- Department of General Surgery, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
Background: Papillary thyroid micro-carcinoma (PTMC) is defined as a tumor with a larger diameter ≤1 cm which has an indolent course and satisfying prognosis. However, the incidence of lymph node metastasis of PTMC cannot be ignored. The aim of this study was to assess the incidence of lymph node metastasis in PTMC patients, as well as to evaluate the risk factors for both central lymph node metastases (CLNM) and lateral lymph node metastases (LLNM).
Methods: Patients who underwent thyroidectomy from January 2017 to October 2020, and pathologically diagnosed with PTMC were enrolled in our study and their medical records were collected and analyzed.
Results: A total of 484 PTMC patients were included. The incidence of central and lateral lymph node metastasis was 49.6% and 9.1%, respectively. Multivariate analysis demonstrated as independent risk factors for CLNM male sex, age <40 years, largest tumor size ≥5 mm and bilaterality. Extrathyroidal extension, presence of CLNM, number of CLNM ≥5 were strong indicators for LLNM.
Conclusion: The incidence of lymph node metastases in PTMC is non-negligible. The identification of potential risk factors for CLNM and LLNM would help tailor individual surgical interventions for patients with PTMC.
Introduction
Thyroid cancer (TC) is considered to be responsible for over 500,000 cancer cases reported from all sources globally in 2018 and ranks ninth among malignant diseases (1). It is well-known that thyroid carcinoma occurs more frequently in females than in males. In 2020, the statistics confirmed TC as the fifth most common malignancy in women (2). Since the late 20th century, the incidence of TC has continued to increase, which is probably attributed to major advances in diagnostic techniques and to ultrasonography, in particular (2).
TC can be categorized into three types, namely: 1) differentiated TC, including papillary (PTC), follicular (FTC), and Hürthle cell (HTC); 2) medullary TC; and 3) anaplastic TC (3). Among these, PTC accounts for 80%–90% of TC (3). Given the indolent nature of PTC, most patients could be cured by surgical resection and radioiodine therapy, with a survival rate reaching over 90% over a 10-year follow-up (4). Papillary thyroid micro-carcinoma (PTMC) is defined as small thyroid papillary cancer with lesions ≤1 cm in diameter (5). Though patients with PTMC are often classified into low-risk population due to its favorable prognosis, it is noteworthy that a characteristic of PTC is the tendency for lymph node metastasis.
According to the 2015 version of American Thyroid Association (ATA) guidelines (6), PTMC lacking aggressive characteristics, including clinically uninvolved lymph nodes (cN0), is classified in the low-risk group and routine prophylactic central lymph node dissection (CLND) is not recommended. However, given the interference with the thyroid gland, the detection of lymph node metastasis in the central compartment remains challenging and largely depends on sonographer skill. Researchers have revealed that a substantial proportion of cN0 patients are confirmed to be pathologically node-involved (pN1) (7–9). Thus, ATA guidelines also indicate that, for patients with predictive factors of disease relapse and poor prognosis, prophylactic CLND should be included as part of the diagnostic workup as the information relative to lymph node status may affect tumor staging and improve clinical decision-making (6).
The objective of this study was to assess the incidence of lymph node metastasis in PTMC patients, and to evaluate risk factors for both central and lateral lymph node metastases, in an attempt to provide clinical evidence for tailored individual surgical management.
Methods
Patient Selection and Perioperative Evaluation
Patients who underwent thyroidectomy at the General Surgery Department of Guangdong Provincial People’s Hospital from January 2017 to October 2020 and were pathologically diagnosed with papillary thyroid micro-carcinoma were enrolled in this study. Patients were then screened based on the following inclusion and exclusion criteria for subsequent data analysis. Inclusion criteria were as follows: 1) pathologically-diagnosed with papillary thyroid micro-carcinoma, confirmed with postoperative paraffin-embedded sections; 2) complete medical records were available; and 3) subjected to thyroidectomy as well as CLND with or without lateral lymph node dissection (LLND). The exclusion criteria were as follows: 1) age under 18 years; 2) postoperative pathology revealed mixed histologic types of carcinoma; and 3) incomplete medical records.
Preoperative evaluation included physical examination, thyroiditis assessment, thyroid function assessment, and high-resolution ultrasonography of the neck. Ultrasonographic features of malignancy included: solid hypoechoic nodule(s), irregular margins, microcalcifications, taller than wide shape, and evidence of capsule discontinuities. Moreover, fine needle cytology aspiration (FNA) was adopted in cases with nodules ≥5 mm and with intermediate malignancy suspicion.
Blood tests for parathyroid hormone (PTH) were performed on the day after surgery as well as at regular out-patient follow-up visits. Hypoparathyroidism, the most common complication of thyroid surgery, was defined as low PTH level accompanied by decreased total serum calcium levels (10). Temporary hypoparathyroidism referred to a duration of less than 6 months after surgery, whereas permanent hypoparathyroidism indicates a duration of greater than 6 months (10).
Surgical Approach and Histopathological Characteristics
Surgical procedures mainly included total thyroidectomy (TT) with CLND and lobo-isthmectomy (LH) with CLND for patients with or without preoperative indications of CLNM, while LLND was only performed in cases diagnosed with LLNM before or during surgery. Cases clinically suspected of having involved central lymph nodes were categorized into cN1 group. Frozen section was performed on all patients during surgical intervention, and the malignancy of thyroid nodule was confirmed before CLND either by preoperative FNA or intraoperative frozen section. Likewise, for those with lateral lymph nodes highly suspicious of metastases with features including increase of lymph node diameter, microcalcifications, hypoechogenicity, cystic changes, peripheral vascularity by ultrasonography (11), FNA before surgery or intraoperative frozen section was adopted to confirmed diagnosis of LLNM.
Micro-carcinoma was defined as a maximum tumor diameter less than or equal to 1 cm. As for multifocal tumors, the tumor size was determined as the largest diameter of the primary tumor. Extrathyroidal invasion and vascular embolus were observed by pathological examination. Hashimoto’s thyroiditis (HT) was confirmed with any of the following: positive thyroglobulin-antibodies (Tg-Ab) and/or thyroid peroxidase-antibodies (TPO-Ab), or positive information at pathological examination. Lymph node yield was defined as the number of lymph nodes dissected, whereas the lymph node ratio was calculated as the ratio of metastatic lymph nodes out of the total lymph nodes removed.
Statistical Analysis
Categorical variables are reported as frequency with a percentage while continuous variables as a mean with standard deviation. SPSS 23.0 software (SPSS, Inc, Chicago, Ill) was employed for statistical analysis. Univariate analysis was used to screen for risk factors of CLNM and LLNM, and a multivariate regression model was adopted for further evaluation. For univariate analysis, p<0.1 was considered statistically significant, while p <0.05 was adopted for multivariate analysis (two-sided).
Results
Baseline Characteristics
A total of 484 patients with PTMC who had met the inclusion criteria were enrolled in this study. All patients underwent central compartment lymph node dissection, while lateral cervical lymph node dissection was only performed for those with ultra-sonographic indication for LLNM before surgery.
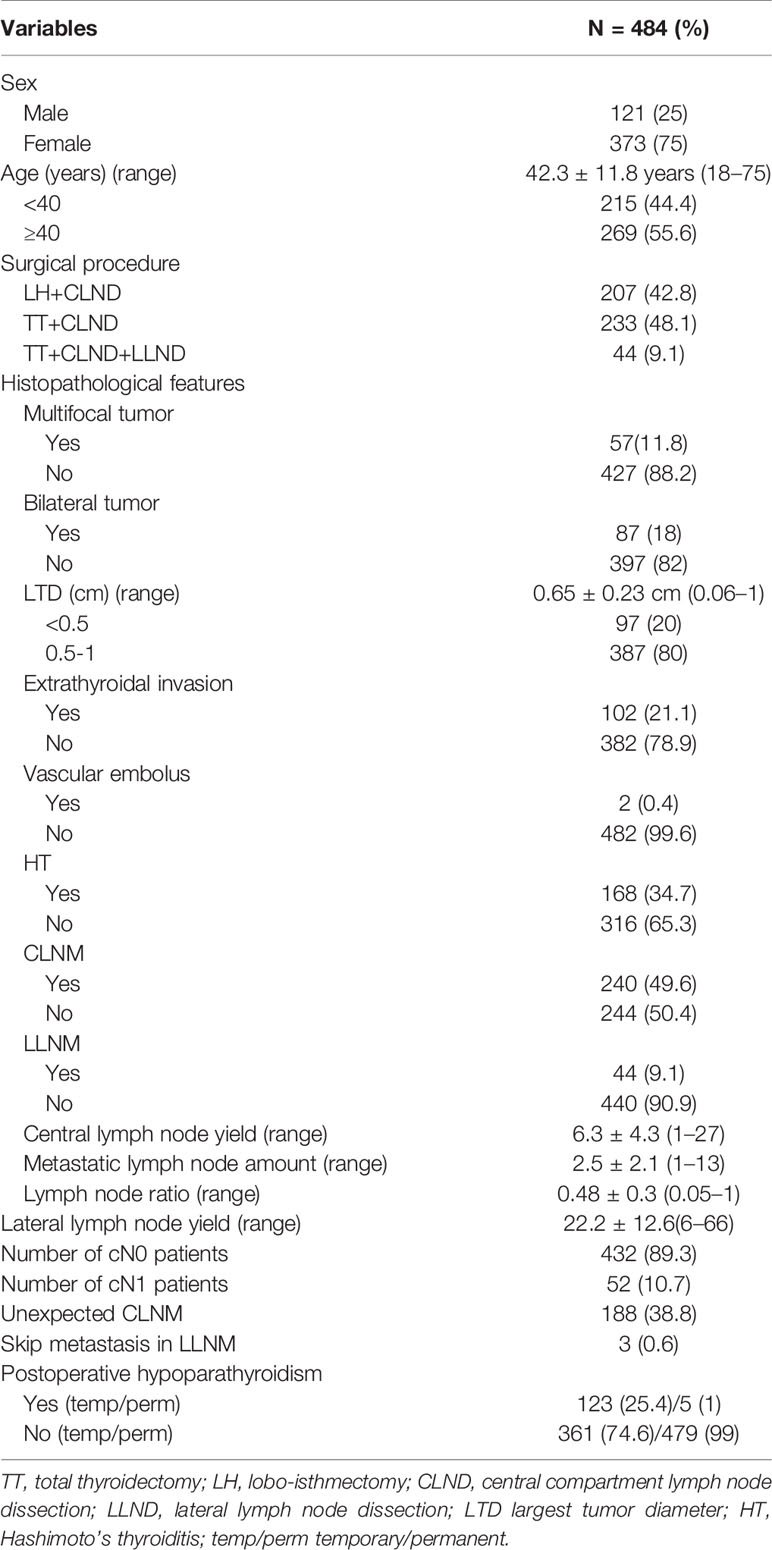
As summarized in Table 1, there were 121 males (25%) and 373 females (75%) included in this study, with an average age of 42.3 ± 11.8 years. Among those patients, LH accompanied with CLND was performed in 207 (42.8%) cases, TT with CLND in 233 (48.1%) cases, and TT with CLND and LLND in 44 (8.9%) cases. Eight (1.7%) patients were incidentally diagnosed with PTMC, whereas for 476 (98.3%) cases patients were indicated by preoperative examinations, among which 82 (16.9%) patients were diagnosed by preoperative FNA.
According to the pathological features obtained from paraffin section, the average tumor diameter was 0.65 ± 0.23 cm. The presence of CLNM was confirmed in 240 (49.6%) cases, with a mean lymph node yield of 6.3 ± 4.3. More specifically, the average number of involved central lymph nodes was 2.5 ± 2.1, with an average lymph node ratio of 0.48 ± 0.3. Interestingly, the number of cN0 and cN1 patients were 432 (89.3%) and 52 (10.7%), respectively. CLNM was discovered incidentally in 188 (38.8%) cases and was pathologically confirmed in 52 (10.7%) cN1 patients. The sensitivity of preoperative ultrasonography was 21.7%.
Conversely, the incidence of LLNM was 9.1%, with a mean lymph node yield of 22.2 ± 12.6, ranging from 6 to 66. Moreover, skip metastasis (presence of LLNM without involving central compartment) was observed in three cases (0.6%).
As for postoperative complications, temporary hypoparathyroidism was observed in 123 patients (25.4%), usually presented with tingling or numbness in fingers, toes, or around the mouth. However, the PTH level gradually recovered within 6 months after surgery in most cases, with five patients (1%) experienced permanent hypoparathyroidism.
Univariate and Multivariate Analyses of Risk Factors for Central Lymph Node Metastases
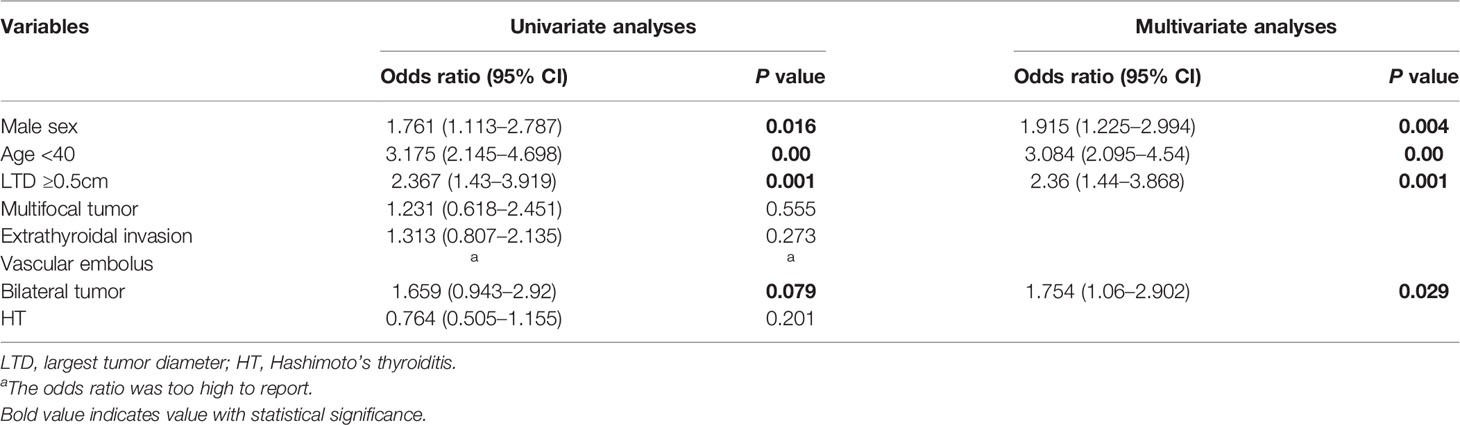
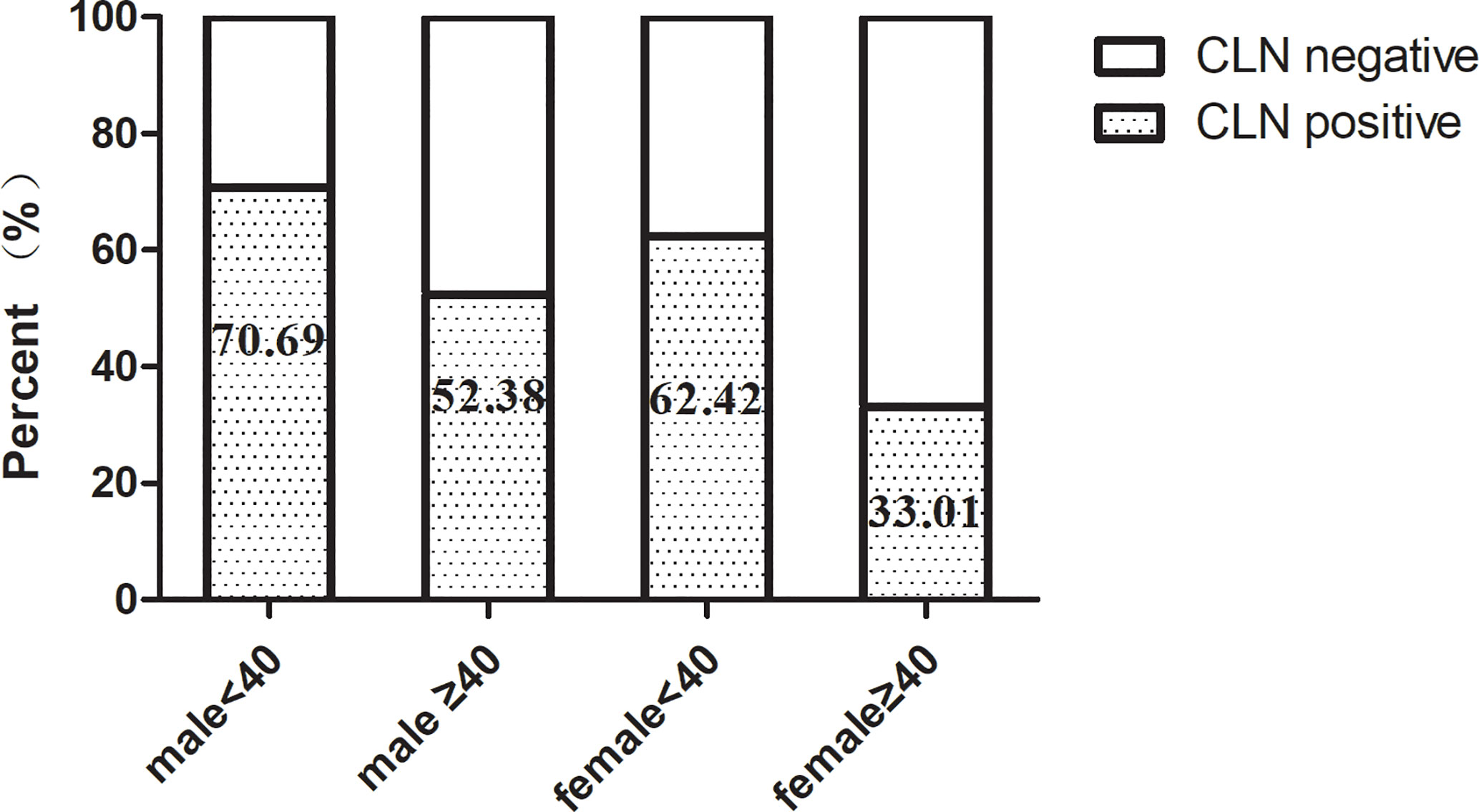
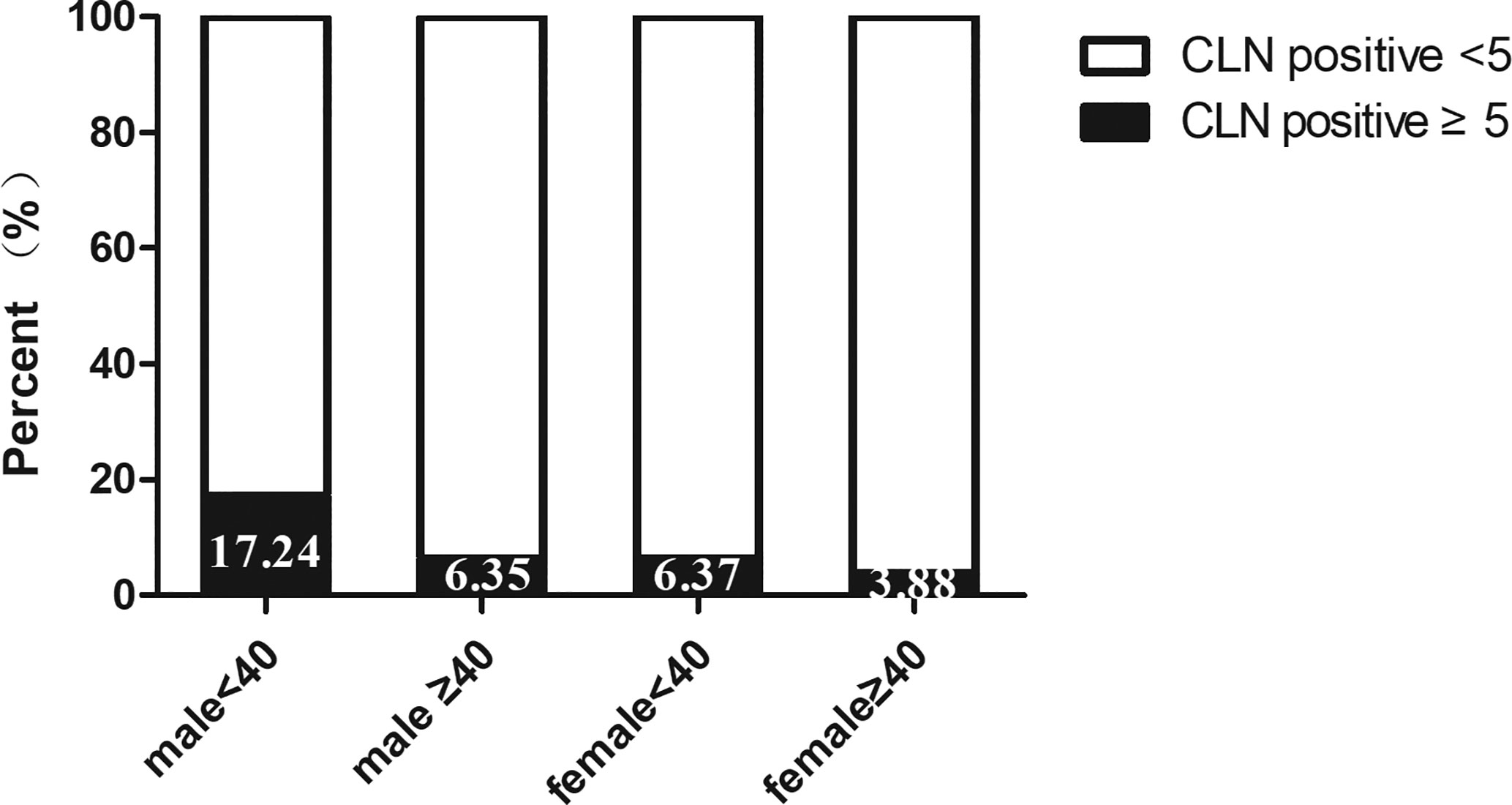
Univariate analysis was used to identify risks factors for CLNM in patients with PTMC. An increased risk of CLNM was associated with male sex, age <40 years, largest tumor diameter (LTD) ≥0.5 cm, and bilaterality (odds ratio [OR]=1.761 [95% confidence interval (CI), 1.113–2.787], p=0.016; OR=3.175 [95% CI, 2.145–4.698], p=0.00, OR=2.367 [95% CI, 1.43–3.919], p=0.001, OR=1.659 [95% CI, 0.943–2.92], p=0.079, respectively) (Table 2). Furthermore, multivariate analyses confirmed that male sex, age <40 years, LTD ≥0.5 cm, and bilaterality were independent risk factors for CLNM in patients with PTMC (OR=1.915 [95% CI, 1.225–2.994], p=0.004; OR=3.084 [95% CI, 2.095–4.54], p=0.00, OR=2.36 [95% CI, 1.44–3.868], p=0.001, OR=1.754 [95% CI, 1.06–2.902], p=0.029, respectively) (Table 2). As shown in Figure 1, male patients aged <40 years had a higher rate of CLNM than female patients aged over 40 years. In addition, the above-mentioned patients had larger volume of involved lymph node (number of CLNM ≥5) than others, as indicated in Figure 2.
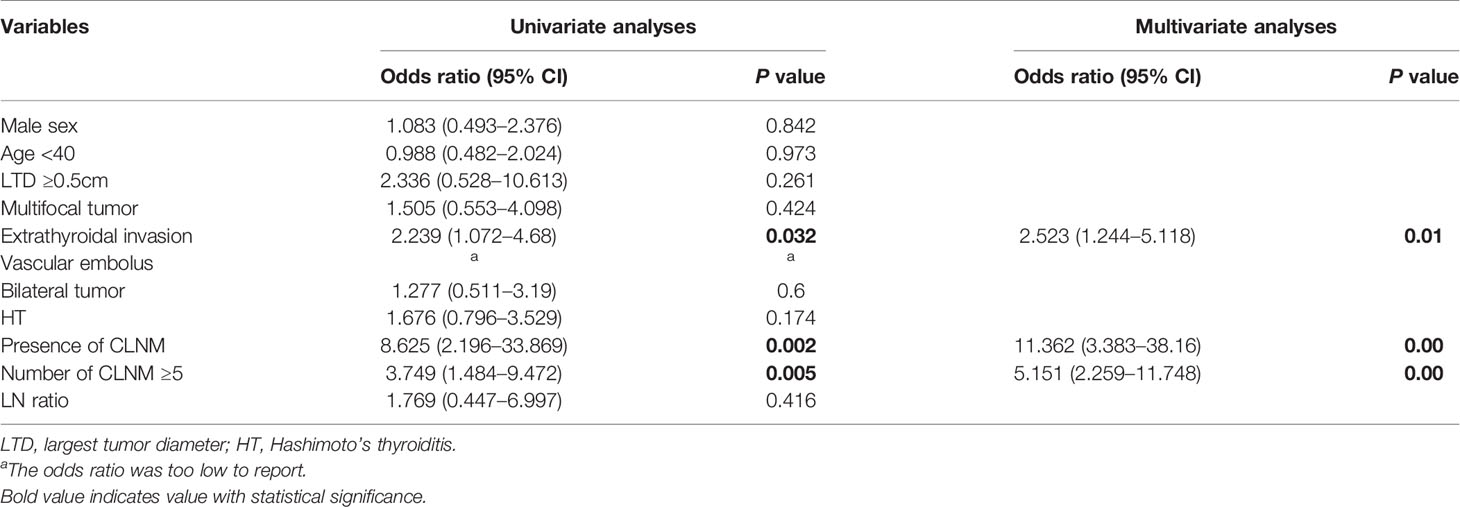
Univariate and Multivariate Analyses of Risk Factors for Lateral Lymph Node Metastases
A total of 9.1% patients experienced LLNM when they were first diagnosed with PTMC. Among patients with PTMC, patients with extrathyroidal invasion, presence of CLNM and CLNM ≥5 were associated with an increased risk of LLNM (OR=2.239 [95% CI 1.072–4.68], p=0.032; OR=8.625 [95% CI 2.196–33.869], p=0.002; and OR=3.749 [95% CI 1.484-9.472], p=0.005, respectively). Moreover, these three factors remained independent risk factors on multivariate analysis (OR=2.523 [95% CI 1.244–5.118], p=0.01; OR=11.362 [95% CI 3.383–38.16], p=0.00; OR=5.151 [95% CI 2.259-11.748], p=0.00, respectively) (Table 3).
Discussion
In our series, the number of female patients was triple those of males, which is consistent with the characteristics of thyroid malignancy of higher prevalence among women (1). Other studies have associated male sex and younger age with an increased rate of CLNM and disease recurrence, leading to poor prognosis (12–15). Accordingly, our data revealed that males and individuals aged under 40 years were more prone to CLNM in patients with PTMC. As compared with female PTMC patients aged 40 or more, large-volume CLNM (≥5), an independent predictor of LLNM, was more frequently observed in male patients aged under 40 years. Moreover, tumor size has been reported to be another essential predictor of CLNM and LLNM (14–16). Similarly, when we adopted tumor size of 5 mm as a threshold, we found that tumors with LTD ≥5 mm was significantly correlated with CLNM.
Bilateral tumors were observed in 18% PTMC cases in our cohort, which was in line with the description of previous studies (17, 18). In 2012, Wang et al. demonstrated that bilateral PTCs presented more advanced tumor stage and poorer prognosis than unilateral PTC (18). Later in 2016, the same conclusions were drawn by comparing the outcomes of bilateral and unilateral-multifocal PTCs, indicating that bilaterality, but not multifocality was associated with higher CLNM rates and shorter disease-free survival in PTC patients (19). Further analysis showed that bilateral PTC shared the same clonal origin, suggesting the presence of intrathyroidal metastasis, which may in part explain the aggressive features and high CLNM rates. However, different opinions have proposed, supporting the predictive role of multifocality for increased risk of CLNM and disease recurrence (20, 21). Our current study focused on PTMCs, and the results suggested that bilaterality had a stronger influence on CLNM than multifocality. Interestingly, investigating further, Wu et al. determined that for patients with unilateral multifocal PTMC, the sum of the diameter of all tumors ≥1.0 cm was an independent risk factor for CLNM (22).
HT refers to a common autoimmune thyroiditis that features lymphocytic infiltration and follicular destruction (23). The correlation between HT and TC is well-established yet debated. Some studies have indicated that PTC patients with concomitant HT exhibit aggressive behavior (14, 23), while others have reported a protective role of HT on cervical lymph node metastasis (24, 25). Further studies have revealed that the association between HT and TC could be age-related (26). Based on the findings of our study, no correlation between HT and CLNM or LLNM was identified in PTMC patients.
Considering the indolent course of PTMC, it is not recommended by the ATA guidelines to perform routine prophylactic central neck dissection in clinically node-negative PTMC patients (6). However, it is noteworthy that neck ultrasound has poor sensitivity in detecting metastases of the central compartment (11, 27). As for our cohort, among the 240 patients pathologically diagnosed as lymph node positive, only 21.7% were indicated by preoperative examination. Our result is consistent with the observation by Wu et al. that ultrasonography usually has sensitivity rates below 30% for CLNM (11). Also, it should be taken into consideration that CLND at re-operation would be complicated, and presents an increased risk of complications including laryngeal recurrent nerve injury and hypoparathyroidism (28).
Moreover, extrathyroidal invasion is indicative of locally advanced behavior, and tumors of any size presenting with extrathyroidal invasion are grouped into stage T4. Of note, our results showed that in over 20% of PTMC patients extrathyroidal invasion was confirmed on pathological examination, which resulted to be an independent risk factor for LLNM. Likewise, other studies have also described gross extrathyroidal invasion as a predictive factor for recurrence and poor outcomes, independently affecting disease-free survival and disease-specific survival (12, 14, 29).
In our series, 44 patients (9.1%) with PTMC presented with LLNM, with three patients exhibiting skip metastasis (i.e., the presence of LLNM without involving the central compartment). During subsequent analysis, we further divided patients into two groups according to the number and ratio of CLNM. Large-volume metastasis was defined by the presence of up to 5 metastatic CLN or a metastatic ratio of more than 50% and indicated adverse outcomes. Shou et al. observed significantly lower 5-year recurrence-free survival rates in cN0 PTMC patients with non-small-volume CLNM (metastatic lymph nodes >5 or ≥2 mm in size), indicating the negative role of large-volume metastasis (30). Likewise, our results demonstrated that both the presence of CLNM and CLNM ≥5 were strong indicators for LLNM in patients with PTMC. Therefore, for those underwent prophylactic central neck dissection and confirmed with positive CLNM or CLNM ≥5 at the pathological diagnosis, close follow-up may be warranted to screen for an increased risk of LLNM.
There are also several limitations to this study. First, this was a single-center retrospective study. Second, patients with CLNM were not differentiated by ipsilateral metastases or contralateral metastases due to the fact that the dissected lymph node samples were collectively defined as central lymph node in most cases; thus, further studies are required to address this issue. Finally, we calculated the incidence of temporary or permanent hypoparathyroidism but not the rates of laryngeal recurrent nerve injury given that the occurrence of hoarseness was not recorded in the medical records. However, based on our observations, no permanent vocal cord paralysis was reported for any of the patients evaluated.
Conclusion
In the present study, we show that the incidence of CLNM in PTMC is not negligible, and is associated with several independent risk factors including male sex, age <40 years, largest tumor diameter ≥0.5 cm, and bilaterality. Furthermore, lateral lymph node metastasis was exhibited in some PTMC patients and was associated with tumor extrathyroidal invasion, presence of CLNM, and CLNM ≥5 lesions. Although active surveillance is recommended as an alternative approach by ATA guidelines for PTMCs with no locally advanced features, based on our findings we proposed that it should be considered more carefully for patients that undergo delayed surgical treatment as they may present with more aggressive features. Given the increased risks of complications during second operation, we believe that identification of potential risk factors for CLNM and LLNM would help tailor individual surgical interventions for patients with PTMC.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
YH contributed to the conception and design of the study. WZ organized the database and acquired the data. YY performed the statistical analysis. WZ and YY wrote the first draft of the manuscript. YH critically revised the manuscript and supplemented the data for the final draft of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the Medical Scientific Research Foundation of Guangdong Province of China (grant#A2020014).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ATA, American Thyroid Association; CI, Confidence interval; CLND, Central lymph node dissection; CLNM, Central lymph node metastases; FNA, Fine needle cytology aspiration; FTC, Follicular thyroid cancer; HT, Hashimoto’s thyroiditis; HTC, Hürthle cell thyroid cancer; LH, Lobo-isthmectomy; LLND, Lateral lymph node dissection; LLNM, Lateral lymph node metastases; LTD, Largest tumor diameter; OR, Odds ratio; PTC, Papillary thyroid cancer; PTH, Parathyroid hormone; PTMC, Papillary thyroid micro-carcinoma; TC, Thyroid cancer; TT, Total thyroidectomy.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin (2020) 70(1):7–30. doi: 10.3322/caac.21590
3. Grimm D. Cell and Molecular Biology of Thyroid Disorders. Int J Mol Sci (2019) 20:2895. doi: 10.3390/ijms20122895
4. Markovina S, Grigsby PW, Schwarz JK, DeWees T, Moley JF, Siegel BA, et al. Treatment approach, surveillance, and outcome of well-differentiated thyroid cancer in childhood and adolescence. Thyroid (2014) 24:1121–6. doi: 10.1089/thy.2013.0297
5. Lim DJ, Baek KH, Lee YS, Park WC, Kim MK, Kang MI, et al. Clinical, histopathological, and molecular characteristics of papillary thyroid microcarcinoma. Thyroid (2007) 17(9):883–8. doi: 10.1089/thy.2007.0001
6. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
7. Xue S, Zhang L, Pang RZ, Wang PS, Jin MS, Guo L, et al. Predictive Factors of Central-Compartment Lymph Node Metastasis for Clinical N0 Papillary Thyroid Carcinoma With Strap Muscle Invasion. Front Endocrinol (Lausanne) (2020) 11:511. doi: 10.3389/fendo.2020.00511
8. So YK, Son YI, Hong SD, Seo MY, Baek CH, Jeong HS, et al. Subclinical lymph node metastasis in papillary thyroid microcarcinoma: a study of 551 resections. Surgery (2010) 148(3):526–31. doi: 10.1016/j.surg.2010.01.003
9. Calò PG, Conzo G, Raffaelli M, Medas F, Gambardella C, De CC, et al. Total thyroidectomy alone versus ipsilateral versus bilateral prophylactic central neck dissection in clinically node-negative differentiated thyroid carcinoma. A retrospective multicenter study. Eur J Surg Oncol (2017) 43(1):126–32. doi: 10.1016/j.ejso.2016.09.017
10. Orloff LA, Wiseman SM, Bernet VJ, Fahey TJ, Shaha AR, Shindo ML, et al. American Thyroid Association Statement on Postoperative Hypoparathyroidism: Diagnosis, Prevention, and Management in Adults. Thyroid (2018) 28(7):830–41. doi: 10.1089/thy.2017.0309
11. Wu X, Li BL, Zheng CJ, He XD. Predictive factors for central lymph node metastases in papillary thyroid microcarcinoma. World J Clin Cases (2020) 8(8):1350–60. doi: 10.12998/wjcc.v8.i8.1350
12. Zheng XQ, Peng C, Gao M, Zhi JT, Hou XK, Zhao JZ, et al. Risk factors for cervical lymph node metastasis in papillary thyroid microcarcinoma: a study of 1,587 patients. Cancer Biol Med (2019) 16(1):121–30. doi: 10.20892/j.issn.2095-3941.2018.0125
13. Siddiqui S, White MG, Antic T, Grogan RH, Angelos P, Kaplan EL, et al. Clinical and Pathologic Predictors of Lymph Node Metastasis and Recurrence in Papillary Thyroid Microcarcinoma. Thyroid (2016) 26(6):807–15. doi: 10.1089/thy.2015.0429
14. Song JL, Yan T, Qiu WW, Fan YB, Yang ZL. Clinical Analysis of Risk Factors for Cervical Lymph Node Metastasis in Papillary Thyroid Microcarcinoma: A Retrospective Study of 3686 Patients. Cancer Manag Res (2020) 12:2523–30. doi: 10.2147/CMAR.S250163
15. Tao Y, Wang CJ, Li LY, Xing HJ, Bai Y, Han B, et al. Clinicopathological features for predicting central and lateral lymph node metastasis in papillary thyroid microcarcinoma: Analysis of 66 cases that underwent central and lateral lymph node dissection. Mol Clin Oncol (2017) 6(1):49–55. doi: 10.3892/mco.2016.1085
16. Kim SK, Park I, Woo JW, Lee JH, Choe JH, Kim JH, et al. Predictive Factors for Lymph Node Metastasis in Papillary Thyroid Microcarcinoma. Ann Surg Oncol (2016) 23(9):2866–73. doi: 10.1245/s10434-016-5225-0
17. Koo BS, Lim HS, Lim YC, Yoon YH, Kim YM, Park YH, et al. Occult contralateral carcinoma in patients with unilateral papillary thyroid microcarcinoma. Ann Surg Oncol (2010) 17(4):1101–5. doi: 10.1245/s10434-009-0906-6
18. Wang WB, Zhao WH, Wang HY, Teng XD, Wang HH, Chen XH, et al. Poorer prognosis and higher prevalence of BRAF (V600E) mutation in synchronous bilateral papillary thyroid carcinoma. Ann Surg Oncol (2012) 19(1):31–6. doi: 10.1245/s10434-011-2096-2
19. Wang WB, Su XY, He KF, Wang YL, Wang HY, Wang HH, et al. Comparison of the clinicopathologic features and prognosis of bilateral versus unilateral multifocal papillary thyroid cancer: An updated study with more than 2000 consecutive patients. Cancer (2016) 122(2):198–206. doi: 10.1002/cncr.29689
20. Choi WR, Roh JL, Gong G, Cho KJ, Choi SH, Nam SY, et al. Multifocality of papillary thyroid carcinoma as a risk factor for disease recurrence. Oral Oncol (2019) 94:106–10. doi: 10.1016/j.oraloncology.2019.05.023
21. Zheng WH, Wang KJ, Wu JZ, Wang WD, Shang JB. Multifocality is associated with central neck lymph node metastases in papillary thyroid microcarcinoma. Cancer Manag Res (2018) 10:1527–33. doi: 10.2147/CMAR.S163263
22. Wu X, Li BL, Zheng CJ, He XD. Predicting factors of central lymph node metastases in patients with unilateral multifocal papillary thyroid microcarcinoma. Gland Surg (2020) 9(3):695–701. doi: 10.21037/gs.2020.03.27
23. Ragusa F, Fallahi P, Elia G, Gonnella D, Paparo SR, Giusti C, et al. Hashimotos’ thyroiditis: Epidemiology, pathogenesis, clinic and therapy. Best Pract Res Clin Endocrinol Metab (2019) 33(6):101367. doi: 10.1016/j.beem.2019.101367
24. Medas F, Canu GL, Cappellacci F, Boi F, Lai ML, Erdas E, et al. Predictive Factors of Lymph Node Metastasis in Patients With Papillary Microcarcinoma of the Thyroid: Retrospective Analysis on 293 Cases. Front Endocrinol (Lausanne) (2020) 11:551. doi: 10.3389/fendo.2020.00551
25. Dvorkin S, Robenshtok E, Hirsch D, Strenov Y, Shimon I, Benbassat CA. Differentiated thyroid cancer is associated with less aggressive disease and better outcome in patients with coexisting Hashimotos thyroiditis. J Clin Endocrinol Metab (2013) 98(6):2409–14. doi: 10.1210/jc.2013-1309
26. Babli S, Payne RJ, Mitmaker E, Rivera J. Effects of Chronic Lymphocytic Thyroiditis on the Clinicopathological Features of Papillary Thyroid Cancer. Eur Thyroid J (2018) 7(2):95–101. doi: 10.1159/000486367
27. Ahn JE, Lee JH, Yi JS, Shong YK, Hong SJ, Lee DH, et al. Diagnostic accuracy of CT and ultrasonography for evaluating metastatic cervical lymph nodes in patients with thyroid cancer. World J Surg (2008) 32(7):1552–8. doi: 10.1007/s00268-008-9588-7
28. Medas F, Tuveri M, Canu GL, Erdas E, Calò PG. Complications after reoperative thyroid surgery: retrospective evaluation of 152 consecutive cases. Updates Surg (2019) 71(4):705–10. doi: 10.1007/s13304-019-00647-y
29. Bortz MD, Kuchta K, Winchester DJ, Prinz RA, Moo-Young TA. Extrathyroidal extension predicts negative clinical outcomes in papillary thyroid cancer. Surgery (2021) 169(1):2–6. doi: 10.1016/j.surg.2020.04.003
Keywords: papillary thyroid carcinoma, micro-carcinoma, lymph node metastasis, thyroidectomy, risk factor
Citation: Huang Y, Yin Y and Zhou W (2021) Risk Factors for Central and Lateral Lymph Node Metastases in Patients With Papillary Thyroid Micro-Carcinoma: Retrospective Analysis on 484 Cases. Front. Endocrinol. 12:640565. doi: 10.3389/fendo.2021.640565
Received: 11 December 2020; Accepted: 01 February 2021;
Published: 05 March 2021.
Edited by:
Loredana Pagano, University of Turin, ItalyReviewed by:
Luigi Oragano, Azienda Sanitaria Locale - Verbano Cusio Ossola, ItalyJeffrey A. Knauf, Cleveland Clinic, United States
Copyright © 2021 Huang, Yin and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yijie Huang, aHlqMTIyOUAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Yijie Huang
Yijie Huang Ying Yin
Ying Yin Wenyi Zhou
Wenyi Zhou