- 1Reproductive Medicine Center, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 2Shanghai Ji Ai Genetics and IVF Institute, Obstetrics and Gynecology Hospital, Fudan University, Shanghai, China
Background: Our study aimed to investigate if serum prolactin (PRL) levels associated with insulin resistance and beta-cell dysfunction in infertile patients with polycystic ovary syndrome (PCOS).
Methods: This was a retrospective cross-sectional study performed in the reproductive medicine center of the first affiliated hospital of Wenzhou Medical University. From January 2007 to August 2018, a total of 792 PCOS and 700 non-PCOS infertile women were included. All patients’ prolactin levels were in the normal range. PCOS was diagnosed according to the Rotterdam Criteria. Anthropometric parameters, blood pressure, serum prolactin levels, sex hormones, fasting lipids, fasting plasma glucose (FPG), fasting insulin (FINS) and hepatic biological parameters were measured in all subjects.
Results: Serum prolactin levels in PCOS women were significantly decreased compared with levels in non-PCOS women after adjusting for age and BMI (P < 0.05). Moreover, we found that prolactin levels were positively associated with high-density lipoprotein cholesterol (HDL-C) and negatively associated with age, BMI, waist circumference (WC), hip circumference (HC), luteinizing hormone/follicle stimulating hormone (LH/FSH), estradiol (E2), FINS, homeostasis model assessment of insulin resistance (HOMA-IR), homeostasis model assessment of β (HOMA-β), triglyceride (TG) and alanine aminotransferase (ALT) (P < 0.05). After adjusting for age and BMI, multiple linear regression analysis revealed that LH, LH/FSH, E2, FINS, HOMA-IR, and HOMA-β were negatively associated with serum PRL (P < 0.05).
Conclusions: Low serum PRL levels within the normal range associates with a higher incidence of insulin resistance and beta-cell dysfunction in infertile women with PCOS.
Background
Prolactin (PRL) is a multifunctional polypeptide that stimulates insulin secretion, beta-cell proliferation and survival (1–5). It is reported that circulating PRL can support islet growth via enhancing hepatic insulin sensitivity and the secretion of 5-hydroxytryptamine and serotonin (6). Moreover, numerous studies have documented that PRL affects metabolism homeostasis through the regulation of key enzymes and transporters related to insulin resistance, hypertension or coronary syndrome (7–12).
It is reported that the role of PRL on glucose metabolism and insulin resistance depends on its circulating concentration. In the clinic, PRL improves glucose homeostasis by increasing beta-cell mass under certain conditions such as pregnancy, whereas excessive high PRL levels in serum indicate a high-risk of obesity and dysmetabolism, such as decreased insulin sensitivity, abnormal glucose tolerance or progressive insulin resistance (13–17). It has previously been observed that high levels of PRL exacerbate insulin resistance and impair the insulin-secretory capacity in diabetic mice, in contrast to the normal adaptive increases in glucose stimulated insulin secretion through expanded beta-cell mass and insulin sensitivity realized with moderately increased PRL levels (18). Additionally, increasing evidence links low PRL levels within the normal range with markers and outcomes of metabolic dysfunction (19–21). Previous studies have shown that serum PRL levels were negatively correlated with insulin sensitivity and glucose in young individuals (22). Low PRL levels may have an adverse effect on the JAK2/STAT5 signaling pathway and depress the function of beta-cells (8, 23–27). The maintenance of high serum PRL levels within the physiological range can improve insulin sensitivity and promote the proper distribution of fat, which ultimately modifies the metabolic dysfunction (20).
Polycystic ovary syndrome (PCOS) is a prevalent endocrine and metabolic condition characterized by the disturbance of reproductive hormones, insulin resistance, abnormal glucose tolerance, hypertension and cardiovascular disease (28, 29). It has been reported that serum PRL levels were significantly decreased in patients with PCOS, possibly leading to insulin resistance and damage of beta-cells (23). Nonetheless, the function of beta-cells and status of metabolism remain unknown in infertile patients with PCOS exhibiting normal serum PRL levels.
Thus, we analyzed the association between serum PRL levels and clinical parameters, such as waist circumference (WC), hip circumference (HC), luteinizing hormone (LH), triglyceride (TG), or the homeostasis model assessment of insulin resistance (HOMA-IR), in infertile PCOS patients by a retrospective cross-sectional study, to explore the status of PRL secretion and association with insulin resistance and beta-cell function.
Methods
Inclusion Criteria
The study was designed as a retrospective observational study of infertile women (792 with PCOS and 700 with tubal infertility) who were initially treated by IVF-ET and referred to the reproductive center, at the First Affiliated Hospital of Wenzhou Medical University during January 2007 to August 2018. All patients’ prolactin levels were in the normal range. The detection lower limit for PRL was 20 mIU/L and if serum PRL is over 530 mIU/L, it is considered to be hyperprolactinemia (30, 31). Due to variation in detection assays and the different kit used, the normal range of PRL varies slightly among hospitals. Based on the assay and kit used at our hospital and published consensus on diagnosis and treatment of hyperprolactinemia, all patients had normal range prolactin levels below the upper reference limit (566.46 mIU/L) and above the lower reference limit (70.81 mIU/L). PCOS was diagnosed according to the Rotterdam criteria (Rotterdam, 2004). Written informed consent was obtained from all participating individuals and the study was approved by an Institutional Review Board that complies with all principles of the Declaration of Helsinki Principles Accord.
Exclusion Criteria
Patients with hormone therapy in three months, smoking, history of ovarian function damage by radiotherapy or chemotherapy, endometriosis, adenomyosis, thyroid disorders, liver disease, kidney disease, high blood pressure, pituitary microadenoma were excluded. Patients were also excluded if she had unexplained infertility, recurrent miscarriage or previous history of adverse pregnancy, congenital abnormalities such as chromosome aberration, congenital adrenal cortex hyperplasia, Cushing’s syndrome or testosterone-secreting tumors.
Clinical Samples
Fasting blood samples were collected between 9 to 11 am in the morning at least 2 h after wake-up and 8 h after fasting on day 2–5 of a menstrual cycle. The body height (m) and weight (kg), waist-circumference and hip-circumference were measured by experienced nurses according to standard protocols. Body mass index(BMI) was calculated as body weight in kilograms divided by body height in meters squared. Blood pressure was taken twice in an interval of 2 min after at least 10 min rest using a mercury sphygmomanometer.
Assay
The PRL, FSH, LH, T, and E2 levels in blood samples were measured using chemiluminescence assay on UniCel® DxI 800 Immunoassay System (Beckman Coulter, USA) with commercial kits according to manufacturer’s and supplier’s instructions. The FPG, TG, TC, LDL-C, HDL-C and hepatic function were measured by a Cobas 8000 modular analyzer kits, and FINS by a Cobas E602 automatic electrochemical luminescence analyzer according to manufacturer’s instructions.
Calculation
1. BMI = Weight (kg)/Height (m2)
2. Waist-hip ratio = Waist circumference (cm)/Hip circumference (cm)
3. Waist-height ratio = Waist circumference (cm)/Height (cm)
4. HOMA-IR = Fasting blood-glucose (FPG, mmol/L) × Fasting insulin (FINS, mIU/L)/22.5
5. HOMA-β = 20 × Fasting insulin (FINS, mIU/L)/[Fasting blood-glucose (FPG, mmol/L) − 3.5] (%)
6. The normal range of prolactin levels: 70.81–566.46 mIU/L.
Statistical Analysis
Parameters were not normally distributed and were therefore described using medians and quartiles. The rank sum test was used to compare differences between patients and controls. The correlation among variables was analyzed using the Spearman correlation analysis. Univariate logistic regression and multiple linear regression analysis were applied to reveal the association between prolactin and the index. All statistical analyses were performed using the SPSS version 22. P values < 0.05 were considered statistically significant.
Results
Baseline Characteristics of Study Population
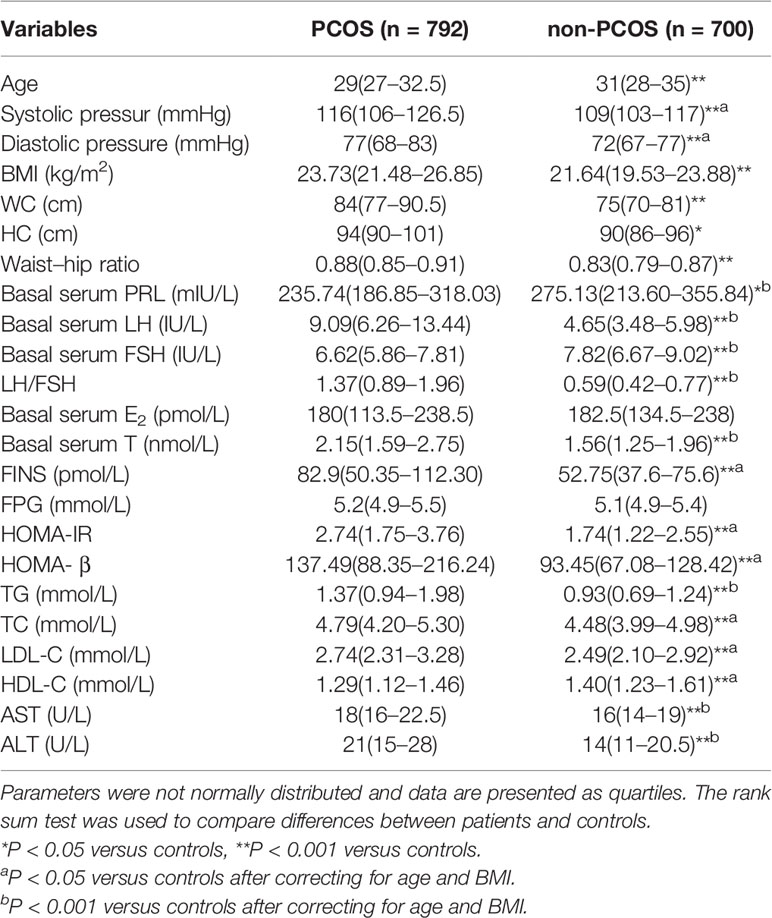
Characteristics of 792 PCOS and 700 non-PCOS women are provided in Table 1. Prolactin, FSH, and HDL-C levels were significantly lower in PCOS compared with non-PCOS women (235.74 versus 275.13 mIU/L, 6.62 versus 7.82 IU/L, and 1.29 versus 1.40 mmol/L, respectively), whereas blood pressure, LH, LH/FSH, testosterone (T), FINS, HOMA-IR, HOMA-β, TG, TC, LDL-C, AST and ALT remained higher in PCOS versus controls after correcting for age and BMI.
Clinical, Hormonal, and Metabolic Characteristics in Patients With Polycystic Ovary Syndrome
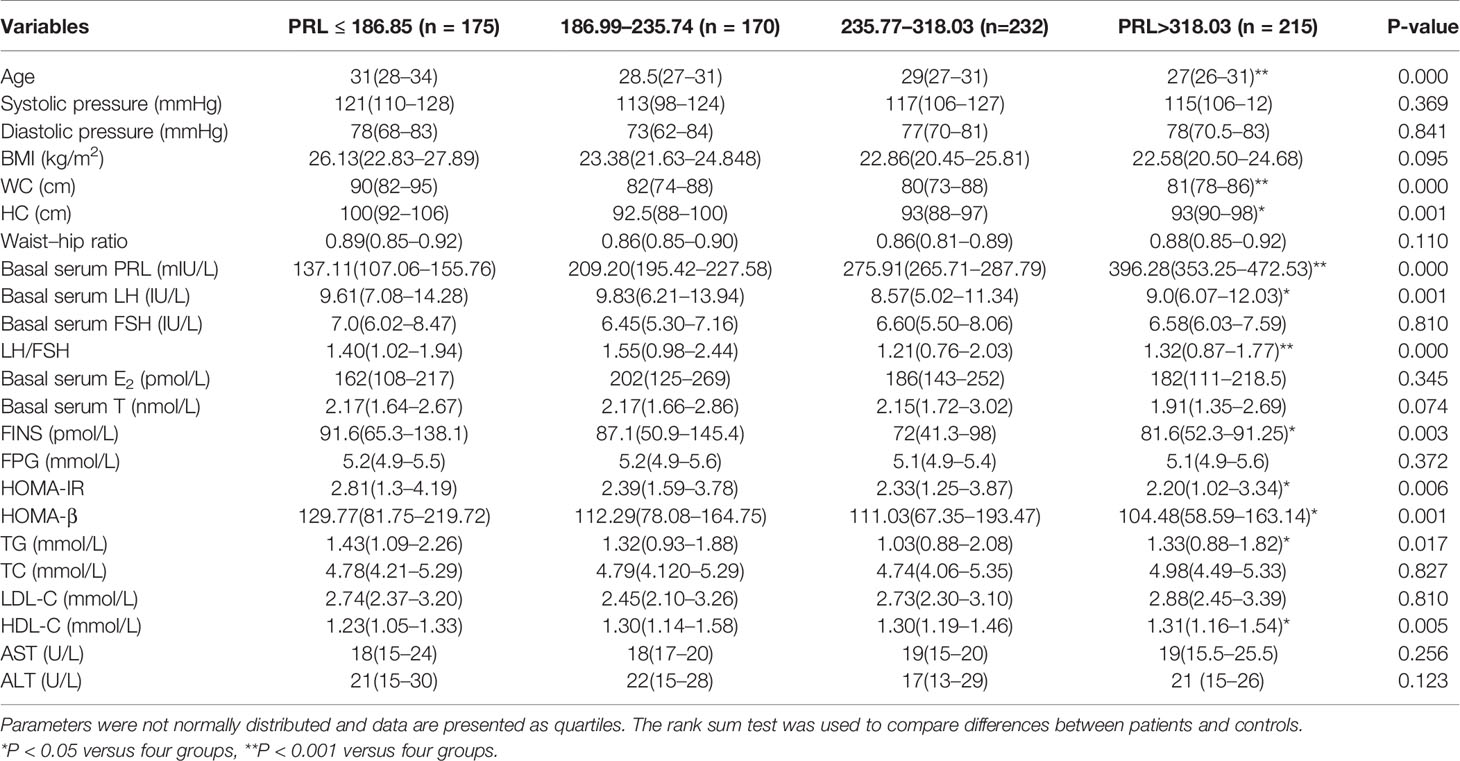
Table 2 shows the clinical and biochemical characteristics of PCOS patients grouped according to the shown quartiles of prolactin levels. Patients’ prolactin levels were inversely association with age, WC, HC, basal serum LH, LH/FSH, FINS, HOMA-IR, HOMA-β, and TG, but showed a positive association with HDL-C.
Associations of Serum Prolactin Levels With Sexual Hormonal and Metabolic Variables in Polycystic Ovary Syndrome Patients
Table 3 shows the analysis of bivariate associations between prolactin and hormonal and metabolic variables in patients with PCOS, which displayed a negative association between prolactin levels and age, WC, HC, Waist-hip ratio, basal serum LH, LH/FSH, E2, FINS, HOMA-IR, HOMA-β, TG, and ALT (P < 0.05 or P < 0.001). Additionally, we found that prolactin levels were positively associated with HDL-C (P < 0.05).
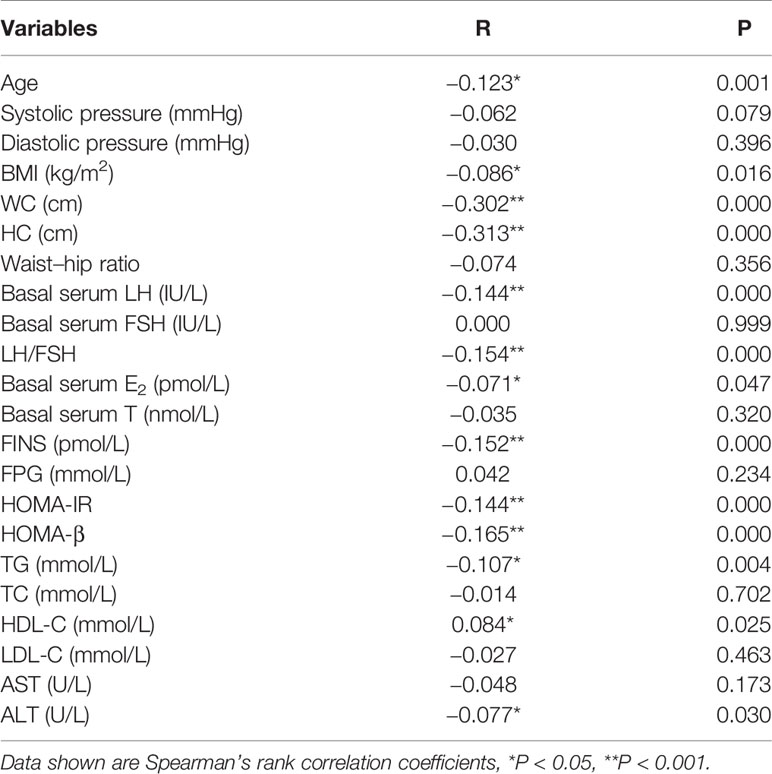
Table 3 Bivariate associations between prolactin and hormonal and metabolic variables in patients with PCOS.
Multiple Linear Regression Analysis on the Effect of Prolactin Upon Hormonal and Metabolic Outcomes in Patients With Polycystic Ovary Syndrome
Table 4 shows serum PRL was inversely associated with waist-hip ratio, LH, LH/FSH, E2, FINS, HOMA-IR and HOMA-beta after excluding the influence of age and BMI in multiple linear regression analysis.
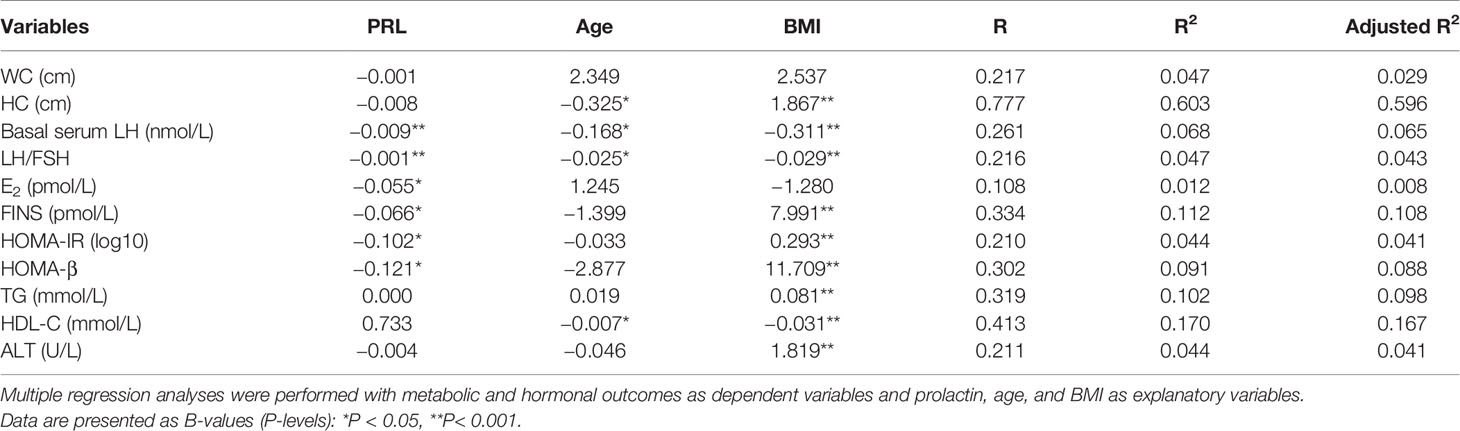
Table 4 Regression analysis on the effect of prolactin upon hormonal and metabolic outcomes in patients with PCOS.
Associations of Serum Prolactin Levels With Sexual Hormonal and Metabolic Variables in Non-Polycystic Ovary Syndrome Patients
Table 5 shows serum PRL was negative association with age, BMI and waist-hip ratio (P < 0.05 or P < 0.001) and positively correlated with basal serum LH, LH/FSH and basal serum T (P < 0.05) in non-PCOS patients. In multiple linear regression analysis, serum PRL was not directly correlated with waist-hip ratio, basal serum LH, LH/FSH, T, FINS, HOMA-IR and HOMA-β after adjusting for age and BMI (Table 6).
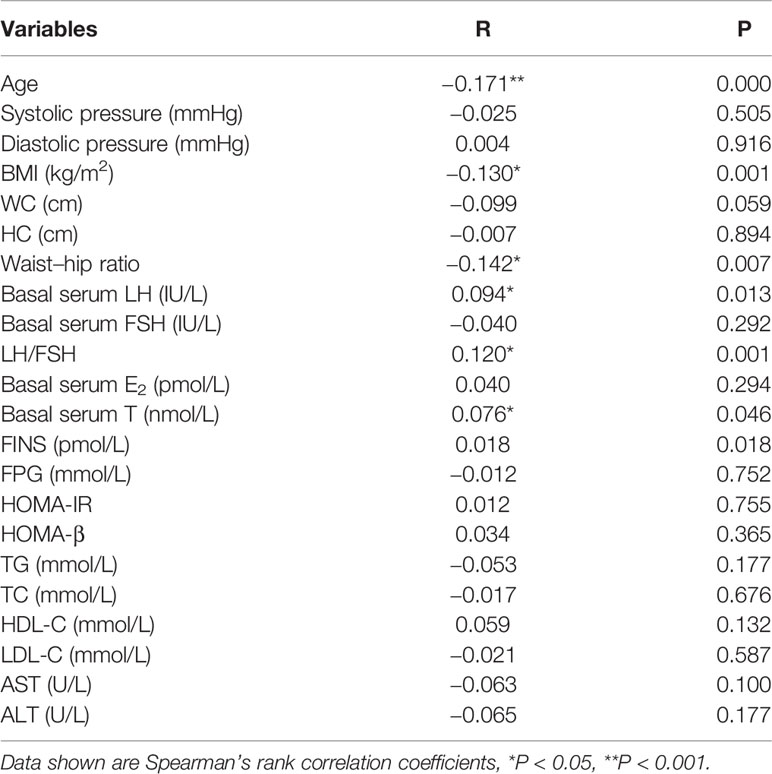
Table 5 Bivariate associations between prolactin and hormonal and metabolic variables in non-PCOS patients.
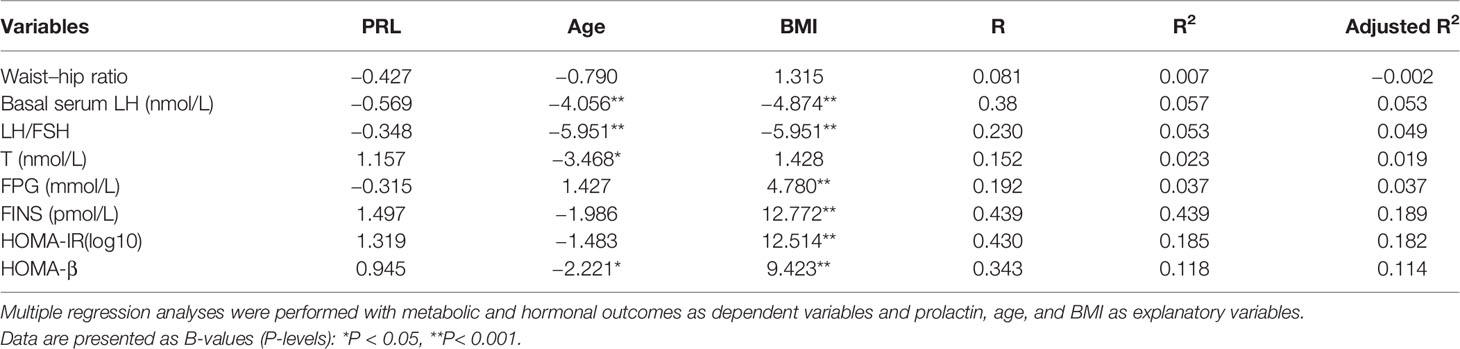
Table 6 Regression analysis on the effect of prolactin upon hormonal and metabolic outcomes in patients with non-PCOS.
Discussion
To the best of our knowledge, this is the first study to report the association between serum PRL levels within the normal range and insulin resistance and beta-cell dysfunction in infertile patients with PCOS. In the present study, we observed that serum PRL levels were correlated with insulin sensitivity and beta-cell function in infertile PCOS patients with normal PRL levels, through analysis of the association of PRL levels with WC/HC, glucose metabolism indexes, lipid metabolism indexes and sexual hormonal regulation indexes. Whereas the correlation between PRL levels and insulin sensitivity or beta-celll function was not observed in infertile non-PCOS patients with normal PRL levels. The consequences of PRL levels in PCOS patients showed a significant decline after excluding the influence of age and BMI (P < 0.001), compared with non-PCOS patients (exhibiting oviductal infertility).
As a clinical diagnostic standard of central obesity, WC reflects the addition of visceral and abdominal fat, which can predict obesity-related health risk and provide a key risk factor for metabolic syndrome (MS) involving the onset of insulin resistance (32–34). In addition, as an insulin resistance-related risk factor, serum PRL levels were found to have an inverse association with WC, similar to results for PCOS or hypertrichosis patients (23, 24). In addition to WC, many studies have investigated the association between HC and type 2 diabetes (T2D), cardiovascular disease, and hypertensive or dysmetabolism disorders (35–39). An epidemiologic survey of urban Tehranian women found that HC was independently and inversely associated with metabolic risk factors (40). However, recent studies have revealed that HC is an independent risk factor for MS and cardiovascular disease (41). A relatively small sample size or imprecision of the measurements cannot be excluded as possible explanations. Our findings showed that PRL levels in women with PCOS were inversely associated with WC, HC, HOMA-IR, or HOMA-β, but not with the waist-hip ratio. Hence, we deduce that low prolactin levels within the normal range may be associated with increased WC and HC and a higher risk for insulin resistance.
Circulating PRL levels exert wide effects upon glucose metabolism. Previous studies showed that high PRL disrupted glucose homeostasis and led to metabolic abnormalities (19, 42). Patients with hyperprolactinemia exhibit more insulin resistance and glucose intolerance compared with normal individuals. However, there are also increased MS- and T2D-related risks when low prolactin levels fall within the physiological range (20, 22, 27, 43–45). Our findings support an inverse association between serum PRL levels and clinical parameters including FINS, HOMA-IR and HOMA-β in women with PCOS, after adjustment of age and BMI. Furthermore, the FINS, HOMA-IR and HOMA-β in infertile women with PCOS were significant increased compared with non-PCOS women with oviductal infertility. Thus, we propose that serum PRL levels in infertile women with PCOS may be a predictor for insulin resistance and a functional deficiency of beta-cells.
In the analysis of reproductive hormones, we found that serum PRL exhibited inverse associations with LH, LH/FSH and E2 levels, but was not directly correlated with either T or FSH levels. Excessive PRL reduces the secretion of FSH and LH via suppression of gonadotrophin-releasing hormone (GnRH) synthesis and release (46–51). Therefore, we predict that higher PRL levels within the normal range may also decrease the production of gonadotrophins. Moreover, emotional changes and a reduced quality-of-life in PCOS patients may promote dopamine secretion, which may reduce PRL levels, and lead to the inverse association between prolactin and LH or LH/FSH (52, 53). Hence, we conclude that low PRL levels may increase the levels of LH and LH/FSH.
Furthermore, we found the PRL levels were also inversely associated with TG and positively associated with HDL-C. TG, TC and LDL-C were significantly higher in PCOS compared with non-PCOS women after correcting the influence of BMI. Thus, there is significant correlation between metabolic abnormalities and serum PRL. We suspect that lower prolactin levels within the normal range may lead to dyslipidemia. Additionally, the index of ALT and AST was higher in PCOS compared with non-PCOS patients (none exhibited hepatitis or liver dysfunction), after controlling for BMI, and PRL was inversely associated with ALT. Hence, low PRL levels within the normal range may have association with higher prevalence of liver damage in PCOS. A recent clinical study into the role of PRL in the development of non-alcoholic fatty liver disease (NAFLED) suggested that there was a negative association between PRL and the presence of NAFLED. Lower PRL levels were found in patients with severe hepatic steatosis compared with those displaying mild and moderate hepatic steatosis (54). Moreover, the results revealed a novel association between the central nervous system and liver, whereby PRL/PRLR improved hepatic steatosis via the CD36 pathway.
With regard to the research methods, some limitations need to be acknowledged. For instance, we cannot draw causality from simple correlations in a retrospective study. Prospective studies are needed to testify their correlation, and future research will allow a more detailed investigation of all parameters such as glucose tolerance testing, insulin releasing test or abdominal ultrasonography. In addition, considering that the secretion of PRL is pulsatile and follows a circadian rhythm with the highest plasma concentration reached during sleep, and the lowest observed in the morning about 2–3 h after waking up (55), several dynamic tests of PRL secretion may be needed.
Conclusions
Our clinical study lend support to the assumption that serum PRL levels within the normal range associates with glucose metabolism changes in infertile women with PCOS, suggesting that PRL may be a sensitive marker to predict insulin resistance and dysfunction of beta-cells. Further studies are warranted to confirm this association.
Data Availability Statement
All relevant data are contained within the article: the original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by The Ethics Committee of The First Affiliated Hospital of Wenzhou Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
HY contributed to the conception, data analysis, and draft writing. JL was involved in the acquisition of data. ZL was involved in the execution. HL provided suggestions on the study design. XC contributed to the conception and design of study. QC contributed to conception and study design and revised the article. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81901551).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Address all correspondence and requests for reprints: QC, MD, Reproductive Medicine Center, The First Affiliated Hospital of Wenzhou Medical University, No.96 Fuxue Road, Wenzhou 325000, People’s Republic of China; Telephone number: +86-13695885092; Fax number: +86-0577-88069786; Email:Y2hhbmN5MTAzMUAxNjMuY29t.
Abbreviations
PRL, Prolactin; PCOS, Polycystic ovary syndrome; FPG, Fasting plasma glucose; FINS, Fasting insulin; HDL-C, High-density lipoprotein cholesterol; LDL-C, Low density lipoprotein-cholesterol; WC, Waist circumference; HC, Hip circumference; LH, Luteinizing hormone; FSH, Follicle stimulating hormone; E2, Estradiol; HOMA-IR, Homeostasis model assessment of insulin resistance; HOMA-β, Homeostasis model assessment of β; TC, Total cholesterol TG, Triglyceride; ALT, Alanine aminotransferase; HPL, Hyperprolactinemia; MS, Metabolic syndrome; T2D, Type 2 diabetes; GnRH, Gonadotrophin-releasing hormone; NAFLED, Non-alcoholic fatty liver disease.
References
1. Grattan DR, Kokay IC. Prolactin: A pleiotropic neuroendocrine hormone. J Neuroendocrinol (2008) 20:752–63. doi: 10.1111/j.1365-2826.2008.01736.x
2. Crabtree JS, Scacheri PC, Ward JM, McNally SR, Swain GP, Montagna C, et al. Of mice and MEN1: Insulinomas in a conditional mouse knockout. Mol Cell Biol (2003) 23:6075–85. doi: 10.1128/MCB.23.17.6075-6085.2003
3. Freemark M, Avril I, Fleenor D, Driscoll P, Petro A, Opara E, et al. Targeted deletion of the PRL receptor: Effects on islet development, insulin production, and glucose tolerance. Endocrinology (2002) 143:1378–85. doi: 10.1210/endo.143.4.8722
4. Huang C, Snider F, Cross JC. Prolactin Receptor Is Required for Normal Glucose Homeostasis and Modulation of β-Cell Mass during Pregnancy. Endocrinology (2009) 150:1618–26. doi: 10.1210/en.2008-1003
5. Yu J, Xiao F, Zhang Q, Liu B, Guo Y, Lv Z, et al. PRLR Regulates Hepatic Insulin Sensitivity in Mice via STAT5. Diabetes (2013) 62:3103–13. doi: 10.2337/db13-0182
6. Kim H, Toyofuku Y, Lynn FC, Chak E, Uchida T, Mizukami H, et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med (2010) 16:804–8. doi: 10.1038/nm.2173
7. Auffret J, Freemark M, Carré N, Mathieu Y, Tourrel-Cuzin C, Lombès M, et al. Defective prolactin signaling impairs pancreatic β-cell development during the perinatal period. Am J Physiol Endocrinol Metab (2013) 305:E1309–18. doi: 10.1152/ajpendo.00636.2012
8. Ben-Jonathan N, Hugo ER, Brandebourg TD, LaPensee CR. Focus on prolactin as a metabolic hormone. Trends Endocrinol Metab (2006) 17:110–6. doi: 10.1016/j.tem.2006.02.005
9. Georgiopoulos GA, Stamatelopoulos KS, Lambrinoudaki I, Lykka M, Kyrkou K, Rizos D, et al. Prolactin and Preclinical Atherosclerosis in Menopausal Women With Cardiovascular Risk Factors. Hypertension (2009) 54:98–105. doi: 10.1161/HYPERTENSIONAHA.109.132100
10. Yavuz D, Deyneli O, Akpinar I, Yildiz E, Gözü H, Sezgin O, et al. Endothelial function, insulin sensitivity and inflammatory markers in hyperprolactinemic pre-menopausal women. Eur J Endocrinol (2003) 149:187–93. doi: 10.1530/eje.0.1490187
11. Raaz D, Wallaschofski H, Stumpf C, Yilmaz A, Cicha I, Klinghammer L, et al. Increased Prolactin in Acute Coronary Syndromes as Putative Co-activator of ADP-stimulated P-Selectin Expression. Horm Metab Res (2006) 38:767–72. doi: 10.1055/s-2006-955090
12. van Zaane B, Squizzato A, Reuwer AQ, van Zanten AP, Twickler MTB, Dekkers OM, et al. Prolactin and Venous Thrombosis. Arterioscler Thromb Vasc Biol (2011) 31:672–7. doi: 10.1161/ATVBAHA.110.209569
13. Tuzcu A, Yalaki S, Arikan S, Gokalp D, Bahcec M, Tuzcu S. Evaluation of insulin sensitivity in hyperprolactinemic subjects by euglycemic hyperinsulinemic clamp technique. Pituitary (2009) 12:330–4. doi: 10.1007/s11102-009-0183-1
14. Bahceci M, Tuzcu A, Bahceci S, Tuzcu S. Is hyperprolactinemia associated with insulin resistance in non-obese patients with polycystic ovary syndrome? J Endocrinol Invest (2003) 26:655–9. doi: 10.1007/BF03347025
15. Peric B, Kruljac I, Sundalic S, Pecina HI, Jovic A, Stefanovic M, et al. Obesity and hypercholesterolemia in patients with prolactinomas: Could DHEA-S and growth hormone be the missing link? Endocr Res (2016) 41:200–6. doi: 10.3109/07435800.2015.1135444
16. Galluzzi F, Salti R, Stagi S, La Cauza F, Chiarelli F. Reversible weight gain and prolactin levels - Long-term follow-up in childhood. J Pediatr Endocr Met (2005) 18:921–4. doi: 10.1515/JPEM.2005.18.9.921
17. Ratner LD, Stevens G, Bonaventura MM, Lux-Lantos VA, Poutanen M, Calandra RS, et al. Hyperprolactinemia induced by hCG leads to metabolic disturbances in female mice. J Endocrinol (2016) 230:157–69. doi: 10.1530/JOE-15-0528
18. Park S, Kim da S, Daily JW, Kim SH. Serum prolactin concentrations determine whether they improve or impair β-cell function and insulin sensitivity in diabetic rats. Diabetes Metab Res Rev (2011) 27:564–74. doi: 10.1002/dmrr.1215
19. Serri O, Li L, Mamputu J, Beauchamp M, Maingrette F, Renier G. The influences of hyperprolactinemia and obesity on cardiovascular risk markers: effects of cabergoline therapy. Clin Endocrinol (2006) 64:366–70. doi: 10.1111/j.1365-2265.2006.02469.x
20. Wang T, Lu J, Xu Y, Li M, Sun J, Zhang J, et al. Circulating Prolactin Associates With Diabetes and Impaired Glucose Regulation: A population-based study. Diabetes Care (2013) 36:1974–80. doi: 10.2337/dc12-1893
21. Corona G, Wu FC, Rastrelli G, Lee DM, Forti G, O’Connor DB, et al. Low Prolactin Is Associated with Sexual Dysfunction and Psychological or Metabolic Disturbances in Middle-Aged and Elderly Men: The European Male Aging Study (EMAS). J Sex Med (2014) 11:240–53. doi: 10.1111/jsm.12327
22. Wagner R, Heni M, Linder K, Ketterer C, Peter A, Böhm A, et al. Age-dependent association of serum prolactin with glycaemia and insulin sensitivity in humans. Acta Diabetol (2014) 51:71–8. doi: 10.1007/s00592-013-0493-7
23. Glintborg D, Altinok M, Mumm H, Buch K, Ravn P, Andersen M. Prolactin is associated with metabolic risk and cortisol in 1007 women with polycystic ovary syndrome. Hum Reprod (2014) 29:1773–9. doi: 10.1093/humrep/deu133
24. Friedrich N, Rosskopf D, Brabant G, Völzke H, Nauck M, Wallaschofski H. Associations of Anthropometric Parameters with Serum TSH, Prolactin, IGF-I, and Testosterone Levels: Results of the Study of Health in Pomerania (SHIP). Exp Clin Endocrinol Diabetes (2010) 118:266–73. doi: 10.1055/s-0029-1225616
25. Balbach L, Wallaschofski H, Völzke H, Nauck M, Dörr M, Haring R. Serum prolactin concentrations as risk factor of metabolic syndrome or type 2 diabetes? BMC Endocr Disord (2013) 13:12. doi: 10.1186/1472-6823-13-12
26. Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev (1998) 19:225–68. doi: 10.1210/edrv.19.3.0334
27. Ruiz-Herrera X, de los Ríos EA, Díaz JM, Lerma-Alvarado RM, Martínez de la Escalera L, Lopez-Barrera F, et al. Prolactin Promotes Adipose Tissue Fitness and Insulin Sensitivity in Obese Males. Endocrinology (2017) 158:56–68. doi: 10.1210/en.2016-1444
28. Glintborg D, Andersen M. An update on the pathogenesis, inflammation, and metabolism in hirsutism and polycystic ovary syndrome. Gynecol Endocrinol (2009) 26:281–96. doi: 10.3109/09513590903247873
29. Mani H, Levy MJ, Davies MJ, Morris DH, Gray LJ, Bankart J, et al. Diabetes and cardiovascular events in women with polycystic ovary syndrome: a 20-year retrospective cohort study. Clin Endocrinol (2013) 78:926–34. doi: 10.1111/cen.12068
30. Casanueva FF, Molitch ME, Schlechte JA, Abs R, Bonert V, Bronstein MD, et al. Guidelines of the pituitary society for the diagnosis and management of prolactinomas. Clin Endocrinol (2006) 65:265–73. doi: 10.1111/j.1365-2265.2006.02562.x
31. Melmed S, Casanueva FF, Hoffffman AR, Kleinberg DL, Montori VM, Schlechte JA, et al. Diagnosis and treatment of hyperprolactinemia: an endocrine society clinical practice guideline. J Clin Endocrinol Metab (2011) 96:273–88. doi: 10.1210/jc.2010-1692
32. Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity- related health risk. Am J Clin Nutr (2004) 79:379–84. doi: 10.1093/ajcn/79.3.379
33. Barroso LN, Farias DR, Soares-Mota M, Bettiol H, Barbieri MA, Foss MC, et al. Waist circumference is an effect modifier of the association between bone mineral density and glucose metabolism. Arch Endocrinol Metab (2018) 62:285–95. doi: 10.20945/2359-3997000000040
34. Da Silva CDC, Vasques ACJ, Zambon MP, Camilo DF, De Bernardi Rodrigues AM, Antonio MARG, et al. Sagittal abdominal diameter resembles waist circumference as a surrogate marker of insulin resistance in adolescents-Brazilian Metabolic Syndrome Study. Pediatr Diabetes (2018) 19:882–91. doi: 10.1111/pedi.12664
35. Parker ED, Pereira MA, Stevens J, Folsom AR. Association of Hip Circumference With Incident Diabetes and Coronary Heart Disease: The Atherosclerosis Risk in Communities Study. Am J Epidemiol (2009) 169:837–47. doi: 10.1093/aje/kwn395
36. Snijder MB, Dekker JM, Visser M, Bouter LM, Stehouwer CD, Kostense PJ, et al. Associations of hip and thigh circumferences independent of waist circumference with the incidence of type 2 diabetes: the Hoorn Study. Am J Clin Nutr (2003) 77:1192–7. doi: 10.1093/ajcn/77.5.1192
37. Snijder M, Zimmet P, Visser M, Dekker J, Seidell J, Shaw J. Independent and opposite associations of waist and hip circumferences with diabetes, hypertension and dyslipidemia: the AusDiab Study. Int J Obes Relat Metab Disord (2004) 28:402–9. doi: 10.1038/sj.ijo.0802567
38. Conway B, Xiang Y, Villegas R, Zhang X, Li H, Wu X, et al. Hip Circumference and the Risk of Type 2 Diabetes in Middle-Aged and Elderly Men and Women: The Shanghai Women and Shanghai Men’s Health Studies. Ann Epidemiol (2011) 21:358–66. doi: 10.1016/j.annepidem.2011.02.005
39. Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. Abdominal Obesity and the Risk of All-Cause, Cardiovascular, and Cancer Mortality. Circulation (2008) 117:1658–67. doi: 10.1161/CIRCULATIONAHA.107.739714
40. Esmaillzadeh A, Mirmiran P, Moeini SH, Azizi F. Larger hip circumference independently contributed to reduced metabolic risks in Tehranian adult women. Int J Cardiol (2006) 108:338–45. doi: 10.1016/j.ijcard.2005.05.019
41. Wang Z, Hoy WE. Waist circumference, body mass index, hip circumference and waist-to-hip ratio as predictors of cardiovascular disease in Aboriginal people. Eur J Clin Nutr (2004) 58:888–93. doi: 10.1038/sj.ejcn.1601891
42. Pala NA, Laway BA, Misgar RA, Dar RA. Metabolic abnormalities in patients with prolactinoma: response to treatment with cabergoline. Diabetol Metab Syndr (2015) 7:99. doi: 10.1186/s13098-015-0094-4
43. Chirico V, Cannavo S, Lacquaniti A, Salpietro V, Mandolfino M, Romeo PD, et al. Prolactin in obese children: a bridge between inflammation and metabolic-endocrine dysfunction. Clin Endocrinol (2013) 79:537–44. doi: 10.1111/cen.12183
44. Wang T, Xu Y, Xu M, Ning G, Lu J, Dai M, et al. Circulating Prolactin and Risk of Type 2 Diabetes: A Prospective Study. Am J Epidemiol (2016) 184:295–301. doi: 10.1093/aje/kwv326
45. Cincotta AH, Wilson JM, DeSouza CJ, Meier AH. Properly timed injections of cortisol and prolactin produce long-term reductions in obesity, hyperinsulinaemia and insulin resistance in the Syrian hamster (Mesocricetus auratus). J Endocrinol (1989) 120:385–91. doi: 10.1677/joe.0.1200385
46. Tay CC, Glasier AF, McNeilly AS. The 24 h pattern of pulsatile luteinizing hormone, follicle stimulating hormone and prolactin release during the first 8 weeks of lactational amenorrhoea in breastfeeding women. Hum Reprod (Oxford England) (1992) 7:951–8. doi: 10.1093/oxfordjournals.humrep.a137777
47. Koike K, Aono T, Miyake A, Tasaka K, Chatani F, Kurachi K. Effect of pituitary transplants on the LH-RH concentrations in the medial basal hypothalamus and hypophyseal portal blood. Brain Res (1984) 301:253–8. doi: 10.1016/0006-8993(84)91093-X
48. Sarkar DK, Frautschy SA, Mitsugi N. Pituitary portal plasma levels of oxytocin during the estrous cycle, lactation, and hyperprolactinemia. Ann N Y Acad Sci (1992) 652:397–410. doi: 10.1111/j.1749-6632.1992.tb34370.x
49. Bouchard P, Lagoguey M, Brailly S, Schaison G. Gonadotropin-Releasing Hormone Pulsatile Administration Restores Luteinizing Hormone Pulsatility and Normal Testosterone Levels in Males with Hyperprolactinemia. J Clin Endocrinol Metab (1985) 60:258–62. doi: 10.1210/jcem-60-2-258
50. Zinaman MJ, Cartledge T, Tomai T, Tippett P, Merriam GR. Pulsatile GnRH stimulates normal cyclic ovarian function in amenorrheic lactating postpartum women. J Clin Endocrinol Metab (1995) 80:2088–93. doi: 10.1210/jcem.80.7.7608260
51. Grattan DR, Selmanoff M. Prolactin- and testosterone-induced inhibition of LH secretion after orchidectomy: role of preoptic and tuberoinfundibular gamma-aminobutyric acidergic neurones. J Endocrinol (1994) 143:165–74. doi: 10.1677/joe.0.1430165
52. Hahn S, Janssen OE, Tan S, Pleger K, Mann K, Schedlowski M, et al. Clinical and psychological correlates of quality-of-life in polycystic ovary syndrome. Eur J Endocrinol (2005) 153:853–60. doi: 10.1530/eje.1.02024
53. Altinok ML, Glintborg D, Depont Christensent R, Hallast J, Andersen M. Prescription of antidepressants is increased in Danish patients with polycystic ovary syndrome and is associated with hyperandrogenism. A population-based cohort study. Clin Endocrinol (2014) 80:884–9. doi: 10.1111/cen.12365
54. Zhang P, Ge Z, Wang H, Feng W, Sun X, Chu X, et al. Prolactin improves hepatic steatosis via CD36 pathway. J Hepatol (2018) 68:1247–55. doi: 10.1016/j.jhep.2018.01.035
Keywords: prolactin, infertility, polycystic ovary syndrome (PCOS), insulin resistance, beta-cell function
Citation: Yang H, Lin J, Li H, Liu Z, Chen X and Chen Q (2021) Prolactin Is Associated With Insulin Resistance and Beta-Cell Dysfunction in Infertile Women With Polycystic Ovary Syndrome. Front. Endocrinol. 12:571229. doi: 10.3389/fendo.2021.571229
Received: 29 June 2020; Accepted: 04 January 2021;
Published: 25 February 2021.
Edited by:
Sara Marchiani, University of Florence, ItalyReviewed by:
Natassia Rodrigo, The University of Sydney, AustraliaThemistoklis Tzotzas, St. Luke’s Hospital, Greece
Copyright © 2021 Yang, Lin, Li, Liu, Chen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qianqian Chen, Nzg1NDQ2MTYzQHFxLmNvbQ==; Xia Chen, Z3JhY2V3eml2ZkAxNjMuY29t
†These authors have contributed equally to this work
 Haiyan Yang1†
Haiyan Yang1† Jie Lin
Jie Lin He Li
He Li Zhangwei Liu
Zhangwei Liu Xia Chen
Xia Chen Qianqian Chen
Qianqian Chen