- 1Department of Endocrinology and Metabolism, The Second Affiliated Hospital Zhejiang University School of Medicine, Hangzhou, China
- 2Department of Neurosurgery, The Second Affiliated Hospital Zhejiang University School of Medicine, Hangzhou, China
Background: Cushing’s disease is associated with an increased risk of pulmonary fungal infection, which could be a relative contraindication for pituitary adenoma excision surgery.
Case: We report a case of a patient with Cushing’s disease and pulmonary Cryptococcus neoformans. A 48-year-old woman was admitted to our hospital because of moon face and edema. Laboratory and radiological findings suggested a diagnosis of Cushing’s disease and pulmonary cryptococcus infection. Fluconazole 400 mg per day was administered intravenously and continued orally for 3 months. Both cryptococcus infection and hypercortisolism relieved and transsphenoidal resection was performed.
Conclusion: Cushing’s disease can be effectively treated with fluconazole to normalize cortisol concentration prior to pituitary surgery. Fluconazole is an alternative treatment especially in Cushing’s disease patients with cryptococcal pneumonia.
Introduction
Cushing’s syndrome (CS) is a rare disorder caused by chronic hypercortisolism with multisystem morbidity, increased mortality and decreased quality of life (1). Surgical excision of the pituitary, adrenal or ectopic lesion is recommended as the first-line therapy of CS, but not all patients are eligible for surgery (2). Guideline recommends medical treatment in patients who are not candidates for surgery, or have recurrent disease, and in patients awaiting the effects of radiotherapy (2). Medication used in patients with CS can be classified into steroidogenesis inhibitors, pituitary-targeting agents, and glucocorticoid receptor antagonists (3). Steroidogenesis inhibitors can block various steps of the steroid synthesis pathway. Ketoconazole, the most widely used medication, is unavailable in most countries and regions because of the risk of severe hepatotoxicity (4). New steroidogenesis inhibitors, including osilodrostat and levoketoconazole, have shown efficacy and acceptable safety profiles in clinical trials (5, 6). Though osilodrostat is approved by US Food and Drug Administration (FDA), it has not been used widely due to unavailability (7). The present work demonstrates the effect of fluconazole in controlling hypercortisolism in a patient with Cushing’s disease (CD) and pulmonary cryptococcus infection. All cases previously reported in the literature are also reviewed. In addition, a comprehensive bioinformatics analysis has been done to identify the potential targets of fluconazole in CD.
Case Presentation
A 48-year-old woman visited the hospital for evaluation of facial swelling and fatigue lasting for more than one year. She reported 5 kg of weight gain, hypertension, insomnia, weakness, and easy bruising. She denied any fever, oligouria, chest distress or shortness of breath. She went to the local hospital and the examinations were unremarkable except “hypokalemia.” She had been treated with irbesartan at a daily dose of 150 mg for 1 year. The social history and family history was unremarkable.
On examination, the blood pressure was 164/84 mm Hg, the pulse 56 beats per minute, the weight 53.7 kg, the height 146 cm and the body mass index (BMI) 25.19 kg/m2. She had moon face, dorsal fat pad, abdominal obesity and ecchymosis, but no striae. The remainder of the examination was normal.
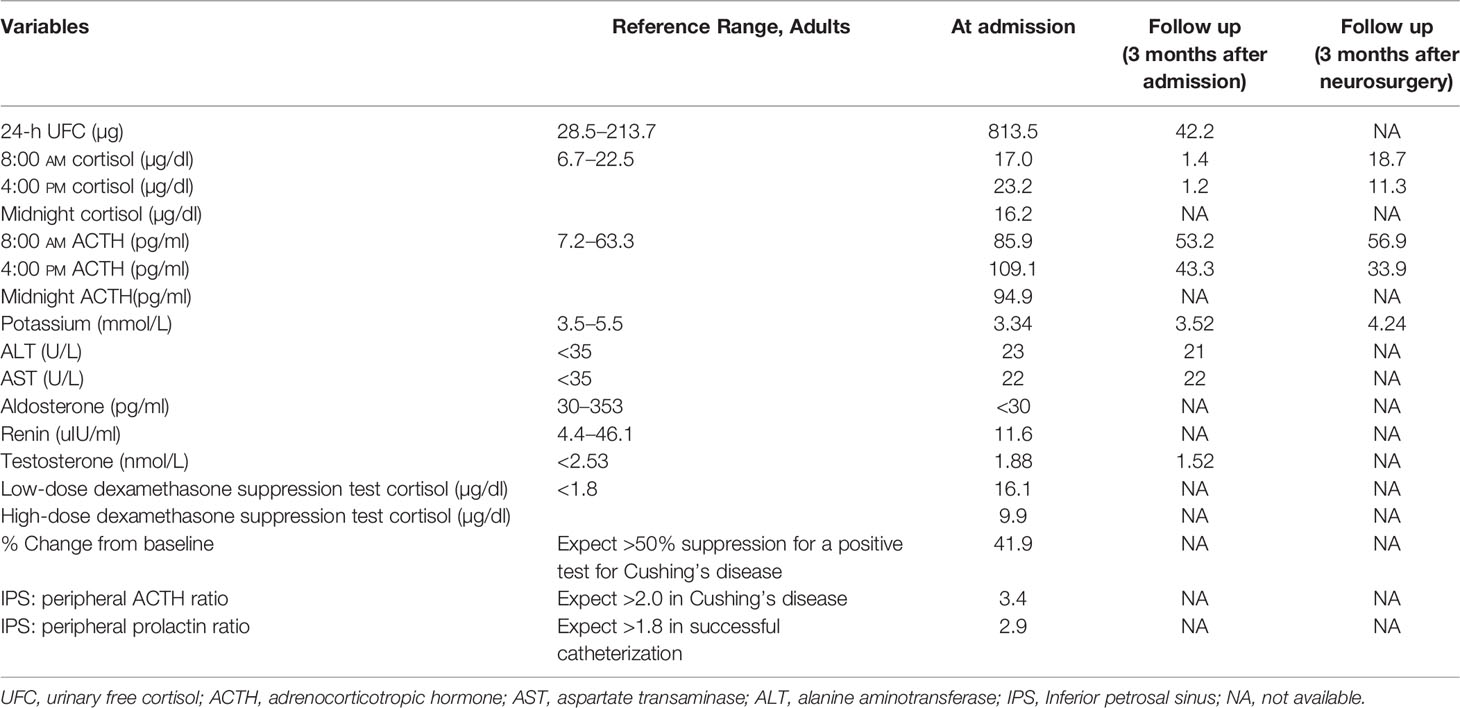
Initial investigations were summarized in Table 1. A midnight serum cortisol was 16.2 ng/dl and adrenocorticotropic hormone (ACTH) was 94.9 pg/ml 24-h urinary free cortisol (UFC) was 813.5 μg/day. Cortisol failed to suppress during a 48-h low-dose dexamethasone suppression test (16.1 ng/dl). These findings were consistent with an ACTH-dependent CS. No suppression was seen with high-dose dexamethasone test. A pituitary magnetic resonance imaging (MRI) demonstrated a 1.2 cm adenoma (Figure 1A). Inferior petrosal sinus (IPS) sampling confirmed a pituitary source of ACTH secretion. At the same time, the chest computed tomography revealed multiple lung nodules (Figure 1B). Bronchoalveolar lavage fluid (BALF) and serum cryptococcal antigen was positive. Lung biopsy histology confirmed pulmonary Cryptococcus neoformans. Cerebrospinal fluid (CSF) cryptococcal antigen was negative.
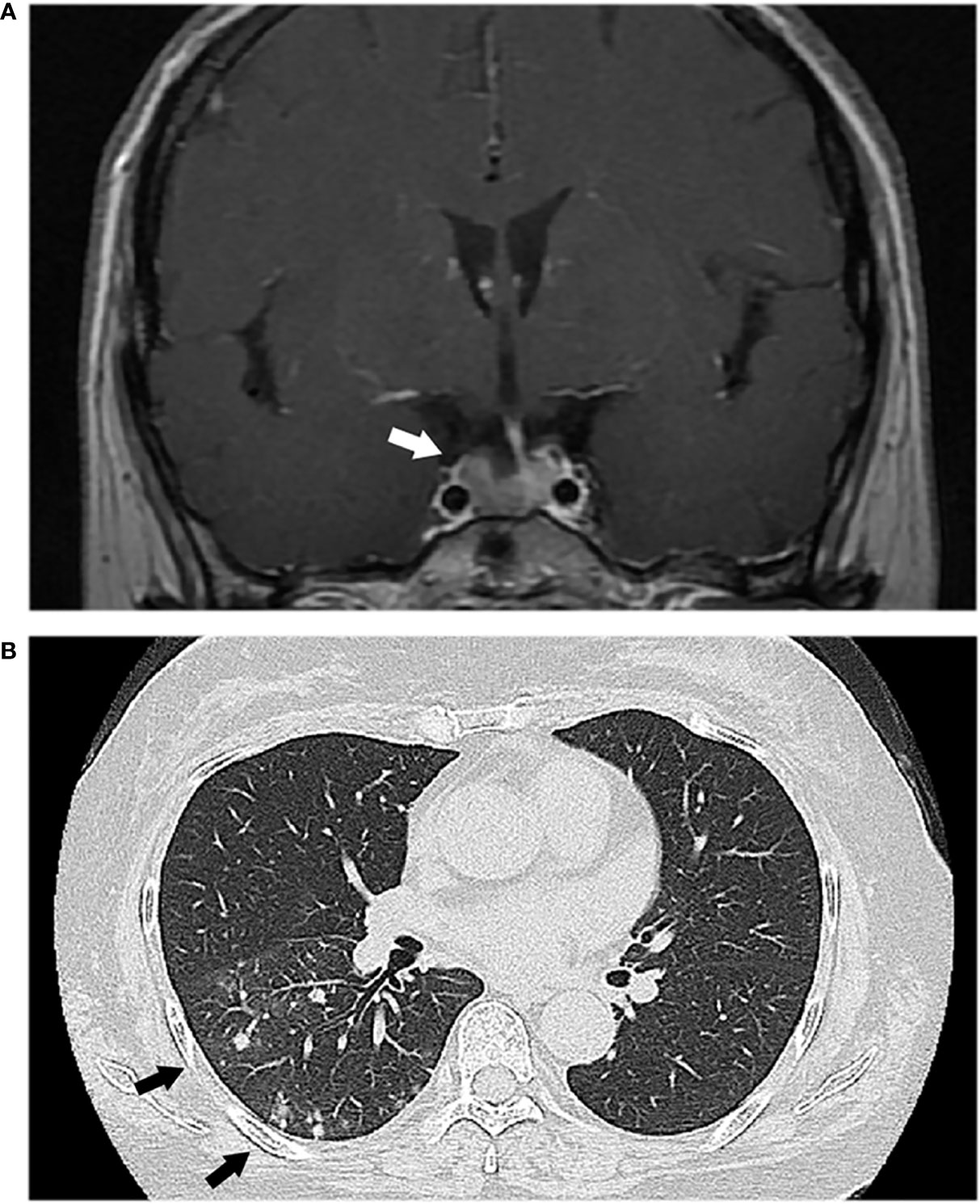
Figure 1 (A) Magnetic resonance imaging showed a pituitary adenoma (white arrow). (B) Chest computed tomography revealed multiple lung nodules (black arrows).
Immediate transsphenoidal selective adenomectomy (TSS) was not an option due to cryptococcus infection and possible postoperative cryptococcal meningitis. Intravenous fluconazole was started with 400 mg daily. One week later, the patient was discharged and continued with oral fluconazole 400 mg per day. At 3 months follow-up the pulmonary infection improved and the UFC was 42.2 μg/day. Fluconazole was withdrawn and TSS was performed. Histopathology confirmed ACTH-secreting pituitary adenoma.
Three months after TSS, the patient was in good general health and her serum cortisol and UFC normalized.
Discussion
TSS performed by an experienced neurosurgeon is the first-line treatment for CD in most patients (2). Patients with CD have an increased risk of cyrptococcal infection (8). Undiagnosed cryptococcal pneumonia can lead to postoperative cryptococcal meningitis and poor prognosis (9). Medical therapy is needed in patients with hypercortisolism when surgery is not possible. Ketoconazole, which is used to control both hypercortisolism and fungal infections, is unavailable due to severe adverse effect. Fluconazole, another azole antifungal, is an alternative to ketoconazole with less hepatotoxicity.
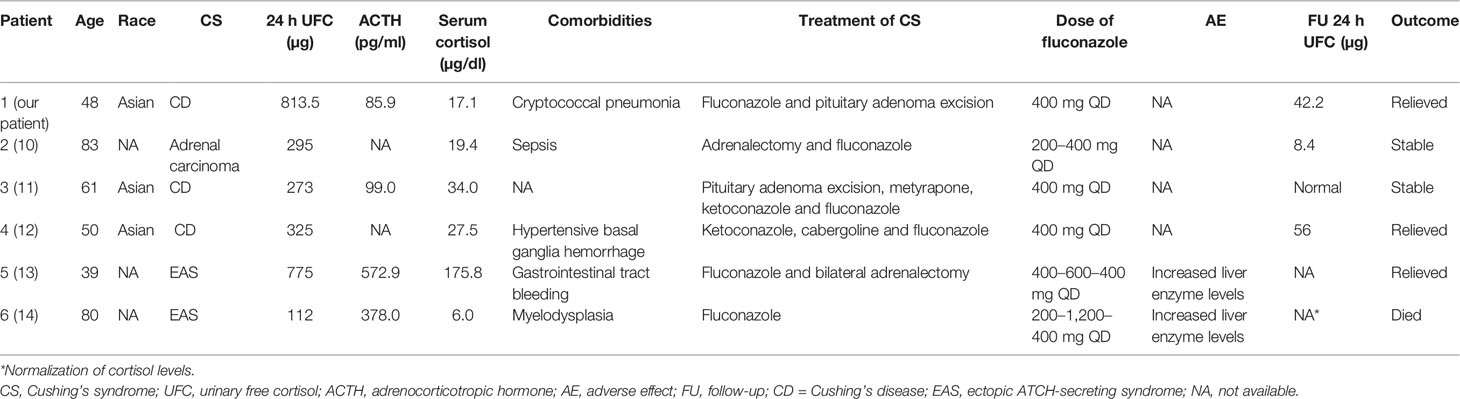
The present work revealed only 5 other CS patients treated successfully by fluconazole when surgery was not possible or was noncurative (as showed in Table 2) (10–14). All of patients were female, which could be attributed to the female predominant prevalence of CS (female-to-male ratio 3:1) (1). Three of them were diagnosed as CD, two with ectopic ATCH-secreting syndrome (EAS), and one had adrenal carcinoma. The median UFC levels was 310 µg/day (range, 112–813.45 µg/day). Hypocortisolism was more prone to occur in CD patients instead of other kinds of CS. The reported dose of fluconazole ranged from 200 to 1200 mg/day. Two of them developed liver dysfunction with the daily dose of fluconazole more than 400 mg. Liver enzymes normalized after the dose decreased to 400 mg/day.
In previous reports, inhibitory effect of fluconazole on glucocorticoid production was controversial and less potent than ketoconazole (10, 15, 16). Some case reports showed adrenal dysfunction with fluconazole in patients with server comorbidities (17–20). However, another study suggested that fluconazole was not associated with adrenal insufficiency compared with placebo (21).
The different in vitro effect of fluconazole could be attributed to the different experimental cell lines and different expression of steroid synthesizing enzymes of the cells. Previous studies demonstrated potent inhibitory effect of fluconazole on cortisol production in several human cell culture lines, but only a weak effect in rat cell culture lines (10, 15, 16). A previous study has revealed that the polymorphism in the CYP17A1 gene is associated with the responsiveness to steroidogenesis inhibitors in CS patients (22). However, the relationship of genetic variants with the efficacy of fluconazole is unknown.
We predict potential targets of fluconazole and obtained a network from STITCH (Figure 2). STITCH (http://stitch.embl.de) is a unique system pharmacological database describing the relationships between drugs, targets, and diseases (23). The results showed brain-derived neurotrophic factor (BDNF) was a target of fluconazole. BDNF is the neurotrophin mediating pro-neuronal survival and plasticity. Fiocco’s study showed BDNF regulated the activity of hypothalamic-pituitary-adrenal axis (24). Similar studies showed BDNF polymorphism had an influence on individual cortisol response to stress (25, 26). In Cushing disease patients, decreased BDNF level was observed after remission indicating a potential role of BDNF in Cushing disease (27). Over the past two decades, studies showed that BDNF and its receptor tropomyosin receptor kinase B (TrkB) were up-regulated in many types of cancers such as breast cancer and cervical cancer (28–30). The activated BDNF/TrkB signal stimulates a series of downstream pathways, including phosphoinositide 3-kinase/protein kinase (PI3K), Ras-Raf-mitogen activated protein kinase kinase-extracellular signal-regulated kinases, the phospholipase-C-γ pathway and the transactivation of epidermal growth factor receptor (28). Dworakowska’s study showed PI3K pathway was upregulated in ACTH- pituitary adenomas (31). Song’s study showed PI3K/AKT signaling pathway could affect migration and invasion of pituitary adenoma (32). In this series, two patients with fluconazole therapy showed decreased ACTH levers, which could not be attributed to effects on adrenocortical steroidogenesis (14). We propose the following hypothesis, BDNF/TrkB and PI3K pathways are potent therapeutic mechanisms for fluconazole in Cushing disease. In the future, more in vitro and in vivo studies are needed to verify our findings.
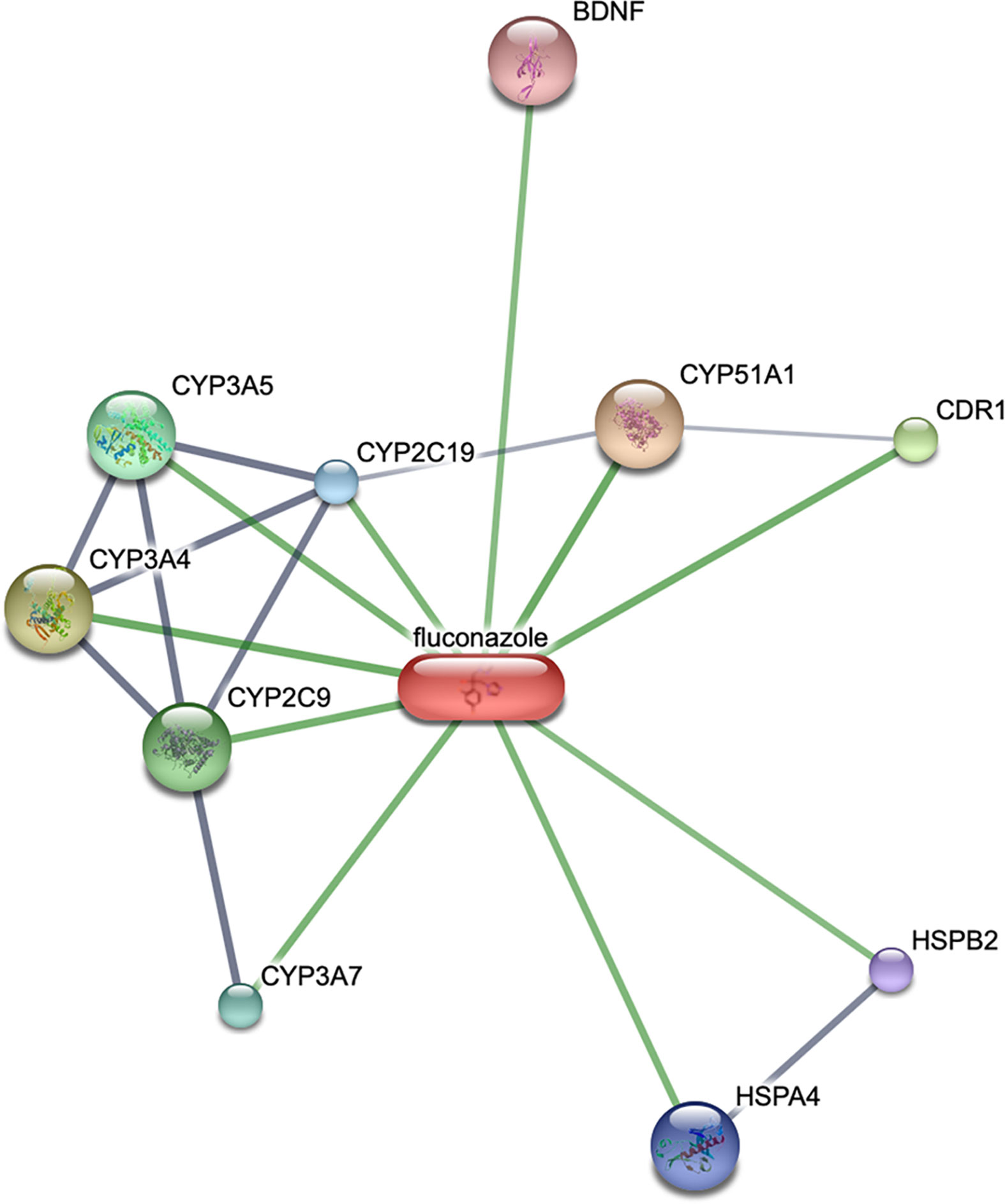
Figure 2 Potential targets of fluconazole and Cushing’s disease from STITCH. The node size reflects the degree of relationship: the smaller the degree value, the smaller the node size is.
Concluding Remarks
In summary, this case report and the bioinformatics analysis suggest that fluconazole might be effective in controlling hypercorticolism in CD patients. Further studies on the mechanism by which fluconazole inhibits cortisol production are needed to develop more potent and less toxic agents.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The patient provided written informed consent for research participation as well as for the publication of indirectly identifiable data (age, gender, and medical history).
Author Contributions
YZ and WL wrote the first draft of the manuscript. YZ, QW, FC, and YW made contributions to the acquisition of the clinical data. YZ and YW made critical revisions and approved final version. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Natural Science Foundation of Zhejiang Province (LQ19H160024).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing’s syndrome. Lancet (2015) 386(9996):913–27. doi: 10.1016/s0140-6736(14)61375-1
2. Nieman LK, Biller BM, Findling JW, Murad MH, Newell-Price J, Savage MO, et al. Treatment of Cushing’s Syndrome: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab (2015) 100(8):2807–31. doi: 10.1210/jc.2015-1818
3. Feelders RA, Newell-Price J, Pivonello R, Nieman LK, Hofland LJ, Lacroix A. Advances in the medical treatment of Cushing’s syndrome. Lancet Diabetes Endocrinol (2019) 7(4):300–12. doi: 10.1016/s2213-8587(18)30155-4
4. McCance DR, Ritchie CM, Sheridan B, Atkinson AB. Acute hypoadrenalism and hepatotoxicity after treatment with ketoconazole. Lancet (1987) 1(8532):573. doi: 10.1016/s0140-6736(87)90222-4
5. Biller BMK, Newell-Price J, Fleseriu M, Bertagna X, Findling J, Shimatsu A, et al. OR16-2 Osilodrostat Treatment in Cushing’s Disease (CD): Results from a Phase III, Multicenter, Double-Blind, Randomized Withdrawal Study (LINC 3). J Endocr Soc (2019) 3(Suppl 1):OR16–2. doi: 10.1210/js.2019-OR16-2
6. Fleseriu M, Pivonello R, Elenkova A, Salvatori R, Auchus RJ, Feelders RA, et al. Efficacy and safety of levoketoconazole in the treatment of endogenous Cushing’s syndrome (SONICS): a phase 3, multicentre, open-label, single-arm trial. Lancet Diabetes Endocrinol (2019) 7(11):855–65. doi: 10.1016/s2213-8587(19)30313-4
7. Voelker R. New Therapy Offers Direct Cortisol Control for Cushing Disease. Jama (2020) 323(15):1436. doi: 10.1001/jama.2020.5157
8. Lu L, Zhao YY, Yang HB, Tian XL, Xu ZJ, Lu ZL. Cushing’s disease with pulmonary Cryptococcus neoformans infection in a single center in Beijing, China: A retrospective study and literature review. J Formosan Med Assoc = Taiwan yi zhi (2019) 118(1 Pt 2):285–90. doi: 10.1016/j.jfma.2018.05.005
9. Liu Y, Feng M, Yao Y, Deng K, Bao X, Liu X, et al. Cryptococcal meningitis after transnasal transsphenoidal pituitary microsurgery of ACTH-secreting pituitary adenoma: A case report. Med (Baltimore) (2017) 96(28):e7124. doi: 10.1097/md.0000000000007124
10. Riedl M, Maier C, Zettinig G, Nowotny P, Schima W, Luger A. Long term control of hypercortisolism with fluconazole: case report and in vitro studies. Eur J Endocrinol (2006) 154(4):519–24. doi: 10.1530/eje.1.02120
11. Burns K, Christie-David D, Gunton JE. Fluconazole in the treatment of Cushing’s disease. Endocrinol Diabetes Metab Case Rep (2016) 2016:150115. doi: 10.1530/edm-15-0115
12. Teng Chai S, Haydar Ali Tajuddin A, N AW, Mustafa N, Sukor N, Kamaruddin NA. Fluconazole as a Safe and Effective Alternative to Ketoconazole in Controlling Hypercortisolism of Recurrent Cushing’s Disease: A Case Report. Int J Endocrinol Metab (2018) 16(3):e65233. doi: 10.5812/ijem.65233
13. Canteros TM, De Miguel V, Fainstein-Day P. Fluconazole treatment in severe ectopic Cushing syndrome. Endocrinol Diabetes Metab Case Rep (2019) 2019(1). doi: 10.1530/edm-19-0020
14. Schwetz V, Aberer F, Stiegler C, RP T, Obermayer-Pietsch B, Pilz S. Fluconazole and acetazolamide in the treatment of ectopic Cushing’s syndrome with severe metabolic alkalosis. Endocrinol Diabetes Metab Case Rep (2015) 2015:150027. doi: 10.1530/edm-15-0027
15. Eckhoff C, Oelkers W, Bähr V. Effects of two oral antimycotics, ketoconazole and fluconazole, upon steroidogenesis in rat adrenal cells in vitro. J Steroid Biochem (1988) 31(5):819–23. doi: 10.1016/0022-4731(88)90291-9
16. van der Pas R, Hofland LJ, Hofland J, Taylor AE, Arlt W, Steenbergen J, et al. Fluconazole inhibits human adrenocortical steroidogenesis in vitro. J Endocrinol (2012) 215(3):403–12. doi: 10.1530/joe-12-0310
17. Albert SG, DeLeon MJ, Silverberg AB. Possible association between high-dose fluconazole and adrenal insufficiency in critically ill patients. Crit Care Med (2001) 29(3):668–70. doi: 10.1097/00003246-200103000-00039
18. Shibata S, Kami M, Kanda Y, Machida U, Iwata H, Kishi Y, et al. Acute adrenal failure associated with fluconazole after administration of high-dose cyclophosphamide. Am J Hematol (2001) 66(4):303–5. doi: 10.1002/ajh.1063
19. Huang YW, Chang CC, Sun HY, Chen MY, Hung CC, Chang SC. Primary adrenal insufficiency in patients with acquired immunodeficiency syndrome: report of four cases. J Microbiol Immunol Infect = Wei mian yu gan ran za zhi (2004) 37(4):250–3.
20. Santhana Krishnan SG, Cobbs RK. Reversible acute adrenal insufficiency caused by fluconazole in a critically ill patient. Postgrad Med J (2006) 82(971):e23. doi: 10.1136/pgmj.2006.047258
21. Magill SS, Puthanakit T, Swoboda SM, Carson KA, Salvatori R, Lipsett PA, et al. Impact of fluconazole prophylaxis on cortisol levels in critically ill surgical patients. Antimicrob Agents Chemother (2004) 48(7):2471–6. doi: 10.1128/aac.48.7.2471-2476.2004
22. Valassi E, Aulinas A, Glad CA, Johannsson G, Ragnarsson O, Webb SM. A polymorphism in the CYP17A1 gene influences the therapeutic response to steroidogenesis inhibitors in Cushing’s syndrome. Clin Endocrinol (Oxf) (2017) 87(5):433–9. doi: 10.1111/cen.13414
23. Szklarczyk D, Santos A, von Mering C, Jensen LJ, Bork P, Kuhn M. STITCH 5: augmenting protein-chemical interaction networks with tissue and affinity data. Nucleic Acids Res (2016) 44(D1):D380–4. doi: 10.1093/nar/gkv1277
24. Fiocco AJ, D’Amico D, de Beaumont L, Poirier J, Lupien S. Association between BDNF Polymorphism and Hypothalamic-Pituitary-Adrenal Activity in Later Adulthood. Gerontology (2020) 66(2):131–7. doi: 10.1159/000502143
25. de Assis GG, Gasanov EV. BDNF and Cortisol integrative system—Plasticity vs. degeneration: Implications of the Val66Met polymorphism. Front Neuroendocrinol (2019) 55:100784. doi: 10.1016/j.yfrne.2019.100784
26. Tsuru J, Tanaka Y, Ishitobi Y, Maruyama Y, Inoue A, Kawano A, et al. Association of BDNF Val66Met polymorphism with HPA and SAM axis reactivity to psychological and physical stress. Neuropsychiatr Dis Treat (2014) 10:2123–33. doi: 10.2147/NDT.S68629
27. Valassi E, Crespo I, Keevil BG, Aulinas A, Urgell E, Santos A, et al. Affective alterations in patients with Cushing’s syndrome in remission are associated with decreased BDNF and cortisone levels. Eur J Endocrinol (2017) 176(2):221–31. doi: 10.1530/EJE-16-0779
28. Meng L, Liu B, Ji R, Jiang X, Yan X, Xin Y. Targeting the BDNF/TrkB pathway for the treatment of tumors. Oncol Lett (2019) 17(2):2031–9. doi: 10.3892/ol.2018.9854
29. Yuan Y, Ye HQ, Ren QC. Proliferative role of BDNF/TrkB signaling is associated with anoikis resistance in cervical cancer. Oncol Rep (2018) 40(2):621–34. doi: 10.3892/or.2018.6515
30. Tajbakhsh A, Mokhtari-Zaer A, Rezaee M, Afzaljavan F, Rivandi M, Hassanian SM, et al. Therapeutic Potentials of BDNF/TrkB in Breast Cancer; Current Status and Perspectives. J Cell Biochem (2017) 118(9):2502–15. doi: 10.1002/jcb.25943
31. Dworakowska D, Wlodek E, Leontiou CA, Igreja S, Cakir M, Teng M, et al. Activation of RAF/MEK/ERK and PI3K/AKT/mTOR pathways in pituitary adenomas and their effects on downstream effectors. Endocr Relat Cancer (2009) 16(4):1329–38. doi: 10.1677/ERC-09-0101
Keywords: Cushing’s syndrome, Cushing’s disease, fluconazole, hypercortisolism, medical treatment
Citation: Zhao Y, Liang W, Cai F, Wu Q and Wang Y (2020) Fluconazole for Hypercortisolism in Cushing’s Disease: A Case Report and Literature Review. Front. Endocrinol. 11:608886. doi: 10.3389/fendo.2020.608886
Received: 21 September 2020; Accepted: 12 November 2020;
Published: 17 December 2020.
Edited by:
Renzhi Wang, Peking Union Medical College Hospital (CAMS), ChinaReviewed by:
Mauro Antonio Czepielewski, Federal University of Rio Grande do Sul, BrazilNina Rosa De Castro Musolino, Universidade de São Paulo, Brazil
Copyright © 2020 Zhao, Liang, Cai, Wu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongjian Wang, V2FuZ3lvbmdqYWluQHpqdS5lZHUuY24=
 Yiming Zhao
Yiming Zhao Weiwei Liang
Weiwei Liang Feng Cai
Feng Cai Qun Wu
Qun Wu Yongjian Wang1*
Yongjian Wang1*