- 1School of Traditional Chinese Medicine, School of Life Sciences, Beijing University of Chinese Medicine, Beijing, China
- 2Beijing Key Laboratory of Acupuncture Neuromodulation, Department of Acupuncture and Moxibustion, Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing, China
- 3School of Biological Sciences, Nanyang Technological University, Singapore, Singapore
- 4Department of Microbiology, Tumor and Cell Biology (MTC), Karolinska Institutet, Biomedicum, Stockholm, Sweden
Sarcopenic obesity and diabetes are two increasing health problems worldwide, which both share many common risk factors, such as aging, and general obesity. The pathogenesis of sarcopenic obesity includes aging, physical inactivity, malnutrition, low-grade inflammation, insulin resistance, and hormonal changes. Nevertheless, there are two major reasons to cause diabetes: impaired insulin secretion and impaired insulin action. Furthermore, the individual diagnosis of obesity and sarcopenia should be combined to adequately define sarcopenic obesity. Also, the diagnosis of diabetes includes fasting plasma glucose test (FPG), 2-h oral glucose tolerance test (OGTT), glycated hemoglobin (A1C), and random plasma glucose coupled with symptoms. Healthy diet and physical activity are beneficial to both sarcopenic obesity and diabetes, but there are only recommended drugs for diabetes. This review consolidates and discusses the latest research in pathogenesis, diagnosis, and treatments of diabetes and sarcopenic obesity.
Introduction
There are two greatest epidemiological trends of world—aging and obesity with the extension of average lifetime span and the changing lifestyle. The two factors dramatically affect body composition, morbidity and mortality (1). Aging is associated with the decrease in muscle mass and strength and the increase in body fat mass, which leads to frailty, falls, disability, social isolation, and hospitalization. The word sarcopenia from Greek refers to age-related loss of muscle mass. Although the impact of sarcopenia has been well-demonstrated, the effect of obesity on it emerges as a new public health problem (2). Therefore, a new term sarcopenic obesity (SO) arises to represent the coexistence of sarcopenia and obesity (3). Compared to simple sarcopenia or obesity alone, the medical sequelae related to SO are much greater, causing significantly higher healthcare costs (4). Data from Health and Nutrition Examination Survey (NHANES) noted SO rates of 12.6% in men and 33.5% in women (5). With the rapid growth of the elderly population globally, it was estimated that SO would affect 100–200 million people from 2016 to 2051 (6). Although SO is more common in older people due to the natural changes in body composition, it is estimated that younger people with class II or III obesity are vulnerable as well. A study assessed the prevalence of SO in 120 young adults using different diagnostic criteria of SO, revealing that 23.3% female and 58.8% male suffered SO according to the definition of appendicular skeletal muscle mass (ASM) by weight × 100 % (7). Due to the chronic progress of SO, the symptoms hardly draw people's attention, resulting in poor diagnosis, which causes negative consequences of quality of life and all-cause mortality (8).
Moreover, aging and obesity are two risk factors of diabetes as well. A variety of studies have the consensus that aging as well as obesity have positive associations with diabetes. A study in America has suggested that the percentage of diabetics rises with age (9). Furthermore, a study has estimated that the number of global diabetic patients would increase from 422 million in 2014 to at least 592 million in 2,035 and it has been shown that more than 50% of the diabetics are obese (10). Hence, there is a good reason to suspect that diabetes and SO have a strong relationship (11). On the one hand, insulin loses the function to enhance cellular glucose uptake and utilization in diabetics, which is defined clinically as insulin resistance which promotes obesity. Furthermore, the increasing fat mass facilitates various cytokines which accelerate the catabolism of muscles. On the other hand, the loss of muscle mass leads to less insulin-responsive target tissue, resulting in a severe condition of insulin resistance (12, 13). Therefore, the vicious cycle continues until more negative health consequences occur (Figure 1). Hence, it is necessary to deduce the pathogenesis, diagnosis and treatments of both diabetes and SO in order to stop the vicious cycle and increase the quality of life. This review consolidates and discusses the latest research in pathogenesis, diagnosis and treatments of diabetes, and sarcopenic obesity.
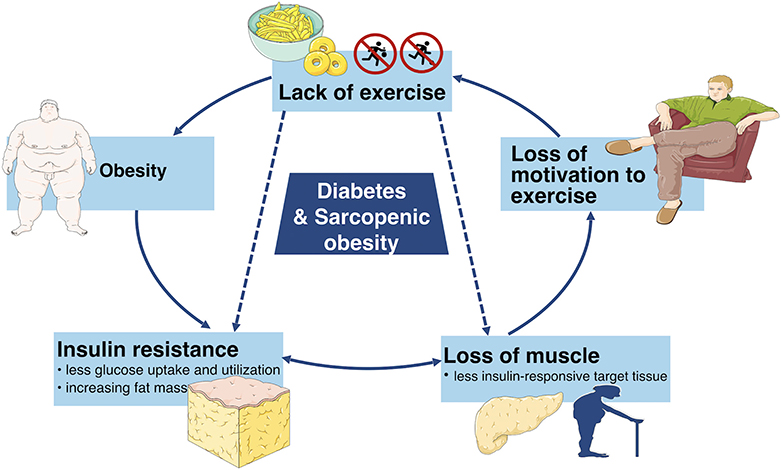
Figure 1. This simplified schematic diagram depicts the vicious cycle of unhealthy lifestyle which can eventually lead to diabetes and sarcopenic obesity as well as other adverse metabolic conditions.
Pathogenesis and Complications of Sarcopenic Obesity
Pathogenesis of Sarcopenic Obesity
There are multiple factors that cause sarcopenic obesity, such as aging, lack of physical activity, malnutrition low-grade inflammation, insulin resistance and hormonal changes, which leads to body composition changes (muscle mass and strength decline and fat mass increase). Moreover, each factor has independent impacts on the quality and quantity of muscle and fat, whereas, the cross-talks of them have stronger influences.
Aging
With aging, the reduction of basal metabolic rates may lead to weight gain and muscle mass decrease, which are associated with SO. Indeed, young people generally have more bone mass and muscle strength than old people. Meanwhile, studies have validated that the amount of bone mass and muscle mass peak around 30 years old, and after that, a gradual loss of muscle mass is accompanied with a parallel gain of fat mass (14). Therefore, weight generally increases in elderly people. However, it is also common that SO occurs in the old without increase of body weight, which could be due to the ectopic redistribution of fat. Studies have found that fat tends to move to viscera, muscle and abdomen with age, and the ectopic fat cause disorders of inflammatory factors, insulin and hormones, thus resulting in SO (15).
Lack of Physical Activity
Age-related SO is usually due to the lack of physical activity. In fact, old people tend to decrease physical activity, which contributes to the severe loss of muscle strength. Then atrophic muscles make even more difficult for elderly people to exercise, further aggravating the sedentary lifestyle. Numerous studies have proven that physical activity is necessary to lose weight and improve muscle strength, which is beneficial to ameliorate the state of SO (16, 17).
Imbalanced Nutrition
The imbalance of energy intake and expenditure is also linked to SO, especially for the old. On one hand, old people obtain inadequate protein in their diet. It is difficult to sustain muscle mass and strength with insufficient amount of protein intake (18). On the other hand, owing to decrease of outdoor physical activities, vitamin D produces less with scanty expose of ultraviolet radiation (19). Hence, inadequate vitamin D accelerates SO (20).
Low-Grade Inflammation
Both aging and physical inactivity have contributions on weight gain, by which an increase in adipose cell size may be determined. Specifically, adipocytes promote macrophage recruitment (21), then adipocytes and immune cells secrete more adipokines such as leptin, chemerin, resistin, and more cytokines such as tumor necrosis factor-α (TNF-α), interleukins (ILs), interferon-γ (INF-γ) (22–25), creating a circumstance of low-grade inflammation. Previous studies have indicated that an inflammatory state plays a significant role in the progression of SO as well as the morbidity and mortality driven by SO. A study conducted in Italy has verified that elevated levels of IL-6 and C-reactive protein (CRP) are related to SO, which in turn have suppressive effects on muscle strength (26). Moreover, a similar result has been shown in another cross-sectional study. The study has found the highest level of monocyte chemoattractant protein-1 (MCP-1) and the lowest level of appendicular lean mass in SO group (27), which supports the theory that low-grade inflammation is linked to SO.
Besides, the increased level of leptin contributes to leptin resistance, resulting in accumulation of free fatty acids. As less amount of fatty acid are oxidized in muscles, more fat mass deposits in visceral such as liver, heart, and spleen. Thereby, dysfunctional insulin production occurs, gradually leading to insulin resistance (28). Furthermore, the reduction of fatty acid consumption is coupled with the increase of reactive oxygen species generation. Oxidative stress is strongly associated with the expression of various inflammatory transcription factors, such as nuclear factor-kB (NF- kB) that modulates proteolytic pathways and promotes inflammation (29–31). Moreover, either low-grade inflammation or ectopic fat distribution generates myokine imbalance and mitochondrial dysfunction (32, 33). Particularly, myokines can exacerbate insulin resistance. Also, mitochondrial dysfunction causes more lipid peroxidation, which reinforces the collections of lipid intermediates and reactive oxygen metabolites, accelerating inflammation, insulin resistance, and oxidative stress (33). Hence, another vicious circle leading to SO forms.
Insulin Resistance
The production and efficiency of insulin decline in elderly and obese people. Meanwhile, obesity is related to a low-grade inflammation, the increased production and secretion of multifarious inflammatory factors including TNF-α, IL-6 modulate insulin sensitivity by altering some key steps in the insulin signaling pathway, which is responsible for the subsequent insulin resistance (34–36). Studies have elucidated that insulin resistance is essential for protein anabolism, thus directly concerns muscle fiber atrophy (37). Obese individuals with insulin resistance have a higher rate of muscle catabolism, which has been evidenced in a study that leg muscle strength and quality decrease distinctly in older diabetics (38). Therefore, insulin resistance is involved in poor muscle mass and muscle strength, progressively resulting in SO. Furthermore, insulin resistance is correlated to mitochondrial dysfunction as well. A down-regulation of genes involving mitochondrial enzymes declines mitochondrial content, which has been found in insulin resistant states (39, 40), augmenting accumulation of fat in muscle and liver. Hence, the loss of muscle strength and the gain of fat which characterize SO is attributed by insulin resistance (41).
Hormonal Changes
As an endocrine organ, the muscle can produce a variety of myokines such as myostatin and irisin. It is believed that myostatin inhibits muscle cell growth and differentiation, and irisin stimulates the increase of muscle mass (42, 43). However, several studies have documented that the content of myostatin is upregulated while irisin is downregulated in sarcopenia (44). At the same time, the increase in myostatin and decrease in irisin are tightly associated with poor browning reaction of white fat, reducing energy expenditure, triggering fat gain (45). Eventually, the crosstalk between muscle and fat leads to muscle damage, eliciting SO.
Other hormones, including insulin-like growth factors-1 (IGF-1), growth hormone, testosterone, and estrogen, also regulate the anabolic and catabolic progressions in muscle (46, 47). The reduction of IGF-1 is accompanied by the downregulation of irisin (45), and high level of free fatty acids in obese people inhibits both IGF-1 and growth hormone (48), which lowers the mass and strength of muscle, leading to muscle impairment and thus to SO (49). Moreover, testosterone and estrogen are essential for muscle health (47), but the production of these hormones decline naturally with aging. Hence, the muscle mass and strength weaken with the reduced testosterone and estrogen concentrations (50). Therefore, aberrant hormonal changes with age exacerbate SO.
Complications of Sarcopenic Obesity
It is acknowledged that either low muscle mass and strength or obesity is an independent risk factor for reduced physical capacity and quality of life. Therefore, it is reasonable to speculate when muscle damage and obesity coexist, they act synergistically on the risk of mortality, metabolic disorders, and quality of life (47, 51). Firstly, SO is overtly associated with an increased risk of all-cause mortality. A meta-analysis of 12 prospective cohort studies has indicated that compare to non-SO participants, subjects with SO have a 24% increased risk of all-cause mortality, especially in men. Noteworthily, according to different definition of SO, the risk of all-cause mortality changes. Specifically, all-cause mortality is higher basing on the criteria of mid-arm muscle circumference or muscle strength (HR 1.46, 95% CI 1.23–1.73 and 1.23, 1.09–1.38, respectively) rather than on the definition of skeletal muscle mass (pooled HR 1.24, 95% CI 1.12–1.37, P < 0.001) (52). Moreover, in a Japanese study, all-cause mortality increases in men with SO defined by waist circumference (HR, 1.19; 95% CI, 1.02–1.38), but not body mass index (BMI) or percent body fat (BF%) (53). Secondly, metabolic disorders including cardiometabolic syndrome (CMS), diabetes, cardiovascular disease (CVD), and cancer are common comorbidities of SO (54, 55). Several Korean studies have indicated that individuals with SO are associated with increased waist circumference, elevated fasting blood glucose, insulin resistance, higher blood pressure, and abnormal blood lipids as compared to sarcopenia or obesity alone (13, 23, 56). The cluster of abdominal obesity, hyperglycemia, hyperinsulinemia, dyslipidemia, and hypertension that composes CMS progressively leads to diabetes and CVD. A meta-analysis including 606 articles has demonstrated that SO increases the risk of T2D by almost 38% compared to individuals with excess weight or obesity alone (11). Furthermore, the characteristic may attribute to physical disability and metabolic syndromes, which has been clarified in another Korean study (57). In addition, studies have also estimated 10-years risk of CVD in SO group, non-sarcopenic group, and non-obese group, and a significant increase has been found in the SO group (58). Moreover, adverse clinical cancer outcomes reported to be relevant to SO, especially with respect to dose-limiting toxicity, surgical complications, physical disability, and shorter survival (59–61). Finally, even if there is no discrepancies between SO group and other groups of physical capacity found in a study (62), most studies support that SO causes physical disability. As the loss of muscle mass and strength makes a higher risk of osteoarthritis (63, 64), this exacerbates physical inactivity, worsening both physical and mental health. A cross-sectional study has evaluated the association between SO and cognitive function in geriatric population, and found that SO is an indicator of probable cognitive impairment (65). Therefore, these factors, which significantly affect the quality of life for elderly people, have been illustrated in another study that the highest fall risk and the lowest muscle function test results in the SO group, confirming an inverse association with health-related quality of life scores (66).
Pathogenesis and Complications of Diabetes
Pathogenesis of Diabetes
Diabetes mellitus is a chronic metabolic disease involving persistently elevated levels of blood glucose. Diabetes can be classified into several types: type 1 diabetes (T1D), type 2 diabetes (T2D), gestational diabetes, maturity-onset diabetes of the youth, neonatal diabetes, and secondary diabetes resulting from endocrinopathies, steroid use. The major subtypes of diabetes are T1D and T2D (67, 68). In 1889, scientists first discovered the role of the pancreas in the pathogenesis of diabetes. There are two main subtypes of endocrine cells in the pancreatic islets: β cells and α cells. β cells are involved in the production insulin, while α cells are responsible for secreting glucagon. The function of β cells and α cells changes with the glucose environment in an individual's body (69, 70). Once the imbalance between the secretion of insulin and glucagon occurs, the levels of blood glucose skew improperly as well. In the case of diabetes, it may be linked to impaired insulin secretion (insulin deficiency), impaired insulin action (insulin resistance), or both (71).
Impaired Insulin Secretion
Impaired insulin secretion is multifactorial, the exact mechanism is still unclear, but commonly develops from glucose toxicity, lipid toxicity, immunoinflammatory response, and oxidative stress, leading to the dysfunction of islet β cells (72). Persistent elevated levels of glucose can swamp the glycolytic process and inhibit glyceraldehyde catabolism, which promotes reactive oxygen species (ROS) production and oxidative damage, inducing β cells apoptosis and inhibiting β cells secretion (73, 74).
The accumulation of free fatty acids in the islet β cells accelerates the production of NO, which causes the apoptosis of β cells. Furthermore, long chain saturated fatty acids can inhibit the expression of adenine nucleotide translocator. Thus, the inner mitochondrial membrane of islet β cells fails to protect, which increases the permeability of mitochondrial membrane, leading to β cells apoptosis (75, 76).
In fact, the chronic activation of the innate immune system, leading to intra-islet inflammation also seems to be the key part of β cells apoptosis (77). Both obesity and hyperglycemia promote the release of inflammatory mediators, like TNF-α, or IL-6, released mediators stimulate macrophages and other innate immune cells, as well as some apoptosis-related signaling pathways, such as Fas/FasL signaling pathway (78, 79). This leads to the destruction and dysfunction of certain cells, like islet β cells that produce insulin. Besides, chronic inflammatory environment is also beneficial for the formation of free radicals such as reactive oxygen species (ROS), exacerbating β cells damage and yielding a positive feedback circle with the secretion of more detrimental cytokines to trigger further damage to β cells (80).
As mentioned above, prolonged as well as elevated levels of glucose blood, high free fatty acids, and chronic inflammatory environment, all increase the levels of ROS, activating the mechanism of oxidative stress (81). Furthermore, the islet β cells are especially sensitive to ROS because of their low inherent level of antioxidant enzymes. Therefore, ROS is capable to directly damage β cells, promoting apoptosis, or it can indirectly regulate insulin signaling pathway to inhibit the function of β cells (82, 83). Moreover, chronically excessive levels of ROS can cause the loss of transcription factors PDX-1 and MsfA, disturbing the expression of insulin gene (84). With the destruction of β cells, the peak of insulin secretion will be delayed, intensifying the fluctuation of blood glucose, resulting in further severe damage of β cells (85). The general mechanism of how glucose toxicity, lipid toxicity, immunoinflammatory response, as well as oxidative stress cause β cell damage, leading to impaired insulin secretion is illustrated in Figure 2.
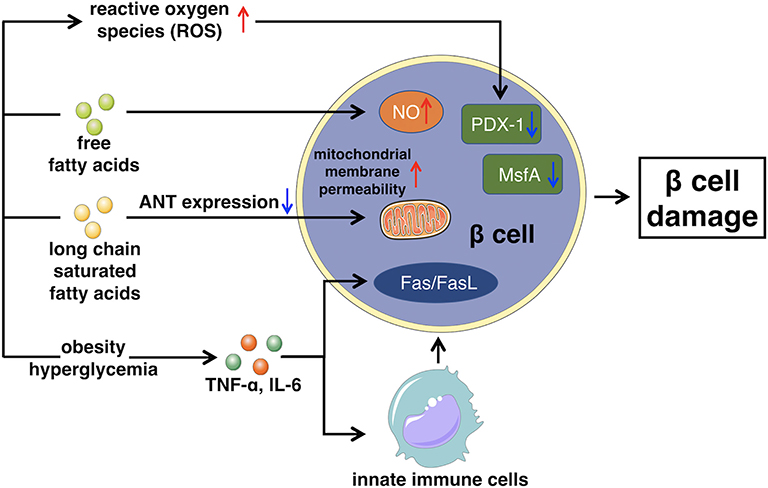
Figure 2. This figure illustrates the main mechanism of impaired insulin secretion that glucose toxicity, lipid toxicity, immunoinflammatory response, and oxidative stress lead to β cell damage. ANT, Adenine nucleotide translocator.
Impaired Insulin Action
Impaired insulin action refers to the reduction of glucose uptake and utilization. In order to sustain a stable levels of blood glucose, islet β cells secret excessive insulin to compensate, leading to hyperinsulinemia (86). Increased insulin content results in less affinity of insulin receptor (IR) thus, cells (major muscle) gradually become insensitive to insulin. IR is a member of the ligand-activated receptor and tyrosine kinase family of transmembrane signaling proteins composed of two α- subunits and two β-subunits linked by disulfide bonds (87). The main function of the two α- subunits is to bind to insulin as they are located at the extracellular surface. While the two β-subunits are distributed at extracellular, transmembrane, and intracellular sites, which regulate insulin- stimulated tyrosine kinase activity. After the α- subunits bind to insulin, insulin receptor tyrosine kinase is activated by phosphorylating of the β-subunits on multiple tyrosine residues. The main physiological role of insulin receptor tyrosine kinase appears to be metabolic regulation. Therefore, any impairments occur on main phosphorylation sites will reduce insulin receptor tyrosine kinase activity, eventually affecting the insulin function (88–90).
Furthermore, there are multiple factors, particular obesity, increase the levels of adipocytes, inflammatory cytokines (IL-1, IL-6, and TNFα) (91). The elevated amount of these components stimulates several signaling pathways such as inhibitor kappa beta kinase beta (IKKβ), Jun N-terminal kinase (JNK), and NF-κB signaling pathways to induce the development of insulin resistance (92, 93). Meanwhile, the transduction of the insulin PI3K/AKT signaling pathway is weakened, which may be the major manifestation of insulin resistance, impacting the downstream mediator—glycogen synthase kinase-3β (GSK3β). GSK3β is one of the few protein kinases of which the activity can be inhibited by phosphorylation (94).
Insulin signaling pathway begins with the binding to its cell surface IR, which has a tyrosine- protein kinase activity, and regulates the insulin response (95). Then, the binding of insulin and its IR stimulates the association between the receptor and downstream mediators, such as insulin receptor substrate-1 (IRS-1) and phosphatidylinositol 3-kinase (PI3K). The insulin receptor can directly activate PI3K by binding to the p85 regulatory subunit, leading to the production of phosphatidylinositol-3,4,5-triphosphate (PIP3). Moreover, the indirect PI3K activation is associated with phosphorylation and activation of AKT, also known as protein kinase B (PKB). Afterwards, AKT inhibits GSK3β by phosphorylating at Ser9 site. When the insulin signaling pathway fails, the expression of GSK3β improves, which reduces the insulin sensitivity and increases the levels of blood glucose, thus subsequently leading to T2D (72, 96, 97). The general mechanism of how excessive insulin secretion, adipocytes, and inflammatory factors directly affect IR or indirectly interfere the insulin signaling pathway, leading to impaired insulin action is illustrated in Figure 3.
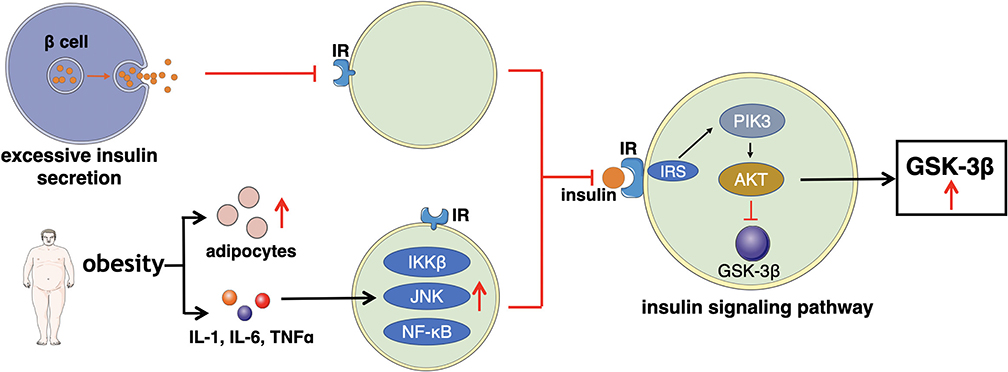
Figure 3. This figure illustrates the main mechanism of impaired insulin action that excessive insulin secretion, adipocytes, and inflammatory factors directly affect IR or indirectly interfere the insulin signaling pathway.
Complications of Diabetes
Unmanaged diabetes can cause multiple complications, most common of which are microvascular complications: nephropathy, retinopathy, and neuropathy, and macrovascular complications: cardiovascular diseases.
Renal Disease
Diabetic kidney disease is defined as persistent albuminuria, accompanied with a persistent reduction of glomerular filtration rate and an increase of arterial blood pressure, which can progress to end-stage renal disease (ESRD) (98). The prevalence of diabetic kidney disease in patients with T1D and T2D is 30 and 40%, respectively (99). A study has found that the risk of diabetic kidney disease is much higher in Asian countries than in Western countries due to different economic outcomes (100). Furthermore, the incidence of ESRD has been estimated to be about 40%-50% in the United States (101). In fact, more than 50% of the patients in America with T1D will eventually receive renal replacement treatments (98). Besides, the prevalence of ESRD is even higher than 60% in Malaysia, Singapore, and Mexico, which may relate to genetic background, lifestyle, health awareness and economic situation (101).
Ocular Disease
There are many diabetes-related ocular diseases, such as cataract, glaucoma, ischemic optic neuropathy, cranial nerve palsies, and recurrent corneal erosion syndrome (102). However, diabetic retinopathy is the most well-known complication, and it is expected to increase from 415 million in 2015 to 642 million by 2040 (103). Moreover, World Health Organization (WHO) has estimated that diabetic retinopathy leads to blindness in 5% of blind people (104). One of the structural changes in diabetic retinopathy is widened retinal arteriolar caliber which might be an early sign of microvascular dysfunction. Also, another change is widened retinal venular caliber which might be independently related to the prevalence and progression in diabetic retinopathy, as well as an indicator of developing retinopathy (105, 106).
Diabetic Neuropathy
Diabetic neuropathy is one of the most prominent complications of diabetes. The true prevalence is unclear but it varies from 10 to 90% in diabetics, according to the different standards and approaches used to define diabetic neuropathy (107). Diabetic neuropathy involves various syndromes, such as mono- and polyneuropathies, plexopathies, radiculopathies and autonomic neuropathy, among which distal symmetric polyneuropathy is very prevalent (108). Meanwhile, distal symmetric polyneuropathy mainly contributes to disability in diabetics, stemming from gait disturbance, fall-related injury, and foot ulceration and amputation (109). It has been reported that neuropathy is associated with a 1.7-fold increase in the risk of amputation, and even higher when coupled with other problems (107). In the UK, approximately one third amputees have a history of diabetes (110), and in Australia, about half of the amputees have diabetes (111). Therefore, diabetic neuropathy extremely influences the quality of life in diabetic people.
Cardiovascular Diseases
Cardiovascular diseases, involving coronary heart disease, peripheral vascular disease and cerebrovascular disease, are the primary contributors of death and disability in diabetic people (112). Individuals with diabetes suffer from a higher risk of cardiovascular diseases compared to those without diabetes, which is associated with a number of common risk factors such as age, obesity, smoking, hypertension, and dyslipidemia (113). Moreover, recent studies have shown that diabetes is considered as an independent risk factor for coronary heart diseases (114). Interestingly, a study has indicated that patients with T2D in Asian countries have a lower risk of coronary diseases than those from eastern Europe or Established Market Economies (100). However, Indian patients with T2D are as twice likely to develop coronary artery diseases as white Europeans who have T2D (115).
Cancer
Numerous epidemiological evidences have indicated that diabetes is considered as an independent risk factor for increased rates of various types of cancer. Cancer is a class of diseases resulted from external factors such as environment, diet and radiation as well as internal factors including obesity and diabetes (31, 116, 117). It has been proven that there is 1–2% of diabetic patients who will develop pancreatic cancer in 3 years (118). Also, diabetes serves as an independent risk factor for colorectal cancer as a study has found that there is a 49% increased risk of colorectal cancer in men with diabetes (119). Moreover, diabetes contributes a 27% increased risk of breast cancer in women and predisposes to more aggressive cancer stages (120). For endometrial cancer, the new-onset diabetics (<5 years) have a two-fold risk compared with those with long-standing diabetes (≥5 years) (121). Besides, diabetic individuals with liver cancer and bladder cancer are associated with a poor survival compared with non-diabetic subjects (122).
Other Complications
Recent studies have regarded Alzheimer's disease as type 3 diabetes because of insulin signaling pathways damage (123). Moreover, sexual dysfunction becomes more and more popular in both men and women with diabetes (124). Besides, high prevalence of depression as one of psychological complications that result from diabetes, makes it harder for diabetics to manage blood glucose, thus leading to further complications (125).
Diagnosis of Sarcopenic Obesity and Diabetes
Diagnosis of Sarcopenic Obesity
There is currently no consistent diagnosis of SO, nevertheless an adequate one should include the individual diagnosis of obesity and sarcopenia. According to the criteria of WHO, BMI ≥ 30 kg/m2 or wrist circumference (men ≥ 102 cm and women ≥ 88 cm) is considered as obesity. However, whether these criteria are appropriate for each individual has been questioned. Alternatively, cutoffs of BF% (Body Fat Percentage) or other adiposity indices have been regarded as useful outcomes measure of obesity (126, 127).
European Working Group on Sarcopenia in Older People (EWGSOP) has proposed that (1) low muscle mass; (2) low muscle strength; (3) low physical performance are the three important parameters to define sarcopenia (128). Various techniques emerge for assessing muscle mass, among which, computed tomography (CT) and magnetic resonance imaging (MRI) are deemed as the gold standard to distinguish fat from other soft tissues, thereby, effectively estimating fat mass and muscle mass. However, it is hard to generalize CT and MRI due to the high cost, and the risk of radiation (for CT) (129). Therefore, another relatively inexpensive and low radiation method called dual-energy-X-ray absorptiometry (DXA) is recommended to estimate the lean and fat mass of the whole body or certain regions of body. Moreover, it also manifests strength in assessment of bone mass and density, thus simultaneously providing the conditions of bone, muscle, and fat (130, 131). In addition, as an affordable and available tool, bioelectrical impedance analysis (BIA) is used to measure muscle mass as well, whereas, the inaccuracy makes it unrecommended to diagnose sarcopenia (132). For it indirectly reflects body composition by evaluating the entire body and segmental reactance as well as resistance influenced by fluid retention and disease-related conditions. Hence, an overestimation is accompanied with a poor distinction between extracellular and intracellular fluid (133). Furthermore, air displacement plethysmography (ADP) measures body volume and body density and hence, total fat and lean tissue (134). In spite of the widespread use of anthropometric measurements, such as mid-upper arm circumference, calf circumference, and skin fold thickness, they are inaccurate (135). Muscle strength can be assessed by handgrip strength, knee flexion/extension, and peak expiratory flow. Handgrip strength is a great predictor of extremity muscle power and mobility. Furthermore, keen flexion/extension is strongly related with certain functional activities. Although peak expiratory flow manifests the strength of respiratory muscles, it is not considered as a separated method. Physical performance is defined by short physical performance battery (SPPB), usual gait speed, timed get-up-and-go test, and stair climb power test, which evaluate an individual's balance, gait, strength and endurance (136, 137).
In 1998, Baumarterner et al. (138) first defined sarcopenia as ASM divided by body height squared [ASM(kg)/height2 (m2)] two or more standard deviations below the mean of a young reference values measured by DXA. Later, Janssen et al. (139) proposed a definition of sarcopenia that the skeletal muscle mass index [skeletal muscle mass (kg)/weight (kg) × 100] is one or two standard deviations below the reference value of younger, healthy individuals measured by BIA. Then, Newman et al. (140) came up with a new criteria for sarcopenia according to appendiculate lean mass (ALM) adjusted for height and fat mass using residuals from linear regression models.
Based on the abovementioned assessments, the calculation of Baumarterner et al. with the addition of the BF% exceeds 60% of population of the peers is one definition of SO (141). Another definition includes that BF% exceeds 60% of population of the peers and muscle mass is inferior to 60% of population of the peers (142). However, the diagnosis of SO varies with the changes of the diagnosis of obesity and sarcopenia. After 2010, when the standard of sarcopenia was updated by EWGSOP, the diagnosis of SO should change too. The evaluation of muscle strength and physical function are supposed to be combined with the assessments mentioned above (4).
Diagnosis of Diabetes
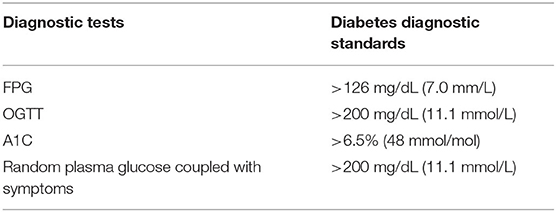
According to American Diabetes Association (ADA), there are four approaches to diagnosis diabetes: Fasting plasma glucose test (FPG), Two-Hour Oral Glucose Tolerance Test (OGTT), Glycated Hemoglobin (A1C), and Random plasma glucose coupled with symptoms (67). Table 1 summarizes these approaches as well as their respective diagnostic standards.
Fasting Plasma Glucose Test (FPG)
The test should be carried out after at least 8 h fast, the level of FPG more than 126 mg/dL (7.0 mm/L) is considered as diabetes (67).
Two-Hour Oral Glucose Tolerance Test (OGTT)
In this test, individuals should consume at least 150 g per day of carbohydrate for 3–5 days without taking any medications. Then, the test is carried out before and 2 h after consuming 75 g of glucose. The 2-h plasma glucose is more than 200 mg/dL (11.1 mmol/L) is consistent with the diagnosis. Although OGTT is a standard test, it is less convenient and affordable than FPG (67).
Glycated Hemoglobin (A1C)
A1C shows the average of blood glucose over last 2–3 months, and it is relative accurate since less influence by acute illness or stress. Diabetes is diagnosed if the result of A1C is more than 6.5% (48 mmol/mol) (67).
Random Plasma Glucose Coupled With Symptoms
In patients with classic symptoms: polydipsia, polyphagia, and polyuria, random plasma glucose more than 200 mg/dL (11.1 mmol/L) is diagnosed as having diabetes (67).
Treatments for Sarcopenic Obesity and Diabetes
Conventional Treatments for Sarcopenic Obesity and Diabetes
The most common treatments of SO and diabetes are dietary intervention and physical activity. Although there are various approved and effective drugs for diabetes, there is currently no recommended pharmacological intervention for SO, not even for diabetics with SO.
Dietary Intervention for Sarcopenic Obesity
The main dietary interventions for SO consist of caloric restriction, protein intake, and micronutrient supplementation. It is noted that lack of proper randomized clinical trials (RCTs) makes expert opinions to be the major guidelines at present of dietary interventions for SO.
Caloric restriction aims to lose weight, inducing body composition changes, and a reasonable weight loss goal should be under 5–8% of the initial body weight (1, 143). As acute caloric restriction could promote proteolysis and negatively affect muscle protein synthesis, resulting in a further reduction of muscle mass, acute caloric restriction is not recommended. In contrast, chronic caloric restriction might increase muscle protein synthesis rather than downregulate (144, 145). Major RCTs in non-sarcopenic women with obesity have suggested that caloric restriction (500 kcal deficit) with at least 0.8 g/kg of protein intake can effectively lose fat and improve physical function (146, 147). Moreover, another study has demonstrated that even caloric restriction might cause loss of muscle mass, and hence, it is still more beneficial to mobility and strength when accompanying with resistance training (148).
Increased dietary protein can prevent weight loss-induced sarcopenia. According to existing evidence, a healthy young individual should take an average daily protein intake of 1 g/kg per day. While in older people, a higher protein intake should be provided in order to promote muscle protein synthesis due to anabolic resistance. Therefore, adequate protein intake of 1–1.2 g/kg per day is recommended for geriatrics, and an even higher intake for older people suffering from SO or other similar diseases. Acceptable protein intake of 1.2–1.5 g/kg per day should be provided for individuals with acute or chronic diseases, which are related to unbalanced body composition. Furthermore, individuals with critical illness or severe malnutrition need an increased protein intake from 2 g/kg per day (149–151). Moreover, oral protein supplements should be considered when ample dietary intake is not practical. A study assessed the effect of a diet moderately rich in proteins on lean mass in SO older women, and has indicated that sufficient protein intake is able to preserve muscle mass in older women with SO (18). A similar result has been shown in another study that high-protein diet improves muscle strength in SO patients and prevent weight loss-induced sarcopenia (152).
Despite that vitamin D supplement has not been properly tested in patients with SO, several studies have suggested that vitamin D deficiency is associated with lower muscle strength, greater body instability, falls and disability in geriatric population (153). Therefore, in order to minimize the adverse effects of weight loss, it may be necessary to increase vitamin D intake (154, 155). Besides, studies have elucidated that vitamin D has the capability of regulating bioactive metabolites, and thus, improving muscle function (156). A study has verified that 25-hydroxyvitamin D3 can indirectly impact muscle function as its free metabolite is more closely related to body fat than muscle gene expression (155).
Dietary Intervention for Diabetes
Dietary intervention plays an important role in the management of diabetes and diabetes-related complications. The intake of low glycemic food including cereal fiber or a mixture of whole grain and bran can decrease 18–40% of risk for diabetics (157). Moreover, the risk of diabetes reduces by 26% due to the consumption of one sugar-containing beverage more than 1 month compared to one per day (158). Furthermore, a systemic review of randomized clinical trials has assessed the effects of low carbohydrate, macrobiotic, vegan, vegetarian, Mediterranean, and intermittent fasting diets, compared to low-fat diets on diabetes control and management. However, there were no evident differences of low carbohydrate diets and low-fat diets in glycemic control, weight and lipids. The macrobiotic and vegan diets were beneficial to glycemic control, while the vegetarian diet demonstrated better weight loss and insulin sensitivity. Besides, the Mediterranean diet showed a greater regulation of A1C levels. Therefore, the study has concluded that vegan, vegetarian and Mediterranean diets are better strategies to control glycemic marker in diabetics (159). In addition, a study has also found that the benefits of Mediterranean diets were greater than low-fat diets in diabetic retinopathy, but no significant differences were found in diabetic nephropathy (160). Furthermore, dietary intervention is an important modifiable factor to reduce the incidence of cardiovascular diseases in diabetics (161). Hence, it may be necessary to implement dietary intervention in public health managements of diabetes.
Physical Activity for Sarcopenic Obesity
A combination of physical activity and dietary intervention is a more effective strategy to treat SO. Indeed, there are multiple biological effects of physical activity: promote insulin sensitivity, improve anabolic response to endogenous amino acids, activate skeletal muscle satellites cells and trigger the proliferation and differentiation of them, amplify irisin production, adjust hormonal milieu, increase mitochondrial biogenesis, ameliorate inflammation and reduce oxidative stress (162–164). Even though various studies have confirmed that exercise has positive effects for SO patients, many professional organizations recommend that a combination of resistance training and aerobic training seems to be the most practical way to improve physical performance (165, 166). For geriatric people, the main goal of physical training is to ameliorate elasticity, strength, and physical endurance. Meanwhile, as resistance training can improve flexibility, muscle strength, and muscle hypertrophy, aerobic training focuses on increasing physical endurance of older people (166). Therefore, two non-consecutive sessions of resistance training coupled with at least 150 min per week of aerobic training is recommended for all older individuals (167, 168). A meta-analysis involving 14 RCTs and a quasi-experimental trial has clarified that exercise, particularly resistance training plays a key part in improving body composition and physical performance in patients with SO (169). Also, an RCT, allocating 60 men and women to four groups (resistance training, aerobic training, combination training, and control), has found that the skeletal muscle mass, body fat mass, ASM/weight % and visceral fat area of treatment groups exhibited better results than control group. Noteworthy, the grip strength and knee extensor performance of resistance group were superior to those of the other groups (170). Furthermore, a clinical study evaluated the effects of resistance training, aerobic training, or combination training in obese older people. It has revealed that the physical performance test score was the highest in the combination group (21% increase) compared to the resistance group (14% increase) and aerobic group (14% increase). But strength increased more in the resistance group (19% increase) than in the combination group (18% increase) and aerobic group (4% increase). While peak oxygen consumption (milliliters per kilogram of body weight per minute) increased most in the aerobic group (18% increase) compared to the combination group (17% increase) and resistance group (8% increase). Thus, resistance training combined aerobic training is the most effective way to alleviated physical disability (171).
Physical Activity for Diabetes
Several studies have demonstrated that physical activity is an effective regimen to lower the risk of developing diabetes by improving the β-cell function (172). A randomized controlled trial has evaluated the effects of moderate to intense exercise on pancreatic fat content and β cell function. The amount of pancreatic fat decreased in both healthy group (from 4.4 to 3.6%) and prediabetes/T2D group (from 8.7 to 6.7%), without significant differences observed for the improvement of β-cell function in different exercise strategies (173). Another study also has confirmed that high-intensity interval training is strongly associated with the improvement of β-cell function in patients with T2D. The total body-fat percentage was reduced, whereas lean body mass was protected. Noteworthy, high-intensity interval training was not able to regulate the levels of fasting plasma glucose, insulin, C-peptide, pro- insulin, and free fatty acids, nor did the levels of first-phase (0–30 min) and late-phase (30–180 min) plasma glucose, insulin, C-peptide, and proinsulin (174). Furthermore, as mentioned previously that the combination of resistance and aerobic training is beneficial to SO, it is a highly effective strategy for diabetes as well. A randomized controlled trial conducted in America has examined the effects of resistance training alone, aerobic training alone, and a combination of both on A1C within diabetics. Compared to control group, there were no significant mean changes of A1C in either the resistance training alone group (−0.16%; 95% CI: −0.46–0.15%; P = 0.32) or the aerobic training alone group (-0.24%; 95% CI: −0.55–0.07%; P = 0.14), however, there was a significant absolute mean change of A1C in the combination training group. Moreover, the combination training group had a maximal progression of oxygen consumption compared to other groups. All three exercise groups decreased waist circumference by 1.9–2.8 cm as compared to the control group. Therefore, in spite of the benefits of resistance training alone and aerobic training alone on diabetics, the combination of both could improve the level of A1C, which could hardly be obtained by each training exercise alone (175).
Pharmacological Intervention for Sarcopenic Obesity and Diabetes
In spite of many novel pharmacological interventions are under investigation, such as testosterone supplement, selective androgen receptor modulators, and myostatin inhibitors, there is currently no approved drugs for SO (176–178). However, there are approved and effectively drugs for diabetes. Metformin is considered as one of the most prevalent medications for diabetes management (179). Based on existing evidence, the National Institute for Health and Care Excellence (NICE) guidance for adults with type 2 diabetes recommends standard-release metformin as the initial drug treatment (180). A considerable number of studies have also found that metformin decreases fasting plasma glucose (PG) concentration and hemoglobin A1c by conserving the β-cell function or by decreasing liver glucose production (hepatic gluconeogenesis) (181–184). Also, thiazolidinediones can promote insulin sensitivity, increase glucose metabolism, and preserve the β-cell function through activating PPAR-γ (185, 186). Meanwhile, they can reduce plasma free fatty acid and intramyocellular lipid content to increase insulin sensitivity and redistribute fats from visceral to subcutaneous adipose to alleviate diabetes. Pioglitazone and troglitazone, belonging to thiazolidinediones, have the effect of controlling the progression on gestational diabetes (187, 188). Besides, α-Glucosidase Inhibitors contribute for prolonging the overall carbohydrate digestion duration and reducing the rate of glucose absorption (189). Whereas, it should be noted that they do not increase insulin sensitization (190), and a systematic review disapproves of acarbose dosages higher than 50 mg (three times a day), as there is no better effects on glycated hemoglobin (191). Therefore, α-Glucosidase Inhibitors should not be applied as initial drugs, and it is more positive to combine them with other types of anti-diabetic drugs. Moreover, incretins can shrink appetite, thus reduce food intake, leading to weight reduction (192). Because of the impact on weight reduction, incretins may find increasing use in diabesity, which is the coexistence of diabetes and obesity (193). In addition, Sodium-Glucose Cotransporter (SGLT) 2 Inhibitors are one of the latest pharmacological interventions to decrease the reabsorption of glucose in kidney and lower FGP and A1C, which enhances urinary glucose elimination and attenuates blood glucose level. Meanwhile, they can also positively affect cardiovascular diseases due to sodium decrease, uric acid absorption, and blood pressure reduction (194).
Complementary and Alternative Treatments for Sarcopenic Obesity and Diabetes
Herbal Medicine and Derivative
With the growing of popularity of herbal medicine, many studies have indicated that herbal medicine or related derivatives may be effective methods to treat SO and diabetes. A study has reported two cases about using wild ginseng complex (WGC) on two patients who only wanted to lose abdominal fat, but not in other parts of body. After 3 weeks of WGC intervention, the two patients had an increase in muscle mass, protein content, and basal metabolic rate. Therefore, WGC intervention may be a new alternative treatment for age-related sarcopenic obesity but more studies using larger samples are required to support this (195). In another study, three major herbal medicine including Zuo Gui Wan, red raspberry leaves, and Orthosiphon stamineus were found beneficial to gestational diabetes by controlling glucose (196). Hence, effective management of diabetics with SO using herbal medicine and derivatives may be plausible but more supportive evidences are still required.
Acupuncture
Acupuncture has been used in diabetes for a long time in Asian countries and recent studies have also suggested that acupuncture may alleviate SO (197–199). A randomized controlled trial, using electrical acupuncture coupled with essential amino acid supplementation to treat SO in male older people, has indicated that both electrical acupuncture with oral essential amino acids group and oral essential amino acids alone group can decrease BF% and increase ASM/H2, with the combination group being more effective than another group. Moreover, the combination group can increase muscle mass in a shorter time (197). Besides, a meta-analysis of randomized controlled trials has confirmed that acupuncture should be recommended as a complementary treatment in T2D control, particularly with obesity or other metabolic disorders (195). Although the underlying mechanism of acupuncture on diabetes remains unclear, studies have suggested that it may be related to adjust nerve conduction, modulate signal pathways, regulate hormonal level, and ameliorate oxidative stress level (200). Further investigations are required to prove that acupuncture is indeed effective for the treatment of patients with diabetes and SO.
Discussion
The occurrence of SO and diabetes has a rapid growth worldwide because of lifestyle changes and longer life expectancy. Indeed, they share many common risk factors, especially in aging and obesity. Moreover, a study has found that in the case of similar BMI, diabetics have decreased lean body mass and increased body fat mass compared with non-diabetics (57), indicating that diabetes is associated with increased risk of SO. Although the underlying mechanism of the association remains unclear, we speculate a bidirectional interplay in obesity, low-grade inflammation, insulin resistance and sarcopenia. SO combines sarcopenia and obesity, and low-grade inflammation plays a crucial role in the pathogenesis of diabetes (11). Therefore, SO may have synergistic effect with low-grade inflammation to exacerbate insulin resistance, further impairing glucose metabolism. Noteworthily, physical activity is helpful for both SO and diabetes. However, it should be treated with caution due to the degeneration of muscle mass for SO and venerable feet for diabetes. The young individuals in good metabolic control are able to do most activities, but the middle-aged and older individuals or patients with other complications are encouraged to check with the doctor to avoid injuries of intensive exercise (201). Generally, for patients with SO and diabetes, an appropriate warm-up and cool-down period should be included before and after physical activity session. A short warm-up session at a low-intensity level helps skeletal muscles, heart, lungs prepare before formal exercise. After the physical activity session, a short cool-down session should be conducted similarly as the warm-up session to gradually bring the heart rate down. Proper stretches should be structured after warm-up and cool-down period to protect muscles. Moreover, suitable footwear should be emphasized to prevent blisters and keep the feet dry because it is easy to cause trauma of the feet for diabetics. Fluid intake affects blood glucose levels and heart function, thus, during physical activity, fluid should be taken early and regularly. Finally, the diabetics should never forget to test blood glucose regularly (202, 203).
Although pharmacological intervention has been widely used in treating diabetes, doctors and patients need to be cautious of the side effects of the drugs. Metformin has been reported to cause deficiency in vitamin 12 and folic acid. Thiazolidinediones can cause bladder cancer and fractures, and combined insulin-thiazolidinediones therapy may lead to heart failure. The common adverse reactions of SGLT2 inhibitors are urinary tract infections, increase in low-density lipoprotein (LDL) cholesterol, bone fractures, and they may sometimes cause ketoacidosis. Therefore, pharmacological intervention needs to be monitored, especially in elderly patients and patients with other complications (204). Additionally, there are some limitations in the complementary and alternative treatments. Although several studies have revealed some positive results, the overall quality of the evidence is low, and the available data are too few to adequately suggest that herbal medicine and derivative and acupuncture are useful. Thus, more large-scale, multicenter, high-quality RCTs are required in the future, which will lead to deeper understandings of complementary and alternative treatments for SO and diabetes. Furthermore, it is important to raise the awareness of the high prevalence of the sarcopenia in the obese population, which seems to be strongly associated with diabetes, and screening for SO in subjects with obesity during clinical practice may be necessary. Therapies for SO and diabetes should be treated carefully and personally in order to minimize adverse effects.
Conclusion
By analyzing research evidence from previous studies, this review identified possible pathogenesis of SO and diabetes, such as malnutrition, insulin resistance, low-grade inflammation, and hormonal changes. Meanwhile, the underlying mechanisms of insulin deficiency and insulin resistance have been discussed to provide a better understanding of the association between SO and diabetes. Also, complications of SO and diabetes have been explored to urge more attention on SO and diabetes. Additionally, we have consolidated the novel diagnostic methods of SO and diabetes. Furthermore, different treatment options have been exhibited that dietary intervention and physical activity are considered as prevalently effective treatments for diabetics with SO, and some complementary and alternative interventions have shown some positive effects on SO and diabetes. As a final suggestion for the prevention and treatment of diabetes and SO, we recommend dietary intervention and regular exercises, coupled with specific drugs prescribed to individuals by the clinicians.
Author Contributions
ZL: conceptualization. YT and PW: resources. MW: writing—original draft preparation. YS: figure preparation. ZL, YT, XW, and PW: writing—review and editing. ZL and PW: supervision. PW: project administration. YT and PW: funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the “Double First-Class” Construction Funds of Discipline of Integrated Traditional Chinese and Western Medicine of Beijing University of Chinese Medicine.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Zamboni M, Rubele S, Rossi AP. Sarcopenia and obesity. Curr Opin Clin Nutr. (2019) 22:13–9. doi: 10.1097/MCO.0000000000000519
2. Koliaki C, Liatis S, Dalamaga M, Kokkinos A. Sarcopenic obesity: epidemiologic evidence, pathophysiology, therapeutic perspectives. Curr Obesity Rep. (2019) 8:458–71. doi: 10.1007/s13679-019-00359-9
3. Nezameddin R, Itani L, Kreidieh D, El Masri D, Tannir H, El Ghoch M. Understanding sarcopenic obesity in terms of definition and health consequences: a clinical review. Curr Diabetes Rev. (2020). doi: 10.2174/1573399816666200109091449. [Epub ahead of print].
4. Xie WQ, Xiao GL, Fan YB, He M, Lv S, Li YS. Sarcopenic obesity: research advances in pathogenesis and diagnostic criteria. Aging Clin Exp Res. (2019). doi: 10.1007/s40520-019-01435-9. [Epub ahead of print].
5. Batsis JA, Mackenzie TA, Emeny RT, Lopez-Jimenez F, Bartels SJ. Low lean mass with and without obesity, and mortality: results from the 1999-2004 national health and nutrition examination survey. J Gerontol A Biol Sci Med Sci. (2017) 72:1445–51. doi: 10.1093/gerona/glx002
6. Lee DC, Shook RP, Drenowatz C, Blair SN. Physical activity and sarcopenic obesity: definition, assessment, prevalence and mechanism. Future Sci OA. (2016) 2:FSO127. doi: 10.4155/fsoa-2016-0028
7. Johnson Stoklossa CA, Sharma AM, Forhan M, Siervo M, Padwal RS, Prado CM. Prevalence of sarcopenic obesity in adults with class II/III obesity using different diagnostic criteria. J Nutr Metab. (2017) 2017:7307618. doi: 10.1155/2017/7307618
8. Stephen WC, Janssen I. Sarcopenic-obesity and cardiovascular disease risk in the elderly. J Nutr Health Aging. (2009) 13:460–6. doi: 10.1007/s12603-009-0084-z
9. Fang M. Trends in the prevalence of diabetes among US adults: 1999-2016. Am J Prevent Med. (2018) 55:497–505. doi: 10.1016/j.amepre.2018.05.018
10. Khan RMM, Chua ZJY, Tan JC, Yang Y, Liao Z, Zhao Y. From pre-diabetes to diabetes: diagnosis, treatments and translational research. Medicina (Kaunas). (2019) 55:546. doi: 10.3390/medicina55090546
11. Khadra D, Itani L, Chebaro Y, Obeid M, Jaber M, Ghanem R, et al. Association between sarcopenic obesity and higher risk of type 2 diabetes in adults: a systematic review and meta-analysis. World J Diabetes. (2019) 10:311–23. doi: 10.4239/wjd.v10.i5.311
12. Dominguez LJ, Barbagallo M. The cardiometabolic syndrome and sarcopenic obesity in older persons . J Cardiometab Syndr. (2007) 2:183–9. doi: 10.1111/j.1559-4564.2007.06673.x
13. Baek SJ, Nam GE, Han KD, Choi SW, Jung SW, Bok AR, et al. Sarcopenia and sarcopenic obesity and their association with dyslipidemia in Korean elderly men: the 2008–2010 Korea National health and nutrition examination survey. J Endocrinol Invest. (2014) 37:247–60. doi: 10.1007/s40618-013-0011-3
14. Mourtzakis M, Pardo CMM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantifica- tion of body composition in cancer patients using computed tomog- raphy images acquired during routine care. Appl Physiol Nutr Metab. (2008) 33:997–1006. doi: 10.1139/H08-075
15. Polyzos SA, Margioris AN. Sarcopenic obesity. Hormones. (2018) 17:321–31. doi: 10.1007/s42000-018-0049-x
16. Patel VS, Chan ME, Rubin J, Rubin CT. Marrow adiposity and hematopoiesis in aging and obesity: exercise as an intervention. Curr Osteoporos Rep. (2018) 16:1–11. doi: 10.1007/s11914-018-0424-1
17. Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. (2012) 8:457–65. doi: 10.1038/nrendo.2012.49
18. Muscariello E, Nasti G, Siervo M, Di Maro M, Lapi D, D'Addio G, et al. Dietary protein intake in sarcopenic obese older women. Clin Interv Aging. (2016) 11:133–40. doi: 10.2147/CIA.S96017
19. Oh C, Jeon BH, Reid S, Shaun Nicholas, Jho S, No JK. The most effective factors to offset sarcopenia and obesity in the older Korean: physical activity, vitamin D, protein intake. Nutrition. (2016) 33:169–73. doi: 10.1016/j.nut.2016.06.004
20. Son J, Yu Q, Seo JS. Sarcopenic obesity can be negatively associated with active physical activity and adequate intake of some nutrients in Korean elderly: findings from the Korea National Health and Nutrition Examination Survey (2008-2011). Nutr Res Pract. (2019) 13:47–57. doi: 10.4162/nrp.2019.13.1.47
21. Neels JG. Inflamed fat: what starts the fire? J Clin Invest. (2005) 116:33–5. doi: 10.1172/JCI27280
22. Polyzos SA, Kountouras J, Mantzoros CS. Adipose tissue, obesity and nonalcoholic fatty liver disease. Minerva Endocrinologica. (2016) 42:92–108. doi: 10.23736/S0391-1977.16.02563-3
23. Kim TN, Park MS, Lim KI, Choi HY, Yang SJ, Yoo HJ, et al. Relationships between sarcopenic obesity and insulin resistance, inflammation, and vitamin D status: the Korean sarcopenic obesity study. Clin Endocrinol. (2013) 78:525–32. doi: 10.1111/j.1365-2265.2012.04433.x
24. Dong R, Tan Y, Fan A, Liao Z, Liu H, Wei P. Molecular dynamics of the recruitment of immunoreceptor signaling module DAP12 homodimer to lipid raft boundary regulated by PIP2. J Phys Chem B. (2019) 124:504–10. doi: 10.1021/acs.jpcb.9b11095
25. Wei P, Zuo LM, Qu J, Chen P, Luo S-Z. Critical residues and motifs for homodimerization of the first transmembrane domain of the plasma membrane glycoprotein CD36. J Biol Chem. (2017) 292:8683–93. doi: 10.1074/jbc.M117.779595
26. Schrager MA, Metter EJ, Simonsick E, Ble A, Bandinelli S, Lauretani F, et al. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol (1985). (2007) 102:919–25. doi: 10.1152/japplphysiol.00627.2006
27. Lim JP, Leung BP, Ding YY, Tay L, Mei SC. Monocyte chemoattractant protein-1: a proinflammatory cytokine elevated in sarcopenic obesity. Clin Intervent Aging. (2015) 10:605–9. doi: 10.2147/CIA.S78901
28. Michio S. Leptin resistance and lipolysis of white adipose tissue: an implication to ectopic fat disposition and its consequences. J Atheroscler Thromb. (2017) 24:1088–9. doi: 10.5551/jat.ED083
29. Jensen GL. Inflammation: roles in aging and sarcopenia. JPEN J Parenter Enteral Nutr. (2008) 32:656–9. doi: 10.1177/0148607108324585
30. Meng SJ, Yu LJ. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci. (2010) 11:1509–26. doi: 10.3390/ijms11041509
31. Liao Z, Chua D, Tan NS. Reactive oxygen species: a volatile driver of field cancerization and metastasis. Mol Cancer. (2019) 18:65. doi: 10.1186/s12943-019-0961-y
32. Goya WS, Atkins JL. Muscle loss and obesity: the health implications of sarcopenia and sarcopenic obesity. Proc Nutr Soc. (2015) 74:405–12. doi: 10.1017/S002966511500169X
33. Kalinkovich A, Livshits G. Sarcopenic obesity or obese sarcopenia: a cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev. (2017) 35:200–21. doi: 10.1016/j.arr.2016.09.008
34. Mcternan PG, Kusminski CM, Kumar S. Recent advances in the relationship between obesity, inflammation, insulin resistance. Eur Cytokine Netw. (2006) 17:4–12. doi: 10.1104/pp.92.4.891
35. Shoelson SE, Herrero L, Naaz Obesity A. Inflammation, insulin resistance. Gastroenterology. (2007) 132:2169–80. doi: 10.1053/j.gastro.2007.03.059
36. Matulewicz N, Karczewska-Kupczewska M. Insulin resistance and chronic inflammation. Postepy Hig Med Dosw. (2016) 70:1245–58. doi: 10.5604/17322693.1226662
37. Nomura T, Ikeda Y, Nakao S, Ito K, Ishida K, Suehiro T, et al. Muscle strength is a marker of insulin resistance in patients with type 2 diabetes: a pilot study. Endocr J. (2007) 54:791–6. doi: 10.1507/endocrj.K07-055
38. Abbatecola AM, Ferrucci L, Ceda G, Russo CR, Lauretani F, Bandinelli S, et al. Insulin resistance and muscle strength in older persons. J Gerontol. (2005) 60:1278–82. doi: 10.1093/gerona/60.10.1278
39. Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. (2003) 34:267–73. doi: 10.1038/ng1180
40. Patti ME, Butte AJ, Crunkhorn S, Cusi K, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci USA. (2003) 100:8466–71. doi: 10.1073/pnas.1032913100
41. Abbatecola AM, Paolisso G, Fattoretti P, Evans WJ, Fiore V, Dicioccio L, et al. Discovering pathways of sarcopenia in older adults: a role for insulin resistance on mitochondria dysfunction. J Nutr Health Aging. (2011) 15:890–5. doi: 10.1007/s12603-011-0366-0
42. Huh JY. The role of exercise-induced myokines in regulating metabolism. Arch Pharm Res. (2018) 41:14–29. doi: 10.1007/s12272-017-0994-y
43. Díaz BB, González DA, Gannar F, Pérez MCR, de León AC. Myokines, physical activity, insulin resistance and autoimmune diseases. Immunol Lett. (2018) 203:1–5. doi: 10.1016/j.imlet.2018.09.002
44. Chang JS, Kim TH, Nguyen TT, Park K-S, Kim N, Kong ID. Circulating irisin levels as a predictive biomarker for sarcopenia: a cross-sectional community-based study. Geriatr Gerontol Int. (2017) 17:2266–73. doi: 10.1111/ggi.13030
45. Colaianni G, Cinti S, Colucci S, Grano M. Irisin and musculoskeletal health. Ann N Y Acad Sci. (2017) 1402:5–9. doi: 10.1111/nyas.13345
46. Wang C, Bai L. Sarcopenia in the elderly: basic and clinical issues. Geriatr Gerontol Int. (2012) 12:388–96 doi: 10.1111/j.1447-0594.2012.00851.x
47. Choi KM. Sarcopenia and sarcopenic obesity. Endocrinol Metab (Seoul). (2013) 28:86–9 doi: 10.3803/EnM.2013.28.2.86
48. Weltman A, Weltman J, Veldhuis JD, Hartman ML. Body composition, physical exercise, growth hormone and obesity. Eat Weight Disord. (2001) 6(3 Suppl):28–37.
49. Waters DL, Qualls CR, Dorin RI, Veldhuis JD, Baumgartner RN. Altered growth hormone, cortisol, and leptin secretion in healthy elderly persons with sarcopenia and mixed body composition phenotypes. J Gerontol. (2008) 63:536–41. doi: 10.1093/gerona/63.5.536
50. Sipilä S, Narici M, Kjaer M, Pöllänen E, Atkinson RA, Hansen M, et al. Sex hormones and skeletal muscle weakness. Biogerontology. (2013) 14:231–45. doi: 10.1007/s10522-013-9425-8
51. Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. (2008) 11:693–700. doi: 10.1097/MCO.0b013e328312c37d
52. Tian S, Xu Y. Association of sarcopenic obesity with the risk of all-cause mortality: a meta-analysis of prospective cohort studies. Geriatr Gerontol Int. (2016) 16:155–66. doi: 10.1111/ggi.12579
53. Sanada K, Chen R, Willcox B, Ohara T, Masaki K. Association of sarcopenic obesity predicted by anthropometric measurements and twenty-four year all-cause mortality in elderly men: the kuakini honolulu heart program. Nutrition. (2017) 46:97. doi: 10.1016/j.nut.2017.09.003
54. Hong S-H, Choi KM. Sarcopenic obesity, insulin resistance, and their implications in cardiovascular and metabolic consequences. Int J Mol Sci. (2020) 21:E494. doi: 10.3390/ijms21020494
55. Liao Z, Tan ZW, Zhu P, Tan NS. Cancer-associated fibroblasts in tumor microenvironment–Accomplices in tumor malignancy. Cell Immunol. (2019) 343:103729. doi: 10.1016/j.cellimm.2017.12.003
56. Kim TN, Yang SJ, Yoo HJ, Lim KI, Kang HJ, Song W, et al. Prevalence of sarcopenia and sarcopenic obesity in Korean adults: the Korean sarcopenic obesity study. Int J Obes (Lond). (2009) 33:885–92. doi: 10.1038/ijo.2009.130
57. Kim TN, Park MS, Yang SJ, Yoo HJ, Kang HJ, Song W, et al. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: the Korean Sarcopenic Obesity Study (KSOS). Diabetes Care. (2010) 33:1497–9. doi: 10.2337/dc09-2310
58. Jeong-Hyeon K, Jin CJ, Soon PY. Relationship between sarcopenic obesity and cardiovascular disease risk as estimated by the framingham risk score. J Korean Med Sci. (2015) 30:264. doi: 10.3346/jkms.2015.30.3.264
59. Baracos VE, Arribas L. Sarcopenic obesity: hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann Oncol. (2018) 29:ii1–9. doi: 10.1093/annonc/mdx810
60. Carneiro IP, Mazurak VC, Prado CM. Clinical implications of sarcopenic obesity in cancer. Curr Oncol Rep. (2016) 18:62. doi: 10.1007/s11912-016-0546-5
61. Gonzalez-Molina J, Gramolelli S, Liao Z, Carlson JW, Ojala PM, Lehti K. MMP14 in sarcoma: a regulator of tumor microenvironment communication in connective tissues. Cells. (2019) 8:991. doi: 10.3390/cells8090991
62. Brochu M. Sarcopenic/obesity and physical capacity in older men and women: data from the nutrition as a determinant of successful aging (NuAge)-the Quebec longitudinal study. Obesity(Sliver Spring). (2012) 17:2082–8. doi: 10.1038/oby.2009.109
63. Lee S, Kim T-N, Kim S-H. Sarcopenic obesity is more closely associated with knee osteoarthritis than is nonsarcopenic obesity: a cross-sectional study. Arthritis Rheum. (2012) 64:3947–54. doi: 10.1002/art.37696
64. Misra D, Fielding RA, Felson DT, Niu J, Brown C, Nevitt M, et al. Risk of knee osteoarthritis with obesity, sarcopenic obesity, and sarcopenia. Arthritis Rheumatol. (2019) 71:232–7. doi: 10.1002/art.40692
65. Magdalena T, Stephanie C, James G. Sarcopenic obesity and cognitive performance. Clin Interv Aging. (2018) 13:1111–9. doi: 10.2147/CIA.S164113
66. Öztürk ZA, Türkbeyler IH, Abiyev A, Kul S, Edizer B, Yakaryilmaz FD, et al. Health-related quality of life and fall risk associated with age-related body composition changes; sarcopenia, obesity and sarcopenic obesity. Inter Med J. (2018) 48:973–81. doi: 10.1111/imj.13935
67. American Diabetes Association. (2) Classification and diagnosis of diabetes. Diabetes Care. (2015) 38:S8–16. doi: 10.2337/dc15-S005
68. Harreiter J, Roden M. Diabetes mellitus-Definition, classification, diagnosis, screening and prevention (Update 2019). Wien Klin Wochenschr. (2019) 131:6–15. doi: 10.1007/s00508-019-1450-4
69. Giwa AM, Ahmed R, Omidian Z, Majety N, Karakus KE, Omer SM, et al. Current understandings of the pathogenesis of type 1 diabetes: genetics to environment. World J Diabetes. (2020) 11:13–25. doi: 10.4239/wjd.v11.i1.13
70. Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev. (2016) 37:278–316. doi: 10.1210/er.2015-1137
71. Liu B, Xiang Y, Liu Z, Zhou Z. Past, present and future of latent autoimmune diabetes in adults. Diabetes Metab Res Rev. (2020) 36:e3205. doi: 10.1002/dmrr.3205
72. Zhang Y, Huang N-Q, Yan F, Jin H, Zhou S-Y, Shi J-S, et al. Diabetes mellitus and Alzheimer's disease: GSK-3β as a potential link. Behav Brain Res. (2018) 339:57–65. doi: 10.1016/j.bbr.2017.11.015
73. Mota M, Banini BA, Cazanave SC, Sanyal AJ. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism. (2016) 65:1049–61. doi: 10.1016/j.metabol.2016.02.014
74. Las G, Oliveira MF, Shirihai OS. Emerging roles of β-cell mitochondria in type-2-diabetes. Mol Aspects Med. (2020) 71:100843. doi: 10.1016/j.mam.2019.100843
75. Nolan CJ. Lipotoxicity, β cell dysfunction, gestational diabetes. Cell Metab. (2014) 19:553–4. doi: 10.1016/j.cmet.2014.03.020
76. Farrell GC, Haczeyni F, Chitturi S. Pathogenesis of NASH: how metabolic complications of overnutrition favour lipotoxicity and pro-inflammatory fatty liver disease. Adv Exp Med Biol. (2018) 1061:19–44. doi: 10.1007/978-981-10-8684-7_3
77. Donath MY, Størling J, Maedler K, Mandrup-Poulsen T. Inflammatory mediators and islet β-cell failure: a link between type 1 and type 2 diabetes. (2003) 81:455–70. doi: 10.1007/s00109-003-0450-y
78. Acharjee S, Ghosh B, Al-Dhubiab BE, Nair AB. Understanding type 1 diabetes: etiology and models. Can J Diabetes. (2013) 37:269–76. doi: 10.1016/j.jcjd.2013.05.001
79. DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. (2004) 88:787–835. doi: 10.1016/j.mcna.2004.04.013
80. Blaser H, Dostert C, Mak TW, Brenner D. TNF and ROS crosstalk in inflammation. Trends Cell Biol. (2016) 26:249–61. doi: 10.1016/j.tcb.2015.12.002
81. Rendra E, Riabov V, Mossel DM, Sevastyanova T, Harmsen MC, Kzhyshkowska J. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology. (2019) 224:242–53. doi: 10.1016/j.imbio.2018.11.010
82. Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem. (2004) 279:42351–4. doi: 10.1074/jbc.R400019200
83. Panigrahy SK, Bhatt R, Kumar A. Reactive oxygen species: sources, consequences and targeted therapy in type 2 diabetes. J Drug Target. (2017) 25:93–101. doi: 10.1080/1061186X.2016.1207650
84. Robertson R, Zhou H, Zhang T, Harmon JS. Chronic oxidative stress as a mechanism for glucose toxicity of the beta cell in type 2 diabetes. Cell Biochem Biophys. (2007) 48:139–46. doi: 10.1007/s12013-007-0026-5
85. Newsholme P, Cruzat VF, Keane KN, Carlessi R, de Bittencourt PIH Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem J. (2016) 473:4527–50. doi: 10.1042/BCJ20160503C
86. Nolan CJ, Prentki M. Insulin resistance and insulin hypersecretion in the metabolic syndrome and type 2 diabetes: time for a conceptual framework shift. Diab Vasc Dis Res. (2019) 16:118–27. doi: 10.1177/1479164119827611
87. Chen Y, Huang L, Qi X, Chen C. Insulin receptor trafficking: consequences for insulin sensitivity and diabetes. Int J Mol Sci. (2019) 20:5007. doi: 10.3390/ijms20205007
88. Abdul-Ghani MA, DeFronzo RA. Pathogenesis of insulin resistance in skeletal muscle. J Biomed Biotechnol. (2010) 2010:476279. doi: 10.1155/2010/476279
89. Kasuga M. Structure and function of the insulin receptor-a personal perspective. Proc JPN Acad B Phys Biol Sci. (2019) 95:581–9. doi: 10.2183/pjab.95.039
90. De Meyts P. Insulin and its receptor: structure, function and evolution. Bioessays. (2004) 26:1351–62. doi: 10.1002/bies.20151
91. Polsky S, Ellis SL. Obesity, insulin resistance, and type 1 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. (2015) 22:277. doi: 10.1097/MED.0000000000000170
92. Akash MSH, Rehman K, Liaqat A. Tumor necrosis factor-alpha: role in development of insulin resistance and pathogenesis of type 2 diabetes mellitus. J Cell Biochem. (2018) 119:105–10. doi: 10.1002/jcb.26174
93. Gomes BF, de Accardo CM. Immunoinflammatory mediators in the pathogenesis of diabetes mellitus. Einstein (Sao Paulo). (2019) 17:eRB4596. doi: 10.31744/einstein_journal/2019RB4596
94. Yaribeygi H, Farrokhi FR, Butler AE, Sahebkar A. Insulin resistance: review of the underlying molecular mechanisms. J Cell Physiol. (2019) 234:8152–61. doi: 10.1002/jcp.27603
95. Posner BI. Insulin signalling: the inside story. Can J Diabetes. (2017) 41:108–13. doi: 10.1016/j.jcjd.2016.07.002
96. Arneth B, Arneth R, Shams M. Metabolomics of type 1 and type 2 diabetes. Int J Mol Sci. (2019) 20:2467. doi: 10.3390/ijms20102467
97. Gao C, Hölscher C, Liu Y, Li L. GSK3: a key target for the development of novel treatments for type 2 diabetes mellitus and Alzheimer disease. Rev Neurosci. (2011) 23:1–11. doi: 10.1515/rns.2011.061
98. Lin Y-C, Chang Y-H, Yang SY, Wu K-D, Chu T-S. Update of pathophysiology and management of diabetic kidney disease. J Formos Med Assoc. (2018) 117:662–75. doi: 10.1016/j.jfma.2018.02.007
99. Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. (2017) 12:2032–45. doi: 10.2215/CJN.11491116
100. Clarke PM, Glasziou P, Patel A, Chalmers J, Woodward M, Harrap SB, et al. Event rates, hospital utilization, and costs associated with major complications of diabetes: a multicountry comparative analysis. PLoS Med. (2010) 7:e1000236. doi: 10.1371/journal.pmed.1000236
101. Lim AK. Diabetic nephropathy - complications and treatment. Int J Nephrol Renovasc Dis. (2014) 7:361–81. doi: 10.2147/IJNRD.S40172
102. Henriques J, Vaz-Pereira S, Nascimento J, Rosa PC. Diabetic eye disease. Acta Med Port. (2015) 28:107–13. doi: 10.20344/amp.5361
103. Simó-Servat O, Hernández C, Simó R. Diabetic retinopathy in the context of patients with diabetes. Ophthalmic Res. (2019) 62:211–7. doi: 10.1159/000499541
104. Tarr JM, Kaul K, Chopra M, Kohner EM, Chibber R. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. (2013) 2013:343560. doi: 10.1155/2013/343560
105. Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. (2010) 376:124–36. doi: 10.1016/S0140-6736(09)62124-3
106. Sosna T. History of diagnosis and therapy of diabetic retinopathy. Vnitr Lek. (2016) 62(11 Suppl 4):S136–41.
107. Vinik AI, Nevoret M-L, Casellini C, Parson H. Diabetic neuropathy. Endocrinol Metab Clin North Am. (2013) 42:747–87. doi: 10.1016/j.ecl.2013.06.001
108. Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, et al. Diabetic neuropathy. Nat Rev Dis Primers. (2019) 5:41. doi: 10.1038/s41572-019-0092-1
109. Lechleitner M, Abrahamian H, Francesconi C, Kofler M, Sturm W, Köhler G. Diabetic neuropathy and diabetic foot syndrome (Update 2019). Wien Klin Wochenschr. (2019) 131:141–50. doi: 10.1007/s00508-019-1487-4
110. Schofield CJ, Libby G, Brennan GM, Macalpine RR, Leese GP. Mortality and hospitalization in patients after amputation: a comparison between patients with and without diabetes. Diabetes Care. (2006) 29:2252–6. doi: 10.2337/dc06-0926
111. Lim TS, Finlayson A, Thorpe JM, Sieunarine K, Mwipatayi BP, Brady A, et al. Outcomes of a contemporary amputation series. ANZ J Surg. (2006) 76:300–5. doi: 10.1111/j.1445-2197.2006.03715.x
112. Henning RJ. Type-2 diabetes mellitus and cardiovascular disease. Future Cardiol. (2018) 14:491–509. doi: 10.2217/fca-2018-0045
113. de Matheus ASM, Tannus LRM, Cobas RA, Palma CCS, Negrato CA, de Gomes MB. Impact of diabetes on cardiovascular disease: an update. Int J Hypertens. (2013) 2013:653789. doi: 10.1155/2013/653789
114. Leon BM, Maddox TM. Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes. (2015) 6:1246–58. doi: 10.4239/wjd.v6.i13.1246
115. Forouhi NG, Sattar N, Tillin T, McKeigue PM, Chaturvedi N. Do known risk factors explain the higher coronary heart disease mortality in South Asian compared with European men? prospective follow-up of the Southall and Brent studies, UK. Diabetologia. (2006) 49:2580–8. doi: 10.1007/s00125-006-0393-2
116. Yang Y, Yang T, Liu S, Cao Z, Zhao Y, Su X, et al. Concentrated ambient PM2. 5 exposure affects mice sperm quality and testosterone biosynthesis. PeerJ. (2019) 7:e8109. doi: 10.7717/peerj.8109
117. Stone TW, McPherson M, Darlington LG. Obesity and cancer: existing and new hypotheses for a causal connection. EBioMedicine. (2018) 30:14–28. doi: 10.1016/j.ebiom.2018.02.022
118. Magruder JT, Elahi D, Andersen DK. Diabetes and pancreatic cancer: chicken or egg? (2016) 40:339–51. doi: 10.1097/MPA.0b013e318209e05d
119. Larsson SC, Giovannucci E, Wolk A. Diabetes and colorectal cancer incidence in the cohort of Swedish men. Diabetes Care. (2005) 28:1805–7. doi: 10.2337/diacare.28.7.1805
120. Boyle P, Boniol M, Koechlin A, Robertson C, Valentini F, Coppens K, et al. Diabetes and breast cancer risk: a meta-analysis. Br J Cancer. (2012) 107:1608–17. doi: 10.1038/bjc.2012.414
121. Saltzman BS, Doherty JA, Hill DA, Beresford SA, Voigt LF, Chen C, et al. Diabetes and endometrial cancer: an evaluation of the modifying effects of other known risk factors. Am J Epidemiol. (2008) 167:607–14. doi: 10.1093/aje/kwm333
122. Wang M, Yang Y, Liao Z. Diabetes and cancer: epidemiological and biological links. World J Diabetes. (2020) 11:227–38. doi: 10.4239/wjd.v11.i6.227
123. Kandimalla R, Thirumala V, Reddy PH. Is alzheimer's disease a type 3 diabetes? a critical appraisal. Biochim Biophys Acta Mol Basis Dis. (2017) 1863:1078–89. doi: 10.1016/j.bbadis.2016.08.018
124. Bak E, Marcisz C, Krzeminska S, Dobrzyn-Matusiak D, Foltyn A, Drosdzol-Cop A. Relationships of sexual dysfunction with depression and acceptance of illness in women and men with type 2 diabetes mellitus. Int J Environ Res Public Health. (2017) 14:1073. doi: 10.3390/ijerph14091073
125. Semenkovich K, Brown ME, Svrakic DM, Lustman PJ. Depression in type 2 diabetes mellitus: prevalence, impact, and treatment. Drugs. (2015) 75:577–87. doi: 10.1007/s40265-015-0347-4
126. Weir CB, Jan A. BMI Classification Percentile And Cut Off Points. Treasure Island, FL: StatPearls, StatPearls Publishing (2020).
127. Panuganti KK, Kshirsagar RK. Obesity. Treasure Island, FL: StatPearls, StatPearls Publishing (2020).
128. Yakabe M, Hosoi T, Akishita M, Ogawa S. Updated concept of sarcopenia based on muscle-bone relationship. J Bone Mineral Metab. (2020) 38:7–13. doi: 10.1007/s00774-019-01048-2
129. Donini LM, Busetto L, Bauer JM, Bischoff S, Boirie Y, Cederholm T, et al. Critical appraisal of definitions and diagnostic criteria for sarcopenic obesity based on a systematic review. Clin Nutr. (2020) 39:2368–88. doi: 10.1016/j.clnu.2019.11.024
130. Ilich JZ, Kelly OJ, Inglis JE. Osteosarcopenic obesity syndrome: what is it and how can it be identified and diagnosed? Curr Gerontol Geriatr Res. (2016) 2016:1–7. doi: 10.1155/2016/7325973
131. Berger-Groch J, Thiesen DM, Ntalos D, Hennes F, Hartel MJ. Assessment of bone quality at the lumbar and sacral spine using CT scans: a retrospective feasibility study in 50 comparing CT and DXA data. Eur Spine J. (2020) 29:1098–104. doi: 10.1007/s00586-020-06292-z
132. Bosy-Westphal A, Jensen B, Braun W, Pourhassan M, Gallagher D, Müller MJ. Quantification of whole-body and segmental skeletal muscle mass using phase-sensitive 8-electrode medical bioelectrical impedance devices. Eur J Clin Nutr. (2017) 71:1061–7. doi: 10.1038/ejcn.2017.27
133. Yu S, Alice P, Kareeann K, Renuka V. The performance of five bioelectrical impedance analysis prediction equations against dual x-ray absorptiometry in estimating appendicular skeletal muscle mass in an adult australian population. Nutrients. (2016) 8:189. doi: 10.3390/nu8040189
134. Kuriyan R. Body composition techniques. Indian J Med Res. (2018) 148:648–58. doi: 10.4103/ijmr.IJMR_1777_18
135. Choi KM. Sarcopenia and sarcopenic obesity. Korean J Inter Med. (2016) 31:1054–60. doi: 10.3904/kjim.2016.193
136. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. (2010) 39:412–23. doi: 10.1093/ageing/afq034
137. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:601. doi: 10.1093/ageing/afz046
138. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. (1998) 147:755–63. doi: 10.1093/oxfordjournals.aje.a009520
139. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (Sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. (2002) 50:889–96. doi: 10.1046/j.1532-5415.2002.50216.x
140. Newman AB, Kupelian V, Visser M, Simonsick E, Harris TB. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. (2003) 51:1602–9. doi: 10.1046/j.1532-5415.2003.51534.x
141. Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. (2000) 904:437–48. doi: 10.1111/j.1749-6632.2000.tb06498.x
142. Davison KK, Ford ES, Cogswell ME, Dietz WH. Percentage of body fat and body mass index are associated with mobility limitations in people aged 70 and older from NHANES III. J Am Geriatr Soc. (2002) 50:1802–9. doi: 10.1046/j.1532-5415.2002.50508.x
143. Trouwborst I, Verreijen A, Memelink R, Massanet P, Boirie Y, Weijs P, et al. Exercise and nutrition strategies to counteract sarcopenic obesity. Nutrients. (2018) 10:605. doi: 10.3390/nu10050605
144. Heymsfield SB, Gonzalez MCC, Shen W, Redman L, Thomas D. Weight loss composition is one-fourth fat-free mass: a critical review and critique of this widely cited rule. Obes Rev. (2014) 15:310–21. doi: 10.1111/obr.12143
145. Freiberger E, Goisser S, Porzel S, Volkert D, Bollheimer C. Sarcopenic obesity and complex interventions with nutrition and exercise in community-dwelling older persons – a narrative review. Clin Intervent Aging. (2015) 10:1267–82. doi: 10.2147/CIA.S82454
146. Backx EM, Tieland M, Borgonjen-van den Berg KJ, Claessen PR, van Loon LJ, de Groot LC. Protein intake and lean body mass preservation during energy intake restriction in overweight older adults. Int J Obesity. (2016) 40:299–304. doi: 10.1038/ijo.2015.182
147. Petroni ML, Caletti MT, Dalle Grave R, Bazzocchi A, Aparisi Gómez MP, Marchesini G. Prevention and treatment of sarcopenic obesity in women. Nutrients. (2019) 11:1302. doi: 10.3390/nu11061302
148. Nicklas BJ, Chmelo E, Delbono O, Carr JJ, Lyles MF, Marsh AP. Effects of resistance training with and without caloric restriction on physical function and mobility in overweight and obese older adults: a randomized controlled trial. Am J Clin Nutr. (2015) 101:991–9. doi: 10.3945/ajcn.114.105270
149. Deutz NEP, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. (2014) 33:929–36. doi: 10.1016/j.clnu.2014.04.007
150. Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Direct Assoc. (2013) 14:542–59. doi: 10.1016/j.jamda.2013.05.021
151. Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Cin Nutr Metab Care. (2009) 12:86–90. doi: 10.1097/MCO.0b013e32831cef8b
152. Sammarco R, Marra M, Di Guglielmo ML, Naccarato M, Contaldo F, Poggiogalle E, et al. Evaluation of hypocaloric diet with protein supplementation in middle-aged sarcopenic obese women: a pilot study. Obesity Facts. (2017) 10:160–7. doi: 10.1159/000468153
153. Bellia A, Garcovich C, D'Adamo M, Lombardo M, Tesauro M, Donadel G, et al. Serum 25-hydroxyvitamin D levels are inversely associated with systemic inflammation in severe obese subjects. Inter Emerg Med. (2013) 8:33–40. doi: 10.1007/s11739-011-0559-x
154. Moyer VA, U.S. Preventive Services Task Force*. Vitamin D and calcium supplementation to prevent fractures in adults: US preventive Services Task Force recommendation statement. Ann Intern Med. (2013) 158:691–6. doi: 10.7326/0003-4819-158-9-201305070-00603
155. Hassan-Smith ZK, Jenkinson C, Smith DJ, Hernandez I, Morgan SA, Crabtree NJ, et al. 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 exert distinct effects on human skeletal muscle function and gene expression. PLoS ONE. (2017) 12:e0170665. doi: 10.1371/journal.pone.0170665
156. Sohl E, de Jongh RT, Heijboer AC, Swart KMA, Brouwer-Brolsma EM, Enneman AW, et al. Vitamin D status is associated with physical performance: the results of three independent cohorts. Osteoporos Int. (2013) 24:187–96. doi: 10.1007/s00198-012-2124-5
157. Cho SS, Qi L, Fahey GC Jr, Klurfeld DM., Consumption of cereal fiber, mixtures of whole grains and bran, and whole grains and risk reduction in type 2 diabetes, obesity, cardiovascular disease. Am J Clin Nutr. (2013) 98:594–619. doi: 10.3945/ajcn.113.067629
158. Malik VS, Popkin BM, Bray GA, Després J-P, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes care. (2010) 33:2477–83. doi: 10.2337/dc10-1079
159. Papamichou D, Panagiotakos DB, Itsiopoulos C. Dietary patterns and management of type 2 diabetes: a systematic review of randomised clinical trials. Nutr Metab Cardiovasc Dis. (2019) 29:531–43. doi: 10.1016/j.numecd.2019.02.004
160. Díaz-López A, Babio N, Martínez-González MA, Corella D, Amor AJ, Fitó M, et al. Mediterranean diet, retinopathy, nephropathy, and microvascular diabetes complications: a post hoc analysis of a randomized trial. Diabetes Care. (2015) 38:2134–41. doi: 10.2337/dc15-1117
161. M.C. Archundia Herrera, Subhan FB, Chan CB. Dietary patterns and cardiovascular disease risk in people with type 2 diabetes. Curr Obes Rep. (2017) 6:405–13. doi: 10.1007/s13679-017-0284-5
162. Barazzoni R, Bischoff S, Boirie Y, Busetto L, Cederholm T, Dicker D, et al. Sarcopenic obesity: time to meet the challenge. Obesity Facts. (2018) 11:294–305. doi: 10.1159/000490361
163. Joseph A-M, Adhihetty PJ, Leeuwenburgh C. Beneficial effects of exercise on age-related mitochondrial dysfunction and oxidative stress in skeletal muscle. J Physiol. (2016) 594:5105–23. doi: 10.1113/JP270659
164. Mooren FC, Krüger K. Exercise autophagy, and apoptosis. Prog Mol Biol Transl Sci. (2015) 135:407–22. doi: 10.1016/bs.pmbts.2015.07.023
165. Lombardo M, Boaria A, Aulisa G, Padua E, Annino G, Pratesi A, et al. Sarcopenic obesity: etiology and lifestyle therapy. Eur Rev Med Pharmacol Sci. (2019) 23:7152–62. doi: 10.26355/eurrev_201908_18761
166. Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. (2018) 14:513–37. doi: 10.1038/s41574-018-0062-9
167. Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. (2011) 43:1334–59. doi: 10.1249/MSS.0b013e318213fefb
168. Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, et al. American association of clinical endocrinologists and american college of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. (2016) 22:1–203. doi: 10.4158/EP161365.GL
169. Hsu K-J, Liao C-D, Tsai M-W, Chen C-N. Effects of exercise and nutritional intervention on body composition, metabolic health, and physical performance in adults with sarcopenic obesity: a meta-analysis. Nutrients. (2019) 11:2163. doi: 10.3390/nu11092163
170. Chen H-T, Chung Y-C, Chen Y-J, Ho S-Y, Wu H-J. Effects of different types of exercise on body composition, muscle strength, and igf-1 in the elderly with sarcopenic obesity. J Am Geriatr Soc. (2017) 65:827–32. doi: 10.1111/jgs.14722
171. Villareal DT, Aguirre L, Gurney AB, Waters DL, Sinacore DR, Colombo E, et al. Aerobic or resistance exercise, or both, in dieting obese older adults. N Engl J Med. (2017) 376:1943–55. doi: 10.1056/NEJMoa1616338
172. Aune D, Norat T, Leitzmann M, Tonstad S, Vatten LJ. Physical activity and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis. Eur J Epidemiol. (2015) 30:529–42. doi: 10.1007/s10654-015-0056-z
173. Heiskanen MA, Motiani KK, Mari A, Saunavaara V, Eskelinen J-J, Virtanen KA, et al. Exercise training decreases pancreatic fat content and improves beta cell function regardless of baseline glucose tolerance: a randomised controlled trial. Diabetologia. (2018) 61:1817–28. doi: 10.1007/s00125-018-4627-x
174. Nieuwoudt S, Fealy CE, Foucher JA, Scelsi AR, Malin SK, Pagadala M, et al. Functional high-intensity training improves pancreatic β-cell function in adults with type 2 diabetes. Am J Physiol Endocrinol Metab. (2017) 313:E314–20. doi: 10.1152/ajpendo.00407.2016
175. Church TS, Blair SN, Cocreham S, Johannsen N, Johnson W, Kramer K, et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA. (2010) 304:2253–62. doi: 10.1001/jama.2010.1710
176. Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, et al. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab. (2002) 282:E601–7. doi: 10.1152/ajpendo.00362.2001
177. Papanicolaou DA, Ather SN, Zhu H, Zhou Y, Lutkiewicz J, Scott BB, et al. A phase IIA randomized, placebo-controlled clinical trial to study the efficacy and safety of the selective androgen receptor modulator (SARM), MK-0773 in female participants with sarcopenia. J Nutr Health Aging. (2013) 17:533–43. doi: 10.1007/s12603-013-0335-x
178. J.-Camporez PG, Petersen MC, Abudukadier A, Moreira GV, Jurczak MJ, Friedman G, et al. Anti-myostatin antibody increases muscle mass and strength and improves insulin sensitivity in old mice. Proc Natl Acad Sci USA. (2016) 113:2212–7. doi: 10.1073/pnas.1525795113
179. Vallianou NG, Stratigou T, Tsagarakis S. Metformin and gut microbiota: their interactions and their impact on diabetes. Hormones (Athens). (2019) 18:141–4. doi: 10.1007/s42000-019-00093-w
180. NICE Guideline Updates Team (UK). Type 2 Diabetes In Adults: Management. London: National Institute for Health and Care Excellence (2019).
181. DeFronzo RA, Barzilai N, Simonson DC. Mechanism of metformin action in obese and lean noninsulin-dependent diabetic subjects. J Clin Endocrinol Metab. (1991) 73:1294–301. doi: 10.1210/jcem-73-6-1294
182. DeFronzo RA, Goodman AM. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. the multicenter metformin study group. N Engl J Med. (1995) 333:541–9. doi: 10.1056/NEJM199508313330902
183. Foretz M, Guigas B, Viollet B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat Rev Endocrinol. (2019) 15:569–89. doi: 10.1038/s41574-019-0242-2
184. Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) group. JAMA. (1999) 281:2005–12. doi: 10.1001/jama.281.21.2005
185. Tyagi S, Sharma S, Gupta P, Saini A, Kaushal C. The peroxisome proliferator-activated receptor: a family of nuclear receptors role in various diseases. J Adv Pharmac Technol Res. (2011) 2:236–40. doi: 10.4103/2231-4040.90879
186. Wendy P, Melissa W, Zehuan L, Nguan T. An aPPARent functional consequence in skeletal muscle physiology via peroxisome proliferator-activated receptors. Int J Mol Sci. (2018) 19:1425. doi: 10.3390/ijms19051425
187. Berkowitz K, Peters R, Kjos SL, Goico J, Marroquin A, Dunn ME, et al. Effect of troglitazone on insulin sensitivity and pancreatic beta-cell function in women at high risk for NIDDM. Diabetes. (1996) 45:1572–9. doi: 10.2337/diabetes.45.11.1572
188. Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, et al. Effect of pioglitazone on pancreatic beta-cell function and diabetes risk in Hispanic women with prior gestational diabetes. Diabetes. (2006) 55:517–22. doi: 10.2337/diabetes.55.02.06.db05-1066
189. Teng H, Chen L. α-Glucosidase and α-amylase inhibitors from seed oil: a review of liposoluble substance to treat diabetes. Crit Rev Food Sci Nutr. (2017) 57:3438–48. doi: 10.1080/10408398.2015.1129309
190. Shimabukuro M, Tanaka A, Sata M, Dai K, Shibata Y, Inoue Y, et al. α-Glucosidase inhibitor miglitol attenuates glucose fluctuation, heart rate variability and sympathetic activity in patients with type 2 diabetes and acute coronary syndrome: a multicenter randomized controlled (MACS) study. Cardiovasc Diabetol. (2017) 16:86. doi: 10.1186/s12933-017-0571-1
191. van de Laar FA, Lucassen PL, Akkermans RP, van de Lisdonk EH, Rutten GE, van Weel C. Alpha-glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care. (2005) 28:154–63. doi: 10.2337/diacare.28.1.154
192. Tasyurek HM, Altunbas HA, Balci MK, Sanlioglu S. Incretins: their physiology and application in the treatment of diabetes mellitus. Diabetes Metab Res Rev. (2014) 30:354–71. doi: 10.1002/dmrr.2501
193. Chia CW, Egan JM. Incretins in obesity and diabetes. Ann N Y Acad Sci. (2020) 1461:104–26. doi: 10.1111/nyas.14211
194. List JF, Whaley JM. Glucose dynamics and mechanistic implications of SGLT2 inhibitors in animals and humans. Kidney Int. (2011) 79:S20–7. doi: 10.1038/ki.2010.512
195. Hwang JH, Jung HW. Effects of pharmacopuncture with wild ginseng complex in 2 elderly patients with obesity: case report. Medicine (Baltimore). (2018) 97:e11534. doi: 10.1097/MD.0000000000011534
196. Xu YXZ, Xi S, Qian X. Evaluating traditional chinese medicine and herbal products for the treatment of gestational diabetes mellitus. J Diabetes Res. (2019) (2019) 9182595. doi: 10.1155/2019/9182595
197. Zhou X, Xing B, He G, Lyu X, Zeng Y. The effects of electrical acupuncture and essential amino acid supplementation on sarcopenic obesity in male older adults: a randomized control study. Obes Facts. (2018) 11:327–34. doi: 10.1159/000491797
198. Liao Z, Zhao Y. Effect of heat-producing needling technique on the local skin temperature: clinical dataset. Data. (2018) 3:65. doi: 10.3390/data3040065
199. Wang M, Liu L, Zhang CS, Liao Z, Jing X, Fishers M, et al. Mechanism of traditional chinese medicine in treating knee osteoarthritis. J Pain Res. (2020) 13:1421–9. doi: 10.2147/JPR.S247827
200. Feng Y, Fang Y, Wang Y, Hao Y. Acupoint therapy on diabetes mellitus and its common chronic complications: a review of its mechanisms. Biomed Res Int. (2018) 2018:3128378. doi: 10.1155/2018/3128378
201. Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, et al. Exercise and type 2 diabetes: the american college of sports medicine and the american diabetes association: joint position statement. Diabetes Care. (2010) 33:e147–67. doi: 10.2337/dc10-9990
202. Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, et al. Physical activity/exercise and diabetes: a position statement of the american diabetes association. Diabetes Care. (2016) 39:2065–79. doi: 10.2337/dc16-1728
203. American Diabetes Association. Physical activity/exercise and diabetes. Diabetes Care. (2004) 27(Suppl. 1):S58–62. doi: 10.2337/diacare.27.2007.S58
Keywords: diabetes, sarcopenic obesity, insulin resistance, aging, inflammation
Citation: Wang M, Tan Y, Shi Y, Wang X, Liao Z and Wei P (2020) Diabetes and Sarcopenic Obesity: Pathogenesis, Diagnosis, and Treatments. Front. Endocrinol. 11:568. doi: 10.3389/fendo.2020.00568
Received: 18 April 2020; Accepted: 13 July 2020;
Published: 25 August 2020.
Edited by:
Indah Suci Widyahening, University of Indonesia, IndonesiaReviewed by:
Guo Chen, Jinan University, ChinaWanzhu Bai, China Academy of Chinese Medical Sciences, China
Copyright © 2020 Wang, Tan, Shi, Wang, Liao and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Wei, d2VpcGVuZ0BidWNtLmVkdS5jbg==; Zehuan Liao, bGlhbzAwNThAZS5udHUuZWR1LnNn
†These authors have contributed equally to this work
 Mina Wang
Mina Wang Yan Tan
Yan Tan Yifan Shi
Yifan Shi Xu Wang1
Xu Wang1 Zehuan Liao
Zehuan Liao Peng Wei
Peng Wei