP-4, helps providing glucose to tissues/organs with high energy demands, such as the brain. Interestingly, the brain-type GLUT-3 facilitative glucose transporter has also been shown to be transcriptionally regulated by HMGA1 (116), thereby supporting further the relevance of this factor in multiple settings of energy demand.
HMGA1 in Adipose and Muscle Cell Differentiation and Function
Both muscle and fat play relevant roles in maintaining euglycemia. In this regard, previous studies from our group demonstrated that INSR expression is reduced in muscle and adipose tissues from both Hmga1-knockout mice and in individuals with reduced levels of HMGA1 (19, 44).
The physiological role of HMGA1 in adipogenesis has been investigated in vitro and in vivo (63, 117), and a critical role of HMGA1 in adipocytic cell growth and differentiation has been demonstrated in murine 3T3-L1 adipocytes (117). Also, HMGA1 may exert a negative role in adipose cell growth by balancing the effects of the cognate HMGA2 protein, another member of the HMGA family (118). Indeed, transgenic mice, overexpressing HMGA1 in both white and brown adipose tissues, showed reduced fat mass and impaired adipogenesis with respect to wild-type mice (63), and were protected against high-fat diet induced obesity and systemic insulin resistance, thus supporting the role of HMGA1 in the maintenance of glucose homeostasis.
In addition to RBP-4, whose regulation has been discussed, other adipokines have been demonstrated to be under the control of HMGA1. In 3T3-L1 adipocytes, visfatin, an insulin-mimetic factor, is transcriptionally regulated by HMGA1 in cooperation with the hypoxia-inducible factor 1, HIF-1 (119), whereas leptin, an adipokine involved in glucose and fatty acid metabolism, is regulated by HMGA1 through a non-canonical mechanism that spares HMGA1 direct binding to DNA and requires the physical interaction and functional cooperation of HMGA1 with the nuclear factor C/EBPbeta (117).
Several reports have also indicated that HMGA1 plays a role in muscle tissue, and HMGA1 is present in mouse C2C12 cultured muscle cells, in which HMGA1 overexpression increases cell proliferation and prevents myotube formation (64). Downregulation of HMGA1 is an early and necessary step for the progression of the myogenic program. In this regard, it has been reported that miR-195/497, by binding the HMGA1 3′UTR, reduces HMGA1 protein abundance in C2C12 cells, thus promoting muscle cell differentiation (120).
The Lin28/let-7 pathway, whose implication in cancer is well known (121), displays also a role in the regulation of glucose metabolism. In fact, mice overexpressing Lin28a and Lin28b show an insulin-sensitized state, with protection against high-fat diet induced diabetes (122). In contrast, muscle-specific loss of Lin28a and overexpression of let-7 resulted in insulin resistance and impaired glucose tolerance (122). As Lin28a directly promotes HMGA1 translation (123), it has been postulated that in muscle-specific Lin28a knockout mice, insulin resistance is, at least in part, due to reduced HMGA1 levels and consequently impaired INSR expression (122). If confirmed in further studies, the relationship between HMGA1 and the Lin28/let-7 pathway may indicate another molecular mechanism for the involvement of HMGA1 in mammalian glucose metabolism.
HMGA1 in Insulin Resistant Diseases
Syndromes of Severe Insulin Resistance
Insulin resistance, defined as a subnormal biological response to the glucose-lowering effect of insulin, is a characteristic of many common disorders, including type 2 diabetes, the metabolic syndrome, fatty liver disease, and obesity (124–126). However, severe forms of insulin resistance may occur as uncommon syndromes, either congenital or acquired, in patients with impaired INSR signaling or lipodistrophy (127, 128). Congenital disorders include the Type A syndrome of insulin resistance, the Rabson-Mendenhall syndrome, leprechaunism, and some syndromes of generalized or partial lipodystrophy. Type A syndrome is an autosomal dominant disorder characterized by the triad of hyperinsulinemia, acanthosis nigricans, and ovarian hyperandrogenism (127–129). Hyperglycemia is not always present at diagnosis. Female patients appear lean and without lipodystrophy, even if a variant of this syndrome has been reported in obese women (130, 131). Male patients may initially exhibit acanthosis nigricans and hypoglycemia, while overt diabetes may not occur until the fourth decade or later (128). In some cases, the syndrome is caused by heterozygous mutations affecting the tyrosine kinase domain of the INSR; however, only 10–25% of female with Type A syndrome have mutations in the INSR gene (132).
As a step toward understanding the molecular basis of regulation of the INSR gene, a nuclear binding protein that specifically interacted with, and activated the INSR gene promoter, was identified previously, during muscle and adipose cell differentiation (88). Later, this DNA binding protein was identified as HMGA1, and its expression was markedly reduced in two unrelated patients with either the Type A syndrome or the common form of type 2 diabetes, in whom cell surface INSRs were decreased and INSR gene transcription was impaired despite the fact that the INSR genes were normal, thus indicating defects in INSR gene regulation (15, 89, 133). Subsequent investigations in both these patients allowed the identification of a novel genetic variant, c.*369del, in the 3′ non-coding region of HMGA1 mRNA, which resulted in a decreased mRNA half-life and reduced HMGA1 protein levels. In other two patients (mother and daughter) with the type A syndrome of insulin resistance, a hemizygous deletion of the HMGA1 gene was also identified (19). Restoration of HMGA1 protein expression in these subjects' cells enhanced INSR gene transcription and restored cell-surface INSR protein expression, thus confirming that defects in HMGA1, by decreasing INSR protein production may indeed induce severe insulin resistance (19).
The mechanistic linkage between HMGA1, insulin resistance and certain less common forms of type 2 diabetes has been further supported by a study in two diabetic patients, in whom aberrant expression of a pseudogene for HMGA1, HMGA1-p, caused destabilization of HMGA1 mRNA with consequent loss of INSR and generation of insulin resistance (44).
These findings demonstrate, therefore, that HMGAl is necessary for proper expression of the INSR. Further, they provide evidence for recognizing “HMGA1opathy” as a novel diabetic subphenotype (134).
Type 2 Diabetes
In its common form, type 2 diabetes is a heterogeneous complex disease in which concomitant insulin resistance and beta-cell dysfunction lead to hyperglycemia (135, 136). From a pathogenetic point of view, both predisposing genetic factors and precipitating environmental factors contribute importantly to the development of the disease (135, 136). So far, about 100 gene variants have been associated with an increased risk for type 2 diabetes (137). Most of these variants are presumed to negatively affect pancreatic beta-cell function and insulin secretion, while some of them appear to impact peripheral insulin sensitivity, thereby impairing tissue glucose uptake (138).
As it concerns HMGA1, on the basis of its involvement in insulin resistance, a role for this nuclear factor in type 2 diabetes has also been postulated and studies in this direction have been performed by us and others (20, 139–141). In particular, by sequencing the entire HMGA1 gene in a large number of diabetic patients and healthy controls, four variants of the HMGA1 gene were identified by us in approximately 10% of diabetics (20). In circulating monocytes and cultured lymphoblasts from diabetic patients carrying these variants, HMGA1 and INSR expressions were markedly decreased and these defects were corrected by transfecting HMGA1 cDNA (20). The most frequent HMGA1 rs139876191 variant (previously named rs146052672), was significantly higher in type 2 diabetic patients from three populations of white European ancestry: Italian, American and French populations (20). Although not replicated in a heterogeneous French population (139), the rs139876191 variant was later associated with type 2 diabetes among Chinese (140) and Americans of Hispanic ancestry (141), thus providing evidence for the implication of the HMGA1 gene locus as one conferring a cross-race risk for the development of type 2 diabetes. More recently, the credibility of an association between the HMGA1 rs139876191 variant and type 2 diabetes was confirmed also in a transethnic meta-analysis that included all available published articles examining this association in different populations (142). Like other polymorphisms located at the intron/exon boundaries, functional analysis of the HMGA1 rs139876191 revealed that this variant is functional and exhibits a dominant negative effect (143).
Metabolic Syndrome
The metabolic syndrome is a common multicomponent disorder, which is associated with increased risk for type 2 diabetes, cardiovascular disease (CVD), and nonalcoholic fatty liver disease (144, 145). As insulin resistance plays a pivotal role in the pathophysiology of metabolic syndrome (125, 146), the impact of HMGA1 has been investigated in two large Italian and Turkish populations, both affected by metabolic syndrome (21). Findings indicated that the HMGA1 rs139876191 variant was significantly associated with metabolic syndrome in both populations, in which this association occurred independently of type 2 diabetes, thus lending credence to the hypothesis that this variant may independently associate with other insulin resistance-related traits. Consistent with this assumption, a strong association of the rs139876191 variant with certain metabolic syndrome-related traits (i.e., high fasting plasma glucose, high body mass index, low HDL-Cholesterol, reduced insulin sensitivity) was observed in affected individuals of European and Hispanic-American ancestry, further supporting the notion that defects that negatively affect HMGA1 can play a role in the pathogenesis of metabolic syndrome and other insulin-resistance related conditions (21, 141). Interestingly, as CVD is a major risk for both type 2 diabetes and the metabolic syndrome, the association of HMGA1 rs139876191 variant with acute myocardial infarction, independently from diabetes and other cardiovascular risk factors, has been reported previously (147, 148), suggesting that HMGA1 may also represent a novel genetic marker of cardiovascular risk.
Discrepancy Between Human and Mouse Phenotypes With HMGA1 Loss-of-Function
An important issue that deserves to be discussed is to which extent Hmga1-knockout mice reflect findings in humans. Although in the broader context of glucose metabolism similarities between the two species are well known (i.e., in both species, insulin and glucagon represent key effectors in the control of glucose homeostasis), differences are likewise described in relation to pancreas development and, in particular, to the late stages of beta cell differentiation and susceptibility to pancreatic beta-cell injury (97, 149, 150). At a molecular level, previous known beta cell species-specificities in ion channel components and membrane transporters, as well as in insulin secretion, have been recently further enriched by data from transcriptome profiles in single human and murine beta cells (150, 151), while evidence of heterogeneity of pancreatic beta cells has been proved to occur in both humans and mice (152). However, interspecies differences do not exclude that in some instances, like in the case of lack of the KCNJ11 gene, the mouse phenotype well recapitulates human neonatal diabetes (153).
Focusing on HMGA1 loss-of-function, three biochemical and metabolic conditions are common to humans and mice: reduced insulin receptor expression, impaired insulin signaling, and insulin resistant diabetes. Instead, insulin levels in humans (hyperinsulinemic) and mice (hypoinsulinemic) are clearly discrepant (19). In fact, in Hmga1-knockout mice, both beta cell mass and insulin secretion are impaired. Differences in pancreatic islet ontogenesis and differentiation, as well as differences in nongenetic environmental elements and susceptibility to genetic modifiers, have been postulated to explain these dissimilarities (19). On the other hand, Hmga1-knockout mice have proved to be insulin hypersensitive, despite the deficit in INSRs. This apparent paradox suggests the presence of adaptive, compensatory mechanisms, in mice, that include the already described cAMP-HMGA1-RBP4 pathway and the IGFBP-1/IGF1 system. Although the latter has proved to be more effective to reduce glycemia in mice than in humans (154), the importance of these systems in both species still deserves further investigations. As an example, recent findings obtained in genetically engineered mice with a specific deletion of the RBP4 gene in the liver, indicating that circulating RBP4 derives mainly from hepatocytes (155), need to be confirmed in humans.
Conclusions
At present, HMGA1 is known to be involved in multiple biological processes. Based on the above-mentioned findings, among the many tasks that HMGA1 can perform, there is its role in the transcriptional regulation of gene and gene networks involved in INSR signaling and glucose metabolism. In this review, we provided an overview of the major contributions that have been made in this area over the last years. Overall, the data obtained so far well support the role of HMGA1 in the regulation of genes implicated in the maintenance of glucose homeostasis and metabolic control, providing new insight into the regulation of glucose metabolism and disposal. Clinically, the importance of HMGA1 gene variability in glucose metabolism is emphasized in a wide range of clinical conditions ranging from rare insulin resistance syndromes to type 2 diabetes and the metabolic syndrome. Besides, being a multifunctional protein, HMGA1 may constitute a molecular link between metabolism and other distinct biological processes, including cell proliferation, and differentiation, viability, autophagy, cell cycle, apoptosis, that need to be sustained by cell energy.
New insights may come from epigenetic studies, including miRNAs, whose common role in both malignancy and metabolism is recently emerging. On the other hand, disentangling the pleiotropic nature of HMGA1 by the identification of distinct molecular partners and networks uniquely implicated in metabolism, still represents a big challenge. A contribution could come from studies on the relationship between HMGA1 and the yet unexplored nuclear metabolic sensors.
Apart from the intrinsic biological and clinical interest of these findings, a deeper understanding of the mechanisms that regulate glucose metabolism in health and disease is of importance for the development of more effective therapies. To fill the gap of our knowledge in this regard, future directions based on the omics-related technologies, combined with bioinformatic tools, can help identify novel proteins and their networks, as well as genes and gene products regulated by, or interacting with HMGA1.
To the best of our knowledge, this is the first review article exclusively dedicated to the role of HMGA1 in this context, and we hope that it will serve as a quickly accessible reference in this important clinical field.
Author Contributions
EC and DF prepared the first draft of the manuscript. RS, SP, MG, and GM contributed to critical revision of the manuscript. BA and FB were involved in the literature search. AB critically revised and edited the final version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor is currently co-organizing a Research Topic with one of the authors AB, and confirms the absence of any other collaboration.
References
1. Gerich JE. Physiology of glucose homeostasis. Diabetes Obes Metab. (2000) 2:345–50. doi: 10.1046/j.1463-1326.2000.00085.x
2. Dashty M. A quick look at biochemistry: carbohydrate metabolism. Clin Biochem. (2013) 46:1339–52. doi: 10.1016/j.clinbiochem.2013.04.027
3. Desvergne B, Michalik L, Wahli W. Transcriptional regulation of metabolism. Physiol Rev. (2006) 86:465–514. doi: 10.1152/physrev.00025.2005
4. Kitamura T. The role of FOXO1 in beta-cell failure and type 2 diabetes mellitus. Nature Rev Endocrinol. (2013) 110:1839–47. doi: 10.1038/nrendo.2013.157
5. Nakae J, Park B-C, Accili D. Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a Wortmannin-sensitive pathway. J Biol Chem. (1999) 274:15982–5. doi: 10.1074/jbc.274.23.15982
6. Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell (2004) 117:421–6. doi: 10.1016/S0092-8674(04)00452-0
7. Cleynen I, Van and de Ven WJ. The HMGA proteins: a myriad of functions (Review). Int J Oncol. (2008) 32:289–305. doi: 10.3892/ijo.32.2.289
8. Reeves R, Beckerbauer L. HMGI/Y proteins: flexible regulators of transcription and chromatin structure. Biochim Biophys Acta (2001) 1519:13–29. doi: 10.1016/S0167-4781(01)00215-9
9. Wisniewski JR, Schwanbeck. High mobility group I/Y: multifunctional chromosomal proteins causally involved in tumor progression and malignant transformation. Int J Mol Med. (2000) 6:409–19. doi: 10.3892/ijmm.6.4.409
10. Sumter TF, Xian Huso T, Koo M, Chang YT, Almasri TN, et al. The high mobility group A1 (HMGA1) transcriptome in cancer and development. Curr Mol Med. (2016) 16:353–93. doi: 10.2174/1566524016666160316152147
11. Sgarra R, Zammitti S, Lo Sardo A, Maurizio E, Arnoldo L, Pegoraro S, et al. HMGA molecular network: from transcriptional regulation to chromatin remodeling. Biochim Biophys Acta (2010) 1799:37–47. doi: 10.1016/j.bbagrm.2009.08.009
12. Sgarra R, Diana F, Rustighi A, Manfioletti G, Giancotti V. Increase of HMGA1a protein methylation is a distinctive characteristic of leukaemic cells induced to undergo apoptosis. Cell Death Differ. (2003) 10:386–9. doi: 10.1038/sj.cdd.4401184
13. Diana F, Sgarra R, Manfioletti G, Rustighi A, Poletto D, Sciortino MT, et al. A link between apoptosis and degree of phosphorylation of high mobility group A1a protein in leukemic cells. J Biol Chem. (2001) 276:11354–61. doi: 10.1074/jbc.M009521200
14. Resar LM. The high mobility group A1 gene: transforming inflammatory signals into cancer? Cancer Res. (2010) 70:436–9. doi: 10.1158/0008-5472.CAN-09-1212
15. Foti D, Iuliano R, Chiefari E, Brunetti A. A nucleoprotein complex containing Sp1, C/EBP beta, and HMGI-Y controls human insulin receptor gene expression. Mol Cell Biol. (2003) 23:2720–32. doi: 10.1128/MCB.23.8.2720-2732.2003
16. Iiritano S, Chiefari E, Ventura V, Arcidiacono B, Possidente K, Nocera A, et al. The HMGA1-IGF-I/IGFBP system: a novel pathway for modulating glucose uptake. Mol Endocrinol. (2012) 6:1578–89. doi: 10.1210/me.2011-1379
17. Bianconcini A, Lupo A, Capone S, Quadro L, Monti M, Zurlo D, et al. Transcriptional activity of the murine retinol-binding protein gene is regulated by a multiprotein complex containing HMGA1, p54 nrb/NonO, protein-associated splicing factor (PSF) and steroidogenic factor 1 (SF1)/liver receptor homologue 1 (LHR-1). Int J Biochem Cell Biol. (2009) 41:2189–203. doi: 10.1016/j.biocel.2009.04.011
18. Arcidiacono B, Iiritano S, Chiefari E, Brunetti FS, Gu G, Foti DP, et al. Cooperation between HMGA1, PDX-1, and MafA is Essential for Glucose-Induced Insulin Transcription in Pancreatic Beta Cells. Front Endocrinol. (2015) 5:237. doi: 10.3389/fendo.2014.00237
19. Foti D, Chiefari E, Fedele M, Iuliano R, Brunetti L, Paonessa F, et al. Lack of the architectural factor HMGA1 causes insulin resistance and diabetes in human and mice. Nat Med. (2005) 7:765–73. doi: 10.1038/nm1254
20. Chiefari E, Tanyolaç S, Paonessa F, Pullinger CR, Capula C, Iiritano S, et al. Functional variants of the HMGA1 gene and type 2 diabetes mellitus. JAMA (2011) 305:9. doi: 10.1001/jama.2011.207
21. Chiefari E, Tanyolaç S, Iiritano S, Sciacqua A, Arcidiacono B, et al. A polymorphism of HMGA1 is associated with increased risk of metabolic syndrome and related components. Sci Rep. (2013) 3:1491. doi: 10.1038/srep01491
22. Bustin M, Lehn DA, Landsman D. Structural features of the HMG chromosomal proteins and their genes. Biochim Biophys Acta (1990) 1049:231–43. doi: 10.1016/0167-4781(90)90092-G
23. Fusco A, Fedele M. Roles of HMGA proteins in cancer. Nat Rev Cancer (2007) 7:899–910. doi: 10.1038/nrc2271
24. Leppek K, Das R, Barna M. Functional 5′ UTR mRNA structures in eukaryotic translation regulation and how to find them. Nat Rev Mol Cel Biol. (2018) 19:158–74. doi: 10.1038/nrm.2017.103
25. Friedmann M, Holth LT, Zoghbi HY, Reeves R. Organization, inducible-expression, and chromosome localization of the human HMG-I(Y) nonhistone protein gene. Nucleic Acids Res. (1993) 21:4259–67. doi: 10.1093/nar/21.18.4259
26. Ogram SA, Reeves R. Differential regulation of a multipromoter gene. Selective 12-O-tetradecanoylphorbol-13-acetate induction of a single transcription start site in the HMG-I/Y gene. J Biol Chem. (1995) 270:14235–42. doi: 10.1074/jbc.270.23.14235
27. Cleynen I, Huysmans C, Sasazuki T, Shirasawa S, Van de Ven W, Peeters K. Transcriptional control of the human high mobility group A1 gene: basal and oncogenic Ras-regulated expression. Cancer Res. (2007) 67:4620–9. doi: 10.1158/0008-5472.CAN-06-4325
28. Wood LJ, Mukherjee M, Dolde CE, Xu Y, Maher JF, Bunton TE, et al. HMG-I/Y, a new c-Myc target gene and potential oncogene. Mol Cell Biol. (2000) 20:5490–502. doi: 10.1128/MCB.20.15.5490-5502.2000
29. Zhong J, Liu C, Zhang QH, Chen L, Shen YY, Chen YJ, et al. TGF-β1 induces HMGA1 expression: The role of HMGA1 in thyroid cancer proliferation and invasion. Int J Oncol. (2017) 50:1567–78. doi: 10.3892/ijo.2017.3958
30. Chiefari E, Arcidiacono B, Possidente K, Iiritano S, Ventura V, et al. Transcriptional regulation of the HMGA1 gene by octamer-binding proteins Oct-1 and Oct-2. PLoS ONE (2013) 8:e83969. doi: 10.1371/journal.pone.0083969
31. Mao L, Wertzler KJ, Maloney SC, Wang Z, Magnuson NS, Reeves R. HMGA1 levels influence mitochondrial function and mitochondrial DNA repair efficiency. Mol Cell Biol (2009) 29:5426–40. doi: 10.1128/MCB.00105-09
32. Xue W, Huang J, Chen H, Zhang Y, Zhu X, Li J, et al. Histone methyltransferase G9a modulates hepatic insulin signaling via regulating HMGA1. Biochim Biophys Acta (2018) 1864:338–46. doi: 10.1016/j.bbadis.2017.10.037
33. Johnson KR, Lehn DA, Reeves R. Alternative processing of mRNAs encoding mammalian chromosomal high-mobility-group proteins HMG-I and HMG-Y. Mol Cell Biol. (1989) 9:2114–23.
34. Huth JR, Bewley CA, Nissen MS, Evans JN, Reeves R, Gronenborn AM, et al. The solution structure of an HMG-I(Y)-DNA complex defines a new architectural minor groove binding motif. Nat Struct Biol. (1997) 4:657–65. doi: 10.1038/nsb0897-657
35. Colombo DF, Burger L, Baubec T, Schübeler D. Binding of high mobility group A proteins to the mammalian genome occurs as a function of AT-content. PLoS Genet. (2017) 13:e1007102. doi: 10.1371/journal.pgen.1007102
36. Harrer M, Lührs H, Bustin M, Scheer U, Hock R. Dynamic interaction of HMGA1a proteins with chromatin. J Cell Sci. (2004) 117:3459–71. doi: 10.1242/jcs.01160
37. Reeves R, Edberg DD, Li Y. Architectural transcription factor HMGI(Y) promotes tumor progression and mesenchimal transition of human epithelial cells. Mol Cell Biol (2001) 21:575-94. doi: 10.1128/MCB.21.2.575-594.2001
38. Arnoldo L, Sgarra R, Chiefari E, Iiritano S, Arcidiacono B, Pegoraro S, et al. A novel mechanism of post-translational modulation of HMGA1 functions by the histone chaperone nucleophosmin. Sci Rep. (2015) 5:8552. doi: 10.1038/srep08552
39. Sgarra R, Rustighi A, Tessari MA, Di Bernardo J, Altamura S, Fusco A, et al. Nuclear phosphoproteins HMGA and their relationship with chromatin structure and cancer. FEBS Lett. (2004) 574:1–8. doi: 10.1016/j.febslet.2004.08.013
40. Catez F, Yang H, Tracey KJ, Reeves R, Misteli T, Bustin M. Network of dynamic interactions between histone H1 and high-mobility group proteins in chromatin. Mol Cell Biol. (2004) 24:4321–8. doi: 10.1128/MCB.24.10.4321-4328.2004
41. Borrmann L, Wilkening S, Bullerdiek J. The expression of HMGA genes is regulated by their 3′UTR. Oncogene (2001) 20:4537–41. doi: 10.1038/sj.onc.1204577
42. D'Angelo D, Esposito F, Fusco A. Epigenetic mechanisms leading to overexpression of HMGA proteins in human pituitary adenomas. Front Med. (2015) 2:39. doi: 10.3389/fmed.2015.00039
43. Fu X, Dong B, Tian Y, Lefebre P, Meng Z, Wang X, et al. MicroRNA-26a regulates insulin sensitivity and metabolism of glucose and lipids. J Clin Invest. (2015) 125:2497–509. doi: 10.1172/JCI75438
44. Chiefari E, Iiritano S, Paonessa F, Le Pera I, Arcidiacono B, Filocamo M, et al. Pseudogene-mediated posttranscriptional silencing of HMGA1 can result in insulin resistance and type 2 diabetes. Nat Commun. (2010) 1:40. doi: 10.1038/ncomms1040
45. Balakirev ES, Ayala FJ. Pseudogenes: are they “junk” or functional DNA? Annu Rev Genet. (2003) 37:123–51. doi: 10.1146/annurev.genet.37.040103.103949
46. Goncalves I, Duret L, Mouchiroud D. Nature and structure of human genes that generate retropseudogenes. Genome Res. (2000) 10:672–78. doi: 10.1101/gr.10.5.672
47. Sakai H, Koyanagi KO, Imanishi T, Gojobori T. Frequent emergence and functional resurrection of processed pseudogenes in the human and mouse genomes. Gene (2007) 389:196–203. doi: 10.1016/j.gene.2006.11.007
48. De Martino M, Forzati F, Arra C, Fusco A, Esposito F. HMGA1-pseudogenes and cancer. Oncotarget (2016) 7:28724–35. doi: 10.18632/oncotarget.7427
49. Zhang Q, Wang Y. High mobility group proteins and their post-translational modifications. Biochim Biophys Acta (2008) 1784:1159–66. doi: 10.1016/j.bbapap.2008.04.028
50. Sgarra R, Maurizio E, Zammitti S, Lo Sardo A, Giancotti V. Macroscopic differences in HMGA oncoproteins posttranslational modifications: C-terminal phosphorylation of HMGA2 affects its DNA binding properties. J Proteome Res. (2009) 8:2978–89. doi: 10.1021/pr900087r
51. Lund T, Laland SG. The metaphase specific phosphorylation of HMG I. Biochem Biophys Res Commun. (1990) 171:342–7. doi: 10.1016/0006-291X(90)91399-D
52. Nissen MS, Langan TA, Reeves R. Phosphorylation by cdc2 kinase modulates DNA binding activity of high mobility group I nonhistone chromatin protein. J Biol. Chem. (1991) 266:19945–52.
53. Xiao DM, Pak JH, Wang X, Sato T, Huang FL, Chen HC, et al. Phosphorylation of HMG-I by protein kinase C attenuates its binding affinity to the promoter regions of protein kinase C gamma and neurogranin/RC3 genes. J Neurochem. (2000) 74:392–9. doi: 10.1046/j.1471-4159.2000.0740392.x
54. Palvimo J, Linnala-Kankkunen A. Identification of sites on chromosomal protein HMG-I phosphorylated by casein kinase II. FEBS Lett. (1989) 257:101-4. doi: 10.1016/0014-5793(89)81796-X
55. Maurizio E, Cravello L, Brady L, Spolaore B, Arnoldo L, Giancotti V, et al. Conformational role for the C-terminal tail of the intrinsically disordered high mobility group A (HMGA) chromatin factors. J Proteome Res. (2011) 10:3283–91. doi: 10.1021/pr200116w
56. Munshi N, Agalioti T, Lomvardas S, Merika M, Chen G, Thanos D. Coordination of a transcriptional switch by HMGI(Y) acetylation. Science (2001) 293:1133–6. doi: 10.1126/science.293.5532.1133
57. Sgarra R, Lee J, Tessari MA, Altamura S, Spolaore B, Giancotti V, et al. The AT-hook of the chromatin architectural transcription factor high mobility group A1a is arginine-methylated by protein arginine methyltransferase 6. J Biol Chem. (2006) 281:3764–72. doi: 10.1074/jbc.M510231200
58. Sgarra R, Diana F, Bellarosa C, Dekleva V, Rustighi A, Toller M, et al. During apoptosis of tumor cells HMGA1a protein undergoes methylation: identification of the modification site by mass spectrometry. Biochemistry (2003) 42:3575–85. doi: 10.1021/bi027338l
59. Fonfría-Subirós E, Acosta-Reyes F, Saperas N, Pous J, Subirana JA, Campos JL. Crystal structure of a complex of DNA with one AT-hook of HMGA1. PLoS ONE (2012) 7:e37120. doi: 10.1371/journal.pone.0037120
60. Chiappetta G, Avantaggiato V, Visconti R, Fedele M, Battista S, Trapasso F, et al. High level expression of the HMGI (Y) gene during embryonic development. Oncogene (1996) 13:2439–46.
61. Yanagisawa BL, Resar LM. Hitting the bull's eye: targeting HMGA1 in cancer stem cells. Expert Rev Anticancer Ther. (2014) 14:23–30. doi: 10.1586/14737140.2013.859988
62. Fedele M, Fidanza V, Battista S, Pentimalli F, Klein-Szanto AJ, Visone R, et al. Haploinsufficiency of the Hmga1 gene causes cardiac hypertrophy and myelolymphoproliferative disorders in mice. Cancer Res (2006) 66:2536-43. doi: 10.1158/0008-5472.CAN-05-1889
63. Arce-Cerezo A, Garcia M, Rodriguez-Nuevo A, Crosa-Bonell M, Enguiz N, Pero A, et al. HMGA1 overexpression in adipose tissue impairs adipogenesis and prevents diet-induced obesity and insulin resistance. Sci Rep. (2015) 5:14487. doi: 10.1038/srep14487
64. Brocher J, Vogel B, Hock R. HMGA1 down-regulation is crucial for chromatin composition and gene expression profile permitting myogenic differentiation. BMC Cell Biol. (2010) 11:64. doi: 10.1186/1471-2121-11-64
65. Sgarra R, Pegoraro S, Ros G, Penzo C, Chiefari E, Foti D, et al. High Mobility Group A (HMGA) proteins: molecular instigators of breast cancer onset and progression. Biochim Biophys Acta (2018)1869:216–29. doi: 10.1016/j.bbcan.2018.03.001
66. Greco M, Arcidiacono B, Chiefari E, Vitagliano T, Ciraco AG, Brunetti FS, et al. HMGA1 and MMP-11 are overexpressed in human non-melanoma skin cancer. Anticancer Res. (2018) 38:771–8. doi: 10.21873/anticanres.12283
67. Pegoraro S, Ros G, Ciani Y, Sgarra R, Piazza S, Manfioletti G. A novel HMGA1-CCNE2-YAP axis regulates breast cancer aggressiveness. Oncotarget (2015) 6:19087–101. doi: 10.18632/oncotarget.4236
68. Pegoraro S, Ros G, Piazza S, Sommaggio R, Ciani Y, Rosato A, et al. HMGA1 promotes metastatic processes in basal-like breast cancer regulating EMT and stemness. Oncotarget (2013) 4:1293–308. doi: 10.18632/oncotarget.1136
69. Maurizio E, Wiśniewski JR, Ciani Y, Amato A, Arnoldo L, Penzo C, et al. Translating proteomic into functional data: an High Mobility Group A1 (HMGA1) proteomic signature has prognostic value in breast cancer. Mol Cell Proteomics (2016) 15:109–23. doi: 10.1074/mcp.M115.050401.
70. Resmini G, Rizzo S, Franchin C, Zanin R, Penzo C, Pegoraro S, et al. HMGA1 regulates the plasminogen activation system in the secretome of breast cancer cells. Sci Rep. (2017) 7:11768. doi: 10.1038/s41598-017-11409-4
71. Shah SN, Cope L, Poh W, Belton A, Roy S, Talbot CC, et al. HMGA1: a master regulator of tumor progression in triple-negative breast cancer cells. PLoS ONE (2013) 8:e63419. doi: 10.1371/journal.pone.0063419
72. Narita M, Narita M, Krizhanovsky V, Nunez S, Chicas A, Hearn SA, et al. A novel role for high-mobility group a proteins in cellular senescence and heterochromatin formation. Cell (2006) 126:503–14. doi: 10.1016/j.cell.2006.05.052
73. Reeves R, Nissen MS. Cell cycle regulation and functions of HMGI(Y). Prog Cell Cycle Res. (1995) 1:337–47.
74. Ozturk N, Singh I, Mehta A, Braun T, Barreto G. HMGA proteins as modulators of chromatin structure during transcriptional activation. Front Cell Dev Biol. (2014) 2:5. doi: 10.3389/fcell.2014.00005
75. Yie J, Merika M, Munshi N, Chen G, Thanos D. The role of HMGI(Y) in the assembly and function of the IFN-beta enhanceosome. EMBO J. (1999) 18:3074–89. doi: 10.1093/emboj/18.11.3074
76. Grosschedl R. Higher-order nucleoprotein complexes in transcription: analogies with site-specific recombination. Curr Opin Cell Biol. (1995) 7:362–70. doi: 10.1016/0955-0674(95)80091-3
77. Merika M, Thanos D. Enhanceosomes. Curr Opin Genet Dev. (2001) 11:205–8. doi: 10.1016/S0959-437X(00)00180-5
78. Sgarra R, Tessari MA, Di Bernardo J, Rustighi A, Zago P, Liberatori S, et al. Discovering high mobility group A molecular partners in tumour cells. Proteomics (2005) 5:1494–506. doi: 10.1002/pmic.200401028
79. Sgarra R, Furlan C, Zammitti S, Lo Sardo A, Maurizio E, Di Bernardo J, et al. Interaction proteomics of the HMGA chromatin architectural factors. Proteomics (2008) 8:4721–32. doi: 10.1002/pmic.200800193
80. Esposito F, Tornincasa M, Chieffi P, De Martino I, Pierantoni GM. Fusco A High-mobility group A1 proteins regulate p53-mediated transcription of the Bcl-2 gene. Cancer Res. (2010) 70:5379–88. doi: 10.1158/0008-5472.CAN-09-4199
81. Costa V, Foti D, Paonessa F, Chiefari E, Palaia L, Brunetti G, Gulletta E, et al. The insulin receptor: a new anticancer target for peroxisome proliferator-activated receptor-gamma (PPARgamma) and thiazolidinedione-PPARgamma agonists. Endocr Relat Cancer (2008) 15:325–35. doi: 10.1677/ERC-07-0226
82. Manabe T, Ohe K, Katayama T, Matsuzak i S, Yanagita T, Okuda H, et al. HMGA1a: sequence-specific RNA-binding factor causing sporadic Alzheimer's disease-linked exon skipping of presenilin-2 pre-mRNA. Genes Cells (2007) 12:1179–91. doi: 10.1111/j.1365-2443.2007.01123.x
83. Eilebrecht S, Benecke BJ, Benecke A. 7 SK snRNA-mediated, gene-specific cooperativity of HMGA1 and P-TEFb. RNA Biol. (2011) 8:1084–93. doi: 10.4161/rna.8.6.17015
84. Benecke AG, Eilebrecht S. RNA-mediated regulation of HMGA1 function. Biomolecules (2015) 5:943–57. doi: 10.3390/biom5020943
85. Thomae AW, Pich D, Brocher J, Spindler MP, Berens C, Hock R, et al. Interaction between HMGA1a and the origin recognition complex creates site-specific replication origins. Proc Natl Acad Sci U S A. (2008) 105:1692–7. doi: 10.1073/pnas.0707260105
86. Araki E, Shimada F, Fukushima H, Mori M, Shichiri M, Ebina Y. Characterization of the promoter region of the human insulin receptor gene. Diabetes Res Clin Pract. (1989) 7:S31–3. doi: 10.1016/0168-8227(89)90085-5
87. Muller-Wieland D, Taub R, Tewari DS, Kriauciunas KM, Sethu S, Reddy K, Kahn CR. Insulin-receptor gene and its expression in patients with insulin resistance. Diabetes (1989) 38:31–8.
88. Brunetti A, Foti D, Goldfine ID. Identification of unique nucler regulatory proteins for the insulin receptor gene, which appear during myocyte and adipocyte differentiation. J Clin Invest. (1993) 92:1288–95. doi: 10.1172/JCI116702
89. Brunetti A, Manfioletti G, Chiefari E, Goldfine ID, Foti D. Transcriptional regulation of human insulin receptor gene by the high-mobility group protein HMGI/Y. FASEB J (2001) 15:492–500. doi: 10.1096/fj.00-0190com
90. Hribal ML, Perego L, Lovari S, Andreozzi F, Menghini R, Perego C, et al. Chronic hyperglycemia impairs insulin secretion by affecting insulin receptor gene expression, splicing, and signaling in RIN beta cell line and human islets of Langerhans. FASEB J. (2003) 17:1340–2. doi: 10.1096/fj.02-0685fje
91. Paonessa F, Foti D, Costa V, Chiefari E, Leone F, Luciano F, et al. Activator protein-2 overexpression accounts for increased insulin receptor expression in human breast cancer. Cancer Res. (2006) 10:5085–93. doi: 10.1158/0008-5472.CAN-05-3678
92. Dey D, Bhattacharya A, Roy S, Bhattacharya S. Fatty acid represses insulin receptor gene expression by impairing HMGA1 through protein kinase Cepsilon. Biochem Biophys Res Commun. (2007) 357:474–9. doi: 10.1016/j.bbrc.2007.03.183
93. Biswas A, Bhattacharya S, Dasgupta S, Kundu R, Roy SS, Pal BC, et al. Insulin resistance due to lipid-induced signaling defects could be prevented by mahanine. Mol Cell Biochem. (2010) 336:97–107. doi: 10.1007/s11010-009-0257-4
94. Dasgupta S, Bhattacharya S, Maitra S, Pal D, Majumdar SS, Datta A, et al. Mechanism of lipid induced insulin resistance: activated PKCε is a key regulator. Biochim Biophys Acta (2011) 1812:495–506. doi: 10.1016/j.bbadis.2011.01.001
95. Gogoi B, Chatterjee P, Mukherjee S, Buragohain AK, Bhattacharya S, Dasgupta S. A polyphenol rescues lipid induced insulin resistance in skeletal muscle cells and adipocytes. Biochem Biophys Res Commun. (2014) 452:382–8. doi: 10.1016/j.bbrc.2014.08.079
96. Ohneda K, Mirmira RG, Wang J, Johnson JD, German MS. The homeodomain of PDX-1 mediates multiple protein-protein interactions in the formation of a transcriptional activation complex on the insulin promoter. Mol Cell Biol. (2000) 20:900–11. doi: 10.1128/MCB.20.3.900-911.2000
97. Eizirik DL, Pipeleers DG, Ling Z, Welsh N, Hellerström C, Andersson A. Major species differences between humans and rodents in the susceptibility to pancreatic beta cell injuriy. Proc Natl Acad Sci USA. (1994) 91:9253–6.
98. Kim SK, Hebrok M. Intercellular signals regulating pancreas development and function. Genes Dev. (2001) 15:111–27. doi: 10.1101/gad.859401
99. Arcidiacono B, Chiefari E, Messineo S, Bilotta FL, Pastore I, Corigliano DM, et al. HMGA1 is a novel transcriptional regulator of the FoxO1 gene. Endocrine (2018) 60:56–64. doi: 10.1007/s12020-017-1445-8
100. Kitamura T, Nakae J, Kitamura Y, Kido Y, Biggs WH III, Wright CV et al. The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic beta cell growth. J Clin Invest. (2002) 110:1839–47. doi: 10.1172/JCI16857
101. Al-Masri M, Krishnamurthy M, Li J, Fellows GF, Dong HH, Goodyer CG, et al. Effect of forkhead box O1 (FOXO1) on beta cell development in the human fetal pancreas. Diabetologia (2010) 53:699–711. doi: 10.1007/s00125-009-1632-0
102. Nakae J, Biggs WH III, Kitamura T, Cavenee WK, Wright CV, Arden KC, et al. Regulation of insulin action and pancreatic beta-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat Genet. (2002) 32:245–53. doi: 10.1038/ng890
103. Kitamura YI, Kitamura T, Kruse JP, Raum JC, Stein R, Gu W, et al. FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab. (2005) 2:153–63. doi: 10.1016/j.cmet.2005.08.004
104. Chiefari E, Nevolo MT, Arcidiacono B, Maurizio E, Nocera A, Iiritano S, et al. HMGA1 is a novel downstream nuclear target of the insulin receptor signaling pathway. Sci Rep. (2012) 2:251. doi: 10.1038/srep00251
105. Barthel A, Schmoll D, Unterman TG. FoxO proteins in insulin action and metabolism. Trends Endocrinol Metab. (2005) 16:183–9. doi: 10.1016/j.tem.2005.03.010
106. Shimomura I, Matsuda M, Hammer RE, Bashmakov Y, Brown MS, Golstein JL. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in lipodystrophic and ob/ob mice. Mol Cell (2000) 6:77–86. doi: 10.1016/S1097-2765(05)00010-9
107. Rane SG, Dubus P, Mettus RV, Galbreath EJ, Boden G, Reddy EP, et al. Loss of Cdk4 expression causes insuliln-deficient diabetes and Cdk4activation results in beta-islet cell hyperplasia. Nat Genet. (1999) 22:44–52. doi: 10.1038/8751
108. Chiefari E, Paonessa F, Iiritano S, Le Pera I, Palmieri D, Brunetti G, et al. The cAMP-HMGA1-RBP4 system: a novel biochemical pathway modulating glucose homeostasis. BMC Biol. (2009) 7:24. doi: 10.1186/1741-7007-7-24
109. Gasparini G, De Gori M, Paonessa F, Chiefari E, Brunetti A, Galasso O. Functional relationship between high mobility group A1 (HMGA1) protein and insulin-like growth factor-binding protein 3 (IGFBP-3) in human chondrocytes. Arthritis Res Ther. (2012) 14:R207. doi: 10.1186/ar4045
110. Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell (1989) 59:675–80. doi: 10.1016/0092-8674(89)90013-5
111. Harootunian AT, Adams SR, Wen W, Meinkoth JL, Taylor SS, Tsien RY. Movement of the free catalytic subunit of cAMP-dependent protein kinase into and out of the nucleus can be explained by diffusion. Mol Biol Cell (1993) 4:993–1002. doi: 10.1091/mbc.4.10.993
112. Montminy M. Transcriptional regulation by cyclic AMP. Annu Rev Biochem. (1997) 66:807–22. doi: 10.1146/annurev.biochem.66.1.807
113. Wondisford AR, Xiong L, Chang E, Meng S, Meyers DJ, Li M, et al. Control of Foxo1 gene expression by co-activator 300. J Biol Chem. (2014) 289:4326–33. doi: 10.1074/jbc.M113.540500
114. Jongbloed F, Saat TC, Verweij M, Payan-Gomez C, Hoeijmakers JH, van den Engel S, et al. A signature of renal stress resistance induced by short-term dietary restriction, fasting, and protein restriction. Sci Rep. (2017) 7:40901. doi: 10.1038/srep40901
115. Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature (2005) 436:356–62. doi: 10.1038/nature03711
116. Ha TK, Her NG, Lee MG, Ryu BK, Lee JH, Han J, et al. Caveolin-1 increases aerobic glycolysis in colorectal cancers by stimulating HMGA1-mediated GLUT3 transcription. Cancer Res. (2012) 72:4097–109. doi: 10.1158/0008-5472.CAN-12-0448
117. Melillo RM, Pierantoni GM, Scala S, Battista S, Fedele M, Stella A, et al. Critical role of the HMGI(Y) proteins in adipocytic cell growth and differentiation. Mol Cell Biol. (2001) 21:2485–95. doi: 10.1128/MCB.21.7.2485-2495.2001
118. Fedele M, Battista S, Manfioletti G, Croce CM, Giancotti V, Fusco A. Role of the high mobility group A proteins in human lipomas. Carcinogenesis (2001) 22:1583–91. doi: 10.1093/carcin/22.10.1583
119. Messineo S, Laria AE, Arcidiacono B, Chiefari E, Luque Huertas RM, Foti DP, et al. Cooperation between HMGA1 and HIF-1 Contributes to Hypoxia-Induced VEGF and Visfatin Gene Expression in 3T3-l1 Adipocytes. Front Endocrinol. (2016) 7:73. doi: 10.3389/fendo.2016.00073
120. Qiu H, Zhong J, Luo L, Tang Z, Liu N, Kang K, et al. Regulatory axis of miR-195/497 and HMGA1-Id3 governs muscle cell proliferation and differentiation. Int J Biol Sci. (2017) 13:157–66. doi: 10.7150/ijbs.17440
121. Balzeau J, Menezes MR, Cao S, Hagan JP. The LIN28/let-7 pathway in cancer. Front Genet. (2017) 8:31. doi: 10.3389/fgene.2017.00031
122. Zhu H, Shyh-Chang N, Ayellet V, Segre AV, Shinoda G, Shah SP, et al. The Lin28/let-7 axis regulates glucose metabolism. Cell (2011) 147:81–94. doi: 10.1016/j.cell.2011.08.033
123. Peng S, Chen LL, Lei XX, Yang L, Lin H, Carmichael GG, et al. Genome-wide studies reveal that lin28 enhances the translation of genes important for growth and survival of human embryonic stem cells. Stem Cells (2011) 29:496–504. doi: 10.1002/stem.591
124. Lebovitz HE. Insulin resistance: definition and consequences. Exp Clin Endocrinol Diabetes (2001) 109:S135–48. doi: 10.1055/s-2001-18576
125. Reaven GM. Pathophysiology of insulin resistance in human disease. Physiol Rev. (1995) 75:473–86. doi: 10.1152/physrev.1995.75.3.473
126. Reaven GM. Insulin resistance: the link between obesity and cardiovascular disease. Med Clin North Am. (2011) 95:875–92. doi: 10.1016/j.mcna.2011.06.002
127. Taylor SI, Accili D, Cama A, Imano E, Kadowaki H, Kadowaki T. Unusual forms of insulin resistance. Annu Rev Med. (1991) 42:373–9. doi: 10.1146/annurev.me.42.020191.002105
128. Semple RK, Savage DB, Cochran EK, Gorden P, O'Rahilly S. Genetic syndromes of severe insulin resistance. Endocr Rev. (2011) 32:498–514. doi: 10.1210/er.2010-0020
129. Kahn CR, Flier JS, Bar RS, Archer JA, Gorden P, Martin MM, Roth J. The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N Engl J Med. (1976) 294:739–45. doi: 10.1056/NEJM197604012941401
130. Musso C, Cochran E, Moran SA, Skarulis MC, Oral EA, Taylor S, et al. Clinical course of genetic diseases of the insulin receptor (type A and Rabson-Mendenhall syndromes): a 30-year prospective. Medicine (Baltimore) (2004) 83:209–22. doi: 10.1097/01.md.0000133625.73570.54
131. Dunaif A, Green G, Phelps RG, Lebwohl M, Futterweit W, Lewy L. Acanthosis Nigricans, insulin action, and hyperandrogenism: clinical, histological, and biochemical findings. J Clin Endocrinol Metab. (1991) 73:590–5. doi: 10.1210/jcem-73-3-590
132. Moller DE, Cohen O, Yamaguchi Y, Assiz R, Grigorescu F, Eberle A, et al. Prevalence of mutations in the insulin receptor gene in subjects with features of the type A syndrome of insulin resistance. Diabetes (1994) 43:247–55.
133. Brunetti A, Brunetti L, Foti D, Accili D, Goldfine ID. Human diabetes associated with defects in nuclear regulatory proteins for the insulin receptor gene. J Clin Invest. (1996) 97:258–62. doi: 10.1172/JCI118400
134. Semple RK. From bending DNA to diabetes: the curious case of HMGA1. J Biol (2009) 8:64. doi: 10.1186/jbiol164
135. Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: pathogenesis and treatment. Lancet (2008) 371:2153–6. doi: 10.1016/S0140-6736(08)60932-0
136. Unger RH. Reinventing type 2 diabetes: pathogenesis, treatment, and prevention. JAMA (2008) 299:1185–7. doi: 10.1001/jama.299.10.1185
137. Fuchsberger C, Flannick J, Teslovich TM, Mahajan A, Agarwala V, Gaulton KJ, et al. The genetic architecture of type 2 diabetes. Nature (2016) 536:41–7. doi: 10.1038/nature18642
138. Brunetti A, Chiefari E, Foti D. Recent advances in the molecular genetics of type 2 diabetes mellitus. World J Diabetes (2014) 5:128–40. doi: 10.4239/wjd.v5.i2.128
139. Marquez M, Huyvaert M, Perry JR, Pearson RD, Falchi M, Morris AP, et al. Low-frequency variants in HMGA1 are not associated with type 2 diabetes risk. Diabetes (2012) 61:524–30. doi: 10.2337/db11-0728
140. Liu L, Ding H, Wang HR, Xu YJ, Cui GL, Wang PH, et al. Polymorphism of HMGA1 is associated with increased risk of type 2 diabetes among Chinese individuals. Diabetologia (2012) 55:1685–8. doi: 10.1007/s00125-012-2518-0
141. Pullinger CR, Goldfine ID, Tanyolaç S Movsesyan I, Faynboym M, Durlach V, et al. Evidence that an HMGA1 gene variant associates with type 2 diabetes, body mass index, and high-density lipoprotein cholesterol in a Hispanic-American population. Metab Syndr Relat Disord. (2014) 12: 25–30. doi: 10.1089/met.2013.0086
142. Bianco A, Chiefari E, Nobile CG, Foti D, Pavia M, Brunetti A. The association between HMGA1 rs146052672 variant and type 2 Diabetes: a transethnic meta-analysis. Plos ONE (2015) 10:8. doi: 10.1371/journal.pone.0136077
143. Chiefari E, Ventura V, Capula C, Randazzo G, Scorcia V, Fedele M, et al. A polymorphism of HMGA1 protects against proliferative diabetic retinopathy by impairing HMGA1-induced VEGFA expression. Sci Rep. (2016) 6:39429. doi: 10.1038/srep39429
144. Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, et al. The metabolic syndrome. Endocr Rev. (2008) 29:777–822. doi: 10.1210/er.2008-0024
145. Greco M, Chiefari E, Montalcini T, Accattato F, Costanzo FS, Pujia A, et al. Early effects of a hypocaloric, mediterranean diet on laboratory parameters in obese individuals. Med Inflamm. (2014) 2014:750860. doi: 10.1155/2014/750860
146. Hanley AJ, Karter AJ, Festa A, D'Agostino R Jr, Wagenknecht LE, Savage P, et al. Factor analysis of metabolic syndrome using directly measured insulin sensitivity: the insulin resistance atherosclerosis study. Diabetes (2002) 51:2642–7. doi: 10.2337/diabetes.51.8.2642
147. De Rosa S, Chiefari E, Salerno N, Ventura V, D'Ascoli GL, Arcidiacono B, et al. HMGA1 is a novel candidate gene for myocardial infarction susceptibility. Int J Cardiol. (2017) 227:331–4. doi: 10.1016/j.ijcard.2016.11.088
148. De Rosa S, Arcidiacono B, Chiefari E, Brunetti A, Indolfi C, Foti DP. Type 2 diabetes mellitus and cardiovascular disease: genetic and epigenetic links. Front Endocrinol (2018) 9:2. doi: 10.3389/fendo.2018.00002
149. Laber S, Cox RD. Mouse models of human GWAS hits for obesity and diabetes in the post genomic era: time for reevaluation. Front Endocrinol. (2017) 8:11. doi: 10.3389/fendo.2017.00011
150. Ashcroft FM, Rorsman P. Diabetes mellitus and the β cell: the last ten years. Cell (2012) 148:1160–71. doi: 10.1016/j.cell.2012.02.010
151. Xin Y, Kim J, Okamoto H, Ni M, Wei Y, Adler C, et al. RNA sequencing of single human islet cells reveals type 2 diabetes genes. Cell Metab. (2016) 24:608–15. doi: 10.1016/j.cmet.2016.08.018
152. Benninger RKP, Hodson DJ. New understanding of β-cell heterogeneity and in situ islet function. Diabetes (2018) 67:537–47. doi: 10.2337/dbi17-0040
153. Girard CA, Wunderlich FT, Shimomura K, Collins S, Kaizik S, Proks P, et al. Expression of an activating mutation in the gene encoding the KATP channel subunit Kir6.2 in mouse pancreatic beta cells recapitulates neonatal diabetes. J Clin Invest. (2009) 119:80–90. doi: 10.1172/JCI35772
154. Di Cola G, Cool MH, Accili D. Hypoglycemic effect of insulin-like growth factor-1 in mice lacking the insulin receptors. J Clin Invest. (1997) 99:2538–44. doi: 10.1172/JCI119438
Keywords: HMGA1, glucose homeostasis, insulin resistance, type 2 diabetes, glucose metabolism
Citation: Chiefari E, Foti DP, Sgarra R, Pegoraro S, Arcidiacono B, Brunetti FS, Greco M, Manfioletti G and Brunetti A (2018) Transcriptional Regulation of Glucose Metabolism: The Emerging Role of the HMGA1 Chromatin Factor. Front. Endocrinol. 9:357. doi: 10.3389/fendo.2018.00357
Received: 22 February 2018; Accepted: 13 June 2018;
Published: 03 July 2018.
Edited by:
Robert Kenneth Semple, University of Edinburgh, United KingdomReviewed by:
Justin J. Rochford, University of Aberdeen, United KingdomRobert Sladek, McGill University, Canada
Copyright © 2018 Chiefari, Foti, Sgarra, Pegoraro, Arcidiacono, Brunetti, Greco, Manfioletti and Brunetti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonio Brunetti, YnJ1bmV0dGlAdW5pY3ouaXQ=
†Equal coauthorship.
 Eusebio Chiefari
Eusebio Chiefari Daniela P. Foti
Daniela P. Foti Riccardo Sgarra
Riccardo Sgarra Silvia Pegoraro
Silvia Pegoraro Biagio Arcidiacono
Biagio Arcidiacono Francesco S. Brunetti
Francesco S. Brunetti Manfredi Greco4
Manfredi Greco4 Guidalberto Manfioletti
Guidalberto Manfioletti Antonio Brunetti
Antonio Brunetti