- 1Fourth Affiliated Hospital, Zhejiang University School of Medicine, Yiwu, China
- 2Department of Gynecology, Second Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, China
- 3Women’s Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 4Department of Obstetrics and Gynecology, First Affiliated Hospital, Heilongjiang University of Chinese Medicine, Harbin, China
- 5Heilongjiang Province Hospital, Harbin, China
Objective: To evaluate associations between serum lipid levels and treatment outcomes in women undergoing assisted reproduction.
Materials and Methods: The study included 2011 women who underwent in vitro fertilization/intracytoplasmic sperm injection with fresh embryo transfer. Serum lipid evaluation included total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG). Ovarian stimulation outcomes included endometrial thickness and the number of oocytes retrieved, and reproductive outcomes included live birth, clinical pregnancy, and miscarriage.
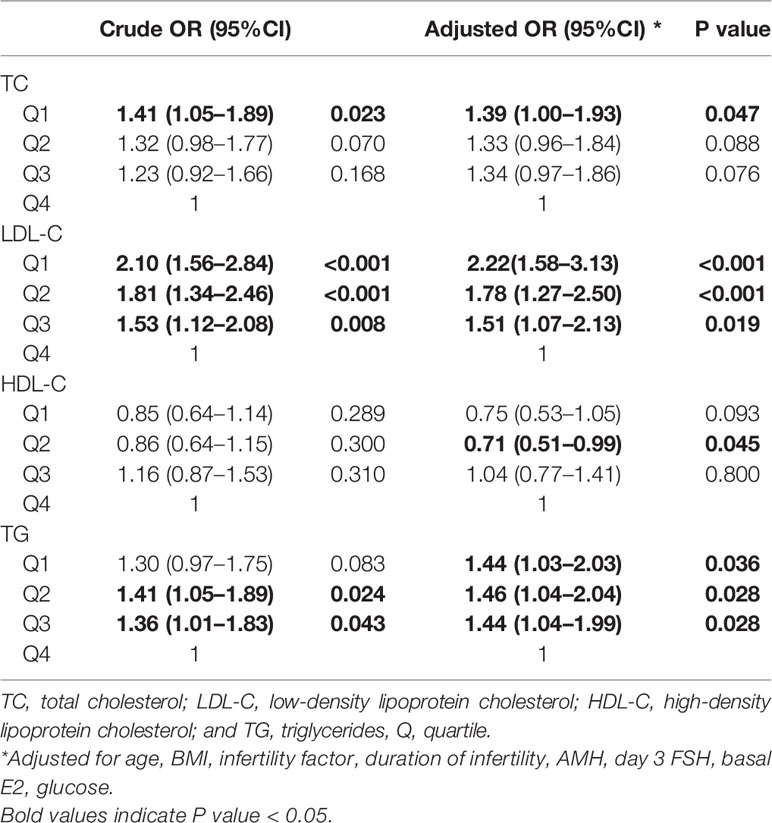
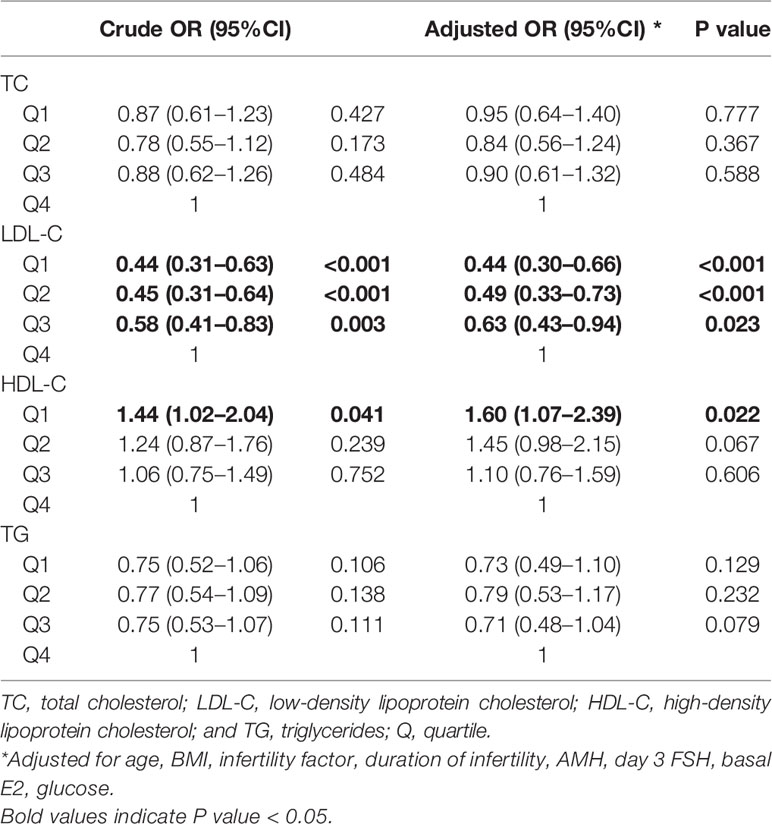
Results: Higher HDL-C quartiles were associated with more oocytes retrieved. Lower TC (quartile 1 odds ratio [OR] 1.59 [1.21–2.08], quartile 3 OR 1.36 [1.04–1.77]), LDL-C (quartile 1 OR 1.41 [1.07–1.86]), and TG (quartile 2 OR 1.39 [1.06–1.84]) were independently associated with clinical pregnancy after adjusting for potential confounders. Lower LDL-C (quartile 1 OR 2.22 [1.58–3.13], quartile 2 OR 1.78 [1.27–2.50], quartile 3 OR 1.51 [1.07–2.13]), TC (quartile 1 OR 1.39 [1.00–1.93]), TG (quartile 1 OR 1.44 [1.03–2.03], quartile 2 OR 1.46 [1.04–2.04], quartile 3 OR 1.44 [1.04–1.99]), and higher HDL-C (quartile 2 OR 0.71 [0.51–0.99]) were independently associated with live birth. Higher LDL-C (quartile 1 OR 0.44 [0.30–0.66], quartile 2 OR 0.49 [0.33–0.73], quartile 3 OR 0.63 [0.43–0.94]) and lower HDL-C (quartile 1 OR 1.60 [1.07–2.39]) were independently associated with miscarriage.
Conclusions: Serum lipid levels were associated with treatment outcomes in women undergoing assisted reproduction.
Introduction
Infertility, defined as the inability to conceive a child after 1 year, is estimated to affect millions of couples worldwide (1). Infertility and assisted reproduction technology are associated with considerable emotional and financial burdens, and assisted reproduction does not have a good success rate (2–4). In conjunction with the worldwide obesity epidemic (5), lipid abnormalities are common (6, 7). The success of assisted reproduction may be affected by the patient’s social, psychological, and physical status. Routine serum lipid screening includes total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG). Lipid disorders are hypothesized to play a role in female reproduction. The synthesis of steroid hormones in reproductive tissues occurs in thecal and granulosa cells, and utilizes cholesterol as the substrate for steroidogenesis (8). HDL-C and LDL-C both play important roles in the transport of cholesterol to ovarian tissue (9). Altered endometrial lipid levels may impair endometrial receptivity and early embryo implantation (10). Acute atherosis and diffuse lipid infiltration can also be found in the placenta, and are pathognomonic signs of potential placental malfunction (11). These observations suggest possible links between serum lipids and reproductive health in women.
Some studies have reported associations between serum lipids and reproductive outcomes in subfertile women. In one prospective cohort study that included couples attempting pregnancy, increased serum free cholesterol concentrations in both men and women led to reduced fecundity (12). In couples with prior pregnancy loss, higher serum TC and TG were associated with less spontaneous pregnancy (13). Another study in women undergoing in vitro fertilization (IVF) suggests that HDL within the follicular fluid may play protective roles in the health of the human oocyte by reducing oocyte fragmentation (14, 15). It has also been reported that follicular fluid HDL had an antioxidative function and was associated with normal oocyte fertilization (16, 17).
Few studies have investigated associations between serum lipid profiles and treatment outcomes in women undergoing assisted reproduction, particularly after ruling out the effects of potential confounders such as body mass index (BMI). At 40%–50% the prevalence of lipid abnormalities is high (6, 7), and understanding associations between serum lipid levels and reproductive outcomes after assisted reproduction may provide an early, modifiable, and inexpensive treatment choice. The objective of the current study was to investigate associations between treatment outcomes and preconception serum TC, LDL-C, HDL-C, and TG levels in a cohort of women undergoing IVF/intracytoplasmic sperm injection (ICSI) cycles.
Materials and Methods
Study Design
The present retrospective study was conducted from January 2018 to October 2020 at a reproductive medical center of a university-affiliated hospital in mainland China. The study was approved by the ethics committee of the hospital (IRB-20200235-R), and was conducted in accordance with the Declaration of Helsinki. Because it was a retrospective study and only de-identified data were analyzed, the ethics committee waived the standard requirement for informed consent from the women in the study.
Study Population
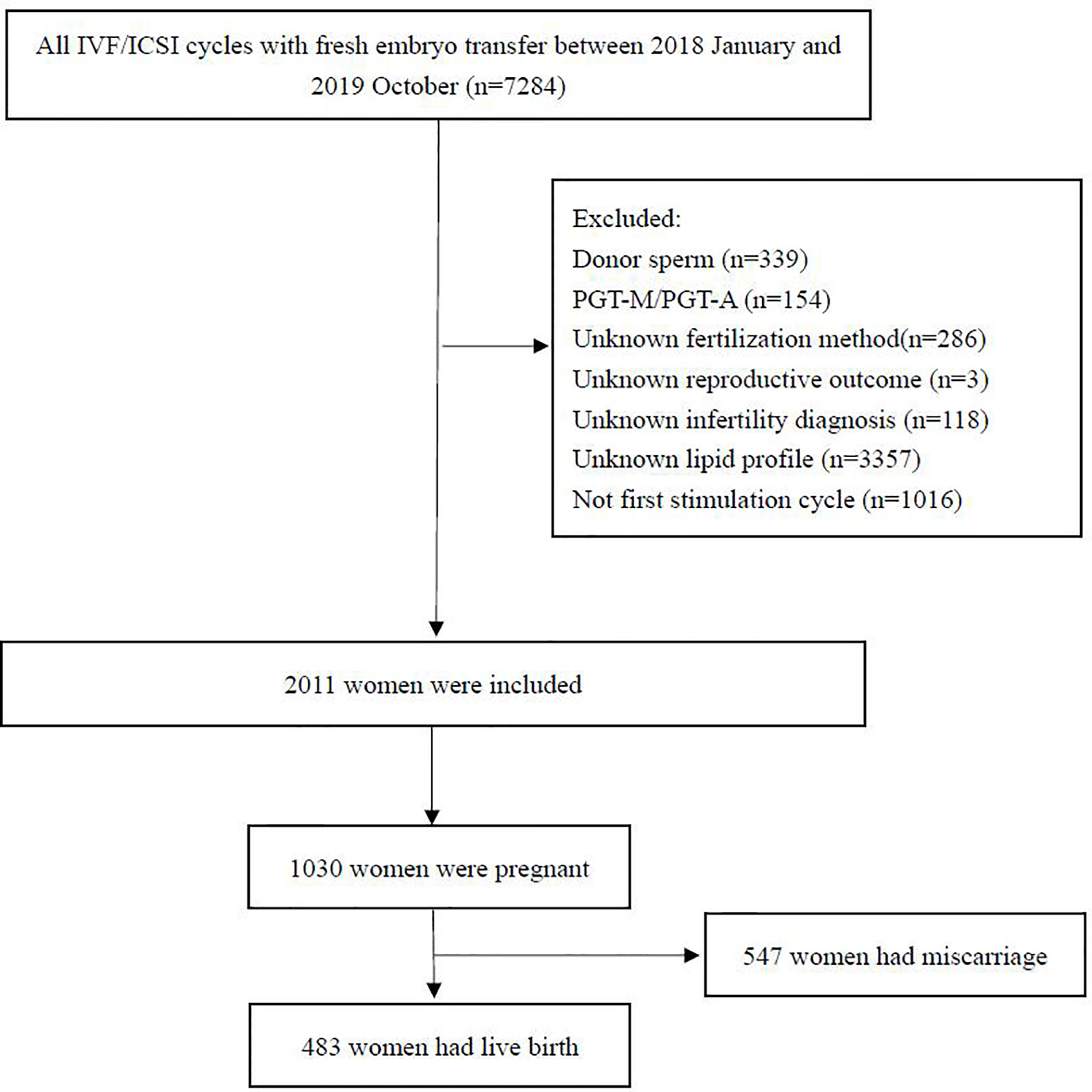
Patients aged between 18 and 45 years who underwent their first IVF/ICSI cycle with fresh embryo transfer from January 2018 to October 2020 were included in the study. Patients with infertility of any cause were included. The inclusion criteria were (1) IVF/ICSI with fresh embryo transfer; and (2) serum lipid levels evaluated before treatment. The exclusion criteria were (1) the use of an egg or sperm donor; (2) preimplantation genetic diagnosis or screening; (3) lack of serum lipid evaluation; (4) lack of treatment outcome data; and (5) incorrect information or important data missing from the database. Data from a total of 7,284 women were initially screened, of which 2011 were included in the final analysis. A flow chart representing the study procedure is shown in Figure 1.
During the initial evaluation age, height, weight, and current smoker status were recorded, and BMI was calculated. Reproductive characteristics included infertility diagnosis, primary infertility, and duration of infertility. Anti-Mullerian hormone (AMH), day 3 follicle-stimulating hormone (FSH), basal estradiol (E2), TC, LDL-C, HDL-C, TG, and glucose were measured. After treatment, ovarian stimulation days, total gonadotropin dose, E2 on the day of human choriogonadotropin (hCG) administration, endometrial thickness, fertilization procedure, number of oocytes retrieved, number of two pronuclei embryos, and number of embryo transfers were recorded.
Laboratory Assessment
Preconception serum samples were collected on days 2–4 of the women’s menstrual cycles. TG, LDL-C, HDL-C, and TG were measured using a Beckman AU5800 chemistry analyzer (Beckman Coulter, United States). TC was analyzed via a cholesterol oxidase enzymatic method (Beckman Coulter, United States), LDL-C and HDL-C were analyzed via a direct enzymatic method (Beckman Coulter, United States), and TG was analyzed via the GPO-PAP method (Beckman Coulter, United States). The intra-assay laboratory coefficients of variation ranged from 0.50% to 1.53%, and the between-assay coefficients of variation ranged from 1.08% to 3.55%. The diagnostic criteria for abnormal serum lipid levels were based on the Chinese Guidelines on Prevention and Treatment of Dyslipidemia in Adults (18), and were as follows: TC ≥ 6.22 mmol/L = abnormal; TG ≥ 2.26 mmol/L = abnormal; HDL-C < 1.04 mmol/L = abnormal; LDL-C ≥ 4.14 mmol/L = abnormal.
Ovarian Stimulation
In the short-acting gonadotrophin-releasing hormone agonist (GnRH-a) (Triptorelin, Ferring AG, Germany) protocol, GnRH-a was administrated daily in the mid-luteal phase of the preceding cycle. After 14 days, serum luteinizing hormone (LH), FSH, and E2 were assessed and 150–300 IU recombinant FSH (Gonal-F, Merck Serono, Switzerland) was administered daily when FSH and LH were <5 IU/L and E2 was <50 pg/ml. GnRH-a was continued until trigger. In the subsequent GnRH-A (Cetrorelix, Merck Serono, France) protocol, 150–300 IU recombinant FSH was initiated on day 2 or day 3 of the menstrual cycle and maintained until trigger, and 0.25 mg GnRH-A was used daily when the leading follicles reached a mean diameter of 14 mm until trigger. During both protocols, if three follicles reached a mean diameter of 17 mm or two follicles reached a mean diameter of 18 mm, recombinant hCG (Ovidrel, Serono, Italy) was administered subcutaneously. Oocyte retrieval was performed 36 h after hCG injection via transvaginal ultrasound-guided single-lumen needle aspiration. Retrieved oocytes were inseminated via conventional IVF, ICSI, or IVF/ICSI (IVF 50%/ICSI 50%). Embryos were cultured in G1-plus medium (Vitrolife, Switzerland) at 37°C in an incubator with 6% carbon dioxide. Embryo transfer was performed on day 3 after fertilization in cleavage stage.
Outcome Measures
The primary outcome was live birth. Secondary outcomes were ovarian stimulation parameters, including endometrial thickness, the number of oocytes retrieved, clinical pregnancy, and miscarriage. All women were followed to determine these reproductive outcomes. Live birth was defined as the delivery of a viable infant after 28 weeks. Clinical pregnancy was confirmed by the visualization of at least one gestational sac on ultrasound. Miscarriage was defined as spontaneous clinical pregnancy loss before 28 weeks of gestation.
Statistical Analysis
SPSS statistics 24.0 (IBM) was used to analyze the data. Means and standard deviations, frequencies, and percentages derived from demographic and reproductive characteristics data were calculated. Patients were divided into four quartiles based on serum lipid levels. To identify possible confounders, demographic characteristics including age, BMI, duration of infertility, initial infertility diagnosis, smoker status, primary infertility, glucose, day 3 FSH level, basal E2, and AMH in the different serum lipid quartiles were compared via analysis of variance.
Linear regression was used to evaluate associations between endometrial thickness, the number of oocytes retrieved, and serum lipid levels before and after adjusting for possible confounders. Adjusted means with 95% confidence intervals (CIs) were calculated. Trends in endometrial thickness and the number of oocytes retrieved in the serum lipid quartiles were determined by entering the median value of each quartile and viewing them as a continuous variable in a generalized linear model. Live birth rates, clinical pregnancy rates, and miscarriage rates in serum TC, LDL-C, HDL-C, and TG quartiles were compared using chi-square tests. Logistic regression analyses were used to identify potential effects of serum lipids on pregnancy, live birth, and miscarriage before and after adjusting for possible confounders. Serum lipid levels were viewed as both continuous and categorical variables. Effects were described as odds ratios (ORs) with 95% CIs. All reported p values are two sided, and p < 0.05 was considered statistically significant.
Results
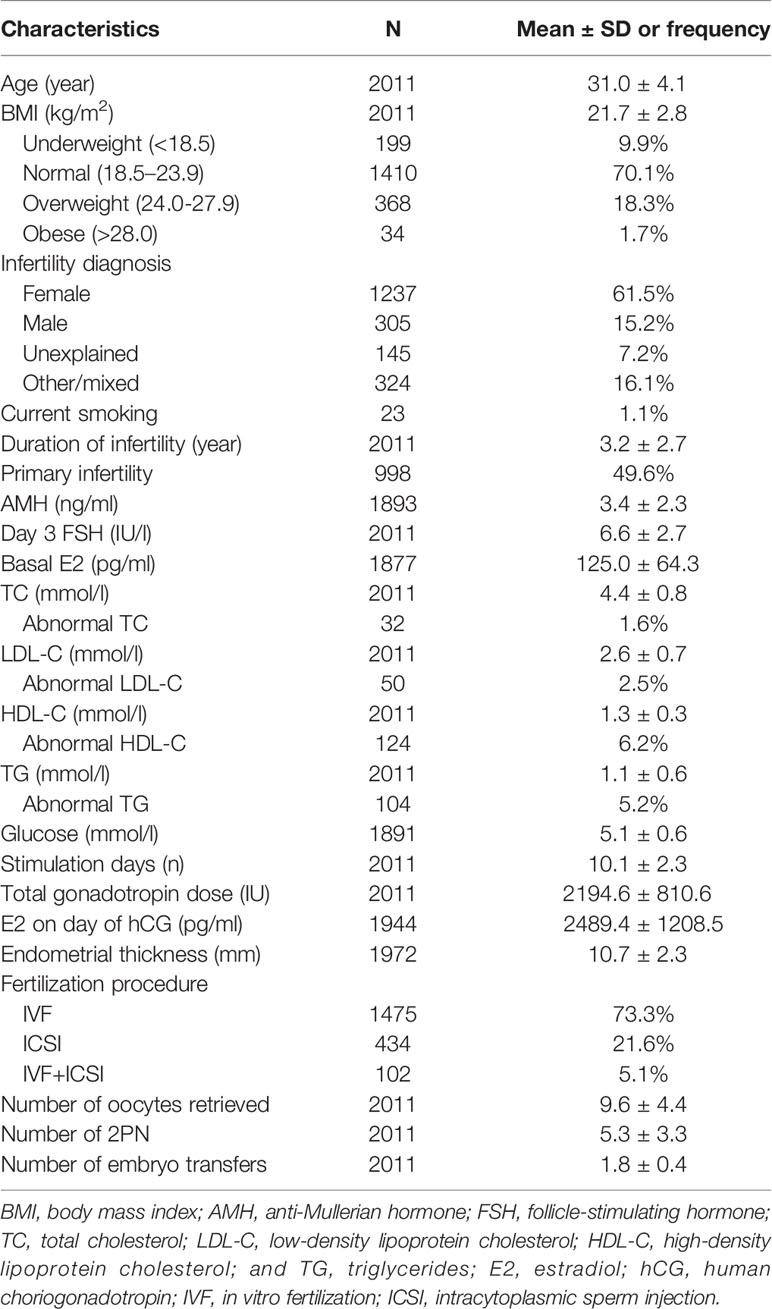
The clinical and demographic characteristics of the 2011 women included in the study are shown in Table 1. The mean age was 31.0 ± 4.1 years, and the mean BMI was 21.7 ± 2.8. With regard to weight 1410 (70.1%) were normal, 199 (9.9%) were underweight, 368 (18.3%) were overweight, and 34 (1.7%) were obese. Infertility diagnosis, smoker status, duration of infertility, primary infertility, AMH, day 3 FSH, TC, LDL-C, HDL-C, TG, glucose, stimulation duration, stimulation dose, E2 level on day of hCG, endometrial thickness, type of fertilization, number of oocytes retrieved, number of two pronuclei embryos, and number of embryo transfers were also recorded. The respective incidences of abnormal TC, TG, LDL-C, and HDL-C were 1.6%, 5.2%, 2.5%, and 6.2%, respectively. To identify possible confounders, patient characteristics in the different serum lipid quartiles were compared (Supplemental Table 1–4). Age, BMI, infertility diagnosis, duration of infertility, AMH, day 3 FSH, basal E2, and glucose were considered potential confounders and were adjusted for in subsequent analyses.
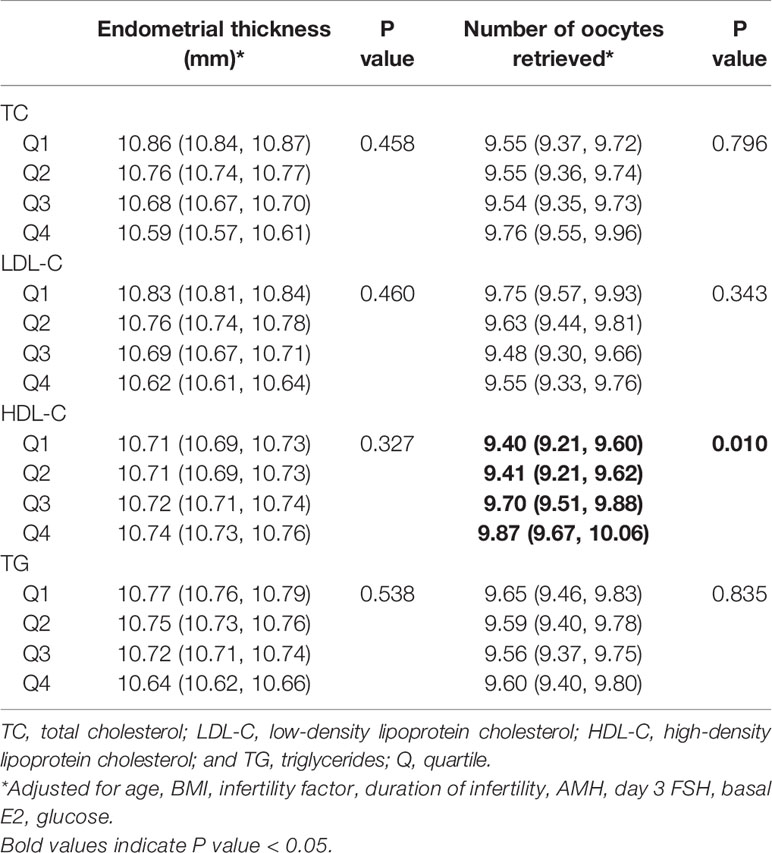
Associations between serum lipid levels, endometrial thickness, and number of oocytes retrieved are shown in Table 2. TC, LDL-C, and TG were not significantly associated with endometrial thickness or the number of oocytes retrieved. Increasing HDL-C quartiles were associated with more oocytes retrieved 9.40 (9.21, 9.60), 9.41 (9.21, 9.62), 9.70 (9.51, 9.88), 9.87 (9.67, 10.06) after adjusting for confounders (p = 0.010). When lipid levels were included in analyses as continuous variables, the associations were similar to those obtained when they were included in analyses as categorical variables (Supplemental Table 5).
A total of 1030/2011 women (51.2%) achieved clinical pregnancy, 483 (24.0%) had live births, and 547 (53.1%) had miscarriages. Reproductive outcomes in the different serum lipid quartiles are shown in Figure 2. Clinical pregnancy rates in the TC quartiles differed significantly (p = 0.017). Live birth rates reduced as LDL-C quartile increased (p < 0.001). Miscarriage rates increased as LDL-C quartile increased (p < 0.001).
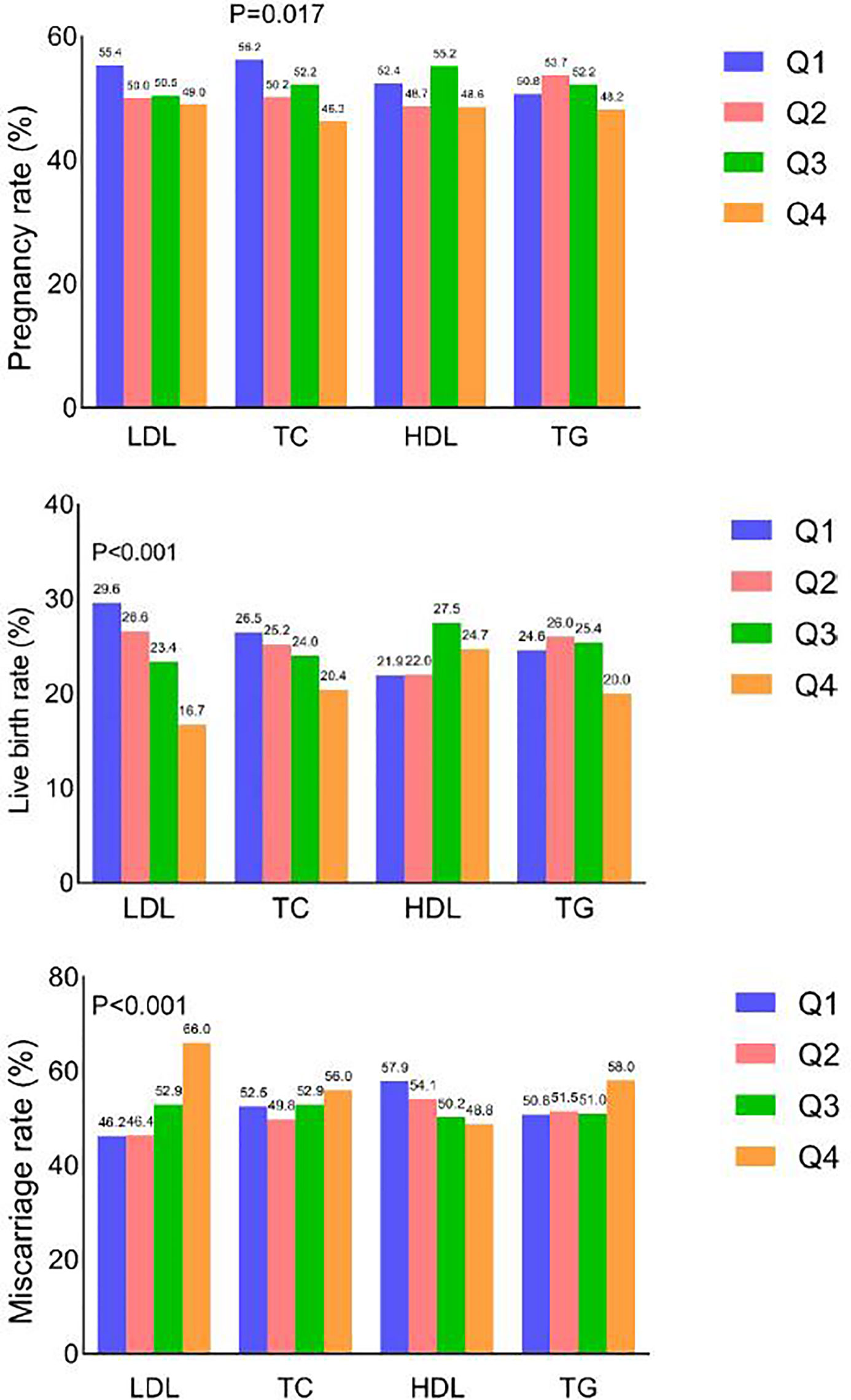
Figure 2 Clinical pregnancy, live birth and miscarriage rates according to quartile of each serum lipid level.
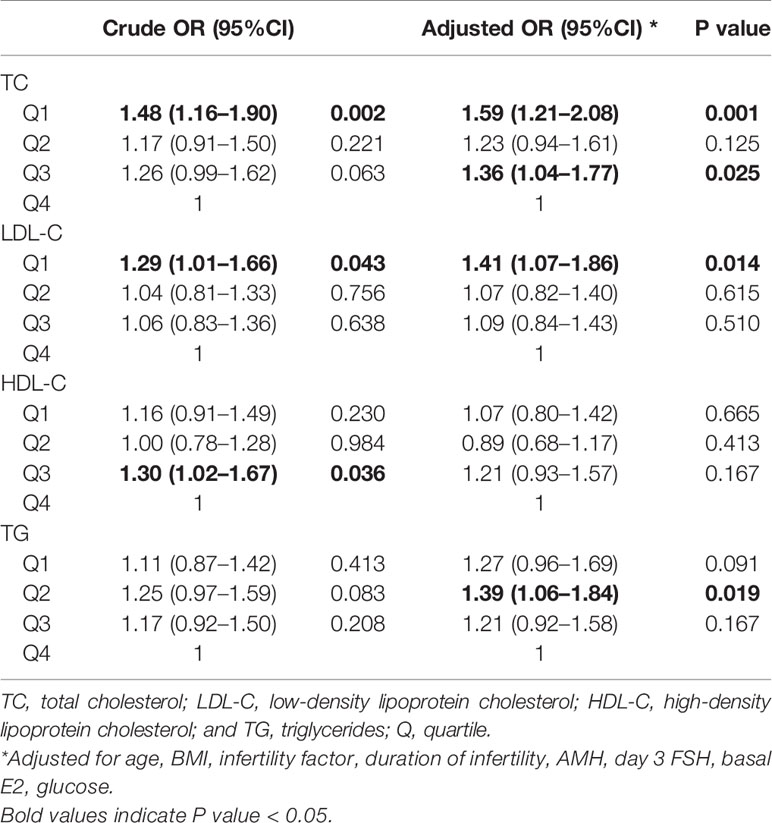
Crude and adjusted ORs of serum lipid levels for clinical pregnancy, live birth, and miscarriage compared to the highest quartile are shown in Tables 3, 4, 5. After adjusting for potential confounders, women in TC quartiles 1 (OR 1.59 [1.21–2.08]) and 3 (OR 1.36 [1.04–1.77]), LDL-C quartile 1 (OR 1.41 [1.07–1.86]), and TG quartile 2 (OR 1.39 [1.06–1.84]) were significantly more likely to achieve clinical pregnancy. After adjusting for potential confounders, women in LDL-C quartiles 1 (OR 2.22 [1.58–3.13]), 2 (OR 1.78 [1.27–2.50]), and 3 (OR 1.51 [1.07–2.13]), TC quartile 1 (OR 1.39 [1.00–1.93]), and TG quartiles 1 (OR 1.44 [1.03–2.03]), 2 (OR 1.46 [1.04–2.04]), and 3 (OR 1.44 [1.04–1.99]) were significantly more likely to have a live birth, whereas HDL-C quartile 2 (OR 0.71 [0.51–0.99]) was associated with a lower chance of live birth. After adjusting for potential confounders, women in LDL-C quartiles 1 (OR 0.44 [0.30–0.66]), 2 (OR 0.49 [0.33–0.73]), and 3 (OR 0.63 [0.43–0.94]) were less likely to have a miscarriage, whereas HDL-C quartile 1 (OR 1.60 [1.07–2.39]) was associated with an increased chance of miscarriage. When serum lipid levels were included in analyses as continuous variables, the directions of associations were similar to those obtained when they were included in analyses as categorical variables (Supplemental Tables 6–8).
Discussion
In the current retrospective cohort study serum lipid levels were associated with treatment outcomes in women undergoing assisted reproduction. Overall, LDL-C had the strongest negative effect on reproduction outcomes including lower pregnancy rate, lower live birth rate, and higher miscarriage rate. TG and TC were also negatively associated with pregnancy and live birth rate. Conversely, higher HDL-C was associated with greater numbers of oocytes retrieved, higher live birth rates, and lower miscarriage rates.
Some previous studies have investigated relationships between lipids and embryos. In the current study, serum HDL-C was positively associated with the number of oocytes retrieved. In female reproduction, follicular fluid is the microenvironment for oocyte maturation and development. The aforementioned association may be partly explained by the anti-inflammatory and antioxidant role of HDL-C (19), which is increasingly considered to be beneficial for reproductive health (20). In the preovulatory period, follicular fluid contains only HDL which originates from blood circulation (21). HDL in follicular fluid reportedly has considerable antioxidative properties and plays an important role in the development of human oocytes (15–17, 22). In a previous study, an altered endometrial lipid profile during the peri-implantation period was associated with endometrial receptivity (10). In the current study, serum lipids were not significantly associated with endometrial thickness, which is associated with the live birth rate (23). Endometrial thickness is not the sole marker of endometrial receptivity (24), and preconception serum lipid may not completely account for local endometrial lipid profile changes during the implantation window.
The results of the current study are similar to those of some previous studies that investigated preconception lipid concentrations and reproductive outcomes in women. In a population-based prospective cohort study that included 501 couples, increased total lipid and free cholesterol concentrations as continuous variables in women were associated with a reduced fecundity OR as assessed by time to hCG-detected pregnancy (12). In another randomized controlled trial that included 1228 women with prior pregnancy loss attempting pregnancy for up to six menstrual cycles, a secondary analysis was performed to investigate associations between preconception lipid levels and fecundity, which was determined by a positive hCG test or ultrasound (13). That study used clinical cutoff points, and fecundability was reduced at all abnormal levels of TC, LDL-C, HDL-C, and TG. In the current study, significant associations between reproductive outcomes and serum lipid levels were evident when they were analyzed as both continuous variables and categorical variables. The study included patients who were young and lean, hence the low incidence of altered serum lipid profiles (ranging from 1.6%–6.2% depending on the lipid assessed). It can be speculated that in older women and obese women, among whom elevated serum lipids are more common (25, 26), associations between lipids and reproductive outcomes will be stronger. These findings further underline the role of serum lipids in reproductive outcomes in women.
The mechanisms behind associations between serum lipids and reproductive outcomes are not fully understood. Serum lipids may influence steroidogenesis. Progesterone, estrogens, androgens, and glucocorticoids are essential from embryo implantation to fetal development (27). The oocyte is unable to produce cholesterol, and therefore relies on maternal serum lipids to provide cholesteryl esters for granulosa cell steroidogenesis. HDL-C is the main transporter of cholesterol to granulosa cells for steroidogenesis (28), which is supplied from maternal circulation. The process of steroidogenesis is gradually taken over by the placenta as it develops during the first trimester. Placental steroid synthesis is dependent on delivery from the maternal circulation. Altered maternal lipids may alter steroidogenesis and ultimately influence pregnancy outcomes. In addition, placental dysfunction is a possible cause of multiple pregnancy complications including miscarriage, preeclampsia, stillbirth, and preterm delivery (29–33). Altered lipid metabolism may affect placental lipid transport and fetal development (34). Maternal, fetal, and placental components form a unit to maintain a healthy pregnancy, and the maternal component includes serum lipids. Combined, these considerations provide plausible explanations for the independent associations between serum lipid levels and reproductive outcomes in the women undergoing assisted reproduction in the current study.
Serum lipids are routinely monitored in clinical settings as indicators of cardiovascular system health. The results of the current study suggest that serum lipids are also predictors of outcomes in women undergoing assisted reproduction. The high prevalence of lipid abnormalities in modern populations facilitates an opportunity to evaluate lipid profiles in an effort to predict reproductive outcomes in these populations. Abnormal serum lipid levels are usually modified by increased physical activity, diet modification, or statins (35–37). The current study suggests that there may be benefits to improving serum lipid levels before assisted reproduction treatment. Future clinical studies should focus on whether pretreatment to optimize lipid levels via interventions such as physical activity, diet modification, or statins can improve reproductive outcomes.
The major strength of the current study was the large sample size. The observed associations remained significant after adjustment for a number of potential confounders. Despite efforts to eliminate sources of bias in the study, it also had several limitations. One is the inevitable bias due to its retrospective nature. Despite the large sample size, the adjustments made, and the multivariable regression analysis conducted, the presence of confounding bias cannot be excluded. In addition, having in mind that our studied population was heterogeneous, more research is needed in specific patient subpopulations where one might expect that altered lipid profiles may affect treatment outcomes, such as polycystic ovary syndrome patients. As only a single measure of preconception serum lipid levels was available, it was not possible to investigate associations between lipid levels in sequential cycles, and due to the study design it was also not possible to investigate whether serum lipids were related to the cumulative live birth rate. Lastly, data on time elapsed from the measurement of serum lipids and the initiation of IVF were unavailable, and future studies could investigate this.
The results of the current study constitute evidence on the role of serum lipids in treatment outcomes in women undergoing assisted reproduction. Among women who aim to achieve good outcomes from assisted reproduction, a lipid panel test may serve as an early, simple, inexpensive, and modifiable predictor. Further studies are needed to expand our understanding of whether interventions aimed at improving serum lipid profiles can optimize assisted pregnancy outcomes.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Hospital Ethics Committee, Women’s Hospital, School of Medicine, Zhejiang University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
X-KW and JX designed the study and critically revised the manuscript. W-YC and XL performed data analysis. W-YC, XL, EC, HL, and KF collected data. W-YC and XL drafted the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
We thank Shao-Qin Sheng for her assistance with language improvement.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.633766/full#supplementary-material
References
1. Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med (2012) 9(12):e1001356. doi: 10.1371/journal.pmed.1001356
2. Wu AK, Elliott P, Katz PP, Smith JF. Time costs of fertility care: the hidden hardship of building a family. Fertil Steril (2013) 99(7):2025–30. doi: 10.1016/j.fertnstert.2013.01.145
3. Rooney KL, Domar AD. The relationship between stress and infertility. Dialog Clin Neurosci (2018) 20(1):41–7. doi: 10.31887/DCNS.2018.20.1/klrooney
4. Kushnir VA, Barad DH, Albertini DF, Darmon SK, Gleicher N. Systematic review of worldwide trends in assisted reproductive technology 2004-2013. Reprod Biol Endocrinol (2017) 15(1):6–. doi: 10.1186/s12958-016-0225-2
5. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet (2017) 390(10113):2627–42. doi: 10.1016/S0140-6736(17)32129-3
6. Tóth PP, Potter D, Ming EE. Prevalence of lipid abnormalities in the United States: the National Health and Nutrition Examination Survey 2003-2006. J Clin Lipidol (2012) 6(4):325–30. doi: 10.1016/j.jacl.2012.05.002
7. Huang Y, Gao L, Xie X, Tan SC. Epidemiology of dyslipidemia in Chinese adults: meta-analysis of prevalence, awareness, treatment, and control. Popul Health Metr (2014) 12(1):28. doi: 10.1186/s12963-014-0028-7
8. Miller WL. Molecular biology of steroid hormone synthesis. Endocr Rev (1988) 9(3):295–318. doi: 10.1210/edrv-9-3-295
9. Huang Q, Liu Y, Yang Z, Xie Y, Mo Z. The Effects of Cholesterol Metabolism on Follicular Development and Ovarian Function. Curr Mol Med (2019) 19(10):719–30. doi: 10.2174/1566524019666190916155004
10. Li J, Gao Y, Guan L, Zhang H, Chen P, Gong X, et al. Lipid Profiling of Peri-implantation Endometrium in Patients With Premature Progesterone Rise in the Late Follicular Phase. J Clin Endocrinol Metab (2019) 104(11):5555–65. doi: 10.1210/jc.2019-00793
11. Brosens I, Brosens JJ, Muter J, Benagiano G. Acute atherosis and diffuse lipid infiltration of the placental bed: A review of historical lipid studies. Placenta (2020) 97:36–41. doi: 10.1016/j.placenta.2020.06.012
12. Schisterman EF, Mumford SL, Browne RW, Barr DB, Chen Z, Louis GMB. Lipid concentrations and couple fecundity: the LIFE study. J Clin Endocrinol Metab (2014) 99(8):2786–94. doi: 10.1210/jc.2013-3936
13. Pugh SJ, Schisterman EF, Browne RW, Lynch AM, Mumford SL, Perkins NJ, et al. Preconception maternal lipoprotein levels in relation to fecundability. Hum Reprod (2017) 32(5):1055–63. doi: 10.1093/humrep/dex052
14. Browne RW, Shelly WB, Bloom MS, Ocque AJ, Sandler JR, Huddleston HG, et al. Distributions of high-density lipoprotein particle components in human follicular fluid and sera and their associations with embryo morphology parameters during IVF. Hum Reprod (2008) 23(8):1884–94. doi: 10.1093/humrep/den183
15. Browne RW, Bloom MS, Shelly WB, Ocque AJ, Huddleston HG, Fujimoto VY. Follicular fluid high density lipoprotein-associated micronutrient levels are associated with embryo fragmentation during IVF. J Assist Reprod Genet (2009) 26(11-12):557–60. doi: 10.1007/s10815-009-9367-x
16. Nagy RA, van Montfoort APA, Groen H, Homminga I, Andrei D, Mistry RH, et al. Anti-oxidative function of follicular fluid HDL and outcomes of modified natural cycle-IVF. Sci Rep (2019) 9(1):12817. doi: 10.1038/s41598-019-49091-3
17. Bacchetti T, Morresi C, Vignini A, Tiano L, Orlando P, Montik N, et al. HDL functionality in follicular fluid in normal-weight and obese women undergoing assisted reproductive treatment. J Assist Reprod Genet (2019) 36(8):1657–64. doi: 10.1007/s10815-019-01523-9
18. Joint Committee for Developing Chinese guidelines on Prevention and Treatment of Dyslipidemia in Adults. [Chinese guidelines on prevention and treatment of dyslipidemia in adults]. Zhonghua Xin Xue Guan Bing Za Zhi (2007) 35(5):390–419. doi: 10.3760/j.issn:0253-3758.2007.05.003
19. Soran H, Schofield JD, Durrington PN. Antioxidant properties of HDL. Front Pharmacol (2015) 6:222. doi: 10.3389/fphar.2015.00222
20. Fujimoto VY, Kane JP, Ishida BY, Bloom MS, Browne RW. High-density lipoprotein metabolism and the human embryo. Hum Reprod Update (2010) 16(1):20–38. doi: 10.1093/humupd/dmp029
21. Jaspard B, Fournier N, Vieitez G, Atger V, Barbaras R, Vieu C, et al. Structural and functional comparison of HDL from homologous human plasma and follicular fluid. A model for extravascular fluid. Arterioscler Thromb Vasc Biol (1997) 17(8):1605–13. doi: 10.1161/01.ATV.17.8.1605
22. Kim K, Bloom MS, Browne RW, Bell EM, Yucel RM, Fujimoto VY. Associations between follicular fluid high density lipoprotein particle components and embryo quality among in vitro fertilization patients. J Assist Reprod Genet (2017) 34(1):1–10. doi: 10.1007/s10815-016-0826-x
23. Simeonov M, Sapir O, Lande Y, Ben-Haroush A, Oron G, Shlush E, et al. The entire range of trigger-day endometrial thickness in fresh IVF cycles is independently correlated with live birth rate. Reprod BioMed Online (2020) 41(2):239–47. doi: 10.1016/j.rbmo.2020.04.008
24. Casper RF. Frozen embryo transfer: evidence-based markers for successful endometrial preparation. Fertil Steril (2020) 113(2):248–51. doi: 10.1016/j.fertnstert.2019.12.008
25. Pan L, Yang Z, Wu Y, Yin R-X, Liao Y, Wang J, et al. The prevalence, awareness, treatment and control of dyslipidemia among adults in China. Atherosclerosis (2016) 248:2–9. doi: 10.1016/j.atherosclerosis.2016.02.006
26. Zhang L, Zhang W-H, Zhang L, Wang P-Y. Prevalence of overweight/obesity and its associations with hypertension, diabetes, dyslipidemia, and metabolic syndrome: a survey in the suburban area of Beijing, 2007. Obes Facts (2011) 4(4):284–9. doi: 10.1159/000331014
27. Morel Y, Roucher F, Plotton I, Goursaud C, Tardy V, Mallet D. Evolution of steroids during pregnancy: Maternal, placental and fetal synthesis. Ann Endocrinol (Paris) (2016) 77(2):82–9. doi: 10.1016/j.ando.2016.04.023
28. Azhar S, Tsai L, Medicherla S, Chandrasekher Y, Giudice L, Reaven E. Human granulosa cells use high density lipoprotein cholesterol for steroidogenesis. J Clin Endocrinol Metab (1998) 83(3):983–91. doi: 10.1210/jcem.83.3.4662
29. Gun BD, Numanoglu G, Ozdamar SO. The comparison of vessels in elective and spontaneous abortion decidua in first trimester pregnancies: importance of vascular changes in early pregnancy losses. Acta Obstet Gynecol Scand (2006) 85(4):402–6. doi: 10.1080/00016340500501731
30. Lambropoulou M, Tamiolakis D, Venizelos J, Liberis V, Galazios G, Tsikouras P, et al. Imbalance of mononuclear cell infiltrates in the placental tissue from foetuses after spontaneous abortion versus therapeutic termination from 8th to 12th weeks of gestational age. Clin Exp Med (2006) 6(4):171–6. doi: 10.1007/s10238-006-0111-x
31. Guzin K, Tomruk S, Tuncay YA, Naki M, Sezginsoy S, Zemheri E, et al. The relation of increased uterine artery blood flow resistance and impaired trophoblast invasion in pre-eclamptic pregnancies. Arch Gynecol Obstet (2005) 272(4):283–8. doi: 10.1007/s00404-005-0005-2
32. Romero R, Kusanovic JP, Chaiworapongsa T, Hassan SS. Placental bed disorders in preterm labor, preterm PROM, spontaneous abortion and abruptio placentae. Best Pract Res Clin Obstet Gynaecol (2011) 25(3):313–27. doi: 10.1016/j.bpobgyn.2011.02.006
33. Wijaya JC, Khanabdali R, Georgiou HM, Kokkinos MI, James PF, Brennecke SP, et al. Functional changes in decidual mesenchymal stem/stromal cells are associated with spontaneous onset of labour. Mol Hum Reprod (2020) 26(8):636–51. doi: 10.1093/molehr/gaaa045
34. Delhaes F, Giza SA, Koreman T, Eastabrook G, McKenzie CA, Bedell S, et al. Altered maternal and placental lipid metabolism and fetal fat development in obesity: Current knowledge and advances in non-invasive assessment. Placenta (2018) 69:118–24. doi: 10.1016/j.placenta.2018.05.011
35. Hasan B, Nayfeh T, Alzuabi M, Wang Z, Kuchkuntla AR, Prokop LJ, et al. Weight Loss and Serum Lipids in Overweight and Obese Adults: A Systematic Review and Meta-Analysis. J Clin Endocrinol Metab (2020) 105(12):3695–703. doi: 10.1210/clinem/dgaa673
36. Ge L, Sadeghirad B, Ball GDC, da Costa BR, Hitchcock CL, Svendrovski A, et al. Comparison of dietary macronutrient patterns of 14 popular named dietary programmes for weight and cardiovascular risk factor reduction in adults: systematic review and network meta-analysis of randomised trials. BMJ (2020) 369:m696. doi: 10.1136/bmj.m696
Keywords: lipid, reproduction, assisted reproduction, live birth, pregnancy, miscarriage
Citation: Cai W-Y, Luo X, Chen E, Lv H, Fu K, Wu X-K and Xu J (2021) Serum Lipid Levels and Treatment Outcomes in Women Undergoing Assisted Reproduction: A Retrospective Cohort Study. Front. Endocrinol. 12:633766. doi: 10.3389/fendo.2021.633766
Received: 26 November 2020; Accepted: 01 February 2021;
Published: 08 March 2021.
Edited by:
Daniele Santi, University Hospital of Modena, ItalyReviewed by:
Alessandro Conforti, University of Naples Federico II, ItalyBiljana Popovic Todorovic, University Hospital Brussels, Belgium
Copyright © 2021 Cai, Luo, Chen, Lv, Fu, Wu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Xu, eHVqQHpqdS5lZHUuY24=; Xiao-Ke Wu, eGlhb2tld3UyMDAyQHZpcC5zaW5hLmNvbQ==
†These authors share first authorship
 Wang-Yu Cai
Wang-Yu Cai Xi Luo
Xi Luo Erxidi Chen
Erxidi Chen Houyi Lv
Houyi Lv Kaiyou Fu
Kaiyou Fu Xiao-Ke Wu4,5*
Xiao-Ke Wu4,5* Jian Xu
Jian Xu