- Diabetes Center, Department of Internal Medicine, Amsterdam University Medical Center, Amsterdam, Netherlands
The glucagon-like peptide-1 receptor agonist (GLP-1RA) semaglutide is the most recently approved agent of this drug class, and the only GLP-1RA currently available as both subcutaneous and oral formulation. While GLP-1RAs effectively improve glycemic control and cause weight loss, potential safety concerns have arisen over the years. For semaglutide, such concerns have been addressed in the extensive phase 3 registration trials including cardiovascular outcome trials for both subcutaneous (SUSTAIN: Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes) and oral (PIONEER: Peptide InnOvatioN for the Early diabEtes tReatment) semaglutide and are being studied in further trials and registries, including real world data studies. In the current review we discuss the occurrence of adverse events associated with semaglutide focusing on hypoglycemia, gastrointestinal side effects, pancreatic safety (pancreatitis and pancreatic cancer), thyroid cancer, gallbladder events, cardiovascular aspects, acute kidney injury, diabetic retinopathy (DRP) complications and injection-site and allergic reactions and where available, we highlight potential underlying mechanisms. Furthermore, we discuss whether effects are specific for semaglutide or a class effect. We conclude that semaglutide induces mostly mild-to-moderate and transient gastrointestinal disturbances and increases the risk of biliary disease (cholelithiasis). No unexpected safety issues have arisen to date, and the established safety profile for semaglutide is similar to that of other GLP-1RAs where definitive conclusions for pancreatic and thyroid cancer cannot be drawn at this point due to low incidence of these conditions. Due to its potent glucose-lowering effect, patients at risk for deterioration of existing DRP should be carefully monitored if treated with semaglutide, particularly if also treated with insulin. Given the beneficial metabolic and cardiovascular actions of semaglutide, and the low risk for severe adverse events, semaglutide has an overall favorable risk/benefit profile for patient with type 2 diabetes.
Introduction
With an alarming increase in type 2 diabetes (T2D) prevalence as well as its associated complications (1), the need for adequate treatment strategies for this devastating disease has never been higher. However, apart from studying the potential beneficial effects of new glucose-lowering agents, regulators and clinicians are increasingly focusing on long-term safety aspects. One of the newer antihyperglycemic drug classes receiving such scrutiny on safety are the glucagon-like peptide-1 (GLP-1) receptor agonists (GLP-1RAs). These agents are based on the gut-derived incretin hormone GLP-1, which is a potent stimulator of insulin, while suppressing glucagon secretion (2). In combination with inhibiting effects on gastric emptying and hepatic gluconeogenesis (3), GLP-1RA effectively reduce glucose levels (4). Several agents are now available after the first agent received marketing approval in 2005. Within the class of GLP-1RAs, substantial differences exist in drug structure, efficacy, dosing interval and even adverse effects (5). Nevertheless, in general, a decrease in HbA1c of 1–1.5% is observed, as well as beneficial effects on body weight, blood pressure and lipid profile (4). However, partly due to the widespread presence of GLP-1 receptors, several adverse effects have been observed, of which pancreatitis, pancreatic cancer and thyroid cancer were initially flagged as safety alerts (6).
The most recently approved GLP-1RAs is semaglutide. This agent is somewhat special among GLP-1RAs given that it is the only drug available as both subcutaneous injection (similar to all other GLP-1RAs) and as an oral formulation. Moreover, with years of development after marketing approval of the first GLP-1RA, the registration trials with semaglutide could focus on the already known potential safety risks of this drug class. In this review, as part of a supplement on semaglutide, we will detail the safety aspects of this drug.
Semaglutide
Semaglutide has been developed based on the vast body of research behind the development of liraglutide (7). Compared to liraglutide, which is administered once daily, semaglutide has an even longer half-life, allowing for once weekly administration. While a significant improvement over once or twice daily subcutaneous administration, the injecting route could be a barrier for some potential users. An absorption enhancer was discovered (sodium N-[8-(2-hydroxybenzoyl) aminocaprylate] or SNAC), which, when co-administered with semaglutide, was demonstrated to give therapeutic levels of the latter (8). SNAC helps to protect semaglutide from proteolytic degradation in the stomach and facilitates its absorption across the gastric mucosa by transient effects on transcellular pathways (8). At equivalent levels of exposure, similar glycemic and weight responses have been seen with both oral and subcutaneous semaglutide (9).
Both the subcutaneous and oral formulations of semaglutide have undergone extensive phase 3 clinical testing (Table 1). For the once-weekly subcutaneous formulation, the SUSTAIN program (Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes) included 13 separate randomized clinical phase 3a and 3b trials (10–13, 22, 25–32) SUSTAIN 1 through 10 were global international trials, while three additional trials were specific for China and Japan. In four studies, semaglutide was compared with placebo, with differing patient populations. SUSTAIN-6 is the cardiovascular outcome trial (CVOT) of subcutaneous semaglutide (28).
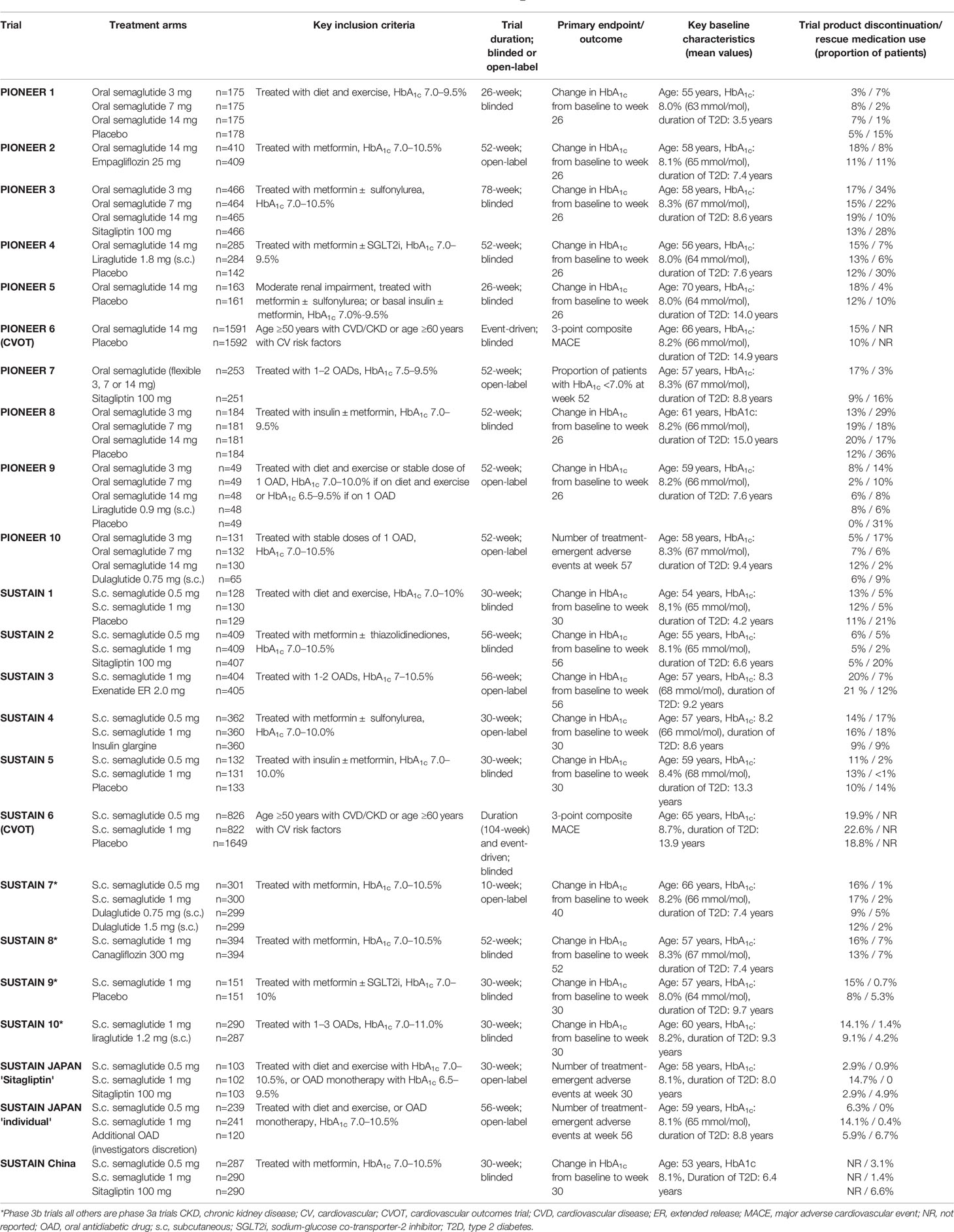
Table 1 Overview of Phase 3 studies of oral semaglutide (PIONEER) and subcutaneous semaglutide (SUSTAIN) (10–32).
The PIONEER program (Peptide InnOvatioN for the Early diabEtes tReatment) comprised 10 individual trials comparing once-daily oral semaglutide with placebo (six studies) or active comparator in different populations (14–21, 23, 24). Similar to the SUSTAIN program, PIONEER 6, was the CVOT (19). PIONEER 9 and 10 are specific to the Japanese population (12, 13). The SOUL (A Heart Disease Study of Semaglutide in Patients with Type 2 Diabetes) study is a larger CVOT with oral semaglutide that is currently ongoing (NCT03914326).
Combining all individual studies, the SUSTAIN program contained almost 12,000 participants, with over 9,500 subjects in the PIONEER program. With treatment duration of at least 26 weeks, this accounts of many patient years of follow-up, allowing an adequate review of the safety of semaglutide.
Adverse Effects of Semaglutide
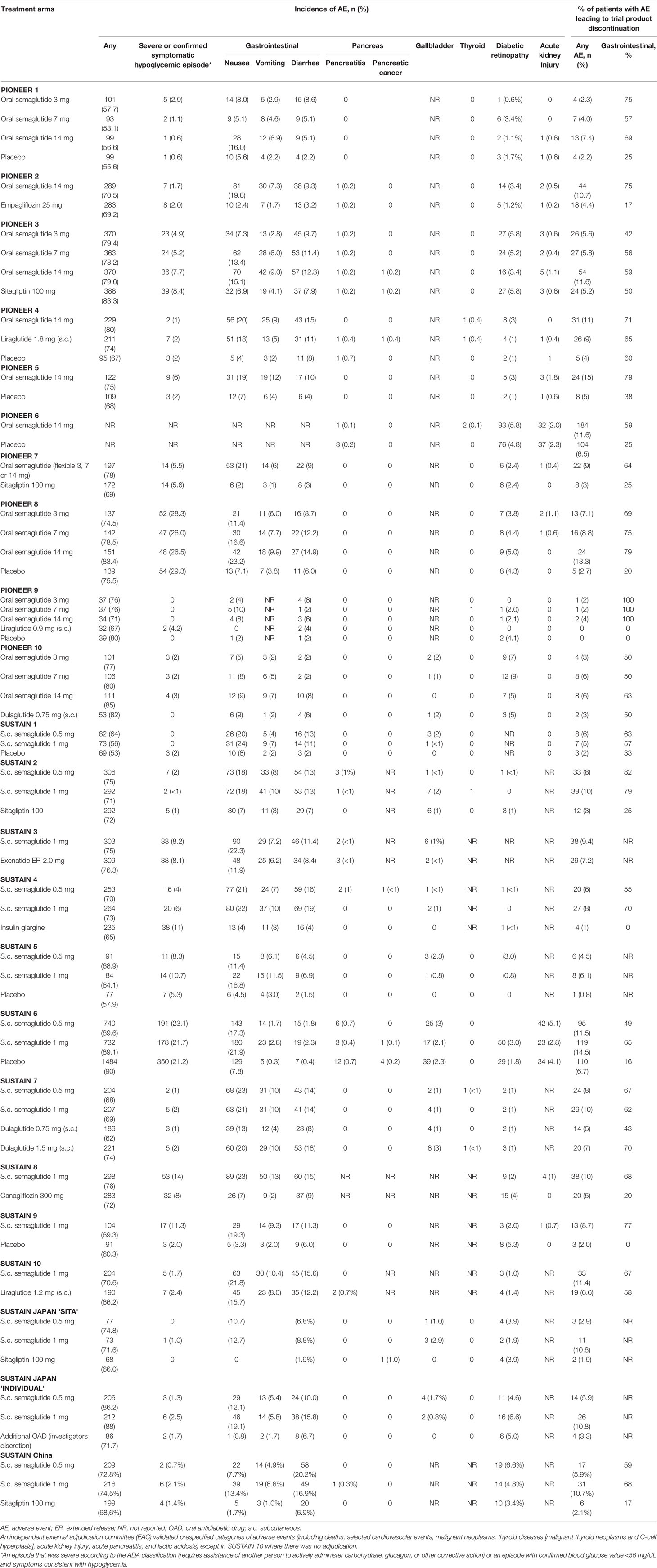
Semantically, the on-target effects of GLP-1RAs are those effects leading to a reduction in glucose levels. Any other effect can be considered as a pleiotropic, off-target effect, or in the case of unwanted actions, adverse effects (Figure 1). Many of the (adverse) class effects are shared among the different GLP-1RA, however, differences do occur. For semaglutide, one could expect a different side-effect profile for the oral versus the subcutaneous formulation. Apart from the obvious—tablets will not induce injection-site reactions—it could be suggested that higher portal levels induce more gastrointestinal disturbances. Moreover, with the maximum oral dosage plasma levels are lower compared with the maximal subcutaneous dose (oral 20 mg yields plasma levels of ~25 nM, subcutaneous 1 mg yields plasma levels of ~45 nM (33, 34)). Worth noting is that no data comparing the pharmacokinetic profile of both formulations against each other are available. In the following sections, the adverse reactions and safety issues of semaglutide, both oral and subcutaneous, will be discussed. We will discuss the risk of hypoglycemia, gastrointestinal side effects including previous reports on increased risk for pancreatitis and pancreas cancer, thyroid cancer, gallbladder stones, effects on the cardiovascular system, acute kidney injury, diabetic retinopathy risks and allergies/injection-site reactions (Table 2).
Hypoglycemia
Given that the aim of GLP-1RA therapy is mainly to reduce blood glucose levels, it is conceivable that these agents could cause hypoglycemia. However, since GLP-1RA mainly lower blood glucose by stimulating glucose-dependent insulin secretion, hypoglycemia is an infrequent problem. In addition, the inhibition of glucagon release does not occur under hypoglycemic conditions (35). In SUSTAIN-6, severe or plasma glucose-confirmed (<56 mg/dl [3.1 mol/L]) hypoglycemia occurred in similar rates between patients with semaglutide (23.1% in the 0.5 mg group and 21.7% in the 1 mg group) and placebo (21.2%) (28). In comparison, in SUSTAIN-4, severe or confirmed hypoglycemia occurred in 11% of insulin glargine-treated patients, compared with 4–6% in the semaglutide-treated patients (26). Importantly, in SUSTAIN-4 it is reported that hypoglycemia predominantly occurred in subjects using sulfonylurea agents (26). To illustrate: in the group of subjects randomized to semaglutide 1 mg, 9% of subjects using a s sulfonylurea had a severe or blood-glucose confirmed hypoglycemia, versus 2% in those not using a sulfonylurea. Similarly, in SUSTAIN-3, the majority of hypoglycemic events were reported in subjects concomitantly receiving sulfonylureas in both the semaglutide 1.0 mg and exenatide ER 2.0 mg groups. For oral semaglutide, the percentage of patients with severe hypoglycemia was 1.4% with oral semaglutide and 0.8% with placebo in PIONEER 6 (19). Here all severe hypoglycemic events occurred in patients receiving concomitant insulin or sulfonylurea therapy at the time of the event. In other phase 3 trials, no increase in hypoglycemia risk was observed versus comparator groups, including other GLP-1RAs (18–21, 23, 33–36).
Real world data with respect to hypoglycemia are limited to a single observational cohort from Canada (36). In 815 individuals who started semaglutide therapy and were followed for 6 months, there was no change in overall reported hypoglycemia. Although the group of concomitant insulin users also reported no change in hypoglycemia occurrence, this could have been mitigated by the on average 10–20% reduction in total daily insulin dosage (36). Sulfonylurea users did not experience an increase in hypoglycemia events.
Thus, the risk of hypoglycemia appears to be low with subcutaneous and oral semaglutide by themselves, yet the risk is increased when combined with sulfonylurea and/or insulin therapy. Several experts advise to lower the dose of sulfonylurea and short-acting and low-acting insulin analogues prior to or during titration of GLP-1RA therapy, to reduce the risk of (severe) hypoglycemia (37).
Gastrointestinal (GI) Adverse Effects
In the phase 3 trials, both oral and subcutaneous semaglutide were associated with gastrointestinal disturbances, such as nausea, vomiting and diarrhea, well-known effects from this drug class. When compared with placebo, subcutaneous semaglutide for 30 weeks induced nausea in 11.4 to 20% of the semaglutide-treated patients (placebo 3.3–8%), vomiting in 4 to 11.5% (placebo 2–3%) and diarrhea in 4.5 to 11.3% (placebo 1.5–6%) (10, 27, 31). In SUSTAIN 6, where generally older patients with comorbid conditions were treated for 104 weeks, the incidence of GI disturbances was somewhat higher (28). For oral semaglutide, the placebo-controlled trials found nausea ranged between 5.1 and 23.2% (placebo 5.6–7.1%), vomiting between 2.9 and 9.9% (placebo 2.2–3.8%) and diarrhea between 5.1 and 15% (placebo 2.2–8%) during the on-treatment period (14, 17, 21). These rates were not different when focusing on Japanese patients [PIONEER 9 (23)], but appeared higher in patients with T2D, reduced kidney function (estimated glomerular filtration rate [GFR] of 30–59 ml/min) and comorbidities in PIONEER 5 (18).
In one phase 2 trial, subcutaneous and oral semaglutide were compared with each other (38). Here, patients were randomized to oral semaglutide (at a dose of 5, 10, 20 or 40 mg once daily), subcutaneous semaglutide (1 mg once weekly) or placebo. As discussed below, this study also assessed the effect of dose escalation in two additional groups. Unfortunately, the currently advocated oral treatment doses of 7 and 14 mg were not included. When comparing oral 20 mg to subcutaneous 1 mg, the total amount of gastrointestinal disturbances was similar (56% versus 54%, respectively). This was also true for nausea (34% versus 32%), vomiting (16% versus 9%) and diarrhea (20% versus 14%). The proportion of patients with premature discontinuation because of adverse events appeared higher for oral semaglutide 20 mg (27%) than for subcutaneous semaglutide 1 mg (14%). All numbers were similar between the 10 and 20 mg oral dose, except for treatment discontinuation, which was 12% for the lower dosage.
Importantly, for both formulations, higher doses are often associated with more frequent GI adverse effects. For this reason, a dose escalation scheme is advised, starting with a low dose (3 mg). As a clear example in the abovementioned phase 2 study (38), 77% of patients experienced GI adverse effects when a fast 2-week dose escalation was used to reach 40 mg compared with 54% in the slower 8-week dose-escalation group. Generally, the GI complaints with semaglutide occur in the first 8–12 weeks of treatment during dose escalation [in contrast to for example liraglutide, where they occur within 2 weeks (17, 32)], and wane over time (Figure 2). Overall, the adverse effects are mild to moderate in severity and often self-limiting.
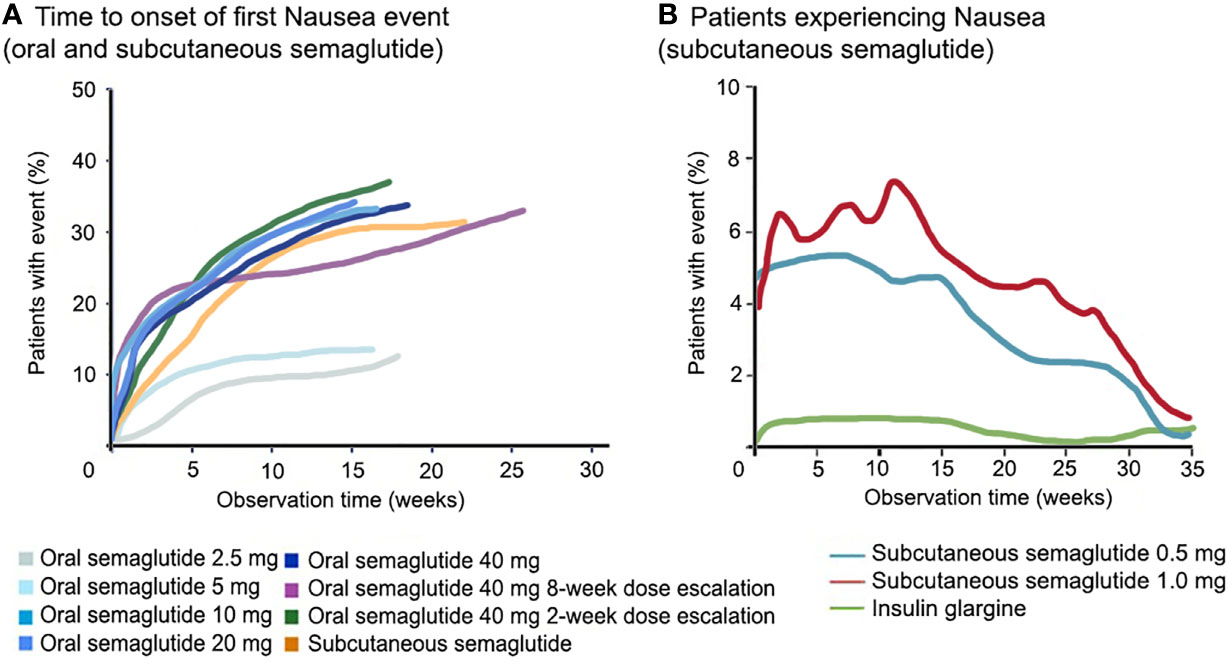
Figure 2 Course of nausea with semaglutide. GLP-1RA, including semaglutide, cause nausea in about one third of treated patients, which is both dose- and time-dependent. In panel (A), a direct comparison between subcutaneous and oral semaglutide is shown, as well as different doses of oral semaglutide, for the first occurrence of nausea. In panel (B), the course of the occurrence of nausea is shown for subcutaneous semaglutide. Data for panel (A) are derived from the phase-2 trial (38), for panel (B) data are shown from (26). GLP-1RA, glucagon-like peptide-1 receptor agonist.
Nevertheless, GI complaints are the main adverse-event related cause of drug discontinuation in the phase-3 trials, with rates up to 12% (Table 2). Moreover, cohorts with real-world data show similar numbers. In one retrospective study where 189 patients with T2DM starting subcutaneous semaglutide, 9.5% discontinued therapy because of GI complaints, while in 5.8% such adverse effects limited uptitration (39). In another cohort where 164 T2DM patients were switched from a different GLP-1RA therapy to semaglutide, 10.4% discontinued semaglutide because of adverse GI effects (40). Combined, data from clinical trials and clinical practice suggest that approximately 10% of patients will discontinue semaglutide because of GI complaints, which may be a bit higher compared to other GLP-1 analogues.
Apart from gradual dose titration, data on how to prevent or treat GI disturbances with GLP-1RA are limited. Patients can be counseled to eat slowly with reduced portion size per meal, stop eating when they experience satiety, and to avoid high-fat food (41). Anti-emetic therapy has been found effective in healthy subjects (42), but are not common practice since long-term data are not available. Interestingly, in one systematic analysis, background use of metformin was associated with more nausea and vomiting when using a GLP-1RA (43). However, whether this is also true for the combination with semaglutide, or whether lowering the dose of metformin has effect, has not been studied.
The mechanisms behind nausea/vomiting and diarrhea are incompletely understood. For nausea, a relation with the inhibiting effects on gastric emptying seems plausible. However, nausea also occurs in the fasting state (44), and is not related to measures of gastric emptying speed after meal ingestion (45). An effect on the central nervous system has been suggested as a recent study with modified exenatide—with reduced brain penetrance—showed less vomiting in musk shrews, despite retaining effects on glucose control (46). For diarrhea, studies are lacking. In one study, osmotic diarrhea occurred 8 h after infusion of GLP-1 peptide (47), and GLP-1RAs have been shown to reduce intestinal uptake of glucose and lipids (48, 49). Also, in patients with type 1 diabetes, liraglutide reduced colon transit time (50). So, hypothetically, semaglutide could induce diarrhea by altering nutrient absorption or intestinal motility.
Finally, although nausea and vomiting are perhaps unwanted effects, they may also be partly responsible for aspects of the drug’s efficacy as indicated above. As such, in some studies, nausea induced by GLP-1RAs is linked to weight loss (51, 52). For example, obese subjects treated with high-dose liraglutide who experienced (transient) nausea had on average 2.9 kg (95%-CI 0.5–5.3) more weight loss compared to those without GI events (51). In a mediation analysis of the SUSTAIN 1 to 5 trials, a small component (0.07 to 0.5 kg) of the total treatment difference in weight loss was explained by nausea or vomiting (52). In contrast, when combining data from SUSTAIN 3, 7 and 10, the occurrence of nausea and vomiting was not associated with superior weight loss (53). Whether this route plays a role in the beneficial effects of GLP-1RA on body weight needs further studying.
Pancreatic Adverse Events: Pancreatitis and Pancreatic Cancer
Within years of the introduction of GLP-1RAs, these agents were linked to the occurrence of acute pancreatitis, and suggested to potentially cause pancreatic cancer (6). In the subsequent years, many pharmacovigilance and database studies followed, with conflicting results (54–60). Given the nature of observational studies, data could have been confounded, since patients with diabetes whom have an indication for GLP-1RA therapy often have concomitant risk factors for pancreatitis (notably obesity, longer diabetes duration and co-medication). As such, the longer term CVOTs were a welcome addition to the discussion. When focusing on semaglutide, no signals of pancreatic AEs were present with blinded adjudication. In SUSTAIN 6, acute pancreatitis occurred in 9 semaglutide-treated patients, and in 12 placebo-treated patients. Pancreatic cancer occurred in one and four patients, respectively (28). In PIONEER 6, acute pancreatitis occurred in one semaglutide-treated patient, and in three placebo-treated patients (19). The incidence of pancreatic cancer was not reported. When combining all phase 3a data, pancreatitis occurred in five semaglutide-treated patients in PIONEER (six in the comparator group), and in 15 patients in SUSTAIN (13 in the comparator group). However, it is possible that for a relatively rare complication (the background incidence of pancreatitis and pancreatic cancer in T2D patients is 422 and 15–24 per 100,000 person-years, respectively (61, 62)), the CVOTs and phase 3 studies are of insufficient power to show differences between groups. When combining all available CVOT data (including those from non-semaglutide GLP-1RAs) in a meta-analysis, a hazard ratio of 1.05 (95% confidence interval [CI] 0.78–1.40) was found for pancreatitis and 1.12 (95% CI 0.77–1.63) for pancreatic cancer (63). These data thus argue against an effect of GLP-1RA on pancreatitis and pancreatic cancer incidence. However, one can wonder whether the follow-up duration in the CVOTs (ranging from a median of 1.3 to 5.4 years) is long enough for patients to develop pancreatic cancer.
While establishing (the absence of) a link with pancreatitis and pancreatic cancer in large clinical studies was one aspect in this field of research, others focused on animal studies and more mechanistic findings. One consistent finding is a subtle and asymptomatic increase in plasma lipase and amylase level (64, 65), which occurs within hours of administration (66). In a 26-week randomized controlled trial, oral semaglutide dose-dependently increased lipase levels by 9 to 55% and subcutaneous semaglutide by 36% (38). An increase in enzyme levels was not associated with occurrence of pancreatic events in trials with liraglutide (67, 68). Moreover, our group previously demonstrated that the liraglutide-induced increase in pancreatic enzymes is not associated with changes in pancreatic exocrine function or pancreas size measured by magnetic resonance imaging (69). Such studies have not yet been conducted for semaglutide.
A handful of preclinical studies showed that GLP-1RAs induce pancreatic inflammation, cellular proliferation and intra-epithelial neoplasia (PanIN) (70–72). However, the majority of animal studies did not find any effect of GLP-1RAs on pancreatic physiology, even with doses up to 240 the normal human dose (73–76). Preclinical studies with semaglutide also found no adverse signals in pancreatic tissue (76). Although pancreatic adverse events are difficult to completely rule out, an assessment by the FDA and the EMA concluded that a causal association between incretin-based drugs and pancreatitis or pancreatic cancer is inconsistent with the current data (77).
Thyroid Cancer
Both formulations of semaglutide have received an official box warning for thyroid C-cell tumors in the US. This caution is solely based on data from rodent studies and is not unique for semaglutide amongst the GLP-1RA. In rodents, the thyroid C-cells (neuroendocrine parafollicular cells which secrete calcitonin) highly express the GLP-1 receptor (78). Stimulation leads to upregulation of the calcitonin gene expression, calcitonin synthesis, C-cell hyperplasia, and increased risk of medullary adenomas and carcinomas (78). Initial studies found expression of the GLP-1 receptor in healthy human thyroid tissue, as well as in medullary thyroid carcinoma (MTC) and C-cell hyperplasia (79, 80). However, these studies were later refuted, as incompletely validated GLP-1 receptor antisera were used (81). When using validated antibodies, the GLP-1 receptor is only marginally expressed in thyroids of non-human primates and humans (78, 82). Supporting this is the observation that monkeys treated with >60 times the human dose of liraglutide do not develop C-cell abnormalities after 20 months (78).
In the SUSTAIN program, three adjudicated events of malignant thyroid neoplasm were identified, two in semaglutide-treated patients (combined n = 5,933), and one in the comparator group (n = 4,736) (10–13, 22, 26–32). None of these were medullary carcinoma. Serum calcitonin was measured during these trials, and no notable difference in mean levels was seen between the treatment arms. In the PIONEER program, four thyroid malignancies occurred in semaglutide-treated patients, versus one in the comparator group (14–21, 23, 24). In one instance, a MTC developed in a patient with preexisting nodules and elevated calcitonin at baseline (19). When looking at long-term data from the LEADER trials, the CVOT for liraglutide, there was no difference between liraglutide and placebo regarding calcitonin levels and C-cell malignancies (83).
It should be noted that MTC is rare (estimated incidence of 0.2 cases per 100,000 patient-years), and as such, it is very difficult to definitively rule out an association between GLP-1RA and thyroid malignancies. Therefore, regulatory authorities required additional pharmacovigilance activities, by systematically monitoring the annual incidence of MTC in the US for at least 15 years (MTC-22341, results expected by 2035–2037). In the meantime, semaglutide is contraindicated in patients with a personal or family history of MTC, as well as in patients with multiple endocrine neoplasia (MEN) type 2 in the US.
Gallbladder
In the SCALE-trial, high-dose liraglutide for the treatment of obesity was associated with an increased risk of gallbladder events compared to placebo (2.5% versus 1.0% of patients, respectively) (84). Based on AEs as reported in the European EudraVigilance database, gallbladder disease is likely not limited to liraglutide, but affects all incretin-based therapies (85). A recent meta-analysis observed an increased risk of 28% for cholelithiasis with GLP-1RA treatment (86), but a breakdown for each agent was not given. In the SUSTAIN program, 83 patients (1.4%) treated with semaglutide developed a gallbladder event, compared with 39 patients (1.9%) in the placebo group (10, 27, 28, 31, 32). The events mainly included cholelithiasis. In the PIONEER program, cholelithiasis occurred more often in the semaglutide-treated group (0.6% versus 0.1% with placebo), while the risk of cholecystitis was similar (data derived from the summary of product characteristics (SmPC) (87), as the manuscripts did not describe these data). Importantly, none of the gallbladder events have been linked to mortality. Cholelithiasis has been included in the SmPC of both subcutaneous and oral semaglutide).
Initially the gallbladder events were attributed to GLP-1RA-induced weight loss, as for example in the SCALE and LEADER trials, the patients with gallbladder events had more than average weight loss (84, 88). However, as gallbladder disease is not an issue with sodium-glucose co-transporter-2 (SGLT-2) inhibitors (with similar weight loss) (89), and gallbladder events also occurred in GLP-1RA-treated patients well after weight reduction (90), other mechanisms are possibly in play. One option could be lower gallbladder motility, which enhances biliary sludge formation and bile stones. In acute intervention studies, exenatide and albiglutide reduced cholecystokinin-induced gallbladder emptying (91, 92). However, after 12-week liraglutide intervention, we were unable to demonstrate an effect on gallbladder emptying (93), while Nexøe-Larsen et al. observed that liraglutide prolonged the time to reach maximum gallbladder emptying (94). Another mechanism is a change in bile salts, leading to supersaturated bile. While we observed changes in deoxycholic acid levels in plasma and fecal samples after liraglutide treatment, the clinical relevance remains unclear (93). Fascinatingly, exendin-4 appears to stimulate cholangiocyte proliferation through the GLP-1 receptor, hereby preventing cholangiocyte apoptosis in models of bile acid-induced damage and models of ductopenic cholangiopathies (95, 96). Although these data are considered beneficial, it also indicates that GLP-1RA could have direct adverse effects on the biliary tree. What the exact mechanism is behind the gallbladder events requires further study, but probably encompasses a combination of factors.
Cardiovascular
All GLP-1RAs increase heart rate, and this is not different for semaglutide. In SUSTAIN 6, a placebo-corrected heart rate increase of 2.75 beats per minute (bpm) was observed for semaglutide 0.5 mg, and 3.2 bpm for the 1.0 mg dosage (97). This increase was not associated with adverse cardiac events.
In addition, no increase in cardiovascular outcomes were observed in SUSTAIN 6 and PIONEER 6, which is reassuring given the initial fear of adverse cardiac events with increased resting heart rates. Large epidemiological studies have found that an increase in 5 bpm is associated with an increase of 17% in mortality (98). It is unclear whether this association holds true for drug-induced heart-rate acceleration. The α-blocking agent doxazosin increases heart rate by ~25% (99), and is associated with an increase in heart failure incidence (compared with the diuretic agent chlorthalidone) (100). In contrast, lowering heart rate by approximately 10 bpm using the cardiac funny-channel inhibitor ivabradine did not affect mortality in patients with stable coronary artery disease. At this point, it is clear that the beneficial effects of GLP-1RA on cardiovascular risk factors and physiology outweigh a potential risk of the associated heart rate increase. Liraglutide has been on the market for 10 years, but cardiovascular safety beyond this has not been studied yet.
The increase in heart rate is also of importance in patients with heart failure (HF). While the semaglutide CVOTs did not show an increased incidence of hospitalization for HF compared to placebo (101), in earlier smaller studies with liraglutide in patients with HF with reduced left ventricular ejection fraction, the GLP-1RA was associated with increased incidence of serious cardiac events (rhythm disorders, worsening of HF) (102, 103). Since patients with HF with New York Heart Association class IV were excluded from the CVOTs, it is unclear whether safety risks could occur in semaglutide-treated patients. However, a recent meta-analysis of all current CVOTs, showed that GLP-1RAs as a group were associated with a (non-significant) reduction in HF (104).
Several clinical mechanistic trials provided conflicting evidence while aiming to understand the GLP-1RA-induced heart rate-increase. Some studies found systemic vasodilation (with likely consequent reflex tachycardia), while others failed to show this (105–107). Similarly, discrepant findings are available for activation of the (cardiac) sympathetic nervous system (106, 108–111). Our own group previously hypothesized a direct effect of GLP-1RAs on sino-atrial cells (106), after exclusion of other potential causes. This postulation was later confirmed in a mouse model, where stimulation of GLP-1 receptors on atrial cells induced a chronotropic effect, but only when neuronal input was present (112).
Most novel drugs also undergo testing for their effect on the QT interval, as QT prolongation is a marker for potential ventricular fibrillation. Compared with placebo, subcutaneous semaglutide had no effect on this ECG measure in healthy volunteers, with doses above what is used in daily practice (113).
Acute Kidney Injury
Initial case reports suggested that GLP-1RA treatment could cause acute kidney injury (AKI) in some patients (114). Mechanistically, this was explained by dehydration caused by nausea, vomiting and diarrhea (see above). Also, very recently it was shown that the GLP-1RA, dulaglutide decreased fluid intake (115). Furthermore, GLP-1RA potentially further compromise fluid homeostasis by increasing renal sodium excretion (116). Combined, this could induce renal failure, especially in frail patients or those with medication such as renin–angiotensin–aldosterone system inhibitors, non-steroidal anti-inflammatory drugs or diuretic drugs.
In the SUSTAIN program, acute kidney failure was only reported in SUSTAIN 6, where its occurrence was similar between semaglutide and placebo (28). In PIONEER, AKI was a safety event of interest, and reported in all papers (14, 17–19, 21, 23). In PIONEER 6, AKI occurred in 2.0% of patients treated with oral semaglutide and 2.3% of placebo-treated patients (19). Whether this is statistically or clinically significant has not been evaluated yet.
In contrast to the incidental cases of AKI, the CVOTs mainly demonstrate a beneficial effect on renal outcomes, likely because of effects on cardiovascular risk factors (117). As recently reviewed, GLP-1RAs reduce progression to macro-albuminuria and lead to (subtle) reductions in the decline in renal function (118). In a recent post-hoc analysis of SUSTAIN 6, semaglutide was associated with less events of nephropathy, independent of baseline blood pressure (119). Thus, while it is conceivable yet not statistically confirmed that semaglutide could cause AKI in selected patients, there is plenty of evidence that it reduces nephropathy in the long term. A dedicated kidney trial (the FLOW study; NCT03819153) is currently ongoing, studying the effects of subcutaneous semaglutide on renal outcomes in people with T2D and chronic kidney disease.
Diabetic Retinopathy
In the SUSTAIN-6 trial, an increase in DRP complications, defined as a composite of need for retinal photocoagulation or treatment with intravitreal agents or vitreous hemorrhage or diabetes-related-blindness, was reported for semaglutide compared to placebo (hazard ratio 1.76; 95% CI 1.11–2.78). In a large systemic review and network analysis, including several GLP-1RAs, subcutaneous semaglutide was the only glucose-lowering drug for which this signal was observed (120). However, in the LEADER trial, a non-significant trend towards DRP was observed for liraglutide (121). In PIONEER 6, unadjudicated DRP occurred in 5.8% of oral semaglutide-treated patients and in 4.8% of the placebo-treated patients (19).
Villsbol and colleagues further investigated the DRP signal in the SUSTAIN program (122). In SUSTAIN-6, nearly 30% of patients had previous documented DRP, with 6% proliferative DRP. This percentage was not surprising given the inclusion of patients with previous cardiovascular disease, usually associated with long-standing diabetes. In semaglutide-treated patients, 3% (versus 1.8% in the placebo group) of patients reached an adjudicated endpoint of DRP. Across all DRP categories as indicated above, more events with semaglutide were noted. Participants that were prone to develop DRP had pre-existing DRP, longer diabetes duration, higher HbA1c levels at baseline, and more often used insulin therapy. Particularly, participants with pre-existing DRP who were using insulin therapy had the highest risk for a new DRP event.
This analysis further assessed whether the increase in DRP was a GLP-1 specific effect, or rather caused by a robust and early glucose lowering as suggested by several other studies, where acute and large reductions in glucose concentrations may initially and transiently worsen DRP, yet prevent or delay onset or progression of DRP in the long term (123–128). Patients that met a DRP endpoint had strongest glucose lowering during the trial, independent of their randomization to semaglutide or placebo. A post-hoc mediation analysis adjusting for HbA1c reduction at week 16 showed that glucose reduction at this time point explained the increased incidence. Limitations of DRP assessment during the trial were the absence of assessment of retinal changes over time, while the severity was not graded on baseline. Nevertheless, based on the data brought forward, it seems safe to conclude that the phenomenon of early worsening of pre-existing DRP was secondary to the initial and rapid improvement in glycemic control that occurred in SUSTAIN-6. This was confirmed in the recent AngioSafe study which showed no effect of GLP-1RA therapy on angiogenesis and no association between GLP-1 exposure and severe DRP was shown (129).
Currently, a large trial is ongoing assessing the long-term effects of semaglutide on DRP in patients with T2D as primary outcome (FOCUS trial, NCT03811561). This study will provide important data with respect to semaglutide safety on the retina. Until that time, caution should be exercised when using semaglutide in patients with DRP. It may be sensible to perform a fundoscopy prior to semaglutide therapy, and existing DRP should be treated where necessary. In addition, given the strong effects of semaglutide on glucose levels, down titrating insulin will prevent rapid decreases in glucose concentrations thereby reducing the risk of acute DRP worsening.
Injection-Site and Allergic Reactions
Although every subcutaneous injection can induce injection-site reactions, there are no signals that this is higher with semaglutide compared with placebo (130). In phase 3 studies, any site reaction was present in 0.6% of patients on the 0.5 mg dose, 0.3% on the 1 mg dose, and 0.8% in the comparator groups. The local site reaction includes bruising, discoloration, induration, and pain (130). In SUSTAIN-6, none of these injection site-reactions was considered severe, and it was never a reason to withhold therapy.
Given the immunogenic potential of protein-based drugs, it is important to monitor allergic reactions with GLP-1RAs. Allergic reactions were reported in four patients in the SUSTAIN program. However, at closer inspection, these reactions were more likely caused by the (concomitant) use of angiotensin-converting enzyme inhibitors or an infection (130). Across the phase 3a PIONEER trials, less subjects with oral semaglutide (2.9%) had allergic reactions compared with the comparators (4.6%) (131). No cases of anaphylactic reactions have yet been attributed to semaglutide; one patient using semaglutide had an anaphylactic shock attributed to cefazolin in SUSTAIN-6.
Effects of Semaglutide Compared to Other GLP-1 Receptor Agonists
The group of GLP-1RA contains several agents, and their adverse effect profile is not identical. This could be due to differences in pharmacokinetic profile (short- vs long-acting) and due to structural differences. Exendin-derived agents, i.e. exenatide and lixisenatide, are based on a protein derived from saliva of the Gila monster, and only share roughly 50% of the homology of GLP-1, which could trigger immunogenicity. The more frequent injection site reactions with exenatide once weekly (22%) compared with semaglutide (1.2%) in SUSTAIN-3 could be a consequence of this (22).
The head-to-head studies within the SUSTAIN and PIONEER programs allow some comparison of the adverse effect profile (Table 2). With these data, the safety profiles of rare potential events (e.g. pancreatitis, thyroid cancer, kidney injury, etc.) and hypoglycemia risk are comparable for semaglutide, dulaglutide, exenatide once weekly and liraglutide. However, semaglutide appears to be associated with more frequent nausea and vomiting. In SUSTAIN-3, 41.8% of patients with subcutaneous semaglutide had GI adverse effects, compared with 33.3% in exenatide once weekly (22). In SUSTAIN-7, nausea or vomiting occurred similarly for semaglutide 0.5 mg and 1 mg and dulaglutide 1.5 mg (43–48%), yet less frequent with dulaglutide 0.75 mg (33%) (29). In SUSTAIN-10, 21.8% of semaglutide-patients had nausea, compared to 15.7% of liraglutide-patients (32).
For oral semaglutide, the data are similar. In PIONEER-9, oral semaglutide induced nausea in up to 10% of patients, whereas none of the liraglutide patients had nausea (liraglutide was low-dose however) (23). Compared with dulaglutide in PIONEER-10, nausea rates were similar, yet oral semaglutide was more frequently associated with vomiting (14 mg dose semaglutide: 7%, dulaglutide 0.75 mg 2%) (24).
Finally, in a network meta-analysis, several short- and long-acting GLP-1RA were compared regarding efficacy and side effect profile. Compared with lixisenatide, exenatide twice daily, liraglutide, albiglutide and dulaglutide, semaglutide is associated with highest nausea and vomiting rates, yet also with highest rates of improvement in glycemic control and weight loss (132).
Whether the more rare adverse events differ between the different agents can only be answered by using observational cohort data from a very large group of patients and a longer follow-up time. Since semaglutide is relatively new, these data are not available yet. It should be stressed that guidelines do not favor the prescription of one GLP-1 RA over another, although clinicians are advised to select a compound with proven cardiovascular benefit.
Discussion
Since the finding that the thiazolidinedione rosiglitazone increased cardiovascular events, much weight has been placed on the safety of novel glucose-lowering drugs. For all new drugs, a thorough safety profile needs to be established, with particular emphasis on cardiovascular safety. While safety within the phase 3 program is sufficient for marketing authorization (although the risk of cardiovascular events should not exceed a hazard ratio of 1.8 according to a guidance document that was released by the FDA at that time), it is the post-marketing phase in which rare adverse events and any other potential safety risks are identified or resolved. The same FDA document mandates post-marketing trials to demonstrate that the novel agent does not increase cardiovascular risk by more than 30% compared to placebo (henceforth these trials were named ‘cardiovascular outcome trial’), if premarketing studies did not already demonstrate this.
Although designed for cardiovascular safety, other safety aspects may also be assessed in CVOTs. Moreover, after marketing approval, several databases can be employed to understand safety risks. In this regard, case reports and studies using adverse event databases (such as the FDA Adverse Event Reporting System and European Eudravigilance) frequently are the first signals of potential safety risks. With GLP-1RA, these encompassed AKI, pancreatitis, pancreatic cancer and thyroid cancer (6, 114). While awaiting the CVOTs, results from several health care database studies (insurance claims, hospital registry, etc.) were published, and were somewhat conflicting. With the totality of the evidence, many of the feared safety risks were nuanced or refuted.
As semaglutide is one of the youngest GLP-1RA, it was possible to prospectively monitor for the rarer adverse events in the phase 3 program and CVOT. As detailed in the current review, semaglutide appears not to increase the risk of pancreatitis (yet nevertheless it has been added to the SmPC to align with health authorities expectations on class labeling), but it is associated with more events of cholelithiasis. Although current data argue against an increased risk of pancreatic cancer and thyroid cancer with semaglutide, it can be debated whether the background incidence of these disorders is too low to fully conclude the absence of an association.
Even though the route of administration, their drug formulation and the dosage differ, the AE profile appears not to be very different between subcutaneous and oral semaglutide. One important co-product in oral semaglutide, SNAC, can be toxic at high doses (133). However, at the SNAC dosage of 300 mg per tablet of oral semaglutide, it is well below the toxic dose of 1.8 g/kg/day observed in monkeys, where it caused nausea and diarrhea (133). Post-marketing surveillance will help to elucidate whether the subcutaneous and oral variant differ in their real-world safety profile.
Most data reported in this review are from phase 3 clinical trials. Whether all of these data can be extrapolated to clinical practice remains a matter of debate. In RCTs, there are tightly regulated cohorts based on stringent in- and exclusion criteria, thereby reducing generalizability. Moreover, the frequent visits and calls during a study could improve patient coherence. However, real-world evidence—where available—has not shown major differences in for example hypoglycemia rate or drug discontinuation (36, 39, 40).
Conclusion
Over the years, the use of GLP-1RAs has first been associated with several adverse events, which were later mostly nuanced or refuted. As one of the newer agents within the class, the safety of semaglutide—both the subcutaneous and oral formulation—has been scrutinized in the phase 3 programs and CVOTs. Compared with placebo and active comparator, semaglutide induces mostly mild and transient gastrointestinal disturbances, and increases the risk of cholelithiasis. However, no major safety concerns have arisen to date, although definitive conclusions for pancreatic cancer, thyroid cancer and DRP complications cannot be drawn at this point. When compared with the beneficial effects of these drugs on glucose metabolism, blood pressure, body weight and cardiovascular (and potentially even renal) endpoints, these agents have an overall beneficial risk/benefit-profile for treatment of patients with T2D.
Author Contributions
The authors drafted all versions of the article, and provided final approval for submission. All authors contributed to the article and approved the submitted version.
Funding
This article was supported by Novo Nordisk, who was provided with the opportunity to perform a medical accuracy review.
Conflict of Interest
DVR has acted as a consultant and received honoraria from Boehringer Ingelheim, Eli Lilly, Merck, Novo Nordisk and Sanofi and has received research operating funds from the Boehringer Ingelheim–Eli Lilly Diabetes Alliance, MSD, AstraZeneca and Novo Nordisk.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that this article received funding from Novo Nordisk. The funder had the following involvement in the article: medical writing support.
References
1. International Diabetes Federation. IDF Diabetes Atlas 2019 (2019). Available at: https://www.diabetesatlas.org/data/en/region/3/eur.html.
2. Holst JJ. The Physiology of Glucagon-Like Peptide 1. Physiol Rev (2007) 87:1409–39. doi: 10.1152/physrev.00034.2006
3. Smits MM, Tonneijck L, Muskiet MHA, Kramer MHH, Cahen DL, van Raalte DH. Gastrointestinal Actions of GLP-1 Based Therapies: Glycaemic Control Beyond the Pancreas. Diabetes Obes Metab (2016) 18:178–85. doi: 10.1111/dom.12593
4. Hussein H, Zaccardi F, Khunti K, Davies MJ, Patsko E, Dhalwani NN, et al. Efficacy and Tolerability of Sodium-Glucose Co-Transporter-2 Inhibitors and Glucagon-Like Peptide-1 Receptor Agonists: A Systematic Review and Network Meta-Analysis. Diabetes Obes Metab (2020) 22:1035–46. doi: 10.1111/dom.14008
5. Lyseng-Williamson KA. Glucagon-Like Peptide-1 Receptor Agonists in Type 2 Diabetes: Their Use and Differential Feature. Clin Drug Investig (2019) 39:805–19. doi: 10.1007/s40261-019-00826-0
6. Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, Pancreatic, and Thyroid Cancer With Glucagon-Like Peptide-1-Based Therapies. Gastroenterology (2011) 141:150–6. doi: 10.1053/j.gastro.2011.02.018
7. Knudsen LB, Lau J. The Discovery and Development of Liraglutide and Semaglutid. Front Endocrinol (Lausanne) (2019) 10:155. doi: 10.3389/fendo.2019.00155
8. Buckley ST, Bækdal TA, Vegge A, Maarbjerg SJ, Pyke C, Ahnfelt-Rønne J, et al. Transcellular Stomach Absorption of a Derivatized Glucagon-Like Peptide-1 Receptor Agonist. Sci Transl Med (2018) 10:eaar7047. doi: 10.1126/scitranslmed.aar7047
9. Overgaard R, Navarria A, Hertz C, Ingwersen S. Similar Efficacy and Gastrointestinal Tolerability Versus Exposure for Oral and Subcutaneous Semaglutide, in: The 55th Annual Meeting of the European Association for the Study of Diabetes (EASD Virtual Meeting), Abstract #777. Diabetologia (2019) 62:1–600. doi: 10.1007/s00125-019-4946-6
10. Sorli C, Harashima S, Tsoukas GM, Unger J, Karsbøl JD, Hansen T, et al. Efficacy and Safety of Once-Weekly Semaglutide Monotherapy Versus Placebo in Patients With Type 2 Diabetes (SUSTAIN 1): A Double-Blind, Randomised, Placebo-Controlled, Parallel-Group, Multinational, Multicentre Phase 3a Trial. Lancet Diabetes Endocrinol (2017) 5:251–60. doi: 10.1016/S2213-8587(17)30013-X
11. Ahrén B, Masmiquel L, Kumar H, Sargin M, Karsbøl JD, Jacobsen SH, et al. Efficacy and Safety of Once-Weekly Semaglutide Versus Once-Daily Sitagliptin as an Add-on to Metformin, Thiazolidinediones, or Both, in Patients With Type 2 Diabetes (SUSTAIN 2): A 56-Week, Double-Blind, Phase 3a, Randomised Trial. Lancet Diabetes Endocrinol (2017) 5:341–54. doi: 10.1016/S2213-8587(17)30092-X
12. Seino Y, Terauchi Y, Osonoi T, Yabe D, Abe N, Nishida T, et al. Safety and Efficacy of Semaglutide Once Weekly vs Sitagliptin Once Daily, Both as Monotherapy in J Apanese People With Type 2 Diabetes. Diabetes Obes Metab (2018) 20:378–88. doi: 10.1111/dom.13082
13. Kaku K, Yamada Y, Watada H, Abiko A, Nishida T, Zacho J, et al. Safety and Efficacy of Once-Weekly Semaglutide vs Additional Oral Antidiabetic Drugs in Japanese People With Inadequately Controlled Type 2 Diabetes: A Randomized Trial. Diabetes Obes Metab (2018) 20:1202–12. doi: 10.1111/dom.13218
14. Aroda VR, Rosenstock J, Terauchi Y, Altuntas Y, Lalic NM, Morales Villegas EC, et al. PIONEER 1: Randomized Clinical Trial of the Efficacy and Safety of Oral Semaglutide Monotherapy in Comparison With Placebo in Patients With Type 2 Diabete. Diabetes Care (2019) 42:1724–32. doi: 10.2337/dc19-0749
15. Rodbard HW, Rosenstock J, Canani LH, Deerochanawong C, Gumprecht J, Lindberg SØ, et al. Oral Semaglutide Versus Empagliflozin in Patients With Type 2 Diabetes Uncontrolled on Metformin: The PIONEER 2 Tria. Diabetes Care (2019) 42:2272–81. doi: 10.2337/dc19-0883
16. Rosenstock J, Allison D, Birkenfeld AL, Blicher TM, Deenadayalan S, Jacobsen JB, et al. Effect of Additional Oral Semaglutide vs Sitagliptin on Glycated Hemoglobin in Adults With Type 2 Diabetes Uncontrolled With Metformin Alone or With Sulfonylure. JAMA (2019) 321:1466. doi: 10.1001/jama.2019.2942
17. Pratley R, Amod A, Hoff ST, Kadowaki T, Lingvay I, Nauck M, et al. Oral Semaglutide Versus Subcutaneous Liraglutide and Placebo in Type 2 Diabetes (PIONEER 4): A Randomised, Double-Blind, Phase 3a Trial. Lancet (2019) 394:39–50. doi: 10.1016/S0140-6736(19)31271-1
18. Mosenzon O, Blicher TM, Rosenlund S, Eriksson JW, Heller S, Hels OH, et al. Efficacy and Safety of Oral Semaglutide in Patients With Type 2 Diabetes and Moderate Renal Impairment (PIONEER 5): A Placebo-Controlled, Randomised, Phase 3a Trial. Lancet Diabetes Endocrinol (2019) 7:515–27. doi: 10.1016/S2213-8587(19)30192-5
19. Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, et al. Oral Semaglutide and Cardiovascular Outcomes in Patients With Type 2 Diabete. N Engl J Med (2019) 381:841–51. doi: 10.1056/NEJMoa1901118
20. Pieber TR, Bode B, Mertens A, Cho YM, Christiansen E, Hertz CL, et al. Efficacy and Safety of Oral Semaglutide With Flexible Dose Adjustment Versus Sitagliptin in Type 2 Diabetes (PIONEER 7): A Multicentre, Open-Label, Randomised, Phase 3a Trial. Lancet Diabetes Endocrinol (2019) 7:528–39. doi: 10.1016/S2213-8587(19)30194-9
21. Zinman B, Aroda VR, Buse JB, Cariou B, Harris SB, Hoff ST, et al. Efficacy, Safety, and Tolerability of Oral Semaglutide Versus Placebo Added to Insulin With or Without Metformin in Patients With Type 2 Diabetes: The PIONEER 8 Tria. Diabetes Care (2019) 42:2262–71. doi: 10.2337/dc19-0898
22. Ahmann AJ, Capehorn M, Charpentier G, Dotta F, Henkel E, Lingvay I, et al. Efficacy and Safety of Once-Weekly Semaglutide Versus Exenatide ER in Subjects With Type 2 Diabetes (SUSTAIN 3): A 56-Week, Open-Label, Randomized Clinical Tria. Diabetes Care (2018) 41:258–66. doi: 10.2337/dc17-0417
23. Yamada Y, Katagiri H, Hamamoto Y, Deenadayalan S, Navarria A, Nishijima K, et al. Dose-Response, Efficacy, and Safety of Oral Semaglutide Monotherapy in Japanese Patients With Type 2 Diabetes (PIONEER 9): A 52-Week, Phase 2/3a, Randomised, Controlled Trial. Lancet Diabetes Endocrinol (2020) 8:377–91. doi: 10.1016/S2213-8587(20)30075-9
24. Yabe D, Nakamura J, Kaneto H, Deenadayalan S, Navarria A, Gislum M, et al. Safety and Efficacy of Oral Semaglutide Versus Dulaglutide in Japanese Patients With Type 2 Diabetes (PIONEER 10): An Open-Label, Randomised, Active-Controlled, Phase 3a Trial. Lancet Diabetes Endocrinol (2020) 8:392–406. doi: 10.1016/S2213-8587(20)30074-7
25. Ji L, Dong X, Li Y, Li Y, Lim S, Liu M, et al. Efficacy and Safety of Once-Weekly Semaglutide vs Once-Daily Sitagliptin as Add-on to Metformin in Patients With Type 2 Diabetes (SUSTAIN China): A 30-Week Double-Blind, Phase 3a, Randomised Trial. Diabetes Obes Metab (2020) 23:404–14. doi: 10.1111/dom.14232
26. Aroda VR, Bain SC, Cariou B, Piletič M, Rose L, Axelsen M, et al. Efficacy and Safety of Once-Weekly Semaglutide Versus Once-Daily Insulin Glargine as Add-on to Metformin (With or Without Sulfonylureas) in Insulin-Naive Patients With Type 2 Diabetes (SUSTAIN 4): A Randomised, Open-Label, Parallel-Group, Multicentre, Mul. Lancet Diabetes Endocrinol (2017) 5:355–66. doi: 10.1016/S2213-8587(17)30085-2
27. Rodbard HW, Lingvay I, Reed J, de la Rosa R, Rose L, Sugimoto D, et al. Semaglutide Added to Basal Insulin in Type 2 Diabetes (SUSTAIN 5): A Randomized, Controlled Tria. J Clin Endocrinol Metab (2018) 103:2291–301. doi: 10.1210/jc.2018-00070
28. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, et al. Semaglutide and Cardiovascular Outcomes in Patients With Type 2 Diabetes. N Engl J Med (2016) 375:1834–44. doi: 10.1056/NEJMoa1607141
29. Pratley RE, Aroda VR, Lingvay I, Lüdemann J, Andreassen C, Navarria A, et al. Semaglutide Versus Dulaglutide Once Weekly in Patients With Type 2 Diabetes (SUSTAIN 7): A Randomised, Open-Label, Phase 3b Trial. Lancet Diabetes Endocrinol (2018) 6:275–86. doi: 10.1016/S2213-8587(18)30024-X
30. Lingvay I, Catarig A-M, Frias JP, Kumar H, Lausvig NL, le Roux CW, et al. Efficacy and Safety of Once-Weekly Semaglutide Versus Daily Canagliflozin as Add-on to Metformin in Patients With Type 2 Diabetes (SUSTAIN 8): A Double-Blind, Phase 3b, Randomised Controlled Trial. Lancet Diabetes Endocrinol (2019) 7:834–44. doi: 10.1016/S2213-8587(19)30311-0
31. Zinman B, Bhosekar V, Busch R, Holst I, Ludvik B, Thielke D, et al. Semaglutide Once Weekly as Add-on to SGLT-2 Inhibitor Therapy in Type 2 Diabetes (SUSTAIN 9): A Randomised, Placebo-Controlled Trial. Lancet Diabetes Endocrinol (2019) 7:356–67. doi: 10.1016/S2213-8587(19)30066-X
32. Capehorn MS, Catarig A-M, Furberg JK, Janez A, Price HC, Tadayon S, et al. Efficacy and Safety of Once-Weekly Semaglutide 1.0 Mg vs Once-Daily Liraglutide 1.2 Mg as Add-on to 1–3 Oral Antidiabetic Drugs in Subjects With Type 2 Diabetes (SUSTAIN 10). Diabetes Metab (2020) 46:100–9. doi: 10.1016/j.diabet.2019.101117
33. Ikushima I, Jensen L, Flint A, Nishida T, Zacho J. Irie S. A Randomized Trial Investigating the Pharmacokinetics, Pharmacodynamics, and Safety of Subcutaneous Semaglutide Once-Weekly in Healthy Male Japanese and Caucasian Subject. Adv Ther (2018) 35:531–44. doi: 10.1007/s12325-018-0677-1
34. Granhall C, Donsmark M, Blicher TM, Golor G, Søndergaard FL, Thomsen M, et al. Safety and Pharmacokinetics of Single and Multiple Ascending Doses of the Novel Oral Human GLP-1 Analogue, Oral Semaglutide, in Healthy Subjects and Subjects With Type 2 Diabete. Clin Pharmacokinet (2019) 58:781–91. doi: 10.1007/s40262-018-0728-4
35. Korsatko S, Jensen L, Brunner M, Sach-Friedl S, Tarp MD, Holst AG, et al. Effect of Once-Weekly Semaglutide on the Counterregulatory Response to Hypoglycaemia in People With Type 2 Diabetes: A Randomized, Placebo-Controlled, Double-Blind, Crossover Trial. Diabetes Obes Metab (2018) 20:2565–73. doi: 10.1111/dom.13422
36. Brown RE, Bech PG, Aronson R. Semaglutide Once Weekly in People With Type 2 Diabetes: Real-World Analysis of the Canadian LMC Diabetes Registry ( SPARE Study). Diabetes Obes Metab (2020) 22:2013–20. doi: 10.1111/dom.14117
37. Romera I, Cebrián-Cuenca A, Álvarez-Guisasola F, Gomez-Peralta F. Reviriego J. A Review of Practical Issues on the Use of Glucagon-Like Peptide-1 Receptor Agonists for the Management of Type 2 Diabete. Diabetes Ther (2019) 10:5–19. doi: 10.1007/s13300-018-0535-9
38. Davies M, Pieber TR, Hartoft-Nielsen M-L, Hansen OKH, Jabbour S, Rosenstock J. Effect of Oral Semaglutide Compared With Placebo and Subcutaneous Semaglutide on Glycemic Control in Patients With Type 2 Diabete. JAMA (2017) 318:1460. doi: 10.1001/jama.2017.14752
39. Williams DM, Ruslan AM, Khan R, Vijayasingam D, Iqbal F, Shaikh A, et al. Real-World Clinical Experience of Semaglutide in Secondary Care Diabetes: A Retrospective Observational Stud. Diabetes Ther (2021) 12:801–11. doi: 10.1007/s13300-021-01015-z
40. Jain AB, Kanters S, Khurana R, Kissock J, Severin N, Stafford SG. Real-World Effectiveness Analysis of Switching From Liraglutide or Dulaglutide to Semaglutide in Patients With Type 2 Diabetes Mellitus: The Retrospective REALISE-DM Stud. Diabetes Ther (2021) 12:527–36. doi: 10.1007/s13300-020-00984-x
41. Shomali M. Optimizing the Care of Patients With Type 2 Diabetes Using Incretin-Based Therapy: Focus on GLP-1 Receptor Agonist. Clin Diabetes (2014) 32:32–43. doi: 10.2337/diaclin.32.1.32
42. Ellero C, Han J, Bhavsar S, Cirincione BB, Deyoung MB, Gray AL, et al. Prophylactic Use of Anti-Emetic Medications Reduced Nausea and Vomiting Associated With Exenatide Treatment: A Retrospective Analysis of an Open-Label, Parallel-Group, Single-Dose Study in Healthy Subjects. Diabetes Med (2010) 27:1168–73. doi: 10.1111/j.1464-5491.2010.03085.x
43. Bettge K, Kahle M, Abd El Aziz MS, Meier JJ, Nauck MA. Occurrence of Nausea, Vomiting and Diarrhoea Reported as Adverse Events in Clinical Trials Studying Glucagon-Like Peptide-1 Receptor Agonists: A Systematic Analysis of Published Clinical Trials. Diabetes Obes Metab (2017) 19:336–47. doi: 10.1111/dom.12824
44. Linnebjerg H, Park S, Kothare PA, Trautmann ME, Mace K, Fineman M, et al. Effect of Exenatide on Gastric Emptying and Relationship to Postprandial Glycemia in Type 2 Diabetes. Regul Pept (2008) 151:123–9. doi: 10.1016/j.regpep.2008.07.003
45. Willms B, Werner J, Holst JJ, Orskov C, Creutzfeldt W, Nauck MA. Gastric Emptying, Glucose Responses, and Insulin Secretion After a Liquid Test Meal: Effects of Exogenous Glucagon-Like Peptide-1 (GLP-1)-(7-36) Amide in Type 2 (Noninsulin-Dependent) Diabetic Patients. J Clin Endocrinol Metab (1996) 81:327–32. doi: 10.1210/jcem.81.1.8550773
46. Borner T, Workinger JL, Tinsley IC, Fortin SM, Stein LM, Chepurny OG, et al. Corrination of a GLP-1 Receptor Agonist for Glycemic Control Without Emesi. Cell Rep (2020) 31:107768. doi: 10.1016/j.celrep.2020.107768
47. Gutzwiller J-P, Hruz P, Huber AR, Hamel C, Zehnder C, Drewe J, et al. Glucagon-Like Peptide-1 is Involved in Sodium and Water Homeostasis in Humans. Digestion (2006) 73:142–50. doi: 10.1159/000094334
48. Deane AM, Chapman MJ, Fraser RJL, Summers MJ, Zaknic AV, Storey JP, et al. Effects of Exogenous Glucagon-Like Peptide-1 on Gastric Emptying and Glucose Absorption in the Critically Ill: Relationship to Glycemia. Crit Care Med (2010) 38:1261–9. doi: 10.1097/CCM.0b013e3181d9d87a
49. Xiao C, Bandsma RHJ, Dash S, Szeto L, Lewis GF. Exenatide, a Glucagon-Like Peptide-1 Receptor Agonist, Acutely Inhibits Intestinal Lipoprotein Production in Healthy Humans. Arterioscler Thromb Vasc Biol (2012) 32:1513–9. doi: 10.1161/ATVBAHA.112.246207
50. Wegeberg A-ML, Hansen CS, Farmer AD, Karmisholt JS, Drewes AM, Jakobsen PE, et al. Liraglutide Accelerates Colonic Transit in People With Type 1 Diabetes and Polyneuropathy: A Randomised, Double-Blind, Placebo-Controlled Trial. United Eur Gastroenterol J (2020) 8(6):695–704. doi: 10.1177/2050640620925968
51. Lean MEJ, Carraro R, Finer N, Hartvig H, Lindegaard ML, Rössner S, et al. Tolerability of Nausea and Vomiting and Associations With Weight Loss in a Randomized Trial of Liraglutide in Obese, non-Diabetic Adults. Int J Obes (2014) 38:689–97. doi: 10.1038/ijo.2013.149
52. Ahrén B, Atkin SL, Charpentier G, Warren ML, Wilding JPH, Birch S, et al. Semaglutide Induces Weight Loss in Subjects With Type 2 Diabetes Regardless of Baseline BMI or Gastrointestinal Adverse Events in the SUSTAIN 1 to 5 Trials. Diabetes Obes Metab (2018) 20:2210–9. doi: 10.1111/dom.13353
53. Lingvay I, Hansen T, Macura S, Marre M, Nauck MA, de la Rosa R, et al. Superior Weight Loss With Once-Weekly Semaglutide Versus Other Glucagon-Like Peptide-1 Receptor Agonists is Independent of Gastrointestinal Adverse Events. BMJ Open Diabetes Res Care (2020) 8:e001706. doi: 10.1136/bmjdrc-2020-001706
54. Dore DD, Bloomgren GL, Wenten M, Hoffman C, Clifford CR, Quinn SG, et al. A Cohort Study of Acute Pancreatitis in Relation to Exenatide Use. Diabetes Obes Metab (2011) 13:559–66. doi: 10.1111/j.1463-1326.2011.01376.x
55. Funch D, Gydesen H, Tornøe K, Major-Pedersen A, Chan KA. A Prospective, Claims-Based Assessment of the Risk of Pancreatitis and Pancreatic Cancer With Liraglutide Compared to Other Antidiabetic Drugs. Diabetes Obes Metab (2014) 16:273–5. doi: 10.1111/dom.12230
56. Garg R, Chen W, Pendergrass M. Acute Pancreatitis in Type 2 Diabetes Treated With Exenatide or Sitagliptin: A Retrospective Observational Pharmacy Claims Analysis. Diabetes Care (2010) 33:2349–54. doi: 10.2337/dc10-0482
57. Giorda CB, Picariello R, Nada E, Tartaglino B, Marafetti L, Costa G, et al. Incretin Therapies and Risk of Hospital Admission for Acute Pancreatitis in an Unselected Population of European Patients With Type 2 Diabetes: A Case-Control Study. Lancet Diabetes Endocrinol (2014) 2:111–5. doi: 10.1016/S2213-8587(13)70147-5
58. Li L, Shen J, Bala MM, Busse JW, Ebrahim S, Vandvik PO, et al. Incretin Treatment and Risk of Pancreatitis in Patients With Type 2 Diabetes Mellitus: Systematic Review and Meta-Analysis of Randomised and Non-Randomised Studies. BMJ (2014) 348:g2366. doi: 10.1136/bmj.g2366
59. Wenten M, Gaebler JA, Hussein M, Pelletier EM, Smith DB, Girase P, et al. Relative Risk of Acute Pancreatitis in Initiators of Exenatide Twice Daily Compared With Other Anti-Diabetic Medication: A Follow-Up Study. Diabetes Med (2012) 29:1412–8. doi: 10.1111/j.1464-5491.2012.03652.x
60. Singh S, Chang H-Y, Richards TM, Weiner JP, Clark JM, Segal JB. Glucagonlike Peptide 1-Based Therapies and Risk of Hospitalization for Acute Pancreatitis in Type 2 Diabetes Mellitus: A Population-Based Matched Case-Control Study. JAMA Intern Med (2013) 173:534–9. doi: 10.1001/jamainternmed.2013.2720
61. Noel RA, Braun DK, Patterson RE, Bloomgren GL. Increased Risk of Acute Pancreatitis and Biliary Disease Observed in Patients With Type 2 Diabetes: A Retrospective Cohort Study. Diabetes Care (2009) 32:834–8. doi: 10.2337/dc08-1755
62. Koo D-H, Han K-D, Park C-Y. The Incremental Risk of Pancreatic Cancer According to Fasting Glucose Levels: Nationwide Population-Based Cohort Stud. J Clin Endocrinol Metab (2019) 104:4594–9. doi: 10.1210/jc.2019-00033
63. Cao C, Yang S, Zhou Z. GLP-1 Receptor Agonists and Pancreatic Safety Concerns in Type 2 Diabetic Patients: Data From Cardiovascular Outcome Trials. Endocrine (2020) 68:518–25. doi: 10.1007/s12020-020-02223-6
64. Lando HM, Alattar M, Dua AP. Elevated Amylase and Lipase Levels in Patients Using Glucagonlike Peptide-1 Receptor Agonists or Dipeptidyl-Peptidase-4 Inhibitors in the Outpatient Setting. Endocr Pract (2012) 18:472–7. doi: 10.4158/EP11290.OR
65. Tokuyama H, Kawamura H, Fujimoto M, Kobayashi K, Nieda M, Okazawa T, et al. A Low-Grade Increase of Serum Pancreatic Exocrine Enzyme Levels by Dipeptidyl Peptidase-4 Inhibitor in Patients With Type 2 Diabetes. Diabetes Res Clin Pract (2013) 100:e66–9. doi: 10.1016/j.diabres.2013.03.034
66. Smits MM, Tonneijck L, Muskiet MHA, Diamant M, Kramer MHH, Cahen DL, et al. Acute Plasma Amylase Increase After Glucagon-Like Peptide -1 Receptor Agonist Exenatide Administration in Type 2 Diabetes. Diabetes Med (2017) 34:591–2. doi: 10.1111/dme.13160
67. Steinberg WM, Rosenstock J, Wadden TA, Donsmark M, Jensen CB, DeVries JH. Impact of Liraglutide on Amylase, Lipase, and Acute Pancreatitis in Participants With Overweight/Obesity and Normoglycemia, Prediabetes, or Type 2 Diabetes: Secondary Analyses of Pooled Data From the SCALE Clinical Development Progra. Diabetes Care (2017) 40:839–48. doi: 10.2337/dc16-2684
68. Steinberg WM, Buse JB, Ghorbani MLM, Ørsted DD, Nauck MA, LEADER Steering Committee, et al. Amylase, Lipase, and Acute Pancreatitis in People With Type 2 Diabetes Treated With Liraglutide: Results From the LEADER Randomized Tria. Diabetes Care (2017) 40:966–72. doi: 10.2337/dc16-2747
69. Smits MM, Tonneijck L, Muskiet MHA, Kramer MHH, Pieters-Van Den Bos IC, Vendrik KEW, et al. Pancreatic Effects of Liraglutide or Sitagliptin in Overweight Patients With Type 2 Diabetes: A 12-Week Randomized, Placebo-Controlled Trial. Diabetes Care (2017) 40:301–8. doi: 10.2337/dc16-0836
70. Nachnani JS, Bulchandani DG, Nookala A, Herndon B, Molteni A, Pandya P, et al. Biochemical and Histological Effects of Exendin-4 (Exenatide) on the Rat Pancreas. Diabetologia (2010) 53:153–9. doi: 10.1007/s00125-009-1515-4
71. Gier B, Matveyenko AV, Kirakossian D, Dawson D, Dry SM, Butler PC. Chronic GLP-1 Receptor Activation by Exendin-4 Induces Expansion of Pancreatic Duct Glands in Rats and Accelerates Formation of Dysplastic Lesions and Chronic Pancreatitis in the Kras(G12D) Mouse Model. Diabetes (2012) 61:1250–62. doi: 10.2337/db11-1109
72. Ellenbroek JH, Töns HAM, Westerouen van Meeteren MJA, de Graaf N, Hanegraaf MA, Rabelink TJ, et al. Glucagon-Like Peptide-1 Receptor Agonist Treatment Reduces Beta Cell Mass in Normoglycaemic Mice. Diabetologia (2013) 56:1980–6. doi: 10.1007/s00125-013-2957-2
73. Nyborg NCB, Mølck A-M, Madsen LW, Knudsen LB. The Human GLP-1 Analog Liraglutide and the Pancreas: Evidence for the Absence of Structural Pancreatic Changes in Three Species. Diabetes (2012) 61:1243–9. doi: 10.2337/db11-0936
74. Tatarkiewicz K, Belanger P, Gu G, Parkes D, Roy D. No Evidence of Drug-Induced Pancreatitis in Rats Treated With Exenatide for 13 Weeks. Diabetes Obes Metab (2013) 15:417–26. doi: 10.1111/dom.12040
75. Vrang N, Jelsing J, Simonsen L, Jensen AE, Thorup I, Søeborg H, et al. The Effects of 13 Wk of Liraglutide Treatment on Endocrine and Exocrine Pancreas in Male and Female ZDF Rats: A Quantitative and Qualitative Analysis Revealing No Evidence of Drug-Induced Pancreatitis. Am J Physiol Endocrinol Metab (2012) 303:E253–64. doi: 10.1152/ajpendo.00182.2012
76. Gotfredsen CF, Mølck A-M, Thorup I, Nyborg NCB, Salanti Z, Knudsen LB, et al. The Human GLP-1 Analogs Liraglutide and Semaglutide: Absence of Histopathological Effects on the Pancreas in Nonhuman Primate. Diabetes (2014) 63:2486–97. doi: 10.2337/db13-1087
77. Egan AG, Blind E, Dunder K, de Graeff PA, Hummer BT, Bourcier T, et al. Pancreatic Safety of Incretin-Based Drugs–FDA and EMA Assessment. N Engl J Med (2014) 370:794–7. doi: 10.1056/NEJMp1314078
78. Bjerre Knudsen L, Madsen LW, Andersen S, Almholt K, de Boer AS, Drucker DJ, et al. Glucagon-Like Peptide-1 Receptor Agonists Activate Rodent Thyroid C-Cells Causing Calcitonin Release and C-Cell Proliferation. Endocrinology (2010) 151:1473–86. doi: 10.1210/en.2009-1272
79. Gier B, Butler PC, Lai CK, Kirakossian D, DeNicola MM, Yeh MW. Glucagon Like Peptide-1 Receptor Expression in the Human Thyroid Glan. J Clin Endocrinol Metab (2012) 97:121–31. doi: 10.1210/jc.2011-2407
80. Körner M, Stöckli M, Waser B, Reubi JC. GLP-1 Receptor Expression in Human Tumors and Human Normal Tissues: Potential for In Vivo Targeting. J Nucl Med (2007) 48:736–43. doi: 10.2967/jnumed.106.038679
81. Pyke C, Knudsen LB. The Glucagon-Like Peptide-1 Receptor–or Not? Endocrinology (2013) 154:4–8. doi: 10.1210/en.2012-2124
82. Waser B, Blank A, Karamitopoulou E, Perren A, Reubi JC. Glucagon-Like-Peptide-1 Receptor Expression in Normal and Diseased Human Thyroid and Pancreas. Mod Pathol (2014) 28:391–402. doi: 10.1038/modpathol.2014.113
83. Hegedüs L, Sherman SI, Tuttle RM, von Scholten BJ, Rasmussen S, Karsbøl JD, et al. No Evidence of Increase in Calcitonin Concentrations or Development of C-Cell Malignancy in Response to Liraglutide for Up to 5 Years in the LEADER Tria. Diabetes Care (2018) 41:620–2. doi: 10.2337/dc17-1956
84. Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A Randomized, Controlled Trial of 3.0 Mg of Liraglutide in Weight Managemen. N Engl J Med (2015) 373:11–22. doi: 10.1056/NEJMoa1411892
85. Pizzimenti V, Giandalia A, Cucinotta D, Russo GT, Smits M, Cutroneo PM, et al. Incretin-Based Therapy and Acute Cholecystitis: A Review of Case Reports and EudraVigilance Spontaneous Adverse Drug Reaction Reporting Database. J Clin Pharm Ther (2016) 41:116–8. doi: 10.1111/jcpt.12373
86. Nreu B, Dicembrini I, Tinti F, Mannucci E, Monami M. Cholelithiasis in Patients Treated With Glucagon-Like Peptide-1 Receptor: An Updated Meta-Analysis of Randomized Controlled Trials. Diabetes Res Clin Pract (2020) 161:108087. doi: 10.1016/j.diabres.2020.108087
87. Committee for Medicinal Products for Human Use (CHMP). Assessment Report “Rybelsu” (EM/95374/2020) (2020). Available at: https://www.ema.europa.eu/en/documents/assessment-report/rybelsus-epar-public-assessment-report_en.pdf.
88. Nauck MA, Muus Ghorbani ML, Kreiner E, Saevereid HA, Buse JB. Effects of Liraglutide Compared With Placebo on Events of Acute Gallbladder or Biliary Disease in Patients With Type 2 Diabetes at High Risk for Cardiovascular Events in the LEADER Randomized Tria. Diabetes Care (2019) 42:1912–20. doi: 10.2337/dc19-0415
89. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabete. N Engl J Med (2019) 380:347–57. doi: 10.1056/NEJMoa1812389
90. Le Roux CW, Astrup AV, Fujioka K, Greenway FL, Lau DCW, Van Gaal L, et al. 3 Years of Liraglutide Versus Placebo for Type 2 Diabetes Risk Reduction and Weight Management in Individuals With Prediabetes: A Randomised, Double-Blind Trial. Lancet (2017) 389:1399–409. doi: 10.1016/S0140-6736(17)30069-7
91. Keller J, Trautmann ME, Haber H, Tham LS, Hunt T, Mace K, et al. Effect of Exenatide on Cholecystokinin-Induced Gallbladder Emptying in Fasting Healthy Subjects. Regul Pept (2012) 179:77–83. doi: 10.1016/j.regpep.2012.08.005
92. Shaddinger BC, Young MA, Billiard J, Collins DA, Hussaini A, Nino A. Effect of Albiglutide on Cholecystokinin-Induced Gallbladder Emptying in Healthy Individuals: A Randomized Crossover Stud. J Clin Pharmacol (2017) 57:1322–9. doi: 10.1002/jcph.940
93. Smits MM, Tonneijck L, Muskiet MHA, Hoekstra T, Kramer MHH, Diamant M, et al. Biliary Effects of Liraglutide and Sitagliptin, a 12-Week Randomized Placebo-Controlled Trial in Type 2 Diabetes Patients. Diabetes Obes Metab (2016) 18:1217–25. doi: 10.1111/dom.12748
94. Nexøe-Larsen CC, Sørensen PH, Hausner H, Agersnap M, Baekdal M, Brønden A, et al. Effects of Liraglutide on Gallbladder Emptying: A Randomized, Placebo-Controlled Trial in Adults With Overweight or Obesity. Diabetes Obes Metab (2018) 20:2557–64. doi: 10.1111/dom.13420
95. Marzioni M, Alpini G, Saccomanno S, Candelaresi C, Venter J, Rychlicki C, et al. Exendin-4, a Glucagon-Like Peptide 1 Receptor Agonist, Protects Cholangiocytes From Apoptosis. Gut (2009) 58:990–7. doi: 10.1136/gut.2008.150870
96. Marzioni M, Alpini G, Saccomanno S, Candelaresi C, Venter J, Rychlicki C, et al. Glucagon-Like Peptide-1 and its Receptor Agonist Exendin-4 Modulate Cholangiocyte Adaptive Response to Cholestasis. Gastroenterology (2007) 133:244–55. doi: 10.1053/j.gastro.2007.04.007
97. Seufert J, Nauck M, Rosenstock J, Hansen T, Vrazic H, Vilsboll T. P2857Increase in Pulse Rate With Semaglutide did Not Result in Increased Adverse Cardiac Events in Subjects With Type 2 Diabetes in the SUSTAIN 6 Cardiovascular Outcomes Trial. Eur Heart J (2018) 39(Supplement 1):39. doi: 10.1093/eurheartj/ehy565.P2857
98. Hozawa A, Ohkubo T, Kikuya M, Ugajin T, Yamaguchi J, Asayama K, et al. Prognostic Value of Home Heart Rate for Cardiovascular Mortality in the General Population: The Ohasama Study. Am J Hypertens (2004) 17:1005–10. doi: 10.1016/j.amjhyper.2004.06.019
99. van Bloemendaal L, ten Kulve JS, La Fleur SE, Ijzerman RG, Diamant M. Effects of Glucagon-Like Peptide 1 on Appetite and Body Weight: Focus on the CNS. J Endocrinol (2014) 221:T1–T16. doi: 10.1530/JOE-13-0414
100. ten Kulve JS, Veltman DJ, van Bloemendaal L, Barkhof F, Drent ML, Diamant M, et al. Liraglutide Reduces CNS Activation in Response to Visual Food Cues Only After Short-Term Treatment in Patients With Type 2 Diabete. Diabetes Care (2016) 39:214–21. doi: 10.2337/dc15-0772
101. Husain M, Bain SC, Jeppesen OK, Lingvay I, Sørrig R, Treppendahl MB, et al. Semaglutide (SUSTAIN and PIONEER) Reduces Cardiovascular Events in Type 2 Diabetes Across Varying Cardiovascular Risk. Diabetes Obes Metab (2020) 22:442–51. doi: 10.1111/dom.13955
102. Margulies KB, Hernandez AF, Redfield MM, Givertz MM, Oliveira GH, Cole R, et al. Effects of Liraglutide on Clinical Stability Among Patients With Advanced Heart Failure and Reduced Ejection Fractio. JAMA (2016) 316:500. doi: 10.1001/jama.2016.10260
103. Jorsal A, Kistorp C, Holmager P, Tougaard RS, Nielsen R, Hänselmann A, et al. Effect of Liraglutide, a Glucagon-Like Peptide-1 Analogue, on Left Ventricular Function in Stable Chronic Heart Failure Patients With and Without Diabetes (LIVE)-A Multicentre, Double-Blind, Randomised, Placebo-Controlled Trial. Eur J Heart Fail (2017) 19:69–77. doi: 10.1002/ejhf.657
104. Ghosh-Swaby OR, Goodman SG, Leiter LA, Cheng A, Connelly KA, Fitchett D, et al. Glucose-Lowering Drugs or Strategies, Atherosclerotic Cardiovascular Events, and Heart Failure in People With or at Risk of Type 2 Diabetes: An Updated Systematic Review and Meta-Analysis of Randomised Cardiovascular Outcome Trials. Lancet Diabetes Endocrinol (2020) 8:418–35. doi: 10.1016/S2213-8587(20)30038-3
105. Mendis B, Simpson E, MacDonald I, Mansell P. Investigation of the Haemodynamic Effects of Exenatide in Healthy Male Subjects. Br J Clin Pharmacol (2012) 74:437–44. doi: 10.1111/j.1365-2125.2012.04214.x
106. Smits MM, Tonneijck L, Muskiet MHA, Hoekstra T, Kramer MHH, Diamant M, et al. Heart Rate Acceleration With GLP-1 Receptor Agonists in Type 2 Diabetes Patients: An Acute and 12-Week Randomised, Double-Blind, Placebo-Controlled Trial. Eur J Endocrinol (2017) 176:77–86. doi: 10.1530/EJE-16-0507
107. Smits MM, Muskiet MHA, Tonneijck L, Hoekstra T, Kramer MHH, Diamant M, et al. Exenatide Acutely Increases Heart Rate in Parallel With Augmented Sympathetic Nervous System Activation in Healthy Overweight Males. Br J Clin Pharmacol (2016) 81:613–20. doi: 10.1111/bcp.12843
108. Cacciatori V, Zoppini G, Bellavere F, Rigolon R, Thomaseth K, Pichiri I, et al. Long-Acting GLP-1 Receptor Agonist Exenatide Influence on the Autonomic Cardiac Sympatho-Vagal Balanc. J Endocr Soc (2018) 2:53–62. doi: 10.1210/js.2017-00300
109. Kumarathurai P, Anholm C, Larsen BS, Olsen RH, Madsbad S, Kristiansen O, et al. Effects of Liraglutide on Heart Rate and Heart Rate Variability: A Randomized, Double-Blind, Placebo-Controlled Crossover Stud. Diabetes Care (2017) 40:117–24. doi: 10.2337/dc16-1580
110. Bharucha AE, Charkoudian N, Andrews CN, Camilleri M, Sletten D, Zinsmeister AR, et al. Effects of Glucagon-Like Peptide-1, Yohimbine, and Nitrergic Modulation on Sympathetic and Parasympathetic Activity in Humans. Am J Physiol Regul Integr Comp Physiol (2008) 295:R874–80. doi: 10.1152/ajpregu.00153.2008
111. Nakatani Y, Kawabe A, Matsumura M, Aso Y, Yasu T, Banba N, et al. Effects of GLP-1 Receptor Agonists on Heart Rate and the Autonomic Nervous System Using Holter Electrocardiography and Power Spectrum Analysis of Heart Rate Variabilit. Diabetes Care (2016) 39:e22–3. doi: 10.2337/dc15-1437
112. Baggio LL, Ussher JR, McLean BA, Cao X, Kabir MG, Mulvihill EE, et al. The Autonomic Nervous System and Cardiac GLP-1 Receptors Control Heart Rate in Mice. Mol Metab (2017) 6:1339–49. doi: 10.1016/j.molmet.2017.08.010
113. Demmel V, Sandberg-Schaal A, Jacobsen JB, Golor G, Pettersson J, Flint A. No QTc Prolongation With Semaglutide: A Thorough QT Study in Healthy Subject. Diabetes Ther (2018) 9:1441–56. doi: 10.1007/s13300-018-0442-0
114. López-Ruiz A, del Peso-Gilsanz C, Meoro-Avilés A, Soriano-Palao J, Andreu A, Cabezuelo J, et al. Acute Renal Failure When Exenatide is Co-Administered With Diuretics and Angiotensin II Blockers. Pharm World Sci (2010) 32:559–61. doi: 10.1007/s11096-010-9423-8
115. Winzeler B, da Conceição I, Refardt J, Sailer CO, Dutilh G, Christ-Crain M. Effects of Glucagon-Like Peptide-1 Receptor Agonists on Fluid Intake in Healthy Volunteers. Endocrine (2020) 70:292–8. doi: 10.1007/s12020-020-02394-2
116. Lovshin JA, Barnie A, DeAlmeida A, Logan A, Zinman B, Drucker DJ. Liraglutide Promotes Natriuresis But Does Not Increase Circulating Levels of Atrial Natriuretic Peptide in Hypertensive Subjects With Type 2 Diabetes. Diabetes Care (2015) 38:132–9. doi: 10.2337/dc14-1958
117. Muskiet MHA, Tonneijck L, Smits MM, van Baar MJB, Kramer MHH, Hoorn EJ, et al. GLP-1 and the Kidney: From Physiology to Pharmacology and Outcomes in Diabetes. Nat Rev Nephrol (2017) 13:605–28. doi: 10.1038/nrneph.2017.123
118. Mosterd CM, Bjornstad P, van Raalte DH. Nephroprotective Effects of GLP-1 Receptor Agonists: Where do We Stand? J Nephrol (2020) 33:965–75. doi: 10.1007/s40620-020-00738-9
119. Leiter LA, Bain SC, Bhatt DL, Buse JB, Mazer CD, Pratley RE, et al. The Effect of Glucagon-Like Peptide-1 Receptor Agonists Liraglutide and Semaglutide on Cardiovascular and Renal Outcomes Across Baseline Blood Pressure Categories: Analysis of the LEADER and SUSTAIN 6 Trials. Diabetes Obes Metab (2020) 22:1690–5. doi: 10.1111/dom.14079
120. Tsapas A, Avgerinos I, Karagiannis T, Malandris K, Manolopoulos A, Andreadis P, et al. Comparative Effectiveness of Glucose-Lowering Drugs for Type 2 Diabete. Ann Intern Med (2020) 173:278–86. doi: 10.7326/M20-0864
121. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JFEE, Nauck MA, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med (2016) 375:311–22. doi: 10.1056/NEJMoa1603827
122. Vilsbøll T, Bain SC, Leiter LA, Lingvay I, Matthews D, Simó R, et al. Semaglutide, Reduction in Glycated Haemoglobin and the Risk of Diabetic Retinopathy. Diabetes Obes Metab (2018) 20:889–97. doi: 10.1111/dom.13172
123. UK Prospective Diabetes Study Group. Intensive Blood-Glucose Control With Sulphonylureas or Insulin Compared With Conventional Treatment and Risk of Complications in Patients With Type 2 Diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Grou. Lancet (1998) 352:837–53. doi: 10.1016/S0140-6736(98)07019-6
124. The Diabetes Control and Complications Trial Research Group. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N Engl J Med (1993) 329:977–86. doi: 10.1056/NEJM199309303291401
125. Gorman DM, le Roux CW, Docherty NG. The Effect of Bariatric Surgery on Diabetic Retinopathy: Good, Bad, or Bot? Diabetes Metab J (2016) 40:354. doi: 10.4093/dmj.2016.40.5.354
126. The Kroc Collaborative Study Group. Blood Glucose Control and the Evolution of Diabetic Retinopathy and Albuminuria. N Engl J Med (1984) 311:365–72. doi: 10.1056/NEJM198408093110604
127. Lauritzen T, Frost-Larsen K, Larsen H-W, Deckert T. Two-Year Experience With Continuous Subcutaneous Insulin Infusion in Relation to Retinopathy and Neuropathy. Diabetes (1985) 34:74–9. doi: 10.2337/diab.34.3.S74
128. Dahl-Jørgensen K. Near-Normoglycemia and Late Diabetic Complications. The Oslo Study. Acta Endocrinol Suppl (Copenh) (1987) 284:1–38. doi: 10.1530/acta.0.115S007
129. Gaborit B, Julla J-B, Besbes S, Proust M, Vincentelli C, Alos B, et al. Glucagon-Like Peptide 1 Receptor Agonists, Diabetic Retinopathy and Angiogenesis: The AngioSafe Type 2 Diabetes Study. J Clin Endocrinol Metab (2020) 105:e1549–60. doi: 10.1210/clinem/dgz069
130. FDA Briefing Document, Endocrinologic and Metabolic Drugs Advisory Committee Meeting (EMDA) (2017). Available at: https://www.fda.gov/media/108291/download.
131. Committee for Medicinal Products for Human Use (CHM). In: . Assessment Report “Rybelsus” (EM/95374/2020). Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/rybelsus
132. Witkowski M, Wilkinson L, Webb N, Weids A, Glah D, Vrazic H. A Systematic Literature Review and Network Meta-Analysis Comparing Once-Weekly Semaglutide With Other GLP-1 Receptor Agonists in Patients With Type 2 Diabetes Previously Receiving Basal Insuli. Diabetes Ther (2018) 9:1233–51. doi: 10.1007/s13300-018-0428-y
Keywords: glucagon-like peptide-1 receptor agonist (GLP-1RA), oral, subcutaneous, semaglutide, type 2 diabetes, safety
Citation: Smits MM and Van Raalte DH (2021) Safety of Semaglutide. Front. Endocrinol. 12:645563. doi: 10.3389/fendo.2021.645563
Received: 23 December 2020; Accepted: 19 April 2021;
Published: 07 July 2021.
Edited by:
Francesco Giorgino, University of Bari Aldo Moro, ItalyReviewed by:
Bo Ahrén, Lund University, SwedenAsimina Mitrakou-Fanariotou, National and Kapodistrian University of Athens, Greece
Burkhard J. Göke, Medical School Hamburg, Germany
Copyright © 2021 Smits and Van Raalte. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniël H. Van Raalte, ZC52YW5yYWFsdGVAYW1zdGVyZGFtdW1jLm5s
 Mark M. Smits
Mark M. Smits Daniël H. Van Raalte
Daniël H. Van Raalte